Abstract
Ischaemia–reperfusion (I/R) injury is a common feature of several diseases associated with high morbidity and mortality, such as stroke and myocardial infarction. The damaged tissue displays cardinal signs of inflammation and microvascular injury that, unless resolved, lead to long-term tissue damage with associated dysfunction. Current therapies are limited and are often associated with many side effects. Increasing evidence suggests that members of the formyl peptide receptor (FPR) family, in particular human FPR2/ALX, might have an important role in the pathophysiology of I/R injury. It was recently demonstrated that several peptides and non-peptidyl small-molecule compounds have anti-inflammatory and pro-resolving properties via their action on members of the FPR family. Here I review this evidence and suggest that FPR ligands, particularly in the brain, could be novel and exciting anti-inflammatory therapeutics for the treatment of a variety of clinical conditions, including stroke.
Introduction
Ischaemia–reperfusion (I/R) contributes to the pathophysiology of many clinical problems such as myocardial infarction, stroke, resuscitation, coronary bypass surgery, frostbite, extension of burn injury and organ transplantation. According to the American Heart Association, more than 1 million people suffer a heart attack each year and approximately 795,000 suffer a stroke. These diseases significantly contribute to the mortality rate and full recovery is unlikely, with the single most important factor being the degree of ischaemic damage at the time of the event. Ischaemia refers to a reduction in blood flow and reperfusion injury is associated with an initial blood-borne neutrophil infiltration, giving rise to an inflammatory response and finally resulting in tissue injury 1, 2, 3.
Although restoration of blood flow to a previously ischaemic region is essential to prevent irreversible tissue damage, reperfusion itself is a double-edged sword and thus is not always beneficial. Although a great deal of damage occurs to the tissue because of reperfusion, a significant amount of injury occurs due to ischaemia itself. During myocardial injury, several events occur that mediate vascular injury, including oxygen free radical production by mitochondrial respiration, activated neutrophils and xanthine oxidase activity [3]. These are all intimately involved in the inflammatory cascade, themselves activating leukocytes, inducing lipid peroxidation and increasing vascular permeability.
Leukocyte recruitment occurs in the microvasculature and involves a complex set of events that can occur both locally and systemically. Both in vivo and in vitro evidence (e.g. antibodies against adhesion molecules; chemotaxis and transmigration assays; flow chamber assays; and real-time studies to visualise cellular interactions in the circulation of anaesthetised animals using intravital microscopy) have demonstrated molecular and cellular pathways involved in this multi-step cascade. The leukocyte adhesion cascade involves: capture, rolling, slow rolling, leukocyte arrest, post-adhesion strengthening, intravascular crawling and paracellular or transcellular transmigration [4] (Figure 1 ).
Figure 1.
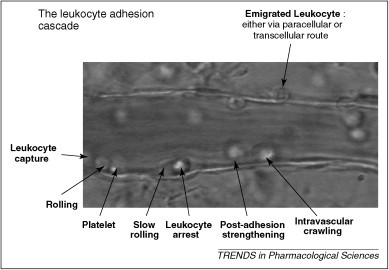
Example of an inflamed murine mesenteric venule. This picture demonstrates the steps involved in the inflammatory cascade: leukocyte capture, rolling, slow rolling, arrest, post-adhesion strengthening, intravascular crawling and either paracellular or ranscellular migration of leukocytes into the surrounding tissue.
Living organisms are capable of recovering from different pathogens and noxious stimuli that enter the system. Injury to the body provokes a host acute inflammatory response of pain, fever, redness, swelling and, in the case of chronic inflammation, loss of function. The inflammatory response is characterised by leukocyte infiltration, which is achieved by integrins, adhesion, selectins and glycoprotein selectin ligands in response to cytokines and chemotactic factor gradients. These factors mediate the inflammatory cascade, involving leukocyte rolling, adhesion and transendothelial migration 3, 4 (Figure 1). Numerous receptors modulate the host inflammatory response. Under abnormal situations, the body's response can assume the character of a disease itself [5], resulting in tissue damage, as observed in pathological conditions such as I/R injury.
Microvascular dysfunctions are observed on reperfusion of ischaemic tissue, including endothelial-dependent dilation of arterioles and increased fluid filtration and leukocyte plugging in capillaries, leading to a no-reflow phenomenon [3]. On the basis of current understanding of I/R, several potential treatments have been suggested based on mechanical (e.g. coronary angioplasty or stenting) or pharmacological (e.g. anti-platelet therapy, tissue plasminogen activator administration, neutralization of already secreted pro-inflammatory cytokines, administration of anti-inflammatory cytokines, factors favouring mesenchymal stem cell implantation and mobilization) restoration of blood flow 2, 6, 7. Other therapeutic approaches have been explored such as targeting of receptors implicated in regulating and resolving the inflammation associated with I/R. One such family of receptors is the G-protein-coupled receptors (GPCRs) known as the formyl peptide receptors (FPRs), whose involvement in I/R injury has been demonstrated in a variety of different tissues, and thus ligands for FPRs might play a role in host defense.
Table 1.
Tissue distribution and IUPHAR nomenclature for human and murine FPRs
| Species | Previous nomenclature | IUPHAR nomenclature | Tissue and cellular distribution |
|---|---|---|---|
| Human | Formyl-peptide receptor; FPR; NFPR; FMLPR | FPR1 | Adrenal glands, adrenal cortical cells, astrocytes, bone marrow, carcinoma cells, CNS, colon, endothelial cells, epithelial cells, eye, fibroblasts, heart, hepatocytes, immature DCs, kidney, Kupffer cells, liver, lung, macrophages, microglial cells, monocytes, neuroblastoma cells, neutrophils, ovary, placenta, platelets, spleen |
| Formyl-peptide receptor like 1; FPRL1; lipoxin A4 receptor (LXA4R); ALXR; FPRH1; HM63; RFP; FMLPX; FPR2A | FPR2/ALX | Astrocytes, bone marrow, brain, endothelial cells, epithelial cells, fibroblasts, hepatocytes, immature DCs, lung, macrophages, microglial cells, monocytes, neuroblastoma cells, neutrophils, placenta, spleen, T and B lymphocytes, testis | |
| Formyl-peptide receptor like 2; FPRL2; FPRH2; FMLPY; RMLP-related receptor 1 | FPR3 | Adrenal gland, DCs, HL-60 cells, liver, lung, lymph nodes, macrophages, monocytes, placenta, small intestine, spleen, trachea | |
| Mouse | Fpr1 | Fpr1 | Adrenal gland, anterior pituitary, DCs, hippocampus, hypothalamus, liver, lung, microglia, mononuclear cells, neutrophils, spleen |
| Fpr-rs2 | Fpr2 | Anterior pituitary, adrenal gland, DCs, hippocampus, hypothalamus, lungs, microglia, neutrophils, spleen | |
| Fpr-rs1; mALXR; fprL1 | Fpr3 | Adrenal gland, anterior pituitary, heart, hippocampus, hypothalamus, liver, lung, microglia, neutrophils, spleen | |
| Fpr-rs3 | Fpr-rs3 | Skeletal muscle | |
| Fpr-rs4 | Fpr-rs4 | ? | |
| Fpr-rs5 | Fpr-rs5 | ? | |
| Fpr-rs6 | Fpr-rs6 | Brain, skeletal muscle, spleen, testis | |
| Fpr-rs7 | Fpr-rs7 | Heart, liver, lung, pancreas, smooth muscle, spleen | |
| Fpr-rs8 | Fpr-rs8 | ? |
The FPRs are promiscuous in their ability to bind different ligands, such as formyl-Met-Leu-Phe (fMLP) and lipoxin A4 (LXA4) (Box 1 , Table 2 ). However, the receptors are expressed in different cells and tissues, raising the possibility that FPRs have far more diverse and complex roles in biology. Interestingly, resolution of inflammation involves the formation of endogenous anti-inflammatory mediators, which signals the termination of recruitment and removal of inflammatory cells from the inflammatory locus [8]. In this review, I summarise the evidence for proposing FPR ligands as novel anti-inflammatory and pro-resolving therapeutics and focus on their role in reducing the detrimental effects associated with I/R injury.
Box 1. FPR signalling and nomenclature.
FPR signalling
Leukocyte responses to chemoattractants, such as fMLP, require binding and activation of pertussin-toxin-sensitive GPCR coupling to Giα2 or Giα3, which triggers multiple second messengers through phospholipase C (PLC), PLD and PLA2 activation [8]. fMLP stimulation of leukocytes induces shape changes, chemotaxis, adhesion, phagocytosis and the release of superoxide anions and granule contents, leading to tissue damage, as observed in inflammation and infarction [86]. All major neutrophil functions stimulated by fMLF can be inhibited by treatment of the cells with pertussis toxin, indicating that the FPRs belong to the Gi family of heterotrimeric G proteins [11].
All three human FPRs are clustered on chromosomal region 19q13.3 [11], whereas the murine equivalents are arranged in two clusters on chromosome 17 (A3.2) together with an additional pseudogene (ΨFpr2) [18] based not on receptor structure, but on receptor agonists (Table 2).
