Abstract
The outpost position of the olfactory bulb (OB) between the direct inputs from sensory neurons of the nasal epithelium and other parts of the brain suggests its highest vulnerability among all brain structures to penetration of exogenous agents. A number of neurotropic viruses have been found to invade the brain through the OB. There is growing evidence that microscopic particles of toxic dusts can propagate from the nasal epithelium to the OB and further into the brain. These harmful agents impair cellular elements of the brain. Apparently, cells in the OB are the most affected, as they are the first to encounter viral infections and toxic particles. It is well known that neuronal and glial progenitors are continuously generated from neuronal stem cells in the subventricular zone of the adult brain and then migrate predominantly into the OB. Therefore, it is feasible to suggest that substitution of injured or dead cells in the OB by new-born neurons, differentiating from progenitors, plays a role in protecting the OB neuronal microcircuits from destruction. Furthermore, some cytokines and chemokines released in response to infection and/or intoxication can modulate different stages of neurogenesis (proliferation, migration, and differentiation). We hypothesize that continuous neurogenesis in the olfactory system throughout adulthood evolved as a protective mechanism to prevent impairment of the most ancient but vitally important sensory system. In addition, differentiation of a substantial portion of progenitors to glial cells, including macrophages and microglia, may create an additional barrier to exogenous agents on their way deep to the brain.
Abbreviations: BrdU, bromodeoxyuridine; CNS, central nervous system; EPL, external plexiform layer; ET, external tufted cells; GL, glomerular layer; GR, granule cells; GRL, granule cell layer; IL, interleukin; INF, interferon; IPL, internal plexiform layer; LIF, leukemia inhibitory factor; M, mitral cells; MCL, mitral cell layer; NSCs, neuronal stem cells; OB, olfactory bulb; ON, olfactory nerve; ONL, olfactory nerve layer; PG, periglomerular cells; PgCs, progenitor cells; RMS, rostral migratory stream; SEL, subependimal layer; SGZ, subgranular zone; SVZ, subventricular zone; T, medium and deep tufted cells; VEGF, vesicular endothelial growth factor
Keywords: Olfactory bulb, Adult neurogenesis, Viral infection, Toxic dust, Interneuron
1. Introduction
The olfactory bulb (OB) is the first brain structure that receives information about odors from the nasal epithelium. It plays an important role in the neurogenesis in the adult mammalian brain. Presently, there is no doubt that intense neurogenesis takes place in the CNS of mammals (including humans) throughout postnatal life, i.e. proliferation of neural stem cells (NSCs), and the following migration and differentiation of progenitor cells (PgCs) into neurons and glia occur in the adult brain (for review see Okano and Sawamoto, 2008, Revishchin et al., 2008, Zhao et al., 2008). The primary known sources of PgCs are the subventricular zone (SVZ) in the lateral ventricles and the subgranular zone (SGZ) in the hippocampal dentate gyrus. The primary destination of PgCs continuously produced in the SVZ is the OB, and in the dentate gyrus PgCs migrate radially to the granule cell layer. Immediately after exit from the SVZ, all PgCs have immunohistochemical characteristics of glial cells (Ihrie and Alvarez-Buylla, 2008, Zhu and Dahlstrom, 2007). In rats, however, the majority of migrating PgCs (∼ 75%) start expressing proteins characteristic to neurons, thus making them neuronal progenitors, whereas the minority of PgCs (∼ 25%) are destined to become glial cells (Doetsch et al., 1997, Luskin, 1993). This does not mean that there is an anatomically defined region within the RMS where glial immunochemical characteristics of progenitors convert into neuronal characteristics. Instead, the SVZ contains three different types of cells: astrocyte-like neural stem cells (B cells), actively proliferating type C cells, and immature neuroblast type A cells. These neuroblasts migrate to the OB via the astrocyte path constituting the rostral migratory stream (RMS). Therefore, the presently accepted term “neurogenesis” is incomplete, and the term “neurogliogenesis” better reflects the nature of the process. We will use both terms: “neurogliogenesis” when discussing production of new cells in the CNS in general, and “neurogenesis” when discussing production of new neurons. Newborn neurons in the mature brain were shown to integrate into functional brain networks (Lledo and Saghatelyan, 2005), and newborn glial cells develop into astrocytes or oligodendrocytes (Marshall et al., 2003, Taupin, 2006). It seems that the fate of progenitor cells depends on the area to which they migrate and the local environmental cues (Ihrie and Alvarez-Buylla, 2008, Jiao et al., 2008). For example, all PgCs labeled in the SGZ with Ascl1 (a bHLH (basic helix–loop–helix) transcription factor) within 30 days matured into granule cells. PgCs in the OB matured into GABAergic interneurons, but in the subcortical gray and in the white matter they differentiated into oligodendrocytes (Kim et al., 2007a). Apparently, it is a local microenvironment that switches the direction of maturation. For instance, in normal mice, all hippocampal dentate gyrus PgCs become granule cells, whereas in the knockout mice lacking the extracellular matrix protein reelin, PgCs differentiate into astrocytes (Zhao et al., 2007). However, another possibility is that all PgCs are heterozygous as soon as they are generated (Merkle et al., 2007), contributing to the great diversity of interneurons inside the olfactory bulb (Lledo et al., 2008a, Lledo et al., 2008b) (Fig. 1 C).
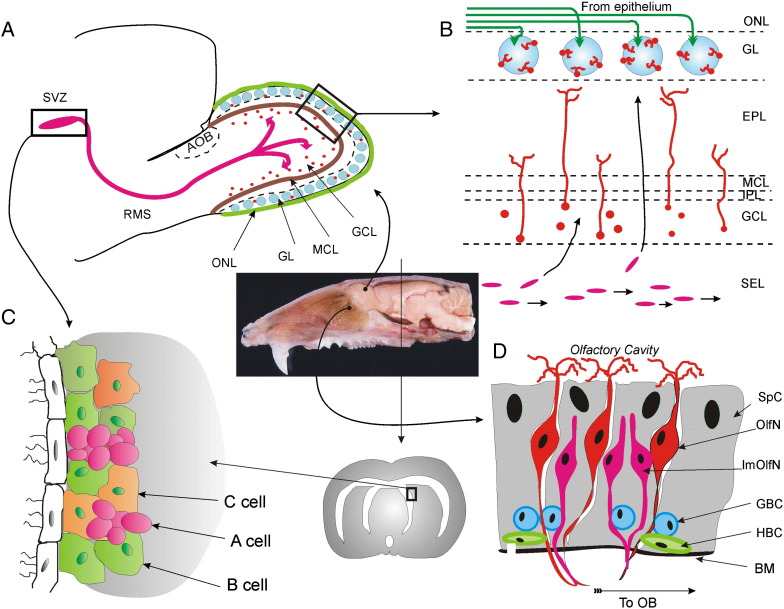
Fig. 1.
Neurogenesis in the mature olfactory system. (A) — Neuronal and glial precursors originating from subventricular zone (SVZ) migrate within the rostral migratory stream (RMS) into the olfactory bulb (OB). (B) — Migrating precursors enter the SEL from the RMS; from SEL they move tangentially either to GCL or to GL, where they differentiate into granule or periglomerular cells correspondingly. Magenta ovals in the SEL designate precursors migrating along the core of the OB; orange circles in the GCL stand for precursors arrived to their destination sites ready to differentiate; orange circles with processes extending to the EPL are differentiated new born granule cells; blue circles in the GL and green lines in the ONL designate glomeruli and olfactory nerve fibers; orange cells in the GL are newly differentiated periglomerular cells. ONL — olfactory nerve layer, GL — glomerular layer, EPL — external plexiform layer, MCL — mitral cell layer, IPL — internal plexiform layer, GCL — granule cell layer, SEL — subependymal layer. (C) — Proliferation in the SVZ takes place in the walls of the lateral ventricle, where stem cells (type B cells, green) divide to generate transit amplifying cells (type C cells, orange), which in turn give rise to immature neuroblasts (type A cells, magenta). The neuroblasts migrate in the RMS (panel A) to their final destination in the OB, where they differentiate into interneurons (panel B). (D) —Neurogenesis in the olfactory epithelium. BM — basal membrane, HBC — horizontal basal cells, GBC — global basal cells, ImOlfN — immature olfactory neuron, OlfN — receptor olfactory neuron, SpC — supporting cell. Basal cells continuously proliferate giving rise to olfactory sensory neurons. The sagittal section of the adult rat head in the center of this figure, illustrates close proximity of the olfactory epithelium to the olfactory bulb. Bundles of axons from sensory neurons project through holes in the cribriform plate of the ethmoid bone forming the olfactory nerve, and establish synaptic contacts in glomeruli of the olfactory bulb. Photo of the rat brain is a courtesy of Dr. Adam Puche.
Dramatic changes in brain size and environmental specializations among different species throughout mammalian evolution required alterations in the process of adult neurogenesis, including the rates and routes to generate the proper number of neurons to maintain brain functions (Abdel-Mannan et al., 2008). Active involvement of newly born cells in functional activity at destination sites gives them a chance to become long-time players in the existing neural circuits. For instance, survival rates of newly integrated neurons inside the olfactory bulb can be increased by sensory stimulations via environmental enrichment (Lledo and Saghatelyan, 2005). Additionally, experiments with anosmic mice (lacking sense of smell) have shown that the presence of sensory afferentation is critical for granule cells to survive between 15 and 45 days and even later after birth (Mandairon et al., 2003, Petreanu and Alvarez-Buylla, 2002). [Technically, anosmic mice can be obtained either by targeted mutations (McEwen et al., 2007, Belluscio et al., 1998) or by destroying olfactory receptor cells with zinc sulfate solution (Crusio and van Abeelen, 1987, Andiné et al., 1995, Uebayashi et al., 2001, McBride et al., 2003)]. Different molecules, including both growth and trophic factors, are also important for insertion of new cells into existing networks (Kuhn et al., 2005). Recent evidence demonstrates that immune factors, such as cytokines, are recruited at all stages of neurogenesis and seemingly serve as upstream signaling arbiters to regulate the course of neurogliogenesis (Taga and Fukuda, 2005). This suggests a possible role of neurogliogenesis in prevention or compensation of impairments caused by viral infections, inflammation, mechanical, or chemical trauma etc.
The propensity for agents to move from the nasal cavity into the brain via the olfactory nerves or surrounding mucosa has been known for many years. Previous studies confirmed the movement of materials from the nasal cavity into the brain and demonstrated that the olfactory nerve cells are, indeed, a route for viruses and a wide variety of other agents from the nasal cavity into the brain (Turner and Esmond, 1926, Clark, 1929, Tisdall et al., 1937; reviewed by Doty, 1997). More recent studies revealed target specificity in propagation of viruses (Baker, 1995, Mori et al., 2005), metal dusts (Tjalve and Henriksson, 1999), soluble instilled agents such as neuronal tracers (Baker and Spencer, 1986, Shipley, 1985) or toxins (Larsson and Tjalve, 2000) from the nasal cavity via olfactory nerve into the brain. In addition, the large surface area of nasal mucosa combined with rapid absorption, was beneficial for intranasal administration of various pharmacological agents, especially for drugs targeting the CNS (Illum, 2004, Vyas et al., 2005).
In the present review, we will discuss the hypothesized link between the adult brain neurogliogenesis and penetration of viruses and toxic dust through the nasal epithelium and the olfactory bulb into the brain. We assume that two facts – (a) migration of the overwhelming majority of neuronal and glial precursor cells into the OB, and (b) the fact that the OB is the brain's outpost directly connected with the environment via axonal fibers from the nasal epithelium – are not a mere coincidence. Instead, the persistent delivery of neural and glial progenitors into the OB may indicate continuous exposure of the OB cells to external invaders, impairment of some cells, and urgent necessity for immediate replacement by nearby progenitors. Thus, neurogliogenesis in the adult mammalian OB could be a mechanism evolved to protect the brain from propagation of harmful agents, and to maintain normal function of the evolutionarily oldest, but still most important sensory system for animal survival. Besides, neurogenesis in the olfactory system occurs outside of the brain as well, immediately in the olfactory epithelium, where damage and death of sensory neurons is highly plausible. Therefore, we begin our tour from the role of neurogenesis in the nasal olfactory epithelium.
