Abstract
Chitin is a linear polysaccharide of N-acetylglucosamine, which is highly abundant in nature and mainly produced by marine crustaceans. Chitosan is obtained by hydrolytic deacetylation. Both polysaccharides are renewable resources, simply and cost-effectively extracted from waste material of fish industry, mainly crab and shrimp shells. Research over the past five decades has revealed that chitosan, in particular, possesses unique and useful characteristics such as chemical versatility, polyelectrolyte properties, gel- and film-forming ability, high adsorption capacity, antimicrobial and antioxidative properties, low toxicity, and biocompatibility and biodegradability features. A plethora of chemical chitosan derivatives have been synthesized yielding improved materials with suggested or effective applications in water treatment, biosensor engineering, agriculture, food processing and storage, textile additives, cosmetics fabrication, and in veterinary and human medicine. The number of studies in this research field has exploded particularly during the last two decades. Here, we review recent advances in utilizing chitosan and chitosan derivatives in different technical, agricultural, and biomedical fields.
Introduction
Chitosan, a polymer of β(1-4)-linked glucosamine (2-amino-2-deoxy-O-glucose) units, is a biopolymer with unique characteristics due to the presence of free amino groups on its backbone. It is obtained by partial deacetylation of chitin, which is found in the cell walls of unicellular and filamenous fungi and in extracellular matrices and skeletal deposits of many protozoan and metazoan organisms including algae, choanoflagellates, sponges, corals, cephalopods, and arthropods. Commercially, chitin is extracted from the waste shells of marine crustaceans such as shrimp and crab. A significant proportion is used to produce chitosan, which, in contrast to chitin, is soluble in water at a slightly acidic pH and is easy to modify chemically to increase solubility at neutral pH and to add new functionalities. Chitosan and its derivatives have many desirable properties such as antioxidative and antimicrobial effects, mucoadhesiveness, biodegradability, and biocompatibility and can be manufactured in various formulations including hydrogels, films, membranes, porous sponges, nanoparticles, and nanofibers. Moreover, chitosan is considered a harmless compound, as it has received the generally recognized as safe (GRAS) status by the US Food and Drug Administration (FDA), and it has been approved as a food additive in several Asian countries (No et al. 2007). In the European Union, chitosan is registered as a basic substance, and the use of chitosan hydrochloride is considered by the European Food Safety Authority (EFSA) as having neither harmful effects on human or animal health nor any negative effects on the environment (European Commission 2014). Therefore, chitosan-based materials have been adopted worldwide in numerous applications in water treatment; food, cosmetic, and textile industry; biosensor engineering; plant protection; pharmaceutical industry; and regenerative medicine. They are used as flocculants, ion exchangers, chelating agents, coating materials, drug carriers, and scaffolds for tissue engineering. During the past years, many companies have started to develop chitosan-based products, and some have already successfully launched them for commercial purposes. This review is intended to summarize recent developments in the use of chitosan-based materials for potential and effective applications in different technical, environmental, agricultural, and biomedical fields.
Chitosan-Based Flocculants and Hydrogels Used in Water Treatment
Pollutants in water, industrial wastewater, and reclaimed wastewater for crop irrigation have presented severe environmental and medical problems all over the world. Such contaminants include various heavy metal ions (copper, cobalt, manganese, chromium, mercury, lead, arsenic, cadmium, and nickel), dyes (mainly azo dyes like malachite green, methyl violet, or methylene blue), oil spills, and a variety of pharmaceuticals and endocrine-disrupting compounds. Among the various methods used as remedial measures to treat polluted water and wastewater, the potential of chitosan-based composites as efficient adsorbent, flocculating and chelating agents has been widely investigated.
The presence of free hydroxyls and amino groups in many structural forms of chitosan-derived composites facilitates adsorption of pollutants such as dyes, metals, and organic compounds. Chitosan derivatives like carboxymethyl chitosan and graft polymerization are a prevalent strategy to add a variety of functional groups to the composite. Magnetic particles are embedded usually as nanoparticles in the complex core to facilitate regeneration and reuse of adsorbent composites by applying external magnetic field.
Removal of Heavy Metal Ions
A large number of chitosan-based composites were investigated for removal of metal ions from aqueous solutions. They include chitosan-polymer macromolecular complexes (as cellulose, cellulosic matrix like cotton fibers, alginate, polyvinyl alcohol, polyvinyl chloride), chitosan ceramics, as well as clay and silicate composites ( bentonite, montmorillonite, perlite, and zeolite) (Wan Ngah et al. 2011). Due to the vast number of scientific publications on chitosan-based adsorption that have been published, only a representative sample is depicted for Cr(VI) and Cu(II). Cognate composites were devised as adsorbents of other metal ions (Cd, As, Fe, Pb, Co, Pb, Hg, Ni, Zn, U) that can be found in the detailed reviews of Reddy and Lee (2013), Liu and Bai (2014), Wang and Chen (2014), Kyzas and Bikiaris (2015), Salehi et al. (2016), and Wang and Wang (2016).
The mutagenic and carcinogenic Cr(VI) is considered as a dangerous pollutant for humans and marine ecosystems. Composites of chitin and chitosan nano-hydroxyapatite hybrids removed Cr(VI) from aqueous solution by electrostatic interactions and reduction to Cr(III) via electron-donating groups present in the scaffold (Kousalya et al. 2010). A nanocomposite cross-linked hybrid of chitosan-alginate was able to remove Cr(VI) from water waste (Gokila et al. 2017). A more complex scaffold resin, where chitosan was mixed with magnetic particles (Fe3O4), modified by ethylenediamine and stabilized by glutaraldehyde as cross-linker, was established as an effective adsorbent of Cr(VI) (Hu et al. 2011). Reducing toxic Cr(VI) to nontoxic Cr(III) was accomplished by zero-valent iron [Fe(0)] embedded in chitosan beads (Geng et al. 2009). The oxidized iron Fe(III) formed a precipitately complex with Cr(III), thus enabling the regeneration of the adsorbing complex. Another method used ceramic aluminum coated with chitosan to remove Cr(VI) by electrostatic attraction of the hydrogen chromate ions to the positively charged amino groups of chitosan (Boddu et al. 2003).
Copper(II) Like chromium, Cu2+ ions found particularly in industrial wastewater are hazardous to human health and the ecosystems. Ingenious absorbance methods using a variety of organic and inorganic compounds have been devised to adsorb and remove the toxic ions. Among them are promising measures based on chitosan composite supra-macromolecular structures. Chitosan-based composites with various organic and inorganic compounds were examined as Cu(II) adsorbents. A recyclable complex composed of L-arginine-chitosan-Fe3O4 for removal of Cu(II) ions (Wu et al. 2016) and magnetic cellulose-chitosan composite microspheres was capable to adsorb heavy metals like Cu(II) but also Cd(II) and Pb(II) from aqueous solutions (Peng et al. 2014). Chitosan-algal biomass composite microbeads (Sargın et al. 2016b), a binary chitosan/silk fibroin composite (Ramya and Sudha 2013), and cotton fibers functionalized by triethylenetetramine (TETA) and carboxymethyl chitosan form composites and hybrids for adsorption of Cu(II) from water (Niu et al. 2017). Microcapsules composed of phytopathogenic (Ustilago sp.) fungal spores immobilized in cross-linked chitosan matrix (Sargın et al. 2016a) and a binary complex of chitosan and emu egg shells (Anantha and Kota 2016) were shown to remove copper ions from aqueous solutions.
Chitosan complexed with clays, ceramic minerals, and carbon-based materials was used to enhance absorbance of heavy metals from aqueous solutions. A nanocomposite that consisted of chitosan-montmorillonite (Pereira et al. 2013) and silica gel/chitin and chitosan with nano-hydroxyapatite was used as adsorbents for Cu(II) (Rajiv Gandhi et al. 2011). Nanocomposites containing chitosan-poly(vinyl alcohol)-attapulgite were also used for removal of Cu(II) from aqueous solutions (Wang and Wang 2016). Furthermore, a recyclable magnetic microsphere composed of cross-linked chitosan-rectorite (a clay mineral) and Fe3O4 was studied for adsorption of Cu(II) and Cd(II) (Xie et al. 2015), and chitosan-zeolite composite hydrogel beads were examined for Cu(II) sorption (Djelad et al. 2016).
A particular interesting recyclable composite with chelating capacity consists of core magnetic (Fe3O4)-silica particles combined with cross-linked chitosan. Its porous and highly specific surface area contributed by activated carbon carrier showed an excellent adsorption capability for Cu2+ ions (Li et al. 2017). A recyclable nanocomposite with a core xanthated Fe3O4 chitosan grafted on graphene oxide introduced sulfur groups to the composite using carbon disulfide (Liu et al. 2016a).
Other sorbent composites that were prepared and studied are a recyclable composites containing chitosan grafted on a core of Fe3O4-hexadecyl trimethoxysilane (Liu et al. 2016b), a flocculant composed of poly(acrylic acid) grafted on chitosan (Saleh et al. 2017) or beads containing chitosan-poly(vinyl alcohol) and ZnO (Xu et al. 2017a). A sophisticated composite was prepared by using magnetic nanoparticles on the surface of polystyrene as core, coated with chitosan cross-linked by glutaraldehyde followed by grafting polyethylenimine on the complex surface (Xiao et al. 2017). This submicron composite is recyclable and exhibits good adsorption capacity for Cu(II) ions.
Highly selective adsorption of copper ions from aqueous solutions was achieved by the ion-imprinting polymer method (Kong et al. 2017). Microspheres of magnetic cores of Fe3O4 with a shell of cross-linked chitosan and graphene oxide were used to imprint Cu2+ ions. Zarghami et al. (2014) prepared Cu(II) ion-imprinted membranes composed of cross-linked chitosan/poly(vinyl alcohol) for adsorption of the metal from aqueous solutions. A similar ion-imprinted technique was reported for selective adsorption of Pb(II) from a recycling wastewater unit (Hande et al. 2016).
Removal of Man-Made Environmental Pollutants
Industrial Dyes
Textile, leather, paper, and food industries discharge a plethora of environmental pollutants such as synthetic dyes. A variety of chitosan-based composites was examined as promising adsorbents of hard to remove industrial dyes. Chitosan per se contains functional groups for interaction with pollutants including dyes. Adding more functional groups by modifying chitosan (cross-linking of chitosan layers, direct chemical modification, or graft polymerization – see Chapter 3) improves adsorption capability. Molecular imprinting technique was devised as selective adsorbent of pollutants. Composites’ core of iron oxide magnetic nanoparticles like maghemite (γ-Fe2O3) and magnetite (Fe3O4) offers a way to recover the adsorbent scaffolds for reuse. Again, since the published articles are enormous in number, only essential parameters and basic blocks of adsorbing chitosan-based composites are included.
Methyl orange as a model anionic azo dye was adsorbed by films of cross-linked chitosan/nanonized maghemite from aqueous solution (Jiang et al. 2012). Improved adsorption of the same anionic dye was achieved by preparing a magnetic chitosan grafted with multi-walled carbon nanotubes (Zhu et al. 2010), and magnetic chitosan grafted with graphite oxide nanocomposite was able to adsorb the toxic azo dye, Reactive Black 5 (Travlou et al. 2013). Chitosan modified by ethylenediamine (Zhou et al. 2011) or polyaniline (Abbasian et al. 2017) grafting was able to adsorb other anionic azo dyes like Orange 7, Acid Orange 10 acid and red 4 and direct red 23, respectively. A magnetic complex of chitosan and zirconium oxide was a potent adsorbent of food anionic azo dyes like amaranth and tetrazine (Jiang et al. 2013a). Moreover, a complex composite adsorbent was prepared by grafting chitosan with poly[poly(ethylene glycol) methyl ether methacrylate] (Tsai et al. 2017). The functionalized groups added to chitosan contributed to improved removal of the azo dye Reactive Orange 16 from water.
Recyclable composite microspheres composed of cross-linked chitosan grafted with glutamic acid and having a core of Fe3O4 nanoparticles coated with silica adsorb cationic dyes like methylene blue, crystal violet, and light yellow 7GL (Yan et al. 2013). Similarly, an amphiphilic N-benzyl-O-carboxymethyl chitosan composite with a core of iron oxide nanoparticles was prepared for adsorption of methylene blue, crystal violet, and malachite green (Debrassi et al. 2012). The cyclic oligosaccharide β-cyclodextrin (β-CD) was added to chitosan-based composites as it provides a hydrophobic inner cavity and a hydrophilic exterior. Magnetic chitosan-β-CD with grafted graphene oxide to enlarge surface area exhibited an improved adsorption of methylene blue as a model dye from water (Fan et al. 2013). Molecular imprinting technique is of interest to selectively remove dyes from aqueous solutions. The molecule or ion used as templates will be subsequently removed, and a recognition site is generated. Alizarin red served as template molecule, and imprinted magnetic chitosan nanoparticles showed improved adsorption of the dye (Fan et al. 2012).
Removal of Micropollutants (Pharmaceuticals, Endocrine Disruptors)
Pharmaceutical, endocrine-disrupting compounds and personal care products have become a new class of hazardous environmental pollutants (Grassi et al. 2013) and have emerged as an extensive global concern. They are discharged as municipal and hospital effluents, from manufacturing industries, and found in water, reclaimed wastewater, and even in crops irrigated by reclaimed water (Paltiel et al. 2016). Pharmaceuticals, endocrine disruptors, and personal care products and their chemical transformation derivatives are characterized as stable, persistent compounds that are biologically active at very low concentrations.
The challenging goal has been to completely remove the above micropollutants from wastewater following conventional cleaning methods. Laboratory research including adsorption by chitosan-based composites has been high on the agenda (Amouzgar and Salamatinia 2015). Zhang et al. (2014) used a rather simple cross-linked magnetic chitosan-Fe3O4 composite to examine the sorption of three pharmaceutical compounds from contaminated water. The absorbance analysis showed effective sorption of diclofenac (a nonsteroidal anti-inflammatory drug) and clofibric acid (an antilipemic agent) but not of carbamazepine (an antiepileptic medication). Pharmaceuticals in water can be present as cationic, anionic, and neutral forms at different pH values. Thus, Zhang et al. (2016) in a more recent study devised an innovative, more complex three-dimensional chitosan-based scaffold. A magnetic core of chitosan-Fe3O4 was grafted with polymeric arms of either the polycation [poly(2-methyl acryoxyethyl trimethyl ammonium chloride], the polyanion poly(acrylic acid), or the neutral polymer poly(methylmethacrylate). The polycationic extension was cost-effective in removal of diclofenac from water due to charge attraction (Zhang et al. 2016). Further, magnetic composite pellets with grafted clay ( bentonite) and activated carbon were prepared to examine possible cost-effective removal of cationic and anionic pharmaceuticals (Arya and Philip 2016). The composite was effective as a sorbent for the beta-blocker ( atenolol), the antibiotic ( ciprofloxacin), and the lipid regulator ( gemfibrozil).
A variety of chitosan composites have been tested for the removal of other drugs. Cross-linked chitosan grafted with sulfonate or N-(2-carboxymethyl) groups was used as a sorbent to remove the dopamine agonist pramipexole dihydrochloride from polluted water (Kyzas et al. 2013). Chitosan-poly(acrylic acid)- graphite oxide nanocomposite showed adsorption of dorsolamide, a carbonic anhydrase inhibitor for eye treatment (Kyzas et al. 2014). Adsorption of nonsteroidal anti-inflammatory drugs ibuprofen and ketoprofen was studied using porous composite beads prepared of Chitosan-MIL 101 (Cr) (Zhuo et al. 2017). Using the antiepilectic carbamazepine as template, the magnetic molecular imprinted technique, based on chitosan-Fe3O4 nanoparticles, was applied for selective sorption of the drug (Zhang et al. 2013c).
Chlorophenols are endocrine-disrupting chemicals, used inter alia in manufacturing pharmaceuticals that are found in wastewaters (Sin et al. 2012). Excellent adsorbing capability was demonstrated using a cross-linked chitosan-salicylic acid-β-CD composite. Composites of chitosan-γ-CD were capable of adsorbing the endocrine disruptors, polychlorophenols, and bisphenol A (Duri and Tran 2013). Composite films prepared by blending microporous carbon fibers with cross-linked chitosan/polyvinyl alcohol were examined as sorbents of bisphenol A from water (Bilgin Simsek et al. 2017).
Finally, Soares et al. (2017b) proposed an interesting and unusual concept of using low-cost magnetic chitosan-based scaffold for absorbing and removing oil spills following initial skimming from water. In addition, the composite, which had a core of magnetic nanoparticles with a shell of chitosan-silica hybrid, effectively adsorbs nonpolar organic solvents.
Biosensors
Biosensors are essentially analytical devices that convert biological reactions or interactions into measurable signals. Basically, the biosensors’ constructs consist of a biological sensing element associated and intimately interfaced with a transducer that converts a signal in one form of energy to a signal of another form. Such signals should be proportional to the amount of analyte within a certain concentration range. Electrochemical biosensor devices, for example, possess advantages as being simple and relatively cheap while offering rapid detection and high sensitivity and further being amenable to miniaturization. Biosensors have been developed not only as analytical tools for medical purposes of clinical detection but also for applications in food industry and environmental monitoring.
Chitosan, and to a much lesser extent chitin, has several advantageous qualities in the design of biosensors. The polysaccharides are biocompatible, have functional groups pliable to chemical modification, and can be easily deposited on the surface of the transducer as adhesive thin films for the immobilization of recognition elements (enzymes, antibodies, DNA, whole cells, and cell organelles). Addition of carbon tubes, graphite, and graphene oxide to the composite increases electron transfer to the transducer and enhanced mechanical strength as well as water permeability and retention. Since there is a vast array of biosensors based on chitosan in their constructs, the following provide only representative devices.
Glucose detection and monitoring is of paramount importance in the medical field. A variety of biosensors, constructed with chitosan and using immobilized glucose oxidase for the detection of glucose levels, were reported. A glucose electrochemical sensor was prepared with glucose oxidase immobilized on the composite of chitosan-carbon nanotubes (Liu et al. 2005). An amperometric glucose biosensor composed of multilayered chitosan biofilms-gold nanoparticles-glucose oxidase on platinum (Pt) electrode was devised (Wu et al. 2007). The biocompatible gold nanoparticles helped in directing the transfer of electrons to the transducer. Yang et al. (2009) devised a different glucose biosensor composed of Pt electrode-glucose oxidase-Fe3O4-chitosan-nafion. Zhang et al. (2015c) prepared an electrochemical biosensor for glucose with chitosan-graphite composite and the addition of magnetic Fe3O4 nanoparticles on Pt-coated indium tin oxide (ITO) glass electrode. Shrestha et al. (2016) devised a glucose biosensor with a glassy carbon electrode on which a nanocomposite film of glucose oxidase immobilized on chitosan and on which a graft of polypyrrole-nafion and multi-walled carbon nanotubes was deposited.
Electrochemical biosensors using other oxidases and various constructs were fabricated to monitor food and medically important compounds. For instance, a lactate biosensor was generated using lactate oxidase and a nanocomposite structure of chitosan-polyvinylimidazole-Os-carbon nanotubes (Cui et al. 2007). Glutamate and xanthine oxidases as recognition elements immobilized on chitosan/graphene oxide-polymerized riboflavin were constructed as glutamate and hypoxanthine biosensors (Celiesiute et al. 2017). In addition, a xanthine biosensor based on immobilization of xanthine oxidase on chitosan-polypyrrole-gold nanoparticles was fabricated by Dervisevic et al. (2017). Tkac et al. (2007) developed a selective galactose biosensor with a rather simple configuration of chitosan-single-walled carbon nanotubes and immobilized galactose oxidase. A sensitive amperometric nanocomposite biosensor for cholesterol detection was constructed using a matrix of Pt nanoparticles deposited on multi-walled chitosan-carbon nanotubes complexes with immobilized cholesterol oxidase (Tsai et al. 2008). A similar construct was proposed by Medyantseva et al. (2014) for the detection of antidepressant monoamine drugs using immobilized monoamine oxidase. Dai et al. (2010) developed an electro-chemiluminiscent biosensor to detect choline by immobilizing choline oxidase on a chitosan/titanate nanotubes composite film. Finally, a biosensor for measuring ethanol was prepared using alcohol oxidase immobilized on chitosan-eggshell film (Wen et al. 2007). The biosensor monitored the decrease in oxygen level vs ethanol concentration.
A number of electrochemical biosensors were similarly constructed to immobilize various dehydrogenase enzymes (Zhang et al. 2004). The nanocomposite scaffold film, attached predominantly to glassy carbon electrodes, consists of chitosan, multi-walled carbon nanotubes, and NAD+ as cofactor. The signal current is based essentially on electrooxidation of the formed NADH. Among the large list of enzymes suffice it to mention NAD-dependent alcohol (Lee and Tsai 2009; Zhang and Gorski 2011), lactate (Tsai et al. 2007) and glutamate (Hughes et al. 2015) dehydrogenases, and FAD-dependent glucose dehydrogenase (Monosik et al. 2012).
In contrast to the above enzyme-based biosensors, a nonenzymatic electrochemical device for monitoring glucose was formulated (Al-Mokaram et al. 2017). The construct, which was based on a nanocomposite film composed of polypyrrole-chitosan-titanium dioxide nanoparticles on ITO glass electrodes, involved redox reactions and exhibited improved glucose oxidation and high electron transfer kinetics.
Other biosensors detecting and measuring diverse compounds were formulated, for example, nitrite biosensor based on Cu-containing nitrite reductase immobilized on viologen-chitosan that catalyzes the reduction of nitrite (Quan and Shin 2010). Horseradish peroxidase immobilized on alumina nanoparticles-chitosan composite was devised to detect phenolic compounds (Liu et al. 2011). Wang et al. (2003) developed a biosensor to detect and measure glucose, galactose, and glutamate in human blood by using their corresponding oxidases immobilized on chitosan-Prussian blue composite film. The biosensor used Prussian blue as a good catalyst to form hydrogen peroxide by electroreduction. Biosensors to detect catechol as well as other phenolic compounds were based on immobilized tyrosinase on a film of chitosan-nickel nanoparticles (Yang et al. 2012a). A biosensor for detection of chlorophenol that includes immobilized laccase on ZnO-chitosan nanocomposite was prepared by Mendes et al. (2017). Nanocomposite of functionalized graphene oxide (enriched with carboxylic moieties)-polypyrrole-chitosan film was constructed to detect hydrogen peroxide using screen-printed carbon electrodes (Akhtar et al. 2017). Such a device was able to electro-catalyze the reduction of hydrogen peroxide. Teepoo et al. (2017) constructed an electrochemical biosensor to detect and monitor hydrogen peroxide by using horseradish peroxidase immobilized on a chitin-gelatin nanofiber composite. Another biosensor for hydrogen peroxide that used immobilized catalase on chitosan-β-cyclodextrin (with ferrocene in its cavity) was fabricated by Dong et al. (2017). It was based on chitosan-functionalized graphene oxide (enriched with carboxylic moieties)-polypyrrole nanocomposite able to electrocatalytically reduce hydrogen peroxide.
Detection and quantification of trace amounts of carcinogenic and toxic metallic ions are of great challenge and importance. A cross-linked chitosan-carbon nanotube sensor was developed for the determination of Cd(II) and Hg(II) (Janegitz et al. 2011). Sugunan et al. (2005) prepared a biosensor made of chitosan-gold nanoparticles to detect Cu(II) and Zn(II), and Ahmed and Fekry (2013) used a construct of chitosan-α-Fe3O4 nanoparticles sensor to detect Ni(II), As(II), and Pb(II). Biosensors were developed to detect and determine organophosphorus (OP) pesticides as well. For instance, Stoytcheva et al. (2018) prepared a device based on OP hydrolase immobilization on a chitosan-carbon-nanoparticles-hydroxyapatite nanocomposite. A nanocomposite immunosensor to monitor the OP compound, chlorpyriphos, is based on immobilized anti-chloropyriphos monoclonal antibody on multi-walled carbon nanotubes-chitosan-thionine (as electronic mediator) (Sun et al. 2012b). An intricate electrochemical immunosensor for the detection and monitoring of the fungal hepatocarcinogen, aflatoxin B1, as model antigen was developed by Masoomi et al. (2013). The construct scaffold involved chitosan-gold nanoparticles, immobilized polyclonal anti-aflatoxin B1, and a magnetite core that can enable regeneration of the immunosensor.
Biosensors based on chitosan/multi-walled carbon nanotubes hybrid films were developed largely by Babaei and colleagues to determine and quantitate drugs and neurotransmitters: acetaminophen and mefenamic acid (Babaei et al. 2010), dopamine and morphine (Babaei et al. 2011a), paracetamol (Babaei et al. 2011b), L-DOPA (Babaei and Babazadeh 2011), and 5- hydroxytryptamine and dopamine (Xu et al. 2015).
The polycationic nature of chitosan films in immunobiosensors is also exploited to immobilize polyanionic polymers such as nucleic acid sequences and proteins. Singh et al. (2013) devised an electrochemical DNA biosensor to detect typhoid which was constructed by surface immobilizing Salmonella typhi single-stranded (ss) DNA on graphene oxide/chitosan/ITO nanocomposite as a bioelectrode. The biosensor was capable of distinguishing between complementary, noncomplementary, and one base mismatch sequences. A similar electrochemical DNA biosensor was developed for the detection of 0157:H7 (Xu et al. 2017b). It was prepared with immobilized E. coli ss-DNA using a graphene oxide/chitosan hybrid nanocomposite. An electrochemical immunobiosensor to detect botulism neurotoxin A was reported by Afkhami et al. (2017). The sensor consisted of a gold nanoparticles/chitosan/graphene nanocomposite with immobilized antibodies to quantify the bound neurotoxin. To detect α-fetoprotein in human serum, an immunosensor was fabricated in which the α-fetoprotein antigen was immobilized on a film of a gold nanoparticles/ carbon nanotubes/chitosan nanocomplex to quantify protein levels using a competitive immunoassay format (Lin et al. 2009). Giannetto et al. (2017) fabricated a competitive electrochemical immunosensor to detect HIV1-related capsid protein p24 in human serum. The p24 antigen was immobilized on gold-free single-walled carbon nanotube-chitosan complex for the interaction with a mouse monoclonal anti-p24, which was used for competitive immunodetection. Liu et al. (2009) developed an immunosensor to detect carcinoembryonic antigen, which is based on corresponding antibodies immobilized on chitosan-gold nanoparticles. Finally, Qiu et al. (2009) reported an immunosensor to detect hepatitis B surface antigen, which was constructed on the basis of a gold nanoparticles/chitosan/ferrocene biofilm with immobilized hepatitis B antibodies.
Beneficial Properties of Chitosan for Possible Use in Agriculture, Food, and Textile Industry
The wide-ranging antimicrobial, antiviral, and antioxidant activities, induction of defense systems in plants, and stimulation of plant growth by chitosan, chitosan oligomers, chemically modified chitosan and their composites have indicated their potential use in agricultural practices (El Hadrami et al. 2010; Malerba and Cerana 2016). Pre- and postharvest treatment of coating seeds, fruits, and vegetables by edible chitosan-based films effectively improve germination and plant vigor and prolonged shelf life and storage quality of food products (No et al. 2007). Preservation by chitosan-based coating also expanded to include meat, eggs, dairy products, and seafood (Friedman and Juneja 2010). Other promising practices such as delivery and slow and sustained release of chitosan-based encapsulated agrochemicals ( fertilizers, micronutrients, pest control agents, and genetic materials) have been widely investigated (Malerba and Cerana 2016).
Antimicrobial and Antioxidant Activities
There are several comprehensive reviews that summarize the potential use of chitosan, its derivatives, and chitooligosaccharides in agriculture as related to their broad-spectrum antimicrobial and antioxidant activities (Aider 2010; Cota-Arriola et al. 2013; Li et al. 2013a; Xing et al. 2015; Liaqat and Eltem 2018). Such beneficial activities were demonstrated in a variety of agricultural products like preservation of vegetables, fruits, cereals, dairy products, eggs, meat, and seafood (No et al. 2007; Friedman and Juneja 2010). Chitosan per se has antimicrobial activity that depends on higher degree of deacetylation, low molecular weight (its oligosaccharides), increased protonation at low pH, and the type of microorganisms (Katiyar et al. 2014). The antimicrobial efficiency is enhanced by adding essential oils (extracted from lemon, lemon grass, cinnamon, or rosemary) (Duan and Zhang 2013; Xing et al. 2016; Yuan et al. 2016) or by adding metal ions like silver or copper (An et al. 2011; Brunel et al. 2013; Kumar-Krishnan et al. 2015; Choudhary et al. 2017a; Sharma 2017) particularly to chitosan-based nanoparticles (Friedman and Juneja 2010; Cota-Arriola et al. 2013). The mode of action is mainly attributed to electrochemical interactions between the positively charged chitosan and the negative surface charge of bacterial cells leading to membranes disruption (Xing et al. 2015). In addition, penetration and binding of nanochitosan with microbial DNA that impact mRNA and protein synthesis were proposed (Rabea et al. 2003, Malerba and Cerana 2016).
Scavenging of free radical and reactive oxygen species by chitosan and its derivatives is responsible for its antioxidative effects (Guo et al. 2005; Ngo and Kim 2014). Scavenging of superoxide and hydroxyl radicals by chitosan and its derivatives was demonstrated by several studies (Xie et al. 2001; Guo et al. 2005; Yen et al. 2008; Wan et al. 2013). Furthermore, chitosan acts as a biogenic elicitor of various enzymes that detoxify reactive oxygen species (Malerba and Cerana 2016) and induces the formation of antioxidant and fungicidal phytoalexins (Yamada et al. 1993; Hadwiger 2013; Xing et al. 2015).
Eliciting Defense Responses in Plants
Chitosan and its derivatives were shown to activate plant immunity enzymes ( catalase, peroxidase, superoxide dismutase, phenyl oxidase, phenylalanine ammonia lyase) that are capable of detoxifying reactive oxygen species (Hadwiger 2013; Xing et al. 2015; Malerba and Cerana 2016). Such activation engages different signal transduction pathways that involve a variety of second messengers. Other defense responses include pathogenesis-related proteins, phytoalexins, proteinase inhibitors, lignin synthesis, or callose formation (El Hadrami et al. 2010; Hadwiger 2013). Induction of programmed cell death and hypersensitivity-associated responses by chitosan and chitooligosaccharides was documented (Zuppini et al. 2004; Vasilêv et al. 2009; Zhang et al. 2012), as well as activation of plant defense genes via the octadecanoid pathway leading to jasmonate synthesis (Doares et al. 1995; Rakwal et al. 2002). Chitosan induces hydrolase enzymes such as chitinase and β-1,3 glucanase able to destroy chitin/glucan-containing fungal cell walls (Ma et al. 2013b; Xing et al. 2015).
Plant Protection and Food Preservation
Controlled and sustained release of chitosan-encapsulated agrochemical such as fertilizers, micronutrient, pesticides, and genetic materials was demonstrated by a plethora of investigations (Kashyap et al. 2015). Food products coating by films of edible chitosan derivatives (plus a variety of additives) prolong their shelf life with concomitant improvements in storage quality (Xing et al. 2016; Yuan et al. 2016).
Pesticides
A number of examples linked to chitosan-coated pesticides given below indicate the potential of the eco-friendly techniques in plant protection against phytopathogens, insects, and weeds: controlled release of insecticides like the botanicals azadirachtin being encapsulated in the complex carboxymethyl chitosan-ricinoleic acid (Feng and Peng 2012) and rotenone wrapped in oleoyl carboxymethyl chitosan (Kamari and Aljafree 2017); nanoparticulate chitosan-β-cyclodextrin, which encapsulated carvacrol and exhibited high acaricidal and repellency activities (Campos et al. 2018); and controlled release of avermectin conjugated to N,O-carboxymethyl chitosan (Li et al. 2016) or avermectin coated by silica cross-linked chitosan composite (He et al. 2013). Encapsulation of the neonicotinoids imidacloprid (Li et al. 2012a; Lim and Ahmad 2017) and acetamiprid (Yan et al. 2014), malathion, and spinosad (El Badawy et al. 2016) by chitosan-alginate capsules exhibited prolonged release of the insecticides. Slow release of the fungicide carbendazim against the phytopathogens Sclerotinia sclerotiorum using chitosan/β-CD-epichlorohydrin (Wang et al. 2017a) and hexaconazole encapsulated by chitosan nanoparticles against Rhizoctonia solani (Chauhan et al. 2017) was demonstrated. Ilk et al. (2017) reported the antifungal and antioxidant activities of kaempferol encapsulated in lecithin-chitosan nanoparticles against .
In addition to their slow release property, chitosan composites also protect pesticides from photodegradation. Nanoparticles of chitosan-beeswax protected deltamethrin from photodegradation (Nguyen et al. 2012), and a similar protective effect of avermectin was demonstrated for a silica/chitosan copolymer (He et al. 2013). Likewise, composites of chitosan with a variety of clays (montmorillonite, attapulgite, bentonite, and kaolinite), safe anionic dyes ( Fast Green and Naphthol Yellow S), and photo-stabilized fungal conidia of the insect biocontrol agent Aschersonia spp. were reported (Cohen et al. 2003). Chitosan composites were found to be useful carriers of herbicides facilitating soil sorption as in the case of paraquat associated with chitosan-alginate nanoparticles (Silva Mdos et al. 2011) or slow release of paraquat encapsulated in tripolyphosphate-generated chitosan nanoparticles (Grillo et al. 2014). Moreover, encapsulation of metolachlor in blended gel beads of cross-linked carboxymethyl cellulose and carboxymethyl chitosan was effective in slow release of the herbicide as a model compound (Dong et al. 2012). Finally, slow release of atrazine encapsulated in carboxymethyl chitosan/bentonite gel was demonstrated (Li et al. 2012a).
Fertilizers
The modulated release of encapsulated fertilizers is important for enhanced growth of plants while reducing environmental problems of their excessive use. Experiments were accompanied by swelling rates of composites, fertilizer loads, and kinetics of release. Examples are chitosan-xanthan tablets (Melaj and Daraio 2013) or chitosan-starch beads (Perez and Francois 2016) as carriers of potassium nitrate that serve as model fertilizer; slow release of NPK fertilizers aggregated on chitosan nanoparticles (Corradini et al. 2010) and application on leaf surfaces enables translocation via stomata into the phloem (Abdel-Aziz et al. 2016); efficient controlled slow release of water soluble NPK fertilizers coated by chitosan with an additional outer coating by poly (acrylic acid–co-acrylamide) (Wu and Liu 2008). This composite also exhibited improved water absorption and retention. Noppakundilograt et al. (2015) examined the controlled release of NPK fertilizer granules embedded in a hydrogel composed of poly(vinyl alcohol) and then chitosan and a third layer of acrylamide and acrylic acid following cross-linking of chitosan by glutaraldehyde. Controlled release of urea by a variety of chitosan-based composites was established. Urea dispersed with humic substances in chitosan (Araújo et al. 2017), urea encapsulated in chitosan-acryamide (Siafu 2017), urea release from adduct of silk fibroin-gelatin-chitosan hydrogels (Rattanamanee et al. 2015), urea smectite clay chitosan composite (Puspita et al. 2017), and urea-kaolinite mixed with chitosan (Roshanravan et al. 2015) were tested for controlled release of the fertilizer.
Chitosan-Coated Plant Materials
Preharvest
Beneficial effects of preharvest chitosan-based seed coating and foliar treatment were reported by El Hadrami et al. (2010). Chitosan-coated artichoke seeds, for example, induced better germination, stimulated root system growth, and were effective against a number of pathogenic fungi (Ziani et al. 2010). Bhaskara Reddy et al. (1999) demonstrated induced resistance to seed-borne followed by improved germination and vigor in wheat seeds coated with chitosan. Soybean seeds coated by chitosan had anti-feeding effects and protected against several insect pests (Zeng et al. 2012), and coating rice seeds increased antifungal effect, stimulated seeding growth, improved root system, and increased crop yield (Zeng and Shi 2009). Tomato seeds coated with chitosan resulted in resistance to infection by inducing plant defense mechanisms (Benhamou et al. 1994). Chickpea seeds treated with chitosan-silver nanoparticles promoted germination and increased biomass, chlorophyll, carotenoids, and protein contents as well as amylase activity and defense enzyme activities (Anusuya and Banu 2016). Similar effects were demonstrated in maize seeds coated with Cu/chitosan nanoparticles (Saharan et al. 2016; Choudhary et al. 2017b).
Postharvest
The antimicrobial activity of chitosan was targeted for use to improve preservation of a large variety of vegetable and fruit crops as well as of eggs, meat, and dairy products (Devlieghere et al. 2004; Friedman and Juneja 2010; Yuan et al. 2016). Chitosan with added compounds such as plant materials and animal proteins (formulations of chitosan with additions of tapioca starch, hydroxypropyl cellulose, pectin, and fish gelatin) was used to develop edible films. Such films in addition to their antimicrobial and antioxidant activities also keep food products from loss of moisture and oxygen penetration (Aider 2010; Duan and Zhang 2013). Postharvest coating of vegetables and fruits with chitosan and additional essential oils (extracts from lemon, rosemary, lemon grass, bergamont, cinnamon, oregano, and thymine), which by themselves exert antimicrobial and antioxidant activities, improved storage quality and prolonged the shelf life of products (Xing et al. 2016). Controlling postharvest decay during storage was reported also for additives such as olive oil, glacial acetic acid, green tea extract, and lactic acid (Xing et al. 2016; Yuan et al. 2016).
Technical Applications in Food Packaging
Microbial contaminations are a serious problem in food industry, because food-borne bacteria and fungi are associated with food spoilage and food poisoning leading to economic losses and human health risks. Using appropriate food packaging materials with antimicrobial properties may prevent or at least slow down bacterial and fungal growth. For this reason, a variety of biopolymers has been tested to identify alternative materials to the classical nondegradable plastic packaging materials, which have caused serious environmental issues due to their inappropriate disposal. Optimal alternative materials should be environmentally safe due to biodegradability and biocompatibility. As chitosan-based material combine antimicrobial properties with biodegradability and biocompatibility, they are the focus of research in food packaging. Moreover, chitosan-based materials have food-preserving antioxidant activity and film-forming ability, which allows the production of transparent foils and bags. Different methods have been established during the past decades to fabricate chitosan films including casting, coating, extrusion, and layer-by-layer synthesis, and the resulting materials have been evaluated for their antimicrobial and antioxidant activity and for their optical, mechanical, barrier, and thermal characteristics. Chitosan has also been combined with other functional materials resulting in composite films with tremendous preservative properties that can be utilized for the packaging of different foods such as vegetables, fruit, and meat. For a comprehensive overview on this topic, the reader is referred to an excellent review article published recently by Wang et al. (2018).
Pure chitosan films are frequently based on dispersions of chitosan nanoparticles (Ali et al. 2014), to which plasticizers, such as glycol (Leceta et al. 2013), and/or surfactants, such as Tween 80 (Martins et al. 2012), are added to modify the mechanical properties and to emulsify auxiliary compounds. In addition, chitosan nanofibers have been fabricated as a packaging material and tested for their antimicrobial activity. For instance, Arkoun et al. (2017) examined the antimicrobial activity of chitosan/polyethylene oxide nanofibers produced by an electrospinning process. They showed that the chitosan nanofibers were efficient in inhibiting growth of E. coli, Staphylococcus aureus, Lysteria innocua, and S. typhimurium, however at pH 5.8, which was below the pKa of chitosan, limiting the applicability to slightly acidic food. Importantly, the authors demonstrated that the antibacterial effects were irreversible, suggesting a bactericidal rather than bacteriostatic mechanism.
Combinations of chitosan and other natural polysaccharides have been frequently used to fabricate functional films with applications in food packaging. These biopolymers comprise of cellulose and various cellulose derivatives, alginate, cyclodextrin, glucan, mannan, pectin, starch, and xylan. Chitosan/cellulose films revealed improved mechanical properties while maintaining excellent antimicrobial properties (Xiao et al. 2013). Also chitosan/hydroxypropyl methylcellulose (HMPC) films exhibit significant antimicrobial activity. For instance, Möller et al. (2004) examined the antimicrobial effects of chitosan/HPMC films against Listeria monocytogenes and found that bacterial growth was completely inhibited on the film. Similarly, chitosan/ carboxymethyl cellulose films showed superb food preservation properties when tested on packaged cheese (Youssef et al. 2016). Antimicrobial chitosan-alginate films have a great potential for food packaging as well, particularly because they show improved gas exchange and water vapor permeability properties when prepared by a layer-by-layer electrostatic deposition approach (Poverenov et al. 2014a). Martiñon et al. (2014) studied the effectiveness of antimicrobial multilayered coatings consisting of chitosan, pectin, and trans-cinnamaldehyde at different concentrations to extend the shelf life of fresh-cut cantaloupe and found that certain compositions were effective in preventing bacterial growth and spoilage. Lorevice et al. (2016) produced chitosan nanoparticles and combined them with different methyl pectin matrices to generate nanocomposite films and tested the mechanical, thermal, and barrier properties. The results showed that the nanocomposite film improved mechanical characteristics when compared with conventionally produced pectin films, making these novel materials promising for food packaging production. Similarly, chitosan/cyclodextrin films with inclusions of essential oil have been reported to possess desirable mechanical properties for food packaging (Sun et al. 2014). Moreover, this material showed significant antimicrobial activities against a variety of pathogenic bacteria.
Chitosan films have been also combined with a variety of proteins including casein (Khwaldia et al. 2014), gelatin (Poverenov et al. 2014b; Noorbakhsh-Soltani et al. 2018), collagen (Ahmad et al. 2016), kidney bean protein (Ma et al. 2013a), lactoferrin (Brown et al. 2008), and lysozyme (Yuceer and Caner 2014), as well as with antibacterial peptides such as nisin (Wang et al. 2015). In addition, chitosan was blended with antimicrobial and antioxidant extracts from bee wax (Velickova et al. 2013) and plants, such as citrus (Iturriaga et al. 2014), thyme (Talon et al. 2017), and maqui berry (Genskowsky et al. 2015), as well as with essential oils including clove bud oil, cinnamon oil, and star anise oil (Wang et al. 2011).
Other approaches in fabricating chitosan-based films employed grafts, blends, or casts using synthetic polymers such as poly(vinyl alcohol) (Wang et al. 2015), poly(lactic acid) (Pal and Katiyar 2016), poly(ethylene) (Reesha et al. 2015), poly(ethylene oxide) (Kohsari et al. 2016), poly(styrene) (Lopez-Carballo et al. 2013), poly(propylene) (Cavallo et al. 2014), poly(caprolactone) (Alix et al. 2013), and poly(acrylonitrile-co-acrylamide) (Kumar et al. 2018) that led to improved mechanical and thermal properties. However, these synthetic polymers are not readily degraded in nature; hence concerns regarding the environmental safety have been raised. Guo et al. (2015) developed new edible antimicrobial films using microemulsions in combination with high-pressure homogenization processing. The films were made of chitosan, allyl isothiocyanide, and barley straw arabinoxylan, which were used as film-forming, antimicrobial, and emulsifying agents, respectively. The material was tested to be efficient in preventing growth of L. innocua.
To improve antibacterial activity, chitosan-based films were synthesized as composites with metals, minerals, and other inorganic compounds. Youssef et al. (2014) produced chitosan-silver and chitosan-gold (CS-Au) nanocomposites films, which showed enhanced antimicrobial activity against Gram-positive (S. aureus) and Gram-negative bacteria (Pseudomonas aerugenosa), fungi (Aspergillus niger), and yeast (Candida albicans) . In another study published by Al-Naamani et al. (2016), poly(ethylene) films were coated with zinc oxide/chitosan nanocomposite, which completely inactivated and prevented the growth of food pathogens. In an approach based on a solution cast method, Sanuja et al. (2015) fabricated a chitosan-based nanocomposite film using nano zinc oxide and neem essential oil, which improved mechanical, physical, barrier, and optical properties. Moreover, Zhang et al. (2017) prepared chitosan/titanium dioxide composite films, which were found to possess significant antimicrobial activity against E. coli, S. aureus, C. albicans, and A. niger. Xu et al. (2017c) employed a different strategy by synthesizing chitosan/ graphene oxide nanocomposites with titanium dioxide and analyzed their antimicrobial and food-preserving efficacies. They showed that the material effectively prevented and A. niger biofilm formation presumably by disrupting cellular membranes. In addition, they demonstrated that the nano-coating could be applied as a cling film, which delays loss of moisture in fruits and vegetables and inhibits polyphenol oxidase activity and thus enzymatic browning but increases superoxide dismutase activity, which protects against reactive oxygen species. Next to these materials, chitosan-montmorillonite composites, chitosan/nanosilica films, and manifold combinations of chitin, metals, and minerals have been tested. In addition, numerous chemical chitosan derivatives have been explored for their properties to screen for new films suitable in food packaging. These derivatives include carboxymethyl chitosan and quaternized chitosan such as (2-N-Hydroxypropyl-3-trimethylammonium chloride) chitosan (Hu et al. 2016).
