Abstract
The features of the chemistry of 4-thiazolidinone and pyrazole/pyrazolines as pharmacologically attractive scaffolds were described in a number of reviews in which the main approaches to the synthesis of mentioned heterocycles and their biological activity were analyzed. However, the pyrazole/pyrazoline–thiazolidine-based hybrids as biologically active compounds is poorly discussed in the context of pharmacophore hybrid approach. Therefore, the purpose of this review is to summarize the data about the synthesis and modification of heterocyclic systems with thiazolidine and pyrazoline or pyrazole fragments in molecules as promising objects of modern bioorganic and medicinal chemistry. The description of biological activity was focused on SAR analysis and mechanistic insights of mentioned hybrids.
Keywords: Synthesis, Biological activity, Pyrazole, Pyrazoline, Thiazolidine
Graphical abstract
Highlights
-
•
Synthesis and chemistry of pyrazole/pyrazoline–thiazolidine-based hybrids.
-
•
A diverse spectrum of pyrazole/pyrazoline–thiazolidine-based hybrids biological activities has been presented.
-
•
Structure activity relationship of pyrazole/pyrazoline–thiazolidine-based hybrids for different activities has been discussed.
1. Introduction
Pharmacophore hybrid approach is a current concept in drug design and development to produce molecules with improved affinity and efficacy. There are some reviews dealing with the concept of molecular hybridization and the promises/challenges associated with these hybrid molecules along with recent advances on anticancer hybrids [1], [2], [3]. Various heteroaryl based hybrids in particular isatin and coumarins [3] or indoline-thiazolidinones [4] have been recently reviewed as conjugates with remarkable inhibitory potential.
Design of new drug-like small molecules based on the pharmacologically attractive scaffolds of thiazolidine (4-thiazolidinone) [5], [6], [7], [8], [9], [10] and pyrazole or pyrazoline [11], [12], [13], [14], [15] is a reasonable and promising direction in modern medicinal chemistry. The chemical approaches for the synthesis of thiazolidinone- and pyrazole-based derivatives and their pharmacological activity were described in a numerous reviews [5], [6], [7], [8], [9], [10], [11], [12]. However, the pyrazole/pyrazoline–thiazolidine-based conjugates as biologically active compounds are poorly reviewed in the context of pharmacophore hybrid approach.
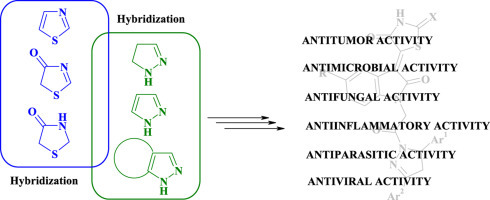
The recent synthetic studies of pyrazole–thiazolidines and related hybrids and biological investigations for their antitumor, antimicrobial, antiviral, antiparasitic, anti-inflammatory activities allowed to identify the promising drug-like compounds. Thus, the pyrazole–thiazolidinones/thiazoles have been patented as inhibitors of necroptosis [16], VHR protein tyrosine phosphatase inhibitors [17], Pin1-modulating compounds [18], compounds for modulating RNA-binding proteins [19] and activators of pro-apoptotic BAX [20]. In addition, the pyrazole–thiazolidinone hybrids have been studied for their possibility to inhibit the TNF-α–TNFRc1 interaction [21], as inhibitors of histone acetyltransferases [22] and inhibitors of COX [31], [39] and ADAMTS-5 enzymes [83]. Therefore, the purpose of this review is to summarize the data about the synthesis and biological activity of heterocyclic systems with thiazolidine and pyrazole/pyrazoline fragments in molecules. The most promising pyrazole–thiazolidinone/thiazole hybrids and target heterocycles that have been reviewed as fragments of hybrid molecules are depicted in Fig. 1 .
Fig. 1.
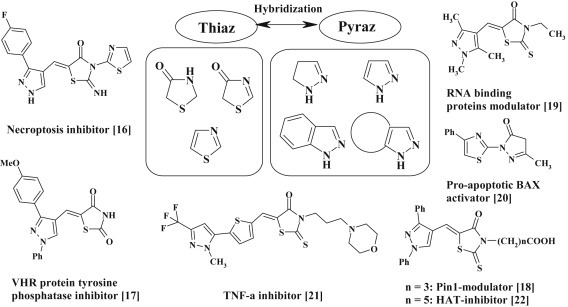
Heterocycles for pyrazole/pyrazoline–thiazole/thiazolidine hybridization and the most promising conjugates.
The reviewed hybrids were classified as 2-, 3- and 5-pyrazole/pyrazoline-substituted thiazolidines based on linkage of target heterocyclic cores. The basic approaches for the synthesis of mentioned derivatives provide a combination of two “small” molecules in aminolysis reactions, acylation and Knoevenagel procedure or heterocyclization of monocyclic compounds via the [2+3]-cyclization reaction yielding the series of non-condensed bicyclic systems.
2. Synthetic approaches for 4-thiazolidinone-based hybrids with pyrazole/pyrazoline fragments in position 2
Detailed biological activity evaluation of pyrazoline–thiazolidinone conjugates 1.2–1.7 and pyrazoline–thiazoles 1.8, 1.9 (Fig. 2 ), synthesized via the [2+3]-cyclocondensation of 4,5-dihydropyrazole-1-carbothioamides 1.1 as S,N-binucleophiles in reactions with equivalents of dielectrophilic synthon [C2]2+, allowed to identify compounds with antimicrobial [23], [24], [25], [26], [27], [28], antiviral [29], [30], anti-inflammatory [31], antitumor [32], [33], [34], [35] and insecticidal [36] activities. For synthesis of target derivatives α-halogenocarboxylic acids [31], [33] and their ethyl esters [23], [24], [25], [26], [27], [28], [29], [35], [36], maleic anhydride [30], maleimides [30], [38], β-aroylacrylic acids [30], dimethyl acetylenedicarboxylate [37], bromoacetophenones [23], [24], [28], [29], [35] and ethyl 4-chloroacetoacetate [35] were used as equivalents of dielectrophilic synthon [C2]2+.
Fig. 2.
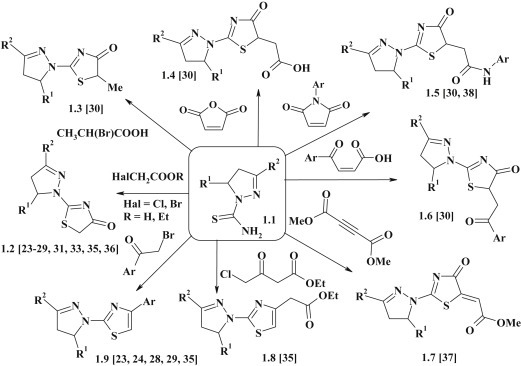
Synthesis of pyrazoline–thiazolidinones via [2+3]-cyclization reactions.
Three-component one-pot reaction that includes [2+3]-cyclocondensation of 4,5-dihydropyrazole-1-carbotioamides 1.1 with chloroacetic acid and the further Knoevenagel reaction with aromatic aldehydes [33], [34] and isatin derivatives [32] is an effective approach for design of new anticancer agents among the pyrazoline–thiazolidinones 1.10, 1.11 (Fig. 3 ).
Fig. 3.
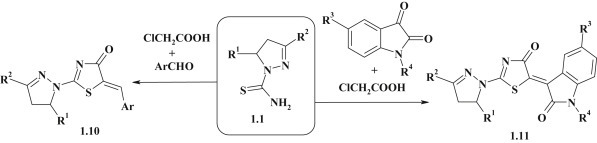
Three-component one-pot reactions in synthesis of 2-pyrazolinyl-4-thiazolidinones.
The reaction between pyrazolyl-imines [39], [40] or pyrazolyl-hydrazones [41] and thioglycolic acid or its esters is widely used approach for the synthesis of 4-thiazolidinone conjugates 1.12, 1.13 with pyrazole moiety in position 2. A. Khodairy proposed a synthesis of 2-substituted 4-thiazolidinone with benzopyrano[2,3-c]pyrazol-3-one fragment 1.14, using N-cyanoacetyl-benzopyranopyrazolone [42] as a starting compound (Fig. 4 ).
Fig. 4.
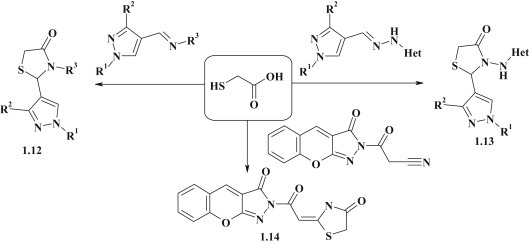
Thioglycolic acid reagent in thiazolidinone ring-formation reactions.
Another direction for synthesis of pyrazole-substituted thiazoline 1.16 (Fig. 5 ) was realized by the creation of pyrazole moiety via the reaction between 5-fluoro-2-hydrazino-4-hydroxy-4,5-bis(trifluoromethyl)-1,3-thiazoline 1.15 with acetoacetone [43], [44].
Fig. 5.
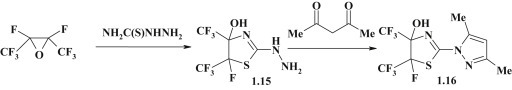
Example of pyrazole formation in the synthesis of pyrazole-thiazolines.
A broad group of pyrazole–thiazolidinone derivatives exemplified by 2-imino-4-thiazolidinones (pseudothiohydantoines) 1.18, 1.19 were synthesized via [2+3]-cyclocondensation of pyrazole substituted asymmetric bis-thioureas 1.17 as S,N-binucleophiles with some equivalents of dielectrophilic synthon [C2]2+ (ethyl 2-chloroacetate [45], [46], dimethyl acetylenedicarboxylate [47]). Simultaneously, bioisosteric pyrazolyl-2-iminothiazolidines 1.20 [46] were obtained by reaction of thioureas 1.17 with α-bromoacetophenones (Fig. 6 ).
Fig. 6.
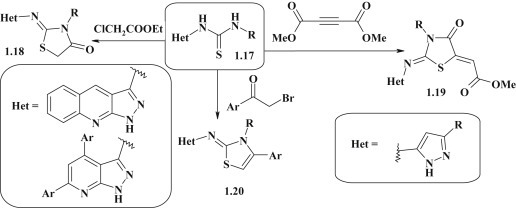
Synthesis of pyrazole–thiazolidinones with imine linker group.
Aiming to search for new anticancer or antimicrobial compounds among pyrazole–thiazolidinone conjugates the pyrazole-sulfonyl-thioureas 1.21 were used as starting materials. The reaction between compounds 1.21 and ethyl 2-chloroacetate or α-bromoacetophenone resulted in formation of the corresponding pyrazole–thiazolidinones 1.22 and pyrazole-thiazolidines 1.23 with sulfonylimine linker group [48], [49], [50], [51], [52], [53] (Fig. 7 ).
Fig. 7.
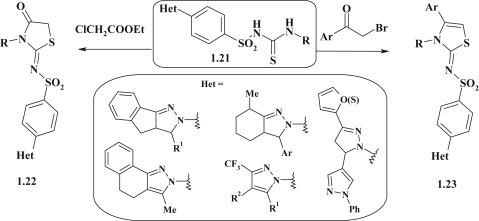
Synthesis of diazole–thiazolidinones/thiazolidines with sulfonylimine linker group.
Another approach for the synthesis of pyrazole derivatives of pseudothiohydantoin 1.26 was suggested by B. Insuasty et al. [54]. The synthesis of target compounds was carried out via aminolysis of 5-arylidene-2-thioxo-4-thiazolidinones (5-arylidenerhodanines) 1.24 by 4,5-diaminopyrazoles 1.25 (Fig. 8 ).
Fig. 8.
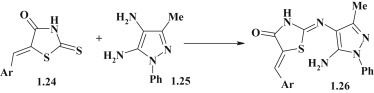
Aminolysis reaction in synthesis of pyrazole–thiazolidinones.
A row of 2-pyrazolyl-hydrazone–thiazolidinones 1.28–1.30 was synthesized via [2+3]-cyclocondensation of thiosemicarbazones 1.27 with α-halogenocarboxylic acids derivatives or maleic anhydride [55], [56], [57], [58], [59], [60]. The pyrazole with bioisosteric 2-thiohydantoin 1.31 [61] and thiazolidine 1.32 [55], [56], [57], [58], [59], [60] fragments were obtained by reaction of thiosemicarbazones 1.27 with chloroacetic acid in pyridine or α-bromoacetophenone. However, the reaction of thiosemicarbazones 1.27 with acetic anhydride or iron chloride was accompanied by the formation of pyrazole–thiadiazole derivatives 1.33, 1.34 [56] (Fig. 9 ).
Fig. 9.
