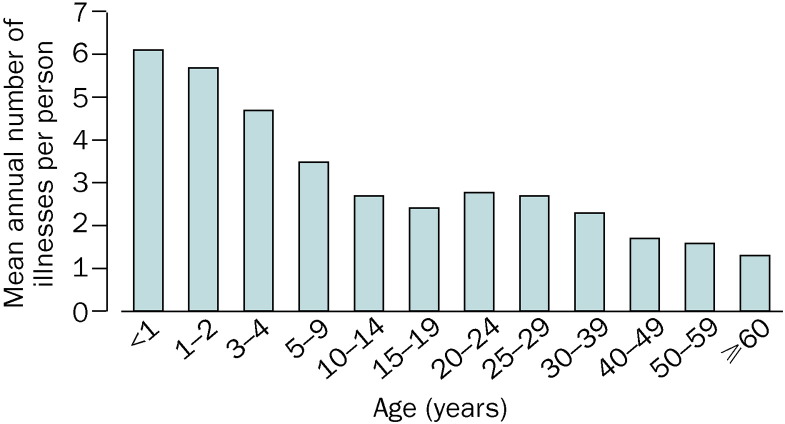Summary
Despite great advances in medicine, the common cold continues to be a great burden on society in terms of human suffering and economic losses. Of the several viruses that cause the disease, the role of rhinoviruses is most prominent. About a quarter of all colds are still without proven cause, and the recent discovery of human metapneumovirus suggests that other viruses could remain undiscovered. Research into the inflammatory mechanisms of the common cold has elucidated the complexity of the virus-host relation. Increasing evidence is also available for the central role of viruses in predisposing to complications. New antivirals for the treatment of colds are being developed, but optimum use of these agents would require rapid detection of the specific virus causing the infection. Although vaccines against many respiratory viruses could also become available, the ultimate prevention of the common cold seems to remain a distant aim.
“I love the doctors—they are dears; But must they spend such years and years Investigating such a lot Of illnesses which no one's got, When everybody, young and old, Is frantic with the common cold? And I will eat my only hat If they know anything of that!”1
The common cold is a conventional term for a mild upper respiratory illness, the hallmark symptoms of which are nasal stuffiness and discharge, sneezing, sore throat, and cough. Although the term tends to imply that there is a single cause for the illness, the common cold is actually a heterogeneous group of diseases caused by numerous viruses that belong to several different families. The common cold is usually a self-limited illness confined to the upper respiratory tract. However, in some patients the viral infection spreads to adjacent organs, resulting in different clinical manifestations, and, occasionally, colds predispose to bacterial complications.
Despite the usually benign nature of the illness, the common cold is an enormous economic burden on society in terms of visits to doctors and other health-care providers, treatments, and absences from work, school, or day care. Every year, in the USA, about 25 million people visit their family doctors with uncomplicated upper respiratory infections,2 and the common cold syndrome results in about 20 million days of absence from work and 22 million days of absence from school.3
Cause
Our knowledge about the cause of the common cold is largely derived from extensive family and community follow-up studies undertaken between the 1960s and the 1980s.4, 5, 6 Intensive research into the cause of respiratory illnesses during the 1950s and 1960s led to the discovery of adenovirus, parainfluenza virus, rhinovirus, respiratory syncytial virus (RSV), enterovirus, and coronavirus, in addition to the influenza viruses that had been identified earlier (figure 1 ). However, because during that era the techniques of viral detection were limited to virus isolation, the viral cause could be proven in only about 25% of patients with respiratory infections.7 Improvements in viral detection techniques during the past two decades, including various viral antigen detection methods and particularly the advent of PCR-based assays, have substantially increased the rates of viral detection in clinical specimens.8, 9
Figure 1.

Timeline of discovery of the common respiratory viruses
Transmission electron micrograph of three adenovirus particles shown in background.
© 2003 Science Photo Library
The relative proportions of different viruses in the cause of the common cold vary dependent on several factors, such as age, season, and viral sampling and detection methods. However, rhinoviruses have been consistently found to be the most common cause in all age groups, irrespective of the viral detection techniques used (panel ).8, 9, 10, 11, 12 Yearly, rhinoviruses account for about 30–50% of all respiratory illnesses,6, 9 but during the autumn peak season these viruses can cause up to 80% of all upper respiratory infections.8 More than 100 different serotypes of rhinoviruses have been identified, the relative prevalences of which seem to vary between different geographical areas and also over the course of time.10
Panel. Viral cause of the common cold6,9,12.
| Virus | Estimated annual proportion of cases |
|---|---|
| Rhinoviruses | 30–50% |
| Coronaviruses | 10–15% |
| Influenza viruses | 5–15% |
| Respiratory syncytial virus | 5% |
| Parainfluenza viruses | 5% |
| Adenoviruses | <5% |
| Enteroviruses | <5% |
| Metapneumovirus | Unknown |
| Unknown | 20–30% |
Although scarce, data are available on the role of coronaviruses as a cause of the common cold. These viruses are found in 7–18% of adults with upper respiratory infections.9, 13, 14 Parainfluenza viruses, RSV, adenoviruses, and enteroviruses all account for minor proportions of the common cold syndrome, but the range of illness caused by each of these viruses also includes other manifestations that can be more typical to these viruses.10, 12 Influenza is often regarded as a disease entity separate from the common cold. However, the clinical presentation of influenza ranges from an asymptomatic infection to severe illness and it therefore overlaps with the common cold. Mild instances of streptococcal pharyngitis are clinically indistinguishable from viral pharyngitis and can be misclassified as colds.
Despite the availability of sophisticated diagnostic methods, about 20–30% of colds remain without a proven viral cause,6, 9 probably due in part to suboptimum methods used in the collection, transportation, and assay of clinical specimens, resulting in underdetection of viruses known to exist. However, many colds could also be caused by infectious agents yet to be identified. This explanation gained much support after the recent discovery of a new virus in young children with respiratory infections.15 The relative effect of this virus, tentatively named human metapneumovirus, by comparison with other respiratory viruses is still undetermined. Findings of serological studies15 indicate that the virus has been circulating in human beings for at least 50 years, and by the age of 5 years virtually all children in the Netherlands are infected by it. Reports16, 17 from other countries suggest that human metapneumovirus has a worldwide distribution.
Two or more viruses are found simultaneously in about 5% of patients with colds.18 However, the rate of dual viral infection increases proportionally with the number of different diagnostic methods used,18 and whether or not all of these cases really represent simultaneous infections by two viruses is unclear. Positive findings by PCR, for instance, do not require the presence of live viruses, and viral genomic materials can be detected in patients long after the subsidence of clinical symptoms.19, 20
Epidemiology
The occurrence of the common cold shows clear seasonality. In temperate regions of the northern hemisphere, the frequency of respiratory infections increases rapidly in the autumn, remains fairly high throughout the winter, and decreases again in the spring. In tropical areas, most colds arise during the rainy season. The incidence of upper respiratory infections is inversely proportional to age (figure 2 ). On average, the youngest children have 6–8 and adults 2–4 colds per year.5, 7 During the first years of life, boys seem to have more respiratory infections than girls, but this difference is reversed later in life.5 Women who work outside the home have fewer infections that those who do not, which might be explained by greater exposure to children in those who stay at home.6 Day-care attendance is another major risk factor for respiratory illnesses in children, and the frequency of colds increases with the number of children in the group.21, 22, 23 However, frequent infections in the preschool years could lower the frequency of the common cold during school years.23 Some genetic factors might also affect an individual's susceptibility to respiratory infections, but any potential mechanisms remain largely unidentified.24, 25 Psychological stress is associated with susceptibility to the common cold in a dose-dependent manner.26 Finally, some reports27 indicate that heavy physical training increases the risk of respiratory infections, whereas moderate physical activity could decrease risk.
Figure 2.
Mean annual incidence of respiratory illnesses per person by age group5
Because of the central role of rhinoviruses in the cause of the common cold, the epidemiology of this condition largely parallels that of rhinoviruses. Although rhinoviruses can be detected throughout the year, the incidence of rhinovirus infections peaks during autumn, with a subsequent smaller outbreak in the spring.28, 29 Results of a follow-up study30 showed the high incidence of rhinovirus infections in children during the first years of life. By age 6 months, more than 20% of children had had a laboratory-confirmed rhinovirus infection. By age 2 years, rhinovirus infection had been documented by virus culture or PCR in 79% of the children, and 91% had antibodies against rhinoviruses. The average annual rate of rhinovirus infection is estimated at about 0·8 per person.28 However, this figure is likely an underestimate, and calculations based on PCR data might yield a substantially higher rate.
