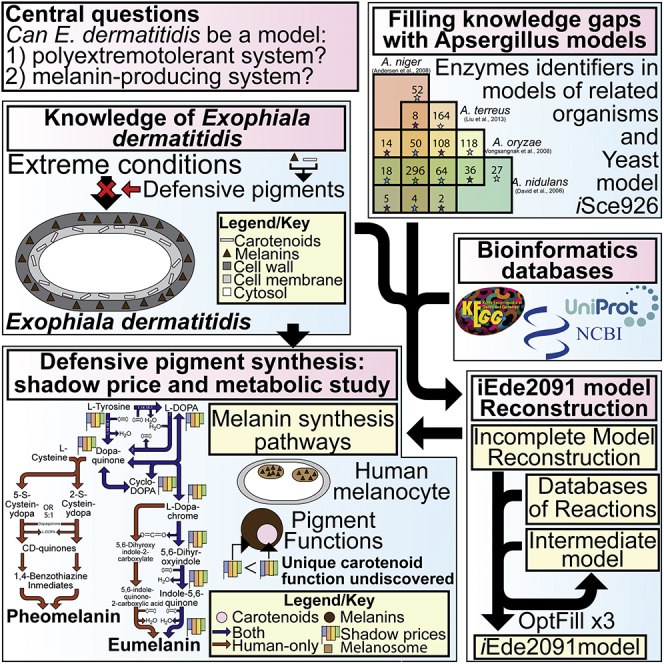
Summary
The polyextremotolerant black yeast Exophiala dermatitidis is a tractable model system for investigation of adaptations that support growth under extreme conditions. Foremost among these adaptations are melanogenesis and carotenogenesis. A particularly important question is their metabolic production cost. However, investigation of this issue has been hindered by a relatively poor systems-level understanding of E. dermatitidis metabolism. To address this challenge, a genome-scale model (iEde2091) was developed. Using iEde2091, carotenoids were found to be more expensive to produce than melanins. Given their overlapping protective functions, this suggests that carotenoids have an underexplored yet important role in photo-protection. Furthermore, multiple defensive pigments with overlapping functions might allow E. dermatitidis to minimize cost. Because iEde2091 revealed that E. dermatitidis synthesizes the same melanins as humans and the active sites of the key tyrosinase enzyme are highly conserved this model may enable a broader understanding of melanin production across kingdoms.
Subject Areas: Microbiology, Bioinformatics, Metabolic Engineering
Graphical Abstract
Highlights
-
•
Exophiala dermatitidis can be a model polyextremotolerant and melaninized organism
-
•
Its genome-scale model is reconstructed to study melanin and carotenoid metabolism
-
•
The shadow price analysis indicates a potential underexplored role of carotenoids
-
•
Comparisons between human and E. dermatitidis melanin synthesis are made
Microbiology; Bioinformatics; Metabolic Engineering
Introduction
Extremophiles are organisms that can live in extreme conditions of temperature, acidity, alkalinity, or salinity. Studying these organisms not only expands our knowledge on the diversity of life but can also provide significant insights into how organisms adapt to stress, particularly metabolic and regulatory responses. Exophiala dermatitidis (hereafter, Exophiala or E. dermatitidis, also known as Wangiella dermatitidis), a highly melanized black fungus and perhaps best known for its H. sapiens (hereafter, human) pathogenic properties (Paolo et al., 2006, Poyntner et al., 2016, Sudhadham et al., 2008), is a potential model extremophile system owing to its small genome of 26.4 Mb (Exophiala dermatitidis NIH/UT8656 Genome, 2011) and its demonstrated extremotolerance with respect to temperature (heat and cold) (Paolo et al., 2006, Sudhadham et al., 2008), acidic pH (Sudhadham et al., 2008, Chen et al., 2014), light (Chen et al., 2014, Nosanchuk and Casadevall, 2006, Geis and Szaniszlo, 1984), radiation (Chen et al., 2014, Nosanchuk and Casadevall, 2006, Geis and Szaniszlo, 1984), oxidative stress (Chen et al., 2014, Geis and Szaniszlo, 1984), and likely tolerance to toxic heavy metals (Nosanchuk and Casadevall, 2006), harmful aromatic compounds (Moreno et al., 2018), various toxins (Moreno et al., 2018), antimicrobial compounds (Nosanchuk and Casadevall, 2006), and other stressors (nutrient, osmotic, and mechanical) (Moreno et al., 2018). The ability of Exophiala to adapt to most of these conditions seemingly results from two classes of defensive pigments: melanins, a class of pigments consisting of six-carbon ring monomers, and carotenoids, a class of polyisoprenoid pigments. Exophiala can produce three different types of melanin: (1) 1,8-dihydroxynaphthalene melanin (hereafter, DHN-melanin), also called naphthalene melanin, (2) DOPA-melanin, also known as eumelanin (Ito and Wakamatsu, 2011), and (3) pyomelanin. Among these, DHN-melanin and pyomelanin are generally produced by fungi (Solano, 2014) including Exophiala, whereas eumelanin is produced by both fungi and animals, including humans (Ito and Wakamatsu, 2011, Solano, 2014). The combination of its small genome (Exophiala dermatitidis NIH/UT8656 Genome, 2011), its ability to be cultured as yeast cells (Chen et al., 2014, Ohkusu et al., 1999), and production of eumelanin (Ito and Wakamatsu, 2011) makes Exophiala a potential model organism for human melanocytes, the cells in humans that produce melanins. Melanocytes are specialized cells in humans that are found primarily in the skin, which produce pheomelanin and eumelanin in specialized subcellular organelles called melanosomes.
A Genome-Scale Model (GSMs) is a mathematical reconstruction of the metabolism of a target organism, generally accomplished using genomic and biochemical databases to define the sets of gene-protein-reaction (GPR) links (Thiele and Palsson, 2010). GSMs have become an indispensable tool of systems biology in a wide variety of applications (Thiele and Palsson, 2010), with perhaps the most common applications being the overproduction of a native metabolite (Khodayari et al., 2015, Lin et al., 2017, Zhang et al., 2016, Feist and Palsson, 2008) or engineering of metabolism to produce a non-native metabolite (Feist and Palsson, 2008, Gudmundsson et al., 2017). Other uses of GSMs have also been to characterize open reading frames (ORFs), determine gene essentiality, and evolutionary studies in Escherichia coli (Feist and Palsson, 2008); investigate the Warburg effect and drug screenings in human cancer cells (Yizhak et al., 2015); study interactions among members of a microbial community (Stolyar et al., 2007, Magnúsdóttir et al., 2016); and investigate plant metabolism under stress (Cheung et al., 2013, Williams et al., 2010, Cramer et al., 2011). Hence, the reconstruction of a GSM of Exophiala can be a useful tool to investigate its potential as a model organism both for polyextremotolerant organism and for human melanocytes. However, GSMs are challenging to reconstruct for under-studied organisms such as Exophiala, where only approximately 43% of genes have some level of annotation (not including hypothetical or putative proteins) and less than 5% of genes are annotated with Enzyme Commission (EC) numbers, which might be used to establish GPR links (Exophiala dermatitidis NIH/UT8656 Genome, 2011, Exophiala dermatitidis (strain ATCC34100/CBS 525.76/NIH/UT8656) (2018) UniProt2.). This lack of annotations often leaves large gaps in metabolic reconstructions, which requires further scrutiny. One tool that we recently have developed is OptFill (Schroeder and Saha, 2020), which performs whole-model thermodynamically infeasible cycle (TIC) free gapfilling. TICs are detrimental to GSMs as they result in the reporting of unrealistic flux results, cause difficulties in using dual formulations of optimization problems (such as in this work), and can make energy costs such as ATP maintenance meaningless (Schroeder and Saha, 2020). OptFill works by first identifying possible TICs that can occur between a database of functionalities proposed to fix the gaps in the model and the model itself. Then the reaction flux in the direction that would allow a TIC is excluded in the second step of OptFill, which attempts to maximize the number of model reactions fixed by adding new reactions (Schroeder and Saha, 2020). Ultimately, this allows for the maximization of model connectivity while minimizing new functionalities added to the model, as well as opportunity to hypothesize functions for un- or poorly annotated genes through the concurrent use of tools such as BLASTp (Altschul et al., 1997, Altschul et al., 2005). Through the process of reconstructing a GSM, metabolic pathways are thoroughly investigated, particularly those related to the subjects of the study, in this case defensive pigments. In addition, this reconstruction provides the basis for comparison between humans and Exophiala, which when supplemented with sequence alignment tools such as COBALT (Papadopoulos and Agarwala, 2007) can provide initial comparisons for determining the suitability of E. dermatitidis as a model organism.
