Abstract
Objective
To report on the experiences with the use of commercial and autologous fibrin glue (AFG) and of an alternative method based on a 3D-printed polycaprolactone (PCL) anchor for the fixation of hydrogel-based scaffolds in an equine model for cartilage repair.
Methods
In a first study, three different hydrogel-based materials were orthotopically implanted in nine horses for 1–4 weeks in 6mm diameter full-thickness cartilage defects in the medial femoral trochlear ridge and fixated with commercially available fibrin glue (CFG). One defect was filled with CFG only as a control. In a second study, CFG and AFG were compared in an ectopic equine model. The third study compared the efficacy of AFG and a 3D-printed PCL-based osteal anchor for fixation of PCL-reinforced hydrogels in three horses for 2 weeks, with a 4-week follow-up to evaluate integration of bone with the PCL anchor. Short-term scaffold integration and cell infiltration were evaluated by microcomputed tomography and histology as outcome parameters.
Results
The first study showed signs of subchondral bone resorption in all defects, including the controls filled with CFG only, with significant infiltration of neutrophils. Ectopically, CFG induced clear inflammation with strong neutrophil accumulation; AFG was less reactive, showing fibroblast infiltration only. In the third study the fixation potential for PCL-reinforced hydrogels of AFG was inferior to the PCL anchor. PCL reinforcement had disappeared from two defects and showed signs of dislodging in the remaining four. All six constructs fixated with the PCL anchor were still in place after 2 weeks. At 4 weeks, the PCL anchor showed good integration and signs of new bone formation.
Conclusions
The use of AFG should be preferred to xenogeneic products in the horse, but AFG is subject to individual variations and laborious to make. The PCL anchor provides the best fixation; however, this technique involves the whole osteochondral unit, which entails a different conceptual approach to cartilage repair.
Keywords: equine model, cartilage repair, hydrogels, fixation, fibrin glue, osteochondral, chondral scaffolds
Introduction
Articular cartilage is a highly specialized tissue in diarthrodial joints. Its roles are to provide smooth motion between joint surfaces, to transmit the forces generated by locomotion, and to attenuate these by redistributing mechanical stress to the underlying bone. However, because of its avascular nature, cartilage has poor intrinsic capacities for self-repair.1 Cartilage injuries in young adults due to sports injuries may, therefore, lead in the long term to degeneration of the tissue; this is one of the causes of osteoarthritis, which is a major cause of disability among the elder.2,3
Many potential solutions for the treatment of cartilage defects have been investigated, leading to a wide variety of repair techniques being proposed over the years.4–6 However, despite the researchers’ efforts, no method for true regeneration of tissue has been found yet, and the quest for the successful induction of native-like hyaline cartilage is still ongoing.7 In this quest, tissue engineering is a promising and appealing approach.
Tissue engineering combines cells, scaffolds, and bioactive molecules to support, guide, and maintain the restoration of native tissue through controlled degradation rates that should balance with the process of tissue regeneration.8 In particular, researchers have focused on providing an appropriate degradable matrix for cells to survive and differentiate. Hydrogels have shown to be suitable biomaterials for this purpose because of their intrinsic hydrated nature, their capacity to incorporate chemical cues, and their potential biocompatibility.8,9 Before possible clinical application, in vitro tissue-engineered constructs need to be tested and, despite growing availability of in silico 10 and ex vivo 11,12 models, large animal models are still deemed essential as final proof of concept. In the case of orthopedic disorders in general and cartilage repair in particular, the equine model has been often described as a very suitable model, as the articular cartilage in the equine stifle joint closely resembles that in the human knee with respect to thickness and biochemical composition, and both species present a very challenging mechanical environment.13,14
A crucial issue in cartilage repair is the fixation of implants. A range of approaches has been reported in literature, but all have significant drawbacks. For example, fascial/periosteal flaps sutured over the defect cause osteoarthritis-like changes in adjacent cartilage,15 and the use of transosseous sutures or biodegradable pins alters the architecture of 3D scaffolds.16 While it is possible to cast directly materials into defects, or to use hydrogels as a glue,17 these techniques are laborious, difficult for translation to human clinics, and limit greatly the possibility to control the design of the implants.
Fibrin glue has been extensively described in literature as a fixation tool for various type of scaffolds for cartilage repair in animal models18,19 and is currently used in human clinics.20 It has the advantage that it does not physically alter either the scaffold or the tissues adjacent to the defect; however, its use is not uncontested. First, there may be a species issue. Brehm et al. described how the use of fibrin glue of human origin caused a massive cell infiltration in the subchondral bone of goats.21 Although thus far not reported in literature, a similar situation may exist in the horse. Problems have been reported in studies where equine fibrin is used as a vehicle for cellular therapies in the horse (McIlwraith, pers. comm. 2016), including demonstration of the lack of ability for mesenchymal stem cells (MSCs) to migrate and proliferate in full strength fibrin and the requirement of dilution of the fibrin to allow these processes,22 and that the addition of MSCs to autologous platelet-enhanced fibrin scaffolds in chondral defects had inferior results to the platelet-enhanced fibrin scaffolds alone, including ossification of repair tissue.23 Second, there is increasing evidence that the use of (fiber) reinforcements in the hydrogels meant for cartilage repair may greatly enhance their efficacy and may even be indispensable.24 This poses an additional challenge to the fixation efficacy of sealants such as fibrin glue.25,26
Given the importance of the equine model for joint-related disorders and the ethical pressure of reducing the number of animal experiments to the minimum, there is, therefore, a need for the critical assessment and, where possible, optimization of fixation methods for hydrogel-based scaffolds, with and without reinforcement, in an equine cartilage defect model.
The data presented in this article emanate from a series of pilot studies focusing on the optimization of the fixation of hydrogel-based scaffolds in (osteo)chondral defects. Starting with a widely described and seemingly harmless commercial fibrin glue (CFG) that is considered the standard for fixation, we discovered that use of the material in the specific equine large animal model is fraught with difficulties (study 1). Based on this observation, we proceeded to comparing commercial and autologous fibrin glue (AFG) from an immunogenic point of view in an equine ectopic model (study 2). Finally, we compared the efficacy of the AFG with a custom-made alternative fixation method for specific fiber-reinforced hydrogel constructs based on a 3D-printed polycaprolactone (PCL)-based anchor for the fixation of PCL-reinforced hydrogels (study 3).
Materials and Methods
Animal experiments
Use of CFG for the fixation of hydrogel-based constructs in an orthotopic equine model
The study had been approved by the ethical and animal welfare of the National University of Costa Rica. Nine criollo horses (age 4–10 years, weight 275–375 kg) were used for surgery. The horses were free of lameness and without any clinical or radiographic evidence of acute or chronic injuries. They were housed in individual boxes and fed a standard maintenance ration of concentrate with hay ad libitum and free access to water.
