Abstract
Experimental evidence suggests that dopamine (DA) modulates refractive eye growth. We evaluated whether increasing endogenous DA activity using pharmacological or genetic approaches decreased myopia susceptibility in mice. First, we assessed the effects of systemic L-3,4-dihydroxyphenylalanine (L-DOPA) injections on form deprivation myopia (FDM) in C57BL/6J (WTC57) mice. WTC57 mice received daily systemic injections of L-DOPA (n=11), L-DOPA + ascorbic acid (AA, n=22), AA (n=20), or Saline (n=16). Second, we tested transgenic mice with increased or decreased expression of vesicular monoamine transporter 2 (VMAT2HI , n=22; WTHI, n=18; VMAT2LO, n=18; or WTLO, n=9), which packages DA into vesicles under normal and form deprivation conditions, affecting DA release. At post-natal day 28 (P28), monocular FD was induced in each genotype. Weekly measurements of refractive error, corneal curvature, and ocular biometry were performed until P42 or P49. WTC57 mice exposed to FD developed a significant myopic shift (treated-contralateral eye) in AA (−3.27±0.73D) or saline (−3.71±0.80D) treated groups that was significantly attenuated by L-DOPA (−0.73±0.90D, p=0.0002) or L-DOPA + AA (−0.11±0.46D, p=0.0103). The VMAT2LO mice, with under-expression of VMAT2, were most susceptible to FDM. VMAT2LO mice developed significant myopic shifts to FD after one week compared to VMAT2HI and WT mice (VMAT2LO: −5.48 ± 0.54D; VMAT2HI: −0.52 ± 0.92D, p<0.05; WT: −2.13 ± 0.78D, p<0.05; ungoggled control: −0.22 ± 0.24D, p<0.001). These results indicate that endogenously increasing DA synthesis and release by genetic and pharmacological methods prevents FDM in mice.
Keywords: myopia, dopamine, form-deprivation, VMAT2, L-DOPA, mouse, retina
1. Introduction
For a growing number of individuals around the world, refractive development is disrupted by either genetic or environmental factors which cause the eye to develop myopia (Fricke et al., 2018; Morgan et al., 2018). Myopia is caused by light focusing in front of the retina, typically caused by axial elongation, which causes a blurred image of objects at a distance. Though relatively easy to treat with corrective lenses, myopia increases the risk for a number of blinding eye diseases later in life such as cataracts, retina detachments, and glaucoma (Morgan et al., 2017; Verkicharla et al., 2015). Myopia dramatically increases the burden on health care systems and negatively impacts educational and economic productivity when left untreated (Resnikoff et al., 2008).
The factors influencing myopia development and the ocular signaling mechanisms controlling it are still largely unknown. The retinal mechanisms by which the eye detects and responds to defocus during development and emmetropization are currently being investigated in animal models with experimentally induced myopia (Schaeffel and Feldkaemper, 2015). Several chemical messengers have been proposed as regulators of refractive eye growth including the neuromodulator dopamine (DA) (Ashby et al., 2007; Nickla et al., 2009; Stone et al., 1989; Stone et al., 1991; Stone et al., 2003). DA is responsible for many important functions of the retina such as gap junction and circadian rhythm regulation (Chakraborty et al., 2018; Jackson et al., 2012; Witkovsky, 2004).
DA has been implicated in myopia and refractive development in many animal studies (Feldkaemper and Schaeffel, 2013; Iuvone et al., 1991; Stone et al., 1989). In chicks with form deprivation (FD), retinas show decreased levels of DA relative to control eyes (Stone et al., 1989). DA receptor agonists generally decrease the response to both FD or lens-induced myopia (Huang et al., 2018; Iuvone et al., 1991; Nickla et al., 2010; Schmid and Wildsoet, 2004), while decreasing DA signaling through receptor antagonists generally exaggerates the response (McCarthy et al., 2007; Rohrer et al., 1993; Schaeffel et al., 1995). While it is unlikely that DA is the only signaling molecule to impact the regulation of ocular growth, it is likely that DA activity is an early signaling mechanism triggering downstream effects on the choroid, sclera, or even cornea (Cavallotti et al., 1999; Grueb et al., 2006; Zhou et al., 2017). Therefore, increasing DA levels and DA activity in the retina may prevent myopia development.
DA in the retina is synthesized when tyrosine hydroxylase (TH) is stimulated by light to convert tyrosine to L-DOPA which is then converted to DA by aromatic amino acid decarboxylase (AADC) in dopaminergic amacrine cells (DACs). Treating animals with L-DOPA could increase the levels of DA synthesized and therefore increase the amounts of DA released into the extracellular space, increase downstream signaling, and prevent myopia. Previous work with L-DOPA to prevent myopia in guinea pigs showed that the drug was effective at increasing DA levels and preventing the response to form deprivation (Mao et al., 2010); however, the effect of L-DOPA has not been tested in mice. In these experiments, we determined the effect of increasing endogenous levels of DA on myopia development by treating form deprived mice with L-DOPA, the DA precursor. We hypothesized that administering L-DOPA to C57BL/6J WT (WTC57) mice would endogenously increase DA activity leading to a protective effect on FDM.
Vesicular monoamine transporter 2 (VMAT2) packages cytosolic DA into vesicles for exocytotic release (Parsons et al., 1993; Reimer et al., 1998; Rudnick et al., 1990). Just as TH is the rate limiting step in DA synthesis, VMAT2 is a critical step in DA release in neural tissue. VMAT2 is vital for maintaining cellular DA capacity and DA cannot be released without VMAT2.
VMAT2HI mice with bacterial artificial chromosome (BAC)-mediated increased expression of VMAT2 have been used to study how altering DA activity could impact DA-related disease states (Lohr et al., 2014). Early characterization of the VMAT2HI mouse showed increased expression of VMAT2 in the striatum, leading to increased vesicle capacity and volume, increased DA levels, and increased DA release upon cellular stimulation (Lohr et al., 2014). This increased DA signaling in VMAT2HI mice resulted in resistance to neurotoxicity from methamphetamines and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (Lohr et al., 2016; Lohr et al., 2015). In contrast, VMAT2LO mice with under-expression of the VMAT2 gene by targeted insertion of a neomycin replacement vector have decreased retinal DA and DOPAC levels and increased nigrostriatal degeneration and motor deficits (Caudle et al., 2007; Taylor et al., 2009). Together, these studies show that VMAT2 has dramatic regulatory effects on DA release and downstream signaling as well as related health outcomes.
Despite its potential role in DA signaling, VMAT2 has not been closely studied in the retina. Studies characterizing VMAT2 in the rat and mouse retina have localized it to dopaminergic amacrine cells (DACs) and have shown how its expression is increased as DACs mature (Burger et al., 2011; Hirasawa et al., 2012; Witkovsky et al., 2005). Some of the initial studies on DA dynamics in myopia were conducted on chick using the non-specific VMAT inhibitor, reserpine, to dampen DA and serotonin activity by depleting its intracellular stores (Diether and Schaeffel, 1997; Ohngemach et al., 1997; Schaeffel et al., 1995). Surprisingly, most of these studies found that reserpine had an inhibitory effect on FDM and LIM. A more recent investigation of the role of VMAT2 in myopia using PET/CT imaging of FD treated guinea pigs, showed that VMAT2 and DA levels were decreased with myopia (Sun et al., 2018).
This study aims to test the hypothesis that increasing endogenous DA can protect against myopia development. WTC57 mice with FD were given L-DOPA to increase DA synthesis and activity in the retina. We also examined whether altered levels of packaging DA into vesicles for release in VMAT2HI and VMAT2LO mice can change susceptibility to FDM. These studies provide novel insights on the role of endogenous DA in refractive development and suggest potential preventative strategies.