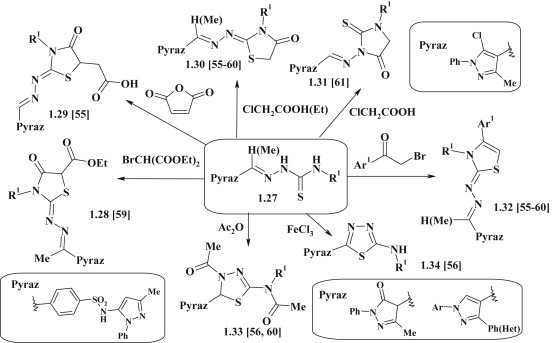
Synthesis of pyrazole derivatives with thiazolidinone, imidazolidine and thiazoline fragments in molecules.
Pyrazole-substituted thiosemicarbazides 1.35 were used for synthesis of the pyrazole–thiazolidinones 1.36 with carbohydrazide linker group [62], [63]. The three-component one-pot reaction between thiosemicarbazides 1.35, chloroacetic acid and aromatic aldehydes accompanied by formation of the corresponding 5-arylidene-4-thiazolidinones 1.37 [63], [64]. The modification of 1.35 in sulfuric acid medium gave pyrazole–thiadiazole 1.38 [64]. The reaction of compounds 1.35 with α-bromoacetophenones was accompanied by the formation of corresponding pyrazole-thiazolidine conjugates 1.39 [62], [63], [64] (Fig. 10 ).
Fig. 10.
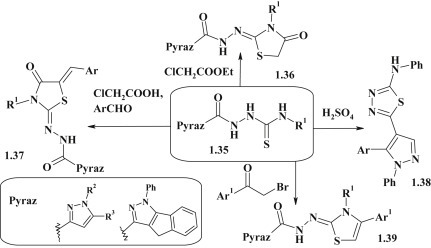
Pyrazole-substituted thiosemicarbazides in synthesis of pyrazole–thiazole hybrids.
3. The main methods for synthesis of 4-thiazolidinones with pyrazole/pyrazoline moiety in position 3
Synthesis of 2-diazole-substituted 4-thiazolidinones was achieved via the [2+3]-cyclocondensation of the pyrazole-based asymmetric thioureas 1.17 as S,N-binucleophiles (see Fig. 6). At the same time, the numerous papers represent the data about obtaining of 4-thiazolidinones with diazole moiety in 3 position (compound 2.1, Fig. 11 ), in particular via the reaction of thioureas with ethylchloroacetate [65].
Fig. 11.
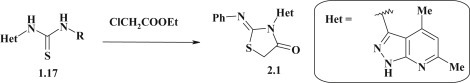
Synthesis of 3-diazole-substituted 4-thiazolidinones.
The dithiocarbamate method of 2-thioxo-4-thiazolidinones (rhodanines) synthesis is a convenient and often used variant of the synthesis of 4-thiazolidinone derivatives based on the [2+3]-cyclocondensation [5]. R.M. Mohareb et al. had successfully applied the dithiocarbamate method for the synthesis of 3-(pyrazol-5-yl)-2-thioxo-4-thiazolidinone via two-stage process based on the reaction between 3-phenyl-5-aminopyrazole 2.2 and carbon disulfide in an alkaline medium and subsequent cyclization with ethyl 2-bromoacetate [66]. The presence of the methylene group in the position 5 of thiazolidine cycle allowed the authors to carry out the structural modification of pyrazole–thiazolidinone 2.3 in the Knoevenagel reaction (ethanol medium, catalyst – piperidine) and diazotization to get the 5-substituted derivatives 2.4, 2.5. Starting from rhodanine 2.3 the alternative methods for the synthesis of condensed pyrano[2,3-d]thiazole system 2.6 with pyrazole moiety in position 3 were proposed, namely the reaction of 5-arylidenerhodanines 2.5 with malononitrile or by heterocyclization of compound 2.3 with benzylidenemalononitrile. Pyrazole–thiazolidinone 2.3 in the presence of hydrazine hydrate undergoes ring transfomation with obtaining of the pyrazole-triazole 2.7 (Fig. 12 ).
Fig. 12.
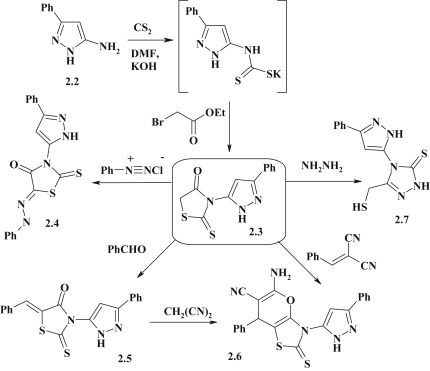
Synthesis and modification of 3-pyrazole-substituted rhodanines.
Various 4-thiazolidinones were synthesized by reaction of α-mercaptocarboxylic acids (especially thioglycolic acid) with isothiocyanates (R–N C S). Thus, a pyrazole–thiazolidinone 2.8 with phenyl sulfonamide linker group was obtained based on the mentioned approach [67]. There were described the synthesis of 3-pyrazole-substituted or polyheterocyclic derivatives of 4-thiazolidinones 2.9, 2.10 based on the reaction between thioglycolic acid and corresponding imines and methylidene-hydrazydes or three-component reaction involving thioglycolic acid, aromatic aldehydes and heterocyclic amines containing diazole fragment [68], [69], [70], [71], [72]. The reaction of isatin with heterocyclic amines led to Schiff's bases which were further reacted with thioglycolic acid to form 3-diazole-substituted indoline-spiro-thiazolidinones 2.11 [73], [74] (Fig. 13 ).
Fig. 13.
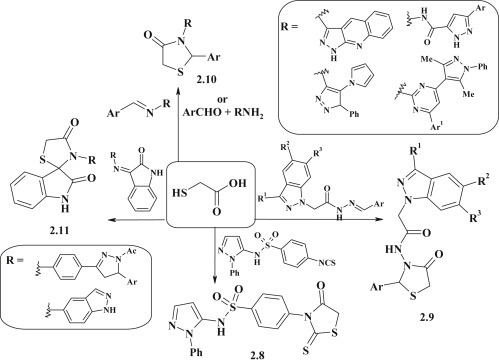
α-Mercaptocarboxylic acids reagent in the synthesis of 3-subtituted thiazolidinones.
The reaction between trithiocarbonyl diglycolic acid and amines in alcohol or alcohol–water medium (Holmberg synthesis) is a convenient and effective method for synthesis of 3-substituted rhodanines, especially based on the aromatic amines and carboxylic acid hydrazides [5]. The mentioned reaction was used by Augustin M. et al. for synthesis of rhodanine 2.12 with benzopyrazole fragment in position 3 [75] (Fig. 14 ).
Fig. 14.
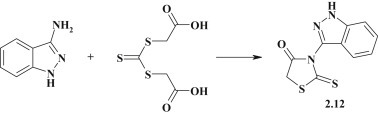
Holmberg synthesis for benzopyrazole–rhodanine.
The method for synthesis of pyrazole–thiazolidinone 2.15 (Fig. 15 ) with acetamide linker group was proposed based on the reaction of 5-arylidenerhodanine-3-acetic acid 2.13 and 5-aminopyrazole 2.14 in DMF in the presence of HATU (1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxid hexafluorophosphate) [76].
Fig. 15.
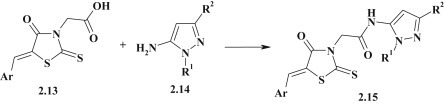
Synthesis of pyrazole–thiazolidinone conjugates via acylation reaction.
Synthesis of structurally related azolidine–pyrazolines 2.16, 2.17 was proposed based on N-alkylation reaction of potassium salts of 2,4-thiazolidinedione, hydantoin and their 5-arylidene derivatives, obtained in situ (Fig. 16 ). 2-Chloro-1-(3,5-diarylpyrazolin-1-yl)-ethanones were successfully used as alkylating agents [77].
Fig. 16.
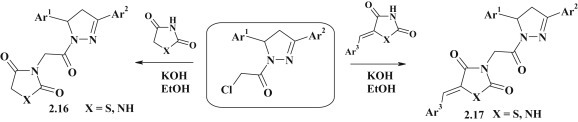
Synthesis of pyrazoline–azolidinone conjugates via alkylation reaction.
4. The main approaches to the synthesis of 4-thiazolidinones with pyrazole/pyrazoline moiety in position 5
One of the main approaches for the synthesis of 5-pyrazole/pyrazoline substituted 4-thiazolidinones is based on Knoevenagel reaction of 4-thiazolidinones [5]. A numerous 1-, 3- and 5-substituted pyrazole-4-carbaldehydes were used as carbonyl compounds and allowed to obtain a series of new 2,4-thiazolidinediones [78], [79], [80], [81], rhodanines [82], [83], [84], [85], [86] and 2-imino-4-thiazolidinones with pyrazole moiety (compounds 3.1) [87], [88], [89]. The synthesis of pyrazolone–thiazolidinones 3.2 was conducted by reaction of 3-arylrhodanines with 4-acetyl-5-methyl-2-phenyl-2,4-dihydropyrazol-3-one [90]. Position 5 of thiazolidine cycle was successfully modified in diazo coupling reaction [91] using pyrazolyl diazonium chloride yielding compound 3.3 (Fig. 17 ).
Fig. 17.
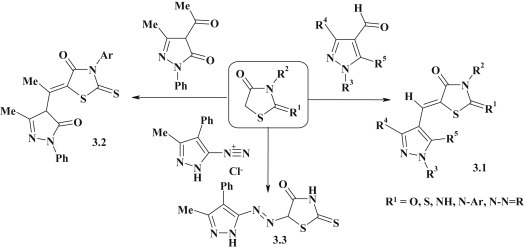
Knoevenagel and diazo coupling reactions for synthesis of pyrazole–thiazolidinones.
The synthesis of pyrazoline–indoline–thiazolidinone conjugates 3.5, 3.6 was realized by the Knoevenagel reaction of pyrazoline–isatines 3.4 with 2,4-thiazolidinedione, 2-thioxo-4-thiazolidinone and 2-amino-4-thiazolidinone in the acetic acid medium and in the presence of sodium acetate [92] (Fig. 18 ).
Fig. 18.
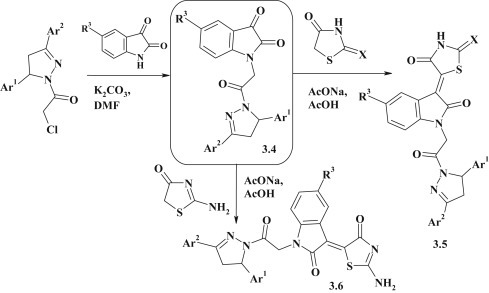
Synthesis of pyrazoline–indoline–thiazolidinone conjugates.
The synthesis of pyrazolone–thiazolidinones 3.7 (Fig. 19 ) was achieved based on the reaction between isonitrosorhodanines and 5-methyl-2,4-dihydropyrazol-3-one using piperidine as basic catalyst [93].
Fig. 19.
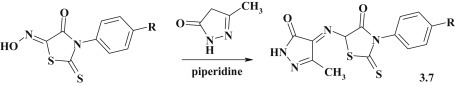
Synthesis of pyrazolone–thiazolidinone conjugates.
Magdy Ahmed Ibrahim et al. investigated a modification of 5-[4-oxo-4H-chromen-3-yl)methylene]-1,3-thiazolidin-2,4-dione 3.8, in particular in the ring-transformation reactions of chromene cycle with hydrazine hydrate and phenylhydrazine. These reactions allowed to obtain the corresponding pyrazole-thiazolidinones 3.9 [94]. Using hydrazine hydrate the structure of final compounds depended on the reaction medium. Thus, 1N-non-substituted pyrazoles were obtained in alcohol medium and in the presence of sodium ethylate, while 1N-acetylderivatives were formed in acetic acid. The reaction of the compound 3.8 and 1-phenylpyrazolidin-3,5-dione in the presence of sodium ethylate led to formation of polyheterocyclic system 3.10 (Fig. 20 ).
Fig. 20.
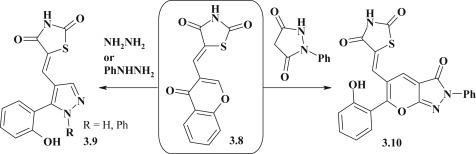
Chromene ring-transformation reactions in synthesis of thiazolidinone–diazole hybrids.
Synthesis of 5-pyrazolyl-4-thiazolidinones with ethylene linker group 3.11 was performed via the [2+3]-cyclocondensation reaction of thiourea with γ-pyrazolyl-α-chlorobutanoates [95] (see Fig. 21 ).
Fig. 21.
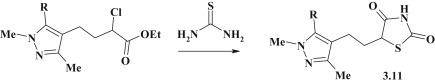
Synthesis of 5-pyrazole-substituted thiazolidinones via [2+3]-cyclocondensation.
Pharmacologically attractive 5-(5-oxo-4,5-dihydro-1H-pyrazol-4-yl)rhodanines 3.12 (Fig. 22 ) were synthesized following heterocyclization of 2-thiazolidinyl-3-oxobutanoates with phenylhydrazine or conjugation of 4-bromopyrazolon-5-ones to 3-arylrhodanines [96].
Fig. 22.