General anesthesia was induced with diazepam (0.05 mg/kg), ketamine (2.2 mg/kg), and lidocaine (2 mg/kg), after premedication with acepromazine (0.025 mg/kg) and xylazin (1.1 mg/kg); anesthesia was maintained with isoflurane in oxygen with an end tidal concentration of 1.0–1.5%. The medial trochlear ridge of the stifle joint was exposed by arthrotomy through a subpatellar approach; two full-thickness cylindrical cartilage defects with a diameter of 6 mm were created using a manual drill guided by a drill sleeve. Remnants of cartilage in the defect were removed using a sharp surgical spoon. In each horse one of the defects was filled with 3D-printed porous constructs made of one of three different hydrogels (M10P10-HAMA,25 star-PEG/heparin,26 and P(AGE/G)-HA-SH,27 which had been previously tested both in vitro and in vivo for safety and biocompatibility and were deemed safe), so N= 3 per gel, and fixated with CFG (Tissucol™, Baxter), the other (control) defect was filled with fibrin glue (~0.3 mL) only (N=9). The glue was allowed 10 min for cross-linking; the wound was then closed in four layers, and full weight bearing was allowed after recovery from anesthesia. Post-operatively, horses received antibiotics for 3 days (procaine penicillin 15,000 IU/kg IM, SID, and gentamicin 6.6 mg/kg IV, BID) and nonsteroidal anti-inflammatory drugs (phenylbutazone 2.2 mg/kg, PO BID) during the first 5 days. The animals were subjected to daily monitoring of clinical parameters (temperature, heart rate, and respiratory rate). After 7, 14, and 28 days (n = 3 with one animal/gel per time point), the horses were euthanized, and the stifle joints were harvested, fixated in formalin 4%, and processed for histology.
Comparison of commercial and AFG in an equine ectopic model
This study aimed at comparing the in vivo tissue reaction, safety, and degradation of commercial and AFG in an ectopic equine model. This study had been approved by the Ethics Committee for Animal Experimentation of Utrecht University and was performed in accordance with the Institutional Guidelines on the Use of Laboratory Animals in compliance with the Dutch Act on Animal Experimentation.
AFG production
The protocol for production of the fibrin component of the fibrin glue was obtained by adapting the method described by Thorn et al.27 with the addition of a cryoprecipitation step to enhance fibrin precipitation.28
A blood sample (40 mL) was collected from each animal in a tube previously filled with heparin (20 IU/mL of blood) and medical grade citrate to obtain a citrate concentration of 3.2%. The plasma was separated by centrifugation for 18 min at 400 g at room temperature. The top half of the plasma layer was then transferred by pipetting into a new tube, carefully avoiding the surface containing platelets. Cryoprecipitation was performed by storing overnight the sample at −20°C.28 The sample was then allowed to thaw at room temperature; next, we initiated precipitation by adding 176 μL of 100% ethanol and, subsequently, mixing by inversion and placing on ice the plasma for 20 min. Centrifugation for 10 min at 1000 g followed to allow formation of a fibrin pellet on the bottom of the tube.27 The pellet was isolated, warmed at 37°C to allow solubilization, and loaded in a two-syringe system together with a commercially available thrombin solution (TISSEEL™; Baxter). The system was kept at 37°C with warm water and transferred to the surgical theater.
Surgical procedure
Two adult equines were sedated with detomidine (10 μg/kg). Under local anesthesia achieved by subcutaneous injection of 1 mL of mepivacaine solution (20 mg/mL) in the dorsal region of the neck, a series of subcutaneous pockets were created through small incisions on the skin (6 ×, ~10 mm in length). A small quantity of 0.3 mL of fibrin glue (commercial or autologous, randomized, n = 3) was deposited in each pocket and allowed to cross-link for 5 min, after which the wounds were sutured. Animals were monitored daily for signs of reaction (temperature, swelling, and general aspect of incision area).
After 14 days, the two animals were euthanized by administration of pentobarbital (50 mg/kg of body weight), and the entire soft tissue area containing the constructs was harvested for analysis.
Comparative fixation study with AFG and 3D-printed osteal PCL anchor in an equine orthotopic model
This study aimed at comparing the fixation potential of two techniques (osteal anchor and AFG) for the fixation of reinforced hydrogel-based scaffolds intended to be used for articular cartilage repair. These studies had been approved by the Local Ethics Committee for Animal Experimentation of Utrecht University and were performed in accordance with the Institutional Guidelines on the Use of Laboratory Animals in compliance with the Dutch Act on Animal Experimentation.
Preparation of osteal anchor
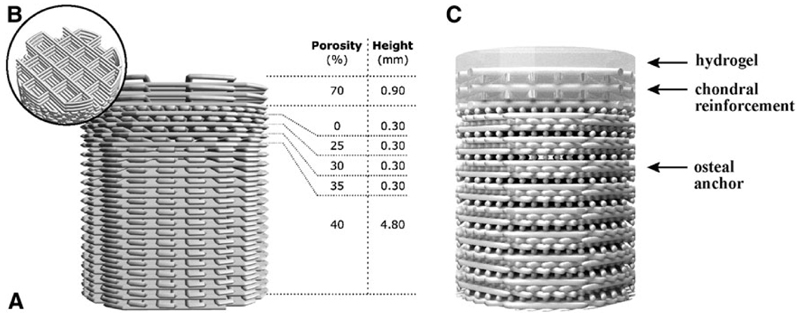
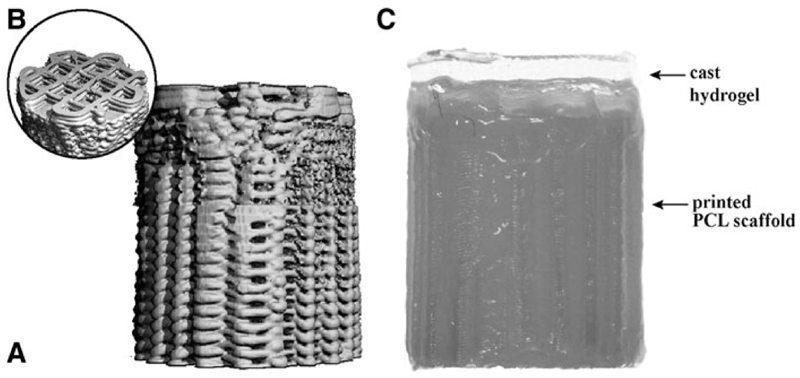
Osteochondral plugs were fabricated by extrusion-based 3D printing of GMP-grade PCL (Purasorb® PC 12, Corbion, The Netherlands) using a screw-based extruder on a 3D Discovery printer (regenHU, Switzerland). The osteochondral plug was designed on BioCAD software (regenHU) as a cylinder with 6 mm diameter, featuring a square-grid scaffold structure with six zones with different porosities. The lower zones were designed for bone osteoconduction and formed a gradient of decreasing porosity from the bottom to the top, mimicking the transition from trabecular to cortical bone. The top zone of the scaffold represented the endochondral interface and was designed as completely closed to separate the hydrogel materials for cartilage repair from the osteal anchor (Fig. 1 A). The uppermost zone in the osteochondral plug was designed for fiber reinforcement of the chondral portion (Fig. 1B), to enhance fixation of the hydrogels and to increase biomechanical resistance of the chondral layer.29
Fig. 1.
The osteal anchor was designed to have a decreasing porosity leading to a closed interface between the osteal and chondral parts of the construct, to mimic the natural architecture of the subchondral bone (A), as showed in the 3D-model of the PCL-based anchor design (A). A fiber reinforcement was added in the chondral layer to enhance fixation of hydrogel materials and biomechanical resistance of the chondral layer (B). The hydrogel materials can be cast on top (C). AFG, autologous fibrin glue; PCL, polycaprolactone.
Before printing, the PCL was first molten in the extruder heating tank at 90°C for at least 30 min to ensure consistent material viscosity. The osteochondral plugs were then fabricated using the following printing parameters: feeding pressure of 0.5 bar, 32G extrusion nozzle, temperature of 80°C, spindle speed of 4rpm, and printing speed of 4mm/s.
