Abstract
Background
Adequate thyroid function during pregnancy is essential for optimal fetal growth. Gestational exposure to perfluoroalkyl substances (PFAS) can negatively affect birth size and disrupt maternal and neonatal thyroid function, although the interrelationship is unclear.
Objective
We aimed to quantify the associations between maternal serum–PFAS concentrations and birth weight, birth length, and cranial circumference. We also aimed to estimate associations between PFAS and thyroid hormone (TH) concentrations, thereby elucidating whether THs potentially mediate the associations between PFAS concentrations and birth size.
Methods
We studied a population-based prospective cohort of 172 mother-singleton pairs from the Faroe Islands. Twelve PFAS were measured in maternal serum obtained at 34 weeks of gestation. THs were measured in maternal and cord serum. Associations between PFAS concentrations and birth size and TH concentrations were estimated using multivariable linear regressions. Sex-stratified analyses along with a mediation analysis were performed to estimate potential mediating effects of THs in the association between PFAS and birth outcomes.
Results
Several PFASs were negatively associated with birth weight, length, and head circumference, and a general positive association between maternal serum–PFASs and cord serum–thyroid-stimulating hormone (TSH; also known as thyrotropin) was found. For instance, a doubling in perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) was associated with a 53% (95% CI, 18%-99%) and 40% (95% CI, 8%-81%) increases in TSH concentrations, respectively. There was little evidence of sexually dimorphic associations. Overall, THs were not found to mediate associations between PFASs and birth size.
Conclusion
In this study, several PFASs were negatively associated with birth size and increased THs; however, this did not explain lower birth weight among children exposed to PFAS.
Keywords: perfluoroalkyl substances, fetal development, thyroid hormones, pregnant women, mediation analysis, birth outcomes
Introduction
Perfluoroalkyl substances (PFAS) are a group of synthetically manufactured chemicals that are highly persistent in the environment and bioaccumulate in food chains (1, 2). Main sources of exposure in the general population include consumer products, seafood, house dust, and drinking water (3–6). Early life stages are particularly vulnerable to PFAS exposure (7), and current evidence suggests that PFAS exposures negatively impact the regulation and development of metabolic, immune, and neurological systems in pregnant women and infants (8–10).
PFAS such as perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) have been found to be inversely associated with fetal growth outcomes such as birth weight, birth length, and/or head circumference in many previous birth cohort studies (10–24), including in an earlier study of the Faroese population (25). Such a relationship between PFAS exposure and birth size may be mediated by thyroid hormones (THs), which play a significant role in regulating metabolic functions (26). One way PFAS can disrupt regulation of THs is through the hypothalamic–pituitary–thyroid (HPT) axis. PFOS can affect TH production through disruption of the HPT axis of zebrafish larvae and alter the secretion and function of major THs that play a key role in energy metabolism, including thyroid-stimulating hormone (TSH; also known as thyrotropin), free thyroxine (FT4), thyroxine (T4), and free triiodothyronine (FT3) (27).
Recent systematic reviews have found that PFAS can potentially impact both maternal TH levels during gestation and newborn TH levels at birth (8, 9, 28). In particular, PFOS and PFOA have been found to be positively associated with maternal and cord TSH levels (29–32). Other PFAS, such as perfluorononanoic acid (PFNA) were reported to be positively associated with maternal TSH (32,33), and inversely associated with total T3, T4, and FT4, whereas perfluoroundecanoic acid (PFUnDA) and perfluorododecanoic acid (PFDoDA) have been found to be negatively associated with maternal FT4 and total T4 (34,35).
From the associations and possible biological pathways between PFAS, thyroid function, and birth size measures, we hypothesize that THs may mediate the relationship between PFAS and birth size. We assessed associations between PFAS exposures and birth size within a prospective cohort study of the Faroese population with a wide range of PFAS exposure levels. We also determined to what extent these associations were mediated by either maternal or cord serum TH concentrations, and the possible modification by infant sex.
Methods
Population and study design
We used data from a cohort study conducted in the Faroe Islands that included measurements of THs (n=182). This population is known to consume pilot whale meat, which thereby causes elevated PFAS exposures (36). Data were collected over a 12-month period in 1994–1995 and were derived from 182 singleton births at the National Hospital in Torshavn. Exclusion criteria included any children who were born before the 36th week of gestation, weighed less than 2,500 grams, or had any congenital neurological disease. Maternal serum was obtained during the last prenatal care visit at 34 weeks of gestation. Cord serum was collected at delivery by drawing blood from the umbilical cord using heparinized syringes. Obstetric data, including birth size, parity, maternal age, pre-pregnancy body mass index (BMI), gestational weight gain, gestational diabetes, and gestational age were extracted from the hospital’s medical records. Demographic and other relevant information was obtained using in-person interviews and standard questionnaires, with questions about the mother’s medical history, indicators of socioeconomic status (SES), and current health, both before and after giving birth. Of note, this study population is rather homogeneous in terms of SES. The study design and methods were approved by the Faroese Ethical Review committee. All participating mothers provided their written informed consent. Additional information about study methods as well as exposure and outcome measurements can be found elsewhere (37, 38).
Birth size outcome measurements.
Weight, length, and head circumference were measured at birth. These variables were standardized to z-scores specific for gestational age and sex using the recent INTERGROWTH-21st fetal growth standard. The INTERGROWTH-21st fetal growth standard is based on a multicenter, international population-based cohort of 20,486 pregnant women and their offspring from 8 countries, including the United States, and utilizes the same methodology as the 2006 WHO Growth Reference to provide growth standard curves specific for fetuses (39). For the weight, length, and head circumference z-scores calculation, we used the Healthy Birth, Growth, and Development (HBGD) package in R (40).
PFAS exposure biomarkers.
Methods for analyzing PFAS exposure have been described previously (37). Separated serum samples were stored at −80 °C until analysis. Seventeen PFAS, including PFAS precursors, were quantified using online solid-phase extraction along with high-pressure liquid chromatography and tandem mass spectrometry (41). The extraction was performed on a Thermo Scientific EQuan MAX system, consisting of two Accela HPLC pumps (Thermo Scientific, San José, CA) and a PAL autosampler module (CTC analysis AG, Zwingen, Switzerland). The tandem mass spectrometer was a TSQ Quantum Ultra Triple Stage Quadropole with heated electrospray ionization (Thermo Scientific, San Jose, CA). A calibration curve serum and solvent blanks, as well as quality control samples were included in each batch of samples analyzed. Imprecision within batches was < 9% for all the analytes and the limit of quantitation (LOQ) was 0.03 ng/mL for all compounds.