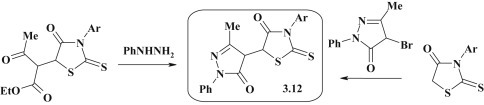
Alternative approaches for the synthesis of thiazolidinone–pyrazolones.
The group of pyrazoline–thiazolidinones 3.13 was obtained by reaction of 5-bromo-2,4-thiazolidindione with 3,5-diarylpyrazolines in alcohol medium (Fig. 23 ). The synthesized compounds were modified in position 3 of thiazolidine cycle by Mannich reaction with secondary cyclic amines and alkylation reactions forming 3.14, 3.15 [97].
Fig. 23.
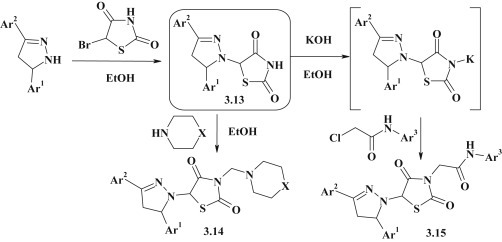
Synthesis and modification of pyrazoline–thiazolidinones.
The following approach to the synthesis of pyrazoline–thiazolidinone conjugates 3.16 with carbonyl methylidene linker group was based on the acylation reaction of 3,5-diarylpyrazolines (Fig. 24 ). The further reaction of substances 3.16 with chloroacetamides via N-alkylation reaction led to derivatives 3.17 [97].
Fig. 24.
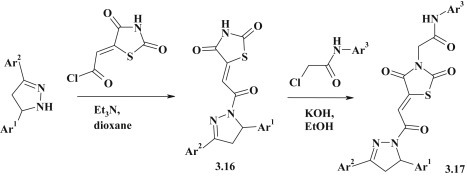
Synthesis and alkylation of thiazolidinone–pyrazolines 3.16.
To explore the structure-antitrypanosomal activity relationship the pyrazoline–thiazolidinone conjugates 3.18 were synthesized through the reaction of the 5-ethoxymethylidenerhodanines with 3,5-diarylpyrazolines (Fig. 25 ). 5-Pyrazoline substituted 4-thioxo-2-thiazolidinones 3.19 were obtained under the same conditions [98].
Fig. 25.
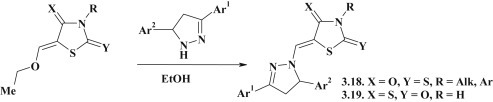
Synthesis of 5-pyrazoline substituted thiazolidinones.
To continue the above mentioned study, 5-pyrazoline derivatives of 4-thiazolidinone 3.21 with oxoethylamino-methylene linker group were synthesized by modification of rhodanine-5-carboxylic acid 3.20 in the reaction with pyrazolines and in the presence of DCC (dicyclohexylcarbodiimide) [98] (Fig. 26 ).
Fig. 26.
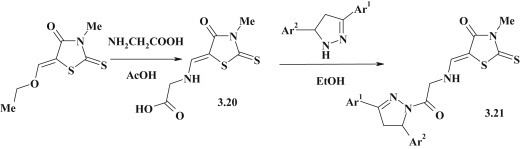
Synthesis of pyrazoline–rhodanine conjugates with oxoethylamino-methylene linker group.
5. Biological activity of pyrazole-thiazoldinones derivatives and reated heterocyclic hybrids
5.1. Antitumor activity of pyrazole/pyrazoline-thiazolidinone hybrids
Antitumor activity evaluation is promising for the thiazolidinones with pyrazoline fragment. The in vitro anticancer activity of thiazolidinones with pyrazoline cycle in position 2 (4.2) or 4 (4.3) of thiazolidine was tested by the National Cancer Institute (NCI) and most of them displayed anticancer activity on leukemia, melanoma, lung, colon, CNS, ovarian, renal, prostate and breast cancers cell lines. The most efficient anticancer compound 4.1 was found to be active with selective influence on colon cancer cell lines, especially on HT 29 (lgGI50 = −6.37) [33]. The SAR study revealed that: (1) anticancer activity of compounds is sensitive to the nature of substituent in position 5 of thiazolone cycle, (2) introduction of p-OH group in 5-benzylidene fragment enhanced potency, and (3) the positional isomers, namely 2-pyrazoline (4.2) and 4-pyrazoline (4.3) substituted thiazolones are promising compounds for further optimization (Fig. 27 ).
Fig. 27.
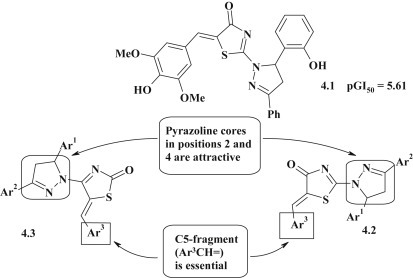
Antitumor activity for pyrazoline–thiazolidinone based hybrids.
Novel pyrazoline–thiazolidinone–isatin 4.4 and pyrazole-indoline-2-one 4.5 conjugates were designed as potential anticancer agents (Fig. 28 ). The most effective anticancer compound 4.6 was found to be active with a mean GI50 and TGI values of 0.071 μM and 0.76 μM, respectively and demonstrated the highest antiproliferative influence on the non-small cell lung cancer cell line HOP-92 (GI50 < 0.01 μM), colon cancer line HCT-116 (GI50 = 0.018 μM), CNS cancer cell line SNB-75 (GI50 = 0.0159 μM), ovarian cancer cell line NCI/ADR-RES (GI50 = 0.0169 μM) and renal cancer cell line RXF 393 (GI50 = 0.0197 μM). The SAR study revealed that: (1) potent anticancer activity of tested compounds depended on the presence of a combination of three heterocycles in one molecule, thus the pyrazoline–thiazolidinone–isatin conjugates were more active in comparison with pyrazoline–thiazolidinone or pyrazole-indoline-2-one systems; (2) attachment of halogen (Br or Cl) to 5-position of isatin scaffold allowed to gain one log unit of activity (GI50 level), in comparison with 5-unsubstituted isatin analogs; (3) introduction of a methyl group or CH2COOH group at 1N-position of isatin fragment led to the loss of activity; (4) the nature of a substituent in the 5-aryl fragment also had an influence on the antitumor activity. Therefore, the introduction of electron-withdrawing group – 4-chlorine improved the antiproliferative activity in comparison with electron-donor 2-OH, 4-OMe and 4-NMe2 groups. This would indicate that decreased of electron density on 5-aryl moiety is important for the inhibitory activity; and (5) substitution of the phenyl fragment in 3 position of pyrazoline cycle on naphthalene-2-yl substituent did not have significant influence on antitumor activity, but introduction of the 4-methoxyphenyl group in the mentioned position limited this effect [32].
Fig. 28.
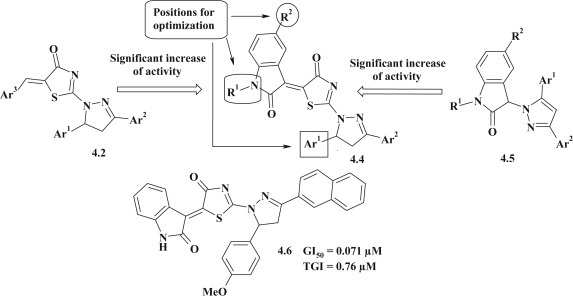
Isatin-based conjugates with antitumor activity.
Y. Rajendra Prasad et al. synthesized a series of pyrazoline–thiazoles 4.7, 4.8, and examined their antitumor activity in vitro on tumor lines: Hela (human cervix carcinoma cell line), A549 (human lung adenocarcinoma cell line), MCF-7 (human breast adenocarcinoma cell line), A2780 (human ovarian cancer cell line) and BGC-823 (human gastric cancer cell line), using the MTT method. As a result of studies two hit-compounds 4.10 and 4.11 were selected. SAR analysis showed that the determining factor for expression of antitumor activity is the structure of the substituent in the 4 position of thiazole (Fig. 29 ). A modification of the ester group into hydrazide and, subsequently, a hydrazone gave the potentiation of antitumor properties. At the same time, pyrazoline–thiazolidinones 4.9 had no significant cytotoxicity [35].
Fig. 29.
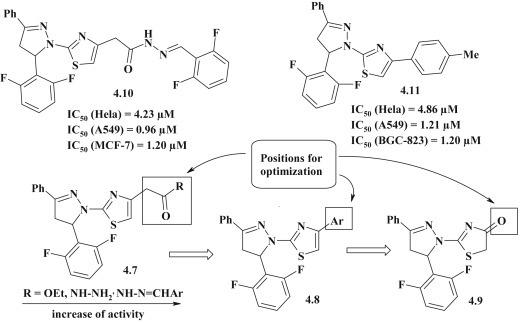
Thiazole–pyrazoline hybrids with antitumor activity.
The series of pyrazoline–thiazolidine-based compounds were tested for antitumor activity according to standard NCI protocol and 4-thiazolidinone derivatives with pyrazoline fragment 4.12, 4.14, 4.17 showed moderate cytostatic effect. The group of publications includes the synthesis of these pyrazoline–thiazolidinones 4.12, 4.14, 4.17 and pyrazoline–thiazolidines 4.13, 4.15, 4.16, 4.18 with diversity of substitution in pyrazole core. The analysis of mentioned results demonstrates that the modification of pyrazoline fragment is not crucial to the implementation of antineoplastic action (Fig. 30 ). This statement is evidenced by the level of efficiency of compounds 4.12–4.18 with moderate rates of effective concentration [48], [49], [50], [51].
Fig. 30.
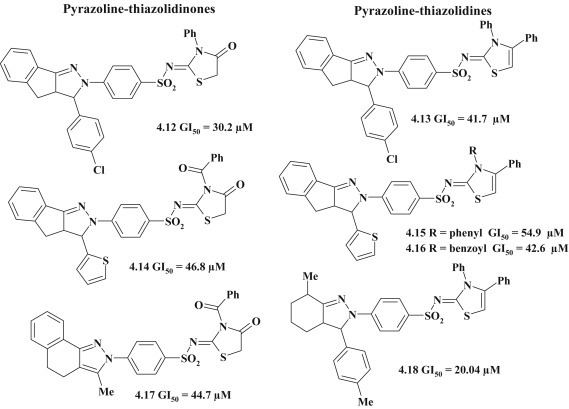
Antitumor thiazolidinone/thiazole derivatives with pyrazoline moiety in 2 position.
Magdy I. El-Zahar et al. [55] synthesized a group of benzofuran–pyrazoles with various heterocycles, including thiazole (4.19) and thiazolidine (4.20) fragments for screening of antitumor activity on lines: HEPG2 (liver carcinoma cell line) and HELA (cervix carcinoma cell line). Interestingly, each of these compounds showed selective effect on one of the lines similar to 5-fluorouracil (Fig. 31 ).
Fig. 31.
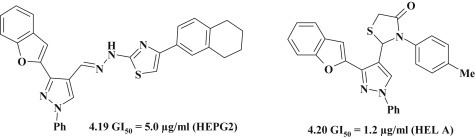
Antitumor azolidine-pyrazolines with thiazolidinone/thiazole fragments.
Synthesis and study of antitumor activity of pyrazole-thiazolidinone–triazoles on lung cancer MCF-7 line were conducted by Arun M. Isloor et al. [40]. Among the studied conjugates the compound 4.21 was the most active with the effective concentration IC50 = 22 μg/mL. The presence of o-tolyl fragment was most favorable for the anticancer effect, while substitution with naphthyl ring caused complete loss of activity (Fig. 32 ).
Fig. 32.
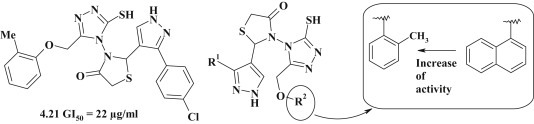
Antitumor activity of pyrazole-thiazolidinone–triazoles on lung cancer.
An important achievement in the study of anticancer pyrazole-thiazolidinone hybrid compounds is the establishing of the necroptosis inhibition for pseudothiohydantoin derivatives with pyrazole moiety in 5 position (Fig. 33 ). Among these derivatives a potential inhibitor Necrostatin-7 (Nec-7) was identified with the activity index of 10.6 μM. Further studies aiming to optimize of Nec-7 molecule, were focused on the modification of thiazole fragment, replacement of substituents in the phenyl ring and replacement of pyrazole cycle by other heterocycles. It was established that the replacement of thiazole fragment by the 1,3,4-thiadiazole led to the complete loss of activity. The introduction of the methyl group in the 4 position of thiazole caused a slight gain of inhibitory action, while presence of methyl in the 5 position of thiazole had a negative effect. The most effective direction for modification of Nec-7 was the replacement of fluorine atom in the 4 position of benzene core. Thus, the introduction of morpholine (4.22), phenol (4.23) and phenyl sulphanilic (4.24) fragments allowed to get 3–7 fold potentiation in comparison to Nec-7. In general, it should be noted that the presence of the substituent in the 4 position and its nature was crucial for the implementation of necroptosis inhibitory activity, because the change of a fluorine atom position was accompanied by loss of activity. The authors synthesized a group of related compounds with aromatic heterocycles (triazole and isooxazole) to establish the impact of pyrazole cycle for activity. This modification resulted in the loss of inhibitory activity, which confirmed the importance of pyrazole moiety in the analogs Nec-7 [87].
