Highlights
-
•
An outbreak of fatal enteritis occurred in a dog litter.
-
•
Major known causes of enteritis of young dogs were excluded.
-
•
A novel canine bocavirus 2 strain was detected by random PCR and NGS.
-
•
CaBoV-2 was detected in the intestinal tract and lymphoid tissue by ISH.
-
•
No additional cases were identified by a small retrospective analysis.
Keywords: Enteritis, Bocavirus, Dog, Canine bocavirus, Parvovirus
Abstract
Bocaviruses are small non-enveloped viruses with a linear ssDNA genome, that belong to the genus Bocaparvovirus of the subfamiliy Parvovirinae. Bocavirus infections are associated with a wide spectrum of disease in humans and various mammalian species. Here we describe a fatal enteritis associated with infection with a novel strain of canine bocavirus 2 (CaBoV-2), that occurred in a litter of German wirehaired pointers. Necropsy performed on three puppies revealed an enteritis reminiscent of canine parvovirus associated enteritis, accompanied with signs of lymphocytolytic disease in bone marrow, spleen, lymph nodes and thymus. While other major causes of enteritis of young dogs, including canine parvovirus, were excluded, by random PCR in combination with next-generation sequencing, a novel CaBoV-2 strain was detected. Phylogenetic analysis of the genome of this novel canine bocavirus strain indicated that this virus was indeed most closely related to group 2 canine bocaviruses. Infection with canine bocavirus was confirmed by in situ hybridization, which revealed the presence of CaBoV-2 nucleic acid in the intestinal tract and lymphoid tissues of the dogs. In a small-scale retrospective analysis concerning the role of CaBoV-2 no additional cases were identified. The findings of this study provide novel insights into the pathogenicity of canine bocaviruses.
1. Introduction
Parvoviruses are small non-enveloped viruses with a linear ssDNA genome of approximately 5 kb. The family of Parvoviridiae consists of two subfamilies, Parvovirinae and Densovirinae. Classification into subfamilies is based on their host range; viruses of the Densovirinae infect insects and arthropods while viruses of the subfamily Parvovirinae infect vertebrates (ICTV, 2013). At present, the International Committee on Taxonomy of Viruses (ICTV) has recognized eight different genera of the subfamily Parvovirinae: Amdoparvovirus, Aveparvovirus, Bocaparvovirus, Copiparvovirus, Dependoparvovirus, Erythroparvovirus, Protoparvovirus and Tetraparvovirus (ICTV, 2013).
Parvovirus infections have been associated with a wide spectrum of disease, including severe gastroenteritis, myocarditis, hepatitis, chronic immune complex disease and meningoencephalitis (Berns and Parrish, pp. 2437–2477, 2007). However, subclinical infections with various parvoviruses are also common (Berns and Parrish, pp. 2437–2477, 2007), indicating that the pathogenicity as well as the underlying mechanisms need further study in humans and animals.
Canine parvovirus 2, belonging to the genus Protoparvovirus, was first identified in dogs in the 1970s as a major cause of viral enteritis in young dogs and has subsequently become endemic among dogs worldwide (Hoelzer and Parrish, 2010). Canine parvovirus 2 targets rapidly proliferating cells, like those in intestinal crypts and lymphoid organs, causing necrosis of crypt epithelia and depletion of lymphoid organs such as spleen, thymus, lymph node and bone marrow (Carman and Povey, 1985, Decaro and Buonavoglia, 2012). Furthermore conspicuous villous shortening and atrophy as well as syncytial giant cell representing crypt regeneration are among typical histological changes (Osterhaus et al., 1980).
In addition to canine parvovirus 2, several viruses from the genus Bocaparvovirus were identified in dogs. This genus was named according to the initial members, bovine parvovirus and Minute virus of canines (MVC) and has an additional open reading frame between the NS1 and VP1 gene (Binn et al., 1970, Manteufel and Truyen, 2008, Spahn et al., 1966, Storz et al., 1978). Besides bovine parvovirus and MVC, several new members of this genus were detected recently in humans and in various animal species with and without respiratory, enteric, or neurological disease symptoms or signs (Allander et al., 2005, Mitui et al., 2012, Schildgen et al., 2008).
MVC was associated with outbreaks of disease in neonatal dogs and fetal deaths in adult infected dogs (Carmichael et al., 1991, Decaro et al., 2012, Harrison et al., 1992, Pollock, 1982). Recently, a novel species of canine bocaviruses (Carnivore bocaparvovirus 2; CaBoV-2) was identified in samples obtained from healthy dogs, dogs with respiratory disease and from fecal samples collected from stray dogs in a surveillance program (Kapoor et al., 2012, Lau et al., 2012). In addition, a third species of canine bocavirus (Canine bocaparvovirus 3) was found in the liver of a dog co-infected with a novel circovirus (Li et al., 2013).
However, in all recent studies the pathogenic role of canine bocaviruses in the observed disease remained unclear. In the present study, we describe the identification, characterization and disease association of a novel canine bocavirus strain belonging to the genetic group of CaBoV-2, that was detected in a dog litter with severe and lethal parvovirus-like enteritis.
2. Materials and methods
2.1. Case description
A litter of 11 day old German wirehaired pointer puppies and dam presented with clinical signs of enteric disease, including vomiting and diarrhea. After a transient improvement of the puppies and the dam following anti-parasitic treatment the first puppy died four days after disease onset. Despite intensive anti-inflammatory and antibiotic treatment as well as sustentative therapy, death of two further puppies could not be prevented. The dam and the remaining three puppies survived. Other dogs and cats on the premise were separated early in the course of disease and remained asymptomatic. The dam had always been fully vaccinated until three years ago. A fresh up-vaccination was performed three months before fecundation.
