Abstract
A series of novel 5-pyrazoline substituted 4-thiazolidinones have been synthesized. Target compounds were evaluated for their anticancer activity in vitro within DTP NCI protocol. Among the tested compounds, the derivatives 4d and 4f were found to be the most active, which demonstrated certain sensitivity profile toward the leukemia subpanel cell lines with GI50 value ranges of 2.12–4.58 μM (4d) and 1.64–3.20 μM (4f). The screening of antitrypanosomal and antiviral activities of 5-(3-naphthalen-2-yl-5-aryl-4,5-dihydropyrazol-1-yl)-thiazolidine-2,4-diones was carried out with the promising influence of the mentioned compounds on Trypanosoma brucei, but minimal effect on SARS coronavirus and influenza types A and B viruses.
Keywords: Synthesis, Pyrazolines, 4-Thiazolidinones, Anticancer activity, Antitrypanosomal activity, Antiviral activity
Graphical abstract

The synthesis and biological activity screening of novel 4-thiazolidinone based conjugates with pyrazoline moiety were performed. Thirteen synthesized compounds were tested for their anticancer activity in NCI60 cell lines and two of them (4d and 4f) were found to be the most active candidates. The screening of antitrypanosomal and antiviral activities of 5-(3-naphthalen-2-yl-5-aryl-4,5-dihydropyrazol-1-yl)-thiazolidine-2,4-diones was carried out.
Highlights
-
•
Synthesis of novel 5-pyrazoline substituted 4-thiazolidinones was performed.
-
•
Compounds 4d and 4f showed promising activity on the leukemia subpanel cell lines.
-
•
Compounds were evaluated for antitrypanosomal and antiviral properties.
1. Introduction
The non-condensed heterocyclic systems with thiazolidinone [1] and pyrazoline [2], [3] moieties have emerged as powerful scaffolds in drug design. Among diazole-substituted 4-thiazolidinones highly active anticancer agents have been identified including inhibitors of necroptosis [4], tumor necrosis factor α [5] and tyrosine phosphatase [6]. Our previous study, based on a hybrid pharmacophore approach, allowed to establish a number of patterns in the structure–activity relationship (SAR) context for 4-thiazolidinones with a pyrazoline fragment in 2, 3 and 4 positions of the thiazolidone cycle, which possessed antitumor activity [7], [8].
On the other hand, thiazolidinones and pyrazolines have occupied a unique position in the design and synthesis of novel biologically active agents that exert trypanocidal activity [9], [10], [11], [12], [13]. The 2-thioxo-4-thiazolidinone-3-acetic acid derivatives were identified as inhibitors of Trypanosoma brucei dolicholphosphate mannose synthase [11]. The 2-hydrazolyl-4-thiazolidinone-5-carboxylic acid derivatives have shown promising activity on the cruzipain protease [12]. The most promising compound in series of aryl-4-oxothiazolylhydrazones was shown to be very active at non-cytotoxic concentrations in in vitro assays against Trypanosoma cruzi cell cultures and exhibited potency comparable with the reference compounds (IC50 (Y strain) = 0.3 μM) [9]. Among pyrazoline derivatives, some novel compounds have been identified as inhibitors of the trypanosomal cysteine protease cruzain with IC50 of 40–230 nM [13].
The antiviral activity of heteryl substituted 4-thiazolidinones is promising. Among thiazole–thiazolidine conjugates [14] and non-condensed derivatives with thiazolidinone and pyridine [15], [16], [17] or pyrimidine [18], [19], [20] cycles, anti-HIV agents were identified. In addition, this group of compounds was active against hepatitis C virus [21], Tobacco Mosaic virus [22] and Vesicular stomatitis virus [23]. Previously we also demonstrated the efficiency of certain pyrazoline–thiazolidinone conjugates on influenza viruses and SARS coronavirus [24].
The present work is an extension of our ongoing efforts toward developing promising biologically active agents using a hybrid pharmacophore approach. We made the design (Fig. 1 ) and synthesized hybrid compounds by linking the main structural unit of the 4-thiazolidinone ring system with the pyrazoline, and examined their antitumor, trypanocidal and antiviral activities in vitro. We have found two compounds from 5-pyrazoline substituted 4-thiazolidinones, which possessed a commensurate antitumor activity compared to the pyrazoline–thiazolidinone analogous compounds reported previously [7], [8] and evaluated anti-trypanosomal activity and antiviral activity of the synthesized compounds.
Fig. 1.

Design of the non-condensed systems with the thiazolidinone and pyrazoline fragments.
2. Results and discussion
2.1. Chemistry
The general methods for synthesis of target thiazolidinone–pyrazoline conjugates are depicted in Schemes 1 and 2 .
Scheme 1.

Synthesis of 5-(3-naphthalen-2-yl-5-aryl-4,5-dihydropyrazol-1-yl)-thiazolidine-2,4-diones. Reagents, conditions and yields: (a) EtOH, reflux 1 h, 78–83%; (b) KOH, EtOH, reflux 5 h, 68–79%; (b) CH2O, EtOH, r.t. 1 h, 69–85%.
Scheme 2.

Synthesis of 5-[2-(3,5-diaryl-4,5-dihydropyrazol-1-yl)-2-oxoethylidene]-thiazolidine-2,4-dione. Reagents, conditions and yields: (d) triethylamine, dioxane, heating to 70–80 °C, 15 min, 69–86%; (e) KOH, EtOH, reflux 5 h, 72–87%.
The starting 3,5-diaryl-4,5-dihydropyrazoles synthesized using known methods from appropriate chalcones [25] easily reacted with 5-bromothiazolidine-2,4-dione [26] yielding 5-(3-naphthalen-2-yl-5-aryl-4,5-dihydropyrazol-1-yl)-thiazolidine-2,4-dione 1a and 1b. It is known that chemical modification of the N3 position of thiazolidinone cycle has an essential influence on the antitumor activity [27], [28]. Relying on these observations we utilized potassium salt of 5-(3-naphthalen-2-yl-5-aryl-4,5-dihydropyrazol-1-yl)-thiazolidine-2,4-dione, generated in situ, in the reactions with 2-chloro-N-arylacetamides. Following the mentioned reaction the new N3-substituted non-condensed thiazolidinone–pyrazoline conjugates 2a–2c were synthesized. Based on the Mannich reaction of 1a and 1b with secondary amines the thiazolidinone analogs 3a–3g were obtained (Scheme 1).
Aiming at the detailed elaboration of SAR, especially the influence of the linking group of thiazolidinone–pyrazoline conjugates on the anticancer activity, 5-[2-(3,5-diaryl-4,5-dihydropyrazol-1-yl)-2-oxoethylidene]-thiazolidine-2,4-diones (4a–4f) were synthesized by the method, described previously [29]. Reaction of 3,5-diaryl-4,5-dihydropyrazoles with (2,4-dioxothiazolidine-5-ylidene)-acetyl chloride [27] afforded in excellent yield and purity the compounds 4a–4f. Following the reaction of generated in situ potassium salts of 4b and 4e with 2-chloro-N-arylacetamides the group of N3-substituted 4-thiazolidinones 5a–5e were synthesized (Scheme 2).
The data characterization of synthesized novel heterocyclic substituted thiazolidones are presented in Experimental part. Analytical and spectral data (1H NMR, 13C NMR) confirmed the structure of the synthesized compounds.
Protons CH2–CH of pyrazoline fragment in the 1H NMR spectra of synthesized compounds showed characteristic patterns of an AMX system. The proton (CH) of thiazolidinone core of 1a–1b, 2a–2c and 3a–3g showed the broad singlet at δ ∼5.59–5.99 and the protons of the methylene group (CH2CO) of 2a–2c and 5a–5e appeared as a broad singlet at δ ∼4.44–4.49 ppm. In the 1H NMR spectra of the 1a–1b and 4a–4f NH proton of thiazolidinone cycle the broad singlet at δ∼12.20–12.72 was found.
2.2. In vitro evaluation of the anticancer activity
Synthesized derivatives 1a, 1b, 2a, 3a–3d, 3f, 4a, 4d, 4e, 4f and 5d were selected by National Cancer Institute (NCI, Bethesda USA) Developmental Therapeutic Program (DTP) and evaluated at the concentration of 10−5 M toward a panel of approximately sixty cancer cell lines (http://dtp.nci.nih.gov). The human tumor cell lines were derived from nine different cancer types: leukemia, melanoma, lung, colon, central nervous system, ovarian, renal, prostate and breast cancers. Primary anticancer assays were performed according to the NCI protocol as described elsewhere [30], [31], [32], [33]. The compounds were added at a single concentration and the cell cultures were incubated for 48 h. The end point determinations were made with a protein binding dye, sulforhodamine B (SRB). The results for each compound are reported as the percent growth (GP%) of treated cells when compared to untreated control cells (Table 1 ). The range of percent growth shows the lowest and the highest percent growth found among the different cancer cell lines.
Table 1.
Anticancer screening data at the concentration of 10 μM.
| Comp | 60 Cell lines assay in 1 dose 10 μM concentration |
|||
|---|---|---|---|---|
| Mean growth % | Range of growth % | The most sensitive cell lines | Growth % of the most sensitive cell lines | |
| 1a | 101.55 | 77.50–113.87 | UO-31 (Renal Cancer) | 77.50 |
| 1b | 104.84 | 74.89–169.65 | HOP-92 (Non-Small Cell Lung Cancer) | 74.89 |
| 2a | 99.23 | 76.58–113.53 | UO-31 (Renal Cancer) | 76.58 |
| 3a | 100.94 | 69.54 to 112.74 | HOP-92 (Non-Small Cell Lung Cancer) | 69.54 |
| 3b | 99.49 | 67.47–122.33 | OVCAR-4 (Ovarian Cancer) | 67.47 |
| 3c | 100.67 | 82.16–126.20 | UO-31 (Renal Cancer) | 82.16 |
| 3d | 102.96 | 74.48–127.35 | UO-31 (Renal Cancer) | 74.48 |
| 3f | 102.12 | 76.37–131.30 | K-562 (Leukemia) | 76.37 |
| 4a | 96.64 | 39.09–147.38 | SR (Leukemia) | 39.09 |
| K-562 (Leukemia) | 58.20 | |||
| RPMI-8226 (Leukemia) | 58.85 | |||
| LOX IMVI (Melanoma): | 55.70 | |||
| 4d | 60.11 | −27.33–160.47 | CCRF-CEM (Leukemia) | 19.50 |
| HL-60(TB) (Leukemia) | −27.33 | |||
| K-562 (Leukemia) | 33.24 | |||
| MOLT-4 (Leukemia) | 16.47 | |||
| HOP-92 (Non-Small Cell Lung Cancer) | 37.75 | |||
| KM12 (Colon Cancer) | 29.89 | |||
| SF-295 (CNS Cancer) | −13.37 | |||
| OVCAR-3 (Ovarian Cancer) | 36.41 | |||
| RXF 393 (Renal Cancer) | 31.39 | |||
| PC-3 (Prostate Cancer) | 33.62 | |||
| MCF7 (Breast Cancer) | 30.34 | |||
| T-47D (Breast Cancer) | 37.99 | |||
| 4e | 99.73 | 78.68–132.27 | T-47D (Breast Cancer) | 78.68 |
| 4f | 51.05 | −5.27–109.26 | CCRF-CEM (Leukemia) | 16.67 |
| HL-60(TB) (Leukemia) | 13.51 | |||
| K-562 (Leukemia) | 24.64 | |||
| MOLT-4 (Leukemia) | 11.47 | |||
| RPMI-8226 (Leukemia) | 2.48 | |||
| SR (Leukemia) | 10.57 | |||
| NCI-H522 (Non-Small Cell Lung Cancer) | 23.21 | |||
| A549/ATCC (Non-Small Cell Lung Cancer) | 30.04 | |||
| NCI-H460 (Non-Small Cell Lung Cancer) | 25.93 | |||
| KM12 (Colon Cancer) | 25.76 | |||
| SF-295 (CNS Cancer) | −5.27 | |||
| UACC-62 (Melanoma) | 32.77 | |||
| MDA-MB-435 (Melanoma) | 39.46 | |||
| PC-3 (Prostate Cancer) | 35.99 | |||
| MCF7 (Breast Cancer) | 34.41 | |||
| HS 578T (Breast Cancer) | 35.58 | |||
| BT-549 (Breast Cancer) | 34.25 | |||
| T-47D | 39.30 | |||
| 5d | 97.97 | 74.66–118.66 | CCRF-CEM (Leukemia) | 74.66 |
The most active compounds 4d and 4f were found to be effective against 12 and 18 cell lines, respectively, compound 4a was found to be moderately effective against few cell lines, while the other compounds (1a, 1b, 2a, 3a–3d, 3f, 4e, 5d) did not show any activity (Table 1).