Maternal and cord serum TH concentrations.
TSH was measured using time-resolved fluoroimmunoassay in maternal and cord serum, while FT4 and FT3 were quantified using a radioimmunoassay after dialysis to equilibrium. Free triiodothyronine resin uptake (T3RU) is also used to measure TH binding capacity, whereas free T4 index (FTI) corrects T4 estimates for abnormalities in serum binding properties (42). Thus, T3RU and total T4 were also quantified by radioimmunoassay, the results of which were then used to calculate FTI values (43).
Covariates.
Complete data on exposures, THs, and birth size measures were available for 172 participants. Confounders were determined a priori based on associations between PFAS and birth size measures from previous studies (14, 15, 18, 25), as illustrated on the directed acyclic graph (DAG) in the Supplemental Material (44). Models were adjusted for the following covariates: sex of the fetus (female/male), gestational age in weeks (treated as a continuous variable), maternal education (coded as having or not having received higher education), maternal pre-pregnancy BMI (treated as a continuous variable), parity (treated as a continuous variable), smoking status during pregnancy (smoker/nonsmoker), alcohol consumption during pregnancy (some/none), along with potential chemical exposures that may affect TH levels or birth size measures (maternal hair-mercury and maternal serum concentrations of the sum of polychlorinated biphenyl [PCB]) congeners 138, 153, and 180.
Data analysis.
Serum PFAS concentrations were included in subsequent analysis if they were detected in at least 30% of participants. PFAS exposures and TH data were considered as continuous variables and log-transformed to approximate a Gaussian distribution and limit the influence of outliers. We generated descriptive tables for THs and PFAS concentrations and conducted a bivariate Pearson correlation test. We used generalized additive models to examine potential nonlinear relationships between PFAS and birth size, but no significant departure from linearity was observed. We therefore performed separate crude and adjusted multiple linear regression models with important covariates between log2-transformed PFAS concentrations and birth weight, birth length, and head circumference z-scores. To convert z-scores back to raw birth size values, the following formula was used:
Where µ is the mean, Z is the z-score, and σ is the standard deviation (SD) of the Faroese cohort (values can be found in Table 3).
Table 3.
Thyroid Parameters and Birth Size of the Faroese Birth Cohort
| Thyroid parameters | n | Geometric mean (SE) | Range |
|---|---|---|---|
| Maternal Serum | |||
| TSH (IU/L) | 172 | 1.34 (0.05) | 0.23 – 3.61 |
| FT4 (pmol/L) | 172 | 8.18 (0.11) | 5.49 – 14.70 |
| FT3 (pmol/L) | 172 | 4.25 (0.05) | 2.94 – 6.62 |
| T4 (nmol/L) | 172 | 121.08 (1.95) | 61.00 – 224.00 |
| T3RU | 172 | 0.66 (0.01) | 0.51 – 1.01 |
| FTI (IU/L) | 172 | 80.47 (1.16) | 53.00 – 164.00 |
| Cord Serum | |||
| TSH (IU/L) | 153 | 6.98 (0.33) | 2.03 – 27.40 |
| FT4 (pmol/L) | 153 | 11.93 (0.36) | 0.38 – 16.60 |
| FT3 (pmol/L) | 153 | 2.35 (0.04) | 2.00 – 5.54 |
| T4 (nmol/L) | 153 | 129.07 (1.92) | 68.00 – 205.00 |
| T3RU | 153 | 0.83 (0.01) | 0.54 – 1.05 |
| FTI (IU/L) | 153 | 107.60 (1.47) | 65.00 – 191.00 |
| Birth size | Arithmetic mean (SD) | Range | |
| Birth Weight, g | 171 | 3672.0 (37.0) | 2500.0 – 4800.0 |
| Birth Length, cm | 171 | 53 (0.2) | 48.0 – 59.0 |
| Head Circumference, cm | 152 | 35.6 (.01) | 31.5 – 39.0 |
Abbreviations: FT3, free triiodothyronine; FT4, free thyroxine; FTI, free T4 index; T3RU, free triiodothyronine resin uptake; T4, thyroxine; TSH, thyrotropin (thyroid-stimulating hormone); SE, standard error; SD, standard deviation.
Additional analyses explored potential sexually dimorphic effects through a cross-product term interaction based on the child’s gender for each of the associations detailed above.
TH concentrations were natural log-transformed to quantify the percent change in mean TH levels for each doubling in PFAS exposure. To calculate the percent change, we used the following formula:
Where β is the regression coefficient.
To investigate the potential for THs for mediating the observed associations between PFAS and birth size measures, we conducted a formal mediation analysis. We calculated: (1) the average causal mediated effect (ACME) which represents the estimated average change in the outcome that is mediated through changes in TH levels, ie, the natural indirect effect; (2) the average direct effect (ADE) which represents the unmediated effect, or the effect of the PFAS exposure on the birth size measures that is not mediated through changes in TH levels (mediated through other mechanisms), ie, the natural direct effect; and (3) the total effect (TE) calculated as the sum of ADE and ACME, and percent mediated (PM) calculated as the ratio of the indirect to the total effect (ACME/TE) x 100% (45). We used Monte Carlo approximation based on the asymptotic sampling distribution (n=1000) (46) to compute confidence intervals. Data analyses were conducted using Stata (47) as well as the Mediation (45) and MGCV (48) packages in R (49).
Results
Table 1 details child and maternal characteristics for the 172 mother-child pairs assessed for PFAS and TH levels. The cohort was 51% boys and 49% girls. The average gestational age was 39.6 weeks (SD = 1.3), the average birth weight was 3672 g (SD = 485), and the average birth length 53.0 cm (SD = 2.1). The average maternal age was 28.1 years (SD = 5.6) and 22% of mothers had received at least college education. The average maternal pre-pregnancy BMI was 23.1 (SD = 3.5), and approximately one-third of the women were primiparous. The percentage of women who consumed alcohol or who smoked during pregnancy was 31.3% and 12.8%, respectively.
