Abstract
Alcoholic liver disease (ALD) is the most prevalent type of chronic liver disease with significant morbidity and mortality worldwide. ALD begins with simple hepatic steatosis and progresses to alcoholic steatohepatitis, fibrosis, and cirrhosis. The severity of hepatic steatosis is highly associated with the development of later stages of ALD. This review explores the disturbances of alcohol-induced hepatic lipid metabolism through altered hepatic lipid uptake, de novo lipid synthesis, fatty acid oxidation, hepatic lipid export, and lipid droplet formation and catabolism. In addition, we review emerging data on the contributions of genetics and bioactive lipid metabolism in alcohol-induced hepatic lipid accumulation.
Keywords: alcoholic liver disease, steatosis, fatty acid oxidation, lipogenesis, fatty acid uptake, lipid droplets
Globally, approximately two billion people consume alcoholic beverages, and alcohol abuse is a leading cause of liver-associated morbidity and mortality (1). The spectrum of alcoholic liver disease (ALD) ranges from simple steatosis to alcoholic steatohepatitis, progressive fibrosis, and cirrhosis. Currently, there are no accepted therapeutics to halt or reverse ALD in patients, despite the profound economic and health impacts of ALD. The earliest and most common hepatic response to alcohol is excess fat accumulation (steatosis). Alcoholic fatty liver is diagnosed when alcohol consumption results in hepatic fat exceeding 5% of the liver weight. Nearly all alcohol consumers develop steatosis (2), widely considered to be a less injurious stage than advanced stages of liver disease but whose severity is highly linked to the development of later stages of ALD (3) and that results from alcohol’s toxic effects on hepatic lipid metabolism. In contradistinction, alcohol intake alone is insufficient to cause significant liver disease in the majority of consumers, although the amount of alcohol consumed is highly associated with the severity of ALD (4). Considering the high incidence of steatosis and lack of approved treatments for ALD, a better understanding of the mechanisms by which steatosis develops is essential to identify early biomarkers and develop ALD therapeutics, especially for those who have been unable to achieve abstinence.
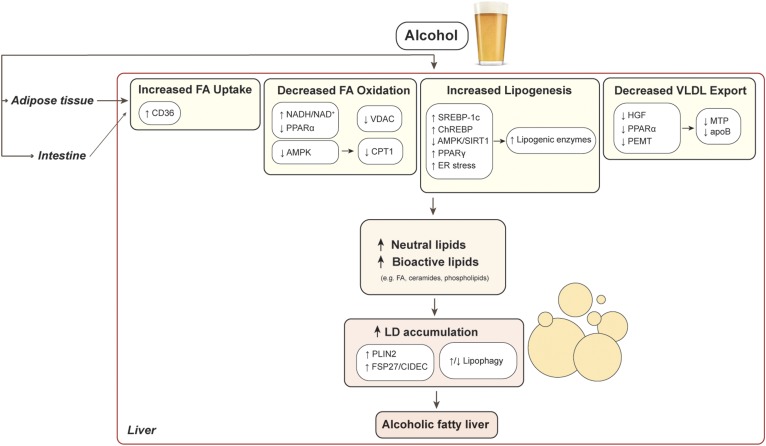
The liver is the major organ responsible for metabolizing ingested alcohol to the toxic metabolite acetaldehyde (5), and most data support that it is alcohol’s metabolism per se that is required for hepatic lipid dysregulation. Once metabolized, alcohol exerts a myriad of effects on hepatic lipid regulation that promote steatosis (Fig. 1). In this review, we focus predominantly on alcohol’s dysregulation of triglyceride metabolism, the predominant lipids implicated in the development of steatosis. From effects on the production of triglyceride to effects on fatty acid uptake and oxidation, lipophagy, triglyceride export, and hepatocellular lipid storage, alcohol impacts cellular lipid homeostasis, which in turn has implications for cellular dysmetabolic stress and inflammation pathways thought to spur liver disease progression. In addition, we explore the role of bioactive lipids in alcohol-induced lipid accumulation, an emerging area of study that has broad implications for the transition from steatosis to more advanced stages of ALD.
Fig. 1.
Mechanisms of alcohol-induced hepatic lipid accumulation.
EFFECTS OF ALCOHOL ON HEPATIC FATTY ACID UPTAKE
As triglycerides are the predominant hepatic neutral lipids that accumulate in ALD, significant attention has been paid to alcohol’s effects on fatty acids whose esterification results in triglyceride synthesis. Exogenous sources of hepatocyte fatty acids include the uptake of circulating NEFAs from adipose tissue lipolysis and intestinally derived chylomicrons. Chronic alcohol consumption exacerbates adipose tissue lipolysis in large part through inciting adipose tissue insulin resistance (6), although other mechanisms, such as alcohol-induced catecholamine release, play a role (7, 8). Indeed, in alcohol-fed rodents (9, 10), peripheral adipose depots are reduced compared with controls. This is in line with our own data demonstrating that alcohol worsens insulin sensitivity in part by inhibiting the lipogenic response of adipocytes to insulin (11), a finding that correlates with observations in clinical studies showing that patients with alcohol addiction have less fat mass and increased NEFA levels (12, 13). In rodents, these effects on adipose tissue lipolysis and subsequent hepatic fatty acid uptake take place as early as within 2 weeks of chronic alcohol ingestion based on high resolution mass spectrometry and in vivo deuterium labeling (10).
Alcohol consumption can also increase the supply of intestine-derived fatty acids from chylomicrons (14). Chylomicrons are formed by intestinal enterocytes and are taken up by hepatocytes as chylomicron remnants via the LDL receptor and the LDL receptor-related protein after undergoing lipolysis by lipases (15, 16). Although this is an established chylomicron clearance pathway, chylomicron-derived fatty acids are not major sources of alcohol-induced hepatic neutral lipid accumulation (17), unlike what is observed with white adipose tissue-derived fatty acids (18).
Upon circulating to the liver, NEFAs enter hepatocytes via hepatic fatty acid transporters. Hepatic plasma membrane fatty acid transporters include fatty acid transporter proteins (FATPs) and fatty acid translocase/CD36. Among six isoforms of FATPs, FATP2 and FATP5 are highly expressed in the liver, while CD36 expression is normally low (19). Although CD36 does not play a significant role in fatty acid transport in normal liver, CD36 is highly inducible and contributes to hepatic steatosis under pathological conditions such as excessive alcohol consumption (20). In fact, chronic alcohol upregulates the expression of CD36 while there is no clear consensus on the expression of FATP2 and FATP5 in the liver (9, 21, 22). Furthermore, CD36 ablation alleviates ethanol-induced hepatic lipid accumulation, corroborating the participation of CD36 in the development of alcoholic steatosis (22). As a consequence of the increased expression of hepatic fatty acid transporters, alcohol consumption increases the hepatic capacity for exogenous fatty acid uptake. Indeed, fatty acid uptake is increased in primary cultured hepatocytes from alcohol-fed rats (23). Together, these alcohol-induced alterations in both hepatic fatty acid delivery and capacity for uptake contribute to increased hepatic lipid accumulation and resultant hepatic steatosis.
EFFECTS OF ALCOHOL ON HEPATIC FATTY ACID OXIDATION
Excessive alcohol consumption impairs fatty acid catabolism predominantly through inhibition of mitochondrial β-oxidation, which we and others have determined is the most significant contribution to alcohol-induced hepatic lipid accumulation (24–28). In addition to the direct toxic effects of alcohol on mitochondria (29), alcohol oxidation increases the NADH:NAD+ ratio, thus promoting alcohol oxidation at the expense of fatty acid oxidation (12, 30, 31). Alcohol also inhibits carnitine palmitoyltransferase (CPT)1 activity, a rate-limiting step in fatty acid translocation for mitochondrial β-oxidation, by reducing AMP-activated protein kinase (AMPK) activity and increasing acetyl-CoA carboxylase (ACC) activity and malonyl-CoA levels (32). Considering that carnitine is an essential cofactor for CPT1, alcohol-induced carnitine deficiency is another plausible mechanism of mitochondrial β-oxidation inhibition. Indeed, lower concentrations of plasma carnitine have been reported in alcoholic patients and chronically alcohol-fed animals (33, 34). In addition to effects on CPT1, ethanol closes voltage-dependent anion channels (VDACs) in the mitochondrial outer membrane (35–37). VDACs are responsible for outer membrane permeability for metabolites including long-chain fatty acyl-CoA, which can lead to suppressed β-oxidation. In cultured rat hepatocytes, ethanol and acetaldehyde inhibit VDAC conductance (36, 37).
PPARα
PPARα is a key transcriptional regulator of many genes involved in mitochondrial oxidation and whose ligands include free fatty acids and fatty acid derivatives (38). On forming a heterodimer with retinoid-X receptor, activated PPARα binds DNA recognition sites to regulate the transcription of target genes. Even with the increased free fatty acids released from adipose tissue, alcohol is able to suppress PPARα signaling by decreasing PPARα binding activity, decreasing protein levels of retinoid-X receptor, and downregulating AMPK. In mice, PPARα agonists (Wy14643 and clofibrate) override these effects of alcohol on PPARα signaling and prevent hepatic steatosis (39). Despite the potential utility of PPARα agonism in alcoholic steatosis, there have been no clinical studies to examine its efficacy in humans with ALD.