2. Methods
2.1. Transgenic and wild-type animals
All animals were bred and raised in the animal facility of the Atlanta VA Medical Center in Atlanta, GA. Mice were given unrestricted access to food and water, kept on a 12:12 hr light:dark cycle at approximately 20-200 lux during the light phase, and their health was checked daily. All mice were used according to the approved Institutional Animal Care and Use Committee protocol and the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Male and female mice of each genotype were included in this study. C57BL/6J wild-type mice (WTC57, n= 66) were ordered from Jackson Laboratories or derived from an in-house breeding colony. VMAT2HI (n=32), VMAT2 WT (n=33), and VMAT2LO (n=19) mice were from in-house breeding colonies established from mice generously shared by Dr. Gary Miller (Emory University). VMAT2HI mice were generated with a BAC-mediated chromosomal insert using pronuclear injections into C57BL6 embryos (Lohr et al., 2014). The BAC-insert contained the entire VMAT2 locus (Slc18a2). VMAT2LO mice were generated with the targeted insertion of a neomycin resistance replacement vector (Mooslehner et al., 2001). This transgenic mouse line expresses a hypomorphic allele of the gene, reducing expression of VMAT2. Each strain was bred separately, but in all cases, VMAT2 WT mice were littermates of the VMAT2HI or VMAT2LO mice. Pups were genotyped for either the BAC or neomycin inserts, and non-carriers were categorized as VMAT2 WTHI or VMAT2 WTLO respectively. All genotyping was done by Transnetyx, Inc. (Cordova, TN). Copy numbers of inserted VMAT2 were kept consistent by mating VMAT2HI mice with C57BL/6 mice obtained from Charles River Labs (Wilmington, MA).
2.2. L-DOPA and ascorbic acid treatment
WTC57 mice were divided into four treatment groups; L-DOPA (1.0 mg/kg body weight), ascorbic acid (AA) only (1.0 mg/kg body weight), L-DOPA + AA (1.0 mg/kg body weight each, Thermo Fisher Scientific, Waltham, MA), and saline (0.9% NaCl). The dose of L-DOPA was based on previous L-DOPA treatment studies in form-deprived guinea pigs (Mao et al., 2010). AA was added to solutions of L-DOPA to prevent oxidation. All drug treatments were prepared in saline immediately before administration, kept in light tight containers, and administered daily via intraperitoneal injection between 9-11 hours after light onset. To prevent added time and oxidation, the pH was not measured and solutions were not titrated. Treatments for all groups began at P28, when a subset of animals were given FD to induce myopia.
2.3. Refractive and ocular measurements
At P28, baseline measurements of refractive error and ocular biometrics were collected for all animals. As previously described, refractive error was measured with a custom-made automated photorefractor (Schaeffel, 2008). Mouse eyes were dilated using 1% tropicamide. The mice were then anesthetized [ketamine (80 mg/kg):xylazine (16 mg/kg)], and the refractive error was measured. Animals that showed a baseline difference between treated right (OD) and naïve, contralateral left (OS) eyes greater than 2.0 diopters (D) in either direction were eliminated from the study.
Following refractive error measurements, the corneal curvature of each eye was recorded using a custom-made keratometer (Schaeffel, 2008). Finally, the axial ocular measurements and retinal thicknesses were measured using a spectral domain optical coherence tomography (SD-OCT) system (Bioptogen Envisu R4300, Leica Microsystems Inc., Wetzlar, Germany). From these images, corneal thickness, lens thickness, and anterior and vitreous chamber depths were measured. After baseline measurements, a subset of animals was treated with form deprivation (FD), as described below. All mice were given atipamezole (Antisedan, 1mg/kg) and saline eye drops to aid in recovery.
Measurements were repeated weekly for FD experiments until P42 (WTC57 mice with L-DOPA) or P49 (VMAT2 mice). A subset of VMAT2 WTHI+LO (n=8), VMAT2HI (n=15), and VMAT2LO (n=7) mice were measured every two weeks beginning at P28 until P112 to determine the effect of VMAT2 expression on refractive development under normal visual conditions.
2.4. Inducing form deprivation
Immediately following baseline measurements (at P28), a subset of mice was exposed to FD by surgically attaching a head mounted diffuser goggle over the right eye (Faulkner et al., 2007). Goggles were removed weekly under darkened conditions for cleaning and to measure changes in refractive error. For FD experiments, WTC57 (n=32), VMAT2 WTHI (n=7), VMAT2HI (n=4), VMAT2 WTLO (n=2), VMAT2LO (n=5) mice were used. Mice from each genotype without FDM were used as littermate controls for each experiment (VMAT2 WTHI: n=7, VMAT2HI: n=5, VMAT2 WTLO: n=3, VMAT2LO: n=6). FD treatment continued until P44 (WTC57) or P51 (VMAT2), two days after the final measurements were taken.
2.5. HPLC analysis of dopamine and DOPAC in the retina
Two days after the final ocular measurements, all mice were sacrificed via cervical dislocation 4-6 hours after light onset. Retinas were immediately frozen on dry ice and stored at −80° C until all tissue samples had been collected. To measure DA and DOPAC content in the retinas, high performance liquid chromatography (HPLC) with electrochemical detection and a 1.4-1.7 mM 1-octanesulfonic acid sodium, 75 mM NaH2PO4, 0.025% triethylamine, and 8% acetonitrile at pH 2.93-3.0. mobile phase was used (Song et al., 2012). The retinas were homogenized in 0.1 N HClO4 solution (0.01% sodium metabisulfite and 50 ng/mL internal standard 3,4-dihydroxybenzylamine hydrobromide) and centrifuged (10,000g for 10 minutes) to separate debris. The supernatant was then filtered (0.22 μm PVDF microcentrifuge filter tube (Thermo Fisher Scientific, Waltham, MA) spun at 5,000g for 2 minutes) and collected for testing. All samples were kept cold during preparation and were randomized for testing. Resulting HPLC peaks were analyzed based on a DA standard curve with 0.1 to 1 ng DA and DOPAC (Sigma Chemical Co., St. Louis, MO). Resulting values were normalized to total protein concentration (ng/mg) as determined by bicinchoninic acid (BCA) assay. Left and right retinas were tested individually.
2.6. Statistical analysis
Analyses were done using Graphpad Prism 7 (La Jolla, CA) and SigmaPlot version 13.0 (Systat Software, San Jose, CA). The response to FD was quantified as a myopic shift: the difference between the refractive error of the FD treated right eye and the naïve contralateral left eye (OD-OS). For comparison, the interocular difference is also calculated for control animals that did not receive FD. The myopic shift and other ocular biometric differences across treatment groups were tested using two-way repeated measures ANOVAs with Holm-Sidak post-hoc comparisons. Final myopic shifts across all mouse genotypes and treatment groups were compared using two-way ANOVAs. Refractive development values under normal conditions in VMAT2 mice were compared across genotypes using two-way repeated measures ANOVAs with Holm-Sidak post-hoc tests to determine significant effects of genotype and age. DA and DOPAC levels were analyzed with one-way ANOVA. The ungoggled left eye was used as an internal control, here referred to as the naïve, contralateral eye. ANOVA effect statistics are described in the text of this paper and post-hoc statistics are included in the figures and captions. All results are plotted as means with standard error.
