Highlights
-
•
1.6% of hospitalized children below 5 years of age had a primary or any secondary discharge diagnosis of rotavirus.
-
•
Incidence of discharge diagnosis of rotavirus was 1071 and 542/100,000 person-years below 2 and 5 years of age, respectively.
-
•
A discharge diagnosis of rotavirus in children below 5 years of age likely under-reports true incidence by 1.59–2.02 times.
-
•
Adjusted and unadjusted incidence of rotavirus and all-cause gastroenteritis trended up, not down, from 1997 to 2011.
Keywords: Rotavirus, Gastroenteritis, Disease burden, Incidence, Surveillance
Abbreviations: ARSN, Asian Rotavirus Surveillance Network; CMS, Clinical Management System; HA, Hospital Authority; ICD9-CM, International Classification of Diseases; LOS, length of stay; PWH, Prince of Wales Hospital; WHO, World Health Organization
Abstract
Sentinel laboratory surveillance from one hospital and passive discharge diagnosis (Clinical Management System, CMS) data from all public Hospital Authority (HA) hospitals were used to estimate disease burden and incidence of rotavirus in hospitalised Hong Kong children over 14 rotavirus seasons (1 July 1997 to 31 March 2011). A primary diagnosis of a gastroenteritis-related disorder was noted in 9.8% of children aged below 5 years, and a primary or secondary diagnosis in 11.8%. Any CMS diagnosis of rotavirus (ICD 008.61) was initially used to derive incidence estimates of rotavirus by age group. Rotavirus was recorded as any primary or any secondary diagnosis in 1.6% of children below 5 years of age. The unadjusted incidence rates per 100,000 person-years based on any CMS diagnosis of rotavirus were: 249 (0 to <1m); 612 (1 to <2m); 1066 (2 to <6m); 1383 (6 to <11m); 959 (1 to <2y); 406 (2 to <3y); 233 (3 to <4y); 124 (4 to <5y). Overall the rotavirus incidence was 1071 in children below 2 years and 542 in children below 5 years of age, with the incidence rates trending up during the time period (p = 0.001). A similar but less marked upward trend (p = 0.046) was noted for the incidence of all-cause gastroenteritis. Laboratory results from a single surveillance hospital (1 July 2000 to 31 March 2011) were then linked to these CMS codes to derive adjustment factors for possible over- and under-diagnosis of rotavirus based on CMS codes alone. This analysis suggested that a CMS diagnosis of rotavirus alone likely under-reported true incidence by a factor of between 1.59 and 2.02 in children below 5 years of age. Despite the availability of rotavirus vaccines in the private sector since 2006, no reduction in the incidence of hospitalisation for either rotavirus or all-cause gastroenteritis was noted in Hong Kong children below 5 years of age over 14 rotavirus seasons (1997–2011).
1. Introduction
In 2013, the World Health Organization (WHO) confirmed its previous 2009 recommendation that rotavirus vaccines should be used in all countries [1]. Country-specific rotavirus disease burden data are important for informing decisions regarding rotavirus vaccine introduction into National Immunisation Programmes. However use of passive surveillance data alone has been shown to underestimate rotavirus disease burden in Hong Kong [2], highlighting the need for active rotavirus surveillance [3]. The first phase of the Asian Rotavirus Surveillance Network (ARSN) used a WHO surveillance protocol and was conducted in China, Hong Kong, Indonesia, Malaysia, Myanmar, South Korea, Taiwan, Thailand and Vietnam between 2001 and 2003 [3], [4]. Overall 30–55% of hospitalizations for diarrhoea in children aged less than 5 years were due to rotavirus [4], with Hong Kong reporting the lowest percentage (30%) [3]. These Hong Kong rotavirus admissions were associated with significant health care and societal costs [5], and an economic evaluation using a Markov model and 2002 cost assumptions estimated that the introduction of routine rotavirus vaccination at a cost of US$40–92 per course could be potentially cost-saving from a government perspective alone [6].
Countries that introduced universal rotavirus vaccination have witnessed a fall in rotavirus-associated hospitalisation rates in children under two years of age of around 70% in both developed countries (Australia [7], [8], Austria [9], Belgium [10], [11], United States [12]) and developing countries (Brazil [13], [14], El Salvador [15], [16], Mexico [17], Nicaragua [18], Panama [19]). Corresponding reductions in all-cause gastroenteritis admissions have been in the region of 35% [20]. Universal rotavirus vaccination may also reduce nosocomial infections [21], and provide indirect protection to unvaccinated older children and adults [22]. Although rotavirus vaccines were licensed in Hong Kong in 2006, they have not been included in the territory's universal childhood immunisation programme. The proportion of Hong Kong children that receive rotavirus vaccine through the private sector is unknown.
