Abstract
The brain renin–angiotensin system continues to be enigmatic more than 40 years after the brain was first recognized to be a site of action of angiotensin II. This review focuses on the enzymatic pathways for the formation and degradation of the growing number of active angiotensins in the brain. A brief description and nomenclature of the peptidases involved in the processing of angiotensin peptides in the brain is given. Of primary interest is the array of enzymes that degrade radiolabeled angiotensins in receptor binding assays. This poses major challenges to studies of brain angiotensin receptors and it is debatable whether an accurate determination of brain angiotensin receptor binding kinetics has yet been made. The quandary facing the investigator of brain angiotensin receptors is the need to protect the radioligand from metabolic alteration while maintaining the characteristics of the receptors in situ. It is the tenet of this review that we have yet to fully understand the binding characteristics of brain angiotensin receptors and the extent of their distribution in the brain because of our inability to fully protect the angiotensins from metabolic alteration until equilibrium binding conditions can be attained.
Keywords: Angiotensin receptors, Metabolism, Radioligand binding assays, Angiotensinases, Peptidases
1. Introduction
The first definitive demonstration of an effect of angiotensin II (Ang II) in the brain was the cross perfusion study of Bickerton and Buckley [1]. However, this effect was produced by blood-borne Ang II later recognized to be mediated by the circumventricular organs of the brain which are outside of the blood–brain-barrier. A subsequent casual observation by Booth [2] revealed that Ang II acts within the brain which initiated an interest in the possible existence of a brain renin–angiotensin system (RAS). A few years later, Ganten et al. [3] reported the existence of renin-like activity in the brain and research on the brain RAS began in earnest.
1.1. Roles of the brain renin–angiotensin system
The influence of the brain RAS on hydromineral balance, arterial blood pressure, neurosecretory functions and overall body homeostasis is well documented. There are several excellent review articles covering early studies of the brain RAS [4], [5], [6].
While these ‘traditional’ functions of angiotensins in the brain have been established for some time, the recognition of the presence of multiple receptor subtypes for angiotensins in the brain as well as novel functions for metabolites of the octa- and heptapeptide angiotensins has greatly extended the range of activities attributed to the brain RAS. More recent reviews cover these aspects of the brain RAS [7], [8], [9], [10], [11], [12].
1.2. Generation and degradation of angiotensin peptides in the brain
The rate of synthesis as well as the rate of degradation of neurotransmitters and neuromodulators is an important factor in initiating and terminating their biological effects. This principle applies to peptide hormones as well. They are regulated by proteases (otherwise called peptidases, proteinases or proteolytic enzymes) that generate and metabolize them [13], [14], [15]. The human genome encodes several hundred proteases, of which the function of many has not yet been determined. Peptidases involved in processing of angiotensin peptides have been collectively termed “angiotensinases”. While the term has largely been used to infer degradation, it is now know that these enzymes can generate active angiotensins. Angiotensinases are comprised of three groups of peptidases: amino-, endo- and carboxypeptidases. Aminopeptidases have traditionally been viewed as the most important group, accounting for 60–90% of angiotensinase activity in various tissues [16] however, it is now known that angiotensin peptides are processed by a broad variety of peptidases.
2. Angiotensin-forming enzymes
The formation of the primary active angiotensin, Ang II is considered to occur via a cascade of enzymatic reactions, starting with a large protein precursor. The fact that the enzyme renin was the first component of the RAS to be discovered has led to the naming of this hormonal system as the renin–angiotensin system. However, as will be seen, renin is certainly not the only angiotensin-forming enzyme. However, out of respect for the discovery of renin by Tigerstedt and Bergstrom [17], use of the term renin–angiotensin system likely will continue. In Section 2 the primary focus will be on formation of Ang II and Ang III from angiotensinogen and Ang I, as they are the angiotensins that act on the classical AT1 and AT2 receptors. The formation of the other active angiotensins from Ang I, Ang II and Ang III, which act on receptors other than AT1 and AT2, are pirmarily addressed in Section 3.
2.1. Renin
The only known substrate for renin is angiotensinogen, which is the only known precursor for the active octapeptide angiotensin II (Ang II). In the brain, the primary source of angiotensinogen is astroglia which are reported to constitutively secrete it into the extracellular fluid of the brain [18]. By the classical pathway of the RAS it is cleaved by renin (EC 3.4.23.15) between Leu10–Leu11 residues to yield angiotensin I (Ang I) (Fig. 1 ) [6], [19], [20]. Renin is an aspartyl peptidase of the A1 family. In the brain of rodents and humans renin is expressed in two forms: one is that secreted either as active renin from the secretory/storage granules, or is formed extracellularly from secreted, inactive prorenin. The other form is a more recently described non-secreted, brain-specific intracellular renin, which in comparison with secreted renin is expressed in much higher amounts in the brain tissue [21], [22], [23], [24], [25], [26].
Fig. 1.
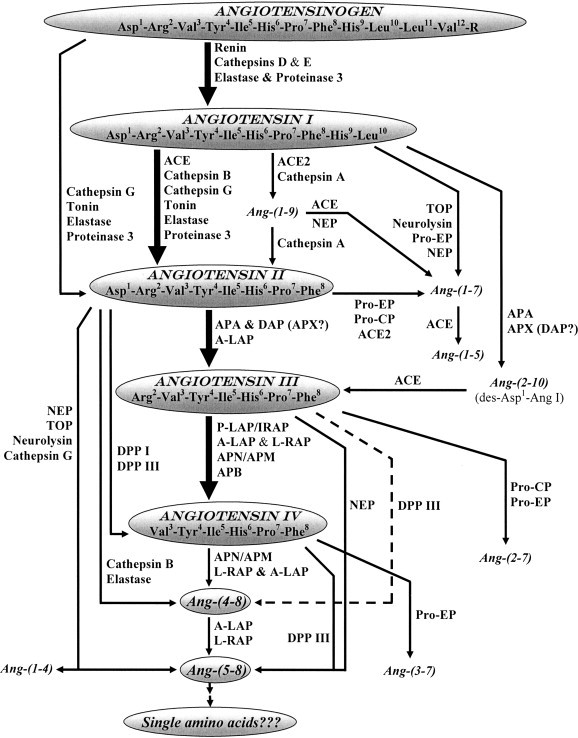
Pathways of formation of angiotensin peptides in the brain. Abbreviations used: ACE — angiotensin converting enzyme; ACE2 — human homolog of angiotensin converting enzyme; APA — aminopeptidase A; A-LAP — adipocyte derived leucine-aminopeptidase; L-RAP — leukocyte-derived arginine aminopeptidase; NEP — neutral endopeptidase; TOP — thimet endopeptidase; Pro-EP — prolyl-endopeptidase; Pro-CP — prolyl-carboxypeptidase; APX — aminopeptidase X; DAP — aspartyl aminopeptidase; P-LAP/IRAP — placental leucine-aminopeptidase/insulin-regulated aminopeptidase; APN/APM — aminopeptidase N/M; APB — aminopeptidase B; DPP I — dipeptidyl peptidase I; DPP III — dipeptidyl peptidase III. Numbering of amino acid residues in all fragments is based on the numbering in angiotensinogen. Larger sized arrows indicate the “classical” metabolic pathways for angiotensin peptides.
2.2. Renin-like enzymes
Several other peptidases, in addition to renin, are capable of forming Ang I from angiotensinogen. Among acid proteases in the brain cathepsins E (EC 3.4.23.34) and D (EC 3.4.23.5), aspartic proteinases belonging to the A1 family, can form Ang I from angiotensinogen substrate [27], [28], [29], [30] (Fig. 1). Cathepsin D is a typical lysosomal enzyme, whereas cathepsin E has been found in the endoplasmic reticulum, in endosomes and other cell compartments. Both enzymes are involved in the processing of various peptide precursors [29].
Two neutral peptidases are also capable of generating Ang I from angiotensinogen in the brain: elastase and proteinase 3 [7], [31], [32] (Fig. 1). Furthermore, two other neutral peptidases — cathepsin G (EC 3.4.21.20) and tonin, together with elastase and proteinase 3, can form Ang II directly from angiotensinogen, without Ang I production as an intermediate, by cleaving the peptide bond between the Phe8–His9 residues [32], [33], [34], [35], [36], [37] (Fig. 1). Cathepsin G, tonin, elastase and proteinase 3 are neutral serine proteinases (S1 family). They are localized mainly to endosomes and lysosomes, and are widely expressed in different mammalian tissues including the brain [31], [34], [35], [36]. They are an essential part of the cell proteolytic machinery with a still growing list of known physiological substrates, and have a role in many biological processes.
2.3. Angiotensin-converting enzyme (ACE)
In the classical RAS cascade, the decapeptide Ang I is converted to Ang II by angiotensin-converting enzyme (ACE, kininase II, EC 3.4.15.1). ACE is one of the most extensively studied mammalian peptidases. It exists in soluble and membrane-bound forms, and is a member of the M2 family of zinc metallopeptidases. ACE possesses dipeptidyl carboxypeptidase activity and can selectively convert Ang I to Ang II, des-Asp-angiotensin I to Ang III, Ang 1–7 to Ang 1–5, and bradykinin to its inactive metabolite, in addition to metabolizing several other peptides [15], [38], [39], [40], [41], [42]. However, ACE does not metabolize Ang II, presumably due to its inability to metabolize the His6–Pro7 bond [43]. The soluble form of ACE is distributed extracellularly while the membrane-bound form is on the external face of the plasma membrane. Thus the formation of Ang II from Ang I by ACE is thought to occur extracellularly only [44].
2.4. Other carboxypeptidases
Two other peptidases — cathepsin A (carboxypeptidase A, lysosomal protective protein, deamidase, EC 3.4.16.5) and human homologue of ACE (ACE2, ACEH) can remove His10 from Ang I, generating Ang-(1–9) [7], [43], [45], [46]. Ang-(1–9) can be further metabolized to Ang II by cathepsin A [7], [45] (Fig. 1). Cathepsin A is a multifunctional lysosomal, acidic, serine carboxypeptidase (S10 family) that also functions as a protective and an activator protein for neuraminidase and beta-galactosidase [47], [48], [49]. Cathepsin A also acts as an esterase/C-terminal deamidase at neutral pH and both carboxypeptidase and esterase/C-terminal deamidase functions are fully separated from its protective function [50]. ACE2, like ACE, is a member of the M2 family of zinc metallopeptidases, existing in a membrane-bound form and widely expressed in a variety of mammalian tissues including the brain [15], [41], [51]. In contrast to ACE, ACE2 cleaves only a single C-terminal residue from its peptide substrates including Ang I and Ang II, but not Ang-(1–9) and Ang-(1–7) [43], [46]. Another differentiating feature of ACE2 from ACE is that it is unable to cleave bradykinin and Hip–His–Leu, and is insensitive to ACE inhibitors, e.g., lisinopril and captopril [15], [41]. In addition to its role as a carboxypeptidase, ACE2 is also the receptor for severe acute respiratory syndrome (SARS) coronavirus [52], [53].
Elastase, proteinase 3, tonin, cathepsin B and G can also cleave the carboxy terminal His–Leu from Ang I to form Ang II [32], [36], [54] (Fig. 1) in a manner similar to the well-characterized formation of Ang II by ACE. Cathepsin B is a member of cysteine cathepsins (C1 family). It is a ubiquitous mammalian lysosomal peptidase, expressed in brain, that functions to convert several peptide precursors into active peptides [54], [55].
