Summary
Causal evidence links modifiable maternal exposures during the periconceptional period with offspring obesity. The interconception period may be an important time to intervene. We systematically identified studies examining change in modifiable maternal exposures between pregnancies and offspring adiposity. We searched for longitudinal studies published between 1990 and 2019, which included measurements taken on at least two occasions in the period from 1 year prior to the conception of the first birth to the time of the second birth, and which included a measure of adiposity in second, or higher order, siblings. Age, ethnicity and genetics were not considered modifiable; all other factors including length of the interpregnancy interval were. Eleven studies satisfied the inclusion criteria. Higher interpregnancy weight gain or loss, maternal smoking inception, mothers smoking in their first pregnancy and quitting, increasing the number of cigarettes smoked and longer interpregnancy intervals were positively associated with adiposity in second or higher order children. Vaginal birth after caesarean delivery was protective. Further research is needed to ascertain whether the risk of adiposity is fixed based on first pregnancy exposures or if interpregnancy change alters the risk for a subsequent child. This can inform the type and effectiveness of interventions for mothers prior to a subsequent pregnancy.
Keywords: adiposity, childhood, interpregnancy, obesity
1. Introduction
The World Health Organization estimated that in 2016 there were 41 million children worldwide under the age of 5 and 340 million children and adolescents aged 5–19 who were affected by overweight or obesity.1 More than 1.9 billion adults were affected by overweight, and, of these, 650 million (11% of men and 15% of women) were affected by obesity.1 Early intervention and prevention is key since childhood weight is strongly associated with adult weight2 and obesity raises the risk of many noncommunicable diseases including cardiovascular disease,3 Type 2 diabetes,4 osteoarthritis,5 other musculo-skeletal disorders6 and a number of different types of cancer.1
The Developmental Origins of Health and Disease (DOHaD) concept links exposures during the periconceptional period and pregnancy to lasting effects on developmental programming of the foetus and to subsequent susceptibility to noncommunicable disease.7 A number of modifiable maternal characteristics and behaviours in the preconception, pregnancy and interpregnancy period (defined by the World Health Organization as the period from the birth of one child until the conception of the subsequent pregnancy8) have been associated with offspring adiposity.9–12 These include, but are not limited to, maternal obesity, with or without maternal gestational diabetes mellitus (GDM),13 gestational weight gain,14 smoking,15 severe pre-pregnancy stress,16 birth by caesarean section17and inequalities in socioeconomic status.18,19
Whilst there is much in the literature examining associations between the exposures in one pregnancy and the outcome for that child, there is little research on whether this risk is then fixed for subsequent children based on those earlier predictors, or whether a change in exposure between pregnancies changes the risk for a subsequent child. The majority of research which has examined inter-pregnancy change, focuses on birth or neonatal outcomes such as large or small-for-gestational-age and premature birth.20–23
We aimed to systematically review the literature for longitudinal studies which characterized modifiable maternal exposures between successive live pregnancies and determine their associations with adiposity in second or higher order siblings.
2. Methods
2.1. Search strategy
An electronic search was conducted using the bibliographic databases EMBASE (via Ovid), MEDLINE (via Ovid), CINAHL (via EBSCO), PsycINFO (via EBSCO) and Web of Science. A specialist librarian was consulted in advance, and the search was run on 30 March 2019. Studies were restricted to those based on human participants and published in English since 1990 to ensure that only the most up-to-date literature was reviewed. The protocol for this review was published on the international prospective register of international reviews, PROSPERO, reference CRD42019124344,24 and this review is reported in line with the PRISMA guidelines.25 The following search strategy was piloted on EMBASE and adapted for the other platforms:
[exp family planning/] OR ((interpregnan* or inter-pregnan* or consecutive pregnan* or interconception or inter-conception or between pregnan* or family planning or inter-natal or multipar* or multigravid* or conception).mp.)] AND
[exp obesity/ OR exp body mass/OR exp overnutrition/OR ((obes* or overweight or overnutrition or adiposity or weight or body mass). mp.)] AND
[exp progeny/OR exp child/or exp sibling/OR ((child* or offspring or infant or sibling* or progeny).mp.)]
2.2. Inclusion and exclusion criteria
We only included longitudinal studies. Case control studies, cross-sectional studies and case reports were excluded.
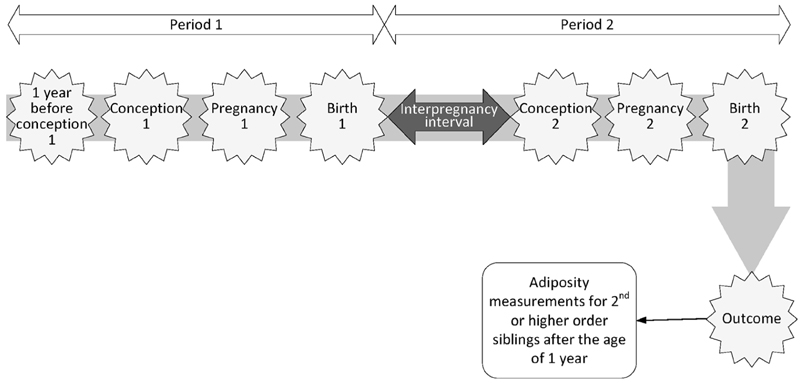
We did not consider age, ethnicity or genetics to be modifiable, but considered all other maternal behaviours and characteristics to be potentially modifiable exposures. Studies where measurements of the main exposure were taken on at least two occasions in the period from 1 year prior to the conception of the first pregnancy up to the time of the second birth were considered. At least one measurement should have been recorded in the period from 1 year prior to the conception of the first pregnancy and the first birth (Period 1) and at least one in the period from the time of the first birth to the time of the second birth (Period 2). Figure 1 represents the relevant timeline.
Figure 1.
Diagram representing the period of time under consideration in this systematic review. At least one exposure measurement should have been recorded in Period 1 and at least one in period 2. Outcome measurements should be recorded in second, or higher order, siblings after the age of 1 year, without upper bound
The length of the interpregnancy interval was specifically included as a modifiable exposure as defined per individual study, even if the World Health Organization definition of the inter-pregnancy interval was not used (the period from the birth of one child to the conception of the subsequent pregnancy).8
We considered studies where outcomes included a measure of adiposity in second or higher order siblings. Appropriate outcome measurements included categorical and continuous offspring BMI, weight for height z-scores, skinfold measurements and body adiposity. All time points were considered after the age of 1, without an upper age limit. The lower bound was given as 1 year to exclude studies focussing on neonatal outcomes, including foetal macrosomia.
2.3. Screening process
The screening management software Rayyan26 was used to screen titles for eligibility. A randomly selected sample of 10% of these titles was independently screened by two reviewers (EJT and SW). A 10% sample was chosen as a recent simulation study has shown that if the fraction being sampled for independent review is increased, there is no decrease in selection bias.27 At the title screening stage, the percentage agreement between the two reviewers was 98.9%, and discrepant titles were included for abstract screening. One author (EJT) reviewed the remaining titles. The same process was then followed for reviewing the abstracts of papers remaining after title screening. The agreement rate between the two reviewers (EJT and SW) was 100%, and accordingly, one author (EJT) reviewed the remaining abstracts.
