Abstract
Glycyrrhetinic acid (GA) is a major constituent of the herb Glycyrrhiza glabra, and many of its derivatives demonstrate a broad spectrum of antiviral activities. In the current study, 18 water-soluble β-cyclodextrin (CD)-GA conjugates, in which GA was covalently coupled to the primary face of β-CD using 1,2,3-triazole moiety along with varying lengths of linker, were synthesized via copper-catalyzed azide-alkyl cycloaddition reaction. Benefited from the attached β-CD moiety, all these conjugates showed lower hydrophobicity (AlogP) compared with their parent compound GA. With the exception of per-O-methylated β-CD-GA conjugate (35), all other conjugates showed no significant cytotoxicity to MDCK cells, and these conjugates were then screened against A/WSN/33 (H1N1) virus using the cytopathic effect assay. The preliminary results indicated that six conjugates showed promising antiviral activity, and the C-3 and C-30 of GA could tolerate some modifications. Our findings suggested that GA could be used as a lead compound for the development of potential anti-influenza virus agents.
Keywords: Glycyrrhetinic acid, Cyclodextrin, Click reaction, Influenza virus, Inhibitors
Graphical abstract
Highlights
-
•
Triterpene natural products are proposed as a new class of compounds for blocking influenza virus entry.
-
•
A total of 18 β-cyclodextrin-glycyrrhetinic acid conjugates were designed and synthesized via click chemistry.
-
•
The structure-activity relationship of their anti-influenza A/WSN/33 (H1N1) viruses was summarized.
1. Introduction
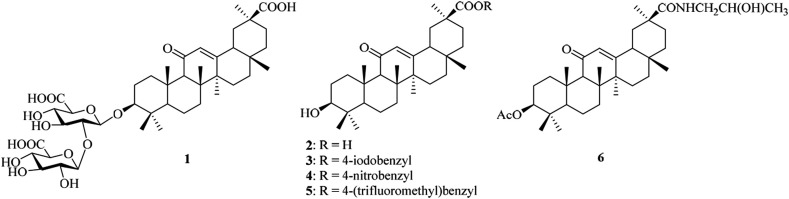
Licorice, the roots of the perennial herb Glycyrrhiza glabra which are endemic to Mediterranean and certain areas of Asia, has been one of the oldest and most extensively used medicinal plants [1]. Pharmacologically active components that have been most studied include triterpenoids (3–5%), with glycyrrhizic acid (also known as glycyrrhizin, 1) being present in the highest concentration, and flavonoids (1–1.5%) [2]. Glycyrrhizic acid (1), composed of one molecule of glycyrrhetinic acid (GA, 2) and two molecules of glucuronic acid (Fig. 1 ), can be hydrolyzed by β-glucuronidases in the intestinal bacteria [3]. The amount of 2 in licorice root is reported to be within the range of 0.1–1.6%, depending on species and growing region [4]. Compounds 1 and 2 have attracted considerable attention from chemists and pharmacologists because of their pharmacological and biological effects, such as anti-inflammatory, antitumor, antiviral and other activities [1,5].
Fig. 1.
Chemical structures of glycyrrhizic acid (1), GA (2) and its semi-synthetic derivatives (3–6) with antiviral activity.
A lot of studies have confirmed the antiviral activity of glycyrrhizic acid (1). In Japan, compound 1 has been used in the treatment of chronic viral hepatitis for more than 40 years as the intravenous drug Stronger Neo-Minophagen C (SNMC). It has shown that the administration of SNMC to patients with hepatitis C virus infection lowers the serum transaminase activity, even in the patients resistant to the interferon therapy [6]. Pompei et al. have reported that compound 1 can inhibit many DNA and RNA viruses, including HBV, HIV and EBV in vitro [7]. The antiviral activities of compound 1 against SARS-associated corona virus and influenza virus have also been demonstrated [8,9]. Recently, it has been noted that the application of compound 1 as a potential antiviral agent can be further improved by using certain drug delivery systems, e.g., mucoadhesive nanoparticles based on poly (methyl vinyl ether-co-maleic anhydride) (PVM/MA) [10].
Compared with glycyrrhizic acid (1), studies of the antiviral activity of its aglycone 2 are limited, but have attracted increasing attention in recent years. Lin et al. have claimed that compound 2 is 7.5-fold more active against EBV (EC50 = 4 μM) than its parent compound 1 (EC50 = 30 μM) [11]. Compound 2 shows significant antiviral activity against rotavirus replication at a step or steps subsequent to virus entry [12]. The semi-synthetic derivatives of 2, such as 4-iodobenzyl ester (3), 4-nitrobenzyl ester (4) and 4-(trifluoromethyl) benzyl ester (5), show potent inhibitory effects on HBV DNA replication activity with IC50 s at the micromolar level [13]. A recent study has indicated that GA derivative (6), with a 2-hydroxypropyl group at C-30 and an acetyl group at C-3, show remarkable antiviral activity against TK+ and TK− strains of HSV-1 with EC50 of 4.95 μM [14]. Despite the recognized pharmacological roles as antiviral agents, the main disadvantage of compound 2 and its derivative for application in the food or pharmaceutical industry is their low aqueous solubility due to its non-polar structure (logP: 6.75 [15]). It has reported that the solubility of compound 2 in water is only 10.6 μg/mL (37 °C), and the water/n-BuOH partition coefficient is 1.02 × 10−2 (37 °C) [16]. This combination of strong lipophilicity, low solubility and partition coefficient indicates its low bioavailability.
Some strategies have been assessed to overcome the limitation. Cyclodextrins (CDs) are a class of highly water-soluble and biocompatible cyclic oligosaccharides, which can reversely form host-guest inclusion complexes with a variety of guest molecules (drugs), thus improving certain properties of drugs, such as solubility, stability and bioavailability [17,18]. The CD-triterpene inclusion complexes have been also synthesized to increase the aqueous solubility of certain pentacyclic triterpenes [[19], [20], [21]]. Ishida et al. have reported that the complex of compound 2 and HP-γ-CD can improve the oral bioavailability and reduce mRNA expressions of TNF-α, IL-1β and IL-6 [22]. However, such a noncovalent complex is disadvantageous when drug targeting is to be attempted because the complex dissociates before it reaches the organs or tissues to which it is to be delivered [23]. Therefore, direct covalent linkage with β-CD has been suggested, which has been widely used in other water insoluble bioactive molecules, such as fullerene (C60), 5-FU, folic acid and artesunate [[24], [25], [26], [27]]. In our recent studies, a series of water-soluble triazole-bridged β-CD-pentacyclic triterpene conjugates have been synthesized via click chemistry [28,29].
As part of our continued interest in the structurally modified pentacyclic triterpene derivatives as antiviral inhibitors [[29], [30], [31], [32], [33]], we thought it value to prepare a wide range of pentacyclic triterpene derivatives to better explore their antiviral structure-activity relationship (SAR). Herein, we reported the synthesis and anti-influenza A/WSN/33 virus activity of a series of 1:1 β-CD-GA conjugates, in which GA (2) was covalently coupled to the primary face of β-CD via C-3 hydroxyl or C-30 carboxylic acid.
2. Results and discussion
2.1. Chemistry
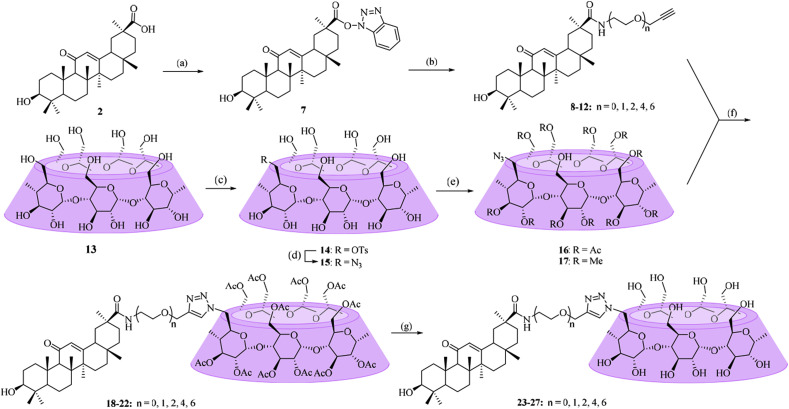
Scheme 1 illustrates the synthesis of β-CD-GA conjugates 23–27. The commercially available GA (2) was reacted with 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium tetrafluoroborate (TBTU) in THF to give the stable intermediate 7 in good yield, which proceeded towards a coupling reaction with different terminal alkynyl-functionalized primary amines under basic condition to install a flexible oligo (ethylene glycol) linker (8–12) in 46–82% yield. Then, 8–12 underwent a “click chemistry” reaction with 6A-azide-6A-deoxy-per-O-acetylated β-CD (16), which was prepared from the known β-CD (13) in three steps using the conventional method as previously described [34], in THF/H2O in the presence of a catalytic amount of copper sulfate and sodium ascorbate as reducing agent to yield 18–22 with yields ranging from 45% to 61%. At last, the acetyl groups of conjugates 18–22 were removed under Zemblén conditions [35] to afford 23–27 in 80–98% yields.
Scheme 1.
Reagents and conditions: (a) TBTU, DIPEA, THF, 90%; (b) Na2CO3, R—NH2, DMF, 60 °C, 46–82%; (c) TsCl, NaOH, H2O, 11%; (d) NaN3, DMF, 80 °C, 81%; (e) Ac2O, pyridine, DMAP, 86% for 16, or NaH, CH3I, DMF, 62% for 17; (f) sodium l-ascorbate, CuSO4, THF-H2O (1:1, v/v), 45–61%; (g) CH3ONa/CH3OH, 80–98%.
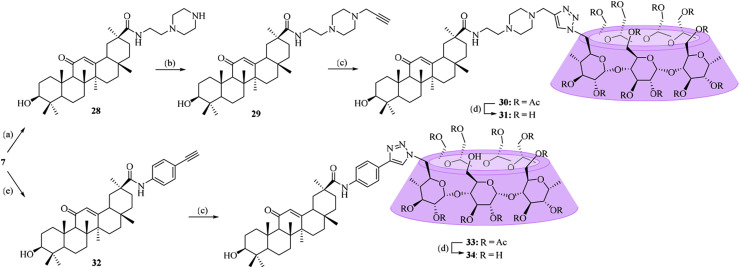
To increase the rigidness of the linker, the benzene and piperazine ring were introduced, and Scheme 2 describes the synthesis route for conjugates 30–31 and 33–34. The intermediate 7 was reacted either with an excess of 1-(2-aminoethyl) piperazine, followed by N-alkylation with propargyl bromide or with an excess of 4-ethynylaniline to give alkynyl-functionalized amides 29 and 32 in moderate yields. Similarly, coupling of compounds 29 and 32 with azide-functionalized per-O-acetylated β-CD (16) was performed via click reaction, followed by de-O-acetylation under Zemplén conditions to give the corresponding conjugates 31 and 34 as the final products.
Scheme 2.
Reagents and conditions: (a) 2-(piperazin-1-yl)ethan-1-amine, Na2CO3, DMF, 60 °C, 45%; (b) propargyl bromide, Na2CO3, DMF, 60 °C, 76%; (c) sodium l-ascorbate, CuSO4, THF-H2O (1:1, v/v), 42% for 30, 59% for 33; (d) CH3ONa/CH3OH, 90% for 31, 86% for 34; (e) 4-ethynylaniline, Na2CO3, DMF, 60 °C, 60%.
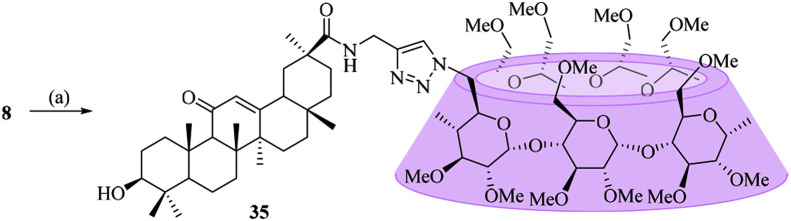
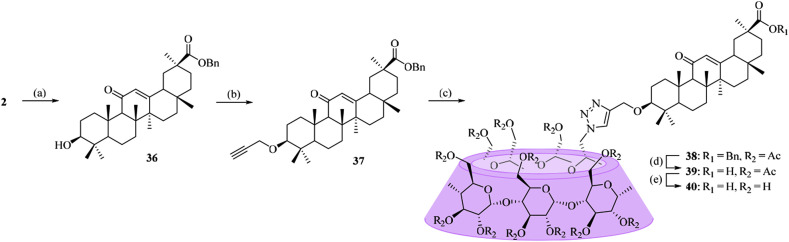
Unlike the β-CD-GA conjugates described above, two other conjugates (35 and 40), in which the per-O-methylated-β-CD was linked to the carboxylic acid at C-30 of GA or β-CD was linked to the hydroxyl at C-3 of GA, were designed. The method used to prepare conjugate 35 was outlined in Scheme 3 , and intermediate 8 was coupled with 6A-azide-6A-deoxy-per-O-methylated-β-CD (17) via click chemistry to furnish conjugate 35. Conjugate 40 was synthesized according to the procedure described in Scheme 4 . In order to synthesize C-3 alkynyl-functionalized compound 37 required for click reaction, the carboxylic acid group at C-30 of GA (2) was first protected by treatment with benzyl bromide in DMF, followed by O-alkylation at C-3 with propargyl bromide in the presence of sodium hydride. Coupling of compound 16 with O-propargyl GA derivative 37 was carried out via click reaction, followed by de-O-benzylation and de-O-acetylation reactions to give the conjugate 40 in 41% yield over three steps.
Scheme 3.
Reagents and conditions: (a) sodium l-ascorbate, 6A-azide-6A-deoxy-per-O-methy-β-CD (17), CuSO4, THF-H2O (1:1, v/v), 59%.
Scheme 4.
Reagents and conditions: (a) benzyl bromide, K2CO3, DMF, 50 °C, 80%; (b) NaH, propargyl bromide, THF, 65 °C, 60%; (c) sodium l-ascorbate, 6A-azide-6A-deoxy-per-O-acetylated-β-CD (16), CuSO4, THF-H2O (1:1, v/v), 60%; (d) Pt/C, H2, CH3OH, 76%; (e) CH3ONa/CH3OH, 81%.