Discharge data has been collected routinely through the Hospital Authority's (Hong Kong's public hospital system, HA) central computerised database (Clinical Management System, CMS) since 1996 [3]. These CMS data are now analysed and combined with sentinel laboratory surveillance from the Prince of Wales Hospital (PWH) to make estimates of disease burden and incidence of rotavirus infection in hospitalised Hong Kong children over 14 rotavirus seasons from 1 July 1997 to 31 March 2011.
2. Methodology
2.1. CMS data
CMS information collected includes patient identifiers, date of birth, sex, a maximum of 15 diagnoses and 15 procedures (classified by International Classification of Diseases ICD9-CM codes), and admission and discharge dates [3]. The CMS was rolled out from 1996, and by mid-1997 this information was available for all HA hospitals. However prior to 2000 the majority of HA hospitals only coded the primary diagnosis for most hospital admissions. Paediatric patients with medical and surgical conditions are admitted to separate wards of government hospitals in Hong Kong. A database of all paediatric patients admitted to general paediatric and neonatal wards from 1 July 1997 to 31 March 2011 was provided by the HA. The ICD9-CM codes were classified as gastroenteritis-associated hospitalisations [2], and these diagnostic groups were assessed by hospital of admission, outcome status (died, discharged home with or without follow-up and discharged with acknowledgement to medical advice) and severity (length of stay).
2.2. Laboratory methods at Prince of Wales Hospital
All stool specimens collected from patients with symptoms of acute gastroenteritis admitted to the PWH were tested for rotavirus (ProSpecT Rotavirus, formerly known as IDEIA Rotavirus), DAKO (sensitivity of 100% and specificity of 99.2%) and cultured for bacteria.
2.3. Linking of CMS with laboratory data for Prince of Wales Hospital
Laboratory data for all paediatric admissions from the PWH were matched on the unique hospital number with the CMS data for the period 1 July 2000 to 31 March 2011. There were 100,330 separate admissions of patients below the age of 18 years in the CMS database and 8705 of these had unique hospital numbers (indicating unique admissions) in the laboratory database of stool specimens. All the analyses were based on the CMS calculated dayage (date of admission minus date of birth in days + 1) and monthage (dayage divided by 30.475). If there was more than one stool specimen for the same hospital admission (same hospital number) in the laboratory dataset, then only the first positive (if any) result was retained for analysis.
2.4. Calculation of rotavirus hospitalisation incidence rates by age group from CMS discharge diagnosis
Incidence rates of hospitalisation for rotavirus for all HA hospitals in Hong Kong were first estimated from the total number of children with any CMS diagnosis of rotavirus (ICD-CM 008.61) (CMS rotavirus+). These initial unadjusted CMS rotavirus incidence rates for each year were calculated:
where
x = number of admissions by age with any CMS rotavirus diagnosis (ICD-CM 008.61).
- y = admissions to public HA hospitals as a percentage of total admissions by age (Appendix 1). These proportions were weighted by the number of admissions when incidence estimates were calculated for different age groups:
where Admissionsj is the number of admissions in the jth age group, and P j is the proportion of admissions to HA hospitals in the jth age group. z = estimated resident population by age (Appendix 2)
Incidence rates were calculated by monthly age groups prior to the application of adjustment factors (see below). These monthly age group estimates were then re-grouped according to the following age ranges: 0d to <1m; 1m to <2m; 2m to <6m; 6m to <12m; 1y to <2y; 2y to <3y; 3y to <4y; 4y to <5y; 5y to <10y; 10y to <14y; 14y to <18y.
2.5. Calculation of adjustment factors
Since a CMS rotavirus diagnosis may reflect both under- and over-diagnosis [2], [3], we then applied adjustment factors to this CMS rotavirus incidence estimate. These factors were derived by linking the PWH laboratory surveillance data (LAB rotavirus + or −) with the PWH CMS data (CMS rotavirus + or −) (Table 1 , Appendix 3). The first factor was derived to adjust for potential under-reporting of rotavirus infection by the CMS system. The second factor was derived to reflect the potential under-estimation of a PWH laboratory diagnosis of rotavirus by accounting for the fact that not all admissions with a primary gastroenteritis-associated diagnosis had a stool specimen sent to the laboratory for testing. The third adjustment factor was the proportion of all admissions to PWH by age group that had a laboratory confirmed diagnosis of rotavirus.
Table 1.
