Abstract
Trichuris trichiura is a parasite that infects 500 million people worldwide, leading to colitis, growth retardation and Trichuris dysentery syndrome. There are no licensed vaccines available to prevent Trichuris infection and current treatments are of limited efficacy. Trichuris infections are linked to poverty, reducing children’s educational performance and the economic productivity of adults. We employed a systematic, multi-stage process to identify a candidate vaccine against trichuriasis based on the incorporation of selected T-cell epitopes into virus-like particles. We conducted a systematic review to identify the most appropriate in silico prediction tools to predict histocompatibility complex class II (MHC-II) molecule T-cell epitopes. These tools were used to identify candidate MHC-II epitopes from predicted ORFs in the Trichuris genome, selected using inclusion and exclusion criteria. Selected epitopes were incorporated into Hepatitis B core antigen virus-like particles (VLPs). Bone marrow-derived dendritic cells and bone marrow-derived macrophages responded in vitro to VLPs irrespective of whether the VLP also included T-cell epitopes. The VLPs were internalized and co-localized in the antigen presenting cell lysosomes. Upon challenge infection, mice vaccinated with the VLPs+T-cell epitopes showed a significantly reduced worm burden, and mounted Trichuris-specific IgM and IgG2c antibody responses. The protection of mice by VLPs+T-cell epitopes was characterised by the production of mesenteric lymph node (MLN)-derived Th2 cytokines and goblet cell hyperplasia. Collectively our data establishes that a combination of in silico genome-based CD4+ T-cell epitope prediction, combined with VLP delivery, offers a promising pipeline for the development of an effective, safe and affordable helminth vaccine.
Author summary
The soil transmitted helminth Trichuris trichiura is a major parasite in developing countries; development of a comprehensive vaccine has been elusive. Here we used a systematic approach based on in silico identification of MHC-II T-cell epitopes from genome sequences, their incorporation into a virus-like particle (VLP), characterization of the assemblies and testing in an in vivo murine infection model. Animals vaccinated with a preparation of four different VLP-antigen fusions showed significant reductions in intestinal worm burdens and associated antibody responses consistent with protection. The results suggest that a pipeline based on in silico prediction of potent MHC-II T-cell epitopes, followed by incorporation into VLPs, could be a strategy which enables rapid translation into a vaccine against Trichuris trichiura.
Introduction
Trichuriasis, caused by the whipworm Trichuris trichiura, is one of the most widespread soil-transmitted helminths (STH) in the world [1]. Global mass drug administration (MDA) programmes are being implemented, but cure rates are low, repeated treatments are costly and may prevent the development of acquired immunity. Further, the existence of drug-resistant parasites is a constant concern [2–4]. Using the mouse model of human trichuriasis, Trichuris muris excretory/secretory (ES) products [5], ES fractions [6], extracellular vesicles (EVs) [7], and, more recently, T. muris whey acidic protein [8] in the context of the adjuvant alum, have shown considerable potential in a number of pre-clinical protection trials. Despite these successes, developing a vaccine based on native antigens is associated with many manufacturing challenges, including cost, time consumption, difficulties in purifying large quantities of worm antigens and control over differences between batches [9, 10]. The advent of the genome era has provided alternative strategies for vaccine development [11]. For example, the reverse vaccinology (RV) approach combines genome information with immunological and bioinformatics tools to overcome some of the limitations of conventional methods of screening vaccine candidates [12–14].
These observations, combined with the economic challenges of producing a low-cost vaccine, prompted us to examine the potential of using virus-like particles (VLPs) as a scaffold for the presentation of predicted Trichuris MHC-II epitopes. The Hepatitis B core protein (HBc) has been widely used as a VLP: it forms stable self-assemblies, which can accommodate T-cell epitopes, is cheap to produce and is safe for human use [15, 16]. Structural analysis has shown that each HBc monomer forms helical structures, which protrude as spikes from the capsid [17]. Antigens, in the form of short T-cell epitopes or whole globular domains, can be inserted at the tip of each spike. Designing vaccines based on 'multi-stage' antigens which are expressed at different stages of infection have shown promising results against several complex pathogens, such as M. tuberculosis and Plasmodium [18, 19]. Further, CD4+ Th2 cells play essential roles in the development of protective immunity against Trichuris spp [20].The principal objective of this study was therefore to develop a novel MHC II T-cell epitope-based vaccine predicted from multi-stage Trichuris proteins, which will induce Th2 protective immunity. To achieve this aim, first, a systematic review was performed to select the optimal MHC class II in silico prediction tools. Second, potential Trichuris MHC-II T-cell epitope vaccine candidates obtained from the Trichuris genome were identified using the selected in silico prediction tool. Third, these epitopes were produced in a commercially viable manner by fusing epitopes in the hepatitis B core antigen (HBc-Ag) virus-like particle (VLP) vaccine delivery system. These VLPs+T-cell epitope vaccine candidates were then tested in vitro for their ability to activate antigen presenting cells (APCs). Finally, in vivo experiments were conducted to test the protective capacity of VLPs expressing different Trichuris T-cell epitopes in vivo using T. muris infection of mice. Collectively the results of this research represent the first significant progress towards identifying a novel, epitope-based vaccine for trichuriasis.
Results
The IEDB and NetMHC-II 2.2 tools exhibited similarly high levels of sensitivity for predicting epitopes with strong affinities
Based on a list of search terms (S1 Table) used on Google and other websites (S2 Table), 88 servers that predict T-cell epitopes based on MHC class I and II binding were identified (S3 Table). Of these 88, only 48 tools which could predict MHC-II epitopes were identified. Since our primary focus was MHC-II T-cell epitope prediction, only tools with that functionality were further evaluated using the inclusion criteria (Fig 1A). Five tools met the inclusion criteria: IEDB, SYPEITHI, NetMHC-II 2.2, Rankpep and ProPred. These tools were then scanned for the ability to predict MHC-II T-cell epitopes for two mouse alleles, I-Ab and I-Ad. The ProPred tool was excluded from further analysis because it only predicted HLA-DR binding sites. The four epitope prediction tools that met all the selection criteria were subsequently evaluated using an epitope training set to calculate the sensitivity with which they could predict MHC II T-cell epitopes. Using the epitope training set (S4 Table), it was observed that IEDB and NetMHC-II 2.2 tools had high levels of sensitivity (~78.00%) for predicting MHC-II T-cell binding epitopes with high affinities, while Rankpep and SYFPETHI exhibited low sensitivity (10.61% and 8.33%, respectively). Collectively, the data indicate that the best prediction tools across all MHC-II T-cell prediction servers considered in this study are IEDB and NetMHC-II 2.2.
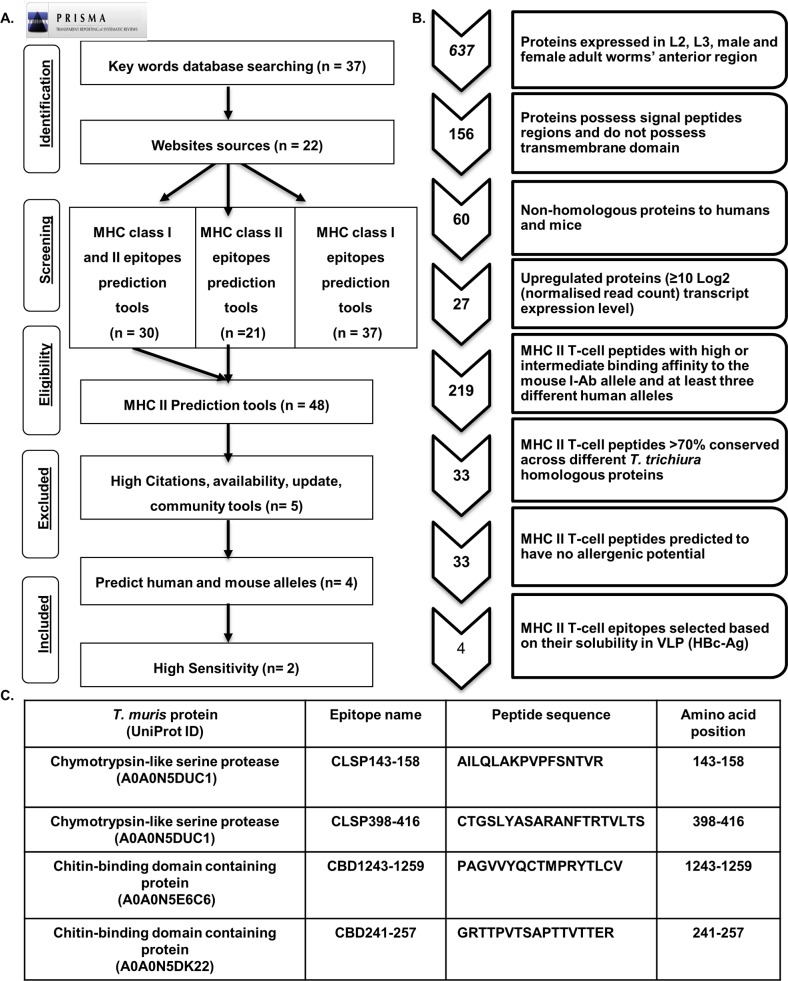
Fig 1.
(A) PRISMA flow diagram of the systematic review screening process for restricted MHC-II T-cell bioinformatics prediction tools. A list of search terms was used in Google website and other website sources to screen for MHC class I and II in silico prediction tools. Aspects relating to the number of citations (>200), number of publications, online availability, last update and community were used as inclusion/exclusion criteria to select the top MHC-II in silico prediction tools. n = number of key words, websites sources and in silico prediction tools. (B) The flow diagram of reverse vaccinology approach to identify potential vaccine candidates (MHC-II T-cell epitopes) from the T. muris genome. The number on the arrow represents the number of proteins or epitopes selected for the next step. (C) List of 4 Trichuris MHC-II T-cell epitopes which have potential as vaccine candidates.
Data acquisition and identification of potential vaccine component proteins
The stichosome, which forms the majority of the whipworm anterior region, is thought to release ES products which trigger the host immune response and induce host immunity after infection [7, 21]. Research conducted by Dixon et al., [22] and Else et al., [23], showed that antibody recognition of high molecular weight proteins in both T. muris adult and larval ES correlated with resistance to T. muris infection. To increase the chances of the identification of antigens which induce a strong immune response, secreted and surface-exposed proteins associated with the anterior region of L2, L3, male and female adult worms were selected for this study [24].
Predicted ORFs from the 85-Mb genome (∼11,004 protein-encoding genes) of T. muris and the 73-Mb genome (∼9650 protein-coding genes) of T. trichiura [24] were scanned for potential vaccine candidates; 637 proteins were selected. From this subset, only proteins that possess signal peptides and did not exhibit transmembrane domains were included, reducing the total to a sample of 156 proteins. Full-length sequences of the Trichuris proteins were obtained from the Universal Protein Resource (UniProt) database in FASTA format http://www.uniprot.org/.