Once a GSM is reconstructed, optimization-based tools of analysis may be applied to investigate E. dermatitidis as a model polyextremotolerant organism. These tools include those that can analyze base metabolism, such as Flux Balance Analysis (FBA) (Orth et al., 2010) and Flux Variability Analysis (FVA) (Gudmundsson and Thiele, 2010); tools that can aid in redesigning metabolism for optimization of a desired phenotype, such as OptKnock (Burgard et al., 2003) and OptForce (Burgard et al., 2003); and tools that elucidate potentially non-intuitive relationships in metabolism such as Flux Coupling Analysis (FCA) (Burgard et al., 2004). This work uses the standard measure of flux of mmol per gDW per h (Orth et al., 2010, Thiele and Palsson, 2010, Maranas and Zomorrodi, 2016). All optimization problems have primal and dual forms, both of which can be enlightening about the problem solution, particularly a quantity determined from the dual problem called the shadow price. The shadow price associated with variable i is defined as the reduction in the optimization objective caused by producing one more unit of i. Generally, shadow price is used in an economic sense to define the cost of some process in terms of currency; however, this metric can also be applied to the cost of some biological objective (e.g., growth) owing to increasing production of a metabolite, such as a defensive pigment, by one unit. This can be determined using dual formulation of the FBA problem. The cost of producing melanins and carotenoids by E. dermatitidis and the associated shadow prices, in particular, have not yet been investigated in this manner.
In this work, a draft GSM of Exophiala dermatitidis was first reconstructed from annotated genome of E. dermatitidis and an enzyme consensus between four GSMs from a related genus, Aspergillus, namely, A. niger (Andersen et al., 2008), A. nidulans (David et al., 2008), A. oryzae (Vongsangnak et al., 2008), and A. terreus (Liu et al., 2013). Enzymes used in these Aspergillus GSMs (Andersen et al., 2008, David et al., 2008, Vongsangnak et al., 2008, Liu et al., 2013) were used in conjunction with bidirectional BLASTp analyses to hypothesize characterizations of open reading frames. In general, the bidirectional BLASTp analyses assigned EC numbers, and the metabolic functionalities that accompany those numbers, to genes already annotated in the NCBI database with non-hypothetical protein names. This draft model next underwent manual and automated curation, the latter through using the tool OptFill (Schroeder and Saha, 2020), to develop the iEde2091 model. iEde2091 was used in computational investigation of the metabolic cost of defensive pigment synthesis through shadow price analysis. This analysis shows that, on both a per-carbon atom and a per-unit (monomer in the case of melanins and molecule in the case of carotenoids), carotenoids are more expensive to produce than melanins. Given that the functions of carotenoids and melanins are generally overlapping, this suggests that carotenoids perform a metabolically valuable protective role that has not been fully explored as of yet, potentially related to absorbance of violet and blue visible light. Finally, the potential of Exophiala as a model eumelanin-producing organism, particularly with respect to human eumelanin production in melanocytes, was investigated based on similarity of metabolic pathways and tyrosinase enzyme sequence similarity. This analysis showed that key amino acid residues are conserved in tyrosinase between Exophiala and humans, including residues whose mutations are associated with oculocutaneous albinism A1 (OCA1), which suggests Exophiala may be used as a model of human eumelanin-production.
Results
Reconstruction of First Draft E. dermatitidis Model
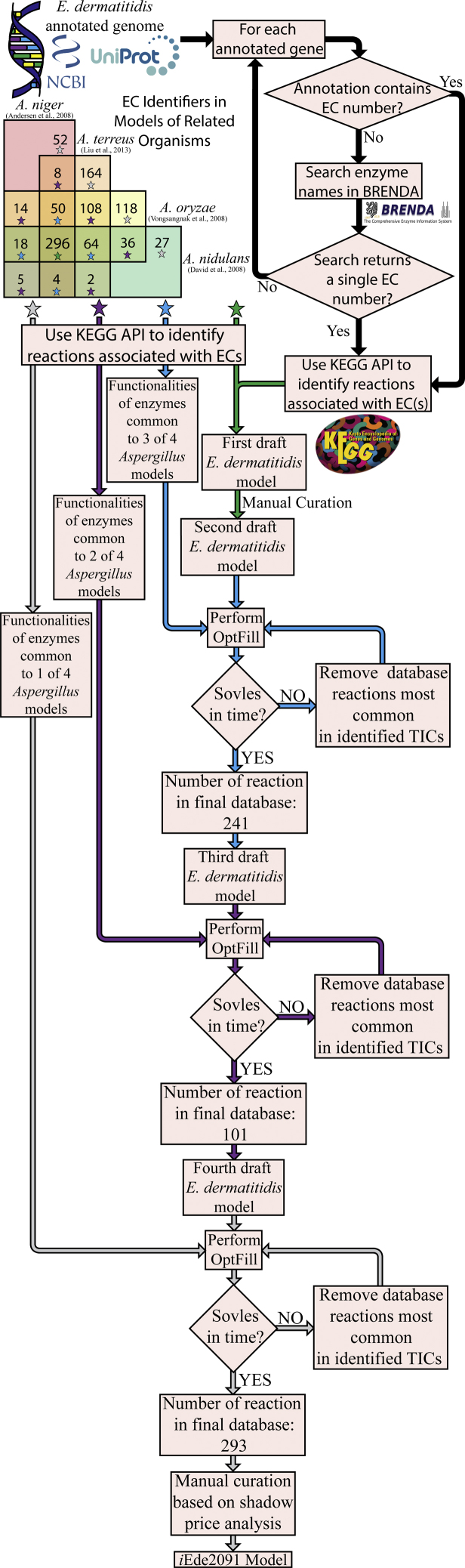
In this work, the first draft GSM of E. dermatitidis was reconstructed using logical Gene-Protein-Reaction (GPR) links to determine the set of metabolic reactions which occur in an organism using publicly available data such as NCBI and UniProt annotated genomes. This initial reconstruction was necessarily incomplete owing to incomplete genome annotation, in that only approximately 43% of genes were annotated and less than 5% had some Enzyme Commission (EC) number annotation (Exophiala dermatitidis NIH/UT8656 Genome, 2011, Exophiala dermatitidis (strain ATCC34100/CBS 525.76/NIH/UT8656) (2018) UniProt2.). EC numbers were used to establish the GPR links, and therefore automated exploration of BRENDA was used to address this incompleteness and to retrieve more EC numbers; see Figure 1 and Transparent Methods for more details of this procedure. From this, approximately 20% of genes were linked to some EC numbers. These proteins were then localized to their respective subcellular compartment through use of the CELLO subcellular localization tool (Yu and Lin, 2004), the results of which can be found in Table S1. This still left major metabolic gaps; therefore, in addition to genome annotation data, a core set of Enzyme Commission (EC) numbers were identified by being common to GSM models of four strains of a closely related genus (Aspergillus), A. niger (Andersen et al., 2008), A. nidulans (David et al., 2008), A. oryzae (Vongsangnak et al., 2008), and A. terreus (Liu et al., 2013), hereafter referred to as a the full consensus of Aspergillus enzymes. These Aspergillus models were chosen as they were the phylogenetically the closest species (Schoch et al., 2009) for which metabolic models were available. This work was limited to using the Aspergillus species models in that the next-closest fungi with GSMs published are at the phylum level, for example, Yarrowia and Saccharomyces species, which are quite phylogenetically distant. Furthermore, all four Aspergillus species considered here have larger genome than E. dermatitidis allowing for greater genome coverage, whereas model Ascomycetes like S. cerevisiae and Y. lipolytica have smaller genomes. This restriction resulted in a more conservative metabolic reconstruction than might have otherwise been created in addition to limiting the number of reactions in the database for OptFill applications. This also limited the number of OptFill applications, as each new model considered would require one additional application. The full consensus of Aspergillus enzymes included 310 EC numbers in total. ECs already identified in E. dermatitidis were removed from the list of EC numbers belonging to the consensus of all four Aspergillus models (Andersen et al., 2008, David et al., 2008, Vongsangnak et al., 2008, Liu et al., 2013), leaving 56 unique ECs. These 56 EC numbers were converted to metabolic functionalities and added to the existing draft model of E. dermatitidis as a set of functionalities likely common to these closely related melanized fungal species (Schoch et al., 2009). See method and the GitHub “E_dermatitidis_model” repository (https://doi.org/10.5281/zenodo.3608172) for how this was accomplished. Steps taken in reconstruction can be found in greater detail in Table S2.
Figure 1.
