Summary
This is an informal review of some of the trends in the infection prevention and control literature since the last Healthcare Infection Society (HIS) conference in late 2012. Google Trends was used to investigate how the volume of interest in various infection control topics had changed over time. Ebola trumped all the others in Google searches, reflecting a surge of publications in the literature. Aside from Ebola, other trends in the infection prevention and control literature covered in this article include Middle East Respiratory Syndrome (MERS) coronavirus, universal versus targeted interventions, faecal microbiota transplantation, whole genome sequencing, carbapenem-resistant Enterobacteriaceae, and some aspects of environmental science. The review ends with an attempt to predict some of the trends in the infection prevention and control literature between now and the next HIS conference in 2016.
Keywords: CRE, Ebola, Environmental science, Faecal microbiota transplantation, Infection prevention and control, MERS, Targeted interventions, Trends, Universal interventions, Whole genome sequencing
Introduction
To track some of the trends in the infection prevention and control literature since the last HIS conference in late 2012, I plugged some search terms into Google Trends (a facility that compares the variation in volume of searches for selected terms over time). One infection control trend trumped all others: Ebola (Figure 1, Figure 2 ). Whereas trends in Google searches may not necessarily correlate with trends in the infection prevention and control literature, in this case it is true that the outbreak of Ebola in West Africa has prompted a lot of publications in the literature – as well as consuming an awful lot of professional time for all who are connected with hospital infection prevention and control! Aside from Ebola, other trends in the infection prevention and control literature covered in this article include Middle East respiratory syndrome coronavirus (MERS-CoV), universal versus targeted interventions, faecal microbiota transplantation, whole genome sequencing, carbapenem-resistant Enterobacteriaceae (CRE), and some aspects of environmental science. Finally, I attempt to predict some of the trends in the infection prevention and control literature between now and the next HIS conference in 2016.
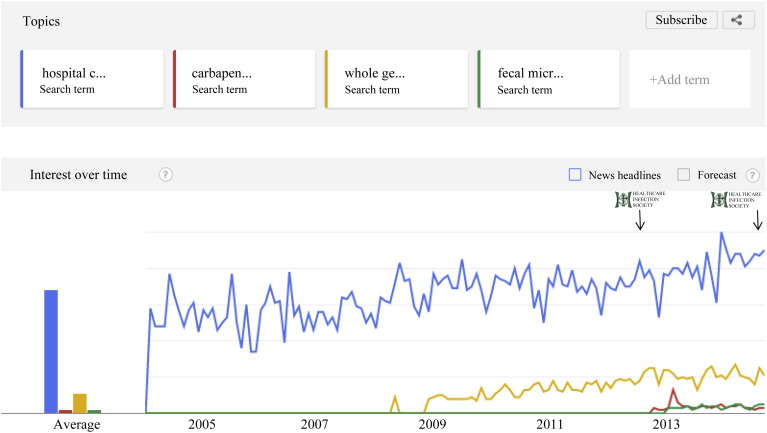
Figure 1.
Google trends for all search terms (excluding viruses) (2004 to present). Logos and arrows represent the time of the HIS 2012 and HIS 2014 conferences. Search terms: hospital cleaning, carbapenem-resistant Enterobacteriaceae, whole genome sequencing, fecal microbiota transplantation. Google Trends does not return the number of searches per se, but gives a relative number of results per unit time on a 0‒100 scale.
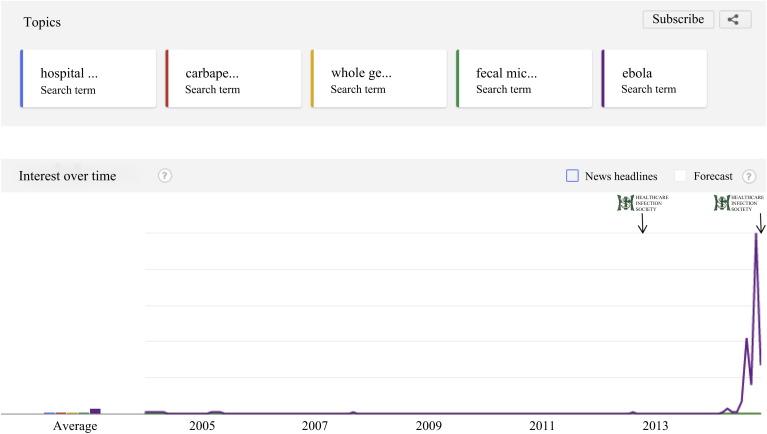
Figure 2.
Google trends for all search terms (2004 to present). Logos and arrows represent the time of the HIS 2012 and HIS 2014 conferences. The first peak corresponds to when Ebola was declared a global public health emergency by the World Health Organization, and the second peak corresponds to the first case of Ebola diagnosed in the USA. Search terms: hospital cleaning, carbapenem resistant Enterobacteriaceae, whole genome sequencing, fecal microbiota transplantation, Ebola. Google Trends does not return the number of searches per se, but gives a relative number of results per unit time on a 0–100 scale.
Ebola, MERS, influenza
A few weeks before HIS 2012, a novel coronavirus was reported in Saudi Arabia that was subsequently named Middle Eastern respiratory syndrome coronavirus (MERS-CoV).1, 2 Much like severe acute respiratory syndrome (SARS) coronavirus before it, MERS-CoV is associated with unnervingly high mortality (∼30%), and its potential for airborne dissemination, gastrointestinal shedding, and asymptomatic carriage present infection prevention and control challenges.3 Most of the 600 cases reported to date have occurred in the Middle East (mainly Saudi Arabia) but a few cases have occurred elsewhere, including the UK and USA.4 The management of MERS in Saudi Arabia has been shrouded in controversy, with ‘missing’ cases reported months after they occurred.5 How to safely manage MERS-CoV in developed healthcare systems has also been controversial, with questions over whether airborne or droplet precautions are necessary to contain the virus. Recent data indicate that small droplet nuclei may be emitted most of the time by influenza-infected patients, which argues for airborne precautions for MERS-CoV.6 A hospital outbreak in Jeddah in Saudi Arabia put the world on red alert in preparation for a pandemic, but that outbreak was contained and few cases are being reported currently.