2.2. Post mortem examination and collection of samples
The three puppies were subsequently submitted to the Department of Pathology, University of Veterinary Medicine Hannover, Germany. A full necropsy was performed and various tissue samples were collected and immersed in 10% non-neutral buffered formalin and routinely processed into paraffin. 3 μm thick sections were stained with hematoxylin and eosin (HE) for histopathological examination. In addition, tissue samples were stored at −20 °C for a few weeks and subsequently stored at −80 °C.
2.3. Routine virological laboratory diagnostic
Collected tissues were tested for the presence of various common viral and bacterial pathogens known to cause diarrhea of young puppies using various methods. PCR primers were selected based on multiple sequence alignments using the hussar software package (DKFZ, Heidelberg, Germany) and the primer express software (ABI, Langen, Germany) or taken from the literature. Unless otherwise specified, conditions for first and second rounds of the PCR were as follows: 25 pmol of each primer, 1 U Taq polymerase (Biotherm), 10 nmol of each dNTP in 1× polymerase buffer (Biotherm), 30 cycles 94 °C 30″, 60 °C 30″, 72 °C 30″ with a final elongation step at 72 °C for 5′.
A sample of small intestine of one puppy (S791/13) was tested for the presence of canine parvovirus 2 and feline parvovirus using primers PV_VP2 1 (5′ GAAAACGGATGGGTGGAAATC 3′) and PV_VP2 4 (5′ AGAAATGGTGGTAAGCCCAATG 3′) in the first round and PV_VP2 5 (5′ ATACTGGAACTAGTGGCACACC 3′) and PV_VP2 4 in the second round of a semi-nested PCR.
In addition, a RT-PCR specific for canine and feline corona virus and a pan-coronavirus specific RT-PCR were performed on this tissue as described previously (Herrewegh et al., 1995, Wise et al., 2006).
Furthermore, samples of thymus, spleen and lymph nodes as well as liver and lung of two affected puppies (S757/13 and S758/13) were tested for the presence of canine herpesvirus 1 (CHV-1) and canine adenovirus (CAV) respectively.
For CHV-1, a nested PCR was performed using primers specific for the thymidine kinase gene: CHV-A (5′ TCCATTTTGTGAGGCGAGTTTC 3′) and CHV-BR (5′ AGGCTTAATTCACCACGCAGC 3′) amplifying a 334 bp product in the first round as well as CHV-C (5′ CGATTGTGGCATCTAATCCCAG 3′) and CHV-DR (5′ ACGCCAGAATGCCATAGGTTC 3′) amplifying a 211 bp product in the second round. The annealing temperature for both rounds of the PCR was chosen at 52 °C.
For CAV, a nested PCR with first round primers amplifying a 704 bp product was performed using primers described previously (Erles et al., 2004). Second round primers were CAV-3 (5′ TTTGAGCCCATGTGCAGACA 3′) and CAV-4 (5′ GCGGGTTAAAGGCCGAAA 3′) amplifying a 121 bp product.
In addition, sample material was inoculated on tissue culture cells including three different canine cell lines (Madin Darby canine kidney cells; ATCC CCL-34; A-72 cells; ATCC CRL-1542 and Walter Reed canine cells (WRCC); kindly supplied by U. Truyen, Leipzig) using routine culture techniques including sub cultivation to demonstrate the presence of infectious virus. Liquid phase immune-electron microscopy was performed on a suspension of intestinal sample material using a high titred polyclonal serum against canine parvovirus (CPV). Immunohistochemistry to detect CPV and canine distemper virus (CDV) antigen was performed on sections of small intestine, spleen, lymph node and bone marrow using a monoclonal mouse anti-CPV1-2A1 antibody (Custom Monoclonal Antibodies International, Sacramento, CA) and a monoclonal mouse anti-CDV nucleoprotein antibody (D110, kindly provided by A. Zurbriggen, Berne, Switzerland) as previously described (Schaudien et al., 2010, von Rüden et al., 2012). In addition, to investigate involvement of bacterial agents, a sample of small intestine of one puppy was incubated on standard agar under aerobic and anaerobic conditions (Department of Microbiology, University of Veterinary Medicine Hannover, Germany).
2.4. Random PCR in combination with next-generation sequencing
Contents of the small and large intestinal tract of one pup (S791/13) were processed for sequence independent RNA and DNA virus screening as described previously (van den Brand et al., 2012, van Leeuwen et al., 2010). In brief, intestinal contents were harvested under aseptical conditions, diluted five times in PBS, vortexed and centrifuged briefly. Supernatants were filtered and treated with Omnicleave endonucleases (Epicenter Biotechnologies) to decrease host DNA and RNA. Subsequently, RNA and DNA were extracted, and after RT amplication of RNA, first and second strand synthesis and random PCR was performed. Amplicons were processed for next-generation sequencing with a 454 GS Junior instrument (Roche). Obtained reads were trimmed and assembled with de novo assembly using CLC Genomics Workbench 5 (CLC Bio), and analyzed by nucleotide and translated nucleotide BLAST searches. Sequences were classified based on the taxonomic origin of the best-hit sequence with MEGAN 4.70.4 (Huson et al., 2011), using E-value cut-offs of 0.001 and 10−10 for BLASTn and BLASTx searches, respectively.