2. Neurogenesis in the olfactory epithelium
One explanation supporting the hypothesized protective function of neurogliogenesis during viral infection or inhalation of toxic particles is that in the course of evolution, the neurogenic areas originated from the epithelium covering surfaces of all organs, protecting them from mechanical, chemical, and biological impairments. An intrinsic feature of epithelial tissues is to keep generating new cells throughout the whole life of the organism. Cell renewal in the olfactory epithelium directly inhibits entry of toxic dusts and viruses into olfactory bulb, as well as into other parts of the brain (Mori et al., 2005b). This suggests that the anti-infection function of neurogliogenesis might be the preservation of the most ancient protective role of epithelium in processes of natural selection, rather than newly developed (“acquired”) function during mammalian evolution.
Repopulation of olfactory sensory epithelium occurs via renewal of sensory neurons (Graziadei, 1973). There are three types of cells inside the olfactory epithelium: supporting cells, basal cells, and sensory neurons (mature and immature) that express odorant receptors (Murdoch and Roskams, 2007) (Fig. 1D). Apoptosis of olfactory sensory neurons increases after bulbectomy, olfactory nerve transection, intranasal chemical lesion, naris occlusion and/or viral infection (Cowan and Roskams, 2002, Murdoch and Roskams, 2007). At the same time, basal cells give birth to new sensory neurons, and the rate of basal cell proliferation increases dramatically under the above damaging conditions (Camara and Harding, 1984). The rate of proliferation is regulated by various molecular factors (Choi et al., 2008, Murdoch and Roskams, 2007). Interestingly, a unique type of involvement into neurogenesis is demonstrated by horizontal basal cells. These cells proliferate neither under normal conditions nor after mild cell loss, but they start proliferating when an extensive damage depletes other progenitors inside the epithelium (Leung et al., 2007). The intermediate period between olfactory sensory neurons' death and their replacement by new sensory neuron is characterized by the loss of olfaction (Henkin et al., 1975). Fast replacement of formerly functional but disabled sensory neurons by new healthy cells represents a useful strategy to prevent viruses and toxic dusts from entry into the brain, given that the impaired cells containing harmful agents are eliminated from the system.
Most frequently, postviral loss of olfaction is associated with infections of the upper respiratory tract with the influenza virus (Welge-Lussen and Wolfensberger, 2006). Other viruses, such as parainfluenza virus, adenovirus, corona virus, poliovirus, enterovirus, rhinovirus, Epstein–Barr virus, respiratory syncytial virus, Coxsackie virus, and herpes virus may also lead to olfaction impairments (Seiden, 2004, Suzuki et al., 2007, Wang et al., 2007a, Welge-Lussen and Wolfensberger, 2006). Apoptosis of olfactory sensory neurons results only in temporary olfaction loss, whereas direct injection of a virus into the olfactory bulb can lead to spread of the virus into many parts of the brain, and can be lethal to animals (Mori et al., 2002, Mori et al., 2004). This suggests that renewal of infected olfactory sensory neurons within the olfactory epithelium does represent an important protective response for the host to survive. Many studies have uncovered the cellular and molecular bases of sensory neuron apoptosis during development and maturation (Choi et al., 2008, Leung et al., 2007, Robinson et al., 2003, Suzuki, 2004). One apoptotic signaling pathway requires the death-receptor (TNFR1; Fas) and activates caspase 3 via caspase 2. It is also possible that mitochondria-released cytochrome C, combined with Apaf-1, activates caspase 3 through caspase 9. Finally, the activated caspase 3 leads to cleavage of various caspase substrate proteins and triggers apoptosis (Suzuki, 2004). However, details of signaling pathways of sensory neuron turnover after viral infection are poorly understood. They are the crucial goals for future studies.
One can divide invasive neuroviruses into the following groups: (1) viruses such as the herpes simplex virus HF10, which lack invasiveness to the peripheral nervous system (chemosensory neurons), but are lethal when directly injected to the brain (Mori et al., 2005a); (2) neurovirulent influenza A viruses, which may affect chemosensory neurons and induce apoptosis, but cannot reach the brain (Mori et al., 2002); (3) parainfluenza viruses, which may infect chemosensory neurons, attenuate virus-induced apoptosis and reach the glomeruli within the OB, but cannot be further transmitted into other brain areas through axons of mitral cells (Mori et al., 1995); (4) viruses such as Herpes simplex virus, Borna disease virus, and rabies virus, which can be transmitted from the olfactory epithelium and through the OB to other brain areas (Hornig et al., 2003, Mori et al., 2004, Mori et al., 2006). Obviously, the apoptosis of sensory neurons within the olfactory epithelium plays an important role in defending peripheral system of the brain from virus propagation. Since viruses of the latter class can propagate through synaptic clefts into many brain areas, they might lead to various types of brain damage, including neurodegenerative diseases (Doty, 2008, Hornig et al., 2001).
Renewal of olfactory sensory neurons inside the olfactory epithelium and SVZ/OB neurogenesis may thus represent two possible mechanisms underlying brain protection from risk factors in the air during normal respiratory activities. The apoptosis of sensory neurons in the olfactory mucosa may prevent the entry of most viruses, while newly born neurons within the OB can efficiently replace damaged neurons to maintain fine odor analysis (Carleton et al., 2002, Gheusi and Lledo, 2007, Lledo and Gheusi, 2003). Notably, newly generated neurons in the olfactory bulb are periglomerular and granule cells, which are not projecting neurons, but represent two types of GABAergic local interneurons (see below). Thus, given that neurotrophic viruses reach the OB, they can propagate into the brain once they penetrate into mitral cells, which project to a number of brain areas, including the piriform cortex, the entorhinal cortex, and the medial amygdala (Mombaerts, 2006). Since only a few viruses (of type 4) are capable of deep brain penetration, it seems feasible that other viruses reaching the OB (type 3) can only affect the smallest and most vulnerable interneurons, whereas large projecting mitral and tufted cells can defend themselves from those viruses.
Throughout evolution, neurogenesis in the SVZ has transformed dramatically as compared to the nasal epithelium, and differences emerged among species. Apparently, this may be due to enlargement of brain volume and changes in its whole morphology (Finlay et al., 1998). For example, the turtle and avian lack the organized SVZ (Cheung et al., 2007). Humans do not have the well pronounced “SVZ” (Sanai et al., 2004), but the existence of RMS in humans has been reported (Curtis et al., 2007). Moreover, combination of immunolabeling with specific cell markers in macaque monkey revealed progenitors migrating from the subependymal zone (SZ) to the OB, where they differentiated into granule interneurons (Kornack and Rakic, 2001). This finding supports the notion that the primate SZ/RMS can serve as a model system for studying neural regenerative mechanisms in the human brain. These variances, together with the multiple roles of SVZ neurogenesis during development and in adulthood, may further complicate understanding of the role of SVZ neurogenesis in protection from environmental risk factors. Currently, there is no direct evidence that species living in high-risk environments, and thus more accessible to viruses or toxic dusts, have evolved some specific types of a neurogenerating zone with increased proliferation rate or more organized structure. Multiple roles of neurogliogenesis in the brain may mask its actual contribution in protection from viral infections or toxic contaminations. One can hope that further studies may elucidate this problem.
3. Structural and functional features of the olfactory bulb
The olfactory bulb is a unique brain structure directly connected with the external world through the olfactory nerve coming from the nasal epithelium. Therefore, various harmful particles, including neuroviruses or microscopic dust, can potentially invade sensory neurons in the olfactory epithelium (viruses in an active manner; dust via endocytosis). They then can be easily transported along the axons into the glomerular layer of the OB, and continue their journey further into the CNS. Therefore the olfactory system represents a particularly sensitive route for transport of viruses, neurotoxins, and toxic particles into the brain which can cause cognitive and motor impairments (Prediger et al., 2006). To better understand a possible response of the OB to a foreign invasion, we need to know its basic structural and functional features.
The OB is an outgrowth of the forebrain, specialized for processing the molecular signals that give rise to the sense of smell. The OB receives the input afferents from the olfactory sensory neurons located in the nasal epithelium, and projects the output axons directly to the olfactory cortex and the amygdala. This basic relationship is characteristic for nearly all vertebrates. Anatomy, structure, and ultrastructure of the OB layers and neuronal elements are well studied (Allison, 1953, Graziadei and Graziadei, 1979, Liu and Shipley, 1994, Schoenfeld et al., 1985, Gil-Carcedo et al., 2000). The OB itself is a distinctly laminated structure, and contains a few sharply distinctive neuronal types. A cross-section of the OB exhibits seven main layers (listed from the surface to the core of OB): 1 — olfactory nerve layer (ONL), 2 — glomerular layer (GL), 3 — external plexiform layer (EPL), 4 — mitral cell layer (MCL), 5 — internal plexiform layer (IPL), 6 — granule cell layer (GRL) and 7 — subependimal layer (SEL). There are a few major cell types characterizing each layer: periglomerular cells (PG) and external tufted cells (ET) in the GL, medium and deep tufted cells (T) in the EPL, mitral cells (M) in the MCL, and granule cells (GR) in the GCL. The SEL is closely related to the RMS, as progenitor cells migrating along the RMS arrive to the SEL and from here they disperse into the GL and GCL (Fig. 1B). Importantly, new PG and GR cells can be generated throughout adulthood, whereas M, ET and T cells differentiate prenatally. Progenitors of PG and GR arrive in the mature bulb within a spatially defined tube, named the rostral migratory stream (RMS), leading from the anterior horn of the lateral ventricle to the ependymal core of the bulb (Kishi, 1987, Lois et al., 1996, Luskin, 1993). The continuous production and migration of these neurons into corresponding layers suggest that remodeling occurs continually among the local GABAergic circuits of the OB, which may be parallel to remodeling of sensory neuron synapses within the glomeruli. The most notable feature of neuronal contacts within the OB is that all neuron types establish dendro-dendritic synapses, where dendrites serve both as presynaptic and postsynaptic structures. PG and GR cells are GABAergic, whereas M/T cells are glutamatergic (Bufler et al., 1992, Halasz and Shepherd, 1983, Ribak et al., 1977, Trombley and Shepherd, 1993). Electron microscopic studies have clearly shown that PG cells within the glomerular neuropil and GR cells in the EPL establish reciprocal dendro-dendritic contacts on M/T cells (Orona et al., 1983, Pinching and Powell, 1971a, Pinching and Powell, 1971b, Price and Powell, 1970). Furthermore, short (100–200 μm) axons of PG cells make inhibitory synapses on M/T, as well as on PG dendrites in the interglomerular space. T cells do not have chemical contacts on each other, but in the glomerular ensemble T cells are well synchronized due to electrical gap-junctions on distal dendrites (Hayar et al., 2004a, Hayar et al., 2004b, Hayar et al., 2005). The so called “short-axon cells” (SA) represent an additional type of intrinsic to the GL glutamatergic interneurons. They have the longest axons among all local neurons in the in the GL (700–1000 μm) creating excitatory interglomerular connections throughout half of the MOB (Aungst et al., 2003). Excitatory interactions within the EPL occur due to T cells' axonal collaterals on M/T lateral dendrites (Kishi et al., 1984, Orona et al., 1984). Axons of sensory neurons enter the brain from the olfactory epithelium through a perphorated bone and synaptically terminate on neuronal dendrites exclusively within the GL. This means that all M/T and some PG cells, but not GR cells, receive direct sensory input and can potentially be the first recipients of harmful particles delivered via the olfactory nerve (ON). Thus, M/T cells receive the ON-input in their glomeruli tufts and give rise to the bulbar output from their cell bodies. PG cells modulate/filter an immediate reception of this input within the GL, whereas GR cells create the second line of modulation/filtering and take part in the final processing of the sensory information stream before it leaves the OB.
