Highlights
-
•
PEDV G1b was not circulating in the Netherlands before November 2014.
-
•
Description of the first PEDV G1b outbreak in the Netherlands in 2014.
-
•
PEDV sequences suggests a one event introduction of PEDV G1b in Europe in 2014.
Keywords: Porcine epidemic diarrhea virus (PEDV), Serological survey, Sequence analysis, Epidemiology, ELISA, Outbreak
Abstract
Porcine epidemic diarrhea virus (PEDV) is the highly contagious, causative agent of an economically important acute enteric disease in pigs of all ages. The disease is characterized by diarrhea and dehydration causing mortality and growth retardation. In the last few decades, only classical PEDV was reported sporadically in Europe, but in 2014 outbreaks of PEDV were described in Germany. Phylogenetic analysis showed a very high nucleotide similarity with a variant of PEDV that was isolated in the US in January 2014. The epidemiological situation of PEDV infections in the Netherlands in 2014 was unknown and a seroprevalence study in swine was performed. In total, 838 blood samples from sows from 267 farms and 101 samples from wild boars were collected from May till November 2014 and tested for antibodies against PEDV by ELISA. The apparent herd prevalence of 0.75% suggests that PEDV was not circulating on a large scale in the Netherlands at this time. However, in November 2014 a clinical outbreak of PEDV was diagnosed in a fattener farm by PCR testing. This was the first confirmed PEDV outbreak since the early nineties. Sequence analyses showed that the viruses isolated in 2014 and 2015 in the Netherlands cluster with recently found European G1b strains. This suggests a one event introduction of PEDV G1b strains in Europe in 2014, which made the Netherlands and other European countries endemic for this type of strains since then.
1. Introduction
Porcine epidemic diarrhea (PED) is an economically important acute enteric disease in pigs of all ages. The disease is characterized by diarrhea and dehydration causing mortality - particularly in neonatal piglets – and growth retardation. The causative agent is porcine epidemic diarrhea virus (PEDV), which is an enveloped, positive single-stranded RNA virus, belonging to the family of Coronaviridae (Pensaert and de Bouck, 1978). The genome of PEDV is approximately 28 kb long and about two-third encodes for non-structural proteins and one-third of structural proteins (Kocherhans et al., 2001). Among these proteins, the main research interest is focused on the Spike (S) gene and its glycoprotein product S, mediating receptor binding and membrane fusion (Li et al., 2016). Although only one serotype has been described, phylogenetic studies of the S gene showed that PEDV can be genetically separated into two groups: genogroup 1 (G1) and genogroup 2 (G2). Each genogroup can be further divided into subgroups 1a and 1b, and 2a and 2b, respectively (Lee, 2015).
Classical PED, now grouped G1a, was first recognized as a severe swine enteric disease separate from Transmissible Gastro Enteritis (TGE) in the United Kingdom in 1971 and first described in Belgium in 1978 (Pensaert and de Bouck, 1978). In the eighties and nineties, the virus was detected in many countries in Europe including the Netherlands and from Europe PEDV spread to Asia, where it caused large outbreaks with considerable losses in the pig industry (Song and Park, 2012). Until 2013, North America was considered to be free of PEDV infections (Cima, 2013), but in that same year highly virulent strains of PEDV emerged in the United States of America (US), causing diarrhea, vomiting and loss of appetite in pigs of all age groups and up to 100% of mortality in suckling piglets (Chen et al., 2014; Huang et al., 2013; Stevenson et al., 2013). This strain, typed as G2b, rapidly spread across the US, Canada, Mexico and several countries in South America (Vlasova et al., 2014).
In the last few decades, only classical PEDV (or G1a) was reported sporadically in Europe (Alborali et al., 2014; Martelli et al., 2008). In 2014, outbreaks of PEDV were described in Germany and phylogenetic analysis showed a very high nucleotide similarity with a variant of PEDV (OH851) containing nucleotide insertions and deletions in the S gene (S-INDEL) that was isolated in the US in January 2014 (Hanke et al., 2015; Stadler et al., 2015). This variant, typed as G1b, caused mild clinical signs and lower mortality rates in suckling piglets compared to other circulating PEDV G2b strains in the US (Wang et al., 2014). Since the reports of outbreaks in Germany, more reports about outbreaks of this particular S-INDEL virus in several European countries have been published, among which France, Belgium, Spain, Portugal and Austria (EFSA, 2016; Grasland et al., 2015; Mesquita et al., 2015; Steinrigl et al., 2015). This suggests that this mild PEDV variant is circulating in Europe since the beginning of 2014.
The aim of this study was to determine the status of PEDV in the Netherlands with a serological survey and to investigate the first PEDV outbreak in the Netherlands since the early nineties.
