Summary
The trend of e-cigarette use among teens is ever increasing. Here we show the dysbiotic oral microbial ecology in e-cigarette users influencing the local host immune environment compared with non-smoker controls and cigarette smokers. Using 16S rRNA high-throughput sequencing, we evaluated 119 human participants, 40 in each of the three cohorts, and found significantly altered beta-diversity in e-cigarette users (p = 0.006) when compared with never smokers or tobacco cigarette smokers. The abundance of Porphyromonas and Veillonella (p = 0.008) was higher among vapers. Interleukin (IL)-6 and IL-1β were highly elevated in e-cigarette users when compared with non-users. Epithelial cell-exposed e-cigarette aerosols were more susceptible for infection. In vitro infection model of premalignant Leuk-1 and malignant cell lines exposed to e-cigarette aerosol and challenged by Porphyromonas gingivalis and Fusobacterium nucleatum resulted in elevated inflammatory response. Our findings for the first time demonstrate that e-cigarette users are more prone to infection.
Subject Areas: In Vitro Toxicology, Microbiome, Oral Microbiology
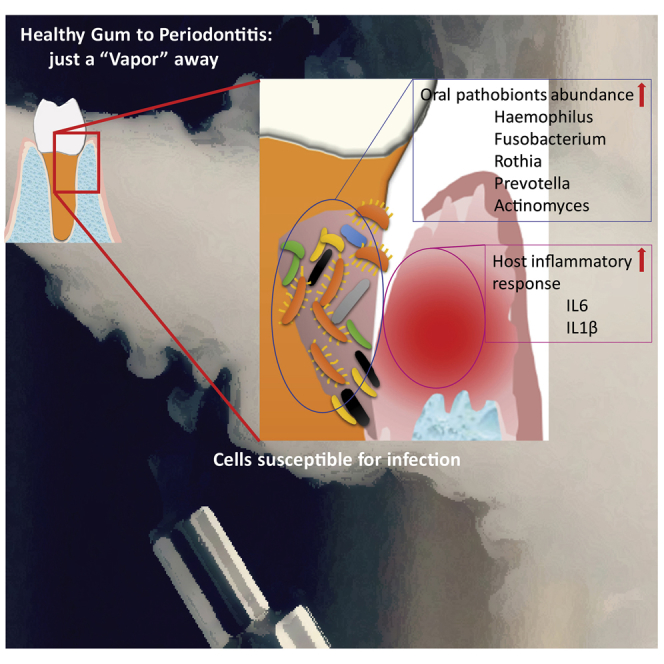
Graphical Abstract
Highlights
-
•
E- cigarette vapors contain high nicotine concentration and other toxic compounds
-
•
E-cigarette modulates oral microbiome and increases the abundance of oral pathobionts
-
•
E-cigarette aerosol alters host response and promotes gum inflammation
-
•
E-cigarette aerosol exposure makes epithelial cells susceptible to infection
In Vitro Toxicology; Microbiome; Oral Microbiology
Introduction
Electronic cigarettes (e-cigarettes), non-combustible battery-operated devices, are considered to be a safe and healthier alternative to conventional combustible cigarette smoke (Beaglehole et al., 2019). However, according to the Centers for Disease Control and Prevention (CDC), the US Food and Drug Administration (FDA), and the National Cancer Institute analysis of the 2011–2018 National Youth Tobacco Surveys data, to estimate tobacco product use in US middle and high school students, it was reported that in 2018, the current use of any tobacco product was 27.1% in high school students (4.04 million) and 7.2% in middle school students (840,000); e-cigarettes were the most commonly used product among high school (20.8%; 3.05 million) and middle school (4.9%; 570,000) students (Gentzke et al., 2019). Interestingly, e-cigarette vaping swelled by 77.8% (from 11.7% to 20.8%) among high school students and 48.5% (from 3.3% to 4.9%) among middle school students in the year 2017–2018 (Gentzke et al., 2019). These figures are of great concern due to the mounting popularity of e-cigarette usage among teenagers having never smoked any combustible products.
Unlike traditional cigarettes with tobacco filling, which approximates 24 mg of nicotine per pack (1.2 mg/cigarette), e-cigarette liquid contains nicotine (Cameron et al., 2013) that varies between 6 and 48 mg/mL (Cameron et al., 2013) and is not meant to be smoked completely in one sitting (Etter and Bullen, 2011). Each nicotine cartridge in an e-cigarette can provide on an average 200 puffs equivalent to one to three packs of cigarettes. The nicotine absorption in the body from e-cigarette use depends on the nicotine concentration in the e-liquid, its aerosol mass quantity and deposition, the chemical form of the nicotine, as well as the vaping volume, frequency, and timing (Etter, 2014). As per a 2014 report, the US FDA detected in one of the cartridges the presence of diethylene glycol, a toxic liquid used in explosives and as an antifreeze agent, in addition to, cancer-causing agents, such as tobacco-specific nitrosamines, aldehydes, metals, volatile organic compounds, phenolic compounds, polycyclic aromatic hydrocarbons, flavors, solvent carriers, tobacco alkaloids, and drugs (amino tadalafil and rimonabant) (Cheng, 2014). Moreover, recent studies showed that nicotine delivery and emission of some toxicant levels from the latest generation of e-cigarettes were comparable to those from tobacco smoke (Eltorai et al., 2019).
The oral cavity is a gateway and a permanent or makeshift harbor for numerous microbial species to colonize the respiratory and gastrointestinal (GI) tracts (Saxena et al., 2012). The multifactorial pathogenicity of chronic periodontal disease involves a complex interaction of microbial pathogens, host immune response, and genetic and environmental factors, stimulating the host tissue destruction and bone loss (Harvey, 2017). Certain oral bacteria, Porphyromonas gingivalis and Fusobacterium nucleatum, are major perpetrators in periodontal destruction, highly associated with disease progression (Tâlvan et al., 2017). The release of host immune mediators as a primary response to these opportunistic pathogens (pathobionts) and its metabolites favors the disease state. The dysbiosis in microbial communities due to impaired homeostasis as a result of environmental and host metabolic factors can contribute to oral diseases, including dental caries, periodontitis, halitosis, or other medical ailments, such as diabetes, cardiovascular diseases, and cancers (He et al., 2015). Elaborate studies have shown the toxicity of conventional tobacco product consumption to periodontal health (Kumar et al., 2011), albeit with very limited understanding on e-cigarette's effect (Stewart et al., 2018). The e-cigarette aerosol interaction with the host ensues largely in the oral cavity and lungs, where the exposure to nicotine and toxic metabolites of the e-liquid components is the highest and can escalate the host susceptibility to infections (Cheng, 2014). Recent reports associate e-cigarette vaping with vascular endothelial dysfunction causing blood-brain barrier damage with higher risk of cerebrovascular diseases (Sivandzade and Cucullo, 2019). Hence, it is imperative to elucidate the detrimental effect of e-liquids on the host inflammatory responses, which is highly challenging and conflicting.
The lucrative e-cigarette vaping delivery systems, available in a variety of flavors and concentrations, can pose a substantial threat to human health (Gentzke et al., 2019). Wu et al. (2014) have shown that e-liquids induce inflammatory responses and alter innate immune defenses in primary airway epithelial cells. The findings of Sussan et al. (2015) indicated that exposure of C57BL/6 mice to e-cigarette aerosols for 2 weeks can cause impairment of pulmonary bacterial and viral clearance. Accordingly, in vitro studies can be extrapolated to recognize responses by electronic nicotine delivery system users, however, without the clinical evidence of smoking history (Kaur et al., 2018). So, to understand the comprehensive health effects of vaping it is essential to conduct clinical research in conjunction with in vitro e-cigarette aerosol-exposed model. This will further help us to associate and understand the impact of vaping on human health by gauging the positive and negative controls.
Here, we report the in vivo effects of e-cigarette aerosol and its influence on human salivary microbiome and immune health. Furthermore, we evaluate in vitro the influence of e-cigarette aerosols on infection efficiency of oral pathogens in pre-cancerous and cancer cell lines using a novel e-cigarette aerosol-generating machine and pro-inflammatory immune mediators.
