Abstract
Research into obtaining a fast, valid, reliable and non-invasive measure of core temperature is of interest in many disciplinary fields. Occupational and sports medicine research has attempted to determine a non-invasive proxy for core temperature particularly when access to participants is limited and thermal safety is of a concern due to protective encapsulating clothing, hot ambient environments and/or high endogenous heat production during athletic competition. This investigation aimed to determine the validity of inner canthus of the eye temperature (TEC) as an alternate non-invasive measure of intestinal core temperature (TC) during rest, exercise and post-exercise conditions. Twelve physically active males rested for 30 min prior to exercise, performed 60 min of aerobic exercise at 60% V̇O2max and passively recovered a further 60 min post-exercise. TEC and TC were measured at 5 min intervals during each condition. Mean differences between TEC and TC were 0.61 °C during pre-exercise, −1.78 °C during exercise and −1.00 °C during post-exercise. The reliability between the methods was low in the pre-exercise (ICC=0.49 [−0.09 to 0.82]), exercise (ICC=−0.14 [−0.65 to 0.44]) and post-exercise (ICC=−0.25 [−0.70 to 0.35]) conditions. In conclusion, poor agreement was observed between the TEC values measured through IRT and TC measured through a gastrointestinal telemetry pill. Therefore, TEC is not a valid substitute measurement to gastrointestinal telemetry pill in sports and exercise science settings.
Abbreviations: ANOVA, Analysis of variance; CI, confidence intervals; HR, heart rate; ICC, Intraclass correlation coefficient; IRT, infrared thermography; SARS, severe acute respiratory syndrome; TC,, core temperature; TEC,, inner canthus of the eye temperature
Keywords: Assessment, Body temperature, Thermal imaging, Thermometry, Thermoregulation
Highlights
-
•
Inner canthus of the eye is not a valid measure of intestinal core temperature.
-
•
Inner canthus of the eye temperature has severe errors in exercises settings.
-
•
The variability is more evident in the aerobic exercise and post-exercise conditions.
1. Introduction
The measurement of core temperature (T C) is fundamental in human thermoregulation studies under resting, exercise and post-exercise conditions (Cuddy et al., 2014, West et al., 2014, Xu et al., 2013). Small T C increases result in specific physiological responses such as increased cutaneous vasodilation and sweating, which helps to dissipate excess body heat during exercise (Romanovsky, 2014, Sawka et al., 2011). Research into obtaining a fast, valid, reliable and non-invasive measure of core temperature is of interest in many disciplinary fields. For example, during the 2009 outbreak of severe acute respiratory syndrome (SARS) generated by Influenza A (H1N1) in Southeast Asia, thermal images obtained through infrared thermography (IRT) were successfully used for screening potential infected individuals at international airports, becoming an important primary step for mass prevention of the spread of SARS (Chan et al., 2004, Lahiri et al., 2012, Ng et al., 2004, Ring and Ammer, 2012, Teunissen and Daanen, 2011). Furthermore, occupational and sports medicine research has attempted to determine a non-invasive proxy for core temperature particularly when access to participants is limited and thermal safety is of a concern due to protective encapsulating clothing (Montain et al., 1994), hot ambient environments (Taylor, 2006) and/or high endogenous heat production during athletic competition (Laursen et al., 2006).
Previous investigations have attempted to locate various regions within the face (Bourlai et al., 2012, Chan et al., 2004, Ng et al., 2004) that correlate to core temperature. Unfortunately, some of these investigations failed to apply sufficient standardization of IRT (Bourlai et al., 2012) or employed core temperature measures prone to bias from ambient conditions or patient morphology (Daanen, 2006) such as tympanic (Ng et al., 2004) or oral temperature (Chan et al., 2004). Recent work by Teunissen and Daanen (2011) has identified the inner canthus of the eye (T EC) as a potential site in which core temperature could be derived in the exercising individual. Using esophageal temperature as a comparison, the T EC was shown to have large mean bias (1.80+0.89 °C) and variability across a number of conditions including rest, exercise, recovery and passive heating. However Teunissen and Daanen (2011) analyzed both sex and did not describe the procedures for extracting and analyzing the data, which can lead an error, given number of factors that may influence infrared thermography (Fernández-Cuevas et al., 2015). Moreover, although esophageal temperature has been reported as a measure that responds quicker than other techniques to changes in T C it can be influenced by swallowing saliva or beverages (Taylor et al., 2014). Additionally, insertion of a probe in the lower third of the esophagus is necessary (Mekjavic and Rempel, 1990), which may cause vomiting, respiratory tract or throat irritation and additionally is difficult to implement in real world applications such as field-based outdoor measurement (Domitrovich et al., 2010, Easton et al., 2007, Lee et al., 2000).
