Highlights
-
•
Hp1036 and Hp1239 are two new cationic host defense peptides from scorpion venom.
-
•
They inhibitory effect on multiple steps of HSV-1 life cycle.
-
•
They adopted α-helix structure in approximate membrane environment.
-
•
They are virucidal of HSV-1 and destroyed the morphology of HSV-1.
-
•
They easily entered Vero cells and reduced the intracellular viral infectivity.
Keywords: Scorpion venom, Antimicrobial peptides, HSV-1, Virucidal
Abstract
Herpes simplex virus type 1 (HSV-1) is a widespread human pathogen that causes severe diseases, but there are not effective and safe drugs in clinical therapy besides acyclovir (ACV) and related nucleoside analogs. In this study, two new venom peptides from the scorpion Heterometrus petersii were identified with effective inhibitory effect on HSV-1 infection in vitro. Both Hp1036 and Hp1239 peptides exhibited potent virucidal activities against HSV-1 (EC50 = 0.43 ± 0.09 and 0.41 ± 0.06 μM, respectively) and effective inhibitory effects when added at the viral attachment (EC50 = 2.87 ± 0.16 and 5.73 ± 0.61 μM, respectively), entry (EC50 = 4.29 ± 0.35 and 4.32 ± 0.47 μM, respectively) and postentry (EC50 = 7.86 ± 0.80 and 8.41 ± 0.73 μM, respectively) steps. Both Hp1036 and Hp1239 peptides adopted α-helix structure in approximate membrane environment and resulted in the destruction of the viral morphology. Moreover, Hp1036 and Hp1239 peptides entered Vero cells and reduced the intracellular viral infectivity. Taken together, Hp1036 and Hp1239 peptides are two anti-viral peptides with effective inhibitory effect on multiple steps of HSV-1 life cycle and therefore are good candidate for development as virucides.
1. Introduction
Herpes simplex virus type 1 (HSV-1) is a widespread human pathogen that infects primarily epithelial tissues and causes severe diseases including mucocutaneous lesions in the oral mucosa (cold sores), encephalitis, meningitis, and blinding keratitis (Gopinath et al., 2012). After an initial infection, HSV-1 spreads to the nervous system and establishes latent infection of neurons in sensory ganglia of the host (Hill et al., 1996). Approximately 80% of the world populations are carriers of HSV-1 and about 40% suffer from recurrent infection (Gold and Corey, 1987, Palem et al., 2011). Current therapeutic drugs against HSV infection are nucleotides, nucleosides or pyrophosphate analogues, such as acyclovir, valacyclovir, penciclovir and famciclovir (Hsiang and Ho, 2008). After uptake by virus-infected cells, these drugs are activated by the viral thymidine kinase and inhibit the viral DNA polymerase. However, HSV infection remains a serious challenge because of the viral resistance and side effect (Crute et al., 2002, Field, 2001). Therefore, the development of new safe and effective anti-HSV molecules is urgently needed.
Many antimicrobial peptides (AMPs) have been shown to have inhibitory activities against HSV infection. According to the structure characteristics, these peptides are classified into five types: α-helix, β-sheet, cyclic β-sheet, β-turn and extended (Jenssen et al., 2006). Proposed anti-viral mechanism of α-helix mainly includes cellular target and viral inactivation, including Magainin, Cecropin, Mellitin, LL-37 and Brevinin-1(Aboudy et al., 1994, Albiol Matanic and Castilla, 2004, Yasin et al., 2000). Two kinds of β-sheet peptides, human and rabbit defensins, were shown to interact with HSV membrane/glycoprotein and cellular targets but not heparan sulfate (Sinha et al., 2003, Yasin et al., 2004). Another β-sheet peptide from frog, dermaseptin, was shown to have potent inhibitory effect when applied to the virus before or during virus adsorption to the target cells (Belaid et al., 2002). Two β-sheet peptides tachyplesin and protegrin were proved to have viral inactivating effect (Yasin et al., 2000). Cyclic β-sheet peptides such as θ-defensin was shown to bound to gB protein and blocked HSV-1 attachment (Yasin et al., 2004). The β-turn peptides lactoferrin (LF) and lactoferricin (Lfcin) are antimicrobial peptides that were found to block HSV entry into Vero cells. LF had no effect against HSV after the virus had entered the cells, while Lfcin exerted anti-viral activity also after the initial binding of the virus to the host cells (Andersen et al., 2004, Jenssen et al., 2004). An extended peptide from bovine, indolicidin, showed a direct inactivation effect on cell-free HSV-1 virons by targeting viral membrane/glycoprotein (Albiol Matanic and Castilla, 2004).
Natural AMPs from scorpion venoms have attracted much attention due to their anti-viral bioactivities. Some of them have been identified as anti-enveloped virus agents in our previous works. The peptide mucroporin-M1 was shown to be virucidal against the measles, SARS-CoV and influenza H5N1 viruses, and it inhibited HBV replication in vitro and in vivo (Li et al., 2011, Zhao et al., 2012). A natural a-helical peptide, Hp1090, was proven to have the property of killing HCV (Yan et al., 2011). Two histidine rich peptides were designed on the molecular template of a short virucidal peptide Ctry2459 and were confirmed to have enhanced bioavailability, which resulted in potent inhibitory effect on HCV proliferation (Hong et al., 2013). Another mutational peptide, Kn2-7, was also effective in inhibiting HIV-1 infection (Chen et al., 2012). These studies indicated that scorpion venom is a rich source of anti-viral peptides. In the present study, we screened and identified two peptides from the venom of scorpion Heterometrus petersii, which exhibited effective inhibitory effect on HSV-1 infection.
2. Materials and methods
2.1. Chemical synthesis
The Hp1035, Hp1036, Hp1165, Hp1239, Hp1412, Hp1478 peptides were from the non-amplified cDNA library of H. petersii venom gland that was constructed in our previous work (Ma et al., 2010). Peptides were chemically synthesized using the solid-phase synthesis method and amidated at the C-terminus (GL Biochem Ltd., China). The synthetic peptides were confirmed by RP-HPLC and MALDI-TOF-MS (purity > 95%).