Thus, the two main functions – input processing and output control – are separated into two distinct levels in relation to the output cells. Input processing occurs exclusively in the GL, whereas output control is accomplished by GR cells. There is increasing evidence that a neuronal ensemble bound to the same glomerulus may act as a functional unit like a cortical column (Jastreboff et al., 1984, Karnup et al., 2006, Leveteau and MacLeod, 1966, Mombaerts et al., 1996, Mori and Yoshihara, 1995). If a neurotropic infection affects a substantial portion of interneurons (PG and/or GR) or even a few principal cells (M and/or T), it would lead to a dramatic change of input/output transduction of olfactory information. Since olfactory information has major significance in the animal kingdom, distortions of it are intolerable. For an adequate behavior in the given environment, and merely for successful survival, an animal must have a highly reliable odor analysis system at any moment, without breaks even for hours. This suggests the necessity of having a pool of spare parts readily available for urgent restoration of a damaged neuronal network. Therefore, the main role of neurogenesis in the OB may be to provide such a firewall of progenitors and prevent failures in odor analysis. Concurrently occurring gliogenesis from the mosaic pool of progenitors may provide substitution of glial elements impaired by viral or toxic dust invasion. Whether the proliferation and differentiation of these diverse interneuron progenitors are differentially regulated under viral invasion is yet to be discovered, and may provide very important clues about the mechanisms underlying brain repair.
4. Neurogenesis in the mature olfactory bulb
The main areas of proliferation in the adult brain are the SVZ, located in the ependyma of lateral ventricles, and the SGZ in the dentate gyrus (for review: (Kempermann et al., 2008, Lim et al., 2008, Lledo, 2008). The fate of neuronal stem cells (NSCs) in the SVZ was uncovered using intraventricular injections of the retrovirus containing beta-galactose gene into the mouse brain. Most of the infected NSCs leave the SVZ as progenitor cells and migrate towards the OB (Craig et al., 1999). Six days after retroviral infection, 60% of labeled cells died, 25% migrated into the OB and 15% stayed in the subependymal layer of the lateral ventricle, demonstrating asymmetric proliferation (Morshead et al., 1998). Another approach to monitor the fate of progenitor cells leaving the SVZ after mitotic division of NSCs is a combination of the intraperitoneal injection of bromodeoxyuridine (BrdU) with markers of various neurogenesis stages. BrdU incorporates into dividing cells within SVZ, whereas other markers can be incorporated at certain stages into migrating progenitor cells, or during their differentiation (Hayes and Nowakowski, 2002, Prickaerts et al., 2004, Sosunov et al., 2002). Progenitor cells migrate from the SVZ towards the OB along the astrocyte path constituting the RMS, which is rich with extracellular matrix (Fig. 1A). Chains of migrating precursor cells move along the RMS within channels formed by a special population of tenascin-R positive astrocytes (Saghatelyan et al., 2004). Migrating cells enter the OB along its central axis, then climb perpendicular to the OB layers to their destination sites, where they differentiate into neurons or glial cells (Fig. 1B) (Alvarez-Buylla and Garcia-Verdugo, 2002, Doetsch et al., 1999, Garcia-Verdugo et al., 2002, Lim and Alvarez-Buylla, 1999, Lois et al., 1996, Luskin, 1998, Pencea et al., 2001, Thomas et al., 1996). Following intraperitoneal injection of BrdU, the number of cells labeled with BrdU peaked in the mouse OB at 11–15 days and in the rat OB at one month. Later, about 50% of labeled cell undergo the apoptotic destruction. The majority (∼ 95%) of remaining labeled neuronal progenitors differentiate into GABAergic GR cells, and a small fraction (∼ 4%) of progenitor cells move further to the OB periphery, reaching the GL (Lim et al., 2008). Differentiation of newborn cells to PG interneurons in the GL takes longer (> 1 month) than differentiation of progenitors into GR cells in the GRL (∼ 15–30 days) (Petreanu and Alvarez-Buylla, 2002). Then number of labeled cells in both layers rapidly decreases. However, those cells that survived for 3 months lived up to 19 months. This means that in the mature OB, neurogenesis results in overproduction of young neurons and fast elimination of redundant ones. This suggests that, under normal conditions, only a few corrupted elements need to be replaced, whereas under conditions causing massive neuronal or glial death, there will be enough new precursors to substitute impaired elements of the network. This process reminds that in the developing brain with the initial excess of precursors and following elimination of redundant cells after the network is completed (Winner et al., 2002). At the same time, the 4:1 ratio of newborn neurons to newborn glial cells suggests that glial cells also require replacement. This assumption is feasible, as glial precursors can differentiate into macrophages, microglia, or astrocytes, taking part in defending the brain against viral infection or toxic particles. Recent studies identified a novel non-neuronal mechanism of regenerative repair by adult SVZ-generated astrocytes (Luo et al., 2008), implying that glial cells play important roles in the regenerative repair by neurogliogenesis.
Some details of neurogenesis in the OB are already known. Differentiation of precursors into neurons occurs predominantly in the granule cell layer, and differentiation into glia takes place in granule and glomerular layers. The data have been accumulated showing that the type of a newborn neuron correlates with the site location in the SVZ from where its precursor originated (Fig. 1C) (Lledo et al., 2008a, Lledo et al., 2008b). Apart from this, there are local stem cells in the OB, but their number is 28 times less than that of precursor cells arriving from the RMS. These local stem cells go through all stages of neurogenesis, and differentiate primarily into glia in the GRL and GL (Fukushima et al., 2002). Five stages of GR cell development are distinguished: (1) tangential migration of neuroblasts from the SVZ to the OB (2–7 days after birth); (2) radial migration of precursor cells (5–7 days); (3) GR cells with non-branching dendrites, which do not reach MCL (11–22 days); (4) GR cells with spineless dendrites in the EPL (11–22 days); (5) mature GR cells (15–30 days) (Petreanu and Alvarez-Buylla, 2002, Zhao et al., 2008). Neuronal content of the OB is subjected to rapid turnover. Combination of radiolabeled thymidine and the neuronal marker calretinin (Kato et al., 2001), as well as genetic labeling (Imayoshi et al., 2008), have demonstrated that 65–77% of neurons in the OB are substituted for new ones during six weeks in adult rodents. Functional significance of this turnover is unclear, but it suggests high vulnerability of neuronal circuits in the OB, impairment of highly vulnerable GR cells, and the necessity for their replacement.
5. Propagation of viruses into the CNS through the olfactory bulb
It is well known that neurotropic viruses can invade the mammalian CNS through the respiratory tract and the OB (Monath et al., 1983, Oliver and Fazakerley, 1998, Ryzhikov et al., 1991, Ryzhikov et al., 1995). The blood–brain barrier in the OB is weaker than in other brain structures (Dvorska et al., 1992, Ermisch, 1992, Nonaka et al., 2008, Yamada et al., 2007). A neurotropic infection can affect a substantial portion of interneurons (PG and/or GR cells) or even a small number of projecting (M and/or T cells) in the OB. This should lead to significant changes in the input/output transformation of olfactory signals. Normal activity of neuronal networks will become pathological (e.g. epileptical) if either feedforward or feedback inhibition is attenuated due to PG/GR cell loss, a decrease in the production of transmitters, or a change in neuronal membrane and receptor properties due to viral infection or toxic contamination. Thus, harmful agents have to be neutralized as early as possible.
Different viruses can infect different layers of the OB and affect different areas of the CNS. For instance, intranasal application of the mouse hepatitis virus (JHM line, coronoviruses) can cause massive impairment in the OB, especially in the mitral cell layer (Lane et al., 1997, Youngentob et al., 2001). The virus penetrates into the brain and even into the spinal cord, affecting neurons and astrocytes. This leads to demyelination, since the activity of myelin producing oligodendrocytes is dependent upon astrocytes (Sun et al., 1995). At the same time, intranasal application of the mouse hepatitis virus (OBVL60 line) is not detected in the brain of immunocompetent animals (Balb/c) 11 days after inoculation, probably due to activity of gamma-interferon (Lane et al., 1997). It has been shown that the mouse hepatitis virus and herpes simplex virus move along different CNS pathways after reaching the OB via the olfactory nerve (Perlman et al., 1993). Another virus, the recombinant virus of vesicular stomatitus (r.VSV, rabdovirus) expressing green fluorescent protein, moved along the olfactory nerve after intranasal injection and penetrated the OB. The virus first infected PG cells, then (by-passing mitral cell layer) GR cells, and finally the SVZ. After 9 days, the virus was detected neither in the OB nor in other brain areas. Upon intraventricular injection, the virus propagated rapidly into some specific periventricular areas of the brain. In other words, the ventricular system can play a key role in virus dissemination in the brain (van den Pol et al., 2002). After intranasal application the herpes virus 9 (EHV-9) penetrated into the brain of Syrian hamsters via the olfactory nerve, then the virus propagated trans-synaptically along the neuronal chain of the olfactory tract (Narita et al., 2001). The flu virus A was detected in various midbrain regions of immunodeficient mice after 10–17 months following an injection into the OB (Aronsson et al., 2003). Two viruses (herpes simplex virus type 1, mice hepatitis virus of JHM line) can penetrate into the brain via the olfactory system but affect different structures. Noradrenergic neurons in the red nucleus were affected only by the herpes virus, whereas both viruses affected dopaminergic neurons in the ventral tegmental area, and the hepatitis virus propagated into other brain areas. Various neurological diseases due to impairments of specific brain regions are assumed to be caused by different viruses (Barnett et al., 1993). Intensity of virus propagation in the brain after intranasal application depends on the animal's age (∼ degree of the immune response). For example, infection of 14-day old mice with Semliki Forest virus was lethal, whereas more mature mice survived and overcame the infection (Oliver and Fazakerley, 1998). Thus, the OB plays a key role in the penetration and propagation of neurotropic viral infections in the CNS.
6. Penetration of toxic dust into the brain through the olfactory bulb
Because of the deteriorating ecological situation in some areas of the world, namely, contamination of air by various toxic dust particles, much attention has been given to the action of these harmful agents on the human organism and to pathways through which they penetrate into different organs. One can find an example of the harmful actions of toxic dust on the brain at the mineral-enriching plant of Akchataussk in central Kazakhstan. Workers of this plant are subjected to prolonged inhalation of polymetal dust with a radioactive component and suffer from encephalopathy, following by development of progressive diffuse scattered and/or focal destruction in the CNS (Dosakhanov et al., 2003). Chronic experiments in rats have shown that, after three months of inhalation with polymetal radioactive dust, animals had sites of degenerative destruction in the vitally important hypothalamo-pituitary-adrenal system. That is, distinctive pathomorphologic changes were revealed in the hypothalamus upon penetration of this dust into the body through the respiratory tract (Tusupbekova et al., 2002).
Toxic dust is likely to invade the brain through the OB. In the recent years, single publications have reported penetration of small toxic dust particles into the OB through the olfactory nerve. Beta-spectrometry and autoradiography showed that after intranasal application of aflotoxin B(1) dust in rats this micotoxin propagated along axons of olfactory neurons from the nasal epithelium to their axonal terminals in the GL of the OB (Larsson and Tjalve, 2000). Some metals (for instance Al, Cd, Co, Hg, Mn, Ni, and Zn) were shown to penetrate into the olfactory receptor neurons and propagate along their axons to the OB. Furthermore, some metals (e.g. Mn, Ni, and Zn) can cross synaptic contacts and migrate through secondary olfactory neurons and further to remote brain areas (Sunderman, 2001).