Workflow of iEde2091 GSM Reconstruction
This figure shows the reconstruction workflow of iEde2091, beginning with the annotated genomes from NCBI and UniProt. These gene names taken from these annotated genomes are then used to automatically search the BRENDA database for the associated Enzyme Commission (EC) number. These data were combined with the consensus of enzymes present in the selected Aspergillus species GSM reconstructions to form the first draft E. dermatitidis GSM model. After manual curation to ensure production of defensive pigments and biomass, this became the second draft E. dermatitidis model. Subsequent draft E. dermatitidis models were created by using the OptFill tool to fill metabolic gaps using non-consensus Aspergillus databases. Once each non-consensus database had been used, the iEde2091 model was complete.
Bidirectional BLASTp of Full Consensus Aspergillus Enzymes onto E. dermatitidis
The list of 56 ECs common to Aspergillus models but not identified in Exophiala were subjected to a bidirectional BLASTp against the Exophiala genome. This was accomplished through the Bidirectional BLAST Program (BBP) developed as part of this work, which can be found in the GitHub “E_dermatitidis_model” repository. The BBP program performs forward and backward BLASTp of amino acid sequences, taken from related species, encoding target ECs against a target genome in order to provide evidence for the presence of certain functionalities. The result of the BBP program when applied to the Aspergillus consensus ECs (Table S3) was that 39 of 56 (69.6%) consensus ECs were identified in Exophiala with 169 unique bidirectional matches using conservative thresholds for the expect (1E-30) and percent positive substitution (60%) values. Many of these matches were between sequences annotated similarly in the reference Aspergillus species and Exophiala. Examples include annotations in Aspergillus species such as “xylulokinase,” “2-aminoadipate transaminase,” and “phosphoadenylyl-sulfate reductase (thioredoxin)” matching to annotations in Exophiala of “D-xylulose kinase A,” “aromatic amino acid aminotransferase I,” and “phosphoadenosine phosphosulfate reductase,” respectively. Other matches assigned EC number to multi-functional enzymes such as the “pentafunctional AROM polypeptide” being assigned to EC numbers 1.1.1.25, 2.5.1.19, and 4.2.1.10 based on strong sequence similarity to specific enzymes such as shikimate dehydrogenase, 3-phosphoshikimate 1-carboxyvinyltransferase, and 3-dehydroquinate dehydratase, respectively. In addition, a total of 22 bidirectional matches to protein sequences currently annotated as “hypothetical proteins” were made. These matches to hypothetical proteins mapped four hypothetical Exophiala protein sequences to seven EC numbers. The used reference Aspergillus sequences of six of these EC numbers, 1.2.1.38, 2.7.2.8, 6.3.3.1, 6.3.4.13, 6.3.4.14, and 6.4.1.2, only produced significant sequence alignment matched to hypothetical proteins in the Exophiala genome, indicating in silico identification of potentially unknown metabolic functionalities. Particularly important to this study is the identification EC 6.4.1.2, acetyl-CoA carboxylase, which produces malonyl-CoA. Malonyl-CoA is an essential precursor for the synthesis of hydroxylated naphthalene compounds, which, when polymerized, produce DHN-melanin. See Figure 2 for DHN-melanin synthesis pathway with the reaction catalyzed by EC 6.4.1.2, which highlights the importance of this functionality.
Figure 2.
Synthesis Pathways of Pyomelanin and DHN-Melanin in E. dermatitidis
This figure shows the synthesis pathways of pyomelanin and DHN-melanin including chemical structures, reaction stoichiometries, catalyzing Enzyme Commission (EC) number, and reaction cofactors.
From First Draft E. dermatitidis Model to Second Draft E. dermatitidis Model
Despite the added functionality of the Aspergillus full consensus enzyme set and subsequent potential identification of new functionalities in the Exophiala genome, there were a number of “holes” in the metabolic reconstruction. These “holes” included lacking full synthesis pathways for defensive pigments and some biomass components. Therefore, the set of enzymes common to three of these Aspergillus models (Andersen et al., 2008, David et al., 2008, Vongsangnak et al., 2008, Liu et al., 2013), the latest model of another ascomycete fungus, Saccharomyces cerevisiae (iSce926) (Chowdhury et al., 2015), and literature information on fungal melanin synthesis (Chen et al., 2014, Eisenman and Casadevall, 2012, Paolo et al., 2006, Schmaler-Ripcke et al., 2009, Toledo et al., 2017), were used to manually address some metabolic gaps. Once this manual step was complete, the model could produce all required defensive pigments and biomass components and all TICs were addressed. In addition, the model was further refined to make sure that Exophiala can grow on carbon sources such as ethanol (Kumar, 2018), glucose (Poyntner et al., 2016, Chen et al., 2014), and sucrose (Dadachova et al., 2007) and to provide the opportunity to study metabolism, specifically pigment costs, under various different growth conditions. Once these objectives had been met, the resulting model was called the second draft Exophiala model. The second draft model had no TICs and consists of 1,591 reactions, of which 711 could carry flux and at best can produce 591 metabolites. For more details on the reconstruction of the first and second draft Exophiala models, see the Transparent Methods.
From Second Draft E. dermatitidis Model to iEde2091
The remainder of the set of enzymes common to three of four Aspergillus models was then converted to their metabolic functionalities (see Transparent Methods), for a total of 344 reactions, and used as a database for the application of OptFill to the second draft model of Exophiala. Unfortunately, the large number of reactions in the model and database, as well as the large number of potential TICs between database and model, required several iterations of performing OptFill and removing from the database reactions participating in the most TICs identified in the allotted solution time (1 week), until the database was reduced to 241 reactions, which allowed reasonable solution times (e.g., under 1 week to produce some gapfilling solutions). This procedure was repeated for the set of enzymes common to two of four Aspergillus models and to those unique to one model. This workflow is highlighted in Figure 1. In total, 43 reactions were added to the Exophiala model. This resulted in unblocking of a total of 82 reactions and 63 metabolites. Once each solution of this workflow was incorporated, the enzymes linked to filling solutions underwent a bidirectional BLASTp between reference Aspergillus sequences and the Exophiala genome, to determine the level of genomic support for these added reactions. This procedure was repeated for the set of enzymes common to two of four Aspergillus models and to the set of enzymes belonging to exactly one Aspergillus model. The resultant model was designated iEde2091. The iEde2091 model contains 1,661 reactions (of which 824 can carry flux as determined by Flux Variability Analysis), 1,856 metabolites, and 2,091 genes. The set of genes includes those used to build the first draft model (861 genes) and those related to added metabolic functionality from the full consensus of Aspergillus model enzymes (33 genes), the set of enzymes common to three of four Aspergillus models (21 genes), the set of enzymes common to two of four Aspergillus models (2 genes), and the set of enzymes unique to an Aspergillus model (18 genes).
Applications of the iEde2091 Model
The iEde2091 model was applied in two investigations. The first is the investigation of the shadow price of defensive pigments to better understand the costs and roles of the defensive pigments in polyextremotolerant systems. The second is the investigation of Exophiala melanin synthesis and comparison with that of humans to investigate the feasibility of using Exophiala as a model of human melanocytes.
Shadow Price of Defensive Pigments and Their Precursors under Various Growth Conditions
The iEde2091 model was subjected to 36 growth conditions based on the available carbon source (sucrose, ethanol, acetate, or glucose), growth-limiting nutrient (carbon, nitrogen, or sulfur), and rate at which that limiting nutrient was made available to the system (low, moderate, or high). In this study, the growth-limiting nutrient or atom was defined as the nutrient that controls the rate of growth through its scarceness, whereas all other nutrients or atoms are provided in at least three order of magnitude excess. The rate of availability of the growth-limiting nutrient to the organism is also arbitrary because no information appears to be published that would suggest biologically relevant uptake rates for E. dermatitidis. Shadow price is the change in the objective value of an optimization problem for one more unit of the desired product. As the model simulations were performed using the objective of maximizing biomass, all shadow prices are negative in value and should be compared against a baseline growth rate of approximately 0.104 h−1 for non-stressed Exophiala growth in nutrient-limited conditions (Dadachova et al., 2007), since, as can be seen in Table S4, the magnitude of the availability of the limiting resource and the growth rate have no effect on the shadow price. Table S4 shows that, under arbitrarily defined high, medium, and low growth-limiting nutrient availability conditions (corresponding to high, medium, and low growth rates), the shadow price is constant. This was chosen as a baseline for comparison with shadow prices derived from the iEde2091 model because no data are at present available to describe the rate of nutrient uptake by E. dermatitidis, which would enable the use of iEde2091 to estimate the growth rate. In the following analyses the per-atom rate of carbon uptake was standardized across the different carbon sources.
