Abstract
Objective
After aneurysmal subarachnoid haemorrhage (aSAH), extracellular haemoglobin (Hb) in the subarachnoid space is bound by haptoglobin, neutralizing Hb toxicity and helping its clearance. Two exons in the HP gene (encoding haptoglobin) exhibit copy number variation (CNV), giving rise to HP1 and HP2 alleles, which influence haptoglobin expression level and possibly haptoglobin function. We hypothesized that the HP CNV associates with long-term outcome beyond the first year after aSAH.
Methods
The HP CNV was typed using quantitative PCR in 1299 aSAH survivors in the Genetics of Subarachnoid Haemorrhage (GOSH) Study, a retrospective multicentre cohort study with a median follow-up of 18 months. To investigate mediation of the HP CNV effect by haptoglobin expression level, as opposed to functional differences, we used rs2000999, a single nucleotide polymorphism associated with haptoglobin expression independent of the HP CNV. Outcome was assessed using modified Rankin and Glasgow Outcome Scores. SAH volume was dichotomized on the Fisher grade. Haemoglobin-haptoglobin complexes were measured in cerebrospinal fluid (CSF) of 44 aSAH patients, and related to the HP CNV.
Results
The HP2 allele associated with a favourable long-term outcome after high-volume, but not low-volume aSAH (multivariable logistic regression). However rs2000999 did not predict outcome. The HP2 allele associated with lower CSF haemoglobin-haptoglobin complex levels. The CSF Hb concentration after high-volume and low-volume aSAH, was respectively higher and lower than the Hb-binding capacity of CSF haptoglobin.
Conclusion
The HP2 allele carries a favourable long-term prognosis after high-volume aSAH. Haptoglobin and the Hb clearance pathway are therapeutic targets after aSAH.
Introduction
Extracellular haemoglobin (Hb) is toxic and is immediately neutralized by the protein haptoglobin (Hp) as a result of a high affinity binding interaction. The Hp-Hb complex is then recognized and endocytosed by the cell surface receptor CD163 1. After aneurysmal subarachnoid haemorrhage (aSAH), Hb is released into the cerebrospinal fluid (CSF) from damaged erythrocytes trapped in the subarachnoid space, where it is toxic to neurones and other cells in the central nervous system 2. The haptoglobin-CD163 Hb clearance mechanism is also present in the central nervous system 3.
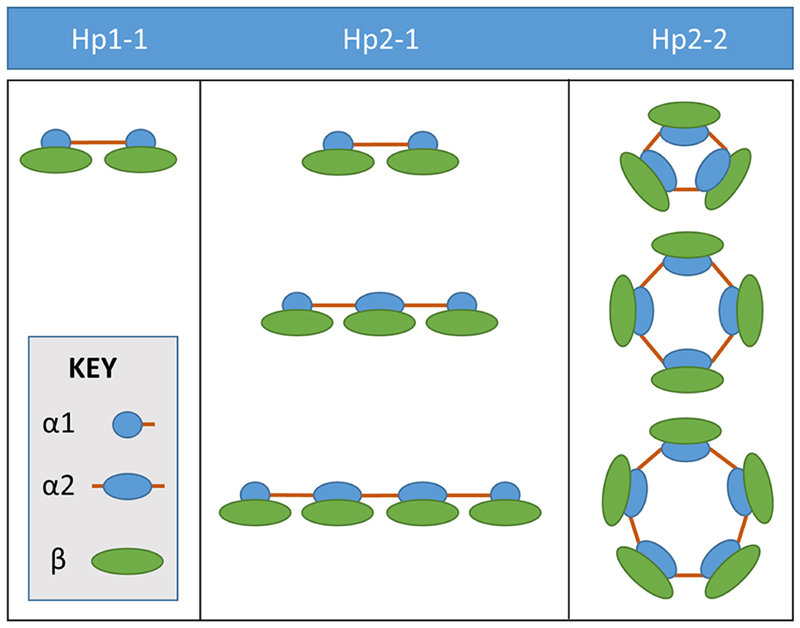
The HP gene codes for the α and β chain of haptoglobin. Two codominant HP alleles exist: HP1 and HP2; the α chain coding region is duplicated in the HP2 allele, so this is a copy number variant (CNV). Three possible HP CNV genotypes: HP1-1, HP2-1 and HP2-2, generate the three types of haptoglobin polymers, Hp1-1, Hp2-1 and Hp2-2 4, illustrated in Figure 1. In HP1-1 individuals, haptoglobin consists of two chains (α1 and β) linked by one disulphide bond. The α1 chain has another free cysteine which leads to dimerization of the haptoglobin molecule, so that the only form present in HP1 homozygotes (HP1-1) is the haptoglobin dimer. In HP2 homozygotes (HP2-2), two free cysteines in the duplicated α2 region endow haptoglobin with the capacity to form cyclic polymers of increasing size. In heterozygotes (HP2-1), linear polymers of increasing size occur, and the dimer is also present.
Figure 1.
Haptoglobin types: Hp1-1, Hp2-1 and Hp2-2. The HP gene codes for the α and β chain of Hp. Two codominant HP alleles exist: HP1 and HP2; the α chain coding region is duplicated in the HP2 allele, so this is a copy number variant (CNV). Three possible HP CNV genotypes: HP1-1, HP2-1 and HP2-2, generate three types of haptoglobin polymers, Hp1-1, Hp2-1 and Hp2-2.
In several small studies, the HP CNV was variably associated with short-term to medium-term outcome after aSAH 5–9, but an individual patient level data analysis did not confirm this 10. An important consideration is that these studies looked at outcome mostly within the first six months after aSAH, and this may not be early enough to allow early brain injury events other than Hb, to settle. Another unresolved question relates to the mechanism of action. HP alleles are associated with differential haptoglobin expression (HP1-1 > HP2-1 > HP2-2 11) as well as haemoglobin-haptoglobin complex scavenging rate by CD163 in vitro 1,12–14. It is not clear which of these two consequences of the HP CNV mediate its effect on aSAH outcome.