Fig. 33.
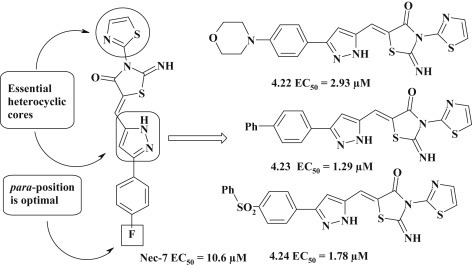
5-Pyrazoline-substituted pseudothiohydantoines as promising necroptosis inhibitors.
The study of antitumor activity for pyrazole-rhodanines allowed to identify the compound 4.25, which proved effective inhibitory effect on the most of the 60 tumor lines at micromolar concentrations according to NCI protocol (Fig. 34 ). The substance 4.25 was characterized by a selective effect on leukemia and lung cancer cell lines – especially on HOP-92 (lung cancer) with the values of effective concentration and cytotoxicity GI50 = 0.62 μM and LC50 > 100 μM, respectively [82].
Fig. 34.

Selective antitumor activity of pyrazole-rhodanine 4.25.
Manal Sarkis et al. carried out the synthesis of new bis-thiazolone derivatives as potential inhibitors of CDC25V phosphatase (specified enzyme involved in cell cycle progression and unregulated tumor development). Aiming to investigate the selectivity, the affinity of the compounds was studied to PTP1B (Protein Tyrosine Phosphatase 1B) and VHR (Vaccinia virus H1 Related – a dual-specificity phosphatase). Overall 31 compounds from bis-thiazolidinone group were synthesized and most of them displayed inhibitory activity with micromolar IC50 values lower than the corresponding monomers. Mentioned group of the compounds included the thiazolidinone–indazole conjugate 4.26 (Fig. 35 ). It was established that the replacement of a 4-hydroxyphenyl fragment with indazole was accompanied by selective inhibition of CDC25B phosphatase and loss of PTP1B inhibitory activity (abolished PTP1B inhibition) [99].
Fig. 35.
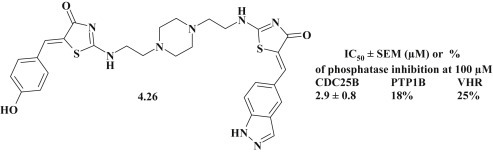
Bis-thiazolone with indazole fragment as potential inhibitor of CDC25V phosphatase.
Screening of in vitro antitumor activity for 5-pyrazoline substituted 4-thiazolidinones (compounds 4.27 and 4.28) was carried out within the DTP NCI [77], [97]. Overall tested substances had a moderate activity, but two compounds were identified with selective effect on leukemia cell lines with effective concentration GI50 2.12–4.58 μM (4.29) and 1.64–3.20 μM (4.30). SAR analysis showed that the introduction of the linker group between heterocycles promoted the potentiation of antitumor activity, however, modification of 3N-position of thiazolidinone cycle via Mannich reaction and alkylation was not effective approach to optimization of hit-compounds (Fig. 36 ).
Fig. 36.
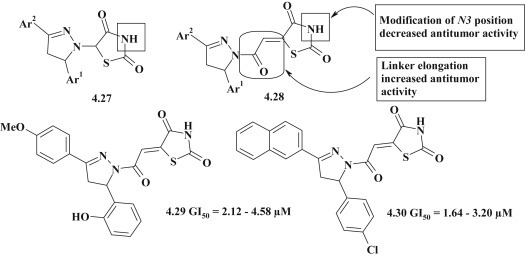
Antitumor activity of 4-thiazolidinones with pyrazolines in 5 position.
5.2. Antimicrobial and antifungal activity of pyrazoline–thiazolidinones
The search for new antimicrobial and antifungal agents among pyrazoline–thiazolidinone related conjugates is a promising direction for biological testing of mentioned compounds. Mervat M. El-Enany et al. synthesized the group of 4-thiazolidinones and thiazoles with pyrazoline fragment in 2 position and studied their antimicrobial activity against Gram-positive (Staphylococcus aureus, Bacillus subtilis), gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa) and fungi Candida albicans [24]. However, the compound 4.31 was inactive to any of the microorganisms, while activity of 4.32 on B. subtilis appeared commensurate with Amoxacillin and Propenicillin (Fig. 37 ). The research of Bakr F. Abdel-Wahab et al. was more successful. The authors synthesized a group of triazole–pyrazoline–thiazoles and corresponding 4-thiazolidinones and studied their activity against gram-positive bacteria (S. aureus ATCC 29213, B. subtilis ATCC6633, Bacillus megaterium ATCC 9885, Sarcina lutea), gram-negative bacteria (Klebseilla pneumonia ATCC13883, P. aeruginosa ATCC27953, E. coli ATCC 25922) and fungi (Saccharomyces cerevisiae and C. albicans NRRL Y-477) compared with Ciprofloxacin and Ketoconazole [23]. Among the tested thiazole–pyrazolines compound 4.33 showed the highest activity for strain P. aeruginosa (MIC = 8.25 μg/mL), which was twice higher than for Ciprofloxacin. Activity of 4.34 appeared commensurate with the drug for bacteria S. aureus and B. subtilis (MIC = 8.25 μg/mL). At the same time 4.35 showed a high antifungal activity (MIC = 8.25 μg/mL). It should be noted that the replacement of thiazole fragment with the 4-thiazolidinone was accompanied by the loss of antimicrobial activity against most types of bacteria and fungi, but compound 4.36 showed the highest activity in B. megaterium and E. coli, which were much less sensitive to the action of thiazole–pyrazolines (Fig. 37). Study of antimicrobial activity for structurally related quinoline–pyrazoline–thiazolidones 4.37 against strains E. coli (MTCC 443), P. aeruginosa (MTCC 1688), S. aureus (MTCC 96), Streptococcus pyogenes (MTCC 442) and antifungal effect on C. albicans (MTCC 227), Aspergillus niger (MTCC 282) and Aspergillus clavatus (MTCC 1323) evidenced of their minor or moderate activity on most types of microorganisms with MIC values range = 50–500 μg/mL [25]. However, among the above groups of compounds 4.38 could be selected, which showed 2–8 times higher bactericidal activity in experiment than Ampicillin and was twice active than Griseofulvin on fungi. The mentioned compound showed the highest activity with the value of MIC 12.5 μg/mL to P. aeruginosa (Fig. 37).
Fig. 37.
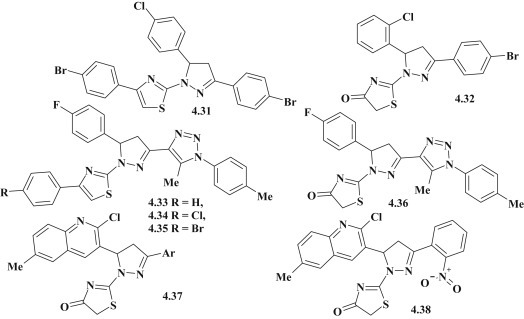
Pyrazoline–thiazolidinone hybrids with antimicrobial activity.
C.S. Reddy et al. performed the synthesis and study of antimicrobial activity of thiazolidinone–pyrazoles 4.39 against the B. subtilis, S. aureus, E. coli and S. pyogenes. It was established the moderate effect of mentioned compounds (MIC = 6.25–50 μg/mL) (Fig. 38 ). The introduction of chlorine, nitro or hydroxyl groups in the para-position of the benzene ring resulted in 2–4 fold potentiation of activity in comparison to 3-phenyl-4-thiazolidinones. However, replacement of the phenyl moiety to pyridine (4.40) or pyrimidine was particularly effective, which allowed to get 4–8 fold enhance of efficiency [100].
Fig. 38.
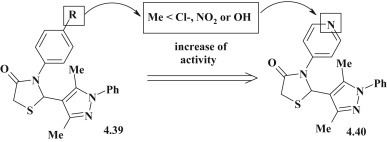
SAR analysis of antimicrobial pyrazole–thiazolidinone hybrids.
The pyrazole–thiazolidinones 4.41 or pyrazole–thiazoles 4.42, coupled by hydrazone linker group were investigated as promising agents with antimicrobial and antifungal activity. It was found that the compound 4.41b (R = Me) showed comparable activity (MIC = 25 μg/mL) with Ampicillin against E. coli, and the compound 4.41c (R = Cl) appeared to be the most active on C. albicans (MIC = 12.5 μg/mL) (Fig. 39 ). Overall, pyrazole-thiazolidinones 4.41 had higher activity than related pyrazole-thiazoles 4.42 [60]. Adnan A. Bekhit et al. synthesized the pyrazoline–thiazoles and thiazolidinones with sulfanilamide group. Among the studied pyrazole-thiazolidinones 4.43 and pyrazole–thiazoles 4.44 the compounds 4.43b (R = Me) and 4.44a (R = H) (Fig. 39) were most effective against E. coli (MIC = 25 μg/mL) [56].
Fig. 39.
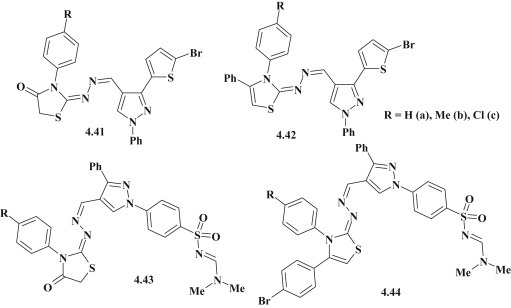
Antimicrobial and antifungal pyrazole–thiazolidinones/thiazoles, coupled by hydrazone linker group.
The study of wide range of antimicrobial and antifungal activities of new benzofuranyl–pyrazoles with thiazolidinone fragment was conducted by Bakr F. Abdel-Wahab et al. The compound 4.45 showed activity against C. albicans and B. subitilis, and thiazole-derivative 4.46 was effective against the E. coli [62] (Fig. 40 ). To continue the research of pyrazole derivatives with different heterocycles, authors conducted the synthesis of new derivatives of pyrazole with thiophene fragment 4.47–4.49, including pyrazole-thiazolidine 4.50 (Fig. 40) and offered a wide range of biological studies, including the evaluation of antimicrobial activity against the following microorganisms: S. aureus ATCC 29213, B. subtilis ATCC6633, Salmonella typhi, Enterobacter Cloaca ATCC3047, K. peneumoniae ATCC13883, P. aeroginosa ATCC27953, E. coli ATCC 25922, Enterococcus faecalis ATCC29212, Mycobacterium phlei, S. cervesiae and C. albicans NRRL Y-477. Among the tested compounds 4.47 and 4.49 showed hihg/good or moderate efficiency against all strains of microorganisms. While compound 4.48 had proved to be the most effective on E. faecalis (MIC = 83.3 μg/mL), compound 4.50 was effective bactericidal agent on the strain S. aureus (MIC = 20.8 μg/mL) and showed significant antifungal effect on S. cervesia (MIC = 41.6 μg/mL) [58].
Fig. 40.
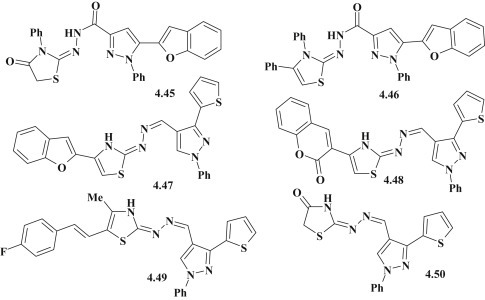
Antimicrobial and antifungal activity for polyheterocyclic systems based on pyrazole-thiazolidinones/thiazoles.
Evaluation of antimicrobial activity for thiazolidines with 5-pyrazolone fragment 4.51–4.53 (Fig. 41 ) revealed their effectiveness on B. subtilis NCTC 10400, S. aureus ATCC 25923, E. coli ATCC 25922, P. aeruginosa ATCC 10415) and fungi C. albicans TMRU 3669, A. niger ATCC 6265 [57].
Fig. 41.
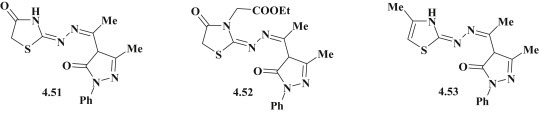
Antimicrobial thiazolidines/thiazoles with 5-pyrazolone fragment.
An antimicrobial screening was carried out for 3-pyrazolinyl-4-thiazolidinones against gram-positive bacteria S. aureus MTCC096, B. subtilis MTCC 441 and Staphylococcus epidermis MTCC435, gram-negative bacteria E. coli MTCC 443, P. aeruginosa MTCC 424, S. typhi MTCC 733 and K. pneumoniae MTCC 432, and antifungal effect was studied on A. niger MTCC 282, Aspergillus fumigatus MTCC 343, Aspergillus flavus MTCC 277 and C. albicans MTCC 227. In general the tested compounds (Fig. 42 ) showed promising antimicrobial activity. However, it could be emphasized that the bis-spiroindolones 4.55 showed better growth inhibition compared with compounds 4.54 [101].