Table 1.
Cohort Description and Covariates of Interest
| Covariate | n | (mean ± SD) / %* |
|---|---|---|
| Child Characteristics | ||
| Sex (boy/girl) | 172 | 50.6/49.4 |
| Gestational age, weeks | 170 | 39.6 ± 1.3 |
| Birth weight, g | 172 | 3671.5 ± 485.2 |
| Birth length, cm | 172 | 53.0 ± 2.1 |
| Head circumference, cm | 153 | 35.6 ± 1.4 |
| Maternal Characteristics | ||
| Maternal age, years | 171 | 28.1 ± 5.6 |
| Maternal education (≥ some higher education) | 167 | 21.6 |
| Maternal BMI, kg/m2 | 172 | 23.1 ± 3.5 |
| Weight gain during pregnancy, kg | 164 | 14.4 ± 4.7 |
| Parity (primiparous/multiparous) | 172 | 28.5/71.5 |
| ∑PCB, ug/g | 169 | 1.6 ± 1.8 |
| Hair mercury, ug/g | 140 | 5.4 ± 3.6 |
| Alcohol consumption during pregnancy (yes/no) | 172 | 12.8/ % for alcohol drinkers |
| Smoking during pregnancy (yes/no) | 172 | 31.3/ % for nonsmokers |
Abbreviations: BMI, body mass index; PCB, polychlorinated biphenyl. *Estimates are presented as (mean ± SD) for continuous variables and percentages (%) for categorical variables.
Table 2 shows the distribution of PFAS concentrations. Twelve PFAS were detected in more than 30% of the samples (Table 2). PFOA and PFOS exhibited higher concentrations than other PFAS. Many PFAS showed significantly positive correlations to other PFAS. The strongest correlations were found between PFNA and perfluorodecanoic acid (PFDA) (0.82) as well as PFDA and PFUnDA (0.87); perfluoroheptanoic acid (PFHpA) and PFDoDA were weakly correlated with most other PFAS (range of correlations, −0.07, 0.33) (44).
Table 2.
Distribution of Different PFAS Concentrations in the Cohort (n = 172)
| Parameter | Acronym | %<LOD | Concentration (µg/g) | |
|---|---|---|---|---|
| Geometric Mean (GSE) | Range | |||
| Carboxylic acids (PFCAs) | ||||
| Perfluoroheptanoic acid | PFHpA | 53% | 0.03 (<0.01) | 0.0 – 0.5 |
| Perfluorooctanoic acid | PFOA | 0% | 2.37 (0.07) | 0.8 – 6.9 |
| Perfluorononanoic acid | PFNA | 0% | 0.60 (0.02) | 0.2 – 1.6 |
| Perfluorodecanoic acid | PFDA | 0% | 0.30 (0.01) | 0.1 – 0.9 |
| Perfluoroundecanoic acid | PFUnDA | 0% | 0.47 (0.02) | 0.1 – 1.8 |
| Perfluorododecanoic acid | PFDoDA | 68% | 0.03 (<0.01) | 0.0 – 0.5 |
| Sulfonic acids (PFSAs) | ||||
| Perfluorohexane sulfonic acid | PFHxS | 0% | 0.55 (0.02) | 0.1 – 2.8 |
| Perfluoroheptane sulfonic acid | PFHpS | 7% | 0.35 (0.03) | 0.0 – 1.4 |
| Perfluorooctane sulfonic acid | PFOS | 0% | 20.86 (0.47) | 6.9 – 47.6 |
| PFAS precursors | ||||
| N-ethyl perfluorooctane sulfonamidoacetate | NEtFOSAA | 0% | 0.65 (0.03) | 0.0 – 1.8 |
| N-methyl perfluorooctane sulfonamidoacetate | NMeFOSAA | 0% | 0.18 (0.01) | 0.0 – 1.8 |
| Perfluorooctane sulfonamide | FOSA | 63% | 0.04 (<0.01) | 0.0 – 1.2 |
Abbreviations: GSE, geometric standard error; LOD, limit of detection. Note: PFDS, PFPeA, PFHxA, PFBA, and PFBS were also quantified but levels did not meet the LOD limit.
Table 3 displays the distribution of maternal and TH concentrations as well as fetal growth outcomes. We did not find mean values outside the normal range. Pearson correlation coefficients between maternal T4 and FTI were highly correlated (0.82), as were cord serum T4 and FTI (0.84). Maternal T4 and T3RU displayed a negative correlation (−0.39), as did cord T4 and T3RU (−0.42). In general, maternal and fetal THs were weakly to moderately correlated (range of correlations, −0.28, 0.31) (44). Among fetal growth outcomes, birth length and birth weight were highly correlated (0.74), as were birth weight and head circumference (0.75) (44).
Associations between PFAS and birth size measures
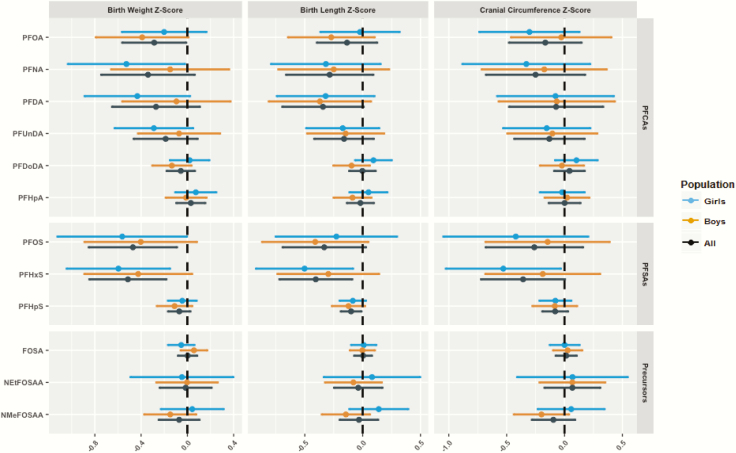
Several PFAS were inversely associated with birth length, birth weight, and cranial circumference (Fig. 1). For instance, a doubling in serum perfluorohexane sulfonate (PFHxS) concentrations was negatively associated with all 3 birth size measures: birth weight (−0.51 SD; 95% CI, −0.85 to 0.18), birth length (−0.40 SD; 95% CI, −0.72 to −0.09), and head circumference (−0.36 SD; 95% CI, −0.72 to 0). This translates into a decrease of 246 g, 0.82 cm, and 0.49 cm, respectively. Likewise, a two-fold increase in PFOS concentrations was negatively associated with birth weight (−0.47 SD; 95% CI, −0.85 to −0.04) and birth length (−0.33 SD; 95% CI, −0.69 to 0.03), which corresponds to a decrease of 227 g and 0.67 cm, respectively. We observed similar yet slightly weaker inverse associations between PFNA, PFDA, and PFOA with birth weight, and between PFDA and perfluoroheptane sulfonate (PFHpS) with birth length (Fig. 1). No other association was observed for other PFAS in relation to the same fetal growth outcomes. In addition, there was no evidence of effect modification by child sex (Fig. 1). Complete estimates for the numerical data are available in Supplemental Material (44).