Preparation of chondral and osteochondral hydrogel reinforced constructs for implantation
To compare fixation potential of AFG and PCL osteal anchor, reinforced hydrogel constructs were prepared. The hydrogels selected for this purpose were M10P10-HAMA,30 starPEG/heparin,31 and P(AGE/G)-HA-SH.32
The osteochondral plugs were inserted into a polytetrafluoroethylene (teflon) custom-made mould of 7.5 mm height. This system allowed to cast hydrogel materials in a confined area, thus obtaining a uniform layer integrated with the osteal anchor (Fig. 1C). Casting of M10P10-HAMA and P(AGE/G)-HA-SH was performed infusing 0.42 μL of polymer solution mixed with a photoinitiator (0.05% w/w, Irgacure®2959; BASF, Ludwigshafen, Germany). Subsequently, chemical cross-linking was induced with a UV lamp at 3 cm distance (UV-Handleuchte Lamp, A. Hartenstein, Germany; wavelength: 365 nm, intensity at 3 cm: 1.2 mW/cm2). Casting of starPEG/heparin was achieved by mixing equal quantities of the two polymer solutions, which then cross-linked by click reaction.
To allow direct comparison of the two fixation methods, also when using AFG the hydrogels were cast in a mould of 1.5 mm height together with the PCL reinforcement following the methodology described previously. The constructs were then transported to the surgical theater for implantation.
Surgical procedure
Three Shetland ponies (age 3–10 years, weight 170–240 kg) were used. The horses were free of lameness and without any clinical evidence of acute or chronic injuries. They were housed in individual boxes and fed a standard maintenance ration of concentrate with hay ad libitum and free access to water.
Two defects were made in the medial trochlear ridge of the femur of each stifle joint. On one side, hydrogel based reinforced constructs were implanted and fixated with AFG (N = 2 per joint, distally and proximally, one material for each animal); on the contralateral side, the same constructs were implanted and fixated with the osteal anchor.
General anesthesia was induced with midazolam and ketamine intravenously (0.06 + 2.2 mg/kg), after premedication with detomidine and morphine (10 mcg/kg +0.1 mg/kg); anesthesia was maintained with isoflurane in oxygen with an end tidal concentration of 1.0–1.5%. The medial trochlear ridge of the stifle joint was exposed by arthrotomy through a subpatellar approach; on one side two full-thickness cylindrical cartilage defects with a diameter of 6 mm were created using a drill guided in a drill sleeve. On the contralateral side two osteochondral defects with a diameter of 6 mm and a depth of 7.5 mm were created using a surgical drill guided with a 7.5 mm custom-made drill.
Defects fixated with fibrin glue were filled with 0.2 mL of AFG, prepared as described previously, after which the hydrogel construct was immersed in the defect and left to sit for 10 min to allow cross-linking and fixation. On the contralateral side, the osteal anchor with the hydrogel constructs was inserted press fit into the defects.
The wounds were then closed in 4 layers, and full weight bearing was allowed after recovery from anesthesia. Postoperatively, the ponies received NSAIDS (meloxicam, 0.6 mg/kg, PO, BID-SID) up to 7 days and opiates (tramadol, 5 mg/kg, PO, BID-SID) up to 3 days postoperatively. For antibiotic prophylaxis, ampicillin (10–15 mg/kg) was administered intravenously once before and procaine penicillin (20 mg/kg, IM) once after surgery.
Clinical parameters of the animals were monitored daily for signs of inflammation and lameness. After 14 days, the animals were euthanized by administration of pentobarbital (50 mg/kg of body weight), and the entire osteochondral area containing the constructs was harvested for analysis with the aid of a surgical bone saw.
With the aim of evaluating the bone integration for a longer period, a follow-up study of 4 weeks was performed on three other ponies (age 4–10 years, weight 190–240 kg), where only the PCL osteal anchor implantation was performed bilaterally, following the methodology described above.
Postmortem processing
Microcomputed tomography
Each construct with osteal anchor was scanned before and after implantation in a micro-CT scanner (μ-CT 80, Scanco Medical AG, Switzerland) at a resolution of 20 μm. All defects were scanned postmortem. The acquisition parameters were set to a voltage of 70kVp, an intensity of 114 μA, and an integration time of 300 ms. Subsequently, the acquired images were processed by first applying a Gauss filter (sigma=2, support = 0.8 voxel) and then segmentation. A global threshold of 55 and 120/mile was used for the constructs before and after implantation, respectively. The segmented images of the constructs before implantation were also used to determine the porosity of the printed chondral reinforcement and osteal region. The adjacent healthy bone tissue was also scanned. The images obtained were processed with ImageJ to obtain the bone volume data before and after implantation.
Histological processing and stainings
Soft tissue samples were fixated in 4% formalin, dehydrated through a graded ethanol series, cleared in xylene, and embedded in paraffin. Osteochondral samples for histology were fixated in 4% formalin, decalcified with Formical-2000 (EDTA/formic acid; Decal Chemical Corporation, Tallman, NY) for 14 days, dehydrated through a graded ethanol series, cleared in xylene, and embedded in paraffin. Samples were sectioned into 5μm slices and stained with hematoxylin and eosin (HE) to allow for morphological analysis and the evaluation of tissue-scaffold integration according to a modified Drobnic’s scoring16,33 and cell infiltration using an Olympus BX51 light microscope. Samples were also stained with picrosirius red and analyzed with polarized light microscopy for visualization of collagen fibril orientation.
Results
The use of CFG for the fixation of hydrogel-based constructs in an orthotopic equine model
Clinical parameters of the animals were normal for the duration of the experiment, with no evidence of lameness or inflammation.
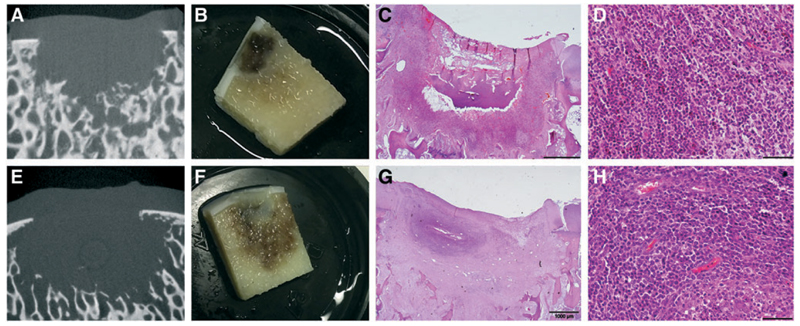
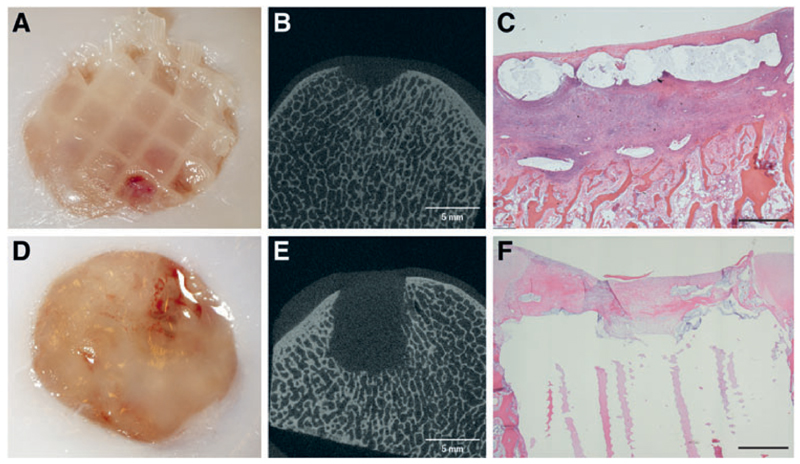
Macroscopically, all defects were filled with tissue. Microcomputed tomography (micro-CT) showed signs of bone loss in the subchondral area directly beneath the defect, irrespective of the hydrogel used (Fig. 2). There was some individual variability, but the phenomenon was seen in all horses. In addition, no correlation with specific implanted materials was found. This bone loss (Fig. 3A–D; Supplementary Table S1 and S2; Supplementary materials available at http://www.liebertpub.com/tec) was also consistently visible in all control defects only filled with the fibrin glue (Fig. 3E–H). At histology, the middle of the defect displayed an area of bone reaction surrounding the site of bone loss (Fig. 3C, G). As shown by the HE staining, this reaction was characterized by infiltration of fibroblasts and predominantly neutrophil granulocytes (Fig. 3D, H).
