Highlights
► We perform a case–case comparison as an improvement of the test-negative design. ► We report IVE estimates with a low probability of bias. ► Influenza vaccination halved the risk of confirmed influenza hospitalization. ► This effect was consistent regardless of age over 60. ► The measured effect was specific for confirmed influenza hospitalizations.
Keywords: Case-control studies; Hospitalizations; Humans; Influenza vaccines; Risk factors; Vaccines, Inactivated; Reverse transcriptase polymerase chain reaction; Influenza, Human/epidemiology; Respiratory syncytial virus infections/epidemiology
Abstract
Introduction
We estimated influenza vaccine effectiveness (IVE) to prevent laboratory-confirmed influenza-related hospitalizations in patients 18 years old or older during the 2010–2011 influenza season.
Methods
We conducted a prospective case-control study in five hospitals, in Valencia, Spain. Study subjects were consecutive emergency hospitalizations for predefined conditions associated with an influenza-like illness episode <8 days before admission. Patients were considered immunized if vaccinated ≥14 days before influenza-like illness onset. Cases were those with a real time reverse transcriptase polymerase chain reaction (RT-PCR) positive for influenza and controls were RT-PCR positive for other respiratory viruses. Adjusted IVE was estimated as 100 × (1 − adjusted odds ratio). To account for indication bias we computed adjusted IVE for respiratory syncytial virus related hospitalizations.
Results
Of 826 eligible hospitalized patients, 102 (12%) were influenza positive and considered cases, and 116 (14%) were positive for other respiratory viruses and considered controls. Adjusted IVE was 54% (95% confidence interval, 11–76%). By subgroup, adjusted IVE was 53% (4–77%) for those with high-risk conditions, 59% (16–79%) for those ≥60 years of age, and, 54% (4–79%) for those ≥60 years of age with high-risk conditions. No influenza vaccine effect was observed against respiratory syncytial virus related hospitalization.
Conclusion
Influenza vaccination was associated with a significant reduction on the risk of confirmed influenza hospitalization, irrespective of age and high-risk conditions.
1. Introduction
Yearly seasonal influenza epidemics are associated with excess morbidity and mortality [1]. Vaccination against influenza is considered the most effective strategy for preventing influenza [2]. As a consequence of antigenic drift, influenza vaccines are to be a produced every year [3]. Despite this achievement, vaccine effectiveness varies from season to season and can be very low one in four influenza seasons [4]. This is due to the unpredictable antigenic distance between vaccine's and the circulating strains [5]. As a consequence, evidence on influenza vaccine effectiveness has been difficult to obtain and is disputed [4], [6], [7], [8].
The reappraisal of the evidence on influenza vaccine effectiveness is possible by the availability of reverse transcriptase polymerase chain reaction (RT-PCR) to diagnose influenza infection [9]. RT-PCR has allowed the development of the test-negative approach for measuring influenza vaccine effectiveness.
In test-negative case-control studies, cases are RT-PCR positive for influenza, and controls those negative for influenza. This approach has been advocated for its practicability, comparability between cases and controls, and the use of laboratory confirmed outcomes [8], [10]. Various authors have used the test-negative case-control study [11], [12], [13], [14], [15], [16], [17], [18]. Under conditions of concurrent circulation an appropriate test-negative control group are patients testing positive for other respiratory viruses, ensuring similarity on quality of sample collection and specificity of outcomes [10], [15], [19].
Using a prospective case–case comparison approach, we have estimated seasonal influenza vaccine effectiveness (IVE) to prevent laboratory confirmed influenza-related hospitalizations in adults.
2. Methods
2.1. Study design
During the 2010–2011 influenza season, we performed a prospective case-control study in five hospitals in Valencia, Spain. The five hospitals provided care to 975,174 inhabitants 18 years of age or older.
The influenza season was defined by the weeks with positive specimens for influenza on enrolled patients. It began on 12 December 2010 (week 50) and ended on 19 March 2011 (week 11). Patients with confirmed influenza by a RT-PCR test were considered cases and patients with an RT-PCR confirmed infection for other respiratory viruses were considered controls.
Influenza vaccines were offered free of charge to health district inhabitants older than 6 months of age with high-risk conditions and to 60 years old or older. Three vaccine formulations were used. Subunit trivalent non-adjuvanted vaccine (Influvac®, Abbot-Solvay, Illinois, USA; batch numbers V4, V20, V23) offered to subjects less that 60 years of age, virosomal trivalent subunit vaccine (Inflexal®-V, Crucell, Leiden, The Netherlands; batch numbers 300187601, 300189301, 300194401) offered to subjects 60 years old or older, and an MF59™-adjuvanted trivalent subunit vaccine (Chiromas®, Novartis Vaccines and Diagnostics, Massachusetts, USA; batch numbers 104603, 104702, 104802, 105001) offered by licensure requirements to those 65 years old or older. Subunit trivalent non-adjuvanted was offered in the five health districts included in the study; virosomal vaccine was used in two, and the MF59™-adjuvanted vaccine was used in three. The strains included in the influenza vaccine for the 2010–2011 season were A/California/7/2009 (H1N1)-like, A/Perth/16/2009 (H3N2)-like, and B/Brisbane/60/2008-like [20].
2.2. Study subjects identification, criteria for inclusion
We established an active surveillance system. Full-time field researchers identified, Monday to Saturday, patients who were hospitalized, coming from the Emergency Department, in the previous 24–48 h. Patients whose indications for admission were any of a predefined set of conditions, described as possibly associated with a recent influenza infection [14], were invited to participate.
Patients were excluded if institutionalized, not permanent residents, with reported egg allergy, had been hospitalized in the previous 30 days, or if they had had a previous laboratory confirmed influenza infection. Patients were included if they reported an ILI episode, defined as at least one of these four systemic symptoms (fever or feverishness, malaise, headache, myalgia) and at least one of these three respiratory symptoms (cough, sore throat, shortness of breath), sudden onset was not a requisite for inclusion [21], less than 8 days preceding their arrival at the Emergency Department. The Ethics Research Committee of the Centro Superior de Investigación en Salud Pública (CSISP) approved the study. All study subjects gave written informed consent before enrollment.