2.5. Genome sequencing and phylogenetic analysis
Specific primers were designed based on obtained 454-sequences (Table 1 ) and conserved regions present in various canine bocaviruses and partially overlapping PCR amplicons were obtained as described previously (Bodewes et al., 2013a). By combining sequences obtained by Sanger sequencing and 454-sequencing, the near full-length genome of a novel canine bocavirus, tentatively called canine bocavirus F13000791S, was obtained. Phylogenetic analysis was performed by creation of multiple alignments using the ClustalW method in MEGA6 (Tamura et al., 2013) based on the nucleotide and deduced amino acid sequences of the VP1 genes of the detected bocavirus and other representative bocaviruses. Neighbor-joining phylogenetic trees were built with the p-distance model, 1000 bootstrap replicates, and otherwise default parameters in MEGA6 (Tamura et al., 2013).
Table 1.
Sequences of primers used in the present study.
| Genome position# | Sequence | Direction |
|---|---|---|
| 1–21 | GATTGGTTGGTGTTTTATGAC | Fw |
| 920–938 | CTGAGGCAAGACCCACTTC | Rev |
| 855–875 | GCAACTCCGTTTGTCTCTCAC | Fw |
| 2084–2104 | CAGTCAAGGCAGCATACAGGC | Rev |
| 2000–2018 | TGGCTACGCTCCTCTGGAG | Fw |
| 3968–3987 | GAGCATGTATAGCGGCGCAC | Rev |
| 3888–3909 | ACTACAGTCTGACCTGACGGAC | Fw |
| 5031–5054 | GCGCAAAACTTTTCGTTTTATGGC | Rev |
| 2447–2467a | TTACACTGCTTCGAAGACATC | Fw |
| 3479–3498a | GATGGGAGCGAACCACTGTC | Rev |
Based on the CaBoV F13000791S genome.
Used for Sanger sequencing only
2.6. Screening of tissues for canine bocavirus 2 DNA
The presence of canine bocavirus 2 in various tissues (Table 2 ) was tested by PCR using primers targeting the partial canine bocavirus VP1 gene as described previously (Kapoor et al., 2012). Tissue pieces were thawed and homogenized using a Fastprep24 tissue homogenizer (MP Biomedicals) in Hank's balanced salt solution containing 0.5% lactalbumin,10% glycerol, 200 U/ml penicillin, 200 mg/ml streptomycin, 100 U/ml polymyxin B sulfate, 250 mg/ml gentamycin and 50 U/ml nystatin (ICN Pharmaceuticals; Transportmedium), centrifuged briefly and total nucleic acids were extracted using the High Pure Viral Nucleic Acid Kit according to the protocol of the manufacturer (Roche). Transportmedium was included as a negative control for the whole procedure.
Table 2.
List of tissues examined by canine bocavirus 2-specific PCR and in situ hybridization and test results.
| Tissue | PCR | In situ hybridization |
|---|---|---|
| Tested in all 3 animals | ||
| Brain | Positive | Negative |
| Kidney | Positive | Negative |
| Liver | Positive | Negative |
| Spleen | Positive | Single positive cells |
| Tested in 2 animals | ||
| Small intestine | Positive | Multifocal positive cells in submucosal lymphoid tissue; single positive enterocytes; |
| Large intestine | Positive | Multifocal positive cells in submucosal lymphoid tissue; |
| Lung | Positive | Negative |
| Tested in 1 animal | ||
| Mediastinum/thymus | Positive | Single positive cells |
| Mesenterial lymph nodes | Positive | Single positive cells |
| Tonsils | Positive | Negative |
| Bone marrow (sternum) | Positive | Negative |
| Pancreas | Positive | Negative |
| Heart | Positive | Negative |
| Urinary bladder | Positive | Negative |
| Stomach | Positive | Negative |
2.7. In situ hybridization
For the generation of a CaBoV-2-specific in situ probe, a synthetic gene construct (MWG Eurofins Operon) was produced encoding a sequenced 124 nt spanning region (nt 1338–1461) of the canine bocavirus F13000791S NS1 gene. This fragment was amplified by PCR with specific NS1 forward (5′ GCTGTACGGATGTGTGAAC 3′) and NS1 reverse (5′ CAGACACTTGGCCTGCTCTA 3′) primers, subcloned into pCR4®TOPO® vector using the TOPO TA Cloning® Kit for Sequencing (Invitrogen) and sequenced (Seqlab). Production of T3 and T7 probe was performed as previously described (Gröters et al., 2005, Hahn et al., 2013). Briefly, a PCR with M13 reverse and CaBoV-2 NS1 reverse or M13 forward and CaBoV-2 NS1 for primers respectively was performed. The fragments and DIG RNA labeling Mix (Roche) were used as template for Digoxigenin labeled probe synthesis with T3 or T7 RNA polymerase (Roche). With the obtained probes various tissues (Table 2) of the infected animals were investigated for the presence of CaBoV-2 nucleic acid as described (Gaedke et al., 1997, Gröters et al., 2005, Zurbriggen et al., 1993). Briefly, tissue sections were de-waxed in xylene, hydrated in graded ethanol, and washed in ultrapure, pyrogen-free, DEPC-treated water. Proteolytic digestion was performed by applying 1 μg/ml proteinase K (Roche Diagnostics). After postfixation, acetylation and pre-hybridization, hybridization was performed overnight in a moist chamber at 52 °C with a probe concentration of 500 ng/100 μl. An anti-DIG-antibody conjugated with alkaline phosphatase (1:200, Roche Diagnostics) and substrates nitrobluetetrazoliumchloride (NBT) and 5-bromo-4-chloro-3-indolyl phosphate (BCIP, X-Phosphate) (both Sigma–Aldrich) were used as detection system. A purple precipitate that was associated with cellular structures and localized at the same level was considered a positive reaction.