Chymase is another enzyme capable of forming Ang II from Ang I [56]. This enzyme is largely associated with mast cells, and aside from the pineal gland and the pituitary, there is little chymase activity in the brain [57].
2.5. Aminopeptidases
Aminopeptidases have traditionally been thought to be inactivators of Ang II, with the exception of aminopeptidase A-mediated formation of the generally short-lived heptapeptide, Ang III [58], [59], [60]. The des–Asp1, des–Arg2 Ang II hexapeptide (Ang IV) has little activity at the classical Ang II receptor mediating the pressor, dipsogenic, salt appetite-inducing and hormone-releasing effects of Ang II and thus was considered to be a weak agonist at best. However, the discovery of high affinity binding sites for Ang IV in the brain [61] and the reported memory enhancing effects of Ang IV [62], yet another active angiotensin was discovered. Thus aminopeptidases are newly cast as angiotensin-synthesizing enzymes in addition to their continuing role as angiotensin inactivators. Indeed, it has been suggested that aminopeptidase A activity is essential for formation of an active angiotensin peptide in the brain [63]. Although recent studies from our laboratory have challenged that hypothesis [64], [65].
2.5.1. Aminopeptidase A (APA)
One of the most known and accepted peptidases responsible for further processing of Ang II is APA (glutamyl aminopeptidase, EC 3.4.11.7), which cleaves Asp1 from Ang II, generating the heptapeptide Ang III [58], [59], [60]. Ang III can also be generated from Ang I without Ang II production as an intermediate through formation of des–Asp1–Ang I (Ang 2–10) by APA and aminopeptidase X (see also Section 2.5.3), and subsequent carboxypeptidase cleavage of Leu–His by ACE [60], [66], [67] (Fig. 1). APA is a widely expressed mammalian membrane-bound aminopeptidase, a member of the M1 family of zinc metallopeptidases [14], [68]. It is abundantly expressed in the brain [69]. The catalytic domain of the peptidase is in the ectodomain of the protein thus it is positioned to metabolize peptides that are in the extracellular milieu. It specifically cleaves acidic residues (aspartic acid or glutamic acid) from the N-terminus of peptide substrates, predominantly from Ang I, Ang II and cholecystokinin-8 [14], [60], [68], [70].
2.5.2. Oxytocinase subfamily
Another aminopeptidase that can cleave Asp1 from Ang II is known as adipocyte-derived leucine aminopeptidase (A-LAP, puromycin insensitive leucyl-specific aminopeptidase, PILSAP) [71], [72]. Moreover, it can sequentially cleave N-terminal amino acids from generated angiotensins up to His–Pro–Phe [72], [73] (Fig. 1). A-LAP is a member of the oxytocinase subfamily of M1 aminopeptidases, found in soluble and membrane-bound forms. It is extensively expressed in the brain and many other tissues [71], [72]. Together with other members of this subfamily: placental leucine aminopeptidase (P-LAP, cysteinyl aminopeptidase, EC 3.4.11.3, EP 11.3), also known as insulin-regulated aminopeptidase (IRAP) and the Ang IV receptor [74], and leukocyte-derived arginine aminopeptidase (L-RAP), it plays an important role in the maintenance of homeostasis during pregnancy, memory retention, blood pressure regulation and antigen presentation [71], [72].
2.5.3. Aspartyl aminopeptidase (DAP)/aminopeptidase X (APX)
Aminopeptidase X (amastatin-, bestatin- and EDTA-insensitive aminopeptidase) activity described by Sim and co-workers [66], [75] can cleave Asp1 from Ang I and Ang II forming the 2–10 nonapeptide fragment of Ang I and Ang III, respectively. This enzyme is likely the same as aspartyl aminopeptidase (DAP, EC 3.4.11.21) first described by Kelly et al. [76] and later characterized by Wilk et al. [67]. It is a member of the M18 family of metalloproteinases. This DTT and o-phenanthroline sensitive, but amastatin, bestatin, relatively EDTA, insensitive enzyme was capable of efficiently metabolizing Ang II to Ang III [67]. As noted above, des–Asp1–Ang I can be processed to Ang III by ACE [38], [60] (Fig. 1).
3. Angiotensin degradation
As noted above, the line between angiotensin synthesis and degradation has blurred due to the discovery of physiological actions and receptors for angiotensin peptides previously considered to be inactive metabolites. However, when considering the primary actions of the RAS, in particular, effects at the AT1 receptor subtype which mediates nearly all of the physiological and pathophysiological effects of Ang II, anything less than the carboxy terminal heptapeptide is considered to be an inactive metabolite.
3.1. Aminopeptidases
Historically aminopeptidases have been viewed as the primary metabolic enzymes for degradation of Ang II [16]. However, it is recognized now that the effects of peptidases are more complex.
3.1.1. Aminopeptidase A (APA) and aspartyl aminopeptidase (DAP)/aminopeptidase X (APX)
APA and DAP have a strong preference for acidic amino acids and are considered to be the primary enzymes responsible for the degradation of Ang II to Ang III. However, since Ang III is thought to be a fully active agonist at AT1 and AT2 receptors, this metabolic step can hardly be considered to be a degradation. On the other hand, Ang III appears to be highly labile and short-lived [77], [78] so the process of formation of Ang III could be considered to be a prelude to inactivation of the peptide, at least as an AT1/AT2 agonist. Of interest is the observation that Ang IV binds to a soluble form of human APA, but is very slowly metabolized by it, thereby acting as a competitive inhibitor [79]. These investigators also reported a small amount of Ang III metabolism to Ang IV by this soluble APA. In addition to its ability to form Ang III from Ang II, the reported ability of APX (likely DAP) to form des Asp1 Ang I from Ang I has been suggested to result in the formation of a physiological antagonist of the AT1 angiotensin receptor subtype [80]. An interesting inference of this observation is that ACE inhibitors could then be prolonging the lifespan of this endogenous AT1 receptor antagonist.
3.1.2. Oxytocinase subfamily
A-LAP (described in Section 2.5.2) is capable of cleaving N-terminal amino acids starting from Ang II up to tripeptide His–Pro–Phe [72], [73]. Thus it is capable of metabolizing angiotensins to completely inactive peptides (Fig. 1). Interestingly, the closely related P-LAP/IRAP and L-RAP are not able to metabolize Ang II [72], [74], [81], [82]. P-LAP/IRAP is reported to metabolize Ang III to IV, whereas L-RAP has ability to metabolize Ang III down to the tripeptide His–Pro–Phe by sequential elimination of N-terminal amino acids [72], [82].
3.1.3. Aminopeptidase N (APN) and aminopeptidase B (APB)
APN (E.C. 3.4.11.2, aminopeptidase M, alanyl aminopeptidase, CD13) and APB (E.C. 3.4.11.6, arginyl aminopeptidase) have also generally been considered to be inactivating enzymes because they form an angiotensin fragment that has negligible affinity for the classical Ang II receptors. In cleaving Arg1 from Ang III they form des–Asp1, des–Arg2 Ang II (Ang IV) [14], [83], [84], which is predominantly involved in learning and memory functions mediated mainly by Ang IV receptors [62]. In addition to its ability to cleave Arg1 from Ang III to form Ang IV, APN can also cleave Val1 from Ang IV to form Ang-(4–8) (Fig. 1), a putatively inactive angiotensin fragment [85]. APN and APB are members of the M1 family of Zn-metallopeptidases. APN is predominantly characterized as a membrane-bound peptidase with its catalytic site in its extracellular domain [14], [84]. APB is both a secreted and membrane-bound protease [86]. Both APN and APB are ubiquitously distributed in different tissues of mammalian organisms, including brain. APN metabolizes regulatory peptides by removing the N-terminal amino acid with a preference towards, neutral residues. APB demonstrates strict specificity for Arg and/or Lys residues at the N terminus of various peptides. This suggests a dominant role of APB, rather than APN, in the processing of Ang III [14], [84], [86], [87]. However it has been suggested that APN is the predominant enzyme responsible for brain Ang III metabolism in the mouse [88] and rat [89] brain. Besides having peptidase activity, APN has also been shown to be a receptor of coronaviruses, a human herpesvirus and Bacillus thuringiensis CryIA(c) toxin in their target tissues [14], [84].
3.1.4. Diaminopeptidases
In addition to APA, DAP and A-LAP, dipeptidyl peptidase I (DPP I, EC 3.4.14.1, previously called cathepsin C or J) and dipeptidyl peptidase III (DPP III, EC 3.4.14.4) can also be responsible for N-terminal amino acid cleavage of Ang II. These aminopeptidases are responsible for processing of various proteins and peptides, including angiotensins [90], [91] They remove the Asp1–Arg2 dipeptide from Ang II forming Ang IV [45], [90], [91], [92] (Fig. 1).
Diaminopeptidases are also reported to cleave Val–Tyr from Ang IV to form the putatively inactive Ang-(5–8) fragment [85], [90]. DPP I and DPP III were first characterized as acidic lysosomal and basic cytosolic cysteine peptidases respectively, however, their membrane-associated forms were also described [91]. As shown in Table 1 , DPP III possesses substantially higher affinity for Ang II than does APA [90]. The affinity of DPP III for Ang III analogs is similar to the affinity of APN for Ang III leading to the suggestion that DPP III also metabolizes Ang III to the inactive Ang-(4–8) fragment [90] (Table 1).
Table 1.
Affinities of some aminopeptidases for angiotensin peptides
| Ang II | Ang III | Ang I | |
|---|---|---|---|
| APA | Ki = 15 μM, Km = 0.13 mM [171];Ki = 24 μM [172];Km = 35.3 μM [59] | No complete inhibition [171] | No complete inhibition [171] |
| APM/N | No complete inhibition [171];no degradation [173] | Ki = 3 μM, Km = 0.24 mM [171];Ki = 0.34 μM, Km = 2 μM [173] | No complete inhibition [171] |
| DPP III | Ki = 0.34 μM [90] | Kia = 0.3 μM [90] |
Analogs of Ang III.
3.2. Endopeptidases
Several endopeptidases metabolize Ang II in the brain. Neprilysin, thimet oligopeptidase, and neutral endopeptidase cleave the Tyr4–Ile5 bond forming two tetrapeptides [93], [94], [95] (Fig. 1). Prolyl endopeptidase can also metabolize Ang II by cleaving the C-terminal Phe8 from Ang II to form Ang 1–7 [7], [96], [97] (Fig. 1). Moreover, all 4 of these endopeptidases are able to cleave Ang I at the Pro7–Phe8 bond to form Ang-(1–7) [45], [95], [98], [99], [100] (Fig. 1). Up until the observation was made that Ang-(1–7) could stimulate vasopressin release from hypothalamic explants [101] the removal of phe8 from Ang II was thought to be a metabolic inactivation of the peptide. However, Ang-(1–7) is widely recognized as having activity on its own right [102], with its own receptor [103] which may play a role in hippocampal plasticity [104]. Thus the formation of Ang-(1–7) by prolyl carboxypeptidase must be viewed as an enzymatic step leading to the formation of an active angiotensin.
