Abstract
Selective amnesia for previously established memories can be induced by administering drugs that impair protein synthesis shortly after memory reactivation. Competing theoretical accounts attribute this selective post-retrieval amnesia to drug-induced engram degradation (reconsolidation blockade) or to incorporation of sensory features of the reactivation experience into the memory representation, hampering later retrieval in a drug-free state (memory integration). Here we present evidence that critically challenges both accounts. In contextual fear conditioning in rats, we find that amnesia induced by administration of midazolam (MDZ) after re-exposure to the training context A generalizes readily to a similar context B. Amnesia is also observed when animals are exposed to the similar context B prior to MDZ administration and later tested for fear to context B, but recovers when instead testing for fear to the original training context A or an equally similar but novel context C. Next to their theoretical implications for the nature of forgetting, our findings raise important questions about the viability of reconsolidation-based interventions for the treatment of emotional disorders.
Keywords: post-retrieval amnesia, reconsolidation, memory generalization, midazolam, prediction error
Under appropriate conditions, retrieval of a consolidated threat memory can return its representation into a labile state. In that state, memory is susceptible to interference by pharmacological agents or other amnestic interventions, a phenomenon known as post-retrieval (retrograde) amnesia (Beckers & Kindt, 2017). Historically, the fact that various post-retrieval manipulations can block the later expression of a threat memory has been explained by two rather different accounts of forgetting, which conceive of post-retrieval amnesia as the result of a storage or a retrieval deficit, respectively. The most widely supported explanation, the reconsolidation blockade account is a storage deficit view (Nader, Schafe, & Le Doux, 2000). According to this account, retrieval-induced protein degradation causes the destabilization of the neural engram into a labile (active) state, after which de-novo protein synthesis is required for the reconsolidation of the mnemonic trace into a stabile state (Nader & Hardt, 2009). Manipulations during the reconsolidation period that block protein synthesis or otherwise impair reconsolidation thus produce a long-lasting deficit in memory performance because they prevent the retention of the original memory (Miller & Matzel, 2000). An alternative view is represented by the memory integration account, which proposes that rather than inducing a need for protein-synthesis dependent reconsolidation, memory reactivation merely results in the integration of salient contextual and internal state features present around the time of retrieval (such as those induced by the administration of ‘amnestic’ drugs) into the memory representation (Gisquet-Verrier & Riccio, 2018). As a result, later memory retrieval becomes critically dependent on the presence of those contextual or state features that were present around the time of memory reactivation (Gisquet-Verrier et al., 2015). Therefore, the larger the congruity between reactivation and testing conditions, the less amnesia is observed (Tulving & Thomson, 1973).
The observation that it is possible to selectively block the expression of reactivated memories through targeted amnestic interventions has raised prospects of exploitation as a new treatment for conditions in which emotional memories play a central role, including anxiety disorders, post-traumatic stress disorder (PTSD) and substance use disorders (Kamboj & Das, 2017; Lee, Nader, & Schiller, 2017). Whereas insight in the conditions and mechanisms governing post-retrieval amnesia comes mostly from laboratory research in animals, proof-of-principle studies have established the phenomenon in experimental studies in humans (e.g., Kindt, Soeter, & Vervliet, 2009) and have suggested that it is governed by the same general principles in humans as in animals (e.g., Sevenster, Beckers, & Kindt, 2013, 2014). Moreover, a number of studies exploiting the logic of drug-induced amnesia in sub-clinical and clinical populations have reported promising findings (for reviews, see, Lonergan, Olivera-figueroa, Pitman, & Brunet, 2013; Walsh et al., 2018), although other trials building on the same logic have met with limited success (Das, Lawn, & Kamboj, 2015; Das, Walsh, Hannaford, Lazzarino, & Kamboj, 2018; Pachas et al., 2015; Saladin et al., 2013). There is a clear need for further insight from basic research into the conditions that constrain post-retrieval amnesia, in order to direct more successful translational efforts in the future.
One outstanding issue here concerns the generalization of post-retrieval amnesia. Memories can be triggered upon confrontation with cues that were present at the time of encoding but also by confrontation with stimuli or situations that merely resemble such cues. For instance, after a threat learning experience involving a particular situation A (Conditioned Stimulus, CS), perceptually similar situations will also come to elicit defensive responses indicative of fear (Dymond, Dunsmoor, Vervliet, Roche, & Hermans, 2015). While those generalization situations (GSs) thus clearly produce strong memory retrieval, it is at present unknown whether amnestic interventions applied after GS-based memory retrieval would impair memory expression to CSs. Conversely, it is unclear whether amnestic interventions applied after CS-elicited memory retrieval impair memory expression to CSs only or extend to GSs as well. The issue is of critical importance, if only because in clinical applications patients typically cannot be exposed to the actual circumstances of initial memory formation (e.g., an elevator in which they experienced a panic attack; a wild dog involved in a biting incident) but will be confronted with a generalization situation (a confined space; a different dog) to trigger memory retrieval instead. Likewise, after treatment, intervention success will be determined in large part by the extent to which future memory retrieval is suppressed not only for the actual situation used in treatment but also for other, similar situations (other small or unescapable spaces; new dogs).
The reconsolidation blockade and memory integration accounts of forgetting introduced above make divergent predictions regarding the generalization of amnesia. According to the reconsolidation blockade account, to the extent that exposure to a GS (e.g., situation B) reactivates the original threat association, the associative threat memory should become vulnerable to disruption by an amnestic intervention just like it would if it was retrieved through exposure to the original situation A. Administration of an amnestic agent should then serve to perturb the memory trace, resulting in diminished memory expression upon subsequent presentation of the original situation A, but also when presented with the GS B used for memory retrieval or with another GS C that is equally similar to A. Thus, amnesia should be observed in ABB (i.e., when B is used for memory reactivation and retention testing after threat learning involving A), but also in ABA (B is used for memory reactivation and A is used for retention testing after threat learning involving A) and ABC (B is used for memory reactivation and C is used for retention testing after threat learning involving A) (Duvarci & Nader, 2004).
According to the memory integration account, amnesia results from a mismatch between the conditions present around the time of memory reactivation and those present at the retention test. Therefore, the more the retention testing situation resembles the memory retrieval situation, the less amnesia should be observed. Thus, less amnesia should be observed in ABB than in ABA or ABC.
To put the divergent predictions from the reconsolidation blockade and memory integration accounts to test, we employed a contextual fear conditioning task in rats to associate a threat memory to context A and then used perceptually similar but discriminable reactivation and test contexts B and C to assess memory generalization. As amnestic agent, we used midazolam (MDZ), a positive allosteric modulator of the GABA-A receptor. It has been suggested that various intracellular signaling cascades that promote de novo protein synthesis, essential for the restabilization of contextual fear memory following memory destabilization, critically depend on a temporary reduction in GABAergic inhibitory transmission in the hippocampus (Makkar, Zhang, & Cranney, 2010). In line with this notion, MDZ, which counteracts such temporary reduction in GABAergic tone, has been shown to interfere with the retention of reactivated contextual fear memories (Bustos, Maldonado, & Molina, 2006; Piñeyro, Ferrer-Monti, Alfei, Bueno, & Urcelay, 2014; Stern, Gazarini, Takahashi, Guimarães, & Bertoglio, 2012; Zhang & Cranney, 2008). In two experiments, we established three contexts A, B and C that promoted strong fear generalization yet were clearly discriminable by the animals (Experiments 1 and 6). In the remaining 5 experiments reported here, we show that when using a context B for threat memory reactivation that elicits full generalization of defensive responding but is discriminable from the original training context A, post-retrieval administration of MDZ induces later amnesia for B but not for A or novel generalization context C. In contrast, amnesia induced by the administration of MDZ after memory reactivation using the original context A does generalize from A to B and C. Our results challenge the reconsolidation blockade as well as the memory integration account of post-retrieval amnesia in their generic form.
Experiment 1
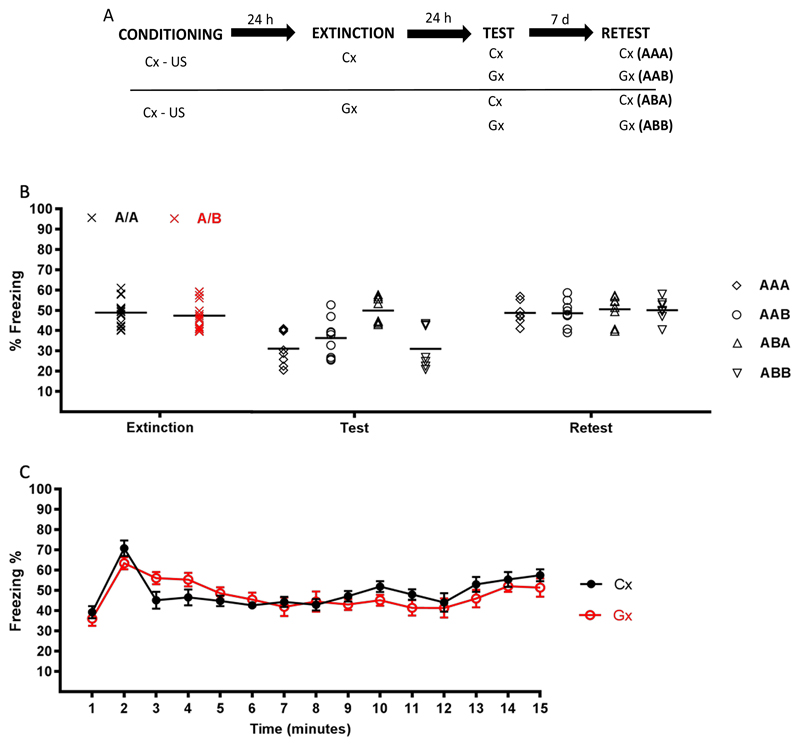
We first conducted an experiment to establish two contexts that would promote strong mutual fear memory generalization yet be clearly discriminable. To this aim, we tested whether in a contextual fear extinction procedure, when conducting extinction training using context B that was similar yet different from the initial fear acquisition context A, contextual fear responding would recover when animals are subsequently tested again in the initial training context A (confirming discrimination ability), despite strong generalization of conditioned fear responding from the end of acquisition to the start of extinction training. The top panel of Figure 1 depicts the experimental protocol. Four groups of animals were trained in a one-trial contextual fear conditioning protocol involving context A. Twenty-four hours later, extinction was carried out by giving the animals prolonged exposure to the same context A (AA groups) or to different context B (AB groups). Another 1 day and 7 days later, two groups of animals were tested for fear responding to the extinction context (AAA and ABB groups, respectively), whereas the other two groups were tested for renewal of fear responding by presenting them with the alternative context (AAB and ABA groups, respectively). Within each group, the physical contexts serving as A and B were fully counterbalanced across animals. Context A and B differed in their background odor, noise, illumination and wall color (see Methods for a detailed overview of the features contained by each context).
Figure 1.
Experiment 1. (A) Design. (B) Conditioned freezing behavior during extinction, retention test and retest, by group. Extinction results in reduced fear expression at test in the AAA, AAB and ABB groups but not in the ABA group. (C) The min-by-min pattern of freezing during the 15-min exposure to A or B (extinction session). Data are expressed as means (+/- SEM in panel C), in panel B symbols represent individual data points.
Methods
All procedures performed were approved by the animal ethics committees at UNC and KU Leuven and were in accordance with the Belgian Royal Decree of 29/05/2013 and European Directive 2010/63/EU.
The design, sample size and analyses for Experiment 1 were preregistered on aspredicted.org, see http://aspredicted.org/blind.php?x=3u53ui. For some of the experiments in the current paper, statistical analyses reported here deviate slightly from the preregistered analyses for reasons of consistency between experiments. However, all preregistered analyses were performed and can be found on the project’s Open Science Framework page at https://osf.io/fqajx/. Of note, these analyses yield the same conclusions as the ones presented here.
Sample size determination
This experiment used the same training and extinction parameters as used in Alfei et al. (2015, Experiment 7), where large effects were observed for the comparison between a regular extinction test and various extinction recovery tests (either within-groups or between-groups; f = 3.71 – 4.12). Our preregistered sample size of 8 animals per group (N total = 32) for the current experiment should allow to detect an effect of similar size with a power of well above .90 (calculated using G*Power, version 3.1.9.4; Faul, Erdfelder, Lang, & Buchner, 2007).