FPR nomenclature
Human: Table 1 demonstrates human FPR nomenclature 11, 19. FPR2/ALX shares 69% sequence homology with FPR1. Both FPR2/ALX and FPR3 possess high degrees of amino acid identity (69%) 15, 20, but are activated by different ligands (Table 2). The FPR3 gene encodes a putative protein with 56% amino acid similarity to FPR1 and 72% to FPR2/ALX.
Non-human: A high degree of specificity to human orthologue genes occurs in non-human primates (95–99%) [11]. The situation is complicated in mice, with gene clusters having undergone differential expansion. The mouse genome encodes for at least seven different receptors (Table 1). It is now agreed that murine Fpr1 is 77% identical to human FPR1 [11] and Fpr3 is 76% identical to human FPRL2. Fpr2 binds fMLF with low affinity 11, 18. Fpr-r3, 4, 6 and 7 seem to have no direct counterparts in the human genome [73]. Fpr-rs4 encodes a receptor with a short COOH-terminal domain, whereas Fpr-rs5 has a stop codon in the putative transmembrane domain 6 (more likely to be a pseudogene) [73]. Two additional genes were characterised from screening of a mouse BAC genomic library, termed Fpr-rs6 and Fpr-rs7 [105]. Other orthologues have been found at a molecular level in species such as rabbit and primates and at a functional level in rat [106], guinea pig and horse (as reviewed in [11]).
It is noteworthy that there is chemoattractant receptor-like molecule (CRTH2) from a human Th2 clone that has high amino-acid homology with members of the FPR family. However, the similarity is insufficient to classify CRTH2 as an FPR member.
Table 2.
Non-exhaustive list of agonists and antagonists of human and murine FPRs and examples of specific effectsa
| Receptor | Agonist or | Ligand | Example of a specific effect | Refs |
|---|---|---|---|---|
| antagonist | ||||
| FPR1 | Agonists | Ac2–12 | Cardio-protective in murine model of myocardial infarction | [53] |
| Ac9–25 | Activates neutrophil release of O2− | [76] | ||
| Ac2–26 | Decreases neutrophil–endothelium interactions in flow chamber | [77] | ||
| Annexin 1 | Decreases neutrophil–endothelium interactions in flow chamber | [77] | ||
| Cathepsin G | Chemoattractant for phagocytic leukocytes | [78] | ||
| fMLP and analogues | Defective PMN chemotaxis in juvenile peridontitis in vivo | [79] | ||
| Chemotaxis, lysozyme release, O2− production, including endothelial interaction, all in neutrophils | ||||
| HIV-1 T20 (DP178) | Chemoattractant and activator of peripheral phagocytes | 47, 48 | ||
| HIV-1 T21 (DP107) | Chemoattractant and activator of peripheral phagocytes (low affinity) | [47] | ||
| HIV gp41 | Induces directional migration and calcium mobilization in human monocytes and neutrophils | [49] | ||
| HSV gG-2p20 | Chemoattractant for monocytes and neutrophils | [50] | ||
| LL-37 | Chemoattractant for human peripheral blood neutrophils, monocytes and T cells | [23] | ||
| SRSRY | Directional cell migration on vitronectin-coated filters | [80] | ||
| WKYMVM | Activates neutrophils (low affinity) | [28] | ||
| WKYMVm peptide | Activates neutrophils (low affinity) | [28] | ||
| FPR1 | Antagonists | CDCA | Inhibits neutrophil chemoattraction and migration (high affinity) | [58] |
| CHIPS | Inhibits chemotaxis in S. aureus infection (low affinity) | [55] | ||
| Coronavirus 229E peptides | Ligand binding studies using transfected CHO cells demonstrated antagonsism of FPR2 | [81] | ||
| Coronavirus peptides | Inhibits fMLP interaction in CHO cells | [81] | ||
| Cyclosporine A | Inhibits fMLF-stimulated degranulation, chemotaxis, calcium mobilization of neutrophils | [82] | ||
| Cyclosporine H | Decreased neutrophil activation (high affinity) | [83] | ||
| DCA | Inhibits fMLP-induced monocyte and neutrophil chemotaxis and calcium mobilization | [57] | ||
| Ebola peptides | Inhibits fMLP interaction in CHO cells | [81] | ||
| FLIPr | FPRL1 inhibitory protein (FLIPr) inhibits leukocyte responses to FPR2 agonists | [56] | ||
| HIV-2 peptides | Inhibits fMLP interaction in CHO cells | [81] | ||
| Isopropylureido-FLFLF | Inhibits chemotaxis | [84] | ||
| Spinorphin | Inhibits calcium mobilization in mouse-FPR transfected human embryonic kidney cells | [85] | ||
| tBOC | Decreased neutrophil activation (low affinity) | [83] | ||
| FPR2/ALX | Agonists | Aβ42 | Chemotaxis of mononuclear cells | [86] |
| Annexin 1 | Decreased neutrophil–endothelium interactions (firm adhesion) in flow chamber | [77] | ||
| Antiflammin 2 | Decreased neutrophil–endothelium interactions | [87] | ||
| CRAMP | Chemoattractant for leukocytes | [88] | ||
| D2D388–274 | Inhibits monocyte chemotaxis and integrin-dependent cell adhesion | [89] | ||
| F peptide | Downregulates expression and function of CCR5 and CXCR4 in monocytes | [50] | ||
| fMLP and analogues | Induces chemotaxis, lysozyme release, O2− production in neutrophils | [11] | ||
| Formylated humanin | Chemotaxis of human FPR2-transfected CHO cells | [90] | ||
| HIV-1 T21 (DP107) | Chemoattractant and activator of peripheral phagocytes (low affinity) | [86] | ||
| Humanin | Chemotaxis of human FPR2-transfected CHO cells | [90] | ||
| Lipoxin A4 | Attenuated hind limb I/R-induced lung injury | [69] | ||
| LL37 | Chemoattractant for monocytes, neutrophils and T cells | [23] | ||
| MMK-1 | Chemotaxis and calcium mobilization in monocytes and neutrophils | [31] | ||
| N36 peptide | Chemotaxis and calcium mobilization in monocytes and neutrophils | [49] | ||
| NADH dehydrogenase | Chemotaxis and calcium mobilization in human FPR2-expressing HL-60 cells | [91] | ||
| PACAP27 | Neutrophil chemotaxis and upregulation of CD11b | [92] | ||
| PRP106-126 | Endocytosis in glial cells | [93] | ||
| Quin-C1 | Chemotaxis and secretion of β-glucuronidase in peripheral neutrophils | [94] | ||
| Rana-6 | Chemoattractant of phagocytes | [57] | ||
| Serum amyloid A (SAA) | Chemoattractant for neutrophils | [41] | ||
| T20 (DP178) | Chemoattractant and activator of peripheral phagocytes | 47, 48 | ||
| T21 (DP107) | Chemoattractant and activator of peripheral phagocytes (high affinity) | [47] | ||
| Temporin A | Chemoattractant of phagocytes | [95] | ||
| uPAR84–95 | Basophil chemotaxis | [96] | ||
| V3 peptide | Inhibits monocytic response to chemokines | [97] | ||
| WKYMVM | Activates neutrophils (high affinity); O2− production | [32] | ||
| WKYMVm peptide | Activates neutrophils (high affinity) | [98] | ||
| sCKb8-1 | Alters protein pattern of PMN cells | [99] | ||
| Hp2–20 | Migration and proliferation of gastric epithelial cell lines MKN-28 and AGS | [96] | ||
| Ac2–26 | Decreased neutrophil–endothelium interactions (firm adhesion) in flow chamber | [77] | ||
| Ac2–12 | Cardioprotective in a murine model of myocardial infarction | [53] | ||
| FPR2/ALX | Antagonists | CDCA | Inhibits neutrophil chemoattraction and migration (low affinity) | [95] |
| Coronavirus 229E peptides | Inhibits neutrophil chemoattraction and migration | [58] | ||
| FLIPr | Inhibits calcium mobilization | [56] | ||
| Isopropylureido-FLFLF | Inhibits chemotaxis | [84] | ||
| PBP10 | Inhibits granule mobilization and oxygen radical secretion | [100] | ||
| tBOC | Decreased neutrophil activation (high affinity) | [83] | ||
| WRWWWW | Inhibits oxidative burst from neutrophils, measured as a release of superoxide anions | [100] | ||
| FPR3 | Agonists | Formylated peptides | Triggers dose-dependent migration of fibroblasts in vitro | [15] |
| WKYMVM | Activates neutrophils | [32] | ||
| WKYMVm peptide | Activates neutrophils | [98] | ||
| Annexin 1 | Initiates chemotactic responses in human monocytes | [39] | ||
| Hp(2–20) | Chemoattractant for basophils | [101] | ||
| Humanin | Induces chemotaxis of mononuclear phagocytes in vitro | [102] | ||
| F2L | Promotes calcium mobilization and chemotaxis of monocytes and monocyte-derived DCs | [103] | ||
| FPR3 | Antagonists | ETYIKPWWWVWL | Inhibits fMLP interaction in CHO cells | [81] |
| (from coronavirus 229E) | ||||
| WRWWWW | Inhibits calcium flux | [32] | ||
| Fpr1 | Agonists | Ac2–26 | Protect against experimental myocardial I/R | [53] |
| Annexin 1 | Protect against experimental myocardial I/R | [53] | ||
| fMLP and analogues | Chemoattractant for leukocytes | [51] | ||
| T20 (DP178) | Induced chemotaxis in primary PMNs and transfected cells in vitro | [48] | ||