2. Materials and methods: serological survey
2.1. Calculation of number of required samples and farms
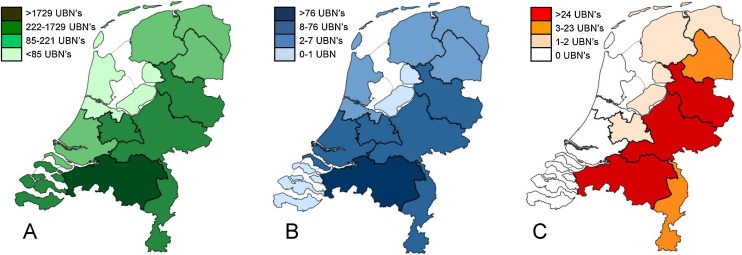
The number of required blood samples from animals and farms to estimate the seroprevalence of PEDV in Dutch sow herds was calculated based on the following assumptions: PEDV is highly contagious and no vaccination against this virus was carried out in the Netherlands. As a result, it was expected that, if PEDV was present in a sow herd, the within herd prevalence would be very high (Bertasio et al., 2016; Goede et al., 2015). The required number of blood samples per herd was calculated using WinEpiscope 2.0 (Thrusfield et al., 2001). To detect infection in a herd with 95% probability, an estimated within herd prevalence of 70%, and an average herd size of 464 sows per farm (WUR, 2016), three blood samples per farm were required. In 2014, there were approximately 2061 sow farms in the Netherlands (WUR, 2016). In order to show with a high probability (95%), that less than 1% of the Dutch farms (N = 20) were infected, 286 farms would need to be tested (Thrusfield et al., 2001). Herds were randomly selected stratified by pig density per province to represent the total Dutch sow herd population (Fig. 1 A and B). Statistical analyses were performed using STATA/SE version 14.1 software (Stata Corporation, 2017).
Fig. 1.
Map of the 12 provinces of the Netherlands. A) Pig herd density in 2014. B) Herd sampled to determine the seroprevalence for infection with PEDV from May till December 2014. C) Areas with PCR positive PEDV herds registered by GD Animal Health from November 2014 till January 2016. The darker the color, the more farms were positive. UBN: Unique Herd Number.
2.2. Collection and storage of samples
For the serological survey, 410 blood samples were selected from samples collected for the obligatory monitoring of Aujeszky’s disease (pseudorabies), swine vesicular disease (SVD) and classical swine fever (CSFV). Additionally, 428 blood samples were collected from sows at slaughter (VION, Groenlo, the Netherlands). Herds were identified based on their Unique Herd Number (UBN). Samples were randomly selected given the availability of enough serum to perform all assays. Samples were collected from May till up to and including November 2014.
Commissioned by the Dutch government, GD Animal Health was also monitoring wild boars for Aujeszky’s disease, SVD, foot and mouth disease, CSFV, Trichinellosis and African swine fever. Because it is suggested that wild boars may play an important role as a PEDV reservoir (Lee et al., 2016), blood samples of wild boar collected as part of this monitor were also tested. These blood samples were collected from regions close to the borders with Germany and Belgium, from April till August 2014.
Blood samples collected were stored at −70 °C, before dispatched to the Virology Division of the Faculty of Veterinary Medicine, at Utrecht University (UU).
2.3. Indirect ELISA
For the detection of PEDV antibodies in serum samples an in-house indirect ELISA based on the viral spike (S) protein S1-part of the G2b strain GDU (Non-S-INDEL, GenBank KU985230.1) was used, similar as the ELISA previously described (Gerber et al., 2014). The S1 antigen used in this study is produced in HEK293 T cells, a mammalian expression system. To facilitate the purification from the cell culture supernatant, the protein is associated with the Fc part of murine IgG. Per well 4 ng of protein was used to coat the plates.
The in-house ELISA was optimized by UU with sera from PEDV-infected (G2b) pigs from the US and with hyperimmune sera of animals vaccinated with the PEDV S1 protein. Sera were tested in duplicate at a 1:100 dilution, the absorbance was measured with an ELISA plate reader at 450 nm. The mean OD value of PEDV-negative sera was 0.11. Sera with OD values of >0.33 (3× OD value PEDV negative sera) were considered positive. The aforementioned PEDV-positive sera from infected animals were high positive (>0.8 OD) in this ELISA (Supplementary Fig. 1). The calculated sensitivity and specificity of the ELISA was 100% (CI 95%: 90–100%; N = 43) and 100% (CI 95%: 92–100%; N = 57), respectively.
2.4. Virus neutralization test
For the detection of virus-specific antibodies in serum samples the virus neutralization test (VNT) was used as an alternative sero-diagnostic assay. A previously described VNT was used (Li et al., 2013) to test all samples positive by ELISA. The validation of this test is shown in Supplementary Table 1.
3. Materials and methods: outbreak investigation
3.1. Samples
Fecal swabs or Eswabs (Copan Diagnostics Incorporated) of individual animals were taken by the local practitioner and sent in the same day for PCR testing. Also three pigs of the index farm with severe diarrhea were euthanized and submitted for post mortem examination.