By contrast, the outbreak of Ebola infection continues unabated in West Africa. There have been more than 10,000 cases in the current outbreak, around five times more cases of Ebola than had ever been reported prior to this outbreak. Furthermore, the three secondary transmissions that have occurred from the 13 cases cared for outside of West Africa to date (two in the USA and one in Spain) have pushed the Ebola crisis to the top of the agenda of many hospitals around the world. This has led to a degree of politicization, where regional and national decisions are made for political reasons rather than based on evidence, resulting in measures such as mandatory quarantine, airport screening, and immigration bans.7, 8
Politicization aside, research performed during the current outbreak has provided some new insight into the epidemiology of Ebola. It is most usually transmitted through direct contact with blood or body fluids including droplet sprays (through broken skin or mucous membranes).9, 10 However, transmission can also occur though indirect contact with contaminated environments.9, 10, 11, 12 Indeed, one outbreak report from Sierra Leone identified incorrect personal protective equipment (PPE) for cleaning staff as a likely contributor to transmission.12 Furthermore, despite being enveloped viruses, Ebola (and indeed MERS-CoV) has the capacity to survive on dry surfaces for days, not hours.13, 14, 15 One study even suggests an Ebola survival time measured in weeks, but this study was done at 4°C, so does not represent field conditions.15 These in-vitro survival times combined with the potential for blood and body fluid contamination argue for careful attention to cleaning and disinfection when caring for patients with Ebola virus disease.
Several studies have evaluated the basic reproductive number (R 0) for Ebola infection in field settings, which seems to be ∼2. One study found that R 0 is significantly higher in non-survivors (2.36) than in survivors (0.66), reinforcing the suggestion that contact with patients in the latter stages of disease is the most important risk factor for acquiring Ebola virus.16
Another area of controversy has been the appropriate type of PPE when dealing with Ebola. Earlier US recommendations were upgraded following the transmissions in Dallas, and now recommend a ‘no skin in the game’ approach, where all skin is covered.17 However, having the right PPE policy is only part of the solution – you also need to ensure PPE supply and that staff know how to don and doff it safely.18, 19
Universal versus targeted interventions
Several high-profile studies have suggested that we should move away from ‘targeted’ interventions, particularly screening and decolonization, towards ‘universal’ interventions such as chlorhexidine daily bathing for all intensive care unit (ICU) patients.20, 21 The most persuasive evidence for a universal approach involves the use of chlorhexidine for daily bathing for all ICU patients. It is worth noting, in passing, that this is not a truly universal approach since it is applied to a targeted population! The studies are impressive, especially in terms of design and to a lesser degree in terms of impact (Table I ).22, 23, 24, 25 Similarly, the use of antibiotics for selective digestive decontamination (SDD) or selective oral decontamination (SOD) has been advocated for all ICU patients. Again, the study design and impact are impressive, with reduced transmission and reduced mortality (Table II ).26, 27, 28, 29 However, indiscriminate use of biocides (such as chlorhexidine) and especially antibiotics is not without ‘collateral damage’.30 Several studies have reported increased antibiotic resistance associated with SOD and SDD, and other studies have reported reduced bacterial susceptibility associated with universal use of chlorhexidine.28, 31, 32, 33, 34 So we need to consider carefully the downside before wholeheartedly adopting universal decolonization.
Table I.
Studies of ‘universal’ chlorhexidine daily bathing, with or without other interventions
| Study | Setting | Design | Intervention | Results |
|---|---|---|---|---|
| Derde et al.22 | ICU | Time-series analysis | Universal CHG plus hand hygiene | Reduction in all MDROs and MRSA (but not VRE or ESBLs) |
| Climo et al.23 | ICU | Cluster RCT | Universal CHG | Reductions in MRSA/VRE acquisition and all BSI; BSI mainly CoNS |
| Milstone et al.24 | Paediatric ICU | Cluster RCT | Universal CHG | BSI reduced; mainly CoNS |
| Huang et al.25 | ICU | Cluster RCT | Universal CHG + mupirocin | Reduction in MRSA clinical isolates and all BSI; MRSA BSI not reduced |
ICU, intensive care unit; CHG, chlorhexidine gluconate; MDROs, multidrug-resistant organisms; MRSA, meticillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococcus; ESBL, extended-spectrum beta-lactamase producer; RCT, randomized controlled trial; BSI, bloodstream infection; CoNS, coagulase-negative staphylococci.
Table II.
Studies of selective digestive decontamination (SDD) or selective oral decontamination (SOD)
| Study | Setting | Design | Intervention | Results |
|---|---|---|---|---|
| de Jong et al.26 | ICU | RCT | SDD | Mortality and acquisition of MDR-GNB reduced |
| de Smet et al.27 | ICU | Cluster RCT | SDD or SOD | Both SOD and SDD reduced mortality |
| Oostdijk et al.28 | ICU | Cluster RCT | SDD vs SOD | No significant difference in mortality, but SDD → more antibiotic resistance |
| Saidel-Odes et al.29 | Adults | RCT | SDD | Reduced, but did not eliminate, CRE colonization |
ICU, intensive care unit; RCT, randomized controlled trial; MDR-GNB, multidrug-resistant Gram-negative bacilli; CRE, carbapenem-resistant Enterobacteriaceae.