Fig. 42.
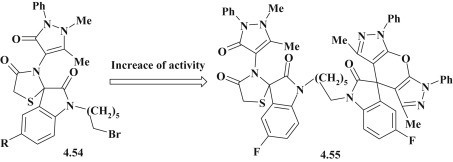
3-Pyrazolinyl-4-thiazolidinones with antimicrobial activity.
S.S. Reddy et al. proposed the synthesis of pyrazole-pyrimidine–thiazolidinones 4.56 (Fig. 43 ) and the study of their antimicrobial (B. subtilis MTCC 441, Bacillus sphaericus MTCC 11, S. aureus MTCC 96, P. aeruginosa MTCC 741, Klobsinella aerogenes MTCC 39 and Chromobacterium violaceum MTCC 2625) and antifungal (C. albicans ATCC 10231, Aspergillus fumigatus HIC 6094, Trichophyton rubrum IFO 9185 and Trichophyton mentagrophytes IFO 40996) activities. Despite the wide range of antimicrobial research, none of the tested compounds was more active than Penicillin (MIC = 1.56–12.5 μg/mL). The range value of the minimum inhibitory concentration was 13–30 μg/mL. However, it should be noted an antifungal activity of pyrazole-pyrimidine–thiazolidinones 4.56. The compounds with furane and 4-dimethylaminophenyl-groups in position 2 of thiazolidinone showed a higher or comparable efficacy (MIC = 14–22 μg/mL) to Fluconazole [72].
Fig. 43.
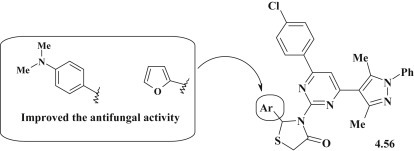
Pyrazole-pyrimidine–thiazolidinones as promising antimicrobial agents.
Among 5-pyrazolyl-4-thiazolidinones the compounds 4.57 (Fig. 44 ) were investigated for their antimicrobial activity. The most effective pyrazole-thiazolidinones included the 4-Br-phenyl or thiophene fragments in molecules and showed 2–4 times higher efficiency (MIC = 16–31 μg/mL) in comparison with other derivatives [80]. The moderate antimicrobial activity towards E. coli and S. aureus was identified for 3N-glycoside substituted 2-thioxo-4-thiazolidinone derivatives 4.58 (Fig. 44). The p-bromophenyl substituted derivative showed the highest efficiency against E. coli (MIC = 143 μg/mL) but was 3 times less active than tetracycline (MIC = 39 μg/mL) [84].
Fig. 44.
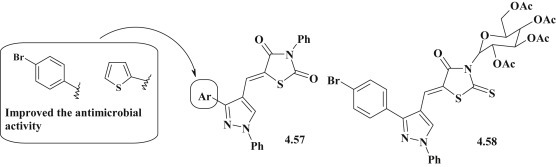
Antimicrobial activity of 4-thiazolidinones with pyrazole in 5 position.
However, the most promising group of antimicrobial agents is 5-pyrazolylrhodanines 4.59–4.63 [85], [86], [102], [103]. Antimicrobial activity of these substances was studied against methicillin- (MRSA) and quinolone-resistant (QRSA) S. aureus. Compounds 4.59–4.61 exhibited stronger activity than the standard drugs, norfloxacin and oxacillin, with MIC values of 1–2 mg/mL. SAR analysis for pyrazole-rhodanine derivatives 4.62, 4.63 revealed the importance of aryl moiety presence in the pyrazole ring for expression of antimicrobial action and a number of substituents that considerably increased effectiveness were identified (Fig. 45 ).
Fig. 45.
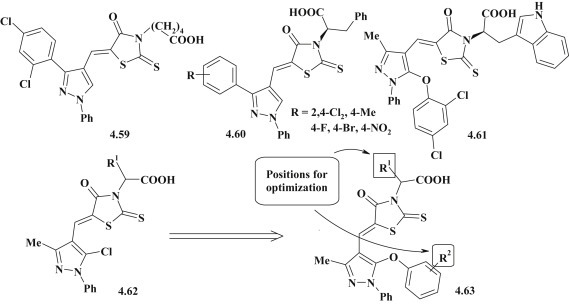
Structure-antimicrobial activity relationship for pyrazole–rhodanine derivatives.
5.3. Antiviral and antiparasitic activity of pyrazole–thiazolidinone hybrids
The search for new chemotherapeutic agents among pyrazole–thiazolidinones is not limited by anticancer and antimicrobial studies. The evaluation of antiviral and antiparasitic activities for mentioned hybrid compounds allowed identifying promising hit-compounds and conducting SAR analysis. Osama I. El-Sabbagh proposed the synthesis of a group of pyrazoline–thiazolidinones 4.64 and structurally related pyrazoline–thiazoles 4.65 (Fig. 46 ) and performed a study of a wide range of antiviral activity. It should be noted that described compounds showed selective activity. However, one derivative (Ar = 4-Me-C6H4) among compounds 4.64 had moderate efficacy against herpes simplex virus type 1, herpes simplex virus type 2, vaccinia virus, vesicular stomatitis virus and thymidine kinase-deficient herpes simplex virus type 1 with value of the effective concentration of 4 μg/mL and cytotoxicity value 20 μg/mL (SI = 5) [29].
Fig. 46.
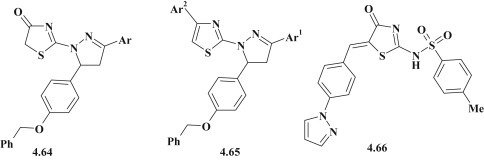
Pyrazole/pyrazolines–thiazolidinone/thiazoles with antiviral activity.
Study of thiazolidinones with sulfonamide fragment against the hepatitis C virus, conducted by Yili Ding et al. allowed to identify a number of promising antiviral agents [104]. Among the studied 4-thiazolidinones the pyrazole derivative 4.66 (Fig. 46) showed moderate effectiveness (IC50 = 10 μg/mL and EC50 = 70 μg/mL and cytotoxicity index CC50 = 90 μg/mL).
5-Pyrazoline substituted 4-thiazolidinones (Fig. 47 ) were studied for their antiviral activity against SARS coronavirus (SARS CoV) and influenza types A and B viruses (Flu A, Flu B), as well as antitrypanosomal effect against Trypanosoma brucei brucei (Tbb) and Trypanosoma brucei gambiense (Tbg). Overall compounds were characterized by the similar values of anti-influenza viruses' activity and cytotoxicity with selectivity indexes 1.0 to 2.1. Compound 4.68 had moderate activity against duck strain of influenza A with 50% effective concentration (EC50) 21.78 μM and selectivity index (SI) > 16.3. In general, tested compounds had no antiviral activity against SARS CoV [97]. The screening was conducted for compounds (Fig. 45) against Trypanosoma brucei brucei (Tbb) and Trypanosoma brucei gambiense (Tbg). The results showed moderate antitrypanosomal activity of compounds 4.67, 4.69 and 4.70 against both strains of tested parasites (activity indexes IC50 (Tbb) = 5.43–13.87 μM and IC50 (Tbg) = 2.53–6.66 μM) [97].
Fig. 47.
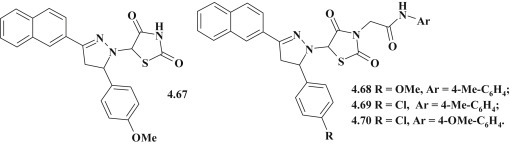
Antiviral and antitrypanosomal 5-pyrazoline substituted 4-thiazolidinones.
Aiming to investigate the trypanocidal activity for pyrazoline–thiazolidinone conjugates the synthesis of new 5-(pyrazolin-1-ylmethylene)-4-thiazolidinones was carried out (Fig. 48 ). Target heterocycles were branched by the methylidene or oxoethylamino-methylidene linker groups. The results of screening against Trypanosoma brucei gambiense allowed to identify 5 compounds with sufficient antitrypanosomal activity (IC50 ≤ 1.2 μM) and moderate or low cytotoxicity (CC50 = 13.6 -> 184.3 μM) towards myoblast derived cell line (L-6). Compounds 4.71 (IC50 = 0.6 μM, CC50 = 175.2 μM, SI = 292) and 4.72 (IC50 = 0.7 μM, CC50 = 40 μM, SI = 57) were significantly more effective than Nifurtimox (IC50 = 4.4 μM, 78.2 μM = CC50, SI = 17.8). SAR analysis has been directed on substitution of pyrazoline core and modification of N3-thiazolidinone position (4.73), elongation of linker group (4.74) and rhodanine-isorhodanine isomerism (4.75) [98].
Fig. 48.
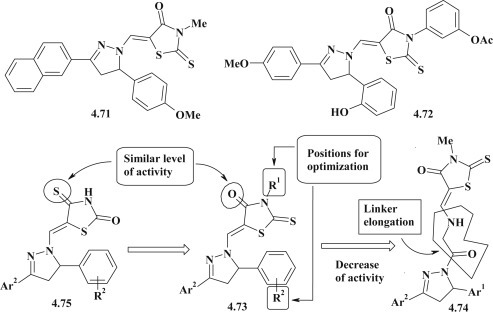
SAR for trypanocidal 5-(pyrazolin-1-ylmethylene)-4-thiazolidinones.
The study of antiparasitic activity for pyrazoline–thiazolidinone 4.76 (Fig. 49 ) was carried out towars Trypanosoma cruzi, Trypanosoma b. rhodesiense, Leishmania donovani and Plasmodium falciparum. The results indicated a moderate inhibitory effect on Trypanosoma b. rhodesiense (IC50 = 12 μg/mL) and L. donovani (IC50 > 30 μg/mL) and a higher influence on P. falciparum (IC50 > 5 μg/mL) with cytotoxicity index CC50 > 90 μg/mL [36].
Fig. 49.
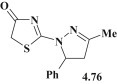
Pyrazoline–thiazolidinone hybrid with antiparasitic activity.
5.4. Design of new anti-inflammatory agents among pyrazole–thiazolidinones
The promising direction for pharmacological investigation of pyrazole–thiazolidinones is the search for new anti-inflammatory agents considering their structural relationship with a known NSAIDs COX-2 inhibitor – celecoxib. Magda N.A. Nasr and Shehta A. Said studied a group of pyrazoline–thiazolidine/thiazoles 4.79, 4.80 and thiazolidine/thiazole derivatives with hexahydroindazole fragment 4.77, 4.78 and carried out in vivo research on carrageenin induced paw edema at dose of 100 mg/kg using ketoprofen as a reference standard [105]. Results showed that pyrazoline–thiazoles/thiazolidinones 4.79, 4.80 possessed the better anti-inflammatory activity (percentage reduction of paw endema from control group was 17–50, and ketoprofen efficiency – 63%) compared with hexahydroindazole 4.77, 4.78 analogues. Comparing the effectiveness of thiazole derivatives (4.78, 4.80) with thiazolidinones (4.77, 4.79) it could be concluded the higher activity of pyrazoline–thiazole hybrids (39–50%) (Fig. 50 ).
Fig. 50.
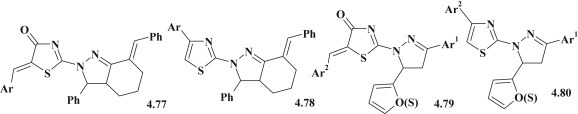
Thiazolidinones and thiazoles with pyrazoline fragments as anti-inflammatory agents.
Detailed study of pyrazoline–thiazolidinones as potential COX-1 and COX-2 inhibitors allowed to identify highly active compound 4.82 (IC50 = 0.5 μM for COX-2) with the best selective index (SI = 84.8), which effectiveness was similar to that of Celecoxib. SAR analysis (Fig. 51 ) of the results showed the dependence of COX-2 inhibition on the nature of aryl substituents in the 3 (A-ring) and 5 (B-ring) pyrazoline positions (structure 4.81). Thus, compounds with electron-donor substituents (methyl group) in the para- and meta-positions of the A-ring had better inhibitory effect compared with chlorine. The nature of the substituent in the para-position of the B-cycle also had a significant impact on the inhibitory activity of COX-2 in the order of: H > Br > Cl > F (for R1 = 3,4-Me2, 4.81); H > Me > OMe (for R1 = 3,4-Cl2, 4.81) [31].
Fig. 51.
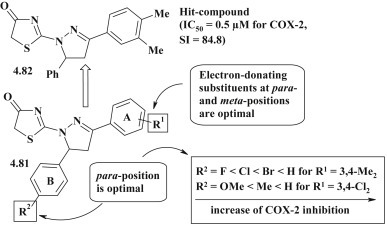
Pyrazoline–thiazolidinone hybrids as COX-2 inhibitors.