Diarrhoea-associated hospitalizations by reported diagnosis (primary diagnosis only) from Clinical Management System (CMS) among 100,330 children aged 0 to <18 years admitted to the Prince of Wales Hospital, Hong Kong (1 July 2000 to 31 March 2011) compared with actual results from the laboratory for patients who had stool specimens sent for rotavirus testing to derive three adjustment factors to account for possible over or under-diagnosis of rotavirus based on CMS ICD diagnosis.
| Age | No. of admission | No. (%) with CMS diagnosis of gastroenteritis (GE)a | No. (%) with rotavirus CMS ICD diagnosis (008.61) (CMSRV+) | No. (%) stool specimen sent to lab | No. (%) with GE and stool specimen sent to lab | No. (%) with GE and stool specimen NOT sent to lab | No. (%) diagnosed rotavirus positive (RVLAB+) | RVLAB+ and CMSRV+ | RVLAB+ and CMSRV− | RVLAB− and CMSRV+ |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 to <12m | 37,488 | 2312 (6.2%) | 342 (0.9%) | 3438 (9.2%) | 1759 (76.1%) | 553 (23.9%) | 552 (1.5%) | 336 | 216 | 4 |
| 12 to <24m | 9049 | 1675 (18.5%) | 384 (4.2%) | 1876 (20.7%) | 1291 (77.1%) | 384 (22.9%) | 586 (6.5%) | 382 | 204 | 1 |
| 24 to <36m | 7189 | 1020 (14.2%) | 183 (2.5%) | 937 (13.0%) | 640 (62.7%) | 380 (37.3%) | 297 (4.1%) | 177 | 120 | 3 |
| 36 to <48m | 6653 | 812 (12.2%) | 115 (1.7%) | 666 (10.0%) | 433 (53.3%) | 379 (46.7%) | 174 (2.6%) | 112 | 62 | 1 |
| 48 to <60m | 4918 | 562 (11.4%) | 61 (1.2%) | 397 (8.1%) | 262 (46.6%) | 300 (53.4%) | 101 (2.1%) | 58 | 43 | 1 |
| 0d to <24m | 46,537 | 3987 (8.6%) | 726 (1.6%) | 5314 (11.4%) | 3050 (76.5%) | 937 (23.5%) | 1138 (2.4%) | 718 | 420 | 5 |
| 0d to <60m | 65,297 | 6381 (9.8%) | 1085 (1.7%) | 7314 (11.2%) | 4385 (68.7%) | 1996 (31.3%) | 1710 (2.6%) | 1065 | 645 | 10 |
| 0 to <18y | 100,330 | 8595 (8.6%) | 1222 (1.2%) | 8705 (8.7%) | 5150 (59.9%) | 3445 (40.1%) | 1954 (1.9%) | 1196 | 758 | 10 |
Primary diagnosis code only.
2.6. Incidence trends
Linear regression analysis was undertaken to determine whether there was a significant increase in incidence of CMS rotavirus, adjusted rotavirus and all-cause gastroenteritis admissions over the 14 seasons.
3. Results
During the study period 1 July 1997 to 31 March 2011, there were 1,312,424 admissions to the paediatric medical wards of Hong Kong's HA hospitals. Three had no gender specified and 118 had missing age data and were excluded. Of the 1,312,303 admissions with valid data, 95.1% (1,248,297) were below 18 years of age and 62.8% (824,514) were below 5 years of age. 1.6% (13,147/824,514) had “any” CMS diagnosis of rotavirus. Gastroenteritis-associated disorders were coded as the primary diagnosis in 9.8% (81,111) and 8.4% (104,527) of admissions of children aged below 5 and 18 years respectively, and as the primary or as one of any 9 secondary diagnoses in 11.8% (97,211) and 10.0% (124,728) respectively (Appendix 4). The rates of gastroenteritis-associated admissions varied considerably between the 12 HA hospitals providing general paediatric services, in both children aged below 5 and 18 years old (Appendix 5). Likewise the proportion of hospitalisations aged below 5 and 18 years of age with “any” diagnosis of rotavirus ranged from 0.1% to 2.8% and 0.1% to 2.0% respectively between these hospitals (Appendix 5). The total number of admissions to all HA hospitals with any CMS rotavirus diagnosis was highest during the first year of life (Appendix 6). Peaks of rotavirus admissions in Hong Kong were from November to March as previously noted [3], and the 2008/9 rotavirus season had the greatest total number of CMS rotavirus positive admissions (Appendix 7).
After linking the CMS data to the sentinel laboratory surveillance at PWH, there were 100,330 children below 18 years of age and 65,297 below 5 years of age admitted during the period 1 July 2000 to 31 March 2011 (Table 1). Rotavirus testing was requested for 8.7% (8705/100,330) of all the admissions below 18 years of age during this period and for 59.9% (5150/8595) of all patients with a primary diagnosis of gastroenteritis. Similarly, for the patients below 5 years of age there were 68.7% (4385/6381) with a primary CMS gastroenteritis diagnosis who had a stool specimen collected. In children below 5 years of age, the rotavirus positive rate for tested specimens was 23.4% (1710/7314), ranging from 13.0% to 33.8% during the 11 year period (Appendix 8). There were 1085 children below 5 years of age with “any” diagnosis coded as rotavirus (Table 1).