Carbon-Limited Conditions
Samples of shadow prices for melanins can be found in Figures 3A and 3B. In general, DHN-melanin is more expensive than eumelanin and pyomelanin both on a per-carbon basis and a per-monomer basis. The higher per-monomer cost of DHN-melanin is due to both the higher per-carbon cost and monomers being composed of ten carbons, as opposed to eight carbons for the other two types of melanin produced by Exophiala. As shown in Figures 3A and 3B, not all carbon sources are equally effective in the production of melanins. Generally, melanins are most expensive, in terms of shadow cost, to produce when Exophiala is grown using sucrose as a sole carbon source, with the exception of producing eumelanin using acetate as a sole carbon source. For all cases, as suggested by the shadow prices in Figures 3A and 3B, producing one additional mmol·gDW−1·h−1 of any melanin monomer would cause Exophiala to cease all growth, and even catabolize existing biomass to meet this demand.
Figure 3.
Shadow Prices of E. dermatitidis Pigments
This figure shows bar graphs of E. dermatitidis defensive pigment shadow prices under carbon-atom limited conditions, using four different carbon sources, on per-limited atom and per-unit basis.
(A) Per-carbon atom shadow costs of the three melanins producible by E. dermatitidis under various carbon-limited growth conditions.
(B) Per-monomer shadow costs of the three melanins producible by E. dermatitidis under various carbon-limited growth conditions.
(C) Per-carbon atom shadow costs of the three carotenoids that are modeled to constitute E. dermatitidis biomass under various carbon-limited growth conditions.
(D) Per-molecule shadow costs of the three carotenoids which are modeled to constitute E. dermatitidis biomass under various carbon-limited growth conditions.
In addition to investigating the pigments themselves, an investigation has been made into the shadow cost of precursor molecules to the pigments. Here, a precursor will be defined as molecules consumed by important enzymes related to pigment production or generally agreed upon as the metabolic branching point to pigment synthesis and all molecules “downstream” of that point. For instance, since tyrosinase is considered important in eumelanin synthesis, tyrosine and all molecules in eumelanin synthesis after tyrosine are considered eumelanin precursors. In this work, these pigment precursors have been included in Figures 2, 4, S1, and S2. With respect to the melanin precursors, per-carbon atom cost of the precursors is generally lower than that of the melanins that these produce. Furthermore, precursor per-carbon atom shadow price is generally consistent from the point at which melanin synthesis pathways branch from other metabolic pathways (the branchpoint being the starting point of the syntheses depicted in Figures 2 and 4). One example can be clearly seen in the DHN-melanin synthesis pathway with 1,3,6,8-tetrahydroxynaphthalene, scytalone, and 1,3,8-trihydroxynaphthalene all having the same shadow cost. This consistency is not seen in those molecules more proximal to core metabolism such as acetate, ATP, CTP, and requisite amino acids to produce these precursors (such as tyrosine and cysteine). The shadow cost of melanin pigments and their precursors is similar between ethanol and acetate growth conditions. This is because nearly the same set of reactions metabolize both these carbon sources, with the primary difference being the generation of two molecules of NADH in the catalysis of ethanol to acetate. This has no effect on the shadow price of molecules such as tyrosinase but has some effect in the shadow price of carotenoids (ethanol-grown E. dermatitidis has a lower shadow price for carotenoids, see Figures S1–S3). It can be noted that, in the shadow prices of melanins and their precursors, these molecules are generally cheaper to produce when grown on sucrose or glucose substrates. This is primarily due to the fact that the precursors to tyrosine synthesis, namely, d-erythrose-4-phosphate (with its own precursors of d-glyceraldehyde-3-phosphate and beta-d-fructose-6-phosphate) and phosphoenolpyruvate, are part of (or proximal to) the glycolysis/gluconeogenesis pathway. From sucrose or glucose, glycolysis is performed to produce these tyrosine precursors. On the other hand, from acetate and ethanol, gluconeogenesis is performed to produce these tyrosine precursors. Gluconeogenesis requires more energy than glycolysis; therefore, the shadow cost of tyrosine-derived pigments is greater for E. dermatitidis when grown on acetate or ethanol in comparison with growth on sucrose or glucose.
Figure 4.
Synthesis Pathways of Eumelanin and Pheomelanin in Humans and E. dermatitidis
This figure shows the synthesis pathways of eumelanin and pheomelanin including chemical structures, reaction stoichiometries, catalyzing Enzyme Commission (EC) number, and reaction cofactors in humans (brown and blue arrows) and E. dermatitidis (blue arrows). The major difference between these species' eumelanin synthesis pathways is the presence of tyrosine-related proteins (TYRPs) in humans that catalyze the reactions indicated by brown arrows. In both species, the key initiating enzyme is tyrosinase, Enzyme Commission 1.14.18.1, which catalyzes the initial steps of eumelanin synthesis. A deficiency in tyrosinase activity may result in oculocutaneous albinism A1 in humans. The second type of human melanin, pheomelanin, is largely produced by spontaneous reactions beyond the tyrosinase-catalyzed production of dopaquinone. The branching of eumelanin and pheomelanin production is accomplished by the presence or absence of cysteine where dopaquinone is concentrated. This suggests that pheomelanin may be inducible in E. dermatitidis.
The per-carbon atom shadow prices of the three carotenoids that are a part of Exophiala biomass as modeled in iEde2091, namely, beta carotene, β-apo-4′-carotenal, and neurosporaxanthin, are approximately equivalent; see Figures 3C and 3D. Synthesis pathways used by E. dermatitidis to produce carotenoids, as well as the shadow prices of carotenoid precursors, can be found in Figures S1–S3. As the per-carbon atom shadow costs are approximately equivalent, the per-molecule differences in shadow cost are due to the difference in number of carbon atoms in the carotenoid molecules, as beta carotene contains 40 carbon atoms, whereas the other two carotenoid compounds contain 35 carbon atoms. Essentially, carotenoids are more expensive for the cell to produce than are melanins on a per-carbon atom basis.
Nitrogen- and Sulfur-Limited Conditions
The nitrogen source used by the model is ammonia and, as with the carbon-limited conditions, the availability of the growth-limiting nutrient has no effect on shadow cost. In this analysis, metabolites that do not contain nitrogen, including DHN melanin, pyomelanin, and all three investigated carotenoids, have no shadow cost under nitrogen-limited conditions. This makes sense in that all other atoms are provided to the system in excess; therefore, utilizing more of those excess atoms would not hamper biomass production. As such, the only melanin compound that has a shadow cost in these conditions is eumelanin, whose monomers contain a single nitrogen. In nitrogen-limited conditions, the per-nitrogen atom shadow cost is approximately 41 times higher than that of the per-carbon atom cost. The reasons for this are likely 2-fold. First, far less nitrogen is needed by Exophiala to produce biomass than carbon (approximately 9.1:1 C:N in the biomass pseudomolecule). Second, not all nitrogen uptaken can be used by Exophiala and utilization of nitrogen is less efficient than utilization of carbon. For instance, waste nitrogen is excreted in a nitrogen compound containing four nitrogen atoms (in urate), as opposed to the majority of waste carbon being expelled as carbon dioxide.
Similarly, in the cases where sulfur is the nutrient limiting model growth, compounds that contain no sulfur atoms have no shadow cost, including all defensive pigments studied. Therefore, only melanin precursors have a shadow cost under these conditions, which includes coenzyme A (CoA), its precursors, and all molecules containing CoA such as malonyl-CoA and acetyl-CoA. These compounds have relatively high per-sulfur atom shadow costs, of −28.73 h−1, since each mole of the biomass pseudomolecule contains approximately 0.035 sulfur atoms, indicating that the sulfur needs of Exophiala are very low. Therefore, to produce one extra mmol·gDW−1·h−1 of a sulfur-containing compound, a large amount of biomass would need to be catabolized.
Comparison of Human and E. dermatitidis Melanin Synthesis
The melanin synthesis pathway of Exophiala and humans was compared in two ways: first, by the series of reactions that produce human melanins (namely, pheomelanin and eumelanin, see Figure 4), and second, by comparison of the tyrosinase enzymes (see Figure 5).
Figure 5.