To more definitively address these issues, we studied the effect of the HP CNV in the Genetic and Observational Subarachnoid Haemorrhage (GOSH) cohort 15 study of long-term outcome in aSAH survivors, assessed at a median time from ictus of 18 months, up to 8 years. We hypothesized that the HP CNV affects long-term outcome after aSAH, and investigated how much of this effect was mediated by haptoglobin expression level using rs2000999, a single nucleotide polymorphism (SNP) associated with haptoglobin expression levels in plasma and tissue (GG > GA > AA), independent of HP CNV 16,17. The combined use of rs2000999 and the HP CNV is a useful genetic epidemiological tool to dissect the mechanism underlying differences between HP1 and HP2 alleles 18. We sought mechanistic evidence supporting our findings by performing biochemical analyses in a separate cohort of aSAH patients with available CSF samples.
Subjects and Method
GOSH study
Clinical data and DNA was collected from patients with aSAH enrolled in the GOSH study, designed to examine the genetic and clinical characteristics of patients with ruptured and unruptured intracranial aneurysms. The GOSH study recruited at 22 tertiary neurosurgical centres in the UK between 2011 and 2014. Written informed consent was obtained from participants, or next of kin if patients lacked capacity. Recruitment was from inpatient and outpatient settings following either a new or previous diagnosis respectively; patients who died early after aSAH were not recruited. Standardized case report forms were completed by trained stroke research practitioners. The study was approved by the National Research Ethics Committee (NRES reference no: 09/H0716/54).
Outcomes, covariates & definitions
The primary outcome measure was the modified Rankin scale (mRS) at follow up, dichotomized into favourable (mRS 0-1) and unfavourable (mRS 2-6) outcomes, administered by qualified research practitioners at the time of assessment. The choice of this instrument and dichotomization threshold was based on data availability in this population of aSAH survivors. The modified version 19 of the Rankin Scale 20 was used throughout in a standardized way, ranging from 0 (no symptoms at all) to 5 (severe disability); mRS 6 (death) was added to include mortality 21.
Covariates included age, sex, admission WFNS score 22, admission Fisher grade 23, hydrocephalus, aneurysmal treatment (coiling, clipping, or none), time since ictus, centre, smoking pack years, presence or absence of nimodipine treatment, diabetes mellitus, hypercholesterolaemia, hypertension, anti-hypertensive medication, and non-SAH related disability affecting the primary outcome measure. We defined hypertension, hypercholesterolaemia and diabetes mellitus as present if the patient or medical records indicated the condition for which either drug treatment, lifestyle, or other advice had been provided.
Control population
A sample of 927 individuals from the ALSPAC cohort 24,25, previously genotyped for the HP CNV (see below), was used as the control population. Plasma haptoglobin level was available for 325 of these individuals. It was measured using an immunoturbimetric haptoglobin assay (Cobas Integra kit catalogue number 03005593 322, Roche, USA) on a Hitachi Cobas c311 autoanalyser. In the ALSPAC study, pregnant women resident in Avon, UK with expected dates of delivery 1st April 1991 to 31st December 1992 were invited to take part in the study. Of the 15,247 pregnancies, there were 14,899 children who were alive at 1 year of age. The ALSPAC study website (http://www.bristol.ac.uk/alspac/researchers/our-data/) contains details of all the data that is available through a fully searchable data dictionary and variable search tool. Ethical approval for the study was obtained from the ALSPAC Ethics and Law Committee and the Local Research Ethics Committees.
Genotyping
Detailed genotyping methods for the HP CNV and rs2000999 are in the online supplementary methods.
Biochemistry – high Fisher grade aSAH
44 Fisher grade III-IV aSAH patients were recruited at the Southampton centre, after approval by the National Research Ethics Committee (reference no: 12/SC/0666). CSF was obtained from external ventricular drains (EVD) on alternate days from insertion and up to two weeks or until the EVD was removed. CSF was spun and frozen within one hour of sampling. We did not use CSF samples in the event of an EVD infection. Further details are in the online supplementary methods.
We performed haemoglobin-haptoglobin complex quantification, irrespective of oxidation state, using size exclusion ultra-performance liquid chromatography (UPLC) with absorbance measurement at 415nm. A 9 point Hb standard curve (0 to 1 mg/ml) was prepared from commercially-available lyophilized human Hb (Sigma) reconstituted to 1 g/L in diluent (9 g/L NaCl, 10 mM EDTA). The concentration of the standard Hb solution was verified independently by spectrophotometric quantification at 570 nm using a HemocueTM (Hemocue, Sweden). We determined accuracy of the standard curve to be 3.3% using a Hb control. 50μL of neat CSF was loaded onto the UPLC column using a running buffer consisting of 50 mM Tris and 150 mM NaCl, at pH 7.5. Bound and free Hb peaks’ area under the curve was quantified against the Hb standard curve. We quality controlled each assay run using three haemoglobin-haptoglobin complex standards (200 μg/ml, 10 μg/ml and 1 μg/ml) covering the dynamic range of the assay. We determined haptoglobin phenotype using two methods: inspection of serum UPLC chromatograms 26 and non-denaturing Western blot using 1:5000 polyclonal rabbit anti-haptoglobin antibody (Sigma, Gillingham, Dorset, UK), with 100% concordance.
CSF/serum albumin ratio (Qalb) was determined after measurement of albumin in serum and CSF by rate nephelometry on an IMMAGE Immunochemistry system (Beckman Coulter). Qalb was only measured on day 4 post-ictus onwards, to ensure reliability as a measure of blood-brain barrier permeability, since preliminary data (not shown) established that three days were required for plasma proteins derived from the bleed to be cleared from the intrathecal compartment. For this reason, Qalb was only available in 19 aSAH patients.
Biochemistry – low Fisher grade aSAH
CSF samples from 8 patients with aSAH Grade I-II were identified retrospectively during an ongoing service evaluation of lumbar puncture at the Southampton centre. We excluded cases with delayed presentation (>10 days) and traumatic/repeat lumbar punctures. Xanthochromia was assessed on a UVIKON XS spectrophotometer using Bio-C software (NorthStar Scientific, Bedfordshire, UK). We determined Hb concentration using the Beer-Lambert equation, using the net Hb absorbance at 415nm and an extinction coefficient of 141.2 27.
