Summary
As an emerging class of porous materials, noble metal aerogels (NMAs) have drawn tremendous attention and displayed unprecedented potential in diverse fields. However, the development of NMAs is impeded by the fabrication methods because of their time- and cost-consuming procedures, limited generality, and elusive understanding of the formation mechanisms. Here, by revealing the self-healing behavior of noble metal gels and applying it in the gelation process at a disturbing environment, an unconventional and conceptually new strategy, i.e., a disturbance-promoted gelation method, is developed by introducing an external force field. It overcomes the diffusion limitation in the gelation process, thus producing monolithic gels within 1–10 min at room temperature, 2–4 orders of magnitude faster than for most reported methods. Moreover, versatile NMAs are acquired by using this method, and their superior (photo-)electrocatalytic properties are demonstrated for the first time in light of combined catalytic and optic properties.
Keywords: noble metals, aerogels, gels, sol-gel, self-healing, rapid, electrocatalysis, photoelectrocatalysis, plasmonics, ethanol oxidation reaction
Graphical Abstract
Highlights
-
•
The self-healing behavior of the noble metal gels is revealed
-
•
An unconventional disturbance-promoted gelation method is proposed
-
•
Various monolithic noble metal gels are created within 1–10 min
-
•
The photoelectrocatalytic properties of noble metal aerogels are pioneered
Progress and Potential
Gels are functional materials almost exclusively prepared in an undisturbed environment, so as to afford an intact three-dimensional network. However, the corresponding retarded mass transfer inevitably leads to sluggish reaction kinetics. Particularly for noble metal systems, the gelation can take a few weeks from a dilute precursor solution at room temperature, discouraging the material synthesis and subsequent applications. Here, revealing the self-healing nature of noble metal gels, an unconventional disturbance-promoted gelation method is developed to overcome the diffusion limit in the gelation process, which produces monolithic gels within 1–10 min and is capable of acquiring gels with various compositions and special structures (e.g., core-shell structure). Moreover, photoelectrocatalysis over noble metal aerogels is pioneered by utilizing their combined catalytic and optical properties. These findings may revolutionize both the fundamental and application-oriented research for broad gel systems.
The unusual self-healing behavior of the noble metal gels is revealed, based on which an unconventional disturbance-promoted gelation method is proposed. On this basis, the diffusion limit in the gelation process is overcome, thus producing various monolithic noble metal gels within 1–10 min, which is 2–4 orders of magnitude faster than most previously reported methods. Moreover, the photoelectrocatalytic properties of noble metal aerogels are pioneered, whereby the light considerably promotes the electrocatalytic properties.
Introduction
Aerogels are a class of self-supported, highly porous, three-dimensional (3D) networked materials.1, 2, 3 Combining their structural features and undefined chemical nature, their functions can be versatilely tailored by tuning their composition (e.g., with silica, polymers, nanocarbons, and metals4, 5, 6, 7), thus unlocking a widespread application potential ranging from thermal insulators, energy storage, and conversion, to environment remediation. Particularly for the recently emerging noble metal aerogels (NMAs), the noble-metal-structured networks afford abundant optically/catalytically active sites as well as 3D electron/mass transfer expressways, thus attracting increasing attention with their unprecedented potential particularly in electrocatalysis, surface-enhanced Raman scattering, and sensing.8, 9, 10, 11, 12, 13, 14
Despite impressive prospects, the fabrication of NMAs remains challenging, which accounts for their sluggish development in the last decade. The core of the aerogel fabrication lies in the preparation of the wet gel via a sol-gel process. For broad material systems, the formation of a monolithic gel usually requires undisturbed conditions to allow the gradual formation of continuous 3D networks, while this undisturbed condition exerts a large diffusion barrier, thus considerably slowing down the gelation process. Especially for the noble metal systems, very long gelation time (up to several weeks) is frequently observed, not to mention other issues, such as the costly concentration process (i.e., ultracentrifugation), the limited generality, and the elusive understanding of the corresponding formation mechanisms.9,11,15, 16, 17, 18, 19, 20, 21 These problems not only impede a facile synthesis and the available material systems but also discourage mechanism-oriented manipulation and tailoring of NMAs, thus hindering both the fundamental research and the practical applications.
In the past few years, the aforementioned issues have been partially resolved by continuous methodology development. For example, a NaBH4-mediated one-step reduction method was developed to obtain Pd and/or Pt gels directly from dilute metal salt solutions (metal salt concentration cM = 0.2 mM) in 3–17 days, avoiding the concentration step.16 Lin's group further cut down the gelation time to 6 h by applying an elevated temperature (333 K).22 Based on Ca2+-induced electrostatic crosslinking, Wen et al.18 fabricated Pd gels in 5 min, but at the expense of using highly concentrated nanoparticle (NP) precursor solutions (cM = 17 mM) prepared by the costly ultracentrifugation technique. Afterward, the same group found that, via unknown mechanisms, dopamine can efficiently trigger the formation of gold gels within 6–72 h from dilute solutions (cM = 0.2 mM) at room temperature.23 Very recently, taking advantage of specific ion effects, we introduced a platform by which the physical picture of the overall gelation process was unveiled, and various tunable noble metal gels were obtained from a wide concentration window (cM = 0.02–2.0 mM) in a few hours to a few days.24
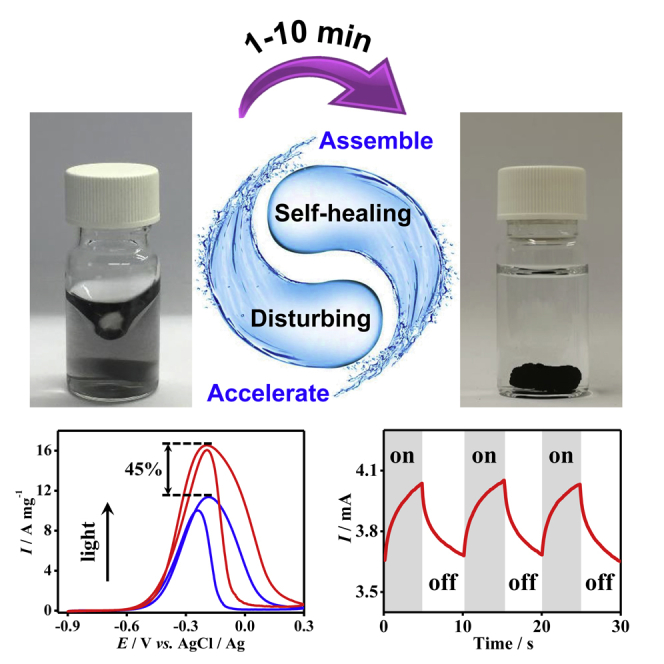
Although considerable progress has been made and various high-performance NMAs have been developed, two serious challenges remain. First, a sufficiently fast gelation process (e.g., <10 min) at a low-to-medium metal concentration (e.g., cM < 1.0 mM) has never been realized at room temperature, thus posing a large barrier blocking facile material synthesis. Second, the application scope of NMAs is largely restricted to electrocatalysis by utilizing their catalytic activities while overlooking their unique optic features. Recently, we noticed long-term reactivity of gold aggregates/gels, that is, they assembled together during and even after the gelation process.24 In this regard, here we further disclose the underlying mechanism behind this phenomenon—the self-healing property—and subtly apply it to the gelation process, with which an unconventional and conceptually new method, i.e., the disturbance-promoted gelation strategy, is developed by introducing an external force field during the preparation process. By applying a disturbing environment (e.g., stirring) to break the diffusion limit during the gelation process and then assembling the gel pieces together mediated by their self-healing properties, a monolithic gel was obtained within 1–10 min from a wide concentration window (cM = 0.02–5.0 mM), which is 2–4 orders of magnitude faster than most reported methods. The presented method is quite universal, fitting diverse initiating approaches, chemical compositions (e.g., Au, Ag, Pd, Rh, Au-Ir, Au-Pd-Pt), and microstructures (e.g., random-distributed and core-shell structures). Moreover, taking advantage of the combined optic and catalytic activities of noble metals, the photoelectrocatalytic properties of NMAs are demonstrated for the first time by using ethanol oxidation reaction (EOR) as a model reaction, displaying an activity increase of up to 45.5% by illumination and realizing a current density of up to 7.3-fold higher than that of commercial Pd/C. Therefore, the present work may open up new space for fundamental and application studies with NMAs as well as other gel systems.