Elimination of closely homologous mouse and human proteins
To eliminate potential autoimmune reactions when tested in mice and humans, the 156-protein subset was checked to determine homology with human and mouse proteins using the basic local alignment search tool (BLAST). All proteins with any degree of homology with humans or mice were excluded, leaving 60 candidate proteins. Of these, only upregulated proteins in the anterior region of L2, L3 and adult worms were selected based on their transcript expression level. This criterion was based on high-throughput transcriptome data generated from the RNA of T. muris and Gene Ontology (GO) term enrichment analyses, a transcriptional upregulation in a particular protein refers to a ≥10 Log2 (normalised read count) transcript expression level [24]. Implementing this criterion, 27 proteins were selected for MHC-II T-cell epitope prediction (Fig 1B).
Prediction of Trichuris MHC-II T-cell binding epitopes
All 27 selected proteins were screened to predict T-cell MHC class II epitopes using the IEDB (consensus method) prediction tool [25]. The analysis was carried out to predict the binding affinity to the MHC class II allele I-Ab mouse strain and the 11 most prevalent human class II HLA allele supertypes [25–28]. The consensus method was used to select only those peptides with a low median percentile rank according to three different prediction methods to reduce the chance of failure during prediction. To cover common global alleles, peptides were selected based on their ability to bind to at least three different human alleles.
Conservation and allergens
To assess how well the predicted MHC-II T-cell peptides were conserved within the T. trichiura genome, the IEDB conservancy analysis tool was used. This tool calculates the degree of conservancy (i.e. similarity) of a peptide within a specified protein sequence [29]. Only peptides that were >70% conserved with at least one homologous T. trichiura protein were selected for further analysis. Of the 219 MHC-II T-cell peptides, only 33 met these criteria. The 33 MHC-II peptides were then assessed for the prediction of IgE epitopes and allergenic potential using the AllerTOP v.2.0 server http://www.ddg-pharmfac.net/AllerTOP/ [30]. The final set of 10 Trichuris MHC-II T-cell epitopes containing 33 overlapping peptides were predicted to have no allergenic potential. The 10 epitopes were further triaged to a final four epitopes based on solubility once expressed on HBc-Ag VLPs. A flow diagram summarising the approach used is shown in Fig 1B, with the final 4 Trichuris MHC II T-cell epitopes detailed in Fig 1C.
Purification and assembly of VLPs expressing Trichuris T-cell epitopes
Four HBc-Ag fusion proteins were designed, incorporating each predicted T-cell epitope into the major immunodominant region. The construct also included a Strep tag at the C-terminus, for affinity purification (S1 Fig). A second round of purification using size exclusion chromatography was performed to produce a homologous population of assembled VLPs. HBc-Ag preparations expressing Trichuris MHC-II T-cell epitopes were pure by SDS PAGE (S1A–S1E Fig). The endotoxin levels in all purified VLPs used in this study were <0.2 endotoxin units (EU) as assessed using the ELISA-based endotoxin detection assay (Data not shown).
VLPs expressing Trichuris T-cell epitopes induced the production of proinflammatory cytokines in vitro and were internalised and co-localized in APCs
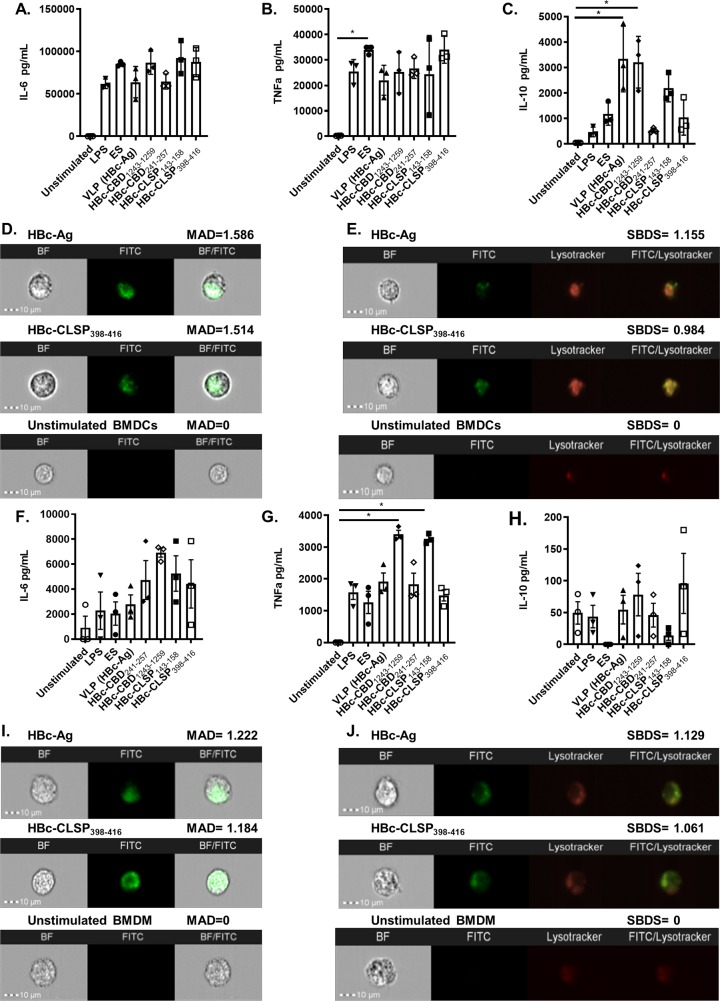
BMDCs stimulated with different VLPs, irrespective of whether they include T-cell epitopes, produced high levels of IL-6 (Fig 2A) and TNF-α (B) at levels equivalent to LPS and ES-stimulated BMDCs. Similiarly, all VLPs activated BMDMs, inducing the secretion of high levels of both IL-6 (Fig 2F), and TNF-α. (G) BMDC-derived IL-10 showed a different pattern. Here VLPs bearing CBD241-257 evoked less IL-10 from BMDC than the control VLP (Fig 2C). The in vivo significance of this is not clear but this may be desirable in order to avoid the induction of regulatory responses. In this study the VLPs bearing the 4 different T-cell epitopes were screened against BMDM and BMDC individually. Whether any synergistic innate cell stimulation is apparent when all four epitope bearing VLPs are added together woould be interesting to explore in the future. To visualise VLP internalisation by APCs, BMDCs and BMDMs were stimulated with or without fluorescein-conjugated VLPs. Images of individual cells taken by merging the brightfield (BF) and FITC (green) channels demonstrated that the fluorescein-conjugated VLPs were internalised by both APCs (Fig 2D & 2I). To confirm that the VLPs were co-localized within the APC lysosome and not the cell surface, BMDCs and BMDMs were stained with the lysosome-specific LysoTracker dye. Cells were then subsequently stimulated with or without fluorescein-conjugated VLPs. Merged images of the brightfield (BF), FITC (green), and Lysotracker (red) channels revealed that the fluorescein-conjugated VLPs were accumulated in the lysosome compartment of the BMDCs (Fig 2E) and BMDMs (Fig 2J).
Fig 2. Inflammatory cytokine production by mouse bone marrow-derived DCs (BMDCs) and mouse bone marrow-derived macrophages (BMDMs) in response to VLPs.
(A-C) BMDCs and BMDMs (F-H) at 1X106/ml were stimulated in vitro with 10 μg/ml VLPs (HBc-Ag, HBc-H112-128, HBc-CBD1243-1259, HBc-CBD241-257, HBc-CLSP143-158 and HBc-CLSP398-416) and with 50 μg/ml ES and 0.1 μg/ml LPS as positive controls. Unstimulated BMDCs and BMDMs served as negative controls. Supernatants were harvested after 24 hours for IL-6, TNF-α and IL-10 cytokine analyses measured by LEGENDplex or CBA. The bars represent mean ± SEM. Statistical analyses were carried out using the Kruskal-Wallis test (multiple comparisons). Significant differences between groups are represented by *(P≤0.05) with a line. Chart bars represent BMDCs and BMDMs grown from three individual mice from one representative experiment of two separate experiments. (D) Representative images of fluorescein-conjugated VLPs internalisation in the BMDCs and BMDMs (I). BMDCs and BMDMs at 1X106/ml were incubated with 10 μg/ml fluorescein-conjugated VLP (HBc-Ag and HBc-CLSP398-416) for 24 hours. As a negative control, unstimulated BMDCs and BMDMs were examined. Cell internalisation was determined by Amnis ImageStreamX cytometer compared to unstimulated BMDCs and BMDMs. Images shown, from left to right, show individual Brightfield images (BF) in the white channel, fluorescent-labelled stimulus (FITC) in the green channel and the combination of both BF/FITC merged channels. The internalisation mean absolute deviation (MAD) is included above its images. The positive MAD value represents internalisation, and negative values represent poor internalisation. (E) Representative images of fluorescein-conjugated VLPs co-localization in the BMDCs and BMDMs (J). BMDCs and BMDMs at 1X106/ml were stained with Lysotracker to visualise the cellular lysosome compartment and subsequently stimulated with 10 μg/ml fluorescein-conjugated VLP (HBc-Ag, and HBc-CLSP398-416) for 24 hours. As a negative control, unstimulated BMDCs and BMDMs were examined. Intracellular co-localization was determined by Amnis ImageStreamX cytometer. Images shown, from left to right, show individual Brightfield images (BF) in the white channel, fluorescent-labelled stimulus (FITC) in the green channel, stained lysosome (Lysotracker) in the red channel and the combination of both FITC/ Lysotracker merged channels. The Similarity bright detail score (SBDS) from the IDEAS quantitative co-localization analysis is included above its image. SBDS values around 1 represent co-localization, and 0 values represent poor co-localization. Scale bars represent 10 μm.
