Abstract
The baculovirus–insect cell expression system is an approved system for the production of viral antigens with vaccine potential for humans and animals and has been used for production of subunit vaccines against parasitic diseases as well. Many candidate subunit vaccines have been expressed in this system and immunization commonly led to protective immunity against pathogen challenge. The first vaccines produced in insect cells for animal use are now on the market. This chapter deals with the tailoring of the baculovirus–insect cell expression system for vaccine production in terms of expression levels, integrity and immunogenicity of recombinant proteins, and baculovirus genome stability. Various expression strategies are discussed including chimeric, virus‐like particles, baculovirus display of foreign antigens on budded virions or in occlusion bodies, and specialized baculovirus vectors with mammalian promoters that express the antigen in the immunized individual. A historical overview shows the wide variety of viral (glyco)proteins that have successfully been expressed in this system for vaccine purposes. The potential of this expression system for antiparasite vaccines is illustrated. The combination of subunit vaccines and marker tests, both based on antigens expressed in insect cells, provides a powerful tool to combat disease and to monitor infectious agents.
I. Introduction to Recombinant Subunit Vaccines
Historically, vaccines have been one of the most cost‐effective and easily administered means of controlling infectious diseases in humans and animals. Vaccine development has its roots in the work of Edward Jenner (1749–1823) who discovered that man could be protected from smallpox by inoculation with cowpox (Fenner, 2000) and the work of Louis Pasteur (1822–1895) who developed the first rabies vaccine (Fu, 1997). These pioneering efforts led to vaccines against diseases that had once claimed millions of lives worldwide (Andre, 2003). Childhood vaccination programs are now common practice and elaborate vaccination programs have been set up by the World Health Organization (WHO), leading to the official eradication of smallpox in 1979 (Fenner, 2000). Today large parts of the world are also declared poliomyelitis free, and measles is the next target for eradication. Vaccines have controlled major bacterial and viral diseases in humans, and effective vaccines are available against many more (Andre 2003, Hansson 2000b). Vaccination also protects our livestock and pet animals (Pastoret et al., 1997). For some diseases, however, such as malaria and acquired immunodeficiency syndrome (AIDS), vaccines are desparately sought.
Most human and animal vaccines are based on killed or live‐attenuated pathogens. Killed vaccines require the production of large amounts of often highly virulent pathogens and these types of vaccines are therefore risky to produce. Another risk lies in the potential for incomplete inactivation of the pathogens. Inactivation on the other hand affects the immunogenic properties of the pathogen, and hence the efficacy as a vaccine, and it is often difficult to find the balance between efficient inactivation and conservation of immunogenicity. Live‐attenuated vaccines consist of pathogens that are reduced in virulence or have been attenuated either by growing them in alternative hosts or under unfavorable growing conditions, or by recombinant DNA technology. These live‐attenuated vaccines can potentially replicate in their host, but are typically attenuated in their pathogenicity to avoid the development of severe disease. Live vaccines elicit humoral and cellular immunity, and may provide lifelong protection with a single or a few doses (Dertzbaugh 1998, Hansson 2000b, Schijns 2003). Such long‐term protection is advantageous in developing countries where individuals are often only immunized once. A drawback of live‐attenuated vaccines is that they can cause side effects, which may be dangerous when used for prophylaxis in immunocompromised persons such as the elderly or individuals with genetic or acquired diseases of the immune system (e.g., AIDS or severe combined immunodeficiency; SCID). Live‐attenuated vaccines may also convert to virulent strains and spread to nonimmunized persons as observed during recent poliomyelitis outbreaks (Kew et al., 2004). Adverse effects with both killed and live‐attenuated vaccines can also be due to allergic reactions to components of the vaccine such as residual egg proteins in the case of influenza vaccines (Kelso and Yunginger, 2003) or gelatin in the measles‐mumps‐rubella (MMR) vaccine (Patja et al., 2001).
The development of vaccines is not easy for all infectious diseases and the medical and veterinary world is challenged frequently by the emergence of novel diseases such as AIDS, severe acute respiratory syndrome (SARS), and West Nile virus infection. The vaccine industry is under constant pressure for rapidly changing pathogens, for which large amounts of vaccines are needed annually, such as influenza viruses (Palese, 2004), and flexible vaccine production techniques are required. For several infectious diseases vaccines cannot be developed using conventional approaches, for instance due to a lack of appropriate animal production systems or the high‐mutation frequency of the pathogen (Human immunodeficiency virus (HIV), malaria). A vaccine against the H5N1 influenza strain that is currently epidemic in Asian poultry could not be produced the classical way, by using embryonized chicken eggs without reducing the virulence of the virus by reverse genetics, due to high‐mortality rates of the chicken embryos (Horimoto et al., 2006).
Recombinant protein production systems may provide good alternatives for the development of vaccines that are more difficult to produce in vivo for manufacture of so‐called subunit vaccines. A pathogen consists of many proteins, frequently with carbohydrate moieties, but these are not all equally important for generation of an adequate immunological response. Subunit vaccines contain the immunodominant components of a pathogen and in the case of viral vaccines these are often (glyco)proteins of the viral coat or envelope such as the hepatitis B surface antigen (Bouma 1999, Valenzuela 1982) or the classical swine fever virus (CSFV) E2 glycoprotein (Bouma 1999, Valenzuela 1982). Viral coat proteins sometimes form virus‐like particles (VLPs) when expressed in heterologous systems (Brown et al., 1991), which are often immunogenic and may induce both humoral and cellular responses. The subunit vaccine against hepatitis B produced in yeast is highly succesful. An extreme example of subunit vaccines are peptide‐based vaccines which consist of small amino acid chains harboring the part of the antigenic protein that is recognized by antibodies. Typically, subunit vaccines do not contain the genetic material of the pathogen or only a small part thereof. Therefore, these vaccines cannot cause disease and do not introduce pathogens into nonendemic regions. An additional advantage of subunit vaccines is that they can be used in combination with specific marker tests, which make it possible to differentiate infected from vaccinated animals, the so‐called DIVA vaccines (Capua 2003, van Oirschot 1999); an important issue in monitoring virus prevalence and virus‐free export of animals and their products.
Immunogenic subunits can be isolated chemically from the pathogen, such as the purified capsular polysaccharides present in the Streptococcus pneumonia vaccine (Pneumovax23; Merck). This process still requires the production of virulent pathogens, which is not without risk. An alternative is the use of recombinant DNA technology to produce protein subunits in a heterologous system, and a variety of expression systems are available (Clark 2005, Hansson 2000b). The yeast system Saccharomyces cerevisiae for instance is used to produce the hepatitis B subunit vaccine (Valenzuela et al., 1982), which is currently the only licensed recombinant subunit vaccine for human use. The yeast Pichia pastoris is used for production of the antitick vaccine Gavac™ (Canales et al., 1997), which protects cattle against the tick Boophilus microplus, the transmitter of Babesia and Anaplasma parasite species. Insect cells are used to produce vaccines against classical swine fever or hog cholera (Depner 2001, van Aarle 2003). For the production of recombinant proteins in higher eukaryotics, mammalian, insect, and plant expression systems are available that either use trangenes or viral vectors for protein expression. Plants have been recognized for the production of so‐called edible subunit vaccines to be administered by ingestion of vegetable foods (Ma 2005, Streatfield 2003).
This chapter concentrates on the use of cultured insect cells or larvae in combination with baculovirus expression vectors for the production of subunit vaccines. The baculovirus expression system is an accepted and well‐developed system for the production of viral antigens with vaccine potential (Dertzbaugh 1998, Hansson 2000a, Vlak 1990). This system has also been explored for development of vaccines against protozoan parasites (Kaba et al., 2005) and for therapeutic vaccines against tumors. A vaccine against prostate‐cancer (Provenge) is in phase II/III clinical trials and is based on combining recombinant prostatic acid phosphatase (characteristic of 95% of prostate cancers) with the patient's own dendritic cells before immunization (Beinart 2005, Rini 2002). Trials have also been initiated for a prophylactic vaccine using VLPs produced in insect cells against cervical cancer caused by Human papillomavirus (HPV) 16 (Mao et al., 2006).
Each expression system has advantages and drawbacks (Table I ) and the system of choice depends very much on the specific requirements for a particular vaccine and is often based, at least partly, on trial and error. Before a definitive choice can be made, the expression levels achieved, the adequacy of posttranslational modifications, the immunological performance, the possibilites for scale‐up, the costs, the risk of contamination, the method of administration, and legal aspects must all be taken into account.
Table I.
Potential of Various Expression Systems for Recombinant Subunit Vaccine Productiona
| Processing/feature | E. coli | Yeast | Mammalian cells | Insect cells | Plants |
|---|---|---|---|---|---|
| Glycosylation | − | + | + + + | + + | + |
| Phosphorylation | − | + | + + | + + | + |
| Acylation | − | + | + | + | + |
| Amidation | − | − | + | + | − |
| Proteolysis | +/− | +/− | + | + | + |
| Folding | +/− | +/− | + + + | + + | + |
| Secretion | +/− | + | + + | + + | +/− |
| Serum free | Not relevant | Not relevant | + | + | Not relevant |
| Yield (%dry mass) | 1–5 | 1 | <1 | Up to 30 | <5 |
| Scale‐up | + + + | + + + | + | + | + + + |
| Downstream processing | + | + | + + | ++ | − − |
| Costs | Low | Low | High | Intermediate | Low |
| Safety | + + | + + | + | + + | + + |
| Versatility | + | + | + + | + + + | + |
II. The Baculovirus–Insect Cell Expression System for Vaccine Production
A. Characteristics
The baculovirus–insect cell expression system (Smith et al., 1983) has been developed for the production of biologically active (glyco)proteins in a well‐established and safe eukaryotic environment (Kost et al., 2005). The family Baculoviridae contains rod‐shaped, invertebrate‐infecting viruses, which have large double‐stranded, covalently closed circular DNA genomes (Table II ). The members of this large virus family are taxonomically divided into the genera Nucleopolyhedrovirus (NPV) and Granulovirus (GV), based on occlusion body morphology (Theilmann et al., 2005). NPVs express two genes, polyhedrin and p10, at very high levels in the very late phase of infection. The polyhedrin protein forms the viral occlusion bodies or polyhedra and p10 is present in fibrillar structures, which function in polyhedron morphology and in breakdown of infected cell‐nuclei to release the polyhedra (Okano 2006, Van Oers 1997). These two genes are not essential for virus replication in cell culture and, therefore, their promoters are exploited to drive foreign gene expression, which forms the basis for the baculovirus–insect cell expression system. Since baculoviruses are rod‐shaped, large amounts of foreign DNA can be accommodated within the virus particle, in contrast to vaccinia and especially adenovirus expression vectors (Table II).
Table II.
Characteristics of Baculovirus Vectors Versus Vaccinia and Adenovirus Vectorsa
| Feature | Baculovirusb | Adenovirus | Vaccinia |
|---|---|---|---|
| Virus morphology | Enveloped, rod shaped | Nonenveloped, icosahedral | Brick shaped |
| Genome structure | Circular dsDNA | Linear dsDNA | Linear dsDNA |
| Genome size | 130 kbp | ±35 kbp | 190 kbp |
| Expandability | Large | Low | Intermediate |
| Particle dimensions | 30−60 × 250−300 nm | 80–110 nm | 250 × 250 × 200 nm |
| Replication site | Nucleus | Nucleus | Cytoplasm |
| Replication in humans | None | Replication competent or defective | Yes |
| Progeny virus | Budding BVs/lysis ODVs | Accumulation in the nucleus | Exocytosis/lysis |
| Pathogenicity for mammals including humans | Nonpathogenic | Low due to host defense and attenuation | Reduced with modified strains |
| Immunological complications | Complement inactivation | Strong protective responses of the host | – |
| Immunological history | – | Preexisting immunity due to natural infections | Preexisting immunity due to smallpox vaccination |
| Protein production system in cell lines | Yes | Less frequently | Yes |
| Applications: | |||
| Antigen display vector | Surface display vectors | No | No |
| Carrier DNA vaccine vector | Yes | Yes | Yes |
| Gene therapy | + | + + | − |
| Vaccine examples | Therapeutic prostate cancer vaccine (see text for further information) | Immunomodulators, therapeutic cancer vaccines | Mucosal immunity against tuberculosis and HIV |
Gherardi 2005, Russell 2000, Young 2006; Universal data base of International Committee on Virus Taxonomy (http://www.ncbi.nlm.nih.gov/ICTVdb/index.htm; January 2006).
AcMNPV, Autographa californica multiple nucleopolyhedrovirus.
The type member of the NPVs is Autographa californica multiple nucleopolyhedrovirus (AcMNPV), a virus with a genome of 133 kilobase pairs (Ayres et al., 1994). This baculovirus is routinely used for foreign gene expression. The baculovirus Bombyx mori NPV is being used for vaccine purposes to a much lesser extent (Choi 2000, Mori 1994). Baculovirus expression vectors replicate in cultured insect cells or larvae and high yields of heterologous protein are generally obtained when the strong viral polyhedrin and p10 promoters are exploited (King 1992, O'Reilly 1992). The insect cell lines used in the baculovirus expression system are derived from lepidopteran insects (moths) and are most often Spodoptera frugiperda lines (Sf9 or Sf21) and Trichoplusia ni (High Five™) cells, which can be used in combination with AcMNPV‐based vectors. B. mori cells (e.g., Bm5) are used for BmNPV. Insect cell lines vary in their characteristics in terms of growth rate, protein production, secretion efficiency and glycosylation pattern, and interference with viral genome stability (Pijlman 2003b, Vlak 1996). These insect cells are relatively easy to maintain and many grow equally well in suspension in large volumes (up to 2000 L reactions) and at high densities as on solid supports, and can be cultivated in serum‐free media which facilitates purification of recombinant proteins. Unlike mammalian cells, they do not require CO2 and can easily withstand temperature fluctuations. An extra advantage is that the chance of contamination with human or mammalian viruses, especially in serum‐free cultures, is small compared to mammalian production systems because these vertebrate viruses do not replicate in lepidopteran cells. These cells do not support the growth of mammalian mycoplasmas either. Instead of insect cells, whole insect larvae may be used as live bioreactors for vaccine production. The use of whole insect larvae has the advantage that the simple insect‐rearing technology and downstream processing can be exploited. Such in vivo production could be performed by small‐scale local industries, especially if the larvae can be fed directly to animals such as for an experimental Newcastle disease vaccine for chickens (Mori et al., 1994). Such vaccines are less well defined however and quality control may therefore be more difficult to achieve.
Expression of proteins in insect cells allows for appropriate folding, posttranslational modification, and oligomerization and therefore, biological activity is normally preserved. Protein glycosylation in insects and mammals is not identical though: the N‐glycan–processing pathway in insects results in glycoproteins with paucimannose glycan groups, in contrast to mammalian glycoproteins which contain complex sialylated glycans (see also Harrison, this volume, pp. 159–191). The exact glycan composition varies between different insect cell lines (Kost 2005, Tomiya 2004). In general, glycan groups are not very immunogenic and therefore this does not seem to be a major disadvantage for subunit vaccines. In situations where more authentic glycosylation is required, for instance for preserving functional activity, transformed “humanized” insect cell lines expressing mammalian glycosylation enzymes are available (Jarvis 2003, Kost 2005, Tomiya 2004). For some insect cell lines it has been reported that fucose groups are added to N‐glycans. The impact of this remains to be determined, but since fucans may cause allergic reactions, it may be a point for consideration when choosing an insect cell line for vaccine production (Long 2006, Tomiya 2004).
B. Baculovirus Vectors
Originally, the baculovirus expression system was based on the allelic exchange of the baculovirus polyhedrin gene for a heterologous gene by recombination in insect cells (Smith et al., 1983). In a similar way, baculovirus vectors have since been developed which exploit the nonessential very late baculovirus p10 promoter (Vlak 1990, Weyer 1991). Vectors that leave the polyhedrin gene intact can be used for the production of recombinant proteins in insect larvae (Fig. 1 ). The in vivo recombination protocol was improved by using linearized viral DNA in the allelic replacement, which resulted in dominant selection and much higher percentages of recombinant viruses (Kitts 1990, Martens 1995). In vectors of this type (BacPAK™ vectors, BaculoGold™, Bac‐N‐Blue™) the linearized viral DNA carries a lethal deletion (ORF1609) and becomes replication competent only after recombination with a transfer plasmid carrying the foreign gene, thereby restoring the deletion (Kitts and Possee, 1993). Baculovirus vectors based on Gateway technology (BaculoDirect™) are linear baculovirus vectors in which foreign genes are introduced through site‐specific in vitro recombination.
Fig 1.
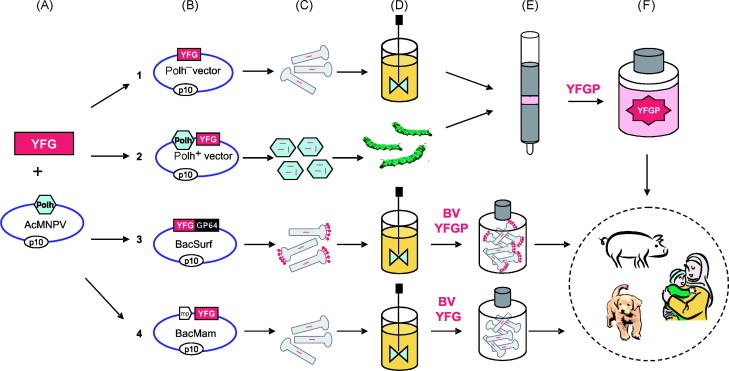
Flow chart showing four different methods to make a vaccine based on your favorite gene (YFG) in the baculovirus expression system: (1) protein expression in insect cell bioreactors using the polyhedrin locus for expression, (2) protein expression in insect larvae leaving the polyhedrin gene intact; expression is driven either by a duplicated p10 promoter or by the original p10 promoter, (3) baculovirus surface display methods where YFG is fused to GP64, and (4) DNA vectors with a mammalian promoter (mp) for synthesis of your favorite gene product (YFGP) in the target species. Subsequent steps in the process are: (A) selection and PCR amplification of YFG, (B) cloning into the appropriate baculovirus vector, (C) generation of recombinant BV particles or occlusion bodies, (D) production in insect cells in bioreactors or larvae, (E) purification of recombinant YFGP or collection of BVs loaded with either YFGP or YFG, and (F) delivery of prophylactic or therapeutic vaccines.
At about the same time, another efficient and rapid method for generation of recombinant baculoviruses was developed (Luckow et al., 1993) that employed transposition of a foreign gene expression cassette from a donor plasmid into a bacterial artificial chromosome (BAC) which contains the entire AcMNPV genome (bacmid). In this system (Bac‐to‐Bac™) recombinant baculovirus genomes are generated in Escherichia coli and then used to transfect insect cells to obtain recombinant baculovirus particles. After generating high‐titer virus stocks, insect cells are infected to produce recombinant proteins. With the bacmid‐based methodology the time to generate recombinant viruses is reduced considerably. Another advantage is that the recombinant bacmid can be stored in E. coli and recovered when needed. A disadvantage is that the bacterial gene cassette present in bacmid‐derived viruses may easily be lost during virus passaging (Pijlman et al., 2003a). In addition to AcMNPV, bacmids have also been constructed for Spodoptera exigua MNPV and Helicoverpa armigera SNPV (Pijlman 2002, Wang 2003). The most recent method combines bacmid technologies with allelic replacement (FlashBac™; Oxford Expression Technologies) and thereby removes the BAC sequences from the viral genome. This latter system is especially suitable for high‐throughput screening.
Over the years, novel baculovirus vectors have been developed with special features and for more specific applications: transfer vectors have been modified to express polyhistidine‐tagged proteins for easy purification (pFastBac‐His™). Transfer vectors with dual, triple, or quadruple promoters usually p10 and polyhedrin, have been developed for allelic replacement (Belyaev 1993, Weyer 1991); dual (pFastBacDual™) and quadruple vectors (Tareilus et al., 2003) have also been developed for bacmid technology. Such multiple vectors can be used to express various proteins simultaneously, and hence are useful for producing multimeric complexes, including viral capsids consisting of more than one viral protein (Belyaev and Roy, 1993). Balancing expression levels is sometimes a problem in these vectors and may require coinfection with a vector expressing only the dominant protein, or a modification of the promoters. One of the promoters in multiple promoter vectors may be used to express a reporter gene, such as green fluorescent protein (GFP), which makes it easy to follow the infection process in cells, perform virus titrations, and track baculovirus infection in the insect (Cha 1997, Kaba 2003). The recently developed vector system (UltraBac) uses the baculovirus late basic protein (P6.9) promoter to express GFP together with the foreign gene to allow earlier monitoring of infection (Philipps et al., 2005).
Baculovirus surface display vectors (Grabherr et al., 2001) expose the antigen on the surface of budded baculovirus particles. This is achieved by fusing the foreign antigen to the baculovirus envelope glycoprotein GP64 (Monsma et al., 1996). The chimeric protein is transported to the cell membrane and is taken up in the viral envelope during budding. This system has also been combined with bacmid technology (Kaba et al., 2003). The recombinant budded virus (BV) particles and lysates of cells infected with a display vector have been shown to evoke protective immune responses (Kaba 2005, Tami 2004, Yoshida 2003). Baculovirus vectors that express foreign genes in fusion with polyhedrin along with wild‐type polyhedrin allow for incorporation of antigens into baculovirus occlusion bodies (Je et al., 2003). These occlusion bodies are stable and easy to purify and can be used directly for immunization (Wilson et al., 2005).
C. Adaptations for Secreted Proteins
Expression of surface (glyco)proteins that go through the export pathway is in general more difficult than expression of soluble cytoplasmic proteins and results in much lower yields (van Oers et al., 2001). To increase the production level, surface proteins are often expressed as secreted proteins by removing hydrophobic transmembrane regions (TMR) that serve to anchor the protein to cell membranes. Removing these domains by recombinant DNA technology leads to secreted proteins which can then be purified from the culture medium. However, some caution is needed because this approach may affect folding and vaccine efficacy.
Not all proteins present at the surface under native conditions are automatically transported to the cell surface when expressed in insect cells, such as the p67 surface protein of the bovine parasite Theileria parva (Nene et al., 1995). When the original signal peptide was replaced with an insect analogue, such as the honeybee mellitin signal peptide (Tessier et al., 1991), p67 was properly routed to the cell surface (Kaba et al., 2004a). A similar routing of p67 to the export pathway could be obtained by fusion to GP64 in a surface display vector (Kaba et al., 2002), where the GP64 signal peptide directed the protein to the cell surface.
Membrane and secreted proteins pass through the endoplasmic reticulum (ER) and the Golgi apparatus on their way to the cell surface and may become glycosylated during this process. The abundant baculovirus protein chitinase is also transported to the ER and accumulates there due to a KDEL retention sequence (Saville 2004, Thomas 1998). Chitinase is expressed in the late phase of baculovirus infection and is involved in the dissolution of the insect chitinous cuticle to enhance the spread of viral occlusion bodies (Hawtin et al., 1997). Deletion of chitinase from the baculovirus vector resulted in higher levels of secreted recombinant protein (Possee et al., 1999) possibly because chitinase “cloggs up” the protein translocation machinery and competes with recombinant secretory proteins. The FlashBac system described earlier lacks this chitinase gene. Another baculovirus protein, v‐cathepsin also accumulates in the ER and is activated on cell death by proteolytic cleavage (Hom et al., 2002). Processing of pro‐v‐cathepsin into active cathepsin is also triggered by chaotropic agents, such as sodium dodecyl sulfate, and this may result in proteolysis of recombinant proteins during extraction and purification (Hom and Volkman, 1998). A bacmid vector that lacked both chitinase and v‐cathepsin (AcBacΔCC) improved the stability of a secreted recombinant protein, thereby increasing the yield of full‐length protein molecules (Kaba et al., 2004a).