3.2.1. Neprilysin
Neutral endopeptidase (EC 3.4.24.11, EP 24.11, neprilysin, NEP) is a member of the membrane-bound, M13 family of zinc-dependent metalloproteases, that cleaves peptide bonds on the amino acid side of hydrophobic amino acid residues [105], [106]. It is constitutively expressed in several tissues including the brain and kidney, but is developmentally regulated in other cell types (e.g. lymphocytes). It terminates the activity of peptides involved in cardiovascular regulation, inflammatory phenomena, and is critical for synaptic neuropeptide metabolism. Neutral endopeptidase has been called the “cholinesterase” of peptidergic synapses by some authors [105], [106]. Of note neutral endopeptidase is reported to cleave Ang-(1–9) to Ang-(1–7), Ang III to Ang-(5–8), and Ang-(1–7) to Ang-(1–4) [43], [107], [108].
3.2.2. Thimet oligopeptidase and Neurolysin
Thimet oligopeptidase (EC 3.4.24.15, EP 24.15, thimet endopeptidase, Pz-peptidase, endo-oligopeptidase A) and neurolysin (EC 3.4.24.16, EP 24.16, “neurotensin-degrading enzyme”) are members of the M3 family of Zn-dependent metalloendopeptidases ubiquitously distributed in the central nervous system and in peripheral organs of mammals [109], [110]. Initially, both proteases were considered to be soluble enzymes, of which thimet oligopeptidase predominantly nuclear, while neurolysin was cytosolic. Later it was demonstrated that in rat brain 20–30% of thimet oligopeptidase activity is associated with membranes, including plasma membranes, endosomes and synaptic vesicles [94], [111], [112], [113], [114]. Moreover, Shivakumar et al. [95] recently showed that thimet oligopeptidase is associated with AT1 and B2 receptors in kidney cells, both at the plasma membrane and after receptor internalization, suggesting a possible mechanism for endosomal disposition of ligand that could facilitate receptor recycling. In the case of neurolysin, it has now been shown that the enzyme is mainly cytosolic in astrocytes, but is largely membrane-associated in neurons [115].
3.2.3. Prolyl endopeptidase
Despite being an endopeptidase, prolyl endopeptidase (3.4.21.26, prolyl oligopeptidase, post-proline cleaving enzyme), a member of the prolyl peptidase subfamily of serine proteases, can cleave Pro–Xaa peptide bonds (where Xaa is any amino acid) even when the Xaa amino acid is the omega amino acid of the peptide [116], [117]. As such, it also has the ability to form Ang-(1–7) from both Ang II and Ang I, Ang-(2–7) from Ang III, and Ang-(3–7) from Ang IV [7], [45], [96], [97], [118], [119]. Prolyl endopeptidase is an intracellular enzyme responsible for degradation of several peptide hormones and neuropeptides, which is highly conserved in mammals and is one of the most abundantly expressed brain peptidases [116], [117], [120], [121].
3.2.4. Other endopeptidases
Cathepsin B and elastase (described above for their ability to form Ang I from angiotensinogen) have also been shown to be able to cleave the Val3–Tyr4 bond of Ang II, whereas cathepsin G cleaves the Tyr4–Ile5 bond [32], [54] (Fig. 1). The various fragments formed by these endopeptidases are virtually devoid of biological activity.
3.3. Carboxypeptidases
3.3.1. ACE2 and ACE
As noted above, ACE2 can cleave Leu10 from Ang I, and Phe8 from Ang II, to make Ang-(1–9) and Ang-(1–7) respectively, and thus is viewed primarily as an angiotensin-forming enzyme [43], [46]. Interestingly the latter two peptides seem to not be metabolized by ACE2. Of note, Ang-(1–7) is a substrate for ACE, APN and DPPIII [85], [90], whereas Ang-(1–9) is metabolized to Ang II by cathepsin A (described above in Section 2.4.) [7], [45] (Fig. 1).
3.3.2. Prolyl carboxypeptidase
Prolyl carboxypeptidase (EC 3.4.16.2, angiotensinase C, peptidyl prolylamino acid hydrolase) is another carboxypeptidase capable of cleaving Ang II and Ang III at the Pro–Phe bond, to yield Ang-(l–7) and Ang-(2–7), respectively [45], [120], [122], [123], [124], [125] (Fig. 1). It exists as membrane-associated and extracellular forms, that remove the omega amino acid from peptides when the penultimate amino acid is a proline [116], [117]. It has an acidic pH optimum (pH = 5.0) when hydrolyzing short synthetic peptide substrates, but retains significant activity in the neutral range with longer, naturally occurring peptides (e.g. Ang II, Ang III, des–Arg9–bradykinin) [122], [124], [126].
4. Protection of radiolabeled angiotensins in binding assays
After the discovery of the brain RAS, binding sites for angiotensin peptides in the brain were extensively studied using receptor binding techniques. The presence of angiotensin binding sites in the brain has been demonstrated in a variety of species [127], [128], [129], [130], [131], [132], [133], [134], [135], [136] and subsequent in vitro autoradiographic studies have directly localized these binding sites to specific brain nuclei [137], [138], [139], [140], [141].
However, from the beginning all these studies faced a major pitfall — severe metabolic degradation of angiotensin peptides, which could and did negatively influence correct generation and interpretation of binding experiments. There were two major problems: 1) impossibility to reach steady-state conditions — the time course for binding of radiolabeled angiotensins has a bell shape (Fig. 2 ), and, 2) identification of different angiotensin fragments, along with intact ligands, bound specifically [133], [134], [142], [143]. There have been three types of attempts to solve these problems: addition of protease inhibitors to the incubation medium, use of purified plasma membranes and development of peptidase-resistant analogs of angiotensins.
Fig. 2.
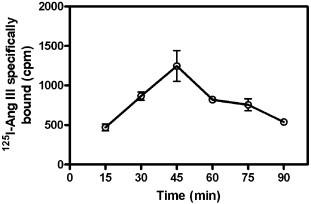
Time course of specific binding of 125I–Ang III to a rat brain membrane preparation. Rat brain membranes (50 mg initial wet weight/ml) were prepared as described previously [169]. The incubation medium contained standard assay buffer 150 mM NaCl, 5 mM EDTA, 0.1 mM bacitracin, and 50 mM NaPO4, pH 7.1–2, plus the following peptidase inhibitors: o-phenanthroline (1 mM), puromycin (3 mM) phenylmethylsulfonyl fluoride (1 mM). A total of 2.5 mg initial wet weight of brain membranes was present in 100 μl for this assay which was carried out at 21–24 °C. Nonspecific binding was determined in the presence of 3 μM Ang II and subtracted from total binding to derive specific binding.
4.1. Peptidase inhibitors
Attempts to overcome the problem of metabolic degradation of angiotensin ligands during receptor binding studies have invariably included a variety of protease inhibitors. The most common of which include: sulfhydryl reagents (dithiothreitol, β-mercaptoethanol), chelating agents (EDTA, EGTA, o-phenanthroline), pure protease inhibitors (leupeptin, pepstatin, bacitracin, amastatin, bestatin, PMSF), unrelated peptides (glucagon, insulin, bovine serum albumin) [128], [136], [143], [144]. Of note, addition of peptidase inhibitors does not always help to protect the ligand from degradation. One of the commonly used peptidase inhibitors in angiotensin receptor binding assays, bacitracin, is reported to activate aspartyl aminopeptidase (DAP) [67]. This would promote its conversion to Ang III, thus distorting the observations of binding kinetics. Moreover, dithiothreitol (DTT) and other disulfide reducing agents are capable of activating thimet oligopeptidase and DPPIII [90], [145], [146], [147].
4.2. Subcellular fractionation
Virtually all brain angiotensin receptor binding assays have used a membrane fraction. In most cases this involves mechanical and osmotic disruption of the cells, centrifugal precipitation of the membrane fraction, with the soluble/cytosolic enzymes as well as the microsomal membrane fractions being discarded in the supernatant. On occasion, synaptosomal preparations have been used for receptor binding assays [142], [148] to focus on receptors expressed on the extracellular side of the plasma membrane. However, additional steps must be taken to ensure that receptor–ligand internalization (receptor-mediated endocytosis) does not alter the binding kinetics and lead to subsequent degradation of receptor-associated angiotensin peptides [149].
4.3. Peptidase-resistant analogs
Initial studies of brain angiotensin receptors used 125I–Ang II. However, subsequent studies using angiotensin analogs modified in the 1 and 8 position gave much better results [140], [143], [144], [150], [151], [152]. The most common amino terminal substitution is sarcosine (N-methyl glycine) for aspartic acid. Because sarcosine is a secondary amine, it resists degradation by aminopeptidases. The lack of a side-chain on the alpha carbon also contributes to its metabolic stability since it is a poor substrate for the acid aminopeptidases (APA, DAP) primarily responsible for removal of Asp1 of Ang II [58], [59], [67]. The most common substitution to the carboxy terminal is the introduction of an alkyl side chain-containing amino acid, with isoleucine being the most often used amino acid. The substitution of the aromatic phenylalanine with an aliphatic amino acid converts the peptide to an antagonist [153], [154], although there are reports suggesting that some agonistic properties of the peptide are retained [155].
The advantage of using antagonistic angiotensin peptides is that the primary angiotensin receptor subtypes: AT1 and AT2 are G protein-coupled receptors, which show different agonist binding affinity states depending on the presence of GTP or GDP [156]. Of note, while the characteristic agonist affinity shift for AT1 receptors has been demonstrated [157], the AT2 receptor does not show this shift [158], except in the presence of dithiothreitol [159]. The AT2 receptor also appears not to internalize [159], [160]. However, it should be considered, that unnatural sequences in angiotensin peptide analogs may not fully represent agonist binding to angiotensin receptors.
4.4. Shortcomings of angiotensin protection in receptor binding assays
Although all the above-mentioned approaches have had a positive impact in preserving angiotensin ligands from degradation, none of them has been completely successful and metabolic degradation still continued [143], [148], [161] (Fig. 3 ). Moreover, both Grover et al. [148] and Abhold et al. [143] suggested that binding assays using angiotensin peptides should include a measurement of ligand metabolism, an identification of specifically bound radioactivity; and a correction of specific binding based on the amount of authentic radioligand bound.
Fig. 3.
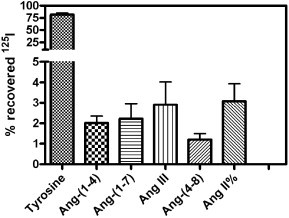
Metabolic fate of 125I–Ang II bound to a rat brain membrane preparation. Rat brain membranes were prepared as described previously [169]. The incubation medium contained 150 mM NaCl, 5 mM EDTA, 0.1 mM bacitracin, and 50 mM NaPO4, pH 7.1–2. A total of 12.5 mg initial wet weight of brain membranes was present in 500 μl for this assay, which was carried out for 60 min at 21–24 °C. After one hour incubation the membrane suspension was centrifuged and the supernatant discarded. The pellet was resuspended in HPLC mobile phase: 21% acetonitrile: 79% triethylamine phosphate (83 mM phosphate, pH 3.0), periodically vortexed during 20 min and recentrifuged. The supernatant was fitered through a 0.22 µm filter, applied to Sep-Pak® C18 (Waters Inc.) column and eluted with 21% acetonitrile: 79% triethylamine phosphate (83 mM phosphate, pH 3.0). The eluate was run on a reverse-phase (C18) column with a mobile phase of either 13% acetonitrile: 87% triethylamine phosphate (83 mM phosphate, pH 3.0), to allow better resolution of smaller fragments, or 21% acetonitrile: 79% triethylamine phosphate (83 mM phosphate, pH 3.0) at a flow rate of 1.2 ml/min. Radiolabeled Ang II and fragments were identified based on the elution times of radioiodinated standards of Ang II and its fragments under the same HPLC conditions. 15 s fractions of the column eluate were collected and counted in a gamma counter.
