Abstract
Infectious bronchitis is one of the most important diseases in poultry and it causes major economic losses. Infectious bronchitis is an acute, highly contagious, viral disease of chickens, characterized by rales, coughing, and sneezing.
Because secreted phospholipases A2 (sPLA2) are involved in inflammatory processes, the gene expressions of sPLA2s were investigated in both healthy chickens and chickens with infectious bronchitis and lung inflammation. The draft chicken genome was first scanned using human sPLA2 sequences to identify chicken sPLA2s (ChPLA2), chicken total mRNA were isolated and RT-PCR experiments were performed to amplify and then sequence orthologous cDNAs. Full-length cDNA sequences of ChPLA2-IB, -IIA, -IIE, -V and -X were cloned. The high degree of sequence identity of 50–70% between the avian and mammalian (human and mouse) sPLA2 orthologs suggests a conservation of important enzymatic functions for these phospholipases.
Quantitation by qPCR of the transcript levels of ChPLA2-IB, -IIA, -IIE, -V and -X in several tissues from healthy chicken indicated that the expression patterns and mRNA levels diverged among the phospholipases tested. In chicken with infectious bronchitis, an over expression of ChPLA2-V was observed in lungs and spleen in comparison with healthy chicken. These findings suggest that ChPLA2-V could be a potential biomarker for lung inflammation. Conversely, a down regulation of ChPLA2-IB, -IIA and -X was observed in lungs and spleen in case of infectious bronchitis. A significant increase in the expression level of ChPLA2-X and ChPLA2-IB was also noticed in pancreas. No or minor changes have been detected in the expression of ChPLA2-IIE in lungs and small intestine, but it shows a significant increase in several infected tissues.
Keywords: Secreted Phospholipase A2, Gene expression, qPCR, Gallus gallus, Avian infectious bronchitis
Abbreviations: sPLA2, secreted phospholipase A2; ChPLA2, Chicken phospholipase A2; hPLA2, human phospholipase A2; mPLA2, mouse phospholipase A2; qPCR, Quantitative real time PCR; ARDS, acute respiratory distress syndrome; Gg, Gallus gallus
Graphical abstract
Highlights
► Infectious bronchitis is one of the most important diseases in poultry. ► Secreted phospholipases A2 (sPLA2) are involved in inflammatory processes. ► Full-length cDNA sequences of Chicken PLA2-IB, -IIA, -IIE, -V and -X were cloned. ► QPCR of ChPLA2-IB, -IIA, -IIE, -V and -X in chicken with infectious bronchitis. ► We suggest that ChPLA2-V could be a potential biomarker for lung inflammation.
1. Introduction
Secreted phospholipases A2 (sPLA2) form a class of structurally related enzymes that catalyze the hydrolysis of glycerophospholipids at the sn-2 position to release free fatty acids and lysophospholipids [1], [2], [3], [4]. Numerous intracellular and secreted PLA2 have been characterized and classified into different groups based on their structural features [5]. They are characterized by a low molecular mass (13–18 kDa), the presence of several disulfide bridges, a broad selectivity for phospholipids with different polar head groups and fatty acid chains and a Ca2+-dependent catalytic mechanism. During the last decade, 11 different groups of sPLA2 have been identified in mammals: IB, IIA, IIC, IID, IIE, IIF, III, V, X, XIIA and XIIB [4], [5], [6], [7].
The molecular diversity of sPLA2 in animal venoms and more recently in mammals raises the possibility that similar and diverse sPLA2s can be found in various species.
Several studies in mammals showed that distinct secreted PLA2 appear in lung cells and some are able to trigger molecular events leading to enhanced inflammation and lung damage causing the acute respiratory distress syndrome (ARDS) [8]. The latter is characterized by arterial hypoxemia, noncardiogenic pulmonary oedema and an alteration of pulmonary surfactant which increases surface tension at the air–liquid interface. sPLA2-IIA, -V, -X can directly hydrolyse lung surfactant phospholipids [8]
Human PLA2-IIA (hPLA2-IIA) has been long considered as the principal group associated to the development of ARDS [9]. However, it was recently demonstrated that only hPLA2-V and -X hydrolyse the pulmonary surfactant [10] and that contrary to hPLA2-IIA, these two PLA2 are expressed in normal human lung. It was concluded that hPLA2-V is the principal sPLA2 implicated in ARDS. Indeed, recent study showed that transgenic mice overexpressing mPLA2-V dies immediately after birth due to the alteration of the surfactant composition, which was not observed in mice overexpressing the mPLA2-X [11].
The growing interest in chicken diseases, such as avian influenza, led us to study bird sPLA2 to further gain some functional and pathological insights. Karray et al. have biochemically characterized an active sPLA2-IB from chicken pancreas (ChPLA2-IB) [12]. Another biochemical and structural study of avian non-pancreatic PLA2 was performed from chicken intestine (ChPLA2-IIA) [13]. We also demonstrated in a recent study that pure ChPLA2-IIA, but not ChPLA2-IB, displays potent bactericidal activity against gram-positive bacteria but lacks bactericidal activity against gram negative ones [14].
Infectious bronchitis is one of the most important viral diseases in poultry and it causes major economic losses to the poultry industry. The etiologic agent in this lung disease is a coronavirus that belongs to the family of coronaviridae, genus Coronavirus, group 3 [15]. In mammals, group 1 coronavirus belonging to the same virus family is responsible for severe respiratory acute syndrome. The group 3 coronavirus infects only avian and the acute bronchitis is characterized by tracheal rales, coughing, and sneezing and it is a highly contagious viral disease of the respiratory tract in chickens [16]. This disease of poultry is common worldwide and has significant economic consequences [17]. Although effective vaccines are available and utilized routinely in commercial poultry production, the virus has the tendency to mutate frequently [16]. The prevalence and severity of respiratory diseases in commercial chicken flocks has increased recently due to the intensification of the poultry industry.
In the last few years, the screening of the exponential number of nucleic acid sequences generated by the genome projects has allowed to clone several mammalian sPLA2 members. The continuing flow of novel sequences and the accession to the genetic information from complete genomes of higher eukaryotes has let to identify novel chicken sPLA2 homologs.
Here, we report the first identification, sequence information, cloning, and expression profiling of several novel ChPLA2s belonging to groups IB, IIA, IIE, V and X. A comparison of the mammal’s sPLA2 tissue distribution is also presented in order to propose functions for these newly identified proteins. We analyzed for the first time the expression pattern of these genes in the intestine, pancreas, heart, liver, colon, lung and spleen from both healthy chicken and chicken with inflammatory lung disease to establish possible gene coregulation and new biomarkers. Our data support the fact that several sPLA2 may contribute to the progression or modulation of infectious bronchitis in poultry.
2. Material and methods
2.1. Chicken tissues, total RNA extraction and cDNA synthesis
Several samples of pancreas, intestine, liver, heart, colon, lung and spleen were collected from both infected and uninfected chicken at the Institute of Tunisian Veterinary Research (IRVT, Sfax). The specimens were from 30 to 45 day-old chickens at the time of diagnosis.
The diagnostic was determined according to the pathologic examinations. The procedures were approved by the Institute of Tunisian Veterinary Research. The histological observations were determined on tissues sections. In addition, several histological normal chicken tissues were collected from animals with no clinical symptoms. These specimens were used as controls.