Statistics
Statistical analyses were conducted in R and SPSS v22. For all studies, two-tailed hypotheses were tested with alpha = 0.05. Detailed statistical methods are in the online supplementary methods.
Results
GOSH study cohort
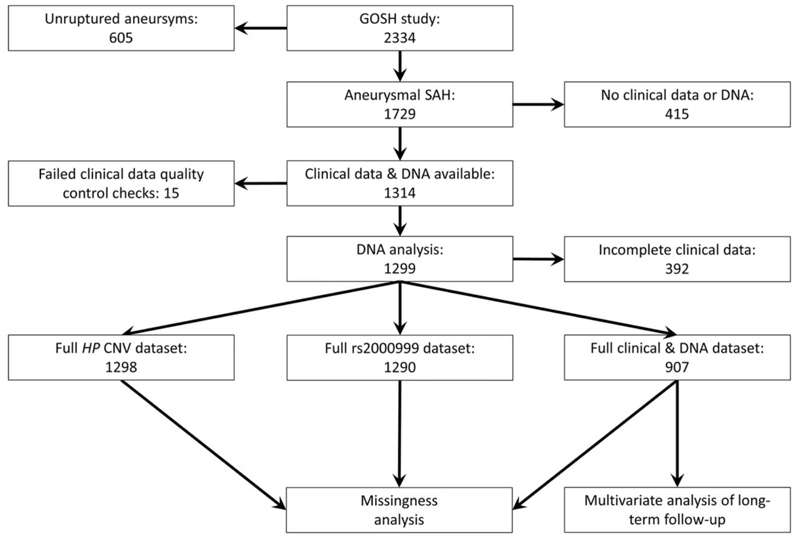
GOSH was a study of long-term outcome in SAH survivors, since patients were assessed after recovery from the acute phase of SAH, with a median time from ictus of 18 months, up to 8 years. A STROBE diagram for the GOSH study participants used in this work is shown in Figure 2. The demographic and clinical characteristics of the GOSH cohort are shown in Table 1 and Supplementary Figure 1.
Figure 2.
STROBE diagram
Table 1.
GOSH demographics and clinical characteristics: whole cohorta and clinical outcome datasetb. Notes: Mean & rangec, number and %d, median & rangee, % reported is of available dataa or of total datab, NA: DNA not available.
| Entire aSAH cohorta | Missingness analysisa | Outcome analysisb | |
|---|---|---|---|
| Number | 1729 | 1299 | 907 |
| Age (years) c | 53.2 (12-92) | 53 (16-92) | 53 (19-92) |
| female | 1215 (70.3%) | 914 (70%) | 646 (71%) |
| 5 | 104 (6.3%) | 77 (6.2%) | 57 (6.3%) |
| 4 | 607 (38.9%) | 457 (38.7%) | 347 (38.3%) |
| Absent | 1121(64.8%) | 840 (65%) | 583 (64%) |
| Supportive | 65 (3.7%) | 14(1%) | 7 (1%) |
| Not classified | 107 (6.19%) | 35(3%) | 7 (1%) |
| Not administered | 117 (6.8%) | 88 (7%) | 37 (4%) |
| Time since ictus (months) e | 15 (0-519) | 18(0-519) | 17 (0-96) |
| Absent | 1187 (68.7%) | 916 (71%) | 633 (70%) |
| Absent | 1660 (96%) | 1246 (96%) | 873 (96%) |
| Smoking (pack-years)c | 20.9 (0-137) | 17(0-137) | 17 (0-137) |
| Absent | 1379 (79.8%) | 1026 (80%) | 711(78%) |
| Absent | 1151(93%) | 1146 (93%) | 843 (93%) |
| HP2-2 | NA | 481 (37%) | 341 (37%) |
| GG | NA | 854(66%) | 598 (66%) |
We considered three essential points to ensure our conclusions are valid. First, because of a potential selection bias toward survivors or those with better functional outcomes in the GOSH study we compared HP genotype frequencies in GOSH versus a young adult control population (with minimal bias as a result of disease, country of origin, sex and healthcare) from a subset of the ALSPAC (Avon Longitudinal Study of Parents and Children) study, previously genotyped for the HP CNV and rs2000999 (n=927). HP CNV and rs2000999 genotype frequencies in GOSH were as expected, when compared to ALSPAC (χ2=2.19, p=0.33 and χ2=0.39, p=0.82, respectively, Supplementary Table 1). Sex was significantly different between GOSH versus ALSPAC (70% versus 51% for females respectively, χ2=81.15, p<0.0001), but there was no sex difference in the HP CNV and rs2000999 genotype frequencies in the ALSPAC cohort (χ2=1.39, p=0.50 and χ2=2.31, p=0.32, respectively).
Second, although the HP CNV and rs2000999 are reported to influence haptoglobin expression levels in other ethnic groups 17,28,29, we confirmed this in a subset of the ALSPAC study in whom the HP CNV, rs2000999 and plasma haptoglobin concentration were all available (n=325). In multivariable linear regression, the HP2 allele and rs2000999 A allele were both associated with a similar decrease in plasma haptoglobin of 0.21 and 0.16 g/L respectively (Supplementary Table 2).
Third, since the clinical dataset sample size was smaller (n=907) compared to the whole GOSH cohort (n=1299) (Table 1), we searched for evidence of bias within the GOSH population with clinical data. There was no missingness of any genotype compared to ALSPAC within these 907 patients (HP CNV: χ2=1.262, p=0.53 and rs 2000999: χ2=0.228, p=0.89, respectively). Moreover the demographic and clinical characteristics of the GOSH participants with available clinical data were similar to those of the whole GOSH cohort (Table 1).
