Abstract
In medium-size, spiny striatal neurons of the direct pathway, dopamine D1- and adenosine A1-receptors are coexpressed and are mutually antagonistic. Recently, a mutation in the gene encoding the A1-receptor (A1R), A1R-G279S7.44, was identified in an Iranian family: two affected offspring suffered from early-onset l-DOPA–responsive Parkinson’s disease. The link between the mutation and the phenotype is unclear. Here, we explored the functional consequence of the G279S substitution on the activity of the A1-receptor after heterologous expression in HEK293 cells. The mutation did not affect surface expression and ligand binding but changed the susceptibility to heat denaturation: the thermodynamic stability of A1R-G279S7.44 was enhanced by about 2 and 8 K when compared with wildtype A1-receptor and A1R-Y288A7.53 (a folding-deficient variant used as a reference), respectively. In contrast, the kinetic stability was reduced, indicating a lower energy barrier for conformational transitions in A1R-G279S7.44 (73 ± 23 kJ/mol) than in wild-type A1R (135 ± 4 kJ/mol) or in A1R-Y288A7.53 (184 ± 24 kJ/mol). Consistent with this lower energy barrier, A1R-G279S7.44 was more effective in promoting guanine nucleotide exchange than wild-type A1R. We detected similar levels of complexes formed between D1-receptors and wild-type A1R or A1R-G279S7.44 by coimmunoprecipitation and bioluminescence resonance energy transfer. However, lower concentrations of agonist were required for half-maximum inhibition of dopamine-induced cAMP accumulation in cells coexpressing D1-receptor and A1R-G279S7.44 than in those coexpressing wild-type A1R. These observations predict enhanced inhibition of dopaminergic signaling by A1R-G279S7.44 in vivo, consistent with a pathogenic role in Parkinson’s disease.
Introduction
The motor symptoms of Parkinson’s disease—i.e., brady-/akinesia, rigor, tremor, and postural instability—result from a loss of dopamine in the striatum (putamen and caudate nucleus) (Ehringer and Hornykiewicz, 1960). Dopamine is supplied by axonal projections of neurons, which reside in the substantia nigra pars compacta (Hornykiewicz et al., 1968). For reasons that still remain enigmatic, these neurons are vulnerable and susceptible to degeneration. Hence, the prevalence of Parkinson’s disease increases with age (Nussbaum and Ellis, 2003). In most instances, neuronal loss is associated with the accumulation of Lewy bodies and Lewy neurites. These fibrillary aggregates contain α-synuclein (Spillantini et al., 1997) and engulfed organelles (Shahmoradian et al., 2019). α-Synuclein is a small protein, which is largely unstructured in solution, but it adopts a α-helical structure in the presence of highly curved membranes containing acidic phospholipids (Davidson et al., 1998). Thus, under physiologic conditions, α-synuclein is distributed between two pools, a largely unstructured soluble monomeric form and an α-helical oligo-meric from, which associates with synaptic vesicles (Burré et al., 2018). α-Synuclein can also form protofibrils composed of β-sheets (Burré et al., 2018). It is not clear what triggers β-sheet formation and fibrillary aggregation of α-synuclein in vivo (Giasson et al., 1999), but point mutations can enhance aggregation (Narhi et al., 1999); the mutated variants also nucleate fibrillation of wild-type α-synuclein (Wood et al., 1999). Hence, it is not surprising that they act in a dominant manner. These missense mutations in α-synuclein occur in patients suffering from early-onset, autosomal dominant Parkinson’s disease; in fact, they were the first genetic cause identified in Parkinson’s disease (Polymeropoulos et al., 1997; Krüger et al., 1998). However, sporadic Parkinson’s disease is substantially more frequent than familial forms. Of the many gene loci that have been linked to Parkinson’s disease over the past two decades (Chang et al., 2017), only a fraction give rise to Mendelian (monogenic) disease, which can be transmitted in an autosomal dominant or recessive form (Zhang et al., 2018). These hereditary forms of Parkinson’s disease have nevertheless shed light on the pathogenesis and on genetic risk factors: mutations in signaling pathways including the endocytotic recycling machinery, in mitochondrial regulators, and in components of the proteostasis network can lead to Parkinson’s disease. Thus, Parkinson’s disease is heterogeneous in both the clinical manifestation and in the underlying cause: in most instances, environmental factors apparently act in combination with a genetic susceptibility (Singleton et al., 2013; Zhang et al., 2018).
Recently, a mutation in the adenosine A1-receptor (A1R), which substituted glycine at the position 279 by serine (A1R-G279S7.44), was identified in a consanguinous Iranian family: two brothers (out of 10 sequenced members) were homozygous for A1R-G279S7.44, and both developed symptoms of Parkinson’s disease during their third decade. None of the other heterozygous members was affected. Hence, A1R-G279S7.44 was proposed as an autosomally recessive transmitted cause of early-onset Parkinson’s disease (Jaberi et al., 2016). The A1-receptor is a prototypical G protein–coupled receptor (GPCR), which is abundantly expressed in the cerebral cortex and the basal ganglia of the human brain (Fastbom et al., 1987). In the striatum, A1-receptors reside on the postsynaptic membrane of the medium-sized spiny neurons of the direct pathway, where they antagonize the dopamine D1-receptor–mediated signaling (Ferré et al., 1994, 1997). In addition, A1-receptors are also present on dopaminergic neurons of the substantia nigra pars compacta, where they reduce dopamine release by presynatic inhibition (Yabuuchi et al., 2006; Borycz et al., 2007). Large prospective studies have shown that consumption of caffeine, which blocks adenosine receptors, protects against the development of Parkinson’s disease (Costa et al., 2010; Palacios et al., 2012). Thus, there is circumstantial evidence to posit a pathogenic role of the mutant A1R-G279S7.44 in the development of Parkinson’s disease. However, the mechanistic basis remains enigmatic. In this study, we explored the functional consequence of the G279S7.44 substitution on the activity of the A1-receptor after heterologous expression in HEK293 cells. We show that the mutation augmented both the basal activity of the receptor and its response to agonist-induced activation due to enhanced conformational flexibility. This translated into more potent inhibition of dopamine-induced cAMP accumulation.
Materials and Methods
Materials
Cell culture media, 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), buffers, salts, and standard reagents were purchased from Sigma Aldrich (St. Louis, MO); FBS from Biowest (Nuaillé, France); 4-(3-butoxy-4-methoxyphenyl)methyl-2-imidazolidone and xanthine amine congener (XAC) from Tocris (Abingdon, UK); adenosine deaminase and Complete protease inhibitor cocktail from Roche (Mannhein, Germany); N6-cyclopentyladenosine (CPA) from Abcam (Cambridge, UK); and Q5 High-Fidelity 2X Master Mix and NEBuilder HiFi DNA Assembly from New England BioLabs (Ipswich, MA). The mouse monoclonal anti-HA antibody immobilized on agarose (A2095) was from Sigma Aldrich; the rabbit monoclonal anti-HA antibody (C29F4) and mouse monoclonal anti-HA antibody (2367) were from Cell Signaling Technology (Cambridge, UK). Mouse monoclonal (M2 clone; F3165) and rabbit polyclonal anti-FLAG antibodies (SC807) were from Sigma Aldrich and from Santa Cruz Biotechnology (Dallas, TX), respectively. AlexaFluor-488–labeled secondary antibody against murine IgG (A32732) for flow cytometry was from Invitrogen (Carlsbad, CA). A fluorescently labeled secondary antibody (donkey anti-rabbit, 926-32213) from Li-Cor (Lincoln, NE) was used for immunoblotting. [3H]Adenine (specific activity 40 Ci/mmol), [3H] DPCPX (specific activity 164Ci/mmol), [3H]7-chloro-3-methyl-1-phenyl-1,2,4,5-tetrahydro-3-benzazepin-8-ol (specific activity 83 Ci/mmol), and [35S]GTPγS (specific activity 1385 Ci/mmol) were purchased from PerkinElmer (Waltham, MA).
The plasmids encoding the human adenosine A1-receptor harboring an N-terminal FLAG epitope, the human dopamine D1-receptor harboring an N-terminal HA-epitope, and NanoLuc luciferase (PNL1.1) were obtained from Sinobiological (Beijing, China), from the cDNA resource center (Bloomsburg University), and from Promega (Fitchburg, WI), respectively. The plasmid encoding the human adenosine A1-receptor with enhanced yellow fluorescent protein (eYFP) fused to its C terminus was a kind gift from Rafael Franco (University of Barcelona, Barcelona, Spain). The G279S7.44 mutation was introduced into the cDNA of the FLAG- and eYFP-tagged A1-receptor by site-directed mutagenesis using the QuikChange II site direction mutagenesis kit (Agilent, Santa Clara, CA). The cDNA coding for the D1-receptor (D1R) was fused in frame to the N-terminal sequence of NanoLuc luciferase to generate D1R-NLuc using NEBuilder HiFi DNA Assembly from New England Biolabs; for polymerase chain reaction amplification of the PNL1.1 vector, standard forward and reverse primers were used; for amplification of the D1R cDNA, primers were designed to have an additional overhang at their 59 end with the primers used to amplify PNL1.1 vector. The transfection reagent was polyethylenimine (PEI; linear 25 kDa; Santa Cruz Biotechnology), and the working stock solution (1 mg/ml in water) was kept at 4°C (maximum for 2 weeks). For long-term storage up to 12 weeks, the PEI stock solution was kept at 220°C.
Molecular Dynamics Simulations
The adenosine A1-receptor was simulated using the active form based on the agonist-liganded, Gi2 protein–bound structure [Protein Data Bank identifier (PDB ID): 6D9H; Draper-Joyce et al., 2018] and starting from the inactive conformation based on the antagonist-bound structure (PDB ID: 5N2S; Cheng et al., 2017). For either conformation, wild-type and mutant receptors were simulated in the presence of absence of adenosine. The missing loop between transmembrane helix (TM) 5 and TM6 (residues 214–222) of the receptor was modeled using Modeler 9.20 (Shen and Sali, 2006; Webb and Sali, 2014) creating 100 structures, which were ranked according to the Discrete Optimized Protein Energy score. The best three were selected for simulations; the G279S mutation was introduced using Pymol (PyMOL Molecular Graphics System, version 1.8.4; Schrödinger, LLC). Eight systems were created for each of these three selected structures, i.e., the receptor with an empty binding site (A1R and A1R-G279S7.44) and bound with adenosine (A1R.ado and A1R-G279S7.44.ado), each in complex with the G protein (A1R.G and A1R-G279S7.44.G; A1R.ado.G and A1R-G279S7.44.ado.G) or in the G protein–free state (A1R and A1R-G279S7.44;A1R.ado and A1R-G279S7.44.ado). Equilibrated membrane embedded systems were created by converting all models into the coarse grain representation of the Martini force field (Monticelli et al., 2008; de Jong et al., 2013; Wassenaar et al., 2015), which allowed for fast membrane equilibration. The proteins were embedded into a phosphatidylcholine (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine) (POPC):cholesterol membrane (70:30 mol%); the simulation box was filled with water and 150 mM NaCl. The coarse grain systems were simulated for 1 microsecond with the protein structure restrained to avoid conformational changes during membrane equilibration. Next, membrane, water, and ions were converted to an all-atom representation (Wassenaar et al., 2014) while the original receptor structure replaced the coarse grain model. Spurious atom overlaps were relaxed using the membed procedure (Wolf et al., 2010). In the all-atom representation, protein, adenosine, and solvent were described using the amber99sb-ildn force field (Lindorff-Larsen et al., 2010), POPC, and cholesterol by Slipid (Jämbeck and Lyubartsev, 2012, 2013). All simulations used GROMACS version 2019.2 (Abraham et al., 2015). The completely assembled systems were energy minimized, and the receptor was released in four steps of 2.5 nanoseconds each by slowly reducing the position restraints (1000, 100, 10, 1 kJ/mol per nanometer) acting on the Ca atoms and on adenosine if present. The production runs were carried for 500 nanoseconds for each independently assembled system. The temperature was maintained at 310 K using the v-rescale (τ = 0.5 picosecond) thermostat (Bussi et al., 2007) while separately coupling protein + adenosine, membrane, and solvent. Pressure was maintained at 1 bar using the Parrinello-Rahman barostat (Parrinello and Rahman, 1981) in a semi-isotropic manner and with a coupling constant of 20.1 picoseconds. Long-range electrostatic interactions were described using the smooth particle mesh Ewald method (Darden et al., 1993) applying a cutoff of 0.9 nm. The van der Waals interactions were described using the Lennard-Jones potentials applying a cutoff of 0.9 nm. Long-range correction for energy and pressure were applied. Coordinates of all atoms were recorded every 50 picoseconds. Data for figures were extracted with the GROMACS package and processed in R and Python scripts using the MD Analysis package, version 0.19.2 (Michaud-Agrawal et al., 2011; Gowers et al., 2019). Visual Molecular Dynamics, version 1.9.3 (Humphrey et al., 1996), and Pymol, version 1.8.4, were used for visualization.
Cell Culture
HEK293 cells were plated in growth medium [Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS] in 15-cm dishes or six-well dishes at 37°C in a humidified atmosphere containing 5% CO2. When the cells were 80% confluent, they were transfected with plasmid(s) of interest using PEI (linear 25 kDa; Santa Cruz Biotechnology) as a transfection reagent. Briefly, DNA and PEI were mixed at a ratio of 1:3 (w:w) in serum-free DMEM and incubated for 15 minutes at 22°C. The mixture was then added in a dropwise manner to the dish. If not otherwise indicated, the total amount of DNA and of PEI used for a typical transfection were 11 μg and 33 μg per 15-cm dish or 2 μg and 6 μg per well, respectively. All assays were done 24 hours after transfection.
Membrane Preparation
HEK293 cells (8 × 106) were seeded in 15-cm dishes. When they were about 80% confluent (about 1.6 × 107/dish), they were transiently transfected with empty plasmid alone (mock; 11 μg/dish) or the combination of empty plasmid (5.5 μg/dish) and plasmids encoding the human wild-type A1R (5.5 μg/dish) or A1R-G279S7.44 (5.5 μg/dish), which carried a FLAG-epitope on their N terminus. After 24 hours, the monolayer was rinsed with ice-cold PBS; subsequently, the cells were mechanically detached with a cell scraper, suspended in 5 ml ice-cold PBS containing 0.5 mM phenyl-methylsulfonyl fluoride (PMSF), and harvested by centrifugation at 300g for 5 minutes at 4°C. The cell pellet was resuspended in ice-cold hypotonic buffer containing 20 mM HEPES/NaOH (pH 7.4), 2 mM MgCl2, 1 mM EDTA, 0.1 mM PMSF, and the Complete protease inhibitor cocktail. Thereafter, the cells were subjected to two freeze-thaw cycles followed by ultrasonication (Sonifier cell disruptor B15,12 pulses of 0.5-second duration at 50% intensity; Branson Ultrasonics, Danbury, CT). Membranes were pelleted by centrifugation for 15 minutes at 38,000g and at 4°C and subsequently resuspended in buffer containing 20 mM HEPES/NaOH (pH 7.4), 2 mM MgCl2, 1 mM EDTA buffer (1 ml per 0.2 g of wet pellet). The protein concentration (about 5 mg/ml) was determined by Coomassie Brilliant Blue binding. Membranes were aliquoted, frozen in liquid nitrogen, and stored at −80°C.