2.3. Laboratory procedures
A nasopharyngeal and a pharyngeal swab were obtained from each included patient. Samples were introduced into vials with viral transport medium and kept at −20 °C until sent to the reference laboratory.
Four multiplex real-time RT-PCR/PCR qualitative amplifications were performed: multiplex # 1 for influenza virus type A [22] and influenza virus type B [23]); multiplex # 2 for coronavirus, metapneumovirus, and bocavirus [24], [25], [26]; multiplex # 3 for respiratory syncytial virus (RSV), adenovirus and parainfluenza virus [27]; and multiplex # 4 for rhinovirus [28]. Negative results for viruses were only considered if human ribonucleoprotein gene amplification was positive. Laboratory procedures to prevent PCR contamination were followed and a series of multiplex assays #1 to #4 negative controls without sample nucleic acid were included in all runs.
2.4. Data collection
Information was obtained on age, sex, indications for inclusion, hospitalization date, time elapsed from symptoms onset to swabbing, presence of major underlying medical conditions, long-term treatments, contact with children, smoking habits, occupation, number of physician encounters in the last three months, number of hospitalizations in the last year, prescription of antivirals, intensive care unit admission, death in hospital and length of stay. Functional status, measured by Barthel index [29], was obtained in study subjects 65 years old or older. Social class was assigned according to occupation [30].
2.5. Influenza vaccination and immunization status
Influenza vaccination status was obtained by asking the patient if he or she had received the current season's influenza vaccine, on which month, and if the vaccine had been administered at least two weeks before the onset of symptoms. In addition, vaccination status was independently ascertained by a researcher blinded to patient characteristics, who consulted Valencia's population-based Vaccine Information System. A patient was considered immunized with the 2010–2011 influenza vaccine if the vaccine was registered as administered 14 or more days before the date of ILI onset or if the patient recalled the month when the vaccine was administered and if it had been administered more than two weeks previous to current ILI episode onset. Information related to the administration of the 2009–2010 seasonal influenza vaccine, the A(H1N1) pandemic vaccine and previous 23-valent polysaccharide plain pneumococcal vaccinations was obtained from the Vaccine Information System.
2.6. Vaccine-effectiveness
IVE was defined as 100 × (1 − adjusted odds ratio [OR])[31], [32]. The adjusted OR was obtained using a logistic regression model using stepwise background selection of the variables, with a criterion of P < 0.1 to remain in the model, starting with a fully saturated model, including being immunized with current season's vaccine, sex, age (in 10 years of age intervals), socioeconomic class, number of high-risk conditions, obesity (IMC ≥ 40), smoking antecedents, number of physician encounters in the last three months, hospitalizations in the last year, pneumococcal vaccination, antiviral prescription, epidemiological week and time from symptoms onset to swabbing.
We defined four groups for IVE estimation: (a) all cases and controls enrolled (overall group); (b) all cases and controls with high-risk conditions regardless of age; (c) cases and controls 60 years old or older and (d) cases and controls 60 years old or older with high-risk conditions. To validate our estimates, we computed IVE against RSV-related hospitalization following the same design and analysis strategy followed for influenza-related hospitalization, but in this instance cases were those positive for RSV and controls those positive for the other respiratory viruses, including influenza.
The significance in differences in the distribution of covariates, between cases and controls, was estimated using the chi-squared, or Fisher's test, for categorical variables; and the t-test, or Kruskal–Wallis test, for continuous variables; P < 0.05 was considered significant. All probabilities were 2-tailed. All analyses were performed with Stata 12.1 (StataCorp, College Station, TX).
3. Results
3.1. Ascertainment of cases and controls
We identified 2286 eligible patients, 826 complied with all inclusion criteria, 102 (12%) were positive for influenza and considered cases; 116 (14%) were positive for other respiratory viruses and considered controls (Fig. 1 ). Swabs were performed 7 days or less after onset of symptoms in 93% of study subjects. Time from onset to swabbing was similar between cases and controls, median was in both instances four days, P = 0.28.
Fig. 1.
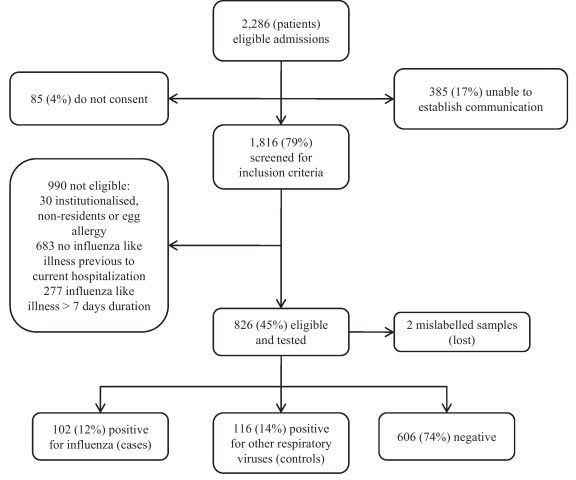
Flow chart of study subjects.
3.2. Hospitalizations related to a viral respiratory infection
Hospitalization rate associated with any of the respiratory viruses assessed was 22 per 100,000 18 years old or older. By age group, hospitalization rate associated to respiratory viruses was 5, 25, 44, and 90 per 100,000 18–49, 50–64, 65–74, and 75 years old or older, respectively. Influenza-related hospitalization rate was 4, 17, 14, and 28 per 100,000 18–49, 50–64, 65–74 and 75 years old and older.