Additionally a section of small intestine of a four days old dog with presumed Minute virus of canines (MVC) infection was investigated by in situ hybridization applying the CaBoV-2 specific probes. The dog was one of the 11 puppies that died few days after birth by caesarian section that revealed 19 puppies. Histologically the jejunal mucosa was intact, but within enterocytes multiple intranuclear eosinophilic inclusion bodies were observed.
In order to explore a potential role of CaBoV-2 for parvovirus-like enteritis in the far and recent past, samples of intestine and various organs of 16 dogs, four wildcats (Felis silvestris), one gray wolf (Canis lupus) and one raccoon (Procyon lotor) dating back to 1972 were included in the study. All animals were presented with enteritis of unknown origin.
3. Results
3.1. Post mortem examination
Grossly and histologically all puppies showed comparable changes. In addition to a moderate exsiccosis the gastrointestinal tract was only sparsely filled with fluid contents. There was thymic atrophy and the femoral bone marrow displayed a fluid texture and a dark red color. Upon microscopic examination severe villus shortening, fusion and atrophy as well as conspicuous crypt regenerations were evident in sections of small intestine (Fig. 1A and B). There was extensive loss of enterocytes. Areas of necrosis were present within villus tips. Superficial bacterial colonization was observed as well as microvessel thrombosis. On the other hand only single crypt dilatations with accumulations of cellular debris were seen in the colon. The bone marrow was severely atrophic. Only a minimal hematopoietic activity as well as a sinus hyperemia was assessed (Supplementary Fig. S1A). Thymus, spleen and various lymph nodes showed moderate lymphoid depletions. Multifocally there was evidence of lymphocytic cell death (apoptosis and necrosis) in the spleen and lymph nodes (Supplementary Fig. S1B–D). A mild alveolar histiocytosis was evident as well as remnants of amniotic fluid. Of note, the cerebellar granular cell layer was intact, lacking signs of hypocellularity.
Fig. 1.
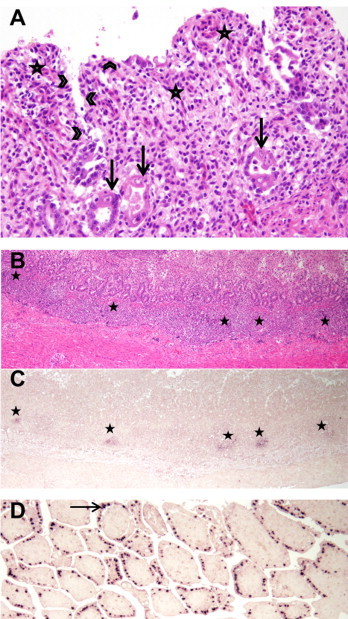
Histological lesions of small intestines and canine bocavirus 2 (CaBoV-2) in situ hybridization. (A) Prominent histological lesions comprised atrophied, shortened and fused villi (asterisks), loss of enterocytes (arrow heads) and large crypt regenerates (arrows) in the small intestine (HE, 200×). (B and C) Within the submucosal lymphoid tissue (asterisks) CaBoV-2 nucleic acid was demonstrated by in situ hybridization ((B) HE, 400×); (C) in situ hybridization with CaBoV-2-specific probes, 400×; (D) Nearly diffuse positive staining of enterocyte nuclei (arrow) by in situ hybridization with CaBoV-2-specific probes in the small intestine of a dog with presumed minute virus of canines infection (400×). (For interpretation of the references to color near the citation of this figure, the reader is referred to the web version of the article.)
3.2. Routine laboratory diagnostics
Parvovirus DNA and coronavirus RNA were not demonstrated in the small intestine by PCR. Using CPV specific immune electron microscopy no virus could be shown in the intestinal content. Immunohistochemical staining of small intestine, spleen, lymph node and bone marrow sections revealed no CPV and CDV antigen. Neither canine herpesvirus-1 nor canine adenovirus DNA was detected in samples of thymus, spleen and lymph nodes as well as in liver and lung respectively. In addition, no infectious virus could be propagated in cell culture. A moderate intestinal content of Escherichia coli (E. coli) was assessed by cultivation.
3.3. Detection of a novel canine bocavirus strain by next-generation sequencing
To identify possible other viral agents, samples were processed for random PCR and next-generation sequencing. In total 34,117 trimmed reads were obtained and using BLAST analysis more than 10 reads were detected that had the highest similarity with canine bocaviruses, while no other viral sequences were detected. The presence of a novel canine bocavirus, tentatively called canine bocavirus F13000791S, was confirmed by PCR and the complete coding sequence of this canine bocavirus was established (GenBank accession number KF771828). Three main open reading frames were identified, coding for the non-structural protein (NS; nt 153–2162), the nucleoprotein (NP; nt 2304–2891) and the viral capsid protein (VP1; nt 2875–5013), with a similar genome organization as described previously for canine bocaviruses (Kapoor et al., 2012, Lau et al., 2012) (Fig. 2A). In addition, a putative RNA splicing site was present at position nt 2078. Phylogenetic analysis of the complete deduced amino acid sequence of the canine bocavirus F13000791S indicated that this virus belongs to the species Canine bocaparvovirus 2 (Fig. 2B). Additional phylogenetic analysis and analysis of pairwise identities of the nucleotide and deduced amino acid sequence of a partial region of the VP1/2 region which was available for various viruses that are closely related to canine bocavirus F13000791S revealed that this novel virus is most closely related to canine bocavirus Dis-017 (pairwise identities of 96% and 97% on the nucleotide and deduced amino acid level respectively), which was detected previously in a sample collected from a dog with respiratory disease of unknown origin (Kapoor et al., 2012) (Fig. 2C).