Results
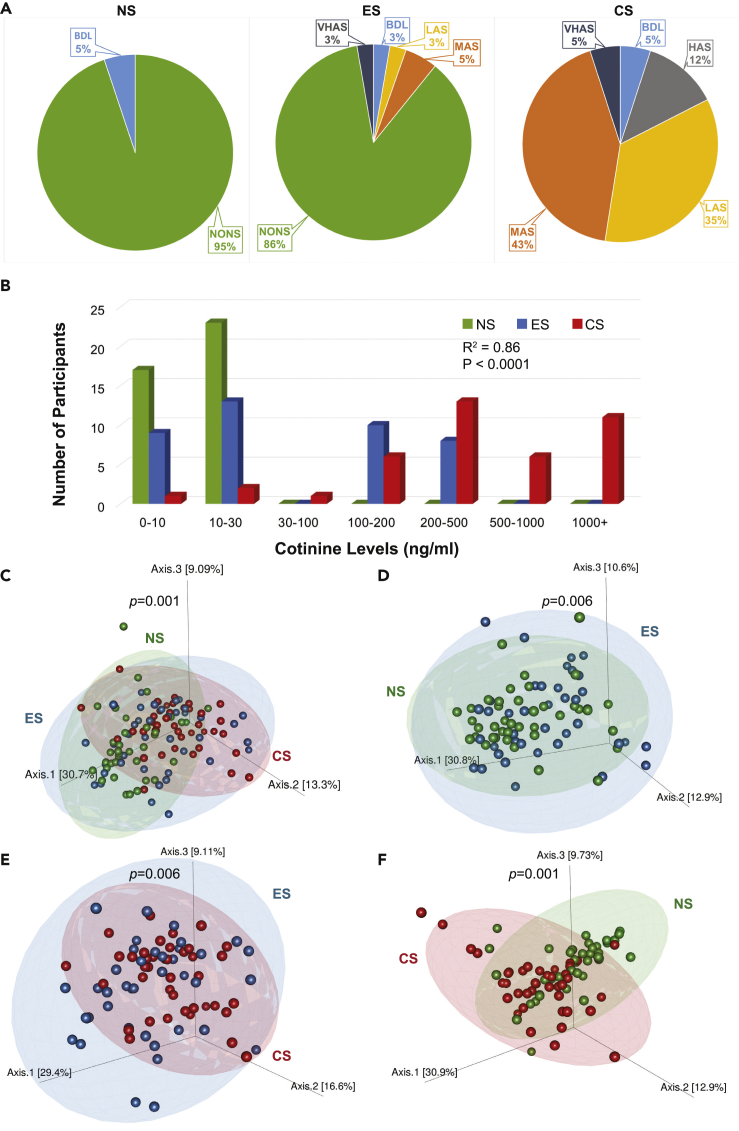
To assess the in vivo influence of smoking on microbial profiles of recruited participants, we stratified 119 subjects according to their smoking status into each of the three cohorts (never smokers [NS, n = 39], e-cigarette users [ES, n = 40], and regular cigarette smokers [CS, n = 40]). The demographic details of all the subjects and smoking history were collected at the baseline for the ES and CS groups (Table S1). The ES and CS cohorts had a nearly equal percentage of male population (∼77%–80%). On the other hand, females were higher at 43.6% in the NS cohort. Participants exclusively using e-cigarettes vaped on an average 0.5 e-cigarettes per day, whereas participants exclusively using combustible cigarettes smoked an average of 11 cigarettes per day. There was no significant change in the salivary flow rate across the participants in different cohorts. The severity index of periodontal disease or infection was significantly higher among CS (72.5%), followed by ES (42.5%), and non-smokers (28.2%), as also reflected by the calculated mean pocket depth among cohorts. This was confirmed by the values of bleeding on probing (BoP), one of the markers for inflammation, elevated in CS and receding in e-cigarette vapers and non-smokers, although with no statistical significance. To determine the participants' smoking status, exhaled breath carbon monoxide (CO) levels (ppm) and salivary cotinine levels (ng/mL) were evaluated. Figures 1A and 1B, respectively, illustrate the cohort-based distribution of the smoking biomarkers for CO levels in exhaled breath and salivary cotinine levels in the participants. The CO and the salivary cotinine levels were the lowest in non-smokers and the highest among traditional cigarette users (Table S1).
Figure 1.
Breath Carbon Monoxide and Salivary Cotinine Levels in NS, ES and CS Cohorts as a Measure of Tobacco Smoke Exposure Resulting in Skewed Bacterial Communities
(A) Levels of breath carbon monoxide (ppm) across the subjects (in percentage) in the non-smokers (NS), e-cigarette users (ES), and cigarette smokers (CS); non-smoker (NONS): 0–6 ppm, borderline (BdL): 7–9 ppm, low addicted smoker (LAS): 10–15 ppm, moderate addicted smoker (MAS): 16–25 ppm, heavily addicted smoker (HAS): 26–35 ppm, and very heavily addicted smoker (VHAS): 36+ ppm.
(B) Distribution of salivary cotinine levels in the participants of the three cohorts.
(C–F) Weighted UniFrac 3D PCoA plots illustrating beta diversity of salivary bacterial taxa across all the samples. (C) Three cohorts, NS, ES, and CS (p < 0.001); (D) between NS and ES cohorts (p = 0.005); (E) between ES and CS cohorts (p = 0.006); and (F) between NS and CS cohorts (p < 0.001). Green, never smokers (NS); blue, e-cigarette vapers (ES); and red, cigarette smokers (CS). Ellipses indicate 95% confidence interval.
Altered Bacterial Richness and Diversity in e-Cigarette Users
The salivary microbiome in 119 participants in the NS, ES, and CS groups was analyzed using 8,254,494 high-quality filtered 16S sequences (mean 68,787 ± 19,758 SD). The salivary microbiome was composed of 11 phyla, 22 classes, 33 orders, 55 families, 99 genera, 162 species, and 911 operational taxonomic units (OTUs).
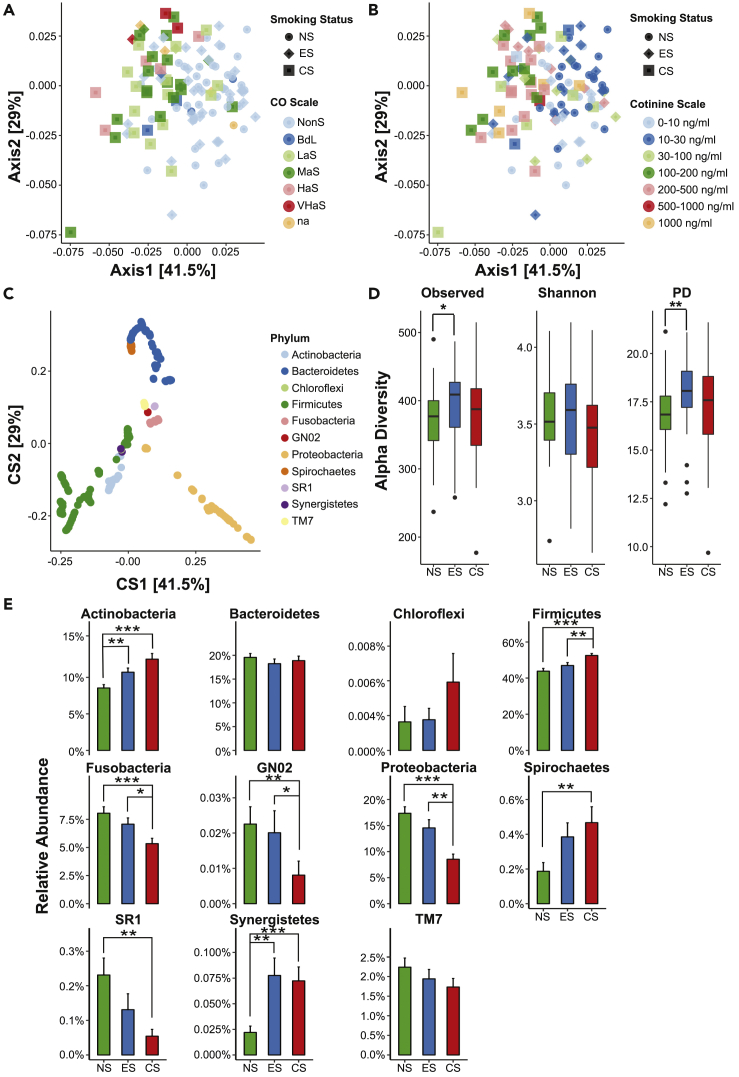
Principal coordinate analyses (PCoA) were performed to determine the overall microbiome composition in the three cohorts. Based on the weighted UniFrac distance matrix generated from all the samples in each cohort, we observed significant differences (p < 0.05, PERMANOVA) in the microbial composition between the three cohorts (Figure 1C) and additionally, between each cohort in an independent combinatorial comparison (Figures 1D–1F). These results suggest that the microbiome structure in each of the three cohorts was distinct. Interestingly, a few of the individual ES cohort samples showed an overlap with some samples in the NS (Figure 1D) and CS (Figure 1E) cohorts, although they were significantly distinct microbiomes. These findings were reiterated by double PCoA that establishes differences in taxonomic signatures (Figure 2C) in context to breath CO levels (Figure 2A) and salivary cotinine levels (Figure 2B) of the participants.
