Abstract
Ever since its initial characterization in the 19th century, tobacco mosaic virus (TMV) has played a prominent role in the development of modern virology and molecular biology. In particular, research on the three-dimensional structure of the virus particles and the mechanism by which these assemble from their constituent protein and RNA components has made TMV a paradigm for our current view of the morphogenesis of self-assembling structures, including viral particles. More recently, this knowledge has been applied to the development of novel reagents and structures for applications in biomedicine and bionanotechnology. In this article, we review how fundamental science has led to TMV being at the vanguard of these new technologies.
Keywords: Self-assembly, Coat protein, Nanostars, Nanorings, Origin of assembly, Peptide presentation, Chemical modification
1. Introduction
Tobacco mosaic virus (TMV) has been an object of intense scientific study for more than a century and was the first virus to be so-named (Beijerinck, 1898). The fact that it could be propagated and purified in large quantities led to TMV being at the forefront of developments in modern virology and molecular biology (recently reviewed by Lomonossoff, 2018). As a result of these fundamental studies, by the 1980s there was a huge amount of data available concerning both the molecular genetics of the virus and the structure of its particles. This led to a burgeoning interest in the use of TMV, and its constituent parts, in bio- and nanotechnology. Such uses have included: the identification and development of translational enhancers for the enhanced expression of heterologous genes (Gallie et al., 1987a; Wilson, 1989), the development of efficient vectors for transient expression and virus-induced gene silencing (VIGS) in plants (reviewed by Peyret and Lomonossoff, 2015), and the use of virus-derived sequences for the creation of virus-resistant lines of plants (Golemboski et al., 1990; Powell Abel et al., 1986). Consideration of all these aspects would require an extremely long article, possibly even a book. Thus, this review will concentrate on the contributions that TMV particles, and their assembly properties, have made to bionanotechnology.
2. Why Are TMV Particles So Suited to Applications in Bionanotechnology?
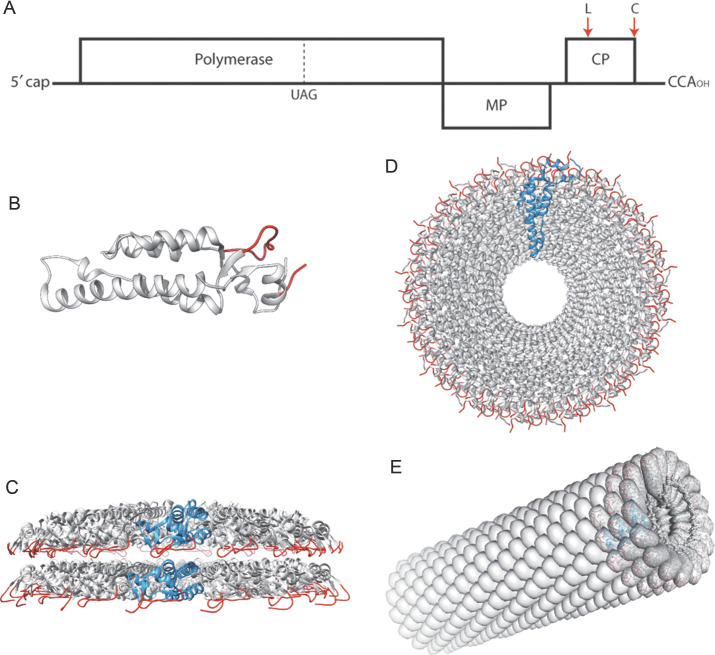
In addition to the ease of its propagation and purification, the sheer simplicity of its genome and particle structure has made TMV a highly tractable experimental system for a number of applications. The genome of TMV consists of one molecule of single-stranded, positive-sense RNA of 6395 nucleotides that contains only three open reading frames (ORFs; Goelet et al., 1982; Fig. 1A; Note: All the numbers quoted here and below refer to the U1 or vulgare strain of TMV unless otherwise specified). The 5′-proximal ORF encodes the 126 and 183 kDa proteins, the longer of which is produced via read-through of a leaky UAG stop codon at the end of the 126 kDa coding sequence; both these proteins are translated from the genomic RNA and are involved in the replication of the viral RNA (Young et al., 1987; Fig. 1A). To the 3′ side of this ORF lie the regions encoding the 30 kDa viral movement protein (MP) and the 17.5 kDa coat protein (CP); both the MP and CP are translated from subgenomic mRNAs (Beachy and Zaitlin, 1977; Hunter et al., 1976).
Fig. 1.
TMV—the basics. (A) Diagram of the genomic RNA showing the three open reading frames (ORFs) for the polymerase, movement protein (MP), and coat protein (CP). The position of the leaky UAG codon within the polymerase ORF is indicated. The sites for insertion into a surface loop (L) and the C-terminus (C) are shown by red arrows above the CP ORF. (B) Tertiary structure of an isolated CP subunit. (C) Structure of the two-layer disk as seen in the crystal structure of the four-layer aggregate described by Bhyravbhatla et al. (1998). (D) View down the assembled nucleoprotein helix. (E) View along the TMV rod. In each case the exposed surface loop and C-terminus of the CP are indicated in red.
The genomic RNA is encapsidated by approximately 2130 copies of the CP to give virus particles with helical symmetry. The particles are hollow cylinders 300 nm in length with external and internal diameters of 18 and 4 nm, respectively. The three-dimensional (3D) structures of both the assembled particles and the CP subunits have been subjected to detailed analysis over many years by electron microscopy, fiber diffraction, and X-ray crystallography. These have resulted in an atomic-resolution picture of the viral particles and the protein–protein and protein–RNA interactions that hold them together (Bloomer et al., 1978; Champness et al., 1976; Namba et al., 1989; Stubbs et al., 1977). Recently, TMV single-particle 3D reconstructions to 3.2 Å resolution have been achieved by cryo-electron microscopy (cryo-EM) using direct electron detectors confirming that fiber diffraction and cryo-EM yield equivalent data (Fromm et al., 2015).
The CP comprises 158 amino acids and is wedge shaped with the wider end at the outer radius of the virus particle (Fig. 1B). Each subunit comprises four α-helices with both the N- and C-termini lying on the external surface of the particle. At pHs above neutrality, the isolated CP assembles into a variety of two-layer structures, including a disk aggregate that has more recently been referred to as a “nanoring” (Bhyravbhatla et al., 1998; Bloomer et al., 1978; Durham et al., 1971; Fig. 1C). Within the fully assembled particles, the subunits form a right-handed helix with a pitch of 2.3 nm with 16⅓ subunits per turn, resulting in an average distance of about 2.5 nm between the N- or C-termini, respectively, of adjacent CPs (Fig. 1D and E). The RNA lies at a radius of 4 nm and three nucleotides are associated with each CP subunit (Franklin, 1956). The purified CP forms helical rods in the absence of RNA at pH less than 6.0. These rods have an identical structure to the virus but are of indeterminate length, suggesting that the RNA effectively acts as a ruler that determines the particle length.
In addition to the detailed structural information about the genome and the virus particles, there is another feature about TMV that has made the virus of great interest for potential applications in bionanotechnology: the availability of a self-assembly system that can operate both in vivo and in vitro. Ever since the discovery that purified CP and RNA can self-assemble to produce infectious virus particles in vitro (Fraenkel-Conrat and Williams, 1955), TMV has become a paradigm for RNA–protein recognition. In the 1970s it was shown that assembly is initiated at a single “origin of assembly sequence” (OAS) positioned approximately 1 kb from the 3′ end of the genomic RNA (Zimmern, 1977; Zimmern and Butler, 1977; Zimmern and Wilson, 1976). Initiation of the process requires that the CP be in the form of a subassembly, consisting of a two-layer disk aggregate, rather than individual subunits or the helical aggregates that the CP can form under acidic conditions (Butler and Klug, 1971); the disks, containing 34 CP subunits (Fig. 1C), interact with a hairpin structure formed by the OAS. Assembly then proceeds bidirectionally (Butler et al., 1977), with assembly toward the 5′ end of the RNA being considerably faster than that toward the 3′ end. This is due to an asymmetric mode of particle growth: further CP disks or smaller two-layer aggregates are added at the 5′-protruding traveling loop of the viral RNA (Fig. 2 ), pulling the 5′ end the RNA through the central channel of the nascent particle. Assembly toward the 3′ end of the RNA occurs through the addition of smaller CP oligomers, and this portion of the RNA remains accessible until assembly is complete. For detailed reviews of these seminal studies on TMV self-assembly, the reader is referred to Butler, 1984, Butler, 1999 and Lomonossoff and Wilson (1985). In addition to controlled assembly of TMV rods, it is also possible to sequentially remove protein subunits to expose the 5′ end of the RNA within rods by treatment at alkaline pH (Perham and Wilson, 1978). The processes of controlled nucleoprotein assembly and disassembly have been exploited to generate TMV-derived nanostructures and hybrid nanoobjects as discussed later (Fig. 2).
Fig. 2.
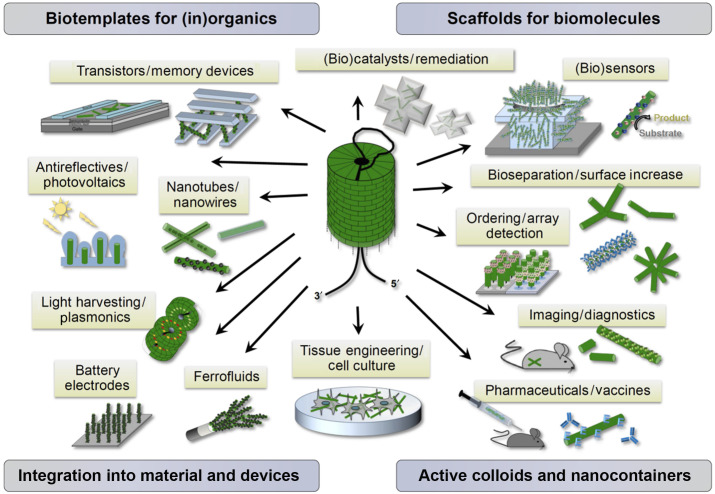
Examples of applications of TMV particles. The image in the center shows a TMV rod assembling via the traveling loop mechanism. The various applications are described throughout the text. Reproduced under the terms of the Creative Commons Attribution 2.0 International Public License from Koch et al. (2016).