However, it should be considered that, metabolism might occur post-binding, in which case radiolabeled metabolites may represent a portion of the specific binding of the radioligand. On the other hand, the suggested correction of the specific binding based on observed metabolism and identification of the specifically bound radioactivity is a technically challenging and cumbersome procedure. This might explain the paucity of reports that have addressed this problem, i.e. correction of the specific binding based on observed metabolism and identification of specifically bound radioactivity to fully determine the binding characteristics of angiotensin ligands in brain tissue.
It should also be noted that metabolic degradation can also affect the ability of non-radiolabeled angiotensin peptides (usually termed cold), used for estimation of the non-specific binding of a redioligand, to fully compete for specific binding sites. Determination of the affinity (expressed as IC50 or KI values) of these non-radiolabeled angiotensin analogs for binding to brain angiotensin receptors can also be differentially affected by their susceptibility to metabolism by brain peptidases.
The most disappointing outcome of the practice of preserving angiotensin ligands from metabolic degradation in receptor binding assays is the case of sulfhydryl reducing agents, in particular DTT. Pioneering studies of Glossmann et al. [127], and Bennett and Snyder [128] indicated that the addition of DTT was required for protection of the angiotensin radioligands from metabolic degradation. Later it was shown that DTT had the ability to increase the binding affinity for Ang II in brain with no change in the density of the binding sites [132], [136]. At that period nearly all angiotensin receptor binding studies conducted in brain were carried out in the presence of up to 5 mM DTT. Printz et al. [136] in their paper mention: “…In studies in our laboratories there is no question but that thiols are essential for optimum binding of Ang II by membranes isolated from brain and adrenal medulla…”
However, again starting from early studies, it was observed that Ang II binding sites in non-neuronal tissues such as vasculature [162], liver [163], and anterior pituitary [136] showed reduced Ang II binding in the presence of DTT. Consistent with this observation is the report by Ellis and Nuenke [145] that β-mercaptoethanol enhances the activity of pituitary DPP III, which converts Ang II to Ang IV. Moreover, activation of thimet oligopeptidase by DTT is also well documented [146], [147].
This mystery continued until the late 80's–early 90's. During this time, sulfhydryl reducing agents continued to be widely used in angiotensin receptor binding assays in neuronal but not peripheral tissues. The discovery of angiotensin receptor subtypes led to the revelation that sulfhydryl agents severely impair binding at AT1, but not at AT2 receptors [164], [165], [166], [167]. Recognizing the fact that sulfhydryl reducing agents impaired binding of angiotensin ligands to AT1 receptors, Speth and co-workers [166] in a paper showing sulfhydryl agent-sensitive and non-sensitive (AT1 and AT2 containing) nuclei distribution in the rat brain noted: “… An inescapable conclusion from these studies, however, is that the vast majority of Ang II receptor binding studies in brain homogenates, and a large number of in vitro receptor autoradiographic studies were carried out under conditions that should have effectively inhibited binding to the AIIα subtype (today known as AT1). Thus only a portion of receptors were characterized for responsivity to various conditions…”
5. Future perspectives
It is clear that the metabolic pathways for angiotensins are complex and that we still have much to learn about the metabolic fate of the various angiotensin peptides in the brain. The relative importance of many of the pathways is unknown due to uncertainties related to their cellular localization, affinity for angiotensin peptides, their catalytic capacity, and their expression levels in the brain. Other factors that can affect the metabolic fate of angiotensins include developmental differences in enzyme expression, the presence of activators and inhibitors of these enzymes, and the redox state of the brain.
It is also apparent that claims of specificity for various enzyme inhibitors must be considered in the light of the expanse of enzymes capable of metabolizing angiotensin peptides. Defining an enzyme inhibitor as a specific inhibitor when only 2 or 3 enzyme preparations are tested is inappropriate because it ignores the possibility that other, untested enzymes could be affected. For example, enzyme inhibitors that interact with sulfhydryl groups are likely to have widespread effects on a large number of proteins, and not just enzymes, as has been noted with the AT1 receptor.
Another interesting development has been the observation that different angiotensin peptides can have opposing actions, e.g., the Ang II counteracting effects of Ang-(1–7) [12], [102] and Ang-(2–10) [80], or complementary actions, e.g., the Ang II protecting effects of Ang IV by virtue of its ability to inhibit aminopeptidases [79]. It may be that other angiotensin fragments are also capable of indirectly interacting with the metabolic processes of angiotensin peptides.
Despite more than 30 years of research on brain angiotensin receptors, the problem of metabolic degradation of angiotensin ligands in brain Ang II receptor binding studies remains unsolved. None of the reported binding studies for angiotensin receptors are immune to the challenge that; 1) the radioligand did not remain intact throughout the incubation period, 2) the bound radioligand may not be representative of the binding of the initial radioligand or 3) the conditions under which the angiotensin peptide was protected from metabolism compromised the ability of the angiotensin receptors to bind angiotensins.
Correct characterization of the receptor binding kinetics of angiotensin peptides can be carried out only in conditions where peptide ligands as well as the receptors are preserved from metabolic degradation, at least until steady state conditions can be attained. However, at the same time, one needs to insure that the ability of angiotensin receptors to interact with ligands will not be impaired by procedures designed to protect ligand integrity.
Continuing studies of brain angiotensin receptors in our laboratory have focused on resolving this problem and are beginning to show some success[168]. However they have also provided some unanticipated results: in the presence of p-chloromurcuribenzoic acid (PCMB), a novel, non-AT1, non-AT2 binding site for angiotensin peptides can be observed in the brain[169]. However, PCMB, like DTT and β-mercaptoethanol inhibits Ang II binding to AT1 receptors, presumably by altering critical cysteine residues in the receptor. Thus it is necessary to continue to be vigilant to alterations in angiotensin receptor behavior while developing effective inhibitors of the peptidases that metabolize angiotensins.
Acknowledgments
Vardan T. Karamyan is supported by the Peptide Radioiodination Service Center at the University of Mississippi. In preparing this manuscript the authors frequently visited the web site of the Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB) http://www.chem.qmul.ac.uk/iubmb/enzyme/ and the web site of MEROPS peptidase database http://merops.sanger.ac.uk/ for background information [170].
References
- 1.Bickerton R.K., Buckley J.P. Evidence for a central mechanism in angiotensin induced hypertension. Proc Soc Exp Biol Med. 1961;106:834–836. [Google Scholar]
- 2.Booth D.A. Mechanism of action of norepinephrine in eliciting an eating response on injection into the rat hypothalamus. J Pharmacol Exp Ther. 1968;160:336–348. [PubMed] [Google Scholar]
- 3.Ganten D., Boucher R., Genest J. Renin activity in brain tissue of puppies and adult dogs. Brain Res. 1971;33:557–559. doi: 10.1016/0006-8993(71)90137-5. [DOI] [PubMed] [Google Scholar]
- 4.Severs W.B., Daniels-Severs A.E. Effects of angiotensin on the central nervous system. Pharmacol Rev. 1973;25:415–449. [PubMed] [Google Scholar]
- 5.Phillips M.I. Angiotensin in the brain. Neuroendocrinology. 1978;25:354–377. doi: 10.1159/000122756. [DOI] [PubMed] [Google Scholar]
- 6.Unger T., Badoer E., Ganten D., Lang R.E., Rettig R. Brain angiotensin: pathways and pharmacology. Circulation. 1988;77:I40–I54. [PubMed] [Google Scholar]
- 7.Saavedra J.M. Brain and pituitary angiotensin. Endocr Rev. 1992;13:329–380. doi: 10.1210/edrv-13-2-329. [DOI] [PubMed] [Google Scholar]
- 8.Saavedra J.M. Brain angiotensin II: new developments, unanswered questions and therapeutic opportunities. Cell Mol Neurobiol. 2005;25:485–512. doi: 10.1007/s10571-005-4011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright J.W., Harding J.W. Brain angiotensin receptor subtypes in the control of physiological and behavioral responses. Neurosci Biobehav Rev. 1994;18:21–53. doi: 10.1016/0149-7634(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 10.Fitzsimons J.T. Angiotensin, thirst, and sodium appetite. Physiol Rev. 1998;78:583–686. doi: 10.1152/physrev.1998.78.3.583. [DOI] [PubMed] [Google Scholar]
- 11.Phillips M.I., Sumners C. Angiotensin II in central nervous system physiology. Regul Pept. 1998;78:1–11. doi: 10.1016/s0167-0115(98)00122-0. [DOI] [PubMed] [Google Scholar]
- 12.Speth R.C., Brown T.E., Barnes R.D., Wright J.W. Brain angiotensinergic activity: the state of our current knowledge. Proc West Pharmacol Soc. 2003;46:11–15. [PubMed] [Google Scholar]