Figure 1.
Estimates and 95% confidence intervals for the associations between perfluoroalkyl substance (PFAS) concentrations and fetal growth outcomes stratified by child sex. Models were adjusted for child gender, parity, maternal body mass index, maternal height, maternal education, maternal age, smoking and drinking alcohol during pregnancy, total PCB, and mercury; sex was not adjusted for in the sex-stratified analyses. PFAS were log2-transformed, while thyroid hormones were log-transformed to fit a normal distribution.
Associations between PFAS and maternal and cord TH concentrations
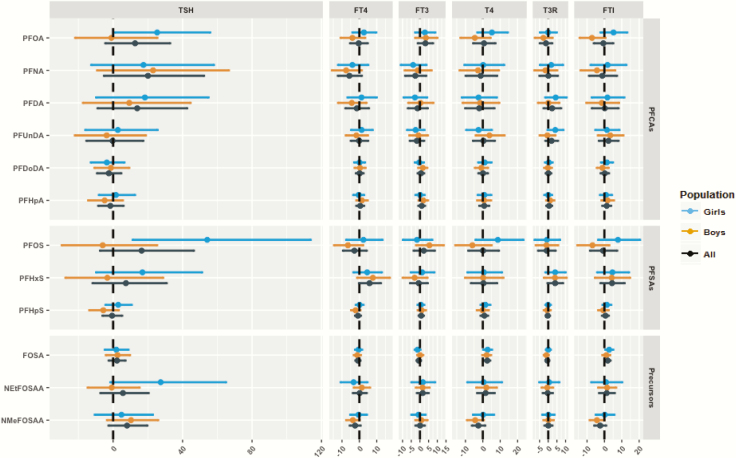
Some PFAS were positively associated with maternal TSH, with PFNA, PFOS, PFDA, and PFOA showing the strongest estimates; however, these increases did not reach the level of significance (Fig. 2). For instance, a doubling in PFNA and PFOS was associated with a 20% (95% CI, −5% to 52%) and 16% (95% CI, −8% to 47%) increase in maternal TSH concentrations, respectively (44).
Figure 2.
Percent change in maternal thyroid hormone levels per doubling of perfluoroalkyl substance (PFAS) concentrations with 95% confidence intervals stratified by child sex. Models were adjusted for child gender, parity, maternal body mass index, maternal education, maternal age, smoking and drinking alcohol during pregnancy, total PCB, and mercury; sex was not adjusted for in the sex-stratified analyses. PFAS were log2-transformed, while thyroid hormones were log-transformed to fit a normal distribution.
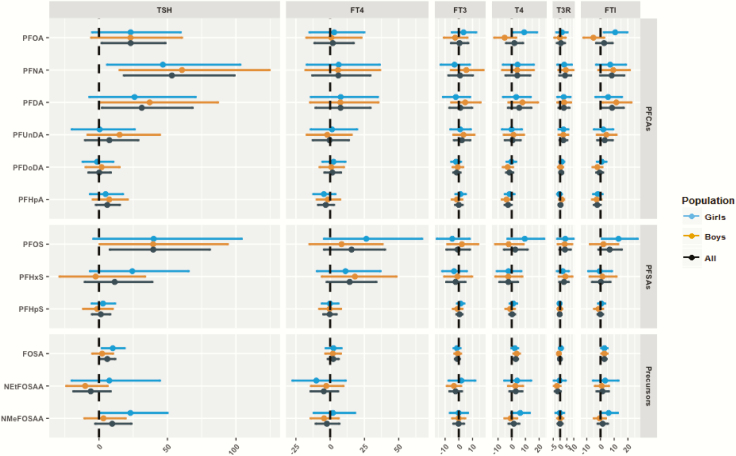
PFOS exhibited sexually dimorphic associations (P-interaction < 0.1 but > 0.05) with maternal TSH and FTI levels (Fig. 3); we also found a similar pattern between PFOA and maternal FTI. In mothers bearing female fetuses, PFOS was positively associated with maternal TSH, T4, and FTI levels, while there was a negative association for mothers bearing male fetuses. For instance, a doubling in gestational PFOS concentrations was associated with a 54% (95% CI, 11%-114%) increase in maternal TSH levels in mothers bearing female fetuses, in comparison to a decrease by 6% (95% CI, −30% to 25%) in mothers bearing male fetuses (P-EM = 0.09) (44). Likewise, higher PFOA concentrations were positively associated with maternal TSH and FTI levels in mothers bearing female fetuses, while the association was negative in mothers bearing male fetuses. A doubling in the gestational PFOA concentrations was associated with a 5% (95% CI, −2% to 13%) increase in maternal FTI levels in mothers bearing female fetuses, in comparison to a decrease by 7% (95% CI, −14% to 1%) in mothers bearing male fetuses (P-EM = 0.02) (44). We did not observe consistent patterns of sexually dimorphic associations for other PFAS concentrations and maternal TH levels.
Figure 3.
Percent change in cord thyroid hormone levels per doubling of perfluoroalkyl substance (PFAS) concentrations with 95% confidence intervals stratified by child sex. Models were adjusted for child gender, parity, maternal body mass index, maternal education, maternal age, smoking and drinking alcohol during pregnancy, total PCB, and mercury; sex was not adjusted for in the sex-stratified analyses. PFAS were log2-transformed, while thyroid hormones were log-transformed to fit a normal distribution.