The Use of Chitosan in the Textile Sector
Due to their versatile and unique physicochemical and biological properties, chitosan, its multiple derivatives, and their adjunct complexes (addition of functional groups) have attracted considerable attention for possible use of eco-friendly materials in the textile industry. They are relatively inexpensive, biocompatible, biodegradable, and nontoxic and readily adhere to textile fabrics and usually demonstrate antibacterial activity. Certain formulations retain moisture as well as impart thermal stability and UV protection.
Chitosan per se or blends of chitosan-based composites deposited onto textiles fabrics were mostly tested for durable antibacterial activity (nearly all antibacterial studies include E. coli and S. aureus that represent correspondingly Gram-negative and Gram-positive bacteria). Coated Thai silk fabric with chitosan using radio frequencies plasma treatment exhibited antibacterial effects (Wongsawaeng et al. 2017), and polyester/cotton fabric treated with chitosan can be used as an alternative to the antibacterial triclosan (Ranganath and Sarkar 2014). Chitosan grafted on cotton (Ferrero et al. 2015) or on wool (Periolatto and Ferrero 2013) fabrics using UV irradiation bestowed antibacterial activity after many washing cycles. Chitosan reduced to nanoparticles and applied onto wool fabric imparted durable antibacterial and bestowed shrink proofing (Yang et al. 2010). Nanonized chitosan applied onto cotton exhibited, in addition to antibacterial activity, also thermal stability, UV protection, as well as improved dye-binding ability (Hebeish et al. 2013). Periolatto et al. (2012) demonstrated antibacterial effects and laundry durability of cotton and silk fabrics by UV curing with 2-hydroxy-2-methylpropylpropane-1-one as photoinitiator of the photochemical reaction.
Chitosan possesses abundant potential, in particular, for use in medical textiles and sportswear. For example, a blend of chitosan (short fibers) with cotton (long fibers) yarn by spinning technology is desirable for medical applications (Lam et al. 2017). Gauze bandages for wound dressing were prepared by electrospinning of chitosan nanofibers and cotton fabric (Nawalakhe et al. 2015). Plasma treatment was applied to improve adhesion by increased cross-linking between the two fiber systems imparting subsequent durability (Nawalakhe et al. 2015). Pure chitosan microfibers produced by wet spinning process was aimed for possible stable 3-D scaffold woven or nonwoven textile fabrics to be used in regenerative medicine such as bone and cartilage engineering (Toskas et al. 2013). Lam et al. (2018) examined a blend of chitin fibrils with cotton jersey fabric and showed reduced rigidity that may provide comfort to patients with epidermolysis bullosa skin disease. Likewise, chitosan-coated textile fabrics improved atopic dermatitis disease by restraining skin microbiome (Lopes et al. 2015). Sonochemical deposition was used by Petkova et al. (2014) to coat cotton fabrics with a hybrid of chitosan and ZnO nanoparticles. This complex showed improved antibacterial activity, slow release of the metal and washing stability, and postulated as effective treatment for hospital textiles to prevent transfer of pathogens. Similarly, the hybrid of chitosan and silver nanoparticles deposited onto cotton fabric demonstrated antibacterial effects and laundry durability befitting their possible use for medical textiles and sportswear (Xu et al. 2016). Ali et al. (2011) proposed to use chitosan nanoparticles that are able to pick and retain silver ions in medical textile applications. A polyester fabric coated with this complex hybrid imparted enhanced antibacterial activity. Nanonized chitosan applied onto cotton exhibited in addition to antibacterial activity, also thermal stability, UV protection as well as improved dye-ability (Hebeish et al. 2013).
A large number of publications signified and reported beneficial properties of chitosan and chitosan-based formulations with possibly great potential to treat textile fabrics. Such valuable features include protecting a variety of fabrics with emphasis on medical textiles, production of aromatic and flame-retarding fabrics, as well as dye removal and treatment of textile wastewater. Table 14.1 summarizes inter alia nanochitosan, chitosan nanometal complexes, or chitosan derivative composites with metals and other substances, which were treated onto textile fabrics (notably cotton), and depicts their conceivable potential for the textile industry.
Table 14.1.
Possible applications of chitosan and chitosan-based composites in the textile industry
| Textile fabric | Chitosan-based composite | Property | Reference |
|---|---|---|---|
| Chitosan derivatives | |||
| Cotton, silk | CS- 2-hydroxy-2-methylpropylpropane-1-onea | AB, laundry durability | Periolatto et al. (2012) |
| Cotton | CS nanoparticles-copper | AB, thermal stability, UV protection | Hebeish et al. (2013) |
| Polyester | CS nanoparticles-silver | AB, sustained release of silver | Ali et al. (2011) |
| Cotton | CS-silver nanoparticles | AB, laundry durability | Xu et al. (2016) |
| Cotton | CS-ZnO | AB, slow release of metal, washing stability | Petkova et al. (2014) |
| Cotton | CS-ZnO nanoparticles | AB, UV blocking | Raza et al. (2016) |
| Cotton | CS-CuO nanoparticles | AB | Dhineshbabu and Rajendran (2016) |
| Cotton/polyester | CS-ZnO, RiO2, SiO2 (nanoparticles) | AB, UV protection, self-cleaning, washing durability | Ibrahim et al. (2017b) |
| Cotton | CS-silver nanoparticles, montmorillonite | AB, thermal stability, flame-retarding activity, UV protection, water retention | Rehan et al. (2018) |
| Cotton | CS-poly(N-isopropylacrylamide) –silver nanoparticles | AB, controlled release of silver | Štular et al. (2017) |
| Cotton | CS-silver-zeolite film | Antimicrobial | Scacchetti et al. (2017) |
| Cotton | LBL CS and graphene oxide | UV protection, laundering durability | Tian et al. (2016) |
| Cotton | CS-poly(2-acrylamide-2-methylpropane sulfonic acid salt). LBL film | AB | Cheng et al. (2016) |
| Cotton | CS-(N,N,N-three methyloxirane methylammonium chloride) | Antimicrobial wound dressing, moisture retention | Yin et al. (2018) |
| Cotton | CS-poly(N-isopropylacrylamide) | AB, thermosensitivity | Wang et al. (2016a) |
| Cotton | CS-N-benzyl-N,N diethyl quaternary ammonium salt | AB | Feng et al. (2016) |
| Wool | CS-poly(propylene) imine | AB, durable washings | Sadeghi-Kiakhani et al. (2013) |
| Silk (Antheraea pernyi) | CS-(N-[(2-hydroxy-3-trimethylammonium) propyl] chloride nanoparticles | AB, durable wrinkle and shrinkage resistant, laundry durability | Lu et al. (2014) |
| Polyester (polylactic acid) | CS-poly(vinyl alcohol) | Thermally stable blend | Grande et al. (2018) |
| Cotton | CS-coating pyrazole compounds | AB | Nada et al. (2018) |
| Wool | CS-cyanuric acid | AB, improved dyeing performance | Zargarkazemi et al. (2015) |
| Polyester | CS covered by nanonized polyaniline | Electrical conductivity, water repellency, stable laundry | Tang et al. (2014) |
| Plant extracts and aromatic textiles | |||
| Cotton | CS-neem seed extract | AB, antiviral | Revathi and Thambidurai (2017) |
| Cotton | CS-beeswax are impregnated with essential oils (Eucalyptus, tea tree, sage) | AB, slow release of fragrant | Cerempei et al. (2015) |
| Cotton | CS microcapsules containing essential oils (Eucalyptus, sandal wood) | AB | Javid et al. (2014) |
| Cotton | CS-β-CD, inclusion of cinnamon oil | AB, slow release of fragrant | Bashari et al. (2017) |
| Cotton | CS β-CD, inclusion of lavender oil | AB, slow release of fragrant | Singh et al. (2017) |
| Cotton | CS-vanillin microcapsules | AB, slow release and retained fragrant after wash cycles | Yang et al. (2014) |
| Flame retardation | |||
| Cotton | CS-diammonium hydrogen phosphate | Durable flame retardation | El-Tahlawy (2008) |
| Cotton | LBL CS and ammonium polyphosphate | Itumescent flame effect | Fang et al. (2015) |
| Cotton | CS phosphate-TiO2 nanoparticles-1,2,3,4-butane tetracarboxylic acid, hypophosphite | AB, flame retardation | El-Shafei et al. (2015) |
| Cotton/polyester | LBL CS and melamine polyphosphate | Flame retardation | Leistner et al. (2015) |
| Cotton | LBL CS and ammonium polyphosphate | Flame retardation | Jimenez et al. (2016) |
| Polyamide 66 fabric | CS-phytic acid, oxidized sodium alginate | Flame retardation | Kundu et al. (2017) |
| Acrylic fabric | LBL CS and montmorillonite | Flame retardant | Carosio and Alongi (2018) |
| Textile wastewater and dye removal | |||
| Cotton | UV-grafted CS | Absorbance and removal of excess dyes | Periolatto and Ferrero (2013) |
| Textile fabrics | CS beads impregnated with ZnO | Photodecolorization of Rhodamine B & Methylene Blue dyes | Farzana and Meenakshi (2015) |
| Textile fabrics | Laccase immobilized on CS-cerium (VI) dioxide microspheres | Decolorization of methyl red and orange II reactive dyes | Lin et al. (2015) |
| Textile fabrics | CS plus ferrous sulfate | Decolorization | Kos (2016) |
| Textile fabrics | CS-coating ZnO nanoparticles-Fe3O4 nanoparticles | Removal of azo dye (reactive blue 198), recyclable composite | Nguyen et al. (2015) |
| Textile fabrics | CS-coating Fe3O4 nanoparticles | Removal of azo dye (Acid Red 2) | Kadam and Lee (2015) |
| Textile fabrics | Acrylic acid grafted on Jute fibers followed by immobilization of CS | Desorption of anthraquinone dye | Hassan (2015) |
| Textile fabrics | Manganese peroxidase immobilized on CS beads | Degradation and detoxification of dyes | Bilal et al. (2016) |
| Textile fabrics | Manganese doped in CS-ZnO | Photocatalytic degradation of azo dye | Nguyen et al. (2016) |
| Textile fabrics | CS-poly(methacrylic acid)-TiO2 microparticles | Removal and degradation of anionic azo dyes | Škorić et al. (2016) |
AB antibacterial effects, CS chitosan, LBL layer by layer deposition, β-CD β-cyclodextrin
aPhotochemical reaction by UV generating cross-linked polymers
Utilization of Chitosan in Cosmetics
Chitin and, in particular, chitosan and its derivatives provide advantageous properties in the cosmetic area. They are biocompatible and adhere to surface components of the skin and hair, forming elastic films with moisturizing and water retention capabilities. They can serve as vehicles for encapsulated cosmetic ingredients and their controlled delivery and release and formation of gels in mixtures with water and alcohol and have some antimicrobial, antioxidant, and anti-inflammatory activities, with the additional important benefit of low cytotoxicity (Lee et al. 2013; Jimtaisong and Saewan 2014; Aranaz et al. 2018).
Chitosan and its derivatives are included in cosmetic formulations and products for mainly care and protection of the skin and hair but inter alia in tooth enamel and tooth lacquer, nail lacquer, lipsticks, cleansing and bath materials, toothpaste, mouthwash, chewing gum, deodorants, and breath refresheners (Dutta et al. 2004). Aging of the skin, viewed as wrinkling, dryness, loss of elasticity, dehydration, and hyperpigmentation, is the result of long-term exposure to sunlight UV, which mainly forms reactive oxygen species. Protection from photoaging is a major drive in the cosmetic industry. For example, chitooligosaccharides per se were able to protect UV-irradiated hairless mouse skin from photoaging damage (Kong et al. 2018). Gel formulation of chitosan microparticles served as a delivery system for the sustained release of the hydrophilic sunscreen, phenylbenzimidazole sulphonic acid (Gomaa et al. 2010). The cosmetic gel formulation of blended chitosan, collagen, and , with antibacterial and antioxidant effects, proved useful in the regeneration and rejuvenation of the skin using cultured mouse fibroblast (Rajashree and Rose 2017). Microspheres composed of carboxymethyl chitosan/collagen peptides-calcium chloride protected mice skin and thymus lymphocytes from UV-B radiation damage (Liu et al. 2015b). A cosmetic cream formulation composed of quaternized carboxymethyl chitosan-montmorillonite nanocomposite bestowed good UV protection and additional moisturizing and water retention effects (Chen et al. 2017). There is a possible cosmetic use of neutralized chitosan in citrate buffer film for skin exfoliation (Libio et al. 2016).
Chitosan and various chitosan derivatives, as active ingredients, were examined in cosmetic hair care products like shampoos, permanent wave agents, hair conditioner, styling lotions, rinses, hair colorant, hair sprays, and hair tonics (Dutta et al. 2004; Aranaz et al. 2018). They can adhere to the negatively charged hair keratin forming a transparent elastic film that covers hair fibers endowing smoothness, softness, and also mechanical strength (Dutta et al. 2004). A blend of chitosan and two other biopolymers like collagen and hyaluronic acid that forms a thin film over hair surface provides enhanced mechanical strength and improved conditioning of the treated hair (Sionkowska et al. 2017). Chitosan as a targeting vehicle to hair follicles in the skin was demonstrated with entrapped minoxidil, a medication to treat hair loss (Gelfuso et al. 2011; Matos et al. 2015). Microparticles and nanoparticles of chitosan-encapsulating minoxidil enabled its controlled release.
A large number of possible applications of cosmetic formulations containing chitosan and its derivatives have been patented. It is noteworthy that formulations containing chitosan are already in the busy cosmetic market.
Biomedical Applications of Chitosan Derivatives
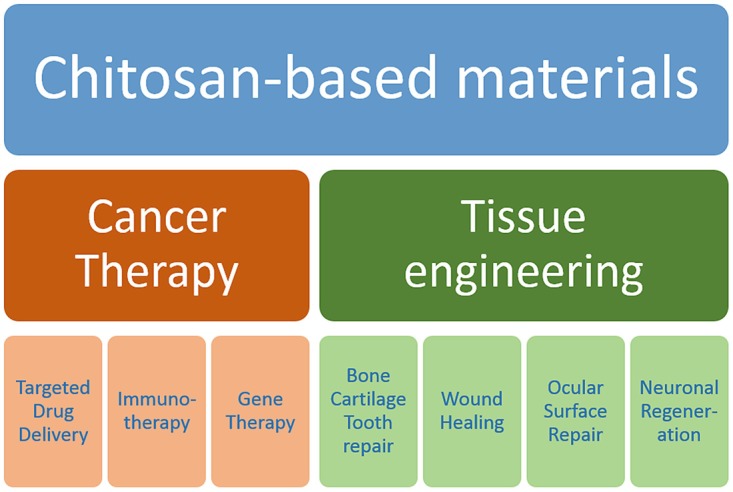
Because chitosan and many of its derivatives are nontoxic, biocompatible, biodegradable, and highly versatile polymers, a large assortment of possible biomedical applications have been explored, of which some were implemented into therapeutic strategies by the pharmaceutical industry. To obtain optimal materials for the delivery of drugs, several factors have to be considered including the stability of the bioactive agents, absorption properties and mucoadhesiveness, gelling properties, particle sizes, permeation and transfection-enhancing properties, efflux pump inhibition, tissue targeting, residual toxicity of the final products, as well as release kinetic profiles. Chitosan derivatives have been developed into different kinds of pharmaceutical excipients used for the production of tablets and capsules (Illum 1998; Werle and Bernkop-Schnurch 2008), suppositories (Caramella et al. 2015), sprays (Osman et al. 2013), ointments (Kang et al. 2016), eye drops (Basaran and Yazan 2012), and wound dressings (Bano et al. 2017). The drugs are usually encapsulated by ionotropic gelation, spray drying, emulsion solvent evaporation, and coacervation (Panos et al. 2008). Chitosan-based excipients have been found useful in tablet disintegration and drug dissolution (Illum 1998) and in enhancing penetration and absorption properties (Thanou et al. 2001; van der Merwe et al. 2004; Sahni et al. 2008). Most importantly, certain dosage forms allow the controlled release of drugs (Jennings et al. 2015; Fonseca-Santos and Chorilli 2017). These include chitosan-based hydrogels (Knapczyk 1993; Kristl et al. 1993; Berger et al. 2004; Ishihara et al. 2006; Elviri et al. 2017) and micro-/nanoparticles for drug delivery (Hamman 2010). Here, we will focus on the applications of chitosan-based matrices in drug delivery for cancer, immune, and gene therapy, and we will summarize some recent advances in tissue engineering (Fig. 14.1).
Fig. 14.1.
Overview on biomedical applications of chitosan-based materials in cancer therapy and tissue engineering
Chitosan-Based Drug Carrier Systems
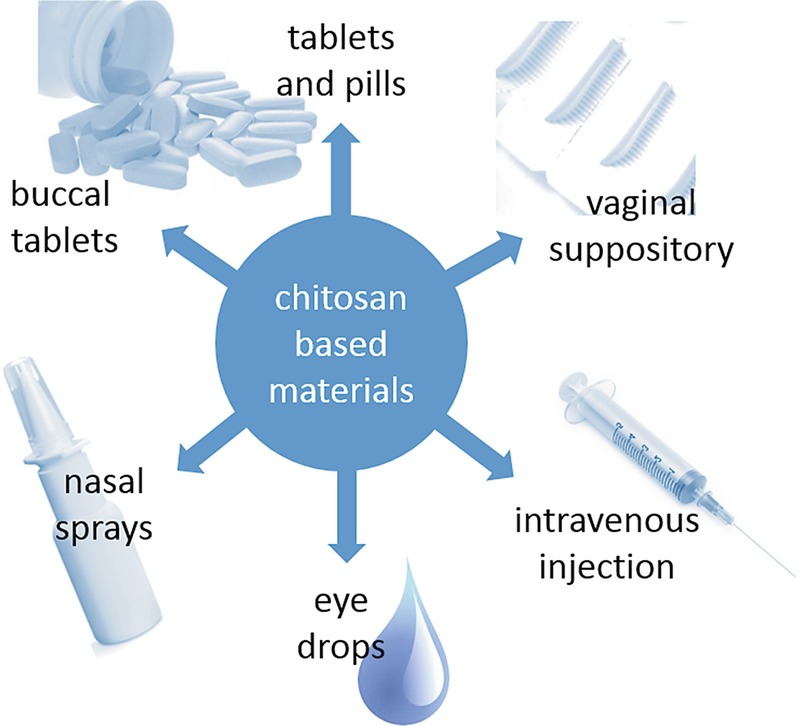
Chitosan-based materials can be used in various forms as drug delivery system (Fig. 14.2). Tablets are probably the most favorable and accurate dosage form, which are moreover easy to fabricate and handle. A simple method for their production is homogenization of the drug and chitosan and compressing the resulting mixture to tablets. However, it has to be considered that due to the alkaline conditions in the distal intestine, drug absorption is restricted to the more proximal regions of the gastrointestinal tract when pure chitosan is used which precipitates at an alkaline pH (Sakkinen et al. 2004; Dhaliwal et al. 2008). Therefore, more pH-insensitive formulations using higher-charged chitosan derivatives such as trimethylated chitosans or thiolated chitosan conjugates have improved absorption properties along the gastrointestinal tract. Although there is still a lack of robust data in human volunteers, some studies indicate that tablet formulations using higher-charged chitosan derivatives increase bioavailability due to improved mucoadhesiveness and better protection of the drug from degrading enzymes (van der Merwe et al. 2004). Chitosan-based tablets have been also examined for their use in vaginal drug delivery, mainly as carriers for antiviral and antifungal therapeutics (El-Kamel et al. 2002; Senyigit et al. 2014; Frank et al. 2017). However, the antimicrobial properties of chitosan may negatively affect the vaginal microflora, and hence long-term treatment should be critically evaluated (Raafat and Sahl 2009).
Fig. 14.2.
Chitosan-based drug delivery systems
As chitosan-based hydrogels facilitate equal distribution and increase mucoadhesiveness, permeation, and bioavailability, they are effective formulations for eye drops to administer therapeutic drugs in ophthalmology (Krishnaswami et al. 2018). Chitosan-based formulations used in eye care include hydrogels, nanoparticles, and liposomal and colloidal systems (De Campos et al. 2001; De Campos et al. 2003; Diebold et al. 2007; Gupta et al. 2010). For similar reasons, they are also in use for nasal drug delivery, which is impaired by high turnover and secretion rates (Illum 2003). Notably, chitosan-coated lipid micro- and nanoparticles have been developed for nose-to-brain delivery of a variety of therapeutic drugs (Casettari and Illum 2014; Sarvaiya and Agrawal 2015). Chitosan-based nanoparticles are also a promising carrier for buccal drug delivery, which has the advantage of avoiding the hepatic first-pass metabolism and degradation in the gastrointestinal system (Sandri et al. 2005). Polymeric carriers generally have the potential advantage of prolonged release times of low-molecular-weight drugs. Because chitosan is additionally susceptible to hydrolysis by lysozyme in the blood serum, which facilitates drug release, and exhibits no toxic or hemolytic effects when applied parenteral (Nordtveit et al. 1994; Richardson et al. 1999), chitosan-based formulations are also suitable carriers for controlled drug release when administered by intravenous injection (Thanoo et al. 1992).
Chitosan-Based Drug Delivery Systems in Chemotherapy
Conventional chemotherapeutics are frequently not very effective in reaching the tumor cells, as solid tumors are not well supplied with blood, and lack lymphatic vessels, which results in and decreased convective flow in the interstitial fluid. To overcome these problems, novel drug delivery systems have been designed. These carriers are capable of encapsulating high concentrations of the cytotoxic compound within a macromolecular matrix that specifically targets the cargo to the tumor cells where the drugs are finally released in a controlled manner. This concept profits from the EPR (enhanced permeability and retention) effect, the phenomenon that macromolecules preferentially accumulate in solid tumors, probably because they have a defective vasculature and lack effective lymphatic drainage (Matsumura and Maeda 1986). Chitosan-based nanoparticles have many properties that make them suitable carriers for anticancer drugs. Next to their great chemical flexibility, allowing the design of selective carriers, chitosan-based materials evidently exhibit also the EPR effect depending on the tumor microenvironment (Yhee et al. 2017). Moreover, they are degraded inside the body into fragments which can be cleared by the kidney (Kean and Thanou 2010), and several studies suggested that chitosan itself has antitumor effects (Qi and Xu 2006; Yao et al. 2013a), making this polymer a highly suitable supplementary antitumor drug and drug carrier. Indeed, chitosan-based nanocomposites can be used to deliver hydrophilic and hydrophobic drugs such as doxorubicin hydrochloride and paclitaxel, respectively (Kim et al. 2006; Yousefpour et al. 2011). Studies analyzing chitosan-based drug delivery systems for cancer treatment are summarized in Table 14.2.
Table 14.2.
In vitro and in vivo studies using chitosan-based nanoparticles in various cancer treatments
| Drug/targeting | Chitosan-based composite | Experimental system, effects | Reference |
|---|---|---|---|
| Chemotherapeutic drug delivery | |||
| Doxorubicin | CS-dextrane conjugate | Mice, AT, prolonged circulation | Mitra et al. (2001) |
| Doxorubicin/trastuzumab | CS cross-linked by succinic anhydrate, Lys thiolation | SKOV-3 cells, AT, targets HER2+ receptors, enhanced uptake | Hebeish et al. (2013) |
| Doxorubicin | CS-pluronic F127 micelles | MCF7 cells, AT, high drug loading capacity | Naruphontjirakul and Viravaidya-Pasuwat (2011) |
| Doxorubicin/luteinizing hormone RH | CS-/poly(methyl vinyl ether maleic acid, magnetic nanoparticles | MCF7 cells, AT, increased cytotoxicity, targeting LHRH receptors | Varshosaz et al. (2016) |
| Doxorubicin/folate | CS-coated magnetic nanoparticles | U87 cells in athymic mice, AT, guide by magnetic field, decreased tumor growth | Yang et al. (2017) |
| Doxorubicin | Aluminosilicate zeolite (ZSM-5) CS core-shell nanodisks | Mice, AT, pH-dependent drug release, reduced Tu growth and increased apoptosis | Yang et al. (2018) |
| Doxorubicin | CS-cobalt-ferrite-titanium oxide nanofibers | B16F10 cells, AT, fast drug release at low pH and alternating magnetic field | Radmansouri et al. (2018) |
| Doxorubicin, verapamil/cRGD | Magnetic CS-poly(lactic acid-co-glycolic acid) nanoparticles | HepG2 and S-180 cells, Tu-bearing mice, AT, accumulation in tumor tissue | Shen et al. (2013a) |
| Paclitaxel | Glycol-CS-β-cholanic acid nanoparticles | Tu-bearing mice, AT, impaired tumor growth after injection | Kim et al. (2006) |
| Paclitaxel | CS-glyceryl monooleate core-shell nanopoparticles | MDA-MB-231cells, AT, 1000-fold reduction in IC50 | Trickler et al. (2008) |
| Glycol-CS-β-cholanic acid nanoparticles | Tu-bearing mice, AT, impaired tumor growth after injection, EPR | Kim et al. (2008) | |
| CS-polyaspartic acid sodium salt | Mice, sustained drug release in vitro and in vivo | Zheng et al. (2007) | |
| 5-Fluorouracil/hyaluronidase | CS-polyethylenglycol-gelatin copolymer | COLO-205 and HT-29 cells, AT, increased cytotoxicity by uptake and controlled drug release | Rajan et al. (2013) |
| 5-Fluorouracil | N-succinyl-CS-g-poly(acrylamide-co-acrylic acid) | Simulated gastric and intestinal fluids, efficient drug loading pH-dependent drug release | Bashir et al. (2017) |
| 5-Fluorouracil/ | cystamine conjugated CS- methoxy poly(ethylene glycol) | MCF7 cells, AT, improved hemocompatibility, high cytotoxicity to cancer cells | Antoniraj et al. (2018) |
| TNF-α/anti-EGFR-2 | CS-silica hollow nanospheres | MCF-7 cells, AT, pH-dependent TNF-α release inside tumor | Deng et al. (2011b) |
| Oxaliplatin/hyaluronic acid | CS nanoparticles encapsulated in Eudragit S100 coated pellets | Mice, HT-29 cells, AT, specific drug delivery in the colon | Jain et al. (2010) |
| Trans-resveratrol/ , | CS nanoparticles | HepG2 cells, cytotoxicity highest when both, avidin and biotin, were coupled | Bu et al. (2013) |
| /anti-EGFR, anti-chitosan | Glycol-CS nanobioconjugate | SW1990 cells, effective inhibition of cell proliferation, colony formation, migration, and invasion | Xiao and Yu (2017) |
| Cancer gene therapy | |||
| Survivin-siRNA/ | N-trimethyl CS-TPP developed for pulmonary delivery | A549 cells, bronchoalveolar lavage fluid, effective gene silencing of the survivin gene resulting in apoptosis | Ni et al. (2018) |
| Midkine-siRNA | CS combined with 2-chloroethylamine and N,N-dimethyl-2-chloroethylamine hydrochloride | HepG2 cells, efficient transfection, significant decrease of cell proliferation | Zhong et al. (2015) |
| psiRNA-hBCL2/dendrimeric RGD | Polyethyleneimine-g-CS | Tu-bearing mice, AT, efficient and specific transfection of tumor cells and silencing of anti-apoptotic hBcl2 | Kim et al. (2017) |
| Ovalbumin | CS nanoparticles | Mice, AT, increased cytokine levels and stimulation of natural killer cells, deacreased tumor growth, detection of ovalalbumin specific cytotoxic T cells | Wen et al. (2011), Highton et al. (2016) |
| IL-12 | CS nanoparticles | Mice, AT, activcation of cytotoxic T cells and natural killer cells, tumor regression, nor recurrence | Zaharoff et al. (2009) |
| GRP | Mannosylated CS nanoparticles | Mice, intranasal application, AT, enhanced tumor regression paralleled by anti-GRP antibody production | Yao et al. (2013b) |
| IP-10 plasmid/folate | CS nanoparticles | Mice, AT, inhibition of cell proliferation, induction of apoptosis, suppression of angiogenesis, and inactivation of regulatory T cells | Lai et al. (2014) |
AT anti-tumor effects, CS chitosan, Tu tumor
To target tumor cells by the EPR effect passively, Mitra et al. (2001) fabricated chitosan-based nanoparticles of about 100 nm carrying a dextran-doxorubicin conjugate and examined the antitumor effects in vivo in macrophage tumor cells implanted into BALB/c mice. The authors observed an improved therapeutic efficacy of dextran-doxorubicin loaded chitosan nanoparticles, which is probably due to the prolonged circulation time and/or drug accumulation at the tumor sites. In another study published by Yousefpour et al. (2011), doxorubicin was conjugated to chitosan using succinic anhydride as a cross-linker. In a second step, the resulting self-assembled chitosan-doxorubicin conjugate nanoparticles were conjugated with trastuzumab, a monoclonal antibody to the human epidermal growth factor receptor 2+ (Her2+), via lysine thiolation and subsequent linking of the derived thiols to chitosan. The Trastuzumab conjugated chitosan-doxorubicin nanoparticles selectively targeted Her2+ cancer cells resulting in enhanced uptake when compared to chitosan-doxorubicin particles and the free drugs. In another study, pluronic F127, a block copolymer of hydrophobic polyoxypropylene flanked by two chains of hydrophilic polyoxyethylene, was grafted onto chitosan to generate a copolymer micelle that can encapsulate doxorubicin (Naruphontjirakul and Viravaidya-Pasuwat 2011). The resulting chitosan-pluronic micelles carrying doxorubicin showed a high drug loading capacity and revealed a higher cytotoxic activity to MCF7 breast cancer cell lines in vitro than the free drug. Another approach to deliver doxorubicin specifically to cancer cells was reported by Varshosaz et al. (2016). The research team fabricated dual targeted nanoparticles loaded with doxorubicin and magnetic nanoparticles to treat breast cancer. For this purpose, the nanoparticles were produced via a layer-by-layer technique and functionalized with a bioconjugate of chitosan/poly(methyl vinyl ether maleic acid) and luteinizing hormone-releasing hormone (LHRH) to target corresponding receptors on the surface of MCF7 breast cancer cells, which presumably take up the particles by endocytosis. The targeted nanoparticles increased the cytotoxicity of doxorubicin about twofold in LHRH-positive cancer cells. In a similar approach, folate-grafted chitosan-coated magnetic nanoparticles were loaded with doxorubicin to target human glioblastoma U87 cells in athymic BALB/c nude mice in a subcutaneous tumor model system (Yang et al. 2017). Guiding the injected nanoparticles to the tumor by a magnetic field significantly decreased tumor growth by controlled delivery of doxorubicin to the cancer cells and demonstrated the feasibility of magnetic nanoparticles to direct the localization of drug release. Mesoporous aluminosilicate zeolite (ZSM-5) chitosan core-shell nanodisks loaded with doxorubicin were used as pH-responsive drug delivery systems against osteosarcoma that release the drug after upon endosomal acidification (Yang et al. 2018). Recently, Radmansouri et al. (2018) showed that doxorubicin-loaded electrospun chitosan/cobalt ferrite/titanium oxide nanofibers could be used for localized melanoma cancer therapy. The fastest release of doxorubicin from prepared magnetic nanofibers was observed at acidic pH when an alternating magnetic field was applied. As mentioned above, chitosan-based nanoparticles can also be modified to carry hydrophobic drugs such as paclitaxel. For this purpose, hydrophobic side chains are grafted onto chitosan. For instance, Kim et al. (2006) used glycol chitosan nanoparticles that were hydrophobically modified with β-cholanic acid and incorporated paclitaxel. The resulting nanoparticles showed sustained drug release, and following injection into the tail vein of tumor-bearing mice, tumor growth was impaired. In a subsequent study, the same research team used this system as carrier for cisplatin, which is also poorly soluble in water. The hydrophobically modified glycol chitosan nanoparticles loaded with cisplatin exhibited the EPR effect, as they accumulated in solid tumors, and was proven to have a high antitumor efficacy in a tumor-bearing mice model (Kim et al. 2008). Paclitaxel was also encapsulated in chitosan-containing glyceryl monooleate core-shell nanoparticles, which were generated by the emulsification/evaporation technique (Trickler et al. 2008). Using this drug delivery system, the authors observed a 1000-fold increase in cytotoxicity, when determining the IC50 values in a human breast cancer cell line. Another common hydrophobic anticancer drug is 5-fluorouracil, which has been widely used to treat different kinds of solid tumors. In a study by Zheng et al. (2007), polyelectrolyte nanoparticles based on chitosan and polyaspartic acid sodium salt were used to encapsulate 5-fluorouracil testing various conditions for nanoparticle preparation such as temperature, ionic strength, pH and cross-linker concentration, and different loading methods. The optimized nanoparticles showed sustained drug release in vitro and in vivo. Rajan et al. (2013) prepared hyaluronidase-5-fluoruracil-loaded chitosan-polyethylenglycol-gelatin copolymers as a targeted drug delivery system and examined particle size, distribution, morphology, and drug loading capacity. The nanoparticles showed less cytotoxicity than free 5-fluorouracil when applied to colon cancer cells for a few hours. Another approach for controlled drug delivery used molecular surface imprinted graft copolymer of chitosan with methyl methacrylate, which was prepared by free-radical polymerization with 5-fluorouracil as template molecule (Zheng et al. 2016). The pH dependency and the kinetics of drug release suggested that this chitosan-based carrier is optimal for orally applied colon-specific drug delivery. A similar strategy to achieve colon specificity was used recently by Bashir et al. (2017). They synthesized pH-responsive semi-interpenetrating network hydrogels of N-succinyl-chitosan via Schiff base mechanism using glutaraldehyde as a cross-linking agent and embedded poly(acrylamide-co-acrylic acid). The hydrogel exhibited a porous structure and pH-dependent swelling properties. The hydrogel was effectively loaded with 5-fluorouracil, and the determined drug release was pH-dependent as well, with high release rates at pH 7.4 and low rates at pH 1.2.
In many cases, chitosan nanoparticles have been conjugated with tumor-specific ligands to mediate active targeting of cancer cells, which is expected to increase therapeutic efficacy, accelerate drug release to selected sites, prevent unwanted drug release before arrival at the target sites, and diminish adverse side effects of chemotherapeutic drugs. Active targeting can be accomplished by functionalizing chitosan-based nanoparticles and hydrogels using tumor-targeting ligands, which bind to specific receptors that are specifically present on the surface of cancer cells. Proper ligands of such kind include cytokines, peptides, folic acid, hyaluronic acid, biotin or avidin, and antibodies (Prabaharan 2015). Here, we will discuss only a one example for each of these ligands to illustrate active targeting.
Deng et al. (2011b) synthesized monodispersed and pH-sensitive chitosan-silica hollow nanospheres, loaded them with antitumorigenic tumor necrosis factor α (TNF-α), and conjugated them with an antibody to epidermal growth factor receptor 2 , which is overexpressed in about 20% of all women suffering from breast cancer (Owens et al. 2004). Subsequent drug release studies demonstrated that the nanospheres delivered cytotoxic TNF-α to MCF-7 breast cancer cells and suppressed tumor with high therapeutic efficacy. Due to the acidic microenvironment inside solid tumors, TNF-α is gradually released from the nanospheres, binds to the TNF-α receptor, and activates a signaling cascade which induces programed cell death.
In a study published by Shen et al. (2013a), doxorubicin and verapamil were combined in chitosan nanoparticles to achieve an integrated treatment for cancer and doxorubicin-induced cardiomyopathy in the process of cancer therapy. For this purpose, chitosan shells coated on magnetic nanoparticles were loaded with both drugs and entrapped into poly(lactic acid-co-glycolic acid) nanoparticles conjugated with a cyclo(Arg-Gly-Asp-D-Phe-Lys) (cRGD) peptide targeting αvβ3 integrin, which is highly expressed on activated endothelial cells of newborn vessels during tumor angiogenesis as well as in some tumor cells (Liu et al. 2008). Near-infrared laser irradiation was sufficient to trigger drug release within an acidic microenvironment. Cytotoxicity assays performed in vitro suggested that cRGD-conjugated nanoparticles exhibited a greater growth inhibitory potential in cancer cell lines than the free drug or control nanoparticles likely due to cRGD-mediated targeting of tumor cells. In vivo imaging and biodistribution studies further showed that the nanoparticles preferentially accumulated in the tumor tissue under magnetic guidance. Finally, in vivo data for tumor regression along with electrocardiogram recordings and histopathology observations indicated that the cRGD-conjugated polymer-coated magnetic nanoparticles could have a high therapeutic potential as a dual-drug delivery system for the treatment of both cancer and doxorubicin-mediated cardiotoxicity.
Recently, a novel disulfide-linked chitosan-g-methoxy poly(ethylene glycol) copolymer was successfully synthesized, which was suggested to have excellent properties for redox-responsive drug delivery (Antoniraj et al. 2018). Redox-responsive 5-fluorouracil-loaded nanoparticles were synthesized by ionic gelation method, and folic acid was used to functionalize the nanoparticles for receptor-targeted drug delivery, as cancer cells commonly express high-affinity folate receptors on their surface. The 5-fluorouracil-free nanoparticles showed improved hemocompatibility, and the 5-fluorouracil-loaded nanoparticles conjugated with folic acid had a high cytotoxicity to MCF7 breast cancer cells, presumably due to intracellular internalization because of folic acid conjugation, which is expected to enhance the cellular uptake of the nanoparticles.
Many types of cancer cells overexpress different isoforms of hyaluronic acid receptors, which leads to enhanced binding and internalization of hyaluronic acid, as reported for instance in breast tumor cells (Bourguignon et al. 2000). To exploit this fact for targeting tumor cells, Jain et al. (2010) prepared hyaluronic acid-conjugated chitosan nanoparticles loaded with oxaliplatin and encapsulated in Eudragit S100-coated pellets for effective delivery to colorectal tumors. In immunodeficient C57BL mice model with HT-29 cancer cells injected into the ascending colon, relatively high local drug concentrations were found in the colon tumors after oral administration of the oxaliplatin nanoparticles, and the concentrations increased with prolonged exposure time. Coupling of hyaluronic acid onto the surface of chitosan nanoparticles was found to make them more specific for delivery of the anticancer drug to the tumor of the colon.
Several studies revealed that also biotin and avidin possess tumor-targeting properties. Biotin receptors are overexpressed in many tumor types characterized by rapid division rates and aggressive growth (Russell-Jones et al. 2004), and avidin (a highly glycosylated protein) is recognized by lectins expressed on the surface of tumor cells (Yao et al. 1998). For this reason, Bu et al. (2013) prepared chitosan nanoparticles conjugated with either biotin or both biotin and avidin as tumor-targeted carrier system for the delivery of trans-resveratrol. Pharmacokinetic experiments revealed that avidin-biotin-loaded nanoparticles rapidly accumulated in the liver after injection, while the delivery nanoparticles conjugated only with biotin was attenuated. Cytotoxicity assays using HepG2 cells further uncovered that compared to trans-resveratrol solution and unconjugated chitosan nanoparticles, both biotin and avidin-biotin loaded nanoparticles significantly improved anticancer activity, but the latter combination exhibited a higher cytotoxicity. Thus, it was proposed that the synthesized nanoparticles conjugated with avidin and biotin may be a potent drug delivery system particularly to targeting hepatic carcinoma.
Finally, Xiao and Yu (2017) developed a glycol/chitosan nanobioconjugate loaded with gemcitabine and conjugated with anti-EGFR and anti-chitosan antibodies to target pancreatic cancer cells and cause aggregation. Administration of the chitosan conjugates efficiently blocked tumor growth and metastatic spread in human pancreatic cancer cells.
Chitosan-Based Vectors for Gene Therapy
Gene therapy requires the transmission of nucleic acids (DNA or RNA) into the target cell to mediate expression of therapeutic genes or to silence gene expression by RNA interference. However, negatively charged phosphates of nucleic acids impair permeation through the plasma membrane, which is negatively charged as well. In addition, unprotected nucleic acids are highly susceptible to degradation by nucleases. Hence, delivery of nucleic acids into cells relies on non-viral or viral vectors, which drastically improves transfection and protects from enzymatic degradation (Wivel and Wilson 1998). As viral vectors have the risk of causing adverse side effects such as immune reactions and malignant transformation, many efforts have been made to develop non-viral vectors for gene delivery, among them are ample examples of different chitosan-based nanoparticles.
Actually, unmodified chitosan is not an effective carrier for the transfer of nucleic acids due to its low solubility in water and instability of DNA/RNA chitosan complexes at physiological pH. Thus, chitosan requires chemical modification or grafting to convey appropriate physicochemical properties to the resulting complex. Chitosan modifications that have been used to design chitosan-based carriers for gene or siRNA delivery include quaternization by alkylation of tertiary amines, reaction with 2-chloroethylamine hydrochloride and N,N-dimethyl-2-chloroethylamine hydrochloride, conjugation with polyethylene glycol, poly(amidoamine) or RGD dendrimer grafting, modification with phosphatidylcholine, or combinations of these modifications.
Among the quaternized chitosan derivatives, N-trimethyl chitosan and its derivatives have been extensively studied for their suitability in gene delivery, because they are reasonably soluble in water, have comparably little tendency to form aggregates, and exhibit a high loading capacity for nucleic acid under physiological conditions. In a systematic study published by Germershaus et al. (2008), the physicochemical properties of chitosan, N-trimethyl chitosan, and poly(ethylenglycol)-N-trimethyl chitosan were analyzed and compared. Using cell lines derived from mouse embryonic fibroblasts as a transfection system for plasmid DNA, the authors observed a significant increase in transfection efficiency when N-trimethyl chitosan nanoparticles were used to deliver plasmid DNA instead of chitosan nanoparticles. In addition, grafting poly(ethylenglycol) onto N-trimethyl chitosan further improved transfection efficiency, stabilized the particles, decreased particle size, and reduced cytotoxicity when compared to unmodified N-trimethyl chitosan nanoparticles. Zheng et al. (2009) prepared folate-conjugated N-trimethyl chitosan nanoparticles and compared cellular uptake and transfection of plasmid DNA (pDNA) in vitro with non-conjugated N-trimethyl chitosan nanoparticles using folate overexpressing KB and SKOV3 cells and folate receptor-deficient A549 and NIH/3T3 cells. The folate-N-trimethyl chitosan/pDNA complex showed a decrease in cytotoxicity in comparison to pDNA complexes made of polyethylenimine. Moreover, folate conjugation increased transfection efficiency and folate receptor-mediated endocytosis by KB cells and SKOV3 cells when compared to non-conjugated N-trimethyl chitosan nanoparticles.
Exploring further possible improvements of N-trimethyl chitosan-based gene delivery systems, Zheng et al. (2015) synthesized arginine, cysteine, and histidine-modified trimethyl chitosan nanoparticles to form complexes with pDNA. Using HEK 239 cells, they evaluated stability, cellular uptake, endosomal escape, release behavior, nuclear localization, and in vitro and in vivo transfection efficiencies. The cysteine-modified N-trimethyl chitosan nanoparticles turned out to be the most promising candidates for gene delivery due to sufficient stability, high cellular uptake, and glutathione-responsive release-favoring mechanism in combination with preferable nuclear distribution. Addition of sodium tripolyphosphate to the cysteine-modified nanoparticles was further effective to compromise certain disadvantageous attributes for pDNA delivery. N-trimethyl chitosan nanoparticles were also employed in drug-siRNA co-delivery using a metastatic breast cancer cell line (Eivazy et al. 2017). In this study, the authors tested simultaneous delivery of siRNA to silence the gene encoding the high mobility antigen (HMGA-2) and the anticancer drug doxorubicin to boost therapeutic anticancer effects. They found that dual delivery of HMGA-2 siRNA and doxorubicin by trimethyl chitosan nanoparticles significantly inhibited breast cancer cells growth.