Inhalation of fine (< 2.5 μm) or ultrafine (< 100 nm) particles is more toxic in the brain compared to coarse particles (Fechter et al., 2002, Zhang et al., 2006). Inhalation of iron particles, unlike manganese particles, (3 μm diameter, 0.31 mg Fe per m3) over 90 min did not result in their transport into the rat brain through the olfactory system, and only 4% of particles accumulated in the OB (Rao et al., 2003). However, histopathological observation showed that the neuron fatty degeneration occurred in the CA3 area of hippocampus after intranasal instillation of fine ferric oxide (Fe2O3, 280 ± 80 nm) (Wang et al., 2007c). Model experiments in rats have shown that inhalation of ultrafine graphite particles (< 36 nm, 160 μg/m3) for 6 h resulted in their accumulation in the OB from the first (0.35 μg/g) until the seventh (0.43 μg/g) day after the exposure to graphite dust (Oberdorster et al., 2004). Depending on the size, more than 50% of inhaled particles precipitated in the nasopharyngeal region, and ∼ 20% of them were transferred into the OB. After a single intranasal administration of ultrafine coal dust (< 14 nm, 25 μg), glutamate and glycine levels were increased in the OB, as well as expression of IL-1 beta mRNA (Tin Tin Win et al., 2008). Since IL-1 beta suppresses proliferation and differentiation of neuronal precursors (see below) (Wang et al., 2007b), one can suggest that exposure to ultrafine coal particles causing IL-1 beta expression can inhibit neurogenesis in the OB. Vapors of toxic substances and some gases also affect the brain and the OB in particular. For example, inhalation of air containing evaporated toluol by rats during 40 days led to death of mitral cells (but not granule cells) in the OB (Gelazonia et al., 2006).
Data have been accumulated demonstrating that workers in the heavy metal industries who breathe with the air containing cobalt dust often suffer with memory impairment. Transport of cobalt particles was studied in rats. In these experiments, autoradiography and gamma-spectroscopy showed that upon intranasal application, small cobalt particles were absorbed by the nasal mucosa and transported into the OB. Metal particles accumulate in the olfactory nerve and its presynaptic terminals in the GL. Small amounts of cobalt particles penetrate deep into the OB, damaging other cell layers, and can even reach the olfactory cortex. It is believed that this path through the olfactory nerve and OB is the major way of cobalt penetration into the brain (Persson et al., 2003). Therefore, protection against fine dust particles must be emphasized whenever risk of cobalt dust inhalation is present. A syndrome known as manganism occurs when individuals are exposed chronically to high levels of Mn. Manganese intoxication is most often associated with occupations in which abnormally high atmospheric concentrations prevail, such as in welding and mining (Roth, 2006). Data are available on small manganese particle accumulation in the OB upon intranasal application of MnO2. Small amounts of these particles penetrated into the neocortex and striatum (Fechter et al., 2002). Similarly, breathing with an aerosol of manganese phosphate (particles 1.68 μm diameter, 0.39 mg/m3) results in high concentration of Mn in the OB and tubercle; striatal Mn concentration was not increased significantly (Dorman et al., 2002). Thus, the OB is a structure that receives the first strike upon inhalation of toxic dust and/or gas. It accumulates harmful particles and prevents their penetration further into the brain. Dust/gas intoxication in the OB triggers changes in the levels of cytokines/chemokines/neurotransmitters that take part in neuronal and glial proliferation and differentiation. Therefore we suggest that neurogliogenesis may represent a mechanism supporting the protective role of the OB as a barrier on the way of toxic substances to the brain. However, this role of the OB is poorly explored, and needs further efforts to elucidate the importance of adult neurogliogenesis as a protective mechanism in the brain against air pollution.
7. Cytokines and neurogenesis
In recent years, convincing evidence has accumulated about the critical role of cytokines, chemokines and growth factors in neurogliogenesis of both the developing and mature brain. This part of review is focused on the importance of these compounds for neurogliogenesis in the mature brain. Since there are very few studies concerning cytokines in the olfactory bulb, we will also discuss relations between cytokines and neurogliogenesis in other brain areas.
Concentration of cytokines and cytokine receptors in the brain is maintained at some optimal level to provide normal brain functions, including persistent neurogliogenesis and angiogenesis. The fibroblast/macrophage networks (named fractones) producing cytokines and growth factors have been discovered in the proliferative zones of the mature brain (Mercier et al., 2002, Mercier et al., 2003). Concentration shifts of these substances can selectively change the course of different neurogenesis phases (proliferation, migration, differentiation). During inflammation, elevated concentration of anti-inflammatory cytokines can reduce the intensity of neurogenesis (Monje et al., 2003). Some cytokines (e.g. IL-1b, INFα, INFγ) inhibit proliferation of precursors in the mature brain, and at the same time accelerate cellular differentiation (Butovsky et al., 2006, Kaneko et al., 2006, Kim et al., 2007b, Koo and Duman, 2008, Vallieres et al., 2002). Other factors can regulate migration of PgCs (Belmadani et al., 2005, Garzotto et al., 2008, Stumm and Hollt, 2007). Anti-inflammatory cytokine IL-6 also decreases proliferation and survival rates of neuronal (but not glial) progenitor cells in the hippocampal dentate gyrus. However, data about its modulatory action on cellular differentiation are controversial: Vallieres et al. (2002) observed decrease and Barkho et al. (2006) observed an increase in the neuronal differentiation rate from neuronal progenitor cells. Another cytokine, TGFα, also attenuates proliferation, but inhibits differentiation (Heldmann et al., 2005, Liu et al., 2005). Vesicular endothelial growth factor (VEGF) enhances neurogenesis in the SVZ and SGZ of the adult brain in normal conditions (Jin et al., 2002) and after focal cerebral ischemia (Sun et al., 2003). Under pathological conditions, certain cytokines can change the direction of their modulatory action. For example, after stroke, TGFα can facilitate survival of newly generated precursors in the rat hippocampus and striatum (Heldmann et al., 2005).
Migration of neural progenitors into the rat striatum or neocortex after stroke is regulated by stromal cell-derived factor-1-alpha (SDF-1α) and its receptor CXCR4 (Stumm and Hollt, 2007, Thored et al., 2006). The virus of human immunodeficiency (HIV) can regulate neurogenesis in the adult hippocampus via binding with CXCR4 receptors (Tran and Miller, 2005, Venkatesan et al., 2007). This suggests that viruses can develop specific mechanisms to impair neurogenesis and facilitate their propagation in the CNS. It also supports the view that persistent neurogenesis in the mature brain creates one of the protective barriers against viral infections.
In vivo and in vitro experiments using leukemia inhibitory factor (LIF) inhibited differentiation of olfactory receptor neurons by activation of STAT3 (Moon et al., 2002), promoted proliferation of olfactory sensory neuron precursors, but did not affect proliferation of non-neuronal cells (Kim et al., 2005). In addition, LIF stimulated mitotic activity of neural progenitors in the olfactory epithelium after olfactory bulb ablation (Bauer et al., 2003). In the latter case, the authors suggested that injured olfactory sensory neurons release LIF to stimulate their own recovery. It seems that by enhancement of progenitor proliferation and suppression of their differentiation into mature cells, LIF provides accumulation of a substantial progenitor pool; this pool might be later used to compensate massive cell loss. Expression of LIF was shown to provide maintenance of neurogenesis in the adult mouse olfactory epithelium (Takaki et al., 2006). A similar effect of LIF has been shown on other types of progenitors. For example, in cultured stem cells, LIF facilitated formation of a progenitor cell pool with characteristics typical of adult SVZ and SGZ stem cells/astrocytes (Bonaguidi et al., 2005). Enhanced expression of LIF resulted in attenuation of neurogliogenesis in the OB and SVZ, acting directly on neural stem cells (Bauer and Patterson, 2006). In this case, LIF also prevented occurrence of differentiated cell types and gave rise to accumulation of the pool of neural stem cells.
Since under viral infection or inflammatory processes, synthesis of many cytokines is greatly enhanced, their influence on neurogliogenesis should be also enhanced. Normally, repeated invasion of small amounts of viruses throughout adult life induces continuous production of cytokines. Most of them boost differentiation of precursors from the available pool for substitution of apoptotic or dead cells, whereas other factors (like VEGF and LIF) stimulate production of new progenitors to refill the pool. Thus, in normal environmental conditions with repeated viral mini-invasions, a specific compensatory response triggered by the virus could evolve: infection, followed by cell impairment or death, then cytokine synthesis, and finally facilitation of neurogenesis.
8. Hypothesis on the protective role on neurogenesis in the olfactory bulb
Despite the fact that the OB is the gate allowing viral infection and toxic dust to invade and propagate in the brain, it is also the path that can become an obstacle for small amounts of various infecting agents on their way into the CNS. As it was stated above, the OB is where the majority of new neurons and glia reach their final destination. Creation of new cells is accompanied by multiple apoptotic events, requiring rapid renewal of cell populations, predominantly in the GL and GRL. In other words, small numbers of infected cells in the OB will quickly be replaced by functionally active neurons and glial cells, provided the course of neurogenesis is normal. The question arises whether only the impairment of intrinsic OB networks by external invaders requires continuous restoration of the network with an ample supply of fresh cells, or regular analysis of sensory information is too exhausting for cells and some of them die being unable to tolerate intensive functioning. The latter seems unlikely, because in other brain structures and sensory systems with similar organization of feedforward and feedback inhibition, a noticeable loss of interneurons was not observed under normal conditions. Therefore, the unique feature of the OB, such as direct physical contact with various environmental agents, seems to be a feasible reason for the delivery of new neuronal and glial elements into the OB. Furthermore, the majority of harmful particles are neutralized in the nasal epithelium and few of them reach the OB, where they encounter the second line of defense. Loss of afferentation was shown to result in apoptosis of GR cells in the OB and PgCs in the SVZ and RMS, but it also increased proliferation of stem cells in the SVZ (Mandairon et al., 2003, Mandairon et al., 2006a, Mandairon et al., 2006b). This does not contradict our hypothesis. Under natural conditions, deafferentation of the OB can occur when sensory cells in the nasal epithelium are damaged, which is a typical consequence of viral infection or toxic inhalation. It indicates that progenitors can receive a signal about viral/toxic attack long before harmful agents reach them, possibly via proinflammatory or inflammatory signaling. Hence, an enhancement of proliferation in the SVZ during this attack can be a forestalling protective response to compensate a foreseeable loss of interneurons in the OB or in other brain areas. Thus, although neurogliogenesis itself does not prevent viral infection or toxic contamination of the brain, it may participate in organization and maintenance of the protective barrier against these agents.
Neurogenesis slows down in aged individuals (Kempermann et al., 2002). Proliferation of neuronal stem cells in the aged brain occurs with intensity similar to that in the adult brain, but apoptosis and differentiation of precursors is substantially inhibited (Cameron and McKay, 1999, Loo et al., 1996), which is thought to result from elongation of the cell cycle (Tropepe et al., 1997). At this time, formation of new neurons suffers more compared to glial cells (Kuhn et al., 1996). Therefore, slowed renewal of the cell population in the OB may lead to failed compensation for the loss of infected/intoxicated cells and can cause higher probability of infectious/toxic damage of the CNS. Similar slowed renewal of the OB neurons has been observed in other toxin and/or virus-related neurodegenerative diseases such as HIV associated dementia (Hult et al., 2008, Kaul and Lipton, 2006), Alzheimer's and Parkinson's diseases (Doty, 2008, Haughey et al., 2002, Höglinger et al., 2004). It may be the normal course of neurogenesis in the OB of adult animals and humans that serves as an important mechanism protecting the brain from invasion of harmful particles or viruses causing various neurodegenerative diseases. This mechanism prevents impairment of the principal OB function: the continuous analysis and transmission of smell information. It is exceedingly important for survival of single organisms and whole species. In other words, due to fast renewal of cell populations, the OB may constitute an outpost against neuroinfections and other harmful agents on their way to the CNS, similar to how lymphoid glands prevent penetration of infectious and toxic particles into different systems and organs.
If the proposed hypothesis about the barrier function of neurogenesis in the OB is correct, then the probability of virus and toxic dust penetration into the brain can be reduced by enhancement of all neurogenesis phases: proliferation of stem cells and precursors, migration into the OB, and differentiation into new neurons and glial cells. One should not exclude the possibility that self-renewal of cellular populations not only in the OB, but in the entire mature brain, can be an additional obstacle for harmful particles. We suggest that, during an acute period of viral disease or after inhalation of toxic dust, the intranasal administration of optimal compositions of cytokines and growth factors enhancing different phases of neurogenesis can bring a positive therapeutic effect. We plan future experiments aimed at testing this hypothesis.