Figure 2.
Abundance of Microbiota with Respect to Carbon Monoxide Concentration, Cotinine Levels and Smoking Status
(A–E) Double PCoA plots showing taxa correlating to the smoking status, in terms of (A) breath CO levels (in percentage), (B) cotinine levels, and (C) phyla taxonomy in the non-smokers (NS), e-cigarette users (ES), and cigarette smokers (CS); non-smoker (NonS): 0–6 ppm, borderline (BdL): 7–9 ppm, low addicted smoker (LaS): 10–15 ppm, moderate addicted smoker (MaS): 16–25 ppm, heavily addicted smoker (HaS): 26–35 ppm, and very heavily addicted smoker (VHaS): 36+ ppm, data not available (na). (D) Alpha diversity as measured by observed species, Shannon diversity index, and phylogenetic diversity (PD) of the salivary microbiome in NS (green), ES (blue), and CS (red) cohorts. The line inside the box represents the median, whereas the whiskers represent the lowest and highest values within the interquartile range. Outliers' individual samples are shown as dots. Analysis was done using Mann-Whitney test. (E) Phylum-level relative abundances of the salivary microbiota based on taxonomic inference of 16S rRNA sequences. Actinobacteria, Firmicutes, Fusobacteria, Proteobacteria, and Spirochaetes were significantly altered. (D and E) Data are represented as mean ± SEM. (*p < 0.05, **p < 0.01, ***p < 0.001).
The alpha diversity indices, such as observed OTUs, Shannon index, and phylogenetic diversity were computed to determine the microbial diversity within the three groups (Figure 2D). We observed a significantly higher number of observed OTUs (p = 0.023) and phylogenetic diversity (p = 0.002) in non-combustible vapers when compared with NS. No significant changes were detected in the diversity indices of the CS cohort when compared with NS and ES users. All the analysis was done using Mann-Whitney test with a confidence level of 99% (p ≤ 0.01) in each index.
Abundance of Oral Microbial Taxa in e-Cigarette Vapers
The relative abundance of salivary microbiome differed between non-combustible vapers and combustible smokers when compared with healthy non-smoking controls. The five most abundant taxa observed were Firmicutes, Proteobacteria, Bacteroidetes, Actinobacteria, and Fusobacteria, which accounted for 97.5% of the total sequences (Figure 2E). Intriguingly, Proteobacteria predominated the ES cohort with significantly lower levels in the CS cohort (p < 0.0001). The CS and ES showed significantly elevated salivary Actinobacteria compared with NS (p < 0.0001 and p < 0.01, respectively). However, Firmicutes were highly enriched in the saliva of the CS cohort compared with those of the ES (p < 0.01) and NS (p < 0.0001) cohorts. On the contrary, Fusobacteria exhibited significant depletion in the CS cohort when compared with the ES (p < 0.024) and NS (p < 0.001) cohorts. Another predominant taxon, Spirochaetes, proliferated in the CS cohort (p < 0.005).
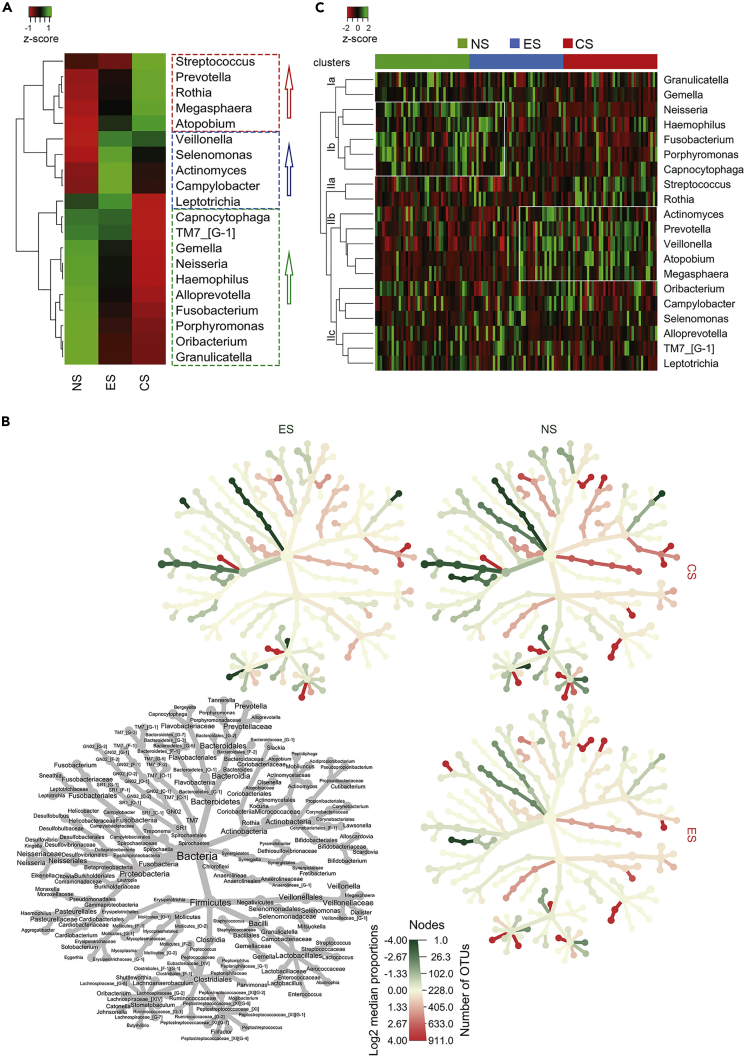
The salivary microbiome in the three cohorts was significantly dominated by eight taxa; Streptococcus, Veillonella, Prevotella, Neisseria, Haemophilus, Porphyromonas, Rothia, and Fusobacterium, which constituted 79.15% of all the sequences (Figures 3A and 3B). Other differentially abundant taxa identified were Leptotrichia, Gemella, and Capnocytophaga (Figures 3A and 3B). A hierarchical cluster analysis showed stratification of taxa into four clusters (Figure 3C). Interestingly, the ES salivary microbiota that harbored Cluster Ib and Cluster IIb showed resemblance to those taxa distinct in NS and CS cohorts, respectively (Figure 3C). A similar pattern was also observed in the weighted PCoA plot (Figure 1C). Further analyses revealed these similarities to be closely associated with the nicotine intake (smoking status) by the ES participants. Cumulatively, the taxa observed to be proliferated were Actinomyces in the ES cohort, TM7 in the ES and NS cohorts, and Granulicatella in the NS cohort. Moreover, Neisseria and Fusobacterium were significantly associated with the ES and NS cohorts compared with the CS cohort, p < 0.0001, whereas the opportunistic pathogens, Streptococcus (p < 0.01), Prevotella (p = 0.01), and Rothia (p = 0.002) were differentially enriched in the CS cohort. Veillonella levels increased significantly by ∼4% in ES (p = 0.008) and ∼4.5% (p = 0.001) in CS cohorts than NS cohorts. These findings suggest microbial dysbiosis in non-combustible vapers and combustible smokers.
Figure 3.
Dysbiotic Salivary Bacterial Genera in NS, ES, and CS Cohorts
(A) Cohort-based microbial abundances observed in NS, ES, and CS cohorts.
(B) Heat tree illustrates the relationship of OTUs up to genus level. Colored branch of the tree denotes significance based on the color of individual cohorts.
