Abstract
A novel mannose-binding lectin (designated CML) was isolated from Clematis montana Buch.-Ham stem (Ranunculaceae) using ion exchange and gel filtration chromatographies on DEAE-Sepharose and Sephacryl S-100. The purified C. montana lectin was a homodimer of 11,968.9 Da subunits as determined by gel filtration and MS. The hemagglutinating activity of CML was inhibited by branched oligomannosides. The N-terminal 15-amino acid sequence of CML, DNVKYSGQVKNTGSA, has not been reported for other lectins. Also, the peptide mass fingerprinting assay confirmed that there is no match result of similar plant lectins for CML, indicating CML may be a novel plant lectin. CML showed marked antiviral activity against various viruses in cell culture. Subsequently, CML was also found to exhibit remarkable inhibitory effect on L929, HeLa, MCF7 and HepG2 cells. Furthermore, CML specially induced L929 cell apoptosis in dose-dependent manner as evidenced by MTT, fluorescent microscopy, LDH activity-based cytotoxicity assays and DNA ladder. Moreover, due to both caspase inhibitors and Western blot analyses, caspase was also found to play the important role in the potential apoptotic mechanism of CML. When the carbohydrate-binding site was fully inhibited by sugars, cytotoxicity was abruptly decreased and apoptotic phenomenon in L929 cells was not observed, suggesting a significant correlation between mannose-binding-specific activity and the antineoplastic mechanism.
Keywords: Clematis montana lectin, Mannose-binding-specific activity, Antiviral activity, Apoptosis, Caspase
1. Introduction
Lectins are carbohydrate-binding proteins that bind carbohydrates reversibly and possess the ability to agglutinate cells or precipitate polysaccharides and glycoconjugates. They are widely distributed in animals, plants and microorganisms [43], [15] and have attracted great interest due to their various biological activities, such as cell agglutination [21], anti-tumor [27], immunomodulatory [40], antifungal [17], antiviral [51] and anti-insect activities [36]. It has been reported that a great number of lectins (e.g., mistletoe lectin, Concanavalin A (ConA), and Polygonatum cyrtonema lectin can induce apoptosis of tumor cells) [6], [39], [30]. Therefore, plant lectins have already been adopted for alternative tumor therapy in Europe for several years [42].
The Clematis genus consists of more than 200 species of climbing shrubs which is mainly distributed in subtropical countries. Most of acrid and poisonous plants in Clematis genus have been used as traditional medicines in India and China. The leaves of Clematis montana have been utilized for the treatment of skin diseases in India [18]. In China, Clematis armandii and C. montana called “mu tong” are also used as traditional Chinese medicines for the treatment of some ailments such as reducing fever to induce urination, stimulating menstrual discharge and promoting lactation [55]. Nevertheless, the two species contain different components: for C. montana, the triterpenoid and its glycoside are major components, while C. armandii mainly contains flavonoids [10].
However, only a few of the investigations have been reported for the bioactive proteins from Clematis genus besides some cyclic compounds (e.g., alkaloids, triterpenoids and flavanones) [10]. It has been reported previously that an N-acetylgalactosamine-specific lectin was purified from winter aconite (Eranthis hyemalis), a representative of the plant family Ranunculaceae. This lectin has been considered to be the first lectin isolated from the family Ranunculaceae [8]. In this study, we purified and characterized a novel mannose-binding lectin from the stem of C. Montana, which belongs to the plant family Ranunculaceae as well. Subsequently, C. montana lectin (CML) was found to bear remarkable antiviral activities in vitro against a variety of DNA and RNA viruses in cell culture. Furthermore, we showed that this lectin possessed the potent anti-tumor activity by direct cytotoxicity in four typical cancer cells and then found apoptosis in L929 cells. More importantly, we further explored that caspase played a crucial role in the potential mechanism of apoptosis. Moreover, through the sugar-inhibitory analysis, a significant correlation was discovered between the sugar-binding specificity of CML and the apoptosis-inducing mechanism. As mentioned above, these results would provide more insightful clues for understanding the key role of this lectin in further cancer investigations.
2. Materials and methods
2.1. Materials
The air-dried slice of Akebia Clematis stem was purchased in local traditional Chinese medicine market, Chengdu, China and identified as C. montana Buch.-Ham (Ranunculaceae) [10]. L929, HeLa, MCF7 and HepG2 cells were provided by Medical Science Center of West China of Sichuan University. DEAE-Sepharose, Sephacryl S-100 and standard molecular weight markers were procured from Pharmacia (Pharmacia, Uppsala, Sweden). Dulbecco's modified Eagle medium (DMEM) and RPMI 1640 medium were supplied by Gibco (Grand Island, NY). Saccharides, glycoproteins, acridine orange (AO), z-DEVD-fmk, z-IETD-fmk, z-VAD-fmk and [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (MTT) were products of Sigma Chemical (St. Louis, MO, USA). ABO blood group erythrocytes were obtained from healthy donors while fresh rabbit and chook blood cells were obtained from the local market.
2.2. Extraction and purification of lectin
Dried stem slices were powdered and extracted overnight in 40 mM, NaAc–HAc buffer (pH 4.6) at 4 °C. After centrifugation (20,000 × g, 15 min), the resulting supernatant obtained was applied to a column of DEAE-Sepharose (1.6 cm × 20 cm). The column was equilibrated with 40 mM, NaAc–HAc buffer (pH 4.6). The active fractions were further concentrated and purified on a Sephacryl S-100 column (2.0 cm × 75 cm) pre-equilibrated with 20 mM, PBS (pH 7.0).
2.3. Protein determination
Protein concentration was determined according to the method of Bradford [7], using bovine serum albumin (BSA) as standard.
2.4. Hemagglutination and inhibition assays
Hemagglutinating activity of CML was determined in 96-well microtiter U plates by the method of serial double dilution method using 2% suspension of rabbit erythrocytes [38]. Lectin (25 μl) was serially diluted 2-fold in 0.15 M NaCl, and an equal volume of erythrocytes in suspension was added to the microtiter plates. The mixture was incubated for 1 h at room temperature before the plate reads. The hemagglutination titer, defined as the reciprocal of the highest dilution exhibiting hemagglutination, was defined as one hemagglutination unit. The specific hemagglutinating activity was defined as unit/mg protein [54]. Specificity of the lectin to human (groups A, B and O) and chook erythrocytes was performed in a similar manner.
Hemagglutination inhibition assays were analyzed in a manner analogous to the hemagglutination assays. Briefly, serial 2-fold dilutions of sugar samples (25 μl) were mixed with an equal volume of the lectin with 8 units in microtiter plates and incubated for 30 min at room temperature. Then 25 μl erythrocytes suspension was added to each well, mixed and the plates read after 1 h. The minimum concentration of the sugar in the final reaction mixture for complete inhibition of eight hemagglutination units of the lectin preparation was calculated [50].
2.5. Characterization of the purified lectin
The tricine-sodium dodecyl sulphate polyacrylamide gel electrophoresis (tricine-SDS-PAGE) was performed according to the method of Schägger and von Jagow [41]. Samples were mixed with tricine-SDS sample buffer in the presence and absence of 2-mercaptoethanol, run on a 16% Tris-tricine gel with tricine-SDS running buffer, and stained with Coomassie Blue R-250.
The apparent molecular mass of the purified lectin was determined using Sephacryl S-100 column (2.0 cm × 75 cm) equilibrated and eluted with 20 mM PBS (pH 7.0) at a flow rate of 0.4 ml/min, monitored at 280 nm. The molecular mass of the eluting lectin was estimated from a plot of the log of the molecular weight versus a distribution coefficient (K av) calculated from the elution volume of the standard markers [3].
Matrix-assisted laser desorption/ionization time-of-flight (MALDI–TOF) mass spectrum was obtained using a Voyager-RP mass spectrometer (PerSeptive Bio-systems) according to the method of Woo et al. [52].
The effect of temperature and pH on hemagglutinating activity was determined after incubating the purified lectin at 20–100 °C for 30 min or at different buffers with pH range of 2.0–12.0 overnight at 4 °C. The vestigial hemagglutinating activity was determined after adjusting the mixtures to pH 7.0 at room temperature.
