While flagella have been studied extensively as motility organelles, with a focus on internal structures such as the axoneme, more recent research has illuminated the roles of the flagellar surface in a variety of biological processes. Parasitic protists of the order Kinetoplastida, which include trypanosomes and Leishmania species, provide a paradigm for probing the role of flagella in host-microbe interactions and illustrate that this interface between the flagellar surface and the host is of paramount importance.
KEYWORDS: flagellar surface, kinetoplastid parasites, microbe-host interactions, sensing, secretion, immune evasion
SUMMARY
While flagella have been studied extensively as motility organelles, with a focus on internal structures such as the axoneme, more recent research has illuminated the roles of the flagellar surface in a variety of biological processes. Parasitic protists of the order Kinetoplastida, which include trypanosomes and Leishmania species, provide a paradigm for probing the role of flagella in host-microbe interactions and illustrate that this interface between the flagellar surface and the host is of paramount importance. An increasing body of knowledge indicates that the flagellar membrane serves a multitude of functions at this interface: attachment of parasites to tissues within insect vectors, close interactions with intracellular organelles of vertebrate cells, transactions between flagella from different parasites, junctions between the flagella and the parasite cell body, emergence of nanotubes and exosomes from the parasite directed to either host or microbial targets, immune evasion, and sensing of the extracellular milieu. Recent whole-organelle or genome-wide studies have begun to identify protein components of the flagellar surface that must mediate these diverse host-parasite interactions. The increasing corpus of knowledge on kinetoplastid flagella will likely prove illuminating for other flagellated or ciliated pathogens as well.
INTRODUCTION
Although flagella and cilia have traditionally been considered motility organelles, it is now recognized that they serve multiple other functions, both in single cell eukaryotes and in metazoa. Notable among these alternate activities is their role in sensing of the external environment (1–4), for which they have been likened to “antennae” that relay information about the extracellular milieu to the interior of the cell, and their activities as secretory organelles (5). Many pathogenic microbes are flagellated (6), with these organelles serving vital functions in microbial activities and in colonization of hosts. Hemoflagellates of the order Kinetoplastida, which include the African and South American trypanosomes Trypanosoma brucei and T. cruzi, as well as many species of Leishmania, are parasitic unicellular eukaryotes that colonize both an insect vector and vertebrate hosts. Flagella are prominent features of parasite morphology in various life cycle stages, and the roles of these organelles in parasite biology have been investigated for decades. Indeed, such parasites, especially T. brucei, have emerged as model organisms for the study of flagellar structure and function (6, 7), with increasingly sophisticated molecular-genetic and cell biological approaches facilitating the dissection of flagellar action, and these flagellated protists can complement more classical systems like Chlamydomonas reinhardtii (8). However, one novel feature of kinetoplastid parasites is the ability to address the function of flagella in host-microbe interactions and disease. Much of the research to date on kinetoplastid flagella has focused upon their motile properties and the structural and biochemical features of internal organellar structures, such as the axoneme, in promoting parasite motility (reviewed in references 6, 7, 9, and 10). In addition, the flagellum plays central roles in defining cell shape, morphogenesis, and polarity (11, 12), and many studies related to this function have also focused upon flagellar internal components. However, equally consequential to the diverse activities of flagella, especially among microbial pathogens, is the organellar surface that is referred to as the flagellar membrane or sheath. This flagellar surface provides a key interface between the parasite and host cells and tissues. The purpose of this review is to highlight recent advances among the kinetoplastid parasites illuminating the role of the flagellar surface in host-microbe interactions.
FLAGELLA IN KINETOPLASTID PARASITES
Kinetoplastid parasites are all flagellated (see Fig. 1 for a schematic diagram of the flagellum of the African trypanosome), but the size and morphology of the flagellum varies depending upon the species and life cycle stage (Fig. 2). Thus, Trypanosoma brucei elaborate a flagellum that is attached to the cell body along most of its length, with only the anterior tip unattached (Fig. 2A). Insect epimastigote forms of Trypanosoma cruzi have an extensive flagellar-cell body attachment but also a long section of free flagellum (Fig. 2B). In contrast, the flagella of Leishmania insect-form promastigotes are largely free of the cell body (Fig. 2C, large image), emerging untethered from the flagellar pocket (13), the invagination of the plasma membrane from which all kinetoplastid flagella extend. Intracellular amastigotes of either Leishmania species (Fig. 2C, small image in inset) or T. cruzi have short flagella that barely emerge from the flagellar pocket. These structural differences in flagella can dictate functional specializations for this organelle between species and life cycle stages. Thus, at least under the conditions tested, the cell shape and attached flagellum of T. brucei insect-stage procyclic forms allow these parasites to swim directionally for extended distances, whereas L. mexicana promastigotes make frequent changes in direction and engage in tumbling activity (14). Nonetheless, two studies (15, 16) on bloodstream forms (BF) rather than procyclic forms (PF) of trypanosomes have demonstrated that swimming behavior can differ depending on the physical composition of the medium, and this life cycle form can also switch or reverse direction and tumble frequently. Cell forms with extended flagella are highly motile, a property that can be critical to colonization of insect (17) or vertebrate (16, 18) hosts. Furthermore, the beating of trypanosome flagella promotes migration through constricted interstitial spaces in host tissues, not simply by allowing directional motility but also by deforming the shape of the parasite cell body, so that it can penetrate though apertures that are smaller than the maximum diameter of the parasite cell body (19).
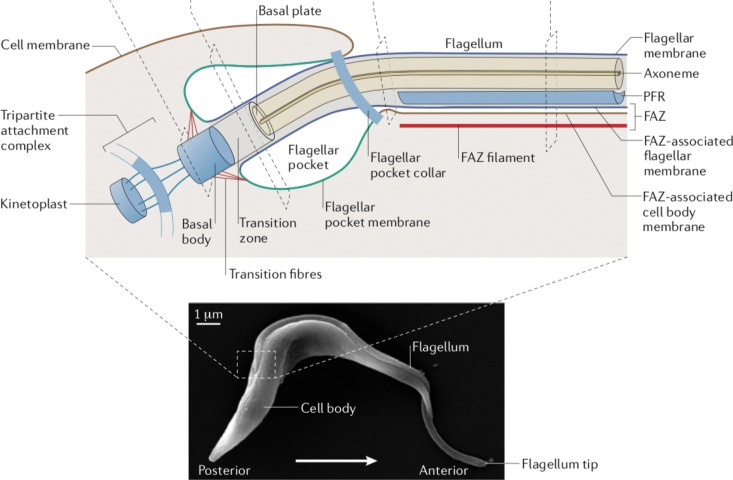
FIG 1.
Diagram of the flagellum and surrounding structures in BF T. brucei. The image on the bottom is a scanning electron micrograph of a BF trypanosome. The section in the dashed box, representing the base of the flagellum and the surrounding cellular components, has been expanded as a schematic diagram at the top of the figure. Subcellular structures associated with the flagellum are indicated by labeling. PFR indicates the paraflagellar rod and FAZ the flagellum attachment zone. (Adapted from reference 6 with permission of Springer.)
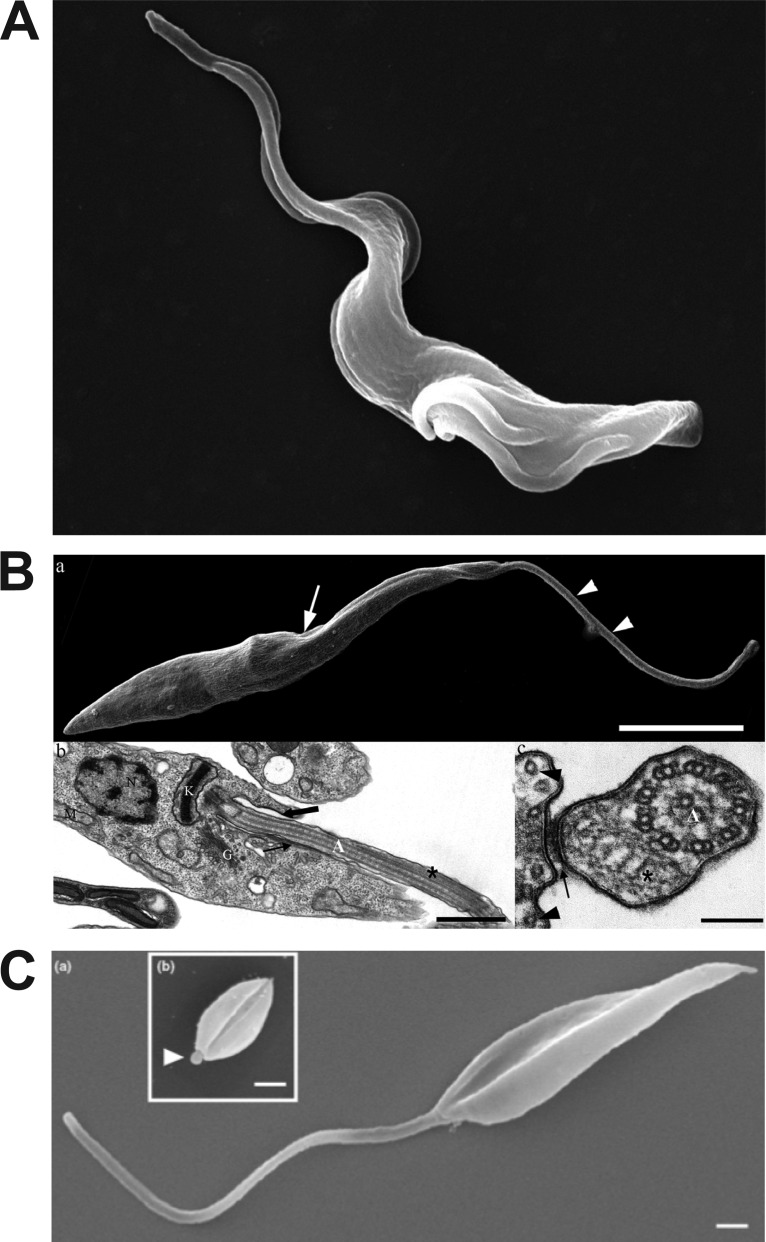
FIG 2.
Images of kinetoplastid parasites and their flagella. (A) Scanning electron micrograph of bloodstream form Trypanosoma brucei. The parasite has begun cell division, and the flagellum has duplicated at the posterior end of the cell (lower right). The anterior tip of the flagellum that is unattached to the cell body can be seen in the upper left. (Reproduced from the Wellcome Collection [https://wellcomecollection.org/works/t3za8n35]; Gull Laboratory, courtesy of Sue Vaughan.) (B) Electron micrographs of epimastigote forms of Trypanosoma cruzi. (a [top]) Scanning electron microscopy image with an arrow pointing to the flagellar pocket and two arrowheads pointing to the region of the flagellum that is free from the cell body; (b [lower left]) TEM image with a thick arrow pointing to the flagellar pocket and a thin arrow pointing to the flagellum attachment zone (FAZ); (c [lower right]) TEM image transverse to the flagellum, with the arrow pointing to the FAZ (connecting the flagellar and cell body membranes) and the flagellar membrane visible as a dark contour surrounding that organelle. (Reproduced from reference 131.) (C) Scanning electron microscopy images of an L. mexicana promastigote (a) and amastigote (b, inset). The arrowhead in subpanel b points to the short amastigote flagellum. (Reproduced from reference 132 with permission of Elsevier.)
The roles of flagella in Leishmania infections of sand flies and vertebrate hosts have also been investigated. Overexpression of a constitutively active Q70L mutant of the small G-protein LdARL-3A in L. amazonensis promastigotes resulted in parasites with very short flagella (20). These “aflagellate” promastigotes were able to infect primary bone marrow-derived murine macrophages with kinetics equivalent to wild-type parasites, suggesting that full-length flagella were not required for host cell invasion. While initial colonization of Lutzomyia longipalpis sand flies was as robust as for wild-type promastigotes, the aflagellar mutants were lost from the midguts following expulsion of the blood meal, consistent with a role for flagellar adhesion and/or motility in successful sand fly infection (21). A second study isolated a “dysflagellar” mutant of L. braziliensis from a patient (22). Although the genetic nature of this mutant is unknown, promastigotes were greatly impaired in motility, and in scanning electron micrographs they exhibited a short protuberance of disorganized material in place of the flagellum. This dysflagellar mutant was able to infect bone marrow-derived macrophages and either BALB/c or C57BL/6 mice as effectively as a reference strain of L. braziliensis, indicating that normal flagella are not required for mammalian infectivity or virulence. This mutant also colonized L. longipalpis sand flies up to 96 h, as well as the reference strain, implying that the flagellum is not required for colonization of the sand fly. The latter result conflicts with the above studies using the LdARL-3A mutant that did not successfully colonize sand flies. Why these two flagellum defective mutants give such different phenotypes in sand fly infections is not clear, although it must be recognized that the genetic lesions and the species of parasite are both distinct. In contrast to promastigotes, amastigotes are not motile, consistent with their intracellular mode of life, but amastigote flagella could nonetheless play potentially central roles in parasite interactions with the infected host cells (see below). Overall, the prominent roles of flagella in the biology of these parasites has ensured that they have been a major focus of research.
FLAGELLAR ATTACHMENT TO HOST CELLS AND TISSUES
One of the earliest roles to be identified for the flagellar surface in Kinetoplastida was attachment of parasites to epithelia in insect vectors. Electron microscopy of Lutzomyia longipalis sand flies infected with Leishmania amazonensis promastigotes revealed two different flagellum-epithelium interactions (23). Four to five days after infection, the microvilli of the posterior midgut lengthened, and nectomonad-form parasites attached to these altered microvilli by their flagella, but no visible junctional complexes were formed between the parasite and insect organelles (Fig. 3A). Nonetheless, this attachment appears to prevent excretion of the parasites during defecation of the blood meal residue. Later in infection, when parasites migrated to the mouthparts, the haptomonad forms attached to the oesophageal or stomodeal valve (24) with the flagellar tips forming electron-dense, foot-like hemidesmosomes with the chitin lining of the valve (Fig. 3B). Adherence and blockage of the stomodeal valve has important consequences for transmission of the parasite from the fly to the vertebrate host (25). In addition to forming a viscous gel-like plug generated by secretion of proteoglycan from the promastigote flagellar pocket, the attached parasites damage the chitin lining of the valve, and together these alterations cause the infected sand fly to engage in repeated pump pulsation that results in regurgitation of nonattached infectious metacyclic parasites into vertebrate tissue. Given the multiple sites of flagellar attachment in the sand fly, it is puzzling that the L. braziliensis dysflagellar mutant was able to colonize sand flies, at least up to 96 h postinfection (22), thus potentially challenging the requirement for such flagellum-vector interactions.