Finally, compounds 4d and 4f were selected for an advanced assay against a panel of approximately sixty tumor cell lines at 10-fold dilutions of five concentrations (100 μM, 10 μM, 1 μM, 0.1 μM and 0.01 μM) [30], [31], [32], [33]. The percentage of growth was evaluated spectrophotometrically versus controls not treated with test agents after 48-h exposure and using SRB protein assay to estimate cell viability or growth. Dose–response parameters were calculated for each cell line: GI50 – molar concentration of the compound that inhibits 50% net cell growth; and TGI – molar concentration of the compound leading to the total inhibition. Furthermore, a mean graph midpoints (MG_MID) were calculated for each of the parameters, giving an average activity parameter over all cell lines for the tested compound. For the MG_MID calculation, insensitive cell lines were included with the highest concentration tested (Table 2 ).
Table 2.
Anticancer activity against a panel of approximately sixty tumor cell lines from nine different cancer types at 10-fold dilutions of five concentrations.
| Compound | End point (μM) | Leukemia | NSC lung cancer | Colon cancer | CNS cancer | Melanoma | Ovarian cancer | Renal cancer | Prostate cancer | Breast cancer | MG_MID |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4d | GI50 | 3.28 | 7.91 | 5.73 | 7.57 | 8.07 | 11.37 | 8.13 | 4.64 | 6.50 | 7.02 |
| TGI | 25.31 | 42.06 | 21.01 | 24.10 | 28.21 | 79.20 | 44.04 | 31.40 | 47.30 | 38.07 | |
| 4f | GI50 | 2.14 | 3.52 | 3.53 | 4.09 | 4.08 | 9.79 | 4.66 | 4.61 | 2.99 | 4.38 |
| TGI | 7.38 | 61.59 | 63.20 | 31.99 | 45.88 | 79.83 | 53.25 | 100.0 | 15.85 | 50.99 |
The tested compounds showed inhibition activity (GI50 < 10 μM) against 47 from 55 (4d) and 56 from 59 (4f) human tumor cells with average GI50/TGI values of 7.02 μM/38.07 μM (4d) and 4.38 μM/50.99 μM (4f) (Table 2). With regard to the sensitivity against some individual cell lines among several subpanel, the compounds 4d and 4f demonstrated a certain sensitivity profile toward the leukemia subpanel tumor cell lines with GI50 values range of 2.12–4.58 μM (4d) and 1.64–3.20 μM (4f) (Table 3 ).
Table 3.
The influence of compounds 4d and 4f on the growth of individual tumor cell lines (GI50 < 5 μM).
| Compound | Disease | Cell line | GI50, μM | TGI, μM |
|---|---|---|---|---|
| 4d | Leukemia | CCRF-CEM | 2.85 | 11.3 |
| Leukemia | RPMI-8226 | 2.12 | 6.91 | |
| Leukemia | SR | 2.85 | 9.02 | |
| Leukemia | HL-60(TB) | 3.35 | 9.14 | |
| Leukemia | K-562 | 4.58 | 100.0 | |
| NSC lung cancer | HOP-62 | 4.85 | 19.8 | |
| NSC lung cancer | NCI-H460 | 4.18 | 18.7 | |
| Colon cancer | HCT-116 | 4.56 | 16.4 | |
| Colon cancer | HCT-15 | 4.85 | 24.6 | |
| Colon cancer | KM12 | 4.76 | 15.3 | |
| CNS cancer | SF-295 | 2.99 | 10.0 | |
| CNS cancer | U251 | 4.29 | 16.6 | |
| Melanoma | LOX IMVI | 3.21 | 17.9 | |
| Melanoma | MDA-MB-435 | 3.91 | 31.7 | |
| Melanoma | UACC-62 | 2.83 | 9.28 | |
| Ovarian cancer | OVCAR-3 | 3.17 | 11.0 | |
| Renal cancer | 786-0 | 2.96 | 13.5 | |
| Prostate Cancer | PC-3 | 4.63 | 44.4 | |
| Prostate Cancer | DU-145 | 4.64 | 18.4 | |
| 4f | Leukemia | HL-60(TB) | 3.20 | 6.48 |
| Leukemia | K-562 | 1.90 | 11.2 | |
| Leukemia | MOLT-4 | 1.64 | 7.41 | |
| Leukemia | RPMI-8226 | 1.97 | 5.88 | |
| Leukemia | SR | 1.98 | 5.92 | |
| NSC lung cancer | A549/ATCC | 3.06 | 100.0 | |
| NSC lung cancer | HOP-92 | 1.17 | 9.72 | |
| NSC lung cancer | NCI-H322M | 2.89 | 100.0 | |
| NSC lung cancer | NCI-H460 | 1.89 | 4.70 | |
| Colon cancer | HCT-116 | 3.01 | 13.9 | |
| Colon cancer | HCT-15 | 2.41 | 100.0 | |
| CNS cancer | SF-295 | 2.45 | 9.12 | |
| CNS cancer | SF-539 | 2.43 | 5.79 | |
| CNS cancer | U251 | 2.58 | 13.2 | |
| Melanoma | MDA-MB-435 | 2.70 | 16.5 | |
| Melanoma | SK-MEL-5 | 2.09 | 22.5 | |
| Melanoma | UACC-62 | 2.66 | 12.8 | |
| Ovarian cancer | OVCAR-3 | 2.90 | 10.6 | |
| Renal cancer | A498 | 1.41 | 5.38 | |
| Prostate Cancer | PC-3 | 4.94 | 100.0 | |
| Prostate Cancer | DU-145 | 4.27 | 100.0 | |
| Breast cancer | MCF-7 | 2.53 | 14.1 | |
| Breast cancer | HS 578T | 1.94 | 8.26 | |
| Breast cancer | BT-549 | 2.16 | 7.75 | |
| Breast cancer | T-47D | 1.53 | 9.40 |
The SAR study revealed that: (1) the level of antitumor activity of active thiazolidinones with pyrazoline fragment in 5 position (4d and 4f) is compatible with effectivity levels of heteryl substituted thiazolidones, described previously [34], [35], [36], [37], [38], [39], [40], [41], [42]; (2) conjugation of pyrazoline and thiazolidinone cycles using oxomethylidene linking group (4f) allowed us to increase the activity, in comparison with the structurally related conjugate representative 5-(3-naphthalen-2-yl-5-aryl-4,5-dihydropyrazol-1-yl)-thiazolidine-2,4-dione 1b; (3) introduction of the substituents in 3N-position of thiazolidine fragment did not have significant influence on the antitumor activity.
2.3. COMPARE analysis
NCI's COMPARE algorithm [30], [31], [32], [33] allows to assume biochemical mechanisms of action of the novel compounds on the basis of their in vitro activity profiles when comparing with those of standard agents. We performed COMPARE computations for the compounds 4d and 4f against the NCI “Standard Agents” database at the GI50 and TGI levels (Table 4 ). However, obtained Pearson correlation coefficients (PCC) did not allow to distinguish cytotoxicity mechanism of tested compounds with high probability. The compound 4d showed the highest correlation at the GI50 level with dihydroorotate dehydrogenase inhibitor brequinar (PCC = 0.651) and compound 4f – with maytansine (RNA/DNA antimetabolite, PCC = 0.636).
Table 4.
COMPARE analysis results for compounds 4d and 4f.
| Compound | End point | PCCa | Target | Target vector NSC | Target mechanism of actionb |
|---|---|---|---|---|---|
| 4d | GI50 | 0.651 | Brequinar | S368390 | Dihydroorotate dehydrogenase inhibitor |
| 0.634 | Dichloroallyl lawsone | S126771 | DNA/RNA antimetabolite | ||
| 0.626 | Trimetrexate | S352122 | Dihydrofolate reductase inhibitor | ||
| 0.62 | l-Cysteine analog | S303861 | Reversible binding inhibitor of the human kinesin Eg5, antimitotic agent | ||
| 0.586 | Soluble Baker's Antifol | S139105 | RNA/DNA antimetabolite | ||
| 0.568 | Glycoxalic acid | S267213 | |||
| 4f | GI50 | 0.636 | Maytansine | S153858 | RNA/DNA antimetabolite |
| 0.573 | Rhizoxin | S749069 | Microtubule polymerization inhibitor | ||
| 0.550 | Macbecin II | S269148 | DNA antimetabolite |
Only correlations with PCC ≥ 0.55 were selected, as significant.
Putative mechanisms of action were identified with the use of literature sources.
2.4. Evaluation of antiviral activity
Antiviral activity of 1a, 1b, 2a–2c, 3d and 3e was determined against SARS coronavirus (SARS CoV) and influenza types A and B viruses (Flu A, Flu B). The obtained results are summarized in Table 5 .
Table 5.