Total mRNAs were isolated from 100-mg tissue samples (stored at −80 °C before RNA extraction) using the single step guanidine isothiocyanate-phenol-chloroform isolation method as described by Chamczynski and Sacchi [18]. ChPLA2 cDNA were obtained from total mRNAs by the reverse transcription procedure. First strand cDNAs were prepared using heat-denaturated (5 min at 70 °C) total mRNAs (10 μg) as template, 200 U MMLV reverse transcriptase (Invitrogen), 20 pmol of each deoxynucleoside triphosphate, and 20 pmol of each primer. Phospholipases genes were searched for in the draft Gallus gallus genomic sequence database (GenBank accession numbers are shown in Table 1 ). The coding sequences of ChPLA2 were determined by reverse transcriptase–polymerase chain reaction (RT-PCR) amplification of mRNA from chicken tissues samples. Several primer pairs were designed to produce overlapping fragments, and finally the pairs shown in Table 2 were used to clone the indicated cDNAs. Reverse transcription was carried out in a total reaction volume of 20 μl for 5 min at room temperature and 60 min at 42 °C. The cDNA/RNA heteroduplex was then denaturated at 70 °C for 15 min and cooled on ice.
Table 1.
Summary of key features of the identified phospholipases from chicken (Gg) and human (Hs) and mouse (M) orthologs.
| Name (s) | Chr.location Gg/Hs/M | cDNA size (pb) Gg/Hs/M | Protein size (aa) Gg/Hs/M | Identity (%) Gg/Hs and Gg/M | mRNA Accession number |
|---|---|---|---|---|---|
| PLA2-IB | 15/12q23.24/5 | 447/435/441 | 147/143/146 | 63–59 | EU 617018 |
| PLA2-IIA | 21/1p34.36/4 | 426/435/441 1 | 141/143/145 | 45–51 | XP 424264 |
| PLA2-IIE | 21/1p34.36/4 | 435/429/429 | 145/143/143 | 42–42 | JF 411005 |
| PLA2-V | 21/1p34/4 | 417/417/414 | 138/139/137 | 45–45 | JF 411004 |
| PLA2-X | 14/16p12.13/16 | 468/570/456 | 156/190/152 | 45–42 | XP 414738 |
The currently known nomenclature of Gallus gallus PLA2s for all gene products is listed under Name(s). Chromosomal locations of the genes, size of the cDNAs and proteins, location of the proteins, % identities between the chicken and human proteins, and accession numbers of the mRNAs, are listed with the relevant references.
Table 2.
Primers used for the cloning of chicken cDNA.
| Gene | Forward/reverse | Sequence (5′–3′) | Melting Temperature (°C) |
|---|---|---|---|
| ChPLA2- IB | forward | 5′-ATGAGACTCTTGGCGTGCTTTTCTTG-3′ | 56 |
| ChPLA2-IB | reverse | 5′-GACAAGAAGAAATACTGCACCAGTTAA-3′ | 56 |
| ChPLA2-IIA | forward | 5′ GTGTGTGTGAATTCATGAACATAGCTCAGTTTGGTATC 3′ | 60 |
| ChPLA2-IIA | reverse | 5′ GTGTGTCTCGAGCTAGCAAGAGGGACGTGAGCC 3′ | 60 |
| ChPLA2-IIE | forward | 5′ GTGTGTGTGTGAATTCATGAGGAATCTCCTGCTCGCC 3′ | 60 |
| ChPLA2-IIE | reverse | 5′ GTGTGTGTTCTAGATCAGCACTGGAGCTTGCTGCC 3′ | 60 |
| ChPLA2-V | forward | 5′ GTGTGTGTGAATTCATGAGCCTCTGGCAGCTGCAGGAG 3′ | 55 |
| ChPLA2-V | reverse | 5′ GTGTGTCTCGAGTCACCTGCACTTGCACCTGGG 3′ | 55 |
| ChPLA2-X | forward | 5′ GTGTGTGTGAATTCATGGGAATTCTTGAATTAGCTGGA 3′ | 58 |
| ChPLA2-X | reverse | 5′ GTGTGTCTCGAGTTAATATCTACACAAAGGTTG 3′ | 58 |
2.2. Cloning of the mature PLA2 gene
Amplification of several specific ChPLA2 cDNAs was carried out by PCR using the single strand cDNAs as template with the forward and reverse primers described in Table 2. PCR was performed in a 0.2 ml Eppendorf tube with a Gene Amp® PCR System 2700. The PCR mixture contained 20 pmol of both primers, 20 pmol of each deoxynucleoside triphosphate, 5 U pfu polymerase and polymerization buffer for a final volume of 100 μl. The single strand cDNAs were directly used as template. The thermal profile involved 35 cycles of denaturation at 94 °C for 1 min, primer annealing at an appropriate and optimized temperature for 1 min, and extension at 72 °C for 3 min.
The amplified products were subjected to 1% agarose gel electrophoresis and stained with ethidium bromide. Subsequently, each PCR product was excised from the gel and DNA was purified with a Gel Extraction Kit from Biogene. Next, the DNA was cloned into the Pet 21 (a+) vector (Invitrogen), the chemically competent DH10B Escherichia coli cells (Invitrogen) were transformed and plated on Luria–Bertani solid medium containing 50 μg/ml ampicillin. Insert-containing bacterial clones were screened with colony PCR using the above described PCR conditions and primers. Positive clones were incubated overnight at 37 °C in Luria–Bertani medium containing 50 μg/ml Ampicillin, and plasmid DNA was purified with Fast-Plasmid Mini kit (Eppendorf). DNA sequencing was confirmed by performing DNA sequencing (GATC, Germany).
2.3. Quantitative real time PCR
Quantitative real time PCR (qPCR) was performed with the Light Cycler 480 system (Roche) using LightCycler FastStart DNA Master SYBR Green I-kit (Roche) according to the manufacturer’s instructions. The qPCR conditions were as follows: 95 °C for 10 min; 45 amplification cycles at 95 °C for 10 s, annealing for 10 s (5 °C above melting temperature of the primer with lower value), elongation at 72 °C for 8 s (25 bp/s + 2 s), measurement of fluorescence at 81–92 °C for 1 s (see Table 3 ). After amplification, melting curve analysis was performed as follows: 98 °C for 10 s; annealing temperature for 20 s; continuous temperature gradient to 95 °C with 5 acquisitions/s. The tissue cDNA samples (from animals and the indicated tissues and cells) were diluted 1/10 with nuclease free water, and the same cDNA dilution was used for all qPCR reactions. A fresh 10−1–10−4 serial dilution was prepared from each target gene to be analyzed starting from the 1/10 dilution of a purified PCR product, and every gene was measured twice independently with duplicate samples. From the serial dilution, 10−1–10−4 dilutions were used as an internal standard. As housekeeping genes, we measured the mRNA expression levels of chicken β-actin. The qPCR primers used are shown in Table 3. In qPCR, every gene had PCR efficiencies ranging from 1.902 to 1.972, as calculated from the internal standard; thus, no efficiency correlation was performed. The Light Cycler 480 Software (release 1.1.0.0520) with absolute quantification was used to calculate the concentrations and standard deviations of the samples. The concentration of the target gene was divided by the concentration of the housekeeping gene thus showing the relative expression of the mRNA in the tissue. This calculation was performed for all tissues analyzed. Standard deviations were calculated by multiplying the obtained relative value with the standard deviation of the target. The resulting relative and standard deviation values were scaled to a range of 0–10 including the standard deviations.
Table 3.
