Abstract
We have reported on aristeromycin (1) and 6′-fluorinated-aristeromycin analogues (2), which are active against RNA viruses such as Middle East respiratory syndrome coronavirus (MERS-CoV), severe acute respiratory syndrome coronavirus (SARS-CoV), Zika virus (ZIKV), and Chikungunya virus (CHIKV). However, these exhibit substantial cytotoxicity. As this cytotoxicity may be attributed to 5′-phosphorylation, we designed and synthesized one-carbon homologated 6′-fluorinated-aristeromycin analogues. This modification prevents 5′-phosphorlyation by cellular kinases, whereas the inhibitory activity towards S-adenosyl-l-homocysteine (SAH) hydrolase will be retained. The enantiomerically pure 6′-fluorinated-5′-homoaristeromycin analogues 3a-e were synthesized via the electrophilic fluorination of the silyl enol ether with Selectfluor, using a base-build up approach as the key steps. All synthesized compounds exhibited potent inhibitory activity towards SAH hydrolase, among which 6′-β-fluoroadenosine analogue 3a was the most potent (IC50 = 0.36 μM). Among the compounds tested, 6′-β-fluoro-homoaristeromycin 3a showed potent antiviral activity (EC50 = 0.12 μM) against the CHIKV, without noticeable cytotoxicity up to 250 μM. Only 3a displayed anti-CHIKV activity, whereas both3a and 3b inhibited SAH hydrolase with similar IC50 values (0.36 and 0.37 μM, respectively), which suggested that 3a’s antiviral activity did not merely depend on the inhibition of SAH hydrolase. This is further supported by the fact that the antiviral effect was specific for CHIKV and some other alphaviruses and none of the homologated analogues inhibited other RNA viruses, such as SARS-CoV, MERS-CoV, and ZIKV. The potent inhibition and high selectivity index make 6′-β-fluoro-homoaristeromycin (3a) a promising new template for the development of antivirals against CHIKV, a serious re-emerging pathogen that has infected millions of people over the past 15 years.
Keywords: 6′-fluorohomoaristeromycin, Anti-RNA virus, Chikungunya, S-adenosylhomocysteine hydrolase, Electrophilic fluorination
1. Introduction
RNA viruses have RNA as their genetic material and many important human pathogens have single-stranded RNA (ssRNA) in their genome [1]. The Baltimore classification distinguishes three groups of RNA viruses based on the nature of their genome and mode of replication: double-stranded RNA viruses, negative-sense ssRNA viruses, and positive-sense ssRNA (+RNA) viruses [1,2]. The +RNA viruses form the largest group and contain many important human pathogens, such as dengue virus, hepatitis C virus, severe acute respiratory syndrome (SARS) coronavirus [3], middle east respiratory syndrome (MERS) coronavirus [4], Zika virus (ZIKV) [[5], (a), (b)], and chikungunya virus (CHIKV) [6]. Antiviral therapy is still lacking for most of these viruses that have a serious impact on human health. Thus, it is desirable to develop new antiviral agents for the treatment of viral diseases caused by RNA viruses.
The genome of a +RNA virus functions as messenger RNA (mRNA) and can be directly translated by host ribosomes into viral (poly)proteins that ultimately form the replication complexes that drive the replication of viral RNA. The 5′-end of the genomes of most +RNA viruses contain a cap structure that is important for translation, RNA stability and evasion of host innate immune responses. Capping of viral RNA is usually carried out by one or multiple viral proteins that use S-adenosylmethionine (SAM) as the methyl donor.
Cellular S-adenosylhomocysteine (SAH) hydrolase is a pivotal enzyme in the regeneration cycle of SAM, which serves as a methyl donor in cellular methylation reactions and is required for viral mRNA capping [7]. Inhibition of SAH hydrolase leads to the accumulation of SAH, which in turn inhibits SAM-dependent methylation reactions, including those required for the maturation of viral RNA [[7], (a), (b)]. Thus, SAH hydrolase is a promising target for the development of broad-spectrum antiviral agents[[8], (a), (b)].
A variety of carbocyclic adenosine analogues with antiviral activity, including aristeromycin are assumed to exert their antiviral action via the inhibition of SAH hydrolase [9]. A close correlation has been detected between the antiviral effects of various carbocyclic and acyclic adenosine analogues and their inhibitory effects on SAH hydrolase in biochemical assays with purified protein [9]. These compounds were observed to be weak inhibitors of flavivirus replication in plaque reduction assays [[10], (b)], whereas they exhibited potent antiviral activity against the replication of ss(−)RNA viruses such as Ebola [(b), [11], (a)]. Among these, aristeromycin (1) (Fig. 1 ) is the representative carbocyclic nucleoside, which was first synthesized in racemic form in 1966 and shortly thereafter, isolated from the fermentation broth of Streptomyces citricolor [(e), (f), (g), (h), [12], (a), (b), (c), (d)]. It displays potent antiviral activity against several RNA viruses, that result from the inhibition of the SAH hydrolase [8]. Although it is a potent SAH hydrolase inhibitor, its therapeutic utility is limited, because of its significant toxicity [(b), [13], (a)]. This cytotoxicity may be caused by 5′-phosphorylation of the compound and its inhibition of cellular RNA polymerases and/or incorporation into cellular RNA[(b), [13], (a)].
Fig. 1.
Rationale for the design of 6′-fluorinated-5′-homoaristeromycin analogues 3a–e.
Based on the potent antiviral activity of 1, we recently reported the synthesis of enantiomerically pure 6′-fluorinated-aristeromycin analogues and their potent anti-RNA virus activities [14]. Among these, 6,6′-difluoro-aristeromycin 2 exhibits potent antiviral activities against MERS-CoV, SARS-CoV, ZIKV, and CHIKV [14]. However, compound 2 still exhibited substantial cytotoxicity, although it was less cytotoxic than 1. This cytotoxicity may be attributed to 5′-phosphorylation, similar to what is presumed for 1. Therefore, it is desirable to design aristeromycin analogues that retain their inhibitory activity towards SAH hydrolase without being 5′-phosphorylated by cellular kinases. Therefore, our strategy was to design 5′-homologated analogues that are expected to displace the phosphate-susceptible 5′-hydroxyl from the phosphate-transfer zone in the kinases. The feasibility of this strategy is supported by the fact that (−)-homoaristeromycin is inactive against HSV-1 and HSV-2, possibly, due to its failure to be phosphorylated [15]. Thus, we designed and synthesized 6′-fluorinated-5′-homoaristeromycin analogues 3a–e, which combined the one-carbon homologation at the 5′-position with the bioisosteric displacement of the hydrogen with the fluorine at the 6′-position. It was expected that compounds 3a–e would retain the inhibitory activity against SAH hydrolase, whereas the lack of 5′-phosphorylation would lead to lower cytotoxicity.
Here, we report the synthesis of enantiomerically pure 6′-fluorinated-5′-homoaristeromycin analogues 3a–e via the electrophilic fluorination [14,16,17] of the silyl enol ether with Selectfluor as the key step and assessment of their antiviral activity.
2. Results and discussion
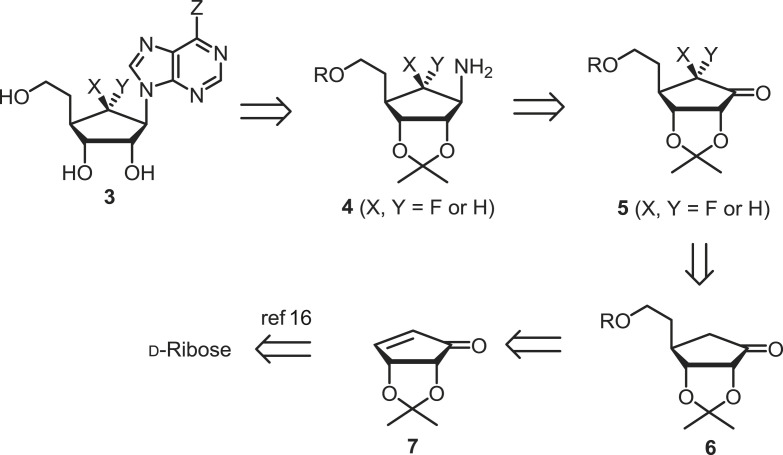
To synthesize the final nucleosides 3a–e, we designed the retrosynthetic analysis, as illustrated in Scheme 1 . Final nucleoside 3 was derived from amino intermediate 4 by the linear purine base build-up approach. Amino derivative 4 was easily derived from ketone 5 via the stereoselective reduction of the ketone and SN2 displacement with azide as key steps. 6-Fluoroketone 5 was synthesized using stereoselective electrophilic fluorination of silyl enol ether, which was obtained from key intermediate 6. Ketone 6 was derived from d-cyclopentenone 7, using a Michael reaction as a key step. Intermediate 7 was efficiently derived from D-ribose according to our previously published procedure with a minor modification [16,17].