The blood temperature measured in the pulmonary artery is a “gold standard” for evaluation of T C; however, the fact of being an invasive method makes other techniques more suitable for studies of thermoregulation (Domitrovich et al., 2010, Easton et al., 2007). Thus, the T C measured in the esophagus, rectum and bowel are the most widely used techniques in scientific studies, being considered less-invasive, valid, reliable and reproducible (Domitrovich et al., 2010, Easton et al., 2007, Lee et al., 2000). Rectal temperature is often used by research groups due to the stability of the measure even in the presence of fluid intake or different humidity and room temperatures (Domitrovich et al., 2010, Easton et al., 2007, Lee et al., 2000). However, it requires a wired connection between the temperature sensor and the recorder that makes difficult to collect the data in real conditions, as well as the associated social stigma accompanying the insertion of a sensor approximately 10 cm past the anal sphincter (Domitrovich et al., 2010, Easton et al., 2007). Intestinal temperature measured by an ingested telemetry pill requires no wires, allowing data collection outdoors, but it has high financial cost and ingested pills can be used only once (Domitrovich et al., 2010, Easton et al., 2007, Fernandes et al., 2014, Lee et al., 2000). Intestinal telemetry pills have been validated against rectal (Byrne and Lim, 2007, Teunissen and Daanen, 2011) and esophageal temperature (Byrne and Lim, 2007) when influencing factors such as calibration and timing of ingestion are controlled for.
In this sense, the T EC obtained by IRT appears as an interesting alternative for thermoregulatory studies during exercise, since this method is non-invasive, requires no contact with the evaluated and allows the collection of data in external environments. Indeed, improvements in the accuracy, functionality and affordability of camera technology have seen IRT become a widely adopted method of temperature measurement in exercise and sports science (Costello et al., 2012). It is important that researchers and clinicians are aware of the potential applications and limitations of IRT provides. Thus, the validation of this technique as a simple non-invasive method of T C measurement could represent an advance for the field of exercise physiology, as researchers may have an interesting alternative to measure T C. Therefore, the objective of this study was to verify the validity of the T EC measured by IRT as a T C indicator under resting, exercise and post-exercise in temperate conditions.
2. Methods
2.1. Participants
The volunteers were informed about the procedures for all stages of the investigation and signed informed consent forms prior to enrolment in the study. This study was approved by the local Ethics Committee on Human Research of Federal University of Viçosa (No 134, 2011), which followed the principles outlined by the World Medical Assembly Declaration of Helsinki. This comparative study involved two temperature measurements. The participants consisted of 12 physically active males (age: 22.4±3.3 years, height: 177.0±0.8 cm, percentage body fat (%BF): 10.3±3.0%, body surface area (BSA): 1.92±0.09 m2 and V̇O2max 48.7±4.9 ml min−1 kg−1). The participants were physical education students and were selected by convenience through e-mail invitation. It was considered as exclusion factors the following characteristics: smoker, history of kidney problems, osteo-myo-articular injury in the last two months or lodge any symptoms; symptoms of pain in any body region; sleep disorders; fever in the last seven days; use of antipyretics or diuretic medications; use of any dietary supplement with potential interference in water homeostasis or body temperature in the last two weeks. All volunteers were apparently healthy through the Physical Activity Readiness Questionnarie (PARq) (Thomas et al., 1992). Considering the dynamics of the proposed exercise, were included subjects classified as “physically active” according to the criteria of the American College of Sports Medicine (ACSM) (Garber et al., 2011) for holding regular physical training sessions at least 3 times a week in the last four months. The sample size was determined with the software GPower (version 3.1.9.2; Franz Faul, Universität Kiel, Germany) (Faul et al., 2007). The following design specifications were taken into account: α=0.05; (1-β) =0.8; effect size f =0.6; test family=F test and statistical test=ANOVA repeated measures, between factor.
2.2. Experimental design
The body mass in grams (Filizola®, Star 300/4), height in centimeters (American Medical®, ES2020) and skinfolds in millimeters (CESCORF®, Scientific) were measured by a trained anthropometrist according to the recommendations of the International Society for the Advancement of Kinanthropometry (Marfell-Jones et al., 2006).
Between 8:00 to 8:30 AM, while at home, participants ingested a thermal pill for evaluating their T C with a telemetry system (HQ CorTemp® Inc., HT150002). This allowed for sufficient time prior to the trials commencing (at least 6 h) for the pill to pass from the stomach to the intestines for optimal T C measurement (Domitrovich et al., 2010). Each pill was properly calibrated and certified by the manufacturer. All procedures followed the recommendations proposed by Byrne and Lim (2007) to ensure the validity of the ingested telemetry pills for recording T C.