To continue the development of structural analogues of Celecoxib, Adnan A. Bekhit et al. synthesized the series of pyrazole–thiazolidines 4.83–4.86 (Fig. 52 ) for anti-inflammatory activity screening using cotton pellet-induced granuloma and carrageenan-induced rat paw edema bioassays. The highly active compounds were tested for their ability to inhibit COX-1/COX-2 as well as ulcerogenic effect and acute toxicity. The first phase of the study (cotton pellet induced granuloma bioassay) allowed to establish that all compounds possessed anti-inflammatory activity with ED50 = 7.86–28.42 μM and the thiazolidine carboxylic acid derivatives (compounds 4.83 – ED50 = 9.74–11.86 μM, compounds 4.84 – ED50 = 7.86–8.11 μM) showed better efficiency than Celecoxib (ED50 = 16.74 μM) and commensurable with Indomethacin (ED50 = 9.64 μM). Further studies of highly active compounds (4.83 and 4.84) allowed to identify their ability to selective inhibition of COX 2 (IC50 = 0.38–0.94 μM) and reaffirmed their low ulcerogenic action and acute toxicity [39].
Fig. 52.
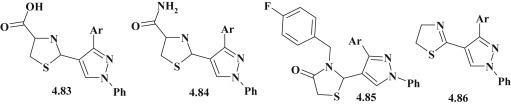
Pyrazole-thiazolidine hybrids as COX-2 inhibitors.
Adam M. Gilbert et al. synthesized a series of 5-pyrazolylmethylidene-2-thioxo-4-thiazolidinones 4.87 (Fig. 53 ) as potential inhibitors of ADAMTS-5 (a disintegrin and metalloproteinase with thrombospondin motifs 5) with significant anti-inflammatory activity. Highly active compound 4.88 was identified with the inhibition of ADAMTS-5 IC50 = 1.1 μM, that demonstrated a strong selectivity (SI > 40) compared to the inhibition of ADAMTS-4. Structural modification of substituents in the pyrazole moiety allowed to establish the main structural criteria to increase the inhibitory effect [83].
Fig. 53.
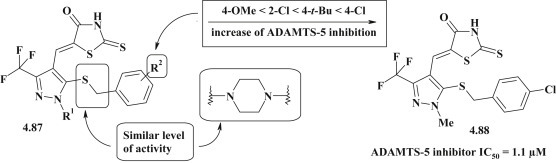
ADAMTS-5 inhibitors among pyrazole-thiazolidinone hybrids.
Amal M. Youssef et al. carried out the synthesis of 5-((1,3-aryl-1H-pyrazol-4-yl)methylene)thiazolidine-2,4-diones as potential anti-inflammatory and neuroprotective agents [79]. The studies conducted in vitro and in vivo allowed to identify a group of highly active anti-inflammatory compounds with a low ulcerogenic impact and satisfactory toxicometry parameters. Based on in vitro experiments four compounds (4.89) were selected for in vivo studies according to screening protocols: the formalin-induced paw edema and turpentine oil-induced granuloma pouch bioassays. It was established that the tested compounds showed activity comparable to Celecoxib at a concentration of 20 mg/kg. The introduction of benzyl moiety in position 3 of thiazolidinone was accompanied by the loss of anti-inflammatory activity (Fig. 54 ).
Fig. 54.
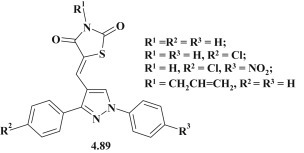
Pyrazole–thiazolidinones with anti-inflammatory activity.
6. Conclusions
This review article presents the rational approaches to the design of chemotherapeutic agents based on molecular hybridization of pharmacologically attractive thiazolidine and pyrazole/pyrazoline heterocycles. Based on linkage of target heterocyclic cores the reviewed hybrids were classified as 2-, 3- and 5-pyrazole/pyrazoline-substituted thiazolidines. The basic approaches for the synthesis of pyrazole-thiazolidinones have been provided by a combination of two “small” molecules via aminolysis, acylation reactions and Knoevenagel procedure or heterocyclization of monocyclic compounds via the [2+3]-cyclization reaction yielding the series of non-condensed bicyclic systems. The review of biological applications was focused on SAR analysis and mechanistic insights of thiazolidine–pyrazole hybrids. Molecular hybridization as a tool of medicinal chemistry has been successfully used for design of biologically active conjugates with antitumor activity, including inhibition of necroptosis, CDC25V phosphatase, TNF-α–TNFRc1 interaction or activation of pro-apoptotic BAX, antimicrobial, antifungal, antiviral and antiparasitic applications, as well as anti-inflammatory properties as inhibitors of COX or ADAMTS-5 enzymes.
Abbreviations
- ADAMTS
a disintegrin and metalloproteinase with thrombospondin motifs
- BAX
Bcl-2-associated X protein
- CC50
half maximal cytotoxic concentration
- CDC25V
dual-specificity phosphatase, the “cdc” in name refers to “cell division cycle”
- DCC
dicyclohexylcarbodiimide
- DTP
Development Therapeutics Program
- EC50
half maximal effective concentration
- ED50
half maximal effective dose
- IC50
half maximal inhibitory concentration
- GI50
drug concentration resulting in a 50% reduction in the net protein increase in control cells during the drug incubation
- HATU
1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxid hexafluorophosphate
- MIC
minimal inhibitory concentration
- MRSA
methicillin-resistant Staphylococcus aureus
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NCI
National Cancer Institute
- PIN1
peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
- PTP1B
protein tyrosine phosphatase 1B
- QRSA
quinolone-resistant Staphylococcus aureus
- SAR
structure-activity relationships
- SARS
severe acute respiratory syndrome
- SI
selectivity index
- Tbb
Trypanosoma brucei brucei
- Tbg
Trypanosoma brucei gambiense
- TGI
drug concentration resulting in total growth inhibition
- TNF-α
tumor necrosis factor alpha
- TNFRc1
type-1 tumor necrosis factor receptor
- VHR
Vaccinia virus H1 Related – a dual-specificity phosphatase
References
- 1.Fortin S., Berube G. Advances in the development of hybrid anticancer drugs. Expert Opin. Drug Discov. 2013;8:1547–1577. doi: 10.1517/17460441.2013.798296. [DOI] [PubMed] [Google Scholar]
- 2.Gediya L.K., Njar V.C. Promise and challenges in drug discovery and development of hybrid anticancer drugs. Expert Opin. Drug Discov. 2009;4:1099–1111. doi: 10.1517/17460440903341705. [DOI] [PubMed] [Google Scholar]
- 3.Nepali K., Sharma S., Sharma M., Bedi P.M.S., Dhar K.L. Rational approaches, design strategies, structure activity relationship and mechanistic insights for anticancer hybrids. Eur. J. Med. Chem. 2014;77:422–487. doi: 10.1016/j.ejmech.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 4.Havrylyuk D., Zimenkovsky B., Lesyk R. Synthesis, biological activity of thiazolidinones bearing indoline moiety and isatin based hybrids. Mini Rev. Org. Chem. 2015;12:66–87. [Google Scholar]
- 5.Lesyk R., Zimenkovsky B. 4-Thiazolidones: centenarian history, current status and perspectives for modern organic and medicinal chemistry. Curr. Org. Chem. 2004;8:1547–1577. [Google Scholar]
- 6.Lesyk R.B., Zimenkovsky B.S., Kaminskyy D.V., Kryshchyshyn A.P., Havrylyuk D.Ya., Atamanyuk D.V., Subtel'na I.Yu., Khyluk D.V. Thiazolidinone motif in anticancer drug discovery. Experience of DH LNMU medicinal chemistry scientific group. Biopolym. Cell. 2011;27:107–117. [Google Scholar]
- 7.Tomasich T., Masich L.P. Rhodanine as a privileged scaffold in drug discovery. Curr. Med. Chem. 2009;16:1596–1629. doi: 10.2174/092986709788186200. [DOI] [PubMed] [Google Scholar]
- 8.Verma A., Saraf S.K. 4-Thiazolidinone - a biologically active scaffold. Eur. J. Med. Chem. 2008;43:897–905. doi: 10.1016/j.ejmech.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 9.Cunico W., Gomes C.R.B., Vellasco W.T. Chemisry and biological activities of 1,3-thiazolidin-4-ones. Mini Rev. Org. Chem. 2008;5:336–344. [Google Scholar]
- 10.Jain A.K., Vaidya A., Ravichandran V., Kashaw S.K., Agrawal R.K. Recent developments and biological activities of thiazolidinone derivatives: a review. Bioorg. Med. Chem. 2012;20:3378–3395. doi: 10.1016/j.bmc.2012.03.069. [DOI] [PubMed] [Google Scholar]
- 11.Ale J.M., Kumar R. 4,5-Dihydro-1H-pyrazole: an indispensable scaffold. J. Enzyme Inhib. Med. Chem. 2013;8:1547–1577. doi: 10.3109/14756366.2013.795956. [DOI] [PubMed] [Google Scholar]
- 12.Shaaban M.R., Mayhoub A.S., Farag A.M. Recent advances in the therapeutic applications of pyrazolines. Expert Opin. Ther. Pat. 2012;22:253–291. doi: 10.1517/13543776.2012.667403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Z.-Z., Yang G.-F. Insecticidal lead identification by screening benzopyrano[4,3-c]-pyrazol-3(2H)-ones library constructed from multiple-parallel synthesis under microwave irradiation. Bioorg. Med. Chem. 2006;14:8666–8674. doi: 10.1016/j.bmc.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 14.Zhao P.-L., Wang F., Zhang M.-Z., Liu Z.-M., Huang W., Yang G.-F. Synthesis, fungicidal, and insecticidal activities of β-methoxyacrylate-containing N-acetyl pyrazoline derivatives. J. Agric. Food Chem. 2008;56:10767–10773. doi: 10.1021/jf802343p. [DOI] [PubMed] [Google Scholar]
- 15.Zhao P.-L., Wang L., Zhu X.-L., Huang X., Zhan C.-G., Wu J.-W., Yang G.-F. Subnanomolar inhibitor of cytochrome bc1 complex designed by optimizing interaction with conformationally flexible residues. J. Am. Chem. Soc. 2010;132:185–194. doi: 10.1021/ja905756c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.J. Yuan, C. Yuan, A. Degterev, Unsaturated heterocyclic inhibitors of necroptosis, PCT Int. Appl. (2010), WO 2010075290.
- 17.T. Mustelin, L. Tautz, VHR protein tyrosine phosphatase inhibitors, compositions and methods of use, U.S. Pat. Appl. Publ. (2009) US20090105254.
- 18.L. Bao, A, Kimzey, Pin1-modulating compounds and methods of use for the treatment of Pin1-associated diseases, including cancer, PCT Int. Appl. (2004), WO 2004093803.
- 19.S. Ryder, Compounds for modulating RNA-binding proteins and uses therefor, PCT Int. Appl. (2010) WO 2010151799.
- 20.L.D. Walensky, Preparation of pyrazol-3-ones as activators of pro-apoptotic BAX, PCT Int. Appl. (2013), WO 2013055949.