2.2. The calculated ALogP
The logarithm of the n-octanol/water partition coefficient (logP) is a well-known measure of molecular lipophilicity [36]. It is used to provide invaluable information for the overall understanding of the uptake, distribution, biotransformation and elimination of a wide variety of chemicals. In our study, the calculated AlogP values were determined using Pipeline Pilot software, Vers. 7.5 (Accelrys Corporation, San Diego, USA) [37]. Due to the introduction of β-CD along with different linkers, all the conjugates showed increased hydrophilicity compared with their parent compound GA (2) (Table 1 ). A decreased ALogP in the order GA (2) » per-O-alkyl-β-CD-GA conjugates (18–22, 30, 33, 35 and 38–39) » β-CD-GA conjugates (23–27, 31, 34 and 40) was observed. Benefited from the per-O-alkyl β-CD moiety, the AlogP value was decreased about ∼2 units compared with GA (2). β-CD-GA conjugates showed the lowest ALogP values within the range of 0.09–0.77, indicating that their solubility in water was about 47,000-fold higher than their parent compound GA (2). As a matter of fact, during the biological assays of the prepared β-CD-GA conjugates, the parent compound GA (2) dissolved in water formed turbid solutions at a concentration of 50 μM and suspensions accompanied by a precipitate even when 5% DMSO (v/v) solutions were used. However, all the β-CD-GA conjugates exhibited greater water solubility generating clear solutions at the same concentration when 1% DMSO (v/v) solutions were used.
Table 1.
Calculated AlogP values of conjugatesa.
| Compd | AlogP | Compd | AlogP | Compd | AlogP |
|---|---|---|---|---|---|
| GA (2) | 5.45 | 24 | 0.11 | 34 | 0.77 |
| 18 | 3.55 | 25 | 0.10 | 35 | 3.28 |
| 19 | 3.63 | 26 | 0.15 | 38 | 4.07 |
| 20 | 3.66 | 27 | 0.26 | 39 | 3.58 |
| 21 | 3.61 | 30 | 3.50 | 40 | 0.34 |
| 22 | 3.61 | 31 | 0.12 | ||
| 23 | 0.09 | 33 | 3.99 |
AlogP values (Ghose and Crippen octanol-water partition coefficient at 25 °C) were calculated using Pipeline Pilot software, version 7.5 (Accelrys Corp., San Diego, CA, USA).
2.3. In vitro cytotoxic activity
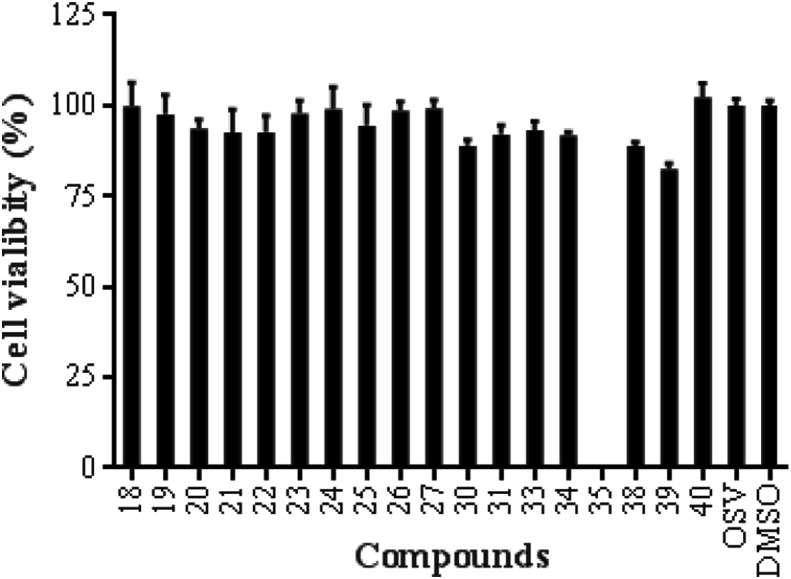
The in vitro cytotoxic activity was evaluated for all of the synthesized conjugates 18–27, 30–31, 33–35 and 38–40 using CellTiter-Glo® Assay. The results showed that most conjugates had no cytotoxicity against uninfected Madin-Daby canine kidney (MDCK) cells at a concentration of 50 μM, except for compound 35 possessing cytotoxicity at the same concentration (Fig. 2 ). This finding, together with our previous observation that per-O-methylated-β-CD derivatives of other pentacyclic triterpenes show different cytotoxicity at a concentration of 5–50 μM [28], indicated that per-O-methylated-β-CD might impart certain degree of cytotoxicity in vitro.
Fig. 2.
The in vitro cytotoxic screening of β-CD-GA conjugates 18–27, 30–31, 33–35 and 38–40 against MDCK cells using CellTiter-Glo® Assay.
2.4. Anti-influenza virus activity of β-CD-GA conjugates
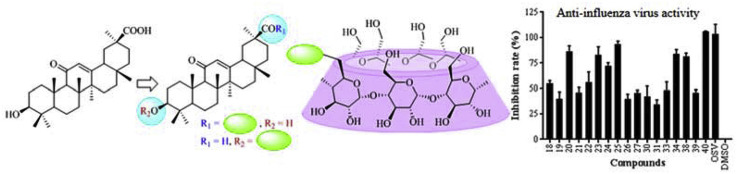
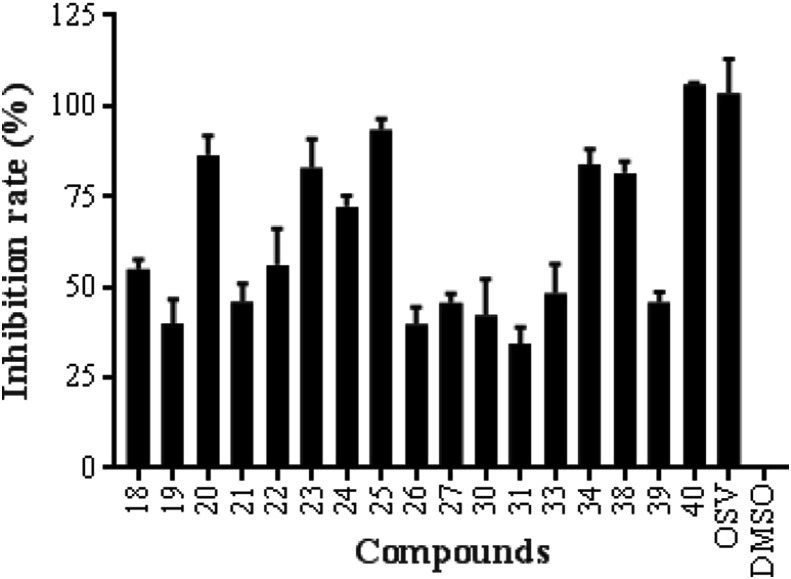
Except for compound 35 with certain cytotoxic activity as described above, the other 17 β-CD-GA conjugates were evaluated against the influenza virus A/WSN/33 (H1N1) that was propagated in MDCK cells at one concentration (50 μM) by the cytopathic effect (CPE) reduction assay. Oseltamivir (OSV) and DMSO were used as positive and negative controls, respectively. Fig. 3 lists the primarily screen results.
Fig. 3.
The CPE-based screen of β-CD-GA conjugates (50 μM). MDCK was utilized as the host cells to test A/WSN/33 virus infection; OSV and DMSO acted as positive and negative control, respectively. Error bars indicate standard deviations of triplicate experiments.
The five conjugates (23–27) with the GA and β-CD groups held constant, but with varying of the oligoethylene glycol linker revealed that compound 25 had the highest antiviral activity to A/WSN/33 (H1N1) virus, indicating 1,2,3-triazole moiety along with diethylene glycol linker could allow a better fit between GA and the proper target. Elongation or shortening of the diethylene glycol chain leads to some decrease of the antiviral activity. Similar results were also observed for conjugates 18–22, in which the β-CD was acetylated, and compound 20 showed the greatest antiviral activity. The diethylene glycol linker of compounds 20 and 25 was further modified by replacing one ethylene glycol moiety with piperazine ring to increase the rigidness of the linker, leading to greatly reduced activity (20 vs. 30, 25 vs. 31). However, the introduction of an aromatic linker (1,2,3-triazol-4-yl)phenyl between β-CD and GA still retained reasonable antiviral activity (23 vs. 34). To our surprise, the introduction of aromatic amino acids methyl esters at C-30 of glycyrrhizin also showed more potent anti-influenza activity than their parent compound [9], indicating an aromatic group at C-30 might be helpful for the binding of GA with its receptor. Evaluation of the three derivatives (18, 23 and 35) with the same linker of (1,2,3-triazol-4-yl)methyl at the C-30 of GA revealed that the substitution of β-CD showed important effect on the activity and cytotoxicity. The O-acetylation of β-CD reduced the activity (18 vs. 23), while the O-methylation of β-CD only resulted in the cytotoxicity (18 and 23 vs. 35). The carboxylic acid derivative 40, shift of the β-CD moiety from C-30 to C-3, had the highest antiviral activity of the new compounds reported in this study, indicating that the C-3 hydroxy of GA was tolerated. Compared with the weak anti-influenza A/H1N1/pdm09 virus activity of glycyrrhizin (1) (EC50 = 364.6 μM) [9], the effect of β-CD on the antiviral activity of GA is obviously more potent than that of two molecules of glucuronic acid.
3. Conclusions
In summary, a series of GA derivatives, altered at position C-3 hydroxy or C-30 carboxylic acid by attachment of β-CD with different linkers, were designed, synthesized and evaluated for their anti-influenza virus activity. The solubility of these β-CD-GA conjugates in water was much higher than their parent compound as deduced from the calculated AlogP values. Our current investigation indicated that the conjugation of per-O-methylated β-CD to GA displayed certain cytotoxicity toward MDCK cells. Six β-CD-GA conjugates showed promising antiviral activity for A/WSN/33 (H1N1) virus, while no cytotoxicity was observed. This study supported that these conjugates were potential candidates of anti-influenza virus agents.
4. Experimental
4.1. Chemistry
4.1.1. General information
High resolution mass spectra (ESI-HRMS) were obtained with a Thermo Scientific Q Exactive spectrometer (Bremen, Germany) in the positive ESI mode. NMR spectra were recorded on a Bruker DRX 400 or DRX 600 spectrometer at ambient temperature. 1H NMR chemical shifts were referenced to the internal standard TMS (δ H = 0.00) or the solvent signal (δ H = 3.31 for the central line of MeOD). 13C NMR chemical shifts were referenced to the solvent signal (δ C = 77.00 for the central line of CDCl3, δ C = 49.00 for the central line of MeOD). Reactions were monitored by thin-layer chromatography (TLC) on a pre-coated silica gel 60 F254 plate (layer thickness of 0.2 mm; E. Merck, Darmstadt, Germany) and detected by staining with a yellow solution containing Ce(NH4)2(NO3)6 (0.5 g) and (NH4)6Mo7O24 ·4H2O (24.0 g) in 6% H2SO4 (500 mL), followed by heating. Flash column chromatography was performed on silica gel 60 (200–300 mesh, Qingdao Haiyang Chemical Co., Ltd.).
The syntheses of 7, 8, 14–17 and 36 was performed as previously reported [34,[38], [39], [40]].
4.1.2. General procedure A for the click reaction
To a solution of alkyne (0.10 mmol) and azide (0.10 mmol) in 1:1 THF-H2O (5 mL), CuSO4 (15.7 mg, 0.10 mmol) and sodium l-ascorbate (40.9 mg, 0.20 mmol) were added. The resulting solution was vigorously stirred for 12 h at room temperature. The reaction mixture was extracted with CH2Cl2 (10 mL × 3). The combined organics were dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography.
4.1.3. General procedure B for the deacetylation reaction
The per-O-acetylated-β-CD-GA conjugate was dissolved in dry methanol (∼5 mL per 100 mg of compound), and a solution of sodium methoxide (30% in methanol, 0.1 eq per mol of acetate) was added. The solution was stirred at room temperature for 4–6 h. After completion (TLC), the reaction mixture was neutralized with Amberlite IR-120 (H+) ion exchange resin, filtered and concentrated. The crude product was purified by RP column chromatography (eluted by CH3OH).
4.1.4. General procedure C for the amidation reaction
To a solution of compound 7 (200 mg, 0.34 mmol) and terminal alkynyl substituted amine (0.43 mmol) in DMF (10 mL), Na2CO3 (72 mg, 0.68 mmol) was added. The resulting solution was vigorously stirred for 24 h at 60 °C. The solvent was removed by steaming. The residue was purified by column chromatography.
4.1.5. Synthesis of N-(2-(2-propyn-1-yloxy)ethyl)-3β-hydroxy-11-oxo-olean-12-en-30-amide (9)
Prepared from 7 and 2-(propyn-1-yloxy)-ethanamine according to general procedure C, the residue was purified by flash chromatography (eluent: petroleum ether:acetone = 4:1) to afford 9 as a white solid with a yield of 46%. Rf = 0.70 (petroleum ether:acetone = 1:1); m.p. 212–214 °C; 1H NMR (400 MHz, CDCl3): δ 6.09 (br s, 1H), 5.62 (s, 1H), 4.17 (d, 2H, J = 2.3 Hz), 3.61–3.39 (m, 4H), 3.20 (dd, 1H, J = 10.7, 5.5 Hz), 2.76 (td, 1H, J = 10.2, 3.1 Hz), 2.48 (t, 1H, J = 2.4 Hz), 2.31 (s, 1H), 2.17–1.02 (m, other aliphatic ring protons), 1.35 (s, 3H, CH3), 1.11 (s, 6H, 2 × CH3), 1.10, 0.98, (s, each 3H, 2 × CH3), 0.92 (dd, 1H, J = 12.9, 4.3 Hz), 0.79, 0.78 (s, each 3H, 2 × CH3), 0.67 (d, 1H, J = 11.7 Hz); 13C NMR (100 MHz, CDCl3): δ 200.03, 175.79, 169.08, 128.47, 79.36, 78.73, 77.05, 75.13, 68.69, 61.83, 58.28, 54.97, 48.07, 45.37, 43.62, 43.18, 41.90, 39.23, 39.14, 39.11, 37.47, 37.07, 32.77, 31.90, 31.44, 29.50, 28.51, 28.12, 27.27, 26.50, 26.41, 23.36, 18.66, 17.48, 16.36, 15.59; ESI-HRMS (m/z) Calcd for C35H53NO4 [M + H]+: 552.4047. Found 552.4041.