A very recent study developed novel strategies in fighting lung cancer by RNA interference mediated gene silencing of the gene encoding the anti-apoptotic protein, Survivin. For this purpose, Ni et al. (2018) designed nanoparticles consisting of baclofen functionalized N-trimethyl chitosan as polymeric carriers, TPP as ionic cross-linker, and siRNA to Survivin. Baclofen was used to target the nanoparticles to non-small lung cancer cells that overexpress the GABAB receptor, which specifically binds baclofen (Zhang et al. 2013b). The siRNA-loaded nanoparticles increased the uptake of Survivin-siRNA through the interaction with GABAB receptor and efficiently induced apoptosis and gene silencing. The authors further encapsulated the siRNA-loaded nanoparticles into mannitol microparticles for dispersion in the HFA-134a aerosol to allow administration by pressurized metered-dose inhalers. Pulmonary delivery of siRNA is expected to avoid serum-induced degradation, reduce systemic side effects, and improve therapeutic efficacy.
Zhong et al. (2015) hypothesized that the addition of amino residues to chitosan could improve stable complex formation with negatively charged siRNA enhancing transfection and gene silencing efficiency. For this purpose, they prepared a novel chitosan derivative (MixNCH) combining 2-chloroethylamine hydrochloride and N,N-dimethyl-2-chloroethylamine hydrochloride with chitosan and examined the physicochemical properties of the resulting nanoparticles. Using a hepatocellular carcinoma cell line (HepG2), gene transfection efficiency of MixNCH/midkine-siRNA nanoparticles and inhibition of HepG2 cell proliferation were analyzed. They found that midkine-siRNA delivered by MixNCH nanoparticles was able to significantly reduce both mRNA and protein levels of the midkine growth factor, resulting in a significant decrease of cell proliferation in HepG2 cells.
Guzman-Villanueva et al. (2014) evaluated the capability of different-sized chitosan derivative-based polyplexes to carry, internalize, and release siRNA in human adenocarcinomic epithelial cells. For this purpose, they first prepared N-phthaloyl-chitosan or N-phthaloyl-oligochitosan, reacted them with polyethylene glycol and hydroxybenzotriazole in DMF, and then cross-linked the polymers using ECD. Finally, the N-phthalimido groups were removed by the reaction with hydrazine monohydrate, and the products were purified by dialysis against water and ethanol. Both the chitosan- and oligochitosan-based polyplexes exhibited biodegradability, low cytotoxicity, and resistance to enzymatic degradation up to 24 h. When loaded with siRNA, the oligochitosan-based polyplexes drastically increased cellular internalization of the siRNA and gene silencing compared to naked siRNA. To improve the transfection efficiency of chitosan-based gene delivery systems, Deng et al. (2011a) fabricated a dendronized chitosan derivative using a copper-catalyzed azide alkyne cyclization reaction of propargyl focal point poly(amidoamine) dendron with 6-azido-6-deoxy-chitosan. The resulting dendronized chitosan nanoparticles exhibited higher water solubility and buffering capacity than native chitosan and showed lower cytotoxicity and enhanced transfection efficiency in transformed human embryonic kidney and nasophyryngeal carcinoma cell lines than commonly used polyethyeneimine.
As already mentioned above, the RGD motif can be used for targeting chitosan-based nanoparticles to tumor sites via the interaction with integrin αvβ3. Utilizing this fact, Kim et al. (2017) produced a dendrimeric RGD peptide/polyethyleneimine grafted chitosan copolymer, which was soluble in water. The copolymer was nontoxic to mammalian cells and erythrocytes in the absence and presence of plasmid DNA. Moreover, it was found to transfect cells involving microtubule-dependent macropinocytosis and clathrin-mediated endocytosis. Finally, injecting copolymers complexed with psiRNA-hBCL2 to silence the gene for the human anti-apoptotic Bcl2 protein into BALB/c-nu mice carrying a PC3 prostate tumor xenografts, markedly inhibited tumor growth. Thus, the copolymer was suggested to be a good candidate to develop a specific targeted gene delivery system.
To confer membrane-like properties to chitosan-based gene delivery systems, Li et al. (2015) grafted phosphorylcholine and macrocyclic polyamine onto chitosan to obtain water-soluble nanoparticles. Chitosan grafted with both compounds were more efficient in binding and protecting plasmid DNA than chitosan grafted only with phosphorylcholine or macrocyclic polyamine. The authors also demonstrated that phosphorylcholine and macrocyclic, polyamine-grafted chitosan had a positive net charge and can, therefore, wrap DNA to yield nanoparticles of about 100 nm in diameter. Finally, the DNA-loaded nanoparticles significantly increased cellular uptake and transfection rates in transformed human embryonic kidney cells when compared to chitosan/DNA complexes. A similar transfection strategy was published by Picola et al. (2016), who inserted phosphorylcholine and increasing numbers of diethylaminoethyl (DEAE) groups into the polymer. The resulting chitosan nanoparticles were water soluble at physiological pH and less cytotoxic than lipofectamine, a commonly used transfection reagent. They further could form complexes with plasmid DNA, and the transfection efficiencies of the nanoparticles with high DEAE substitution rates tested in HeLa cells were in the same range as determined for lipofectamine. When the nanoparticles were loaded with siRNA, they were able to induce gene silencing, with efficiencies highly dependent on the N/P ratio.
Chitosan-Based Adjuvants for Vaccine in Immunotherapy
Chitosan-based materials have been recognized to be potent adjuvants for immunotherapy, because they non-specifically stimulate immune responses in the host organism and therefore have antiviral, antimicrobial, and antitumor properties (Li et al. 2013b). The adjuvant potency of chitosan is comparable to incomplete Freund’s adjuvant, and it has stronger immune-stimulatory effect than aluminum hydroxide, which is frequently used in vaccines though it shows adverse side effects such as neurotoxicity (Zaharoff et al. 2007). The mechanism of how chitosan triggers immune responses involves phagocytosis-dependent activation of the NOD-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome, which finally induces a robust interleukin-1β response (Bueter et al. 2011, 2014). Carroll et al. (2016) described another mechanism by which chitosan stimulates the activation of dendritic cells inducing cellular immunity. They found that chitosan promotes the intracellular release of DNA, which involves the cGAS-STING pathway. As a result, type I interferon is secreted activating the expression of interferon-controlled genes. Due to the release of the cytokines that stimulate dendritic cells, the cellular immune system is elicited.
Due to its mucoadhesiveness, chitosan and its derivatives are considered effective for mucosal administration, which includes oral, nasal, as well as ocular antigen-delivery routes. However, other routes are also anticipated to be effective in provoking immune response including subcutaneous (Borges et al. 2008; Scherliess et al. 2013), intraperitoneal (Chang et al. 2010), intravenous (Shi et al. 2011), and intratumoral injections (Zaharoff et al. 2010). Evidently, innate immune responses are stimulated by chitin, chitosan, or derivatives (Peluso et al. 1994; Tokura et al. 1999; Lee et al. 2008; Lee 2009). This includes the activation of alveolar macrophages with the release of cytokines such as interleukin (IL)-12, tumor necrosis factor-α, or IL-18, leading to INF-γ production predominantly released by natural killer cells (Shibata et al. 1997a, b). However, also humoral and cellular adaptive immune responses are triggered by some antigens when co-administered or encapsulated in chitosan-containing micro- and nanoparticles (Tokura et al. 1999; van der Lubben et al. 2001; Arca et al. 2009; Mori et al. 2012). In particular, Wen et al. (2011) found that the stimulatory effect on the humoral and cellular immune system by chitosan results in a balanced Th1/Th2 response. However, care has to be taken in assessing the properties of chitosan-based adjuvants, as many studies do not provide sufficient data on the chemical and physical characteristics, preparation and formulation procedures, as well as potential impurities (Vasiliev 2015). This is particularly critical, as immune responses appear to depend on these parameters as uncovered by Scherliess et al. (2013), who reported that the degree of immune response varied when chitosans of different qualities were used. The immune-stimulatory effect of chitosan is affected by the combination of molecular weight, solubility, particle size, and viscosity as well as deacetylation degree.
Preclinical studies performed predominantly in mice models suggested that chitosan-containing antigen-delivery systems are promising adjuvant platforms for mucosal vaccination against human pathogenic viruses such as influenza (Read et al. 2005; Svindland et al. 2012; Sawaengsak et al. 2014; Liu et al. 2015a); hepatitis A, B, and E (Jiang et al. 2007; Tao et al. 2017a; Tao et al. 2017b; Soares et al. 2018); human papilloma virus (Ma et al. 2015); and poliovirus (Ghendon et al. 2011). Combinations of chitosan and heat-inactivated human herpes viruses (HSV) were further tested as an immunomodulating adjuvant in T cells and antigen-presenting cells in HSV-infected mice (Choi et al. 2016). Using chitosan nanoparticles targeted to dendritic cells via antibodies to the DEC-205 surface receptor, Raghuwanshi et al. (2012) successfully delivered plasmid DNA carrying the cDNA for the N protein to trigger immunization against the severe acute respiratory syndrome coronavirus (SARS-CoV). Simultaneous comparison of targeted formulations using intramuscular and intranasal routes revealed that intramuscular administration induced a more potent systemic IgG response compared to intranasal administration. Solid evidence substantiating the advantages of chitosan as an efficient adjuvant for nasal vaccination originates from clinical examinations on a norovirus vaccine, which demonstrated the ability of a chitosan/monophosphoryl lipid-based antigen delivery system (ChiSys®) to induce immunity against the gastroenteric norovirus infections after immunization (Smith et al. 2014).
Chitosan-derived adjuvants were also used in combination with antigens derived from bacterial toxins, such as diphtheria toxoids (McNeela et al. 2000; Schipper et al. 2017), tetanus toxoids (Ahire et al. 2007; Pirouzmand et al. 2017), and dermonecrotoxin (Jiang et al. 2004). In addition, the potential of various vaccine formulations against anthrax were evaluated in female BALB/c mice (Malik et al. 2018). Encapsulating protective antigens (PA) in trimethyl-chitosan nanoparticles and administering them by subcutaneous, intramuscular, and intraperitoneal injections resulted in a strong IgG antibody response (Th1-biased) when combined with immune-stimulatory CpG oligodeoxynucleotides or polyinosinic-polycytidylic acids. Interestingly, without the immune-stimulatory nucleic acids, the PA-loaded trimethyl-chitosan nanoparticles led to a Th2-biased immune response.
Many studies have explored the adjuvant properties of chitosan in vaccines against cancer. In a study published by Wen et al. (2011), the effects of chitosan nanoparticles on the immune response triggered by an ovalbumin antigen in mice were analyzed. As the administration of the chitosan nanoparticles did not only increase cytokine levels of Th1 (IL-2 and IFN-γ) and Th2 (Il-10) cells, but also stimulated natural killer cells, the authors suggested that chitosan is a promising adjuvant for cancer immunotherapy by promoting both humoral and cellular immune responses. This hypothesis was confirmed by Highton et al. (2016), who demonstrated that immunization with an ovalbumin/chitosan hydrogel had antitumor effects in an intracaecal mice cancer model. After subcutaneous injection of the ovalbumin/chitosan vaccine, the authors detected CD8+ T memory cells specific for ovalbumin and observed decreased tumor growth in contrast to unvaccinated control mice or mice that were vaccinated with dendritic cells and ovalbumin.
Zaharoff et al. (2009) analyzed antitumor effects in mice using a bioluminescent orthotopic bladder cancer model, after repeatedly administering chitosan/IL-12 into the bladder and comparing the antitumor efficacy of this treatment with that of an established adjuvant therapy applied to treat bladder cancer based on attenuated mycobacteria (bacillus Calmette-Guerin therapy). Determination of the urinary cytokine spectrum and immunohistochemical analysis resulted in the identification of cytotoxic T cells and natural killer cells as effector cells responsible for tumor regression. In contrast to the Bacillus Calmette-Guerin therapy, chitosan/IL-12 treatment utterly prevented recurrence of the disease.
More recently, Yao et al. (2013b) prepared mannosylated chitosan nanoparticles and loaded it with a plasmid to produce a vaccine against gastrin-releasing peptide (GRP), whose receptor is overexpressed in various cancer cells. The nanoparticles were intranasally administered in a subcutaneous mice prostate carcinoma model to evaluate the efficacy on inhibition of the growth of tumor cells. Cell binding and cellular uptake assays revealed that the mannosylated chitosan nanoparticles facilitate targeting to antigen-presenting cells, promoting receptor-mediated endocytosis via the mannose receptor. Due to antigen representation, enhanced tumor regression was observed as a result of the production of high titers of anti-GRP antibodies. A similar strategy was finally used by Lai et al. (2014) to test an immune therapy against hepatocellular carcinoma in a H22 tumor-bearing mice model. They synthesized folate-conjugated chitosan nanoparticles and loaded them with a plasmid-encoding mouse interferon-γ-inducible protein-10 (IP-10). They found that IP-10 plasmid exhibited efficient antitumor activity, prolonging the survival time in H22 tumor-bearing mice. The antitumor effects were likely due to different effects. Next to the secretion of IFN-γ and IP-10, inhibition of regulatory T cells, suppression of angiogenesis, inhibition of cancer cell proliferation, and activation of apoptosis contributed to tumor growth inhibition.
Tissue Engineering
Tissue engineering is an increasingly important interdisciplinary field in regenerative medicine, which aims to create replacements for damaged tissues based on the combined knowledge provided by physicians, biologists, and engineers. Most approaches employ scaffolds made from biocompatible polymers, which are colonized by cells of the respective tissue. Ideally, the scaffold increases adherence, proliferation, and differentiation of colonizing cells. Chitosan and its derivatives offer ample benefits to generate cell and tissue supporting matrices, which include chemical versatility, antimicrobial activity, biocompatibility, biodegradability, and neglible toxicity (Ahsan et al. 2018). Chitosan can be produced to form sponge-like scaffolds using rather simple phase separation techniques including freeze-drying (Aranaz et al. 2014), gas foaming (Kaynak Bayrak et al. 2017), and electrospinning procedures (Qasim et al. 2018). The presence of a system of interconnected pores with appropriate diameters facilitates vascularization and tissue integration. Moreover, chitosan-based scaffolds can be synthesized in combinations with ample natural and synthetic polymers resulting in matrices exhibiting special characteristics. Due to their positive surface charges they open the possibility to fabricate polyelectrolyte complexes with anionic polymers such as glutamic acid (Fang et al. 2014), hyaluronic acid (Lalevee et al. 2016), dextrane sulfate (Kulkarni et al. 2016), heparin (Almodovar and Kipper 2011), dermatan sulfate (Rasente et al. 2016), and chondroitin sulfate (Tsai et al. 2011). Particularly the presence of glycosaminoglycans, which are naturally found in extracellular matrices, is known to modulate the activity of cytokines and growth factors by binding to the polymers (Zaman et al. 2016). Otherwise, chitosan-based scaffolds can be loaded with cytokines and growth factors to attract cells and stimulate tissue regeneration (Sun et al. 2012a; Bader et al. 2015; Choi et al. 2015). Finally, they further open the possibility of controlled degradation and resorption in physiological environments, designing mechanical properties that match the conditions found in the respective tissue, and determination of desired sizes and shape by easy fabrication procedures. Therefore, chitosan-based scaffolds have numerous applications in tissue engineering, and we will review recent progress in using these materials for tissue regeneration and wound healing.
Bone, Cartilage, and Tooth Repair
Bone defects can either be congenital or result from trauma, infection, cancer, or failed orthopedic surgical procedures (Venkatesan and Kim 2010). The grafts used to bridge bone defects can be autografts (bone material from other body regions of the same patient) or allografts (bone material from decedents). However, both materials have disadvantages: autografts require bone harvesting from healthy tissues and may cause complications of wound healing and pain, and allografts may result in immunogenic rejection and have the risk of transmitting viral diseases from the donor to the recipient. Due to these concerns, scientists around the world are searching for alternative materials as bone graft substitutes. As described before, chitosan-based materials have valuable properties for orthopedic applications. Chitosan itself has the capacity to increase bone regeneration rates (Muzzarelli et al. 1993b); however, it cannot fully substitute natural bone material. Therefore, different composite scaffolds have been developed to assure porosity for vascularization and nutrition, facilitate the formation of new bone material (osteoconductivity), guarantee structural integrity during ingrowth at the site of implantation, and orchestrate biodegradation with bone regeneration (Venkatesan and Kim 2010). In addition, chitosan-based composites can be loaded with cells and growth factors that promote osteoconductivity and hence facilitate bone regeneration. One of the most important chitosan grafting that has been used in bone tissue engineering is hydroxyapatite, which by itself stimulates bone regeneration, provided that the scaffold has a microporous structure (Woodard et al. 2007). Hydroxyapatite grafting of chitosan can be easily achieved by coprecipitation from homogeneous mixtures of precursor (Deepthi et al. 2016, and references therein). One of the first researchers who tested combinations of chitosan and hydroxyapatite was Michio Ito, who examined the use of chitosan-bonded hydroxyapatite paste for treatment of periodontal defects (Ito 1991). In 2004, Ge et al. (2004) published a remarkable study, in which they tested different combinations of air- and freeze-dried chitosan/hydroxyapatite materials that were colonized by osteoblasts and implanted into rats. The material was found to be nontoxic and biodegradable and to stimulate mineralization. The explanted material that was colonized by osteoblasts before implantation showed newly formed bone material containing proliferating osteoblasts that recruited surrounding tissue to grow in. In another study published by Oliveira et al. (2006), three-dimensional macroporous hydroxyapatite/chitosan bilayered scaffolds of inorganic and organic deposits were produced in a stepwise procedure and examined with regard to their mechanical properties and cytotoxicity to mouse fibroblast-like cells. Moreover, in vitro cell culture studies using goat marrow stromal cells revealed that the macroporous hydroxyapatite/chitosan composite is a suitable material that promotes attachment, proliferation, and differentiation into osteoblasts and chondrocytes. Additionally, three-layered porous materials of collagen, hydroxyapatite, and chitosan were produced and characterized as an artificial bone matrix (Wang et al. 2008b). When testing murine pre-osteoblast cell line (MC3T3-E1) grown on this matrix, the cells proliferated significantly more rapidly than cells grown on a pure chitosan matrix (Teng et al. 2008). In addition, higher levels of alkaline phosphatase (secreted by osteoblasts) were determined, which is indicative for bone regeneration. Similar results were obtained when osteoblasts were cultured on hydroxyapatite/chitosan nanocomposites and osteocalcin as a marker for late osteoblastic differentiation, and mineralized bone matrix formation was determined (Chesnutt et al. 2009). Further studies characterized hydroxyapatite/chitosan hybrids with additional blend materials such as montmorillonite (Katti et al. 2008), polylactic acid (Cai et al. 2009), cellulose and carboxymethyl cellulose (Liuyun et al. 2009; Jiang et al. 2013b), gelatin (Sellgren and Ma 2012; Maji et al. 2015; Lee et al. 2017), nylon 66 (Huang et al. 2011), polygalacturonic acid (Khanna et al. 2011), marine sponge collagen (Pallela et al. 2012), collagen (Wang et al. 2009), alginate (Jin et al. 2012; Kim et al. 2015; Liao et al. 2018), chondroitin sulfate (Venkatesan et al. 2012a; Hu et al. 2017), hyaluronic acid (Hu et al. 2017), fibroin (Lima et al. 2013; Ran et al. 2016; Ye et al. 2017), poly-3-hydroxybutyrate-co-3-hydroxyvalerate (Zhang et al. 2015b), fucoidan (Lowe et al. 2016), β-tricalcium phosphate (Shavandi et al. 2015; Oryan et al. 2017), graphene oxide (Yu et al. 2017), β-cyclodextrin (Shakir et al. 2016), β-1,3-glucan (Przekora and Ginalska 2017; Przekora et al. 2017), whitlockite (Zhou et al. 2017a), zoledronic acid (Lu et al. 2018), and zirconium dioxide (Balagangadharan et al. 2018). Also, three-dimensional hydroxyapatite/chitosan-carbon nanotube scaffolds were shown to be promising materials for bone regeneration (Im et al. 2012). Naturally, many of these combinations have been tested also in the absence of hydroxyapatite (Park et al. 2000a; Li et al. 2005; Jiang et al. 2006; Arpornmaeklong et al. 2008; Venkatesan et al. 2012b; Deng et al. 2013; Azevedo et al. 2014; Dinescu et al. 2014; Listoni et al. 2015; Georgopoulou et al. 2018; Koç Demir et al. 2018).
Several studies showed that various growth factors such as transforming growth factors (TGFs), vascular endothelial growth factors (VGEFs), bone morphogenic proteins (BMPs), insulin-like growth factors (IGFs), and platelet-derived growth factors (PDGFs) have major impacts on vascularization and osteoblast activities and thus have been employed to stimulate bone regeneration (Yun et al. 2012). However, there are limitations in maintaining therapeutic concentrations due to the short half-life of the growth factors in vivo. This can be effectively prevented by the controlled release of the growth factors from porous chitosan composite matrices, which have been demonstrated to stimulate bone formation. For instance, PDGF-BB is an important osteogenic growth factor in the process of bone regeneration, as it stimulates mesenchymal cell proliferation and differentiation and mediates chemotaxis of osteoblast. Park et al. (2000a, b) produced a porous chitosan or chondroitin-4-sulfate/chitosan sponges releasing PDGF-BB to stimulate bone regeneration. The release rate of PDGF-BB increased proportionally with increasing concentrations loaded onto the sponge, and PDGF-BB retained its chemotactic activity regardless of being loaded onto the sponge or added freely to test solution (Park et al. 2000a). Finally, osteoblast proliferation was found to be stimulated in PDGF-BB-loaded chondroitin-4-sulfate/chitosan sponge compared with that of the unloaded sponge. PGFs have also been used in combination with other growth factors. As VEGF is known to prolong cell survival, osteoblast proliferation, differentiation, and migration next to its effects on angiogenesis, it has been suggested that the combined action of VEGF and PDGF can accelerate the bone healing process even more efficiently. De la Riva et al. (2010) established a system based on brushite-chitosan capable of controlling release kinetics for these two growth factors. After implanting the chitosan scaffolds loaded with the growth factors into rabbits with femur defects, release kinetics and tissue distribution of radiolabeled VEGF and PDGF were determined. Analyzing bone repair histologically revealed that the combined use of VEGF and PDGF promoted bone regeneration most effectively.
Also, the controlled release of IGF-1 and BMP-2 by the enzymatic degradation of the porous chitosan scaffold stimulates bone healing and regeneration in rabbits considerably (Nandi et al. 2013). When the chitosan particles were loaded only with one of the two growth factors, the effect was found to be more pronounced for IGF-1 than for BMP-2 infiltrated matrices. In a very recent study, chitosan/biphasic calcium phosphate scaffolds functionalized with BMP-2-encapsulated nanoparticles and the RGD tripeptide were produced using a desolvation technique (Gan et al. 2018). In vitro cell culture and in vivo implantation tests demonstrated that RGD and BMP-2 synergistically increased cell adhesion and spreading via integrin binding triggering differentiation of osteoblasts. Increased bone healing was also observed, when porous chitosan scaffolds were loaded with resolvin D1, a potent lipid immune modulator derived from both eicosapentaenoic acid, and implanted into rats with a femur defect (Vasconcelos et al. 2018). Obviously, resolving D1 administration in the acute phase of the innate immune response to the bio-implant had beneficial effects during bone tissue repair.
Impairment of the articular cartilage is frequently due to sport-related injury, disease, trauma, and tumor. If not treated successfully, it may result in osteoarthritis, which increasingly affects also younger individuals (Muzzarelli et al. 2012). In contrast to bone regeneration, cartilage healing is limited by the lack of vascularization and poor proliferation rates of chondrocytes. Injection of hyaluronan into the joints of arthritic patients is known to improve their function, as it restores viscoelasticity and flow of the synovial fluid, helps to normalize hyaluronan production, and finally reduces pain and inflammation. Chitosan easily forms polyelectrolyte complexes with hyaluronan and chondroitin sulfate, which are important building blocks particularly of the hyaline cartilage found on the surface of joints. Therefore, combinations of chitosan and hyaluronan and/or chondroitin sulfate may be useful in cartilage healing. In a first attempt to realize this idea, Kuo et al. (2015) synthesized a highly elastic, macroporous, and chitosan-containing gelatin/chondoitin-6-sulfate/hyaluronan (GCH) cryogel scaffold, which mimics the extracellular matrix composition of the cartilage. Furthermore, in vitro cell culture studies suggest that chondrocytes proliferate and redifferentiate within the porous matrix of the cryogels. Although chitosan reduces cell proliferation, it stimulates the secretion of sulfated glycosaminoglycans and type II collagen. In addition, they performed in vivo studies culturing chondrocytes on the GCH chitosan cryogel and implanting the material into rabbits with an articular cartilage defect (Kuo et al. 2015). After 3 months, the defect in the chondrocytes/cryogel group was completely covered with semitransparent tissue, which had similar characteristics as the native cartilage. A large variety of other chitosan-based materials lacking glycosaminoglycans have been suggested as suitable scaffolds for cartilage tissue engineering and some of them have been tested as mesenchymal stem cell carriers. Such materials include scaffolds containing N,N-dicarboxymethyl chitosan (Mattioli-Belmonte et al. 1999), chitosan/ gelatin and/or alginate complex (Xia et al. 2004; Li and Zhang 2005; Bhat et al. 2011), poly(L-lactide)/chitosan microspheres (Lao et al. 2008; Haaparanta et al. 2014), polyethylene oxide/chitin/chitosan scaffolds (Kuo and Ku 2008), genipin-cross-linked chitosan/silk fibroin sponges (Silva et al. 2008; Vishwanath et al. 2016), chitosan/polyester-based scaffolds (Alves da Silva et al. 2010), chitosan/collagen type I scaffolds (Gong et al. 2010), chitosan/poly(epsilon-caprolactone) blend scaffolds (Neves et al. 2011; Filova et al. 2016), chitosan/poly(l- glutamic acid) scaffolds (Zhang et al. 2013a, 2015a), polyvinyl alcohol/chitosan composite hydrogels (Dashtdar et al. 2015), poly(N-isopropylacrylamide)/chitosan hydrogels (Mellati et al. 2016), viscoelastic silk/chitosan microcomposite scaffolds (Chameettachal et al. 2017), and chitosan/graphene oxide polymer nanofibers (Cao et al. 2017).
As in the case of bone repair, strategies using various chitosan-based scaffolds loaded with growth factors such as TGFs (Kim et al. 2003; Choi et al. 2015), IGFs (Zhao et al. 2010), BMPs (Mattioli-Belmonte et al. 1999), and basic fibroblast growth factor (bFGF) (Tan et al. 2007) have been tested for cartilage repair. In an interesting pilot study, Qi et al. (2013) produced an injectable chitosan/polyvinyl alcohol gel and examined its structure and physicochemical properties. The resulting material exhibited low cytotoxicity and good biocompatibility. Next, the gel was mixed with rabbit bone marrow stromal cells (BMSCs) that were transfected with an adenovirus to produce TGF-b1, and rabbits with cartilage defects were injected with this mixture. After 16 weeks, the defects appeared to be fully repaired. The regenerated tissue was almost indistinguishable from the native cartilage. In another study, a demineralized bone matrix was conjugated with mesenchymal stem cell (MSC) E7 affinity peptide (EPLQLKM) and combined with a chitosan hydrogel for cartilage engineering. Cell culture and implantation experiments demonstrated that the developed material has a high chondrogenic capacity facilitating tissue repair of cartilage defects (Meng et al. 2015). In a more recently published study, the proliferation and differentiation of multipotent dental pulp stem cells into chondrocytes were investigated to generate cartilage-like material. In this case, a porous chitosan-xanthan gum matrix was employed as a scaffold and loaded with kartogenin to promote chondrogenic differentiation (Westin et al. 2017). The manufactured scaffold exhibited favorable characteristics for cartilage tissue engineering, such as high porosity, low cytotoxicity, and mechanical properties compatible with those characteristic of cartilage.
Very recently, Agrawal et al. (2018) reported the in vitro generation of cartilage-like material by seeding human mesenchymal stem cells on freeze-dried porous silk fibroin/chitosan scaffolds and culturing them in a spinner flask bioreactor under dynamic conditions. The team was successful in preparing a cartilage construct of 5 mm thickness, which roughly corresponds to the thickness of a native articular cartilage.
Due to its unique properties, chitosan has also emerged as a scaffold for potential applications in dental medicine. Chitosan-based hydrogels and nanocomposites have been used as anti-erosive and enamel-repairing additives in dentifrices and chewing gums (Shibasaki et al. 1994; Arnaud et al. 2010; Ganss et al. 2011; Ruan et al. 2014), for reduction of dental bacterial biofilm formation (Jahanizadeh et al. 2017), for guided tissue regeneration to treat periodontal diseases such as periodontitis (Ma et al. 2014; Lotfi et al. 2016), as dentin-bonding agent (Fawzy et al. 2013), as modification of dental restorative materials and implants (Petri et al. 2007; Ali et al. 2017; Ibrahim et al. 2017a), and as scaffold for stem cell-based tissue regeneration (Yang et al. 2012b; Asghari Sana et al. 2017; Soares et al. 2017a). Periodontitis is a chronic inflammation of the gum, which ultimately may lead to the loss of periodontal tissues and teeth (Pihlstrom et al. 2005). Current therapeutic strategies mainly rely on good oral hygiene (brushing and flossing), plaque removal, and in more severe cases local application of antibiotics and surgical intervention including open flap debridement, osseous surgery, as well as guided tissue and bone regeneration. Chitosan-based materials turned out to be very useful for periodontal tissue regeneration. Such materials comprise methylpyrrolidinone chitosan (Muzzarelli et al. 1993a), chitosan scaffolds coated with a bioactive hydroxyapatite (Ang et al. 2002; Coimbra et al. 2011; Fraga et al. 2011; Miranda et al. 2016), injectable thermosensitive chitosan/β-glycerophosphate/hydroxyapatite hydrogels (Chen et al. 2016), chitosan-based risedronate/zinc-hydroxyapatite intrapocket dental films (Khajuria et al. 2018), asymmetric chitosan/tripolyphosphate cross-linked membranes (Ma et al. 2014), porous chitosan/ collagen scaffolds (Yang et al. 2012b), mucoadhesive electrospun chitosan and thiolated chitosan nanofibers (Samprasit et al. 2015), chitosan-coated titanium surfaces (Campos et al. 2015), chitosan modified glass ionomer restoratives (Petri et al. 2007), chitosan-intercalated montmorillonite/poly(vinyl alcohol) nanofibers (Ghasemi Hamidabadi et al. 2017), and polyhydroxybutyrate/chitosan/nano-bioglass nanofiber scaffolds (Hashemi-Beni et al. 2018).
In one of the first studies that used growth factors to promote dental pulp stem cell differentiation, porous chitosan/collagen scaffolds prepared by freeze-drying were used and loaded with a plasmid vector encoding the human BMP-7 gene (Yang et al. 2012b). The stem cells grown in this scaffold were successfully transfected by the plasmid vector, which led to the formation of BMP-7 triggering odontoblastic differentiation as indicated by the activation of specific marker genes encoding steocalcin, bone sialoprotein, dentin sialophosphoprotein, and dentin matrix protein 1. The chitosan/ collagen scaffolds with stem cells were subcutaneously implanted into the back of BALB/c mice. After 4 weeks, the material was explanted and evaluated by immunohistochemistry. In the gene-activated scaffold group, there were still transfected cells detectable showing the upregulated gene expression when compared to pure scaffold groups.
Cutaneous Wound Healing
Cutaneous wound healing is a complex process in which the skin repairs itself. The process is divided into different stages, which include blood clotting ( hemostasis), inflammation, cell migration and proliferation, and tissue remodeling (maturation). The wound healing process may be delayed or completely fail leading to non-healing chronic wounds, which are frequently found in patients with diabetes, venous or arterial diseases, infections, and age-related metabolic deficiencies. Bedsore and burns behave differently, as the healing process is complicated by coagulation, necrosis, and infections. Small, non-severe wounds may be treated with chitosan-containing ointments (Kweon et al. 2003), topical gels (Alsarra 2009), and/or wound dressings (Jayakumar et al. 2011). For instance, Kang et al. (2016) reported the synthesis of silver chloride nanoparticles stabilized with chitosan oligomer for an ointment that was tested on burn wound healing in a rat model. Burn wound healing of rats treated with this ointment was superior to rats treated with pure Vaseline or chitosan ointments. More severe wounds may require removal of necrotic tissue and surgical wound closure using suturing techniques. If the defects are too large to be covered in this way, autologous avascular mesh grafts, microvascular flap grafts, mikroskin grafts, and/or cultured epithelial grafts are transplanted to the wound site (Chua et al. 2016). However, surgical intervention is only possible up to a critical size. To cover large-sized skin defects, artificial grafts produced by tissue engineering techniques are required. This type of wound dressings must protect from infections, absorb excess exudates, and facilitate oxygen and nutrient exchange. In addition, the material must be nontoxic, non-allergenic, non-adherent, and biocompatible.
Chitosan-based materials are a good choice for wound dressings, as they fullfil most of the criteria mentioned above, and they are known to promote wound healing by activating platelets when getting into contact with blood (Periayah et al. 2013). Moreover, chitosan-based scaffolds can be loaded with growth factors to facilitate skin repair by promoting cell adhesion and proliferation (Lu et al. 2016). Several in vitro, preclinical and clinical studies actually demonstrated that chitosan-based hydrogels films, powders, and dressings, as well as artificial skins, accelerate wound healing and reepithelialization (Patrulea et al. 2015). However, the precise mechanism of action in promoting wound healing is still under debate. Next to chitosan-mediated immunomodulation, the type of functionalization contributes to wound healing.
Chitosan-based scaffolds used to promote wound healing comprise hydrogels, films, micro- and nanoparticles, nanocomposite materials, and micelles, and many biocompatible chitosan derivatives have been tested including N-carboxybutyl chitosan (Dias et al. 2010), hydroxybutyl chitosan (Hu et al. 2018), fluorinated methacrylamide chitosan (Wijekoon et al. 2013; Patil et al. 2016; Akula et al. 2017), and chitosan/polyvinyl alcohol materials (Charernsriwilaiwat et al. 2014; Wang et al. 2016b).
In addition, numerous chitosan hybrid materials have been synthesized for wound healing, which comprise chitosan or carboxymethyl chitosan/gelatin hydrogels (Huang et al. 2013; Patel et al. 2018), chitosan/heparin/poly(γ-glutamic acid) composite hydrogels (Zhang et al. 2018), chitosan-hyaluronan composite sponge scaffolds (Sanad and Abdel-Bar 2017; Tamer et al. 2018), heparin-chitosan complexes (Kratz et al. 1997; Kweon et al. 2003), chitosan-fibrin nanocomposites (Vedakumari et al. 2015), polyvinyl alcohol/chitosan/fibroin-blended sponges (Yeo et al. 2000), polyvinyl alcohol/starch/chitosan hydrogels with nano zinc oxide (Baghaie et al. 2017), poly(caprolactone)/chitosan/poly(vinyl alcohol) nanofibrous sponges (Gholipour-Kanani et al. 2014), chitosan/poly(ethylene glycol)-tyramine hydrogels (Lih et al. 2012), chitosan-alginate polyelectrolyte complexes (Wang et al. 2002; Hong et al. 2008; Caetano et al. 2015; Kong et al. 2016), porous keratin/chitosan scaffolds without and with zinc oxide (Tan et al. 2015; Zhai et al. 2018), nano-titanium oxide/chitosan complexes (Peng et al. 2008), chitosan/collagen hydrogels and sponges (Wang et al. 2008a; Cui et al. 2011; Ti et al. 2015), chitosan green tea polyphenol complexes (Qin et al. 2010, 2013), dextran hydrogels loaded with chitosan microparticles (Ribeiro et al. 2013), chitosan/polycaprolactone scaffolds (Bai et al. 2014; Zhou et al. 2017b), chitosan oleate ionic micelles (Dellera et al. 2014), castor oil polymeric films reinforced with chitosan/zinc oxide nanoparticles (Diez-Pascual and Diez-Vicente 2015), sponge-like nano-silver/zinc oxide-loaded chitosan composites (Lu et al. 2017), gellan gum-chitosan hydrogels (Shukla et al. 2016), chitosan-silica hybrid dressing materials (Park et al. 2017), chitosan/bentonite or tourmaline nanocomposites (Devi and Dutta 2017; Zou et al. 2017), chitosan/gelatin/chondroitin-4-sulfate films with and without zinc oxide (Cahu et al. 2017), chitosan/polyvinylpyrrolidone/cellulose nanowhiskers nanocomposites (Hasan et al. 2017), α-tocopherol-loaded chitosan oleate nanoemulsions (Bonferoni et al. 2018), chitosan-based liposome formulations (Mengoni et al. 2017), and electrospun chitosan/polyethylene oxide/fibrinogen biocomposites (Yuan et al. 2018).
Topical application of anti-inflammatory and antioxidant curcumin, which is a component of many curry powders, has been shown to promote wound healing, significantly preventing oxidative damage in tissues (Gopinath et al. 2004). Chitosan-alginate sponges have been used to deliver curcumin for dermal wound healing in rat. Loading curcumin onto the chitosan sponge enhanced the therapeutic healing effect when compared to other carriers like cotton gauze. Similarly, injectable nanocomposite hydrogels composed of curcumin, N,O- carboxymethyl chitosan, and oxidized alginate possess many characteristics that promote wound healing including exudate absorption and immobilization and activation of growth factors. Nano-curcumin, which is released slowly from the hydrogel in a sustained manner, evidently stimulates fibroblast proliferation, angiogenesis, and collagen production, supporting the healing process when tested in a mice model (Li et al. 2012b). Wound dressings made of chitosan/poly-γ-glutamic acid/pluronic/curcumin nanoparticles also promoted collagen formation and tissue regeneration (Lin et al. 2017), and collagen-alginate scaffolds impregnated with curcumin-loaded chitosan nanoparticles proved promising in the treatment of various pathological manifestations of diabetic wounds (Karri et al. 2016). Most recently, Zhao et al. (2018) prepared a thermosensitive chitosan/β-glycerophosphate hydrogel loaded with β-cyclodextrin-curcumin and demonstrated improved healing of infected cutaneous wounds in rats, which may be due to the combination of antioxidative, antimicrobial, and anti-NF-κB signaling effects.
As silver has been demonstrated to have potent antimicrobial activities with no reports on bacterial resistances, several laboratories prepared chitosan wound dressings impregnated with silver to prevent wound infections and promote wound healing (Graham 2005). Indeed, a wound dressing composed of nano-silver and chitosan improved wound healing in rats better than a silver sulfadiazine dressing, which led to unwanted higher silver levels in the blood than the chitosan-silver dressing (Lu et al. 2008). In another study, Abdelgawad et al. (2014) combined silver nanoparticles that were embedded in chitosan with polyvinyl alcohol to produce antimicrobial nanofibrous material for wound dressing. The material with the highest chitosan-silver nanoparticle content was tested against E. coli and showed significant antibacterial activity. A combined antibacterial/tissue regeneration response triggered by functional chitosan-silver nanocomposites was also reported for thermal burns (Luna-Hernandez et al. 2017). To increase antibacterial activity wound dressings, several groups combined chitosan-silver-based materials with sulfadiazine, a sulfonamide antibiotic. Topical administration of chitosan-based hydrogels containing silver sulfadiazine improved burn and wound healing capacities in different studies (Nascimento et al. 2009; Chakavala et al. 2012; Aguzzi et al. 2014; Lee et al. 2014; El-Feky et al. 2017a). Besides inhibiting Gram-negative bacteria such as E. coli, chitosan-based dressings carrying silver sulfadiazine also inhibited the growth of Gram-positive bacteria as well as fungi such as C. albicans on an infected wound (El-Feky et al. 2017b).
Several studies reported that wound healing is accelerated when chitosan hydrogels are combined with adipose-derived or mesenchymal stem cells. Altman et al. (2009) grew human adipose-derived stem cells onto a chitosan/silk fibroin scaffolds and used it in a cutaneous wound healing model. They found that this regimen significantly enhanced wound healing, increasing micro-vascularization and differentiation into epidermal epithelial cells. In another study, an artificial dermis was fabricated by culturing human adipose-derived stem cells on a poly(L-glutamic acid)/chitosan scaffold (Shen et al. 2013b). Notably, the seeded stem cells maintained their capability to proliferate, produce extracellular matrix, and secrete cytokines including transforming growth factor β1 and vascular endothelial growth factor. The artificial dermis was used to cover wounds that have been generated before in streptozotocin-induced diabetic mice. The artificial dermis significantly accelerated wound closure and healing in diabetic mice. Tong et al. (2016) used a different stem cell-based strategy to generate a skin substitute promoting wound healing. They manufactured a collagen-chitosan sponge scaffold to culture bone marrow-derived stem cells, which were pre-treated by hypoxia to induce the expression of pro-angiogenic cytokines. When the skin substitute was used to treat wounds generated in diabetic rats with hindlimb ischemia, wound healing was enhanced in comparison to scaffold-only controls or skin substitutes that were generated with normoxic stem cells.
A novel strategy for wound healing involving exosomes was reported recently. Exosomes are small secretory membrane vesicles that are involved in cell-to-cell communication. Stem cell-derived exosomes can improve wound healing and promote skin regeneration by stimulating cell proliferation and migration, angiogenesis, and reepithelization and modulating immune responses (Phinney and Pittenger 2017). Based on these observations, Shi et al. (2017b) isolated exosomes derived from gingival mesenchymal stem cells and encapsulated them in chitosan/silk hydrogel sponge. The combination of the exosomes and hydrogel was effective in promoting skin wound healing in a diabetic rat model by inducing reepithelialization, vascularization, and neuronalization paralleled by the remodeling of the extracellular matrix.
Ocular Surface Reconstruction
Corneal damage can be the result of different diseases and injuries and may lead to a reduction or even loss of vision. Currently, the only therapy to cure vision loss after irreversible corneal damage is a surgical procedure where the cornea is replaced by donated corneal tissue. Frequently, the entire cornea is replaced in a surgical intervention called penetrating keratoplasty. As there is a shortage of corneal donors and there is a certain risk associated with the surgery and graft rejection, new types of corneal replacements are examined including materials containing chitosan. Actually, topical application of chitosan or chitosan/N-acetylcysteine to the eye is known to enhance corneal epithelial proliferation and migration during the wound healing in rabbits (Fischak et al. 2017). This process appears to involve the activation of the extracellular signal-regulated kinases (ERK) pathway (Cui et al. 2017). Another study evaluated the effects on corneal epithelium regeneration by combination of exogenous recombinant human serum-derived factor-1α (rhSDF-1α) with a thermosensitive chitosan/gelatin hydrogel and analyzed the underlying mechanism (Tang et al. 2017). Conducting in vitro experiments, the team showed that rhSDF-1α enhanced stem cell proliferation, chemotaxis, and migration, as well as the expression of related genes in limbal epithelial and mesenchymal stem cells (LESCs and MSCs). In vivo experiment using an alkali burn-injury rat model further revealed enhanced corneal epithelium regeneration and increased local expression of growth factors known to be essential for corneal epithelium repair. The underlying mechanism by which rhSDF-1α released from the chitosan/gelatin hydrogel stimulates corneal regeneration may involve activation of C-X-C chemokine receptor type 4 (CXCR4) expressing cells (LESCs and MSCs) and chemotactic attraction of these cells to the sites of lesion via the binding of rhSDF-1α to the CXCR4 receptor.
