Abstract
This review summarizes the key results of recently published studies on the effects of dietary change and nutritional intervention on the human microbiome from around the world, focusing on the USA, Canada, Europe, Asia, and Africa. It first explores mechanisms that might explain the ability of fiber-rich foods to suppress the incidence and mortality from westernized diseases, notably cancers of the colon, breast, liver, cardiovascular, infectious, and respiratory diseases, diabetes, and obesity (1,2). It summarizes studies from Africa which suggest that disturbance of the colonic microbiome may exacerbate chronic malnutrition and growth failure in impoverished communities and highlights the importance of breast feeding. The American section discusses the role of the microbiome in the swelling population of patients with obesity and type 2 diabetes, and examines the effects of race, ethnicity, geography and climate on microbial diversity and metabolism. The studies from Europe and Asia extoll the benefits of whole foods and plant-based diets. The Asian studies examine the worrying changes from low fat high carbohydrate diets to high fat low carbohydrate and the increasing appearance of westernized diseases as in Africa and documents the ability of high fiber traditional Chinese diets to reverse type-2 diabetes and control weight loss. In conclusion, most of the studies reviewed demonstrate clear changes in microbe abundances and in the production of fermentation products, such as short chain fatty acids and phytochemicals following dietary change, but the significance of the microbiota changes to human health, with the possible exception of the stimulation of butyrogenic taxa by fiber rich foods, is generally implied and not measured. Further studies are needed to determine how these changes in microbiota composition and metabolism can improve our health and be used to prevent and treat disease.
Keywords: Diet, Microbiome, Metabolome, Fiber, Fat, colon cancer, obesity, type 2 diabetes, westernized diseases, Prebiotics, Probiotics, Short chain fatty acids, Bile acids
The Human Gut Microbiome and its Metabolites in Health and Disease
The human gut contains trillions of bacterial cells that are reported to be at a ratio of approximately 1:1 with our own cells (3). Thus, they form a vibrant living population that has a metabolic activity similar to the liver. There is consequently a move to describe the microbiota as a whole a new human organ. Their intimate association with the intestinal mucosa, which is also highly biologically active with a high cellular turnover rate, can be expected to have a major impact on human health and the prevention or precipitation of disease. The gut microbiota has a symbiotic relationship with the host, playing a role in maintaining health and metabolic homeostasis through the production of many metabolites. The collective genomes of these micro-organisms that inhabit the gut refer to the metagenome. The development of the gut microbiome begins at birth and in healthy individuals is largely completed within three years, but can be modified by environmental factors, particularly diet composition and volume, and antibiotic therapy (4). This review will focus on the modulatory effects of diet.
Research on the microbiome has expanded rapidly worldwide providing novel insights into both human and animal diseases and interventions designed to alleviate them (5,6). Bacterial populations inhabit the entire length of the human gut from the oral cavity to the rectum. The density and composition of the resident microbial communities, however, differ markedly between anatomical sites and depend on transit rates, host secretions, environmental conditions, substrate availability, and the integrity of the gut wall (7). Overall the predominant bacterial groups in the microbiome are gram positive Firmicutes and gram negative Bacteroidetes (8). The bacteria that reside in the distal parts of the gut contribute to host health through biosynthesis of vitamins and essential amino acids and by production of important metabolites from nondigestible food components (9). Short chain fatty acid (SCFA) metabolites, notably butyrate, propionate and acetate are the byproducts of bacterial fermentation of indigestible carbohydrates, mostly fiber, and act as the major energy source for the colonic epithelial cells and therefore strengthen the mucosal barrier and defense (10).
Dietary Fiber and The Microbiome
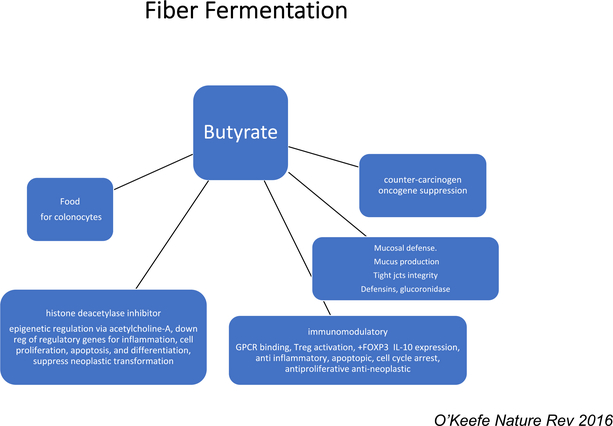
The microbiota is dependent on food residues for survival and metabolism. Recent reviews by ourselves (11) have shown that the fermentation of carbohydrate residues, namely fiber releases metabolites that not only provide food for the colonic epithelium, but also exert a remarkable variety of regulatory effects on colonic mucosal inflammation and proliferation. This helps explain the epidemiological studies from throughout the world that have shown that high fiber consumption maintains colonic health and prevents colon cancer (11). Furthermore, high fiber intakes provide high rates of butyrogenesis which exceed the metabolic requirements of the colonic mucosal and enter the blood stream to exert epigenetic and immunomodulatory effects on other organs in the body. This, in turn, explains the association between high fiber intakes and a reduction in incidence and mortality from all westernized diseases, including cancers of the colon, breast, liver (1,2), cardiovascular, infectious, and respiratory diseases, diabetes, and obesity (12–14). Similar conclusions were drawn by Reynolds based on nearly 135 million person-years of data from 185 prospective studies and 58 clinical trials with 4635 adult participants comparing the highest dietary fiber consumers with the lowest. Here they found a 15–30% lower rate in all-cause and cardiovascular-related mortality, in addition to a lower incidence and mortality in colorectal and breast cancer, coronary heart disease, stroke, and type 2 diabetes. These studies were recently quoted by O’Keefe in his viewpoint for Lancet GI (1) where he updated the evidence supporting Burkitts fiber hypothesis that the deficiency of fiber in the westernized diet can explain much of the morbidity and mortality associated with westernized diseases.
Nutrition and the Gut Microbiome in Africa
Diseases of undernutrition and overnutrition that pose widespread health challenges in Africa are viewed as being ‘microbiota-dependent’ and hence, their mitigation would benefit from ‘microbiota-directed’ interventions (15). Understanding the role of the microbiota in their pathogenesis is indispensable in informing intervention strategies. Research of the past five years on human microbiome and nutrition in Africa, as reviewed in this section, is dominated by intervention trials of childhood undernutrition and exploration of the effect of urbanization on the rising incidences of westernized diseases. In general, studies on undernutrition (summarized in Table 1) investigate impairments of the developing gut microbiome associated with malnutrition and its treatment sequelae while research on westernized diseases aims at finding how the changes in diet and lifestyle affect the gut microbiome and contribute to the increasing risk of these diseases in communities where the incidences were historically low.