Many other viruses that cause the common cold tend to have their own patterns of seasonality. In most temperate countries, RSV usually causes outbreaks around the turn of the year,31 but other patterns have also been documented.32 Influenza epidemics also typically occur in the winter in the northern hemisphere, often overlapping with RSV.31, 33
The transmission of viruses that cause upper respiratory infections can occur by any of the three major mechanisms: 1) hand contact with secretions that contain the virus, either directly from an infected person or indirectly from environmental surfaces; 2) small-particle aerosols lingering in the air for an extended time; or 3) direct hit by large-particle aerosols from an infected person. Although all these mechanisms are likely to be involved in the spread of any respiratory virus, the primary routes of transmission do differ between viruses. For instance, influenza viruses are thought to be spread mainly via small-particle aerosols,34 whereas hand contact followed by self-inoculation with the virus into the nose or eye has been reported as the most efficient way of transmission for rhinoviruses.35 However, aerosol transmission of rhinoviruses has also been clearly documented.36
Pathogenesis
The pathogenesis of the common cold involves a complex interplay between replicating viruses and the host's inflammatory response. The detailed pathogenetic mechanisms of the various respiratory viruses can be very different from each other, as indicated by the fact that the primary site of replication of influenza viruses is in the tracheobronchial epithelium,34 whereas rhinovirus replication starts predominantly in the nasopharynx.37 The available evidence,38 albeit scarce, does not lend support to the popular belief that colds are associated with chilling or exposure to a cold environment.
Much of our understanding of the pathogenetic events in the common cold is derived from studies of volunteers infected with rhinoviruses.35, 36, 37 Rhinovirus infection begins with the deposition of viruses in the anterior nasal mucosa or in the eye, from where they get to the nose via the lacrimal duct. The viruses are then transported to the posterior nasopharynx by mucociliary action. In the adenoid area, the viruses gain entrance to epithelial cells by binding to specific receptors on the cells. About 90% of rhinovirus serotypes use intercellular adhesion molecule-1 (ICAM-1) as their receptor.39, 40 Once inside the epithelial cell, the virus starts to replicate rapidly. Progeny viruses can be detected within 8–10 h after intranasal inoculation of rhinoviruses.41 The infectious dose of rhinovirus is small,42 and up to 95% of individuals without antibodies against the specific viral serotype are infected after intranasal challenge.43 However, for reasons still unknown, all infections do not lead to clinical illness; symptomatic colds develop in only 75% of infected persons.43 The shedding of rhinoviruses peaks on the second day after intranasal inoculation and decreases rapidly thereafter, but small amounts of viruses can be discovered in nasal secretions for up to 3 weeks after infection.37, 44
Viral infection of the nasal mucosa results in vasodilation and increased vascular permeability, which in turn cause nasal obstruction and rhinorrhoea, which are the main clinical symptoms of the common cold. Cholinergic stimulation leads to increased mucous gland secretion and sneezing. The detailed mechanisms by which viral infection causes such changes in the nasal mucosa are still incompletely understood. Distinct differences exist in the degree of epithelial destruction between various respiratory viruses. Whereas influenza viruses and adenoviruses cause extensive damage to the respiratory epithelium,45, 46 no histopathological changes are observed in nasal biopsy specimens from individuals infected with rhinoviruses.37 The absence of epithelial destruction during rhinovirus infections has led to the idea that the clinical symptoms of the common cold might not be caused by a direct cytopathic effect of the viruses, but instead are primarily caused by the inflammatory response of the host. Extensive research into the role of inflammatory mediators in the pathogenesis of the common cold has produced evidence for increased concentrations of several mediators, such as kinins, leukotrienes, histamine, interleukins 1, 6, and 8, tumour necrosis factor, and RANTES (regulated by activation normal T cell expressed and secreted) in the nasal secretions of patients with colds.47, 48, 49, 50, 51, 52 The concentrations of interleukin 6 and interleukin 8 in nasal secretions correlate with the severity of the symptoms.53, 54 The host response mechanisms triggered by viral infection are, however, interrelated, extremely complex, and far from resolved. For example, in respiratory epithelial cells infected with RSV, interleukin 1 β and tumour necrosis factor α induce the synthesis of interleukin 8 at 24 h, but partly inhibit the synthesis of this cytokine at 48 h.55
Results of studies have shown that the effect of the common cold in the upper respiratory tract is not limited to the nasal cavity, but that paranasal sinuses are also frequently affected. CT scans and plain radiographs of sinuses obtained during the early course of illness in adults with colds show substantial abnormalities that usually resolve spontaneously without antibiotic treatment.56, 57 These findings imply that most sinus abnormalities observed during the common cold are not evidence of a bacterial complication, but are instead part of the normal course of illness. Findings of an experimental study58 showed that blowing of the nose creates such a high intranasal pressure that it could propel fluid from the nasal cavity to the paranasal sinuses. Furthermore, rhinovirus RNA has been detected in sinus aspirates even in the absence of bacteria,59 and results of a study60 that used in-situ hybridisation provide evidence for the presence of rhinovirus in the epithelial cells of maxillary sinuses in patients with acute sinusitis.
In adults and children, viral infection of the upper respiratory tract often causes dysfunction of the Eustachian tube, which is considered the most important factor in the pathogenesis of acute otitis media.61 Great middle ear negative pressures are recorded in most preschool and school-aged children with colds.62, 63 In adult volunteers challenged with rhinoviruses or influenza A viruses, normal Eustachian tube function deteriorated in 50–80% of individuals.64, 65
Several respiratory viruses—eg, influenza viruses, RSV, and parainfluenza viruses—can also infect the lower respiratory tract, but the ability of rhinoviruses to replicate in the lower airways has been much debated. Although rhinoviruses have been detected in secretions obtained from the lower airways by bronchoscopy,66 potential viral contamination from the upper respiratory tract was not ruled out until recently, when investigators of one study67 of adult volunteers infected with rhinoviruses avoided potential contamination from the upper airways by use of in-situ hybridisation on bronchial biopsies. The findings of the study showed conclusively that rhinoviruses are able to replicate in the lower airways.