3.2. PCR and sequencing
RNA was isolated from fecal samples and the detection PCR was performed as previously described (Kim et al., 2007; Lowe et al., 2014). In order to type the viruses the PEDV S gene was sequenced with primers previously described (Chen et al., 2014; Oka et al., 2014) and designed in this study (Table 1 ), and compared with sequences available in GenBank. A phylogenetic tree was constructed using MEGA6 software by Neighbor-Joining method maximum likelihood method. Branch lengths represent the predicted number of substitutions and are proportional to the differences between the isolates (Tamura et al., 2013).
Table 1.
PED sequence primers used in this study.
| Primer name | Direction | Sequence 5' to 3' | Positiona | Reference |
|---|---|---|---|---|
| p20320-F | forward | AACACGTCATCGTCAGAGGC | 20320– 20339 | Oka et al. (2014) |
| p21638 | forward | CACTGCTTTAGGAACAAATC | 21638–21657 | this study |
| p22273 | reverse | GTAAATTGTCTAGTGTCAAC | 22254–22273 | Chen et al. (2014) |
| p22324 | forward | CACAGGACAGTAATTGCCCT | 22324– 22343 | this study |
| S2F | forward | GGCCAAGTCAAGATTGCACC | 22932– 22951 | Chen et al. (2014) |
| p23592 | forward | GATGTTCTACAGCGGAACCA | 23592– 23611 | this study |
| p24071 | reverse | CTGAGGTGCTGCCTGTACCA | 24025– 24071 | this study |
| p24558 | forward | GTTGACCTTGAGTGGCTCAA | 24558– 24577 | this study |
| S2R | reverse | AGCTCCAACTCTTGGACAGC | 24863– 24882 | Chen et al. (2014) |
Primer positions are based on the PED genome of L00721/GER/2014, GenBank LM645057.
3.3. Follow up infected farms
After confirmation of the first G1b outbreak in the Netherlands it was unquestionable to try to prevent the further spread of the outbreak. Therefore, it was decided to monitoring and follow up of specific management advices to the farmers. Five fattening herds, five sow herds and one nursery herd were selected after PEDV infection was confirmed by PCR on feces. For control and eradication of PEDV, a tailor made advice per farm, mainly based on biosecurity measures, was given. Advise was mainly focused on the separation between PEDV-infected and non-infected healthy animals. This separation was applied in stables, animal categories and compartments. It was made using the following measures: dress code (change between compartments), additional disinfection cleaning and disinfection of compartments and corridors and improving pest control. Furthermore, all professional herd visitors were informed and required to take measures, in particular those aimed at cleaning and disinfection, which would prevent the transfer of the disease to another pig farm. Regular testing of pooled fecal samples was done to monitor the effect of interventions. In sow herds, nursery piglets and replacement gilts were sampled; in fattening herds a random sampling in all age groups was performed. After introduction of PEDV, the virus could be detected in a compartment for 4–6 weeks. At farm level the virus was detectable much longer due to transmission to new susceptible animals. Farms were presumed negative for PEDV if three sampling rounds with at least 2-weeks interval, of thirty randomly taken individual fecal samples each, proved to be PCR negative.
4. Results
4.1. Serological survey
In total, sera from 838 sows originating from 267 farms, and 101 sera from wild boar were collected. The blood samples came from all provinces in the Netherlands and are fairly representative for the distribution of farms across provinces (Fig. 1B). All sera were tested in the indirect PEDV S1-based ELISA (group 1, Table 2 ). Nine samples, originating from nine different farms located in four different provinces, tested seropositive. The OD values were low in all seropositive samples (OD: 0.3-0.8) relative to positive control sera (convalescent or hyperimmune sera) with values OD: 0.8-3.2. Two of the nine ELISA-positive sera from the serological study also tested positive in the VNT. With two confirmed positive samples of the 838 samples the animal prevalence was 0.24% (CI 95%: 0 – 0.9%). The herd prevalence with 2 out of 267 farms was 0.75% (CI 95%: 0–2.7%).
Table 2.
Summary of the PEDV antibodies of the serological survey of the Dutch pig and wild boar population and the pigs of the index farm.
| Group | Identification | Date of sampling | S1-ELISA (#pos/#total) | VNT validation ELISA (#pos/#total) |
|---|---|---|---|---|
| 1 | Serosurvey | May-Nov 2014 | 9/838 (1.07%) | 2/9 |
| 2 | Wild boar | Apr-July 2014 | 0/101 (0%) | n.d. |
| 3 | Index farm | 17-11-2014 | 10/15 (67%) | 10/10 |
| 4 | Index farm | 26-11-2014 | 16/16 (100%) | n.d. |
| 5 | Index farm | 19-12-2014 | 16/16 (100%) | n.d. |
n.d. = not determined.