Tyrosinase and Tyrosinase-Related Protein Sequence Alignments Between Humans and E. dermatitidis
This figure shows portions of the sequence alignments performed by NCBI's COBALT tool using the amino acid sequences of human tyrosinase-related protein 1 (Has_TYRP1, accession NP_000541.1), 2 (Has_TYRP2, accession NP_01913.2), a reference allele human tyrosinase sequence (Has_ref, accession AAK00805.1), an oculocutaneous albinism A1 allele (Has_alb, accession EAW59356.1), an allele from an individual of the Bantu peoples (Has_ban, accession AGV39054.1), and reference sequences for the four tyrosinase gene copies of E. dermatitidis (Ede_un1, accession XP_009160170.1; Ede_un2, accession XP_009156893.1; Ede_co1, accession XP_009157733.1; and Ede_co2, accession XP_009155657.1). The portions of the alignments shown concern the two parts of the active site of tyrosinase, Copper-Binding Domains A and B (CuA and CuB, respectively). It is shown that all amino acid residues thought to be critical to active site function (key residues) are highly conserved between the two species (García-Borrón and Solano, 2002). Furthermore, many sites where amino acid substitutions are associated with oculocutaneous albinism A1 (residues boxed in black) are conserved between species. Also shown is the Multiple Sequence Alignment (MSA) view, which shows that the active sites and many sequences between the active sites are preserved between the aligned sequences. This further shows that the large differences in sequence lengths between genes are largely due to sequences flanking the active sites.
In building the iEde2091 model, we recognized that fungal melanins are typically transported in exocytic vesicles to the cell surface, where they are then attached to the cell wall (Camacho et al., 2019, Upadhyay et al., 2016). This pathway shares features with that observed in melanocytes, whereby synthesis occurs in specialized melanosomes. Moreover, the Exophiala and human pathways to produce the indole-5,6-quinone monomer of eumelanin are identical. Furthermore, the production of pheomelanin in humans appears replicable in Exophiala should cysteine be added to the extracellular environment. The 5,6-indolequinone-2-carboxilic acid eumelanin monomer is not producible by Exophiala owing to its lack of a tyrosinase-related protein. In investigating the potential for Exophiala to produce pheomelanin, the shadow price of cysteine was also investigated. With respect to carbon-limited conditions (see Figure 4), cysteine is more expensive than most other precursors on a per-carbon basis, particularly in cases of growth on sucrose and glucose. With respect to nitrogen-limited growth cases (see Table S4), cysteine is very similar in cost to other amino acids. With respect to sulfur-limited growth cases (see Table S4), the per-sulfur atom cost is high (around 28 h−1) and is similar in cost to coenzyme A.
The four tyrosinase gene copies in E. dermatitidis where identified through genome annotation. Furthermore, these sequences were used as a BLASTp query against the E. dermatitidis genome to confirm that these four were the only tyrosinase gene copies in E. dermatitidis. A non-redundant BLASTp analysis was performed by using the tyrosinase amino acid sequences of Exophiala as the search sequence against the human genome to determine the sequence similarity. This produced no matches of acceptable expect value (e.g., less than 1E-10), indicating large sequence dissimilarities. However, a COBALT alignment (Papadopoulos and Agarwala, 2007) of the amino acid sequences of three human tyrosinase alleles, human tyrosinase-related proteins, and the four gene copies of Exophiala produces more nuanced results. Tyrosinase-related proteins (TYRPs) have the same evolutionary origin as tyrosinase and are still very similar and were therefore included in this analysis (Furumura et al., 1998). The major catalytic difference between TYRPs and tyrosinases is that they act upon L-Dopachrome differently, one producing 5,6-indolequinone-2-carboxylic acid eumelanin monomers and the other producing indole-5,6-quinone eumelanin monomers. Portions of this alignment, namely, the sequences related to the copper-binding domains A (CuA) and B (CuB) that constitute the active side of tyrosinase, are shown in Figure 5 using the 3-bit highlighting method. This method highlights in red aligned residues that have the same or very similar chemical structure, in blue somewhat conserved regions, and in gray unconserved regions. When highlighting key structural (orange squares located above the residue number), functional (blue squares), and active site (purple squares) residues, it appears that these key residues are highly conserved between human tyrosinase-related proteins and tyrosinase and Exophiala tyrosinases. Poor BLASTp alignment scores appear to be due to substitutions, deletions, or lack of sequence conservation of non-critical residues, gaps in less critical regions of tyrosinase (such as residues that are not a part of secondary structures, such as the gap in CuA), and significant differences in enzyme length. This is shown in the Multiple Sequence Alignment (MSA) view shown in Figure 5. As an example of the length differences, human tyrosinase has a primary structure of 529 amino acids, and tyrosinase-related proteins 1 and 2 have structures of 537 and 519 amino acids, respectively, whereas Exophiala tyrosinase lengths range from 381 to 614 amino acids. Much of the differences in length are in those sequences upstream of CuA and downstream of CuB; see the sequence identity summary shown in Figure 5. Interestingly, Figure 5 highlights residue mutations that trigger the switch between tyrosinase and tyrosinase-related proteins (green squares at 214, 219, 389, and 393 [García-Borrón and Solano, 2002]). In some gene copies of Exophiala tyrosinase, particularly the copy labeled as “Ede_un1,” key residues that when mutated cause the switch between tyrosinase and tyrosinase-related protein are particularly well conserved, suggesting that Exophiala could be engineered to have a tyrosinase-related protein. Should this additional monomer synthesis pathway be engineered in Exophiala, through gene insertion or selective mutation, the melanin synthesis pathways between Exophiala and humans could be very similar. In addition to this analysis, a sequence alignment analysis to the Hidden Markov Model (HMM) using the Pfam tool (El-Gebali et al., 2019) was performed. This tool acknowledged the strong sequence similarity of E. dermatitidis tyrosinase enzymes with that of the general pattern of tyrosinase enzymes. The results of this analysis can be found in Data S1.
In considering the uses of Exophiala as a model system of human melanin production, some amino acid residue positions where residue substitutions are associated with oculocutaneous albinism A1 (OCA1), which accounts for approximately 50% of cases of albinism worldwide and is caused by a non-functional tyrosinase in humans (Kamaraj and Purohit, 2014), are shown in black rectangles (Spritz, 1994) to highlight the potential for Exophiala as a model system to study OCA1.
Discussion
In this work, a stoichiometric GSM of E. dermatitidis (iEde2091) consisting of 1,661 reactions, 1,856 metabolites, and 2,091 genes was developed in order to investigate Exophiala as a potential model organism for extremotolerant fungi and human melanocytes. Several issues were encountered in the metabolic reconstruction. First, the low levels of genome annotation (43% annotated but less than 5% with associated enzyme commission numbers) represented knowledge gaps in the understanding of Exophiala metabolism that lead to many gaps and blocked reactions throughout the stages of reconstruction. This was dealt with by using four metabolic models from the related Aspergillus genus (Andersen et al., 2008, David et al., 2008, Vongsangnak et al., 2008, Liu et al., 2013) in addition to the OptFill tool (Schroeder and Saha, 2020) for TIC-free gapfilling of models; see Figure 1. The low levels of genome annotation also hindered the ability to create GPR links, which was addressed by using Aspergillus protein sequences as enzyme reference sequences for use in BLASTp analyses. This resulted in a large number of previously annotated genes being linked with enzyme commission numbers and the functional identification of four sequences that may not yet have been identified.
In the shadow price investigation of melanins (Figure 3), it was noted that DHN-melanin has a higher per-unit cost than other melanins. This appears to be due simply to the larger number of carbon molecules present in each monomer unit when compared with other melanins (see Figures 2 and 4). Furthermore, the difference between DHN-melanin, eumelanin, and pyomelanin in media where sucrose is the limited carbon source is that the latter two are synthesized from l-tyrosine, whereas DHN-melanin is synthesized from malonyl-CoA. The higher shadow price appears to be due to the higher per-carbon atom cost to produce acetate from sucrose, which is perpetuated through the DHN-synthesis pathway. As shown in Figure 3, both melanin and carotenoid pigments are “cheapest” to produce in carbon-limited cases when glucose is the carbon source. This is due to the lack of preprocessing needed (e.g., other carbon sources may require gluconeogenesis or other metabolic transformations before being shunted to major energy-harvesting pathways).
The changing shadow prices for these defensive pigments under different growth conditions suggest that the profile of pigments (i.e., the type and quantity of defensive pigments) as produced by Exophiala varies by nutrient availability. In other words, the “cheaper” defensive pigments may be produced more than the expensive pigments. Having a range of defensive pigments (e.g., three melanin types and various carotenoids) with differing synthesis pathways makes them to be more or less expensive depending on available nutrients. This, in turn, may help minimize the cost of the extremotolerant nature of Exophiala by allowing the organism to preferentially produce the least expensive defensive pigment(s). The relatively high fractions of biomass accounted for by defensive pigments, 1.3 wt% for melanin (Geis, 1981) and 3.5 wt% for carotenoids (Strobel et al., 2009), as well as their high shadow prices suggest that these pigments are continually produced and stockpiled because increasing production of these pigments to meet cell need if the environment were to quickly become extreme is untenable.