Following abstract screening, the full texts of the remaining titles were accessed and reviewed by two reviewers (EJT and SW), with one exception where the discrepant title was only considered by one reviewer (EJT), due to copyright restrictions, and was excluded in any event.
2.4. Data extraction, quality assessment and analysis
A modified version of the Cochrane Collaboration data extraction form was used to conduct data extraction,28 and this was undertaken by two reviewers (EJT and SW or EJT and NZ).
All eligible data were extracted, including for subgroup analysis and exposure multiple time points where these were reported. Where reported, confidence intervals which did not overlap the null were used to identify statistically significant associations. If these where not reported, p values were used.
Quality assessment was also undertaken by two reviewers (EJT and SW). Key strengths and weaknesses of each of the cohort studies29–38 were formed using the National Heart Lung and Blood Institute assessment tool for observational cohort and cross-sectional studies.39 The remaining study is an individual patient data meta-analysis,40 and in this instance, we used the PRISMA-IPD checklist.41 The overall rating for addressing the risk of bias (good/fair/poor) is included in Table 1.
Table 1.
Included study characteristics
| First author and year | Study design, location and sample size | Maternal exposure(s) | Relevant child outcome(s) and age(s) | Quality assessment and overall rating for addressing the risk of bias (good/fair/poor)a |
|---|---|---|---|---|
| Adane 201829 | Population-based prospective cohort study Australia n = 714 sibling pairs of children |
Interpregnancy BMI changeb
Calculated by taking BMI measures prior to the conception of each child and categorized: Stable, small gain, moderate gain, high gain |
BMI categories based on age and sex-specific cut-offs for childrenb
Second born children aged between 2 and 13 years |
Positive Adjusts for maternal confounders Negative All exposure and outcome measures self-reported Lack of data on gestational age and gestational age gain Only 34% of women invited responded to the survey about their children Rating: Fair |
| Albers 201840 | Individual patient data meta-analysis of 5 datasets from studies reporting BMI of children and the number of cigarettes smoked in pregnancy and then scanned for sibling data America (×3) Australia Canada n = 45 299 children from 14 231 families |
Smoking status Number of cigarettes smoked across pregnancies b The number of siblings per family ranged from 2 to 16 children. There were two children for n = 10 576 families |
BMI z-scores using the World Health Organization’s Child Growth Standardsd
Age range of children in included studies 2–19 years. Mean age 5.61 (SD 2.37) years |
Positive All five underlying studies assessed by the authors as being of good quality Adjusts for maternal confounders Negative Only able to use confounders used in most studies Rating: Fair |
| Aucott 201730 | Population-based cohort (linkage) study Scotland n = 5863 mothers with more than one linked pregnancy and 718 sibling pairs of children |
1. Smoking statusbRecorded at the start of each pregnancy and categorized: Never smoked, quit, started smoking, always smoked 2. Maternal weight change cBroken down into 7 categories from 10% or greater loss to 10% or greater gain. Reference category ±3.0%. |
BMI categories using the International Obesity Task Force criteria for weight categories c
BMI z-scores (derived using the 1990 UK standard)c Children mean age 5.6 (SD 0.6) years |
Positive Routinely collected population level data for exposure and outcome Objectively measured outcome Adjusts for maternal confounders Excludes mothers >16 weeks pregnant at first antenatal appointment in order to exclude weight gain due to pregnancy Negative Smoking status self-reported at a single point in pregnancy only Maternal and child records only linked in 44% of cases Rating: Good |
| Barclay 201831 | Population-based cohort (linkage) study Sweden n = 102 000 |
Length of the interpregnancy intervalc
Defined here as the birth-to birth interval |
BMI categories using standard cut-offs c
Outcome measurements in men only, aged between 17 and 20 years at conscription Study based on full siblings only and in sibling groups of at least 3 Whole sibling groups used for measures of birth spacing, not just men |
Positive Routinely collected population level data for exposure and outcome Objectively measured outcome Sibling groups included only where neither partner has children with a third partner Negative Men only for outcome measurement Based on sibling groups with at least 3 children Only maternal confounder controlled for is age Rating: Good |
| Devakumar 201632 | Prospective cohort Brazil n = 2239 |
Length of the interpregnancy intervalb
Defined as the period from the birth of the previous chid to the beginning of the pregnancy of the index child |
Body composition; fat mass, fat-free mass, visceral fat, subcutaneous fatc
BMIc Offspring mean age 30.2 years |
Positives 68% follow-up rate at offspring age 30 years Objectively measured outcome Adjusts for maternal confounders Negatives Self-reported birth interval length based on maternal recall Rating: Fair |
| Huttly 199233 | Longitudinal population-based cohort Brazil n = 2952 |
Length of the interpregnancy intervalb
Here the interval since the preceding birth was recorded and is referred to as the ‘birth interval’ |
Weight for age and height z-scores, standardized to National Centre for health statistics standardsc
Mean age 19 months |
Positives 82% of eligible children have outcome data at mean age 19 months Objectively measured outcome Adjusts for maternal confounders Negatives Self-reported birth interval length based on maternal recall Does not report variance Rating: Fair |
| Iliadou 201034 | Population-based cohort (linkage) study Sweden n = 8441 sibling pairs |
Smoking statusb
Recorded at the start of each pregnancy and categorized: non-smoker, smoking 1–9 cigarettes a day, smoking ≥10 cigarettes a day |
BMIc
Men only aged approximately 18 years at conscription |
Positive Routinely collected population level data for outcome Objectively measured outcome Adjusts for maternal confounders Negative Men only for outcome measurement Smoking status self-reported at a single point in pregnancy only Rating: Good |
| Li 201835 | Longitudinal cohort study America n = 2119 |
Length of the interpregnancy intervalc
Defined as the date of the previous birth to the conception of the subsequent pregnancy |
BMI z-scores standardized using US national reference datac
Children aged 7 years |
Positives Objectively measured outcome Adjusts for maternal confounders Negatives Study based on very old data with very young mothers; final child measurements taken in 1972 Total duration from recruitment to last birth only 6 years, limiting length of available birth intervals Rating: Poor |
| Smithers 201736 | Population-based cohort (linkage) study Australia n = 4099 |
Birth by elective caesarean or by vaginal birth in the second pregnancy where the previous birth was by caesarean sectionc | Age and sex-specific BMI z-scores using the World Health Organization’s Child Growth Standardsc
Children aged between 3 and 6 years |
Positives Routinely collected population level data for exposure and outcome Objectively measured outcome Adjusts for maternal confounders Negatives No information on low linkage rates Rating: Good |
| Willmer 201337 | Population-based cohort (linkage) study Sweden n = 71 sibling pairs |
Maternal weight loss due to bariatric surgery between pregnanciesc | Measurements from child health Centre height and weight recorded at comprehensive check-up at age 4, and then predicted BMI at 4th birthday c
Models also tried with BMIs converted to z-scores and with standardization against a British reference populationc |
Positives Routinely collected population level data for exposure and outcome Objectively measured outcome Negatives Only 71 child–woman triads with complete data available for analysis Limited adjustments for maternal confounders Rating: Fair |
| Yuan 201638 | Prospective cohort America n = 15 630 |
Birth method across successive pregnancies; caesarean or vaginalc | BMI for individuals under 18 years calculated using the International Obesity Task Force criteria for weight categoriesb
For individuals over 18 years, obesity calculated using World Health Organization cut-offsb Offspring followed from age 9–14 years through age 20–28 years |
Positives Controls for maternal confounders Negatives Self-reported outcome Rating: Fair |
Abbreviations: BMI, body mass index (kg/m2); SD, standard deviation.