Chen et al. (2005) had considered a tissue-engineering scaffold made of collagen, chitosan, and hyaluronic acid as a potential replacement for corneal tissue. To study cytocompatibility in vitro, they cultured rabbit limbal corneal epithelial cells, corneal endothelial cells, and keratocytes on the polymer complexes and demonstrated that the corneal cells were able to attach, migrate, and proliferate. To evaluate biocompatibility in vivo, they implanted the polymer complex into the corneal stroma of rabbit eyes and inspected ocular reactions. Overall, the polymer complexes exhibited transparency and good biocompatibility, as they were degraded and absorbed within the corneal tissue while maintaining transparency. In another study, poly(ethylene glycol)-stabilized carbodiimide-cross-linked collagen-chitosan hydrogels were tested for biocompatibility and host-graft integration. For this purpose, Rafat et al. (2008) performed in vitro and in vivo studies demonstrating excellent biocompatibility when analyzing human corneal cells, dorsal root ganglia from chick embryos, or subcutaneous implants. The hydrogel scaffold was also studied as corneal substitute by implanting it into the cornea of pig eyes and monitoring them for 12 months. The substitute was seamlessly integrated into the cornea with regeneration of host corneal epithelium, stroma, and nerves. Liang et al. (2011) prepared a blend membrane composed of hydroxyethyl-chitosan, gelatin, and chondroitin sulfate. The membrane exhibited good transparency, ion and glucose permeability, and cytocompatibility for corneal endothelial cells, which formed a monolayer on the membrane in cell culture. In vivo animal experiments revealed that the membranes were characterized by biodegradability and a good histocompatibility suggesting that the membranes may be employed as carriers for corneal endothelial cell transplantation. Similar results were obtained for chitosan/polycaprolactone, chitosan/poly(ethylene glycol), silk fibroin/chitosan, carboxymethyl chitosan/gelatin/ hyaluronic acid, as well as hydroxyethyl-chitosan blend membranes, which were tested as potential scaffolds and carriers for bovine, ovine, and rabbit corneal endothelial cells, respectively (Wang et al. 2012b; Guan et al. 2013; Ozcelik et al. 2013; Liang et al. 2014; Xu et al. 2018).
Using an allogeneic rabbit model of stromal destruction caused by bacterial keratitis, Chou et al. (2018) tested the hypothesis that intra-stromal injection of keratocyte spheroids manufactured on chitosan coatings has higher therapeutic efficacies than eye drop instillations or isolated cell injections. The results of clinical observations and histological studies performed 2 weeks after the surgical intervention showed that, in comparison to a treatment relying only on antibiotics, intra-stromal grafting of keratocytes provides additional benefits due to improved preservation of cellular phenotypes, secretion of collagen matrix, and retention of the graft.
In a stem cell therapeutic approach published by Chien et al. (2012), human corneal fibroblasts (keratocytes) were reprogrammed into human- induced pluripotent stem cells (iPSC) using a feeder cell-free culturing system. To increase iPSC delivery and engraftment, the researchers generated an injectable thermogelling carboxymethyl-hexanoyl chitosan nanogel with seeded iPSCs and showed that viability and pluripotent properties of the reprogrammed iPSCs were maintained in the hydrogel system. They further demonstrated that the reprogrammed iPSCs grown on the hydrogel could be used to enhance corneal wound healing efficiently. This strategy opens the possibility for a personalized therapy for human corneal damage when iPSCs are reprogrammed from cells derived from corneal surgical residues.
Neuronal Regeneration
The plethora of favorable characteristics of chitosan outlined in this chapter prompted many researchers around the world to employ chitosan-based materials also in the reconstruction of peripheral nerves to improve healing of nerve damage caused by accidents or diseases. Although therapeutic interventions to peripheral nerve repair have yielded some progress during the past years, a full recovery of nerve function is usually not achieved. Current therapies mostly rely on microsurgical techniques, which either try to directly establish a tension-free connection between the ends of severed nerves (epineural, fascicular, and grouped fascicular repair) or bridge larger nerve defects by autologous grafts (cable grafts, trunk grafts, and vascularized nerve grafts) (Matsuyama et al. 2000; Houschyar et al. 2016). The various neurosurgical techniques used to connect nerve ends are challenging, and the therapeutic results are frequently not satisfactory. Many studies have provided evidence that various types of conduits, such as veins, pseudo-sheaths, and bioabsorbable tubes, are helpful in bridging shorter gaps by promoting nerve regeneration. After bridging nerve gaps with hollow conduits, the lumen between the nerve ends becomes filled with fibrin, and macrophages and other cells are attracted, which create a favorable microenvironment for vascularization and neuronalization.
Chitosan-based conduits have been extensively analyzed for this purpose. An early electrophysiological and histological study on nerve regeneration using rat sciatic nerve defects demonstrated that pure chitosan/collagen conduits were superior in bridging 1 cm nerve defects to that of control groups (Wei et al. 2003). The chitosan/collagen film was found to be degraded about 3 months after the surgery. In a methodologically similar study, Wang et al. (2005) generated an artificial nerve graft composed of a chitosan conduit and tested them to bridge a 3 cm dog sciatic nerve defect. In contrast to the previous study, the conduit was filled with longitudinally arranged filaments of polyglcyolic acid. The team found that the sciatic nerve trunk was successfully reconstructed in dogs treated with the chitosan/polyglycolic acid graft with reinnervation of the target skeletal muscle. In a case report on a 55-year-old man with a 3 cm median nerve defect in the distal forearm, implantation of chitosan/polyglycolic acid graft promoted nerve regeneration and functional reconstruction, so that the patient was able after 36 months to fully use the injured hand during daily activities (Gu et al. 2012). Other chitosan-based conduits have been successfully used to guide and promote nerve generation in various in vitro and in vivo models. These materials include chitosan/ gelatin and chitosan/poly(L-lysine) polyelectrolyte-based scaffolds (Martin-Lopez et al. 2012), chitosan-gold nanocomposites (Lin et al. 2008), chitosan/polylactic acid films (Xie et al. 2008), chitosan/poly(3-hydroxybutyrate-co-3-hydroxyvalerate) nanofibers (Biazar and Heidari Keshel 2014), porous chitosan-poly(p-dioxanone)/silk fibroin copolymers (Wu et al. 2015), poly(D,L-lactide-co-glycolide) sleeves with multifilament chitosan yarn or a microcrystalline chitosan sponge core (Wlaszczuk et al. 2016), chitosan/hyaluronic acid hybrid materials (Li et al. 2018a), porous chitosan-γ-glycidoxypropyltrimethoxysilane hybrid membranes (Shirosaki et al. 2014), hydroxyapatite-coated tendon chitosan tubes with adsorbed laminin peptides (Itoh et al. 2003), and hyaluronic acid doped-poly(3,4-ethylenedioxythiophene) nanoparticles in a chitosan/gelatin matrix (Wang et al. 2017b).
An evident upgrade of chitosan-based conduits is lumenal loading with neurotrophic factors such as nerve growth factor (NGF), ciliary neurotrophic factor (CNF), or brain-derived neurotrophic factor (BDNF), which are all secreted by Schwann cells that support growth of neuronal cells (Houschyar et al. 2016). In addition, fibroblast growth factor (FGF), glial growth factor (GGF), and vascular endothelial growth factor (VEGF) were reported to have positive effects on nerve regeneration.
Yang et al. (2011) immobilized NGF on genipin-cross-linked chitosan and tested the material for cytotoxicity using primary cultured Schwann cells and for neuronal differentiation of PC12 cells in response to NGF release. Subsequently, Wang et al. (2012a) demonstrated that genipin-cross-linked chitosan conduits loaded with NGF can be successfully used to bridge 1-cm-long sciatic nerve defects in rats as revealed by electrophysiological assessment, behavioral analysis, and histological examination 24 weeks after the surgery. Similar results were obtained, when NGF-containing microspheres were implanted into chitosan conduits to repair a 1 cm defect of the facial nerve in rabbits (Liu et al. 2013). In another NGF-based approach, Chao et al. (2016) combined an autologous vein conduit with a chitosan-β-glycerophosphate-NGF hydrogel. The researchers surgically reconstructed a 5-mm-long defect of a rat facial nerve with an autologous vein and then injected the chitosan-β-glycerophosphate-NGF hydrogel into the lumen of the conduit. Facial nerve regeneration was as efficient as in control groups, which were transplanted with an autologous nerve, but significantly better than in control groups where the vein conduit was injected with NGF only.
Shen et al. (2010) used a polylactic/polyglycolic acid chitosan nerve conduit loaded with CNF to repair larger canine tibial nerve defects in crossbred dogs and evaluated nerve regeneration by general inspection, electrophysiological, immunological, and histological analyses 3 months after the surgery. Nerve regeneration was significantly improved in animals that were treated with CNF-loaded polylactic/polyglycolic acid chitosan conduits when compared to groups treated with the polylactic/polyglycolic acid chitosan conduits. The results were similar to controls groups that were treated with autologous nerve grafts, suggesting that the artificial nerve conduit is a promising alternative for bridging nerve defects.
Furthermore, Zhao et al. (2014) hypothesized that tacrolismus-loaded chitosan enhances peripheral nerve regeneration through modulation of the expression profiles of neurotrophic factors. To test this hypothesis, they loaded tacrolismus onto chitosan conduits and examined nerve regeneration of sciatic nerve injury in a rat model. They found significant regeneration of sciatic nerves with normal morphology but higher density of myelinated nerve fibers in rats treated with tacrolismus-loaded chitosan. The underlying mechanism seems to involve BDNF signaling, because nerve regeneration was paralleled by an increased expression of BDNF and its corresponding receptor (TrkB) in the motor neurons in the spinal cord.
The membrane-bound cell adhesion molecule L1 is known to promote neurite growth and prevent neuronal apoptosis, a function which can be mimicked by a recombinant chimeric version of this molecule called L1-Fc (Roonprapunt et al. 2003). Loading L1-Fc to an artificial chitosan/polyglycolic acid conduit, Xu et al. (2004) studied guided regeneration of rat optic nerves. They found that the implanted chitosan/polyglycolic acid conduit was degraded and absorbed. When L1-Fc loaded conduits were implanted to bridge a defect caused by surgical intervention, axonal regeneration and remyelination were significantly improved when compared to control groups that were treated with conduits lacking L1-Fc.
Nerve regeneration can be additionally promoted using chitosan-based scaffolds as conduits seeded with stem cells that express neurotrophic factors and can differentiate into nerve cells. Zheng and Cui (2010) tested chitosan conduits of such kind combined with rat bone marrow mesenchymal stem cells to evaluate their potential for the reconstruction of 8-mm-long rat sciatic nerve defects. They demonstrated that the combination of chitosan and mesenchymal stem cells alone was sufficient to improve nerve regeneration and functional recovery. Moreover, some of the mesenchymal stem cells were found to have differentiated into neural stem cells. Similar results were obtained when injured rat sciatic nerves were treated in this way, and the nerve repair was monitored electrophysiologically and histomorphologically (Moattari et al. 2018) or by noninvasive magnetic resonance neurographic imaging (Liao et al. 2012). In addition, chitosan-coated poly-3-hydroxybutyrate conduits combined with human bone marrow mesenchymal stem cells were recently shown to be efficient in promoting nerve regeneration in this rat model of sciatic nerve injury (Ozer et al. 2018). Improved nerve regeneration was also reported for chitosan/poly(lactic-co-glycolic acid) scaffolds seeded with autologous bone marrow mesenchymal stem cells to treat injuries of dog sciatic nerves and rhesus monkey median nerves (Xue et al. 2012; Hu et al. 2013). In another approach, Zhu et al. (2015) used chitosan conduits filled with bone marrow mesenchymal stem cells and evaluated nerve regeneration and neuronal survival when injured lumbosacral nerves were bridged with this material. They found that this treatment enhanced sacral nerve regeneration and motor function 6 and 12 weeks after the surgery. Moreover, the mesenchymal stem cells prevented cell death of motor neurons in the anterior horn of the spinal cord, thereby improving the motor function in rats treated with the mesenchymal stem cell-seeded chitosan conduit. Finally, a clinical study performed with 14 patients suggests that defects in chronic spinal cord injury can be successfully bridged with peripheral nerve grafts combined with a chitosan-laminin scaffold and co-transplanted bone marrow-derived mesenchymal stem cells, which enhanced recovery (Amr et al. 2014).
Using chitosan/silk fibroin scaffolds grafts seeded with adipose-derived stem cells, Wei et al. (2011) examined regeneration of surgically injured rat sciatic nerves. Implantation of this conduit significantly improved axonal regeneration and functional recovery in comparison to control groups. The positive effect was partially attributed to the differentiation of adipose-derived stem cells into Schwann cells, which additionally secrete neurotrophic factors and prevent apoptosis. Nie et al. (2014) investigated axonal regeneration and remyelination using a chitosan/gelatin-based conduit combined with TGF-β1 and Schwann cells. For this purpose, they bridged a 10-mm defect of a rat sciatic nerve and examined nerve regeneration based on functional recovery, electrophysiological measurements, retrograde labeling, and immunohistochemical analysis. The obtained data indicate satisfactory functional recovery of the injured sciatic nerve.
Meyer et al. (2016) filled chitosan (5% degree of acetylation) conduits with a gel containing hyaluronic acid and laminin (NVR-gel) and added genetically modified neonatal rat Schwann cells as cellular delivery system for neurotrophic factors. Testing the chitosan conduits in the rat sciatic nerve model revealed that the chitosan conduit, which only is filled with the NVR-gel, was insufficient to promote nerve regeneration in contrast to autologous nerve grafts. Notably, delivery of FGF by seeded Schwann cells genetically modified to overexpress this factor improved nerve regeneration significantly. Unexpectedly, Schwann cells expressing GDNF did not show positive effects in this experimental setup. Recently, Zhu et al. (2017) used skin-derived precursor Schwann cells to seed chitosan/silk scaffolds for bridging a 10-mm-long rat sciatic nerve gap. The artificial graft exhibited significant promoting effects on peripheral nerve repair and hence constitutes an alternative to other stem cell-based approach promoting nerve regeneration.
Concluding Remarks
Numerous studies reported favorable effects of chitosan-based materials for a wide range of applications. Doubtless, the controlled and targeted delivery of drugs to specific tissues has a great potential in biomedicine, and first clinical trials with chitosan-based drug carrier systems revealed promising results for the therapy of a variety of diseases including diabetes and cancer and also mainly because adverse side effects are reduced. The antimicrobial activity of chitosan and its derivatives is particularly important when the polymer is used for textile fabrication, food packaging, wound dressings, and tissue engineering. However, the underlying mechanism of antimicrobial activity is not fully understood. One prominent explanation is the assumed interaction of chitosan’s positively charged amine groups with the negatively charged surface of bacteria and fungi, which might impair the movement of ions across membranes and hence disrupt cellular integrity. Although the proposed mechanisms seem plausible, there is a clear lack of experimental data that would provide evidence at a molecular level reminding us to continue basic research on the mode of actions. The studies conducted so far indicate that the antimicrobial activity of pure chitosan is not sufficient to prevent microbial infections completely in vivo. However, chitosan and its derivatives can be combined with other antimicrobial compounds including essential oils (Krausz et al. 2015), polyphenols (Madureira et al. 2015), tretinoin (Ridolfi et al. 2012), metal ions (Sanpui et al. 2008; Tran et al. 2010), lysozyme (Wu et al. 2017), or antibodies (Jamil et al. 2016), to prevent bacterial of fungal infections. Thus, chitosan appears to be an ideal adjuvant polymer for design and production of new materials exhibiting intrinsic antimicrobial properties for a large variety of potential applications in the chemical, pharmaceutical, food, and textile industry. Similarly, the observed immune-stimulatory effects of chitosan need further investigation, as there may be a certain risk to develop allergic or even anaphylactic reactions after oral ingestion (Kato et al. 2005). However, it has to be noted that overall the beneficial characteristics exceed possible side effects due to some allergic potential. Finally, the antitumor activity of chitosan also needs to be analyzed in more detail. Recently, Li et al. (2018b) provided some evidence indicating that chitosan activates dendritic cells, which subsequently secrete pro-inflammatory cytokines and thereby enhance immune surveillance by natural killer cells. Accordingly, the antitumor effects of chitosan can be enhanced by specifically targeting dendritic cells by attaching mannose to the surface of chitosan nanoparticles (Shi et al. 2017a). Different pattern recognition receptors that are expressed on the surface of dendritic cells are potential receptors for chitosan. This includes Toll-like receptors, C-type lectin receptors, and other molecules, which are known to recognize specific molecular patterns, particularly those associated with pathogens. However, currently it is not known how dendritic cells recognize chitosan. In summary, it has to be noted that chitosan is a highly promising material for a variety of applications in industry and medicine. While chitosan-based materials have been commercially launched as packaging and coating material in food industry, as an ingredient in cosmetics, and as ion exchanger in water treatment and are approved for human dietary use and wound dressing, their commercial applications in medicine as drug delivery systems or scaffold for tissue engineering are pending. Nevertheless, there are clinical phase 2/3 trials, and depending on their outcome, some products may reach first approval by the health authorities in near future.
Acknowledgment
The authors are grateful to Subbaratnam Muthukrishnan for critically reading the manuscript.
Contributor Information
Ephraim Cohen, Email: ephraim.cohen@mail.huji.ac.il.
Hans Merzendorfer, Phone: +4949492717403917, Email: hans.merzendorfer@uni-siegen.de.
Hans Merzendorfer, Email: merzendorfer@chemie-bio.uni-siegen.de.
Ephraim Cohen, Email: ephraim.cohen@mail.huji.ac.il.
References
- Abbasian M, Jaymand M, Niroomand P, Farnoudian-Habibi A, Karaj-Abad SG. Grafting of aniline derivatives onto chitosan and their applications for removal of reactive dyes from industrial effluents. Int J Biol Macromol. 2017;95:393–403. doi: 10.1016/j.ijbiomac.2016.11.075. [DOI] [PubMed] [Google Scholar]
- Abdel-Aziz HMM, Hasaneen MNA, Omer AM. Nano chitosan-NPK fertilizer enhances the growth and productivity of wheat plants grown in sandy soil. Span J Agric Res. 2016;14:e0902. doi: 10.5424/Sjar/2016141-8205. [DOI] [Google Scholar]
- Abdelgawad AM, Hudson SM, Rojas OJ. Antimicrobial wound dressing nanofiber mats from multicomponent (chitosan/silver-NPs/polyvinyl alcohol) systems. Carbohydr Polym. 2014;100:166–178. doi: 10.1016/j.carbpol.2012.12.043. [DOI] [PubMed] [Google Scholar]
- Afkhami A, Hashemi P, Bagheri H, Salimian J, Ahmadi A, Madrakian T. Impedimetic immunosensor for the label-free and direct detection of botulinum neurotoxin serotype A using Au nanoparticles/graphene-chitosan composite. Biosens Bioelectron. 2017;93:124–131. doi: 10.1016/j.bios.2016.09.059. [DOI] [PubMed] [Google Scholar]
- Agrawal P, Pramanik K, Biswas A, Ku Patra R. In vitro cartilage construct generation from silk fibroin- chitosan porous scaffold and umbilical cord blood derived human mesenchymal stem cells in dynamic culture condition. J Biomed Mater Res A. 2018;106:397–407. doi: 10.1002/jbm.a.36253. [DOI] [PubMed] [Google Scholar]
- Aguzzi C, Sandri G, Bonferoni C, Cerezo P, Rossi S, Ferrari F, Caramella C, Viseras C. Solid state characterisation of silver sulfadiazine loaded on montmorillonite/chitosan nanocomposite for wound healing. Colloid Surf B. 2014;113:152–157. doi: 10.1016/j.colsurfb.2013.08.043. [DOI] [PubMed] [Google Scholar]
- Ahire VJ, Sawant KK, Doshi JB, Ravetkar SD. Chitosan microparticles as oral delivery system for tetanus toxoid. Drug Dev Ind Pharm. 2007;33:1112–1124. doi: 10.1080/03639040701377847. [DOI] [PubMed] [Google Scholar]
- Ahmad M, Nirmal NP, Danish M, Chuprom J, Jafarzedeh S. Characterisation of composite films fabricated from collagen/chitosan and collagen/soy protein isolate for food packaging applications. RSC Adv. 2016;6:82191–82204. doi: 10.1039/C6RA13043G. [DOI] [Google Scholar]
- Ahmed RA, Fekry AM. Preparation and characterization of a nanoparticles modified chitosan sensor and its application for the determination of heavy metals from different aqueous media. Int J Electrochem Sci. 2013;8:6692–6708. [Google Scholar]
- Ahsan SM, Thomas M, Reddy KK, Sooraparaju SG, Asthana A, Bhatnagar I. Chitosan as biomaterial in drug delivery and tissue engineering. Int Biol Macromol. 2018;110:97–109. doi: 10.1016/j.ijbiomac.2017.08.140. [DOI] [PubMed] [Google Scholar]
- Aider M. Chitosan application for active bio-based films production and potential in the food industry: review. LWT – Food Sci Technol. 2010;43:837–842. doi: 10.1016/j.lwt.2010.01.021. [DOI] [Google Scholar]
- Akhtar MA, Hayat A, Iqbal N, Marty JL, Nawaz MH. Functionalized graphene oxide-polypyrrole-chitosan (fGO-PPy-CS) modified screen-printed electrodes for non-enzymatic hydrogen peroxide detection. J Nanopart Res. 2017;19:334. doi: 10.1007/S11051-017-4029-X. [DOI] [Google Scholar]
- Akula S, Brosch IK, Leipzig ND. Fluorinated methacrylamide chitosan hydrogels enhance cellular wound healing processes. Ann Biomed Eng. 2017;45:2693–2702. doi: 10.1007/s10439-017-1893-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali SW, Rajendran S, Joshi M. Synthesis and characterization of chitosan and silver loaded chitosan nanoparticles for bioactive polyester. Carbohydr Polym. 2011;83:438–446. doi: 10.1016/j.carbpol.2010.08.004. [DOI] [Google Scholar]
- Ali A, Zahid N, Manickam S, Siddiqui Y, Alderson PG. Double layer coatings: a new technique for maintaining physico-chemical characteristics and antioxidant properties of dragon fruit during storage. Food Bioprocess Technol. 2014;7:2366–2374. doi: 10.1007/s11947-013-1224-3. [DOI] [Google Scholar]
- Ali S, Sangi L, Kumar N. Exploring antibacterial activity and hydrolytic stability of resin dental composite restorative materials containing chitosan. Technol Health Care. 2017;25:11–18. doi: 10.3233/THC-161238. [DOI] [PubMed] [Google Scholar]
- Alix S, et al. Active pseudo-multilayered films from polycaprolactone and starch based matrix for food-packaging applications. Eur Polym J. 2013;49:1234–1242. doi: 10.1016/j.eurpolymj.2013.03.016. [DOI] [Google Scholar]
- Almodovar J, Kipper MJ. Coating electrospun chitosan nanofibers with polyelectrolyte multilayers using the polysaccharides heparin and N,N,N-trimethyl chitosan. Macromol Biosci. 2011;11:72–76. doi: 10.1002/mabi.201000261. [DOI] [PubMed] [Google Scholar]
- Al-Mokaram AMAAA, Yahya R, Abdi MM, Mahmud HNME. The development of non-enzymatic glucose biosensors based on electrochemically prepared polypyrrole-chitosan-titanium dioxide nanocomposite films. Nanomaterials. 2017;7:129. doi: 10.3390/Nano7060129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Naamani L, Dobretsov S, Dutta J. Chitosan-zinc oxide nanoparticle composite coating for active food packaging applications. Innovative Food Sci Emerg Technol. 2016;38:231–237. doi: 10.1016/j.ifset.2016.10.010. [DOI] [Google Scholar]
- Alsarra IA. Chitosan topical gel formulation in the management of burn wounds. Int Biol Macromol. 2009;45:16–21. doi: 10.1016/j.ijbiomac.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Altman AM, Yan Y, Matthias N, Bai X, Rios C, Mathur AB, Song YH, Alt EU. IFATS collection: Human adipose-derived stem cells seeded on a silk fibroin-chitosan scaffold enhance wound repair in a murine soft tissue injury model. Stem Cells. 2009;27:250–258. doi: 10.1634/stemcells.2008-0178. [DOI] [PubMed] [Google Scholar]
- Alves da Silva ML, Crawford A, Mundy JM, Correlo VM, Sol P, Bhattacharya M, Hatton PV, Reis RL, Neves NM. Chitosan/polyester-based scaffolds for cartilage tissue engineering: assessment of extracellular matrix formation. Acta Biomater. 2010;6:1149–1157. doi: 10.1016/j.actbio.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Amouzgar P, Salamatinia B. A short review on presence of pharmaceuticals in water bodies and the potential of chitosan and chitosan derivatives for elimination of pharmaceuticals. J Mol Genet Med. 2015;S4:001. doi: 10.4172/1747-0862.S4-001. [DOI] [Google Scholar]
- Amr SM, Gouda A, Koptan WT, Galal AA, Abdel-Fattah DS, Rashed LA, Atta HM, Abdel-Aziz MT. Bridging defects in chronic spinal cord injury using peripheral nerve grafts combined with a chitosan-laminin scaffold and enhancing regeneration through them by co-transplantation with bone-marrow-derived mesenchymal stem cells: case series of 14 patients. J Spinal Cord Med. 2014;37:54–71. doi: 10.1179/2045772312Y.0000000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An J, Luo Q, Yuan X, Wang D, Li X. Preparation and characterization of silver-chitosan nanocomposite particles with antimicrobial activity. J Appl Polymer Sci. 2011;120:3180–3189. doi: 10.1002/app.33532. [DOI] [Google Scholar]
- Anantha RK, Kota S. An evaluation of the major factors influencing the removal of copper ions using the egg shell (Dromaius novaehollandiae): chitosan (Agaricus bisporus) composite. 3 Biotech. 2016;6:83. doi: 10.1007/s13205-016-0381-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang TH, Sultana FSA, Hutmacher DW, Wong YS, Fuh JYH, Mo XM, Loh HT, Burdet E, Teoh SH. Fabrication of 3D chitosan–hydroxyapatite scaffolds using a robotic dispensing system. Mater Sci Eng C. 2002;20:35–42. doi: 10.1016/S0928-4931(02)00010-3. [DOI] [Google Scholar]
- Antoniraj MG, Tisha SA, Mahesh A, Shanmugarathinam A, Kandasamy R. Synthesis and characterization of cystamine-conjugated chitosan-SS-mPEG based 5-Fluorouracil loaded polymeric nanoparticles for redox responsive drug release. Eur J Pharm Sci. 2018;116:37–47. doi: 10.1016/j.ejps.2017.10.035. [DOI] [PubMed] [Google Scholar]
- Anusuya S, Banu KN. Silver-chitosan nanoparticles induced biochemical variations of chickpea (Cicer arietinum L.) Biocatal Agric Biotechnol. 2016;8:39–44. doi: 10.1016/j.bcab.2016.08.005. [DOI] [Google Scholar]
- Aranaz I, Gutierrez MC, Ferrer ML, del Monte F. Preparation of chitosan nanocomposites with a macroporous structure by unidirectional freezing and subsequent freeze-drying. Mar Drugs. 2014;12:5619–5642. doi: 10.3390/md12115619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranaz I, Acosta N, Civera C, Elorza B, Mingo J, Castro C, Gandía DM, Heras Caballero A. Cosmetics and cosmeceutical applications of chitin, chitosan and their derivatives. Polymers. 2018;10:213. doi: 10.3390/polym10020213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo BR, Romao LPC, Doumer ME, Mangrich AS. Evaluation of the interactions between chitosan and humics in media for the controlled release of nitrogen fertilizer. J Environ Manage. 2017;190:122–131. doi: 10.1016/j.jenvman.2016.12.059. [DOI] [PubMed] [Google Scholar]
- Arca HC, Gunbeyaz M, Senel S. Chitosan-based systems for the delivery of vaccine antigens. Expert Rev Vaccines. 2009;8:937–953. doi: 10.1586/erv.09.47. [DOI] [PubMed] [Google Scholar]
- Arkoun M, Daigle F, Heuzey MC, Ajji A. Mechanism of action of electrospun chitosan-based nanofibers against meat spoilage and pathogenic bacteria. Molecules. 2017;22:585. doi: 10.3390/molecules22040585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud TM, de Barros Neto B, Diniz FB. Chitosan effect on dental enamel de-remineralization: an in vitro evaluation. J Dent. 2010;38:848–852. doi: 10.1016/j.jdent.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Arpornmaeklong P, Pripatnanont P, Suwatwirote N. Properties of chitosan-collagen sponges and osteogenic differentiation of rat-bone-marrow stromal cells. Int J Oral Max Surg. 2008;37:357–366. doi: 10.1016/j.ijom.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Arya V, Philip L. Adsorption of pharmaceuticals in water using Fe3O4 coated polymer clay composite. Microporous Mesoporous Mater. 2016;232:273–280. doi: 10.1016/j.micromeso.2016.06.033. [DOI] [Google Scholar]
- Asghari Sana F, Capkin Yurtsever M, Kaynak Bayrak G, Tuncay EO, Kiremitci AS, Gumusderelioglu M. Spreading, proliferation and differentiation of human dental pulp stem cells on chitosan scaffolds immobilized with RGD or fibronectin. Cytotechnology. 2017;69:617–630. doi: 10.1007/s10616-017-0072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo AS, Sá MJ, Fook MV, Neto PI, Sousa OB, Azevedo SS, Teixeira MW, Costa FS, Araújo AL. Use of chitosan and beta-tricalcium phosphate, alone and in combination, for bone healing in rabbits. J Mater Sci Mater Med. 2014;25:481–486. doi: 10.1007/s10856-013-5091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaei A, Babazadeh M. Multi-walled carbon nanotubes/chitosan polymer composite modified glassy carbon electrode for sensitive simultaneous determination of levodopa and morphine. Anal Methods. 2011;3:2400–2405. doi: 10.1039/c1ay05241a. [DOI] [Google Scholar]
- Babaei A, Afrasiabi M, Babazadeh M. A glassy carbon electrode modified with multiwalled carbon nanotube/chitosan composite as a new sensor for simultaneous determination of acetaminophen and mefenamic acid in pharmaceutical preparations and biological samples. Electroanal. 2010;22:1743–1749. doi: 10.1002/elan.200900578. [DOI] [Google Scholar]
- Babaei A, Babazadeh M, Momeni HR. A sensor for simultaneous determination of dopamine and morphine in biological samples using a multi-walled carbon nanotube/chitosan composite modified glassy carbon electrode. Int J Electrochem Sci. 2011;6:1382–1395. [Google Scholar]
- Babaei A, Garrett DJ, Downard AJ. Selective simultaneous determination of paracetamol and uric acid using a glassy carbon electrode modified with multiwalled carbon nanotube/chitosan composite. Electroanal. 2011;23:417–423. doi: 10.1002/elan.201000406. [DOI] [Google Scholar]
- Bader AR, Li T, Wang W, Kohane DS, Loscalzo J, Zhang YY (2015) Preparation and characterization of SDF-1alpha-chitosan-dextran sulfate nanoparticles. J Vis Exp:52323. 10.3791/52323 [DOI] [PMC free article] [PubMed]
- Baghaie S, Khorasani MT, Zarrabi A, Moshtaghian J. Wound healing properties of PVA/starch/chitosan hydrogel membranes with nano Zinc oxide as antibacterial wound dressing material. J Biomater Sci Polym Ed. 2017;28:2220–2241. doi: 10.1080/09205063.2017.1390383. [DOI] [PubMed] [Google Scholar]
- Bai MY, Chou TC, Tsai JC, Yu WC. The effect of active ingredient-containing chitosan/polycaprolactone nonwoven mat on wound healing: in vitro and in vivo studies. J Biomed Mater Res A. 2014;102:2324–2333. doi: 10.1002/jbm.a.34912. [DOI] [PubMed] [Google Scholar]
- Balagangadharan K, Viji Chandran S, Arumugam B, Saravanan S, Devanand Venkatasubbu G, Selvamurugan N. Chitosan/nano-hydroxyapatite/nano-zirconium dioxide scaffolds with miR-590-5p for bone regeneration. Int Biol Macromol. 2018;111:953–958. doi: 10.1016/j.ijbiomac.2018.01.122. [DOI] [PubMed] [Google Scholar]
- Bano I, Arshad M, Yasin T, Ghauri MA, Younus M. Chitosan: A potential biopolymer for wound management. Int Biol Macromol. 2017;102:380–383. doi: 10.1016/j.ijbiomac.2017.04.047. [DOI] [PubMed] [Google Scholar]
- Basaran E, Yazan Y. Ocular application of chitosan. Expert Opin Drug Deliv. 2012;9:701–712. doi: 10.1517/17425247.2012.681775. [DOI] [PubMed] [Google Scholar]
- Bashari A, Hemmatinejad N, Pourjavadi A. Smart and fragrant garment via surface modification of cotton fabric with cinnamon oil/stimuli responsive PNIPAAm/chitosan nano hydrogels. IEEE Trans Nanobiosci. 2017;16:455–462. doi: 10.1109/TNB.2017.2710630. [DOI] [PubMed] [Google Scholar]
- Bashir S, Teo YY, Naeem S, Ramesh S, Ramesh K. pH responsive N-succinyl chitosan/Poly (acrylamide-co-acrylic acid) hydrogels and in vitro release of 5-fluorouracil. PloS One. 2017;12:e0179250. doi: 10.1371/journal.pone.0179250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhamou N, Lafontaine PJ, Nicole M. Induction of systemic resistance to Fusarium crown and root-rot in tomato plants by seed treatment with chitosan. Phytopathology. 1994;84:1432–1444. doi: 10.1094/Phyto-84-1432. [DOI] [Google Scholar]
- Berger J, Reist M, Mayer JM, Felt O, Peppas NA, Gurny R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur J Pharm Biopharm. 2004;57:19–34. doi: 10.1016/S0939-6411(03)00161-9. [DOI] [PubMed] [Google Scholar]
- Bhaskara Reddy MV, Arul J, Angers P, Couture L. Chitosan treatment of wheat seeds induces resistance to Fusarium graminearum and improves seed quality. J Agric Food Chem. 1999;47:1208–1216. doi: 10.1021/jf981225k. [DOI] [PubMed] [Google Scholar]
- Bhat S, Tripathi A, Kumar A. Supermacroprous chitosan-agarose-gelatin cryogels: in vitro characterization and in vivo assessment for cartilage tissue engineering. J R Soc Interface. 2011;8:540–554. doi: 10.1098/rsif.2010.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biazar E, Heidari Keshel S. Development of chitosan-crosslinked nanofibrous PHBV guide for repair of nerve defects. Artif Cells Nanomed Biotechnol. 2014;42:385–391. doi: 10.3109/21691401.2013.832686. [DOI] [PubMed] [Google Scholar]
- Bilal M, Asgher M, Iqbal M, Hu H, Zhang X. Chitosan beads immobilized manganese peroxidase catalytic potential for detoxification and decolorization of textile effluent. Int Biol Macromol. 2016;89:181–189. doi: 10.1016/j.ijbiomac.2016.04.075. [DOI] [PubMed] [Google Scholar]
- Bilgin Simsek E, Saloglu D, Ozcan N, Novak I, Berek D. Carbon fiber embedded chitosan/PVA composites for decontamination of endocrine disruptor bisphenol-A from water. J Taiwan Inst Chem E. 2017;70:291–301. doi: 10.1016/j.jtice.2016.11.008. [DOI] [Google Scholar]
- Boddu VM, Abburi K, Talbott JL, Smith ED. Removal of hexavalent chromium from wastewater using a new composite chitosan biosorbent. Environ Sci Technol. 2003;37:4449–4456. doi: 10.1021/es021013a. [DOI] [PubMed] [Google Scholar]
- Bonferoni MC, et al. Alpha tocopherol loaded chitosan oleate nanoemulsions for wound healing. Evaluation on cell lines and ex vivo human biopsies, and stabilization in spray dried Trojan microparticles. Eur J Pharm Biopharm. 2018;123:31–41. doi: 10.1016/j.ejpb.2017.11.008. [DOI] [PubMed] [Google Scholar]
- Borges O, Silva M, de Sousa A, Borchard G, Junginger HE, Cordeiro-da-Silva A. Alginate coated chitosan nanoparticles are an effective subcutaneous adjuvant for hepatitis B surface antigen. Int Immunopharmacol. 2008;8:1773–1780. doi: 10.1016/j.intimp.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Bourguignon LYW, Zhu H, Shao L, Chen YW. CD44 Interaction with Tiam1 promotes Rac1 signaling and hyaluronic acid-mediated breast tumor cell migration. J Biol Chem. 2000;275:1829–1838. doi: 10.1074/jbc.275.3.1829. [DOI] [PubMed] [Google Scholar]
- Brown CA, Wang B, Oh JH. Antimicrobial activity of lactoferrin against foodborne pathogenic bacteria incorporated into edible chitosan film. J Food Prot. 2008;71:319–324. doi: 10.4315/0362-028X-71.2.319. [DOI] [PubMed] [Google Scholar]
- Brunel F, El Gueddari NE, Moerschbacher BM. Complexation of copper(II) with chitosan nanogels: toward control of microbial growth. Carbohydr Polym. 2013;92:1348–1356. doi: 10.1016/j.carbpol.2012.10.025. [DOI] [PubMed] [Google Scholar]
- Bu L, Gan LC, Guo XQ, Chen FZ, Song Q, Qi-Zhao GXJ, Hou SX, Yao Q. Trans-resveratrol loaded chitosan nanoparticles modified with biotin and avidin to target hepatic carcinoma. Int J Pharm. 2013;452:355–362. doi: 10.1016/j.ijpharm.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Bueter CL, Lee CK, Rathinam VA, Healy GJ, Taron CH, Specht CA, Levitz SM. Chitosan but not chitin activates the inflammasome by a mechanism dependent upon phagocytosis. J Biol Chem. 2011;286:35447–35455. doi: 10.1074/jbc.M111.274936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueter CL, Lee CK, Wang JP, Ostroff GR, Specht CA, Levitz SM. Spectrum and mechanisms of inflammasome activation by chitosan. J Immunol. 2014;92:5943–5951. doi: 10.4049/jimmunol.1301695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano GF, Frade MA, Andrade TA, Leite MN, Bueno CZ, Moraes AM, Ribeiro-Paes JT. Chitosan-alginate membranes accelerate wound healing. J Biomed Mater Res B. 2015;103:1013–1022. doi: 10.1002/jbm.b.33277. [DOI] [PubMed] [Google Scholar]