Clinical manifestations
The symptoms of the common cold arise after an incubation period that can vary considerably between different viruses. In experimental rhinovirus infections, the onset of symptoms has been reported to occur as soon as 10–12 h after intranasal inoculation of the virus,41 whereas the incubation period of influenza ranges from 1 to 7 days.34 Generally, the severity of the symptoms increases rapidly, peaks within 2–3 days after infection, and decreases soon after. The mean duration of the common cold is 7–10 days, but in a proportion of patients some symptoms can still be present after 3 weeks.8, 68, 69
Rhinovirus infections typically start with a sore throat, which is soon accompanied by nasal stuffiness and discharge, sneezing, and cough. The soreness of the throat usually disappears quickly, whereas the initial watery rhinorrhoea turns thicker and more purulent.70 The purulence of the nasal discharge is not associated with changes in the nasopharyngeal bacterial flora71 and is not considered to indicate a simultaneous bacterial infection of the nasal mucosa. Fever is an infrequent finding during rhinovirus infections in adults, but it is fairly common in children with upper respiratory infections of any cause72, 73 Other symptoms associated with the cold syndrome include hoarseness, headache, malaise, and lethargy. Myalgia is an occasional complaint in patients with colds, although it is a more typical feature of influenza infection.34
Although the common cold is usually a self-limited illness of short duration, the viral infection is sometimes accompanied by a bacterial complication. In children, the most common bacterial complication is acute otitis media, which occurs in about 20% of children with viral upper respiratory infections.74, 75 The seasonal incidence rates of otitis media closely parallel the general occurrence of viral respiratory infections,76, 77 and the complication is diagnosed most frequently on days 3 or 4 after the onset of upper respiratory symptoms.78, 79 Findings of studies indicate that respiratory viruses play a crucial part in the development of acute otitis media,61 and the detection rates of different viruses in the middle-ear fluid suggest that at least some viruses actively invade the middle ear and contribute to the inflammatory process in the middle ear mucosa.80, 81, 82, 83
Other common bacterial complications of viral upper respiratory infections include sinusitis and pneumonia. Sinusitis has been estimated to occur as a complication in 0·5–2% of colds,12 but on the basis of recent evidence for the high incidence of sinus abnormalities during apparently uncomplicated colds it is difficult to ascertain whether changes in paranasal sinuses represent real bacterial complications or whether they are part of the natural history of the common cold.56, 57 Pneumonia associated with a viral upper respiratory infection is often a true bacterial complication of the predisposing viral illness, but it can also be a pure extension of the viral illness to the pulmonary level. Research into the microbial cause of pneumonia has suggested that mixed viral-bacterial infections are common, especially in children.84, 85
Findings of several studies indicate a clear association between viral respiratory infections and acute exacerbations of asthma in both adults and children. In a study86 of adult patients with asthma, symptoms of the common cold were reported in 80% of episodes of wheezing or dyspnoea, and rhinoviruses accounted for about 60% of those asthma exacerbations for which the viral cause could be identified. The central role of rhinoviruses in triggering acute exacerbations of asthma has also been well documented in children.87 In the elderly population, the overall morbidity due to respiratory viruses other than influenza is often under-rated, although results of surveillance studies13 indicate that two thirds of elderly patients with colds can be expected to develop lower respiratory illness. Individuals with chronic obstructive pulmonary disease (COPD) form another important risk group for viral infections.88 Although the frequencies of colds are similar in patients with and without COPD, the use of medical resources, including hospital admissions and visits to emergency clinics, during viral respiratory illnesses is substantially increased in patients with COPD.89 In immunocompromised patients, RSV is usually the most common cause of severe viral respiratory illness, but rhinovirus infections have also been associated with severe and even fatal lower respiratory tract disease.90
Diagnosis
In most instances, the clinical diagnosis of the common cold is simple and can be made reliably by adult patients themselves.8 However, diagnosis is sometimes problematic in infants and young children who are not capable of expressing their symptoms. Diagnosis in infants is especially difficult in cases in which fever is the leading symptom during the early phase of the infection, and the doctor is challenged to distinguish benign viral infections from severe invasive bacterial infections. Allergic or vasomotor rhinitis can sometimes mimic the common cold, but usually these conditions can be differentiated easily. The soreness of the throat caused by streptococcal pharyngitis often resembles the initial symptoms of the common cold. However, nasal stuffiness and discharge, which are the primary symptoms of the common cold, are untypical to streptococcal pharyngitis. Although most cases of exudative tonsillitis in children are caused by viruses, the clinical findings on inspection of the pharynx cannot reliably distinguish bacterial from viral tonsillitis.91 In children, intranasal foreign bodies should be searched for in cases of persistent nasal discharge, particularly if the discharge is unilateral.
Although respiratory infections caused by different viruses tend to have some variations in their typical clinical presentations, the wide range of the clinical manifestations of each virus makes it virtually impossible to ascertain the specific virus causing the problem in an individual patient with the common cold on clinical grounds alone.14, 34, 92 Even for influenza, which is often regarded as a distinct disease entity among respiratory viral infections, the positive predictive value of clinical signs and symptoms has ranged between 27% and 79%.93, 94
Methods for identification of viruses include viral culture, antigen detection, and PCR. Isolation of viruses in cell cultures is considered the gold standard for detection, but has little value for clinical practice because of the slowness of the process. Immunoperoxidase staining of the cultures with monoclonal antibodies speeds up viral identification substantially, with results usually available within 48 h.95 Various antigen detection tests are frequently used to identify influenza viruses, parainfluenza viruses, RSV, and adenoviruses,96, 97 but these techniques cannot be routinely used to detect rhinoviruses because of the huge number of different serotypes that exist. Recently developed rapid antigen detection kits for influenza and RSV can provide results within 15–30 min,98, 99 but there are concerns about the real-life sensitivity and specificity of these tests.100 PCR has proved valuable in diagnosis of viral infections in general, and especially rhinovirus infections, for which other methods have been suboptimum.8, 9, 101, 102 However, PCR-based techniques are still too laborious for use in everyday clinical practice, and the extreme sensitivity of PCR can pose problems for the interpretation of results.19, 20
Nasopharyngeal aspirates and nasal wash specimens are usually considered the specimens of choice for the detection of respiratory viruses, but nasal and throat swabs are also often used because of their greater feasibility.103, 104, 105, 106 Scarce data are available on the optimum sampling methods, and the best sites to collect specimens from for viral detection could differ between viruses.
Treatment
Since the common cold is caused by a multitude of different virus types with varying pathogenetic mechanisms, that an effective universal treatment for this disorder has not been developed is understandable. The symptomatic treatment of colds has been aimed at relieving the most disturbing symptoms of the illness, and hundreds of different over-the-counter preparations are available.107, 108 Although antibiotics are not effective against viruses, they are widely used in the treatment of uncomplicated viral upper respiratory infections.2, 109
Most patients find nasal stuffiness and discharge the most bothersome symptoms of the common cold.8 Nasal blockage can be effectively reduced with intranasally or orally administered decongestants.110 First-generation (but not second-generation) antihistamines reduce sneezing and rhinorrhoea, probably because of their anticholinergic rather than antihistamine effects.111 Results of several studies have also shown the efficacy of local ipratropium in reducing rhinorrhoea.112 Theoretically, corticosteroids as potent anti-inflammatory agents could be thought to effectively reduce nasal symptoms, but results of clinical studies of either intranasal or oral steroids have shown no clinical benefit.44, 69, 113 The use of intranasal steroids in children during rhinovirus infection could even increase the risk of acute otitis media.114 Cough medications, both antitussives and mucolytic agents, are frequently used, although their efficacy has been poorly shown.115 Nonsteroidal anti-inflammatory drugs reduce fever and soreness of the throat, and might also have some beneficial effect on cough.116 Data on the efficacy of zinc in reducing the severity and duration of colds are still inconclusive.117

At present, specific antiviral treatments for respiratory viruses are commercially available only for influenza viruses. The usability of amantadine and rimantadine is limited by their side-effects, their inefficacy against influenza B viruses, and the rapid development of resistant viral strains during treatment.118 The new influenza-specific antivirals, zanamivir and oseltamivir, have fewer side-effects and are effective against both influenza A and B viruses. When treatment is initiated within 48 h of the onset of symptoms, the duration of the clinical illness is reduced by 1–2 days with either of these drugs.119, 120 Scarce evidence exists for the efficacy of these drugs in the prevention of bacterial complications, especially in high-risk patients,121 but early treatment of influenza with oseltamivir does reduce the development of acute otitis media in children by more than 40%.122
Because of the leading role of rhinoviruses in the common cold, effective antivirals against rhinoviruses could be expected to have the greatest effect in the treatment of this disease. In the 1980s, much hope was laid on the use of interferon, but disappointingly it provided no benefit in the treatment of naturally occurring colds or experimental rhinovirus infections.123, 124 The discovery of ICAM-1 as the main cellular receptor for rhinoviruses led to attempts to block the attachment of the virus to the receptor, using a recombinant soluble decoy ICAM-1. Findings of clinical trials showed that this approach could reduce the severity of experimental rhinovirus infections, but the effect was modest.125
Recent advances in antirhinoviral drugs include the development of pleconaril, a novel viral capsid binder, and ruprintrivir, a human rhinovirus 3C protease inhibitor.126, 127, 128 Pleconaril is administered orally and is active against a wide range of rhinoviruses and enteroviruses. Findings of early clinical trials have indicated that when pleconaril treatment is instituted within 24–36 h of the onset of symptoms, the duration of illness is reduced by 1–1·5 days.129
Increasing knowledge about the central role of the host inflammatory response in producing the symptoms of the common cold has led to attempts to treat colds with combinations of antiviral and anti-inflammatory agents.130 In a recent trial131 in adults challenged with rhinoviruses, the combination of intranasal interferon with oral chlorphenamine and ibuprofen showed effect in reducing not only nasal but also several other symptoms of the illness.