4.1.6. Synthesis of N-(2-(2-(2-propyn-1-yloxy)ethoxy)ethyl)-3β-hydroxy-11-oxo-olean-12-en-30-amide (10)
Prepared from 7 and 2-(2-(2-propyn-1-yloxy)ethoxy)-ethanamine according to general procedure C, the residue was purified by flash chromatography (eluent: petroleum ether:acetone = 3:1) to afford 10 as a white solid with a yield of 82%. Rf = 0.55 (petroleum ether:acetone = 1:1); m.p. 141–143 °C; 1H NMR (400 MHz, CDCl3): δ 6.22 (br s, 1H), 5.65 (s, 1H), 4.16 (d, 2H, J = 2.9 Hz), 3.69–3.39 (m, 8H), 3.19 (dd, 1H, J = 10.7, 5.4 Hz), 2.75 (td, 1H, J = 10.4, 3.4 Hz), 2.41 (t, 1H, J = 2.2 Hz), 2.30 (s, 1H), 2.14 (dd, 1H, J = 13.2, 3.3 Hz), 2.06–1.55 (m, 10H), 1.42–1.01 (m, other aliphatic ring protons), 1.35 (s, 3H, CH3), 1.10 (s, 9H, 3 × CH3), 0.97 (s, 3H, CH3), 0.91 (dd, 1H, J = 12.8, 3.9 Hz), 0.78 (2 × s, each 3H, 2 × CH3), 0.66 (d, 1H, J = 11.4 Hz); 13C NMR (100 MHz, CDCl3): δ 199.98, 175.71, 169.17, 128.29, 79.44, 78.56, 74.63, 69.88, 69.83, 68.91, 61.69, 58.24, 54.82, 47.96, 45.24, 43.48, 43.04, 41.62, 39.10, 39.03, 38.87, 37.34, 36.90, 32.61, 31.75, 31.31, 29.39, 28.43, 28.02, 27.11, 26.34, 26.26, 23.27, 18.52, 17.35, 16.27, 15.53; ESI-HRMS (m/z) Calcd for C37H57NO5 [M + H]+: 596.4310. Found 596.4300.
4.1.7. Synthesis of N-(3,6,9,12-tetraoxapentadec-14-yn-1-yl)-3β-hydroxy-11-oxo-olean-12-en-30-amide (11)
Prepared from 7 to 3,6,9,12-tetraoxapentadec-14-yn-1-amine according to general procedure C, the residue was purified by flash chromatography (eluent: DCM: MeOH = 20:1) to afford 11 as a white solid with a yield of 52%. Rf = 0.40 (eluent: DCM:MeOH = 10:1); m.p. 52–54 °C; 1H NMR (400 MHz, CDCl3): δ 6.22 (t, 1H, J = 5.5 Hz), 5.67 (s, 1H), 4.19 (d, 2H, J = 2.4 Hz), 3.71–3.61 (m, 12H), 3.57–3.54 (m, 2H), 3.52–3.39 (m, 2H), 3.22 (dd, 1H, J = 10.6, 5.6 Hz), 2.78 (td, 1H, J = 10.2, 3.4 Hz), 2.43 (d, 1H, J = 2.4 Hz), 2.32 (s, 1H), 2.19–2.15 (m, 1H), 2.07–2.00 (m, 1H), 1.95–1.92 (m, 1H), 1.87–0.99 (m, other aliphatic ring protons), 1.37 (s, 3H, CH3), 1.12 (s, 9H, 3 × CH3), 1.00 (s, 3H, CH3), 0.80 (2 × s, 6H, 2 × CH3), 0.69 (d, 1H, J = 11.7 Hz); 13C NMR (100 MHz, CDCl3): δ 199.94, 175.74, 169.11, 128.47, 79.59, 78.76, 74.56, 70.58, 70.50, 70.46, 70.37, 70.21, 70.02, 69.09, 61.82, 58.39, 54.95, 48.03, 45.34, 43.57, 43.19, 41.87, 39.16, 39.13, 37.49, 37.07, 32.77, 31.86, 31.48, 29.46, 28.53, 28.09, 27.29, 26.49, 26.43, 23.38, 18.67, 17.48, 16.35, 15.56; ESI-HRMS (m/z) Calcd for C41H65NO7 [M + NH4]+: 701.5099. Found 701.5090.
4.1.8. Synthesis of N-(3,6,9,12,15,18-hexaoxaheneicos-20-yn-1-yl)-3β-hydroxy-11-oxo-olean-12-en-30-amide (12)
Prepared from 7 to 3,6,9,12,15,18-hexaoxaheneicos-20-yn-1-amine according to general procedure C, the residue was purified by flash chromatography (eluent: petroleum ether:acetone = 4:1) to afford 12 as a yellow oil with a yield of 52%. Rf = 0.35 (petroleum ether:acetone = 1:1); 1H NMR (400 MHz, CDCl3): δ 6.30 (t, 1H, J = 5.5 Hz), 5.64 (s, 1H), 4.17–4.16 (m, 2H), 3.67–3.40 (m, 25H), 3.18 (dd, 1H, J = 10.8, 4.7 Hz), 2.74 (td, 1H, J = 10.4, 2.6 Hz), 2.41 (d, 1H, J = 2.4 Hz), 2.29 (s, 1H), 2.15–0.88 (m, other aliphatic ring protons), 1.34 (s, 3H, CH3), 1.09 (s, 9H, 3 × CH3), 0.97 (s, 3H, CH3),0.77 (s, 6H, 2 × CH3), 0.64 (d, 1H, J = 11.4 Hz); 13C NMR (100 MHz, CDCl3): δ 199.84, 175.71, 169.05, 128.35, 79.57, 78.60, 74.49, 70.48, 70.46, 70.44, 70.37, 70.29, 70.10, 69.98, 68.99, 61.73, 58.29, 54.89, 47.96, 45.26, 43.47, 43.10, 41.73, 39.14, 39.04, 37.41, 36.98, 32.69, 31.77, 31.37, 29.35, 28.45, 28.04, 27.18, 26.41, 26.35, 23.29, 18.58, 17.40, 16.26, 15.52; ESI-HRMS (m/z) Calcd for C45H73NO9 [M + NH4]+: 789.5624. Found 789.5611.
4.1.9. Synthesis of N-(1-(6A-deoxy-per-O-acetylated-β-cyclodextrin-6-yl)-1H-1,2,3-triazol-4-yl)methyl-3β-hydroxy-11-oxo-olean-12-en-30-amide (18)
Prepared from 8 and 16 according to general procedure A, the residue was purified by flash chromatography (eluent: petroleum ether:acetone = 1:1) to afford 18 as a white foam with a yield of 52%. Rf = 0.12 (petroleum ether:acetone = 1:1); 1H NMR (600 MHz, CDCl3): δ 7.51 (s, 1H), 6.64 (t, 1H, J = 5.2 Hz), 5.61 (s, 1H), 5.46 (d, 1H, J = 3.9 Hz), 5.35–5.16 (m, 7H), 5.15 (d, 1H, J = 3.5 Hz), 5.11 (d, 1H, J = 4.0 Hz), 5.08 (d, 1H, J = 4.0 Hz), 5.04 (t, 2H, J = 4.1 Hz), 4.99 (d, 1H, J = 3.4 Hz), 4.93–4.72 (m, 8H), 4.66 (d, 1H, J = 11.7 Hz), 4.62 (dd, 1H, J = 15.4, 6.1Hz), 4.57–4.51 (m, 5H), 4.46 (dd, 1H, J = 15.3, 4.9 Hz), 4.34–4.08 (m, 13H), 3.98 (d, 1H, J = 9.1 Hz), 3.87 (dd, 1H, J = 12.4, 3.4 Hz), 3.74–3.68 (m, 5H), 3.63 (t, 1H, J = 8.8 Hz), 3.53 (t, 1H, J = 9.1 Hz), 3.20 (d, 1H, J = 10.3 Hz), 2.74 (td, 1H, J = 10.4, 3.3 Hz), 2.29 (s, 1H), 2.19–1.80 (m, 75H), 1.68–1.56 (m, 5H), 1.44–0.91 (m, other aliphatic ring protons), 1.35 (s, 3H, CH3), 1.10 (s, 9H, 3 × CH3), 0.98, 0.79, 0.78 (s, each 3H, 3 × CH3), 0.67 (d, 1H, J = 11.6 Hz); 13C NMR (150 MHz, CDCl3): 199.84, 175.94, 170.84, 170.79, 170.72, 170.65, 170.60, 170.59, 170.57, 170.53, 170.51, 170.39, 170.36, 170.25, 169.50, 169.44, 169.39, 169.35, 169.32, 169.30, 169.19, 168.95, 144.82, 128.43, 124.48, 97.11, 96.91, 96.89, 96.82, 96.79, 96.57, 96.40, 78.70, 78.25, 77.12, 77.03, 76.94, 76.56, 76.47, 75.92, 71.68, 71.27, 71.19, 71.01, 70.76, 70.61, 70.33, 70.23, 70.17, 70.05, 69.92, 69.86, 69.71, 69.64, 69.48, 69.29, 69.27, 62.79, 62.75, 62.61, 62.46, 62.33, 62.17, 61.73, 54.88, 49.74, 48.01, 45.26, 43.49, 43.15, 41.43, 39.11, 39.07, 37.44, 37.01, 35.04, 32.73, 31.85, 31.37, 29.32, 28.44, 28.05, 27.21, 26.44, 26.39, 23.32, 20.90, 20.77, 20.74, 20.72, 20.70, 20.66, 20.62, 18.66, 17.45, 16.30, 15.53; ESI-HRMS (m/z) Calcd for C115H159N4O57 [M + H]+: 2507.9661. Found 2507.9614.
4.1.10. Synthesis of N-(2-((1-(6A-deoxy-per-O-acetylated-β-cyclodextrin-6-yl)-1H-1,2,3-triazol-4-yl)methoxy)ethyl)-3β-hydroxy-11-oxo-olean-12-en-30-amide (19)
Prepared from 9 and 16 according to general procedure A, the residue was purified by flash chromatography (eluent: petroleum ether:acetone = 1:1) to afford 19 as a white foam with a yield of 60%. Rf = 0.20 (petroleum ether:acetone = 1:1); 1H NMR (600 MHz, CDCl3): δ 7.69 (s, 1H), 6.26 (t, 1H, J = 5.3 Hz), 5.63 (s, 1H), 5.59 (d, 1H, J = 3.8 Hz), 5.35–5.16 (m, 9H), 5.09 (d, 1H, J = 4.1 Hz), 5.07 (d, 1H, J = 4.0 Hz), 5.04 (d, 1H, J = 3.6 Hz), 5.03 (d, 1H, J = 3.7 Hz), 4.98 (d, 1H, J = 3.5 Hz), 4.92 (dd, 1H, J = 8.6, 4.0 Hz), 4.83–4.79 (m, 3H), 4.73 (dd, 1H, J = 9.8, 3.6 Hz), 4.67–4.51 (m, 9H), 4.47 (dd, 1H, J = 10.4, 3.5 Hz), 4.43 (d, 1H, J = 11.5 Hz), 4.30–4.05 (m, 14H), 3.77–3.42 (m, 11H), 3.18 (dd, 1H, J = 11.3, 4.8 Hz), 2.74 (td, 1H, J = 10.3, 3.4 Hz), 2.30 (s, 1H), 2.17–1.93 (m, 75H), 1.81–1.00 (m, other aliphatic ring protons), 1.34, 1.10 (s, each 3H, 2 × CH3), 1.09 (s, 6H, 2 × CH3), 0.97 (s, 3H, CH3), 0.92 (dd, 1H, J = 13.4, 3.2 Hz), 0.78, 0.77 (s, each 3H, 2 × CH3), 0.66 (d, 1H, J = 11.3 Hz); 13C NMR (150 MHz, CDCl3): δ 199.92, 175.73, 170.80, 170.69, 170.66, 170.63, 170.59, 170.52, 170.45, 170.43, 170.35, 170.31, 170.22, 170.19, 169.50, 169.33, 169.31, 169.25, 169.22, 169.18, 169.11, 144.31, 128.33, 125.67, 97.02, 96.89, 96.72, 96.66, 96.55, 96.52, 96.31, 78.55, 77.34, 76.95, 76.81, 76.57, 76.50, 75.65, 71.54, 71.36, 71.26, 70.92, 70.86, 70.36, 70.29, 70.11, 69.97, 69.90, 69.82, 69.71, 69.59, 69.55, 69.52, 69.40, 69.22, 64.18, 62.67, 62.61, 62.53, 62.34, 62.15, 61.72, 54.87, 49.21, 47.97, 45.26, 43.50, 43.12, 41.74, 39.14, 39.11, 39.05, 37.41, 37.02, 32.69, 31.77, 31.41, 29.33, 28.48, 28.03, 27.21, 26.42, 26.36, 20.79, 20.77, 20.75, 20.72, 20.69, 20.67, 20.64, 20.59, 20.56, 18.62, 17.41, 16.30, 15.52; ESI-HRMS (m/z) Calcd for C117H162N4O58 [M + H]+: 2551.9923. Found 2551.9897.