2.6. The N-terminal amino acid sequence analysis
Following tricine-SDS-PAGE, the lectin was transferred to a polyvinylidene difluoride (PVDF) membrane (BIO-RAD) stained with Coomassie Brilliant Blue R-250. Band corresponding to this lectin was excised from the membrane. Then N-terminal amino acid sequence was determined by using a Hewlett-Packard HP G1000A Edman degradation unit and an HP 1000 HPLC System [23]. BLASTp method from NCBI and manually data mining were both used to search its homology sequence [1].
2.7. In-gel tryptic digestion
In-gel digestion of CML was carried out using mass spectrometry grade Trypsin Gold (Promega, Madison, WI) according to the manufacturer's instructions. Briefly band of CML was excised out of the SDS-PAGE gel stained with Coomassie Brilliant Blue R-250 (Merck) and cut into small pieces, and then destained twice with 100 mM NH4HCO3, 50% acetonitrile (ACN) at 37 °C for 45 min. After dehydration with 100% ACN and drying, the gel was pre-incubated in 10–20 μl of trypsin solution (10 ng/ul) for 1 h. Sufficient digestion buffer (40 mM NH4HCO3, 10% ACN) was then added to cover the gel, which was incubated and mildly shaken overnight at 37 °C (12–14 h). Tryptic digests were extracted using Milli-Q water followed by double extraction with 50% ACN, 5% TFA for 1 h each time. The combined extracts were dried in a SpeedVac concentrator (Thermo Scientific) at 4 °C. The samples were then subjected to mass spectrometry analysis.
2.8. MS/MS analysis and protein identification
Mass spectra were performed by using a Q-TOF mass spectrometer (Micromass, Manchester, UK) fitted with a MALDI source (Micromass). Matrix-assisted laser desorption ionisation time-of-flight mass spectrometry (MALDI-Q-TOF) analyses the tryptic fragments of CML was performed as described previously [48]. Spectra were accumulated until a satisfactory S/N had been obtained. Parent mass peaks with the range from 400 to 1600 m/z were picked out for MS/MS analysis. The peptide mass fingerprintings of CML and MS/MS data were acquired and processed using MassLynx V 4.1 software (Micromass) and were converted to PKL files by the ProteinLynx 2.2.5 software (Waters). The PKL files were analyzed using the MASCOT search engine (http://www.matrixscience.com). The search parameters were defined as follows: database, Swiss-Prot; taxonomy, Homo sapiens; enzyme, trypsin; and allowance of one missed cleavage. Carbamidomethylation was selected as a fixed modification and oxidation of methionine was allowed to be variable. The peptide and fragment mass tolerance were set at 0.1 and 0.05 Da for MS and MS/MS, respectively.
2.9. Antiviral assays
Using previously established procedures [5], the in vitro antiviral activities of CML were determined against a variety of DNA and RNA viruses, and their cytotoxicities for the host cell lines were assayed in parallel with those of standard drugs with known antiviral activities. The viruses and cells used were herpes simplex virus type 1 (HSV-1) (strain KOS), herpes simplex virus type 2 (HSV-2) (strain G), vaccinia virus, vesicular stomatitis virus and thymidine-kinase-deficient HSV-1 (TK-) (strain KOS) in HEL cells; vesicular stomatitis virus, coxsackie virus B4, respiratory syncytial virus in HeLa cells; Feline Corona Virus (FIPV) and Feline Herpes Virus in Crandell-Rees Feline Kidney (CRFK) cells; Influenza A (H1N1 and H3N2 subtypes) and influenza B virus in Madin Darby canine kidney (MDCK) cells; Para-influenza virus type 3, reovirus type 1, sindbis virus, coxsackie B4 virus and Punta Toro virus in Vero cells; and HIV-1 (IIIB) and HIV-2 (strain ROD) in human T-lymphocyte (CEM) cells (at compound concentrations up to 100 μg/ml). The antiviral activities were determined as percent inhibition of microscopically visible virus-induced cytopathicity or by measuring the cell viability with the colorimetric formazan-based MTS assay in the cell cultures.
2.10. Assay for antineoplastic activity
2.10.1. Cell culture
The L929 and MCF-7 cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM) with 10% fetal bovine serum, MEM nonessential amino acids, 100 kU/l penicillin and 100 g/l streptomycin in the presence of 5% of CO2 at 37 °C. HepG2 and HeLa cells were grown in RPMI-1640 medium supplemented with 10% (v/v) heat inactivated (56 °C, 30 min) fetal bovine serum (FBS), 0.03% l-glutamine, 100 kU/l penicillin and 100 g/l streptomycin and maintained at 37 °C in a humidified atmosphere containing 5% CO2 and 95% air. For the experiments, cells were plated 24 h before the treatments to allow adherence.
2.10.2. Inhibition of cell growth
Tests were performed according to the method of Liu et al. [32]. L929, HeLa, MCF-7 or HepG2 cells were seeded into 96-well microtiter plates at 1.5 × 104 cells/well (MCF7 and HepG2 cells), 1 × 105 cells/well (L929) or 1.0 × 104 cells/well (HeLa cells) (100 μl final volume). After 24 h incubation, varying concentrations of the lectin were added for the indicated times and then, cells were incubated with 10 μl of the reagent solution MTT (5 mg/ml) and incubated at 37 °C for 4 h. Absorbance at 490 nm was recorded using a spectrophotometer [Model 3550 Microplate Reader (BIO-RAD)]. The percentage of cell growth inhibition was calculated as follows: cell proliferation inhibition (%) = (A490, control-A490, sample)/(A490, control-A490, blank) × 100.
2.10.3. The effect of carbohydrates and inhibitors on CML-induced L929 cell death
The L929 cells were seeded at a density of 1 × 105 cell/well into 96-well culture plate. After 24 h incubation, the cells were treated with or without general caspase inhibitor (z-VAD-fmk), caspase-3 inhibitor (z-DEVD-fmk), caspase-8 inhibitor (z-IETD-fmk) at given concentrations 1 h prior to the administration of the lectin. Then, MTT assay was determined as described above.
To assess the effect of carbohydrates on CML-induced L929 cell death, MTT assay was determined as above except that the lectin was pre-incubated at 37 °C for 30 min with carbohydrates which can half or fully inhibit hemagglutinating activity prior to application on the test.
2.10.4. Observations of cell morphological changes
The L929 cells were divided into three groups and placed on culture plates for 24 h incubation. The first group was treated with the control medium, the second group with 1 pmol/l (pM) purified lectin and the third group with 1 pM lectin whose hemagglutinating activity was half of fully inhibited by carbohydrates. The cellular morphology was observed using phase contrast microscopy (Leica, Wetzlar, Germany).
2.10.5. Nuclear damage observed by acridine orange (AO) staining
The changes in nuclear morphology of apoptotic cells were investigated by labeling cells with the fluorescent, selective DNA- and RNA-binding dye AO as well as examining them under fluorescent microscopy [13]. After being treated with or without the lectin (1 pM) for 24 h, the cells were stained with 20 mg/ml AO for 15 min and then the nuclear morphology was observed under fluorescence microscopy (Olympus, Tokyo).
2.10.6. Lactate dehydrogenase (LDH) activity-based cytotoxicity assays
LDH activity was assessed using a standardized kinetic determination kit (Zhongsheng LDH kit, Beijing, China). LDH activity was measured in both floating dead cells and viable adherent cells. The floating cells were collected from the culture medium by centrifugation (240 × g) at 4 °C for 5 min, and the LDH content from the pellets was used as an index of apoptotic cell death (LDHp) [28]. The LDH released in the culture supernatant (extracellular LDH, or LDHe), was used as an index of necrotic death, and the LDH present in the adherent viable cells as intracellular LDH (LDHi). The percentage of apoptotic and necrotic cell death was calculated as follows:
2.11. DNA extraction and detection of DNA fragmentation
Cellular DNA was extracted according to the method as previously reported [34]. Briefly, L929 cells (1 × 105) were incubated with or without CML for 12 and 24 h, washed twice with PBS, and incubated in digestion buffer (10 mmol/l Tris–HCl pH 7.4, 10 mmol/l EDTA, 0.5% Triton-X-100) and kept at 4 °C for 10 min. The lysate was centrifuged at 25,000 × g for 20 min. The supernatant was incubated with RNase A (20 mg/ml) at 37 °C for 1 h and then incubated with proteinase K (0.1 mg/ml) at 37 °C for 1 h. The supernatant was again mixed with 0.5 M NaCl and isopropanol at −20 °C overnight, followed by centrifugation at 1000 × g for 15 min. After drying, the DNA was dissolved in TE buffer, separated by 2% agarose gel electrophoresis containing 0.1 mg/ml ethidium bromide at 100 V for 40 min, and then visualized and photographed under UV light.