Fig. 2.
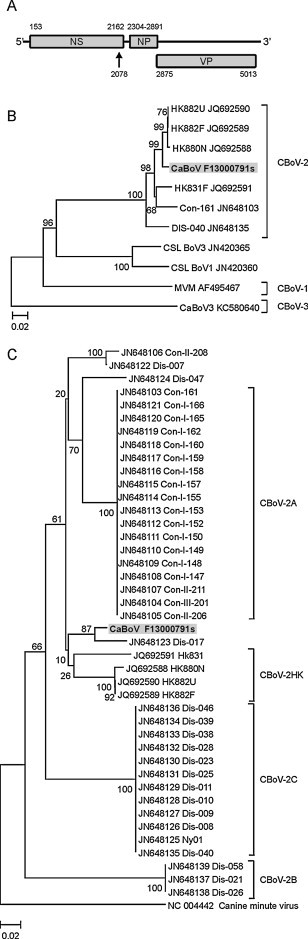
Genome organization and phylogenetic analysis of canine bocavirus F13000791S. A. Genome organization of canine bocavirus F13000791S. Indicated were the location of the major ORFs (gray) and the location of the start- and stopcodons on the nucleotide level counted from the 5′ end of the partial canine bocavirus F13000791S genome. B. Phylogenetic neighbor-joining tree with p-distance and 1000 bootstrap replicates of the deduced amino acid sequences of the VP1 genes of various viruses of the genus Bocaparvovirus. C. Phylogenetic neighbor-joining tree with p-distance and 1000 bootstrap replicates of the nucleotide sequences of the partial VP1/2 genes of various viruses of the genus Bocaparvovirus that are closely related to canine bocavirus F13000791S. Indicated are the GenBank accession number and the strain name. CSL: California sea lion, MVC: minute virus of canines.
3.4. Detection of canine bocavirus 2 DNA in other tissues
Other available samples (Table 2) of the puppies were tested for CaBoV-2 DNA using a specific PCR and a band of the expected size was detected in all samples of all three puppies, while no PCR product was detected in the negative control. A proportion of the obtained PCR products were sequenced and analysis of the obtained sequences revealed that it was identical in samples of all three puppies.
3.5. In situ hybridization
In situ hybridization of the small and large intestine revealed cytoplasmic CaBoV 2 nucleic acid in submucosal lymphoid tissue as well as in few small intestinal enterocytes of two animals (Table 2, Fig. 1C, Supplementary Fig. S2A). The third puppy displayed only minimal positive signals in small intestinal enterocytes. In addition, in situ hybridization of the other tissues revealed single CaBoV-2 nucleic acid positive cells in the spleen, thymus and mesenterial lymph nodes of all three animals (Table 2, Supplementary Fig. S2B–D).
In situ hybridization performed on control enteric tissue of a case of MVC infection revealed small intestinal enterocytes displaying a compact nuclear reaction that was distributed nearly diffusely within the investigated sections (Fig. 1D).
3.6. Retrospective investigation of other canids and felids with parvovirus-like enteritis
Historical cases of parvovirus-like enteritis were tested from 16 dogs, 5 wildcats, 1 wolf and 1 raccoon. There was no evidence of CaBoV-2 nucleic acid in intestinal sections and sections of various other organs.
4. Discussion
Canine viral enteritis is a major cause of death among dogs of very young age. The leading causes of canine viral enteritis include canine parvovirus 2, canine coronavirus and rotaviruses (Decaro and Buonavoglia, 2011, Osterhaus et al., 1980). More recent studies also attribute a role to caliciviruses and astroviruses (Castro et al., 2013, Martella et al., 2008).
In the present manuscript, we describe the detection and characterization of a novel CaBoV-2 strain that was detected in the enteric tract and lymphoid tissues of puppies with parvovirus-like enteritis. Although observed histologic lesions correlated with canine parvovirus 2-infection, both by specific PCR, immunohistochemistry and random PCR in combination with next-generation sequencing of the contents of the small and large intestine of one pup, no canine parvovirus but a novel CaBoV-2 strain was detected. Screening of various tissues of the other puppies indicated that all puppies were infected with this virus. The presence of canine bocavirus nucleic acid in a proportion of the tissues was confirmed by in situ hybridization, while the positive results of the PCR of the other tissues are most likely due to spread of virus via the blood stream.