From 11:00 to 12:00 h, the participants consumed a lunch consisting of foods usually consumed in their daily routine. To avoid physical and thermal stress, the volunteers were transported by car to the laboratory. The volunteers entered the laboratory by 13:30 h and adapted to room temperature for one hour. This room was properly equipped with artificial fluorescent lamps, and the environmental temperature was maintained through a heating/cooling air conditioner (Komeco®, Hi-wall Split). The average temperature remained at 24.9±0.6 °C, and the relative humidity was 62.3±5.7%; both measures were recorded with a digital weather station and anemometer (Instrutherm®, AD-250), which characterized the environment as temperate with null (≅0.2 m/s) wind speed (Bain and Jay, 2011). These environmental conditions are in accordance with the recommendations of James and Ring (2009) for collecting IRT data in fever screening. The heart rate (HR) was monitored at all stages of the study using a heart monitor (Polar Team2 Pro®). The maximal oxygen uptake (V̇O2max) was estimated using a submaximal incremental treadmill test according to the recommendations of the American College of Sports Medicine ACSM (2013) obtained in a pre-session experiment two days before the experiment day.
2.3. Experimental testing
The participants remained standing for 30 min in the test room, both thermal pill temperature and IRT scan were recorded every 5 min, totaling seven collections during the resting condition. For the IRT scan, the volunteer remained in anatomical position in front of the imager at a distance of 1 m for the measurement of thermogram the face in accordance with the recommendations of Ring and Ammer (2000). An infrared imager (FLIR®, T420), with a measurement range from −20 to +120 °C, 2% accuracy, sensitivity ≤0.05 °C, IR spectral band of 7.5–13 µm, refresh rate of 60 Hz, auto-focus and a resolution of 320×240 pixels was used to obtain the thermograms.
The participants completed an interval test on a treadmill consisting of 12 blocks of 5 min each separated by an interval of 1 min. The T EC and T C were recorded during the interval time, totaling 12 collections during the exercise condition. The intensity of the blocks of exercise was individually fixed to 60% of the V̇O2max speed obtained in the pre-session experiment. Participants remained standing for 60 min in the test room, and every 5 min, T EC and T C values were recorded using the IRT or thermal pill for a total of 12 measurements during the entire resting phase. The remaining variables were measured in the same manner as during the pre-exercise period.
2.4. Thermal image processing
After collecting IRT images, the region of interest within the eye was highlighted manually using an ellipse tool available in the software used. This procedure was done observing International Standards Organization recommendations (Ring and Ammer, 2012), with an area exceeding the minimum 9 pixels (225±52) for greater accuracy and representation of temperature, as shown in Fig. 1. In this analysis, each pixel is a measure of temperature, and the median temperature of the area was considered within the analyzed region. Temperatures were recorded in right eye inner canthus of the participant using specific software (FLIR Tools®). The emissivity value adopted for human skin was 0.98 (Lahiri et al., 2012, Ring and Ammer, 2012) and the reflected the room temperature was 25 °C.
Fig. 1.
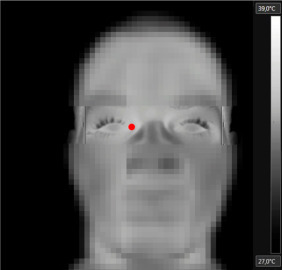
De-identified thermogram showing the location of the inner canthus of the eye where the maximal temperature was registered (red dot). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
2.5. Statistical analysis
The Shapiro-Wilk test was used to assess the normality of the data and Levene's test for homogeneity of variances. The data have shown normal distribution and homogeneity. Considering the assumptions required (dependence of observations, normality of sampling distribution, and uniformity of residuals) (Hopkins, 1997), a two-way repeated measures ANOVA with Bonferroni correction was performed between T EC and T C data. For comparing the difference between methods in each moment, Bonferroni post hoc test was applied. The sphericity was assumed in all calculations. The limits of agreement between pairs of measurements of T EC compared with T C were searched according to the method suggested by Bland and Altman (1986). For reliability of the pairs of values obtained, the Intraclass Correlation Coeficient (ICC) one-way random was used. In this setting, the ICC is the proportion of the total variance that can be ascribed to true differences. Values for the ICC range from 0 to 1. In addition, typical error of the measure which is the standard deviation of the individual values and its range were also calculated. The use of these approaches follows the recommendations of (Atkinson and Nevill, 1998) because there are advantages and disadvantages to each case. Analyses were performed using Microsoft Excel® (Microsoft, Redmond, USA) and IBM SPSS (Version 21.0, Chicago, USA). Statistical significance was set at p<0.05. All data are reported as the mean±SD unless otherwise stated.