Immunization of mice with VLPs expressing Trichuris T-cell epitopes induced a significant reduction in worm burden following challenge infection
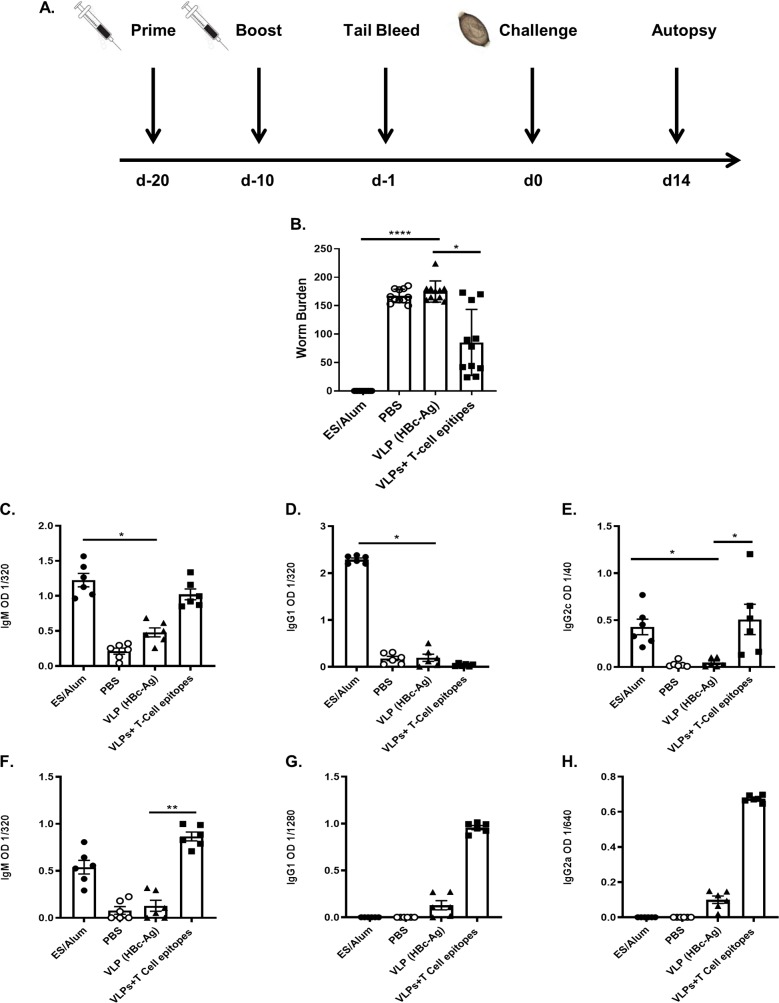
The protective capacity of the 4 T-cell epitopes was assessed in the T. muris–mouse accelerated expulsion model [31] (Fig 3A) where significant reductions in worm burden and elevation in parasite specific antibodies and cytokines can be readily detected at day 14 post infection. Mice vaccinated with pre-mixed four VLPs+T-cell epitopes showed a statistically significant (P<0.01) reduction in worm burden by day 14 p.i. compared to the native VLP (HBc-Ag). In comparison, ES/Alum immunised mice harboured no parasites (Fig 3B).
Fig 3. Experimental schedule, worm burden, parasite-specific and VLP-specific antibody levels of mice vaccinated and challenged with a T. muris infection.
(A) 6-week old male C57BL/6 mice were vaccinated subcutaneously with 50 μg of pre-mixed of VLPs+T-cell epitopes (HBc-(A)CBD1243-1259, HBc-CBD241-257, HBc-CLSP143-158, and HBc-CLSP398-416), 50 μg of VLP (HBc-Ag), 50 μg ES/Alum, or PBS on day -20 and boosted on day -10. Mice were infected by oral gavage with approximately 150 infective T. muris eggs on day 0 and sacrificed at d14 post-infection. (B) Comparison of worm burden at day 14 post-infection. n = 11 mice per group. The results presented are from two separated experiments pooled together. Day 14 p.i. sera were titrated against T. muris ES antigens to assess parasite-specific IgM (C) IgG1 (D), and IgG2c (E) levels in VLPs+T-cell epitopes, VLP (HBc-Ag), PBS and in ES/Alum vaccinated mice by ELISA (reading at 405 nm). Day 14 p.i. sera were titrated against premixed VLPs+T-cell epitopes antigens to assess VLPs+T- cell epitopes-specific IgM (F) IgG1 (G), and IgG2c (H) levels in VLPs+T-cell epitopes, VLP (HBc-Ag), PBS and in ES/Alum vaccinated mice. Statistical analyses were carried out using Kruskal-Wallis test (multiple comparisons compared to the VLP (HBc-Ag)). Significant differences between groups are represented by (*P≤0.05, **P≤0.01, ****P≤0.0001) with a line. Results are shown as mean ± SEM. n = 6 mice per group. This experiment was repeated two times, and the ELISA results shown here are representative of the two experiments.
Immunization of VLPs expressing Trichuris T-cell epitopes induced humoral immunity following challenge infection
To evaluate T. muris-specific serum antibody responses induced by vaccination with VLPs+T-cell epitopes, parasite-specific IgM, IgG1, and IgG2c serum antibody levels were determined at d14 p.i. Following vaccination and infection, VLPs+T-cell epitopes and ES/Alum vaccinated mice had statistically significant higher levels of parasite-specific IgM (Fig 3C) and IgG2c (E) compared to the control PBS and native VLP (HBc-Ag) injected mice. However, high levels of parasite-specific IgG1 were only detected in the serum of mice vaccinated with ES/Alum following Trichuris infection (Fig 3D). There was no or very low levels of parasite-specific IgM, IgG1, and IgG2c detected in the serum of native VLP (HBc-Ag) and PBS/alum injected mice at day 14 p.i., as shown in (Fig 3F–3H). Similarly, statistically significant higher levels of VLPs+T-cell epitopes-specific IgM (Fig 3F), IgG1 (G) and IgG2c (H) were produced following Trichuris infection of mice vaccinated with VLPs+T-cell epitopes, compared to mice given native VLP (HBc-Ag) or PBS. Notably, mice vaccinated with ES/Alum also produced high levels of VLPs+T-cell epitopes-specific IgM following Trichuris infection, as shown in Fig 3F.
Immunisation of mice with VLPs expressing Trichuris T-cell epitopes induces a mixed Th1/Th2 immune response following challenge infection
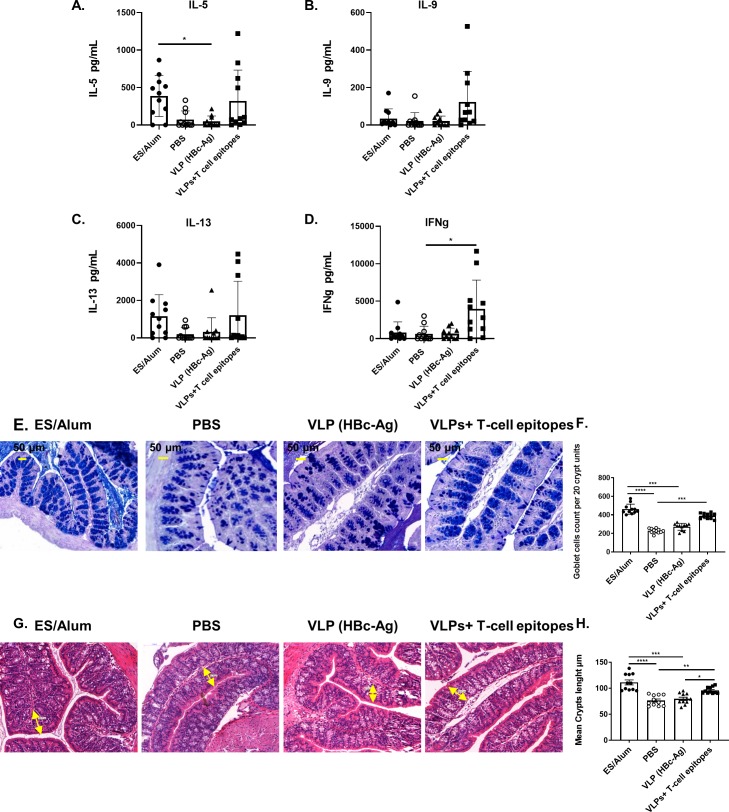
To analyse the cellular immune responses at the primary site of adaptive immune cell activation following T. muris infection [32], MLN cells of mice vaccinated with VLPs+T-cell epitopes were re-stimulated in vitro with Trichuris ES. The MLN is the most appropriate lymph node to assay for the presence of antigen-specific cytokines given that it drains the site of infection. Supernatants were assayed for Th2 cytokines (IL-4, IL-5, IL-9 and IL-13), Th1/Th17 cytokines (IL-2, IFN-γ and IL-17), proinflammatory cytokines (IL-6 and TNF-α), and the anti-inflammatory cytokine (IL-10) production by CBA (Fig 4 and S2 Fig). Levels reached significance versus control vaccinated mice for IL-5 (Fig 4A; ES vaccinated mice) and IFN-γ (Fig 4D; VLPs+T-cell epitope vaccinated mice) with non-significant increases in IL-9 and IL-13 (Fig 4B and 4C). MLN cells from all vaccinated mouse groups also produced IL-4, IL-6, IL-10, IL-17 and TNF-α upon their re-stimulation in vitro (S2A–S2E Fig). Collectively these data support the in vivo immunogenicity of the novel VLPs+T-cell epitope vaccine, evidencing the presence in the MLN of a mixed Th1/Th2 immune response and are in keeping with the worm expulsion and antibody data. Proximal colon goblet cells were quantified in mice vaccinated with ES/Alum, PBS, VLP (HBc-Ag) and VLPs+T-cell epitopes day 14 post T. muris infection. (Fig 4E). Interestingly, VLPs+T-cell epitopes and ES/Alum vaccinated mice exhibited a significantly elevated goblet cell hyperplasia (P<0.05) compared to mice injected with PBS or native VLP (HBc-Ag) (Fig 4F). Control vaccinated (mice injected with VLP only) had shorter crypts than vaccine-protected groups (mice vaccinated with VLP plus T-cell epitopes and mice vaccinate with ES/alum) (Fig 4G & 4H). Thus, these data provide no evidence for increased epithelial cell turnover in vaccine mediated immunity, which would have predicted shorted crypts in the more resistant mice. Further, quantification of serum mMCPT-1 levels (S2(F) Fig). as a surrogate of the mucosal mast cell response [33], and serum IgE levels (S2(G) Fig) did not differ between mice protected by the VLPs+T-cell epitope vaccine and control VLP vaccinated mice. This was in contrast to the ES/alum vaccinated mice where levels of both IgE and mMCPT-1 were elevated over control PBS treated mice. It is possible therefore that the protective mechanism induced by ES in alum differs from that induced by the VLP plus T-cell epitope vaccine; alternatively, both IgE and mMCPT-1 may simply reflect the strength of the Th2 immune response.
Fig 4. Cytokine productions by mesenteric lymph node cells, quantification of goblet cell numbers and changes in intestinal pathology in T. muris infected mice vaccinated with VLPs+T-cell epitopes and control mice.
(A) MLNs from mice vaccinated with 50 μg of pre-mixed of VLPs+T-cell epitopes (HBc-CBD1243-1259, HBc-CBD241-257, HBc-CLSP143-158 and HBc-CLSP398-416), 50 μg of VLP (HBc-Ag), 50 μg ES/Alum, or PBS on day -20 and boosted on day -10 were stimulated at 5x106/ml with 50 μg/ml T. muris ES. After 48 hours of stimulation, cell culture supernatants were harvested and assayed by cytometric bead array for IL-5 (A), IL-9 (B), IL-13 (C), and IFN-γ (D) production. (E) Representative photographs of gut sections stained with Periodic acid–Schiff staining (PAS). The slides were scanned, and the goblet cells were quantified using Panoramic Viewer software. All photographs were taken with 20X magnification. (F) Goblet cells were quantified by counting the number of alcian blue-stained cells per crypt unit in three fields of view from each section and are shown as mean cell numbers per 20 crypt units (cu) ±SEM. (G) Gut pathology assessed by measuring crypt lengths using Panoramic Viewer software. The yellow double-headed arrows show how the crypt lengths were measured. (H) Results are shown as mean crypt length from 20 crypts units per mouse and shown as the mean values per mouse ±SEM. Statistical analyses were carried out using the Kruskal-Wallis test (multiple comparisons). Significant differences between groups are represented by (*P≤0.05, **P≤0.01, ***P≤0.001, ****P≤0.0001) with a line. n = 11 mice per group. The results presented are from two separate experiments pooled together.