- Cahu TB, et al. Evaluation of chitosan-based films containing gelatin, chondroitin 4-sulfate and ZnO for wound healing. Appl Biochem Biotechnol. 2017;183:765–777. doi: 10.1007/s12010-017-2462-z. [DOI] [PubMed] [Google Scholar]
- Cai X, Tong H, Shen X, Chen W, Yan J, Hu J. Preparation and characterization of homogeneous chitosan-polylactic acid/hydroxyapatite nanocomposite for bone tissue engineering and evaluation of its mechanical properties. Acta Biomater. 2009;5:2693–2703. doi: 10.1016/j.actbio.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Campos DM, Toury B, D’Almeida M, Attik GN, Ferrand A, Renoud P, Grosgogeat B. Acidic pH resistance of grafted chitosan on dental implant. Odontology. 2015;103:210–217. doi: 10.1007/s10266-014-0162-5. [DOI] [PubMed] [Google Scholar]
- Campos EVR, Proença PLF, Oliveira JL, Melville CC, Della Vechia JF, de Andrade DJ, Fraceto LF. Chitosan nanoparticles functionalized with β-cyclodextrin: a promising carrier for botanical pesticides. Sci Rep. 2018;8:2067. doi: 10.1038/s41598-018-20602-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Zhang F, Wang Q, Wu X. Fabrication of chitosan/graphene oxide polymer nanofiber and its biocompatibility for cartilage tissue engineering. Mater Sci Eng C Mater Biol Appl. 2017;79:697–701. doi: 10.1016/j.msec.2017.05.056. [DOI] [PubMed] [Google Scholar]
- Caramella CM, Rossi S, Ferrari F, Bonferoni MC, Sandri G. Mucoadhesive and thermogelling systems for vaginal drug delivery. Adv Drug Deliv Rev. 2015;92:39–52. doi: 10.1016/j.addr.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Carosio F, Alongi J. Flame retardant multilayered coatings on acrylic fabrics prepared by one-step deposition of chitosan/montmorillonite complexes. Fibers. 2018;6:36. doi: 10.3390/fib6020036. [DOI] [Google Scholar]
- Carroll EC, et al. The vaccine adjuvant chitosan promotes cellular immunity via DNA sensor cGAS-STING-dependent induction of Type I interferons. Immunity. 2016;44:597–608. doi: 10.1016/j.immuni.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casettari L, Illum L. Chitosan in nasal delivery systems for therapeutic drugs. J Control Release. 2014;190:189–200. doi: 10.1016/j.jconrel.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Cavallo JA, Strumia MC, Gomez CG. Preparation of a milk spoilage indicator adsorbed to a modified polypropylene film as an attempt to build a smart packaging. J Food Eng. 2014;136:48–55. doi: 10.1016/j.jfoodeng.2014.03.021. [DOI] [Google Scholar]
- Celiesiute R, Radzevic A, Zukauskas A, Vaitekonis S, Pauliukaite R. A strategy to employ polymerised riboflavin in the development of electrochemical biosensors. Electroanal. 2017;29:2071–2082. doi: 10.1002/elan.201700218. [DOI] [Google Scholar]
- Cerempei A, Guguianu E, Muresan EI, Horhogea C, Rîmbu C, Borhan O. Antimicrobial controlled release systems for the knitted cotton fabrics based on natural substances. Fiber Polym. 2015;16:1688–1695. doi: 10.1007/s12221-015-4551-3. [DOI] [Google Scholar]
- Chakavala SR, Patel NG, Pate NV, Thakkar VT, Patel KV, Gandhi TR. Development and in vivo evaluation of silver sulfadiazine loaded hydrogel consisting polyvinyl alcohol and chitosan for severe burns. J Pharm Bioallied Sci. 2012;4:S54–S56. doi: 10.4103/0975-7406.94131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chameettachal S, Murab S, Vaid R, Midha S, Ghosh S. Effect of visco-elastic silk-chitosan microcomposite scaffolds on matrix deposition and biomechanical functionality for cartilage tissue engineering. J Tissue Eng Regen Med. 2017;11:1212–1229. doi: 10.1002/term.2024. [DOI] [PubMed] [Google Scholar]
- Chang H, Li X, Teng Y, Liang Y, Peng B, Fang F, Chen Z. Comparison of adjuvant efficacy of chitosan and aluminum hydroxide for intraperitoneally administered inactivated influenza H5N1 vaccine. DNA Cell Biol. 2010;29:563–568. doi: 10.1089/dna.2009.0977. [DOI] [PubMed] [Google Scholar]
- Chao X, Xu L, Li J, Han Y, Li X, Mao Y, Shang H, Fan Z, Wang H. Facilitation of facial nerve regeneration using chitosan-beta-glycerophosphate-nerve growth factor hydrogel. Acta Otolaryngol. 2016;136:585–591. doi: 10.3109/00016489.2015.1136432. [DOI] [PubMed] [Google Scholar]
- Charernsriwilaiwat N, Rojanarata T, Ngawhirunpat T, Opanasopit P. Electrospun chitosan/polyvinyl alcohol nanofibre mats for wound healing. Int Wound J. 2014;11:215–222. doi: 10.1111/j.1742-481X.2012.01077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan N, Dilbaghi N, Gopal M, Kumar R, Kim KH, Kumar S. Development of chitosan nanocapsules for the controlled release of hexaconazole. Int Biol Macromol. 2017;97:616–624. doi: 10.1016/j.ijbiomac.2016.12.059. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Q, Xu J, Huang Y, Ding Y, Deng H, Zhao S, Chen R. Study on biocompatibility of complexes of collagen-chitosan-sodium hyaluronate and cornea. Artif Organs. 2005;29:104–113. doi: 10.1111/j.1525-1594.2005.29021.x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhang F, Fu Q, Liu Y, Wang Z, Qi N. In vitro proliferation and osteogenic differentiation of human dental pulp stem cells in injectable thermo-sensitive chitosan/beta-glycerophosphate/hydroxyapatite hydrogel. J Biomater Appl. 2016;31:317–327. doi: 10.1177/0885328216661566. [DOI] [PubMed] [Google Scholar]
- Chen K, Guo B, Luo J. Quaternized carboxymethyl chitosan/organic montmorillonite nanocomposite as a novel cosmetic ingredient against skin aging. Carbohydr Polym. 2017;173:100–106. doi: 10.1016/j.carbpol.2017.05.088. [DOI] [PubMed] [Google Scholar]
- Cheng X, Li R, Li X, Umair MM, Ren X, Huang T. Preparation and characterization of antimicrobial cotton fabrics via N-halamine chitosan derivative/poly(2-acrylamide-2-methylpropane sulfonic acid sodium salt) self-assembled composite films. J Ind Text. 2016;46:1039–1052. doi: 10.1177/1528083715612232. [DOI] [Google Scholar]
- Chesnutt BM, Yuan Y, Buddington K, Haggard WO, Bumgardner JD. Composite chitosan/nano-hydroxyapatite scaffolds induce osteocalcin production by osteoblasts in vitro and support bone formation in vivo. Tissue Eng Part A. 2009;15:2571–2579. doi: 10.1089/ten.tea.2008.0054. [DOI] [PubMed] [Google Scholar]
- Chien Y, et al. Corneal repair by human corneal keratocyte-reprogrammed iPSCs and amphiphatic carboxymethyl-hexanoyl chitosan hydrogel. Biomaterials. 2012;33:8003–8016. doi: 10.1016/j.biomaterials.2012.07.029. [DOI] [PubMed] [Google Scholar]
- Choi B, Kim S, Fan J, Kowalski T, Petrigliano F, Evseenko D, Lee M. Covalently conjugated transforming growth factor-beta1 in modular chitosan hydrogels for the effective treatment of articular cartilage defects. Biomater Sci. 2015;3:742–752. doi: 10.1039/C4BM00431K. [DOI] [PubMed] [Google Scholar]
- Choi B, Jo DH, Anower AK, Islam SM, Sohn S. Chitosan as an immunomodulating adjuvant on T-cells and antigen-presenting cells in Herpes Simplex virus type 1 infection. Mediat Inflamm. 2016;2016:4374375. doi: 10.1155/2016/4374375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou SF, Lee CH, Lai JY. Bioengineered keratocyte spheroids fabricated on chitosan coatings enhance tissue repair in a rabbit corneal stromal defect model. J Tissue Eng Regen Med. 2018;12:316–320. doi: 10.1002/term.2456. [DOI] [PubMed] [Google Scholar]
- Choudhary RC, Kumaraswamy RV, Kumari S, Sharma SS, Pal A, Raliya R, Biswas P, Saharan V. Cu-chitosan nanoparticle boost defense responses and plant growth in maize (Zea mays L.) Sci Rep. 2017;7:9754. doi: 10.1038/s41598-017-08571-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary SMJ, Joshi A, Saharan V. Assessment of Cu-chitosan nanoparticles for its antibacterial activity against Pseudomonas syringae pv. Glycinea. Int J Curr Microbiol App Sci. 2017;6:1335–1350. [Google Scholar]
- Chua AWC, Khoo YC, Tan BK, Tan KC, Foo CL, Chong SJ. Skin tissue engineering advances in severe burns: review and therapeutic applications. Burns Trauma. 2016;4:3. doi: 10.1186/s41038-016-0027-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E, Joseph T, Kahana F, Magdassi S. Photostabilization of an entomopathogenic fungus using composite clay matrices. Photochem Photobiol. 2003;77:180–185. doi: 10.1562/0031-8655(2003)077<0180:POAEFU>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Coimbra P, Alves P, Valente TA, Santos R, Correia IJ, Ferreira P. Sodium hyaluronate/chitosan polyelectrolyte complex scaffolds for dental pulp regeneration: synthesis and characterization. Int Biol Macromol. 2011;49:573–579. doi: 10.1016/j.ijbiomac.2011.06.011. [DOI] [PubMed] [Google Scholar]
- Corradini E, de Moura MR, Mattoso LHC. A preliminary study of the incorporation of NPK fertilizer into chitosan nanoparticles. Express Polym Lett. 2010;4:509–515. doi: 10.3144/expresspolymlett.2010.64. [DOI] [Google Scholar]
- Cota-Arriola O, Cortez-Rocha MO, Burgos-Hernandez A, Ezquerra-Brauer JM, Plascencia-Jatomea M. Controlled release matrices and micro/nanoparticles of chitosan with antimicrobial potential: development of new strategies for microbial control in agriculture. J Sci Food Agric. 2013;93:1525–1536. doi: 10.1002/jsfa.6060. [DOI] [PubMed] [Google Scholar]
- Cui XQ, Li CM, Zang JF, Yu SC. Highly sensitive lactate biosensor by engineering chitosan/PVI-Os/CNT/LOD network nanocomposite. Biosens Bioelectron. 2007;22:3288–3292. doi: 10.1016/j.bios.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Cui F, Li G, Huang J, Zhang J, Lu M, Lu W, Huan J, Huang Q. Development of chitosan-collagen hydrogel incorporated with lysostaphin (CCHL) burn dressing with anti-methicillin-resistant Staphylococcus aureus and promotion wound healing properties. Drug Deliv. 2011;18:173–180. doi: 10.3109/10717544.2010.509363. [DOI] [PubMed] [Google Scholar]
- Cui R, Lu Q, Teng Y, Li K, Li N. Chitosan promoted the corneal epithelial wound healing via activation of erk pathway. Curr Eye Res. 2017;42:21–27. doi: 10.3109/02713683.2016.1145235. [DOI] [PubMed] [Google Scholar]
- Dai H, Chi YW, Wu XP, Wang YM, Wei MD, Chen GN. Biocompatible electrochemiluminescent biosensor for choline based on enzyme/titanate nanotubes/chitosan composite modified electrode. Biosens Bioelectron. 2010;25:1414–1419. doi: 10.1016/j.bios.2009.10.042. [DOI] [PubMed] [Google Scholar]
- Dashtdar H, Murali MR, Abbas AA, Suhaeb AM, Selvaratnam L, Tay LX, Kamarul T. PVA-chitosan composite hydrogel versus alginate beads as a potential mesenchymal stem cell carrier for the treatment of focal cartilage defects. Knee Surg Sports Traumatol Arthrosc. 2015;23:1368–1377. doi: 10.1007/s00167-013-2723-5. [DOI] [PubMed] [Google Scholar]
- De Campos AM, Sanchez A, Alonso MJ. Chitosan nanoparticles: a new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to cyclosporine A. Int J Pharm. 2001;224:159–168. doi: 10.1016/S0378-5173(01)00760-8. [DOI] [PubMed] [Google Scholar]
- De Campos AM, Sanchez A, Gref R, Calvo P, Alonso MJ. The effect of a PEG versus a chitosan coating on the interaction of drug colloidal carriers with the ocular mucosa. Eur J Pharm Sci. 2003;20:73–81. doi: 10.1016/S0928-0987(03)00178-7. [DOI] [PubMed] [Google Scholar]
- De la Riva B, et al. Local controlled release of VEGF and PDGF from a combined brushite–chitosan system enhances bone regeneration. J Control Release. 2010;143:45–52. doi: 10.1016/j.jconrel.2009.11.026. [DOI] [PubMed] [Google Scholar]
- Debrassi A, Corrêa AF, Baccarin T, Nedelko N, Ślawska-Waniewska A, Sobczak K, Dłużewski P, Greneche J-M, Rodrigues CA. Removal of cationic dyes from aqueous solutions using N-benzyl-O-carboxymethylchitosan magnetic nanoparticles. Chem Eng J. 2012;183:284–293. doi: 10.1016/j.cej.2011.12.078. [DOI] [Google Scholar]
- Deepthi S, Venkatesan J, Kim S-K, Bumgardner JD, Jayakumar R. An overview of chitin or chitosan/nano ceramic composite scaffolds for bone tissue engineering. Int Biol Macromol. 2016;93:1338–1353. doi: 10.1016/j.ijbiomac.2016.03.041. [DOI] [PubMed] [Google Scholar]
- Dellera E, Bonferoni MC, Sandri G, Rossi S, Ferrari F, Del Fante C, Perotti C, Grisoli P, Caramella C. Development of chitosan oleate ionic micelles loaded with silver sulfadiazine to be associated with platelet lysate for application in wound healing. Eur J Pharm Biopharm. 2014;88:643–650. doi: 10.1016/j.ejpb.2014.07.015. [DOI] [PubMed] [Google Scholar]
- Deng J, Zhou Y, Xu B, Mai K, Deng Y, Zhang LM. Dendronized chitosan derivative as a biocompatible gene delivery carrier. Biomacromolecules. 2011;12:642–649. doi: 10.1021/bm101303f. [DOI] [PubMed] [Google Scholar]
- Deng Z, Zhen Z, Hu X, Wu S, Xu Z, Chu PK. Hollow chitosan–silica nanospheres as pH-sensitive targeted delivery carriers in breast cancer therapy. Biomaterials. 2011;32:4976–4986. doi: 10.1016/j.biomaterials.2011.03.050. [DOI] [PubMed] [Google Scholar]
- Deng J, She R, Huang W, Dong Z, Mo G, Liu B. A silk fibroin/chitosan scaffold in combination with bone marrow-derived mesenchymal stem cells to repair cartilage defects in the rabbit knee. J Mater Sci Mater Med. 2013;24:2037–2046. doi: 10.1007/s10856-013-4944-z. [DOI] [PubMed] [Google Scholar]
- Dervisevic M, Dervisevic E, Cevik E, Senel M. Novel electrochemical xanthine biosensor based on chitosan-polypyrrole-gold nanoparticles hybrid bio-nanocomposite platform. J Food Drug Anal. 2017;25:510–519. doi: 10.1016/j.jfda.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi N, Dutta J. Preparation and characterization of chitosan-bentonite nanocomposite films for wound healing application. Int Biol Macromol. 2017;104:1897–1904. doi: 10.1016/j.ijbiomac.2017.02.080. [DOI] [PubMed] [Google Scholar]
- Devlieghere F, Vermeulen A, Debevere J. Chitosan: antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables. Food Microbiol. 2004;21:703–714. doi: 10.1016/j.fm.2004.02.008. [DOI] [Google Scholar]
- Dhaliwal S, Jain S, Singh HP, Tiwary AK. Mucoadhesive microspheres for gastroretentive delivery of acyclovir: in vitro and in vivo evaluation. AAPS J. 2008;10:322–330. doi: 10.1208/s12248-008-9039-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhineshbabu NR, Rajendran V. Antibacterial activity of hybrid chitosan-cupric oxide nanoparticles on cotton fabric. IET Nanobiotechnol. 2016;10:13–19. doi: 10.1049/iet-nbt.2014.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias AM, Seabra IJ, Braga MM, Gil MH, de Sousa HC. Supercritical solvent impregnation of natural bioactive compounds in N-carboxybutyl chitosan membranes for the development of topical wound healing applications. J Control Release. 2010;148:e33–e35. doi: 10.1016/j.jconrel.2010.07.043. [DOI] [PubMed] [Google Scholar]
- Diebold Y, Jarrín M, Sáez V, Carvalho EL, Orea M, Calonge M, Seijo B, Alonso MJ. Ocular drug delivery by liposome-chitosan nanoparticle complexes (LCS-NP) Biomaterials. 2007;28:1553–1564. doi: 10.1016/j.biomaterials.2006.11.028. [DOI] [PubMed] [Google Scholar]
- Diez-Pascual AM, Diez-Vicente AL. Wound healing bionanocomposites based on castor oil polymeric films reinforced with chitosan-modified ZnO nanoparticles. Biomacromolecules. 2015;16:2631–2644. doi: 10.1021/acs.biomac.5b00447. [DOI] [PubMed] [Google Scholar]
- Dinescu S, Ionita M, Pandele AM, Galateanu B, Iovu H, Ardelean A, Costache M, Hermenean A. In vitro cytocompatibility evaluation of chitosan/graphene oxide 3D scaffold composites designed for bone tissue engineering. Biomed Mater Eng. 2014;24:2249–2256. doi: 10.3233/BME-141037. [DOI] [PubMed] [Google Scholar]
- Djelad A, Morsli A, Robitzer M, Bengueddach A, di Renzo F, Quignard F. Sorption of Cu(II) ions on chitosan-Zeolite x composites: impact of gelling and drying conditions. Molecules. 2016;21:E109. doi: 10.3390/molecules21010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doares SH, Syrovets T, Weiler EW, Ryan CA. Oligogalacturonides and chitosan activate plant defensive genes through the octadecanoid pathway. Proc Natl Acad Sci USA. 1995;92:4095–4098. doi: 10.1073/pnas.92.10.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Li F, Li J, Li Y. Characterizations of blend gels of carboxymethylated polysaccharides and their use for the controlled release of herbicide. J Macromol Sci A. 2012;49:235–241. doi: 10.1080/10601325.2012.649204. [DOI] [Google Scholar]
- Dong WB, Wang KY, Chen Y, Li WP, Ye YC, Jin SH. Construction and characterization of a chitosan-immobilized-enzyme and beta-cyclodextrin-included-ferrocene-based electrochemical biosensor for H2O2 detection. Materials. 2017;10:868. doi: 10.3390/Ma10080868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan J, Zhang S. Application of chitosan based coating in fruit and vegetable preservation: a review. J Food Process Technol. 2013;4:227. doi: 10.4172/2157-7110.1000227. [DOI] [Google Scholar]
- Duri S, Tran CD. Supramolecular composite materials from cellulose, chitosan and cyclodextrin: facile preparation and their selective inclusion complex formation with endocrine disruptors. Langmuir. 2013;29:5037–5049. doi: 10.1021/la3050016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta PK, Dutta J, Tripathi VS. Chitin and chitosan: Chemistry, properties and applications. J Sci Ind Res. 2004;63:20–31. [Google Scholar]
- Eivazy P, Atyabi F, Jadidi-Niaragh F, Aghebati Maleki L, Miahipour A, Abdolalizadeh J, Yousefi M. The impact of the codelivery of drug-siRNA by trimethyl chitosan nanoparticles on the efficacy of chemotherapy for metastatic breast cancer cell line (MDA-MB-231) Artif Cells Nanomed Biotechnol. 2017;45:889–896. doi: 10.1080/21691401.2016.1185727. [DOI] [PubMed] [Google Scholar]
- El Badawy M, Taktak NEM, Awad OM, Elfiki SA, Abou El-Ela NE. Evaluation of released malathion and spinosad from chitosan/alginate/gelatin capsules against Culex pipiens larvae. Res Rep Trop Med. 2016;7:23–38. doi: 10.2147/RRTM.S108881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hadrami A, Adam LR, El Hadrami I, Daayf F. Chitosan in plant protection. Mar Drugs. 2010;8:968–987. doi: 10.3390/md8040968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Feky GS, El-Banna ST, El-Bahy GS, Abdelrazek EM, Kamal M. Alginate coated chitosan nanogel for the controlled topical delivery of Silver sulfadiazine. Carbohydr Polym. 2017;177:194–202. doi: 10.1016/j.carbpol.2017.08.104. [DOI] [PubMed] [Google Scholar]
- El-Feky GS, Sharaf SS, El Shafei A, Hegazy AA. Using chitosan nanoparticles as drug carriers for the development of a silver sulfadiazine wound dressing. Carbohydr Polym. 2017;158:11–19. doi: 10.1016/j.carbpol.2016.11.054. [DOI] [PubMed] [Google Scholar]
- El-Kamel A, Sokar M, Naggar V, Al Gamal S. Chitosan and sodium alginate-based bioadhesive vaginal tablets. AAPS PharmSci. 2002;4:E44. doi: 10.1208/ps040444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shafei A, ElShemy M, Abou-Okeil A. Eco-friendly finishing agent for cotton fabrics to improve flame retardant and antibacterial properties. Carbohydr Polym. 2015;118:83–90. doi: 10.1016/j.carbpol.2014.11.007. [DOI] [PubMed] [Google Scholar]
- El-Tahlawy K. Chitosan phosphate: a new way for production of eco-friendly flame-retardant cotton textiles. J Text Inst. 2008;99:185–191. doi: 10.1080/00405000701584311. [DOI] [Google Scholar]
- Elviri L, Bianchera A, Bergonzi C, Bettini R. Controlled local drug delivery strategies from chitosan hydrogels for wound healing. Expert Opin Drug Deliv. 2017;14:897–908. doi: 10.1080/17425247.2017.1247803. [DOI] [PubMed] [Google Scholar]
- European Commission SCotFCaAH (2014) Chitosan hydrochloride SANCO 12388:rev. 2
- Fan L, Zhang Y, Li X, Luo C, Lu F, Qiu H. Removal of alizarin red from water environment using magnetic chitosan with Alizarin Red as imprinted molecules. Colloids Surf B Biointerfaces. 2012;91:250–257. doi: 10.1016/j.colsurfb.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Fan L, Luo C, Sun M, Qiu H, Li X. Synthesis of magnetic β-cyclodextrin–chitosan/graphene oxide as nanoadsorbent and its application in dye adsorption and removal. Colloids Surf B Biointerfaces. 2013;103:601–607. doi: 10.1016/j.colsurfb.2012.11.023. [DOI] [PubMed] [Google Scholar]
- Fang J, Zhang Y, Yan S, Liu Z, He S, Cui L, Yin J. Poly(L-glutamic acid)/chitosan polyelectrolyte complex porous microspheres as cell microcarriers for cartilage regeneration. Acta Biomater. 2014;10:276–288. doi: 10.1016/j.actbio.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Fang F, Zhang X, Meng Y, Gu Z, Bao C, Ding X, Li S, Chen X, Tian X. Intumescent flame retardant coatings on cotton fabric of chitosan and ammonium polyphosphate via layer-by-layer assembly. Surf Coat Technol. 2015;262:9–14. doi: 10.1016/j.surfcoat.2014.11.011. [DOI] [Google Scholar]
- Farzana MH, Meenakshi S. Visible light-driven photoactivity of zinc oxide impregnated chitosan beads for the detoxification of textile dyes. Appl Catal A. 2015;503:124–134. doi: 10.1016/j.apcata.2014.12.034. [DOI] [Google Scholar]
- Fawzy AS, Nitisusanta LI, Iqbal K, Daood U, Beng LT, Neo J. Chitosan/Riboflavin-modified demineralized dentin as a potential substrate for bonding. J Mech Behav Biomed Mater. 2013;17:278–289. doi: 10.1016/j.jmbbm.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Feng B-H, Peng L-F. Synthesis and characterization of carboxymethyl chitosan carrying ricinoleic functions as an emulsifier for azadirachtin. Carbohydr Polym. 2012;88:576–582. doi: 10.1016/j.carbpol.2012.01.002. [DOI] [Google Scholar]
- Feng X, Zheng K, Wang C, Chu F, Chen Y. Durable antibacterial cotton fabrics with chitosan based quaternary ammonium salt. Fiber Polym. 2016;17:371–379. doi: 10.1007/s12221-016-5919-8. [DOI] [Google Scholar]
- Ferrero F, Periolatto M, Ferrario S. Sustainable antimicrobial finishing of cotton fabrics by chitosan UV-grafting: from laboratory experiments to semi industrial scale-up. J Clean Prod. 2015;96:244–252. doi: 10.1016/j.jclepro.2013.12.044. [DOI] [Google Scholar]
- Filova E, et al. Polycaprolactone foam functionalized with chitosan microparticles – a suitable scaffold for cartilage regeneration. Physiol Res. 2016;65:121–131. doi: 10.33549/physiolres.932998. [DOI] [PubMed] [Google Scholar]
- Fischak C, Klaus R, Werkmeister RM, Hohenadl C, Prinz M, Schmetterer L, Garhofer G. Effect of topically administered chitosan-N-acetylcysteine on corneal wound healing in a rabbit model. J Ophthalmol. 2017;2017:5192924. doi: 10.1155/2017/5192924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca-Santos B, Chorilli M. An overview of carboxymethyl derivatives of chitosan: Their use as biomaterials and drug delivery systems. Mater Sci Eng C Mater Biol Appl. 2017;77:1349–1362. doi: 10.1016/j.msec.2017.03.198. [DOI] [PubMed] [Google Scholar]
- Fraga AF, Filho EA, Rigo ECS, Boschi AO. Synthesis of chitosan/hydroxyapatite membranes coated with hydroxycarbonate apatite for guided tissue regeneration purposes. Appl Surf Sci. 2011;257:3888–3892. doi: 10.1016/j.apsusc.2010.11.104. [DOI] [Google Scholar]
- Frank LA, Chaves PS, D’Amore CM, Contri RV, Frank AG, Beck RC, Pohlmann AR, Buffon A, Guterres SS. The use of chitosan as cationic coating or gel vehicle for polymeric nanocapsules: increasing penetration and adhesion of imiquimod in vaginal tissue. Eur J Pharm Biopharm. 2017;114:202–212. doi: 10.1016/j.ejpb.2017.01.021. [DOI] [PubMed] [Google Scholar]
- Friedman M, Juneja VK. Review of antimicrobial and antioxidative activities of chitosans in food. J Food Prot. 2010;73:1737–1761. doi: 10.4315/0362-028X-73.9.1737. [DOI] [PubMed] [Google Scholar]
- Gan Donglin, Liu Min, Xu Tong, Wang Kefeng, Tan Hui, Lu Xiong. Chitosan/biphasic calcium phosphate scaffolds functionalized with BMP‐2‐encapsulated nanoparticles and RGD for bone regeneration. Journal of Biomedical Materials Research Part A. 2018;106(10):2613–2624. doi: 10.1002/jbm.a.36453. [DOI] [PubMed] [Google Scholar]
- Ganss C, Lussi A, Grunau O, Klimek J, Schlueter N. Conventional and anti-erosion fluoride toothpastes: effect on enamel erosion and erosion-abrasion. Caries Res. 2011;45:581–589. doi: 10.1159/000334318. [DOI] [PubMed] [Google Scholar]
- Ge Z, Baguenard S, Lim LY, Wee A, Khor E. Hydroxyapatite–chitin materials as potential tissue engineered bone substitutes. Biomaterials. 2004;25:1049–1058. doi: 10.1016/S0142-9612(03)00612-4. [DOI] [PubMed] [Google Scholar]
- Gelfuso GM, Gratieri T, Simao PS, de Freitas LA, Lopez RF. Chitosan microparticles for sustaining the topical delivery of minoxidil sulphate. J Microencapsul. 2011;28:650–658. doi: 10.3109/02652048.2011.604435. [DOI] [PubMed] [Google Scholar]
- Geng B, Jin Z, Li T, Qi X. Kinetics of hexavalent chromium removal from water by chitosan-Fe0 nanoparticles. Chemosphere. 2009;75:825–830. doi: 10.1016/j.chemosphere.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Genskowsky E, Puente LA, Pérez-Álvarez JA, Fernandez-Lopez J, Muñoz LA, Viuda-Martos M. Assessment of antibacterial and antioxidant properties of chitosan edible films incorporated with maqui berry (Aristotelia chilensis) LWT – Food Sci Technol. 2015;64:1057–1062. doi: 10.1016/j.lwt.2015.07.026. [DOI] [Google Scholar]
- Georgopoulou A, Papadogiannis F, Batsali A, Marakis J, Alpantaki K, Eliopoulos AG, Pontikoglou C, Chatzinikolaidou M. Chitosan/gelatin scaffolds support bone regeneration. J Mater Sci Mater Med. 2018;29:59. doi: 10.1007/s10856-018-6064-2. [DOI] [PubMed] [Google Scholar]
- Germershaus O, Mao S, Sitterberg J, Bakowsky U, Kissel T. Gene delivery using chitosan, trimethyl chitosan or polyethylenglycol-graft-trimethyl chitosan block copolymers: establishment of structure-activity relationships in vitro. J Control Release. 2008;125:145–154. doi: 10.1016/j.jconrel.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Ghasemi Hamidabadi H, et al. Chitosan-intercalated montmorillonite/poly(vinyl alcohol) nanofibers as a platform to guide neuronlike differentiation of human dental pulp stem cells. ACS Appl Mater Interfaces. 2017;9:11392–11404. doi: 10.1021/acsami.6b14283. [DOI] [PubMed] [Google Scholar]
- Ghendon Y, Markushin S, Akopova I, Koptiaeva I, Krivtsov G. Chitosan as an adjuvant for poliovaccine. J Med Virol. 2011;83:847–852. doi: 10.1002/jmv.22030. [DOI] [PubMed] [Google Scholar]
- Gholipour-Kanani A, Bahrami SH, Joghataie MT, Samadikuchaksaraei A, Ahmadi-Taftie H, Rabbani S, Kororian A, Erfani E. Tissue engineered poly(caprolactone)-chitosan-poly(vinyl alcohol) nanofibrous scaffolds for burn and cutting wound healing. IET Nanobiotechnol. 2014;8:123–131. doi: 10.1049/iet-nbt.2012.0050. [DOI] [PubMed] [Google Scholar]
- Giannetto M, Costantini M, Mattarozzi M, Careri M. Innovative gold-free carbon nanotube/chitosan-based competitive immunosensor for determination of HIV-related p24 capsid protein in serum. RSC Adv. 2017;7:39970–39976. doi: 10.1039/C7RA07245G. [DOI] [Google Scholar]
- Gokila S, Gomathi T, Sudha PN, Anil S. Removal of the heavy metal ion chromiuim(VI) using Chitosan and Alginate nanocomposites. Int Biol Macromol. 2017;104:1459–1468. doi: 10.1016/j.ijbiomac.2017.05.117. [DOI] [PubMed] [Google Scholar]
- Gomaa YA, El-Khordagui LK, Boraei NA, Darwish IA. Chitosan microparticles incorporating a hydrophilic sunscreen agent. Carbohydr Polym. 2010;81:234–242. doi: 10.1016/j.carbpol.2010.02.024. [DOI] [Google Scholar]
- Gong Z, Xiong H, Long X, Wei L, Li J, Wu Y, Lin Z. Use of synovium-derived stromal cells and chitosan/collagen type I scaffolds for cartilage tissue engineering. Biomed Mater. 2010;5:055005. doi: 10.1088/1748-6041/5/5/055005. [DOI] [PubMed] [Google Scholar]
- Gopinath D, Ahmed MR, Gomathi K, Chitra K, Sehgal PK, Jayakumar R. Dermal wound healing processes with curcumin incorporated collagen films. Biomaterials. 2004;25:1911–1917. doi: 10.1016/S0142-9612(03)00625-2. [DOI] [PubMed] [Google Scholar]
- Graham Caroline. The role of silver in wound healing. British Journal of Nursing. 2005;14(Sup5):S22–S28. doi: 10.12968/bjon.2005.14.Sup5.19954. [DOI] [PubMed] [Google Scholar]
- Grande R, Pessan LA, Carvalho AJF. Thermoplastic blends of chitosan: A method for the preparation of high thermally stable blends with polyesters. Carbohydr Polym. 2018;191:44–52. doi: 10.1016/j.carbpol.2018.02.087. [DOI] [PubMed] [Google Scholar]
- Grassi M, Rizzo L, Farina A. Endocrine disruptors compounds, pharmaceuticals and personal care products in urban wastewater: implications for agricultural reuse and their removal by adsorption process. Environ Sci Pollut Res. 2013;20:3616–3628. doi: 10.1007/s11356-013-1636-7. [DOI] [PubMed] [Google Scholar]
- Grillo R, Pereira AE, Nishisaka CS, de Lima R, Oehlke K, Greiner R, Fraceto LF. Chitosan/tripolyphosphate nanoparticles loaded with paraquat herbicide: an environmentally safer alternative for weed control. J Hazard Mater. 2014;278:163–171. doi: 10.1016/j.jhazmat.2014.05.079. [DOI] [PubMed] [Google Scholar]
- Gu J, Hu W, Deng A, Zhao Q, Lu S, Gu X. Surgical repair of a 30 mm long human median nerve defect in the distal forearm by implantation of a chitosan-PGA nerve guidance conduit. J Tissue Eng Regen Med. 2012;6:163–168. doi: 10.1002/term.407. [DOI] [PubMed] [Google Scholar]
- Guan L, Ge H, Tang X, Su S, Tian P, Xiao N, Zhang H, Zhang L, Liu P. Use of a silk fibroin-chitosan scaffold to construct a tissue-engineered corneal stroma. Cells Tissues Organs. 2013;198:190–197. doi: 10.1159/000355944. [DOI] [PubMed] [Google Scholar]
- Guo Z, Xing R, Liu S, Yu H, Wang P, Li C, Li P. The synthesis and antioxidant activity of the Schiff bases of chitosan and carboxymethyl chitosan. Bioorganic Med Chem Lett. 2005;15:4600–4603. doi: 10.1016/j.bmcl.2005.06.095. [DOI] [PubMed] [Google Scholar]
- Guo M, Jin TZ, Yadav MP, Yang R. Antimicrobial property and microstructure of micro-emulsion edible composite films against Listeria. Int J Food Microbiol. 2015;208:58–64. doi: 10.1016/j.ijfoodmicro.2015.03.018. [DOI] [PubMed] [Google Scholar]
- Gupta H, Velpandian T, Jain S. Ion- and pH-activated novel in-situ gel system for sustained ocular drug delivery. J Drug Target. 2010;18:499–505. doi: 10.3109/10611860903508788. [DOI] [PubMed] [Google Scholar]
- Guzman-Villanueva D, El-Sherbiny IM, Vlassov AV, Herrera-Ruiz D, Smyth HD. Enhanced cellular uptake and gene silencing activity of siRNA molecules mediated by chitosan-derivative nanocomplexes. Int J Pharm. 2014;473:579–590. doi: 10.1016/j.ijpharm.2014.07.026. [DOI] [PubMed] [Google Scholar]
- Haaparanta AM, Jarvinen E, Cengiz IF, Ella V, Kokkonen HT, Kiviranta I, Kellomaki M. Preparation and characterization of collagen/PLA, chitosan/PLA, and collagen/chitosan/PLA hybrid scaffolds for cartilage tissue engineering. J Mater Sci Mater Med. 2014;25:1129–1136. doi: 10.1007/s10856-013-5129-5. [DOI] [PubMed] [Google Scholar]
- Hadwiger LA. Multiple effects of chitosan on plant systems: solid science or hype Plant science: an international. J Exp Plant Biol. 2013;208:42–49. doi: 10.1016/j.plantsci.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Hamman JH. Chitosan based polyelectrolyte complexes as potential carrier materials in drug delivery systems. Mar Drugs. 2010;8:1305–1322. doi: 10.3390/md8041305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hande PE, Kamble S, Samui AB, Kulkarni PS. Chitosan-based lead ion-imprinted interpenetrating polymer network by simultaneous polymerization for selective extraction of lead(II) Ind Eng Chem Res. 2016;55:3668–3678. doi: 10.1021/acs.iecr.5b04889. [DOI] [Google Scholar]
- Hasan A, Waibhaw G, Tiwari S, Dharmalingam K, Shukla I, Pandey LM. Fabrication and characterization of chitosan, polyvinylpyrrolidone, and cellulose nanowhiskers nanocomposite films for wound healing drug delivery application. J Biomed Mater Res A. 2017;105:2391–2404. doi: 10.1002/jbm.a.36097. [DOI] [PubMed] [Google Scholar]
- Hashemi-Beni B, Khoroushi M, Foroughi MR, Karbasi S, Khademi AA. Cytotoxicity assessment of polyhydroxybutyrate/chitosan/nano- bioglass nanofiber scaffolds by stem cells from human exfoliated deciduous teeth stem cells from dental pulp of exfoliated deciduous tooth. Dent Res J. 2018;15:136–145. doi: 10.4103/1735-3327.226524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan MS. Removal of reactive dyes from textile wastewater by immobilized chitosan upon grafted Jute fibers with acrylic acid by gamma irradiation. Radiat Phys Chem. 2015;115:55–61. doi: 10.1016/j.radphyschem.2015.05.038. [DOI] [Google Scholar]
- He S, Zhang W, Li D, Li P, Zhu Y, Ao M, Lia J, Cao Y. Preparation and characterization of double-shelled avermectin microcapsules based on copolymer matrix of silica-glutaraldehyde-chitosan. J Mater Chem B. 2013;1:1270–1278. doi: 10.1039/c2tb00234e. [DOI] [PubMed] [Google Scholar]
- Hebeish A, Sharaf S, Farouk A. Utilization of chitosan nanoparticles as a green finish in multifunctionalization of cotton textile. Int Biol Macromol. 2013;60:10–17. doi: 10.1016/j.ijbiomac.2013.04.078. [DOI] [PubMed] [Google Scholar]
- Highton AJ, Girardin A, Bell GM, Hook SM, Kemp RA. Chitosan gel vaccine protects against tumour growth in an intracaecal mouse model of cancer by modulating systemic immune responses. BMC Immunol. 2016;17:39. doi: 10.1186/s12865-016-0178-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong HJ, Jin SE, Park JS, Ahn WS, Kim CK. Accelerated wound healing by smad3 antisense oligonucleotides-impregnated chitosan/alginate polyelectrolyte complex. Biomaterials. 2008;29:4831–4837. doi: 10.1016/j.biomaterials.2008.08.023. [DOI] [PubMed] [Google Scholar]
- Houschyar KS, Momeni A, Pyles MN, Cha JY, Maan ZN, Duscher D, Jew OS, Siemers F, van Schoonhoven J. The role of current techniques and concepts in peripheral nerve repair. Plast Surg Int. 2016;2016:4175293. doi: 10.1155/2016/4175293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Wang JS, Liu YG, Li X, Zeng GM, Bao ZL, Zeng XX, Chen AW, Long F. Adsorption of chromium (VI) by ethylenediamine-modified cross-linked magnetic chitosan resin: Isotherms, kinetics and thermodynamics. J Hazard Mater. 2011;185:306–314. doi: 10.1016/j.jhazmat.2010.09.034. [DOI] [PubMed] [Google Scholar]
- Hu N, Wu H, Xue C, Gong Y, Wu J, Xiao Z, Yang Y, Ding F, Gu X. Long-term outcome of the repair of 50 mm long median nerve defects in rhesus monkeys with marrow mesenchymal stem cells-containing, chitosan-based tissue engineered nerve grafts. Biomaterials. 2013;34:100–111. doi: 10.1016/j.biomaterials.2012.09.020. [DOI] [PubMed] [Google Scholar]
- Hu D, Wang H, Wang L. Physical properties and antibacterial activity of quaternized chitosan/carboxymethyl cellulose blend films. LWT – Food Sci Technol. 2016;65:398–405. doi: 10.1016/j.lwt.2015.08.033. [DOI] [Google Scholar]
- Hu Y, Chen J, Fan T, Zhang Y, Zhao Y, Shi X, Zhang Q. Biomimetic mineralized hierarchical hybrid scaffolds based on in situ synthesis of nano-hydroxyapatite/chitosan/chondroitin sulfate/hyaluronic acid for bone tissue engineering. Colloid Surf B. 2017;157:93–100. doi: 10.1016/j.colsurfb.2017.05.059. [DOI] [PubMed] [Google Scholar]
- Hu S, Bi S, Yan D, Zhou Z, Sun G, Cheng X, Chen X. Preparation of composite hydroxybutyl chitosan sponge and its role in promoting wound healing. Carbohydr Polym. 2018;184:154–163. doi: 10.1016/j.carbpol.2017.12.033. [DOI] [PubMed] [Google Scholar]
- Huang D, Zuo Y, Zou Q, Zhang L, Li J, Cheng L, Shen J, Li Y. Antibacterial chitosan coating on nano-hydroxyapatite/polyamide66 porous bone scaffold for drug delivery. J Biomater Sci Polym Ed. 2011;22:931–944. doi: 10.1163/092050610X496576. [DOI] [PubMed] [Google Scholar]
- Huang X, Zhang Y, Zhang X, Xu L, Chen X, Wei S. Influence of radiation crosslinked carboxymethyl-chitosan/gelatin hydrogel on cutaneous wound healing. Mater Sci Eng C Mater Biol Appl. 2013;33:4816–4824. doi: 10.1016/j.msec.2013.07.044. [DOI] [PubMed] [Google Scholar]
- Hughes G, Pemberton RM, Fielden PR, Hart JP. Development of a novel reagentless, screen-printed amperometric biosensor based on glutamate dehydrogenase and NAD(+), integrated with multi-walled carbon nanotubes for the determination of glutamate in food and clinical applications. Sensor Actuat B-Chem. 2015;216:614–621. doi: 10.1016/j.snb.2015.04.066. [DOI] [Google Scholar]
- Ibrahim MA, Meera Priyadarshini B, Neo J, Fawzy AS. Characterization of chitosan/TiO2 cano-powder modified glass-ionomer cement for restorative dental applications. J Esthet Restor Dent. 2017;29:146–156. doi: 10.1111/jerd.12282. [DOI] [PubMed] [Google Scholar]
- Ibrahim NA, Eid BM, El-Aziz EA, Elmaaty TMA, Ramadan SM. Loading of chitosan – nano metal oxide hybrids onto cotton/polyester fabrics to impart permanent and effective multifunctions. Int Biol Macromol. 2017;105:769–776. doi: 10.1016/j.ijbiomac.2017.07.099. [DOI] [PubMed] [Google Scholar]
- Ilk S, Saglam N, Ozgen M. Kaempferol loaded lecithin/chitosan nanoparticles: preparation, characterization, and their potential applications as a sustainable antifungal agent. Artif Cells Nanomed Biotechnol. 2017;45:907–916. doi: 10.1080/21691401.2016.1192040. [DOI] [PubMed] [Google Scholar]
- Illum L. Chitosan and its use as a pharmaceutical excipient. Pharm Res. 1998;15:1326–1331. doi: 10.1023/A:1011929016601. [DOI] [PubMed] [Google Scholar]
- Illum L. Nasal drug delivery-possibilities, problems and solutions. J Control Release. 2003;87:187–198. doi: 10.1016/S0168-3659(02)00363-2. [DOI] [PubMed] [Google Scholar]
- Im O, Li J, Wang M, Zhang LG, Keidar M. Biomimetic three-dimensional nanocrystalline hydroxyapatite and magnetically synthesized single-walled carbon nanotube chitosan nanocomposite for bone regeneration. Int J Nanomedicine. 2012;7:2087–2099. doi: 10.2147/IJN.S29743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara M, et al. Chitosan hydrogel as a drug delivery carrier to control angiogenesis. J Artif Organs. 2006;9:8–16. doi: 10.1007/s10047-005-0313-0. [DOI] [PubMed] [Google Scholar]
- Ito M. In vitro properties of a chitosan-bonded hydroxyapatite bone-filling paste. Biomaterials. 1991;12:41–45. doi: 10.1016/0142-9612(91)90130-3. [DOI] [PubMed] [Google Scholar]
- Itoh S, Yamaguchi I, Suzuki M, Ichinose S, Takakuda K, Kobayashi H, Shinomiya K, Tanaka J. Hydroxyapatite-coated tendon chitosan tubes with adsorbed laminin peptides facilitate nerve regeneration in vivo. Brain Res. 2003;993:111–123. doi: 10.1016/j.brainres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Iturriaga L, Olabarrieta I, Castellan A, Gardrat C, Coma V. Active naringin-chitosan films: impact of UV irradiation. Carbohydr Polym. 2014;110:374–381. doi: 10.1016/j.carbpol.2014.03.062. [DOI] [PubMed] [Google Scholar]