(C) Top 20 taxa in the saliva of the subject population across NS, ES, and CS cohorts; rectangular boxes represents two clusters where several taxa in the samples of ES cohort overlap with the samples of NS and CS cohorts.
A heat tree illustrates the relationships of species-specific OTUs in the NS, e-cigarette vapers, and CS (Figure S1). Streptococcus oralis subsp. tigurinus clade 071, Porphyromonas pasteri, Fusobacterium periodonticum, and Oribacterium parvum were depleted significantly in the ES and CS cohorts (Figures S2A and S2B). On the contrary, abundance of Veillonella rogosae, Granulicatella adiacens, and Prevotella sp. HMT 317 was higher in the NS cohort. Veillonella dispar, Porphyromonas endodontalis, Fusobacterium nucleatum subsp. vincentii, Prevotella oris, and Parvimonas micra predominated substantially in the ES and CS cohorts (Figures S2A and S2B). Veillonella atypica, Megasphaera micronuciformis, Streptococcus parasanguinis clade 411, Prevotella sp. HMT 311, and Actinomyces lingnae, although higher in the ES cohort, significantly expanded in the CS cohort (Figure S2C). P. gingivalis, Alloprevotella tannerae, Dialister invisus, Corynebacterium durum, and Leptotrichia wadei levels were proliferated in the ES cohort, whereas Streptococcus salivarius was higher in the CS cohort.
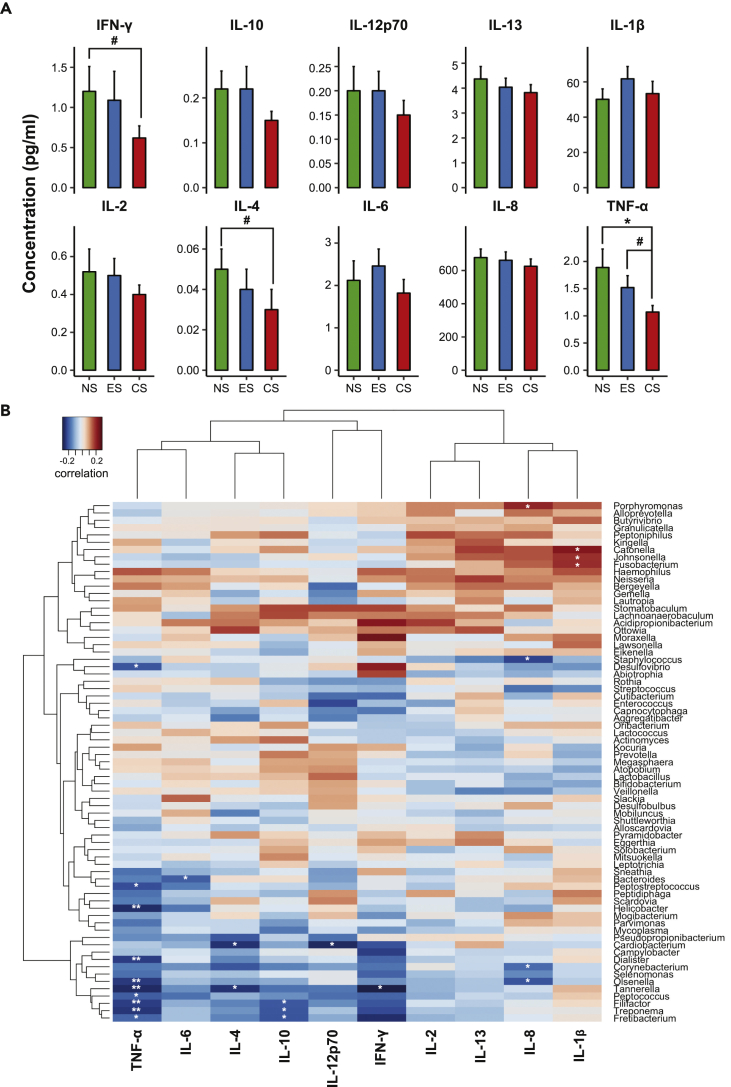
In vivo Interactions among Oral Taxa and Salivary Cytokines
To explore the salivary inflammatory markers in all participants, 39 in NS cohort and 40 in each of ES and CS cohorts, we analyzed 10 different cytokines using a human multiplex immune assay pro-inflammatory panel. Salivary interleukin (IL)-6 and IL-1β were elevated, although not significant in ES when compared with NS and CS (Figure 4A). Significant reduction in levels of interferon (IFN)-γ and IL-4 were observed in traditional CS compared with NS. Tumor necrosis factor (TNF)-α concentrations were altered significantly in non-smokers (p < 0.05) and considerably in ES (p < 0.1) compared with regular CS. However, other salivary cytokines showed no significant difference among cohorts. We further evaluated the association between inflammatory cytokines and salivary bacteria. Spearman correlation analysis indicated Porphyromonas, Haemophilus, Catonella, and Niesseria to be positively correlated with cytokines IL-2, IL-13, IL-8, and IL-1β (Figure 4B). In addition, Lachnoanaerobaculum and Stomatobaculum displayed positive correlation toward inflammatory immune indicators such as IL-2, IL-4, IL-8, IL-13, IL-10, IL-12p70, and IFN-γ (Figure 4B). Positive correlations were observed among Parvimonas, Peptostreptococcus, and Mogibacterium with IL-8 and IL-1β, and significant correlations were observed in Fusobacterium, Johnsonella, and Catonella with IL-1β. On the contrary, among a few taxa inverse correlation was observed, i.e., Desulfovibrio, Dialister, Helicobacter, Peptostreptococcus, Olsenella, and Treponema with TNF-α; Bacteroides with IL-6; Tannerella with TNF-α, IL-4, and IFN-γ; and Corynebacterium and Olsenella with IL-8.
Figure 4.
Salivary Inflammatory Cytokine Expression and Its Correlation with Abundant Taxa
(A and B) (A) Levels of 10 different pro-inflammatory salivary cytokines and chemokines in three cohorts, NS, ES, and CS. Data are represented as mean ± SEM. (#p < 0.1, *p < 0.05). (B) Correlation matrices indicate association of cytokines with the bacterial taxa. Values are represented by colors from blue (negative correlation) to red (positive correlation). Clustering is based on the Spearman rank correlation similarity between cytokines and microbial genera. (*p < 0.05, **p < 0.01)
To confirm and expand the relevance of the aforementioned in vivo findings, we next performed in vitro studies using the premalignant (Leuk-1) and malignant (Fadu) cell lines exposed to e-cigarette aerosols. We evaluated the altered pro-inflammatory cytokine response at mRNA and protein levels when co-cultured with the periodontal pathogens, P. gingivalis and F. nucleatum, the taxa observed to be expanded in vivo in the saliva of ES, and with E. coli GFP, one of the most established models of infection.