| WKYMVm | Induced chemotaxis of transfected cells in vitro | [99] | ||
| Fpr1 | Antagonists | Isopropylureido-FLFLF | Inhibits chemotaxis | [84] |
| Spinorphin | Inhibits chemotaxis | [84] | ||
| tBOC | Prevents the protective effect of AnxA1 peptides in murine mesenteric I/R preparations in vivo | [36] | ||
| Fpr2 | Agonists | Aβ42 | Induced chemotaxis of transfected cells in vitro | [102] |
| Ac2–26 | Decreases adhesion and emigration in inflamed mesentery in vivo | [36] | ||
| Annexin 1 | Cerebroprotective in a murine stroke model in vivo | [54] | ||
| CRAMP | Chemoattractant for leukocytes | [88] | ||
| F2L | Induced chemotaxis in transfected cells and primary PMNs in vitro | [74] | ||
| fMLP and analogues | Chemoattractant for neutrophils | [105] | ||
| Humanin | Induces chemotaxis of mononuclear phagocytes in vitro | [102] | ||
| MMK-1 | Induced chemotaxis of PMNs into air pouch in vivo | [20] | ||
| SAA | Induced chemotaxis of primary PMNs in vitro | [85] | ||
| T20 (DP178) | Induced chemotaxis in transfected cells but not primary PMNs in vitro | [48] | ||
| V3 peptide | Chemoattractant | [14] | ||
| WKYMVm | Potent stimulant for murine neutrophils | [51] | ||
| Fpr2 | Antagonists | Isopropylureido-FLFLF | Inhibits chemotaxis | [84] |
| tBOC | Decreased neutrophil activation (high affinity) | [83] | ||
| Aspirin-triggered lipoxins | Inhibited migration of PMNs into inflamed air pouch in vivo | [20] | ||
| Fpr3 |
CHIPS,chemotaxis inhibitory protein of S. aureus; CHO, Chinese hamster ovary; Hp(2–20), H. pylori peptide Hp(2–20); N36, synthetic peptide derived from HIV-1; PMN, polymorphonucleocyte; spinorphin, LVVYPWT; V3, synthetic peptide derived from HIV-1.
The FPR family: tissue and cellular distribution
FPR1 is one of the best-studied GPCRs, dating back to the early 1970s [9], and its activation produces a range of inflammatory responses associated with I/R, such as stimulation of leukocyte migration [8]. FPR1 has been found in a variety of different tissues and cells (Table 2) involved in inflammation, such as endothelial cells, platelets and dendritic cells (DCs) [10] (possibly important for modulation of T-cell activation) [11]. FPR2/ALX is equally widely distributed, with an interesting presence, in addition to peripheral cells and tissues, in brain and spinal cord, and is functionally expressed in glial cells, fibroblasts and astrocytes 12, 13, 14, 15.
Functional FPRs are widely expressed in non-lymphoid tissues, including normal human lung and skin fibroblasts and the human fibrosarcoma cell line HT-1080. Van Compernolle demonstrated that fibroblasts express FPRs that respond in a chemoattractant manner on treatment with fMLP [15], thus opening up a new area for a role of non-leukocyte cell types in innate immune responses (recently reviewed in [11]). FPR3 seems to be present not in neutrophils, but in monocytes [11]. Fascinatingly, this tissue distribution varies with the monocyte differentiation stage 10, 11, with mature DCs mainly expressing FPR3 [11].
Cui et al. [14] used primary microglia cells to demonstrate expression of FPR1 and FPR2/ALX genes; on stimulation with bacterial lipopolysaccharide (LPS), these cells undergo FPR2/ALX-mediated activation, leading to the suggestion that the blood brain barrier (BBB), under normal conditions, protects microglial cells. When endotoxaemia occurs, however, microvessels form an incomplete BBB and LPS is able to stimulate microglial cells, which assume macrophage characteristics and thus play a role in the inflammatory process [16]. Increased inflammatory events, such as leukocyte rolling and adhesion, occur in the murine cerebral microcirculation after endotoxaemia (something not observed under normal conditions) and seem to be mediated by FPR2/ALX (Gavins et al., unpublished observations). The increase in inflammatory events such as leukocyte rolling and adhesion observed following endotoxaemia also occurs during stroke. The role of FPR2/ALX in stroke is of great interest to my research team and the importance of this receptor as a potential therapeutic target is currently being investigated. The role of the FPRs in cerebral I/R is discussed in more detail later in this review.
A variety of different agonists and antagonists are currently availbale for the FPR family (Table 2). It is not possible to cover all of them within this review, so I have focussed on the more widely used ones.
FPR pharmacology
Agonists
The emergence of more sensitive techniques, such as computer-assisted 3D modelling, has helped us to understand how ligands bind to different receptors. Several natural formylated peptides have been purified from bacterial supernatants and exhibit the ability to activate human leukocytes [17]. fMLP is a widely used agonist of the FPR family and binds to FPR1 with approximately 1000-fold higher affinity than FPR2/ALX (K d values in the picomolar to low nanomolar range), resulting in calcium mobilization and neutrophil activation [18]. Evidence suggests that FPR1 and FPR2/ALX activate the same signal response downstream of the receptor, resulting from activation produced by different ligands 11, 19. FPR2/ALX is a low-affinity receptor for fMLP (K d 430 nM) 11, 20 that produces a chemotactic response at micromolar concentrations. (FPR3 has a K d value of ∼10 μM [21]). LXA4 also interacts with FPR2/ALX (K d ∼0.5 nM) 22, 23, inhibiting the pro-inflammatory responses induced by both FPR2/ALX ligands in neutrophils and non-FPR2/ALX ligands in epithelial cell lines 23, 24, 25. These low K d values demonstrate high affinity for the receptor.
To characterise further FPR interactions in phagocytes and subsequent cellular activation, several fMLP-OMe analogues have been synthesized [26] including for-Met-Leu-Cys(OMe)-Cys-Leu-Met-fpr, which binds to FPR1 [27]. Recently, fMLP-OMe analogues with receptor affinity greater than that of the parent fMLP-OMe have been synthesized, creating the potential for use as carriers for drugs [11].
Construction and screening of random peptide libraries have become useful tools in developing biologically active agents such as WKYMVm, which stimulates human B lymphocyte and monocytic cell lines and neutrophils 28, 29 through both FPR1 and FPR2/ALX. In 1998, Klein isolated many small peptide sequences that react with FPR1 and FPR2/ALX [30], such as MMK-1 (LESIFRSLLFRVM), a potent FPR2/ALX-specific agonist [31]. Other synthetic peptides such as His-Phe-Tyr-Leu-Pro-Met-NH2 (HFYLPM) stimulate both monocytes and neutrophils via FPR2/ALX binding [32]. The peptide fragment of NADH dehydrogenase subunit 1 (MYFINILTL) is an agonist specific for FPR2/ALX. Other recent screening studies include a 152 GPCR screen that identified a novel 21-amino-acid peptide agonist of FPR2/ALX and FPR3 termed CGEN-855A (TIPMFVPESTSKLQKFTSWFM-amide) [33] and a screen for unique non-peptide agonists of FPR1/FPR2/ALX [33]. These screens demonstrate that agonists of FPR1/FPR-ALX have wide chemical diversity and analysis of these and future agonists will enhance our knowledge of ligand–FPR interactions [34].
Both exogenous and endogenous ligands interact with the FPR family. One particular endogenous ligand that has been widely studied is the glucocorticoid-regulated protein annexin A1 (AnxA1), which counteracts leukocyte extravasation (Figure 1) 35, 36, 37. Using calcium binding assays and L-selectin shedding protocols, Walther et al. demonstrated that AnxA1 N-terminal-derived peptides act on human neutrophils through FPR1 [38]. It was then shown that AnxA1 peptides initiate chemotactic responses in human monocytes, desensitizing cells to subsequent stimulations [39].
Other endogenous ligands with physiological relevance because of their involvement in pathological conditions are the acute-phase protein serum amyloid A (SAA) 40, 41, and Aβ42 14, 42 and PrP106–126 (a prion protein fragment) [43], found in prion disease, all of which are required in high micromolar concentrations to activate FPR2/ALX. Serum SAA concentrations increase during acute-phase responses, causing tissue and organ amyloidosis in chronic inflammation 44, 45 by inducing chemotaxis of phagocytic leukocytes through FPR2/ALX 40, 41. It is thought that pathophysiological concentrations of SAA activate FPR2/ALX and could therefore be used as biomarker for inflammatory disease 46, 47.