For all herds, three samples were tested. The additional samples of seven herds with a seropositive sample of which one VNT positive, were examined. These samples, 36 in total, were all negative in the PEDV ELISA. The proportion of seropositive samples (7/838) falls within the expected proportion of false positives given the specificity of the ELISA which is estimated at >99%. So we either detected PEDV outbreaks that had not spread yet or the seven original samples were false positives. For two herds no additional samples from the monitoring programs were available (including one sow farm with a VNT positive sample).
All tested sera obtained from wild boar were negative for PEDV antibodies in the used indirect ELISA (group 2, Table 2).
4.2. Case study
In the first week of November 2014, GD Animal Health received a report of pigs showing lethargy and anorexia for up to 24 h after which profuse diarrhea occurred in almost all pigs in nine compartments within the fattening barn. Diarrhea was watery, light greyish, sometimes yellow or green colored. Body temperature in the clinical phase reached 39.8 °C. After the first days of disease lethargy subsided and lack of appetite diminished, a profuse diarrhea became more prominent. In later stages of the infection the consistency of the diarrhea changed to slightly more solid. Pigs did not seem to suffer much and no animals died, although some pigs did not grow for a week and within the group body weights started to differ. After an extra week of feeding, pigs did recover and had a normal weight at slaughter.
The fattening barn, located in a pig dense area of the Netherlands, consisted of 18 compartments with 104 pigs each, divided over 8 pens. First symptoms occurred on October 26th 2014 in one compartment followed by symptoms in consecutive compartments in the following days. Initial diagnostic tests for the presence of E. coli, Salmonella, Lawsonia intracellularis and Brachyspira pilosicoli were, except for low numbers of pathogenic E. coli, negative. Based on the low numbers of pathogenic E. coli and the age of the pigs involved, E. coli was ruled out as causative agent in this case. Subsequently, it was decided to test for porcine deltacoronavirus (PDCoV) and PEDV. Six fecal samples were found to be negative for PDCoV, but positive for PEDV RNA on November 14th. On the same day, three pigs with severe diarrhea were euthanized and were submitted for post mortem examination. Pathological examination showed severe villus atrophy in the small intestine, and PCR tested positive for PEDV. During the outbreak, in total 67% of the animals (12 compartments) were affected by PEDV as confirmed by PCR on fecal samples.
4.3. Follow up infected farms
Based on the results of the serological survey, over 99% of the Dutch farms were PEDV negative in 2014; it was decided to try to prevent the further spread of the outbreak. To control and eradicate PEDV the biosecurity measures taken, as described in the Materials and methods section, seemed to be of great importance. Also pet control was applied since some farms suffered from mice and rats. Furthermore, it became clear that in compartments in which the infection was present, after thorough cleaning and disinfection, PEDV free piglets could be introduced and that those stayed free from PEDV infection. Three fattening and three sow herds were presumed free of PEDV within 6 months after the diagnosis of PED was confirmed. The nursery herd was depopulated, and, after double cleaning and disinfection of all compartments, repopulated.
4.4. PED spread in the Netherlands
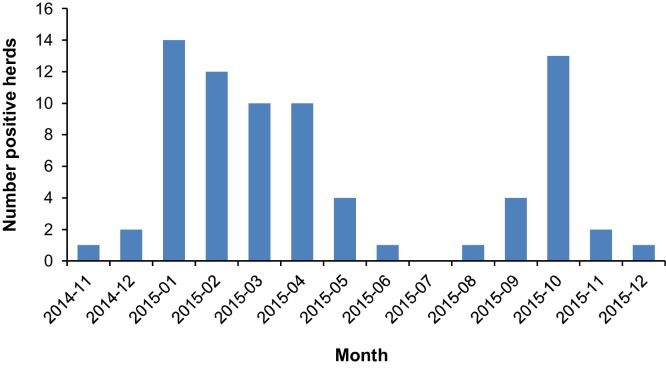
Despite the immediate action of all parties in the Dutch pig production industry to optimize their hygiene measures to ensure that infection by PEDV would be avoided as much as possible, it could not be prevented that PEDV spread to other farms. Most infected farms were located on the east side of the Netherlands at the border of Germany (Fig. 1C). At the end of 2015, in total 75 farms were confirmed PEDV positive by GD Animal Health. In Fig. 2 the number of new PEDV PCR positive farms per month since the beginning of the outbreak is shown.
Fig. 2.
Overview of new PED PCR positive farms confirmed by GD Animal Health per month from November 2014 up to and including December 2015.