- 13.Hooper N.M. Proteases: a primer. Essays Biochem. 2002;38:1–8. doi: 10.1042/bse0380001. [DOI] [PubMed] [Google Scholar]
- 14.Albiston A.L., Ye S., Chai S.Y. Membrane bound members of the M1 family: more than aminopeptidases. Protein Pept Lett. 2004;11:491–500. doi: 10.2174/0929866043406643. [DOI] [PubMed] [Google Scholar]
- 15.Lew R.A. The zinc metallopeptidase family: new faces, new functions. Protein Pept Lett. 2004;11:407–414. doi: 10.2174/0929866043406481. [DOI] [PubMed] [Google Scholar]
- 16.Regoli D., Riniker B., Brunner H. The enzymatic degradation of various angiotensin II derivatives by serum, plasma or kidney homogenate. Biochem Pharmacol. 1963;12:637–646. doi: 10.1016/0006-2952(63)90039-x. [DOI] [PubMed] [Google Scholar]
- 17.Tigerstedt R., Bergmann P.G. Niere und kreislauf. Scand Arch Physiol. 1898;8:223–271. [Google Scholar]
- 18.Sernia C. Location and secretion of brain angiotensinogen. Regul Pept. 1995;57:1–18. doi: 10.1016/0167-0115(95)00015-4. [DOI] [PubMed] [Google Scholar]
- 19.Skeggs L.T., Jr., Kahn J.R., Lentz K., Shumway N.P. The preparation, purification, and amino acid sequence of a polypeptide renin substrate. J Exp Med. 1957;106:439–453. doi: 10.1084/jem.106.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peach M.J. Renin–angiotensin system: biochemistry and mechanisms of action. Physiol Rev. 1977;57:313–370. doi: 10.1152/physrev.1977.57.2.313. [DOI] [PubMed] [Google Scholar]
- 21.Dzau V.J., Brenner A., Emmett N.L. Evidence for renin in rat brain: differentiation from other reninlike enzymes. Am J Physiol. 1982;242:E292–E297. doi: 10.1152/ajpendo.1982.242.5.E292. [DOI] [PubMed] [Google Scholar]
- 22.Morris B.J. Molecular biology of renin. I: Gene and protein structure, synthesis and processing. J Hypertens. 1992;10:209–214. doi: 10.1097/00004872-199203000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Lee-Kirsch M.A., Gaudet F., Cardoso M.C., Lindpaintner K. Distinct renin isoforms generated by tissue-specific transcription initiation and alternative splicing. Circ Res. 1999;84:240–246. doi: 10.1161/01.res.84.2.240. [DOI] [PubMed] [Google Scholar]
- 24.Sinn P.L., Sigmund C.D. Identification of three human renin mRNA isoforms from alternative tissue-specific transcriptional initiation. Physiol Genomics. 2000;3:25–31. doi: 10.1152/physiolgenomics.2000.3.1.25. [DOI] [PubMed] [Google Scholar]
- 25.Lavoie J.L., Cassell M.D., Gross K.W., Sigmund C.D. Adjacent expression of renin and angiotensinogen in the rostral ventrolateral medulla using a dual-reporter transgenic model. Hypertension. 2004;43:1116–1119. doi: 10.1161/01.HYP.0000125143.73301.94. [DOI] [PubMed] [Google Scholar]
- 26.Lavoie J.L., Liu X., Bianco R.A., Beltz T.G., Johnson A.K., Sigmund C.D. Evidence supporting a functional role for intracellular renin in the brain. Hypertension. 2006;47:461–466. doi: 10.1161/01.HYP.0000203308.52919.dc. [DOI] [PubMed] [Google Scholar]
- 27.Day RPReid IA Renin activity in dog brain: enzymological similarity to cathepsin D. Endocrinology. 1976;99:93–100. doi: 10.1210/endo-99-1-93. [DOI] [PubMed] [Google Scholar]
- 28.Hackenthal E., Hackenthal R., Hilgenfeldt U. Isorenin, pseudorenin, cathepsin D and renin. A comparative enzymatic study of angiotensin-forming enzymes. Biochim Biophys Acta. 1978;522:574–588. doi: 10.1016/0005-2744(78)90089-x. [DOI] [PubMed] [Google Scholar]
- 29.Kageyama T., Ichinose M., Yonezawa S. Processing of the precursors to neurotensin and other bioactive peptides by cathepsin E. J Biol Chem. 1995;270:19135–19140. doi: 10.1074/jbc.270.32.19135. [DOI] [PubMed] [Google Scholar]
- 30.Nakanishi H., Yamamoto K. Cathepsin E in the central nervous system. Adv Exp Med Biol. 1998;436:213–217. doi: 10.1007/978-1-4615-5373-1_30. [DOI] [PubMed] [Google Scholar]
- 31.Davies B.J., Pickard B.S., Steel M., Morris R.G., Lathe R. Serine proteases in rodent hippocampus. J Biol Chem. 1998;273:23004–23011. doi: 10.1074/jbc.273.36.23004. [DOI] [PubMed] [Google Scholar]
- 32.Ramaha A., Patston P.A. Release and degradation of angiotensin I and angiotensin II from angiotensinogen by neutrophil serine proteinases. Arch Biochem Biophys. 2002;397:77–83. doi: 10.1006/abbi.2001.2687. [DOI] [PubMed] [Google Scholar]
- 33.Grise C., Boucher R., Thibault G., Genest J. Formation of angiotensin II by tonin from partially purified human angiotensinogen. Can J Biochem. 1981;59:250–255. doi: 10.1139/o81-034. [DOI] [PubMed] [Google Scholar]
- 34.Abraham C.R., Kanemaru K., Mucke L. Expression of cathepsin G-like and alpha 1-antichymotrypsin-like proteins in reactive astrocytes. Brain Res. 1993;621:222–232. doi: 10.1016/0006-8993(93)90110-9. [DOI] [PubMed] [Google Scholar]
- 35.Savage M.J., Iqbal M., Loh T., Trusko S.P., Scott R., Siman R. Cathepsin G: localization in human cerebral cortex and generation of amyloidogenic fragments from the beta-amyloid precursor protein. Neuroscience. 1994;60:607–619. doi: 10.1016/0306-4522(94)90490-1. [DOI] [PubMed] [Google Scholar]
- 36.Lopes E.S., Sumitani M., Juliano L., Beraldo W.T., Pesquero J.L. Distribution of tonin- and kallikrein-like activities in rat brain. Brain Res. 1997;769:152–157. doi: 10.1016/s0006-8993(97)00785-3. [DOI] [PubMed] [Google Scholar]
- 37.Araujo R.C., Lima M.P., Lomez E.S., Bader M., Pesquero J.B., Sumitani M., Pesquero J.L. Tonin expression in the rat brain and tonin-mediated central production of angiotensin II. Physiol Behav. 2002;76:327–333. doi: 10.1016/s0031-9384(02)00720-5. [DOI] [PubMed] [Google Scholar]
- 38.Garcia D.R., Smellie W.S., Morton J.J. des–Asp–angiotensin I: its identification in rat blood and confirmation as a substrate for converting enzyme. Endocrinology. 1981;108:406–412. doi: 10.1210/endo-108-2-406. [DOI] [PubMed] [Google Scholar]
- 39.Chappell M.C., Pirro N.T., Sykes A., Ferrario C.M. Metabolism of angiotensin-(1–7) by angiotensin-converting enzyme. Hypertension. 1998;31:362–367. doi: 10.1161/01.hyp.31.1.362. [DOI] [PubMed] [Google Scholar]
- 40.Skidgel R.A., Erdos E.G. Angiotensin converting enzyme (ACE) and neprilysin hydrolyze neuropeptides: a brief history, the beginning and follow-ups to early studies. Peptides. 2004;25:521–525. doi: 10.1016/j.peptides.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 41.Guy J.L., Lambert D.W., Warner F.J., Hooper N.M., Turner A.J. Membrane-associated zinc peptidase families: comparing ACE and ACE2. Biochim Biophys Acta. 2005;1751:2–8. doi: 10.1016/j.bbapap.2004.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pagliaro PPenna C. Rethinking the renin–angiotensin system and its role in cardiovascular regulation. Cardiovasc Drugs Ther. 2005;19:77–87. doi: 10.1007/s10557-005-6900-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rice G.I., Thomas D.A., Grant P.J., Turner A.J., Hooper N.M. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J. 2004;383:45–51. doi: 10.1042/BJ20040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Corvol P., Michaud A., Soubrier F., Williams T.A. Recent advances in knowledge of the structure and function of the angiotensin I converting enzyme. J Hypertension. 1995;13:S3–S10. doi: 10.1097/00004872-199509003-00002. [DOI] [PubMed] [Google Scholar]
- 45.Erdos E.G., Skidgel R.A. Renal metabolism of angiotensin I and II. Kidney Inter Suppl. 1990;30:S24–S27. [PubMed] [Google Scholar]
- 46.Vickers C., Hales P., Kaushik V., Dick L., Gavin J., Tang J., Godbout K., Parsons T., Baronas E., Hsieh F., Acton S., Patane M., Nichols A., Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- 47.Sleat D.E., Sohar I., Lackland H., Majercak J., Lobel P. Rat brain contains high levels of mannose-6-phosphorylated glycoproteins including lysosomal enzymes and palmitoyl–protein thioesterase, an enzyme implicated in infantile neuronal lipofuscinosis. J Biol Chem. 1996;271:19191–19198. doi: 10.1074/jbc.271.32.19191. [DOI] [PubMed] [Google Scholar]
- 48.Hiraiwa M. Cathepsin A/protective protein: an unusual lysosomal multifunctional protein. Cell Mol Life Sci. 1999;56:894–907. doi: 10.1007/s000180050482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sohma O., Mizuguchi M., Takashima S., Satake A., Itoh K., Sakuraba H., Suzuki Y., Oyanagi K. Expression of protective protein in human tissue. Pediatr Neurol. 1999;20:210–214. doi: 10.1016/s0887-8994(98)00151-9. [DOI] [PubMed] [Google Scholar]
- 50.Galjart N.J., Morreau H., Willemsen R., Gillemans N., Bonten E.J., d'Azzo A. Human lysosomal protective protein has cathepsin A-like activity distinct from its protective function. J Biol Chem. 1991;266:14754–14762. [PubMed] [Google Scholar]
- 51.Tipnis S.R., Hooper N.M., Hyde R., Karran E., Christie G., Turner A.J. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 52.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turner A.J., Hiscox J.A., Hooper N.M. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol Sci. 2004;25:291–294. doi: 10.1016/j.tips.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Azaryan A., Barkhudaryan N., Galoyan A., Lajtha A. Action of brain cathepsin B, cathepsin D, and high-molecular-weight aspartic proteinase on angiotensins I and II. Neurochem Res. 1985;10:1525–1532. [PubMed] [Google Scholar]
- 55.Stoka V., Turk B., Turk V. Lysosomal cysteine proteases: structural features and their role in apoptosis. IUBMB Life. 2005;57:347–353. doi: 10.1080/15216540500154920. [DOI] [PubMed] [Google Scholar]
- 56.Bacani CFrishman W.H. Chymase: a new pharmacologic target in cardiovascular disease. Cardiol Rev. 2006;14:187–193. doi: 10.1097/01.crd.0000195220.62533.c5. [DOI] [PubMed] [Google Scholar]
- 57.Baltatu O., Nishimura H., Hoffmann S., Stoltenburg G., Haulica I.D., Lippoldt A., Ganten D., Urata H. High levels of human chymase expression in the pineal and pituitary glands. Brain Res. 1997;752:269–278. doi: 10.1016/s0006-8993(96)01474-6. [DOI] [PubMed] [Google Scholar]