Type and number of virus identified were: H1N1pdm09, 74 (34%); RSV, 59 (27%); rhinovirus, 22 (10%); coronavirus, 18 (8%); influenza B, 16 (7%); parainfluenza virus, 6 (3%); mixed infections, 10 (5%); H3N2, 7 (3%); and human metaneumovirus, 6 (3%). Including mixed infections, influenza accounted for 47% (n = 102) of all respiratory viruses identified. Influenza subtypes identified were H1N1pdm09 (n = 78; 76%), influenza B (n = 17; 17%), and H3N2 (n = 7; 7%). Influenza viruses circulated concurrently with RSV, rhinovirus and coronavirus (Fig. 2 ).
Fig. 2.
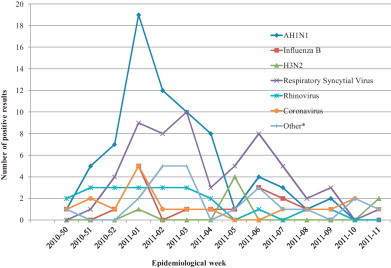
Number and type of viruses identified by epidemiological week, 2010–2011 influenza season. * Other: mixed infections, parainfluenza virus, and metaneumovirus.
3.3. Emergency admission diagnoses
There were no differences regarding emergency admission diagnoses between cases and controls; with the exception of heart failure, that was 3% in cases compared to 10% in controls (P = 0.057) (Table 1 ). In 17% of influenza patients the presenting complain was not for a respiratory condition (Table 1).
Table 1.
Indications for admission and influenza-like illness manifestations in cases and controls.a
| Cases N = 102 % |
Controls N = 116 % |
P value | |
|---|---|---|---|
| Emergency admission diagnoses (any)b | |||
| Acute respiratory infection | 46.1 | 39.7 | 0.3390 |
| Pneumonia | 31.4 | 24.1 | 0.2330 |
| COPD | 18.6 | 21.6 | 0.5910 |
| Dyspnea | 38.2 | 44.0 | 0.3910 |
| Myalgias | 2.9 | 1.7 | 0.6670 |
| Acute coronary syndrome | 2.0 | 6.9 | 0.1090 |
| Heart failure | 2.9 | 9.5 | 0.0570 |
| Acute cerebrovascular disease | 0.0 | 1.72 | 0.5000 |
| Metabolic failurec | 2.9 | 0.9 | 0.3420 |
| Confusion, convulsion | 5.9 | 2.6 | 0.3100 |
| Sepsis, SIRS | 2.0 | 3.5 | 0.6870 |
| Influenza like illness manifestations | |||
| Duration: mean days (rage) | 3 (0–7) | 3 (0–7) | 0.6503 |
| Sudden onset | 74.5 | 69.83 | 0.4420 |
| Fever or feverishness | 89.2 | 73.28 | 0.0030 |
| Headache, malaise or myalgia | 91.2 | 88.79 | 0.5600 |
| Cough | 95.1 | 92.2 | 0.3910 |
| Shortness of breath | 90.2 | 93.1 | 0.4360 |
| Sore throat | 43.1 | 49.1 | 0.3750 |
Cases (influenza positive) and controls (positive for other than influenza respiratory virus).
COPD: chronic pulmonary obstructive disease. SIRS: systemic inflammatory response syndrome.
Metabolic failure encompasses hyperglycemic or hypoglycemic commas, acute renal failure, and disorders of fluid, electrolyte and acid–base balance.
3.4. Influenza-like illness
The duration of symptoms previous to admission was evenly distributed between cases and controls, P = 0.65 (Table 1). There were no differences in the percentage of sudden onset of symptoms, malaise, myalgia, headache, sore throat or shortness of breath; the only significant difference was the frequency of fever in influenza cases, P = 0.003 (Table 1).
3.5. Case-control characteristics
When compared to controls, cases were younger, had fewer high-risk conditions, were of higher social class, more frequently smokers, and consulted their general practitioners or had been hospitalized in fewer occasions (Table 2 ). There were no differences between cases and controls aged 65 years or more in their Barthel index scores (Table 2).
Table 2.
Cases and controlsa characteristics and vaccination history by group: overall, high-risk conditions, and 60 years old or older.
| Overall | Cases N = 102 % |
Controls N = 116 % |
P value |
|---|---|---|---|
| Age ≥ 65 | 42.2 | 80.2 | <0.0001 |
| Age group | |||
| 18–49 | 24.5 | 4.3 | <0.0001 |
| 50–64 | 33.3 | 15.5 | |
| 65–74 | 13.7 | 25.0 | |
| ≥75 | 28.4 | 55.2 | |
| Male | 55.9 | 49.1 | 0.3200 |
| High risk conditionsb | |||
| None | 22.6 | 10.3 | 0.0360 |
| One | 31.4 | 31.0 | |
| Two or more | 46.1 | 58.6 | |
| GP consultations | |||
| None | 25.5 | 19.0 | 0.0110 |
| One | 34.3 | 20.7 | |
| Two or more | 40.2 | 60.3 | |
| Hospitalized last 12 months | 27.5 | 43.1 | 0.0160 |
| Barthel index (over 136 subjects 65 years old or older) | N = 43 | N = 93 | |
| <20 Total | 2.3 | 0 | 0.1600 |
| 20–35 Severe | 0 | 1.1 | |
| 40–55 Moderate | 4.7 | 14.0 | |
| ≥60 Mild | 93.2 | 85.0 | |
| Social classc | |||
| I-III N | 25.9 | 11.2 | 0.0230 |
| III-M | 12.8 | 14.7 | |
| IV-V | 61.4 | 73.9 | |
| Never smoker | 42.2 | 56.9 | 0.0300 |
| Contact with children | 38.2 | 33.6 | 0.4780 |
| ICU admission | 5.9 | 1.7 | 0.1510 |
| Death | 2.0 | 4.3 | 0.4520 |
| Length of stay mean (SE) | 0.45 | 0.44 | 0.4303 |
| Vaccine | |||
| 23-valent Pneumococcal | 12.8 | 25.9 | 0.0150 |
| Influenza seasonal 2009 | 35.3 | 64.7 | <0.0001 |
| Influenza pandemic | 21.6 | 30.2 | 0.0162 |
| Influenza seasonal 2010d | 36.3 | 66.4 | <0.0001 |
| High-risk conditions | N = 79% | N = 104% | |
|---|---|---|---|
| Age group | |||
| 18–49 | 16.5 | 2.9 | <0.0001 |
| 50–64 | 36.7 | 14.4 | |
| 65–74 | 13.9 | 25.0 | |
| ≥75 | 32.9 | 57.7 | |