2.2. Cell and virus
African green monkey kidney cells (Vero) were grown in minimum essential medium (MEM) supplemented with 10% (vol/vol) fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin sulfate at 37 °C in a 5% CO2 incubator. Cells infected with virus were grown in MEM supplemented with 2% serum. To prepare high-titer stocks of HSV-1 (F strain) virus, Vero cells were infected at a low multiplicity of infection (MOI) of 0.1 in a T25 flask (NEST Biotechnology Co. Ltd. China). When the cytopathic effect (CPE) was 90–100%, the infected monolayers were harvested and subjected to three freeze–thaw cycles using a dry ice–ethanol bath, centrifuged at 1,000g for 5 min to remove debris, and stored at −80 °C. Viral titers were determined by plaque forming assay on Vero cells.
2.3. MTT assay
Cells were seeded in a 96-well plate (7000–10,000 cells per well) and cultured at 37 °C for 24 h. A series of concentrations of peptides were added into the medium, and the plate was incubated for at 37 °C for 48 h, at which time 20 μl of MTT solution (5 mg/ml in PBS buffer; Invitrogen) was added to each well, and the plate was incubated at 37 °C for 4 h. The medium was removed, 100 μl DMSO was added, and then the plate was shaken for 20 min at room temperature to completely dissolve the crystal purple formazan. The absorbance was measured at 570 nm.
2.4. Hemolysis
Freshly obtained human red blood cells were washed three times with HEPES buffer (pH 7.2) by centrifugation for 10 min at 1200g. The cells were then resuspended in 0.9% saline and seeded in a 96-well plate with 107–108 cells per well. A series of concentrations of peptides were added and incubated at 37 °C for 1 h. A 0.9% saline solution was used as a negative control, and 0.1% Triton X-100 was used as a positive control. The plate was centrifuged for 5 min at 1000g, and the absorbance of hemoglobin released in the supernatant was measured at 570 nm.
2.5. Plaque forming assay
Six-well plates containing Vero cell monolayers with about 85% confluence were infected with virus. After 1 h of absorption at 37 °C, the inocula were removed. Cells were washed three times with phosphate-buffered saline (PBS) and replenished with a maintenance cover layer (MEM with 2% FBS and 0.75% carboxymethylcellulose). After an incubation period of 3 days, the cells were stained with 1% crystal violet containing 10% methanal and the plaques were counted. The viral titer was calculated according to the plaque number and dilution.
2.6. Anti-viral assay
Six-well plates containing Vero cell monolayers with about 85% confluence were infected with virus to yield about 60 plaques per well. The anti-viral effects of peptides were determined in the following assays.
2.6.1. Viral inactivation assay
Peptides at appointed concentrations were incubated with virus at indicated conditions. At the indicated time, the virus–peptide mixtures were diluted and added to Vero cell monolayers. After 1 h of absorption at 37 °C, the inocula were removed. Cells were rinsed and replenished with cover layer as described above. After 3 days, the inhibitory effects were determined by plaque reduction assay.
2.6.2. Cell inactivation assay
Vero cells were incubated with peptides for 1 h at 37 °C, at which time the peptides were removed and the cells were washed with PBS for three times. The peptide-treated cells were then infected with virus at 37 °C for 1 h. Cells were rinsed and replenished with cover layer as described above. After 3 days, the inhibitory effects were determined by plaque reduction assay.
2.6.3. Viral attachment assay
Vero cells were cooled at 4 °C for 30 min and peptides were added to Vero cells together with virus at 4 °C for 1 h. At the indicated time the cells were rinsed with PBS for three times and shifted to 37 °C for viral entry. After 1 h incubation, cells were rinsed and replenished with cover layer as described above. After 3 days, the inhibitory effects were determined by plaque reduction assay.
2.6.4. Viral entry assay
Vero cells were infected with virus at 4 °C for 1 h, at which time cells were rinsed with PBS for three times and replenished with complete MEM containing peptides. Cells were then shifted to 37 °C for viral entry. After 1 h incubation, cells were rinsed and replenished with cover layer as described above. After 3 days, the inhibitory effects were determined by plaque reduction assay.
2.6.5. Postentry assay
Vero cells were infected with virus at 37 °C for 1 h, at which time the cells were rinsed with PBS for three times and replenished with cover layer containing peptides. After 3 days, the inhibitory effects were determined by plaque reduction assay.
2.7. Circular dichroism analysis
The secondary structure of peptides was measured by circular dichroism (CD) spectroscopy. Measurements were performed in the UV range of 250–190 nm at 25 °C in water and 50% TFE using a Jasco-810 spectropolarimeter, at a concentration of 0.1 mg/ml. Spectra were collected from three separate recordings and averaged after subtracting the blank spectrum of pure water.
2.8. Transmission electron microscopy
A large quantity of HSV-1 supernatant was prepared and cleared by differential centrifugation method or passed through a 0.45-μm filter unit. The viral particles in the media were concentrated by ultracentrifugation at 35,000 rpm at 4 °C for 2 h in a Beckman SW41 rotor. The obtained HSV-1 pellets were dissolved in PBS for subsequent studies. Virus was treated with peptides and incubated at 37 °C. Samples were placed on 200-mesh form var/carbon-coated copper grids by dipping the grids into the virus sample solutions. Each sample was then negatively stained by placing a drop of 2% phosphotungstic acid (pH 7, adjusted with NaOH) on the grid. The sample grid was then dried in air for approximately 10 min. Finally, various areas of the grid were examined and photographs were taken from HITACHI H-8100 Transmission Electron Microscope operating at 100 kV.
2.9. Confocal microscopy
N-terminus FITC-labeled peptide was added to the cells at a final concentration of 10 μM, and then was incubated at 37 °C. After incubating for the indicated time, cells were washed with PBS, fixed with 4% paraformaldehyde, and washed twice. Cell nuclei were stained with DAPI (diluted 1: 500 in PBS). Cells were rinsed with PBS for three times. The cellular localization of the peptide was analyzed by confocal microscopy.
2.10. Intracellular anti-viral mechanism
Vero cells were infected with HSV-1 (moi 0.1) at 37 °C for 1 h. At which time the cells were rinsed with PBS for three times and replenished with medium containing peptide. Infected cells were harvested at 12 and 24 h postinfection and the intracellular viruses were by freeze–thaw method. The intracellular infectivity was determined by plaque forming assay.
2.11. Statistical analysis
Data are expressed as the mean ± standard deviation from at least three separate experiments.