3. Results
On the data collection, the 12 participants had a T EC of 36.57±0.41; 36,08±0.45; 36.43±0.49 and a T C of 37.17±0.25; 37.66±0.38; 37.39±0.33 for rest, exercise and post exercise conditions respectively.
The validity of the T EC compared with T C was verified by a Bland-Altman analysis, showing low levels of agreement between pairs of measures ( Fig. 2). Pairwise comparisons with Bonferroni post hoc test showed significant differences (p<0.01) between T EC and T C in all analyzed moments ( Fig. 3).
Fig. 2.
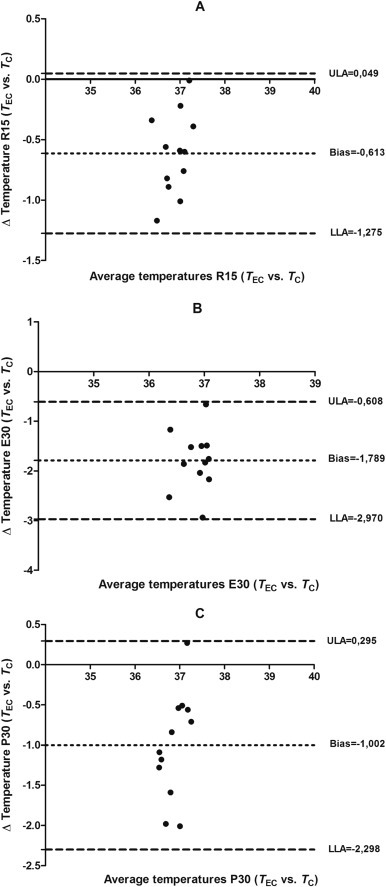
Plot of the bias (mean difference) and limits of agreement (±1.96 95% CI) between TEC and TC. (A) 15 min before exercise (R15), (B) after 30 min of exercise (E30) and (C) 30 min after exercise (P30), according to the procedures of Bland-Altman (n=12).
Fig. 3.
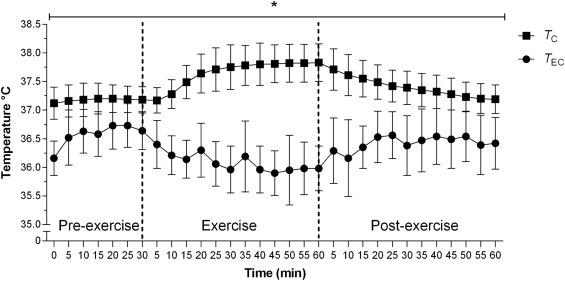
Dynamics of the Core temperature (TC) and eye corner temperature (TEC) at different times before, during and after exercise. The results are presented as the mean±SD. *(p<0.01) between TECand TC in all the measures.
The intraclass correlation coeficient (ICC) and confidence intervals (CI) for T EC compared with T C values showed 0.49 (CI: −0.09; 0.82), −0.14 (CI: −0.65; 0.44), and −0.25 (CI: −0.70; 0.25), respectively for R15, E30 and P30 moments. These results revealed that the validity of T EC compared with T C can be considered as unsatisfactory following the principles of Lee et al. (1989). The obtained typical errors of the measure intervals were large, (ie, ±0.5 °C; ±0.8 °C and ±0.9 °C respectively for R15, E30 and P30 moments).
4. Discussion
This study examined the validity of the T EC as T C indicator in resting, exercise and post-exercise conditions. Based on the analysis of the results, it can be said that T EC had severe errors which did not have a constant course in rest, exercise and post-exercise, providing the technique a poor validity. Thus, it is suggested that the measurement of the T EC may not be a substitute technique for T C in the three conditions studied. It was also noted that the variability is even more evident in the exercise and post-exercise conditions.
Delving into ICC results in the moments before (0.49), during (−0.14) and after exercise (−0.25), the agreement between pairs of measures (T EC vs. T C), mainly during and after exercise are not acceptable (Lee et al., 1989). This was also evident by the plots of the residual scores of Bland-Altman (Fig. 2). According to Bland and Altman (1986), this analysis establish the level of agreement between the measured values and the reference values, expressing the accuracy of the values and the limits of agreement. This difference between the two measures in the three moments was also evident in Fig. 3, where comparisons were made every 5 min. Furthermore, the low agreement between T EC as T C is reinforced in the ICC analysis, according to Atkinson and Nevill (1998) recommendations.
