Abstract
This article summarizes progress in research on Middle East Respiratory Syndrome (MERS) since a FAO-OIE-WHO Global Technical Meeting held at WHO Headquarters in Geneva on 25–27 September 2017. The meeting reviewed the latest scientific findings and identified and prioritized the global activities necessary to prevent, manage and control the disease. Critical needs for research and technical guidance identified during the meeting have been used to update the WHO R&D MERS-CoV Roadmap for diagnostics, therapeutics and vaccines and a broader public health research agenda. Since the 2017 meeting, progress has been made on several key actions in animal populations, at the animal/human interface and in human populations. This report also summarizes the latest scientific studies on MERS since 2017, including data from more than 50 research studies examining the presence of MERS-CoV infection in dromedary camels.
Keywords: MERS-CoV, Research, Animal-human interface, Dromedary camels, Zoonosis, Vaccine
1. Background: Middle East Respiratory Syndrome
Since its identification in the Kingdom of Saudi Arabia (KSA) (Zaki et al., 2012) and Jordan (Hijawi et al., 2013) in 2012, Middle East Respiratory Syndrome (MERS) has become a global public health threat. Typical of an emerging zoonosis, Middle East respiratory syndrome coronavirus (MERS-CoV) has an animal reservoir, i.e. dromedary camels in which the virus causes little to no disease (Mohd et al., 2016). Many details about the extent of circulation and the mechanisms of transmission within dromedary camel herds, or factors related to zoonotic transmission and differences in circulating MERS-CoV strains, remain unknown. The virus has repeatedly spilled over from dromedary camels to humans, principally in countries on the Arabian Peninsula, causing significant morbidity and mortality (World Health Organization, 2017a; Azhar et al., 2014). Clusters of cases in the community and among family members are rare (World Health Organization, 2017a; Drosten et al., 2014). However, delays in diagnosis in hospitals has sometimes led to secondary cases among health care workers, patients sharing rooms or family members as a result of unprotected direct contact with a patient before isolation. This human-to-human transmission in health care facilities can sometimes be amplified, causing very large outbreaks, as has been seen in the Middle East and in the Republic of Korea, with significant public health and economic impacts (Hijawi et al., 2013; Assiri et al., 2013; Al-Abdallat et al., 2014; Drosten et al., 2015; Al Hosani et al., 2016; Ki, 2015; Park et al., 2015). As of August 2018, more than 2249 human cases from 27 countries have been reported to the World Health Organization (WHO) (World Health Organization, 2017a).
The FAO, OIE and WHO Tripartite have regularly brought together affected member states, public health and animal officials, and academics to discuss what is known and unknown about the zoonotic origin of MERS-CoV (World Health Organization, 2016; FAO, 2016, 2014; WHO Regional office for the Eastern Mediterranean, 2013a). The purposes of these meetings and workshops have been to advocate for more surveillance and research on MERS-CoV in animals and humans, to share information about how MERS-CoV is transmitted between animals, from animals to humans and between humans, to describe the diseases it causes, and to develop policies and guidelines for detection, reporting of animal and human infections, and prevention of human cases and clusters.
In the two years since the last international technical consultation on MERS-CoV in 2016 13, there have been notable improvements in surveillance and reporting of human cases, multidisciplinary research, cross-sectoral collaboration at country level, public awareness about the disease, and laboratory and surveillance capacity in affected countries. In addition, a number of countries in the Arabian Peninsula and in Africa have engaged in research activities and surveillance of camel populations to shed light on the wider distribution of this virus or investigate transmission patterns and routes for viral shedding. As a follow-up to previous meetings (World Health Organization, 2016; FAO, 2016, 2014; WHO Regional office for the Eastern Mediterranean, 2014; WHO Regional office for the Eastern Mediterranean, 2013b, 2013c), FAO, OIE and WHO Tripartite held a Global Technical Meeting on MERS-CoV with representatives from Ministries of Health and Ministries of Agriculture, subject matter experts, researchers, funders and industrial partners from 25 to 27 September 2017 in Geneva, Switzerland (see Supplementary Information) (World Health Organization et al., 2017). The objectives were to review the latest scientific evidence on MERS-CoV, further enhance cross-sectoral collaboration and communication during preparedness and response activities, and identify research priorities given the advancements in our knowledge.
With 130 participants, this was the largest MERS-CoV Technical Meeting to date and the first meeting attended by representatives from both affected and at risk countries. That is, countries which have reported human infection, countries with evidence of MERS-CoV in dromedary camels but no reported human cases, and countries at risk for importation (countries without infected camels that have close ties to affected countries through expatriate workers, travel to affected countries for medical procedures and/or frequent international travel).
2. Findings from the global technical meeting
There is strong consensus among all stakeholders that dromedary camels are the main source of transmission to humans. In 2014, OIE identified MERS-CoV as an emerging disease with zoonotic potential in camels and thereby creating expectations of reporting positive camels by countries (OIE, 2014a) and recently published a MERS-CoV case definition (OIE, 2017) for the reporting of confirmed and suspected infection in camels.
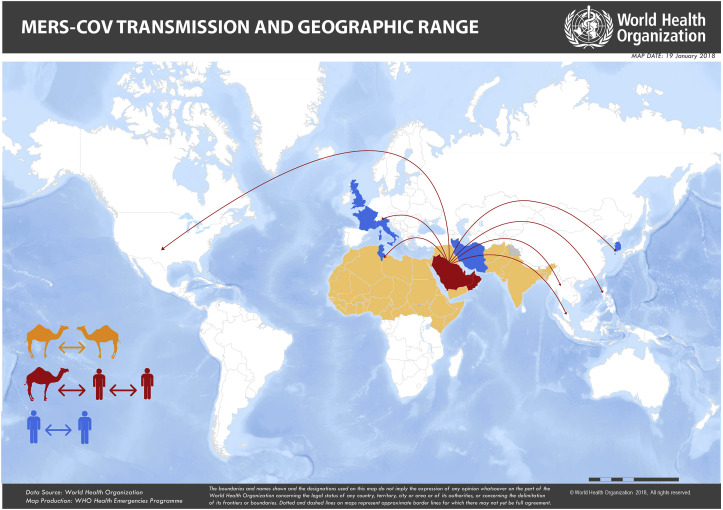
Not all countries face the same risks. For example, countries that have the infected reservoir (dromedary camels) differ from those countries in which dromedary camels show no evidence of current or past infection (Fig. 1 ). There may also be differences in spillover potential in countries with documented zoonotic transmission, compared to those without, due to several factors including potential differences in husbandry practices, cultural, social, medicinal, occupational exposures, prevalence of underlying chronic medical conditions, or genetic factors in human populations, and MERS-CoV viral differences (Wong et al., 2015). As such, technical and risk mitigation guidance to protect human health and research priorities differ by region.
Fig. 1.
MERS-CoV transmission and geographic range. Countries highlighted in red and orange indicate the geographic range of MERS-CoV in dromedary camels. Those in red have had documented spillover (camel-to-human) transmission with subsequent human-to-human transmission. Countries in blue are those with reported human-to-human transmission. (Source: WHO). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The findings from the Global Technical Meeting are summarized below:
-
i.
Surveillance needs: Surveillance in animals and humans to limit zoonotic transmission
Routine human surveillance for MERS-CoV in KSA (Abdulaziz et al., 2017) and throughout the Middle East has improved since the identification of the virus in humans in 2012, but there is significant variation in the quality and extent of surveillance between countries. In other parts of the world, surveillance is limited. Since it is known that MERS-CoV is enzootic in areas of Africa and Asia where dromedary camels are found, heighted awareness and surveillance for zoonotic MERS is required. This is currently lacking and remains a knowledge gap.
One exception is the notable effort to identify potential MERS-CoV infection among pilgrims travelling back from the Middle East. Since 2012, event-based surveillance among pilgrims returning from Hajj, Umrah and other religious events in KSA has been conducted by KSA and countries sending pilgrims. While many return reporting respiratory symptoms, no MERS-CoV infections have been identified among returning pilgrims (Muraduzzaman et al., 2018; Barasheed et al., 2014; Atabani et al., 2016; Koul et al., 2017; Annan et al., 2015; Ma et al., 2017; Memish et al., 2014a, 2014b; Refaey et al., 2017; Al-Abdallat et al., 2017; Matthew et al., 2015; Alqahtani et al., 2016; Win et al., 2016; Yavarian et al., 2018; Kapoor et al., 2014).