3. Results
3.1. L-DOPA prevents form deprivation myopia in WTC57 mice
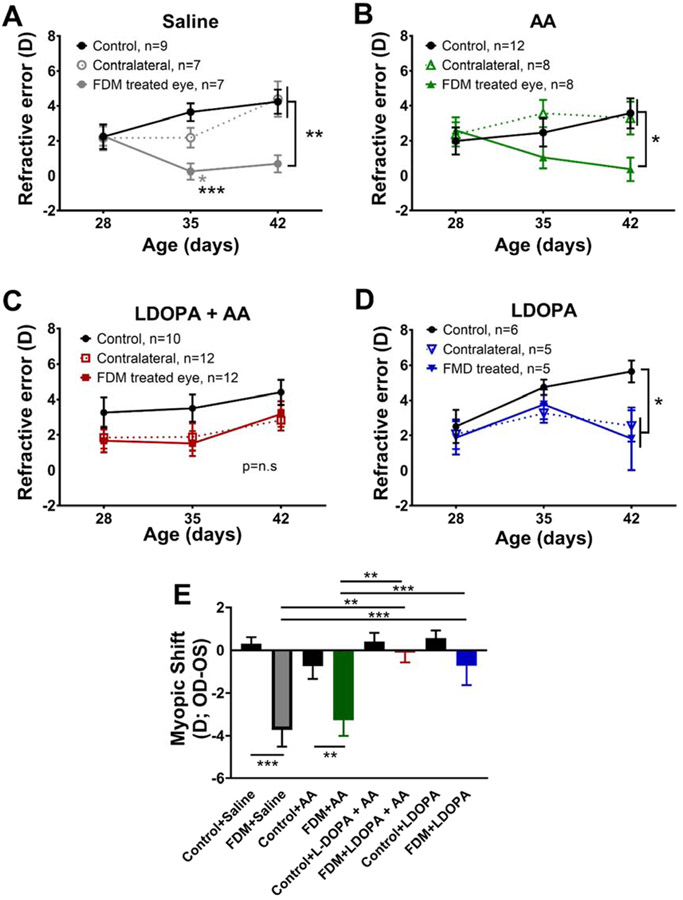
Systemic L-DOPA treatment beginning at P28, the day FD treatment began, was sufficient to prevent myopia development in WT mice (Figure 1). After 2 weeks of treatment, saline and AA mice showed less hyperopic refractive errors compared to both contralateral and control eyes (Figure 1A and B). At P42, mice treated with saline had significant relative myopia in response to FD (0.68±0.56D) compared to the contralateral and control eyes [contralateral: 4.99y±0.40D; control: 4.08±0.52D; Two-way RM ANOVA, interaction effect, F(4,40)=6.78, p<0.001; Figure 1A]. In AA treated mice, the FDM eyes (0.07±0.75D) developed significant relative myopic refractive errors by P42 than both the contralateral eyes (3.35±1.05D) and the eyes of control mice [3.97±0.71D; Two-way RM ANOVA, interaction effect, F(4,44)=3.66, p<0.05, Figure 1B]. WT mice given L-DOPA + AA or L-DOPA showed a low susceptibility to FDM after one week of treatment (Figure 1C and D). After two weeks, FD mice treated with L-DOPA + AA showed no significant differences in refractive error (3.04±0.75D) compared to their contralateral, untreated eyes (2.83±0.68D) and eyes from control mice with L-DOPA + AA (4.40±0.46D, Figure 1C). With L-DOPA treatment, both the FDM (1.81±1.63D) and contralateral eyes (2.54±0.90D) were significantly less hyperopic than control mice [5.64±0.62D; RM Two-way ANOVA, main effect of age, F(2,30)=4.69, p<0.05], but the differences between the eyes in the FD mice were not statistically significant (Figure 1D).
Figure 1. L-DOPA and L-DOPA + AA prevented FDM in WT mice.
FD was induced in WT mice treated with either Saline, AA, L-DOPA + AA, or L-DOPA, in daily systemic injections. The refractive error of all FD treated eyes were compared to the contralateral, treated eyes, and control eyes. (A) Saline injected eyes developed significant myopic growth in the FD eye compared to both the contralateral and control eyes at P35 and 42 [Two-way RM ANOVA, interaction effect, F(4,40)=6.78, p<0.001] (B) After two weeks of AA treatment, FD resulted in significant myopia relative to both contralateral and control eyes [RM Two-way ANOVA, interaction effect, F(4,44)=3.66, p<0.05] (C) With L-DOPA+AA treatment, no significant differences were found between FD and control eyes. (D) With L-DOPA treatment, FD and contralateral eyes showed relative myopia compared to eyes of control mice at P42, but did not differ from each other [RM Two-way ANOVA, main effect of age, F(2,30)=4.69, p<0.05] (E) Comparing the myopic shifts of control and FDM animals in each treatment group shows significant myopia in animals treated with AA or Saline [Two-way ANOVA, interaction effect, F(3,58)=3.65, p<0.05]. Data are mean ± SEM, *p<0.05, **p<0.01, ***p<0.001.
The effect of L-DOPA treatment on myopia susceptibility was measured by comparing the myopic shifts of FD and ungoggled control animals. Significantly different effects were found after 2 weeks of drug treatment [Two-way ANOVA, interaction effect, F(3,58)=3.65, p<0.05]. Significant myopic shifts were found for both saline (FD: −3.71±0.80D, controls: 0.31±0.31D, post-hoc comparison p<0.001) and AA (FD: −3.27±0.73D, controls: −0.75±0.59D, post-hoc comparison p<0.01, Figure 1E) treated animals. In contrast, treatments containing L-DOPA prevented myopic shifts such that there were no statistical differences with the control groups (L-DOPA + AA FD: −0.11±0.46D, controls: 0.39±0.36D, p=0.99; L-DOPA FD: −0.73±0.90D, controls: 0.60±0.037D, p=0.92; Figure 1E). Among the FDM animals, saline treated mice had significantly higher myopic shifts than L-DOPA + AA (p=0.002) and L-DOPA mice (p=0.035). FD mice treated with AA were significantly more myopic than FD mice with L-DOPA + AA (p=0.011). No significant differences between control groups were found.
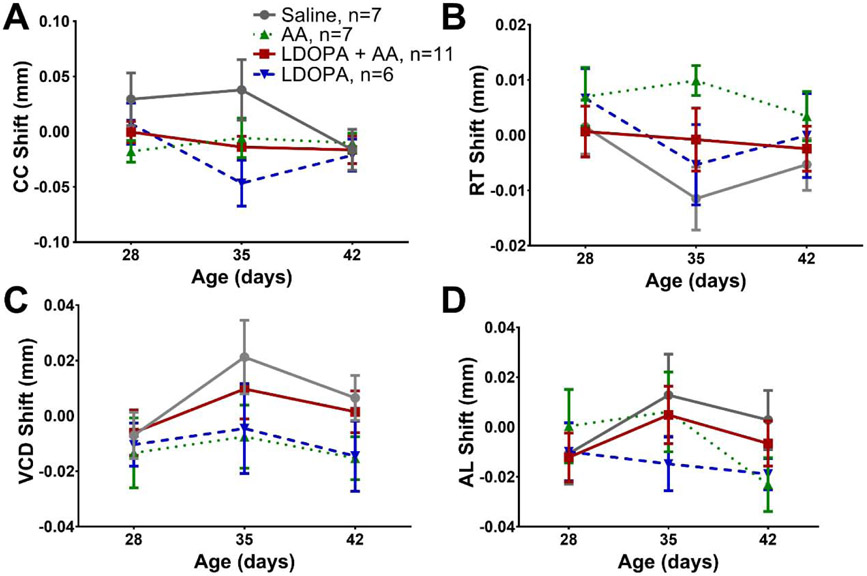
To isolate the effects of drug treatment on the response to FD, the difference between FD and contralateral eye biometrics, termed “shift”, was compared across drug treatment groups (Figure 2). No differences in corneal curvature shifts were found in FD mice after drug treatment (Figure 2A). The retinal thickness shifts were not statistically different between FDM groups (Figure 2B). Axial length and vitreous chamber shifts were also not significantly different across FDM mice (Figure 2C and D). No differences were found in these parameters across treatment group for the ungoggled control mice (Supplemental Figure 1). Additionally, changes in corneal thickness, anterior chamber depth, and lens thickness were not detected in FD or control mice (Supplemental Table 1).