SIRT1-AMPK axis
AMPK is a serine-threonine kinase protein complex that helps maintain cellular energy homeostasis. Once activated by allosteric binding of AMP and by phosphorylation via one of its upstream kinases, AMPK rewires metabolism to inhibit anabolic processes and augment catabolism (40). Activated AMPK inhibits lipid synthesis via inhibitory phosphorylation of ACC. Inactivation of ACC leads to decreased levels of malonyl-CoA, an inhibitor of CPT1, which promotes fatty acid oxidation. AMPK also inhibits lipogenesis by inhibiting SREBP-1c and carbohydrate response element-binding protein (ChREBP) as discussed later. In ethanol-fed rodents, AMPK activity is decreased, which increases hepatocellular lipid accumulation through the activation of ACC, SREBP-1c, and ChREBP (41).
Sirtuin 1 (SIRT1) is an NAD+-dependent deacetylase that acts as a master sensor of NAD+ and modulates cellular metabolism. In the liver, SIRT1 plays a pivotal role in the regulation of lipid metabolism by modifying the acetylation status of various targets (42). The interdependent regulation between SIRT1 and AMPK in hepatic lipid metabolism is well established (43). SIRT1 stimulates AMPK by modulating liver kinase B1 (LKB1), an upstream AMPK kinase; conversely, AMPK increases cellular NAD+ levels, which subsequently activates SIRT1. Hence, the SIRT1-AMPK axis is a central signaling system that controls lipid metabolism. Chronic ethanol administration impairs the hepatic SIRT1-AMPK axis in several animal models (32, 44), thus stimulating lipogenesis. Resveratrol, an agonist of both SIRT1 and AMPK, shows its protective action against hepatic lipid accumulation by stimulating the hepatic SIRT1-AMPK axis in alcohol-fed mice (45).
EFFECTS OF ALCOHOL ON HEPATIC DE NOVO LIPOGENESIS
Besides uptake of fatty acids from extrahepatic sources, hepatocytes can synthesize fatty acids from nonlipid precursors such as sugars and proteins (46). De novo lipogenesis employs several lipogenic enzymes including ACC, FAS, and stearoyl-CoA desaturase 1 (SCD1) that are controlled coordinately by transcriptional factors and metabolic regulators including clock genes (47). In addition, a series of key enzymes including phosphatidyl phosphatases (PAPs) (e.g., lipin) and acyltransferases [e.g., diacylglycerol acyltransferase (DGAT)] are involved in glycerolipid synthesis, which further contribute to triglyceride and phospholipid production (48). Alcohol exposure modulates many of the lipid regulatory factors and lipogenic enzymes and, in doing so, augments hepatic triglyceride accumulation as reviewed below.
SREBP-1c
SREBP-1c is a transcription factor that regulates expression of ACC, FAS, and SCD1 (49). SREBP-1c is implicated in the pathogenesis of alcohol-induced steatosis, as SREBP-1c knockout mice are protected from alcohol-induced hepatic steatosis (50). SREBP-1c protein and its transcripts are increased in the livers of acutely and chronically ethanol-fed mice (51, 52), but unlike the lipogenic genes it regulates (i.e., ACC and FAS), SREBP-1c does not appear to be influenced by alcohol-specific effects on circadian genes (47). The activation of SREBP-1c in ALD is mediated through several pathways. Acetaldehyde directly induces the maturation of SREBP-1c (52). Alcohol also indirectly activates SREBP-1c via multiple factors, including ER stress, adenosine, and endocannabinoid production (53–56). SREBP-1c is also activated by lipopolysaccharide and TNFα, both of which are elevated in ALD patients (57, 58). Finally, alcohol downregulates AMPK, SIRT1, and signal transducer and activator of transcription 3 (STAT3), factors that suppress SREBP-1c expression (32, 44, 59).
ChREBP
ChREBP is a key transcription factor that acts similarly to SREBP-1c, regulating lipogenic target genes (i.e., FAS, SCD1, elongase 6, glycerol phosphate acyltransferase). ChREBP is activated in mice fed chronically with alcohol by a mechanism that involves dephosphorylation of ChREBP through the inhibition of AMPK and protein phosphatase 2A (PP2A) (60). Acute alcohol feeding also increases ChREBP activity via acetylation by SIRT1 (61). In addition, ChREBP silencing prevents ethanol-induced hepatic steatosis in mice, indicating an important role of ChREBP in steatosis under binge drinking conditions.
PPARγ
PPARγ, a member of the nuclear hormone receptor superfamily involved in lipid metabolism (62), also contributes to the pathogenesis of ALD. The role of PPARγ in ALD is less established than the contributions of other pathways. Namely, ethanol upregulates protein expression of both PPARγ1 and PPARγ2 isoforms in the steatotic livers of mice (63) and knockdown of hepatic PPARγ alleviates alcohol-induced lipid accumulation in mouse livers by blunting SREBP-1c and lipogenic genes such as Fas, Dgat1, and Dgat2. However, pioglitazone, a systemic PPARγ agonist that promotes adipocyte lipogenesis, alleviates hepatic lipid accumulation in alcohol-fed rodents (64, 65). These data demonstrate that there are likely tissue-specific effects of alcohol on PPARγ that merit further investigation before modulation of this pathway can be recommended as a therapeutic strategy in alcoholic steatosis.
EFFECTS OF ALCOHOL ON GLYCEROLIPID SYNTHESIS
Among a series of enzymes involved in glycerolipid synthesis, lipin-1 has gained attention as one key enzyme explaining the pathogenesis of ALD. Lipin-1 plays dual functions in lipid metabolism as a PAP enzyme and as a transcriptional coactivator depending on its subcellular localization (66). When in the cytoplasm, lipin-1 can transiently translocate to the ER, interact with substrates, and generate diacylglycerol, a precursor of triglycerides and phospholipids. In the nucleus, lipin-1 functions as a transcriptional coactivator to increase fatty acid oxidation by activating PPARα and PPARγ coactivator-1 and suppressing SREBP-1c. Lipin-1 subcellular localization can be modulated by an alternative mRNA splicing process that produces two lipin-1 isoforms (α and β) and posttranslational modifications (e.g., phosphorylation, sumoylation, and acetylation) (66).
Accumulating evidence suggests that lipin-1 plays a pivotal role in the development of ALD. Ethanol exposure upregulates total hepatic lipin-1 expression in cultured hepatocytes and mouse livers (67, 68). In particular, ethanol increases cytosolic localization of lipin-1, resultant PAP activity, and triglyceride synthesis, whereas ethanol reduces its nuclear entry and disturbs fatty acid oxidation and VLDL secretion in mouse liver. The expression and localization of lipin-1 are modulated by multiple signaling molecules including miR-217, SIRT1-AMPK, SREBP-1c, and adiponectin in ALD models (67, 69–71). The underlying mechanisms of altered subcellular localization of lipin-1 in response to ethanol involve the increased hepatic ratio of lipin-1β/α as well as disturbed posttranslational modifications (67, 72).
The final step of triglyceride biosynthesis is catalyzed by DGAT enzymes, whose gene is encoded by DGAT1 and DGAT2. To catalyze the esterification reaction between a fatty-acyl CoA and diacylglycerol, DGAT1 preferentially targets exogenous fatty acids while DGAT2 utilizes mostly de novo synthesized fatty acids (73, 74). Chronic alcohol exposure upregulates hepatic expression of both DGAT1 and DGAT2 in mice (75, 76); however, the specific mechanistic role of DGAT in alcohol-induced triglyceride formation is unknown.
EFFECTS OF ALCOHOL ON HEPATIC LIPID EXPORT
Hepatocytes export neutral lipids by packaging them into VLDL, which prevents intrahepatic triglyceride accumulation. VLDL secretion depends on the availability of hepatic lipids and the hepatocytes’ capacity for VLDL assembly. VLDL particles are generated in the ER upon apoB100 lipidation, which is facilitated by microsomal triglyceride transfer protein. Nascent VLDL is then transferred to the Golgi apparatus where mature VLDL is formed.
Alcohol impairs VLDL assembly and secretion. Ethanol-fed rodents have decreased apoB synthesis and hepatic microsomal triglyceride transfer protein expression and activity, effects reversed by pharmacological hepatocyte growth factor administration and PPARα agonism, respectively (77–79). Alcohol’s alteration of methionine metabolism is an additional mechanism by which VLDL secretion is impaired. Namely, ethanol reduces S-adenosyl methionine (SAM) levels. The SAM-dependent enzyme phosphatidylethanolamine methyltransferase (PEMT) is responsible for the production of the VLDL lipid phosphatidylcholine (PC), and PEMT inhibition causes steatosis in mice (80, 81). Consequently, alcohol’s inhibitory effects on SAM impair VLDL production indirectly through PEMT inhibition and PC reduction (82).
EFFECTS OF ALCOHOL ON NEUTRAL LIPID STORAGE IN LIPID DROPLETS
In the liver, neutral lipids (triglycerides and cholesterol esters) are stored in lipid droplets (LDs) (83). Compared with the effects on triglyceride metabolism, the effect of alcohol on hepatic cholesterol esters is less well established but may be increased (84). Nascent LDs are thought to form from budding of the outer membrane of the ER, and as such, nascent LDs and outer ER membrane have several membrane proteins in common. The triglyceride synthetic enzyme DGAT is noteworthy (85, 86). The core of hepatocyte LD lipids is enveloped by a phospholipid monolayer of proteins involved in lipid and glucose homeostasis (83, 85). The major hepatocyte LD family of proteins is the perilipin (PLIN) family. In addition to PLINs, LD membranes incorporate a diverse set of surface proteins including proteins involved in LD growth and triglyceride synthesis, the cell death-inducing DNA fragmentation factor-α-like effector (CIDE) protein family (87, 88), and DGAT enzymes (48), respectively. Alcohol’s regulation of these LD proteins and the contribution of LD-associated genetic factors are discussed below.