Antiviral activity of the synthesized compounds.
| Compound | Virus | Virus strain | EC50a μM | SDb | CC50c μM | SD | SId |
|---|---|---|---|---|---|---|---|
| 1a | Flu A (H1N1) | California/07/2009 | 9.82 | 6.23 | 10.30 | 5.75 | 1.1 |
| Flu A (H3N2) | Perth/16/2009 | 4.31 | 3.35 | 6.23 | 4.55 | 1.4 | |
| Flu A (H5N1) | Duck/MN/1525/81 | 7.90 | 0.45 | 12.46 | 6.95 | 1.6 | |
| Flu B | Florida/4/2006 | 4.55 | 2.63 | 8.38 | 5.75 | 1.8 | |
| SARS CoV | Urbani | >23.47 | 6.95 | 23.47 | 6.95 | 0 | |
| 1b | Flu A (H1N1) | California/07/2009 | 4.27 | 3.08 | 4.50 | 3.32 | 1.0 |
| Flu A (H3N2) | Perth/16/2009 | 2.84 | 0.71 | 3.08 | 0.71 | 1.0 | |
| Flu A (H5N1) | Duck/MN/1525/81 | 5.21 | 2.13 | 6.40 | 2.61 | 1.2 | |
| Flu B | Florida/4/2006 | 3.08 | 1.66 | 4.98 | 2.13 | 1.6 | |
| SARS CoV | Urbani | >23.23 | 6.87 | 23.23 | 6.87 | 0 | |
| 2a | Flu A (H1N1) | California/07/2009 | 141.66 | 40.73 | 219.60 | 90.32 | 1.6 |
| Flu A (H3N2) | Perth/16/2009 | 53.13 | 53.13 | 74.38 | 88.55 | 1.4 | |
| Flu A (H5N1) | Duck/MN/1525/81 | 21.78 | 20.01 | >354.19 | 0 | >16.3 | |
| Flu B | Florida/4/2006 | 79.69 | 70.84 | 170.01 | 141.68 | 2.1 | |
| SARS CoV | Urbani | >313.46 | 70.84 | 313.46 | 70.84 | 0 | |
| 2b | Flu A (H1N1) | California/07/2009 | 54.47 | 24.60 | 73.80 | 22.84 | 1.3 |
| Flu A (H3N2) | Perth/16/2009 | >13.35 | 6.68 | 13.35 | 6.68 | 0 | |
| Flu A (H5N1) | Duck/MN/1525/81 | 50.96 | 33.39 | 56.23 | 28.12 | 1.1 | |
| Flu B | Florida/4/2006 | 35.14 | 36.90 | 42.17 | 35.14 | 1.3 | |
| SARS CoV | Urbani | >351.44 | 0 | >351.44 | 0 | 0 | |
| 2c | Flu A (H1N1) | California/07/2009 | 119.64 | 100.84 | 176.04 | 119.64 | 1.5 |
| Flu A (H3N2) | Perth/16/2009 | 18.80 | 7.52 | 25.64 | 18.80 | 1.3 | |
| Flu A (H5N1) | Duck/MN/1525/81 | 179.46 | 111.09 | 264.92 | 94.00 | 1.5 | |
| Flu B | Florida/4/2006 | 95.71 | 164.08 | 131.60 | 158.95 | 1.4 | |
| SARS CoV | Urbani | >341.83 | 0 | >341.83 | 0 | 0 | |
| 3d | Flu A (H1N1) | California/07/2009 | 13.70 | 16.34 | 14.69 | 15.68 | 1.1 |
| Flu A (H3N2) | Perth/16/2009 | >6.60 | 3.63 | 6.60 | 3.63 | 0 | |
| Flu A (H5N1) | Duck/MN/1525/81 | >24.76 | 24.76 | 24.76 | 24.76 | 0 | |
| Flu B | Florida/4/2006 | 57.78 | 75.94 | 59.43 | 75.94 | 1.0 | |
| SARS CoV | Urbani | 21.46 | 5.78 | 34.67 | 19.81 | 1.6 | |
| 3e | Flu A (H1N1) | California/07/2009 | 4.01 | 1.82 | 4.56 | 2.37 | 1.1 |
| Flu A (H3N2) | Perth/16/2009 | >2.37 | 0.73 | 2.37 | 0.73 | 0 | |
| Flu A (H5N1) | Duck/MN/1525/81 | 4.38 | 2.19 | 6.20 | 4.74 | 1.4 | |
| Flu B | Florida/4/2006 | 4.01 | 4.20 | 8.39 | 10.22 | 2.1 | |
| SARS CoV | Urbani | >16.78 | 3.47 | 16.78 | 3.47 | 0 | |
| Ribavirin | Flu A (H1N1) | California/07/2009 | 23.75 | 2.46 | >348.07 | 122.85 | >15 |
| Flu A (H3N2) | Perth/16/2009 | 18.43 | 11.88 | >368.54 | 69.61 | >20 | |
| Flu A (H5N1) | Duck/MN/1525/81 | 18.43 | 7.37 | >323.50 | 90.09 | >20 | |
| Flu B | Florida/4/2006 | 5.73 | 2.05 | >405.39 | 12.28 | >69 | |
| Oseltamirvir-carboxylate | Flu A (H1N1) | California/07/2009 | 18.99 | 10.90 | >35.17 | 0.0 | >1.9 |
| Flu A (H3N2) | Perth/16/2009 | 3.06 | 2.81 | >35.17 | 0.0 | >11 | |
| Flu A (H5N1) | Duck/MN/1525/81 | 0.11 | 0.007 | >35.17 | 0.0 | >326 | |
| Flu B | Florida/4/2006 | 3.52 | 4.22 | >35.17 | 0.0 | >10 | |
| M128533 Protease inhibitor | SARS CoV | Urbani | 1.56 | 1.18 | >236.41 | 0.0 | >152 |
50% Effective (virus-inhibitory) concentration.
50% Cytotoxic concentration, determined in uninfected cells.
SD – standard deviation.
Selectivity index (CC50/EC50), derived from three independent experiments.
Although antiviral activity was evident, virus inhibition occurred at or near the cytotoxic concentration. The compounds showed insignificant activities against the four strains of influenza virus with the range levels of selectivity index from 1.0 to 2.1. Compound 2a had moderate activity against the duck strain of influenza A with a 50% effective concentration (EC50) of 21.78 μM and selective index (SI) of >16.3; but did not have significant activity against other influenza strains. The majority of the compounds showed no activity against SARS CoV. The positive control compounds ribavirin, oseltamivir carboxylate, and M128533 were active as expected in the assays.
2.5. In vitro evaluation of antitrypanosomal activity
The compounds 1a, 2b and 2c were selected in advanced in vitro assay against Trypanosoma brucei brucei (Tbb) and Trypanosoma brucei gambiense (Tbg). The dose–response curves with drug concentrations ranging from 10 μg/ml to 0.625 μg/ml are depicted on Fig. 2 .
Fig. 2.
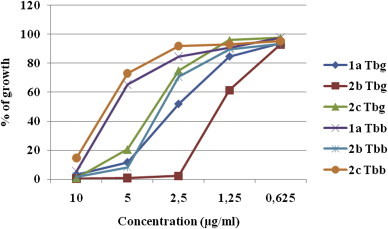
The dose–response curves of compounds 1a, 2b and 2c on Trypanosoma brucei brucei (Tbb) and Trypanosoma brucei gambiense (Tbg) growth.
The results showed a moderated activity of compounds (Table 6 ) on both parasite strains, namely IC50 (Tbb) = 5.43–13.87 μM and IC50 (Tbg) = 2.53–6.66 μM.
Table 6.
Anti-trypanosomal activity of 5-(3-naphthalen-2-yl-5-aryl-4,5-dihydropyrazol-1-yl)-thiazolidine-2,4-dione derivatives (1a, 2b, 2c).
| Comp | Trypanosoma B.B. |
Trypanosoma B.G. |
||
|---|---|---|---|---|
| IC50, μM | SD | IC50, μM | SD | |
| 1a | 13.87 | 0.36 | 6.66 | 1.15 |
| 2b | 5.43 | 0.09 | 2.53 | 0.12 |
| 2c | 11.26 | 0.31 | 6.10 | 0.43 |
| Pentamidine | 0.0032 | 0.0003 | 0.0053 | 0.0009 |
IC50 value is the mean +/− the standard deviation (SD) of three independent experiments.
3. Conclusions
In the present paper new 4-thiazolidinone based conjugates with pyrazoline moiety at 5 position are described. Antitumor activity assay of thirteen synthesized compounds allowed us to identify highly active thiazolidinone–pyrazoline hybrids 4d and 4f, which demonstrated certain sensitivity profile toward the leukemia subpanel tumor cell lines with GI50 values range of 2.12–4.58 μM (4d) and 1.64–3.20 μM (4f). The antitrypanosomal and antiviral activities screening of 5-(3-naphthalen-2-yl-5-aryl-4,5-dihydropyrazol-1-yl)-thiazolidine-2,4-diones was carried out and demonstrated the promising influence of mentioned compounds on T. brucei, and no activity to minimal effect on SARS coronavirus and influenza types A and B viruses. Further investigations of such thiazolidinone derivatives could be interesting with the hope to get more selective anticancer, antiviral and antiprotozoal agents among thiazolidinone–pyrazoline hybrid analogs.
4. Experimental
4.1. Materials and methods
The starting 3,5-diaryl-4,5-dihydro-1H-pyrazole [25], 5-bromothiazolidine-2,4-dione [26], and (2,4-dioxothiazolidine-5-ylidene)-acetyl chloride [27] were obtained according to the methods described previously. Preparation of compounds 4a–4d and 4f was described in our previous report [29].
Melting points were measured in open capillary tubes on a BŰCHI B-545 melting point apparatus and are uncorrected. The elemental analyses (C, H, N) were performed using the Perkin–Elmer 2400 CHN analyzer. Analyses indicated by the symbols of the elements or functions were within ±0.4% of the theoretical values. The 1H NMR spectra were recorded on Varian Gemini 400 MHz and 13C NMR spectra on Varian Mercury-400 100 MHz in DMSO-d 6 or DMSO-d 6 + CCl4 mixture using tetramethylsilane (TMS) as an internal standard. Chemical shifts are reported in ppm units with use of δ scale.
4.2. Chemistry
4.2.1. General procedure for synthesis of 5-(3-naphthalen-2-yl-5-aryl-4,5-dihydropyrazol-1-yl)-thiazolidine-2,4-diones (1a, 1b)
A mixture of 50 mmol 5-bromothiazolidine-2,4-dione and 50 mmol of appropriate 3,5-diaryl-4,5-dihydropyrazole was refluxed in 100 ml of ethanol during 1 h. The crystalline products were separated by filtration, washed with ethanol, and dried. Recrystallization from acetic acid rendered desired products in pure form.
4.2.1.1. 5-[5-(4-Methoxyphenyl)-3-naphthalen-2-yl-4,5-dihydropyrazol-1-yl]-thiazolidine-2,4-dione (1a)
Yield 78%, mp 208–210 °C. 1H NMR (400 MHz, DMSO-d 6 + CCl4): δ 12.20 (s, 1H, NH), 8.08 (s, 1H, arom), 7.87–7.97 (m, 4H, arom), 7.55–7.58 (m, 2H, arom), 7.50 (d, 2H, J = 8.4 Hz, arom), 7.02 (d, 2H, J = 8.4 Hz, arom), 5.74 (s, 1H, CH, thiazol), 4.29 (dd, 1H, CH 2CH, J = 13.5, 10.1 Hz), 3.83 (dd, 1H, CH 2CH, J = 16.8, 10.1 Hz), 3.78 (s, 3H, OCH3), 3.16 (dd, 1H, CH2 CH, J = 16.8, 13.5 Hz). 13C NMR (100 MHz, DMSO-d 6): δ 172.6 (C O), 171.7 (C O), 159.9, 154.7 (C N), 133.9, 133.3, 130.1, 130.0, 129.6, 128.9, 128.7, 128.2, 127.5, 127.2, 127.0, 123.5, 114.8, 70.1 (CH), 66.1 (CHCH2), 55.7 (OCH3), 42.2 (CHCH2). Calcd. for C23H19N3O3S: C, 66.17; H, 4.59; N, 10.06; Found: C, 66.38; H, 4.77; N, 10.28%.
4.2.1.2. 5-[5-(4-Chlorophenyl)-3-naphthalen-2-yl-4,5-dihydropyrazol-1-yl]-thiazolidine-2,4-dione (1b)
Yield 83%, mp 218–220 °C. 1H NMR (400 MHz, DMSO-d 6 + CCl4): δ 12.21 (s, 1H, NH), 8.06 (s, 1H, arom), 7.81–7.97 (m, 4H, arom), 7.49–7.62 (m, 5H, arom), 7.42 (s, 1H, arom), 5.79 (s, 1H, CH, thiazol), 4.41 (dd, 1H, CH 2CH, J = 13.5, 10.3 Hz), 3.82 (dd, 1H, CH 2CH, J = 16.5, 10.3 Hz), 3.14 (dd, 1H, CH2 CH, J = 16.5, 13.5 Hz). 13C NMR (100 MHz, DMSO-d 6): δ 172.5 (C O), 171.6 (C O), 154.6 (C N), 137.9, 133.9, 133.4, 133.2, 130.1, 129.3, 129.0, 128.9, 128.7, 128.2, 127.5, 127.2, 127.1, 123.6, 74.0 (CH), 69.4 (CHCH2), 42.5 (CHCH2). Calcd. for C22H16ClN3O2S: C, 62.63; H, 3.82; N, 9.96; Found: C, 62.48; H, 3.98; N, 9.75%.
