Abstract
BACKGROUND:
Among preterm infants, neurodevelopmental outcomes are influenced by both medical and sociodemographic factors. Less is known about the impact on these factors on neonatal neurobehavioral patterns.
OBJECTIVE:
To determine associations between demographic, psychosocial and medical risk factors and neonatal neurobehavior.
METHODS:
Multi-center observational study of infants born <30 weeks enrolled in the Neonatal Neurobehavior and Outcomes in Very Preterm Infants (NOVI) Study between April 2014-May 2016. Maternal medical, demographic, and psychological variables and infant medical variables were prospectively collected. Demographic, substance, psychological and medical risk indices were developed. Neurobehavioral assessment was performed using the NICU Network Neurobehavioral Scale (NNNS) at NICU discharge.
RESULTS:
709 infants were enrolled in the NOVI study, and for 679 infants with neurobehavioral assessments, 6 NNNS behavioral profiles were calculated using latent profile analysis. Profile 6 infants (n=47/679, 7%) were atypical, having poor attention, self-regulation and movement quality, hypertonia and increased stress signs. After adjustment for site, profile 6 infants had significantly smaller head circumferences at birth (β−0.87; −1.59, −0.14), and higher rates of late sepsis (OR 3.38; CI 1.66, 6.92) compared to Profiles 1–5 infants. There were no significant differences in other neonatal morbidities between the two groups. Profile 6 infants had a higher prenatal demographic risk score (1.46 vs 1.07;β 0.34; CI 0.06, 0.61) compared to Profiles 1–5 infants.
CONCLUSION:
NNNS behavioral profiles identify an atypical behavioral pattern that is associated with early influences of demographic and medical variables. Such behavioral patterns may be seen as early as NICU discharge.
Keywords: NICU Network Neurobehavioral Scale (NNNS), prematurity, neurobehavior
BACKGROUND
Infants born less than 30 weeks post-menstrual age (PMA) are at highest risk for neurodevelopmental delays and impairment. Medical morbidities increasing this risk include brain injury, sepsis and chronic lung disease (CLD). 1–4 In addition, social determinants including poverty, low parental education and social support, and poor caregiver mental health are associated with unfavorable cognitive and behavior outcomes.5–11
Predicting which preterm infants will develop impairments remains challenging. Atypical infant neurobehavior can be identified in the neonate using the NICU Network Neurobehavioral Scale (NNNS), a standardized, comprehensive evaluation that incorporates neurologic and behavioral measures and signs of stress. 12 In preterm infants, abnormalities on the NNNS were associated with morbidities, including lung and brain injury.13–15 Atypical NNNS patterns or profiles that predict early motor, cognitive and behavior problems were found more frequently among infants born <32 weeks compared to older infants. 16,17
Less is known about associations between sociodemographic risks and neurobehavior, particularly in the preterm infant. In term infants, abnormal NNNS scores are associated with prenatal exposure to intrauterine growth restriction,18 young maternal age,19 illicit substances,20 and maternal depression.21 Among preterm infants, prenatal maternal stress has been linked to altered brain growth and reduced neural connectivity when compared to term controls. 22 Similar pathways are influenced by postnatal medical morbidities and intensive care environmental stress, yet the additive burden on neurologic development and function remains unclear. 23–25 The current NOVI study is the first to assess the impact of prenatal environmental risks on neurobehavior, and specifically NNNS behavior profiles, in the context of the unique perinatal medical risks of very preterm birth. The aim of this study was to examine relations between sociodemographic, psychological and medical risks and NNNS profiles at NICU discharge in a multisite cohort of infants born < 30 weeks PMA.
METHODS
Design
The Neonatal Neurobehavior and Outcomes in Very Preterm Infants (NOVI) Study was conducted at 9 university affiliated NICUs Vermont Oxford Network (VON) from April 2014 through June 2016 who were also Vermont Oxford Network (VON) participants. Enrollment and consent procedures were approved by local institutional review boards. Inclusion criteria included: 1) birth at <30 weeks PMA; 2) parental ability to read and speak English, Spanish, Japanese, or Chinese; 3) residence within 3 hours of the NICU and follow-up clinic. Gestation estimates to determine birth <30 weeks PMA were based on the Extremely Low Gestational Age Newborns (ELGAN) Study criteria. 26 Exclusion criteria included maternal age <18 years, maternal cognitive impairment, or infants with major congenital anomalies. 27,28 Parents of eligible infants were invited to participate in the study when infants reached 31–32 weeks PMA, or when survival was deemed likely by the attending neonatologist.
Measures
Maternal Medical, Demographic and Psychologic Data:
Once an infant was enrolled, NOVI-trained personnel collected maternal intrapartum variables from the infant medical record including maternal risk factors for preterm birth, such as growth measures, diabetes, hypertension, infection, sexually transmitted diseases/HIV. Demographic variables, including race/ethnicity, marital status, insurance, education level, income, and occupation and self-reported substance use were obtained during standardized maternal enrollment interviews. Maternal psychological distress was prospectively assessed during the week of NICU discharge using the Brief Symptom Inventory (BSI), 29 which identifies psychological symptom patterns for nine dimensions: somatization, obsessive–compulsive, interpersonal sensitivity, depression, anxiety, hostility, phobic anxiety, paranoid ideation, psychoticism.
Neonatal Medical Data:
The VON Patient Data Booklet 27 criteria were used to collect neonatal medical variables. Standardized data collection procedures were completed by VON staff at each site. Infant data included infections, retinopathy of prematurity (ROP), respiratory, CNS, renal, cardiac, gastrointestinal, genetic, and hematologic disorders, as well as respiratory and surgical interventions.