Acknowledgment
We thank Mr. Daniel Nagode for technical support in preparation of the manuscript.
References
- Abdel-Mannan O., Cheung A.F., Molnar Z. Evolution of cortical neurogenesis. Brain Res. Bull. 2008;75:398–404. doi: 10.1016/j.brainresbull.2007.10.047. [DOI] [PubMed] [Google Scholar]
- Allison A.C. The structure of the olfactory bulb and its relationship to the olfactory pathways in the rabbit and the rat. J. Comp. Neurol. 1953;98:309–353. doi: 10.1002/cne.900980206. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A., Garcia-Verdugo J.M. Neurogenesis in adult subventricular zone. J. Neurosci. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andiné P., Axelsson R., Jacobson I. The effect of anosmia on MK-801-induced behaviour in mice. Neurosci. Lett. 1995;190:113–116. doi: 10.1016/0304-3940(95)11515-x. [DOI] [PubMed] [Google Scholar]
- Aronsson F., Robertson B., Ljunggren H.G., Kristensson K. Invasion and persistence of the neuroadapted influenza virus A/WSN/33 in the mouse olfactory system. Viral Immunol. 2003;16:415–423. doi: 10.1089/088282403322396208. [DOI] [PubMed] [Google Scholar]
- Aungst J.L., Heyward P.M., Puche A.C., Karnup S.V., Hayar A., Szabo G., Shipley M.T. Centre-surround inhibition among olfactory bulb glomeruli. Nature. 2003;426:623–629. doi: 10.1038/nature02185. [DOI] [PubMed] [Google Scholar]
- Baker H. Transport phenomena within the olfactory system. In: Doty R.L., editor. Handbook of Olfaction and Gustation. Marcel Dekker; New York: 1995. pp. 173–190. [Google Scholar]
- Baker H., Spencer R.F. Transneuronal transport of peroxidase-conjugated wheat germ agglutinin (WGA-HRP) from the olfactory epithelium to the brain of the adult rat. Exp. Brain Res. 1986;63:461–473. doi: 10.1007/BF00237470. [DOI] [PubMed] [Google Scholar]
- Barkho B.Z., Song H., Aimone J.B., Smrt R.D., Kuwabara T., Nakashima K., Gage F.H., Zhao X. Identification of astrocyte-expressed factors that modulate neural stem/progenitor cell differentiation. Stem Cells Dev. 2006;15:407–421. doi: 10.1089/scd.2006.15.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett E.M., Cassell M.D., Perlman S. Two neurotropic viruses, herpes simplex virus type 1 and mouse hepatitis virus, spread along different neural pathways from the main olfactory bulb. Neuroscience. 1993;57:1007–1025. doi: 10.1016/0306-4522(93)90045-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer S., Patterson P.H. Leukemia inhibitory factor promotes neural stem cell self-renewal in the adult brain. J. Neurosci. 2006;26:12089–12099. doi: 10.1523/JNEUROSCI.3047-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer S., Rasika S., Han J., Mauduit C., Raccurt M., Morel G., Jourdan F., Benahmed M., Moyse E., Patterson P.H. Leukemia inhibitory factor is a key signal for injury-induced neurogenesis in the adult mouse olfactory epithelium. J. Neurosci. 2003;23:1792–1803. doi: 10.1523/JNEUROSCI.23-05-01792.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluscio L., Gold G.H., Nemes A., Axel R. Mice deficient in G(olf) are anosmic. Neuron. 1998;20:69–81. doi: 10.1016/s0896-6273(00)80435-3. [DOI] [PubMed] [Google Scholar]
- Belmadani A., Tran P.B., Ren D., Assimacopoulos S., Grove E.A., Miller R.J. The chemokine stromal cell-derived factor-1 regulates the migration of sensory neuron progenitors. J. Neurosci. 2005;25:3995–4003. doi: 10.1523/JNEUROSCI.4631-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi M.A., McGuire T., Hu M., Kan L., Samanta J., Kessler J.A. LIF and BMP signaling generate separate and discrete types of GFAP-expressing cells. Development. 2005;132:5503–5514. doi: 10.1242/dev.02166. [DOI] [PubMed] [Google Scholar]
- Bufler J., Zufall F., Franke C., Hatt H. Patch-clamp recordings of spiking and nonspiking interneurons from rabbit olfactory bulb slices: GABA- and other transmitter receptors. J. Comp. Physiol. [A] 1992;170:153–159. doi: 10.1007/BF00196897. [DOI] [PubMed] [Google Scholar]
- Butovsky O., Landa G., Kunis G., Ziv Y., Avidan H., Greenberg N., Schwartz A., Smirnov I., Pollack A., Jung S., Schwartz M. Induction and blockage of oligodendrogenesis by differently activated microglia in an animal model of multiple sclerosis. J. Clin. Invest. 2006;116:905–915. doi: 10.1172/JCI26836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camara C.G., Harding J.W. Thymidine incorporation in the olfactory epithelium of mice: early exponential response induced by olfactory neurectomy. Brain Res. 1984;308:63–68. doi: 10.1016/0006-8993(84)90917-x. [DOI] [PubMed] [Google Scholar]
- Cameron H.A., McKay R.D. Restoring production of hippocampal neurons in old age. Nat. Neurosci. 1999;2:894–897. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- Carleton A., Rochefort C., Morante-Oria J., Desmaisons D., Vincent J.D., Gheusi G., Lledo P.M. Making scents of olfactory neurogenesis. J. Physiol. Paris. 2002;96:115–122. doi: 10.1016/s0928-4257(01)00087-0. [DOI] [PubMed] [Google Scholar]
- Cheung A.F., Pollen A.A., Tavare A., DeProto J., Molnar Z. Comparative aspects of cortical neurogenesis in vertebrates. J. Anat. 2007;211:164–176. doi: 10.1111/j.1469-7580.2007.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi P.S., Zakhary L., Choi W.Y., Caron S., Alvarez-Saavedra E., Miska E.A., McManus M., Harfe B., Giraldez A.J., Horvitz R.H., Schier A.F., Dulac C. Members of the miRNA-200 family regulate olfactory neurogenesis. Neuron. 2008;57:41–55. doi: 10.1016/j.neuron.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan C.M., Roskams A.J. Apoptosis in the mature and developing olfactory neuroepithelium. Microsc. Res. Tech. 2002;58:204–215. doi: 10.1002/jemt.10150. [DOI] [PubMed] [Google Scholar]
- Clark W.E.L. Anatomical investigation into the routes by which infections may pass from the nasal cavities into the brain. Rep. Public Health Med. 1929;No.54:1–27. London. [Google Scholar]
- Craig C.G., D'sa R., Morshead C.M., Roach A., van der Kooy D. Migrational analysis of the constitutively proliferating subependyma population in adult mouse forebrain. Neuroscience. 1999;93:1197–1206. doi: 10.1016/s0306-4522(99)00232-8. [DOI] [PubMed] [Google Scholar]
- Crusio W.E., van Abeelen J.H. Zinc-induced peripheral anosmia and behavioral responses to novelty in mice: a quantitative-genetic analysis. Behav. Neural Biol. 1987;48:63–82. doi: 10.1016/s0163-1047(87)90589-9. [DOI] [PubMed] [Google Scholar]
- Curtis M.A., Kam M., Nannmark U., Anderson M.F., Axell M.Z., Wikkelso C., Holtas S., van Roon-Mom W.M., Bjork-Eriksson T., Nordborg C., Frisen J., Dragunow M., Faull R.L., Eriksson P.S. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315:1243–1249. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- Doetsch F., Caille I., Lim D.A., Garcia-Verdugo J.M., Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Doetsch F., Garcia-Verdugo J.M., Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J. Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman D.C., Brenneman K.A., McElveen A.M., Lynch S.E., Roberts K.C., Wong B.A. Olfactrory transport: a direct route of delivery of inhaled manganese phosphate to the rat brain. J. Toxicol. Environ. Health A. 2002;65:1493–1511. doi: 10.1080/00984100290071630. [DOI] [PubMed] [Google Scholar]
- Dosakhanov A.K., Nadirova G.K., Akhmetova D.A. Clinical characteristic of toxic encephalopathy under action of polymetallic radioactive dust. Medicine (Russian) 2003;1:79–80. [Google Scholar]
- Doty R.L. Studies of Human Olfaction from the University of Pennsylvania Smell and Taste Center. Chem. Senses. 1997;22:565–586. doi: 10.1093/chemse/22.5.565. [DOI] [PubMed] [Google Scholar]
- Doty R.L. The olfactory vector hypothesis of neurodegenerative disease: is it viable? Ann. Neurol. 2008;63:7–15. doi: 10.1002/ana.21327. [DOI] [PubMed] [Google Scholar]
- Dvorska I., Brust P., Hrbas P., Ruhle H.J., Barth T., Ermisch A. On the blood–brain barrier to peptides: effects of immobilization stress on regional blood supply and accumulation of labelled peptides in the rat brain. Endocr. Regul. 1992;26:77–82. [PubMed] [Google Scholar]
- Ermisch A. Peptide receptors of the blood–brain barrier and substrate transport into the brain. Prog. Brain Res. 1992;91:155–161. doi: 10.1016/s0079-6123(08)62330-4. [DOI] [PubMed] [Google Scholar]
- Fechter L.D., Johnson D.L., Lynch R.A. The relationship of particle size to olfactory nerve uptake of a non-soluble form of manganese into brain. Neurotoxicology. 2002;23:177–183. doi: 10.1016/s0161-813x(02)00013-x. [DOI] [PubMed] [Google Scholar]
- Finlay B.L., Hersman M.N., Darlington R.B. Patterns of vertebrate neurogenesis and the paths of vertebrate evolution. Brain Behav. Evol. 1998;52:232–242. doi: 10.1159/000006566. [DOI] [PubMed] [Google Scholar]
- Fukushima N., Yokouchi K., Kawagishi K., Moriizumi T. Differential neurogenesis and gliogenesis by local and migrating neural stem cells in the olfactory bulb. Neurosci. Res. 2002;44:467–473. doi: 10.1016/s0168-0102(02)00173-6. [DOI] [PubMed] [Google Scholar]
- Garcia-Verdugo J.M., Ferron S., Flames N., Collado L., Desfilis E., Font E. The proliferative ventricular zone in adult vertebrates: a comparative study using reptiles, birds, and mammals. Brain Res. Bull. 2002;57:765–775. doi: 10.1016/s0361-9230(01)00769-9. [DOI] [PubMed] [Google Scholar]
- Garzotto D., Giacobini P., Crepaldi T., Fasolo A., De Marchis S. Hepatocyte growth factor regulates migration of olfactory interneuron precursors in the rostral migratory stream through Met-Grb2 coupling. J. Neurosci. 2008;28:5901–5909. doi: 10.1523/JNEUROSCI.1083-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelazonia L., Japaridze N., Maglakelidze G., Svanidze I. Influence of toluene intoxication on the number of mitral and granular neurons in olfactory bulbs of rats. Georgian Med. News. 2006:99–101. [PubMed] [Google Scholar]
- Gil-Carcedo L.M., Vallejo L.A., Gil-Carcedo E. Structure of the principal olfactory tract. Otolaryngol. Head Neck Surg. 2000;122:129–138. doi: 10.1016/S0194-5998(00)70161-6. [DOI] [PubMed] [Google Scholar]
- Gheusi G., Lledo P.M. Control of early events in olfactory processing by adult neurogenesis. Chem. Senses. 2007;32:397–409. doi: 10.1093/chemse/bjm012. [DOI] [PubMed] [Google Scholar]
- Graziadei P.P. Cell dynamics in the olfactory mucosa. Tissue Cell. 1973;5:113–131. doi: 10.1016/s0040-8166(73)80010-2. [DOI] [PubMed] [Google Scholar]
- Graziadei P.P., Graziadei G.A. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J. Neurocytol. 1979;8:1–18. doi: 10.1007/BF01206454. [DOI] [PubMed] [Google Scholar]
- Halasz N., Shepherd G.M. Neurochemistry of the vertebrate olfactory bulb. Neuroscience. 1983;10:579–619. doi: 10.1016/0306-4522(83)90206-3. [DOI] [PubMed] [Google Scholar]