4.1.11. Synthesis of N-(2-(2-((1-(6A-deoxy-per-O-acetylated-β-cyclodextrin-6-yl)-1H-1,2,3-triazol-4-yl)methoxy)ethoxy)ethyl)-3β-hydroxy-11-oxo-olean-12-en-30-amide (20)
Prepared from 10 and 16 according to general procedure A, the residue was purified by flash chromatography (eluent: petroleum ether: acetone = 1:1) to afford 20 as a white foam with a yield of 50%. Rf = 0.30 (petroleum ether:acetone = 1:1); 1H NMR (600 MHz, CDCl3): δ 7.64 (s, 1H), 6.19 (t, 1H, J = 5.4 Hz), 5.67 (s, 1H), 5.63 (d, 1H, J = 3.8 Hz), 5.37–5.17 (m, 9H), 5.12 (d, 1H, J = 4.1 Hz), 5.09 (d, 1H, J = 4.0 Hz), 5.06 (d, 1H, J = 3.6 Hz), 5.04 (d, 1H, J = 3.7 Hz), 5.00 (d, 1H, J = 3.5 Hz), 4.93 (dd, 1H, J = 8.6, 4.0 Hz), 4.84–4.81 (m, 3H), 4.76–4.73 (m, 2H), 4.69–4.66 (m, 3H), 4.62 (dd, 1H, J = 15.0, 4.4 Hz), 4.58–4.46 (m, 6H), 4.31–4.06 (m, 14H), 3.77–3.40 (m, 15H), 3.20 (dd, 1H, J = 11.3, 4.9 Hz), 2.76 (td, 1H, J = 10.2, 3.3 Hz), 2.32 (s, 1H), 2.16–2.00 (m, 73H), 1.92–1.57 (m, 12H), 1.43–1.01 (m, other aliphatic ring protons), 1.36, 1.11 (s, each 3H, 2 × CH3), 1.10 (s, 6H, 2 × CH3), 0.99 (s, 3H, CH3), 0.94 (dd, 1H, J = 13.2, 3.9 Hz), 0.79 (2 × s, each 3H, 2 × CH3), 0.68 (d, 1H, J = 11.3 Hz); 13C NMR (150 MHz, CDCl3): δ 199.93, 175.73, 170.84, 170.73, 170.69, 170.63, 170.57, 170.55, 170.49, 170.38, 170.35, 170.26, 170.24, 169.58, 169.40, 169.36, 169.29, 169.26, 169.16, 169.11, 144.53, 128.43, 125.68, 97.05, 97.01, 96.76, 96.65, 96.53, 96.38, 78.66, 77.02, 76.79, 76.59, 76.47, 75.73, 71.57, 71.39, 71.29, 70.99, 70.89, 70.46, 70.40, 70.32, 70.19, 70.14, 70.00, 69.96, 69.85, 69.65, 69.63, 69.55, 69.51, 69.46, 69.26, 64.39, 62.73, 62.62, 62.56, 62.42, 62.19, 61.77, 54.91, 49.16, 47.99, 45.30, 43.54, 43.16, 41.79, 39.11, 39.08, 37.45, 37.04, 32.74, 31.84, 31.46, 30.87, 29.41, 28.50, 28.06, 27.27, 26.46, 26.40, 23.33, 20.82, 20.80, 20.79, 20.76, 20.73, 20.71, 20.69, 20.67, 20.62, 20.59, 18.65, 17.45, 16.33, 15.55; ESI-HRMS (m/z) Calcd for C119H166N4O59 [M + H]+: 2596.0185. Found 2596.0154.
4.1.12. Synthesis of N-(1-(1-(6A-deoxy-per-O-acetylated-β-cyclodextrin-6-yl)-1H-1,2,3-triazol-4-yl)-2,5,8,11-tetraoxatridecan-13-yl)-3β-hydroxy-11-oxo-olean-12-en-30-amide (21)
Prepared from 11 and 16 according to general procedure A, the residue was purified by flash chromatography (eluent: petroleum ether: acetone = 1:1) to afford 21 as a white foam with a yield of 61%. Rf = 0.70 (petroleum ether:acetone = 1:2); 1H NMR (600 MHz, CDCl3): δ 7.62 (s, 1H), 6.16 (t, 1H, J = 5.2 Hz), 5.66 (s, 1H), 5.64 (d, 1H, J = 3.8 Hz), 5.36–5.25 (m, 7H), 5.18–5.16 (m, 2H), 5.12 (d, 1H, J = 4.1 Hz), 5.09 (d, 1H, J = 4.0 Hz), 5.06 (d, 1H, J = 3.6 Hz), 5.04 (d, 1H, J = 3.7 Hz), 5.01 (d, 1H, J = 3.6 Hz), 4.94 (dd, 1H, J = 8.6, 4.0 Hz), 4.84–4.81 (m, 3H), 4.75 (dd, 2H, J = 9.3, 3.4 Hz), 4.69–4.65 (m, 3H), 4.61 (dd, 1H, J = 15.6, 3.1 Hz), 4.58–4.53 (m, 4H), 4.47–4.19 (m, 2H), 4.30–4.06 (m, 13H), 3.76–3.46 (m, 21H), 3.20 (dd, 1H, J = 11.3, 4.9 Hz), 2.76 (td, 1H, J = 13.4, 3.2 Hz), 2.31 (s, 1H), 2.18–2.01 (m, 62H), 1.92–1.01 (m, other aliphatic ring protons), 1.36 (s, 3H, CH3), 1.11 (s, 6H, 2 × CH3), 1.10, 0.99 (s, each 3H, 2 × CH3), 0.95 (td, 1H, J = 16.1, 4.1 Hz), 0.80, 0.79 (s, each 3H, 2 × CH3), 0.68 (d, 1H, J = 11.3 Hz); 13C NMR (150 MHz, CDCl3): δ 199.89, 175.68, 170.83, 170.73, 170.69, 170.62, 170.55, 170.49, 170.46, 170.37, 170.35, 170.26, 170.24, 169.59, 169.39, 169.36, 169.28, 169.26, 169.17, 169.06, 144.62, 128.43, 125.64, 97.03, 96.76, 96.64, 96.53, 96.50, 96.38, 78.66, 77.12, 77.04, 76.99, 76.78, 76.58, 76.48, 75.78, 71.58, 71.37, 71.29, 70.98, 70.90, 70.51, 70.45, 70.42, 70.33, 70.18, 70.13, 70.00, 69.95, 69.92, 69.83, 69.66, 69.62, 69.59, 69.53, 69.51, 69.46, 69.45, 69.25, 64.49, 62.75, 62.61, 62.57, 62.52, 62.43, 62.20, 61.78, 54.91, 49.10, 48.00, 45.30, 43.54, 43.15, 41.83, 39.12, 39.09, 39.07, 37.46, 37.04, 32.74, 31.84, 31.46, 29.44, 28.50, 28.06, 27.26, 26.45, 26.40, 23.36, 20.82, 20.79, 20.76, 20.73, 20.70, 20.68, 20.67, 20.62, 20.58, 18.64, 17.45, 16.32, 15.55; ESI-HRMS (m/z) Calcd for C123H174N4O61 [M + H]+: 2684.0709. Found 2684.0696.
4.1.13. Synthesis of N-(1-(1-(6A-deoxy-per-O-acetylated-β-cyclodextrin-6-yl)-1H-1,2,3-triazol-4-yl)-2,5,8,11,14,17-hexaoxanonadecan-19-yl)-3β-hydroxy-11-oxo-olean-12-en-30-amide (22)
Prepared from 12 and 16 according to general procedure C, the residue was purified by flash chromatography (eluent: petroleum ether:acetone = 1:1) to afford 22 as a white foam with a yield of 45%. Rf = 0.10 (petroleum ether:acetone = 1:2); 1H NMR (600 MHz, CDCl3): δ 7.62 (s, 1H), 6.20 (t, 1H, J = 4.8 Hz), 5.66 (s, 1H), 5.65 (d, 1H, J = 4.0 Hz), 5.36–5.24 (m, 8H), 5.20–5.16 (m, 2H), 5.12 (d, 1H, J = 4.0 Hz), 5.09 (d, 1H, J = 4.0 Hz), 5.07 (d, 1H, J = 3.6 Hz), 5.05 (d, 1H, J = 3.6 Hz), 5.01 (d, 1H, J = 3.6 Hz), 4.94 (dd, 1H, J = 8.6, 4.0 Hz), 4.85–4.80 (m, 3H), 4.75 (dd, 2H, J = 9.7, 3.6 Hz), 4.71–4.46 (m, 10H), 4.31–4.06 (m, 13H), 3.77–3.39 (m, 29H), 3.21 (dd, 1H, J = 10.6, 5.3 Hz), 2.77 (td, 1H, J = 10.3, 3.3 Hz), 2.32 (s, 1H), 2.20–1.57 (m, 81H), 1.43–1.02 (m, other aliphatic ring protons), 1.36 (s, 3H, CH3), 1.11 (s, 9H, 3 × CH3), 0.99 (s, 3H, CH3), 0.94 (dd, 1H, J = 13.6, 4.0 Hz), 0.80, 0.79 (s, each 3H, 2 × CH3), 0.69 (d, 1H, J = 11.2 Hz); 13C NMR (150 MHz, CDCl3):δ 199.92, 175.73, 170.84, 170.75, 170.71, 170.64, 170.57, 170.51, 170.47, 170.39, 170.37, 170.28, 170.26, 169.62, 169.41, 169.31, 169.29, 169.21, 169.09, 144.68, 129.51, 128.45, 125.66, 97.05, 96.78, 96.65, 96.55, 96.50, 96.41, 78.69, 77.12, 77.05, 77.00, 76.79, 76.61, 76.47, 75.80, 71.60, 71.37, 71.29, 70.99, 70.91, 70.51, 70.44, 70.35, 70.18, 70.16, 70.03, 69.96, 69.85, 69.70, 69.66, 69.64, 69.58, 69.55, 69.52, 69.48, 69.28, 64.52, 62.76, 62.62, 62.58, 62.52, 62.44, 62.22, 61.80, 54.93, 53.78, 49.12, 48.00, 45.32, 43.55, 43.17, 41.84, 39.14, 39.11, 37.47, 37.05, 32.75, 31.85, 31.70, 31.47, 30.64, 29.24, 28.52, 28.07, 27.27, 26.47, 26.42, 23.37, 20.83, 20.80, 20.77, 20.74, 20.72, 20.70, 20.68, 20.64, 20.60, 18.65, 17.46, 16.33, 15.56; ESI-HRMS (m/z) Calcd for C127H182N4O63 [M + H]+: 2772.1233. Found 2772.1228.
4.1.14. Synthesis of N-(1-(6A-deoxy-β-cyclodextrin-6-yl)-1H-1,2,3-triazol-4-yl)methyl-3β-hydroxy-11-oxo-olean-12-en-30-amide (23)
Prepared from 18 according to general procedure B, the residue was purified by RP flash chromatography (eluent: methanol) to afford 23 as a white foam with a yield of 86%. 1H NMR (600 MHz, MeOD:CDCl3 = 2:1): δ 7.83 (s, 1H), 5.64 (s, 1H), 5.11 (d, 1H, J = 3.0 Hz), 4.99–4.97 (m, 6H), 4.93 (d, 2H, J = 2.3 Hz), 4.66–4.59 (m, 1H), 5.48 (s, 1H), 5.09 (t, 1H, J = 9.2 Hz), 3.89–3.71 (m, 22H), 3.60–3.40 (m, 15H), 3.19–3.09 (m, 2H), 2.73 (d, 1H, J = 13.2 Hz), 2.44 (s, 1H), 2.20–0.89 (m, other aliphatic ring protons), 1.42 (s, 3H, CH3), 1.14 (s, 9H, 3 × CH3), 0.99, 0.82, 0.80 (s, each 3H, 3 × CH3), 0.76 (d, 1H, J = 11.6 Hz); 13C NMR (150 MHz, MeOD:CDCl3 = 2:1): δ 201.19, 171.09, 157.03, 145.12, 127.78, 124.72, 102.65, 102.55, 102.47, 102.39, 102.11, 83.59, 81.78, 81.75, 81.7, 81.58, 81.48, 78.11, 78.03, 77.89, 77.67, 73.41, 73.34, 73.25, 73.05, 72.88, 72.84, 72.72, 72.52, 72.50, 72.31, 72.28, 71.89, 70.64, 61.76, 60.45, 60.38, 60.03, 54.82, 50.85, 48.21, 45.32, 43.42, 43.20, 41.18, 39, 38.84, 37.36, 36.94, 34.56, 32.46, 31.56, 30.55, 29.44, 29.34, 29.24, 29.21, 29.07, 28.93, 28.17, 28.10, 27.87, 27.31, 26.44, 26.21, 26.07, 22.38, 17.98, 17.24, 15.61, 14.96; ESI-HRMS (m/z) Calcd for C75H118N4O37 [M + H]+: 1667.7475. Found 1667.7522.
4.1.15. Synthesis of N-(2-((1-(6A-deoxy-β-cyclodextrin-6-yl)-1H-1,2,3-triazol-4-yl)methoxy)ethyl)-3β-hydroxy-11-oxo-olean-12-en-30-amide (24)
Prepared from 19 according to general procedure B, the residue was purified by RP flash chromatography (eluent: methanol) to afford 24 as a white foam with a yield of 80%. 1H NMR (600 MHz, MeOD:CDCl3 = 2:1): δ 8.03 (s, 1H), 5.63 (s, 1H), 5.10 (d, 1H, J = 3.6 Hz), 5.05 (dd, 1H, J = 14.2, 2.0 Hz), 4.99 (d, 1H, J = 3.7 Hz), 4.99–4.96 (m, 3H), 4.92 (dd, 1H, J = 7.6, 3.5 Hz), 4.65 (s, 2H), 4.58 (dd, 1H, J = 14.3, 8.5 Hz), 4.11–4.08 (m, 1H), 3.92–3.72 (m, 21H), 3.64 (t, 2H, J = 5.4 Hz), 3.57 (dd, 1H, J = 9.8, 3.5 Hz), 3.54–3.38 (m, 14H), 3.33–3.29 (m, 1H), 3.18 (dd, 1H, J = 11.8, 4.4 Hz), 3.03 (dd, 1H, J = 12.1, 3.1 Hz), 2.74 (td, 1H, J = 10.1, 3.3 Hz), 2.18–2.14 (m, 2H), 1.97–1.23 (m, other aliphatic ring protons), 1.43, 1.15, 1.14, 1.11 (s, each 3H, 4 × CH3), 1.02 (dd, 1H, J = 13.9, 1.9 Hz), 1.00, 0.81, 0.78 (s, each 3H, 3 × CH3), 0.77 (d, 1H, J = 11.7 Hz); 13C NMR (150 MHz, MeOD:CDCl3 = 2:1): δ 202.57, 178.93, 172.64, 145.66, 129.09, 127.21, 104.13, 104.00, 103.89, 103.87, 103.84, 103.79, 103.40, 85.18, 83.07, 82.97, 82.90, 82.84, 79.49, 79.40, 79.27, 79.05, 74.80, 74.72, 74.56, 74.42, 74.24, 74.21, 74.10, 73.91, 73.69, 73.64, 73.20, 72.13, 70.39, 64.74, 63.16, 61.85, 61.79, 61.11, 56.20, 52.46, 49.63, 46.71, 44.82, 44.59, 42.58, 40.42, 40.22, 40.19, 38.72, 38.33, 33.83, 32.89, 32.02, 29.55, 29.51, 29.23, 28.70, 27.84, 27.59, 27.44, 23.81, 19.36, 18.62, 17.01, 16.36; ESI-HRMS (m/z) Calcd for C77H122N4O38 [M + H]+: 1711.7810. Found 1711.7784.