Among animals, field surveys conducted to date have included several domestic and wildlife species including dromedary camels (Camelus dromedarius) and Bactrian camels (Camelus bactrianus), goats, bats, cattle, sheep, chickens, swine, ducks, buffalo and equids. Field studies in dromedary camels have been conducted in a number of countries (Table 1 ). To date, MERS-CoV RNA or MERS-CoV-specific antibodies have been identified in dromedary camels a number of countries (Table 1) except Australia (Hemida et al., 2014), Kazakhstan (Miguel et al., 2016), and the Netherlands (Reusken et al., 2013a). Other livestock such as alpacas (Vicugna pacos), llamas (Llama pacos), young goats, rabbits and pigs have been shown to be susceptible to experimental infection (Crameri et al., 2016; Adney et al., 2016; Vergara-Alert et al., 2017).
Table 1.
Field studies of evidence of MERS-CoV infection in dromedary camels. ppNT: pseudoparticle neutralization; MN: microneutralization; CI: confidence interval; VNT: virus neutralizing antibodies test.
| Country | Number of camels | Evidence of MERS-CoV infection |
|---|---|---|
| Australia | 25 | No evidence for MERS-CoV infection in dromedary camels (Hemida et al., 2014) |
| Bangladesh | 55 | 17 (31%) samples were seropositive (Ariful et al., 2018) |
| Burkina Faso | 525 | Seropositivity rates ranged from 73.2% (95% CI): 48.6–88.8) to 84.6% (95% CI: 77.2–89.9) and virus detection from 0% (95% CI: 0–0) to 12.2% (95%CI: 7–20.4) (Miguel et al., 2017) |
| Egypt | 2825 | Of 2825 nasal swabs, RNA detection rate was 15% by RT-PCR. Of 2541 sera samples, the overall seroprevalence was 71%. (Ali et al., 2017) |
| 1078 | Of 1031 serological tests, 871 (84.5%) had MERS-CoV neutralizing antibodies. Of 1078 nasal samples, 41 (3.8%) were positive for MERS-CoV using MERS-CoV PCR (Ali et al., 2015) | |
| 110 | 4 (3.6%) nasal swab specimens tested positive for presence of MERS-CoV RNA. Antibodies against MERS-CoV were detected in 48 (92.3%) of 52 serum samples (ref 48) (Chu et al., 2014) | |
| 110 | 103 (93.6%) sera collected neutralized MERS-CoV using ppNT (Perera et al., 2013b) | |
| 43 | 34 (79.1%) dromedary camels were positive for MERS-CoV antibodies using MN (Muller MA et al., 2014) | |
| Ethiopia | 632 | Seropositivity rates ranged from 85.1% (95% CI: 71.8–92.7) to 99.4% (95% CI: 95.4–99.9) and the viral RNA detection rates from 0% (95% CI: 0–0) to 15.7% (95% CI: 8.2–28.0)95 |
| 188 | Seropositivity was 93% in adult dromedary camels and 97% for juvenile dromedary camels (Chantal et al., 2014) | |
| Iran | 186 | 8 (4.3%) samples positive using RT-PCR (OIE, 2014b) |
| Israel | 411 | 254 (61.8%) were positive for MERS-CoV antibodies using VNT; All nasal samples were negative for the presence of MERS-CoV RNA (David et al., 2018) |
| 71 | 51 (71.8%) sera samples had MERS-CoV neutralizing antibodies (Harcourt et al., 2018) | |
| Jordan | 11 | Neutralizing antibodies to MERS-CoV were found in all sera from dromedary camels (Reusken et al., 2013c) |
| 45 | 42 nasal swabs tested positive for the presence of MERS-CoV nucleic acid (van Doremalen et al., 2017) | |
| Kazakhstan | 455 | No evidence for MERS-CoV infection in dromedary camels (Miguel et al., 2016) |
| Kenya | 774 | 228 (29.5%) were positive using rELISA test (Corman et al., 2014) |
| 335 | Seroprevalence of MERS-CoV antibodies in the sampled population was 46.9% (95% CI 41.4–52.5) (Deem et al., 2015) | |
| Kingdom of Saudi Arabia (Azhar et al., 2014; Hemida et al., 2013, 2014, 2017; Alagaili et al., 2014; Briese et al., 2014; Maged et al., 2014, 2015; Kasem et al., 2017; Ziad et al., 2014) | 203 | 150 (74%) sampled were found to have antibodies to MERS-CoV by ELISA (Alagaili et al., 2014) |
| 1309 | 158 (12.1%) nasal swabs were positive for MERS-CoV (Sabir et al., 2016) | |
| 698 | The overall prevalence of MERS infection in camels in animal markets and slaughterhouses by rtRT-PCR was 56.4% | |
| 99 | High levels of seropositivity in two herds demonstrated – in on herd, all samples had MERS-CoV antibodies (Hemida et al., 2017) | |
| 310 | 280 (90.3%) samples were positive using ppNT (Hemida et al., 2013) | |
| 171 | 144 (84.2%) sera samples had specific antibodies against MERS-CoV (Harrath and Abu Duhier, 2018) | |
| 9 | 2 (22.2%) nasal samples were positive using RT-PCR (Ziad et al., 2014) | |
| 9 | 1 (11.1%) nasal samples were negative for MERS-CoV RNA. All serum samples had high titers of MER-CoV antibodies (Azhar et al., 2014) | |
| 131 archived sera | 118 (90.1%) had detectable ppNT antibody titres to MERS-CoV (Hemida et al., 2014) | |
| Kuwait | 63 | 5 (7.9%) nasal samples were positive using RT-PCR (WAHIS Interface, 2014) |
| Mali | 570 | 502 (88.1%) were positive for antibodies against MERS-CoV (Falzarano et al., 2017) |
| Morocco | 343 | Seropositivity rates ranged from 48.3% (95% CI: 18.3–79.5) to 100% (95% CI:100-100) and viral RNA detection rates from 0% (95% CI: 0–0) to 7.6% (95% CI: 1.9–26.1)95 |
| Nigeria | 358 | Seropositivity was 94% in adult dromedary camels and 93% for juvenile dromedary camels (Chantal et al., 2014; Chu et al., 2015) |
| 132 | 14 (11%) nasal swabs were positive using RT-qPCR (Chantal et al., 2014; Chu et al., 2015) | |
| 2529 | MERS-CoV RNA was detected in 4/38 (10.5%) of camels aged < 2 years, in 31/1400 (2.2%) aged 2–4 years and in 20/1091 (1.8%) aged > 4 years (So et al., 2018) | |
| Oman | 76 | 5 (6.6%) proved positive in all applied RT-qPCR and RT-PCR assays. (Nowotny and Kolodziejek, 2014) |
| 50 | 50 (100%) had protein-specific antibodies against MERS-CoV (Reusken et al., 2013d) | |
| Pakistan | 565 | 315 (55.8%) samples exceeded the ELISA signal cutoff. Of these, 223 (39.5%) were confirmed using MN (Saqib et al., 2017) |
| Qatar | 14 | 3 (21.4%) nasal samples were positive using RT-PCR (Haagmans et al., 2014) |
| 105 | 62 (59.0%) camels showed evidence for virus shedding in at least one type of swab at the time of slaughter (Farag et al., 2015) Antibodies to MERS-CoV S1 were found in 100 of 103 (97.1%) dromedary camels tested by micro-array technology (Farag et al., 2015) |
|
| 53 | 1 (1.9%) nasal swab had full viral genome isolated (Raj et al., 2014) | |
| 33 | 7 (21.1%) showed evidence for active virus shedding and 5 (15.2%) had viral RNA in camel milk (Reusken et al., 2014) | |
| 10 | 9 (90%) sera samples had MERS-CoV–specific antibodies. All nasal swab specimens were negative by PCR (Chantal et al., 2016) | |
| Spain (Canary Islands) | 105 | 15 (14%) had protein-specific antibodies against MERS-CoV (Reusken et al., 2013d) |
| Somalia | 86 | 25 (87.5%) dromedary camels were positive for MERS-CoV antibodies using MN (Muller MA et al., 2014) |
| Sudan | 60 | 49 (81.7%) dromedary camels were positive for MERS-CoV antibodies using MN (Muller MA et al., 2014) |
| Tunisia | 204 | Seropositivity was 30% for animals ≤2 years of age and 54% for adult dromedary camels (Chantal et al., 2014) |
| UAE | 1113 | 42 (3.7%) nasal swabs yielded positive results (Muhairi et al., 2016) |
| 843 | 786 (93.2%) sera samples were positive for antibodies against MERS-CoV (Sung Sup et al., 2015) | |
| 11 | 9 (81.8%) sera samples were positive for antibodies supported by similar results in a MERS-CoV recombinant partial spike protein antibody ELISA (Alexandersen et al., 2014) | |
| 651 | 632 (97.1%) had antibodies against MERS-CoV (Meyer et al., 2014) | |
| 376 | 108 (28.7%) nasopharyngeal samples positive for MERS-CoV (Li et al., 2017) | |
| 254 | 234 (92.1%) sera samples were positive for MERS-CoV IgG (Sung Sup et al., 2015) | |
| 6 | 6 (100%) nasopharyngeal swabs tested positive for MERS-CoV (Paden et al., 2018) |
Despite improvements, routine surveillance in dromedary populations is limited. The lack of surveillance information about MERS-CoV circulation in dromedary camels restricts our understanding of the transmission dynamics and epidemiology in dromedary camel populations. Meeting participants agreed that surveillance should be integrated into existing surveillance systems, particularly in at-risk countries, similar to One Health approaches developed for avian influenza, and existing human respiratory disease surveillance systems set up for influenza-like illness (ILI) or severe acute respiratory infections (SARI).