PLINs
PLIN proteins (formerly PAT proteins, named for perilipin adipocyte differentiation-related protein and tail-interacting protein of 47 kDa) are an ancient family of LD binding proteins whose regulation is important for LD formation and degradation (89). In mammals, there are five isoforms of PLINs (PLIN1–5) with shared sequence homology but with unique tissue-specific roles in LD biology. PLIN1 and PLIN2 are constitutively bound to the LD membrane while PLINs 3–5 are considered exchangeable proteins that can attach to and detach from the LD membrane. Normal liver expresses PLIN2, -3, and -5 (90–94), while there is increased expression of PLIN1, -2, -3, and -5 in fatty liver (93–96). Among the PLIN family proteins, only PLIN2 and PLIN3 have been implicated in alcohol-induced hepatic lipid accumulation. Namely, we and others demonstrated that PLIN2 upregulation occurs in alcohol-fed rodents and humans (93, 97, 98). Our laboratory additionally observed that hepatic PLIN2 upregulation coincides with the onset of steatosis and insulin resistance in an experimental rodent model of chronic alcohol consumption (11) and conversely, that alcohol-fed PLIN2-null mice are refractory to hepatic steatosis and glucose intolerance (98). These findings establish a critical role of PLIN2 in alcoholic steatosis development and alcohol-induced glucose dysregulation, the latter of which is associated with the risk of progressive ALD (6, 99).
In contrast to the constitutively bound PLIN2, PLIN3 is an exchangeable protein, which is stable in the cytosol in the quiescent state and able to be recruited to nascent LDs (100, 101). Although some rodent models have not demonstrated a significant role of PLIN3 in ALD pathogenesis (11, 98), other investigations point to an inhibitory role of alcohol in PLIN3-mediated lipid export from the ER and resultant ER lipotoxic stress (102). These data may suggest a potentially protective role of PLIN3 against ALD, but further studies are warranted to clarify this relationship.
CIDEC
Once nascent LDs form, they can expand via local triglyceride synthesis. So-called macrosteatosis results from the coalescence of smaller LDs, thought to be mediated by the CIDE family of proteins (103). Hepatic expression of Fsp27/Cidec (but not Cidea and Cideb) is upregulated in chronic-plus-binge ethanol-fed mice. Moreover, hepatic expression of FSP27/CIDEC correlates with the degree of steatosis in human alcoholic hepatitis patients (104).
LD-associated genetic factors
Emerging evidence suggests that LD-associated genetic factors account for the susceptibility of alcoholic patients to ALD. Several genome-wide association studies demonstrated that patatin-like phospholipase domain-containing protein 3 (PNPLA3), transmembrane 6 superfamily member 2 (TM6SF2), and membrane bound O-acyltransferase domain-containing 7 (MBOAT7) are important genetic determinants of the risk and severity of ALD (105–107). Of these, the PNPLA3 missense I148M mutation is considered the most important risk allele for the development of ALD and the related condition, nonalcoholic fatty liver disease (108). PNPLA3 has been shown to have triglyceride hydrolase and transacylase activity, but its physiological role is not completely understood due to discordant findings in mouse models (109–111). The risk variant of PNPLA3 accumulates on LDs in mice (112) and, in vitro, impairs lipophagy (113).
Like polymorphisms in PNPLA3, the rs641738 C>T variant in MBOAT7 is linked to an increased risk of alcohol-related cirrhosis (108). This MBOAT variant is also associated with an increased risk of steatosis and fibrosis (114). MBOAT7 is a multi-spanning transmembrane protein and works in the remodeling cycle of phospholipids, involved in the transfer of fatty acids between phospholipids and lysophospholipids (115, 116), but the precise role of MBOAT7 in hepatic lipid accumulation remains unclear.
A polymorphism of the PLIN2 gene has been reported (117). A minor allele of this missense polymorphism, Ser251Pro, impairs formation of a PLIN2 α-helix, causing reduced plasma triglyceride and VLDL levels in humans. Although there is a plausible impact of the PLIN2 polymorphism on alcoholic fatty liver, its role, like those of the aforementioned polymorphisms, remains unclear.
EFFECTS OF ALCOHOL ON LD CATABOLISM
Catabolism of LDs takes two forms: lipolysis by cytosolic lipases such as ATGL and autophagy-mediated mobilization. Autophagy is a recycling process by which cellular components are sequestered within vesicles and fuse with lysosomes for degradation. Autophagic degradation of LDs is referred to as lipophagy (118). Lipophagy has been established to play an important role in experimental alcoholic steatosis (119); however, acute alcohol feeding and chronic alcohol feeding have differential effects on lipophagy. Namely, acute ethanol administration induces autophagy in animal models (120–122), while chronic alcohol inhibits lipophagy. This inhibition of lipophagy by chronic alcohol results from lysosomal damage, suppression of lysosomal proteolytic activity, and inhibition of autolysosome formation (123, 124). These data emphasize the importance of differentiating the acute versus chronic effects of alcohol on lipid metabolism and tailoring potential therapies accordingly.
EFFECTS OF ALCOHOL ON BIOACTIVE LIPIDS
Lipidomics analysis has demonstrated that chronic alcohol consumption promotes the accumulation of both neutral and bioactive lipids within hepatocytes (125–128). Neutral lipids such as cholesterol esters and triglycerides (discussed above) lack a charge, have no signaling properties, and are often enclosed within LDs (129, 130). Bioactive lipids are signaling lipid mediators that effect cellular homeostatic and immune pathways (131). Examples include fatty acids, ceramides, and phospholipids, all of which have been implicated in ALD pathogenesis.
Fatty acids
Increased hepatic fatty acids are one notable feature in ALD, reported by many lipidomic studies in experimental ALD rodent models (126, 132–135). As mentioned above, there are multiple mechanisms to increase hepatic fatty acids in ALD. Interestingly, alcohol exposure alters not only the quantity but also the composition of fatty acids in the liver. Alcohol shifts the fatty acid composition from saturated fatty acids toward unsaturated (mono- and polyunsaturated) fatty acids in alcohol-fed rodents (126–128, 136). In addition, 18-carbon fatty acids with different degrees of saturation (C18:0, C18:1, C18:2, and C18:3) as well as docosahexaenoic acid (C22:6) are notably elevated in alcohol-exposed livers (126, 132, 133). Even with these consistent findings in hepatic fatty acid composition in ALD animal models and several studies demonstrating efficacy of a saturated fatty acid diet in experimental ALD (137–139), data in humans are lacking and limit progress toward applying dietary strategies to human ALD.
Ceramides
Altered sphingolipid metabolism is an emerging area of ALD pathogenesis. In particular, the bioactive lipid ceramide accumulates in the livers of patients with ALD and in animal models of ALD (140, 141). Ceramides are bioactive lipids that can impair insulin signaling, induce oxidative stress, impair fat oxidation, and increase lipoprotein aggregation (142–144), all of which are linked to the pathogenesis of ALD as discussed above. Ceramide de novo synthesis is modulated by alcohol exposure, and the mRNA levels of three ceramide synthases (CERS1, CERS5, and CERS6) and the major subunit of serine palmitoyl transferase (SPT) are increased in human livers with advanced ALD (141). In addition, we have demonstrated that CERS6 positively regulates LD accumulation and is upregulated in experimental in vivo and in vitro models of ALD as well as in humans with alcoholic steatosis (145). We and others have demonstrated further that pharmacologic inhibition of ceramide synthesis blunts ethanol-induced steatosis and improves glucose tolerance (145–149). Together, these investigations demonstrate that dysregulated sphingolipid metabolism plays a critical role in alcoholic steatosis and ALD pathogenesis and present a novel therapeutic opportunity for ALD management.
Phospholipids
Alcohol exposure disturbs phospholipid metabolism in the livers of ALD patients and animal models (127, 150). Among various phospholipids, PC and phosphatidylethanolamine (PE) are two major phospholipids that are distributed in the plasma membrane. Hepatic PC is made from choline catalyzed by the CDP-choline pathway and is also made by PEMT that converts PE to PC via three sequential steps of methylation (151, 152). Alcohol exposure decreases hepatic PC as well as the ratio of PC/PE in ALD patients and animal models (127, 150). Alcohol-induced reduction in hepatic PC levels occurs by choline deficiency, decreased PEMT activity, and reduced availability of the methyl groups (153–156). Dietary PC supplementation attenuates ethanol-induced fibrosis in baboons (157, 158); dietary betaine administration also attenuates alcoholic steatosis by promoting PEMT activity in mice (82). Given that the PC/PE ratio determines cellular membrane integrity and plays a role in the development of steatosis and steatohepatitis (159), the ethanol-induced perturbations in phospholipid metabolism are likely to promote the development of ALD.