Regarding cord TH levels, there was a consistent positive association between PFAS and cord TSH. A doubling in PFOS, PFOA, PFDA, PFNA, and perfluorooctane sulfonamide (FOSA) was associated with 53% (95% CI, 18%-99%), 40% (95% CI, 8%-81%), 31% (95% CI, 2%-68%), 23% (95% CI, 2%-49%), and 6% (95% CI, 1%-12%) increase in cord TSH concentrations, respectively (Fig. 3) (44). We also observed an increase in cord T3RU and FT4 concentrations in relation to some PFAS, though most of these associations did not reach the level of significance. Moreover, higher concentrations of PFOS, PFDA, PFNA, and FOSA were associated with higher cord FTI levels. For instance, a two-fold increase in PFNA and PFDA was associated with an 8% (95% CI, 0%-17%) and 8% (95% CI, 1%-17%) increase in cord FTI, respectively.
We observed significant effect modification (P-interaction < 0.1) by child sex in the association between PFOA and cord serum FTI and T4 concentrations (Fig. 3) (44). A doubling in gestational PFOA concentration was associated with an 11% (95% CI, 2%-20%) increase in cord FTI levels in girls, in comparison to a decrease by 5% (95% CI, −13% to 3%) in boys (P-EM = 0.02) (44). The same pattern was observed for associations between PFOA and cord T4 levels.
Mediation analyses
We investigated whether THs potentially mediate some of the effects found between PFAS and birth size. Although a few THs, such as FTI and T4, were found to partially mediate the effect of certain PFAS on birth weight and length (ACME of FTI in the association between PFNA and birth weight = 0.10; 95% CI, 0.00-0.25), overall, values of the mediated effects (ACME) and the proportion mediated (PM) were consistently nonsignificant and close to null for all outcomes and PFAS exposures (44). Thus, THs did not appear to explain the observed associations between PFAS and birth outcomes in this Faroese cohort.
Discussion
In this study of 172 mother-infant pairs from the Faroe Islands, we found negative associations between gestational exposure to certain PFAS and birth weight, birth length, and, to a lesser extent, head circumference. Of the PFAS that were associated with these birth size outcomes, we also found PFAS to be consistently associated with increased cord and maternal serum TSH concentrations, thus suggesting that alterations in TSH levels may be a potential pathway linking PFAS exposure to reduced birth weight, birth length, and head circumference. We did not find a generalized pattern for associations between PFAS and other THs. THs did not appear to mediate the associations between any PFAS and fetal growth outcome.
Recent studies and systematic reviews of epidemiological evidence reported that increases in PFAS concentrations during pregnancy were associated with lower birth weight (10-17, 19-24), also in line with our findings in this study as well as our study in a younger Faroese cohort born in 1997–2000 (25). In the previous study, we found nonsignificant inverse associations between PFAS exposure and birth weight in both single-PFAS linear regression analysis and using structural equation models (mean change per doubling of PFAS exposure: −169 g, 95% CI, −359 to 21), which is slightly lower than the estimates found in this current study. In addition, a study conducted on a Japanese cohort found similar results, as they reported a decrease of −197 g (95% CI, −391 to −3) in birth weight in association to PFOA (16). Another study found that PFOA and PFNA were associated with a decrease of approximately 50 g in birth weight after adjustment for covariates (7, 15). Furthermore, a cohort study conducted on a Swedish population, a population comparable to that in the Faroe Islands, reported a decrease of −359 g (95% CI, −596 to −122) and −292 g (95% CI, −500 to −84) in birth weight per ln-unit increase in PFOA and PFOS, respectively (50). Our results on PFOA, PFOS, and birth weight are consistent with these results, although the magnitude of change in birth weight is lower than that in the Swedish study.
We did not observe the associations between PFAS and birth weight to differ by child sex. Some studies, including our previous investigation in the younger Faroese cohort, showed that prenatal exposure to PFOA and/or PFOS was negatively associated with birth weight in boys but not in girls (14, 25, 50, 51). However, other studies have reported no clear effect modification by sex (15) or associations only in girls (21). It is thus unclear whether the absence of significant modification by sex in the present study reflects the true effect or whether it is due to the small sample size and limited power.
Fewer studies have examined associations between PFAS and either birth length or head circumference. In our previous study of a younger Faroese cohort, we found that PFAS tended to be inversely associated with head circumference, although these estimates did not reach significance (25). A study that was conducted in Sweden reported an inverse association of PFAS exposure with birth length and head circumference, although only associations with birth length were significant. For each ln-unit increase in PFOA, birth length decreased by −1.3 cm (95% CI, −2.3 to −0.3), while for each ln-unit increase in PFOS, birth length decreased by −1.2 cm (95% CI, −2.1 to −0.3) in head circumference (50). A study of a Spanish cohort concluded that PFAS such as PFHxS, PFOA, and PFNA and PFOS were not significantly associated with either birth length or head circumference (14). A further study found that PFDoDA was inversely associated with head circumference, although only in girls (52). Our findings are in line with the findings from the Swedish cohort, as we also found negative yet statistically insignificant associations between PFOA and PFOS with head circumference.
Previous studies have identified PFAS to be a potential thyroid disruptor during pregnancy, influencing maternal and offspring TH levels (8, 9, 29–35). Two systematic reviews concluded that, in general, PFAS were positively associated with TSH, while the associations between PFAS and total and free T3 and T4 were less consistent (8, 9). Our results are consistent with these findings, as we observed that higher PFAS concentrations were associated with changes in certain TH levels, namely TSH, during pregnancy and at birth, and less consistently with free and total T3 and T4. PFAS are thought to affect TH homeostasis by competing with T3 and T4 for their binding proteins (53, 54), which may result in a decrease of TH levels, and which in turn may induce a compensatory increase in TSH levels (55). This hypothesis is supported by positive associations shown between PFAS and TSH in this investigation and previous studies (8, 9).
We also observed the effect of PFAS exposure on THs to differ based on whether the mother was carrying a male or female fetus, as we found that PFOS and PFOA were positively associated with THs in mothers carrying female fetuses and negatively associated with mothers carrying male fetuses. While it is yet unclear how the sex of the child may influence PFAS-associated maternal TH dysfunction, it is worth corroborating these findings in future studies examining other populations. Further analysis should be undertaken to determine the mechanisms for such differences in maternal hormone levels and whether they have any health implications. Another consideration is that any potential sex-specific effects may also reduce the power for detecting significant mediating effects.