4.1.16. Synthesis of N-(2-[2-((1-(6A-deoxy-β-cyclodextrin-6-yl)-1H-1,2,3-triazol-4-yl)methoxy))ethoxy]ethyl)-3β-hydroxy-11-oxo-olean-12-en-30-amide (25)
Prepared from 20 according to general procedure B, the residue was purified by RP flash chromatography (eluent: methanol) to afford 25 as a white foam with a yield of 82%. 1H NMR (600 MHz, MeOD:CDCl3 = 2:1): δ 8.13 (s, 1H), 5.67 (s, 1H), 5.12 (d, 1H, J = 3.6 Hz), 5.07 (dd, 1H, J = 14.9, 1.9 Hz), 4.99–4.96 (m, 4H), 4.93 (dd, 1H, J = 7.7, 3.5 Hz), 4.68 (s, 2H), 4.67 (dd, 1H, J = 14.6, 8.2 Hz), 4.12 (dt, 1H, J = 10.0, 2.2 Hz), 3.94–3.71 (m, 25H), 3.67 (t, 2H, J = 4.5 Hz), 3.54–3.38 (m, 17H), 3.37–3.29 (m, 3H), 3.16 (dd, 1H, J = 11.8, 4.4 Hz), 3.07 (dd, 1H, J = 12.2, 3.4 Hz), 2.73 (td, 1H, J = 10.2, 3.2 Hz), 2.46 (s, 1H), 2.19–2.14 (m, 2H), 1.97–1.02 (m, other aliphatic ring protons), 1.43 (s, 3H, CH3), 1.14 (s, 6H, 2 × CH3), 1.11, 1.00, 0.82, 0.80 (s, each 3H, 4 × CH3), 0.77 (d, 1H, J = 11.4 Hz); 13C NMR (150 MHz, MeOD:CDCl3 = 2:1): δ 202.55, 178.88, 172.58, 145.26, 130.58, 130.39, 129.13, 127.60, 127.18, 120.46, 116.19, 104.06, 104.02, 103.87, 103.83, 103.78, 103.40, 85.05, 83.22, 83.07, 83.01, 82.90, 79.41, 74.82, 74.76, 74.73, 74.57, 74.42, 74.25, 74.22, 74.09, 73.98, 73.90, 73.69, 73.66, 73.29, 71.97, 71.21, 71.15, 70.79, 64.70, 63.16, 62.00, 61.90, 61.82, 61.24, 56.20, 52.80, 49.61, 49.57, 46.71, 44.83, 44.59, 42.61, 40.37, 40.31, 40.23, 38.77, 38.33, 33.83, 32.92, 32.02, 29.55, 29.51, 29.28, 28.70, 27.85, 27.60, 27.45, 23.79, 19.33, 18.63, 16.99, 16.35; ESI-HRMS (m/z) Calcd for C79H126N4O39 [M + H]+: 1755.8077. Found 1755.8071.
4.1.17. Synthesis of N-(1-(1-(6A-deoxy-β-cyclodextrin-6-yl)-1H-1,2,3-triazol-4-yl)-2,5,8,11-tetraoxatridecan-13-yl)-3β-hydroxy-11-oxo-olean-12-en-30-amide (26)
Prepared from 21 according to general procedure B, the residue was purified by RP flash chromatography (eluent: methanol) to afford 26 as a white foam with a yield of 98%. 1H NMR (600 MHz, MeOD): δ 8.02 (s, 1H), 5.66 (s, 1H), 5.12 (d, 1H, J = 3.6 Hz), 4.99–4.96 (m, 4H), 4.93 (dd, 1H, J = 8.8, 3.4 Hz), 4.68–4.61 (m, 3H), 4.12 (t, 1H, J = 8.5 Hz), 3.93–3.62 (m, 37H), 3.58–3.44 (m, 18H), 3.37–3.33 (m, 2H), 3.18 (dd, 1H, J = 11.8, 4.5 Hz), 3.06 (dd, 1H, J = 12.4, 2.4 Hz), 2.72 (td, 1H, J = 10.1, 3.2 Hz), 2.45 (s, 1H), 2.21–1.02 (m, other aliphatic ring protons), 1.43 (s, 3H, CH3), 1.14 (s, 6H, 2 × CH3), 1.12, 1.00, 0.83, 0.81 (s, each 3H, 4 × CH3), 0.76 (d, 1H, J = 11.3 Hz); 13C NMR (150 MHz, MeOD): δ 202.39, 178.80, 172.42, 145.72, 130.84, 129.14, 127.19, 104.04, 103.89, 103.84, 103.38, 85.08, 83.21, 83.06, 83.00, 82.87, 82.77, 79.39, 74.80, 74.72, 74.70, 74.53, 74.45, 74.26, 74.23, 74.08, 73.91, 73.68, 73.64, 73.26, 72.07, 71.56, 71.52, 71.21, 70.97, 70.73, 65.01, 63.14, 61.92, 61.86, 61.83, 61.77, 56.19, 52.37, 49.48, 46.68, 44.82, 44.57, 42.61, 40.35, 40.22, 38.76, 38.32, 36.52, 33.83, 32.91, 32.03, 30.31, 29.51, 29.30, 28.71, 28.10, 27.83, 27.59, 27.45, 23.80, 19.34, 18.62, 16.98, 16.36; ESI-HRMS (m/z) Calcd for C83H134N4O41 [M + H]+: 1843.8596. Found 1843.8595.
4.1.18. Synthesis of N-(1-(1-(6A-deoxy-β-cyclodextrin-6-yl)-1H-1,2,3-triazol-4-yl)-2,5,8,11,14,17-hexaoxanonadecan-19-yl)-3β-hydroxy-11-oxo-olean-12-en-30-amide (27)
Prepared from 22 according to general procedure B, the residue was purified by RP flash chromatography (eluent: methanol) to afford 27 as a white foam with a yield of 90%. 1H NMR (600 MHz, MeOD): δ 8.07 (s, 1H), 5.66 (s, 1H), 5.12 (d, 1H, J = 3.5 Hz), 5.05 (d, 1H, J = 13.6 Hz), 4.99–4.96 (m, 4H), 4.93 (dd, 1H, J = 6.7, 3.4 Hz), 4.70–4.64 (m, 3H), 4.12 (t, 1H, J = 8.9 Hz), 3.93–3.63 (m, 38H), 3.58–3.44 (m, 16H), 3.37–3.33 (m, 2H), 3.18 (dd, 1H, J = 11.8, 4.5 Hz), 3.07 (dd, 1H, J = 12.1, 2.9 Hz), 2.73 (td, 1H, J = 10.1, 3.2 Hz), 2.45 (s, 1H), 2.21–1.01 (m, other aliphatic ring protons), 1.43 (s, 3H, CH3), 1.14 (s, 6H, 2 × CH3), 1.12, 1.00, 0.83, 0.80 (s, each 3H, 4 × CH3), 0.76 (d, 1H, J = 11.3 Hz); 13C NMR (150 MHz, MeOD): δ 202.41, 178.81, 172.45, 145.55, 130.85, 129.15, 127.39, 104.03, 103.88, 103.83, 103.39, 85.05, 83.22, 83.06, 83.01, 82.89, 82.84, 79.41, 74.82, 74.76, 74.73, 74.56, 74.44, 74.26, 74.23, 74.09, 73.96, 73.90, 73.69, 73.66, 73.30, 72.01, 71.54, 71.22, 71.04, 70.73, 64.85, 63.15, 61.97, 61.86, 61.80, 61.18, 56.20, 52.58, 49.59, 46.69, 44.83, 44.58, 42.63, 40.36, 40.23, 38.77, 38.33, 36.47, 33.83, 33.05, 32.92, 32.03, 30.32, 29.50, 29.30, 28.71, 28.11, 27.84, 27.60, 27.46, 26.92, 23.80, 19.33, 18.63, 16.98, 16.36; ESI-HRMS (m/z) Calcd for C87H142N4O43 [M + H]+: 1931.9121. Found 1931.9117.
4.1.19. N-(2-(piperazin-1-yl)-ethyl)-3β-hydroxy-11-oxo-olean-12-en-30-amide (28)
Prepared from 7 and 2-(piperazin-1-yl)ethan-1-amine according to general procedure C, the residue was purified by flash chromatography (eluent: DCM:MeOH:NH3·H2O = 20:1:0.2) to afford 28 as a white solid with a yield of 45%. Rf = 0.35 (eluent: DCM:MeOH:NH3·H2O = 20:1:0.2); m.p. 157–158 °C; 1H NMR (400 MHz, CDCl3): δ 6.35 (br s, 1H), 5.65 (s, 1H), 3.40–3.14 (m, 3H), 2.90 (t, 1H, J = 4.3 Hz), 2.71 (d, 1H, J = 13.1 Hz), 2.47–2.43 (m, 6H), 2.29 (s, 1H), 2.12 (t, 1H, J = 8.3 Hz), 2.00–1.00 (m, other aliphatic ring protons), 1.33 (s, 3H, CH3), 1.07(s, 9H, 3 × CH3), 0.95 (s, 3H, CH3), 0.89 (d, 1H, J = 13.6 Hz), 0.76, 0,75 (s, each 3H, 2 × CH3), 0.64 (d, 1H, J = 11.3 Hz); 13C NMR (100 MHz, CDCl3): δ 199.98, 175.75, 169.34, 128.35, 78.53, 61.85, 57.19, 54.97, 53.53, 48.11, 45.59, 45.37, 43.60, 43.20, 41.81, 39.28, 39.15, 37.49, 37.04, 35.60, 32.73, 31.86, 31.41, 29.62, 28.58, 28.15, 27.28, 26.43, 26.36, 23.42, 18.66, 17.46, 16.36, 15.67; ESI-HRMS (m/z) Calcd for C36H59N3O3 [M + H]+: 582.4629. Found 582.4621.
4.1.20. Synthesis of N-(2-(4-(prop-2-yn-1-yl)piperzain-1-yl)ethyl)-3β-hydroxy-11-oxo-olean-12-en-30-amide (29)
To a solution of 28 (90 mg, 0.16 mmol) and bromopropyne (21.2 mg, 0.18 mmol) in dried THF, K2CO3 (40 mg, 0.29 mmol) was added. The resulting solution was vigorously stirred for 24 h at room temperature. The solvent was removed by steaming. The residue was purified by column chromatography to afford compound 29 as a yellow solid with a yield of 76%. Rf = 0.45 (DCM:MeOH:NH3·H2O = 10:1:0.01); m.p. 121–123 °C; 1H NMR (400 MHz, CDCl3): δ 6.28 (br s, 1H), 5.69 (s, 1H), 3.43–3.31 (m, 2H), 3.28 (d, 2H, J = 2.4 Hz), 3.21 (dd, 1H, J = 10.3, 5.2 Hz), 2.77 (td, 1H, J = 10.2, 3.2 Hz), 2.60–2.50 (m, 10H), 2.32 (s, 1H), 2.22 (t, 1H, J = 2.4 Hz), 2.18 (dd, 1H, J = 12.0, 5.8 Hz), 2.07–1.57 (m, 10H), 1.47–1.03 (m, other aliphatic ring protons), 1.37, 1.12 (s, each 3H, 2 × CH3), 1.11(s, 6H, 2 × CH3), 0.99 (s, 3H, CH3), 0.93 (dd, 1H, J = 12.8, 4.4 Hz), 0.80, 0.79 (s, each 3H, 2 × CH3), 0.68 (d, 1H, J = 11.6 Hz); 13C NMR (100 MHz, CDCl3): δ 199.83, 175.78, 169.10, 128.51, 78.87, 78.72, 73.04, 61.80, 56.46, 54.96, 52.67, 51.81, 48.00, 46.71, 45.35, 43.59, 43.17, 41.80, 39.17, 39.12, 37.51, 37.06, 35.81, 32.73, 31.87, 31.46, 29.63, 28.59, 28.09, 27.29, 26.42, 26.38, 23.41, 18.65, 17.45, 16.33, 15.57; ESI-HRMS (m/z) Calcd for C39H61N3O3 [M + H]+: 620.4786. Found 620.4775.
4.1.21. Synthesis of N-(2-(4-((1-(6A-deoxy-per-O-acetylated-β-cyclodextrin-6-yl)-1H-1,2,3-triazol-4-yl)methyl)piperazin-1-yl)-ethyl)-3β-hydroxy-11-oxo-olean-12-en-30-amide (30)
Prepared from 29 and 16 according to general procedure A, the residue was purified by flash chromatography (eluent: petroleum ether: acetone = 1:2) to afford 30 as a white foam with a yield of 42%. Rf = 0.10 (petroleum ether:acetone = 1:2); 1H NMR (600 MHz, CDCl3): δ 7.56 (s, 1H), 6.27 (br s, 1H), 5.71 (s, 1H), 5.66 (d, 1H, J = 2.6 Hz), 5.37–5.16 (m, 10H), 5.12 (d, 1H, J = 4.0 Hz), 5.10 (d, 1H, J = 4.0 Hz), 5.07 (d, 1H, J = 3.6 Hz), 5.05 (d, 1H, J = 3.7 Hz), 5.01 (d, 1H, J = 3.5 Hz), 4.94 (dd, 1H, J = 8.6, 4.0 Hz), 4.85–4.81 (m, 3H), 4.75 (dd, 2H, J = 9.7, 3.6 Hz), 4.68 (dd, 1H, J = 12.1, 1.3 Hz), 4.60–4.45 (m, 7H), 4.32–4.06 (m, 13H), 3.78–3.62 (m, 8H), 3.58 (t, 1H, J = 9.1 Hz), 3.34 (d, 2H, J = 26.2 Hz), 3.21 (dd, 1H, J = 11.2, 4.9 Hz), 2.77 (td, 1H, J = 10.1, 3.7 Hz), 2.50 (s, 9H), 2.34 (s, 1H), 2.15–2.01 (m, 84H), 1.92–1.58 (m, 10H), 1.44–1.03 (m, other aliphatic ring protons), 1.38, 1.13, 1.12, 1.11, 1.00 (s, each 3H, 5 × CH3), 0.96 (dd, 1H, J = 13.2, 3.7 Hz), 0.80, 0.79 (s, each 3H, 2 × CH3), 0.69 (d, 1H, J = 11.5 Hz); 13C NMR (150 MHz, CDCl3): δ 199.9, 170.85, 170.75, 170.70, 170.63, 170.56, 170.51, 170.39, 170.27, 170.25, 169.60, 169.40, 169.37, 169.29, 169.27, 169.16, 144.25, 128.53, 125.73, 97.04, 96.78, 96.66, 96.53, 96.49, 96.38, 78.69, 77.03, 76.96, 76.80, 76.54, 76.49, 76.78, 71.61, 71.39, 71.34, 71.03, 70.94, 70.52, 70.41, 70.32, 70.13, 70.05, 69.99, 69.84, 69.67, 69.63, 69.53, 69.47, 69.25, 68.12, 62.78, 62.61, 62.58, 62.43, 62.21, 61.80, 56.37, 54.94, 52.64, 49.11, 47.95, 45.35, 43.59, 43.20, 41.83, 39.12, 39.10, 37.51, 37.07, 35.79, 32.75, 31.87, 31.52, 29.62, 28.59, 28.08, 27.30, 26.45, 26.42, 23.42, 20.84, 20.78, 20.74, 20.72, 20.70, 20.63, 20.60, 18.68; ESI-HRMS (m/z) Calcd for C121H170N6O57 [M + H]+: 2620.0661. Found 2620.0632.