Prevention
The diversity of the viral cause of the common cold has hampered prevention as well as treatment initiatives. In the absence of a suitable common antigen across the wide range of rhinovirus serotypes, the prospects for development of a vaccine against rhinoviruses seem poor. Influenza is the only respiratory infection for which a vaccine is commercially available. In addition to the present inactivated influenza vaccine, which is administered intramuscularly, new types of influenza vaccines for intranasal administration have been developed.132, 133, 134 Several types of vaccines against RSV and parainfluenza viruses are also being developed and are in early clinical trials.135, 136, 137
The antiviral drug approach to prevention of respiratory infections is currently to influenza, for which the efficacy of the specific antivirals has been shown for both seasonal prophylaxis and post-exposure prophylaxis within families.138, 139, 140, 141, 142 Contrary to the lack of effect in the treatment of rhinovirus infections, the prophylactic efficacy of intranasally administered interferon has been well demonstrated, but the unacceptably high rate of nasal adverse events during extended administration has decreased enthusiasm about its use for prevention of colds.143 In a proof of concept study,144 intranasally administered immunoglobulin reduced episodes of rhinitis in children, but no further investigations of passive immunisation of the nasal mucosa have been published.
Despite the widespread use of vitamin C and extracts of the plant Echinacea for the prevention of the common cold, conclusive evidence for such an effect is still lacking.145, 146 In view of the available data, it seems obvious that complete prevention of colds would only be possible by total long-term isolation from the community.147 While waiting for the next ship bound for Antarctica, however, many people might find solace in a report148 that suggests that intake of wine, especially red wine, may have a protective effect against the common cold.
Future considerations
Together with the substantial proportion of colds without a proven microbial cause, the recent discovery of human metapneumovirus suggests that other important respiratory viruses might still remain undetected, and the use of highly sophisticated molecular techniques could lead to new discoveries. Further research into the host inflammatory response and viral-bacterial interaction during the common cold might reveal pathways that could serve as targets for intervention to better alleviate the symptoms and to prevent the development of complications. Optimum use of the newly available and forthcoming antiviral agents is likely to increase the need for simple, rapid, inexpensive, and accurate point-of-care tests to identify the specific virus causing the infection on an individual level. In this era of ever-increasing antimicrobial resistance of bacteria, efforts aimed at the education of people about the ineffectiveness of antibiotics in the treatment of uncomplicated colds should be intensified.
Search strategy
The data for this seminar were identified by computer-aided searches of PubMed and Cochrane Library databases, using keywords relevant to the different sections. We also reviewed the journal reference lists and standard textbooks and used our existing knowledge of the primary publications in this area and new data presented at international scientific meetings. We then selected reports that, in our understanding, have contributed substantially to the current knowledge of various aspects of the common cold, and that also have potential for further reading.
Conflict of interest statement
T Heikkinen has received support for research on the epidemiology of respiratory viral infections from Wyeth and GlaxoSmithKline; has participated as one of the local investigators in Finland in international multicentre studies of zanamivir sponsored by GlaxoSmithKline; and has received honoraria for lectures in the area of respiratory infections at academic meetings organised by various pharmaceutical companies. None of these sources have contributed to the content of this Seminar. A Järvinen has no conflict of interest with respect to this Seminar.
References
- 1.Herbert AP. The common cold. In: Look back and laugh. Methuen; London: 1960. pp. 115–117. [Google Scholar]
- 2.Gonzales R, Malone DC, Maselli JH, Sande MA. Excessive antibiotic use for acute respiratory infections in the United States. Clin Infect Dis. 2001;33:757–762. doi: 10.1086/322627. [DOI] [PubMed] [Google Scholar]
- 3.Adams PF, Hendershot GE, Marano MA. Current estimates from the National Health Interview Survey. Vital Health Stat. 1999;10:1996. [PubMed] [Google Scholar]
- 4.Fox JP, Hall CE, Cooney MK, Luce RE, Kronmal RA. The Seattle virus watch, 2: objectives, study population and its observation, data processing and summary of illnesses. Am J Epidemiol. 1972;96:270–285. doi: 10.1093/oxfordjournals.aje.a121458. [DOI] [PubMed] [Google Scholar]
- 5.Monto AS, Ullman BM. Acute respiratory illness in an American community: the Tecumseh study. JAMA. 1974;227:164–169. [PubMed] [Google Scholar]
- 6.Monto AS, Sullivan KM. Acute respiratory illness in the community: frequency of illness and the agents involved. Epidemiol Infect. 1993;110:145–160. doi: 10.1017/s0950268800050779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monto AS. Studies of the community and family: acute respiratory illness and infection. Epidemiol Rev. 1994;16:351–373. doi: 10.1093/oxfordjournals.epirev.a036158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arruda E, Pitkäranta A, Witek TJ, Jr, Doyle CA, Hayden FG. Frequency and natural history of rhinovirus infections in adults during autumn. J Clin Microbiol. 1997;35:2864–2868. doi: 10.1128/jcm.35.11.2864-2868.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mäkelä MJ, Puhakka T, Ruuskanen O. Viruses and bacteria in the etiology of the common cold. J Clin Microbiol. 1998;36:539–542. doi: 10.1128/jcm.36.2.539-542.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monto AS, Bryan ER, Ohmit S. Rhinovirus infections in Tecumseh, Michigan: frequency of illness and numbers of serotypes. J Infect Dis. 1987;156:43–49. doi: 10.1093/infdis/156.1.43. [DOI] [PubMed] [Google Scholar]
- 11.Vesa S, Kleemola M, Blomqvist S. Epidemiology of documented viral respiratory infections and acute otitis media in a cohort of children followed from two to twenty-four months of age. Pediatr Infect Dis J. 2001;20:574–581. doi: 10.1097/00006454-200106000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Gwaltney JM., Jr . The common cold. In: Mandell GL, Bennett JE, Dolin R, editors. 5th edn. Churchill Livingstone; Philadelphia: 2000. pp. 651–665. (Principles and practice of infectious diseases). [Google Scholar]