Folding of complicated transmembrane glycoproteins can be improved by coexpression of molecular chaperones. The serotonin transporter (SERT) protein is a brain glycoprotein with 12 predicted transmembrane domains. Coexpression of the chaperones calnexin and, to a lesser extent, of immunoglobulin heavy chain‐binding protein (BiP) or calreticulin increased the yield of functional SERT threefold. The foldase ERp57 did not have this effect (Tate et al., 1999). Calreticulin and calnexin were also shown to increase the level of active lipoprotein lipase when coexpressed in insect cells, and to stimulate dimerization of the recombinant protein (Zhang et al., 2003). Expression of calnexin and calreticulin in a stable transgenic insect cell line, which was than infected with a recombinant baculovirus, resulted in a lower ratio of secreted versus intracellular recombinant protein than when cells were coinfected with two baculoviruses, one carrying the gene of interest and the other a chaperone (Kato et al., 2005). This result suggests that chaperone expression levels should be of the same order as recombinant protein levels.
D. Baculovirus Vectors with Mammalian Promoters
Another special adaptation is the incorporation of mammalian promoters in baculovirus vectors to drive foreign gene expression. Baculovirus vectors with mammalian promoters (BacMam™ viruses) have the potential to serve as gene delivery vectors in gene therapy (Huser 2003, Kost 2002) and have also been tested for vaccination purposes (Abe 2003, Aoki 1999, Facciabene 2004, Poomputsa 2003). In this case, a mammalian promoter or a viral promoter active in mammalian cells, such as the human cytomegalovirus (HCMV) IE1 promoter, drives intracellular expression of the antigen. Exposure on the cell surface via the major histocompatibility complex (MHC) activates the cellular immune system and in this respect, these types of vaccines resemble DNA vaccines. BacMam™ vectors are produced in insect cells and are replication incompetent in mammalian cells (Table II). A further advantage is that multiple genes can be inserted simultaneously into the baculovirus genome allowing for multivalent vaccines. Expression of multiple proteins is an advantage of the baculovirus expression system over other systems, especially adenovirus vectors, where the maximal increase in genome size is more limited due to packaging restrictions.
E. Adaptations for Vector Genome Stability
For manufacturing subunit vaccines, large‐scale production units will be needed, for instance for the production of malaria or the annual influenza subunit vaccines. Baculovirus–insect cell systems have been scaled‐up to large‐scale cultures in either fermentors (bioreactors) or cellbag devices (WAVE reactors). Insect cell bioreactors up to 2000 L have been reported. The bioprocess technology behind this large scale production has been reviewed by others (Hunt 2005, Ikonomou 2003, Vlak 1996). A problem repeatedly encountered when expressing recombinant proteins with baculovirus vectors is a drop in expression levels with increasing virus passage (reviewed in Krell, 1996). This so‐called “passage effect” is intrinsic to baculovirus replication in cell culture, but is less critical for small laboratory‐scale protein production when the number of virus passages is low (<10). It is a significant problem though for large‐scale industrial production of vaccines in insect cell bioreactors (Van Lier et al., 1996) and prevents the use of continous bioreactors. The major causes of loss of recombinant protein expression are (1) mutations in the FP25K gene, reducing the activity of the polyhedrin promoter (Harrison et al., 1996), (2) the generation of defective interfering particles (DIs) which replicate at the expense of the full‐length recombinant virus (Kool 1991, Pijlman 2001, Wickham 1991), (3) the intracellular accumulation of concatenated viral sequences, for example, non‐hr (homologous repeat) origins of DNA replication) which interfere with replication of full‐length genomes (Lee 1994, Pijlman 2002), and (4) spontaneous deletion of the heterologous gene from the baculovirus vector. The latter aspect is especially seen in bacmid‐derived vectors, which are extremely sensitive to spontaneous removal of the expression cassette, a large piece of DNA which is not under selection (Pijlman et al., 2003a). To prevent the amplification of DIs, baculovirus vectors must be used at low multiplicities of infection (MOI) (de Gooijer 1992, Wickham 1991) and it is now common practice to keep the number of viral passages to a minimum and establish low‐passage virus banks as seed stocks for production purposes.
In recent years, several approaches have been used to improve the stability of the baculovirus genome. The accumulation of non‐hr–containing sequences can easily be prevented by removing this sequence from the baculovirus backbone (Pijlman et al., 2002). Reducing the distance between origins of replication in the bacmid system by insertion of an extra hr sequence within the expression cassette also resulted in prolonged foreign gene expression in a test bioreactor (Pijlman et al., 2004). In the FlashBac system, all destabilizing bacterially derived sequences are removed on recombination with the transfer vector. To prevent loss of the foreign gene cassette, a bicistronic vector was developed that contained the foreign gene and the baculovirus essential gene GP64 on a single bicistronic transcriptional unit linked by an internal ribosome entry site (IRES). GP64 was deleted from its original locus. In this bicistronic vector, loss of the foreign gene would automatically result in loss of expression of the essential gene, which is needed for the generation of complete virus particles as well as for DIs. GFP expression levels were kept at a high level for at least 20 passages with this vector providing dominant selection for GP64 (Pijlman et al., 2006). This system awaits testing for expression of proteins of medical importance. By combining several of the methods described in this section, it is likely that genome stability will be further improved.
III. Viral Subunits Expressed in the Baculovirus System
Since its recognition as a production system for subunit vaccines (Vlak and Keus, 1990), the baculovirus–insect cell expression system has been used extensively for the expression of candidate vaccine antigens. A comprehensive overview of the viral antigens from viruses of vertebrates that have been expressed in this system is provided in Table III . Only those antigens that were tested for their ability to induce protective immune responses are included. In addition, many viral antigens have successfully been expressed in insect cells for the development of diagnostics, and to perform structural and functional studies, but these studies are excluded from this chapter. Various viral antigens ranging from capsid and envelope proteins to nonstructural proteins have been chosen for the development of subunit vaccines. These viral antigens can be divided into those that are expressed as single or oligomeric protein subunits, and those that self‐assemble into VLPs. Different approaches to vaccination are described in the examples later, with special attention paid to influenza subunit vaccines.
Table III.
Vertebrate Immune Response Studies with Viral Proteins Expressed in the Baculovirus–Insect Cell Systema
| Virus family or genus | Abbreviation | Host | Antigen(s)b | Neutralizing antibodiesc | T cells/cytokines | Protection host/model | References |
|---|---|---|---|---|---|---|---|
| Asfarviridae | |||||||
| African swine fever virus | ASFV | Pigs | p22, p30, p54, p72 | Yes | – | No | Neilan et al., 2004 |
| p30–p54 fusion | Yes | – | Yes | Barderas et al., 2001 | |||
| HA | Yes | – | Yes | Ruiz‐Gonzalvo et al., 1996 | |||
| Arenaviridae | |||||||
| Lymphocytic choriomeningitis virus | LCMV | Humans, rodents | GP, NP | – | T cells | Yes | Bachmann et al., 1994 |
| Arteriviridae | |||||||
| Porcine reproductive and respiratory syndrome virus | PRRSV | Pigs | 3, 5 (7) | Yes | – | Yes | Plana Duran et al., 1997 |
| Birnaviridae | |||||||
| Infectious pancreatic necrosis virus | IPNV | Fish | Structural proteins (VLPd) | – | – | Partial | Shivappa et al., 2005 |
| Infectious bursal disease virus | IBDV | Birds | VP2 (VLP) | Yes | – | Yes | Pitcovski 1996, Wang 2000 |
| VP2 (VLP), VPX, PP | Yes | – | Yes | Martinez‐Torrecuadrada et al., 2003 | |||
| VP2 + VP3 + VP4 (chimeric) | Yes | – | Yes | Snyder et al., 1994 | |||
| VP2 + VP3 + VP4 | Yes | – | Yes | Vakharia et al., 1994 | |||
| VP3 | No | – | No | Pitcovski et al., 1999 | |||
| Yellowtail ascites virus | YAV | Fish | VP2, VP3, NS | Yes | – | Yes | Sato et al., 2000 |
| Bunyaviridae | |||||||
| La Crosse virus | LACV | Humans | G1 | Yes | – | Yes | Pekosz et al., 1995 |
| Hantaan virus | HTNV | Humans, rodents | G1, G2, NP | Yes | – | Yes | Schmaljohn et al., 1990 |
| Rift Valley fever virus | RVFV | Humans, ruminants | G1, G2 | Yes | – | Yes | Schmaljohn et al., 1989 |
| Caliciviridae | |||||||
| Hepatitis E virus | HEV | Humans | Capsid (VLP) | Yes | – | Yes | Li 2001, Li 2004 |
| Norwalk virus, Genogroup I | NWV | Humans | Capsid (VLP) | Antibodies | – | – | Ball 1996, Ball 1998, Ball 1999, Guerrero 2001 |
| Norwalk virus, Genogroup II | NWV | Humans | Capsid (VLP) | Antibodies | Yes | – | Nicollier‐Jamot et al., 2004 |
| Circoviridae | |||||||
| Chicken anaemia virus | CAV | Birds | VP1, VP2 | Yes | – | Yes | Koch et al., 1995 |
| Porcine circovirus 2 | PCV2 | Pigs | ORF2 | Yes | – | Yes | Blanchard et al., 2003 |
| Coronaviridae | |||||||
| Avian infectious bronchitis virus | IBV | Chicken | S1 | Yes | – | Partial | Cavanagh 2003, Song 1998 |
| Feline infectious peritonitis virus | FIPV | Cats | N | No | Yes | Yes | Hohdatsu et al., 2003 |
| SARS corona virus | SARS | Humans | Spike GP | Yes | – | Yes | Bisht et al., 2005 |
| Transmissible gastroenteritis virus | TGEV | Pigs | S + N + M | Yes | Yes | Partial | Sestak et al., 1999 |
| Deltavirus | |||||||
| Hepatitis deltavirus | – | Humans/rodents | HD Ag | No | – | – | Karayiannis et al., 1993 |
| HD Ag p24, p27 | Antibodies | – | No | Fiedler and Roggendorf, 2001 | |||
| Filoviridae | |||||||
| Ebola virus | EBOV | Humans | GP | Yes | T cells | Partial | Mellquist‐Riemenschneider et al., 2003 |
| Marburg virus | MBGV | Humans | GP | Yes | – | Yes | Hevey et al., 1997 |
| Flaviviridae | |||||||
| Bovine viral diarrhea virus | BVDV | Cows | E2 | Yes | – | Yes | Bolin and Ridpath, 1996 |
| Classical swine fever virus | CSFV | Pigs | E2 | Yes | – | Yes | Ahrens 2000, Bouma 1999, Hulst 1993 |
| Dengue 2 virus | DEN 2 | Humans | E | Yes | – | – | Kelly et al., 2000 |
| E | Yes | – | Partial | Delenda 1994, Velzing 1999 | |||
| E | No | – | Partial | Feighny et al., 1994 | |||
| NS1 | Yes | – | Partial | Qu et al., 1993 | |||
| Dengue 4 virus | DEN 4 | Humans | Cocktail | Yes | – | Yes | Zhang et al., 1988 |
| Cocktail, E | Yes | – | Partial | Eckels et al., 1994 | |||
| Dengue virus 2 + 3 | DEN2/3 | Humans | E protein hybrid | Yes | T cells | – | Bielefeldt‐Ohmann et al., 1997 |
| Japanese encephalitis virus | JEV | prME, E, NS1 | Yes | – | Yes/No | Yang et al., 2005 | |
| E, NS1 | Yes | – | Yes | McCown et al., 1990 | |||
| West Nile virus | WNV | Humans/birds | prME (VLP) | Yes | – | Yes | Qiao et al., 2004 |
| Hepatitis C virus | HCV | Humans | E1 + E2 (VLP) | Yes | T cells/cytokines | Yes | Jeong et al., 2004 |
| St Louis encephalitis virus | SLEV | Humans | prME | Yes | – | Yes | Venugopal et al., 1995 |
| Tick‐borne encaphalitis virus | TBEV | Humans | E, C | – | T cells/ cytokines | – | Gomez et al., 2003 |
| Yellow fever virus | YFV | Humans | E, E + NS1 | Yes | – | Yes | Despres et al., 1991 |
| Hepadnaviridae | |||||||
| Hepatitis B virus | HBV | Humans | HBsAg | Antibodies | – | – | Attanasio et al., 1991 |
| Herpesviridae | |||||||
| Bovine herpesvirus 1 | BHV‐1 | Cows | gIII | Yes | – | – | Okazaki et al., 1994 |
| gIV | Yes | – | Yes | van Drunen Littel‐van den Hurk 1991, van Drunen Littel‐van den Hurk 1993 | |||
| Canine herpesvirus | CHV | Dogs | gC | Yes | – | – | Xuan et al., 1996 |
| Equine herpesvirus 1 | EHV‐1 | Horses | gB | Yes | – | Yes | Kukreja et al., 1998 |
| gB, gC, gD | Yes/No | T cells | Yes/No | Packiarajah et al., 1998 | |||
| gC | Yes | T cells | Yes | Stokes et al., 1996a | |||
| gC, gD | Yes | T cells | Yes | Whalley et al., 1995 | |||
| gD | Yes | – | – | Foote et al., 2005 | |||
| gD (DNA prime) | Yes | T cells | Yes | Ruitenberg et al., 2000 | |||
| gD, gH | Yes/No | – | Yes/No | Stokes et al., 1997 | |||
| gH, gL | – | – | Partial/No | Stokes et al., 1996b | |||
| Feline herpes virus 1 | FHV‐1 | Cats | gD | Yes | – | – | Maeda et al., 1996 |
| Guinea pig cytomegalovirus | GPCMV | Rodents | gB | Yes | – | Yes | Schleiss et al., 2004 |
| Herpes simpex virus 1 | HSV‐1 | Humans | gD | Yes | T cells | Yes | Krishna et al., 1989 |
| gE | Yes | – | – | Lin et al., 2004 | |||
| gB‐gI cocktail | Yes | – | Yes | Ghiasi et al., 1996 | |||
| gD | Yes | – | Yes | Ghiasi et al., 1991 | |||
| gD, gG, gK | – | Cytokines | – | Ghiasi et al., 1999 | |||
| gE | Yes | T cells/cytokines | Yes | Ghiasi et al., 1995 | |||
| gK | No | – | ADEe | Ghiasi et al., 2000 | |||
| gL | No | – | No | Ghiasi et al., 1994 | |||
| Human cytomegalovirus | HCMV | Humans | gB | Yes | – | – | Marshall et al., 2000 |
| IE1‐pp65 | – | T cells | – | Vaz‐Santiago et al., 2001 | |||
| Phocid herpes virus 1 | PhHV‐1 | Seals | gB | Yes | – | Yes | Harder and Osterhaus, 1997 |
| Pseudorabies virus | PrV | Pigs | gII | Yes | – | Yes | Xuan et al., 1995 |
| gIII | Yes | – | – | Inumaru and Yamada, 1991 | |||
| Orthomyxoviridae | |||||||
| Equine influenza virus | H3N8 | Horses | H3 | No | – | Partial | Olsen et al., 1997 |
| Human influenza A | H1N1 | Humans | H1 (proteosomes) | Yes | – | Yes | Jones et al., 2003 |
| H2N2 | M2 | Yes | – | Yes | Slepushkin et al., 1995 | ||
| H3N2 | H3 + M1 (VLP) | Yes | – | Yes | Galarza et al., 2005b | ||
| H3N2 | H3 | Yes | Yes | Yes | Powers 1995, Powers 1997 | ||
| H3N2 | H3 | Yes | – | – | Treanor et al., 1996 | ||
| H3N2 | H3, N2 | Yes | – | Yes | Brett 2005, Johansson 1999 | ||
| H3N2 | N2 | Yes | – | Yes | Deroo et al., 1996 | ||
| H6N2 | N2 | Yes | – | Yes | Kilbourne et al., 2004 | ||
| Multiple | H1, H3 | Yes | – | – | Lakey et al., 1996 | ||
| Avian influenza virus | H5N1 | Birds | H5 | No/– | – | Yes | Katz 2000, Swayne 2001 |
| H5N1 | H5 | In humans | – | – | Treanor et al., 2001 | ||
| Multiple | H5, H7 | Yes | Yes | Crawford et al., 1999 | |||
| Papovaviridae | |||||||
| Bovine papillomavirus | BPV | Cows | L1 | Yes | – | – | Kirnbauer et al., 1992 |
| Cottontail rabbit papillomavirus | CRPV | Rodents | L1 (VLP) | Yes | – | Yes | Breitburd 1995, Christensen 1996 |
| L1, L1 + L2 (VLP) | Yes | – | Yes | ||||
| Human papillomavirus 16 | HPV‐16 | Humans | L1 (VLP) | Yes | – | – | Harro et al., 2001 |
| L1 + L2 + E7 (VLP) | Yes | – | Yes | Greenstone et al., 1998 | |||
| L1 (VLP) | – | Yes | – | Dupuy et al., 1997 | |||
| Paramyxoviridae | |||||||
| Bovine parainfluenza virus | BPIV‐3 | Cattle | HN | Yes | – | Yes | Haanes et al., 1997 |
| Bovine repiratoiry syncytial virus | BRSV | Cattle | F | Yes | Yes | Yes | Sharma et al., 1996 |
| F partial | Yes | Yes | Yes | Werle et al., 1998 | |||
| Human parainfluenza virus | HPIV‐3 | Humans | F | Low | – | Partial | Hall et al., 1991 |
| F | Yes | – | Yes | Ray et al., 1989 | |||
| HN | Yes | – | Yes | van Wyke Coelingh et al., 1987 | |||
| HN (+ RSV F) | Yes | – | Yes | Du 1994, Homa 1993 | |||
| HN‐F fusion | Yes | – | Yes | Brideau et al., 1993 | |||
| HN, F, HN‐F | Yes | – | Yes | Lehman et al., 1993 | |||
| Human respiratory syncytial virus | HRSV | Humans | F (+HPIV‐HN) | Yes | – | Yes | Du 1994, Homa 1993 |
| FG fusion | Low | – | Partial | Connors et al., 1992 | |||
| FG fusion | Yes | – | Yes | Brideau 1989, Oien 1993, Wathen 1991 | |||
| Newcastle disease virus | NDV | Birds | F | – | – | Yes | Mori et al., 1994 |
| HN | Yes | – | Yes | Nagy et al., 1991 | |||
| Peste‐des‐petits‐ruminants virus | PPRV | Ruminants | HN | Yes | Yes | – | Sinnathamby et al., 2001a |
| Rinderpest virus | RPV | Cows | F, H | Yes | – | No | Bassiri et al., 1993 |
| H | – | Yes | – | Sinnathamby et al., 2001b | |||
| Rubella virus | RV | Humans | E2, C | – | – | No | Cusi et al., 1995 |
| Parvoviridae | |||||||
| B19 virus | B19V | Humans | VP1, VP2 (VLP) | Yes | – | – | Kajigaya et al., 1991 |
| Canine parvovirus | CPV | Dogs | VP2 (VLP) | Yes | – | Yes | Lopez de Turiso 1992, Saliki 1992 |
| Duck parvovirus | DPV | Birds | VP1, VP2 (VLP) | Yes | – | – | Le Gall‐Recule et al., 1996 |
| Mink enteritis parvovius | MEV | Mink | VP2 (VLP) | Yes | – | Yes | Christensen et al., 1994 |
| Porcine parvovirus | PPV | Pigs | VP2 (VLP) | Antibodies | – | – | Martinez et al., 1992 |
| Picornaviridae | |||||||
| Foot‐and‐mouth disease virus | FMDV | Cattle | Epitopes fused to GP64 | Yes | – | Yes | Tami et al., 2004 |
| P1–2A + part P2 | – | – | Partial | Grubman et al., 1993 | |||
| Hepatitis A virus | HAV | Humans | polyprotein | Yes | – | – | Rosen et al., 1993 |
| Polyomaviridae | |||||||
| Simian virus 40 | SV40 | Primates | Large T | Antibodies | – | Yes | Shearer et al., 1993 |
| Yes | No | Yes | Bright 1998, Watts 1999 | ||||
| Reoviridae | |||||||
| African horse sickness virus | AHSV | Horses | VP2 (VLP) | – | – | Yes | Roy and Sutton, 1998 |
| Bluetongue virus | BTV | Sheep, cattle | VP2 (VLP) | Yes | – | Yes | Roy et al., 1994 |
| VP2, VP5 (VLP) | Yes | – | – | Loudon et al., 1991 | |||
| VP2, VP5, VP3, and VP7 (VLP) | Yes | – | Yes | French 1990, Pearson 1993, Roy 2003, van Dijk 1993 | |||
| Bovine rotavirus | BoRV | Cows | VP2 + VP4 + VP6 + | Yes | – | Yes | Conner 1996a, Conner 1996b |
| VP7 (VLP) | |||||||
| Human rotavirus | HRV | Humans | VP2 + VP4 + VP6 + | Yes | – | Yes | Conner et al., 1996a |
| VP7 (VLP) | |||||||
| Simian rotavirus | SiRV | Primates | VP2 + VP4 + VP6 + | Yes | – | Yes | Conner 1996a, Conner 1996b |
| VP7 (VLP) | |||||||
| Retroviridae | |||||||
| Feline immunodeficiency virus | FIV | Cats | gp120 | Yes | – | Partial | Leutenegger et al., 1998 |
| Human immunodeficiency virus | HIV‐1 | Humans | p24 | – | T cells | – | Fyfe et al., 1993 |
| gp41 MEPRf/ PERVg p15E fusion | Yes | – | – | Luo et al., 2006 | |||
| gp41 + V3 loop | Yes | – | – | Luo et al., 1992 | |||
| gp55 (VLP) | Boost | – | – | Jaffray et al., 2004 | |||
| gp55–gp120 (VLP) | Yes | – | – | Arico et al., 2005 | |||
| Yes | T cells | – | Buonaguro 2002, Tobin 1997 | ||||
| gp120 | Antibodies | – | – | Peet et al., 1997 | |||
| No | – | – | Bristow et al., 1994 | ||||
| – | No CTL | – | Perales et al., 1995 | ||||
| – | CTL | – | Doe et al., 1994 | ||||
| gp160 | Partial | – | – | Keefer et al., 1994 | |||
| No | – | – | Akerblom et al., 1993 | ||||
| Boosth | – | – | Gorse 1994, Graham 1993, Lubeck 1994, Montefiori 1992 | ||||
| Boost, partial | CTL | – | Cooney et al., 1993 | ||||
| Antibodies | T cells | – | Lundholm et al., 1994 | ||||
| Memory B cells | – | – | Reuben et al., 1992 | ||||
| No | T cells | – | McElrath et al., 1994 | ||||
| HIV‐1 | Humans | gp160 | – | T cells | – | Gorse 1992, Keefer 1991 | |
| HIV‐2 | Humans | gp41 HIV‐1 + HIV‐2 V3 loop | Yes | – | – | Luo et al., 1992 | |
| Simian immunodeficiency virus | SIV | Primates | Env on gag VLP | Yes | Yes | – | Yao 2000, Yao 2002 |
| gp160 | Boost | – | Yes | Hu et al., 1992 | |||
| Rhabdoviridae | |||||||
| Rabies virus | RABV | Mammals | G | Yes | Yes | Yes | Prehaud et al., 1989 |
| G | Yes | – | Yes | Fu et al., 1993 | |||
| N, G | Yes | – | Yes | Drings et al., 1999 | |||
| Mokola virus | MOKV | Mammals | G | Antibodies | – | Yes | Tordo et al., 1993 |
Dashes in the table mean not analysed in this study.