- 21.Carter P.H., Scherle P.A., Muckelbauer J.A., Voss M.E., Liu R.-Q., Thompson L.A., Tebben A.J., Solomon K.A., Lo Y.C., Li Z., Strzemienski P., Yang G., Falahatpisheh N., Xu M., Wu Z., Farrow N.A., Ramnarayan K., Wang J., Rideout D., Yalamoori V., Domaille P., Underwood D.J., Trzaskos J.M., Friedman S.M., Newton R.C., Decicco C.P. Photochemically enhanced binding of small molecules to the tumor necrosis factor receptor-1 inhibits the binding of TNF-α. Proc. Natl. Acad. Sci. U. S. A. 2001;98:11879–11884. doi: 10.1073/pnas.211178398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furdas S.D., Shekfeh S., Kannan S., Sippl W., Jung M. Rhodanine carboxylic acids as novel inhibitors of histoneacetyltransferases. Med. Chem. Commun. 2012;3:305–311. [Google Scholar]
- 23.Abdel-Wahab B.F., Abdel-Latif E., Mohamed H.A., Awad G.E.A. Design and synthesis of new 4-pyrazolin-3-yl-1,2,3-triazoles and 1,2,3-triazol-4-yl-pyrazolin-1-ylthiazoles as potential antimicrobial agents. Eur. J. Med. Chem. 2012;52:263–268. doi: 10.1016/j.ejmech.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 24.El-Enany M.M., El-Meligie S.E.M., Abdou N.A., El-Nassan H.B. Synthesis and antimicrobial activity of some 3,5-diaryl-4,5-dihydropyrazole derivatives. Orient. J. Chem. 2010;26:1265–1270. [Google Scholar]
- 25.Desai N.C., Joshi V.V., Rajpara K.M. Synthesis of new quinoline-2-pyrazoline-based thiazolinone derivatives as potential antimicrobial agents. Med. Chem. Res. 2013;22:3663–3674. [Google Scholar]
- 26.Desai N.C., Joshi V.V., Rajpara K.M., Vaghani H.V., Satodiya H.M. Facile synthesis of novel fluorine containing pyrazole based thiazole derivatives and evaluation of antimicrobial activity. J. Fluor. Chem. 2012;142:67–78. [Google Scholar]
- 27.Desai N.C., Rajpara K.M., Joshi V.V. Synthesis and characterization of some new quinoline based derivatives endowed with broad spectrum antimicrobial potency. Bioorg. Med. Chem. Lett. 2012;22:6871–6875. doi: 10.1016/j.bmcl.2012.09.039. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y.-S., Zhang F., Gao C., Zhang Y.-B., Wang X.-L., Tang J.-F., Sun J., Gong H.-B., Zhu H.-L. Discovery and modification of sulfur-containing heterocyclic pyrazoline derivatives as potential novel class of b-ketoacyl-acyl carrier protein synthase III (FabH) inhibitors. Bioorg. Med. Chem. Lett. 2012;22:4619–4624. doi: 10.1016/j.bmcl.2012.05.091. [DOI] [PubMed] [Google Scholar]
- 29.El-Sabbagh O.I., Baraka M.M., Ibrahim S.M., Pannecouque C., Andrei G., Snoeck R., Balzarini J., Rashad A.A. Synthesis and antiviral activity of new pyrazole and thiazole derivatives. Eur. J. Med. Chem. 2009;44:3746–3753. doi: 10.1016/j.ejmech.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 30.Havrylyuk D., Zimenkovsky B., Vasylenko O., Lesyk R. Synthesis, anticancer and antiviral activity of new 2-pyrazoline substituted 4-thiazolidinones. J. Heterocycl. Chem. 2013;50:E55–E62. doi: 10.1016/j.ejmech.2013.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiu K.-M., Yan R., Xing M., Wang H.-H., Cui H.-E., Gong H.-B., Zhu H.-L. Synthesis, biological evaluation and molecular modeling of dihydro-pyrazolyl-thiazolinone derivatives as potential COX-2 inhibitors. Bioorg. Med. Chem. 2012;20:6648–6654. doi: 10.1016/j.bmc.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 32.Havrylyuk D., Zimenkovsky B., Vasylenko O., Gzella A., Lesyk R. Synthesis of new 4-thiazolidinone-, pyrazoline-, and isatin-based conjugates with promising antitumor activity. J. Med. Chem. 2012;55:8630–8641. doi: 10.1021/jm300789g. [DOI] [PubMed] [Google Scholar]
- 33.Havrylyuk D., Zimenkovsky B., Vasylenko O., Zaprutko L., Gzella A., Lesyk R. Synthesis of novel thiazolone-based compounds containing pyrazoline moiety and evaluation of their anticancer activity. Eur. J. Med. Chem. 2009;44:1396–1404. doi: 10.1016/j.ejmech.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 34.Khalil N.A., Ahmed E.M., El-Nassan H.B. Synthesis, characterization, and biological evaluation of certain 1,3-thiazolone derivatives bearing pyrazoline moiety as potential anti-breast cancer agents. Med. Chem. Res. 2013;22:1021–1027. [Google Scholar]
- 35.Prasad Y.R., Kumar G.V.S., Chandrashekar S.M. Synthesis and biological evaluation of novel 4,5-dihydropyrazole derivatives as potent anticancer and antimicrobial agents. Med. Chem. Res. 2013;22:2061–2078. [Google Scholar]
- 36.Seebacher W., Belaj F., Saf R., Brun R., Weis R. New 1,3-thiazoles and 1,3-thiazines from 1-thiocarbamoylpyrazoles. Monatsh. Chem. 2003;134:1623–1628. [Google Scholar]
- 37.Danilkina N.A., Mikhaylov L.E., Ivin B.A. Reaction of acetylenedicarboxylic acids esters with 4,5-dihydro-1H-pyrazole-1-carbothioamides and 3,4,5,6-tetrahydro-2H-1,2,4-triazepine-3-thiones. Chem. Heterocycl. Comp. 2011;47:886–900. [Google Scholar]
- 38.Rudenko R.V., Komykhov S.A., Desenko S.M. New direction in the reaction of thiocarboxamides with N-substituted maleimides. Chem. Heterocycl. Comp. 2009;45:1017–1018. [Google Scholar]
- 39.Bekhit A.A., Fahmy H.T.Y., Rostom S.A.F., Bekhit A.E.-D.A. Synthesis and biological evaluation of some thiazolylpyrazole derivatives as dual anti-inflammatory antimicrobial agents. Eur. J. Med. Chem. 2010;45:6027–6038. doi: 10.1016/j.ejmech.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Isloor A.M., Sunil D., Shetty P., Malladi S., Pai K.S.R., Maliyakkl N. Synthesis, characterization, anticancer, and antioxidant activity of some new thiazolidin-4-ones in MCF-7 cells. Med. Chem. Res. 2013;22:758–767. [Google Scholar]
- 41.Patil S.B., Goudgaon N.M. Synthesis of 3-(1-benzyl-1H-benzo[d]imidazol-2-yl amino)-2-(3-aryl-1-phenyl-1H-pyrazol-4-yl)thiazolidin-4-ones and their antimicrobial activities. Int. J. Pharm. Sci. Res. 2010;1:50–56. [Google Scholar]
- 42.Khodairy A. Synthesis of some new heterocyclic compounds from benzopyrano[2,3-c]pyrazol-3-one derivatives. Synth. Commun. 2001;31:2697–2712. [Google Scholar]
- 43.Saloutina L.V., Zapevalov A.Ya., Kodess M.I., Saloutin V.I., Aleksandrov G.G., Chupakhin O.N. Polyfluoroalkylated 1,3-thiazolines: synthesis from polyfluoro-2,3-epoxyalkanes. J. Fluor. Chem. 2000;104:155–165. [Google Scholar]
- 44.Saloutina L.V., Zapevalov A.Ya., Kodess M.I., Saloutin V.I., Aleksandrov G.G., Chupakhin O.N. Polyfluoro-2,3-epoxyalkanes in reactions with thiourea and thiosemicarbazide. Russ. J. Org. Chem. 2000;36:887–899. [Google Scholar]
- 45.El-Sayed O.A., Aboul-Enein H.Y. Synthesis and antimicrobial activity of novel pyrazolo[3,4-b]quinoline derivatives. Arch. Pharm. Pharm. Med. Chem. 2001;334:117–120. doi: 10.1002/1521-4184(200104)334:4<117::aid-ardp117>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 46.Elneairy M.A.A., Attaby F.A., Elsayed M.S. Synthesis of thiazole, triazole, pyrazolo[3,4-b]-pyridinyl-3-phenylthiourea, aminopyrazolo[3,4-b]pyridine derivatives and their biological evaluation. Phosphorus Sulfur Silicon Relat. Elem. 2000;167:161–179. [Google Scholar]
- 47.Hassan A.A., Ibrahim Y.R., El-Sheref E.M., Aly A.A., Braese S., Brown A.B. Novel synthesis of pyrazolyloxothiazolidine derivatives. J. Heterocycl. Chem. 2012;49:1380–1385. [Google Scholar]
- 48.Faidallah H.M., Al-Saadi M.S., Rostom S.A.F., Fahmy H.T.Y. Synthesis of some sulfonamides, disubstituted sulfonylureas or thioureas and some structurally related variants. A class of promising antitumor agents. Med. Chem. Res. 2007;16:300–318. [Google Scholar]
- 49.Faidallah H.M., Khan K.A., Rostom S.A.F., Asiri A.M. Synthesis and in vitro antitumor and antimicrobial activity of some 2,3-diaryl-7-methyl-4,5,6,7-tetrahydroindazole and 3,3a,4,5,6,7-hexahydroindazole derivatives. J. Enzym. Inhib. Med. Chem. 2013;28:495–508. doi: 10.3109/14756366.2011.653354. [DOI] [PubMed] [Google Scholar]
- 50.Al-Saadi M.S., Rostom S.A.F., Faidallah H.M. 3-Methyl-2-(4-substituted phenyl)-4,5-dihydronaphtho[1,2-c]-pyrazoles: synthesis and in-vitro biological evaluation as antitumour agents. Arch. Pharm. Chem. Life Sci. 2008;341:181–190. doi: 10.1002/ardp.200700178. [DOI] [PubMed] [Google Scholar]
- 51.Rostom S.A.F. Synthesis and in vitro antitumor evaluation of some indeno[1,2-c]pyrazol(in)es substituted with sulfonamide, sulfonylurea(-thiourea) pharmacophores, and some derived thiazole ring systems. Bioorg. Med. Chem. 2006;14:6475–6485. doi: 10.1016/j.bmc.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 52.Faidallah H.M., Khan K.A., Asiri A.M. Synthesis and biological evaluation of new 3- trifluoromethylpyrazolesulfonyl-urea and thiourea derivatives as antidiabetic and antimicrobial agents. J. Fluor. Chem. 2011;132:131–137. [Google Scholar]
- 53.Faidallah H.M., Khan K.A., Asiri A.M., Zayed M.E.M. Synthesis and biological evaluation of some new bipyrazolylbenzenesulfonamides as possible antimicrobial and chemotherapeutic agents. Indian J. Chem. B. 2012;51:1114–1122. [Google Scholar]
- 54.Insuasty B., Tigreros A., Martinez H., Quiroga J., Abonia R., Gutierrez A., Nogueras M., Cobo J. An efficient two-step synthesis of novel thiazolo[2,3-b]pyrazolo[3,4-f][1, 3,5]triazepines. J. Heterocycl. Chem. 2009;46:756–761. [Google Scholar]
- 55.El-Zahar M.I., Abd El-Karim S.S., Anwar M.M. Synthesis and cytotoxicity screening of some novel benzofuranoyl-pyrazole derivatives against liver and cervix carcinoma cell lines. S. Afr. J. Chem. 2009;62:189–199. [Google Scholar]
- 56.Bekhit A.A., Ashour H.M.A., Abdel Ghany Y.S., Bekhit A.E.-D.A., Baraka A. Synthesis and biological evaluation of some thiazolyl and thiadiazolyl derivatives of 1H-pyrazole as anti-inflammatory antimicrobial agents. Eur. J. Med. Chem. 2008;43:456–463. doi: 10.1016/j.ejmech.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 57.Abdelall M.M. A convenient route to 1,3,4-thiadiazoles, thiazolidinone, thiazoles, pyridones, coumarins, triazolo[5,1-c]triazines, and pyrazolo[5,1-c]triazines incorporating pyrazolone moiety and their use as antimicrobial agents. Phosphorus Sulfur Silicon Relat. Elem. 2009;184:2208–2226. [Google Scholar]
- 58.Abdel-Wahab B.F., Abdel-Gawad H., Awad G.E.A., Badria F.A. Synthesis, antimicrobial, antioxidant, anti-inflammatory, and analgesic activities of some new 3-(2-thienyl)pyrazole-based heterocycles. Med. Chem. Res. 2012;21:1418–1426. [Google Scholar]
- 59.El-Emary T.I., Al-Muaikel N., Moustafa O.S. Synthesis and antimicrobial activity of some new heterocycles based on 3-methyl-1-phenyl-5-benzene sulfonamido pyrazole. Phosphorus Sulfur Silicon Relat. Elem. 2002;177:195–210. [Google Scholar]
- 60.Farghaly A.A., Bekhit A.A., Park J.Y. Design and synthesis of some oxadiazolyl, thiadiazolyl, thiazolidinyl, and thiazolyl derivatives of 1H-pyrazole as anti-inflammatory antimicrobial agents. Arch. Pharm. Pharm. Med. Chem. 2000;333:53–57. doi: 10.1002/(sici)1521-4184(200002)333:2/3<53::aid-ardp53>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 61.Farghaly A.-R., Esmail S., Abdel-Hafez A., Vanelle P., El-Kashef H. New pyrazole derivatives of potential biological activity. Arkivoc. 2012;7:228–241. [Google Scholar]