Table 1:
Studies of the gut microbiome in undernourished children in Africa from 2015 to 2019
| Location of study and reference | Disease or condition | Age of participants | Number and/or nutritional status of participants | Study design | Study outcomes |
|---|---|---|---|---|---|
|
Uganda Atukunda et al. 2019 |
Undernutrition | 20–24 months | n=77 intervention; n=78 control | Follow-up of open cluster-randomized intervention trial | Cognitive development, growth, gut microbiota |
|
Kenya Paganini et al. 2019 |
Diarrhea and Iron deficiency anaemia | 8–10 months | n=28: 4 groups (with and without iron and antibiotics) | Sub-study of a double-blind randomized controlled intervention trial | Gut microbiome, enteropathogens, gut inflammation, fecal pH, morbidity |
|
Kenya Tang et al. 2017 |
Iron deficiency and iron deficiency anaemia | 6 months | n=45: 3 groups (with and without iron and placebo) | Double-blind individually-randomized controlled trial | Gut microbiome, gut inflammation, Zinc absorption |
|
Gambia Davis et al. 2017 |
Undernutrition | 1–5 months | 33 mother/infant pairs at 4, 16, and 20 weeks postpartum | Sub-study of a randomized trial | Human milk oligosaccharides (HMO), infant gut microbiota, gut inflammation, growth, morbidity |
| Malawi Ordiz et al. 2017 | Environmental enteric dysfunction (EED) | 12–23 months | n=81; 47 boys and 34 girls | Legume intervention trial | Gut microbiota, gut absorption, morbidity |
|
Niger & Senegal Million et al. 2016 |
SAM | <60 months | n=86; not in feeding programs; prior to supplements | 2 prospective case-control studies | Gut microbiota, Aerotolerant odds ratio, gut redox potential, Methanobrevibacter smithii |
|
Malawi Charbonneau et al. 2016 |
Undernutrition | 6 months | n=29 healthy; n=59 severe stunting | Sub-study of 2 randomized controlled single-blind parallel group trials Germ-free mouse and piglet studies |
HMO composition, infant gut microbiota, growth |
|
Uganda Kristensen et al. 2016 |
SAM | 6–24 months | n=87: all hospitalized with SAM | Sub-study of an observational study | Gut microbiota, nutritional outcomes |
|
Malawi Reyes et al. 2015 |
SAM | 0–30 months | 8 monozygotic healthy twin pairs; 12 dizygotic twin pairs (6 twin pairs discordant for kwashiorkor; and the other 6 discordant for marasmus) | Longitudinal studies, follow-up of the Malawian discordant twin trial Germ-free mouse study |
Gut microbiota DNA viromes, nutritional outcomes |
|
Malawi Dewey et al. 2015 |
Diet-dependent enteropathy in SAM | 21 months | One monozygotic twin pair discordant for kwashiorkor | Longitudinal studies, follow-up of the Malawian discordant twin trial Germ-free mouse study |
IgA+ bacterial taxa of the fecal microbiota, Weight loss, sepsis |
|
Kenya Jaeggi et al. 2015 |
Iron deficiency and iron deficiency anaemia | 6 months | n=115, consuming home-fortified maize porridge with or without iron | 2 double-blind randomized controlled trials | Gut microbiome, gut inflammation, morbidity |
Undernutrition and the Gut Microbiome
Undernutrition is a global health challenge accounting for 45% of childhood mortality and, higher morbidities in sub-Saharan Africa and South Asia (16). Malnutrition is a complex of disorders arising from inadequate nutrition that include gut microbiota dysbiosis, metabolic, immune and intestinal barrier dysfunction, as well as micronutrient deficiencies. It is often exacerbated by enteropathogenic and HIV infections, enteropathy, diarrhea, and chronic inflammation. It manifests as severe or moderate, acute or chronic, edematous or nonedematous forms, all of which are managed through hospitalization and subsequent nutritional rehabilitation coupled with antibiotic therapy (17). The challenge in Africa is that, despite the implementation of the World Health Organization (WHO)-recommended treatment protocol, there is still high mortality among children undergoing treatment. It is thought that severe acute malnutrition (SAM) is complicated by co-occurrence in a vicious cycle with infections culminating in death (18). Malnutrition results in adverse health outcomes such, stunting, immune dysfunction and cognitive impairments (15). In 2011, Gordon and colleagues proposed a hypothetical model of relationships between the gut microbiota, the immune system and diet, which underlie the development of malnutrition (19). Years later, they provided evidence that gut microbiota dysbiosis is causally linked to childhood undernutrition, based on longitudinal studies involving Malawian infants and children with varying degrees of undernutrition and corresponding growth rates of gnotobiotic mice to which the kids’ fecal samples had been transplanted (15,20). Since then, several intervention and observational studies (21), completed (Table 1) (22–32) and ongoing (33) have sought to understand the role of gut microbiota in nutritional outcomes, childhood morbidities and enteropathy associated with malnutrition in limited resources settings to improve outcomes. Vray and associates embarked on alternative ‘renutrition’ strategies for the management of moderate acute malnutrition among children across Madagascar, Niger, Central African Republic, and Senegal (34).
Iron-containing micronutrients powders (MNP) are widely used in sub-Saharan Africa in the prevention of iron deficiency anemia and micronutrient deficiencies in infants (23). However there is concern that their iron content may have adverse effects in children simultaneously treated with antibiotics. Antibiotic use is remarkably common in impoverished communities in Kenya, with an average of 4.9 antibiotic courses per child year. In rural Tanzania, more than 50% of infants receive antibiotics by the age of 6 months, chiefly for diarrheal and respiratory diseases. In a controlled intervention trial, Paganini et al. gave small groups (n 6–8) healthy infants who were still being breast-fed various combinations of MNP with and without iron (5mg), and with and without antibiotics (amoxicillin 125 mg/day; ampicillin 125 mg/day + cloxacillin 125 mg/day; or trimethoprim 40 mg/ day + sulphamethoxazole 200mg/day). They were concerned to find that iron supplementation increased the appearance of pathogens such as pathogenic E. coli and decreased Bifidobacterium abundance. In other separate studies Jaeggi et al. and Tang et al. made similar observations that iron supplementation increased entero-pathogen levels and intestinal inflammation in weaning infants (24,25). Notably, a randomized clinical trial investigating the effect of antibiotics on the gut microbiome diversity in Burkina Faso pre-school children also reported that the use of Azithromycin affected the composition of the intestinal microbiome (22). Although these findings discourage use of iron-containing micronutrient powders during antibiotic therapy, they raise concern over the empiric use of antibiotics and nutritional supplements in impoverished communities, where more attention should arguably be paid to improving food, sanitation and housing.
Malawi has exceedingly high rates of infant mortality from malnutrition (35). Consequently there is a desperate need for developing efficacious interventions to ameliorate SAM. To investigate this, Malawian infant twin pairs discordant and concordant for marasmus and kwashiorkor have been involved as microbiota donors in a series of longitudinal studies designed to understand underlying mechanisms of SAM (20,31,32). Remarkably, undernutrition-associated phenotypes such as stunting (29) and diet-dependent enteropathy (32) could be transferred to gnotobiotic mouse models through transplantation of fecal samples or culture-purified bacteria obtained from sick infants. Bearing in mind the previously described low efficacy of ready-to-use-therapeutic foods (RUTFs) (20), there is hope that identification of distinct microbial biomarkers that characterize different states of malnutrition, such as those indicative of stunting and enteropathy, as well as those that can ameliorate these pathologies, is indispensable in evaluating interventions, in different socio-economic settings and geographic locations (4,32). Indeed, the gut microbiota maturity, defined using bacterial taxonomic biomarkers, discriminatory for age and predictive for growth, has been described (4). Its potential as a lead in studying the effects of host and environmental factors on the gut microbiota development and associated diseases, has been demonstrated (15, 32). Such studies portray the coupling of infant twin microbiota donors with gnotobiotic mouse models as powerful tools in investigating the role of gut microbiome in childhood undernutrition and its interventions.
Breast milk is a source of nutrients and bioactive substances required for normal growth and development of the infant (36). It contains pro-, and prebiotics such as milk oligosaccharides (37) that are viewed as potential microbiota-directed therapeutics for childhood undernutrition. These oligosaccharides are beneficial substrates to the gut microbiota contributing to enhanced gut bacteria function, protection from pathogen infection and improved immune response. Gordon and co-workers analyzed human milk oligosaccharides (HMOs) from breast milk of 6-month-postpartum mothers in two independent randomized controlled clinical trials of undernourishment in Malawi (29). The first birth cohort study tested the efficacy of lipid-based nutrient supplements (LNS) to prevent severe stunting among infants (LCNI-5 study), while the second investigated supplementing maternal and infant diet with micronutrient fortified LNS (iLiNS-DYAD-M study).
Initially, breast milk samples were selected from mothers in the LCNI-5 study whose children exhibited healthy growth (height-for-age Z score [HAZ] > 0; n=29) or severe stunting (HAZ < −3; n=59). The HMO abundance and composition were found to associate with infant growth since the breast milk from mothers whose children were severely stunted had significantly lower levels of total, sialylated, and fucosylated HMOs compared to that from mothers whose infants had healthy growth. Among the mothers lacking the FUT2 gene, i.e. unable to produce alpha-1,2-linked fucosylated HMOs (“non-secretor” phenotype), those whose infants were severely stunted produced milk HMOs deficient in fucosylated and sialylated components compared to milk HMOs from mothers of healthy infants. Similar observations were made in mothers enrolled in the iLiNS-DYAD-M study. However, in “secretor” mothers in the latter study, only the sialylated milk HMOs, neither total nor fucosylated, were elevated significantly in those with healthy infants (n=20) compared to the ones with stunted infants (n=40).
To investigate the role of sialylated milk HMOs in infant growth, the researchers used bacteria from fecal microbiota of a stunted infant donor to colonize germ-free mice and piglets feeding on prototypic Malawian diet (control) selectively supplemented with sialylated bovine milk oligosaccharides (S-BMOs) or inulin. Their objective was to determine preclinically if the sialylated HMOs are causally related to healthy growth. After colonizing 5-week-old male germ-free mice with a defined community of 25 bacterial strains, and monitoring body weight and composition for 5 weeks, weight and lean body mass gain were significantly increased by S-BMO supplementation and not inulin. Of the 25 bacterial strains, only Escherichia coli and Bacteroides fragilis responded transcriptionally to S-BMO supplementation in vivo, however, interaction with all the community members was necessary to mediate growth promotion. Further, observations of decreased levels of serum and liver acylcarnitines and increased concentrations of serum insulin, leptin and triglycerides in S-BMO-treated mice compared to controls, over the initial 5-week period, suggested that S-BMO supplementation modulated metabolism in host tissues. Overall, this study demonstrated the causal relationship between S-BMOs and growth as well as the potential of S-BMOs as supplements that have to be tested for improving growth outcomes. In a randomized trial, Davis et al. investigated the relationships between HMOs, microbiota, infant morbidity and growth in Gambian mother-infant dyads (26). Without providing causation mechanisms, the fucosylated HMOs were found to be associated with morbidity while sialylated HMOs were predictive of growth. Prevotella was associated with decreased morbidity, as had been observed in a previous Malawian children cohort study cited (38).