Primers used for the qPCR analyzes (f) : forward; (r) : reverse.
| Primer name | Sequence (5′–3′) | PCR product size | Melting temperature; Temperature of fluorescence measurement; |
|---|---|---|---|
| ChPLA2-IB (f) | AAGACTCTTGGCGTGCTTTTCTTGCTG | 200 | 56;89 |
| ChPLA2-IB (r) | GCTTGACAGCACCTGTCCAGTTCATCCA | 200 | 56;89 |
| ChPLA2-IIA (f) | AAGCTCTGGTGCTCCTCGTTGCCTT | 200 | 60;90.5 |
| ChPLA2-IIA (r) | CAGTCGTGGGCATGGCAGCACCAGTCG | 200 | 60;90.5 |
| ChPLA2-IIE (f) | GGCAGAGGGACCCCAGTGGA | 200 | 70;92 |
| ChPLA2-IIE (r) | CACGAAGCCACGGCCCTGTC | 200 | 70;92 |
| ChPLA2-V (f) | AATGCTCTCCTTGCATTGGCCATACTG | 200 | 49;90 |
| ChPLA2-V (r) | AGCTGGCAGCACTGTCTGTGCATCC | 200 | 49;90 |
| ChPLA2-X (f) | AAAAATCCTTGTCAGCTGCCGCTCGTG | 200 | 60;88 |
| ChPLA2-X (r) | TTGGGCCATCCCCTTCCTCCCAGGCCG | 200 | 60;88 |
| Beta Actine (f) | CACAGATCATGTTTGAGACCTT | 60 | 60;85 |
| Beta Actine (r) | CACAATACCAGTGGTACG | 60 | 60;85 |
2.4. Statistical analysis
Results are expressed as the mean +/− SEM for the indicated number of independently performed experiments. Comparisons between values were analyzed by Student’s t test for unpaired data, and p < 0.05 was considered significant.
3. Results
3.1. Identification and sequencing of novel chicken sPLA2, structural features
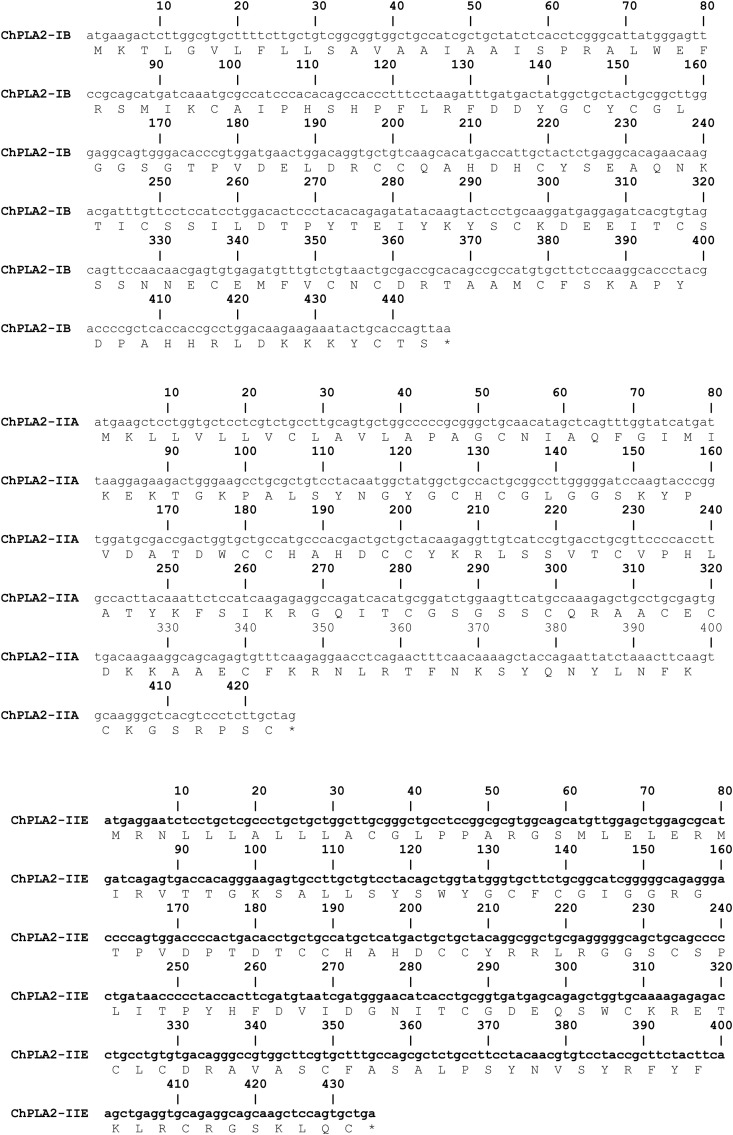
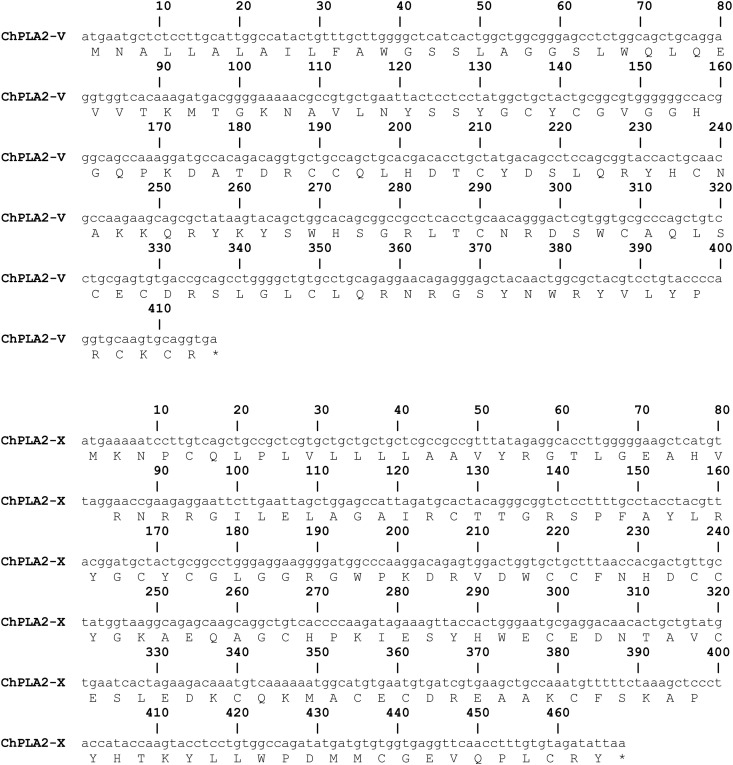
Chicken phospholipases A2 were identified from the draft chicken genomic database using sequences from the human orthologs. The nomenclature of the human PLA2s as suggested by Wilson and Schusterwoldan [19] is adopted throughout this text. PCRs were then performed to amplify the corresponding cDNAs using total mRNA from chicken pancreas, intestine, heart, colon, liver, lung and spleen tissues as template. The resulting DNA fragments were cloned and sequenced. Using this approach we obtained the full-length cDNA coding ChPLA2-IB, ChPLA2-IIA, ChPLA2-IIE, ChPLA2-V, and chPLA2-X. The cDNA sequences of these chicken PLA2s (Fig. 1 ) were deposited in the GenBank database under the accession numbers EU617018 (ChPLA2-IB), GU474517 (ChPLA2-IIA), JF411005 (ChPLA2-IIE) JF411004 (ChPLA2-V) and GU474518 (ChPLA2-X). The data concerning the newly identified chicken cDNAs and proteins are summarized in Table 1, together with the available informations on human and mouse orthologs. When full-length clones could be obtained, the sizes of the predicted proteins were found to be similar to those of human sPLA2s, lending support to the assigned designations of the galline phospholipases A2.