Aβ42 is an enzymatic cleavage fragment of the amyloid precursor protein (APP). Its aggregated form is the major component of senile plaques, found in brain tissue of patients with Alzheimer's disease (AD). Aβ42 has been implicated in neurodegeneration and pro-inflammatory responses observed in AD; it activates FPR2/ALX, suggesting that FPR2/ALX might represent a potential therapeutic target for AD treatment 14, 42. PrP106–126 stimulation of FPR2ALX on human monocytes caused internalization of the receptor and a release of pro-inflammatory mediators [43].
Other potential ligands, including HIV-envelope proteins gp41 (T20/DP178, T21/DP107 and N36) and gp120 (F and V3 peptides), interact with FPR1 and/or FPR2/ALX. T20/DP178 interacts with FPR1 in vitro [47] and mouse Fpr1 in vivo [48]; T21/DP107 uses both FPR1 and FPR2/ALX (higher efficacy FPR2/ALX) [47] and N36 (overlaps with T21/DP107) signals through FPR2/ALX [49]. In terms of the gp120-derived peptides, both F peptide (20 amino acids) and V3 peptide (33 amino acids) interact with FPR2/ALX [50]. The Helicobacter pylori peptide Hp(2–20) also acts via FPR2/ALX, inducing NADPH oxidase activation in human neutrophils [51].
It is clear from the above that a large amount of research (still ongoing) has been performed to design a successful ligand for the FPR family. A key aspect for this process relies on target specificity, namely a ligand with high affinity for FPR2/ALX and almost no affinity for FPR1. Ligand target specificity can also reduce the risk of harmful side effects produced by, for example, non-specific binding.
Antagonists
There are various antagonists, such as those formed by replacing the N-formyl group of fMLP with t-butyloxycarbonyl (tBOC) or isopropyl ureido, yielding peptides that block fMLP interaction with its receptor 14, 52. Several studies have used these Boc derivatives (that are non-selective antagonists to all FPR family members) to demonstrate FPR family involvement in diseases such as myocardial infarction and stroke 53, 54.
More selective antagonists are available, such as the chemotaxis inhibitory protein (C5aR) of Staphylococcus aureus (CHIPS), which is present in over 50% of clinical strains of S. aureus and antagonises FPR2/ALX and the complement component 5a receptor 1. More specifically, a peptide fragment of CHIPS (FTFEPFPTNEEIESN) is selective for FPR2 and not C5aR [55]. Another protein of S. aureus that exhibits selective binding of and activation by MMK-1 is FPRL1 inhibitory protein (FLIPr), which binds directly to FPR1 and FPR2, but not FPR3 [56].
The bile acids deoxycholic acid (DCA) and chenodeoxycholic acid (CDCA) antagonise both FPR1 and FPR2/ALX [57], possibly suppressing anti-bacterial responses in individuals with cholestasis [58]. DCA inhibits fMLP-induced monocyte and neutrophil migration and calcium mobilization though fMLP binding to its receptors, whereas CDCA selectively inhibits monocyte chemotaxis and calcium flux induced by fMLP and W peptide, suggesting a mechanism for inhibition of inflammation and suppression of innate immune response 57, 58. Quin-c7, a synthetic non-peptide antagonist of FPR2/ALX, has been developed through chemical modification of Quin-C7 (an FPR2/ALX agonist) [59].
Although different ligands continue to be identified for the FPRs, there is a clear need to develop more potent and specific agonists and antagonists to offer a greater therapeutic potential devoid of major side effects.
Involvement of FPRs in I/R
Our understanding of the complexity and need for a balance between pro- and anti-inflammatory pathways to retain a homeostatic environment 60, 61, 62, 63 has given rise to a great number of possible drug targets of the FPR family. In particular, targets activated by endogenous anti-inflammatory mediators could be used for drug discovery for both I/R and other disease states because they are likely to have fewer side effects and their application would be similar to mechanisms that the body uses to reject inflammation [60]. One particular endogenous ligand of interest is the 37-kDa protein AnxA1, whose involvement with the FPR family has been widely studied and which affords protection in a number of I/R models. Initially, cardioprotection by AnxA1 was observed in rat myocardial I/R injury [64]. It was not until 2001, however, that La et al. [53] investigated the mechanistic and molecular basis of AnxA1 action and demonstrated that the pharmacophore responsible for cardioprotection lies within amino acids 2–12, with correct alignment being crucial [53]. A clinical application was hypothesised on the basis of cardioprotection being retained by the AnxA1 N-terminal peptide Ac2–26 (also termed AnxA1Ac2–26) when administered 60 min into reperfusion [53]. Boc2 administration demonstrated for the first time that FPRs are involved in the cardioprotective properties of AnxA1. Histology demonstrated that neutrophil influx into the myocardium induces AnxA1 expression, which was absent in sham and naïve animals.
Although rat models provided novel results, murine models are particularly useful given our current knowledge of the genome and the ease of genetic manipulation (Table 3 ). The Fpr1-null mouse provided evidence that FPR1 does not mediate the cardioprotection afforded by peptide AnxA1Ac2–26 [65]. Neutrophil depletion studies did not affect the extent of acute heart injury, but affected protection afforded by peptide AnxA1Ac2–26. This study provided evidence of the role of mouse Fpr2 and/or Fpr3 and circulating neutrophils in mediating AnxA1-induced cardioprotection.
Table 3.
Examples of agonists used in different I/R modelsa
| Species | I/R model | Agonist | Protective | Identified as ligand for which FPR family member? | Ref. |
|---|---|---|---|---|---|
| hBLTR TG mice | Hind-limb I/R-induced second-organ lung injury (3-h I+3-h R) | ATL analogue | Yes | ALX/FPR2 | [67] |
| LXA4 | ALX/FPR2 | [67] | |||
| Male FVB mice | Hind-limb I/R-induced second-organ lung injury (1-h I+1-h R) | ATL analogue ZK-994 | Yes | ALX/FPR2 | [69] |
| ATL analogue ZK-142 | Yes | ALX/FPR2 | [69] | ||
| ATLa | No | ALX/FPR2 | [69] | ||
| NIH Swiss mice | Ischaemic acute renal failure (30-min I+24-h R) | 15-Epi-16-(p-fluorophenoxy)-lipoxin A4-methyl ester | Yes | ALX/FPR2 | [68] |
| Male Sprague-Dawley rats | Myocardial I/R (25-min I + 2-h R) | Human recombinant annexin 1 | Yes | FPR1 | [60] |
| Annexin 1 peptide Ac2–26 | Yes | FPR1 | [60] | ||
| Annexin 1 peptide Ac2–12 | Yes | FPR1 | [60] | ||
| Annexin 1 peptide scrambled Ac2–12 | No | [60] | |||
| Annexin 1 peptide Ac2–6 | No | [60] | |||
| fMLP | Yes | FPR1 | [60] | ||
| Male Sprague-Dawley rats | Myocardial I/R (25-min I+2-h R) | Annexin 1/5 | No | [7] | |
| Denatured preparation of annexin 1 | No | [7] | |||
| Human recombinant annexin 1 | Yes | Unknown receptor | [7] | ||
| Male C57bl/6 mice | Myocardial I/R (25-min I+1-h R) | Annexin 1 peptide Ac2–26 | Yes | FPR3 | [65] |
| W peptide | Yes | ALX/FPR2, Fpr3 | [65] | ||
| ATLa-ME | Yes | ALX/FPR2, Fpr3 | [65] | ||
| Male Fpr1-null mice | Myocardial I/R (25-min I+1-h R) | Peptide Ac2–26 | Yes | Excluded involvement of Fpr1 | [65] |
| W peptide | Yes | Excluded involvement of Fpr1 | [65] | ||
| ATLa-ME | Yes | ALX/FPR2, Fpr3 | [65] | ||
| Male albino mice | Myocardial I/R (25-min I+2-h R) | CGEN-855A | Yes | ALX/FPR2 | [33] |
| Male Sprague-Dawley rats | Myocardial I/R (30-min I+3-h R) | CGEN-855A | Yes | ALX/FPR2 | [33] |
| Male C57bl/6 mice | Mesenteric I/R (30-min I+45-min R) | Annexin 1 peptide Ac2–26 | Yes | Fpr1 and Fpr3 | [36] |
| Male Fpr1-null mice | Annexin 1 peptide Ac2–26 | Yes, 50% | Fpr1 and Fpr3 | [36] | |
| ATLa | Yes | Fpr1 and Fpr3 | [36] | ||
| fMLP | No | [36] | |||
| Male Sprague-Dawley rats | Splanchnic artery I/R | Annexin 1 peptide Ac2–26 | Yes | Fpr1 | [104] |
| Male Fpr2-null mice | Mesenteric I/R (30-min I+45-min R) | None | Yes | Fpr2 | [71] |
ATL, aspirin-triggered lipoxin; ATLa, 15-epi-16-(p-fluoro)-phenoxy lipoxin A4; ATLa-ME, ATLa methyl ester; hBLTR, human leukotriene B4 receptor; ATL analogue, 15-epi-16-(p-fluoro)-phenoxy-LXA4 [15(S)-16-(p-fluoro)-phenoxy-LXA4 methyl ester]; peptide Ac2–6, acetyl-AMVSE; peptide Ac2–12, acetyl-AMVSEFLKQAW; peptide scrambled Ac2–12, acetyl-SVEQKMWALFA; peptide Ac2–26, acetyl-AMVSEFLKQAWIENEEQEYVVQTVK; W peptide, WKYMVm (Trp-Lys-Tyr-Met-Val-D-Met).