Discussion
Trichuris trichiura is one of the most common human STH parasites and remains a major health concern for humans worldwide [1]. A number of pre-clinical vaccines against trichuriasis have been reported, containing whole Trichuris antigens or fractions [6, 7, 22]. However, developing a vaccine for Trichuris based on native antigens has several limitations, such as cost, time consumption and difficulty in purifying large quantities of worm antigens. It is also challenging to control for differences in batches in order to develop a commercially stable vaccine [10]. An alternative strategy embraces the use of informatics to predict and assess MHC-II T-cell epitopes using criteria to maximize their in vivo protective potential. The fast-growing application of VLP-based vaccines against several parasites, including Plasmodium spp [34, 35], Leishmania infantum [36], Toxoplasma gondii [37], Trichinella spiralis [38], and Clonorchis sinensis [39] prompted our interest in developing a VLP-based vaccine against trichuriasis.
Identification of novel Trichuris MHC-II T-cell epitopes as promising vaccine candidates
The expulsion of T. muris is known to be CD4+ T-cell dependent [40]. Thus we focussed our study on identifying protective CD4+ T-cell epitopes. Initiation of an antigen-specific immune response requires presentation of antigenic peptides to CD4+ T-cells in the context of MHC Class II molecules [41]. Selection of appropriate MHC Class II binding peptides is therefore a critical first step in developing an epitope-based vaccine. There are more than 80 computer-based prediction tools for identifying peptides that bind to MHC class I and II molecules, but not all are equivalent; some epitope prediction tools may fail to predict all significant epitopes [42]. The performance of in silico prediction tools is affected by several factors. For example, MHC-II CD4+ T-cell epitope prediction tools have much lower accuracy than MHC-I tools because the MHC-I binding groove is closed, while the MHC-II groove is open at both ends [42, 43]. Also, the use of a limited training dataset to evaluate the tools may affect performance [25, 44, 45]. Further, the low performance of MHC class II prediction tools may not only be due to poor algorithm performance; the genetic diversity of human populations presents additional challenges [46]. Thus, choosing the best bioinformatics tool to predict MHC class II T-cell epitopes is critical when designing epitope-based vaccines [47, 48]. This study presents a systematic review of existing MHC-II restricted T-cell epitope prediction tools and evaluated four tools in order to establish the most appropriate bioinformatics tools currently available for predicting Trichuris MHC-II T-cell epitopes.
The IEDB and NetMHC-II 2.2 tools achieved similarly high levels of sensitivity of predicting binding epitopes with high affinities, while Rankpep and SYFPETHI exhibited low sensitivity. Each tool applies different methods of prediction; the IEDB tool uses a quantitative consensus method that combines the strengths of various methods [25]; the NetMHC-II 2.2, another quantitative tool, uses an NN-align algorithm and weight matrix [28]; and both SYFPEITHI [49] and Rankpep [50] are qualitative tools that use motif PSSMs. All the tools were ‘user-friendly’ but SYFPETHI was limited in its coverage of mouse and human MHC-II alleles. The IEDB tool has features that the other three do not, including a browser to input protein sequence formats in a National Centre for Biotechnology Information (NCBI) database, seven different prediction methods and an easy method of downloading the prediction output into an Excel spreadsheet.
Several studies have compared the performance of different MHC class II peptide binding prediction tools [51–53], but the comparison presented in this study is different for two main reasons. First, the MHC-II prediction tools were selected in a systematic way using inclusion/exclusion criteria. Second, a unique dataset consisting of a new 'test' dataset which has not been used to build or evaluate IEDB was used. For example, Zhao and Sher (2018) evaluated the MHC-II prediction tools hosted on the IEDB analysis resource server using newly available, untested data of both synthetic and naturally processed epitopes. Among the 18 predictors that were benchmarked, NetMHC-II outperformed all other tools, including NetMHCIIpan and the consensus method for both MHC class I and class II predictions [54]. Furthermore, Andreatta et al. (2018) created an automated platform to benchmark six commonly used MHC class II peptide binding prediction tools using new 59 datasets. Their evaluation suggested that NetMHCIIpan is currently the most accurate tool, followed by NN-align and the IEDB (consensus) tool [55]. Despite differences in the datasets used for comparison, these studies agree with the comparison study conducted in our study, which found that the IEDB (consensus) and NETMHC-II 2.2 (ANN) tools are among the best MHC-II prediction tools. However, NetMHCIIpan could not be included in this study because it did not meet the inclusion criteria. Collectively, we recommend the use of IEDB and NETMHC-II 2.2 prediction tools in any MHC class II epitope prediction study to reduce the experimental cost of identifying epitopes. However, the output of these tools needs to be carefully evaluated in vitro and in vivo before they are used to bring an epitope-based vaccine to trial.
Since the advent of immunoinformatics tools for prediction of antigenic epitopes and protein analysis, several VLP-based vaccines have been engineered to carry foreign antigens and have proven to be highly immunogenic [56]. To our knowledge, the data presented in this study is the first to identify novel Trichuris MHC-II T-cell epitopes as potential vaccine antigen candidates. The framework used here to identify potential epitope vaccine candidates within the T. muris and T. trichiura genome could be used in the future to identify potential vaccine candidates for other parasite species. The final set of Trichuris MHC-II T-cell epitope vaccine candidates were derived from chitin-binding domain-containing proteins and chymotrypsin-like serine proteases (Fig 1C).
Chitin-binding domains genes are highly expressed in different life stages in many nematodes including the parasites T. trichiura, Ascaris lumbricoides and Ancylostoma ceylanicum and the free-living nematode Caenorhabditis elegans, [57–60]. In particular, these proteins are thought to be associated with eggshell formation and early development at the single-cell stage [61, 62]. Furthermore, given that, in addition to T. muris, more than 40 other helminth species express high levels of chymotrypsin-like serine proteases, these proteins may also be promising vaccine candidates for other helminths [24, 63]. Chymotrypsin-like serine proteases are thought to play central roles in either the invasion process or modulation of the host immune response to enhance parasite survival [64–67]. In addition, numerous publications, using pre-clinical models of parasitic infection, have noted that protective immunity can be consistently achieved using helminth protease molecules [68]. For example, vaccinating BALB/c mice with the whole recombinant serine protease of T. spiralis prior to challenge infection led to a reduction in worm burden and induced a mixed Th1/Th2 immune responses [69–71]. Furthermore, Shears et al. [6] showed that vaccinating mice with the T. muris ES fraction containing serine proteases induced high parasite-specific antibody responses. Remarkably all the proteins selected in this study have been identified within the most immunogenic fractions of T. muris ES following vaccination of mice [6].
Identification of a novel VLP-based vaccine against trichuriasis
All VLP recombinant proteins stimulated a non-specific inflammatory response characterized by the secretion of high levels of proinflammatory cytokines. Furthermore, by identification of intracellular co-localization with lysosomes, VLPs were shown to be taken up by both BMDC and BMDMs. These results are consistent with those of Serradell et al. [72] and Wahl-Jensen et al. [73] who examined the activation of APCs in response to VLPs. For example, Serradell et al. developed a (VLP-HA/VSP-G) vaccine composed of an influenza virus expressing influenza virus hemagglutinin (HA) antigen, co-expressed with the extracellular region of Giardia lamblia variant-specific surface proteins (VSPs) as an adjuvant. The recombinant protein (VLP-HA/VSP-G) induced the secretion of high levels of TNF-α, IL-10, and IL-6 cytokines in vitro. They also showed that oral vaccination with the recombinant protein protected mice from influenza infection and generated protective humoral and cellular immunity. These results suggest that the VLPs are well placed to act as delivery systems to drive immune responses. Further, the data raises the exciting prospect of modifying the VLPs using adhesion molecules and/or cytokines co-displayed on the VLP surface, in order to target the epitopes to specific APC subsets, thus enhancing the activation of antigen-specific T-cells [74].
Remarkably, upon challenge with T. muris infection, mice vaccinated with 50 μg of pre-mixed VLPs+T-cell epitopes (HBc-CBD1243-1259, HBc-CBD241-257, HBc-CLSP143-158, and HBc-CLSP398-416), in the absence of any additional adjuvant, showed a significantly reduced worm burden. Parasite-specific IgM and IgG2c were detected in the sera of mice vaccinated with the VLPs+T-cell epitopes. Levels were equivalent to the control ES in alum-vaccinated mice, and significantly higher than control vaccinated and infected mice. These results suggest that these VLPs+T-cell epitopes are antigenic and can boost antibody response sufficiently to recognize specific small peptides in the T. muris ES. Importantly, analysis of the serum from immunised mice showed that vaccination with the VLPs+T-cell epitopes elicited high levels of IgM, IgG1 and IgG2c to the VLPs+T-cell epitope recombinant protein pool (HBc-CBD1243-1259, HBc-CBD241-257, HBc-CLSP143-158, and HBc-CLSP398-416) with limited recognition of the native VLP (HBc-Ag) protein. In keeping with these data, the malaria VLP-based vaccine (Malarivax) [75–77] composed of HBc-Ag expressing P. falciparum T cell and B-cell epitopes identified from the circumsporozoite protein developed long-lasting immunity, eliciting a CD4+ T-cell immune response and is currently undergoing a clinical trial [15, 78, 79]. The results of these studies support some critical insights into VLP-based vaccines, including confirming that an HBc virus-like particle, in particular, is an excellent delivery system for developing potential vaccine candidates for parasites.
A proportion of mice vaccinated with VLPs+T-cell epitopes produced detectable levels of MLN-derived Th2 cytokines IL-5, IL-9, and IL-13 in response to re-stimulation in vitro with Trichuris ES. The Th1 cytokine IFN−γ was also significantly elevated above levels detected in control mice. These data indicate that vaccine-induced protective immunity is characterized by a mixed Th1/Th2 immune response, as has been previously reported [21, 80]. Similarly, Gu et al. [80], reported that the protective immunity to Trichinella spiralis infection induced by vaccination with CD4+ T-cell epitopes was associated with both Th1 and Th2 cytokines.
Whilst the mechanism by which vaccination protects mice from T. muris infection remains unclear, this study reveals that vaccination of mice with VLPs+T-cell epitopes or ES/Alum promoted a marked goblet cell hyperplasia [81]. Goblet cells, and the mucins they produce, have been implicated in Th2-mediated defence in mice resistant to a primary T. muris infection [82, 83]. However, it remains to be determined whether the goblet cell hyperplasia seen here in vaccinated mice is simply a Th2 correlate or is functionally important in the protection observed. Future work will include determining the effector mechanism(s) at play in vaccine-mediated protective immunity. Using a whole ES vaccine preparation Dixon et al has previously hypothesised that the effector response is distinct from that seen in a primary infection [21]. Thus this study reported no elevation in epithelial cell turnover in vaccine-protected mice, but rather an accumulation of cell-bound IgG1 in the lamina propria. Prior to elucidating mechanism however we aim to improve the efficacy of our VLPs+T-cell epitope based vaccine, as the 50% protection offered by the current preparation is a limitation. We will adopt a number of strategies. We will assess each VLPs+T-cell epitope in vivo for its ability to drive Th1, Th2, Th17 or Regulatory T-cells and exclude any epitope that might promote Th2-antagonistic Th1, Th17 or regulatory responses. We also aim to modify our VLP delivery platform by incorporating targeting antibodies which will preferentially deliver the T-cell epitopes to Th2-inducing antigen presenting cells [72]. In summary, the current study describes the development and efficacy of a novel epitope-based vaccine against trichuriasis. VLPs expressing different Trichuris MHC-II T-cell epitopes, predicted from chitin-binding domain containing proteins and chymotrypsin-like serine proteases, were shown to promote protective immunity in vivo. Collectively, given the right combination of immunoinformatics and immunogenicity screening tools, epitope-based vaccines will undoubtedly limit the cost and effort associated with bringing a Trichuris vaccine to trial.