The higher per-carbon shadow prices of carotenoids compared with melanins might help to expand the current understanding of the role of carotenoids. First, carotenoids are a secondary line of defense against external extreme conditions as they are deposited in the cell membrane (Chen et al., 2014, Kumar, 2018, whereas melanins are deposited in the cell wall (Chen et al., 2014, Geis, 1981, Szaniszlo, 2002). Second, melanins are known to provide protection against antifungal and antimicrobial compounds (Nosanchuk and Casadevall, 2006, Paolo et al., 2006, Toledo et al., 2017); lytic enzymes (Paolo et al., 2006); heat and cold stress (Paolo et al., 2006, Toledo et al., 2017); rapid freezing (Paolo et al., 2006); ionizing radiation (Kumar, 2018, Dadachova et al., 2007); oxidative stress (Toledo et al., 2017); UV radiation (Toledo et al., 2017); heavy metals (Kumar, 2018, Singh et al., 2013); light (Chen et al., 2014); and immune responses (Chen et al., 2014). Furthermore, several genes related to both eumelanin and DHN-melanin synthesis are upregulated under low pH stress, suggesting that melanins are also produced under pH stress (Chen et al., 2014). At present, it is known that carotenoids protect against stress conditions such as oxidative stress (such as free radicals) (Kumar, 2018, Avalos and Carmen Limón, 2015, Strobel et al., 2009), UV radiation (Avalos and Carmen Limón, 2015, Geis and Szaniszlo, 1984, Kumar, 2018, Strobel et al., 2009), and light (Kumar, 2018, Avalos and Carmen Limón, 2015, Strobel et al., 2009). Each function of carotenoids is already accounted for by melanins. It has been suggested that carotenoids do not play a physiological role in fungi, but rather function as precursors to the synthesis of other biomolecules (Avalos and Carmen Limón, 2015). However, this appears inconsistent with their higher shadow cost in comparison with melanin compounds, which can accomplish the same functions with deposition in the cell membrane and high weight fraction in some fungal species (Strobel et al., 2009). Although several previous works hinted about the possibility of carotenoids having unexplored functions in fungi (Chen et al., 2014, Avalos and Carmen Limón, 2015), this is the first study that provides a computational and systems biology perspective. One study has postulated that perhaps carotenoids protect against light that passes through the melanin in the cell wall (Chen et al., 2014). This seems a likely function as melanin absorbance of electromagnetic radiation is high in the UV spectrum to approximately 400 nm in wavelength and exponentially declines in the wavelength range of 400–500 nm (Mahmood et al., 2015, Ou-Yang et al., 2004), whereas this latter range constitutes the peak absorbance of carotenoids (Yamamoto and Bangham, 1978, Zaghdoudi et al., 2017). Thus, the combination of these two pigments would protect Exophiala cell from the UV spectrum through higher-energy visible light (namely, violet and blue light). The high cost of producing carotenoids along with high fraction of cell weight does suggest that the violet and/or blue light is particularly disruptive to some high-value metabolic process in Exophiala, which should be further investigated.
In exploring the suitability of Exophiala as a model organism for human melanocytes, the sequence alignment results of Exophiala and human tyrosinase enzymes show that CuB is the best-conserved portion of tyrosinase active site, through all key amino acids, and therefore likely the essential structures of CuA is also preserved. As tyrosinase is the key enzyme in eumelanin synthesis in both E. dermatitidis and human, several residues associated with OCA1 are persevered between the species. Since Exophiala has a significantly smaller genome (26.4 Mb compared with 3253.8 Mb for human), Exophiala may be used as a model system for human eumelanin production. As OCA1 is the most prevalent type of albinism worldwide, Exophiala may be used as a model system for studying causal mechanisms of OCA1 and potentially to identify treatment options. Unfortunately, African populations, where albinism is a more pressing social and health problem (Brilliant, 2015), would benefit less from Exophiala eumelanin studies, than Caucasian and Asian populations, as approximately 77% of albinism cases in African populations result from oculocutaneous albinism A2 (OCA2), with most of the remainder being attributed to OCA1. OCA2 is a result of the lack of a tyrosinase transporter proteins, called P protein, which is necessary to transport tyrosinase into human melanosomes (a subcellular compartment dedicated to melanin synthesis in melanocytes) and/or stabilize tyrosinase (Kamaraj and Purohit, 2014), which was not identifiable through in silico methods in Exophiala, such as through BLASTp or annotated genomes. Therefore, further study of Exophiala is warranted to3 identify this transporter protein and improve the potential for Exophiala as a model system.
Furthermore, it was determined that one type of melanin produced by human is not produced by Exophiala. Pheomelanin is a red-brown to yellow pigment (Ito and Wakamatsu, 2011), and it is likely that Exophiala could produce this type of melanin. No additional enzymes are needed to produce pheomelanin beyond that which Exophiala already possesses (see Figure 4), rather free cysteine in the location of eumelanin synthesis is required (Ito, 2003). Growing Exophiala in a cysteine-rich culture or engineering a cysteine pump to the extracellular space of Exophiala could result in pheomelanin production, allowing production of both human melanin types. Alternatively, if Exophiala were to provide the cysteine for pheomelanin synthesis, given its high shadow price and the shadow price of dopaquinone, it is reasonable to hypothesize that the resultant pheomelanin would be the costliest melanin produced by E. dermatitidis. This is perhaps why E. dermatitidis does not natively produce pheomelanin.
Overall, the results of this work suggest several potential interesting in vivo follow-up studies that will increase our understanding of extremotolerant fungi using Exophiala as a model system. Key predictions arising from the iEde2091 model that are currently being tested include assessing the effects of different carbon sources on melanin and carotenoid accumulation, as well as determining the effects of mutations that abrogate specific metabolic pathways on pigment production. In addition, phenotypic profiling of mutants defective in melanin and/or carotenoid synthesis is underway to better evaluate the roles of each pigment in stress tolerance. Although detailed in vivo investigation may be needed to further establish Exophiala as a potential model organism for human melanocytes including demonstrating the production of pheomelanin, this work attempts to enable a broader understanding of melanin production across kingdoms.
Limitations of the Study
The major limitation of this study is that no in vivo experiments accompany this work; therefore, this work focuses on an in silico systems level analysis of metabolism. Furthermore, a number of reactions in the model, approximately 50%, cannot carry flux. This limitation is due in large part to lack of system knowledge that this work was not entirely able to address.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work has been completed utilizing the Holland Computing Center of the University of Nebraska, which receives support from the Nebraska Research Initiative (United States of America). The authors gratefully acknowledge funding from University of Nebraska-Lincoln (United States of America) Faculty Startup Grant to R.S.. S.D.H. wishes to acknowledge support from the Natural Sciences and Engineering Research Council of Canada (NSERC) of Canada Discovery Grants program (Canada).
Author Contributions
Conceptualization, W.L.S., S.D.H., and R.S.; Data curation, W.L.S.; Formal analysis, W.L.S.; Funding Acquisition, R.S.; Investigation, W.L.S.; Methodology, W.L.S.; Project administration, R.S.; Resources, R.S. and S.D.H.; Software, W.L.S.; Supervision, R.S.; Validation, W.L.S.; Visualization, W.L.S.; Writing – original draft, W.L.S. and R.S.; Writing – reviewing & editing – W.L.S., S.D.H., and R.S.
Declaration of Interests
The authors declare no competing interests.
Published: April 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.100980.
Data and Code Availability
The published article does not include all datasets and code generated or analyzed during this study. The datasets and code generated during this study are available at GitHub in the ssbio/OptFill repository (https://doi.org/10.5281/zenodo.3608172).
Supplemental Information
This Microsoft Excel workbook contains CELLO Predictions of E. dermatitidis enzyme subcellular localization as well as KEGG reactions and KEGG-defined stoichiometry. A table of contents is included to help readers navigate this supplemental file.
This Microsoft Excel workbook contains various data sources used to build the draft models of E. dermatitidis. This includes E. dermatitidis biomass information (including growth rates), sources for the Aspergillus models used, Aspergillus model stoichiometries, overlap summaries between Aspergillus model EC numbers, E. dermatitidis genome annotations, and OptFill results.
The results of BLASTs of Aspergillus sequences linked to enzyme numbers added to the E. dermatitidis models at various stages in the model-building process, in addition to OptFill results used to curate the models.
This Microsoft Excel workbook contains bar graphs showing the shadow price of various metabolites in the iEde2091 model. Many of these shadow prices are given on both a per-metabolite and a per-limiting atom basis.