4.2.2. General procedure for synthesis of 2-{5-[5-aryl-3-naphthalen-2-yl-4,5-dihydropyrazol-1-yl]-2,4-dioxothiazolidin-3-yl}-N-arylacetamides (2a–2c)
A suspension of compound 1a or 1b (3 mmol) and potassium hydroxide (3 mmol) was stirred at r.t. during 5 min, later appropriate 2-chloro-N-arylacetamide (3.3 mmol) was added and the mixture was refluxed for 5 h in EtOH (10 ml). Obtained powders were filtered off, washed with ethanol and recrystallized with DMF:ethanol (1:2) mixtures.
4.2.2.1. 2-{5-[5-(4-Methoxyphenyl)-3-naphthalen-2-yl-4,5-dihydropyrazol-1-yl]-2,4-dioxothiazolidin-3-yl}-N-p-tolylacetamide (2a)
Yield 73%, mp 216–218 °C. 1H NMR (400 MHz, DMSO-d 6 + CCl4): δ 10.03 (s, 1H, NH), 8.14 (s, 1H, arom), 7.87–7.97 (m, 4H, arom), 7.55–7.58 (m, 2H, arom), 7.52 (d, 2H, J = 8.6 Hz, arom), 7.30 (d, 2H, J = 8.3 Hz, arom), 7.17 (d, 2Н, J = 8.3 Hz, arom), 7.03 (d, 2Н, J = 8.6 Hz, arom), 5.91 (s, 1H, CH, thiazol), 4.45 (br. s, 2H, CH2), 4.40 (dd, 1Н, CH 2CН, J = 13.8, 10.5 Hz), 3.83 (dd, 1Н, CH 2CН, J = 16.1, 10.5 Hz), 3.79 (s, 3H, OCH3), 3.19 (dd, 1Н, CН2 CH, J = 16.1, 13.8 Hz), 2.28 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d 6): δ 172.6 (C O), 171.7 (C O), 163.8 (C O), 159.9, 154.7 (C N), 133.9, 133.3, 132.7, 131.5, 130.8, 130.1, 130.0, 129.7, 129.6, 129.4, 128.7, 128.2, 127.5, 127.2, 127.0, 123.5, 114.8, 70.1 (CH), 66.1 (CHCH2), 55.7 (OCH3), 44.1 (CH2), 42.2 (CHCH2), 20.9 (CH3). Calcd. for C32H28N4O4S: C, 68.07; H, 5.00; N, 9.92; Found: C, 67.88; H, 5.15; N, 9.68%.
4.2.2.2. 2-{5-[5-(4-Chlorophenyl)-3-naphthalen-2-yl-4,5-dihydropyrazol-1-yl]-2,4-dioxothiazolidin-3-yl}-N-p-tolylacetamide (2b)
Yield 68%, mp 220–222 °C. 1H NMR (400 MHz, DMSO-d 6 + CCl4): δ 10.05 (s, 1H, NH), 7.99 (s, 1H, arom), 7.83–7.89 (m, 3H, arom), 7.63 (d, 2Н, J = 8.3 Hz, arom), 7.43–7.50 (m, 4H, arom), 7.07 (d, 2Н, J = 8.3 Hz, arom), 5.81 (s, 1H, CH, thiazol), 4.52 (dd, 1Н, CH 2CН, J = 13.8, 9.9 Hz), 4.47 (br. s, 2H, CH2), 3.85 (dd, 1Н, CH 2CН, J = 16.2, 9.9 Hz), 3.16 (dd, 1Н, CН2 CH, J = 16.8, 13.8 Hz), 2.31 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d 6): δ 172.5 (C O), 171.6 (C O), 164.0 (C O), 154.6 (C N), 137.9, 133.9, 133.4, 133.2, 132.7, 131.5, 130.8, 130.1, 129.7, 129.3, 129.0, 128.9, 128.7, 128.2, 127.5, 127.2, 127.1, 123.6, 74.0 (CH), 69.4 (CHCH2), 44.1 (CH2), 42.5 (CHCH2), 21.0 (CH3). Calcd. for C31H25ClN4O3S: C, 65.43; H, 4.43; N, 9.85; Found: C, 65.62; H, 4.61; N, 9.68%.
4.2.2.3. 2-{5-[5-(4-Chlorophenyl)-3-naphthalen-2-yl-4,5-dihydropyrazol-1-yl]-2,4-dioxothiazolidin-3-yl}-N-(4-methoxyphenyl)-acetamide (2c)
Yield 79%, mp 212–214 °C. 1H NMR (400 MHz, DMSO-d 6 + CCl4): δ 10.21 (s, 1H, NH), 8.10 (s, 1H, arom), 7.88–7.95 (m, 4H, arom), 7.49–7.63 (m, 8H, arom), 6.93 (d, 2Н, J = 8.6 Hz, arom), 5.99 (s, 1H, CH, thiazol), 4.50 (dd, 1Н, CH 2CН, J = 13.3, 9.7 Hz), 4.45 (br. s, 2H, CH2), 3.85 (dd, 1Н, CH 2CH, J = 16.8, 9.7 Hz), 3.72 (s, 3H, OCH3), 3.16 (dd, 1H, CH2 CH, J = 16.8, 13.3 Hz). 13C NMR (100 MHz, DMSO-d 6): δ 170.8 (C O), 170.6 (C O), 163.6 (C O), 155.9, 154.6 (C N), 138.0, 133.9, 133.3, 133.2, 132.2, 130.1, 129.3, 129.0, 128.8, 128.6, 128.2, 127.5, 127.2, 127.1, 123.9, 121.2, 114.4, 72.6 (CH), 69.2 (CHCH2), 55.7 (OCH3), 44.3 (CH2), 42.6 (CHCH2). Calcd. for C31H25ClN4O4S: C, 63.64; H, 4.31; N, 9.58; Found: C, 63.79; H, 4.55; N, 9.71%.
4.2.3. General procedure for synthesis of 5-[5-aryl-3-naphthalen-2-yl-4,5-dihydropyrazol-1-yl]-3-R-methylthiazolidine-2,4-diones (3a–3g)
A mixture of compound 1a or 1b (3 mmol), appropriate amine (3.3 mmol) and formaldehyde (3 mmol) was stirred at r.t. during 1 h in EtOH (10 ml). Obtained powders were filtered off, washed with ethanol and recrystallized with DMF:ethanol (1:2) mixtures.
4.2.3.1. 5-[5-(4-Methoxyphenyl)-3-naphthalen-2-yl-4,5-dihydropyrazol-1-yl]-3-piperidin-1-ylmethylthiazolidine-2,4-dione (3a)
Yield 75%, mp 202–204 °C. 1H NMR (400 MHz, DMSO-d 6 + CCl4): δ 7.94–7.97 (m, 2H, arom), 7.76–7.83 (m, 3H, arom), 7.49–7.51 (m, 4H, arom), 6.97 (d, 2H, J = 7.7 Hz, arom), 5.54 (s, 1H, CH, thiazol), 4.59 (br. s, 2H, NCH2N), 4.34 (dd, 1H, CH 2CH, J = 12.6, 9.9 Hz), 3.83 (s, 3H, OCH3), 3.75 (dd, 1H, CH 2CH, J = 16.4, 9.9 Hz), 3.15 (dd, 1H, CH2CH, J = 16.4, 12.6 Hz), 2.67–2.72 (m, 4H, 2*CH2), 1.52–1.57 (m, 6H, 3*CH2). 13C NMR (100 MHz, DMSO-d 6): δ 170.8 (C O), 170.6 (C O), 159.9, 154.6 (C N), 133.9, 133.3, 130.0, 129.6, 129.5, 128.8, 128.4, 128.2, 127.5, 127.3, 127.2, 123.9, 114.7, 72.6 (CH), 70.0 (CHCH2), 55.7 (OCH3), 51.7 (CH2), 42.2 (CHCH2), 40.9, 40.8, 26.1. Calcd. for C29H30N4O3S: C, 67.68; H, 5.88; N, 10.89; Found: C, 67.49; H, 5.62; N, 10.72%.
4.2.3.2. 5-[5-(4-Methoxyphenyl)-3-naphthalen-2-yl-4,5-dihydropyrazol-1-yl]-3-morpholin-4-ylmethylthiazolidine-2,4-dione (3b)
Yield 85%, mp 195–196 °C. 1H NMR (400 MHz, DMSO-d 6 + CCl4): δ 8.13 (s, 1H, arom), 7.92–7.97 (m, 4H, arom), 7.52–7.56 (m, 2H, arom), 7.34 (d, 2H, J = 8.1 Hz, arom), 6.94 (d, 2H, J = 8.1 Hz, arom), 5.74 (s, 1H, CH, thiazol), 5.09 (br. s, 2H, NCH2N), 4.33 (dd, 1H, CH 2CH, J = 13.1, 9.7 Hz), 3.74–3.81 (m, 4H), 3.51–3.54 (m, 4H, 2*CH2) 3.38 (dd, 1H, CH2 CH, J = 16.7, 13.1 Hz), 2.45–2.47 (m, 4H, 2*CH2). 13C NMR (100 MHz, DMSO-d 6): δ 172.5 (C O), 171.5 (C O), 159.1, 153.6 (C N), 133.8, 133.4, 130.1, 129.6, 129.5, 128.8, 128.6, 128.2, 127.5, 127.3, 127.1, 123.8, 114.8, 70.9 (CH), 69.6 (CHCH2), 66.6, 62.9, 55.6 (OCH3), 50.7 (CH2), 41.0 (CHCH2). Calcd. for C28H28N4O4S: C, 65.10; H, 5.46; N, 10.84; Found: C, 65.32; H, 5.69; N, 10.69%.
4.2.3.3. 3-(4-Acetylpiperazin-1-ylmethyl)-5-[5-(4-methoxyphenyl)-3-naphthalen-2-yl-4,5-dihydro-pyrazol-1-yl]-thiazolidine-2,4-dione (3c)
Yield 79%, mp 185–187 °C. 1H NMR (400 MHz, DMSO-d 6 + CCl4): δ 7.84–7.88 (m, 5H, arom), 7.49–7.51 (m, 4H, arom), 6.97 (d, 2H, J = 7.6 Hz, arom), 5.59 (s, 1H, CH, thiazol), 4.70 (d, 1H, J = 12.9 Hz, NCH2N), 4.65 (d, 1H, J = 12.9 Hz, NCH2N), 4.33 (dd, 1H, CH 2CH, J = 12.9, 9.7 Hz), 3.73–3.83 (m, 4H), 3.44–3.46 (m, 4H, 2*CH2) 3.15 (dd, 1H, CH2 CH, J = 15.8, 12.9 Hz), 2.69–2.80 (m, 4H, 2*CH2), 1.99 (s, 3H, COCH3). 13C NMR (100 MHz, DMSO-d 6): δ 172.6 (C O), 171.7 (C O), 168.8 (C O), 159.9, 154.7 (C N), 133.9, 133.3, 130.1, 130.0, 129.6, 128.9, 128.7, 128.2, 127.5, 127.2, 127.0, 123.5, 114.8, 70.1 (CH), 66.1 (CHCH2), 63.0, 55.7 (OCH3), 51.0, 50.1, 46.1, 42.2 (CHCH2), 41.2. Calcd. for C30H31N5O4S: C, 64.61; H, 5.60; N, 12.56; Found: C, 64.78; H, 5.46; N, 12.40%.