Fig. 1.
Nucleotide sequences of the cDNA of ChPLA2s and deduced amino acid sequences. Sequencing was performed in triplicate with three independent PCRs. A: ChPLA2-IB, B: ChPLA2-IIA, C: ChPLA2-IIE, D: ChPLA2-V and E: ChPLA2-X.
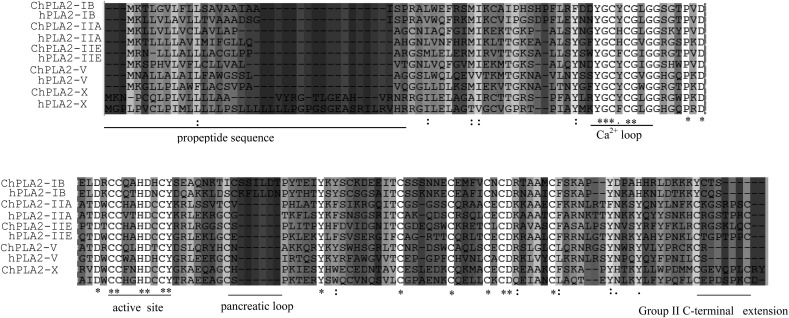
Amino acid sequence alignment of the five chicken sPLA2 that have been cloned in this work and the corresponding human sPLA2 is presented in Fig. 2 , and the degrees of sequence identity are shown in Table 1. Based on their structural features, chicken group IIA and group IIE sPLA2 are clearly members of the group II collection of sPLA2 [1], [4], [5], [6], [20], [21], [22]. Indeed, both sPLA2 display the specific features of group II sPLA2, including a cystein residue at position 50 and a cystein residue that terminates the group II-specific C-terminal extension of 7 residues (Fig. 2). A BLAST search for homology with the ChPLA2-X protein sequence revealed that this protein has the highest identity level (45%) with hPLA2-X [21]. In contrast, the identity level of ChPLA2-X to other Gallus gallus sPLA2 is lower than 38%. In addition, ChPLA2-X shares with hPLA2-X the same structural features [23]. It is predicted that ChPLA2-X consists of 31-amino acid prepropeptide sequence ending with a basic dipeptide (R–R) and a mature protein of 124 residues compared with the human (same dipeptide R–R) and the mouse PLA2-X which is made of a 28-amino acid prepropeptide sequence ending with a basic dipeptide (K–R) and a mature protein of 123 residues. Like hPLA2-X and mPLA2-X, ChPLA2-X has 8 conserved disulfides bridges but it has a group II like C-terminal extension of 9 residues instead of 8 (Fig. 2).
Fig. 2.
Comparison of the conserved chicken and human phospholipase A2 core modules. The GenBank accession numbers for the chicken, mouse and human sequences are provided in Table 1. The identities between full-length PLA2 sequences of the human and chicken proteins are for sPLA2-IB, 63%; sPLA2-IIA, 45%; sPLA2-IIE, 42%; sPLA2-V, 54% and sPLA2-X, 45%. Overall, the sequences show little conservation. The active-site motifs, however, are well conserved. The phospholipase consensus motif with the catalytic HD catalytic diad, CCXXHDX, is fully conserved. In addition, the calcium loop consensus sequences YGCXCGXGG is conserved in several sPLA2 sequence. We note also a conserved arrangement of Disulfides Bridge. Identical amino acids in all 5 proteins are marked with an asterisk (∗), conservative substitutions with a colon (:), and semi-conservative substitutions with a period (.).
The sequence identity among the active site domain and calcium binding segment is low. However, the central catalytic domain with the active site and the calcium loop motif are well conserved (Fig. 2). The histidine of HD catalytic diad is thought to function as a general base to deprotonate a water molecule as it attacks the substrate ester carbonyl carbon, and the ß carboxyl aspartate coordinates directly to the catalytic Ca2+ cofactor [24], [25]. Conserved active site CCXXHDXC and calcium loop YGCXCGXGG consensus sequences are present like in several mammal sPLA2. A sequence alignment of the core modules of galline and human sPLA2 is shown in Fig. 2.
Within the PLA2 super family, the sequences in the vicinity of the active site motifs are well conserved (Fig. 2). Because the structural arrangement of disulfides has been the main bases for designating the different sPLA2 group numbers, the naming of the new chicken sPLA2 as group IB,IIA and IIE (with 14 cysteines), group V (with 12 cysteines) and group X (with 16 cysteines) seems to be appropriate. Moreover, the high levels of identity with the human orthologs support the names given to the chicken enzymes identified in this work.
The core module of human, mouse and chicken phospholipases shows that the residues of the active site of mPLA2-IB and ChPLA2-IB are identical; only a threonine residue (T47) is replaced by an alanine residue (A 47) in chicken sPLA2. Moreover, the residues R 43, V 46 and T47 in human and mouse PLA2-IIA are different from the chicken one and they are substituted by W43, H46 and A47. Furthermore, Cysteine 50 is preceded by H49 in hPLA2-V, R49 in mouse and T49 in chicken ChPLA2-V. The active site is well conserved in the case of group IIE PLA2, except W43 (in human and mouse) which is replaced by T43 in chicken. Interestingly, the calcium loop contains identical sequences in mammals and bird PLA2 group IB, IIA, IIE, V and X. Only Y28 in human and mPLA2-IIE is replaced by F28 in chicken.
3.2. Expression levels of sPLA2 genes in normal chicken tissues
To analyze the tissue expression profiles of sPLA2, we measured the relative abundance of mRNA coding for ChPLA2-IB, -IIA, -IIE, -V and -X in several organs and tissues using the quantitative real time PCR (Fig. 3 ). The data were normalized relatively to the levels of β-actine used as the house keeping gene. The results show marked differences between sPLA2s with the highest relative expression levels for group IB and group IIA sPLA2 in the pancreas (Fig. 3A) and the intestine (Fig. 3B), respectively. The pancreatic-type sPLA2-IB showed a significant widespread tissue distribution, including lung where its relative expressed level reached 4.038. The relative quantification results show that the ChPLA2-IB is expressed at very high levels in pancreas (43.152) and at lower levels in lungs (4.038), liver (3.934), colon (1.375) and intestine (1.016) (Fig. 3A).
Fig. 3.
Comparison of mRNA expression profiles of PLA2s in normal and infection bronchitis tissues. a: ChPLA2-IB, b: ChPLA2-IIA, c: ChPLA2-IIE, d: ChPLA2-V and e: ChPLA2-X. Gene-specific primers were used to determine the expression profiles of the avian PLA2. Each chicken gene was analyzed twice independently using the Light Cycler 480 system. Error bars represent the observed standard deviation. The relative expression values were scaled to a range of 0–100. The values shown were calculated using β-actin as the housekeeping gene. Results are expressed as the mean +/− SEM percentage of the control value (n _ 3).∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
ChPLA2-IIA was found to be expressed at very high levels in the small intestine with a relative expression level of 9.442, and at lower levels in heart (2.034), liver (2.473) and lungs (1.049). A low expression level was also detected in colon (0.081) and there was no expression in the pancreas (Fig. 3B).
The highest relative expression level of ChPLA2-IIE was observed in lungs (Fig. 3C). The expression level in other tissues was found to be similar to that of ChPLA2-IIA since it was detected at a high level in the small intestine (6.334) but at a moderate level in heart (1.365) and liver (0.574).