CONCLUSIONS
In summary, alcohol dysregulates many aspects of hepatic lipid metabolism. Alcohol-induced hepatic fatty acid uptake, impairment of fatty acid oxidation, promotion of de novo lipid synthesis and neutral lipid storage, and inhibition of lipid export and LD catabolism are all pathways that converge to cause hepatocellular LD accumulation. In addition to these relatively well-established mechanisms, several emerging areas of alcohol-induced hepatocellular LD regulation have gained attention. In particular, the role of bioactive lipids, such as sphingolipids, and genetics are likely to unveil novel biomarkers and create opportunities for early therapeutic intervention for those at risk of advanced disease.
Acknowledgments
The authors thank the Center for Molecular Studies in Digestive and Liver Diseases (National Institutes of Health Grant P30 DK050306) and its core facilities (Molecular Pathology and Imaging Core, Molecular Biology/Gene Expression Core, and Transgenic and Chimeric Mouse Core).
Footnotes
Abbreviations:
- ACC
- acetyl-CoA carboxylase
- ALD
- alcoholic liver disease
- AMPK
- AMP-activated protein kinase
- ChREBP
- carbohydrate response element-binding protein
- CIDE
- cell death-inducing DNA fragmentation factor-α-like effector
- CPT
- carnitine palmitoyltransferase
- DGAT
- diacylglycerol acyltransferase
- FATP
- fatty acid transporter protein
- LD
- lipid droplet
- MBOAT7
- membrane bound O-acyltransferase domain-containing 7
- PAP
- phosphatidyl phosphatase
- PC
- phosphatidylcholine
- PE
- phosphatidylethanolamine
- PEMT
- phosphatidylethanolamine methyltransferase
- PLIN
- perilipin
- PNPLA3
- patatin-like phospholipase domain-containing protein 3
- SAM
- S-adenosyl methionine
- SCD1
- stearoyl-CoA desaturase 1
- SIRT1
- sirtuin 1
- VDAC
- voltage-dependent anion channel
This work was supported by National Institutes of Health Grants R01 AA026302-02 and P30 DK0503060. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. R.C. receives research salary support from Intercept and has received compensation as an invited speaker at Boehringer. S.J. declares no conflicts of interest with the contents of this article.
REFERENCES
- 1.Asrani S. K., Devarbhavi H., Eaton J., and Kamath P. S.. 2019. Burden of liver diseases in the world. J. Hepatol. 70: 151–171. [DOI] [PubMed] [Google Scholar]
- 2.Edmondson H. A., Peters R. L., Frankel H. H., and Borowsky S.. 1967. The early stage of liver injury in the alcoholic. Medicine (Baltimore). 46: 119–129. [DOI] [PubMed] [Google Scholar]
- 3.Powell E. E., Jonsson J. R., and Clouston A. D.. 2005. Steatosis: co-factor in other liver diseases. Hepatology. 42: 5–13. [DOI] [PubMed] [Google Scholar]
- 4.Lazo M., and Mitchell M. C.. 2016. Epidemiology and risk factors for alcoholic liver disease. In Alcoholic and Non-Alcoholic Fatty Liver Disease: Bench to Bedside. N. Chalasani and G. Szabo, editors. Springer International Publishing, Cham, Switzerland. 1–20. [Google Scholar]
- 5.Zakhari S. 2006. Overview: how is alcohol metabolized by the body? Alcohol Res. Health. 29: 245–254. [PMC free article] [PubMed] [Google Scholar]
- 6.Carr R. M., and Correnti J.. 2015. Insulin resistance in clinical and experimental alcoholic liver disease. Ann. N. Y. Acad. Sci. 1353: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao C., Liu Y., Xiao J., Liu L., Chen S., Mohammadi M., McClain C. J., Li X., and Feng W.. 2015. FGF21 mediates alcohol-induced adipose tissue lipolysis by activation of systemic release of catecholamine in mice. J. Lipid Res. 56: 1481–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leggio L., Malandrino N., Ferrulli A., Cardone S., Miceli A., Gasbarrini G., Capristo E., and Addolorato G.. 2009. Is cortisol involved in the alcohol-related fat mass impairment? A longitudinal clinical study. Alcohol Alcohol. 44: 211–215. [DOI] [PubMed] [Google Scholar]
- 9.Zhong W., Zhao Y., Tang Y., Wei X., Shi X., Sun W., X. Sun, X. Yin, X. Sun, S. Kim, et al. 2012. Chronic alcohol exposure stimulates adipose tissue lipolysis in mice: role of reverse triglyceride transport in the pathogenesis of alcoholic steatosis. Am. J. Pathol. 180: 998–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei X., Shi X., Zhong W., Zhao Y., Tang Y., Sun W., X. Yin, B. Bogdanov, S. Kim, C. McClain, et al. 2013. Chronic alcohol exposure disturbs lipid homeostasis at the adipose tissue-liver axis in mice: analysis of triacylglycerols using high-resolution mass spectrometry in combination with in vivo metabolite deuterium labeling. PLoS One. 8: e55382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carr R. M., Dhir R., Yin X., Agarwal B., and Ahima R. S.. 2013. Temporal effects of ethanol consumption on energy homeostasis, hepatic steatosis, and insulin sensitivity in mice. Alcohol. Clin. Exp. Res. 37: 1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Addolorato G., Capristo E., Greco A. V., Stefanini G. F., and Gasbarrini G.. 1997. Energy expenditure, substrate oxidation, and body composition in subjects with chronic alcoholism: new findings from metabolic assessment. Alcohol. Clin. Exp. Res. 21: 962–967. [PubMed] [Google Scholar]
- 13.Gandhi B. M., and Raina N.. 1984. Alcohol-induced changes in lipids and lipoproteins. Alcohol. Clin. Exp. Res. 8: 29–32. [DOI] [PubMed] [Google Scholar]
- 14.Lieber C. S., and Savolainen M.. 1984. Ethanol and lipids. Alcohol. Clin. Exp. Res. 8: 409–423. [DOI] [PubMed] [Google Scholar]
- 15.Hussain M. M., Maxfield F. R., Mas-Oliva J., Tabas I., Ji Z. S., Innerarity T. L., and R. W. Mahley. 1991. Clearance of chylomicron remnants by the low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor. J. Biol. Chem. 266: 13936–13940. [PubMed] [Google Scholar]
- 16.Willnow T. E. 1997. Mechanisms of hepatic chylomicron remnant clearance. Diabet. Med. 14 (Suppl. 3): S75–S80. [DOI] [PubMed] [Google Scholar]
- 17.Baraona E., and Lieber C. S.. 1970. Effects of chronic ethanol feeding on serum lipoprotein metabolism in the rat. J. Clin. Invest. 49: 769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donnelly K. L., Smith C. I., Schwarzenberg S. J., Jessurun J., Boldt M. D., and Parks E. J.. 2005. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 115: 1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stahl A., Gimeno R. E., Tartaglia L. A., and Lodish H. F.. 2001. Fatty acid transport proteins: a current view of a growing family. Trends Endocrinol. Metab. 12: 266–273. [DOI] [PubMed] [Google Scholar]
- 20.Zhou J., Febbraio M., Wada T., Zhai Y., Kuruba R., He J., J. H. Lee, S. Khadem, S. Ren, S. Li, et al. 2008. Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPARgamma in promoting steatosis. Gastroenterology. 134: 556–567. [DOI] [PubMed] [Google Scholar]
- 21.Ronis M. J., Hennings L., Stewart B., Basnakian A. G., Apostolov E. O., Albano E., T. M. Badger, and D. R. Petersen. 2011. Effects of long-term ethanol administration in a rat total enteral nutrition model of alcoholic liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 300: G109–G119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clugston R. D., Yuen J. J., Hu Y., Abumrad N. A., Berk P. D., Goldberg I. J., W. S. Blaner, and L. S. Huang. 2014. CD36-deficient mice are resistant to alcohol- and high-carbohydrate-induced hepatic steatosis. J. Lipid Res. 55: 239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berk P. D., Zhou S., and Bradbury M. W.. 2005. Increased hepatocellular uptake of long chain fatty acids occurs by different mechanisms in fatty livers due to obesity or excess ethanol use, contributing to development of steatohepatitis in both settings. Trans. Am. Clin. Climatol. Assoc. 116: 335–344. [PMC free article] [PubMed] [Google Scholar]
- 24.Blomstrand R., Kager L., and Lantto O.. 1973. Studies on the ethanol-induced decrease of fatty acid oxidation in rat and human liver slices. Life Sci. 13: 1131–1141. [DOI] [PubMed] [Google Scholar]
- 25.Cederbaum A. I., Lieber C. S., Beattie D. S., and Rubin E.. 1975. Effect of chronic ethanol ingestion on fatty acid oxidation by hepatic mitochondria. J. Biol. Chem. 250: 5122–5129. [PubMed] [Google Scholar]
- 26.Correnti J. M., Gottshall L., Lin A., Williams B., Oranu A., Beck J., J. Chen, M. J. Bennett, and R. M. Carr. 2018. Ethanol and C2 ceramide activate fatty acid oxidation in human hepatoma cells. Sci. Rep. 8: 12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reboucas G., and Isselbacher K. J.. 1961. Studies on the pathogenesis of the ethanol-induced fatty liver. I. Synthesis and oxidation of fatty acids by the liver. J. Clin. Invest. 40: 1355–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ontko J. A. 1973. Effects of ethanol on the metabolism of free fatty acids in isolated liver cells. J. Lipid Res. 14: 78–86. [PubMed] [Google Scholar]