Figure 2. Ocular parameters of WTC57 FD mice are not altered by L-DOPA or AA treatment.
The effects of FD with drug treatment were measured by studying the shifts (OD-OS) of corneal curvature (CC; A), retinal thickness (RT; B), axial length (AL; C) and vitreous chamber length (VCD; D) of each group from P28 to P42. No differences were found between groups for any of the ocular parameters. Data are means ± SEM. L-DOPA + AA (red solid), AA (green dotted), L-DOPA (blue dashed), Saline (grey solid).
3.2. Alternations in DA levels and DA activity with VMAT2 expression
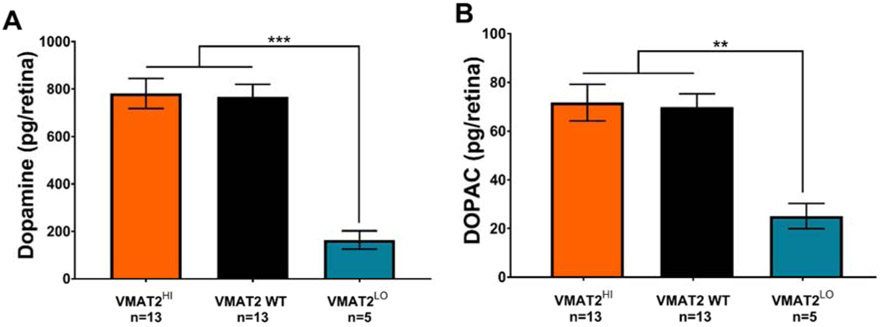
Levels of DA and DOPAC did not vary significantly with FD in any of the genotypes used here, therefore only data from ungoggled, control mice (between P28-P49) are reported. VMAT2 WT includes both VMAT2 WTHI and VMAT2 WTLO mice which were not significantly different. VMAT2 WT (786.5±52.6 pg/retina) and VMAT2HI (781.6±63.4 pg/retina) mice had statistically similar levels of DA in the retina. In contrast, VMAT2LO mice showed significantly decreased retinal DA (164.1±38.4 pg/retina, One-way ANOVA, F(2,28)=20.12, p<0.001, Figure 3A). Levels of DOPAC, the DA metabolite, followed a similar trend between the three genotypes (VMAT2 WTHI: 69.9±5.4 pg/retina; VMAT2HI: 71.7±7.5 pg/retina, VMAT2LO: 25.2±5.2 pg/retina; One-way ANOVA, F(2.28)=8.82, p<0.01, Figure 3B).
Figure 3. Decreased VMAT2 expression reduced retinal DA and DOPAC levels.
(A) Retinal DA content was significantly lower in VMATLO (teal) mice compared to VMAT2HI (orange) and VMAT2 WT (black) (One-way ANOVA, F(2, 28)=20.12, p<0.001). (B) Retinal DOPAC levels were also significantly decreased in VMAT2LO mice (One-way ANOVA, F(2, 28)=8.82, p<0.01). Data shown are mean ± SEM. **p<0.01, ***p<0.001.
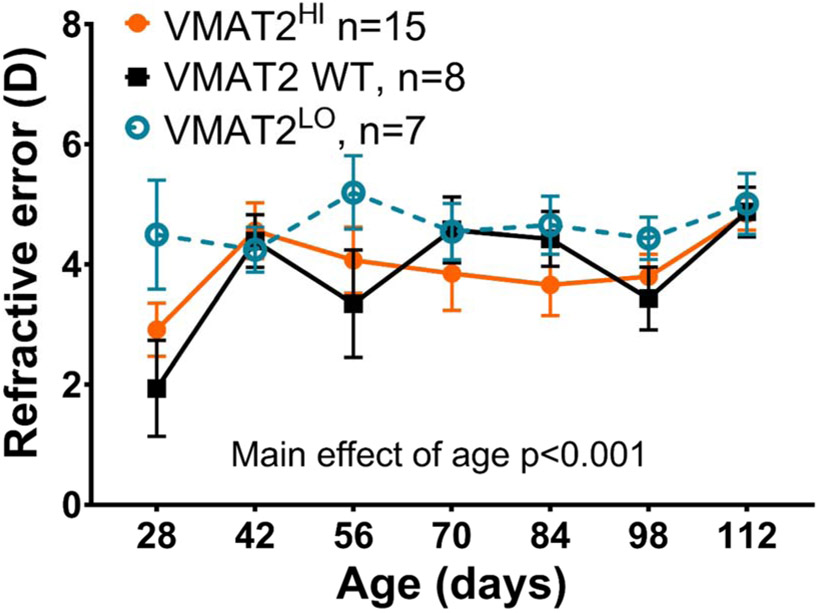
3.3. Refractive development normal in VMAT2 mutant mice
Refractive error in VMAT2 WT and VMAT2HI mice became more hyperopic with age [RM Two-way ANOVA, main effect of age, F(6,162)=4.27, p<0.001, Figure 4]. While not statistically different from the other genotypes, VMAT2LO mice had similar refractions across all ages tested (Figure 4). Here VMAT2 WT includes both VMAT2 WTHI and VMAT2 WTLO mice as there were no statistical differences between groups. By P112, VMAT2 WT (4.88±0.42D), VMAT2HI (4.84±0.27D) and VMAT2LO (5.01±0.51D) mice had similar refractive errors. .
Figure 4. Refractive development of VMAT2 WT, VMAT2 HI, and VMAT2 LO mice.
Both VMAT2HI (orange) and VMAT2 WT (black) mice became more hyperopic at P42 and remained at approximately 4D through the experimental period [RM Two-way ANOVA, main effect of age, F(6,162)=4.27, p<0.001). VMAT2LO (teal) mice had refractions between 4-5D across all ages tested. There were no significant differences between genotypes. Data show mean ± SEM.
3.4. Increased susceptibility to FDM in VMAT2LO mice
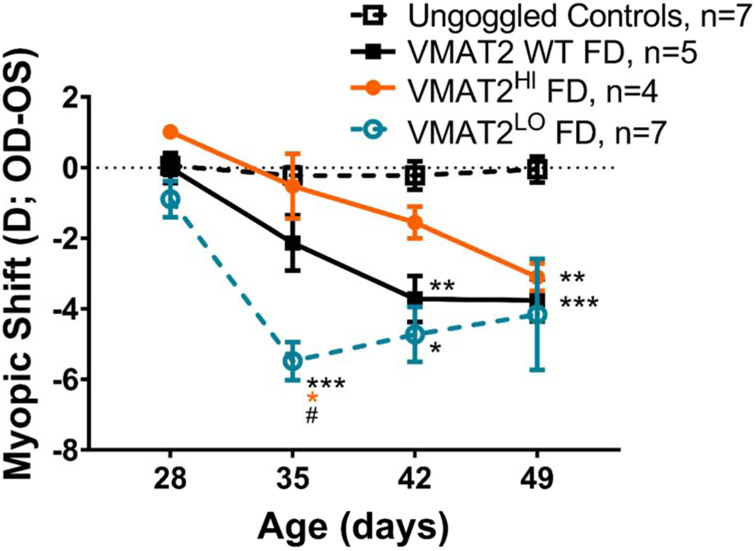
Both VMAT2HI and VMAT2LO mice showed a response to FD, but the magnitude of the response was significantly different. (RM Two-way ANOVA, interaction effect, F (9, 95) = 4.87, p<0.001, Figure 5). There were no significant differences between the ungoggled, control mice of any genotype, therefore these groups were combined for analysis and referred to as Control mice. After one week of FD, VMAT2LO mice showed the largest myopic shift in response to FD (−5.48±0.54D, p<0.001 compared to controls), while VMAT2 WT and VMAT2HI mice had a myopic shift of approximately −1D (p>0.05 compared to Control mice). After two weeks of FD the VMAT2LO mice were significantly more myopic than control mice (VMAT2LO: −4.96±0.96D, Control: −0.22±0.40D, p<0.05). VMAT2 WT mice developed a myopic shift after two weeks (−3.72±0.65D) relative to control mice (p<0.01). The significant response in VMAT2LO mice grew less robust after three weeks of FD such that after 3 weeks of goggling the myopic shifts were not different across genotypes (VMAT2LO: −4.07±2.03D, VMAT2HI: −3.10±0.39D, VMAT2 WT: −3.76±0.59D).