4.5. Virus characterization
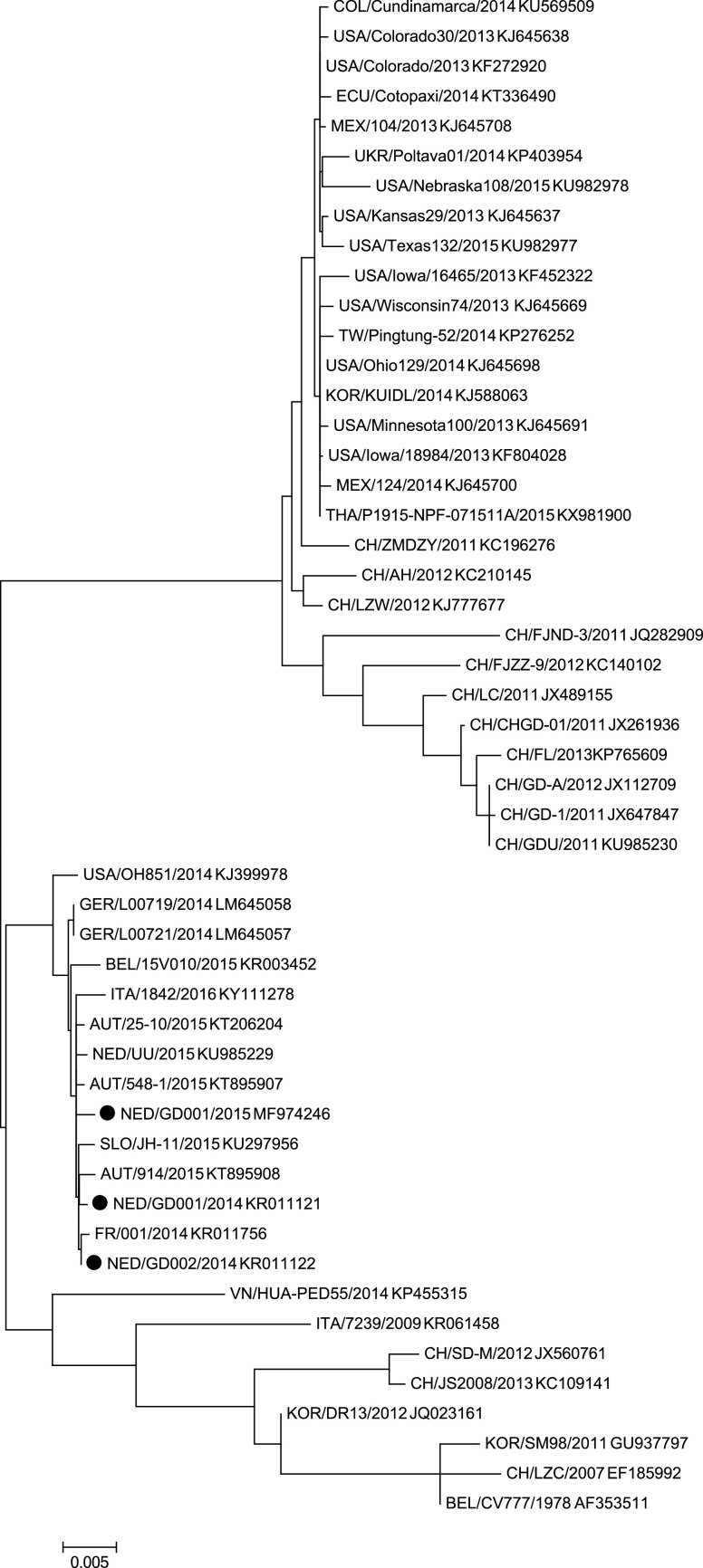
In order to characterize the virus originating from outbreaks in 2014 and 2015, the S gene of three isolates of different farms was sequenced and the sequences NED/GD001/2014, NED/GD002/2014 and NED/GD001/2015 were deposited in the GenBank database and received accession numbers KR011121 (index farm), KR011122 and MF974246, respectively. Together with sequences available in GenBank, the isolates were compared in a phylogenetic tree (Fig. 3 ). Sequence analyses showed that the isolates had a 99.5% homology with the USA/OH851 strain and a 99.8% homology with the 2014 German G1b strains. Furthermore, the European strains available in GenBank (2014–2016) cluster together with this OH851 strain, or the so called S-INDEL strain (Fig. 3) (Wang et al., 2014).
Fig. 3.
Phylogenetic analysis of PEDV strains based on the S gene nucleotide sequences by the maximum likelihood method using 1000 bootstrapping replicates. Branch lengths represent the predicted number of substitutions and are proportional to the differences between the isolates. Evolutionary analyses were conducted in MEGA6 [21]. For each sequence, Country/Identifier/Year GenBank accession number is given. Isolates determined in this study are indicated with ●. The upper cluster are the high virulent G2 strains from Asia and America and the lower cluster are the vaccine strains, mild virulent and European G1 strains.