Since reports providing theoretical frameworks for considering ALX/FPR2 as a putative target for I/R injury were published 65, 66, 67, 68, a number of novel ligands have been identified, such as the peptide and ALX/FPR2 agonist CGEN-855A, which has cardioprotective effects in rat and murine myocardial I/R, reducing both infarct size (by 25% and 36%, respectively) and murine troponin I levels [36]. Thus, CGEN-855A elicits cardioprotection similar to the effect reported for other ALX/FPR2 agonists such as AnxA1 [65]. This study provides further evidence of a beneficial effect of the ALX/FPR2 pathway in inflammation [36].
Other agonists of ALX/FPR2 exhibit protective effects in I/R models, such as15-epi-16-(p-fluorophenoxy)-LXA4-methyl ester [15-epi-16-(FPhO)-LXA4-Me], which is protective in renal I/R in part by modulating cytokine and chemokine expression and neutrophil recruitment [66]. The first evidence of ALX/FPR2 activation by aspirin-triggered lipoxins was provided by Chiang et al., who demonstrated protection from second-organ reperfusion injury [67].
The novel 3-oxa-aspirin-triggered 15-epi-lipoxin analogues ZK-994 and ZK-142 have exhibited inhibition of neutrophil accumulation in murine hind-limb I/R-induced second-organ lung injury [69].
Intravital microscopy (IVM) has been used in I/R studies to assess the effect of FPR ligands (Figure 1). IVM is a real-time qualitative and quantitative method for observing cell–cell interactions in the microcirculation of an anaesthetized animal [70]. Our own I/R studies in the mesentery (30 min I+45 min R, followed by mesenteric visualization using IVM) of C57BL/6 and Fpr1-null mice demonstrated that more than one FPR family member mediates the effects of peptide AnxA1Ac2–26, possibly including mouse Fpr2. These effects are similar to those observed in LPS-induced damage of the mouse mesentery (Hughes et al., unpublished data).
The availability of genetically modified mice has facilitated characterisation and understanding of the FPR family, but differences between species remains an issue. However, very recent studies using Fpr2-null mice and IVM have revealed a marked increase in inflammation, as demonstrated by an increase in cell adhesion and emigration following mesenteric I/R [71]. These Fpr2-null mice also had an augmented response to carrageenan-induced paw edema in comparison to their wild-type counterparts [71]. These studies, along with studies in my own laboratory looking at the effects of stroke in Fpr2-null mice (Gavins, unpublished data), further demonstrate that FPR2/ALX is an anti-inflammatory receptor and serves a function in the host defence response.
It is clear that I/R elicits an acute inflammatory response that is characterised by inflammatory cell recruitment, oxidative stress and the failure of the endothelial barrier, and contributes to the pathogenesis of many diseases. One area in which targeting of inflammatory markers has failed to yield successful therapeutic drug candidates is stroke. Evidence of FPRs and their agonists as possible novel therapeutic targets for the treatment of cerebral I/R injury is now discussed.
FPRs in cerebral I/R
When cerebral blood flow is restored following ischaemia, several cascades are initiated, leading to activation of blood-borne and resident cells (microglia, astrocytes and neurons) [72]. The actual contribution of resident cells versus blood-borne cells has yet to be fully quantified and might explain why clinical trials targeting one specific aspect of the inflammatory cascade have failed. A neuroprotective role for AnxA1, mediated via FPRs, has been demonstrated in studies using a middle cerebral artery occlusion model combined with IVM to quantify cerebral cellular interactions following stroke [54]. Peptide AnxA1Ac2–26 decreased infarct volume, leukocyte adhesion and markers of inflammation. Microglia might, in part, contribute to these inflammatory responses [54]. Both blood cells and microglia express mouse Fpr2, which might have mediated the effects of peptide AnxA1Ac2–26 in this study. Further studies are being performed to validate this theory, with current data suggesting that microglial cell inhibition reduces brain inflammation post-I/R (Gavins and Perretti, unpublished data). Figure 2 demonstrates how AnxA1 could act in an autocrine and/or paracrine fashion with the members of the FPR family to reduce inflammation by (i) causing detachment of leukocytes within the microvasculature and (ii) inhibiting the production of cytotoxic proinflammatory mediators by activated microglial cells, both of which are thought to play a role in cerebrovascular events such as stroke [54].
Figure 2.
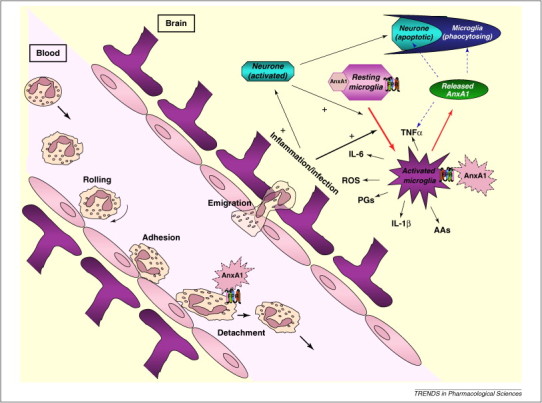
Schematic of how the FPR agonist AnxA1 could reduce inflammation in the brain following I/R injury and exert neuroprotection. Neutrophils are activated after I/R injury. They roll, adhere and migrate into the tissue. AnxA1 is released from neutrophil cytosolic granules to the cell surface, where it interacts with FPRs in an autocrine or paracrine fashion. Administration of AnxA1 (or the N-terminal peptide Ac2–26) causes the leukocyte detachment from endothelial cells. Resident cells, such as microglia, are also activated by I/R injury and, like blood-borne cells, release a plethora of damaging mediators such as reactive oxygen species, cytokines (TNF-α, IL-1β) and leukotrienes. The involvement of AnxA1 in these processes might be similar to that observed in the peripheral microvasculature, namely to promote resolution. The receptors that mediate this process in stroke remain unknown, although evidence suggests that FPR/ALX plays a role.
Whether the mechanisms that mediate inflammatory responses in the brain are the same as those observed in the periphery remains to be determined. It is likely that similar endogenous pathways are available throughout the host, the mechanism(s) of action of which is to inhibit molecular and cellular responses to (I/R) injury, such as for CGEN-855A and AnxA1 peptides. These bioactive peptides and associated FPRs could represent potential therapeutic targets [33].
The neuroprotective peptide humanin is an agonist of FPR2/ALX and an endogenous ligand of FPR3 [73]. Another endogenous ligand of FPR3 is a peptide isolated from the human haem-binding protein termed F2L (Ac MLGMIKNSLFFGSVETWPWQVLNH2). F2L has weak activity for FPR2/ALX and is not active for FPR1 [74]. However, it is an agonist for FPR3, binding and activating the receptor at concentrations in the low nanomolar range [75].
Concluding remarks
This review has drawn together compelling evidence to suggest that ligands to the FPRs could provide novel anti-inflammatory and pro-resolving therapeutics that reduce the damage associated with I/R injury. The promiscuity of these receptors in binding different ligands, coupled with their presence in different cells and tissue, indicates a diverse role in multiple biological settings. Improved understanding of these fundamental functions would instruct therapeutic target identification and drug development. Thus, further study of endogenous anti-inflammatory agonists, such as AnxA1, and the lipoxins could lead to the identification and development of better therapeutics capable of limiting the detrimental effects of I/R injury and promoting resolution, with fewer side effects.
Glossary
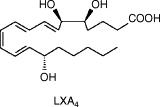
- Lipoxin A4 (LXA4)
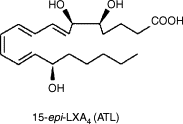
- 15-Epi-LXA4
an aspirin-triggered lipoxin
- Annexin Ac2–26
N-terminal domain of annexin A1, representing the bioactive peptide spanning amino acids 2–26.
- Complement 5a (C5a) receptor (C5AR)
also known as complement component 5a receptor 1 and CD88 (cluster of differentiation 88); a G-protein-coupled receptor for C5a.
- Dissociation constant (Kd)
measure of how tightly a ligand binds to a particular receptor. Low ligand Kd values reflect a high affinity for the receptor.
- G-protein-coupled receptors (GPCRs)
proteins with an extracellular domain and an intracellular C-terminal tail, separated by three extracellular loops and three intracellular loops. They are the largest family of membrane receptors and couple to intracellular effector systems via a G protein.