2.12. Western blot assay
The L929 cells were treated with 1 pM lectin for 6, 12, 24 and 36 h. Both adherent and floating cells were collected, and Western blot analysis was carried out as described previously [11], [14]. Briefly, the cell pellets were resuspended with lysis buffer consisting of 50 mmol/l Hepes (pH 7.4), 1% Triton-X-100, 2 mM sodium orthovanada, 100 mM sodium fluoride, 1 mM edetic acid, 1 mM PMSF, 10 mg/ml aprotinin, 10 mg/ml leupeptin and lysed at 4 °C for 1 h. After 12,000 × g centrifugation for 15 min, the protein content of supernatant was determined by the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA, USA), and then separated by electrophoresis in 12% SDS-polyacrylamide gel electrophoresis, and blotted onto nitrocellulose membranes (Amersham Biosciences, Piscataway, NJ). The membranes were soaked in blocking buffer (5% skimmed milk) and proteins were detected using polyclonal antibodies and visualized using anti-rabbit or anti-mouse IgG conjugated with peroxidase (HRP) and 3,3-diaminobenzidine tetrahydrochloride (DAB) as the HRP substrate.
2.13. Statistical analysis
All the presented data and results were confirmed in at least three independent experiments. The data are expressed as means ± S.D. Statistical comparisons were made by Student's t-test. P < 0.05 was considered statistically significant.
3. Results
3.1. Purification of lectin
Ion exchange and gel filtration chromatographic steps were applied to the purification of this lectin. When the crude extract of proteins was loaded onto DEAE-Sepharose column, the unbound fraction (A) was eluted with the equilibrating buffer and the bound fractions (B and C) were eluted with a linear gradient of NaCl (0–0.3 M) (Fig. 1A). Hemagglutinating activity test which was employed to monitor all the purification procedure showed that the lectin was enriched in fraction C. Then active fraction was pooled and applied to Sephacryl S-100 column, it was resolved into two fractions D and E (Fig. 1B). The fraction E with major hemagglutinating activity demonstrated a molecular mass of 12.5 kDa in tricine-SDS-PAGE and was designated as C. montana lectin (CML) (Fig. 2A). A summary of its purification was provided in Table 1 . The yield of the purified CML from crude protein extract was 3.2% and its specific activity increased 14.4-folds.
Fig. 1.
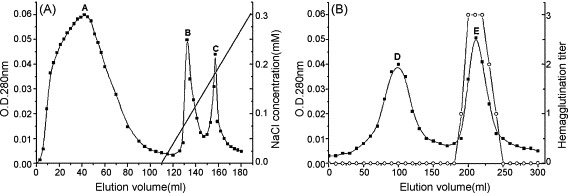
The purification of the lectin by ion exchange chromatography and gel chromatography. The elution profiles were monitored at 280 nm. Active fractions were detected by hemagglutination assay. (A) Ion exchange chromatography of crude extracts on DEAE-Sepharose column pre-equilibrated with NaAc–HAc buffer (pH 4.6). The bound protein was eluted with a linear gradient of 0–0.3 M NaCl at a flow rate of 1 ml/min. (B) Gel chromatography of fraction C on Sephacryl S-100 column pre-equilibrated with PBS (20 mM, pH 7.0) at a flow rate of 18 ml/h. (■) absorbance at 280 nm; (○) hemagglutination titer.
Fig. 2.
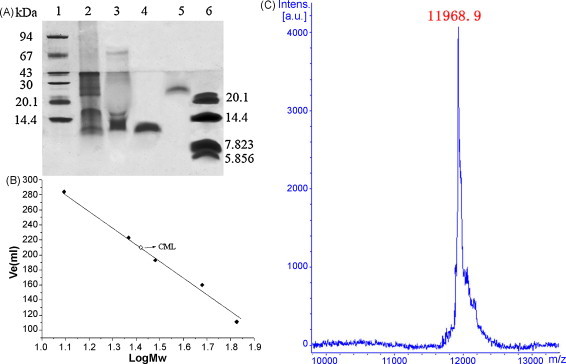
Molecular mass determination. (A) Tricine-SDS-PAGE analysis. Lane 1, molecular weight standards (in kDa): phosphorylase b (94 kDa), bovine serum albumin (66 kDa), ovalbumin (43 kDa), carbonicanhydrase (30 kDa), trypsin inhibitor (20.1 kDa), and lactalbumin (14.4 kDa); lane 2, crude extract; lane 3, fraction B on DEAE-Sepharose; lane 4, fraction E on Sephacryl S-100 with 2-mercaptoethanol; lane 5, fraction E without 2-mercaptoethanol; lane 6, low molecular weight standards (in kDa): trypsin inhibitor (20.1 kDa), lactalbumin (14.4 kDa), ABI-80 (7.823) and ABI-81 (5.856). (B) Native molecular mass estimation of the lectin by gel filtration. Standard markers: BSA (67 kDa), Galanthus nivalis agglutinin (48 kDa), cytochrome C (12.5 kDa), soybean trypsin inhibitor (30.2 kDa) and trypsin (23.3 kDa). The molecular weight size markers (■) and the lectin (□) are indicated. (C) Molecular mass determination by MALDI-TOF.
Table 1.
Specific hemagglutinating activities and yields of chromatographic fractions obtained at different steps of purification of lectin.
| Steps | Total protein (mg) | Specific hemagglutinating activity (HU/mg) | Total hemagglutination activity (HU)a | Purification foldb | Recovery of protein (%) |
|---|---|---|---|---|---|
| Crude extract | 270 | 47.4 | 12798 | 1 | 100 |
| DEAE-Sepharose fraction C | 88.5 | 128 | 11329 | 2.7 | 32.7 |
| SephacrylS-100 fraction E | 8.9 | 682 | 6069 | 14.4 | 3.2 |
The crude extract was obtained from 200 g Clematis montana Buch.-Ham.
Hemagglutinating unit (HU) represents the highest exhibiting complete hemagglutination. 2% rabbit erythrocytes were used for hemagglutinating activity.
3.2. Properties of purified lectin
Tricine-SDS-PAGE analysis showed that the molecular mass of the purified lectin was approximately 12.5 and 25.8 kDa in the presence and absence of 2-mercaptoethanol, respectively (Fig. 2A). Gel filtration of CML on Sephacryl S-100 gave a symmetrical single peak and the molecular weight was approximately 25.4 kDa (Fig. 2B). The mass spectrometry analysis appeared as a peak corresponding to m/z 11,968.9 Da (Fig. 2C). These results indicated that CML is a homodimer consisting of two identical subunits of 11,968.9 Da which are held together by disulphide linkages.
The hemagglutinating activity of CML was stable up to 80 °C for 30 min, but it decreased sharply between 90 and 100 °C and was completely abolished when incubated at 100 °C for less than 10 min. Agglutination was not markedly affected by pH in the range of 6.0–10.0; whereas 50%, 12.5% and 62.5% of the activity was observed at pH 4.0, 2.0 and 12.0, respectively.
3.3. Hemagglutinating activity and carbohydrates specificity
CML agglutinated rabbit erythrocytes at 3.9 μg/ml and showed no agglutination towards chook and human blood groups (A, AB, B and O) erythrocytes even at 500 μg/ml. The preliminary carbohydrates specificity assays showed that of all saccharides and glycoproteins tested only yeast mannan rich in oligomannosidic-type glycans and thyroglobulin, a glycoprotein inhibited the agglutination of rabbit erythrocytes (date not shown). To refine the carbohydrate-binding specificity, much more saccharides with the different branched oligomannosides were studied in detail (Table 2 ). It indicated that the agglutination was strongly inhibited by yeast mannan and Man-a(1,3:1,6)-mannotriose. The minimum inhibitory concentration (MIC) was 1.56 μg/ml and 2.5 mM, respectively. Man-a(1,3)-Man, Man-a(1,2)-Man and Man-a(1,6)-Man were slightly inhibitory whereas a-methyl-mannoside and 2-deoxy-d-Man were not inhibitory at all, even at concentrations up to 160 mM. These results suggested that yeast mannan and Man-a (1,3:1,6)-mannotriose were the most potent inhibitors. A few of other lectins have been reported with hemagglutinating activity only inhibited by complex carbohydrates as from T. divaricatum [35], V. volvacea [44] and G. lucidum [46].