- Haughey N.J., Liu D., Nath A., Borchard A.C., Mattson M.P. Disruption of neurogenesis in the subventricular zone of adult mice, and in human cortical neuronal precursor cells in culture, by amyloid beta-peptide: implications for the pathogenesis of Alzheimer's disease. Neuromol. Med. 2002;1:125–135. doi: 10.1385/NMM:1:2:125. [DOI] [PubMed] [Google Scholar]
- Hayar A., Karnup S., Ennis M., Shipley M.T. External tufted cells: a major excitatory element that coordinates glomerular activity. J. Neurosci. 2004;24:6676–6685. doi: 10.1523/JNEUROSCI.1367-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A., Karnup S., Shipley M.T., Ennis M. Olfactory bulb glomeruli: external tufted cells intrinsically burst at theta frequency and are entrained by patterned olfactory input. J. Neurosci. 2004;24:1190–1199. doi: 10.1523/JNEUROSCI.4714-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A., Shipley M.T., Ennis M. Olfactory bulb external tufted cells are synchronized by multiple intraglomerular mechanisms. J. Neurosci. 2005;25:8197–8208. doi: 10.1523/JNEUROSCI.2374-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes N.L., Nowakowski R.S. Dynamics of cell proliferation in the adult dentate gyrus of two inbred strains of mice. Brain Res. Dev. Brain Res. 2002;134:77–85. doi: 10.1016/s0165-3806(01)00324-8. [DOI] [PubMed] [Google Scholar]
- Heldmann U., Thored P., Claasen J.H., Arvidsson A., Kokaia Z., Lindvall O. TNF-alpha antibody infusion impairs survival of stroke-generated neuroblasts in adult rat brain. Exp. Neurol. 2005;196:204–208. doi: 10.1016/j.expneurol.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Henkin R.I., Larson A.L., Powell R.D. Hypogeusia, dysgeusia, hyposmia, and dysosmia following influenza-like infection. Ann. Otol. Rhinol. Laryngol. 1975;84:672–682. doi: 10.1177/000348947508400519. [DOI] [PubMed] [Google Scholar]
- Höglinger G.U., Rizk P., Muriel M.P., Duyckaerts C., Oertel W.H., Caille I., Hirch E.C. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat. Neurosci. 2004;7:726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- Hornig M., Solbrig M., Horscroft N., Weissenbock H., Lipkin W.I. Borna disease virus infection of adult and neonatal rats: models for neuropsychiatric disease. Curr. Top. Microbiol. Immunol. 2001;253:157–177. doi: 10.1007/978-3-662-10356-2_8. [DOI] [PubMed] [Google Scholar]
- Hornig M., Briese T., Lipkin W.I. Borna disease virus. J. Neurovirol. 2003;9:259–273. doi: 10.1080/13550280390194064. [DOI] [PubMed] [Google Scholar]
- Hult B., Chana G., Masliah E., Everall I. Neurobiology of HIV. Int. Rev. Psychiatry. 2008;20:3–13. doi: 10.1080/09540260701862086. [DOI] [PubMed] [Google Scholar]
- Ihrie R.A., Alvarez-Buylla A. Cells in the astroglial lineage are neural stem cells. Cell Tissue Res. 2008;331:179–191. doi: 10.1007/s00441-007-0461-z. [DOI] [PubMed] [Google Scholar]
- Jastreboff P.J., Pedersen P.E., Greer C.A., Stewart W.B., Kauer J.S., Benson T.E., Shepherd G.M. Specific olfactory receptor populations projecting to identified glomeruli in the rat olfactory bulb. Proc. Natl. Acad. Sci. U. S. A. 1984;81:5250–5254. doi: 10.1073/pnas.81.16.5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illum L. Is nose-to-brain transport of drugs in man a reality? J. Pharm. Pharmacol. 2004;56:3–17. doi: 10.1211/0022357022539. [DOI] [PubMed] [Google Scholar]
- Imayoshi I., Sakamoto M., Ohtsuka T., Takao K., Miyakawa T., Yamaguchi M., Mori K., Ikeda T., Itohara S., Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat. Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Jiao J.W., Feldheim D.A., Chen D.F. Ephrins as negative regulators of adult neurogenesis in diverse regions of the central nervous system. Proc. Natl. Acad. Sci. U. S. A. 2008;105:8778–8783. doi: 10.1073/pnas.0708861105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K., Zhu Y., Sun Y., Mao X.O., Xie L., Greenberg D.A. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc. Natl. Acad. Sci. U. S. A. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko N., Kudo K., Mabuchi T., Takemoto K., Fujimaki K., Wati H., Iguchi H., Tezuka H., Kanba S. Suppression of cell proliferation by interferon-alpha through interleukin-1 production in adult rat dentate gyrus. Neuropsychopharmacology. 2006;31:2619–2626. doi: 10.1038/sj.npp.1301137. [DOI] [PubMed] [Google Scholar]
- Karnup S.V., Hayar A., Shipley M.T., Kurnikova M.G. Spontaneous field potentials in the glomeruli of the olfactory bulb: the leading role of juxtaglomerular cells. Neuroscience. 2006;142:203–221. doi: 10.1016/j.neuroscience.2006.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T., Yokouchi K., Fukushima N., Kawagishi K., Li Z., Moriizumi T. Continual replacement of newly-generated olfactory neurons in adult rats. Neurosci. Lett. 2001;307:17–20. doi: 10.1016/s0304-3940(01)01914-0. [DOI] [PubMed] [Google Scholar]
- Kaul M., Lipton S.A. Mechanisms of neuroimmunity and neurodegeneration associated with HIV-1 infection and AIDS. J. Neuroimmune Pharmacol. 2006;1:138–151. doi: 10.1007/s11481-006-9011-9. [DOI] [PubMed] [Google Scholar]
- Kempermann G., Gast D., Gage F.H. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann. Neurol. 2002;52:135–143. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- Kempermann G., Song H., Gage F.H. Neurogenesis in the Adult Hippocampus. In: Gage F.H., Kempermann G., Song H., editors. Vol. Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 2008. pp. 159–174. (Adult Neurogenesis). [Google Scholar]
- Kim E.J., Simpson P.J., Park D.J., Liu B.Q., Ronnett G.V., Moon C. Leukemia inhibitory factor is a proliferative factor for olfactory sensory neurons. Neuroreport. 2005;16:25–28. doi: 10.1097/00001756-200501190-00007. [DOI] [PubMed] [Google Scholar]
- Kim E.J., Leung C.T., Reed R.R., Johnson J.E. In vivo analysis of Ascl1 defined progenitors reveals distinct developmental dynamics during adult neurogenesis and gliogenesis. J. Neurosci. 2007;27:12764–12774. doi: 10.1523/JNEUROSCI.3178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.J., Son T.G., Kim K., Park H.R., Mattson M.P., Lee J. Interferon-gamma promotes differentiation of neural progenitor cells via the JNK pathway. Neurochem. Res. 2007;32:1399–1406. doi: 10.1007/s11064-007-9323-z. [DOI] [PubMed] [Google Scholar]
- Kishi K. Golgi studies on the development of granule cells of the rat olfactory bulb with reference to migration in the subependymal layer. J. Comp. Neurol. 1987;258:112–124. doi: 10.1002/cne.902580109. [DOI] [PubMed] [Google Scholar]
- Kishi K., Mori K., Ojima H. Distribution of local axon collaterals of mitral, displaced mitral, and tufted cells in the rabbit olfactory bulb. J. Comp. Neurol. 1984;225:511–526. doi: 10.1002/cne.902250404. [DOI] [PubMed] [Google Scholar]
- Koo J.W., Duman R.S. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc. Natl. Acad. Sci. U. S. A. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornack D.R., Rakic P. The generation, migration, and differentiation of olfactory neurons in the adult primate brain. Proc. Natl. Acad. Sci. U. S. A. 2001;98:4752–4757. doi: 10.1073/pnas.081074998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn H.G., Dickinson-Anson H., Gage F.H. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn H.G., Cooper-Kuhn C., Eriksson P., Nilsson M. Signals regulating neurogenesis in the adult olfactory bulb. Chem. Senses. 2005;30(Suppl 1):i109–i110. doi: 10.1093/chemse/bjh138. [DOI] [PubMed] [Google Scholar]
- Lane T.E., Paoletti A.D., Buchmeier M.J. Disassociation between the in vitro and in vivo effects of nitric oxide on a neurotropic murine coronavirus. J. Virol. 1997;71:2202–2210. doi: 10.1128/jvi.71.3.2202-2210.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson P., Tjalve H. Intranasal instillation of aflatoxin B(1) in rats: bioactivation in the nasal mucosa and neuronal transport to the olfactory bulb. Toxicol. Sci. 2000;55:383–391. doi: 10.1093/toxsci/55.2.383. [DOI] [PubMed] [Google Scholar]
- Leung C.T., Coulombe P.A., Reed R.R. Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nat. Neurosci. 2007;10:720–726. doi: 10.1038/nn1882. [DOI] [PubMed] [Google Scholar]
- Leveteau J., MacLeod P. Olfactory discrimination in the rabbit olfactory glomerulus. Science. 1966;153:175–176. doi: 10.1126/science.153.3732.175. [DOI] [PubMed] [Google Scholar]
- Lim D.A., Alvarez-Buylla A. Interaction between astrocytes and adult subventricular zone precursors stimulates neurogenesis. Proc. Natl. Acad. Sci. U. S. A. 1999;96:7526–7531. doi: 10.1073/pnas.96.13.7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim D.A., Huang Y.C., Alvarez-Buylla A. Adult Subventricular Zone and Olfactory Bulb Neurogenesis. In: Gage F.H., Kempermann G., Song H., editors. Vol. Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 2008. pp. 175–206. (Adult Neurogenesis). [Google Scholar]
- Liu W.L., Shipley M.T. Intrabulbar associational system in the rat olfactory bulb comprises cholecystokinin-containing tufted cells that synapse onto the dendrites of GABAergic granule cells. J. Comp. Neurol. 1994;346:541–558. doi: 10.1002/cne.903460407. [DOI] [PubMed] [Google Scholar]
- Liu Y.P., Lin H.I., Tzeng S.F. Tumor necrosis factor-alpha and interleukin-18 modulate neuronal cell fate in embryonic neural progenitor culture. Brain Res. 2005;1054:152–158. doi: 10.1016/j.brainres.2005.06.085. [DOI] [PubMed] [Google Scholar]
- Lledo P.M. Adult neurogenesis in the olfactory bulb. In: Gage F.H., Kempermann G., Song H., editors. Vol. Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 2008. pp. 425–443. (Adult Neurogenesis). [Google Scholar]
- Lledo P.M., Gheusi G. Olfactory processing in a changing brain. Neuroreport. 2003;14:1655–1663. doi: 10.1097/00001756-200309150-00001. [DOI] [PubMed] [Google Scholar]
- Lledo P.M., Saghatelyan A. Integrating new neurons into the adult olfactory bulb: joining the network, life-death decisions, and the effects of sensory experience. Trends Neurosci. 2005;28:248–254. doi: 10.1016/j.tins.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Lledo P.-M., Merkle F.T., Alvarez-Buylla A. Origin and function of olfactory bulb interneuron diversity. TINS. 2008;625:9. doi: 10.1016/j.tins.2008.05.006. [E-pub. ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo P.M., Merkle F.T., Alvarez-Buylla A. Origin and function of olfactory bulb interneuron diversity. Trends Neurosci. 2008;31:392–400. doi: 10.1016/j.tins.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C., Garcia-Verdugo J.M., Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- Loo A.T., Youngentob S.L., Kent P.F., Schwob J.E. The aging olfactory epithelium: neurogenesis, response to damage, and odorant-induced activity. Int. J. Dev. Neurosci. 1996;14:881–900. doi: 10.1016/s0736-5748(96)00046-9. [DOI] [PubMed] [Google Scholar]