Westernized diseases and the gut microbiome
Nutrition and the gut microbiome have been implicated in the etiology of many westernized diseases (11,39). However, the role of the gut microbiome in the rising incidences of colorectal cancer (CRC) in countries undergoing dietary and lifestyle transitions, typical of sub-Saharan Africa, is just unfolding. The age standardized incidence of CRC per 100,000 has been traditionally <5 (40), but, despite variations between African countries, larger incidences of >10 have been reported in some countries (41). In Zimbabwe, an observational study was recently undertaken to assess the link between CRC risk and dietary changes from traditional to Western during urbanization (41). Owing to retention of fiber intake in the urban population, the levels of butyrate and butyrogenic bacteria were similar between rural and urban participants, yet the levels of bile acids, indicative of high-fat diet were higher among urban participants. This study demonstrated the potential to prevent a CRC epidemic through retention of traditional high fiber foods, a sustained adjustment of diet that would reverse the pattern of metabolic markers associated with CRC risk.
In a previous comparative diet switch study of rural Africans to a high-fat, low-fiber diet, and African Americans to a high-fiber, low-fat diet, reciprocal changes in microbiota, its metabolic activity and mucosal biomarkers of CRC risk were observed within two weeks (42). In yet another comparative metagenomic study, not related to a disease, but attempting to understand how the microbiome has evolved to support hosts living in different environments, the gut microbiome of the Hadza hunter-gatherers of Tanzania was compared with that of the urban Italians (43). The Hadza gut microbiome was enriched with genes with the functional capacity to fermenting carbohydrates into short-chain fatty acids (SCFAs) and synthesize SCFAs from amino acids, while the urban Italian gut microbiome showed functional potential in xenobiotics metabolism (43,44). It has been assessed that the current diet of the Hazda is rich in fiber, containing 75–100 grams of fiber per day mostly from pulp and seeds of baobab fruits (45). Their microbiome features reflected the diet, lifestyle and environment of the study participants, that is, the unindustrialized and traditional rural lifestyle and foraging diet of the hunter-gatherers versus the industrialized and westernized diet and lifestyle of urban Italians. Taken together, there is strong evidence that the gut microbiota metabolizes the plant- and animal-based diet into products that may respectively protect against and promote westernized diseases in African populations (42), and will shift compositionally and functionally in favor of the diet and environment at hand (43) thus affecting health.
Nutrition and the Gut Microbiome in USA and Canada
Nutrition can impact the general well-being of people of every age, race, and ethnicity. Notably, industrialized nations bear witness to an obesity epidemic, setting the stage for vast increases of serious chronic illnesses, such as non-alcoholic fatty liver disease leading to liver failure and metabolic syndrome, which predisposes individuals to diabetes and its multiple micro- and macro-vascular complications. While lifestyle changes through diet and exercise are encouraged to combat the metabolic syndrome, several researchers in Canada and the U.S. have focused on modulation of the gut microbiota as a viable strategy to manage weight and improve metabolic health. These studies are described below.
Prebiotics/Probiotics
Prebiotics, non-digestible fermentable oligo- and polysaccharides that act as a food source for probiotics (living microbes) were used as possible means of modulating the gut microbiota to manage obesity and improve metabolic health. Three studies investigated the effects of prebiotics administered in different formulations on body composition, appetite control, metabolic markers, and gut microbiota in adults and children. Reimer et al. studied the effect of 3 kinds of isocaloric snack bars, one containing the prebiotic, 8 grams inulin-type fructans (ITF), one containing whey protein, a control bar, and combination of ITF + whey protein bars administered twice daily for 12 weeks in 96 overweight or obese adults (46). In a separate study, a daily dose of prebiotic oligofructose-enriched inulin (OI) or an isocaloric amount of control maltodextrin as powder dissolved in water was consumed prior to the evening meal for 16 weeks in 38 overweight and obese but otherwise healthy children (47). ITF, whey protein, and the combination snack bars all improved long-term appetite control but only whey protein was associated with modest reduced body fat in the adult participants. There was no significant additive effect in weight loss or appetite regulation when ITF and whey protein were administered in combination. A significant increase in Bifidobacterium, was observed only with the ITF-containing snack bars. Among children who consumed the OI prebiotic, researchers observed normalized weight gain, reduced whole body and trunk body fat, and decreased primary fecal bile acids, cholic acid and chenodeoxycholic acid compared to placebo. However, there was no reduction in absolute body weight. Bifidobacterium also increased with OI intake compared to placebo. A criticism of the study in children is the distribution of race of the participants was 82% Caucasian and 18% African American and Hispanic. In the third prebiotic study, the effect of a daily dose of 15 grams of yellow pea fiber consumed prior to large meals for 12 weeks in overweight/obese adults with stable weight was examined (48). The consumption of the yellow pea fiber resulted in small improvements in body fat and glucose tolerance along with a reduction of body weight primarily as fat mass but no significant changes in microbiota were detected at this dose.
Probotics have been used to increase the proportion of intestinal microbes known to have beneficial effects on human gut mucosa. However, due to their short colonization time their beneficial effects are limited. In an attempt to produce a probiotic with long-term sustainability, Maldonado-Gómez et al. investigated orally administered Bifidobactrium longum (49). In this double-blind, placebo-controlled, human crossover study, 23 adults consumed a daily dose of 1010 viable cells of B. longum AH1206 or maltodextrin placebo in random succession with 1 to 3 weeks washout between 2 week treatments and 4 weeks test of persistence. In 30% of participants, the strain remained detectable in feces for at least 6 months without causing gastrointestinal symptoms or impacting the resident gut microbiota. The long-term persistence of AH1206 was dependent on individualized features of the resident gut microbiota. These results suggest that bacterial species can be established and sustained in the gut of certain individuals, given the opportunity and suitable environment. Krumbeck, et al. (50,51) describe testing of a probiotic preparation involving an in vivo selection (IVS) method compared with commercially prepared probiotic administered with (synbiotic) and without a prebiotic. In the first study, researchers isolated a bacterial strain of Bifidobacterium (IVS-1) in fecal samples following 9-week administration of increasing doses of prebiotic galactooligosaccharides (GOS) to volunteers. A retrospective analysis of fecal microbiota in one participant demonstrated eight-fold enrichment in Bifidobacterium post intake. The autochthonist (bacteria already resident in the host) IVS-1 functionality was tested in rats administered the bacterial strain alone, the GOS prebiotic alone, or the combination of the pre- and probiotics. IVS-1 out-performed the resident Bifidobacterium in terms of replication in the presence of GOS. Investigators concluded that IVS can be used to successfully formulate a synergistic synbiotic that will overtake other strains naturally resident in the gut (50). In the second study, researchers used a randomized, double-blind, placebo-controlled, parallel arm clinical trial to evaluate IVS bifidobacterial strains and commercial bifidobacterial probiotic preparations with and without GOS prebiotic vs. placebo in improving intestinal permeability among 94 obese adults. Improvements in intestinal permeability were shown with both probiotic treatments and GOS. The treatment effects were primarily directed to permeability of the colon. This 6-arm study did not demonstrate a synergic effect in the two products containing both pre- and probiotics. However, authors stated that the IVS probiotic preparation was more effective than the commercial probiotic in establishing residence in the human gut (51). The latter two studies represent research examining autochthonist vs. commercially prepared probiotics and exploring potential synergism with specific prebiotics. Clearly, these investigators promote the use of individually-isolated probiotic strains naturally resident in the gut over the introduction of foreign strains that are commercially prepared. This practice would be closely aligned with holistic approaches to health care and traditional indigenous healing practices.
Traditional Foods and Ethnicity
While host genetics and lifestyle influence the gut microbiome, we know that diet is the major factor shaping it. Investigators in this study were interested in how seasonality affects the gut microbiome among Canadian Inuit people, assuming that variations in food sources available during different seasons would contribute to large shifts in gut microbiome composition (52).