Methods
Search strategy
A protocol was designed to identify the bioinformatics tools that can predict MHC-II T-cell epitopes in accordance with the well-defined Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [84]. The search was limited to the English language and used the search terms shown in (S1 Table). A list of the search terms was first used in December 2015 on Google and other websites (S2 Table) to screen for MHC class I and II in silico prediction tools. The number of citations (>200), number of publications (>200), online availability, last update and community were considered to determine whether the tools would be selected for further analysis. The number of publications and citations for each tool were obtained from Google Scholar. In the case of duplicate citations, the highest number of citations was used. A flow diagram of the systematic review screening process for MHC-II T-cell bioinformatics prediction tools is shown in Fig 1A.
Construction of an epitope training set
To evaluate the performance of the tools, a literature search for MHC-II CD4+ T-cell peptide binding datasets was performed using the search terms ‘universal T-cell epitopes’ and ‘MHC class II T-cell epitopes’ on Google Scholar in December 2015. These datasets included epitopes with publicly available sequences that the literature had experimentally validated to be immunogenic in mice. Because training sets of peptides were used for the development of epitope prediction tools, care was taken to exclude the training datasets used for tool development [85–87].
In order to find the optimal set of epitopes, two different sets of epitopes were analysed. The first set was composed of publicly available series of peptide sequences from 15 different proteins that can bind to MHC class II molecules. The second set included one untested protein of T. muris, which served as a control to evaluate the performance of the tools. Collectively, the training dataset was composed of 145 epitopes from 16 different proteins (S4 Table).
Prediction of Trichuris MHC-II T-cell epitopes
Full-length sequences of proteins containing T-cell epitopes were obtained from the Universal Protein Resource (UniProt) database http://www.uniprot.org in FASTA format. The Immune Epitope Database (IEDB), NetMHC-II 2.2, Rankpep and SYFPEITHI tools were used to predict T-cell epitopes for mouse strains with I-Ab and I-Ad mouse alleles. Each tool applied a different prediction method and scoring system to generate the predictions output. For instance, the IEDB tool uses the consensus method for prediction, which combines the NN-align, SMM-align, combinatorial library and Sturniolo methods [25], while the NetMHC 2.2 tool uses ANNs [28]. The median percentile rank (%) of the three prediction methods was used to generate the rank for the consensus method. A small numbered percentile rank (%) indicates that a peptide has a high binding affinity to MHC class II alleles [25].The output of the IEDB and NetMHC 2.2 tools (the binding affinities) were expressed as half-maximal inhibitory concentration IC50 (nM) values. Epitopes that bind with an affinity of <50 nM are considered to have high affinity, those that bind with <500 nM have an intermediate affinity and those that bind with <5000 nM have a low affinity [28]. All epitopes with high or intermediate affinity are considered ‘true binders’, while epitopes with low affinity are considered ‘nonbinders’. No known T-cell epitope has an IC50 value of >5000 nM [25]. The scoring system of the SYFPEITHI prediction tool depends on whether peptide amino acids are frequently occur in anchor positions. Optimal anchor residues are given the value 15 and scores of -1 or -3 points are given to amino acids that have a negative effect on epitopes’ binding ability at a certain sequence position. Epitopes that bind strongly are among the top 2% of all peptides predicted in 80% of all predictions results [49]. The Rankpep tool uses position-specific scoring matrices (PSSMs) to predict MHC-II T-cell epitopes [50]. A high peptide score percentage indicates that the epitope is likely to bind to the set of aligned peptides that bind to a given MHC-II molecule [50]. The peptide lengths in all the resulting sets were based on the complex of 15 mer core region peptides of MHC class II molecules.
Evaluation and statistical analysis
Using the training set of epitopes, the performance of the four MHC-II epitope prediction tools, selected through our inclusion/exclusion criteria, were assessed as weak, intermediate or high binders. The prediction results were classified into two categories, true positive (TP) and false negative (FN), based on the threshold values [88]. In addition, the evaluation assessed sensitivity (TP/ [TP+FN]). Nonparametric Spearman correlation and Bland-Altman analyses were performed to show the relationship and agreement between the scores derived from the NetMHC-II 2.2 and IEDB tools. The level of significance was set at p < 0.05 for the correlation test.
Mice and parasites
6–8 weeks male C57BL/6 mice (Envigo) were fed autoclaved food and water and were maintained under specific pathogen-free conditions. Parasite maintenance, ES collection from adult T. muris worms and the method used for infection and evaluation of worm burden were carried out as described previously [89].
Ethics statement
All animal experiments were approved by the University of Manchester Animal Welfare and Ethical Review Board and performed under the regulation of the Home Office Scientific Procedures Act (1986), and the Home Office approved licence 70/8127.
Plasmid construction
The coding sequence for native VLP (HBc-Ag) was engineered with BamHI + EcoRI sites to allow insertion of peptide antigen sequences into the major immunodominant region (MIR). The entire HBc-Ag coding sequence was inserted into the pET17b expression vector between the NdeI (CATATG) and XhoI (CTCGAG) restriction sites. The insertion of each MHC-II T-cell epitope into the MIR was obtained by annealing the relevant oligonucleotide primers (S4 Table) with BamHI + EcoRI restriction sites and ligation into BamHI/EcoRI cut HBc-Ag in the pET-17b vector. Constructs containing MHC-II T-cell epitopes were confirmed by DNA sequencing.
Production and purification of VLP recombinant proteins
The recombinant plasmids were transformed into ClearColi-BL21 (DE3) electrocompetent cells (Lucigen) by heat-shock transformation. The bacterial culture was grown in LB media containing 100 μg/mL ampicillin and incubated at 37°C for 14 hours in a shaker incubator. The transformed cells at a starting optical density (0.1 OD600) were inoculated into LB liquid media supplemented with 100 μg/mL ampicillin for 3–4 hours until the optical density at 595 nm (OD600) reached 0.6. Isopropyl-b-D-thiogalactopyranoside (IPTG) was added to the culture to a final concentration of 0.4 mM, and the culture was grown by continuous shaking for 12–16 hours at 16°C. The cells were then harvested by centrifugation, resuspended and sonicated in Strep-Tag washing buffer (100 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA) contaning 1 tablet of cOmplete TM EDTA- free protease inhibitor cocktail (Sigma-Aldrich) per 50 mls of the resuspended cells along with 5 μg/ml DNase I (Sigma-Aldrich). The cell suspension was then disrupted by ultra-sonication on ice using Banddelinuw 3200 (Sonoplus) amplitude (Am) in 35% for 5 sec on and 10 sec off for 5–8 minutes. The supernatant containing soluble recombinant protein was harvested following centrifugation at 18,900 x g for 40 mins at 4°C using Sorvall RC Plus with the Fiber-Lite F21 8x50y rotor. Finally, the soluble supernatant was filtered through 0.22 μm pore size filters. After filtration, Strep(II)-tag proteins were purified using affinity column chromatography using a StrepTrap column prepacked with StrepTactin Sepharose by following the manufacturer’s protocol (GE Healthcare). The VLP recombinant proteins were further purified by size exclusion chromatography (Superose 6, 10/300 GL;GE Healthcare). The level of endotoxin in all the purified VLPs was measured with an ELISA-based endotoxin detection assay (Hyglos) following the manufacturer’s protocol.
SDS-PAGE
The VLP recombinant protein samples were subjected to 10% SDS-PAGE and subsequently characterized by TEM as described previously [20]. Briefly, the VLP recombinant proteins were separated on 10% polyacrylamide gels and stained with instant blue protein stain (Expedeon).
In vitro stimulation of BMDCs and BMDMs
For BMDCs, bone marrow cells were harvested from mice into RPMI 1640 (Sigma-Aldrich) supplemented with 5% heat-inactivated FCS (Hyclone, Logon, UT), 100 μg /mL penicillin and 100 μg/mL streptomycin (Sigma-Aldrich), 2 mM L-Glutamine (Sigma-Aldrich), 50 μM β-mercaptoethanol (Gibco) and 20 ng/ml granulocyte-macrophage colony-stimulating factor (GM-CSF) (eBioscience). For BMDMs, BM cells were harvested from mice into modified Eagle′s Medium (DMEM)–high glucose (Sigma-Aldrich) supplemented with 5% heat-inactivated FCS (Hyclone, Logon, UT), 100 μg/mL penicillin and 100 μg/mL streptomycin (Sigma-Aldrich), Eagle′s minimum essential medium (MEM) non-essential amino acid solution (Sigma-Aldrich), and 0.1 mg/ml macrophage colony-stimulating factor (M-CSF) (eBioscience). BMDMs and BMDCs on day 8 were collected at 1X106/ml and incubated with 10 μg/ml VLP recombinant protein for overnight at 37°C. ES at a final concentration 50 μg/ml and lipopolysaccharide (LPS) at final concentration 0.1 μg/ml were used as positive controls, and untreated cells were used as a negative control. After 24 hours, the supernatants were harvested separately and stored at -20°C until analysed for cytokine content by Legendplex or CBA according to the manufacturer’s instructions.
Fluorescein-conjugated VLP internalization and localization in APCs
BMDCs and BMDMs on day 8 were collected at 1X106/ml and incubated with 10 μg/ml with fluorescein-conjugated VLP separately (HBc-CBD1243-1259, HBc-CBD241-257, HBc-CLSP143-158 and HBc-CLSP398-416) for overnight at 37°C in 5% CO2. Unstimulated BMDCs and BMDMs were used as negative controls. Next day, cells were incubated with 50 mM LysoTracker dye obtained from (Invitrogen) for 45 min prior to harvesting to visualise the lysosomal localisation of fluorescein-conjugated VLPs by ImageStreamX cytometry. A Brightfield-1 filter was employed to image dendritic cells and macrophages, a fluorescein isothiocyanate (FITC-488 nm) filter to image fluorescein-conjugated VLPs, and an (APC-592 nm) filter to image lysosomes. The data were analysed using IDEAS software version 6.2.187.0. The degree of co-localization was measured by the Bright Detail Similarity (BDS-R3) on a cell-by-cell basis. A Bright Detail Similarity value of 1.0 indicates a high degree of similarity between two images in the same spatial location (correlated) and a value around 0 has no significant similarity (uncorrelated).