Scheme 1.
Retrosynthetic analysis of target nucleoside 3.
Key intermediate 6 was synthesized according to our previously published procedure, as shown in Scheme 2 [17]. Briefly, D-ribose was converted to diene 8 in four steps without purification in a 64% yield. The ring-closing metathesis (RCM) reaction of 6 with Neolyst M2 instead of Grubbs’ catalyst, followed by pyridinium dichromate (PDC) oxidation afforded the cyclopentenone 7 in a 56% yield. This modified procedure created desired intermediate 7 in six steps with a 36% overall yield [16].
Scheme 2.
Synthesis of key intermediate 6 [17].
The Michael addition of 7 with vinylmagnesium bromide in the presence of copper(I) bromide afforded vinyl ketone 9 [18] in a 76% yield. Reduction of 9 with sodium borohydride yielded α-alcohol 10a as the single stereoisomer, which was protected with a tert-butyldiphenylsilyl (TBDPS) group to yield 10b. The hydroboration-oxidation of 10b with a borane-dimethyl sulphide complex afforded primary alcohol 11. The removal of the TBDPS group of 11 with tetra-n-butylammonium fluoride (TBAF) yielded diol 12, in which the primary hydroxyl group was selectively protected with a TBDPS group to afford 13. Oxidation of the remaining secondary alcohol in 13 with tetra-n-propylammonium perruthenate (TPAP) and N-methylmorpholine N-oxide (NMO) afforded ketone 6 [17], which served as the key starting material for the electrophilic fluorination.
Ketone 6 was converted to the amines 18a and 18b, which were the key precursors for the synthesis of the final nucleosides, as shown in Scheme 3 . Ketone 6 was treated with lithium bis(trimethylsilyl)amide (LiHMDS), followed by trapping the resulting enolate with chlorotriethylsilane (TESCl) to produce silyl enol ether. The silyl enol ether was treated with electrophilic fluorine, Selectfluor (1-chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane ditetrafluoroborate) to produce desired 6-β-fluoro-cyclopentanone 14 as the single stereoisomer, which was equilibrated to geminal diol because of the electronegative fluorine atom [14,16,17]. The β stereochemistry of fluorine was obtained because of the steric hindrance by the 2,3-isopropylidene group, which was confirmed by long-range coupling between H-8 and 6′-β-F and X-ray crystallography after conversion to adenosine derivative 3a. 6,6-Difluoro ketone 15 was synthesized from 14 in a 50% yield using the same procedure. Fluoro ketones 14 and 15 were reduced with sodium borohydride to produce alcohols 16a and 16b, respectively. First, we attempted a direct condensation reaction with 6-chloropurine under the Mitsunobu conditions to obtain the desired nucleoside, but it failed to yield the desired condensed product. Direct SN2 reactions of triflates synthesized from 16a and 16b with a 6-chloropurine anion were also unsuccessful. Thus, we decided to utilize the linear purine base build-up approach [14]. The SN2 displacement of triflates of 16a and 16b with a linear, less bulky and more powerful nucleophile, NaN3 proceeded smoothly to yield azido derivatives 17a and 17b, respectively. Catalytic reduction of 17a and 17b with 10% Pd/C yielded amines 18a and 18b, respectively.
Scheme 3.
Synthesis of precursors 18a and 18b for purine base build-up.
6-Fluoroamine 18a was treated with 5-amino-4,6-dichloropyrimidine and triethylamine in n-butanol under microwave irradiation to yield 19a (Scheme 4 ) [14,16]. However, weaker nucleophile, 6,6-difluoroamine 18b did not give desired product 19b under the same microwave conditions. Thus, we used a stronger electrophile, 4,6-dichloro-5-formamidopyrimidine rather than 5-amino-4,6-dichloropyrimidine, which afforded the desired product 19b in a good yield. The cyclisation of 19a and 19b with diethoxymethyl acetate yielded 6-chloropurine nucleosides 20a and 20b, respectively. The H-8 proton in the 1H NMR spectrum of 20a was split as a doublet with a small coupling constant (J = 2.4 Hz), which resulted from the long-range coupling between H-8 and 6′-β-F, which confirmed the desired stereochemistry of 6′-F, as well as 6-chloropurine.
Scheme 4.
Synthesis of 6′-fluorinated homoaristeromycin analogues 3a–3e.
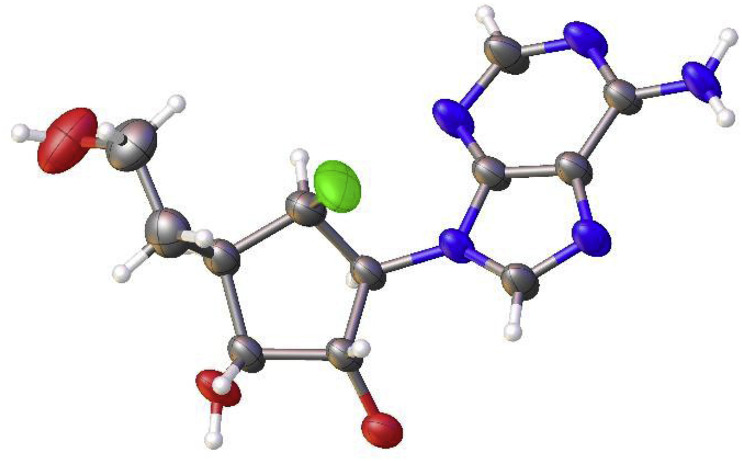
Treatment of 20a and 20b with 50% aqueous trifluoroacetic acid yielded 6-chloropurine nucleosides 21a and 21b, respectively. For the synthesis of adenosine derivatives, 21a was treated with saturated tert-butanolic ammonia to yield 3a, whereas 21b was converted to 3d using saturated methanolic ammonia solution in a steel bomb. Treatment of 21a and 21b with 40% aqueous methylamine afforded N [6]-methyladenosine nucleosides 3b and 3e, respectively. Hydrolysis of 21a with 1 N HCl in 1,4-dioxane yielded inosine derivative 3c. The structure of 3a was confirmed by single-crystal X-ray crystallography [19] (Fig. 2 ).
Fig. 2.
The X-ray crystal structure of 3a.
2.1. Biological activity
The synthesized nucleosides 3a-3e were assayed for their inhibitory activity against SAH hydrolase as well as their antiviral activity against the +RNA viruses, MERS-CoV, SARS-CoV, ZIKV, and CHIKV using cytopathic effect (CPE) reduction assays (Table 1 ) [14].
Table 1.