4.1.22. Synthesis of N-(2-(4-((1-(6A-deoxy-β-cyclodextrin-6-yl)-1H-1,2,3-triazol-4-yl)methyl)piperazin-1-yl)-ethyl)-3β-hydroxy-11-oxo-olean-12-en-30-amide (31)
Prepared from 30 according to general procedure B, the residue was purified by RP flash chromatography (eluent: methanol) to afford 31 as a white foam with a yield of 90%. 1H NMR (600 MHz, MeOD:CDCl3 = 2:1): δ 7.97 (s, 1H), 5.67 (s, 1H), 5.08 (d, 1H, J = 3.1 Hz), 5.03 (d, 1H, J = 13.6 Hz), 4.96–4.94 (m, 4H), 4.91 (t, 2H, J = 4.3 Hz), 4.57 (dd, 3H, J = 13.7, 7.3 Hz), 4.13 (t, 1H, J = 8.6 Hz), 3.92–3.72 (m, 23H), 3.58–3.42 (m, 15H), 3.34–3.28 (m, 3H), 3.17 (dd, 1H, J = 11.8, 4.5 Hz), 2.79 (br s, 5H), 2.72 (td, 1H, J = 13.5, 3.6 Hz), 2.65 (t, 3H, J = 5.6 Hz), 2.43 (s, 1H), 2.17–2.10 (m, 2H), 1.99 (s, 1H), 1.94 (dd, 1H, J = 10.8, 2.5 Hz), 1.89–1.86 (m, 2H), 1.75–1.03 (m, other aliphatic ring protons), 1.42, 1.14, 1.13, 1.11, 0.99, 0.82, 0.79 (s, each 3H, 7 × CH3), 0.74 (d, 1H, J = 11.7 Hz); 13C NMR (150 MHz, MeOD:CDCl3 = 2:1): δ 202.40, 178.76, 172.29, 128.95, 103.94, 103.87, 103.75, 103.68, 103.63, 103.28, 84.97, 82.90, 82.85, 82.70, 74.50, 74.44, 74.30, 74.13, 74.01, 73.97, 73.81, 73.62, 73.39, 73.34, 72.88, 71.83, 62.97, 61.60, 60.85, 57.75, 56.00, 53.01, 52.84, 46.54, 44.61, 44.38, 42.34, 40.21, 40.04, 38.54, 38.11, 36.89, 33.65, 32.73, 31.85, 29.55, 29.28, 28.59, 27.59, 27.38, 27.25, 23.84, 19.28, 18.39, 16.93, 16.23; ESI-HRMS (m/z) Calcd for C81H130N6O37 [M + H]+: 1779.8554. Found 1779.8523.
4.1.23. Synthesis of N-(4-ethynylphenyl)-3β-hydroxy-11-oxo-olean-12-en-30-amide (32)
To a solution of 2 (669 mg, 1.42 mmol) and benzenamine (111 mg, 0.95 mmol) in DMF, DMAP (55 mg, 0.45 mmol) and EDC (362 mg, 1.90 mmol) were added. The resulting solution was vigorously stirred for 24 h at room temperature. The solvent was removed by steaming. The residue was purified by column chromatography to afford compound 32 as a white solid with a yield of 60%. Rf = 0.50 (petroleum ether:ethyl acetate = 1:1); m.p. 146–148 °C; 1H NMR (400 MHz, CDCl3): δ 7.50–7.43 (m, 5H), 5.68 (s, 1H), 3.22 (dd, 1H, J = 10.3, 5.8 Hz), 3.03 (s, 1H), 2.79 (dt, 1H, J = 13.3, 3.0 Hz), 2.34 (s, 1H), 2.22 (dd, 1H, J = 13.8, 3.2 Hz), 2.10–1.59 (m, 11H), 1.50–0.85 (m, other aliphatic ring protons), 1.39, 1.24 (s, each 3H, 2 × CH3), 1.12 (s, 6H, 2 × CH3), 1.00, 0.82, 0.80 (s, each 3H, 3 × CH3), 0.70 (d, 1H, J = 11.7 Hz); 13C NMR (100 MHz, CDCl3): δ 200.06, 174.08, 168.94, 138.39, 132.91, 128.61, 119.66, 117.72, 83.34, 78.75, 76.85, 61.85, 54.96, 48.14, 45.38, 44.66, 43.22, 41.73, 39.15, 39.12, 37.39, 37.08, 32.75, 31.99, 31.62, 29.28, 28.37, 28.09, 27.27, 26.41, 26.39, 23.40, 18.65, 17.45, 16.31, 15.54; ESI-HRMS (m/z) Calcd for C38H51NO3 [M + H]+: 570.3942. Found 570.3935.
4.1.24. Synthesis of N-(4-(1-(6A-deoxy-per-O-acetylated-β-cyclodextrin-6-yl)-1H-1,2,3-triazol-4-yl)phenyl)-3β-hydroxy-11-oxo-olean-12-en-30-amide (33)
Prepared from 32 and 16 according to general procedure A, the residue was purified by flash chromatography (eluent: petroleum ether:acetone = 1:1) to afford 33 as a white foam with a yield of 59%. Rf = 0.20 (petroleum ether:acetone = 1:1); 1H NMR (600 MHz, CDCl3): δ 7.91 (s, 1H), 7.85 (d, 2H, J = 8.3 Hz), 7.60 (d, 2H, J = 8.5 Hz), 7.42 (br s, 1H), 5.69 (s, 1H), 5.62 (d, 1H, J = 3.7 Hz), 5.37–5.20 (m, 8H), 5.19 (d, 1H, J = 4.0 Hz), 5.14 (d, 1H, J = 4.0 Hz), 5.09 (d, 1H, J = 4.0 Hz), 5.08–5.06 (m, 2H), 5.01 (d, 1H, J = 3.6 Hz), 4.97 (dd, 1H, J = 8.8, 4.0 Hz), 4.84–4.81 (m, 3H), 4.77 (dd, 1H, J = 9.7, 3.7 Hz), 4.74 (dd, 1H, J = 9.8, 3.6 Hz), 4.71–4.69 (m, 2H), 4.61 (d, 1H, J = 11.5 Hz), 4.56–4.45 (m, 5H), 4.37–4.12 (m, 12H), 4.09 (dt, 1H, J = 9.4, 2.9 Hz), 3.79 (dd, 1H, J = 9.3, 7.3 Hz), 3.76–3.65 (m, 6H), 3.22 (dd, 1H, J = 11.0, 5.2 Hz), 2.83 (s, 1H), 2.79 (td, 1H, J = 13.6, 3.5 Hz), 2.28–2.25 (m, 1H), 2.15–1.97(m, 61H), 1.92–1.20 (m, other aliphatic ring protons), 1.39, 1.26 (s, each 3H, 2 × CH3), 1.12 (s, 6H, 2 × CH3), 1.06–1.04 (m, 1H), 1.00 (s, 3H, CH3), 0.97 (dt, 1H, J = 12.7, 3.8 Hz), 0.83, 0.80 (s, each 3H, 2 × CH3), 0.70 (d, 1H, J = 11.5 Hz); 13C NMR (150 MHz, CDCl3): δ 199.92, 173.94, 170.84, 170.72, 170.68, 170.63, 170.57, 170.54, 170.50, 170.41, 170.37, 170.34, 170.30, 169.61, 169.37, 169.35, 169.30, 169.19, 168.73, 146.93, 137.75, 128.66, 126.64, 126.36, 122.38, 120.26, 97.09, 97.05, 96.83, 96.68, 96.60, 96.48, 96.40, 78.74, 77.41, 77.29, 76.78, 76.71, 76.19, 75.82, 71.55, 71.52, 71.15, 70.98, 70.78, 70.43, 70.38, 70.33, 70.25, 70.18, 70.07, 69.98, 69.74, 69.64, 69.54, 69.53, 69.48, 69.44, 69.34, 62.75, 62.69, 62.57, 62.52, 62.33, 61.84, 54.94, 49.49, 48.03, 45.31, 44.56, 43.21, 41.80, 39.12, 37.46, 37.09, 32.76, 32.00, 31.70, 29.40, 28.40, 28.08, 27.29, 26.43, 23.40, 20.84, 20.83, 20.78, 20.74, 20.71, 20.66, 20.62, 18.67, 17.47, 16.32, 15.54; ESI-HRMS (m/z) Calcd for C120H160N4O57 [M + H]+: 2569.9823. Found 2569.9827.
4.1.25. Synthesis of N-(4-(1-(6A-deoxy-β-cyclodextrin-6-yl)-1H-1,2,3-triazol-4-yl)phenyl)-3β-hydroxy-11-oxo-olean-12-en-30-amide (34)
Prepared from 33 according to general procedure B, the residue was purified by RP flash chromatography (eluent: methanol) to afford 39 as a white foam with a yield of 86%. 1H NMR (600 MHz, DMSO): δ 9.37 (s, 1H), 8.40 (s, 1H), 7.78 (d, 2H, J = 8.6 Hz), 7.71 (d, 2H, J = 8.8 Hz), 5.50 (s, 1H), 5.10 (d, 1H, J = 3.5 Hz), 4.93 (d, 1H, J = 12.8 Hz), 4.87–4.85 (m, 4H), 4.80 (dd, 1H, J = 9.0, 3.1 Hz), 4.61 (dd, 1H, J = 14.2, 8.2 Hz), 4.10 (d, 1H, J = 10.1 Hz), 3.76–3.52 (m, 22H), 3.42–3.30 (m, 13H), 3.25 (dd, 1H, J = 9.8, 3.0 Hz), 3.06–2.99 (m, 3H), 2.60 (td, 1H, J = 10.1, 3.2 Hz), 2.35 (s, 1H), 2.18–0.96 (m, other aliphatic ring protons), 1.40, 1.21 (s, each 3H, 2 × CH3), 1.05 (2 × s, 6H, 2 × CH3), 0.93, 0.77 (s, each 3H, 2 × CH3), 0.72 (d, 1H, J = 10.6 Hz), 0.71 (s, 3H, CH3); 13C NMR (150 MHz, DMSO): δ 199.15, 174.63, 169.70, 146.18, 138.82, 127.50, 126.00, 125.44, 121.92, 120.78, 102.23, 102.10, 101.99, 101.89, 101.34, 83.53, 82.06, 81.55, 81.44, 80.86, 76.63, 73.21, 73.11, 73.06, 73.02, 72.95, 72.60, 72.48, 72.41, 72.34, 72.25, 72.18, 72.08, 71.87, 69.87, 61.24, 60.19, 60.03, 59.94, 59.85, 59.76, 58.66, 54.13, 50.57, 47.89, 44.88, 44.05, 42.97, 40.73, 38.81, 38.55, 37.33, 36.68, 32.18, 31.56, 30.42, 28.38, 28.17, 28.07, 26.98, 26.10, 25.92, 23.02, 18.38, 17.18, 16.21, 16.02; ESI-HRMS (m/z) Calcd for C80H120N4O37 [M + H]+: 1729.7704. Found 1729.7715.
4.1.26. Synthesis of N-(1-(6A-deoxy-per-O-methylated-β-cyclodextrin-6-yl)-1H-1,2,3-triazol-4-yl)methyl-3β-hydroxy-11-oxo-olean-12-en-30-amide (35)
Prepared from 8 and 17 according to general procedure A, the residue was purified by flash chromatography (eluent: petroleum ether:acetone = 1:1) to afford 35 as a white foam with a yield of 59%. Rf = 0.15 (petroleum ether:acetone = 1:1); 1H NMR (600 MHz, CDCl3): δ 7.62 (s, 1H), 6.34 (br s, 1H), 5.66 (s, 1H), 5.33 (d, 1H, J = 3.2 Hz), 5.17 (d, 1H, J = 3.4 Hz), 5.16 (d, 1H, J = 3.5 Hz), 5.13–5.12 (m, 3H), 5.11 (d, 1H, J = 3.6 Hz), 4.94 (dd, 1H, J = 14.0, 3.7 Hz), 4.79 (d, 1H, J = 13.4 Hz), 4.50 (dq, 2H, J = 14.9, 4.9 Hz), 4.05–4.03 (m, 1H), 3.95–3.93 (m, 1H), 3.89–3.72 (m, 10H), 3.65–3.16 (m, 98H), 3.00 (dd, 2H, J = 9.8, 3.4 Hz), 2.78 (dt, 1H, J = 13.6, 3.5 Hz), 2.31 (s, 1H), 2.18–2.16 (m, 1H), 2.05–2.00 (m, 2H), 1.85–1.79 (m, 3H), 1.67–1.58 (m, 5H), 1.47–1.17 (m, other aliphatic ring protons), 1.36, 1.12, 1.11, 1.08 (s, each 3H, 4 × CH3), 1.02–1.01 (m, 1H), 1.00 (s, 3H, CH3), 0.96 (dt, 1H, J = 12.8, 4.0 Hz), 0.80, 0.79 (s, each 3H, 2 × CH3), 0.69 (d, 1H, J = 11.3 Hz); 13C NMR (150 MHz, CDCl3): δ 199.90, 175.89, 168.84, 144.00, 128.57, 124.26, 99.17, 98.92, 98.85, 98.79, 98.76, 98.29, 82.34, 82.17, 82.02, 81.97, 81.90, 81.81, 81.77, 81.47, 81.08, 80.41, 80.25, 80.23, 79.82, 79.79, 79.03, 78.77, 71.60, 71.34, 71.31, 71.23, 71.05, 70.98, 70.96, 70.92, 70.82, 70.50, 70.23, 61.79, 61.73, 61.48, 61.45, 61.35, 61.32, 59.19, 59.09, 59.01, 58.97, 58.95, 58.68, 58.64, 58.59, 58.45, 58.36, 58.31, 54.95, 51.11, 47.92, 45.33, 43.52, 43.17, 41.60, 39.18, 39.12, 37.45, 37.07, 35.03, 32.77, 31.91, 31.87, 31.52, 29.34, 28.47, 28.09, 27.28, 26.45, 26.42, 23.35, 18.68, 17.48, 16.35, 15.55; ESI-HRMS (m/z) Calcd for C95H158N4O37 [M + H]+: 1948.0678. Found 1948.0671.