Fig. 2.
Bone volume loss from study 1. The area beneath the defects and healthy adjacent tissue was scanned postmortem with micro-CT imaging. Images obtained were processed with ImageJ to obtain bone volume values before and after implantation. Statistical analysis showed no correlation of bone loss with any of the materials. micro-CT, microcomputed tomography. Color images available online at www.liebertpub.com/tec
Fig. 3.
Inflammatory reaction in control defects filled with CFG (14 days postoperatively). First and second rows show representative examples from two different animals. micro-CT imaging showed loss of the trabecular structure and bone resorption (A, E). Upon sectioning the bone loss was confirmed, and a reaction of the surrounding area with inflammation was visible to the naked eye (B, F). HE staining showed a focal reaction (C, G) with recruitment of neutrophil granulocytes with loss of architecture and bone structure (D, H). CFG, commercial fibrin glue; HE, hematoxylin and eosin. Color images available online at www.liebertpub.com/tec
Comparison of commercial (CFG) and AFG in an equine ectopic model
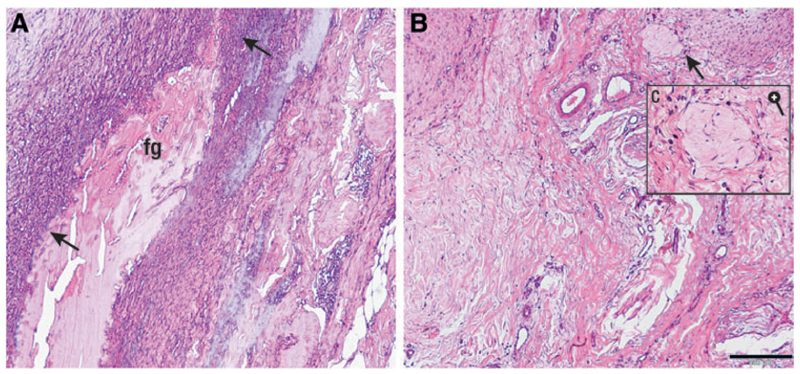
No local or systemic adverse reactions were observed in the horses during the experiment. Upon harvest, the excised tissue appeared macroscopically normal. Histological evaluation with HE staining of the CFG showed that the glue was clearly identifiable within the tissue (Fig. 4; Supplementary Fig. S2). It appeared contracted and was surrounded by a front of neutrophil granulocytes (Fig. 4A, arrow). The AFG was not clearly identifiable through different sections of the implantation area, although a structure compatible with its expected appearance was detected in the soft tissue (Fig. 4B, C). The neighboring tissue did not display any abnormal cell infiltration.
Fig. 4.
Histological sections of commercial (A, left) and autologous (B, right) fibrin glue, implanted in an equine ectopic model for 14 days. CFG appears contracted and is easily recognizable (A, fg); the glue is surrounded by a front of neutrophil granulocytes (A, black arrows); this is not present in the area where the autologous fibrin was implanted (B), where some macrophages and fibroblasts are present (C) (black bar = 200 μ). Color images available online at www.liebertpub.com/tec
Comparative fixation study with AFG and 3D-printed osteal PCL anchor in an equine orthotopic model
PCL osteal anchor and constructs for implantation
3D osteochondral constructs with a hierarchical porous architecture were successfully fabricated by means of an extrusion-based 3D printing technique (Fig 5). Design (Fig. 1) and built architecture (Fig. 5A) revealed the high accuracy of the printing technique. From the micro-CT data of the printed constructs porosity was also assessed and compared to the design values. Chondral and osteal regions showed porosities of 32%±3% (designed 40%) and 66%±2% (designed 70%), respectively. Furthermore, the 3D reconstructions showed that interconnected pores are presented on the lateral surface of the constructs (Fig. 5A). This is an important factor for the successful mechanical interlocking between the osteal anchor and surrounding natural bone tissue at construct/bone interface.
Fig. 5.
micro-CT 3D render of the osteochondral anchor before implantation (lateral view, A and top view, B) showing the closure of the layers to allow casting of hydrogels. Aspect of the PCL-based osteal anchor with cast hydrogel on top before implantation (C).
Animal study
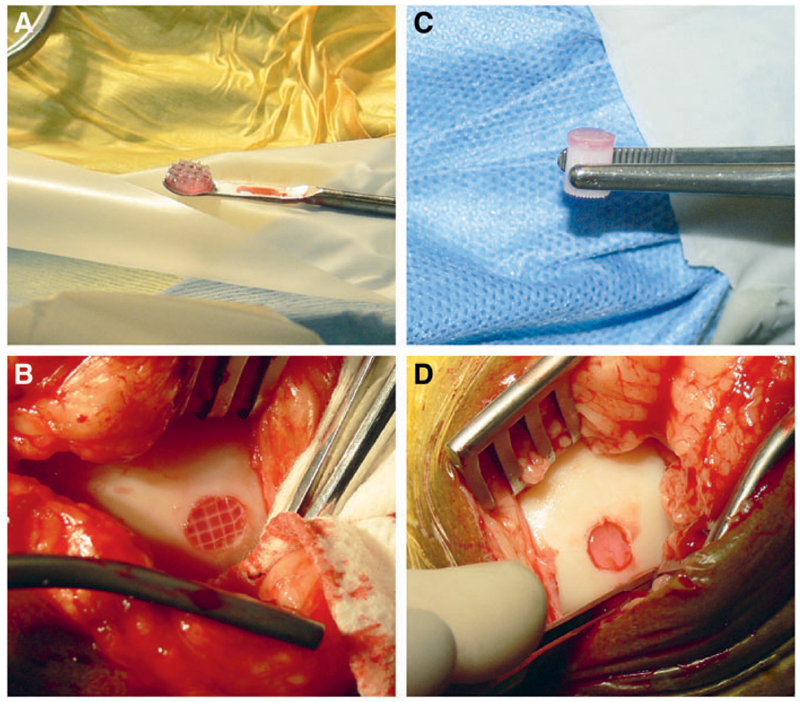
Surgical implantation of the constructs was successful. AFG was kept at 37°C before implantation, and mixed with the thrombin solution in the defect, to allow immersion of the hydrogel reinforced constructs (Fig. 6A, B). Osteochondral constructs were fixated by press fit, exerting pressure with a forceps on the PCL osteal anchor to avoid damage to the hydrogel portions (Fig. 6C, D).