Various previously unknown bocaviruses were recently identified in the enteric tract of different animal species and humans, with and without disease association (Bodewes et al., 2013b, Kapoor et al., 2010, Lee et al., 2007, Li et al., 2011, Shan et al., 2011). To understand the role of bocaviruses in enteric disease, demonstration of virus replication in tissue and association with histologic lesions is an important first step. In the present study, viral nucleic acid could be detected by in situ hybridization mainly in the cytoplasm of lymphoid cells of the submucosal lymphoid tissue of the enteric tract. Only low amounts were present in epithelial cells lining the villi of the small intestinal tract. The presence of viral nucleic acid in the cytoplasm suggests that virus transcription and replication occurred in these cells. However, parvovirus DNA replicates in the nucleus and additional studies are required to elucidate why in the present study viral nucleic acid was detected mainly in the cytoplasm. Of interest, observed in situ hybridization results for the canine bocavirus F13000791s cases were in contrast to small intestinal tissue of a dog infected with MVC. In this control case, viral nucleic acid could be detected exclusively in the nucleus of epithelial cells lining the villi. The divergent histological lesions and apparent viral cell tropism of the CaBoV-2- and the MVC-infected control case may be related to different levels of virulence of both viruses. However, in the cases of CaBoV-2 infection tissue samples were collected from puppies that had died four to five days after onset of disease. Thus, infection and replication of the virus in the enteric tract might have declined while viral nucleic acid could still be detected. Alternatively, CaBoV-2 may have infected only low numbers of epithelial cells of the intestinal tract but played a role in the observed intestinal disease due to infection and partial destruction of the intestinal immune system (including the Peyer patches). Subsequent immunosuppression might have allowed pathogenic bacteria to colonize the intestinal tract and thus contributed to the severe lesions. Therefore, only by fulfilling Koch's postulates the exact role of CaBoV-2 in the observed disease can be demonstrated.
Upon a retrospective in situ hybridization study on tissues of several canids and felids with parvovirus-like enteritis no CaBoV-2 nucleic acid could be demonstrated, suggesting that canine bocaviruses are not often a cause of severe enteritis similar to suggested previously (Harrison et al., 1992). However, only a relatively small number of animals was tested and no definitive conclusions can be drawn.
Analysis of the sequences of the major open reading frames revealed that canine bocavirus F13000791S was most closely related to canine bocaviruses of group 2. Since the identity of the NS1 gene was more than 95% with various canine bocaviruses on the nucleotide level, canine bocavirus F13000791S does not belong to a novel species but is a novel strain within the species Carnivore bocaparvovirus 2 (ICTV, 2013). In addition, since phylogenetic analysis indicated that canine bocavirus F13000791S had the highest identity with viruses Canine bocavirus Dis-017 and canine bocaviruses of the genetic group of canine bocaviruses detected in Hong Kong (CBoV-HK), this virus may either belong to a novel genetic group or belong to the same group as canine bocaviruses detected in Hong Kong (Kapoor et al., 2012).
Only a few amino acid changes at certain key positions of the capsid protein can induce a host switch and induce antigenic variants of canine parvoviruses (Decaro and Buonavoglia, 2012, Kaelber et al., 2012). Interestingly, the deduced amino acid sequence of canine bocavirus F13000791s VP1 had four unique amino acid substitutions compared to closely related canine bocaviruses 2 viruses HK882U, HK882F, HK880N, DIS-040, Con-161 and HK831F (Supplementary Fig. S3). In addition, the VP1 gene of canine bocavirus F13000791s differed at two amino acid positions from the partial sequenced canine bocavirus Dis-017 that was detected in a dog with respiratory disease. Additional research is necessary to elucidate if these amino acids substitutions played a role in the virulence and tissue tropism of this virus strain.
In conclusion, the present study suggests that group 2 canine bocaviruses should be taken into consideration as a cause of enteritis of puppies.
Conflict of interests
A.D.M.E. Osterhaus is part-time chief scientific officer of Viroclinics Biosciences B.V, a spin-off contract research organization of Erasmus MC that collaborates with pharmaceutical companies. A patent application of a previously undescribed possible enteropathogenic variant of Canine bocavirus 2, canine bocavirus F13000791s, was filed in the Netherlands (No. 2011751).
Acknowledgements
The outstanding technical support of Danuta Waschke, Department of Pathology, University of Veterinary Medicine Hannover is acknowledged. Further gratitude is expressed to Dr. Feuerherdt from the small animal practice in Suhlendorf, Germany for committed sample acquisitions. This work was financially supported in part by a grant from the Niedersachsen-Research Network on Neuroinfectiology (N-RENNT) of the Ministry of Science and Culture of Lower Saxony, Germany and the Virgo Consortium. The funding source had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