| High risk conditionsb | |||
| At least one | 40.5 | 34.6 | 0.4140 |
| Two or more | 59.5 | 65.4 | |
| Visits to GP | |||
| None | 29.1 | 18.3 | 0.0790 |
| One | 26.6 | 21.2 | |
| Two or more | 44.3 | 60.6 | |
| Vaccines administered | |||
| 23-valent Pneumococcal | 16.5 | 28.9 | 0.0500 |
| Influenza seasonal 2009 | 40.5 | 66.4 | <0.0001 |
| Influenza pandemic | 27.9 | 33.7 | 0.4010 |
| Influenza seasonal 2010d | 41.8 | 68.3 | <0.0001 |
| 60 years old or older | N = 58% | N = 103% | |
|---|---|---|---|
| Age group | |||
| 60–64 | 25.9 | 9.7 | 0.0250 |
| 65–74 | 24.2 | 28.2 | |
| ≥75 | 50 | 62.1 | |
| High risk conditions b | |||
| None | 12.7 | 6.8 | 0.5070 |
| At least one | 27.6 | 31.1 | |
| Two or more | 60.3 | 62.1 | |
| Visits to GP | |||
| None | 20.7 | 18.5 | 0.2180 |
| One | 34.5 | 23.3 | |
| Two or more | 44.8 | 58.3 | |
| Vaccines administered | |||
| 23-valent Pneumococcal | 19.0 | 29.1 | 0.1550 |
| Influenza seasonal 2009 | 51.7 | 70.9 | 0.0150 |
| Influenza pandemic | 29.3 | 31.2 | 0.8160 |
| Influenza seasonal 2010d | 51.7 | 72.8 | 0.0007 |
GP: General practitioner, ICU: intensive care unit, Barthel Index: index of functional daily life activities, SE: standard error.
Cases (influenza positive) and controls (positive for other than influenza respiratory virus).
High-risk conditions: cardiovascular disease, chronic pulmonary disease, chronic hepatic disease, chronic renal disease, diabetes mellitus, neuromuscular disorders, neoplasm, immunodeficiency or immunosuppressive therapies, obesity (body mass index, 40 and over).
I: Professional, II: Managerial and technical, III: Skilled: (N) Non-manual, (M) Manual, IV: Partly skilled, V: Unskilled.
Vaccinated 14 or more days before symptoms onset.
When restricting the comparison, between cases and controls, by the presence of high-risk conditions, the differences that remained significant were age, 23-valent pneumococcal vaccination, and having been vaccinated with the previous or current season influenza vaccines (Table 2). When restricted to those 60 years old or older, age and influenza vaccination with the previous or current seasonal influenza vaccine remained as significant differences between cases and controls (Table 2).
3.6. Vaccination status
Compared to 36.3% cases, 66.4% controls were immunized with the 2010 influenza seasonal vaccine; P < 0.0001 (Table 2). Controls also had more often been vaccinated with the 23-valent pneumococcal polysaccharide vaccine (P = 0.015), 2009 seasonal influenza (P < 0.0001), and 2009 pandemic (P = 0.0162) vaccines (Table 2). When high-risk conditions or age were taken into account, no differences were observed in the percentage of cases and controls vaccinated with the pandemic vaccine, and only in those with high-risk conditions, irrespective of age, there was a difference (P = 0.05) in pneumococcal vaccination (17% cases vs. 29% controls) (Table 2). This difference was not observed between cases and controls 60 years old or older (Table 2). According to the presence of high-risk conditions or age, the percentage of controls who had been vaccinated with the 2010 vaccine compared to cases remained significantly higher (Table 2). Current influenza season vaccination was highly associated with previous influenza-seasonal vaccination (P < 0.0001) and age 60 or older (P < 0.0001).
3.7. Influenza vaccine effectiveness
Adjusted vaccine effectiveness to prevent confirmed influenza-associated hospitalization was 54% (95%CI, 11–76%) (Table 3 ). For the subgroup analysis, in those with high-risk conditions influenza vaccine effectiveness estimate was 53% (95%CI, 4–77%); for those 60 years old or older, it was 59% (95%CI, 16–79%); and for those 60 years old or older with high-risk conditions it was 54% (95%CI, 4–79%) (Table 3). The overall adjusted OR of RSV-associated hospitalization of 2010 seasonal influenza vaccination was 1.2 (95%CI, 0.6–2.4); for those with high-risk conditions it was 1.4 (95%CI, 0.7–3.0); for those 60 years old or older, 1.5 (95%CI, 0.5–3.1); and for those 60 years old or older with high-risk conditions, 1.7 (95%CI, 0.8–3.7).
Table 3.
Non-adjusted and adjusted 2010–2011 seasonal influenza vaccine effectiveness (IVE) to prevent influenza-related hospitalizations.a
| Group at risk | Cases vaccinated |
Controls vaccinated |
IVE (95%CI) Non-adjusted | IVE (95%CI) adjustedb | ||
|---|---|---|---|---|---|---|
| n/N (%) | n/N (%) | |||||
| All (≥18 years old or older) | 37/102 | (36.3) | 77/116 | (66.4) | 71.2% (48.2–84.0%) | 53.9% (11.4–76.0%) |
| All with at least one HRC | 33/79 | (41.8) | 71/104 | (68.3) | 66.7% (37.2–82.3%) | 53.4% (4.1–77.3%) |
| 60 years or older | 30/58 | (51.7) | 75/103 | (72.8) | 60.0% (20.1–80.0%) | 58.5% (16.1–79.4%) |
| 60 years or older with at least one HRC | 28/51 | (54.9) | 70/96 | (72.9) | 54.8% (6.5–78.1%) | 53.7% (3.6–77.8%) |
HRC: High-risk condition.