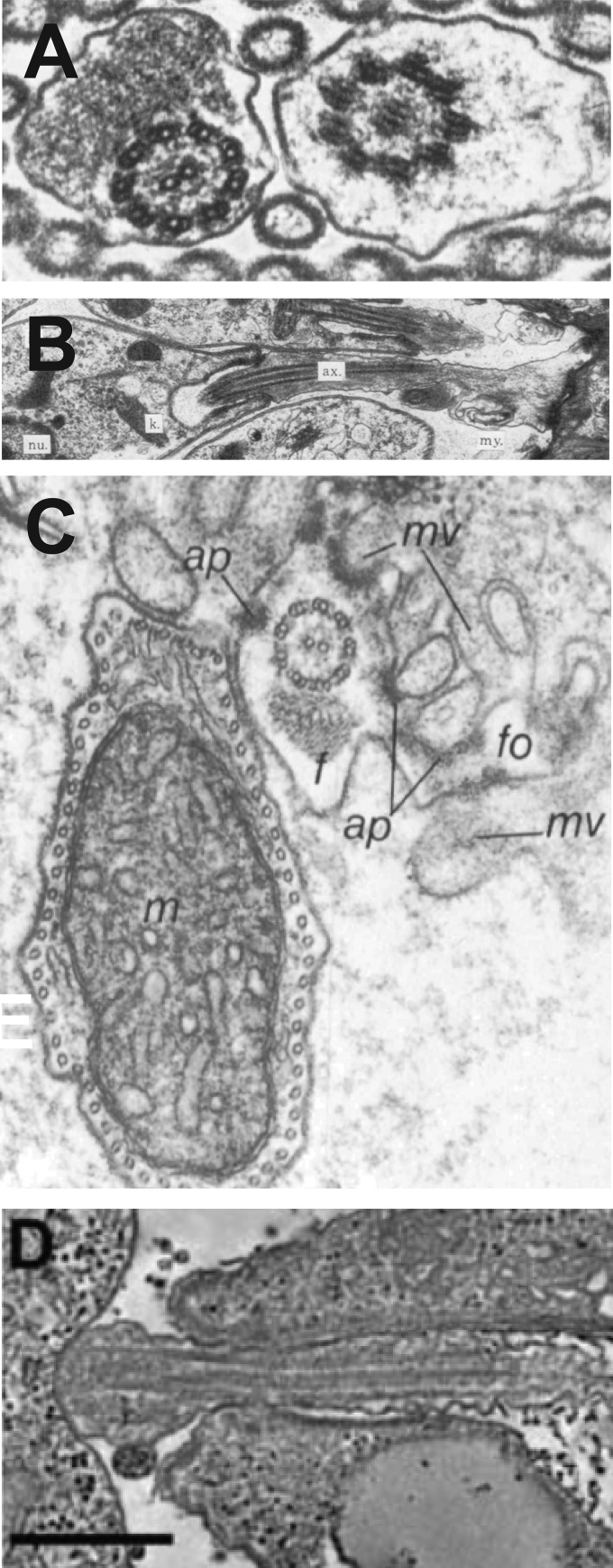
FIG 3.
Images of flagella from kinetoplastid parasites interacting with tissues of insect vectors (A to C) or with internal organelles of host cells (D). (A) Transverse view of two flagella from nectomonads of L. amazonensis interacting with the midgut microvilli of a sand fly. (Reproduced from reference 23 with permission of the Royal Society of London.) (B) Flagellum of a haptomonad of L. amazonensis forming a foot-like adhesion (extreme right of image) with the stomodeal valve of a sand fly. Labels represent the flagellar axoneme (ax), the kinetoplast (k), and the nucleus (nu). (Reproduced from reference 23 with permission of the Royal Society of London.) (C) Flagellum (f) of a T. brucei epimastigote attached to salivary gland microvilli (mv) of a tsetse fly. Labels represent flagellar outgrowth (fo), attachment plaque (ap), and trypanosome mitochondrion (m). (Reproduced from reference 26 with permission of the Company of Biologists.) (D) Attachment of the tip of an amastigote flagellum to the parasitophorous vacuole membrane of a host macrophage. (Reproduced from reference 29 with permission of the Federation of American Societies for Experimental Biology.)
Similarly, the flagellar surface is critical for the adherence of dividing epimastigote forms of T. brucei to the salivary gland of the tsetse fly (26). The epimastigote flagellum exhibits branched protrusions that intercalate into host cell microvilli and form hemidesmosome-like attachment plaques to the surfaces of the microvilli (Fig. 3C). Late in tsetse fly infection, attached epimastigotes undergo an asymmetric division, with one daughter cell being released from the epithelium and differentiating into nondividing infectious metacyclic forms in the saliva (27). Hence, both sequential development and transmission of insect-stage Leishmania and T. brucei are critically dependent upon the modification and interaction of the flagellar surface with vector tissues. What these flagellar modifications are and the precise nature of the adhesive interactions remain to be determined and will likely be challenging to define, given the limited number of adhered parasites available. However, a recent study employing the model kinetoplastid protist Crithidia fasciculata, a parasite of mosquitos, has identified genes that may be involved in adherence of these organisms to both mosquito gut and to plastic surfaces in vitro (28). Most of these genes are conserved in other kinetoplastids, suggesting that they could be involved in shared mechanisms of adherence.
Intracellular amastigotes of Leishmania and T. cruzi have short flagella (∼1.5 μm) that barely emerge from the flagellar pocket (Fig. 2C, inset, white arrowhead). While such “residual” flagella were considered by many workers to be primarily generative structures to allow outgrowth of bona fide flagella when the parasites developed into flagellated insect forms, this view of the organelle has been transformed dramatically in recent years with the recognition that this shorter flagellum doubtless plays critical roles in amastigote biology. A detailed electron microscopic study of L. mexicana amastigotes inside J774 macrophages resulted in two significant discoveries (29).
First, the axoneme of the amastigote flagellum was structurally similar to that of the mammalian primary cilium, an organelle with demonstrated sensory function (30). Instead of the 9 + 2 arrangement of microtubules characteristic of motile cilia and flagella, both the amastigote flagellum and the primary cilium have an atypical 9v (variable) axoneme in which one or more of the microtubule doublets progressively occupy a central position, thus breaking the 9-fold symmetry. Second, a large percentage of amastigotes exhibited flagella that were in intimate contact with the host cell parasitophorous membrane that surrounds the intracellular parasites (Fig. 3D). The authors of that study hypothesized that the amastigote flagellum is a sensory organelle that may bind ligands in the vacuolar membrane and may thus play a key role in host-parasite interaction during infection. This hypothesis is intriguing but remains to be definitively tested. In addition, they suggest that the flagellum could also serve as a portal for delivery of parasite proteins (effectors?) into the cytosol of the host macrophage. This suggestion would be consistent with observations of secretory functions for flagella in other microorganisms, including trypanosomes (see below). Nonetheless, both studies on aflagellate or dysflagellar Leishmania mutants cited above found that these mutants were able to infect macrophages and/or mice as well as wild-type parasites, thus challenging whether the amastigote-host cell interactions are critical for infectivity and virulence. This issue is especially relevant for the L. braziliensis mutant, where electron microscopy has confirmed that the short amastigote flagellum is indeed morphologically aberrant and terminated by a large amorphous body (22) in this isolate, so that the flagellar defects are not confined to promastigotes.
Another fascinating recent discovery is the physical interaction detected between amastigote flagella and host cell mitochondria in T. cruzi (31). Unlike Leishmania amastigotes, these trypanosomes live unenclosed within the cytosol of many differentiated mammalian cells, and they thus have direct access to host cell cytosolic components and organelles. Human dermal fibroblasts were infected with T. cruzi amastigotes and fluorescently labeled with antibodies or fluorescent proteins directed against the host cell mitochondria and against the T. cruzi flagellar calcium-binding protein, FCaBP. Fluorescence microscopy showed close contact between the amastigote flagella and the host mitochondria, and this proximity was confirmed by transmission electron microscopy (TEM). The authors suggested that host cell mitochondria may play a functional role in the infection process and that the amastigote flagellum may be an active participant in pathogenesis. As for the hypothesized functions of amastigote flagella in Leishmania, the latter inference remains to be tested.
INTERACTIONS OF THE FLAGELLAR AND CELL BODY MEMBRANES
Most eukaryotic flagella extend out from a cell into the surrounding medium. However, the flagellum of T. brucei exits the flagellar pocket and is attached along the long axis of the cell body by the flagellum attachment zone or FAZ (Fig. 1 and 4A), which is an extended structure that connects the flagellar cytoskeleton and membrane to the cell body cytoskeleton and membrane (32–34). An extensive body of literature has shown that the T. brucei FAZ is a key cytoskeletal/membrane structure orchestrating cell length and organelle position, including positioning of the cleavage furrow during cell division, and is therefore crucial for cell division and viability.
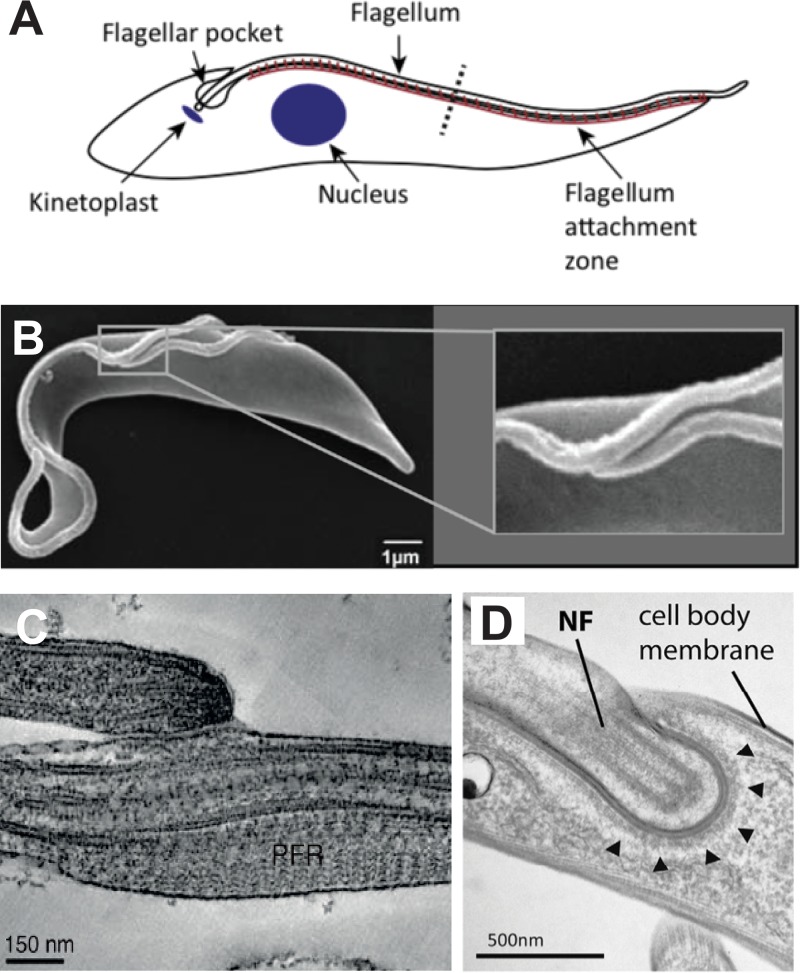
FIG 4.
Interactions of the T. brucei flagellum with other components of the parasite. (A) Schematic diagram of a BF parasite showing the flagellum attachment zone (brown line) attaching the flagellum to the cell body. (Reproduced from reference 32 with permission of Elsevier.) (B) Scanning electron micrograph of a replicating PF trypanosome showing the shorter new flagellum attached at its growing end to the longer old flagellum. The inset at the right shows an expanded view of the boxed region containing the two attached flagella. (Reproduced from reference 52.) (C) Transmission electron micrograph of the flagellum connector attaching the growing tip of the new flagellum (top) to the lateral membrane of the old flagellum (bottom). (Reproduced from reference 52.) (D) Transmission electron micrograph of the flagellar groove of a replicating BF trypanosome. NF indicates the tip of the new flagellum embedded in a groove within the cell body membrane. Arrowheads indicate electron dense material lining the cytosolic side of the groove membrane. (Reproduced from reference 55 with permission of the Company of Biologists.)
Studies on adhesion of the flagellar membrane to the cell body began with the identification of glycoprotein 72 (GP72) in T. cruzi, an immunodominant protein localized to the junction between cell body and flagellum (35, 36). Deletion of both copies of the GP72 gene generated viable epimastigotes with flagella that were detached from the cell bodies (35). Subsequently, knockdown of the T. brucei ortholog of GP72, FLA1, demonstrated that it is required for flagellum attachment to the cell body in African trypanosomes (37, 38). Moreover, knockdown of FLA1, together with the related FLA2 RNA in BF trypanosomes (see below), caused flagellar detachment and blocked cytokinesis but did not inhibit mitosis (37), leading to multinucleate cells that were not viable and indicating a link between flagellar-cell body attachment and cytokinesis. Subsequently, the He laboratory demonstrated that FLA1 is a component of the cell body membrane FAZ and that it interacts with the FLA1 binding protein (FLA1BP), located in the flagellar membrane FAZ, thus creating a tight zipper-like junction between both membranes (39). Additional studies (see Table 1 in reference 32) identified several more structurally related membrane-associated adhesins, FLA2 (37) and two additional different proteins (39, 40), one a FLA1 and the other a FLA1BP homolog, that were unfortunately both given the name FLA3. All of these FLA1- and FLA1BP-related proteins are glycoproteins, have NHL (NCL-1, HT2A, LIN-41) repeats (Pfam number PF01436), and are thought to act as adhesins that tether together the flagellar and cell body membranes in the FAZ. FLA1 and FLA1BP are preferentially expressed in PF trypanosomes, whereas the FLA1-related FLA2 and FLA3 are preferentially expressed in BF trypanosomes, so different adhesins appear to play similar roles in both life cycle stages.