We did not find THs to mediate the relationship between PFAS exposure and birth size. This may be due to a number of factors, including small sample size, as well as the effect of THs to explain only a very small part of the PFAS-birth size association. Furthermore, TH concentrations were measured at a single point in time during pregnancy and at birth, which may have introduced some measurement error due to physiological variations. Other potential hormones should be further explored as mediators, such as adipokine hormones that also play an important role in growth and development, and that have also been associated to prenatal PFAS exposure (16, 19, 56).
To our knowledge, this is the first study examining the potential of THs to mediate associations between PFAS and birth size. Important strengths of this study include our usage of a population-based birth cohort with elevated PFAS exposures from seafood intake to evaluate a wide range of thyroid parameters in mother-singleton birth pairs as well as a larger panel of PFAS than commonly examined. Due to the high intake of seafood, this population is believed to be without iodine deficiency that might have affected thyroid functions. Moreover, we included analyses on T3RU and FTI as indirect measures of TH binding in relation to PFAS exposure. Furthermore, since the Faroe Islands has a relatively homogenous population in terms of SES, results are less likely to be due to residual confounding.
Potential study limitations include the small sample size used in this analysis. Furthermore, TH concentrations measured from cord blood during late gestation may not be an accurate marker of fetal TH levels, because cord TH measurement can be influenced by maternal TH levels, can change until shortly after birth, and can be influenced by mode and time of delivery (9, 57). We also found associations between FT4 and TH levels to be nonsignificant and associations between FTI and TH levels to be significant. There are some discussions about the inclusion and reliability of FTI calculations to assess TH levels during pregnancy (58–61); thus, associations between FTI and TH levels should be treated with caution. Regardless of significance, however, associations were found to be in the same direction for both TH markers, and thus results should not be considered contradictory or inconsistent. The null findings for FT4 and significant findings for FTI can be potentially attributed to differences in measurement error, which may have attenuated the findings for FT4. Imprecision may not have been an important issue for the PFAS measurement, as PFAS examined in the main analyses have relatively long half-lives and thus these measurements can be considered as reliable estimates. However, we did observe low assay detection of a few PFAS and their precursors, including PFHpA, PFDoDA, and FOSA. Thus, results for these PFAS should be treated with caution.
Other limitations include the lack of information on iodine status that may confound the TH-birth size associations. However, we can assume that because the Faroese population generally consumes a high quantity of seafood, they should not be iodine-deficient (38, 43). In addition, as we included only mostly term births and infants with birth weights greater than 2500 g, our findings may not be generalizable to preterm or low-birth weight births. Finally, because several PFAS were found to be highly intercorrelated, our results may be subject to confounding among PFAS. We also did not consider whether PFAS mixtures can exhibit different effects on fetal growth outcomes and TH concentrations, when mothers and fetuses are exposed to multiple PFAS.
Conclusions
In an ethnically homogenous Faroese birth cohort, we found that prenatal exposures to several PFAS were inversely associated with birth weight, birth length, and cranial circumference. PFAS were generally positively associated with cord serum TSH concentrations; however, the effect of PFAS concentrations on birth size measures did not appear to be mediated through changes in THs.
Acknowledgments
We thank Ulrike Steuerwald for performing the clinical examinations and the cohort families for their support of the study.
Financial Support: This work was supported by the National Institute of Environmental Health Sciences (ES011681, ES012199 and ES027706) and the Danish Environmental Protection Agency as part of the environmental support program DANCEA (Danish Cooperation for Environment in the Arctic).
Author Contributions: C.X. conducted the data analysis, prepared the figures and tables, and wrote the manuscript under supervision of YO. C.X., Y.O., and P.G. conceptualized the study. P.G. and P.W. initiated the cohort, recruited the participants, and conducted subsequent follow-up. F.N. conducted the PFAS analyses. C.X., P.G., D.V., FN., T.K.J., and Y.O. contributed in data interpretation, validated the findings, and critically reviewed the manuscript.
Glossary
Abbreviations
- ACME
average causal mediated effect
- ADE
average direct effect
- BMI
body mass index
- FOSA
perfluorooctane sulfonamide
- FT3
free triiodothyronine
- FT4
free thyroxine
- FTI
free T4 index
- HPT
hypothalamic–pituitary–thyroid
- LOQ
limit of quantitation
- PCB
polychlorinated biphenyl
- PFAS
perfluoroalkyl substance
- PFDA
perfluorodecanoic acid
- PFDoDA
perfluorododecanoic acid
- PFHpA
perfluoroheptanoic acid
- PFHpS
perfluoroheptane sulfonate
- PFHxS
perfluorohexane sulfonate
- PFNA
perfluorononanoic acid
- PFOA
perfluorooctanoic acid
- PFOS
perfluorooctane sulfonate
- PFUnDA
perfluoroundecanoic acid
- PM
percent mediated
- SES
socioeconomic status
- T3
triiodothyronine
- T3RU
free triiodothyronine resin uptake
- T4
thyroxine
- TE
total effect
- TH
thyroid hormone
- TSH
thyrotropin (thyroid-stimulating hormone)
Additional Information
Disclosure Summary: P.G. served as a health expert for the State of Minnesota in a lawsuit against a PFAS-producing company. All other authors declare they have no actual or potential competing financial interests.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Birnbaum LS, Grandjean P. Alternatives to PFASs: perspectives on the science. Environ Health Perspect. 2015;123(5):A104–A105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Buck RC, Franklin J, Berger U, et al. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag. 2011;7(4):513–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vestergren R, Berger U, Glynn A, Cousins IT. Dietary exposure to perfluoroalkyl acids for the Swedish population in 1999, 2005 and 2010. Environ Int. 2012;49:120–127. [DOI] [PubMed] [Google Scholar]