- 13.Larson HE, Reed SE, Tyrrell DAJ. Isolation of rhinoviruses and coronaviruses from 38 colds in adults. J Med Virol. 1980;5:221–229. doi: 10.1002/jmv.1890050306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicholson KG, Kent J, Hammersley V, Cancio E. Acute viral infections of upper respiratory tract in elderly people living in the community: comparative, prospective, population based study of disease burden. BMJ. 1997;315:1060–1064. doi: 10.1136/bmj.315.7115.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Hoogen BG, de Jong JC, Groen J. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nissen MD, Siebert DJ, Mackay IM, Sloots TP, Withers SJ. Evidence of human metapneumovirus in Australian children. Med J Aust. 2002;176:188. doi: 10.5694/j.1326-5377.2002.tb04354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peret TCT, Boivin G, Li Y. Characterization of human metapneumoviruses isolated from patients in North America. J Infect Dis. 2002;185:1660–1663. doi: 10.1086/340518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drews AL, Atmar RL, Glezen WP. Dual respiratory virus infections. Clin Infect Dis. 1997;25:1421–1429. doi: 10.1086/516137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnston SL, Sanderson G, Pattemore PK. Use of polymerase chain reaction for diagnosis of picornavirus infection in subjects with and without respiratory symptoms. J Clin Microbiol. 1993;31:111–117. doi: 10.1128/jcm.31.1.111-117.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nokso-Koivisto J, Kinnari TJ, Lindahl P, Hovi T, Pitkäranta A. Human picornavirus and coronavirus RNA in nasopharynx of children without concurrent respiratory symptoms. J Med Virol. 2002;66:417–420. doi: 10.1002/jmv.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wald ER, Dashefsky B, Byers C, Guerra N, Taylor F. Frequency and severity of infections in day care. J Pediatr. 1988;112:540–546. doi: 10.1016/s0022-3476(88)80164-1. [DOI] [PubMed] [Google Scholar]
- 22.Alho OP, Koivu M, Sorri M, Rantakallio P. Risk factors for recurrent acute otitis media and respiratory infection in infancy. Int J Pediatr Otorhinolaryngol. 1990;19:151–161. doi: 10.1016/0165-5876(90)90221-c. [DOI] [PubMed] [Google Scholar]
- 23.Ball TM, Holberg CJ, Aldous MB, Martinez FD, Wright AL. Influence of attendance at day care on the common cold from birth through 13 years of age. Arch Pediatr Adolesc Med. 2002;156:121–126. doi: 10.1001/archpedi.156.2.121. [DOI] [PubMed] [Google Scholar]
- 24.Casselbrant ML, Mandel EM, Fall PA. The heritability of otitis media: a twin and triplet study. JAMA. 1999;282:2125–2130. doi: 10.1001/jama.282.22.2125. [DOI] [PubMed] [Google Scholar]
- 25.Pitkäranta A, Nokso-Koivisto J, Jäntti V. Lowered yields of virus-induced interferon production in leukocyte cultures and risk of recurrent respiratory infections in children. J Clin Virol. 1999;14:199–205. doi: 10.1016/S1386-6532(99)00056-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen S, Tyrrell DAJ, Smith AP. Psychological stress and susceptibility to the common cold. N Engl J Med. 1991;325:606–612. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- 27.Nieman DC. Exercise, upper respiratory tract infection, and the immune system. Med Sci Sports Exerc. 1994;26:128–139. doi: 10.1249/00005768-199402000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Gwaltney JM, Jr, Hendley JO, Simon G, Jordan WS., Jr Rhinovirus infections in an industrial population, 1:the occurrence of illness. N Engl J Med. 1966;275:1261–1268. doi: 10.1056/NEJM196612082752301. [DOI] [PubMed] [Google Scholar]
- 29.Monto AS, Shope TC, Schwartz SA, Albrecht JK. Intranasal interferon-alpha 2b for seasonal prophylaxis of respiratory infection. J Infect Dis. 1986;154:128–133. doi: 10.1093/infdis/154.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blomqvist S, Roivainen M, Puhakka T, Kleemola M, Hovi T. Virological and serological analysis of rhinovirus infections during the first two years of life in a cohort of children. J Med Virol. 2002;66:263–268. doi: 10.1002/jmv.2140. [DOI] [PubMed] [Google Scholar]
- 31.Monto AS, Cavallaro JJ. The Tecumseh study of respiratory illness, 2: patterns of occurrence of infection with respiratory pathogens, 1965–1969. Am J Epidemiol. 1971;94:280–289. doi: 10.1093/oxfordjournals.aje.a121321. [DOI] [PubMed] [Google Scholar]
- 32.Waris M. Pattern of respiratory syncytial virus epidemics in Finland: two-year cycles with alternating prevalence of groups A and B. J Infect Dis. 1991;163:464–469. doi: 10.1093/infdis/163.3.464. [DOI] [PubMed] [Google Scholar]
- 33.Izurieta HS, Thompson WW, Kramarz P. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med. 2000;342:232–239. doi: 10.1056/NEJM200001273420402. [DOI] [PubMed] [Google Scholar]
- 34.Nicholson KG. Human influenza. In: Nicholson KG, Webster RG, Hay AJ, editors. Blackwell Science; Oxford: 1998. pp. 219–264. (Textbook of influenza). [Google Scholar]
- 35.Gwaltney JM, Jr, Moskalski PB, Hendley JO. Hand-to-hand transmission of rhinovirus colds. Ann Intern Med. 1978;88:463–467. doi: 10.7326/0003-4819-88-4-463. [DOI] [PubMed] [Google Scholar]
- 36.Dick EC, Jennings LC, Mink KA, Wartgow CD, Inhorn SL. Aerosol transmission of rhinovirus colds. J Infect Dis. 1987;156:442–448. doi: 10.1093/infdis/156.3.442. [DOI] [PubMed] [Google Scholar]
- 37.Winther B, Gwaltney JM, Jr, Mygind N, Turner RB, Hendley JO. Sites of rhinovirus recovery after point inoculation of the upper airway. JAMA. 1986;256:1763–1767. [PubMed] [Google Scholar]
- 38.Douglas RG, Jr, Lindgren KM, Couch RB. Exposure to cold environment and rhinovirus common cold: failure to demonstrate effect. N Engl J Med. 1968;279:742–747. [Google Scholar]
- 39.Staunton DE, Merluzzi VJ, Rothlein R. A cell adhesion molecule, ICAM-1, is the major surface receptor for rhinoviruses. Cell. 1989;56:849–853. doi: 10.1016/0092-8674(89)90689-2. [DOI] [PubMed] [Google Scholar]
- 40.Winther B, Arruda E, Witek TJ. Expression of ICAM-1 in nasal epithelium and levels of soluble ICAM-1 in nasal lavage fluid during human experimental rhinovirus infection. Arch Otolaryngol Head Neck Surg. 2002;128:131–136. doi: 10.1001/archotol.128.2.131. [DOI] [PubMed] [Google Scholar]
- 41.Harris JM, II, Gwaltney JM., Jr Incubation periods of experimental rhinovirus infection and illness. Clin Infect Dis. 1996;23:1287–1290. doi: 10.1093/clinids/23.6.1287. [DOI] [PubMed] [Google Scholar]
- 42.Hendley JO, Gwaltney JM., Jr Mechanisms of transmission of rhinovirus infections. Epidemiol Rev. 1988;10:243–258. [PubMed] [Google Scholar]
- 43.Gwaltney JM, Jr, Hayden FG. Psychological stress and the common cold. N Engl J Med. 1992;326:644–645. doi: 10.1056/NEJM199202273260915. [DOI] [PubMed] [Google Scholar]
- 44.Gustafson LM, Proud D, Hendley JO, Hayden FG, Gwaltney JM., Jr Oral prednisone therapy in experimental rhinovirus infections. J Allergy Clin Immunol. 1996;97:1009–1014. doi: 10.1016/s0091-6749(96)80077-7. [DOI] [PubMed] [Google Scholar]
- 45.Treanor JJ. Influenza virus. In: Mandell GL, Bennett JE, Dolin R, editors. 5th edn. Churchill Livingstone; Philadelphia: 2000. pp. 1823–1849. (Principles and practice of infectious diseases). [Google Scholar]
- 46.Cherry JD. Adenoviruses. In: Feigin RD, Cherry JD, editors. 4th edn. W B Saunders; Philadelphia: 1998. pp. 1666–1684. (Textbook of pediatric infectious diseases). [Google Scholar]
- 47.Naclerio RM, Proud D, Lichtenstein LM. Kinins are generated during experimental rhinovirus colds. J Infect Dis. 1988;157:133–142. doi: 10.1093/infdis/157.1.133. [DOI] [PubMed] [Google Scholar]
- 48.Welliver RC, Wong DT, Sun M. The development of respiratory syncytial virus-specific IgE and the release of histamine in nasopharyngeal secretions after infection. N Engl J Med. 1981;305:841–846. doi: 10.1056/NEJM198110083051501. [DOI] [PubMed] [Google Scholar]
- 49.Volovitz B, Faden H, Ogra PL. Release of leukotriene C4 in respiratory tract during acute viral infection. J Pediatr. 1988;112:218–222. doi: 10.1016/s0022-3476(88)80058-1. [DOI] [PubMed] [Google Scholar]
- 50.Noah TL, Henderson FW, Wortman IA. Nasal cytokine production in viral acute upper respiratory infection of childhood. J Infect Dis. 1995;171:584–592. doi: 10.1093/infdis/171.3.584. [DOI] [PubMed] [Google Scholar]