- Jahanizadeh S, Yazdian F, Marjani A, Omidi M, Rashedi H. Curcumin-loaded chitosan/carboxymethyl starch/montmorillonite bio-nanocomposite for reduction of dental bacterial biofilm formation. Int Biol Macromol. 2017;105:757–763. doi: 10.1016/j.ijbiomac.2017.07.101. [DOI] [PubMed] [Google Scholar]
- Jain A, Jain SK, Ganesh N, Barve J, Beg AM. Design and development of ligand-appended polysaccharidic nanoparticles for the delivery of oxaliplatin in colorectal cancer. Nanomedicine. 2010;6:179–190. doi: 10.1016/j.nano.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Jamil B, Habib H, Abbasi S, Nasir H, Rahman A, Rehman A, Bokhari H, Imran M. Cefazolin loaded chitosan nanoparticles to cure multi drug resistant Gram-negative pathogens. Carbohydr Polym. 2016;136:682–691. doi: 10.1016/j.carbpol.2015.09.078. [DOI] [PubMed] [Google Scholar]
- Janegitz BC, Figueiredo LCS, Marcolino LH, Souza SPN, Pereira ER, Fatibello O. Development of a carbon nanotubes paste electrode modified with crosslinked chitosan for cadmium(II) and mercury(II) determination. J Electroanal Chem. 2011;660:209–216. doi: 10.1016/j.jelechem.2011.07.001. [DOI] [Google Scholar]
- Javid A, Raza ZA, Hussain T, Rehman A. Chitosan microencapsulation of various essential oils to enhance the functional properties of cotton fabric. J Microencapsul. 2014;31:461–468. doi: 10.3109/02652048.2013.879927. [DOI] [PubMed] [Google Scholar]
- Jayakumar R, Prabaharan M, Sudheesh Kumar PT, Nair SV, Tamura H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol Adv. 2011;29:322–337. doi: 10.1016/j.biotechadv.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Jennings JA, Wells CM, McGraw GS, Velasquez Pulgarin DA, Whitaker MD, Pruitt RL, Bumgardner JD. Chitosan coatings to control release and target tissues for therapeutic delivery. Ther Deliv. 2015;6:855–871. doi: 10.4155/tde.15.31. [DOI] [PubMed] [Google Scholar]
- Jiang HL, Park IK, Shin NR, Yoo HS, Akaike T, Cho CS. Controlled release of Bordetella bronchiseptica dermonecrotoxin (BBD) vaccine from BBD-loaded chitosan microspheres in vitro. Arch Pharm Res. 2004;27:346–350. doi: 10.1007/BF02980071. [DOI] [PubMed] [Google Scholar]
- Jiang T, Abdel-Fattah WI, Laurencin CT. In vitro evaluation of chitosan/poly(lactic acid-glycolic acid) sintered microsphere scaffolds for bone tissue engineering. Biomaterials. 2006;27:4894–4903. doi: 10.1016/j.biomaterials.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Jiang L, Qian F, He X, Wang F, Ren D, He Y, Li K, Sun S, Yin C. Novel chitosan derivative nanoparticles enhance the immunogenicity of a DNA vaccine encoding hepatitis B virus core antigen in mice. J Gene Med. 2007;9:253–264. doi: 10.1002/jgm.1017. [DOI] [PubMed] [Google Scholar]
- Jiang R, Fu YQ, Zhu HY, Yao J, Xiao L. Removal of methyl orange from aqueous solutions by magnetic maghemite/chitosan nanocomposite films: Adsorption kinetics and equilibrium. J Appl Polymer Sci. 2012;125:E540–E549. doi: 10.1002/app.37003. [DOI] [Google Scholar]
- Jiang H, Chen P, Luo S, Luo X, Tu X, Cao Q, Zhou Y, Zhang W. Synthesis of novel biocompatible composite Fe3O4/ZrO2/chitosan and its application for dye removal. J Inorg Organomet Polym Mater. 2013;23:393–400. doi: 10.1007/s10904-012-9792-7. [DOI] [Google Scholar]
- Jiang H, Zuo Y, Zou Q, Wang H, Du J, Li Y, Yang X. Biomimetic spiral-cylindrical scaffold based on hybrid chitosan/cellulose/nano-hydroxyapatite membrane for bone regeneration. ACS Appl Mater Interfaces. 2013;5:12036–12044. doi: 10.1021/am4038432. [DOI] [PubMed] [Google Scholar]
- Jimenez M, Guin T, Bellayer S, Dupretz R, Bourbigot S, Grunlan JC (2016) Microintumescent mechanism of flame-retardant water-based chitosan–ammonium polyphosphate multilayer nanocoating on cotton fabric. J Appl Polymer Sci 133. 10.1002/app.43783
- Jimtaisong A, Saewan N. Utilization of carboxymethyl chitosan in cosmetics. Int J Cosmet Sci. 2014;36:12–21. doi: 10.1111/ics.12102. [DOI] [PubMed] [Google Scholar]
- Jin HH, Kim DH, Kim TW, Shin KK, Jung JS, Park HC, Yoon SY. In vivo evaluation of porous hydroxyapatite/chitosan-alginate composite scaffolds for bone tissue engineering. Int Biol Macromol. 2012;51:1079–1085. doi: 10.1016/j.ijbiomac.2012.08.027. [DOI] [PubMed] [Google Scholar]
- Kadam AA, Lee DS. Glutaraldehyde cross-linked magnetic chitosan nanocomposites: Reduction precipitation synthesis, characterization, and application for removal of hazardous textile dyes. Bioresour Technol. 2015;193:563–567. doi: 10.1016/j.biortech.2015.06.148. [DOI] [PubMed] [Google Scholar]
- Kamari A, Aljafree NFA. Amphiphilic chitosan derivatives as carrier agents for rotenone. AIP Conf Proc. 2017;1868:020001. doi: 10.1063/1.4995087. [DOI] [Google Scholar]
- Kang Yun, Jung Ju-Young, Cho Donghwan, Kwon Oh, Cheon Ja, Park Won. Antimicrobial Silver Chloride Nanoparticles Stabilized with Chitosan Oligomer for the Healing of Burns. Materials. 2016;9(4):215. doi: 10.3390/ma9040215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karri VV, Kuppusamy G, Talluri SV, Mannemala SS, Kollipara R, Wadhwani AD, Mulukutla S, Raju KR, Malayandi R. Curcumin loaded chitosan nanoparticles impregnated into collagen-alginate scaffolds for diabetic wound healing. Int Biol Macromol. 2016;93:1519–1529. doi: 10.1016/j.ijbiomac.2016.05.038. [DOI] [PubMed] [Google Scholar]
- Kashyap PL, Xiang X, Heiden P. Chitosan nanoparticle based delivery systems for sustainable agriculture. Int Biol Macromol. 2015;77:36–51. doi: 10.1016/j.ijbiomac.2015.02.039. [DOI] [PubMed] [Google Scholar]
- Katiyar D, Hemantaranjan A, Singh B, Bhanu AN. A future perspective in crop protection: chitosan and its oligosaccharides. Adv Plants Agric Res. 2014;1:23–30. [Google Scholar]
- Kato Y, Yagami A, Matsunaga K. A case of anaphylaxis caused by the health food chitosan. Arerugi. 2005;54:1427–1429. [PubMed] [Google Scholar]
- Katti KS, Katti DR, Dash R. Synthesis and characterization of a novel chitosan/montmorillonite/hydroxyapatite nanocomposite for bone tissue engineering. Biomed Mater. 2008;3:034122. doi: 10.1088/1748-6041/3/3/034122. [DOI] [PubMed] [Google Scholar]
- Kaynak Bayrak G, Demirtas TT, Gumusderelioglu M. Microwave-induced biomimetic approach for hydroxyapatite coatings of chitosan scaffolds. Carbohydr Polym. 2017;157:803–813. doi: 10.1016/j.carbpol.2016.10.016. [DOI] [PubMed] [Google Scholar]
- Kean T, Thanou M. Biodegradation, biodistribution and toxicity of chitosan. Adv Drug Deliv Rev. 2010;62:3–11. doi: 10.1016/j.addr.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Khajuria DK, Zahra SF, Razdan R. Effect of locally administered novel biodegradable chitosan based risedronate/zinc-hydroxyapatite intra-pocket dental film on alveolar bone density in rat model of periodontitis. J Biomater Sci Polym Ed. 2018;29:74–91. doi: 10.1080/09205063.2017.1400145. [DOI] [PubMed] [Google Scholar]
- Khanna R, Katti KS, Katti DR. Bone nodules on chitosan-polygalacturonic acid-hydroxyapatite nanocomposite films mimic hierarchy of natural bone. Acta Biomater. 2011;7:1173–1183. doi: 10.1016/j.actbio.2010.10.028. [DOI] [PubMed] [Google Scholar]
- Khwaldia K, Basta AH, Aloui H, El-Saied H. Chitosan-caseinate bilayer coatings for paper packaging materials. Carbohydr Polym. 2014;99:508–516. doi: 10.1016/j.carbpol.2013.08.086. [DOI] [PubMed] [Google Scholar]
- Kim SE, Park JH, Cho YW, Chung H, Jeong SY, Lee EB, Kwon IC. Porous chitosan scaffold containing microspheres loaded with transforming growth factor-beta1: implications for cartilage tissue engineering. J Control Release. 2003;91:365–374. doi: 10.1016/S0168-3659(03)00274-8. [DOI] [PubMed] [Google Scholar]
- Kim JH, et al. Hydrophobically modified glycol chitosan nanoparticles as carriers for paclitaxel. J Control Release. 2006;111:228–234. doi: 10.1016/j.jconrel.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Kim JH, et al. Antitumor efficacy of cisplatin-loaded glycol chitosan nanoparticles in tumor-bearing mice. J Control Release. 2008;127:41–49. doi: 10.1016/j.jconrel.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Kim HL, Jung GY, Yoon JH, Han JS, Park YJ, Kim DG, Zhang M, Kim DJ. Preparation and characterization of nano-sized hydroxyapatite/alginate/chitosan composite scaffolds for bone tissue engineering. Mater Sci Eng C Mater Biol Appl. 2015;54:20–25. doi: 10.1016/j.msec.2015.04.033. [DOI] [PubMed] [Google Scholar]
- Kim Y-M, Park S-C, Jang M-K. Targeted gene delivery of polyethyleneimine-grafted chitosan with RGD dendrimer peptide in αvβ3 integrin-overexpressing tumor cells. Carbohydr Polym. 2017;174:1059–1068. doi: 10.1016/j.carbpol.2017.07.035. [DOI] [PubMed] [Google Scholar]
- Knapczyk J. Chitosan hydrogel as a base for semisolid drug forms. Int J Pharm. 1993;93:233–237. doi: 10.1016/0378-5173(93)90182-F. [DOI] [Google Scholar]
- Koç Demir A, Elçin AE, Elçin YM. Strontium-modified chitosan/montmorillonite composites as bone tissue engineering scaffold. Mater Sci Eng C. 2018;89:8–14. doi: 10.1016/j.msec.2018.03.021. [DOI] [PubMed] [Google Scholar]
- Kohsari I, Shariatinia Z, Pourmortazavi SM. Antibacterial electrospun chitosan-polyethylene oxide nanocomposite mats containing ZIF-8 nanoparticles. Int Biol Macromol. 2016;91:778–788. doi: 10.1016/j.ijbiomac.2016.06.039. [DOI] [PubMed] [Google Scholar]
- Kong Y, Xu R, Darabi MA, Zhong W, Luo G, Xing MM, Wu J. Fast and safe fabrication of a free-standing chitosan/alginate nanomembrane to promote stem cell delivery and wound healing. Int J Nanomedicine. 2016;11:2543–2555. doi: 10.2147/IJN.S102861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong D, Wang N, Qiao N, Wang Q, Wang Z, Zhou Z, Ren Z. Facile preparation of ion-imprinted chitosan microspheres enwrapping Fe3O4 and graphene oxide by inverse suspension cross-linking for highly selective removal of copper(II) ACS Sustain Chem Eng. 2017;5:7401–7409. doi: 10.1021/acssuschemeng.7b01761. [DOI] [Google Scholar]
- Kong SZ, et al. Anti-photoaging effects of chitosan oligosaccharide in ultraviolet-irradiated hairless mouse skin. Exp Gerontol. 2018;103:27–34. doi: 10.1016/j.exger.2017.12.018. [DOI] [PubMed] [Google Scholar]
- Kos L. Use of chitosan for textile wastewater decolourization fibres. Text East Eur. 2016;24:130–135. [Google Scholar]
- Kousalya GN, Gandhi MR, Meenakshi S. Removal of toxic Cr(VI) ions from aqueous solution using nano-hydroxyapatite-based chitin and chitosan hybrid composites. Adsorpt Sci Technol. 2010;28:49–64. doi: 10.1260/0263-6174.28.1.49. [DOI] [Google Scholar]
- Kratz G, Arnander C, Swedenborg J, Back M, Falk C, Gouda I, Larm O. Heparin-chitosan complexes stimulate wound healing in human skin. Scand J Plastic Reconstructi Surg Hand Surg. 1997;31:119–123. doi: 10.3109/02844319709085478. [DOI] [PubMed] [Google Scholar]
- Krausz AE, et al. Curcumin-encapsulated nanoparticles as innovative antimicrobial and wound healing agent. Nanomedicine. 2015;11:195–206. doi: 10.1016/j.nano.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnaswami V, Kandasamy R, Alagarsamy S, Palanisamy R, Natesan S. Biological macromolecules for ophthalmic drug delivery to treat ocular diseases. Int Biol Macromol. 2018;110:7–16. doi: 10.1016/j.ijbiomac.2018.01.120. [DOI] [PubMed] [Google Scholar]
- Kristl J, Šmid-Korbar J, Štruc E, Schara M, Rupprecht H. Hydrocolloids and gels of chitosan as drug carriers. Int J Pharm. 1993;99:13–19. doi: 10.1016/0378-5173(93)90317-9. [DOI] [Google Scholar]
- Kulkarni AD, Vanjari YH, Sancheti KH, Patel HM, Belgamwar VS, Surana SJ, Pardeshi CV. New nasal nanocomplex self-assembled from charged biomacromolecules: N,N,N-Trimethyl chitosan and dextran sulfate. Int Biol Macromol. 2016;88:476–490. doi: 10.1016/j.ijbiomac.2016.03.045. [DOI] [PubMed] [Google Scholar]
- Kumar D, Kumar P, Pandey J. Binary grafted chitosan film: synthesis, characterization, antibacterial activity and prospects for food packaging. Int Biol Macromol. 2018;115:341–348. doi: 10.1016/j.ijbiomac.2018.04.084. [DOI] [PubMed] [Google Scholar]
- Kumar-Krishnan S, Prokhorov E, Hernández-Iturriaga M, Mota-Morales JD, Vázquez-Lepe M, Kovalenko Y, Sanchez IC, Luna-Bárcenas G. Chitosan/silver nanocomposites: Synergistic antibacterial action of silver nanoparticles and silver ions. Eur Polym J. 2015;67:242–251. doi: 10.1016/j.eurpolymj.2015.03.066. [DOI] [Google Scholar]
- Kundu CK, Wang W, Zhou S, Wang X, Sheng H, Pan Y, Song L, Hu Y. A green approach to constructing multilayered nanocoating for flame retardant treatment of polyamide 66 fabric from chitosan and sodium alginate. Carbohydr Polym. 2017;166:131–138. doi: 10.1016/j.carbpol.2017.02.084. [DOI] [PubMed] [Google Scholar]
- Kuo YC, Ku IN. Cartilage regeneration by novel polyethylene oxide/chitin/chitosan scaffolds. Biomacromolecules. 2008;9:2662–2669. doi: 10.1021/bm800651r. [DOI] [PubMed] [Google Scholar]
- Kuo CY, Chen CH, Hsiao CY, Chen JP. Incorporation of chitosan in biomimetic gelatin/chondroitin-6-sulfate/hyaluronan cryogel for cartilage tissue engineering. Carbohydr Polym. 2015;117:722–730. doi: 10.1016/j.carbpol.2014.10.056. [DOI] [PubMed] [Google Scholar]
- Kweon DK, Song SB, Park YY. Preparation of water-soluble chitosan/heparin complex and its application as wound healing accelerator. Biomaterials. 2003;24:1595–1601. doi: 10.1016/S0142-9612(02)00566-5. [DOI] [PubMed] [Google Scholar]
- Kyzas GZ, Bikiaris DN. Recent modifications of chitosan for adsorption applications: a critical and systematic review. Mar Drugs. 2015;13:312–337. doi: 10.3390/md13010312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyzas GZ, Kostoglou M, Lazaridis NK, Lambropoulou DA, Bikiaris DN. Environmental friendly technology for the removal of pharmaceutical contaminants from wastewaters using modified chitosan adsorbents. Chem Eng J. 2013;222:248–258. doi: 10.1016/j.cej.2013.02.048. [DOI] [Google Scholar]
- Kyzas GZ, Bikiaris DN, Seredych M, Bandosz TJ, Deliyanni EA. Removal of dorzolamide from biomedical wastewaters with adsorption onto graphite oxide/poly(acrylic acid) grafted chitosan nanocomposite. Bioresour Technol. 2014;152:399–406. doi: 10.1016/j.biortech.2013.11.046. [DOI] [PubMed] [Google Scholar]
- Lai C, et al. Anti-tumor immune response of folate-conjugated chitosan nanoparticles containing the IP-10 gene in mice with hepatocellular carcinoma. J Biomed Nanotechnol. 2014;10:3576–3589. doi: 10.1166/jbn.2014.2051. [DOI] [PubMed] [Google Scholar]
- Lalevee G, Sudre G, Montembault A, Meadows J, Malaise S, Crépet A, David L, Delair T. Polyelectrolyte complexes via desalting mixtures of hyaluronic acid and chitosan-Physicochemical study and structural analysis. Carbohydr Polym. 2016;154:86–95. doi: 10.1016/j.carbpol.2016.08.007. [DOI] [PubMed] [Google Scholar]
- Lam NYK, Zhang M, Guo H-f, Ho CP, Li L. Effect of fiber length and blending method on the tensile properties of ring spun chitosan–cotton blend yarns. Text Res J. 2017;87:244–257. doi: 10.1177/0040517516629141. [DOI] [Google Scholar]
- Lam NYK, Zhang M, Yang CX, Ho CP, Li L. A pilot intervention with chitosan/cotton knitted jersey fabric to provide comfort for epidermolysis bullosa patients. Text Res J. 2018;88:704–716. doi: 10.1177/0040517516688625. [DOI] [Google Scholar]
- Lao L, Tan H, Wang Y, Gao C. Chitosan modified poly(L-lactide) microspheres as cell microcarriers for cartilage tissue engineering. Colloid Surf B. 2008;66:218–225. doi: 10.1016/j.colsurfb.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Leceta I, Guerrero P, Ibarburu I, Dueñas MT, de la Caba K. Characterization and antimicrobial analysis of chitosan-based films. J Food Eng. 2013;116:889–899. doi: 10.1016/j.jfoodeng.2013.01.022. [DOI] [Google Scholar]
- Lee CG. Chitin, chitinases and chitinase-like proteins in allergic inflammation and tissue remodeling. Yonsei Med J. 2009;50:22–30. doi: 10.3349/ymj.2009.50.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CA, Tsai YC. Preparation of multiwalled carbon nanotube-chitosan-alcohol dehydrogenase nanobiocomposite for amperometric detection of ethanol. Sensor Actuators B-Chem. 2009;138:518–523. doi: 10.1016/j.snb.2009.01.001. [DOI] [Google Scholar]
- Lee CG, Da Silva CA, Lee J-Y, Hartl D, Elias JA. Chitin regulation of immune responses: an old molecule with new roles. Curr Opin Immunol. 2008;20:684–689. doi: 10.1016/j.coi.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Liu KH, Liu YY, Chang YP, Lin CC, Chen YS. Chitosonic((R)) acid as a novel cosmetic ingredient: evaluation of its antimicrobial, antioxidant and hydration activities. Materials. 2013;6:1391–1402. doi: 10.3390/ma6041391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, et al. Chitosan/polyurethane blended fiber sheets containing silver sulfadiazine for use as an antimicrobial wound dressing. J Nanosci Nanotechnol. 2014;14:7488–7494. doi: 10.1166/jnn.2014.9581. [DOI] [PubMed] [Google Scholar]
- Lee CM, Yang SW, Jung SC, Kim BH. Oxygen plasma treatment on 3D-printed chitosan/gelatin/hydroxyapatite scaffolds for bone tissue engineering. J Nanosci Nanotechnol. 2017;17:2747–2750. doi: 10.1166/jnn.2017.13337. [DOI] [PubMed] [Google Scholar]
- Leistner M, Abu-Odeh AA, Rohmer SC, Grunlan JC. Water-based chitosan/melamine polyphosphate multilayer nanocoating that extinguishes fire on polyester-cotton fabric. Carbohydr Polym. 2015;130:227–232. doi: 10.1016/j.carbpol.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Li Z, Zhang M. Chitosan-alginate as scaffolding material for cartilage tissue engineering. J Biomed Mater Res A. 2005;75:485–493. doi: 10.1002/jbm.a.30449. [DOI] [PubMed] [Google Scholar]
- Li Z, Ramay HR, Hauch KD, Xiao D, Zhang M. Chitosan-alginate hybrid scaffolds for bone tissue engineering. Biomaterials. 2005;26:3919–3928. doi: 10.1016/j.biomaterials.2004.09.062. [DOI] [PubMed] [Google Scholar]
- Li J, Yao J, Li Y, Shao Y. Controlled release and retarded leaching of pesticides by encapsulating in carboxymethyl chitosan/bentonite composite gel. J Environ Sci Health B. 2012;47:795–803. doi: 10.1080/03601234.2012.676421. [DOI] [PubMed] [Google Scholar]
- Li X, Chen S, Zhang B, Li M, Diao K, Zhang Z, Li J, Xu Y, Wang X, Chen H. In situ injectable nano-composite hydrogel composed of curcumin, N,O-carboxymethyl chitosan and oxidized alginate for wound healing application. Int J Pharm. 2012;437:110–119. doi: 10.1016/j.ijpharm.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Li B, Shan CL, Ge MY, Wang L, Fang Y, Wang YL, Xie GL, Sun GC. Antibacterial mechanism of chitosan and its applications in protection of plant from bacterial disease. Asian J Chem. 2013;25:10033–10036. doi: 10.14233/ajchem.2013.15217. [DOI] [Google Scholar]
- Li X, Min M, Du N, Gu Y, Hode T, Naylor M, Chen D, Nordquist RE, Chen WR. Chitin, chitosan, and glycated chitosan regulate immune responses: the novel adjuvants for cancer vaccine. Clin Dev Immunol. 2013;2013:387023. doi: 10.1155/2013/387023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zhao F, Zhao B, Zhang J, Li C, Qiao R. Chitosan grafted with phosphorylcholine and macrocyclic polyamine as an effective gene delivery vector: Preparation, characterization and in vitro transfection. Macromol Biosci. 2015;15:912–926. doi: 10.1002/mabi.201400518. [DOI] [PubMed] [Google Scholar]
- Li Y, Qin Y, Liu S, Xing R, Yu H, Li K, Li P. Preparation, characterization, and insecticidal activity of avermectin-grafted-carboxymethyl chitosan. BioMed Res Int. 2016;2016:9805675. doi: 10.1155/2016/9805675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Jiang B, Liu Y, Qiu C, Hu J, Qian G, Guo W, Ngo HH. Preparation and adsorption properties of magnetic chitosan composite adsorbent for Cu2+ removal. J Clean Prod. 2017;158:51–58. doi: 10.1016/j.jclepro.2017.04.156. [DOI] [Google Scholar]
- Li R, et al. Chitosan conduit combined with hyaluronic acid prevent sciatic nerve scar in a rat model of peripheral nerve crush injury. Mol Med Rep. 2018;17:4360–4368. doi: 10.3892/mmr.2018.8388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, et al. The natural product chitosan enhances the anti-tumor activity of natural killer cells by activating dendritic cells. Oncoimmunology. 2018;7:e1431085. doi: 10.1080/2162402x.2018.1431085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Liu W, Han B, Yang C, Ma Q, Zhao W, Rong M, Li H. Fabrication and characters of a corneal endothelial cells scaffold based on chitosan. J Mater Sci Mater Med. 2011;22:175–183. doi: 10.1007/s10856-010-4190-6. [DOI] [PubMed] [Google Scholar]
- Liang Y, Xu W, Han B, Li N, Zhao W, Liu W. Tissue-engineered membrane based on chitosan for repair of mechanically damaged corneal epithelium. J Mater Sci Mater Med. 2014;25:2163–2171. doi: 10.1007/s10856-014-5248-7. [DOI] [PubMed] [Google Scholar]
- Liao CD, Zhang F, Guo RM, Zhong XM, Zhu J, Wen XH, Shen J. Peripheral nerve repair: monitoring by using gadofluorine M-enhanced MR imaging with chitosan nerve conduits with cultured mesenchymal stem cells in rat model of neurotmesis. Radiology. 2012;262:161–171. doi: 10.1148/radiol.11110911. [DOI] [PubMed] [Google Scholar]
- Liao J, Li Y, Li H, Liu J, Xie Y, Wang J, Zhang Y. Preparation, bioactivity and mechanism of nano-hydroxyapatite/sodium alginate/chitosan bone repair material. J Appl Biomater Funct Mater. 2018;16:28–35. doi: 10.5301/jabfm.5000372. [DOI] [PubMed] [Google Scholar]
- Liaqat F, Eltem R. Chitooligosaccharides and their biological activities: a comprehensive review. Carbohydr Polym. 2018;184:243–259. doi: 10.1016/j.carbpol.2017.12.067. [DOI] [PubMed] [Google Scholar]
- Libio IC, Demori R, Ferrao MF, Lionzo MIZ, da Silveira NP. Films based on neutralized chitosan citrate as innovative composition for cosmetic application. Mater Sci Eng C Mater Biol Appl. 2016;67:115–124. doi: 10.1016/j.msec.2016.05.009. [DOI] [PubMed] [Google Scholar]
- Lih E, Lee JS, Park KM, Park KD. Rapidly curable chitosan-PEG hydrogels as tissue adhesives for hemostasis and wound healing. Acta Biomater. 2012;8:3261–3269. doi: 10.1016/j.actbio.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Lim G-P, Ahmad MS. Development of Ca-alginate-chitosan microcapsules for encapsulation and controlled release of imidacloprid to control dengue outbreaks. J Ind Eng Chem. 2017;56:382–393. doi: 10.1016/j.jiec.2017.07.035. [DOI] [Google Scholar]
- Lima PA, Resende CX, Soares GD, Anselme K, Almeida LE. Preparation, characterization and biological test of 3D-scaffolds based on chitosan, fibroin and hydroxyapatite for bone tissue engineering. Mater Sci Eng C Mater Biol Appl. 2013;33:3389–3395. doi: 10.1016/j.msec.2013.04.026. [DOI] [PubMed] [Google Scholar]
- Lin YL, Jen JC, Hsu SH, Chiu IM. Sciatic nerve repair by microgrooved nerve conduits made of chitosan-gold nanocomposites. Surg Neurol. 2008;70(S1):9–18. doi: 10.1016/j.surneu.2008.01.057. [DOI] [PubMed] [Google Scholar]
- Lin JH, He CY, Zhang LJ, Zhang SS. Sensitive amperometric immunosensor for alpha-fetoprotein based on carbon nanotube/gold nanoparticle doped chitosan film. Anal Biochem. 2009;384:130–135. doi: 10.1016/j.ab.2008.09.033. [DOI] [PubMed] [Google Scholar]
- Lin J, Fan L, Miao R, Le X, Chen S, Zhou X. Enhancing catalytic performance of laccase via immobilization on chitosan/CeO2 microspheres. Int Biol Macromol. 2015;78:1–8. doi: 10.1016/j.ijbiomac.2015.03.033. [DOI] [PubMed] [Google Scholar]
- Lin YH, Lin JH, Hong YS. Development of chitosan/poly-gamma-glutamic acid/pluronic/curcumin nanoparticles in chitosan dressings for wound regeneration. J Biomed Mater Res B. 2017;105:81–90. doi: 10.1002/jbm.b.33394. [DOI] [PubMed] [Google Scholar]
- Listoni AJ, Arruda I, Maia L, Barberini DJ, Martins I, Vasconcellos FC, Landim-Alvarenga FC. Differentiation potential of mesenchymal stem cells from equine bone marrow cultured on hyaluronic acid-chitosan polyelectrolyte multilayer biofilm. J Stem Cells. 2015;10:69–77. [PubMed] [Google Scholar]
- Liu C, Bai R. Recent advances in chitosan and its derivatives as adsorbents for removal of pollutants from water and wastewater. Curr Opin Chem Eng. 2014;4:62–70. doi: 10.1016/j.coche.2014.01.004. [DOI] [Google Scholar]
- Liu Y, Wang MK, Zhao F, Xu ZA, Dong SJ. The direct electron transfer of glucose oxidase and glucose biosensor based on carbon nanotubes/chitosan matrix. Biosens Bioelectron. 2005;21:984–988. doi: 10.1016/j.bios.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Liu Z, Wang F, Chen X. Integrin α(v)β(3)-targeted cancer therapy. Drug Dev Res. 2008;69:329–339. doi: 10.1002/ddr.20265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YX, Yuan R, Chai YQ, Hong CL, Liu KG, Guan S. Ultrasensitive amperometric immunosensor for the determination of carcinoembryonic antigen based on a porous chitosan and gold nanoparticles functionalized interface. Microchim Acta. 2009;167:217–224. doi: 10.1007/s00604-009-0243-2. [DOI] [Google Scholar]
- Liu XJ, Luo LQ, Ding YP, Xu YH. Amperometric biosensors based on alumina nanoparticles-chitosan-horseradish peroxidase nanobiocomposites for the determination of phenolic compounds. Analyst. 2011;136:696–701. doi: 10.1039/C0AN00752H. [DOI] [PubMed] [Google Scholar]
- Liu H, Wen W, Hu M, Bi W, Chen L, Liu S, Chen P, Tan X. Chitosan conduits combined with nerve growth factor microspheres repair facial nerve defects. Neural Regen Res. 2013;8:3139–3147. doi: 10.3969/j.issn.1673-5374.2013.33.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Zheng X, Zhang C, Shao X, Zhang X, Zhang Q, Jiang X. Conjugating influenza a (H1N1) antigen to N-trimethylaminoethylmethacrylate chitosan nanoparticles improves the immunogenicity of the antigen after nasal administration. J Med Virol. 2015;87:1807–1815. doi: 10.1002/jmv.24253. [DOI] [PubMed] [Google Scholar]
- Liu X-Y, Gohi BFCA, Zeng H-Y, Liao M-C, Sun J-W. Effect of collagen peptides-carboxymethyl chitosan microspheres on ultraviolet induced damages. Mater Express. 2015;5:497–504. doi: 10.1166/mex.2015.1264. [DOI] [Google Scholar]
- Liu J, Liu W, Wang Y, Xu M, Wang B. A novel reusable nanocomposite adsorbent, xanthated Fe3O4-chitosan grafted onto graphene oxide, for removing Cu(II) from aqueous solutions. Appl Surf Sci. 2016;367:327–334. doi: 10.1016/j.apsusc.2016.01.176. [DOI] [Google Scholar]
- Liu Y, Chen L, Yang Y, Li M, Li Y, Dong Y. The efficient removal of Cu(II) from aqueous solutions by Fe3O4@hexadecyl trimethoxysilane@chitosan composites. J Mol Liq. 2016;219:341–349. doi: 10.1016/j.molliq.2016.03.015. [DOI] [Google Scholar]
- Liuyun J, Yubao L, Chengdong X. Preparation and biological properties of a novel composite scaffold of nano-hydroxyapatite/chitosan/carboxymethyl cellulose for bone tissue engineering. J Biomed Sci. 2009;16:65. doi: 10.1186/1423-0127-16-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes C, et al. Chitosan coated textiles may improve atopic dermatitis severity by modulating skin staphylococcal profile: a randomized controlled trial. PloS One. 2015;10:e0142844. doi: 10.1371/journal.pone.0142844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Carballo G, Higueras L, Gavara R, Hernandez-Munoz P. Silver ions release from antibacterial chitosan films containing in situ generated silver nanoparticles. J Agric Food Chem. 2013;61:260–267. doi: 10.1021/jf304006y. [DOI] [PubMed] [Google Scholar]
- Lorevice MV, Otoni CG, MRd M, Mattoso LHC. Chitosan nanoparticles on the improvement of thermal, barrier, and mechanical properties of high- and low-methyl pectin films. Food Hydrocolloid. 2016;52:732–740. doi: 10.1016/j.foodhyd.2015.08.003. [DOI] [Google Scholar]
- Lotfi G, Shokrgozar MA, Mofid R, Abbas FM, Ghanavati F, Baghban AA, Yavari SK, Pajoumshariati S. Biological evaluation (in vitro and in vivo) of bilayered collagenous coated (nano electrospun and solid wall) chitosan membrane for periodontal guided bone regeneration. Ann Biomed Eng. 2016;44:2132–2144. doi: 10.1007/s10439-015-1516-z. [DOI] [PubMed] [Google Scholar]
- Lowe B, Venkatesan J, Anil S, Shim MS, Kim SK. Preparation and characterization of chitosan-natural nano hydroxyapatite-fucoidan nanocomposites for bone tissue engineering. Int Biol Macromol. 2016;93:1479–1487. doi: 10.1016/j.ijbiomac.2016.02.054. [DOI] [PubMed] [Google Scholar]
- Lu S, Gao W, Gu HY. Construction, application and biosafety of silver nanocrystalline chitosan wound dressing. Burns. 2008;34:623–628. doi: 10.1016/j.burns.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Lu Y, Cheng D, Lu S, Huang F, Li G. Preparation of quaternary ammonium salt of chitosan nanoparticles and their textile properties on Antheraea pernyi silk modification. Text Res J. 2014;84:2115–2124. doi: 10.1177/0040517514538691. [DOI] [Google Scholar]
- Lu B, Wang T, Li Z, Dai F, Lv L, Tang F, Yu K, Liu J, Lan G. Healing of skin wounds with a chitosan–gelatin sponge loaded with tannins and platelet-rich plasma. Int Biol Macromol. 2016;82:884–891. doi: 10.1016/j.ijbiomac.2015.11.009. [DOI] [PubMed] [Google Scholar]
- Lu Z, Gao J, He Q, Wu J, Liang D, Yang H, Chen R. Enhanced antibacterial and wound healing activities of microporous chitosan-Ag/ZnO composite dressing. Carbohydr Polym. 2017;156:460–469. doi: 10.1016/j.carbpol.2016.09.051. [DOI] [PubMed] [Google Scholar]
- Lu Y, et al. High-activity chitosan/nano hydroxyapatite/zoledronic acid scaffolds for simultaneous tumor inhibition, bone repair and infection eradication. Mater Sci Eng C Mater Biol Appl. 2018;82:225–233. doi: 10.1016/j.msec.2017.08.043. [DOI] [PubMed] [Google Scholar]
- Luna-Hernandez E, et al. Combined antibacterial/tissue regeneration response in thermal burns promoted by functional chitosan/silver nanocomposites. Int Biol Macromol. 2017;105:1241–1249. doi: 10.1016/j.ijbiomac.2017.07.159. [DOI] [PubMed] [Google Scholar]
- Ma W, Tang C-H, Yang X-Q, Yin S-W. Fabrication and characterization of kidney bean (Phaseolus vulgaris L.) protein isolate–chitosan composite films at acidic pH. Food Hydrocolloid. 2013;31:237–247. doi: 10.1016/j.foodhyd.2012.10.007. [DOI] [Google Scholar]
- Ma Z, Yang L, Yan H, Kennedy JF, Meng X. Chitosan and oligochitosan enhance the resistance of peach fruit to brown rot. Carbohydr Polym. 2013;94:272–277. doi: 10.1016/j.carbpol.2013.01.012. [DOI] [PubMed] [Google Scholar]
- Ma S, et al. Guided bone regeneration with tripolyphosphate cross-linked asymmetric chitosan membrane. J Dent. 2014;42:1603–1612. doi: 10.1016/j.jdent.2014.08.015. [DOI] [PubMed] [Google Scholar]
- Ma F, Zhang Q, Zheng L. Interleukin/chitosan (JY) adjuvant enhances the mucosal immunity of human papillomavirus 16 L1 virus-like particles in mice. Biotechnol Lett. 2015;37:773–777. doi: 10.1007/s10529-014-1739-3. [DOI] [PubMed] [Google Scholar]
- Madureira AR, Pereira A, Pintado M. Current state on the development of nanoparticles for use against bacterial gastrointestinal pathogens. Focus on chitosan nanoparticles loaded with phenolic compounds. Carbohydr Polym. 2015;130:429–439. doi: 10.1016/j.carbpol.2015.05.030. [DOI] [PubMed] [Google Scholar]
- Maji K, Dasgupta S, Kundu B, Bissoyi A. Development of gelatin-chitosan-hydroxyapatite based bioactive bone scaffold with controlled pore size and mechanical strength. J Biomater Sci Polym Ed. 2015;26:1190–1209. doi: 10.1080/09205063.2015.1082809. [DOI] [PubMed] [Google Scholar]
- Malerba Massimo, Cerana Raffaella. Chitosan Effects on Plant Systems. International Journal of Molecular Sciences. 2016;17(7):996. doi: 10.3390/ijms17070996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik A, Gupta M, Mani R, Gogoi H, Bhatnagar R. Trimethyl chitosan nanoparticles encapsulated protective antigen protects the mice against anthrax. Front Immunol. 2018;9:562. doi: 10.3389/fimmu.2018.00562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Lopez E, Alonso FR, Nieto-Diaz M, Nieto-Sampedro M. Chitosan, gelatin and poly(L-lysine) polyelectrolyte-based scaffolds and films for neural tissue engineering. J Biomater Sci Polym Ed. 2012;23:207–232. doi: 10.1163/092050610X546426. [DOI] [PubMed] [Google Scholar]
- Martiñon ME, Moreira RG, Castell-Perez ME, Gomes C. Development of a multilayered antimicrobial edible coating for shelf-life extension of fresh-cut cantaloupe (Cucumis melo L.) stored at 4 °C. LWT – Food Sci Technol. 2014;56:341–350. doi: 10.1016/j.lwt.2013.11.043. [DOI] [Google Scholar]
- Martins JT, Cerqueira MA, Vicente AA. Influence of α-tocopherol on physicochemical properties of chitosan-based films. Food Hydrocolloid. 2012;27:220–227. doi: 10.1016/j.foodhyd.2011.06.011. [DOI] [Google Scholar]
- Masoomi L, Sadeghi O, Banitaba MH, Shahrjerdi A, Davarani SSH. A non-enzymatic nanomagnetic electro-immunosensor for determination of Aflatoxin B-1 as a model antigen. Sensor Actuat B-Chem. 2013;177:1122–1127. doi: 10.1016/j.snb.2012.11.067. [DOI] [Google Scholar]
- Matos BN, Reis TA, Gratieri T, Gelfuso GM. Chitosan nanoparticles for targeting and sustaining minoxidil sulphate delivery to hair follicles. Int Biol Macromol. 2015;75:225–229. doi: 10.1016/j.ijbiomac.2015.01.036. [DOI] [PubMed] [Google Scholar]
- Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- Matsuyama T, Mackay M, Midha R. Peripheral nerve repair and grafting techniques: a review. Neurol Med Chir. 2000;40:187–199. doi: 10.2176/nmc.40.187. [DOI] [PubMed] [Google Scholar]
- Mattioli-Belmonte M, Gigante A, Muzzarelli RA, Politano R, De Benedittis A, Specchia N, Buffa A, Biagini G, Greco F. N,N-dicarboxymethyl chitosan as delivery agent for bone morphogenetic protein in the repair of articular cartilage. Med Biol Eng Comput. 1999;37:130–134. doi: 10.1007/BF02513279. [DOI] [PubMed] [Google Scholar]
- McNeela EA, O’Connor D, Jabbal-Gill I, Illum L, Davis SS, Pizza M, Peppoloni S, Rappuoli R, Mills KH. A mucosal vaccine against diphtheria: formulation of cross reacting material (CRM(197)) of diphtheria toxin with chitosan enhances local and systemic antibody and Th2 responses following nasal delivery. Vaccine. 2000;19:1188–1198. doi: 10.1016/S0264-410X(00)00309-1. [DOI] [PubMed] [Google Scholar]
- Medyantseva EP, Brusnitsyn DV, Varlamova RM, Baibatarova MA, Budnikov GK, Fattakhova AN. determination of antidepressants using monoamine oxidase amperometric biosensors based on screen-printed graphite electrodes modified with multi-walled carbon nanotubes. Pharm Chem J. 2014;48:478–482. doi: 10.1007/s11094-014-1135-2. [DOI] [Google Scholar]
- Melaj MA, Daraio ME. Preparation and characterization of potassium nitrate controlled-release fertilizers based on chitosan and xanthan layered tablets. J Appl Polymer Sci. 2013;130:2422–2428. doi: 10.1002/app.39452. [DOI] [Google Scholar]
- Mellati A, Kiamahalleh MV, Madani SH, Dai S, Bi J, Jin B, Zhang H. Poly(N-isopropylacrylamide) hydrogel/chitosan scaffold hybrid for three-dimensional stem cell culture and cartilage tissue engineering. J Biomed Mater Res A. 2016;104:2764–2774. doi: 10.1002/jbm.a.35810. [DOI] [PubMed] [Google Scholar]
- Mendes RK, Arruda BS, de Souza EF, Nogueira AB, Teschke O, Bonugli LO, Etchegaray A. Determination of chlorophenol in environmental samples using a voltammetric biosensor based on hybrid nanocomposite. J Brazil Chem Soc. 2017;28:1212–1219. [Google Scholar]
- Meng Q, et al. A composite scaffold of MSC affinity peptide-modified demineralized bone matrix particles and chitosan hydrogel for cartilage regeneration. Sci Rep. 2015;5:17802. doi: 10.1038/srep17802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengoni Tamara, Adrian Manuela, Pereira Susana, Santos-Carballal Beatriz, Kaiser Mathias, Goycoolea Francisco. A Chitosan—Based Liposome Formulation Enhances the In Vitro Wound Healing Efficacy of Substance P Neuropeptide. Pharmaceutics. 2017;9(4):56. doi: 10.3390/pharmaceutics9040056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer C, et al. Peripheral nerve regeneration through hydrogel-enriched chitosan conduits containing engineered Schwann cells for drug delivery. Cell Transplant. 2016;25:159–182. doi: 10.3727/096368915X688010. [DOI] [PubMed] [Google Scholar]
- Miranda DG, Malmonge SM, Campos DM, Attik NG, Grosgogeat B, Gritsch K. A chitosan-hyaluronic acid hydrogel scaffold for periodontal tissue engineering. J Biomed Mater Res B. 2016;104:1691–1702. doi: 10.1002/jbm.b.33516. [DOI] [PubMed] [Google Scholar]
- Mitra S, Gaur U, Ghosh PC, Maitra AN. Tumour targeted delivery of encapsulated dextran–doxorubicin conjugate using chitosan nanoparticles as carrier. J Control Release. 2001;74:317–323. doi: 10.1016/S0168-3659(01)00342-X. [DOI] [PubMed] [Google Scholar]
- Moattari M, Kouchesfehani HM, Kaka G, Sadraie SH, Naghdi M, Mansouri K. Chitosan-film associated with mesenchymal stem cells enhanced regeneration of peripheral nerves: a rat sciatic nerve model. J Chem Neuroanat. 2018;88:46–54. doi: 10.1016/j.jchemneu.2017.10.003. [DOI] [PubMed] [Google Scholar]
- Möller H, Grelier S, Pardon P, Coma V. Antimicrobial and physicochemical properties of chitosan−HPMC-based films. J Agric Food Chem. 2004;52:6585–6591. doi: 10.1021/jf0306690. [DOI] [PubMed] [Google Scholar]
- Monosik R, Stred’ansky M, Luspai K, Magdolen P, Sturdik E. Amperometric glucose biosensor utilizing FAD-dependent glucose dehydrogenase immobilized on nanocomposite electrode. Enzym Microb Technol. 2012;50:227–232. doi: 10.1016/j.enzmictec.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Mori A, et al. The vaccine adjuvant alum inhibits IL-12 by promoting PI3 kinase signaling while chitosan does not inhibit IL-12 and enhances Th1 and Th17 responses. Eur J Immunol. 2012;42:2709–2719. doi: 10.1002/eji.201242372. [DOI] [PubMed] [Google Scholar]
- Muzzarelli RA, Biagini G, Bellardini M, Simonelli L, Castaldini C, Fratto G. Osteoconduction exerted by methylpyrrolidinone chitosan used in dental surgery. Biomaterials. 1993;14:39–43. doi: 10.1016/0142-9612(93)90073-B. [DOI] [PubMed] [Google Scholar]
- Muzzarelli RAA, Zucchini C, Ilari P, Pugnaloni A, Mattioli Belmonte M, Biagini G, Castaldini C. Osteoconductive properties of methylpyrrolidinone chitosan in an animal model. Biomaterials. 1993;14:925–929. doi: 10.1016/0142-9612(93)90134-N. [DOI] [PubMed] [Google Scholar]
- Muzzarelli RA, Greco F, Busilacchi A, Sollazzo V, Gigante A. Chitosan, hyaluronan and chondroitin sulfate in tissue engineering for cartilage regeneration: a review. Carbohydr Polym. 2012;89:723–739. doi: 10.1016/j.carbpol.2012.04.057. [DOI] [PubMed] [Google Scholar]