4.2.3.4. 3-(4-Benzylpiperazin-1-ylmethyl)-5-[5-(4-methoxyphenyl)-3-naphthalen-2-yl-4,5-dihydropyrazol-1-yl]-thiazolidine-2,4-dione (3d)
Yield 80%, mp 168–170 °C. 1H NMR (400 MHz, DMSO-d 6 + CCl4): δ 8.09 (s, 1H, arom), 7.79–7.80 (m, 4H, arom), 7.50–7.55 (m, 4H, arom), 7.19–7.28 (m, 5H, arom), 7.01 (d, 2H, J = 8.4 Hz, arom), 5.72 (s, 1H, CH, thiazol), 4.55 (br. s, 2H, NCH2N), 4.33 (dd, 1H, CH 2CH, J = 13.0, 9.8 Hz), 3.77–3.84 (m, 4H), 3.45–3.51 (m, 4H, 2*CH2) 3.18 (dd, 1H, CH2 CH, J = 15.0, 13.0 Hz), 2.65–2.69 (m, 2H, CH2), 2.38–2.41 (m, 4H, 2*CH2). 13C NMR (100 MHz, DMSO-d 6): δ 172.2 (C O), 172.1 (C O), 159.9, 155.1 (C N), 133.9, 133.3, 130.1, 130.0, 129.6, 129.5, 128.9, 128.8, 128.6, 128.5, 128.1, 127.6, 127.3, 127.2, 127.1, 123.7, 114.8, 72.5 (CH), 69.9 (CHCH2), 55.7 (OCH3), 55.6, 50.7, 42.3 (CHCH2), 40.9. Calcd. for C35H35N5O3S: C, 69.40; H, 5.82; N, 11.56; Found: C, 69.23; H, 5.96; N, 11.40%.
4.2.3.5. 5-[5-(4-Chlorophenyl)-3-naphthalen-2-yl-4,5-dihydropyrazol-1-yl]-3-(4-ethylpiperazin-1-ylmethyl)-thiazolidine-2,4-dione (3e)
Yield 72%, mp 182–184 °C. 1H NMR (400 MHz, DMSO-d 6 + CCl4): δ 8.11 (s, 1H, arom), 7.92–7.96 (m, 4H, arom), 7.54–7.61 (m, 5H, arom), 7.43 (br. s, 1H, arom), 5.78 (s, 1H, CH, thiazol), 4.58 (d, 1H, J = 12.9 Hz, NCH2N), 4.54 (d, 1H, J = 12.9 Hz, NCH2N), 4.42 (dd, 1H, CH 2CH, J = 13.3, 10.0 Hz), 3.85 (dd, 1H, CH 2CH, J = 16.0, 10.0 Hz), 3.47–3.55 (m, 4H, 2*CH2), 3.16 (dd, 1H, CH2 CH, J = 16.0, 13.3 Hz), 3.64–3.67 (m, 2H, CH2), 2.39–2.43 (m, 4H, 2*CH2), 1.03 (t, 3H, J = 7.1 Hz, CH3). 13C NMR (100 MHz, DMSO-d 6): δ 172.9 (C O), 171.7 (C O), 154.6 (C N), 137.9, 133.9, 133.3, 133.2, 130.1, 129.3, 128.9, 128.7, 128.6, 128.2, 127.6, 127.2, 127.1, 123.6, 74.0 (CH), 69.4 (CHCH2), 62.8, 52.3, 51.9, 42.5 (CHCH2), 40.9. Calcd. for C29H30ClN5O2S: C, 63.55; H, 5.52; N, 12.78; Found: C, 63.69; H, 5.38; N, 12.56%.
4.2.3.6. 3-(4-Acetylpiperazin-1-ylmethyl)-5-[5-(4-chlorophenyl)-3-naphthalen-2-yl-4,5-dihydropyrazol-1-yl]-thiazolidine-2,4-dione (3f)
Yield 79%, mp 211–212 °C. 1H NMR (400 MHz, DMSO-d 6 + CCl4): δ 8.02 (s, 1H, arom), 7.78–7.93 (m, 5H, arom), 7.61 (d, 2H, J = 7.8 Hz, arom), 7.50–7.54 (m, 4H, arom), 7.43 (br. s, 1H, arom), 5.80 (s, 1H, CH, thiazol), 4.62 (br. s, 2H, NCH2N), 4.66 (dd, 1H, CH 2CH, J = 13.0, 10.2 Hz), 3.88 (dd, 1H, CH 2CH, J = 15.9, 10.2 Hz), 3.44–3.55 (m, 4H, 2*CH2), 3.16 (dd, 1H, CH2 CH, J = 15.9, 13.0 Hz), 2.60–2.66 (m, 4H, 2*CH2), 2.00 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d 6): δ 172.2 (C O), 172.1 (C O), 168.8 (C O), 155.0 (C N), 137.9, 133.9, 133.4, 133.2, 130.1, 129.3, 129.2, 128.9, 128.8, 128.2, 127.7, 127.5, 127.3, 123.2, 72.6 (CH), 69.2 (CHCH2), 63.0, 51.0, 50.1, 46.1, 42.6 (CHCH2), 41.2. Calcd. for C29H28ClN5O3S: C, 61.97; H, 5.02; N, 12.46; Found: C, 61.72; H, 5.21; N, 12.34%.
4.2.3.7. 3-(4-Benzylpiperazin-1-ylmethyl)-5-[5-(4-chlorophenyl)-3-naphthalen-2-yl-4,5-dihydropyrazol-1-yl]-thiazolidine-2,4-dione (3g)
Yield 69%, mp 194–196 °C. 1H NMR (400 MHz, DMSO-d 6 + CCl4): δ 8.06 (s, 1H, arom), 7.87–7.93 (m, 5H, arom), 7.60 (d, 2H, J = 8.1 Hz, arom), 7.40–7.54 (m, 4H, arom), 7.22–7.26 (m, 4H, arom), 5.79 (s, 1H, CH, thiazol), 4.45–4.56 (m, 3H), 3.86 (dd, 1H, CH 2CH, J = 16.8, 9.8 Hz), 3.47–3.54 (m, 4H, 2*CH2), 3.28 (dd, 1H, CH2 CH, J = 16.8, 13.2 Hz), 2.56–2.68 (m, 2H, CH2), 2.32–2.41 (m, 4H, 2*CH2). 13C NMR (100 MHz, DMSO-d 6): δ 172.2 (C O), 172.1 (C O), 155.0 (C N), 137.9, 133.9, 133.4, 133.2, 130.5, 130.1, 129.3, 129.2, 128.9, 128.7, 128.6, 128.5, 128.1, 127.6, 127.4, 127.3, 127.2, 123.7, 72.6 (CH), 69.3 (CHCH2), 63.0, 53.1, 50.1, 42.6 (CHCH2), 41.0. Calcd. for C34H32ClN5O2S: C, 66.93; H, 5.29; N, 11.48; Found: C, 66.68; H, 5.10; N, 11.22%.
4.2.4. General procedure for synthesis of 5-[2-(3,5-diaryll-4,5-dihydropyrazol-1-yl)-2-oxoethylidene]-thiazolidine-2,4-diones (4a–4f)
A solution of (2,4-dioxothiazolidin-5-ylidene)-acetyl chloride (3 mmol) in 5 ml of dioxane was added to a mixture of appropriate 3,5-diaryl-4,5-dihydro-1H-pyrazole (3 mmol) and triethylamine (3 mmol) in 5 ml of dioxane and later was heated to 70–80 °C during 15 min, cooled and poured water (50 ml). Obtained powder was filtered off, washed with water and recrystallized with DMF:ethanol (1:2) mixtures.
4.2.4.1. 5-{2-[5-(4-Methoxyphenyl)-3-phenyl-4,5-dihydropyrazol-1-yl]-2-oxoethylidene}-thiazolidine-2,4-dione (4a)
Yield 75%, mp 240–242 °C. 1H NMR (400 MHz, DMSO-d 6 + CCl4): δ 12.64 (br. s, 1H, NH), 7.81–7.86 (m, 3H, arom), 7.48–7.51 (m, 3H, arom, CH), 7.14 (d, 2H, J = 8.6 Hz, arom), 6.87 (d, 2H, J = 8.6 Hz, arom), 5.59 (dd, 1H, CH2 CH, J = 11.2, 4.3 Hz), 3.93 (dd, 1H, CH 2CH, J = 18.3, 11.2 Hz), 3.70 (s, 3H, OCH3), 3.21 (dd, 1H, CH 2CH, J = 18.3, 4.3 Hz). 13C NMR (100 MHz, DMSO-d 6): δ 171.3 (C O), 167.1 (C O), 160.9 (C O), 159.2, 157.4 (C N), 139.9, 133.8, 131.4, 131.0, 129.4, 127.6, 127.5, 117.8, 114.6, 60.2 (CHCH2), 55.6 (OCH3), 42.7 (CHCH2). Calcd. for C21H17N3O4S: C, 61.91; H, 4.21; N, 10.31; Found: C, 61.78; H, 4.08; N, 10.13%.
4.2.4.2. 5-{2-[5-(4-Chlorophenyl)-3-phenyl-4,5-dihydropyrazol-1-yl]-2-oxoethylidene}-thiazolidine-2,4-dione (4b)
Yield 86%, mp 250–252 °C. 1H NMR (400 MHz, DMSO-d 6 + CCl4): δ 12.72 (br. s, 1H, NH), 7.84–7.87 (m, 3H, arom), 7.50–7.54 (m, 3H, arom, CH), 7.41 (d, 2H, J = 8.1 Hz, arom), 7.28 (d, 2H, J = 8.1 Hz, arom), 5.68 (dd, 1H, CH2 CH, J = 11.4, 4.3 Hz), 3.97 (dd, 1H, CH 2CH, J = 18.0, 11.4 Hz), 3.29 (dd, 1H, CH 2CH, J = 18.0, 4.3 Hz). 13C NMR (100 MHz, DMSO-d 6): δ 171.4 (C O), 167.1 (C O), 160.9 (C O), 157.7 (C N), 137.9, 133.4, 133.2, 131.2, 129.5, 129.3, 129.0, 127.4, 117.9, 116.0, 57.2 (CHCH2), 41.6 (CHCH2). Calcd. for C20H14ClN3O3S: C, 58.32; H, 3.43; N, 10.20; Found: C, 58.58; H, 3.28; N, 10.03%.
4.2.4.3. 5-{2-[5-(2-Hydroxyphenyl)-3-phenyl-4,5-dihydropyrazol-1-yl]-2-oxoethylidene}-thiazolidine-2,4-dione (4c)
Yield 82%, mp 232–234 °C. 1H NMR (400 MHz, DMSO-d 6 + CCl4): δ 12.67 (br. s, 1H, NH), 9.72 (s, 1H, OH), 7.80–7.85 (m, 3H, arom), 7.46–7.49 (m, 3H, arom, CH), 7.07 (t, 1H, J = 7.8 Hz, arom), 6.90 (d, 1H, J = 6.7 Hz, arom), 6.80 (d, 1H, J = 7.8 Hz, arom), 6.71 (t, 1H, J = 7.4 Hz, arom), 5.72 (dd, 1H, CH2 CH, J = 11.6, 4.6 Hz), 3.88 (dd, 1H, CH 2CH, J = 17.9, 11.6 Hz), 3.14 (dd, 1H, CH 2CH, J = 17.9, 4.6 Hz). 13C NMR (100 MHz, DMSO-d 6): δ 171.4 (C O), 167.1 (C O), 160.9 (C O), 157.7 (C N), 154.7, 139.7, 131.2, 129.3, 128.9, 127.4, 127.0, 119.4, 117.9, 116.0, 57.2 (CHCH2), 41.6 (CHCH2). Calcd. for C20H15N3O4S: C, 61.06; H, 3.84; N, 10.68; Found: C, 61.27; H, 3.71; N, 10.53%.