Adjustment factor 1 was not age related (Appendix 3) and a mean value of 1.59 was used for all age groups below 5 years of age. However adjustment factor 2 and adjustment factor 3 varied with age so a smoothed value was used for each age group below 5 years of age (Appendix 3). 1.7% of all admissions below 5 years of age had a CMS rotavirus diagnosis. Applying adjustment factor 1 (1.59) would increase this proportion to 2.7%. The proportion of all admissions at PWH due to rotavirus (adjustment factor 3) peaked between about 12–18 months (Appendix 3). Overall 2.4% of admissions to PWH below 2 years and 2.6% below 5 years of age had laboratory confirmation of rotavirus (Table 1 and Appendix 8). The proportion of all admissions that had laboratory confirmed rotavirus also varied by year of admission (Appendix 8).
The incidence rates of hospitalisation for rotavirus per 100,000 person-years were lowest in the first two months of life, then peaked between 7 and 8 months, and then declined from about 18 to 24 months (Fig. 1 ). Similar patterns were observed over all 14 rotavirus seasons of the study. The unadjusted incidence rates per 100,000 person-years based on any CMS rotavirus diagnosis (ICD = 008.61) were: 249 (0 to <1m); 612 (1 to <2m); 1066 (2 to <6m); 1383 (6 to <11m); 959 (1 to <2y); 406 (2 to <3y); 233 (3 to <4y); 124 (4 to <5y) (Table 2 ). Overall the incidence was 1071 in children below 2 years old and 542 in children below 5 years of age. Adjusted incidence rates were generally higher than the unadjusted rates (Table 2, Fig. 1). In the older age groups the effect of adjustment factor 3 was less i.e. this factor assumes that the proportion of rotavirus positive admissions as a proportion of all admissions by age group were the same in all HA hospitals as they were in PWH. Incidence rates of both unadjusted CMS rotavirus diagnosis (p = 0.001, standardised coefficient = 0.778) and adjusted rotavirus diagnosis (adjustment factor 1, p = 0.001, standardised coefficient = 0.778; adjustment factor 2, p = 0.001, standardised coefficient = 0.778; adjustment factor 3, p < 0.0001, standardised coefficient = 0.959) trended up over the 14 rotavirus seasons (Fig. 2 ). Although there was a similar upward trend for the incidence of all-cause gastroenteritis based on primary CMS diagnosis alone (p = 0.046, standardised coefficient = 0.540), this was less marked than that seen for rotavirus (Fig. 2).
Fig. 1.
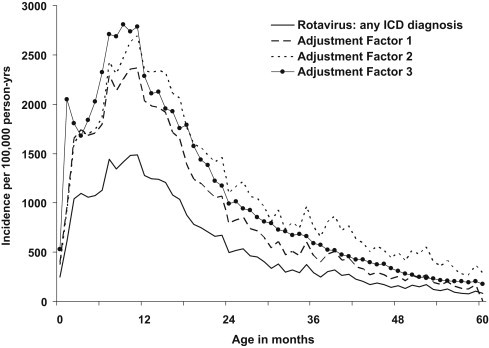
Incidence of hospitalisation by age for children aged below 5 years of age for rotavirus gastroenteritis (primary or secondary ICD code = 008.61) with and without application of adjustment factors derived from laboratory surveillance at one hospital, Hong Kong (1 July 1997 to 31 March 2011).
Table 2.
Incidence rates for hospitalisation with rotavirus and gastroenteritis per 100,000 person-years in Hong Kong (1 July 1997 to 31 March 2011).
| Estimates based on ANY CMS diagnosis of rotavirus (ICD9-CM 008.61) with NO adjustment | Estimates based on ANY CMS diagnosis of rotavirus (ICD9-CM 008.61) with adjustment for under- and over-CMS RV diagnosis based on data from Prince of Wales Hospital (adjustment factor 1)a | Estimates based on ANY CMS diagnosis of rotavirus (ICD9-CM 008.61) with adjustment for under- and over-CMS RV diagnosis and failure to send stool on all gastroenteritis-related admissions based on data from Prince of Wales Hospital (smoothed adjustment factor 2 omitting first month of life)a | Estimates based on assumption that laboratory confirmed rotavirus admissions as a proportion of all admissions by age group identified in Prince of Wales Hospital (laboratory confirmation) is the same proportion of all admissions to all HA hospitals (smoothed adjustment factor 3 with unsmoothed value for first month of life)a | Estimates based on primary ICD diagnosis of gastroenteritis | Estimates based on ANY primary OR secondary ICD diagnosis of gastroenteritis | |
|---|---|---|---|---|---|---|
| 0m | 249 | 396 | – | 529 | 2520 | 4380 |
| 1m | 612 | 973 | 925 | 2047 | 8745 | 9923 |
| 2 to <6m | 1066 | 1695 | 1684 | 1837 | 7224 | 8683 |
| 6m to <1y | 1383 | 2200 | 2389 | 2674 | 8428 | 10,376 |
| 1 to <2y | 959 | 1526 | 1908 | 1727 | 4473 | 5284 |
| 2 to <3y | 406 | 646 | 965 | 814 | 2532 | 3007 |
| 3 to <4y | 233 | 371 | 655 | 455 | 1775 | 2099 |
| 4 to <5y | 124 | 198 | 406 | 233 | 1143 | 1338 |
| 5 to <10y | 33 | 446 | 515 | |||
| 10 to <14y | 5 | 136 | 163 | |||
| 14 to <18y | 1 | 65 | 84 | |||
| Total | 117 | 842 | 1004 | |||
| <24m | 1071 | 1704 | 1798 | 2000 | 6108 | 7378 |
| <60m | 542 | 863 | 1093 | 1029 | 3347 | 4011 |
See Appendix 3 for description of adjustment factors 1, 2, and 3.