Results and Discussion
Stirring-Promoted Rapid Sol-Gel Process
Conventionally the sol-gel process proceeds under undisturbed conditions for all material systems to ensure a static environment, allowing the formation of intact and self-supported 3D networks. However, the static environment can strongly retard the mass transfer, thus placing a large diffusion barrier that slows down the gelation process. Only for a few organic gel systems, the sol-gel transition was induced by disturbances (i.e., sonication) due to the morphology change of the aggregates during this process.25,26 Here, we show that in the inorganic world, e.g., for noble metal systems, various disturbing conditions can be applied to overcome the diffusion-limited gelation process and to yield self-supported gels by taking advantage of their self-healing properties. The self-healing behavior, in fact, is also rarely observed in sole inorganic systems without deliberately introducing reversible covalent27 or non-covalent bonds.28,29
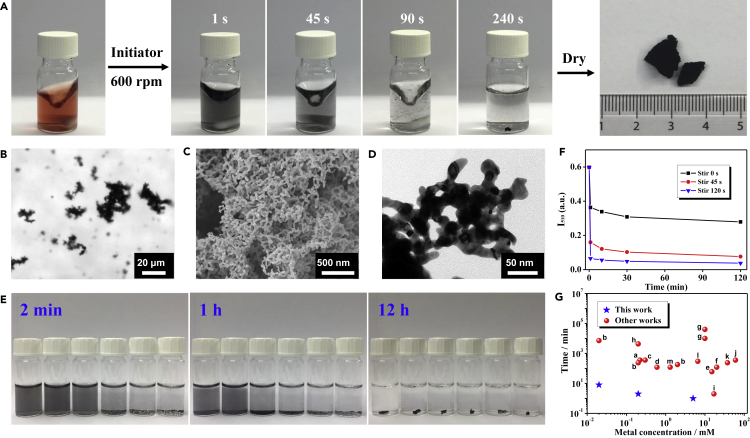
In the present work, as an example, the disturbance-promoted gelation process is demonstrated for a dilute gold NP system (gold salt concentration cM = 0.2 mM). Stirring, which can produce a shearing field, was introduced to create a disturbing environment because of its facileness and good controllability. As illustrated in Figure 1A and Videos S1 and S2, upon initiation of the destabilization by a salting-out method (NH4F was used as the initiator), the gold NP solution instantly transferred from red to black, suggesting the formation of nanostructured gold aggregates. With further stirring, visible aggregates appeared after ca. 45 s, the reaction was almost completed at 90 s, and a monolithic gel evolved within 4 min assisted by manually assembling the as-formed self-healable “gel pieces.” Following purification and freeze-drying, self-supported aerogels were obtained with a yield of 86.9%. The formation of large gold aggregates after 45 s is also confirmed by optical microscopy imaging, as shown in Figure 1B. A close-up inspection of the resulting gel by scanning electron microscopy (SEM) and transmission electron microscopy (TEM) revealed the highly porous structure, which is similar to those from gels fabricated in an undisturbed environment (Figures 1C, 1D, S1, and S2).
Figure 1.
Demonstration and Characterization of the Stirring-Promoted Rapid Fabrication of Gold Gels
(A) Photographs of the stirring-directed gelation process of a gold NP solution (0.2 mM, 10 mL, left) induced by NH4F and the corresponding aerogel (right). For a better demonstration, the aerogel was created from ~800-mL solutions.
(B) Optical microscopy image of the as-formed gold aggregates after stirring for 45 s.
(C and D) SEM image (C) and TEM image (D) of the obtained gold aerogel.
(E) The gelation process of gold NP solutions under stirring at 600 rpm for 0, 10, 30, 60, 90, and 120 s.
(F) Time-lapse UV-vis absorption intensities obtained at 510 nm for the gelation system under stirring at 600 rpm for 0, 45, and 120 s.