In Vitro mRNA Levels of Pro-inflammatory Cytokines Are Altered in the Presence of e-Cigarette Aerosols
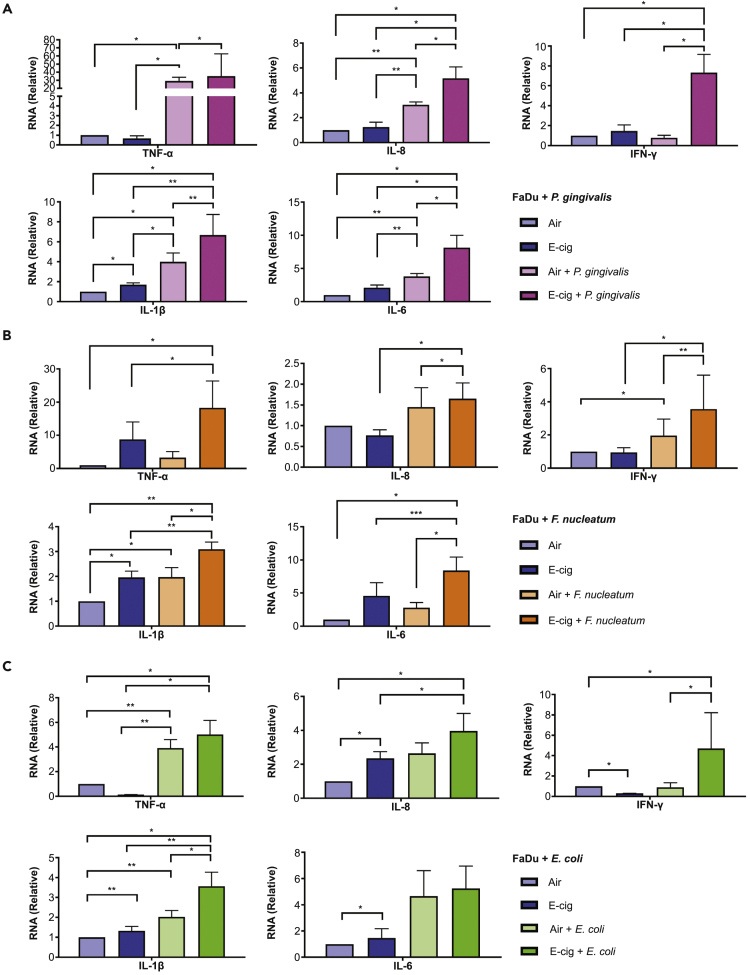
Fadu and Leuk-1 cells were exposed to e-cigarette aerosol or air for 40 min followed by infection with P. gingivalis and F. nucleatum for 2 h (Figures 5A and 5B). Initially, we exposed malignant Fadu cells to either air or e-cigarette aerosols followed by infection with each of these bacteria. We quantified the mRNA levels of the cytokines, IL-1β, TNF-α, IFN-γ, IL-6, and IL-8 by qPCR. A significant rise in all the five cytokine mRNA levels was detected in e-cigarette aerosol-exposed Fadu cells co-infected with P. gingivalis when compared with those exposed to only air co-infected with same bacteria. Intriguingly, TNF-α showed almost a 30-fold increase in expression when compared with the cells exposed only to air. Similarly, higher mRNA levels of cytokines were observed when e-cigarette aerosol-exposed Fadu cells were co-infected with F. nucleatum. The expansion in mRNA expression of IFN-γ was highly significant, followed by IL-6, IL-8, and IL-1β, and moderate for TNF-α upon e-cigarette aerosol exposure for Fadu cells. We further assessed the mRNA levels of TNF-α and IL-8 in the Leuk-1 cell line infected with P. gingivalis and F. nucleatum and observed results similar to that with Fadu cell line (Figures S3A and S3B). Significantly high mRNA expression of IL-8 was observed in e-cigarette aerosol-exposed cells with both bacteria.
Figure 5.
mRNA Expression Levels of Various Cytokines, TNF-α, IL-8, IFN-γ, IL-1β, and IL-6 in Fadu Cells in the Presence of Bacteria and e-Cigarette Aerosols as Determined by qPCR.
(A–C) Significant increase in expression of all cytokine mRNAs was observed with (A) P. gingivalis (B) F. nucleatum, and (C) E. coli GFP. Cells exposed to only air or e-cigarette aerosol were used as controls. Data are represented as mean ± SEM. (*p < 0.05, **p < 0.01, ***p < 0.001).
Furthermore, we examined whether a similar influence on cytokines is observed when Fadu cells were infected with the classical microbe, E. coli GFP (Figure 5C). Upon co-infection with this bacteria, the e-cigarette aerosol-exposed Fadu cells exhibited significantly higher mRNA levels of IL-1β and IFN-γ in contrast to air-exposed bacterial co-infected cells. In parallel, the IL-8 mRNA expression was up-regulated significantly in Fadu cells exposed to e-cigarette aerosol and E. coli GFP when compared with cells exposed singly to either air or e-cigarette. TNF-α, IL-8, IFN-γ, IL-1β, and IL-6 mRNA expression was also measured in Leuk-1 cells in the presence of E. coli GFP, and e-cigarette aerosols caused a significant rise in cytokine levels (Figure S3C).
The findings suggested augmented cytokine mRNA expression in Fadu as well as Leuk-1 cells upon e-cigarette aerosol exposure, and even more so when co-infected with bacteria, indicating increased susceptibility to infection in cells when exposed to e-cigarette aerosols.
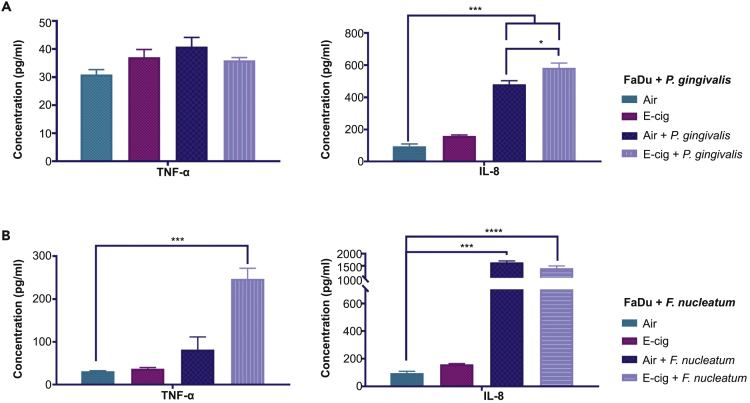
e-Cigarette Aerosol Exposure Increased the Protein Levels of Pro-inflammatory Cytokines In Vitro
Fadu cells infected with P. gingivalis, showed significant up-regulation of IL-8 protein in the presence of e-cigarette aerosol when compared with air exposure. However, TNF-α protein levels did not significantly change in the cells infected with P. gingivalis and F. nucleatum (Figures 6A and 6B). On the contrary, under similar conditions with F. nucleatum, IL-8 and TNF-α protein levels were differentially up-regulated when compared with air only. However, these protein cytokine levels were not in the detectable range with E. coli GFP (data not shown).
Figure 6.
Protein Cytokine Concentration of TNF-α and IL-8 in Bacteria-Treated Fadu Cell-Free Medium upon e-Cigarette Aerosol Exposure as Quantified by ELISA Assay
Cytokine protein levels were up-regulated with e-cigarette aerosol exposure: (A) IL-8 only with P. gingivalis and (B) TNF-α only with F. nucleatum. Data are represented as mean ± SEM. (*p < 0.05, ***p < 0.001, ****p < 0.0001).
In addition, when another cell line Leuk-1 was tested, up-regulated IL-8 level protein upon e-cigarette aerosol exposure was observed. Conversely, co-infection of Leuk-1 with P. gingivalis did not show a similar effect, where TNF-α levels were not altered (Figure S4A). However, infection with F. nucleatum showed higher protein concentration of both TNF-α and IL-8 in e-cigarette aerosol-exposed cells (Figure S4B). Leuk-1 cells co-infected with E.coli GFP in the presence of e-cigarette aerosols significantly up-regulated IL-8 protein. However, the TNF-α protein levels could not be detected with ELISA due to the very low levels of expression (Figure S4C).
Our in vitro results clearly indicated that e-cigarette aerosols altered the cytokine concentration at mRNA and protein levels in the presence of periodontal pathogens, as was also confirmed with E. coli GFP.
e-Cigarette Aerosols Accelerate Oral Bacterial Infection
To affirm that the e-cigarette aerosol influenced the rate of microbial infection in vitro, FaDu cells were co-cultured with either P. gingivalis or F. nucleatum pre-labeled with fluorescein isothiocyanate and then exposed to air or e-cigarette aerosol, and the infected FaDu cell population was evaluated by flow cytometry. It was observed that P. gingivalis and F. nucleatum infection in Fadu cells with e-cigarette aerosol contact increased significantly (p < 0.05) by about 65% and 16%, respectively, compared with air only (Figures S5A and S5B). Furthermore, FaDu cells were also infected with E.coli GFP in a 1:50 ratio after treating with either air or e-cigarette aerosol. Infection efficiency determined by GFP expression with flow cytometry showed a significant elevation (p < 0.048) of almost 21% in FaDu cells exposed to e-cigarette aerosol than in those exposed only to air (Figure S5C).
Discussion
Although e-cigarettes are conjectured to be an effective alternative to conventional cigarette smoking, statistical data insinuate an exponential rise in e-cigarette usage among college students, and its co-use with alcohol can contribute to negative consequences in the current younger generation (Littlefield et al., 2015). Also, there is limited information on the effects of nicotine content in the e-cigarette on oral microbiota and periodontal infection. This study assessed the influence of different host smoking environments on periodontal health analogous to host immune response and salivary microbial profiles.