Subjects
In this and all following experiments, subjects were experimentally naive male Wistar rats (60-65 days old, weighing 290 - 340 g at the start of training) obtained from Centro de Medicina Comparada (Esperanza, Santa Fe, Argentina). They were housed in groups of 4 in standard laboratory Plexiglas cages (60 cm long x 40 cm wide x 20 cm high) in a climate-controlled animal room in the Laboratorio de Psicología Experimental, Facultad de Psicología, Universidad Nacional de Córdoba, Argentina. For each experiment, new animals were obtained from the supplier and housed in the vivarium for at least 11 days before the start of the experiment to allow acclimation. Food and water were available ad libitum. Animals were maintained on a 12-h light/dark cycle (lights on at 8 a.m.) at a room temperature of 21°C.
Apparatus
Contextual Fear Conditioning (CFC) was conducted in a 250 x 250 x 250 mm chamber with 2 modular and removable grey opaque aluminum walls, a transparent Plexiglas ceiling and rear wall and a hinged front door (PanLab, Harvard Apparatus, US, controlled by PackWin V2.0 software). The floor consisted of 18 parallel stainless-steel rods, each measuring 4 mm in diameter, spaced 1.5 cm apart and connected to a device to provide adjustable footshocks (Unconditioned Stimulus, US). Variations in background odor, white noise, ventilation fan operation, house lights and wall color were employed to create distinct contexts. Recording of behavior (for offline analysis) was done with digital video cameras mounted in front of the conditioning chamber. The chamber was enclosed in a sound-attenuating cubicle in a well-lit sound-attenuated experimental room.
Context 1
One context contained a standard grid floor, uniformly colored walls and a white house light (three 5500K – 6500K LEDs with a 120˚ beam angle) mounted to the upper middle part of the left wall. A ventilation fan (65 dB) located in the upper back part of the cubicle was turned on and the chamber was cleaned with 80% ethanol prior to the session. The testing room light remained on throughout.
Context 2
The alternative context contained the same standard grid floor, a grey opaque left wall and vertically striped (black/white) right wall. An infrared light (three bright 850nm/940nm wavelength LEDs with a 120˚ beam angle) mounted to the upper middle part of the right wall was turned on. The white house light and the ventilation fan were turned off. The chamber was cleaned with a household cleaning product prior to the session and the drop pan below the grid floor was scented with a thin layer of the same product. The testing room light remained on throughout.
Procedure
Figure 1 (top panel) depicts the experimental design. Four groups of animals received contextual fear conditioning to context A. Twenty-four hours later the animals were given 15 min of non-reinforced exposure to the A or B context (extinction training). One day and again 1 week after the extinction session, animals were given an extinction retention test in the A or B context. Animals were randomly assigned to groups (AAA: n = 8, AAB: n = 8, ABA: n = 8, ABB: n = 8).
In all experiments, rats were first individually labelled, weighed (one day before the start of handling and on the fourth day of handling) and handled for 5 min on four separate days to habituate them to the experimenter. Handling was performed in a different room than the one used for conditioning. Two contexts were created as described above and used interchangeably as A (original context) and B (generalization context). The assignment of the different physical contexts to the roles of A and B was fully counterbalanced in this and all subsequent experiments. Transportation from the animal room to the experimental rooms always took place in a yellow plastic box filled with bedding and covered with a white cloth. All procedures were performed during the light phase of the diurnal cycle, between 10.00 am and 6.30 pm.
Contextual Fear Conditioning (CFC)
24 h after the last day of handling, rats were taken individually from their home cage and transported to the conditioning chamber. The animals were exposed to context A for 1 min after which two footshocks (1.0 mA, 3-s duration, with an inter-shock interval of 30 s) were delivered. The total length of the CFC session was 1 min 36 s (Alfei, Ferrer-Monti, Molina, Bueno, & Urcelay, 2015; Ferrer-Monti et al., 2017).
Extinction
Extinction training always occurred 24 h after conditioning. Rats were exposed to context A or B during 15 minutes without footshock.
Retention Test
Fear retention was tested 24 h after extinction training. It consisted of a 5-min exposure to the chamber, without shocks. Animals were either exposed to the same context as used for extinction (AAA and ABB groups) or to a different context (AAB and ABA groups).
Retest
7 days after the retention test, a second test was conducted to evaluate spontaneous recovery of fear responding (Bouton, 2004). It consisted of a 5-min exposure to the same context as used for retention testing.
Scoring of freezing behavior
In this and all following experiments, freezing was used as index of fear memory expression. It was defined as the total absence of body and head movements except for those associated with breathing. Given that previous reports have shown that software-scored freezing cannot reliably be compared across different contexts (Luyten, Schroyens, Hermans, & Beckers, 2014), freezing was scored manually, min-by-min, with a stopwatch and expressed as percentage of time. For all but one of the experiments below, percentage of freezing per min during memory reactivation (non-reinforced memory retrieval, retraining or extinction) and retention test was scored by two experienced raters and inter-observer reliability was found to be very high (Pearson’s r = .95). Researchers were blinded to pharmacological treatments (but not physical contexts) during all behavioral testing procedures and fully blinded to group allocation during scoring of freezing behavior (all data files were randomized prior to scoring of freezing behavior).
Exclusion Criteria
The pre-specified behavioral exclusion criterion stated that animals showing less than 20% freezing during the first 5 minutes of the extinction session were considered as non-learners. In addition, some animals were excluded due to technical issues such as apparatus malfunction (USs were not delivered) or because the footshocks during training were blocked by the presence of a rat’s faeces on the grid floor. The exclusions are listed by experiment and group in Appendix A. Importantly, for all the experiments reported in the current paper, excluded animals were replaced in order to obtain the prespecified group sizes. All animals were given a number upon arrival in the lab, and replacement animals were included consecutively, following this numbering, until the preregistered sample size was reached in each experiment.
Data Analysis
Results in this and the following experiments are expressed as mean +/- the standard error of the mean (SEM) percentage of time the animals spent freezing. Data were analyzed by means of independent-samples t-tests (extinction session) or ANOVAs (test and retest sessions) and effect sizes calculated as Cohen´s d (for t-tests) or η2p (ANOVAs). Significant one-way ANOVAs were followed up with Tukey tests. In case of deviation from normality, non-parametric Mann-Whitney U-tests were used rather than t-tests, and rank biserial correlation (rpb) was used to estimate effect sizes. Min-by-min mixed ANOVAs were used to assess temporal control of behavior during the extinction session. Deviations from sphericity were corrected using Greenhouse-Geisser correction if ε < 0.75 and Huynh-Feldt correction if ε > 0.75. When there was a specific within-subjects effect, Tukey post-hoc tests based on specific one-way ANOVAs are reported to compare the second minute with the fourth and fifth minutes during the test (Alfei et al., 2015).
All statistical analyses for the present and following experiments were carried out using JASP 0.9.0.1 (JASP Team, 2018) and all graphs were made with GraphPad Prism 7 (GraphPad Software Inc, La Jolla, CA, USA).
Results and Discussion
Raw data for this and the following experiments are available on the Open Science Framework at https://osf.io/fqajx/. Animals that received extinction of CFC through exposure to the initial training context A showed almost equal expression of extinction when tested in generalization context B (AAB group) as when tested in the initial context A (AAA group). Animals that received extinction of CFC through exposure to generalization context B likewise showed clear expression of extinction when subsequently tested for fear for context B (ABB group). However, animals that received extinction of CFC through exposure to the generalization context B showed preserved expression of contextual fear when tested in initial training context A (ABA renewal)1 (see Fig. 1B). Analysis of freezing during the extinction phase revealed no difference between the AA and AB groups, u = 152, p = .37, rpb = .18. Analyses on the retention test and retest data yielded an effect of group for the test phase (F (3,28) = 8.11, p < .001, η2p = .46) but not the retest phase (F (3,28) = .09, p = .96, η2p = .01). Planned comparisons revealed that the ABA group showed significantly higher levels of freezing than the other three groups at test (p < .02 for all comparisons). The strong renewal of fear responding when tested in A after extinction to B indicates that the animals readily discriminated between A and B. The difference between the ABA group and the other groups was not retained on the retest one week later, due to spontaneous recovery in the latter groups, which is consistent with previous extinction findings (Bouton, 2004).
The min-by-min pattern of freezing during the 15-min exposure to A or B (extinction session, see Fig. 1C) revealed strong generalization of contextual fear from A to B. A mixed ANOVA on total freezing behavior during extinction (group x time as factors) revealed no effect of group (F (1,15) = .14, p = .71, η2p = .009), a significant effect of time (F (11.91,175.09) = 9.40, p < .001, η2p = .38), and no interaction (F (11.67,175.09) = 1.55, p = .11, η2p = .09). Planned comparisons revealed that levels of freezing were higher during the 2nd min of extinction than during the 4th and 5th min (p <.01 in both cases; η2p = .21 and η2p = .38 for the comparison of min 2 vs 4 and 2 vs 5, respectively). The temporal pattern of conditioned freezing during extinction training for the generalization context indicates a temporally well-defined expectation of the US during extinction, which is as precise and strong as for the original training context, suggesting a similar degree of expectancy violation or prediction error during exposure to the generalization context as during exposure to the original training context. It is worth noting that the 15 min extinction session did not result in a strong within-session attenuation of the CR (also see Fig. 6C). Extinction learning was evident however from the clear reduction of CR at test, which recovered spontaneously a week later. A lack of within-session attenuation of CR during extinction training is in line with other studies using single-session extinction training in rodents (Cain, Blouin, & Barad, 2003) and humans (Tsao & Craske, 2000) and with previous findings in our laboratory (Alfei et al., 2015; Ferrer-Monti et al., 2017). Of note, within-session reduction of fear during extinction training does not necessarily predict between-session fear extinction as expressed on subsequent tests (Craske, Treanor, Conway, Zbozinek, & Vervliet, 2014).
Taken together, our data indicate that despite full generalization from context A to B, evident in the ability of context B to support full expression of the contextual fear memory acquired in A and to promote similar within-session fear extinction as exposure to A, there is a strong fear recovery when animals extinguished to B are re-exposed to context A (ABA group). This renewal effect demonstrates that, despite the strong generalization between them, A and B constitute discriminably different contexts for the animals. The results of the present experiment are consistent with previous demonstrations in animals and humans of renewal after a change in context or stimulus features (Boddez et al., 2012; Bouton & Bolles, 1979; Bouton & King, 1983; Vervliet, Vansteenwegen, Baeyens, Hermans, & Eelen, 2005) and fit with the notion that extinction training promotes new (context-dependent) inhibitory learning, rather than the modification of the original fear memory (Bouton, 2002). More important for the present purposes, our results provide the basis for exploring in the following experiments whether a contextual fear memory involving context A that is reactivated through exposure to context B would nonetheless become malleable and sensitive to disruption by injection of an amnestic agent, as may be expected on the basis of the reconsolidation blockade account of post-retrieval amnesia.
Experiment 2
The goal of Experiment 2 was to evaluate whether amnesia for contextual fear, as can be produced by administering an amnestic agent (MDZ) after re-exposure to the training context, can also be achieved by exposing animals to a generalization context known to support strong memory retrieval (i.e., the context B established in Experiment 1) rather than to the original training context (A) prior to MDZ administration.
Previous research suggests that, to render a memory vulnerable to an amnestic intervention upon retrieval, the retrieval experience needs to be accompanied by an appropriate degree of prediction error (PE), understood as a discrepancy between expected and actual events at the time of retrieval (Exton-McGuinness, Lee, & Reichelt, 2015; Fernández, Boccia, & Pedreira, 2016; Krawczyk, Fernández, Pedreira, & Boccia, 2017). We previously demonstrated in rats (Alfei et al., 2015; Ferrer-Monti et al., 2017) and humans (Sevenster, Beckers, & Kindt, 2012; Sevenster et al., 2013, 2014) that in a Pavlovian conditioning situation, memory malleability is obtained upon memory retrieval when either (a) the temporal relationship between the conditioned stimulus (CS) and unconditioned stimulus (US) is modified relative to training or (b) if the US is omitted altogether at retrieval. Critically, however, in all those studies, the same cue or context (A) that was presented as CS during initial training was used as the memory reactivation stimulus (Beckers & Kindt, 2017).