- 4. Hu XC, Andrews DQ, Lindstrom AB, et al. Detection of poly- and perfluoroalkyl substances (PFASs) in U.S. drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants. Environ Sci Technol Lett. 2016;3(10):344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Christensen KY, Raymond M, Blackowicz M, et al. Perfluoroalkyl substances and fish consumption. Environ Res. 2017;154:145–151. [DOI] [PubMed] [Google Scholar]
- 6. Schaider LA, Balan SA, Blum A, et al. Fluorinated compounds in U.S. fast food packaging. Environ Sci Technol Lett. 2017;4(3):105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. ATSDR. Toxicological Profile for Perfluoroalkyls. Agency for Toxic Substances and Disease Registry: Atlanta, Georgia; 2018. https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=1117&tid=237. Accessed November 12, 2018. [Google Scholar]
- 8. Ballesteros V, Costa O, Iñiguez C, Fletcher T, Ballester F, Lopez-Espinosa MJ. Exposure to perfluoroalkyl substances and thyroid function in pregnant women and children: a systematic review of epidemiologic studies. Environ Int. 2017;99:15–28. [DOI] [PubMed] [Google Scholar]
- 9. Lee JE, Choi K. Perfluoroalkyl substances exposure and thyroid hormones in humans: epidemiological observations and implications. Ann Pediatr Endocrinol Metab. 2017;22(1):6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liew Z, Goudarzi H, Oulhote Y. Developmental exposures to perfluoroalkyl substances (PFASs): an update of associated health outcomes. Curr Environ Health Rep. 2018;5(1):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Apelberg BJ, Witter FR, Herbstman JB, et al. Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth. Environ Health Perspect. 2007;115(11):1670–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johnson PI, Sutton P, Atchley DS, et al. The navigation guide - evidence-based medicine meets environmental health: systematic review of human evidence for PFOA effects on fetal growth. Environ Health Perspect. 2014;122(10):1028–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sagiv SK, Rifas-Shiman SL, Fleisch AF, Webster TF, Calafat AM, Ye X, Gillman MW, Oken E. Early-pregnancy plasma concentrations of perfluoroalkyl substances and birth outcomes in project viva: confounded by pregnancy hemodynamics? Am J Epidemiol. 2018;187(4):793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manzano-Salgado CB, Casas M, Lopez-Espinosa MJ, et al. Prenatal exposure to perfluoroalkyl substances and birth outcomes in a Spanish birth cohort. Environ Int. 2017;108:278–284. [DOI] [PubMed] [Google Scholar]
- 15. Starling AP, Adgate JL, Hamman RF, et al. Perfluoroalkyl substances during pregnancy and offspring weight and adiposity at birth: examining mediation by maternal fasting glucose in the Healthy Start Study. Environ Health Perspect. 2017;125(6):067016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Minatoya M, Itoh S, Miyashita C, et al. Association of prenatal exposure to perfluoroalkyl substances with cord blood adipokines and birth size: the Hokkaido Study on environment and children’s health. Environ Res. 2017;156:175–182. [DOI] [PubMed] [Google Scholar]
- 17. Chen M-H, Ha E-H, Wen T-W, et al. Perfluorinated compounds in umbilical cord blood and adverse birth outcomes. PLoS One. 2012;7(8):e42474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fei C, McLaughlin JK, Tarone RE, Olsen J. Fetal growth indicators and perfluorinated chemicals: a study in the Danish National Birth Cohort. Am J Epidemiol. 2008;168(1):66–72. [DOI] [PubMed] [Google Scholar]
- 19. Ashley-Martin J, Dodds L, Arbuckle TE, et al. Maternal concentrations of perfluoroalkyl substances and fetal markers of metabolic function and birth weight. Am J Epidemiol. 2017;185(3):185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marks KJ, Cutler AJ, Jeddy Z, Northstone K, Kato K, Hartman TJ. Maternal serum concentrations of perfluoroalkyl substances and birth size in British boys. Int J Hyg Environ Health. 2019;222(5):889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kishi R, Nakajima T, Goudarzi H, et al. The association of prenatal exposure to perfluorinated chemicals with maternal essential and long-chain polyunsaturated fatty acids during pregnancy and the birth weight of their offspring: the Hokkaido Study. Environ Health Perspect. 2015;123(10):1038–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shoaff J, Papandonatos GD, Calafat AM, et al. Prenatal exposure to perfluoroalkyl substances: infant birth weight and early life growth. Environ Epidemiol. 2018;2(2):e010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meng Q, Inoue K, Ritz B, Olsen J, Liew Z. Prenatal exposure to perfluoroalkyl substances and birth outcomes; an updated analysis from the Danish National Birth Cohort. Int J Environ Res Public Health. 2018;15(9):1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bach CC, Bech BH, Brix N, Nohr EA, Bonde JP, Henriksen TB. Perfluoroalkyl and polyfluoroalkyl substances and human fetal growth: a systematic review. Crit Rev Toxicol. 2015;45(1):53–67. [DOI] [PubMed] [Google Scholar]
- 25. Valvi D, Oulhote Y, Weihe P, et al. Gestational diabetes and offspring birth size at elevated environmental pollutant exposures. Environ Int. 2017;107:205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94(2):355–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shi X, Liu C, Wu G, Zhou B. Waterborne exposure to PFOS causes disruption of the hypothalamus-pituitary-thyroid axis in zebrafish larvae. Chemosphere. 2009;77(7):1010–1018. [DOI] [PubMed] [Google Scholar]
- 28. Kim MJ, Moon S, Oh BC, et al. Association between perfluoroalkyl substances exposure and thyroid function in adults: a meta-analysis. Plos One. 2018;13(5):e0197244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Berg V, Nøst TH, Hansen S, et al. Assessing the relationship between perfluoroalkyl substances, thyroid hormones and binding proteins in pregnant women; a longitudinal mixed effects approach. Environ Int. 2015;77:63–69. [DOI] [PubMed] [Google Scholar]
- 30. Kato S, Itoh S, Yuasa M, et al. Association of perfluorinated chemical exposure in utero with maternal and infant thyroid hormone levels in the Sapporo cohort of Hokkaido Study on the Environment and Children’s Health. Environ Health Prev Med. 2016;21(5):334–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim S, Choi K, Ji K, Seo J, et al. Trans-placental transfer of thirteen perfluorinated compounds and relations with fetal thyroid hormones. Environ Sci Technol. 2011;45(17):7465–7472. [DOI] [PubMed] [Google Scholar]