- 58.Tonnaer J.A., Engels G.M., Wiegant V.M., Burbach J.P., De Jong W., De Wied D. Proteolytic conversion of angiotensins in rat brain tissue. Eur J Biochem. 1983;131:415–421. doi: 10.1111/j.1432-1033.1983.tb07279.x. [DOI] [PubMed] [Google Scholar]
- 59.Bausback H.H., Churchill L., Ward P.E. Angiotensin metabolism by cerebral microvascular aminopeptidase A. Biochem Pharmacol. 1988;37:155–160. doi: 10.1016/0006-2952(88)90712-5. [DOI] [PubMed] [Google Scholar]
- 60.Hermann K., Phillips M.I., Raizada M.K. Metabolism of angiotensin peptides by neuronal and glial cultures from rat brain. J Neurochem. 1989;52:863–868. doi: 10.1111/j.1471-4159.1989.tb02534.x. [DOI] [PubMed] [Google Scholar]
- 61.Swanson G.N., Hanesworth J.M., Sardinia M.F., Coleman J.K., Wright J.W., Hall K.L., Miller-Wing A.V., Stobb J.W., Cook V.I., Harding E.C. Discovery of a distinct binding site for angiotensin II (3–8), a putative angiotensin IV receptor. Regul Pept. 1992;40:409–419. doi: 10.1016/0167-0115(92)90527-2. [DOI] [PubMed] [Google Scholar]
- 62.Wright J.W., Harding J.W. The brain angiotensin system and extracellular matrix molecules in neural plasticity, learning, and memory. Prog Neurobiol. 2004;72:263–293. doi: 10.1016/j.pneurobio.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 63.Reaux A., Iturrioz X., Vazeux G., Fournie-Zaluski M.C., David C., Roques B.P., Corvol P., Llorens-Cortes C. Aminopeptidase A, which generates one of the main effector peptides of the brain renin–angiotensin system, angiotensin III, has a key role in central control of arterial blood pressure. Biochem Soc Trans. 2000;28:435–440. [PubMed] [Google Scholar]
- 64.Wilson W.L., Roques B.P., Llorens-Cortes C., Speth R.C., Harding J.W., Wright J.W. Roles of brain angiotensins II and III in thirst and sodium appetite. Brain Res. 2005;1060:108–117. doi: 10.1016/j.brainres.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 65.Kokje RJ, Wilson WL, Brown TE, Karamyan VT, Wright JW, Speth RC. Pressor Actions of Aminopeptidase-Resistant Analogs of Angiotensin II in the Rat Brain: Challenging the Angiotensin III Hypothesis. Hypertension (in press). [DOI] [PubMed]
- 66.Sim M.K., Choo M.H., Qiu X.S. Degradation of angiotensin I to [des–Asp1]angiotensin I by a novel aminopeptidase in the rat hypothalamus. Biochem Pharmacol. 1994;48:1043–1046. doi: 10.1016/0006-2952(94)90376-x. [DOI] [PubMed] [Google Scholar]
- 67.Wilk S., Wilk E., Magnusson R.P. Purification, characterization, and cloning of a cytosolic aspartyl aminopeptidase. J Biol Chem. 1998;273:15961–15970. doi: 10.1074/jbc.273.26.15961. [DOI] [PubMed] [Google Scholar]
- 68.Ofner L.D., Hooper N.M. The C-terminal domain, but not the interchain disulphide, is required for the activity and intracellular trafficking of aminopeptidase A. Biochem J. 2002;362:191–197. doi: 10.1042/0264-6021:3620191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Healy D.P., Wilk S. Localization of immunoreactive glutamyl aminopeptidase in rat brain. II. Distribution and correlation with angiotensin II. Brain Res. 1993;606:295–303. doi: 10.1016/0006-8993(93)90997-2. [DOI] [PubMed] [Google Scholar]
- 70.Migaud M., Durieux C., Viereck J., Soroca-Lucas E., Fournie-Zaluski M.C., Roques B.P. The in vivo metabolism of cholecystokinin (CCK-8) is essentially ensured by aminopeptidase A. Peptides. 1996;17:601–607. doi: 10.1016/0196-9781(96)00036-8. [DOI] [PubMed] [Google Scholar]
- 71.Schomburg L., Kollmus H., Friedrichsen S., Bauer K. Molecular characterization of a puromycin-insensitive leucyl-specific aminopeptidase, PILS-AP. Eur J Biochem. 2000;267:3198–3207. doi: 10.1046/j.1432-1327.2000.01348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsujimoto M., Hattori A. The oxytocinase subfamily of M1 aminopeptidases. Biochim Biophys Acta. 2005;1751:9–18. doi: 10.1016/j.bbapap.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 73.Hattori A., Kitatani K., Matsumoto H., Miyazawa S., Rogi T., Tsuruoka N., Mizutani S., Natori Y., Tsujimoto M. Characterization of recombinant human adipocyte-derived leucine aminopeptidase expressed in Chinese hamster ovary cells. J Biochem (Tokyo) 2000;128:755–762. doi: 10.1093/oxfordjournals.jbchem.a022812. [DOI] [PubMed] [Google Scholar]
- 74.Albiston A.L., McDowall S.G., Matsacos D., Sim P., Clune E., Mustafa T., Lee J., Mendelsohn F.A., Simpson R.J., Connolly L.M., Chai S.Y. Evidence that the angiotensin IV (AT(4)) receptor is the enzyme insulin-regulated aminopeptidase. J Biol Chem. 2001;276:48623–48626. doi: 10.1074/jbc.C100512200. [DOI] [PubMed] [Google Scholar]
- 75.Sim M.K., Lim B.C. Determination of aminopeptidase X activity in tissues of normo- and hypertensive rats by capillary electrophoresis. J Chromatogr B Biomed Sci Appl. 1997;697:259–262. doi: 10.1016/s0378-4347(97)00005-4. [DOI] [PubMed] [Google Scholar]
- 76.Kelly J.A., Neidle E.L., Neidle A. An aminopeptidase from mouse brain cytosol that cleaves N-terminal acidic amino acid residues. J Neurochem. 1983;40:1727–1734. doi: 10.1111/j.1471-4159.1983.tb08148.x. [DOI] [PubMed] [Google Scholar]
- 77.Harding J.W., Yoshida M.S., Dilts R.P., Woods T.M., Wright J.W. Cerebroventricular and intravascular metabolism of [125I]angiotensins in rat. J Neurochem. 1986;46:1292–1297. doi: 10.1111/j.1471-4159.1986.tb00652.x. [DOI] [PubMed] [Google Scholar]
- 78.Wright J.W., Bechtholt A.J., Chambers S.L., Harding J.W. Angiotensin III and IV activation of the brain AT1 receptor subtype in cardiovascular function. Peptides. 1996;17:1365–1371. doi: 10.1016/s0196-9781(96)00226-4. [DOI] [PubMed] [Google Scholar]
- 79.Goto Y., Hattori A., Ishii Y., Mizutani S., Tsujimoto M. Enzymatic properties of human aminopeptidase A. Regulation of its enzymatic activity by calcium and angiotensin IV. J Biol Chem. 2006;281:23503–23513. doi: 10.1074/jbc.M603191200. [DOI] [PubMed] [Google Scholar]
- 80.Sim M.K., Min L. Des–aspartate–angiotensin I and angiotensin AT1 receptors in rat cardiac ventricles. Regul Pept. 2005;129:133–137. doi: 10.1016/j.regpep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 81.Tsujimoto M., Mizutani S., Adachi H., Kimura M., Nakazato H., Tomoda Y. Identification of human placental leucine aminopeptidase as oxytocinase. Arch Biochem Biophys. 1992;292:388–392. doi: 10.1016/0003-9861(92)90007-j. [DOI] [PubMed] [Google Scholar]
- 82.Tanioka T., Hattori A., Masuda S., Nomura Y., Nakayama H., Mizutani S., Tsujimoto M. Human leukocyte-derived arginine aminopeptidase. The third member of the oxytocinase subfamily of aminopeptidases. J Biol Chem. 2003;278:32275–32283. doi: 10.1074/jbc.M305076200. [DOI] [PubMed] [Google Scholar]
- 83.Reaux A., de Mota N., Zini S., Cadel S., Fournie-Zaluski M.C., Roques B.P., Corvol P., Llorens-Cortes C. PC18, a specific aminopeptidase N inhibitor, induces vasopressin release by increasing the half-life of brain angiotensin III. Neuroendocrinology. 1999;69:370–376. doi: 10.1159/000054439. [DOI] [PubMed] [Google Scholar]
- 84.Bauvois B., Dauzonne D. Aminopeptidase-N/CD13 (EC 3.4.11.2) inhibitors: chemistry, biological evaluations, and therapeutic prospects. Med Res Rev. 2006;26:88–130. doi: 10.1002/med.20044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chansel D., Czekalski S., Vandermeersch S., Ruffet E., Fournie-Zaluski M.C., Ardaillou R. Characterization of angiotensin IV-degrading enzymes and receptors on rat mesangial cells. Am J Physiol. 1998;275:F535–F542. doi: 10.1152/ajprenal.1998.275.4.F535. [DOI] [PubMed] [Google Scholar]
- 86.Ramirez M., Arechaga G., Garcia S., Sanchez B., Lardelli P., Gandarias J.M. Soluble and membrane-bound leucyl- and arginyl-aminopeptidase activities in subcellular fractions of young and adult rat brains. Rev Esp Fisiol. 1990;46:393–397. [PubMed] [Google Scholar]
- 87.Foulon T., Cadel S., Cohen P. Aminopeptidase B (EC 3.4.11.6) Int J Biochem Cell Biol. 1999;31:747–750. doi: 10.1016/s1357-2725(99)00021-7. [DOI] [PubMed] [Google Scholar]
- 88.Zini S., Fournie-Zaluski M.C., Chauvel E., Roques B.P., Corvol P., Llorens-Cortes C. Identification of metabolic pathways of brain angiotensin II and III using specific aminopeptidase inhibitors: predominant role of angiotensin III in the control of vasopressin release. Proc Natl Acad Sci U S A. 1996;93:11968–11973. doi: 10.1073/pnas.93.21.11968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reaux A., Fournie-Zaluski M.C., David C., Zini S., Roques B.P., Corvol P., Llorens-Cortes C. Aminopeptidase A inhibitors as potential central antihypertensive agents. Proc Natl Acad Sci U S A. 1999;96:13415–13420. doi: 10.1073/pnas.96.23.13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee C.M., Snyder S.H. Dipeptidyl-aminopeptidase III of rat brain. Selective affinity for enkephalin and angiotensin. J Biol Chem. 1982;257:12043–12050. [PubMed] [Google Scholar]
- 91.Alba F., Arenas J.C., Lopez M.A. Properties of rat brain dipeptidyl aminopeptidases in the presence of detergents. Peptides. 1995;16:325–329. doi: 10.1016/0196-9781(94)00186-3. [DOI] [PubMed] [Google Scholar]
- 92.Smyth MO'Cuinn G. Dipeptidyl aminopeptidase III of guinea-pig brain: specificity for short oligopeptide sequences. J Neurochem. 1994;63:1439–1445. doi: 10.1046/j.1471-4159.1994.63041439.x. [DOI] [PubMed] [Google Scholar]
- 93.Gafford J.T., Skidgel R.A., Erdos E.G., Hersh L.B. Human kidney “enkephalinase”, a neutral metalloendopeptidase that cleaves active peptides. Biochemistry. 1983;22:3265–3271. doi: 10.1021/bi00282a035. [DOI] [PubMed] [Google Scholar]
- 94.Dahms P., Mentlein R. Purification of the main somatostatin-degrading proteases from rat and pig brains, their action on other neuropeptides, and their identification as endopeptidases 24.15 and 24.16. Eur J Biochem. 1992;208:145–154. doi: 10.1111/j.1432-1033.1992.tb17168.x. [DOI] [PubMed] [Google Scholar]