According to an unqualified version of the reconsolidation blockade view presented in the introduction, if a generalization situation (GS) effectively and fully retrieves the original CS memory, and provided that this memory retrieval is accompanied by an appropriate degree of PE, the memory should become sensitive to disruption; subsequent administration of an amnestic agent should then block retention of the engram. Based on previous work (Alfei et al., 2015; Ferrer-Monti et al., 2017), we expected that a 2-min non-reinforced exposure to the context (be it original training context A or generalization context B) during the reactivation session should be sufficient to trigger the detection of a relevant difference with the training experience (in which the US was presented in min 2). We assessed whether administration of MDZ after non-reinforced reactivation using a GS (context B) would cause a similar memory deficit (amnesia) for the original CS (context A) at test (ABA) as would administration of MDZ after non-reinforced reactivation using the CS (context A).
Method
Contextual fear conditioning was conducted similarly as in Experiment 1, but instead of a 15-min extinction session, a 2-min reactivation session followed by administration of MDZ or saline was conducted 24 h after contextual fear conditioning. The subsequent retention test was supplemented by a memory reacquisition and retest procedure (see below for details). Scoring of freezing behavior and exclusion criteria were the same as for Experiment 1. The design, sample size and analyses of Experiment 2 were preregistered on aspredicted.org, see http://aspredicted.org/blind.php?x=wx98u2.
Sample size determination
Based on the effect size of 1.80 (Cohen’s d) for the significant difference in responding between an MDZ and SAL group in a study using similar procedures and parameters as used here (Alfei et al., 2015, Experiment 5), we preregistered a sample size of 8 animals per group for Experiments 2-5, which should yield a power of .92 to detect a similar effect in pair-wise comparisons (calculated using G*Power, version 3.1.9.4; Faul, Erdfelder, Lang, & Buchner, 2007).
Procedure
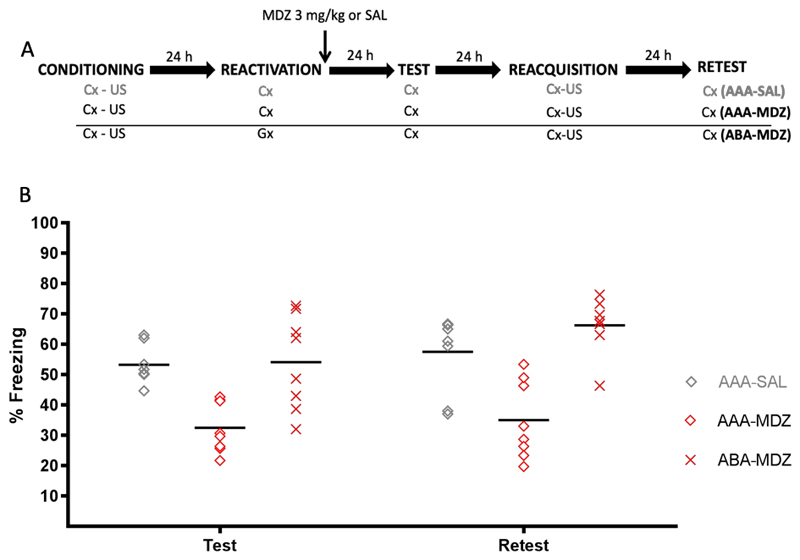
The top panel of Fig. 2 depicts the experimental design. Three groups of rats received contextual fear conditioning (CFC) in context A. Twenty-four h later, two groups received memory reactivation through re-exposure to context A, immediately followed by injection of MDZ or SAL. A third group of animals received memory reactivation through exposure to context B, which was capable of retrieving the CFC memory with the same strength and temporal precision as context A (see Experiment 1, Fig. 1B-C), immediately followed by injection of MDZ. All groups then received a retention test (identical to that in Experiment 1) followed by reacquisition and retest (identical to the first retention test), all in context A, on consecutive days. Which contexts served as A and B was fully counterbalanced. Animals were randomly assigned to groups (AAA-MDZ: n=8, AAA-SAL: n=8, ABA-MDZ: n=8).
Figure 2.
Experiment 2. (A) Design. (B) Conditioned freezing behavior during retention test and retest, by group. Amnesia was observed in the AAA-MDZ group (relative to the AAA-SAL group). No attenuation in memory expression was observed in the ABA-MDZ group. Data are expressed as means, symbols represent individual data points.
Memory Retrieval
Memory reactivation always occurred 24 h after conditioning. Rats were exposed to context A or B during 2 min without footshock.
Drug Administration
Midazolam (MDZ, Gobbi Novag SA, Buenos Aires, Argentina) was diluted in sterile isotonic saline (SAL, 0.9% w/v) to a concentration of 3 mg/ml and administered intraperitoneally (i.p.). The total volume of drug or equivalent amount of SAL was 1.0 ml/kg in all cases. Injections were given immediately after the reactivation session. Rats were also injected with 1ml/kg SAL at the end of the second handling session, to habituate them to the injection procedure. The room where the injections were given was different from the conditioning room. The dose of 3 mg/kg MDZ was previously demonstrated to block the retention of reactivated contextual fear memories in Wistar rats (Alfei et al., 2015; Bustos et al., 2006; Ferrer-Monti et al., 2016; Monti et al., 2017; Piñeyro et al., 2014).
Reacquisition
Contextual fear for context A was reconditioned, 24 h after the retention test. Contextual fear conditioning consisted of a 1 min 33 s pre-shock period followed by a single mild (0.5 mA) 3-s footshock, after which rats were immediately removed from the chamber and taken back to their home cages in the animal room. The number and intensity of shocks was reduced relative to initial training of A to prevent ceiling effects of fear responding during the subsequent test from masking any differences between treatments. Rapid and strong reacquisition typically occurs when the reinforcer is presented at full strength again in the original training context (Bouton, 2002).
Data Analysis
Reactivation, test, reacquisition and retest data were analyzed by means of ANOVAs and effect sizes calculated as η2p. Significant one-way ANOVAs were followed up with Tukey tests. In case of deviation from normality, nonparametric one-way ANOVAs (Kruskal-Wallis test) were used, followed up by post-hoc analysis with Dunn’s test.
Results and Discussion
The bottom panel of Figure 2 shows memory performance during the retention test and retest (data of the reactivation and reacquisition phases for this and following experiments are in Appendix B and C, respectively). A nonparametric one-way ANOVA (Kruskal-Wallis test) on the retention test data yielded a significant effect of group (H (2) = 12.68, p = .002), and Dunn’s post-hoc test indicated that the animals in the AAA-MDZ group exhibited significantly less freezing than those in the AAA-SAL and ABA-MDZ groups (p < .002 for both comparisons). A one-way ANOVA on the retest data similarly found a significant group effect, F (2,21) = 15.39, p < .001, η2p = .59. Post-hoc comparisons showed the same pattern as in the test phase, with the AAA-MDZ group expressing significantly less freezing than the AAA-SAL and ABA-MDZ groups (p < .002 for both comparisons). We thus replicate previous results of our lab showing that a 2-min re-exposure to the original training context is sufficient to trigger memory malleability, as inferred from the long-lasting deficit in memory performance in the AAA-MDZ group relative to the AAA-SAL group (Alfei et al., 2015; Ferrer-Monti et al., 2017). Critically, the results indicate no attenuation of contextual fear responding in the ABA-MDZ group.
The absence of a memory deficit in the ABA-MDZ group is open to at least two explanations. Two minutes of non-reinforced exposure to context B may have been sufficient to trigger a PE signal, due to the difference in conditions between training (i.e., presentation of the US during the 2nd minute) and reactivation (i.e., absence of US at the expected time). Memory malleability may thus have been triggered, but the amnestic effect may have failed to generalize to the original training context A. That is, upon presentation of context A at test, the MDZ-induced amnesia was disrupted by renewal. If this is the case, presenting context B rather than A at test should reveal a memory deficit (ABB). Alternatively, memory malleability may not have been triggered in the generalization context at all, perhaps due to a lack of sufficient PE. In the next experiment, we attempted to discriminate between these two possibilities.
Experiment 3
In light of the failure to obtain a memory impairment in the ABA-MDZ group in Experiment 2 (see Fig. 2B), we next set out to determine whether this result was due to an overall inability to trigger memory vulnerability to an amnestic intervention by means of exposure to generalization context B or whether it was due to the return to the original training context A. To this aim, we tested whether an amnestic effect would be observed when reactivation and retention testing both involved the generalization context (ABB). In addition, we examined whether MDZ-induced amnesia could be generalized towards a generalization context at all (AAB).
Method
The general methods for contextual fear conditioning, memory retrieval, drug administration, retention test, reacquisition and retest, as well as scoring of freezing behavior and exclusion criteria were the same as for Experiment 2. The design, sample size and analyses of Experiment 3 were preregistered on aspredicted.org, see http://aspredicted.org/blind.php?x=br9tw3.
Procedure
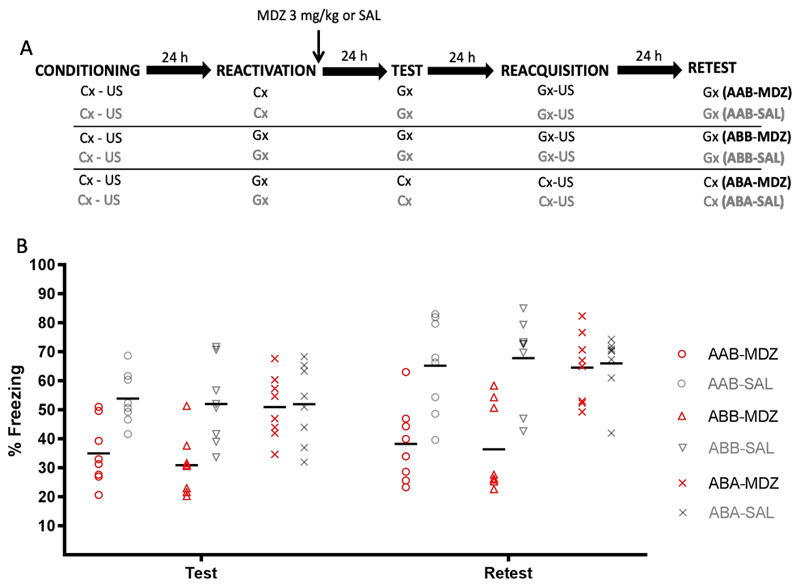
The top panel of Fig. 3 depicts the experimental design. Six groups of rats received CFC to context A. Twenty-four h later, two groups received memory reactivation through re-exposure to context A. The other 4 groups received memory reactivation through exposure to a context B that, although discriminable from context A, was capable of eliciting the contextual fear memory with the same strength and precision as context A (see Fig 1B-C). Immediately after reactivation, half of the groups were injected with MDZ (3 mg/kg, i.p.) whereas the other group were injected with SAL (i.p.). The first four groups then received a retention test followed by reacquisition and retest, all in context B, on consecutive days. In the other 2 groups, the retention test, reacquisition and retest were conducted for the original training context A. Which contexts served as A and B was fully counterbalanced. Animals were randomly assigned to groups (AAB-MDZ: n = 8, AAB-SAL: n = 8, ABB-MDZ: n = 8, ABB-SAL: n = 8, ABA-MDZ: n = 8, ABA-SAL: n = 8).
Figure 3.
Experiment 3. (A) Design. (B) Conditioned freezing behavior during test and retest, by group. Rats that received reactivation through exposure to the initial training context and given MDZ showed a generalized memory impairment when tested in novel context B (AAB-MDZ). A similar memory deficit was observed in animals that received reactivation through exposure to context B prior to MDZ administration and were then tested in B (ABB-MDZ). However, when after memory reactivation using B, rats were re-exposed to the originally trained context (ABA groups), no MDZ-induced amnesia was found. Data are expressed as means, symbols represent individual data points.
Data Analysis
Reactivation data were analyzed by means of independent-samples t-tests and effect sizes calculated as Cohen´s d. Test, reacquisition and retest data were analyzed by means of ANOVAs and effect sizes calculated as η2p. Factorial ANOVAs were followed up with planned t-tests comparing MDZ- versus SAL- treated animals per group.
Results and Discussion
Figure 3 (panel B) shows memory performance during the retention test and retest. A factorial ANOVA on the retention test data (group x treatment as factors) revealed no main effect of group (F (2,42) = 3.17, p = .052, η2p = .13), a significant main effect of treatment (F (1,42) = 16.90, p < .001, η2p = .28), and a group x treatment interaction (F (2,42) = 3.66, p = .03, η2p = .14). Planned comparisons indicated that MDZ produced a memory deficit in the AAB-MDZ group (relative to AAB-SAL), t (14) = 3.81, p = .002, d = 1.90, and in the ABB-MDZ group (relative to ABB-SAL), t (14) = 3.44, p = .004, d = 1.72, whereas no effect was observed for the ABA groups, t (14) = .16, p = .87, d = .08. A factorial ANOVA on the retest data revealed main effects of group (F (2,42) = 4.98, p = .01, η2p = .19) and treatment (F (1,42) = 24.90, p < .001, η2p = .37), and a group x treatment interaction (F (2,42) = 5.42, p = .008, η2p = .20). In line with the retention test data, planned comparisons indicated that MDZ-treated animals expressed significantly less freezing than SAL-treated animals in the AAB and ABB groups, t (14) = 3.63, p = .003, d = 1.81 and u = 6, p = .007, d = .81, respectively, but not in the ABA group, t (14) = .02, p = .98, d = .01.