Currently, a limitation in our ability to mitigate spillover from dromedary camels to humans is a lack of clarity on the mode(s) of transmission between dromedary camels and humans, the extent and epidemiology of MERS-CoV circulation in dromedary camels in large parts of Africa and South Asia, and on why zoonotic transmission is limited across Africa, large parts of the Middle East, and some parts of South Asia despite high seroprevalence in dromedary camels (Chu et al., 2018) (Table 1).
FAO has outlined the meeting participants conclusions on priorities for MERS-CoV surveillance and management of PCR positive dromedary camels, coordinated outbreak investigation of community acquired cases with dromedary exposure, testing of animals at quarantine and entry points, food safety and environmental contamination, risk communication and awareness raising for MERS-CoV among animal owners and intersectoral collaboration and coordination in an updated Doha Declaration first published in 2015 (FAO, 2015) (REF/hyperlink). In dromedary camels, longitudinal studies to evaluate the natural history, shedding profile and immunity were highlighted as key research priorities. Meeting participants agreed that further understanding of differences in viral strains and transmission dynamics, including the role of immunity in acquiring infection and shedding virus, the geographic range of spillover events, and environmental, behavioral or host-related risk factors for zoonotic transmission should be prioritized.
-
ii.
Research needs: Hospital transmission and infection prevention and control
Countries face significant challenges in the early identification and diagnosis of MERS in humans due to the non-specificity of clinical symptoms (Arabi et al., 2017a; TheS-Coesearch, 2013; Arabi et al., 2017b; Al-Tawfiq et al., 2017; Al-Tawfiq and Hinedi, 2018; Hui et al., ). The spectrum of illness ranges from no symptoms (or asymptomatic infection) to severe disease including pneumonia, acute respiratory disease syndrome, organ failure and death, with a case fatality ratio 35.5% among reported cases (World Health Organization, 2012). The delay in identification and recognition of signs and symptoms compatible with MERS and delay in early isolation of patients has reduced the ability to prevent transmission between people in health care settings, notably in emergency departments, cardiac care centers and renal dialysis units (Hijawi et al., 2013; Assiri et al., 2013; Al-Abdallat et al., 2014; Drosten et al., 2015; Al Hosani et al., 2016; Ki, 2015; Park et al., 2015; Ahmed et al., 2018; Amer et al., 2018).
Our understanding of human-to-human transmission in health care settings has improved through experimental and observational studies conducted in countries during such outbreaks. For example, studies of respiratory pathogens (Yu et al., 2007; Tran et al., 2012; Thompson et al., 2013) and MERS-CoV conducted in the Middle East (Assiri et al., 2013; Oboho et al., 2015; Hunter et al., 2016; Balkhy et al., 2016) and the Republic of Korea (Bin et al., 2016; Kim et al., 2016a, 2016b; Nam et al., 2017) illustrate that aerosol-generating procedures and non-invasive ventilation, combined with inappropriate infection prevention and control practices and lack of adherence to standard practices had an important role in facilitating human-to-human transmission in health care settings. The role of environmental contamination has been evaluated in a number of hospitals following the 2015 outbreak in the Republic of Korea and collaborative, experimental studies are being conducted to evaluate the viability and persistence of MERS-CoV on surfaces and in the air (Bin et al., 2016; Kim et al., 2016a; van Doremalen et al., 2013). The role of mild or asymptomatic cases in transmission chains, however, remains unclear (Omrani et al., 2013; Memish et al., 2014c; Al-Gethamy et al., 2015; Moon and Son, 2017; Al-Abdely et al., 2018), warranting targeted epidemiological and clinical studies to be conducted among contacts during outbreaks, especially in health care facilities.
Countries which have reported health care-associated outbreaks have implemented a variety of strategies to improve infection prevention and control and reduce human-to-human transmission in hospitals, including the introduction of a visual triage system prior to entrance to the emergency departments, the restructure of emergency department layouts for better triage of patients with respiratory symptoms, the standardization and training and re-training of infection prevention and control practices at facilities with high hospital staff turnover, and the auditing of health care facilities for adherence to infection prevention and control measures.
-
iii.
Product research and development needs: Clinical management, diagnostics and medical interventions
The WHO R&D Blueprint, a global strategy and preparedness plan that allows the rapid activation of R&D of epidemic pathogens, aims to fast-track the development and use of effective point-of-care diagnostic tests, vaccines and medicines that can be used to save lives and avert large scale crises. Since 2015, MERS-CoV has been included in the annual WHO R&D Blueprint list of prioritized pathogens for accelerated research and development on diagnostics, vaccines and therapeutics (World Health Organization). In addition, MERS-CoV has a specific roadmap for product research and development, outlined by WHO in 2015 (Modjarrad et al., 2016). Recent updates on product research and development, presented at the Global Technical Meeting, are below.
2.1. Diagnostics
The nonspecific, and sometimes unusual, clinical presentation of MERS in humans, makes early diagnosis difficult in health care facilities. While several highly specific and sensitive molecular and serologic assays exist for diagnosis in animals and humans (Drosten et al., 2014; Corman et al., 2012a, 2012b; Lu et al., 2014; Perera et al., 2013a; Reusken et al., 2013b; Müller et al., 2015; Song et al., 2015), there was a clear call from representatives from affected countries for the development of a rapid diagnostic test to improve identification and isolation of primary human cases in health care facilities. A full landscape analysis of MERS-CoV diagnostics will be published separately (Van Kerkhove, personal communication).
2.1.1. Therapeutics
At the Global Technical Meeting, several therapeutics (including convalescent plasma, lopinavir/ritonavir, ribavirin, interferon and novel therapies including polyclonal antibodies and broad-spectrum antivirals) in development were presented. However, small case numbers make the evaluation of their impact on morbidity and mortality from MERS-CoV infection difficult (Arabi et al., 2017a; Arabi et al., 2017b; Arabi et al.; Al-Dorzi et al., 2016; Sheahan et al., 2017; Ko et al., 2018; Arabi et al., 2017c). Several pre-clinical and phase I-III studies are under way or in the design phase (outlined by the WHO R&D Blueprint: http://who-blueprint-mapping-tool.surge.sh/). WHO is currently evaluating all available evidence on therapeutics to update guidance on clinical management of patients and in the process to develop standardized clinical trial protocols that could be used in affected countries to evaluate promising therapeutic candidates.
2.2. Vaccines for humans
WHO has identified target product profiles for MERS-CoV vaccines which include a dromedary camel vaccine for the reduction of zoonotic transmission, a human vaccine for long term protection of high risk individuals, such as those working with infected dromedary camels or health care workers, and a human vaccine for reactive use in outbreak settings (World Health Organization, 2017b).
Currently, no MERS-CoV-specific or licensed human vaccines are available (Modjarrad et al., 2016; World Health Organization, 2017c). Several human vaccine candidates for coronaviruses, including MERS-CoV, are at various stages of development and five general vaccine technology platforms have been developed and target the MERS-CoV spike protein (Modjarrad et al., 2016; Okba et al., 2017). WHO, the Ministry of Health in KSA and the International Vaccine Institute (IVI) have continued to further align efforts to develop coronavirus vaccines (Excler et al., 2016) and the Coalition for Epidemic Preparedness and Innovation (CEPI) has included MERS-CoV as one of three priority pathogens for financing of a human vaccine. Understanding correlates for protection and having a reliable animal model remain essential for evaluating coronavirus vaccine candidates, including MERS-CoV.
2.3. Vaccines for camels
There was a clear call from Global Technical Meeting participants to accelerate the development of a dromedary camel vaccine in order to evaluate the potential to reduce spillover transmission to humans. The acceptability, cost-effectiveness and feasibility of a dromedary camel vaccine will also need to be evaluated and compared to other intervention strategies, such as human vaccination of high risk groups (e.g., those with occupational exposure). Because MERS-CoV is endemic in dromedary camel populations in the Middle East and elsewhere, multiple intervention strategies, including personal protective measures and the strategic implementation of a camel vaccine, are likely needed to reduce transmission from dromedary camels to humans.