Quality was assessed using an adapted version of the National Heart, Lung and Blood Institute assessment tool for observational cohort and cross-sectional studies39 and the PRISMA-IPD checklist.41
Outcome/exposure based on self-reported information
Outcome/exposure objectively measured
Details of whether outcome/exposure measurements were self-reported or objectively measured are not stated
Since we expected significant variation in study exposures and design, a qualitative synthesis was planned a priori rather than a meta-analysis. Studies have been grouped based on the exposures being considered, and we summarize the effect sizes and precision for each included study.
3. Results
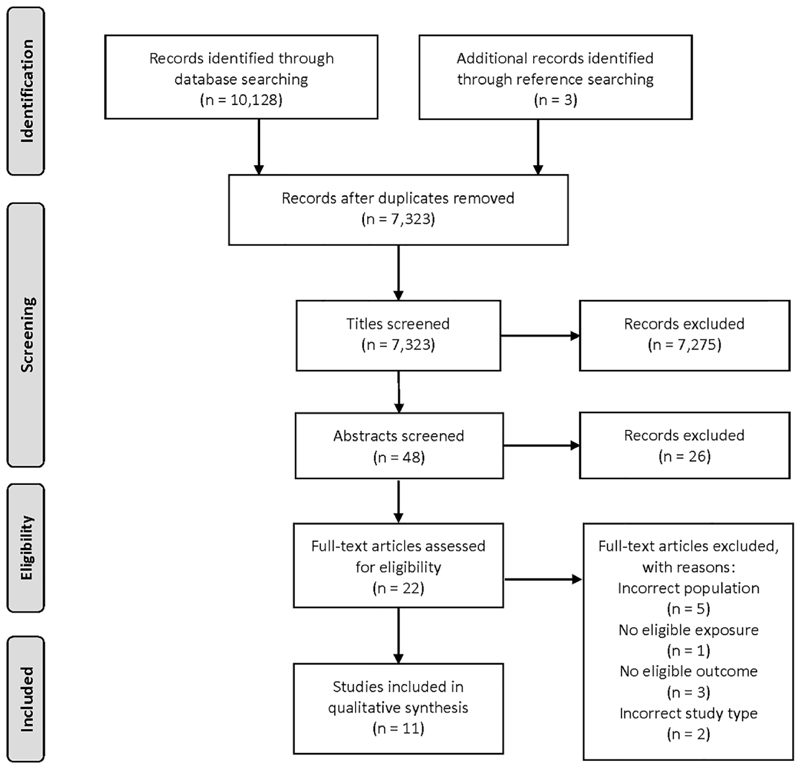
All stages of data screening were documented using a PRISMA flow diagram25 (Figure 2). Our searches identified 10 131 records of which 2808 were duplicates. A total of 7323 titles were screened and of these, 48 abstracts were screened. Full texts of 22 papers were assessed for inclusion, and of these, 11 have been included in the qualitative synthesis.
Figure 2.
PRISMA flow diagram
Three of the included studies were based on populations in Sweden,31,34,37 two each in America,35,38 Australia29,36 and Brazil32,33 and one in Scotland.30 The remaining study40 used data from five underlying datasets from Australia, Canada and America; one of which was the Collaborative Perinatal Project which was also used by one of the other studies.35 Three studies included maternal interpregnancy weight change as the exposure,29,30,37 three examined maternal smoking status,30,34,40 two considered mode of birth36,38 and four the length of the interpregnancy interval.31–33,35 Further study characteristics are detailed in Table 1. Associations between change in exposures and the risk of offspring adiposity are summarized in Table 2. A summary of the statistical analysis undertaken, adjustments made and significant results reported in each study is given in Table 3.
Table 2.
Associations between interpregnancy change in maternal exposures and childhood adiposity in second or higher order siblings across the 11 included studies
| First author and year | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Maternal exposure | Adane 201829 | Albers 201840 | Aucott 201730 | Barclay 201831 | Devakumar 201632 | Huttly 199233 | Iliadou 201034 | Li 201835 | Smithers 201736 | Willmer 201337 | Yuan 201638 |
| Interpregnancy weight change | |||||||||||
| ● Highest loss categorya | x | + | |||||||||
| ● Loss | x | x | |||||||||
| ● Small/moderate gains | x | x | |||||||||
| ● Highest gain categoryb | + | + | |||||||||
| ● Change in BMI at week 10 of pregnancy | x | ||||||||||
| Smoking status | |||||||||||
| ● Smoking PG2 only | + | x | |||||||||
| ● Smoking PG1 only | + | x | |||||||||
| ● Smoked PG1 and PG2 | + | + | |||||||||
| ● Increase in each additional cigarette smoked | + | ||||||||||
| Mode of birth | |||||||||||
| ● Elective caesarean compared to vaginal birth after a caesarean birth | x | ||||||||||
| ● Vaginal birth after a caesarean birth | - | ||||||||||
| ● Caesarean birth after a vaginal birth | x | ||||||||||
| Length of the interpregnancy interval | |||||||||||
| ● Longer preceding birth intervalsc | + | x | + | x | |||||||
| ● Shorter preceding birth intervalsd | x | - | |||||||||
| ● Preceding interval<18 months | x | ||||||||||
| ● Continuous variable | x | x | |||||||||
| ● Continuous variable—Female offspring | + | ||||||||||
| ● Continuous variable—Male offspring | x | ||||||||||
| ● Binary variablee x | |||||||||||
Notes: (+) factor associated with greater childhood adiposity; (−) factor associated with reduced childhood adiposity; (x) confidence interval for association includes the null (1.0 for odds and risk ratios, 0.0 for linear coefficients), or p values >0.05 where these are unavailable.
Abbreviations: BMI, body mass index; PG1, Pregnancy 1; PG2, Pregnancy 2.
Adane et al. used loss of ≥1 BMI units; Aucott et al. used loss ≥−10%.
Adane et al. used gain of ≥4 BMI units; Aucott et al. used gain ≥10%.
Barclay and Kolk 31 months plus; Devakumar et al. 18 months plus; Huttly et al. 24 months plus; Li et al. 6 months plus.
Barclay and Kolk 19–24 months; Huttly et al. 18–23 months.
The only association found was with fat-free mass (see Table 3).
Table 3.