3. Use of Particles Produced via Infection
Since TMV is mechanically transmissible, it is very easy to produce large quantities of wild-type particles by simply inoculating plants with sap from infected plants or with purified virus. However, the advent of reverse genetic systems for the manipulation of the TMV genome (Dawson et al., 1986; Meshi et al., 1986) opened up the possibility of genetically manipulating the CP sequence. Subsequent advances in the technology for efficiently initiating infections, such as the use of agroinfiltration (Lindbo, 2007; Marillonnet et al., 2004) or the use of plant-infectious plasmids, in which the viral cDNA is cloned between a strong promoter and a self-cleaving ribozyme to trim the 3′-terminus of the in vivo-transcribed RNA (Bittner et al., 2013; Kadri et al., 2011), mean that it is now relatively straightforward to produce genetically modified particles in plants. Furthermore, developments in the field of chemistry have opened up possibilities for the chemical modification of both wild-type and genetically altered particles (Strable and Finn, 2009; Wen and Steinmetz, 2016). These developments are outlined in Fig. 2.
3.1. Peptide Display
Their highly repetitive structure and size, coupled with detailed knowledge of their 3D structure, make TMV particles attractive candidates for the display of multiple copies of peptides. To avoid disrupting CP intersubunit contacts and thereby interfering with particle formation, it is essential that any inserted peptides be presented on a solvent-exposed surface; this should also ensure an effective interaction with the immune system if the displayed peptides are intended to provoke an immune response. The most commonly used position for the fusion of peptides to the CP is the C-terminus (Fig. 1), which lies on the outer surface of assembled virions. Though the N-terminus is also surface-exposed, attempts at fusing peptides to this position have generally prevented particle formation, probably because of the proximity of amino acids, such as the buried invariant tyrosine at position 2, critical for particle formation (Altschuh et al., 1987).
An early report of the use TMV particles to present peptides involved the CP expressed in Escherichia coli (Haynes et al., 1986; discussed in Section 4). The first attempt to produce TMV particles containing a modified CP in plants was reported by Takamatsu et al. (1990). A sequence encoding Leu-enkephalin was fused to the C-terminus of the viral CP in an infectious cDNA clone of the virus. Inoculation of tobacco plants gave only local lesions, and no particles could be purified despite the efficient synthesis of the modified CP. This was the first indication of a significant problem with using TMV particles to display foreign peptides. Although the large number of subunits within a virus particle makes TMV very attractive as a potential peptide display system, it has the drawback that the subunits are very tightly packed, allowing little space on the virus surface for the accommodation of multiple copies of a heterologous peptide. In addition, mutations near the C-terminus adversely affect the ability of CP subunits to oligomerize (Li et al., 2013), thereby compromising assembly. Nonetheless, the successful formation of particles was later reported when an 11-amino acid epitope from VP1 of foot and mouth disease virus (FMDV) was fused to the extreme C-terminus of the CP (Wu et al., 2003). The purified particles could protect a number of target animals, including pigs, against challenge with FMDV. Hexahistidine tags were also shown to be tolerated as 3′-terminal extensions to all TMV CPs but substantially reduced particle formation (Mueller et al., 2010).
An initial approach to solving the problem of steric crowding was the creation of mosaic particles containing a mixture of wild-type and modified subunits. This was achieved by incorporating a leaky termination codon at the C-terminus of the CP gene and placing the sequence of the target, antigenic peptides immediately downstream (Hamamoto et al., 1993; Sugiyama et al., 1995; Turpen et al., 1995). This resulted in the production of particles in which up to 5% of the subunits were fusion proteins containing the heterologous peptide at their C-termini. However, in each case the presented peptide was quite short (less than 20 amino acids). A conceptually similar, but mechanistically different, approach to creating mosaic particles was adopted by Röder et al. (2017) to express longer peptides. In this case the sequence of the 113-amino acid fluorescent protein iLOV was fused to the C-terminus of the CP via a 16-amino acid 2A “ribosomal skip” peptide from FMDV (Donnelly et al., 2001). Particles isolated from plants infected with this construct contained iLOV fusion subunits, suggesting this approach could be used to express whole proteins on the surface of TMV particles.
The disadvantage of the mosaic approach is that it is difficult to control the number and spatial distribution of modified subunits within the particles. Thus, several alternative approaches for peptide insertion into the CP have been developed to produce particles in which all the CP subunits are modified while retaining virus viability. Turpen et al. (1995) investigated the use of a surface-exposed loop, downstream of proline 63, between two of the α-helices (Fig. 1A and B), to express malarial epitopes on the virus surface, and demonstrated particle formation. A similar site was later used to express a 10-amino acid metal-binding peptide that catalyzed the deposition of gold on the surface of purified particles (Love et al., 2015). Using a TMV vector that allowed peptides to be inserted just upstream of the C-terminus of the CP (after amino acid 154), Fitchen et al. (1995) expressed a 13-amino acid peptide from the glycoprotein ZP3 from the murine zona pellucida. A detailed analysis of the size and charge of peptides that could be successfully displayed at this site (Bendahmane et al., 1999) showed that sequences of up to 23 amino acids were tolerated provided their isoelectric point was below 7.0. Using this information, Koo et al. (1999) displayed peptides of either 10 or 15 amino acids from the spike protein of the coronavirus murine hepatitis virus on the surface of assembled particles. When purified virions were used to immunize mice, animals with high antibody titers were protected from subsequent challenge with infectious murine hepatitis virus. This was a highly significant result as it showed, for the first time, that TMV particles expressing a foreign epitope could act as effective candidate vaccines. It also cemented the idea that modified plant virus particles can act as candidate vaccines as originally demonstrated with cowpea mosaic virus (Dalsgaard et al., 1997). Subsequently, Staczek et al. (2000) demonstrated protection using an epitope from the outer membrane protein F of Pseudomonas aeruginosa expressed at the same site in the virus particles, and, more recently, Petukhova et al. (2013) were able to demonstrate protective immunity in mice using TMV particles displaying the influenza virus M2e epitope at a site near the C-terminus of the CP.
Despite these successes, it is reasonable to conclude that the direct fusion of peptides at or near the C-terminus, or at an exposed loop within it, imposes severe limitations on the size of sequence that can be displayed on assembled particles, probably a consequence of the close-packed nature of the subunits. This sparked the development of a number of approaches to increasing the size of peptides that can be presented by modifying the C-terminus of the CP. By deleting four to six amino acids from the C-terminus amino acids of the CP, Jiang et al. (2006) were able to present a peptide of 25 amino acids from FMDV VP1 on the virus surface—a modest increase over the previously reported maximum. An alternative approach has been to insert linker sequences between the C-terminus and the sequence to be presented. By incorporating a 15-amino acid linker, Werner et al. (2006) were able to display a 133-amino acid segment of protein A, on the surface of particles of a close relative of TMV, the tobamovirus turnip vein clearing virus—a dramatic increase over what had been reported previously. The protein A fragment could bind IgG indicating that the modified virus particles could potentially be used to purify antibodies. Using a similar linker strategy, Frolova et al. (2010) were able to display trastuzumab-binding peptides of HER2/neu on the surface of virus particles and showed that the peptides retained their trastuzumab-binding capacity.
The development of TMV-based systems for the display of increasingly large peptides has led to a number of detailed immunological studies and the commercial development of the technology. These aspects are beyond the scope of this review and the reader is referred to articles by McCormick and Palmer (2008), Smith et al. (2009), Lee et al. (2016), and Steele et al. (2017) for a detailed discussion of progress in these areas.
3.2. Chemical Modification of Particles
3.2.1. External Surface
The outer surface of natural TMV particles has relatively few chemically reactive amino acids such as cysteine and lysine, making the chemical functionalization of such particles problematic. However, Schlick et al. (2005) reported the derivatization of exterior-exposed tyrosine residues with diazonium salts, resulting in a conjugate that could react with a wide range of other molecules including polyethylene glycol derivatives.
To overcome the lack of reactive amino acid chains on the virus surface, several mutants displaying reactive cysteine or lysine residues on the solvent-exposed exterior of the virus have been made, allowing decoration via thiol- or amine-selective chemistry (Demir and Stowell, 2002; Geiger et al., 2013; Yi et al., 2005, Yi et al., 2007). However, in several cases the presence of these added residues adversely affected virus yield. To address this, Smith et al. (2006) screened a collection of random TMV mutants that had additional amino acids, including a single lysine, inserted near the N-terminus of the CP. By selecting those mutants that grew well, the authors were able to identify a mutant suitable for the chemical coupling of a variety of peptides (McCormick and Palmer, 2008).
The external surface of TMV has also been used as a template for a diversity of chemical deposition reactions. These include the cocrystallization of CdS and PbS, oxidative hydrolysis yielding iron oxides, condensation of SiO2, and (NH4)2CO3 decomposition producing electrocatalytically active Co3O4 (Fowler et al., 2001; Schenk et al., 2017; Shenton et al., 1999). Such deposition is often carried out using particles displaying mineralization-directing peptides either genetically or chemically attached to the particle surface (Altintoprak et al., 2015; Love et al., 2015). In some cases, striking mesostructured superassemblies such as composite threads, nanoparticles with radial channels, and hierarchically arranged microtubes were obtained (Fowler et al., 2001; Schenk et al., 2017). In fact, the use of plant virus particles, including TMV (Douglas and Young, 1999; Shenton et al., 1999) as mineralization-guiding agents, may be regarded the dawn of “structural TMV nanotechnology” (Fig. 2).
The deposition approach has included the creation of metal nanoparticles and nanotubes (Dujardin et al., 2003; Knez et al., 2002, Knez et al., 2003, Knez et al., 2004b). Such tubes can be grown from deposited clusters of palladium, platinum, and gold on the exterior TMV surface and result in a metallic coat on the virion that serves as a basal layer for electroless plating of other metals including nickel and cobalt. For example, Royston et al. (2008) reported the deposition of nickel and cobalt on the exterior surface of the virus to create metallic coatings up to 40 nm in thickness. When the nickel-coated virions were incorporated into a nickel–zinc battery system, the electrode capacity of the battery more than doubled due to the large increase of its active surface area through the TMV-derived nanostructures. Similarly, TMV nanorods immobilized on semiconducting electrodes and coated with nickel from solution, and subsequently with indium–tin oxide and copper oxide layers by sputtering and annealing, yielded excellently performing photoelectrochemical cells. The performance of these structures also profited from the antireflective properties of the 3D nanorod structures (Chiang et al., 2012). Hydrogen generation in these water-splitting solar cells resulted in higher photocurrent densities than reported previously for any other system based on similarly sized copper oxide structures.
3.2.2. Modification of the Internal Channel
TMV particles are hollow cylinders with an internal diameter of 4 nm (Fig. 1E). The interior channel is lined with aspartic and glutamic acid residues and these have been labeled with a variety of small molecules, such as biotin, using carbodiimide coupling reactions (Schlick et al., 2005). Nanowires consisting of bimetallic alloys of CoPt, CoPt3, and FePt3 with lengths up to 100 and 4 nm diameter have been synthesized within the TMV capsid channel (Tsukamoto et al., 2007), and the formation of small isolated nanoparticles of silver and nickel within the channel has also been reported (Dujardin et al., 2003). Taken together, these examples demonstrate effective templating of inorganic solid and tubular structures by TMV, and the process has now reached the state of being competitive with conventional production processes for several applications (Fig. 2). These range from conductive to electronically, plasmonically, and catalytically (Manocchi et al., 2010) active parts (for reviews, see Culver et al., 2015; Fan et al., 2013).