At least two promising camel vaccine candidates are currently in development and being evaluated in field trials (Haagmans et al., 2016; Alharbi et al., 2017). Stakeholders agreed that the current funding mechanisms need to include risk-mitigating options that target the animal-human interface to prevent zoonotic transmission. These funding pathways would enable the development of camel vaccine candidates. Stakeholders also agreed that prior to camel vaccine implementation, consultation with camel owners and government agencies, feasibility studies including exploring opportunities for commercial manufacturing and incentives for camel vaccination and an assessment of potential trade implications, would all be critical.
In June 2018, WHO and the International Vaccine Institut (IVI) held a joint workshop update the status of human and animal MERS-CoV vaccine development, and identify and prioritize activities to accelerate vaccine research and development. The meeting was held in Seoul, Korea on 26–27 June and included 120 experts and professionals from industry, academia, international organizations and government agencies around the world, including the Coalition for Epidemic Preparedness Innovations (CEPI), the Korean Ministry of Food and Drug Safety (KMFDS) and the Korea Centers for Disease Control and Prevention (KCDC).
3. The way forward
The Global Technical Meeting served as an opportunity to review the available evidence and best practices for control of this epidemic threat six years after the virus was first detected in humans. This covered our understanding of the virus, our ability to detect and respond to cases in animal and human populations, how we communicate our findings and how our work impacts policy decisions to protect animals and prevent human infections.
During the Global Technical Meeting, the latest findings from scientific studies and knowledge gained from collaborative research and surveillance were shared across animal, environmental and human sectors. FAO, OIE and WHO strongly believe that to effectively address zoonoses, including MERS-CoV, a One Health approach to prevent, detect, contain, eliminate and respond to animal and public health risks from zoonotic high threat respiratory pathogens such as MERS-CoV and should involve all relevant sectors, the public and animal health and academic research community, industry and affected communities. We acknowledged the progress that has been made, and importantly, discussed the challenges that need to be addressed so that we can minimize the future public health and economic impacts of this epidemic prone virus. Our aim was to articulate a clear action plan to address these remaining unknowns and to foster better collaboration between sectors and with subject matter experts willing to support member states.
Given the marked expansion in research related to MERS-CoV conducted in the past two years, FAO, OIE and WHO agree that global surveillance and research activities must now be focused on achieving the following major public health goals: reducing zoonotic transmission, detecting and identifying suspected cases early, providing safe and effective treatment to reduce human morbidity and mortality, and significantly reducing human-to-human transmission in health care settings.
The critical needs for research and technical guidance identified during the Global Technical Meeting have been used to inform the WHO R&D MERS-CoV Roadmap (Modjarrad et al., 2016) and a broader Research Agenda for MERS-CoV and other high threat coronaviruses. This research agenda serves as a catalyst to focus, align and mobilize partners to address outstanding knowledge gaps in relation to MERS-CoV across five technical areas:
-
i)
virus origin and characteristics,
-
ii)
epidemiology and transmission,
-
iii)
clinical management and infection prevention and control,
-
iv)
product development and implementation (Modjarrad et al., 2016) and
-
v)
impact of interventions and operational research. The meeting identified a large number of remaining priorities and the organizing committee has summarized the main research needs in Table 2 .
Table 2.
List of prioritized research and progress on MERS-CoV research, as discussed at the September 2017 meeting. *Based on an enhanced understanding of the virus, the Doha Declaration (FAO, 2015) is undergoing revision with a focus on guiding surveillance techniques, management of dromedary camels shedding the virus, research, regional and inter-sectoral coordination, risk communication, food and environmental safety practices, and biosecurity measures. The update includes explicit guidance on import testing, quarantine procedures, and management of shedding animals. These recommendations and priority actions in the Doha Declaration will be delivered as a separate document after validation by stakeholders in affected and at risk countries.
| Population focus | Prioritized research* | Progress since the September, 2017 Meeting |
|---|---|---|
| In dromedary camel populations* |
|
|
| At the animal-human interface |
|
|
| In human populations |
|
|
Now is the time to devote more effort to long term planning for MERS-CoV. We believe more focused efforts in our activities and investments to address scientific and public health research questions, accelerate promising medical interventions and are more strategic on where activities are conducted globally will go further to address remaining public health unknowns. While there have been improvements in the ability to prevent human-to-human transmission once a MERS case is identified, these are not sufficient to prevent a large event with substantial public health and economic consequences.
Competing financial interests
The authors have no competing financial interests.
Acknowledgements
We gratefully acknowledge the input from all those who attended the FAO-OIE-WHO Global Technical Meeting on MERS-CoV in September 2017. The authors also thank Malik Peiris for his review of the final manuscript. The opinions expressed in this article are those of the authors and do not necessarily reflect those of the institutions or organizations with which they are affiliated.
Footnotes
Supplementary data to this article can be found online at https://doi.org/mmcdoino.
Contributor Information
FAO-OIE-WHO MERS Technical Working Group:
Ryan Aguanno, Ahmed ElIdrissi, Amgad A. Elkholy, Peter Ben Embarek, Emma Gardner, Rebecca Grant, Heba Mahrous, Mamunur Rahman Malik, Gounalan Pavade, Sophie VonDobschuetz, Lidewij Wiersma, and Maria D. Van Kerkhove
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- Abdulaziz A.B.S., Glen R.A., Abdullah G.A., et al. Surveillance and testing for Middle East respiratory syndrome coronavirus, Saudi Arabia, April 2015–February 2016. Emerg. Infect. Dis. J. 2017;23:682. doi: 10.3201/eid2304.161793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adney D.R.B.-O.H., Hartwig A.E., Bowen R.A. Infection, replication, and transmission of Middle East respiratory syndrome coronavirus in alpacas. Emerg. Infect. Dis. 2016;22:1031–1037. doi: 10.3201/2206.160192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A.E., Al-Jahdali H., Alshukairi A.N., et al. Early identification of pneumonia patients at increased risk of Middle East respiratory syndrome coronavirus infection in Saudi Arabia. Int. J. Infect. Dis. 2018;70:51–56. doi: 10.1016/j.ijid.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Hosani F., Pringle K., Al Mulla M., et al. Response to emergence of Middle East respiratory syndrome coronavirus, Abu Dhabi, United Arab Emirates, 2013-2014. Emerg. Infect. Dis. 2016 Jul;22(7):1162–1168. doi: 10.3201/eid2207.160040. 2016;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Abdallat M.M., Payne D.C., Alqasrawi S., et al. Hospital-associated outbreak of Middle East respiratory syndrome coronavirus: a serologic, epidemiologic, and clinical description. Clin. Infect. Dis. 2014;59:1225–1233. doi: 10.1093/cid/ciu359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Abdallat M.M., Rha B., Alqasrawi S., et al. Acute respiratory infections among returning Hajj pilgrims—Jordan, 2014. J. Clin. Virol. 2017;89:34–37. doi: 10.1016/j.jcv.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Abdely H.M., Midgley C.M., Alkhamis A.M., et al. Infectious MERS-CoV isolated from a mildly Ill patient, Saudi Arabia. Open Forum Infect. Dis. 2018;5:ofy111–ofy. doi: 10.1093/ofid/ofy111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Dorzi H.M., Aldawood A.S., Khan R., et al. The critical care response to a hospital outbreak of Middle East respiratory syndrome coronavirus (MERS-CoV) infection: an observational study. Ann. Intensive Care. 2016;6:101. doi: 10.1186/s13613-016-0203-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Gethamy M., Corman V.M., Hussain R., Al-Tawfiq J.A., Drosten C., Memish Z.A. A case of long-term excretion and subclinical infection with Middle East respiratory syndrome coronavirus in a healthcare worker. Clin. Infect. Dis. 2015;60:973–974. doi: 10.1093/cid/ciu135. Epub 2014 Dec 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Tawfiq J.A., Hinedi K. The calm before the storm: clinical observations of Middle East respiratory syndrome (MERS) patients. J. Chemother. 2018;30:179–182. doi: 10.1080/1120009X.2018.1429236. [DOI] [PubMed] [Google Scholar]