Brain Injury:
Brain injury diagnoses were based on routine site ultrasonograms and readings were categorized as early (day 7–10) or late (between 36 weeks GA and discharge). All site cranial ultrasounds were performed using high-frequency transducers with six standard quasi-coronal views and five para-sagittal views. Study neuro-radiologists were identified as central radiologists and classified observations according to ELGAN study criteria. 30 Sonograms were read initially as part of site-specific clinical care and subsequently read by a Study neuro-radiologist. A third reading, by a Study neuro-radiologist, was performed if the initial and second readings disagreed about the presence of one or more of the following: IVH, parenchymal echodensity, parenchymal echolucency, or moderate-to-severe ventricular enlargement. Ultrasound abnormalities were classified as present if identified by at least two readers.
Neurobehavior:
Neonatal neurobehavior was assessed using the NNNS, which is a standardized tool that includes measures of active and passive tone, primitive reflexes, items that reflect physical maturity, social and behavioral functioning including visual and auditory tracking, cuddling and consolability, and a checklist of stress signs organized by organ system.12 With preterm infants, the NNNS takes approximately 20 minutes to complete. Exams were scheduled to be administered during the week of NICU discharge by site examiners (trained to reliability and certified by central NNNS trainers).31 Individual items were converted to 13 summary scores; habituation, attention, handling, regulation, arousal, excitability, lethargy, hypertonia, hypotonia, non-optimal reflexes, asymmetric reflexes, quality of movement and stress abstinence. A higher summary score reflects a higher level of the construct. Habituation was omitted from analyses, as 50% of infants were not in the appropriate state during initiation of exam. The remaining 12 summary scores were converted to NNNS profiles, which are mutually exclusive, discrete categories representing the infant’s pattern of performance across the summary scores.17
Risk Indices:
Prenatal maternal demographic, substance use, and medical risk indices were created as the sum of a set of known and suspected binary indicators of risk associated with prematurity as highlighted by the March of Dimes.32 A neonatal medical risk index, based on published work, 2,3 included brain injury (defined as PVL, moderate-severe ventriculomegaly with or without grade 3–4 IVH, or parenchymal echodensity), CLD, severe ROP, necrotizing enterocolitis (NEC), and/or culture positive sepsis. The Maternal Psychological Distress risk variable was determined based on scores in the clinical range that reflected the number and severity of symptoms included in the BSI Global Severity Index (GSI; Table 1).29
Table 1.
Prenatal and Perinatal Maternal and Infant Risk
| Prenatal | |
| Demographic Risk Index | low socioeconomic status (SES)a, minorityb, no partner, maternal age >35, no prenatal care |
| Substance Risk Index | prenatal alcohol, tobacco, and marijuana use, other illicit drug use |
| Maternal Medical Risk Index | diabetes, infections (vaginal or urinary), chronic or pregnancy induced hypertension, sexually transmitted diseases or HIV, obesity, underweight, fetal congenital anomaly |
| Prenatal | |
| Maternal Psychological Distress | Brief Symptom Inventory Global Severity Index (GSI) |
| Infant Medical Risk | brain injury, severe ROP, CLD, NEC/sepsis |
Socioeconomic status was calculated using the four-factor Hollingshead Index (based on marital status, employment status, educational attainment, and occupation) which has been adapted to single parent and non-nuclear families with Hollingshead level V indicating low SES. (Hollingshead 1975)
hispanic or non-white
Statistics
Latent profile analysis (LPA, Mplus version 8.1) of the NNNS summary scores was used to group infants into mutually exclusive categories that represent heterogeneous subgroups. LPA models with different numbers of profiles were fitted and the model containing the optimal number of profiles identified. 33 Determination of the best model fit was assessed via Bayesian information criteria (BIC) adjusted for sample size, whereby the smallest BIC value indicates the best fit as well as minimization of cross classification probabilities, the bootstrapped likelihood ratio test, and the number of cases in each profile.
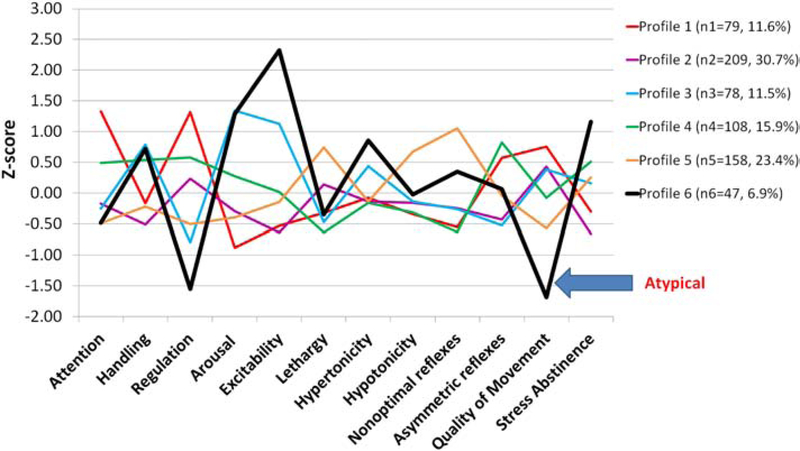
The number and composition of profiles derived using LPA depend on sample specific factors such as characteristics and size, as well as statistical criteria. In this NOVI sample six distinct NNNS profiles were identified. Infants in profile 6 had scores that reflect the most extremely poor functioning in multiple domains, compared to the other profiles. This was consistent with findings from a previously identified profile 17 in a sample of high risk full term and preterm infants, where a comparably negative profile was also associated with impaired developmental and behavioral outcomes. Thus, infants in profile 6 were compared to infants in profiles 1–5. Infant medical characteristics and risk indices differences between profile 6 vs profiles 1–5 were tested using chi-square (or Fisher’s exact test) and ANOVA. NNNS profiles were examined by neonatal medical data and prenatal and perinatal maternal and infant risk using logistic and linear regression as appropriate. Categorical medical outcomes were adjusted for study site if there was sufficient cell size (≥5 per group) and statistically significant associations with NNNS profiles (p<.05).