GenBank accession number canine bocavirus F13000791S: KF771828.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vetmic.2014.08.025.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- Allander T., Tammi M.T., Eriksson M., Bjerkner A., Tiveljung-Lindell A., Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc. Natl. Acad. Sci. U. S. A. 2005;102:12891–12896. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns K., Parrish C.R. 5th ed. Lippincott, Williams and Wilkins; Philadelphia: 2007. Parvoviridae. Fields Virology; pp. 2437–2477. [Google Scholar]
- Binn L.N., Lazar E.C., Eddy G.A., Kajima M. Recovery and characterization of a minute virus of canines. Infect. Immun. 1970;1:503–508. doi: 10.1128/iai.1.5.503-508.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodewes R., van de Bildt M.W., Schapendonk C.M., van Leeuwen M., van Boheemen S., de Jong A.A., Osterhaus A.D., Smits S.L., Kuiken T. Identification and characterization of a novel adenovirus in the cloacal bursa of gulls. Virology. 2013;440:84–88. doi: 10.1016/j.virol.2013.02.011. [DOI] [PubMed] [Google Scholar]
- Bodewes R., van der Giessen J., Haagmans B.L., Osterhaus A.D., Smits S.L. Identification of multiple novel viruses, including a parvovirus and a hepevirus, in feces of red foxes. J. Virol. 2013;87:7758–7764. doi: 10.1128/JVI.00568-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman P.S., Povey R.C. Pathogenesis of canine parvovirus-2 in dogs: histopathology and antigen identification in tissues. Res. Vet. Sci. 1985;38:141–150. [PubMed] [Google Scholar]
- Carmichael L.E., Schlafer D.H., Hashimoto A. Pathogenicity of minute virus of canines (MVC) for the canine fetus. Cornell Vet. 1991;81:151–171. [PubMed] [Google Scholar]
- Castro T.X., Cubel Garcia R.C., Costa E.M., Leal R.M., Xavier Mda P., Leite J.P. Molecular characterisation of calicivirus and astrovirus in puppies with enteritis. Vet. Rec. 2013;172:557. doi: 10.1136/vr.101566. [DOI] [PubMed] [Google Scholar]
- Decaro N., Amorisco F., Lenoci D., Lovero A., Colaianni M.L., Losurdo M., Desario C., Martella V., Buonavoglia C. Molecular characterization of Canine minute virus associated with neonatal mortality in a litter of Jack Russell terrier dogs. J. Vet. Diagn. Invest. 2012;24:755–758. doi: 10.1177/1040638712445776. [DOI] [PubMed] [Google Scholar]
- Decaro N., Buonavoglia C. Canine coronavirus: not only an enteric pathogen. Vet. Clin. N. Am. Small Anim. Pract. 2011;41:1121–1132. doi: 10.1016/j.cvsm.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Buonavoglia C. Canine parvovirus—a review of epidemiological and diagnostic aspects, with emphasis on type 2c. Vet. Microbiol. 2012;155:1–12. doi: 10.1016/j.vetmic.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erles K., Dubovi E.J., Brooks H.W., Brownlie J. Longitudinal study of viruses associated with canine infectious respiratory disease. J. Clin. Microbiol. 2004;42:4524–4534. doi: 10.1128/JCM.42.10.4524-4529.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaedke K., Zurbriggen A., Baumgärtner W. In vivo and in vitro detection of canine distemper virus nucleoprotein gene with digoxigenin-labelled RNA, double-stranded DNA probes and oligonucleotides by in situ hybridization. J. Vet. Med.Zentralblatt fur Veterinarmedizin. Reihe B. 1997;44:329–340. doi: 10.1111/j.1439-0450.1997.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Gröters S., Alldinger S., Baumgärtner W. Up-regulation of mRNA for matrix metalloproteinases-9 and -14 in advanced lesions of demyelinating canine distemper leukoencephalitis. Acta Neuropathol. (Berl.) 2005;110:369–382. doi: 10.1007/s00401-005-1055-z. [DOI] [PubMed] [Google Scholar]
- Hahn K., Habierski A., Herder V., Wohlsein P., Peters M., Hansmann F., Baumgartner W. Schmallenberg virus in central nervous system of ruminants. Emerg. Infect. Dis. 2013;19:154–155. doi: 10.3201/eid1901.120764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison L.R., Styer E.L., Pursell A.R., Carmichael L.E., Nietfeld J.C. Fatal disease in nursing puppies associated with minute virus of canines. J. Vet. Diagn. Invest. 1992;4:19–22. doi: 10.1177/104063879200400105. [DOI] [PubMed] [Google Scholar]
- Herrewegh A.A., de Groot R.J., Cepica A., Egberink H.F., Horzinek M.C., Rottier P.J. Detection of feline coronavirus RNA in feces, tissues, and body fluids of naturally infected cats by reverse transcriptase PCR. J. Clin. Microbiol. 1995;33:684–689. doi: 10.1128/jcm.33.3.684-689.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelzer K., Parrish C.R. The emergence of parvoviruses of carnivores. Vet. Res. 2010;41:39. doi: 10.1051/vetres/2010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huson D.H., Mitra S., Ruscheweyh H.J., Weber N., Schuster S.C. Integrative analysis of environmental sequences using MEGAN4. Genome Res. 2011;21:1552–1560. doi: 10.1101/gr.120618.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Committee on Taxonomy of Viruses (ICTV) 2013. Virus Taxonomy: 2013. http://www.ictvonline.org/virusTaxonomy.asp. [Google Scholar]