- Al-Tawfiq J.A., Alfaraj S.H., Altuwaijri T.A., Memish Z.A. A cohort-study of patients suspected for MERS-CoV in a referral hospital in Saudi Arabia. J. Infect. 2017;75:378–379. doi: 10.1016/j.jinf.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagaili A.N., Briese T., Mishra N., et al. Middle East respiratory syndrome coronavirus infection in dromedary camels in Saudi Arabia. mBio. 2014;5(2) doi: 10.1128/mBio.00884-14. (2014). Web. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersen S., Kobinger G.P., Soule G., Wernery U. Middle East respiratory syndrome coronavirus antibody reactors among camels in Dubai, United Arab Emirates, in 2005. Transboundary Emerg. Dis. 2014;61:105–108. doi: 10.1111/tbed.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alharbi N.K., Padron-Regalado E., Thompson C.P., et al. ChAdOx1 and MVA based vaccine candidates against MERS-CoV elicit neutralising antibodies and cellular immune responses in mice. Vaccine. 2017;35:3780–3788. doi: 10.1016/j.vaccine.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M, El-Shesheny R, Kandeil A, et al.. Cross-sectional surveillance of Middle East respiratory syndrome coronavirus (MERS-CoV) in dromedary camels and other mammals in Egypt, August 2015 to January 2016. Eurosurveillance;22:30487. [DOI] [PMC free article] [PubMed]
- Ali M.A., Shehata M.M., Gomaa M.R., et al. Systematic, active surveillance for Middle East respiratory syndrome coronavirus in camels in Egypt. Emerg. Microb. Infect. 2017;6:e1. doi: 10.1038/emi.2016.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alqahtani A.S., Wiley K.E., Mushta S.M., et al. Association between Australian Hajj Pilgrims' awareness of MERS-CoV, and their compliance with preventive measures and exposure to camels. J. Trav. Med. 2016;23:taw046–taw. doi: 10.1093/jtm/taw046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amer H., Alqahtani A.S., Alzoman H., Aljerian N., Memish Z.A. Unusual presentation of Middle East respiratory syndrome coronavirus leading to a large outbreak in Riyadh during 2017. Am. J. Infect. Contr. 2018 doi: 10.1016/j.ajic.2018.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annan A., Owusu M., Marfo K.S., et al. High prevalence of common respiratory viruses and no evidence of Middle East Respiratory Syndrome Coronavirus in Hajj pilgrims returning to Ghana, 2013. Trop. Med. Int. Health. 2015;20:807–812. doi: 10.1111/tmi.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabi Y.M., Al-Omari A., Mandourah Y., et al. Critically Ill patients with the Middle East respiratory syndrome: a multicenter retrospective cohort study. Crit. Care Med. 2017;45:1683–1695. doi: 10.1097/CCM.0000000000002621. [DOI] [PubMed] [Google Scholar]
- Arabi Y.M., Balkhy H.H., Hayden F.G., et al. Middle East respiratory syndrome. N. Engl. J. Med. 2017;376:584–594. doi: 10.1056/NEJMsr1408795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabi Y.M., Mandourah Y., Al-Hameed F., et al. Corticosteroid therapy for critically Ill patients with Middle East respiratory syndrome. Am. J. Respir. Crit. Care Med. 2017;197:757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- Arabi DYM, Mandourah DY, Al-Hameed DF, et al.. Corticosteroid Therapy for Critically Ill Patients with the Middle East Respiratory Syndrome. American Journal of Respiratory and Critical Care Medicine;0:null. [DOI] [PubMed]
- Ariful I., Jonathan H.E., Melinda K.R., et al. Middle East respiratory syndrome coronavirus antibodies in dromedary camels, Bangladesh, 2015. Emerg. Infect. Dis. J. 2018;24:926. doi: 10.3201/eid2405.171192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assiri A., McGeer A., Perl T.M., et al. Hospital outbreak of Middle East respiratory syndrome coronavirus. N. Engl. J. Med. 2013;369:407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atabani S.F.W.S., Overton-Lewis C., Workman J., Kidd I.M., Petersen E., Zumla A., Smit E., Osman H. Active screening and surveillance in the United Kingdom for Middle East respiratory syndrome coronavirus in returning travellers and pilgrims from the Middle East: a prospective descriptive study for the period 2013-2015. Int. J. Infect. Dis. 2016;47:10–14. doi: 10.1016/j.ijid.2016.04.016. https://doi.org/1016/j.ijid.2016.04.016 Epub Apr 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhar E.I., El-Kafrawy S.A., Farraj S.A., et al. Evidence for camel-to-human transmission of MERS coronavirus. N. Engl. J. Med. 2014;370:2499–2505. doi: 10.1056/NEJMoa1401505. [DOI] [PubMed] [Google Scholar]
- Balkhy H.H., Alenazi T.H., Alshamrani M.M., et al. Description of a hospital outbreak of Middle East respiratory syndrome in a large tertiary care hospital in Saudi Arabia. Infect. Contr. Hosp. Epidemiol. 2016;37 doi: 10.1017/ice.2016.132. 1147-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barasheed O.R.H., Alfelali M., Tashani M., Azeem M., Bokhary H., Kalantan N., Samkari J., Heron L., Kok J., Taylor J., El Bashir H., Memish Z.A., Haworth E., Holmes E.C., Dwyer D.E., Asghar A., Booy R., Hajj Research Team Viral respiratory infections among Hajj pilgrims in 2013. Virol. Sin. 2014;29:364–371. doi: 10.1007/s12250-014-3507-x. Epub 2014 Nov 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bin S.Y., Heo J.Y., Song M.-S., et al. Environmental contamination and viral shedding in MERS patients during MERS-CoV outbreak in South Korea. Clin. Infect. Dis. 2016;62:755–760. doi: 10.1093/cid/civ1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briese T., Mishra N., Jain K., et al. Middle East respiratory syndrome coronavirus quasispecies that include homologues of human Isolates revealed through whole-genome analysis and virus cultured from dromedary camels in Saudi Arabia. mBio. 2014;5 doi: 10.1128/mBio.01146-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantal B.E.M.R., Lilia M., Ashenafi F., et al. Geographic distribution of MERS coronavirus among dromedary camels, Africa. Emerg. Infect. Dis. J. 2014;20:1370. doi: 10.3201/eid2008.140590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantal B.E.M.R., Chrispijn S., Raj V.S., et al. MERS-CoV infection of alpaca in a region where MERS-CoV is endemic. Emerg. Infect. Dis. J. 2016;22:1129. doi: 10.3201/eid2206.152113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D.K., Poon L.L., Gomaa M.M., et al. MERS coronaviruses in dromedary camels, Egypt. Emerg. Infect. Dis. 2014;20:1049–1053. doi: 10.3201/eid2006.140299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D.K., Oladipo J.O., Perera R.A., et al. Middle East respiratory syndrome coronavirus (MERS-CoV) in dromedary camels in Nigeria. Euro Surveill. 2015;20:30086. doi: 10.2807/1560-7917.ES.2015.20.49.30086. [DOI] [PubMed] [Google Scholar]
- Chu D.K.W., Hui K.P.Y., Perera R.A.P.M., et al. MERS coronaviruses from camels in Africa exhibit region-dependent genetic diversity. Proc. Natl. Acad. Sci. Unit. States Am. 2018 doi: 10.1073/pnas.1718769115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V.M., Eckerle I., Bleicker T., et al. Detection of a novel human coronavirus by real-time reverse-transcription polymerase chain reaction. Euro Surveill. 2012;17 doi: 10.2807/ese.17.39.20285-en. [DOI] [PubMed] [Google Scholar]
- Corman V.M., Müller M.A., Costabel U., et al. Assays for laboratory confirmation of novel human coronavirus (hCoV-EMC) infections. Euro Surveill. 2012;17(49) doi: 10.2807/ese.17.49.20334-en. http://wwweurosurveillanceorg/ViewArticleaspx?ArticleId=20334 pii=20334 Available from: [DOI] [PubMed] [Google Scholar]
- Corman V.M., Jores J., Meyer B., et al. Antibodies against MERS coronavirus in dromedary camels, Kenya, 1992–2013. Emerg. Infect. Dis. 2014:20. doi: 10.3201/eid2008.140596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crameri G.D.P., Klein R., Foord A., Yu M., Riddell S., Haining J., Johnson D., Hemida M.G., Barr J., Peiris M., Middleton D., Wang L.F. Experimental infection and response to rechallenge of alpacas with Middle East respiratory syndrome coronavirus. Emerg. Infect. Dis. 2016;22:1071–1074. doi: 10.3201/eid2206.160007. Epub 2016 Jun 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David D., Rotenberg D., Khinich E., et al. Middle East respiratory syndrome coronavirus specific antibodies in naturally exposed Israeli llamas, alpacas and camels. One Health. 2018;5:65–68. doi: 10.1016/j.onehlt.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deem S.L., Fèvre E.M., Kinnaird M., et al. Serological Evidence of MERS-CoV Antibodies in Dromedary Camels (Camelus dromedaries) in Laikipia County, Kenya. PLoS One. 2015;10 doi: 10.1371/journal.pone.0140125. e0140125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C., Meyer B., Muller M.A., et al. Transmission of MERS-coronavirus in household contacts. N. Engl. J. Med. 2014;371:828–835. doi: 10.1056/NEJMoa1405858. [DOI] [PubMed] [Google Scholar]
- Drosten C., Muth D., Corman V.M., et al. An observational, laboratory-based study of outbreaks of middle East respiratory syndrome coronavirus in Jeddah and Riyadh, kingdom of Saudi Arabia, 2014. Clin. Infect. Dis. 2015;60:369–377. doi: 10.1093/cid/ciu812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Excler J.L., Delvecchio C.J., Wiley R.E., et al. Toward developing a preventive MERS-CoV vaccine—report from a workshop organized by the Saudi Arabia Ministry of health and the international vaccine Institute, Riyadh, Saudi Arabia, November 14–15, 2015. Emerg. Infect. Dis. 2016 doi: 10.3201/eid2208.160229. 2016 Aug [16/01/2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falzarano D., Kamissoko B., de Wit E., et al. Dromedary camels in northern Mali have high seropositivity to MERS-CoV. One Health. 2017;3:41–43. doi: 10.1016/j.onehlt.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO FAO calls for more surveillance and research on MERS. Vet. Rec. 2014;174:620. doi: 10.1136/vr.g3816. [DOI] [PubMed] [Google Scholar]
- FAO . 2015. OIE, WHO. Doha Declaration. Regional Workshop on MERS-cov and One Health 27-29 May 2015, Doha Qatar.http://www.fao.org/ag/againfo/programmes/en/empres/documents/docs/Doha_Declaration_2015.pdf Available at: [Google Scholar]
- FAO. Understanding MERS-CoV and the Animal-Human Interface. Technical Meeting 21-11 January 2016, Rome Italy. Available at: http://www.fao.org/3/a-i5682e.pdf. 2016.