- 51.Fritz RS, Hayden FG, Calfee DP. Nasal cytokine and chemokine responses in experimental influenza A virus infection: results of a placebo-controlled trial of intravenous zanamivir treatment. J Infect Dis. 1999;180:586–593. doi: 10.1086/314938. [DOI] [PubMed] [Google Scholar]
- 52.Saito T, Deskin RW, Casola A. Respiratory syncytial virus induces selective production of the chemokine RANTES by upper airway epithelial cells. J Infect Dis. 1997;175:497–504. doi: 10.1093/infdis/175.3.497. [DOI] [PubMed] [Google Scholar]
- 53.Zhu Z, Tang W, Ray A. Rhinovirus stimulation of interleukin-6 in vivo and in vitro: evidence for nuclear factor B-dependent transcriptional activation. J Clin Invest. 1996;97:421–430. doi: 10.1172/JCI118431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turner RB, Weingand KW, Yeh CH, Leedy DW. Association between interleukin-8 concentration in nasal secretions and severity of symptoms of experimental rhinovirus colds. Clin Infect Dis. 1998;26:840–846. doi: 10.1086/513922. [DOI] [PubMed] [Google Scholar]
- 55.Patel JA, Jiang Z, Nakajima N, Kunimoto M. Autocrine regulation of interleukin-8 by interleukin-1 in respiratory syncytial virus-infected pulmonary epithelial cells in vitro. Immunology. 1998;95:501–506. doi: 10.1046/j.1365-2567.1998.00640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gwaltney JM, Jr, Phillips CD, Miller RD, Riker DK. Computed tomographic study of the common cold. N Engl J Med. 1994;330:25–30. doi: 10.1056/NEJM199401063300105. [DOI] [PubMed] [Google Scholar]
- 57.Puhakka T, Mäkelä MJ, Alanen A. Sinusitis in the common cold. J Allergy Clin Immunol. 1998;102:403–408. doi: 10.1016/S0091-6749(98)70127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gwaltney JM, Jr, Hendley JO, Phillips CD. Nose blowing propels nasal fluid into the paranasal sinuses. Clin Infect Dis. 2000;30:387–391. doi: 10.1086/313661. [DOI] [PubMed] [Google Scholar]
- 59.Pitkäranta A, Arruda E, Malmberg H, Hayden FG. Detection of rhinovirus in sinus brushings of patients with acute community-acquired sinusitis by reverse transcription-PCR. J Clin Microbiol. 1997;35:1791–1793. doi: 10.1128/jcm.35.7.1791-1793.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pitkäranta A, Starck M, Savolainen S. Rhinovirus RNA in the maxillary sinus epithelium of adult patients with acute sinusitis. Clin Infect Dis. 2001;33:909–911. doi: 10.1086/322678. [DOI] [PubMed] [Google Scholar]
- 61.Heikkinen T. The role of respiratory viruses in otitis media. Vaccine. 2000;19(suppl 1):S51–S55. doi: 10.1016/s0264-410x(00)00278-4. [DOI] [PubMed] [Google Scholar]
- 62.Sanyal MA, Henderson FW, Stempel EC, Collier AM, Denny FW. Effect of upper respiratory tract infection on Eustachian tube ventilatory function in the preschool child. J Pediatr. 1980;97:11–15. doi: 10.1016/s0022-3476(80)80121-1. [DOI] [PubMed] [Google Scholar]
- 63.Winther B, Hayden FG, Arruda E. Viral respiratory infection in schoolchildren: effects on middle ear pressure. Pediatrics. 2002;109:826–832. doi: 10.1542/peds.109.5.826. [DOI] [PubMed] [Google Scholar]
- 64.McBride TP, Doyle WJ, Hayden FG, Gwaltney JM., Jr Alterations of the eustachian tube, middle ear, and nose in rhinovirus infection. Arch Otolaryngol Head Neck Surg. 1989;115:1054–1059. doi: 10.1001/archotol.1989.01860330044014. [DOI] [PubMed] [Google Scholar]
- 65.Doyle WJ, Skoner DP, Hayden F. Nasal and otologic effects of experimental influenza A virus infection. Ann Otol Rhinol Laryngol. 1994;103:59–69. doi: 10.1177/000348949410300111. [DOI] [PubMed] [Google Scholar]
- 66.Halperin SA, Eggleston PA, Hendley JO. Pathogenesis of lower respiratory tract symptoms in experimental rhinovirus infection. Am Rev Respir Dis. 1983;128:806–810. doi: 10.1164/arrd.1983.128.5.806. [DOI] [PubMed] [Google Scholar]
- 67.Papadopoulos NG, Bates PJ, Bardin PG. Rhinoviruses infect the lower airways. J Infect Dis. 2000;181:1875–1884. doi: 10.1086/315513. [DOI] [PubMed] [Google Scholar]
- 68.Gwaltney JM, Jr, Hendley JO, Simon G, Jordan WS., Jr Rhinovirus infections in an industrial population, 2: characteristics of illness and antibody response. JAMA. 1967;202:494–500. [PubMed] [Google Scholar]
- 69.Puhakka T, Mäkelä MJ, Malmström K. The common cold: effects of intranasal fluticasone propionate treatment. J Allergy Clin Immunol. 1998;101:726–731. doi: 10.1016/S0091-6749(98)70301-X. [DOI] [PubMed] [Google Scholar]
- 70.Igarashi Y, Skoner DP, Doyle WJ. Analysis of nasal secretions during experimental rhinovirus upper respiratory infections. J Allergy Clin Immunol. 1993;92:722–731. doi: 10.1016/0091-6749(93)90016-9. [DOI] [PubMed] [Google Scholar]
- 71.Winther B, Brofeldt S, Gronborg H. Study of bacteria in the nasal cavity and nasopharynx during naturally acquired common colds. Acta Otolaryngol. 1984;98:315–320. doi: 10.3109/00016488409107569. [DOI] [PubMed] [Google Scholar]
- 72.Putto A, Ruuskanen O, Meurman O. Fever in respiratory virus infections. Am J Dis Child. 1986;140:1159–1163. doi: 10.1001/archpedi.1986.02140250085040. [DOI] [PubMed] [Google Scholar]
- 73.Dick EC, Inhorn SL, Glezen WP. Rhinoviruses. In: Feigin RD, Cherry JD, editors. 4th edn. W B Saunders; Philadelphia: 1998. pp. 1839–1865. (Textbook of pediatric infectious diseases). [Google Scholar]
- 74.Heikkinen T, Ruuskanen O, Ziegler T, Waris M, Puhakka H. Short-term use of amoxicillin-clavulanate during upper respiratory tract infection for prevention of acute otitis media. J Pediatr. 1995;126:313–316. doi: 10.1016/s0022-3476(95)70569-4. [DOI] [PubMed] [Google Scholar]
- 75.Tapiainen T, Luotonen L, Kontiokari T, Renko M, Uhari M. Xylitol administered only during respiratory infections failed to prevent acute otitis media. Pediatrics. 2002;109:e19. doi: 10.1542/peds.109.2.e19. [DOI] [PubMed] [Google Scholar]
- 76.Henderson FW, Collier AM, Sanyal MA. A longitudinal study of respiratory viruses and bacteria in the etiology of acute otitis media with effusion. N Engl J Med. 1982;306:1377–1383. doi: 10.1056/NEJM198206103062301. [DOI] [PubMed] [Google Scholar]
- 77.Ruuskanen O, Arola M, Putto-Laurila A. Acute otitis media and respiratory virus infections. Pediatr Infect Dis J. 1989;8:94–99. [PubMed] [Google Scholar]
- 78.Heikkinen T, Ruuskanen O. Temporal development of acute otitis media during upper respiratory tract infection. Pediatr Infect Dis J. 1994;13:659–661. doi: 10.1097/00006454-199407000-00016. [DOI] [PubMed] [Google Scholar]
- 79.Koivunen P, Kontiokari T, Niemelä M, Pokka T, Uhari M. Time to development of acute otitis media during an upper respiratory tract infection in children. Pediatr Infect Dis J. 1999;18:303–305. doi: 10.1097/00006454-199903000-00023. [DOI] [PubMed] [Google Scholar]
- 80.Chonmaitree T, Owen MJ, Howie VM. Respiratory viruses interfere with bacteriologic response to antibiotic in children with acute otitis media. J Infect Dis. 1990;162:546–549. doi: 10.1093/infdis/162.2.546. [DOI] [PubMed] [Google Scholar]
- 81.Arola M, Ziegler T, Ruuskanen O. Respiratory virus infection as a cause of prolonged symptoms in acute otitis media. J Pediatr. 1990;116:697–701. doi: 10.1016/s0022-3476(05)82650-2. [DOI] [PubMed] [Google Scholar]
- 82.Pitkäranta A, Virolainen A, Jero J, Arruda E, Hayden FG. Detection of rhinovirus, respiratory syncytial virus, and coronavirus infections in acute otitis media by reverse transcriptase polymerase chain reaction. Pediatrics. 1998;102:291–295. doi: 10.1542/peds.102.2.291. [DOI] [PubMed] [Google Scholar]