Another universal strategy that has been evaluated recently is the universal use of gloves and gowns for the care of ICU patients.35 This impressive cluster-randomized controlled trial failed to meet the primary endpoint [a reduction in a composite measure of MRSA and vancomycin-resistant enterococci (VRE)], although MRSA was reduced. Furthermore, compliance with glove and gowning was high in the study (85%) whereas another study monitoring glove and gown use in the real world recorded much lower compliance (29%).36 Compliance with correct use of gloves and gowns fell as the proportion of patients on contact precautions increased.36 Also, the ‘over-use’ of gloves can result in unexpected risks, for example, wearing the same pair of gloves for too long.37 So there is unlikely to be a rush of hospitals implementing universal glove and gown policies.
The question of whether all patients should be given a single room was the subject of a recent BMJ debate.38 Single rooms are associated with lower rates of healthcare-associated infection, some patients report high levels of satisfaction, and the reduction in interruptions during patient contact leads to fewer ‘mix-up’ errors.39, 40, 41, 42, 43, 44, 45 On the other hand, caring for patients in multi-occupancy bays is associated with a reduced risk of adverse events, patients in single rooms often report less social contact and feelings of isolation, and bays require a lower staffing ratio.45, 46, 47, 48, 49 So, should hospitals provide all patients with single rooms? The jury is out.
Faecal microbiota transplantation
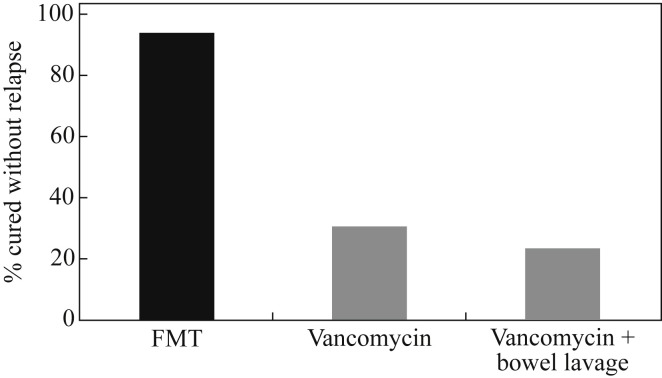
A stunning study published in the New England Journal of Medicine evaluated the impact of faecal microbiota transplantation (FMT) for the treatment of recurrent C. difficile infection (CDI) (Figure 3 ).50 Patients were randomized to receive either FMT or standard vancomycin treatment. The cure rate with FMT was >90% compared with ∼30% for vancomycin. The difference was so stark that the trial was stopped early. It therefore seems that FMT will soon become the standard of care for recurrent CDI. However, FMT as it stands is currently crude, in every sense, involving an infusion of donor stool. Another option is an oral dose of donor stool in carefully developed capsules, known colloquially as ‘crapsules’, which is as effective as a duodenal infusion.51 Finally, we are moving ever closer to a ‘synthetic’ FMT, using a carefully configured live bacterial cocktail.52
Figure 3.
Faecal microbiota transplant (FMT) for recurrent Clostridium difficile infection (CDI). Patients with recurrent CDI randomized to FMT (n = 16), vancomycin (n = 12) or vancomycin plus bowel lavage (n = 13).50
However, recurrent CDI is just the first of many potential applications for FMT. As well as aiming to develop microbiome-sparing therapy, we should be advancing techniques to maintain and restore indigenous microbiota.30 This could be applied not only for the treatment of disease, but also for decolonization of people carrying pathogens in the gastrointestinal tract, without the use of antibiotics.53 In the wider field of medicine, the importance of a healthy gut microbiome is becoming apparent, and may be key in new understanding of inflammatory bowel disease, obesity, diabetes, neurologic disorders, and so on.30
Whole genome sequencing
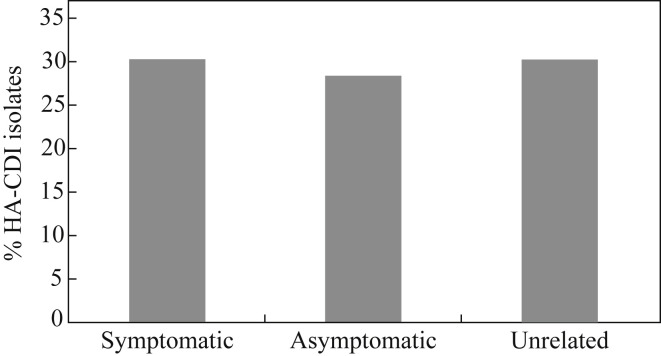
A landmark study published in the New England Journal of Medicine has challenged the status quo of our understanding of the epidemiology of CDI.54 The researchers used whole genome sequencing (WGS) to type C. difficile from each of their 1250 CDI cases over five years. Only around a third of the cases were closely related genetically, suggesting that transmission from other symptomatic cases occurs much less frequently than previously thought. However, another recent study used multi-locus variable number of tandem repeat analysis (MLVA) to type C. difficile from each of their hospital-acquired CDI cases, and from other sources, and found that around a third of CDI cases were genetically related to symptomatic cases (as with the Oxford study54) but an additional third of cases were genetically related to asymptomatic carriers of C. difficile (Figure 4 ).55 So is it time to start screening and isolating asymptomatic carriers of toxigenic C. difficile?
Figure 4.