- 29.Hoek J. B., Cahill A., and Pastorino J. G.. 2002. Alcohol and mitochondria: a dysfunctional relationship. Gastroenterology. 122: 2049–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sonko B. J., Prentice A. M., Murgatroyd P. R., Goldberg G. R., van de Ven M. L., and Coward W. A.. 1994. Effect of alcohol on postmeal fat storage. Am. J. Clin. Nutr. 59: 619–625. [DOI] [PubMed] [Google Scholar]
- 31.Suter P. M., Schutz Y., and Jequier E.. 1992. The effect of ethanol on fat storage in healthy subjects. N. Engl. J. Med. 326: 983–987. [DOI] [PubMed] [Google Scholar]
- 32.You M., Matsumoto M., Pacold C. M., Cho W. K., and Crabb D. W.. 2004. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology. 127: 1798–1808. [DOI] [PubMed] [Google Scholar]
- 33.Sachan D. S., Rhew T. H., and Ruark R. A.. 1984. Ameliorating effects of carnitine and its precursors on alcohol-induced fatty liver. Am. J. Clin. Nutr. 39: 738–744. [DOI] [PubMed] [Google Scholar]
- 34.Kępka A., Zwierz P., Chojnowska S., Ochocinska A., Skorupa E., Szczepanski M., S. D. Szaida, and N. Waszkiewicz. 2019. Relation of plasma carnitine and aminotransferases to alcohol dose and time of dependence. Alcohol. 81: 62–69. [DOI] [PubMed] [Google Scholar]
- 35.Lemasters J. J., and Holmuhamedov E.. 2006. Voltage-dependent anion channel (VDAC) as mitochondrial governator–thinking outside the box. Biochim. Biophys. Acta. 1762: 181–190. [DOI] [PubMed] [Google Scholar]
- 36.Holmuhamedov E., and Lemasters J. J.. 2009. Ethanol exposure decreases mitochondrial outer membrane permeability in cultured rat hepatocytes. Arch. Biochem. Biophys. 481: 226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holmuhamedov E. L., Czerny C., Beeson C. C., and Lemasters J. J.. 2012. Ethanol suppresses ureagenesis in rat hepatocytes: role of acetaldehyde. J. Biol. Chem. 287: 7692–7700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mandard S., Müller M.and Kersten S.. 2004. Peroxisome proliferator-activated receptor α target genes. Cell. Mol. Life Sci. 61: 393–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fischer M., You M., Matsumoto M., and Crabb D. W.. 2003. Peroxisome proliferator-activated receptor α (PPARα) agonist treatment reverses PPARα dysfunction and abnormalities in hepatic lipid metabolism in ethanol-fed mice. J. Biol. Chem. 278: 27997–28004. [DOI] [PubMed] [Google Scholar]
- 40.Herzig S., and Shaw R. J.. 2018. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 19: 121–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shearn C. T., Smathers R. L., Jiang H., Orlicky D. J., Maclean K. N., and Petersen D. R.. 2013. Increased dietary fat contributes to dysregulation of the LKB1/AMPK pathway and increased damage in a mouse model of early-stage ethanol-mediated steatosis. J. Nutr. Biochem. 24: 1436–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang H-C., and Guarente L.. 2014. SIRT1 and other sirtuins in metabolism. Trends Endocrinol. Metab. 25: 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruderman N. B., Xu X. J., Nelson L., Cacicedo J. M., Saha A. K., Lan F., and Y. Ido. 2010. AMPK and SIRT1: a long-standing partnership? Am. J. Physiol. Endocrinol. Metab. 298: E751–E760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.You M., Liang X., Ajmo J. M., and Ness G. C.. 2008. Involvement of mammalian sirtuin 1 in the action of ethanol in the liver. Am. J. Physiol. Gastrointest. Liver Physiol. 294: G892–G898. [DOI] [PubMed] [Google Scholar]
- 45.Ajmo J. M., Liang X., Rogers C. Q., Pennock B., and You M.. 2008. Resveratrol alleviates alcoholic fatty liver in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 295: G833–G842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanders F. W., and Griffin J. L.. 2016. De novo lipogenesis in the liver in health and disease: more than just a shunting yard for glucose. Biol. Rev. Camb. Philos. Soc. 91: 452–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou P., Ross R. A., Pywell C. M., Liangpunsakul S., and Duffield G. E.. 2014. Disturbances in the murine hepatic circadian clock in alcohol-induced hepatic steatosis. Sci. Rep. 4: 3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yen C-L. E., Stone S. J., Koliwad S., Harris C., and Farese R. V.. 2008. DGAT enzymes and triacylglycerol biosynthesis. J. Lipid Res. 49: 2283–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Foufelle F., and Ferre P.. 2002. New perspectives in the regulation of hepatic glycolytic and lipogenic genes by insulin and glucose: a role for the transcription factor sterol regulatory element binding protein-1c. Biochem. J. 366: 377–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ji C., Chan C., and Kaplowitz N.. 2006. Predominant role of sterol response element binding proteins (SREBP) lipogenic pathways in hepatic steatosis in the murine intragastric ethanol feeding model. J. Hepatol. 45: 717–724. [DOI] [PubMed] [Google Scholar]
- 51.Yin H-Q., Kim M., Kim J-H., Kong G., Kang K-S., Kim H-L., B. I. Yoon, M. O. Lee, and B. H. Lee. 2007. Differential gene expression and lipid metabolism in fatty liver induced by acute ethanol treatment in mice. Toxicol. Appl. Pharmacol. 223: 225–233. [DOI] [PubMed] [Google Scholar]
- 52.You M., Fischer M., Deeg M. A., and Crabb D. W.. 2002. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP). J. Biol. Chem. 277: 29342–29347. [DOI] [PubMed] [Google Scholar]
- 53.Esfandiari F., Medici V., Wong D. H., Jose S., Dolatshahi M., Quinlivan E., S. Dayal, S. R. Lentz, H. Tsukamoto, Y. H. Zhang, et al. 2010. Epigenetic regulation of hepatic endoplasmic reticulum stress pathways in the ethanol-fed cystathionine beta synthase-deficient mouse. Hepatology. 51: 932–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dara L., Ji C., and Kaplowitz N.. 2011. The contribution of endoplasmic reticulum stress to liver diseases. Hepatology. 53: 1752–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peng Z., Borea P. A., Wilder T., Yee H., Chiriboga L., Blackburn M. R., G. Azzena, G. Resta, and B. N. Cronstein. 2009. Adenosine signaling contributes to ethanol-induced fatty liver in mice. J. Clin. Invest. 119: 582–594. [Erratum. 2009. J. Clin. Invest. 119: 1052.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jeong W. I., Osei-Hyiaman D., Park O., Liu J., Batkai S., Mukhopadhyay P., N. Horiguchi, J. Harvey-White, G. Marsicano, B. Lutz, et al. 2008. Paracrine activation of hepatic CB1 receptors by stellate cell-derived endocannabinoids mediates alcoholic fatty liver. Cell Metab. 7: 227–235. [DOI] [PubMed] [Google Scholar]
- 57.Bode C., Kugler V., and Bode J. C.. 1987. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J. Hepatol. 4: 8–14. [DOI] [PubMed] [Google Scholar]
- 58.Endo M., Masaki T., Seike M., and Yoshimatsu H.. 2007. TNF-α induces hepatic steatosis in mice by enhancing gene expression of sterol regulatory element binding protein-1c (SREBP-1c). Exp. Biol. Med. (Maywood). 232: 614–621. [PubMed] [Google Scholar]
- 59.Horiguchi N., Wang L., Mukhopadhyay P., Park O., Jeong W. I., Lafdil F., D. Osei-Hyiaman, A. Moh, X. Y. Fu, P. Pacher, et al. 2008. Cell type-dependent pro- and anti-inflammatory role of signal transducer and activator of transcription 3 in alcoholic liver injury. Gastroenterology. 134: 1148–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liangpunsakul S., Ross R. A., and Crabb D. W.. 2013. Activation of carbohydrate response element-binding protein by ethanol. J. Investig. Med. 61: 270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marmier S., Dentin R., Daujat-Chavanieu M., Guillou H., Bertrand-Michel J., Gerbal-Chaloin S., J. Girard, S. Lotersztajn, and C. Postic. 2015. Novel role for carbohydrate responsive element binding protein in the control of ethanol metabolism and susceptibility to binge drinking. Hepatology. 62: 1086–1100. [DOI] [PubMed] [Google Scholar]
- 62.Gavrilova O., Haluzik M., Matsusue K., Cutson J. J., Johnson L., Dietz K. R., C. J. Nicol, C. Vinson, F. J. Gonzalez, and M. L. Reitman. 2003. Liver peroxisome proliferator-activated receptor γ contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J. Biol. Chem. 278: 34268–34276. [DOI] [PubMed] [Google Scholar]