Radioligand Binding
For [3H]DPCPX saturation and displacement experiments, membranes (2–5 μg/assay) were incubated in 0.1 ml buffer containing 50 mM Tris-HCl (pH 7.4), 2 mM MgCl2, 1 mM EDTA, 0.1 mM GTPgS, and 5 U/ml adenosine deaminase in the absence and presence of ligands (CPA or XAC) and [3H]DPCPX (covering the range of 0.2–8 nM for saturation experiments and ~3 nM for displacement experiments) for 30 minutes at 25°C. The reaction was terminated by rapid filtration through GF/C glass fiber filter (Sartorius Stedim, Göttingen, Germany) followed by three washes with ice-cold wash buffer (10 mM Tris/HCl, pH 7.4, 1 mM MgCl2) using a Skatron cell harvester. The radioactivity retained on the filters was measured by liquid scintillation. Nonspecific binding was determined in the presence of 10 μM XAC and represented <<10% of total binding in the KD concentration range. In saturation experiments, the KD and Bmax were determined by subjecting the data to nonlinear least-squares curve fitting to the equation for a rectangular hyperbola. In displacement experiments, the IC50 was estimated by fitting the data to the equation for a monophasic displacement curve. The Ki was calculated using the Cheng-Prusoff approximation (Ki = IC50/(1 + [L]/KD,L). For binding of [35S]GTPγS, membranes (10 μg/assay) were incubated in a total volume of 80 μl containing 50 mM Tris/HCl (pH 7.4), 5 mM MgCl2, 1 mM EDTA, 100 mM NaCl, 10 μM GDP, and 5 U/ml adenosine deaminase in the absence and presence of CPA or DPCPX for 30 minutes at 25°C. Thereafter, a solution (20 μl) containing [35S]GTPγS (to ~1 nM final concentration, buffer composition otherwise identical) was added, and the incubation was continued for 1 minute at 25°C. The incubation was terminated by rapid filtration followed by three washes with ich-cold buffer containing 10 mM Tris/HCl (pH 7.4), 1 mM MgCl2, and 100 mM NaCl. The radioactivity trapped on the filter was determined as outlined above.
Heat Denaturation
Membrane aliquots (5 μg/assay) were incubated in a total volume of 50 μl buffer containing 50 mM Tris-HCl, 2 mM MgCl2,1 mM EDTA, and 5 U/ml adenosine deaminase, pH 7.4 at temperatures ranging from 40°C to 65°C for time intervals ranging from 1 to 120 minutes. Thereafter, the reactions were placed on ice for 15 minutes and subsequently incubated in the presence of 3 nM [3H] DPCPX for 1 hour on ice in a final volume of 0.1 ml containing the same buffer as described above.
Flow Cytometry
HEK293 cells (0.5 × 106/well) were seeded into six-well dishes; when the cells were about 80% confluence (1 × 106/well), they were transiently transfected with empty plasmid alone (mock; 2 μg/well) or cotransfected with the combination of empty plasmid (1 or 1.5 μg/well) and plasmid (0.5 or 1 μg/well) encoding the human wild-type or mutant A1-receptor (A1R-G279S7.44), which carried a FLAG epitope on their N termini. Cells were also cotransfected with plasmids encoding the D1-receptor (1 μg/well) and wild-type or mutant A1-receptor (1 μg/well). The transiently transfected HEK293 cells were washed with PBS containing 0.1% bovine serum albumin (BSA) and thereafter incubated with PBS containing 1 mM EDTA for 10 minutes at 37°C to detach the cells and then suspended in ice-cold PBS containing 0.1% BSA to a density of 1 × 10 cells/ml. The single cell suspension was sequentially incubated with the primary mouse M2 monoclonal anti-FLAG antibody (1:2000; Sigma) and the secondary mouse anti-mouse IgG1 antibody conjugated to AlexaFluor-488 fluorophore from Invitrogen (1:2000) for 20 minutes on ice. Thereafter, cells were pelleted by centrifugation (200g for 5 minutes at 4°C), resuspended in PBS containing 0.1% BSA, and injected into the flow cytometer (BD FACSCanto II; BD Biosciences, Franklin Lakes, NJ). Forward versus side scatter was used to identify cell populations and to exclude debris, which was found at the bottom left-hand corner of the forward scatter versus side scatter density plot (data not shown). In addition, backgating was used to ensure that debris and dead cells were not included in the analysis. Single parameter histograms were generated to quantify the staining by AlexaFluor-488 in the gated area; the specific area under the curve was calculated by subtracting the nonspecific area under the curve obtained from cells transfected with empty vector (mock transfection control).
Immunoprecipitation
HEK293 cells transiently coexpressing the HA-tagged D1-receptor and the FLAG-tagged wild-type or mutant A1-receptor (A1R-G279S7.44) were washed thrice with ice-cold PBS, collected in 5 ml PBS containing 0.1 mM PMSF, and harvested by centrifugation for 5 minutes at 300g and at 4°C. The cell pellet was suspended in buffer containing 25 mM Tris-HCl (pH 7.4), 2 mM MgCl2, 1 mM EDTA, 100 mM NaCl, 1% dodecylmaltoside, 0.1 mM PMSF, and EDTA-free Complete protease inhibitor cocktail. Cell lysis was achieved by incubation for 1 hour with end-over-end rotation at 4°C. Thereafter, the solubilized material was retrieved by centrifugation (16,000g for 15 minutes at 4°C). Preequilibrated beaded agarose (0.1 ml of a 50% slurry) containing immobilized anti-HA antibody was added to an aliquot of the lysate (1 mg); the suspension was incubated with end-over-end rotation for 16 hours with at 4°C. Samples were centrifuged (1 minute at 5000g and at 4°C) and washed three times. The bound proteins were eluted with 0.1 ml denaturing sample buffer containing 20 mM dithiothreitol by heating at 60°C for 15 minutes. Thereafter, aliquots (10 μl) were separated by electrophoresis on SDS-polyacrylamide gels; proteins were transferred onto nitrocellulose membrane. Nonspecific binding was blocked by incubating the membranes in 25 mM Tris-buffered saline (pH 7.5), 0.1% Tween 20, and 5% bovine serum albumin for 1 hour at room temperature. After sequential incubation with anti-FLAG or anti-HA antibodies (1:1000 dilution) and fluorescently labeled donkey anti-rabbit antibody (1:1000 dilution), the immunoreactive bands were visualized and quantified on an Odyssey Clx infrared fluorescent imaging system (LI-COR Biosciences). Aliquots of the cell lysates (20 μg) were also electrophoretically resolved and transferred to nitrocellulose to verify comparable levels of receptor expression in the starting material. The pertinent blots were also probed with a rabbit antiserum, which recognizes all G protein b-subunits (Hohenegger et al., 1996), to control for equal loading of individual lanes (1:2000 dilution).
Bioluminescence Resonance Energy Transfer
HEK293 cells (0.5 × 106/well) were seeded into six-well dishes. When the cells were about 80% (1 × 106/well) confluent, the cells were transiently cotransfected with a constant amount of plasmid encoding the human D1-receptor tagged on its C terminus with NanoLuc luciferase (D1R-NLuc 0.2 μg/well) and increasing amounts (0–1.8 μg/well) of plasmid coding for the wild-type or mutant A1-receptor, which were tagged at their C terminus with eYFP. The total amount of plasmid (2 μg/dish) was kept constant by adding the appropriate amount of empty plasmid. After 8 hours, cells were detached, seeded into 96-well dishes (5 × 104/well), and allowed to adhere for 16 hours. After serum withdrawal for 1 hour, vehicle (control) or the indicated ligands (i.e., 10 μM CPA, 10 μM dopamine, or their combination) and luciferase substrate (furimazine = Nano-Glo, Promega; 1:200 dilution) were added, and bioluminescence was recorded for up to 20 minutes. Bioluminescence resonance energy transfer (BRET) readings were taken by simultaneously measuring light emission at 460 nm and at 530 nm in the microplate reader (FlexStation3; Molecular Devices). The BRET unit (BRET) signal was calculated by the ratio of emission at 530 nm [A1R–yellow fluorescent protein (YFP)] to 460 nm (D1R-NLuc). Cells expressing BRET donor alone (D1R-NLuc) were used to determine background. BRET specificity was tested by using human β-arrestin-2 fused at its C termini to NLuc as a donor and A1R-YFP as an acceptor, which gave equivalent values to that of the cells expressing donor alone. The net-BRET unit was calculated by subtracting background BRET. The data are presented as milli BRET units (net BRET × 1000). Parallel incubations were done with cells solely expressing D1-receptor tagged with NanoLuc, and the emission recorded from these cells was subtracted.
Accumulation of cAMP
Eight hours after transfection, cells were replated into six-well plates (3 × 105 cells/well) and incubated for 16 hours in DMEM containing 1 μCi/ml [3H]adenine (Waldhoer et al., 1999). Cells were then stimulated with 10 μM dopamine alone or in combination with the indicated concentrations of CPA and DPCPX in a total volume of 1 ml DMEM containing 5 U/ml adenosine deaminase for 20 minutes at 25°C. Thereafter, the cells were lysed in 1 ml ice-cold 2.5% perchloric acid containing 100 mM cAMP for 15 minutes on ice. The cell extract was then neutralized with 4.2 M KOH. [3H]cAMP was separated by double column chromatography (Johnson et al., 1994).
Statistical Analysis
The first part of the study was exploratory in nature: the pharmacology of the mutant receptor and its expression were characterized without any working hypothesis. Three (CV ≤ 25%) to six experiments (CV ≤ 60%) were considered enough to verify the reproducibility of experimental findings. For experiments examining the hypothesis of constitutive activity (generated by molecular dynamics simulations and the analysis of thermal stability), the number of experiments was adjusted based on the variation observed: if three experiments did not suffice to show statistical significance, the number of required experiments was estimated with a power calculation (>90% probability of finding a statistically significant difference with P < 0.025) based on the observed variation. Statistical comparisons were done by paired t test (for comparison of two groups), by Friedman test (for paired comparison of multiple groups) followed by Holm-Sidak post hoc testing, or by F-test to compare two curves. Transient transfections with plasmids encoding wild-type and mutant receptors and the subsequent measurements were done in parallel. These parallel samples were considered as paired data because transfection efficiency varied on a day-to-day basis (cf. also Fig. 1D).
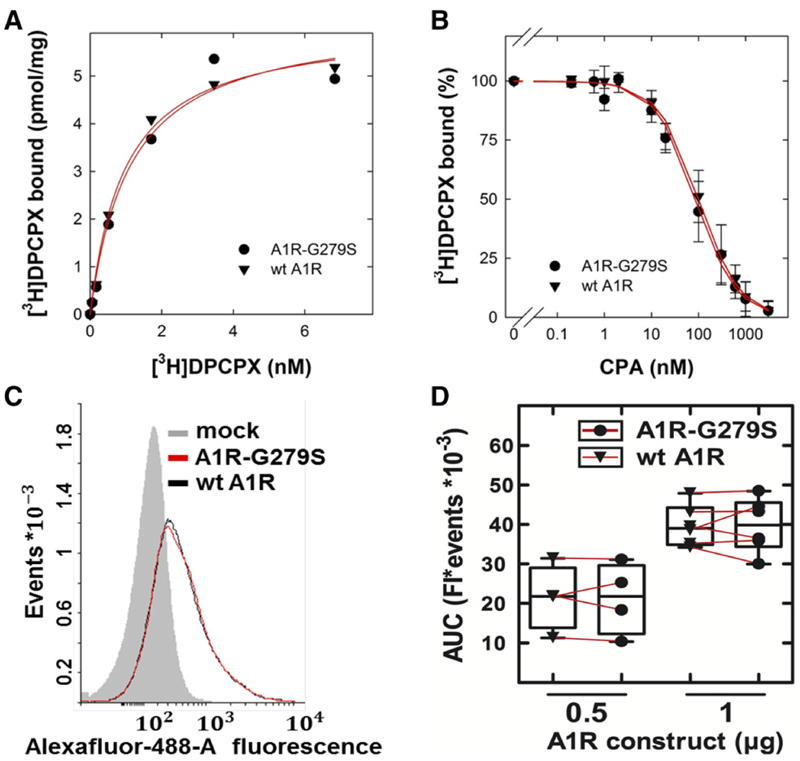
Fig. 1.
Binding of the antagonist/inverse agonist radioligand [3H]DPCPX (A) and of the agonist CPA (B) to and surface expression of wild-type (wt A1R) and mutant A1-receptors (C and D). (A) Saturation curve. Membranes (5 mg/assay) prepared from HEK293 cells transiently expressing the FLAG epitope–tagged human wild-type A1R (triangle down) or A1R-G279S7.44 (full circle) were incubated with the indicated concentrations of [3H]DPCPX for 30 minutes at 25°C in 0.1 ml buffer. Nonspecific binding was determined in the presence of 10 μM XAC and subtracted. The data are means from duplicate determinations in a representative experiment. The curves were drawn by fitting the data to the equation for a rectangular hyperbola. (B) Displacement curve. As described for (A), membranes (2–3 μg/assay) were incubated in the presence of [3H]DPCPX (2.7 nM) in 0.1 ml buffer containing the indicated concentrations of CPA. Data are means ± S.D. from three independent experiments carried out in duplicate with membranes prepared from paired transfections. The curves were drawn by fitting the data to the equation for a monophasic displacement. (C and D) Flow cytometry histograms and their quantification. HEK293 cells were transiently transfected with the empty plasmid alone (mock, shaded histogram) or the combination of empty plasmid and plasmid encoding the FLAG epitope–tagged human wild-type A1R [0.5 or 1 μg/well, black histogram in (C) and full circle in (D)] or A1R-G279S7.44 [0.5 or 1 μg/well, red histogram in (C) and triangle down in (D)]. The total amount of plasmid (2 mg/disk) was kept constant by adding the appropriate amount of empty plasmid. After 24 hours, cells were stained by sequential incubation in the presence of an antibody directed against the FLAG-epitope (1:2000 dilution) and an AlexaFluor-488–conjugated antibody directed against murine IgG1 (1:2000 dilution). The cell-associated fluorescence (Fl) was quantified by flow cytometry. The histogram shows a representative result from a paired transfection. The results from four to six paired transfections with 0.5 and 1 μg plasmid DNA encoding wild-type A1R or A1R-G279S7.44, respectively, are summarized in the spaghetti plot shown in (D). AUC, area under the curve.