5. Discussion
This study showed that most likely PEDV and particularly the genogroup 1b (S-INDEL) strains did not circulate in the Netherlands on a large scale till the end of 2014. Only a very small part of the Dutch sow farms tested positive on antibodies against PEDV (0.75% (CI 95%: 0–2.7%)) and no PEDV antibodies were detected in wild boars. The used ELISA based on the S1 protein of a G2b strain showed a high sensitivity and specificity against antibodies raised against G1a, G1b en G2b strains. However, the ELISA was validated with a limited amount of samples (Supplementary Fig. 1) and field samples may react differently, just like in a similar ELISA recently described (Gerber et al., 2014). The viral spike (S) protein is prominent on the virus surface and is very immunogenic. All animals that have been infected with PEDV have antibodies against the S protein and particularly against the S1-part. The S1-part of the spike protein is the most variable part between related coronaviruses and there is no cross reactivity with other coronaviruses such as Transmissible gastroenteritis coronavirus (TGEV), Porcine respiratory coronavirus (PRCoV) and Porcine Delta Coronavirus (PDCoV) as previously described (Gimenez-Lirola et al., 2017; Lin et al., 2015b). Because results of immunoassays based on the S protein correlate well with viral neutralization (Paudel et al., 2014), as shown in Supplementary Table 1, we decided to use the VNT as an alternative sero-diagnostic assay.
Based on case reports from Germany (Hanke et al., 2015; Stadler et al., 2015), Austria (Steinrigl et al., 2015) and the Netherlands (GD Animal Health) a PEDV infection with the European circulating strain (G1b) will spread very rapidly within a herd and a single PEDV positive animal on a farm is not likely to occur. The number of ELISA positive animals (67%, Table 2, group 3) on the index farm after the clinical signs started, seemed to justify the assumption of the high within herd prevalence of 70% for sample size calculation for the serological survey. Eventually, there were fewer herds sampled than planned (267 instead of 286) since it was decided to stop the serological survey, because the first case of PEDV was diagnosed in the Netherlands on November 14th 2014.
Sequence analyses of the viruses isolated in 2014–2015 showed a 99% homology with OH851, a less virulent PEDV strain found in the US and in Germany in 2014 (Hanke et al., 2015). Although the G1b virus strain present in Europe is less virulent (EFSA, 2016; Grasland et al., 2015; Hanke et al., 2015; Mesquita et al., 2015; Steinrigl et al., 2015) compared to the strain that caused the US outbreaks in 2013 (Wang et al., 2014), this European strain showed to be very contagious and still could cause severe economic damage in PEDV naive herds. After infection on sow farms loss off piglets could range up to 100% in the sucking piglets ((Lin et al., 2015a) and individual case reports, GD Animal Health).
In the foreseeable future, vaccination will not be possible. Therefore, the authorities in the Netherlands advised all parties in the pig production industry to optimize their hygiene measures to ensure that infection by this virus would be avoided as much as possible. The strict preventive biosecurity measures taken in these herds demonstrated that most herds that were monitored could prevent the transfer to PEDV naive compartments within the herd and some were able to obtain a presumed PEDV free status for the whole farm. Additionally, a higher biosecurity level helps preventing the introduction of other pathogens and controls the spread of infection within that herd (FAO, 2010). However, an increasing number of herds became infected (Fig. 2) and PEDV spread across the country after the initial outbreak (Fig. 1C). The transport trucks with positive animals between herds seemed to have the highest transmission risk (data not shown). The number of new PEDV PCR positive farms per month since the beginning of the outbreak is most likely an underestimation (Fig. 2). Since PED is not a notifiable disease, nor have all veterinarians performed diagnostic testing in all cases, as the clinical signs of an outbreak are very typical.
It seems that PEDV G1b became endemic in the Netherlands since its initial outbreak in November 2014, just like in most countries in Europe. That G1b viruses isolated in Europe in 2014–2016 phylogenetically cluster together with a high similarity (Fig. 3) suggests a onetime introduction event. However, PEDV is endemic and many other coronaviruses are circulating that may result in coronavirus variants through mutation or recombination (Fehr and Perlman, 2015). Therefore, it is of upmost importance that the presence of circulating PEDV is being monitored and genetically analyzed, in order to update diagnostic tools where necessary.
Conflict of interest
We declare no conflict of interest.
Acknowledgements
The authors would like to thank D. Oorburg en C. Sima of VION, Groenlo, The Netherlands, for providing blood samples from slaughter sows, G. Spierts and laboratory staff for collecting and archiving blood samples and N. Schuurman and J. de Jong for performing the ELISA. Furthermore, R. Dijkman for implementing the PCR at the GD Animal Health lab, A. van Lenthe for advice during the survey, M. Meijerink for assisting in sample collection during herd visits and A. Veldhuis and H. Brouwer-Middelesch for making the maps of the Netherlands (Fig. 1). The authors thank the owner of the index farm and the practitioner involved in this farm for their information and their cooperation in collecting the samples.