Genetic relatedness of hospital-acquired Clostridium difficile (HA-CDI) isolates. Relatedness of 56 hospital-acquired C. difficile infection (CDI) cases to other CDI cases using multi-locus variable number of tandem repeat analysis.55
Another application of WGS that has emerged in recent years is to dissect outbreak epidemiology with unprecedented detail. For example, during an outbreak of carbapenem-resistant Enterobacteriaceae, a US team was able to turn a ‘plate of spaghetti’ map of epidemiologically related contacts into a clear transmission map by laying WGS data over epidemiological contacts.56
Carbapenem-resistant Enterobacteriaceae
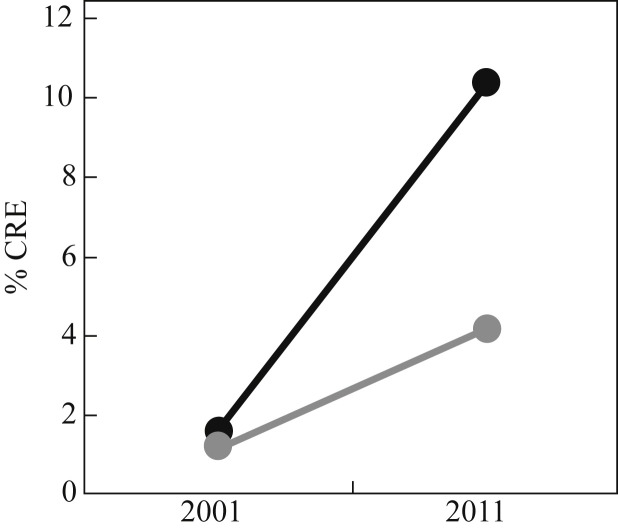
Carbapenem-resistant Enterobacteriaceae (CRE) have prompted an enormous amount of interest in the last few years. CRE more than any other organism have led to dire warning from world leaders on the impending post-antibiotic era. Both the UK and US governments have published CRE toolkits so that hospitals can prepare and manage problems due to CRE effectively.57, 58 The prevalence of CRE in the UK seems to be low at present, judging by the prevalence of carbapenem resistance in invasive K. pneumoniae isolates reported to the European Antimicrobial Resistance Surveillance Network (EARS-Net) (<1%).59 However, the number of referrals to Public Health England is increasing, and there are parts of the UK with more established problems.58 The picture from elsewhere is more disturbing. For example, there has been a sharp increase in CRE in Italy since 2009, and one recent study reported a high level of colistin resistance (43% of 191 CRE isolates from 21 laboratories across Italy).60 There has been a progressive increase in CRE in the USA, with the rate of carbapenem resistance in Klebsiella species increasing from <2% in 2001 to >10% in 2011 (Figure 5 ).61 Furthermore, there have been some shocking reports from some US regions. For example, ∼30% of patients in Chicago long-term acute care hospitals carried CRE in a point prevalence survey, and the rate in individual facilities ranged from ∼10% to almost 60%.62
Figure 5.
US national survey of resistance in Enterobacteriaceae in 2001 (n = 2631) and 2011 (n = 6573).61 Black line: Klebsiella pneumoniae/oxytoca; grey line: all Enterobacteriaceae. CRE, carbapenemase-resistant Enterobacteriaceae.
What should we do to prevent and control the spread of CRE and other multidrug-resistant Gram-negative organisms? The European Society of Clinical Microbiology and Infectious Diseases (ESCMID) has published comprehensive guidelines outlining the core principles of prevention and control, including active surveillance, contact precautions, antibiotic stewardship, hand hygiene, and cleaning and disinfection.63 However, we still do not really know what is effective in controlling multidrug-resistant Gram-negatives in general, and CRE in particular. Some interesting analysis of Gram-negative outbreak reports found that bundled interventions were less likely to fail than single interventions.64 Put another way, when in doubt, throw in the kitchen sink!
Environmental science
A key question for some time has been whether automated room disinfection systems are able to reduce the rate of transmission compared with conventional cleaning and disinfection.65 A US study found that patients admitted to rooms decontaminated using hydrogen peroxide vapour (HPV) were 64% less likely to acquire multidrug-resistant organisms than patients admitted to rooms disinfected using standard methods.66 Although this study is not without its problems, as outlined in accompanying editorial, it does reinforce the need to do a better job of cleaning and disinfection, particularly at the time of patient discharge.67
There has been an explosion of interest in the potential use of ultraviolet (UV)-based systems for automated room disinfection.65 Our understanding of these systems is evolving rapidly, and the following now seem clear. UV-C is fundamentally different to pulsed-xenon UV (PX-UV).65 UV systems are more effective than conventional cleaning and disinfection.68, 69 UV systems are faster and easier, but less effective than HPV.70 UV systems are less effective out of direct line of sight; using multiple locations in the room helps to mitigate this.70, 71 Some evidence is emerging that UV room disinfection reduces transmission.72 The results of a US Centers for Disease Control and Prevention-funded study in progress into the clinical impact of UV for terminal room disinfection are eagerly awaited.
There has been much interest in the potential for antimicrobial surfaces to provide a continuous reduction in the level of microbes on hospital surfaces.73 A US study evaluated the impact of introducing copper hand-touch surfaces into ICU rooms.74 The randomized study of 614 patients in three ICUs found that patients admitted to ‘copperized’ rooms had significantly less healthcare-associated infection or colonization. The study has come under fire for some aspects of its design and reporting, but it does provide compelling data that antimicrobial surfaces should be evaluated further.75, 76
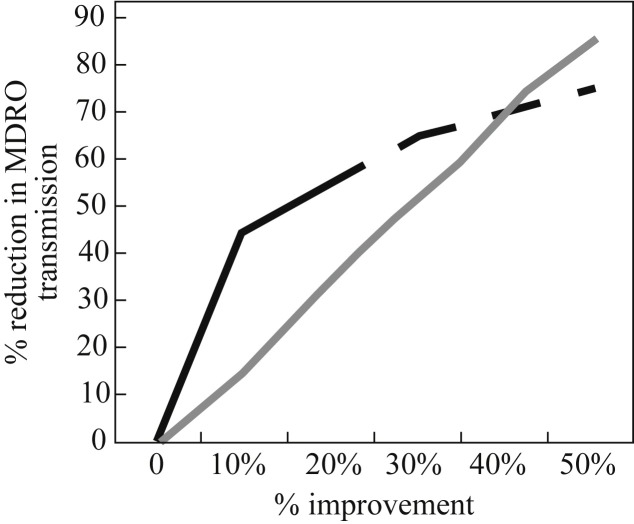
An age-old question is whether contaminated hands or surfaces are more important in the transmission of healthcare-associated infection. A model provides new insight into this question.77 The model simulates patient-to-patient transmission in a 20-bed ICU; 100 simulations were run for each pathogen of interest, evaluating the impact of stepwise changes in hand hygiene or terminal cleaning compliance. The key finding is that improvements in hand hygiene compliance are more or less twice as effective in preventing the transmission of MDR Acinetobacter baumannii, MRSA or VRE, i.e. a 20% improvement in terminal cleaning is required to ‘match’ a 10% improvement in hand hygiene compliance. However, although this may be overstretching the model, it appears that improving terminal cleaning may make more impact than improving hand hygiene at higher levels of compliance (Figure 6 ).77
Figure 6.