Taken together, those data demonstrate that retrieval-induced memory vulnerability to an amnestic intervention can effectively be triggered by exposure to a generalization situation (ABB-MDZ), but the resulting MDZ-induced amnesia fails to generalize towards the originally trained context (ABA-MDZ). In contrast, when the memory is reactivated by exposure to the original training context, MDZ-induced amnesia does generalize readily to the generalization situation (AAB-MDZ). Collectively, these results would seem to pose a challenge for the rivalling accounts of forgetting mentioned in the introduction, at least in their generic form. The lack of amnesia in an ABA procedure appears at odds with the reconsolidation blockade account, whereas the strong amnesia observed in the ABB procedure does not fit readily with a memory integration account of post-retrieval amnesia. In the next experiments, we evaluated whether the result of Experiments 2 and 3 could be accounted for by the unique features of A, which had not undergone reactivation through exposure to B, supporting sufficiently strong memory retrieval upon test in A to promote full recovery of contextual fear.
Experiment 4
Given that context B shares only some of its features with the original training context A, exposure to B will result in the direct, first-order retrieval of those shared features’ association with the US only; the association of A’s unique features to the US will be retrieved only indirectly (i.e., through their link with the directly retrieved US or through pattern completion). It has been suggested that manipulations that interfere with reconsolidation will affect memory associations retrieved through first-order relations only (Debiec, Doyere, Nader, & LeDoux, 2006; Debiec, Diaz-Mataix, Bush, Doyère, & LeDoux, 2013). Therefore, one could argue that MDZ administration in Experiments 2 and 3 affected only the association of the shared features of A and B with the US, leaving the association of A’s unique features with the US unaffected. This might have been sufficient to drive the strong memory retrieval observed when animals were subsequently tested in A (ABA recovery; Fig. 2-3).
Instead of non-reinforced exposure to the CS, also reinforced exposure to the CS can be used to induce memory reactivation and the ensuing vulnerability to amnestic interventions (Debiec, Díaz-Mataix, Bush, Doyère, & Ledoux, 2010; Zeng et al., 2014). Given that the relative timing of the CS and US is encoded during acquisition, a shift in this timing during reactivation can provide sufficient PE to trigger sensitivity to interference even when the CS is effectively followed by the US (Alfei et al., 2015; Díaz-Mataix, Ruiz Martinez, Schafe, Ledoux, & Doyère, 2013; López et al., 2016; Tallot et al., 2017). Critically, US presentation during retrieval should serve to directly retrieve all elements that were associated with the US during initial fear training, including also A’s unique features (Dębiec, Díaz-Mataix, Bush, Doyère, & Ledoux, 2010; Díaz-Mataix, Dębiec, LeDoux, & Doyere, 2011; Liu et al., 2014; Luo et al., 2015; Xue et al., 2017). We first tested the effectiveness of this procedure in an ABB procedure.
Method
The general methods for contextual fear conditioning, drug administration, retention test, reacquisition and retest, as well as scoring of freezing behavior and exclusion criteria were the same as for Experiments 2 and 3. However, instead of non-reinforced context exposure, animals were submitted to a retraining procedure for memory retrieval (see below for details). The design, sample size and analyses of Experiment 4 were preregistered on aspredicted.org, see http://aspredicted.org/blind.php?x=up5yz9.
Procedure
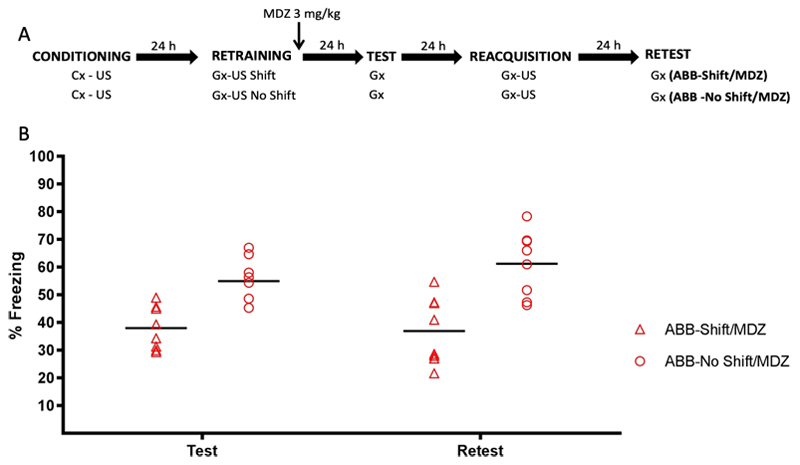
Figure 4 (panel A) depicts the experimental design. All rats received CFC to context A. Twenty-four h later both groups were given a reminder/retraining session involving context B. The timing of US presentation was identical as during initial training in group ABB-No Shift/MDZ (and therefore consistent with the animals’ peak in conditioned freezing, see Fig 1C), but was shifted in time in group ABB-Shift/MDZ (thereby violating training-based expectancies). All animals were injected with MDZ (3 mg/kg, i.p.) immediately after the reminder/retraining session. Twenty-four h later both groups received a retention test followed by reacquisition and retest, all in context B, on consecutive days. Animals were randomly assigned to groups (ABB-Shift/MDZ: n = 8, ABB-No Shift MDZ: n = 8).
Figure 4.
Experiment 4. (A) Design. (B) Conditioned freezing behavior during test and retest, by group. Animals in the ABB-Shift/MDZ group expressed significantly less fear than those in the AAB-No Shift/MDZ group at test and retest. Data are expressed as means, symbols represent individual data points.
Memory Retrieval
Rats received additional US exposure during a retraining session in context B. In the ABB-No Shift condition, this retraining was identical to the CFC session one day earlier. In the Shift group, both US onsets occurred 30 s earlier than during initial training. Since all animals were removed from the chamber immediately after the second US, session duration for the Shift condition was 30 s shorter than for the No Shift condition. We have previously demonstrated in an AAA protocol that changing the retraining parameters induces a US temporal prediction-error (Alfei et al., 2015), a finding that has also been observed in other procedures (Díaz-Mataix et al., 2013; Tallot et al., 2017) and species (López et al., 2016; Sevenster et al., 2013).
Data Analysis
Reactivation, test, reacquisition and retest data were analyzed by means of independent-samples t-tests and effect sizes calculated as Cohen´s d.
Results and Discussion
The bottom panel of Fig. 4 depicts memory performance during the retention test and retest. T-tests on the retention test (t (14) = 4.22, p = .001, d = 1.96) and retest data (t (14) = 4.08, p = .001, d = 2.04) revealed that the ABB-Shift/MDZ group expressed significantly less freezing than the ABB-No Shift/MDZ group. Critically, this suggests that a shift in the timing of US administration during retrieval generates a temporal PE about the US when a contextual fear memory is retrieved through reinforced presentation of a generalization context. When the timing of the US during retrieval matches the original contextual fear learning episode (no PE), however, mere memory retrieval is obtained.
Overall, these data support the notion that the occurrence of memory vulnerability to MDZ upon retrieval in a generalization situation is critically a PE-driven process, as is the case for memories retrieved by exposure to the original training situation (Alfei et al., 2015; Sevenster et al., 2013). Importantly, given that the presentation of the US in a generalization context should allow for the direct retrieval and reactivation of all the representational features associated with the US (i.e., all features of A, including its unique features, and their association with the US), subsequent reconsolidation interference should affect memory expression in ABA as much as it does in AAA or ABB. Testing this prediction was the aim of Experiment 5.
Experiment 5
In Experiment 5, we tested whether presenting the US during GS-based memory retrieval, with a shift in US timing, might allow to retrieve and reactivate the memory for the original training context and its association with shock in a more complete way, thus allowing for MDZ-induced amnesia not only for the GS but also the CS (i.e., generalized amnesia) in an ABA procedure.
Method
The general methods were the same as for Experiment 4. Design, sample size and analyses of Experiment 5 were preregistered on aspredicted.org, see http://aspredicted.org/blind.php?x=mr7dj2.
Procedure
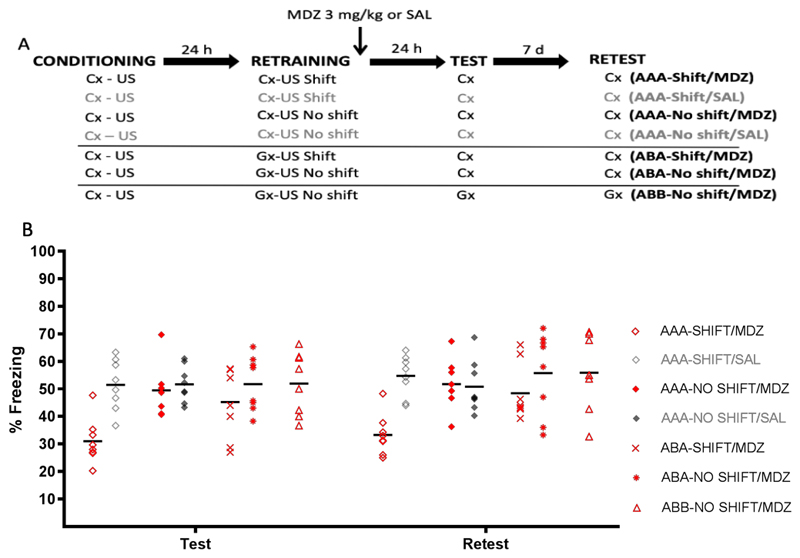
The top panel of Fig. 5 depicts the experimental design. Seven groups of rats were submitted to CFC to context A. Twenty-four h later, four groups were given a retraining session involving context A in which US timing was either identical (AAA-No Shift) or shifted (AAA-Shift) relative to initial training, after which one group of each was injected with MDZ (3 mg/kg, i.p.) and one with SAL (i.p.). In three further groups, retraining involved context B, with timing of the US shifted in the first group but identical to initial training in the other two groups, after which animals in all three groups were injected with MDZ (3 mg/kg, i.p.). In all but the last group, animals were tested and retested for freezing to context A 1 day and 1 week after the retraining session. Test and retest involved context B in the last group2. Animals were randomly assigned to groups (AAA-Shift/MDZ: n = 8, AAA-Shift/SAL: n = 8, AAA-No Shift/MDZ: n = 8, AAA-No Shift/SAL: n = 8, ABA-Shift/MDZ: n = 8, ABA-No Shift/MDZ: n = 8, ABB-No Shift/MDZ: n = 8).
Figure 5.
Experiment 5. (A) Design. (B) Conditioned freezing behavior during test and retest, by group. Only rats that experienced a shift in US timing during retraining to the originally trained context A showed a long-lasting impairment of memory retention (AAA-Shift/MDZ). When US timing was preserved for retraining, animals showed intact memory performance at test and retest (AAA-No shift groups, ABA-No shift/MDZ and ABB-No shift/MDZ). Critically, a shift in US timing during retraining in the generalization context did not produce generalized MDZ-induced amnesia for the original training context (ABA-Shift/MDZ). Data are expressed as means, symbols represent individual data points.
Data Analysis
Retraining, test and retest data were analyzed by means of one-way ANOVAs and effect sizes calculated as η2p. Significant one-way ANOVAs were followed up with Tukey tests.
Results and Discussion
Figure 5 (bottom panel) shows the results for the retention test and retest. One-way ANOVAs revealed significant effects of group in the test (F (6,49) = 4.99, p < .001, η2p = .37) and retest phase (F (6,49) = 4.34, p = .001, η2p = .34). Planned comparisons indicated that the only MDZ group to exhibit significantly lower levels of freezing during test and retest than its appropriate control was the AAA-Shift/MDZ group (p = .002, p = .005 for test and retest, respectively).