Because of their size and biocompatibility, for the past decade or so plant virus particles have been considered as promising drug delivery vehicles, particularly in relation to anticancer drugs (Franzen and Lommel, 2009). Much of the research in this area has focussed on spherical viruses since the capsid subunits form closed shells (Czapar and Steinmetz, 2017; Wen and Steinmetz, 2016); by contrast, the open-ended tubular particles are less obvious candidates. However, Czapar et al. (2016) made use of the density of negative charges lining the inner channel to load TMV particles with a potent platinum-based anticancer agent, phenanthriplatin (PhenPt). When TMV rods were incubated with a cationic form of the drug, approximately 2000 PhenPt2 + cations were incorporated, consistent with the idea that binding occurs through ionic interaction with the carboxylate groups lining the inner channel of the virus particles. The drug could be released by lowering the pH of the medium. Subsequent work using TMV particles loaded with a related drug, cisplatin, showed that TMV particles are an efficient way of delivering anticancer therapeutics (Franke et al., 2017).
4. Production of Virus Particles In Vivo in the Absence of Infection
Although infection of plants can lead to the production of very high levels of assembled particles, this approach has some limitations. For example, the length of the genomic RNA inevitably governs the length of the particles. Although the genomic RNA can be modified to a certain extent by inserting or deleting viral sequences, the range of variation is quite limited if infectivity is to be retained. Additional issues include the very infectious nature of the particles meaning that some degree of containment is required during their production and the fact modifications to the CP can make virions difficult to purify. For these reasons, there have been many attempts to produce virus particles in a variety of expression systems in the absence of infection.
4.1. Expression in Bacteria
An early attempt to exploit TMV particles as a platform for the display of foreign peptides involved expressing a version of the CP, modified to carry an eight-amino acid poliovirus epitope at its C-terminus, in E. coli (Haynes et al., 1986). Particles were formed and purified after acidification of bacterial extracts expressing soluble CP. The polymerized protein-only rods were able to elicit the production of higher titers of neutralizing antibodies in rats compared to the same modified CP that had been disaggregated by treatment at pH 8.0, indicating the importance of a repetitive structure for the stimulation of a strong immune reaction. The authors also stated that the bacterially expressed protein was capable of assembly with added TMV RNA in vitro, though no data were provided.
Expression of TMV CP in E. coli was initially considered to be a highly promising method of producing variants that could subsequently be used to generate particles with varying properties. However, Shire et al. (1990) found that wild-type TMV CP produced in E. coli was unable to assemble with TMV RNA in vitro, a problem also encountered when expression of modified forms of CP was attempted (Bruckman et al., 2011; Wnek et al., 2013). The alternative approach of coexpressing the CP and RNA molecules containing the OAS within bacteria or yeast cells was only partially successful, with only a small number of virus-like particles or correctly formed rods being obtained (Hwang et al., 1994; Kadri et al., 2013). The aberrant assembly properties of CP expressed in bacteria have been ascribed to the lack of acetylation of the N-terminal serine, which prevents the formation of the disk structures necessary to initiate the process (Shire et al., 1990; Wnek et al., 2013). This problem could be alleviated by spiking the E. coli-produced protein with a minimum of 20% of plant-made TMV CP, an approach that enabled efficient RNA-guided assembly of TMV-CPHis6 into particles of the expected length (Eiben et al., 2014). This approach, akin to the production of mosaic particles in plants, provides a further option for tailoring the properties and addressability of TMV-like particles assembled in vitro.
Bacterial production has been successfully used to produce a number of CP variants. For example, expression of mutant T103C allowed the formation of disulfide bonds between the exposed loops of adjacent subunits in the central channel of assembled TMV-like nanotubes (Zhou et al., 2013). This expanded the spectrum of metal fabrication within the channel to include gold nanobeads and -rods (Zhou et al., 2015). Likewise, Finbloom et al. (2016) produced a mutant CP in E. coli in which two lysine residues (positions 53 and 68) were mutated to arginines. The modified CP was able to form stable disk structures that were subsequently modified for drug delivery purposes.
4.2. Expression in Plants
The first attempt to produce the TMV CP in plants involved the transformation of tobacco plants with a copy of the CP, with expression being driven by the cauliflower mosaic virus 35S promoter (Bevan et al., 1985). Although CP was detected immunologically, no attempt was made to assess its aggregation state. It soon transpired that tobacco plants transgenic for the TMV CP were resistant to subsequent challenge with virus (Powell Abel et al., 1986), and the concept of “coat protein-mediated resistance” was born (Beachy, 1999). As part of their studies on the mechanism of CP-mediated resistance, Asurmendi et al. (2007) examined the aggregation state of the TMV CP in transgenic tobacco and showed that the subunits formed higher aggregates, suggesting that they are assembly competent. However, the levels of CP produced in transgenic plants were too low for this system to be considered a practical method of CP production.
To increase the levels of TMV CP in plants, Saunders and Lomonossoff (2017) used transient expression of the CP coding sequence in Nicotiana benthamiana. No detectable material accumulated when the CP was expressed alone, indicating that assembly is crucial for CP accumulation in plants. However, when the CP was expressed in the presence of RNA molecules containing the OAS, virus-like rods readily formed, the length of which was controlled by the length of the RNA. Furthermore, it was possible to fuse a nine-amino acid cobalt–platinum (CoPt)-binding peptide to the C-terminus of the CP without abolishing particle assembly in planta. CoPt could be deposited on the surface of particles harboring the peptide but not on particles containing wild-type CP (Saunders and Lomonossoff, 2017). Thus, plant-based expression may be an effective approach to the creation of defined-length conducting nanowires.
4.3. Other Expression Systems
Mueller et al. (2011) demonstrated that it is possible to express both wild-type and variant versions of TMV CP in the yeast, Schizosaccharomyces pombe, and the yeast-expressed CP retained its ability to assemble with TMV RNA in vitro. Subsequently, Kadri et al. (2013) demonstrated the ability of the yeast-expressed material to assemble with coexpressed OAS-containing RNA similarly to CP coexpressed with RNA in E. coli. The results suggested that the yeast-expressed CP was more similar to that expressed in plants than was CP expressed in bacteria, probably as a result of the correct acetylation of the N-terminus.
Experiments designed to develop an efficient TMV-based VIGS system for the phytopathogenic fungus, Colletotrichum acutatum, incidentally demonstrated efficient assembly of particles within the hyphal cells (Mascia et al., 2014). These results suggest that it is likely that assembly competent TMV CP can be generated in most eukaryotic expression systems. Indeed, work on the identification of the TMV CP subgenomic mRNA showed that CP produced via in vitro translation in wheat germ extracts could assemble with exogenously added TMV RNA (Hunter et al., 1976).
5. In Vitro Assembly
Following the detailed characterization of the OAS, it was shown that it is possible to efficiently encapsidate essentially any RNA molecule provided it contains the OAS (Gallie et al., 1987b; Sleat et al., 1986; Turner et al., 1989). The initial experiments involved the use of wild-type CP purified from TMV particles produced by infection although they also demonstrated that RNA containing an OAS transcribed from a transgene could assemble with CP produced via TMV infection (Sleat et al., 1988).
Jupin et al. (1989) proposed that the ability to package transcripts containing the OAS might provide a means of protecting labile RNA molecules during shipping. A similar protective role of the CP has been used in the development of positive controls for PCR-based diagnosis of Ebola infections (Lam et al., 2016). In vitro assembled rods also have been shown to be an effective method of delivering specific transcripts since they uncoat to allow translation of incorporated RNA within target cells (Gallie et al., 1987b). Smith et al. (2007) made use of this phenomenon to deliver RNA encoding the nonstructural proteins from Semliki forest virus into mammalian cells. They showed that the encapsidated RNA was uncoated and translated within the cells, and that the expressed protein stimulated an immune response in mice.
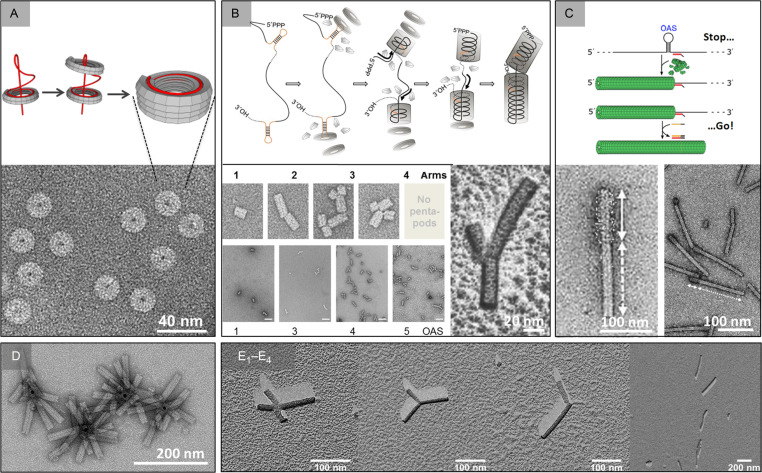
While natural TMV RNA has only a single copy of the OAS resulting in the production of linear particles, it is possible to generate more complex structures through the incorporation of more than one OAS on the same transcript (Gallie et al., 1987c; Fig. 3 ). Systematic studies have shown that the incorporation of multiple OAS sequences can generate TMV-based rods with a number of different morphologies including kinked “nanoboomerangs” as well as tri- and tetrapod structures (Eber et al., 2015; Fig. 3). The applications of such variants are described in Section 6.
Fig. 3.
Redesigning the shape of TMV-derived nanoobjects. RNA-directed growth and combination of nanotubular structures using in vitro technology. (A) Nanorings assembled and stabilized by help of a 204-nt long, OAS-containing RNA. (B) Kinked and branched boomerang up to tetrapod products accessed through colliding nanotube domain ends growing on RNA scaffolds with two up to five OAS. (C) TMV-like nanorods with selectively addressable longitudinal subdomains, obtained either by serial assembly with limited amounts of distinct CP species or in a nanometrically defined manner by a DNA blocking element-enabled stop-and-go procedure. Bottom row: Hybrid assemblies with nonbiomolecular core structures: (D) nanostars with TMV-deduced arms, grown by bottom-up technology on DNA-programmed gold beads. (E1–E4) Expanded nanoarchitectures with up to four TMV arms linked covalently to tetrahedral adamantane-based organic cores.