RESULTS
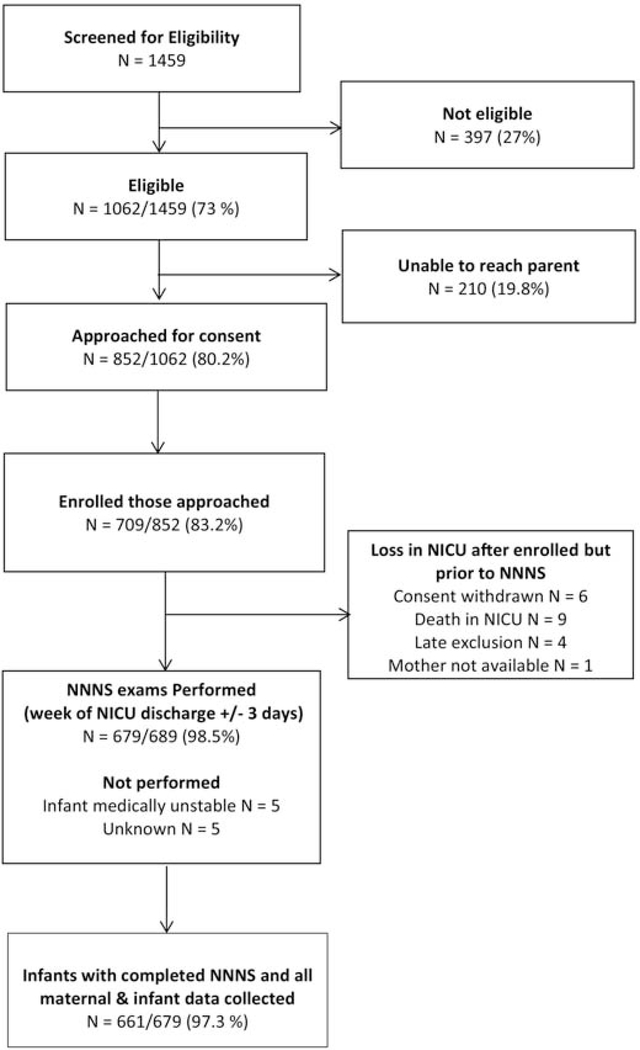
Of 1062 infants eligible for enrollment, 709 infants were enrolled and 679 NNNS exams were performed during the week of NICU discharge (+/− 3 days). The majority (95%) of NNNS exams were completed in the NICU, the remaining (5%) were conducted after discharge due to scheduling conflicts. The final study sample includes 661 infants with an NNNS assessments and complete data collection (Figure 1).
Figure 1.
Study Derivation
NNNS Profiles:
NNNS profile patterns are illustrated in Figure 2. Infants in profile 1 had the best attention and regulation scores and required average handling. Profile 2 infants had the lowest stress scores, and mostly average performance on the remaining summary scores. Profile 3 infants required more handling, had less regulation, and higher arousal and excitability. Profile 4 infants also needed increased handling to maintain attention, but had better self-regulation. Profile 5 infants had the most lethargy and non-optimal reflexes, as well as poor quality of movements. Profile 6 infants had the most extreme findings. They were the least regulated, required substantial handling, had exceptionally high arousal, excitability, and stress signs, along with hypertonia and poor quality of movements. For this study, profile 6 is defined as the “atypical profile”, and profiles 1–5 consist of infants with more optimal, less extreme behavior patterns than profile 6. Table A.1 (appendix) presents means and standard deviations of individual NNNS scores across profiles.
Figure 2.
NNNS Profiles (n=679)
Medical Outcomes:
Infant’s with the atypical profile 6 were more likely to have smaller head circumference (HC) at birth, and sepsis when compared to infants with profiles 1–5 (Table 2). There were no differences between the two groups for morbidities such as CLD, NEC and severe ROP. Additionally, profile 6 infants were more likely to have IVH or PVL noted on late cranial ultrasound, while no differences were seen for other brain injuries between infants in profile 6 vs 1–5. After adjustment for study site, associations between profile 6 and smaller HC and late onset sepsis remained statistically significant.
Table 2.