Table 2.
Carbohydrate-binding specificity.
| Carbohydrate/glycoproteina | Minimum inhibitory concentration (MIC)c |
|---|---|
| D-Man | NI |
| a-Methyl-mannoside | NI |
| 2-Deoxy-d-Man | NI |
| Man-a(1,2)-Man | 40 mM |
| Man-a(1,3)-Man | 20 mM |
| Man-a(1,6)-Man | 40 mM |
| Man-a(1,3:1,6)-mannotriose | 2.5 mM |
| Yeast mannanb | 1.56 μg/ml |
Hemagglutinating inhibition test was used to study carbohydrate-binding specificity. The minimum inhibitory concentration (MIC) of sugar was exhibited in the table.
d-Galactose, d-glucose, maltose, d-galactosamine, d-fructose, lactose, fetuin, sucrose, N-acetyl-d-glucosamine, 2-methyl-d-glucoside, N-acetylgalactosamine and N-acetylactosamine failed to inhibit the hemagglutinating activity of CML even at 160 mM.
The initial concentration of yeast mannan was 4 mg/ml.
The minimum inhibitory concentration of sugar required for complete inhibition. NI = not inhibitory until a concentration of 160 mM.
3.4. The N-terminal amino acid sequence analysis
N-terminal amino acid sequence of CML was DNVKYSGQVKNTGSA. No homology sequence was found by BLASTP searching and also by manually data searching and comparing the N-terminal amino acid sequence of all lectins reported previously, suggesting that CML might be a novel lectin which did not show similarity at N-terminal with any known lectin.
3.5. The identification of CML by mass spectrometry and database search
CML was further identified by MALDI-Q-TOF MS/MS analysis (Fig. 3 ). The peptide mass fingerprinting of CML was obtained by MALDI-Q-TOF MS analysis. Then, MS/MS profile of the peptide fragment with a mass of 791.9557 was analyzed by using the MASCOT search engine against the Swiss-Prot protein database. For all the total 124 queries, no value of MOWSE score was higher than that indicates homology (data not shown). These results showed that CML matched with no protein (or lectin) from database searching.
Fig. 3.
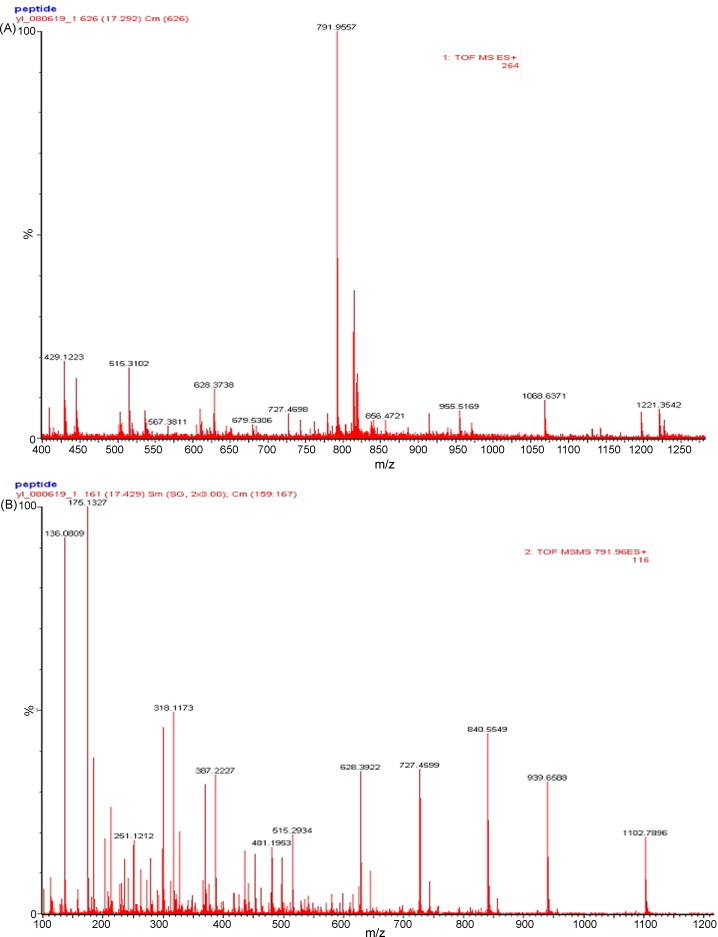
The result of the MALDI-TOF MS/MS analysis of CML. (A) Peptide mass fingerprint of the tryptic digest of CML. (B) MS/MS profile of the peptide with a mass of 791.9557 Da.
3.6. The antiviral activity of CML in vitro
To measure the inhibitory effect on the viral cytopathogenic effects (CPE) in cell culture, CML was exposed to different cell cultures that were infected by a variety of DNA and RNA viruses. It is found that CML showed a relatively low cytotoxicity to the uninfected control cells. The anti-HIV activity of CML was examined by using HIV-1 and HIV-2 in CEM cell culture, the results showed obvious anti-HIV activity at EC50 (μg/ml) values of 11 ± 3.9 and 71 ± 41 of HIV-1 and HIV-2, respectively (Table 3 ). CML also exerted marked activity towards Influenza A H1N1 subtype, Influenza A H3N2 subtype and Influenza B with EC50 (μg/ml) values of 34, 45 and 45 visual CPE score in each virus. The MTS values in these types of viruses are 37.2, 51.0, and 27.6, respectively (Table 4 ). CML also possessed activity towards some other viruses such as Para-influenza-3 and virus reovirus-1, even thought the activity is not very strong (Table 5 ).
Table 3.
Anti-HIV-1 and -HIV-2 activity and cytostatic properties of Clematis montana lectin (CML) in human T-lymphocyte (CEM) cells.
| Compound | EC50 (μg/ml) |
CC50 (μg/ml) | |
|---|---|---|---|
| HIV-1 | HIV-2 | ||
| CML | 11 ± 3.9 | 71 ± 41 | >100 |
| GNA | 0.92 | 0.78 | 6.9 |
| LRA | 0.79 | 0.59 | 7.1 |
| SCA | 4.6 | 8 | 83 |
GNA, Galanthus nivalis agglutinin; LRA, Lycoris radiate agglutinin; SCA, Scilla campanulata agglutinin [42].
EC50 = effective concentration or concentration required to protect CEM cells against the cytopathogenicity of HIV by 50%.
CC50 = cytotoxic concentration or concentration required to reduce CEM cell viability by 50%.
Table 4.
Anti-influenza virus activity and cytotoxicity of Clematis montana lectin (CML) in MDCK cell cultures.
| Compound | Cytotoxicity |
Antiviral EC50c |
||||||
|---|---|---|---|---|---|---|---|---|
| CC50a | Minimum cytotoxic concentrationb | Influenza A H1N1 subtype |
Influenza A H3N2 subtype |
Influenza B |
||||
| Visual CPE |
Visual CPE |
Visual CPE |
||||||
| Score | MTS | Score | MTS | Score | MTS | |||
| CML (μg/ml) | >100 | >100 | >100 | >100 | 45 | 35.0 | 100 | 23.5 |
| Oseltamivir | ||||||||
| Carboxylate (μM) | >100 | >100 | 2.0 | 4.3 | 9 | 5.2 | 8 | 15.0 |
| Ribavirin (μM) | >100 | >100 | 9 | 9.9 | 9 | 9.3 | 9 | 4.3 |
| Amantadine (μM) | >1000 | >1000 | 447 | 445 | >1000 | >1000 | >1000 | >1000 |
| Rimantadine (μM) | 525 | 1000 | 18 | 13.8 | >200 | >200 | >200 | >200 |
50% cytotoxic concentration, as determined by measuring the cell viability with the colorimetric formazan-based MTS assay.