Stool samples collected from toilet tissue were analyzed for microbiome composition among Canadian Inuit volunteers living in Resolute Bay, Nunavut and Canadian individuals of primarily European descent living in Montreal, Quebec. Important findings were that 45–61% (the greatest proportion) of variability in microbiome composition was due to individual identity. Examining both groups together, 11% of the variability over the course of 12 months was explained by diet. Among Inuit alone, 17% of the variability was due to diet. Sex explained 3–4% of the variability in microbiomes and geography contributed 3–5% to the variability. Microbiomes of women varied more than men and microbiomes among Nunavut residents were more varied than Montreal residents. Unexpectedly, there were no significant seasonal shifts in microbiome composition in either the Inuit in Nunavut or the European descent Montreal groups. Aside from the above results, the study revealed that Canada’s indigenous population living in its northern remote areas is no longer solely reliant on “traditional” food items. Westernized foods are available year-around, which could be the reason why seasonality did not seem to affect microbiome composition in this study. However, the Inuit microbiome did vary over time more than the Montreal diet. Authors suspect greater food security in Montreal and the fact that all foods are available year-round in that area were the reasons for less variability over time in that group. Circumstances in Nunavut resemble those of other indigenous communities in the circumpolar north. Elders and other community residents report less reliance on subsistence lifestyle with the advent of big box stores (e.g., Costco, Walmart, Sam’s Club) in more urbanized areas, increased mechanization and commercial air transport, internet orders through companies that provide free or substantially reduced shipping fees, and the availability of “westernized” prepared foods in the local community stores. A second important study outcome was the assessment of dietary intake measured as mean frequency of consumption (number of times eaten per participant) of each food category per day. The Nunavut group consumed substantially more eggs (mean frequency 0.91 vs. 0.18) than the Montreal group, while the Montreal group consumed more dairy products (mean frequency 1.9 vs. 0.88) and alcoholic beverages (mean frequency 0.96 vs. 0.22). There was also notably less consumption of fruits and vegetables (mean frequency 0.85 vs. 2.72) among the Nunavut group than the Montreal group. Importantly, the Nunavut group diet consisted largely of game meats – raw, cooked, and fermented. The foods traditionally fermented include walrus and whale meat, fish, seal, and caribou. The meat and fat that is caught in the summer is buried in the ground over the autumn and winter months ready for consumption in the next year. These traditional foods are similar to traditional foods consumed by indigenous peoples (Inupiat and Yup’ik) in northern circumpolar regions of Alaska. In Alaska, raw, cooked, and fermented game sources include moose, caribou, and marine mammals, such as whale meat and blubber, sea lion, walrus, seal and seal oil, wild birds and bird eggs, and multiple ocean and river fish. However, all communities have local markets, which stock “western” prepared/preserved foods with long shelf life, decreasing reliance on traditional indigenous food sources.
The developing gastrointestinal microbiota in the first years of life is important for immune function, nutrient metabolism and protection from pathogens (53,54). Early evidence suggests that diet influences the infant gut microbiome (55). There is preliminary evidence that gut microbial composition in adults and children varies by age, dietary intake, ethnicity, geography, and adoption of western life-styles (56,57). In 2017, Stearns et al. explored the effects of race/ethnicity separate from geographical region on the gut microbiota in early life (58). Participants from two prospective Canadian birth cohorts were included. Researchers used 1-year fecal samples from 173 Caucasian infants and 182 infants of South Asian ancestry living in Canada in the main analysis. Results demonstrated that the gut microbiome of infants is influenced by race/ethnicity, age, weight gain, and breastfeeding. South Asian mothers lived in Canada for an average of 8 years (vs. a lifetime for Caucasian mothers), were younger, more likely to be vegetarian, and more likely to be diagnosed with gestational diabetes than the Caucasian mothers. South Asian infants had higher abundance of several members of lactic acid bacteria (LAB) contained in the feces, specifically Bifidobacterium, Lactococcus, Streptococcus, and Enterococcus than in the Caucasian infants feces. LAB are responsible for the breakdown of carbohydrates to lactate and acetate (57,59). The abundance of the Atopobium cluster of Actinobacteria was also higher in South Asian vs. Caucasian infants. This group of bacteria are saccharolytic and have been observed in abundance in the microbiome of individuals with a fiber-rich diet (60). Caucasian infants showed greater abundance of members of Firmicutes from the order Clostridiales, shown to increase in response to diets rich in animal protein and fat (61). This study demonstrates race/ethnicity and infant feeding practices play important roles in the development of the infant gut microbiome. While race/ethnicity are not modifiable, the infant diet is and its alteration can assist in promoting healthy gut microbiota.
Nutrition and the gut microbiome in Europe
Numerous recent clinical studies performed by research groups in Europe examine the effects of whole foods and additives on the gut microbiome and human health. While European diets are quite diverse, the general trend towards ‘Westernization’ of diet (e.g., high intake of fat and low consumption of milk) causes similar health problems in most European countries (e.g., rising incidences of obesity, cardiovascular diseases and colon cancer). In this context of nutrition, gut microbiome and human health, a selection of recent studies is discussed.
Nuts (walnuts in particular) are known to have health benefits in that their consumption was shown to lower plasma lipid levels (62). The aim of one study was to investigate how walnut intake alters the gut microbiome composition and diversity. In a randomized controlled trial (cross-over design) of 96 healthy participants, a walnut-enriched diet was administered for 8 weeks followed by a switch to nut-free diet. (63). A second group of 98 participants followed the dietary pattern in reverse order and fecal samples were collected for 16S rRNA gene sequencing analysis. No difference was found in alpha-diversity, but with regard to beta-diversity a distinct clustering of the walnut and control groups was observed. However, the walnut diet explained only about 5% of observed difference compared to the control diet. In the walnut group, a significantly increased abundance of Ruminococcaceae and Bifidobacteria coincided with a decrease in Clostridium sp. Cluster XIVa species compared to control diet. Thus, walnut intake may promote compositional shifts of the gut microbiota to potentially probiotic and SCFA-producing species that may account for health benefits associated with walnut consumption. However, the link to health-promoting, walnut-dependent metabolites remains speculative, since this study reported compositional differences and did not quantify actual microbial metabolites.
A vegetarian diet rich in vitamins and fibers has been shown to be beneficial to the immune system (64). The aim of another recent study was to investigate the effect of a 3-months lacto-ovo-vegetarian diet on the gut microbiota and the immune system in healthy omnivorous volunteers (65). Fifteen healthy omnivorous volunteers switched to a lacto-ovo-vegetarian diet for 3 months, and blood and fecal samples were collected pre- (month 0) and post-intervention (month 3). Control groups consisted of healthy volunteers staying on their omnivorous diet or vegetarian diet, respectively. All fecal samples were analyzed by metagenomic shotgun-sequencing. While no difference in alpha-diversity was detected, a few genus-level changes were associated with the vegetarian diet intervention. After 3 months of lacto-ovo-vegetarian diet, the abundance of Alistipes was reduced, coinciding with increased abundance of Roseburia inunilivorans, Ruminococcus lactis, Lactobacillus plantarum and Streptococcus thermophiles in the fecal microbiota. However, it needs to be noted that the authors did neither comment on compliance of study participants that switched from an omnivorous to a vegetarian diet nor provided details on how/if dietary intake was recorded, which are crucial parameters to evaluate the obtained results. An additional comparison of both control groups (long-term omnivores and vegetarians) revealed compositional differences at genus and species levels, supporting the idea that long-term dietary patterns are a major driver of gut microbiota assembly. In conclusion, a switch to the vegetarian diet had an impact on gut microbiota composition, but its functional relevance on gut microbial co-metabolism remains to be elucidated.
It is reported that Bifidobacteria can have functional benefits by cross-feeding other members of gut microbiota specialized on production of butyrate, an essential short chain fatty acid for colon epithelial cells (66). The aim of this recent study was to analyze how consumption of fermented dairy products (FDP) – a yogurt fortified with the probiotic Bifidobacterium animalis subsp. lactis, BB-12, may be associated with positive impact on human health (67). In this prospective intervention study, 150 healthy participants received FDP fortified with BB-12 twice a day for 30 days in total. Fecal samples were collected at day 0 and 30 and 16S rRNA gene sequencing analysis performed. While no change in alpha-diversity after FDP intake was shown, a correlation analysis of species occurring together identified 5 major microbial networks and distinct compositional shifts following FDP intake were observed. A paired comparison revealed that abundance of 39 taxa increased and 24 taxa decreased after FDP intake. An increased abundance after FDP intake was demonstrated for Coriobacteriaceae (ability to metabolize lactose to lactate), Bifidobacteriaceae (probiotic, anti-inflammatory), Staphylococcaceae, and Erysipelotrichaceae. Decreased numbers were demonstrated for Lachnoclostridium_unclassified (involved in bile acid metabolism) and Roseburia genera (potential SCFA producers), for example. When investigating changes in lactose-fermenting taxa (e.g., Bifidobacterium, Lactobacillus, Lactococcus), which are likely to be affected by altered FDP intake, the authors identified 2 clusters of study participants: cluster 1 showed a weaker increase in relative abundance of lactose-fermenting bacteria compared to the individuals from cluster 2 and and less changes in overall fecal microbiota composition. In contrast, a higher number of taxa in the fecal microbiota of individuals from cluster 2 was affected by FDP intake, suggesting that cluster 2 can be termed “responders”. Interestingly, these responders showed a lower abundance of lactose-fermenting bacteria in the fecal microbiota at baseline compared to cluster 1 (or “non-responders”), which may explain the observed cluster-specific increase in lactose-fermenting bacteria. Overall, significant shifts in gut microbiota composition suggest changes induced by 30 days of FDP intake, but the effect on microbial metabolism, in particular with regard to human health, remains unclear.