Inhibitory activity towards SAH hydrolase and the anti-CHIKV activity in Vero E6 cells of the nucleosides 3a-3e and 2a-2b.
| Compound No | SAH hydrolase IC50 (μM) | CHIKV |
|
|---|---|---|---|
| EC50 (μM) | CC50 (μM) | ||
| 3a (n = 2, X = F, Y = H, Z = NH2) | 0.36 | 0.12 | >250 |
| 3b (n = 2, X = F, Y = H, Z = NHMe) | 0.37 | >100 | >100 |
| 3c (n = 2, X = F, Y = H, Z = OH) | >100 | >100 | >100 |
| 3d (n = 2, X = F, Y = F, Z = NH2) | 2.03 | >100 | >25 |
| 3e (n = 2, X = F, Y = F, Z = NHMe) | 3.05 | >100 | >100 |
| 2a (n = 1, X = F, Y = H, Z = NH2) | 0.37 | >100 | >100 |
| 2b (n = 1, X = F, Y = F, Z = NH2) | 1.06 | 0.13 | >1.25 |

The 6′-fluorinated adenosine analogues (n = 2) 3a and 3b exhibited potent inhibitory activity against SAH hydrolase. 6,6′-Difluoroadenosine analogues 3d and 3e also inhibited SAH hydrolase, but to a lesser extent. For example, 6′-β-fluoroadenosine analogue 3a was 5.6 times more potent than 6,6′-difluoroadenosine analogue 3d. A similar trend was observed with 6′-fluorinated-adenosine analogues (n = 1), 2a and 2b. 6′-β-Fluoro-N [6]-methyladenosine 3b showed similar inhibitory activity to that of 6′-β-fluoroadenosine 3a. A similar trend was observed with 6,6′-difluoro-adenosine 3d and 6,6′-difluoro-N [6]-methyladenosine 3e. However, the inosine analogue 3c did not inhibit SAH hydrolase, as no inhibitory activity was observed at the highest dose tested (100 μM).
Among the compounds tested, 6′-β-fluoro-homoaristeromycin 3a showed potent antiviral activity (EC50 = 0.12 μM) against CHIKV without noticeable cytotoxicity up to 250 μM (highest dose tested). This antiviral activity did not merely seem to depend on the inhibition of SAH hydrolase, as 3a and 3b inhibited the enzyme with similar IC50 values (0.36 and 0.37 μM, respectively), although only 3a displayed anti-CHIKV activity. Regular 6′-β-fluoro-aristeromycin 2a was inactive against CHIKV. In addition, although 6,6′-difluoro-aristeromycin 2b displayed broad-spectrum antiviral activity against several RNA viruses, including SARS-CoV, MERS-CoV, CHIKV, and ZIKV, the corresponding homologated analogue 3d did not exhibit antiviral activity, despite its potent inhibitory activity towards SAH hydrolase. These results demonstrate that other factors besides the inhibitory activity towards SAH hydrolase may be involved in the antiviral activity of this series. More detailed studies on the antiviral activity of 3a and its mode of action will be published elsewhere (Kovacikova et al., submitted).
3. Conclusion
To reduce the cytotoxicity of 6′-fluorinated aristeromycin analogues 2a and 2b, which is likely due to their 5′-phosphorylation by cellular kinases, we designed and synthesized 6′-fluorinated homoaristeromycin analogues 3a–3e. These nucleosides were synthesized from D-ribose, using the Michael reaction, stereoselective electrophilic fluorination, and linear purine base build-up as key steps. Among the compounds tested, 6′-β-fluoro-homoaristeromycin (3a) showed potent anti-CHIKV activity (EC50 = 0.12 μM) without noticeable cytotoxicity at concentrations up to 250 μM, which resulted in a high selectivity index (SI) of more than 2083. This indicates that one carbon homologation may prevent 5′-phosphorylation, which results in low cytotoxicity. This homologation had no effect on the inhibitory activity towards SAH hydrolase, as compounds 3a and 2a inhibited this enzyme with similar IC50 values (0.36 and 0.37 μM). The antiviral activity of 3a was specific for CHIKV and to a lesser extent some other alphaviruses, whereas other viruses have been shown to be sensitive to the SAH hydrolase inhibitors 2a and 2b. This suggests that the potent anti-CHIKV activity of 3a is based on more than merely the inhibition of SAH hydrolase. This study shows that 6′-β-fluoro-homoaristeromycin (3a) is a promising new template for the development of anti-CHIKV agents.
4. Experimental section
4.1. General methods
Proton (1H) and carbon 13C{1H} NMR spectra were obtained on a Bruker AV 400 (400/100 MHz), AMX 500 (500/125 MHz), Jeol JNM-ECA 600 (600/150 MHz) or Bruker AVANCE III 800 (800/200 MHz) spectrometer. The 1H NMR data were reported as peak multiplicities: s for singlet; d for doublet; dd for doublet of doublets; t for triplet; td for triplet of doublet; q for quartet; quin for quintet; bs for broad singlet and m for multiplet. Coupling constants were reported in Hertz. The chemical shifts were reported as ppm (δ) relative to the solvent peak. All reactions were routinely carried out under an inert atmosphere of dry nitrogen. IKA RCT basic type heating mantle was used to provide a constant heat source. Microwave-assisted reactions were carried out in sealed vessels using a Biotage Initiator+ US/JPN (part no. 356007) microwave reactor and the reaction temperatures were monitored by an external surface IR sensor. High resolution mass spectra were measured with electrospray-ionization quadrupole time-of-flight (ESI-Q-TOF) techniques. Melting points were recorded on Barnstead electrothermal 9100 instrument and are uncorrected. Reactions were checked by thin layer chromatography (Kieselgel 60 F254, Merck). Spots were detected by viewing under a UV light, and by colorizing with charring after dipping in a p-anisaldehyde solution. The crude compounds were purified by column chromatography on a silica gel (Kieselgel 60, 70–230 mesh, Merck). All the anhydrous solvents were redistilled over CaH2, or P2O5, or sodium/benzophenone prior to the reaction.
4.1.1. 3aS,4R,5R,6R,6aR)-6-(2-((tert-Butyldiphenylsilyl)oxy)ethyl)-5-fluoro-2,2-dimethyltetrahydro-4H-cyclopenta[d][1,3]dioxol-4-ol (16a)
To a stirred solution of 6 [17] (6.4 g, 14.5 mmol) in anhydrous tetrahydrofuran (105 mL) at −78 °C, under nitrogen atmosphere were added chlorotrietylsilane (9.73 mL, 58.0 mmol) and lithium bis (trimethylsilyl)amide (1.0 M solution in tetrahydrofuran, 29.0 mL, 29.0 mmol) and the reaction mixture was stirred at −78 °C for 1 h, warmed to 0 °C and quenched using saturated aqueous NH4Cl (30 mL). The solution was extracted with ethyl acetate (2 × 100 mL). The organic layer was dried using anhydrous MgSO4 and concentrated in vacuo to give the crude silyl enol ether, which was used immediately for the next step without further purification.
To a stirred solution of the crude silyl enol ether in anhydrous acetonitrile (120 mL) was added Selectfluor® (7.7 g, 21.8 mmol) at 0 °C and the reaction mixture was stirred for 16 h at 0 °C, diluted with brine (50 mL) and extracted with ethyl acetate (2 × 100). The organic layer was dried over anhydrous MgSO4, filtered and concentrated in vacuo. The residue was purified by silica gel column chromatography (hexanes:ethyl acetate = 5.6:1) to give 14 [17] (3.7 g, 84%) as colorless oil, which was equilibrated to the 6-fluorogeminal diol.
To a stirred solution of 14 (4.06 g, 8.89 mmol) in tetrahydrofuran (120 mL) was slowly added sodium borohydride (0.5 mg, 13.34 mmol) at −40 °C and the reaction mixture was stirred at −40 °C for 1 h and then at 0 °C for 3 h. The mixture was diluted with water (50 mL) and extracted with ethyl acetate (2 × 100 mL). The organic layer was dried over anhydrous MgSO4, filtered and concentrated in vacuo. The residue was purified by silica gel chromatography (hexanes:ethyl acetate = 11.5:1) to give 16a as colorless oil (2.62 g, 64%); [α]D 25 − 43.5 (c 0.14, CH3OH): 1H NMR (400 MHz, CDCl3) δ 7.68 (m, 4H), 7.40 (m, 6H), 4.9 (ddd, J = 3.6, 4.4, 50.8 Hz, 1H), 4.64 (m, 1H), 4.54 (m, 1H), 4.08 (m, 1H), 3.75 (m, 1H), 2.73 (dd, J = 2.8, 4.4 Hz, 1H), 2.48 (m, 1H), 1.89 (m, 1H), 1.66 (m, 1H), 1.51 (s, 3H), 1.34 (s, 3H), 1.05 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 135.8, 133.9, 129.8, 113.8, 101.6, 99.8, 84.0, 78.2, 72.3 (J = 27.5 Hz), 62.5, 43.5 (J = 72.0 Hz), 29.6 (J = 5.9 Hz), 26.5, 24.7, 19.3; 19F NMR (376 MHz, CDCl3) δ −204.7; HRMS (FAB) found 459.2366 [calcd for C26H36FO4Si+ (M + H)+ 459.2361].