- 83.Heikkinen T, Thint M, Chonmaitree T. Prevalence of various respiratory viruses in the middle ear during acute otitis media. N Engl J Med. 1999;340:260–264. doi: 10.1056/NEJM199901283400402. [DOI] [PubMed] [Google Scholar]
- 84.Juvén T, Mertsola J, Waris M. Etiology of community-acquired pneumonia in 254 hospitalized children. Pediatr Infect Dis J. 2000;19:293–298. doi: 10.1097/00006454-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 85.Heiskanen-Kosma T, Korppi M, Jokinen C. Etiology of childhood pneumonia: serologic results of a prospective, population-based study. Pediatr Infect Dis J. 1998;17:986–991. doi: 10.1097/00006454-199811000-00004. [DOI] [PubMed] [Google Scholar]
- 86.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993;307:982–986. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Johnston SL, Pattemore PK, Sanderson G. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Seemungal T, Harper-Owen R, Bhowmik A. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1618–1623. doi: 10.1164/ajrccm.164.9.2105011. [DOI] [PubMed] [Google Scholar]
- 89.Greenberg SB, Allen M, Wilson J, Atmar RL. Respiratory viral infections in adults with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162:167–173. doi: 10.1164/ajrccm.162.1.9911019. [DOI] [PubMed] [Google Scholar]
- 90.Ghosh S, Champlin R, Couch R. Rhinovirus infections in myelosuppressed adult blood and marrow transplant recipients. Clin Infect Dis. 1999;29:528–532. doi: 10.1086/598627. [DOI] [PubMed] [Google Scholar]
- 91.Putto A. Febrile exudative tonsillitis: viral or streptococcal? Pediatrics. 1987;80:6–12. [PubMed] [Google Scholar]
- 92.Walsh EE, Falsey AR, Hennessey PA. Respiratory syncytial and other virus infections in persons with chronic cardiopulmonary disease. Am J Respir Crit Care Med. 1999;160:791–795. doi: 10.1164/ajrccm.160.3.9901004. [DOI] [PubMed] [Google Scholar]
- 93.Carrat F, Tachet A, Rouzioux C, Housset B, Valleron AJ. Evaluation of clinical case definitions of influenza: detailed investigation of patients during the 1995–1996 epidemic in France. Clin Infect Dis. 1999;28:283–290. doi: 10.1086/515117. [DOI] [PubMed] [Google Scholar]
- 94.Monto AS, Gravenstein S, Elliott M, Colopy M, Schweinle J. Clinical signs and symptoms predicting influenza infection. Arch Intern Med. 2000;160:3243–3247. doi: 10.1001/archinte.160.21.3243. [DOI] [PubMed] [Google Scholar]
- 95.Waris M, Ziegler T, Kivivirta M, Ruuskanen O. Rapid detection of respiratory syncytial virus and influenza A virus in cell cultures by immunoperoxidase staining with monoclonal antibodies. J Clin Microbiol. 1990;28:1159–1162. doi: 10.1128/jcm.28.6.1159-1162.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim HW, Wyatt RG, Fernie BF. Respiratory syncytial virus detection by immunofluorescence in nasal secretions with monoclonal antibodies against selected surface and internal proteins. J Clin Microbiol. 1983;18:1399–1404. doi: 10.1128/jcm.18.6.1399-1404.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nikkari S, Halonen P, Kharitonenkov I. One-incubation time-resolved fluoroimmunoassay based on monoclonal antibodies in detection of influenza A and B viruses directly in clinical specimens. J Virol Methods. 1989;23:29–40. doi: 10.1016/0166-0934(89)90086-4. [DOI] [PubMed] [Google Scholar]
- 98.Cox NJ, Subbarao K. Influenza. Lancet. 1999;354:1277–1282. doi: 10.1016/S0140-6736(99)01241-6. [DOI] [PubMed] [Google Scholar]
- 99.Krilov LR, Lipson SM, Barone SR. Evaluation of a rapid diagnostic test for respiratory syncytial virus (RSV): potential for bedside diagnosis. Pediatrics. 1994;93:903–906. [PubMed] [Google Scholar]
- 100.Monto AS, Herlocher ML, Rotthoff J, Bidol SA. Detection and control of influenza outbreaks in Michigan nursing homes. Program and Abstracts of the 39th Annual Meeting of the Infectious Diseases Society of America; San Francisco, USA: 2001. abstr 888. [Google Scholar]
- 101.Hyypiä T, Puhakka T, Ruuskanen O. Molecular diagnosis of human rhinovirus infections: comparison with virus isolation. J Clin Microbiol. 1998;36:2081–2083. doi: 10.1128/jcm.36.7.2081-2083.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fan J, Henrickson KJ, Savatski LL. Rapid simultaneous diagnosis of infections with respiratory syncytial viruses A and B, influenza viruses A and B, and human parainfluenza virus types 1, 2, and 3 by multiplex quantitative reverse transcription-polymerase chain reaction-enzyme hybridization assay (Hexaplex) Clin Infect Dis. 1998;26:1397–1402. doi: 10.1086/516357. [DOI] [PubMed] [Google Scholar]
- 103.McIntosh K, Halonen P, Ruuskanen O. Report of a workshop on respiratory viral infections: epidemiology, diagnosis, treatment, and prevention. Clin Infect Dis. 1993;16:151–164. doi: 10.1093/clinids/16.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hall CB, Douglas RG., Jr Clinically useful method for the isolation of respiratory syncytial virus. J Infect Dis. 1975;131:1–5. doi: 10.1093/infdis/131.1.1. [DOI] [PubMed] [Google Scholar]
- 105.Covalciuc KA, Webb KH, Carlson CA. Comparison of four clinical specimen types for detection of influenza A and B viruses by optical immunoassay (FLU OIA test) and cell culture methods. J Clin Microbiol. 1999;37:3971–3974. doi: 10.1128/jcm.37.12.3971-3974.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Heikkinen T, Salmi AA, Ruuskanen O. Comparative study of nasopharyngeal aspirate and nasal swab specimens for detection of influenza. BMJ. 2001;322:138. doi: 10.1136/bmj.322.7279.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Smith MB, Feldman W. Over-the-counter cold medications: a critical review of clinical trials between 1950 and 1991. JAMA. 1993;269:2258–2263. doi: 10.1001/jama.269.17.2258. [DOI] [PubMed] [Google Scholar]
- 108.Lowenstein SR, Parrino TA. Management of the common cold. Adv Intern Med. 1987;32:207–233. [PubMed] [Google Scholar]
- 109.Arroll B, Kenealy T. Antibiotics for the common cold (Cochrane Review).The Cochrane Library Issue 3. Update Software; Oxford: 2002. [DOI] [PubMed] [Google Scholar]
- 110.Sperber SJ, Sorrentino JV, Riker DK, Hayden FG. Evaluation of an alpha agonist alone and in combination with a nonsteroidal antiinflammatory agent in the treatment of experimental rhinovirus colds. Bull N Y Acad Med. 1989;65:145–160. [PMC free article] [PubMed] [Google Scholar]
- 111.Gwaltney JM, Jr, Druce HM. Efficacy of brompheniramine maleate for the treatment of rhinovirus colds. Clin Infect Dis. 1997;25:1188–1194. doi: 10.1086/516105. [DOI] [PubMed] [Google Scholar]
- 112.Hayden FG, Diamond L, Wood PB, Korts DC, Wecker MT. Effectiveness and safety of intranasal ipratropium bromide in common colds: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1996;125:89–97. doi: 10.7326/0003-4819-125-2-199607150-00002. [DOI] [PubMed] [Google Scholar]
- 113.Farr BM, Gwaltney JM, Jr, Hendley JO. A randomized controlled trial of glucocorticoid prophylaxis against experimental rhinovirus infection. J Infect Dis. 1990;162:1173–1177. doi: 10.1093/infdis/162.5.1173. [DOI] [PubMed] [Google Scholar]
- 114.Ruohola A, Heikkinen T, Waris M, Puhakka T, Ruuskanen O. Intranasal fluticasone propionate does not prevent acute otitis media during viral upper respiratory infection in children. J Allergy Clin Immunol. 2000;106:467–471. doi: 10.1067/mai.2000.108912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schroeder K, Fahey T. Over-the-counter medications for acute cough in children and adults in ambulatory settings (Cochrane Review).The Cochrane LibraryIssue 2. Update Software; Oxford: 2002. [Google Scholar]