The authors would like to thank the Dutch Ministry of Economic Affairs and the Product Board for Livestock and Meat for funding the Monitoring system for Pig Health in The Netherlands.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.vetmic.2018.05.014.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Alborali G.L., Boniotti B., Lavazza A. Surveillance and control of PED coronavirus in pigs in Italy. International Conference on Swine Enteric Coronavirus Diseases (nSECD); Chicago, Illinois, USA; 2014. p. 18. [Google Scholar]
- Bertasio C., Giacomini E., Lazzaro M., Perulli S., Papetti A., Lavazza A., Lelli D., Alborali G., Boniotti M.B. Porcine epidemic diarrhea virus shedding and antibody response in swine farms: a longitudinal study. Front. Microbiol. 2016;7:2009. doi: 10.3389/fmicb.2016.02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Li G., Stasko J., Thomas J.T., Stensland W.R., Pillatzki A.E., Gauger P.C., Schwartz K.J., Madson D., Yoon K.J., Stevenson G.W., Burrough E.R., Harmon K.M., Main R.G., Zhang J. Isolation and characterization of porcine epidemic diarrhea viruses associated with the 2013 disease outbreak among swine in the United States. J. Clin. Microbiol. 2014;52:234–243. doi: 10.1128/JCM.02820-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cima G. Viral disease affects U.S. Pigs: porcine epidemic diarrhea found in at least 11 states. J. Am Vet. Med. Assoc. 2013;243:30–31. [PubMed] [Google Scholar]
- EFSA, 2016, Updated epidemiological data on PED, Journal, E., ed. (Parma, Italy, European Food Safety Authority), 52.
- FOA Good practices for biosecurity in the pig sector – issues and options in developing and transition countries. Anim. Prod. Health. 2010;169:89. [Google Scholar]
- Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol. Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber P.F., Gong Q., Huang Y.W., Wang C., Holtkamp D., Opriessnig T. Detection of antibodies against porcine epidemic diarrhea virus in serum and colostrum by indirect ELISA. Vet. J. 2014;202:33–36. doi: 10.1016/j.tvjl.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez-Lirola L.G., Zhang J., Carrillo-Avila J.A., Chen Q., Magtoto R., Poonsuk K., Baum D.H., Pineyro P., Zimmerman J. Reactivity of porcine epidemic diarrhea virus structural proteins to antibodies against porcine enteric coronaviruses: diagnostic implications. J. Clin. Microbiol. 2017;55:1426–1436. doi: 10.1128/JCM.02507-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goede D., Murtaugh M.P., Nerem J., Yeske P., Rossow K., Morrison R. Previous infection of sows with a “mild” strain of porcine epidemic diarrhea virus confers protection against infection with a “severe” strain. Vet. Microbiol. 2015;176 doi: 10.1016/j.vetmic.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Grasland B., Bigault L., Bernard C., Quenault H., Toulouse O., Fablet C. Complete genome sequence of a porcine epidemic diarrhea S gene indel strain isolated in France in december 2014. Genome Announc. 2015;3 doi: 10.1128/genomeA.00535-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke D., Jenckel M., Petrov A., Ritzmann M., Stadler J., Akimkin V., Blome S., Pohlmann A., Schirrmeier H., Beer M., Hoper D. Comparison of porcine epidemic diarrhea viruses from Germany and the United States, 2014. Emerg. Infect. Dis. 2015;21:493–496. doi: 10.3201/eid2103.141165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.W., Dickerman A.W., Pineyro P., Li L., Fang L., Kiehne R., Opriessnig T., Meng X.J. Origin, evolution, and genotyping of emergent porcine epidemic diarrhea virus strains in the United States. mBio. 2013;4:e00737–00713. doi: 10.1128/mBio.00737-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.H., Kim I.J., Pyo H.M., Tark D.S., Song J.Y., Hyun B.H. Multiplex real-time RT-PCR for the simultaneous detection and quantification of transmissible gastroenteritis virus and porcine epidemic diarrhea virus. J. Virol. Methods. 2007;146:172–177. doi: 10.1016/j.jviromet.2007.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocherhans R., Bridgen A., Ackermann M., Tobler K. Completion of the porcine epidemic diarrhoea coronavirus (PEDV) genome sequence. Virus Genes. 2001;23 doi: 10.1023/A:1011831902219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. Porcine epidemic diarrhea virus: an emerging and re-emerging epizootic swine virus. Virol. J. 2015;12:1–16. doi: 10.1186/s12985-015-0421-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.U., Kwon T., Je S.H., Yoo S.J., Seo S.W., Sunwoo S.Y., Lyoo Y.S. Wild boars harboring porcine epidemic diarrhea virus (PEDV) may play an important role as a PEDV reservoir. Vet. Microbiol. 2016;192:90–94. doi: 10.1016/j.vetmic.