Only those antigens are included that were tested in immunization experiments.
If not known whether neutralizing indicated as “antibodies.”
VLP, virus‐like particle.
ADE, antibody‐dependent enhancement by nonneutralizing antibodies (resulting in chronic infections).
MEPR, membrane‐proximal region.
PERV, porcine endogenous retrovirus.
Boost, boost with baculovirus‐produced recombinant protein, prime form other origin.
A. Viral Envelope Proteins
Envelope proteins are synthesized as single or oligomeric subunits. The expressed envelope proteins are often functionally active and have been reported to oligomerize, an indication that they are correctly folded (Crawford et al., 1999). Commonly, viral envelope glycoproteins are glycosylated in insect cells. Examples of baculovirus‐produced subunit vaccine candidates (Table III) are the fusion proteins and hemagglutinins of paramyxoviruses, such as Newcastle disease virus, and the E proteins of Flaviviridae, including West Nile virus, dengue viruses, and CSFV. Two commercially available veterinary subunit vaccines against classical swine fever (BAYOVAC CSF E2™ and PORCILIS PESTI™) are based on the CSFV E2 glycoprotein produced in insect cells (Ahrens 2000, Bouma 1999, Bouma 2000, Depner 2001, van Aarle 2003). The E2 envelope glycoprotein of CSFV was expressed as a secreted protein by removing the TMR and this resulted in a threefold increase in expression levels, and allowed for purification of E2 from the culture medium (Hulst et al., 1993). In a similar way, the related Bovine diarrhea virus (BVDV) E2 protein was expressed in insect cells (Bolin and Ridpath, 1996). Recent research showed that the BVDV E2 protein needs to be glycosylated to be effectively secreted from baculovirus‐infected cells (Pande et al., 2005) and that the glycosylated protein was able to block BVDV infection better in an in vitro assay. Whether the glycosylated E2 protein also performs better as a vaccine is not known. The spike glycoprotein of the SARS coronavirus is one of the most recently expressed proteins in insect cells and protected mice against intranasal SARS infection (Bisht et al., 2005).
Influenza presents a serious risk for both human and animal health. The single‐stranded RNA of the influenza virus changes quickly through an accumulation of mutations and frequent recombination events, requiring annual vaccine updates (Palese, 2004). The most threatening recent example is the outbreak of avian influenza of the H5N1 serotype which has killed birds and humans in the Far East since 2003 (WHO) and which caused the first human casualties outside this area in East Turkey in January 2006. The big fear is that such an avian virus will change into a virus that can be transmitted directly from man to man, which may then lead to an influenza outbreak of pandemic dimensions (Palese, 2004). The most widely used influenza vaccines, e g., Fluzone (Sanofi Pasteur) and Fluvirin (Chiron), consist of chemically inactivated split virus or purified virus subunits. These vaccines have several disadvantages which have recently been reviewed (Cox 2005, Cox 2004), including reduced efficacy in the elderly, where vaccination does reduce mortality rates but is not very effective in preventing disease. In addition, an enormous number of eggs are needed each year (one egg per dose) which will very likely lead to a shortage of vaccine in the event of a pandemic; some strains grow poorly in eggs requiring coinfections with other strains or genetic adaptations (e.g., H5N1) (Horimoto et al., 2006); and these vaccines can cause strong allergic reactions in some individuals. Live, attenuated influenza vaccines have the advantage of inducing secretory and systemic immunity and are applied intranasally, preventing virus replication in the respiratory tracts (Cox et al., 2004). However, all of these vaccines still need to be grown in chicken embryos, which are ironically also the target for a potentially pandemic virus like H5N1.
To overcome these drawbacks, various cell‐based vaccines for influenza are under development as well as recombinant protein vaccines. Clinical trials of vaccines based on influenza virus produced in mammalian cell cultures, such as Madin Darby canine kidney (MDCK) cells, have been described (Brands 1999, Percheson 1999) and trials with influenza vaccines produced in the human retina cell line Per.C6® (Pau et al., 2001) are ongoing. These products still require inactivation of the influenza virus which may reduce immunogenicity as seen for inactivated vaccines. In response to human casualties of H5 and H7 influenza viruses in Asia in the late 1990s, the immunogenicity and safety of baculovirus recombinant H5 and H7 hemagglutinin (HA) proteins was tested in chickens and resulted in 100% protection against disease symptoms (Crawford et al., 1999). The immunogenicity of the baculovirus‐derived H5 vaccine was subsequently evaluated in over 200 healthy human adults. The vaccine was well tolerated and provided neutralizing antibody responses equivalent to those observed in convalescent sera in ∼50% of the individuals after two doses (Treanor et al., 2001). A clinical trial with baculovirus‐produced recombinant H3 antigens in 127 adult volunteers showed protective neutralizing antibody levels and a reduction in influenza rates in the following epidemic season compared to a placebo group (Powers et al., 1995). This HA‐based vaccine induced both B and T memory cells (Powers et al., 1997). A clinical study of 399 individuals with an average age of 70 years was completed in 2003–2004 with an experimental vaccine (FluBlØk, Protein Sciences corporation) containing the same three HA antigen variants as present in the licensed inactivated vaccine of that flu season (Treanor et al., 2006). Compared to the licensed vaccine, the recombinant vaccine produced higher antibody titers against the H3 strain, the strain responsible for the majority of influenza deaths each year (Cox, 2005). This result suggests that this vaccine can be especially useful for reduction of the annual number of influenza‐related deaths in the elderly, where H3 antibody titers induced by conventional vaccines are too low to be protective. Phase III trials in healthy adults have been completed and showed a 100% protective effcicacy even against H3N2 influenza viruses (Manon Cox, personal communication) (http://www.proteinsciences.com/, Jan 2006). Preparation of a recombinant influenza virus vaccine cocktail for the coming flu season may take about 3 months to complete from the moment the new vaccine composition is announced by the World Health Organization (WHO).
The inactivated conventional vaccine and the trivalent recombinant HA‐based vaccine under development are based on antibody responses against the HA surface protein and require annual modifications to the vaccine due to antigenic drift of the influenza virus. A baculovirus recombinant vaccine with both HA and neuraminidase (NA) subunits resulted in a bivalent seroconversion with antibodies against both HA and NA (Johansson, 1999). The efficacy of an H3N2 vaccine based on both HA and NA produced with a recombinant baculovirus was analyzed in a murine model and compared with a conventional killed and a live‐attenuated vaccine preparation and an HA single‐subunit vaccine (Brett and Johansson, 2005). The NA in the baculovirus‐derived vaccine was much more immunogenic than in the conventional vaccines. The advantage of inducing an immune response to both surface proteins is illustrated by the fact that the recombinant vaccine containing both HA and NA did not only prevent infection with homotypic and closely related viruses, but also showed a strong reduction in pulmonary virus titers in infections with a more distantly related virus (H3N2 A/Panama/2007/99 versus A/Fuijan/411/2002), in contrast to a vaccine based on HA only. These results suggest that a vaccine containing intact NA tolerates more antigenic drift, thereby reducing the chance of virus escaping the immune system during the flu season.
B. Virus‐like Particles
Viral capsid proteins produced in insect cells often self‐assemble into VLPs. The advantage of VLPs is that they resemble the natural virus but are not infectious because they lack genetic material. VLPs are also an excellent tool for study of virus structure. VLPs can easily be purified by extraction, centrifugation, or precipitation (Brown et al., 1991) and often give strong immune reactions even in the absence of adjuvants due to their particulate nature. In addition, humoral, cell‐mediated, and mucosal immune responses have been reported (Roy, 1996). An example of a vaccine consisting of recombinant VLPs produced with a baculovirus vector is a patented Canine parvovirus vaccine (Lopez de Turiso 1992, Valdes 1999). Sometimes the expression of more than one viral coat protein is needed to make immunogenic VLPs, either due to the complexity of the capsid structure (Bluetongue virus: BTV, Reoviridae) or presence of crucial epitopes on several coat proteins. Multicomponent VLPs can be produced by using vectors with multiple promoters or by coinfections with several baculovirus vectors that each encode one or more viral proteins. One difficulty in making complex VLPs is to achieve appropriate expression levels of each protein present in the viral capsid.
The capsid protein of Hepatitis E virus (Caliciviridae) forms VLPs and these VLPs induce both systemic and mucosal immunity after oral administration in a mouse model. They also protect cynomolgus monkeys when challenged with HEV against infection and hepatitis (Li 2001, Li 2004). Infectious bursal disease (IBDV, Birnaviridae) of birds can also be prevented by vaccination with single component VLPs (Martinez‐Torrecuadrada 2003, Wang 2000). The major capsid protein L1 of Papovaviridae forms VLPs and has been shown to protect cottontail rabbits against Cottontail rabbit papillomavirus. Combinations of the HPV‐16 L1 and L2 capsid proteins and the oncogenic protein E7 protected against tumor formation in a mouse model (Greenstone et al., 1998). Multivalent VLP preparations containing BTV (Reoviridae) VP2 subunits of various serotypes were made by coinfections of several baculovirus vectors and induced long‐lasting protection in sheep (Roy et al., 1994). Vaccine candidates in the form of VLPs with up to four different VPs have also been successfully produced for BTV (Pearson 1993, van Dijk 1993) as well as for several other Reoviridae (Conner 1996a, Conner 1996b). Immunization of mice with an influenza VLP containing the two matrix proteins M1 and M2, and the surface proteins HA and NA showed almost complete protection against an H3N2 virus via both intramuscular and intranasal immunization routes (Galarza et al., 2005a).
The rationale for using VLPs as vaccine candidates is obvious for nonenveloped viruses, because in these viruses the capsid proteins are directly exposed to the immune system. However, they may also be useful for displaying epitopes of enveloped viruses. HIV is an enveloped virus and in this case VLPs have been produced based on gp55 (gag) to which immunogenic segments of the envelope protein gp120 were coupled (Arico 2005, Buonaguro 2002, Tobin 1997). An extension of these chimeric VLP‐based vaccines is to use VLPs of one virus to display epitopes of heterologous proteins that do not form VLPs by themselves. Examples of such systems are Human parvovirus B19 VLPs which carry linear epitopes in fusion with the viral VP2 protein. This system was used to display epitopes of Murine hepatitis virus A59 (MHV; Coronaviridae) and Herpes simplex virus (HSV; Herpesviridae) (Brown et al., 1994). Such chimeric VLPs protected mice against a lethal challenge with MHV of HSV. Epitope‐presenting chimeric VLPs have also been developed based on Mouse papillomavirus (Tegerstedt et al., 2005) and Flock house virus VLPs (Scodeller et al., 1995).
C. Inclusion of Recombinant Cytokines in the Vaccine
Mono‐ and oligomeric protein subunits are often less potent and need to be formulated carefully before administration to extend their half‐life and to present them in a proper form to the immune system, for instance by uptake by antigen‐presenting cells (APCs) (Dertzbaugh 1998, Schijns 2003). Adjuvant possibilities for human application are very limited because of safety considerations and this may limit the application of monomeric subunit vaccines in humans. VLPs on the other hand have been shown to induce protection even without the addition of adjuvants (Li 2004, Roy 1996). An alternative way to modulate the immune response is by the addition of recombinant cytokines as vaccine adjuvants. Cytokines can either be added separately to the vaccine or may be included in VLPs. This approach may not only modulate the magnitude but also the type of immune response (Lofthouse et al., 1996). By carefully choosing which cytokine is added the immune response can be driven in a certain direction. Interferon gamma (IFN‐γ) may be added to stimulate macrophages, while addition of interleukin‐12 (IL‐12) promotes cell‐mediated adaptive immunity (Abbas and Lichtman, 2005). IL‐12 can be efficiently produced with baculovirus vectors as functional dimers that shift the immunogenic balance to Th1 cells in bovine calves (Takehara et al., 2002). IL‐12 added to influenza VLPs enhances antibody responses but in this case VLPs alone already result in 100% protection (Galarza et al., 2005a). Immune reactions to helminths involve Th2 responses. Interleukin‐4 (IL‐4) drives the immune response to differentiation of Th2 cells and to the production of heminth‐specific IgE antibodies (Abbas and Lichtman, 2005). Addition of IL‐4 may therefore be helpful for vaccines against helminths (Lofthouse et al., 1996). For baculovirus‐derived products the addition of cytokines has not been fully exploited, but it is commonly used for DNA vaccines. The addition of costimulators, such as B7 or CD40, to baculovirus‐produced vaccines has not been reported.
D. Baculoviruses as DNA Vaccines
Baculoviral vectors with mammalian promoters driving the expression of viral genes have been used in a limited number of vaccine trials. A candidate Pseudorabies virus vaccine expressing its glycoprotein B from a recombinant baculovirus vector with a mammalian promoter resulted in seroconversion in immunized mice (Aoki et al., 1999). Intramuscular injection with baculovirus BVs expressing the E2 glycoprotein of Hepatitis C virus controlled by the CMV immediate‐early promoter‐enhancer provided specific humoral and cellular responses (Facciabene et al., 2004). Similar results were obtained with the carcinoembryonic antigen (CEA) indicating that these types of vaccines can also be effective against tumors. The addition of the Vesicular stomatis virus (VSV) G protein to the baculovirus envelope increased immunogenicity in this experiment, possibly by enhancement of virus fusion. BacMam™ vectors have also been used to produce mutated, attenuated influenza virus in mammalian cells by delivery of an altered NS1 gene (Poomputsa et al., 2003). Surprisingly, a baculovirus vector with the influenza hemagglutinin gene (H1) controlled by the chicken beta‐actin promoter gave a similar level of protection as a wild‐type baculovirus against a lethal influenza challenge in intranasally immunized mice (Abe et al., 2003). The authors ascribe this to the induction of a strong innate immune response by the baculovirus, protecting the mice from a subsequent lethal challenge with influenza virus.
A possible drawback to the use of baculoviruses directly for vaccination and possibly also for surface display and polyhedra‐incorporation vectors is the accumulation of anti‐baculovirus antibodies upon repeated vaccination, resulting in rapid inactivation of subsequent vaccines of the same type. Pigs for instance have been shown to produce high levels of baculovirus‐neutralizing antibodies after injection of baculovirus BVs (Tuboly et al., 1993). A solution to this problem could be to design multivalent vaccines, since baculoviruses can take up a large amount of foreign DNA. Another problem with the use of baculovirus particles as vaccines is rapid degradation by the complement system which also affects gene therapy applications. Pseudotyping of BVs with the VSV glycoprotein instead of GP64 resulted in a reduction in complement inactivation (Tani et al., 2003). Incorporation of human decay‐accelerating factor (DAF), a complement‐regulatory protein, in the BV envelope has been shown to protect baculovirus gene therapy vectors against complement‐mediated inactivation (Huser et al., 2001). This strategy could also be applied for vaccine purposes.
E. Combinations of Vaccine Strategies
HIV VLPs containing only gp55 (gag) have been used to boost immunization induced by a gp55 DNA vaccine (Jaffray et al., 2004). In this case, a capsid protein of an enveloped virus is a reasonable subunit for vaccination because DNA vaccines are expressed intracellularly and fragments of the resulting proteins are presented by MHC complexes to induce cellular immune responses. In this way the natural situation of intracellular expression of viral genes is mimicked. T cell responses, especially the action of cytotoxic T lymphocytes (CTL) are crucial in defense against HIV. HIV combination vaccines where adenovirus, vaccinia (HIVAC‐1e), or vesicular stomatitis virus vectors were used for immunization in combination with a boost with a protein subunit (gp160, gp41) have been tested in phase I trials in humans (Cooney 1993, Graham 1993, Lubeck 1994, Luo 2006, Perales 1995, Zheng 1999). Viral carriers also express HIV antigens inside cells, and these antigens are displayed either by specialized APCs or other cells to the immune system. The aim of this regimen with a DNA/carrier vaccine and a protein boost regimen is therefore to induce both neutralizing antibodies and T cell responses.
F. Viral Marker Vaccines and Differential Diagnosis Technology
Nonvaccination policies exist for many animal diseases because of the risk that vaccinated animals may be protected against disease but may still be carriers of the virus. In cases of outbreaks, ring vaccination is sometimes applied, but large‐scale vaccination is generally not allowed. A prerequisite for the broader use of animal vaccines is the development of marker vaccines that enable differentiation between vaccinated and infected animals. This is especially important for endemic diseases in animals where monitoring is of crucial importance to avoid spread of the virus to nonendemic regions or to other host species such as humans or wild animals. Marker vaccines also have good prospects for eradication of animal diseases in general (van Aarle, 2003). For such purposes, marker vaccines do not have to give 100% herd immunity, because reducing susceptibility and transmission can be sufficient to have a major effect in controlling animal disease (Henderson, 2005). Marker vaccines need to be accompanied by specific diagnostic tests that are commonly based on determining serum titers of a viral component that is absent in the vaccine but present in the pathogen, to which antibodies can be raised.
The baculovirus expression system has proven to be useful in producing not only the protein subunits for marker vaccines but also the recombinant polypeptides for these diagnostic tests. The commercially available CSFV vaccine based on the E2 glycoprotein is a marker vaccine because only antibodies against E2 are generated. An enzyme‐linked immunosorbent assay (ELISA) test for serum antibodies against the other immunogenic surface protein ERNS can be used to discriminate immunized animals from virus carriers (Langedijk 2001, van Aarle 2003). Another possibility is to use only some of the epitopes of an antigen for immunization and others for the diagnostic analysis, as demonstrated for CSFV (van Rijn et al., 1999). The coexistence of a subunit vaccine and a discriminative diagnostic test enabled registration of CSFV vaccines in Europe. Marker vaccines will also be very useful for immunization of poultry against avian influenza (Crawford et al., 1999), where monitoring for the presence of virus is essential. Animals immunized with HA or HA/NA vaccines could be screened for antibodies against the viral matrix proteins. Similar assays have been developed for other subunit vaccines, such as one that discriminates between VSV‐infected and immunized animals, where the marker vaccine is based on the glycoprotein and the assay on the nucleocapsid protein produced in insect larvae (Ahmad et al., 1993).
IV. Baculovirus‐Produced Vaccines Against Protozoan Parasites and Helminths
Parasites of the genera Plasmodium, Theileria, and Babesia are protozoan blood parasites causing malaria, theileriosis, and babesiosis. These parasites have a complex life cycle and are transmitted by either mosquito or tick vectors. Candidate subunit vaccines against these parasites can be roughly separated into preblood (preerythrocyte or prelymphocyte) stage vaccines, blood stage vaccines, transmission‐blocking vaccines, and multistage vaccines. An overview of parasite subunits expressed in the baculovirus insect cell system for vaccine purposes is given in Table IV .
Table IV.
Vaccine Trials for Parasitic Diseases Based on Subunits Expressed in the Baculovirus–Insect Cell System
| Pathogen | Antigen | Trial | Immunologic response | References |
|---|---|---|---|---|
| Protozoa | ||||
| Babesia rodhaini | P26 surface protein | Immunization of rats | 40–100% protection | Igarashi et al., 2000 |
| Plasmodium berghei | Ookinete surface protein 21 | Injection in mice | Antibodies, oocyst formation blocked in Anopheles stephensi mosquitoes | Matsuoka et al., 1996 |
| Plasmodium cynmolgi | Merozoite surface protein (MSP)‐1 | Challenge in primates | Protection | Perera et al., 1998 |
| Plasmodium falciparum | Circumsporozoite protein (CSP) | Human safety and immunity trial | No response to native CSP | Herrington et al., 1992 |
| Theileria parva | Sporozoite surface protein p67 | Challenge in cattle | 50% protection | Nene et al., 1995 |
| Sporozoite surface protein P67 (GFp fusion, surface display) | Challenge in cattle | Upto 80% protection | Kaba 2004b, Kaba 2005 | |
| Theileria sergenti | P32 | Challenge in cattle | Protection | Onuma et al., 1997 |
| Trypanosoma cruzi | TolT | Mice immunization/in vitro inhibition by CD4 cells | 50–60% reduction of parasite numbers in infected macrophages | Quanquin et al., 1999 |
| Helminths | ||||
| Fasciola hepatica | Procathepsin L3 | Challenge in rats | 50% protection | Dalton et al., 2003 |
| Ostertagia ostertagi | Metalloprotease 1 | Challenge in cattle | No protection | De Maere et al., 2005a |
| Aspartyl‐protease inhibitor | Challenge in cattle | No protection | De Maere et al., 2005b | |
| Schistosoma mansoni | Calpain (Sm‐p80) | Challenge in mice | 29–39% reduction in worms | Hota‐Mitchell et al., 1997 |
A. Plasmodium
A comprehensive record of all subunit and recombinant carrier vaccines under development for human malaria is maintained by WHO (Reed, 2005). Most of these vaccines are still in a preclinical stage but several Plasmodium falciparum vaccines are in phase I and phase II trials in malaria endemic countries. Plasmodium is transmitted in the form of sporozoites by Anopheles mosquitoes. A vaccine candidate based on the circumsporozoite protein (CSP) is aimed at blocking these sporozoites. Both B and T cell responses appear to be essential for protective immunity based on the CSP protein. CSP has been produced in insect cells but was minimally immunogenic when tested in 20 volunteers (Herrington et al., 1992). Alternative approaches to experimental CSP vaccines, which facilitate T cell responses, are in phase II trials (Ballou et al., 2004). These vaccines include CSP displayed on HBsAg VLP particles, modified vaccinia Ankara virus as recombinant carrier, or DNA vaccines.
The merozoite is the extracellular, erythrocyte‐invasive form of the Plasmodium parasite. Merozoite surface proteins are promising vaccine candidates due to their accessibility for antibodies and their expected role in erythrocyte invasion. The major merozoite surface protein (MSP‐1) is an important prebloodstage candidate vaccine with homology to epidermal growth factor (EGF) and antibodies directed against this protein block erythrocyte invasion (Holder and Blackman, 1994). Plasmodium cynomolgi functions as a model system for the highly similar Plasmodium vivax in humans. An active, C‐terminally processed form of P. cynomolgi MSP‐1, was produced in insect cells and protected primates in a challenge experiment (Perera et al., 1998). The C‐terminally mature MSP‐1 of P. falciparum was also successfully expressed in insect cells and used for ultrastructural studies (Chitarra 1999, Pizarro 2003), but has not been tested in human trials. Meanwhile, E. coli‐expressed MSP‐1 has entered phase II clinical trials (Ballou et al., 2004).
Plasmodium parasites in the ookinete stage are taken up by mosquitoes and antigens specific for this stage can function as transmission‐blocking subunit vaccines. The major ookinete surface antigen Pbs21 (P28) of Plasmodium berghei was expressed in B. mori larvae, preserving conformational B cell epitopes which were lost upon expression in E. coli. The recombinant Pbs21 antigen produced in insect cells blocked oocyte formation in Anopheles mosquitoes fed on immunized mice (Matsuoka et al., 1996). The immunogenicity of the recombinant protein was strongly reduced when the protein was expressed as a secreted protein by removing its glycosylphosphatidylinositol (GPI) anchor signal (Martinez et al., 2000). This protein provides a good example of loss of immunogenicity by expressing a membrane protein in a secreted form. Subunit vaccines based on the Plasmodium‐induced erythrocyte membrane protein 1 (EMP‐1) are aimed at blocking vertical transmission from mother to child (maternal malaria) via the placenta. This transmission involves the sequestration of P. falciparum‐infected erythrocytes through EMP‐1, which binds to chondroitin sulphate A in the placenta. EMP‐1 expressed in the baculovirus system induces inhibitory antibodies which react with both homologous and heterologous EMP‐1 proteins (Costa et al., 2003).