- Intravital microscopy (IVM)
real-time qualitative and quantitative method for observing cell–cell interactions in the microcirculation of an anaesthetized animal.
- Neutrophil
white blood cell.
References
- 1.Romson J.L. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983;67:1016–1023. doi: 10.1161/01.cir.67.5.1016. [DOI] [PubMed] [Google Scholar]
- 2.Steffens S. The inflammatory response as a target to reduce myocardial ischaemia and reperfusion injury. Thromb. Haemost. 2009;102:240–247. doi: 10.1160/TH08-12-0837. [DOI] [PubMed] [Google Scholar]
- 3.Carden D.L., Granger D.N. Pathophysiology of ischaemia–reperfusion injury. J. Pathol. 2000;190:255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Ley K. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 5.Flower R.J. Lipocortin and the mechanism of action of the glucocorticoids. Br. J. Pharmacol. 1988;94:987–1015. doi: 10.1111/j.1476-5381.1988.tb11614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexandrov A.V. Current and future recanalization strategies for acute ischemic stroke. J. Intern. Med. 2010;267:209–219. doi: 10.1111/j.1365-2796.2009.02206.x. [DOI] [PubMed] [Google Scholar]
- 7.Gavins F. The evolving paradigm for blood cell–endothelial cell interactions in the cerebral microcirculation. Microcirculation. 2007;14:667–681. doi: 10.1080/10739680701404903. [DOI] [PubMed] [Google Scholar]
- 8.Ali H. Chemoattractant receptor cross-desensitization. J. Biol. Chem. 1999;274:6027–6030. doi: 10.1074/jbc.274.10.6027. [DOI] [PubMed] [Google Scholar]
- 9.Schiffmann E. N-Formylmethionyl peptides as chemoattractants for leucocytes. Proc. Natl. Acad. Sci. U. S. A. 1975;72:1059–1062. doi: 10.1073/pnas.72.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang D. Differential regulation of formyl peptide receptor-like 1 expression during the differentiation of monocytes to dendritic cells and macrophages. J. Immunol. 2001;166:4092–4098. doi: 10.4049/jimmunol.166.6.4092. [DOI] [PubMed] [Google Scholar]
- 11.Ye R.D. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol. Rev. 2009;61:119–161. doi: 10.1124/pr.109.001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lacy M. Expression of the receptors for the C5a anaphylatoxin, interleukin-8 and FMLP by human astrocytes and microglia. J. Neuroimmunol. 1995;61:71–78. doi: 10.1016/0165-5728(95)00075-d. [DOI] [PubMed] [Google Scholar]
- 13.Le Y. Expression of functional formyl peptide receptors by human astrocytoma cell lines. J. Neuroimmunol. 2000;111:102–108. doi: 10.1016/s0165-5728(00)00373-8. [DOI] [PubMed] [Google Scholar]
- 14.Cui Y. Potential role of the formyl peptide receptor-like 1 (FPRL1) in inflammatory aspects of Alzheimer's disease. J. Leukoc. Biol. 2002;72:628–635. [PubMed] [Google Scholar]
- 15.VanCompernolle S.E. Expression and function of formyl peptide receptors on human fibroblast cells. J. Immunol. 2003;171:2050–2056. doi: 10.4049/jimmunol.171.4.2050. [DOI] [PubMed] [Google Scholar]
- 16.Panaro M.A. Biological role of the N-formyl peptide receptors. Immunopharmacol. Immunotoxicol. 2006;28:103–127. doi: 10.1080/08923970600625975. [DOI] [PubMed] [Google Scholar]
- 17.Czapiga M. Human platelets exhibit chemotaxis using functional N-formyl peptide receptors. Exp. Hematol. 2005;33:73–84. doi: 10.1016/j.exphem.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Prossnitz E.R., Ye R.D. The N-formyl peptide receptor: a model for the study of chemoattractant receptor structure and function. Pharmacol. Ther. 1997;74:73–102. doi: 10.1016/s0163-7258(96)00203-3. [DOI] [PubMed] [Google Scholar]
- 19.Brink C. International Union of Pharmacology XXXVII. Nomenclature for leukotriene and lipoxin receptors. Pharmacol. Rev. 2003;55:195–227. doi: 10.1124/pr.55.1.8. [DOI] [PubMed] [Google Scholar]
- 20.Chiang N. Activation of lipoxin A4 receptors by aspirin-triggered lipoxins and select peptides evokes ligand-specific responses in inflammation. J. Exp. Med. 2000;191:1197–1208. doi: 10.1084/jem.191.7.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serhan C.N. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu. Rev. Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 22.Fiore S. Identification of a human cDNA encoding a functional high affinity lipoxin A4 receptor. J. Exp. Med. 1994;180:253–260. doi: 10.1084/jem.180.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang D. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J. Exp. Med. 2000;192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serhan C.N. Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution. Prostaglandins Leukot. Essent. Fatty Acids. 2005;73:141–162. doi: 10.1016/j.plefa.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Bonnans C. Synthesis and anti-inflammatory effect of lipoxins in human airway epithelial cells. Biomed. Pharmacother. 2007;61:261–267. doi: 10.1016/j.biopha.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 26.Fabbri E. Studies on fMLP–receptor interaction and signal transduction pathway by means of fMLP-OMe selective analogues. Cell Signal. 2000;12:391–398. doi: 10.1016/s0898-6568(00)00075-9. [DOI] [PubMed] [Google Scholar]
- 27.Cavicchioni G. Biological variation responses in fMLP-OMe analogs, introducing bulky protecting groups on the side-chain of hydrophilic residues at position 2. J. Pept. Res. 2002;60:223–231. doi: 10.1034/j.1399-3011.2002.21019.x. [DOI] [PubMed] [Google Scholar]
- 28.Le Y. Utilization of two seven-transmembrane, G protein-coupled receptors, formyl peptide receptor-like 1 and formyl peptide receptor, by the synthetic hexapeptide WKYMVm for human phagocyte activation. J. Immunol. 1999;163:6777–6784. [PubMed] [Google Scholar]
- 29.Seo J.K. A peptide with unique receptor specificity: stimulation of phosphoinositide hydrolysis and induction of superoxide generation in human neutrophils. J. Immunol. 1997;158:1895–1901. [PubMed] [Google Scholar]
- 30.Klein C. Identification of surrogate agonists for the human FPRL-1 receptor by autocrine selection in yeast. Nat. Biotechnol. 1998;16:1334–1337. doi: 10.1038/4310. [DOI] [PubMed] [Google Scholar]
- 31.Hu J.Y. Synthetic peptide MMK-1 is a highly specific chemotactic agonist for leukocyte FPRL1. J. Leukoc. Biol. 2001;70:155–161. [PubMed] [Google Scholar]
- 32.Bae Y.S. Novel chemoattractant peptides for human leukocytes. Biochem. Pharmacol. 2003;66:1841–1851. doi: 10.1016/s0006-2952(03)00552-5. [DOI] [PubMed] [Google Scholar]
- 33.Hecht I. A novel peptide agonist of formyl-peptide receptor-like 1 (ALX) displays anti-inflammatory and cardioprotective effects. J. Pharmacol. Exp. Ther. 2009;328:426–434. doi: 10.1124/jpet.108.145821. [DOI] [PubMed] [Google Scholar]
- 34.Kirpotina L.N. Identification of novel small-molecule agonists for human formyl peptide receptors and pharmacophore models of their recognition. Mol. Pharmacol. 2010;77:159–170. doi: 10.1124/mol.109.060673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perretti M. Mobilizing lipocortin 1 in adherent human leukocytes downregulates their transmigration. Nat. Med. 1996;2:1259–1262. doi: 10.1038/nm1196-1259. [DOI] [PubMed] [Google Scholar]
- 36.Gavins F.N. Leukocyte antiadhesive actions of annexin 1: ALXR- and FPR-related anti-inflammatory mechanisms. Blood. 2003;101:4140–4147. doi: 10.1182/blood-2002-11-3411. [DOI] [PubMed] [Google Scholar]
- 37.Lim L.H. Promoting detachment of neutrophils adherent to murine postcapillary venules to control inflammation: effect of lipocortin 1. Proc. Natl. Acad. Sci. U. S. A. 1998;95:14535–14539. doi: 10.1073/pnas.95.24.14535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walther A. A novel ligand of the formyl peptide receptor: annexin 1 regulates neutrophil extravasation by interacting with the FPR. Mol. Cell. 2000;5:831–840. doi: 10.1016/s1097-2765(00)80323-8. [DOI] [PubMed] [Google Scholar]