Figure 5. VMAT2LO mice are more susceptible to FDM than both VMAT2 WT and VMAT2HI mice.
In response to FD treatment, both VMAT2 WT (includes WTLO and WTHI littermates, solid black), VMAT2HI (orange), and VMAT2LO (teal) mice develop a myopic shift (RM Two-way ANOVA, interaction effect, F(9,95)=4.87, p<0.001). Control mice without FD treatment (dashed black) did not develop a myopic shift (all genotypes are combined). VMAT2LO mice develop a significant myopic shift after 1 week of treatment relative to control (p<0.001, indicated by black asterisks), WT FD (p<0.05) and VMAT2HI FD (p<0.05). This difference between VMAT2LO FDM treated mice and controls continued after 2 weeks of treatment (p<0.05). VMAT2 WT FD mice develop a myopic shift relative to control mice after 2 weeks of treatment (p<0.01). VMAT2HI FDM treated mice became significantly myopic after 3 weeks of treatment (p<0.01). There were no significant differences found between the WT FD and VMAT2HI FD treated mice. All data shown are mean ± SEM. Comparisons to Controls: *p<0.05, **p<0.01, ***p<0.001; Comparison to VMAT2HI: orange asterisk, p<0.05; comparison to VMAT2 WT FD: # p<0.05.
4. Discussion
4.1. Pharmacologically increasing endogenous DA prevents FDM in mice
The administration of L-DOPA, the DA precursor, prevented the myopigenic effects of FD in C57BL/6J mice. This finding supports the hypothesis that DA activity plays an important role in myopia development, specifically as a stop signal for myopic eye growth (Feldkaemper and Schaeffel, 2013; Stone et al., 1989). This finding also suggests myopia can be prevented by increasing endogenous DA synthesis in the retina. The results presented here confirm previous studies that showed protective effects of systemic L-DOPA administration in guinea pigs with FDM (Mao et al., 2010) and topical L-DOPA treatment in form-deprived chicks (Thomson et al., 2019). While direct measurements of retinal DA content after systemic L-DOPA treatments were not completed here, these previous studies have demonstrated that systemic or topical L-DOPA treatment will increase retinal DA levels (Mao et al., 2010; Thomson et al., 2019). Additionally, increased DA levels are predicted to increase DA receptor activity which has been reported to alter the effects of FDM or LIM (McCarthy et al., 2007; Nickla et al., 2010; Schaeffel et al., 1995). This is the first study to show prevention of myopia with L-DOPA treatment in mice.
We have shown that L-DOPA administration alone is sufficient to completely prevent the effects of FDM. Thus, while other eye growth signaling may occur in the retina, the effects of DA may dominate or preclude other signaling. It seems unlikely that DA is directly impacting the development of the choroid or sclera. However, it is possible that by increasing DA signaling, the downstream effects of FDM on those tissues are prevented. Here, systemic injections, rather than previously used, more invasive, intravitreal injections, prevented FDM (Ward et al., 2016).
4.2. Normal refractive development in VMAT2 mutant mice
VMAT2 over-expression or under-expression did not alter refractive development with normal visual conditions. The VMAT2 mice had similar refractive development curves as other WT mice (Figure 4) (Pardue et al., 2008; Park et al., 2014; Park et al., 2013). These results indicate that refractive development was unaffected despite potentially altering DA release in the retina due to modulation of VMAT2 expression. This is particularly surprising for VMAT2LO mice which had reduced retinal DA content and DA turnover.
Visual function testing in VMAT2HI and VMAT2LO using optomotor response showed no significant differences in spatial frequency or contrast sensitivity thresholds (Supplemental Figure 2). This finding is similar to previous work with VMAT2LO mice showing no effect on electroretinogram responses (Taylor et al., 2009). However, mice with reduced DA levels from a conditional retinal-specific knock-out of TH show significant deficits in both contrast sensitivity and spatial frequency thresholds (Jackson et al., 2012). Studies have also linked the effects of DA on circadian rhythms to daily changes in contrast sensitivity (Hwang et al., 2013; Jackson et al., 2014).
In this study, DA levels were measured from whole retina tissue using HPLC. This method does not differentiate where DA resides in the tissue: intracellular vs extracellular. Therefore, while DA levels in VMAT2LO retinas are decreased compared to VMAT2 WT retinas, the localization of DA is unknown. Unfortunately, due to lack of established techniques for measuring retinal DA, it is not possible to determine if these results are masking changes to DA localization caused by VMAT2. Measurement of extracellular DA after light stimulation through the eye cup perfusion assay described previously (Cameron et al., 2018; Perez-Fernandez et al., 2019) may be useful for determining how DA release might be altered with VMAT2 over-expression or under-expression. Given the absence of visual deficits in VMAT2LO mice, it is unlikely that VMAT2 expression has a similar detrimental effect in the retina, as in the brain (Taylor et al., 2009). In tests of VMAT2HI striatal tissue, small but significant increases in DA were found (Lohr et al., 2014). VMAT2LO mice have significant decreases in striatal DA as well as decreases in the dopamine transporter (DAT) and TH (Caudle et al., 2007).
Without a myopiagenic factor, such as FD, DA activity may not significantly impact refractive development. However, other transgenic mice, such as the retinal-specific TH mice, with low levels of DA (7% of WT) show myopic refractive errors throughout development (Bergen et al., 2016). Thus, it is also possible that dopamine packaging and release have less of an impact on visual function and/or refractive development than dopamine synthesis or that there is a critical level of DA release that is needed for normal refractive development and the retinal DA levels in VMAT2LO mice are above this critical value (20% of VMAT2WT).
4.3. Altering VMAT2 modulates the early response to FDM in mice
In the early stages of FD (1 or 2 weeks post-goggling) the VMAT2HI mice showed the smallest myopic shift while the VMAT2LO mice showed the greatest susceptibility to FD with a nearly 6 D myopic shift after 1 week of goggling. By three weeks of FD, the myopic shifts between the three genotypes were indistinguishable. This data suggests that the level of DA release in the retina may alter the initial response to myopigenic visual stimuli.
Interestingly, the VMAT2HI mice did not show sustained protection from FD. A potential explanation for these findings could be an incomplete mutation in the VMAT2HI mouse that results in normal levels of VMAT2 in the retina. Previously, this BAC-mediated mutation was assumed to be complete; however, Western blots of adult VMAT2 WT and VMAT2HI retinas showed no clear differences in band size or intensity (data not shown). An analysis of the copy number of the VMAT2 gene (Slc18a2) showed a significant increase in gene copies in VMAT2HI mice relative to their WTHI littermates (Supplementary Figure 3). VMAT2HI brain regions have similarly shown higher western blot intensity and 2.5 fold higher gene copies than VMAT2 WT mice (Lohr et al., 2014). Another potential explanation for the lack of sustained refractive changes with increasing VMAT2 expression would be a simultaneous change in other proteins involved in DA signaling to compensate and bring DA activity back to WT levels. Due to the cytotoxicity of DA and the importance of small changes in DA signaling, the proteins which control the dopaminergic system are highly adaptable, and therefore, a closer study of the mechanisms of action in DA signaling is necessary to fully determine the effects of VMAT2 in the retina.