4.1.27. Synthesis of 3β-(prop-2-yn-1-yloxy)-11-oxo-olean-12-en-30-oic acid benzyl ester (37)
A solution of 36 (342 mg, 0.61 mmol) in dry THF (20 mL) was cooled to 0 °C. Sodium hydride (96 mg, 2.4 mmol) was added, and the mixture was stirred for 1 h at 0 °C. Then, 3-bromopropyne was added, and the solution was stirred for 24 h at room temperature. The solvent of THF was evaporated, and the residue was purified by column chromatography to afford compound 37 as a white solid with a yield of 60%. Rf = 0.65 (petroleum ether:ethyl acetate = 4:1); m.p. 204–206 °C; 1H NMR (400 MHz, CDCl3): δ 7.40–7.31 (m, 5H), 5.54 (s, 1H), 5.20 (d, 1H, J = 12.2 Hz), 5.09 (d, 1H, J = 12.2 Hz), 4.26–4.16 (m, 2H), 3.04 (dd, 1H, J = 4.3, 11.7 Hz), 2.81 (td, 1H, J = 3.1, 10.2 Hz), 2.36 (t, 1H, J = 2.3 Hz), 2.31 (s, 1H), 2.04–1.73 (m, 8H), 1.64–0.83 (m, other aliphatic ring protons), 1.34, 1.16, 1.13, 1.10, 1.01, 0.80, 0.73 (s, each 3H, 7 × CH3), 0.70 (d, 1H, J = 10.1 Hz); 13C NMR (100 MHz, CDCl3): δ 200.24, 176.23, 169.07, 128.61, 128.50, 128.30, 128.25, 85.38, 80.92, 73.42, 66.22, 61.75, 56.21, 55.47, 48.18, 45.37, 43.97, 43.13, 41.02, 38.88, 38.77, 37.62, 36.98, 32.72, 31.75, 31.13, 28.39, 28.28, 28.06, 26.88, 26.36, 23.31, 18.65, 17.40, 16.36, 16.33; ESI-HRMS (m/z) Calcd for C40H54O4 [M + H]+: 599.4022. Found 599.4095.
4.1.28. Synthesis of 3β-((1-(6A-deoxy-per-O-acetylated-β-cyclodextrin-6-yl)-1H-1,2,3-triazol-4-yl)methoxy)-11-oxo-olean-12-en-30-oic acid benzyl ester (38)
Prepared from 37 and 16 according to general procedure A, the residue was purified by flash chromatography (eluent: petroleum ether: acetone = 1:1) to afford 38 as a white foam with a yield of 60%. Rf = 0.49 (petroleum ether:acetone = 1:1); 1H NMR (600 MHz, CDCl3): δ 7.60 (s, 1H), 7.39–7.32 (m, 5H), 5.71 (d, 1H, J = 3.9 Hz), 5.52 (s, 1H), 5.38–5.26 (m, 7H), 5.20–5.18 (m, 3H), 5.13 (d, 1H, J = 4.1 Hz), 5.10–5.07 (m, 3H), 5.05 (d, 1H, J = 3.6 Hz), 5.01 (d, 1H, J = 3.6 Hz), 4.95 (dd, 1H, J = 3.9, 8.6 Hz), 4.85–4.81 (m, 3H), 4.78–4.74 (m, 3H), 4.67 (br d, 1H, J = 10.8 Hz), 4.61–4.50 (m, 7H), 4.46 (dd, 1H, J = 3.6, 10.5 Hz), 4.32–4.07 (m, 13H), 3.77–3.65 (m, 6H), 3.57 (t, 1H, J = 9.0 Hz), 3.00 (dd, 1H, J = 4.4, 11.7 Hz), 2.77 (td, 1H, J = 3.0, 13.5 Hz), 2.30 (s, 1H), 2.17–1.97 (m, 61H), 1.92 (dt, 1H, J = 3.6, 13.6 Hz), 1.84–1.72 (m, 8H), 1.63–1.53 (m, 5H), 1.33, 1.15, 1.12, 1.08, 0.90 (s, each 3H, 5 × CH3), 1.43–0.86 (m, other aliphatic ring protons), 0.80, 0.72 (s, each 3H, 2 × CH3), 0.68 (d, 1H, J = 11.3 Hz); 13C NMR (150 MHz, CDCl3): δ 200.17, 176.22, 170.85, 170.77, 170.74, 170.66, 170.59, 170.57, 170.53, 170.43, 170.39, 170.31, 170.28, 169.65, 169.45, 169.42, 169.33, 169.28, 168.95, 145.94, 136.10, 128.59, 128.47, 128.27, 128.22, 125.47, 97.23, 97.06, 96.78, 96.63, 96.53, 96.45, 96.40, 85.87, 77.04, 76.72, 76.63, 76.39, 75.78, 71.70, 71.35, 71.27, 71.03, 70.96, 70.53, 70.35, 70.13, 70.05, 69.82, 69.66, 69.64, 69.56, 69.48, 69.28, 66.20, 62.92, 62.82, 62.59, 62.54, 62.43, 62.24, 61.72, 55.23, 49.00, 48.19, 45.36, 43.97, 43.14, 41.02, 39.03, 38.88, 37.63, 36.98, 32.72, 31.75, 31.14, 28.38, 28.27, 28.04, 26.41, 26.37, 23.31, 22.74, 20.86, 20.85, 20.81, 20.80, 20.76, 20.74, 20.70, 20.65, 20.61, 18.65, 17.39, 16.44, 16.36; ESI-HRMS (m/z) Calcd for C122H163N3O58 [M + H]+: 2598.9970. Found 2598.9980.
4.1.29. Synthesis of 3β-((1-(6A-deoxy-per-O-acetylated-β-cyclodextrin-6-yl)-1H-1,2,3-triazol-4-yl)methoxy)-11-oxo-olean-12-en-30-oic acid (39)
A solution of compound 38 (50 mg, 0.019 mmol) in methanol was added catalytic amounts of Pd–C. The resulting solution was vigorously stirred for 24 h at 0.4 MPa under hydrogen environment. The solvent was removed by steaming. The residue was purified by column chromatography to afford compound 39 as a white foam with a yield of 76%. Rf = 0.10 (PE:Acetone = 1:1); 1H NMR (600 MHz, CDCl3): δ 7.60 (s, 1H), 5.71 (d, 1H, J = 3.9 Hz), 5.67 (s, 1H), 5.38–5.27 (m, 7H), 5.19–5.17 (m, 2H), 5.13 (d, 1H, J = 4.0 Hz), 5.09 (dd, 1H, J = 4.0, 9.8 Hz), 5.05 (d, 1H, J = 3.7 Hz), 5.01 (d, 1H, J = 3.6 Hz), 4.95 (dd, 1H, J = 4.0, 8.6 Hz), 4.86–4.74 (m, 7H), 4.67 (d, 1H, J = 11.0 Hz), 4.61–4.50 (m, 7H), 4.47 (dd, 1H, J = 3.6, 10.4 Hz), 4.32–4.24 (m, 7H), 4.20–4.08 (m, 8H), 3.78–3.66 (m, 7H), 3.58 (d, 1H, J = 9.1 Hz), 3.01 (dd, 1H, J = 4.2, 11.8 Hz), 2.78 (td, 1H, J = 3.3, 13.6 Hz), 2.33 (s, 1H), 2.19–2.01 (m, 68H), 1.93–1.90 (m, 1H), 1.84–1.73 (m, 3H), 1.36, 1.21, 1.13, 1.11, 0.91 (s, each 3H, 5 × CH3), 1.64–1.00 (m, other aliphatic ring protons), 0.82, 0.80 (s, each 3H, 2 × CH3), 0.70 (d, 1H, J = 11.9 Hz); 13C NMR (150 MHz, CDCl3): δ 200.40, 180.08, 170.86, 170.78, 170.75, 170.66, 170.60, 170.57, 170.53, 170.43, 170.39, 170.31, 170.28, 169.66, 169.45, 169.42, 169.33, 169.29, 129.90, 129.87, 128.47, 125.47, 97.23, 97.07, 96.80, 96.64, 96.55, 96.47, 96.41, 85.96, 77.41, 77.30, 76.78, 76.70, 76.40, 75.82, 71.71, 71.36, 71.28, 71.04, 70.96, 70.55, 70.37, 70.33, 70.16, 70.06, 69.83, 69.67, 69.65, 69.58, 69.53, 69.49, 69.30, 62.95, 62.84, 62.60, 62.55, 62.45, 62.26, 61.75, 55.27, 49.02, 48.27, 45.45, 43.75, 43.19, 41.03, 39.05, 38.89, 37.76, 37.02, 32.75, 31.85, 31.03, 29.75, 29.67, 29.59, 29.53, 29.50, 29.45, 29.35, 29.29, 29.21, 28.52, 28.44, 28.06, 26.46, 26.41, 23.37, 22.77, 20.87, 20.85, 20.79, 20.76, 20.74, 20.73, 20.70, 20.65, 20.61, 18.68, 17.40, 16.45; ESI-HRMS (m/z) Calcd for C115H157N3O58 [M+H]+: 2508.9501. Found 2508.9487.
4.1.30. Synthesis of 3β-((1-(6A-deoxy-β-cyclodextrin-6-yl)-1H-1,2,3-triazol-4-yl)methoxy)-11-oxo-olean-12-en-30-oic acid (40)
Prepared from 39 according to general procedure B, the residue was purified by RP flash chromatography (eluent: methanol) to afford 40 as a white foam with a yield of 81%. 1H NMR (600 MHz, MeOD:CDCl3 = 2:1): δ 7.93 (s, 1H), 5.61 (s, 1H), 5.09 (d, 1H, J = 3.7 Hz), 4.97–4.94 (m, 5H), 4.92 (d, 1H, J = 3.5 Hz), 4.90 (d, 1H, J = 3.6 Hz), 4.63 (dd, 1H, J = 14.5, 7.5 Hz), 4.13–4.10 (m, 1H), 3.92–3.71 (m, 20H), 3.56 (dd, 1H, J = 9.8, 3.5 Hz), 3.54–3.43 (m, 11H), 3.36 (dd, 1H, J = 10.8, 1.5 Hz), 3.11 (dd, 1H, J = 12.2, 3.1 Hz), 3.03 (dd, 1H, J = 11.7, 4.3 Hz), 2.77 (td, 1H, J = 10.0, 3.5 Hz), 2.43 (s, 1H), 2.20 (dd, 1H, J = 12.9, 3.6 Hz), 2.11 (dt, 1H, J = 13.6, 4.3 Hz), 1.96–1.22 (m, other aliphatic ring protons), 1.41, 1.17, 1.14, 1.13 (s, each 3H, 4 × CH3), 1.04 (dd, 1H, J = 11.5, 2.3 Hz), 0.99 (dd, 1H, J = 13.8, 3.0 Hz), 0.97, 0.83, 0.81, (s, each 3H, 3 × CH3), 0.76 (d, 1H, J = 11.5 Hz); 13C NMR (150 MHz, MeOD:CDCl3 = 2:1): δ 202.46, 180.23, 172.57, 146.19, 128.78, 126.85, 103.85, 103.82, 103.72, 103.66, 103.64, 103.20, 87.61, 84.62, 82.92, 82.79, 82.77, 82.74, 82.63, 82.54, 74.47, 74.40, 74.25, 74.18, 73.99, 73.95, 73.78, 73.59, 73.56, 73.34, 73.32, 73.29, 72.95, 71.69, 63.33, 62.87, 61.57, 61.50, 60.88, 56.34, 51.91, 49.85, 49.30, 49.09, 54, 44.65, 44.37, 42.14, 39.95, 39.86, 38.73, 38.05, 33.60, 32.75, 31.82, 29.11, 28.77, 28.64, 27.36, 27.19, 23.87, 23.54, 19.25, 18.28, 16.91; ESI-HRMS (m/z) Calcd for C75H117N3O38 [M + H]+: 1668.7388. Found 1668.7393.
4.2. Cell culture and virus
H1N1 influenza virus A/WSN/33 (H1N1) was used in antiviral studies. It was propagated in MDCK cells in serum-free Eagle's minimum essential medium supplemented with 2 μg/μL trypsin and 1.2 mM bicarbonate. Titers of virus stocks were determined according to Reed and Muench (1938) in MDCK cells [41].
4.3. In vitro cytotoxicity assay
All of the reported conjugates were evaluated for cytotoxicity in MDCK cells. The cells (1 × 104 cells per well) were seeded into 96-well tissue culture plates and incubated at 37 °C for 24 h in an atmosphere of 5% CO2 to allow the cells to adhere to the surface of the wells. Subsequently, the culture medium was replaced with fresh medium containing the compounds at the concentration of 50 μM in triplicate, and control wells contained the equivalent volume of the medium containing 1% DMSO. After 36 h of incubation at 37 °C in an atmosphere of 5% CO2, CellTiter-Glo reagent was added, and the plates were read using a Tecan Infinite M2000 PRO™ plate reader.