(G) Summary of the gelation time of noble metal gels reported in the literature and in this work versus the initial metal salt concentrations (cM). References denoted by red dots: a, Wen et al.;23 b, Du et al.;24 c, Zhu et al.;22 d, Shi et al.;30 e, Naskar et al.;20 f, Tang et al.;8 g, Bigall et al.;10 h, Liu et al.;15 i, Wen et al.;18 j, Cai et al.;19 k, Gao et al.;17 l,Yazdan-Abad et al.;31 m, Shi et al.21
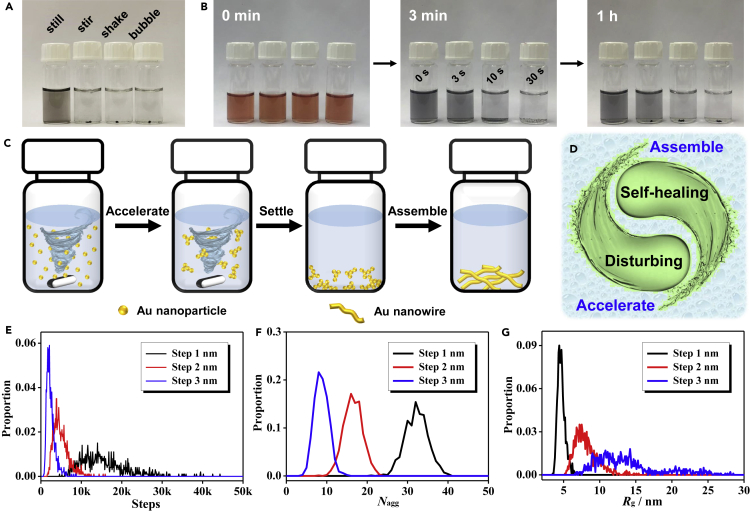
The disturbance can considerably enhance the mass transfer, thus reducing the diffusion barrier and greatly promoting the gelation process. To clarify the effect of disturbance, we investigated the input “disturbing power,” expressed by the stirring time and stirring speed. As seen from Figures 1E and S3, both the elongation of the stirring time and increase of the stirring speed considerably promoted the destabilization process. A similar trend was also observed by using other initiating methods (Figure S4). The acceleration behavior was further semi-quantitatively characterized by UV-visible (UV-vis) absorption spectroscopy (Figures 1F and S5), whereby 39%, 73%, and 89% absorption intensity decay (recorded at a wavelength of 510 nm) were observed after successive NH4F initiation, stirring (for 0, 45, and 120 s, respectively), and grounding for 1 min. With prolonged grounding time until 120 min, the absorption intensity decay of the undisturbed system was only 53%, much less than that of the stirred systems (87% and 94% for 45-s and 120-s stirring, respectively). The above results unambiguously point out that the destabilization process of a gold NP solution is strongly promoted by applying a disturbing environment, where the gelation speed is positively correlated with the degree of disturbance. More impressively, the disturbance-promoted gelation process adapts to a broad concentration window cM, spanning from 0.02 to 5.0 mM and keeping the gelation time within 1–10 min. As summarized in Figure 1G and Table S1, compared with most previously reported strategies, the presented method remarkably accelerates the gelation speed of the gold system by 2–4 orders of magnitude, allowing the ultrafast and facile acquirement of noble metal gels without elevated temperatures or a concentrating step. Notably, besides introducing a shearing field by stirring, various other disturbance methods, such as manually shaking and bubbling, can be applied to prepare monolithic gels and display similar results (Figures 2A and 2B).
Figure 2.
The Mechanisms for the Disturbance-Promoted Gelation
(A) Gold NP solutions destabilized by NH4F in different environments (undisturbed, stirring for 2 min, shaking for 20 s, and bubbling for 2 min with N2). The photo was taken 2 min after the corresponding treatment.
(B) Time-lapse photographs of the disturbing-promoted gelation process of gold NP solutions (triggered by NH4F) by manually shaking for 0, 3, 10, and 30 s.
(C and D) Proposed model for stirring-promoted gelation (C) and the role of the self-healing properties and the disturbing environment played in this process (D).
(E–G) MC simulation under different disturbing conditions (simulated for different maximum step lengths). Distribution of (E) the required steps to form one single aggregate, (F) the number of aggregates after 5,000 MC steps, and (G) the average radius of gyration (Rg) of the resulting aggregates after 15,000 MC steps.
Mechanisms of Disturbance-Promoted Gelation Mediated by Self-Healing Properties
It is not surprising that a disturbing environment can accelerate the reaction by prompting collisions between the reactants, while it is fascinating to see that the fragmented products—visible large aggregates (called gel pieces)—can assemble into a monolithic gel after terminating the disturbance. Previously we found that the gold species (intermediate aggregates or as-formed gels) are highly active throughout the gelation process, thus enabling the final formation of a monolithic gel at the bottom of the vessel due to gravity-driven aggregate sedimentation and assembly.24 The high-reactivity-enabled assembly of the gold species let us recall the well-known self-healing behavior. Inspired by this, the idea comes naturally that by taking advantage of the high reactivity of the gold species, it is possible to accelerate the reaction in a disturbing environment and subsequently reassemble the resulting products into a monolithic gel under undisturbed conditions, thus harnessing the contradiction between the reaction kinetics and the self-supported materials.
In the first place, the self-healing behavior of noble metal hydrogels is studied. The self-healing properties are almost exclusively found in organic or organic-inorganic hybrid systems that involve specially devised reversible crosslinking forces (e.g., hydrogen bonds and dynamic chemical bonds),27,29 while it is rarely observed in sole inorganic systems without delicately introduced interactions. Here, the NH4F-induced gelation of trisodium citrate (NaCA)-stabilized gold NPs is taken as an example for the discussion. As seen from Figures S6 and S7, the vigorous mechanical disturbance during the gelation did not affect the final formation of a monolithic gold hydrogel, inferring that the gold aggregates/hydrogels maintain high reactivity, i.e., the self-healing properties, during and after the gelation process. Moreover, the gold hydrogels are capable of healing themselves in either the original gelation solution, deionized water, or ethanol, implying that instead of interacting with “healing agents” (e.g., gold species, ligands, reductants) from the initial gelation environment, the self-healing properties should arise from the reversible interactions between the gold building blocks. However, due to the uncontrollable shape of the gold hydrogel because of its mechanical fragility, the quantification of such self-healing properties by rheology and other techniques remains to be tackled.
Second, combining the intriguing self-healing behavior of the gold hydrogels and the disturbance-induced acceleration during the gelation process, a model depicting the formation of monolithic gels is schemed in Figures 2C and 2D. The addition of initiators induces the aggregation and assembly of gold NPs, the process of which is greatly accelerated by applying a disturbing environment such as vigorous stirring. The disturbance can on the one hand enhance the mass transfer and accelerate the reaction speed, while on the other hand restricting the unlimited growth of the gold aggregates due to mechanical crushing, thus leading to the formation of many small gel pieces in the bulk solution. Afterward, the disturbance is removed and the gel pieces can be manually gathered together to form a monolithic gel due to their self-healing properties. In this way, the synergy of disturbance-induced acceleration and self-healing-mediated assembly affords an ultrafast fabrication of monolithic gels in a few minutes.