- Farag E.A.B.A., Reusken C.B.E.M., Haagmans B.L., et al. High proportion of MERS-CoV shedding dromedaries at slaughterhouse with a potential epidemiological link to human cases, Qatar 2014. Infect. Ecol. Epidemiol. 2015;5 doi: 10.3402/iee.v5.28305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haagmans B.L., Al Dhahiry S.H.S., Reusken C.B.E.M., et al. Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect. Dis. 2014;14:140–145. doi: 10.1016/S1473-3099(13)70690-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haagmans B.L., van den Brand J.M.A., Raj V.S., et al. An orthopoxvirus-based vaccine reduces virus excretion after MERS-CoV infection in dromedary camels. Science. 2016;351:77–81. doi: 10.1126/science.aad1283. [DOI] [PubMed] [Google Scholar]
- Harcourt J.L., Rudoler N., Tamin A., et al. The prevalence of Middle East respiratory syndrome coronavirus (MERS-CoV) antibodies in dromedary camels in Israel. Zoonoses Publ. Health. 2018;65:749–754. doi: 10.1111/zph.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrath R., Abu Duhier F.M. Sero-prevalence of Middle East respiratory syndrome coronavirus (MERS-CoV) specific antibodies in dromedary camels in Tabuk, Saudi Arabia. J. Med. Virol. 2018;90:1285–1289. doi: 10.1002/jmv.25186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemida M.G., Perera R.A., Wang P., et al. Middle East Respiratory Syndrome (MERS) coronavirus seroprevalence in domestic livestock in Saudi Arabia, 2010 to 2013. Euro Surveill. 2013;18:20659. doi: 10.2807/1560-7917.es2013.18.50.20659. [DOI] [PubMed] [Google Scholar]
- Hemida M.G., Perera R.A., Al Jassim R.A., et al. Seroepidemiology of Middle East respiratory syndrome (MERS) coronavirus in Saudi Arabia (1993) and Australia (2014) and characterisation of assay specificity. Euro Surveill. 2014;19 doi: 10.2807/1560-7917.es2014.19.23.20828. pii: 20828. PubMed PMID: 24957744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemida M.G., Alnaeem A., Chu D.K.W., et al. Longitudinal study of Middle East Respiratory Syndrome coronavirus infection in dromedary camel herds in Saudi Arabia, 2014–2015. Emerg. Microb. Infect. 2017;6:e56. doi: 10.1038/emi.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijawi B., Abdallat M., Sayaydeh A., et al. Novel coronavirus infections in Jordan, April 2012: epidemiological findings from a retrospective investigation. East. Mediterr. Health J. 2013;19:S12–S18. [PubMed] [Google Scholar]
- Hui DS, Azhar EI, Kim Y-J, Memish ZA, Oh M-d, Zumla A. Middle East respiratory syndrome coronavirus: risk factors and determinants of primary, household, and nosocomial transmission. Lancet Infect. Dis.. [DOI] [PMC free article] [PubMed]
- Hunter J.C., Nguyen D., Aden B., et al. Transmission of Middle East respiratory syndrome coronavirus infections in healthcare settings, Abu Dhabi. Emerg. Infect. Dis. 2016;22:647–656. doi: 10.3201/eid2204.151615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor M., Pringle K., Kumar A., et al. Clinical and laboratory findings of the first imported case of Middle East respiratory syndrome coronavirus to the United States. Clin. Infect. Dis. 2014;59:1511–1518. doi: 10.1093/cid/ciu635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasem S., Qasim I., Al-Hufofi A., et al. Cross-sectional study of MERS-CoV-specific RNA and antibodies in animals that have had contact with MERS patients in Saudi Arabia. J. Infect. Publ. Health. 2017 doi: 10.1016/j.jiph.2017.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ki M. MERS outbreak in Korea: hospital-to-hospital transmission. Epidemiol. Health. 2015;37 doi: 10.4178/epih/e2015033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.-H., Chang S.Y., Sung M., et al. Extensive viable Middle East respiratory syndrome (MERS) coronavirus contamination in air and surrounding environment in MERS outbreak units. Clin. Infect. Dis. 2016 doi: 10.1093/cid/ciw239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.W., Park J.W., Jung H.D., et al. Risk factors for transmission of Middle East respiratory syndrome coronavirus infection during the 2015 outbreak in South Korea. Clin. Infect. Dis. 2016;64:551–557. doi: 10.1093/cid/ciw768. Epub 2016 Dec 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J.H., Seok H., Cho S.Y., et al. Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: a single centre experience. Antivir. Ther. 2018;Jun 20 doi: 10.3851/IMP3243. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Koul P.A.M.H., Saha S., Chadha M.S., Potdar V., Widdowson M.A., Lal R.B., Krishnan A. Influenza not MERS CoV among returning Hajj and Umrah pilgrims with respiratory illness, Kashmir, north India, 2014-15. Trav. Med. Infect. Dis. 2017;15:45–47. doi: 10.1016/j.tmaid.2016.12.002. Epub Dec 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Khalafalla A.I., Paden C.R., et al. Identification of diverse viruses in upper respiratory samples in dromedary camels from United Arab Emirates. PLoS One. 2017;12 doi: 10.1371/journal.pone.0184718. e0184718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Whitaker B., Sakthivel S.K., et al. Real-time reverse transcription-PCR assay panel for Middle East respiratory syndrome coronavirus. J. Clin. Microbiol. 2014;52:67–75. doi: 10.1128/JCM.02533-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Liu F., Liu L., et al. No MERS-CoV but positive influenza viruses in returning Hajj pilgrims, China, 2013–2015. BMC Infect. Dis. 2017;17:715. doi: 10.1186/s12879-017-2791-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maged G.H., Daniel K.W.C., Leo L.M.P., et al. MERS coronavirus in dromedary camel herd, Saudi Arabia. Emerg. Infect. Dis. J. 2014;20:1231. doi: 10.3201/eid2007.140571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maged G.H., Abdulmohsen A.-N., Ranawaka A.P.M.P., Alex W.H.C., Leo L.M.P., Malik P. Lack of Middle East respiratory syndrome coronavirus transmission from infected camels. Emerg. Infect. Dis. J. 2015;21:699. doi: 10.3201/eid2104.141949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew G., Romy O., Erik K., et al. Acute respiratory infections in travelers returning from MERS-CoV–affected areas. Emerg. Infect. Dis. J. 2015;21:1654. doi: 10.3201/eid2109.150472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memish Z.A., Almasri M., Turkestani A., Al-Shangiti A.M., Yezli S. Etiology of severe community-acquired pneumonia during the 2013 Hajj—part of the MERS-CoV surveillance program. Int. J. Infect. Dis. 2014;25:186–190. doi: 10.1016/j.ijid.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memish Z.A., Assiri A., Almasri M., et al. Prevalence of MERS-CoV Nasal carriage and compliance with the Saudi health recommendations among pilgrims attending the 2013 Hajj. J. Infect. Dis. 2014;210:1067–1072. doi: 10.1093/infdis/jiu150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memish Z.A., Assiri A.M., Al-Tawfiq J.A. Middle East respiratory syndrome coronavirus (MERS-CoV) viral shedding in the respiratory tract: an observational analysis with infection control implications. Int. J. Infect. Dis. 2014;29:307–308. doi: 10.1016/j.ijid.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B.M.M., Corman V.M., Reusken C.B.E.M., Ritz D., Godeke G.-D., et al. Antibodies against MERS coronavirus in dromedary camels, United Arab Emirates, 2003 and 2013. Emerg. Infect. Dis. 2014;April doi: 10.3201/eid2004.131746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel E., Perera R.A., Baubekova A., et al. Absence of Middle East respiratory syndrome coronavirus in Camelids, Kazakhstan, 2015. Emerg. Infect. Dis. 2016;22:555–557. doi: 10.3201/eid2203.151284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel E.C.V., Ayelet G., Ben Bencheikh M., Boussini H., Chu D., et al. Risk factors for MERS coronavirus infection in dromedary camels in Burkina Faso, Ethiopia, and Morocco, 2015. Euro Surveill. 