Fig. 6.
Surgical implantation of materials for comparison of fixation with fibrin glue (left) versus osteal anchor (right). The reinforced hydrogel (A) was implanted in a full-thickness chondral defect and fixated with autologous fibrin glue (B). The hydrogel with PCL osteal anchor (C) was inserted in the osteochondral defect and secured by press fit (D). Color images available online at www.liebertpub.com/tec
In the 2-week study all animals had an uneventful course of the experiment, temperature and other parameters were well within normal range. However, when challenged by a flexion test, one animal showed some signs of discomfort on the limb in which the scaffolds had been fixated with the osteal anchor.
At postmortem, the 2-week study comparing the two fixation techniques showed that the fixation potential of AFG was inferior compared to the PCL osteal anchor. Although in two out of three animals AFG could keep the reinforced constructs in place, in one animal both defects were empty. Moreover, in the remaining four defects, the constructs displayed evidence of slipping out of the defects (Fig. 7A). All six hydrogels with PCL reinforcement fixated by means of the PCL anchor were still in place after 2 weeks (Fig. 7D). In the single animal that showed discomfort at the flexion test, the soft tissue opposing the defect showed signs of inflammation.
Fig. 7.
Fixation potential of two techniques, autologous fibrin glue (top) and PCL osteal anchor (bottom), 14 days after implantation. Reinforced chondral constructs appeared still in place in two of three cases; however, the scaffolds looked as if they were starting to slip out proximally (A). micro-CT of AFG fixation showed some bone resorption (B), confirmed by the HE staining (C), which showed loss of architecture directly underneath the defect with significant infiltration of neutrophil granulocytes and fibroblasts. Constructs fixated with the PCL anchor were all still in place (D), and micro-CT imaging showed a conserved trabecular architecture surrounding the construct (E). The chondral portion of the defect appears filled with repair tissue with a predominance of fibroblasts (F) (Black bar = 1mm). Color images available online at www.liebertpub.com/tec
Micro-CT of the defects and surrounding area in the AFG group (Fig. 7B) showed again the subchondral inflammatory reaction that had been observed in the first study in which CFG had been used, although to a lesser extent. The defects in the PCL group showed no signs of bone reaction (Fig. 7E).
Semiquantitative fixation scores showed that PCL gave better results both from an attachment point of view and from a scaffold integrity aspect (Table 1). One-way ANOVA confirmed a significant difference between the two groups (p < 0.05).
Table 1. Criteria for Fixation Scoring According to a Modified Drobnic Scoring.
| Outline attachment | Area coverage | Scaffold integrity |
|---|---|---|
| Unchanged (5) | Unchanged (5) | Unchanged (5) |
| <25% (4) | <75–100% (4) | Minor deformities unrelated to fixation (4) |
| 25–50% (3) | 50–75% (3) | Minor cracks close to fixation site (3) |
| 50–75% (2) | 25–50% (2) | Fissures that jeopardize the fixation (2) |
| 75–100% (1) | <25% (1) | Fissures and scaffold disorganization in outer area (1) |
| 100% (0) | 0% (0) | Fissures and scaffold disorganization in general (0) |
|
| ||
| AFG | PCL | |
|
| ||
| Outline attachment | 1.7 ± 1.50 | 4.8 ± 0.41 |
| Area coverage | 2.7 ± 2.06 | 4.8 ± 0.41 |
| Scaffold integrity | 2 ± 1.79 | 4±0 |
| Total score | 6.3 ± 5.12 | 13.7 ± 0.82* |
Scores show the difference between AFG and PCL efficacy in fixation. AFG shows high variability in results; overall efficacy for fixation with PCL was significantly higher than with AFG (*p < 0.05).
AFG, autologous fibrin glue; PCL, polycaprolactone.
Histological analysis confirmed the findings from the micro-CT. In the AFG group, an inflammatory response was observed in the bone underlying the defect, characterized by the presence of fibroblastic cells, neutrophils, and multinucleated cells (Fig. 7C). Bone resorption was consistently present in all defects. In the PCL group, a fibroblast-based infiltration from the bone surrounding the osteal part of the plug was observed, to a degree that can be expected after implanting an osteal scaffold (Fig. 7F; Supplementary Fig. S1).
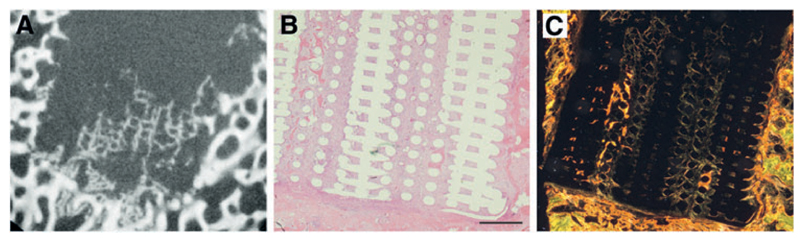
In the follow-up 4-week study none of the animals showed any clinical sign. micro-CT imaging showed signs of mineralization within the PCL osteal anchor (Fig. 8A), which was confirmed upon histology (Fig. 8B, C).
Fig. 8.
Bone regeneration after 28 days. micro-CT imaging showed signs of mineralization and new bone formation within the PCL osteal anchor (A). HE staining showed good integration of the anchor with the surrounding tissue (B) and picrosirius red staining under polarized light confirmed presence of new bone (C) (Black bar = 1 mm). Color images available online at www.liebertpub.com/tec
Discussion
In the quest for the optimal tissue-engineered construct that can be implanted in an articular cartilage defect to incite regeneration of the tissue, the fixation of such constructs within the surrounding non-degraded native tissue is a pivotal issue. Improper fixation will inevitably lead to implant failure, irrespective of the qualities of the materials and/or cells that are to be tested.34 In large animal models, fixation is especially important as loading is almost immediate after surgery and as constructs are exposed to substantial biomechanical compressive and shear forces. Thus far, the focus in the field has predominantly been directed at material development, production methods (e.g., 3D bio-printing), structural aspects of the construct (e.g., zonal composition), and cellular aspects (e.g., use of differentiated versus undifferentiated cells),35,36 but surprisingly little attention has been given to the fixation of the implants.
In the advanced techniques used for cartilage defect repair in human healthcare, such as autologous chondrocyte implantation (ACI) and matrix-assisted ACI, the use of fibrin glue is regarded as the gold standard for fixation of implanted cells and/or materials.20,37,38 For this purpose, commercially available fibrin glue obtained from human blood components is used. In many animal studies in which various types of implants for cartilage repair were assessed, this practice has been copied without further investigation, notwithstanding the fact that by doing so the use of fibrin glue changed from allogeneic in humans to xenogeneic in any other animal.19,39,40 Long-term results in some studies did not give rise to suspicions that the use of CFG might have negative side effects,18 but no short-term assessments of a possible acute reaction to the product have been performed and there were some indications that the use of the product might be less innocuous than assumed in both goats and horses21 (McIlwraith, pers. comm. 2016).
In this article we present data from a series of short-term studies that were originally executed as preliminary work for long-term experiments focusing on the assessment of hydrogels for cartilage repair. (Supplementary Table S3) Well aware of the limitations of these studies that were originally never meant to be stand-alone studies, we nevertheless felt it useful to report the outcome to the scientific community, given the unexpected events at short term after implantation that came to light, the importance of construct fixation in cartilage repair, and the need to avoid as much as possible unnecessary wastage of experimental animals.