HP genotype and long-term outcome
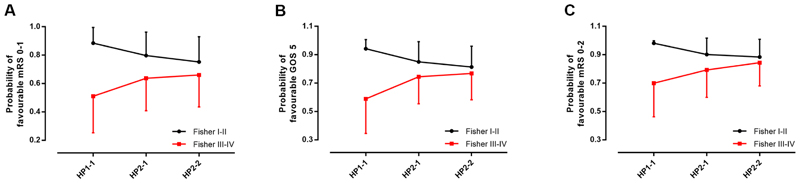
Next, we investigated the effect of HP genotype on favourable functional outcome (defined as modified Rankin scale 0-1) using multivariable logistic regression (Table 2). Favourable outcome was predicted by lower aSAH severity assessed by the clinical World Federation of Neurosurgical Societies score, lower haemorrhage burden as assessed by Fisher category (grades I-II), coiling versus clipping, and absence of hydrocephalus, diabetes, hypercholesterolaemia and non-SAH related neurological disability, but not rs2000999. There was a strong interaction between the haemorrhage volume (Fisher category) and the HP CNV (Tables 2&3, Figure 3A). HP CNV predicted long-term outcome in high Fisher category patients (HP2-2 versus HP1-1, Odds ratio of favourable outcome = 2.6, 95% CI 1.4-4.9, p = 0.003), but not low Fisher category patients (Odds ratio = 2.0, 95% CI 0.71-5.6, p = 0.194). On the other hand, the Fisher category predicted long-term outcome in HP1-1, but not in HP2-2 patients (Tables 2&3, Figure 3A). In essence, the poor prognostic effect of a high Fisher category was attenuated by HP2-2, while the Fisher category effect dominated in patients with HP1-1.
Table 2.
Logistic regression model for primary outcome (favourable mRS 0-1). Logistic regression model fit was excellent (log-likelihood chi-squared test p<10-27; Hosmer & Lemeshow test p=0.305). The model explained 32% of the variance in functional outcome. WFNS = World Federation of Neurosurgical Societies; OR = Odds ratio; CI = confidence interval.
| p (overall effect) | OR | 95% CI | Contrast (vs reference) | P (contrast) | ||
|---|---|---|---|---|---|---|
| Age | 0.490 | 0.995 | 0.980 | 1.010 | ||
| Sex | 0.446 | 1.156 | 0.797 | 1.677 | Female (vs male) | |
| WFNS | <0.001 | 4.787 | 2.404 | 9.531 | WFNS 1 (vs 5) | <0.001 |
| Hydrocephalus | <0.001 | 2.004 | 1.386 | 2.897 | Absent (vs present) | <0.001 |
| Aneurysmal treatment | 0.005 | 1.817 | 1.194 | 2.763 | Coilingvs clipping | 0.014 |
| Nimodipine | 0.111 | 1.914 | 0.862 | 4.249 | Given vs not given | |
| Followup time | 0.121 | 1.007 | 0.998 | 1.015 | ||
| Centre | <0.001 | <0.001 | ||||
| Hypertension | 0.761 | 0.951 | 0.650 | 1.392 | Absent (vs present) | |
| Diabetes | 0.035 | 2.529 | 1.068 | 5.986 | Absent (vs present) | |
| Smoking (pack-years) | 0.441 | 1.003 | 0.995 | 1.012 | ||
| Hypercholesterolemia | 0.023 | 1.636 | 1.070 | 2.502 | Absent (vs present) | |
| Non-aSAH related disability | <0.001 | 5.536 | 2.984 | 10.271 | Absent (vs present) | |
| rs2000999 | 0.359 | 1.469 | 0.646 | 3.341 | GG vs AA | 0.154 |
| Fisher x HP | 0.013 | |||||
| Fisher | 0.009 | 4.105 | 1.428 | 11.806 | Low vs high Fisher in HP 1-1 | |
| HP | 0.011 | 2.602 | 1.381 | 4.904 | HP2-2 vs HP1-1 at high Fisher | 0.003 |
Table 3.
The effects of haemorrhage burden (Fisher category) and HP CNV on favourable outcome (mRS 0-1) are mutually dependent.
| n (HP 1-1 versus HP2-2) | OR | 95% C.I. | P | ||
|---|---|---|---|---|---|
| Low Fisher (III) | 181 (52 vs 129) | 1.991 | 0.705 | 5.628 | 0.194 |
| High Fisher (III-IV) | 302 (90 vs212) | 0.384 | 0.204 | 0.724 | 0.003 |
| n (HP2-1 versus HP2-2) | OR | 95% C. I. | P | ||
| Low Fisher (III) | 302(173 vs 129) | 1.433 | 0.771 | 2.665 | 0.255 |
| High Fisher (III-IV) | 463 (251 vs 212) | 0.660 | 0.418 | 1.043 | 0.075 |
| n (HP2-1 versus HP1-1) | OR | 95% C.I. | P | ||
| Low Fisher (III) | 225 (173 vs 52) | 0.502 | 0.178 | 1.419 | 0.194 |
| High Fisher (III-IV) | 341 (251 vs 90) | 1.718 | 0.951 | 3.102 | 0.073 |
| n (low versus high Fisher) | OR | 95% C.I. | P | ||
|---|---|---|---|---|---|
| HP1-1 | 142 (52 vs 90) | 4.105 | 1.428 | 11.806 | 0.009 |
| HP2-2 | 341 (129 vs 212) | 1.262 | 0.703 | 2.265 | 0.435 |
Figure 3.
HP association with outcome after aSAH depends on clot volume. A: The mean predicted probability of favourable outcome (mRS: 0-1) ± standard deviation, by HP CNV and Fisher category. The HP CNV predicted long-term outcome in high Fisher category patients (HP2-2 versus HP1-1, Odds ratio (OR) of favourable outcome = 2.6, 95% CI 1.4-4.9, p = 0.007). In the low Fisher category, there is a trend suggesting that the reverse might be happening (i.e. that HP1-1 confers a favourable outcome versus HP2-2), but this was not significant (OR=2.0, 95% CI 0.71-5.6, p = 0.194), despite the lower standard deviations in the low Fisher category. B: The mean predicted probability of favourable outcome (GOS: 5) ± standard deviation, by HP CNV and Fisher category. At high Fisher grade: p=0.003, OR=2.74 (95% CI: 1.4-5.3) for HP2-2 versus HP1-1. At low Fisher grade: p=0.045, OR=0.26 (95% CI: 0.07-0.97) for HP2-2 versus HP1-1. C: The mean predicted probability of favourable outcome (mRS: 0-2) ± standard deviation, by HP CNV and Fisher category. At high Fisher: p=0.002, OR=3.26 (95% CI: 1.5-6.9) for HP2-2 versus HP1-1. At low Fisher: p=0.149, OR=0.211 (95% CI: 0.03-1.7) for HP2-2 versus HP1-1.