Minimum compound concentration that causes a microscopically detectable alteration of normal cell morphology.
50% effective concentration, or concentration producing 50% inhibition of virus-induced cytopathic effect, as determined by visual scoring of the CPE, or by measuring the cell viability with the colorimetric formazan-based MTS assay. MDCK cells: Madin Darby canine kidney cells.
Table 5.
Cytotoxicity and antiviral activity of Clematis montana lectin (CML) in Vero cell cultures.
| Compound | Minimum cytotoxic concentrationa | EC50b (μg/ml) |
||||
|---|---|---|---|---|---|---|
| Para-influenza-3 virus | Reovirus-1 | Sindbis virus | Coxsackie virus B4 | Punta Toro virus | ||
| CML (μg/ml) | 100 | >20 | >20 | >20 | >20 | >20 |
| DS-5000 (μg/ml) | >100 | >100 | >100 | 20 | 12 | 20 |
| (S)-DHPA (μM) | >250 | >250 | >250 | >250 | >250 | >250 |
| Ribavirin (μM) | >250 | 146 | 250 | >250 | >250 | 146 |
Required to cause a microscopically detectable alteration of normal cell morphology.
Required to reduce virus-induced cytopathogenicity by 50%.
3.7. Growth inhibition
Inhibition of cell proliferation was measured by MTT assay. It found that from 1.0 × 10−4 to 10 pM CML exerted a potent cytotoxic effect on L929, MCF-7, HeLa and HepG2 cells and showed a concentration-dependent manner. After 24 h incubation at 37 °C, 1 pM CML inhibited HeLa cells proliferation in a 28 ± 5% and MEF-7 in a 21 ± 4% (Fig. 4A). CML had a stronger effect on L929 cells and caused 55 ± 3% cell growth reductions at 1 pM. Whereas even at 10 pM CML, inhibitory ratio of HepG2 cells was 21 ± 7%. Thus, it was particularly interesting to investigate a potent effect on L929 and 24 h incubation with 1 pM CML is sufficient for 50% inhibition of cell growth (IC50).
Fig. 4.
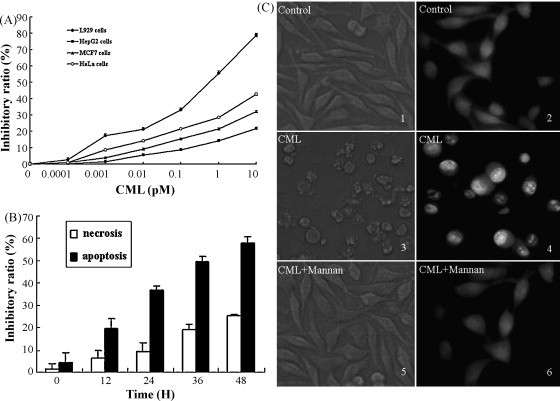
Antineoplastic activity. (A) Determination of inhibition ratios of L929, MCF-7, HeLa and HepG2 cells growth by MTT assay, the cells were cultured for different concentrations of CML. Values are expressed as mean ± S.D. (B) Lactate dehydrogenase (LDH) activity, L929 cells were treated with CML (1 pM) for 12, 24, 36, 48 h. The cell inhibitory ratio was measured by MTT assay, n = 5, means ± S.D. (C) Morphological observation of L929 cells. The cellular morphologic changes were observed after the cells were incubated with medium, 1 pM CML or 1 pM CML pre-incubated with Man-a(1,3:1,6)-mannotriose (2.5 mM) for 24 h under a phase contrast microscope (1, medium; 3, 1 pM CML; 5, CML pre-incubated with Man-a (1,3:1,6)-mannotriose at 37 °C for 30 min; 200 magnification) or under a fluorescence microscope by AO staining (2, medium; 4, 1 pM CML; 6, CML pre-incubated with Man-a(1,3:1,6)-mannotriose at 37 °C for 30 min; 200 magnification).
3.8. Morphological observation
After stained by AO, L929 cells were observed under the fluorescent microscopy. As shown in Fig. 4C, RNA in cytoplasm and nucleolus appeared red while DNA in nucleus was green or chartreuse as observed. While among CML (1 pM) treated cells, dense chartreuse nucleolus, condensed chromatin, and fragmented nuclei were observed. Also, there was a reduction in nuclear volume. Some of the nucleus degenerated into discretely spherical fragments of highly condensed chromatin (Fig. 4C).
3.9. The balance between apoptosis and necrosis
To further characterize lectin-induced L929 cell death, the ratio of LDH released from viable cells, floating dead cells and the culture medium were compared. The number of apoptotic and necrotic cells was 40% and 11% at 24 h, respectively, suggesting that the number of apoptotic cells was unregulated, with lower number of necrotic cells, after progressively increasing concentration of CML, especially incubation of the cells with 1 pM CML. It demonstrated that the major reason of CML-induced L929 cell death was apoptosis rather than necrocytosis (Fig. 4B).
3.10. DNA ladder assay of apoptosis in L929 cells
The most distinct biochemical hallmark of apoptosis is the activation of the endogenous Ca2+-/Mg2+-dependent endonuclease and endonuclease-mediated cleavage of internucleosomes to generate oligonucleotide fragments with about 180–200 bp length or their polymers. Characteristic ladder bands can be obtained by agarose gel electrophoresis of DNA extracted from apoptotic cells. In this study, DNA ladder bands could be observed in 1 pM CML-treated group after 24 h, whereas in the presence of CML for 12 h or in control group, smear-like DNA degradation was observed as shown in Fig. 5A. These phenomena demonstrated that CML-induced L929 apoptosis in a time-dependent manner.
Fig. 5.
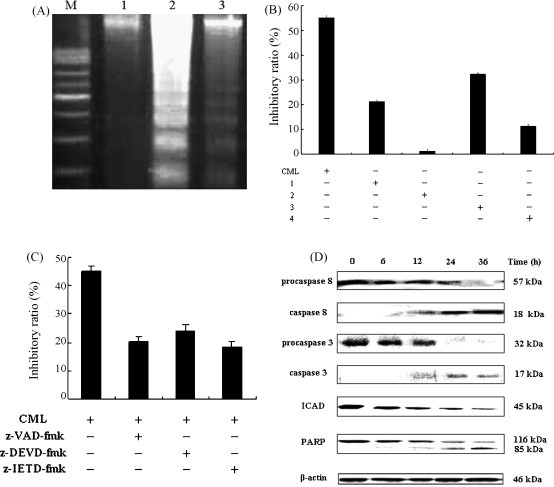
(A) DNA fragmentation induced apoptosis in L929 cells. M: marker, lane 1: medium control, lane 2: CML treatment for 12 h, lane 3: CML treatment for 24 h. (B) Effects of carbohydrates on CML-induced L929 cell death. Control, the cells were treated with 1 pM CML for 24 h, 1 and 2, the cells were treated with 1 pM CML for 24 h which the hemagglutinating activity was half and fully inhibited by Man-a(1,3:1,6)-mannotriose, 3 and 4, the cells were treated with 1 pM CML for 24 h, half and fully inhibited by Man-a(1,6)-Man. The cell inhibitory ratio was measured by MTT assay (x ± S.D., n = 3). (C) Effects of inhibitors on CML-induced L929 cell death. L929 cells were cultured in the absence or presence of z-VAD-fmk (20 μmol l−1), z-DEVD-fmk (20 μmol l−1) or z-IETD-fmk (20 μmol l−1) 1 h prior to the addition of CML, then incubated for 24 h, n = 5. Mean ± S.D. (D) CML-induced procaspase-8, procaspase-3 cleaving and ICAD, PARP expressing. The cells were treated with 1 pM CML for the indicated time periods followed by Western blot analysis for detecting procaspase-8, procaspase-3, ICAD and PARP expressions. β-Actin was used as an equal loading control.