Results
Heterologous Expression of Wild-Type (A1R) and Mutant Adenosine A1-Receptor (A1R-G279S7.44)
Many mutations affect the ability of GPCRs to undergo folding in the endoplasmic reticulum (Nanoff and Freissmuth, 2012). In fact, a substantial portion of the heterologously expressed wildtype A1-adenosine receptor is retained and degraded in the endoplasmic reticulum (Pankevych et al., 2003; Kusek et al., 2015). Accordingly, we first examined the impact of the G279S mutation on receptor levels by transient transfection. Transient rather than stable expression was chosen because this approach eliminated possible distortions arising from clonal selection of cells. Comparable levels of receptors were detected with the antagonist radioligand (Fig. 1A): in three independent experiments the number of binding competent receptors Bmax was 5.1 ± 1.1 and 5.9 ± 1.3 pmol/mg (means ± S.D.) for wild-type A1R and A1R-G279S7.44, respectively. It is also evident from Figure 1A that mutant and wild-type receptor did not differ in their affinity for the radioligand (KD = 1.4 ± 0.3 and 1.5 ± 0.4 nM for wild-type A1R and A1R-G279S7.44, respectively). Similarly, as exemplified in Figure 1B for the A1-selective agonist CPA, the G279S7.44 mutation did not affect agonist affinity (Ki = 0.4 ± 0.1 and 0.3 ± 0.1 μM for wildtype A1R and A1R-G279S7.44, respectively). We stress that incubations were done in the presence of GTPgS. In the absence of guanine nucleotides, high-affinity agonist binding sites are expected to exist, which reflect ternary complex formation of agonist, receptor, and heterotrimeric G protein. In a buffer devoid of GTPgS, CPA displaced the radioligand with a biphasic curve (data not shown). However, the proportion of high-affinity sites was too low to provide reliable estimates for agonist affinity in the ternary complex. This is to be expected in transient transfections with high expression levels: upon membrane preparation, a large fraction of the receptor accumulates in vesicles, where receptor molecules outnumber G proteins. We also used flow cytometry by detecting the receptors via their N-terminal FLAG-epitope tag to verify that equivalent amounts were delivered to the plasma membrane: there was a variation in surface levels in individual transfections, but in paired experiments there was no appreciable difference between wild-type and mutant receptor (Fig. 1C). In addition, the amount of receptors, which was detected on the cells, was related to the amount of plasmid DNA (Fig. 1D).
Thermal Stability of Wild-Type and Mutant A1-Adenosine Receptor
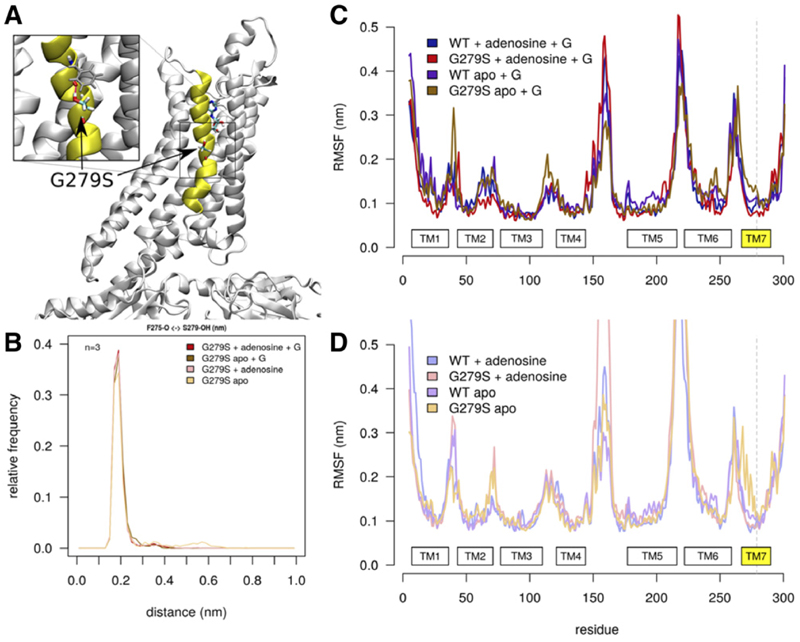
The substitution of G2797.44 by serine introduces an additional hydrogen bond donor within TM7. We performed molecular dynamics simulations to obtain structural and dynamic insights into the flexibility of wildtype A1-receptor and the changes caused by the G279S7.44 mutation. We used the solved structure of the adenosine-bound human A1-receptor in complex with Gi2 as a starting point (Draper-Joyce et al., 2018). Three parallel 500-nanosecond-long simulations were carried out for wild-type and mutant receptor, with and without the G protein and in the presence and absence of adenosine. Figure 2A shows the membrane exposed orientation of the G279S mutation, located in the middle of TM7. The side chain of G279S7.44 interacts strongly with the backbone carbonyl of F2757.40 (Fig. 2B). A hydrogen bond is much stronger in a hydrophobic environment, where it can provide binding energies up to ∼20–25 kJ/mol (Bowie, 2011). The energetic penalty for opening the hydrogen bond is much higher in a hydrophobic environment because it cannot be replaced by an alternative interaction as would occur in an aqueous environment. We also analyzed the root mean square fluctuations (RMSFs) of the A1-receptor to quantify the global mobility of the receptor and to detect local changes in protein flexibility (Fig. 2, C and D). The global mobility of the A1-receptor was similar for all systems in complex with Gi2 (Fig. 2C) and without Gi2 (Fig. 2D). The pattern of mobility reflects the secondary structure of the receptor: the RMSF declines to low values over transmembrane helices, which reflect their rigidity. In contrast, the loops are much more flexible resulting in local maxima of RMSF. Complex formation with Gi2 has an ordering effect on the intracellular loop 3: the mobility of intracellular loop 3 is strongly reduced when interacting with the Gα subunit. The G279S7.44 mutation exerts mostly a local effect on TM7 mobility: in the absence of adenosine (i.e., in the apo state), the mobility of TM7 is larger in the mutant than in the wild-type receptor. This is seen in both the receptor complexed to the G protein (cf. brown and dark violet trace in Fig. 2D) and in the absence of the G protein, where the mobility of TM7 is even more pronounced (cf. amber and violet trace in Fig. 2D).
Fig. 2.
The G279S mutation enhances TM7 flexibility. (A) Structural overview of the A1-receptor. TM7 is highlighted in yellow. The inset shows a zoom-in into the structure of the mutant receptor, which highlights the hydrogen bond formed between the hydroxyl group of G279S and the backbone of F2757.40 (gray sticks). (B) Frequency distribution of the distance between the carbonyl oxygen of F2757.40 and the hydrogen of the hydroxyl group of S2797.44. The histogram shows that—in all simulations (i.e., over 3 × 500 nanoseconds with 1 nanosecond sampling interval)—this hydrogen bond opens very rarely regardless of the state (ligand-bound vs. empty apo state; complexed to G protein or free receptor). (C and D) RMSFs of A1R with and without G protein, respectively. RMSF plots show average data of three replications, measured with 1 nanosecond temporal resolution. WT and G279S refer to the wild-type and the mutant A1-receptor, respectively. The apo state corresponds to the empty receptor (binding site devoid of ligand); + G indicates the mutant and wild-type receptor in complex with the G protein Gi2. In the apo state [brown and amber traces in (C) and (D), respectively], the upper part of TM7 of A1R-G279S7.44 deviates from all other conformations regardless of whether examined with (C) or without (D) G protein.
Hydrogen bonds are an important factor contributing to the forces stabilizing membrane proteins (Bowie, 2000; Stockner et al., 2004). Thus, the additional hydrogen bond in TM7 is predicted to increase thermal stability of the A1-R-G279S7.44. However, TM7 is kinked (cf. Fig. 7A). This bending must be stabilized by helical packing. The additional hydrogen bond introduces a counteracting force, which results in destabilization and hence enhanced flexibility, which is evident from the molecular dynamics simulations, in particular of the G protein–free apo state (amber trace in Fig. 2D). Because of this enhanced flexibility, the mutant receptor also ought to incur a penalty in thermal stability.
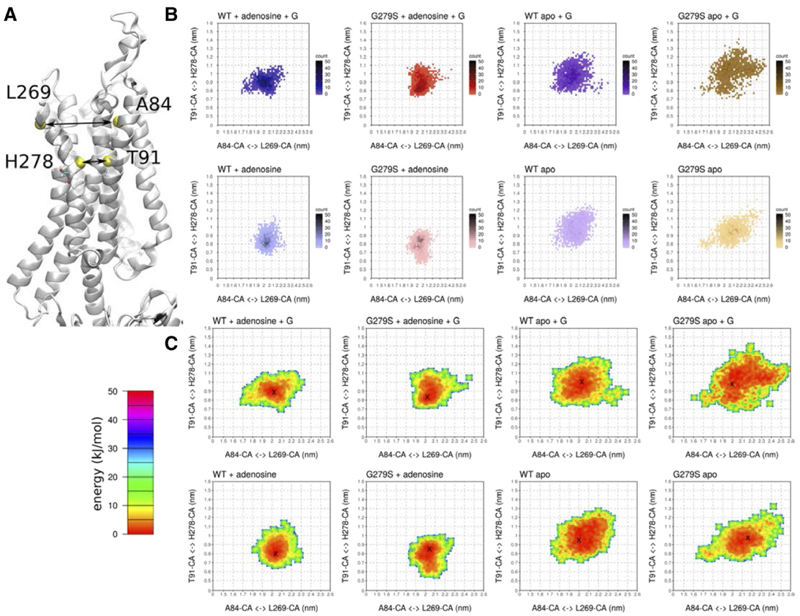
Fig. 7.
The mutation G279S7.44 lowers the free-energy barrier for TM7 to change conformation. (A) The distances, which were measured during the simulations, are highlighted in the structure of the A1-receptor. (B) Two-dimensional histogram of the distances between the Cα atoms (CA) of T913.36 and H2787.43 (x-axes) and of A843.29 andL2697.34 (y-axes). (C) Free-energy estimate associated with conformational changes in the distances shown in (B). The minimum position in the free-energy basin is indicated by ×. Each plot shows the average of three independent 500-nanosecond simulations with data points sampled at 1-nanosecond intervals.WT and G279S refer to the wild-type and the mutant A1-receptor, respectively. The apo state corresponds to the empty receptor (binding site devoid of ligand); + G indicates the mutant and wild-type receptor in complex with the G protein Gi2. CA,
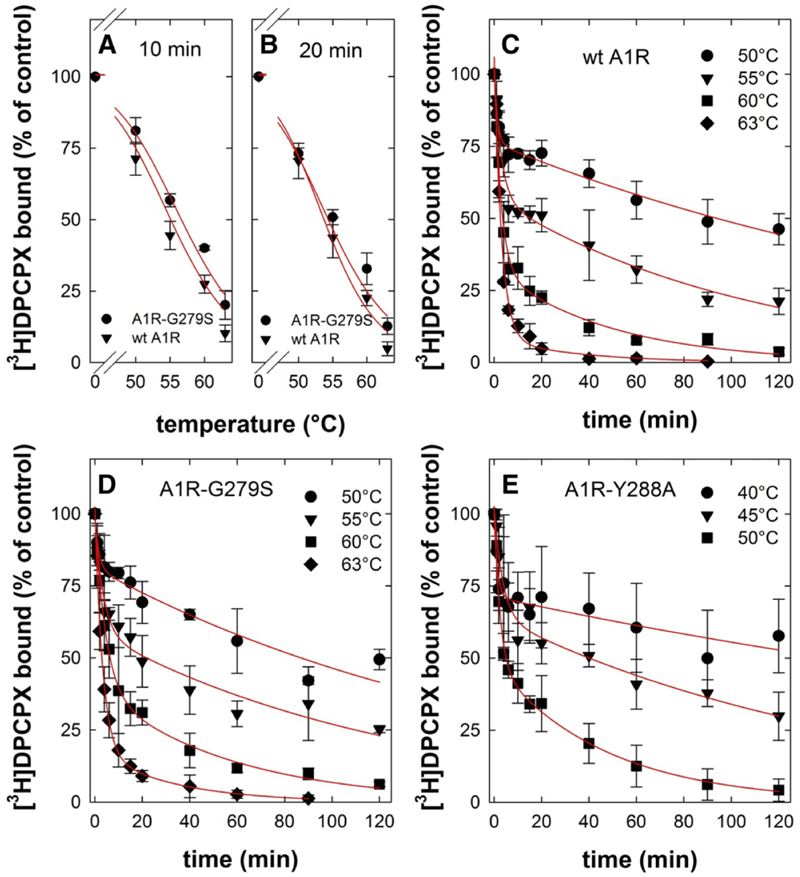
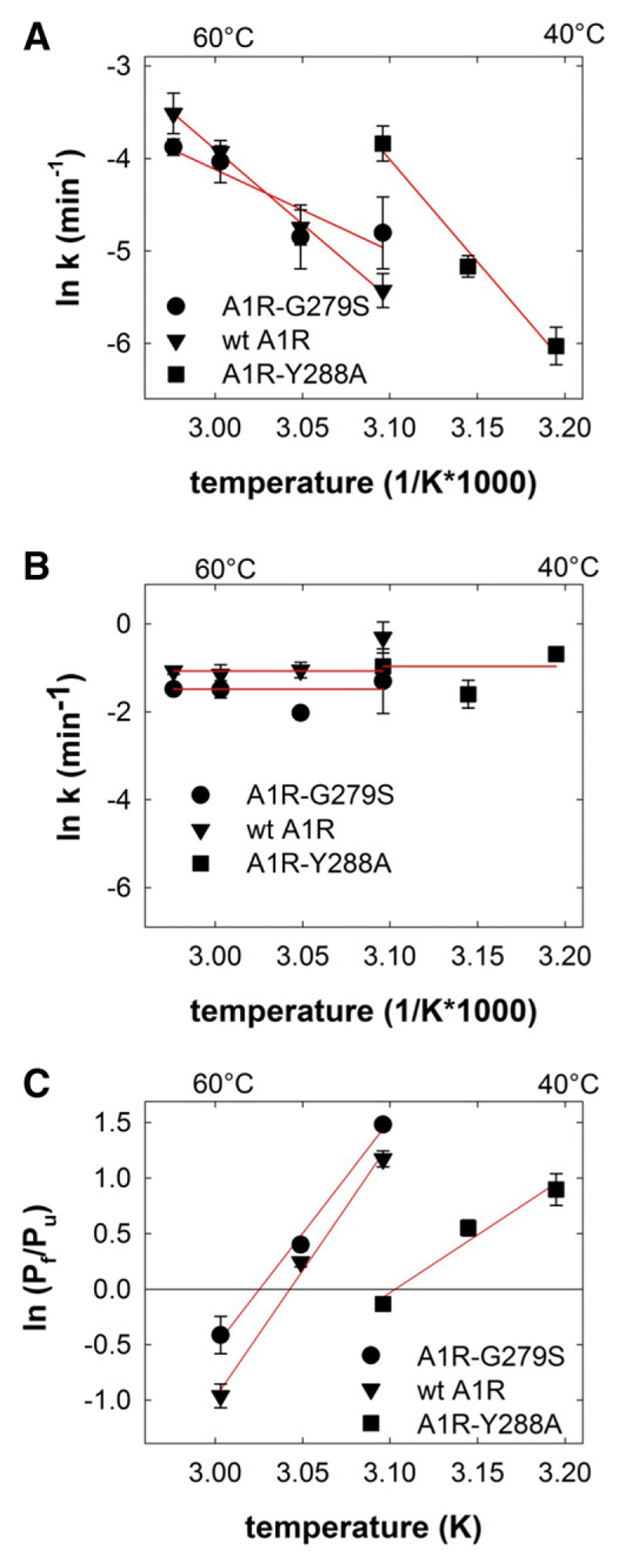