4.1.2. (3aS,4R,6R,6aR)-6-(2-((tert-Butyldiphenylsilyl)oxy)ethyl)-5,5-difluoro-2,2-dimethyltetrahydro-4H-cyclopenta[d][1,3]dioxol-4-ol (16b)
To a stirred solution of 14 [17] (5.7 g, 10.03 mmol) in anhydrous tetrahydrofuran (100 mL) at 0 °C, under N2 atmosphere were added triethylamine (7.0 mL, 50.15 mmol), chlorotriethylsilane (8.4 mL, 50.15 mmol) and lithium bis trimethylsilyl amide (1.0 M solution in tetrahydrofuran, 25.1 mL, 25.1 mmol) and the reaction mixture was stirred at room temperature for 1 h, warmed to 0 °C and quenched using saturated aqueous ammonium chloride solution. The solution was extracted with ethyl acetate (2 × 100 mL). The organic layer was dried over anhydrous MgSO4 and concentrated in vacuo to give the crude silyl enol ether, which was used immediately for the next step without further purification.
To a stirred solution of the crude silyl enol ether in anhydrous acetonitrile (100 mL) was added a solution of Selectfluor® (7.11 g, 20.06 mmol) in acetonitrile (85 mL) at 0 °C. The reaction mixture was stirred overnight at room temperature, concentrated in vacuo and diluted with ethyl acetate (2 × 100 mL). The organic layer was dried over anhydrous MgSO4 and concentrated in vacuo. The residue was purified by silica gel column chromatography (hexanes:ethyl acetate = 2.3:1) to give 15 (2.37 g, 50%) as the 6,6-difluorogeminal diol.
To a stirred solution of 15 (2.19 g, 4.61 mmol) in tetrahydrofuran (45 mL) was added sodium borohydride (0.21 g, 5.53 mmol) at 0 °C over a period of 15 min. The reaction mixture was stirred at 0 °C for 3 h, neutralized with glacial acetic acid, diluted with water (30 mL), and extracted with ethyl acetate (2 × 40 mL). The organic layer was washed with brine (30 mL), dried over anhydrous MgSO4 and concentrated in vacuo. The residue was purified by silica gel column chromatography (hexanes:ethyl acetate = 9:1) to give 16b as colorless oil (1.79 g, 81%): [α]D 25 +18.8 (c 0.340, CHCl3); 1H NMR (500 MHz, CDCl3) δ 7.69–7.66 (m, 4H), 7.44–7.36 (m, 6H), 4.54 (m, 1H), 4.36–4.34 (m, 1H), 3.97 (ddd, J = 6.4, 12.5 Hz, 1H), 3.79–3.74 (m, 2H), 2.87 (d, J = 5.6 Hz, 1H), 2.65–2.60 (m, 1H), 1.97–1.91 (m, 1H), 1.58–1.52 (m, 4H), 1.32 (s, 3H), 1.05 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 135.6, 133.5, 129.6, 128.0 (J = 248.8, 263.5 Hz), 127.6, 112.9, 81.1, 75.2, 71.0 (J = 12.4, 19.8 Hz), 61.5, 44.9 (J = 20.5 Hz), 28.8, 26.8, 26.0, 24.4, 19.1; 19F NMR (CDCl3, 376 MHz) δ −112.7 (dd, J = 16.1, 241.9 Hz), −115.8 (dd, J = 24.8, 242.7 Hz); HRMS (FAB) found 494.2540 [calcd for C26H38F2NO4Si+ (M + NH4)+ 494.2533].
4.1.3. (2-((3aR,4R,5R,6S,6aS)-6-Azido-5-fluoro-2,2-dimethyltetrahydro-4H-cyclopenta[d][1,3]dioxol-4-yl)ethoxy)(tert-butyl)diphenylsilane (17a)
To a stirred solution of 16a (2.35 g, 5.12 mmol) in anhydrous pyridine (50 mL) was added triflic anhydride (1.72 mL, 10.24 mmol) at 0 °C and the reaction mixture was stirred at 0 °C for 1 h, quenched with water (3 mL) and concentrated in vacuo. The residue was diluted with ethyl acetate (100 mL) and washed with 15% aqueous CuSO4 solution (3 × 30 mL). The organic layer was dried over anhydrous MgSO4, filtered and concentrated in vacuo to get the crude triflate, which was used immediately for the next step without further purification.
To a stirred solution of the crude triflate in anhydrous DMF (50 mL) was added sodium azide (3.08 g, 51.2 mmol) at room temperature and the reaction mixture was stirred at 100 °C for 12 h, cooled to room temperature and diluted with water (10 mL). The solution was extracted with diethyl ether (100 mL). The organic layer was washed with water (5 × 50 mL), dried anhydrous MgSO4, filtered and concentrated in vacuo. The residue was purified by silica gel column chromatography (hexanes:ethyl acetate = 32.3:1) to give 17a as colorless oil (0.36 g, 90%): [α]D 25 − 98.7 (c 0.155, CH3OH); 1H NMR (400 MHz, CDCl3) δ 7.69 (m, 4H), 7.41 (m, 6H), 4.92 (dt, J = 3.2, 52.8 Hz, 1 H), 4.68 (m, 1H), 4.45 (m, 1H), 3.79 (m, 2H), 3.67 (m, 1H), 2.34 (m, 1H), 1.87 (m, 1H), 1.52 (s, 3H), 1.32 (s, 3H), 1.07 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 135.8, 133.8, 129.9, 127.8, 114.2, 99.4, 97.6, 83.6, 82.3, 68.3 (J = 15.7 Hz), 61.9, 45.5 (J = 17.7 Hz), 30.3 (J = 4.6 Hz), 27.4, 24.9, 19.3; 19F NMR (376 MHz, CDCl3) δ −205.5; HRMS (FAB) found 506.2254 [calcd for C26H34FN3NaO3Si+ (M + Na)+ 506.2246].
4.1.4. (2-((3aR,4R,6S,6aS)-6-azido-5,5-difluoro-2,2-dimethyltetrahydro-4H-cyclopenta[d][1,3]dioxol-4-yl)ethoxy)(tert-butyl)diphenylsilane (17b)
To a stirred solution of 16b (1.65 g, 3.46 mmol) in anhydrous pyridine (30 mL) was added triflic anhydride (1.16 mL 6.92 mmol) at 0 °C. The reaction mixture was stirred at 0 °C for 1 h, quenched with saturated aqueous sodium bicarbonate solution (5 mL), concentrated in vacuo to get the crude residue, which was diluted with ethyl acetate (40 mL), washed with 15% aqueous CuSO4 solution and extracted with ethyl acetate (2 × 30 mL). The organic layer was washed with water, dried over MgSO4 and concentrated in vacuo. The residue was purified by silica gel column chromatography (hexanes:ethyl acetate = 2.5:1) to give the triflate, which was used immediately for the next step.