3.11. Effects of carbohydrates and inhibitors on CML-induced L929 cell death
To assess the effect of sugars on CML-induced L929 cell death, CML was pre-incubated at 37 °C for 30 min in buffer containing Man-a(1,3:1,6)-mannotriose (1.25 or 2.5 mM) or Man-a(1,6)-Man (10 or 20 mM), respectively. The cell inhibitory ratio was measured by MTT assay (x ± S.D., n = 3). The result showed that Man-a(1,6)-Man was a slightly more effective inhibitor than Man-a(1,3:1,6)-mannotriose (Fig. 5B). When CML was pre-incubated with Man-a(1,3:1,6)-mannotriose at a concentration of 2.5 mM abrogated almost all CML binding, the cell inhibitory ratio was abruptly decreased and apoptotic phenomenon was not observed at all (Fig. 5B). Furthermore, the inhibitors for caspase-8 (z-IETD-fmk, 20 μM), caspase-3 (z-DEVD-fmk, 20 μM) and pan-caspase inhibitor (z-VAD-fmk, 20 μM) partially blocked apoptosis in L929 cells (Fig. 5C).
3.12. Participation of caspase-8 and caspase-3 in apoptosis
Western blot analysis was carried out to further confirm the participation of caspase-8 and caspase-3. As shown in Fig. 5D, when L929 cells were treated with CML for 6, 12, 24, 36 h, the expressions of procaspase-8 and procaspase-3 were decreased, but the expressions of caspase-8 and caspase-3 were increased with culturing time, suggesting the activation of caspase-8 and caspase-3. It has been reported that poly(ADPribose) polymerase (PARP) and inhibitor of a caspase-dependent Dnase (ICAD) were caspase-3 substrates [53]. Subsequently, ICAD and PARP expressions were examined. After exposure to CML, ICAD was degraded. Cleavage of PARP was examined and the 116-kDa protein expression was declined and the 85-kDa degraded product was increased with culturing time.
4. Discussion
In the present study, a novel mannose-binding lectin with obvious antiviral and potent antineoplastic activities has been purified from C. montana Buch.-Ham stem (Ranunculaceae) for the first time though an N-acetylgalactosamine-specific lectin was purified previously from winter aconite (E. hyemalis), a representative of the plant family Ranunculaceae [8]. However, there is a significant difference on their caulomes although winter aconite (E. hyemalis) and C. montana belong to the same family. Winter aconite (E. hyemalis) is identified as a herbaceous plant while C. montana is identified as a woody plant. So CML may differ largely from the lectin purified from winter aconite due to the remarkable difference of the two plants and the extraordinary nature of C. montana.
Purification of CML from raw material is a time-consuming process, demanding large amounts of plant materials. Even after the procedure of anion exchange chromatography and gel filtration chromatography, the final yield of CML is merely 3.2%, being approximately 44.5 mg lectin per kilogram dried stem material. However, the yield of lectins from the plant seeds was reportedly in the ranges 9.5–66% [39], [22]. In addition, another similar yield has been reported that the lectin from jasmonic acid treated tobacco leaves is approximately 30 mg lectin per kilogram fresh leaves [24], which indicated that the content of CML is quite low.
The assay of carbohydrate-binding specificity indicated that CML had high affinity with complex carbohydrates. Yeast mannan, containing oligomannosidic-type glycans with a (1,2) and a (1,3) terminal non-reducing mannosyl residues [49], and Man-a(1,3:1,6)-mannotriose were the most effective inhibitors for hemagglutination activity of this lectin. Nevertheless, the agglutination was not inhibited by d-mannose, a-methyl-mannoside and 2-deoxy-d-Man. These results indicated that CML only recognized oligosaccharidic sequences containing branched oligomannosides. It demonstrated that this lectin possesses an extended carbohydrate-binding site, which can accommodate highly branched oligomannosides. This phenomenon has been observed in monocot mannose-binding lectins, such as H. tuberosus and A. constricta lectins [49], [16].
Some mannose-specific plant lectins showed antiviral activity, especially the monocot mannose-binding lectins, such as the anti-HIV activities of P. cyrtonema lectin and Galanthus nivalis (Snowdrop) agglutinin (GNA) [2], [4], [20], the anti-HSV activity of Ophiopogon japonicus lectin [47]. CML also showed marked antiviral activity against various viruses in cell cultures like some other aforementioned mannose-binding lectins. However, CML exhibited no sequence similarity with other known lectins, especially all the previously reported mannose-binding lectins according to the results of N-terminal homology searching, MALDI-Q-TOF MS/MS analysis and database search. Thus, CML might be a novel mannose-binding plant lectin, which has not been reported in any lectin family. However, only the N-terminal homology searching by BLASTp method from NCBI and manually data mining, and further by peptide mass fingerprintings analysis are not remarkable enough to prove conclusively that CML is a completely novel lectin. Furthermore, this lectin may also possess more unknown significant biological activities to further explore our following investigations of CML. So, future studies will be aimed at the molecular cloning and sequence analysis of CML. And based on the cloning results, more information will be obtained and the classification of this lectin would be further clearly defined.
Recently, the remarkable antineoplastic activity of plant lectins has driven much attention to cancer studies [19]. Con A, a widely studied mannose-/glucose-binding lectin, is cytotoxic or inhibitory to numerous cancer cell lines, which has been reported for its remarkable antineoplastic activity recently [25], [9]. A great number of studies have reported that some ‘ideal’ anti-cancer candidate drugs can induce apoptosis in susceptible cancer cells [37]. Like these anti-cancer drugs, CML executed dose-dependent growth-inhibitory effect on four typical cancer cells. Based upon the morphologic observation, it was found that CML-induced L929 cell apoptosis; moreover, by MTT assay, it also indicated that CML is active in exerting cytotoxicity against L929 cells. It is axiomatic that cancer therapies can work in two different ways, by induction of apoptosis as well as by direct cytoxicity [12].
Thus, further study about the mechanism of CML-induced L929 cells apoptosis has been performed. The inspiring finding was that CML was able to activate the caspase-8. Subsequently, caspase-8 activated caspase-3 whose substrates were PARP and ICAD. The ICAD is a caspase substrate that is cleaved to be inactivated and allow CAD to execute the characteristic fragmentation of DNA. Thus, the ICAD expression in L929 cells was examined to confirm the activation of caspase-3. The roles of caspase-8 and caspase-3 are not only substantiated to be associated with participation of caspase inhibitors, but also seems to be restricted to apoptosis mediated by upstream death receptor pathway or mitochondrial pathway [6], [45]. Consequently, based upon the observations reported, we suggested that the above-mentioned two major pathways are likely existing in apoptosis induced by CML. Further investigation of these possible mechanisms would clearly affect the outcomes of this lectin, including a wide variety of agents currently used in cancer treatment and pharmaceutical application [31], [33].
In addition, Con A has also been found to be mediated by apoptosis and autophagy (programmed cell death II) in the mitochondrial pathways in cancer cells [29], [30]. In the current study, we found a typical caspase-dependent apoptosis through participation of three caspase inhibitors and Western blot analysis. The distinction of their antineoplastic mechanisms might be due to the fine differences of their carbohydrate-binding sites. In our study, it indicated that the anti-tumor activity decreased abruptly and the phenomenon of apoptosis almost disappeared when the carbohydrate-binding site was fully inhibited. Thus, the CML binding might be the predominant reason which sparks off the significant anti-tumor biological characteristics, even casting a crucial impact on its potential apoptotic mechanism. In recent years, some research has reported the PCL-induced apoptosis in A375 cells [27], [28] and comparisons of the antiproliferative and apoptosis-inducing activities among PCL, OJL and LNL in MCF-7 cells [31]. In addition, other major lectin families, such as RIPs II, have also been reported to induce apoptosis in various cancer cells for several years [26]. These results indicate that the various lectin families-induced apoptotic mechanism is a caspase-dependent pathway and would provide more clues for further elucidating their anti-tumor activity and mechanisms in lectin studies. Accordingly, this interesting finding would drive us a principal force to further explore the significant correlation between the carbohydrate-binding-specificity and the antineoplastic molecular mechanism.
In summary, we purified CML from Clematis genus and characterized some significant biological characteristics, especially its antiviral and apoptosis-inducing activities for the first time. Due to accumulating evidence, the findings of CML exhibiting remarkable antiviral and apoptosis-inducing activities, would open up a new exploration for plant lectins as potential candidate drugs. Furthermore, it might provide more promising insights into pharmaceutical exploitation in treatment of different human diseases in the near future.