2017;22 doi: 10.2807/1560-7917.ES.2017.22.13.30498. http://www.eurosurveillance.org/images/dynamic/EE/V22N13/art22754.pdf Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modjarrad K.M.V., Ben Embarek P., Van Kerkhove M., Kim J., Kieny M.P. A roadmap for MERS-CoV research and product development: report from a World Health Organization consultation. Nat. Med. 2016 Jul 7;22(7):701–705. doi: 10.1038/nm.4131. 2016;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohd H.A., Al-Tawfiq J.A., Memish Z.A. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) origin and animal reservoir. Virol. J. 2016;13:87. doi: 10.1186/s12985-016-0544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon S.Y., Son J.S. Infectivity of an asymptomatic patient with Middle East respiratory syndrome coronavirus infection. Clin. Infect. Dis. 2017;64:1457–1458. doi: 10.093/cid/cix170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhairi S.A., Hosani F.A., Eltahir Y.M., et al. Epidemiological investigation of Middle East respiratory syndrome coronavirus in dromedary camel farms linked with human infection in Abu Dhabi Emirate, United Arab Emirates. Virus Gene. 2016;52:848–854. doi: 10.1007/s11262-016-1367-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M.A., Meyer B., Corman V.M., et al. Presence of Middle East respiratory syndrome coronavirus antibodies in Saudi Arabia: a nationwide, cross-sectional, serological study. Lancet Infect. Dis. 2015;15:629. doi: 10.1016/S473-3099(15)00029-8. Epub 2015 May 17. [DOI] [PubMed] [Google Scholar]
- Muller MA V.M.C., Jores J., et al. MERS coronavirus neutralizing antibodies in camels, Eastern Africa, 1983-1997. Emerg. Infect. Dis. 2014;20:2093–2095. doi: 10.3201/eid2012.141026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraduzzaman A.K.M., Khan M.H., Parveen R., et al. Event based surveillance of Middle East Respiratory Syndrome Coronavirus (MERS- CoV) in Bangladesh among pilgrims and travelers from the Middle East: an update for the period 2013–2016. PLoS One. 2018;13 doi: 10.1371/journal.pone.0189914. e0189914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam H.-S., Park J.W., Ki M., Yeon M.-Y., Kim J., Kim S.W. High fatality rates and associated factors in two hospital outbreaks of MERS in Daejeon, the Republic of Korea. Int. J. Infect. Dis. 2017;58:37–42. doi: 10.1016/j.ijid.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowotny N., Kolodziejek J. Middle East respiratory syndrome coronavirus (MERS-CoV) in dromedary camels, Oman, 2013. Euro Surveill. 2014;19:20781. doi: 10.2807/1560-7917.es2014.19.16.20781. [DOI] [PubMed] [Google Scholar]
- Oboho I.K., Tomczyk S.M., Al-Asmari A.M., et al. 2014 MERS-CoV outbreak in Jeddah - a link to health care facilities. N. Engl. J. Med. 2015;372:846–854. doi: 10.1056/NEJMoa1408636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE . 2014. OIE AD HOC Group on MERS-cov Infection in Animals July 2014.http://www.oie.int/en/standard-setting/specialists-commissions-working-groups/scientific-commission-reports/ad-hoc-groups-reports/ Available at. [Google Scholar]
- OIE . 2014. Infection with Coronavirus in Camels, Iran.http://www.oie.int/wahis_2/public/wahid.php/Reviewreport/Review?page_refer=MapFullEventReport&reportid=16411 Available at: [Google Scholar]
- OIE . May 2017. Middle East Respiratory Syndrome Coronavirus (MERS-cov) Case Definition for Reporting to OIE.http://www.oie.int/en/scientific-expertise/specific-information-and-recommendations/mers-cov/. 2017 Available at: [Google Scholar]
- Okba N.M.A., Raj V.S., Haagmans B.L. Middle East respiratory syndrome coronavirus vaccines: current status and novel approaches. Curr. Opin. Virol. 2017;23:49–58. doi: 10.1016/j.coviro.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omrani A.S., Matin M.A., Haddad Q., Al-Nakhli D., Memish Z.A., Albarrak A.M. A family cluster of Middle East Respiratory Syndrome Coronavirus infections related to a likely unrecognized asymptomatic or mild case. Int. J. Infect. Dis. 2013;17:e668–e672. doi: 10.1016/j.ijid.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paden C.R., Yusof M.F.B.M., Al Hammadi Z.M., et al. Zoonotic origin and transmission of Middle East respiratory syndrome coronavirus in the UAE. Zoonoses Publ. Health. 2018;65:322–333. doi: 10.1111/zph.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.Y., Lee E.J., Ryu Y.W., et al. Epidemiological investigation of MERS-CoV spread in a single hospital in South Korea, May to June 2015. Euro Surveill. 2015;20:1–6. doi: 10.2807/1560-7917.es2015.20.25.21169. [DOI] [PubMed] [Google Scholar]
- Perera R.A., Wang P., Gomaa M.R., et al. Seroepidemiology for MERS coronavirus using microneutralisation and pseudoparticle virus neutralisation assays reveal a high prevalence of antibody in dromedary camels in Egypt, June 2013. Euro Surveill. 2013;18 doi: 10.2807/1560-7917.es2013.18.36.20574. pii=20574. [DOI] [PubMed] [Google Scholar]
- Perera R.A., Wang P., Gomaa M.R., et al. Seroepidemiology for MERS coronavirus using microneutralisation and pseudoparticle virus neutralisation assays reveal a high prevalence of antibody in dromedary camels in Egypt, June 2013. Euro Surveill. 2013;18:20574. doi: 10.2807/1560-7917.es2013.18.36.20574. [DOI] [PubMed] [Google Scholar]
- Raj V.S., Farag E., Reusken C.B.E.M., et al. Isolation of MERS coronavirus from a dromedary camel, Qatar, 2014. Emerg. Infect. Dis. 2014;20:1339–1342. doi: 10.3201/eid2008.140663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refaey S., Amin M.M., Roguski K., et al. Cross‐sectional survey and surveillance for influenza viruses and MERS‐CoV among Egyptian pilgrims returning from Hajj during 2012‐2015. Infl. Other Respir. Viruses. 2017;11:57–60. doi: 10.1111/irv.12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusken C.B., Haagmans B.L., Müller M.A., et al. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: a comparative serological study. Lancet Infect. Dis. 2013;13:70164–70166. doi: 10.1016/S1473-3099(13)-6. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusken C., Mou H., Godeke G.J., et al. Specific serology for emerging human coronaviruses by protein microarray. Euro Surveill. 2013;18:20441. doi: 10.2807/1560-7917.es2013.18.14.20441. [DOI] [PubMed] [Google Scholar]
- Reusken C.B., Ababneh M., Raj V.S., et al. Middle East Respiratory Syndrome coronavirus (MERS-CoV) serology in major livestock species in an affected region in Jordan, June to September 2013. Euro Surveill. 2013;18:20662. doi: 10.2807/1560-7917.es2013.18.50.20662. [DOI] [PubMed] [Google Scholar]
- Reusken C.B.E.M., Haagmans B.L., Müller M.A., et al. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: a comparative serological study. Lancet Infect. Dis. 2013;13:859–866. doi: 10.1016/S1473-3099(13)70164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusken C.B., Farag E.A., Jonges M., et al. Middle East respiratory syndrome coronavirus (MERS-CoV) RNA and neutralising antibodies in milk collected according to local customs from dromedary camels, Qatar, April 2014. Euro Surveill. 2014;19:20829. doi: 10.2807/1560-7917.es2014.19.23.20829. [DOI] [PubMed] [Google Scholar]
- Sabir J.S.M., Lam T.T.-Y., Ahmed M.M.M., et al. Co-circulation of three camel coronavirus species and recombination of MERS-CoVs in Saudi Arabia. Science. 2016;351:81–84. doi: 10.1126/science.aac8608. [DOI] [PubMed] [Google Scholar]