Summary of statistical analysis, adjustments made and significant results reported across the 11 included studies
| First author and year | Statistical analysis | Significant results reported* | |
|---|---|---|---|
| Exposure | Outcome | ||
| Adane 201829 | Logistic regression | Interpregnancy weight gain (≥ 4 kg/m2) (Ref: Stable interpregnancy weight (± 1 kg/m2)) | Obesity (aOR 2.20, 95% CI [1.02, 4.75]) |
| Adjustments: Maternal characteristics prior to the second birth (age, area of residence, education, smoking and physical activity), interpregnancy interval and BMI prior to the first birth | |||
| Albers 201840 | Individual patient data meta-analysis on five studies. Multilevel model separating within-family and between-family effects | Each additional cigarette smoked a day in sibling pregnancies | BMI z-score (β = 0.007, 95% CI [0.006, 0.009]) |
| Adjustments: Maternal weight status, breastfeeding and maternal education attained by the start of the respective pregnancy | |||
| Aucott 201730 | Multinomial multilevel logistic modelling and a two level multivariate model. Analysis undertaken with and without sibling analysis | Maternal weight loss >10% (Ref: weight change ±3%) | Overweight (aOR 1.3, 95% CI [1.0, 1.6]) |
| Obesity (aOR 1.9, 95% CI [1.4, 2.7]) | |||
| Maternal weight gain ≥10% (Ref: weight change ±3%) | Mean BMI z-score (β = 0.13, 95% CI [0.05, 0.20]) | ||
| Maternal smoking: | |||
| With sibling analysis: | |||
| ● Starting to smoke between pregnancies | Mean BMI z-score (β = 0.19, 95% CI [0.01, 0.36]) | ||
| ● Smoking in both pregnancies | Mean BMI z-score (β = 0.10, 95% CI [0.01, 0.20]) | ||
| Without sibling analysis: | |||
| ● Starting to smoke between pregnancies | Mean BMI z-score (β = 0.22, 95% CI [0.07, 0.36]) | ||
| ● Smoking in both pregnancies | Mean BMI z-score (β = 0.32, 95% CI [0.24, 0.40]) | ||
| ● Quitting between pregnancies | Mean BMI z-score (β = 0.29, 95% CI [0.18, 0.40]) | ||
| Odds ratios (Ref: never smokers) | |||
| ● Starting to smoke between pregnancies | Overweight (aOR 1.5, (95% CI) 1.0, 2.2]) | ||
| Obesity (aOR 2.0, 95% CI [1.1, 3.6]) | |||
| ● Smoking in both pregnancies | Overweight (aOR 1.5, 95% CI [1.2, 2.8]) Obesity (aOR 1.8, 95% CI [1.3, 2.6]) | ||
| ● Quit between pregnancies | |||
| Overweight (aOR 1.5, 95% CI [1.0, 2.0]) | |||
| Obesity (aOR 1.6, 95% CI [1.0, 2.5]) | |||
| Adjustments: Child sex, maternal weight category, maternal smoking (for weight change exposure)/percentage weight change (for smoking change exposure), socio-economic status, parity, maternal age and birthweight z-score | |||
| Barclay 201831 | Analysis: Between-family analysis using linear regression and within-family analysis, using sibling fixed effects | Preceding interval length (Ref: 25–30 months) | |
| ● Between-family analysis: (months) | Overweight/obesity: | ||
| 31–36 | (β = 0.012, 95% CI [0.005, 0.019]) | ||
| 37–42 | (β = 0.013, 95% CI [0.005, 0.020]) | ||
| 43–48 | (β = 0.016, 95% CI [0.008, 0.024]) | ||
| 49–54 | (β = 0.018, 95% CI [0.009, 0.027]) | ||
| 55–60 | (β = 0.033, 95% CI [0.023, 0.043]) | ||
| 61–66 | (β = 0.036, 95% CI [0.024, 0.047]) | ||
| 67–72 | (β = 0.042, 95% CI [0.029, 0.055]) | ||
| 73–78 | (β = 0.044, 95% CI [0.030, 0.059]) | ||
| 79–84 | (β = 0.057, 95% CI [0.040, 0.073]) | ||
| 85–90 | (β = 0.057, 95% CI [0.038, 0.076]) | ||
| 91–96 | (β = 0.075, 95% CI [0.052, 0.097]) | ||
| 97+ | (β = 0.084, 95% CI [0.070, | ||
| 0.098]) | |||
| ● Within-family analysis: (months) | Overweight/obesity: | ||
| 31–36 | (β = 0.014, 95% CI [0.000, 0.028]) | ||
| 55–60 | (β = 0.021, 95% CI [0.002, 0.040]) | ||
| 67–72 | (β = 0.034. 95% CI [0.009, 0.059]) | ||
| 79–84 | (β = 0.062, 95% CI [0.031, 0.093]) | ||
| 97+ | (β = 0.039, 95% CI [0.009, 0.068]) | ||
| Adjustments: Birth order, maternal age, birth year, sibling group size, age at and year of conscription | |||
| Devakumar 201632 | Multivariable linear regression | Birth interval ≥ 18 months (ref: <18 months) | Fat free mass (kg) (β = 1.717, 95% CI [0.242, 3.193]) |
| Continuous birth interval (females) | Fat free mass (kg) (β = 0.014, 95% CI [0.000, 0.027]) | ||
| Visceral fat (cm) (β = 0.004, 95% CI [0.000, 0.007]) | |||
| Adjustments: Maternal age, education, BMI at the beginning of pregnancy, family income at birth and birth order | |||
| Huttly 199233 | ANOVA (p-value < 0.001) | Birth interval in months | Mean weight-for-height z-scores |
| <18 | 0.07 | ||
| 18–23 | -0.03 | ||
| 24–35 | 0.06 | ||
| 36–47 | 0.23 | ||
| 48–71 | 0.19 | ||
| >71 | 0.27 | ||
| Adjustments: Maternal income, race, education, cohabitation, age and parity | |||
| Iliadou 201034 | Analysis: Logistic regression | Smoking in both male pregnancies (Ref: non-smokers in pregnancy) | Overweight (aOR 1.71, 95% CI [1.39, 2.09]) |
| Adjustments: Maternal age, height, BMI, pregnancy weight gain, maternal and paternal socio-economic category and education, offspring birthweight, head circumference, gestational age, urban living and age at conscription | |||
| Li 201835 | Generalized linear regression models | Length of the interpregnancy interval | No significant results reported |
| Adjustments: Infant gender, maternal race, maternal marriage status, SES, mother’s educational level, smoking status, pregestational/gestational diabetes, pregestational/gestational hypertension (in the continuous model only), gestational age, prepregnancy BMI, weight gain in pregnancy, mode of labour onset, birthweight for gestational age, Apgar score at 5 minutes, birthweight (in the categorical model only), feeding pattern, rapid weight gain in the first year of life, and maternal age at birth | |||
| Smithers 201736 | Augmented inverse probability weighted analysis | Mode of birth | No significant results reported |
| Adjustments: Maternal age, type of antenatal care, number of antenatal visits, medical conditions in pregnancy (asthma, diabetes, hypertension), smoking in pregnancy, gestational age, birthweight for gestational age z-scores, maternal partnership status, maternal ethnicity, maternal occupation, neighbourhood-level indicators of socioeconomic disadvantage and remote residence | |||
| Willmer 201337 | Fixed-effects regression | Maternal weight change | No significant results reported |
| Adjustments: Sex of siblings, birth order, mother’s age and smoking in pregnancy | |||
| Yuan 201638 | Within-family analysis using conditional logistic regression | Vaginal birth after caesarean birth | Obesity (RR 0.69, 95% CI [0.53, 0.83]) |
| Maternal age at birth, race, prepregnancy BMI group, maternal height, gestational diabetes, pre-eclampsia, pregnancy induced hypertension, child sex, year of birth, gestational age at birth, birth order, birth weight group, prepregnancy smoking and region of residence at birth | |||
Abbreviations: aOR, adjusted odds ratio; BMI, body mass index (kg/m2); CI, confidence interval; RR, relative risk.