According to the National Health and Nutrition Examination Survey of the U.S.A, dietary phosphate intake of Americans exceeds the daily recommended intake of 700 mg phosphorus for adults and the elderly (68). This is mainly a result of the increased consumption of food prepared or treated with phosphate additives, ranging from baked goods to cola beverages (69). A recent study by Trautvetter et al. aimed at analyzing the impact of high phosphorus intake on gut-related parameters (70). The randomized controlled trial, double-blinded intervention (monosodium phosphate with/without calcium carbonate supplements) with 62 healthy participants consisted of a pre-intervention phase (consumption of placebo for 2 weeks) and an intervention for 8 weeks (monosodium phosphate with or without calcium carbonate). Fecal samples were collected after placebo and intervention and 16S rRNA gene sequencing, fecal water cytotoxicity and targeted short-chain fatty acids were analyzed. Significantly higher levels of total short-chain fatty acids and acetate in feces were detected after 8 weeks on monosodium phosphate with calcium carbonate compared to monosodium phosphate without calcium carbonate. However, no differences in epithelial cell cytotoxicity and only minor differences in gut microbiota composition between men from both groups, but not women were found (e.g., higher abundance of Clostridium XVIII in men receiving the highest levels of monosodium phosphate and calcium carbonate compared to the other groups). These results suggest that there is no strong effect of a high phosphorus diet on gut microbiota composition and metabolism, despite small increases in short-chain fatty acid production.
Nutrition and the gut microbiome in Asia
Weight management, obesity, and metabolic disorders are also a concern in Asian populations as diet and life styles change. The following Asian studies break down the effects of food components and different diets on the gut microbiome and how they may influence health.
Fat
In China, the nutritional transition from a traditional low-fat, high-carbohydrate diet to a diet relatively higher in fat and lower in carbohydrate has been associated with a dramatic increase in the risk of obesity, type 2 diabetes, cardiovascular diseases and colon cancer in the past 30 years (71). Studies have shown that human gut microbiota diversity and richness are reduced when comparing high fat diets with more traditional diets of relatively higher proportions of carbohydrates (72,73). Wan et al. performed a 6-month randomized controlled-feeding trial of 217 healthy young adults, varying the amounts of fat in isocaloric diets containing lower-fat (20% energy), moderate-fat (30% energy), and higher-fat (40% energy) (74). At the phylum level, the moderate and higher fat diets decreased the ratio of Firmicutes to Bacteriodetes after intervention. At the genus level, the higher fat diet decreased the abundance of Faecalibacterium, increased the abundance of Alistipes and Bacteroides, while the lower fat diet increased the Faecalibacterium and Blautia abundance after the intervention. Concentration of fecal butyrate was increased after the lower-fat diet intervention and decreased after the higher-fat intervention. The changes in relative abundance of Blautia was negatively associated with the changes in serum total cholesterol, low-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol, whereas the change in Bacteroides abundance was positively correlated with the changes in these blood lipid markers. Higher-fat consumption by healthy young adults whose diet is in a state of nutrition transition appeared to be associated with unfavorable changes in gut microbiota, faecal metabolomic profiles and plasma proinflammatory factors, which might confer adverse consequences for long-term health outcomes (74). It should however, be noted that these diets were kept isocaloric, so it would be fair to replace low fat with high carbohydrate, and high fat with low carbohydrate. Further the low carbohydrate diet would have lower fiber. In this context, international studies that reflect different environmental conditions are important to identify the effects of diet composition on the gut microbiota. For example, Ocvirk et al. recently showed that the high fat, low carbohydrate and fiber diet of the Alaska Native people (AN) was associated with a very different colonic microbiota composition. In this study, the fecal microbiota showed a compositional predominance of Blautia and fecal levels of short-chain fatty acids were significantly lower compared to a low risk rural African population. The importance of this is that AN have the highest documented risk of colon cancer in the world, and the incidence of other westernized diseases such as obesity, diabetes, and cardiovascular diseases is also high. This highlights the concern about conclusions that dysbiotic signatures may play a causative role in westernized diseases (75).
Fiber
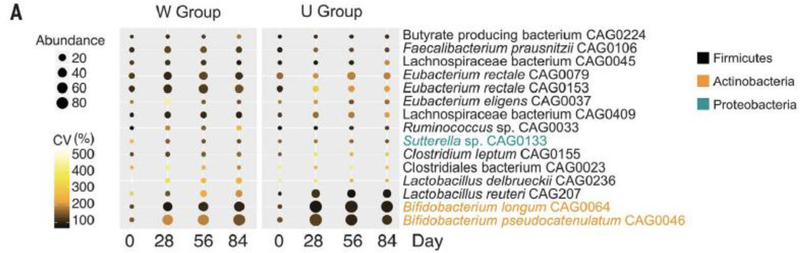
The need to determine whether associations between diet, the microbiome and westernized diseases were indeed causal led to very interesting human trial-experimental animal mechanistic studies reported by Zhao et al. in Science. In a randomized clinical study of Chinese patients with Type 2 diabetes mellitus, 43 patients received either the usual care based on the 2013 recommendations of the Chinese Diabetes Society (control) or a high-fiber diet (approximately 50 grams/day) composed of whole grains, traditional Chinese medicinal foods and prebiotics (WTP) for 84 days (76). Daily energy and macronutrients were similar across groups. Both groups received acarbose as the standardized medication (transforms part of the starch in the diet into a fiber by reducing its digestion and making it more available as fermentable carbohydrate in the colon). A group of 15 SCFA producing strains that were selectively promoted by the WTP diet were detected (76). Promoting this active group of SCFA producers not only enhanced a beneficial function but also maintained a gut environment that keeps detrimental bacteria such as indole- and hydrogen sulfide- producing strains at bay. Hemoglobin A1c (HbA1c) levels (primary outcome measure in this study) were significantly decreased in both groups from baseline in a time-dependent manner, but by day 28 of the study the decrease was greater in the WTP group, partly via increased glucagon-like peptide-1. There was a temporal difference in fasting blood glucose levels—only the WTP group achieved a significant reduction by day 28, although at the end of the intervention there was no difference between groups. The WTP group also showed greater reduction in body weight and better blood lipid profiles than the control group. In another Chinese study, Zhang et al. performed a hospitalized dietary interventional trial for 30 days of 40 children diagnosed with genetic (diagnosed with Prader-Willi syndrome (PWS)) and simple obesity. Some of the children in the PWS group continued on the diet intervention for 60 days. A WTP diet in combination with appropriate amounts of vegetables, fruits and nuts induced significant weight loss and concomitant structural changes of the gut microbiota together with reduction of serum antigen load and alleviation of inflammation (77). This dietary intervention shifted the dysbiotic gut microbiota to a healthier structure with relatively lower levels of bacteria that can produce potentially toxic metabolites from the fermentation of dietary fats and proteins, and higher level of bacteria that can produce potentially beneficial products from the fermentation of carbohydrates.
Chinese Traditional herbal formulas
A traditional Chinese herbal formula, Gegen Qinlian Decoction (GQD), is believed to alleviate type 2 diabetes. A 12 week randomized double-blinded placebo controlled clinical trial in 187 patients with type 2 diabetes by Xu et al. found that low (48 grams), moderate (144 grams), and high doses (240 grams) of GQD induced structural changes of the gut microbiota composition and enriched the amounts of ‘beneficial’ bacteria, such as Faecalibacterium spp., high butyrate producers, in a dose dependent manner (78). Another herbal formulation, the prebiotic Deshipu stachyose granules (DSG), a mixture of α-galacto-oligosaccharides derived from the dietary roots of Lycopus lucidus Turcz, was reported to facilitate intestinal peristalsis and defecation relieving constipation in mice (79). A study designed to investigate the effect of DSG on gut microbiota and bowel function in 100 adult volunteers found that DSG at a dosage of 5 grams per day for 14 days could instructively regulate the gut microbiota with significant increases in ‘probiotic’ species bifidobacteria and lactobacilli and a remarkable decrease in the potential pathobiont Clostridium perfringens. DSG also could effectively improve the bowel function of patients suffering from constipation with a marked increase in defecation frequency from 1.78 times per week to 3.02 times per week (80).
Green tea has been claimed to hold health benefits including anti-colorectal cancer activity and is characterized by the presence of large amounts of polyphenols, also known as catechins, such as epigallocatechin-3-gallate, epigallocatechin, epicatechin gallate, and epicatechin (81). Yuan et al. (82) investigated the effect of green tea liquid (GTL) consumption (400 ml per day for 2 weeks) on the gut and oral microbiome in 12 healthy volunteers. They found an increased FIR:BAC (Firmicutes to Bacteroidetes ratio), elevated SCFA producing genera, and reduction of bacterial LPS synthesis in feces following a 1 week washout period. GTL also altered the salivary microbiota and reduced the functional pathways abundance relevant to carcinogenesis and reduced the fecal levels of Fusobacterium.