Fig. 2.
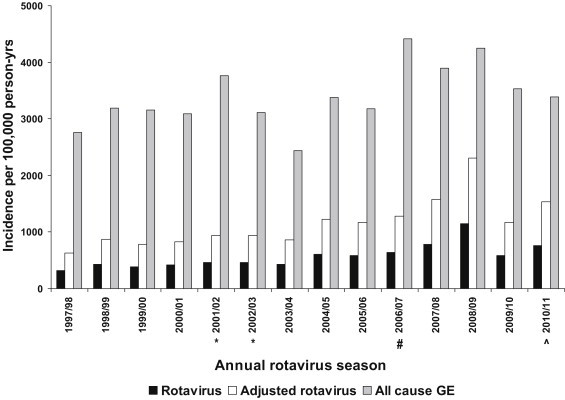
Incidence of hospitalisation by rotavirus season for children aged below 5 years of age for all-cause gastroenteritis (GE) (primary diagnosis only, grey bars), rotavirus gastroenteritis (primary or secondary ICD code = 008.61, black bars), or with application of adjustment factor 2 derived from laboratory surveillance at one hospital (see text, white bars), Hong Kong (1 July 1997 to 31 March 2011). *Asian Rotavirus Surveillance Network study period. #Rotavirus vaccine licensed for private sector use in Hong Kong. ^Population adjusted to account for shorter final study year 1 July 2010 to 31 March 2011 (9 months).
The length of stay (LOS, date of discharge minus date of admission plus one) is one measure of severity of illness. The median LOS for rotavirus diarrhoea (4 days) was longer than that of other viral gastroenteritis (3 days) for children aged both below 5 years and below 18 years of age (Appendix 9). The LOS in rotavirus was similar to that of bacterial and parasitic gastroenteritis. In PWH, there were two deaths in children below 18 years old during the period July 2000 to March 2011. Both children were over 5 years old and were coded respectively as gastroenteritis of presumed infectious and non-infectious diarrhoea and vomiting and gastritis. No patient coded as rotavirus gastroenteritis died. Most of the patients were discharged without follow up, and 13.5% of the patients coded as rotavirus gastroenteritis below 5 years of age had a follow-up visit scheduled after discharge. This proportion was similar to that of patients with other viral and non-specific gastroenteritis but less than that for patients with bacterial and parasitic gastroenteritis (Appendix 9). Similar patterns were seen for all patients admitted to other HA hospitals, although the proportions of patients with follow-up was greater. There were 5 deaths in all HA hospitals coded as a gastroenteritis-related illness, and three of these were below 5 years of age.
4. Discussion
The unadjusted incidence rates per 100,000 person-years based on any CMS diagnosis of rotavirus hospitalisation were 542 for children below 5 years of age. However after applying adjustment factors 1, 2 and 3 (derived from laboratory data from one hospital) these estimates increased to 863, 1093 and 1029 respectively, indicating the potential magnitude of under-coding and/or under-testing. Stool specimens were actively collected at four hospitals during the two-year period 2001–2003 as part of the Asian Rotavirus Surveillance Network (ARSN) [4]. We previously reported for children below 5 years of age rotavirus incidence rates per 100,000 of 810–880 during this two-year active surveillance period [3]. The corresponding CMS rotavirus diagnosis incidence during this same two-year period was 300 per 100,000. Although our current analysis includes data from the ARSN study period, our results suggest that the adjusted rotavirus incidence estimates were similar during the same period but trended up during the subsequent rotavirus seasons (Fig. 2). The current analysis suggests that between 1.7% (unadjusted CMS diagnosis of rotavirus at one hospital), 2.6% (laboratory confirmed rotavirus confirmed at one hospital), and 3.4% (1.7% × 2.02) (CMS diagnosis adjusted for under-diagnosis and under-testing with adjustment factor 2) of hospital admissions below 5 years of age were due to rotavirus infection. The ARSN surveillance data estimated a cumulative risk of admission by five years of age of 4.2% (1 in 24 children) [3]. Estimates of cumulative risk of admission by five years of age from the current analysis ranged from 1.4% (1 in 69 children) to 4.5% (1 in 22 children) during the 11 year period from 1 April 2000 to 31 March 2011 (Fig. 3 ). The cumulative risk of admission by five years of age was higher (4.5% and 4.2% or 1 in 22 and 1 in 24 children) during the period of ARSN surveillance (1 April 2001–31 March 2003) [3], and lowest (1.4% or 1 in 69 children) following a 2008/9 peak of rotavirus admissions (Fig. 3, Appendix 7). The possible reasons for the 2008/9 peak are unknown.