To intuitively demonstrate the proposed acceleration effect, we apply random-walk Monte Carlo (MC) simulations (Figures 2E, 2F, and S8). In brief, non-interacting particles are allowed to move randomly in a spherical space, and particles that meet during movement are considered as a single aggregate. The algorithm is terminated when all particles are aggregated into a single aggregate, and statistical analysis is performed over 1,000 simulations per parameter set. The disturbing condition is simulated by using different maximum step lengths (x, representing the maximum value of one step) for the particles, while the gelation time is reflected by the required MC steps for all particles to form one aggregate. As seen from Figure 2E, increasing the maximum step length (x = 1, 2, 3 nm) results in a smaller number of MC steps to form a single aggregate as evidenced from the respective probability distribution curves, which reflect a shorter gelation time with a more intensive disturbance. For a fixed step number, i.e., a specific period of reaction time, the amount of remaining aggregates (Nagg) and the average radius of gyration (Rg) are negatively and positively correlated with x, respectively (Figures 2F, 2G, and S8), suggesting that an enhanced disturbance can accelerate the reaction and thus form larger aggregates, which further portrays the disturbance-directed acceleration of the gelation process. In addition, after formation of a single aggregate, it is found that a larger maximum step length x resulted in a smaller Rg, inferring an increase of density for the gel fabricated under disturbance (Figure S9). This is in line with the experimental results showing that the stirring-produced gold aerogel exhibited a density much larger than the one prepared under non-disturbed conditions24 (309.1 mg cm−3 versus 108.7 mg cm−3).
Generality of the Disturbance-Promoted Gelation Method
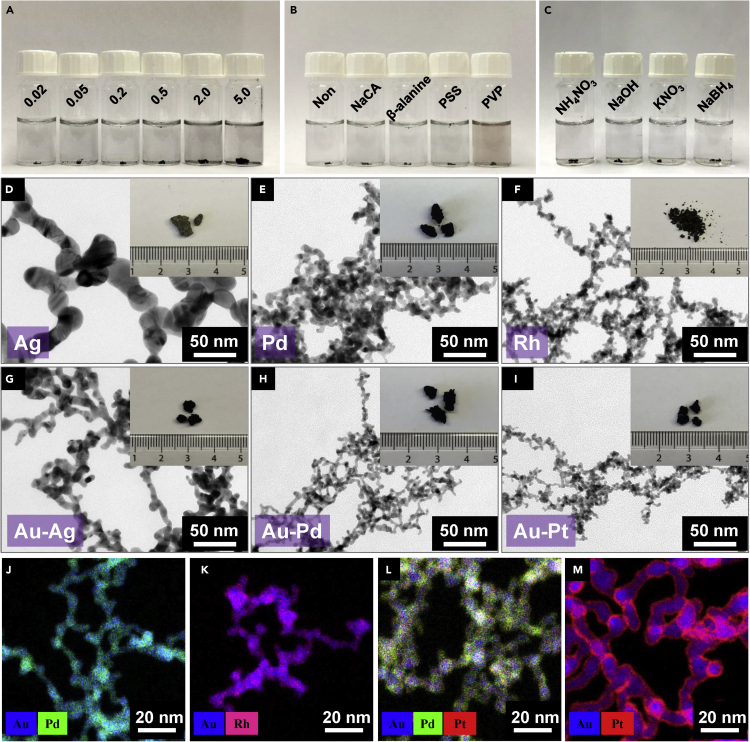
Introducing a disturbing environment to substantially accelerate the gelation process is a general strategy that is compatible with various initiation approaches and fits diverse systems. As seen from Figures 3A–3C, the stirring-promoted gelation of gold gels works well for a broad concentration range (cM = 0.02–5 mM), various ligand-stabilized gold NPs (e.g., ligand-free, NaCA, β-alanine, polystyrene sulfonate, polyvinylpyrrolidone [PVP]), and versatile initiators (e.g., NH4NO3, NaOH, KNO3, and NaBH4). In this way, the structure parameters of the resulting gold aerogels can be flexibly tuned (Figure S10 and Table S2). For example, compared with the NH4F-induced gelation of NaCA-stabilized gold NPs, the aerogel made from NaBH4-destabilized PVP-coordinated gold NPs displayed a reduced ligament size from 19.8 ± 3.7 nm to 10.4 ± 3.1 nm, presumably due to the PVP-directed oriented growth,32 thus resulting in a high specific surface area (SSA) of 42.6 m2 g−1, surpassing that of most previously reported porous gold materials (<1 to 30 m2 g−1)10,12,24,33, 34, 35 and only slightly lower than that of a gold-β-cyclodextrin aerogel (50.1 m2 g−1, with ∼18 wt % β-cyclodextrin23).
Figure 3.
Generality of the Disturbance-Promoted Gelation
(A–C) Stirring-promoted gelation of diverse gold gels. (A) NaBH4-initiated gelation from NaCA-stabilized gold NP solutions with cM ranging from 0.02 mM to 5.0 mM, (B) NaBH4-initiated gelation from gold NP solutions stabilized by different ligands (cM = 0.2 mM), and (C) gelation of NaCA-stabilized gold NP solutions triggered by different initiators (cM = 0.2 mM).
(D–I) NMAs with different compositions. (D) Ag, (E) Pd, (F) Rh, (G) Au-Ag, (H) Au-Pd, and (I) Au-Pt. The inset photograph in (F) shows the residual of the Rh aerogel after spontaneously burning in air.
(J–M) Energy-dispersive X-ray mapping of multimetallic aerogels made of (J) Au-Pd, (K) Au-Rh, (L) Au-Pd-Pt, and (M) core-shell Au-Pt. (J), (K), and (L) were prepared by a two-step method, while (M) was prepared by the dynamic shelling approach.
Moreover, the disturbance-promoted acceleration can be extended to NMAs with versatile compositions such as Ag, Pd, Rh, Au-Ag, Au-Pd, Au-Pt, Au-Rh, and Au-Pd-Pt, with a reasonable yield of >75% for all mentioned systems, which is acceptable compared with the yield obtained under undisturbed conditions (typically >90%). The yield might be further increased by a more careful operation or a prolonged time for the gel-assembling process. The resulting aerogels were characterized by electron microscopy, X-ray diffraction, and X-ray photoelectron spectroscopy (XPS) (Figures 3D–3I and S11–S16; Table S2). Similar to previous observations,24 the Ag aerogel manifested a large ligament size (18.1 ± 3.0 nm), considerably thicker than that of the single-metallic Pd (5.8 ± 1.2 nm) and Rh (4.1 ± 0.5 nm) aerogels, as well as the multimetallic Au-Ag (7.1 ± 1.0 nm), Au-Pd (3.9 ± 0.6 nm), Au-Pt (3.7 ± 0.7 nm), Au-Rh (4.6 ± 1.2 nm), Au-Ir (7.2 ± 1.8 nm), and Au-Pd-Pt (4.3 ± 0.6 nm) aerogels. The various ligament sizes along with the chemical compositions further influenced the SSAs and the pore volumes of NMAs, which are located in the range of 5.6–83.6 m2 g−1 and 0.1–0.9 cm3 g−1, respectively (Figures S17–S19 and Table S2). As seen from the pore-size distribution, the presence of predominant mesopores (mostly 3–10 nm) should offer numerous active sites while simultaneously maintaining a fast mass transfer, which is beneficial for applications such as electrocatalysis.