Neonatal Medical Data
| N (%) of Mean (SD) | Profile 6 N=46 | Profiles 1–5 N=615 | Unadjusted β or Odds Ratio (95%CI) | Adjusted β or Odds Ratio (95%CI) |
|---|---|---|---|---|
| Birth GA <27 weeks | 23 (50.0) | 267 (43.4) | 1.30 (0.72, 2.37) | 1.24 (0.68, 2.27) |
| Birth HC (cm) | 23.6 (2.3) | 24.6 (2.4) | −1.00 (−1.72, −0.27) | −0.87 (−1.59, −0.14) |
| Length of NICU stay (days) | 103 (45) | 92 (42) | 11.00 (−1.71, 23.70) | 11.24 (−1.55, 24.03) |
| Discharge weight (g) | 3036 (846) | 3000 (891) | 36 (−233, 305) | 118 (−148, 384) |
| Post menstrual age (wks) at discharge | 41.6 (5.1) | 40.3 (5.1) | 1.32 (−0.22, 2.87) | 1.38 (−0.18, 2.93) |
| Chronic Lung Diseasea | 21 (45.7) | 314 (51.1) | 0.81 (0.44, 1.47) | 0.82 (0.45, 1.51) |
| Necrotizing enterocolitis (NEC)b | 4 (8.7) | 39 (6.3) | 1.41 (0.48, 4.12) | * |
| Bacterial Sepsis (<= day 3) | 4 (8.7) | 15 (2.4) | 3.80 (1.21, 11.97) | * |
| Bacterial Sepsis (> day 3) | 13 (28.3) | 56 (9.1) | 3.93 (1.96, 7.90) | 3.39 (1.66, 6.92) |
| Severe ROP c | 1 (2.2) | 39 (6.3) | 0.33 (0.04, 2.45) | * |
| Germinal Matrix Hemorrhage (GMH) | 9 (19.6) | 138 (22.4) | 0.84 (0.40, 1.79) | 0.85 (0.40, 1.82) |
| GMH Early | 9 (20.0) | 113 (18.5) | 1.10 (0.52, 2.35) | 1.16 (0.54, 2.48) |
| GMH Late | 4 (8.7) | 62 (10.7) | 0.80 (0.28, 2.30) | * |
| Any Intraventricular Hemorrhage (IVH) | 10 (21.7) | 107 (17.4) | 1.32 (0.64, 2.74) | 1.31 (0.63, 2.73) |
| IVH Early | 8 (17.8) | 98 (16.1) | 1.13 (0.51, 2.50) | 1.17 (0.53, 2.61) |
| IVH Late | 9 (19.6) | 58 (10.0) | 2.19 (1.01, 4.77) | 1.98 (0.90, 4.36) |
| Ventricular Dilation | 4 (8.7) | 46 (7.5) | 1.18 (0.41, 3.43) | * |
| Ventricular Dilation Early | 4 (9.5) | 33 (5.5) | 1.80 (0.61, 5.35) | * |
| Ventricular Dilation Late | 4 (8.7) | 35 (6.1) | 1.46 (0.50, 4.32) | * |
| Parenchymal echolucency (PVL) | 6 (13.0) | 37 (6.0) | 2.34 (0.93, 5.88) | 2.27 (0.89, 5.75) |
| Parenchymal echodensity | 6 (13.0) | 46 (7.5) | 1.86 (0.75, 4.61) | 1.82 (0.73, 4.56) |
oxygen requirement at 36 weeks PMA
Bell’s classification ≥ stage 2
Stage 4 or 5 or requiring surgery
Categorical medical outcomes were adjusted for study site if there was sufficient cell size (≥5 per group) and statistically significant associations with NNNS profiles (p<.05).
Notes: For continuous medical data, data are unstandardized coefficients from linear regression analysis; for categorical medical data, data are odds ratios from logistic regression analyses
Risk Indices:
Profile 6 infants were born to mothers with a higher mean prenatal demographic risk index score compared to profile 1–5 infants (Table 3). No differences were seen between groups for prenatal exposures including the substance risk index and the maternal medical risk index. Perinatal exposures of maternal psychological distress and infant medical risk index were also similar between NNNS profile groups.
Table 3.
Prenatal and Perinatal Maternal and Infant Risk
| Mean (SD) | Profile 6 N=46 | Profiles 1–5 N=615 | Unadjusted B (95%CI) | Adjusted β (95% CI) |
|---|---|---|---|---|
| Prenatal | ||||
| Demographic Risk Index | 1.46 (0.94) | 1.07 (0.90) | 0.38 (0.11, 0.65 ) | 0.34 (0.06, 0.61 ) |
| Substance Risk Index | 0.33 (0.72) | 0.29 (0.66) | 0.04 (−0.17, 0.24) | 0.02 (−0.18, 0.23) |
| Maternal Medical Risk Index | 0.89 (0.85) | 0.95 (0.87) | −0.06 (−0.32, 0.20) | −0.10 (−0.36, 0.16) |
| Perinatal | ||||
| Maternal Psychological Distress (GSI Total) | 0.27 (0.32) | 0.29 (0.35) | −0.02 (−0.12, 0.09) | −0.00 (−0.11, 0.10) |
| Infant Medical Risk Index * | 1.07 (0.85) | 0.86 (0.87) | 0.21 (−0.05, 0.47) | 0.20 (−0.06, 0.46) |
Infant Medical Risk Index consists of CLD, Severe ROP, Brain Injury, NEC or sepsis
DISCUSSION
Among infants born <30 weeks gestation, we identified an extreme, “atypical” neurobehavioral profile prior to discharge influenced by both prenatal sociodemographic and perinatal medical variables. Specifically, profile 6 infants were more likely to have a small HC at birth and late onset sepsis compared to profile 1–5 infants. Additionally, a maternal demographic risk composite was associated with profile 6.