Panel (A) reproduced according to the copyright terms from Altintoprak, K., Seidenstücker, A., Krolla-Sidenstein, P., Plettl, A., Jeske, H., Gliemann, H., Wege, C., 2017. RNA-stabilized protein nanorings: high-precision adapters for biohybrid design. Bioinspired Biomimetic Nanobiomater. 6, 208; panel (B) reproduced according to the copyright permission terms in modified arrangements from Eber, F.J., Eiben, S., Jeske, H., Wege, C., 2015. RNA-controlled assembly of tobacco mosaic virus-derived complex structures: from nanoboomerangs to tetrapods. Nanoscale 7, 344; panel (C) left: reproduced from Geiger, F.C., Eber, F.J., Eiben, S., Mueller, A., Jeske, H., Spatz, J.P., Wege, C., 2013. TMV nanorods with programmed longitudinal domains of differently addressable coat proteins. Nanoscale 5, 3803; scheme and right: reproduced from Schneider, A., Eber, F.J., Wenz, N., Altintoprak, K., Jeske, H., Eiben, S., Wege, C., 2016. Dynamic DNA-controlled "stop-and-go" assembly of well-defined protein domains on RNA-scaffolded TMV-like nanotubes. Nanoscale 8, 19853; panel (D) reproduced with permission from Eber, F.J., Eiben, S., Jeske, H., Wege, C., 2013. Bottom-up-assembled nanostar colloids of gold cores and tubes derived from tobacco mosaic virus. Angew. Chem. Int. Ed. 52, 7203; panel (E1–E4) reproduced in agreement with the copyright terms from Wenz, N., Piasecka, S., Kalinowski, M., Schneider, A., Richert, C., Wege, C., 2018. Building expanded structures from tetrahedral DNA branching elements, RNA and TMV protein. Nanoscale 10, 6496.
6. Supramolecular Structures
TMV and TMV-like particles have been coupled to a number of organic molecules, including chromophores, polymers, or certain heavy metal complexes, to create classes of TMV-derived effector colloids. Such hybrid objects are envisaged for biomedical applications inside living organisms. As such applications are many and varied, this section is intended more to indicate the possible range of applications rather than to provide a comprehensive account. For those readers interested in this aspect, there are several excellent recent reviews (e.g., Koch et al., 2016; Wen and Steinmetz, 2016).
6.1. Display of Active Enzymes
The display of active enzymes of the surface of TMV particles is of great interest to many fields, including medicine, biodetection, the development of sensors, and even small-scale enzymatic conversions (Fig. 2). The surface of TMV has a strong stabilizing effect on different enzymes, including glucose oxidase, horseradish peroxidase (Baecker et al., 2017; Koch et al., 2015), and penicillinase (Koch et al., 2018; Poghossian et al., 2018). The use of TMV adapter rods on sensor surfaces (see below) also has enabled bioaffinity-based display of streptavidin conjugates of these enzymes at surface densities not achievable on TMV-free supports, with the additional advantages of increased reusability and enhanced target detection ranges of such devices. Thus, it is anticipated that high-performance biosensors employing TMV rods will soon be used in proof-of-concept applications.
6.2. TMV-Based Arrays on Solid Supports and in Miniaturized Devices
The adsorption properties of TMV on various surfaces such as gold, mica, glass, and silicon wafers have been investigated in detail (Knez et al., 2004a). Techniques such as convective deposition, microcontact printing (e.g., Kuncicky et al., 2006), and a number of evaporation-based methods for rapid and large-scale assembly of thin film coatings and ordered fibers consisting of aligned TMV particles also have been reported. The effects of divalent metal ions (Nedoluzhko and Douglas, 2001) and other additives also have been evaluated in detail, resulting in the development of long-range deposition techniques as well as several unique approaches. These comprise, among others, the spatially directed assembly of patterned TMV structures in capillary tubes (Lin et al., 2010) and the oriented growth of TMV fiber bundles on nanopillar structured superhydrophobic surfaces (Marinaro et al., 2015).
Of particular importance for close-to-application formulations is the observation that nanorods containing cysteine-exposed TMV CP mutants adopt a primarily vertical orientation on certain substrates, such as gold, under suitable conditions (Peng et al., 2011; Royston et al., 2008). This has been exploited for increasing the efficiency of energy storage and conversion devices as outlined earlier. To generate controlled arrays of TMV particles, Yi et al., 2005, Yi et al., 2007 partially disassembled the CP from TMV particles to expose the RNA at the 5′ end of the rods. Oriented assembly of TMV on solid supports was then achieved in a controlled manner via nucleic acid hybridization using complementary oligonucleotides. By this approach, the immobilization of fluorescently labeled TMV onto electrodes could be demonstrated. Furthermore, by using differentially labeled TMV particles in conjunction with a micropatterned substrate, it was possible to construct a patterned TMV microarray (Tan et al., 2008). An alternative approach made use of the viral self-assembly mechanism predicted to function not only in solution but also on 3′-terminally immobilized RNAs containing the viral OAS sequence. Indeed it was possible to grow nanostick arrays bottom-up on different solid supports, including prepatterned flat substrates, with covalently conjugated RNA scaffolds (Mueller et al., 2011), and on DNA-programmed gold nanobeads after sequence-selective hybridization of RNAs via their 3′ ends. This resulted in the formation of nanostar colloids with an adjustable number of TMV arms with varying lengths (Eber et al., 2013). Such composite structures suggest themselves as carrier colloids for functional molecules, with applications ranging from separation to biodetection methods.
Initial attempts to incorporate TMV particles into multilayers using electrostatic interactions revealed that, unlike spherical cowpea mosaic virus particles, the rods floated on top of the structures (Steinmetz et al., 2008). This problem was solved by sequentially alternating layer-by-layer application of two differently charged TMV variants yielding stable multilayer films that could be converted into free-standing TMV membranes that, in turn, could be used as tissue engineering supports (Tiu et al., 2017). One of the most rapidly advancing applications of TMV carrier templates is the preparation of surfaces that foster cell attachment and differentiation. TMV-coated culture supports appear to have advantages for the cultivation of certain cells; they also enable the spatially defined presentation of peptide ligands over nanometric distances. Applications, such as the osteogenesis of bone marrow stem cells on arginine–glycine–aspartic acid peptide-fashioned TMV layers, have been extensively explored (Kaur et al., 2010; Sitasuwan et al., 2014). Tissue engineering approaches, making use of TMV as a carrier for cell-binding peptide motifs to mimic extracellular matrix proteins, also include the production of mats made from electrospun composite polymer–virus nanorod fibers for improved handling (Wu et al., 2011) and layouts combining 3D nanostructures with TMV-based effector rods (reviewed by Wen and Steinmetz, 2016; Zhao et al., 2015; Fig. 2).
7. TMV Particles With Novel Morphology: From Boomerangs to Spheres
7.1. Variations on the Rod-Shaped Theme
Knowledge of the mechanism of assembly of TMV particles has allowed the generation of rod-shaped particles with altered length and morphology by RNA-guided assembly in vitro. Sets of shortened TMV rods of defined size classes were initially produced to evaluate their ability to stabilize and regulate the properties of ferrofluids (Wu et al., 2010). The TMV rods enhanced and fine-tuned magnetoviscosity of the fluids to an unexpected extent and suppressed shear thinning in a length- and surface charge-dependent manner (Fig. 2). As rheological fluids are employed for such purposes as vacuum-tight sealing of rotating shafts or for damping or heat transfer tasks in technical equipment, the study suggested previously unforeseen applications for TMV-based particles. TMV-like particles of differing length grown in situ on solid flat or bead supports (see above) yielded high-surface density arrays of carrier rods in nanobrush and nanostar layouts (Azucena et al., 2012; Eber et al., 2013; Mueller et al., 2011; Fig. 3).
The reliable in vitro fabrication of TMV-based nanorods of other size classes (Rego et al., 2013) has opened unprecedented opportunities for biomedicine. Upon delivery into mice via the bloodstream, fluorescently labeled TMV derivatives in distinct length classes underwent a selective, aspect ratio-dependent uptake into tumor cells that could be modulated by an additional display of cell surface receptor-targeting arginine–glycine–aspartic acid ligands (Shukla et al., 2015). The concomitant good rate of clearance suggests that there are good prospects for TMV-based imaging and therapies as a result the shape of the viral carrier particle. These results were extended by Liu et al. (2016) and might provide the basis for future tumor-targeting treatments. RNA-scaffolded four-turn helices constitute the lower size limit of TMV-derived assemblies. Such nanorings of 9.2 nm thickness, stabilized by a 204 nt OAS-RNA and containing blends of distinct CP types, were not only used for attaching functional molecules to technical substrates (Altintoprak et al., 2017; Fig. 3) but recently have been employed as “pore-in-pore” inlays to implant their central 4 nm nanopores into solid-state membrane templates (Farajollahi et al., 2018).
As described in Section 5, straight TMV-like particles are not the only structures accessible by RNA-mediated CP assembly as the presence of more than a single OAS on an RNA can be used to generate angular nanostructures. These branched and kinked structures (Fig. 3) are interesting candidates for subsequent processing into bioinorganic hybrids with unusual physical properties, and supra(bio)molecular complexes for multimodal delivery and display purposes.
7.2. Going Spherical
While TMV particles usually have a rod-shaped helical structure, it is possible to convert both RNA-containing and protein-only rods to a spherical morphology by heat treatment (Atabekov et al., 2011; Bruckman et al., 2015; Hart, 1956). This conversion is accompanied by a change in the secondary structure of the subunits from a primarily α-helical structure to one that contains a significant proportion of β-sheet (Dobrov et al., 2014). These spherical particles have proved to be an effective platform for displaying antigens (Karpova et al., 2012) and have been investigated as delivery vehicles for anticancer drugs (Bruckman et al., 2016). The molecular organization of these TMV derivatives retains the right-side-out orientation of the viral components with CP termini accessible on the outer surface of the nanospheres.
8. Conclusions
As we hope we have shown in this review, the study of TMV particles has come a long way since the virus was first described in the late 19th century. In some ways it seems amazing that so simple a structure, consisting of multiple copies of a single type of protein and a single molecule of RNA, could be the object of such intense study for over 100 years. However, it is this very simplicity that has made TMV such a tractable system both for fundamental studies and, latterly, for exploitation for applications in bionanotechnology. Indeed, the number of publications regarding the uses of TMV particles continues to increase rapidly, so much so that it is difficult to keep pace with all the developments and certainly to do them all justice in a review of this length. However, we hope that we have at least given our readers an insight into how basic research has been translated into possible applications.