The lower difference between methods was observed before exercise, with an average error 0.61 °C (range between 0.04 °C to −1.27 °C; 95% CI) (Fig. 2a). Studies of Ng et al. (2004), Sumriddetchkajorn and Chaitavon (2009) and Teunissen and Daanen (2011) also registered T EC comparing with other gold standard methods showing also smaller values than for T C as the obtained in our study. For example, in the moment R15 there was a difference of 0.6 °C, being T EC (36.6 °C) and T C (37.2 °C).
The aim of the works of Ng et al. (2004) and Sumriddetchkajorn and Chaitavon (2009) was to test the validity of the T EC to detect people with fever and the method used for evaluation of T C was the ear thermometer. In these two studies, the authors concluded that the T EC can be used as a measure for the detection of fever. It should be considered that under conditions of fever the T EC could be higher and closer to the T C and this fact could explain why our results differ from those of the aforementioned investigations. On the other hand, Teunissen and Daanen (2011) compared the T EC to esophageal temperature of 10 subjects in four conditions (rest, exercise, recovery and passive heating) concluding that the measure obtained as T EC was not as reliable as the obtained for T C, pointing out an agreement with the findings of our study.
In the exercise condition major differences between the measures were observed, with a bias of −1.79 and a standard deviation of 0.60, resulting in 95% limits of agreement of −0.61and −2.97. So that, the bias increased with the completion of the exercise (E30; Fig. 2b). It is worth noting that in exercise condition, T C increased in comparison with the rest as occurred in different previous studies (Bain and Jay, 2011, Byrne and Lim, 2007, Domitrovich et al., 2010, Easton et al., 2007, Lee et al., 2000), while the T EC measured by IRT was reduced compared to rest (Fig. 3). Thus, at E30 moment the difference was 1.8 °C [T EC (35.9 °C) and T C (37.7 °C)] i.e. an increment of 1.2 °C after exercise considering the resting values (0.61 °C of difference between T EC and T C). A similar result was found in the study of Teunissen and Daanen (2011) where a difference of 1.3 °C [(T EC (35.6 °C) and T C (36.9 °C)] was registered at rest and a difference 2.9 °C [T EC (35.5 °C) and T C (38.4 °C)] after 30 min of biking, resulting a relative increment of 1.6 °C after exercise.
The statistical analysis of the data after exercise also indicates a low agreement between T EC and the T C, which is worst than at rest, showing average error of −1.00 °C (range between −0.29 °C and −2.29; 95% CI) (Fig. 3c). At the moment P30, the difference between methods was 1.0 °C [T EC (36.4 °C) and T C (37.4 °C). In the study of Teunissen and Daanen (2011) the T EC values obtained by IRT were inconsistent with the T C values during rest. We should also consider a point in order to understand the divergence on the evolution of the T EC and T C during exercise (i.e., while the T C increase in a uniform way, the T EC decrease with higher variability). On the other hand, the present research differs from Cholewka et al. (2016) which studied twelve male cyclists during a incremental test and found a constant increase of T EC as the exercise intensity increased. However, this difference can be explained mainly because of exercise type, since the present study the participants performed continuous exercise fixed to 60% of the V̇O2max speed, while the study of Cholewka et al. (2016) carried out an incremental test increasing the intensity every 3 min until maximal effort. Thus, it is suggested that T EC has a different response in continuous and incremental exercise. Indeed, sweat could potentially affect the T EC results because, given that sweat level depends on exercise intensity (Machado-Moreira et al., 2008), the sweat accumulated on the forehead during exercise is not blocked between the eyebrows and it may precipitate as beads of sweat on both sides of the nose cooling the area of inner canthus of the eye and influence IRT measurements (Bach et al., 2015, Bernard et al., 2013).
In this sense, it seems that T EC, as recorded in our study, it is not a measure that can replace the traditional methods used in studies of thermoregulation to measure T C during exercise. It is important to note that there are several methods for T C measurement in humans during resting or exercise conditions and each of the various forms of T C measurement have their own inherent limitations (Domitrovich et al., 2010). Although ingestible telemetry pill have been shown to be valid and reliable alternative to rectal T C (Byrne and Lim, 2007, Ganio et al., 2009), evidence suggests that telemetry pills consistently overestimate T C by >0.1 °C compared to rectal thermometers (Byrne and Lim, 2007). As a result, it is plausible to suggest that differences observed between T EC and T C seen in this study would be lower and therefore more agreeable if T C were measured by rectal thermometer. However, it is unclear whether rectal T C would provide sufficient agreement to T EC. This investigation attempted to control for individual rates of gastrointestinal motility that prevent a fixed anatomical position to measure intestinal T C by instructing participants to ingest the pill at least 6 h prior to data collection to allow for the pill to pass through the stomach into the intestines (Byrne and Lim, 2007, Domitrovich et al., 2010). Furthermore, we instructed our participants to maintain typical meal intake in the 24 h preceding testing to minimize changes to normal gastrointestinal motility. However, the gastrointestinal motility may be influenced by many factors including diurnal variation (Goodman et al., 2009), exercise (Leiper et al., 2005), age (Madsen and Graff, 2004), among others (Koffler et al., 1992). Thus, intestinal pill position at the time of measurement could not be guaranteed, consequently this variation may contribute to differences observed in this study.