4.2.4.4. 5-{2-[5-(2-Hydroxyphenyl)-3-(4-methoxyphenyl)-4,5-dihydropyrazol-1-yl]-2-oxoethylidene}-thiazolidine-2,4-dione (4d)
Yield 69%, mp 245–247 °C. 1H NMR (400 MHz, DMSO-d 6 + CCl4): δ 12.57 (br. s, 1H, NH), 10.08 (s, 1H, OH), 7.82 (s, 1H, CH), 7.76 (d, 2H, J = 8.6 Hz, arom), 7.24 (d, 1H, J = 8.5 Hz, arom), 6.99–7.04 (m, 3H, arom), 6.77 (d, 1H, J = 8.6 Hz, arom), 5.64 (dd, 1H, CH2 CH, J = 11.4, 4.8 Hz), 3.77–3.91 (m, 4H), 3.14 (dd, 1H, CH 2CH, J = 18.2, 4.8 Hz). 13C NMR (100 MHz, DMSO-d 6): δ 171.3 (C O), 167.2 (C O), 162.7 (C O), 161.9, 160.8, 157.5 (C N), 154.2, 139.7, 131.6, 129.8, 129.5, 129.2, 123.6, 118.3, 117.9, 114.8, 110.5, 56.8 (CHCH2), 55.9 (OCH3), 41.4 (CHCH2). Calcd. for C21H17N3O5S: C, 59.57; H, 4.05; N, 9.92; Found: C, 59.73; H, 4.21; N, 9.78%.
4.2.4.5. 5-{2-[5-(4-Methoxyphenyl)-3-naphthalen-2-yl-4,5-dihydropyrazol-1-yl]-2-oxoethylidene}-thiazolidine-2,4-dione (4e)
Yield 78%, mp 271–273 °C. 1H NMR (400 MHz, DMSO-d 6 + CCl4): δ 12.71 (br. s, 1H, NH), 8.27 (s, 1H, arom), 8.08 (d, 1H, J = 8.6 Hz, arom), 7.96–8.02 (m, 3H, arom), 7.87 (s, 1H, CH), 7.56–7.61 (m, 2H, arom), 7.18 (d, 2H, J = 8.3 Hz, arom), 6.89 (d, 2H, J = 8.3 Hz, arom), 5.64 (dd, 1H, CH2 CH, J = 11.4, 4.0 Hz), 4.03 (dd, 1H, CH 2CH, J = 18.0, 11.4 Hz), 3.72 (s, 3H, OCH3), 3.39 (dd, 1H, CH 2CH, J = 18.0, 4.0 Hz). 13C NMR (100 MHz, DMSO-d 6): δ 171.4 (C O), 167.2 (C O), 160.9 (C O), 159.2, 157.5 (C N), 140.1, 134.4, 133.8, 133.2, 129.1, 128.9, 128.7, 128.6, 128.2, 128.1, 127.6, 127.4, 123.6, 117.7, 114.6, 60.3 (CHCH2), 55.6 (OCH3), 42.7 (CHCH2). Calcd. for C25H19N3O4S: C, 65.63; H, 4.19; N, 9.18; Found: C, 65.49; H, 4.03; N, 9.32%.
4.2.4.6. 5-{2-[5-(4-Chlorophenyl)-3-naphthalen-2-yl-4,5-dihydropyrazol-1-yl]-2-oxoethylidene}-thiazolidine-2,4-dione (4f)
Yield 82%, mp 268–270 °C. 1H NMR (400 MHz, DMSO-d 6 + CCl4): δ 12.71 (br. s, 1H, NH), 8.27 (s, 1H, arom), 7.95–8.11 (m, 4H, arom), 7.88 (s, 1H, CH), 7.56–7.60 (m, 2H, arom), 7.40 (d, 2H, J = 8.5 Hz, arom), 7.29 (d, 2H, J = 8.5 Hz, arom), 5.71 (dd, 1H, CH2 CH, J = 11.8, 4.7 Hz), 4.07 (dd, 1H, CH 2CH, J = 18.2, 11.8 Hz), 3.40 (dd, 1H, CH 2CH, J = 18.2, 4.7 Hz). 13C NMR (100 MHz, DMSO-d 6): δ 171.2 (C O), 167.1 (C O), 161.2 (C O), 157.4 (C N), 140.7, 140.3, 134.4, 133.2, 132.7, 129.2, 129.0, 128.9, 128.8, 128.4, 128.3, 128.2, 128.1, 127.4, 123.6, 117.6, 60.3 (CHCH2), 42.6 (CHCH2). Calcd. for C24H16ClN3O3S: C, 62.40; H, 3.49; N, 9.10; Found: C, 62.18; H, 3.62; N, 9.02%.
4.2.5. General procedure for synthesis of 2-{5-[2-(3,5-diaryl-4,5-dihydropyrazol-1-yl)-2-oxoethylidene]-2,4-dioxothiazolidin-3-yl}-N-arylacetamides (5a–5e)
A suspension of compound 4b or 4f (3 mmol) and potassium hydroxide (3 mmol) was stirred at r.t. during 5 min, later appropriate 2-chloro-N-arylacetamide (3.3 mmol) was added and the mixture was refluxed for 5 h in EtOH (10 ml). Obtained powders were filtered off, washed with ethanol and recrystallized with DMF:ethanol (1:2) mixtures.
4.2.5.1. 2-(5-{2-[5-(4-Chlorophenyl)-3-phenyl-4,5-dihydropyrazol-1-yl]-2-oxoethylidene}-2,4-dioxothiazolidin-3-yl)-N-p-tolylacetamide (5a)
Yield 76%, mp 234–236 °C. 1H NMR (400 MHz, DMSO-d 6 + CCl4): δ 10.07 (s, 1H, NH), 8.02 (s, 1H, COCH), 7.82–7.84 (m, 2H, arom), 7.39–7.46 (m, 5H, arom), 7.31 (d, 2H, J = 8.2 Hz, arom), 7.26 (d, 2H, J = 8.2 Hz, arom), 7.04 (d, 2H, J = 7.9 Hz, arom), 5.70 (dd, 1H, CH2 CH, J = 11.3, 4.2 Hz), 4.47 (s, 2H, CH2), 3.98 (dd, 1H, CH 2CH, J = 18.1, 11.3 Hz), 3.24 (dd, 1H, CH 2CH, J = 18.1, 4.2 Hz), 2.29 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d 6): δ 170.1 (C O), 166.1 (C O), 163.8 (C O), 160.9 (C O), 157.8 (C N), 140.5, 137.5, 136.3, 133.2, 132.7, 131.5, 130.8, 129.7, 129.4, 129.3, 128.2, 127.6, 119.7, 119.1, 60.2 (CHCH2), 44.1 (CH2), 42.7 (CHCH2), 20.9 (CH3) Calcd. for C29H23ClN4O4S: C, 62.31; H, 4.15; N, 10.02; Found: C, 62.49; H, 4.00; N, 9.89%.
4.2.5.2. 2-(5-{2-[5-(4-Chlorophenyl)-3-phenyl-4,5-dihydropyrazol-1-yl]-2-oxoethylidene}-2,4-dioxothiazolidin-3-yl)-N-(4-methoxyphenyl)-acetamide (5b)
Yield 72%, mp 228–230 °C. 1H NMR (400 MHz, DMSO-d 6 + CCl4): δ 10.04 (s, 1H, NH), 8.02 (s, 1H, COCH), 7.82–7.84 (m, 2H, arom), 7.43–7.52 (m, 5H, arom), 7.32 (d, 2H, J = 7.7 Hz, arom), 7.26 (d, 2H, J = 7.7 Hz, arom), 6.79 (d, 2H, J = 8.6 Hz, arom), 5.69 (dd, 1H, CH2 CH, J = 11.6, 4.2 Hz), 4.44 (s, 2H, CH2), 3.98 (dd, 1H, CH 2CH, J = 17.8, 11.6 Hz), 3.75 (s, 3H, CH3), 3.24 (dd, 1H, CH 2CH, J = 17.8, 4.2 Hz). 13C NMR (100 MHz, DMSO-d 6): δ 170.1 (C O), 166.1 (C O), 163.5 (C O), 160.9 (C O), 157.7 (C N), 156.0, 140.5, 137.5, 132.7, 131.9, 131.5, 130.8, 129.4, 129.3, 128.3, 127.6, 121.3, 119.1, 114.4, 60.2 (CHCH2), 55.7 (OCH3), 44.1 (CH2), 42.7 (CHCH2). Calcd. for C29H23ClN4O5S: C, 60.57; H, 4.03; N, 9.74; Found: C, 60.39; H, 3.88; N, 9.58%.
4.2.5.3. 2-(5-{2-[5-(4-Chlorophenyl)-3-phenyl-4,5-dihydropyrazol-1-yl]-2-oxoethylidene}-2,4-dioxothiazolidin-3-yl)-N-(4-chlorophenyl)-acetamide (5c)
Yield 79%, mp 289–290 °C. 1H NMR (400 MHz, DMSO-d 6 + CCl4): δ 10.51 (s, 1H, NH), 7.98 (s, 1H, COCH), 7.83–7.86 (m, 2H, arom), 7.48–7.58 (m, 5H, arom), 7.34–7.42 (m, 3H, arom), 7.28 (d, 2H, J = 8.3 Hz, arom), 5.69 (dd, 1H, CH2 CH, J = 11.2, 4.2 Hz), 4.49 (s, 2H, CH2), 3.97 (dd, 1H, CH 2CH, J = 17.5, 11.2 Hz), 3.25 (dd, 1H, CH 2CH, J = 17.5, 4.2 Hz). 13C NMR (100 MHz, DMSO-d 6): δ 170.1 (C O), 165.1 (C O), 164.3 (C O), 160.9 (C O), 157.8 (C N), 140.5, 137.7, 137.4, 132.7, 131.5, 130.8, 129.4, 129.3, 129.2, 128.3, 127.9, 127.6, 121.3, 119.3, 60.2 (CHCH2), 44.2 (CH2), 42.7 (CHCH2). Calcd. for C28H20ClN4O4S: C, 58.04; H, 3.48; N, 9.67; Found: C, 58.19; H, 3.28; N, 9.45%.