Acknowledgments
G.P.L. is extremely grateful to the Fulbright Commission for the Scholarship that enabled him to spend a sabbatical year in Prof. Zaitlin's laboratory at Cornell University, USA, between 1987 and 1988. He also acknowledges the support of the John Innes Foundation, the UK Biotechnology and Biological Sciences Research Council (BBSRC) Institute Strategic Programme Grant “Molecules from Nature” (BB/P012523/1) and Dr. John Steele for help with the figures.
C.W. acknowledges financial support by the Carl Zeiss Stiftung and the University of Stuttgart (Projekthaus NanoBioMater), and subsidiary funding by the DFG and the Baden-Württemberg Stiftung. Many thanks also to Holger Jeske for inspiring debates and pleasant discussions on all aspects of molecular and applied plant virology.
References
- Altintoprak K., Seidenstücker A., Welle A., Eiben S., Atanasova P., Stitz N., Plettl A., Bill J., Gliemann H., Jeske H., Rothenstein D., Geiger F., Wege C. Peptide-equipped tobacco mosaic virus templates for selective and controllable biomineral deposition. Beilstein J. Nanotechnol. 2015;6:1399. doi: 10.3762/bjnano.6.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altintoprak K., Seidenstücker A., Krolla-Sidenstein P., Plettl A., Jeske H., Gliemann H., Wege C. RNA-stabilized protein nanorings: high-precision adapters for biohybrid design. Bioinspired Biomimetic Nanobiomater. 2017;6:208. [Google Scholar]
- Altschuh D., Lesk A.M., Bloomer A.C., Klug A. Correlation of co-ordinated amino acid substitutions with function in viruses related to tobacco mosaic virus. J. Mol. Biol. 1987;193:693. doi: 10.1016/0022-2836(87)90352-4. [DOI] [PubMed] [Google Scholar]
- Asurmendi S., Berg R.H., Smith T.J., Bendhamane M., Beachy R.N. Aggregation of TMV CP plays a role in CP functions and in coat-protein mediated resistance. Virology. 2007;366:98. doi: 10.1016/j.virol.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atabekov J., Nikitin N., Arkhipenko M., Chirkov S., Karpova O. Thermal transition of native tobacco mosaic virus and RNA-free viral proteins into spherical nanoparticles. J. Gen. Virol. 2011;92:453. doi: 10.1099/vir.0.024356-0. [DOI] [PubMed] [Google Scholar]
- Azucena C., Eber F.J., Trouillet V., Hirtz M., Heissler S., Franzreb M., Fuchs H., Wege C., Gliemann H. New approaches for bottom-up assembly of tobacco mosaic virus-derived nucleoprotein tubes on defined patterns on silica- and polymer-based substrates. Langmuir. 2012;28 doi: 10.1021/la302774h. [DOI] [PubMed] [Google Scholar]
- Baecker M., Koch C., Eiben S., Geiger F., Eber F.J., Gliemann H., Poghossian A., Wege C., Schoening M.J. Tobacco mosaic virus as enzyme nanocarrier for electrochemical biosensors. Sens. Actuators B. 2017;238:716. [Google Scholar]
- Beachy R.N. Coat-protein-mediated resistance to tobacco mosaic virus: discovery mechanisms and exploitation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999;354:659. doi: 10.1098/rstb.1999.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachy R.N., Zaitlin M. Characterization and in vitro translation of the RNAs from less-than-full-length, virus-related, nucleoprotein rods present in tobacco mosaic virus preparations. Virology. 1977;81:160. doi: 10.1016/0042-6822(77)90068-x. [DOI] [PubMed] [Google Scholar]
- Beijerinck M.W. Concerning contagium vivum fluidum as a cause of the spot disease of tobacco leaves. In: Johnson J., editor. Phytopathological Classics, no.7. American Phytopathological Society; 1898. p. 33. English translation. [Google Scholar]
- Bendahmane M., Koo M., Karrer E., Beachy R.N. Display of epitopes on the surface of tobacco mosaic virus: impact of charge and isoelectric point of the epitope on virus-host interactions. J. Mol. Biol. 1999;290:9. doi: 10.1006/jmbi.1999.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan M.W., Mason S.E., Goelet P. Expression of tobacco mosaic virus coat protein by a cauliflower mosaic virus promoter in plants transformed by agrobacterium. EMBO J. 1985;4:1921. doi: 10.1002/j.1460-2075.1985.tb03871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhyravbhatla B., Watowich S.J., Caspar D.L.D. Refined atomic model of the four-layer aggregate of the tobacco mosaic virus coat protein at 2.4 Å resolution. Biophys. J. 1998;74:604. doi: 10.1016/S0006-3495(98)77819-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner A.M., Alonso J.M., Górzny M.L., Wege C. Nanoscale science and technology with plant viruses and bacteriophages. In: Mateu M.G., editor. Structure and Physics of Viruses: An Integrated Textbook. Springer Science + Business Media; Dordrecht: 2013. p. 667. [Google Scholar]
- Bloomer A.C., Champness J.N., Bricogne G., Staden R., Klug A. Protein disk of tobacco mosaic virus at 2.8 Å resolution showing the interactions within and between subunits. Nature. 1978;276:362. doi: 10.1038/276362a0. [DOI] [PubMed] [Google Scholar]
- Bruckman M.A., Soto C.M., McDowell H., Liu J.L., Ratna B.R., Korpany K.V., Zahr O.K., Blum A.S. Role of hexahistidine in directed nanoassemblies of tobacco mosaic virus coat protein. ACS Nano. 2011;5:1606. doi: 10.1021/nn1025719. [DOI] [PubMed] [Google Scholar]
- Bruckman M.A., VanMeter A., Steinmetz N.F. Nanomanufacturing of tobacco mosaic virus-based spherical biomaterials using a continuous flow method. ACS Biomater. Sci. Eng. 2015;1:13. doi: 10.1021/ab500059s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckman M.A., Czapar A.E., VanMeter A., Randolph L.N., Steinmetz N.F. Tobacco mosaic virus-based protein nanoparticles and nanorods for chemotherapy delivery targeting breast cancer. J. Control. Release. 2016;231:103. doi: 10.1016/j.jconrel.2016.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler P.J.G. The current picture of the structure and assembly of tobacco mosaic virus. J. Gen. Virol. 1984;65:253. doi: 10.1099/0022-1317-65-2-253. [DOI] [PubMed] [Google Scholar]
- Butler P.J.G. Self-assembly of tobacco mosaic virus: the role of an intermediate aggregate in generating both specificity and speed. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999;354:537. doi: 10.1098/rstb.1999.0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler P.J.G., Klug A. Assembly of the particle of tobacco mosaic virus from RNA and disks of protein. Nat. New Biol. 1971;229:47. doi: 10.1038/newbio229047a0. [DOI] [PubMed] [Google Scholar]
- Butler P.J.G., Finch J.T., Zimmern D. Configuration of tobacco mosaic virus RNA during virus assembly. Nature. 1977;265:217. doi: 10.1038/265217a0. [DOI] [PubMed] [Google Scholar]
- Champness J.N., Bloomer A.C., Bricogne G., Butler P.J.G., Klug A. The structure of the protein disk of tobacco mosaic virus to 5 Å resolution. Nature. 1976;259:20. doi: 10.1038/259020a0. [DOI] [PubMed] [Google Scholar]
- Chiang C.-Y., Epstein J., Brown A., Munday J.N., Culver J.N., Ehrman S. Biological templates for antireflective current collectors for photo-electrochemical cell applications. Nano Lett. 2012;12:6005. doi: 10.1021/nl303579z. [DOI] [PubMed] [Google Scholar]
- Culver J.N., Brown A.D., Zang F., Gnerlich M., Gerasopoulos K., Ghodssi R. Plant virus directed fabrication of nanoscale materials and devices. Virology. 2015;200:479. doi: 10.1016/j.virol.2015.03.008. [DOI] [PubMed] [Google Scholar]
- Czapar A.E., Steinmetz N.F. Plant viruses and bacteriophages for drug delivery in medicine and biotechnology. Curr. Opin. Chem. Biol. 2017;38:108. doi: 10.1016/j.cbpa.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czapar A.E., Zheng Y.R., Riddell I.A., Shukla S., Awuah S.G., Lippard S.J., Steinmetz N.F. Tobacco mosaic virus delivery of phenanthriplatin for cancer therapy. ACS Nano. 2016;10:4119. doi: 10.1021/acsnano.5b07360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard K., Uttenthal Å., Jones T.D., Xu F., Merryweather A., Hamilton W.D.O., Langeveld J.P.M., Boshuizen R.S., Kamstrup S., Lomonossoff G.P., Porta C., Vela C., Casal J.I., Meloen R.H., Rodgers P.B. Plant-derived vaccine protects target animals against a virus disease. Nature Biotechnol. 1997;15:248. doi: 10.1038/nbt0397-248. [DOI] [PubMed] [Google Scholar]
- Dawson W.O., Beck D.L., Knorr D.A., Grantham G.L. cDNA cloning of the complete genome of tobacco mosaic virus and production of infectious transcripts. Proc. Natl. Acad. Sci. U.S.A. 1986;83:1832. doi: 10.1073/pnas.83.6.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir M., Stowell M.H.B. A chemoselective biomolecular template for assembling diverse nanotubular materials. Nanotechnology. 2002;13:541. [Google Scholar]
- Dobrov E.N., Nikitin N.A., Trifonova E.A., Parshina E.Y., Makarov V.V., Maksimov G.V., Karpova O.V., Atabekov J.G. β-Structure of the coat protein subunits in spherical particles generated by tobacco mosaic virus thermal denaturation. J. Biomol. Struct. Dyn. 2014;32:701. doi: 10.1080/07391102.2013.788983. [DOI] [PubMed] [Google Scholar]
- Donnelly M.L.L., Luke G., Mehrotra A., Li X., Hughes L.E., Gani D., Ryan M.D. Analysis of the aphthovirus 2A/2B polyprotein ‘cleavage’ mechanism indicates not a proteolytic reaction, but a novel translational effect: a putative ribosomal ‘skip’. J. Gen. Virol. 2001;82:1013. doi: 10.1099/0022-1317-82-5-1013. [DOI] [PubMed] [Google Scholar]
- Douglas T., Young M. Virus particles as templates for materials synthesis. Adv. Mater. 1999;11:679. [Google Scholar]