Interpretation of this data is subject to certain limitations including the relatively moderate exercise intensity performed in intervals to allow for data collection. Future studies should build upon the findings of the current investigation by assessing continuous exercise at higher intensities. Furthermore, the study population was limited to healthy young adult men, and the thermal response may be different for other age groups, unhealthy populations and women. Another limitation of the current study was the small sample which leads a low statistical power. Future research should also implement the use of other commonly used T C measures such as rectal and esophageal temperature. Finally, it may be of interest to determine if of prevention sweat pooling around the eye would yield similar findings to the current investigation.
5. Conclusion
In conclusion, poor agreement was observed between the T EC values measured through IRT and T C measured through a gastrointestinal telemetry pill. Additionally, significant differences and mean bias across all tested time points demonstrated insufficient agreement between these two forms of body temperature measurement during rest, exercise and recovery, with augmented differences seen during exercise and post-exercise conditions. Thus, the T EC is not a valid substitute measurement to traditional methods of T C assessment used in sports and exercise science settings.
Conflict of interest
The authors have no conflict of interest to declare.
Acknowledgements
We appreciate the efforts of the participants during these experiments and thank them for their participation in the various experimental protocols. This research was supported by Agency for Improvement of Higher Education Personnel (Agency for Improvement of Higher Education Personnel www.capes.gov.br) for the scholarship to AAF, the National Council of Scientific and Technologic Development (National Council of Scientific and Technologic Development www.cnpq.br) for the phD Scholarship number 205815/2014-6 for DGM and Post-Doctoral Scholarship 234243/2014-7 for CJB, and to the Foundation for Research Support of the State of Minas Gerais (Foudation for Research Support of the State of Minas Gerais www.fapemig.br) for funding the Project number 00356-11.
Footnotes
This research was conducted in Department of Physical Education, Human Performance Laboratory, Federal University of Viçosa, Viçosa, Brazil.
References
- ACSM . American College of Sports Medicine’s Guidelines for Exercise Testing and Prescription. Lippincott Williams & Wilkins; Philadelphia: 2013. [Google Scholar]
- Atkinson G., Nevill A.M. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med. 1998;26:217–238. doi: 10.2165/00007256-199826040-00002. [DOI] [PubMed] [Google Scholar]
- Bach A.J., Stewart I.B., Disher A.E., Costello J.T. A comparison between conductive and infrared devices for measuring mean skin temperature at rest, during exercise in the heat, and recovery. PLoS One. 2015;10:e0117907. doi: 10.1371/journal.pone.0117907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain A.R., Jay O. Does summer in a humid continental climate elicit an acclimatization of human thermoregulatory responses? Eur. J. Appl. Physiol. 2011;111:1197–1205. doi: 10.1007/s00421-010-1743-9. [DOI] [PubMed] [Google Scholar]
- Bernard V., Staffa E., Mornstein V., Bourek A. Infrared camera assessment of skin surface temperature – effect of emissivity. Phys. Med.: PM: Int. J. Devot. Appl. Phys. Med. Biol.: Off. J. Ital. Assoc. Biomed. Phys. 2013;29:583–591. doi: 10.1016/j.ejmp.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Bland J.M., Altman D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327:307–310. [PubMed] [Google Scholar]
- Bourlai T., Pryor R.R., Suyama J., Reis S.E., Hostler D. Use of thermal imagery for estimation of core body temperature during precooling, exertion, and recovery in wildland firefighter protective clothing. Prehosp. Emerg. Care. 2012;16:390–399. doi: 10.3109/10903127.2012.670689. [DOI] [PubMed] [Google Scholar]