There was no evidence of missingness within high or low Fisher category groups that could have biased the results, as shown by several analyses: (1) HP CNV genotype frequency was not significantly different between low and high Fisher category groups (χ2=1.112, p=0.57); (2) HP CNV genotype frequency in the high and low Fisher category groups was not significantly different from the ALSPAC control cohort (χ2=1.685, p=0.43 and χ2=0.794, p=0.67 respectively); (3) HP genotype frequency of patients excluded from the regression due to data availability was not significantly different from that of the included patients (HP CNV: χ2=0.378, p=0.97 and rs 2000999: χ2=0.288, p=0.87, respectively) or the ALSPAC control cohort (HP CNV: χ2=2.181, p=0.34 and rs 2000999: χ2=0.562, p=0.76, respectively).
Sensitivity analyses
A similar pattern was confirmed in five sensitivity analyses: (1) using the Glasgow Outcome Scale 30 (Figure 3B); (2) using an alternative dichotomization of the modified Rankin scale, with a favourable outcome defined as 0-2 (Figure 3C); (3) using non-dichotomized Fisher grade (Supplementary Figure 2); (4) using multiple imputation on the whole GOSH cohort (Supplementary Table 4); and (5) analyses across decreasing follow-up intervals (Table 4). The finding that the HP2 allele predicted long-term outcome in high Fisher category patients was robust to decreasing follow-up time intervals, except at one year. This was not due to smaller sample sizes since the 3-8 epoch had a similar sample size to the ≤ 1 year epoch.
Table 4.
Sensitivity analysis at different follow-up intervals. Findings are largely robust, except at shorter follow-up of one year or less.
| Sample size | Time since ictus | HP CNV1 | HP CNV1 | rs20009992 |
|---|---|---|---|---|
| Low Fisher grade | High Fisher grade | |||
| 907 | ≤ 8 years | 0.5,0.2-1.4,0.194 | 2.6, 1.4-4.9, 0.003 | NS, p = 0.359 |
| 863 | ≤ 6 years | 0.4, 0.1-1.2,0.106 | 2.8, 1.5-5.4, 0.002 | NS, p = 0.263 |
| 776 | ≤ 4 years | 0.5,0.2-1.5,0.193 | 2.8, 1.4-5.4, 0.003 | NS, p = 0.258 |
| 575 | ≤ 2 years | 0.5,0.2-1.7,0.258 | 2.0, 1.1-3.4,0.100 | NS, p = 0.807 |
| 349 | ≤ 1 year | 0.5,0.1-2.4,0.367 | 1.6,0.5-5.1,0.415 | NS, p = 0.967 |
| 204 | ≤ 6 months | 0.2, 0.02-1.5, 0.114 | 0.8,0.1-4.8,0.777 | NS, p = 0.929 |
| 87 | ≤ 3 months | NS,p = 1.0 | NS,p = 1.0 | NS, p= 1.0 |
| 332 | 3-8 years | 0.4, 0.04-4.3, 0.404 | 4.4, 1.3-14.4, 0.014 | NS, p = 0.215 |
| Sample size | Time since ictus | HP CNV1 | HP CNV1 | rs20009992 |
|---|---|---|---|---|
| Low Fisher grade | High Fisher grade | |||
| 907 | ≤ 8 years | 0.3,0.1-1.0, 0.045 | 2.7, 1.4-5.3, 0.003 | NS,p = 0.415 |
| 863 | ≤ 6 years | 0.3,0.1-1.0, 0.047 | 2.8, 1.4-5.5, 0.002 | NS, p = 0.472 |
| 776 | ≤ 4 years | 0.3,0.1-1.3,0.119 | 2.9, 1.4-5.9, 0.003 | NS,p = 0.532 |
| 575 | ≤ 2 years | 0.3,0.1-1.4,0.132 | 2.8, 1.2-6.4, 0.019 | NS, p = 0.627 |
| 349 | ≤ 1 year | 0.4, 0.1-2.7,0.370 | 2.8,0.7-10.6, 0.128 | NS, p = 0.856 |
| 204 | ≤ 6 months | 0.09, 0.01-1.5,0.91 | 6.4,0.5-87,0.165 | NS, p = 0.677 |
| 87 | ≤ 3 months | NS,p = 1.0 | NS,p = 1.0 | NS,p= 1.0 |
| 332 | 3-8 years | 0.0, 0.0-0.0, 0.998 | 4.0, 1.1-15.1, 0.039 | NS,p = 0.512 |
HP2-2 versus HP1-1, for favourable outcome
rs2000999 G versus A, for favourable outcome
Biochemical studies
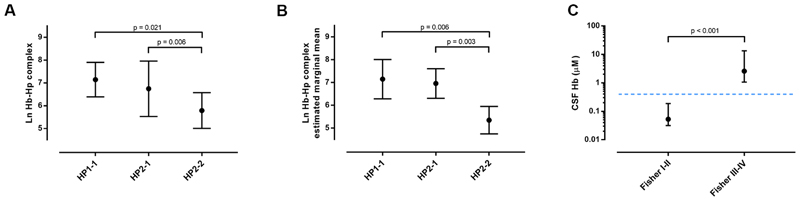
Although the HP CNV and rs2000999 affect haptoglobin expression level to a similar extent 18, only the HP CNV associated with outcome after aSAH, suggesting that functional differences between Hp1-1 and Hp2-2 proteins, perhaps relating to Hb scavenging rather than expression, are likely to be more important. Hence we measured haemoglobin-haptoglobin complexes in serial CSF samples taken from an external ventricular drain after high-grade aSAH (Fisher grade III-IV, n=44, Supplementary Table 3), using ultra-performance size-exclusion liquid chromatography coupled with absorption detection at 415nm. The patients’ HP CNV status was: HP1-1=9, HP2-1=19, HP2-2=16. All samples contained haemoglobin-haptoglobin complexes, in keeping with saturation of membrane CD163 binding sites in the brain after aSAH, as previously reported 3. The CSF concentration of haemoglobin-haptoglobin complexes was compared across HP CNV types using ANOVA, and was lower in HP2-2 patients than those with HP1-1 (Figure 4A). In an analysis of covariance of CSF haemoglobin-haptoglobin complex concentration across HP CNV genotype, controlling for age, sex, clot volume, and CSF/serum albumin quotient, the HP CNV genotype was the dominant determinant, explaining 50% of variance in CSF haemoglobin-haptoglobin complex concentration, out of a total of 57% by the whole model (p=0.001, Figure 4B).