- Dujardin E., Peet C., Stubbs G., Culver J.N., Mann S. Organization of metallic nanoparticles using tobacco mosaic virus templates. Nano Lett. 2003;3:413. [Google Scholar]
- Durham A.C.H., Finch J.T., Klug A. States of aggregation of tobacco mosaic virus protein. Nat. New Biol. 1971;229:37. doi: 10.1038/newbio229037a0. [DOI] [PubMed] [Google Scholar]
- Eber F.J., Eiben S., Jeske H., Wege C. Bottom-up-assembled nanostar colloids of gold cores and tubes derived from tobacco mosaic virus. Angew. Chem. Int. Ed. 2013;52:7203. doi: 10.1002/anie.201300834. [DOI] [PubMed] [Google Scholar]
- Eber F.J., Eiben S., Jeske H., Wege C. RNA-controlled assembly of tobacco mosaic virus-derived complex structures: from nanoboomerangs to tetrapods. Nanoscale. 2015;7:344. doi: 10.1039/c4nr05434b. [DOI] [PubMed] [Google Scholar]
- Eiben S., Stitz N., Eber F., Wagner J., Atanasova P., Bill J., Wege C., Jeske H. Tailoring the surface properties of tobacco mosaic virions by the integration of bacterially expressed mutant coat protein. Virus Res. 2014;180:92. doi: 10.1016/j.virusres.2013.11.019. [DOI] [PubMed] [Google Scholar]
- Fan X.Z., Pomerantseva E., Gnerlich M., Brown A., Gerasopoulos K., McCarthy M., Culver J., Ghodssi R. Tobacco mosaic virus: a biological building block for micro/nano/biosystems. J. Vac. Sci. Technol. A. 2013;31 [Google Scholar]
- Farajollahi F., Seidenstücker A., Altintoprak K., Walther P., Ziemann P., Plettl A., Marti O., Wege C., Gliemann H. Electrochemically-driven insertion of biological nanodiscs into solid state membrane pores as a basis for “pore-in-pore” membranes. Nanomaterials. 2018;8:237. doi: 10.3390/nano8040237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finbloom A., Han K., Aanei I.L., Hartman E.C., Finley D.T., Dedeo M.T., Fishman M., Downing K.H., Francis M.B. Stable disk assemblies of a tobacco mosaic virus mutant as nanoscale scaffolds for applications in drug delivery. Bioconjug. Chem. 2016;27:2480. doi: 10.1021/acs.bioconjchem.6b00424. [DOI] [PubMed] [Google Scholar]
- Fitchen J., Beachy R.N., Hein M.B. Plant virus expressing hybrid coat protein with added murine epitope elicits autoantibody response. Vaccine. 1995;13:1051. doi: 10.1016/0264-410x(95)00075-c. [DOI] [PubMed] [Google Scholar]
- Fowler C.E., Shenton W., Stubbs G., Mann S. Tobacco mosaic virus liquid crystals as templates for the interior design of silica mesophases and nanoparticles. Adv. Mater. 2001;13:1266. [Google Scholar]
- Fraenkel-Conrat H., Williams R.C. Reconstitution of active tobacco mosaic virus from its inactive protein and nucleic acid components. Proc. Natl. Acad. Sci. U.S.A. 1955;41:690. doi: 10.1073/pnas.41.10.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke C.E., Czapar A.E., Patel R.B., Steinmetz N.F. Tobacco mosaic virus-delivered cisplatin restores efficacy in platinum-resistant ovarian cancer cells. Mol. Pharm. 2017 doi: 10.1021/acs.molpharmaceut.7b00466. [DOI] [PubMed] [Google Scholar]
- Franklin R.E. Structure of tobacco mosaic virus: location of the ribonucleic acid in the tobacco mosaic virus particle. Nature. 1956;177:928. [Google Scholar]
- Franzen S., Lommel S.A. Targeting cancer with ‘smart bombs’: equipping plant virus nanoparticles for a ‘seek and destroy’ mission. Nanomedicine. 2009;4:575. doi: 10.2217/nnm.09.23. [DOI] [PubMed] [Google Scholar]
- Frolova O.Y., Petrunia I.V., Komarova T.V., Kosorukov V.S., Sheval E.V., Gleba Y.Y., Dorokhov Y.L. Trastuzumab-binding peptide display by tobacco mosaic virus. Virology. 2010;407:7. doi: 10.1016/j.virol.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Fromm S.A., Bharat T.A., Jakobi A.J., Hagen W.J., Sachse C. Seeing tobacco mosaic virus through direct electron detectors. J. Struct. Biol. 2015;189:87. doi: 10.1016/j.jsb.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie D.R., Sleat D.E., Watts J.W., Turner P.C., Wilson T.M.A. The 5′-leader sequence of tobacco mosaic virus RNA enhances the expression of foreign gene transcripts in vitro and in vivo. Nucleic Acids Res. 1987;15:3257. doi: 10.1093/nar/15.8.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie D.R., Sleat D.E., Watts J.W., Turner P.C., Wilson T.M.A. In vivo uncoating and efficient expression of foreign mRNAs packaged in TMV-like particles. Science. 1987;236:1122. doi: 10.1126/science.3472350. [DOI] [PubMed] [Google Scholar]
- Gallie D.R., Plaskitt K.A., Wilson T.M.A. The effect of multiple dispersed copies of the origin-of-assembly sequence from TMV RNA on the morphology of pseudovirus particles assembled in vitro. Virology. 1987;158:473. doi: 10.1016/0042-6822(87)90225-x. [DOI] [PubMed] [Google Scholar]
- Geiger F.C., Eber F.J., Eiben S., Mueller A., Jeske H., Spatz J.P., Wege C. TMV nanorods with programmed longitudinal domains of differently addressable coat proteins. Nanoscale. 2013;5:3808. doi: 10.1039/c3nr33724c. [DOI] [PubMed] [Google Scholar]
- Goelet P., Lomonossoff G.P., Butler P.J.G., Akam M.E., Gait M.J., Karn J. Nucleotide sequence of tobacco mosaic virus RNA. Proc. Natl. Acad. Sci. U.S.A. 1982;79:5818. doi: 10.1073/pnas.79.19.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golemboski D.B., Lomonossoff G.P., Zaitlin M. Plants transformed with a tobacco mosaic virus nonstructural gene sequence are resistant to the virus. Proc. Natl. Acad. Sci. U.S.A. 1990;87:6311. doi: 10.1073/pnas.87.16.6311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamamoto H., Sugiyama Y., Nakagawa N., Hashida E., Matsunaga Y., Takemoto S., Watanabe Y., Okada Y. A new tobacco mosaic virus vector and its use for the systemic production of angiotensin-I-converting enzyme inhibitor in transgenic tobacco and tomato. Biotechnology. 1993;11:930. doi: 10.1038/nbt0893-930. [DOI] [PubMed] [Google Scholar]
- Hart R.G. Morphological changes accompanying thermal denaturation of tobacco mosaic virus. Biochim. Biophys. Acta. 1956;20:388. doi: 10.1016/0006-3002(56)90301-8. [DOI] [PubMed] [Google Scholar]
- Haynes J.R., Cunningham J., von Seefried A., Lennick M., Garvin R.T., Shen S.-H. Development of a genetically engineered, candidate polio vaccine employing the self-assembling properties of the tobacco mosaic virus coat protein. Biotechnology. 1986;4:637. doi: 10.1038/nbt0786-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T.R., Hunt T., Knowland J., Zimmern D. Messenger RNA for the coat protein of tobacco mosaic virus. Nature. 1976;260:759. doi: 10.1038/260759a0. [DOI] [PubMed] [Google Scholar]
- Hwang D.J., Roberts I.M., Wilson T.M.A. Expression of tobacco mosaic virus coat protein and assembly of pseudovirus particles in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 1994;91:9067. doi: 10.1073/pnas.91.19.9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Li Q., Li M., Zhou Z., Wu L., Fan J., Zhang Q., Zhu H., Xu Z. A modified TMV-based vector facilitates the expression of longer foreign epitopes in tobacco. Vaccine. 2006;24:109. doi: 10.1016/j.vaccine.2005.09.060. [DOI] [PubMed] [Google Scholar]
- Jupin I., Sleat D.E., Watkins P.A., Wilson T.M.A. Direct recovery of in vitro transcripts in a protected form suitable for prolonged storage and shipment at ambient temperatures. Nucleic Acids Res. 1989;7:815. doi: 10.1093/nar/17.2.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadri A., Maiß E., Amsharov N., Bittner A.M., Balci S., Kern K., Jeske H., Wege C. Engineered tobacco mosaic virus mutants with distinct physical characteristics in planta and enhanced metallization properties. Virus Res. 2011;157:35. doi: 10.1016/j.virusres.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Kadri A., Wege C., Jeske H. In vivo self-assembly of TMV-like particles in yeast and bacteria for nanotechnological applications. J. Virol. Meth. 2013;189:328. doi: 10.1016/j.jviromet.2013.02.017. [DOI] [PubMed] [Google Scholar]
- Karpova O., Nikitin N., Chirkov S., Trifonova E., Sheveleva A., Lazareva E., Atabekov J.G. Immunogenic compositions assembled from tobacco mosaic virus-generated spherical particle platforms and foreign antigens. J. Gen. Virol. 2012;93:400. doi: 10.1099/vir.0.036293-0. [DOI] [PubMed] [Google Scholar]
- Kaur G., Wang C., Sun J., Wang Q. The synergistic effects of multivalent ligand display and nanotopography on osteogenic differentiation of rat bone marrow stem cells. Biomaterials. 2010;31:5813. doi: 10.1016/j.biomaterials.2010.04.017. [DOI] [PubMed] [Google Scholar]
- Knez M., Sumser M., Bittner A.M., Wege C., Jeske H., Kooi S., Burghard M., Kern K. Electrochemical modification of individual nano-objects. J. Electroanal. Chem. 2002;522:70. [Google Scholar]
- Knez M., Bittner A.M., Boes F., Wege C., Jeske H., Maiß E., Kern K. Biotemplate synthesis of 3-nm nickel and cobalt nanowires. Nano Lett. 2003;3:1079. [Google Scholar]
- Knez M., Sumser M.P., Bittner A.M., Wege C., Jeske H., Hoffmann D.M.P., Kuhnke K., Kernl K. Binding the tobacco mosaic virus to inorganic surfaces. Langmuir. 2004;20:441. doi: 10.1021/la035425o. [DOI] [PubMed] [Google Scholar]
- Knez M., Sumser M., Bittner A.M., Wege C., Jeske H., Martin P., Kern K. Spatially selective nucleation of metal clusters on the tobacco mosaic virus. Adv. Funct. Mater. 2004;14:116. [Google Scholar]