- 32. Wang Y, Starling AP, Haug LS, et al. Association between perfluoroalkyl substances and thyroid stimulating hormone among pregnant women: a cross-sectional study. Environ Health. 2013;12(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Webster GM, Venners SA, Mattman A, Martin JW. Associations between perfluoroalkyl acids (PFASs) and maternal thyroid hormones in early pregnancy: a population-based cohort study. Environ Res. 2014;133:338–347. [DOI] [PubMed] [Google Scholar]
- 34. Wang Y, Rogan WJ, Chen P-C, et al. Association between maternal serum perfluoroalkyl substances during pregnancy and maternal and cord thyroid hormones: Taiwan maternal and infant cohort study. Environ Health Perspect. 2014;122(5):529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang L, Li J, Lai J, et al. Placental transfer of perfluoroalkyl substances and associations with thyroid hormones: Beijing Prenatal Exposure Study. Sci Rep. 2016;6(1):21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weihe P, Kato K, Calafat AM, et al. Serum concentrations of polyfluoroalkyl compounds in Faroese whale meat consumers. Environ Sci Technol. 2008;42(16):6291–6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Grandjean P, Andersen EW, Budtz-Jørgensen E, et al. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. Jama. 2012;307(4):391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Julvez J, Debes F, Weihe P, Choi AL, Grandjean P. Thyroid dysfunction as a mediator of organochlorine neurotoxicity in preschool children. Environ Health Perspect. 2011;119(10):1429–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Villar J, Cheikh Ismail L, Victora CG, et al. ; International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st) International standards for newborn weight, length, and head circumference by gestational age and sex: The Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384(9946):857–868. [DOI] [PubMed] [Google Scholar]
- 40. Hafen R, Anderson C, Schloerke B.. hbgd: Healthy Birth, Growth & Development. Version 0.3.8. 2017. [Google Scholar]
- 41. Haug LS, Thomsen C, Becher G. A sensitive method for determination of a broad range of perfluorinated compounds in serum suitable for large-scale human biomonitoring. J Chromatogr A. 2009;1216(3):385–393. [DOI] [PubMed] [Google Scholar]
- 42. Dunlap DB. Thyroid Function Tests (Chapter 142). In: Walker HK, Hall WD, Hurst JW, eds. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition. Boston: Butterworths; 1990. http://www.ncbi.nlm.nih.gov/pubmed/21250093. Accessed June 14, 2018. [PubMed] [Google Scholar]
- 43. Steuerwald U, Weihe P, Jørgensen PJ, et al. Maternal seafood diet, methylmercury exposure, and neonatal neurologic function. J Pediatr. 2000;136(5):599–605. [DOI] [PubMed] [Google Scholar]
- 44. Xiao C, Grandjean P, Valvi D, et al. Data from: associations of exposure to perfluoroalkyl substances with thyroid hormone concentrations and birth size. Figshare. 2019. doi:10.6084/m9.figshare.9855833.v1. Deposited on September 16, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. mediation: R package for causal mediation analysis. J Stat Softw. 2014;59(5):1–38.26917999 [Google Scholar]
- 46. King G, Tomz M, Wittenberg J.Making the most of statistical analyses: improving interpretation and presentation. Am J Pol Sci 2000;44(2):347. [Google Scholar]
- 47. StataCorp. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP; 2015. [Google Scholar]
- 48. Wood SN. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc. 2011;73(1):3–36. [Google Scholar]
- 49. R Core Team. R: A Language and Environment for Statistical Computing 2018. http://www.r-project.org/. Accessed March 18, 2018.
- 50. Lauritzen HB, Larose TL, Øien T, et al. Maternal serum levels of perfluoroalkyl substances and organochlorines and indices of fetal growth: a Scandinavian case-cohort study. Pediatr Res. 2017;81(1-1):33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. de Cock M, De Boer MR, Lamoree M, Legler J, Van De Bor M. Prenatal exposure to endocrine disrupting chemicals and birth weight-a prospective cohort study. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2016;51(2):178–185. [DOI] [PubMed] [Google Scholar]
- 52. Wang Y, Adgent M, Su PH, et al. Prenatal Exposure to Perfluorocarboxylic Acids (PFCAs) and fetal and postnatal growth in the Taiwan Maternal and Infant Cohort Study. Environ Health Perspect. 2016;124(11):1794–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Boas M, Feldt-Rasmussen U, Main KM. Thyroid effects of endocrine disrupting chemicals. Mol Cell Endocrinol. 2012;355(2):240–248. [DOI] [PubMed] [Google Scholar]
- 54. Weiss JM, Andersson PL, Lamoree MH, Leonards PE, van Leeuwen SP, Hamers T. Competitive binding of poly- and perfluorinated compounds to the thyroid hormone transport protein transthyretin. Toxicol Sci. 2009;109(2):206–216. [DOI] [PubMed] [Google Scholar]
- 55. Li Y, Cheng Y, Xie Z, Zeng F. Perfluorinated alkyl substances in serum of the southern Chinese general population and potential impact on thyroid hormones. Sci Rep. 2017;7(1):43380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shelly C, Grandjean P, Oulhote Y, et al. Early life exposures to perfluoroalkyl substances in relation to adipokine hormone levels at birth and during childhood. J Clin Endocrinol Metab. 2019;104(11):5338–5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Herbstman J, Apelberg BJ, Witter FR, Panny S, Goldman LR. Maternal, infant, and delivery factors associated with neonatal thyroid hormone status. Thyroid. 2008;18(1):67–76. [DOI] [PubMed] [Google Scholar]
- 58. Gronowski AM. Evaluation of thyroid function during pregnancy: have we taken a wrong turn? Clin Chem. 2018;64(3):439–441. [DOI] [PubMed] [Google Scholar]
- 59. McNeil AR, Stanford PE. Reporting thyroid function tests in pregnancy. Clin Biochem Rev. 2015;36(4):109–126. [PMC free article] [PubMed] [Google Scholar]
- 60. De Groot L, Abalovich M, Alexander EK, et al. Management of thyroid dysfunction during pregnancy and postpartum: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(8):2543–2565. [DOI] [PubMed] [Google Scholar]
- 61. Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27(3):315–389. [DOI] [PubMed] [Google Scholar]