- Kaelber J.T., Demogines A., Harbison C.E., Allison A.B., Goodman L.B., Ortega A.N., Sawyer S.L., Parrish C.R. Evolutionary reconstructions of the transferrin receptor of Caniforms supports canine parvovirus being a re-emerged and not a novel pathogen in dogs. PLoS Pathog. 2012;8:e1002666. doi: 10.1371/journal.ppat.1002666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A., Mehta N., Dubovi E.J., Simmonds P., Govindasamy L., Medina J.L., Street C., Shields S., Lipkin W.I. Characterization of novel canine bocaviruses and their association with respiratory disease. J. Gen. Virol. 2012;93:341–346. doi: 10.1099/vir.0.036624-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A., Mehta N., Esper F., Poljsak-Prijatelj M., Quan P.L., Qaisar N., Delwart E., Lipkin W.I. Identification and characterization of a new bocavirus species in gorillas. PLoS ONE. 2010;5:e11948. doi: 10.1371/journal.pone.0011948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S.K., Woo P.C., Yeung H.C., Teng J.L., Wu Y., Bai R., Fan R.Y., Chan K.H., Yuen K.Y. Identification and characterization of bocaviruses in cats and dogs reveals a novel feline bocavirus and a novel genetic group of canine bocavirus. J. Gen. Virol. 2012;93:1573–1582. doi: 10.1099/vir.0.042531-0. [DOI] [PubMed] [Google Scholar]
- Lee J.I., Chung J.Y., Han T.H., Song M.O., Hwang E.S. Detection of human bocavirus in children hospitalized because of acute gastroenteritis. J. Infect. Dis. 2007;196:994–997. doi: 10.1086/521366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Pesavento P.A., Leutenegger C.M., Estrada M., Coffey L.L., Naccache S.N., Samayoa E., Chiu C., Qiu J., Wang C., Deng X., Delwart E. A novel bocavirus in canine liver. Virol. J. 2013;10:54. doi: 10.1186/1743-422X-10-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Shan T., Wang C., Cote C., Kolman J., Onions D., Gulland F.M., Delwart E. The fecal viral flora of California sea lions. J. Virol. 2011;85:9909–9917. doi: 10.1128/JVI.05026-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manteufel J., Truyen U. Animal bocaviruses: a brief review. Intervirology. 2008;51:328–334. doi: 10.1159/000173734. [DOI] [PubMed] [Google Scholar]
- Martella V., Lorusso E., Decaro N., Elia G., Radogna A., D’Abramo M., Desario C., Cavalli A., Corrente M., Camero M., Germinario C.A., Banyai K., Di Martino B., Marsilio F., Carmichael L.E., Buonavoglia C. Detection and molecular characterization of a canine norovirus. Emerg. Infect. Dis. 2008;14:1306–1308. doi: 10.3201/eid1408.080062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitui M.T., Tabib S.M., Matsumoto T., Khanam W., Ahmed S., Mori D., Akhter N., Yamada K., Kabir L., Nishizono A., Soderlund-Venermo M., Ahmed K. Detection of human bocavirus in the cerebrospinal fluid of children with encephalitis. Clin. Infect. Dis. 2012;54:964–967. doi: 10.1093/cid/cir957. [DOI] [PubMed] [Google Scholar]
- Osterhaus A.D., Drost G.A., Wirahadiredja R.M., van den Ingh T.S. Canine viral enteritis: prevalence of parvo-, corona- and rotavirus infections in dogs in the Netherlands. Vet. Q. 1980;2:181–190. doi: 10.1080/01652176.1980.9693779. [DOI] [PubMed] [Google Scholar]
- Pollock R.V. Experimental canine parvovirus infection in dogs. Cornell Vet. 1982;72:103–119. [PubMed] [Google Scholar]
- Schaudien D., Polizopoulou Z., Koutinas A., Schwab S., Porombka D., Baumgärtner W., Herden C. Leukoencephalopathy associated with parvovirus infection in Cretan hound puppies. J. Clin. Microbiol. 2010;48:3169–3175. doi: 10.1128/JCM.01582-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildgen O., Muller A., Allander T., Mackay I.M., Volz S., Kupfer B., Simon A. Human bocavirus: passenger or pathogen in acute respiratory tract infections? Clin. Microbiol. Rev. 2008;21:291–304. doi: 10.1128/CMR.00030-07. (table of contents) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan T., Lan D., Li L., Wang C., Cui L., Zhang W., Hua X., Zhu C., Zhao W., Delwart E. Genomic characterization and high prevalence of bocaviruses in swine. PLoS ONE. 2011;6:e17292. doi: 10.1371/journal.pone.0017292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spahn G.J., Mohanty S.B., Hetrick F.M. Experimental infection of calves with hemadsorbing enteric (HADEN) virus. Cornell Vet. 1966;56:377–386. [PubMed] [Google Scholar]
- Storz J., Leary J.J., Carlson J.H., Bates R.C. Parvoviruses associated with diarrhea in calves. J. Am. Vet. Med. Assoc. 1978;173:624–627. [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brand J.M., van Leeuwen M., Schapendonk C.M., Simon J.H., Haagmans B.L., Osterhaus A.D., Smits S.L. Metagenomic analysis of the viral flora of pine marten and European badger feces. J. Virol. 2012;86:2360–2365. doi: 10.1128/JVI.06373-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen M., Williams M.M., Koraka P., Simon J.H., Smits S.L., Osterhaus A.D. Human picobirnaviruses identified by molecular screening of diarrhea samples. J. Clin. Microbiol. 2010;48:1787–1794. doi: 10.1128/JCM.02452-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Rüden E.L., Avemary J., Zellinger C., Algermissen D., Bock P., Beineke A., Baumgärtner W., Stein V.M., Tipold A., Potschka H. Distemper virus encephalitis exerts detrimental effects on hippocampal neurogenesis. Neuropathol. Appl. Neurobiol. 2012;38:426–442. doi: 10.1111/j.1365-2990.2011.01218.x. [DOI] [PubMed] [Google Scholar]
- Wise A.G., Kiupel M., Maes R.K. Molecular characterization of a novel coronavirus associated with epizootic catarrhal enteritis (ECE) in ferrets. Virology. 2006;349:164–174. doi: 10.1016/j.virol.2006.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurbriggen A., Müller C., Vandevelde M. In situ hybridization of virulent canine distemper virus in brain tissue, using digoxigenin-labeled probes. Am. J. Vet. Res. 1993;54:1457–1461. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