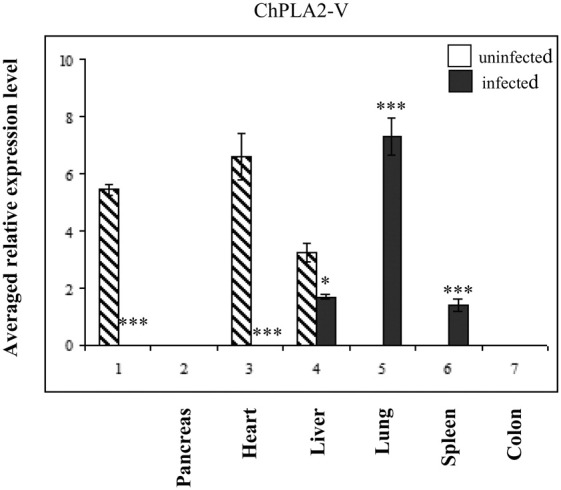
Fig. 3D shows that the expression patterns of ChPLA2-V resemble to that of mammals with the highest expression levels in the heart (6.83) and intestine (5.235), and to a lower extent in the liver (3.01). ChPLA2-V was not detected in lung, spleen and colon.
Finally chicken sPLA2 group X (Fig. 3E) was found to be expressed in lung (4.32), in small intestine (2.067), in spleen (3.116), and in heart (2.421). The lowest relative expression level was observed in colon (2.16) and its expression was not detectable in pancreas nor in the liver (Fig. 3E).
3.3. Expression levels of sPLA2 genes in chicken with infectious bronchitis
We have analyzed the expression pattern of sPLA2 group IB, IIA, IIE, V and X in chicken with infectious bronchitis disease. The expression levels of each sPLA2 gene in the infected birds vs healthy ones are represented in Fig. 3. Interestingly, the expression levels of ChPLA2-IB and ChPLA2-IIA in lungs decreased about 30- and -12 folds, respectively (Fig. 3A and B). The same behavior was observed in liver. Conversely, we found that ChPLA2-V was expressed in lungs of infected chicken whereas this sPLA2 was not expressed in the lungs of healthy chickens. In addition to the sudden expression of ChPLA2 group V in infected lungs, ChPLA2 also appeared to be expressed at lower levels in the spleen to lower extent, whereas ChPLA2-V expression in the small intestine and the heart was abolished. Only the expression of ChPLA2-IIE did not change in inflamed chicken lung compared to normal ones.
Whereas ChPLA2-IIA in normal chickens was expressed at significant levels in several tissues including intestine, heart, liver, lung and spleen, the expression of this enzyme in the pulmonary infected chicken’s tissues was very low. The relative expression levels were 0.974 and 0.882 in the intestine and heart of infected chicken, respectively. However, the expression was below the limit of detection in many infected tissues (0.0875 and 0.0152 in lung and spleen, respectively) (Fig. 3). ChPLA2-IIA expression only increased in the colon.
The expression level of ChPLA2-IB was significantly increased (Fig. 3A) and a high expression of ChPLA2-X appeared (Fig. 3E) in the pancreas of infected chicken.
Contrary to all the other sPLA2 genes tested, quantitative PCR showed no significant variations in the expression levels of ChPLA2-IIE in lungs and small intestine, but an increase of its expression level was significantly observed in heart, liver, spleen and colon (Fig. 3C).
4. Discussion
4.1. sPLA2 gene expression level in normal chicken organs
To our knowledge, this is the first study providing insights into the avian secreted phospholipases A2. The significant degree of sequence conservation between the chicken and mammalian (human and mouse) secreted PLA2s suggests that these enzymes play important roles in both birds and mammals. The sequence alignment of the various chicken sPLA2 with cloned human and mouse orthologs shows that they share similar features, including conserved positions of cysteines, a strong similarity of the putative Ca2+ binding loop consensus sequences YGCXCGXGG and the active site consensus sequence CCXXHDXC.
A crucial question that remains to be answered concerns the tissues and cells distribution, the physiological roles and the mechanisms of action of bird sPLA2. In this study, the mRNA levels of various sPLA2 in chicken were investigated. Our data show significant differences in the relative expression of these sPLA2. Group IB and group IIA sPLA2 were found to be the most abundant sPLA2, at least in some chicken tissues including pancreas and small intestine (43.15 and 9.45 relative expression level, respectively, in healthy chickens). This is probably the reason why ChPLA2-IB and ChPLA2-IIA could be purified and characterized from pancreas and intestine respectively [12], [13]. A similar distribution of sPLA2-IB and -IIA has been also observed in human and mouse tissues at the mRNA level [26] and at the protein level [27], [28], [29], [30].
Outside the gastrointestinal tract, ChPLA2-IB was found to be expressed at moderate levels in lung and liver and at low levels in spleen. These results are in agreement with a previous study in the mouse showing that both mPLA2-IB mRNA and protein were present in lung, spleen and thymus [31]. Although sPLA2-IB has long been proposed to act mainly as a digestive enzyme in the gastrointestinal tract [27], qPCR results suggest that this enzyme can play other physiological roles. Gene knock-out mice suggested a role of this enzyme in some obesity mechanism [32]. Mammalian sPLA2-IB seems also involved in cell proliferation, lipid mediators release and acute lung injury [33], [34], [35], [36].
Concerning ChPLA2-IIA, it is by far mainly expressed in the small intestine. This expression level was found to be in agreement with previous data in all tested species [37]. In mammals, the expression levels of sPLA2-IIA were very low in both spleen and colon, while moderate in heart, liver, lung and pancreas.
Surprisingly, the gene encoding ChPLA2-IIA enzyme was not found to be expressed in chicken pancreas, whereas it was detected by qPCR in the mouse [31] and by northern blotting in humans [6]. In agreement with previous data showing strong expression of human, rat and mouse group V sPLA2 in heart [38], ChPLA2-V was found to be expressed at high levels in heart. Interestingly, it was also detected with moderate relative expression level in the small intestine and liver of bird. In contrast, it was not detected in lung, spleen nor in colon, whereas, analysis of sPLA2-V gene expression in the mouse using qPCR showed its expression in lung spleen and colon [31]. This sPLA2 was also detected in murine macrophages and mastocytes, where it plays a role in lipid mediator production [39], [40], [41].
Finally, ChPLA2-X was found to be expressed with moderate levels in lung, small intestine, colon, heart and spleen (Fig. 3E). Using qPCR, similar results were observed in mammals with a highest mRNA expression level in colon [31]. It is worth noticing that ChPLA2-X was neither detected in the pancreas nor in the liver. A fairly expression of mPLA2-X was also observed in stomach, indicating that mPLA2-X expression is not exclusive to testis but present in a few number of tissues. Surprisingly, the tissue distribution pattern of hPLA2-X appears very different from that of mPLA2-X and it is expressed in spleen, thymus, blood leucocytes, lung, colon, and pancreas [21]. Using in situ studies, hybridization of human lung biopsies showed that only hPLA2-V and -X were expressed in airway epithelium [42]. More recently, it has been shown that the levels of sPLA2-IIA was shown to be present at high levels in lungs of ARDS patients [43].
Our current results obtained at the mRNA levels clearly indicate that ChPLA2-IB and ChPLA2-IIA are by far the most abundant enzymes at least in some chicken tissues including intestine and pancreas. Whereas, ChPLA2-V and ChPLA2-X, are expressed at fairly high levels. In addition, the low mRNA expression level of ChPLA2-IIE explain our incapacity in measuring of the sPLA2 activities in chicken organs.