- 63.Zhang W., Sun Q., Zhong W., Sun X., and Zhou Z.. 2016. Hepatic peroxisome proliferator-activated receptor gamma signaling contributes to alcohol-induced hepatic steatosis and inflammation in mice. Alcohol. Clin. Exp. Res. 40: 988–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Enomoto N., Takei Y., Hirose M., Konno A., Shibuya T., Matsuyama S., S. Suzuki, K. I. Kitamura, and N. Sato. 2003. Prevention of ethanol-induced liver injury in rats by an agonist of peroxisome proliferator-activated receptor-γ, pioglitazone. J. Pharmacol. Exp. Ther. 306: 846–854. [DOI] [PubMed] [Google Scholar]
- 65.Tomita K., Azuma T., Kitamura N., Nishida J., Tamiya G., Oka A., S. Inokuchi, T. Nishimura, M. Suematsu, and H. Ishii. 2004. Pioglitazone prevents alcohol-induced fatty liver in rats through up-regulation of c-Met. Gastroenterology. 126: 873–885. [DOI] [PubMed] [Google Scholar]
- 66.Bou Khalil M., Blais A., Figeys D., and Yao Z.. 2010. Lipin - the bridge between hepatic glycerolipid biosynthesis and lipoprotein metabolism. Biochim. Biophys. Acta. 1801: 1249–1259. [DOI] [PubMed] [Google Scholar]
- 67.Hu M., Wang F., Li X., Rogers C. Q., Liang X., Finck B. N., M. S. Mitra, R. Zhang, D. A. Mitchell, and M. You. 2012. Regulation of hepatic lipin-1 by ethanol: role of AMP-activated protein kinase/sterol regulatory element-binding protein 1 signaling in mice. Hepatology. 55: 437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu M., Yin H., Mitra M. S., Liang X., Ajmo J. M., Nadra K., R. Chrast, B. N. Finck, and M. You. 2013. Hepatic-specific lipin-1 deficiency exacerbates experimental alcohol-induced steatohepatitis in mice. Hepatology. 58: 1953–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yin H., Hu M., Zhang R., Shen Z., Flatow L., and You M.. 2012. MicroRNA-217 promotes ethanol-induced fat accumulation in hepatocytes by down-regulating SIRT1. J. Biol. Chem. 287: 9817–9826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yin H., Hu M., Liang X., Ajmo J. M., Li X., Bataller R., G. Odena, S. M. Stevens, Jr., and M. You. 2014. Deletion of SIRT1 from hepatocytes in mice disrupts lipin-1 signaling and aggravates alcoholic fatty liver. Gastroenterology. 146: 801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shen Z., Liang X., Rogers C. Q., Rideout D., and You M.. 2010. Involvement of adiponectin-SIRT1-AMPK signaling in the protective action of rosiglitazone against alcoholic fatty liver in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 298: G364–G374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Everitt H., Hu M., Ajmo J. M., Rogers C. Q., Liang X., Zhang R., H. Yin, A. Choi, E. S. Bennett, and M. You. 2013. Ethanol administration exacerbates the abnormalities in hepatic lipid oxidation in genetically obese mice. Am. J. Physiol. Gastrointest. Liver Physiol. 304: G38–G47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wurie H. R., Buckett L., and Zammit V. A.. 2012. Diacylglycerol acyltransferase 2 acts upstream of diacylglycerol acyltransferase 1 and utilizes nascent diglycerides and de novo synthesized fatty acids in HepG2 cells. FEBS J. 279: 3033–3047. [DOI] [PubMed] [Google Scholar]
- 74.Qi J., Lang W., Geisler J. G., Wang P., Petrounia I., Mai S., C. Smith, H. Askari, G. T. Struble, R. Williams, et al. 2012. The use of stable isotope-labeled glycerol and oleic acid to differentiate the hepatic functions of DGAT1 and -2. J. Lipid Res. 53: 1106–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wada S., Yamazaki T., Kawano Y., Miura S., and Ezaki O.. 2008. Fish oil fed prior to ethanol administration prevents acute ethanol-induced fatty liver in mice. J. Hepatol. 49: 441–450. [DOI] [PubMed] [Google Scholar]
- 76.Wang Z., Yao T., and Song Z.. 2010. Involvement and mechanism of DGAT2 upregulation in the pathogenesis of alcoholic fatty liver disease. J. Lipid Res. 51: 3158–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sugimoto T., Yamashita S., Ishigami M., Sakai N., Hirano K-i., Tahara M., K. Matsumoto, T. Nakamura, and Y. Matsuzawa. 2002. Decreased microsomal triglyceride transfer protein activity contributes to initiation of alcoholic liver steatosis in rats. J. Hepatol. 36: 157–162. [DOI] [PubMed] [Google Scholar]
- 78.Nanji A. A., Dannenberg A. J., Jokelainen K., and Bass N. M.. 2004. Alcoholic liver injury in the rat is associated with reduced expression of peroxisome proliferator-α (PPARα)-regulated genes and is ameliorated by PPARα activation. J. Pharmacol. Exp. Ther. 310: 417–424. [DOI] [PubMed] [Google Scholar]
- 79.Améen C., Edvardsson U., Ljungberg A., Asp L., Åkerblad P., Tuneld A., Olofsson S. O., Lindén D., and Oscarsson J.. 2005. Activation of peroxisome proliferator-activated receptor α increases the expression and activity of microsomal triglyceride transfer protein in the liver. J. Biol. Chem. 280: 1224–1229. [DOI] [PubMed] [Google Scholar]
- 80.Noga A. A., Zhao Y., and Vance D. E.. 2002. An unexpected requirement for phosphatidylethanolaminen-methyltransferase in the secretion of very low density lipoproteins. J. Biol. Chem. 277: 42358–42365. [DOI] [PubMed] [Google Scholar]
- 81.Vance J. E., and Vance D. E.. 2005. Metabolic insights into phospholipid function using gene-targeted mice. J. Biol. Chem. 280: 10877–10880. [DOI] [PubMed] [Google Scholar]
- 82.Kharbanda K. K., Mailliard M. E., Baldwin C. R., Beckenhauer H. C., Sorrell M. F., and Tuma D. J.. 2007. Betaine attenuates alcoholic steatosis by restoring phosphatidylcholine generation via the phosphatidylethanolamine methyltransferase pathway. J. Hepatol. 46: 314–321. [DOI] [PubMed] [Google Scholar]
- 83.Carr R. M., and Ahima R. S.. 2016. Pathophysiology of lipid droplet proteins in liver diseases. Exp. Cell Res. 340: 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li Q., Zhong W., Qiu Y., Kang X., Sun X., Tan X., Y. Zhao, X. Sun, W. Jia, and Z. Zhou. 2013. Preservation of hepatocyte nuclear factor-4alpha contributes to the beneficial effect of dietary medium chain triglyceride on alcohol-induced hepatic lipid dyshomeostasis in rats. Alcohol. Clin. Exp. Res. 37: 587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Walther T. C., and Farese R. V. Jr.. 2009. The life of lipid droplets. Biochim. Biophys. Acta. 1791: 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kilwein M. D., and Welte M. A.. 2019. Lipid droplet motility and organelle contacts. Contact (Thousand Oaks). 2: doi:10.1177/2515256419895688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gluchowski N. L., Becuwe M., Walther T. C., and Farese R. V. Jr.. 2017. Lipid droplets and liver disease: from basic biology to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 14: 343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou L., Xu L., Ye J., Li D., Wang W., Li X., Wu L., Wang H., Guan F, and Li P.. 2012. Cidea promotes hepatic steatosis by sensing dietary fatty acids. Hepatology. 56: 95–107. [DOI] [PubMed] [Google Scholar]
- 89.Bickel P. E., Tansey J. T., and Welte M. A.. 2009. PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim. Biophys. Acta. 1791: 419–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dalen K. T., Schoonjans K., Ulven S. M., Weedon-Fekjaer M. S., Bentzen T. G., Koutnikova H., J. Auwerx, and H. I. Nebb. 2004. Adipose tissue expression of the lipid droplet–associating proteins S3-12 and perilipin is controlled by peroxisome proliferator-activated receptor-γ. Diabetes. 53: 1243–1252. [DOI] [PubMed] [Google Scholar]
- 91.Brasaemle D. L., Barber T., Wolins N. E., Serrero G., Blanchette-Mackie E. J., and Londos C.. 1997. Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J. Lipid Res. 38: 2249–2263. [PubMed] [Google Scholar]
- 92.Wolins N. E., Quaynor B. K., Skinner J. R., Tzekov A., Croce M. A., Gropler M. C., V. Varma, A. Yao-Borengasser, N. Rasoouli, P. A. Kern, et al. 2006. OXPAT/PAT-1 is a PPAR-induced lipid droplet protein that promotes fatty acid utilization. Diabetes. 55: 3418–3428. [DOI] [PubMed] [Google Scholar]
- 93.Straub B. K., Stoeffel P., Heid H., Zimbelmann R., and Schirmacher P.. 2008. Differential pattern of lipid droplet-associated proteins and de novo perilipin expression in hepatocyte steatogenesis. Hepatology. 47: 1936–1946. [DOI] [PubMed] [Google Scholar]
- 94.Carr R. M., Dhir R., Mahadev K., Comerford M., Chalasani N. P., and Ahima R. S.. 2017. Perilipin staining distinguishes between steatosis and nonalcoholic steatohepatitis in adults and children. Clin. Gastroenterol. Hepatol. 15: 145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Motomura W., Inoue M., Ohtake T., Takahashi N., Nagamine M., Tanno S., Y. Kohgo, and T. Okumura. 2006. Up-regulation of ADRP in fatty liver in human and liver steatosis in mice fed with high fat diet. Biochem. Biophys. Res. Commun. 340: 1111–1118. [DOI] [PubMed] [Google Scholar]