Fig. 3.
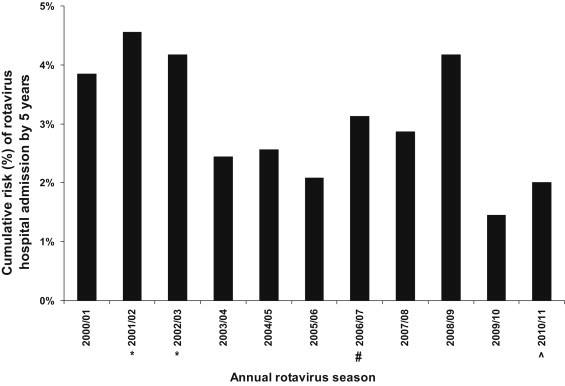
Cumulative risk of a Hong Kong child being admitted with rotavirus diarrhoea by the age of 5 years (assumes that proportion of laboratory confirmed rotavirus positive admissions at Prince of Wales Hospital is the same in all Hospital Authority hospitals in Hong Kong). *Asian Rotavirus Surveillance Network study period. #Rotavirus vaccine licensed for private sector use in Hong Kong. ^Population adjusted to account for shorter final study year 1 July 2010 to 31 March 2011 (9 months).
Our current analysis shows the potential of combining passive discharge diagnostic coding surveillance with sentinel laboratory surveillance to monitor disease burden of pathogens that are vaccine-preventable or potentially vaccine-preventable [2], [3], [23]. However even though all children admitted with gastroenteritis to PWH were anticipated to have stool specimens sent for rotavirus testing, this did not happen. In our analysis we have made estimates of possible increased disease burden had all children had specimens taken.
A number of assumptions have been made that should be considered when interpreting our results. First our incidence estimates assumed the proportion of admissions to public HA hospitals and the resident Hong Kong population was constant over the entire study period. In our previous analyses it was assumed that public hospitals catered for 90% of paediatric admissions and that all Hong Kong births utilised the Hong Kong health system [3]. However in recent years there have been two significant changes that challenge these assumptions. With improvements in the economy since the 1997 economic downturn, there has been an increased use of private hospital facilities (Appendix 1). Second there has been a significant increase in the number of cross-border births and these have accounted for up to 50% of all Hong Kong births in recent years. All children born in Hong Kong are eligible to obtain a Hong Kong identity card and access to Hong Kong medical care. There is limited data on the subsequent residence and health care utilisation of these cross border births i.e. some live with parent(s)/relatives in Hong Kong and others return to live in the Mainland, but still potentially seeking medical care in Hong Kong. PWH is in close proximity to the Hong Kong-Mainland border and likely caters for a greater proportion of children moving frequently to-and-fro for medical care, than do other more distant HA hospitals. These limitations mean that our incidence estimates (Table 2) are likely under-estimates rather than over-estimates since they exclude rotavirus admissions to hospitals in mainland China. However countering this is the fact that this analysis did not determine the proportion of children readmitted for the same diarrheal episode and those with nosocomial rotavirus infection, factors that could result in an overestimation of incidence. Also our adjustment for under-testing (adjustment factor 2) assumed that the rotavirus positive rate in untested stools was equal to that in tested stools and could therefore over-estimate true incidence since it is possible that children who are not tested have more mild disease and lower likelihood of rotavirus infection. Population census data were mid-year estimates (January to December), whereas the rotavirus admission data was calculated by rotavirus season (July to the following June). We have also assumed that the adjustment factors derived from one institution, PWH, can be applied uniformly across all the HA hospitals, and that these factors are stable over time. This may not be a valid assumption. Although PWH is one of the largest HA hospitals and one of the two university teaching hospitals in Hong Kong, it accounts for only about 10% of all the public hospital paediatric admissions. It is possible that there may be differences in clinical practices, admission policies and laboratory services between PWH and other HA hospitals and also over time. During the period 2001 to 2003 PWH was one of four HA hospitals to participate in active rotavirus surveillance as part of the ARSN, and it is likely that testing for rotavirus is more frequent at PWH than at some of the other HA hospitals. Ideally having laboratory data from other sentinel surveillance hospitals, that have a policy of routine testing for rotavirus, could strengthen our incidence estimates in future studies.