3. Results
3.1. Screening of anti-HSV-1 agents from scorpion venom peptides
Sequence alignments of these peptides were performed using Genedoc software (Fig. 1 A) and showed the typical characters of cationic host defense peptides. The anti-HSV-1 activities of these scorpion venom peptides were determined by plaque reduction assay (PRA) (Fig. 1B). The result showed that among the six peptides, Hp1036 and Hp1239 exhibited the most effective anti-viral activities. They could significantly inhibit the plaque forming unit (PFU) of HSV-1, with an inhibition rate > 60% at a concentration of 10 μM (Fig. 1C). These results indicated that Hp1036 and Hp1239 were the most potential anti-HSV-1 agents, and then they were chosen for further studies.
Fig. 1.
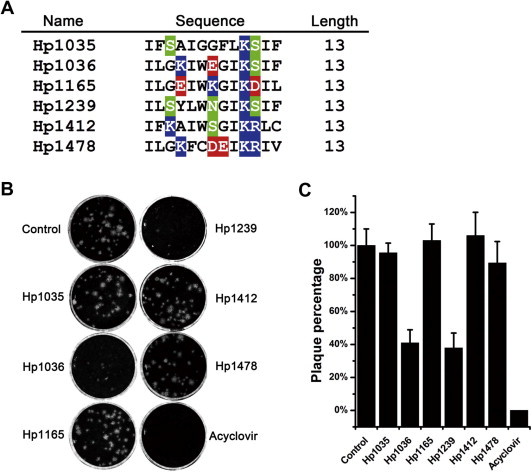
Screening of anti-HSV-1 agents from scorpion venom peptides. (A) Sequence alignments of scorpion cationic host defense peptides. The alignments were performed using Genedoc software. The residues shaded in colors were hydrophilic and the residues without a background color were hydrophobic. (B) Plaque reduction assay of the 6 peptides from the scorpion Heterometrus petersii. Peptides with a final concentration of 10 μM were used to screen for the ability to inhibit HSV-1 proliferation in Vero cells by plaque reduction assay. (C) Inhibitory rate of the 6 peptides against HSV-1 proliferation. The plaque numbers were counted and the inhibitory rates were calculated. ACV (10 μM) was used as a positive control.
3.2. Cytotoxic and hemolytic activities of Hp1036 and Hp1239
The cytotoxicities of Hp1036 and Hp1239 peptides on Vero cells were determined using MTT assay. The concentrations of Hp1036 and Hp1239 that inhibited 50% of cell growth (CC50) were 46.71 ± 3.80 and 26.15 ± 1.91 μM, respectively. At the concentration of 10 μM, the viability of the peptide-treated Vero cells was greater than 90%, indicating that 10 μM or less peptides was minimally cytotoxic to Vero cells (Fig. 2 A). Thus, this peptide concentration was chosen for further anti-viral studies. Hemolytic assay indicated that the HC50 of Hp1036 and Hp1239 peptides were 34.91 ± 0.47 and 33.32 ± 0.96 μM, respectively (Fig. 2B).
Fig. 2.
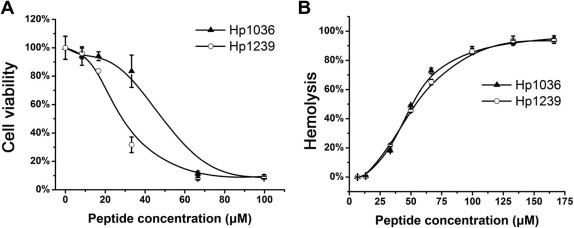
Cytotoxic and hemolytic activities of Hp1036 and Hp1239 peptides. (A) Cytotoxic activities of Hp1036 and Hp1239 peptides. Cytotoxic activities of Hp1036 and Hp1239 peptides against Vero cells were measured by MTT assay. The concentrations ranged from 0 to 100 μM. (B) Hemolytic activities of Hp1036 and Hp1239 peptides against human erythrocytes. The concentrations ranged from 0 to 160 μM.
3.3. Hp1036 and Hp1239 block multiple steps of HSV-1 proliferation
Since having shown that Hp1036 and Hp1239 inhibited the plaque forming unit of HSV-1 (Fig. 1) in vitro, we sought to determine the exact step that was blocked by the two peptides. To determine whether Hp1036 and Hp1239 could inhibit HSV-1 infection or replication in Vero cells, 10 μM of each peptide was added to the virus or cells at different times relative to incubation (Fig. 3 A). As shown in Fig. 3B and C, both Hp1036 and Hp1239 exhibit potent inhibitory activities when added to the virus before infection or added to the cells together with viral attachment and entry step, suggesting an initial inhibitory effect of viral infection. In contrast, they displayed less inhibitory activities when added to the cells 1 h after viral infection and much less inhibitory activities when added to the cells for 1 h and removed before the virus was added. These results suggest that the peptides Hp1036 and Hp1239 could potently inhibit the initial infection of HSV-1, but with less inhibitory activities of the viral replication.
Fig. 3.
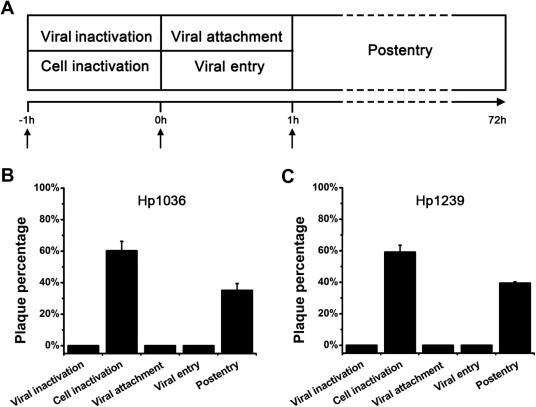
Comprehensive antiviral activities of Hp1036 and Hp1239 peptides. (A) Schematic overview of the time of addition experiments. Hp1036 and Hp1239 peptides were added at a final concentration of 10 μM under the following conditions: (i) Viral inactivation assay. Peptides were added to the virus for 1 h at 37 °C, and then the mixtures were used to infect cells for 1 h at 37 °C. The virus-peptide mixtures were removed, and the cells were rinsed and replenished with cover layer without peptide. (ii) Cell inactivation assay. Peptide was added to cells for 1 h at 37 °C and the peptide-treated cells were rinsed before virus infection; (iii) Viral attachment assay. Cells were precooled at 4 °C for 30 min and peptides were added together with virus for 1 h at 4 °C. At the indicated time the cells were rinsed and shifted to 37 °C for viral entry. (iv) Viral entry assay. Virus was attached to cells for 1 h at 4 °C and the cells were rinsed before peptides were added. The cultures were then shifted to 37 °C for viral entry. (v) Postentry assay. Cells were infected with virus for 1 h at 37 °C, at which time the virus was removed. The cells were rinsed and replenished with cover layer containing peptides throughout the experiment. (B, C) Antiviral activities of Hp1036 and Hp1239 peptides in time of addition assay. After 1 h postinfection, all cells were replenished with cover layer and cultured for 72 h, at which time, the inhibitory effects of peptides were determined by plaque reduction assay.