We observed that distinct microbial communities harbor the oral cavity of ES, albeit exhibiting some community overlap with either NS or conventional CS participants. Interestingly, the e-cigarette vapers that had overlapping taxa clustering with either the NS or CS cohorts presented moderate to severe periodontitis. In addition, the e-cigarette participants who shared higher abundance of certain microbial communities (Figure 3C) with the non-smoker cohort and with conventional smokers had BoP of 56.09% and 65.49%, respectively, which reflects high inflammatory fingerprint in CS participants. An earlier study has shown an association between periodontal status and the microbiota, with increased counts of several bacteria including periodonto-pathogens, P. gingivalis, Treponema denticola, Tannerella forsythia, and Niesseria mucosa with increased inflammatory biomarkers in the saliva of periodontal disease-positive non-smokers (n = 366) (Lira-Junior et al., 2018). This connotes dependencies for dysbiotic microbial communes on periodontal health status, as signified by pocket depth and inflammatory responses, a measure of bleeding on probing and observed cytokine levels, in addition to salivary cotinine levels. The exposure to nicotine or other components from e-cigarette vaping expanded the abundance of periodontal pathogens. Periodontal microbes are a potential source of oral bacterial consortia that is altered by smoking, as reported earlier in the intervention studies for treatments of periodontal disease and tobacco dependence (Delima et al., 2010).
It is well established that smoking-associated periodontitis is not just a deliberation of poor oral hygiene but extends to provide an appropriate niche for colonization of bacteria such as P. gingivalis, Prevotella intermedia, and Actinobacillus actinomycetemcomitans, facilitating development of periodontal lesions (Eggert et al., 2001). It has been reported that exposure to nicotine modulated the immunological functions via P. gingivalis, promoting biofilm formation by interacting with commensal oral bacteria Streptococcus gordonii (Hanioka et al., 2019). Moreover, the salivary microbiome exemplifies bacteria from different surfaces in the oral cavity (Gao et al., 2018). Socransky and Haffajee (2005) observed a higher prevalence of eight species, including P. gingivalis, in current smokers. Socransky et al. (1998) showed that several bacterial complexes are involved in the etiology of periodontitis. The red complex (P. gingivalis, T. forsythia, and T. denticola) and orange complex (F. nucleatum subspecies, F. periodonticum, Peptostreptococcus micros, Prevotella intermedia, Prevotella nigrescens, and Campylobacter rectus) perio-pathogens indicate high and moderate risks, respectively, of periodontitis (Socransky et al., 1998) as detected in our study.
Moreover, we observed a significant proliferation of specific salivary taxa, to note, Veillonella in ES and regular CS when compared with NS. Nonetheless, Porphyromonas expanded significantly in ES, whereas Prevotella did in conventional CS. The taxon Granulicatella was predominant in all the three cohorts. The predominance of these periodontal pathogens in the oral cavity of ES and combustible cigarette users is a reflection of severely compromised periodontal health (p = 0.001) as depicted in Table S1. It has been shown that nicotine and its metabolite cotinine, as well as treatment with a cigarette smoke extract, can alter the function of key periodontal pathogens such as P. gingivalis and promote biofilm formation, colonization, and infection (Hanioka et al., 2019). Granulicatella is a known commensal of the human oral microbiome, however, which has been implicated in endodontic infection (Siqueira and Rôças, 2006) and is linked to increased risk of systemic diseases, such as pancreatic cancer related to oral inflammation (Farrell et al., 2012).
Cigarette smoking is known to escalate the infection risk of pathogenic or opportunistic bacteria by bringing about physiological and structural changes, increases in bacterial virulence, and immune dysfunction (Bagaitkar et al., 2008). A sophisticated cytokine network regulates the cross talk between periodontal pathogens and immune mediators. The extent of the host inflammatory response to a microbial challenge defines the severity of periodontal disease (Harvey, 2017). IL-1β concentrations were found to be significantly higher along with the progression of periodontal disease (Tâlvan et al., 2017). Prevotella was found to be positively associated with the cytokine IL-1β, which suggests a high pro-inflammatory state (Acharya et al., 2017). Previous studies have indicated Peptostreptococcus (Riggio et al., 2001) as well as Mogibacterium and Catonella to be associated with periodontal and endodontic infections (Siqueira and Rôças, 2006). In the present study, Veillonella was found to be a highly abundant taxon, specifically with species V. atypica and V. rogosae being highly elevated in ES and CS cohorts. Veillonella are largely commensals, abundantly existing in saliva and on dorsal and lateral surfaces of the tongue (Mager et al., 2003), and have conflicting (i.e., positive, negative, or no) associations with cigarette smoking in patients with periodontitis (Kato et al., 2016). Previous reports suggest that V. atypica specifically converts ingested nitrate, largely present in the saliva of tobacco users, to nitrite, which can further be converted to potentially toxic carcinogenic nitrosamines and pro-inflammatory nitric oxide (Stepanov et al., 2008). These compounds can have pathophysiological consequences on oral and systemic health. Furthermore, tobacco exposure suppresses overall immune responsiveness to bacteria and bacterial by-products such as lipopolysaccharides (LPS) (Bagaitkar et al., 2008).
Similar to our in vivo results showing increased salivary IL-6 production in ES, our in vitro study also asserted up-regulation of IL-6 after exposure to e-cigarette aerosols in Fadu and Leuk-1 cells. Moreover, in the presence of P. gingivalis alone (without e-cigarette aerosols), increased IL-6 expression in Fadu and Leuk-1 cells was detected. P. gingivalis is known to affect leukocyte distribution near microbial colonization sites disrupting the innate host surveillance (Hajishengallis et al., 2012, How et al., 2016). The observed enhanced IL-6 expression after infection with bacteria, including P. gingivalis, F. nucleatum, and E. coli GFP, suggests that e-cigarette aerosols enhanced susceptibility to periodontal disease. Both P. gingivalis and F. nucleatum play pivotal roles in periodontal disease and act on macrophages, neutrophils, and monocytes to induce TNF-α, IL-6, and IL-8 production (Petković et al., 2010). TNF-α secreted by immune cells can block bone formation and inhibit collagen synthesis (Fleetwood et al., 2017). IL-1β synergistically acts with TNF-α in the bone resorption process, and relatively high IL-1β concentrations are reported in the gingival crevicular fluid of patients with severe periodontal disease (Boström et al., 2000). Also, F. nucleatum, ubiquitous in oral cavity, is associated with chronic aggressive periodontitis, gingivitis, and endodontic infections, with additional effects such as adverse pregnancy outcomes, GI disorders, and other infections (Han, 2015). Moreover, the LPS of F. nucleatum stimulates improved IL-1β and TNF-α production when compared with that of P. gingivalis (Lagha and Grenier, 2016). Our in vivo results indicated expanded IL-1β levels in the saliva of e-cigarette vapers that parallel the proliferated presence of certain periodontal pathogenic taxa, such as Porphyromonas and Fusobacteria, in this participating cohort. In addition, F. nucleatum, known to stimulate nuclear factor-κB-driven pro-inflammatory responses, induces colon cancer (Lagha and Grenier, 2016). Increasing evidence revealed IL-6, IL-1β, and TNF-α involvement in dental and periodontal disease including inflamed pulp and periapical lesions (Brekalo Pršo et al., 2007) and in stimulation of tissue degradation due to an increase in matrix metalloproteinases (Alevizos et al., 2001). Studies have indicated high IL-6 expression at both transcriptional and translational levels in gingival mononuclear cells of patients with periodontitis (Fujihashi et al., 1993).
The periodontal microbes in tissues likewise stimulate chemokine IL-8 production, which induces a signal for accumulation and activation of neutrophils in local sites (Wilson et al., 1985). Importantly, IL-8 levels were expanded in Fadu cells after co-infection with E. coli GFP, P. gingivalis, or F. nucleatum and increased maximally in cells treated with bacteria and exposed to e-cigarette aerosols. Also, our results have shown an up-regulation of IFN-γ upon co-incubation with e-cigarette aerosols and E. coli GFP when compared with air only, e-cigarette only, and combination of air and bacteria. IFN-γ is a key player in immune responses in periodontal disease (Gemmell and Seymour, 2004) and in the activation of immunomodulation in mesenchymal stem cells. In vivo studies reported that mice, in the absence of IFN-γ, showed decreased bone loss after P. gingivalis infection in oral cavity (Meyle and Chapple, 2015). The limitations of this experimental design are that all in vitro experiments were done on cell culture models using oral pathogens, and it will be valuable to include primary cell or 3D oral tissue models and other canonical intracellular pathogen (e.g., Listeria) in our future experiments.