These predictions were examined by incubating membranes harboring wild-type and mutant receptors at temperatures ranging from 50°C to 63°C. Subsequently, the level of residual binding was determined by incubating the membranes with [3H]DPCPX on ice. If the heat-induced denaturation was allowed to proceed for 10 minutes, there was a small but consistent difference between wild-type and mutant receptor (Fig. 3A; T50 = 55.0°C ± 0.6°C and 56.7°C ± 0.5°C for wild-type A1R and A1R-G279S7.44, respectively). This difference was less evident if the incubation time was increased to 20 minutes (Fig. 3B; T50 = 53.7°C ± 0.5°C and 54.3°C± 0.8°C for wild-type A1R and A1R-G279S7.44, respectively). There are two components of protein stability, thermodynamic stability and kinetic stability (Sanchez-Ruiz, 2010): thermodynamic stability refers to the equilibrium between the amount of native functional protein and that of unfolded and partially unfolded states. It is high if—at a given temperature—the equilibrium is tilted in favor of the native protein. Kinetic stability is imparted by a high-energy barrier, which prevents the native state from visiting (partially) unfolded states. This energy barrier corresponds to an activation energy and can therefore be extracted from Arrhenius plots. We examined which component was affected by the G279S mutation by measuring the time-dependent loss of binding at different temperatures for both wild-type A1R (Fig. 3C) and A1R-G279S7.44 (Fig. 3D).Asacontrol, we used A1R-Y288A7.53 (Fig. 2E). This receptor variant has a folding defect, butit can be rescued by pharmacochaperoning (Málaga-Diéguez et al., 2010; Kusek et al., 2015). Accordingly, HEK293 cells were transiently transfected with a plasmid driving the expression of A1R-Y288A7.53 and incubated with 100 mM 3-isobutyl-1-methylxanthine for 16 hours prior to membrane preparation. This suffices to restore folding and cell surface expression of functionally active A1R-Y288A7.53; i.e., the pharmacochaperoned A1R-Y288A7.53 binds the radio-ligand [3H]DPCPX and engages Gi in a manner comparable to wild-type A1R (Málaga-Diéguez et al., 2010). In all instances, heat led to a biphasic loss of binding competent receptors (Fig. 3, C–E): the curves were adequately described by fitting them to the equation for a biexponential decay. We extracted the rate constants for both the fast and the slow component to generate Arrhenius plots (Fig. 4, A and B). It is evident from Figure 3, C–E, that the rate of the slow component increased with rising temperatures. The corresponding Arrhenius plots in Figure 4A show that this temperature-dependent increase was less pronounced with A1R-G279S7.44 than with the wildtype receptor (cf. circles and triangles in Fig. 4A). From the slope of the Arrhenius plot we calculated an activation energy of 135 ± 4 and 73 ± 23 kJ/mol for wild-type A1R and A1R-G279S7.44, respectively. As predicted, A1R-Y288A7.53 was inactivated at lower temperatures than wild-type A1R or A1R-G279S7.44 (cf. Fig. 3, C–E). However, the slope of the Arrhenius plot was actually steeper, and hence the activation energy (184 ± 24 kJ/mol) was larger than that of A1R-G279S7.44 (cf. squares and circles in Fig. 4A). Thus, the G279S7.44 mutation reduced the kinetic stability of the receptor.
Fig. 3.
Heat-induced denaturation of wild-type and mutant adenosine A1-receptors. (A and B) Membranes were prepared from HEK293 cells transiently expressing the FLAG-tagged human wildtype (wt A1R, closed triangles) and mutant A1-receptor (A1R-G279S7.44, closed circles). Membranes (3–5 μg/assay) were subjected to heat-induced denaturation and incubated for 10 minutes (A) or 20 minutes (B) at the indicated temperatures. Thereafter, the membranes were placed on ice. Binding of the [3H]DPCPX (3 nM) was determined for 1 hour at 0°C. The curves in (A and B) were drawn by fitting the data to a three-parameter logistic equation. (C–E) Membranes harboring FLAG-tagged A1R-Y288A7.53 (closed squares) were prepared from transiently transfected HEK293 cells, which had been incubated in the presence of 100 μM 3-isobutyl-1-methylxanthine to restore folding and surface expression of the receptor prior to cell lysis. Membranes (3–5 μg/assay for wt A1R and A1R-G279S7.44; 10–15 μg/assay for A1R-Y288A7.53) were subjected to de-naturation at the indicated temperatures and for the indicated time intervals. Thereafter, binding of [3H]DPCPX (3 nM) was determined as outlined for (A and B). Nonspecific binding was defined in the presence of 10 μM XAC; it was <10% of total binding, did not change with temperature or time, and was subtracted. The 100% reference value is binding to control membranes, which were held on ice throughout the experiment. This binding was 10–15 fmol/assay. Data are means ± S.D. from three independent experiments, which were done in parallel. The curves in (C–E) were drawn by fitting the data to the equation for a biexponential decay.
Fig. 4.
Arrhenius plots for the slow (A) and fast component (B) of heat-induced denaturation and the temperature-dependent change of their ratio (C) for wild-type (wt A1R) and mutant A1-adenosine receptors. (A and B) The rates of the slow (A) and the fast component (B) were calculated from the individual biexponential decay curves summarized in Figure 2, C–E, and their natural logarithm (means ± S.E.M.) plotted as a function of the reciprocal of the absolute temperature. (C) The relative proportion of the slowly (Pf) and of the rapidly denaturing (Pu) component were calculated from the individual biexponential decay curves summarized in Figure 2, C–E. The natural logarithms of their ratios (means ± S.E.M.) were plotted as a function of the reciprocal of the absolute temperature. The lines were drawn by linear regression.
Regardless of which variant of the A1-receptor was examined, the rate of the fast component did not show any appreciable dependence on temperature (Fig. 4B), reflecting the low energy barrier of thermodynamic stability (Sanchez-Ruiz, 2010). Plotting the ratio of the slowly denaturing component over the rapidly unfolding component (Pf/Pu) as a function of temperature (1/K) allows for comparing the thermodynamic stability of the receptor variants: it is evident from Figure 4C that the x-intercept of A1R-G279S7.44 is shifted to the left (i.e.,to a higher temperature) of that of wild-type A1R; the difference of about 2 K is consistent with the difference in melting temperature seen in Figure 3A. In contrast and as predicted for a folding-deficient mutant, the melting temperature of A1R-Y288A7.53 was lower by some 6.5 K than that of wild-type A1R (cf. squares and triangles in Fig. 4C). Taken together, these observations show that A1R-G279S7.44 has an enhanced thermodynamic stability but a reduced kinetic stability, whereas the reverse is true for A1R-Y288A7.53.
Complex Formation between the Dopamine D1- and Wild-Type and Mutant A1-Receptors
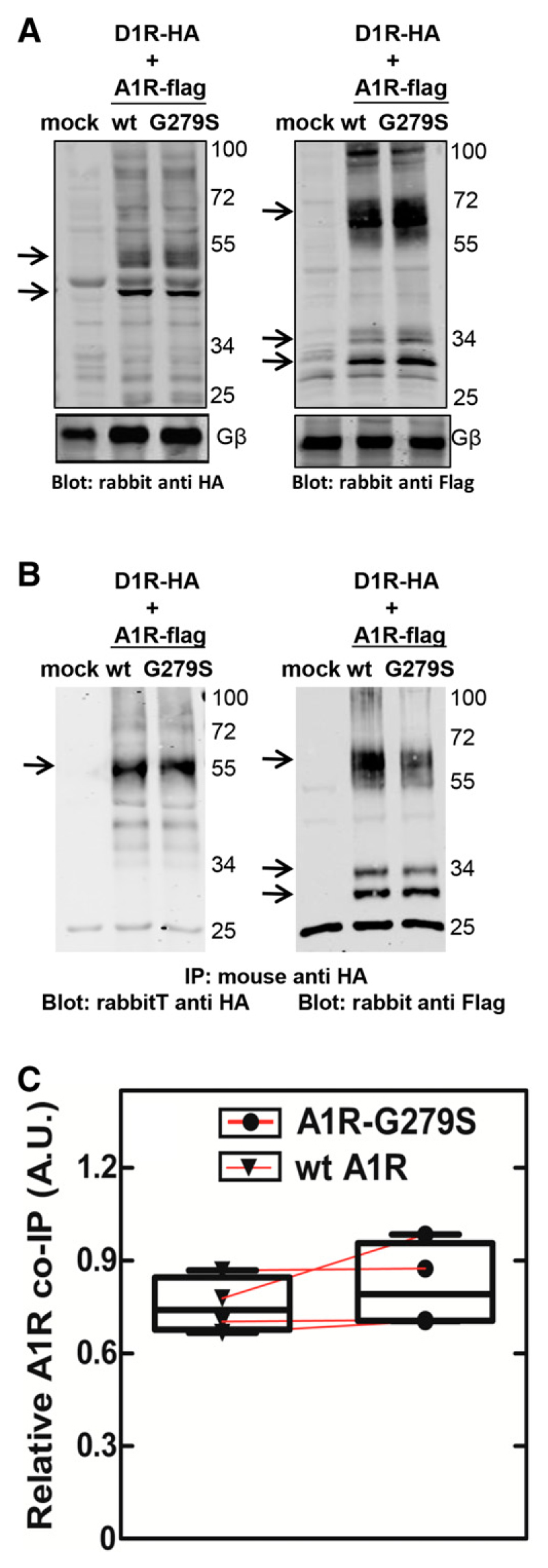
Adenosine A1- and dopamine D1-receptors form heteromeric complexes (Ginés et al., 2000; Rivera-Oliver et al., 2019). When transiently coexpressed with either A1R or A1R-G297S7.44, D1-receptors accumulated to comparable levels as assessed by binding of the antagonist radioligand [3H]7-chloro-3-methyl-1-phenyl-1,2,4,5-tetrahydro-3-benzazepin-8-ol (Bmax = 1.2 ± 0.2 and 1.4 ± 0.2 pmol/mg in the presence of wild-type A1R and A1R-G297S7.44, respectively). Similarly, equivalent amounts of receptors were detected by immunoblotting detergent lysates prepared from cotransfected cells (Fig. 5A). We first assessed complex formation by immunoprecipitating the D1-receptor via its N-terminal HA tag: in the immunoprecipitated, equivalent levels of wild-type A1R and A1R-G297S7.44 were visualized by immunoblotting for the N-terminal FLAG-epitope (Fig. 5B).
Fig. 5.
Coimmunoprecipitation of the wild-type adenosine A1-receptor or the mutant A1R-G279S7.44 with the dopamine D1-receptor. HEK293 cells (1.6 × 107/15-cm dish) were transiently cotransfected with plasmids encoding the HA-tagged D1-receptor (5.5 μg/15-cm dish) and FLAG-tagged wild-type (wt A1R) or mutant (A1R-G279S7.44) A1-receptors (5.5 μg/15-cm dish). After 24 hours, cells were detached and lysed as described in the Materials and Methods section. An aliquot of the lysate (20 μg/lane) was used to assess the expression of the receptors by immunoblotting with antibodies directed against the epitope tags (A). Lysates were also prepared from HEK293 cells subjected to transfection with empty plasmid (lanes labeled mock). The lysates (1 mg) were incubated with beaded agarose containing immobilized HA-antibody. An aliquot (10%) of the immunoprecipitate was resolved by denaturing electrophoresis and transferred to nitrocellulose membranes. The immunoreactive bands of the D1-receptor [left-hand blot in (B)] and of wild-type and mutant A1-receptors [right-hand blot in (B)] were visualized by blotting for the HA- and FLAG-epitope tags, respectively. Arrows point to the receptor-specific immunoreactivity; the lower bands correspond to the ER-resident core glycosylated forms of the D1-receptor and of the A1-receptor. We note that there are also receptor aggregates, in particular, of the A1-receptors (immunoreactive bands at about 70 kDa highlighted by an arrow). Data are from a representative experiment, which was replicated four times in independent, paired transfections. The D1- and A1-receptor immunoreactivity was quantified by densitometry, and the ratio seen in these four experiments is shown in (C). AUC, area under the curve; A.U., arbitrary unit; IP, immunoprecipitation.