Significant results are those where the confidence interval for association does not include the null (1.0 for odds and risk ratios, 0.0 for linear coefficients), or where p values <0.05 if these are unavailable.
3.1. Characteristics of the included studies
Table 1 summarizes the included studies. With the exception of the individual patient data meta-analysis,40 all included studies use data from cohorts29,32,33,35,38 and data-linkage studies.30,31,34,36,37 These studies showed considerable variation in terms of the exposure and outcome measurements.
Studies where the exposure was interpregnancy weight change,29,30,37 smoking status30,34,40 or mode of birth36,38 had a measurement taken during Period 1 and a further measurement in Period 2, as defined above (Figure 1). Most studies included only one time-point for outcome measurement and the timings varied by study; the youngest mean age for offspring was 19 months33 and the highest 30.2 years.32 Both of these studies32,33 followed children whose mothers formed part of the 1982 Pelotas (Brazil) Birth Cohort Study.42 One study from America followed offspring from ages 9–14 years through ages 20–28 years, considering annual time points for 5 years and biennial time points thereafter.38 One study from Sweden used measurements taken at comprehensive health checks when the child was 4 years of age and used these to predict BMI on the child’s actual fourth birthday.37 Two studies utilized Swedish conscription data and only included outcome measurements for males.31,34
Across the 11 studies, there was one categorical and a number of continuous outcomes. Three studies considered more than one relevant outcome30,32,37 (Table 1). For studies other than the meta-analysis,40 these outcome measurements were taken by school nurses,30 military personnel,31,34 research centre staff,32,33 clinic staff35,37 and nurses.36 In one case, measurements were self-reported,38 and in another, mothers were provided with measuring tapes and instructions on how to take measurements.29
The four maternal exposures which emerged from this review are reviewed in turn below and summarized in Tables 1–3.
3.2. Length of the interpregnancy interval
Of the four studies, which considered the length of the interpregnancy interval as the exposure, one was judged to be good,31 two fair32,33 and one poor35 in terms of their risk of bias (Table 1).
Barclay and Kolk31 considered the long-term consequences of the length of both preceding and subsequent (not considered here) inter-pregnancy intervals on offspring health. They found that, compared with a birth interval of 25 to 30 months, men born after an interval of at least 31 months had a higher probability of being affected by overweight/obesity in young adulthood. Between-family analysis showed an increasing trend as the interpregnancy interval increased, and within-family analysis showed a broadly similar pattern31 (Table 3).
Both Devakumar et al.32 and Huttly et al.33 followed children whose mothers formed the 1982 Pelotas (Brazil) Birth Cohort Study,42 but both studies considered different outcome measurements and time points (Table 1). Huttly et al.33 reported mean weight-for height z-scores which generally increased with the length of the interval (Table 3). Devakumar et al.32 found a positive association for fat-free mass and visceral fat32 for female offspring where the birth interval was treated as a continuous variable and a positive association with offspring fat-free mass when the birth interval was treated as a binary variable (Table 3).
In contrast, Li et al.35 did not find an association between the length of the interpregnancy period and offspring BMI z-score at age 7 years.
These four studies had objectively measured outcomes,31–33,35 but in the case of two, the exposure was based on maternal recall.32,33 One study was limited to considering shorter birth intervals since the duration from recruitment to the last birth was 6 years.35
3.3. Maternal weight change
Three studies considered maternal interpregnancy weight change as the exposure and these were all judged to be fair29,37 or good30 in terms of their risk of bias (Table 1).
Adane et aI.29 found twice the odds of being affected by obesity where there was high interpregnancy weight gain (≥4 kg/m2) but reported no increase in odds of offspring obesity for interpregnancy weight loss (>1 kg/m2), small gain (1 to <2 kg/m2) or moderate gain (2 to <4 kg/m2)29 (Table 3).
Willmer et al.37 examined interpregnancy weight loss due to bariatric surgery between pregnancies. They found no association between the differences in BMI in week 10 of the pregnancy prior to, and the pregnancy after, surgery with differences in sibling’s BMI at age 4 years.
The primary focus of the paper by Aucott et al. was maternal smoking, but they also provide results for children being affected by overweight/obesity based on percentage maternal weight change between pregnancies.30 They found for a loss of ≥10% (compared with a change of ±3%) the odds of children being affected by overweight or obesity were increased by 30% and 90%, respectively, and that mean BMI z-score was also higher for a gain of ≥10% (compared with a change of ±3%)30 (Table 3).
The studies by Aucott et al.30 and Willmer et al.37 utilized routinely collected population level data in Scotland and Sweden respectively, and included objectively measured outcomes, but in the former, maternal and child records were linked in only 44% of cases while in the latter, full details were only available for 71 child–woman triads. All the exposure and outcome measurements in the Adane et al.29 study were self-reported, and only 34% of women responded to the survey asking for their children’s details.
3.4. Maternal smoking status
Three studies considered maternal smoking status as the exposure and these were judged to be good30,34 and fair40 in terms of their risk of bias (Table 1).
Aucott et al.30 found that siblings born after a mother had started smoking between pregnancies (i.e., she did not smoke in her first pregnancy but did in her second) had higher mean BMI z-scores at age 5 than older siblings who were unexposed30 (Table 3). BMI z-score was also higher in the younger sibling compared to the older sibling where a mother smoked in both pregnancies, but they found no significant difference where a mother quit smoking between pregnancies (i.e., where she was a smoker in the first but not the second pregnancy).30
Without sibling analysis, and compared to never smokers, mean offspring BMI z-score and the odds of overweight/obesity where mothers started smoking between pregnancies, always smoked or quit between pregnancies were higher30 (Table 3).
Iliadou et al.34 found increased odds of being affected by overweight only where the mother smoked in both male pregnancies, compared with mothers who did not smoke during either pregnancy34 (Table 3). The odds were not increased where a mother smoked only in her first or only in her second pregnancy. They found no effect where the association was evaluated within full and half sibling pairs.34
Albers et al.40 performed an individual patient data meta-analysis on five studies to analyse differences in the number of cigarettes smoked across successive pregnancies and the dose-dependent relationship with offspring BMI (Table 1). They found that each additional cigarette smoked a day in sibling pregnancies increased offspring BMI z-score40 (Table 3).
In both the Aucott et al.30 and Iliadou et al.34 studies, smoking status was self-reported at a single point during pregnancy. Three of the underlying studies used by Albers et al.40 had multiple self-reported measures of the numbers of cigarettes smoked during different stages of pregnancy and they used the maximum number at any time point in their analysis.
3.5. Mode of birth
Two studies considered mode of birth as the exposure and these were judged to be good36 and fair38 in terms of their risk of bias (Table 1).