B. Theileria and Babesia
T. parva is the causative agent of East Coast fever, a deadly cattle disease endemic in large parts of Africa. Immunization with recombinant sporozoite surface protein p67 is aimed at blocking invasion of lymphocytes by this parasite. P67 is an example of a protein that was not easy to express in a native form in insect cells as well as in many other systems. In insect cells, it was expressed at low levels and in contrast to expectations was not present on the cell surface. Similar to E. coli‐expressed p67, it did not react with a monoclonal antibody against native p67, indicating that the folding of the protein was not correct (Nene et al., 1995). Several adaptations were therefore made to the expression system. Expression of p67 coupled to the honeybee mellitin signal instead of the original signal peptide resulted in correct routing of this protein to the cell surface, but the folding was still not optimal (Kaba et al., 2004a). Fusion of p67 to the C terminus of GFP drastically increased expression levels and resulted in recognition by the conformation‐sensitive monoclonal antibody (Kaba et al., 2002). A similar effect was also seen when parts of this protein were fused to the baculovirus GP64 glycoprotein in a surface display vector, which led to expression on the cell surface and on baculovirus BVs (Kaba et al., 2003). The recombinant GFP‐p67 protein and the C terminal half of p67 coupled to GP64 induced high levels of sporozoite‐neutralizing serum antibodies and showed up to 80% protection against lethal T. parva challenge in a double‐blind placebo‐controlled experiment (Kaba 2004b, Kaba 2005). The next phase will be to evaluate the quality of these experimental vaccines under field conditions in countries in which East Coast fever is endemic.
Babesia species are a major cause of parasitemias in cattle and dogs. Babesia divergens is the major cause of bovine babesiosis in Europe and the increased incidence of this disease is correlated with an increase in the numbers of ticks (Ixodus ricinus) that transmit this parasite. B. divergens is also responsible for zoonotics in immunocompromised humans (Zintl et al., 2003). The soluble parasite antigen (SPA) of bovine and canine Babesia species has been developed as a vaccine against clinical manifestations in dogs and is produced in mammalian cells infected with Babesia (Schetters, 2005). Several other Babesia antigens have been expressed in the baculovirus expression system to develop ELISA tests for diagnosis. The baculovirus‐expressed Babesia radhaini P26 protein was shown to induce protection against the disease in rats (Igarashi et al., 2000).
Theileria and Babesia parasites are transmitted by ioxidic ticks and in the future candidate antiparasite vaccines may be combined with vaccines directed against the tick vector (Bishop et al., 2004). These vaccines may be tick antigens directly exposed to the host immune system, such as vitellin, the most abundant B. microplus egg protein (Tellam et al., 2002) or cement proteins involved in the attachment of the tick to the skin of the host. Concealed antigens can also give good results, such as the B. microplus Bm86 gut antigen (Gavac™), which results in binding of antibodies taken up from immunized animals to a gut transmembrane protein (Willadsen et al., 1995). Ticks are known to immunomodulate their host by secreting specific immunomodulators and transmitted parasites also profit from the reduction in immune response. Tick vaccines may therefore be aimed at reducing the chance of transmission by directly affecting the feeding process, by interacting with immunomodulators, or by reducing tick populations.
C. Trypanosoma and Leishmania
Chagas’ disease in the Americas is caused by Trypanosoma cruzi and is found in humans, dogs, cats, and rodents. T. cruzi is a macrophage‐invading protozoan, and complicating factors in the development of vaccines are immune escape and autoimmunity due to molecular mimickry (Girones et al., 2005). Macrophages are also the target for Leishmania parasites, which use complex immune evasion strategies that affect host cell signaling (Olivier et al., 2005). Immunization with the T. cruzi Tol A‐like protein (TolT) expressed in insect cells resulted in T cell‐dependent antiparasitic activity (Quanquin et al., 1999). For Leishmania several candidate subunit vaccine antigens with protective potential have been identified, including the surface protein gp63 (Coler and Reed, 2005), but these proteins have not been expressed with baculovirus vectors in insect cells.
D. Helminths
Helminths or parasitic worms that are a serious threat to human and animal health worldwide, are divided into the Annelida (segmented worms), Platyhelminthes (flatworms including flukes), and Nematoda (roundworms). These worms have complex life cycles, which often involve more than one host. Because helminths vary widely among populations, vaccines are not competitive so far with chemical broad spectrum antihelminths (Bos and Schetters, 1990). Helminths may also modulate the immune system as exemplified by the filarial nematodes, which are present in 150–200 million humans worldwide and cause river blindness for example. These filarial nematodes are difficult to combat because they modulate T cell responses leading to chronic helminth infections (reviewed by Hoerauf et al., 2005). This T cell modulation is not restricted to the response to filarial larvae but also affects allergies, the response to other pathogens and vaccines through modulation of not only antigen‐specific T cells but also APCs, which affects immune responses in general.
There are a few examples of baculovirus‐expressed helminth proteins but few of these have been tested in immunization studies (Table IV). However, the baculovirus expression system may be a valuable tool for these parasitic worms as illustrated by the following examples: a baculovirus‐derived subunit vaccine against liver fluke (Fasciola hepatica) based on procathepsin L3 conferred 50% protection to rats, in contrast to the yeast‐expressed protein which did not confer any protection (Reszka et al., 2005). Bilharzia or schistosomiasis is caused by Schistosoma spp. (Platyhelminthes), blood parasites of humans in tropical areas. Several Schistosoma genes have been expressed in insect cells, but primarily for analysis of enzymatic functions. The large subunit of calpain (SmP80) produced in the baculovirus expression system reduced the worm burden in mice (Hota‐Mitchell et al., 1997). Antigens of the tapeworm Taenia solium have been expressed with baculovirus vectors for diagnostic purposes (Lee 2005, Levine 2004).
V. Conclusions and Prospects
There are many examples in the literature where immunization with recombinant proteins produced in the baculovirus–insect cell expression system conferred good protection against infectious disease. Subunit vaccine development begins with careful identification of the antigen, which is related to whether neutralizing adaptive immune responses are raised against the particular protein in natural infections. Once selected, the open reading frame (ORF) of the antigen is cloned while keeping flanking DNA sequences to a minimum, which can best be achieved with a proof‐reading PCR enzyme. The sequence around the ATG translational start site is best modified to that of the polyhedrin or p10 gene in the wild‐type baculovirus, with at least an adenosine residue at the −3 position (Chang et al., 1999). Tags may be added to facilitate purification, preferably in such a way that they can subsequently be removed. Tags are not required for VLPs because they can be purified by centrifugation. Transmembrane (glyco)proteins are best produced in a secreted form by removal of TMRs to increase expression levels. However, this may occasionally affect the folding of the protein. An alternative approach is to fuse the immunogenic domains of envelope proteins to GP64. Vectors that lack chitinase and v‐cathepsin genes are preferable for expression of envelope proteins. During the preparation of seed stocks, care should be taken to keep the virus passage number low and to use a multiplicity of infection of <0.1 to minimize the formation of DIs. Careful checking of the purity of recombinant bacmids or plaque purified recombinant viruses by PCR is crucial to avoid loss of recombinant virus in subsequent passages due to empty vectors that out‐compete the recombinants.
Only two baculovirus‐produced products are approved for veterinary practice, namely, two vaccines against classical swine fever consisting of the E2 surface glycoprotein. With these vaccines in the market (although a nonvaccination policy still exists) the confidence in this type of subunit vaccine will grow as well as the possibilities for registration, thereby increasing the likelihood that more vaccines of this kind will appear in the market. This expectation of an increasing number of products may be expanded to human applications when registration of a trivalent recombinant influenza vaccine, for which phase III clinical trials in humans in the United States have been completed recently, can be achieved. Therapeutic anticancer vaccines, such as a vaccine against prostate cancer, may be more readily accepted, in view of the severe side effects of anticancer drugs and irradiation techniques. The application of baculovirus display or BacMam™ vectors for vaccines against infectious disease may be applied in the future for animal use. Because more foreign proteins are incorporated into the vaccine than just the targeted antigen, such vaccines for human use are likely to be in the more distant future because of safety considerations.
Baculovirus expression systems compete with other cheaper production systems, which are more widely used and which scale‐up more easily, such as E. coli and yeast (Table I), and once a good protection level is achieved there is no commercial interest to switch to the more expensive insect cell system. For those cases though, where folding or posttranslational modifications are crucial to epitope formation the baculovirus–insect cell system is a versatile expression system and many candidate vaccines have been successfully tested. Alternative methods are provided by the development of DNA vaccine technology and recombinant carrier vaccines based on vaccinia, adenovirus, or BacMam™ vectors (Table II). These methods are more prone to induce cellular immune responses than many protein subunit vaccines. The combination of a primary vaccine with an intracellular delivery system (recombinant carrier vaccines, DNA vaccines, BacMam™ vectors) followed by a boost immunization with recombinant protein subunits appears to be a promising approach, aimed at both cellular and humoral immune responses. This approach can be further strengthened through the addition of recombinant cytokines that drive the immune response in a specific direction. A major challenge now is to broaden the array of viral vaccines produced in insect cells and to develop effective vaccines against more complex organisms, such as protozoan parasites and multicellular worms, for which the baculovirus expression system also holds promise. With the increasing insight into immunology leading to new methods of vaccine production and delivery, accompanied by a wealth of genomic and proteomic data, many new generation vaccines are expected within the foreseeable future.
Acknowledgments
I thank Manon Cox, Dick Schaap, and Stephen Kaba for assistance with the sections on viral vaccines, parasite vaccines, and malaria vaccines respectively; Christina van Houte for verifying Table III, and Just Vlak for review of the manuscript and for helpful suggestions.
References
- Abbas A.K., Lichtman A.H. “Cellular and Molecular Immunology.”. Elsevier Saunders; Philadelphia, USA: 2005. [Google Scholar]
- Abe T., Takahashi H., Hamazaki H., Miyano‐Kurosaki N., Matsuura Y., Takaku H. Baculovirus induces an innate immune response and confers protection from lethal influenza virus infection in mice. J. Immunol. 2003;171:1133–1139. doi: 10.4049/jimmunol.171.3.1133. [DOI] [PubMed] [Google Scholar]
- Ahmad S., Bassiri M., Banerjee A.K., Yilma T. Immunological characterization of the VSV nucleocapsid (N) protein expressed by recombinant baculovirus in Spodoptera exigua larva: Use in differential diagnosis between vaccinated and infected animals. Virology. 1993;192:207–216. doi: 10.1006/viro.1993.1023. [DOI] [PubMed] [Google Scholar]
- Ahrens U., Kaden V., Drexler C., Visser N. Efficacy of the classical swine fever (CSF) marker vaccine Porcilis Pesti in pregnant sows. Vet. Microbiol. 2000;77:83–97. doi: 10.1016/s0378-1135(00)00265-0. [DOI] [PubMed] [Google Scholar]
- Akerblom L., Nara P., Dunlop N., Putney S., Morein B. HIV experimental vaccines based on the iscom technology using envelope and GAG gene products. Biotechnol. Ther. 1993;4:145–161. [PubMed] [Google Scholar]
- Andre F.E. Vaccinology: Past achievements, present roadblocks and future promises. Vaccine. 2003;21:593–595. doi: 10.1016/s0264-410x(02)00702-8. [DOI] [PubMed] [Google Scholar]
- Aoki H., Sakoda Y., Jukuroki K., Takada A., Kida H., Fukusho A. Induction of antibodies in mice by a recombinant baculovirus expressing pseudorabies virus glycoprotein B in mammalian cells. Vet. Microbiol. 1999;68:197–207. doi: 10.1016/s0378-1135(99)00110-8. [DOI] [PubMed] [Google Scholar]
- Arico E., Wang E., Tornesello M., Tagliamonte M., Lewis G.K., Marincola F.M., Buonaguro F.M., Buonaguro L. Immature monocyte derived dendritic cells gene expression profile in response to virus‐like particles stimulation. J. Transl. Med. 2005;3:45. doi: 10.1186/1479-5876-3-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attanasio R., Lanford R.E., Dilley D., Stunz G.W., Notvall L., Henderson A.B., Kennedy R.C. Immunogenicity of hepatitis B surface antigen derived from the baculovirus expression vector system: A mouse potency study. Biologicals. 1991;19:347–353. doi: 10.1016/s1045-1056(05)80024-7. [DOI] [PubMed] [Google Scholar]
- Ayres M.D., Howard S.C., Kuzio J., Lopez‐Ferber M., Possee R.D. The complete DNA sequence of Autographa californica nuclear polyhedrosis virus. Virology. 1994;202:586–605. doi: 10.1006/viro.1994.1380. [DOI] [PubMed] [Google Scholar]
- Bachmann M.F., Kundig T.M., Freer G., Li Y., Kang C.Y., Bishop D.H., Hengartner H., Zinkernagel R.M. Induction of protective cytotoxic T cells with viral proteins. Eur. J. Immunol. 1994;24:2228–2236. doi: 10.1002/eji.1830240944. [DOI] [PubMed] [Google Scholar]
- Ball J.M., Estes M.K., Hardy M.E., Conner M.E., Opekun A.R., Graham D.Y. Recombinant Norwalk virus‐like particles as an oral vaccine. Arch. Virol. Suppl. 1996;12:243–249. doi: 10.1007/978-3-7091-6553-9_26. [DOI] [PubMed] [Google Scholar]
- Ball J.M., Hardy M.E., Atmar R.L., Conner M.E., Estes M.K. Oral immunization with recombinant Norwalk virus‐like particles induces a systemic and mucosal immune response in mice. J. Virol. 1998;72:1345–1353. doi: 10.1128/jvi.72.2.1345-1353.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball J.M., Graham D.Y., Opekun A.R., Gilger M.A., Guerrero R.A., Estes M.K. Recombinant Norwalk virus‐like particles given orally to volunteers: Phase I study. Gastroenterology. 1999;117:40–48. doi: 10.1016/s0016-5085(99)70548-2. [DOI] [PubMed] [Google Scholar]
- Ballou W.R., Arevalo‐Herrera M., Carucci D., Richie T.L., Corradin G., Diggs C., Druilhe P., Giersing B.K., Saul A., Heppner D.G., Kester K.E., Lanar D.E. Update on the clinical development of candidate malaria vaccines. Am. J. Trop. Med. Hyg. 2004;71:239–247. [PubMed] [Google Scholar]
- Barderas M.G., Rodriguez F., Gomez‐Puertas P., Aviles M., Beitia F., Alonso C., Escribano J.M. Antigenic and immunogenic properties of a chimera of two immunodominant African swine fever virus proteins. Arch. Virol. 2001;146:1681–1691. doi: 10.1007/s007050170056. [DOI] [PubMed] [Google Scholar]
- Bassiri M., Ahmad S., Giavedoni L., Jones L., Saliki J.T., Mebus C., Yilma T. Immunological responses of mice and cattle to baculovirus‐expressed F and H proteins of rinderpest virus: Lack of protection in the presence of neutralizing antibody. J. Virol. 1993;67:1255–1261. doi: 10.1128/jvi.67.3.1255-1261.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beinart G., Rini B.I., Weinberg V., Small E.J. Antigen‐presenting cells 8015 (Provenge) in patients with androgen‐dependent, biochemically relapsed prostate cancer. Clin. Prostate Cancer. 2005;4:55–60. doi: 10.3816/cgc.2005.n.013. [DOI] [PubMed] [Google Scholar]
- Belyaev A.S., Roy P. Development of baculovirus triple and quadruple expression vectors: Co‐expression of three or four bluetongue virus proteins and the synthesis of bluetongue virus‐like particles in insect cells. Nucleic Acids Res. 1993;21:1219–1223. doi: 10.1093/nar/21.5.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielefeldt‐Ohmann H., Beasley D.W., Fitzpatrick D.R., Aaskov J.G. Analysis of a recombinant dengue‐2 virus‐dengue‐3 virus hybrid envelope protein expressed in a secretory baculovirus system. J. Gen. Virol. 1997;78:2723–2733. doi: 10.1099/0022-1317-78-11-2723. [DOI] [PubMed] [Google Scholar]
- Bishop R., Musoke A., Morzaria S., Gardner M., Nene V. Theileria: Intracellular protozoan parasites of wild and domestic ruminants transmitted by ixodid ticks. Parasitology. 2004;129(Suppl.):S271–S283. doi: 10.1017/s0031182003004748. [DOI] [PubMed] [Google Scholar]
- Bisht H., Roberts A., Vogel L., Subbarao K., Moss B. Neutralizing antibody and protective immunity to SARS coronavirus infection of mice induced by a soluble recombinant polypeptide containing an N‐terminal segment of the spike glycoprotein. Virology. 2005;334:160–165. doi: 10.1016/j.virol.2005.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard P., Mahe D., Cariolet R., Keranflec'h A., Baudouard M.A., Cordioli P., Albina E., Jestin A. Protection of swine against post‐weaning multisystemic wasting syndrome (PMWS) by porcine circovirus type 2 (PCV2) proteins. Vaccine. 2003;21:4565–4575. doi: 10.1016/s0264-410x(03)00503-6. [DOI] [PubMed] [Google Scholar]
- Bolin S.R., Ridpath J.F. Glycoprotein E2 of bovine viral diarrhea virus expressed in insect cells provides calves limited protection from systemic infection and disease. Arch. Virol. 1996;141:1463–1477. doi: 10.1007/BF01718248. [DOI] [PubMed] [Google Scholar]
- Bos H.J., Schetters T. Is there a future for vaccines against gastrointestinal helminths? Tijdschr. Diergeneeskd. 1990;115:1102–1110. [PubMed] [Google Scholar]
- Bouma A., de Smit A.J., de Kluijver E.P., Terpstra C., Moormann R.J. Efficacy and stability of a subunit vaccine based on glycoprotein E2 of classical swine fever virus. Vet. Microbiol. 1999;66:101–114. doi: 10.1016/s0378-1135(99)00003-6. [DOI] [PubMed] [Google Scholar]
- Bouma A., De Smit A.J., De Jong M.C., DeKluijver E.P., Moormann R.J. Determination of the onset of the herd‐immunity induced by the E2 sub‐unit vaccine against classical swine fever virus. Vaccine. 2000;18:1374–1381. doi: 10.1016/s0264-410x(99)00398-9. [DOI] [PubMed] [Google Scholar]
- Brands R., Visser J., Medema J., Palache A.M., van Scharrenburg G.J. Influvac: A safe madin darby canine kidney (MDCK) cell culture‐based influenza vaccine. Dev. Biol. Stand. 1999;98:93–100. discussion 111. [PubMed] [Google Scholar]
- Breitburd F., Kirnbauer R., Hubbert N.L., Nonnenmacher B., Trin‐Dinh‐Desmarquet C., Orth G., Schiller J.T., Lowy D.R. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J. Virol. 1995;69:3959–3963. doi: 10.1128/jvi.69.6.3959-3963.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett I.C., Johansson B.E. Immunization against influenza A virus: Comparison of conventional inactivated, live‐attenuated and recombinant baculovirus produced purified hemagglutinin and neuraminidase vaccines in a murine model system. Virology. 2005;339:273–280. doi: 10.1016/j.virol.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Brideau R.J., Walters R.R., Stier M.A., Wathen M.W. Protection of cotton rats against human respiratory syncytial virus by vaccination with a novel chimeric FG glycoprotein. J. Gen. Virol. 1989;70:2637. doi: 10.1099/0022-1317-70-10-2637. [DOI] [PubMed] [Google Scholar]
- Brideau R.J., Oien N.L., Lehman D.J., Homa F.L., Wathen M.W. Protection of cotton rats against human parainfluenza virus type 3 by vaccination with a chimeric FHN subunit glycoprotein. J. Gen. Virol. 1993;74:471–477. doi: 10.1099/0022-1317-74-3-471. [DOI] [PubMed] [Google Scholar]
- Bright R.K., Shearer M.H., Pass H.I., Kennedy R.C. Immunotherapy of SV40 induced tumours in mice: A model for vaccine development. Dev. Biol. Stand. 1998;94:341–353. [PubMed] [Google Scholar]