- Luo J., Shook B.A., Daniels S.B., Conover J.C. Subventricular zone-mediated ependyma repair in the adult mammalian brain. J. Neurosci. 2008;28:3804–3813. doi: 10.1523/JNEUROSCI.0224-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luskin M.B. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Luskin M.B. Neuroblasts of the postnatal mammalian forebrain: their phenotype and fate. J. Neurobiol. 1998;36:221–233. [PubMed] [Google Scholar]
- Mandairon N., Jourdan F., Didier A. Deprivation of sensory inputs to the olfactory bulb up-regulates cell death and proliferation in the subventricular zone of adult mice. Neuroscience. 2003;119:507–516. doi: 10.1016/s0306-4522(03)00172-6. [DOI] [PubMed] [Google Scholar]
- Mandairon N., Sacquet J., Garcia S., Ravel N., Jourdan F., Didier A. Neurogenic correlates of an olfactory discrimination task in the adult olfactory bulb. Eur. J. Neurosci. 2006;24:3578–3588. doi: 10.1111/j.1460-9568.2006.05235.x. [DOI] [PubMed] [Google Scholar]
- Mandairon N., Sacquet J., Jourdan F., Didier A. Long-term fate and distribution of newborn cells in the adult mouse olfactory bulb: influences of olfactory deprivation. Neuroscience. 2006;141:443–451. doi: 10.1016/j.neuroscience.2006.03.066. [DOI] [PubMed] [Google Scholar]
- Marshall C.A., Suzuki S.O., Goldman J.E. Gliogenic and neurogenic progenitors of the subventricular zone: who are they, where did they come from, and where are they going? Glia. 2003;43:52–61. doi: 10.1002/glia.10213. [DOI] [PubMed] [Google Scholar]
- McBride K., Slotnick B., Margolis F.L. Does intranasal application of zink sulfate produce anosmia in the mouse? An olfactometric and anatomical study. Chem. Senses. 2003;28:659–670. doi: 10.1093/chemse/bjg053. [DOI] [PubMed] [Google Scholar]
- McEwen D.P., Koenekoop R.K., Khanna H., Jenkins H., Lopez I., Swaroop A., Martens J.R. Hypomorphic CEP290/NPHP6 mutations result in anosmia caused by the selective loss of G proteins in cilia of olfactory sensory neurons. Proc. Natl. Acad. Sci. U. S. A. 2007;104:15917–15922. doi: 10.1073/pnas.0704140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier F., Kitasako J.T., Hatton G.I. Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J. Comp. Neurol. 2002;451:170–188. doi: 10.1002/cne.10342. [DOI] [PubMed] [Google Scholar]
- Mercier F., Kitasako J.T., Hatton G.I. Fractones and other basal laminae in the hypothalamus. J. Comp. Neurol. 2003;455:324–340. doi: 10.1002/cne.10496. [DOI] [PubMed] [Google Scholar]
- Merkle F.T., Mirzadeh Z., Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- Mombaerts P. Axonal wiring in the mouse olfactory system. Annu. Rev. Cell Dev. Biol. 2006;22:713–737. doi: 10.1146/annurev.cellbio.21.012804.093915. [DOI] [PubMed] [Google Scholar]
- Mombaerts P., Wang F., Dulac C., Chao S.K., Nemes A., Mendelsohn M., Edmondson J., Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- Monath T.P., Cropp C.B., Harrison A.K. Mode of entry of a neurotropic arbovirus into the central nervous system. Reinvestigation of an old controversy. Lab. Invest. 1983;48:399–410. [PubMed] [Google Scholar]
- Monje M.L., Toda H., Palmer T.D. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Moon C., Yoo J.Y., Matarazzo V., Sung Y.K., Kim E.J., Ronnett G.V. Leukemia inhibitory factor inhibits neuronal terminal differentiation through STAT3 activation. Proc. Natl. Acad. Sci. U. S. A. 2002;99:9015–9020. doi: 10.1073/pnas.132131699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K., Yoshihara Y. Molecular recognition and olfactory processing in the mammalian olfactory system. Prog. Neurobiol. 1995;45:585–619. doi: 10.1016/0301-0082(94)00058-p. [DOI] [PubMed] [Google Scholar]
- Mori I., Komatsu T., Takeuchi K., Nakakuki K., Sudo M., Kimura Y. Parainfluenza virus type 1 infects olfactory neurons and establishes long-term persistence in the nerve tissue. J. Gen. Virol. 1995;76(Pt 5):1251–1254. doi: 10.1099/0022-1317-76-5-1251. [DOI] [PubMed] [Google Scholar]
- Mori I., Goshima F., Imai Y., Kohsaka S., Sugiyama T., Yoshida T., Yokochi T., Nishiyama Y., Kimura Y. Olfactory receptor neurons prevent dissemination of neurovirulent influenza A virus into the brain by undergoing virus-induced apoptosis. J. Gen. Virol. 2002;83:2109–2116. doi: 10.1099/0022-1317-83-9-2109. [DOI] [PubMed] [Google Scholar]
- Mori I., Nishiyama Y., Yokochi T., Kimura Y. Virus-induced neuronal apoptosis as pathological and protective responses of the host. Rev. Med. Virol. 2004;14:209–216. doi: 10.1002/rmv.426. [DOI] [PubMed] [Google Scholar]
- Mori I., Nishiyama Y., Yokochi T., Kimura Y. Olfactory transmission of neurotropic viruses. J. Neurovirol. 2005;11:129–137. doi: 10.1080/13550280590922793. [DOI] [PubMed] [Google Scholar]
- Mori I., Liu B., Goshima F., Ito H., Koide N., Yoshida T., Yokochi T., Kimura Y., Nishiyama Y. HF10, an attenuated herpes simplex virus (HSV) type 1 clone, lacks neuroinvasiveness and protects mice against lethal challenge with HSV types 1 and 2. Microbes Infect. 2005;7:1492–1500. doi: 10.1016/j.micinf.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Mori I., Nishiyama Y., Yokochi T., Kimura Y. Olfactory transmission of neurotropic viruses. J. Neurovirol. 2005;11:129–137. doi: 10.1080/13550280590922793. [DOI] [PubMed] [Google Scholar]
- Mori I., Goshima F., Watanabe D., Ito H., Koide N., Yoshida T., Liu B., Kimura Y., Yokochi T., Nishiyama Y. Herpes simplex virus US3 protein kinase regulates virus-induced apoptosis in olfactory and vomeronasal chemosensory neurons in vivo. Microbes. Infect. 2006;8:1806–1812. doi: 10.1016/j.micinf.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Morshead C.M., Craig C.G., van der Kooy D. In vivo clonal analyses reveal the properties of endogenous neural stem cell proliferation in the adult mammalian forebrain. Development. 1998;125:2251–2261. doi: 10.1242/dev.125.12.2251. [DOI] [PubMed] [Google Scholar]
- Murdoch B., Roskams A.J. Olfactory epithelium progenitors: insights from transgenic mice and in vitro biology. J. Mol. Histol. 2007;38:581–599. doi: 10.1007/s10735-007-9141-2. [DOI] [PubMed] [Google Scholar]
- Narita M., Uchimura A., Kawanabe M., Fukushi H., Hirai K. Invasion and spread of equine herpesvirus 9 in the olfactory pathway of pigs after intranasal inoculation. J. Comp. Pathol. 2001;124:265–272. doi: 10.1053/jcpa.2000.0461. [DOI] [PubMed] [Google Scholar]
- Nonaka N., Farr S.A., Kageyama H., Shioda S., Banks W.A. Delivery of galanin-like peptide to the brain: targeting with intranasal delivery and cyclodextrins. J. Pharmacol. Exp. Ther. 2008;325:513–519. doi: 10.1124/jpet.107.132381. [DOI] [PubMed] [Google Scholar]
- Oberdorster G., Sharp Z., Atudorei V., Elder A., Gelein R., Kreyling W., Cox C. Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol. 2004;16:437–445. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- Okano H., Sawamoto K. Neural stem cells: involvement in adult neurogenesis and CNS repair. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008;363:2111–2122. doi: 10.1098/rstb.2008.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver K.R., Fazakerley J.K. Transneuronal spread of Semliki Forest virus in the developing mouse olfactory system is determined by neuronal maturity. Neuroscience. 1998;82:867–877. doi: 10.1016/s0306-4522(97)00309-6. [DOI] [PubMed] [Google Scholar]
- Orona E., Scott J.W., Rainer E.C. Different granule cell populations innervate superficial and deep regions of the external plexiform layer in rat olfactory bulb. J. Comp. Neurol. 1983;217:227–237. doi: 10.1002/cne.902170209. [DOI] [PubMed] [Google Scholar]
- Orona E., Rainer E.C., Scott J.W. Dendritic and axonal organization of mitral and tufted cells in the rat olfactory bulb. J. Comp. Neurol. 1984;226:346–356. doi: 10.1002/cne.902260305. [DOI] [PubMed] [Google Scholar]
- Pencea V., Bingaman K.D., Freedman L.J., Luskin M.B. Neurogenesis in the subventricular zone and rostral migratory stream of the neonatal and adult primate forebrain. Exp. Neurol. 2001;172:1–16. doi: 10.1006/exnr.2001.7768. [DOI] [PubMed] [Google Scholar]
- Perlman S., Barnett E., Jacobsen G. Mouse hepatitis virus and herpessimplex virus move along different CNS pathways. Adv. Exp. Med. Biol. 1993;342:313–318. doi: 10.1007/978-1-4615-2996-5_48. [DOI] [PubMed] [Google Scholar]
- Persson E., Henriksson J., Tjalve H. Uptake of cobalt from the nasal mucosa into the brain via olfactory pathways in rats. Toxicol. Lett. 2003;145:19–27. doi: 10.1016/s0378-4274(03)00266-2. [DOI] [PubMed] [Google Scholar]
- Petreanu L., Alvarez-Buylla A. Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. J. Neurosci. 2002;22:6106–6113. doi: 10.1523/JNEUROSCI.22-14-06106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinching A.J., Powell T.P. The neuropil of the glomeruli of the olfactory bulb. J. Cell. Sci. 1971;9:347–377. doi: 10.1242/jcs.9.2.347. [DOI] [PubMed] [Google Scholar]
- Pinching A.J., Powell T.P. The neuropil of the periglomerular region of the olfactory bulb. J. Cell. Sci. 1971;9:379–409. doi: 10.1242/jcs.9.2.379. [DOI] [PubMed] [Google Scholar]
- Prediger R.D., Batista L.C., Medeiros R., Pandolfo P., Florio J.C., Takahashi R.N. The risk is in the air: intranasal administration of MPTP to rats reproducing clinical features of Parkinson's disease. Exp. Neurol. 2006;202:391–403. doi: 10.1016/j.expneurol.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Price J.L., Powell T.P. The synaptology of the granule cells of the olfactory bulb. J. Cell. Sci. 1970;7:125–155. doi: 10.1242/jcs.7.1.125. [DOI] [PubMed] [Google Scholar]
- Prickaerts J., Koopmans G., Blokland A., Scheepens A. Learning and adult neurogenesis: survival with or without proliferation? Neurobiol. Learn. Mem. 2004;81:1–11. doi: 10.1016/j.nlm.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Rao D.B., Wong B.A., McManus B.E., McElveen A.M., James A.R., Dorman D.C. Inhaled iron, unlike manganese, is not transported to the rat brain via the olfactory pathway. Toxicol. Appl. Pharmacol. 2003;193:116–126. doi: 10.1016/s0041-008x(03)00340-5. [DOI] [PubMed] [Google Scholar]
- Revishchin A.V., Korochkin L.I., Okhotin V.E., Pavlova G.V. Neural stem cells in the mammalian brain. Int. Rev. Cytol. 2008;265:55–109. doi: 10.1016/S0074-7696(07)65002-5. [DOI] [PubMed] [Google Scholar]