- Byrne C., Lim C.L. The ingestible telemetric body core temperature sensor: a review of validity and exercise applications. Br. J. Sports Med. 2007;41:126–133. doi: 10.1136/bjsm.2006.026344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan L.S., Cheung G.T.Y., Lauder I.J., Kumana C.R. Screening for fever by remote-sensing infrared thermographic camera. J. Travel Med. 2004;11:273–279. doi: 10.2310/7060.2004.19102. [DOI] [PubMed] [Google Scholar]
- Cholewka A., Kasprzyk T., Stanek A., Sieroń-Stołtny K., Drzazga Z. May thermal imaging be useful in cyclist endurance tests? J. Therm. Anal. Calorim. 2016;123:1973–1979. [Google Scholar]
- Costello J.T., McInerney C.D., Bleakley C.M., Selfe J., Donnelly A.E. The use of thermal imaging in assessing skin temperature following cryotherapy: a review. J. Therm. Biol. 2012;37:103–110. [Google Scholar]
- Cuddy J.S., Hailes W.S., Ruby B.C. A reduced core to skin temperature gradient, not a critical core temperature, affects aerobic capacity in the heat. J. Therm. Biol. 2014;43:7–12. doi: 10.1016/j.jtherbio.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Daanen H. Infrared tympanic temperature and ear canal morphology. J. Med. Eng. Technol. 2006;30:224–234. doi: 10.1080/03091900600711613. [DOI] [PubMed] [Google Scholar]
- Domitrovich J.W., Cuddy J.S., Ruby B.C. Core-temperature sensor ingestion timing and measurement variability. J. Athl. Train. 2010;45:594–600. doi: 10.4085/1062-6050-45.6.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton C., Fudge B.W., Pitsiladis Y.P. Rectal, telemetry pill and tympanic membrane thermometry during exercise heat stress. J. Therm. Biol. 2007;32:78–86. [Google Scholar]
- Faul F., Erdfelder E., Lang A.G., Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Fernandes Ad.A., Amorim P.R.S., Brito C.J., Moura A.Gd, Moreira D.G., Costa C.M.A., Sillero-Quintana M., Marins J.C.B. Measuring skin temperature before, during and after exercise: a comparison of thermocouples and infrared thermography. Physiol. Meas. 2014;35:189. doi: 10.1088/0967-3334/35/2/189. [DOI] [PubMed] [Google Scholar]
- Fernández-Cuevas I., Marins J.C.B., Lastras J.A., Carmona P.M.G., Cano S.P., García-Concepción M.Á., Sillero-Quintana M. Classification of factors influencing the use of infrared thermography in humans: a review. Infrared Phys. Technol. 2015;71:28–55. [Google Scholar]
- Ganio M.S., Brown C.M., Casa D.J., Becker S.M., Yeargin S.W., McDermott B.P., Boots L.M., Boyd P.W., Armstrong L.E., Maresh C.M. Validity and reliability of devices that assess body temperature during indoor exercise in the heat. J. Athl. Train. 2009;44:124–135. doi: 10.4085/1062-6050-44.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber C.E., Blissmer B., Deschenes M.R., Franklin B.A., Lamonte M.J., Lee I.M., Nieman D.C., Swain D.P., American College of Sports Medicine American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- Goodman D.A., Kenefick R.W., Cadarette B.S., Cheuvront S.N. Influence of sensor ingestion timing on consistency of temperature measures. Med. Sci. Sports Exerc. 2009;41:597–602. doi: 10.1249/MSS.0b013e31818a0eef. [DOI] [PubMed] [Google Scholar]
- Hopkins W.G. Will G; Hopkins: 1997. A New View of Statistics. [Google Scholar]
- James B.M., Ring E.F.J. Fever screening and infrared thermal imaging: concerns and guidelines. Therm. Int. 2009;19:67–69. [Google Scholar]
- Koffler K.H., Menkes A., Redmond R.A., Whitehead W.E., Pratley R.E., Hurley B.F. Strength training accelerates gastrointestinal transit in middle-aged and older men. Med. Sci. Sports Exerc. 1992;24:415–419. [PubMed] [Google Scholar]
- Lahiri B.B., Bagavathiappan S., Jayakumar T., Philip J. Medical applications of infrared thermography: a review. Infrared Phys. Technol. 2012;55:221–235. doi: 10.1016/j.infrared.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen P.B., Suriano R., Quod M.J., Lee H., Abbiss C.R., Nosaka K., Martin D.T., Bishop D. Core temperature and hydration status during an Ironman triathlon. Br. J. Sports Med. 2006;40:320–325. doi: 10.1136/bjsm.2005.022426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Koh D., Ong C. Statistical evaluation of agreement between two methods for measuring a quantitative variable. Comput. Biol. Med. 1989;19:61–70. doi: 10.1016/0010-4825(89)90036-x. [DOI] [PubMed] [Google Scholar]