- 96.Granneman J. G., Moore H-P. H., Mottillo E. P., Zhu Z., and Zhou L.. 2011. Interactions of perilipin-5 (plin5) with adipose triglyceride lipase. J. Biol. Chem. 286: 5126–5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mak K. M., Ren C., Ponomarenko A., Cao Q., and Lieber C. S.. 2008. Adipose differentiation-related protein is a reliable lipid droplet marker in alcoholic fatty liver of rats. Alcohol. Clin. Exp. Res. 32: 683–689. [DOI] [PubMed] [Google Scholar]
- 98.Carr R. M., Peralta G., Yin X., and Ahima R. S.. 2014. Absence of perilipin 2 prevents hepatic steatosis, glucose intolerance and ceramide accumulation in alcohol-fed mice. PLoS One. 9: e97118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Raynard B., Balian A., Fallik D., Capron F., Bedossa P., Chaput J. C., and S. Naveau. 2002. Risk factors of fibrosis in alcohol-induced liver disease. Hepatology. 35: 635–638. [DOI] [PubMed] [Google Scholar]
- 100.Bulankina A. V., Deggerich A., Wenzel D., Mutenda K., Wittmann J. G., Rudolph M. G., Burger K. N.and Höning S.. 2009. TIP47 functions in the biogenesis of lipid droplets. J. Cell Biol. 185: 641–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Carr R. M., Patel R. T., Rao V., Dhir R., Graham M. J., Crooke R. M., and R. S. Ahima. 2012. Reduction of TIP47 improves hepatic steatosis and glucose homeostasis in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302: R996–R1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gu Y., Yang Y., Cao X., Zhao Y., Gao X., Sun C., F. Zhang, Y. Yuan, Y. Xu, J. Zhang, et al. 2019. Plin3 protects against alcoholic liver injury by facilitating lipid export from the endoplasmic reticulum. J. Cell. Biochem. 120: 16075–16087. [DOI] [PubMed] [Google Scholar]
- 103.Jambunathan S., Yin J., Khan W., Tamori Y., and Puri V.. 2011. FSP27 promotes lipid droplet clustering and then fusion to regulate triglyceride accumulation. PLoS One. 6: e28614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xu M-J., Cai Y., Wang H., Altamirano J., Chang B., Bertola A., G. Odena, J. Lu, N. Tanaka, K. Matsusue, et al. 2015. Fat-specific protein 27/CIDEC promotes development of alcoholic steatohepatitis in mice and humans. Gastroenterology. 149: 1030–1041.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tian C., Stokowski R. P., Kershenobich D., Ballinger D. G., and Hinds D. A.. 2010. Variant in PNPLA3 is associated with alcoholic liver disease. Nat. Genet. 42: 21–23. [DOI] [PubMed] [Google Scholar]
- 106.Stickel F., Buch S., Lau K., zu Schwabedissen H. M., Berg T., Ridinger M., M. Rietschel, C. Schafmayer, F. Braun, J. Hinrichsen, et al. 2011. Genetic variation in the PNPLA3 gene is associated with alcoholic liver injury in Caucasians. Hepatology. 53: 86–95. [DOI] [PubMed] [Google Scholar]
- 107.Buch S., Stickel F., Trépo E., Way M., Herrmann A., Nischalke H. D., M. Brosch, J. Rosendahl, T. Berg, M. Ridinger, et al. 2015. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat. Genet. 47: 1443–1448. [DOI] [PubMed] [Google Scholar]
- 108.Stickel F., Moreno C., Hampe J., and Morgan M. Y.. 2017. The genetics of alcohol dependence and alcohol-related liver disease. J. Hepatol. 66: 195–211. [DOI] [PubMed] [Google Scholar]
- 109.Basantani M. K., Sitnick M. T., Cai L., Brenner D. S., Gardner N. P., Li J. Z., Schoiswohl G., Yang K., Kumari M., Gross R. W., et al. 2011; Pnpla3/adiponutrin deficiency in mice does not contribute to fatty liver disease or metabolic syndrome. J. Lipid Res. 52: 318–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen W., Chang L., and Chan L.. 2010. Patatin-like phospholipase domain-containing 3/adiponutrin deficiency in mice is not associated with fatty liver disease. Hepatology. 52: 1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li J. Z., Huang Y., Karaman R., Ivanova P. T., Brown H. A., Roddy T., J. Perez-Castro, J. C. Cohen, and H. H. Hobbs. 2012. Chronic overexpression of PNPLA3I148M in mouse liver causes hepatic steatosis. J. Clin. Invest. 122: 4130–4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.BasuRay S., Y. Wang, E. Smagris, J. C. Cohen, and H. H. Hobbs. 2019. Accumulation of PNPLA3 on lipid droplets is the basis of associated hepatic steatosis. Proc. Natl. Acad. Sci. USA. 116: 9521–9526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Negoita F., Blomdahl J., Wasserstrom S., Winberg M. E., Osmark P., Larsson S., K. G. Stenkula, M. Ekstedt, S. Kechagias, C. Holm, et al. 2019. PNPLA3 variant M148 causes resistance to starvation-mediated lipid droplet autophagy in human hepatocytes. J. Cell. Biochem. 120: 343–356. [DOI] [PubMed] [Google Scholar]
- 114.Teo K., Abeysekera K. W. M., Adams L., E., Banerjee R., Basu P., Berg T., Bhatnagar P., Buch S., Canbay A., et al. 2020. rs641738C>T near MBOAT7 promotes steatosis, NASH, fibrosis and hepatocellular carcinoma in non-alcoholic fatty liver disease: a meta-analysis . medRxiv. doi:10.1101/19013623. [Google Scholar]
- 115.Caddeo A., Jamialahmadi O., Solinas G., Pujia A., Mancina R. M., Pingitore P., and S. Romeo. 2019. MBOAT7 is anchored to endomembranes by six transmembrane domains. J. Struct. Biol. 206: 349–360. [DOI] [PubMed] [Google Scholar]
- 116.Yamashita A., Watanabe M., Sato K., Miyashita T., Nagatsuka T., Kondo H., N. Kawagishi, H. Nakanishi, R. Kamata, T. Sugiura, et al. 2003. Reverse reaction of lysophosphatidylinositol acyltransferase functional reconstitution of coenzyme A-dependent transacylation system. J. Biol. Chem. 278: 30382–30393. [DOI] [PubMed] [Google Scholar]
- 117.Magné J., Aminoff A., Sundelin J. P., Mannila M. N., Gustafsson P., Hultenby K., A. Wernerson, G. Bauer, L. Listenberger, M. J. Neville, et al. 2013. The minor allele of the missense polymorphism Ser251Pro in perilipin 2 (PLIN2) disrupts an α-helix, affects lipolysis, and is associated with reduced plasma triglyceride concentration in humans. FASEB J. 27: 3090–3099. [DOI] [PubMed] [Google Scholar]
- 118.Singh R., Kaushik S., Wang Y., Xiang Y., Novak I., Komatsu M., Tanaka K., Cuervo A. M., and Czaja M. J.. 2009. Autophagy regulates lipid metabolism. Nature. 458: 1131–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lin C-W., Zhang H., Li M., Xiong X., Chen X., Chen X., X. C. Dong, and X. M. Yin. 2013. Pharmacological promotion of autophagy alleviates steatosis and injury in alcoholic and non-alcoholic fatty liver conditions in mice. J. Hepatol. 58: 993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ding W. X., Li M., Chen X., Ni H. M., Lin C. W., Gao W., B. Lu, D. B. Stolz, D. L. Clemens, and X. M. Yin. 2010. Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice. Gastroenterology. 139: 1740–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ni H-M., Du K., You M., and Ding W-X.. 2013. Critical role of FoxO3a in alcohol-induced autophagy and hepatotoxicity. Am. J. Pathol. 183: 1815–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Thomes P. G., Trambly C. S., Fox H. S., Tuma D. J., and Donohue T. M. Jr.. 2015. Acute and chronic ethanol administration differentially modulate hepatic autophagy and transcription factor EB. Alcohol. Clin. Exp. Res. 39: 2354–2363. [DOI] [PubMed] [Google Scholar]