Cases (influenza positive) and controls (positive for other than influenza respiratory virus).
Adjusted estimates were obtained by stepwise logistic regression selection, P for exclusion ≥0.1, with all relevant covariates included in the model. VE: vaccine effectiveness: (1 − odds ratio) × 100. Variables remaining in the model by group at risk analysis: (a) All: age, epidemiological week and number of general practitioner consultations in the last three months consultations; (b) All with high risk conditions: age, epidemiological week and number of general practitioner consultations in the last three months consultations; (c) Sixty years old or older: never smoker and social class; (d): Sixty years old or older with high risk conditions: never smoker.
4. Discussion
Subjects vaccinated experienced a risk of influenza-related hospitalization two times lower compared to the unvaccinated. The vaccine preventive effect was specific for influenza.
4.1. How our findings relate to previous knowledge
Three recent systematic reviews of studies reporting influenza vaccine efficacy or effectiveness [4], [7], [33] reach the conclusion that evidence on IVE to prevent influenza in older adults is scarce, elusive or non-existent. Osterholm et al. [4] looked for studies published between January 1967 and February 2011 with outcomes confirmed by RT-PCR or viral culture, as estimates based on serologic outcomes [34] overestimate inactivated vaccine efficacy [9]. They identified only two observational studies in older adults [16], [35]. Talbot et al. [16] studied three consecutive seasons (2006–2009), using a test-negative case-control design, and reported a pooled adjusted IVE of 61% for influenza-related hospitalizations; however, these estimates were only significant when pooled over three seasons. More recently, Castilla et al. [18] conclude that IVE for preventing laboratory-confirmed influenza-related hospitalization in adults 60 years of age or older, during the 2010–2011 influenza season, is 58% to 59%.
4.2. Viral match and vaccine effectiveness
Influenza vaccine effectiveness depends closely on the match of the vaccine strain to the circulating strain [4], [5]. During the 2010–2011 influenza season, 36% of hospitalized subjects with confirmed influenza had been immunized with the seasonal vaccine. This was in clear contrast to what was observed during the 2009–2010 autumn pandemic wave, when, in presence of a good match between the circulating and the vaccine strain, vaccine failures were rare [14]. The percentage of vaccine failures observed during the 2010–2011 influenza season can be interpreted considering that 20% of specimens collected in Europe showed a reduced activity against the A/California/7/2009 vaccine virus strain [36].
5. Limitations
We tried to minimize selection bias by an enrollment strategy based on an active surveillance system, the use of broad eligibility requirements for inclusion, completeness of inclusion, and enrolling subjects without previous knowledge of their vaccination or case-control status.
We reduced classification bias by the use of two independent sources to ascertain vaccination, performing RT-PCR for influenza diagnosis, and by the case–case comparison.
Patients’ recall is considered a valid source of influenza vaccination status [37], but is limited by recall bias and uncertainty on date of vaccine administration, or type of vaccine administered. Record of vaccination reliably indicates immunization, but absence of record is not informative. We estimate electronic Vaccine Information System sensitivity as 90%, and specificity as 99%, during the 2009–2010 autumn pandemic wave [38]. With the data collected in the present study, and using a capture recapture method [39], completeness of ascertainment was 93% for electronic Vaccine Information System, 98% for patient recall, and 99% for both sources. We aimed to reduce classification bias considering a study subject as vaccinated or non-vaccinated adding the information provided by both sources.
5.1. Case-control status ascertainment
RT-PCR is the preferred diagnostic test for influenza [9]; but, case status misclassification may contribute to underestimation of IVE because of false-negative RT-PCR results [10], [15], [40]. To maximize RT-PCR sensitivity, we included patients with onset of symptoms seven or less days before hospitalization. PCR positivity is, with a similar swabbing strategy, 88% and 70%, at 4 and 6 days after symptoms onset [40]. A non-differential misclassification of true positives as negatives cannot be ruled out and underestimation of vaccine effectiveness is to be expected if a test-negative design is used. This was minimized by case–case comparison [10], [15].
5.2. Specimen collection method
Although nasopharyngeal aspirate is considered the best specimen for detection of influenza viruses [41], we opted for pharyngeal (throat) and nasopharyngeal swabbing to reduce patients discomfort and performance easiness. In children nasal swabs are comparable to that of nasopharyngeal aspirates for the detection of all major respiratory viruses, except RSV [42]. In adults, swabbing has been used to study respiratory virus disease [43], [44], and IVE [14], [18], [40], [45], [46]. We obtained a yield of positives similar to other studies on hospitalized adults [16], [43], and the timing of the epidemic wave and types and subtypes we identified were consistent with those reported by Spain's surveillance system [47]. Swabbing is a reliable and convenient alternative to obtain specimens for RT-PCR testing [42], and accounting for days elapsed from symptoms onset to swabbing, should limit the effect of misclassification of true positives as negatives [10], [15].
5.3. Case–case approach
The case–case analysis approach design assures comparability of controls to cases [10], [15]. In a case–case comparison approach, cases and controls should mainly differ in the exposure (and its correlates) associated to the outcome of interest [19]. All this is even more plausible if influenza and other respiratory viruses co-circulate concurrently (Fig. 2).
5.4. Impact of age as a confounder and age-related protection due to previous exposure
Age effect was taken into account by adjustment, and by performing an analysis restricted to those 60 years old or older.