In Experiment 4, we demonstrated that the contextual fear memory malleability conferred by exposure to a generalization context is a PE-driven process (see Fig 4B, ABB-Shift/MDZ versus ABB-No Shift/MDZ). In the present experiment, we therefore reactivated contextual fear memory by presenting animals with a temporally unpredictable US in generalization context B. This should serve to maximize the similarity between the acquisition (A) and reactivation (B) context and allow direct associative retrieval of the original training context by the US. Nonetheless, amnestic drug administration after memory reactivation failed to produce generalized amnesia towards the original training context A. Again, the lack of amnesia in an ABA procedure challenges a reconsolidation account, whereas the significant impairment of performance in an ABB procedure appears hard to reconcile with a memory integration account of amnesia.
Experiment 6
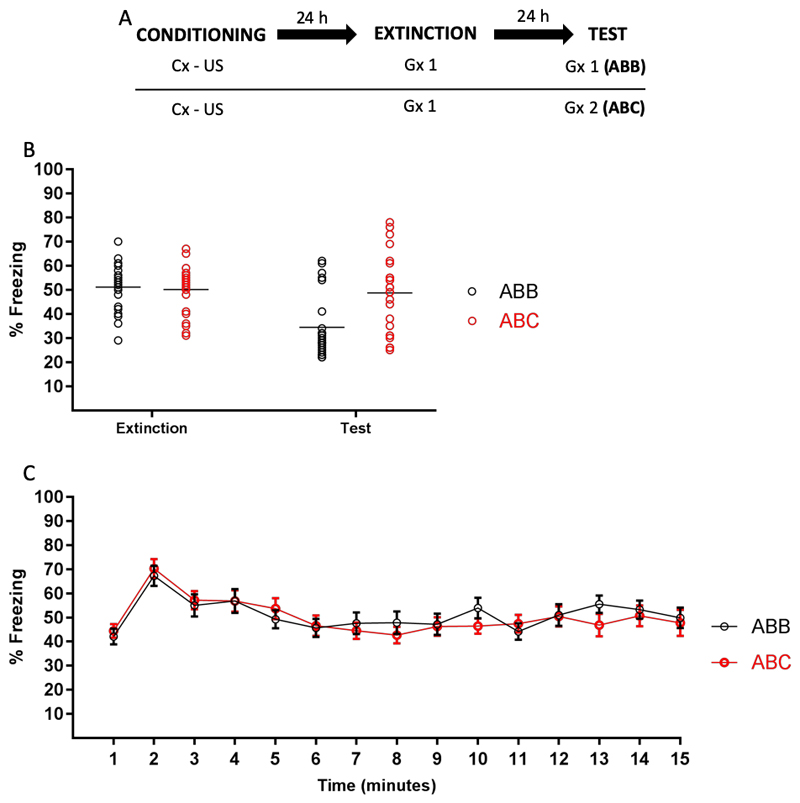
As indicated, the lack of amnesia in an ABA procedure could in principle be attributed to unique features of A, not shared by B, remaining unaffected by manipulations at the time of retrieval. In Experiments 4 and 5, we tried to exclude this possibility by presenting the US during GS-based retrieval, on the basis of the notion that this should ensure the direct retrieval of any of A’s unique features associated with the US. As a further means of exploring the possibility that unique features of A not shared by B might be driving recovery from amnesia in ABA, we set out to establish a new generalization context C that was similar to but distinguishable from both the context in which the animals were trained (A) and the context used for retrieval (B). Critically, A, B and C were designed to be composed of shared features common to all three contexts and of non-shared features unique to each, but they did not contain obvious features shared by only two of the three contexts. Before testing the fate of MDZ-induced amnesia in an ABC procedure (Experiment 7), we ascertained that contexts B and C would promote strong generalization yet be distinguishable to the animals, using an ABC extinction experiment along the same logic as Experiment 1. Generalization should be expressed as strong fear responding during prolonged exposure (extinction training) to either of the generalization contexts. Discrimination would in turn be implied by significantly stronger fear responding upon a subsequent test involving the alternative generalization context (ABC) than if animals were tested in the extinction context (ABB).
Figure 6.
Experiment 6. (A) Design. (B) Conditioned freezing during extinction and retention test, by group. Extinction of generalized contextual fear was expressed only when subsequently tested in the extinction context (ABB). When the animals were tested in a different context than the one used for extinction, fear renewal was observed (ABC). (C) The min-by-min pattern of freezing during the 15-min exposure to context B (extinction session). Data are expressed as means (+/- SEM in panel B), symbols in panel B represent individual data points.
Method
The general methods for contextual fear conditioning, extinction training and retention test were the same as in Experiment 1, be it that a third context was introduced (see below for details). The design, sample size and analyses of Experiment 6 were preregistered on aspredicted.org, see http://aspredicted.org/blind.php?x=hd5p9t.
Sample size determination
An independent-samples t-test specifically comparing the ABA and ABB extinction groups of Experiment 1 at test yielded an effect size of 2.25 (Cohen’s d). Given that ABC renewal is typically markedly smaller than ABA renewal (Harris, Jones, Bailey, & Westbrook, 2000; Laborda, Witnauer, & Miller, 2011), we adopted a conservative approach and pre-determined a sample size of n = 36 animals per group (N total = 72). A sensitivity analysis indicated that this should yield a power of at least .9 to detect an effect of d = .78 or more (calculated using G*Power, version 3.1.9.4; Faul, Erdfelder, Lang, & Buchner, 2007).
Apparatus
The apparatus and two of the contexts were identical to those used in the previous experiments. In addition, in this and the following experiment, a third context was introduced, which was composed of shared features common to the two other contexts and unique features not contained in either of the other contexts. In this and the following experiment, which physical context served as A, B or C was fully counterbalanced.
Context 3
The additional context consisted of a standard grid floor, a grey opaque left wall and a right wall containing red circles on a white background. A blue light (three 11000K – 12000K LEDs with a 120˚ beam angle) mounted to the upper front part of the left wall was turned on, as was a white noise generator located on the upper back part of the wall. The ventilation fan and house light were turned off. The chamber was cleaned with 80% ethanol and the drop pan was scented with a thin layer of banana essence prior to the session. The testing room light was turned off.
Procedure
Figure 6 (panel A) depicts the experimental design. Rats received CFC to context A. Twenty-four h later animals were given 15 min of non-reinforced exposure to generalization context B (extinction training). One day after the extinction session, animals were given a retention test that involved exposure to either B or a second generalization context C. Animals were randomly assigned to groups (ABB: n = 36, ABC: n = 36).
Data Analysis
Extinction and test data were analyzed by means of independent-samples t-tests and effect sizes were calculated as Cohen´s d. As for Experiment 1, a min-by-min mixed ANOVA was used to assess temporal control of behavior during the extinction session.
Results and Discussion
We observed no differences in extinction between the ABB and ABC groups (see Figure 6B), t (46) = .34, p = .72, d =.10, but critically, levels of freezing were significantly different between the groups at test, u = 430, p = .003, rpb = .49: Freezing was strongly attenuated in animals that were tested in the context in which extinction took place (ABB), but in animals that were tested in the alternative generalization context, fear renewal was observed (ABC group). This observation is consistent with prevailing theories of extinction learning (Bouton, 1993, 2002, 2004) and with previous reports of stimulus renewal in rodents (Boddez et al., 2012). Moreover, the temporal pattern of conditioned freezing during extinction suggested a similar temporal precision in the prediction of US arrival as was observed in Experiment 1 (compare Fig. 6C to Fig. 1C). A mixed ANOVA on freezing during the extinction session (group x time as factors) revealed no effect of group (F (1,23) = .104, p = .750, η2p = .004), a significant effect of time (F (11.05,254.35) = 5.87, p < .001, η2p = .20), and no interaction (F (11.52,265.009) = .57, p = .85, η2p = .02). Planned comparisons revealed that freezing levels during the 2nd min of extinction were higher than during the 4th and 5th min (p = .007, η2p = .07, and p = .001, η2p = .16, for the comparison of min 2 vs 4 and 2 vs 5, respectively).
Experiment 7
In the final experiment, we used the newly validated context from Experiment 6 to test whether a MDZ-induced memory deficit would be sensitive to recovery if animals that had received contextual fear conditioning for context A and memory reactivation through exposure to generalization context B were tested in another generalization context C. The results of this experiment should help to illuminate the underlying mechanisms of the GS-specific amnesia that we observed in the preceding experiments. If recovery from MDZ-induced amnesia in an ABA procedure is driven by unique features of A having retained their association with the US, recovery should not be observed when animals are exposed to a context C that does not contain any shared features with A that it does not also share with B.
Method
The general methods for contextual fear conditioning, memory reactivation, drug administration and retention test were the same as for Experiments 2 and 3, be it that an additional context was used (i.e., the third context validated in Experiment 6; see below for details). The design, sample size and analyses of Experiment 7 were preregistered on aspredicted.org, see http://aspredicted.org/blind.php?x=vg7qa4.
Sample size determination
We anticipated that the addition of an extra context to the design would increase variability in freezing between subjects. In order to maintain sufficient power, we preregistered a sample size of 18 animals per group (N total = 108) for the current experiment. Assuming an attenuated effect size of d = 1.20, this should still yield a power of well over .90 to detect a similar effect (calculated using G*Power, version 3.1.9.4; Faul, Erdfelder, Lang, & Buchner, 2007).
Procedure
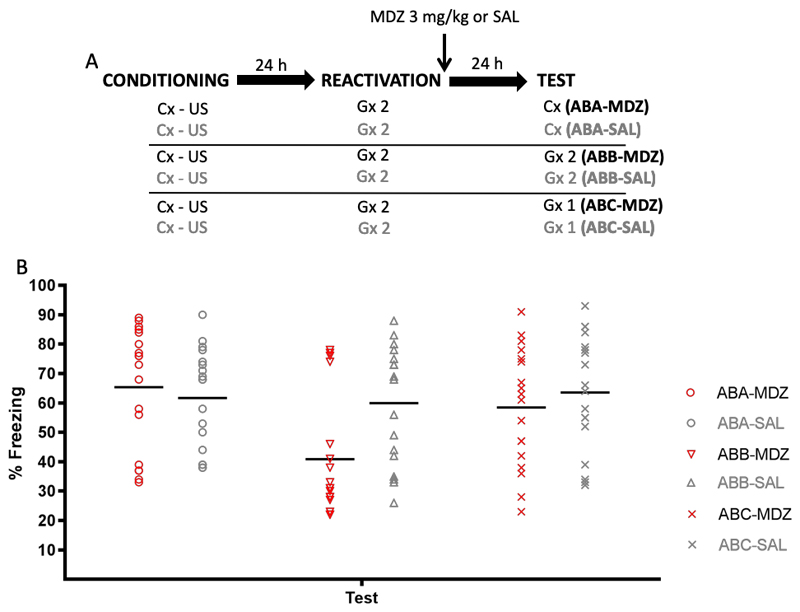
The top panel of Fig. 7 depicts the experimental design. Six groups of rats received CFC to context A. Twenty-four h later all groups received memory reactivation through exposure to generalization context B, after which half were injected with MDZ (3 mg/kg, i.p.) and half were injected with SAL (i.p.). One day later, all groups were given a retention test involving either initially trained context A, the generalization context used for reactivation B, or another generalization context C. A, B and C were clearly discriminable, yet exposure to B and C promoted equally strong and temporally precise memory retrieval as exposure to the initially trained context A (see Fig 6B). Animals were randomly assigned to groups (ABA-MDZ: n = 18, ABA-SAL: n = 18, ABB-MDZ: n = 18, ABB-SAL: n = 18, ABC-MDZ: n = 18, ABC-SAL: n = 18).
Figure 7.
Experiment 7. (A) Design. (B) Conditioned freezing behavior during test, by group. Only in animals in which memory retention was tested for the same generalization context as used for reactivation, MDZ-induced amnesia was observed. Data are expressed as means, symbols represent individual data points.
Data Analysis
Reactivation data were analyzed by means of independent-samples t-tests and effect sizes calculated as Cohen´s d. Test data were analyzed by a means of a factorial ANOVA and effect sizes calculated as η2p and followed up with planned t-tests comparing MDZ- versus SAL- treated animals per group. In case of deviation from normality, non-parametric Mann-Whitney U-tests were used rather than t-tests, and rank biserial correlation (rpb) was used to estimate effect sizes.