- 116.Sperber SJ, Hendley JO, Hayden FG. Effects of naproxen on experimental rhinovirus colds: a randomized, double-blind, controlled trial. Ann Intern Med. 1992;117:37–41. doi: 10.7326/0003-4819-117-1-37. [DOI] [PubMed] [Google Scholar]
- 117.Marshall I. Zinc for the common cold (Cochrane Review).The Cochrane LibraryIssue 2. Update Software; Oxford: 2002. [Google Scholar]
- 118.Hayden FG, Belshe RB, Clover RD. Emergence and apparent transmission of rimantadine-resistant influenza A virus in families. N Engl J Med. 1989;321:1696–1702. doi: 10.1056/NEJM198912213212502. [DOI] [PubMed] [Google Scholar]
- 119.The MIST Study Group Randomised trial of efficacy and safety of inhaled zanamivir in treatment of influenza A and B virus infections. Lancet. 1998;352:1877–1881. [PubMed] [Google Scholar]
- 120.Nicholson KG, Aoki FY, Osterhaus AD. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Lancet. 2000;355:1845–1850. doi: 10.1016/s0140-6736(00)02288-1. [DOI] [PubMed] [Google Scholar]
- 121.Kaiser L, Keene ON, Hammond JMJ, Elliott M, Hayden FG. Impact of zanamivir on antibiotic use for respiratory events following acute influenza in adolescents and adults. Arch Intern Med. 2000;160:3234–3240. doi: 10.1001/archinte.160.21.3234. [DOI] [PubMed] [Google Scholar]
- 122.Whitley RJ, Hayden FG, Reisinger KS. Oral oseltamivir treatment of influenza in children. Pediatr Infect Dis J. 2001;20:127–133. doi: 10.1097/00006454-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 123.Hayden FG, Gwaltney JM., Jr Intranasal interferon-alpha 2 treatment of experimental rhinoviral colds. J Infect Dis. 1984;150:174–180. doi: 10.1093/infdis/150.2.174. [DOI] [PubMed] [Google Scholar]
- 124.Hayden FG, Kaiser DL, Albrecht JK. Intranasal recombinant alfa-2b interferon treatment of naturally occurring common colds. Antimicrob Agents Chemother. 1988;32:224–230. doi: 10.1128/aac.32.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Turner RB, Wecker MT, Pohl G. Efficacy of tremacamra, a soluble intercellular adhesion molecule 1, for experimental rhinovirus infection: a randomized clinical trial. JAMA. 1999;281:1797–1804. doi: 10.1001/jama.281.19.1797. [DOI] [PubMed] [Google Scholar]
- 126.Schiff GM, Sherwood JR. Clinical activity of pleconaril in an experimentally induced coxsackievirus A21 respiratory infection. J Infect Dis. 2000;181:20–26. doi: 10.1086/315176. [DOI] [PubMed] [Google Scholar]
- 127.Kaiser L, Crump CE, Hayden FG. In vitro activity of pleconaril and AG7088 against selected serotypes and clinical isolates of human rhinoviruses. Antiviral Res. 2000;47:215–220. doi: 10.1016/s0166-3542(00)00106-6. [DOI] [PubMed] [Google Scholar]
- 128.Hsyu PH, Pithavala YK, Gersten M, Penning CA, Kerr BM. Pharmacokinetics and safety of an antirhinoviral agent, ruprintrivir, in healthy volunteers. Antimicrob Agents Chemother. 2002;46:392–397. doi: 10.1128/AAC.46.2.392-397.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hayden FG, Coats T, Kim K. Oral pleconaril treatment of picornavirus-associated viral respiratory illness in adults: efficacy and tolerability in phase II clinical trials. Antivir Ther. 2002;7:53–65. [PubMed] [Google Scholar]
- 130.Gwaltney JM., Jr Combined antiviral and antimediator treatment of rhinovirus colds. J Infect Dis. 1992;166:776–782. doi: 10.1093/infdis/166.4.776. [DOI] [PubMed] [Google Scholar]
- 131.Gwaltney JM, Jr, Winther B, Patrie JT, Hendley JO. Combined antiviral-antimediator treatment for the common cold. J Infect Dis. 2002;186:147–154. doi: 10.1086/341455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Belshe RB, Mendelman PM, Treanor J. The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenzavirus vaccine in children. N Engl J Med. 1998;338:1405–1412. doi: 10.1056/NEJM199805143382002. [DOI] [PubMed] [Google Scholar]
- 133.Nichol KL, Mendelman PM, Mallon KP. Effectiveness of live, attenuated intranasal influenza virus vaccine in healthy, working adults: a randomized controlled trial. JAMA. 1999;282:137–144. doi: 10.1001/jama.282.2.137. [DOI] [PubMed] [Google Scholar]
- 134.Glueck R. Pre-clinical and clinical investigation of the safety of a novel adjuvant for intranasal immunization. Vaccine. 2001;20(suppl 1):S42–S44. doi: 10.1016/s0264-410x(01)00292-4. [DOI] [PubMed] [Google Scholar]
- 135.Crowe JE., Jr Respiratory syncytial virus vaccine development. Vaccine. 2001;20(suppl 1):S32–S37. doi: 10.1016/s0264-410x(01)00287-0. [DOI] [PubMed] [Google Scholar]
- 136.Lee MS, Greenberg DP, Yeh SH. Antibody responses to bovine parainfluenza virus type 3 (PIV3) vaccination and human PIV3 infection in young infants. J Infect Dis. 2001;184:909–913. doi: 10.1086/323150. [DOI] [PubMed] [Google Scholar]
- 137.Skiadopoulos MH, Tatem JM, Surman SR. The recombinant chimeric human parainfluenza virus type 1 vaccine candidate, rHPIV3–1cp45, is attenuated, immunogenic, and protective in African green monkeys. Vaccine. 2002;20:1846–1852. doi: 10.1016/s0264-410x(02)00038-5. [DOI] [PubMed] [Google Scholar]
- 138.Dolin R, Reichman RC, Madore HP. A controlled trial of amantadine and rimantadine in the prophylaxis of influenza A infection. N Engl J Med. 1982;307:580–584. doi: 10.1056/NEJM198209023071002. [DOI] [PubMed] [Google Scholar]
- 139.Monto AS, Robinson DP, Herlocher ML. Zanamivir in the prevention of influenza among healthy adults: a randomized controlled trial. JAMA. 1999;282:31–35. doi: 10.1001/jama.282.1.31. [DOI] [PubMed] [Google Scholar]
- 140.Hayden FG, Atmar RL, Schilling M. Use of the selective oral neuraminidase inhibitor oseltamivir to prevent influenza. N Engl J Med. 1999;341:1336–1343. doi: 10.1056/NEJM199910283411802. [DOI] [PubMed] [Google Scholar]
- 141.Hayden FG, Gubareva LV, Monto AS. Inhaled zanamivir for the prevention of influenza in families. N Engl J Med. 2000;343:1282–1289. doi: 10.1056/NEJM200011023431801. [DOI] [PubMed] [Google Scholar]
- 142.Welliver R, Monto AS, Carewicz O. Effectiveness of oseltamivir in preventing influenza in household contacts: a randomized controlled trial. JAMA. 2001;285:748–754. doi: 10.1001/jama.285.6.748. [DOI] [PubMed] [Google Scholar]
- 143.Douglas RM, Moore BW, Miles HB. Prophylactic efficacy of intranasal alpha2-interferon against rhinovirus infections in the family setting. N Engl J Med. 1986;314:65–70. doi: 10.1056/NEJM198601093140201. [DOI] [PubMed] [Google Scholar]
- 144.Heikkinen T, Ruohola A, Ruuskanen O. Intranasally administered immunoglobulin for the prevention of rhinitis in children. Pediatr Infect Dis J. 1998;17:367–372. doi: 10.1097/00006454-199805000-00004. [DOI] [PubMed] [Google Scholar]
- 145.Douglas RM, Chalker EB, Treacy B. Vitamin C for preventing and treating the common cold (Cochrane Review).The Cochrane LibraryIssue 2. Update Software; Oxford: 2002. [DOI] [PubMed] [Google Scholar]
- 146.Melchart D, Linde K, Fischer P, Kaesmayr J. Echinacea for preventing and treating the common cold (Cochrane Review).The Cochrane LibraryIssue 2. Update Software; Oxford: 2002. [DOI] [PubMed] [Google Scholar]
- 147.Warshauer DM, Dick EC, Mandel AD, Flynn TC, Jerde RS. Rhinovirus infections in an isolated Antarctic station: transmission of the viruses and susceptibility of the population. Am J Epidemiol. 1989;129:319–340. doi: 10.1093/oxfordjournals.aje.a115136. [DOI] [PubMed] [Google Scholar]
- 148.Takkouche B, Regueira-Méndez C, Garcia-Closas R. Intake of wine, beer, and spirits and the risk of clinical common cold. Am J Epidemiol. 2002;155:853–858. doi: 10.1093/aje/155.9.853. [DOI] [PubMed] [Google Scholar]