- 62.Abdel-Wahab B.F., Abdel-Aziz H.A., Ahmed E.M. Convenient synthesis and antimicrobial activity of new 3-substituted 5-(benzofuran-2-yl)-pyrazole derivatives. Arch. Pharm. Chem. Life Sci. 2008;341:734–739. doi: 10.1002/ardp.200800119. [DOI] [PubMed] [Google Scholar]
- 63.Hegazi B., Mohamed H.A., Dawood K.M., Badria F.A.-R. Cytotoxicity and utility of 1-indanone in the synthesis of some new heterocycles. Chem. Pharm. Bull. 2010;58:479–483. doi: 10.1248/cpb.58.479. [DOI] [PubMed] [Google Scholar]
- 64.Dawood K.M., Abdel-Gawad H., Mohamed H.A., Badria F.A. Synthesis, anti-HSV-1, and cytotoxic activities of some new pyrazole- and isoxazole-based heterocycles. Med. Chem. Res. 2011;20:912–919. [Google Scholar]
- 65.Metwally M.A., Etman H.A., Gafer H.E., Khalil A.M. The use of 3-amino-4,6-dimethylpyrazolo[3,4-b]pyridine in the synthesis of novel heterocycles of pharmaceutical interest. Chem. Heterocycl. Compd. 2008;44:715–721. [Google Scholar]
- 66.Mohareb R.M., Zohdi H.F., Wardakhan W.W. Reaction of 3-phenyl-5-aminopyrazole with carbon disulfide: a novel synthesis of 3-(3′-phenylpyrazol-5'-yl)-4-phenylpyrazol-2-thione as well as of pyrazolo[3,4-d]thiazole and pyrano[2,3-d]thiazole derivatives. Monatsh. Chem. 1995;126:1391–1400. [Google Scholar]
- 67.Aly H.M. Synthesis and antitumor activity of some novel pyrazole and thienopyrimidine derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2010;185:211–221. [Google Scholar]
- 68.Ashour H.M.A., Wahab A.E.A. Synthesis and biological evaluation of novel pyrazoles and pyrazolo[3,4-d]pyrimidines incorporating a benzenesulfonamide moiety. Arch. Pharm. Chem. Life Sci. 2009;342:238–252. doi: 10.1002/ardp.200800178. [DOI] [PubMed] [Google Scholar]
- 69.Shetgiri N.P., Chitre A.D., Kokitkar S.V., Ghate S.M., Patil S.S., Kelaskar R.C. Synthesis and biological activity of 4′-oxathiazolidinyl benzopyrazoles. Indian J. Chem. B. 2006;45:1308–1311. [Google Scholar]
- 70.Pathak R.B., Chovatia P.T., Parekh H.H. Synthesis, antitubercular and antimicrobial evaluation of 3-(4-chlorophenyl)-4-substituted pyrazole derivatives. Bioorg. Med. Chem. Lett. 2012;22:5129–5133. doi: 10.1016/j.bmcl.2012.05.063. [DOI] [PubMed] [Google Scholar]
- 71.Low J.N., Cobo J., Insuasty B., Orozco F., Glidewell C. Rac-3-(5-amino-3-methyl-1-phenyl-1H-pyrazol-4-yl)-2-phenylthiazolidin-4-one: sheets built from N-H…N and C-H…pi(arene) hydrogen bonds. Acta Crystallogr. C. 2004;60:o486–o488. doi: 10.1107/S0108270104011540. [DOI] [PubMed] [Google Scholar]
- 72.Reddy C.S., Devi M.V., Sunitha M., Nagaraj A. Synthesis and antimicrobial study of linked heterocyclics containing pyrazole-pyrimidine-thiazolidin-4-one. Chem. Pharm. Bull. 2010;58:1622–1626. doi: 10.1248/cpb.58.1622. [DOI] [PubMed] [Google Scholar]
- 73.Jain S.C., Khanna P., Bhagat S., Jain M., Sakhuja R. Novel fluorinated spiro [indole-indazolyl-thiazolidine]-2,4′-diones: design and synthesis. Phosphorus Sulfur Silicon Relat. Elem. 2005;180:1829–1839. [Google Scholar]
- 74.Bhambi D., Sharma C., Sharma S., Salvi V.K., Talesara G.L. Synthesis and pharmacological studies of some phthalimidoxy substituted spiro-thiazolidinone derivatives of isatin. Indian J. Chem. B. 2009;48:1006–1012. [Google Scholar]
- 75.Augustin M., Rudorf W.-D. Synthese substituierter rhodanine. J. Prakt. Chem. 1974;316:520–524. [Google Scholar]
- 76.Chandrappa S., Chandru H., Sharada A.C., Vinaya K., Kumar C.S.A., Thimmegowda N.R., Nagegowda P., Kumar M.K., Rangappa K.S. Synthesis and in vivo anticancer and antiangiogenic effects of novel thioxothiazolidin-4-one derivatives against transplantable mouse tumor. Med. Chem. Res. 2010;19:236–249. [Google Scholar]
- 77.Havrylyuk D., Kovach N., Zimenkovsky B., Lesyk R. Synthesis of new 4-azolidinones with 3,5-diaryl-4,5-dihydropyrazole moiety and evaluation of their antitumor activity in vitro. Ann. Univ. Mariae Curie-Sklodowska. 2010;23:173–177. [Google Scholar]
- 78.Jawale D.V., Lingampalle D.L., Mane R.A., Pratap U.R. Dicationic ionic liquid mediated synthesis of 5-arylidine-2,4-thiazolidinediones. Chin. J. Chem. 2011;29:942–946. [Google Scholar]
- 79.Youssef A.M., White M.S., Villanueva E.B., El-Ashmawy I.M., Klegeris A. Synthesis and biological evaluation of novel pyrazolyl-2,4-thiazolidinediones as anti-inflammatory and neuroprotective agents. Bioorg. Med. Chem. 2010;18:2019–2028. doi: 10.1016/j.bmc.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 80.Prakash O., Aneja D.K., Arora S., Sharma C., Aneja K.R. Synthesis and antimicrobial activity of some new of 5-((3-(aryl)-1-phenyl-1H-pyrazol-4-yl)methylene)-3-phenylthiazolidine-2,4-diones. Med. Chem. Res. 2012;21:10–15. [Google Scholar]
- 81.Prakash O., Aneja D.K., Lohan P., Hussain K., Arora S., Sharma C., Aneja K.R. Synthesis and antimicrobial activity of 5-((3-aryl-1-phenyl-1H-pyrazol-4-yl)methylene)thiazolidine-2,4-diones. Med. Chem. Res. 2012;21:2961–2968. [Google Scholar]
- 82.Insuasty A., Ramirez J., Raimondi M., Echeverry C., Quiroga J., Abonia R., Nogueras M., Cobo J., Rodriguez M.V., Zacchino S.A., Insuasty B. Synthesis, antifungal and antitumor activity of novel (Z)-5-hetarylmethylidene-1,3-thiazol-4-ones and (Z)-5-ethylidene-1,3-thiazol-4-ones. Molecules. 2013;18:5482–5497. doi: 10.3390/molecules18055482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gilbert A.M., Bursavich M.G., Lombardi S., Georgiadis K.E., Reifenberg E., Flannery C.R., Morris E.A. 5-((1H-Pyrazol-4-yl)methylene)-2-thioxothiazolidin-4-one inhibitors of ADAMTS-5. Bioorg. Med. Chem. Lett. 2007;17:1189–1192. doi: 10.1016/j.bmcl.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 84.Metwally N.H., Abdalla M.A., Mosselhi M.A.N., El-Desoky E.A. Synthesis and antimicrobial activity of some new N-glycosides of 2-thioxo-4-thiazolidinone derivatives. Carbohydr. Res. 2010;345:1135–1141. doi: 10.1016/j.carres.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 85.Zheng C.-J., Song M.-X., Sun L.-P., Wu Y., Hong L., Piao H.-R. Synthesis and biological evaluation of 5-aryloxypyrazole derivatives bearing a rhodanine-3-aromatic acid as potential antimicrobial agents. Bioorg. Med. Chem. Lett. 2012;22:7024–7028. doi: 10.1016/j.bmcl.2012.09.107. [DOI] [PubMed] [Google Scholar]
- 86.Zheng C.-J., Xu L.-L., Sun L.-P., Miao J., Piao H.-R. Synthesis and antibacterial activity of novel 1,3-diphenyl-1H-pyrazoles functionalized with phenylalanine-derived rhodanines. Eur. J. Med. Chem. 2012;58:112–116. doi: 10.1016/j.ejmech.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 87.Zheng W., Degterev A., Hsu E., Yuan J., Yuan C. Structure-activity relationship study of a novel necroptosis inhibitor, Necrostatin-7. Bioorg. Med. Chem. Lett. 2008;18:4932–4935. doi: 10.1016/j.bmcl.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 88.B'Bhatt H., Sharma S. Synthesis and antimicrobial evaluation of some novel 2-(4-chlorophenylimino)thiazolidin-4-one derivatives. J. Korean Chem. Soc. 2012;56:341–347. [Google Scholar]
- 89.Abdel-Wahab B.F., Awad G.E.A., Badria F.A. Synthesis, antimicrobial, antioxidant, anti-hemolytic and cytotoxic evaluation of new imidazole-based heterocycles. Eur. J. Med. Chem. 2011;46:1505–1511. doi: 10.1016/j.ejmech.2011.01.062. [DOI] [PubMed] [Google Scholar]
- 90.Mitra P., Nayak A., Mittra A.S. Studies on 2-pyrazolin-5-one derivatives. J. Indian Chem. Soc. 1982;59:1005–1007. [Google Scholar]
- 91.Ledenyova I.V., Didenko V.V., Shestakov A.S., Shikhaliev K.S. Synthesis of new azocompounds and fused pyrazolo[5,1-c][1,2,4]triazines using heterocyclic components. J. Heterocycl. Chem. 2013;50:573–578. [Google Scholar]
- 92.Havrylyuk D., Kovach N., Zimenkovsky B., Vasylenko O., Lesyk R. Synthesis and anticancer activity of isatin-based pyrazolines and thiazolidines conjugates. Arch. Pharm. Chem. Life Sci. 2011;344:514–522. doi: 10.1002/ardp.201100055. [DOI] [PubMed] [Google Scholar]
- 93.Husain M.I., Shukla S. Synthesis and biological activity of 4-(3-aryl-4-oxo-2-thioxothiazolidin-5-ylimino)-3-methyl-1-(N,N-disubstituted aminomethyl)pyrazolin-5-ones. Indian J. Chem. 1986;25B:983–985. [Google Scholar]
- 94.Ibrahim M.A., Abdel-Hamed M.A.-M., El-Gohary N.M. A new approach for the synthesis of bioactive heteroaryl thiazolidine-2,4-diones. J. Braz. Chem. Soc. 2011;22:1130–1139. [Google Scholar]
- 95.T. Tawaraishi, H. Imoto, N. Cho, Preparation of fused ring compounds for treatment of diabetes, US2008/194617 A1 (2008).
- 96.Mittra P., Mittra A.S. Syntheis and fungitoxicity of some bicyclic compounds. J. Indian Chem. Soc. 1981;58:923–925. [Google Scholar]
- 97.Havrylyuk D., Zimenkovsky B., Vasylenko O., Day C.W., Smee D.F., Grellier P., Lesyk R. Synthesis and biological activity evaluation of 5-pyrazoline substituted 4-thiazolidinones. Eur. J. Med. Chem. 2013;66:228–237. doi: 10.1016/j.ejmech.2013.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Havrylyuk D., Zimenkovsky B., Karpenko O., Grellier P., Lesyk R. Synthesis of pyrazoline-thiazolidinone hybrids with trypanocidal activity. Eur. J. Med. Chem. 2014;85:245–254. doi: 10.1016/j.ejmech.2014.07.103. [DOI] [PubMed] [Google Scholar]
- 99.Sarkis M., Tran D.N., Miteva M.A., Kolb S., Villoutreix B.O., Garbay C., Braud E. Design and synthesis of novel bis-thiazolone derivatives as micromolar CDC25 phosphatase inhibitors: effect of dimerisation on phosphatase inhibition. Bioorg. Med. Chem. Lett. 2012;22:7345–7350. doi: 10.1016/j.bmcl.2012.10.072. [DOI] [PubMed] [Google Scholar]
- 100.Reddy C.S., Kumar G.R., Devi M.V., Nagaraj A. Synthesis of novel linked pyrazolyl-thiazolidinone heterocycles as potent antibacterial agents. Acta Chim. Slov. 2011;58:576–581. [PubMed] [Google Scholar]
- 101.Sakhuja R., Panda S.S., Khanna L., Khurana S., Jain S.C. Design and synthesis of spiro[indole-thiazolidine]spiro[indole-pyrans] as antimicrobial agents. Bioorg. Med. Chem. Lett. 2011;21:5465–5469. doi: 10.1016/j.bmcl.2011.06.121. [DOI] [PubMed] [Google Scholar]
- 102.Song M.-X., Zheng C.-J., Deng X.-Q., Sun L.-P., Wu Y., Hong L., Li Y.-J., Liu Y., Wei Z.-Y., Jin M.-J., Piao H.-R. Synthesis and antibacterial evaluation of rhodanine-based 5-aryloxy pyrazoles against selected methicillin resistant and quinoloneresistant Staphylococcus aureus (MRSA and QRSA) Eur. J. Med. Chem. 2013;60:376–385. doi: 10.1016/j.ejmech.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 103.Xu L.-L., Zheng C.-J., Sun L.-P., Miao J., Piao H.-R. Synthesis of novel 1,3-diaryl pyrazole derivatives bearing rhodanine-3-fatty acid moieties as potential antibacterial agents. Eur. J. Med. Chem. 2012;48:174–178. doi: 10.1016/j.ejmech.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 104.Ding Y., Smith K.L., Varaprasad C.V.N.S., Chang E., Alexander J., Yao N. Synthesis of thiazolone-based sulfonamides as inhibitors of HCV NS5B polymerase. Bioorg. Med. Chem. Lett. 2007;17:841–845. doi: 10.1016/j.bmcl.2006.08.104. [DOI] [PubMed] [Google Scholar]
- 105.Nasr M.N.A., Said S.A. Novel 3,3a,4,5,6,7-hexahydroindazole and arylthiazolylpyrazoline derivatives as anti-inflammatory agents. Arch. Pharm. Pharm. Med. Chem. 2003;336:551–559. doi: 10.1002/ardp.200300796. [DOI] [PubMed] [Google Scholar]