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Li Z., Zou Y., Wicht O., van Kuppeveld F.J., Rottier P.J., Bosch B.J. Manipulation of the porcine epidemic diarrhea virus genome using targeted RNA recombination. PloS One. 2013;8:e69997. doi: 10.1371/journal.pone.0069997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., van Kuppeveld F.J.M., He Q., Rottier P.J.M., Bosch B.J. Cellular entry of the porcine epidemic diarrhea virus. Virus Res. 2016;226:117–127. doi: 10.1016/j.virusres.2016.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.M., Annamalai T., Liu X., Gao X., Lu Z., El-Tholoth M., Hu H., Saif L.J., Wang Q. Experimental infection of a US spike-insertion deletion porcine epidemic diarrhea virus in conventional nursing piglets and cross-protection to the original US PEDV infection. Vet. Res. 2015;46:134. doi: 10.1186/s13567-015-0278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.M., Gao X., Oka T., Vlasova A.N., Esseili M.A., Wang Q., Saif L.J. Antigenic relationships among porcine epidemic diarrhea virus and transmissible gastroenteritis virus strains. J. Virol. 2015;89:3332–3342. doi: 10.1128/JVI.03196-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe J., Gauger P., Harmon K., Zhang J., Connor J., Yeske P. Role of transportation in spread of porcine epidemic diarrhea virus infection. U.S. Emerg. Infect. Dis. 2014;20 doi: 10.3201/eid2005.131628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelli P., Lavazza A., Nigrelli A.D., Merialdi G., Alborali L.G., Pensaert M.B. Epidemic of diarrhoea caused by porcine epidemic diarrhoea virus in Italy. Vet. Rec. 2008;162:307–310. doi: 10.1136/vr.162.10.307. [DOI] [PubMed] [Google Scholar]
- Mesquita J.R., Hakze-van der Honing R., Almeida A., Lourenco M., van der Poel W.H., Nascimento M.S. Outbreak of porcine epidemic diarrhea virus in Portugal, 2015. Transbound. Emerg. Dis. 2015;62:586–588. doi: 10.1111/tbed.12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T., Saif L.J., Marthaler D., Esseili M.A., Meulia T., Lin C.M., Vlasova A.N., Jung K., Zhang Y., Wang Q. Cell culture isolation and sequence analysis of genetically diverse US porcine epidemic diarrhea virus strains including a novel strain with a large deletion in the spike gene. Vet. Microbiol. 2014;173:258–269. doi: 10.1016/j.vetmic.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paudel S., Park J.E., Jang H., Shin H.J. Comparison of serum neutralization and enzyme-linked immunosorbent assay on sera from porcine epidemic diarrhea virus vaccinated pigs. Vet. Q. 2014;34:218–223. doi: 10.1080/01652176.2014.979512. [DOI] [PubMed] [Google Scholar]
- Pensaert M.B., de Bouck P. A new coronavirus-like particle associated with diarrhea in swine. Arch. Virol. 1978;58:243–247. doi: 10.1007/BF01317606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D., Park B. Porcine epidemic diarrhoea virus: a comprehensive review of molecular epidemiology, diagnosis, and vaccines. Virus Genes. 2012;44:167–175. doi: 10.1007/s11262-012-0713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler J., Zoels S., Fux R., Hanke D., Pohlmann A., Blome S., Weissenbock H., Weissenbacher-Lang C., Ritzmann M., Ladinig A. Emergence of porcine epidemic diarrhea virus in southern Germany. BMC Vet. Res. 2015;11:142. doi: 10.1186/s12917-015-0454-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinrigl A., Fernandez S.R., Stoiber F., Pikalo J., Sattler T., Schmoll F. First detection, clinical presentation and phylogenetic characterization of porcine epidemic diarrhea virus in Austria. BMC Vet. Res. 2015;11:310. doi: 10.1186/s12917-015-0624-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson G.W., Hoang H., Schwartz K.J., Burrough E.R., Sun D., Madson D., Cooper V.L., Pillatzki A., Gauger P., Schmitt B.J., Koster L.G., Killian M.L., Yoon K.J. Emergence of porcine epidemic diarrhea virus in the United States: clinical signs, lesions, and viral genomic sequences. J. Vet. Diagn. Investig. 2013;25:649–654. doi: 10.1177/1040638713501675. [DOI] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrusfield M., Ortega C., de Blas I., Noordhuizen J.P., Frankena K. WIN EPISCOPE 2.0: improved epidemiological software for veterinary medicine. Vet. Rec. 2001;148:567–572. doi: 10.1136/vr.148.18.567. [DOI] [PubMed] [Google Scholar]
- Vlasova A.N., Marthaler D., Wang Q., Culhane M.R., Rossow K.D., Rovira A. Distinct characteristics and complex evolution of PEDV strains, North America, May 2013–February 2014. Emerg. Infect. Dis. 2014;20 doi: 10.3201/eid2010.140491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Byrum B., Zhang Y. New variant of porcine epidemic diarrhea virus, United States, 2014. Emerg. Infect. Dis. 2014;20:917–919. doi: 10.3201/eid2005.140195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WUR . In Agro & Food Portal. Wageningen University & Research; 2016. Pig farming. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.