To a stirred solution of the triflate in anhydrous DMF (30 mL) was added sodium azide (0.62 g, 10.38 mmol) at room temperature and the reaction mixture was stirred at 60 °C for 12 h, cooled to room temperature and diluted with water (10 mL). The mixture was extracted with diethyl ether (2 × 40 mL) and the organic layer was washed with water (5 × 30 mL), dried over anhydrous MgSO4 and concentrated in vacuo. The residue was purified by silica gel column chromatography (hexanes:ethyl acetate = 19:1) to give 17b as colorless oil (1.12 g, 64%): [α]D 25 +14.6 (c 0.335, CHCl3); 1H NMR (500 MHz, CDCl3) δ 7.69–7.67 (m, 4H), 7.44–7.36 (m, 6H), 4.40–4.38 (m, 1H), 4.30–4.27 (m, 1H), 3.94 (dt, J = 5.3, 17.6 Hz, 1H), 3.77 (t, J = 6.2 Hz, 2H), 2.66–2.59 (m, 1H), 2.02–1.96 (m, 1H), 1.75–1.68 (m, 1H), 1.52 (s, 3H), 1.28 (s, 3H), 1.04 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 135.6, 133.5, 129.6, 127.6, 127.3(J = 270.0, 275.0 Hz), 113.5, 80.1 (J = 8.6 Hz), 79.5 (J = 7.0 Hz), 68.6 (J = 18.9, 22.8 Hz), 60.9, 46.5 (J = 20.1 Hz), 28.7 (J = 4.3 Hz), 29.0, 26.8, 24.7, 19.2; 19F NMR (376 MHz, CDCl3) δ −104.5, −120.2; HRMS (FAB) found 524.2161 [calcd for C26H33F2N3NaO3Si+ (M + Na)+ 524.2151].
4.1.5. N [4]-((3aS,4S,5R,6R,6aR)-6-(2-((tert-Butyldiphenylsilyl)oxy)ethyl)-5-fluoro-2,2-dimethyltetrahydro-4H-cyclopenta[d][1,3]dioxol-4-yl)-6-chloropyrimidine-4,5-diamine (19a)
To a stirred solution of 17a (0.991 g, 2.04 mmol) in methanol (50 mL) was added 10% palladium on carbon (wet with ca. 55% water) (250 mg) at room temperature and the reaction mixture was stirred under H2 for 3 h. After completion of reaction (TLC), the suspension was filtered through a pad of Celite and concentrated to give the amine 18a as colorless syrup, which was used for the next step without further purification.
To a solution of the crude 18a (0.933 g, 2.04 mmol) in n-butanol (20 mL) were added 5-amino-4,6-dichloropyrimidine and N,N-diisopropylethylamine at room temperature and the reaction mixture was subjected to microwave irradiation at 170 °C for 10 h. After cooled to room temperature, the reaction mixture was concentrated in vacuo, diluted with ethyl acetate (30 mL), washed with saturated aqueous NaHCO3, dried over anhydrous MgSO4, filtered, and concentrated in vacuo. The residue was purified by silica gel chromatography (hexanes:ethyl acetate = 4.6:1) to give 19a as colorless oil (0.91 g, 76%): [α]D 25 − 19.7 (c 0.167, CHCl3); 1H NMR (500 MHz, CDCl3) δ 8.07 (s, 1H), 7.60–7.64 (m, 4H), 7.44–7.34 (m, 6H), 5.33 (d, J = 7.9 Hz), 5.05 (dt, J = 3.0, 53.3 Hz, 1H), 4.75–4.64 (m, 1H), 4.61 (merged dd, J 1 = J 2 = 6.7 Hz, 1H), 4.44 (merged dd, J 1 = J 2 = 6.7 Hz, 1H), 3.82–3.71 (m, 2H), 3.60–3.45 (brs, 2 H), 2.51–2.37 (m, 1H), 1.94–1.80 (m, 2H), 1.54 (s, 3H), 1.30 (s, 3H), 1.03 (s, 9H); 13C NMR (150 MHz, CDCl3) δ 154.3, 149.3, 143.2, 135.5, 133.6, 129.6, 127.6, 122.3, 114.1, 98.3 (J = 211.8 Hz), 84.1, 83.4, 61.8, 60.3 (J = 19.4 Hz), 45.6 (J = 21.8 Hz), 30.4 (J = 5.6), 27.3, 26.8, 24.9, 19.1 19F NMR (376 MHz, CDCl3) δ −208.0; HRMS (FAB) found 585.2468 [calcd for C30H39ClFN4O3Si+ (M + H)+ 585.2458].
4.1.6. 9-((3aS,4S,5R,6R,6aR)-6-(2-((tert-Butyldiphenylsilyl)oxy)ethyl)-5-fluoro-2,2-Dimethyltetrahydro-4H-cyclopenta[d][1,3]dioxol-4-yl)-6-chloro-9H-purine (20a)
A stirred mixture of 19a (0.91 g, 1.56 mmol) and diethoxymethyl acetate (15 mL) was heated at 120 °C for 14 h and cooled to room temperature. The solvent was removed under reduced pressure and the residue was purified by silica gel chromatography (hexanes:ethyl acetate = 9:1) to give 20a as a white foam (0.87 g, 93%): [α]D 25 − 12.3 (c 0.162, CH3OH); UV (CH3OH) λ max 263 nm; 1H NMR (400 MHz, CDCl3) δ 8.78 (s, 1H), 8.32 (d, J = 2.4 Hz, 1H), 7.68 (m, 4H), 7.4 (m, 6H), 5.18 (m, 1H), 5.08 (m, 2H), 4.62 (m, 1H), 3.81 (m, 2H), 2.64 (m, 1H), 1.95 (m, 2H), 1.59 (s, 3H), 1.34 (s, 3H), 1.06 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 152.3, 144.2, 135.7, 133.7, 129.9, 127.9, 115.3, 99.2, 97.4, 83.4, 83.0, 63.3, 61.7, 46.0, 30.5, 27.6, 27.0, 25.1, 19.3; 19F NMR (376 MHz, CDCl3) δ −200.72; HRMS (FAB) found 595.2290 [calcd for C31H37ClFN4O3Si+ (M + H)+ 595.2302].
4.1.7. 9-((3aS,4S,6R,6aR)-6-(2-((tert-Butyldiphenylsilyl)oxy)ethyl)-5,5-difluoro-2,2-dimethyltetrahydro-4H-cyclopenta[d][1,3]dioxol-4-yl)-6-chloro-9H-purine (20b)
To solution of 17b (0.93 g, 1.85 mmol) in methanol (18 mL) was added 10% palladium on carbon (wet with ca. 55% water) (200 mg) at room temperature and the reaction mixture was stirred under H2 atmosphere at room temperature overnight. The suspension was filtered through a Celite and concentrated to give the amine 18b as colorless syrup which was used for the next step without further purification.
To a stirred solution of the crude 18b in 1,4-dioxane (15 mL) were added 4,6-dichloro-5-formamidopyrimidine (0.71 g, 3.70 mmol) and triethylamine (1.9 mL, 13.60 mmol) and the reaction mixture was heated at reflux for 2 d and cooled to room temperature. The solvent was removed under reduced pressure and the residue 19b was used for the next step without further purification. To the crude 19b was added diethoxymethyl acetate (15 mL) and the reaction mixture was stirred at 120 °C overnight. The solvent was removed under reduced pressure and the residue was purified by silica gel column chromatography (hexane:ethyl acetate = 5:1) to give 20b (0.35 g, 30%) as a white foam: [α]D 25 − 10.1 (c 0.250, CHCl3); 1H NMR (600 MHz, CDCl3) δ 8.78 (s, 1H), 8.24 (d, J = 1.9 Hz, 1H), 7.70–7.67 (m, 4H), 7.43–7.36 (m, 6H), 5.38–5.32 (m, 1H), 5.14 (t, J = 6.9 Hz, 1H), 4.52 (t, J = 6.4 Hz, 1H), 3.85–3.80 (m, 2H), 2.98–2.90 (m, 1H), 2.09–2.03 (m, 1H), 1.86–1.81 (m, 1H), 1.59 (s, 3H), 1.31 (s, 3H), 1.05 (s, 9H); 13C NMR (150 MHz, CDCl3) δ 152.5, 152.4, 151.6, 143.7 (J = 4.3 Hz), 135.6, 133.4, 131.4, 129.7, 127.7, 126.1(J = 247.5, 262.5 Hz), 114.5, 79.9 (J = 10.1 Hz), 78.8 (J = 7.2 Hz), 63.8 (J = 22.3 Hz), 60.6, 46.6 (J = 20.1 Hz), 28.6 (J = 4.3 Hz), 27.2, 26.8, 24.9, 19.2; 19F NMR (376 MHz, CDCl3) δ −107.7, −117.1; HRMS (FAB) found 613.2213 [calcd for C31H36ClF2N4O3Si+ (M + H)+ 613.2208].