- Saqib M., Sieberg A., Hussain M.H., et al. Serologic evidence for MERS-CoV infection in dromedary camels, Punjab, Pakistan, 2012–2015. Emerg. Infect. Dis. 2017;23:550–551. doi: 10.3201/eid2303.161285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T.P., Sims A.C., Graham R.L., et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So R.T., Perera R.A., Oladipo J.O., et al. Lack of serological evidence of Middle East respiratory syndrome coronavirus infection in virus exposed camel abattoir workers in Nigeria, 2016. Euro Surveill. 2018;23 doi: 10.2807/1560-7917.ES.2018.23.32.1800175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D., Ha G., Serhan W., et al. Development and validation of a rapid immunochromatographic assay for detection of Middle East respiratory syndrome coronavirus antigen in dromedary camels. J. Clin. Microbiol. 2015;53:1178–1182. doi: 10.28/JCM.03096-14. Epub 2015 Jan 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung Sup P., Ulrich W., Victor M.C., et al. Acute Middle East respiratory syndrome coronavirus infection in livestock dromedaries, Dubai, 2014. Emerg. Infect. Dis. J. 2015;21:1019. doi: 10.3201/eid2106.150038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The WHO MERS-CoV Research Group State of knowledge and data gaps of Middle East respiratory syndrome coronavirus (MERS-CoV) in humans. PLoS Curr. Outbreaks. 2013 doi: 10.1371/currents.outbreaks.0bf719e352e7478f8ad85fa30127ddb8. Published, 2013 Nov 12. Edition 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson K.-A., Pappachan J.V., Bennett A.M., et al. Influenza Aerosols in UK Hospitals during the H1N1 (2009) Pandemic – the Risk of Aerosol Generation during Medical Procedures. PLoS One. 2013;8 doi: 10.1371/journal.pone.0056278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran K., Cimon K., Severn M., Pessoa-Silva C.L., Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0035797. Epub 2012 Apr 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Munster V.J. Stability of Middle East respiratory syndrome coronavirus (MERS-CoV) under different environmental conditions. Euro Surveill. 2013;18 doi: 10.2807/1560-7917.es2013.18.38.20590. http://www.eurosurveillance.org/View Article.aspx?ArticleId= pii=20590. Av ailable online: [DOI] [PubMed] [Google Scholar]
- van Doremalen N., Hijazeen Z.S.K., Holloway P., et al. High prevalence of Middle East respiratory coronavirus in young dromedary camels in Jordan. Vector Borne Zoonotic Dis. 2017;17:155–159. doi: 10.1089/vbz.2016.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara-Alert J., van den Brand J.M., Widagdo W., et al. Livestock susceptibility to infection with Middle East respiratory syndrome coronavirus. Emerg. Infect. Dis. 2017;23:232–240. doi: 10.3201/eid2302.161239. Epub 2017 Feb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAHIS Database Interface O.I.E. 2014. Immediate Notification of MERS-CoV in Kuwait. Information Received on 11/06/2014 from Mrs. Hanadi Ghuloom Abdul Rahman Mohammad, Deputy Director General for Animal Health, Animal Health Department, Public Authority for Agriculture Affairs & Fish Resources, Safat, Kuwait, June 2014.http://www.oie.int/wahis_2/public/wahid.php/Reviewreport/Review?page_refer=MapFullEventReport&reportid=15407 Available: [Google Scholar]
- WHO Regional office for the Eastern Mediterranean . 2013. Report on the Technical Consultative Meeting on Novel Coronavirus. Cairo, Egypt. 14-15 January 2013.http://applications.emro.who.int/docs/IC_Meet_Rep_2013_EN_14936.pdf?ua=1 Available at: [PubMed] [Google Scholar]
- WHO Regional office for the Eastern Mediterranean . 2013. Report on the Intercountry Meeting on the Middle East Respiratory Syndrome Coronavirus (MERS-cov) Outbreak in the Eastern Mediterranean Region, Cairo, Egypt. 20-22 June 2013.http://applications.emro.who.int/docs/IC_Meet_Rep_2013_EN_15164.pdf?ua=1 Available at: [Google Scholar]
- WHO Regional office for the Eastern Mediterranean . 2013. Press Release: Experts Meet to Discuss Novel Coronavirus Infection in Humans, 14–15 January 2013.http://www.emro.who.int/surveillance-forecasting-response/surveillance-events/consultation-ncv-january-2013.html Available at: [Google Scholar]
- WHO Regional office for the Eastern Mediterranean . 2014. Press Release: Countries Discuss Stepping up Preparedness for MERS-cov and Ebola.http://www.emro.who.int/surveillance-forecasting-response/surveillance-events/preparedness-mers-ebola.html Available at: [Google Scholar]
- Win M.K., Chow A., Ho H.J., Tay S.Y., Leo Y.S. Risk assessment and laboratory investigation of respiratory illness in travellers returning to Singapore 2012–2015: experience from the MERS-CoV Surveillance Programme. Epidemiol. Infect. 2016;145:285–288. doi: 10.1017/S0950268816002326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G., Liu W., Liu Y., Zhou B., Bi Y., Gao George F. MERS, SARS, and Ebola: the role of super-spreaders in infectious disease. Cell Host Microbe. 2015;18:398–401. doi: 10.1016/j.chom.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. WHO R&D Blueprint List of Priority Diseases. Available at: http://www.who.int/blueprint/priority-diseases/en/.
- World Health Organization. Middle East respiratory syndrom coronavirus (MERS-CoV). Available at http://www.who.int/emergencies/mers-cov/en/. . 2012-2018.
- World Health Organization. Summary report on the Intrenational scientific meeting on Middle East respiratory syndrome coronavirus (MERS-CoV). WHO Regional Office for the Eastern Mediterranean. Available at: http://applications.emro.who.int/docs/IC_Meet_Rep_2016_EN_18725.pdf?ua=1. 2016.
- World Health Organization . 2017. Middle East Respiratory Syndrome Coronavirus (MERS-cov)http://www.who.int/emergencies/mers-cov/en/ Available at: [Google Scholar]
- World Health Organization . May 2017. WHO Target Product Profiles for MERS-cov Vaccines.http://www.who.int/blueprint/what/research-development/MERS_CoV_TPP_15052017.pdf?ua=1. 2017 Available at: [Google Scholar]
- World Health Organization . 2017. Landscape Analysis of MERS-cov Research and Project Development for Diagnostics, Theraputics and Preventives.http://www.who.int/blueprint/priority-diseases/key-action/mers-landscape.pdf?ua=1 Available at: [Google Scholar]
- World Health Organization, Food and Agriculture Organization of the United Nations, World Organisation for Animal Health . 2017. Press Release: Countries Agree Next Steps to Combat Global Health Threat by MERS-cov.http://www.who.int/emergencies/mers-cov/accelerating-response/en/ Available at: [Google Scholar]
- Yavarian J.S.J.N., Naseri M., Hemmati P., Dadras M., Gouya M.M., Mokhtari Azad T. Influenza virus but not MERS coronavirus circulation in Iran, 2013-2016: comparison between pilgrims and general population. Trav. Med. Infect. Dis. 2018 Jan - Feb:21. doi: 10.1016/j.tmaid.2017.10.007. 2018;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I.T., Xie Z.H., Tsoi K.K.C.Y., Lok S.W., Tang X.P., Hui D.S., Lee N., Li Y.M., Huang Z.T., Liu T., Wong T.W., Zhong N.S., Sung J.J. Why did outbreaks of severe acute respiratory syndrome occur in some hospital wards but not in others? Clin. Infect. Dis. 2007;44:1017–1025. doi: 10.1086/512819. Epub 2007 Mar 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- Ziad A.M., Matthew C., Benjamin M., et al. Human infection with MERS coronavirus after exposure to infected camels, Saudi Arabia, 2013. Emerg. Infect. Dis. J. 2014;20:1012. doi: 10.3201/eid2006.140402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.