Despite the availability of rotavirus vaccines in Hong Kong since 2006, an upward trend of incidence rate of both rotavirus gastroenteritis and all-cause gastroenteritis is observed. Possible reasons for this could include the limited use of vaccine within the private sector. Increased awareness of rotavirus following the ARSN active surveillance period (2001–2003) and increased surveillance and infection control measures for respiratory and diarrhoeal diseases following the outbreak of severe acute respiratory syndrome in 2003, could have contributed to the increased testing for rotavirus during the latter part of the study period and improved accuracy of CMS coding. More permissive admission policies could be another explanation for the increased trends in incidence of rotavirus and all-cause gastroenteritis observed.
5. Conclusion
Estimates of the incidence of rotavirus that requires hospital admission were highest in children aged 6 months to below 2 years. After adjustment for likely under-coding and under-testing our overall estimates of incidence of rotavirus hospitalisation over 14 rotavirus seasons are similar to our previous estimates made during the active surveillance period 2001–2003 but trended up over the study period. Our data suggest that estimates of rotavirus disease burden and incidence can be derived from passive discharge data combined with data from laboratory surveillance but should ideally include data from more than one sentinel hospital.
Conflict of interest
EASN has received funding and support from Merck and Pfizer for diarrhoeal and respiratory disease surveillance studies, has participated in vaccine studies funded by Baxter, GlaxoSmithKline, MedImmune and Wyeth, and has received lecture fees and travel support from GlaxoSmithKline, Merck, Intercell and Pfizer. MI has received funding and support from Pfizer for respiratory disease surveillance studies. PKSC has participated in vaccine studies funded by Baxter, GlaxoSmithKline, MedImmune and Wyeth, and has received lecture fees and travel support from GlaxoSmithKline, Merck and Roche.
Acknowledgements
The Statistics and Workforce Planning Department in the Strategy and Planning Division of Hospital Authority provided the paediatrics hospital admission dataset from the HA clinical data repository for this study.
Footnotes
Supplementary material related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2014.01.065.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.World Health Organization Rotavirus vaccines: WHO position paper – January 2013. Wkly Epidemiol Rec. 2013;88(January (5)):49–64. [PubMed] [Google Scholar]
- 2.Nelson E.A., Tam J.S., Yu L.M., Glass R.I., Parashar U.D., Fok T.F. Surveillance of childhood diarrhoeal disease in Hong Kong, using standardized hospital discharge data. Epidemiol Infect. 2004;132(August (4)):619–626. doi: 10.1017/s0950268804002250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson E.A., Tam J.S., Bresee J.S., Poon K.H., Ng C.H., Ip K.S. Estimates of rotavirus disease burden in Hong Kong: hospital-based surveillance. J Infect Dis. 2005;192(September (Suppl. 1)):S71–S79. doi: 10.1086/431492. [DOI] [PubMed] [Google Scholar]
- 4.Nelson E.A., Bresee J.S., Parashar U.D., Widdowson M.A., Glass R.I. Asian Rotavirus SN Rotavirus epidemiology: the Asian Rotavirus Surveillance Network. Vaccine. 2008;26(June (26)):3192–3196. doi: 10.1016/j.vaccine.2008.03.073. [DOI] [PubMed] [Google Scholar]
- 5.Nelson E.A., Tam J.S., Yu L.M., Ng Y.C., Bresee J.S., Poon K.H. Hospital-based study of the economic burden associated with rotavirus diarrhea in Hong Kong. J Infect Dis. 2005;192(September (Suppl. 1)):S64–S70. doi: 10.1086/431493. [DOI] [PubMed] [Google Scholar]
- 6.Ho A.M., Nelson E.A., Walker D.G. Rotavirus vaccination for Hong Kong childr an economic evaluation from the Hong Kong Government perspective. Arch Dis Child. 2008;93(January (1)):52–58. doi: 10.1136/adc.2007.117879. [DOI] [PubMed] [Google Scholar]
- 7.Buttery J.P., Lambert S.B., Grimwood K., Nissen M.D., Field E.J., Macartney K.K. Reduction in rotavirus-associated acute gastroenteritis following introduction of rotavirus vaccine into Australia's National Childhood vaccine schedule. Pediatr Infect Dis J. 2011;30(1 Suppl.):S25–S29. doi: 10.1097/INF.0b013e3181fefdee. [DOI] [PubMed] [Google Scholar]
- 8.Field E.J., Vally H., Grimwood K., Lambert S.B. Pentavalent rotavirus vaccine and prevention of gastroenteritis hospitalizations in Australia. Pediatrics. 2010;126(3):e506–e512. doi: 10.1542/peds.2010-0443. [DOI] [PubMed] [Google Scholar]