4.1.8. (1R,2S,3S,4R,5R)-3-(6-Chloro-9H-purin-9-yl)-4-fluoro-5-(2-hydroxyethyl)cyclopentane-1,2-diol (21a)
To a stirred solution of 20a (0.57 g, 0.966 mmol) in methanol (3 mL) was added 50% aqueous trifluoroacetic acid (15 mL) at 0 °C. The reaction mixture was stirred at room temperature for 2 h. The solvent was removed under reduced pressure and the residue was purified by column chromatography (methylene chloride:methanol = 9:1) to give 21a (0.23 g, 75%), which was crystallized from diethyl ether/methanol as a white solid: mp 147–149 °C; [α]D 25 − 32.7 (c 0.107, CH3OH); UV (CH3OH) λ max 263.5 nm; 1H NMR (400 MHz, CD3OD) δ 8.75 (s, 1H), 8.71 (d, J = 2.4 Hz, 1H), 5.18 (dt, J = 4.0, 51.6 Hz, 1H), 5.15, (m, 1H), 4.80 (m, 1H), 4.06 (t, 5.4 Hz, 1H), 3.72 (m, 2H), 2.41 (m, 1H), 1.9 (m, 2H); 13C NMR (100 MHz, CD3OD) δ 154.0, 153.2, 151.4, 147.4 (J = 3.62 Hz), 94.3, 92.5, 74.5, 73.7, 64.3 (J = 17.3 Hz), 31.8 (J = 7.1 Hz); 19F NMR (376 MHz, CD3OD) δ −204.34; HRMS (FAB) found 317.0810 [calcd for C12H12ClFN4O3 + (M + H)+ 317.0811].
4.1.9. 2-((3aR,4R,6S,6aS)-6-(6-Chloro-9H-purin-9-yl)-5,5-difluoro-2,2-dimethyltetrahydro-4H-cyclopenta[d][1,3]dioxol-4-yl)ethan-1-ol (21b)
To a stirred solution of 20b (0.28 g, 0.45 mmol) in 1,4-dioxane (4 mL) was added 70% aqueous trifluoroacetic acid solution (12 mL) at 0 °C and the reaction mixture was stirred at room temperature for 2 h. The mixture was concentrated in vacuo and the residue was purified by silica gel column chromatography (methylene chloride:methanol = 9:1) to give 21b (0.12 g, 81%), which was crystallized from diethyl ether/methanol as a white solid: mp 80–82 °C [α]D 25 +53.2 (c 0.130, CH3OH); UV (CH3OH) λ max 264 nm; 1H NMR (500 MHz, CD3OD) δ 8.77 (s, 1H), 8.76 (d, J = 1.9 Hz, 1H), 5.45 (ddd, J 1 = J 2 = 8.7 Hz, J 3 = 17.9 Hz, 1H), 4.91–4.88 (m, 1H), 4.10–4.09 (m, 1H), 3.74–3.69 (m, 2H), 2.70–2.67 (m, 1H), 2.01–1.95 (m, 1H), 1.83, 1.78 (m, 1H); 13C NMR (125 MHz, CDCl3) δ 155.0, 154.13, 152.42, 148.46, 133.03, 126.16 (J = 251.0, 259.1 Hz), 74.3 (J = 7.1 Hz), 72.8 (J = 6.6 Hz), 65.9 (J = 23.0 Hz), 61.2, 31.3 (J = 6.8 Hz), 61.2, 31.3 (J = 6.8 Hz) (1 carbon hidden by methanol peak); 19F NMR (376 MHz, CD3OD) δ −103.3, −115.9; HRMS (FAB) found 335.0713 [calcd for C12H14ClF2N4O3 + (M + H)+ 335.0717].
4.1.10. (1R,2S,3S,4R,5R)-3-(6-Amino-9H-purin-9-yl)-4-fluoro-5-(2-hydroxyethyl)cyclopentane-1,2-diol (3a)
A solution of 21a (70 mg, 0.22 mmol) in saturated tert-butanolic ammonia in steel bomb was heated at 90 °C for 3 h. After cooling to room temperature, the solvent was removed under reduced pressure and the residue was purified by silica gel chromatography (methylene chloride:methanol = 5:1) to give 3a (44 mg, 68%), which was crystallized from diethyl ether/methanol as a white solid: mp 168–170 °C; [α]D 25 − 49.3 (c 0.146, CH3OH); UV (CH3OH) λ max 259 nm, 1H NMR (400 MHz, CD3OD) δ 8.26 (d, J = 2.0 Hz, 1H), 8.20 (s, 1H), 5.11 (dt, J = 3.6, 55.2 Hz, 1H), 4.99 (ddd, J = 3.2, 9.6, 26.4 Hz, 1H), 4.75 (m, 1H), 4.04 (m, 1H), 3.71 (m, 2H), 2.38 (m, 1H), 1.89 (m, 2H); 13C NMR (100 MHz, CD3OD) δ 157.4, 153.9, 151.5, 141.7 (J = 4.4 Hz), 94.2, 92.6, 74.5, 73.7, 63.7 (J = 17.1 Hz), 61.1, 47.8, (J = 17.9 Hz), 31.8 (J = 6.6 Hz); 19F NMR (376 MHz, CD3OD) δ −204.9; HRMS (FAB) found 298.1310 [calcd for C12H17FN5O3 + (M + H)+ 298.1310].
4.1.11. (1S,2R,3R,4R,5S)-4-fluoro-3-(2-hydroxyethyl)-5-(6-(methylamino)-9H-purin-9-yl)cyclopentane-1,2-diol (3b)
A solution of 21a (55 mg, 0.17 mmol) in methanol was added 40% methylamine aqueous solution and the reaction mixture was heated at 80 °C for 5 h in steel bomb. After cooling to room temperature, the solvent was removed under reduced pressure and the residue was purified by silica gel chromatography (methylene chloride:methanol = 5:1) to give 3b (37 mg, 68%), which was crystallized from diethyl ether/methanol as a white solid: mp 171–173 °C; [α]D 25 − 1.06 (c 0.160, CH3OH); UV (CH3OH) λ max 265 nm; 1H NMR (400 MHz, CD3OD) δ 8.25 (s, 1H), 8.2 (d, J = 1.4 Hz, 1H), 5.09 (dt, J = 3.6, 54.8 Hz, 1H), 4.96 (ddd, J = 3.0, 9.4, 26.7 Hz, 1H), 4.74 (t, J = 7.4 Hz, 1H), 4.02 (t, J = 6.0 Hz, 1H), 3.74–3.67 (m, 2H), 3.20–3.00 (brs, 3H), 2.42–2.31 (m, 1H), 1.94–1.84 (m, 2H); 13C NMR (125 MHz, CD3OD) δ 157.5, 154.6, 151.1, 141.8 (J = 3.6 Hz), 121.1, 94.2 (J = 180.5 Hz), 75.3, 74.4, 64.4 (J = 17.0 Hz), 61.8, 48.5 (J = 18.5 Hz), 32.6 (J = 6.6 Hz), 28.6; 19F NMR (376 MHz, CD3OD) δ −206.4; HRMS (FAB) found 312.1460 [calcd for C13H19FN5O3 + (M + H)+ 312.1466].