Smithers et al.36 examined the association between mothers who had previously given birth by caesarean section and who subsequently went on to give birth vaginally or by elective caesarean with the second child’s BMI. They found no association between elective caesarean section for the subsequent birth and BMI z-score.36 Those who gave birth to their subsequent child by emergency caesarean, or who had an instrumental birth, were excluded from the analysis36 but over 85% of the subsequent deliveries were by caesarean section.
The association between caesarean section birth and the risk of offspring being affected by obesity also formed part of the study undertaken by Yuan et al.38 They compared siblings who were discordant in their mode of birth and found that amongst women with a previous caesarean birth, the risk of offspring being affected by obesity after a subsequent vaginal birth was reduced by 31% (compared to a repeat caesarean birth)38 (Table 3). For women who had a previous vaginal birth, the estimated risk of offspring being affected by obesity for a successive caesarean birth was not significant.38 Their study does not make a distinction between emergency and elective caesarean sections and includes no data on intrapartum indicators of caesarean birth, or any detailed labour/birth information.38
4. Discussion
This systematic review included eleven studies that assessed maternal interpregnancy weight change, differences in maternal smoking status, changes in mode of birth and the length of the interpregnancy interval between successive pregnancies in relation to adiposity in second or higher order siblings from age one onwards. Higher interpregnancy weight gain or loss, starting smoking between pregnancies, smoking at the first pregnancy only, or an increase in the number of cigarettes smoked during successive pregnancies and longer interpregnancy intervals showed a positive association. Vaginal birth after caesarean had a protective effect. To our knowledge, this is the first time that evidence on this subject has been systematically reviewed.
The quality assessment and overall rating for addressing the risk of bias has been judged to be either good or fair for all but one of the included studies (Table 1). The study judged as poor reported no results of significance.35 There was much variability in the confounders controlled for in the different analyses (Table 3). All but one40 of the included studies control for maternal age and the majority for maternal smoking29,35–38 and maternal weight in some form,29,30,32,34,35,38,40 where these were not the exposures under consideration. Most studies included at least one socio-economic factor.13,29,30,32–36,38,40 Only a minority of studies were able to take account of exposures in pregnancy such as diabetes and hypertension.35,36,38 Only one study included any adjustment for paternal confounders.34 Most studies did not specifically state which pregnancy the confounders related to.
A number of studies have made adjustments for covariates which are potential mediators between the exposure and offspring adiposity. Examples of such adjustments made in the included studies are breastfeeding,40 gestational weight gain34 and birthweight30,34–36,38 and both of the included studies which consider mode of birth adjust for birthweight in some form36,38 even though this is a potential mediator (Table 3). Children exposed to diabetes (including gestational diabetes mellitus) in utero are at high risk of childhood adiposity, although breastfeeding has been shown to attenuate this risk.43,44 Gestational weight gain has been shown to mediate the relationship between a mother’s BMI and offspring adiposity.45 Misclassification of confounders and mediators affects the magnitude of the total effect of the exposure on the outcome;46 both the direct effect, which is not through the mediator, and the indirect effect, through the mediator.47 Over-adjustment bias can affect the direct effect of the strength of associations and arises when controlling for variables which are on the causal pathway between exposure and outcome.48 Unnecessary adjustments of other variables on the causal pathway can affect the precision.47,48
All the studies were based on data from high-income or upper middle income countries, which will have had an effect on the prevalence of adiposity49 and individual risk factors, including the level of antenatal care,50 rates of birth by caesarean section51 and smoking.52 This limits our ability to generalize the findings to lower income countries. In addition, potential biases may have arisen because many of the included studies had low response rates to questionnaires or surveys asking for details about children,29 low rates of data linkage30,36,53 or only included male outcomes.31,34 Further, the data used in one study were relatively old, with only 6 years from recruitment to the final birth and with final outcome measurements taken in 1972.35
Most of the exposure and outcome measurements were objectively recorded by professionals, but where these were self-reported, for example, in the case of smoking status in pregnancy, potential information bias due to under-reporting54 may have arisen. Longitudinal cohort studies which use population level data and are linked to objectively measured child outcomes are needed to avoid bias in recruitment and in measurement. A number of the included studies have low response or linkage rates29,30,36 or only have details available for very small numbers of participants.37
This review has a number of strengths. The initial search was adapted and run through a variety of different online databases. It is unlikely that this search was too narrow since a very large number of studies were retrieved for consideration (Figure 2). Our search was, however, limited to studies published in English, and it is possible that there may be literature published in other languages which could have formed part of this review.
We did not include grey literature in our search, and there may therefore be an element of publication bias towards statistically significant associations.55 This does not appear to have been the case, as although most of the studies reported at least one non-null finding, a number only reported nonsignificant associations34–37 (Table 2).
Agreement between two authors (EJT and SW) at the screening stage of a random 10% sample of titles and abstracts was very high (98.9% and 100%), and two reviewers considered the full texts of studies remaining after abstract screening (EJT and SW). Data extraction and risk of bias assessments were undertaken by two authors (EJT and SW or EJT and NZ). We then followed a recent approach and listed strengths and weaknesses for each study using two commonly used checklists.18 This allowed identification of important criteria in each study.
There are a number of exposures, which are known to have an impact on offspring adiposity, which were not found in the literature searched for this review, for example, gestational weight gain,14 maternal prepregnancy stress,16 supplement intake and lower maternal vitamin D status,56 and measures of social and economic status such as education and area of residence.18 Further research into changes in exposures and into combinations and interactions between exposures and the association with the magnitude of the risk of children being affected by overweight/obesity is recommended. We specifically recommend studies which examine differences in vitamin D, folic acid and other vitamin supplementation and those which consider different levels of gestational weight gain. We do not know whether a change in exposure between pregnancies modifies the risk of adiposity for a second or subsequent child or whether the risk is based on past exposures. The World Health Organization recommends a birth to conception interval of at least 24 months in order to avoid a range of poor maternal, perinatal, neonatal and birth outcomes which are particularly associated with intervals under 18 months.8 A number of included studies have found associations between the longest interpregnancy intervals and childhood adiposity, and research which combines the effect of the length of the preceding birth interval with other changes in maternal exposures is also recommended.
5. Conclusion
Change in a number of exposures across successive pregnancies showed associations with greater adiposity in second or higher order siblings; substantial interpregnancy weight gain or loss, smoking in the first or second pregnancy only, or increasing the number of cigarettes smoked and longer interpregnancy intervals. Vaginal birth after a caesarean birth had a protective effect. There was considerable heterogeneity in the quality and methodology of the included studies.
Further research into changes in modifiable maternal exposures and behaviours between successive pregnancies is recommended to see if the risk of adiposity for second or subsequent children is fixed based on earlier predictors or if this risk changes as exposures and behaviours change.
Acknowledgements
This research is supported by an NIHR Southampton Biomedical Research Centre and University of Southampton Primary Care, Population Sciences and Medical Education PhD studentship to E. J. T. and an Academy of Medical Sciences and Wellcome Trust grant to N.A.A. (Grant AMS_HOP001\1060). K. M. G. is supported by the UK Medical Research Council (MC_UU_12011/4), the National Institute for Health Research (NIHR Senior Investigator (NF-SI-0515-10042), NIHR Southampton 1000DaysPlus Global Nutrition Research Group) and NIHR Southampton Biomedical Research Centre), the European Union (Erasmus+ Programme Early Nutrition eAcademy Southeast Asia-573651-EPP-1-2016-1-DE-EPPKA2-CBHE-JP), the US National Institute On Aging of the National Institutes of Health (Award No. U24AG047867) and the UK ESRC and BBSRC (Award No. ES/M00919X/1) and the British Heart Foundation (RG/15/17/3174). The research funders had no input on research design or on manuscript drafting. The authors wish to thank Paula Sands (Site and Research Engagement Librarian, University of Southampton) for her contribution to the search design.