Two other non-FLA-related proteins, FAZ5 (41) and a putative Ca2+ channel designated either FS179 (42) or TbCaCh (43), are located either in the cell body membrane (FAZ5) or in the flagellar membrane (FS179) component of the FAZ. Both proteins have multiple transmembrane segments, and knocking down their respective RNAs leads to flagellar detachment. While flagellar detachment for RNAi directed against the FLA1- or FLA1BP-like adhesins is in line with their roles in mediating membrane-membrane attachment, the reason for organellar detachment for knockdown of FAZ5 or FS179/TbCaCh is less clear. Do these latter proteins also have direct adhesive functions, or are other functional properties, such as Ca2+ influx through the channel, required for attachment of the flagellum to the cell body? Notably, in citrated blood, where Ca2+ is probably chelated by citrate, the daughter flagellum of dividing BF trypanosomes is released free of the FAZ (44). This observation would be consistent with a possible role for Ca2+ import through the channel in flagellar adhesion. In addition, knockdown of different FAZ membrane proteins can have different phenotypes regarding cell viability. Thus, knockdown of FLA1 or FS179/TbCaCh is lethal, generating parasites that cannot undergo cytokinesis, whereas knockdown of FLA1BP produces cells with detached flagella and epimastigote morphology that still divide. It is notable that knockdown of many cytoskeletal components of the FAZ, such as cell body-associated FAZ1 and flagellar FLAM3 (45), also disrupt the FAZ and cause flagellar detachment, indicating that many constituents of this complex structure are critical for attachment of the flagellar membrane to the cell body.
While the FAZ was first identified and studied in T. brucei, most of the FAZ proteins are conserved in Leishmania and T. cruzi (46), and the FAZ has been identified at both morphological (47, 48) and molecular (47) levels in L. mexicana. L. mexicana FAZ protein orthologs FAZ5, FLA1-BP, and ClpGM6 (49) are localized in a discrete and limited region connecting the flagellar membrane and the flagellar pocket membrane, as demonstrated by fluorescence and electronic microscopy. Thus, the Leishmania FAZ is much less extended than that of trypanosomes. Deletion of the FAZ5 gene results in a more bulbous morphology for the flagellar pocket, but culture form promastigotes are not impaired in growth. In contrast, Δfaz5 knockout L. mexicana are unable to develop late-stage infections in sand flies, and they exhibit >97% reduction in the parasite burden following infection of BALB/c mice, establishing a critical role for this discrete adhesive junction in vivo.
CONNECTIONS BETWEEN FLAGELLAR MEMBRANES IN DIVIDING TRYPANOSOMES
In African trypanosomes, duplication and growth of a new flagellum occurs early during cell replication. In PF trypanosomes, the new flagellum emerges from the flagellar pocket but remains attached to the side of the old flagellum as it grows (50) (Fig. 4B), and this attachment is maintained until the new flagellum has attained full length. The resulting strict positioning of the new flagellum and FAZ, following the left-hand helical pattern of the old flagellum around the replicating cell, establishes the helical arrangement of the cytoskeleton, the axis and polarity for cell division, and the position of subcellular organelles in the new cell. How the new flagellum is constrained to follow this geometric pattern emerged from examination of negatively stained detergent-extracted cytoskeletons from dividing PFs (50). A distinctive PF-specific structure was identified, designated the “flagellum connector” (FC), that attached the distal tip of the new flagellum to the side of the old flagellum (Fig. 4C). This mobile junction between the two flagellar membranes was further examined by transmission electron microscopy of negatively stained cytoskeletons, which revealed a trilaminar structure consisting of plate like electron densities in the two flagellar membranes and discontinuous electron densities in the intermembrane space (51). Further ultrastructural analysis by three-dimensional electron tomography of fixed, stained sections and subsequently cryo-electron tomography of high-pressure frozen preparations (52) revealed a similar, albeit more compact, tripartite structure for the FC, containing interstitial material between the two membranes that had a clear periodicity and fibrous structures that appear to attach the membrane components of the FC to the two flagellar axonemes.
Two recent studies have identified molecular components of the FC. FC1 (53), is a putative protein kinase that is a probable substrate for the polo-like kinase of T. brucei that associates transiently with the FC. Varga et al. (54) employed a structure-immunoprecipitation/mass spectrometry approach to identify seven constituent proteins that occupy different domains of the FC. FCP1 contains predicted transmembrane domains and is thus likely a constituent of the interstitial zone where the two membranes will be in close proximity. FCP4 is a member of the kinesin-15 family of plus end-directed microtubule motors that associates with the dynamic end of the new flagellum axoneme, and FCP2 is a different kinesin that likely attaches the FC to the axoneme of the old flagellum. These two kinesins are postulated to be connected and operating across a membrane junction. Interestingly, both of the above studies find that RNA interference (RNAi) knockdown of FC components often results in disconnection of the tip of the new from the old flagellum, but, remarkably, the positioning of the new flagellum on the cell body is not affected by this structural disruption. This result may imply (54) that the FAZ, rather than the FC, plays the dominant role in positioning of the new flagellum.
Notably, the FC does not occur in BF trypanosomes, Leishmania, or T. cruzi (51). Nonetheless, the new flagellum of dividing BFs does track close to the old flagellum despite the absence of an attachment between the surfaces of the two flagella. In contrast, a different mode of attachment has been identified between the tip of the new flagellum and the cell body membrane in BFs, at a position close to the old flagellum, and this juncture has been designated the cell body groove (55). The authors of that study used a combination of TEM, serial block-face scanning electron microscopy, and electron tomography to show that the tip of the new flagellum is embedded in an invagination of the cell body membrane (Fig. 4D). The microtubule corset in the cell body is disrupted at this moving groove but reforms as the groove progresses toward the anterior end of the cell. The tip of the new flagellum is always in close contact with the cell body plasma membrane, and there is electron dense material that accumulates on the cytosolic side of the groove (Fig. 4D), suggesting that there is a specific mode of attachment of the flagellar membrane tip to the cell body. This groove is located near the microtubule quartet (an array of 4 parallel microtubules, located close to the FAZ, with polarity opposite that of the other cell body microtubules) and the adjacent FAZ filament, consistent with the tracking of the new flagellum along the route of the old flagellum and its attachment to the cell body. Thus, the surface of the replicating flagellum interacts with either the flagellar or cell body membrane using different structures in PF and BF trypanosomes, but both interactions promote trafficking of the new flagellum along the path of the old flagellum.
FLAGELLUM-MEDIATED PHYSICAL INTERACTIONS BETWEEN PARASITES
Flagella can also mediate interactions between parasites by a number of distinct modalities. One notable example is genetic exchange between T. brucei parasites that occurs by sexual recombination within the tsetse fly salivary gland (56–58). The process of parasite mating was shrouded in secrecy for many years, but Peacock et al. (59) found that haploid parasites with short, wide bodies and very long flagella could be isolated from salivary glands, and when mixed in vitro these haploid forms intertwine their flagella and exchange cytoplasmic contents (Fig. 5A). This flagellar interaction is reminiscent of the flagellar adhesion that occurs during mating by Chlamydomonas, which is mediated by glycoproteins on the flagellar surface (60). Genetic evidence indicates that Leishmania spp. also undergo genetic exchange within the sand fly vector, but mating parasites have not been observed directly (61, 62), so the role of flagella in the formation of Leishmania genetic hybrids is currently unknown.
FIG 5.
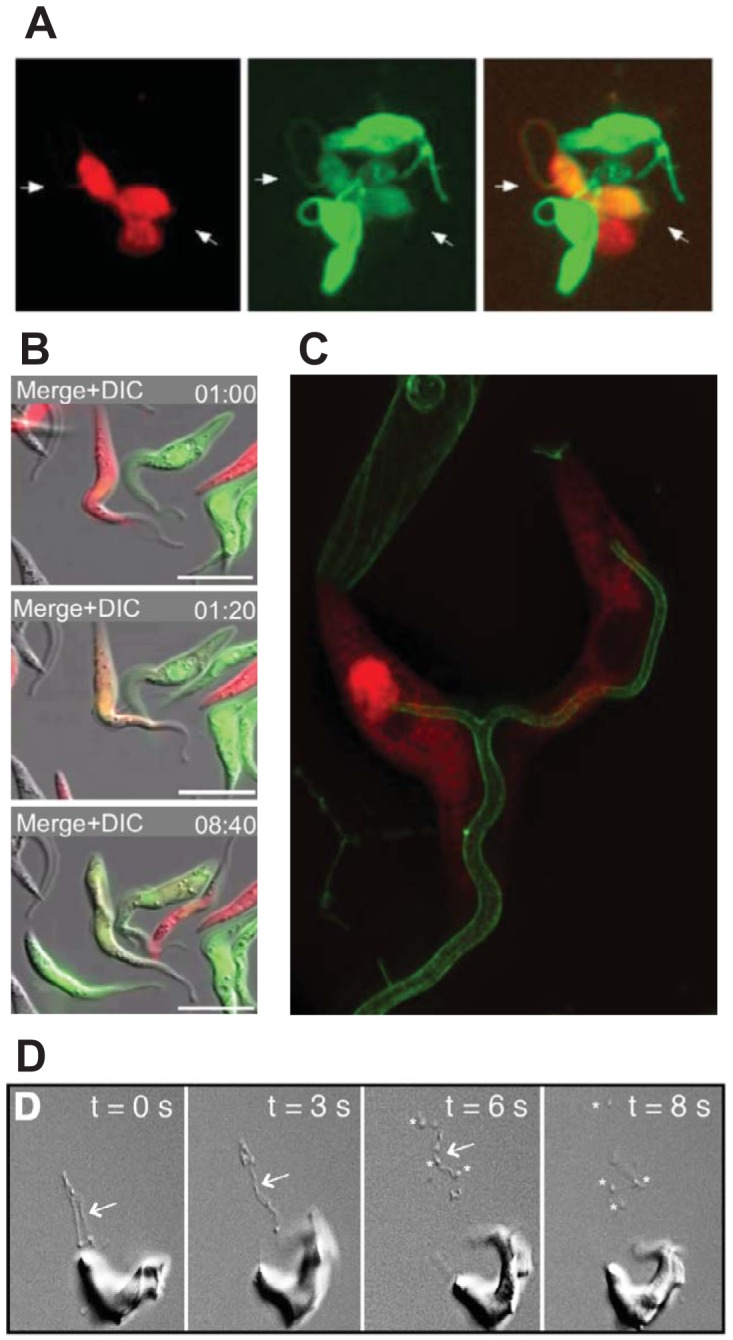
Flagellar interactions in T. brucei. (A) Gametes of insect-derived trypanosomes labeled with either red or green fluorescent proteins interact via their flagella (two parasites indicated by white arrows) and exchange their cytosolic contents, resulting in dually red and green labeled parasites. (Reproduced from reference 59.) (B) Time-lapse fluorescence microscopy of mixed red and green fluorescently labeled PF trypanosomes (top panel, 1:00 min) shows that some parasites interact via their flagella and exchange cytosolic contents, producing dually labeled (orange) parasites (middle panel, 1:20 min). (Reproduced from reference 63.) (C) Structured illumination fluorescence microscopy shows two PF parasites labeled in the cytosol with DsRed (red) and on the flagellar membrane with calflagin44-GFP (green). The two flagella have fused to form a single wider flagellar structure (bottom of the image). (Reproduced from reference 63.) (D) Video microscopy of differential interference images of BF T. brucei show the emergence of nanotubes (arrows) from the flagellar membrane (0 to 6 s), followed by fragmentation into extracellular vesicles (asterisks, 6 and 8 s). (Reproduced from reference 73 with permission from Elsevier.)
In addition to this observed interaction between haploid cells, trypanosome flagella are also involved in nonsexual protein exchange. When procyclic parasites expressing diffuse red or green fluorescent proteins were mixed, parasites containing both fluorescent proteins were observed at low frequency, comprising approximately 1% of the population (63). Video microscopy showed that this protein transfer occurred (Fig. 5B) when two parasites, each expressing a different fluorescent protein, interact via their flagella (Fig. 5C) in a process dependent on extracellular calcium but not dependent on GPI-anchored surface procyclins, the major coat proteins of PF trypanosomes. The protein exchange is not part of sexual reproduction, since genetic material is not exchanged, and the parasites uncouple and resume growth as individual cells, while the transferred protein is eventually lost via protein turnover (Fig. 5B, 8:40 min). The molecular mechanism of this flagellar interaction is unknown, but electron microscopy and high-resolution fluorescence microscopy (Fig. 5C) show flagellar fusion. The biological function of flagellar fusion and cytosolic exchange is also unclear.
SECRETION OF PROTEINS FROM TRYPANOSOME FLAGELLA
Parasitic unicellular eukaryotes use extracellular vesicles (EVs) as vehicles for intercellular communication and host manipulation (64, 65). EVs can help pathogenic protists establish infective niches, modulate the immune system of the host, and cause disease. Historically, multiple groups have observed thread-like or filamentous appendages, fibrils, secretory filaments, and similar processes emerging from various points along the trypanosome cell body or flagella, and sometimes fragmenting into vesicles, and have suggested that they might represent routes for exporting material from the parasite (66–71). More recently, a study reported BF T. brucei produce long membrane nanotubes, confirmed by transmission electron microscopy to arise through budding and extension of the flagellar membrane (72, 73), and these nanotubes subsequently fragment into vesicles and produce free EVs ∼80 nm in diameter that are enriched in flagellar proteins (Fig. 5D). This process exhibits some parallels to the release of EVs from the tips of flagella that was discovered in Chlamydomonas reinhardtii (5, 74), an observation that first established that flagella can act as secretory organelles (75). Notably, Szempruch and collaborators demonstrated that trypanosome EVs transfer the serum resistance-associated protein (SRA) from the human-pathogenic T. brucei rhodesiense to the non-human-pathogenic T. brucei brucei, enabling the latter parasite to infect and persist in a primate host where it would not otherwise be viable. Moreover, nanotube-derived EVs fuse with host erythrocyte membranes, and such remodeling changes the physical properties of the erythrocyte membrane, causing clearance of infected red blood cells by macrophages in the liver and spleen, thus generating anemia and often death of the murine experimental host. Notably, proteomic analysis of purified EVs showed the presence of abundant nonflagellar components, such as ribosomal proteins and some glycosomal enzymes, in addition to the flagellar proteins that were strongly enriched. As discussed by Szempruch and coworkers (72, 73), these results suggest that EVs may arise by different mechanisms from various sites in the parasite, not just from the flagellum. This conclusion would be consistent with the earlier studies cited above in which filaments were observed potentially originating from different sites on the cell surface. Leishmania species also produce EVs that can affect virulence (64, 65). L. major and L. infantum have been shown to produce extracellular vesicles while in the lumen of the sand fly, but, unlike the flagellum-derived extracellular vesicles from T. brucei, these bud from the flagellar pocket or from multivesicular bodies on the membrane of the cell body (76).