4.1.12. 9-((1S,2R,3R,4R,5S)-2-fluoro-4,5-dihydroxy-3-(2-hydroxyethyl)cyclopentyl)-1,9-dihydro-6H-purin-6-one (3c)
To a stirred solution of 21a (50 mg, 0.158 mmol) in 1,4-dioxane (3 mL) was added 1 N HCl (3 mL) at room temperature and the reaction mixture was heated at reflux overnight, cooled to room temperature and concentrated in vacuo. The residue was purified by C-18 reverse-phase silica gel column chromatography (H2O) to give 3c (33 mg, 70%), which was crystallized from diethyl ether/methanol as a white solid: mp 184–186 °C; [α]D 25 − 33.25 (c 0.160, H2O); UV (H2O) λ max 250 nm; 1H NMR (800 MHz, D2O) δ 8.20 (s, 1H), 8.08 (s, 1H), 5.07 (dt, J = 3.7, 54.2 Hz, 1H), 4.91 (ddd, J = 3.2, 9.8, 30.2 Hz, 1H), 4.74–4.72 (m, 1H), 4.05 (t, J = 5.9 Hz, 1H), 3.65 (t, J = 6.6 Hz, 2H), 2.33–2.25 (m, 1H), 1.87–1.77 (m, 2H); 13C NMR (150 MHz, D2O) δ 158.4, 149.4, 145.7, 141.0, 123.0, 91.9 (J = 179.9 Hz), 72.5, 72.1, 61.8 (J = 16.9 Hz), 59.6, 45.5 (J = 18.5 Hz), 29.4 (J = 6.9 Hz)); 19F NMR (376 MHz, CD3OD) δ −204.9; HRMS (FAB) found 299.1150 [calcd for C12H16FN4O5 + (M + H)+ 299.1150].
4.1.13. 2-((3aR,4R,6S,6aS)-6-(6-Amino-9H-purin-9-yl)-5,5-difluoro-2,2-dimethyltetrahydro-4H-cyclopenta[d][1,3]dioxol-4-yl)ethan-1-ol (3d)
A solution of 21b (33 mg g, 0.099 mmol) in saturated methanolic ammonia (20 mL) was heated at 70 °C overnight in steel bomb. After cooled to room temperature, the solvent was removed under reduced pressure and the residue was purified by silica gel column chromatography (methylene chloride:methanol = 9:1) to obtain 3d (0.012 g, 55% based on recovered starting material), which was crystallized from diethyl ether/methanol as a white solid: mp 109–111 °C; [α]D 25 +131.8 (c 0.040, CH3OH); UV (CH3OH) λ max 261 nm; 1H NMR (500 MHz, CD3OD) δ 8.27 (d, J = 1.8 Hz, 1H), 8.20 (s, 1H), 5.29 (ddd, J 1 = J 2 = 8.4 Hz, J 3 = 17.8 Hz, 1H), 4.77 (dd, J = 6.6, 8.8 Hz, 1H), 4.07 (t, J = 4.8 Hz, 1H), 3.74–3.69 (m, 2H), 2.69–2.62 (m, 1H), 2.00–1.94 (m, 1H), 1.83–1.78 (m, 1H); 13C NMR (125 MHz, CDCl3) δ 158.2, 154.8, 152.6, 142.7, 126.1 (J = 251.3, 258.5 Hz), 120.6, 74.0 (J = 7.9 Hz), 73.0 (J = 6.5 Hz), 65.2 (J = 21.6 Hz), 61.3, 50.7, 31.3 (J = 6.5 Hz); 19F NMR (376 MHz, CD3OD) δ −103.3, −115.9; HRMS (FAB) found 316.1220 [calcd for C12H16F2N5O3 + (M + H)+ 316.1216].
4.1.14. 2-((3aR,4R,6S,6aS)-5,5-difluoro-2,2-dimethyl-6-(6-(methylamino)-9H-purin-9-yl)tetrahydro-4H-cyclopenta[d][1,3]dioxol-4-yl)ethan-1-ol (3e)
A solution of 21b (10 mg, 0.03 mmol) in methanol (2 mL) was added 40% methylamine aqueous solution (0.25 mL, 3.22 mmol). The reaction mixture was heated at 50 °C for 5 h in steel bomb. After cooled to room temperature, the solvent was removed under reduced pressure and the residue was purified by silica gel column chromatography (methylene chloride:methanol = 9:1) to give 3e (0.008 g, 81%), which was crystallized from diethyl ether/methanol as a white solid: mp 89–91 °C; [α]D 25 +2.0 (c 0.160, CH3OH); UV (CH3OH) λ max 265 nm; 1H NMR (500 MHz, CD3OD) δ 8.25 (s, 1H), 8.21 (s, 1H), 5.27 (ddd, J 1 = J 2 = 8.4, 17.8 Hz, 1H), 4.76 (dd, J = 6.6, 8.7 Hz, 1H), 4.06 (t, J = 4.7 Hz, 1H), 3.74–3.68 (m, 2H), 3.11 (brs, 3H), 2.66–2.61 (m, 1H), 2.00–1.94 (m, 1H), 1.83–1.77 (m, 1H); 13C NMR (125 MHz, CDCl3) δ 157.6, 154.8, 151.5, 142.0, 126.1 (J = 250.1, 258.38 Hz), 121.2, 74.2 (J = 7.5 Hz), 73.0 (J = 7.0 Hz), 65.1 (J = 21.4 Hz), 61.3, 50.7, 31.3 (J = 6.5 Hz), 28.6; 19F NMR (376 MHz, CD3OD) δ −103.3, −115.9; HRMS (FAB) found 330.1386 [calcd for C13H18F2N5O3 + (M +H)+ 330.1372].
4.2. Antiviral activity and inhibition of purified SAH hydrolase
The determination of the antiviral activity of the synthesized compounds and the calculation of the EC50 and CC50 was done as described before [14].
The inhibition of purified SAH hydrolase by the different compounds and determination of IC50 values was done as previously described [14].
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by a grant from the Mid-career Research program (NRF-2016R1A2B3010164 ) of National Research Foundation (NRF), Korea.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejmech.2019.111956.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Payne S. Academic Press; 2018. Viruses from Understanding to Investigation; pp. 97–105. (Chapter 10), Introduction to RNA viruses. [Google Scholar]
- 2.Baltimore D. Expression of animal virus genomes. Bacteriol. Rev. 1971;35:235–241. doi: 10.1128/br.35.3.235-241.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thiel V., editor. Coronaviruses: Molecular and Cellular Biology. first ed. Caister Academic Press; 2007. [Google Scholar]
- 4.Zumla A., Hui D.S., Perlman S. Middle east respiratory syndrome. Lancet. 2015;386:995–1007. doi: 10.1016/S0140-6736(15)60454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Musso D., Gubler D.J. Zika virus. Clin. Microbiol. Rev. 2016;29:487–524. doi: 10.1128/CMR.00072-15. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Agumadu V.C., Ramphul K. Zika virus: a review of literature. Cureus. 2018;10 doi: 10.7759/cureus.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caglioti C., Lalle E., Castilletti C., Carletti F., Capobianchi M.R., Bordi L. Chikungunya virus infection: an overview. New Microbiol. 2013;36:211–227. [PubMed] [Google Scholar]
- 7.(a) Turner M.A., Yang X., Yin D., Kuczera K., Borchardt R.T., Howell P.L. Structure and function of S-adenosylhomocysteine hydrolase. Cell Biochem. Biophys. 2000;33:101–125. doi: 10.1385/CBB:33:2:101. [DOI] [PubMed] [Google Scholar]; (b) Cantoni G.L. The centrality of S-adenosylhomocysteinase in the regulation of the biological utilization of S-adenosylmethionine. In: Borchardt R.T., Creveling C.R., Ueland P.M., editors. Biological Methylation and Drug Design. Humana Press; Clifton, NJ: 1986. pp. 227–238. [Google Scholar]
- 8.(a) Wolfe M.S., Borchardt R.T., Adenosyl-L-homocysteine S.- Hydrolase as a target for antiviral chemotherapy. J. Med. Chem. 1991;34:1521–1530. doi: 10.1021/jm00109a001. [DOI] [PubMed] [Google Scholar]; (b) De Clercq E. Strategies in the design of antiviral drugs. Nat. Rev. Drug Discov. 2002;1:13–25. doi: 10.1038/nrd703. [DOI] [PubMed] [Google Scholar]