Ketogenic Diets
A ketogenic diet (KD) consists of a diet very low in carbohydrates and high in fat. This reduction in carbohydrates puts the body in a state of ketosis due to the need to increase the mobilization and oxidation of fat for energy. A KD has been advocated for the treatment of chronic epilepsy. Xie et al. performed a comparison between 14 infants with epilepsy and 30 healthy control infants before and after KD treatment to explore if the gut microbiota of infants with refractory epilepsy differed with that of age-matched healthy infants (83). The therapeutic effect of the KD on refractory epilepsy and the changes in the gut microbiota were also studied. After being on the KD treatment for a week, 64% of epileptic infants showed an improvement with a 50% decrease in seizure frequency. The gut microbiome structure in epileptic infants differed dramatically from that in healthy infants. Proteobacteria, which was significantly higher in epileptic infants, decreased dramatically after the KD. Cronobacter predominated in the epileptic infants group and remained at a low level both in the healthy infants and in the epileptic infants after the KD. Bacteroides increased significantly in the epileptic infants after KD, in which Prevotella and Bifidobacterium also grew in numbers and kept increasing (83).
Summary
The studies discussed provide irrefutable evidence from around the world that the human microbiome can be modified by dietary change in children and adults. Whilst the associations between specific dietary components and health and disease are strong, a lot more work on causality is needed before specific microbes, or groups of microbes, can be used therapeutically. The evidence for a balanced diet and balanced microbiota being able to prevent westernized diseases and extend good quality lifespan is, however, compelling2.
Figure 1:
Illustration of some of the actions of butyrate released by colonic microbial fermentation of fiber on host metabolism, inflammation and carcinogenesis.
Figure 2:
From the study of Zhjao et al: 43 patients with T2DM were randomized for 84 days to a traditional Chinese high fiber diet. Eighty nine percent of the high fiber group showed normalization of their HbA1c compared to 29% of the control group. Shotgun sequencing identified 15 specific SCFA-producing strains (see above) that were promoted by dietary fiber which were also associated with better improvement in hemoglobin A1c levels and diminished indole and hydrogen sulfide producers.
Table 2:
Studies of the gut microbiome in USA and Canada from 2015 to 2019
| Location of study and reference | Disease or condition | Age of participants | Number and/or nutritional status of participants | Study design | Study outcomes |
|---|---|---|---|---|---|
|
Chicago, USA Krumbeck et al. 2018 |
Obesity | 18–65 years | n=94, 6 treatment groups placebo, probiotic and prebiotic combinations | Randomized, double-blind, placebo-controlled | Gut microbiota |
|
Toronto, Canada DaSilva et al. 2018 |
Non-alcoholic fatty liver disease | 21–68 years | n=39 with biopsy-verified NAFLD, n=28 normal controls | Cross-sectional study | Gut microbiota, glucose, HbA1c, lipid profile |
|
Calgary, Canada Reimer et al. 2017 |
Obesity | 18–75 years | n=125; n=27 control, n=26 inulin, n=21 whey, n=22 inulin+whey | Single center, placebo-controlled, double blind study | Gut microbiota |
|
Calgary, Canada Nicolucci et al. 2017 |
Obesity | 7–12 years; male and female | n=38; n=20 oligofructose-enriched inulin, n=18 placebo maltodextrin | Randomized, controlled study | Gut microbiota |
|
Vancouver, Edmonton, Winnipeg/Winkler-Morden, and Toronto, Canada Stearns et al. 2017 |
Normal health | 1 year | n=173 white Caucasian; n=182 South Asian | Sub-study | Gut microbiota, fecal fat, short chain fatty acids |
|
Montreal, Canada Dubois et al. 2017 |
Normal health | 24–67 years (Inuit) 23–48 years (non-Inuit) |
n=15 Inuit participants; n=9 non-Inuit participants | Observational study | Gut microbiota |
|
Canada/USA Maldonado-Gómez et al. 2016 |
Normal health | 22 and 38 years | n=23 placebo or AH1206 | Double-blind, placebo-controlled, human crossover study | Gut microbiota |
|
Calgary, Canada Lambert et al. 2015 |
Obesity | 44 ± 15 years | n=50)9 male, 41 female); placebo or pea fiber | Randomized controlled-feeding trial | Gut microbiota, body composition, metabolic markers of obesity |
|
Florida, USA Hanifi et al. 2015 |
Normal health | 18–50 years | n=81; 4 treatments, placebo, B.subtilis doses | Double-blind placebo-controlled randomized trial | Gut microbiota |
|
USA/Canada Krumbeck et al. 2015 |
Non-alcoholic fatty liver disease model | 4 week old Sprague-Dawley rats | N=3–6 rats per 5 treatments groups | Bifidobacteria isolated from human stool | Gut microbiota |
|
Toronto, Canada Rahat-Rozenbloom et al. 2014 |
Obesity | 35.8± 4.2 (lean) 42.5±3.9 (overweight and obese) |
n=22; 11 lean and 11 overweight and obese | Observational study | Rate SCFA absorption, gut microbiota, dietary intake |
Table 3:
Studies of the gut microbiome in Europe and Asia from 2015 to 2019
| Location of study and reference | Disease or condition | Age of participants | Number and/or nutritional status of participants | Study design | Study outcomes |
|---|---|---|---|---|---|
|
Russia Volokh et al. 2019 |
Normal health | 18–40 years; male and female | n=150 fermented dairy product | Part of large open prospective controlled study | Gut microbiota |
|
Germany Bamberger et al. 2018 |
Normal health | 63 ± 7 years; male and female | n=96 intervention (walnuts) first; n=98 control first | Randomized, controlled, prospective, cross-over study | Gut microbiota, |
|
Germany Trautvetter et al. 2018 |
Normal health | 29 ± 7 years; male and female | n=62 intervention (phosphorus & calcium); n=62 control first | Double-blind randomized controlled intervention trial | Gut microbiota, fecal fat, short chain fatty acids |
|
Sweden Zhang et al. 2018 |
Normal health | 39 ± 10.1; 34.7 ± 10.4; 33.5 ± 7.4 | n=17 switched to vegetarian diet n=14 control diets (7 omnivorous, 7 vegetarian) |
Sub-study of a randomized trial | Gut microbiota, immune analysis |
|
China Wan et al. 2019 |
Normal health | 18–35 years | n=217; n=73 lower-fat, n=73 moderate-fat, n=71 higher-fat diets | Randomized controlled-feeding trial | Gut microbiota, fecal metabolomics, plasma proinflammatory factors |
|
Japan Kushida et al. 2019 |
Normal health | 20–29 years | n=11 1975 Japanese diet, n=10 modern Japanese diet | Randomized controlled clinical trial | Gut microbiota |
|
China Zhao et al. 2018 |
Type 2 diabetes | Adults | n=43 type 2 diabetes; n=16 control diet or n=27 WTP diet | Randomized clinical trial | Gut microbiota, hemoglobin A1c (HbA1c) |
|
China Yuan et al. 2018 |
Normal health or obese | 27–46 years | n=12, green tea liquid | Observational study | Gut microbiota, oral microbiota |
|
China Zhang et al. 2017 |
Obesity | 3–16 years | n=17 Prader-Willi syndrome; n=21 simple obesity intervention: diet of whole grains, traditional Chinese medicinal foods, prebiotics (WTP) | Open-labeled and self-controlled clinical trial | Gut microbiota, urinary metabolites, fecal metabolites, inflammation analysis |
|
China Xie et al. 2017 |
Epilepsy | 1.95 ± 3.10 years (epilepsy), up to 3 years (healthy control) | n=14 epilepsy, n=30 control before and after ketogenic diet | Controlled clinical trial | Gut microbiota |
|
China Li et al. 2017 |
Constipation | Adults under 65 years | n=100; n=50 (Deshipu stachyose granules), n=50 (control) | Non-blind randomized control trial | Gut microbiota, bowel function |
|
China Xu et al. 2015 |
Type 2 diabetes | 54.23 ± 8.56 (placebo), 55.06 ± 7.49 (low dose), 53.18 ± 8.89 (median dose), 51.24 ± 9.10 (high dose) | n=187; n=44 high dose, n=52 moderate dose, n=50 low dose Gegen Qinlin Decoction, n=41 placebo | Randomized double-blinded, placebo-controlled clinical trial | Gut microbiota, fasting blood glucose, HbA1c, |
Biography












Footnotes
Source and Selection of Articles Reviewed: The aim of this review is to summarize recently published research from human studies on the gut microbiota-modulating effects of diet. It includes sections on microbiome research of populations from around the globe in the USA, Canada, Europe, Asia, and Africa that address specific public health challenges. References for this review were identified through searches of PubMed for peer-reviewed articles published in English from 2014 to August 31, 2019 by use of the terms “Human Nutrition (or Nutrition) and the (Human) Microbiome in USA, Canada, Europe, Asia, and Africa,” appearing in the title or abstract. Articles resulting from these searches, as well as relevant references cited in those articles, were reviewed. The conclusions highlight the future needs and implications for scientists and clinicians in this fast-developing field of research, and the need for high-quality, large-scale controlled human dietary intervention studies.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Bibliography