4.2. Down regulation of sPLA2 group IB, IIA and X gene expression in chicken lung inflammation
The infectious bronchitis virus, a group 3 coronavirus (CoV), causes a costly viral disease in chicken [16]. It is responsible of acute respiratory disease syndrome (ARDS) in chickens at all ages and then a loss of production. Upon infection, the virus is present in the respiratory system, the gastrointestinal tract, liver, spleen and kidney and it induces serious lung injury [16]. The CoV infecting humans is responsible for severe respiratory acute syndrome [17] characterized by hypoxemia and non cardiogenic pulmonary oedema [8]. The comparative gene expression of sPLA2 in healthy chickens and chickens with infectious bronchitis was performed here for the first time. A marked decrease by 30-, 12- and 2-fold in the respective expression levels of ChPLA2-IB, ChPLA2-IIA and ChPLA2-X was observed in infected chicken lung. Indeed, ChPLA2-IIA was found to be present in several tissues at high and moderate level of normal intestine, heart liver and lung, and its expression was markedly reduced in avian infectious bronchitis (Fig. 3). These findings suggest a down regulation of sPLA2 gene expression as an immunity response, to limit for instance the hydrolysis of lung surfactant by these enzymes. In a previous work, Vial et al. [44] reported that the expression of sPLA2-IIA in alveolar macrophages is down-regulated by an inflammatory stimulus suggesting a possible negative control of sPLA2-IIA during alveolar macrophages activation. In several other studies, Touqui et al. have suggested a mechanism of down regulation of sPLA2-IIA by the lung surfactant and intracellular AMPc in alveolar macrophages [45].
4.3. Over expression of sPLA2-IB and appearance of a high sPLA2-X expression in the pancreas of infected chickens
We noticed a marked increase in the expression level of ChPLA2-IB (from 43,15 to 107.43) in the pancreas of infected chickens. This observation could be related to an anatomical particularity in chickens, in which there is no sharp boundary between digestive and respiratory system due to the absence of diaphragm as the case in mammals [46]. It is worth noticing that hPLA2-IB has been detected in serum of patients with ARDS but not in healthy controls [47] and it can be used as a diagnostic and prognostic marker for future development of ARDS [48]. The enzyme has also been implicated in complications of severe acute pancreatitis [47], [49], a recognized risk factor in ARDS. ChPLA2-X, totally absent in healthy chicken pancreas, was found to be highly expressed in infected chicken pancreas (104.78). The sPLA2-X could therefore be considered as a good marker in pulmonary disease. Since pancreatic enzymes such as sPLA2-IB can be found in the blood of patients, it would be interesting to investigate the presence of sPLA2-X in the blood of infected chicken using specific antibodies.
4.4. Drastic changes in the tissue-specific expression of sPLA2-V gene in lung inflammation
A marked increase in ChPLA2-V expression level was observed in infected chicken lungs. In fact, no or very weak sPLA2 expression was observed in normal chicken lungs and spleen, suggesting a specific role of this group of sPLA2 in pathological situation.
Because of its up regulation in infected lungs, and the negative variations observed in the expression levels of ChPLA2-IB, -IIA and -X in the lungs, only PLA2-V can be considered as a specific biomarker of the infectious bronchitis disease in chicken.
The majority of sPLA2 are up-regulated by proinflammatory stimuli such as bacterial lipopolysaccharide (LPS), which predominantly increases the expression of group V sPLA2. Furthermore, it has been recently shown that sPLA2-V is a critical messenger in the regulation of cell migration. Lapointe et al. [50] investigated the effect of sPLA2-V on lipopolysaccharide mediated leukocyte recruitment supporting the contribution of sPLA2-V in the development of inflammatory innate immune response and its ability to modulate adhesion molecule expression. Indeed, immunohistochemistry studies revealed that sPLA2-V, is expressed in the airways of patients with pneumonia but not those of normal individuals [51].
Regarding sPLA2-V, it has a specific function related to phagocytosis in humans. The ability of sPLA2-V to regulate phagocytosis is specific and not shared with other phospholipases such as cPLA2α nor sPLA2-IIA [52]. Moreover, it was shown that activated cells secrete sPLA2-V which exert transcellular lipolytic activity on neighboring inflammatory cells [53]. The elevation of sPLA2-V expression in mice lungs with severe inflammation can be associated with an ongoing surfactant hydrolysis often observed in lung dysfunction [11]. In addition to the increase of gene expression level of ChPLA2-V in lungs, we observed an increase in its expression level in spleen, as well as, the decrease in ChPLA2-IB, -IIA and -X in lungs followed by a decrease in the expression level in spleen. These observations give evidence for a role of regulation of their expression by an immune system in case of inflammation.
4.5. Group IIE expression level in uninfected and infected chicken
ChPLA2-IIE expression was increased significantly in heart liver and spleen of infected chicken. In the lung, pancreas and intestine, ChPLA2-IIE expression did not change upon lung inflammation. These results are in agreement with previous works showing a constitutive expression of mPLA2-IIE in the spleen, heart and colon in control mice, its lipopolysaccharide-dependent induction in the liver, kidney, small intestine and testis, as well as a low expression in the lung and stomach [54].
5. Conclusion
In conclusion, this work presents the first comprehensive data regarding the expression of several sPLA2s in chicken infectious bronchitis. The distinct expression pattern observed for ChPLA2 genes suggests that mRNA profiling of the full set of chicken sPLA2s could be useful to detect lung inflammation in poultry. We have observed a dramatic increase in ChPLA2-V expression in both lungs and spleen which strongly supports that sPLA2-V may represent a novel broad molecular biomarker of infectious bronchitis. It will be now of interest to search for the presence of the ChPLA2-V protein and other sPLA2 in chicken serum using specific antibodies raised against the recombinant sPLA2 that have been cloned.
Acknowledgment
Our thanks are due to Mr Yann Denis (Institut de Microbiologie de la Méditerranée IMM-IFR88 Transcriptomics platform, Marseille, France) for his technical assistance during Q-PCR experiments.