In the first orthotopic study the panel of cells that had infiltrated the defects in which CFG had been used for fixation showed a large predominance of neutrophils that suggest a recruitment of inflammatory cells far beyond expectations 1–4 weeks post-implantation. The most striking point was that this inflammatory reaction, which was accompanied by severe bone loss, was similar in all defects, irrespective of the hydrogel used. Two possible explanations for this phenomenon were formulated based on the observations and literature. The first potential explanation was that the use of xenogeneic material (human origin glue in an equine model) might have led to an inflammatory reaction based on an immune response, as had been supposed by Brehm et al. 21 in their goat model. Alternatively, the subchondral bone reaction might have been caused by the mechanical stress exerted by weight bearing on the exposed bone triggering a biological response.41 Vasara et al.42 described how a subchondral reaction with bone resorption was detected in goats, 3 months after implantation. Moreover, Kold et al.43 reported the formation of cystic lesions in the femoral condyle of horses after creating a slit-like lesion in the articular cartilage. Timewise, it might have been the case that in our study the bone did not have sufficient time to complete remodeling, and hence, the bone resorption was more evident than in other reported studies. Since bone remodeling occurs starting from the third or fourth week,44 it may even be possible that this phenomenon is more common than suspected, but may go undetected, as most large animal studies are longer than 4 weeks.45 However, in our pilot study lesions were created on the medial trochlear ridge of the femur, which is an area that is not or hardly loaded during rest and only affected by principally shear forces during locomotion. For this reason, we focused first on the CFG as potential cause of the heavy reaction.
To realize a study on the potential inflammatory effect of CFG, a procedure for the production of AFG had to be developed. The use of partially AFG was deemed acceptable, as the most immunogenic component was suspected to be the one containing fibrinogen.46 We, therefore, elected to still use the commercial thrombin component. While being successful, the efforts to develop a reproducible protocol yielding a usable product confirmed that the synthesis of AFG is laborious and subject to individual variation in concentration and characteristics, based on the animal’s health, nutrition, and immune condition at the moment of blood collection.47 This results in a final product that is not standardized and that may introduce additional variation to the experiments. This is a disadvantage compared to the use of a commercially available fibrin glue that offers a ready-to-use consistent product.
The difference in cell recruitment observed in the ectopic model between the CFG and AFG strongly suggested that indeed the xenogeneic nature of the CFG resulted in an undesired inflammatory response. This response was clearly less prominent when using AFG, although not entirely nonexistent. This outcome suggests that the fibrin component may indeed have had a predominant role in the provocation of this response; however, it also hinted at the presence of some residual immunogenic effect, possibly caused by the still xenogeneic thrombin.
Given the fact that also the use of this form of AFG was not entirely reaction free and the inherent difficulty of the use of any type of fibrin glue for the fixation of fiber-reinforced scaffolds, a different approach was considered. The first part of the third study compared the efficacy of AFG versus an entirely different osteochondral concept for the fixation of fiber reinforced hydrogels for cartilage repair. Multicomposite scaffolds for cartilage regeneration envisage solving the problem of the relatively low stiffness that is inherent to hydrogels by altering the architecture of the scaffolds.
One of the approaches to overcome this is the use of fiber reinforcement.29 While this technology allows for the creation of scaffolds of which the stiffness approaches that of native cartilage,24 it poses an ulterior problem for the fixation of the scaffold. This is particularly important when treating chondral defects that are usually not more than 1–2 mm deep and in which the risk of the reinforcing mesh being swept out by shear forces is real. For this reason, in this study the AFG-based fixation was compared to fixation of the mesh and hydrogel to an osteal anchor that itself was placed press fit in an osteochondral defect. The PCL osteal anchor was designed to mimic the natural architecture and transition of subchondral bone to cartilage. The reinforcement in the chondral portion was added to enhance integration and mechanical properties of the hydrogel cast on top. In this case, casting was used, but the design can easily be adapted to allow 3D co-printing of the hydrogel and reinforcement fibers.29
The hydrogels used were the same as in the first study and all of them had been previously tested for safety and biocompatibility in the horse (data not shown), suggesting that the materials were not likely to cause reaction. The last fixation study showed that bone resorption was still an issue in the AFG, although the bone destruction seemed considerably less than in the case of CFG. It was concluded that apparently only replacing the fibrin component of the glue by an autologous product was not enough to avoid this unwanted side effect and/or that the influence of the mechanical component was larger than anticipated.
Another important finding was that the fixation potential of the AFG was apparently not enough to keep the fiber reinforcement in place in all cases. After 14 days 33% of the defects were empty, and the remaining constructs were in place but showed early signs of the mesh slipping out. In that aspect, the PCL fixation group showed more promising results, with a good integration of the anchor with the surrounding tissue at early stages, which were confirmed by the second, 4-week follow-up, study. The infiltration of the PCL osteal anchor by tissue and the formation of new bone suggest a good integration and adequate response of the native tissue to the construct. However, a long-term evaluation is necessary to confirm these preliminary findings and draw more definitive conclusions.48
The PCL anchor technique solves the fixation issue, as the construct is kept in place by the press fit fixation of the anchor in the osteochondral defect, but it should be realized that this is a conceptually different approach that is valid for osteochondral defects only and not for chondral ones. There has been debate in literature whether chondral or osteochondral defects lead to better results in cartilage repair,43,49 but there is no consensus. An equine study in which the spontaneous repair after 1 year of standardized chondral and osteochondral lesions in the same animal was compared showed better cartilage healing in the osteochondral defects, but these also featured almost invariably subchondral bone changes, which were seen to a much lesser extent in the chondral lesions (Salonius, E. et al. Insufficient spontaneous repair of even small cartilage lesions in the horse. submitted 2017).
Taken together, it can be concluded from the pilot investigations reported in this study that the use of xenogeneic fibrin glue for the fixation of scaffolds intended for regeneration of articular cartilage can be advised against. The use of AFG reduces the unwanted side effects seen with the xenogeneic glue, but its fabrication process is tedious, susceptible to variability, and should probably focus on the manufacturing of an entirely autologous product, as use of a partly autologous product did not result in the complete avoidance of the inflammatory response. Even if this would be solved, the limited capacity of any type of fibrin glue to fixate composite scaffolds featuring fiber reinforcement remains an issue. The use of osteochondral plugs that are implanted press fit solves the fixation issue, but this versatile and surgically relatively easy approach implies the application of an entirely different concept of cartilage healing that includes the total osteochondral unit and not only the chondral part of the joint surface.
It is well possible that in the near future, with the advent of sophisticated bioreactor systems that will be able to mimic physiological loading of joint tissues for prolonged periods, the need for large animal in vivo studies for the assessment of potential regenerative therapies for articular cartilage defects will decline. However, at this stage of orthopedic research, no valid alternatives that can fully replace large animal models have been described yet50,51 and the equine model is still considered as one of the most representative models available.14,52 In these large animal models, fixation represents a fundamental issue and will continue to do so particularly in the perspective of future translation of composite scaffolds to clinical use, where obviously humans will be in first place, followed however by companion animals such as horses and dogs, which are in this respect not only experimental animals but also patients in their own right.