4.4. Challenges and future directions
Several animal models have been used to study refractive eye growth, each with advantages and disadvantages [see review (Schaeffel and Feldkaemper, 2015)]. The mouse model has been used extensively in studies of retinal biology and visual processing. The ability to manipulate both genes and environment in mice provides a powerful opportunity to advance our understanding of the mechanisms underlying visually driven refractive development and myopia. While the mouse model of myopia has been shown to develop relative myopic shifts in refractive error with form deprivation and lens defocus [see review (Pardue et al., 2013)], it can be difficult to obtain accurate axial measurement in the small mouse eye. In the mouse, it is estimated that 1D of refractive error corresponds to ~6 microns of axial length change (Schmucker and Schaeffel, 2004). This small difference is at the resolution limit of our SD-OCT instrument (~5 microns). With the overall small myopic shifts that develop with FD, several of our studies have not measured significant differences in axial length or other axial parameters, despite the development of significant myopic shifts (Bergen et al., 2016; Chakraborty et al., 2014; Chakraborty et al., 2015; Chakraborty et al., 2019). Thus, advances in current SD-OCT resolution to measure axial parameters may be necessary to confirm that the mouse eye elongates with experimental myopia development.
The role of DA in myopia has been established using different experimental models of myopia, in which retinal or vitreal DA levels are decreased with myopia development (Iuvone et al., 1991; Stone et al., 1989). In the mouse model, FD does not always induce changes in retinal DA and DOPAC (Chakraborty et al., 2014; Chakraborty et al., 2015; Park et al., 2014; Park et al., 2013), as observed in this study with the VMAT2 mice. However, DA does seem to influence refractive eye growth in the mouse. In this study, the use of both L-DOPA and VMAT2 transgenic mice to pharmacologically and genetically alter endogenous DA levels altered the response to form deprivation with higher levels of DA consistently providing protective effects. Several other mouse studies have also shown that altered endogenous DA levels due to mutations are associated with altered refractive errors (Bergen et al., 2016; Park et al., 2013). The lack of differences found with FD treatment in mice may be due to the homeostasis in DA production and signaling commonly seen in neuronal tissue (Cooper et al., 2003). Since retinal tissue for DA analysis was taken 2-3 weeks following FD induction, a shorter time span between FD induction and DA and DOPAC measurements may provide an opportunity to observe this homeostasis process in the mouse model.
While the response to FD seems to be highly conserved across species, the mechanism of that response and the role of DA may differ. Further investigation into the mechanisms behind myopic eye growth in these animal models are needed to increase our understanding of human myopia.
4.5. Potential clinical applications for increasing endogenous retinal dopamine
The protective effects of L-DOPA treatment against FDM highlights the importance of DA activity in myopia and the potential for increasing DA activity as a preventative strategy to treat myopia clinically. While several studies have associated DA activity with myopia, this study clearly shows how increasing DA levels might directly benefit those at risk of myopia.
Systemic treatment with L-DOPA for children at risk of developing myopia is unlikely as it can have dramatic impacts on movement and other DA related neural functioning. However, a more localized delivery of L-DOPA to the retina might provide some benefit. A recent study found that topical delivery of levodopa inhibited myopia progression in chickens with FD and decreased axial eye growth without any detectable side effects (Thomson et al., 2019), offering potential for L-DOPA treatment to be developed as a topical eye drop for clinical treatment of myopia.
Alternatively, increasing endogenous levels of DA in the retina can also be achieved by increasing exposure to bright light (Iuvone, 1984). Higher intensity light increases the activity of TH and therefore the synthesis of DA (Iuvone et al., 1978). Bright light also reduces experimental myopia in animal models - likely by increasing DA synthesis and signaling (Ashby et al., 2009; Siegwart Jr et al., 2012; Smith et al., 2012). Early studies in human populations have shown that spending more time outdoors in bright sunlight reduces the incidence and progression of myopia (Jones et al., 2007; Rose et al., 2008; Wu et al., 2018). The mechanism behind this bright light protection is not completely known, although DA has been implicated [for review (Norton and Siegwart, 2013)]. Future investigations of how increased DA activity alters refractive error development are necessary to uncover safe and effective prevention strategies.
5. Conclusions
Systemic L-DOPA administration prevented the effects of FD in mice. These results support the hypothesis that DA activity is involved in myopic eye growth and that myopia could be prevented by increasing DA in the retina. Our second approach to increasing DA activity by potentially increasing or decreasing DA release through increased and decreased VMAT2 expression showed the potential of DA packaging and presumed release to impact FDM. VMAT2 levels may play a role in the early onset of FDM with increased expression providing protection and decreased expression increasing susceptibility. A more careful characterization of these mouse models will be necessary to confirm its effect on retinal DA release and myopia and further investigate how the dopamine signaling pathway modulates myopia development.
Supplementary Material
Highlights.
Systemic L-DOPA administration prevents form-deprivation myopia in mice
The dopamine packaging protein VMAT2 may affect early form-deprivation myopia
Endogenously increased dopamine activity prevents form-deprivation myopia in mice
Acknowledgements
This study was supported in part by the Emory HPLC Bioanalytical Core (EHBC), which was supported by the Department of Pharmacology, Emory University School of Medicine and the Georgia Clinical & Translational Science Alliance of the National Institutes of Health under Award Number UL1TR002378. We would also like to acknowledge the Miller Lab at Emory University (Gary Miller) for sharing their VMAT2 mouse colonies.