- Nada A, Al-Moghazy M, Soliman AAF, Rashwan GMT, Eldawy THA, Hassan AAE, Sayed GH. Pyrazole-based compounds in chitosan liposomal emulsion for antimicrobial cotton fabrics. Int Biol Macromol. 2018;107:585–594. doi: 10.1016/j.ijbiomac.2017.09.031. [DOI] [PubMed] [Google Scholar]
- Nandi SK, Kundu B, Basu D. Protein growth factors loaded highly porous chitosan scaffold: a comparison of bone healing properties. Mater Sci Eng C. 2013;33:1267–1275. doi: 10.1016/j.msec.2012.12.025. [DOI] [PubMed] [Google Scholar]
- Naruphontjirakul P, Viravaidya-Pasuwat K (2011) Development of doxorubicin – core shell chitosan nanoparticles to treat cancer. Int Conf Biomed Eng Technol, IPCBEE, 11, Singapore [DOI] [PMC free article] [PubMed]
- Nascimento EG, Sampaio TB, Medeiros AC, Azevedo EP. Evaluation of chitosan gel with 1% silver sulfadiazine as an alternative for burn wound treatment in rats. Acta Cir Bras. 2009;24:460–465. doi: 10.1590/S0102-86502009000600007. [DOI] [PubMed] [Google Scholar]
- Nawalakhe R, Shi Q, Vitchuli N, Bourham MA, Zhang X, McCord MG. Plasma-assisted preparation of high-performance chitosan nanofibers/gauze composite bandages. Int J Polym Mater Polym. 2015;64:709–717. doi: 10.1080/00914037.2014.1002098. [DOI] [Google Scholar]
- Neves SC, Moreira Teixeira LS, Moroni L, Reis RL, Van Blitterswijk CA, Alves NM, Karperien M, Mano JF. Chitosan/poly(epsilon-caprolactone) blend scaffolds for cartilage repair. Biomaterials. 2011;32:1068–1079. doi: 10.1016/j.biomaterials.2010.09.073. [DOI] [PubMed] [Google Scholar]
- Ngo DH, Kim SK. Antioxidant effects of chitin, chitosan, and their derivatives. Adv Food Nutr Res. 2014;73:15–31. doi: 10.1016/B978-0-12-800268-1.00002-0. [DOI] [PubMed] [Google Scholar]
- Nguyen HM, Hwang IC, Park JW, Park HJ. Photoprotection for deltamethrin using chitosan-coated beeswax solid lipid nanoparticles. Pest Manag Sci. 2012;68:1062–1068. doi: 10.1002/ps.3268. [DOI] [PubMed] [Google Scholar]
- Nguyen VC, Nguyen NLG, Pho QH. Preparation of magnetic composite based on zinc oxide nanoparticles and chitosan as a photocatalyst for removal of reactive blue 198. Adv Nat Sci Nanosci Nanotechnol. 2015;6:035001. doi: 10.1088/2043-6262/6/3/035001. [DOI] [Google Scholar]
- Nguyen NLG, Pho Q-H, Nguyen V-C. A high photo-catalytic activity of magnetic composite based on chitosan and manganese-doped zinc oxide nanoparticles for removal of dyeing wastewater. J Nanosci Nanotechnol. 2016;16:7959–7967. doi: 10.1166/jnn.2016.12763. [DOI] [Google Scholar]
- Ni S, Liu Y, Tang Y, Chen J, Li S, Pu J, Han L. GABAB receptor ligand-directed trimethyl chitosan/tripolyphosphate nanoparticles and their pMDI formulation for survivin siRNA pulmonary delivery. Carbohydr Polym. 2018;179:135–144. doi: 10.1016/j.carbpol.2017.09.075. [DOI] [PubMed] [Google Scholar]
- Nie X, Deng M, Yang M, Liu L, Zhang Y, Wen X. Axonal regeneration and remyelination evaluation of chitosan/gelatin-based nerve guide combined with transforming growth factor-beta1 and Schwann cells. Cell Biochem Biophys. 2014;68:163–172. doi: 10.1007/s12013-013-9683-8. [DOI] [PubMed] [Google Scholar]
- Niu Y, Li K, Ying D, Wang Y, Jia J. Novel recyclable adsorbent for the removal of copper(II) and lead(II) from aqueous solution. Bioresour Technol. 2017;229:63–68. doi: 10.1016/j.biortech.2017.01.007. [DOI] [PubMed] [Google Scholar]
- No HK, Meyers SP, Prinyawiwatkul W, Xu Z. Applications of chitosan for improvement of quality and shelf life of foods: a review. J Food Sci. 2007;72:R87–R100. doi: 10.1111/j.1750-3841.2007.00383.x. [DOI] [PubMed] [Google Scholar]
- Noorbakhsh-Soltani SM, Zerafat MM, Sabbaghi S. A comparative study of gelatin and starch-based nano-composite films modified by nano-cellulose and chitosan for food packaging applications. Carbohydr Polym. 2018;189:48–55. doi: 10.1016/j.carbpol.2018.02.012. [DOI] [PubMed] [Google Scholar]
- Noppakundilograt S, Pheatcharat N, Kiatkamjornwong S (2015) Multilayer-coated NPK compound fertilizer hydrogel with controlled nutrient release and water absorbency. J Appl Polymer Sci 132. 10.1002/App.41249
- Nordtveit RJ, Varum KM, Smidsrod O. Degradation of fully water-soluble, partially N-acetylated chitosans with lysozyme. Carbohydr Polym. 1994;23:253–260. doi: 10.1016/0144-8617(94)90187-2. [DOI] [Google Scholar]
- Oliveira JM, et al. Novel hydroxyapatite/chitosan bilayered scaffold for osteochondral tissue-engineering applications: Scaffold design and its performance when seeded with goat bone marrow stromal cells. Biomaterials. 2006;27:6123–6137. doi: 10.1016/j.biomaterials.2006.07.034. [DOI] [PubMed] [Google Scholar]
- Oryan A, Alidadi S, Bigham-Sadegh A, Meimandi-Parizi A. Chitosan/gelatin/platelet gel enriched by a combination of hydroxyapatite and beta-tricalcium phosphate in healing of a radial bone defect model in rat. Int Biol Macromol. 2017;101:630–637. doi: 10.1016/j.ijbiomac.2017.03.148. [DOI] [PubMed] [Google Scholar]
- Osman R, Kan PL, Awad G, Mortada N, El-Shamy AE, Alpar O. Spray dried inhalable ciprofloxacin powder with improved aerosolisation and antimicrobial activity. Int J Pharm. 2013;449:44–58. doi: 10.1016/j.ijpharm.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin Breast Cancer. 2004;5:63–69. doi: 10.3816/CBC.2004.n.011. [DOI] [PubMed] [Google Scholar]
- Ozcelik B, Brown KD, Blencowe A, Daniell M, Stevens GW, Qiao GG. Ultrathin chitosan-poly(ethylene glycol) hydrogel films for corneal tissue engineering. Acta Biomater. 2013;9:6594–6605. doi: 10.1016/j.actbio.2013.01.020. [DOI] [PubMed] [Google Scholar]
- Ozer H, Bozkurt H, Bozkurt G, Demirbilek M. Regenerative potential of chitosan-coated poly-3-hydroxybutyrate conduits seeded with mesenchymal stem cells in a rat sciatic nerve injury model. Int J Neurosci. 2018;128:828–834. doi: 10.1080/00207454.2018.1435536. [DOI] [PubMed] [Google Scholar]
- Pal AK, Katiyar V. Nanoamphiphilic chitosan dispersed poly(lactic acid) bionanocomposite films with improved thermal, mechanical, and gas barrier properties. Biomacromolecules. 2016;17:2603–2618. doi: 10.1021/acs.biomac.6b00619. [DOI] [PubMed] [Google Scholar]
- Pallela R, Venkatesan J, Janapala VR, Kim SK. Biophysicochemical evaluation of chitosan-hydroxyapatite-marine sponge collagen composite for bone tissue engineering. J Biomed Mater Res A. 2012;100:486–495. doi: 10.1002/jbm.a.33292. [DOI] [PubMed] [Google Scholar]
- Paltiel O, Fedorova G, Tadmor G, Kleinstern G, Maor Y, Chefetz B. Human exposure to wastewater-derived pharmaceuticals in fresh produce: a randomized controlled trial focusing on carbamazepine. Environ Sci Technol. 2016;50:4476–4482. doi: 10.1021/acs.est.5b06256. [DOI] [PubMed] [Google Scholar]
- Panos I, Acosta N, Heras A. New drug delivery systems based on chitosan. Curr Drug Discov Technol. 2008;5:333–341. doi: 10.2174/157016308786733528. [DOI] [PubMed] [Google Scholar]
- Park YJ, Lee YM, Lee JY, Seol YJ, Chung CP, Lee SJ. Controlled release of platelet-derived growth factor-BB from chondroitin sulfate–chitosan sponge for guided bone regeneration. J Control Release. 2000;67:385–394. doi: 10.1016/S0168-3659(00)00232-7. [DOI] [PubMed] [Google Scholar]
- Park YJ, Lee YM, Park SN, Sheen SY, Chung CB, Lee SJ. Platelet derived growth factor releasing chitosan sponge for periodontal bone regeneration. Biomaterials. 2000;21:153–159. doi: 10.1016/S0142-9612(99)00143-X. [DOI] [PubMed] [Google Scholar]
- Park JU, Jung HD, Song EH, Choi TH, Kim HE, Song J, Kim S. The accelerating effect of chitosan-silica hybrid dressing materials on the early phase of wound healing. J Biomed Mater Res B. 2017;105:1828–1839. doi: 10.1002/jbm.b.33711. [DOI] [PubMed] [Google Scholar]
- Patel S, Srivastava S, Singh MR, Singh D. Preparation and optimization of chitosan-gelatin films for sustained delivery of lupeol for wound healing. Int Biol Macromol. 2018;107:1888–1897. doi: 10.1016/j.ijbiomac.2017.10.056. [DOI] [PubMed] [Google Scholar]
- Patil PS, Fountas-Davis N, Huang H, Michelle Evancho-Chapman M, Fulton JA, Shriver LP, Leipzig ND. Fluorinated methacrylamide chitosan hydrogels enhance collagen synthesis in wound healing through increased oxygen availability. Acta Biomater. 2016;36:164–174. doi: 10.1016/j.actbio.2016.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrulea V, Ostafe V, Borchard G, Jordan O. Chitosan as a starting material for wound healing applications. Eur J Pharm Biopharm. 2015;97:417–426. doi: 10.1016/j.ejpb.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Peluso G, Petillo O, Ranieri M, Santin M, Ambrosio L, Calabró D, Avallone B, Balsamo G. Chitosan-mediated stimulation of macrophage function. Biomaterials. 1994;15:1215–1220. doi: 10.1016/0142-9612(94)90272-0. [DOI] [PubMed] [Google Scholar]
- Peng CC, Yang MH, Chiu WT, Chiu CH, Yang CS, Chen YW, Chen KC, Peng RY. Composite nano-titanium oxide-chitosan artificial skin exhibits strong wound-healing effect-an approach with anti-inflammatory and bactericidal kinetics. Macromol Biosci. 2008;8:316–327. doi: 10.1002/mabi.200700188. [DOI] [PubMed] [Google Scholar]
- Peng S, Meng H, Ouyang Y, Chang J. Nanoporous magnetic cellulose–chitosan composite microspheres: preparation, characterization, and application for Cu(II) adsorption. Ind Eng Chem Res. 2014;53:2106–2113. doi: 10.1021/ie402855t. [DOI] [Google Scholar]
- Pereira FAR, Sousa KS, Cavalcanti GRS, Fonseca MG, de Souza AG, Alves APM. Chitosan-montmorillonite biocomposite as an adsorbent for copper (II) cations from aqueous solutions. Int Biol Macromol. 2013;61:471–478. doi: 10.1016/j.ijbiomac.2013.08.017. [DOI] [PubMed] [Google Scholar]
- Perez JJ, Francois NJ. Chitosan-starch beads prepared by ionotropic gelation as potential matrices for controlled release of fertilizers. Carbohydr Polym. 2016;148:134–142. doi: 10.1016/j.carbpol.2016.04.054. [DOI] [PubMed] [Google Scholar]
- Periayah MH, Halim AS, Hussein AR, Saad AZ, Rashid AH, Noorsal K. In vitro capacity of different grades of chitosan derivatives to induce platelet adhesion and aggregation. Int J Biol Macromol. 2013;52:244–249. doi: 10.1016/j.ijbiomac.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Periolatto M, Ferrero F. Cotton filter fabrics functionalization by chitosan UV-grafting for removal of dyes. Chem Eng Trans. 2013;32:85–90. [Google Scholar]
- Periolatto M, Ferrero F, Vineis C. Antimicrobial chitosan finish of cotton and silk fabrics by UV-curing with 2-hydroxy-2-methylphenylpropane-1-one. Carbohydr Polym. 2012;88:201–205. doi: 10.1016/j.carbpol.2011.11.093. [DOI] [Google Scholar]
- Petkova P, Francesko A, Fernandes MM, Mendoza E, Perelshtein I, Gedanken A, Tzanov T. Sonochemical coating of textiles with hybrid ZnO/chitosan antimicrobial nanoparticles. ACS Appl Mater Interfaces. 2014;6:1164–1172. doi: 10.1021/am404852d. [DOI] [PubMed] [Google Scholar]
- Petri DF, Donega J, Benassi AM, Bocangel JA. Preliminary study on chitosan modified glass ionomer restoratives. Dent Mater. 2007;23:1004–1010. doi: 10.1016/j.dental.2006.06.038. [DOI] [PubMed] [Google Scholar]
- Phinney DG, Pittenger MF. Concise review: msc-derived exosomes for cell-free therapy. Stem Cells. 2017;35:851–858. doi: 10.1002/stem.2575. [DOI] [PubMed] [Google Scholar]
- Picola IP, Shi Q, Fernandes JC, Petronio MS, Lima AM, de Oliveira Tiera VA, Tiera MJ. Chitosan derivatives for gene transfer: effect of phosphorylcholine and diethylaminoethyl grafts on the in vitro transfection efficiency. J Biomater Sci Polym Ed. 2016;27:1611–1630. doi: 10.1080/09205063.2016.1225333. [DOI] [PubMed] [Google Scholar]
- Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- Pirouzmand H, Khameneh B, Tafaghodi M. Immunoadjuvant potential of cross-linked dextran microspheres mixed with chitosan nanospheres encapsulated with tetanus toxoid. Pharm Biol. 2017;55:212–217. doi: 10.1080/13880209.2016.1257032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poverenov E, Danino S, Horev B, Granit R, Vinokur Y, Rodov V. Layer-by-layer electrostatic deposition of edible coating on fresh cut melon model: anticipated and unexpected effects of alginate–chitosan combination. Food Bioprocess Technol. 2014;7:1424–1432. doi: 10.1007/s11947-013-1134-4. [DOI] [Google Scholar]
- Poverenov E, Zaitsev Y, Arnon H, Granit R, Alkalai-Tuvia S, Perzelan Y, Weinberg T, Fallik E. Effects of a composite chitosan–gelatin edible coating on postharvest quality and storability of red bell peppers. Postharvest Biol Technol. 2014;96:106–109. doi: 10.1016/j.postharvbio.2014.05.015. [DOI] [Google Scholar]
- Prabaharan M. Chitosan-based nanoparticles for tumor-targeted drug delivery. Int Biol Macromol. 2015;72:1313–1322. doi: 10.1016/j.ijbiomac.2014.10.052. [DOI] [PubMed] [Google Scholar]
- Przekora A, Ginalska G. Chitosan/beta-1,3-glucan/hydroxyapatite bone scaffold enhances osteogenic differentiation through TNF-alpha-mediated mechanism. Mater Sci Eng C Mater Biol Appl. 2017;73:225–233. doi: 10.1016/j.msec.2016.12.081. [DOI] [PubMed] [Google Scholar]
- Przekora A, Vandrovcova M, Travnickova M, Pajorova J, Molitor M, Ginalska G, Bacakova L. Evaluation of the potential of chitosan/beta-1,3-glucan/hydroxyapatite material as a scaffold for living bone graft production in vitro by comparison of ADSC and BMDSC behaviour on its surface. Biomed Mater. 2017;12:015030. doi: 10.1088/1748-605X/aa56f9. [DOI] [PubMed] [Google Scholar]
- Puspita A, Prawati G, Fatimah I. Chitosan-modified smectite clay and study on adsorption-desorption of urea. Chem Eng Trans. 2017;56:1645–1650. [Google Scholar]
- Qasim SB, Zafar MS, Najeeb S, Khurshid Z, Shah AH, Husain S, Rehman IU. Electrospinning of chitosan-based solutions for tissue engineering and regenerative medicine. Int J Mol Sci. 2018;19:407. doi: 10.3390/ijms19020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L, Xu Z. In vivo antitumor activity of chitosan nanoparticles. Bioorg Med Chem Lett. 2006;16:4243–4245. doi: 10.1016/j.bmcl.2006.05.078. [DOI] [PubMed] [Google Scholar]
- Qi BW, Yu AX, Zhu SB, Zhou M, Wu G. Chitosan/poly(vinyl alcohol) hydrogel combined with Ad-hTGF-beta1 transfected mesenchymal stem cells to repair rabbit articular cartilage defects. Exp Biol Med. 2013;238:23–30. doi: 10.1258/ebm.2012.012223. [DOI] [PubMed] [Google Scholar]
- Qin Y, Wang HW, Karuppanapandian T, Kim W. Chitosan green tea polyphenol complex as a released control compound for wound healing. Chin J Traumatol. 2010;13:91–95. [PubMed] [Google Scholar]
- Qin Y, Guo XW, Li L, Wang HW, Kim W. The antioxidant property of chitosan green tea polyphenols complex induces transglutaminase activation in wound healing. J Med Food. 2013;16:487–498. doi: 10.1089/jmf.2012.2623. [DOI] [PubMed] [Google Scholar]
- Qiu JD, Liang RP, Wang R, Fan LX, Chen YW, Xia XH. A label-free amperometric immunosensor based on biocompatible conductive redox chitosan-ferrocene/gold nanoparticles matrix. Biosens Bioelectron. 2009;25:852–857. doi: 10.1016/j.bios.2009.08.048. [DOI] [PubMed] [Google Scholar]
- Quan D, Shin W. A nitrite biosensor based on co-immobilization of nitrite reductase and viologen-modified chitosan on a glassy carbon electrode. Sensors. 2010;10:6241–6256. doi: 10.3390/s100606241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raafat D, Sahl HG. Chitosan and its antimicrobial potential-a critical literature survey. Microb Biotechnol. 2009;2:186–201. doi: 10.1111/j.1751-7915.2008.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabea EI, Badawy MET, Stevens CV, Smagghe G, Steurbaut W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules. 2003;4:1457–1465. doi: 10.1021/bm034130m. [DOI] [PubMed] [Google Scholar]
- Radmansouri M, Bahmani E, Sarikhani E, Rahmani K, Sharifianjazi F, Irani M. Doxorubicin hydrochloride – Loaded electrospun chitosan/cobalt ferrite/titanium oxide nanofibers for hyperthermic tumor cell treatment and controlled drug release. Int Biol Macromol. 2018;116:378–384. doi: 10.1016/j.ijbiomac.2018.04.161. [DOI] [PubMed] [Google Scholar]
- Rafat M, Li F, Fagerholm P, Lagali NS, Watsky MA, Munger R, Matsuura T, Griffith M. PEG-stabilized carbodiimide crosslinked collagen-chitosan hydrogels for corneal tissue engineering. Biomaterials. 2008;29:3960–3972. doi: 10.1016/j.biomaterials.2008.06.017. [DOI] [PubMed] [Google Scholar]
- Raghuwanshi D, Mishra V, Das D, Kaur K, Suresh MR. Dendritic cell targeted chitosan nanoparticles for nasal DNA immunization against SARS CoV nucleocapsid protein. Mol Pharm. 2012;9:946–956. doi: 10.1021/mp200553x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan M, Raj V, Al-Arfaj AA, Murugan AM. Hyaluronidase enzyme core-5-fluorouracil-loaded chitosan-PEG-gelatin polymer nanocomposites as targeted and controlled drug delivery vehicles. Int J Pharm. 2013;453:514–522. doi: 10.1016/j.ijpharm.2013.06.030. [DOI] [PubMed] [Google Scholar]
- Rajashree S, Rose C. Studies on an anti-aging formulation prepared using Aloe vera blended collagen and chitosan. Intl J Pharm Sci Res. 2017;19:582–588. [Google Scholar]
- Rajiv Gandhi M, Kousalya GN, Meenakshi S. Removal of copper(II) using chitin/chitosan nano-hydroxyapatite composite. Int Biol Macromol. 2011;48:119–124. doi: 10.1016/j.ijbiomac.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Rakwal R, Tamogami S, Agrawal GK, Iwahashi H. Octadecanoid signaling component “burst” in rice (Oryza sativa L.) seedling leaves upon wounding by cut and treatment with fungal elicitor chitosan. Biochem Biophys Res Commun. 2002;295:1041–1045. doi: 10.1016/S0006-291X(02)00779-9. [DOI] [PubMed] [Google Scholar]
- Ramya R, Sudha PN. Adsorption of cadmium (II) and copper (II) ions from aqueous solution using chitosan composite. Polym Compos. 2013;34:233–240. doi: 10.1002/pc.22407. [DOI] [Google Scholar]
- Ran J, Hu J, Sun G, Chen S, Jiang P, Shen X, Tong H. A novel chitosan-tussah silk fibroin/nano-hydroxyapatite composite bone scaffold platform with tunable mechanical strength in a wide range. Int Biol Macromol. 2016;93:87–97. doi: 10.1016/j.ijbiomac.2016.08.062. [DOI] [PubMed] [Google Scholar]
- Ranganath AS, Sarkar AK. Evaluation of durability to laundering of triclosan and chitosan on a textile substrate. J Text. 2014;2014:5. doi: 10.1155/2014/812303. [DOI] [Google Scholar]
- Rasente RY, Imperiale JC, Lazaro-Martinez JM, Gualco L, Oberkersch R, Sosnik A, Calabrese GC. Dermatan sulfate/chitosan polyelectrolyte complex with potential application in the treatment and diagnosis of vascular disease. Carbohydr Polym. 2016;144:362–370. doi: 10.1016/j.carbpol.2016.02.046. [DOI] [PubMed] [Google Scholar]
- Rattanamanee A, Niamsup H, L-o S, Punyodom W, Watanesk R, Watanesk S. Role of chitosan on some physical properties and the urea controlled release of the silk fibroin/gelatin hydrogel. J Polym Environ. 2015;23:334–340. doi: 10.1007/s10924-014-0703-6. [DOI] [Google Scholar]
- Raza ZA, Anwar F, Ahmad S, Aslam M. Fabrication of ZnO incorporated chitosan nanocomposites for enhanced functional properties of cellulosic fabric. Mater Res Express. 2016;3:115001. doi: 10.1088/2053-1591/3/11/115001. [DOI] [Google Scholar]
- Read RC, Naylor SC, Potter CW, Bond J, Jabbal-Gill I, Fisher A, Illum L, Jennings R. Effective nasal influenza vaccine delivery using chitosan. Vaccine. 2005;23:4367–4374. doi: 10.1016/j.vaccine.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Reddy DHK, Lee S-M. Application of magnetic chitosan composites for the removal of toxic metal and dyes from aqueous solutions. Adv Colloid Interface Sci. 2013;201-202:68–93. doi: 10.1016/j.cis.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Reesha KV, Panda SK, Bindu J, Varghese TO Development and characterization of an LDPE/chitosan composite antimicrobial film for chilled fish storage. Int Biol Macromol. 2015;79:934–942. doi: 10.1016/j.ijbiomac.2015.06.016. [DOI] [PubMed] [Google Scholar]
- Rehan M, El-Naggar ME, Mashaly HM, Wilken R. Nanocomposites based on chitosan/silver/clay for durable multi-functional properties of cotton fabrics. Carbohydr Polym. 2018;182:29–41. doi: 10.1016/j.carbpol.2017.11.007. [DOI] [PubMed] [Google Scholar]
- Revathi T, Thambidurai S. Synthesis of chitosan incorporated neem seed extract (Azadirachta indica) for medical textiles. Int Biol Macromol. 2017;104:1890–1896. doi: 10.1016/j.ijbiomac.2017.02.081. [DOI] [PubMed] [Google Scholar]
- Ribeiro MP, Morgado PI, Miguel SP, Coutinho P, Correia IJ. Dextran-based hydrogel containing chitosan microparticles loaded with growth factors to be used in wound healing. Mater Sci Eng C Mater Biol Appl. 2013;33:2958–2966. doi: 10.1016/j.msec.2013.03.025. [DOI] [PubMed] [Google Scholar]
- Richardson SC, Kolbe HV, Duncan R. Potential of low molecular mass chitosan as a DNA delivery system: biocompatibility, body distribution and ability to complex and protect DNA. Int J Pharm. 1999;178:231–243. doi: 10.1016/S0378-5173(98)00378-0. [DOI] [PubMed] [Google Scholar]
- Ridolfi DM, Marcato PD, Justo GZ, Cordi L, Machado D, Duran N. Chitosan-solid lipid nanoparticles as carriers for topical delivery of tretinoin. Colloid Surf B. 2012;93:36–40. doi: 10.1016/j.colsurfb.2011.11.051. [DOI] [PubMed] [Google Scholar]
- Roonprapunt C, Huang W, Grill R, Friedlander D, Grumet M, Chen S, Schachner M, Young W. Soluble cell adhesion molecule L1-Fc promotes locomotor recovery in rats after spinal cord injury. J Neurotrauma. 2003;20:871–882. doi: 10.1089/089771503322385809. [DOI] [PubMed] [Google Scholar]
- Roshanravan B, Soltani SM, Rashid SA, Mahdavi F, Yusop MK. Enhancement of nitrogen release properties of urea-kaolinite fertilizer with chitosan binder. Chem Spec Bioavailab. 2015;27:43–50. doi: 10.1080/09542299.2015.1023090. [DOI] [Google Scholar]
- Ruan Q, Siddiqah N, Li X, Nutt S, Moradian-Oldak J. Amelogenin-chitosan matrix for human enamel regrowth: effects of viscosity and supersaturation degree. Connect Tissue Res. 2014;55(Suppl 1):150–154. doi: 10.3109/03008207.2014.923856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell-Jones G, McTavish K, McEwan J, Rice J, Nowotnik D. Vitamin-mediated targeting as a potential mechanism to increase drug uptake by tumours. J Inorg Biochem. 2004;98:1625–1633. doi: 10.1016/j.jinorgbio.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Sadeghi-Kiakhani M, Arami M, Gharanjig K. Application of a biopolymer chitosan-poly(propylene)imine dendrimer hybrid as an antimicrobial agent on the wool fabrics. Iran Polym J. 2013;22:931–940. doi: 10.1007/s13726-013-0193-8. [DOI] [Google Scholar]
- Saharan V, Kumaraswamy RV, Choudhary RC, Kumari S, Pal A, Raliya R, Biswas P. Cu-chitosan nanoparticle mediated sustainable approach to enhance seedling growth in maize by mobilizing reserved food. J Agric Food Chem. 2016;64:6148–6155. doi: 10.1021/acs.jafc.6b02239. [DOI] [PubMed] [Google Scholar]
- Sahni JK, Chopra S, Ahmad FJ, Khar RK. Potential prospects of chitosan derivative trimethyl chitosan chloride (TMC) as a polymeric absorption enhancer: synthesis, characterization and applications. J Pharm Pharmacol. 2008;60:1111–1119. doi: 10.1211/jpp.60.9.0001. [DOI] [PubMed] [Google Scholar]
- Sakkinen M, Marvola J, Kanerva H, Lindevall K, Ahonen A, Marvola M. Scintigraphic verification of adherence of a chitosan formulation to the human oesophagus. Eur J Pharm Biopharm. 2004;57:145–147. doi: 10.1016/S0939-6411(03)00098-5. [DOI] [PubMed] [Google Scholar]
- Saleh AS, Ibrahim AG, Abdelhai F, Elsharma EM, Metwally E, Siyam T. Preparation of poly(chitosan-acrylamide) flocculant using gamma radiation for adsorption of Cu(II) and Ni(II) ions. Radiat Phys Chem. 2017;134:33–39. doi: 10.1016/j.radphyschem.2017.01.019. [DOI] [Google Scholar]
- Salehi E, Daraei P, Arabi Shamsabadi A. A review on chitosan-based adsorptive membranes. Carbohydr Polym. 2016;152:419–432. doi: 10.1016/j.carbpol.2016.07.033. [DOI] [PubMed] [Google Scholar]
- Samprasit W, Kaomongkolgit R, Sukma M, Rojanarata T, Ngawhirunpat T, Opanasopit P. Mucoadhesive electrospun chitosan-based nanofibre mats for dental caries prevention. Carbohydr Polym. 2015;117:933–940. doi: 10.1016/j.carbpol.2014.10.026. [DOI] [PubMed] [Google Scholar]
- Sanad RA, Abdel-Bar HM. Chitosan-hyaluronic acid composite sponge scaffold enriched with Andrographolide-loaded lipid nanoparticles for enhanced wound healing. Carbohydr Polym. 2017;173:441–450. doi: 10.1016/j.carbpol.2017.05.098. [DOI] [PubMed] [Google Scholar]
- Sandri G, Rossi S, Bonferoni MC, Ferrari F, Zambito Y, Di Colo G, Caramella C. Buccal penetration enhancement properties of N-trimethyl chitosan: Influence of quaternization degree on absorption of a high molecular weight molecule. Int J Pharm. 2005;297:146–155. doi: 10.1016/j.ijpharm.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Sanpui P, Murugadoss A, Prasad PV, Ghosh SS, Chattopadhyay A. The antibacterial properties of a novel chitosan-Ag-nanoparticle composite. Int J Food Microbiol. 2008;124:142–146. doi: 10.1016/j.ijfoodmicro.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Sanuja S, Agalya A, Umapathy MJ. Synthesis and characterization of zinc oxide–neem oil–chitosan bionanocomposite for food packaging application. Int Biol Macromol. 2015;74:76–84. doi: 10.1016/j.ijbiomac.2014.11.036. [DOI] [PubMed] [Google Scholar]
- Sargın İ, Arslan G, Kaya M. Efficiency of chitosan–algal biomass composite microbeads at heavy metal removal. React Funct Polym. 2016;98:38–47. doi: 10.1016/j.reactfunctpolym.2015.11.007. [DOI] [Google Scholar]
- Sargın İ, Arslan G, Kaya M. Microfungal spores (Ustilago maydis and U. digitariae) immobilised chitosan microcapsules for heavy metal removal. Carbohydr Polym. 2016;138:201–209. doi: 10.1016/j.carbpol.2015.11.065. [DOI] [PubMed] [Google Scholar]
- Sarvaiya J, Agrawal YK. Chitosan as a suitable nanocarrier material for anti-Alzheimer drug delivery. Int Biol Macromol. 2015;72:454–465. doi: 10.1016/j.ijbiomac.2014.08.052. [DOI] [PubMed] [Google Scholar]
- Sawaengsak C, Mori Y, Yamanishi K, Srimanote P, Chaicumpa W, Mitrevej A, Sinchaipanid N. Intranasal chitosan-DNA vaccines that protect across influenza virus subtypes. Int J Pharm. 2014;473:113–125. doi: 10.1016/j.ijpharm.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Scacchetti FAP, Pinto E, Soares GMB. Preparation and characterization of cotton fabrics with antimicrobial properties through the application of chitosan/silver-zeolite film. Procedia Eng. 2017;200:276–282. doi: 10.1016/j.proeng.2017.07.039. [DOI] [Google Scholar]
- Scherliess R, Buske S, Young K, Weber B, Rades T, Hook S. In vivo evaluation of chitosan as an adjuvant in subcutaneous vaccine formulations. Vaccine. 2013;31:4812–4819. doi: 10.1016/j.vaccine.2013.07.081. [DOI] [PubMed] [Google Scholar]
- Schipper P, et al. Diphtheria toxoid and N-trimethyl chitosan layer-by-layer coated pH-sensitive microneedles induce potent immune responses upon dermal vaccination in mice. J Control Release. 2017;262:28–36. doi: 10.1016/j.jconrel.2017.07.017. [DOI] [PubMed] [Google Scholar]
- Sellgren KL, Ma T. Perfusion conditioning of hydroxyapatite-chitosan-gelatin scaffolds for bone tissue regeneration from human mesenchymal stem cells. J Tissue Eng Regen Med. 2012;6:49–59. doi: 10.1002/term.396. [DOI] [PubMed] [Google Scholar]
- Senyigit ZA, Karavana SY, Erac B, Gursel O, Limoncu MH, Baloglu E. Evaluation of chitosan based vaginal bioadhesive gel formulations for antifungal drugs. Acta Pharm. 2014;64:139–156. doi: 10.2478/acph-2014-0013. [DOI] [PubMed] [Google Scholar]
- Shakir M, Jolly R, Khan MS, Rauf A, Kazmi S. Nano-hydroxyapatite/beta-CD/chitosan nanocomposite for potential applications in bone tissue engineering. Int Biol Macromol. 2016;93:276–289. doi: 10.1016/j.ijbiomac.2016.08.046. [DOI] [PubMed] [Google Scholar]
- Sharma S. Enhanced antibacterial efficacy of silver nanoparticles immobilized in a chitosan nanocarrier. Int Biol Macromol. 2017;104:1740–1745. doi: 10.1016/j.ijbiomac.2017.07.043. [DOI] [PubMed] [Google Scholar]
- Shavandi A, Bekhit Ael D, Sun Z, Ali A, Gould M. A novel squid pen chitosan/hydroxyapatite/beta-tricalcium phosphate composite for bone tissue engineering. Mater Sci Eng C Mater Biol Appl. 2015;55:373–383. doi: 10.1016/j.msec.2015.05.029. [DOI] [PubMed] [Google Scholar]
- Shen H, Shen ZL, Zhang PH, Chen NL, Wang YC, Zhang ZF, Jin YQ. Ciliary neurotrophic factor-coated polylactic-polyglycolic acid chitosan nerve conduit promotes peripheral nerve regeneration in canine tibial nerve defect repair. J Biomed Mater Res B. 2010;95:161–170. doi: 10.1002/jbm.b.31696. [DOI] [PubMed] [Google Scholar]
- Shen J-M, Gao F-Y, Yin T, Zhang H-X, Ma M, Yang Y-J, Yue F. cRGD-functionalized polymeric magnetic nanoparticles as a dual-drug delivery system for safe targeted cancer therapy. Pharmacol Res. 2013;70:102–115. doi: 10.1016/j.phrs.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Shen T, Pan ZG, Zhou X, Hong CY. Accelerated healing of diabetic wound using artificial dermis constructed with adipose stem cells and poly (L-glutamic acid)/chitosan scaffold. Chin Med J. 2013;126:1498–1503. [PubMed] [Google Scholar]
- Shi Q, Wang H, Tran C, Qiu X, Winnik FM, Zhang X, Dai K, Benderdour M, Fernandes JC. Hydrodynamic delivery of chitosan-folate-DNA nanoparticles in rats with adjuvant-induced arthritis. J Biomed Biotechnol. 2011;2011:148763. doi: 10.1155/2011/148763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi GN, et al. Enhanced antitumor immunity by targeting dendritic cells with tumor cell lysate-loaded chitosan nanoparticles vaccine. Biomaterials. 2017;113:191–202. doi: 10.1016/j.biomaterials.2016.10.047. [DOI] [PubMed] [Google Scholar]
- Shi Q, Qian Z, Liu D, Sun J, Wang X, Liu H, Xu J, Guo X. GMSC-derived exosomes combined with a chitosan/silk hydrogel sponge accelerates wound healing in a diabetic rat skin defect model. Front Physiol. 2017;8:904. doi: 10.3389/fphys.2017.00904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki K, Sano H, Matsukubo T, Takaesu Y. pH response of human dental plaque to chewing gum supplemented with low molecular chitosan. Bull Tokyo Dent College. 1994;35:61–66. [PubMed] [Google Scholar]
- Shibata Y, Foster LA, Metzger WJ, Myrvik QN. Alveolar macrophage priming by intravenous administration of chitin particles, polymers of N-acetyl-D-glucosamine, in mice. Inf Immun. 1997;65:1734–1741. doi: 10.1128/iai.65.5.1734-1741.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata Y, Metzger WJ, Myrvik QN. Chitin particle-induced cell-mediated immunity is inhibited by soluble mannan: mannose receptor-mediated phagocytosis initiates IL-12 production. J Immunol. 1997;159:2462–2467. [PubMed] [Google Scholar]
- Shirosaki Y, Hayakawa S, Osaka A, Lopes MA, Santos JD, Geuna S, Mauricio AC. Challenges for nerve repair using chitosan-siloxane hybrid porous scaffolds. BioMed Res Int. 2014;2014:153808. doi: 10.1155/2014/153808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha BK, Ahmad R, Mousa HM, Kim IG, Kim JI, Neupane MP, Park CH, Kim CS. High-performance glucose biosensor based on chitosan-glucose oxidase immobilized polypyrrole/Nafion/functionalized multi-walled carbon nanotubes bio-nanohybrid film. J Colloid Interface Sci. 2016;482:39–47. doi: 10.1016/j.jcis.2016.07.067. [DOI] [PubMed] [Google Scholar]
- Shukla R, Kashaw SK, Jain AP, Lodhi S. Fabrication of Apigenin loaded gellan gum-chitosan hydrogels (GGCH-HGs) for effective diabetic wound healing. Int Biol Macromol. 2016;91:1110–1119. doi: 10.1016/j.ijbiomac.2016.06.075. [DOI] [PubMed] [Google Scholar]
- Siafu Sempeho Ibahati. Silicone Doped Chitosan-Acrylamide Coencapsulated Urea Fertilizer: An Approach to Controlled Release Fertilizers. Journal of Nanotechnology. 2017;2017:1–7. doi: 10.1155/2017/8490730. [DOI] [Google Scholar]
- Silva Mdos S, Cocenza DS, Grillo R, de Melo NF, Tonello PS, de Oliveira LC, Cassimiro DL, Rosa AH, Fraceto LF. Paraquat-loaded alginate/chitosan nanoparticles: preparation, characterization and soil sorption studies. J Hazard Mater. 2011;190:366–374. doi: 10.1016/j.jhazmat.2011.03.057. [DOI] [PubMed] [Google Scholar]
- Silva SS, Motta A, Rodrigues MT, Pinheiro AF, Gomes ME, Mano JF, Reis RL, Migliaresi C. Novel genipin-cross-linked chitosan/silk fibroin sponges for cartilage engineering strategies. Biomacromolecules. 2008;9:2764–2774. doi: 10.1021/bm800874q. [DOI] [PubMed] [Google Scholar]
- Sin Jin-Chung, Lam Sze-Mun, Mohamed Abdul Rahman, Lee Keat-Teong. Degrading Endocrine Disrupting Chemicals from Wastewater by Photocatalysis: A Review. International Journal of Photoenergy. 2012;2012:1–23. doi: 10.1155/2012/185159. [DOI] [Google Scholar]
- Singh A, Sinsinbar G, Choudhary M, Kumar V, Pasricha R, Verma HN, Singh SP, Arora K. Graphene oxide-chitosan nanocomposite based electrochemical DNA biosensor for detection of typhoid. Sensor Actuat B-Chem. 2013;185:675–684. doi: 10.1016/j.snb.2013.05.014. [DOI] [Google Scholar]
- Singh N, Yadav M, Khanna S, Sahu O. Sustainable fragrance cum antimicrobial finishing on cotton: indigenous essential oil. Sustain Chem Pharm. 2017;5:22–29. doi: 10.1016/j.scp.2017.01.003. [DOI] [Google Scholar]
- Sionkowska Alina, Kaczmarek Beata, Michalska Marta, Lewandowska Katarzyna, Grabska Sylwia. Preparation and characterization of collagen/chitosan/hyaluronic acid thin films for application in hair care cosmetics. Pure and Applied Chemistry. 2017;89(12):1829–1839. doi: 10.1515/pac-2017-0314. [DOI] [Google Scholar]
- Škorić ML, Terzić I, Milosavljević N, Radetić M, Šaponjić Z, Radoičić M, Krušić MK. Chitosan-based microparticles for immobilization of TiO2 nanoparticles and their application for photodegradation of textile dyes. Eur Polym J. 2016;82:57–70. doi: 10.1016/j.eurpolymj.2016.06.026. [DOI] [Google Scholar]
- Smith A, Perelman M, Hinchcliffe M. Chitosan: a promising safe and immune-enhancing adjuvant for intranasal vaccines. Hum Vaccin Immunother. 2014;10:797–807. doi: 10.4161/hv.27449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares DG, Rosseto HL, Scheffel DS, Basso FG, Huck C, Hebling J, de Souza Costa CA. Odontogenic differentiation potential of human dental pulp cells cultured on a calcium-aluminate enriched chitosan-collagen scaffold. Clin Oral Invest. 2017;21:2827–2839. doi: 10.1007/s00784-017-2085-3. [DOI] [PubMed] [Google Scholar]
- Soares SF, Rodrigues MI, Trindade T, Daniel-da-Silva AL. Chitosan-silica hybrid nanosorbents for oil removal from water. Colloids Surf A. 2017;532:305–313. doi: 10.1016/j.colsurfa.2017.04.076. [DOI] [Google Scholar]
- Soares E, Jesus S, Borges O. Oral hepatitis B vaccine: chitosan or glucan based delivery systems for efficient HBsAg immunization following subcutaneous priming. Int J Pharm. 2018;535:261–271. doi: 10.1016/j.ijpharm.2017.11.009. [DOI] [PubMed] [Google Scholar]
- Stoytcheva M, Zlatev R, Montero G, Velkova Z, Gochev V. A nanotechnological approach to biosensors sensitivity improvement: application to organophosphorus pesticides determination. Biotechnol Biotechnol Equip. 2018;32:213–220. doi: 10.1080/13102818.2017.1389618. [DOI] [Google Scholar]
- Štular D, Jerman I, Naglič I, Simončič B, Tomšič B. Embedment of silver into temperature- and pH-responsive microgel for the development of smart textiles with simultaneous moisture management and controlled antimicrobial activities. Carbohydr Polym. 2017;159:161–170. doi: 10.1016/j.carbpol.2016.12.030. [DOI] [PubMed] [Google Scholar]
- Sugunan A, Thanachayanont C, Dutta J, Hilborn JG. Heavy-metal ion sensors using chitosan-capped gold nanoparticles. Sci Technol Adv Mater. 2005;6:335–340. doi: 10.1016/j.stam.2005.03.007. [DOI] [Google Scholar]
- Sun H, Wang X, Hu X, Yu W, You C, Hu H, Han C. Promotion of angiogenesis by sustained release of rhGM-CSF from heparinized collagen/chitosan scaffolds. J Biomed Mater Res B. 2012;100:788–798. doi: 10.1002/jbm.b.32512. [DOI] [PubMed] [Google Scholar]
- Sun X, Cao YY, Gong ZL, Wang XY, Zhang Y, Gao JM. An amperometric immunosensor based on multi-walled carbon nanotubes-thionine-chitosan nanocomposite film for chlorpyrifos detection. Sensors. 2012;12:17247–17261. doi: 10.3390/s121217247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Sui S, Ference C, Zhang Y, Sun S, Zhou N, Zhu W, Zhou K. Antimicrobial and mechanical properties of beta-cyclodextrin inclusion with essential oils in chitosan films. J Agric Food Chem. 2014;62:8914–8918. doi: 10.1021/jf5027873. [DOI] [PubMed] [Google Scholar]
- Svindland SC, Jul-Larsen Å, Pathirana R, Andersen S, Madhun A, Montomoli E, Jabbal-Gill I, Cox RJ. The mucosal and systemic immune responses elicited by a chitosan-adjuvanted intranasal influenza H5N1 vaccine. Influenza Other Respir Viruses. 2012;6:90–100. doi: 10.1111/j.1750-2659.2011.00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talon E, Trifkovic KT, Nedovic VA, Bugarski BM, Vargas M, Chiralt A, Gonzalez-Martinez C. Antioxidant edible films based on chitosan and starch containing polyphenols from thyme extracts. Carbohydr Polym. 2017;157:1153–1161. doi: 10.1016/j.carbpol.2016.10.080. [DOI] [PubMed] [Google Scholar]
- Tamer Tamer, Collins Maurice, Valachová Katarina, Hassan Mohamed, Omer Ahmed, Mohy-Eldin Mohamed, Švík Karol, Jurčík Rastislav, Ondruška Ľubomír, Biró Csaba, Albadarin Ahmad, Šoltés Ladislav. MitoQ Loaded Chitosan-Hyaluronan Composite Membranes for Wound Healing. Materials. 2018;11(4):569. doi: 10.3390/ma11040569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H, Gong Y, Lao L, Mao Z, Gao C. Gelatin/chitosan/hyaluronan ternary complex scaffold containing basic fibroblast growth factor for cartilage tissue engineering. J Mater Sci Mater Med. 2007;18:1961–1968. doi: 10.1007/s10856-007-3095-5. [DOI] [PubMed] [Google Scholar]
- Tan HB, Wang FY, Ding W, Zhang Y, Ding J, Cai DX, Yu KF, Yang J, Yang L, Xu YQ. Fabrication and evaluation of porous keratin/chitosan (KCS) scaffolds for effectively accelerating wound healing. Biomed Environ Sci. 2015;28:178–189. doi: 10.3967/bes2015.024. [DOI] [PubMed] [Google Scholar]
- Tang X, Tian M, Qu L, Zhu S, Guo X, Han G, Sun K, Hu X, Wang Y, Xu X. A facile fabrication of multifunctional knit polyester fabric based on chitosan and polyaniline polymer nanocomposite. Appl Surf Sci. 2014;317:505–510. doi: 10.1016/j.apsusc.2014.08.105. [DOI] [Google Scholar]
- Tang Q, Luo C, Lu B, Fu Q, Yin H, Qin Z, Lyu D, Zhang L, Fang Z, Zhu Y, Yao K. Thermosensitive chitosan-based hydrogels releasing stromal cell derived factor-1 alpha recruit MSC for corneal epithelium regeneration. Acta Biomater. 2017;61:101–113. doi: 10.1016/j.actbio.2017.08.001. [DOI] [PubMed] [Google Scholar]