The presented data suggest that vaping e-cigarettes causes oral environmental shifts and highly influences the colonization of complex heterogeneous microbial biofilms. More elaborate studies would help in identifying the detrimental effects of e-cigarette aerosols and its toxic components, albeit taking into consideration other confounding factors such as vaping behavior and the dual use of e-cigarettes and conventional cigarettes. With the advent of more advanced versions of e-cigarettes, users have greater control over the quantity of e-liquid usage, power, and airflow settings. In one study, higher plasma nicotine levels were detected in newer generation of e-cigarettes compared with a first-generation e-cigarette device (Farsalinos et al., 2014). Studies are also warranted to evaluate the long-term adverse effects of metal fumes and other contaminants associated with e-cigarette usage. As e-cigarettes have become commercially available only in the past 10–12 years, there is no study that assesses the long-term health hazards associated with their use. However, very recently, the CDC and FDA reported about 215 cases of mysterious lung illnesses, linked to e-cigarette usage and vaping among teens and young adults in 25 states, currently under investigation with state and federal health officials (https://www.cdc.gov/media/releases/2019/s0830-statement-e-cigarette.html, August 30, 2019). Consequently, detailed studies will help to elucidate the mechanism and pathways of host-microbe interactions when in contact with e-cigarette aerosols that could compromise oral, respiratory, and cardiovascular health and its implication in DNA damage with cancer as a potential consequence.
Limitations of the Study
The limitations of this experimental design are that all in vitro experiments were done on cell culture models using oral pathogens, and it will be valuable to include primary cell or 3D oral tissue models. We have used three bacteria to study the increase in infection after aerosol exposure; however, it will be helpful if other canonical intracellular pathogens (e.g., Listeria) are used in future experiments.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This research project was supported by NIH grants DE025992 (D.S., X.L.), DE027074 (D.S., X.L.), CA206105 (D.S.), P30ES000260 (T.G.), and the NYU Mega grant initiative (D.S., X.L.).
Author Contributions
S.P. carried out sample collection, study design, in vivo microbiology and immunology experiments, DNA extraction and 16S sequencing, data analyses and interpretation, and manuscript preparation; B.P. performed in vitro cell culture and immunology experiments, study design, qPCR analysis and interpretation, and manuscript preparation; Q.L. performed computational analyses, manuscript preparation, and critical review; J.Y. carried out in vitro cell culture and immunology experiments; R.V. carried out subject recruitment; S.M. carried out in vitro cell culture and immunology experiments; J.M.G. carried out in vitro cell culture experiments and interpretation; S.S. performed DNA extraction; C.X. performed in vitro cell culture experiments; M.N.J. performed statistical analyses and critical review; E.Q. carried out subject recruitment; M.B. carried out clinical data analysis; J.L. performed flow cytometry experiments; J.S. performed DNA extraction; F.X. carried out REDCap data entry, technical laboratory work, and statistical analyses; E.A. performed REDCap data entry; Y.G. performed in vitro cell culture experiments; D.A. performed oral examination and clinical sample collection; C.G. performed oral examination and clinical sample collection; A.K. performed oral examination, clinical sample collection, and analyses; D.S. performed subject recruitment; Y.A. managed REDCap, clinical data, and electronic messaging system; C.B. provided technical assistance; T.G. assisted in aerosol-generating machine and manuscript preparation; P.C. provided assistance in subject recruitment and clinical sample collection; X.L. and D.S. conceived, designed, supervised, analyzed, and interpreted the study, provided critical review, and prepared the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: February 26, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.100884.
Contributor Information
Xin Li, Email: xl15@nyu.edu.
Deepak Saxena, Email: ds100@nyu.edu.
Supplemental Information
References
- Acharya A., Chan Y., Kheur S., Kheur M., Gopalakrishnan D., Watt R., Mattheos N. Salivary microbiome of an urban Indian cohort and patterns linked to subclinical inflammation. Oral Dis. 2017;23:926–940. doi: 10.1111/odi.12676. [DOI] [PubMed] [Google Scholar]
- Alevizos I., Mahadevappa M., Zhang X., Ohyama H., Kohno Y., Posner M., Gallagher G.T., Varvares M., Cohen D., Kim D. Oral cancer in vivo gene expression profiling assisted by laser capture microdissection and microarray analysis. Oncogene. 2001;20:6196. doi: 10.1038/sj.onc.1204685. [DOI] [PubMed] [Google Scholar]
- Bagaitkar J., Demuth D.R., Scott D.A. Tobacco use increases susceptibility to bacterial infection. Tob. Induc. Dis. 2008;4:12. doi: 10.1186/1617-9625-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaglehole R., Bates C., Youdan B., Bonita R. Nicotine without smoke: fighting the tobacco epidemic with harm reduction. Lancet. 2019;394:718–720. doi: 10.1016/S0140-6736(19)31884-7. [DOI] [PubMed] [Google Scholar]
- Boström L., Linder L.E., Bergström J. Smoking and GCF levels of IL-1β and IL-1ra in periodontal disease. J. Clin. Periodontol. 2000;27:250–255. doi: 10.1034/j.1600-051x.2000.027004250.x. [DOI] [PubMed] [Google Scholar]
- Brekalo Pršo I., Kocjan W., Šimic H., Brumini G., Pezelj-Ribaric S., Borcic J., Ferreri S., Miletic Karlovic I. Tumor necrosis factor-alpha and interleukin 6 in human periapical lesions. Mediators Inflamm. 2007;2007:38210. doi: 10.1155/2007/38210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron J.M., Howell D.N., White J.R., Andrenyak D.M., Layton M.E., Roll J.M. Variable and potentially fatal amounts of nicotine in e-cigarette nicotine solutions. Tob. Control. 2013;23:77–78. doi: 10.1136/tobaccocontrol-2012-050604. [DOI] [PubMed] [Google Scholar]
- Cheng T. Chemical evaluation of electronic cigarettes. Tob. Control. 2014;23(Suppl 2) doi: 10.1136/tobaccocontrol-2013-051482. ii11–i17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delima S.L., Mcbride R.K., Preshaw P.M., Heasman P.A., Kumar P.S. Response of subgingival bacteria to smoking cessation. J. Clin. Microbiol. 2010;48:2344–2349. doi: 10.1128/JCM.01821-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert F.-M., Mcleod M.H., Flowerdew G. Effects of smoking and treatment status on periodontal bacteria: evidence that smoking influences control of periodontal bacteria at the mucosal surface of the gingival crevice. J. Periodontol. 2001;72:1210–1220. doi: 10.1902/jop.2000.72.9.1210. [DOI] [PubMed] [Google Scholar]
- Eltorai A.E., Choi A.R., Eltorai A.S. Impact of electronic cigarettes on various organ systems. Respir. Care. 2019;64:328–336. doi: 10.4187/respcare.06300. [DOI] [PubMed] [Google Scholar]
- Etter J.-F. Levels of saliva cotinine in electronic cigarette users. Addiction. 2014;109:825–829. doi: 10.1111/add.12475. [DOI] [PubMed] [Google Scholar]
- Etter J.F., Bullen C. Saliva cotinine levels in users of electronic cigarettes. Eur. Respir. J. 2011;38:1219–1220. doi: 10.1183/09031936.00066011. [DOI] [PubMed] [Google Scholar]