- Bristow R.G., Douglas A.R., Skehel J.J., Daniels R.S. Analysis of murine antibody responses to baculovirus‐expressed human immunodeficiency virus type 1 envelope glycoproteins. J. Gen. Virol. 1994;75:2089–2095. doi: 10.1099/0022-1317-75-8-2089. [DOI] [PubMed] [Google Scholar]
- Brown C.S., Van Lent J.W., Vlak J.M., Spaan W.J. Assembly of empty capsids by using baculovirus recombinants expressing human parvovirus B19 structural proteins. J. Virol. 1991;65:2702–2706. doi: 10.1128/jvi.65.5.2702-2706.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C.S., Welling‐Wester S., Feijlbrief M., Van Lent J.W., Spaan W.J. Chimeric parvovirus B19 capsids for the presentation of foreign epitopes. Virology. 1994;198:477–488. doi: 10.1006/viro.1994.1059. [DOI] [PubMed] [Google Scholar]
- Buonaguro L., Racioppi L., Tornesello M.L., Arra C., Visciano M.L., Biryahwaho B., Sempala S.D., Giraldo G., Buonaguro F.M. Induction of neutralizing antibodies and cytotoxic T lymphocytes in Balb/c mice immunized with virus‐like particles presenting a gp120 molecule from a HIV‐1 isolate of clade A. Antiviral Res. 2002;54:189–201. doi: 10.1016/s0166-3542(02)00004-9. [DOI] [PubMed] [Google Scholar]
- Canales M., Enriquez A., Ramos E., Cabrera D., Dandie H., Soto A., Falcon V., Rodriguez M., de la Fuente J. Large‐scale production in Pichia pastoris of the recombinant vaccine Gavac against cattle tick. Vaccine. 1997;15:414–422. doi: 10.1016/s0264-410x(96)00192-2. [DOI] [PubMed] [Google Scholar]
- Capua I., Terregino C., Cattoli G., Mutinelli F., Rodriguez J.F. Development of a DIVA (differentiating infected from vaccinated animals) strategy using a vaccine containing a heterologous neuraminidase for the control of avian influenza. Avian Pathol. 2003;32:47–55. doi: 10.1080/0307945021000070714. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. Severe acute respiratory syndrome vaccine development: Experiences of vaccination against avian infectious bronchitis coronavirus. Avian Pathol. 2003;32:567–582. doi: 10.1080/03079450310001621198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha H.J., Gotoh T., Bentley W.E. Simplification of titer determination for recombinant baculovirus by green fluorescent protein marker. Biotechniques. 1997;23:782–784. doi: 10.2144/97235bm03. 786. [DOI] [PubMed] [Google Scholar]
- Chang M., Kuzio J., Blissard G. Modulation of translational efficiency by contextual nucleotides flanking a baculovirus initiator AUG codon. Virology. 1999;259:369–383. doi: 10.1006/viro.1999.9787. [DOI] [PubMed] [Google Scholar]
- Chitarra V., Holm I., Bentley G.A., Petres S., Longacre S. The crystal structure of C‐terminal merozoite surface protein 1 at 1.8 A resolution, a highly protective malaria vaccine candidate. Mol. Cell. 1999;3:457–464. doi: 10.1016/s1097-2765(00)80473-6. [DOI] [PubMed] [Google Scholar]
- Choi J.Y., Woo S.D., Lee H.K., Hong H.K., Je Y.H., Park J.H., Song J.Y., An S.H., Kang S.K. High‐level expression of canine parvovirus VP2 using Bombyx mori nucleopolyhedrovirus vector. Arch. Virol. 2000;145:171–177. doi: 10.1007/s007050050014. [DOI] [PubMed] [Google Scholar]
- Christensen J., Alexandersen S., Bloch B., Aasted B., Uttenthal A. Production of mink enteritis parvovirus empty capsids by expression in a baculovirus vector system: A recombinant vaccine for mink enteritis parvovirus in mink. J. Gen. Virol. 1994;75:149–155. doi: 10.1099/0022-1317-75-1-149. [DOI] [PubMed] [Google Scholar]
- Christensen N.D., Reed C.A., Cladel N.M., Han R., Kreider J.W. Immunization with viruslike particles induces long‐term protection of rabbits against challenge with cottontail rabbit papillomavirus. J. Virol. 1996;70:960–965. doi: 10.1128/jvi.70.2.960-965.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark T.G., Cassidy‐Hanley D. Recombinant subunit vaccines: Potentials and constraints. Dev. Biol. (Basel) 2005;121:153–163. [PubMed] [Google Scholar]
- Coler R.N., Reed S.G. Second‐generation vaccines against leishmaniasis. Trends Parasitol. 2005;21:244–249. doi: 10.1016/j.pt.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Conner M.E., Crawford S.E., Barone C., O'Neal C., Zhou Y.J., Fernandez F., Parwani A., Saif L.J., Cohen J., Estes M.K. Rotavirus subunit vaccines. Arch. Virol. 1996;12(Suppl.):199–206. doi: 10.1007/978-3-7091-6553-9_21. [DOI] [PubMed] [Google Scholar]
- Conner M.E., Zarley C.D., Hu B., Parsons S., Drabinski D., Greiner S., Smith R., Jiang B., Corsaro B., Barniak V., Madore H.P., Crawford S. Virus‐like particles as a rotavirus subunit vaccine. J. Infect. Dis. 1996;174(Suppl. 1):S88–S92. doi: 10.1093/infdis/174.supplement_1.s88. [DOI] [PubMed] [Google Scholar]
- Connors M., Collins P.L., Firestone C.Y., Sotnikov A.V., Waitze A., Davis A.R., Hung P.P., Chanock R.M., Murphy B.R. Cotton rats previously immunized with a chimeric RSV FG glycoprotein develop enhanced pulmonary pathology when infected with RSV, a phenomenon not encountered following immunization with vaccinia—RSV recombinants or RSV. Vaccine. 1992;10:475–484. doi: 10.1016/0264-410x(92)90397-3. [DOI] [PubMed] [Google Scholar]
- Cooney E.L., McElrath M.J., Corey L., Hu S.L., Collier A.C., Arditti D., Hoffman M., Coombs R.W., Smith G.E., Greenberg P.D. Enhanced immunity to human immunodeficiency virus (HIV) envelope elicited by a combined vaccine regimen consisting of priming with a vaccinia recombinant expressing HIV envelope and boosting with gp160 protein. Proc. Natl. Acad. Sci. USA. 1993;90:1882–1886. doi: 10.1073/pnas.90.5.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa F.T., Fusai T., Parzy D., Sterkers Y., Torrentino M., Douki J.B., Traore B., Petres S., Scherf A., Gysin J. Immunization with recombinant duffy binding‐like‐gamma3 induces pan‐reactive and adhesion‐blocking antibodies against placental chondroitin sulfate A‐binding plasmodium falciparum parasites. J. Infect. Dis. 2003;188:153–164. doi: 10.1086/375800. [DOI] [PubMed] [Google Scholar]
- Cox M.M. Cell‐based protein vaccines for influenza. Curr. Opin. Mol. Ther. 2005;7:24–29. [PubMed] [Google Scholar]
- Cox R.J., Brokstad K.A., Ogra P. Influenza virus: Immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand. J. Immunol. 2004;59:1–15. doi: 10.1111/j.0300-9475.2004.01382.x. [DOI] [PubMed] [Google Scholar]
- Crawford J., Wilkinson B., Vosnesensky A., Smith G., Garcia M., Stone H., Perdue M.L. Baculovirus‐derived hemagglutinin vaccines protect against lethal influenza infections by avian H5 and H7 subtypes. Vaccine. 1999;17:2265–2274. doi: 10.1016/s0264-410x(98)00494-0. [DOI] [PubMed] [Google Scholar]
- Cusi M.G., Valassina M., Bianchi S., Wunner W., Valensin P.E. Evaluation of rubella virus E2 and C proteins in protection against rubella virus in a mouse model. Virus Res. 1995;37:199–208. doi: 10.1016/0168-1702(95)00037-q. [DOI] [PubMed] [Google Scholar]
- Dalton J.P., Neill S.O., Stack C., Collins P., Walshe A., Sekiya M., Doyle S., Mulcahy G., Hoyle D., Khaznadji E., Moire N., Brennan G. Fasciola hepatica cathepsin L‐like proteases: Biology, function, and potential in the development of first generation liver fluke vaccines. Int. J. Parasitol. 2003;33:1173–1181. doi: 10.1016/s0020-7519(03)00171-1. [DOI] [PubMed] [Google Scholar]
- de Gooijer C.D., Koken R.H.M., van Lier F.L.J., Kool M., Vlak J.M., Tramper J. A structured dynamic model for the baculovirus infection process in insect cell reactor configurations. Biotechnol. Bioeng. 1992;40:537–548. doi: 10.1002/bit.260400413. [DOI] [PubMed] [Google Scholar]
- De Maere V., Vercauteren I., Geldhof P., Gevaert K., Vercruysse J., Claerebout E. Molecular analysis of astacin‐like metalloproteases of Ostertagia ostertagi. Parasitology. 2005;130:89–98. doi: 10.1017/s0031182004006274. [DOI] [PubMed] [Google Scholar]
- De Maere V., Vercauteren I., Gevaert K., Vercruysse J., Claerebout E. An aspartyl protease inhibitor of Ostertagia ostertagi: Molecular cloning, analysis of stage and tissue specific expression and vaccine trial. Mol. Biochem. Parasitol. 2005;141:81–88. doi: 10.1016/j.molbiopara.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Delenda C., Frenkiel M.P., Deubel V. Protective efficacy in mice of a secreted form of recombinant dengue‐2 virus envelope protein produced in baculovirus infected insect cells. Arch. Virol. 1994;139:197–207. doi: 10.1007/BF01309465. [DOI] [PubMed] [Google Scholar]
- Depner K.R., Bouma A., Koenen F., Klinkenberg D., Lange E., de Smit H., Vanderhallen H. Classical swine fever (CSF) marker vaccine. Trial II. Challenge study in pregnant sows. Vet. Microbiol. 2001;83:107–120. doi: 10.1016/s0378-1135(01)00410-2. [DOI] [PubMed] [Google Scholar]
- Deroo T., Jou W.M., Fiers W. Recombinant neuraminidase vaccine protects against lethal influenza. Vaccine. 1996;14:561–569. doi: 10.1016/0264-410x(95)00157-v. [DOI] [PubMed] [Google Scholar]
- Dertzbaugh M.T. Genetically engineered vaccines: An overview. Plasmid. 1998;39:100–113. doi: 10.1006/plas.1997.1329. [DOI] [PubMed] [Google Scholar]
- Despres P., Dietrich J., Girard M., Bouloy M. Recombinant baculoviruses expressing yellow fever virus E and NS1 proteins elicit protective immunity in mice. J. Gen. Virol. 1991;72:2811–2816. doi: 10.1099/0022-1317-72-11-2811. [DOI] [PubMed] [Google Scholar]
- Doe B., Steimer K.S., Walker C.M. Induction of HIV‐1 envelope (gp120)‐specific cytotoxic T lymphocyte responses in mice by recombinant CHO cell‐derived gp120 is enhanced by enzymatic removal of N‐linked glycans. Eur. J. Immunol. 1994;24:2369–2376. doi: 10.1002/eji.1830241017. [DOI] [PubMed] [Google Scholar]
- Drings A., Jallet C., Chambert B., Tordo N., Perrin P. Is there an advantage to including the nucleoprotein in a rabies glycoprotein subunit vaccine? Vaccine. 1999;17:1549–1557. doi: 10.1016/s0264-410x(98)00357-0. [DOI] [PubMed] [Google Scholar]
- Du R.P., Jackson G.E., Wyde P.R., Yan W.Y., Wang Q., Gisonni L., Sanhueza S.E., Klein M.H., Ewasyshyn M.E. A prototype recombinant vaccine against respiratory syncytial virus and parainfluenza virus type 3. Biotechnology (NY) 1994;12:813–818. doi: 10.1038/nbt0894-813. [DOI] [PubMed] [Google Scholar]
- Dupuy C., Buzoni‐Gatel D., Touze A., Le Cann P., Bout D., Coursaget P. Cell mediated immunity induced in mice by HPV 16 L1 virus‐like particles. Microb. Pathog. 1997;22:219–225. doi: 10.1006/mpat.1996.0113. [DOI] [PubMed] [Google Scholar]
- Eckels K.H., Dubois D.R., Summers P.L., Schlesinger J.J., Shelly M., Cohen S., Zhang Y.M., Lai C.J., Kurane I., Rothman A., Hasty S., Howard B. Immunization of monkeys with baculovirus‐dengue type‐4 recombinants containing envelope and nonstructural proteins: Evidence of priming and partial protection. Am. J. Trop. Med. Hyg. 1994;50:472–478. doi: 10.4269/ajtmh.1994.50.472. [DOI] [PubMed] [Google Scholar]
- Facciabene A., Aurisicchio L., La Monica N. Baculovirus vectors elicit antigen‐specific immune responses in mice. J. Virol. 2004;78:8663–8672. doi: 10.1128/JVI.78.16.8663-8672.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feighny R., Burrous J., Putnak R. Dengue type‐2 virus envelope protein made using recombinant baculovirus protects mice against virus challenge. Am. J. Trop. Med. Hyg. 1994;50:322–328. doi: 10.4269/ajtmh.1994.50.322. [DOI] [PubMed] [Google Scholar]
- Fenner F. Adventures with poxviruses of vertebrates. FEMS Microbiol. Rev. 2000;24:123–133. doi: 10.1016/S0168-6445(00)00027-9. [DOI] [PubMed] [Google Scholar]
- Fiedler M., Roggendorf M. Vaccination against hepatitis delta virus infection: Studies in the woodchuck (Marmota monax) model. Intervirology. 2001;44:154–161. doi: 10.1159/000050042. [DOI] [PubMed] [Google Scholar]
- Foote C.E., Love D.N., Gilkerson J.R., Rota J., Trevor‐Jones P., Ruitenberg K.M., Wellington J.E., Whalley J.M. Serum antibody responses to equine herpesvirus 1 glycoprotein D in horses, pregnant mares and young foals. Vet. Immunol. Immunopathol. 2005;105:47–57. doi: 10.1016/j.vetimm.2004.12.012. [DOI] [PubMed] [Google Scholar]
- French T.J., Marshall J.J., Roy P. Assembly of double‐shelled, viruslike particles of bluetongue virus by the simultaneous expression of four structural proteins. J. Virol. 1990;64:5695–5700. doi: 10.1128/jvi.64.12.5695-5700.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z.F. Rabies and rabies research: Past, present and future. Vaccine. 1997;15(Suppl.):S20–S24. doi: 10.1016/s0264-410x(96)00312-x. [DOI] [PubMed] [Google Scholar]
- Fu Z.F., Rupprecht C.E., Dietzschold B., Saikumar P., Niu H.S., Babka I., Wunner W.H., Koprowski H. Oral vaccination of racoons (Procyon lotor) with baculovirus‐expressed rabies virus glycoprotein. Vaccine. 1993;11:925–928. doi: 10.1016/0264-410x(93)90379-c. [DOI] [PubMed] [Google Scholar]
- Fyfe L., Maingay J.P., Howie S.E. Murine HIV‐1 p24 specific T lymphocyte activation by different antigen presenting cells: B lymphocytes from immunized mice present core protein to T cells. Immunol. Lett. 1993;35:45–50. doi: 10.1016/0165-2478(93)90146-s. [DOI] [PubMed] [Google Scholar]
- Galarza J.M., Latham T., Cupo A. Virus‐like particle (VLP) vaccine conferred complete protection against a lethal influenza virus challenge. Viral Immunol. 2005;18:244–251. doi: 10.1089/vim.2005.18.244. [DOI] [PubMed] [Google Scholar]
- Galarza J.M., Latham T., Cupo A. Virus‐like particle vaccine conferred complete protection against a lethal influenza virus challenge. Viral Immunol. 2005;18:365–372. doi: 10.1089/vim.2005.18.365. [DOI] [PubMed] [Google Scholar]
- Gherardi M.M., Esteban M. Recombinant poxviruses as mucosal vaccine vectors. J. Gen. Virol. 2005;86:2925–2936. doi: 10.1099/vir.0.81181-0. [DOI] [PubMed] [Google Scholar]
- Ghiasi H., Nesburn A.B., Kaiwar R., Wechsler S.L. Immunoselection of recombinant baculoviruses expressing high levels of biologically active herpes simplex virus type 1 glycoprotein D. Arch. Virol. 1991;121:163–178. doi: 10.1007/BF01316752. [DOI] [PubMed] [Google Scholar]
- Ghiasi H., Kaiwar R., Slanina S., Nesburn A.B., Wechsler S.L. Expression and characterization of baculovirus expressed herpes simplex virus type 1 glycoprotein L. Arch. Virol. 1994;138:199–212. doi: 10.1007/BF01379126. [DOI] [PubMed] [Google Scholar]
- Ghiasi H., Wechsler S.L., Kaiwar R., Nesburn A.B., Hofman F.M. Local expression of tumor necrosis factor alpha and interleukin‐2 correlates with protection against corneal scarring after ocular challenge of vaccinated mice with herpes simplex virus type 1. J. Virol. 1995;69:334–340. doi: 10.1128/jvi.69.1.334-340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiasi H., Nesburn A.B., Wechsler S.L. Vaccination with a cocktail of seven recombinantly expressed HSV‐1 glycoproteins protects against ocular HSV‐1 challenge more efficiently than vaccination with any individual glycoprotein. Vaccine. 1996;14:107–112. doi: 10.1016/0264-410x(95)00169-2. [DOI] [PubMed] [Google Scholar]
- Ghiasi H., Hofman F.M., Cai S., Perng G.C., Nesburn A.B., Wechsler S.L. Vaccination with different HSV‐1 glycoproteins induces different patterns of ocular cytokine responses following HSV‐1 challenge of vaccinated mice. Vaccine. 1999;17:2576–2582. doi: 10.1016/s0264-410x(99)00056-0. [DOI] [PubMed] [Google Scholar]
- Ghiasi H., Perng G.C., Nesburn A.B., Wechsler S.L. Antibody‐dependent enhancement of HSV‐1 infection by anti‐gK sera. Virus Res. 2000;68:137–144. doi: 10.1016/s0168-1702(00)00165-9. [DOI] [PubMed] [Google Scholar]
- Girones N., Cuervo H., Fresno M. Trypanosoma cruzi‐induced molecular mimicry and Chagas’ disease. Curr. Top. Microbiol. Immunol. 2005;296:89–123. doi: 10.1007/3-540-30791-5_6. [DOI] [PubMed] [Google Scholar]
- Gomez I., Marx F., Saurwein‐Teissl M., Gould E.A., Grubeck‐Loebenstein B. Characterization of tick‐borne encephalitis virus‐specific human T lymphocyte responses by stimulation with structural TBEV proteins expressed in a recombinant baculovirus. Viral Immunol. 2003;16:407–414. doi: 10.1089/088282403322396190. [DOI] [PubMed] [Google Scholar]
- Gorse G.J., Belshe R.B., Newman F.K., Frey S.E. Lymphocyte proliferative responses following immunization with human immunodeficiency virus recombinant GP160. The NIAID AIDS vaccine clinical trials network. Vaccine. 1992;10:383–388. doi: 10.1016/0264-410x(92)90068-u. [DOI] [PubMed] [Google Scholar]
- Gorse G.J., Frey S.E., Patel G., Newman F.K., Belshe R.B. Vaccine‐induced antibodies to native and recombinant human immunodeficiency virus type 1 envelope glycoproteins. NIAID AIDS vaccine clinical trials network. Vaccine. 1994;12:912–918. doi: 10.1016/0264-410x(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Grabherr R., Ernst W., Oker‐Blom C., Jones I. Developments in the use of baculoviruses for the surface display of complex eukaryotic proteins. Trends Biotechnol. 2001;19:231–236. doi: 10.1016/s0167-7799(01)01610-9. [DOI] [PubMed] [Google Scholar]
- Graham B.S., Matthews T.J., Belshe R.B., Clements M.L., Dolin R., Wright P.F., Gorse G.J., Schwartz D.H., Keefer M.C., Bolognesi D.P., Corey L., Stablein D.M. Augmentation of human immunodeficiency virus type 1 neutralizing antibody by priming with gp160 recombinant vaccinia and boosting with rgp160 in vaccinia‐naive adults. The NIAID AIDS vaccine clinical trials network. J. Infect. Dis. 1993;167:533–537. doi: 10.1093/infdis/167.3.533. [DOI] [PubMed] [Google Scholar]
- Greenstone H.L., Nieland J.D., de Visser K.E., De Bruijn M.L., Kirnbauer R., Roden R.B., Lowy D.R., Kast W.M., Schiller J.T. Chimeric papillomavirus virus‐like particles elicit antitumor immunity against the E7 oncoprotein in an HPV16 tumor model. Proc. Natl. Acad. Sci. USA. 1998;95:1800–1805. doi: 10.1073/pnas.95.4.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubman M.J., Lewis S.A., Morgan D.O. Protection of swine against foot‐and‐mouth disease with viral capsid proteins expressed in heterologous systems. Vaccine. 1993;11:825–829. doi: 10.1016/0264-410x(93)90357-4. [DOI] [PubMed] [Google Scholar]
- Guerrero R.A., Ball J.M., Krater S.S., Pacheco S.E., Clements J.D., Estes M.K. Recombinant Norwalk virus‐like particles administered intranasally to mice induce systemic and mucosal (fecal and vaginal) immune responses. J. Virol. 2001;75:9713–9722. doi: 10.1128/JVI.75.20.9713-9722.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haanes E.J., Guimond P., Wardley R. The bovine parainfluenza virus type‐3 (BPIV‐3) hemagglutinin/neuraminidase glycoprotein expressed in baculovirus protects calves against experimental BPIV‐3 challenge. Vaccine. 1997;15:730–738. doi: 10.1016/s0264-410x(96)00231-9. [DOI] [PubMed] [Google Scholar]
- Hall S.L., Murphy B.R., van Wyke Coelingh K.L. Protection of cotton rats by immunization with the human parainfluenza virus type 3 fusion (F) glycoprotein expressed on the surface of insect cells infected with a recombinant baculovirus. Vaccine. 1991;9:659–667. doi: 10.1016/0264-410x(91)90192-9. [DOI] [PubMed] [Google Scholar]
- Hansson M., Nygren P.A., Stahl S. Design and production of recombinant subunit vaccines. Biotechnol. Appl. Biochem. 2000;32(Pt 2):95–107. doi: 10.1042/ba20000034. [DOI] [PubMed] [Google Scholar]
- Hansson M., Nygren P.A., Stahl S. Design and production of recombinant subunit vaccines. Biotechnol. Appl. Biochem. 2000;32:95–107. doi: 10.1042/ba20000034. [DOI] [PubMed] [Google Scholar]
- Harder T.C., Osterhaus A.D. Molecular characterization and baculovirus expression of the glycoprotein B of a seal herpesvirus (phocid herpesvirus‐1) Virology. 1997;227:343–352. doi: 10.1006/viro.1996.8332. [DOI] [PubMed] [Google Scholar]
- Harrison R.L., Jarvis D.L., Summers M.D. The role of the AcMNPV 25K gene ‘FP25’ in baculovirus polh and p10 expression. Virology. 1996;226:34–46. doi: 10.1006/viro.1996.0625. [DOI] [PubMed] [Google Scholar]
- Harro C.D., Pang Y.Y., Roden R.B., Hildesheim A., Wang Z., Reynolds M.J., Mast T.C., Robinson R., Murphy B.R., Karron R.A., Dillner J., Schiller J.T. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus‐like particle vaccine. J. Natl. Cancer Inst. 2001;93:284–292. doi: 10.1093/jnci/93.4.284. [DOI] [PubMed] [Google Scholar]
- Hawtin R.E., Zarkowska T., Arnold K., Thomas C.J., Gooday G.W., King L.A., Kuzio J.A., Possee R.D. Liquefaction of Autographa californica nucleopolyhedrovirus‐infected insects is dependent on the integrity of virus‐encoded chitinase and cathepsin genes. Virology. 1997;238:243–253. doi: 10.1006/viro.1997.8816. [DOI] [PubMed] [Google Scholar]
- Henderson L.M. Overview of marker vaccine and differential diagnostic test technology. Biologicals. 2005;33:203–209. doi: 10.1016/j.biologicals.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Herrington D.A., Losonsky G.A., Smith G., Volvovitz F., Cochran M., Jackson K., Hoffman S.L., Gordon D.M., Levine M.M., Edelman R. Safety and immunogenicity in volunteers of a recombinant Plasmodium falciparum circumsporozoite protein malaria vaccine produced in lepidopteran cells. Vaccine. 1992;10:841–846. doi: 10.1016/0264-410x(92)90047-n. [DOI] [PubMed] [Google Scholar]