For the multimetallic systems, the spatial element distribution cannot be well predicted by using the two-step method as detailed in Supplemental Information. As seen from Figures 3J–3L, S20, and S21, the Au-Ag and Au-Rh gels displayed core-shell-like structures while the others exhibited random element distributions. The spontaneous formation of the core-shell structure might be attributed to the segregation of low-surface-energy metals on the high-surface-energy ones.36 To increase the utilization efficiency of the active component, the Au-Pt aerogel with a pronounced core-shell structure was devised by combining our previously reported dynamic shelling approach (DSA)24 with the currently developed stirring-promoted gelation strategy (Figures 3M and S21), by simply altering the feeding order of the reactants. The successful application of the DSA offers an extremely facile and rapid fabrication strategy toward monolithic noble metal gels with sophisticated microstructures, which is much easier and extremely time-saving compared with previously reported approaches, such as pre-NP-engineering or sequential gel fabrication-underpotential decomposition-galvanic replacement methods.14,19
Electrocatalysis and Photoelectrocatalysis by NMAs
Featuring stable 3D networks, continuous electron/mass transfer highways, controlled spatial element distribution, and abundant active catalytic sites, NMAs have been widely explored in various electrocatalytic processes, such as alcohol oxidation, oxygen reduction reaction, and oxygen evolution reaction.14,21,22,30,37,38 On the other hand, noble metal nanostructures feature unique optic properties, which allows coupling between the collective surface electron oscillations and the incident electromagnetic field,39 thus yielding a dramatically enhanced localized electric field for surface-enhanced Raman scattering.8 However, the plasmonic-assisted photoelectrocatalysis properties, which entered the vision of the scientific community in recent years, have not been explored for NMAs so far.
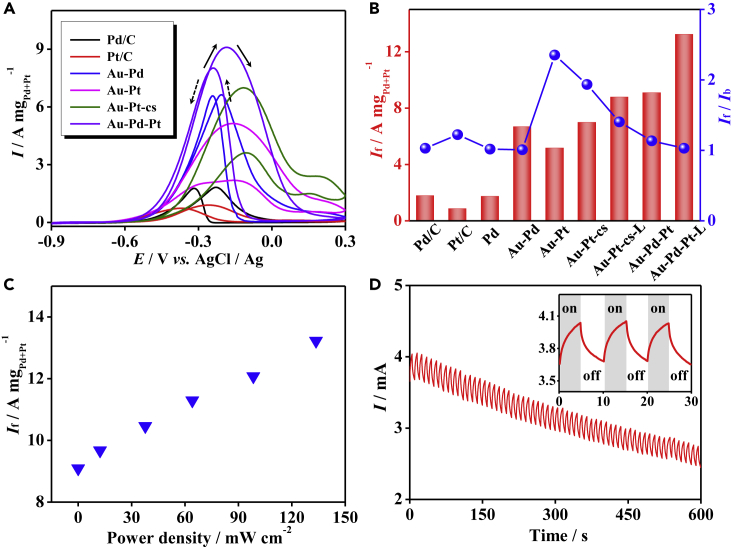
Here, the ethanol oxidation is adopted as a model reaction, whereby the electrocatalytic performance under dark conditions is investigated first. As shown in Figure 4A, the peak appearing in the forward scan and the backward scan mark the oxidation of the ethanol and the oxidation of the intermediate generated during the forward scan, respectively, where the forward current density (If) and the ratio of the forward/backward current density (If/Ib) are the two important parameters accounting for the EOR performance.30 The difference of the peak positions obtained from various catalysts can be attributed to the different reaction pathways presented in the respective systems, while the details remain to be deeply investigated. As summarized in Figure 4B, except for the Pd aerogel, all other Pd- and Pt-based multimetallic aerogels displayed considerably higher activity than that of the commercial Pd/C or Pt/C catalysts (1.8 A mgPd−1 and 0.9 A mgPt−1). In particular, the delicately designed Au-Pt core-shell aerogel exhibited an If of 7.3 A mgPt−1, and the Au-Pd-Pt trimetallic aerogel showed an even higher If of 9.1 A mgPd+Pt−1, indicating the advantages of the delicately devised core-shell structure and/or the synergistic effect from multiple elements. On the other hand, a substantially high If/Ib is observed for the Au-Pt (2.35) and the Au-Pt core-shell aerogel (1.93), roughly double that of the other commercial and aerogel catalysts (1.00–1.22), thus indicating high efficiency in the forward oxidation step for the gold-platinum bimetallic systems. Although a rapid current decay was observed for all systems, NMA-based electrocatalysts manifested a much better stability compared with that of the commercial ones (current retention of 19.6%–32.9% versus 1.2%–5.7% for a 2,000-s operation), which can be attributed to the stable 3D networks feature of NMAs (Figure S22).
Figure 4.
Application of NMAs in Electrocatalysis and Photoelectrocatalysis
(A) Electrocatalytic performance of the EOR with commercial and various aerogel catalysts.
(B) Summarized If and If/Ib of various catalysts. Au-Pt-cs denotes the core-shell-structured Au-Pt aerogel, and the suffix “L” denotes that the test was conducted under white-light illumination at 133.6 mW cm−2.
(C) Dependence of If of the Au-Pd-Pt aerogel on the input light power density.
(D) Light response behavior on an Au-Pd-Pt aerogel electrode in the course of a chronoamperometry test. All tests were performed in nitrogen-saturated 1.0 M KOH + 1.0 M ethanol aqueous solution.
Taking advantage of the unique optical properties of the noble metal nanostructures (especially Au and Ag),39,40 the photoelectrocatalytic properties of EOR have been primarily investigated. With a commercial white-light LED source, the electrocatalytic EOR on commercial- and aerogel-based noble metal catalysts has been explored under dark and light conditions (133.6 mW cm−2), as shown in Figures 4B and S23. In virtue of the light-assisted performance enhancement, 12.4% and 15.9% increase in If was observed for Pt/C and Pd/C, respectively, while improvements of >20% were observed for the aerogel systems. However, the If/Ib ratio is slightly reduced for all systems, except for Pt/C and the Pd aerogel. Impressively, the Au-Pd-Pt aerogel manifested a 45.5% current density enhancement upon illumination, reaching a remarkably high If of 13.2 A mgPd+Pt−1, which is 7.3-fold higher than that of commercial Pd/C and surpasses most NMAs such as Pd, Pd-Ni, and Au-Pd-Pt aerogels (2.3–6.1 times higher than Pd/C),15,19,24,31 and being only lower than the Pd-ensembles-anchored Au-Cu aerogel (11.6 times higher than Pd black).30 The Au-Pt core-shell aerogel also displayed a substantially high If of 8.8 A mgPd+Pt−1, and concurrently maintained a fairly high If/Ib of 1.4. The high performance of these NMAs can be attributed to the synergy of high catalytic activity of Pd/Pt, and high electrical conductivity of Au, as well as strong optic absorption of all nanostructured metals. Further experiments on the Au-Pd-Pt aerogel indicate that the If is positively correlated with the light power density, and the presented light-promoted catalysis might be applied for photosensing with a sensitivity of >40.2 μA mW−1 (Figures 4C and 4D). In contrast, on a bare glassy carbon electrode, no visible oxidation peak was observed (Figure S23), either in darkness or under light, indicating the indispensable role of the noble metal nanostructures.