Our findings of abnormal neurobehavior among preterm infants are consistent with prior work. Poor term-equivalent performance on NNNS summary scores have been reported for NICU infants, 13,15 and associated with increased risk for cognitive, motor, and language delay at 2 years. 34,35 Incorporating individual NNNS summary scores into neurobehavioral profiles allows integration of the infant’s full repertoire of neurobehavior, classifies behavior as typical (normal) or atypical (which may reflect abnormal or impaired behavior), and offers an overall “neurobehavioral picture”. The atypical profile 6 infants identified in this study are virtually identical to previously reported profiles. Liu et al, were the first to describe a NNNS profiles, and interestingly, the high risk profile 6 group for this cohort consisted of infants low gestation (≤32 weeks) and birthweight (<1500 grams). 17 Similarly, in a small cohort of infants 23–35 weeks gestation, the high-risk NNNS profile group also had behavior patterns resembling the profile 6 infants of this much larger NOVI cohort.16 Early identification of profiles is of considerable interest, as Liu reported significant association between high-risk NNNS profile infants and neurodevelopmental delay beyond 2 years, including cerebral palsy at 3 years, behavior problems at 3 years, and abnormal cognitive scores persisting until 4.5 years of age. 17
As medical morbidities are also markers of impairment, we explored the contribution of biologic factors to infant neurobehavior. Significant differences in sepsis rates were seen between study groups, a finding of clinical importance, as sepsis is a contributor to mortality and morbidity in preterm infants. Despite recent reductions in neonatal sepsis, one-quarter to one-third of extremely preterm infants are diagnosed with late onset sepsis.36,37 Additionally, sepsis has been linked to neurodevelopmental delay in the preterm population. 38,39 In a Swiss cohort, sepsis was associated with a three-fold increase in the risk of CP and a two-fold increase in the risk of neurodevelopmental impairment at 2 years of age. 37
Infection and subsequent inflammation has multi-organ effects, making it difficult to isolate the effects of sepsis from other neonatal morbidities, including neuronal injury. Downstream effects of the inflammatory cascade can disrupt brain development during critical growth periods40 and impair neurobehavioral performance. Liu reported that infants in the high-risk NNNS profile were more likely to have abnormal cranial ultrasound readings.17 In the current NOVI study, profile 6 infants were more likely to have a diagnosis of late IVH or PVL compared to profile 1–5 infants, although the difference was not significant after adjustment for covariates. Of concern, NOVI profile 6 infants had smaller birth HCs. Alteration in early brain growth could reflect intrauterine insult or stress, potentially impacting neurologic integrity and function at term-equivalent age. Our findings suggest that, among preterm infants, neurobehavioral manifestations of brain integrity may be reflected and categorized by NNNS behavior patterns.
No differences between study groups were seen for CLD, a condition with an underlying inflammatory component. For this NOVI cohort, CLD was defined as oxygen requirement at 36 weeks; perhaps if we quantified into degree of severity41 differences may have been uncovered. However, when we utilized a count of neonatal morbidities, which has been shown to have predictive value for later impairment 2,3, mean infant medical risk scores were similar between NNNS profile groups.
Identifying socioeconomic risks for atypical neurobehavior is particularly relevant since prenatal psychosocial and environmental stress exposures modulate infant physiologic and behavioral functioning.42–44 In a small cohort of preterm infants, a high-risk infant NNNS behavior profile pattern was established and was associated with lower maternal education. 16 This aligns with the current NOVI findings, where the demographic risk index that included factors such as an absent partner, no prenatal care, and lower education, predicted the atypical profile 6. In related NOVI cohort work, we reported epigenetic DNA methylation differences between the atypical profile 6 infants and infants with the most optimal profile (profile 1) infants.45 It was not surprising that some of the genes identified, which were linked to stress and cortisol-regulating sites, have been associated with neurologic structure, function, or neurobehavioral disorders. 19,21,46,47 This not only provides clues to the molecular basis for neurobehavioral findings, but offers insight into the complex interplay of biology, genetics and environmental exposures.
The NOVI study has several strengths, including standardized and detailed neurobehavioral assessments by examiners who were blinded to infant and parent history, prospectively collected data on prenatal and perinatal environmental exposures, extensive efforts to increase the validity of brain ultrasound interpretations, and an innovative derivation of behavioral profiles to provide an overall “picture” of high risk infants which may be useful to clinicians and families. Weaknesses of the study include maternal medical and substance use being recorded by infant medical chart abstraction, thus information may have been incomplete. Additionally, the requisite use of interviews for retrospective reporting of risk, such as substance use, may have resulted in under-reporting. Limited details about respiratory therapies precluded a classification of infants based on CLD severity.
This NOVI study allows for identification of prenatal and antenatal risk factors associated with the most at-risk infants on the NNNS. This may provide early alerts to neonatal care teams, potentially allowing for enhanced neurodevelopmental and behavioral support for both infant and parent. Recent findings from studies of family-centered care show that developmental support, maternal bedside involvement and skin-to-skin/kangaroo care are predictive of improved neurobehavior on the NNNS.48–52 In addition, opportunities exist for targeted care to be transitioned into the post-discharge environment. By following this NOVI cohort through childhood, we plan to assess the ability of NNNS profiling to predict motor, language, cognitive and behavioral trajectories in the context of varied prenatal, antenatal and postnatal environmental exposures.
In summary, the NNNS demonstrates potential in identifying preterm infants who show impaired neurobehavior at NICU discharge. By profiling neurobehavior, an atypical, high risk group of infants may be identified. Importantly, this atypical profile is associated with both demographic and medical variables, and offers insight into factors prior to NICU discharge that alter early infant behavior and developmental pathways, thus allowing for early identification of such risks.
Highlights.
Neurobehavioral assessment can capture signs of atypical behavior in preterm infants
Sepsis and small head circumference are associated with atypical neurobehavior
Increased sociodemographic risk may also alter infant neurobehavior
Prior to hospital discharge such neurobehavioral changes may be identified
Recognition of atypical neurobehavioral patterns offer insight brain developmental
Acknowledgements
We are grateful for the participation of our study infants and their families, and thank the study teams at each site for their commitment to our NOVI participants and study goals. We appreciate the contributions of individuals who enhanced many aspects of our study: Linda LaGasse, PhD, for her early leadership with the NOVI Data Center, Study Site Co-PIs, Study Coordinators, Site NNNS examiners, and Ultrasound Consultants as listed below:
Brown Alpert Medical School and Women and Infants Hospital, Providence, RI: Amy Salisbury, PhD, Elizabeth Danella MOT, OTR/L, Lynne Andreozzi, PhD, Erica Oliveira, BA, Brenda Rosario Perez, BA, BS.
Children’s Mercy Hospital, Kansas City, MO: Howard Kilbride, MD, Anne Holmes, RN, MSN, CCRC, Allison Knutson, RNC-NIC, Denise Smith, RN, MSN, RNC, NNP.