3.4. Viral inactivation activities of Hp1036 and Hp1239
The above results suggest that Hp1036 and Hp1239 peptides have potent inhibitory activities against the initial viral infection. We further determined the detailed viral inactivation activities of them. The inhibitory activities against HSV-1 infection were time-dependent at the concentration 10 μM (Fig. 4 A and B). When treated for 5 min with Hp1036 or Hp1239, the PFU of HSV-1 decreased to about 50%, suggesting a rapid viral inactivating effect. When treated for 1 h, the inhibitory activity against HSV-1 was the most effective. Under this condition, the inhibitory effect on HSV-1 infection was dose- and temperature-dependent (Fig. 4C and D). The most effective temperature is 37 °C and when treated at 37 °C for 1 h, the 50% effective concentrations (EC50) of Hp1036 and Hp1239 were 0.43 ± 0.09 and 0.41 ± 0.06 μM, approximate 106-fold and 63-fold lower than their CC50 against Vero cells, respectively (Table 1 ).
Fig. 4.
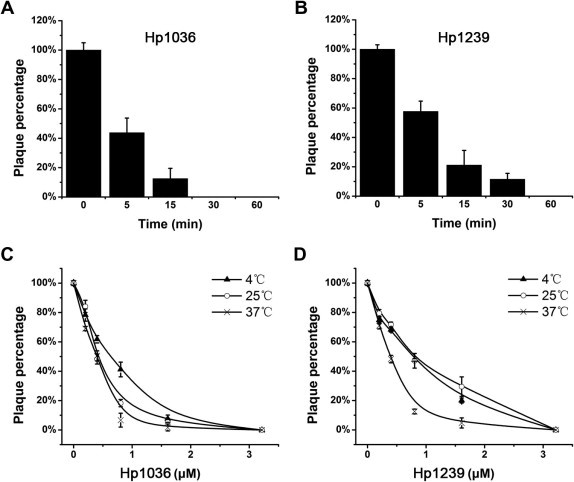
Viral inactivation activities of HSV-1 by Hp1036 and Hp1239 peptides. (A, B) Time-dependent inactivation activities of HSV-1 by Hp1036 and Hp1239 peptides. HSV-1 (60 PFU per well) was incubated with peptides at a final concentration of 10 μM for 5, 15, 30, 60 and 120 min at 37 °C. At the indicated time, the virus-peptide mixtures were diluted and added to Vero cell monolayers. After incubated at 37 °C for 1 h, the cultures were rinsed with complete MEM and replenished with covering layer. (C, D) Dose-and temperature-dependent inactivation activities of HSV-1 by Hp1036 and Hp1239 peptides. HSV-1 (60 PFU per well) was incubated with various concentrations of Hp1036 and Hp1239 at 4, 25, 37 °C for 1 h. The virus-peptide mixtures were diluted and added to Vero cell monolayers. After incubated at 37 °C for 1 h, the cultures were rinsed with complete MEM and replenished with covering layer. After 72 h postinfection, the inhibitory effects were determined by plaque reduction assay.
Table 1.
Pharmacological profiles of Hp1036 and Hp1239 peptides.
| Peptide | EC50 (μM)a in the following assay |
CC50 (μM)b | HC50 (μM)c | |||
|---|---|---|---|---|---|---|
| Viral inactivation | Viral attachment | Viral entry | Postentry | |||
| Hp1036 | 0.43 ± 0.09 | 2.87 ± 0.16 | 4.29 ± 0.35 | 7.86 ± 0.80 | 46.71 ± 3.80 | 34.91 ± 0.47 |
| Hp1239 | 0.41 ± 0.06 | 5.73 ± 0.61 | 4.32 ± 0.47 | 8.41 ± 0.73 | 26.15 ± 1.91 | 33.32 ± 0.96 |
EC50 (50% effective concentration) value of HSV-1 was determined on Vero cells.
CC50 (50% cytotoxic concentration) value of Vero cells was determined by MTT assay.
HC50 (50% hemolysis concentration) value was determined as described above.
3.5. Comprehensive anti-viral activities of Hp1036 and Hp1239
Various concentrations of peptides were added to Vero cells 1 h before HSV-1 infection and the inhibitory effects were determined by plaque assay. As shown in Fig. 5 A, Hp1036 and Hp1239 slightly inhibited HSV-1 infection when preincubated with Vero cells. In addition, Hp1036 and Hp1239 peptides inhibited HSV-1 attachment and entry in a dose-dependent manner, although weaker than their virucidal activities. In the viral attachment assay, the 50% effective (EC50) concentrations of Hp1036 and Hp1239 were 2.87 ± 0.16 and 5.73 ± 0.61 μM, respectively (Fig. 5B), approximate 16-fold and 5-fold lower than their CC50 against Vero cells. In the viral entry assay, the 50% effective (EC50) concentrations of Hp1036 and Hp1239 were 4.29 ± 0.35 and 4.32 ± 0.47 μM, respectively (Fig. 5C). Finally, when added 1 h postinfection, Hp1036 and Hp1239 inhibited HSV-1 proliferation with EC50 of 7.86 ± 0.80 and 8.41 ± 0.73 μM, respectively (Fig. 5D). The pharmacological profiles of Hp1036 and Hp1239 peptides were listed in Table 1.
Fig. 5.