- 39.Ernst S. An annexin 1 N-terminal peptide activates leukocytes by triggering different members of the formyl peptide receptor family. J. Immunol. 2004;172:7669–7676. doi: 10.4049/jimmunol.172.12.7669. [DOI] [PubMed] [Google Scholar]
- 40.Badolato R. Serum amyloid A induces calcium mobilization and chemotaxis of human monocytes by activating a pertussis toxin sensitive signaling pathway. J. Immunol. 1995;155:4004–4010. [PubMed] [Google Scholar]
- 41.Su S.B. A seven-transmembrane, G protein-coupled receptor, FPRL1, mediates the chemotactic activity of serum amyloid A for human phagocytic cells. J. Exp. Med. 1999;189:395–402. doi: 10.1084/jem.189.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yazawa H. Beta amyloid peptide (Abeta42) is internalized via the G-protein-coupled receptor FPRL1 and forms fibrillar aggregates in macrophages. FASEB J. 2001;15:2454–2462. doi: 10.1096/fj.01-0251com. [DOI] [PubMed] [Google Scholar]
- 43.Le Y. The neurotoxic prion peptide fragment PrP(106-126) is a chemotactic agonist for the G protein-coupled receptor formyl peptide receptor-like 1. J. Immunol. 2001;166:1448–1451. doi: 10.4049/jimmunol.166.3.1448. [DOI] [PubMed] [Google Scholar]
- 44.Ali-Khan Z. Echinococcus multilocularis: relationship between persistent inflammation, serum amyloid A protein response and amyloidosis in four mouse strains. Exp. Parasitol. 1988;67:334–345. doi: 10.1016/0014-4894(88)90080-x. [DOI] [PubMed] [Google Scholar]
- 45.Malle E., De Beer F.C. Human serum amyloid A (SAA) protein: a prominent acute-phase reactant for clinical practice. Eur. J. Clin. Invest. 1996;26:427–435. doi: 10.1046/j.1365-2362.1996.159291.x. [DOI] [PubMed] [Google Scholar]
- 46.Ureili-Shoval S. Expression and function of serum amyloid A, a major acute-phase protein, in normal and disease states. Curr. Opin. Hematol. 2000;7:64–69. doi: 10.1097/00062752-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 47.Su S.B. T20/DP178, an ectodomain peptide of human immunodeficiency virus type 1 gp41, is an activator of human phagocyte N-formyl peptide receptor. Blood. 1999;93:3885–3892. [PubMed] [Google Scholar]
- 48.Hartt J.K. The HIV-1 cell entry inhibitor T-20 potently chemoattracts neutrophils by specifically activating the N-formylpeptide receptor. Biochem. Biophys. Res. Commun. 2000;272:699–704. doi: 10.1006/bbrc.2000.2846. [DOI] [PubMed] [Google Scholar]
- 49.Le Y. N36, a synthetic N-terminal heptad repeat domain of the HIV-1 envelope protein gp41, is an activator of human phagocytes. Clin. Immunol. 2000;96:236–242. doi: 10.1006/clim.2000.4896. [DOI] [PubMed] [Google Scholar]
- 50.Deng X. A synthetic peptide derived from human immunodeficiency virus type 1 gp120 downregulates the expression and function of chemokine receptors CCR5 and CXCR4 in monocytes by activating the 7-transmembrane G protein-coupled receptor FPR1/LXA4R. Blood. 1999;94:1165–1173. [PubMed] [Google Scholar]
- 51.Bylund J. Lipopolysaccharide-induced granule mobilization and priming of the neutrophil response to Helicobacter pylori peptide Hp(2-20), which activates formyl peptide receptor-like 1. Infect. Immun. 2002;70:2908–2914. doi: 10.1128/IAI.70.6.2908-2914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dalpiaz A. Formylpeptide receptor antagonists: structure and activity. Boll. Chim. Farm. 1999;138:44–48. [PubMed] [Google Scholar]
- 53.La M. Annexin 1 peptides protect against experimental myocardial ischemia–reperfusion: analysis of their mechanism of action. FASEB J. 2001;15:2247–2256. doi: 10.1096/fj.01-0196com. [DOI] [PubMed] [Google Scholar]
- 54.Gavins F.N. Activation of the annexin 1 counter-regulatory circuit affords protection in the mouse brain microcirculation. FASEB J. 2007;21:1751–1758. doi: 10.1096/fj.06-7842com. [DOI] [PubMed] [Google Scholar]
- 55.Haas P.J. N-Terminal residues of the chemotaxis inhibitory protein of Staphylococcus aureus are essential for blocking formylated peptide receptor but not C5a receptor. J. Immunol. 2004;173:5704–5711. doi: 10.4049/jimmunol.173.9.5704. [DOI] [PubMed] [Google Scholar]
- 56.Prat C. A new staphylococcal anti-inflammatory protein that antagonizes the formyl peptide receptor-like 1. J. Immunol. 2006;177:8017–8026. doi: 10.4049/jimmunol.177.11.8017. [DOI] [PubMed] [Google Scholar]
- 57.Chen X. Regulatory effects of deoxycholic acid, a component of the anti-inflammatory traditional Chinese medicine Niuhuang, on human leukocyte response to chemoattractants. Biochem. Pharmacol. 2002;63:533–541. doi: 10.1016/s0006-2952(01)00917-0. [DOI] [PubMed] [Google Scholar]
- 58.Chen X. Characterization of chenodeoxycholic acid as an endogenous antagonist of the G-coupled formyl peptide receptors. Inflamm. Res. 2000;49:744–755. doi: 10.1007/s000110050656. [DOI] [PubMed] [Google Scholar]
- 59.Zhou C. Pharmacological characterization of a novel nonpeptide antagonist for formyl peptide receptor-like 1. Mol. Pharmacol. 2007;72:976–983. doi: 10.1124/mol.107.037564. [DOI] [PubMed] [Google Scholar]
- 60.Perretti M. Endogenous mediators that inhibit the leukocyte–endothelium interaction. Trends Pharmacol. Sci. 1997;18:418–425. doi: 10.1016/s0165-6147(97)01116-4. [DOI] [PubMed] [Google Scholar]
- 61.Serhan C.N. Resolution phases of inflammation: novel endogenous anti-inflammatory and pro-resolving lipid mediators and pathways. Annu. Rev. Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 62.Gilroy D.W. Inflammatory resolution: new opportunities for drug discovery. Nat. Rev. Drug Discov. 2004;3:401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- 63.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 64.D’Amico M. Lipocortin 1 reduces myocardial ischemia–reperfusion injury by affecting local leukocyte recruitment. FASEB J. 2000;14:1867–1869. doi: 10.1096/fj.99-0602fje. [DOI] [PubMed] [Google Scholar]
- 65.Gavins F.N. Formyl-peptide receptor is not involved in the protection afforded by annexin 1 in murine acute myocardial infarct. FASEB J. 2005;19:100–102. doi: 10.1096/fj.04-2178fje. [DOI] [PubMed] [Google Scholar]
- 66.Leonard M.O. 15-Epi-16-(para-fluorophenoxy)-lipoxin A4-methyl ester, a synthetic analogue of 15-epi-lipoxin A4, is protective in experimental ischemic acute renal failure. J. Am. Soc. Nephrol. 2002;13:1657–1662. doi: 10.1097/01.asn.0000015795.74094.91. [DOI] [PubMed] [Google Scholar]
- 67.Chiang N. Leukotriene B4 receptor transgenic mice reveal novel protective roles for lipoxins and aspirin-triggered lipoxins in reperfusion. J. Clin. Invest. 1999;104:309–316. doi: 10.1172/JCI7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Perretti M. Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A4 receptor. Nat. Med. 2002;8:1296–1302. doi: 10.1038/nm786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bannenberg G. Lipoxins and novel 15-epi-lipoxin analogs display potent anti-inflammatory actions after oral administration. Br. J. Pharmacol. 2004;143:43–52. doi: 10.1038/sj.bjp.0705912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gavins F.N., Chatterjee B.E. Intravital microscopy for the study of mouse microcirculation in anti-inflammatory drug research: focus on the mesentery and cremaster preparations. J. Pharmacol. Toxicol. Methods. 2004;49:1–14. doi: 10.1016/S1056-8719(03)00057-1. [DOI] [PubMed] [Google Scholar]
- 71.Dufton N. Anti-inflammatory role of the murine formyl-peptide receptor 2: ligand-specific effects on leukocyte responses and experimental inflammation. J. Immunol. 2010;184:2611–2619. doi: 10.4049/jimmunol.0903526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Farooqui A.A. Modulation of inflammation in brain: a matter of fat. J. Neurochem. 2007;101:577–599. doi: 10.1111/j.1471-4159.2006.04371.x. [DOI] [PubMed] [Google Scholar]