The photoelectrocatalytic process has long been focused on semiconductor-based systems, while studies on noble metal nanostructures, which are usually used as co-catalysts in conjunction with semiconductors to enhance light harvesting or promote charge transfer, only appeared recently.38,39,41,42 The enhancement mechanism by noble metal nanostructures can be attributed to localized surface plasmon resonances (LSPR), light absorption by nanostructures-induced trapping, and the intraband transition.42 As opposed to most other materials, the surface electrons of noble metal nanostructures can be oscillated upon irradiation with incident light of appropriate wavelength (i.e., LSPR), strongly enhancing the light field and generating hot carriers (electron-hole pair), or by electron-phonon coupling (i.e. heat dissipation) upon de-excitation.39 Hence, three mechanisms including (1) enhanced photon density, (2) hot-carrier transfer, and (3) plasmonic thermal effect may account for the increase in plasmon-assisted performance.40 Because of the application of a white-light source (wavelength 350–800 nm) in this study (covering a wide wavelength range), none of those mechanisms can be ruled out. On the other hand, for the non-plasmon-absorption region, light trapping by metal nanostructures may incur a heat effect via the multiple absorption and scattering of the incident light,24 also contributing to the catalytic enhancement. The decoupling and careful characterization of the contributions in the photoelectrocatalytic process, the further enhancement of the light-assisted catalytic performance possibly by engineering the plasmonic absorption behavior of the aerogels, and the expansion of available catalytic reactions will be future directions for NMA-based photoelectrocatalysis research.
Conclusion
In the field of NMAs, many synthesis-related challenges, including cost- and time-consuming procedures and the debated gel-formation mechanisms, have largely discouraged the material design and the application diversity of NMAs. In this work, by disclosing the self-healing behavior of the noble metal hydrogels and subtly applying it in the gelation process, an unconventional and conceptually new method, i.e., disturbance-promoted gelation, is developed by introducing an external force field. This approach is capable of breaking the diffusion barrier in the gelation process and thus acquiring monolithic gels within 1–10 min without elevated temperatures or pre-concentrating step, which is 2–4 orders of magnitude faster than for most methods reported. To understand the self-healing-property-mediated disturbance-promoted process, MC simulations have been performed, whereby the statistics of the aggregation behaviors unambiguously point out the acceleration mechanisms. Notably, the developed strategy is compatible with various initiation methods, thus affording a library of monolithic gels with widespread ligament sizes (3.7–19.8 nm), compositions (Au, Ag, Pd, Rh, and versatile multimetallic alloys), and spatial element distributions (e.g., random-distributed and core-shell structures). Finally, taking advantage of the unique optical properties of nanostructured noble metals, photoelectrocatalytic ethanol oxidation has been studied, whereby the Au-Pd-Pt and the Au-Pt core-shell aerogels, under illumination at 133.6 mW cm−2, exhibited If of 7.3- and 4.9-fold, and If/Ib ratios 1.05- and 1.40-fold higher than those of commercial Pd/C. The present work not only provides a counterintuitive yet very efficient gelation method that may be expanded to various gel systems, but also pioneers the exploration of photoelectrocatalysis on NMAs, thus opening up new space for both fundamental and application-oriented studies of noble metal gels and other systems.
Experimental Procedures
Synthesis Methodologies
Noble metal hydrogels were synthesized by either a one-step method or a two-step method at ambient temperature (∼293 K), starting from a metal salt solution and a metal nanoparticle (NP) solution, respectively. As an example for the preparation of gold aerogels via a two-step method, an aqueous solution of NH4F (10.0 M, 50 μL) was added to the as-prepared trisodium citrate-stabilized gold NP solution (4.95 mL, cM = 0.2 mM). After continuously stirring for ca. 90 s, the resulting gel pieces were manually assembled together to form a monolithic hydrogel. The as-prepared hydrogel was washed by a large amount of water four to five times with a total duration of 2–3 days to remove possible residues, followed by solvent exchange with tert-butanol. Afterward, the wet gel was flash-frozen by liquid nitrogen and subjected to vacuum drying for 12–24 h. The yield of NMAs was calculated based on the mass of the final aerogels with respect to the mass of the corresponding precursor metal salts.
Electrocatalysis and Photoelectrocatalysis
All electrochemical tests were performed with a three-electrode system, with a glassy carbon electrode (3 mm in diameter), an Ag/AgCl (saturated KCl aqueous solution) electrode, and a platinum foil as the working electrode, reference electrode, and counter electrode, respectively. For the electro-oxidation of ethanol, the test was performed under N2 atmosphere in 1.0 M KOH aqueous solution containing 1.0 M ethanol. CV curves were recorded between −0.9 and 0.3 V (versus AgCl/Ag) with a scanning rate of 50 mV s−1, and the stability test was conducted at a potential of −0.23 V (versus AgCl/Ag). For photoelectrocatalysis, a white LED source was placed perpendicular to the working electrode, affording an adjustable light power density between 0 and 133.6 mW cm−2.
Acknowledgments
R.D. acknowledges the support from the Alexander von Humboldt Foundation. We thank Dr. Julio Bastos Arrieta for assisting in optical imaging, Dr. Minghao Yu for assisting with the Fourier transform infrared tests, and Shishu Zhang for assisting in XPS measurements. Furthermore, the use of the HZDR Ion Beam Center TEM facilities is acknowledged. The computations were performed on an HPC system at the Center for Information Services and High Performance Computing (ZIH) at TU Dresden within the project QDSIM. This work was supported by the research funding of the Humboldt fellowship, the ERC AdG AEROCAT, the German Federal Ministry of Education of Research (BMBF, 03SF0451), the China Scholarship Council, the National Natural Science Foundation of China (51972237), and the Natural Science Foundation of Zhejiang Province (LY19E020008).