Harbor-UCLA Medical Center, Torrance, CA: Lucinda Santos, MHA, Jennifer Huynh, RN,
Miller Children’s and Women’s Hospital Long Beach, Long Beach, CA: Lucinda Santos, MHA, Aimee Burdick, PT, DPT, PCS, Alison Yamaguchi, PT
Spectrum Health-Helen Devos Hospital, Grand Rapids, MI: Edgar J. Beaumont, MD, Virginia DeWitt, BSN, RN, BS, Stephanie Fagerman, MS, MB, Kathy Nystrom, BSN, RN, Emily Gleason, BSN, RN, Karen Pawloski, RN, Rebecca McCurdy, PNP-PC, Jason Powell, PT
University of Hawaii John A. Burns School of Medicine, Honolulu, HI: Venkataraman Balaraman MBBS, Mari Uehara, MD, Joann Cheung, MA, CCRC, Micah Tong, CCRP, Pattaraporn Chun, MD, Eydie Nakasone, Jayna Lee.
Wake Forest School of Medicine, Winston Salem, NC: Jennifer Check, MD, MS, Shannon Green Hanson, PhD, MPH, April Stewart, Heather Vye, PT, MPT, PCS, Kerry Dudziak MS, OTR/L, Kristi Lanier, RN, BSN, Nancy Peters, RN, Caroline Ludwig, BS, Melissa Tuttle.
Ultrasound Consultants: Steve Bezinque, DO, Heather Borders, MD, Joseph Junewick, MD, Brad Betz, MD, Barbara Specter, MD.
Funding Source: Funded by National Institutes of Health (NIH)/ Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) grant R01HD072267.
Abbreviations:
- (PMA)
post-menstrual age
- (CLD)
chronic lung disease
- (HC)
head circumference
- (IVH)
intraventricular hemorrhage
- (LPA)
latent profile analysis
- (PVL)
periventricular leukomalacia
- (NEC)
necrotizing enterocolitis
- (NNNS)
NICU Network Neurobehavioral Scale
- (NOVI)
Neonatal Neurobehavior and Outcomes in Very Preterm Infants
- (ROP)
retinopathy of prematurity
- (LOS)
late onset bacterial sepsis
Table A.1(appendix 1):
Means and standard deviations of individual NNNS assessment scores across the NNNS profiles identified by latent profile analysis (n=679).
| NNNS Summary Scales | Profiles | p-value | |||||
|---|---|---|---|---|---|---|---|
| 1 (n=79) | 2 (n=209) | 3 (n=78) | 4 (n=108) | 5 (n=158) | 6 (n=47) | ||
| Attention | 7.26 (1.12) | 5.02 (1.21) | 4.90 (0.96) | 6.01 (1.16) | 4.55 (1.44) | 4.56 (1.35) | <.001 |
| Handling | 0.37 (0.24) | 0.27 (0.22) | 0.62 (0.24) | 0.55 (0.22) | 0.35 (0.24) | 0.60 (0.26) | <.001 |
| Self Regulation | 6.67 (0.60) | 5.81 (0.45) | 4.99 (0.51) | 6.08 (0.54) | 5.22 (0.54) | 4.38 (0.67) | <.001 |
| Arousal | 3.17 (0.47) | 3.55 (0.37) | 4.61 (0.50) | 3.92 (0.49) | 3.48 (0.51) | 4.58 (0.48) | <.001 |
| Excitability | 1.38 (1.18) | 1.17 (0.91) | 4.79 (1.34) | 2.51 (1.35) | 2.16 (1.10) | 7.26 (1.57) | <.001 |
| Lethargy | 3.87 (1.55) | 4.9 (1.87) | 3.56 (1.53) | 3.19 (1.54) | 6.13 (2.23) | 3.81 (1.91) | <.001 |
| Hypertonicity | 0.32 (0.71) | 0.27 (0.54) | 0.69 (1.06) | 0.26 (0.48) | 0.27 (0.56) | 1.00 (1.27) | <.001 |
| Hypotonicity | 0.05 (0.22) | 0.14 (0.35) | 0.15 (0.40) | 0.06 (0.28) | 0.55 (0.75) | 0.21 (0.41) | <.001 |
| Non Optimal Reflexes | 4.20 (1.59) | 4.85 (1.53) | 4.81 (1.80) | 4.04 (1.47) | 7.53 (1.67) | 6.06 (1.90) | <.001 |
| Asymmetrical Reflexes | 1.72 (1.48) | 0.42 (0.72) | 0.29 (0.56) | 2.05 (1.54) | 0.92 (1.17) | 0.98 (1.30) | <.001 |
| Quality of Movement | 5.11 (0.57) | 4.88 (0.46) | 4.85 (0.56) | 4.53 (0.59) | 4.20 (0.50) | 3.43 (0.63) | <.001 |
| Stress Abstinence | 0.11 (0.06) | 0.09 (0.05) | 0.15 (0.07) | 0.17 (0.06) | 0.16 (0.06) | 0.22 (0.08) | <.001 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Singer L, Yamashita T, Lilien L, Collin M, Baley J. A longitudinal study of developmental outcome of infants with bronchopulmonary dysplasia and very low birth weight. Pediatrics. 1997;100(6):987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt B, Asztalos EV, Roberts RS, Robertson CM, Sauve RS, Whitfield MF. Impact of bronchopulmonary dysplasia, brain injury, and severe retinopathy on the outcome of extremely low-birth-weight infants at 18 months: results from the trial of indomethacin prophylaxis in preterms. Jama. 2003;289(9):1124–1129. [DOI] [PubMed] [Google Scholar]
- 3.Bassler D, Stoll BJ, Schmidt B, et al. Using a count of neonatal morbidities to predict poor outcome in extremely low birth weight infants: added role of neonatal infection. Pediatrics. 2009;123(1):313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kono Y, Mishina J, Yonemoto N, Kusuda S, Fujimura M. Neonatal correlates of adverse outcomes in very low-birthweight infants in the NICU Network. Pediatrics international : official journal of the Japan Pediatric Society. 2011;53(6):930–935. [DOI] [PubMed] [Google Scholar]
- 5.Asztalos EV, Church PT, Riley P, Fajardo C, Shah PS. Association between Primary Caregiver Education and Cognitive and Language Development of Preterm Neonates. American journal of perinatology. 2017;34(4):364–371. [DOI] [PubMed] [Google Scholar]