Figure 4.
Haptoglobin-haemoglobin complex scavenging in CSF varies with the HP CNV in high-grade aSAH. A. ANOVA of CSF haemoglobin-haptoglobin complex concentration across HP CNV types (n=44, p=0.003, F=6.58, df=43). Post-hoc group comparisons were performed using Bonferroni adjustment. Plot shows means ± standard deviation. B. ANCOVA of CSF haemoglobin-haptoglobin complex concentration across HP CNV types controlling for age, clot volume, CSF/serum albumin quotient and sex (n=19, p=0.006 and partial eta squared=0.566 for model). We performed group comparisons with Bonferroni adjustment. The plot shows estimated marginal means ± 95% confidence intervals. C. CSF Hb concentration in Fisher grade I-II (n=8) and III-IV (n=44). Plot shows medians ± interquartile range. Mann-Whitney U test. Dotted line represents the Hb-binding capacity of haptoglobin in CSF.
The effect of the HP CNV on long-term outcome varied with the volume of aSAH. It is known that haptoglobin in the CSF is present at very low concentrations in both healthy controls and after aSAH, such that after high-grade aSAH, haptoglobin is saturated with Hb 3. We confirmed this observation in our patients; median CSF haptoglobin was 0.29μM (interquartile range: 0.11-0.58μM, expressed as Hb dimer binding capacity) and it was fully saturated with Hb. The low haptoglobin concentration in the CSF has a potential to set up a situation where the system could operate differently depending on Hb concentration. After low-volume aSAH, Hb concentration may be low such that there is sufficient haptoglobin to bind all the Hb, while after high-volume aSAH, the system may be overwhelmed. Ideally one would study haptoglobin saturation with Hb in the CSF from high and low Fisher aSAH patients. However it was challenging to prospectively identify CSF samples from Fisher I-II aSAH patients, since CSF drainage has no place in their clinical management. Nevertheless, we were able to study retrospective data from Fisher I-II aSAH cases referred for spectrophotometric testing for xanthochromia (median days post-ictus = 2 days, interquartile range 1-3 days, Supplementary Table 3). The median Hb concentration in the CSF of patients with Fisher III-IV aSAH was 2.58μM (interquartile range: 1.07-13.5μM, n=44), i.e. well above the 0.29μM Hb-binding capacity of Hp. In the CSF of patients with Fisher I-II aSAH, the mean Hb CSF concentration was 0.053μM (0.032-0.189μM, n=8), i.e. well below 0.29μM (p < 0.001, Figure 4C). These findings provide a potential explanation for the observation that the HP2-2 genotype is only protective after high-volume aSAH.
Discussion
This is the largest study of HP genotype and outcome after SAH, and provides a number of novel insights. The HP allele does not associate with outcome after aSAH if this is measured early after aSAH, within the first year 10. We argue that the HP influence on outcome is overshadowed by the effect of early brain injury on outcome in the first year after aSAH, i.e. it takes longer than previously thought for early brain injury effect to settle. In support of this interpretation, we show that the HP2 allele’s association with good functional outcome was only detectable two years or more after aSAH (Table 4).
We found that after low-volume aSAH, CSF Hb concentration was within the Hb-binding capacity of CSF haptoglobin, while it exceeded this concentration in high-volume patients. Hence high-volume patients have unbound Hb available to impact on outcome, so that functional differences between HP genotypes makes a difference after high-volume aSAH.
Collectively, these data suggest that the association of the HP2 allele on long-term outcome after aSAH depends on the haemorrhage burden (Fisher category) and Hb concentration in the CSF. In the presence of high CSF Hb concentration, the HP2 allele is superior to the HP1 allele, being associated with lower haemoglobin-haptoglobin complexes in the CSF and a better functional outcome. At low haemorrhage burden and CSF Hb concentration, the HP CNV does not associate with long-term outcome. That the differential clinical effect of the HP CNV is mediated via mechanisms other than haptoglobin expression level is supported by the fact that while both the HP CNV and rs2000999 associate with haptoglobin expression, only the HP CNV is linked to long-term outcome. A recent study has found that lumbar CSF drainage improves outcome in high but not low modified Fisher grade patients 31, which resonates with our findings here.
There is conflicting evidence in the literature regarding the relative efficacy of haptoglobin types in CD163-mediated cellular uptake of haemoglobin-haptoglobin complexes. Although one study suggested that haemoglobin-haptoglobin complex uptake is better with Hp1-1 12, two subsequent studies have reported that Hp2-2 is better 13,14 which would be in keeping with the results from biochemical binding studies 1,12. Although the differences between these in vitro studies may be due to experimental technicalities, the conflicting results suggest that the difference between the two alleles may not be marked. However it is possible that a subtle difference between HP1 and HP2 allele protein products is amplified in the brain where the low CD163 expression level is a limiting factor in Hb scavenging 3,32. The low haemoglobin-haptoglobin complex concentration in the CSF of HP2 carriers could be due to lower haptoglobin expression in the CSF, as would be expected for the HP2 allele. However lower CSF haptoglobin levels in HP2 carriers would carry a worse outcome after SAH, not a better one. Also rs2000999 did not associate with outcome. It is therefore more likely that haemoglobin-haptoglobin complex scavenging after high-grade SAH is better in HP2 carriers, versus HP1. The higher valency of Hp2-containing complexes likely improves clustering of CD163 receptors 33. The larger size of the Hb-Hp2-2 complexes (compared to the smaller Hb-Hp1-1 complexes), may also prevent their entry into the brain parenchyma, thereby reducing neurotoxicity. These explanations need further careful study.