- Hevey M., Negley D., Geisbert J., Jahrling P., Schmaljohn A. Antigenicity and vaccine potential of Marburg virus glycoprotein expressed by baculovirus recombinants. Virology. 1997;239:206–216. doi: 10.1006/viro.1997.8883. [DOI] [PubMed] [Google Scholar]
- Hoerauf A., Satoguina J., Saeftel M., Specht S. Immunomodulation by filarial nematodes. Parasite Immunol. 2005;27:417–429. doi: 10.1111/j.1365-3024.2005.00792.x. [DOI] [PubMed] [Google Scholar]
- Hohdatsu T., Yamato H., Ohkawa T., Kaneko M., Motokawa K., Kusuhara H., Kaneshima T., Arai S., Koyama H. Vaccine efficacy of a cell lysate with recombinant baculovirus‐expressed feline infectious peritonitis (FIP) virus nucleocapsid protein against progression of FIP. Vet. Microbiol. 2003;97:31–44. doi: 10.1016/j.vetmic.2003.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder A.A., Blackman M.J. What is the function of MSP‐I on the malaria merozoite? Parasitol. Today. 1994;10:182–184. doi: 10.1016/0169-4758(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Hom L.G., Volkman L.E. Preventing proteolytic artifacts in the baculovirus expression system. Biotechniques. 1998;25:18–20. doi: 10.2144/98251bm01. [DOI] [PubMed] [Google Scholar]
- Hom L.G., Ohkawa T., Trudeau D., Volkman L.E. Autographa californica M nucleopolyhedrovirus ProV‐CATH is activated during infected cell death. Virology. 2002;296:212–218. doi: 10.1006/viro.2002.1378. [DOI] [PubMed] [Google Scholar]
- Homa F.L., Brideau R.J., Lehman D.J., Thomsen D.R., Olmsted R.A., Wathen M.W. Development of a novel subunit vaccine that protects cotton rats against both human respiratory syncytial virus and human parainfluenza virus type 3. J. Gen. Virol. 1993;74:1995–1999. doi: 10.1099/0022-1317-74-9-1995. [DOI] [PubMed] [Google Scholar]
- Horimoto T., Takada A., Fujii K., Goto H., Hatta M., Watanabe S., Iwatsuki‐Horimoto K., Ito M., Tagawa‐Sakai Y., Yamada S., Ito H., Ito T. The development and characterization of H5 influenza virus vaccines derived from a 2003 human isolate. Vaccine. 2006;24:3669–3676. doi: 10.1016/j.vaccine.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Hota‐Mitchell S., Siddiqui A.A., Dekaban G.A., Smith J., Tognon C., Podesta R.B. Protection against Schistosoma mansoni infection with a recombinant baculovirus‐expressed subunit of calpain. Vaccine. 1997;15:1631–1640. doi: 10.1016/s0264-410x(97)00081-9. [DOI] [PubMed] [Google Scholar]
- Hu S.L., Abrams K., Barber G.N., Moran P., Zarling J.M., Langlois A.J., Kuller L., Morton W.R., Benveniste R.E. Protection of macaques against SIV infection by subunit vaccines of SIV envelope glycoprotein gp160. Science. 1992;255:456–459. doi: 10.1126/science.1531159. [DOI] [PubMed] [Google Scholar]
- Hulst M.M., Westra D.F., Wensvoort G., Moormann R.J. Glycoprotein E1 of hog cholera virus expressed in insect cells protects swine from hog cholera. J. Virol. 1993;67:5435–5442. doi: 10.1128/jvi.67.9.5435-5442.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt I. From gene to protein: A review of new and enabling technologies for multi‐parallel protein expression. Protein Expr. Purif. 2005;40:1–22. doi: 10.1016/j.pep.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Huser A., Hofmann C. Baculovirus vectors: Novel mammalian cell gene‐delivery vehicles and their applications. Am. J. Pharmacogenomics. 2003;3:53–63. [PubMed] [Google Scholar]
- Huser A., Rudolph M., Hofmann C. Incorporation of decay‐accelerating factor into the baculovirus envelope generates complement‐resistant gene transfer vectors. Nat. Biotechnol. 2001;19:451–455. doi: 10.1038/88122. [DOI] [PubMed] [Google Scholar]
- Igarashi I., Asaba U., Xuan X., Omata Y., Saito A., Nagasawa H., Fujisaki K., Suzuki N., Iwakura Y., Mikami T. Immunization with recombinant surface antigens p26 with Freund's adjuvants against Babesia rodhaini infection. J. Vet. Med. Sci. 2000;62:717–723. doi: 10.1292/jvms.62.717. [DOI] [PubMed] [Google Scholar]
- Ikonomou L., Schneider Y.J., Agathos S.N. Insect cell culture for industrial production of recombinant proteins. Appl. Microbiol. Biotechnol. 2003;62:1–20. doi: 10.1007/s00253-003-1223-9. [DOI] [PubMed] [Google Scholar]
- Inumaru S., Yamada S. Characterization of pseudorabies virus neutralization antigen glycoprotein gIII produced in insect cells by a baculovirus expression vector. Virus Res. 1991;21:123–139. doi: 10.1016/0168-1702(91)90003-e. [DOI] [PubMed] [Google Scholar]
- Jaffray A., Shephard E., van Harmelen J., Williamson C., Williamson A.L., Rybicki E.P. Human immunodeficiency virus type 1 subtype C Gag virus‐like particle boost substantially improves the immune response to a subtype C gag DNA vaccine in mice. J. Gen. Virol. 2004;85:409–413. doi: 10.1099/vir.0.19396-0. [DOI] [PubMed] [Google Scholar]
- Jarvis D.L. Developing baculovirus‐insect cell expression systems for humanized recombinant glycoprotein production. Virology. 2003;310:1–7. doi: 10.1016/s0042-6822(03)00120-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Je Y.H., Jin B.R., Park H.W., Roh J.Y., Chang J.H., Seo S.J., Olszewski J.A., O'Reilly D.R., Kang S.K. Baculovirus expression vectors that incorporate the foreign protein into viral occlusion bodies. Biotechniques. 2003;34:81–87. doi: 10.2144/03341st04. [DOI] [PubMed] [Google Scholar]
- Jeong S.H., Qiao M., Nascimbeni M., Hu Z., Rehermann B., Murthy K., Liang T.J. Immunization with hepatitis C virus‐like particles induces humoral and cellular immune responses in nonhuman primates. J. Virol. 2004;78:6995–7003. doi: 10.1128/JVI.78.13.6995-7003.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson B.E. Immunization with influenza A virus hemagglutinin and neuraminidase produced in recombinant baculovirus results in a balanced and broadened immune response superior to conventional vaccine. Vaccine. 1999;17:2073–2080. doi: 10.1016/s0264-410x(98)00413-7. [DOI] [PubMed] [Google Scholar]
- Jones T., Allard F., Cyr S.L., Tran S.P., Plante M., Gauthier J., Bellerose N., Lowell G.H., Burt D.S. A nasal proteosome influenza vaccine containing baculovirus‐derived hemagglutinin induces protective mucosal and systemic immunity. Vaccine. 2003;21:3706–3712. doi: 10.1016/s0264-410x(03)00387-6. [DOI] [PubMed] [Google Scholar]
- Kaba S.A., Nene V., Musoke A.J., Vlak J.M., van Oers M.M. Fusion to green fluorescent protein improves expression levels of Theileria parva sporozoite surface antigen p67 in insect cells. Parasitology. 2002;125:497–505. doi: 10.1017/s003118200200241x. [DOI] [PubMed] [Google Scholar]
- Kaba S.A., Hemmes J.C., van Lent J.W., Vlak J.M., Nene V., Musoke A.J., van Oers M.M. Baculovirus surface display of Theileria parva p67 antigen preserves the conformation of sporozoite‐neutralizing epitopes. Protein Eng. 2003;16:73–78. doi: 10.1093/proeng/gzg004. [DOI] [PubMed] [Google Scholar]
- Kaba S.A., Salcedo A.M., Wafula P.O., Vlak J.M., van Oers M.M. Development of a chitinase and v‐cathepsin negative bacmid for improved integrity of secreted recombinant proteins. J. Virol. Methods. 2004;122:113–118. doi: 10.1016/j.jviromet.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Kaba S.A., Schaap D., Roode E.C., Nene V., Musoke A.J., Vlak J.M., van Oers M.M. Improved immunogenicity of novel baculovirus‐derived Theileria parva p67 subunit antigens. Vet. Parasitol. 2004;121:53–64. doi: 10.1016/j.vetpar.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Kaba S.A., Musoke A.J., Schaap D., Schetters T., Rowlands J., Vermeulen A.N., Nene V., Vlak J.M., van Oers M.M. Novel baculovirus‐derived p67 subunit vaccines efficacious against East Coast fever in cattle. Vaccine. 2005;23:2791–2800. doi: 10.1016/j.vaccine.2004.10.043. [DOI] [PubMed] [Google Scholar]
- Kajigaya S., Fujii H., Field A., Anderson S., Rosenfeld S., Anderson L.J., Shimada T., Young N.S. Self‐assembled B19 parvovirus capsids, produced in a baculovirus system, are antigenically and immunogenically similar to native virions. Proc. Natl. Acad. Sci. USA. 1991;88:4646–4650. doi: 10.1073/pnas.88.11.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayiannis P., Saldanha J., Jackson A.M., Luther S., Goldin R., Monjardino J., Thomas H.C. Partial control of hepatitis delta virus superinfection by immunisation of woodchucks (Marmota monax) with hepatitis delta antigen expressed by a recombinant vaccinia or baculovirus. J. Med. Virol. 1993;41:210–214. doi: 10.1002/jmv.1890410308. [DOI] [PubMed] [Google Scholar]
- Kato T., Murata T., Usui T., Park E.Y. Improvement of the production of GFPuv‐ß1,3‐N‐acetylglucosaminyltransferase 2 fusion protein using a molecular chaperone‐assisted insect‐cell‐based expression system. Biotechnol. Bioeng. 2005;89:424–433. doi: 10.1002/bit.20362. [DOI] [PubMed] [Google Scholar]
- Katz J.M., Lu X., Frace A.M., Morken T., Zaki S.R., Tumpey T.M. Pathogenesis of and immunity to avian influenza A H5 viruses. Biomed. Pharmacother. 2000;54:178–187. doi: 10.1016/S0753-3322(00)89024-1. [DOI] [PubMed] [Google Scholar]
- Keefer M.C., Bonnez W., Roberts N.J., Jr., Dolin R., Reichman R.C. Human immunodeficiency virus (HIV‐1) gp160‐specific lymphocyte proliferative responses of mononuclear leukocytes from HIV‐1 recombinant gp160 vaccine recipients. J. Infect. Dis. 1991;163:448–453. doi: 10.1093/infdis/163.3.448. [DOI] [PubMed] [Google Scholar]
- Keefer M.C., Graham B.S., Belshe R.B., Schwartz D., Corey L., Bolognesi D.P., Stablein D.M., Montefiori D.C., McElrath M.J., Clements M.L., Gorse G.G., Wright P.F. Studies of high doses of a human immunodeficiency virus type 1 recombinant glycoprotein 160 candidate vaccine in HIV type 1‐seronegative humans. AIDS Res. Hum. Retroviruses. 1994;10:1713–1723. doi: 10.1089/aid.1994.10.1713. [DOI] [PubMed] [Google Scholar]
- Kelly E.P., Greene J.J., King A.D., Innis B.L. Purified dengue 2 virus envelope glycoprotein aggregates produced by baculovirus are immunogenic in mice. Vaccine. 2000;18:2549–2559. doi: 10.1016/s0264-410x(00)00032-3. [DOI] [PubMed] [Google Scholar]
- Kelso J.M., Yunginger J.W. Immunization of egg‐allergic individuals with egg‐ or chicken‐derived vaccines. Immunol. Allergy Clin. North Am. 2003;23:635–648. doi: 10.1016/s0889-8561(03)00091-2. [DOI] [PubMed] [Google Scholar]
- Kew O.M., Wright P.F., Agol V.I., Delpeyroux F., Shimizu H., Nathanson N., Pallansch M.A. Circulating vaccine‐derived polioviruses: Current state of knowledge. Bull. World Health Organ. 2004;82:16–23. [PMC free article] [PubMed] [Google Scholar]
- Kilbourne E.D., Pokorny B.A., Johansson B., Brett I., Milev Y., Matthews J.T. Protection of mice with recombinant influenza virus neuraminidase. J. Infect. Dis. 2004;189:459–461. doi: 10.1086/381123. [DOI] [PubMed] [Google Scholar]
- King L.K., Possee R.D. “The Baculovirus Expression System: A Laboratory Guide.”. Chapman and Hall; London: 1992. [Google Scholar]
- Kirnbauer R., Booy F., Cheng N., Lowy D.R., Schiller J.T. Papillomavirus L1 major capsid protein self‐assembles into virus‐like particles that are highly immunogenic. Proc. Natl. Acad. Sci. USA. 1992;89:12180–12184. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitts P.A., Ayres M.D., Possee R.D. Linearization of baculovirus DNA enhances the recovery of recombinant virus expression vectors. Nucleic Acids Res. 1990;18:5667–5672. doi: 10.1093/nar/18.19.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitts P.A., Possee R.D. A method for producing recombinant baculovirus expression vectors at high frequency. Biotechniques. 1993;14:810–817. [PubMed] [Google Scholar]
- Koch G., van Roozelaar D.J., Verschueren C.A., van der Eb A.J., Noteborn M.H. Immunogenic and protective properties of chicken anaemia virus proteins expressed by baculovirus. Vaccine. 1995;13:763–770. doi: 10.1016/0264-410x(94)00034-k. [DOI] [PubMed] [Google Scholar]
- Kool M., Voncken J.W., van Lier F.L., Tramper J., Vlak J.M. Detection and analysis of Autographa californica nuclear polyhedrosis virus mutants with defective interfering properties. Virology. 1991;183:739–746. doi: 10.1016/0042-6822(91)91003-y. [DOI] [PubMed] [Google Scholar]
- Kost T.A., Condreay J.P. Recombinant baculoviruses as mammalian cell gene‐delivery vectors. Trends Biotechnol. 2002;20:173–180. doi: 10.1016/s0167-7799(01)01911-4. [DOI] [PubMed] [Google Scholar]
- Kost T.A., Condreay J.P., Jarvis D.L. Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat. Biotechnol. 2005;23:567–575. doi: 10.1038/nbt1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krell P.J. Vol. 2. Kluwer, Dordrecht; The Netherlands: 1996. Passage effect of virus infection in insect cells; pp. 125–137. (“Insect Cell Cultures Fundamental and Applied Aspects,”). [DOI] [PubMed] [Google Scholar]
- Krishna S., Blacklaws B.A., Overton H.A., Bishop D.H., Nash A.A. Expression of glycoprotein D of herpes simplex virus type 1 in a recombinant baculovirus: Protective responses and T cell recognition of the recombinant‐infected cell extracts. J. Gen. Virol. 1989;70:1805–1814. doi: 10.1099/0022-1317-70-7-1805. [DOI] [PubMed] [Google Scholar]
- Kukreja A., Walker C., Fitzmaurice T., Awan A., Love D.N., Whalley J.M., Field H.J. Protective effects of equine herpesvirus‐1 (EHV‐1) glycoprotein B in a murine model of EHV‐1‐induced abortion. Vet. Microbiol. 1998;62:303–311. doi: 10.1016/s0378-1135(98)00211-9. [DOI] [PubMed] [Google Scholar]
- Lakey D.L., Treanor J.J., Betts R.F., Smith G.E., Thompson J., Sannella E., Reed G., Wilkinson B.E., Wright P.F. Recombinant baculovirus influenza A hemagglutinin vaccines are well tolerated and immunogenic in healthy adults. J. Infect. Dis. 1996;174:838–841. doi: 10.1093/infdis/174.4.838. [DOI] [PubMed] [Google Scholar]
- Langedijk J.P.M., Middel W.G.J., Meloen R.H., Kramps J.A., de Smit J.A. Enzyme‐linked immunosorbent assay using a virus type‐specific peptide based on a subdomain of envelope protein Erns for serologic diagnosis of pestivirus infections in swine. J. Clin. Microbiol. 2001;39:906–912. doi: 10.1128/JCM.39.3.906-912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall‐Recule G., Jestin V., Chagnaud P., Blanchard P., Jestin A. Expression of muscovy duck parvovirus capsid proteins (VP2 and VP3) in a baculovirus expression system and demonstration of immunity induced by the recombinant proteins. J. Gen. Virol. 1996;77:2159–2163. doi: 10.1099/0022-1317-77-9-2159. [DOI] [PubMed] [Google Scholar]
- Lee E.G., Lee M.Y., Chung J.Y., Je E.Y., Bae Y.A., Na B.K., Kim T.S., Eom K.S., Cho S.Y., Kong Y. Feasibility of baculovirus‐expressed recombinant 10‐kDa antigen in the serodiagnosis of Taenia solium neurocysticercosis. Trans. R. Soc. Trop. Med. Hyg. 2005;99:919–926. doi: 10.1016/j.trstmh.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Lee H., Krell P.J. Reiterated DNA fragments in defective genomes of Autographa californica nuclear polyhedrosis virus are competent for AcMNPV‐ dependent DNA replication. Virology. 1994;202:418–442. doi: 10.1006/viro.1994.1358. [DOI] [PubMed] [Google Scholar]
- Lehman D.J., Roof L.L., Brideau R.J., Aeed P.A., Thomsen D.R., Elhammer A.P., Wathen M.W., Homa F.L. Comparison of soluble and secreted forms of human parainfluenza virus type 3 glycoproteins expressed from mammalian and insect cells as subunit vaccines. J. Gen. Virol. 1993;74:459–469. doi: 10.1099/0022-1317-74-3-459. [DOI] [PubMed] [Google Scholar]
- Leutenegger C.M., Hofmann‐Lehmann R., Holznagel E., Cuisinier A.M., Wolfensberger C., Duquesne V., Cronier J., Allenspach K., Aubert A., Ossent P., Lutz H. Partial protection by vaccination with recombinant feline immunodeficiency virus surface glycoproteins. AIDS Res. Hum. Retroviruses. 1998;14:275–283. doi: 10.1089/aid.1998.14.275. [DOI] [PubMed] [Google Scholar]
- Levine M.Z., Calderon J.C., Wilkins P.P., Lane W.S., Asara J.M., Hancock K., Gonzalez A.E., Garcia H.H., Gilman R.H., Tsang V.C. Characterization, cloning, and expression of two diagnostic antigens for Taenia solium tapeworm infection. J. Parasitol. 2004;90:631–638. doi: 10.1645/GE-189R. [DOI] [PubMed] [Google Scholar]
- Li T., Takeda N., Miyamura T. Oral administration of hepatitis E virus‐like particles induces a systemic and mucosal immune response in mice. Vaccine. 2001;19:3476–3484. doi: 10.1016/s0264-410x(01)00059-7. [DOI] [PubMed] [Google Scholar]
- Li T.C., Suzaki Y., Ami Y., Dhole T.N., Miyamura T., Takeda N. Protection of cynomolgus monkeys against HEV infection by oral administration of recombinant hepatitis E virus‐like particles. Vaccine. 2004;22:370–377. doi: 10.1016/j.vaccine.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Lin X., Lubinski J.M., Friedman H.M. Immunization strategies to block the herpes simplex virus type 1 immunoglobulin G Fc receptor. J. Virol. 2004;78:2562–2571. doi: 10.1128/JVI.78.5.2562-2571.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofthouse S.A., Andrews A.E., Elhay M.J., Bowles V.M., Meeusen E.N., Nash A.D. Cytokines as adjuvants for ruminant vaccines. Int. J. Parasitol. 1996;26:835–842. doi: 10.1016/s0020-7519(96)80052-x. [DOI] [PubMed] [Google Scholar]
- Long G., Westenberg M., Wang H.L., Vlak J.M., Hu Z.H. Function, oligomerization and N‐linked glycosylation of the Helicoverpa armigera single nucleopolyhedrovius envelope fusion protein. J. Gen. Virol. 2006;87:839–846. doi: 10.1099/vir.0.81592-0. [DOI] [PubMed] [Google Scholar]
- Lopez de Turiso J.A., Cortes E., Martinez C., Ruiz de Ybanez R., Simarro I., Vela C., Casal I. Recombinant vaccine for canine parvovirus in dogs. J. Virol. 1992;66:2748–2753. doi: 10.1128/jvi.66.5.2748-2753.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loudon P.T., Hirasawa T., Oldfield S., Murphy M., Roy P. Expression of the outer capsid protein VP5 of two bluetongue viruses, and synthesis of chimeric double‐shelled virus‐like particles using combinations of recombinant baculoviruses. Virology. 1991;182:793–801. doi: 10.1016/0042-6822(91)90620-q. [DOI] [PubMed] [Google Scholar]
- Lubeck M.D., Natuk R.J., Chengalvala M., Chanda P.K., Murthy K.K., Murthy S., Mizutani S., Lee S.‐G., Wade M.S., Bhat B.M., Bhat R., Dheer S.K. Immunogenicity of recombinant adenovirus‐human immunodeficiency virus vaccines in chimpanzees following intranasal administration. AIDS Res. Hum. Retroviruses. 1994;10:1443–1449. doi: 10.1089/aid.1994.10.1443. [DOI] [PubMed] [Google Scholar]
- Luckow V.A., Lee S.C., Barry G.F., Olins P.O. Efficient generation of infectious recombinant baculoviruses by site‐specific transposon‐mediated insertion of foreign genes into a baculovirus genome propagated in Escherichia coli. J. Virol. 1993;67:4566–4579. doi: 10.1128/jvi.67.8.4566-4579.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundholm P., Wahren M., Sandstrom E., Volvovitz F., Wahren B. Autoreactivity in HIV‐infected individuals does not increase during vaccination with envelope rgp160. Immunol. Lett. 1994;41:147–153. doi: 10.1016/0165-2478(94)90125-2. [DOI] [PubMed] [Google Scholar]
- Luo L., Li Y., Cannon P.M., Kim S., Kang C.Y. Chimeric gag‐V3 virus‐like particles of human immunodeficiency virus induce virus‐neutralizing antibodies. Proc. Natl. Acad. Sci. USA. 1992;89:10527–10531. doi: 10.1073/pnas.89.21.10527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M., Yuan F., Liu Y., Jiang S., Song X., Jiang P., Yin X., Ding M., Deng H. Induction of neutralizing antibody against human immunodeficiency virus type 1 (HIV‐1) by immunization with gp41 membrane‐proximal external region (MPER) fused with porcine endogenous retrovirus (PERV) p15E fragment. Vaccine. 2006;24:435–442. doi: 10.1016/j.vaccine.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Ma J.K., Barros E., Bock R., Christou P., Dale P.J., Dix P.J., Fischer R., Irwin J., Mahoney R., Pezzotti M., Schillberg S., Sparrow P. Molecular farming for new drugs and vaccines. Current perspectives on the production of pharmaceuticals in transgenic plants. EMBO Rep. 2005;6:593–599. doi: 10.1038/sj.embor.7400470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K., Ono M., Kawaguchi Y., Niikura M., Okazaki K., Yokoyama N., Tokiyoshi Y., Tohya Y., Mikami T. Expression and properties of feline herpesvirus type 1 gD (hemagglutinin) by a recombinant baculovirus. Virus Res. 1996;46:75–80. doi: 10.1016/s0168-1702(96)01376-7. [DOI] [PubMed] [Google Scholar]
- Mao C., Koutsky L.A., Ault K.A., Wheeler C.M., Brown D.R., Wiley D.J., Alvarez F.B., Bautista O.M., Jansen K.U., Barr E. Efficacy of human papillomavirus‐16 vaccine to prevent cervical intraepithelial neoplasia: A randomized controlled trial. Obstet. Gynecol. 2006;107:18–27. doi: 10.1097/01.AOG.0000192397.41191.fb. [DOI] [PubMed] [Google Scholar]