- Koch C., Wabbel K., Eber F.J., Krolla-Sidenstein P., Azucena C., Gliemann H., Eiben S., Geiger F., Wege C. Modified TMV particles as beneficial scaffolds to present sensor enzymes. Front. Plant Sci. 2015;6:1137. doi: 10.3389/fpls.2015.01137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C., Eber F.J., Azucena C., Förste A., Walheim S., Schimmel T., Bittner A., Jeske H., Gliemann H., Eiben S., Geiger F., Wege C. Novel roles for well-known players: from tobacco mosaic virus pests to enzymatically active assemblies. Beilstein J. Nanotechnol. 2016;7:613. doi: 10.3762/bjnano.7.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C., Poghossian A., Schoening M.J., Wege C. Penicillin detection by tobacco mosaic virus-assisted colorimetric biosensors. Nanotheranostics. 2018;2:184. doi: 10.7150/ntno.22114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo M., Bendahmane M., Lettieri G.A., Paoletti A.D., Lane T.E., Fitchen J.H., Buchmeier M.J., Beachy R.N. Protective immunity against murine hepatitis virus (MHV) induced by intranasal or subcutaneous administration of hybrids of tobacco mosaic virus that carries an MHV epitope. Proc. Natl. Acad. Sci. U.S.A. 1999;96:7774. doi: 10.1073/pnas.96.14.7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuncicky D.M., Naik R.R., Velev O.D. Rapid deposition and long-range alignment of nanocoatings and arrays of electrically conductive wires from tobacco mosaic virus. Small. 2006;2:1462. doi: 10.1002/smll.200600399. [DOI] [PubMed] [Google Scholar]
- Lam P., Gulati N.M., Stewart P.L., Keri R.A., Steinmetz N.F. Bioengineering of tobacco mosaic virus to create a non-infectious positive control for Ebola diagnostic assays. Sci. Rep. 2016;6 doi: 10.1038/srep23803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.L., Twyman R.M., Fiering S., Steinmetz N.F. Virus-based nanoparticles as platform technologies for modern vaccines. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016;8:554. doi: 10.1002/wnan.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Song B., Chen X., Wang Z., Zeng M., Yu D., Hu D., Chen Z., Jin L., Yang S., Yang C., Chen B. Crystal structure of a four-layer aggregate of engineered TMV CP implies the importance of terminal residues for oligomer assembly. PLoS One. 2013;8 doi: 10.1371/journal.pone.0077717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Balizan E., Lee L.A., Niu Z., Wang Q. Self-assembly of rod-like bio-nanoparticles in capillary tubes. Ange. Chem. Internat. Ed. 2010;49:868. doi: 10.1002/anie.200904993. [DOI] [PubMed] [Google Scholar]
- Lindbo J.A. High-efficiency protein expression in plants from agroinfection-compatible tobacco mosaic virus expression vectors. BMC Biotechnol. 2007;7:52. doi: 10.1186/1472-6750-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Wu F., Tian Y., Wu M., Zhou Q., Jiang S., Niu Z. Size dependent cellular uptake of rod-like bionanoparticles with different aspect ratios. Sci. Rep. 2016;6 doi: 10.1038/srep24567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomonossoff G.P. So what have plant viruses ever done for virology and molecular biology? Adv. Virus Res. 2018;100:145. doi: 10.1016/bs.aivir.2017.12.001. [DOI] [PubMed] [Google Scholar]
- Lomonossoff, G.P., Wilson, T.M.A., 1985. Structure and in vitro assembly of tobacco mosaic virus. In: “Molecular Plant Virology”, Vol. 1 (J.W. Davies ed.), CRC Press, Florida, USA, pp. 43.
- Love A.J., Makarov V.V., Sinitsyna O.V., Shaw J., Yaminsky I.V., Kalinina N.O., Taliansky M.E. A genetically modified tobacco mosaic virus that can produce gold nanoparticles from a metal salt precursor. Front. Plant Sci. 2015;6:984. doi: 10.3389/fpls.2015.00984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manocchi A.K., Horelik N.E., Lee B., Yi H. Simple, readily controllable palladium nanoparticle formation on surface-assembled viral nanotemplates. Langmuir. 2010;26:3670. doi: 10.1021/la9031514. [DOI] [PubMed] [Google Scholar]
- Marillonnet S., Giritch A., Gils M., Kandzia R., Klimyuk V., Gleba Y. In planta engineering of viral RNA replicons: efficient assembly by recombination of DNA modules delivered by agrobacterium. Proc. Natl. Acad. Sci. U.S.A. 2004;101:6852. doi: 10.1073/pnas.0400149101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinaro G., Burghammer M., Costa L., Dane T., De Angelis F., Di Fabrizio E., Riekel C. Directed growth of virus nanofilaments on a superhydrophobic surface. ACS Appl. Mat. Inter. 2015;7 doi: 10.1021/am507509z. [DOI] [PubMed] [Google Scholar]
- Mascia T., Nigro F., Abdallah A., Ferrara M., De Stradis A., Faedda R., Palukaitis P., Gallitelli D. Gene silencing and gene expression in phytopathogenic fungi using a plant virus vector. Proc. Natl. Acad. Sci. U.S.A. 2014;111:4291. doi: 10.1073/pnas.1315668111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick A., Palmer K.E. Genetically engineered tobacco mosaic virus as nanoparticle vaccines. Expert Rev. Vaccines. 2008;7:33. doi: 10.1586/14760584.7.1.33. [DOI] [PubMed] [Google Scholar]
- Meshi T., Ishikawa M., Motoyoshi F., Semba K., Okada Y. In vitro transcription of infectious RNAs from full-length cDNAs of tobacco mosaic virus. Proc. Natl. Acad. Sci. U.S.A. 1986;83:5043. doi: 10.1073/pnas.83.14.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller A., Kadri A., Jeske H., Wege C. In vitro assembly of tobacco mosaic virus coat protein variants derived from fission yeast expression clones or plants. J. Virol. Meth. 2010;166:77. doi: 10.1016/j.jviromet.2010.02.026. [DOI] [PubMed] [Google Scholar]
- Mueller A., Eber F.J., Azucena C., Petershans A., Bittner A.M., Gliemann H., Jeske H., Wege C. Inducible site-selective bottom-up assembly of virus-derived nanotube arrays on RNA-equipped wafers. ACS Nano. 2011;5:4512. doi: 10.1021/nn103557s. [DOI] [PubMed] [Google Scholar]
- Namba K., Pattanayek R., Stubbs G. Visualization of protein-nucleic acid interactions in a virus. Refined structure of intact tobacco mosaic virus at 2.9 Å resolution by X-ray fiber diffraction. J. Mol. Biol. 1989;208:307. doi: 10.1016/0022-2836(89)90391-4. [DOI] [PubMed] [Google Scholar]
- Nedoluzhko A., Douglas T. Ordered association of tobacco mosaic virus in the presence of divalent metal ions. J. Inorg. Biochem. 2001;84:233. doi: 10.1016/s0162-0134(01)00174-x. [DOI] [PubMed] [Google Scholar]
- Peng B., Liu N., Lin Y., Wang L., Zhang W., Niu Z., Wang Q., Su Z. Self-assembly of anisotropic tobacco mosaic virus nanoparticles on gold substrate. Sci. China Chem. 2011;54:137. [Google Scholar]
- Perham R.N., Wilson T.M.A. The characterization of intermediates formed during the disassembly of tobacco mosaic virus at alkaline pH. Virology. 1978;84:293. doi: 10.1016/0042-6822(78)90249-0. [DOI] [PubMed] [Google Scholar]
- Petukhova N.V., Gasanova T.V., Stepanova L.A., Rusova O.A., Potapchuk M.V., Korotkov A.V., Skurat E.V., Tsybalova L.M., Kiselev O.I., Ivanov P.A., Atabekov J.G. Immunogenicity and protective efficacy of candidate universal influenza A nanovaccines produced in plants by tobacco mosaic virus-based vectors. Curr. Pharm. Des. 2013;19:5587. doi: 10.2174/13816128113199990337. [DOI] [PubMed] [Google Scholar]
- Peyret H., Lomonossoff G.P. When plant virology met agrobacterium: the rise of the deconstructed clones. Plant Biotechnol. J. 2015;13:1121. doi: 10.1111/pbi.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poghossian A., Jablonski M., Koch C., Bronder T.S., Rolka D., Wege C., Schöning M.J. Field-effect biosensor using virus particles as scaffolds for enzyme immobilization. Biosens. Bioelectron. 2018;110:168. doi: 10.1016/j.bios.2018.03.036. [DOI] [PubMed] [Google Scholar]
- Powell Abel P., Nelson R.S., De B., Hofmann N., Rogers S.G., Fraley R.T., Beachy R.N. Delay of disease development in transgenic plants that express the tobacco mosaic virus coat-protein gene. Science. 1986;232:738. doi: 10.1126/science.3457472. [DOI] [PubMed] [Google Scholar]
- Rego J.M., Lee J.H., Lee D.H., Yi H. Biologically inspired strategy for programmed assembly of viral building blocks with controlled dimensions. Biotechnol. J. 2013;8:237. doi: 10.1002/biot.201100504. [DOI] [PubMed] [Google Scholar]
- Röder J., Fischer R., Commandeur U. Adoption of the 2A ribosomal skip principle to tobacco mosaic virus for peptide display. Front. Plant Sci. 2017;8:1125. doi: 10.3389/fpls.2017.01125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royston E., Ghosh A., Kofinas P., Harris M.T., Culver J.N. Self-assembly of virus-structured high surface area nanomaterials and their application as battery electrodes. Langmuir. 2008;24:906. doi: 10.1021/la7016424. [DOI] [PubMed] [Google Scholar]
- Saunders K., Lomonossoff G.P. In planta synthesis of designer-length tobacco mosaic virus-based nano-rods that can be used to fabricate nano-wires. Front. Plant Sci. 2017;8:1335. doi: 10.3389/fpls.2017.01335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk A., Eiben S., Goll M., Reith L., Kulak A.N., Meldrum F., Jeske H., Wege C., Ludwigs S. Virus-directed formation of electrocatalytically active nanoparticle-based Co3O4 tubes. Nanoscale. 2017;9:6334. doi: 10.1039/c7nr00508c. [DOI] [PubMed] [Google Scholar]
- Schlick T.L., Ding Z.B., Kovacs E.W., Francis M.B. Dual-surface modification of the tobacco mosaic virus. J. Am. Chem. Soc. 2005;127:3718. doi: 10.1021/ja046239n. [DOI] [PubMed] [Google Scholar]
- Shenton W., Douglas T., Young M., Stubbs G., Mann S. Inorganic-organic nanotube composites from template mineralization of tobacco mosaic virus. Adv. Mater. 1999;11:253. [Google Scholar]