Immunization schedule and challenge infection
Mice were divided randomly into 4 groups: mice were inoculated s.c. with overnight ES antigens emulsified with an equal volume of Alum-an aluminum salt adjuvant [90] obtained from Thermo Scientific to achieve a vaccine dose of 100 μg ES in 100 μl Alum as a positive control. The negative group was inoculated s.c. with 200 μl of sterile PBS. Alternatively, mice were vaccinated with 200 μl of 50 μg VLP (HBc-Ag). The vaccinated mouse group was administrated with 200 μl of 50 μg pre-mixed VLPs+T-cell epitopes (HBc-CBD1243-1259, HBc-CBD241-257, HBc-CLSP143-158 and HBc-CLSP398-416). All experimental groups were vaccinated in the scruff of the neck at (day -20) and were boosted with the same dose at (day -10) in the same site. At day 10 post vaccination, mice were challenged orally with a high dose of T. muris (200 eggs) per mouse. Mice were sacrificed 14 days after challenge infection by an overdose of CO2.
Ag-specific antibody detection in the serum
Blood samples were collected immediately from mice by cardiac puncture and left at room temperature to clot. Parasite-specific and VLP recombinant protein-specific antibodies (IgM, IgG1 and IgG2c) were determined in sera by enzyme-linked immunosorbent assay (ELISA) as previously described [91]. Briefly, 96-well plates were coated with 50 μg/well of the overnight T. muris E/S antigen at 5 μg/ml or with 50 μg/well of the purified VLP recombinant protein in 0.5 M carbonate-bicarbonate buffer for overnight at 4°C. The plates were washed and then blocked with 3% BSA (Sigma-Aldrich) in PBS Tween-20 (PBS-T20) (0.05% Tween 20, Sigma-Aldrich) for 1 h at 37°C. After washing, diluted sera were added and incubated for 1 h at 37°C. Antibody responses were detected using the Biotinylated rat anti-mouse IgM, IgG1 and IgG2a/c (BD Biosciences). After washing, streptavidin peroxidase (Sigma-Aldrich) was added to the plates and incubated for 1 hour at room temperature. The TMB ELISA substrate (3, 3’, 5, 5’-tetramethylbenzidine- Thermo) was used to develop color and stopped with 0.003% (H2SO4). The optical density was measured with a Dynex MRX11 plate reader (DYNEX Technologies) at 450 nm with a reference of 570 nm.
IgE ELISA
Serum was assayed for total IgE antibody production. 96 well plates were coated with purified anti-mouse IgE (2ug/ml, Biolegend, Clone: RME-1) in 0.05M carbonate/ bicarbonate buffer and incubated overnight at 4°C. Following coating, plates were washed in PBS-Tw and non-specific binding blocked with 3% BSA (Sigma-Aldrich) in PBS for 1 hour at room temperature. Plates were washed and diluted serum (1:10) added to the plate and incubated for 2hrs at 37°C. After washing HRP conjugated goat anti-mouse IgE (1ug/ml; Bio-rad) was added to the plates for 1 hour. Finally, plates were washed and developed with TMB substrate kit (BD Biosciences, Oxford, UK) according to the manufacturer’s instructions. The reaction was stopped using 0.18M H2SO4, when sufficient colour had developed. The plates were read by a MRX II microplate reader (DynexTechnologies, VA, USA) at 450nm, with reference of 570nm subtracted.
MCPT-1 ELISA
Serum MCPT-1 levels were assayed at a 1:100 dilution utilising a mouse MCPT-1 ELISA kit (Invitrogen) according to manufacturer’s instructions.
In vitro stimulation of MLN
MLNs were harvested from mice at autopsy and dissociated in RPMI-1640 medium (Sigma-Aldrich) supplemented with 10% foetal calf serum (FCS), 1% L-Glutamine, and 1% penicillin/streptomycin (all Invitrogen Ltd., Paisley, UK). 5x106/ml MLN cells were re-stimulated in vitro in 96-well plates with 50 μg/ml T. muris E/S antigens and with 10 μg/ml pre-mixed VLPs+T-cell epitopes proteins (HBc-CBD1243-1259, HBc-CBD241-257, HBc-CLSP143-158, and HBc-CLSP398-416) at 37°C for 48 hours. Cytokine concentration (IL-4, IL-5, IL-9, IL-13, IFN-ϒ, TNF-α, IL-6, IL-17 and IL-10) was quantified in the supernatants by cytometric bead array (CBA) according to the manufacturer’s instructions from the Mouse/Rat Soluble Protein Flex Set System kit (BD Biosciences Pharmingen, Oxford, UK). CBA data was analysed on a MACSQuant analyser with MACSQuantify Software and the CBA analysis software package (BD Biosciences).
Histology
At autopsy, colonic tissue samples were removed and fixed at room temperature for overnight in 10% neutral buffered formalin, prior to storage in 70% EtOh and processing and embedding in paraffin wax. 5 μm thick serial sections were cut on a Microrn HM325 microtome (Microm International, Germany), de-waxed in citroclear and rehydrated prior to periodic acid–Schiff and Hematoxylin and Eosin staining. Stained slides of proximal colon sections were scanned using 3D Histech Pannoramic 250 flash slide scanner. Photographs of the sections were taken at 100X magnification using panoramic viewer version 1.15.4 software. The number of goblet cells was determined as the total number of PAS-positive cells in 60 randomly selected crypts in three fields of view from each section. All samples were counted in a blinded fashion.
Statistics
Statistical analyses were performed using Graph Pad Prism, version 7.00 software. In all tests, P≤0.05 were considered statistically significant and were determined using the Kruskal–Wallis non-parametric ANOVA for comparing multiple groups.
Supporting information
Left: Chromatogram of the VLP recombinant proteins HBc-Ag (A), HBc-CBD1243-1259 (B), HBc-CBD241-257 (C), HBc-CLSP143-158, (D), and HBc-CLSP398-416 (E) purified using SEC. Separation was carried out on a Superose 6, 10/300 GL (GE Healthcare), at a flow rate of 0.2 ml/min with 100 mM Tris-HCl, 150 mM NaCl, 1 mM EDTAatpH 8. VLP recombinant proteins were eluted in 0.5 ml fraction and a sample of SEC the elution peak was visualised by SDS-PAGE. Vertical red lines indicate the elution volume of mass standards, from left to right: 2MDa, 670kDa. Right: Purification visualised by SDS-PAGE (10% gel) analysis stained with Coomassie blue. M: protein marker (Precision Plus protein standard, unstained, Bio-Rad); Lane 1: VLP recombinant protein after Strep-Tag affinity purification using the StrepTrap HP, and Lane 2: VLP recombinant protein after SEC purification. The arrow represents VLP recombinant protein monomer with an expected molecular mass of ~22 kDa.
(PPTX)
(A-E) Cytokine productions by mesenteric lymph node cells in mice vaccinated with VLPs+T-cell epitopes and control mice. (A) MLNs from mice vaccinated with 50 μg of pre-mixed of VLPs+T-cell epitopes (HBc-CBD1243-1259, HBc-CBD241-257, HBc-CLSP143-158 and HBc-CLSP398-416), 50 μg of VLP (HBc-Ag), 50 μg ES/Alum, or PBS on day -20 and boosted on day -10 were stimulated at 5x106/ml with 50 μg/ml T. muris ES. After 48 hours of stimulation, cell culture supernatants were harvested and assayed by cytometric bead array for IL-4 (A), IL-6 (B), IL-10 (C), IL-17 (D), and TNF-α (E) production. Results are shown as mean ± SEM. n = 11 mice per group. The results presented are from two separated experiment pooled together. (F) Serum mMCPT-1 levels assayed at a 1:100 dilution utilising a mouse mMCPT-1 ELISA kit (Invitrogen) according to manufacturer’s instructions. (G) Day 14 p.i. sera were titrated against purified anti-mouse IgE to assess serum IgE antibody levels in VLPs+T-cell epitopes, VLP (HBc-Ag), PBS and in ES/Alum vaccinated mice by ELISA (reading at 405 nm, with reference of 570nm subtracted). Statistical analyses were carried out using the Kruskal-Wallis test (multiple comparisons). Significant differences between groups are represented by (*P≤0.05, **P≤0.01, ***P≤0.001) with a line. This experiment was repeated two times, and the ELISA results shown here are representative of the two experiments.