Author Contributions
R.D. conceived the experiments and performed the materials synthesis. J.-O.J. carried out the computational part. Y.H. partially performed SEM, TEM, and XPS analysis. X.F., R.D., and D.S. performed EOR electrocatalysis and photoelectrocatalysis. R.H. performed the scanning TEM energy-dispersive X-ray spectroscopy analysis. R.D. performed all other experimental characterizations or measurements as well as the data analysis. R.D. drafted the manuscript, R.H., J.-O.J., and A.E. revised the manuscript, and all authors commented on the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: January 29, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.matt.2020.01.002.
Contributor Information
Ran Du, Email: dr1581@foxmail.com.
Alexander Eychmüller, Email: alexander.eychmueller@chemie.tu-dresden.de.
Data and Code Availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or Supplemental Information. Additional data related to this paper can be requested from the authors.
Supplemental Information
References
- 1.Gesser H., Goswami P. Aerogels and related porous materials. Chem. Rev. 1989;89:765–788. [Google Scholar]
- 2.Hüsing N., Schubert U. Aerogels—airy materials: chemistry, structure, and properties. Angew. Chem. Int. Ed. 1998;37:22–45. doi: 10.1002/(SICI)1521-3773(19980202)37:1/2<22::AID-ANIE22>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 3.Ziegler C., Wolf A., Liu W., Herrmann A.-K., Gaponik N., Eychmüller A. Modern inorganic aerogels. Angew. Chem. Int. Ed. 2017;56:13200–13221. doi: 10.1002/anie.201611552. [DOI] [PubMed] [Google Scholar]
- 4.Kistler S.S. Coherent expanded aerogels and jellies. Nature. 1931;127:741. [Google Scholar]
- 5.Du R., Zhang N., Xu H., Mao N., Duan W., Wang J., Zhao Q., Liu Z., Zhang J. CMP aerogels: ultrahigh-surface-area carbon-based monolithic materials with superb sorption performance. Adv. Mater. 2014;26:8053–8058. doi: 10.1002/adma.201403058. [DOI] [PubMed] [Google Scholar]
- 6.Cai B., Sayevich V., Gaponik N., Eychmüller A. Emerging hierarchical aerogels: self-assembly of metal and semiconductor nanocrystals. Adv. Mater. 2018;30:1707518. doi: 10.1002/adma.201707518. [DOI] [PubMed] [Google Scholar]
- 7.Du R., Zhao Q., Zhang N., Zhang J. Macroscopic carbon nanotube-based 3D monoliths. Small. 2015;11:3263–3289. doi: 10.1002/smll.201403170. [DOI] [PubMed] [Google Scholar]
- 8.Tang S., Vongehr S., Wang Y., Cui J., Wang X., Meng X. Versatile synthesis of high surface area multi-metallic nanosponges allowing control over nanostructure and alloying for catalysis and SERS detection. J. Mater. Chem. A. 2014;2:3648–3660. [Google Scholar]
- 9.Du R., Fan X., Jin X., Hübner R., Hu Y., Eychmüller A. Emerging noble metal aerogels: state of the art and a look forward. Matter. 2019;1:39–56. [Google Scholar]
- 10.Bigall N.C., Herrmann A.K., Vogel M., Rose M., Simon P., Carrillo-Cabrera W., Dorfs D., Kaskel S., Gaponik N., Eychmüller A. Hydrogels and aerogels from noble metal nanoparticles. Angew. Chem. Int. Ed. 2009;48:9731–9734. doi: 10.1002/anie.200902543. [DOI] [PubMed] [Google Scholar]
- 11.Du R., Jin X., Hübner R., Fan X., Hu Y., Eychmüller A. Engineering self-supported noble metal foams toward electrocatalysis and beyond. Adv. Energy Mater. 2019 [Google Scholar]
- 12.Nahar L., Farghaly A.A., Esteves R.J.A., Arachchige I.U. Shape controlled synthesis of Au/Ag/Pd nanoalloys and their oxidation-induced self-assembly into electrocatalytically active aerogel monoliths. Chem. Mater. 2017;29:7704–7715. [Google Scholar]
- 13.Tappan B.C., Steiner S.A., Luther E.P. Nanoporous metal foams. Angew. Chem. Int. Ed. 2010;49:4544–4565. doi: 10.1002/anie.200902994. [DOI] [PubMed] [Google Scholar]
- 14.Cai B., Hübner R., Sasaki K., Zhang Y., Su D., Ziegler C., Vukmirovic M.B., Rellinghaus B., Adzic R.R., Eychmüller A. Core-shell structuring of pure metallic aerogels towards highly efficient platinum utilization for the oxygen reduction reaction. Angew. Chem. Int. Ed. 2018;57:2963–2966. doi: 10.1002/anie.201710997. [DOI] [PubMed] [Google Scholar]
- 15.Liu W., Herrmann A.K., Geiger D., Borchardt L., Simon F., Kaskel S., Gaponik N., Eychmüller A. High-performance electrocatalysis on palladium aerogels. Angew. Chem. Int. Ed. 2012;51:5743–5747. doi: 10.1002/anie.201108575. [DOI] [PubMed] [Google Scholar]
- 16.Liu W., Rodriguez P., Borchardt L., Foelske A., Yuan J., Herrmann A.K., Geiger D., Zheng Z., Kaskel S., Gaponik N. Bimetallic aerogels: high-performance electrocatalysts for the oxygen reduction reaction. Angew. Chem. Int. Ed. 2013;52:9849–9852. doi: 10.1002/anie.201303109. [DOI] [PubMed] [Google Scholar]
- 17.Gao X., Esteves R.J., Luong T.T.H., Jaini R., Arachchige I.U. Oxidation-induced self-assembly of Ag nanoshells into transparent and opaque Ag hydrogels and aerogels. J. Am. Chem. Soc. 2014;136:7993–8002. doi: 10.1021/ja5020037. [DOI] [PubMed] [Google Scholar]
- 18.Wen D., Herrmann A.-K., Borchardt L., Simon F., Liu W., Kaskel S., Eychmüller A. Controlling the growth of palladium aerogels with high-performance toward bioelectrocatalytic oxidation of glucose. J. Am. Chem. Soc. 2014;136:2727–2730. doi: 10.1021/ja412062e. [DOI] [PubMed] [Google Scholar]
- 19.Cai B., Wen D., Liu W., Herrmann A.K., Benad A., Eychmüller A. Function-led design of aerogels: self-assembly of alloyed PdNi hollow nanospheres for efficient electrocatalysis. Angew. Chem. Int. Ed. 2015;54:13101–13105. doi: 10.1002/anie.201505307. [DOI] [PubMed] [Google Scholar]