Vaccine effectiveness could be explained in the elderly by acquired protection due to distant exposure to similar H1N1 strains. We consider this pre-existing protection bias in our results as debatable. First, we observed the third H1N1pdm09 wave, this repeated circulation levels exposure to H1N1pdm09 over the age range [48]. Second, age-specific H1N1pdm09 influenza-related hospitalization rates were in our population two to seven times higher in the 75 years or more age group. Third, seroepidemiology studies [48], [49], [50] have described the persistence of protective antibody titers against H1N1pmd09 only in a small fraction of subjects 80 years old or older [48], [49], [50]. Fourth, when T cell epitopes are compared between H1N1pdm09 and seasonal H1N1, 41% and 69% for CD4+ and CD8+, respectively, are conserved [51], hence a less dependent on age protection for severe episodes should be expected in those 18 years old or older [14]. Fifth, vaccine effectiveness did not differ when age was considered.
5.5. Sample size
The main weakness of our study was the number of influenza-related hospitalization. Although we were able to assess adjusted IVE on large groups, this was done with broad confidence intervals, and we did not attain a sufficient number of cases to provide robust IVE estimates by virus strain or vaccine type.
6. Conclusions
We report IVE estimates with a low probability of bias and the current vaccines provided a significant health benefit. Any single IVE study results are difficult to generalize. Variability of the factors involved, such as circulating strains, vaccine types and composition, match between vaccine's and circulating strains, population characteristics, and outcomes measured are limitations to generalizability.
Future studies should be planned, after taking into consideration the strengths and limitations exposed, to attain the necessary statistical power to obtain robust IVE estimates by virus antigenic subtypes, comparing the different vaccines available, and for relevant high-risk groups.
Acknowledgements
The authors thank the staff of the Hospital La Plana, in Vila-real; Arnau de Vilanova, in Valencia; La Ribera, in Alzira; San Juan, in Alicante; and Hospital de Elda, in Elda. As well, we thank all the study participants and their families.
We wish to express our recognition to Prof Juan García-de-Lomas for his support and contribution on the laboratory methods section. We also acknowledge the dedication and commitment of researchers in the field Begoña Escribano-López, Verónica Alcarria-García, Ester Huet-Trujillo, Ángela López-Doménech, Montserrat Cano-Armenteros M and Consuelo Calvo-Mas.Funding: The study was funded in part by contract code GRT109 between Sanofi-Pasteur and Centro Superior de Investigación en Salud Pública (CSISP). Sanofi-Pasteur did not participate in the design or conduct of the study, collection, management, analysis, or interpretation of the data, writing the report, and the decision to submit the report for publication.
References
- 1.Simonsen L., Fukuda K., Schonberger L.B., Cox N.J. The impact of influenza epidemics on hospitalizations. J Infect Dis. 2000;181(3):831–837. doi: 10.1086/315320. [DOI] [PubMed] [Google Scholar]
- 2.Influenza vaccines. WHO position paper. Wkly Epidemiol Rec 2005; 80(33):279–87. [PubMed]
- 3.Gerdil C. The annual production cycle for influenza vaccine. Vaccine. 2003;21(16):1776–1779. doi: 10.1016/s0264-410x(03)00071-9. [DOI] [PubMed] [Google Scholar]
- 4.Osterholm M.T., Kelley N.S., Sommer A., Belongia E.A. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2011;12(1):36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 5.Carrat F., Flahault A. Influenza vaccine: the challenge of antigenic drift. Vaccine. 2007;25(39–40):6852–6862. doi: 10.1016/j.vaccine.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 6.Jefferson T. Influenza vaccination: policy versus evidence. BMJ. 2006;333(7574):912–915. doi: 10.1136/bmj.38995.531701.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michiels B., Govaerts F., Remmen R., Vermeire E., Coenen S. A systematic review of the evidence on the effectiveness and risks of inactivated influenza vaccines in different target groups. Vaccine. 2011;29(49):9159–9170. doi: 10.1016/j.vaccine.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Kelly H., Valenciano M. Estimating the effect of influenza vaccines. Lancet Infect Dis. 2012;12(1):5–6. doi: 10.1016/S1473-3099(11)70289-4. [DOI] [PubMed] [Google Scholar]
- 9.Petrie J.G., Ohmit S.E., Johnson E., Cross R.T., Monto A.S. Efficacy studies of influenza vaccines: effect of end points used and characteristics of vaccine failures. J Infect Dis. 2011;203(9):1309–1315. doi: 10.1093/infdis/jir015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puig-Barbera J. 2010–2011 influenza seasonal vaccine, preliminary mid-season effectiveness estimates: reason for concern, confounding or are we following the right track? Euro Surveill 2011;16(11):pii=19821. [DOI] [PubMed]
- 11.Belongia E.A., Kieke B.A., Donahue J.G., Greenlee R.T., Balish A., Foust A. Effectiveness of inactivated influenza vaccines varied substantially with antigenic match from the 2004–2005 season to the 2006–2007 season. J Infect Dis. 2009;199(2):159–167. doi: 10.1086/595861. [DOI] [PubMed] [Google Scholar]
- 12.Skowronski D.M., De Serres G., Crowcroft N.S., Janjua N.Z., Boulianne N., Hottes T.S. Association between the 2008–09 seasonal influenza vaccine and pandemic H1N1 illness during Spring-Summer 2009: four observational studies from Canada. PLoS Med. 2010;7(4):e1000258. doi: 10.1371/journal.pmed.1000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savulescu C., Valenciano M., de Mateo S., Larrauri A., cycEVA Study Team Estimating the influenza vaccine effectiveness in elderly on a yearly basis using the Spanish influenza surveillance network – pilot case-control studies using different control groups, 2008–2009 season, Spain. Vaccine. 2010;28(16):2903–2907. doi: 10.1016/j.vaccine.2010.01.054. [DOI] [PubMed] [Google Scholar]
- 14.Puig-Barberà J., Arnedo-Pena A., Pardo-Serrano F., Tirado-Balaguer M.D., Pérez-Vilar S., Silvestre-Silvestre E. Effectiveness of seasonal 2008–2009, 2009–2010 and pandemic vaccines, to prevent influenza hospitalizations during the autumn 2009 influenza pandemic wave in Castellón, Spain. A test-negative, hospital-based, case-control study. Vaccine. 2010;28(47):7460–7467. doi: 10.1016/j.vaccine.2010.09.042. [DOI] [PubMed] [Google Scholar]