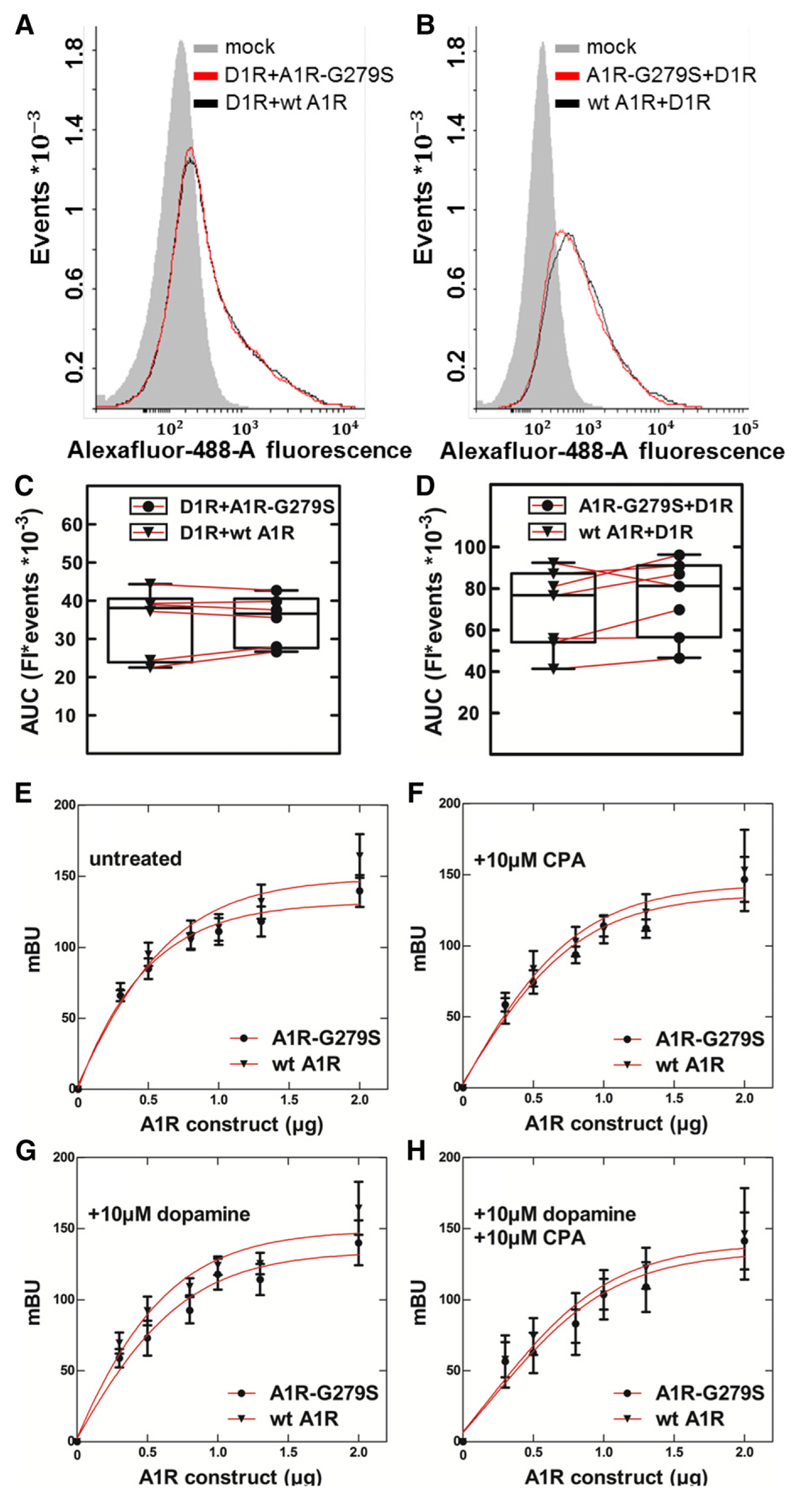
In addition, we examined complex formation in intact cells by bioluminescence resonance energy transfer between a fixed amount of D1-receptors, which were C-terminally tagged with a luciferase (NanoLuc), and increasing amounts of YFP-tagged A1-receptors. This approach allowed for monitoring complex formation in the absence of receptor activation (Fig. 6A) or after stimulation of the receptors’ activation by their cognate agonists, i.e., by the A1-selective agonist CPA (Fig. 6B), dopamine (Fig. 6C), or the combination thereof (Fig. 6D). It is evident that the curves are comparable, i.e., there was no any appreciable difference in the interaction of the dopamine receptor with wild-type or mutant A1-receptor regardless of whether receptors were activated or not (Table 1). This indicates that the receptor heteromers form in a constitutive manner, an interpretation that is also supported by the coimmunoprecipitation in the absence of receptor activation (Fig. 5B).
Fig. 6.
BRET between the luciferase-tagged dopamine D1-receptor and the wild-type adenosine A1-receptor or the mutant A1R-G279S7.44. HEK293 cells (1 × 106/well) were transiently cotransfected with a constant amount of plasmid encoding D1R [1 and 0.2 μg/well for (A–D) and (E–H), respectively], which was tagged either on its N terminus with an HA-epitope (A–D) or on its C terminus with a lucif-erase (NanoLuc) (E – H), and either 1 μg/well (A–D) or increasing amounts [0–1.8 μg/well; (E – H)] of plasmid coding for the wild-type (wt A1R) or the mutant A1-receptor (A1R-G279S7.44), which were tagged either on their N terminus with the FLAG-epitope (A–D) or on their C terminus with eYFP (E – H). The total amount of plasmid (2 μg/dish) was kept constant by adding the appropriate amount of empty plasmid. (A–D) Flow cytometry histograms and their quantification. After 24 hours, cells were detached and divided into two aliquots, which were incubated with murine M2 anti-FLAG antibody (1:2000) or murine anti-HA antibody (1:2000) and then with the secondary anti-mouse IgG antibody conjugated to AlexaFluor-488 (1:2000). The resulting receptor-associated immunofluorescence was quantified by flow cytometry as outlined under Materials and Methods. (E –H) BRET recordings in the absence and presence of agonists. Eight hours after transfection, cells were seeded into 96-well dishes (5 × 104/well) and allowed to adhere for 15 hours. After serum withdrawal for 1 hour, vehicle (control) or the indicated ligands (i.e., 10 μM CPA, 10 μM dopamine, or their combination) and lucif-erase substrate (furimazine, 1:200 dilution) were added, and bioluminescence was recorded for up to 20 minutes. Parallel incubations were done with cells solely expressing D1-receptor tagged with NanoLuc, and the luminescence recorded from these cells was subtracted. Data are means ± S.D. from three independent experiments done in parallel and carried out in duplicate. AUC, area under the curve; Fl, fluorescence; mBU, milli BRET unit.
Table 1.
Complex formation between dopamine D1-receptor and wild-type adenosine A1-receptor or the mutant A1R-G279S7.44 BRET50 refers to the amount of plasmid encoding wild-type or mutant adenosine A1-receptor giving half-maximum bioluminescence resonance energy transfer; maximum BRET is the response estimated at saturation. The values (means ± S.D.) were calculated by fitting the data from three independent experiments (summarized in Fig. 4) to the equation of a rectangular hyperbola.
| Incubation | A1-receptor variant | BRET50 | Maximum BRET signal |
|---|---|---|---|
| μg A1R plasmid | milli BRET | ||
| Control | Wild-type A1R | 0.6 ± 0.1 | 177 ± 9 |
| A1R-G279S7.44 | 0.5 ± 0.1 | 160 ± 30 | |
| CPA (10 μM) | Wild-type A1R | 0.9 ± 0.2 | 218 ± 64 |
| A1R-G279S7.44 | 0.9 ± 0.2 | 202 ± 37 | |
| Dopamine (10 μM) | Wild-type A1R | 0.7 ± 0.1 | 207 ± 27 |
| A1R-G279S7.44 | 0.8 ± 0.1 | 190 ± 28 | |
| CPA and dopamine (10 μM each) | Wild-type A1R | 1.1 ± 0.3 | 226 ± 82 |
| A1R-G279S7.44 | 1.4 ± 0.6 | 229 ± 23 |
Comparison of Gi Activation by Wild-Type and Mutant A1-Receptor
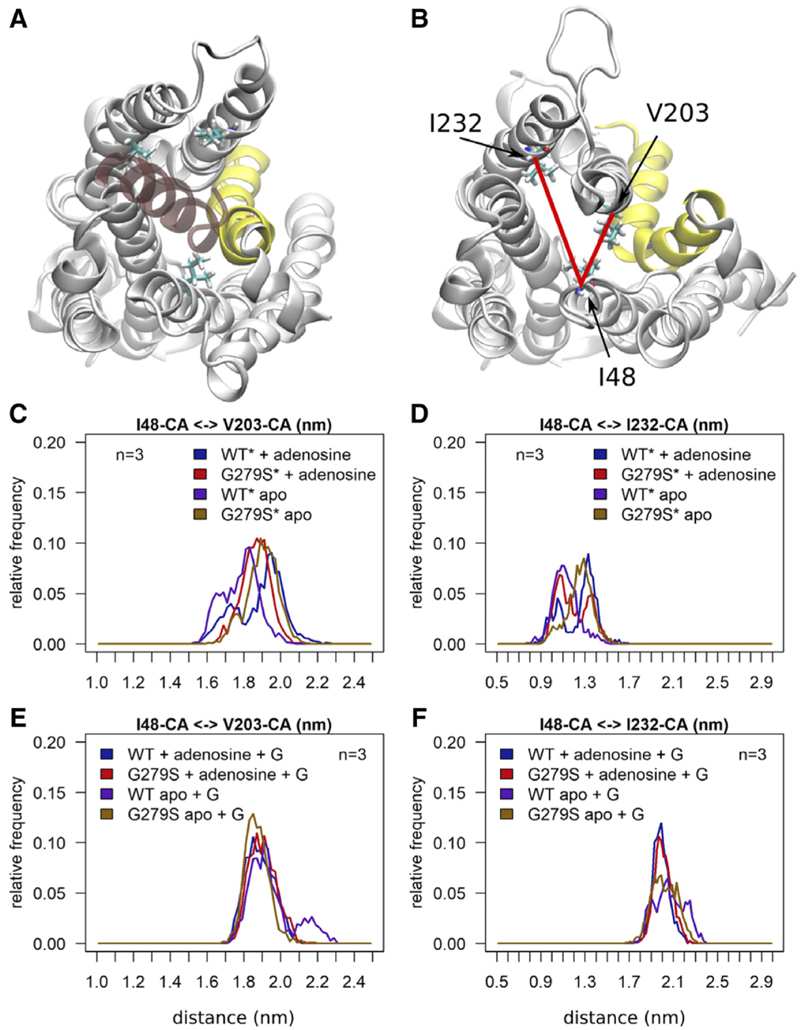
The analysis of the thermostability suggested that the G279S mutation lowered the energy barrier for conformational changes because its kinetic stability was lower than that of the wild-type receptor (cf. Fig. 4A). The RMSF plots summarized in Figure 2 showed a higher mobility of TM7 in A1R-G279S7.44. We interrogated the molecular dynamics simulations to search for changes in the energy landscape associated with movements of TM7: we quantified the increased mobility of TM7 by measuring the distances between TM3 and TM7 at the extracellular face of the receptor, i.e., the distance between L2697.34 and A843.29, and at the site of the mutation site, i.e., the distance between T913.36 and H2787.43 (Fig. 7A). These measurements captured the changes in stability, dynamics, and conformations induced by the G279S7.44 mutation. The two-dimensional histogram (Fig. 7B) and the associated free-energy map (Fig. 7C) visualized the movements of TM7. It is evident that in the apo (i.e., ligand-free) state of the receptor, TM7 visits many more positions distant from TM3 than in the adenosine-bound state: this is true for both the wild-type receptor in the presence (cf. first and third two-dimensional histogram in the top row of Fig. 7B) and absence of Gi2 (cf. first and third two-dimensional histogram in the bottom row of Fig. 7B) and for the mutant receptor (cf. corresponding second and fourth two-dimensional histograms in Fig. 7B). This observation shows that binding of adenosine restrains the movement of TM7. In fact, the distance between TM3 and TM7 becomes shorter at the bottom of the ligand-binding site as TM7 closes in onto the agonist adenosine. The structural change is visible as a shift in the T913.36–H2787.43 distance (y-axis in Fig. 7, B and C): in the energy basin, the minimum (indicated by an “x” in Fig. 7C) is located at, 0.9 nm in the presence of adenosine (two-dimensional histograms in the first and second column of Fig. 7C). In contrast, in the absence of adenosine, the most stable distance is larger than <0.9 nm (two-dimensional histograms in the first and second column of Fig. 7C). The most conspicuous difference between the mutant and the wild-type receptor can be appreciated by comparing the energy minima of the apo state in the presence of Gi2: in the A1R-G279S7.44, the basin of low-energy states covers a substantially larger area than in the wild-type receptor (cf. fourth and third two-dimensional histogram in the top row of Fig. 7C). This observation is consistent with a low-energy barrier imparted by the mutation, which allows TM7 and thus the mutant receptor to sample many more conformational states than the wild-type receptor.
In the active state, the receptor has to accommodate the C terminus of its cognate G protein α-subunit in a cavity, which forms on the intracellular side within the transmembrane bundle. A comparison between the active Gα protein–bound conformation and the receptor without the G protein shows large movements of TM5 to create the space that allows for Gα protein binding (cf. Fig. 8, A and B). We also examined the effect of G279S7.44 mutation on the intracellular face of the receptor by measuring distances between I482.43 and V2035.61 (TM2–TM5) and between I482.43 and I2326.33 (TM2 – TM6) (highlighted as red lines in Fig. 8B). Simulations starting from the inactive conformation reveal that the size of the cavity is sensitive to the mutation: in the apo state, the G279S7.44 mutation (brown trace in Fig. 8, C and D) leads to an opening of the binding site for the Gα protein. In contrast, the wild-type A1-receptor remains close to its starting structure in the absence of adenosine (red trace in Fig. 8, C and D). Figure 8, E and F, shows that the bound Gα protein restricts receptor movements in both the wild-type and the mutant receptor. In contrast, the inactive conformation of the A1-receptor is sensitive to the mutation and the presence of the adenosine ligand. Figure 8, C and D, shows that the mutation leads to an opening of the Gα protein binding site as compared with the wild-type A1-receptor, which remains close to its starting structure in the absence of the adenosine ligand. Addition of the adenosine ligand to the wild-type receptor induces a similar conformational change. A comparison between panels D and F of Figure 8 indicates that the time window of the molecular dynamics simulation of 0.5 microsecond is not long enough to observe a complete conversion from the inactive to the active conformation.
Fig. 8.
Geometry of the Gα protein binding cavity of the wild-type adenosine A1-receptor and of the mutant A1-R-G279S7.44. Snapshots taken at the end of the simulations shows the wild-type A1-receptor from the cytoplasmic side with (A) and without (B) bound Gα. The A1-receptor is represented as white cartoon and TM7 is highlighted in yellow, the bound C-terminal helix of the Gαi2 protein is shown in transparent red color. Residues I482.43, I2326.33 and V2035.61 are shown as sticks. Red lines indicate the distances measured in (C–F). The inactive receptor is indicated by an asterisk (*). Histograms summarize the frequency at which the indicated distances between the Cα atoms (CA) of I482.43 (TM2) and V2035.61 (TM5) (C and E) and of I482.43 (TM2) and I2326.33 (TM6) (D and F) were observed. Each histogram includes three independent simulations of 0.5 microsecond each. Data points were sampled at 1-nanosecond intervals. The A1-receptor was simulated using the active form based of the agonist-liganded, G protein–bound structure (+G; PDB ID: 6D9H) and starting from the inactive conformation based on the antagonist-bound structure (PDB ID: 5N2S). For either conformation, wild-type (WT) and mutant receptor A1-R-G279S7.44 (G279S) were simulated in the absence (apo) and presence of adenosine.