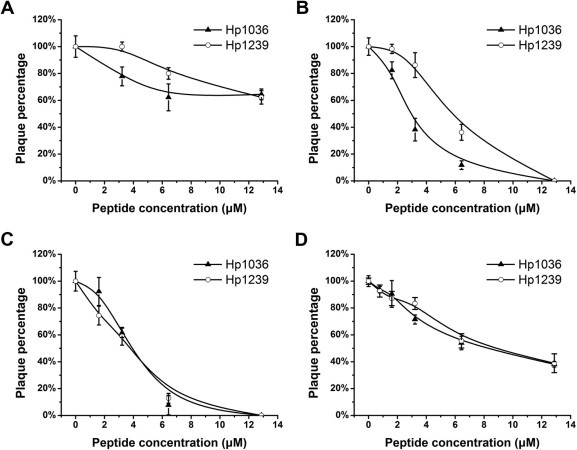
Anti-viral activities of Hp1036 and Hp1239 peptides on multiple steps of HSV-1 proliferation. (A) Cell inactivation activities of Hp1036 and Hp1239 peptides. Vero cells were incubated with various concentrations of peptide for 1 h at 37 °C, rinsed, and then infected with HSV-1. (B) Viral attachment inhibitory activities of Hp1036 and Hp1239 peptides. Vero cells were cooled at 4 °C for 30 min. The cultures were then incubated with virus in the presence of various concentrations of peptide at 4 °C for 1 h. The cells were washed and incubated at 37 °C for viral entry. (C) Viral entry inhibitory activities of Hp1036 and Hp1239 peptides. Virus was attached to cells for 1 h at 4 °C, various concentrations of peptide were added, and the cultures were shifted to 37 °C. (D) Postentry inhibitory activities of Hp1036 and Hp1239 peptides. Vero cells were infected with virus for 1 h at 37 °C, rinsed and replenished with medium containing various concentrations of peptide.
3.6. Structure analysis of Hp1036 and Hp1239
Circular dichroism (CD) analysis was used to analyze the secondary structures of both Hp1036 and Hp1239 peptides. The result showed that the synthetic peptides Hp1036 and Hp1239 both exhibited α-helix structure in TFE (Fig. 6 A and C). When plotted as a helical wheel projection (Fig. 6B and D), Hp1036 and Hp1239 were divided into two parts: one part was the hydrophobic face, and the other was the hydrophilic face. These data indicated that Hp1036 and Hp1239 are amphipathic peptide molecules.
Fig. 6.
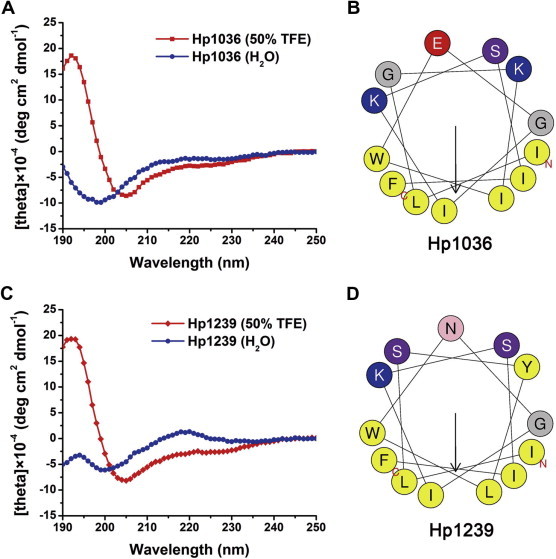
Structure analyses of Hp1036 and Hp1239 peptides. (A) CD spectra of the Hp1036 and Hp1239 peptides. Measurements were performed in the UV range of 250–190 nm at 25 °C in water on a Jasco-810 spectropolarimeter. (B) Helical wheels of Hp1036 and Hp1239 peptides. Helical wheels of Hp1036 and Hp1239 showed the hydrophilic and hydrophobic faces.
3.7. Morphological changes of HSV-1 by Hp1036 and Hp1239
To confirm whether the viral structural integrity was destabilized by the treatment of Hp1036 or Hp1239, we performed transmission electron microscopy (TEM) experiments to monitor the morphological changes of HSV-1. As shown in Fig. 7 , the morphology of untreated HSV-1 virions were mostly integrated, which contains compact envelop (∼200 nm). Treatment by either Hp1036 or Hp1239 significantly changed the structure of HSV-1, including rupture of viral envelop and dissociation of proteins from the virons, generally displayed incompact morphology. These results strongly supported the underlying mechanism of action of Hp1036 and Hp1239 that destabilized the integrity of HSV-1 and inhibited its infectivity.
Fig. 7.
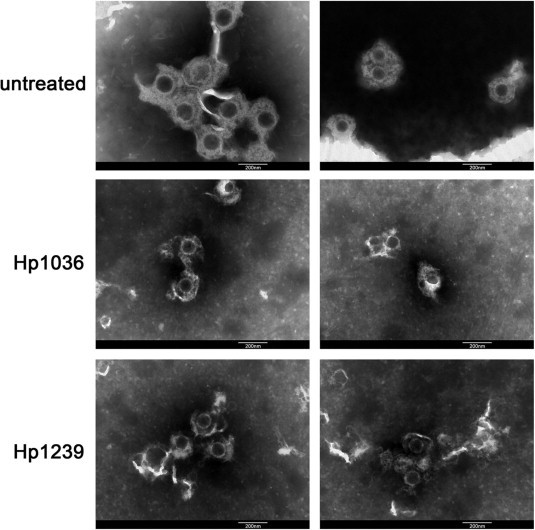
TEM images of HSV-1 before and after treated by Hp1036 peptide or Hp1239 peptide. HSV-1 was treated with Hp1036 or Hp1239 (final concentration, 0.3 mM) at 37 °C for 5 min (left) or 15 min (right) before negatively stained with 2% phosphotungstic acid. Pictures shown are taken from different areas of the sample grid. Scale bar, 200 nm.
3.8. Intracellular anti-viral mechanism of Hp1036 and Hp1239 against HSV-1
To explore the intracellular anti-viral mechanism of Hp1036 and Hp1239 against HSV-1, confocal microscopy was used to investigate whether Hp1036 and Hp1239 peptides could penetrate the cell membrane and enter the Vero cells. As shown in Fig. 8 A and C, FITC-labeled Hp1036 and Hp1239 peptides slightly entered the Vero cells and accumulated in granular structures in the cytoplasm after incubated for 1 h. However, they exhibited a higher amount of cellular uptake after incubated for 24 h, with a dispersed distribution in the cytoplasm. Moreover, when infected cells were incubated with Hp1036 and Hp1239 for 12 and 24 h, the infectivity of intracellular virus significantly decreased (Fig. 8B and D). These results suggest that Hp1036 and Hp1239 peptides enter the Vero cells and inactivated intracellular viral particles.
Fig. 8.