Acknowledgements
We thank Professor Jan Balzarini (Rega Institute for Medical Research, Katholieke University Leuven, Belgium) for his kind help to provide the antiviral tests and Miss He-jiao Bian (Sichuan University) for her critical review of this manuscript. This work was supported in part by grants from the National Natural Science Foundation of China (General Programs: No. 30270331 and No. 30670469), Director Fund of State Key Laboratory of Oral Diseases (Sichuan University), the Science and Technology Fund for Distinguished Young Scholars of Sichuan Province (No. 06ZQ026-035) and the Key Technologies R&D Program of Sichuan Province (2006Z08-010).
References
- 1.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.An J., Liu J.Z., Wu C.F., Li J., Dai L., Van Damme E. Anti-HIV I/II activity and molecular cloning of a novel mannose/sialic acid-binding lectin from rhizome of Polygonatum cyrtonema Hua. Acta Biochim Biophys Sin (Shanghai) 2006;38:70–78. doi: 10.1111/j.1745-7270.2006.00140.x. [DOI] [PubMed] [Google Scholar]
- 3.Andrews P. Estimation of molecular size and molecular weights of biological compounds by gel filtration. Methods Biochem Anal. 1970;18:1–53. [PubMed] [Google Scholar]
- 4.Balzarini J., Hatse S., Vermeire K., Princen K., Aquaro S., Perno C.F. Mannose-specific plant lectins from the Amaryllidaceae family qualify as efficient microbicides for prevention of human immunodeficiency virus infection. Antimicrob Agents Chemother. 2004;48:3858–3870. doi: 10.1128/AAC.48.10.3858-3870.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balzarini J., Van Laethem K., Hatse S., Vermeire K., De Clercq E., Peumans W. Profile of resistance of human immunodeficiency virus to mannose-specific plant lectins. J Virol. 2004;78:10617–10627. doi: 10.1128/JVI.78.19.10617-10627.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bantel H., Engels I.H., Voelter W., Schulze-Osthoff K., Wesselborg S. Mistletoe lectin activates caspase-8/FLICE independently of death receptor signaling and enhances anticancer drug-induced apoptosis. Cancer Res. 1999;59:2083–2090. [PubMed] [Google Scholar]
- 7.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utlizing the principle of protein–dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 8.Cammue B.P., Peeters B., Peumans W.J. Isolation and partial characterization of an N-acetylgalactosamine-specific lectin from winter-aconite (Eranthis hyemalis) root tubers. Biochem J. 1985;227:949–955. doi: 10.1042/bj2270949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang C.P., Yang M.C., Liu H.S., Lin Y.S., Lei H.Y. Concanavalin A induces autophagy in hepatoma cells and has a therapeutic effect in a murine in situ hepatoma model. Hepatology. 2007;45:286–296. doi: 10.1002/hep.21509. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y.F., Liu J., Davidson R.S., Howarth O.W. Isolation and structure of clematine, a new flavanone glycoside from Clematis armandii franch. Tetrahedron. 1993;49:5169–5176. [Google Scholar]
- 11.Chen Z., Merta P.J., Lin N.H., Tahir S.K., Kovar P., Sham H.L. A-432411, a novel indolinone compound that disrupts spindle pole formation and inhibits human cancer cell growth. Mol Cancer Ther. 2005;4:562–568. doi: 10.1158/1535-7163.MCT-04-0229. [DOI] [PubMed] [Google Scholar]
- 12.Cheng Y., Qiu F., Huang J., Tashiro S., Onodera S., Ikejima T. Apoptosis-suppressing and autophagy-promoting effects of calpain on oridonin-induced L929 cell death. Arch Biochem Biophys. 2008;475:148–155. doi: 10.1016/j.abb.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 13.Cheng Y., Qiu F., Tashiro S., Onodera S., Ikejima T. ERK and JNK mediate TNFalpha-induced p53 activation in apoptotic and autophagic L929 cell death. Biochem Biophys Res Commun. 2008;376:483–488. doi: 10.1016/j.bbrc.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Cheng Y., Qiu F., Ye Y.C., Guo Z.M., Tashiro S., Onodera S. Autophagy inhibits reactive oxygen species-mediated apoptosis via activating p38-nuclear factor-kappa B survival pathways in oridonin-treated murine fibrosarcoma L929 cells. FEBS J. 2009;276:1291–1306. doi: 10.1111/j.1742-4658.2008.06864.x. [DOI] [PubMed] [Google Scholar]
- 15.de Mejia E.G., Bradford T., Hasler C. The anticarcinogenic potential of soybean lectin and lunasin. Nutr Rev. 2003;61:239–246. doi: 10.1301/nr.2003.jul.239-246. [DOI] [PubMed] [Google Scholar]
- 16.Guzmán-Partida A.M., Robles-Burgueño M.R., Ortega-Nieblas M., Vázquez-Moreno I. Purification and characterization of complex carbohydrate specific isolectins from wild legume seeds: Acacia constricta is (vinorama) highly homologous to Phaseolus vulgaris lectins. Biochimie. 2004;86:335–342. doi: 10.1016/j.biochi.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Herre J., Willment J.A., Gordon S., Brown G.D. The role of Dectin-1 in antifungal immunity. Crit Rev Immunol. 2004;24:193–203. doi: 10.1615/critrevimmunol.v24.i3.30. [DOI] [PubMed] [Google Scholar]
- 18.Hooker J.D. L. Reeve; London: 1875. Flora of British India. pp. 2–3. [Google Scholar]
- 19.Jung E.C., Kim K.D., Bae C.H., Kim J.C., Kim D.K., Kim H.H. A mushroom lectin from ascomycete Cordyceps militaris. Biochim Biophys Acta. 2007;1770:833–838. doi: 10.1016/j.bbagen.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Keyaerts E., Vijgen L., Pannecouque C., Van Damme E., Peumans W., Egberink H. Plant lectins are potent inhibitors of coronaviruses by interfering with two targets in the viral replication cycle. Antiviral Res. 2007;75:179–187. doi: 10.1016/j.antiviral.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan F., Ahmad A., Khan M.I. Purification and characterization of a lectin from endophytic fungus Fusarium solani having complex sugar specificity. Arch Biochem Biophys. 2007;457:243–251. doi: 10.1016/j.abb.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 22.Konozy E.H., Bernardes E.S., Rosa C., Faca V., Greene L.J., Ward R.J. Isolation, purification, and physicochemical characterization of a d-galactose-binding lectin from seeds of Erythrina speciosa. Arch Biochem Biophys. 2003;410:222–229. doi: 10.1016/s0003-9861(02)00695-1. [DOI] [PubMed] [Google Scholar]
- 23.Lam S.S., Wang H., Ng T.B. Purification and characterization of novel ribosome inactivating proteins, alpha- and beta-pisavins, from seeds of the garden pea Pisum sativum. Biochem Biophys Res Commun. 1998;253:135–142. doi: 10.1006/bbrc.1998.9764. [DOI] [PubMed] [Google Scholar]
- 24.Lannoo N., Vervecken W., Proost P., Rougé P., Van Damme E.J. Expression of the nucleocytoplasmic tobacco lectin in the yeast Pichia pastoris. Protein Expr Purif. 2007;53:275–282. doi: 10.1016/j.pep.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Lei H.Y., Chang C.P. Induction of autophagy by concanavalin A and its application in anti-tumor therapy. Autophagy. 2007;3:402–404. doi: 10.4161/auto.4280. [DOI] [PubMed] [Google Scholar]
- 26.Liu B., Bian H.J., Bao J.K. Plant lectins: potential novel antineoplastic drugs from bench to clinic. Cancer Lett. 2009 doi: 10.1016/j.canlet.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 27.Liu B., Cheng Y., Bian H.J., Bao J.K. Molecular mechanisms of Polygonatum cyrtonema lectin induced apoptosis and autophagy in cancer cells. Autophagy. 2009;5:253–255. doi: 10.4161/auto.5.2.7561. [DOI] [PubMed] [Google Scholar]