In most, if not all, GPCRs TM7 is bent; this is also true for the A1-receptor. This kink is energetically not optimal and used by the receptor to sense agonist binding. The G279S7.44 mutation introduces an additional hydrogen bond donor. This ought to stabilize the protein provided that it optimally fits into the structure. However, the traces in Figure 8D show that, in the absence of the G protein, the additional hydrogen bond prefers or needs a different conformation to fulfill its bonding interactions. As a consequence, in the presence of adenosine, helix TM7 of A1R-G279S7.44 oscillates between two conformations (red trace in Fig. 8D).
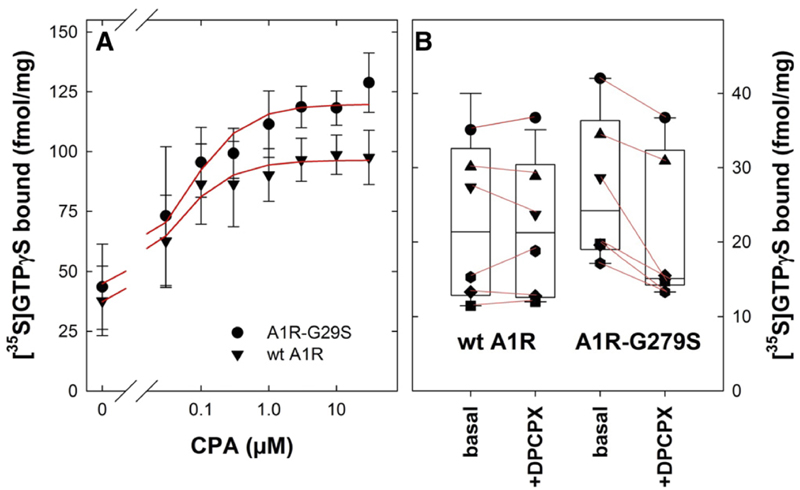
We surmised that the increased conformational flexibility (Fig. 7C) and the partial opening of the G protein binding site (Fig. 8, C and D) ought to translate into more effective agonist-induced G protein activation and/or higher basal— i.e., agonist-independent—activity. This prediction was verified by 1) measuring agonist-induced binding of [35S]GTPγS (Fig. 9A) and 2) the effect of an inverse agonist on the basal binding of [35S]GTPγS (Fig. 9B) under initial rate conditions (i.e., after 1 minute): when stimulated with the agonist CPA at saturating concentrations, the mutant A1R-G279S7.44 caused a larger increase in [35S]GTPγS binding than the wild-type A1-receptor (Fig. 9A). In contrast, the concentration required for half-maximum stimulation did not differ (EC50 = 38 ± 9 and 58 ± 19 nM for wild-type A1R and A1R-G279S7.44, respectively). On average, the basal rate of [35S]GTPγS binding was slightly higher in membranes prepared from HEK293 cells transiently expressing A1R-G279S7.44 than those transiently expressing wild-type A1R. Most G protein–coupled receptors have some basal (i.e., constitutive, agonist-independent) activity, which can be blocked by antagonists, which are in most instances inverse agonists (Schütz and Freissmuth, 1992; Leff, 1995). This is also true for the A1-receptor (Freissmuth et al., 1991a). Accordingly, we examined the extent to which a saturating concentration of the antagonist/inverse agonist DPCPX reduced basal [35S]GTPγS binding in membranes from HEK293 cells transiently expressing wild-type A1R and A1R-G279S7.44.It is evident from Figure 8B that DPCPX caused a statistically significant inhibition of basal [35S]GTPγS binding to membranes harboring A1R-G279S7.44 by on average 23.4% (95% confidence interval 12.4%–34.5%). In contrast, there was no appreciable effect of DPCPX on basal [35S]GTPγS binding to membranes harboring wild-type A1R (on average 1.1% lower than in the absence of DPCPX; 95% confidence interval from 11.8% lower to 9.7% higher). These observations indicate that the constitutive activity of A1R-G279S7.44 is more pronounced than that of the wild-type receptor.
Fig. 9.
[35S]GTPγS binding to membranes prepared from HEK293 cells transiently expressing wild-type or mutant adenosine A1-receptors. HEK293 cells were transfected with plasmids driving the expression of the wild-type (wt A1R, triangle down, 5.5 μg/15-cm dish) or the mutant (A1R-G279S7.44, full circles, 5.5 mg/15-cm dish) adenosine A1-receptor and membranes were prepared as outlined in the legend to Figure 1A. (A) Membranes (10 μg) were preincubated at 25°C in the absence and presence of the indicated concentrations of the agonist CPA for 30 minutes; the reaction started by adding [35S] GTPγS to a final concentration of 1 nM and stopped after 1 minute by rapid filtration as outlined under Materials and Methods. Data are means ± S.D. from three independent experiments (with different membranes from paired transfections) carried out in duplicate. The curves were drawn by fitting the data to the equation describing the hyperbolic concentration-dependent stimulation of a basal activity. The two curves are better described by separate fits rather than by a fit with shared parameters (F-test based on the extra sum of squares-principle; F = 16.03, P = 0.0004) because of the difference in the maximal [35S]GTPγS binding (95% confidence interval 91.5–101.3 and 111.5–128.1 fmol/mg for wt A1R and A1R-G279S7.44, respectively). (B) The assay was done as described for (A) in the absence (basal) and presence of the antagonist/inverse agonist DPCPX (10 μM). Shown are the results from six independent paired transient transfections; each individual experiment is represented by the same symbol. The lines connect basal binding to the corresponding binding in the presence DPCPX to illustrate the consistent inhibition by DPCPX in membranes harboring the mutant receptor A1R-G279S7.44 and the absence thereof in membranes harboring the wild-type A1-receptor (wt A1R). The box plot shows the median and interquartile range; the whiskers correspond to the 95% confidence interval. In membranes carrying A1R-G279S7.44, the difference between basal [35S]GTPγS binding and binding in the presence of DPCPX was statistically significant (P < 0.02, Friedman test followed by Holm-Sidak post hoc testing).
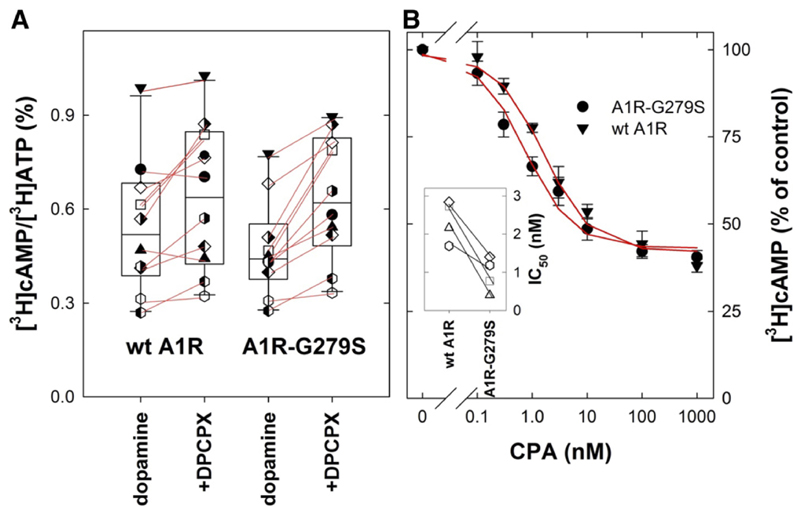
Taken together the data in summarized in Figure 9 suggested that A1R-G279S7.44 was more effective in promoting nucleotide exchange on cognate G proteins than the wild-type receptor. The A1-receptor is a prototypical Gi/Go-coupled receptor, which engages all isoforms of Gai and Gao (Freissmuth et al., 1991b; Jockers et al., 1994). The bidirectional regulation of cAMP formation is the major effector pathway, which is regulated in a mutually antagonistic manner by D1- and A1-receptors (Ferré et al., 1998). We therefore explored if wildtype A1R and A1R-G279S7.44 differed in their ability to inhibit cAMP accumulation induced by the D1-receptor in transiently cotransfected cells. We first measured dopamine-induced cAMP accumulation in the absence and presence of DPCPX to address the question of whether the different level of constitutive nucleotide exchange activity detected by [35S] GTPγS binding (Fig. 8B) translated into modulation of cAMP production. This was the case: in HEK293 cells coexpressing the D1-receptor and the mutant A1R-G279S7.44, DPCPX produced a consistent and statistically significantly increased cAMP response to dopamine (by 1.4 ± 0.2-fold; Fig. 10A). In contrast, in HEK293 cells coexpressing the D1-receptor and the wild-type A1-receptor, the effect of DPCPX was less pronounced and did not reach statistical significance in spite of the large number of paired observations (increase by 1.2 ± 0.2-fold; Fig. 10A). Similarly, in cells expressing A1R-G279S7.44, the concentration-response curve for CPA was shifted to the left of that seen in cells expressing wild-type A1R (Fig. 10B; IC50 = 2.4 ± 0.5 and 0.9 ± 0.4 nM for wild-type A1-receptor and the mutant A1R-G279S7.44, respectively). Although the shift was modest, the difference was consistently observed in paired experiments, where cells were subjected to transient cotransfection with plasmids encoding the D1-receptor and the wildtype or mutant A1-receptor (cf. inset in Fig. 10B). This difference between wild-type and mutant A1-receptor is consistent with the higher efficacy of the agonist-stimulated A1R-G279S7.44 in promoting guanine nucleotide exchange (Fig. 9A).
Fig. 10.
Effect of the A1-antagonis t/inverse agonist DPCPX (A) and of the A1-agonist CPA (B) on dopamine-induced cAMP accumulation in HEK293 cells co-expressing dopamine D1 and wild-type or mutant adenosine A1-receptors. HEK293 cells were transiently co-transfected with plasmids encoding D1R (5.5 μg/15-cm dish), and wild-type A1-receptor (wt A1R; 5.5 μg/15-cm dish, triangle down) or the mutant A1R-G279S7.44 (5.5 μg/15-cm dish, full circle). After 8 hours, cells were replated into 6-well dishes (0.5 × 106/well) in DMEM medium containing 1 μCi/ml [3H]adenine and incubated for 16 hours and subsequently stimulated in medium containing 1 μM dopamine alone or combination of 1 μM dopamine and 10 μM DPCPX (A) or increasing concentration of CPA (B) for 20 minutes as outlined under Materials and Methods. (A) Shown are the results from 11 independent paired transient transfections; each individual experiment is represented by the same symbol. The lines connect basal cAMP levels to the corresponding level in the presence DPCPX: addition of DPCPX caused a statistically significant increase in basal cAMP levels in cells expressing A1R-G279S7.44 but not in cells expressing wt A1R (P = 0.001 and 0.059, respectively, Friedmann test followed by Holm-Sidak post hoc testing for multiple comparisons). The box plot shows the median and interquartile range; the whiskers correspond to the 95% confidence interval. (B) Data represent means ± S.D. from four independent experiments and the spaghetti plot in the inset shows the IC50 values for wt A1R and A1R-G279S7.44 (paired experiments are indicated by the same symbols). IC50-values differ in a statistically significant manner (P = 0.011, t test for paired data). The curves were drawn by fitting the data to a monophasic inhibition curve. The two curves are better described by separate fits rather than by a fit to a common curve with shared parameters (F-test based on the extra sum of squares-principle; F = 13.57, P = 0.0001).
Discussion
GPCRs represent the largest family of mammalian proteins. Hence their genes collectively occupy a large fraction of the protein-coding genome. A survey that covered 107 GPCRs targeted by approved drugs documented that, on average, an apparently healthy individual harbors 68 nonsynonymous coding variations (missense variations) in about one third of these receptors (Hauser et al., 2018). In a data base search (www.ensembl.org), we identified 134 missense variations (relative to the reference genome GRCH38.p13) at 96 positions in the coding sequence of the A1-receptor. Fourteen of these variations (at 12 positions) were found in the 2504 apparently healthy individuals covered by the 1000 Genomes Project (Auton et al., 2015). However, the only source describing the G279S7.44 mutation in the human A1-receptor is the report by Jaberi et al. (2016), which linked the mutated receptor to early-onset Parkinson’s disease. Our experiments were designed to explore the impact of the mutation on the activity of the receptor. Based on our findings, we conclude that the variant A1R-G279S7.44 has an enhanced conformational flexibility, which translates into a higher basal (i.e., agonist-independent, constitutive) activity and an enhanced agonist-induced response. This conclusion is based on three independent lines of evidence. 1) The kinetic stability of A1R-G279S7.44 was about 50% lower than that of the wild-type receptor. Thus the mutation lowered the energy barrier for conformational transitions, and this finding was recapitulated in molecular dynamics simulations. 2) When we probed the receptor in the presence and absence of an antagonist/inverse agonist to define the constitutive activity of the receptor, we consistently observed a larger effect of the antagonist/inverse agonist for A1R-G279S7.44 than for the wild-type receptor, regardless of whether guanine nucleotide exchange or by cAMP accumulation was assessed. 3) The agonist-liganded A1R-G279S7.44 was more efficacious than the wild-type receptor in catalyzing guanine nucleotide exchange. Accordingly, in cells expressing A1R-G279S7.44, the agonist concentration-response curve for lowering cAMP levels was shifted to the left of that seen in cells expressing the wild-type receptor.
Residues in TM7 contribute to the orthosteric binding site of the A1-receptor; in fact, T270 is the amino acid critical for binding of ligands, which discriminate between the A1- and the A2A-receptor (Cheng et al., 2017; Glukhova et al., 2017). G279 is about 2.5 helical turns distal to T270. Our experiments rule out that the G279S mutation has indirect effects on the geometry of the ligand-binding cavity: both the A1-selective antagonist/inverse agonist DPCPX and the agonist CPA bound with similar affinity to the wild-type A1-receptor and to A1R-G279S7.44. Similarly, G279S7.44 is about 2.5 helical turns from Y2887.53, which is critical for folding of the A1-receptor in the endoplasmic reticulum: during the conformational search, TM7 and the C terminus must be correctly positioned to allow for emergence of the native conformation (Pankevych et al., 2003; Málaga-Diéguez et al., 2010). The G279S7.44 mutation does not interfere with the folding trajectory of the A1-receptor. This conclusion is based on our observations that equivalent levels of wild-type and mutant receptors were found on the cell surface. The A1-receptor can form homodimers (Gracia et al., 2013) and heteromers, in particular with the D1-receptor (Ginès et al., 2000; Rivera-Oliver et al., 2019), which allows for their reciprocal, mutually antagonistic modulation (Ferré et al., 1998). The interface in the A1-/D1-receptor heteromer is not known, but our observations indicate that the G279S7.44 mutation does not affect this interface: wild-type and mutant A1-receptors did not differ in their ability to form complexes with the D1-receptor, regardless of whether the interaction was assessed by coimmunoprecipitation or monitored by BRET.