Model simulating the impact of improvements in hand or environmental hygiene on patient-to-patient transmission in a 20-bed intensive care unit.77 Black line: hand hygiene; grey line: terminal cleaning. MDRO, multidrug-resistant organism. Dashed line represents my approximate extrapolation from visual inspection of the data.
A recent Australian study discovered biofilms, some containing viable MRSA, on dry hospital surfaces.78 There are important implications if the presence of biofilms on dry hospital surfaces turns out to be a widespread occurrence. Biofilms could explain why vegetative bacteria can survive on dry hospital surfaces for so long, be part of the reason why they are so difficult to remove or inactivate using disinfectants, and explain, to some degree, the difficulty in recovering environmental pathogens by surface sampling.79, 80 Further work is required to explore the prevalence and composition of biofilms on dry hospital surfaces.
What will be trending at HIS 2016?
Looking into my crystal ball for HIS in 2016, it seems unlikely that the Ebola outbreak will be ongoing in West Africa, and I hope that we will have seen the last of MERS-CoV. However, pandemic influenza will be around for at least as long as mankind! It does seem likely that there will be a move towards universal interventions. The impressive data on FMT is likely to continue; it will quickly become the standard of care for recurrent CDI, and other applications will come through. WGS is unlikely to be so topical, only because it will increasingly become a standard tool used for day-to-day hospital epidemiology. I am sure that environmental science will continue to grow, and the global CRE epidemic curve will only go one way: upwards. Finally, as financial constraints continue to squeeze us, studies of cost-effectiveness and healthcare economics will become increasingly important to direct our precious resources.
And finally …
A team from Imperial College, London, evaluated Twitter trends relating to antibiotics, finding that peak Twitter activity correlated neatly with various antibiotic-related national and international announcements.81 Regardless of how you feel about social media, it is good to see that the issue of antibiotic resistance is receiving considerable public attention.
Conflict of interest statement
The author is employed part-time by Bioquell.
Funding sources
None.
References
- 1.de Groot R.J., Baker S.C., Baric R.S. Middle East Respiratory Syndrome Coronavirus (MERS-CoV); announcement of the Coronavirus Study Group. J Virol. 2013;87:7790–7792. doi: 10.1128/JVI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 3.Hui D.S., Memish Z.A., Zumla A. Severe acute respiratory syndrome vs. the Middle East respiratory syndrome. Curr Opin Pulm Med. 2014;20:233–241. doi: 10.1097/MCP.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 4.Zumla A., Hui D.S. Infection control and MERS-CoV in health-care workers. Lancet. 2014;31:1869–1871. doi: 10.1016/S0140-6736(14)60852-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holmes D. MERS-CoV enigma deepens as reported cases surge. Lancet. 2014;383:1793. doi: 10.1016/S0140-6736(14)60866-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bischoff W.E., Swett K., Leng I., Peters T.R. Exposure to influenza virus aerosols during routine patient care. J Infect Dis. 2013;207:1037–1046. doi: 10.1093/infdis/jis773. [DOI] [PubMed] [Google Scholar]
- 7.Mabey D., Flasche S., Edmunds W.J. Airport screening for Ebola. BMJ. 2014;349:g6202. doi: 10.1136/bmj.g6202. [DOI] [PubMed] [Google Scholar]
- 8.Drazen J.M., Kanapathipillai R., Campion E.W. Ebola and quarantine. N Engl J Med. 2014;371:2029–2030. doi: 10.1056/NEJMe1413139. [DOI] [PubMed] [Google Scholar]
- 9.Ftika L., Maltezou H.C. Viral haemorrhagic fevers in healthcare settings. J Hosp Infect. 2013;83:185–192. doi: 10.1016/j.jhin.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Lee L.M., Henderson D.K. Emerging viral infections. Curr Opin Infect Dis. 2001;14:467–480. doi: 10.1097/00001432-200108000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Bausch D.G., Towner J.S., Dowell S.F. Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. J Infect Dis. 2007;196(Suppl. 2):S142–S147. doi: 10.1086/520545. [DOI] [PubMed] [Google Scholar]
- 12.Forrester J.D., Hunter J.C., Pillai S.K. Cluster of Ebola cases among Liberian and US health care workers in an Ebola treatment unit and adjacent hospital ‒ Liberia, 2014. Morb Mortal Wkly Rep. 2014;63:925–929. [PMC free article] [PubMed] [Google Scholar]
- 13.van Doremalen N., Bushmaker T., Munster V.J. Stability of Middle East respiratory syndrome coronavirus (MERS-CoV) under different environmental conditions. Euro Surveill. 2013;18 doi: 10.2807/1560-7917.es2013.18.38.20590. pii:20590. [DOI] [PubMed] [Google Scholar]
- 14.Sagripanti J.L., Rom A.M., Holland L.E. Persistence in darkness of virulent alphaviruses, Ebola virus, and Lassa virus deposited on solid surfaces. Arch Virol. 2010;155:2035–2039. doi: 10.1007/s00705-010-0791-0. [DOI] [PubMed] [Google Scholar]
- 15.Piercy T.J., Smither S.J., Steward J.A., Eastaugh L., Lever M.S. The survival of filoviruses in liquids, on solid substrates and in a dynamic aerosol. J Appl Microbiol. 2010;109:1531–1539. doi: 10.1111/j.1365-2672.2010.04778.x. [DOI] [PubMed] [Google Scholar]