- 15.Kelly H., Jacoby P., Dixon G.A., Carcione D., Williams S., Moore H.C. Vaccine effectiveness against laboratory-confirmed influenza in healthy young children: a case-control study. Pediatr Infect Dis J. 2010;30(2):107–111. doi: 10.1097/INF.0b013e318201811c. [DOI] [PubMed] [Google Scholar]
- 16.Talbot H.K., Griffin M.R., Chen Q., Zhu Y., Williams J.V., Edwards K.M. Effectiveness of seasonal vaccine in preventing confirmed influenza-associated hospitalizations in community dwelling older adults. J Infect Dis. 2011;203(4):500–508. doi: 10.1093/infdis/jiq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng A.C., Kotsimbos T., Kelly H.A., Irving L.B., Bowler S.D., Brown S.G. Effectiveness of H1N1/09 monovalent and trivalent influenza vaccines against hospitalization with laboratory-confirmed H1N1/09 influenza in Australia: a test-negative case control study. Vaccine. 2011;29(43):7320–7325. doi: 10.1016/j.vaccine.2011.07.087. [DOI] [PubMed] [Google Scholar]
- 18.Castilla J., Martínez-Artola V., Salcedo E., Martínez-Baz I., Cenoz M.G., Guevara M. Vaccine effectiveness in preventing influenza hospitalizations in Navarre, Spain, 2010–2011: cohort and case-control study. Vaccine. 2012;30(2):195–200. doi: 10.1016/j.vaccine.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 19.McCarthy N., Giesecke J. Case-case comparisons to study causation of common infectious diseases. Int J Epidemiol. 1999;28(4):764–768. doi: 10.1093/ije/28.4.764. [DOI] [PubMed] [Google Scholar]
- 20.Recommended viruses for influenza vaccines for use in the 2010–2011 northern hemisphere influenza season. Wkly Epidemiol Rec 2010; 85(10):81–92. [PubMed]
- 21.Influenza Surveillance Influenza case definition. ECDC. Available: http://ecdc.europa.eu/en/activities/surveillance/EISN/surveillance/Pages/influenza_case_definitions.aspx; [accessed 28.09.11].
- 22.He J., Bose M.E., Beck E.T., Fan J., Tiwari S., Metallo J. Rapid multiplex reverse transcription-PCR typing of influenza A and B virus, and subtyping of influenza A virus into H1, 2, 3, 5, 7, 9, N1 (human), N1 (animal), N2, and N7, including typing of novel swine origin influenza A (H1N1) virus, during the 2009 outbreak in Milwaukee, Wisconsin. J Clin Microbiol. 2009;47(9):2772–2778. doi: 10.1128/JCM.00998-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suwannakarn K., Payungporn S., Chieochansin T., Samransamruajkit R., Amonsin A., Songserm T. Typing (A/B) and subtyping (H1/H3/H5) of influenza A viruses by multiplex real-time RT-PCR assays. J Virol Methods. 2008;152(1-2):25–31. doi: 10.1016/j.jviromet.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Kuypers J., Martin E.T., Heugel J., Wright N., Morrow R., Englund J.A. Clinical disease in children associated with newly described coronavirus subtypes. Pediatrics. 2007;119(1):e70–e76. doi: 10.1542/peds.2006-1406. [DOI] [PubMed] [Google Scholar]
- 25.Matsuzaki Y., Takashita E., Okamoto M., Mizuta K., Itagaki T., Katsushima F. Evaluation of a new rapid antigen test using immunochromatography for detection of human metapneumovirus in comparison with real-time PCR assay. J Clin Microbiol. 2009;47(9):2981–2984. doi: 10.1128/JCM.00321-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neske F., Blessing K., Tollmann F., Schubert J., Rethwilm A., Kreth H.W. Real-time PCR for diagnosis of human bocavirus infections and phylogenetic analysis. J Clin Microbiol. 2007;45(7):2116–2122. doi: 10.1128/JCM.00027-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van de Pol A.C., van Loon A.M., Wolfs T.F., Jansen N.J., Nijhuis M., Breteler E.K. Increased detection of respiratory syncytial virus, influenza viruses, parainfluenza viruses, and adenoviruses with real-time PCR in samples from patients with respiratory symptoms. J Clin Microbiol. 2007;45(7):2260–2262. doi: 10.1128/JCM.00848-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tapparel C., Cordey S., Van Belle S., Turin L., Lee W.M., Regamey N. New molecular detection tools adapted to emerging rhinoviruses and enteroviruses. J Clin Microbiol. 2009;47(6):1742–1749. doi: 10.1128/JCM.02339-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puig-Barberà J., Diez-Domingo J., Pérez Hoyos S., Belenguer Varea A., González Vidal D. Effectiveness of the MF59-adjuvanted influenza vaccine in preventing emergency admissions for pneumonia in the elderly over 64 years of age. Vaccine. 2004;23(3):283–289. doi: 10.1016/j.vaccine.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 30.Domingo-Salvany A., Regidor E., Alonso J., Alvarez-Dardet C. Proposal for a social class measure, Working Group of the Spanish Society of Epidemiology and the Spanish Society of Family and Community Medicine. Aten Primaria. 2000;25(5):350–363. [PubMed] [Google Scholar]
- 31.Miettinen O.S. Proportion of disease caused or prevented by a given exposure, trait or intervention. Am J Epidemiol. 1974;99(5):325–332. doi: 10.1093/oxfordjournals.aje.a121617. [DOI] [PubMed] [Google Scholar]
- 32.Pearce N. What does the odds ratio estimate in a case-control study? Int J Epidemiol. 1993;22(6):1189–1192. doi: 10.1093/ije/22.6.1189. [DOI] [PubMed] [Google Scholar]
- 33.Jefferson T., Di Pietrantonj C., Al-Ansary L.A., Ferroni E., Thorning S., Thomas R.E. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev. 2010;2(2):CD004876. doi: 10.1002/14651858.CD004876.pub3. [DOI] [PubMed] [Google Scholar]
- 34.Govaert T.M., Thijs C.T., Masurel N., Sprenger M.J., Dinant G.J., Knottnerus J.A. The efficacy of influenza vaccination in elderly individuals. A randomized double-blind placebo-controlled trial. JAMA. 1994;272(21):1661–1665. [PubMed] [Google Scholar]
- 35.Kissling E., Valenciano M., Falcao J., Larrauri A., Widgren K., Pitigoi D. I-MOVE towards monitoring seasonal and pandemic influenza vaccine effectiveness: lessons learnt from a pilot multi-centric case-control study in Europe, 2008–9. Euro Surveill. 2009;14(44) [PubMed] [Google Scholar]
- 36.Community Network of Reference Laboratories (CNRL) for Human Influenza in Europe. Influenza virus characterisation, summary Europe, March 2011. ECDC and Community Network of Reference Laboratories (CNRL) for Human Influenza in Europe; 13 April 2011. Available: http://ecdc.europa.eu/en/publications/Publications/1304_Influenza_virus_characterisation_2011_April.pdf; [accessed 26.02.12].