References
- 1.Dennis E.A. Diversity of group types, regulation, and function of phospholipase A(2) J. Biol. Chem. 1994;269:13057–13060. [PubMed] [Google Scholar]
- 2.Aoubala M., Douchet I., Bezzine S., Hirn M., Verger R., De Caro A. Immunological techniques for the characterization of digestive lipases. In: Dennis E., Rubin B., editors. Methods in Enzymology. Academic Press, INC; San Diego: 1997. pp. 126–149. [DOI] [PubMed] [Google Scholar]
- 3.Murakami M., Nakatani Y., Atsumi G., Inoue K., Kudo I. Regulatory functions of phospholipase A2. Crit. Rev. Immunol. 1997;17:225–283. doi: 10.1615/critrevimmunol.v17.i3-4.10. [DOI] [PubMed] [Google Scholar]
- 4.Lambeau G., Lazdunski M. Receptors for a growing family of secreted phospholipases A2. Trends Pharmacol. Sci. 1999;20:162–170. doi: 10.1016/s0165-6147(99)01300-0. [DOI] [PubMed] [Google Scholar]
- 5.Dennis E.A. The growing phospholipase A2 superfamily of signal transduction enzymes. Trends Biochem. Sci. 1997;22:1–2. doi: 10.1016/s0968-0004(96)20031-3. [DOI] [PubMed] [Google Scholar]
- 6.Valentin E., Ghomashchi F., Gelb M., Lazdunski M., Lambeau G. On the diversity of secreted phospholipases A(2). Cloning, tissue distribution, and functional expression of two novel mouse group II enzymes. J. Biol. Chem. 1999;274:31195–31202. doi: 10.1074/jbc.274.44.31195. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki N., Ishizaki J., Yokota Y., Higashino K., Ono T., Ikeda M., Fujii N., Kawamoto K., Hanasaki K. Structures, enzymatic properties, and expression of novel human and mouse secretory phospholipase A(2)s. J. Biol. Chem. 2000;275:5785–5793. doi: 10.1074/jbc.275.8.5785. [DOI] [PubMed] [Google Scholar]
- 8.Kitsiouli E., Nakos G., Lekka M.E. Phospholipase A2 subclasses in acute respiratory distress syndrome. Biochim. Biophys. Acta. 2009;1792:941–953. doi: 10.1016/j.bbadis.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Arbibe L., Koumanov K., Vial D., Rougeot C., Faure G., Havet N., Longacre S., Vargaftig B.B., Bereziat G., Voelker D.R., Wolf C., Touqui L. Generation of lyso-phospholipids from surfactant in acute lung injury is mediated by type-II phospholipase A2 and inhibited by a direct surfactant protein A-phospholipase A2 protein interaction. J. Clin. Invest. 1998;102:1152–1160. doi: 10.1172/JCI3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chabot S., Koumanov K., Lambeau G., Gelb M.H., Balloy V., Chignard M., Whitsett J.A., Touqui L. Inhibitory effects of surfactant protein A on surfactant phospholipid hydrolysis by secreted phospholipases A2. J. Immunol. 2003;171:995–1000. doi: 10.4049/jimmunol.171.2.995. [DOI] [PubMed] [Google Scholar]
- 11.Ohtsuki M., Taketomi Y., Arata S., Masuda S., Ishikawa Y., Ishii T., Takanezawa Y., Aoki J., Arai H., Yamamoto K., Kudo I., Murakami M. Transgenic expression of group V, but not group X, secreted phospholipase A2 in mice leads to neonatal lethality because of lung dysfunction. J. Biol. Chem. 2006;281:36420–36433. doi: 10.1074/jbc.M607975200. [DOI] [PubMed] [Google Scholar]
- 12.Karray A., Frikha F., Ben Bacha A., Ben Ali Y., Gargouri Y., Bezzine S. Biochemical and molecular characterization of purified chicken pancreatic phospholipase A2. Febs J. 2009;276:4545–4554. doi: 10.1111/j.1742-4658.2009.07160.x. [DOI] [PubMed] [Google Scholar]
- 13.Karray A., Frikha F., Ben Ali Y., Gargouri Y., Bezzine S. Purification and biochemical characterization of a secreted group IIA chicken intestinal phospholipase A2. Lipids Health Dis. 2011;10:27. doi: 10.1186/1476-511X-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karray A., Ali Y.B., Gargouri Y., Bezzine S. Antibacterial properties of chicken intestinal phospholipase A2. Lipids Health Dis. 2011;10:4. doi: 10.1186/1476-511X-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emery S.L., Erdman D.D., Bowen M.D., Newton B.R., Winchell J.M., Meyer R.F., Tong S., Cook B.T., Holloway B.P., McCaustland K.A., Rota P.A., Bankamp B., Lowe L.E., Ksiazek T.G., Bellini W.J., Anderson L.J. Real-time reverse transcription-polymerase chain reaction assay for SARS-associated coronavirus. Emerg. Infect. Dis. 2004;10:311–316. doi: 10.3201/eid1002.030759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gelb J., Cavanagh D. twelveth ed. Wiley-Blackwell Publishing; Ames (IA): 2008. Infectious Bronchitis. [Google Scholar]
- 17.Cavanagh D. Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus. Avian Pathol. 2003;32:567–582. doi: 10.1080/03079450310001621198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 19.Wilson T., Schusterwoldan N. Isolation, characterization and regulation of gravidin, a phospholipase a2 inhibitor. Protein Eng. 1995;8:48. [Google Scholar]
- 20.Valentin E., Koduri R., Scimeca J., Carle G., Gelb M., Lazdunski M., Lambeau G. Cloning and recombinant expression of a novel mouse-secreted phospholipase A2. J. Biol. Chem. 1999;274:19152–19160. doi: 10.1074/jbc.274.27.19152. [DOI] [PubMed] [Google Scholar]
- 21.Cupillard L., Koumanov K., Mattei M., Lazdunski M., Lambeau G. Cloning, chromosomal mapping, and expression of a novel human secretory phospholipase A2. J. Biol. Chem. 1997;272:15745–15752. doi: 10.1074/jbc.272.25.15745. [DOI] [PubMed] [Google Scholar]
- 22.Tischfield J. A reassessment of the low molecular weight phospholipase A2 gene family in mammals. J. Biol. Chem. 1997;272:17247–17250. doi: 10.1074/jbc.272.28.17247. [DOI] [PubMed] [Google Scholar]
- 23.Murakami M., Kudo I. Secretory phospholipase A2. Biol. Pharm. Bull. 2004;27:1158–1164. doi: 10.1248/bpb.27.1158. [DOI] [PubMed] [Google Scholar]
- 24.Pan Y., Epstein T., Jain M., Bahnson B. Five coplanar anion binding sites on one face of phospholipase a2: relationship to interface binding. Biochemistry. 2001;40:609–617. doi: 10.1021/bi002514g. [DOI] [PubMed] [Google Scholar]
- 25.Berg O., Gelb M., Tsai M., Jain M. Interfacial enzymology: the secreted phospholipase A(2)-paradigm. Chem. Rev. 2001;101:2613–2654. doi: 10.1021/cr990139w. [DOI] [PubMed] [Google Scholar]
- 26.Valentin E., Lambeau G. Increasing molecular diversity of secreted phospholipases A(2) and their receptors and binding proteins. Biochim. Biophys. Acta. 2000;1488:59–70. doi: 10.1016/s1388-1981(00)00110-4. [DOI] [PubMed] [Google Scholar]
- 27.Verheij H.M., Slotboom A.J., De Haas G. Structure and function of phospholipase A2. Rev. Physiol. Biochem. Pharmacol. 1981;91:91–203. doi: 10.1007/3-540-10961-7_3. [DOI] [PubMed] [Google Scholar]
- 28.Seilhamer J.J., Randall T.L., Yamanaka M., Johnson L.K. Pancreatic phospholipase A2: isolation of the human gene and cDNAs from porcine pancreas and human lung. DNA. 1986;5:519–527. doi: 10.1089/dna.1.1986.5.519. [DOI] [PubMed] [Google Scholar]