- 95.Shivakumar B.R., Wang Z., Hammond T.G., Harris R.C. EP24.15 interacts with the angiotensin II type I receptor and bradykinin B2 receptor. Cell Biochem Funct. 2005;23:195–204. doi: 10.1002/cbf.1176. [DOI] [PubMed] [Google Scholar]
- 96.Welches W.R., Santos R.A., Chappell M.C., Brosnihan K.B., Greene L.J., Ferrario C.M. Evidence that prolyl endopeptidase participates in the processing of brain angiotensin. J Hypertens. 1991;9:631–638. doi: 10.1097/00004872-199107000-00008. [DOI] [PubMed] [Google Scholar]
- 97.O'Leary R.M., Gallagher S.P., O'Connor B. Purification and characterization of a novel membrane-bound form of prolyl endopeptidase from bovine brain. Int J Biochem Cell Biol. 1996;28:441–449. doi: 10.1016/1357-2725(95)00154-9. [DOI] [PubMed] [Google Scholar]
- 98.McDermott J.R., Gibson A.M., Turner J.D. Involvement of endopeptidase 24.15 in the inactivation of bradykinin by rat brain slices. Biochem Biophys Res Commun. 1987;146:154–158. doi: 10.1016/0006-291x(87)90704-2. [DOI] [PubMed] [Google Scholar]
- 99.Welches W.R., Brosnihan K.B., Ferrario C.M. A comparison of the properties and enzymatic activities of three angiotensin processing enzymes: angiotensin converting enzyme, prolyl endopeptidase and neutral endopeptidase 24.11. Life Sci. 1993;52:1461–1480. doi: 10.1016/0024-3205(93)90108-f. [DOI] [PubMed] [Google Scholar]
- 100.Rioli V., Kato A., Portaro F.C., Cury G.K., te K.K., Vincent B., Checler F., Camargo A.C., Glucksman M.J., Roberts J.L., Hirose S., Ferro E.S. Neuropeptide specificity and inhibition of recombinant isoforms of the endopeptidase 3.4.24.16 family: comparison with the related recombinant endopeptidase 3.4.24.15. Biochem Biophys Res Commun. 1998;250:5–11. doi: 10.1006/bbrc.1998.8941. [DOI] [PubMed] [Google Scholar]
- 101.Schiavone M.T., Santos R.A., Brosnihan K.B., Khosla M.C., Ferrario C.M. Release of vasopressin from the rat hypothalamo-neurohypophysial system by angiotensin-(1–7) heptapeptide. Proc Natl Acad Sci U S A. 1988;85:4095–4098. doi: 10.1073/pnas.85.11.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ferrario C.M., Chappell M.C. Novel angiotensin peptides. Cell Mol Life Sci. 2004;61:2720–2727. doi: 10.1007/s00018-004-4243-4. [DOI] [PubMed] [Google Scholar]
- 103.Santos R.A., Simoes e Silva A.C., Maric C., Silva D.M., Machado R.P., de B., I, Heringer-Walther S., Pinheiro S.V., Lopes M.T., Bader M., Mendes E.P., Lemos V.S., Campagnole-Santos M.J., Schultheiss H.P., Speth R., Walther T. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci U S A. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hellner K., Walther T., Schubert M., Albrecht D. Angiotensin-(1–7) enhances LTP in the hippocampus through the G-protein-coupled receptor Mas. Mol Cell Neurosci. 2005;29:427–435. doi: 10.1016/j.mcn.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 105.Roques B.P., Noble F., Dauge V., Fournie-Zaluski M.C., Beaumont A. Neutral endopeptidase 24.11: structure, inhibition, and experimental and clinical pharmacology. Pharmacol Rev. 1993;45:87–146. [PubMed] [Google Scholar]
- 106.Turner A.J., Tanzawa K. Mammalian membrane metallopeptidases: NEP, ECE, KELL, and PEX. FASEB J. 1997;11:355–364. doi: 10.1096/fasebj.11.5.9141502. [DOI] [PubMed] [Google Scholar]
- 107.Abhold R.H., Harding J.W. Metabolism of angiotensins II and III by membrane-bound peptidases from rat brain. J Pharmacol Exp Ther. 1988;245:171–177. [PubMed] [Google Scholar]
- 108.Allred A.J., Diz D.I., Ferrario C.M., Chappell M.C. Pathways for angiotensin-(1–7) metabolism in pulmonary and renal tissues. Am J Physiol Renal Physiol. 2000;279:F841–F850. doi: 10.1152/ajprenal.2000.279.5.F841. [DOI] [PubMed] [Google Scholar]
- 109.Dauch P., Vincent J.P., Checler F. Molecular cloning and expression of rat brain endopeptidase 3.4.24.16. J Biol Chem. 1995;270:27266–27271. doi: 10.1074/jbc.270.45.27266. [DOI] [PubMed] [Google Scholar]
- 110.Ferro E.S., Carreno F.R., Goni C., Garrido P.A., Guimaraes A.O., Castro L.M., Oliveira V., Araujo M.C., Rioli V., Gomes M.D., Fontenele-Neto J.D., Hyslop S. The intracellular distribution and secretion of endopeptidases 24.15 (EC 3.4.24.15) and 24.16 (EC 3.4.24.16) Protein Pept Lett. 2004;11:415–421. doi: 10.2174/0929866043406706. [DOI] [PubMed] [Google Scholar]
- 111.Chu T.G., Orlowski M. Soluble metalloendopeptidase from rat brain: action on enkephalin-containing peptides and other bioactive peptides. Endocrinology. 1985;116:1418–1425. doi: 10.1210/endo-116-4-1418. [DOI] [PubMed] [Google Scholar]
- 112.Acker G.R., Molineaux C., Orlowski M. Synaptosomal membrane-bound form of endopeptidase-24.15 generates Leu-enkephalin from dynorphin1–8, alpha- and beta-neoendorphin, and Met-enkephalin from Met-enkephalin-Arg6-Gly7-Leu8. J Neurochem. 1987;48:284–292. doi: 10.1111/j.1471-4159.1987.tb13160.x. [DOI] [PubMed] [Google Scholar]
- 113.Healy D.P., Orlowski M. Immunocytochemical localization of endopeptidase 24.15 in rat brain. Brain Res. 1992;571:121–128. doi: 10.1016/0006-8993(92)90517-d. [DOI] [PubMed] [Google Scholar]
- 114.Fontenele-Neto J.D., Massarelli E.E., Gurgel Garrido P.A., Beaudet A., Ferro E.S. Comparative fine structural distribution of endopeptidase 24.15 (EC3.4.24.15) and 24.16 (EC3.4.24.16) in rat brain. J Comp Neurol. 2001;438:399–410. doi: 10.1002/cne.1323. [DOI] [PubMed] [Google Scholar]
- 115.Vincent B., Beaudet A., Dauch P., Vincent J.P., Checler F. Distinct properties of neuronal and astrocytic endopeptidase 3.4.24.16: a study on differentiation, subcellular distribution, and secretion processes. J Neurosci. 1996;16:5049–5059. doi: 10.1523/JNEUROSCI.16-16-05049.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Polgar L. The prolyl oligopeptidase family. Cell Mol Life Sci. 2002;59:349–362. doi: 10.1007/s00018-002-8427-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rosenblum J.S., Kozarich J.W. Prolyl peptidases: a serine protease subfamily with high potential for drug discovery. Curr Opin Chem Biol. 2003;7:496–504. doi: 10.1016/s1367-5931(03)00084-x. [DOI] [PubMed] [Google Scholar]
- 118.Andrews P.C., Hines C.M., Dixon J.E. Characterization of proline endopeptidase from rat brain. Biochemistry. 1980;19:5494–5500. doi: 10.1021/bi00565a005. [DOI] [PubMed] [Google Scholar]
- 119.Fruitier-Arnaudin I., Cohen M., Coitoux C., Piot J.M. In vitro metabolism of LVV-Hemorphin-7 by renal cytosol and purified prolyl endopeptidase. Peptides. 2003;24:1201–1206. doi: 10.1016/j.peptides.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 120.Walter R., Simmons W.H., Yoshimoto T. Proline specific endo- and exopeptidases. Mol Cell Biochem. 1980;30:111–127. doi: 10.1007/BF00227927. [DOI] [PubMed] [Google Scholar]
- 121.Wilk S. Prolyl endopeptidase. Life Sci. 1983;33:2149–2157. doi: 10.1016/0024-3205(83)90285-0. [DOI] [PubMed] [Google Scholar]
- 122.Odya C.E., Marinkovic D.V., Hammon K.J., Stewart T.A., Erdos E.G. Purification and properties of prolylcarboxypeptidase (angiotensinase C) from human kidney. J Biol Chem. 1978;253:5927–5931. [PubMed] [Google Scholar]
- 123.Orawski A.T., Simmons W.H. Degradation of bradykinin and its metabolites by rat brain synaptic membranes. Peptides. 1989;10:1063–1073. doi: 10.1016/0196-9781(89)90191-5. [DOI] [PubMed] [Google Scholar]
- 124.Tan F., Morris P.W., Skidgel R.A., Erdos E.G. Sequencing and cloning of human prolylcarboxypeptidase (angiotensinase C). Similarity to both serine carboxypeptidase and prolylendopeptidase families. J Biol Chem. 1993;268:16631–16638. [PubMed] [Google Scholar]
- 125.Moreira C.R., Schmaier A.H., Mahdi F., da Motta G., Nader H.B., Shariat-Madar Z. Identification of prolylcarboxypeptidase as the cell matrix-associated prekallikrein activator. FEBS Lett. 2002;523:167–170. doi: 10.1016/s0014-5793(02)02980-0. [DOI] [PubMed] [Google Scholar]
- 126.Skidgel R.A., Erdos E.G. Cellular carboxypeptidases. Immunol Rev. 1998;161:129–141. doi: 10.1111/j.1600-065x.1998.tb01577.x. [DOI] [PubMed] [Google Scholar]
- 127.Glossmann H., Baukal A.J., Catt K.J. Properties of angiotensin II receptors in the bovine and rat adrenal cortex. J Biol Chem. 1974;249:825–834. [PubMed] [Google Scholar]
- 128.Bennett J.P., Jr., Snyder S.H. Angiotensin II binding to mammalian brain membranes. J Biol Chem. 1976;251:7423–7430. [PubMed] [Google Scholar]
- 129.Sirett N.E., McLean A.S., Bray J.J., Hubbard J.I. Distribution of angiotensin II receptors in rat brain. Brain Res. 1977;122:299–312. doi: 10.1016/0006-8993(77)90296-7. [DOI] [PubMed] [Google Scholar]
- 130.Cole F.E., Blakesley H.L., Graci K.A., Frohlich E.D., MacPhee A.A. Brain angiotensin II receptor affinity and capacity in SHR and WKY rats: effects of acute dietary changes in NaCl. Brain Res. 1980;190:272–277. doi: 10.1016/0006-8993(80)91179-8. [DOI] [PubMed] [Google Scholar]
- 131.Harding J.W., Stone L.P., Wright J.W. The distribution of angiotensin II binding sites in rodent brain. Brain Res. 1981;205:265–274. doi: 10.1016/0006-8993(81)90338-3. [DOI] [PubMed] [Google Scholar]
- 132.Chen F.M., Hawkins R., Printz M.P. Evidence for a functional, independent brain–angiotensin system: correlation between regional distribution of brain angiotensin receptors, brain angiotensin and drinking during the estrous cycle of rats. Exp Brain Res Suppl. 1982;40:157–168. [Google Scholar]
- 133.Petersen E.P., Camara C.G., Abhold R.H., Wright J.W., Harding J.W. Characterization of angiotensin binding to gerbil brain membranes using [125I]angiotensin III as the radioligand. Brain Res. 1984;321:225–235. doi: 10.1016/0006-8993(84)90176-8. [DOI] [PubMed] [Google Scholar]