- 16.Yamin D., Gertler S., Ndeffo-Mbah M.L. Effect of Ebola progression on transmission and control in Liberia. Ann Intern Med. 2015;162:11–17. doi: 10.7326/M14-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edmond M.B., Diekema D.J., Perencevich E.N. Ebola virus disease and the need for new personal protective equipment. JAMA. 2014;312:2495–2496. doi: 10.1001/jama.2014.15497. [DOI] [PubMed] [Google Scholar]
- 18.Klompas M., Diekema D.J., Fishman N.O., Yokoe D.S. Ebola fever: reconciling Ebola planning with Ebola risk in U.S. hospitals. Ann Intern Med. 2014;161:751–752. doi: 10.7326/M14-1918. [DOI] [PubMed] [Google Scholar]
- 19.Fischer W.A., II, Hynes N.A., Perl T.M. Protecting health care workers from Ebola: personal protective equipment is critical but is not enough. Ann Intern Med. 2014;161:753–754. doi: 10.7326/M14-1953. [DOI] [PubMed] [Google Scholar]
- 20.Septimus E., Weinstein R.A., Perl T.M., Goldman D.A., Yokoe D.S. Approaches for preventing healthcare-associated infections: go long or go wide? Infect Control Hosp Epidemiol. 2014;35:797–801. doi: 10.1086/676535. [DOI] [PubMed] [Google Scholar]
- 21.Wenzel R.P., Edmond M.B. Infection control: the case for horizontal rather than vertical interventional programs. Int J Infect Dis. 2010;14(Suppl. 4):S3–S5. doi: 10.1016/j.ijid.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Derde L.P., Cooper B.S., Goossens H. Interventions to reduce colonisation and transmission of antimicrobial-resistant bacteria in intensive care units: an interrupted time series study and cluster randomised trial. Lancet Infect Dis. 2014;14:31–39. doi: 10.1016/S1473-3099(13)70295-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Climo M.W., Yokoe D.S., Warren D.K. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med. 2013;368:533–542. doi: 10.1056/NEJMoa1113849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milstone A.M., Elward A., Song X. Daily chlorhexidine bathing to reduce bacteraemia in critically ill children: a multicentre, cluster-randomised, crossover trial. Lancet. 2013;381:1099–1106. doi: 10.1016/S0140-6736(12)61687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang S.S., Septimus E., Kleinman K. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med. 2013;368:2255–2265. doi: 10.1056/NEJMoa1207290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Jonge E., Schultz M.J., Spanjaard L. Effects of selective decontamination of digestive tract on mortality and acquisition of resistant bacteria in intensive care: a randomised controlled trial. Lancet. 2003;362:1011–1016. doi: 10.1016/S0140-6736(03)14409-1. [DOI] [PubMed] [Google Scholar]
- 27.de Smet A.M., Kluytmans J.A., Cooper B.S. Decontamination of the digestive tract and oropharynx in ICU patients. N Engl J Med. 2009;360:20–31. doi: 10.1056/NEJMoa0800394. [DOI] [PubMed] [Google Scholar]
- 28.Oostdijk E.A., Kesecioglu J., Schultz M.J. Effects of decontamination of the oropharynx and intestinal tract on antibiotic resistance in ICUs: a randomized clinical trial. JAMA. 2014;312:1429–1437. doi: 10.1001/jama.2014.7247. [DOI] [PubMed] [Google Scholar]
- 29.Saidel-Odes L., Polachek H., Peled N. A randomized, double-blind, placebo-controlled trial of selective digestive decontamination using oral gentamicin and oral polymyxin E for eradication of carbapenem-resistant Klebsiella pneumoniae carriage. Infect Control Hosp Epidemiol. 2012;33:14–19. doi: 10.1086/663206. [DOI] [PubMed] [Google Scholar]
- 30.Tosh P.K., McDonald L.C. Infection control in the multidrug-resistant era: tending the human microbiome. Clin Infect Dis. 2012;54:707–713. doi: 10.1093/cid/cir899. [DOI] [PubMed] [Google Scholar]
- 31.Lubbert C., Faucheux S., Becker-Rux D. Rapid emergence of secondary resistance to gentamicin and colistin following selective digestive decontamination in patients with KPC-2-producing Klebsiella pneumoniae: a single-centre experience. Int J Antimicrob Agents. 2013;42:565–570. doi: 10.1016/j.ijantimicag.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Buelow E., Gonzalez T.B., Versluis D. Effects of selective digestive decontamination (SDD) on the gut resistome. J Antimicrob Chemother. 2014;69:2215–2223. doi: 10.1093/jac/dku092. [DOI] [PubMed] [Google Scholar]
- 33.Otter J.A., Patel A., Cliff P.R., Halligan E.P., Tosas O., Edgeworth J.D. Selection for qacA carriage in CC22 but not CC30 MRSA bloodstream infection isolates during a successful institutional infection control programme. J Antimicrob Chemother. 2013;68:992–999. doi: 10.1093/jac/dks500. [DOI] [PubMed] [Google Scholar]
- 34.Suwantarat N., Carroll K.C., Tekle T. High prevalence of reduced chlorhexidine susceptibility in organisms causing central line-associated bloodstream infections. Infect Control Hosp Epidemiol. 2014;35:1183–1186. doi: 10.1086/677628. [DOI] [PubMed] [Google Scholar]
- 35.Harris A.D., Pineles L., Belton B. Universal glove and gown use and acquisition of antibiotic-resistant bacteria in the ICU: a randomized trial. JAMA. 2013;310:1571–1580. doi: 10.1001/jama.2013.277815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dhar S., Marchaim D., Tansek R. Contact precautions: more is not necessarily better. Infect Control Hosp Epidemiol. 2014;35:213–221. doi: 10.1086/675294. [DOI] [PubMed] [Google Scholar]
- 37.Loveday H.P., Lynam S., Singleton J., Wilson J. Clinical glove use: healthcare workers' actions and perceptions. J Hosp Infect. 2014;86:110–116. doi: 10.1016/j.jhin.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Pennington H., Isles C. Should hospitals provide all patients with single rooms? BMJ. 2013;347:f5695. doi: 10.1136/bmj.f5695. [DOI] [PubMed] [Google Scholar]