- 29.Nevalainen T.J., Kortesuo P.T., Rintala E., Marki F. Immunochemical detection of group I and group II phospholipases A2 in human serum. Clin. Chem. 1992;38:1824–1829. (Winston-Salem, N. C.) [PubMed] [Google Scholar]
- 30.Fourcade O., Simon M., Viode C., Rugani N., Leballe F., Ragab A., Fournie B., Sarda L., Chap H. Secretory phospholipase A2 generates the novel lipid mediator lysophosphatidic acid in membrane microvesicles shed from activated cells. Cell. 1995;80:919–927. doi: 10.1016/0092-8674(95)90295-3. [DOI] [PubMed] [Google Scholar]
- 31.Eerola L.I., Surrel F., Nevalainen T.J., Gelb M.H., Lambeau G., Laine V.J. Analysis of expression of secreted phospholipases A2 in mouse tissues at protein and mRNA levels. Biochim. Biophys. Acta. 2006;1761:745–756. doi: 10.1016/j.bbalip.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Hua X., Enerbaeck S., Hudson J., Youkhana K., Gimble J.M. Cloning and characterization of the promoter of the murine lipoprotein lipase-encoding gene: structural and functional analysis. Gene. 1991;107:247–258. doi: 10.1016/0378-1119(91)90325-6. [DOI] [PubMed] [Google Scholar]
- 33.Ohara O., Ishizaki J., Arita H. Structure and function of phospholipase A(2) receptor. Prog. Lipid Res. 1995;34:117–138. doi: 10.1016/0163-7827(94)00009-b. [DOI] [PubMed] [Google Scholar]
- 34.Hanasaki K., Yokota Y., Ishizaki J., Itoh T., Arita H. Resistance to endotoxic shock in phospholipase A2 receptor-deficient mice. J. Biol. Chem. 1997;272:32792–32797. doi: 10.1074/jbc.272.52.32792. [DOI] [PubMed] [Google Scholar]
- 35.Kundu G.C., Mukherjee A.B. Evidence that porcine pancreatic phospholipase A2 via its high affinity receptor stimulates extracellular matrix invasion by normal and cancer cells. J. Biol. Chem. 1997;272:2346–2353. [PubMed] [Google Scholar]
- 36.Rae D., Beechey-Newman N., Burditt L., Sumar N., Hermon-Taylor J. Activation of human granulocyte type 1-prophospholipase A2. Scand. J. Gastroenterol. Suppl. 1996;219:24–27. doi: 10.3109/00365529609104995. [DOI] [PubMed] [Google Scholar]
- 37.Mulherkar R., Rao R.S., Wagle A.S., Patki V., Deo M.G. Enhancing factor, a Paneth cell specific protein from mouse small intestines: predicted amino acid sequence from RT-PCR amplified cDNA and its expression. Biochem. Biophys. Res. Commun. 1993;195:1254–1263. doi: 10.1006/bbrc.1993.2179. [DOI] [PubMed] [Google Scholar]
- 38.Chen J., Engle S.J., Seilhamer J.J., Tischfield J.A. Cloning, expression and partial characterization of a novel rat phospholipase A2. Biochim. Biophys. Acta. 1994;1215:115–120. doi: 10.1016/0005-2760(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 39.Shinohara H., Ishida H., Fernandez E.J., Amabe Y., Nagata T., Wakano Y. Phospholipase-A2 in rat gingival tissue. J. Period Res. 1992;27:528–533. doi: 10.1111/j.1600-0765.1992.tb01827.x. [DOI] [PubMed] [Google Scholar]
- 40.Reddy S.T., Winstead M.V., Tischfield J.A., Herschman H.R. Analysis of the secretory phospholipase A2 that mediates prostaglandin production in mast cells. J. Biol. Chem. 1997;272:13591–13596. doi: 10.1074/jbc.272.21.13591. [DOI] [PubMed] [Google Scholar]
- 41.Balboa M.A., Balsinde J., Winstead M.V., Tischfield J.A., Dennis E.A. Novel group V phospholipase A2 involved in arachidonic acid mobilization in murine P388D1 macrophages. J. Biol. Chem. 1996;271:32381–32384. doi: 10.1074/jbc.271.50.32381. [DOI] [PubMed] [Google Scholar]
- 42.Seeds M.C., Jones K.A., Duncan Hite R., Willingham M.C., Borgerink H.M., Woodruff R.D., Bowton D.L., Bass D.A. Cell-specific expression of group X and group V secretory phospholipases A(2) in human lung airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2000;23:37–44. doi: 10.1165/ajrcmb.23.1.4034. [DOI] [PubMed] [Google Scholar]
- 43.Nakos G., Kitsiouli E., Hatzidaki E., Koulouras V., Touqui L., Lekka M.E. Phospholipases A2 and platelet-activating-factor acetylhydrolase in patients with acute respiratory distress syndrome. Crit. Care Med. 2005;33:772–779. doi: 10.1097/01.ccm.0000158519.80090.74. [DOI] [PubMed] [Google Scholar]
- 44.Vial D., Senoralepose M., Havet N., Molio L., Vargaftig B.B., Touqui L. Expression of the type-II phospholipase A(2) in alveolar macrophages – down-regulation by an inflammatory signal. J. Biol. Chem. 1995;270:17327–17332. doi: 10.1074/jbc.270.29.17327. [DOI] [PubMed] [Google Scholar]
- 45.Touqui L., Arbibe L. A role for phospholipase A2 in ARDS pathogenesis. Mol. Med. Today. 1999;5:244–249. doi: 10.1016/s1357-4310(99)01470-7. [DOI] [PubMed] [Google Scholar]
- 46.M. bouzouaia, in al baytary, 2001 pp. 1–36, Tunisia.
- 47.Wilson P.G., Manji M., Neoptolemos J.P. Acute pancreatitis as a model of sepsis. J. Antimicrob. Chemother. 1998;41(Suppl. A):51–63. doi: 10.1093/jac/41.suppl_1.51. [DOI] [PubMed] [Google Scholar]
- 48.Rae D., Porter J., Beechey-Newman N., Sumar N., Bennett D., Hermon-Taylor J. Type 1 prophospholipase A2 propeptide in acute lung injury. Lancet. 1994;344:1472–1473. doi: 10.1016/s0140-6736(94)90291-7. [DOI] [PubMed] [Google Scholar]
- 49.Nevalainen T.J., Hietaranta A.J., Gronroos J.M. Phospholipase A2 in acute pancreatitis: new biochemical and pathological aspects. Hepatogastroenterology. 1999;46:2731–2735. [PubMed] [Google Scholar]
- 50.Lapointe S., Brkovic A., Cloutier I., Tanguay J.F., Arm J.P., Sirois M.G. Group V secreted phospholipase A2 contributes to LPS-induced leukocyte recruitment. J. Cell Physiol. 2010;224:127–134. doi: 10.1002/jcp.22106. [DOI] [PubMed] [Google Scholar]
- 51.Masuda S., Murakami M., Mitsuishi M., Komiyama K., Ishikawa Y., Ishii T., Kudo I. Expression of secretory phospholipase A2 enzymes in lungs of humans with pneumonia and their potential prostaglandin-synthetic function in human lung-derived cells. Biochem. J. 2005;387:27–38. doi: 10.1042/BJ20041307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Balestrieri B., Arm J.P. Group V sPLA2: classical and novel functions. Biochim. Biophys. Acta. 2006;1761:1280–1288. doi: 10.1016/j.bbalip.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 53.Wijewickrama G.T., Kim J.H., Kim Y.J., Abraham A., Oh Y., Ananthanarayanan B., Kwatia M., Ackerman S.J., Cho W. Systematic evaluation of transcellular activities of secretory phospholipases A2. High activity of group V phospholipases A2 to induce eicosanoid biosynthesis in neighboring inflammatory cells. J. Biol. Chem. 2006;281:10935–10944. doi: 10.1074/jbc.M512657200. [DOI] [PubMed] [Google Scholar]
- 54.Hamaguchi K., Kuwata H., Yoshihara K., Masuda S., Shimbara S., Oh-ishi S., Murakami M., Kudo I. Induction of distinct sets of secretory phospholipase A(2) in rodents during inflammation. Biochim. Biophys. Acta. 2003;1635:37–47. doi: 10.1016/j.bbalip.2003.10.004. [DOI] [PubMed] [Google Scholar]