- Farrell J.J., Zhang L., Zhou H., Chia D., Elashoff D., Akin D., Paster B.J., Joshipura K., Wong D.T.W. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut. 2012;61:582. doi: 10.1136/gutjnl-2011-300784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos K.E., Spyrou A., Tsimopoulou K., Stefopoulos C., Romagna G., Voudris V. Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Sci. Rep. 2014;4:4133. doi: 10.1038/srep04133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleetwood A.J., Lee M.K.S., Singleton W., Achuthan A., Lee M.-C., O'brien-Simpson N.M., Cook A.D., Murphy A.J., Dashper S.G., Reynolds E.C. Metabolic remodeling, inflammasome activation, and pyroptosis in macrophages stimulated by porphyromonas gingivalis and its outer membrane vesicles. Front. Cell. Infect. Microbiol. 2017;7:351. doi: 10.3389/fcimb.2017.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihashi K., Beagley K.W., Kono Y., Aicher W.K., Yamamoto M., Difabio S., Xu-Amano J., Mcghee J.R., Kiyono H. Gingival mononuclear cells from chronic inflammatory periodontal tissues produce interleukin (IL)-5 and IL-6 but not IL-2 and IL-4. Am. J. Pathol. 1993;142:1239–1250. [PMC free article] [PubMed] [Google Scholar]
- Gao L., Xu T., Huang G., Jiang S., Gu Y., Chen F. Oral microbiomes: more and more importance in oral cavity and whole body. Protein Cell. 2018;9:488–500. doi: 10.1007/s13238-018-0548-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemmell E., Seymour G.J. Immunoregulatory control of Th1/Th2 cytokine profiles in periodontal disease. Periodontol. 2000. 2004;35:21–41. doi: 10.1111/j.0906-6713.2004.003557.x. [DOI] [PubMed] [Google Scholar]
- Gentzke A.S., Creamer M., Cullen K.A., Ambrose B.K., Willis G., Jamal A., King B.A. Vital signs: tobacco product use among middle and high school students - United States, 2011-2018. MMWR Morb. Mortal. Wkly. Rep. 2019;68:157–164. doi: 10.15585/mmwr.mm6806e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G., Darveau R.P., Curtis M.A. The keystone-pathogen hypothesis. Nat. Rev Microbiol. 2012;10:717. doi: 10.1038/nrmicro2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y.W. Fusobacterium nucleatum: a commensal-turned pathogen. Curr. Opin. Microbiol. 2015;23:141–147. doi: 10.1016/j.mib.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanioka T., Morita M., Yamamoto T., Inagaki K., Wang P.-l., Ito H., Morozumi T., Takeshita T., Suzuki N., Shigeishi H. Smoking and periodontal microorganisms. Jpn. Dent Sci. Rev. 2019;55:88–94. doi: 10.1016/j.jdsr.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J.D. Periodontal microbiology. Dent Clin. North Am. 2017;61:253–269. doi: 10.1016/j.cden.2016.11.005. [DOI] [PubMed] [Google Scholar]
- He J., Li Y., Cao Y., Xue J., Zhou X. The oral microbiome diversity and its relation to human diseases. Folia Microbiol. (Praha) 2015;60:69–80. doi: 10.1007/s12223-014-0342-2. [DOI] [PubMed] [Google Scholar]
- How K.Y., Song K.P., Chan K.G. Porphyromonas gingivalis: an overview of periodontopathic pathogen below the gum line. Front. Microbiol. 2016;7:53. doi: 10.3389/fmicb.2016.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato I., Vasquez A.A., Moyerbrailean G., Land S., Sun J., Lin H.-S., Ram J.L. Oral microbiome and history of smoking and colorectal cancer. J. Epidemiol. Res. 2016;2:92–101. doi: 10.5430/jer.v2n2p92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G., Pinkston R., Mclemore B., Dorsey W.C., Batra S. Immunological and toxicological risk assessment of e-cigarettes. Eur. Respir. Rev. 2018;27:170119. doi: 10.1183/16000617.0119-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P.S., Matthews C.R., Joshi V., De Jager M., Aspiras M. Tobacco smoking affects bacterial acquisition and colonization in oral biofilms. Infect. Immun. 2011;79:4730–4738. doi: 10.1128/IAI.05371-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagha A.B., Grenier D. Tea polyphenols inhibit the activation of NF-κB and the secretion of cytokines and matrix metalloproteinases by macrophages stimulated with Fusobacterium nucleatum. Sci. Rep. 2016;6:34520. doi: 10.1038/srep34520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lira-Junior R., Åkerman S., Klinge B., Boström E.A., Gustafsson A. Salivary microbial profiles in relation to age, periodontal, and systemic diseases. PLoS One. 2018;13:e0189374. doi: 10.1371/journal.pone.0189374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlefield A.K., Gottlieb J.C., Cohen L.M., Trotter D.R.M. Electronic cigarette use among college students: links to gender, race/ethnicity, smoking, and heavy drinking. J. Am. Coll. Health. 2015;63:523–529. doi: 10.1080/07448481.2015.1043130. [DOI] [PubMed] [Google Scholar]
- Mager D.L., Ximenez-Fyvie L.A., Haffajee A.D., Socransky S.S. Distribution of selected bacterial species on intraoral surfaces. J. Clin. Periodontol. 2003;30:644–654. doi: 10.1034/j.1600-051x.2003.00376.x. [DOI] [PubMed] [Google Scholar]
- Meyle J., Chapple I. Molecular aspects of the pathogenesis of periodontitis. Periodontol. 2000. 2015;69:7–17. doi: 10.1111/prd.12104. [DOI] [PubMed] [Google Scholar]
- Petković A.B., Matić S.M., Stamatović N.V., Vojvodić D.V., Todorović T.M., Lazić Z.R., Kozomara R.J. Proinflammatory cytokines (IL-1β and TNF-α) and chemokines (IL-8 and MIP-1α) as markers of peri-implant tissue condition. Int. J. Oral Maxillofac. Surg. 2010;39:478–485. doi: 10.1016/j.ijom.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Riggio M.P., Lennon A., Smith A. Detection of Peptostreptococcus micros DNA in clinical samples by PCR. J. Med. Microbiol. 2001;50:249–254. doi: 10.1099/0022-1317-50-3-249. [DOI] [PubMed] [Google Scholar]
- Saxena D., Li Y., Yang L., Pei Z., Poles M., Abrams W.R., Malamud D. Human microbiome and HIV/AIDS. Curr. HIV/AIDS Rep. 2012;9:44–51. doi: 10.1007/s11904-011-0103-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira J.F., Jr., Rôças I.N. Catonella morbi and Granulicatella adiacens: new species in endodontic infections. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006;102:259–264. doi: 10.1016/j.tripleo.2005.09.021. [DOI] [PubMed] [Google Scholar]
- Sivandzade F., Cucullo L. Assessing the protective effect of rosiglitazone against electronic cigarette/tobacco smoke-induced blood-brain barrier impairment. BMC Neurosci. 2019;20:15. doi: 10.1186/s12868-019-0497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky S.S., Haffajee A.D. Periodontal microbial ecology. Periodontol. 2000. 2005;38:135–187. doi: 10.1111/j.1600-0757.2005.00107.x. [DOI] [PubMed] [Google Scholar]
- Socransky S.S., Haffajee A.D., Cugini M.A., Smith C., Kent R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- Stepanov I., Jensen J., Hatsukami D., Hecht S.S. New and traditional smokeless tobacco: comparison of toxicant and carcinogen levels. Nicotine Tob. Res. 2008;10:1773–1782. doi: 10.1080/14622200802443544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C.J., Auchtung T.A., Ajami N.J., Velasquez K., Smith D.P., De La Garza R., 2nd, Salas R., Petrosino J.F. Effects of tobacco smoke and electronic cigarette vapor exposure on the oral and gut microbiota in humans: a pilot study. Peer J. 2018;6:e4693. doi: 10.7717/peerj.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussan T.E., Gajghate S., Thimmulappa R.K., Ma J., Kim J.-H., Sudini K., Consolini N., Cormier S.A., Lomnicki S., Hasan F. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One. 2015;10:e0116861. doi: 10.1371/journal.pone.0116861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tâlvan E.T., Mohor C., Chisnoiu D., Cristea V., Câmpian R.S. Expression of interleukin (IL)-1β, IL-8, IL-10 and IL-13 in chronic adult periodontitis progression. Arch. Med. 2017;9:4. [Google Scholar]
- Wilson M.E., Zambon J.J., Suzuki J.B., Genco R.J. Generalized juvenile periodontitis, defective neutrophil chemotaxis and Bacteroides gingivalis in a 13-year-old female. J. Periodontol. 1985;56:457–463. doi: 10.1902/jop.1985.56.8.457. [DOI] [PubMed] [Google Scholar]
- Wu Q., Jiang D., Minor M., Chu H.W. Electronic cigarette liquid increases inflammation and virus infection in primary human airway epithelial cells. PLoS One. 2014;9:e108342. doi: 10.1371/journal.pone.0108342. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.