- 39.Teltsch D.Y., Hanley J., Loo V., Goldberg P., Gursahaney A., Buckeridge D.L. Infection acquisition following intensive care unit room privatization. Archs Intern Med. 2011;171:32–38. doi: 10.1001/archinternmed.2010.469. [DOI] [PubMed] [Google Scholar]
- 40.Levin P.D., Golovanevski M., Moses A.E., Sprung C.L., Benenson S. Improved ICU design reduces acquisition of antibiotic-resistant bacteria: a quasi-experimental observational study. Crit Care. 2011;15:R211. doi: 10.1186/cc10446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borg M.A. Bed occupancy and overcrowding as determinant factors in the incidence of MRSA infections within general ward settings. J Hosp Infect. 2003;54:316–318. doi: 10.1016/s0195-6701(03)00153-1. [DOI] [PubMed] [Google Scholar]
- 42.Haill C.F., Newell P., Ford C. Compartmentalization of wards to cohort symptomatic patients at the beginning and end of norovirus outbreaks. J Hosp Infect. 2012;82:30–35. doi: 10.1016/j.jhin.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 43.Moore G., Ali S., FitzGerald G. Ward assessment of SmartIdeas Project: bringing source isolation to the patient. J Hosp Infect. 2010;76:103–107. doi: 10.1016/j.jhin.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 44.Barlas D., Sama A.E., Ward M.F., Lesser M.L. Comparison of the auditory and visual privacy of emergency department treatment areas with curtains versus those with solid walls. Ann Emerg Med. 2001;38:135–139. doi: 10.1067/mem.2001.115441. [DOI] [PubMed] [Google Scholar]
- 45.Maben J. Splendid isolation? The pros and cons of single rooms for the NHS. Nurs Manag. 2009;16:18–19. doi: 10.7748/nm2009.05.16.2.18.c7010. [DOI] [PubMed] [Google Scholar]
- 46.Stelfox H.T., Bates D.W., Redelmeier D.A. Safety of patients isolated for infection control. JAMA. 2003;290:1899–1905. doi: 10.1001/jama.290.14.1899. [DOI] [PubMed] [Google Scholar]
- 47.Tarzi S., Kennedy P., Stone S., Evans M. Methicillin-resistant Staphylococcus aureus: psychological impact of hospitalization and isolation in an older adult population. J Hosp Infect. 2001;49:250–254. doi: 10.1053/jhin.2001.1098. [DOI] [PubMed] [Google Scholar]
- 48.Young P., Yarandipour R. Examining the case for single rooms. Health Estate. 2007;61:85–86. [PubMed] [Google Scholar]
- 49.Mooney H. Single rooms: a blueprint for better care? Nursing Times. 2008;104:14–16. [PubMed] [Google Scholar]
- 50.van Nood E., Vrieze A., Nieuwdorp M. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 51.Youngster I., Russell G.H., Pindar C., Ziv-Baran T., Sauk J., Hohmann E.L. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA. 2014;312:1772–1778. doi: 10.1001/jama.2014.13875. [DOI] [PubMed] [Google Scholar]
- 52.Lawley T.D., Clare S., Walker A.W. Targeted restoration of the intestinal microbiota with a simple, defined bacteriotherapy resolves relapsing Clostridium difficile disease in mice. PLoS Pathog. 2012;8:e1002995. doi: 10.1371/journal.ppat.1002995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freedman A, Eppes S. Use of stool transplant to clear fecal colonization with carbapenem-resistant Enterobacteriaceae (CRE): proof of concept. Abstract #1805. ID Week 2014.
- 54.Eyre D.W., Cule M.L., Wilson D.J. Diverse sources of C. difficile infection identified on whole-genome sequencing. N Engl J Med. 2013;369:1195–1205. doi: 10.1056/NEJMoa1216064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Curry S.R., Muto C.A., Schlackman J.L. Use of multilocus variable number of tandem repeats analysis genotyping to determine the role of asymptomatic carriers in Clostridium difficile transmission. Clin Infect Dis. 2013;57:1094–1102. doi: 10.1093/cid/cit475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Snitkin E.S., Zelazny A.M., Thomas P.J. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3004129. 148ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.US Centers for Disease Control and Prevention . 2012. CRE Toolkit ‒ Guidance for control of carbapenem-resistant Enterobacteriaceae (CRE) [Google Scholar]
- 58.Public Health England . PHE; London: 2013. Acute trust toolkit for the early detection, management and control of carbapenemase-producing Enterobacteriaceae. [Google Scholar]
- 59.European Antimicrobial Resistance Surveillance Network (EARS-Net) European Centre for Disease Prevention and Control; Stockholm: 2012. Antimicrobial resistance surveillance in Europe 2012. [Google Scholar]
- 60.Monaco M., Giani T., Raffone M. Colistin resistance superimposed to endemic carbapenem-resistant Klebsiella pneumoniae: a rapidly evolving problem in Italy, November 2013 to April 2014. Euro Surveill. 2014;19 doi: 10.2807/1560-7917.es2014.19.42.20939. pii: 20939. [DOI] [PubMed] [Google Scholar]
- 61.Centers for Disease Control and Prevention Vital signs: carbapenem-resistant Enterobacteriaceae. Morb Mortal Wkly Rep. 2013;62:165–170. [PMC free article] [PubMed] [Google Scholar]
- 62.Lin M.Y., Lyles-Banks R.D., Lolans K. The importance of long-term acute care hospitals in the regional epidemiology of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae. Clin Infect Dis. 2013;57:1246–1252. doi: 10.1093/cid/cit500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tacconelli E., Cataldo M.A., Dancer S.J. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin Microbiol Infect. 2014;20(Suppl. 1):1–55. doi: 10.1111/1469-0691.12427. [DOI] [PubMed] [Google Scholar]
- 64.Cataldo MA, Foschi F, De Angelis G, et al. Main failings of intervention aimed at minimising the hospital spread of multidrug-resistant Gram-negative bacteria. Abstract #O125. ECCMID 2014.