4.2.5.4. N-(4-Methoxyphenyl)-2-(5-{2-[5-(4-methoxyphenyl)-3-naphthalen-2-yl-4,5-dihydropyrazol-1-yl]-2-oxoethylidene}-2,4-dioxothiazolidin-3-yl)-acetamide (5d)
Yield 86%, mp 268–269 °C. 1H NMR (400 MHz, DMSO-d 6 + CCl4): δ 10.24 (s, 1H, NH), 8.29 (s, 1H, COCH), 8.00–8.09 (m, 5H, arom), 7.59 (br. s, 2H, arom), 7.46 (d, 2H, J = 6.4 Hz, arom), 7.21 (d, 2H, J = 6.4 Hz, arom), 6.90 (br. s, 3H, arom), 5.68 (dd, 1H, CH2 CH, J = 11.1, 4.2 Hz), 4.47 (s, 2H, CH2), 4.05 (dd, 1H, CH 2CH, J = 15.6, 11.1 Hz), 3.71 (s, 6H, 2*OCH3), 3.42 (dd, 1H, CH 2CH, J = 15.6, 4.2 Hz). 13C NMR (100 MHz, DMSO-d 6): δ 171.4 (C O), 167.2 (C O), 164.3 (C O), 160.9 (C O), 159.2, 157.5 (C N), 156.0, 140.1, 137.5, 134.4, 133.8, 133.2, 131.9, 129.1, 128.9, 128.7, 128.6, 128.2, 128.1, 127.6, 127.4, 123.6, 117.7, 114.6, 60.3 (CHCH2), 55.7 (OCH3), 55.6 (OCH3), 44.1 (CH2), 42.7 (CHCH2). Calcd. for C34H28N4O6S: C, 65.79; H, 4.55; N, 9.03; Found: C, 65.96; H, 4.32; N, 8.75%.
4.2.5.5. N-(4-Chlorophenyl)-2-(5-{2-[5-(4-methoxyphenyl)-3-naphthalen-2-yl-4,5-dihydropyrazol-1-yl]-2-oxoethylidene}-2,4-dioxothiazolidin-3-yl)-acetamide (5e)
Yield 87%, mp 279–280 °C. 1H NMR (400 MHz, DMSO-d 6 + CCl4): δ 10.33 (s, 1H, NH), 8.09–8.14 (m, 3H, COCH, arom), 7.87–7.93 (m, 3H, arom), 7.53–7.58 (m, 4H, arom), 7.25 (d, 2H, J = 7.4 Hz, arom), 7.19 (d, 2H, J = 6.4 Hz, arom), 6.84 (d, 2H, J = 6.4 Hz, arom), 5.68 (dd, 1H, CH2 CH, J = 12.1, 3.8 Hz), 4.48 (s, 2H, CH2), 4.03 (dd, 1H, CH 2CH, J = 16.6, 12.1 Hz), 3.76 (s, 3H, OCH3), 3.40 (dd, 1H, CH 2CH, J = 16.6, 3.8 Hz). 13C NMR (100 MHz, DMSO-d 6): δ 170.2 (C O), 165.1 (C O), 164.3 (C O), 160.8 (C O), 159.2, 157.9 (C N), 137.8, 137.3, 134.4, 133.6, 133.2, 129.2, 129.1, 129.0, 128.8, 128.5, 128.2, 128.1, 127.9, 127.6, 127.4, 123.7, 121.4, 119.4, 114.7, 60.4 (CHCH2), 55.6 (OCH3), 44.2 (CH2), 42.8 (CHCH2). Calcd. for C33H25ClN4O5S: C, 63.41; H, 4.03; N, 8.96; Found: C, 63.59; H, 4.18; N, 9.09%.
4.3. Pharmacology
4.3.1. Primary anticancer assay
Primary anticancer assay was performed on a panel of approximately sixty human tumor cell lines derived from nine neoplastic diseases, in accordance with the protocol of the Drug Evaluation Branch, National Cancer Institute, Bethesda [30], [31], [32], [33]. Tested compounds were added to the culture at a single concentration (10−5 M) and the cultures were incubated for 48 h. End point determinations were made with a protein binding dye, sulforhodamine B (SRB). Results for each tested compound were reported as the percent of growth of the treated cells when compared to the untreated control cells. The percentage growth was evaluated spectrophotometrically versus controls not treated with test agents. The cytotoxic and/or growth inhibitory effects of the most active selected compounds were tested in vitro against the full panel of human tumor cell lines at concentrations ranging from 10−4 to 10−8 M. 48-h continuous drug exposure protocol was followed and an SRB protein assay was used to estimate cell viability or growth.
Using absorbance measurements [time zero (Tz), control growth in the absence of drug (C), and test growth in the presence of drug (Ti)], the percentage growth was calculated for each drug concentration. Percentage growth inhibition was calculated as:
Dose response parameters (GI50, TGI) were calculated for each compound. Growth inhibition of 50% (GI50) was calculated from [(Ti − Tz)/(C − Tz)] × 100 = 50, which is the drug concentration resulting in a 50% lower net protein increase in the treated cells (measured by SRB staining) as compared to the net protein increase seen in the control cells. The drug concentration resulting in total growth inhibition (TGI) was calculated from Ti = Tz. Values were calculated for each of these parameters if the level of activity was reached; however, if the effect was not reached or was excessive, the value for that parameter was expressed as more or less than the maximum or minimum concentration tested. The lowest values were obtained with the most sensitive cell lines. Compounds having GI50 values ≤100 μM were declared to be active.
4.3.2. Methods for assay of antiviral activity
Primary antiviral assay was performed on a respiratory viruses panel (Flu A (H1N1), Flu A (H3N2), Flu A (H5N1), Flu B, SARS CoV) [43]. Compounds were diluted to 20 mg/ml in DMSO then eight half-log dilutions were prepared in MEM solution with 50 mg/ml gentamicin. Each dilution was added to 5 wells of a 96-well plate with 80–100% confluent cells, and three wells of each dilution were then infected with the test virus using a multiplicity of infection of <0.006 CCID50 per cell for each virus. Two wells remained uninfected as toxicity controls. A known active compound was run in parallel as a control. After cytopathic effect (CPE) was observed microscopically, plates were stained with neutral red dye for approximately 2 h, then supernatant dye was removed from the wells and the incorporated dye was extracted in 50:50 Sorensen citrate buffer/ethanol and read on a spectrophotometer at 540 nm. The optical density of test wells was converted to percent of cell and virus controls, then the concentration of test compound required to inhibit viral CPE by 50% (EC50) was calculated by regression analysis. The concentration of compound that would cause 50% cytotoxicity in the uninfected cells was similarly calculated (CC50). EC50 and CC50 were presented in μM. The selective index (SI) is the CC50 divided by EC50.
4.3.3. Anti-trypanosomal activity assay
Bloodstream forms of T. brucei brucei strain 90-13 and T. brucei gambiense Feo strain were cultured in HMI9 medium supplemented with 10% FCS at 37 °C under an atmosphere of 5% CO2 [44]. In all experiments, log-phase parasite cultures were harvested by centrifugation at 3000×g and immediately used. Drug assays were based on the conversion of a redox-sensitive dye (resazurin) to a fluorescent product by viable cells as previously described [45]. Drug stock solutions were prepared in pure DMSO. T. brucei bloodstream forms (105 cells/ml) were cultured in 96-well plates either in the absence or in the presence of different concentrations of inhibitors in a final volume of 200 μl. After a 72-h incubation, resazurin solution was added in each well at the final concentration of 45 μM and fluorescence was measured at 530 nm and 590 nm absorbance after a further 4-h incubation. The percentage of inhibition of parasite growth rate was calculated by comparing the fluorescence of parasites maintained in the presence of drug to that of in the absence of drug. DMSO was used as control. Concentration inhibiting 50% of parasite growth (IC50) was determined from the dose–response curve with a drug concentrations ranging from 10 μg/ml to 0.625 μg/ml and presented in μM. IC50 value is the mean +/− the standard deviation of three independent experiments.
Acknowledgments
We are grateful to Dr. V.L. Narayanan from Drug Synthesis and Chemistry Branch, National Cancer Institute, Bethesda, MD, USA, for in vitro evaluation of anticancer activity. Evaluations of compounds for antiviral activity were supported by funds from contract N01-AI-15435 from the Virology Branch, Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
References
- 1.Lesyk R., Zimenkovsky B. 4-Thiazolidones: centenarian history, current status and perspectives for modern organic and medicinal chemistry. Curr. Org. Chem. 2004;8:1547–1577. [Google Scholar]
- 2.Kumar S., Bawa S., Drabu S., Kumar R., Gupta H. Biological activities of pyrazoline derivatives – a recent development. Recent Pat. Anti-Infect. Drug Discovery. 2009;4:154–163. doi: 10.2174/157489109789318569. [DOI] [PubMed] [Google Scholar]
- 3.Shaaban M.R., Mayhoub A.S., Farag A.M. Recent advances in the therapeutic applications of pyrazolines. Expert Opin. Ther. Pat. 2012;22(3):253–291. doi: 10.1517/13543776.2012.667403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng W., Degterev A., Hsu E., Yuan J., Yuan C. Structure–activity relationship study of a novel necroptosis inhibitor, necrostatin-7. Bioorg. Med. Chem. Lett. 2008;18:4932–4935. doi: 10.1016/j.bmcl.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 5.Carter P.H., Scherle P.A., Muckelbauer J.K., Voss M.E., Liu R.Q., Thompson L.A., Tebben A.J., Solomon K.A., Lo Y.C., Li Z., Strzemienski P., Yang G., Falahatpisheh N., Xu M., Wu Z., Farrow N.A., Ramnarayan K., Wang J., Rideout D., Yalamoori D.V., Domaille P., Underwood P.D.J., Trzaskos J.M., Friedman S.M., Newton R.C., Decicco C.P. Photochemically enhanced binding of small molecules to the tumor necrosis factor receptor-1 inhibits the binding of TNF-α. Proc. Natl. Acad. Sci. 2001;98:11879–11884. doi: 10.1073/pnas.211178398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geronikaki A., Eleftheriou P., Vicini P., Alam I., Dixit A., Saxena A.K. 2-Thiazolylimino/heteroarylimino-5-arylidene-4-thiazolidinones as new agents with SHP-2 inhibitory action. J. Med. Chem. 2008;51:5221–5228. doi: 10.1021/jm8004306. [DOI] [PubMed] [Google Scholar]
- 7.Havrylyuk D., Zimenkovsky B., Vasylenko O., Zaprutko L., Gzella A., Lesyk R. Synthesis of novel thiazolone-based compounds containing pyrazoline moiety and evaluation of their anticancer activity. Eur. J. Med. Chem. 2009;44:1396–1404. doi: 10.1016/j.ejmech.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 8.Havrylyuk D., Kovach N., Zimenkovsky B., Lesyk R. Synthesis of new 4-azolidinones with 3,5-diaryl-4,5-dihydropyrazole moiety and evaluation of their antitumor activity in vitro. Ann. Univ. Mariae. Curie. Sklodowska. 2010;23:107–110. [Google Scholar]
- 9.Leite A.C.L., Moreira D.R.M., Cardoso M.V.O., Hernandes M.Z., Pereira V.R.A., Silva R.O., Kiperstok A.C., Lima M.S., Soares M.B.P. Synthesis, cruzain docking, and in vitro studies of aryl-4-oxothiazolylhydrazones against Trypanosoma cruzi. Chem. Med. Chem. 2007;2:1339–1345. doi: 10.1002/cmdc.200700022. [DOI] [PubMed] [Google Scholar]