FLAGELLA AND SOCIAL MOTILITY
Trypanosomes also communicate information about neighboring parasites via flagellar membrane proteins. Social motility (SoMo) in trypanosomatids was initially observed when early procyclic forms of T. brucei plated on semisolid agar migrated away from a site of inoculation in radial projections (77), and these projections also diverged in direction as they encountered each other, suggesting that populations of parasites sense each other’s presence and avoid close contact. This process was dependent on flagellar motility and regulated by cyclic AMP (cAMP). The T. brucei genome encodes a large number of membrane-bound adenylate cyclases (referred to as either ACs or ACPs), enzymes that catalyze the conversion of ATP to cAMP, and several members of this family of transmembrane proteins are localized to the flagella (78) (Fig. 6A). RNAi directed either against both AC1 and AC2 or against AC6, all of which are flagellar membrane proteins (79), induced hyperactive social motility, indicating a role for these flagellar surface proteins in SoMo. Furthermore, the cAMP-specific phosphodiesterase PDEB1 is also required for SoMo (79) and is localized to the flagella as well (80). Although growth on a semisolid agar plate might appear to be distant from the natural environment of trypanosomes, light sheet fluorescence microscopy of trypanosomes in tsetse flies (81) has shown that they encounter multiple surfaces and can exhibit collective motility involving swarms of parasites in confined areas; hence, SoMo is likely to reflect activities undertaken by trypanosomes in nature. Furthermore, recent work (17) has shown that SoMo-deficient PDEB1 mutants cannot complete colonization of the insect host, failing to migrate across the peritrophic membrane that surrounds the blood meal into the ectoperitrophic space. Hence, an enzyme that is required for in vitro SoMo is essential for the completion of the parasite life cycle in the insect vector. Thus, signaling that occurs at the flagellar membrane may induce changes in motility that drive parasite migration across the peritrophic matrix within the tsetse fly. Indeed, a plausible interpretation of the results is that the PDEB1 mutant disrupts a sensory arm of the SoMo pathway, resulting in failure of the mutant parasites to respond to a chemotactic signal and migrate out of the peritrophic membrane.
FIG 6.
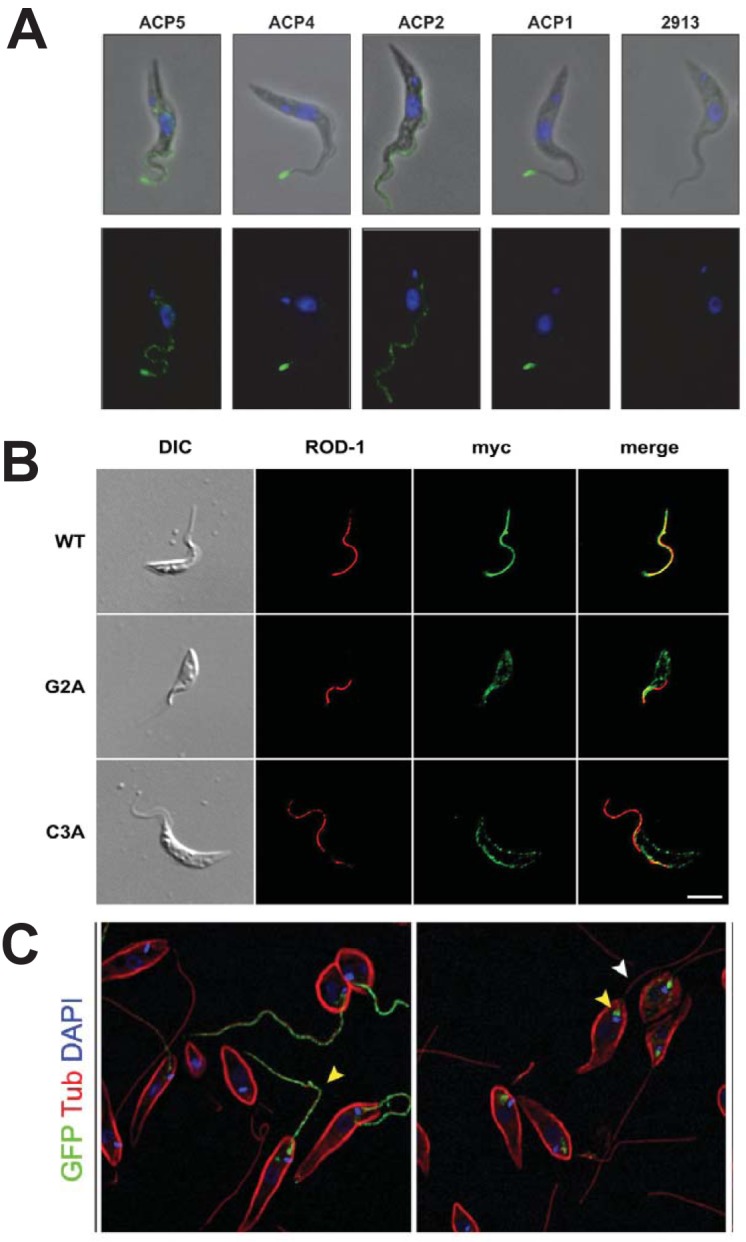
Flagellar localization of several membrane proteins. (A) Flagellar localization of 4 hemagglutinin (HA)-tagged adenylate cyclase proteins (ACPs) in PF T. brucei. Green fluorescence indicates localization of the relevant HA-fusion proteins, and blue is DAPI (4′,6′-diamidino-2-phenylindole) fluorescence from nuclear and kinetoplast DNA. The top panels are superpositions of phase-contrast and immunofluorescence images, and the bottom panels are immunofluorescence images. The panels marked 2913 represent a parasite not expressing any HA fusion protein. (Reproduced from reference 78 with permission.) (B) The location of calflagin Tb44 in PF T. brucei was monitored using antibody against the myc epitope tag (green) and antibody against an endogenous paraflagellar rod protein (ROD-1, red). Wild-type (WT) Tb44 colocalized with ROD-1 to the flagellum, whereas the Tb44 mutants G2A (nonacylated) and C3A (nonpalmitoylated) did not localize to the flagellum. (Reproduced from reference 133 with permission from the Company of Biologists.) (C) Flagellar localization of GT1-GFP (green, yellow arrowhead) was observed in wild-type L. mexicana promastigotes (left panel), but the fusion protein arrested in the flagellar pocket (yellow arrowhead, right panel) in the Δkharon mutant, showing dependency of flagellar trafficking upon the LmxKHARON protein. Red fluorescence represents staining with anti-α-tubulin, which stains the subpellicular microtubules and flagellar axoneme (white arrowhead). (Reproduced from reference 111 with permission of the American Society for Biochemistry and Molecular Biology.)
SENSORY ACTIVITY OF FLAGELLA
Primary cilia of multicellular organisms are sensory organelles, displaying receptors on the ciliary membrane that mediate transduction of extracellular signals to the interior of the cell (30). Motile flagella, of the type found in kinetoplastid parasites, are related to primary cilia in structure and are also capable of sensing the environment (2). One example is the flagellum of C. reinhardtii, where flagellar adhesins on gametes of one mating type engage cognate receptors on the flagella of gametes of the other mating type to initiate a developmental program that leads to the induction of cAMP, the expression of genes involved in mating, gamete fusion, and zygote formation (60).
The life cycle of kinetoplastid parasites is characterized by growth in a wide variety of conditions, as the parasites pass through different anatomical compartments in the insect vector and the mammalian host. Parasites are scavengers and are auxotrophic for many common metabolic building blocks, so they need to modify nutrient uptake and metabolic pathways in response to nutrient availability. Indeed, these parasites can regulate expression of mRNAs and proteins in response to changes in environmental conditions, such as alterations in levels of glucose, purines, iron, and amino acids (82–85). In most cases it is unknown how the parasite senses the change in nutrients, but a few recent examples suggest that transporters localized to the flagella could be acting as sensors to monitor the extracellular levels of their cargo. A sensory role for transporters has been established in some other eukaryotes, and such dual function transporter-receptors have been designated “transceptors” (86–88).
L. mexicana parasites are exposed to various levels of sugars as they move through the sand fly (89). One glucose transporter, GT1, is localized selectively on the flagellar membrane (90, 91), and the expression of GT1 is upregulated in response to reduced extracellular glucose (85). A potential role for GT1 in glucose sensing is supported by the observation that promastigotes of the Δgt1 null mutant fail to transition from logarithmic to stationary phase when glucose is exhausted from the growth medium, as observed for wild-type promastigotes, but the mutant instead undergoes a catastrophic loss in viability. This failure of the null mutant to respond to glucose depletion at high cell density by transitioning to stationary phase suggests that the GT1 permease could be acting as a glucose sensor whose activity is required to promote this phase transition.
Arginine is an essential amino acid in Leishmania parasites, and in L. donovani it is taken up by AAP3, a high-affinity arginine transporter that is located in both the flagellar and glycosomal membranes (92). When L. donovani promastigotes are starved for arginine, AAP3 protein abundance increases (93), as does the abundance of mRNAs encoding several other transporters, and this response requires the mitogen-activated protein (MAP) kinase MAPK2 (82). The parasites also display changes in the phosphorylation of AAP3 and approximately 100 other proteins in response to arginine deprivation, indicating a coordinated cellular response to amino acid starvation mediated, at least in part, by phosphorylation. The parasites also upregulate AAP3 when they enter macrophages and transform from promastigotes to amastigotes, possibly to compete with the mammalian host for arginine. Further work has indicated that some inhibitors of arginine transport induce this arginine deprivation response, while others do not, indicating that arginine transport per se is not the primary signal for the arginine sensing pathway (94), and the permease likely has separate transport and sensory functions. Thus, this flagellar-membrane-localized transporter may act as a sensor for extracellular levels of arginine.
Another example of flagellar protein involvement in response to the environment is osmotic regulation through the transport of small neutral substrates and trivalent metalloids mediated by the aquaglyceroporin channel AQP1 (95). In L. major, overexpression of AQP1 confers sensitivity to Sb(III) and As(III) and rapid uptake of these metalloids. AQP1 is permeable to water, glycerol, methyl glyoxal, and other nonionic solutes (96). Interestingly, AQP1 localizes to the flagellar membrane in promastigotes and to the flagellar pocket, likely to the shortened amastigote flagellum located within the flagellar pocket, in amastigotes. Leishmania promastigotes migrate up an osmotic gradient, and taxis may be important during their development in the insect vector (97). The osmotaxis of L. donovani promastigotes toward higher glucose was more rapid in parasites overexpressing AQP1, but not in parasites that expressed mutated nonfunctional channels, suggesting a role for AQP1 in sensing and responding to osmotic gradients. At present it is unclear whether the localization of AQP1 to the flagellar membrane is required for its function in osmotaxis.
For each of these flagellar transmembrane proteins in Leishmania parasites, the protein functions as a transporter or pore but is also involved, directly or indirectly, in sensing the environment. Another example of flagellar membrane proteins that are likely involved in signaling involves the adenylate cyclases (ACs) of T. brucei that affect social motility, as discussed above. Although it is not clear how these ACs are activated, their large extracellular domains suggest they may bind some extracellular ligand that induces AC activity in the flagellum. In summary, the flagellar surface serves as a specialized sensory organelle mediating communication between the external environment and the interior of the parasite. This conclusion is in line with the observation that motile cilia, as well as smaller sensory cilia such as the primary cilium of mammalian cells, can display sensory functions (2).
IMMUNE EVASION
T. brucei flagellar receptor type ACs (98) play an unanticipated role in evasion of host innate immunity (99). Expression of a dominant-negative (DN) mutant of one such AC, ESAG4, reduced parasitemia and increased the host survival time following infection of mice, suggesting that functional ACs were important for successful colonization of the host. The increased survival of mice infected with the DN-expressing parasites was associated with host phagocytic liver myeloid cells, which produced increased amounts of tumor necrosis factor alpha (TNF-α) when infected with the ESAG4-DN-expressing compared to wild-type trypanosomes. A body of experimental results supported a model whereby African trypanosomes are ingested by liver myeloid cells early in infection and these immune cells internalize trypanosome ACs from the dying parasites. The resulting parasite-induced increase in cAMP within these phagocytes stimulates protein kinase A, which inhibits synthesis of TNF-α, a cytokine that would otherwise stimulate further phagocytosis of trypanosomes leading to control of the infection. Hence, one function of parasite ACs is to blunt the host innate immune response by increasing cAMP in myeloid cells and impairing synthesis of TNF-α. Thus, components of the parasite flagellar membrane have a dedicated role in immune evasion, underscoring another route whereby this organelle mediates host-parasite interactions.
A second example of flagellar membrane proteins that protect trypanosomes against the immune response involves the calflagins (100), three Ca2+-binding proteins of T. brucei that are related to the flagellar Ca2+-binding protein from T. cruzi, FCaBP (101). These proteins are bound to the inner surface of the flagellar membrane by a dual acylation near their N terminus, and they undergo a conformational change upon binding Ca2+ that allows the acyl groups to insert into the flagellar membrane (102, 103). To address the biological function of the calflagins in BF T. brucei, expression of all three proteins was knocked down using an RNAi target sequence that was shared by all three open reading frames. Strikingly, induction of RNAi against the calflagins during a mouse infection resulted in an abrupt drop in parasitemia at day 7 postinfection. Wild-type parasites increased in number and killed all of the infected mice by day 10, but mice infected with the RNAi-induced parasites survived much longer, with about one-third of them living until the end of the 21-day experiment. Remarkably, induction of RNAi against the calflagins did not impair growth of BF parasites in vitro, nor did it affect their motility or morphology. The mechanism whereby the calflagins protect parasites and promote virulence in vivo is not clear.