Funding information
NIHR Southampton Biomedical Research Centre and University of Southampton Primary Care, Population Sciences and Medical Education; Academy of Medical Sciences and Wellcome Trust, Grant/Award Number: AMS_HOP001\1060
Footnotes
Conflict of Interest
K. M. G. has received reimbursement for speaking at conferences sponsored by companies selling nutritional products and is part of an academic consortium that has received research funding from BenevolentAIBio Ltd., Abbott Nutrition, Nestec and Danone. All other authors declare no conflicts of interest.
Author Contributions
N. A. A. conceived the review idea. All authors contributed to the design of the study and to the protocol, and all have edited and approved the final manuscript. E. J. T. was responsible for drafting the protocol, conducting the search, screening, quality assessment and drafting of the manuscript. S. W. and N. Z. contributed to screening, data extraction and quality assessment.
References
- 1.World Health Organisation. Obesity and Overweight. [Accessed July 19, 2019];2018 https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight.
- 2.Parsons TJ, Power C, Logan S, Summerbell CD. Childhood predictors of adult obesity: a systematic review. Int J Obes Relat Metab Disord. 1999;23(Suppl 8):S1–S107. [PubMed] [Google Scholar]
- 3.Umer A, Kelley GA, Cottrell LE, Giacobbi P, Jr, Innes KE, Lilly CL. Childhood obesity and adult cardiovascular disease risk factors: a systematic review with meta-analysis. BMC Public Health. 2017;17(1):683–683. doi: 10.1186/s12889-017-4691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui X, You L, Zhu L, et al. Change in circulating microRNA profile of obese children indicates future risk of adult diabetes. Metabolism. 2018;78:95–105. doi: 10.1016/j.metabol.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Lementowski PW, Zelicof SB. Obesity and osteoarthritis. Am J Orthop (Belle Mead NJ) 2008;37(3):148–151. [PubMed] [Google Scholar]
- 6.Paulis WD, Silva S, Koes BW, van Middelkoop M. Overweight and obesity are associated with musculoskeletal complaints as early as childhood: a systematic review. Obes Rev. 2014;15(1):52–67. doi: 10.1111/obr.12067. [DOI] [PubMed] [Google Scholar]
- 7.Gluckman PD, Hanson MA, Beedle AS. Early life events and their consequences for later disease: a life history and evolutionary perspective. Am J Hum Biol. 2007;19(1):1–19. doi: 10.1002/ajhb.20590. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organisation. Report of a WHO technical consultation on birth spacing: Geneva, Switzerland 13–15 June 2005 World Health Organization. World Health Organization; 2007. [Google Scholar]
- 9.Stephenson J, Heslehurst N, Hall J, et al. Before the beginning: nutrition and lifestyle in the preconception period and its importance for future health. The Lancet. 2018;391(10132):1830–1841. doi: 10.1016/S0140-6736(18)30311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleming TP, Watkins AJ, Velazquez MA, et al. Origins of lifetime health around the time of conception: causes and consequences. The Lancet. 2018;391(10132):1842–1852. doi: 10.1016/S0140-6736(18)30312-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aris IM, Bernard JY, Chen LW, et al. Modifiable risk factors in the first 1000 days for subsequent risk of childhood overweight in an Asian cohort: significance of parental overweight status. Int J Obes (Lond) 2018;42(1):44–51. doi: 10.1038/ijo.2017.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson SM, Crozier SR, Harvey NC, et al. Modifiable early-life risk factors for childhood adiposity and overweight: an analysis of their combined impact and potential for prevention. Am J Clin Nutr. 2015;101(2):368–375. doi: 10.3945/ajcn.114.094268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desai M, Beall M, Ross MG. Developmental origins of obesity: programmed adipogenesis. Curr Diab Rep. 2013;13(1):27–33. doi: 10.1007/s11892-012-0344-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol. 2007;196(4):322.e321–322.e328. doi: 10.1016/j.ajog.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rayfield S, Plugge E. Systematic review and meta-analysis of the association between maternal smoking in pregnancy and childhood overweight and obesity. J Epidemiol Community Health. 2017;71(2):162–173. doi: 10.1136/jech-2016-207376. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Olsen J, Vestergaard M, Obel C, Baker JL, Sørensen TIA. Prenatal stress exposure related to maternal bereavement and risk of childhood overweight. PLoS ONE. 2010;5(7):e11896. doi: 10.1371/journal.pone.0011896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuhle S, Tong OS, Woolcott CG. Association between caesarean section and childhood obesity: a systematic review and meta-analysis. Obes Rev. 2015;16(4):295–303. doi: 10.1111/obr.12267. [DOI] [PubMed] [Google Scholar]
- 18.Wilding S, Ziauddeen N, Smith D, Roderick P, Alwan NA. Maternal and early-life area-level characteristics and childhood adiposity: a systematic review. Obes Rev. 2019;20(8):1093–1105. doi: 10.1111/obr.12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Sayed AM, Scarborough P, Galea S. Socioeconomic inequalities in childhood obesity in the United Kingdom: a systematic review of the literature. Obes Facts. 2012;5(5):671–692. doi: 10.1159/000343611. [DOI] [PubMed] [Google Scholar]
- 20.Ziauddeen N, Wilding S, Roderick PJ, Macklon NS, Alwan NA. Is maternal weight gain between pregnancies associated with risk of large-for-gestational age birth? Analysis of a UK population-based cohort. BMJ Open. 2019;9(7):e026220. doi: 10.1136/bmjopen-2018-026220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grove G, Ziauddeen N, Harris S, Alwan NA. Maternal inter-pregnancy weight change and premature birth: findings from an English population-based cohort study. PLoS ONE. 2019;14(11):e0225400. doi: 10.1371/journal.pone.0225400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teulings NEWD, Masconi KL, Ozanne SE, Aiken CE, Wood AM. Effect of interpregnancy weight change on perinatal outcomes: systematic review and meta-analysis. BMC Pregnancy Childbirth. 2019;19(1):386–386. doi: 10.1186/s12884-019-2566-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Timmermans YEG, van de Kant KDG, Oosterman EO, et al. The impact of interpregnancy weight change on perinatal outcomes in women and their children: a systematic review and meta-analysis. Obes Rev. 2020;21(3):e12974. doi: 10.1111/obr.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.PROSPERO. International prospective register of systematic reviews. https://www.crd.york.ac.uk/prospero/
- 25.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:332–336.b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210–210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.If Sikich NN, Ye C. Quality control tool for screening titles and abstracts by second reviewer: QCTSTAR. 2015;06 [Google Scholar]
- 28.Cochrane Collaboration. Data extraction and assessment form. [Accessed 12 November 2018]; data collection form for intervention reviews for RCTs and non-RCTs.doc https://community.cochrane.org/sites/default/files/uploads/inline-files/ERC.