- Marshall G.S., Li M., Stout G.G., Louthan M.V., Duliege A.M., Burke R.L., Hunt L.A. Antibodies to the major linear neutralizing domains of cytomegalovirus glycoprotein B among natural seropositives and CMV subunit vaccine recipients. Viral Immunol. 2000;13:329–341. doi: 10.1089/08828240050144653. [DOI] [PubMed] [Google Scholar]
- Martens J.W., van Oers M.M., van de Bilt B.D., Oudshoorn P., Vlak J.M. Development of a baculovirus vector that facilitates the generation of p10‐based recombinants. J. Virol. Methods. 1995;52:15–19. doi: 10.1016/0166-0934(94)00129-5. [DOI] [PubMed] [Google Scholar]
- Martinez A.P., Margos G., Barker G., Sinden R.E. The roles of the glycosylphosphatidylinositol anchor on the production and immunogenicity of recombinant ookinete surface antigen Pbs21 of Plasmodium berghei when prepared in a baculovirus expression system. Parasite Immunol. 2000;22:493–500. doi: 10.1046/j.1365-3024.2000.00329.x. [DOI] [PubMed] [Google Scholar]
- Martinez C., Dalsgaard K., Lopez de Turiso J.A., Cortes E., Vela C., Casal J.I. Production of porcine parvovirus empty capsids with high immunogenic activity. Vaccine. 1992;10:684–690. doi: 10.1016/0264-410x(92)90090-7. [DOI] [PubMed] [Google Scholar]
- Martinez‐Torrecuadrada J.L., Saubi N., Pages‐Mante A., Caston J.R., Espuna E., Casal J.I. Structure‐dependent efficacy of infectious bursal disease virus (IBDV) recombinant vaccines. Vaccine. 2003;21:3342–3350. doi: 10.1016/s0264-410x(02)00804-6. [DOI] [PubMed] [Google Scholar]
- Matsuoka H., Kobayashi J., Barker G.C., Miura K., Chinzei Y., Miyajima S., Ishii A., Sinden R.E. Induction of anti‐malarial transmission blocking immunity with a recombinant ookinete surface antigen of Plasmodium berghei produced in silkworm larvae using the baculovirus expression vector system. Vaccine. 1996;14:120–126. doi: 10.1016/0264-410x(95)00162-t. [DOI] [PubMed] [Google Scholar]
- McCown J., Cochran M., Putnak R., Feighny R., Burrous J., Henchal E., Hoke C. Protection of mice against lethal Japanese encephalitis with a recombinant baculovirus vaccine. Am. J. Trop. Med. Hyg. 1990;42:491–499. doi: 10.4269/ajtmh.1990.42.491. [DOI] [PubMed] [Google Scholar]
- McElrath M.J., Corey L., Berger D., Hoffman M.C., Klucking S., Dragavon J., Peterson E., Greenberg P.D. Immune responses elicited by recombinant vaccinia‐human immunodeficiency virus (HIV) envelope and HIV envelope protein: Analysis of the durability of responses and effect of repeated boosting. J. Infect. Dis. 1994;169:41–47. doi: 10.1093/infdis/169.1.41. [DOI] [PubMed] [Google Scholar]
- Mellquist‐Riemenschneider J.L., Garrison A.R., Geisbert J.B., Saikh K.U., Heidebrink K.D., Jahrling P.B., Ulrich R.G., Schmaljohn C.S. Comparison of the protective efficacy of DNA and baculovirus‐derived protein vaccines for EBOLA virus in guinea pigs. Virus Res. 2003;92:187–193. doi: 10.1016/s0168-1702(02)00338-6. [DOI] [PubMed] [Google Scholar]
- Monsma S.A., Oomens A.G., Blissard G.W. The GP64 envelope fusion protein is an essential baculovirus protein required for cell‐to‐cell transmission of infection. J. Virol. 1996;70:4607–4616. doi: 10.1128/jvi.70.7.4607-4616.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montefiori D.C., Graham B.S., Kliks S., Wright P.F. Serum antibodies to HIV‐1 in recombinant vaccinia virus recipients boosted with purified recombinant gp160. NIAID AIDS vaccine clinical trials network. J. Clin. Immunol. 1992;12:429–439. doi: 10.1007/BF00918855. [DOI] [PubMed] [Google Scholar]
- Mori H., Tawara H., Nakazawa H., Sumida M., Matsubara F., Aoyama S., Iritani Y., Hayashi Y., Kamogawa K. Expression of the Newcastle disease virus (NDV) fusion glycoprotein and vaccination against NDV challenge with a recombinant baculovirus. Avian Dis. 1994;38:772–777. [PubMed] [Google Scholar]
- Nagy E., Krell P.J., Dulac G.C., Derbyshire J.B. Vaccination against newcastle disease with a recombinant baculovirus hemagglutinin‐neuraminidase subunit vaccine. Avian Dis. 1991;35:585–590. [PubMed] [Google Scholar]
- Neilan J.G., Zsak L., Lu Z., Burrage T.G., Kutish G.F., Rock D.L. Neutralizing antibodies to African swine fever virus proteins p30, p54, and p72 are not sufficient for antibody‐mediated protection. Virology. 2004;319:337–342. doi: 10.1016/j.virol.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Nene V., Inumaru S., McKeever D., Morzaria S., Shaw M., Musoke A. Characterization of an insect cell‐derived Theileria parva sporozoite vaccine antigen and immunogenicity in cattle. Infect. Immun. 1995;63:503–508. doi: 10.1128/iai.63.2.503-508.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicollier‐Jamot B., Ogier A., Piroth L., Pothier P., Kohli E. Recombinant virus‐like particles of a norovirus (genogroup II strain) administered intranasally and orally with mucosal adjuvants LT and LT(R192G) in BALB/c mice induce specific humoral and cellular Th1/Th2‐like immune responses. Vaccine. 2004;22:1079–1086. doi: 10.1016/j.vaccine.2003.10.004. [DOI] [PubMed] [Google Scholar]
- O'Reilly D.R., Miller L.K., Luckow V.A. “Baculovirus Expression Vectors, A Laboratory Manual.”. Oxford University Press; New York, USA: 1992. [Google Scholar]
- Oien N.L., Brideau R.J., Thomsen D.R., Homa F.L., Wathen M.W. Vaccination with a heterologous respiratory syncytial virus chimeric FG glycoprotein demonstrates significant subgroup cross‐reactivity. Vaccine. 1993;11:1040–1048. doi: 10.1016/0264-410x(93)90131-g. [DOI] [PubMed] [Google Scholar]
- Okano K., Vanarsdall A.L., Mikhailov V.S., Rohrmann G.F. Conserved molecular systems of the Baculoviridae. Virology. 2006;344:77–87. doi: 10.1016/j.virol.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Okazaki K., Honda E., Kono Y. Expression of bovine herpesvirus 1 glycoprotein gIII by a recombinant baculovirus in insect cells. J. Gen. Virol. 1994;75:901–904. doi: 10.1099/0022-1317-75-4-901. [DOI] [PubMed] [Google Scholar]
- Olivier M., Gregory D.J., Forget G. Subversion mechanisms by which Leishmania parasites can escape the host immune response: A signaling point of view. Clin. Microbiol. Rev. 2005;18:293–305. doi: 10.1128/CMR.18.2.293-305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen C.W., McGregor M.W., Dybdahl‐Sissoko N., Schram B.R., Nelson K.M., Lunn D.P., Macklin M.D., Swain W.F., Hinshaw V.S. Immunogenicity and efficacy of baculovirus‐expressed and DNA‐based equine influenza virus hemagglutinin vaccines in mice. Vaccine. 1997;15:1149–1156. doi: 10.1016/s0264-410x(96)00309-x. [DOI] [PubMed] [Google Scholar]
- Onuma M., Kubota S., Kakuda T., Sako Y., Asada M., Kabeya H., Sugimoto C. Control of Theileria sergenti infection by vaccination. Trop. Anim. Health Prod. 1997;29:119S–123S. doi: 10.1007/BF02632949. [DOI] [PubMed] [Google Scholar]
- Packiarajah P., Walker C., Gilkerson J., Whalley J.M., Love D.N. Immune responses and protective efficacy of recombinant baculovirus‐expressed glycoproteins of equine herpesvirus 1 (EHV‐1) gB, gC and gD alone or in combinations in BALB/c mice. Vet. Microbiol. 1998;61:261–278. doi: 10.1016/s0378-1135(98)00189-8. [DOI] [PubMed] [Google Scholar]
- Palese P. Influenza: Old and new threats. Nat. Med. 2004;10:S82–S87. doi: 10.1038/nm1141. [DOI] [PubMed] [Google Scholar]
- Pande A., Carr B.V., Wong S.Y., Dalton K., Jones I.M., McCauley J.W., Charleston B. The glycosylation pattern of baculovirus expressed envelope protein E2 affects its ability to prevent infection with bovine viral diarrhoea virus. Virus Res. 2005;114:54–62. doi: 10.1016/j.virusres.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Pastoret P.P., Blancou J., Vannier P., Verschueren C. “Veterinary Vaccinology.”. Elsevier; Amsterdam: 1997. [Google Scholar]
- Patja A., Makinen‐Kiljunen S., Davidkin I., Paunio M., Peltola H. Allergic reactions to measles‐mumps‐rubella vaccination. Pediatrics. 2001;107:E27. doi: 10.1542/peds.107.2.e27. [DOI] [PubMed] [Google Scholar]
- Pau M.G., Ophorst C., Koldijk M.H., Schouten G., Mehtali M., Uytdehaag F. The human cell line PER.C6 provides a new manufacturing system for the production of influenza vaccines. Vaccine. 2001;19:2716–2721. doi: 10.1016/s0264-410x(00)00508-9. [DOI] [PubMed] [Google Scholar]
- Pearson L.D., Roy P. Genetically engineered multi‐component virus‐like particles as veterinary vaccines. Immunol. Cell Biol. 1993;71:381–389. doi: 10.1038/icb.1993.44. [DOI] [PubMed] [Google Scholar]
- Peet N.M., McKeating J.A., Ramos B., Klonisch T., De Souza J.B., Delves P.J., Lund T. Comparison of nucleic acid and protein immunization for induction of antibodies specific for HIV‐1 gp120. Clin. Exp. Immunol. 1997;109:226–232. doi: 10.1046/j.1365-2249.1997.4411339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekosz A., Griot C., Stillmock K., Nathanson N., Gonzalez‐Scarano F. Protection from La Crosse virus encephalitis with recombinant glycoproteins: Role of neutralizing anti‐G1 antibodies. J. Virol. 1995;69:3475–3481. doi: 10.1128/jvi.69.6.3475-3481.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perales M.A., Schwartz D.H., Fabry J.A., Lieberman J. A vaccinia‐gp160‐based vaccine but not a gp160 protein vaccine elicits anti‐gp160 cytotoxic T lymphocytes in some HIV‐1 seronegative vaccinees. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1995;10:27–35. [PubMed] [Google Scholar]
- Percheson P.B., Trepanier P., Dugre R., Mabrouk T. A phase I, randomized controlled clinical trial to study the reactogenicity and immunogenicity of a new split influenza vaccine derived from a non‐tumorigenic cell line. Dev. Biol. Stand. 1999;98:127–132. discussion 133–124. [PubMed] [Google Scholar]
- Perera K.L., Handunnetti S.M., Holm I., Longacre S., Mendis K. Baculovirus merozoite surface protein 1 C‐terminal recombinant antigens are highly protective in a natural primate model for human Plasmodium vivax malaria. Infect. Immun. 1998;66:1500–1506. doi: 10.1128/iai.66.4.1500-1506.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipps B., Rotmann D., Wicki M., Mayr L.M., Forstner M. Time reduction and process optimization of the baculovirus expression system for more efficient recombinant protein production in insect cells. Protein Expr. Purif. 2005;42:211–218. doi: 10.1016/j.pep.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Pijlman G.P., van den Born E., Martens D.E., Vlak J.M. Autographa californica baculoviruses with large genomic deletions are rapidly generated in infected insect cells. Virology. 2001;283:132–138. doi: 10.1006/viro.2001.0854. [DOI] [PubMed] [Google Scholar]
- Pijlman G.P., Dortmans J.C., Vermeesch A.M., Yang K., Martens D.E., Goldbach R.W., Vlak J.M. Pivotal role of the non‐hr origin of DNA replication in the genesis of defective interfering baculoviruses. J. Virol. 2002;76:5605–5611. doi: 10.1128/JVI.76.11.5605-5611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijlman G.P., Van Schijndel J.E., Vlak J.M. Spontaneous excision of BAC vector sequences from bacmid‐derived baculovirus expression vectors upon passage in insect cells. J. Gen. Virol. 2003;84:2669–2678. doi: 10.1099/vir.0.19438-0. [DOI] [PubMed] [Google Scholar]
- Pijlman G.P., Vermeesch A.M., Vlak J.M. Cell line‐specific accumulation of the baculovirus non‐hr origin of DNA replication in infected insect cells. J. Invertebr. Pathol. 2003;84:214–219. doi: 10.1016/j.jip.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Pijlman G.P., de Vrij J., van den End F.J., Vlak J.M., Martens D.E. Evaluation of baculovirus expression vectors with enhanced stability in continuous cascaded insect‐cell bioreactors. Biotechnol. Bioeng. 2004;87:743–753. doi: 10.1002/bit.20178. [DOI] [PubMed] [Google Scholar]
- Pijlman G.P., Roode E.C., Fan X., Roberts L.O., Belsham G.J., Vlak J.M., van Oers M.M. Stabilized baculovirus vector expressing a heterologous gene and GP64 from a single bicistronic transcript. J. Biotechnol. 2006;123:13–21. doi: 10.1016/j.jbiotec.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Pitcovski J., Di‐Castro D., Shaaltiel Y., Azriel A., Gutter B., Yarkoni E., Michael A., Krispel S., Levi B.Z. Insect cell‐derived VP2 of infectious bursal disease virus confers protection against the disease in chickens. Avian Dis. 1996;40:753–761. [PubMed] [Google Scholar]
- Pitcovski J., Levi B.Z., Maray T., Di‐Castro D., Safadi A., Krispel S., Azriel A., Gutter B., Michael A. Failure of viral protein 3 of infectious bursal disease virus produced in prokaryotic and eukaryotic expression systems to protect chickens against the disease. Avian Dis. 1999;43:8–15. [PubMed] [Google Scholar]
- Pizarro J.C., Chitarra V., Verger D., Holm I., Petres S., Dartevelle S., Nato F., Longacre S., Bentley G.A. Crystal structure of a Fab complex formed with PfMSP1–19, the C‐terminal fragment of merozoite surface protein 1 from Plasmodium falciparum: A malaria vaccine candidate. J. Mol. Biol. 2003;328:1091–1103. doi: 10.1016/s0022-2836(03)00376-0. [DOI] [PubMed] [Google Scholar]
- Plana Duran J., Climent I., Sarraseca J., Urniza A., Cortes E., Vela C., Casal J.I. Baculovirus expression of proteins of porcine reproductive and respiratory syndrome virus strain Olot/91. Involvement of ORF3 and ORF5 proteins in protection. Virus Genes. 1997;14:19–29. doi: 10.1023/a:1007931322271. [DOI] [PubMed] [Google Scholar]
- Poomputsa K., Kittel C., Egorov A., Ernst W., Grabherr R. Generation of recombinant influenza virus using baculovirus delivery vector. J. Virol. Methods. 2003;110:111–114. doi: 10.1016/s0166-0934(03)00084-3. [DOI] [PubMed] [Google Scholar]
- Possee R.D., Thomas C.J., King L.A. The use of baculovirus vectors for the production of membrane proteins in insect cells. Biochem. Soc. Trans. 1999;27:928–932. doi: 10.1042/bst0270928. [DOI] [PubMed] [Google Scholar]
- Powers D.C., Smith G.E., Anderson E.L., Kennedy D.J., Hackett C.S., Wilkinson B.E., Volvovitz F., Belshe R.B., Treanor J.J. Influenza A virus vaccines containing purified recombinant H3 hemagglutinin are well tolerated and induce protective immune responses in healthy adults. J. Infect. Dis. 1995;171:1595–1599. doi: 10.1093/infdis/171.6.1595. [DOI] [PubMed] [Google Scholar]
- Powers D.C., McElhaney J.E., Florendo O.A., Jr., Manning M.C., Upshaw C.M., Bentley D.W., Wilkinson B.E. Humoral and cellular immune responses following vaccination with purified recombinant hemagglutinin from influenza A (H3N2) virus. J. Infect. Dis. 1997;175:342–351. doi: 10.1093/infdis/175.2.342. [DOI] [PubMed] [Google Scholar]
- Prehaud C., Takehara K., Flamand A., Bishop D.H. Immunogenic and protective properties of rabies virus glycoprotein expressed by baculovirus vectors. Virology. 1989;173:390–399. doi: 10.1016/0042-6822(89)90551-5. [DOI] [PubMed] [Google Scholar]
- Qiao M., Ashok M., Bernard K.A., Palacios G., Zhou Z.H., Lipkin W.I., Liang T.J. Induction of sterilizing immunity against West Nile virus (WNV), by immunization with WNV‐like particles produced in insect cells. J. Infect. Dis. 2004;190:2104–2108. doi: 10.1086/425933. [DOI] [PubMed] [Google Scholar]
- Qu X., Chen W., Maguire T., Austin F. Immunoreactivity and protective effects in mice of a recombinant dengue 2 Tonga virus NS1 protein produced in a baculovirus expression system. J. Gen. Virol. 1993;74:89–97. doi: 10.1099/0022-1317-74-1-89. [DOI] [PubMed] [Google Scholar]
- Quanquin N.M., Galaviz C., Fouts D.L., Wrightsman R.A., Manning J.E. Immunization of mice with a TolA‐like surface protein of Trypanosoma cruzi generates CD4(+) T‐cell‐dependent parasiticidal activity. Infect. Immun. 1999;67:4603–4612. doi: 10.1128/iai.67.9.4603-4612.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray R., Galinski M.S., Compans R.W. Expression of the fusion glycoprotein of human parainfluenza type 3 virus in insect cells by a recombinant baculovirus and analysis of its immunogenic property. Virus Res. 1989;12:169–180. doi: 10.1016/0168-1702(89)90062-2. [DOI] [PubMed] [Google Scholar]
- Reed Z. Portfolio of candidate malaria vaccines currently in development, March 2005. Initiative on Vaccine Research; World Health Organization: 2005. [Google Scholar]
- Reszka N., Cornelissen J.B., Harmsen M.M., Bienkowska‐Szewczyk K., de Bree J., Boersma W.J., Rijsewijk F.A. Fasciola hepatica procathepsin L3 protein expressed by a baculovirus recombinant can partly protect rats against fasciolosis. Vaccine. 2005;23:2987–2993. doi: 10.1016/j.vaccine.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Reuben J.M., Liang L., Atmar R.L., Greenberg S. B‐cell activation and differentiation by HIV‐1 antigens among volunteers vaccinated with VaxSyn HIV‐1. J. Acquir. Immune Defic. Syndr. 1992;5:719–725. [PubMed] [Google Scholar]
- Rini B.I. Technology evaluation: APC‐8015, Dendreon. Curr. Opin. Mol. Ther. 2002;4:76–79. [PubMed] [Google Scholar]
- Rosen E., Stapleton J.T., McLinden J. Synthesis of immunogenic hepatitis A virus particles by recombinant baculoviruses. Vaccine. 1993;11:706–712. doi: 10.1016/0264-410x(93)90253-t. [DOI] [PubMed] [Google Scholar]
- Roy P. Genetically engineered particulate virus‐like structures and their use as vaccine delivery systems. Intervirology. 1996;39:62–71. doi: 10.1159/000150476. [DOI] [PubMed] [Google Scholar]
- Roy P. Nature and duration of protective immunity to bluetongue virus infection. Dev. Biol. (Basel) 2003;114:169–183. [PubMed] [Google Scholar]
- Roy P., Sutton G. New generation of African horse sickness virus vaccines based on structural and molecular studies of the virus particles. Arch. Virol. Suppl. 1998;14:177–202. doi: 10.1007/978-3-7091-6823-3_17. [DOI] [PubMed] [Google Scholar]
- Roy P., Bishop D.H., LeBlois H., Erasmus B.J. Long‐lasting protection of sheep against bluetongue challenge after vaccination with virus‐like particles: Evidence for homologous and partial heterologous protection. Vaccine. 1994;12:805–811. doi: 10.1016/0264-410x(94)90289-5. [DOI] [PubMed] [Google Scholar]
- Ruitenberg K.M., Walker C., Love D.N., Wellington J.E., Whalley J.M. A prime‐boost immunization strategy with DNA and recombinant baculovirus‐expressed protein enhances protective immunogenicity of glycoprotein D of equine herpesvirus 1 in naive and infection‐primed mice. Vaccine. 2000;18:1367–1373. doi: 10.1016/s0264-410x(99)00400-4. [DOI] [PubMed] [Google Scholar]
- Ruiz‐Gonzalvo F., Rodriguez F., Escribano J.M. Functional and immunological properties of the baculovirus‐expressed hemagglutinin of African swine fever virus. Virology. 1996;218:285–289. doi: 10.1006/viro.1996.0193. [DOI] [PubMed] [Google Scholar]
- Russell W.C. Update on adenovirus and its vectors. J. Gen. Virol. 2000;81:2573–2604. doi: 10.1099/0022-1317-81-11-2573. [DOI] [PubMed] [Google Scholar]
- Saliki J.T., Mizak B., Flore H.P., Gettig R.R., Burand J.P., Carmichael L.E., Wood H.A., Parrish C.R. Canine parvovirus empty capsids produced by expression in a baculovirus vector: Use in analysis of viral properties and immunization of dogs. J. Gen. Virol. 1992;73:369–374. doi: 10.1099/0022-1317-73-2-369. [DOI] [PubMed] [Google Scholar]
- Sato H., Nakajima K., Maeno Y., Kamaishi T., Kamata T., Mori H., Kamei K., Takano R., Kudo K., Hara S. Expression of YAV proteins and vaccination against viral ascites among cultured juvenile yellowtail. Biosci. Biotechnol. Biochem. 2000;64:1494–1499. doi: 10.1271/bbb.64.1494. [DOI] [PubMed] [Google Scholar]
- Saville G.P., Patmanidi A.L., Possee R.D., King L.A. Deletion of the Autographa californica nucleopolyhedrovirus chitinase KDEL motif and in vitro and in vivo analysis of the modified virus. J. Gen. Virol. 2004;85:821–831. doi: 10.1099/vir.0.19732-0. [DOI] [PubMed] [Google Scholar]
- Schetters T. Vaccination against canine babesiosis. Trends Parasitol. 2005;21:179–184. doi: 10.1016/j.pt.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Schijns V.E. Mechanisms of vaccine adjuvant activity: Initiation and regulation of immune responses by vaccine adjuvants. Vaccine. 2003;21:829–831. doi: 10.1016/s0264-410x(02)00527-3. [DOI] [PubMed] [Google Scholar]
- Schleiss M.R., Bourne N., Stroup G., Bravo F.J., Jensen N.J., Bernstein D.I. Protection against congenital cytomegalovirus infection and disease in guinea pigs, conferred by a purified recombinant glycoprotein B vaccine. J. Infect. Dis. 2004;189:1374–1381. doi: 10.1086/382751. [DOI] [PubMed] [Google Scholar]
- Schmaljohn C.S., Parker M.D., Ennis W.H., Dalrymple J.M., Collett M.S., Suzich J.A., Schmaljohn A.L. Baculovirus expression of the M genome segment of Rift Valley fever virus and examination of antigenic and immunogenic properties of the expressed proteins. Virology. 1989;170:184–192. doi: 10.1016/0042-6822(89)90365-6. [DOI] [PubMed] [Google Scholar]