Funding Information
National Institutes of Health (NIH) [Grants R01 EY016435 (MTP) and T32 EY007092 (EGL)], and a Department of Veterans Affairs Rehabilitation R&D Service Research Career Scientist Award (MTP)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashby R, McCarthy CS, Maleszka R, Megaw P, Morgan IG, 2007. A muscarinic cholinergic antagonist and a dopamine agonist rapidly increase ZENK mRNA expression in the form-deprived chicken retina. Exp Eye Res 85, 15–22. [DOI] [PubMed] [Google Scholar]
- Ashby R, Ohlendorf A, Schaeffel F, 2009. The Effect of Ambient Illuminance on the Development of Deprivation Myopia in Chicks. Investigative Ophthalmology & Visual Science 50, 5348–5354. [DOI] [PubMed] [Google Scholar]
- Bergen MA, Park HN, Chakraborty R, Landis EG, Sidhu C, He L, Iuvone PM, Pardue MT, 2016. Altered Refractive Development in Mice With Reduced Levels of Retinal Dopamine. Invest Ophthalmol Vis Sci 57, 4412–4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger S, Doring B, Hardt M, Beuerlein K, Gerstberger R, Geyer J, 2011. Co-expression studies of the orphan carrier protein Slc10a4 and the vesicular carriers VAChT and VMAT2 in the rat central and peripheral nervous system. Neuroscience 193, 109–121. [DOI] [PubMed] [Google Scholar]
- Cameron MA, Perez Fernandez V, Milosavljevic N, Morley JW, 2018. Rod input drives, and supresses, dopamine release in the mouse retina. Investigative Ophthalmology & Visual Science 59, 3099–3099. [Google Scholar]
- Caudle WM, Richardson JR, Wang MZ, Taylor TN, Guillot TS, McCormack AL, Colebrooke RE, Di Monte DA, Emson PC, Miller GW, 2007. Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J Neurosci 27, 8138–8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallotti C, Pescosolido N, Artico M, Feher J, 1999. Localization of dopamine receptors in the rabbit cornea. Cornea 18, 721–728. [DOI] [PubMed] [Google Scholar]
- Chakraborty R, Ostrin LA, Nickla DL, Iuvone PM, Pardue MT, Stone RA, 2018. Circadian rhythms, refractive development, and myopia. Ophthalmic Physiol Opt 38, 217–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty R, Park H, Aung MH, Tan CC, Sidhu CS, Iuvone PM, Pardue MT, 2014. Comparison of refractive development and retinal dopamine in OFF pathway mutant and C57BL/6J wild-type mice. Mol Vis 20, 1318–1327. [PMC free article] [PubMed] [Google Scholar]
- Chakraborty R, Park HN, Hanif AM, Sidhu CS, Iuvone PM, Pardue MT, 2015. ON pathway mutations increase susceptibility to form-deprivation myopia. Exp Eye Res 137, 79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty R, Yang V, Park HN, Landis EG, Dhakal S, Motz CT, Bergen MA, Iuvone PM, Pardue MT, 2019. Lack of cone mediated retinal function increases susceptibility to form-deprivation myopia in mice. Exp Eye Res 180, 226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JR, Bloom FE, Roth RH, 2003. The biochemical basis of neuropharmacology. Oxford University Press, New York. [Google Scholar]
- Diether S, Schaeffel F, 1997. Local changes in eye growth induced by imposed local refractive error despite active accommodation. Vision Res 37, 659–668. [DOI] [PubMed] [Google Scholar]
- Faulkner AE, Kim MK, Iuvone PM, Pardue MT, 2007. Head-mounted goggles for murine form deprivation myopia. J Neurosci Methods 161, 96–100. [DOI] [PubMed] [Google Scholar]
- Feldkaemper M, Schaeffel F, 2013. An updated view on the role of dopamine in myopia. Exp Eye Res 114, 106–119. [DOI] [PubMed] [Google Scholar]
- Fricke TR, Jong M, Naidoo KS, Sankaridurg P, Naduvilath TJ, Ho SM, Wong TY, Resnikoff S, 2018. Global prevalence of visual impairment associated with myopic macular degeneration and temporal trends from 2000 through 2050: systematic review, meta-analysis and modelling. Br J Ophthalmol 102, 855–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueb M, Wallenfels-Thilo B, Denk O, Mielke J, Reinthal E, Rohrbach JM, Bartz-Schmidt KU, 2006. Monoamine receptors in human corneal epithelium and endothelium. Acta Ophthalmol Scand 84, 110–115. [DOI] [PubMed] [Google Scholar]
- Hirasawa H, Betensky RA, Raviola E, 2012. Corelease of dopamine and GABA by a retinal dopaminergic neuron. J Neurosci 32, 13281–13291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Zhang L, Wang Q, Yang Y, Li Q, Wu Y, Chen J, Qu J, Zhou X, 2018. Dopamine D1 Receptors Contribute Critically to the Apomorphine-Induced Inhibition of Form-Deprivation Myopia in Mice. Invest Ophthalmol Vis Sci 59, 2623–2634. [DOI] [PubMed] [Google Scholar]
- Hwang CK, Chaurasia SS, Jackson CR, Chan GC, Storm DR, Iuvone PM, 2013. Circadian rhythm of contrast sensitivity is regulated by a dopamine-neuronal PAS-domain protein 2-adenylyl cyclase 1 signaling pathway in retinal ganglion cells. J Neurosci 33, 14989–14997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuvone PM, 1984. Regulation of retinal dopamine biosynthesis and tyrosine hydroxylase activity by light. Fed Proc 43, 2709–2713. [PubMed] [Google Scholar]
- Iuvone PM, Galli CL, Garrison-Gund CK, Neff NH, 1978. Light stimulates tyrosine hydroxylase activity and dopamine synthesis in retinal amacrine neurons. Science 202, 901–902. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Tigges M, Stone RA, Lambert S, Laties AM, 1991. Effects of apomorphine, a dopamine receptor agonist, on ocular refraction and axial elongation in a primate model of myopia. Invest Ophthalmol Vis Sci 32, 1674–1677. [PubMed] [Google Scholar]
- Jackson CR, Capozzi M, Dai H, McMahon DG, 2014. Circadian perinatal photoperiod has enduring effects on retinal dopamine and visual function. J Neurosci 34, 4627–4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CR, Ruan GX, Aseem F, Abey J, Gamble K, Stanwood G, Palmiter RD, Iuvone PM, McMahon DG, 2012. Retinal dopamine mediates multiple dimensions of light-adapted vision. J Neurosci 32, 9359–9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LA, Sinnott LT, Mutti DO, Mitchell GL, Moeschberger ML, Zadnik K, 2007. Parental History of Myopia, Sports and Outdoor Activities, and Future Myopia. Invest Ophthalmol Vis Sci 48, 3524–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr KM, Bernstein AI, Stout KA, Dunn AR, Lazo CR, Alter SP, Wang M, Li Y, Fan X, Hess EJ, Yi H, Vecchio LM, Goldstein DS, Guillot TS, Salahpour A, Miller GW, 2014. Increased vesicular monoamine transporter enhances dopamine release and opposes Parkinsonian disease-related neurodegeneration in vivo. PNAS 111, 9977–9982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr KM, Chen M, Hoffman CA, McDaniel MJ, Stout KA, Dunn AR, Wang M, Bernstein AI, Miller GW, 2016. Vesicular Monoamine Transporter 2 (VMAT2) Level Regulates MPTP Vulnerability and Clearance of Excess Dopamine in Mouse Striatal Terminals. Toxicol Sci 153, 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr KM, Stout KA, Dunn AR, Wang M, Salahpour A, Guillot TS, Miller GW, 2015. Increased Vesicular Monoamine Transporter 2 (VMAT2; Slc18a2) Protects against Methamphetamine Toxicity. ACS Chem Neurosci 6, 790–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Liu S, Qin W, Li F, Wu X, Tan Q, 2010. Levodopa inhibits the development of form-deprivation myopia in guinea pigs. Optom Vis Sci 87, 53–60. [DOI] [PubMed] [Google Scholar]
- McCarthy CS, Megaw P, Devadas M, Morgan IG, 2007. Dopaminergic agents affect the ability of brief periods of normal vision to prevent form-deprivation myopia. Exp Eye Res 84, 100–107. [DOI] [PubMed] [Google Scholar]
- Mooslehner KA, Chan PM, Xu W, Liu L, Smadja C, Humby T, Allen ND, Wilkinson LS, Emson PC, 2001. Mice with very low expression of the vesicular monoamine transporter 2 gene survive into adulthood: potential mouse model for parkinsonism. Mol Cell Biol 21, 5321–5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan IG, French AN, Ashby RS, Guo X, Ding X, He M, Rose KA, 2018. The epidemics of myopia: Aetiology and prevention. Prog Retin Eye Res 62, 134–149. [DOI] [PubMed] [Google Scholar]