References
- Altschul S.F., Madden T.L., Schäffer A.A., Zhang J., Zhang Z., Miller W., Lipman D.J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S.F., Wootton J.C., Gertz E.M., Agarwala R., Morgulis A., Schäffer A.A., Yu Y.K. Protein database searches using compositionally adjusted substitution matrices. FEBS J. 2005;272:5101–5109. doi: 10.1111/j.1742-4658.2005.04945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen M.R., Nielsen M.L., Nielsen J. Metabolic model integration of the bibliome, genome, metabolome and reactome of Aspergillus niger. Mol. Syst. Biol. 2008;4:178. doi: 10.1038/msb.2008.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avalos J., Carmen Limón M. Biological roles of fungal carotenoids. Curr. Genet. 2015;61:309–324. doi: 10.1007/s00294-014-0454-x. [DOI] [PubMed] [Google Scholar]
- Brilliant M.H. Albinism in Africa: a medical and social emergency. Int. Health. 2015;7:223–225. doi: 10.1093/inthealth/ihv039. [DOI] [PubMed] [Google Scholar]
- Burgard A.P., Nikolaev E.V., Schilling C.H., Maranas C.D. Flux coupling analysis of genome-scale metabolic network reconstructions. Genome Res. 2004;14:301–312. doi: 10.1101/gr.1926504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgard A.P., Pharkya P., Maranas C.D. OptKnock: a bilevel programming framework for identifying gene knockout strategies for microbial strain optimization. Biotechnol. Bioeng. 2003;84:647–657. doi: 10.1002/bit.10803. [DOI] [PubMed] [Google Scholar]
- Camacho E., Vij R., Chrissian C., Prados-Rosales R., Gil D., O'Meally R.N., Cordero R.J.B., Cole R.N., McCaffery J.M., Stark R.E., Casadevall A. The structural unit of melanin in the cell wall of the fungal pathogen Cryptococcus neoformans. J. Biol. Chem. 2019;294:10471–10489. doi: 10.1074/jbc.RA119.008684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Martinez D.A., Gujja S., Sykes S.M., Zeng Q., Szaniszlo P.J., Wang Z., Cuomo C.A. Comparative genomic and transcriptomic analysis of Wangiella dermatitidis, a major cause of phaeohyphomycosis and a model black yeast human pathogen. G3 (Bethesda, Md) 2014;4:561–578. doi: 10.1534/g3.113.009241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung C.Y.M., Williams T.C., Poolman M.G., Fell D.A., Ratcliffe R.G., Sweetlove L.J. A method for accounting for maintenance costs in flux balance analysis improves the prediction of plant cell metabolic phenotypes under stress conditions. Plant J. 2013;75:1050–1061. doi: 10.1111/tpj.12252. [DOI] [PubMed] [Google Scholar]
- Chowdhury R., Chowdhury A., Maranas C.D. Using gene essentiality and synthetic lethality information to correct yeast and CHO cell genome-scale models. Metabolites. 2015;5:536–570. doi: 10.3390/metabo5040536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer G.R., Urano K., Delrot S., Pezzotti M., Shinozaki K. Effects of abiotic stress on plants: a systems biology perspective. BMC Plant Biol. 2011;11:163. doi: 10.1186/1471-2229-11-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadachova E., Bryan R.A., Huang X., Moadel T., Schweitzer A.D., Aisen P., Nosanchuk J.D., Casadevall A. Ionizing radiation changes the electronic properties of melanin and enhances the growth of melanized fungi. PLoS One. 2007;2:e457. doi: 10.1371/journal.pone.0000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David H., Ozçelik I.S., Hofmann G., Nielsen J. Analysis of Aspergillus nidulans metabolism at the genome-scale. BMC Genomics. 2008;9:163. doi: 10.1186/1471-2164-9-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman H.C., Casadevall A. Synthesis and assembly of fungal melanin. Appl. Microbiol. Biotechnol. 2012;93:931–940. doi: 10.1007/s00253-011-3777-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Gebali S., Mistry J., Bateman A., Eddy S.R., Luciani A., Potter S.C., Qureshi M., Richardson L.J., Salazar G.A., Smart A. The Pfam protein families database in 2019. Nucleic Acids Res. 2019;47:D427–D432. doi: 10.1093/nar/gky995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exophiala dermatitidis (strain ATCC34100/CBS 525.76/NIH/UT8656) (2018) UniProt2.
- Exophiala dermatitidis NIH/UT8656 Genome. Assembly NCBI. 2011 https://www.ncbi.nlm.nih.gov/assembly/GCF_000230625.1 [Google Scholar]
- Feist A.M., Palsson B. The growing scope of applications of genome-scale metabolic reconstructions using Escherichia coli. Nat. Biotechnol. 2008;26:659–667. doi: 10.1038/nbt1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furumura M., Solano F., Matsunaga N., Sakai C., Spritz R.A., Hearing V.J. Metal ligand-binding specificities of the tyrosinase-related proteins. Biochem. Biophys. Res. Commun. 1998;242:579–585. doi: 10.1006/bbrc.1997.8007. [DOI] [PubMed] [Google Scholar]
- García-Borrón J.C., Solano F. Molecular anatomy of tyrosinase and its related proteins: beyond the histidine-bound metal catalytic center. Pigment Cell Res. 2002;15:162–173. doi: 10.1034/j.1600-0749.2002.02012.x. [DOI] [PubMed] [Google Scholar]
- Geis P.A. University of Texas at Austin; 1981. Chemical Composition of the Yeast and Sclerotic Cell Walls of Wangiella dermatitidis. [Google Scholar]
- Geis P.A., Szaniszlo P.J. Carotenoid pigments of the dematiaceous fungus Wangiella dermatitidis. Mycologia. 1984;76:268–273. doi: 10.1007/BF00410729. [DOI] [PubMed] [Google Scholar]
- Gudmundsson S., Agudo L., Nogales J. Elsevier Ltd; 2017. Applications of Genome-Scale Metabolic Models of Microalgae and Cyanobacteria in Biotechnology, Microalgae-Based Biofuels and Bioproducts: From Feedstock Cultivation to End-Products. [Google Scholar]
- Gudmundsson S., Thiele I. Computationally efficient flux variability analysis. BMC Bioinformatics. 2010;11:2–4. doi: 10.1186/1471-2105-11-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S. IFPCS presidential lecture: a chemist’s view of melanogenesis. Pigment Cell Res. 2003;16:230–236. doi: 10.1034/j.1600-0749.2003.00037.x. [DOI] [PubMed] [Google Scholar]
- Ito S., Wakamatsu K. Diversity of human hair pigmentation as studied by chemical analysis of eumelanin and pheomelanin. J. Eur. Acad. Dermatol. Venereol. 2011;25:1369–1380. doi: 10.1111/j.1468-3083.2011.04278.x. [DOI] [PubMed] [Google Scholar]
- Kamaraj B., Purohit R. Mutational analysis of oculocutaneous albinism: a compact review. Biomed. Res. Int. 2014;2014:905472. doi: 10.1155/2014/905472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodayari A., Chowdhury A., Maranas C.D. Succinate overproduction: a case study of computational strain design using a comprehensive Escherichia coli kinetic model. Front. Bioeng. Biotechnol. 2015;2:76. doi: 10.3389/fbioe.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar J. Adaptations of Exophiala dermatitidis in Stressful Environments. ETD collection for University of Nebraska - Lincoln; 2018. https://digitalcommons.unl.edu/dissertations/AAI10792984 AAI10792984. [Google Scholar]
- Lin P.C., Saha R., Zhang F., Pakrasi H.B. Metabolic engineering of the pentose phosphate pathway for enhanced limonene production in the cyanobacterium Synechocysti s sp. PCC 6803. Sci. Rep. 2017;7:17503. doi: 10.1038/s41598-017-17831-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Gao Q., Xu N., Liu L. Genome-scale reconstruction and in silico analysis of Aspergillus terreus metabolism. Mol. BioSyst. 2013;9:1939–1948. doi: 10.1039/c3mb70090a. [DOI] [PubMed] [Google Scholar]
- Magnúsdóttir S., Heinken A., Kutt L., Ravcheev D.A., Bauer E., Noronha A., Greenhalgh K., Jäger C., Baginska J., Wilmes P. Generation of genome-scale metabolic reconstructions for 773 members of the human gut microbiota. Nat. Biotechnol. 2016;35:81–89. doi: 10.1038/nbt.3703. [DOI] [PubMed] [Google Scholar]
- Mahmood Alaa, Mohammed Huda, Flayyih May. Purification and Physoichemical Characteristics of Pyomelanin Pigment Produced from Local Pseudomona aeruginosa Isolates. World Journal of Pharmaceutical Research. 2015;4:289–299. [Google Scholar]
- Maranas C.D., Zomorrodi A.R. Wiley; 2016. Optimization Methods in Metabolic Networks. [Google Scholar]
- Moreno L.F., Vicente V.A., de Hoog S. Black yeasts in the omics era: Achievements and challenges. Med. Mycol. 2018;56:32–41. doi: 10.1093/mmy/myx129. [DOI] [PubMed] [Google Scholar]