(PPTX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank the University of Manchester Histology, Bio-imaging and Flow Cytometry Core Facility for facilitating the image analyses.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
AZ was awarded a PhD studentship from King Abdul Aziz University in Jeddah, Saudi Arabia. RF is supported by Medical Research Council UK grant MR/NO22661/1 to KE (https://mrc.ukri.org/). HS is supported by Medical Research Council UK grant: MR/NO13751/1 (https://mrc.ukri.org/). IM is supported by Biotechnology and Biological Sciences grant BB/P018157/1 to KE (https://bbsrc.ukri.org/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Alexander T.Y. and Blackburn B.G., Soil-Transmitted Helminths: Ascaris, Trichuris, and Hookworm Infections. Water and Sanitation-Related Diseases and the Environment: In the Age of Climate Change, 2019: p. 95. [Google Scholar]
- 2.WHO, W.h.O., Soil-transmitted helminthiasis: Deworming campaign improves child health, school attendance in Rwanda Geneva: WHO Library, 2015. [Google Scholar]
- 3.Farrell S.H., et al. , Investigating the Effectiveness of Current and Modified World Health Organization Guidelines for the Control of Soil-Transmitted Helminth Infections. Clinical Infectious Diseases, 2018. 66(suppl_4): p. S253–S259. 10.1093/cid/ciy002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Speich B., et al. , Efficacy and safety of albendazole plus ivermectin, albendazole plus mebendazole, albendazole plus oxantel pamoate, and mebendazole alone against Trichuris trichiura and concomitant soil-transmitted helminth infections: a four-arm, randomised controlled trial. The Lancet infectious diseases, 2015. 15(3): p. 277–284. 10.1016/S1473-3099(14)71050-3 [DOI] [PubMed] [Google Scholar]
- 5.Jenkins S.N. and Wakelin D., The source and nature of some functional antigens of Trichuris muris. Parasitology, 1977. 74(2): p. 153–161. [DOI] [PubMed] [Google Scholar]
- 6.Shears R.K., et al. , Vaccination Against Whipworm: Identification of Potential Immunogenic Proteins in Trichuris muris Excretory/Secretory Material. Scientific reports, 2018. 8(1): p. 4508 10.1038/s41598-018-22783-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shears R., et al. , Extracellular vesicles induce protective immunity against Trichuris muris. Parasite immunology, 2018: p. e12536 10.1111/pim.12536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Briggs N., et al. , Trichuris muris whey acidic protein induces type 2 protective immunity against whipworm. PLoS pathogens, 2018. 14(8): p. e1007273 10.1371/journal.ppat.1007273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geldhof P., et al. , Recombinant expression systems: the obstacle to helminth vaccines? Trends in parasitology, 2007. 23(11): p. 527–532. 10.1016/j.pt.2007.08.012 [DOI] [PubMed] [Google Scholar]
- 10.Hewitson J.P. and Maizels R.M., Vaccination against helminth parasite infections. Expert review of vaccines, 2014. 13(4): p. 473–487. 10.1586/14760584.2014.893195 [DOI] [PubMed] [Google Scholar]
- 11.Del Tordello E., Rappuoli R., and Delany I., Reverse Vaccinology: Exploiting Genomes for Vaccine Design. Human Vaccines: Emerging Technologies in Design and Development, 2017: p. 65–86. [Google Scholar]
- 12.Capecchi B., et al. , The genome revolution in vaccine research. Current issues in molecular biology, 2004. 6: p. 17–28. [PubMed] [Google Scholar]
- 13.Moise L., et al. , Harnessing the power of genomics and immunoinformatics to produce improved vaccines. Expert opinion on drug discovery, 2011. 6(1): p. 9–15. 10.1517/17460441.2011.534454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnon R. and Ben-Yedidia T., Old and new vaccine approaches. International immunopharmacology, 2003. 3(8): p. 1195–1204. 10.1016/S1567-5769(03)00016-X [DOI] [PubMed] [Google Scholar]
- 15.Gregson A.L., et al. , Phase I trial of an alhydrogel adjuvanted hepatitis B core virus-like particle containing epitopes of Plasmodium falciparum circumsporozoite protein. PloS one, 2008. 3(2): p. e1556 10.1371/journal.pone.0001556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roose K., et al. , Hepatitis B core–based virus–like particles to present heterologous epitopes. Expert review of vaccines, 2013. 12(2): p. 183–198. 10.1586/erv.12.150 [DOI] [PubMed] [Google Scholar]
- 17.Wynne S., Crowther R., and Leslie A., The crystal structure of the human hepatitis B virus capsid. Molecular cell, 1999. 3(6): p. 771–780. 10.1016/s1097-2765(01)80009-5 [DOI] [PubMed] [Google Scholar]
- 18.Mosavat A., et al. , Fused Mycobacterium tuberculosis multi-stage immunogens with an Fc-delivery system as a promising approach for the development of a tuberculosis vaccine. Infection, Genetics and Evolution, 2016. 39: p. 163–172. 10.1016/j.meegid.2016.01.027 [DOI] [PubMed] [Google Scholar]
- 19.Cabrera-Mora M., et al. , A recombinant chimeric Ad5/3 vector expressing a multistage plasmodium antigen induces protective immunity in mice using heterologous prime-boost immunization regimens. The Journal of Immunology, 2016: p. 1501926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouchery T., et al. , The Differentiation of CD4(+) T-Helper Cell Subsets in the Context of Helminth Parasite Infection. Front Immunol, 2014. 5: p. 487 10.3389/fimmu.2014.00487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dixon H., Little M.C., and Else K.J., Characterisation of the protective immune response following subcutaneous vaccination of susceptible mice against Trichuris muris. International journal for parasitology, 2010. 40(6): p. 683–693. 10.1016/j.ijpara.2009.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dixon H., Johnston C., and Else K., Antigen selection for future anti‐Trichuris vaccines: a comparison of cytokine and antibody responses to larval and adult antigen in a primary infection. Parasite immunology, 2008. 30(9): p. 454–461. 10.1111/j.1365-3024.2008.01035.x [DOI] [PubMed] [Google Scholar]
- 23.Else K., et al. , MHC-restricted antibody responses to Trichuris muris excretory/secretory (E/S) antigen. Parasite immunology, 1990. 12(4‐5): p. 509–527. [DOI] [PubMed] [Google Scholar]
- 24.Foth B.J., et al. , Whipworm genome and dual-species transcriptome analyses provide molecular insights into an intimate host-parasite interaction. Nature genetics, 2014. 46(7): p. 693 10.1038/ng.3010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang P., et al. , A systematic assessment of MHC class II peptide binding predictions and evaluation of a consensus approach. PLoS Comput Biol, 2008. 4(4): p. e1000048 10.1371/journal.pcbi.1000048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang P., et al. , Peptide binding predictions for HLA DR, DP and DQ molecules. BMC bioinformatics, 2010. 11: p. 568 10.1186/1471-2105-11-568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nielsen M., et al. , NetMHCIIpan-2.0-Improved pan-specific HLA-DR predictions using a novel concurrent alignment and weight optimization training procedure. Immunome Res, 2010. 6(9): p. 7580–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nielsen M. and Lund O., NN-align. An artificial neural network-based alignment algorithm for MHC class II peptide binding prediction. BMC bioinformatics, 2009. 10(1): p. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bui H.H., et al. , Development of an epitope conservancy analysis tool to facilitate the design of epitope-based diagnostics and vaccines. BMC Bioinformatics, 2007. 8: p. 361 10.1186/1471-2105-8-361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dimitrov I., et al. , AllerTOP v. 2—a server for in silico prediction of allergens. Journal of molecular modeling, 2014. 20(6): p. 1–6. [DOI] [PubMed] [Google Scholar]
- 31.Bancroft A.J., et al. , The effect of challenge and trickle Trichuris muris infections on the polarisation of the immune response. International journal for parasitology, 2001. 31(14): p. 1627–1637. 10.1016/s0020-7519(01)00281-8 [DOI] [PubMed] [Google Scholar]
- 32.King I.L., et al. , Intestinal helminth infection impacts the systemic distribution and function of the naive lymphocyte pool. Mucosal immunology, 2017. 10(5): p. 1160 10.1038/mi.2016.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benedé S. and Berin M.C., Mast cell heterogeneity underlies different manifestations of food allergy in mice. PloS one, 2018. 13(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nardin E.H., et al. , A totally synthetic polyoxime malaria vaccine containing Plasmodium falciparum B cell and universal T cell epitopes elicits immune responses in volunteers of diverse HLA types. The Journal of Immunology, 2001. 166(1): p. 481–489. 10.4049/jimmunol.166.1.481 [DOI] [PubMed] [Google Scholar]
- 35.Salman A.M., et al. , Rational development of a protective P. vivax vaccine evaluated with transgenic rodent parasite challenge models. Scientific reports, 2017. 7: p. 46482 10.1038/srep46482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moura A.P.V., et al. , Virus-like particle display of the α-Gal carbohydrate for vaccination against Leishmania infection. ACS central science, 2017. 3(9): p. 1026–1031. 10.1021/acscentsci.7b00311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo J., et al. , Immunogenicity of a Virus-Like-Particle Vaccine Containing Multiple Antigenic Epitopes of Toxoplasma gondii Against Acute and Chronic Toxoplasmosis in Mice. Front Immunol, 2019. 10: p. 592 10.3389/fimmu.2019.00592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee S.-H., et al. , Evaluation of protective efficacy induced by virus-like particles containing a Trichinella spiralis excretory-secretory (ES) protein in mice. Parasites & vectors, 2016. 9(1): p. 384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee D.-H., et al. , Virus-like particles vaccine containing Clonorchis sinensis tegumental protein induces partial protection against Clonorchis sinensis infection. Parasites & vectors, 2017. 10(1): p. 626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Else K.J. and Grencis R.K., Antibody-independent effector mechanisms in resistance to the intestinal nematode parasite Trichuris muris. Infection and immunity, 1996. 64(8): p. 2950–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dhanda S.K., et al. , Novel in silico tools for designing peptide-based subunit vaccines and immunotherapeutics. Brief Bioinform, 2017. 18(3): p. 467–478. 10.1093/bib/bbw025 [DOI] [PubMed] [Google Scholar]
- 42.Yang X. and Yu X., An introduction to epitope prediction methods and software. Reviews in medical virology, 2009. 19(2): p. 77–96. 10.1002/rmv.602 [DOI] [PubMed] [Google Scholar]
- 43.Godkin A.J., et al. , Naturally processed HLA class II peptides reveal highly conserved immunogenic flanking region sequence preferences that reflect antigen processing rather than peptide-MHC interactions. The Journal of Immunology, 2001. 166(11): p. 6720–6727. 10.4049/jimmunol.166.11.6720 [DOI] [PubMed] [Google Scholar]
- 44.Lin HH Z.G., Tongchusak S, Reinherz EL, Brusic Evaluation of MHC-II peptide binding prediction servers: applications for vaccine research. BMC Bioinformatics, 2008. 9: p. Suppl 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hegde N.R., et al. , The use of databases, data mining and immunoinformatics in vaccinology: where are we? Expert Opinion on Drug Discovery, 2018. 13(2): p. 117–130. 10.1080/17460441.2018.1413088 [DOI] [PubMed] [Google Scholar]
- 46.Paul S., et al. , Development and validation of a broad scheme for prediction of HLA class II restricted T cell epitopes. Journal of immunological methods, 2015. 422: p. 28–34. 10.1016/j.jim.2015.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schumacher F.R., et al. , Building proteomic tool boxes to monitor MHC class I and class II peptides. Proteomics, 2017. 17(1–2). [DOI] [PubMed] [Google Scholar]
- 48.Pappalardo F., et al. , Computational modelling approaches to vaccinology. Pharmacol Res, 2015. 92: p. 40–5. 10.1016/j.phrs.2014.08.006 [DOI] [PubMed] [Google Scholar]
- 49.Rammensee H.-G., et al. , SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics, 1999. 50(3–4): p. 213–219. 10.1007/s002510050595 [DOI] [PubMed] [Google Scholar]
- 50.Reche P.A., Glutting J.-P., and Reinherz E.L., Prediction of MHC class I binding peptides using profile motifs. Human immunology, 2002. 63(9): p. 701–709. 10.1016/s0198-8859(02)00432-9 [DOI] [PubMed] [Google Scholar]