- 6.Beaino G, Khoshnood B, Kaminski M, et al. Predictors of the risk of cognitive deficiency in very preterm infants: the EPIPAGE prospective cohort. Acta paediatrica (Oslo, Norway : 1992). 2011;100(3):370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joseph RM, O’Shea TM, Allred EN, Heeren T, Kuban KK. Maternal educational status at birth, maternal educational advancement, and neurocognitive outcomes at age 10 years among children born extremely preterm. Pediatric research. 2018;83(4):767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Potijk MR, de Winter AF, Bos AF, Kerstjens JM, Reijneveld SA. Behavioural and emotional problems in moderately preterm children with low socioeconomic status: a population-based study. European child & adolescent psychiatry. 2015;24(7):787–795. [DOI] [PubMed] [Google Scholar]
- 9.Huhtala M, Korja R, Lehtonen L, Haataja L, Lapinleimu H, Rautava P. Parental psychological well-being and behavioral outcome of very low birth weight infants at 3 years. Pediatrics. 2012;129(4):e937–944. [DOI] [PubMed] [Google Scholar]
- 10.Huhtala M, Korja R, Lehtonen L, et al. Parental psychological well-being and cognitive development of very low birth weight infants at 2 years. Acta paediatrica (Oslo, Norway : 1992). 2011;100(12):1555–1560. [DOI] [PubMed] [Google Scholar]
- 11.Treyvaud K, Lee KJ, Doyle LW, Anderson PJ. Very preterm birth influences parental mental health and family outcomes seven years after birth. The Journal of pediatrics. 2014;164(3):515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lester BM, Tronick EZ, Brazelton TB. The Neonatal Intensive Care Unit Network Neurobehavioral Scale procedures. Pediatrics. 2004;113(3 Pt 2):641–667. [PubMed] [Google Scholar]
- 13.Brown NC, Doyle LW, Bear MJ, Inder TE. Alterations in neurobehavior at term reflect differing perinatal exposures in very preterm infants. Pediatrics. 2006;118(6):2461–2471. [DOI] [PubMed] [Google Scholar]
- 14.Brown NC, Inder TE, Bear MJ, Hunt RW, Anderson PJ, Doyle LW. Neurobehavior at term and white and gray matter abnormalities in very preterm infants. The Journal of pediatrics. 2009;155(1):32–38, 38.e31. [DOI] [PubMed] [Google Scholar]
- 15.Pineda RG, Tjoeng H, Vavasseur C, Kidokoro H, Neil J, Inder T. Patterns of Altered Neurobehavior in Preterm Infants within the NICU. The Journal of pediatrics. 2013;162(3):470–476.e471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lester BM, Marsit CJ, Giarraputo J, Hawes K, LaGasse LL, Padbury JF. Neurobehavior related to epigenetic differences in preterm infants. Epigenomics. 2015;7(7):1123–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Bann C, Lester B, et al. Neonatal neurobehavior predicts medical and behavioral outcome. Pediatrics. 2010;125(1):e90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Moraes Barros MC, Guinsburg R, Mitsuhiro SS, Chalem E, Laranjeira RR. Neurobehavior of full-term small for gestational age newborn infants of adolescent mothers. Jornal de pediatria. 2008;84(3):217–223. [DOI] [PubMed] [Google Scholar]
- 19.de Moraes Barros MC, Guinsburg R, Mitsuhiro S, Chalem E, Laranjeira RR. Neurobehavioral profile of healthy full-term newborn infants of adolescent mothers. Early human development. 2008;84(5):281–287. [DOI] [PubMed] [Google Scholar]
- 20.Lester BM, Lagasse L, Seifer R, et al. The Maternal Lifestyle Study (MLS): effects of prenatal cocaine and/or opiate exposure on auditory brain response at one month. The Journal of pediatrics. 2003;142(3):279–285. [DOI] [PubMed] [Google Scholar]
- 21.Salisbury AL, Lester BM, Seifer R, et al. Prenatal cocaine use and maternal depression: effects on infant neurobehavior. Neurotoxicology and teratology. 2007;29(3):331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheinost D, Kwon SH, Lacadie C, et al. Prenatal stress alters amygdala functional connectivity in preterm neonates. NeuroImage. Clinical. 2016;12:381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogers CE, Smyser T, Smyser CD, Shimony J, Inder TE, Neil JJ. Regional white matter development in very preterm infants: perinatal predictors and early developmental outcomes. Pediatric research. 2016;79(1–1):87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith GC, Gutovich J, Smyser C, et al. Neonatal intensive care unit stress is associated with brain development in preterm infants. Annals of neurology. 2011;70(4):541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strahle JM, Triplett RL, Alexopoulos D, et al. Impaired hippocampal development and outcomes in very preterm infants with perinatal brain injury. NeuroImage. Clinical. 2019;22:101787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Shea TM, Allred EN, Dammann O, et al. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early human development. 2009;85(11):719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vermont Oxford Network, Manual of Operations (Part 2). http://www.vtoxford.org/tools/manualofoperationspart2.pdf.