Supplementary Material
Acknowledgements
The authors thank C. Wayne McIlwraith, BVSc, PhD Dipl ACVS/ECVS/ACVSMR for his much appreciated help and constructive comments and A. Bouwman for the help in data collection of study 1. The research leading to these results has received funding from the Ministerio de Ciencia, Tecnología y Telecomunicaciones de Costa Rica (MICITT), the Consejo Nacional para Investigaciones Científicas y Tecnológicas de Costa Rica (CONICIT), the European Community’s Seventh Framework Programme (FP7/2007–2013) under grant agreement 309962 (HydroZONES), the European Research Council under grant agreement 647426 (3D-JOINT), and the Dutch Arthritis Foundation (LLP-12 and LLP-22).
Footnotes
Disclosure Statement
No competing financial interests exist.
References
- 1.Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009;1:461. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Z, Shi Y, Wei X, He J, Yang S, Dickson G, Tang J, Xiang J, Song C, Li G. Fabrication and repair of cartilage defects with a novel acellular cartilage matrix scaffold. Tissue Eng Part C Methods. 2009;16:865. doi: 10.1089/ten.TEC.2009.0444. [DOI] [PubMed] [Google Scholar]
- 3.Steinert AF, Ghivizzani SC, Rethwilm A, Tuan RS, Evans CH, No¨th U. Major biological obstacles for persistent cell-based regeneration of articular cartilage. Arthritis Res Ther. 2007;9:213. doi: 10.1186/ar2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Temenoff JS, Mikos AG. Review: tissue engineering for regeneration of articular cartilage. Biomaterials. 2000;21:431. doi: 10.1016/s0142-9612(99)00213-6. [DOI] [PubMed] [Google Scholar]
- 5.Smith G, Knutsen G, Richardson J. A clinical review of cartilage repair techniques. Bone Joint J. 2005;87:445. doi: 10.1302/0301-620X.87B4.15971. [DOI] [PubMed] [Google Scholar]
- 6.Makris EA, Gomoll AH, Malizos KN, Hu JC, Athanasiou KA. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. 2015;11:21. doi: 10.1038/nrrheum.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunziker EB, Lippuner K, Keel M, Shintani N. An educational review of cartilage repair: precepts & practice–myths & misconceptions–progress & prospects. Osteoarthritis Cartilage. 2015;23:334. doi: 10.1016/j.joca.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Kuo CK, Li W-J, Mauck RL, Tuan RS. Cartilage tissue engineering: its potential and uses. Curr Opin Rheumatol. 2006;18:64. doi: 10.1097/01.bor.0000198005.88568.df. [DOI] [PubMed] [Google Scholar]
- 9.Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA. Hydrogels in regenerative medicine. Adv Mater. 2009;21:3307. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kock L, van Donkelaar CC, Ito K. Tissue engineering of functional articular cartilage: the current status. Cell Tissue Res. 2012;347:613. doi: 10.1007/s00441-011-1243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LeBaron R, Athanasiou KA. Ex vivo synthesis of articular cartilage. Biomaterials. 2000;21:2575. doi: 10.1016/s0142-9612(00)00125-3. [DOI] [PubMed] [Google Scholar]
- 12.Schwab A, Kock L, Mouser VHM, Stichler S, Abbadessa A, Schro¨n F, Hansmann F, Ehlicke F, Walles F. Screening novel hydrogels in an ex vivo cartilage defect model. Eur Cells Mater. 2016;31:7. [Google Scholar]
- 13.McIlwraith CW, Fortier LA, Frisbie DD, Nixon AJ. Equine models of articular cartilage repair. Cartilage. 2011;2:317. doi: 10.1177/1947603511406531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malda J, Benders K, Klein T, De Grauw J, Kik M, Hutmacher D, Saris D, Van Weeren P, Dhert W. Comparative study of depth-dependent characteristics of equine and human osteochondral tissue from the medial and lateral femoral condyles. Osteoarthritis Cartilage. 2012;20:1147. doi: 10.1016/j.joca.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Hunziker EB, Stähli A. Surgical suturing of articular cartilage induces osteoarthritis-like changes. Osteoarthritis Cartilage. 2008;16:1067. doi: 10.1016/j.joca.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bekkers J, Tsuchida A, Malda J, Creemers L, Castelein R, Saris D, Dhert W. Quality of scaffold fixation in a human cadaver knee model. Osteoarthritis Cartilage. 2010;18:266. doi: 10.1016/j.joca.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Kerker JT, Leo AJ, Sgaglione NA. Cartilage repair: synthetics and scaffolds: basic science, surgical techniques, and clinical outcomes. Sports Med Arthrosc Rev. 2008;16:208. doi: 10.1097/JSA.0b013e31818cdbaa. [DOI] [PubMed] [Google Scholar]
- 18.Nixon A, Rickey E, Butler T, Scimeca M, Moran N, Matthews G. A chondrocyte infiltrated collagen type I/III membrane (MACI® implant) improves cartilage healing in the equine patellofemoral joint model. Osteoarthritis Cartilage. 2015;23:648. doi: 10.1016/j.joca.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 19.Lind M, Larsen A, Clausen C, Osther K, Everland H. Cartilage repair with chondrocytes in fibrin hydrogel and MPEG polylactide scaffold: an in vivo study in goats. Knee Surg Sports Traumatol Arthrosc. 2008;16:690. doi: 10.1007/s00167-008-0522-1. [DOI] [PubMed] [Google Scholar]
- 20.Bekkers JE, Tsuchida AI, van Rijen MH, Vonk LA, Dhert WJ, Creemers LB, Saris DB. Single-stage cell-based cartilage regeneration using a combination of chondrons and mesenchymal stromal cells comparison with microfracture. Am J Sports Med. 2013;41:2158. doi: 10.1177/0363546513494181. [DOI] [PubMed] [Google Scholar]
- 21.Brehm W, Aklin B, Yamashita T, Rieser F, Tru¨b T, Jakob R, Mainil-Varlet P. Repair of superficial osteochondral defects with an autologous scaffold-free cartilage construct in a caprine model: implantation method and short-term results. Osteoarthritis Cartilage. 2006;14:1214. doi: 10.1016/j.joca.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Hale BW, Goodrich LR, Frisbie DD, McIlwraith CW, Kisiday JD. Effect of scaffold dilution on migration of mesenchymal stem cells from fibrin hydrogels. Am J Vet Res. 2012;73:313. doi: 10.2460/ajvr.73.2.313. [DOI] [PubMed] [Google Scholar]
- 23.Goodrich LR, Chen AC, Werpy NM, Williams AA, Kisiday JD, Su AW, Cory E, Morley PS, McIlwraith CW, Sah RL. Addition of Mesenchymal stem cells to autologous platelet-enhanced fibrin scaffolds in chondral defects. J Bone Joint Surg Am. 2016;98:23. doi: 10.2106/JBJS.O.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Visser J, Melchels FP, Jeon JE, Van Bussel EM, Kimpton LS, Byrne HM, Dhert WJ, Dalton PD, Hutmacher DW, Malda J. Reinforcement of hydrogels using three-dimensionally printed microfibres. Nat Commun. 2015;6 doi: 10.1038/ncomms7933. [DOI] [PubMed] [Google Scholar]
- 25.Frenkel SR, Di Cesare PE. Scaffolds for articular cartilage repair. Ann Biomed Eng. 2004;32:26. doi: 10.1023/b:abme.0000007788.41804.0d. [DOI] [PubMed] [Google Scholar]