- 73.Migeotte I. Formyl peptide receptors: a promiscuous subfamily of G protein-coupled receptors controlling immune responses. Cytokine Growth Factor Rev. 2006;17:501–519. doi: 10.1016/j.cytogfr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 74.Gao J.L. F2L, a peptide derived from heme-binding protein, chemoattracts mouse neutrophils by specifically activating Fpr2, the low-affinity N-formylpeptide receptor. J. Immunol. 2007;178:1450–1456. doi: 10.4049/jimmunol.178.3.1450. [DOI] [PubMed] [Google Scholar]
- 75.Shin E.H. Trp-Arg-Trp-Trp-Trp-Trp antagonizes formyl peptide receptor like 2-mediated signaling. Biochem. Biophys. Res. Commun. 2006;341:1317–1322. doi: 10.1016/j.bbrc.2006.01.098. [DOI] [PubMed] [Google Scholar]
- 76.Karlsson J. Neutrophil NADPH-oxidase activation by an annexin AI peptide is transduced by the formyl peptide receptor (FPR), whereas an inhibitory signal is generated independently of the FPR family receptors. J. Leukoc. Biol. 2005;78:762–771. doi: 10.1189/jlb.0305153. [DOI] [PubMed] [Google Scholar]
- 77.Hayhoe R.P. Annexin 1 and its bioactive peptide inhibit neutrophil-endothelium interactions under flow: indication of distinct receptor involvement. Blood. 2006;107:2123–2130. doi: 10.1182/blood-2005-08-3099. [DOI] [PubMed] [Google Scholar]
- 78.Sun R. Identification of neutrophil granule protein cathepsin G as a novel chemotactic agonist for the G protein-coupled formyl peptide receptor. J. Immunol. 2004;173:428–436. doi: 10.4049/jimmunol.173.1.428. [DOI] [PubMed] [Google Scholar]
- 79.Perez H.D. Defective polymorphonuclear leukocyte formyl peptide receptor(s) in juvenile periodontitis. J. Clin. Invest. 1991;87:971–976. doi: 10.1172/JCI115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gargiulo L. Cross-talk between fMLP and vitronectin receptors triggered by urokinase receptor-derived SRSRY peptide. J. Biol. Chem. 2005;280:25225–25232. doi: 10.1074/jbc.M412605200. [DOI] [PubMed] [Google Scholar]
- 81.Mills J.S. Peptides derived from HIV-1, HIV-2, Ebola virus, SARS coronavirus and coronavirus 229E exhibit high affinity binding to the formyl peptide receptor. Biochim. Biophys. Acta. 2006;1762:693–703. doi: 10.1016/j.bbadis.2006.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yan P. The immunosuppressant cyclosporin A antagonizes human formyl peptide receptor through inhibition of cognate ligand binding. J. Immunol. 2006;177:7050–7058. doi: 10.4049/jimmunol.177.10.7050. [DOI] [PubMed] [Google Scholar]
- 83.Stenfeldt A.L. Cyclosporin H, Boc-MLF and Boc-FLFLF are antagonists that preferentially inhibit activity triggered through the formyl peptide receptor. Inflammation. 2007;30:224–229. doi: 10.1007/s10753-007-9040-4. [DOI] [PubMed] [Google Scholar]
- 84.Higgins J.D. N-Terminus urea-substituted chemotactic peptides: new potent agonists and antagonists toward the neutrophil fMLF receptor. J. Med. Chem. 1996;39:1013–1015. doi: 10.1021/jm950908d. [DOI] [PubMed] [Google Scholar]
- 85.Liang T.S. The endogenous opioid spinorphin blocks fMet-Leu-Phe-induced neutrophil chemotaxis by acting as a specific antagonist at the N-formylpeptide receptor subtype FPR. J. Immunol. 2001;167:6609–6614. doi: 10.4049/jimmunol.167.11.6609. [DOI] [PubMed] [Google Scholar]
- 86.Le Y. Pleiotropic roles of formyl peptide receptors. Cytokine Growth Factor Rev. 2001;12:91–105. doi: 10.1016/s1359-6101(01)00003-x. [DOI] [PubMed] [Google Scholar]
- 87.Kamal A.M. Antiflammin-2 activates the human formyl peptide receptor like 1. Sci. World J. 2006;6:1375–1384. doi: 10.1100/tsw.2006.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kurosaka K. Mouse cathelin-related antimicrobial peptide chemoattracts leukocytes using formyl peptide receptor-like 1/mouse formyl peptide receptor-like 2 as the receptor and acts as an immune adjuvant. J. Immunol. 2005;174:6257–6265. doi: 10.4049/jimmunol.174.10.6257. [DOI] [PubMed] [Google Scholar]
- 89.Furlan F. The soluble D2D388-274 fragment of the urokinase receptor inhibits monocyte chemotaxis and integrin-dependent cell adhesion. J. Cell Sci. 2004;117:2909–2916. doi: 10.1242/jcs.01149. [DOI] [PubMed] [Google Scholar]
- 90.Harada M. N-Formylated humanin activates both formyl peptide receptor-like 1 and 2. Biochem. Biophys. Res. Commun. 2004;324:255–261. doi: 10.1016/j.bbrc.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 91.Rabiet M.J. Human mitochondria-derived N-formylated peptides are novel agonists equally active on FPR and FPRL1, while Listeria monocytogenes-derived peptides preferentially activate FPR. Eur. J. Immunol. 2005;35:2486–2495. doi: 10.1002/eji.200526338. [DOI] [PubMed] [Google Scholar]
- 92.Kim Y. Pituitary adenylate cyclase-activating polypeptide 27 is a functional ligand for formyl peptide receptor-like 1. J. Immunol. 2006;176:2969–2975. doi: 10.4049/jimmunol.176.5.2969. [DOI] [PubMed] [Google Scholar]
- 93.Brandenburg L.O. Internalization of PrP106-126 by the formyl-peptide-receptor-like-1 in glial cells. J. Neurochem. 2007;101:718–728. doi: 10.1111/j.1471-4159.2006.04351.x. [DOI] [PubMed] [Google Scholar]
- 94.Nanamori M. A novel nonpeptide ligand for formyl peptide receptor-like 1. Mol. Pharmacol. 2004;66:1213–1222. doi: 10.1124/mol.104.004309. [DOI] [PubMed] [Google Scholar]
- 95.Chen Q. Temporin A and related frog antimicrobial peptides use formyl peptide receptor-like 1 as a receptor to chemoattract phagocytes. J. Immunol. 2004;173:2652–2659. doi: 10.4049/jimmunol.173.4.2652. [DOI] [PubMed] [Google Scholar]
- 96.Paulis A. Urokinase induces basophil chemotaxis through a urokinase receptor epitope that is an endogenous ligand for formyl peptide receptor-like 1 and -like 2. J. Immunol. 2004;173:5739–5748. doi: 10.4049/jimmunol.173.9.5739. [DOI] [PubMed] [Google Scholar]
- 97.Shen W. Activation of the chemotactic peptide receptor FPRL1 in monocytes phosphorylates the chemokine receptor CCR5 and attenuates cell responses to selected chemokines. Biochem. Biophys. Res. Commun. 2000;272:276–283. doi: 10.1006/bbrc.2000.2770. [DOI] [PubMed] [Google Scholar]
- 98.Wan H.X. Discovery of Trp-Nle-Tyr-Met as a novel agonist for human formyl peptide receptor-like 1. Biochem. Pharmacol. 2007;74:317–326. doi: 10.1016/j.bcp.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 99.He R. The synthetic peptide Trp-Lys-Tyr-Met-Val-D-Met is a potent chemotactic agonist for mouse formyl peptide receptor. J. Immunol. 2000;165:4598–4605. doi: 10.4049/jimmunol.165.8.4598. [DOI] [PubMed] [Google Scholar]
- 100.Fu H. The two neutrophil members of the formylpeptide receptor family activate the NADPH-oxidase through signals that differ in sensitivity to a gelsolin derived phosphoinositide-binding peptide. BMC Cell Biol. 2004;5:50. doi: 10.1186/1471-2121-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.de Paulis A. Basophils infiltrate human gastric mucosa at sites of Helicobacter pylori infection, and exhibit chemotaxis in response to H. pylori-derived peptide Hp(2-20) J. Immunol. 2004;172:7734–7743. doi: 10.4049/jimmunol.172.12.7734. [DOI] [PubMed] [Google Scholar]
- 102.Ying G. Humanin, a newly identified neuroprotective factor, uses the G protein-coupled formylpeptide receptor-like-1 as a functional receptor. J. Immunol. 2004;172:7078–7085. doi: 10.4049/jimmunol.172.11.7078. [DOI] [PubMed] [Google Scholar]
- 103.Migeotte I. Identification and characterization of an endogenous chemotactic ligand specific for FPRL2. J. Exp. Med. 2005;201:83–93. doi: 10.1084/jem.20041277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cuzzocrea S. Lipocortin 1 protects against splanchnic artery occlusion and reperfusion injury by affecting neutrophil migration. J. Immunol. 1997;159:5089–5097. [PubMed] [Google Scholar]
- 105.Wang Z.G., Ye R.D. Characterization of two new members of the formyl peptide receptor gene family from 129S6 mice. Gene. 2002;299:57–63. doi: 10.1016/s0378-1119(02)01012-0. [DOI] [PubMed] [Google Scholar]
- 106.Chiang N. A novel rat lipoxin A4 receptor that is conserved in structure and function. Br. J. Pharmacol. 2003;139:89–98. doi: 10.1038/sj.bjp.0705220. [DOI] [PMC free article] [PubMed] [Google Scholar]