- 29.Adane AA, Dobson A, Tooth L, Mishra GD. Maternal preconception weight trajectories are associated with offsprings’ childhood obesity. Int J Obes (Lond) 2018;42(7):1265–1274. doi: 10.1038/s41366-018-0078-1. [DOI] [PubMed] [Google Scholar]
- 30.Aucott L, Bhattacharya S, McNeill G, Turner S. Differences in body mass index between siblings who are discordant for exposure to antenatal maternal smoking. Paediatr Perinat Epidemiol. 2017;31(5):402–408. doi: 10.1111/ppe.12386. [DOI] [PubMed] [Google Scholar]
- 31.Barclay KJ, Kolk M. Birth intervals and health in adulthood: a comparison of siblings using Swedish register data. Demography. 2018;55(3):929–955. doi: 10.1007/s13524-018-0673-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Devakumar D, Hallal PC, Horta BL, Barros FC, Wells JCK. Association between birth interval and cardiovascular outcomes at 30 years of age: a prospective cohort study from Brazil. Plos One. 2016;11(2):e0149054. doi: 10.1371/journal.pone.0149054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huttly SRA, Victora CG, Barros FC, Vaughan JP. Birth spacing and child health in urban Brazilian children. Pediatrics. 1992;89(6):1049–1054. [PubMed] [Google Scholar]
- 34.Iliadou AN, Koupil I, Villamor E, et al. Familial factors confound the association between maternal smoking during pregnancy and young adult offspring overweight. Int J Epidemiol. 2010;39:1193–1202. doi: 10.1093/ije/dyq064. [DOI] [PubMed] [Google Scholar]
- 35.Li S, Hua J, Hong H, Wang Y, Zhang J. Interpregnancy interval, maternal age, and offspring’s BMI and blood pressure at 7 years of age. J Hum Hypertens. 2018;32(5):349–358. doi: 10.1038/s41371-018-0035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smithers LG, Lynch JW, Mol BW, Jamieson L. Cesarean birth is not associated with early childhood body mass index. Pediatr Obes. 2017;12:120–124. doi: 10.1111/ijpo.12180. [DOI] [PubMed] [Google Scholar]
- 37.Willmer M, Berglind D, Sorensen TIA, Naslund E, Tynelius P, Rasmussen F. Surgically induced interpregnancy weight loss and prevalence of overweight and obesity in offspring. PLoS ONE. 2013;8(12):e82247. doi: 10.1371/journal.pone.0082247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan C, Gaskins AJ, Blaine AI, et al. Association between cesarean birth and risk of obesity in offspring in childhood, adolescence, and early adulthood cesarean birth and risk of obesity in offspring cesarean birth and risk of obesity in offspring. JAMA Pediatr. 2016;170(11) doi: 10.1001/jamapediatrics.2016.2385. e162385-e162385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Heart Lung and Blood Institute. Study quality assessment tools. [Accessed 15 January 2019]; https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- 40.Albers L, von Kries R, Sobotzki C, et al. Differences in maternal smoking across successive pregnancies - dose-dependent relation to BMI z-score in the offspring: an individual patient data (IPD) meta-analysis. Obes Rev. 2018;19(9):1248–1255. doi: 10.1111/obr.12698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stewart LA, Clarke M, Rovers M, et al. Preferred reporting items for a systematic review and meta-analysis of individual participant data: the PRISMA-IPD statement. JAMA. 2015;313(16):1657–1665. doi: 10.1001/jama.2015.3656. [DOI] [PubMed] [Google Scholar]
- 42.Barros FC, Victora CG, Horta BL, Gigante DP. Methodology of the Pelotas birth cohort study from 1982 to 2004-5, Southern Brazil. Rev Saude Publica. 2008;42(Suppl 2):7–15. doi: 10.1590/s0034-89102008000900003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crume TL, Ogden L, Maligie M, et al. Long-term impact of neonatal breastfeeding on childhood adiposity and fat distribution among children exposed to diabetes in utero. Diabetes Care. 2011;34(3):641–645. doi: 10.2337/dc10-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dugas C, Perron J, Kearney M, et al. Postnatal prevention of childhood obesity in offspring prenatally exposed to gestational diabetes mellitus: where are we now. Obes Facts. 2017;10(4):396–406. doi: 10.1159/000477407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Josey MJ, McCullough LE, Hoyo C, Williams-DeVane C. Overall gestational weight gain mediates the relationship between maternal and child obesity. BMC Public Health. 2019;19(1):1062. doi: 10.1186/s12889-019-7349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang T, Li H, Su P, et al. Sensitivity analysis for mistakenly adjusting for mediators in estimating total effect in observational studies. BMJ Open. 2017;7(11):e015640. doi: 10.1136/bmjopen-2016-015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pearl J. Proceedings of the seventeenth conference on uncertainty in artificial intelligence. Seattle; Washington: 2001. Direct and indirect effects; pp. 411–420. [Google Scholar]
- 48.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidimiology. 2009;20(4):488–495. doi: 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the global burden of disease study 2013. The Lancet. 2014;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moller A-B, Petzold M, Chou D, Say L. Early antenatal care visit: a systematic analysis of regional and global levels and trends of coverage from 1990 to 2013. Lancet Glob Health. 2017;5(10):e977–e983. doi: 10.1016/S2214-109X(17)30325-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Betran AP, Ye J, Moller AB, Zhang J, Gulmezoglu AM, Torloni MR. The increasing trend in caesarean section rates: global, regional and National Estimates: 1990-2014. PLoS ONE. 2016;11(2):e0148343. doi: 10.1371/journal.pone.0148343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lange S, Probst C, Rehm J, Popova S. National, regional, and global prevalence of smoking during pregnancy in the general population: a systematic review and meta-analysis. Lancet Glob Health. 2018;6(7):e769–e776. doi: 10.1016/S2214-109X(18)30223-7. [DOI] [PubMed] [Google Scholar]
- 53.Berglind D, Willmer M, Naeslund E, Tynelius P, Sorensen TIA, Rasmussen F. Differences in gestational weight gain between pregnancies before and after bariatric surgery: correlation with birth weight but not childhood BMI. Pediatr Obes. 2013;9(6):427–434. doi: 10.1111/j.2047-6310.2013.00205.x. [DOI] [PubMed] [Google Scholar]
- 54.Dukic VM, Niessner M, Pickett KE, Benowitz NL, Wakschlag LS. Calibrating self-reported measures of maternal smoking in pregnancy via bioassays using a Monte Carlo approach. Int J Environ Res Public Health. 2009;6(6):1744–1759. doi: 10.3390/ijerph6061744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paez A. Gray literature: an important resource in systematic reviews. J Evid Based Med. 2017;10(3):233–240. doi: 10.1111/jebm.12266. [DOI] [PubMed] [Google Scholar]
- 56.Crozier SR, Harvey NC, Inskip HM, et al. Maternal vitamin D status in pregnancy is associated with adiposity in the offspring: findings from the Southampton women’s survey. Am J Clin Nutr. 2012;96(1):57–63. doi: 10.3945/ajcn.112.037473. [DOI] [PMC free article] [PubMed] [Google Scholar]