- 26.Knecht S, Erggelet C, Endres M, Sittinger M, Kaps C, Stu¨ssi E. Mechanical testing of fixation techniques for scaffold-based tissue-engineered grafts. J Biomed Mater Res B Appl Biomater. 2007;83:50. doi: 10.1002/jbm.b.30765. [DOI] [PubMed] [Google Scholar]
- 27.Thorn J, Sørensen H, Weis-Fogh U, Andersen M. Autologous fibrin glue with growth factors in reconstructive maxillofacial surgery. Int J Oral Maxillofac Surg. 2004;33:95. doi: 10.1054/ijom.2003.0461. [DOI] [PubMed] [Google Scholar]
- 28.Cavichiolo JB, Buschle M, Carvalho B. Comparison of fibrin adhesives prepared by 3 different methods. Int Arch Otorhinolaryngol. 2013;17:62. doi: 10.7162/S1809-97772013000100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schuurman W, Khristov V, Pot MW, van Weeren PR, Dhert WJ, Malda J. Bioprinting of hybrid tissue constructs with tailorable mechanical properties. Biofabrication. 2011;3 doi: 10.1088/1758-5082/3/2/021001. 021001. [DOI] [PubMed] [Google Scholar]
- 30.Mouser VHM, Abbadessa A, Levato R, Hennink WE, Vermonden T, Gawlitta D, Malda J. Development of a thermosensitive HAMA-containing bio-ink for the fabrication of composite cartilage repair constructs. Biofabrication. 2017;9 doi: 10.1088/1758-5090/aa6265. 015026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hesse E, Freudenberg U, Niemietz T, Greth C, Weisser M, Hagmann S, Binner M, Werner C, Richter W. Peptide-functionalized starPEG/heparin hydrogels direct mitogenicity, cell morphology and cartilage matrix distribution in vitro and in vivo. J Tissue Eng Regen Med. 2017 doi: 10.1002/term.2404. [DOI] [PubMed] [Google Scholar]
- 32.Stichler S, Bertlein S, Jüngst T, Böck T, Hesse E, Renz Y, Seebach E, Mancini I, Van Weeren R, Richter W. Thiol-ene cross-linked polyglycidol-hyaluronic acid hybrid hydrogels: preparation, cell loading, 3D printing, and in vivo evaluation. Front Bioeng Biotechnol; Conference abstract: 10th World Biomaterials Congress; [DOI] [Google Scholar]
- 33.Drobnič M, Radosavljevič D, Ravnik D, Pavlovčič V, Hribernik M. Comparison of four techniques for the fixation of a collagen scaffold in the human cadaveric knee. Osteoarthritis Cartilage. 2006;14:337. doi: 10.1016/j.joca.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 34.Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21:2529. doi: 10.1016/s0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 35.Groen WM, Diloksumpan P, van Weeren PR, Levato R, Malda J. From intricate to integrated: biofabrication of articulating joints. J Orthop Res. 2017 doi: 10.1002/jor.23602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huey DJ, Hu JC, Athanasiou KA. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338:917. doi: 10.1126/science.1222454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cucchiarini M, Madry H, Guilak F, Saris D, Stoddart M, Koon Wong M, Roughley P. A vision on the future of articular cartilage repair. Eur Cell Mater. 2014;27:12. doi: 10.22203/ecm.v027sa03. [DOI] [PubMed] [Google Scholar]
- 38.Kon E, Filardo G, Di Martino A, Marcacci M. ACI and MACI. J Knee Surg. 2012;25:017. doi: 10.1055/s-0031-1299651. [DOI] [PubMed] [Google Scholar]
- 39.Marmotti A, Bruzzone M, Bonasia DE, Castoldi F, Von Degerfeld MM, Bignardi C, Mattia S, Maiello A, Rossi R, Peretti GM. Autologous cartilage fragments in a composite scaffold for one stage osteochondral repair in a goat model. Eur Cell Mater. 2013;26:15. doi: 10.22203/ecm.v026a02. [DOI] [PubMed] [Google Scholar]
- 40.Nixon AJ, Fortier LA, Williams J, Mohammed H. Enhanced repair of extensive articular defects by insulin-like growth factor-I-laden fibrin composites. J Orthop Res. 1999;17:475. doi: 10.1002/jor.1100170404. [DOI] [PubMed] [Google Scholar]
- 41.Fisher MB, Belkin NS, Milby AH, Henning EA, Bostrom M, Kim M, Pfeifer C, Meloni G, Dodge GR, Burdick JA. Cartilage repair and subchondral bone remodeling in response to focal lesions in a mini-pig model: implications for tissue engineering. Tissue Eng Part A. 2014;21:850. doi: 10.1089/ten.tea.2014.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vasara AI, Hyttinen MM, Lammi MJ, Lammi PE, Laångsjoö TK, Lindahl A, Peterson L, Kellomäki M, Konttinen YT, Helminen HJ. Subchondral bone reaction associated with chondral defect and attempted cartilage repair in goats. Calcif Tissue Int. 2004;74:107. doi: 10.1007/s00223-002-2153-8. [DOI] [PubMed] [Google Scholar]
- 43.Kold S, Hickman J, Melsen F. An experimental study of the healing process of equine chondral and osteochondral defects. Equine Vet J. 1986;18:18. doi: 10.1111/j.2042-3306.1986.tb03529.x. [DOI] [PubMed] [Google Scholar]
- 44.White AA, Panjabi M, Southwick W. The four biomechanical stages of fracture repair. J Bone Joint Surg Am. 1977;59:188. [PubMed] [Google Scholar]
- 45.Ahern B, Parvizi J, Boston R, Schaer T. Preclinical animal models in single site cartilage defect testing: a systematic review. Osteoarthritis Cartilage. 2009;17:705. doi: 10.1016/j.joca.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 46.Seegers WH. Blood Clotting Enzymology. New York, London: Academic Press; 2013. [Google Scholar]
- 47.Meade T, Chakrabarti R, Haines A, North W, Stirling Y. Characteristics affecting fibrinolytic activity and plasma fibrinogen concentrations. Br Med J. 1979;1:153. doi: 10.1136/bmj.1.6157.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bolaños RV, Cokelaere S, McDermott JE, Benders K, Gbureck U, Plomp S, Weinans H, Groll J, van Weeren P, Malda J. The use of a cartilage decellularized matrix scaffold for the repair of osteochondral defects: the importance of long-term studies in a large animal model. Osteoarthritis Cartilage. 2017;25:413. doi: 10.1016/j.joca.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frisbie DD, Oxford JT, Southwood L, Trotter GW, Rodkey WG, Steadman JR, Goodnight JL, McIlwraith CW. Early events in cartilage repair after subchondral bone microfracture. Clin Orthop Relat Res. 2003;407:215. doi: 10.1097/00003086-200302000-00031. [DOI] [PubMed] [Google Scholar]
- 50.Cook J, Hung C, Kuroki K, Stoker A, Cook C, Pfeiffer F, Sherman S, Stannard J. Animal models of cartilage repair. Bone Joint Res. 2014;3:89. doi: 10.1302/2046-3758.34.2000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chu CR, Szczodry M, Bruno S. Animal models for cartilage regeneration and repair. Tissue Eng Part B Rev. 2010;16:105. doi: 10.1089/ten.teb.2009.0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCoy A. Animal models of osteoarthritis comparisons and key considerations. Vet Pathol. 2015;52:803. doi: 10.1177/0300985815588611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.