The association of the HP2 allele with good long-term outcome in high Fisher grade patients is in contrast to the findings from a mouse model of SAH where HP2-2-transgenic animals had a worse outcome compared to HP1-1 wild-type mice 34. It is important to bear in mind that there are marked differences in the biochemistry of Hb scavenging between mouse and man. In particular, the haptoglobin receptor CD163 has a higher affinity for haemoglobin-haptoglobin complexes in man, but not in mice 35. Also, human CD163 is cleaved during inflammation, releasing soluble CD163, but this does not happen with mouse CD163 36. For these two reasons, differences in haptoglobin types with respect to CD163 binding are more likely to be important in humans than in mice.
In conclusion, in patients with aSAH who have a high haemorrhage burden, the HP2 allele is associated with favourable long-term functional outcome, possibly via improved haemoglobin-haptoglobin complex clearance. Our findings suggest that preclinical trials of haptoglobin supplementation should consider testing Hp1-1 versus Hp2-2. Also, the HP CNV genotype and its interaction with Fisher grade should be considered when designing prognostic algorithms and clinical trials in aSAH.
Supplementary Material
Acknowledgements.
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC and GOSH teams, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses.
Funding.
The GOSH study was funded by The Stroke Association and supported by the National Institute of Health Research (NIHR) Stroke Research Network. This research was also funded by UK MRC grant MR/L01453X/1 (MM, IG) and by Cancer Research UK program grant C18281/A19169 (NK). TG receives funding from the UK MRC (MRC Integrative Epidemiology Unit, MC_UU_00011/4). The UK Medical Research Council (MRC) and Wellcome Trust (Grant ref: 102215/2/13/2) and the University of Bristol provide core support for ALSPAC. GWAS data was generated by Sample Logistics and Genotyping Facilities at Wellcome Sanger Institute and LabCorp (Laboratory Corporation of America) using support from 23andMe. A comprehensive list of grant funding is available on the ALSPAC website (http://www.bristol.ac.uk/alspac/external/documents/grant-acknowledgements.pdf). This publication is the work of the authors who will serve as guarantors for the contents of this paper.
Footnotes
Author contributions
Concept: DJW, IG. Design: MJM, NK, TG, ICH, DB, DJW, IG. Data contributors: DJW, DB, all GOSH investigators and ALSPAC. Analysis: MJM, NK, ICH, IG. Manuscript: all authors.
Competing Interests
Nothing to report.
References
- 1.Kristiansen M, Graversen JH, Jacobsen C, et al. Identification of the haemoglobin scavenger receptor. Nature. 2001;409(6817):198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 2.Bulters D, Gaastra B, Zolnourian A, et al. Haemoglobin scavenging in intracranial bleeding: biology and clinical implications. Nature Reviews Neurology. 2018;14(7):416–32. doi: 10.1038/s41582-018-0020-0. [DOI] [PubMed] [Google Scholar]
- 3.Galea J, Cruickshank G, Teeling JL, et al. The intrathecal CD163-haptoglobin-hemoglobin scavenging system in subarachnoid hemorrhage. J Neurochem. 2012;121(5):785–92. doi: 10.1111/j.1471-4159.2012.07716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larsson M, Cheng T-M, Chen C-Y, et al. Unique Assembly Structure of Human Haptoglobin Phenotypes 1-1, 2-1, and 2-2 and a Predominant Hp 1 Allele Hypothesis. In: Janciauskiene S, editor. Acute Phase Proteins. Ch. 7 Rijeka: InTech; 2013. [Google Scholar]
- 5.Borsody M, Burke A, Coplin W, et al. Haptoglobin and the development of cerebral artery vasospasm after subarachnoid hemorrhage. Neurology. 2006;66(5):634–40. doi: 10.1212/01.wnl.0000200781.62172.1d. [DOI] [PubMed] [Google Scholar]
- 6.Galea J, Cruickshank G, Teeling JL, et al. The intrathecal CD163-haptoglobin-hemoglobin scavenging system in subarachnoid hemorrhage. Journal of neurochemistry. 2012;121(5):785–92. doi: 10.1111/j.1471-4159.2012.07716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kantor E, Bayır H, Ren D, et al. Haptoglobin genotype and functional outcome after aneurysmal subarachnoid hemorrhage. Journal of neurosurgery. 2014;120(2):386–90. doi: 10.3171/2013.10.JNS13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leclerc JL, Blackburn S, Neal D, et al. Haptoglobin phenotype predicts the development of focal and global cerebral vasospasm and may influence outcomes after aneurysmal subarachnoid hemorrhage. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(4):1155–60. doi: 10.1073/pnas.1412833112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohnishi H, Iihara K, Kaku Y, et al. Haptoglobin phenotype predicts cerebral vasospasm and clinical deterioration after aneurysmal subarachnoid hemorrhage. Journal of stroke and cerebrovascular diseases: the official journal of National Stroke Association. 2013;22(4):520–6. doi: 10.1016/j.jstrokecerebrovasdis.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Gaastra B, Ren D, Alexander S, et al. Haptoglobin genotype and aneurysmal subarachnoid hemorrhage: Individual patient data analysis. Neurology. 2019 doi: 10.1212/WNL.0000000000007397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaastra B, Glazier J, Bulters D, et al. Haptoglobin Genotype and Outcome after Subarachnoid Haemorrhage: New Insights from a Meta-Analysis. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/6747940. 6747940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asleh R, Marsh S, Shilkrut M, et al. Genetically determined heterogeneity in hemoglobin scavenging and susceptibility to diabetic cardiovascular disease. Circulation research. 2003;92(11):1193–200. doi: 10.1161/01.RES.0000076889.23082.F1. [DOI] [PubMed] [Google Scholar]