- 9.Snoeck R., Andrei G., Neyts J., Schols D., D, Cools M., Balzarini J., J, De Clercq E. Inhibitory activity of S-adenosylhomocysteine hydrolase inhibitors against human cytomegalovirus replication. Antivir. Res. 1993;21:197–216. doi: 10.1016/0166-3542(93)90028-h. [DOI] [PubMed] [Google Scholar]
- 10.Chambers T.J., Monath T.P., editors. The Flaviviruses: Detection, Diagnosis and Vaccine Development. Academic press; 2003. pp. pp3–577. [Google Scholar]; (b) Tseng C.K., Marquez V.E., Fuller R.W., Goldstein B.M., Haines D.R., Mcpherson H., Parsons J.L., Shannon W.M., Arnett G., Hollingshead M., Driscoll J.S. Synthesis of 3-deazaneplanocin A, a powerful inhibitor of S-adenosylhomocysteine hydrolase with potent and selective in vitro and in vivo antiviral activities. J. Med. Chem. 1989;32:1442–1446. doi: 10.1021/jm00127a007. [DOI] [PubMed] [Google Scholar]
- 11.(a) Bray M., Driscoll J.S., Huggins J.W. Treatment of lethal Ebola virus infection in mice with a single dose of an S-adenosyl-L-homocysteine hydrolase inhibitor. Antivir. Res. 2000;45:135–147. doi: 10.1016/s0166-3542(00)00066-8. [DOI] [PubMed] [Google Scholar]; (b) Bray M., Davis K., Geisbert T., Schmaljohn C., Huggins J.A. Mouse model for evaluation of prophylaxis and therapy of Ebola hemorrhagic fever. J. Infect. Dis. 1999;179:S248–S258. doi: 10.1086/514292. [DOI] [PubMed] [Google Scholar]
- 12.(a) Kusaka T., Yamamoto H., Shibata M., Muro M., Kishi T., T, Mizuno K. Streptomyces citricolor nov. sp. and a new antibiotic. J. Antibiot. (Tokyo) 1968;21:255–263. doi: 10.7164/antibiotics.21.255. [DOI] [PubMed] [Google Scholar]; (b) Shealy Y.F., Clayton J.D. 9-[β-DL-2α,3α-Dihydroxy-4β-(hydroxymethyl)- cyclopentyl]adenine, the carbocyclic analog of adenosine. J. Am. Chem. Soc. 1966;88:3885–3887. [Google Scholar]; (c) Shealy Y.F., Clayton J.D. Synthesis of carbocyclic analogs of purine ribonucleosides. J. Am. Chem. Soc. 1969;91:3075–3083. [Google Scholar]; (d) Shealy Y.F., Thorpe M.C., Coburn W.C., Clayton J.D. Identity of the synthetic carbocylic analog of adenosine and aristeromycin. Chem. Pharm. Bull. 1980;28:3114–3117. [Google Scholar]; (e) Arita M., Adachi K., Ito Y., Sawai H., Ohno M., M Enantioselective synthesis of the carbocyclic nucleosides (−)-aristeromycin and (−)-neplanocin A by a chemicoenzymatic approach. J. Am. Chem. Soc. 1983;105:4049–4055. [Google Scholar]; (f) Yoshikawa M., Okaichi Y., Cha B.C., Kitagawa I. Synthesis of (−)-aristeromycin from D-glucose. Tetrahedron. 1990;46:7459–7470. [Google Scholar]; (g) Wolfe M.S., Lee Y., Bartlett W.J., Borcherding D.R., Borchardt R.T. 4’-Modified analogs of aristeromycin and neplanocin A: synthesis and inhibitory activity toward S-adenosyl-L-homocysteine hydrolase. J. Med. Chem. 1992;35:1782–1791. doi: 10.1021/jm00088a013. [DOI] [PubMed] [Google Scholar]; (h) Madhavan G.V., Martin J.C. A novel and stereospecific synthesis of (±)- and (−)-aristeromycin. J. Org. Chem. 1986;51:1287–1293. [Google Scholar]
- 13.(a) Bennett L.L., Jr., Allan P.W., Rose L.M., Comber R.N., Secrist J.A., III Differences in the metabolism and metabolic effects of the carbocyclic adenosine analogs, neplanocin A and aristeromycin. Mol. Pharmacol. 1986;29:383–390. [PubMed] [Google Scholar]; (b) Bennett L.L., Bowdon B.J., Allan P.W., Rose L.M. Evidence that the carbocyclic analog of adenosine has different mechanisms of cytotoxicity to cells with adenosine kinase activity and to cells lacking this enzyme. Biochem. Pharmacol. 1986;35:4106–4109. doi: 10.1016/0006-2952(86)90036-5. [DOI] [PubMed] [Google Scholar]
- 14.Yoon J.-s., Kim G., Jarhad D.B., Kim H.-R., Shin Y.S., Sahu P.K., Kim H.O., Lee H.W., Wang S.B., Kong Y.J., Chang T.-S., Ogando N.S., Kovacikova K., Snijder E.J., Posthuma C.C., van Hemert M.J., Jeong L.S. Design, synthesis and anti-RNA virus activity of 6’-fluorinated-aristeromycin analogues. J. Med. Chem. 2019;62:6346–6362. doi: 10.1021/acs.jmedchem.9b00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang M., Schneller S.W., 5′-Homoaristeromycin Synthesis and antiviral activity against orthopox viruses. Bioorg. Med. Chem. Lett. 2005;15:149–151. doi: 10.1016/j.bmcl.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 16.Kim G., Yoon J.-s., Jarhad D.B., Shin Y.S., Majik M.S., Mulamoottil V.A., Hou X., Qu S., Park J., Baik M.-H., Jeong L.S. Asymmetric synthesis of (−)-6’-fluoro-aristeromycin via stereoselective electrophilic fluorination. Org. Lett. 2017;19:5732–5735. doi: 10.1021/acs.orglett.7b02470. [DOI] [PubMed] [Google Scholar]
- 17.Jarhad D.B., Jang M.H., Shin Y.S., Kim G., Kim H.-R., Hyun Y.E., Yoon J.s., Jeong L.S. An efficient synthesis of fluoro-neplanocin A analogs using electrophilic fluorination and palladium-catalyzed dehydrosilylation. Org. Chem. Front. 2019;6:959–966. [Google Scholar]
- 18.Yang M., Ye W., Schneller S.W. Preparation of carbocyclic S-adenosylazamethionine accompanied by a practical synthesis of (−)-aristeromycin. J. Org. Chem. 2004;69:3993–3996. doi: 10.1021/jo040119g. [DOI] [PubMed] [Google Scholar]
- 19.Crystal structure data for C12H16FN5O3 (3a) are as follows: Mr = 297.30, T = 296.5(2) K, orthorhombic, space group P212121, a = 10.34289(14) Å, b = 11.70521(15) Å, c = 15.2643(3) Å, α = 90°, β = 90°, γ = 90°, V = 1847.98(5) Å3, Z = 4, ρcalc = 1.069 g/cm3, μ = 0.728 mm-1, F(000) = 624.0, crystal size 0.146 × 0.086 × 0.013 mm3, radiation CuKα (λ = 1.54184). Of 22855 reflections collected in the 2θ range from 9.522 to 153.208° using an ω scan on a SuperNova, Dual, Cu at zero, AtlasS2 diffractometer, 3856 were unique reflections (Rint = 0.0310, Rsigma = 0.0180). Using Olex2, the structure was solved with the ShelXT structure solution program using Direct Methods and refined with the ShelXL refinement package using Least Squares minimization. Final R indexes [all data] R1 = 0.0367, wR2 = 0.1054, GOF = 1.057, and maxmin-1 residual electron density 0.23/-0.18 eÅ-3. Flack parameter = 0.12(7). Further details of the crystal structure investigation(s) may be obtained from the Cambridge Crystallographic Data Centre (CCDC, 12 Union Road, Cambridge, CB2 1EZ (UK); Tel: (+44)1223-336-408, Fax: (+44)1223-336033, e-mail: deposit@ccdc.cam.ac.kr) using no. CCDC 1963491.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.