- 65.Otter J.A., Yezli S., Perl T.M., Barbut F., French G.L. Is there a role for “no-touch” automated room disinfection systems in infection prevention and control? J Hosp Infect. 2013;83:1–13. doi: 10.1016/j.jhin.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 66.Passaretti C.L., Otter J.A., Reich N.G. An evaluation of environmental decontamination with hydrogen peroxide vapor for reducing the risk of patient acquisition of multidrug-resistant organisms. Clin Infect Dis. 2013;56:27–35. doi: 10.1093/cid/cis839. [DOI] [PubMed] [Google Scholar]
- 67.McDonald L.C., Arduino M. Climbing the evidentiary hierarchy for environmental infection control. Clin Infect Dis. 2013;56:36–39. doi: 10.1093/cid/cis845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jinadatha C., Quezada R., Huber T.W., Williams J.B., Zeber J.E., Copeland L.A. Evaluation of a pulsed-xenon ultraviolet room disinfection device for impact on contamination levels of methicillin-resistant Staphylococcus aureus. BMC Infect Dis. 2014;14:187. doi: 10.1186/1471-2334-14-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anderson D.J., Gergen M.F., Smathers E. Decontamination of targeted pathogens from patient rooms using an automated ultraviolet-C-emitting device. Infect Control Hosp Epidemiol. 2013;34:466–471. doi: 10.1086/670215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Havill N.L., Moore B.A., Boyce J.M. Comparison of the microbiological efficacy of hydrogen peroxide vapor and ultraviolet light processes for room decontamination. Infect Control Hosp Epidemiol. 2012;33:507–512. doi: 10.1086/665326. [DOI] [PubMed] [Google Scholar]
- 71.Mahida N., Vaughan N., Boswell T. First UK evaluation of an automated ultraviolet-C room decontamination device (Tru-D) J Hosp Infect. 2013;44:332–335. doi: 10.1016/j.jhin.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 72.Levin J., Riley L.S., Parrish C., English D., Ahn S. The effect of portable pulsed xenon ultraviolet light after terminal cleaning on hospital-associated Clostridium difficile infection in a community hospital. Am J Infect Control. 2013;41:746–748. doi: 10.1016/j.ajic.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 73.Otter J.A. An overview of options for antimicrobial hard surfaces in hospitals. In: Borkow G., editor. Use of biocidal surfaces for reduction of healthcare acquired infections. Springer; Berlin: 2014. [Google Scholar]
- 74.Salgado C.D., Sepkowitz K.A., John J.F. Copper surfaces reduce the rate of healthcare-acquired infections in the intensive care unit. Infect Control Hosp Epidemiol. 2013;34:479–486. doi: 10.1086/670207. [DOI] [PubMed] [Google Scholar]
- 75.Salgado C.D., Sepkowitz K.A., John J.F. Reply to Harbarth, et al. Infect Control Hosp Epidemiol. 2013;34:997–999. doi: 10.1086/671938. [DOI] [PubMed] [Google Scholar]
- 76.Harbarth S., Maiwald M., Dancer S.J. The environment and healthcare-acquired infections: why accurate reporting and evaluation of biological plausibility are important. Infect Control Hosp Epidemiol. 2013;34:996–997. doi: 10.1086/671741. [DOI] [PubMed] [Google Scholar]
- 77.Barnes S.L., Morgan D.J., Harris A.D., Carling P.C., Thom K.A. Preventing the transmission of multidrug-resistant organisms: modeling the relative importance of hand hygiene and environmental cleaning interventions. Infect Control Hosp Epidemiol. 2014;35:1156–1162. doi: 10.1086/677632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vickery K., Deva A., Jacombs A., Allan J., Valente P., Gosbell I.B. Presence of biofilm containing viable multiresistant organisms despite terminal cleaning on clinical surfaces in an intensive care unit. J Hosp Infect. 2012;80:52–55. doi: 10.1016/j.jhin.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 79.Espinal P., Marti S., Vila J. Effect of biofilm formation on the survival of Acinetobacter baumannii on dry surfaces. J Hosp Infect. 2012;80:56–60. doi: 10.1016/j.jhin.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 80.Otter J.A., Vickery K., Walker J. Surface-attached cells, biofilms and biocide susceptibility: implications for hospital cleaning and disinfection. J Hosp Infect. 2015;89:16–27. doi: 10.1016/j.jhin.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 81.Dyar O.J., Castro-Sanchez E., Holmes A.H. What makes people talk about antibiotics on social media? A retrospective analysis of Twitter use. J Antimicrob Chemother. 2014;69:2568–2572. doi: 10.1093/jac/dku165. [DOI] [PMC free article] [PubMed] [Google Scholar]