Results and Discussion
The bottom panel of Figure 7 shows the test results. A factorial (group x treatment) ANOVA on the test data revealed an effect of group (F (2,102) = 4.46, p = .01, η2p = .08), no effect of treatment (F (1,102) = 3.21, p = .07, η2p = .03), and a marginally significant group x treatment interaction (F (2,102) = 3.03, p = .05, η2p = .05). Planned comparisons revealed that the MDZ and SAL groups differed when the reactivation and test contexts were identical (ABB groups), u = 78.50, p = .009, rpb = .51, but not when animals were tested in a different context than the one used for memory reactivation, be it the initial training context, ABA-MDZ vs SAL, u = 183, p = .51, rpb = .13, or a new generalization context, ABC-MDZ vs SAL, t (34) = .75, p = .45, d = .25.
Overall the data are in line with the previous experiments, suggesting that MDZ-induced post-reactivation amnesia for learned contextual fear cannot be generalized from the reactivation context to the original training context (ABA) or a new generalization context (ABC), as demonstrated by intact levels of freezing in the ABA-MDZ and ABC-MDZ groups during the retention test. Critically, the observed ABC recovery poses a challenge for the reconsolidation blockade account of forgetting. Given that all of the features that context C shared with A were also shared with B, and that MDZ administration after exposure to B should have disrupted the reconsolidation of those shared features’ association to the US (hence the observed ABB amnesia), there should be no basis for fear responding in context C (any unique features of A that could have retained an association with the US are not present in C); the unique features of C have never been paired with the US. Hence, according to a reconsolidation blockade logic, a similar deficit in memory performance should be observed in ABC-MDZ animals as in ABB-MDZ animals. Problematic for the memory integration theory of amnesia, on the other hand, is the combination of memory recovery in ABC groups with suppression of memory performance in ABB; clearly, the test in C in the former group recreates less of the circumstances present at the time of memory retrieval than the test in B in the latter, while being equally similar or dissimilar to the initial training experience in A. If sensory aspects of the retrieval experience would be integrated in the memory representation, the test in B should allow more readily for strong retrieval of the memory than the test in C, even in the absence of MDZ.
General Discussion
Administration of MDZ after contextual fear memory reactivation attenuated defensive responding to a generalization context if memory reactivation involved exposure to the initial training context (AAB, see Experiment 3), or if the reactivation and test contexts were identical (ABB, see Experiments 3, 4, 7). The latter finding demonstrates that memory vulnerability to amnestic interventions for experimentally established memories can effectively be triggered through exposure to a generalization situation (Kroes, Dunsmoor, Lin, Evans, & Phelps, 2017; Soeter & Kindt, 2015). Critically, however, MDZ-induced amnesia following exposure to a generalization context failed to generalize back to the original training context (ABA, see Experiments 2, 3, 5, 7) or towards a novel situation (ABC, see Experiment 7), despite clear evidence that exposure to the generalization context retrieved the memory for the contextual fear learning episode with great strength and temporal precision (see Figures 1 and 6). We further demonstrated that presentation of the US during GS-based memory reactivation, while able to support MDZ-induced amnesia for the generalization context (Experiment 4), still failed to facilitate generalization of amnesia towards the initial training context A (Experiment 5), despite evidence that US presentation during reactivation promotes full retrieval of the original training context (Dębiec et al., 2010; Díaz-Mataix et al., 2011; Liu et al., 2014; Luo et al., 2015; Xue et al., 2017). The recovery from amnesia observed in ABC provides further evidence that the lack of generalized amnesia after GS-based memory reactivation is not due to unique features of the original training context escaping destabilization.
Based on a large number of previous studies (Lee et al., 2017), a concept has emerged in which post-reactivation amnesia arises from a restorage failure, due to disruption of the process that converts a fragile, active memory engram into a stable engram again. Our findings appear to contradict that view (see below) and are strikingly similar to what is known about the suppression of conditioned fear responding obtained through extinction training, which is widely accepted to reflect a retrieval deficit rather than the undoing of the original fear memory trace. Overwhelming evidence has indeed shown that Pavlovian extinction does not result from the unlearning of the original CS-US association, but should be conceived of as reflecting the formation of an inhibitory CS-NoUS association that competes with the excitatory CS-US trace for retrieval (Bouton, 1993; Tronson et al., 2009). Critical evidence for this trace competition theory comes from experiments that demonstrate recovery from extinction in renewal manipulations. For example, one might train subjects in context A and conduct extinction in a different context (B). Thereafter, if subjects are tested in a different context from the one in which extinction occurred (A or C, for ABA or ABC renewal, respectively), retrieval of the inhibitory extinction trace is impaired and the excitatory fear memory trace drives behavioral responding again (Bouton, 1993; Bouton & Bolles, 1979). However, if testing is conducted in the context in which extinction took place (AAA or ABB), extinction retrieval is preserved and fear attenuation is maintained. Expression of extinction learning thus tends to remain limited to the context of extinction training and/or the actual cue or situation presented during extinction training (Boddez et al., 2012; Vervliet et al., 2005) (see also the current Experiments 1 and 6). Likewise, in Experiments 2-5 and 7 reported here, amnesia (i.e., impaired contextual fear memory expression) was observed when testing involved the reactivation context (AAA or ABB) but not when testing involved a different context than the one used to memory reactivation (ABA or ABC), unless acquisition and reactivation involved the same context (AAB) – but note that in Experiment 1 we observed no renewal from extinction in AAB either. The similarity between the laws of extinction and the laws of amnesia observed here suggests that, like extinction, amnesia may reflect a retrieval deficit rather than a (re)storage deficit.
A prime example of a retrieval interference account of post-retrieval amnesia is the memory integration theory. Under this account, retrograde amnesia is considered to be due to a retrieval deficit caused by the mismatch between reactivation and test conditions (Gisquet-Verrier & Riccio, 2018). This perspective is consistent with recent studies showing that amnesia induced by post-retrieval drug administration (some of which notably do not interfere with protein synthesis) can be reversed through experimental manipulations that recreate the conditions that were present during memory retrieval (e.g., administration of the same drug prior to test) (Briggs & Olson, 2013; Flint, Noble, & Ulmen, 2013; Gisquet-Verrier et al., 2015; Nikitin, Kozyrev, & Solntseva, 2019; Nikitin, Solntseva, & Nikitin, 2019; Rossato et al., 2015; Sierra et al., 2013). Despite being in support of a retrieval interference account of post-retrieval amnesia, however, our findings are difficult to reconcile with the memory integration account in its generic form. According to the integration logic, less of a deficit in memory performance should be observed in ABB animals (even if treated with MDZ upon reactivation) than in equally drug-treated animals that experienced a context shift between memory reactivation and test (i.e., ABA, ABC and AAB animals). In other words, we should have observed, if anything, less amnesia in ABB than in ABA, ABC and AAB. We actually found the opposite pattern of results, that is, equivalent post-retrieval attenuation of responding in ABB and AAB, and intact memory performance in ABA (Fig. 3) and ABC (Fig. 7). Likewise problematic for this account is that we obtained similar amnesia in AAB and ABB groups, whereas greater amnesia in AAB than ABB should arguably be anticipated from the memory integration account.
As said, an alternative view is represented by the memory reconsolidation account, which conceives of reconsolidation as an adaptive mechanism to maintain memory relevance via updating or strengthening of a memory trace (Khalaf et al., 2018; Lee, 2013). Failures to obtain reconsolidation experimentally, termed ‘reconsolidation resistance’, have been attributed to ‘boundary’ conditions for the induction of reconsolidation (Finnie & Nader, 2012). A plethora of research has determined that memory destabilization-and-reconsolidation requires that memory retrieval is accompanied by an optimal degree of PE (Exton-McGuinness et al., 2015; Fernández et al., 2016; Krawczyk et al., 2017; Lee, 2013). Findings presented here and previous results from our laboratory systematically support this notion in rats (Alfei et al., 2015; Ferrer Monti et al., 2017) and humans (Sevenster et al., 2012, 2013, 2014). Here in particular, we showed that the memory destabilization following exposure to a generalization context can be driven by either a negative PE (US absent, see Fig. 3) or a positive PE (unexpected change in US timing, see Fig. 4). However, as said, GS-based memory retrieval accompanied by the appropriate degree of PE does not allow for generalization of MDZ-induced post-retrieval amnesia, given that also under these circumstances, amnesia is exclusively observed when memory retention is tested using the same GS (group ABB; see Figures 4 and 5).
Clearly, the notion that reconsolidation-mediated impairment of contextual fear memory retention necessarily results from an attenuation of the original memory trace is difficult to reconcile with the lack of post-retrieval amnesia in ABA (see Fig. 2, 3, 5, 7) and ABC (see Fig. 7), because rewriting of the original memory trace should similarly affect memory expression when tested in ABA and ABC as when tested in ABB. Instead, what we observed was intact fear memory expression (equal responding in MDZ and SAL-treated animals) in ABA and ABC groups, despite robust attenuation of memory expression in ABB (lower fear responding in MDZ than SAL-treated animals). Perhaps the discrepant results in ABA and ABC versus ABB groups necessitate to shift focus from reconsolidation to additional consolidation as an explanatory mechanism. Whereas the presence of new contextual information at the time of memory retrieval can boost destabilization (Winters, Tucci, & DaCosta-Furtado, 2009; Jarome, Ferrara, Kwapis, & Helmstetter, 2015), it can also produce a switch from reconsolidation of an existing memory trace to encoding of a new memory trace (consolidation) (Hupbach, Hardt, Gomez, & Nadel, 2008). Hippocampal pattern completion processes allow the retrieval of an existing memory on the basis of partial cues (giving rise to generalization) (Lissek et al., 2014). At the same time, to the extent that the sensory input does not fully match the original learning situation, pattern separation processes will transmit a mismatch signal via a descending hippocampal pathway that can trigger activation of dopaminergic neurons in the ventral tegmental area and support the PE-driven encoding of a new memory trace (Lisman & Grace, 2005; Reichelt, Exton-McGuinness, & Lee, 2013). Building on these notions, we propose the following speculation. While hippocampal pattern completion drives memory retrieval of fear acquired for context A during subsequent exposure to context B, based on their physical similarity, hippocampal pattern separation may in parallel cause any PE signal that is encountered in B to be attributed specifically to the unique features of B. This pattern separation-mediated attribution may prevent the PE signal from inducing destabilization of the initial fear memory for context A, and instead induce consolidation of a separate episodic memory involving context B as a situation that is associated with fear (by virtue of the aforementioned pattern completion signal). Here then, MDZ might block the consolidation of a contextual fear memory regarding B instead of the reconsolidation of the contextual fear memory regarding A. At the same time, it must be assumed that the mere fact of experiencing B as different from A (which may be enhanced by the PE experienced in B being attributed to pattern separation) will prevent later generalization of the intact fear memory for A to context B on a later retention test (but not to C). Somewhat problematic for this speculation is that in previous work, MDZ was not found to prevent the consolidation of an excitatory fear memory trace (Bustos et al., 2006; Pain, Launoy, Fouquet, & Oberling, 2002) (although it has been shown to disrupt the consolidation of an extinction fear memory trace; e.g., Alfei et al., 2015; Bustos, Maldonado, & Molina, 2009; Ferrer-Monti et al., 2017). Further research will therefore be needed to evaluate whether this is a viable hypothesis.
To recap, It has been fiercely debated whether experimentally-induced post-retrieval memory impairment reflects a storage or a retrieval deficit (Beckers & Kindt, 2017). Our data challenge both types of accounts. Unless a number of additional and highly speculative assumptions are made (see above), a deficit in memory restorage seems particularly hard to reconcile with our observation that at the behavioral level amnesia can be rescued or reversed under a variety of conditions. While also problematic for a memory integration account, our observations are more generally consistent with a retrieval deficit view and with recent reports that used learning-dependent cell labelling and optogenetics to artificially revive forgotten contextual fear memories (Roy, Muralidhar, Smith, & Tonegawa, 2017; Ryan, Roy, Pignatelli, Arons, & Tonegawa, 2015).