MECHANISMS OF FLAGELLAR TARGETING
The unique functional properties of the flagellar surface are likely conferred by proteins that are selectively targeted to the flagellar membrane and ensure that it is differentiated from the rest of the cell surface. What mechanisms are responsible for targeting such proteins selectively to the flagellar membrane? One example that has been investigated in some detail includes the flagellar Ca2+ binding proteins, the 24-kDa FCaBP (Fig. 6B) of T. cruzi and the three homologous calflagins of T. brucei mentioned above under the section on immune evasion. These proteins are dually acylated, with a myristate covalently attached to G2, the N-terminal residue of the mature protein, and a palmitate attached to the sulfhydryl moiety of C4 (103). These FCaBP proteins are tethered to the intracellular surface of the flagellar membrane and so are not directly exposed to the flagellar surface, but they are nonetheless functionally important components of the flagellar membrane. It was subsequently demonstrated that the myristate is sufficient to confer membrane localization upon these proteins but that the palmitate is essential for partitioning from the cell body into the flagellum (104). Furthermore, Ca2+ binding to the EF hand sites was required for association of TcFCaBP with the membrane (103). Hence, the FCaBPs are considered to be calcium switch proteins in which flagellar localization depends upon Ca2+ binding, and a drop in intraflagellar Ca2+ results in removal of the protein from the membrane. The authors suggested that, similar to recoverin in mammalian retinal rod cells, the FCaBPs may modulate the activity of other flagellar membrane proteins in a Ca2+-dependent manner.
How does the dual acylation mediate flagellar targeting of this and presumably other dually acylated flagellar proteins? In initial studies (105), Engman and coworkers showed that the T. brucei flagellar membrane has increased concentrations of sterols, glycolipids, and sphingolipids, indicating a high level of lipid rafts. Furthermore, the calflagin Tb34 was found in detergent-resistant membranes that floated to the top of a sucrose gradient, which is characteristic for raft-associated proteins. When β-cyclodextrin was employed to deplete BF parasite membranes of cholesterol, the flagellar localization of Tb34 was lost. These results underscore the interaction of the acyl groups with rafts in trafficking of the FCaBPs to the flagellum. Nonetheless, dual acylation alone is not sufficient to induce trafficking to the flagellar membrane, since not all dually acylated proteins traffic to the flagellum (106). The CAP5.5 protein of T. brucei, the HASPB protein of L. major, and a phosphoinositide-specific phospholipase C of T. cruzi are all myristoylated and palmitoylated, but they do not localize to the flagellum. To address what else might be required for flagellar targeting, Engman and coworkers fused the first 12 and the first 24 amino acids of TcFCaBP to green fluorescent protein (GFP) and discovered that while residues 1 to 12 could mediate dual acylation and membrane binding, residues 13 to 24 were required for flagellar trafficking (106). Furthermore, mutagenesis of several conserved K residues in the latter region indicated that these amino acids were essential for flagellar targeting. Overall, these data indicate that dually acylated proteins do not simply diffuse into the raft-rich flagellar membrane by thermodynamic partitioning but that they likely require interactions with other currently unknown proteins to ensure localization to this organelle. Whether such interactions operate by actively trafficking the acylated proteins into the flagellum or by anchoring them there once they have arrived is an open question, as discussed previously (107).
Other examples of dually acylated flagellar proteins include the Small Myristolyated Protein 1 (SMP-1), which is a member of a family of related proteins discovered initially in L. major (108) but also present in T. brucei and T. cruzi. SMP-1 is present in detergent-resistant membranes and is discretely localized in the flagellar membrane. Nonetheless, treatment of L. major promastigotes with a combination of ketoconazole and myriocin, inhibitors of sterol and sphingolipid biosynthesis, respectively, did not cause mislocalization of SMP-1 away from the flagellum despite resulting in massive distension of the flagellar membrane and major disruption of the flagellar axoneme and paraflagellar rod structures. These results suggest that SMP-1 targets to the flagellar membrane independently of the axoneme or intraflagellar transport, the motility process that traffics many flagellar components along the axonemal microtubules (109), because flagellar trafficking still occurs when the microtubule-based structure for intraflagellar transport has been massively disrupted.
Other flagellar membrane components that are integral membrane proteins, rather than lipid anchored moieties, include receptor adenylate cyclases (98), transporters for glucose (90, 110) and arginine (82), an aquaglyceroporin from L. major (96), several proteins from T. brucei, including a putative Ca2+ channel (42), and others that are listed in Table 1. As discussed above, it is possible that many of these proteins serve sensory functions, as ciliary and flagellar membrane proteins often monitor the levels of solutes in the vicinity of the cell (2–4). Among these integral membrane proteins, the one that has been dissected most extensively with regard to flagellar trafficking is the L. mexicana glucose transporter GT1 (Fig. 6C). Deletion and site-directed mutagenesis of GT1 identified three sequential amino acids within the N-terminal lumenal hydrophilic domain, N95-P96-M97, that were critical for flagellar targeting and which strongly impaired trafficking to the flagellum when mutated to A residues (91). However, to target another normally nonflagellar glucose transporter, GT2, to the flagellar membrane, it was necessary to fuse to its N terminus a larger segment of LmxGT1 that encompassed amino acids 81 to 113.
TABLE 1.
List of known or probable flagellar membrane proteins in T. brucei, Leishmania species, and T. cruzia
| Gene ID | Name(s) | Reference(s) | Comments |
|---|---|---|---|
| (A) Flagellar membrane proteins identified by fluorescence microscopy | |||
| Tb927.8.4050/4100 | FLA1BP | 39 | PF, 1 TM, FAZ attachment |
| Tb927.5.4570/4580 | FLA1BP | 40 | BF, 1 TM, FAZ attachment |
| Tb927.11.17040, 10.16190, 10.13040, 11.13740, respectively | ACP1, ACP2, ACP4, ACP5, respectively | 78 | PF, 1 TM, adenylate cyclases |
| Tb927.4.3750 | ESAG4 | 98 | BF, 1 TM, adenylate cyclase |
| Tb927.2880 | FS179, TbCaCh | 42, 43 | BF, 24 TM, putative Ca2+ channel |
| Tb927.8.6010 | TbHrg | 128 | PF and BF, 4 TM, heme transporter |
| Tb927.6.1520 | TbAQP1 | 129 | PF and BF, 6 TM, aquaporin |
| Tb927.7.4270 | Hypothetical protein | 120 | PF and BF, 1 TM |
| Tb927.11.1830 | Hypothetical protein | 120 | PF and BF, 6 TM |
| Tb927.1.2120 | TbCALP1.3 | 130 | PF and BF, dual acylation, calpain-like protein |
| Tb927.1.2160 | TbSKCRP1.5 | 130 | PF and BF, dual acylation, calpain-like protein |
| Tb927.9.6170 | Arginine kinase | 122 | PF and BF, dual acylation |
| TcCLB.509391.20 | FCaBP | 101 | All life cycle stages, dual acylation, flagellar Ca2+ binding protein |
| LmxM.36.6300 | GT1 | 110 | Glucose transporter, potential glucose sensor |
| LmjF.20.1310 | SMP-1 | 108 | Myristolyated protein that stabilizes the flagellar membrane |
| LinJ.31.0910 | AAP3 | 82 | Arginine transporter, potential arginine sensor |
| LmjF.31.0020 | AQP1 | 96 | Involved in drug sensitivity, osmotic response |
| (B) Flagellar membrane proteins in L. mexicana promastigotes identified by proteomics | |||
| LmxM.23.0840 | Hypothetical protein | 121 | 1 TM |
| LmxM.04.0630 | Hypothetical protein | 121 | 1 TM |
| LmxM.08_29.2440 | Cyclic nucleotide phosphodiesterase | 121 | 1 TM |
| LmxM.27.0970 | ABC transporter, putative | 121 | 14 TM |
| LmxM.07.0830 | Hypothetical protein | 121 | 3 TM |
| LmxM.12.0891 | Surface antigen protein 2 | 121 | 1 TM |
| LmxM.29.0850 | Surface protein amastin, putative | 121 | 4 TM |
| LmxM.36.3180 | Receptor-type adenylate cyclase A-like protein | 121 | 1 TM |
| LmxM.04.0190 | Surface antigen-like protein | 121 | 1 TM |
| LmxM.33.0910 | Hypothetical protein | 121 | 1 TM |
| LmxM.36.2590 | Membrane-bound acid phosphatase 2, putative | 121 | 2 TM |
| LmxM.25.2090 | Hypothetical protein | 121 | 1 TM |
| LmxM.10.0390 | GP63, leishmanolysin | 121 | 1 TM |
| LmxM.05.1030 | Hypothetical protein | 121 | 1 TM |
| LmxM.36.2710 | Mitochondrial ATP-dependent zinc metallopeptidase | 121 | 1 TM |
| LmxM.32.0900 | DnaJ chaperone-like protein | 121 | 8 TM |
| LmxM.30.0350 | AATP11, putative amino acid transporter | 121 | 11 TM |
| LmxM.30.2310 | 3′-Nucleotidase/nuclease | 121 | 2 TM |
Many of the proteins in Part A were localized by fluorescence microscopy in the referenced publications, and a few of the T. brucei flagellar membrane proteins were identified by TrypTag (120) as flagellar proteins with predicted TM domains. Part B lists proteins from the flagellar proteome of L. mexicana (121). This list includes all proteins that met the following criteria: ≥1 predicted transmembrane domain; log2 solubility in detergent soluble fraction >0; and enriched in the flagellar versus cell body fraction by log2 ≥ 1. PF and BF refer to expression detected in procyclic forms or bloodstream forms, and TM indicates the number of predicted transmembrane domains.
To detect proteins that may interact with this flagellar targeting domain, both wild type and the nonflagellar Δ84-100 internal deletion mutant of GT1 were tandem affinity tagged, and candidate proteins that associated with the wild-type but not mutant permease were identified by mass spectrometry (111). A novel kinetoplastid protein designated KHARON was identified, and deletion of the corresponding gene prevented trafficking of the GT1-GFP fusion protein to the flagellum. Instead, GT1 arrested in the flagellar pocket (Fig. 6C), the invagination of the plasma membrane at the base of the flagellum where all secreted and integral membrane proteins arrive at the cell surface during biosynthesis (Fig. 1). Hence, KHARON is essential for trafficking of GT1 from the flagellar pocket membrane into the flagellar membrane and thus may mediate passage across a “ciliary gate” or “perciliary barrier” (112–115) at the base of the flagellum that prevents free mixing of the cell body and flagellar membrane components (116). The observation that KHARON is located, in part, at the base of the flagellum places this protein at a position where it could mediate transport across such a barrier. The precise distribution of KHARON along the flagellar axoneme has not been defined at high resolution, but immunoelectron microscopy suggests that it may be located from the basal body into the proximal domains of the axoneme (111). A proteomic study of the T. brucei transition zone did not detect KHARON as a component of that structure (117).
The failure to traffic GT1 into the flagellum was the only defect identified in the Δkharon mutant promastigotes. Strikingly, Δkharon mutant amastigotes failed to undergo cytokinesis and died within several days after entry of the parasites into mammalian macrophages. KHARON is a cytoskeletal protein that is localized in three compartments, the base of the flagellum, the subpellicular microtubules that are attached to the cytosolic side of the plasma membrane, and the mitotic spindle. Several critical questions remain to be answered regarding the role of KHARON and flagellar trafficking. How does this protein, and other partners with which it may interact, mediate passage of GT1 from the flagellar pocket to the flagellar membrane? Are there other flagellar membrane proteins that are targeted to the flagellar membrane by this same machinery? Is flagellar trafficking of some other membrane components critical for amastigote viability, given that GT1 is not expressed in amastigotes (85) and the failure of GT1 to traffic to the flagellum thus cannot explain lethality of the Δkharon mutant in amastigotes? Or, alternatively, is the critical function of KHARON unrelated to flagellar trafficking and mediated by another activity associated with the subpellicular microtubules and/or mitotic spindle?
The KHARON protein appears to be unique to the kinetoplastid parasites and is not identifiable outside this order, but KHARON orthologs are present in all of the sequenced kinetoplastid genomes. Furthermore, bioinformatic analysis has so far failed to identify conserved domains that might suggest structural or functional elements of this unique protein. Molecular genetic studies on the KHARON ortholog from T. brucei (43) demonstrated that it is required for flagellar trafficking of a putative Ca2+ channel, designated FS179 (42) or TbCaCh (43). Furthermore, knockdown of KHARON mRNA was lethal for both BF and PF trypanosomes. In both cases, the flagellum detached from the cell body, as it does for RNAi knockdowns of TbCaCh (42), and parasites failed to undergo cytokinesis, generating multiflagellated, multinucleate cells that subsequently died. It is likely that one reason for the lethal consequences of KHARON knockdown is the failure to traffic TbCaCh to the flagellar membrane, followed by flagellar detachment and failure of cytokinesis, a common but not universal phenotype of mutants that produce flagellar dissociation from the cell body (37). Overall, these studies confirmed that flagellar membrane trafficking by KHARON orthologs is shared among multiple kinetoplastid parasites and different flagellar membrane proteins and that KHARON is an essential protein in these microbes.
Some intriguing explorations relevant to flagellar trafficking have also been undertaken for the receptor adenylate cyclases (78). Four ACs that are expressed in PF trypanosomes were localized using either epitope tags or selectively reactive antibodies (Fig. 6A). ACP1 and ACP4 were located at the anterior tip of the flagellum, ACP5 was concentrated at the tip but also present along the length of the flagellum, and ACP2 was distributed more or less uniformly along the length. Despite their difference in flagellar distribution, ACP1 and ACP2 were almost identical in sequence except for their C-terminal 45 or 46 amino acids, suggesting that this region was critical for organellar targeting. Only five of these C-terminal residues were conserved between the two tip-specific ACs, ACP1 and ACP4, and these amino acids are thus likely to confer localization to the flagellar tip. The distinct localizations of multiple ACs suggests that flagellar subdomains exist, both with regard to membrane protein composition and the likely signaling function (79, 118) of these integral membrane proteins. Whether differential flagellar distribution represents tethering or active targeting remains to be determined.