- 9.Paulke-Korinek M., Rendi-Wagner P., Kundi M., Kronik R., Kollaritsch H. Universal mass vaccination against rotavirus gastroenteritis: impact on hospitalization rates in Austrian children. Pediatr Infect Dis J. 2010;29(4):319–323. doi: 10.1097/INF.0b013e3181c18434. [DOI] [PubMed] [Google Scholar]
- 10.Hanquet G., Ducoffre G., Vergison A., Neels P., Sabbe M., Van Damme P., Van Herck K. Impact of rotavirus vaccination on laboratory confirmed cases in Belgium. Vaccine. 2011;29(29–30):4698–4703. doi: 10.1016/j.vaccine.2011.04.098. [DOI] [PubMed] [Google Scholar]
- 11.Raes M., Strens D., Vergison A., Verghote M., Standaert B. Reduction in pediatric rotavirus-related hospitalizations after universal rotavirus vaccination in Belgium. Pediatr Infect Dis J. 2011;30(7):e120–e125. doi: 10.1097/INF.0b013e318214b811. [DOI] [PubMed] [Google Scholar]
- 12.Tate J.E., Mutuc J.D., Panozzo C.A., Payne D.C., Cortese M.M., Cortes J.E. Sustained decline in rotavirus detections in the United States following the introduction of rotavirus vaccine in 2006. Pediatr Infect Dis J. 2011;30(1 Suppl.):S30–S34. doi: 10.1097/INF.0b013e3181ffe3eb. [DOI] [PubMed] [Google Scholar]
- 13.Gurgel R.Q., Ilozue C., Correia J.B., Centenari C., Oliveira S.M.T., Cuevas L.E. Impact of rotavirus vaccination on diarrhoea mortality and hospital admissions in Brazil. Trop Med Int Health. 2011;16(9):1180–1184. doi: 10.1111/j.1365-3156.2011.02844.x. [DOI] [PubMed] [Google Scholar]
- 14.do Carmo G.M.I., Yen C., Cortes J., Siqueira A.A., de Oliveira W.K., Cortez-Escalante J.J. Decline in diarrhea mortality and admissions after routine childhood rotavirus immunization in Brazil: a time-series analysis. PLoS Med. 2011;8(4):e1001024. doi: 10.1371/journal.pmed.1001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yen C., Armero Guardado J.A., Alberto P., Rodriguez Araujo D.S., Mena C., Cuellar E. Decline in rotavirus hospitalizations and health care visits for childhood diarrhea following rotavirus vaccination in El Salvador. Pediatr Infect Dis J. 2011;30(1 Suppl.):S6–S10. doi: 10.1097/INF.0b013e3181fefa05. [DOI] [PubMed] [Google Scholar]
- 16.dePalma O., Cruz L., Ramos H., de Baires A., Villatoro N., Pastor D. Effectiveness of rotavirus vaccination against childhood diarrhoea in El Salvador: case–control study. BMJ. 2010;340:c2825. doi: 10.1136/bmj.c2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quintanar-Solares M., Yen C., Richardson V., Esparza-Aguilar M., Parashar U.D., Patel M.M. Impact of rotavirus vaccination on diarrhea-related hospitalizations among children <5 years of age in Mexico. Pediatr Infect Dis J. 2011;30(1 Suppl.):S11–S15. doi: 10.1097/INF.0b013e3181fefb32. [DOI] [PubMed] [Google Scholar]
- 18.Patel M., Pedreira C., de Oliveira L.H., Tate J., Orozco M., Mercado J. Association between pentavalent rotavirus vaccine and severe rotavirus diarrhea among children in Nicaragua. JAMA. 2009;301(21):2243–2251. doi: 10.1001/jama.2009.756. [DOI] [PubMed] [Google Scholar]
- 19.Molto Y., Cortes J.E., de Oliveira L.H., Mike A., Solis I., Suman O. Reduction of diarrhea-associated hospitalizations among children aged <5 years in Panama following the introduction of rotavirus vaccine. Pediatr Infect Dis J. 2011;30(1 Suppl.):S16–S20. doi: 10.1097/INF.0b013e3181fefc68. [DOI] [PubMed] [Google Scholar]
- 20.Patel M.M., Glass R., Desai R., Tate J.E., Parashar U.D. Fulfilling the promise of rotavirus vaccines: how far have we come since licensure? Lancet Infect Dis. 2012;12(7):561–570. doi: 10.1016/S1473-3099(12)70029-4. [DOI] [PubMed] [Google Scholar]
- 21.Macartney K.K., Porwal M., Dalton D., Cripps T., Maldigri T., Isaacs D. Decline in rotavirus hospitalisations following introduction of Australia's national rotavirus immunisation programme. J Paediatr Child Health. 2011;47(5):266–270. doi: 10.1111/j.1440-1754.2010.01953.x. [DOI] [PubMed] [Google Scholar]
- 22.Lopman B.A., Curns A.T., Yen C., Parashar U.D. Infant rotavirus vaccination may provide indirect protection to older children and adults in the United States. J Infect Dis. 2011;204(7):980–986. doi: 10.1093/infdis/jir492. [DOI] [PubMed] [Google Scholar]
- 23.Nelson E.A.S. Disease burden of diarrhoeal and respiratory disorders in children: Hong Kong perspectives. In: Preedy V.R., Watson R.R., editors. Handbook of disease burdens and quality of life measures. Springer; New York: 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