- Morgan IG, He M, Rose KA, 2017. EPIDEMIC OF PATHOLOGIC MYOPIA: What Can Laboratory Studies and Epidemiology Tell Us? RETINA 37, 989–997. [DOI] [PubMed] [Google Scholar]
- Nickla DL, Damyanova P, Lytle G, 2009. Inhibiting the neuronal isoform of nitric oxide synthase has similar effects on the compensatory choroidal and axial responses to myopic defocus in chicks as does the non-specific inhibitor L-NAME. Exp Eye Res 88, 1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickla DL, Totonelly K, Dhillon B, 2010. Dopaminergic agonists that result in ocular growth inhibition also elicit transient increases in choroidal thickness in chicks. Exp Eye Res 91, 715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, Siegwart JT, 2013. Light levels, refractive development, and myopia - A speculative review. Exp Eye Res 114, 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohngemach S, Hagel G, Schaeffel F, 1997. Concentrations of biogenic amines in fundal layers in chickens with normal visual experience, deprivation, and after reserpine application. Vis Neurosci 14, 493–505. [DOI] [PubMed] [Google Scholar]
- Pardue MT, Faulkner AE, Fernandes A, Yin H, Schaeffel F, Williams RW, Pozdeyev N, Iuvone PM, 2008. High susceptibility to experimental myopia in a mouse model with a retinal on pathway defect. Invest Ophthalmol Vis Sci 49, 706–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardue MT, Stone RA, Iuvone PM, 2013. Investigating mechanisms of myopia in mice. Exp Eye Res 114, 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Jabbar SB, Tan CC, Sidhu CS, Abey J, Aseem F, Schmid G, Iuvone PM, Pardue MT, 2014. Visually driven ocular growth in mice requires functional rod photoreceptors. Invest Ophthalmol Vis Sci 55, 6272–6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Tan CC, Faulkner A, Jabbar SB, Schmid G, Abey J, Iuvone PM, Pardue MT, 2013. Retinal degeneration increases susceptibility to myopia in mice. Mol Vis 19, 2068–2079. [PMC free article] [PubMed] [Google Scholar]
- Parsons SM, Bahr BA, Rogers GA, Clarkson ED, Noremberg K, Hicks BW, 1993. Acetylcholine transporter--vesamicol receptor pharmacology and structure. Prog Brain Res 98, 175–181. [DOI] [PubMed] [Google Scholar]
- Perez-Fernandez V, Milosavljevic N, Allen AE, Vessey KA, Jobling AI, Fletcher EL, Breen PP, Morley JW, Cameron MA, 2019. Rod Photoreceptor Activation Alone Defines the Release of Dopamine in the Retina. Curr Biol 29, 763–774.e765. [DOI] [PubMed] [Google Scholar]
- Reimer RJ, Fon EA, Edwards RH, 1998. Vesicular neurotransmitter transport and the presynaptic regulation of quantal size. Curr Opin Neurobiol 8, 405–412. [DOI] [PubMed] [Google Scholar]
- Resnikoff S, Pascolini D, Mariotti SP, Pokharel GP, 2008. Global magnitude of visual impairment caused by uncorrected refractive errors in 2004. Bull World Health Organ 86, 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer B, Spira AW, Stell WK, 1993. Apomorphine blocks form-deprivation myopia in chickens by a dopamine D2-receptor mechanism acting in retina or pigmented epithelium. Vis Neurosci 10, 447–453. [DOI] [PubMed] [Google Scholar]
- Rose KA, Morgan IG, Ip J, Kifley A, Huynh S, Smith W, Mitchell P, 2008. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology 115, 1279–1285. [DOI] [PubMed] [Google Scholar]
- Rudnick G, Steiner-Mordoch SS, Fishkes H, Stern-Bach Y, Schuldiner S, 1990. Energetics of reserpine binding and occlusion by the chromaffin granule biogenic amine transporter. Biochemistry 29, 603–608. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, 2008. Test systems for measuring ocular parameters and visual function in mice. Frontiers in bioscience: a journal and virtual library 13, 4904–4911. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Bartmann M, Hagel G, Zrenner E, 1995. Studies on the role of the retinal dopamine/melatonin system in experimental refractive errors in chickens. Vision Res 35, 1247–1264. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Feldkaemper M, 2015. Animal models in myopia research. Clinical and Experimental Optometry 98, 507–517. [DOI] [PubMed] [Google Scholar]
- Schmid KL, Wildsoet CF, 2004. Inhibitory effects of apomorphine and atropine and their combination on myopia in chicks. Optometry and Vision Science 81, 137–147. [DOI] [PubMed] [Google Scholar]
- Schmucker C, Schaeffel F, 2004. A paraxial schematic eye model for the growing C57BL/6 mouse. Vision Res 44, 1857–1867. [DOI] [PubMed] [Google Scholar]
- Siegwart JT Jr, Ward AH, Norton TT, 2012. Moderately elevated fluorescent light levels slow form deprivation and minus lens-induced myopia development in tree shrews. Invest Ophthalmol Vis Sci 53, 3457–3457. [Google Scholar]
- Smith EL 3rd, Hung LF, Huang J, 2012. Protective effects of high ambient lighting on the development of form-deprivation myopia in rhesus monkeys. Invest Ophthalmol Vis Sci 53, 421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C-H, Fan X, Exeter CJ, Hess EJ, Jinnah HA, 2012. Functional analysis of dopaminergic systems in a DYT1 knock-in mouse model of dystonia. Neurobiology of disease 48, 66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone RA, Lin T, Iuvone PM, 1989. Retinal dopamine and form-deprivation myopia. Proc Natl Acad Sci U S A 86, 704–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone RA, Lin T, Laties AM, 1991. Muscarinic antagonist effects on experimental chick myopia. Exp Eye Res 52, 755–758. [DOI] [PubMed] [Google Scholar]
- Stone RA, Liu J, Sugimoto R, Capehart C, Zhu X, Pendrak K, 2003. GABA, experimental myopia, and ocular growth in chick. Invest Ophthalmol Vis Sci 44, 3933–3946. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zhao N, Liu W, Liu M, Ju Z, Li J, Cheng Z, Liu X, 2018. Study of Vesicular Monoamine Transporter 2 in Myopic Retina Using [18F]FP-(+)-DTBZ. Molecular Imaging and Biology 20, 771–779. [DOI] [PubMed] [Google Scholar]
- Taylor TN, Caudle WM, Shepherd KR, Noorian A, Jackson CR, Iuvone PM, Weinshenker D, Greene JG, Miller GW, 2009. Nonmotor symptoms of Parkinson's disease revealed in an animal model with reduced monoamine storage capacity. J Neurosci 29, 8103–8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson K, Karouta C, Morgan I, Kelly T, Ashby R, 2019. Effectiveness and safety of topical levodopa in a chick model of myopia. Sci Rep 9, 18345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkicharla PK, Ohno-Matsui K, Saw SM, 2015. Current and predicted demographics of high myopia and an update of its associated pathological changes. Ophthalmic Physiol Opt 35, 465–475. [DOI] [PubMed] [Google Scholar]
- Ward AH, Siegwart JT Jr., Frost MR, Norton TT, 2016. The effect of intravitreal injection of vehicle solutions on form deprivation myopia in tree shrews. Exp Eye Res 145, 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkovsky P, 2004. Dopamine and retinal function. Doc Ophthalmol 108, 17–39. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Arango-Gonzalez B, Haycock JW, Kohler K, 2005. Rat retinal dopaminergic neurons: differential maturation of somatodendritic and axonal compartments. J Comp Neurol 481, 352–362. [DOI] [PubMed] [Google Scholar]
- Wu PC, Chen CT, Lin KK, Sun CC, Kuo CN, Huang HM, Poon YC, Yang ML, Chen CY, Huang JC, Wu PC, Yang IH, Yu HJ, Fang PC, Tsai CL, Chiou ST, Yang YH, 2018. Myopia Prevention and Outdoor Light Intensity in a School-Based Cluster Randomized Trial. Ophthalmology 125, 1239–1250. [DOI] [PubMed] [Google Scholar]
- Zhou X, Pardue MT, Iuvone PM, Qu J, 2017. Dopamine signaling and myopia development: What are the key challenges. Prog Retin Eye Res 61, 60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.