- 28.Liu B., Cheng Y., Zhang B., Bian H.J., Bao J.K. Polygonatum cyrtonema lectin induces apoptosis and autophagy in human melanoma A375 cells through a mitochondria-mediated ROS-p38-p53 pathway. Cancer Lett. 2009;275:54–60. doi: 10.1016/j.canlet.2008.09.042. [DOI] [PubMed] [Google Scholar]
- 29.Liu B., Li C.Y., Bian H.J., Min M.W., Chen L.F., Bao J.K. Antiproliferative activity and apoptosis-inducing mechanism of concanavalin A on human melanoma A375 cells. Arch Biochem Biophys. 2009;482:1–6. doi: 10.1016/j.abb.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Liu B., Min M.W., Bao J.K. Induction of apoptosis by concanavalin A and its molecular mechanisms in cancer cells. Autophagy. 2009;5:432–433. doi: 10.4161/auto.5.3.7924. [DOI] [PubMed] [Google Scholar]
- 31.Liu B., Peng H., Yao Q., Li J., Van Damme E., Balzarini J. Bioinformatics analyses of the mannose-binding lectins from Polygonatum cyrtonema, Ophiopogon japonicus and Liparis noversa with antiproliferative and apoptosis-inducing activities. Phytomedicine. 2009;16:601–608. doi: 10.1016/j.phymed.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Liu B., Xu X.C., Cheng Y., Huang J., Liu Y.H., Liu Z. Apoptosis-inducing effect and structural basis of Polygonatum cyrtonema lectin and chemical modification properties on its mannose-binding sites. BMB Rep. 2008;41:369–375. doi: 10.5483/bmbrep.2008.41.5.369. [DOI] [PubMed] [Google Scholar]
- 33.Liu B., Zhang B., Min M.W., Bian H.J., Chen L.F., Liu Q. Induction of apoptosis by Polygonatum odoratum lectin and its molecular mechanisms in murine fibrosarcoma L929 cells. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbagen.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 34.Liu Z., Liu B., Zhang Z.T., Zhou T.T., Bian H.J., Min M.W. A mannose-binding lectin from Sophora flavescens induces apoptosis in HeLa cells. Phytomedicine. 2008;15:867–875. doi: 10.1016/j.phymed.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 35.Luo Y., Xu X., Liu J., Li J., Sun Y., Liu Z. A novel mannose-binding tuber lectin from Typhonium divaricatum (L.) Decne (family Araceae) with antiviral activity against HSV-II and anti-proliferative effect on human cancer cell lines. J Biochem Mol Biol. 2007;40:358–367. doi: 10.5483/bmbrep.2007.40.3.358. [DOI] [PubMed] [Google Scholar]
- 36.Macedo M.L., Damico D.C., Freire M.G., Toyama M.H., Marangoni S., Novello J.C. Purification and characterization of an N-acetylglucosamine-binding lectin from Koelreuteria paniculata seeds and its effect on the larval development of Callosobruchus maculatus (Coleoptera: Bruchidae) and Anagasta kuehniella (Lepidoptera: Pyralidae) J Agric Food Chem. 2003;51:2980–2986. doi: 10.1021/jf034013i. [DOI] [PubMed] [Google Scholar]
- 37.Nicholson D.W. From bench to clinic with apoptosis-based therapeutic agents. Nature. 2000;407:810–816. doi: 10.1038/35037747. [DOI] [PubMed] [Google Scholar]
- 38.Oda Y., Minami K. Isolation and characterization of a lectin from tulip bulbs, Tulipa gesneriana. Eur J Biochem. 1986;159:239–245. doi: 10.1111/j.1432-1033.1986.tb09859.x. [DOI] [PubMed] [Google Scholar]
- 39.Pérez G. Isolation and characterization of a novel lectin from Dioclea lehmanni (Fabaceae) seeds. Int J Biochem Cell Biol. 1998;30:843–853. doi: 10.1016/s1357-2725(98)00045-4. [DOI] [PubMed] [Google Scholar]
- 40.Rubinstein N., Ilarregui J.M., Toscano M.A., Rabinovich G.A. The role of galectins in the initiation, amplification and resolution of the inflammatory response. Tissue Antigens. 2004;64:1–12. doi: 10.1111/j.0001-2815.2004.00278.x. [DOI] [PubMed] [Google Scholar]
- 41.Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 42.Schumacher K., Schneider B., Reich G., Stiefel T., Stoll G., Bock P.R. Influence of postoperative complementary treatment with lectin-standardized mistletoe extract on breast cancer patients. A controlled epidemiological multicentric retrolective cohort study. Anticancer Res. 2003;23:5081–5087. [PubMed] [Google Scholar]
- 43.Sharon N., Lis H. Lectins as cell recognition molecules. Science. 1989;246:227–234. doi: 10.1126/science.2552581. [DOI] [PubMed] [Google Scholar]
- 44.Strazza S., Sherry A.D. Concanavalin A will not assume the sugar binding conformation in the complete absence of metal ions. Biochem Biophys Res Commun. 1982;106:1291–1297. doi: 10.1016/0006-291x(82)91253-0. [DOI] [PubMed] [Google Scholar]
- 45.Tan Y., Wu C., De Veyra T., Greer P.A. Ubiquitous calpains promote both apoptosis and survival signals in response to different cell death stimuli. J Biol Chem. 2006;281:17689–17698. doi: 10.1074/jbc.M601978200. [DOI] [PubMed] [Google Scholar]
- 46.Thakur A., Rana M., Lakhanpal T.N., Ahmad A., Khan M.I. Purification and characterization of lectin from fruiting body of Ganoderma lucidum: lectin from Ganoderma lucidum. Biochim Biophys Acta. 2007;1770:1404–1412. doi: 10.1016/j.bbagen.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 47.Tian Q., Wang W., Miao C., Peng H., Liu B., Leng F.W. Purification, characterization and molecular cloning of a novel mannose-binding lectin from rhizomes of Ophiopogon japonicus with antiviral and antifungal activities. Plant Sci. 2008;175:877–884. [Google Scholar]
- 48.Tong A., Wu L., Lin Q., Lau Q.C., Zhao X., Li J. Proteomic analysis of cellular protein alterations using a hepatitis B virus-producing cellular model. Proteomics. 2008;8:2012–2023. doi: 10.1002/pmic.200700849. [DOI] [PubMed] [Google Scholar]
- 49.Van Damme E.J., Barre A., Mazard A.M., Verhaert P., Horman A., Debray H. Characterization and molecular cloning of the lectin from Helianthus tuberosus. Eur J Biochem. 1999;259:135–142. doi: 10.1046/j.1432-1327.1999.00013.x. [DOI] [PubMed] [Google Scholar]
- 50.Wang H.X., Ng T.B., Liu W.K., Ooi V.E., Chang S.T. Isolation and characterization of two distinct lectins with antiproliferative activity from the cultured mycelia of the mushroom Tricholoma mongolicum. Int J Pept Protein Res. 1995;46:508–513. doi: 10.1111/j.1399-3011.1995.tb01606.x. [DOI] [PubMed] [Google Scholar]
- 51.Wong J.H., Ng T.B. Isolation and characterization of a glucose/mannose/rhamnose-specific lectin from the knife bean Canavalia gladiata. Arch Biochem Biophys. 2005;439:91–98. doi: 10.1016/j.abb.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 52.Woo B.H., Lee J.T., Na D.H., Lee K.C. Sepharose-unbinding ricin E as a source for ricin A chain immunotoxin. J Immunol Methods. 2001;249:91–98. doi: 10.1016/s0022-1759(00)00330-6. [DOI] [PubMed] [Google Scholar]
- 53.Xia M., Wang M., Tashiro S., Onodera S., Minami M., Ikejima T. Dracorhodin perchlorate induces A375-S2 cell apoptosis via accumulation of p53 and activation of caspases. Biol Pharm Bull. 2005;28:226–232. doi: 10.1248/bpb.28.226. [DOI] [PubMed] [Google Scholar]
- 54.Yan Q., Jiang Z., Yang S., Deng W., Han L. A novel homodimeric lectin from Astragalus mongholicus with antifungal activity. Arch Biochem Biophys. 2005;442:72–81. doi: 10.1016/j.abb.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 55.Zhang J., Chen D.C., Lin H.R., Zhang W.G. Color Pictures of Chinese Crude Drug. Guangdong Sciences & Techology Publishing House; Guangzhou: 1990. The pharmaceutical dictionary of China. p. 33. [Google Scholar]