- 28.Walden RV, Taylor SC, Hansen NI, et al. Major congenital anomalies place extremely low birth weight infants at higher risk for poor growth and developmental outcomes. Pediatrics. 2007;120(6):e1512–1519. [DOI] [PubMed] [Google Scholar]
- 29.Deragotis LR. The Brief Symptom Inventory (BSI): Administration, Scoring and Procedures Manual, 3rd Ed. Minneoapolis, MD: National Comoputer Systems; 1993. [Google Scholar]
- 30.Kuban K, Adler I, Allred EN, et al. Observer variability assessing US scans of the preterm brain: the ELGAN study. Pediatric radiology. 2007;37(12):1201–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tronick EZ, Olson K, Rosenberg R, Bohne L, Lu J, Lester BM. Normative neurobehavioral performance of healthy infants on the Neonatal Intensive Care Unit Network Neurobehavioral Scale. Pediatrics. 2004;113(3 Pt 2):676–678. [PubMed] [Google Scholar]
- 32.March of Dimes Data Book for Policy Makers: Maternal and Infant Health in the United States 2016. [Google Scholar]
- 33.Nylund KL, Asparouhov T, Muthén BO. Deciding on the Number of Classes in Latent Class Analysis and Growth Mixture Modeling: A Monte Carlo Simulation Study. Structural Equation Modeling: A Multidisciplinary Journal. 2007;14(4):535–569. [Google Scholar]
- 34.Spittle AJ, Walsh JM, Potter C, et al. Neurobehaviour at term-equivalent age and neurodevelopmental outcomes at 2 years in infants born moderate-to-late preterm. Developmental medicine and child neurology. 2017;59(2):207–215. [DOI] [PubMed] [Google Scholar]
- 35.El-Dib M, Massaro AN, Glass P, Aly H. Neurobehavioral assessment as a predictor of neurodevelopmental outcome in preterm infants. Journal of perinatology : official journal of the California Perinatal Association. 2012;32(4):299–303. [DOI] [PubMed] [Google Scholar]
- 36.Stoll BJ, Hansen NI, Bell EF, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA. 2015;314(10):1039–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlapbach LJ, Aebischer M, Adams M, et al. Impact of sepsis on neurodevelopmental outcome in a Swiss National Cohort of extremely premature infants. Pediatrics. 2011;128(2):e348–357. [DOI] [PubMed] [Google Scholar]
- 38.Martin CR, Dammann O, Allred EN, et al. Neurodevelopment of extremely preterm infants who had necrotizing enterocolitis with or without late bacteremia. The Journal of pediatrics. 2010;157(5):751–756.e751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hentges CR, Silveira RC, Procianoy RS, et al. Association of late-onset neonatal sepsis with late neurodevelopment in the first two years of life of preterm infants with very low birth weight. Jornal de pediatria. 2014;90(1):50–57. [DOI] [PubMed] [Google Scholar]
- 40.Novak CM, Ozen M, Burd I. Perinatal Brain Injury: Mechanisms, Prevention, and Outcomes. Clinics in perinatology. 2018;45(2):357–375. [DOI] [PubMed] [Google Scholar]
- 41.Ehrenkranz RA, Walsh MC, Vohr BR, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116(6):1353–1360. [DOI] [PubMed] [Google Scholar]
- 42.Hofheimer JA, Smith LM, McGowan EC, et al. Psychosocial and medical adversity associated with neonatal neurobehavior in infants born before 30 weeks gestation. Pediatric research. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wadhwa PD, Entringer S, Buss C, Lu MC. The contribution of maternal stress to preterm birth: issues and considerations. Clinics in perinatology. 2011;38(3):351–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van den Bergh BRH, van den Heuvel MI, Lahti M, et al. Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy. Neuroscience and biobehavioral reviews. 2017. [DOI] [PubMed] [Google Scholar]
- 45.Everson TM, Marsit CJ, Michael O’Shea T, et al. Epigenome-wide Analysis Identifies Genes and Pathways Linked to Neurobehavioral Variation in Preterm Infants. Scientific reports. 2019;9(1):6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Moraes Barros MC, Guinsburg R, de Araujo Peres C, Mitsuhiro S, Chalem E, Laranjeira RR. Exposure to marijuana during pregnancy alters neurobehavior in the early neonatal period. The Journal of pediatrics. 2006;149(6):781–787. [DOI] [PubMed] [Google Scholar]
- 47.Conradt E, Lester BM, Appleton AA, Armstrong DA, Marsit CJ. The roles of DNA methylation of NR3C1 and 11beta-HSD2 and exposure to maternal mood disorder in utero on newborn neurobehavior. Epigenetics. 2013;8(12):1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lester BM, Hawes K, Abar B, et al. Single-family room care and neurobehavioral and medical outcomes in preterm infants. Pediatrics. 2014;134(4):754–760. [DOI] [PubMed] [Google Scholar]
- 49.Silva MG, Barros MC, Pessoa UM, Guinsburg R. Kangaroo-mother care method and neurobehavior of preterm infants. Early human development. 2016;95:55–59. [DOI] [PubMed] [Google Scholar]
- 50.Montirosso R, Del Prete A, Bellu R, Tronick E, Borgatti R. Level of NICU quality of developmental care and neurobehavioral performance in very preterm infants. Pediatrics. 2012;129(5):e1129–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reynolds LC, Duncan MM, Smith GC, et al. Parental presence and holding in the neonatal intensive care unit and associations with early neurobehavior. Journal of perinatology : official journal of the California Perinatal Association. 2013;33(8):636–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pineda R, Bender J, Hall B, Shabosky L, Annecca A, Smith J. Parent participation in the neonatal intensive care unit: Predictors and relationships to neurobehavior and developmental outcomes. Early human development. 2018;117:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]