Our observation that GS-based induction of amnesia fails to generalize beyond the retrieval situation has important implications for clinical translation. Reconsolidation-based procedures have been touted as a promising avenue for the treatment of emotional memory disorders, including post-traumatic stress disorder, anxiety-related disorders and addiction. In addition to the observation that the induction of memory destabilization depends on a finely calibrated degree of prediction error during memory retrieval that may be hard to achieve in a reliable way clinically (Alfei et al., 2015; Kindt, 2018; Merlo, Milton, Goozée, Theobald, & Everitt, 2014; Ferrer-Monti et al., 2017), our results further complicate the translation of ‘reconsolidation-based’ procedures to clinical practice in that they suggest that amnestic interventions upon memory reactivation are unlikely to result in a robust attenuation of fear responding unless reactivation is achieved through exposure to the actual situation of initial learning. In practice, however, revisiting the original situation in which for instance a trauma was experienced may be practically or ethically impossible. A major challenge for future basic and clinical research will therefore be to uncover ways to enhance the generalizability of reactivation-mediated amnesia. In this regard, it may be helpful to consider the mechanisms that regulate generalization of fear at early time points in comparison to those at more remote time points (Asok, Kandel, & Rayman, 2019). It has been suggested that associative fear memories are detailed and precise shortly after training but tend to lose detail and become less specific with time (Riccio, Richardson, & Ebner, 1984; Wiltgen & Silva, 2007). Future studies could thus explore whether GS-based memory retrieval is more successful in producing the opportunity for generalized amnesia if such retrieval happens at time points further removed from initial memory acquisition. If so, this might salvage the prospect of harnessing amnestic procedures to the treatment of emotional memory disorders.
Context
Emotional memories are notoriously difficult to disrupt, often recovering over time. Reconsolidation theory states that, following retrieval, consolidated memories can be permanently modified or erased, through pharmacological interventions. Over the last two decades, considerable evidence has been garnered in support of this theory, with important translational implications for treatment of anxiety and other disorders. Our experiments in rats show that fear memories, reactivated through exposure to a situation resembling the original fear learning situation, indeed become sensitive to pharmacological disruption. Yet we find no evidence of amnesia when animals are later tested in a different situation than the one used for memory reactivation. This lack of generalization of post-retrieval amnesia is at odds with the reconsolidation-blockade theory of forgetting and presents a major hurdle for clinical translation.
Supplementary Material
Footnotes
While strictly speaking, renewal or recovery of conditioned responding would suggest a within-subjects comparison of conditioned responding over time, we here use the terms renewal and recovery also to refer to a return of memory expression inferred from a between-group difference in conditioned responding at test between groups having received similar training prior to test, in keeping with the broader literatures on extinction (e.g., Bouton & Bolles, 1979; Bouton & King, 1983) and amnesia (e.g., Miller & Springer, 1972).
On reflection, the latter group (ABB-No Shift/MDZ) is an unnecessary condition that does not add anything to the critical comparisons between the 6 other groups. Nonetheless, as the group was included in the pre-registered design and we deemed it important to be complete in our reporting, we have chosen to not remove it from the paper.
References
- Alfei JM, Ferrer Monti RI, Molina VA, Bueno AM, Urcelay GP. Prediction error and trace dominance determine the fate of fear memories after post-training manipulations. Learning and Memory. 2015;22(8):385–400. doi: 10.1101/lm.038513.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfei JM, Luyten L, Beckers T, Ferrer Monti RI, Molina VA, De Bundel D. Generalization and recovery of post-retrieval amnesia. 2019 Nov 7; doi: 10.1037/xge0000765. Retrieved from osf.io/fqajx. [DOI] [PMC free article] [PubMed]
- Asok A, Kandel ER, Rayman JB. The Neurobiology of Fear Generalization. Frontiers in Behavioral Neuroscience. 2019;12(329):1–15. doi: 10.3389/fnbeh.2018.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckers T, Kindt M. Memory Reconsolidation Interference as an Emerging Treatment for Emotional Disorders: Strengths, Limitations, Challenges, and Opportunities. Annual Review of Clinical Psychology. 2017;13:99–121. doi: 10.1146/annurev-clinpsy-032816-045209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddez Y, Callaerts-Vegh Z, Vervliet B, Baeyens F, D’Hooge R, Hermans D, Beckers T. Stimulus generalization and return of fear in C57BL/6J mice. Frontiers in Behavioral Neuroscience. 2012 Jul;6:1–5. doi: 10.3389/fnbeh.2012.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Context time and memory retrieval in the interference paradigms of pavlovian learning. Psychological Bulletin. 1993;114(1):80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biological Psychiatry. 2002 doi: 10.1016/S0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learning and Memory. 2004;11(5):485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Contextual control of the extinction of conditioned fear. Learning and Motivation. 1979;10(4):445–466. doi: 10.1016/0023-9690(79)90057-2. [DOI] [Google Scholar]
- Bouton ME, King DA. Contextual control of the extinction of conditioned fear: Tests for the associative value of the context. Journal of Experimental Psychology: Animal Behavior Processes. 1983;9(3):248–265. doi: 10.1029/2004TC001688. [DOI] [PubMed] [Google Scholar]
- Briggs JF, Olson BP. Reexposure to the amnestic agent alleviates cycloheximide-induced retrograde amnesia for reactivated and extinction memories. Learning and Memory. 2013;20(5):285–288. doi: 10.1101/lm.030270.113. [DOI] [PubMed] [Google Scholar]
- Bustos S, Maldonado H, Molina VA. Midazolam disrupts fear memory reconsolidation. Neuroscience. 2006;139(3):831–842. doi: 10.1016/j.neuroscience.2005.12.064. [DOI] [PubMed] [Google Scholar]
- Bustos S, Maldonado H, Molina VA. Disruptive effect of midazolam on fear memory reconsolidation: Decisive influence of reactivation time span and memory age. Neuropsychopharmacology. 2009;34(2):446–457. doi: 10.1038/npp.2008.75. [DOI] [PubMed] [Google Scholar]
- Cain CK, Blouin AM, Barad M. Temporally Massed CS Presentations Generate More Fear Extinction Than Spaced Presentations. 2003;29(4):323–333. doi: 10.1037/0097-7403.29.4.323. [DOI] [PubMed] [Google Scholar]
- Craske MG, Treanor M, Conway CC, Zbozinek T, Vervliet B. Behaviour Research and Therapy Maximizing exposure therapy : An inhibitory learning approach. Behaviour Research and Therapy. 2014;58:10–23. doi: 10.1016/j.brat.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das RK, Lawn W, Kamboj SK. Rewriting the valuation and salience of alcohol-related stimuli via memory reconsolidation. Translational Psychiatry. 2015;5(9):e645–9. doi: 10.1038/tp.2015.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das RK, Walsh K, Hannaford J, Lazzarino AI, Kamboj SK. Nitrous oxide may interfere with the reconsolidation of drinking memories in hazardous drinkers in a prediction-error-dependent manner. European Neuropsychopharmacology. 2018;28(7):828–840. doi: 10.1016/j.euroneuro.2018.05.001. [DOI] [PubMed] [Google Scholar]
- Dȩbiec J, Díaz-Mataix L, Bush DEA, Doyère V, Ledoux JE. The amygdala encodes specific sensory features of an aversive reinforcer. Nature Neuroscience. 2010;13(5):536–537. doi: 10.1038/nn.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, Doyere V, Nader K, LeDoux JE. Directly reactivated, but not indirectly reactivated, memories undergo reconsolidation in the amygdala. Proceedings of the National Academy of Sciences. 2006;103(9):3428–3433. doi: 10.1073/pnas.0507168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec Jacek, Diaz-Mataix L, Bush DEA, Doyère V, LeDoux JE. The selectivity of aversive memory reconsolidation and extinction processes depends on the initial encoding of the Pavlovian association. Learning and Memory. 2013;20(12):695–699. doi: 10.1101/lm.031609.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Mataix L, Debiec J, LeDoux J, Doyere V. Sensory-Specific Associations Stored in the Lateral Amygdala Allow for Selective Alteration of Fear Memories. Journal of Neuroscience. 2011;31(26):9538–9543. doi: 10.1523/JNEUROSCI.5808-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Mataix L, Ruiz Martinez RC, Schafe GE, Ledoux JE, Doyère V. Detection of a temporal error triggers reconsolidation of amygdala-dependent memories. Current Biology. 2013;23(6):467–472. doi: 10.1016/j.cub.2013.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Nader K. Characterization of Fear Memory Reconsolidation. Journal of Neuroscience. 2004;24(42):9269–9275. doi: 10.1523/JNEUROSCI.2971-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dymond S, Dunsmoor JE, Vervliet B, Roche B, Hermans D. Fear Generalization in Humans: Systematic Review and Implications for Anxiety Disorder Research. Behavior Therapy. 2015;46(5):561–582. doi: 10.1016/j.beth.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Exton-McGuinness MTJ, Lee JLC, Reichelt AC. Updating memories-The role of prediction errors in memory reconsolidation. Behavioural Brain Research. 2015;278:375–384. doi: 10.1016/j.bbr.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang A-G, Buchner A. G * Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39(2):175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- Fernández RS, Boccia MM, Pedreira ME. The fate of memory: Reconsolidation and the case of Prediction Error. Neuroscience and Biobehavioral Reviews. 2016 doi: 10.1016/j.neubiorev.2016.06.004. Elsevier Ltd. [DOI] [PubMed] [Google Scholar]
- Ferrer Monti RI, Alfei JM, Mugnaini M, Bueno AM, Beckers T, Urcelay GP, Molina VA. A comparison of behavioral and pharmacological interventions to attenuate reactivated fear memories. Learning and Memory. 2017;24(8):369–374. doi: 10.1101/lm.045385.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer Monti RI, Giachero M, Alfei JM, Bueno AM, Cuadra G, Molina VA. An appetitive experience after fear memory destabilization attenuates fear retention: Involvement GluN2B-NMDA receptors in the basolateral amygdala complex. Learning and Memory. 2016;23(9):465–478. doi: 10.1101/lm.042564.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnie PSB, Nader K. The role of metaplasticity mechanisms in regulating memory destabilization and reconsolidation. Neuroscience and Biobehavioral Reviews. 2012;36(7):1667–1707. doi: 10.1016/j.neubiorev.2012.03.008. [DOI] [PubMed] [Google Scholar]
- Flint RW, Noble LJ, Ulmen AR. NMDA receptor antagonism with MK-801 impairs consolidation and reconsolidation of passive avoidance conditioning in adolescent rats: Evidence for a state dependent reconsolidation effect. Neurobiology of Learning and Memory. 2013;101:114–119. doi: 10.1016/j.nlm.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Gisquet-Verrier P, Lynch JF, Cutolo P, Toledano D, Ulmen A, Jasnow AM, Riccio DC. Integration of New Information with Active Memory Accounts for Retrograde Amnesia: A Challenge to the Consolidation/Reconsolidation Hypothesis? Journal of Neuroscience. 2015;35(33):11623–11633. doi: 10.1523/JNEUROSCI.1386-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisquet-Verrier P, Riccio D. Memory Integration: an alternative to the consolidation/reconsolidation hypothesis. Progess in Neurobiology. 2018 doi: 10.1016/j.pneurobio.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Harris JA, Jones ML, Bailey GK, Westbrook RF. Contextual Control Over Conditioned Responding in an Extinction Paradigm. Journal of Experimental Psychology. Animal Behavior Processes. 2000;26(2):174–185. doi: 10.1037//0097-7403.26.2.174. [DOI] [PubMed] [Google Scholar]
- Hupbach A, Hardt O, Gomez R, Nadel L. The dynamics of memory: Context-dependent updating. Learning and Memory. 2008;15(8):574–579. doi: 10.1101/lm.1022308. [DOI] [PubMed] [Google Scholar]
- Jarome TJ, Ferrara NC, Kwapis JL, Helmstetter FJ. Contextual Information Drives the Reconsolidation-Dependent Updating of Retrieved Fear Memories. Neuropsychopharmacology. 2015;40(13):3044–3052. doi: 10.1038/npp.2015.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamboj SK, Das RK. Behavioral and Pharmacological Strategies for Weakening Maladaptive Reward Memories A New Approach to Treating a Core Disease Mechanism in Tobacco Use Disorder. JAMA Psychiatry. 2017;74:209–211. doi: 10.1001/jamapsychiatry.2016.3937. [DOI] [PubMed] [Google Scholar]