- 37.Irving S.A., Donahue J.G., Shay D.K., Ellis-Coyle T.L., Belongia E.A. Evaluation of self-reported and registry-based influenza vaccination status in a Wisconsin cohort. Vaccine. 2009;27(47):6546–6549. doi: 10.1016/j.vaccine.2009.08.050. [DOI] [PubMed] [Google Scholar]
- 38.Puig-Barberà J, Pérez-Vilar S, Pérez-Breva L, Alemán-Sánchez S, Morant-Talamante N, Martínez-Úbeda S, et al. Validez del registro de vacunas nominal para conocer el estado de vacunación frente a la gripe en adultos ingresados en hospitales de la comunidad valenciana. 6° Congreso de la Asociación Española de Vacunología (AEV). Santiago de Compostela, España, 23–26 November 2011. (P 34).
- 39.Hook E.B., Regal R.R. Capture-recapture methods in epidemiology: methods and limitations. Epidemiol Rev. 1995;17(2):243–264. doi: 10.1093/oxfordjournals.epirev.a036192. [DOI] [PubMed] [Google Scholar]
- 40.Lee N., Chan P.K., Hui D.S., Rainer T.H., Wong E., Choi K.W. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis. 2009;200(4):492–500. doi: 10.1086/600383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zambon M. Laboratory diagnosis of influenza. In: Nicholson K.J., Webster R.G., Hay J., editors. Textbook of influenza. Blackwell Science; Oxford: 1998. pp. 291–313. [Google Scholar]
- 42.Abu-Diab A., Azzeh M., Ghneim R., Ghneim R., Zoughbi M., Turkuman S. Comparison between pernasal flocked swabs and nasopharyngeal aspirates for detection of common respiratory viruses in samples from children. J Clin Microbiol. 2008;46(7):2414–2417. doi: 10.1128/JCM.00369-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Falsey A.R., Hennessey P.A., Formica M.A., Cox C., Walsh E.E. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352(17):1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 44.Walsh E.E., Peterson D.R., Falsey A.R. Human metapneumovirus infections in adults: another piece of the puzzle. Arch Intern Med. 2008;168(22):2489–2496. doi: 10.1001/archinte.168.22.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Talbot H.K., Falsey A.R. The diagnosis of viral respiratory disease in older adults. Clin Infect Dis. 2010;50(5):747–751. doi: 10.1086/650486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valenciano M., Kissling E., Cohen J.M., Oroszi B., Barret A.S., Rizzo C. Estimates of pandemic influenza vaccine effectiveness in Europe, 2009–2010: results of influenza monitoring vaccine effectiveness in Europe (I-MOVE) multicentre case-control study. PLoS Med. 2011;8(1):2424–2435. doi: 10.1371/journal.pmed.1000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delgado C., Jiménez-Jorge S., Ledesma J., Pozo F., León I., de Mateo S., Larrauri A Vigilancia de la gripe en España, Temporada 2010–11. (Desde la semana 40/2010 hasta la semana 20/2011) Boletín Epidemiológico Semanal (BES) 2011;19(Semanas 28–29):117–130. [Google Scholar]
- 48.Miller E., Hoschler K., Hardelid P., Stanford E., Andrews N., Zambon M. Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross-sectional serological study. Lancet. 2010;375(9720):1100–1108. doi: 10.1016/S0140-6736(09)62126-7. [DOI] [PubMed] [Google Scholar]
- 49.Chen H., Wang Y., Liu W., Zhang J., Dong B., Fan X. Serologic survey of pandemic (H1N1) 2009 virus, Guangxi Province, China. Emerg Infect Dis. 2009;15(11):1849–1850. doi: 10.3201/eid1511.090868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ikonen N., Strengell M., Kinnunen L., Osterlund P., Pirhonen J., Broman M. High frequency of cross-reacting antibodies against 2009 pandemic influenza A (H1N1) virus among the elderly in Finland. Euro Surveill. 2010;15(5) pii: 19478. [PubMed] [Google Scholar]
- 51.Greenbaum J.A., Kotturi M.F., Kim Y., Oseroff C., Vaughan K., Salimi N. Pre-existing immunity against swine-origin H1N1 influenza viruses in the general human population. Proc Natl Acad Sci U S A. 2009;106(48):20365–20370. doi: 10.1073/pnas.0911580106. [DOI] [PMC free article] [PubMed] [Google Scholar]