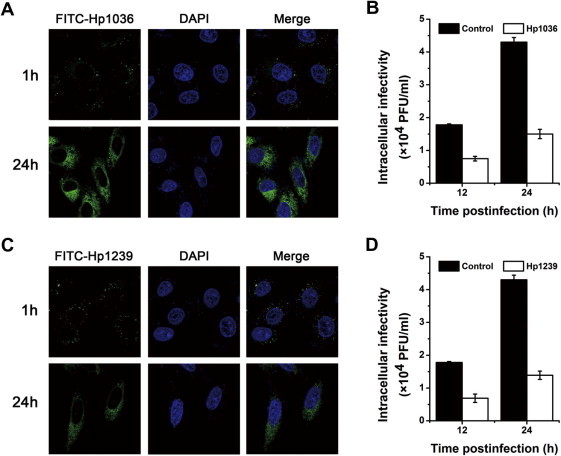
Intracellular anti-viral activities and mechanisms of Hp1036 and Hp1239 peptides against HSV-1. (A) Cellular localization of Hp1036 and Hp1239 peptides in Vero cells. The cellular localization of Hp1036 and Hp1239 peptides were determined by confocal microscopy after incubation for 1 and 24 h. (B) Intracellular viral inactivation effects of Hp1036 and Hp1239 peptides. Vero cells were infected with HSV-1 (moi 0.1) and Hp1036 and Hp1239 (10 μM) were added at indicated time postinfection. Infected cells were treated for 12 h and 24 h, the intracellular viruses were harvested by freeze–thaw method. The intracellular infectivity was determined by plaque forming assay.
4. Discussion
Currently, various virucidal agents have been identified and they provided a rich source of developing effective anti-viral drugs. But the challenge is how to solve the lack of inhibitory effect on viral postentry step. Inhibition of cell-to-cell spread is important for topical microbicide development because a source of sexual acquisition also may be cell-associated virus (Sinha et al., 2003). The derived virucidal peptide, C5A, disturbs the integrity of the viral membrane and exhibits potent virucidal activities against HCV, HIV-1 and HSV-1. It is worth mentioning that C5A has a property of suppressing viral entry and established infection, which differs from common virucidal agents (Bobardt et al., 2008, Cheng et al., 2008, de Witte et al., 2011).
In this study, we screened and identified two new anti-viral peptides from the peptide library of scorpion venom. The peptides, Hp1036 and Hp1239, with potent inhibitory effect on the proliferation of HSV-1 (Fig. 1), were selected for further analysis. We proved that both Hp1036 and Hp1239 inhibited multiple steps of HSV-1 life cycle (Fig. 3). At noncytotoxic concentration, Hp1036 and Hp1239 peptides effectively inactivated HSV-1 virons in time-, dose- and temperature-dependent manners in vitro (Fig. 4). Hp1036 and Hp1239 also blocked HSV-1 attachment and entry to Vero cells. It was worthy to be attention that Hp1036 and Hp1239 could prevent viral proliferation in the postentry assay (Fig. 5).
In the comprehensive anti-viral assay, we found that Hp1036 and Hp1239 induced poor cellular resistance to infection, suggesting that Hp1036 and Hp1239 might not effectively target to cellular components. However, Hp1036 and Hp1239 displayed effective inhibitory activity in the viral attachment and entry stages. According to the time-dependent virucidal experiment in Fig. 4A and B, the peptides Hp1036 and Hp1239 inactivated more than 40% virus in 5 min and 80% in 15 min, indicating a rapid action mode of Hp1036 and Hp1239. For this property, we can speculate that Hp1036 and Hp1239 may inactivate HSV-1 when they attach to the cell surface and also effectively inactivate the virus that has already bound to the cells but not entry yet.
In our previous studies, a serious of anti-viral peptides from scorpion venom have been identified, such as Ctry2459 (Hong et al., 2013), Hp1090 (Yan et al., 2011) and Kn2-7 [21]. These peptides shared the same α-helix structure and similar virucidal activities against enveloped virus. However, they lacked the inhibitory activities when added to the cells after viral infection. This disadvantage badly reduced the medical value of this type of anti-viral peptide. In this study, we found that both Hp1036 and Hp1239 peptides exhibited potent inhibitory effects against HSV-1 proliferation in the postentry assay. Obviously, they are different from the above virucidal peptides, but are similar to the mutant virucidal peptide Ctry2459-H3 (Hong et al., 2013).
The virucidal activities of Hp1036 and Hp1239 mostly depend on the sequence and structure characters. Recent studies had shown that sialic acid on HSV-1 envelope glycoprotein was required for efficient infection and a β-peptide possibly binded to sialic acid, which resulted in inhibitory effect on the infection of HSV-1.(Akkarawongsa et al., 2008, Teuton and Brandt, 2007) In this study, CD analysis displayed that both Hp1036 and Hp1239 peptides adopted amphipathic α-helix structure in approximate membrane environment, with positive charged amino acids exposed outside the helix wheel (Fig. 6). Therefore, we can speculate that the peptides Hp1036 and Hp1239 interact directly with viral membranes via binding to the negatively charged sialic acid units on the virus and inactivate the viral particles. Nevertheless, future studies will be needed to identify the target on the virus for Hp1036 and Hp1239.
Transmission electron microscopy has been used to monitor the morphological changes of adenovirus type 5, showing that the conformational changes of the viral proteins result in the destruction of the viral morphology (Yoon et al., 2011). In the present study, we also observed the significant structure change of HSV-1, especially the rupture of viral envelop and the incompact morphology of virons (Fig. 7). This is the first time to show the morphology change of enveloped virus by anti-viral peptides, providing a direction of revealing the mechanism of virucidal drugs.
To find the intracellular mechanism of the inhibitory effect on viral proliferation, we firstly determined the cellular localization of Hp1036 and Hp1239. As shown in Fig. 8A and C, FITC-labeled Hp1036 and Hp1239 peptides slightly entered Vero cells at 1 h postincubation, but displayed a big amount of uptake and equally dispersed distribution in the cytoplasm after 24 h. Moreover, Hp1036 and Hp1239 had inhibitory activities against intracellular viral infectivity (Fig. 8B and D). The intracellular mature HSV-1 virions rapidly accumulated to high levels at 12 and 24 h postinfection, at which time the intracellular virucidal activities of Hp1036 and Hp1239 were significantly reflected. Thus, we can conclude that Hp1036 Hp1239 enter the Vero cells, target to the mature viral particles and inactivated them. Previously, we designed two histidine rich peptides with enhanced cellular uptake and advanced intracellular distribution, which resulted in potent anti-viral activities against HCV (Hong et al., 2013). While in the present study, we found two natural peptides with the same properties and anti-viral functions, indicating the diversity of biomolecules from scorpion venom.