APPROACHING A COMPREHENSIVE LIST OF FLAGELLAR MEMBRANE PROTEINS
Many flagellar functions depend upon the activities of proteins that are selectively localized to the flagellar membrane. Several examples discussed above include: the GT1 glucose transporter, the AQP1 aquaporin, the TbCaCh calcium channel, various ACs in trypanosomes, the CaBPs of T. brucei and T. cruzi, the AAP3 arginine permease of L. donovani, and the small acetylated protein SMP-1 in L. mexicana (108, 119). These and other established flagellar membrane proteins are tabulated in Table 1, part A. A comprehensive proteome-wide localization of proteins in PF trypanosomes was undertaken recently by the TrypTag consortium (120), and the results made accessible at http://tryptag.org. This global undertaking has identified several other proteins that are preferentially localized to the flagellar membrane of these insect stage parasites, and these are also listed in Table 1, part A. Most recently, a proteomic analysis of flagella isolated from L. mexicana promastigotes (121) has implicated another group of proteins as likely components of the flagellar membrane, listed in Table 1, part B. These and earlier (42, 122) global approaches have been especially welcome, since the piecemeal identification of flagellar membrane proteins has been relatively slow and arduous, and these “genome scale” undertakings offer the prospect of cataloging a comprehensive “parts list” of the flagellar membrane. Notable among the newer arrivals in this company are additional putative transporters, but there are also many proteins of unknown function that will present both challenges and opportunities to define novel functions of the flagellar surface. What is required now are functional studies to dissect the activities of individual candidates. The ability to rapidly generate gene knockouts or RNAi-based knockdowns, especially with CRISPR-Cas technology (123), facilitates such investigations. However, application of a range of sometimes imaginative phenotypic tests will likely be necessary to maximize the yield in terms of biological insights.
SUMMARY AND FUTURE DIRECTIONS
The flagellar surface carries out a multiplicity of functions that are critical for the ability of kinetoplastid parasites to successfully colonize their insect vectors and vertebrate hosts. Functions for flagella and their surfaces identified to date include the following: motility, attachment to host tissues, secretion of parasite material into hosts, establishing cell morphology and initiation of cytokinesis, sensing the extracellular environment, and altering the host immune response. Some of these flagellar mediated microbe-host interactions have been appreciated for decades, but many of them have emerged recently. Much remains to be learned about how these processes work, especially at the molecular level.
Similarly, sensory functions have been suggested for parasite flagella, consistent with the demonstrated sensory activities of cilia and flagella in other eukaryotes. However, it is not clear precisely what pathways engage in flagellar sensing or what the components are that constitute the sensory pathways. Perhaps the best characterized sensory response to date is that for the arginine deprivation in L. donovani, where a flagellar arginine transporter and a MAP kinase have been implicated (82), but there are certainly other components that must constitute this pathway. A major mystery continues to surround the presumed function of flagellar ACs in sensing. The structure of these proteins suggests that the large extracellular domains are likely to be involved in binding ligands that stimulate AC activity, but no such ligands have been identified and the mechanisms for regulating AC activity are obscure.
Our understanding of trafficking of flagellar membrane proteins to that organelle is rudimentary, with one pathway for dually acylated proteins engaging lipids rafts and probably some unknown flagellar proteins and another pathway dependent upon the cytoskeletal protein KHARON. It is not yet clear how many flagellar membrane proteins may access each of these pathways, nor is it known mechanistically how proteins like KHARON address membrane proteins to the flagellum. How many distinct mechanisms there may be for flagellar membrane trafficking is also currently unknown.
It is likely that secretion from the flagellar membrane is more widely active than the limited number of currently known examples might suggest. Do intracellular parasites export proteins to their host cells via flagellar pathways, and if so, what molecular components are exported and to what purpose?
Finally, cilia and flagella are organelles present in various other types of eukaryotic pathogen. Notable examples include protists such as Giardia (124) and Trichomonas (125) and male gametes of Plasmodium (126). Metazoan pathogens, including nematodes and flatworms, have differentiated cells that are ciliated (127), such as neurons. Hence, it is likely that flagella and their surfaces will prove to be broadly important for microbe-host interactions across a wide range of eukaryotic pathogens. To date, these organelles have been best studied in the kinetoplastid parasites, but the lessons being learned in these hemoflagellates may prove to be instructive for other pathogens.
It has not been possible in this review to reference every article of relevance to the topic. We apologize to any authors whose work may have been overlooked inadvertently or not cited due to the necessity to be selective.
ACKNOWLEDGMENT
Research in the Landfear laboratory relevant to this topic has been supported by a grant from the National Institutes of Health (AI121160).
Biographies

Felice D. Kelly obtained her B.S. in Biology and Psychology from Bradley University, in Peoria, IL. She then studied transposon insertion preferences with Dr. Henry Levin at the National Institutes of Health as part of their postbaccalaureate intramural program. For her Ph.D. work at The Rockefeller University, she identified genetic determinants of fission yeast cell shape with Dr. Paul Nurse. In her postdoctoral studies with Dr. John Boothroyd at Stanford University School of Medicine she determined molecular mechanisms of Toxoplasma gondii interactions with the host cell mitochondria. Currently, she is studying the control of intracellular protein trafficking and cell division in Leishmania mexicana at Oregon Health and Sciences University.

Marco A. Sanchez obtained his B.S. in chemistry from the National University of Mexico. Later, he obtained his M.S. in biochemistry from the National Polytechnic Institute in Mexico City. His Ph.D. research on the molecular biology of Entamoeba histolytica was performed at CINVESTAV-Mexico and Harvard University, where he identified, cloned, and characterized the alpha-tubulin genes from E. histolytica. Then, he moved to the Oregon Health and Science University to conduct postdoctoral training on the molecular biology and biochemistry of receptor adenylate cyclases in Leishmania donovani. He has transitioned to different positions at the OHSU investigating the molecular biology, genetics, and biochemistry of nucleoside/nucleobase, glucose, and pyruvate plasma membrane transporters in L. major and Trypanosoma brucei. Currently, he is a Staff Scientist at OHSU engaged in studying fundamental biological processes in T. brucei, especially on how an essential cytoskeletal protein, KHARON, is involved in a plethora of cellular functions, among them, trafficking of flagellar membrane proteins to the flagellum, mitotic spindle formation and stability, cellular morphology, and cytokinesis.

Scott M. Landfear obtained his B.A. in chemistry from the University of Chicago. He then received his M.A. and Ph.D. in biochemistry from Harvard University, studying the mechanism of allosteric regulation of bacterial aspartate transcarbamoylase. He subsequently did postdoctoral work at the Massachusetts Institute of Technology with Dr. Harvey Lodish, working on the molecular biology of development in Dictyostelium discoideum, and at the Harvard School of Public Health with Dr. Dyann Wirth, studying gene expression in Leishmania parasites. He is University Professor at the Oregon Health and Science University, where he studies the molecular and cell biology of parasitic protozoa including Leishmania parasites and Trypanosoma brucei.
REFERENCES
- 1.Berbari NF, O’Connor AK, Haycraft CJ, Yoder BK. 2009. The primary cilium as a complex signaling center. Curr Biol 19:R526–R535. doi: 10.1016/j.cub.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloodgood RA. 2010. Sensory reception is an attribute of both primary cilia and motile cilia. J Cell Sci 123:505–509. doi: 10.1242/jcs.066308. [DOI] [PubMed] [Google Scholar]
- 3.Pazour GJ, Witman GB. 2003. The vertebrate primary cilium is a sensory organelle. Curr Opin Cell Biol 15:105–110. doi: 10.1016/s0955-0674(02)00012-1. [DOI] [PubMed] [Google Scholar]
- 4.Singla V, Reiter JF. 2006. The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science 313:629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- 5.Wood CR, Huang K, Diener DR, Rosenbaum JL. 2013. The cilium secretes bioactive ectosomes. Curr Biol 23:906–911. doi: 10.1016/j.cub.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langousis G, Hill KL. 2014. Motility and more: the flagellum of Trypanosoma brucei. Nat Rev Microbiol 12:505–518. doi: 10.1038/nrmicro3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ralston KS, Kabututu ZP, Melehani JH, Oberholzer M, Hill KL. 2009. The Trypanosoma brucei flagellum: moving parasites in new directions. Annu Rev Microbiol 63:335–362. doi: 10.1146/annurev.micro.091208.073353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris EH. 2001. Chlamydomonas as a model organism. Annu Rev Plant Physiol Plant Mol Biol 52:363–406. doi: 10.1146/annurev.arplant.52.1.363. [DOI] [PubMed] [Google Scholar]
- 9.Kohl L, Bastin P. 2005. The flagellum of trypanosomes. Int Rev Cytol 244:227–285. doi: 10.1016/S0074-7696(05)44006-1. [DOI] [PubMed] [Google Scholar]
- 10.Vaughan S, Gull K. 2003. The trypanosome flagellum. J Cell Sci 116:757–759. doi: 10.1242/jcs.00287. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Q, Liu B, Sun Y, He CY. 2011. A coiled-coil- and C2-domain-containing protein is required for FAZ assembly and cell morphology in Trypanosoma brucei. J Cell Sci 124:3848–3858. doi: 10.1242/jcs.087676. [DOI] [PubMed] [Google Scholar]
- 12.Kohl L, Robinson D, Bastin P. 2003. Novel roles for the flagellum in cell morphogenesis and cytokinesis of trypanosomes. EMBO J 22:5336–5346. doi: 10.1093/emboj/cdg518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wheeler RJ, Gluenz E, Gull K. 2011. The cell cycle of Leishmania: morphogenetic events and their implications for parasite biology. Mol Microbiol 79:647–662. doi: 10.1111/j.1365-2958.2010.07479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wheeler RJ. 2017. Use of chiral cell shape to ensure highly directional swimming in trypanosomes. PLoS Comput Biol 13:e1005353. doi: 10.1371/journal.pcbi.1005353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bargul JL, Jung J, McOdimba FA, Omogo CO, Adung’a VO, Krüger T, Masiga DK, Engstler M. 2016. Species-specific adaptations of trypanosome morphology and motility to the mammalian host. PLoS Pathog 12:e1005448. doi: 10.1371/journal.ppat.1005448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimogawa MM, Ray SS, Kisalu N, Zhang Y, Geng Q, Ozcan A, Hill KL. 2018. Parasite motility is critical for virulence of African trypanosomes. Sci Rep 8:9122. doi: 10.1038/s41598-018-27228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw S, DeMarco SF, Rehmann R, Wenzler T, Florini F, Roditi I, Hill KL. 2019. Flagellar cAMP signaling controls trypanosome progression through host tissues. Nat Commun 10:803. doi: 10.1038/s41467-019-08696-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffiths S, Portman N, Taylor PR, Gordon S, Ginger ML, Gull K. 2007. RNA interference mutant induction in vivo demonstrates the essential nature of trypanosome flagellar function during mammalian infection. Eukaryot Cell 6:1248–1250. doi: 10.1128/EC.00110-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun SY, Kaelber JT, Chen M, Dong X, Nematbakhsh Y, Shi J, Dougherty M, Lim CT, Schmid MF, Chiu W, He CY. 2018. Flagellum couples cell shape to motility in Trypanosoma brucei. Proc Natl Acad Sci U S A 115:E5916–E5925. doi: 10.1073/pnas.1722618115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cuvillier A, Redon F, Antoine JC, Chardin P, DeVos T, Merlin G. 2000. LdARL-3A, a Leishmania promastigote-specific ADP-ribosylation factor-like protein, is essential for flagellum integrity. J Cell Sci 113:2065–2074. [DOI] [PubMed] [Google Scholar]
- 21.Cuvillier A, Miranda JC, Ambit A, Barral A, Merlin G. 2003. Abortive infection of Lutzomyia longipalpis insect vectors by aflagellated LdARL-3A-Q70L overexpressing Leishmania amazonensis parasites. Cell Microbiol 5:717–728. doi: 10.1046/j.1462-5822.2003.00316.x. [DOI] [PubMed] [Google Scholar]
- 22.Zauli RC, Yokoyama-Yasunaka JK, Miguel DC, Moura AS, Pereira L, da Silva IA Jr, Lemes LG, Dorta ML, de Oliveira MA, Pitaluga AN, Ishikawa EA, Rodrigues JC, Traub-Cseko YM, Bijovsky AT, Ribeiro-Dias F, Uliana SR. 2012. A dysflagellar mutant of Leishmania (Viannia) braziliensis isolated from a cutaneous leishmaniasis patient. Parasit Vectors 5:11. doi: 10.1186/1756-3305-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Killick-Kendrick R, Molyneux DH, Ashford RW. 1974. Leishmania in phlebotomid sandflies. I. Modifications of the flagellum associated with attachment to the mid-gut and oesophageal valve of the sandfly. Proc R Soc Lond B Biol Sci 187:409–419. doi: 10.1098/rspb.1974.0085. [DOI] [PubMed] [Google Scholar]
- 24.Kamhawi S. 2006. Phlebotomine sand flies and Leishmania parasites: friends or foes? Trends Parasitol 22:439–445. doi: 10.1016/j.pt.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 25.Volf P, Hajmova M, Sadlova J, Votypka J. 2004. Blocked stomodeal valve of the insect vector: similar mechanism of transmission in two trypanosomatid models. Int J Parasitol 34:1221–1227. doi: 10.1016/j.ijpara.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Tetley L, Vickerman K. 1985. Differentiation in Trypanosoma brucei: host-parasite cell junctions and their persistence during acquisition of the variable antigen coat. J Cell Sci 74:1–19. [DOI] [PubMed] [Google Scholar]