- Schmaljohn C.S., Chu Y.K., Schmaljohn A.L., Dalrymple J.M. Antigenic subunits of Hantaan virus expressed by baculovirus and vaccinia virus recombinants. J. Virol. 1990;64:3162–3170. doi: 10.1128/jvi.64.7.3162-3170.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scodeller E.A., Tisminetzky S.G., Porro F., Schiappacassi M., De Rossi A., Chiecco‐Bianchi L., Baralle F.E. A new epitope presenting system displays a HIV‐1 V3 loop sequence and induces neutralizing antibodies. Vaccine. 1995;13:1233–1239. doi: 10.1016/0264-410x(95)00058-9. [DOI] [PubMed] [Google Scholar]
- Sestak K., Meister R.K., Hayes J.R., Kim L., Lewis P.A., Myers G., Saif L.J. Active immunity and T‐cell populations in pigs intraperitoneally inoculated with baculovirus‐expressed transmissible gastroenteritis virus structural proteins. Vet. Immunol. Immunopathol. 1999;70:203–221. doi: 10.1016/S0165-2427(99)00074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A.K., Woldehiwet Z., Walrevens K., Letteson J. Immune responses of lambs to the fusion (F) glycoprotein of bovine respiratory syncytial virus expressed on insect cells infected with a recombinant baculovirus. Vaccine. 1996;14:773–779. doi: 10.1016/0264-410x(95)00248-y. [DOI] [PubMed] [Google Scholar]
- Shearer M.H., Bright R.K., Lanford R.E., Kennedy R.C. Immunization of mice with baculovirus‐derived recombinant SV40 large tumour antigen induces protective tumour immunity to a lethal challenge with SV40‐transformed cells. Clin. Exp. Immunol. 1993;91:266–271. doi: 10.1111/j.1365-2249.1993.tb05893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivappa R.B., McAllister P.E., Edwards G.H., Santi N., Evensen O., Vakharia V.N. Development of a subunit vaccine for infectious pancreatic necrosis virus using a baculovirus insect/larvae system. Dev. Biol. (Basel) 2005;121:165–174. [PubMed] [Google Scholar]
- Sinnathamby G., Renukaradhya G.J., Rajasekhar M., Nayak R., Shaila M.S. Immune responses in goats to recombinant hemagglutinin‐neuraminidase glycoprotein of Peste des petits ruminants virus: Identification of a T cell determinant. Vaccine. 2001;19:4816–4823. doi: 10.1016/s0264-410x(01)00210-9. [DOI] [PubMed] [Google Scholar]
- Sinnathamby G., Renukaradhya G.J., Rajasekhar M., Nayak R., Shaila M.S. Recombinant hemagglutinin protein of rinderpest virus expressed in insect cells induces cytotoxic T‐cell responses in cattle. Viral Immunol. 2001;14:349–358. doi: 10.1089/08828240152716592. [DOI] [PubMed] [Google Scholar]
- Slepushkin V.A., Katz J.M., Black R.A., Gamble W.C., Rota P.A., Cox N.J. Protection of mice against influenza A virus challenge by vaccination with baculovirus‐expressed M2 protein. Vaccine. 1995;13:1399–1402. doi: 10.1016/0264-410x(95)92777-y. [DOI] [PubMed] [Google Scholar]
- Smith G.E., Summers M.D., Fraser M.J. Production of human beta interferon in insect cells infected with a baculovirus expression vector. Mol. Cell. Biol. 1983;3:2156–2165. doi: 10.1128/mcb.3.12.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder D.B., Vakharia V.N., Mengel‐Whereat S.A., Edwards G.H., Savage P.K., Lutticken D., Goodwin M.A. Active cross‐protection induced by a recombinant baculovirus expressing chimeric infectious bursal disease virus structural proteins. Avian Dis. 1994;38:701–707. [PubMed] [Google Scholar]
- Song C.S., Lee Y.J., Lee C.W., Sung H.W., Kim J.H., Mo I.P., Izumiya Y., Jang H.K., Mikami T. Induction of protective immunity in chickens vaccinated with infectious bronchitis virus S1 glycoprotein expressed by a recombinant baculovirus. J. Gen. Virol. 1998;79:719–723. doi: 10.1099/0022-1317-79-4-719. [DOI] [PubMed] [Google Scholar]
- Stokes A., Alber D.G., Cameron R.S., Marshall R.N., Allen G.P., Killington R.A. The production of a truncated form of baculovirus expressed EHV‐1 glycoprotein C and its role in protection of C3H (H‐2Kk) mice against virus challenge. Virus Res. 1996;44:97–109. doi: 10.1016/0168-1702(96)01339-1. [DOI] [PubMed] [Google Scholar]
- Stokes A., Alber D.G., Greensill J., Amellal B., Carvalho R., Taylor L.A., Doel T.R., Killington R.A., Halliburton I.W., Meredith D.M. The expression of the proteins of equine herpesvirus 1 which share homology with herpes simplex virus 1 glycoproteins H and L. Virus Res. 1996;40:91–107. doi: 10.1016/0168-1702(95)01256-7. [DOI] [PubMed] [Google Scholar]
- Stokes A., Cameron R.S., Marshall R.N., Killington R.A. High level expression of equine herpesvirus 1 glycoproteins D and H and their role in protection against virus challenge in the C3H (H‐2Kk) murine model. Virus Res. 1997;50:159–173. doi: 10.1016/s0168-1702(97)00067-1. [DOI] [PubMed] [Google Scholar]
- Streatfield S.J., Howard J.A. Plant production systems for vaccines. Expert Rev. Vaccines. 2003;2:763–775. doi: 10.1586/14760584.2.6.763. [DOI] [PubMed] [Google Scholar]
- Swayne D.E., Beck J.R., Perdue M.L., Beard C.W. Efficacy of vaccines in chickens against highly pathogenic Hong Kong H5N1 avian influenza. Avian Dis. 2001;45:355–365. [PubMed] [Google Scholar]
- Takehara K., Kikuma R., Ishikawa S., Kamikawa M., Nagata T., Yokomizo Y., Nakamura M. Production and in vivo testing of a recombinant bovine IL‐12 as an adjuvant for Salmonella typhimurium vaccination in calves. Vet. Immunol. Immunopathol. 2002;86:23–30. doi: 10.1016/s0165-2427(02)00009-0. [DOI] [PubMed] [Google Scholar]
- Tami C., Peralta A., Barbieri R., Berinstein A., Carrillo E., Taboga O. Immunological properties of FMDV‐gP64 fusion proteins expressed on SF9 cell and baculovirus surfaces. Vaccine. 2004;23:840–845. doi: 10.1016/j.vaccine.2004.03.070. [DOI] [PubMed] [Google Scholar]
- Tani H., Limn C.K., Yap C.C., Onishi M., Nozaki M., Nishimune Y., Okahashi N., Kitagawa Y., Watanabe R., Mochizuki R., Moriishi K., Matsuura Y. In vitro and in vivo gene delivery by recombinant baculoviruses. J. Virol. 2003;77:9799–9808. doi: 10.1128/JVI.77.18.9799-9808.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tareilus E., van Oers M.M., Vlak J. Salt Enhancer Assay, European patent number 0229807.3. 2003. [Google Scholar]
- Tate C.G., Whiteley E., Betenbaugh M.J. Molecular chaperones stimulate the functional expression of the cocaine‐sensitive serotonin transporter. J. Biol. Chem. 1999;274:17551–17558. doi: 10.1074/jbc.274.25.17551. [DOI] [PubMed] [Google Scholar]
- Tegerstedt K., Franzen A.V., Andreasson K., Joneberg J., Heidari S., Ramqvist T., Dalianis T. Murine polyomavirus virus‐like particles (VLPs) as vectors for gene and immune therapy and vaccines against viral infections and cancer. Anticancer Res. 2005;25:2601–2608. [PubMed] [Google Scholar]
- Tellam R.L., Kemp D., Riding G., Briscoe S., Smith D., Sharp P., Irving D., Willadsen P. Reduced oviposition of Boophilus microplus feeding on sheep vaccinated with vitellin. Vet. Parasitol. 2002;103:141–156. doi: 10.1016/s0304-4017(01)00573-8. [DOI] [PubMed] [Google Scholar]
- Tessier D.C., Thomas D.Y., Khouri H.E., Laliberte F., Vernet T. Enhanced secretion from insect cells of a foreign protein fused to the honeybee melittin signal peptide. Gene. 1991;98:177–183. doi: 10.1016/0378-1119(91)90171-7. [DOI] [PubMed] [Google Scholar]
- Theilmann D.A., Blissard G.W., Bonning B., Jehl J., O'Reilly D.R., Rohrman G.F., Thiem S., Vlak J.M. In: 8th Ed. Fauquet C.M., Mayo M.A., Maniloff J., Desselberger U., Ball L.A., editors. Elsevier/Academic Press; London: 2005. (“Family Baculoviridae”). Virus Taxonomy, VIIIth Report of the ICTV. [Google Scholar]
- Thomas C.J., Brown H.L., Hawes C.R., Lee B.Y., Min M.K., King L.A., Possee R.D. Localization of a baculovirus‐induced chitinase in the insect cell endoplasmic reticulum. J. Virol. 1998;72:10207–10212. doi: 10.1128/jvi.72.12.10207-10212.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin G.J., Li G.H., Fong S.E., Nagashima K., Gonda M.A. Chimeric HIV‐1 virus‐like particles containing gp120 epitopes as a result of a ribosomal frameshift elicit Gag‐ and SU‐specific murine cytotoxic T‐lymphocyte activities. Virology. 1997;236:307–315. doi: 10.1006/viro.1997.8745. [DOI] [PubMed] [Google Scholar]
- Tomiya N., Narang S., Lee Y.C., Betenbaugh M.J. Comparing N‐glycan processing in mammalian cell lines to native and engineered lepidopteran insect cell lines. Glycoconj. J. 2004;21:343–360. doi: 10.1023/B:GLYC.0000046275.28315.87. [DOI] [PubMed] [Google Scholar]
- Tordo N., Bourhy H., Sather S., Ollo R. Structure and expression in baculovirus of the Mokola virus glycoprotein: An efficient recombinant vaccine. Virology. 1993;194:59–69. doi: 10.1006/viro.1993.1235. [DOI] [PubMed] [Google Scholar]
- Treanor J.J., Betts R.F., Smith G.E., Anderson E.L., Hackett C.S., Wilkinson B.E., Belshe R.B., Powers D.C. Evaluation of a recombinant hemagglutinin expressed in insect cells as an influenza vaccine in young and elderly adults. J. Infect. Dis. 1996;173:1467–1470. doi: 10.1093/infdis/173.6.1467. [DOI] [PubMed] [Google Scholar]
- Treanor J.J., Wilkinson B.E., Masseoud F., Hu‐Primmer J., Battaglia R., O'Brien D., Wolff M., Rabinovich G., Blackwelder W., Katz J.M. Safety and immunogenicity of a recombinant hemagglutinin vaccine for H5 influenza in humans. Vaccine. 2001;19:1732–1737. doi: 10.1016/s0264-410x(00)00395-9. [DOI] [PubMed] [Google Scholar]
- Treanor J.J., Sciff G.M., Couch R.B., Cate T.R., Brady R.C., Hay C.M., Wolff M., She D., Cox M.M. Dose‐related safety and immunogenicity of a tivalent baculvoirus‐expressed infleunzavirus hemagglutinin vaccine in elderly adults. J. Inf. Dis. 2006;193:1223–1228. doi: 10.1086/503050. [DOI] [PubMed] [Google Scholar]
- Tuboly T., Nagy E., Derbyshire J.B. Potential viral vectors for the stimulation of mucosal antibody responses against enteric viral antigens in pigs. Res. Vet. Sci. 1993;54:345–350. doi: 10.1016/0034-5288(93)90133-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakharia V.N., Snyder D.B., Lutticken D., Mengel‐Whereat S.A., Savage P.K., Edwards G.H., Goodwin M.A. Active and passive protection against variant and classic infectious bursal disease virus strains induced by baculovirus‐expressed structural proteins. Vaccine. 1994;12:452–456. doi: 10.1016/0264-410x(94)90124-4. [DOI] [PubMed] [Google Scholar]
- Valdes E., Vela C., Alvarez J.I. United States patent number 5,882,652. 1999. Empty canine parvovirus capsids having CPV recombinant VP2 and vaccines having such capsids. [Google Scholar]
- Valenzuela P., Medina A., Rutter W.J., Ammerer G., Hall B.D. Synthesis and assembly of hepatitis B virus surface antigen particles in yeast. Nature. 1982;298:347–350. doi: 10.1038/298347a0. [DOI] [PubMed] [Google Scholar]
- van Aarle P. Suitability of an E2 subunit vaccine of classical swine fever in combination with the E(rns)‐marker‐test for eradication through vaccination. Dev. Biol. (Basel) 2003;114:193–200. [PubMed] [Google Scholar]
- van Dijk A.A. Development of recombinant vaccines against bluetongue. Biotechnol. Adv. 1993;11:1–12. doi: 10.1016/0734-9750(93)90407-e. [DOI] [PubMed] [Google Scholar]
- van Drunen Littel‐van den Hurk S., Parker M.D., Fitzpatrick D.R., Zamb T.J., van den Hurk J.V., Campos M., Harland R., Babiuk L.A. Expression of bovine herpesvirus 1 glycoprotein gIV by recombinant baculovirus and analysis of its immunogenic properties. J. Virol. 1991;65:263–271. doi: 10.1128/jvi.65.1.263-271.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Drunen Littel‐van den Hurk S., Parker M.D., Massie B., van den Hurk J.V., Harland R., Babiuk L.A., Zamb T.J. Protection of cattle from BHV‐1 infection by immunization with recombinant glycoprotein gIV. Vaccine. 1993;11:25–35. doi: 10.1016/0264-410x(93)90336-v. [DOI] [PubMed] [Google Scholar]
- Van Lier F.L.J., van den Hombergh J.P.T.W., de Gooijer C.D., den Boer M.M., Vlak J.M., Tramper J. Long‐term semi‐continuous production of recombinant baculovirus protein in a repeated (fed‐) batch two‐stage reactor system. Enzyme Microb. Technol. 1996;18:460–466. [Google Scholar]
- Van Oers M.M., Vlak J.M. The baculovirus 10‐kDa protein. J. Invertebr. Pathol. 1997;70:1–17. doi: 10.1006/jipa.1997.4675. [DOI] [PubMed] [Google Scholar]
- van Oers M.M., Thomas A.A., Moormann R.J., Vlak J.M. Secretory pathway limits the enhanced expression of classical swine fever virus E2 glycoprotein in insect cells. J. Biotechnol. 2001;86:31–38. doi: 10.1016/s0168-1656(00)00403-x. [DOI] [PubMed] [Google Scholar]
- van Oirschot J.T. Diva vaccines that reduce virus transmission. J. Biotechnol. 1999;73:195–205. doi: 10.1016/s0168-1656(99)00121-2. [DOI] [PubMed] [Google Scholar]
- van Rijn P.A., van Gennip H.G., Moormann R.J. An experimental marker vaccine and accompanying serological diagnostic test both based on envelope glycoprotein E2 of classical swine fever virus (CSFV) Vaccine. 1999;17:433–440. doi: 10.1016/s0264-410x(98)00215-1. [DOI] [PubMed] [Google Scholar]
- van Wyke Coelingh K.L., Murphy B.R., Collins P.L., Lebacq‐Verheyden A.M., Battey J.F. Expression of biologically active and antigenically authentic parainfluenza type 3 virus hemagglutinin‐neuraminidase glycoprotein by a recombinant baculovirus. Virology. 1987;160:465–472. doi: 10.1016/0042-6822(87)90018-3. [DOI] [PubMed] [Google Scholar]
- Vaz‐Santiago J., Lule J., Rohrlich P., Jacquier C., Gibert N., Le Roy E., Betbeder D., Davignon J.L., Davrinche C. Ex vivo stimulation and expansion of both CD4(+) and CD8(+) T cells from peripheral blood mononuclear cells of human cytomegalovirus‐seropositive blood donors by using a soluble recombinant chimeric protein, IE1‐pp65. J. Virol. 2001;75:7840–7847. doi: 10.1128/JVI.75.17.7840-7847.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velzing J., Groen J., Drouet M.T., van Amerongen G., Copra C., Osterhaus A.D., Deubel V. Induction of protective immunity against Dengue virus type 2: Comparison of candidate live attenuated and recombinant vaccines. Vaccine. 1999;17:1312–1320. doi: 10.1016/s0264-410x(98)00393-4. [DOI] [PubMed] [Google Scholar]
- Venugopal K., Jiang W.R., Gould E.A. Immunity to St. Louis encephalitis virus by sequential immunization with recombinant vaccinia and baculovirus derived PrM/E proteins. Vaccine. 1995;13:1000–1005. doi: 10.1016/0264-410x(95)00015-s. [DOI] [PubMed] [Google Scholar]
- Vlak J.M., Keus R.J. Baculovirus expression vector system for production of viral vaccines. Adv. Biotechnol. Processes. 1990;14:91–128. [PubMed] [Google Scholar]
- Vlak J.M., Schouten A., Usmany M., Belsham G.J., Klinge‐Roode E.C., Maule A.J., Van Lent J.W., Zuidema D. Expression of cauliflower mosaic virus gene I using a baculovirus vector based upon the p10 gene and a novel selection method. Virology. 1990;179:312–320. doi: 10.1016/0042-6822(90)90299-7. [DOI] [PubMed] [Google Scholar]
- Vlak J.M., de Gooijer C.D., Tramper J., Miltenburger H.G., editors. Current applications of cell culture engineering. Vol. 2. Kluwer, Dordrecht; The Netherlands: 1996. (“Insect Cell Cultures, Fundamental and Applied Aspects,”). [Google Scholar]
- Wang H., Deng F., Pijlman G.P., Chen X., Sun X., Vlak J.M., Hu Z. Cloning of biologically active genomes from a Helicoverpa armigera single‐nucleocapsid nucleopolyhedrovirus isolate by using a bacterial artificial chromosome. Virus Res. 2003;97:57–63. doi: 10.1016/j.virusres.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Wang M.Y., Kuo Y.Y., Lee M.S., Doong S.R., Ho J.Y., Lee L.H. Self‐assembly of the infectious bursal disease virus capsid protein, rVP2, expressed in insect cells and purification of immunogenic chimeric rVP2H particles by immobilized metal‐ion affinity chromatography. Biotechnol. Bioeng. 2000;67:104–111. doi: 10.1002/(sici)1097-0290(20000105)67:1<104::aid-bit12>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Wathen M.W., Kakuk T.J., Brideau R.J., Hausknecht E.C., Cole S.L., Zaya R.M. Vaccination of cotton rats with a chimeric FG glycoprotein of human respiratory syncytial virus induces minimal pulmonary pathology on challenge. J. Infect. Dis. 1991;163:477–482. doi: 10.1093/infdis/163.3.477. [DOI] [PubMed] [Google Scholar]
- Watts A.M., Shearer M.H., Pass H.I., Bright R.K., Kennedy R.C. Comparison of simian virus 40 large T antigen recombinant protein and DNA immunization in the induction of protective immunity from experimental pulmonary metastasis. Cancer Immunol. Immunother. 1999;47:343–351. doi: 10.1007/s002620050540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werle B., Bourgeois C., Alexandre A., Massonneau V., Pothier P. Immune response to baculovirus expressed protein fragment amino acids 190–289 of respiratory syncytial virus (RSV) fusion protein. Vaccine. 1998;16:1127–1130. doi: 10.1016/s0264-410x(98)80109-6. [DOI] [PubMed] [Google Scholar]
- Weyer U., Possee R.D. A baculovirus dual expression vector derived from the Autographa californica nuclear polyhedrosis virus polyhedrin and p10 promoters: Co‐expression of two influenza virus genes in insect cells. J. Gen. Virol. 1991;72:2967–2974. doi: 10.1099/0022-1317-72-12-2967. [DOI] [PubMed] [Google Scholar]
- Whalley J.M., Love D.N., Tewari D., Field H.J. Characteristics of equine herpesvirus 1 glycoproteins expressed in insect cells. Vet. Microbiol. 1995;46:193–201. doi: 10.1016/0378-1135(95)00083-m. [DOI] [PubMed] [Google Scholar]
- Wickham T.J., Davis T., Granados R.R., Hammer D.A., Shuler M.L., Wood H.A. Baculovirus defective interfering particles are responsible for variations in recombinant protein production as a function of multiplicity of infection. Biotechnol. Lett. 1991;13:483–488. [Google Scholar]
- Willadsen P., Bird P., Cobon G.S., Hungerford J. Commercialisation of a recombinant vaccine against Boophilus microplus. Parasitology. 1995;110(Suppl.):S43–S50. doi: 10.1017/s0031182000001487. [DOI] [PubMed] [Google Scholar]
- Wilson R., Je Y.H., Bugeon L., Straschil U., O'Reilly D.R., Olszweski J.A. Display of foreign proteins using recombinant baculovirus occlusion bodies: A novel vaccination tool. 38th Annual Meeting of the Society of Invertebrate Virology, Anchorage, Alaska, 2005; 2005. Abstract 174. [Google Scholar]
- Xuan X., Nakamura T., Ihara T., Sato I., Tuchiya K., Nosetto E., Ishihama A., Ueda S. Characterization of pseudorabies virus glycoprotein gII expressed by recombinant baculovirus. Virus Res. 1995;36:151–161. doi: 10.1016/0168-1702(94)00112-p. [DOI] [PubMed] [Google Scholar]
- Xuan X., Maeda K., Mikami T., Otsuka H. Characterization of canine herpesvirus glycoprotein C expressed by a recombinant baculovirus in insect cells. Virus Res. 1996;46:57–64. doi: 10.1016/s0168-1702(96)01374-3. [DOI] [PubMed] [Google Scholar]
- Yang D.K., Kweon C.H., Kim B.H., Lim S.I., Kwon J.H., Kim S.H., Song J.Y., Han H.R. Immunogenicity of baculovirus expressed recombinant proteins of Japanese encephalitis virus in mice. J. Vet. Sci. 2005;6:125–133. [PubMed] [Google Scholar]
- Yao Q., Kuhlmann F.M., Eller R., Compans R.W., Chen C. Production and characterization of simian–human immunodeficiency virus‐like particles. AIDS Res. Hum. Retroviruses. 2000;16:227–236. doi: 10.1089/088922200309322. [DOI] [PubMed] [Google Scholar]
- Yao Q., Vuong V., Li M., Compans R.W. Intranasal immunization with SIV virus‐like particles (VLPs) elicits systemic and mucosal immunity. Vaccine. 2002;20:2537–2545. doi: 10.1016/s0264-410x(02)00160-3. [DOI] [PubMed] [Google Scholar]
- Yoshida S., Kondoh D., Arai E., Matsuoka H., Seki C., Tanaka T., Okada M., Ishii A. Baculovirus virions displaying Plasmodium berghei circumsporozoite protein protect mice against malaria sporozoite infection. Virology. 2003;316:161–170. doi: 10.1016/j.virol.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Young L.S., Searle P.F., Onion D., Mautner V. Viral gene therapy strategies: From basic science to clinical applications. J. Pathol. 2006;208:299–318. doi: 10.1002/path.1896. [DOI] [PubMed] [Google Scholar]
- Zhang L., Wu G., Tate C.G., Lookene A., Olivecrona G. Calreticulin promotes folding/dimerization of human lipoprotein lipase expressed in insect cells (Sf21) J. Biol. Chem. 2003;278:29344–29351. doi: 10.1074/jbc.M300455200. [DOI] [PubMed] [Google Scholar]
- Zhang Y.M., Hayes E.P., McCarty T.C., Dubois D.R., Summers P.L., Eckels K.H., Chanock R.M., Lai C.J. Immunization of mice with dengue structural proteins and nonstructural protein NS1 expressed by baculovirus recombinant induces resistance to dengue virus encephalitis. J. Virol. 1988;62:3027–3031. doi: 10.1128/jvi.62.8.3027-3031.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng R. Technology evaluation: HIVAC‐1e. Curr. Opin. Mol. Ther. 1999;1:121–125. [PubMed] [Google Scholar]
- Zintl A., Mulcahy G., Skerrett H.E., Taylor S.M., Gray J.S. Babesia divergens, a bovine blood parasite of veterinary and zoonotic importance. Clin. Microbiol. Rev. 2003;16:622–636. doi: 10.1128/CMR.16.4.622-636.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