- 123.Rasineni K., Donohue T. M. Jr., Thomes P. G., Yang L., Tuma D. J., McNiven M. A., and C. A. Casey. 2017. Ethanol-induced steatosis involves impairment of lipophagy, associated with reduced Dynamin2 activity. Hepatol. Commun. 1: 501–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schulze R. J., Rasineni K., Weller S. G., Schott M. B., Schroeder B., Casey C. A., and M. A. McNiven. 2017. Ethanol exposure inhibits hepatocyte lipophagy by inactivating the small guanosine triphosphatase Rab7. Hepatol. Commun. 1: 140–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Clugston R. D., Gao M. A., and Blaner W. S.. 2017. The hepatic lipidome: a gateway to understanding the pathogenes is of alcohol-induced fatty liver. Curr. Mol. Pharmacol. 10: 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Clugston R. D., Jiang H., Lee M. X., Piantedosi R., Yuen J. J., Ramakrishnan R., M. J. Lewis, M. E. Gottesman, L. S. Huang, I. J. Goldberg, et al. 2011. Altered hepatic lipid metabolism in C57BL/6 mice fed alcohol: a targeted lipidomic and gene expression study. J. Lipid Res. 52: 2021–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fernando H., Kondraganti S., Bhopale K. K., Volk D. E., Neerathilingam M., Kaphalia B. S., B. A. Luxon, P. J. Boor, and G. A. Shakeel Ansari. 2010. 1H and 31P NMR lipidome of ethanol-induced fatty liver. Alcohol. Clin. Exp. Res. 34: 1937–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fernando H., Bhopale K. K., Kondraganti S., Kaphalia B. S., and Shakeel Ansari G. A.. 2011. Lipidomic changes in rat liver after long-term exposure to ethanol. Toxicol. Appl. Pharmacol. 255: 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bosner M. S., Gulick T., Riley D. J., Spilburg C. A., and Lange L. G. III.. 1988. Receptor-like function of heparin in the binding and uptake of neutral lipids. Proc. Natl. Acad. Sci. USA. 85: 7438–7442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Athenstaedt K., and Daum G.. 2006. The life cycle of neutral lipids: synthesis, storage and degradation. Cell. Mol. Life Sci. 63: 1355–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shimizu T. 2009. Lipid mediators in health and disease: enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu. Rev. Pharmacol. Toxicol. 49: 123–150. [DOI] [PubMed] [Google Scholar]
- 132.Bradford B. U., O’Connell T. M., Han J., Kosyk O., Shymonyak S., Ross P. K., J. Winnike, H. Kono, and I. Rusyn. 2008. Metabolomic profiling of a modified alcohol liquid diet model for liver injury in the mouse uncovers new markers of disease. Toxicol. Appl. Pharmacol. 232: 236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Loftus N., Barnes A., Ashton S., Michopoulos F., Theodoridis G., Wilson I., C. Ji, and N. Kaplowitz. 2011. Metabonomic investigation of liver profiles of nonpolar metabolites obtained from alcohol-dosed rats and mice using high mass accuracy MSn analysis. J. Proteome Res. 10: 705–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhao Z., Yu M., Crabb D., Xu Y., and Liangpunsakul S.. 2011. Ethanol-induced alterations in fatty acid-related lipids in serum and tissues in mice. Alcohol. Clin. Exp. Res. 35: 229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jang Z. H., Chung H. C., Ahn Y. G., Kwon Y. K., Kim J. S., Ryu J. H., C. H. Kim, and G. S. Hwang. 2012. Metabolic profiling of an alcoholic fatty liver in zebrafish (Danio rerio). Mol. Biosyst. 8: 2001–2009. [DOI] [PubMed] [Google Scholar]
- 136.Fernando H., Bhopale K. K., Boor P. J., Ansari G. A., and Kaphalia B. S.. 2012. Hepatic lipid profiling of deer mice fed ethanol using (1)H and (3)(1)P NMR spectroscopy: a dose-dependent subchronic study. Toxicol. Appl. Pharmacol. 264: 361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nanji A. A., Sadrzadeh S. M., Yang E. K., Fogt F., Meydani M., and Dannenberg A. J.. 1995. Dietary saturated fatty acids: a novel treatment for alcoholic liver disease. Gastroenterology. 109: 547–554. [DOI] [PubMed] [Google Scholar]
- 138.You M., Considine R. V., Leone T. C., Kelly D. P., and Crabb D. W.. 2005. Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology. 42: 568–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen P., Torralba M., Tan J., Embree M., Zengler K., Starkel P., van Pijkeren J. P., DePew J., Loomba R., Ho S. B., et al. 2015. Supplementation of saturated long-chain fatty acids maintains intestinal eubiosis and reduces ethanol-induced liver injury in mice. Gastroenterology. 148: 203–214.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ramirez T., Longato L., Dostalek M., Tong M., Wands J. R., and de la Monte S. M.. 2013. Insulin resistance, ceramide accumulation and endoplasmic reticulum stress in experimental chronic alcohol-induced steatohepatitis. Alcohol Alcohol. 48: 39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Longato L., Ripp K., Setshedi M., Dostalek M., Akhlaghi F., Branda M., Wands J. R., and de la Monte S. M.. 2012. Insulin resistance, ceramide accumulation, and endoplasmic reticulum stress in human chronic alcohol-related liver disease. Oxid. Med. Cell. Longev. 2012: 479348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chavez J. A., and Summers S. A.. 2012. A ceramide-centric view of insulin resistance. Cell Metab. 15: 585–594. [DOI] [PubMed] [Google Scholar]
- 143.Liangpunsakul S., Sozio M. S., Shin E., Zhao Z., Xu Y., Ross R. A., Y. Zeng, and D. W. Crabb. 2010. Inhibitory effect of ethanol on AMPK phosphorylation is mediated in part through elevated ceramide levels. Am. J. Physiol. Gastrointest. Liver Physiol. 298: G1004–G1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hannun Y. A., and Obeid L. M.. 2011. Many ceramides. J. Biol. Chem. 286: 27855–27862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Williams B., Correnti J., Oranu A., Lin A., Scott V., Annoh M., J. Beck, E. Furth, V. Mitchell, C. E. Senkal, et al. 2018. A novel role for ceramide synthase 6 in mouse and human alcoholic steatosis. FASEB J. 32: 130–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tong M., Longato L., Ramirez T., Zabala V., Wands J. R., and de la Monte S. M.. 2014. Therapeutic reversal of chronic alcohol-related steatohepatitis with the ceramide inhibitor myriocin. Int. J. Exp. Pathol. 95: 49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Deaciuc I. V., Nikolova-Karakashian M., Fortunato F., Lee E. Y., Hill D. B., and McClain C. J.. 2000. Apoptosis and dysregulated ceramide metabolism in a murine model of alcohol-enhanced lipopolysaccharide hepatotoxicity. Alcohol. Clin. Exp. Res. 24: 1557–1565. [PubMed] [Google Scholar]
- 148.Liangpunsakul S., Rahmini Y., Ross R. A., Zhao Z., Xu Y., and Crabb D. W.. 2012. Imipramine blocks ethanol-induced ASMase activation, ceramide generation, and PP2A activation, and ameliorates hepatic steatosis in ethanol-fed mice. Am. J. Physiol. Gastrointest. Liver Physiol. 302: G515–G523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fernandez A., Matias N., Fucho R., Ribas V., Von Montfort C., Nuño N., A. Baulies, L. Martinez, N. Tarrats, M. Mari, et al. 2013. ASMase is required for chronic alcohol induced hepatic endoplasmic reticulum stress and mitochondrial cholesterol loading. J. Hepatol. 59: 805–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Schlemmer H-P. W., Sawatzki T., Sammet S., Dornacher I., Bachert P., van Kaick G., R. Waldherr, and H. K. Seitz. 2005. Hepatic phospholipids in alcoholic liver disease assessed by proton-decoupled 31P magnetic resonance spectroscopy. J. Hepatol. 42: 752–759. [DOI] [PubMed] [Google Scholar]
- 151.Vance D. E., and Ridgway N. D.. 1988. The methylation of phosphatidylethanolamine. Prog. Lipid Res. 27: 61–79. [DOI] [PubMed] [Google Scholar]
- 152.Cui Z., and Vance D. E.. 1996. Expression of phosphatidylethanolamine n-methyltransferase-2 is markedly enhanced in long term choline-deficient rats. J. Biol. Chem. 271: 2839–2843. [DOI] [PubMed] [Google Scholar]
- 153.Barak A. J., Tuma D. J., and Sorrell M. F.. 1973. Relationship of ethanol to choline metabolism in the liver: a review. Am. J. Clin. Nutr. 26: 1234–1241. [DOI] [PubMed] [Google Scholar]
- 154.Duce A. M., Ortíz P., Cabrero C., and Mato J. M.. 1988. S-adenosyl-L-methionine synthetase and phospholipid methyltransferase are inhibited in human cirrhosis. Hepatology. 8: 65–68. [DOI] [PubMed] [Google Scholar]
- 155.Lieber C. S., Robins S. J., and Leo M. A.. 1994. Hepatic phosphatidylethanolamine methyltransferase activity is decreased by ethanol and increased by phosphatidylcholine. Alcohol. Clin. Exp. Res. 18: 592–595. [DOI] [PubMed] [Google Scholar]
- 156.Lu S. C., Huang Z-Z., Yang H., Mato J. M., Avila M. A., and Tsukamoto H.. 2000. Changes in methionine adenosyltransferase and S-adenosylmethionine homeostasis in alcoholic rat liver. Am. J. Physiol. Gastrointest. Liver Physiol. 279: G178–G185. [DOI] [PubMed] [Google Scholar]
- 157.Lieber C. S., Robins S. J., Jianjun L., DeCarli L. M., Mak K. M., Fasulo J. M., and M. A. Leo. 1994. Phosphatidylcholine protects against fibrosis and cirrhosis in the baboon. Gastroenterology. 106: 152–159. [DOI] [PubMed] [Google Scholar]
- 158.Lieber C. S., Weiss D. G., Groszmann R., Paronetto F., and Schenker S.; Veterans Affairs Cooperative Study 391 Group . 2003. II. Veterans Affairs Cooperative Study of polyenylphosphatidylcholine in alcoholic liver disease. Alcohol. Clin. Exp. Res. 27: 1765–1772. [DOI] [PubMed] [Google Scholar]
- 159.Li Z., Agellon L. B., Allen T. M., Umeda M., Jewell L., Mason A., and D. E. Vance. 2006. The ratio of phosphatidylcholine to phosphatidylethanolamine influences membrane integrity and steatohepatitis. Cell Metab. 3: 321–331. [DOI] [PubMed] [Google Scholar]