- Khalaf O, Resch S, Dixsaut L, Gorden V, Glauser L, Gräff J. Reactivation of recall-induced neurons contributes to remote fear memory attenuation. Science. 2018;360(6394):1239–1242. doi: 10.1126/science.aas9875. [DOI] [PubMed] [Google Scholar]
- Kindt M. The surprising subtleties of changing fear memory: A challenge for translational science. Philosophical Transactions of the Royal Society B: Biological Sciences. 2018;373(1742):1–8. doi: 10.1098/rstb.2017.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt M, Soeter M, Vervliet B. Beyond extinction : erasing human fear responses and preventing the return of fear. Nature Neuroscience. 2009;12:256–258. doi: 10.1038/nn.2271. [DOI] [PubMed] [Google Scholar]
- Krawczyk MC, Fernández RS, Pedreira ME, Boccia MM. Toward a better understanding on the role of prediction error on memory processes : From bench to clinic. Neurobiology of Learning and Memory. 2017;142:13–20. doi: 10.1016/j.nlm.2016.12.011. [DOI] [PubMed] [Google Scholar]
- Kredlow AM, Unger LD, Otto MW. Harnessing reconsolidation to weaken fear and appetitive memories: A meta-analysis of post-retrieval extinction effects. Psychological Bulletin. 2016;142(3):314–336. doi: 10.1037/bul0000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroes MCW, Dunsmoor JE, Lin Q, Evans M, Phelps EA. A reminder before extinction strengthens episodic memory via reconsolidation but fails to disrupt generalized threat responses. Scientific Reports. 2017;7(1):1–14. doi: 10.1038/s41598-017-10682-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laborda MA, Witnauer JE, Miller RR. Contrasting AAC and ABC renewal : the role of context associations. Learning and Behaviorn. 2011;39:46–56. doi: 10.3758/s13420-010-0007-1. [DOI] [PubMed] [Google Scholar]
- Lee JLC. Reconsolidation : maintaining memory relevance. Trends in Cognitive Sciences. 2013;32(8):413–420. doi: 10.1016/j.tins.2009.05.002.Reconsolidation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JLC, Nader K, Schiller D. An Update on Memory Reconsolidation Updating. Trends in Cognitive Sciences. 2017 doi: 10.1016/j.tics.2017.04.006. Elsevier Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: Controlling the entry of information into long-term memory. Neuron. 2005 doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Lissek S, Bradford DE, Alvarez RP, Burton P, Espensen-Sturges T, Reynolds RC, Grillon C. Neural substrates of classically conditioned fear-generalization in humans: A parametric fMRI study. Social Cognitive and Affective Neuroscience. 2014;9(8):1134–1142. doi: 10.1093/scan/nst096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhao L, Xue Y, Shi J, Suo L, Luo Y, et al. Lu L. An unconditioned stimulus retrieval extinction procedure to prevent the return of fear memory. Biological Psychiatry. 2014;76(11):895–901. doi: 10.1016/j.biopsych.2014.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonergan MH, Olivera-figueroa LA, Pitman RK, Brunet A. Propranolol ’ s effects on the consolidation and reconsolidation of long-term emotional memory in healthy participants : a meta-analysis. Journal of Psychiatry & Neuroscience (JPN) 2013;38(4):222–231. doi: 10.1503/jpn.120111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López AM, Santos JM, Cortasa S, Fernández RS, Carbó Tano M, Pedreira ME. Different dimensions of the prediction error as a decisive factor for the triggering of the reconsolidation process. Neurobiology of Learning and Memory. 2016;136:210–219. doi: 10.1016/j.nlm.2016.10.016. [DOI] [PubMed] [Google Scholar]
- Luo YX, Xue YX, Liu JF, Shi HS, Jian M, Han Y, et al. Lu L. A novel UCS memory retrieval-extinction procedure to inhibit relapse to drug seeking. Nature Communications. 2015;6:1–14. doi: 10.1038/ncomms8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyten L, Schroyens N, Hermans D, Beckers T. Parameter optimization for automated behavior assessment: plug-and-play or trial-and-error? Frontiers in Behavioral Neuroscience. 2014;8(February):1–4. doi: 10.3389/fnbeh.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkar SR, Zhang SQ, Cranney J. Behavioral and neural analysis of GABA in the acquisition, consolidation, reconsolidation, and extinction of fear memory. Neuropsychopharmacology. 2010;35(8):1625–1652. doi: 10.1038/npp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo E, Milton AL, Goozée ZY, Theobald DEH, Everitt BJ. Reconsolidation and extinction are dissociable and mutually exclusive processes: behavioral and molecular evidence. Journal of Neuroscience. 2014;34(7):2422–2431. doi: 10.1523/JNEUROSCI.4001-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RR, Matzel LD. Memory involves far more than "consolidation. Nature Reviews Neuroscience. 2000;1(December):214–216. doi: 10.1038/35044578. [DOI] [PubMed] [Google Scholar]
- Miller RR, Springer AD. Induced recovery of memory in rats following electroconvulsive shock. Physiology & Behavior. 1972;8(4):645–651. doi: 10.1016/0031-9384(72)90089-3. [DOI] [PubMed] [Google Scholar]
- Nader K, Hardt O. A single standard for memory: The case for reconsolidation. Nature Reviews Neuroscience. 2009;10(3):224–234. doi: 10.1038/nrn2590. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406(6797):722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Nikitin VP, Kozyrev SA, Solntseva SV. Administration of Protein Synthesis Inhibitor before Reminder Reverses Amnesia Induced by Memory Reconsolidation Impairment with 5-HT Receptors Antagonist. Bulletin of Experimental Biology and Medicina. 2019;167(1):1–6. doi: 10.1007/s10517-019-04448-6. [DOI] [PubMed] [Google Scholar]
- Nikitin VP, Solntseva SV, Nikitin PV. Protein synthesis inhibitors induce both memory impairment and its recovery. Behavioural Brain Research. 2019;360:202–208. doi: 10.1016/j.bbr.2018.11.046. [DOI] [PubMed] [Google Scholar]
- Pachas GN, Gilman J, Orr SP, Hoeppner B, Carlini SV, Loebl T, et al. Evins AE. Single dose propranolol does not affect physiologic or emotional reactivity to smoking cues. Psychopharmacology. 2015;232:1619–1628. doi: 10.1007/s00213-014-3797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pain L, Launoy A, Fouquet N, Oberling P. Mechanisms of action of midazolam on expression of contextual fear in rats. British Journal of Anaesthesia. 2002;89(4):614–621. doi: 10.1093/bja/aef228. [DOI] [PubMed] [Google Scholar]
- Piñeyro ME, Ferrer Monti RI, Alfei JM, Bueno AM, Urcelay GP. Memory destabilization is critical for the success of the reactivation-extinction procedure. Learning and Memory. 2014;21(1):46–54. doi: 10.1101/lm.032714.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybyslawski J, Sara SJ. Reconsolidation of memory after its reactivation. Behavioural Brain Research. 1997;84(1–2):241–246. doi: 10.1016/S0166-4328(96)00153-2. [DOI] [PubMed] [Google Scholar]
- Reichelt AC, Exton-McGuinness MT, Lee JLC. Ventral Tegmental Dopamine Dysregulation Prevents Appetitive Memory Destabilization. Journal of Neuroscience. 2013;33(35):14205–14210. doi: 10.1523/JNEUROSCI.1614-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio DC, Richardson R, Ebner DL. Memory Retrieval Deficits Based Upon Altered Contextual Cues : A Paradox. Psychological Bulletin. 1984;96(1):152–165. [PubMed] [Google Scholar]
- Rossato JI, Köhler CA, Radiske A, Lima RH, Bevilaqua LRM, Cammarota M. State-dependent effect of dopamine D1/D5receptors inactivation on memory destabilization and reconsolidation. Behavioural Brain Research. 2015;285:194–199. doi: 10.1016/j.bbr.2014.09.009. [DOI] [PubMed] [Google Scholar]
- Roy DS, Muralidhar S, Smith LM, Tonegawa S. Silent memory engrams as the basis for retrograde amnesia. Proceedings of the National Academy of Sciences. 2017 doi: 10.1073/pnas.1714248114. 201714248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan TJ, Roy DS, Pignatelli M, Arons A, Tonegawa S. Engram cells retain memory under retrograde amnesia. Science. 2015;348(6238):1007–1013. doi: 10.1126/science.aaa5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saladin ME, Gray KM, Mcrae-clark AL, LaRowe SD, Yeatts SD, Baker NL, et al. Brady KT. A double blind, placebo-controlled study of the effects of post-retrieval propranolol on reconsolidation of memory for craving and cue reactivity in cocaine dependent humans. 2013;226:721–737. doi: 10.1007/s00213-013-3039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevenster D, Beckers T, Kindt M. Retrieval per se is not sufficient to trigger reconsolidation of human fear memory. Neurobiology of Learning and Memory. 2012;97(3):338–345. doi: 10.1016/j.nlm.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Sevenster D, Beckers T, Kindt M. Prediction error governs pharmacologically induced amnesia for learned fear. Science. 2013;339(6121):830–833. doi: 10.1126/science.1231357. [DOI] [PubMed] [Google Scholar]
- Sevenster D, Beckers T, Kindt M. Prediction error demarcates the transition from retrieval, to reconsolidation, to new learning. Learning and Memory. 2014;21(11):580–584. doi: 10.1101/lm.035493.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra RO, Cassini LF, Santana F, Crestani AP, Duran JM, Haubrich J, et al. Quillfeldt JA. Reconsolidation may incorporate state-dependency into previously consolidated memories. Learning and Memory. 2013;20(7):379–387. doi: 10.1101/lm.030023.112. [DOI] [PubMed] [Google Scholar]
- Soeter M, Kindt M. Retrieval cues that trigger reconsolidation of associative fear memory are not necessarily an exact replica of the original learning experience. Frontiers in Behavioral Neuroscience. 2015;9(May):1–10. doi: 10.3389/fnbeh.2015.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CAJ, Gazarini L, Takahashi RN, Guimarães FS, Bertoglio LJ. On disruption of fear memory by reconsolidation blockade: Evidence from cannabidiol treatment. Neuropsychopharmacology. 2012;37(9):2132–2142. doi: 10.1038/npp.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallot L, Diaz-Mataix L, Perry RE, Wood K, LeDoux JE, Mouly AM, et al. Doyère V. Updating of aversive memories after temporal error detection is differentially modulated by mTOR across development. Learning and Memory. 2017;24(3):115–122. doi: 10.1101/lm.043083.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronson NC, Schrick C, Guzman YF, Huh KH, Srivastava DP, Penzes P, et al. Radulovic J. Segregated Populations of Hippocampal Principal CA1 Neurons Mediating Conditioning and Extinction of Contextual Fear. Journal of Neuroscience. 2009;29(11):3387–3394. doi: 10.1523/JNEUROSCI.5619-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao JC, Craske MG. Timing of Treatment and Return of Fear : Effects of Massed, Uniform- , and Expanding-Spaced Exposure Schedules. Behavior Therapy. 2000;31:479–497. [Google Scholar]
- Tulving E, Thomson MD. Encoding Specificity and retrieval processes in episodic memory. Psychological Review. 1973;80(5):352–373. doi: 10.1037/h0020071. [DOI] [Google Scholar]
- Vervliet B, Vansteenwegen D, Baeyens F, Hermans D, Eelen P. Return of fear in a human differential conditioning paradigm caused by a stimulus change after extinction. Behaviour Research and Therapy. 2005;43(3):357–371. doi: 10.1016/j.brat.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Walsh KH, Das RK, Saladin ME, Kamboj SK. Modulation of naturalistic maladaptive memories using behavioural and pharmacological reconsolidation-interfering strategies : a systematic review and meta-analysis of clinical and ‘ sub-clinical ’ studies. Psychopharmacology. 2018;235:2507–2527. doi: 10.1007/s00213-018-4983-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen BJ, Silva AJ. Memory for context becomes less specific with time. Learning and Memory. 2007;14:313–317. doi: 10.1101/lm.430907.this. [DOI] [PubMed] [Google Scholar]
- Winters BD, Tucci MC, DaCosta-Furtado M. Older and stronger object memories are selectively destabilized by reactivation in the presence of new information. Learning and Memory. 2009;16(9):545–553. doi: 10.1101/lm.1509909. [DOI] [PubMed] [Google Scholar]
- Xue Y, Chen Y, Zhang L, Zhang L, Huang G, Deng J, et al. Lu L. Selective inhibition of amygdala neuronal ensembles encoding nicotine-associated memories prevents nicotine seeking and relapse. Biological Psychiatry. 2017;82(11):781–793. doi: 10.1016/j.biopsych.2017.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng XX, Du J, Zhuang CQ, Zhang JH, Jia YL, Zheng XF. Unconditioned stimulus revaluation to promote conditioned fear extinction in the memory reconsolidation window. PLoS ONE. 2014;9(7):1–5. doi: 10.1371/journal.pone.0101589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Cranney J. The Role of GABA and Anxiety in the Reconsolidation of Conditioned Fear. Behavioral Neuroscience. 2008;122(6):1295–1305. doi: 10.1037/a0013273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.