- Ribak C.E., Vaughn J.E., Saito K., Barber R., Roberts E. Glutamate decarboxylase localization in neurons of the olfactory bulb. Brain Res. 1977;126:1–18. doi: 10.1016/0006-8993(77)90211-6. [DOI] [PubMed] [Google Scholar]
- Robinson A.M., Conley D.B., Kern R.C. Olfactory neurons in bax knockout mice are protected from bulbectomy-induced apoptosis. Neuroreport. 2003;14:1891–1894. doi: 10.1097/00001756-200310270-00002. [DOI] [PubMed] [Google Scholar]
- Roth J.A. . Homeostatic and toxic mechanisms regulating manganese uptake, retention, and elimination. Biol. Res. 2006;39:45–57. doi: 10.4067/s0716-97602006000100006. [DOI] [PubMed] [Google Scholar]
- Ryzhikov A.B., Tkacheva N.V., Sergeev A.N., Ryabchikova E.I. Venezuelan equine encephalitis virus propagation in the olfactory tract of normal and immunized mice. Biomed. Sci. 1991;2:607–614. [PubMed] [Google Scholar]
- Ryzhikov A.B., Ryabchikova E.I., Sergeev A.N., Tkacheva N.V. Spread of Venezuelan equine encephalitis virus in mice olfactory tract. Arch. Virol. 1995;140:2243–2254. doi: 10.1007/BF01323243. [DOI] [PubMed] [Google Scholar]
- Sanai N., Tramontin A.D., Quinones-Hinojosa A., Barbaro N.M., Gupta N., Kunwar S., Lawton M.T., McDermott M.W., Parsa A.T., Manuel-Garcia Verdugo J., Berger M.S., Alvarez-Buylla A. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- Saghatelyan A., de Chevigny A., Schachner M., Lledo P.M. Tenascin-R mediated activity-dependent recruitment of neuroblasts in the adult mouse forebrain. Nat. Neurosci. 2004;7:346–356. doi: 10.1038/nn1211. [DOI] [PubMed] [Google Scholar]
- Schoenfeld T.A., Marchand J.E., Macrides F. Topographic organization of tufted cell axonal projections in the hamster main olfactory bulb: an intrabulbar associational system. J. Comp. Neurol. 1985;235:503–518. doi: 10.1002/cne.902350408. [DOI] [PubMed] [Google Scholar]
- Seiden A.M. Postviral olfactory loss. Otolaryngol. Clin. North Am. 2004;37:1159–1166. doi: 10.1016/j.otc.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Shipley M.T. Transport of molecules from nose to brain: transneuronal anterograde and retrograde labeling in the rat olfactory system by wheat germ agglutinin-horseradish peroxidase applied to the nasal epithelium. Brain Res. Bull. 1985;15:129–142. doi: 10.1016/0361-9230(85)90129-7. [DOI] [PubMed] [Google Scholar]
- Sosunov A.A., Chelyshev Iu A., McKhann G., Krugliakov P.P., Balykova O.P., Shikhanov N.P. [Neurogenesis in the adult mammalian brain] Ontogenez. 2002;33:405–420. [PubMed] [Google Scholar]
- Stumm R., Hollt V. CXC chemokine receptor 4 regulates neuronal migration and axonal pathfinding in the developing nervous system: implications for neuronal regeneration in the adult brain. J. Mol. Endocrinol. 2007;38:377–382. doi: 10.1677/JME-06-0032. [DOI] [PubMed] [Google Scholar]
- Sun N., Grzybicki D., Castro R.F., Murphy S., Perlman S. Activation of astrocytes in the spinal cord of mice chronically infected with a neurotropic coronavirus. Virology. 1995;213:482–493. doi: 10.1006/viro.1995.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Jin K., Xie L., Childs J., Mao X.O., Logvinova A., Greenberg D.A. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J. Clin. Invest. 2003;111:1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderman F.W., Jr. Nasal toxicity, carcinogenicity, and olfactory uptake of metals. Ann. Clin. Lab. Sci. 2001;31:3–24. [PubMed] [Google Scholar]
- Suzuki Y. Fine structural aspects of apoptosis in the olfactory epithelium. J. Neurocytol. 2004;33:693–702. doi: 10.1007/s11068-005-3337-8. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Saito K., Min W.P., Vladau C., Toida K., Itoh H., Murakami S. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007;117:272–277. doi: 10.1097/01.mlg.0000249922.37381.1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taga T., Fukuda S. Role of IL-6 in the neural stem cell differentiation. Clin. Rev. Allergy Immunol. 2005;28:249–256. doi: 10.1385/CRIAI:28:3:249. [DOI] [PubMed] [Google Scholar]
- Takaki E., Fujimoto M., Sugahara K., Nakahari T., Yonemura S., Tanaka Y., Hayashida N., Inouye S., Takemoto T., Yamashita H., Nakai A. Maintenance of olfactory neurogenesis requires HSF1, a major heat shock transcription factor in mice. J. Biol. Chem. 2006;281:4931–4937. doi: 10.1074/jbc.M506911200. [DOI] [PubMed] [Google Scholar]
- Taupin P. Neural progenitor and stem cells in the adult central nervous system. Ann. Acad. Med. 2006;35:814–820. Singapore. [PubMed] [Google Scholar]
- Thomas L.B., Gates M.A., Steindler D.A. Young neurons from the adult subependymal zone proliferate and migrate along an astrocyte, extracellular matrix-rich pathway. Glia. 1996;17:1–14. doi: 10.1002/(SICI)1098-1136(199605)17:1<1::AID-GLIA1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Thored P., Arvidsson A., Cacci E., Ahlenius H., Kallur T., Darsalia V., Ekdahl C.T., Kokaia Z., Lindvall O. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24:739–747. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- Tin Tin Win S., Mitsushima D., Yamamoto S., Fukushima A., Funabashi T., Kobayashi T., Fujimaki H. Changes in neurotransmitter levels and proinflammatory cytokine mRNA expressions in the mice olfactory bulb following nanoparticle exposure. Toxicol. Appl. Pharmacol. 2008;226:192–198. doi: 10.1016/j.taap.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Tisdall E.F., Brown A., Defries R.D., Ross M.A., Sellers A.H. Nasal spraying as preventive of poliomyelitis. Can. Public Health J. 1937;28:431–434. [Google Scholar]
- Tjalve H., Henriksson J. Uptake of metals in the brain via olfactory pathways. Neurotoxicology. 1999;20:181–195. [PubMed] [Google Scholar]
- Tran P.B., Miller R.J. HIV-1, chemokines and neurogenesis. Neurotox. Res. 2005;8:149–158. doi: 10.1007/BF03033826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombley P.Q., Shepherd G.M. Synaptic transmission and modulation in the olfactory bulb. Curr. Opin. Neurobiol. 1993;3:540–547. doi: 10.1016/0959-4388(93)90053-2. [DOI] [PubMed] [Google Scholar]
- Tropepe V., Craig C.G., Morshead C.M., van der Kooy D. Transforming growth factor-alpha null and senescent mice show decreased neural progenitor cell proliferation in the forebrain subependyma. J. Neurosci. 1997;17:7850–7859. doi: 10.1523/JNEUROSCI.17-20-07850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A.L., Esmond R.E. Study of the paths of infection of the brain, meninges and venous blood sinuses from neighboring peripheral foci of inflammation. III. Nasal mucous polypi; intra-nasal operation on the ethmoidal air cells; purulent leptomeningitis; death; autopsy. J. Laryngol. Otol. 1926;41:717–731. [Google Scholar]
- Tusupbekova M.M., Dosakhanov A.K., Akhmetova D.A., KG N. Morphologic equivalent of neuroendocrinal syndromes under action of polymetallic radioactive dust. Med. & Ecol. (Russian) 2002;2:45–47. [Google Scholar]
- Uebayashi H., Hatanaka T., Kanemura F., Tonosaki K. Acute anosmia in the mouse: behavioral discrimination among the four basic taste substances. Physiol. Behav. 2001;72:291–296. doi: 10.1016/s0031-9384(00)00441-8. [DOI] [PubMed] [Google Scholar]
- Vallieres L., Campbell I.L., Gage F.H., Sawchenko P.E. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J. Neurosci. 2002;22:486–492. doi: 10.1523/JNEUROSCI.22-02-00486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol A.N., Dalton K.P., Rose J.K. Relative neurotropism of a recombinant rhabdovirus expressing a green fluorescent envelope glycoprotein. J. Virol. 2002;76:1309–1327. doi: 10.1128/JVI.76.3.1309-1327.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan A., Nath A., Ming G.L., Song H. Adult hippocampal neurogenesis: regulation by HIV and drugs of abuse. Cell. Mol. Life Sci. 2007;64:2120–2132. doi: 10.1007/s00018-007-7063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas T.K., Shahiwala A., Marathe S., Misra A. Intranasal drug delivery for brain targeting. Curr. Drug Deliv. 2005;2:165–175. doi: 10.2174/1567201053586047. [DOI] [PubMed] [Google Scholar]
- Wang J.H., Kwon H.J., Jang Y.J. Detection of parainfluenza virus 3 in turbinate epithelial cells of postviral olfactory dysfunction patients. Laryngoscope. 2007;117:1445–1449. doi: 10.1097/MLG.0b013e318063e878. [DOI] [PubMed] [Google Scholar]
- Wang X., Fu S., Wang Y., Yu P., Hu J., Gu W., Xu X.M., Lu P. Interleukin-1beta mediates proliferation and differentiation of multipotent neural precursor cells through the activation of SAPK/JNK pathway. Mol. Cell. Neurosci. 2007;36:343–354. doi: 10.1016/j.mcn.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Wang B., Feng W.Y., Wang M., Shi J.M., Zhang F., Ouyang H., Zhao Y.L., Chai Z.F., Huang Y.Y., Xie Y.N., Wang H.F., Wang J. Transport of intranasally instilled fine Fe2O3 particles into the brain: micro-distribution, chemical states, and histopathological observations. Biol. Trace Elem. Res. 2007;118:233–243. doi: 10.1007/s12011-007-0028-6. [DOI] [PubMed] [Google Scholar]
- Welge-Lussen A., Wolfensberger M. Olfactory disorders following upper respiratory tract infections. Adv. Otorhinolaryngol. 2006;63:125–132. doi: 10.1159/000093758. [DOI] [PubMed] [Google Scholar]
- Winner B., Cooper-Kuhn C.M., Aigner R., Winkler J., Kuhn H.G. Long-term survival and cell death of newly generated neurons in the adult rat olfactory bulb. Eur. J. Neurosci. 2002;16:1681–1689. doi: 10.1046/j.1460-9568.2002.02238.x. [DOI] [PubMed] [Google Scholar]
- Yamada K., Hasegawa M., Kametani S., Ito S. Nose-to-brain delivery of TS-002, prostaglandin D2 analogue. J. Drug Target. 2007;15:59–66. doi: 10.1080/10611860601029496. [DOI] [PubMed] [Google Scholar]
- Youngentob S.L., Schwob J.E., Saha S., Manglapus G., Jubelt B. Functional consequences following infection of the olfactory system by intranasal infusion of the olfactory bulb line variant (OBLV) of mouse hepatitis strain JHM. Chem. Senses. 2001;26:953–963. doi: 10.1093/chemse/26.8.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Chai X., Frotscher M. Balance between neurogenesis and gliogenesis in the adult hippocampus: role for reelin. Dev. Neurosci. 2007;29:84–90. doi: 10.1159/000096213. [DOI] [PubMed] [Google Scholar]
- Zhao C., Deng W., Gage F.H. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zhang Q.Z., Zha L.S., Zhang Y., Jiang W.M., Lu W., Shi Z.Q., Jiang X.G., Fu S.K. The brain targeting efficiency following nasally applied MPEG-PLA nanoparticles in rats. J. Drug Target. 2006;14:281–290. doi: 10.1080/10611860600721051. [DOI] [PubMed] [Google Scholar]
- Zhu H., Dahlstrom A. Glial fibrillary acidic protein-expressing cells in the neurogenic regions in normal and injured adult brains. J. Neurosci. Res. 2007;85:2783–2792. doi: 10.1002/jnr.21257. [DOI] [PubMed] [Google Scholar]