Many anti-viral drugs readily lead to the generation of drug-resistant virus variants. For more than two decades, anti-viral drugs targeting to the viral DNA polymerase such as acyclovir (ACV) have been widely used for the treatment of HSV infection. Unfortunately, the extensive clinical use of these drugs has led to the emergence of drug-resistant viral strains.(Greco et al., 2007, Krawczyk et al., 2013) Using the virucidal peptides such as Hp1036 and Hp1239 for the treatment of HSV infection, the generation of drug-resistant virus variants is likely to be reduced. Amphipathic α-helix peptides, such as Hp1036 and Hp1239, have potent virucidal activity and inhibitory effect on viral proliferation, exhibiting potential medical value for the treatment of viral infection.
Furthermore, because of the advantage of therapeutic alliance, anti-viral strategy became diversification and already achieved remarkable successes. In this way, the virucidal molecules may act as a potential member to participate the therapeutic alliance, with its unique anti-viral pattern. In brief, this kind of membrane-targeting peptides could be combined with drugs that target intracellular components of the viral life cycle. Thus, a virucidal peptide used in combination with protease and/or polymerase inhibitors targeting multiple steps of viral infection cycle would have advantages over single drug therapy in reducing viral titers as well as in suppressing the emergence of viral resistance (Liu et al., 2010).
In summary, the present study revealed for the first time that scorpion venom cationic peptides are indeed effective against HSV-1 infection in vitro. The screened peptides Hp1036 and Hp1239 not only exhibited extracellular virucidal activities against HSV-1, but also had potent inhibitory effect when added at the viral attachment and entry steps. The virucidal activities depended on the morphological changes of HSV-1 virons by the treatment of Hp1036 and Hp1239. Their properties of amphipathic α-helix conformation under appropriate membrane environment possibly contributed to their membrane penetrating and virucidal activities, resulting in the intracellular anti-viral effect. Hp1036 and Hp1239 easily entered Vero cells. Naturally, both peptides also inactivated intracellular viral particles and inhibited viral proliferation at the postinfection step. Our work provided a new direction of developing potential biofunctional molecules from animal venoms and opened new avenues for discovering anti-viral agents or sources.
Acknowledgments
This work was supported by grants from the National Key Basic Research Program in China (Nos. 2010CB529800 and 2010CB530100), the China Specific Project for Developing New Drugs (Nos. 2011ZX09401-302 and 2011ZX09102-001-32), and the Fundamental Research Funds for the Central Universities in China.
References
- Aboudy Y., Mendelson E., Shalit I., Bessalle R., Fridkin M. Activity of two synthetic amphiphilic peptides and magainin-2 against herpes simplex virus types 1 and 2. Int. J. Pept. Protein Res. 1994;43:573–582. doi: 10.1111/j.1399-3011.1994.tb00559.x. [DOI] [PubMed] [Google Scholar]
- Akkarawongsa R., Potocky T.B., English E.P., Gellman S.H., Brandt C.R. Inhibition of herpes simplex virus type 1 infection by cationic beta-peptides. Antimicrob. Agents. Chemother. 2008;52:2120–2129. doi: 10.1128/AAC.01424-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albiol Matanic V.C., Castilla V. Antiviral activity of antimicrobial cationic peptides against Junin virus and herpes simplex virus. Int. J. Antimicrob. Agents. 2004;23:382–389. doi: 10.1016/j.ijantimicag.2003.07.022. [DOI] [PubMed] [Google Scholar]
- Andersen J.H., Jenssen H., Sandvik K., Gutteberg T.J. Anti-HSV activity of lactoferrin and lactoferricin is dependent on the presence of heparan sulphate at the cell surface. J. Med. Virol. 2004;74:262–271. doi: 10.1002/jmv.20171. [DOI] [PubMed] [Google Scholar]
- Belaid A., Aouni M., Khelifa R., Trabelsi A., Jemmali M., Hani K. In vitro antiviral activity of dermaseptins against herpes simplex virus type 1. J. Med. Virol. 2002;66:229–234. doi: 10.1002/jmv.2134. [DOI] [PubMed] [Google Scholar]
- Bobardt M.D., Cheng G., de Witte L., Selvarajah S., Chatterji U., Sanders-Beer B.E., Geijtenbeek T.B., Chisari F.V., Gallay P.A. Hepatitis C virus NS5A anchor peptide disrupts human immunodeficiency virus. Proc. Natl. Acad. Sci. U.S.A. 2008;105:5525–5530. doi: 10.1073/pnas.0801388105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Cao L., Zhong M., Zhang Y., Han C., Li Q., Yang J., Zhou D., Shi W., He B., Liu F., Yu J., Sun Y., Cao Y., Li Y., Li W., Guo D., Cao Z., Yan H. Anti-HIV-1 activity of a new scorpion venom peptide derivative Kn2-7. PLoS One. 2012;7:e34947. doi: 10.1371/journal.pone.0034947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G., Montero A., Gastaminza P., Whitten-Bauer C., Wieland S.F., Isogawa M., Fredericksen B., Selvarajah S., Gallay P.A., Ghadiri M.R., Chisari F.V. A virocidal amphipathic {alpha}-helical peptide that inhibits hepatitis C virus infection in vitro. Proc. Natl. Acad. Sci. U.S.A. 2008;105:3088–3093. doi: 10.1073/pnas.0712380105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crute J.J., Grygon C.A., Hargrave K.D., Simoneau B., Faucher A.M., Bolger G., Kibler P., Liuzzi M., Cordingley M.G. Herpes simplex virus helicase-primase inhibitors are active in animal models of human disease. Nat. Med. 2002;8:386–391. doi: 10.1038/nm0402-386. [DOI] [PubMed] [Google Scholar]
- Lot de Witte L., Bobardt M.D., Chatterji U., van Loenen F.B., Verjans G.M., Geijtenbeek T.B., Gallay P.A. HSV neutralization by the microbicidal candidate C5A. PLoS One. 2011;6:e18917. doi: 10.1371/journal.pone.0018917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H.J. Herpes simplex virus antiviral drug resistance–current trends and future prospects. J. Clin. Virol. 2001;21:261–269. doi: 10.1016/s1386-6532(00)00169-4. [DOI] [PubMed] [Google Scholar]