- 27.Rotureau B, Subota I, Buisson J, Bastin P. 2012. A new asymmetric division contributes to the continuous production of infective trypanosomes in the tsetse fly. Development 139:1842–1850. doi: 10.1242/dev.072611. [DOI] [PubMed] [Google Scholar]
- 28.Filosa JN, Berry CT, Ruthel G, Beverley SM, Warren WC, Tomlinson C, Myler PJ, Dudkin EA, Povelones ML, Povelones M. 2019. Dramatic changes in gene expression in different forms of Crithidia fasciculata reveal potential mechanisms for insect-specific adhesion in kinetoplastid parasites. PLoS Negl Trop Dis 13:e0007570. doi: 10.1371/journal.pntd.0007570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gluenz E, Hoog JL, Smith AE, Dawe HR, Shaw MK, Gull K. 2010. Beyond 9 + 0: noncanonical axoneme structures characterize sensory cilia from protists to humans. FASEB J 24:3117–3121. doi: 10.1096/fj.09-151381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wheway G, Nazlamova L, Hancock JT. 2018. Signaling through the primary cilium. Front Cell Dev Biol 6:8. doi: 10.3389/fcell.2018.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lentini G, Dos Santos Pacheco N, Burleigh BA. 2018. Targeting host mitochondria: a role for the Trypanosoma cruzi amastigote flagellum. Cell Microbiol 20:e12807. doi: 10.1111/cmi.12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sunter JD, Gull K. 2016. The flagellum attachment zone: “the cellular ruler” of trypanosome morphology. Trends Parasitol 32:309–324. doi: 10.1016/j.pt.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson DR, Sherwin T, Ploubidou A, Byard EH, Gull K. 1995. Microtubule polarity and dynamics in the control of organelle positioning, segregation, and cytokinesis in the trypanosome cell cycle. J Cell Biol 128:1163–1172. doi: 10.1083/jcb.128.6.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Q, Hu H, He CY, Li Z. 2015. Assembly and maintenance of the flagellum attachment zone filament in Trypanosoma brucei. J Cell Sci 128:2361–2372. doi: 10.1242/jcs.168377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooper R, Inverso JA, Espinosa M, Nogueira N, Cross GA. 1991. Characterization of a candidate gene for GP72, an insect stage-specific antigen of Trypanosoma cruzi. Mol Biochem Parasitol 49:45–59. doi: 10.1016/0166-6851(91)90129-t. [DOI] [PubMed] [Google Scholar]
- 36.Haynes PA, Russell DG, Cross GA. 1996. Subcellular localization of Trypanosoma cruzi glycoprotein Gp72. J Cell Sci 109:2979–2988. [DOI] [PubMed] [Google Scholar]
- 37.LaCount DJ, Barrett B, Donelson JE. 2002. Trypanosoma brucei FLA1 is required for flagellum attachment and cytokinesis. J Biol Chem 277:17580–17588. doi: 10.1074/jbc.M200873200. [DOI] [PubMed] [Google Scholar]
- 38.LaCount DJ, Bruse S, Hill KL, Donelson JE. 2000. Double-stranded RNA interference in Trypanosoma brucei using head-to-head promoters. Mol Biochem Parasitol 111:67–76. doi: 10.1016/s0166-6851(00)00300-5. [DOI] [PubMed] [Google Scholar]
- 39.Sun SY, Wang C, Yuan YA, He CY. 2013. An intracellular membrane junction consisting of flagellum adhesion glycoproteins links flagellum biogenesis to cell morphogenesis in Trypanosoma brucei. J Cell Sci 126:520–531. doi: 10.1242/jcs.113621. [DOI] [PubMed] [Google Scholar]
- 40.Woods K, Nic a’Bhaird N, Dooley C, Perez-Morga D, Nolan DP. 2013. Identification and characterization of a stage specific membrane protein involved in flagellar attachment in Trypanosoma brucei. PLoS One 8:e52846. doi: 10.1371/journal.pone.0052846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sunter JD, Varga V, Dean S, Gull K. 2015. A dynamic coordination of flagellum and cytoplasmic cytoskeleton assembly specifies cell morphogenesis in trypanosomes. J Cell Sci 128:1580–1594. doi: 10.1242/jcs.166447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oberholzer M, Langousis G, Nguyen HT, Saada EA, Shimogawa MM, Jonsson ZO, Nguyen SM, Wohlschlegel JA, Hill KL. 2011. Independent analysis of the flagellum surface and matrix proteomes provides insight into flagellum signaling in mammalian-infectious Trypanosoma brucei. Mol Cell Proteomics 10:M111.010538. doi: 10.1074/mcp.M111.010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanchez MA, Tran KD, Valli J, Hobbs S, Johnson E, Gluenz E, Landfear SM. 2016. KHARON is an essential cytoskeletal protein involved in the trafficking of flagellar membrane proteins and cell division in African trypanosomes. J Biol Chem 291:19760–19773. doi: 10.1074/jbc.M116.739235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vickerman K. 1969. On the surface coat and flagellar adhesion in trypanosomes. J Cell Sci 5:163–193. [DOI] [PubMed] [Google Scholar]
- 45.Rotureau B, Blisnick T, Subota I, Julkowska D, Cayet N, Perrot S, Bastin P. 2014. Flagellar adhesion in Trypanosoma brucei relies on interactions between different skeletal structures in the flagellum and cell body. J Cell Sci 127:204–215. doi: 10.1242/jcs.136424. [DOI] [PubMed] [Google Scholar]
- 46.Sunter JD, Benz C, Andre J, Whipple S, McKean PG, Gull K, Ginger ML, Lukes J. 2015. Modulation of flagellum attachment zone protein FLAM3 and regulation of the cell shape in Trypanosoma brucei life cycle transitions. J Cell Sci 128:3117–3130. doi: 10.1242/jcs.171645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sunter JD, Yanase R, Wang Z, Catta-Preta CMC, Moreira-Leite F, Myskova J, Pruzinova K, Volf P, Mottram JC, Gull K. 2019. Leishmania flagellum attachment zone is critical for flagellar pocket shape, development in the sand fly, and pathogenicity in the host. Proc Natl Acad Sci U S A 116:6351–6360. doi: 10.1073/pnas.1812462116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weise F, Stierhof YD, Kühn C, Wiese M, Overath P. 2000. Distribution of GPI-anchored proteins in the protozoan parasite Leishmania, based on an improved ultrastructural description using high-pressure frozen cells. J Cell Sci 113:4587–4603. [DOI] [PubMed] [Google Scholar]
- 49.Wheeler RJ, Sunter JD, Gull K. 2016. Flagellar pocket restructuring through the Leishmania life cycle involves a discrete flagellum attachment zone. J Cell Sci 129:854–867. doi: 10.1242/jcs.183152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moreira-Leite FF, Sherwin T, Kohl L, Gull K. 2001. A trypanosome structure involved in transmitting cytoplasmic information during cell division. Science 294:610–612. doi: 10.1126/science.1063775. [DOI] [PubMed] [Google Scholar]
- 51.Briggs LJ, McKean PG, Baines A, Moreira-Leite F, Davidge J, Vaughan S, Gull K. 2004. The flagella connector of Trypanosoma brucei: an unusual mobile transmembrane junction. J Cell Sci 117:1641–1651. doi: 10.1242/jcs.00995. [DOI] [PubMed] [Google Scholar]
- 52.Hoog JL, Lacomble S, Bouchet-Marquis C, Briggs L, Park K, Hoenger A, Gull K. 2016. 3D architecture of the Trypanosoma brucei flagella connector, a mobile transmembrane junction. PLoS Negl Trop Dis 10:e0004312. doi: 10.1371/journal.pntd.0004312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McAllaster MR, Ikeda KN, Lozano-Nunez A, Anrather D, Unterwurzacher V, Gossenreiter T, Perry JA, Crickley R, Mercadante CJ, Vaughan S, de Graffenried CL. 2015. Proteomic identification of novel cytoskeletal proteins associated with TbPLK, an essential regulator of cell morphogenesis in Trypanosoma brucei. Mol Biol Cell 26:3013–3029. doi: 10.1091/mbc.E15-04-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Varga V, Moreira-Leite F, Portman N, Gull K. 2017. Protein diversity in discrete structures at the distal tip of the trypanosome flagellum. Proc Natl Acad Sci U S A 114:E6546–E6555. doi: 10.1073/pnas.1703553114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hughes L, Towers K, Starborg T, Gull K, Vaughan S. 2013. A cell-body groove housing the new flagellum tip suggests an adaptation of cellular morphogenesis for parasitism in the bloodstream form of Trypanosoma brucei. J Cell Sci 126:5748–5757. doi: 10.1242/jcs.139139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jenni L, Marti S, Schweizer J, Betschart B, Le Page RW, Wells JM, Tait A, Paindavoine P, Pays E, Steinert M. 1986. Hybrid formation between African trypanosomes during cyclical transmission. Nature 322:173–175. doi: 10.1038/322173a0. [DOI] [PubMed] [Google Scholar]
- 57.Peacock L, Ferris V, Sharma R, Sunter J, Bailey M, Carrington M, Gibson W. 2011. Identification of the meiotic life cycle stage of Trypanosoma brucei in the tsetse fly. Proc Natl Acad Sci U S A 108:3671–3676. doi: 10.1073/pnas.1019423108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gibson W, Peacock L, Ferris V, Williams K, Bailey M. 2008. The use of yellow fluorescent hybrids to indicate mating in Trypanosoma brucei. Parasit Vectors 1:4. doi: 10.1186/1756-3305-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peacock L, Bailey M, Carrington M, Gibson W. 2014. Meiosis and haploid gametes in the pathogen Trypanosoma brucei. Curr Biol 24:181–186. doi: 10.1016/j.cub.2013.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pan J, Snell WJ. 2000. Signal transduction during fertilization in the unicellular green alga, Chlamydomonas. Curr Opin Microbiol 3:596–602. doi: 10.1016/s1369-5274(00)00146-6. [DOI] [PubMed] [Google Scholar]
- 61.Inbar E, Shaik J, Iantorno SA, Romano A, Nzelu CO, Owens K, Sanders MJ, Dobson D, Cotton JA, Grigg ME, Beverley SM, Sacks D. 2019. Whole-genome sequencing of experimental hybrids supports meiosis-like sexual recombination in Leishmania. PLoS Genet 15:e1008042. doi: 10.1371/journal.pgen.1008042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Romano A, Inbar E, Debrabant A, Charmoy M, Lawyer P, Ribeiro-Gomes F, Barhoumi M, Grigg M, Shaik J, Dobson D, Beverley SM, Sacks DL. 2014. Cross-species genetic exchange between visceral and cutaneous strains of Leishmania in the sand fly vector. Proc Natl Acad Sci U S A 111:16808–16813. doi: 10.1073/pnas.1415109111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Imhof S, Fragoso C, Hemphill A, von Schubert C, Li D, Legant W, Betzig E, Roditi I. 2016. Flagellar membrane fusion and protein exchange in trypanosomes: a new form of cell-cell communication? F1000Res 5:682. doi: 10.12688/f1000research.8249.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dong G, Filho AL, Olivier M. 2019. Modulation of host-pathogen communication by extracellular vesicles (EVs) of the protozoan parasite Leishmania. Front Cell Infect Microbiol 9:100. doi: 10.3389/fcimb.2019.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Silverman JM, Clos J, de’Oliveira CC, Shirvani O, Fang Y, Wang C, Foster LJ, Reiner NE. 2010. An exosome-based secretion pathway is responsible for protein export from Leishmania and communication with macrophages. J Cell Sci 123:842–852. doi: 10.1242/jcs.056465. [DOI] [PubMed] [Google Scholar]
- 66.Baudieri B, Tomassini N. 1962. Fine struttura dei tripanosomi. Parassitologia 4:89–95. [Google Scholar]
- 67.Ellis DS, Ormerod WE, Lumsden W. 1976. Filaments of “Trypanosoma brucei”: some notes on differences in origin and structure in two strains of “Trypanosoma (Trypanozoon) brucei rhodesiense.” Acta Tropica 33:151–168. [PubMed] [Google Scholar]
- 68.Molloy JO, Ormerod WE. 1964. A fibril emerging from the posterior end of Trypanosoma rhodesiense. Philos Trans R Soc Lond B Biol Sci 58:2. [Google Scholar]
- 69.Schepilewsky E. 1912. Fadenförmige anhängsel bei den trypanosomen. Zentralbl Bakteriol 65:79–83. [Google Scholar]
- 70.Vickerman K, Luckins AG. 1969. Localization of variable antigens in the surface coat of Trypanosoma brucei using ferritin conjugated antibody. Nature 224:1125–1126. doi: 10.1038/2241125a0. [DOI] [PubMed] [Google Scholar]
- 71.Wright KA, Lumsden WH, Hales H. 1970. The formation of filopodium-like processes by Trypanosoma. J Cell Sci 6:285–297. [DOI] [PubMed] [Google Scholar]
- 72.Szempruch AJ, Dennison L, Kieft R, Harrington JM, Hajduk SL. 2016. Sending a message: extracellular vesicles of pathogenic protozoan parasites. Nat Rev Microbiol 14:669–675. doi: 10.1038/nrmicro.2016.110. [DOI] [PubMed] [Google Scholar]
- 73.Szempruch AJ, Sykes SE, Kieft R, Dennison L, Becker AC, Gartrell A, Martin WJ, Nakayasu ES, Almeida IC, Hajduk SL, Harrington JM. 2016. Extracellular vesicles from Trypanosoma brucei mediate virulence factor transfer and cause host anemia. Cell 164:246–257. doi: 10.1016/j.cell.2015.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baldari CT, Rosenbaum J. 2010. Intraflagellar transport: it’s not just for cilia anymore. Curr Opin Cell Biol 22:75–80. doi: 10.1016/j.ceb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Avasthi P, Marshall W. 2013. Ciliary secretion: switching the cellular antenna to ‘transmit’. Curr Biol 23:R471–R473. doi: 10.1016/j.cub.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 76.Atayde VD, Aslan H, Townsend S, Hassani K, Kamhawi S, Olivier M. 2015. Exosome secretion by the parasitic protozoan Leishmania within the sand fly midgut. Cell Rep 13:957–967. doi: 10.1016/j.celrep.2015.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oberholzer M, Lopez MA, McLelland BT, Hill KL. 2010. Social motility in African trypanosomes. PLoS Pathog 6:e1000739. doi: 10.1371/journal.ppat.1000739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saada EA, Kabututu ZP, Lopez M, Shimogawa MM, Langousis G, Oberholzer M, Riestra A, Jonsson ZO, Wohlschlegel JA, Hill KL. 2014. Insect stage-specific receptor adenylate cyclases are localized to distinct subdomains of the Trypanosoma brucei flagellar membrane. Eukaryot Cell 13:1064–1076. doi: 10.1128/EC.00019-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lopez MA, Saada EA, Hill KL. 2015. Insect stage-specific adenylate cyclases regulate social motility in African trypanosomes. Eukaryot Cell 14:104–112. doi: 10.1128/EC.00217-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oberholzer M, Marti G, Baresic M, Kunz S, Hemphill A, Seebeck T. 2007. The Trypanosoma brucei cAMP phosphodiesterases TbrPDEB1 and TbrPDEB2: flagellar enzymes that are essential for parasite virulence. FASEB J 21:720–731. doi: 10.1096/fj.06-6818com. [DOI] [PubMed] [Google Scholar]