We used thermal denaturation to probe the conformational flexibility of mutant and wild-type A1-receptors. Heating resulted in irreversible loss of binding. Under our experimental conditions, it was not possible to capture the initial reversible unfolding. Hence, a Lumry-Eyring model is applicable, which in its simplest version posits a two-step process N ⇆ U → D, where N, U, and D are the native, reversibly unfolded, and irreversibly denatured states, respectively (Lumry and Eyring, 1954). Because the reversibly unfolded state is inaccessible, it is not possible to extract the energy change DG (or DH) associated with initial unfolding/refolding, i.e., the N ⇆ U transition. However, the rates of denaturation do shed light on the underlying processes. The rapidly denaturing component reflects a fraction of the A1-receptor, which visits conformational states that are separated by a low-energy barrier from the unfolded state. This fraction rather than the rate of denaturation increased with temperature. Hence, the resulting Arrhenius plots were flat. We posit that this rapid rate reflects the thermodynamic stability of the protein. Substitution of G279 by serine introduces an additional hydrogen bond donor into TM7. The most likely acceptor is the backbone carbonyl of F2757.40 (Cheng et al., 2017; Glukhova et al., 2017): this conjecture was substantiated by the molecular dynamics simulations. When compared with the wild-type A1-receptor, the thermodynamic stability of the mutant A1R-G279S7.44 was enhanced: this increase can be accounted for by the extra hydrogen bond donated by the serine residue, which stabilizes TM7. The importance of TM7 for the stability of the receptor is highlighted by the reduced thermodynamic stability of the mutant A1R-Y288A7.53. In contrast, the second component of thermal denaturation proceeded with a slow rate, which was accelerated with increasing temperature, resulting in steep Arrhenius plots. This second component reflects the kinetic stability of the receptor. The relationship between thermodynamic and kinetic stability is complex: it may range from a perfect correlation to total independence (Sanchez-Ruiz, 2010). Our observations show that, in the A1-receptor, thermodynamic stability and kinetic stability are not correlated. This is exemplified by both A1R-G279S7.44 and A1R-Y288A7.53: the energy barrier, which separated the wild-type A1-receptor in its ground state from the denatured state(s), was larger than that of A1R-G279S7.44 and smaller than that of A1R-Y288A7.53. We note that the kinetic barrier is substantially smaller in the A1-receptor (135 kJ/mol) than in rhodopsin (670 kJ/mol; Hubbard, 1958; Corley et al., 2011). This difference is not surprising. Rhodopsin supports vision in dim light and thus requires a high thermal barrier to spontaneous conformational transitions (Guo et al., 2014). However, the kinetic barrier for thermal denaturation of the A1-receptor is larger than that required for productive ternary complex formation (Waldhoer et al., 1999). In the active, G protein–bound state of the A1-receptor, the ligand-binding site collapses on the agonist (Draper-Joyce et al., 2018). Our molecular dynamics simulations show that this is due to the movement of several helices, including TM7, which is facilitated by substituting serine for G2797.44. The G279S7.44-induced increased flexibility also allows for rationalizing the reduced kinetic stability, the increase in constitutive activity, and the enhanced agonist-induced response because they can all be linked to lower energy barriers between conformational states.
In Parkinson’s disease, the G279S7.44 variant of the A1-receptor is a rare mutation because it does not occur in the large set of whole exome sequencing data of the International Parkinson’s Disease Genomics Consortium (Blauwendraat et al., 2017). The interpretation is also confounded by the fact that the affected individuals also harbor a mutation (C52Y) on both alleles of the gene encoding peptidyl-t-RNA hydrolase domain containing-1 (PTRHD1) (Elahi, 2018). PTRHD1 is a protein of unknown function, which lacks its eponymous activity: although it binds peptidyl-t-RNA, it does not hydrolyze it (Burks et al., 2016). The role of PTRHD1 in autosomal recessive Parkinson’s disease is supported by two additional reports: an adjacent mutation (H53Y) was found in Iranian patients (Khodadadi et al., 2017), and mutations in PTRHD1, which result in truncation of the protein, were identified in African patients (Kuipers et al., 2018). There are two arguments that support a pathogenic role of A1R-G279S7.44. First, in the striatum there is mutual antagonism between signaling pathways controlled by dopamine and adenosine; in the direct and indirect pathways, A1- and A2A-receptors counteract the actions of D1- and D2-receptors, respectively (Ferré et al., 1994, 1997; Yabuuchi et al., 2006). Second, although adenosine-induced stimulation of A1-receptors has been posited to be a priori neuroprotective, this may not be the case upon prolonged stimulation (Cunha, 2016; Stockwell et al., 2017). In fact, prolonged stimulation of A1-receptors promotes the accumulation of α-synuclein in dopaminergic neurons of the substantia nigra and impairs motor control of the animals (Lv et al., 2020). Duplication (Chartier-Harli et al., 2004) and triplication of the α-synuclein gene (Singleton et al., 2003) results in Parkinson’s disease, suggesting that increased expression of the protein per se suffices to trigger its fibrillation and Lewy body formation. In the brain, including the striatum, A1-receptors are expressed to high levels; nevertheless, they do not have an appreciable constitutive activity (Savinainen et al., 2003). We observed that by comparison with the wild-type A1-receptor, the mutated variant A1R-G279S7.44 had a measurable constitutive activity. This may translate to a tonic long-term activation of signaling pathways favoring neurodegeneration. Thus, at the very least, the A1R-G279S7.44 may represent a disease-modifying gene, which renders individuals more susceptible to insults that impair the activity of the nigrostriatal dopaminergic neurons.
Significance Statement.
Parkinson’s disease is caused by a loss of dopaminergic input from the substantia nigra to the caudate nucleus and the putamen. Activation of the adenosine A1-receptor antagonizes responses elicited by dopamine D1-receptor. We show that this activity is more pronounced in a mutant version of the A1-receptor (A1R-G279S7.44), which was identified in individuals suffering from early-onset Parkinson’s disease.
Acknowledgements
We thank Petra Schaffer and Luka Schmölz for their participation in experiments, which assessed the heat stability of the A1-receptor and guanine nucleotide exchange, respectively.
This work is part of a dissertation submitted in partial fulfillment of the requirements of the Ph.D. degree.
This work was supported by the doctoral program Cell Communication in Health and Disease funded the Austrian Science Fund/FWF [Grant W1205] and by the Medical University of Vienna.
Abbreviations
- A1R
adenosine A1-receptor
- BRET
bioluminescence resonance energy transfer
- BSA
bovine serum albumin
- CPA
N6-cyclopentyladenosine
- D1R
D1-receptor
- DMEM
Dulbecco’s modified Eagle’s medium
- DPCPX
8-cyclopentyl-1,3-dipropylxanthine
- eYFP
enhanced yellow fluorescent protein
- GPCR
G protein–coupled receptor
- PDB ID
Protein Data Bank identifier
- PEI
polyethylenimine
- PMSF
phenylmethylsulfonyl fluoride
- PTRHD1
peptidyl-t-RNA hydrolase domain containing-1
- RMSF
root mean square fluctuation
- TM
transmembrane helix
- XAC
xanthine amine congener
- YFP
yellow fluorescent protein
Footnotes
The authors declare that they have no conflicts of interest with the contents of this article.
Authorship Contributions
Participated in research design: Nasrollahi-Shirazi, Stockner, Nanoff, Freissmuth.
Conducted experiments: Nasrollahi-Shirazi, Szöllösi, Yang.
Contributed new reagents or analytic tools: Muratspahic, El-Kasaby, Sucic.
Performed data analysis: Nasrollahi-Shirazi, Szöllösi, Stockner, Nanoff, Freissmuth.
Wrote or contributed to the writing of the manuscript: Nasrollahi-Shirazi, Szöllösi, Freissmuth.
References
- Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindahl E. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 2015;1-2:19–25. [Google Scholar]
- Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL, McCarthy S, McVean GA, Abecasis GR, 1000 Genomes Project Consortium A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauwendraat C, Nalls MA, Federoff M, Pletnikova O, Ding J, Letson C, Geiger JT, Gibbs JR, Hernandez DG, Troncoso JC, et al. International Parkinson’s Disease Genomics Consortium ADORA1 mutations are not a common cause of Parkinson’s disease and dementia with Lewy bodies. Mov Disord. 2017;32:298–299. doi: 10.1002/mds.26886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borycz J, Pereira MF, Melani A, Rodrigues RJ, Köfalvi A, Panlilio L, Pedata F, Goldberg SR, Cunha RA, Ferré S. Differential glutamate-dependent and glutamate-independent adenosine A1 receptor-mediated modulation of dopamine release in different striatal compartments. J Neurochem. 2007;101:355–363. doi: 10.1111/j.1471-4159.2006.04386.x. [DOI] [PubMed] [Google Scholar]
- Bowie JU. Understanding membrane protein structure by design. Nat Struct Biol. 2000;7:91–94. doi: 10.1038/72454. [DOI] [PubMed] [Google Scholar]
- Bowie JU. Membrane protein folding: how important are hydrogen bonds? Curr Opin Struct Biol. 2011;21:42–49. doi: 10.1016/j.sbi.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burks GL, McFeeters H, McFeeters RL. Expression, purification, and buffer solubility optimization of the putative human peptidyl-tRNA hydrolase PTRHD1. Protein Expr Purif. 2016;126:49–54. doi: 10.1016/j.pep.2016.05.011. [DOI] [PubMed] [Google Scholar]
- Burré J, Sharma M, Südhof TC. Cell biology and pathophysiology of α-synuclein. Cold Spring Harb Perspect Med. 2018;8:a024091. doi: 10.1101/cshperspect.a024091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussi G, Donadio D, Parrinello M. Canonical sampling through velocity rescaling. J Chem Phys. 2007;126:14101. doi: 10.1063/1.2408420. [DOI] [PubMed] [Google Scholar]
- Chang D, Nalls MA, Hallgrímsdóttir IB, Hunkapiller J, van der Brug M, Cai F, Kerchner GA, Ayalon G, Bingol B, Sheng M, et al. International Parkinson’s Disease Genomics Consortium 23andMe Research Team A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci. Nat Genet. 2017;49:1511–1516. doi: 10.1038/ng.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, et al. α-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- Cheng RKY, Segala E, Robertson N, Deflorian F, Doré AS, Errey JC, Fiez-Vandal C, Marshall FH, Cooke RM. Structures of human A1 and A2A adenosine receptors with xanthines reveal determinants of selectivity. Structure. 2017;25:1275–1285.e4. doi: 10.1016/j.str.2017.06.012. [DOI] [PubMed] [Google Scholar]
- Corley SC, Sprangers P, Albert AD. The bilayer enhances rhodopsin kinetic stability in bovine rod outer segment disk membranes. Biophys J. 2011;100:2946–2954. doi: 10.1016/j.bpj.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa J, Lunet N, Santos C, Santos J, Vaz-Carneiro A. Caffeine exposure and the risk of Parkinson’s disease: a systematic review and meta-analysis of observational studies. J Alzheimers Dis. 2010;20(Suppl 1):S221–S238. doi: 10.3233/JAD-2010-091525. [DOI] [PubMed] [Google Scholar]
- Cunha RA. How does adenosine control neuronal dysfunction and neuro-degeneration? J Neurochem. 2016;139:1019–1055. doi: 10.1111/jnc.13724. [DOI] [PubMed] [Google Scholar]
- Darden T, York D, Pedersen L. Particle mesh Ewald: an N×log(N) method for Ewald sums in large systems. J Chem Phys. 1993;98:10089–10092. [Google Scholar]
- Davidson WS, Jonas A, Clayton DF, George JM. Stabilization of α-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem. 1998;273:9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- de Jong DH, Singh G, Bennett WF, Arnarez C, Wassenaar TA, Schäfer LV, Periole X, Tieleman DP, Marrink SJ. Improved parameters for the Martini coarsegrained protein force field. J Chem Theory Comput. 2013;9:687–697. doi: 10.1021/ct300646g. [DOI] [PubMed] [Google Scholar]
- Draper-Joyce CJ, Khoshouei M, Thal DM, Liang YL, Nguyen ATN, Furness SGB, Venugopal H, Baltos JA, Plitzko JM, Danev R, et al. Structure of the adenosine-bound human adenosine A 1 receptor-G i complex. Nature. 2018;558:559–563. doi: 10.1038/s41586-018-0236-6. [DOI] [PubMed] [Google Scholar]
- Ehringer H, Hornykiewicz O. Distribution of noradrenaline and dopamine (3-hydroxytyramine) in the human brain and their behavior in diseases of the extrapyramidal system. Klin Wochenschr. 1960;38:1236–1239. doi: 10.1007/BF01485901. [DOI] [PubMed] [Google Scholar]
- Elahi E. PTRHD1 and possibly ADORA1 mutations contribute to Parkinsonism with intellectual disability. Mov Disord. 2018;33:174. doi: 10.1002/mds.27126. [DOI] [PubMed] [Google Scholar]
- Fastbom J, Pazos A, Probst A, Palacios JM. Adenosine A1 receptors in the human brain: a quantitative autoradiographic study. Neuroscience. 1987;22:827–839. doi: 10.1016/0306-4522(87)92962-9. [DOI] [PubMed] [Google Scholar]
- Ferré S, Fredholm BB, Morelli M, Popoli P, Fuxe K. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 1997;20:482–487. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- Ferré S, Popoli P, Giménez-Llort L, Finnman U-B, Martínez E, Scotti de Carolis A, Fuxe K. Postsynaptic antagonistic interaction between adenosine A1 and dopamine D1 receptors. Neuroreport. 1994;6:73–76. doi: 10.1097/00001756-199412300-00020. [DOI] [PubMed] [Google Scholar]