- Lee S.M., Williams W.J., Schneider S.M.F. Core temperature measurement during supine exercise: esophageal, rectal, and intestinal temperatures. Aviat. Sp. Environ. Med. 2000;71:939–945. [PubMed] [Google Scholar]
- Leiper J.B., Nicholas C.W., Ali A., Williams C., Maughan R.J. The effect of intermittent high-intensity running on gastric emptying of fluids in man. Med. Sci. Sports Exerc. 2005;37:240–247. doi: 10.1249/01.mss.0000152730.74596.50. [DOI] [PubMed] [Google Scholar]
- Machado-Moreira C.A., Wilmink F., Meijer A., Mekjavic I.B., Taylor N.A. Local differences in sweat secretion from the head during rest and exercise in the heat. Eur. J. Appl. Physiol. 2008;104:257–264. doi: 10.1007/s00421-007-0645-y. [DOI] [PubMed] [Google Scholar]
- Madsen J.L., Graff J. Effects of ageing on gastrointestinal motor function. Age Ageing. 2004;33:154–159. doi: 10.1093/ageing/afh040. [DOI] [PubMed] [Google Scholar]
- Marfell-Jones M., Olds T.S., Stewart A., Carter L. International Standards for Anthropometric Assessment (ISAK) Potchefstroom; South Africa: 2006. [Google Scholar]
- Mekjavic I.B., Rempel M.E. Determination of esophageal probe insertion length based on standing and sitting height. J. Appl. Physiol. 1990;69:376–379. doi: 10.1152/jappl.1990.69.1.376. [DOI] [PubMed] [Google Scholar]
- Montain S.J., Sawka M.N., Cadarette B.S., Quigley M.D., McKay J.M. Physiological tolerance to uncompensable heat stress: effects of exercise intensity, protective clothing, and climate. J. Appl. Physiol. 1994;77:216–222. doi: 10.1152/jappl.1994.77.1.216. [DOI] [PubMed] [Google Scholar]
- Ng E.Y.K., Kawb G.J.L., Chang W.M. Analysis of IR thermal imager for mass blind fever screening. Microvasc. Res. 2004;68:104–109. doi: 10.1016/j.mvr.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Ring E.F., Ammer K. Infrared thermal imaging in medicine. Physiol. Meas. 2012;33:33–46. doi: 10.1088/0967-3334/33/3/R33. [DOI] [PubMed] [Google Scholar]
- Ring E.F.J., Ammer K. The technique of infra red imaging in medicine. Thermol. Int. 2000;10:7–14. [Google Scholar]
- Romanovsky A. Skin temperature: its role in thermoregulation. Acta Physiol. 2014;210:498–507. doi: 10.1111/apha.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawka M.N., Leon L.R., Montain S.J., Sonna L.A. Integrated physiological mechanisms of exercise performance, adaptation, and maladaptation to heat stress. Compr. Physiol. 2011;1:1883–1928. doi: 10.1002/cphy.c100082. [DOI] [PubMed] [Google Scholar]
- Sumriddetchkajorn S., Chaitavon K. Field test studies of our infrared-based human temperature screening system embedded with a parallel measurement approach. Infrared Phys. Technol. 2009;52:119–123. [Google Scholar]
- Taylor N.A. Challenges to temperature regulation when working in hot environments. Ind. Health. 2006;44:331–344. doi: 10.2486/indhealth.44.331. [DOI] [PubMed] [Google Scholar]
- Taylor N.A., Tipton M.J., Kenny G.P. Considerations for the measurement of core, skin and mean body temperatures. J. Therm. Biol. 2014;46:72–101. doi: 10.1016/j.jtherbio.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Teunissen L.P.J., Daanen H.A.M. Infrared thermal imaging of the inner canthus of the eye as an estimator of body core temperature. J. Med. Eng. Technol. 2011;35:134–138. doi: 10.3109/03091902.2011.554595. [DOI] [PubMed] [Google Scholar]
- Thomas S., Reading J., Shephard R.J. Revision of the physical activity readiness questionnaire (PAR-Q) Can. J. Sport Sci. 1992;17:338–345. [PubMed] [Google Scholar]
- West D.J., Cook C.J., Beaven M.C., Kilduff L.P. The influence of the time of day on core temperature and lower body power output in elite rugby union sevens players. J. Strength Cond. Res. 2014;28:1524–1528. doi: 10.1519/JSC.0000000000000301. [DOI] [PubMed] [Google Scholar]
- Xu X., Karis A.J., Buller M.J., Santee W.R. Relationship between core temperature, skin temperature, and heat flux during exercise in heat. Eur. J. Appl. Physiol. 2013;113:2381–2389. doi: 10.1007/s00421-013-2674-z. [DOI] [PubMed] [Google Scholar]