- 51.Gowthaman U. and Agrewala J.N., In silico tools for predicting peptides binding to HLA-class II molecules: more confusion than conclusion. Journal of proteome research, 2007. 7(01): p. 154–163. [DOI] [PubMed] [Google Scholar]
- 52.Borrás-Cuesta F., et al. , Specific and general HLA-DR binding motifs: comparison of algorithms. Human immunology, 2000. 61(3): p. 266–278. 10.1016/s0198-8859(99)00153-6 [DOI] [PubMed] [Google Scholar]
- 53.Lin H.H., et al. , Evaluation of MHC-II peptide binding prediction servers: applications for vaccine research. BMC bioinformatics, 2008. 9((Suppl 12)): p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao W. and Sher X., Systematically benchmarking peptide-MHC binding predictors: From synthetic to naturally processed epitopes. PLoS Comput Biol, 2018. 14(11): p. e1006457 10.1371/journal.pcbi.1006457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Andreatta M., et al. , An automated benchmarking platform for MHC class II binding prediction methods. Bioinformatics, 2018. 34(9): p. 1522–1528. 10.1093/bioinformatics/btx820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dobano C., et al. , Concentration and avidity of antibodies to different circumsporozoite epitopes correlate with RTS,S/AS01E malaria vaccine efficacy. Nat Commun, 2019. 10(1): p. 2174 10.1038/s41467-019-10195-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Veronico P., et al. , Nematode chitin synthases: gene structure, expression and function in Caenorhabditis elegans and the plant parasitic nematode Meloidogyne artiellia. Molecular Genetics and Genomics, 2001. 266(1): p. 28–34. 10.1007/s004380100513 [DOI] [PubMed] [Google Scholar]
- 58.Wei J., et al. , The hookworm Ancylostoma ceylanicum intestinal transcriptome provides a platform for selecting drug and vaccine candidates. Parasites & vectors, 2016. 9(1): p. 518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Foor W.E., Ultrastructural aspects of oocyte development and shell formation in Ascaris lumbricoides. The Journal of parasitology, 1967: p. 1245–1261. [PubMed] [Google Scholar]
- 60.Santos L.N., et al. , De novo assembly and characterization of the Trichuris trichiura adult worm transcriptome using Ion Torrent sequencing. Acta tropica, 2016. 159: p. 132–141. 10.1016/j.actatropica.2016.03.036 [DOI] [PubMed] [Google Scholar]
- 61.Johnston W.L., Krizus A., and Dennis J.W., The eggshell is required for meiotic fidelity, polar-body extrusion and polarization of the C. elegans embryo. BMC biology, 2006. 4(1): p. 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnston W.L., Krizus A., and Dennis J.W., Eggshell chitin and chitin-interacting proteins prevent polyspermy in C. elegans. Current Biology, 2010. 20(21): p. 1932–1937. 10.1016/j.cub.2010.09.059 [DOI] [PubMed] [Google Scholar]
- 63.Cuesta-Astroz Y., et al. , Helminth secretomes reflect different lifestyles and parasitized hosts. International journal for parasitology, 2017. 47(9): p. 529–544. 10.1016/j.ijpara.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 64.Balasubramanian N., Toubarro D., and Simoes N., Biochemical study and in vitro insect immune suppression by a trypsin‐like secreted protease from the nematode Steinernema carpocapsae. Parasite immunology, 2010. 32(3): p. 165–175. 10.1111/j.1365-3024.2009.01172.x [DOI] [PubMed] [Google Scholar]
- 65.Santos L.N., et al. , A proteomic approach to identify proteins from Trichuris trichiura extract with immunomodulatory effects. Parasite immunology, 2013. 35(5–6): p. 188–193. 10.1111/pim.12025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang Y., et al. , Serine proteases of parasitic helminths. The Korean journal of parasitology, 2015. 53(1): p. 1 10.3347/kjp.2015.53.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Toubarro D., et al. , Serine protease-mediated host invasion by the parasitic nematode Steinernema carpocapsae. Journal of biological chemistry, 2010: p. jbc. M110. 129346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dalton J.P., et al. , Helminth vaccines: from mining genomic information for vaccine targets to systems used for protein expression. International journal for parasitology, 2003. 33(5–6): p. 621–640. 10.1016/s0020-7519(03)00057-2 [DOI] [PubMed] [Google Scholar]
- 69.Wang B., et al. , Cloning, expression and characterization of a Trichinella spiralis serine protease gene encoding a 35.5 kDa protein. Experimental parasitology, 2013. 134(2): p. 148–154. 10.1016/j.exppara.2013.03.004 [DOI] [PubMed] [Google Scholar]
- 70.Feng S., et al. , Vaccination of mice with an antigenic serine protease-like protein elicits a protective immune response against Trichinella spiralis infection. The Journal of parasitology, 2013. 99(3): p. 426–432. 10.1645/12-46.1 [DOI] [PubMed] [Google Scholar]
- 71.Xu J., et al. , Immune responses in mice vaccinated with a DNA vaccine expressing serine protease-like protein from the new-born larval stage of Trichinella spiralis. Parasitology, 2017. 144(6): p. 712–719. 10.1017/S0031182016002493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Serradell M.C., et al. , Efficient oral vaccination by bioengineering virus-like particles with protozoan surface proteins. Nature Communications, 2019. 10(1): p. 361 10.1038/s41467-018-08265-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wahl-Jensen V., et al. , Role of Ebola virus secreted glycoproteins and virus-like particles in activation of human macrophages. Journal of virology, 2005. 79(4): p. 2413–2419. 10.1128/JVI.79.4.2413-2419.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lahoud M.H., Fatma Kitsoulis, SusieSan Wan, SooVremec DavidLee, Chin-Nien Phipson, Belinda Shi, Wei Smyth, Gordon K and A.M Lew, Targeting antigen to mouse dendritic cells via Clec9A induces potent CD4 T cell responses biased toward a follicular helper phenotype. The Journal of Immunology, 2011(2): p. 842–850. 10.4049/jimmunol.1101176 [DOI] [PubMed] [Google Scholar]
- 75.Birkett A., et al. , Hepatitis B virus core antigen particles containing minimal T and B cell epitopes of Plasmodium falciparum CS protein elicit high levels of malaria specific immune responses in mice and non-human primates. Am. J. Trop. Med. Hyg, 2001. 65: p. 258. [Google Scholar]
- 76.Birkett A., et al. , A modified hepatitis B virus core particle containing multiple epitopes of the Plasmodium falciparum circumsporozoite protein provides a highly immunogenic malaria vaccine in preclinical analyses in rodent and primate hosts. Infection and immunity, 2002. 70(12): p. 6860–6870. 10.1128/IAI.70.12.6860-6870.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Milich D.R., et al. , Conversion of poorly immunogenic malaria repeat sequences into a highly immunogenic vaccine candidate. Vaccine, 2001. 20(5–6): p. 771–788. 10.1016/s0264-410x(01)00400-5 [DOI] [PubMed] [Google Scholar]
- 78.Nardin E.H., et al. , Phase I testing of a malaria vaccine composed of hepatitis B virus core particles expressing Plasmodium falciparum circumsporozoite epitopes. Infection and immunity, 2004. 72(11): p. 6519–6527. 10.1128/IAI.72.11.6519-6527.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oliveira G.A., et al. , Safety and enhanced immunogenicity of a hepatitis B core particle Plasmodium falciparum malaria vaccine formulated in adjuvant Montanide ISA 720 in a phase I trial. Infection and immunity, 2005. 73(6): p. 3587–3597. 10.1128/IAI.73.6.3587-3597.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gu Y., et al. , Identification and characterization of CD4+ T cell epitopes present in Trichinella spirals paramyosin. Veterinary parasitology, 2016. 231: p. 59–62. 10.1016/j.vetpar.2016.06.022 [DOI] [PubMed] [Google Scholar]
- 81.Datta R., et al. , Identification of novel genes in intestinal tissue that are regulated after infection with an intestinal nematode parasite. Infection and immunity, 2005. 73(7): p. 4025–4033. 10.1128/IAI.73.7.4025-4033.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hasnain S.Z., et al. , Mucin gene deficiency in mice impairs host resistance to an enteric parasitic infection. Gastroenterology, 2010. 138(5): p. 1763–1771. e5. 10.1053/j.gastro.2010.01.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hasnain S.Z., et al. , Serine protease(s) secreted by the nematode Trichuris muris degrade the mucus barrier. 2012. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moher D., et al. , Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. International journal of surgery, 2010. 8(5): p. 336–341. 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 85.Yasser E.-M., Dobbs D., and Honavar V., On evaluating MHC-II binding peptide prediction methods. PLoS One, 2008. 3(9): p. e3268 10.1371/journal.pone.0003268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Murugan N. and Dai Y., Prediction of MHC class II binding peptides based on an iterative learning model. Immunome research, 2005. 1(1): p. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nielsen M., et al. , Improved prediction of MHC class I and class II epitopes using a novel Gibbs sampling approach. Bioinformatics, 2004. 20(9): p. 1388–1397. 10.1093/bioinformatics/bth100 [DOI] [PubMed] [Google Scholar]
- 88.Altman D.G. and Bland J.M., Diagnostic tests. 1: Sensitivity and specificity. BMJ: British Medical Journal, 1994. 308(6943): p. 1552 10.1136/bmj.308.6943.1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wakelin D.J.P., Acquired immunity to Trichuris muris in the albino laboratory mouse. 1967. 57(3): p. 515–524. [DOI] [PubMed] [Google Scholar]
- 90.HogenEsch H., Mechanism of immunopotentiation and safety of aluminum adjuvants. Frontiers in immunology, 2013. 3: p. 406 10.3389/fimmu.2012.00406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Else K., Entwistle G., and Grencis R., Correlations between worm burden and markers of Th1 and Th2 cell subset induction in an inbred strain of mouse infected with Trichuris muris. Parasite immunology, 1993. 15(10): p. 595–600. 10.1111/pim.1993.15.10.595 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Left: Chromatogram of the VLP recombinant proteins HBc-Ag (A), HBc-CBD1243-1259 (B), HBc-CBD241-257 (C), HBc-CLSP143-158, (D), and HBc-CLSP398-416 (E) purified using SEC. Separation was carried out on a Superose 6, 10/300 GL (GE Healthcare), at a flow rate of 0.2 ml/min with 100 mM Tris-HCl, 150 mM NaCl, 1 mM EDTAatpH 8. VLP recombinant proteins were eluted in 0.5 ml fraction and a sample of SEC the elution peak was visualised by SDS-PAGE. Vertical red lines indicate the elution volume of mass standards, from left to right: 2MDa, 670kDa. Right: Purification visualised by SDS-PAGE (10% gel) analysis stained with Coomassie blue. M: protein marker (Precision Plus protein standard, unstained, Bio-Rad); Lane 1: VLP recombinant protein after Strep-Tag affinity purification using the StrepTrap HP, and Lane 2: VLP recombinant protein after SEC purification. The arrow represents VLP recombinant protein monomer with an expected molecular mass of ~22 kDa.
(PPTX)
(A-E) Cytokine productions by mesenteric lymph node cells in mice vaccinated with VLPs+T-cell epitopes and control mice. (A) MLNs from mice vaccinated with 50 μg of pre-mixed of VLPs+T-cell epitopes (HBc-CBD1243-1259, HBc-CBD241-257, HBc-CLSP143-158 and HBc-CLSP398-416), 50 μg of VLP (HBc-Ag), 50 μg ES/Alum, or PBS on day -20 and boosted on day -10 were stimulated at 5x106/ml with 50 μg/ml T. muris ES. After 48 hours of stimulation, cell culture supernatants were harvested and assayed by cytometric bead array for IL-4 (A), IL-6 (B), IL-10 (C), IL-17 (D), and TNF-α (E) production. Results are shown as mean ± SEM. n = 11 mice per group. The results presented are from two separated experiment pooled together. (F) Serum mMCPT-1 levels assayed at a 1:100 dilution utilising a mouse mMCPT-1 ELISA kit (Invitrogen) according to manufacturer’s instructions. (G) Day 14 p.i. sera were titrated against purified anti-mouse IgE to assess serum IgE antibody levels in VLPs+T-cell epitopes, VLP (HBc-Ag), PBS and in ES/Alum vaccinated mice by ELISA (reading at 405 nm, with reference of 570nm subtracted). Statistical analyses were carried out using the Kruskal-Wallis test (multiple comparisons). Significant differences between groups are represented by (*P≤0.05, **P≤0.01, ***P≤0.001) with a line. This experiment was repeated two times, and the ELISA results shown here are representative of the two experiments.
(PPTX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.