- Gold D., Corey L. Acyclovir prophylaxis for herpes simplex virus infection. Antimicrob. Agents Chemother. 1987;31:361–367. doi: 10.1128/aac.31.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath S.C., Hayashi K., Kumar P.K. Aptamer that binds to the gD protein of herpes simplex virus 1 and efficiently inhibits viral entry. J. Virol. 2012;86:6732–6744. doi: 10.1128/JVI.00377-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco A., Diaz J.J., Thouvenot D., Morfin F. Novel targets for the development of anti-herpes compounds. Infect. Disord.: Drug Targets. 2007;7:11–18. doi: 10.2174/187152607780090766. [DOI] [PubMed] [Google Scholar]
- Hill J.M., Gebhardt B.M., Wen R., Bouterie A.M., Thompson H.W., O’Callaghan R.J., Halford W.P., Kaufman H.E. Quantitation of herpes simplex virus type 1 DNA and latency-associated transcripts in rabbit trigeminal ganglia demonstrates a stable reservoir of viral nucleic acids during latency. J. Virol. 1996;70:3137–3141. doi: 10.1128/jvi.70.5.3137-3141.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W., Zhang R., Di Z., He Y., Zhao Z., Hu J., Wu Y., Li W., Cao Z. Design of histidine-rich peptides with enhanced bioavailability and inhibitory activity against hepatitis C virus. Biomaterials. 2013;34:3511–3522. doi: 10.1016/j.biomaterials.2013.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiang C.Y., Ho T.Y. Emodin is a novel alkaline nuclease inhibitor that suppresses herpes simplex virus type 1 yields in cell cultures. Br. J. Pharmacol. 2008;155:227–235. doi: 10.1038/bjp.2008.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenssen H., Andersen J.H., Uhlin-Hansen L., Gutteberg T.J., Rekdal O. Anti-HSV activity of lactoferricin analogues is only partly related to their affinity for heparan sulfate. Antiviral Res. 2004;61:101–109. doi: 10.1016/j.antiviral.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Jenssen H., Hamill P., Hancock R.E. Peptide antimicrobial agents. Clin. Microbiol. Rev. 2006;19:491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk A., Arndt M.A., Grosse-Hovest L., Weichert W., Giebel B., Dittmer U., Hengel H., Jager D., Schneweis K.E., Eis-Hubinger A.M., Roggendorf M., Krauss J. Overcoming drug-resistant herpes simplex virus (HSV) infection by a humanized antibody. Proc. Natl. Acad. Sci. U.S.A. 2013;110:6760–6765. doi: 10.1073/pnas.1220019110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Zhao Z., Zhou D., Chen Y., Hong W., Cao L., Yang J., Zhang Y., Shi W., Cao Z., Wu Y., Yan H., Li W. Virucidal activity of a scorpion venom peptide variant mucroporin-M1 against measles, SARS-CoV and influenza H5N1 viruses. Peptides. 2011;32:1518–1525. doi: 10.1016/j.peptides.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R., Tewari M., Kong R., Zhang R., Ingravallo P., Ralston R. A peptide derived from hepatitis C virus E2 envelope protein inhibits a post-binding step in HCV entry. Antiviral Res. 2010;86:172–179. doi: 10.1016/j.antiviral.2010.02.316. [DOI] [PubMed] [Google Scholar]
- Ma Y., Zhao Y., Zhao R., Zhang W., He Y., Wu Y., Cao Z., Guo L., Li W. Molecular diversity of toxic components from the scorpion Heterometrus petersii venom revealed by proteomic and transcriptome analysis. Proteomics. 2010;10:2471–2485. doi: 10.1002/pmic.200900763. [DOI] [PubMed] [Google Scholar]
- Palem J.R., Bedadala G.R., El Sayed K.A., Hsia S.C. Manzamine A as a novel inhibitor of herpes simplex virus type-1 replication in cultured corneal cells. Planta Med. 2011;77:46–51. doi: 10.1055/s-0030-1250093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S., Cheshenko N., Lehrer R.I., Herold B.C. NP-1, a rabbit alpha-defensin, prevents the entry and intercellular spread of herpes simplex virus type 2. Antimicrob. Agents Chemother. 2003;47:494–500. doi: 10.1128/AAC.47.2.494-500.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuton J.R., Brandt C.R. Sialic acid on herpes simplex virus type 1 envelope glycoproteins is required for efficient infection of cells. J. Virol. 2007;81:3731–3739. doi: 10.1128/JVI.02250-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R., Zhao Z., He Y., Wu L., Cai D., Hong W., Wu Y., Cao Z., Zheng C., Li W. A new natural alpha-helical peptide from the venom of the scorpion Heterometrus petersii kills HCV. Peptides. 2011;32:11–19. doi: 10.1016/j.peptides.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Yasin B., Pang M., Turner J.S., Cho Y., Dinh N.N., Waring A.J., Lehrer R.I., Wagar E.A. Evaluation of the inactivation of infectious Herpes simplex virus by host-defense peptides. Eur. J. Clin. Microbiol. Infect. Dis. 2000;19:187–194. doi: 10.1007/s100960050457. [DOI] [PubMed] [Google Scholar]
- Yasin B., Wang W., Pang M., Cheshenko N., Hong T., Waring A.J., Herold B.C., Wagar E.A., Lehrer R.I. Theta defensins protect cells from infection by herpes simplex virus by inhibiting viral adhesion and entry. J. Virol. 2004;78:5147–5156. doi: 10.1128/JVI.78.10.5147-5156.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J., Jekle A., Najafi R., Ruado F., Zuck M., Khosrovi B., Memarzadeh B., Debabov D., Wang L., Anderson M. Virucidal mechanism of action of NVC-422, a novel antimicrobial drug for the treatment of adenoviral conjunctivitis. Antiviral Res. 2011;92:470–478. doi: 10.1016/j.antiviral.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Zhao Z., Hong W., Zeng Z., Wu Y., Hu K., Tian X., Li W., Cao Z. Mucroporin-M1 inhibits hepatitis B virus replication by activating the mitogen-activated protein kinase (MAPK) pathway and down-regulating HNF4alpha in vitro and in vivo. J. Biol. Chem. 2012;287:30181–30190. doi: 10.1074/jbc.M112.370312. [DOI] [PMC free article] [PubMed] [Google Scholar]