4.4. CPE reduction assay
The assay was performed as described by Noah et al. [38] with some modifications. MDCK cells were seeded into 96-well plates, incubated overnight and infected with influenza virus (MOI = 0.1) suspended in DMEM supplemented with 1% FBS, test compound and 2 μg/mL TPCK-treated trypsin, with a final DMSO concentration of 1% in each well, followed by 40 h of incubation, CellTiter-Glo reagent was added, and the plates were read using a Tecan Infinite M2000 PRO™ plate reader.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant no. 81573269, 21572015, 21877007, 91753202 and 21702007) and the open funding of the State Key Laboratory of Phytochemistry and Plant Resources in West China.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejmech.2019.01.074.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Yang R., Wang L.Q., Yuan B.C., Liu Y. The pharmacological activities of Licorice. Planta Med. 2015;81:1654–1669. doi: 10.1055/s-0035-1557893. [DOI] [PubMed] [Google Scholar]
- 2.Paolino D., Lucania G., Mardente D., Alhaique F., Fresta M. Ethosomes for skin delivery of ammonium glycyrrhizinate: In vitro percutaneous permeation through human skin and in vivo anti-inflammatory activity on human volunteers. J. Contr. Release. 2005;106:99–110. doi: 10.1016/j.jconrel.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Takeda S., Ishihara K., Wakui Y., Amagaya S., Maruno M., Akao T., Kobashi K. Bioavailability study of glycyrrhetic acid after oral administration of glycyrrhizin in rats; Relevance to the intestinal bacterial hydrolysis. J. Pharm. Pharmacol. 1996;48:902–905. doi: 10.1111/j.2042-7158.1996.tb05998.x. [DOI] [PubMed] [Google Scholar]
- 4.Sabbioni C., Mandrioli R., Ferranti A., Bugamelli F., Saracino M.A., Forti G.C., Fanali S., Raggi M.A. Separation and analysis of glycyrrhizin, 18β-glycyrrhetic acid and 18α-glycyrrhetic acid in liquorice roots by means of capillary zone electrophoresis. J. Chromatogr. A. 2005;1081:65–71. doi: 10.1016/j.chroma.2005.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hussain H., Green I.R., Shamraiz U., Saleem M., Badshah A., Abbas G., Rehman N.U., Irshad M. Therapeutic potential of glycyrrhetinic acids: a patent review (2010-2017) Expert Opin. Ther. Pat. 2018;28:383–398. doi: 10.1080/13543776.2018.1455828. [DOI] [PubMed] [Google Scholar]
- 6.van Rossum T.G.J., Vulto A.G., Hop W.C.J., Schalm S.W. Pharmacokinetics of intravenous glycyrrhizin after single and multiple doses in patients with chronic hepatitis C infection. Clin. Therapeut. 1999;21:2080–2090. doi: 10.1016/s0149-2918(00)87239-2. [DOI] [PubMed] [Google Scholar]
- 7.Pompei R., Flore O., Marccialis M.A., Pani A., Loddo B. Glycyrrhizic acid inhibits virus growth and inactivates virus-particles. Nature. 1979;281:689–690. doi: 10.1038/281689a0. [DOI] [PubMed] [Google Scholar]
- 8.Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361:2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baltina L.A., Zarubaev V.V., Baltina L.A., Orshanskaya I.A., Fairushina A.I., Kiselev O.I., Yunusov M.S. Glycyrrhizic acid derivatives as influenza A/H1N1 virus inhibitors. Bioorg. Med. Chem. Lett. 2015;25:1742–1746. doi: 10.1016/j.bmcl.2015.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aguilar-Rosas I., Alcala-Alcala S., Llera-Rojas V., Ganem-Rondero A. Preparation and characterization of mucoadhesive nanoparticles of poly (methyl vinyl ether-co-maleic anhydride) containing glycyrrhizic acid intended for vaginal administration. Drug Dev. Ind. Pharm. 2015;41:1632–1639. doi: 10.3109/03639045.2014.980425. [DOI] [PubMed] [Google Scholar]
- 11.Lin J.C., Cherng J.M., Hung M.S., Baltina L.A., Baltina L., Kondratenko R. Inhibitory effects of some derivatives of glycyrrhizic acid against Epstein-Barr virus infection: structure-activity relationships. Antivir. Res. 2008;79:6–11. doi: 10.1016/j.antiviral.2008.01.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardy M.E., Hendricks J.M., Paulson J.M., Faunce N.R. 18β-glycyrrhetinic acid inhibits rotavirus replication in culture. Virol. J. 2012;9 doi: 10.1186/1743-422X-9-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L.J., Geng C.A., Ma Y.B., Huang X.Y., Luo J., Chen H., Zhang X.M., Chen J.J. Synthesis, biological evaluation and structure-activity relationships of glycyrrhetinic acid derivatives as novel anti-hepatitis B virus agents. Bioorg. Med. Chem. Lett. 2012;22:3473–3479. doi: 10.1016/j.bmcl.2012.03.081. [DOI] [PubMed] [Google Scholar]
- 14.Zigolo M.A., Salinas M., Alche L., Baldessari A., Linares G.G. Chemoenzymatic synthesis of new derivatives of glycyrrhetinic acid with antiviral activity. Molecular docking study. Bioorg. Chem. 2018;78:210–219. doi: 10.1016/j.bioorg.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 15.Hao J.J., Sun Y.M., Wang Q., Tong X.A., Zhang H., Zhang Q. Effect and mechanism of penetration enhancement of organic base and alcohol on Glycyrrhetinic acid in vitro. Int. J. Pharm. 2010;399:102–108. doi: 10.1016/j.ijpharm.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Takiura K., Honda S., Yamamoto M., Takai H., Kii M., Yuki H. Studies of oligosaccharides .12. Hydrophilization of glycyrrhetinic acid by coupling to gentio oligosaccharides. Chem. Pharm. Bull. 1974;22:1618–1623. doi: 10.1248/cpb.22.1618. [DOI] [PubMed] [Google Scholar]
- 17.Suvarna V., Gujar P., Murahari M. Complexation of phytochemicals with cyclodextrin derivatives - an insight. Biomed. Pharmacother. 2017;88:1122–1144. doi: 10.1016/j.biopha.2017.01.157. [DOI] [PubMed] [Google Scholar]
- 18.Jambhekar S.S., Breen P. Cyclodextrins in pharmaceutical formulations II: solubilization, binding constant, and complexation efficiency. Drug Discov. Today. 2016;21:363–368. doi: 10.1016/j.drudis.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 19.Wang H.M., Soica C.M., Wenz G. A comparison investigation on the solubilization of betulin and betulinic acid in cyclodextrin derivatives. Nat Prod Commun. 2012;7:289–291. [PubMed] [Google Scholar]
- 20.Loopez-Miranda S., Guardiola L., Hernandez-Sanchez P., Nunez-Delicado E. Complexation between oleanolic and maslinic acids with native and modified cyclodextrins. Food Chem. 2018;240:139–146. doi: 10.1016/j.foodchem.2017.07.092. [DOI] [PubMed] [Google Scholar]
- 21.Soica C., Trandafirescu C., Danciu C., Muntean D., Dehelean C., Simu G. New improved drug delivery technologies for pentacyclic triterpenes: a review. Protein Pept. Lett. 2014;21:1137–1145. doi: 10.2174/0929866521666140807115109. [DOI] [PubMed] [Google Scholar]
- 22.Ishida T., Miki I., Tanahashi T., Yagi S., Kondo Y., Inoue J., Kawauchi S., Nishiumi S., Yoshida M., Maeda H., Tode C., Takeuchi A., Nakayama H., Azuma T., Mizuno S. Effect of 18 β-glycyrrhetinic acid and hydroxypropyl gamma cyclodextrin complex on indomethacin-induced small intestinal injury in mice. Eur. J. Pharmacol. 2013;714:125–131. doi: 10.1016/j.ejphar.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Uekama K., Minami K., Hirayama F. 6A-O-[(4-biphenylyl)acetyl]-α-, -β-, and -γ-cyclodextrins and 6A-deoxy-6A-[[(4-biphenylyl)acetyl]amino]-α-, -β-, and -γ-cyclodextrins: potential prodrugs for colon-specific delivery. J. Med. Chem. 1997;40:2755–2761. doi: 10.1021/jm970130r. [DOI] [PubMed] [Google Scholar]
- 24.Udo K., Hokonohara K., Motoyama K., Arima H., Hirayama F., Uekama K. 5-Fluorouracil acetic acid/β-cyclodextrin conjugates: drug release behavior in enzymatic and rat cecal media. Int. J. Pharm. 2010;388:95–100. doi: 10.1016/j.ijpharm.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 25.Onodera R., Motoyama K., Okamatsu A., Higashi T., Arima H. Potential use of folate-appended methyl-β-cyclodextrin as an anticancer agent. Sci. Rep. 2013;3:1104. doi: 10.1038/srep01104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao S.L., Wang Q., Yu F., Peng Y.Y., Yang M., Sollogoub M., Sinay P., Zhang Y.M., Zhang L.H., Zhou D.M. Conjugation of cyclodextrin with fullerene as a new class of HCV entry inhibitors. Bioorg. Med. Chem. 2012;20:5616–5622. doi: 10.1016/j.bmc.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 27.Jiang R.J., Zhao Y.L., Chen Y.J., Xiao D., Wang F., Han B., Yang J., Liao X.L., Yang L.J., Gao C.Z., Yang B. Synthesis, characterization, and in vitro evaluation of artesunate-β-cyclodextrin conjugates as novel anti-cancer prodrugs. Carbohydr. Res. 2014;400:19–25. doi: 10.1016/j.carres.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 28.Xiao S., Wang Q., Si L., Shi Y., Wang H., Yu F., Zhang Y., Li Y., Zheng Y., Zhang C., Wang C., Zhang L., Zhou D. Synthesis and anti-HCV entry activity studies of β-cyclodextrin-pentacyclic triterpene conjugates. ChemMedChem. 2014;9:1060–1070. doi: 10.1002/cmdc.201300545. [DOI] [PubMed] [Google Scholar]
- 29.Xiao S., Si L., Tian Z., Jiao P., Fan Z., Meng K., Zhou X., Wang H., Xu R., Han X., Fu G., Zhang Y., Zhang L., Zhou D. Pentacyclic triterpenes grafted on CD cores to interfere with influenza virus entry: a dramatic multivalent effect. Biomaterials. 2016;78:74–85. doi: 10.1016/j.biomaterials.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 30.Yu F., Wang Q., Zhang Z., Peng Y., Qiu Y., Shi Y., Zheng Y., Xiao S., Wang H., Huang X., Zhu L., Chen K., Zhao C., Zhang C., Yu M., Sun D., Zhang L., Zhou D. Development of oleanane-type triterpenes as a new class of HCV entry inhibitors. J. Med. Chem. 2013;56:4300–4319. doi: 10.1021/jm301910a. [DOI] [PubMed] [Google Scholar]
- 31.Yu M., Si L., Wang Y., Wu Y., Yu F., Jiao P., Shi Y., Wang H., Xiao S., Fu G., Tian K., Wang Y., Guo Z., Ye X., Zhang L., Zhou D. Discovery of pentacyclic triterpenoids as potential entry inhibitors of influenza viruses. J. Med. Chem. 2014;57:10058–10071. doi: 10.1021/jm5014067. [DOI] [PubMed] [Google Scholar]
- 32.Xiao S., Tian Z., Wang Y., Si L., Zhang L., Zhou D. Recent progress in the antiviral activity and mechanism study of pentacyclic triterpenoids and their derivatives. Med. Res. Rev. 2018;38:951–976. doi: 10.1002/med.21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H., Yu F., Peng Y., Wang Q., Han X., Xu R., Zhou X., Wan C., Fan Z., Jiao P., Zhang Y., Zhang L., Zhou D., Xiao S. Synthesis and biological evaluation of ring A and/or C expansion and opening echinocystic acid derivatives for anti-HCV entry inhibitors. Eur. J. Med. Chem. 2015;102:594–599. doi: 10.1016/j.ejmech.2015.08.034. [DOI] [PubMed] [Google Scholar]
- 34.Vizitiu D., Walkinshaw C.S., Gorin B.I., Thatcher G.R.J. Synthesis of monofacially functionalized cyclodextrins bearing amino pendent groups. J. Org. Chem. 1997;62:8760–8766. [Google Scholar]
- 35.Zemplén G., Pascu E. The saponification acetyl sugar and relative substances. Ber. Dtsch. Chem. Ges. 1929;62:1613–1614. [Google Scholar]
- 36.Tetko I.V., Tanchuk V.Y., Villa A.E.P. Prediction of n-octanol/water partition coefficients from PHYSPROP database using artificial neural networks and E-state indices. J. Chem. Inf. Comput. Sci. 2001;41:1407–1421. doi: 10.1021/ci010368v. [DOI] [PubMed] [Google Scholar]
- 37.Lovering F., Bikker J., Humblet C. Escape from Flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 2009;52:6752–6756. doi: 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]
- 38.Schwarz S., Lucas S.D., Sommerwerk S., Csuk R. Amino derivatives of glycyrrhetinic acid as potential inhibitors of cholinesterases. Bioorg. Med. Chem. 2014;22:3370–3378. doi: 10.1016/j.bmc.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 39.Beseda I., Czollner L., Shah P.S., Khunt R., Gaware R., Kosma P., Stanetty C., del Ruiz-Ruiz M.C., Amer H., Mereiter K., Da Cunha T., Odermatt A., Classen-Houben D., Jordis U. Synthesis of glycyrrhetinic acid derivatives for the treatment of metabolic diseases. Bioorg. Med. Chem. 2010;18:433–454. doi: 10.1016/j.bmc.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 40.Csuk R., Schwarz S., Siewert B., Kluge R., Strohl D. Synthesis and antitumor activity of ring A modified glycyrrhetinic acid derivatives. Eur. J. Med. Chem. 2011;46:5356–5369. doi: 10.1016/j.ejmech.2011.08.038. [DOI] [PubMed] [Google Scholar]
- 41.Ree L.J., Muench H. A simple method of estimating fifty percent endpoint. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.