- 10.Greenbaum D.C., Mackey Z., Hansell E., Doyle P., Gut J., Caffrey C.R., Lehrman J., Rosenthal P.J., McKerrow J.H., Chibale K. Synthesis and structure–activity relationships of parasiticidal thiosemicarbazone cysteine protease inhibitors against plasmodium falciparum, Trypanosoma brucei, and Trypanosoma cruzi. J. Med. Chem. 2004;47:3212–3219. doi: 10.1021/jm030549j. [DOI] [PubMed] [Google Scholar]
- 11.Smith T.K., Young B.L., Denton H., Hughes D.L., Wagner G.K. First small molecular inhibitors of T. brucei dolicholphosphate mannose synthase (DPMS), a validated drug target in African sleeping sickness. Bioorg. Med. Chem. Lett. 2009;19:1749–1752. doi: 10.1016/j.bmcl.2009.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pizzo C., Saiz C., Talevi A., Gavernet L., Palestro P., Bellera C., Blanch L.B., Benitez D., Cazzulo J.J., Chidichimo A., Wipf P., Mahler S.G. Synthesis of 2-hydrazolyl-4-thiazolidinones based on multicomponent reactions and biological evaluation against T. Cruzi. Chem. Biol. Drug Des. 2011;77:166–172. doi: 10.1111/j.1747-0285.2010.01071.x. [DOI] [PubMed] [Google Scholar]
- 13.Du X., Guo C., Hansell E., Doyle P.S., Caffrey C.R., Holler T.P., McKerrow J.H., Cohen F.E. Synthesis and structure–activity relationship study of potent trypanocidal thio semicarbazone inhibitors of the trypanosomal cysteine protease cruzain. J. Med. Chem. 2002;45:2695–2707. doi: 10.1021/jm010459j. [DOI] [PubMed] [Google Scholar]
- 14.Rawal R.K., Tripathi R.K., Katti S.B., Pannecouque C., De Clercq E. Design, synthesis, and evaluation of 2-aryl-3-heteroaryl-1,3-thiazolidin-4-ones as anti-HIV agents. Bioorg. Med. Chem. 2007;15:1725–1731. doi: 10.1016/j.bmc.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Balzarini J., Orzeszko B., Maurin J.K., Orzeszko A. Synthesis and anti-HIV studies of 2-adamantyl-substituted thiazolidin-4-ones. Eur. J. Med. Chem. 2007;42:993–1003. doi: 10.1016/j.ejmech.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Barreca M.L., Chimirri A., De Luca L., Monforte A.M., Monforte P., Rao A., Zappala M., Balzarini J., De Clercq E., Pannecouque C., Witvrouw M. Discovery of 2,3-diaryl-1,3-thiazolidin-4-ones as potent anti-HIV-1 agents. Bioorg. Med. Chem. Lett. 2001;11:1793–1796. doi: 10.1016/s0960-894x(01)00304-3. [DOI] [PubMed] [Google Scholar]
- 17.Rawal R.K., Tripathi R., Katti S.B., Pannecouque C., De Clercq E. Design and synthesis of 2-(2,6-dibromophenyl)-3-heteroaryl-1,3-thiazolidin-4-ones as anti-HIV agents. Eur. J. Med. Chem. 2008;43:2800–2806. doi: 10.1016/j.ejmech.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Rawal R.K., Tripathi R., Katti S.B., Pannecouque C., De Clercq E. Synthesis and evaluation of 2-(2,6-dihalophenyl)-3-pyrimidinyl-1,3-thiazolidin-4-one analogues as anti-HIV-1 agents. Bioorg. Med. Chem. 2007;15:3134–3142. doi: 10.1016/j.bmc.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 19.Ravichandran V., Prashantha Kumar B.R., Sankar S., Agrawal R.K. Predicting anti-HIV activity of 1,3,4-thiazolidinone derivatives: 3D-QSAR approach. Eur. J. Med. Chem. 2009;44:1180–1187. doi: 10.1016/j.ejmech.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 20.Chen H., Bai J., Jiao L., Guo Z., Yin Q., Li X. Design, microwave-assisted synthesis and HIV-RT inhibitory activity of 2-(2,6-dihalophenyl)-3-(4,6-dimethyl-5-(un)substituted-pyrimidin-2-yl)thiazolidin-4-ones. Bioorg. Med. Chem. 2009;17:3980–3986. doi: 10.1016/j.bmc.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 21.Rawal R.K., Katti S.B., Kaushik-Basu N., Arora P., Pan Z. Non-nucleoside inhibitors of the hepatitis C virus NS5B RNA-dependant RNA polymerase: 2-aryl-3-heteroaryl-1,3-thiazolidin-4-one derivatives. Bioorg. Med. Chem. Lett. 2008;18:6110–6114. doi: 10.1016/j.bmcl.2008.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Husain M.I., Shukla S. Synthesis and biological activity of 4-(3-aryl-4-oxo-2-thioxothiazolidin-5-ylimino)-3-methyl-1-(N,N-disubstituted aminomethyl)pyrazolin-5-ones. Indian J. Chem. 1986;25B:983–985. [Google Scholar]
- 23.El-Sabbagh O.I., Baraka M.M., Ibrahim S.M., Pannecouque C., Andrei G., Snoeck R., Balzarini J., Rashad A.A. Synthesis and antiviral activity of new pyrazole and thiazole derivatives. Eur. J. Med. Chem. 2009;44:3746–3753. doi: 10.1016/j.ejmech.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 24.Havrylyuk D.Ya., Zimenkovsky B.S., Vasylenko O.M., Lesyk R.B. Synthesis and anticancer and antiviral activities of new 2-pyrazoline-substituted 4-thiazolidinones. J. Heterocyclic Chem. 2013;50:E55–E62. [Google Scholar]
- 25.Palaska E., Aytemir V., Uzbay I.T., Erol D. Synthesis and antidepressant activities of some 3,5-diphenyl-2-pyrazolines. Eur. J. Med. Chem. 2001;36:539–543. doi: 10.1016/s0223-5234(01)01243-0. [DOI] [PubMed] [Google Scholar]
- 26.Zask A., Jirkovsky I., Nowicki J.W., McCaleb M.L. Synthesis and antihyperglycemic activity of novel 5-(naphthalenylsulfonyl)-2,4-thiazolidinediones. J. Med. Chem. 1990;33:1418–1423. doi: 10.1021/jm00167a022. [DOI] [PubMed] [Google Scholar]
- 27.Kaminskyy D., Zimenkovsky B., Lesyk R. Synthesis and in vitro anticancer activity of 2,4-azolidinedione-acetic acids derivatives. Eur. J. Med. Chem. 2009;44:3627–3636. doi: 10.1016/j.ejmech.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 28.Kaminskyy D.V., Lesyk R.B. Structure–anticancer activity relationships among 4-azolidinone-3-carboxylic acids derivatives. Biopolym. Cell. 2010;26:136–145. [Google Scholar]
- 29.Havrylyuk D.Ya., Lesyk R.B. Synthesis and evaluation of antitumor activity of 5-[2-(3,5-diaryl-4,5-dihydropyrazol-1-yl)-2-oxoethylidene]-2,4-thiazolidinediones. Farmatsevtychnyy Zhurnal. 2009;3:51–55. (in Ukrainian) Chem Abstr 2009, 152:381257. [Google Scholar]
- 30.Monks A., Scudiero D., Skehan P., Shoemaker R., Paull K., Vistica D., Hose C., Langley J., Cronise P., Vaigro-Wolff A., Gray-Goodrich M., Campbell H., Mayo J., Boyd M. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Nat. Cancer Inst. 1991;83(11):757–766. doi: 10.1093/jnci/83.11.757. [DOI] [PubMed] [Google Scholar]
- 31.Boyd M.R., Paull K.D. Some practical considerations and applications of the national cancer institute in vitro anticancer drug discovery screen. Drug Dev. Res. 1995;34:91–109. [Google Scholar]
- 32.Boyd M.R. In: Teicher B.A., editor. Vol. 2. Humana Press; 1997. pp. 23–43. (Cancer Drug Discovery and Development). [Google Scholar]
- 33.Shoemaker R.H. The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. Cancer. 2006;6:813–823. doi: 10.1038/nrc1951. [DOI] [PubMed] [Google Scholar]
- 34.Havrylyuk D., Zimenkovsky B., Lesyk R. Synthesis and anticancer activity of novel nonfused bicyclic thiazolidinone derivatives. Phosphorus, Sulfur Silicon. 2009;184:638–650. [Google Scholar]
- 35.Zimenkovsky B., Kazmirchuk G., Zaprutko L., Paraskiewicz A., Melzer E., Lesyk R. New 5-arylidene-4-thiazolidinones and their anticancer activity. Ann. Polish Chem. Soc. 2005;1:69–72. [Google Scholar]
- 36.Kaminskyy D., Khyluk D., Vasylenko O., Zaprutko L., Lesyk R. A facile synthesis and anticancer activity evaluation of spiro[thiazolidinone-isatin] conjugates. Sci. Pharm. 2011;79:763–777. doi: 10.3797/scipharm.1109-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lesyk R.B., Zimenkovsky B.S., Kaminskyy D.V., Kryshchyshyn A.P., Havrylyuk D.Ya., Atamanyuk D.V., Subtel'na I.Yu., Khyluk D.V. Thiazolidinone motif in anticancer drug discovery. Experience of DH LNMU medicinal chemistry scientific group. Biopolym. Cell. 2011;27:107–117. [Google Scholar]
- 38.Havrylyuk D., Mosula L., Zimenkovsky B., Vasylenko O., Gzella A., Lesyk R. Synthesis and anticancer activity evaluation of 4-thiazolidinones containing benzothiazole moiety. Eur. J. Med. Chem. 2010;45:5012–5021. doi: 10.1016/j.ejmech.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 39.Havrylyuk D., Kovach N., Zimenkovsky B., Vasylenko O., Lesyk R. Synthesis and anticancer activity of isatin-based pyrazolines and thiazolidines conjugates. Arch. Pharm. Chem. Life Sci. 2011;344:514–522. doi: 10.1002/ardp.201100055. [DOI] [PubMed] [Google Scholar]
- 40.Mosula L., Zimenkovsky B., Havrylyuk D., Missir A.-V., Chirita I.C., Lesyk R. Synthesis and antitumor activity of novel 2-thioxo-4-thiazolidinones with benzothiazole moieties. Farmacia. 2009;57:321–330. [Google Scholar]
- 41.Havrylyuk D., Zimenkovsky B., Vasylenko O., Gzella A., Lesyk R. Synthesis of new 4–thiazolidinone-, pyrazoline-, and isatin-based conjugates with promising antitumor activity. J. Med. Chem. 2012;55:8630–8641. doi: 10.1021/jm300789g. [DOI] [PubMed] [Google Scholar]
- 42.Panchuk R.R., Chumak V.V., Fil' M.R., Havrylyuk D.Ya., Zimenkovsky B.S., Lesyk R.B., Stoika R.S. Study of molecular mechanisms of proapoptotic action of novel heterocyclic 4-thiazolidone derivatives. Biopolym. Cell. 2012;28(2):121–128. [Google Scholar]
- 43.Sidwell R.W., Smee D.F. In vitro and in vivo assay systems for study of influenza virus inhibitors. Antiviral. Res. 2000;48:1–16. doi: 10.1016/s0166-3542(00)00125-x. [DOI] [PubMed] [Google Scholar]
- 44.Bastos I., Motta F., Charneau S., Santana J., Dubost L., Augustyns K., Grellier P. Prolyl oligopeptidase of Trypanosoma brucei hydrolyzes native collagen, peptide hormones and is active in the plasma of infected mice. Microbe. Infect. 2010;12:457–466. doi: 10.1016/j.micinf.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 45.Lethu S., Bosc D., Mouray E., Grellier P., Dubois J. New protein farnesyltransferase inhibitors in the 3-arylthiophene 2-carboxylic acid series: diversification of the aryl moiety by solid-phase synthesis. J. Enzyme Inhib. Med. Chem. 2013;1:163–171. doi: 10.3109/14756366.2011.643302. [DOI] [PubMed] [Google Scholar]