- 1.O’Keefe SJ. The association between dietary fibre deficiency and high-income lifestyle-associated diseases: Burkitt’s hypothesis revisited. Lancet Gastroenterol Hepatol. 2019. December;4(12):984–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Keefe SJD. Plant-based foods and the microbiome in the preservation of health and prevention of disease. Am J Clin Nutr. 2019. July 3; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016. August 19;14(8):e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Subramanian S, Huq S, Yatsunenko T, Haque R, Mahfuz M, Alam MA, et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature. 2014. June 19;510(7505):417–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold JW, Roach J, Azcarate-Peril MA. Emerging technologies for gut microbiome research. Trends Microbiol. 2016. July 15;24(11):887–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reid G, Nduti N, Sybesma W, Kort R, Kollmann TR, Adam R, et al. Harnessing microbiome and probiotic research in sub-Saharan Africa: recommendations from an African workshop. Microbiome. 2014. April 16;2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graf D, Di Cagno R, Fåk F, Flint HJ, Nyman M, Saarela M, et al. Contribution of diet to the composition of the human gut microbiota. Microb Ecol Health Dis. 2015. February 4;26:26164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker AW, Ince J, Duncan SH, Webster LM, Holtrop G, Ze X, et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011. February 1;5(2):220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005. March 25;307(5717):1915–20. [DOI] [PubMed] [Google Scholar]
- 10.Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001. July;81(3):1031–64. [DOI] [PubMed] [Google Scholar]
- 11.O’Keefe SJD. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol. 2016. December;13(12):691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim Y, Je Y. Dietary fibre intake and mortality from cardiovascular disease and all cancers: A meta-analysis of prospective cohort studies. Arch Cardiovasc Dis. 2016. January;109(1):39–54. [DOI] [PubMed] [Google Scholar]
- 13.Park Y, Subar AF, Hollenbeck A, Schatzkin A. Dietary fiber intake and mortality in the NIH-AARP diet and health study. Arch Intern Med. 2011. June 27;171(12):1061–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aune D, Chan DSM, Lau R, Vieira R, Greenwood DC, Kampman E, et al. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2011. November 10;343:d6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanton LV, Barratt MJ, Charbonneau MR, Ahmed T, Gordon JI. Childhood undernutrition, the gut microbiota, and microbiota-directed therapeutics. Science. 2016. June 24;352(6293):1533. [DOI] [PubMed] [Google Scholar]
- 16.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013. August 3;382(9890):427–51. [DOI] [PubMed] [Google Scholar]
- 17.Nel E Severe acute malnutrition. Curr Opin Clin Nutr Metab Care. 2018;21(3):195–9. [DOI] [PubMed] [Google Scholar]
- 18.Jones KD, Thitiri J, Ngari M, Berkley JA. Childhood malnutrition: toward an understanding of infections, inflammation, and antimicrobials. Food Nutr Bull. 2014. June;35(2 Suppl):S64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011. June 16;474(7351):327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith MI, Yatsunenko T, Manary MJ, Trehan I, Mkakosya R, Cheng J, et al. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science. 2013. February 1;339(6119):548–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oldenburg CE, Sié A, Coulibaly B, Ouermi L, Dah C, Tapsoba C, et al. Effect of commonly used pediatric antibiotics on gut microbial diversity in preschool children in burkina faso: A randomized clinical trial. Open Forum Infect Dis. 2018. November 2;5(11):ofy289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atukunda P, Muhoozi GKM, van den Broek TJ, Kort R, Diep LM, Kaaya AN, et al. Child development, growth and microbiota: follow-up of a randomized education trial in Uganda. J Glob Health. 2019. June;9(1):010431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paganini D, Uyoga MA, Kortman GAM, Cercamondi CI, Winkler HC, Boekhorst J, et al. Iron-containing micronutrient powders modify the effect of oral antibiotics on the infant gut microbiome and increase post-antibiotic diarrhoea risk: a controlled study in Kenya. Gut. 2019;68(4):645–53. [DOI] [PubMed] [Google Scholar]
- 24.Jaeggi T, Kortman GAM, Moretti D, Chassard C, Holding P, Dostal A, et al. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut. 2015. May;64(5):731–42. [DOI] [PubMed] [Google Scholar]
- 25.Tang M, Frank DN, Hendricks AE, Ir D, Esamai F, Liechty E, et al. Iron in micronutrient powder promotes an unfavorable gut microbiota in kenyan infants. Nutrients. 2017. July 19;9(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis JCC, Lewis ZT, Krishnan S, Bernstein RM, Moore SE, Prentice AM, et al. Growth and morbidity of gambian infants are influenced by maternal milk oligosaccharides and infant gut microbiota. Sci Rep. 2017. January 12;7:40466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ordiz MI, Stephenson K, Agapova S, Wylie KM, Maleta K, Martin J, et al. Environmental enteric dysfunction and the fecal microbiota in malawian children. Am J Trop Med Hyg. 2017. February 8;96(2):473–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Million M, Tidjani Alou M, Khelaifia S, Bachar D, Lagier J-C, Dione N, et al. Increased gut redox and depletion of anaerobic and methanogenic prokaryotes in severe acute malnutrition. Sci Rep. 2016. May 17;6:26051. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Charbonneau MR, O’Donnell D, Blanton LV, Totten SM, Davis JCC, Barratt MJ, et al. Sialylated Milk Oligosaccharides Promote Microbiota-Dependent Growth in Models of Infant Undernutrition. Cell. 2016. February 25;164(5):859–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kristensen KHS, Wiese M, Rytter MJH, Özçam M, Hansen LH, Namusoke H, et al. Gut Microbiota in Children Hospitalized with Oedematous and Non-Oedematous Severe Acute Malnutrition in Uganda. PLoS Negl Trop Dis. 2016. January 15;10(1):e0004369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reyes A, Blanton LV, Cao S, Zhao G, Manary M, Trehan I, et al. Gut DNA viromes of Malawian twins discordant for severe acute malnutrition. Proc Natl Acad Sci USA. 2015. September 22;112(38):11941–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kau AL, Planer JD, Liu J, Rao S, Yatsunenko T, Trehan I, et al. Functional characterization of IgA-targeted bacterial taxa from undernourished Malawian children that produce diet-dependent enteropathy. Sci Transl Med. 2015. February 25;7(276):276ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bwakura-Dangarembizi M, Amadi B, Bourke CD, Robertson RC, Mwapenya B, Chandwe K, et al. Health Outcomes, Pathogenesis and Epidemiology of Severe Acute Malnutrition (HOPE-SAM): rationale and methods of a longitudinal observational study. BMJ Open. 2019. February 1;9(1):e023077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vray M, Hedible BG, Adam P, Tondeur L, Manirazika A, Randremanana R, et al. A multicenter, randomized controlled comparison of three renutrition strategies for the management of moderate acute malnutrition among children aged from 6 to 24 months (the MALINEA project). Trials. 2018. December 4;19(1):666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kane AV, Dinh DM, Ward HD. Childhood malnutrition and the intestinal microbiome. Pediatr Res. 2015. January;77(1–2):256–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gordon JI, Dewey KG, Mills DA, Medzhitov RM. The human gut microbiota and undernutrition. Sci Transl Med. 2012. June 6;4(137):137ps12. [DOI] [PubMed] [Google Scholar]
- 37.Williams JE, Price WJ, Shafii B, Yahvah KM, Bode L, McGuire MA, et al. Relationships among microbial communities, maternal cells, oligosaccharides, and macronutrients in human milk. J Hum Lact. 2017. August;33(3):540–51. [DOI] [PubMed] [Google Scholar]
- 38.Gough EK, Stephens DA, Moodie EEM, Prendergast AJ, Stoltzfus RJ, Humphrey JH, et al. Linear growth faltering in infants is associated with Acidaminococcus sp. and community-level changes in the gut microbiota. Microbiome. 2015. June 13;3:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sonnenburg ED, Sonnenburg JL. Starving our microbial self: the deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab. 2014. November 4;20(5):779–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferlay J, Shin H-R, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010. December 15;127(12):2893–917. [DOI] [PubMed] [Google Scholar]
- 41.Katsidzira L, Ocvirk S, Wilson A, Li J, Mahachi CB, Soni D, et al. Differences in Fecal Gut Microbiota, Short-Chain Fatty Acids and Bile Acids Link Colorectal Cancer Risk to Dietary Changes Associated with Urbanization Among Zimbabweans. Nutr Cancer. 2019. April 22;71(8):1313–24. [DOI] [PubMed] [Google Scholar]
- 42.O’Keefe SJD, Li JV, Lahti L, Ou J, Carbonero F, Mohammed K, et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat Commun. 2015. April 28;6:6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rampelli S, Schnorr SL, Consolandi C, Turroni S, Severgnini M, Peano C, et al. Metagenome Sequencing of the Hadza Hunter-Gatherer Gut Microbiota. Curr Biol. 2015. June 29;25(13):1682–93. [DOI] [PubMed] [Google Scholar]