- 81.Schuster S, Kruger T, Subota I, Thusek S, Rotureau B, Beilhack A, Engstler M. 2017. Developmental adaptations of trypanosome motility to the tsetse fly host environments unravel a multifaceted in vivo microswimmer system. Elife 6:e27656. doi: 10.7554/eLife.27656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Goldman-Pinkovich A, Balno C, Strasser R, Zeituni-Molad M, Bendelak K, Rentsch D, Ephros M, Wiese M, Jardim A, Myler PJ, Zilberstein D. 2016. An arginine deprivation response pathway Is induced in Leishmania during macrophage invasion. PLoS Pathog 12:e1005494. doi: 10.1371/journal.ppat.1005494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Laranjeira-Silva MF, Wang W, Samuel TK, Maeda FY, Michailowsky V, Hamza I, Liu Z, Andrews NW. 2018. A MFS-like plasma membrane transporter required for Leishmania virulence protects the parasites from iron toxicity. PLoS Pathog 14:e1007140. doi: 10.1371/journal.ppat.1007140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martin JL, Yates PA, Soysa R, Alfaro JF, Yang F, Burnum-Johnson KE, Petyuk VA, Weitz KK, Camp DG 2nd, Smith RD, Wilmarth PA, David LL, Ramasamy G, Myler PJ, Carter NS. 2014. Metabolic reprogramming during purine stress in the protozoan pathogen Leishmania donovani. PLoS Pathog 10:e1003938. doi: 10.1371/journal.ppat.1003938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rodriguez-Contreras D, Aslan H, Feng X, Tran K, Yates PA, Kamhawi S, Landfear S. 2015. Regulation and biological function of a flagellar glucose transporter in Leishmania mexicana: a potential glucose sensor. FASEB J 29:11–24. doi: 10.1096/fj.14-251991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Diallinas G. 2017. Transceptors as a functional link of transporters and receptors. Microb Cell 4:69–73. doi: 10.15698/mic2017.03.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schothorst J, Zeebroeck GV, Thevelein JM. 2017. Identification of Ftr1 and Zrt1 as iron and zinc micronutrient transceptors for activation of the PKA pathway in Saccharomyces cerevisiae. Microb Cell 4:74–89. doi: 10.15698/mic2017.03.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thevelein JM, Voordeckers K. 2009. Functioning and evolutionary significance of nutrient transceptors. Mol Biol Evol 26:2407–2414. doi: 10.1093/molbev/msp168. [DOI] [PubMed] [Google Scholar]
- 89.Schlein Y. 1986. Sandfly diet and Leishmania. Parasitol Today 2:175–177. doi: 10.1016/0169-4758(86)90150-x. [DOI] [PubMed] [Google Scholar]
- 90.Piper RC, Xu X, Russell DG, Little BM, Landfear SM. 1995. Differential targeting of two glucose transporters from Leishmania enriettii is mediated by an NH2-terminal domain. J Cell Biol 128:499–508. doi: 10.1083/jcb.128.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tran KD, Rodriguez-Contreras D, Shinde U, Landfear SM. 2012. Both sequence and context are important for flagellar targeting of a glucose transporter. J Cell Sci 125:3293–3298. doi: 10.1242/jcs.103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shaked-Mishan P, Suter-Grotemeyer M, Yoel-Almagor T, Holland N, Zilberstein D, Rentsch D. 2006. A novel high-affinity arginine transporter from the human parasitic protozoan Leishmania donovani. Mol Microbiol 60:30–38. doi: 10.1111/j.1365-2958.2006.05060.x. [DOI] [PubMed] [Google Scholar]
- 93.Darlyuk I, Goldman A, Roberts SC, Ullman B, Rentsch D, Zilberstein D. 2009. Arginine homeostasis and transport in the human pathogen Leishmania donovani. J Biol Chem 284:19800–19807. doi: 10.1074/jbc.M901066200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pawar H, Puri M, Fischer Weinberger R, Madhubala R, Zilberstein D. 2019. The arginine sensing and transport binding sites are distinct in the human pathogen Leishmania. PLoS Negl Trop Dis 13:e0007304. doi: 10.1371/journal.pntd.0007304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gourbal B, Sonuc N, Bhattacharjee H, Legare D, Sundar S, Ouellette M, Rosen BP, Mukhopadhyay R. 2004. Drug uptake and modulation of drug resistance in Leishmania by an aquaglyceroporin. J Biol Chem 279:31010–31017. doi: 10.1074/jbc.M403959200. [DOI] [PubMed] [Google Scholar]
- 96.Figarella K, Uzcategui NL, Zhou Y, LeFurgey A, Ouellette M, Bhattacharjee H, Mukhopadhyay R. 2007. Biochemical characterization of Leishmania major aquaglyceroporin LmAQP1: possible role in volume regulation and osmotaxis. Mol Microbiol 65:1006–1017. doi: 10.1111/j.1365-2958.2007.05845.x. [DOI] [PubMed] [Google Scholar]
- 97.Leslie G, Barrett M, Burchmore R. 2002. Leishmania mexicana: promastigotes migrate through osmotic gradients. Exp Parasitol 102:117–120. doi: 10.1016/s0014-4894(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 98.Paindavoine P, Rolin S, Van Assel S, Geuskens M, Jauniaux J, Dinsart C, Huet G, Pays E. 1992. A gene from the variant surface glycoprotein expression site encodes one of several transmembrane adenylate cyclases located on the flagellum of Trypanosoma brucei. Mol Cell Biol 12:1218–1225. doi: 10.1128/mcb.12.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Salmon D, Vanwalleghem G, Morias Y, Denoeud J, Krumbholz C, Lhomme F, Bachmaier S, Kador M, Gossmann J, Dias FB, De Muylder G, Uzureau P, Magez S, Moser M, De Baetselier P, Van Den Abbeele J, Beschin A, Boshart M, Pays E. 2012. Adenylate cyclases of Trypanosoma brucei inhibit the innate immune response of the host. Science 337:463–466. doi: 10.1126/science.1222753. [DOI] [PubMed] [Google Scholar]
- 100.Emmer BT, Daniels MD, Taylor JM, Epting CL, Engman DM. 2010. Calflagin inhibition prolongs host survival and suppresses parasitemia in Trypanosoma brucei infection. Eukaryot Cell 9:934–942. doi: 10.1128/EC.00086-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Engman DM, Krause K-H, Blumin JH, Kim KS, Kirchhoff LV, Donelson JE. 1989. A novel flagellar Ca+2-binding protein in trypanosomes. J Biol Chem 264:18627–18631. [PubMed] [Google Scholar]
- 102.Buchanan KT, Ames JB, Asfaw SH, Wingard JN, Olson CL, Campana PT, Araujo AP, Engman DM. 2005. A flagellum-specific calcium sensor. J Biol Chem 280:40104–40111. doi: 10.1074/jbc.M505777200. [DOI] [PubMed] [Google Scholar]
- 103.Godsel LM, Engman DM. 1999. Flagellar protein localization mediated by a calcium-myristoyl/palmitoyl switch mechanism. EMBO J 18:2057–2065. doi: 10.1093/emboj/18.8.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Emmer BT, Maric D, Engman DM. 2010. Molecular mechanisms of protein and lipid targeting to ciliary membranes. J Cell Sci 123:529–536. doi: 10.1242/jcs.062968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tyler KM, Fridberg A, Toriello KM, Olson CL, Cieslak JA, Hazlett TL, Engman DM. 2009. Flagellar membrane localization via association with lipid rafts. J Cell Sci 122:859–866. doi: 10.1242/jcs.037721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Maric D, McGwire BS, Buchanan KT, Olson CL, Emmer BT, Epting CL, Engman DM. 2011. Molecular determinants of ciliary membrane localization of Trypanosoma cruzi flagellar calcium-binding protein. J Biol Chem 286:33109–33117. doi: 10.1074/jbc.M111.240895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maric D, Epting CL, Engman DM. 2010. Composition and sensory function of the trypanosome flagellar membrane. Curr Opin Microbiol 13:466–472. doi: 10.1016/j.mib.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tull D, Vince JE, Callaghan JM, Naderer T, Spurck T, McFadden GI, Currie G, Ferguson K, Bacic A, McConville MJ. 2004. SMP-1, a member of a new family of small myristoylated proteins in kinetoplastid parasites, is targeted to the flagellum membrane in Leishmania. Mol Biol Cell 15:4775–4786. doi: 10.1091/mbc.e04-06-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rosenbaum J. 2002. Intraflagellar transport. Curr Biol 12:R125. doi: 10.1016/s0960-9822(02)00703-0. [DOI] [PubMed] [Google Scholar]
- 110.Burchmore RJS, Rodriguez-Contreras D, McBride K, Merkel P, Barrett MP, Modi G, Sacks DL, Landfear SM. 2003. Genetic characterization of glucose transporter function in Leishmania mexicana. Proc Natl Acad Sci U S A 100:3901–3906. doi: 10.1073/pnas.0630165100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tran KD, Rodriguez-Contreras D, Vieira DP, Yates PA, David L, Beatty W, Elferich J, Landfear SM. 2013. KHARON1 mediates flagellar targeting of a glucose transporter in Leishmania mexicana and is critical for viability of infectious intracellular amastigotes. J Biol Chem 288:22721–22733. doi: 10.1074/jbc.M113.483461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Garcia-Gonzalo FR, Corbit KC, Sirerol-Piquer MS, Ramaswami G, Otto EA, Noriega TR, Seol AD, Robinson JF, Bennett CL, Josifova DJ, Garcia-Verdugo JM, Katsanis N, Hildebrandt F, Reiter JF. 2011. A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat Genet 43:776–784. doi: 10.1038/ng.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hu Q, Milenkovic L, Jin H, Scott MP, Nachury MV, Spiliotis ET, Nelson WJ. 2010. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science 329:436–439. doi: 10.1126/science.1191054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ishikawa H, Marshall WF. 2011. Ciliogenesis: building the cell’s antenna. Nat Rev Mol Cell Biol 12:222–234. doi: 10.1038/nrm3085. [DOI] [PubMed] [Google Scholar]
- 115.Reiter JF, Blacque OE, Leroux MR. 2012. The base of the cilium: roles for transition fibres and the transition zone in ciliary formation, maintenance, and compartmentalization. EMBO Rep 13:608–618. doi: 10.1038/embor.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Garcia G, Raleigh DR, Reiter JF. 2018. How the ciliary membrane is organized inside-out to communicate outside-in. Curr Biol 28:R421–R434. doi: 10.1016/j.cub.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dean S, Moreira-Leite F, Varga V, Gull K. 2016. Cilium transition zone proteome reveals compartmentalization and differential dynamics of ciliopathy complexes. Proc Natl Acad Sci U S A 113:E5135–E5143. doi: 10.1073/pnas.1604258113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Salmon D. 2018. Adenylate cyclases of Trypanosoma brucei, environmental sensors and controllers of host innate immune response. Pathogens 7:48. doi: 10.3390/pathogens7020048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tull D, Naderer T, Spurck T, Mertens HD, Heng J, McFadden GI, Gooley PR, McConville MJ. 2010. Membrane protein SMP-1 is required for normal flagellum function in Leishmania. J Cell Sci 123:544–554. doi: 10.1242/jcs.059097. [DOI] [PubMed] [Google Scholar]
- 120.Dean S, Sunter JD, Wheeler RJ. 2017. TrypTag.org: a trypanosome genome-wide protein localization resource. Trends Parasitol 33:80–82. doi: 10.1016/j.pt.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Beneke T, Demay F, Hookway E, Ashman N, Jeffery H, Smith J, Valli J, Becvar T, Myskova J, Lestinova T, Shafiq S, Sadlova J, Volf P, Wheeler RJ, Gluenz E. 2019. Genetic dissection of a Leishmania flagellar proteome demonstrates requirement for directional motility in sand fly infections. PLoS Pathog 15:e1007828. doi: 10.1371/journal.ppat.1007828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Subota I, Julkowska D, Vincensini L, Reeg N, Buisson J, Blisnick T, Huet D, Perrot S, Santi-Rocca J, Duchateau M, Hourdel V, Rousselle JC, Cayet N, Namane A, Chamot-Rooke J, Bastin P. 2014. Proteomic analysis of intact flagella of procyclic Trypanosoma brucei cells identifies novel flagellar proteins with unique sub-localization and dynamics. Mol Cell Proteomics 13:1769–1786. doi: 10.1074/mcp.M113.033357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Beneke T, Madden R, Makin L, Valli J, Sunter J, Gluenz E. 2017. A CRISPR Cas9 high-throughput genome editing toolkit for kinetoplastids. R Soc Open Sci 4:170095. doi: 10.1098/rsos.170095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dawson SC, House SA. 2010. Life with eight flagella: flagellar assembly and division in Giardia. Curr Opin Microbiol 13:480–490. doi: 10.1016/j.mib.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kusdian G, Gould SB. 2014. The biology of Trichomonas vaginalis in the light of urogenital tract infection. Mol Biochem Parasitol 198:92–99. doi: 10.1016/j.molbiopara.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 126.Sinden RE, Talman A, Marques SR, Wass MN, Sternberg MJ. 2010. The flagellum in malarial parasites. Curr Opin Microbiol 13:491–500. doi: 10.1016/j.mib.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 127.Inglis PN, Ou G, Leroux MR, Scholey JM. 2007. The sensory cilia of Caenorhabditis elegans. WormBook doi: 10.1895/wormbook.1.126.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Horáková E, Changmai P, Vancová M, Sobotka R, Van Den Abbeele J, Vanhollebeke B, Lukeš J. 2017. The Trypanosoma brucei TbHrg protein is a heme transporter involved in the regulation of stage-specific morphological transitions. J Biol Chem 292:6998–7010. doi: 10.1074/jbc.M116.762997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bassarak B, Uzcategui NL, Schonfeld C, Duszenko M. 2011. Functional characterization of three aquaglyceroporins from Trypanosoma brucei in osmoregulation and glycerol transport. Cell Physiol Biochem 27:411–420. doi: 10.1159/000327968. [DOI] [PubMed] [Google Scholar]
- 130.Liu W, Apagyi K, McLeavy L, Ersfeld K. 2010. Expression and cellular localization of calpain-like proteins in Trypanosoma brucei. Mol Biochem Parasitol 169:20–26. doi: 10.1016/j.molbiopara.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 131.Rocha GM, Teixeira DE, Miranda K, Weissmuller G, Bisch PM, de Souza W. 2010. Structural changes of the paraflagellar rod during flagellar beating in Trypanosoma cruzi. PLoS One 5:e11407. doi: 10.1371/journal.pone.0011407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gluenz E, Ginger ML, McKean PG. 2010. Flagellum assembly and function during the Leishmania life cycle. Curr Opin Microbiol 13:473–479. doi: 10.1016/j.mib.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 133.Emmer BT, Souther C, Toriello KM, Olson CL, Epting CL, Engman DM. 2009. Identification of a palmitoyl acyltransferase required for protein sorting to the flagellar membrane. J Cell Sci 122:867–874. doi: 10.1242/jcs.041764. [DOI] [PMC free article] [PubMed] [Google Scholar]