- 134.Petersen E.P., Abhold R.H., Camara C.G., Wright J.W., Harding J.W. Characterization of angiotensin binding in the African green monkey. Brain Res. 1985;341:139–146. doi: 10.1016/0006-8993(85)91481-7. [DOI] [PubMed] [Google Scholar]
- 135.Speth R.C., Wamsley J.K., Gehlert D.R., Chernicky C.L., Barnes K.L., Ferrario C.M. Angiotensin II receptor localization in the canine CNS. Brain Res. 1985;326:137–143. doi: 10.1016/0006-8993(85)91392-7. [DOI] [PubMed] [Google Scholar]
- 136.Printz M.P., Chen F.M., Slivka S., Maciejewski A.R. Comparison of neural and peripheral angiotensin II receptors. In: Buckley J.P., Ferrario C.M., editors. Brain Peptides and Catecholamines in Cardiovascular Regulation. Raven Press; New York: 1987. pp. 233–243. [Google Scholar]
- 137.Gehlert D.R., Speth R.C., Healy D.P., Wamsley J.K. Autoradiographic localization of angiotensin II receptors in the rat brainstem. Life Sci. 1984;34:1565–1571. doi: 10.1016/0024-3205(84)90611-8. [DOI] [PubMed] [Google Scholar]
- 138.Gehlert D.R., Speth R.C., Wamsley J.K. Autoradiographic localization of angiotensin II receptors in the rat brain and kidney. Eur J Pharmacol. 1984;98:145–146. doi: 10.1016/0014-2999(84)90122-5. [DOI] [PubMed] [Google Scholar]
- 139.Mendelsohn F.A., Quirion R., Saavedra J.M., Aguilera G., Catt K.J. Autoradiographic localization of angiotensin II receptors in rat brain. Proc Natl Acad Sci U S A. 1984;81:1575–1579. doi: 10.1073/pnas.81.5.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hwang B.H., Harding J.W., Liu D.K., Hibbard L.S., Wieczorek C.M., Wu J.Y. Quantitative autoradiography of 125I-[Sar1, Ile8]-angiotensin II binding in the brain of spontaneously hypertensive rats. Brain Res Bull. 1986;16:75–82. doi: 10.1016/0361-9230(86)90014-6. [DOI] [PubMed] [Google Scholar]
- 141.Rowe B.P., Saylor D.L., Speth R.C. Novel angiotensin II binding sites in the mesopontine area of the rat brain. Brain Res. 1990;534:129–134. doi: 10.1016/0006-8993(90)90122-r. [DOI] [PubMed] [Google Scholar]
- 142.Harding J.W., Erickson J.B., Camara C.C., Abhold R.H., Wright J.W. High-performance liquid chromatographic analysis of ‘specifically bound’ label after [125I]angiotensin II binding to rat brain membranes. Neurosci Lett. 1986;65:23–28. doi: 10.1016/0304-3940(86)90114-x. [DOI] [PubMed] [Google Scholar]
- 143.Abhold R.H., Sullivan M.J., Wright J.W., Harding J.W. Binding, degradation and pressor activity of angiotensins II and III after aminopeptidase inhibition with amastatin and bestatin. J Pharmacol Exp Ther. 1987;242:957–962. [PubMed] [Google Scholar]
- 144.Bennett J.P., Jr., Snyder S.H. Receptor binding interactions of the angiotensin II antagonist, 125I-[sarcosine1,leucine8]angiotensin II, with mammalian brain and peripheral tissues. Eur J Pharmacol. 1980;67:11–25. doi: 10.1016/0014-2999(80)90003-5. [DOI] [PubMed] [Google Scholar]
- 145.Ellis S., Nuenke J.M. Dipeptidyl arylamidase III of the pituitary. Purification and characterization. J Biol Chem. 1967;242:4623–4629. [PubMed] [Google Scholar]
- 146.Dando P.M., Brown M.A., Barrett A.J. Human thimet oligopeptidase. Biochem J. 1993;294(2):451–457. doi: 10.1042/bj2940451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shrimpton C.N., Glucksman M.J., Lew R.A., Tullai J.W., Margulies E.H., Roberts J.L., Smith A.I. Thiol activation of endopeptidase EC 3.4.24.15. A novel mechanism for the regulation of catalytic activity. J Biol Chem. 1997;272:17395–17399. doi: 10.1074/jbc.272.28.17395. [DOI] [PubMed] [Google Scholar]
- 148.Grover A.K., Kwan C.Y., Kostka P., Daniel E.E. Binding and degradation of angiotensin II by mesenteric artery subcellular membranes. Eur J Pharmacol. 1985;112:137–143. doi: 10.1016/0014-2999(85)90489-3. [DOI] [PubMed] [Google Scholar]
- 149.Wang J.M., Baudhuin P., Courtoy P.J., de Potter W. Conversion of angiotensin II into active fragments by an endosomal pathway in bovine adrenal medullary cells in primary culture. Endocrinology. 1995;136:5274–5282. doi: 10.1210/endo.136.12.7588271. [DOI] [PubMed] [Google Scholar]
- 150.Mendelsohn F.A., Aguilera G., Saavedra J.M., Quirion R., Catt K.J. Characteristics and regulation of angiotensin II receptors in pituitary, circumventricular organs and kidney. Clin Exp Hypertens Part A Theory Pract. 1983;5:1081–1097. doi: 10.3109/10641968309048843. [DOI] [PubMed] [Google Scholar]
- 151.Israel A., Plunkett L.M., Saavedra J.M. Quantitative autoradiographic characterization of receptors for angiotensin II and other neuropeptides in individual brain nuclei and peripheral tissues from single rats. Cell Mol Neurobiol. 1985;5:211–222. doi: 10.1007/BF00711007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Speth R.C., Bumpus F.M., Husain A. Identification of angiotensin II receptors in the rat ovary. Eur J Pharmacol. 1986;130:351–352. doi: 10.1016/0014-2999(86)90293-1. [DOI] [PubMed] [Google Scholar]
- 153.Marshall G.R., Vine W., Needlemann P. A specific competitive inhibitor of angiotensin II. Proc Natl Acad Sci U S A. 1970;67:1624–1630. doi: 10.1073/pnas.67.3.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Khairallah P.A., Toth A., Bumpus F.M. Analogs of angiotensin II. II. Mechanism of receptor interaction. J Med Chem. 1970;13:181–184. doi: 10.1021/jm00296a003. [DOI] [PubMed] [Google Scholar]
- 155.Noda K., Saad Y., Karnik S.S. Interaction of Phe(8) of angiotensin II with Lys(199) and His(256) of AT(1) receptor in agonist activation. J Biol Chem. 1995;270:28511–28514. doi: 10.1074/jbc.270.48.28511. [DOI] [PubMed] [Google Scholar]
- 156.Rodbell M., Krans H.M., Pohl S.L., Birnbaumer L. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. IV. Effects of guanylnucleotides on binding of 125I-glucagon. J Biol Chem. 1971;246:1872–1876. [PubMed] [Google Scholar]
- 157.Crane J.K., Campanile C.P., Garrison J.C. The hepatic angiotensin II receptor II. Effect of guanine nucleotides and interaction with cyclic AMP production. J Biol Chem. 1982;257:4959–4965. [PubMed] [Google Scholar]
- 158.Speth R.C., Kim K.H. Discrimination of two angiotensin II receptor subtypes with a selective agonist analogue of angiotensin II, p-aminophenylalanine6 angiotensin II. Biochem. Biophys Res Commun. 1990;169:997–1006. doi: 10.1016/0006-291x(90)91993-3. [DOI] [PubMed] [Google Scholar]
- 159.Mukoyama M., Horiuchi M., Nakajima M., Pratt R.E., Dzau V.J. Characterization of a rat type 2 angiotensin II receptor stably expressed in 293 cells. Mol Cell Endocrinol. 1995;112:61–68. doi: 10.1016/0303-7207(95)03586-v. [DOI] [PubMed] [Google Scholar]
- 160.Ouali R., LeBrethon M.C., Saez J.M. Identification and characterization of angiotensin-II receptor subtypes in cultured bovine and human adrenal fasciculata cells and PC12W cells. Endocrinology. 1993;133:2766–2772. doi: 10.1210/endo.133.6.8243303. [DOI] [PubMed] [Google Scholar]
- 161.Regoli D., Rioux F., Park W.K., Choi C. Role of the N-terminal amino acid for the biological activities of angiotensin and inhibitory analogues. Can J Physiol Pharm. 1974;52:39–49. doi: 10.1139/y74-006. [DOI] [PubMed] [Google Scholar]
- 162.Gunther S., Gimbrone M.A., Jr., Alexander R.W. Identification and characterization of the high affinity vascular angiotensin II receptor in rat mesenteric artery. Circ Res. 1980;47:278–286. doi: 10.1161/01.res.47.2.278. [DOI] [PubMed] [Google Scholar]
- 163.Gunther S. Characterization of angiotensin II receptor subtypes in rat liver. J Biol Chem. 1984;259:7622–7629. [PubMed] [Google Scholar]
- 164.Whitebread S., Mele M., Kamber B., de Gasparo M. Preliminary biochemical characterization of two angiotensin II receptor subtypes. Biochem Biophys Res Commun. 1989;163:284–291. doi: 10.1016/0006-291x(89)92133-5. [DOI] [PubMed] [Google Scholar]
- 165.Chiu A.T., McCall D.E., Nguyen T.T., Carini D.J., Duncia J.V., Herblin W.F., Uyeda R.T., Wong P.C., Wexler R.R., Johnson A.L. Discrimination of angiotensin II receptor subtypes by dithiothreitol. Eur J Pharmacol. 1989;170:117–118. doi: 10.1016/0014-2999(89)90145-3. [DOI] [PubMed] [Google Scholar]
- 166.Speth R.C., Rowe B.P., Grove K.L., Carter M.R., Saylor D. Sulfhydryl reducing agents distinguish two subtypes of angiotensin II receptors in the rat brain. Brain Res. 1991;548:1–8. doi: 10.1016/0006-8993(91)91098-l. [DOI] [PubMed] [Google Scholar]
- 167.Gehlert D.R., Gackenheimer S.L., Schober D.A. Angiotensin II receptor subtypes in rat brain: dithiothreitol inhibits ligand binding to AII-1 and enhances binding to AII-2. Brain Res. 1991;546:161–165. doi: 10.1016/0006-8993(91)91173-x. [DOI] [PubMed] [Google Scholar]
- 168.Karamyan VT, Gadepalli R, Rimoldi J, Speth RC. Preservation of 125-I-Angiotensin II in brain AT-1 receptor binding assays. 2007 Experimental Biology meeting abstracts [on CD-ROM], Abstract #571.3.
- 169.Karamyan V., Speth R. Identification of a novel non-AT1, non-AT2 angiotensin binding site in the rat brain. Brain Res. 2007;1143:83–91. doi: 10.1016/j.brainres.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 170.Rawlings N.D., Morton F.R., Barrett A.J. MEROPS: the peptidase database. Nucleic Acids Res. 2006;34:D270–D272. doi: 10.1093/nar/gkj089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kugler P. Aminopeptidase A is angiotensinase A. II. Biochemical studies on aminopeptidase A and M in rat kidney homogenate. Histochemistry. 1982;74:247–261. doi: 10.1007/BF00495834. [DOI] [PubMed] [Google Scholar]
- 172.Sakura H., Kobayashi H., Mizutani S., Sakura N., Hashimoto T., Kawashima Y. Kinetic properties of placental aminopeptidase A: N-terminal degradation of angiotensin II. Biochem Int. 1983;6:609–615. [PubMed] [Google Scholar]
- 173.Palmieri F.E., Bausback H.H., Ward P.E. Metabolism of vasoactive peptides by vascular endothelium and smooth muscle aminopeptidase M. Biochem Pharmacol. 1989;38:173–180. doi: 10.1016/0006-2952(89)90165-2. [DOI] [PubMed] [Google Scholar]