- 44.Turroni S, Fiori J, Rampelli S, Schnorr SL, Consolandi C, Barone M, et al. Fecal metabolome of the Hadza hunter-gatherers: a host-microbiome integrative view. Sci Rep. 2016. September 14;6:32826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Vrieze J Gut instinct. Science. 2014. January 17;343(6168):241–3. [DOI] [PubMed] [Google Scholar]
- 46.Reimer RA, Willis HJ, Tunnicliffe JM, Park H, Madsen KL, Soto-Vaca A. Inulin-type fructans and whey protein both modulate appetite but only fructans alter gut microbiota in adults with overweight/obesity: A randomized controlled trial. Mol Nutr Food Res. 2017. August 29;61(11). [DOI] [PubMed] [Google Scholar]
- 47.Nicolucci AC, Hume MP, Martínez I, Mayengbam S, Walter J, Reimer RA. Prebiotics reduce body fat and alter intestinal microbiota in children who are overweight or with obesity. Gastroenterology. 2017. June 5;153(3):711–22. [DOI] [PubMed] [Google Scholar]
- 48.Lambert JE, Parnell JA, Tunnicliffe JM, Han J, Sturzenegger T, Reimer RA. Consuming yellow pea fiber reduces voluntary energy intake and body fat in overweight/obese adults in a 12-week randomized controlled trial. Clin Nutr. 2017;36(1):126–33. [DOI] [PubMed] [Google Scholar]
- 49.Maldonado-Gómez MX, Martínez I, Bottacini F, O’Callaghan A, Ventura M, van Sinderen D, et al. Stable Engraftment of Bifidobacterium longum AH1206 in the Human Gut Depends on Individualized Features of the Resident Microbiome. Cell Host Microbe. 2016. October 12;20(4):515–26. [DOI] [PubMed] [Google Scholar]
- 50.Krumbeck JA, Maldonado-Gomez MX, Martínez I, Frese SA, Burkey TE, Rasineni K, et al. In vivo selection to identify bacterial strains with enhanced ecological performance in synbiotic applications. Appl Environ Microbiol. 2015. April;81(7):2455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krumbeck JA, Rasmussen HE, Hutkins RW, Clarke J, Shawron K, Keshavarzian A, et al. Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Microbiome. 2018. June 28;6(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dubois G, Girard C, Lapointe F-J, Shapiro BJ. The Inuit gut microbiome is dynamic over time and shaped by traditional foods. Microbiome. 2017. November 16;5(1):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Falk PG, Hooper LV, Midtvedt T, Gordon JI. Creating and maintaining the gastrointestinal ecosystem: what we know and need to know from gnotobiology. Microbiol Mol Biol Rev. 1998. December;62(4):1157–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guarner F, Malagelada J-R. Gut flora in health and disease. Lancet. 2003. February 8;361(9356):512–9. [DOI] [PubMed] [Google Scholar]
- 55.Bokulich NA, Chung J, Battaglia T, Henderson N, Jay M, Li H, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016. June 15;8(343):343ra82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. 2012. May 9;486(7402):222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schnorr SL. The diverse microbiome of the hunter-gatherer. Nature. 2015. February 26;518(7540):S14–5. [DOI] [PubMed] [Google Scholar]
- 58.Stearns JC, Zulyniak MA, de Souza RJ, Campbell NC, Fontes M, Shaikh M, et al. Ethnic and diet-related differences in the healthy infant microbiome. Genome Med. 2017. March 29;9(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duncan SH, Louis P, Flint HJ. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl Environ Microbiol. 2004. October;70(10):5810–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martínez I, Lattimer JM, Hubach KL, Case JA, Yang J, Weber CG, et al. Gut microbiome composition is linked to whole grain-induced immunological improvements. ISME J. 2013. February;7(2):269–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen Y-Y, Keilbaugh SA, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011. October 7;334(6052):105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bamberger C, Rossmeier A, Lechner K, Wu L, Waldmann E, Stark RG, et al. A Walnut-Enriched Diet Reduces Lipids in Healthy Caucasian Subjects, Independent of Recommended Macronutrient Replacement and Time Point of Consumption: a Prospective, Randomized, Controlled Trial. Nutrients. 2017. October 6;9(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bamberger C, Rossmeier A, Lechner K, Wu L, Waldmann E, Fischer S, et al. A Walnut-Enriched Diet Affects Gut Microbiome in Healthy Caucasian Subjects: A Randomized, Controlled Trial. Nutrients. 2018. February 22;10(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tanaka T, Kouda K, Kotani M, Takeuchi A, Tabei T, Masamoto Y, et al. Vegetarian diet ameliorates symptoms of atopic dermatitis through reduction of the number of peripheral eosinophils and of PGE2 synthesis by monocytes. J Physiol Anthropol Appl Human Sci. 2001. November;20(6):353–61. [DOI] [PubMed] [Google Scholar]
- 65.Zhang C, Björkman A, Cai K, Liu G, Wang C, Li Y, et al. Impact of a 3-Months Vegetarian Diet on the Gut Microbiota and Immune Repertoire. Front Immunol. 2018. April 27;9:908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rivière A, Selak M, Lantin D, Leroy F, De Vuyst L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front Microbiol. 2016. June 28;7:979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Volokh O, Klimenko N, Berezhnaya Y, Tyakht A, Nesterova P, Popenko A, et al. Human gut microbiome response induced by fermented dairy product intake in healthy volunteers. Nutrients. 2019. March 4;11(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Adatorwovor R, Roggenkamp K, Anderson JJB. Intakes of Calcium and Phosphorus and Calculated Calcium-to-Phosphorus Ratios of Older Adults: NHANES 2005–2006 Data. Nutrients. 2015. November 19;7(11):9633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ritz E, Hahn K, Ketteler M, Kuhlmann MK, Mann J. Phosphate additives in food--a health risk. Dtsch Arztebl Int. 2012. January 27;109(4):49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Trautvetter U, Camarinha-Silva A, Jahreis G, Lorkowski S, Glei M. High phosphorus intake and gut-related parameters - results of a randomized placebo-controlled human intervention study. Nutr J. 2018. February 16;17(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Du SF, Wang HJ, Zhang B, Zhai FY, Popkin BM. China in the period of transition from scarcity and extensive undernutrition to emerging nutrition-related non-communicable diseases, 1949–1992. Obes Rev. 2014. January;15 Suppl 1:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. 2010. August 17;107(33):14691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ou J, Carbonero F, Zoetendal EG, DeLany JP, Wang M, Newton K, et al. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am J Clin Nutr. 2013. July;98(1):111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wan Y, Wang F, Yuan J, Li J, Jiang D, Zhang J, et al. Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: a 6-month randomised controlled-feeding trial. Gut. 2019. February 19;68(8):1417–29. [DOI] [PubMed] [Google Scholar]
- 75.Zackular JP, Rogers MAM, Ruffin MT, Schloss PD. The human gut microbiome as a screening tool for colorectal cancer. Cancer Prev Res (Phila Pa). 2014. November;7(11):1112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao L, Zhang F, Ding X, Wu G, Lam YY, Wang X, et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018. March 9;359(6380):1151–6. [DOI] [PubMed] [Google Scholar]
- 77.Zhang C, Yin A, Li H, Wang R, Wu G, Shen J, et al. Dietary modulation of gut microbiota contributes to alleviation of both genetic and simple obesity in children. EBioMedicine. 2015. August;2(8):968–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu J, Lian F, Zhao L, Zhao Y, Chen X, Zhang X, et al. Structural modulation of gut microbiota during alleviation of type 2 diabetes with a Chinese herbal formula. ISME J. 2015. March;9(3):552–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li T, Lu X, Yang X. Stachyose-enriched α-galacto-oligosaccharides regulate gut microbiota and relieve constipation in mice. J Agric Food Chem. 2013. December 4;61(48):11825–31. [DOI] [PubMed] [Google Scholar]
- 80.Li T, Lu X, Yang X. Evaluation of clinical safety and beneficial effects of stachyose-enriched α-galacto-oligosaccharides on gut microbiota and bowel function in humans. Food Funct. 2017. January 25;8(1):262–9. [DOI] [PubMed] [Google Scholar]
- 81.Stoner GD, Mukhtar H. Polyphenols as cancer chemopreventive agents. J Cell Biochem Suppl. 1995;22:169–80. [DOI] [PubMed] [Google Scholar]
- 82.Yuan X, Long Y, Ji Z, Gao J, Fu T, Yan M, et al. Green tea liquid consumption alters the human intestinal and oral microbiome. Mol Nutr Food Res. 2018. June 10;62(12):e1800178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xie G, Zhou Q, Qiu C-Z, Dai W-K, Wang H-P, Li Y-H, et al. Ketogenic diet poses a significant effect on imbalanced gut microbiota in infants with refractory epilepsy. World J Gastroenterol. 2017. September 7;23(33):6164–71. [DOI] [PMC free article] [PubMed] [Google Scholar]