- Shire S.J., McKay P., Leung D.W., Cachianes G.J., Jackson E., Wood W.I., Raghavendra K., Khairallah L., Schuster T.M. Preparation and properties of recombinant DNA derived tobacco mosaic virus coat protein. Biochemistry. 1990;29:5119. doi: 10.1021/bi00473a017. [DOI] [PubMed] [Google Scholar]
- Shukla S., Eber F., Nagarajan A.S., DiFranco N.A., Schmidt N., Wen A.M., Eiben S., Twyman R.M., Wege C., Steinmetz N.F. The impact of aspect ratio on biodistribution and tumor homing of rigid soft-matter nanorods. Adv. Healthc. Mater. 2015;4:874. doi: 10.1002/adhm.201400641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitasuwan P., Lee L.A., Li K., Nguyen H.G., Wang Q. RGD-conjugated rod-like viral nanoparticles on 2D scaffold improve bone differentiation of mesenchymal stem cells. Front. Chem. 2014;2:31. doi: 10.3389/fchem.2014.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleat D.E., Turner P.C., Finch J.T., Butler P.J.G., Wilson T.M.A. Packaging of recombinant RNA molecules into pseudovirus particles directed by the origin-of-assembly sequence from tobacco mosaic virus RNA. Virology. 1986;155:299. doi: 10.1016/0042-6822(86)90194-7. [DOI] [PubMed] [Google Scholar]
- Sleat D.E., Gallie D.R., Watts J.W., Deom C.M., Turner P.C., Beachy R.N., Wilson T.M.A. Selective recovery of foreign gene transcripts as virus-like particles in TMV-infected transgenic tobaccos. Nucleic Acids Res. 1988;16:3127. doi: 10.1093/nar/16.8.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.L., Lindbo J.A., Dillard-Telm S., Brosio P.M., Lasnik A.B., McCormick A.A., Nguyen L.V., Palmer K.E. Modified tobacco mosaic virus particles as scaffolds for display of protein antigens for vaccine applications. Virology. 2006;34:475. doi: 10.1016/j.virol.2005.12.039. [DOI] [PubMed] [Google Scholar]
- Smith M.L., Corbo T., Bernales J., Lindbo J.A., Pogue G.P., Palmer K.E., McCormick A.A. Assembly of trans-encapsidated recombinant viral vectors engineered from tobacco mosaic virus and Semliki Forest virus and their evaluation as immunogens. Virology. 2007;358:321. doi: 10.1016/j.virol.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Smith M.L., Fitzmaurice W.P., Turpen T.H., Palmer K.E. Display of peptides on the surface of tobacco mosaic virus particles. Current Topics Microbiol. Immunol. 2009;332:13. doi: 10.1007/978-3-540-70868-1_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staczek J., Bendahmane M., Gilleland L.B., Beachy R.N., Gilleland H.E. Immunization with a chimeric tobacco mosaic virus containing an epitope of outer membrane protein F of Pseudomonas aeruginosa provides protection against challenge with P. aeruginosa. Vaccine. 2000;18:2266. doi: 10.1016/s0264-410x(99)00571-x. [DOI] [PubMed] [Google Scholar]
- Steele J.F.C., Peyret H., Saunders K., Castells-Graells R., Marsian J., Meshcheriakova Y., Lomonossoff G.P. Synthetic plant virology for nanobiotechnology and nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017;9:e1447. doi: 10.1002/wnan.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz N.F., Findlay K.C., Noel T.R., Parker R., Lomonossoff G.P., Evans D.J. Layer-by-layer assembly of viral nanoparticles and polyelectrolytes: the film architecture is different for spheres versus rods. Chembiochem. 2008;9:1662. doi: 10.1002/cbic.200800070. [DOI] [PubMed] [Google Scholar]
- Strable E., Finn M.G. Chemical modification of viruses and virus-like particles. Current Topics Microbiol. Immunol. 2009;332:1. doi: 10.1007/978-3-540-69379-6_1. [DOI] [PubMed] [Google Scholar]
- Stubbs G., Warren S., Holmes K. Structure of RNA and RNA binding site in tobacco mosaic virus from 4-Å map calculated from X-ray fibre diagrams. Nature. 1977;267:216. doi: 10.1038/267216a0. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y., Hamamoto H., Takemoto S., Watanabe Y., Okada Y. Systemic production of foreign peptides on the particle surface of tobacco mosaic virus. FEBS Lett. 1995;359:247. doi: 10.1016/0014-5793(95)00054-d. [DOI] [PubMed] [Google Scholar]
- Takamatsu N., Watanabe Y., Yanagi H., Meshi T., Shiba T., Okada Y. Production of enkephalin in tobacco protoplasts using tobacco mosaic virus RNA vector. FEBS Lett. 1990;269:73. doi: 10.1016/0014-5793(90)81121-4. [DOI] [PubMed] [Google Scholar]
- Tan W.S., Lewis C.L., Horelik N.E., Pregibon D.C., Doyle P.S., Yi H. Hierarchical assembly of viral nanotemplates with encoded microparticles via nucleic acid hybridization. Langmuir. 2008;24 doi: 10.1021/la802089q. [DOI] [PubMed] [Google Scholar]
- Tiu B.D.B., Kernan D.L., Tiu S.B., Wen A.M., Zheng Y., Pokorski J.K., Advincula R.C., Steinmetz N.F. Electrostatic layer-by-layer construction of fibrous TMV biofilms. Nanoscale. 2017;9:1580. doi: 10.1039/c6nr06266k. [DOI] [PubMed] [Google Scholar]
- Tsukamoto R., Muraoka M., Seki M., Tabata H., Yamashita I. Synthesis of CoPt and FePt3 nanowires using the central channel of tobacco mosaic virus as a biotemplate. Chem. Mater. 2007;19:2389. [Google Scholar]
- Turner D.R., McGuigan C.J., Butler P.J.G. Assembly of hybrid RNAs with tobacco mosaic virus coat protein. Evidence for incorporation of disks in 5′-elongation along the major RNA tail. J. Mol. Biol. 1989;209:407. doi: 10.1016/0022-2836(89)90006-5. [DOI] [PubMed] [Google Scholar]
- Turpen T.H., Reinl S.J., Charoenvit Y., Hoffman S.L., Fallarme V., Grill L.K. Malarial epitopes expressed on the surface of recombinant tobacco mosaic virus. Biotechnology. 1995;13:53. doi: 10.1038/nbt0195-53. [DOI] [PubMed] [Google Scholar]
- Wen A.M., Steinmetz N.F. Design of virus-based nanomaterials for medicine, biotechnology, and energy. Chem. Soc. Rev. 2016;45:4035. doi: 10.1039/c5cs00287g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S., Marillonnet S., Hause G., Klimyuk V., Gleba Y. Immunoabsorbent nanoparticles based on a tobamovirus displaying protein A. Proc. Natl. Acad. Sci. U.S.A. 2006;103 doi: 10.1073/pnas.0608869103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T.M.A. Plant viruses: a tool-box for genetic engineering and crop protection. Bioessays. 1989;10:179. doi: 10.1002/bies.950100602. [DOI] [PubMed] [Google Scholar]
- Wnek M., Gorzny Ł., Ward M.B., Walti C., Davies A.G., Brydson R., Evans S.D., Stockley P.G. Fabrication and characterization of gold nano-wires template on virus-like arrays of tobacco mosaic virus coat proteins. Nanotechnology. 2013;24 doi: 10.1088/0957-4484/24/2/025605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Jiang L., Zhou Z., Fan J., Zhang Q., Zhu H., Han Q., Xu Z. Expression of foot-and-mouth disease virus epitopes in tobacco by a tobacco mosaic virus-based vector. Vaccine. 2003;21:4390. doi: 10.1016/s0264-410x(03)00428-6. [DOI] [PubMed] [Google Scholar]
- Wu Z., Mueller A., Degenhard S., Ruff S.E., Geiger F., Bittner A., Wege C., Krill C., III Enhancing the magnetoviscosity of ferrofluids by the addition of biological nanotubes. ACS Nano. 2010;4:4531. doi: 10.1021/nn100645e. [DOI] [PubMed] [Google Scholar]
- Wu L.Y., Zang J.F., Lee L.A., Niu Z.W., Horvatha G.C., Braxtona V., Wibowo A.C., Bruckman M.A., Ghoshroy S., zur Loye H.C., Li X.D., Wang Q. Electrospinning fabrication, structural and mechanical characterization of rod-like virus-based composite nanofibers. J. Mater. Chem. 2011;21:8550. [Google Scholar]
- Yi H.M., Nisar S., Lee S.Y., Powers M.A., Bentley W.E., Payne G.F., Ghodssi R., Rubloff G.W., Harris M.T., Culver J.N. Patterned assembly of genetically modified viral nanotemplates via nucleic acid hybridization. Nano Lett. 2005;5:1931. doi: 10.1021/nl051254r. [DOI] [PubMed] [Google Scholar]
- Yi H.M., Rubloff G.W., Culver J.N. TMV microarrays: hybridization-based assembly of DNA-programmed viral nanotemplates. Langmuir. 2007;23:2663. doi: 10.1021/la062493c. [DOI] [PubMed] [Google Scholar]
- Young N., Forney J., Zaitlin M. Tobacco mosaic virus replicase and replicative structures. J. Cell Sci. Suppl. 1987;7:277. doi: 10.1242/jcs.1987.supplement_7.19. [DOI] [PubMed] [Google Scholar]
- Zhao X., Lin Y., Wang Q. Virus-based scaffolds for tissue engineering applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015;7:534. doi: 10.1002/wnan.1327. [DOI] [PubMed] [Google Scholar]
- Zhou K., Li F., Dai G., Meng C., Wang Q. Disulfide bond: dramatically enhanced assembly capability and structural stability of tobacco mosaic virus nanorods. Biomacromolecules. 2013;14:2593. doi: 10.1021/bm400445m. [DOI] [PubMed] [Google Scholar]
- Zhou K., Zhang J., Wang Q. Site-selective nucleation and controlled growth of gold nanostructures in tobacco mosaic virus nanotubulars. Small. 2015;11:2505. doi: 10.1002/smll.201401512. [DOI] [PubMed] [Google Scholar]
- Zimmern D. The nucleotide sequence at the origin for assembly on tobacco mosaic virus RNA. Cell. 1977;11:463. doi: 10.1016/0092-8674(77)90065-4. [DOI] [PubMed] [Google Scholar]
- Zimmern D., Butler P.J.G. The isolation of tobacco mosaic virus RNA fragments containing the origin for viral assembly. Cell. 1977;11:455. doi: 10.1016/0092-8674(77)90064-2. [DOI] [PubMed] [Google Scholar]
- Zimmern D., Wilson T.M.A. Location of the origin for viral reassembly on tobacco mosaic virus RNA and its relation to stable fragment. FEBS Lett. 1976;71:294. doi: 10.1016/0014-5793(76)80954-4. [DOI] [PubMed] [Google Scholar]