- Ferré S, Torvinen M, Antoniou K, Irenius E, Civelli O, Arenas E, Fredholm BB, Fuxe K. Adenosine A1 receptor-mediated modulation of dopamine D1 receptors in stably cotransfected fibroblast cells. J Biol Chem. 1998;273:4718–4724. doi: 10.1074/jbc.273.8.4718. [DOI] [PubMed] [Google Scholar]
- Freissmuth M, Schütz W, Linder ME. Interactions of the bovine brain A1-adenosine receptor with recombinant G protein alpha-subunits. Selectivity for rGi alpha-3. J Biol Chem. 1991b;266:17778–17783. [PubMed] [Google Scholar]
- Freissmuth M, Selzer E, Schütz W. Interactions of purified bovine brain A1-adenosine receptors with G-proteins. Reciprocal modulation of agonist and antagonist binding. Biochem J. 1991a;275:651–656. doi: 10.1042/bj2750651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giasson BI, Uryu K, Trojanowski JQ, Lee VM. Mutant and wild type human α-synucleins assemble into elongated filaments with distinct morphologies in vitro. J Biol Chem. 1999;274:7619–7622. doi: 10.1074/jbc.274.12.7619. [DOI] [PubMed] [Google Scholar]
- Ginés S, Hillion J, Torvinen M, Le Crom S, Casadó V, Canela EI, Rondin S, Lew JY, Watson S, Zoli M, et al. Dopamine D1 and adenosine A1 receptors form functionally interacting heteromeric complexes. Proc Natl Acad Sci USA. 2000;97:8606–8611. doi: 10.1073/pnas.150241097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glukhova A, Thal DM, Nguyen AT, Vecchio EA, Jörg M, Scammells PJ, May LT, Sexton PM, Christopoulos A. Structure of the adenosine A1 receptor reveals the basis for subtype selectivity. Cell. 2017;168:867–877.e13. doi: 10.1016/j.cell.2017.01.042. [DOI] [PubMed] [Google Scholar]
- Gowers Linke RJM, Barnoud J, Reddy TJE, Melo MN, Seyler SL, Domanski J, Dotson DL, Buchoux S, IMKenney, Beckstein O. MDAnalysis: a Python package for the rapid analysis of molecular dynamics simulations. Proceedings of the 15th Python in Science Conference; Austin, TX. 2019. pp. 98–105. [Google Scholar]
- Gracia E, Moreno E, Cortés A, Lluís C, Mallol J, McCormick PJ, Canela EI, Casadó V. Homodimerization of adenosine A1 receptors in brain cortex explains the biphasic effects of caffeine. Neuropharmacology. 2013;71:56–69. doi: 10.1016/j.neuropharm.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Guo Y, Sekharan S, Liu J, Batista VS, Tully JC, Yan EC. Unusual kinetics of thermal decay of dim-light photoreceptors in vertebrate vision. Proc Natl Acad Sci USA. 2014;111:10438–10443. doi: 10.1073/pnas.1410826111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser AS, Chavali S, Masuho I, Jahn LJ, Martemyanov KA, Gloriam DE, Babu MM. Pharmacogenomics of GPCR drug targets. Cell. 2018;172:41–54.e19. doi: 10.1016/j.cell.2017.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohenegger M, Mitterauer T, Voss T, Nanoff C, Freissmuth M. Thiophosphorylation of the G protein b subunit in human platelet membranes: evidence against a direct phosphate transfer reaction to G a subunits. Mol Pharmacol. 1996;49:73–80. [PubMed] [Google Scholar]
- Hornykiewicz O, Lisch HJ, Springer A. Homovanillic acid in different regions of the human brain: attempt at localizing central dopamine fibres. Brain Res. 1968;11:662–671. doi: 10.1016/0006-8993(68)90154-6. [DOI] [PubMed] [Google Scholar]
- Hubbard R. The thermal stability of rhodopsin and opsin. J Gen Physiol. 1958;42:259–280. doi: 10.1085/jgp.42.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J Mol Graph. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. 27-28. [DOI] [PubMed] [Google Scholar]
- Jaberi E, Rohani M, Shahidi GA, Nafissi S, Arefian E, Soleimani M, Moghadam A, Arzenani MK, Keramatian F, Klotzle B, et al. Mutation in ADORA1 identified as likely cause of early-onset parkinsonism and cognitive dysfunction. Mov Disord. 2016;31:1004–1011. doi: 10.1002/mds.26627. [DOI] [PubMed] [Google Scholar]
- Jämbeck JP, Lyubartsev AP. An extension and further validation of an all-atomistic force field for biological membranes. J Chem Theory Comput. 2012;8:2938–2948. doi: 10.1021/ct300342n. [DOI] [PubMed] [Google Scholar]
- Jämbeck JP, Lyubartsev AP. Another piece of the membrane puzzle: extending slipids further. J Chem Theory Comput. 2013;9:774–784. doi: 10.1021/ct300777p. [DOI] [PubMed] [Google Scholar]
- Jockers R, Linder ME, Hohenegger M, Nanoff C, Bertin B, Strosberg AD, Marullo S, Freissmuth M. Species difference in the G protein selectivity of the human and bovine A1-adenosine receptor. J Biol Chem. 1994;269:32077–32084. [PubMed] [Google Scholar]
- Johnson RA, Alvarez R, Salomon Y. Determination of adenylyl cyclase catalytic activity using single and double column procedures. Meth Enzymol. 1994;238:31–56. doi: 10.1016/0076-6879(94)38005-8. [DOI] [PubMed] [Google Scholar]
- Khodadadi H, Azcona LJ, Aghamollaii V, Omrani MD, Garshasbi M, Taghavi S, Tafakhori A, Shahidi GA, Jamshidi J, Darvish H, et al. PTRHD1 (C2orf79) mutations lead to autosomal-recessive intellectual disability and parkinsonism. Mov Disord. 2017;32:287–291. doi: 10.1002/mds.26824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger R, Kuhn W, Müller T, Woitalla D, Graeber M, Kösel S, Przuntek H, Epplen JT, Schöls L, Riess O. Ala30Pro mutation in the gene encoding α-synuclein in Parkinson’s disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Kuipers DJS, Carr J, Bardien S, Thomas P, Sebate B, Breedveld GJ, van Minkelen R, Brouwer RWW, van Ijcken WFJ, van Slegtenhorst MA, et al. PTRHD1 Loss-of-function mutation in an african family with juvenile-onset Parkinsonism and intellectual disability. Mov Disord. 2018;33:1814–1819. doi: 10.1002/mds.27501. [DOI] [PubMed] [Google Scholar]
- Kusek J, Yang Q, Witek M, Gruber CW, Nanoff C, Freissmuth M. Chaperoning of the A1-adenosine receptor by endogenous adenosine - an extension of the retaliatory metabolite concept. Mol Pharmacol. 2015;87:39–51. doi: 10.1124/mol.114.094045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff P. The two-state model of receptor activation. Trends Pharmacol Sci. 1995;16:89–97. doi: 10.1016/s0165-6147(00)88989-0. [DOI] [PubMed] [Google Scholar]
- Lindorff-Larsen K, Piana S, Palmo K, Maragakis P, Klepeis JL, Dror RO, Shaw DE. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins. 2010;78:1950–1958. doi: 10.1002/prot.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumry R, Eyring H. Conformational changes in proteins. J Phys Chem. 1954;58:110–120. [Google Scholar]
- Lv YC, Gao AB, Yang J, Zhong LY, Jia B, Ouyang SH, Gui L, Peng TH, Sun S, Cayabyab FS. Long-term adenosine A1 receptor activation-induced sortilin expression promotes α-synuclein upregulation in dopaminergic neurons. Neural Regen Res. 2020;15:712–723. doi: 10.4103/1673-5374.266916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Málaga-Diéguez L, Yang Q, Bauer J, Pankevych H, Freissmuth M, Nanoff C. Pharmacochaperoning of the A1 adenosine receptor is contingent on the endoplasmic reticulum. Mol Pharmacol. 2010;77:940–952. doi: 10.1124/mol.110.063511. [DOI] [PubMed] [Google Scholar]
- Michaud-Agrawal N, Denning EJ, Woolf TB, Beckstein O. MDAnalysis: a toolkit for the analysis of molecular dynamics simulations. J Comput Chem. 2011;32:2319–2327. doi: 10.1002/jcc.21787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monticelli L, Kandasamy SK, Periole X, Larson RG, Tieleman DP, Marrink SJ. The MARTINI coarse-grained force field: extension to proteins. J Chem Theory Comput. 2008;4:819–834. doi: 10.1021/ct700324x. [DOI] [PubMed] [Google Scholar]
- Nanoff C, Freissmuth M. ER-bound steps in the biosynthesis of G proteincoupled receptors. Subcell Biochem. 2012;63:1–21. doi: 10.1007/978-94-007-4765-4_1. [DOI] [PubMed] [Google Scholar]
- Narhi L, Wood SJ, Steavenson S, Jiang Y, Wu GM, Anafi D, Kaufman SA, Martin F, Sitney K, Denis P, et al. Both familial Parkinson’s disease mutations accelerate α-synuclein aggregation. J Biol Chem. 1999;274:9843–9846. doi: 10.1074/jbc.274.14.9843. [DOI] [PubMed] [Google Scholar]
- Nussbaum RL, Ellis CE. Alzheimer’s disease and Parkinson’s disease. N Engl J Med. 2003;348:1356–1364. doi: 10.1056/NEJM2003ra020003. [DOI] [PubMed] [Google Scholar]
- Palacios N, Gao X, McCullough ML, Schwarzschild MA, Shah R, Gapstur S, Ascherio A. Caffeine and risk of Parkinson’s disease in a large cohort of men and women. Mov Disord. 2012;27:1276–1282. doi: 10.1002/mds.25076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankevych H, Korkhov V, Freissmuth M, Nanoff C. Truncation of the A1 adenosine receptor reveals distinct roles of the membrane-proximal carboxyl terminus in receptor folding and G protein coupling. J Biol Chem. 2003;278:30283–30293. doi: 10.1074/jbc.M212918200. [DOI] [PubMed] [Google Scholar]
- Parrinello M, Rahman A. Polymorphic transitions in single crystals: a new molecular dynamics method. J Appl Phys. 1981;52:7182–7190. [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, et al. Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Rivera-Oliver M, Moreno E, Álvarez-Bagnarol Y, Ayala-Santiago C, Cruz-Reyes N, Molina-Castro GC, Clemens S, Canela EI, Ferré S, Casadó V, et al. Adenosine A1-dopamine D1 receptor heteromers control the excitability of the spinal motoneuron. Mol Neurobiol. 2019;56:797–811. doi: 10.1007/s12035-018-1120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Ruiz JM. Protein kinetic stability. Biophys Chem. 2010;148:1–15. doi: 10.1016/j.bpc.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Savinainen JR, Saario SM, Niemi R, Järvinen T, Laitinen JT. An optimized approach to study endocannabinoid signaling: evidence against constitutive activity of rat brain adenosine A1 and cannabinoid CB1 receptors. Br J Pharmacol. 2003;140:1451–1459. doi: 10.1038/sj.bjp.0705577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütz W, Freissmuth M. Reverse intrinsic activity of antagonists on G protein-coupled receptors. Trends Pharmacol Sci. 1992;13:376–380. doi: 10.1016/0165-6147(92)90116-n. [DOI] [PubMed] [Google Scholar]
- Shahmoradian SH, Lewis AJ, Genoud C, Hench J, Moors TE, Navarro PP, Castaño-Díez D, Schweighauser G, Graff-Meyer A, Goldie KN, et al. Lewy pathology in Parkinson’s disease consists of crowded organelles and lipid membranes. Nat Neurosci. 2019;22:1099–1109. doi: 10.1038/s41593-019-0423-2. [DOI] [PubMed] [Google Scholar]
- Shen MY, Sali A. Statistical potential for assessment and prediction of protein structures. Protein Sci. 2006;15:2507–2524. doi: 10.1110/ps.062416606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, et al. α-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Singleton AB, Farrer MJ, Bonifati V. The genetics of Parkinson’s disease: progress and therapeutic implications. Mov Disord. 2013;28:14–23. doi: 10.1002/mds.25249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. α-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Stockner T, Ash WL, MacCallum JL, Tieleman DP. Direct simulation of transmembrane helix association: role of asparagines. Biophys J. 2004;87:1650–1656. doi: 10.1529/biophysj.104.045310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell J, Jakova E, Cayabyab FS. Adenosine A1 and A2A receptors in the brain: current research and their role in neurodegeneration. Molecules. 2017;22:E676. doi: 10.3390/molecules22040676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldhoer M, Wise A, Milligan G, Freissmuth M, Nanoff C. Kinetics of ternary complex formation with fusion proteins composed of the A(1)-adenosine receptor and G protein α-subunits. J Biol Chem. 1999;274:30571–30579. doi: 10.1074/jbc.274.43.30571. [DOI] [PubMed] [Google Scholar]
- Wassenaar TA, Ingólfsson HI, Böckmann RA, Tieleman DP, Marrink SJ. Computational lipidomics with insane: a versatile tool for generating custom membranes for molecular simulations. J Chem Theory Comput. 2015;11:2144–2155. doi: 10.1021/acs.jctc.5b00209. [DOI] [PubMed] [Google Scholar]
- Wassenaar TA, Pluhackova K, Böckmann RA, Marrink SJ, Tieleman DP. Going backward: a flexible geometric approach to reverse transformation from coarse grained to atomistic models. J Chem Theory Comput. 2014;10:676–690. doi: 10.1021/ct400617g. [DOI] [PubMed] [Google Scholar]
- Webb B, Sali A. Protein structure modeling with MODELLER. Methods Mol Biol. 2014;1137:1–15. doi: 10.1007/978-1-4939-0366-5_1. [DOI] [PubMed] [Google Scholar]
- Wolf MG, Hoefling M, Aponte-Santamaría C, Grubmüller H, Groenhof G. g_membed: efficient insertion of a membrane protein into an equilibrated lipid bilayer with minimal perturbation. J Comput Chem. 2010;31:2169–2174. doi: 10.1002/jcc.21507. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Wypych J, Steavenson S, Louis JC, Citron M, Biere AL. α-synuclein fibrillogenesis is nucleation-dependent. Implications for the pathogenesis of Parkinson’s disease. J Biol Chem. 1999;274:19509–19512. doi: 10.1074/jbc.274.28.19509. [DOI] [PubMed] [Google Scholar]
- Yabuuchi K, Kuroiwa M, Shuto T, Sotogaku N, Snyder GL, Higashi H, Tanaka M, Greengard P, Nishi A. Role of adenosine A1 receptors in the modulation of dopamine D1 and adenosine A2A receptor signaling in the neostriatum. Neuroscience. 2006;141:19–25. doi: 10.1016/j.neuroscience.2006.04.047. [DOI] [PubMed] [Google Scholar]
- Zhang PL, Chen Y, Zhang CH, Wang YX, Fernandez-Funez P. Genetics of Parkinson’s disease and related disorders. J Med Genet. 2018;55:73–80. doi: 10.1136/jmedgenet-2017-105047. [DOI] [PubMed] [Google Scholar]