- 13.Lipiski M, Deuel JW, Baek JH, et al. Human Hp1-1 and Hp2-2 phenotype-specific haptoglobin therapeutics are both effective in vitro and in guinea pigs to attenuate hemoglobin toxicity. Antioxid Redox Signal. 2013;19(14):1619–33. doi: 10.1089/ars.2012.5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Na N, Ouyang J, Taes YE, et al. Serum free hemoglobin concentrations in healthy individuals are related to haptoglobin type. Clin Chem. 2005;51(9):1754–5. doi: 10.1373/clinchem.2005.055657. [DOI] [PubMed] [Google Scholar]
- 15.Hostettler IC, Alg VS, Shahi N, et al. Characteristics of Unruptured Compared to Ruptured Intracranial Aneurysms: A Multicenter Case–Control Study. Neurosurgery. 2017 doi: 10.1093/neuros/nyx365. nyx365-nyx65. [DOI] [PubMed] [Google Scholar]
- 16.Boettger LM, Salem RM, Handsaker RE, et al. Recurring exon deletions in the HP (haptoglobin) gene contribute to lower blood cholesterol levels. Nature Genetics. 2016;48:359. doi: 10.1038/ng.3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Froguel P, Ndiaye NC, Bonnefond A, et al. A Genome-Wide Association Study Identifies rs2000999 as a Strong Genetic Determinant of Circulating Haptoglobin Levels. Plos One. 2012;7(3):e32327. doi: 10.1371/journal.pone.0032327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kazmi N, Koda Y, Ndiaye NC, et al. Genetic determinants of circulating haptoglobin concentration. Clinica Chimica Acta. 2019;494:138–42. doi: 10.1016/j.cca.2019.03.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farrell B, Godwin J, Richards S, et al. The United Kingdom transient ischaemic attack (UK-TIA) aspirin trial: final results. J Neurol Neurosurg Psychiatry. 1991;54(12):1044–54. doi: 10.1136/jnnp.54.12.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rankin J. Cerebral vascular accidents in patients over the age of 60. II. Prognosis. Scott Med J. 1957;2(5):200–15. doi: 10.1177/003693305700200504. [DOI] [PubMed] [Google Scholar]
- 21.Quinn TJ, Dawson J, Walters MR, et al. Reliability of the modified Rankin Scale: a systematic review. Stroke. 2009;40(10):3393–5. doi: 10.1161/STROKEAHA.109.557256. [DOI] [PubMed] [Google Scholar]
- 22.Teasdale GM, Drake CG, Hunt W, et al. A universal subarachnoid hemorrhage scale: report of a committee of the World Federation of Neurosurgical Societies. J Neurol Neurosurg Psychiatry. 1988;51(11):1457. doi: 10.1136/jnnp.51.11.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6(1):1–9. doi: 10.1227/00006123-198001000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Boyd A, Golding J, Macleod J, et al. Cohort Profile: the 'children of the 90s'--the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol. 2013;42(1):111–27. doi: 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fraser A, Macdonald-Wallis C, Tilling K, et al. Cohort Profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol. 2013;42(1):97–110. doi: 10.1093/ije/dys066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delanghe J, Allcock K, Langlois M, et al. Fast determination of haptoglobin phenotype and calculation of hemoglobin binding capacity using high pressure gel permeation chromatography. Clinica Chimica Acta. 2000;291(1):43–51. doi: 10.1016/s0009-8981(99)00194-1. [DOI] [PubMed] [Google Scholar]
- 27.Meng F, Alayash AI. Determination of extinction coefficients of human hemoglobin in various redox states. Analytical Biochemistry. 2017;521:11–19. doi: 10.1016/j.ab.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bjornsson E, Helgason H, Halldorsson G, et al. A rare splice donor mutation in the haptoglobin gene associates with blood lipid levels and coronary artery disease. Human Molecular Genetics. 2017;26(12):2364–76. doi: 10.1093/hmg/ddx123. [DOI] [PubMed] [Google Scholar]
- 29.Soejima M, Sagata N, Komatsu N, et al. Genetic factors associated with serum haptoglobin level in a Japanese population. Clinica Chimica Acta. 2014;433:54–57. doi: 10.1016/j.cca.2014.02.029. [DOI] [PubMed] [Google Scholar]
- 30.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1(7905):480–4. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 31.Fang Y, Shao Y, Lu J, et al. The effectiveness of lumbar cerebrospinal fluid drainage in aneurysmal subarachnoid hemorrhage with different bleeding amounts. Neurosurg Rev. 2019 doi: 10.1007/s10143-019-01116-1. [DOI] [PubMed] [Google Scholar]
- 32.Durnford A, Dunbar J, Galea J, et al. Haemoglobin scavenging after subarachnoid haemorrhage. Acta Neurochir Suppl. 2015;120:51–4. doi: 10.1007/978-3-319-04981-6_9. [DOI] [PubMed] [Google Scholar]
- 33.Andersen CB, Torvund-Jensen M, Nielsen MJ, et al. Structure of the haptoglobin-haemoglobin complex. Nature. 2012;489(7416):456–9. doi: 10.1038/nature11369. [DOI] [PubMed] [Google Scholar]
- 34.Chaichana KL, Levy AP, Miller-Lotan R, et al. Haptoglobin 2-2 genotype determines chronic vasospasm after experimental subarachnoid hemorrhage. Stroke. 2007;38(12):3266–71. doi: 10.1161/STROKEAHA.107.490003. [DOI] [PubMed] [Google Scholar]
- 35.Etzerodt A, Kjolby M, Nielsen MJ, et al. Plasma clearance of hemoglobin and haptoglobin in mice and effect of CD163 gene targeting disruption. Antioxid Redox Signal. 2013;18(17):2254–63. doi: 10.1089/ars.2012.4605. [DOI] [PubMed] [Google Scholar]
- 36.Etzerodt A, Rasmussen MR, Svendsen P, et al. Structural basis for inflammation-driven shedding of CD163 ectodomain and tumor necrosis factor-alpha in macrophages. J Biol Chem. 2014;289(2):778–88. doi: 10.1074/jbc.M113.520213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.