- Nosanchuk J.D., Casadevall A. Impact of melanin on microbial virulence and clinical resistance to antimicrobial compounds. Antimicrob. Agents Chemother. 2006;50:3519–3528. doi: 10.1128/AAC.00545-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkusu M., Yamaguchi K., Hata K., Yoshida S., Tanaka R., Nishimura K., de Hoog G.S., Takeo K. Cellular and nuclear characteristics of Exophiala dermatitidis. Stud. Mycol. 1999;43:143–150. [Google Scholar]
- Orth J.D., Thiele I., Palsson B.O. What is flux balance analysis? Nat. Biotechnol. 2010;28:245–248. doi: 10.1038/nbt.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou-Yang H., Stamatas G., Kollias N. Spectral responses of melanin to ultraviolet a irradiation. J. Invest. Dermatol. 2004;122:492–496. doi: 10.1046/j.0022-202X.2004.22247.x. [DOI] [PubMed] [Google Scholar]
- Paolo W.F., Dadachova E., Mandal P., Casadevall A., Szaniszlo P.J., Nosanchuk J.D. Effects of disrupting the polyketide synthase gene WdPKS1 in Wangiella [Exophiala] dermatitidis on melanin production and resistance to killing by antifungal compounds, enzymatic degradation, and extreme temperature. BMC Microbiol. 2006;6:55. doi: 10.1186/1471-2180-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos J.S., Agarwala R. COBALT: Constraint-based alignment tool for multiple protein sequences. Bioinformatics. 2007;23:1073–1079. doi: 10.1093/bioinformatics/btm076. [DOI] [PubMed] [Google Scholar]
- Poyntner C., Blasi B., Arcalis E., Mirastschijski U., Sterflinger K., Tafer H. The transcriptome of Exophiala dermatitidis during ex-vivo skin model infection. Front. Cell Infect. Microbiol. 2016;6:136. doi: 10.3389/fcimb.2016.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaler-Ripcke J., Sugareva V., Gebhardt P., Winkler R., Kniemeyer O., Heinekamp T., Brakhage A.A. Production of pyomelanin, a second type of melanin, via the tyrosine degradation pathway in Aspergillus fumigatus. Appl. Environ. Microbiol. 2009;75:493–503. doi: 10.1128/AEM.02077-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch C.L., Sung G.H., López-Giráldez F., Townsend J.P., Miadlikowska J., Hofstetter V., Robbertse B., Matheny P.B., Kauff F., Wang Z. The ascomycota tree of life: a phylum-wide phylogeny clarifies the origin and evolution of fundamental reproductive and ecological traits. Syst. Biol. 2009;58:224–239. doi: 10.1093/sysbio/syp020. [DOI] [PubMed] [Google Scholar]
- Schroeder W.L., Saha R. OptFill: a tool for infeasible cycle-free gapfilling of stoichiometric metabolic models. iScience. 2020;23:100783. doi: 10.1016/j.isci.2019.100783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Malhotra A.G., Pandey A., Pandey K.M. Computational model for pathway reconstruction to unravel the evolutionary significance of melanin synthesis. Bioinformation. 2013;9:94–100. doi: 10.6026/97320630009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano F. Melanins: skin pigments and much more—types, structural models, biological functions, and formation routes. New J. Sci. 2014;2014:1–28. [Google Scholar]
- Spritz Richard. Molecular genetics of oculocutaneous albinism. Human Molecular Genetics. 1994;3(Review):1469–1475. doi: 10.1093/hmg/3.suppl_1.1469. [DOI] [PubMed] [Google Scholar]
- Stolyar S., Van Dien S., Hillesland K.L., Pinel N., Lie T.J., Leigh J.A., Stahl D.A. Metabolic modeling of a mutualistic microbial community. Mol. Syst. Biol. 2007;3:92. doi: 10.1038/msb4100131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel I., Breitenbach J., Scheckhuber C.Q., Osiewacz H.D., Sandmann G. Carotenoids and carotenogenic genes in Podospora anserina: engineering of the carotenoid composition extends the life span of the mycelium. Curr. Genet. 2009;55:175–184. doi: 10.1007/s00294-009-0235-0. [DOI] [PubMed] [Google Scholar]
- Sudhadham M., Prakitsin S., Sivichai S., Chaiyarat R., Dorrestein G.M., Menken S.B., de Hoog G.S. The neurotropic black yeast Exophiala dermatitidis has a possible origin in the tropical rain forest. Stud. Mycol. 2008;61:145–155. doi: 10.3114/sim.2008.61.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaniszlo P.J. Molecular genetic studies of the model dematiaceous pathogen Wangiella dermatitidis. Int. J. Med. Microbiol. 2002;292:381–390. doi: 10.1078/1438-4221-00221. [DOI] [PubMed] [Google Scholar]
- Thiele I., Palsson B.Ø. A protocol for generating a high-quality genome-scale metabolic reconstruction. Nat. Protoc. 2010;5:93–121. doi: 10.1038/nprot.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo Andrea Vanesa, Franco Mario Emilio Ernesto, Lopez Silvina Marianela Yanil, Troncozo Maria Ines, Saparrat Mario Carlos Nazareno, Balatti Pedro Alberto. Melanins in fungi: types, localization and putative biological roles. Physiol. Mol. Plant Pathol. 2017;99:2–6. [Google Scholar]
- Upadhyay S., Xu X., Lowry D., Jackson J.C., Roberson R.W., Lin X. Subcellular compartmentalization and trafficking of the biosynthetic machinery for fungal melanin. Cell Rep. 2016;14:2511–2518. doi: 10.1016/j.celrep.2016.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vongsangnak W., Olsen P., Hansen K., Krogsgaard S., Nielsen J. Improved annotation through genome-scale metabolic modeling of Aspergillus oryzae. BMC Genomics. 2008;9:245. doi: 10.1186/1471-2164-9-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T.C.R., Poolman M.G., Howden A.J., Schwarzlander M., Fell D.A., Ratcliffe R.G., Sweetlove L.J. A genome-scale metabolic model accurately predicts fluxes in central carbon metabolism under stress conditions. Plant Physiol. 2010;154:311–323. doi: 10.1104/pp.110.158535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H.Y., Bangham A.D. Carotenoid organization in membranes. Thermal transition and spectral properties of carotenoid-containing liposomes. Biochim. Biophys. Acta. 1978;507:119–127. doi: 10.1016/0005-2736(78)90379-6. [DOI] [PubMed] [Google Scholar]
- Yizhak K., Chaneton B., Gottlieb E., Ruppin E. Modeling cancer metabolism on a genome scale. Mol. Syst. Biol. 2015;11:817. doi: 10.15252/msb.20145307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C., Lin C. Predicting subcellular localization of proteins for Gram-negative bacteria by support vector machines based on n -peptide compositions. Protein Sci. 2004;13:1402–1406. doi: 10.1110/ps.03479604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaghdoudi K., Ngomo O., Vanderesse R., Arnoux P., Myrzakhmetov B., Frochot C., Guiavarc'h Y. Extraction, identification and photo-physical characterization of Persimmon (Diospyros kaki L.) carotenoids. Foods. 2017;6:4. doi: 10.3390/foods6010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Tervo C.J., Reed J.L. Metabolic assessment of E. coli as a Biofactory for commercial products. Metab. Eng. 2016;35:64–74. doi: 10.1016/j.ymben.2016.01.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This Microsoft Excel workbook contains CELLO Predictions of E. dermatitidis enzyme subcellular localization as well as KEGG reactions and KEGG-defined stoichiometry. A table of contents is included to help readers navigate this supplemental file.
This Microsoft Excel workbook contains various data sources used to build the draft models of E. dermatitidis. This includes E. dermatitidis biomass information (including growth rates), sources for the Aspergillus models used, Aspergillus model stoichiometries, overlap summaries between Aspergillus model EC numbers, E. dermatitidis genome annotations, and OptFill results.
The results of BLASTs of Aspergillus sequences linked to enzyme numbers added to the E. dermatitidis models at various stages in the model-building process, in addition to OptFill results used to curate the models.
This Microsoft Excel workbook contains bar graphs showing the shadow price of various metabolites in the iEde2091 model. Many of these shadow prices are given on both a per-metabolite and a per-limiting atom basis.
Data Availability Statement
The published article does not include all datasets and code generated or analyzed during this study. The datasets and code generated during this study are available at GitHub in the ssbio/OptFill repository (https://doi.org/10.5281/zenodo.3608172).