- 20.Naskar S., Freytag A., Deutsch J., Wendt N., Behrens P., Köckritz A., Bigall N.C. Porous aerogels from shape-controlled metal nanoparticles directly from nonpolar colloidal solution. Chem. Mater. 2017;29:9208–9217. [Google Scholar]
- 21.Shi Q., Zhu C., Zhong H., Su D., Li N., Engelhard M.H., Xia H., Zhang Q., Feng S., Beckman S.P. Nanovoid incorporated IrxCu metallic aerogels for oxygen evolution reaction catalysis. ACS Energy Lett. 2018;3:2038–2044. [Google Scholar]
- 22.Zhu C., Shi Q., Fu S., Song J., Xia H., Du D., Lin Y. Efficient synthesis of MCu (M= Pd, Pt, and Au) aerogels with accelerated gelation kinetics and their high electrocatalytic activity. Adv. Mater. 2016;28:8779–8783. doi: 10.1002/adma.201602546. [DOI] [PubMed] [Google Scholar]
- 23.Wen D., Liu W., Haubold D., Zhu C., Oschatz M., Holzschuh M., Wolf A., Simon F., Kaskel S., Eychmüller A. Gold aerogels: three-dimensional assembly of nanoparticles and their use as electrocatalytic interfaces. ACS Nano. 2016;10:2559–2567. doi: 10.1021/acsnano.5b07505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du R., Hu Y., Hübner R., Joswig J.-O., Fan X., Eychmüller A. Specific ion effects directed noble metal aerogels: versatile manipulation for electrocatalysis and beyond. Sci. Adv. 2019;5:eaaw4590. doi: 10.1126/sciadv.aaw4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Z.-X., Feng Y., Yan Z.-C., He Y.-M., Liu C.-Y., Fan Q.-H. Multistimuli responsive dendritic organogels based on azobenzene-containing poly(aryl ether) dendron. Chem. Mater. 2012;24:3751–3757. [Google Scholar]
- 26.Zhang S., Yang S., Lan J., Tang Y., Xue Y., You J. Ultrasound-induced switching of sheetlike coordination polymer microparticles to nanofibers capable of gelating solvents. J. Am. Chem. Soc. 2009;131:1689–1691. doi: 10.1021/ja808210z. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y., Yang B., Zhang X., Xu L., Tao L., Li S., Wei Y. A magnetic self-healing hydrogel. Chem. Commun. 2012;48:9305–9307. doi: 10.1039/c2cc34745h. [DOI] [PubMed] [Google Scholar]
- 28.Wang Q., Mynar J.L., Yoshida M., Lee E., Lee M., Okuro K., Kinbara K., Aida T. High-water-content mouldable hydrogels by mixing clay and a dendritic molecular binder. Nature. 2010;463:339–343. doi: 10.1038/nature08693. [DOI] [PubMed] [Google Scholar]
- 29.Du R., Wu J., Chen L., Huang H., Zhang X., Zhang J. Hierarchical hydrogen bonds directed multi-functional carbon nanotube-based supramolecular hydrogels. Small. 2014;10:1387–1393. doi: 10.1002/smll.201302649. [DOI] [PubMed] [Google Scholar]
- 30.Shi Q., Zhu C., Tian M., Su D., Fu M., Engelhard M.H., Chowdhury I., Feng S., Du D., Lin Y. Ultrafine Pd ensembles anchored-Au2Cu aerogels boost ethanol electrooxidation. Nano Energy. 2018;53:206–212. [Google Scholar]
- 31.Yazdan-Abad M.Z., Noroozifar M., Alam A.R.M., Saravani H. Palladium aerogel as a high-performance electrocatalyst for ethanol electro-oxidation in alkaline media. J. Mater. Chem. A. 2017;5:10244–10249. [Google Scholar]
- 32.Koczkur K.M., Mourdikoudis S., Polavarapu L., Skrabalak S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015;44:17883–17905. doi: 10.1039/c5dt02964c. [DOI] [PubMed] [Google Scholar]
- 33.Burpo F.J., Nagelli E.A., Morris L.A., McClure J.P., Ryu M.Y., Palmer J.L. Direct solution-based reduction synthesis of Au, Pd, and Pt aerogels. J. Mater. Res. 2017;32:4153–4165. doi: 10.3791/57875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu C., Su J., Xu X., Liu P., Zhao H., Tian F., Ding Y. Low temperature CO oxidation over unsupported nanoporous gold. J. Am. Chem. Soc. 2007;129:42–43. doi: 10.1021/ja0675503. [DOI] [PubMed] [Google Scholar]
- 35.Freytag A., Sánchez-Paradinas S., Naskar S., Wendt N., Colombo M., Pugliese G., Poppe J., Demirci C., Kretschmer I., Bahnemann D.W. Versatile aerogel fabrication by freezing and subsequent freeze-drying of colloidal nanoparticle solutions. Angew. Chem. Int. Ed. 2016;55:1200–1203. doi: 10.1002/anie.201508972. [DOI] [PubMed] [Google Scholar]
- 36.Feng J., Yin Y. Self-templating approaches to hollow nanostructures. Adv. Mater. 2019;31:1802349. doi: 10.1002/adma.201802349. [DOI] [PubMed] [Google Scholar]
- 37.Shi Q., Zhu C., Du D., Bi C., Xia H., Feng S. Kinetically controlled synthesis of AuPt bi-metallic aerogels and their enhanced electrocatalytic performances. J. Mater. Chem. A. 2017;5:19626–19631. [Google Scholar]
- 38.Wang H., Wu Y., Luo X., Jiao L., Wei X., Gu W., Du D., Lin Y., Zhu C. Ternary PtRuCu aerogels for enhanced methanol electrooxidation. Nanoscale. 2019;11:10575–10580. doi: 10.1039/c9nr02712b. [DOI] [PubMed] [Google Scholar]
- 39.Linic S., Aslam U., Boerigter C., Morabito M. Photochemical transformations on plasmonic metal nanoparticles. Nat. Mater. 2015;14:567–576. doi: 10.1038/nmat4281. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Z., Zhang C., Zheng H., Xu H. Plasmon-driven catalysis on molecules and nanomaterials. Acc. Chem. Res. 2019;52:2506–2515. doi: 10.1021/acs.accounts.9b00224. [DOI] [PubMed] [Google Scholar]
- 41.Chang X., Wang T., Yang P., Zhang G., Gong J. The development of cocatalysts for photoelectrochemical CO2 reduction. Adv. Mater. 2019;31:1804710. doi: 10.1002/adma.201804710. [DOI] [PubMed] [Google Scholar]
- 42.Liu L., Zhang X., Yang L., Ren L., Wang D., Ye J. Metal nanoparticles induced photocatalysis. Natl. Sci. Rev. 2017;4:761–780. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data needed to evaluate the conclusions in the paper are present in the paper and/or Supplemental Information. Additional data related to this paper can be requested from the authors.