- Tao W, Fu T, He Z, Hu R, Jia L, Hong Y. Evaluation of immunostimulatory effects of N-(2-hydroxy) propyl-3-trimethylammonium chitosan chloride for improving live attenuated hepatitis a virus vaccine efficacy. Viral Immunol. 2017;30:120–126. doi: 10.1089/vim.2016.0099. [DOI] [PubMed] [Google Scholar]
- Tao W, Zheng HQ, Fu T, He ZJ, Hong Y. N-(2-hydroxy) propyl-3-trimethylammonium chitosan chloride: An immune-enhancing adjuvant for hepatitis E virus recombinant polypeptide vaccine in mice. Hum Vaccin Immunotherap. 2017;13:1818–1822. doi: 10.1080/21645515.2017.1331191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teepoo S, Dawan P, Barnthip N. electrospun chitosan-gelatin biopolymer composite nanofibers for horseradish peroxidase immobilization in a hydrogen peroxide biosensor. Biosensors. 2017;7:47. doi: 10.3390/Bios7040047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng SH, Lee EJ, Wang P, Shin DS, Kim HE. Three-layered membranes of collagen/hydroxyapatite and chitosan for guided bone regeneration. J Biomed Mater Res B. 2008;87:132–138. doi: 10.1002/jbm.b.31082. [DOI] [PubMed] [Google Scholar]
- Thanoo BC, Sunny MC, Jayakrishnan A. Cross-linked chitosan microspheres: preparation and evaluation as a matrix for the controlled release of pharmaceuticals. J Pharm Pharmacol. 1992;44:283–286. doi: 10.1111/j.2042-7158.1992.tb03607.x. [DOI] [PubMed] [Google Scholar]
- Thanou M, Verhoef JC, Junginger HE. Oral drug absorption enhancement by chitosan and its derivatives. Adv Drug Deliv Rev. 2001;52:117–126. doi: 10.1016/S0169-409X(01)00231-9. [DOI] [PubMed] [Google Scholar]
- Ti D, et al. Controlled release of thymosin beta 4 using a collagen-chitosan sponge scaffold augments cutaneous wound healing and increases angiogenesis in diabetic rats with hindlimb ischemia. Tissue Eng Part A. 2015;21:541–549. doi: 10.1089/ten.tea.2013.0750. [DOI] [PubMed] [Google Scholar]
- Tian M, Hu X, Qu L, Du M, Zhu S, Sun Y, Han G. Ultraviolet protection cotton fabric achieved via layer-by-layer self-assembly of graphene oxide and chitosan. Appl Surf Sci. 2016;377:141–148. doi: 10.1016/j.apsusc.2016.03.183. [DOI] [Google Scholar]
- Tkac J, Whittaker JW, Ruzgas T. The use of single walled carbon nanotubes dispersed in a chitosan matrix for preparation of a galactose biosensor. Biosens Bioelectron. 2007;22:1820–1824. doi: 10.1016/j.bios.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Tokura S, Tamura H, Azuma I. Immunological aspects of chitin and chitin derivatives administered to animals. EXS. 1999;87:279–292. doi: 10.1007/978-3-0348-8757-1_20. [DOI] [PubMed] [Google Scholar]
- Tong C, et al. Hypoxia pretreatment of bone marrow-derived mesenchymal stem cells seeded in a collagen-chitosan sponge scaffold promotes skin wound healing in diabetic rats with hindlimb ischemia. Wound Repair Regen. 2016;24:45–56. doi: 10.1111/wrr.12369. [DOI] [PubMed] [Google Scholar]
- Toskas G, Brünler R, Hund H, Hund R-D, Hild M, Aibibu D, Cherif C. Pure chitosan microfibres for biomedical applications. Autex Res J. 2013;13:134. doi: 10.2478/v10304-012-0041-5. [DOI] [Google Scholar]
- Tran N, Mir A, Mallik D, Sinha A, Nayar S, Webster TJ. Bactericidal effect of iron oxide nanoparticles on Staphylococcus aureus. Int J Nanomedicine. 2010;5:277–283. doi: 10.2147/ijn.s9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travlou NA, Kyzas GZ, Lazaridis NK, Deliyanni EA. Graphite oxide/chitosan composite for reactive dye removal. Chem Eng J. 2013;217:256–265. doi: 10.1016/j.cej.2012.12.008. [DOI] [Google Scholar]
- Trickler WJ, Nagvekar AA, Dash AK. A novel nanoparticle formulation for sustained paclitaxel delivery. AAPS PharmSciTech. 2008;9:486–493. doi: 10.1208/s12249-008-9063-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai YC, Chen SY, Liaw HW. Immobilization of lactate dehydrogenase within multiwalled carbon nanotube-chitos anhanocompo site for application to lactate biosensors. Sensor Actuators B-Chem. 2007;125:474–481. doi: 10.1016/j.snb.2007.02.052. [DOI] [Google Scholar]
- Tsai YC, Chen SY, Lee CA. Amperometric cholesterol biosensors based on carbon nanotube-chitosan-platinum-cholesterol oxidase nanobiocomposite. Sensor Actuators B-Chem. 2008;135:96–101. doi: 10.1016/j.snb.2008.07.025. [DOI] [Google Scholar]
- Tsai HY, Chiu CC, Lin PC, Chen SH, Huang SJ, Wang LF. Antitumor efficacy of doxorubicin released from crosslinked nanoparticulate chondroitin sulfate/chitosan polyelectrolyte complexes. Macromol Biosci. 2011;11:680–688. doi: 10.1002/mabi.201000456. [DOI] [PubMed] [Google Scholar]
- Tsai B, Garcia-Valdez O, Champagne P, Cunningham M. Poly(poly(ethylene glycol) methyl ether methacrylate) grafted chitosan for dye removal from water. Processes. 2017;5:12. doi: 10.3390/pr5010012. [DOI] [Google Scholar]
- van der Lubben IM, Verhoef JC, Borchard G, Junginger HE. Chitosan for mucosal vaccination. Adv Drug Deliv Rev. 2001;52:139–144. doi: 10.1016/S0169-409X(01)00197-1. [DOI] [PubMed] [Google Scholar]
- van der Merwe SM, Verhoef JC, Verheijden JH, Kotze AF, Junginger HE. Trimethylated chitosan as polymeric absorption enhancer for improved peroral delivery of peptide drugs. Eur J Pharm Biopharm. 2004;58:225–235. doi: 10.1016/j.ejpb.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Varshosaz J, Hassanzadeh F, Aliabadi HS, Khoraskani FR, Mirian M, Behdadfar B. Targeted delivery of doxorubicin to breast cancer cells by magnetic LHRH chitosan bioconjugated nanoparticles. Int Biol Macromol. 2016;93:1192–1205. doi: 10.1016/j.ijbiomac.2016.07.025. [DOI] [PubMed] [Google Scholar]
- Vasconcelos DP, Costa M, Neves N, Teixeira JH, Vasconcelos DM, Santos SG, Águas AP, Barbosa MA, Barbosa JN. Chitosan porous 3D scaffolds embedded with resolvin D1 to improve in vivo bone healing. J Biomed Mater Res A. 2018;106:1626–1633. doi: 10.1002/jbm.a.36370. [DOI] [PubMed] [Google Scholar]
- Vasilêv LA, Dzyubinskaya EV, Zinovkin RA, Kiselevsky DB, Lobysheva NV, Samuilov VD. Chitosan-induced programmed cell death in plants. Biochemistry (Mosc) 2009;74:1035–1043. doi: 10.1134/S0006297909090120. [DOI] [PubMed] [Google Scholar]
- Vasiliev YM. Chitosan-based vaccine adjuvants: incomplete characterization complicates preclinical and clinical evaluation. Expert Rev Vaccines. 2015;14:37–53. doi: 10.1586/14760584.2015.956729. [DOI] [PubMed] [Google Scholar]
- Vedakumari WS, Prabu P, Sastry TP. Chitosan-fibrin nanocomposites as drug delivering and wound healing materials. J Biomed Nanotechnol. 2015;11:657–667. doi: 10.1166/jbn.2015.1948. [DOI] [PubMed] [Google Scholar]
- Velickova E, Winkelhausen E, Kuzmanova S, Alves VD, Moldão-Martins M. Impact of chitosan-beeswax edible coatings on the quality of fresh strawberries (Fragaria ananassa cv Camarosa) under commercial storage conditions. LWT – Food Sci Technol. 2013;52:80–92. doi: 10.1016/j.lwt.2013.02.004. [DOI] [Google Scholar]
- Venkatesan J, Kim S-K. Chitosan composites for bone tissue engineering—an overview. Mar Drugs. 2010;8:2252–2266. doi: 10.3390/md8082252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan J, Pallela R, Bhatnagar I, Kim SK. Chitosan-amylopectin/hydroxyapatite and chitosan-chondroitin sulphate/hydroxyapatite composite scaffolds for bone tissue engineering. Int Biol Macromol. 2012;51:1033–1042. doi: 10.1016/j.ijbiomac.2012.08.020. [DOI] [PubMed] [Google Scholar]
- Venkatesan J, Ryu B, Sudha PN, Kim SK. Preparation and characterization of chitosan-carbon nanotube scaffolds for bone tissue engineering. Int Biol Macromol. 2012;50:393–402. doi: 10.1016/j.ijbiomac.2011.12.032. [DOI] [PubMed] [Google Scholar]
- Vishwanath V, Pramanik K, Biswas A. Optimization and evaluation of silk fibroin-chitosan freeze-dried porous scaffolds for cartilage tissue engineering application. J Biomater Sci Polym Ed. 2016;27:657–674. doi: 10.1080/09205063.2016.1148303. [DOI] [PubMed] [Google Scholar]
- Wan Ngah WS, Teong LC, Hanafiah MAKM. Adsorption of dyes and heavy metal ions by chitosan composites: a review. Carbohydr Polym. 2011;83:1446–1456. doi: 10.1016/j.carbpol.2010.11.004. [DOI] [Google Scholar]
- Wan A, Xu Q, Sun Y, Li H. Antioxidant activity of high molecular weight chitosan and N,O-quaternized chitosans. J Agric Food Chem. 2013;61:6921–6928. doi: 10.1021/jf402242e. [DOI] [PubMed] [Google Scholar]
- Wang J, Chen C. Chitosan-based biosorbents: Modification and application for biosorption of heavy metals and radionuclides. Bioresour Technol. 2014;160:129–141. doi: 10.1016/j.biortech.2013.12.110. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang C. Chitosan-poly(vinyl alcohol)/attapulgite nanocomposites for copper(II) ions removal: pH dependence and adsorption mechanisms. Colloids and Surfaces A. 2016;500:186–194. doi: 10.1016/j.colsurfa.2016.04.034. [DOI] [Google Scholar]
- Wang L, Khor E, Wee A, Lim LY. Chitosan-alginate PEC membrane as a wound dressing: assessment of incisional wound healing. J Biomed Mater Res. 2002;63:610–618. doi: 10.1002/jbm.10382. [DOI] [PubMed] [Google Scholar]
- Wang YT, Zhu JZ, Zhu RJ, Zhu ZQ, Lai ZS, Chen ZY. Chitosan/Prussian blue-based biosensors. Meas Sci Technol. 2003;14:831–836. doi: 10.1088/0957-0233/14/6/317. [DOI] [Google Scholar]
- Wang X, Hu W, Cao Y, Yao J, Wu J, Gu X. Dog sciatic nerve regeneration across a 30-mm defect bridged by a chitosan/PGA artificial nerve graft. Brain. 2005;128:1897–1910. doi: 10.1093/brain/awh517. [DOI] [PubMed] [Google Scholar]
- Wang W, Lin S, Xiao Y, Huang Y, Tan Y, Cai L, Li X. Acceleration of diabetic wound healing with chitosan-crosslinked collagen sponge containing recombinant human acidic fibroblast growth factor in healing-impaired STZ diabetic rats. Life Sci. 2008;82:190–204. doi: 10.1016/j.lfs.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang L, Hu M, Liu H, Wen W, Xiao H, Niu Y. Synthesis and characterization of collagen-chitosan-hydroxyapatite artificial bone matrix. J Biomed Mater Res A. 2008;86:244–252. doi: 10.1002/jbm.a.31758. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang X, Tan Y, Zhang B, Gu Z, Li X. Synthesis and evaluation of collagen-chitosan-hydroxyapatite nanocomposites for bone grafting. J Biomed Mater Res A. 2009;89:1079–1087. doi: 10.1002/jbm.a.32087. [DOI] [PubMed] [Google Scholar]
- Wang L, Liu F, Jiang Y, Chai Z, Li P, Cheng Y, Jing H, Leng X. Synergistic antimicrobial activities of natural essential oils with chitosan films. J Agric Food Chem. 2011;59:12411–12419. doi: 10.1021/jf203165k. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhao Q, Zhao W, Liu Q, Gu X, Yang Y. Repairing rat sciatic nerve injury by a nerve-growth-factor-loaded, chitosan-based nerve conduit. Biotechnol Appl Biochem. 2012;59:388–394. doi: 10.1002/bab.1031. [DOI] [PubMed] [Google Scholar]
- Wang TJ, Wang IJ, Lu JN, Young TH. Novel chitosan-polycaprolactone blends as potential scaffold and carrier for corneal endothelial transplantation. Mol Vis. 2012;18:255–264. [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhang R, Zhang H, Jiang S, Liu H, Sun M, Jiang S. Kinetics and functional effectiveness of nisin loaded antimicrobial packaging film based on chitosan/poly(vinyl alcohol) Carbohydr Polym. 2015;127:64–71. doi: 10.1016/j.carbpol.2015.03.058. [DOI] [PubMed] [Google Scholar]
- Wang B, Wu X, Li J, Hao X, Lin J, Cheng D, Lu Y. Thermosensitive behavior and antibacterial activity of cotton fabric modified with a chitosan-poly(n-isopropylacrylamide) interpenetrating polymer network hydrogel. Polymers. 2016;8:110. doi: 10.3390/polym8040110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Roy AK, Webster TJ. Development of chitosan/poly(vinyl alcohol) electrospun nanofibers for infection related wound healing. Front Physiol. 2016;7:683. doi: 10.3389/fphys.2016.00683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Jia M, Wang L, Song S, Feng J, Zhang X. Chitosan and β-cyclodextrin-epichlorohydrin polymer composite film as a plant healthcare material for carbendazim-controlled release to protect rape against Sclerotinia sclerotiorum (Lib.) de Bary. Materials. 2017;10:343. doi: 10.3390/ma10040343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Guan S, Zhu Z, Li W, Liu T, Ma X. Hyaluronic acid doped-poly(3,4-ethylenedioxythiophene)/chitosan/gelatin (PEDOT-HA/Cs/Gel) porous conductive scaffold for nerve regeneration. Mater Sci Eng C Mater Biol Appl. 2017;71:308–316. doi: 10.1016/j.msec.2016.10.029. [DOI] [PubMed] [Google Scholar]
- Wang H, Qian J, Ding F. Emerging chitosan-based films for food packaging applications. J Agric Food Chem. 2018;66:395–413. doi: 10.1021/acs.jafc.7b04528. [DOI] [PubMed] [Google Scholar]
- Wei X, Lao J, Gu YD. Bridging peripheral nerve defect with chitosan-collagen film. Chin J Traumatol. 2003;6:131–134. [PubMed] [Google Scholar]
- Wei Y, Gong K, Zheng Z, Wang A, Ao Q, Gong Y, Zhang X. Chitosan/silk fibroin-based tissue-engineered graft seeded with adipose-derived stem cells enhances nerve regeneration in a rat model. J Mater Sci Mater Med. 2011;22:1947–1964. doi: 10.1007/s10856-011-4370-z. [DOI] [PubMed] [Google Scholar]
- Wen GM, Zhang Y, Shuang SM, Dong C, Choi MMF. Application of a biosensor for monitoring of ethanol. Biosens Bioelectron. 2007;23:121–129. doi: 10.1016/j.bios.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Wen Z-S, Xu Y-L, Zou X-T, Xu Z-R. Chitosan nanoparticles act as an adjuvant to promote both Th1 and Th2 immune responses induced by ovalbumin in mice. Mar Drugs. 2011;9:1038–1055. doi: 10.3390/md9061038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werle M, Bernkop-Schnurch A. Thiolated chitosans: useful excipients for oral drug delivery. J Pharm Pharmacol. 2008;60:273–281. doi: 10.1211/jpp.60.3.3001. [DOI] [PubMed] [Google Scholar]
- Westin CB, Trinca RB, Zuliani C, Coimbra IB, Moraes AM. Differentiation of dental pulp stem cells into chondrocytes upon culture on porous chitosan-xanthan scaffolds in the presence of kartogenin. Mater Sci Eng C Mater Biol Appl. 2017;80:594–602. doi: 10.1016/j.msec.2017.07.005. [DOI] [PubMed] [Google Scholar]
- Wijekoon A, Fountas-Davis N, Leipzig ND. Fluorinated methacrylamide chitosan hydrogel systems as adaptable oxygen carriers for wound healing. Acta Biomater. 2013;9:5653–5664. doi: 10.1016/j.actbio.2012.10.034. [DOI] [PubMed] [Google Scholar]
- Wivel NA, Wilson JM. Methods of gene delivery. Hematol Oncol Clinics North Am. 1998;12:483–501. doi: 10.1016/S0889-8588(05)70004-6. [DOI] [PubMed] [Google Scholar]
- Wlaszczuk A, Marcol W, Kucharska M, Wawro D, Palen P, Lewin-Kowalik J. Poly(D,L-lactide-co-glycolide) tubes with multifilament chitosan yarn or chitosan sponge core in nerve regeneration. J Oral Maxillofac Surg. 2016;74:2327.e1–2327.e12. doi: 10.1016/j.joms.2016.07.009. [DOI] [PubMed] [Google Scholar]
- Wongsawaeng D, Khemngern S, Somboonna NEJVI. Environmentally-friendly RF plasma treatment of Thai silk fabrics with chitosan for durable antibacterial property. Eng J. 2017;21:29–43. doi: 10.4186/ej.2017.21.1.29. [DOI] [Google Scholar]
- Woodard JR, et al. The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials. 2007;28:45–54. doi: 10.1016/j.biomaterials.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Wu L, Liu M. Preparation and properties of chitosan-coated NPK compound fertilizer with controlled-release and water-retention. Carbohydr Polym. 2008;72:240–247. doi: 10.1016/j.carbpol.2007.08.020. [DOI] [Google Scholar]
- Wu BY, Hou SH, Yin F, Li J, Zhao ZX, Huang JD, Chen Q. Amperometric glucose biosensor based on layer-by-layer assembly of multilayer films composed of chitosan, gold nanoparticles and glucose oxidase modified Pt electrode. Biosens Bioelectron. 2007;22:838–844. doi: 10.1016/j.bios.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Wu H, Zhang J, Luo Y, Wan Y, Sun S. Mechanical properties and permeability of porous chitosan-poly(p-dioxanone)/silk fibroin conduits used for peripheral nerve repair. J Mech Behav Biomed Mater. 2015;50:192–205. doi: 10.1016/j.jmbbm.2015.06.016. [DOI] [PubMed] [Google Scholar]
- Wu Z-C, Wang Z-Z, Liu J, Yin J-H, Kuang S-P. Removal of Cu(II) ions from aqueous water by l-arginine modifying magnetic chitosan. Colloids Surf A. 2016;499:141–149. doi: 10.1016/j.colsurfa.2016.04.012. [DOI] [Google Scholar]
- Wu T, Wu C, Fu S, Wang L, Yuan C, Chen S, Hu Y. Integration of lysozyme into chitosan nanoparticles for improving antibacterial activity. Carbohydr Polym. 2017;155:192–200. doi: 10.1016/j.carbpol.2016.08.076. [DOI] [PubMed] [Google Scholar]
- Xia W, Liu W, Cui L, Liu Y, Zhong W, Liu D, Wu J, Chua K, Cao Y. Tissue engineering of cartilage with the use of chitosan-gelatin complex scaffolds. J Biomed Mater Res B. 2004;71:373–380. doi: 10.1002/jbm.b.30087. [DOI] [PubMed] [Google Scholar]
- Xiao J, Yu H. Gemcitabine conjugated chitosan and double antibodies (Abc-GC-gemcitabine nanoparticles) enhanced cytoplasmic uptake of gemcitabine and inhibit proliferation and metastasis in human SW1990 pancreatic cancer cells. Med Sci Monit. 2017;23:1613–1620. doi: 10.12659/MSM.901292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Xu J, Liu X, Hu Q, Huang J. Antibacterial hybrid materials fabricated by nanocoating of microfibril bundles of cellulose substance with titania/chitosan/silver-nanoparticle composite films. J Mater Chem B. 2013;1:3477–3485. doi: 10.1039/c3tb20303d. [DOI] [PubMed] [Google Scholar]
- Xiao C, Liu X, Mao S, Zhang L, Lu J. Sub-micron-sized polyethylenimine-modified polystyrene/Fe3O4/chitosan magnetic composites for the efficient and recyclable adsorption of Cu(II) ions. Appl Surf Sci. 2017;394:378–385. doi: 10.1016/j.apsusc.2016.10.116. [DOI] [Google Scholar]
- Xie W, Xu P, Liu Q. Antioxidant activity of water-soluble chitosan derivatives. Bioorg Med Chem Lett. 2001;11:1699–1701. doi: 10.1016/S0960-894X(01)00285-2. [DOI] [PubMed] [Google Scholar]
- Xie F, Li QF, Gu B, Liu K, Shen GX. In vitro and in vivo evaluation of a biodegradable chitosan-PLA composite peripheral nerve guide conduit material. Microsurgery. 2008;28:471–479. doi: 10.1002/micr.20514. [DOI] [PubMed] [Google Scholar]
- Xie M, Zeng L, Zhang Q, Kang Y, Xiao H, Peng Y, Chen X, Luo J. Synthesis and adsorption behavior of magnetic microspheres based on chitosan/organic rectorite for low-concentration heavy metal removal. J Alloys Compd. 2015;647:892–905. doi: 10.1016/j.jallcom.2015.06.065. [DOI] [Google Scholar]
- Xing K, Zhu X, Peng X, Qin S. Chitosan antimicrobial and eliciting properties for pest control in agriculture: a review. Agron Sustain Dev. 2015;35:569–588. doi: 10.1007/s13593-014-0252-3. [DOI] [Google Scholar]
- Xing Y, Xu Q, Li X, Chen C, Ma L, Li S, Che Z, Lin H. Chitosan-based coating with antimicrobial agents: preparation, property, mechanism, and application effectiveness on fruits and vegetables international. J Polym Sci. 2016;2016:24. [Google Scholar]
- Xu G, et al. Optic nerve regeneration in polyglycolic acid-chitosan conduits coated with recombinant L1-Fc. Neuroreport. 2004;15:2167–2172. doi: 10.1097/00001756-200410050-00004. [DOI] [PubMed] [Google Scholar]
- Xu HR, Wang L, Luo JP, Song YL, Liu JT, Zhang S, Cai XX. Selective recognition of 5-hydroxytryptamine and dopamine on a multi-walled carbon nanotube-chitosan hybrid film-modified microelectrode array. Sensors. 2015;15:1008–1021. doi: 10.3390/s150101008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Wu Y, Zhang Y, Fu F, Liu X. Durable antibacterial cotton modified by silver nanoparticles and chitosan derivative binder. Fiber Polym. 2016;17:1782–1789. doi: 10.1007/s12221-016-6609-2. [DOI] [Google Scholar]
- Xu J, Zhang Y, Gutha Y, Zhang W. Antibacterial property and biocompatibility of Chitosan/Poly(vinyl alcohol)/ZnO (CS/PVA/ZnO) beads as an efficient adsorbent for Cu(II) removal from aqueous solution. Colloid Surf B: Biointerfaces. 2017;156:340–348. doi: 10.1016/j.colsurfb.2017.05.028. [DOI] [PubMed] [Google Scholar]
- Xu SC, Zhang YY, Dong K, Wen JN, Zheng CM, Zhao SH. Electrochemical DNA biosensor based on graphene oxide-chitosan hybrid nanocomposites for detection of Escherichia coli O157:H7. Int J Electrochem Sc. 2017;12:3443–3458. doi: 10.20964/2017.04.16. [DOI] [Google Scholar]
- Xu W, Xie W, Huang X, Chen X, Huang N, Wang X, Liu J. The graphene oxide and chitosan biopolymer loads TiO2 for antibacterial and preservative research. Food Chem. 2017;221:267–277. doi: 10.1016/j.foodchem.2016.10.054. [DOI] [PubMed] [Google Scholar]
- Xu W, Wang Z, Liu Y, Wang L, Jiang Z, Li T, Zhang W, Liang Y. Carboxymethyl chitosan/gelatin/hyaluronic acid blended-membranes as epithelia transplanting scaffold for corneal wound healing. Carbohydr Polym. 2018;192:240–250. doi: 10.1016/j.carbpol.2018.03.033. [DOI] [PubMed] [Google Scholar]
- Xue C, Hu N, Gu Y, Yang Y, Liu Y, Liu J, Ding F, Gu X. Joint use of a chitosan/PLGA scaffold and MSCs to bridge an extra large gap in dog sciatic nerve. Neurorehab Neural Repair. 2012;26:96–106. doi: 10.1177/1545968311420444. [DOI] [PubMed] [Google Scholar]
- Yamada A, Shibuya N, Kodama O, Akatsuka T. Induction of phytoalexin formation in suspension-cultured rice cells by N-acetyl-chitooligosaccharides. Biosci Biotechnol Biochem. 1993;57:405–409. doi: 10.1271/bbb.57.405. [DOI] [Google Scholar]
- Yan H, Li H, Yang H, Li A, Cheng R. Removal of various cationic dyes from aqueous solutions using a kind of fully biodegradable magnetic composite microsphere. Chem Eng J. 2013;223:402–411. doi: 10.1016/j.cej.2013.02.113. [DOI] [Google Scholar]
- Yan H, Chen X, Wu T, Feng Y, Wang C, Li J, Lin Q. Mechanochemical modification of kaolin surfaces for immobilization and delivery of pesticides in alginate-chitosan composite beads. Polym Bull. 2014;71:2923–2944. doi: 10.1007/s00289-014-1231-1. [DOI] [Google Scholar]
- Yang LQ, Ren XL, Tang FQ, Zhang L. A practical glucose biosensor based on Fe3O4 nanoparticles and chitosan/nafion composite film. Biosens Bioelectron. 2009;25:889–895. doi: 10.1016/j.bios.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Yang H-C, Wang W-H, Huang K-S, Hon M-H. Preparation and application of nanochitosan to finishing treatment with anti-microbial and anti-shrinking properties. Carbohydr Polym. 2010;79:176–179. doi: 10.1016/j.carbpol.2009.07.045. [DOI] [Google Scholar]
- Yang Y, Zhao W, He J, Zhao Y, Ding F, Gu X. Nerve conduits based on immobilization of nerve growth factor onto modified chitosan by using genipin as a crosslinking agent. Eur J Pharm Biopharm. 2011;79:519–525. doi: 10.1016/j.ejpb.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Yang L, Xiong H, Zhang X, Wang S. A novel tyrosinase biosensor based on chitosan-carbon-coated nickel nanocomposite film. Bioelectrochemistry. 2012;84:44–48. doi: 10.1016/j.bioelechem.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Yang X, Han G, Pang X, Fan M (2012b) Chitosan/collagen scaffold containing bone morphogenetic protein-7 DNA supports dental pulp stem cell differentiation in vitro and in vivo. J Biomed Mater Res A. 10.1002/jbm.a.34064 [DOI] [PubMed]
- Yang Z, Zeng Z, Xiao Z, Ji H. Preparation and controllable release of chitosan/vanillin microcapsules and their application to cotton fabric. Flavour Fragr J. 2014;29:114–120. doi: 10.1002/ffj.3186. [DOI] [Google Scholar]
- Yang CL, Chen JP, Wei KC, Chen JY, Huang CW, Liao ZX. Release of doxorubicin by a folate-grafted, chitosan-coated magnetic nanoparticle. Nanomaterials. 2017;7:85. doi: 10.3390/nano7040085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Wen X, Ke QF, Xie XT, Guo YP. pH-responsive mesoporous ZSM-5 zeolites/chitosan core-shell nanodisks loaded with doxorubicin against osteosarcoma. Mater Sci Eng C Mater Biol Appl. 2018;85:142–153. doi: 10.1016/j.msec.2017.12.024. [DOI] [PubMed] [Google Scholar]
- Yao Z, Zhang M, Sakahara H, Saga T, Arano Y, Konishi J. Avidin targeting of intraperitoneal tumor xenografts. J Natl Cancer Inst. 1998;90:25–29. doi: 10.1093/jnci/90.1.25. [DOI] [PubMed] [Google Scholar]
- Yao Q, et al. Preparation, characterization, and cytotoxicity of various chitosan nanoparticles. J Nanomaterials. 2013;2013:6. doi: 10.1155/2013/183871. [DOI] [Google Scholar]
- Yao W, Peng Y, Du M, Luo J, Zong L. Preventative vaccine-loaded mannosylated chitosan nanoparticles intended for nasal mucosal delivery enhance immune responses and potent tumor immunity. Mol Pharm. 2013;10:2904–2914. doi: 10.1021/mp4000053. [DOI] [PubMed] [Google Scholar]
- Ye P, Yu B, Deng J, She RF, Huang WL. Application of silk fibroin/chitosan/nano-hydroxyapatite composite scaffold in the repair of rabbit radial bone defect. Exp Therapeut Med. 2017;14:5547–5553. doi: 10.3892/etm.2017.5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen M-T, Yang J-H, Mau J-L. Antioxidant properties of chitosan from crab shells. Carbohydr Polym. 2008;74:840–844. doi: 10.1016/j.carbpol.2008.05.003. [DOI] [Google Scholar]
- Yeo JH, Lee KG, Kim HC, Oh HYL, Kim AJ, Kim SY. The effects of Pva/chitosan/fibroin (PCF)-blended spongy sheets on wound healing in rats. Biol Pharm Bull. 2000;23:1220–1223. doi: 10.1248/bpb.23.1220. [DOI] [PubMed] [Google Scholar]
- Yhee JY, et al. Effects of tumor microenvironments on targeted delivery of glycol chitosan nanoparticles. J Control Release. 2017;267:223–231. doi: 10.1016/j.jconrel.2017.09.015. [DOI] [PubMed] [Google Scholar]
- Yin X, et al. Enhanced wettability and moisture retention of cotton fabrics coated with self-suspended chitosan derivative. Cellulose. 2018;25:2721–2732. doi: 10.1007/s10570-018-1707-5. [DOI] [Google Scholar]
- Yousefpour P, Atyabi F, Vasheghani-Farahani E, Movahedi A-AM, Dinarvand R. Targeted delivery of doxorubicin-utilizing chitosan nanoparticles surface-functionalized with anti-Her2 trastuzumab. Int J Nanomedicine. 2011;6:1977–1990. doi: 10.2147/IJN.S21523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef AM, Abdel-Aziz MS, El-Sayed SM. Chitosan nanocomposite films based on Ag-NP and Au-NP biosynthesis by Bacillus subtilis as packaging materials. Int Biol Macromol. 2014;69:185–191. doi: 10.1016/j.ijbiomac.2014.05.047. [DOI] [PubMed] [Google Scholar]
- Youssef AM, El-Sayed SM, El-Sayed HS, Salama HH, Dufresne A. Enhancement of Egyptian soft white cheese shelf life using a novel chitosan/carboxymethyl cellulose/zinc oxide bionanocomposite film. Carbohydr Polym. 2016;151:9–19. doi: 10.1016/j.carbpol.2016.05.023. [DOI] [PubMed] [Google Scholar]
- Yu P, Bao RY, Shi XJ, Yang W, Yang MB. Self-assembled high-strength hydroxyapatite/graphene oxide/chitosan composite hydrogel for bone tissue engineering. Carbohydr Polym. 2017;155:507–515. doi: 10.1016/j.carbpol.2016.09.001. [DOI] [PubMed] [Google Scholar]
- Yuan G, Chen X, Li D. Chitosan films and coatings containing essential oils: The antioxidant and antimicrobial activity, and application in food systems. Food Res Int. 2016;89:117–128. doi: 10.1016/j.foodres.2016.10.004. [DOI] [PubMed] [Google Scholar]
- Yuan TT, DiGeorge Foushee AM, Johnson MC, Jockheck-Clark AR, Stahl JM. Development of Electrospun chitosan-polyethylene oxide/fibrinogen biocomposite for potential wound healing applications. Nanoscale Res Lett. 2018;13:88. doi: 10.1186/s11671-018-2491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuceer M, Caner C. Antimicrobial lysozyme-chitosan coatings affect functional properties and shelf life of chicken eggs during storage. J Sci Food Agric. 2014;94:153–162. doi: 10.1002/jsfa.6322. [DOI] [PubMed] [Google Scholar]
- Yun YR, Jang JH, Jeon E, Kang W, Lee S, Won JE, Kim HW, Wall I. Administration of growth factors for bone regeneration. Regen Med. 2012;7:369–385. doi: 10.2217/rme.12.1. [DOI] [PubMed] [Google Scholar]
- Zaharoff DA, Rogers CJ, Hance KW, Schlom J, Greiner JW. Chitosan solution enhances both humoral and cell-mediated immune responses to subcutaneous vaccination. Vaccine. 2007;25:2085–2094. doi: 10.1016/j.vaccine.2006.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaharoff DA, et al. Intravesical immunotherapy of superficial bladder cancer with chitosan/interleukin-12. Cancer Res. 2009;69:6192–6199. doi: 10.1158/0008-5472.CAN-09-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaharoff DA, Hance KW, Rogers CJ, Schlom J, Greiner JW. Intratumoral immunotherapy of established solid tumors with chitosan/IL-12. J Immunother. 2010;33:697–705. doi: 10.1097/CJI.0b013e3181eb826d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman P, Wang J, Blau A, Wang W, Li T, Kohane DS, Loscalzo J, Zhang YY. Incorporation of heparin-binding proteins into preformed dextran sulfate-chitosan nanoparticles. Int J Nanomedicine. 2016;11:6149–6159. doi: 10.2147/IJN.S119174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zargarkazemi A, Sadeghi-Kiakhani M, Arami M, Bahrami SH. Modification of wool fabric using prepared chitosan-cyanuric chloride hybrid. J Text Inst. 2015;106:80–89. doi: 10.1080/00405000.2014.906097. [DOI] [Google Scholar]
- Zarghami S, Kazemimoghadam M, Mohammadi T. Cu(II) removal enhancement from aqueous solutions using ion-imprinted membrane technique. Chem Pap. 2014;68:809–815. [Google Scholar]
- Zeng D, Shi Y. Preparation and application of a novel environmentally friendly organic seed coating for rice. J Sci Food Agric. 2009;89:2181–2185. doi: 10.1002/jsfa.3700. [DOI] [Google Scholar]
- Zeng D, Luo X, Tu R. Application of bioactive coatings based on chitosan for soybean seed protection international. J Carbohydr Chem. 2012;2012:5. doi: 10.1155/2012/104565. [DOI] [Google Scholar]
- Zhai M, Xu Y, Zhou B, Jing W. Keratin-chitosan/n-ZnO nanocomposite hydrogel for antimicrobial treatment of burn wound healing: characterization and biomedical application. J Photochem Photobiol B. 2018;180:253–258. doi: 10.1016/j.jphotobiol.2018.02.018. [DOI] [PubMed] [Google Scholar]
- Zhang MG, Gorski W. Amperometric Ethanol biosensors based on chitosan-NAD(+)-alcohol dehydrogenase films. Electroanal. 2011;23:1856–1862. doi: 10.1002/elan.201100078. [DOI] [Google Scholar]
- Zhang MG, Smith A, Gorski W. Carbon nanotube-chitosan system for electrochemical sensing based on dehydrogenase enzymes. Anal Chem. 2004;76:5045–5050. doi: 10.1021/ac049519u. [DOI] [PubMed] [Google Scholar]
- Zhang H, Wang W, Yin H, Zhao X, Du Y. Oligochitosan induces programmed cell death in tobacco suspension cells. Carbohydr Polym. 2012;87:2270–2278. doi: 10.1016/j.carbpol.2011.10.059. [DOI] [Google Scholar]
- Zhang K, Zhang Y, Yan S, Gong L, Wang J, Chen X, Cui L, Yin J. Repair of an articular cartilage defect using adipose-derived stem cells loaded on a polyelectrolyte complex scaffold based on poly(l-glutamic acid) and chitosan. Acta Biomater. 2013;9:7276–7288. doi: 10.1016/j.actbio.2013.03.025. [DOI] [PubMed] [Google Scholar]
- Zhang X, et al. Expression of gamma-aminobutyric acid receptors on neoplastic growth and prediction of prognosis in non-small cell lung cancer. J Translat Med. 2013;11:102. doi: 10.1186/1479-5876-11-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y-L, Zhang J, Dai C-M, Zhou X-F, Liu S-G. Sorption of carbamazepine from water by magnetic molecularly imprinted polymers based on chitosan-Fe3O4. Carbohydr Polym. 2013;97:809–816. doi: 10.1016/j.carbpol.2013.05.072. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Shen Z, Dai C, Zhou X. Removal of selected pharmaceuticals from aqueous solution using magnetic chitosan: sorption behavior and mechanism. Environ Sci Pollut R. 2014;21:12780–12789. doi: 10.1007/s11356-014-3212-1. [DOI] [PubMed] [Google Scholar]
- Zhang K, Yan S, Li G, Cui L, Yin J. In-situ birth of MSCs multicellular spheroids in poly(L-glutamic acid)/chitosan scaffold for hyaline-like cartilage regeneration. Biomaterials. 2015;71:24–34. doi: 10.1016/j.biomaterials.2015.08.037. [DOI] [PubMed] [Google Scholar]
- Zhang S, Prabhakaran MP, Qin X, Ramakrishna S. Biocomposite scaffolds for bone regeneration: role of chitosan and hydroxyapatite within poly-3-hydroxybutyrate-co-3-hydroxyvalerate on mechanical properties and in vitro evaluation. J Mech Behav Biomed Mater. 2015;51:88–98. doi: 10.1016/j.jmbbm.2015.06.032. [DOI] [PubMed] [Google Scholar]
- Zhang WJ, Li XJ, Zou RT, Wu HZ, Shi HY, Yu SS, Liu Y (2015c) Multifunctional glucose biosensors from Fe3O4 nanoparticles modified chitosan/graphene nanocomposites. Sci Rep 5. 10.1038/Srep11129 [DOI] [PMC free article] [PubMed]
- Zhang S, Dong Y, Yang Z, Yang W, Wu J, Dong C. Adsorption of pharmaceuticals on chitosan-based magnetic composite particles with core-brush topology. Chem Eng J. 2016;304:325–334. doi: 10.1016/j.cej.2016.06.087. [DOI] [Google Scholar]
- Zhang X, Xiao G, Wang Y, Zhao Y, Su H, Tan T. Preparation of chitosan-TiO2 composite film with efficient antimicrobial activities under visible light for food packaging applications. Carbohydr Polym. 2017;169:101–107. doi: 10.1016/j.carbpol.2017.03.073. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ma Y, Pan X, Chen S, Zhuang H, Wang S. A composite hydrogel of chitosan/heparin/poly (gamma-glutamic acid) loaded with superoxide dismutase for wound healing. Carbohydr Polym. 2018;180:168–174. doi: 10.1016/j.carbpol.2017.10.036. [DOI] [PubMed] [Google Scholar]
- Zhao R, Ren Y, Sun B, Zhang R, Liang D. Experimental study on chitosan mediated insulin-like growth factor gene transfection repairing injured articular cartilage in rabbits. Chin J Reparat Reconstr Surg. 2010;24:1372–1375. [PubMed] [Google Scholar]
- Zhao J, Zheng X, Fu C, Qu W, Wei G, Zhang W. FK506-loaded chitosan conduit promotes the regeneration of injured sciatic nerves in the rat through the upregulation of brain-derived neurotrophic factor and TrkB. J Neurol Sci. 2014;344:20–26. doi: 10.1016/j.jns.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Liu JG, Chen WM, Yu AX. Efficacy of thermosensitive chitosan/beta-glycerophosphate hydrogel loaded with beta-cyclodextrin-curcumin for the treatment of cutaneous wound infection in rats. Exp Therapeut Med. 2018;15:1304–1313. doi: 10.3892/etm.2017.5552. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zheng L, Cui HF. Use of chitosan conduit combined with bone marrow mesenchymal stem cells for promoting peripheral nerve regeneration. J Mater Sci Mater Med. 2010;21:1713–1720. doi: 10.1007/s10856-010-4003-y. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Yang W, Wang C, Hu J, Fu S, Dong L, Wu L, Shen X. Nanoparticles based on the complex of chitosan and polyaspartic acid sodium salt: preparation, characterization and the use for 5-fluorouracil delivery. Eur J Pharm Biopharm. 2007;67:621–631. doi: 10.1016/j.ejpb.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Cai Z, Song X, Yu B, Bi Y, Chen Q, Zhao D, Xu J, Hou S. Receptor mediated gene delivery by folate conjugated N-trimethyl chitosan in vitro. Int J Pharm. 2009;382:262–269. doi: 10.1016/j.ijpharm.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Zheng H, Tang C, Yin C. Exploring advantages/disadvantages and improvements in overcoming gene delivery barriers of amino acid modified trimethylated chitosan. Pharm Res. 2015;32:2038–2050. doi: 10.1007/s11095-014-1597-7. [DOI] [PubMed] [Google Scholar]
- Zheng XF, Lian Q, Yang H, Wang X. Surface molecularly imprinted polymer of chitosan grafted poly(methyl methacrylate) for 5-fluorouracil and controlled release. Sci Rep. 2016;6:21409. doi: 10.1038/srep21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Huang HL, Li J, Qian FC, Li LQ, Niu PP, Dai LC. Development of hybrid-type modified chitosan derivative nanoparticles for the intracellular delivery of midkine-siRNA in hepatocellular carcinoma cells. Hepatobiliary Pancreat Dis Int. 2015;14:82–89. doi: 10.1016/S1499-3872(15)60336-8. [DOI] [PubMed] [Google Scholar]
- Zhou L, Jin J, Liu Z, Liang X, Shang C. Adsorption of acid dyes from aqueous solutions by the ethylenediamine-modified magnetic chitosan nanoparticles. J Hazard Mater. 2011;185:1045–1052. doi: 10.1016/j.jhazmat.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Zhou D, Qi C, Chen YX, Zhu YJ, Sun TW, Chen F, Zhang CQ. Comparative study of porous hydroxyapatite/chitosan and whitlockite/chitosan scaffolds for bone regeneration in calvarial defects. Int J Nanomedicine. 2017;12:2673–2687. doi: 10.2147/IJN.S131251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Wang H, Zhang J, Li X, Wu Y, Wei Y, Ji S, Kong D, Zhao Q. Functional poly(epsilon-caprolactone)/chitosan dressings with nitric oxide-releasing property improve wound healing. Acta Biomater. 2017;54:128–137. doi: 10.1016/j.actbio.2017.03.011. [DOI] [PubMed] [Google Scholar]
- Zhu HY, Jiang R, Xiao L, Zeng GM. Preparation, characterization, adsorption kinetics and thermodynamics of novel magnetic chitosan enwrapping nanosized γ-Fe2O3 and multi-walled carbon nanotubes with enhanced adsorption properties for methyl orange. Bioresour Technol. 2010;101:5063–5069. doi: 10.1016/j.biortech.2010.01.107. [DOI] [PubMed] [Google Scholar]
- Zhu L, Liu T, Cai J, Ma J, Chen AM. Repair and regeneration of lumbosacral nerve defects in rats with chitosan conduits containing bone marrow mesenchymal stem cells. Injury. 2015;46:2156–2163. doi: 10.1016/j.injury.2015.08.035. [DOI] [PubMed] [Google Scholar]
- Zhu C, Huang J, Xue C, Wang Y, Wang S, Bao S, Chen R, Li Y, Gu Y. Skin derived precursor Schwann cell-generated acellular matrix modified chitosan/silk scaffolds for bridging rat sciatic nerve gap. Neurosci Res pii. 2017;S0168-0102(17):30584–30589. doi: 10.1016/j.neures.2017.12.007. [DOI] [PubMed] [Google Scholar]
- Zhuo N, Lan Y, Yang W, Yang Z, Li X, Zhou X, Liu Y, Shen J, Zhang X. Adsorption of three selected pharmaceuticals and personal care products (PPCPs) onto MIL-101(Cr)/natural polymer composite beads. Sep Purif Technol. 2017;177:272–280. doi: 10.1016/j.seppur.2016.12.041. [DOI] [Google Scholar]
- Ziani K, Ursua B, Mate JI. Application of bioactive coatings based on chitosan for artichoke seed protection. Crop Prot. 2010;29:853–859. doi: 10.1016/j.cropro.2010.03.002. [DOI] [Google Scholar]
- Zou Q, Cai B, Li J, Li J, Li Y. In vitro and in vivo evaluation of the chitosan/Tur composite film for wound healing applications. J Biomater Sci Polym Ed. 2017;28:601–615. doi: 10.1080/09205063.2017.1289036. [DOI] [PubMed] [Google Scholar]
- Zuppini A, Baldan B, Millioni R, Favaron F, Navazio L, Mariani P. Chitosan induces Ca2+-mediated programmed cell death in soybean cells. New Phytol. 2004;161:557–568. doi: 10.1046/j.1469-8137.2003.00969.x. [DOI] [PubMed] [Google Scholar]


