Spores of a variety of bacteria are resistant to common decontamination agents, and many of them are major causes of food spoilage and some serious human diseases, including anthrax caused by spores of Bacillus anthracis. Consequently, there is an ongoing need for efficient methods for spore eradication, in particular methods that have minimal deleterious effects on people or the environment. UV radiation at 254 nm (UV254) is sporicidal and commonly used for surface decontamination but can cause deleterious effects in humans. Recent work, however, suggests that 222-nm UV (UV222) may be less harmful to people than UV254 yet may still kill bacteria and at lower fluences than UV254. The present work has identified the damage by UV222 that leads to the killing of growing cells and spores of some bacteria, many of which are human pathogens, and UV222 also inactivates a herpesvirus.
KEYWORDS: Bacillus, decontamination, spores, ultraviolet radiation
ABSTRACT
This study examined the microbicidal activity of 222-nm UV radiation (UV222), which is potentially a safer alternative to the 254-nm UV radiation (UV254) that is often used for surface decontamination. Spores and/or growing and stationary-phase cells of Bacillus cereus, Bacillus subtilis, Bacillus thuringiensis, Staphylococcus aureus, and Clostridioides difficile and a herpesvirus were all killed or inactivated by UV222 and at lower fluences than with UV254. B. subtilis spores and cells lacking the major DNA repair protein RecA were more sensitive to UV222, as were spores lacking their DNA-protective proteins, the α/β-type small, acid-soluble spore proteins. The spore cores’ large amount of Ca2+-dipicolinic acid (∼25% of the core dry weight) also protected B. subtilis and C. difficile spores against UV222, while spores’ proteinaceous coat may have given some slight protection against UV222. Survivors among B. subtilis spores treated with UV222 acquired a large number of mutations, and this radiation generated known mutagenic photoproducts in spore and cell DNA, primarily cyclobutane-type pyrimidine dimers in growing cells and an α-thyminyl-thymine adduct termed the spore photoproduct (SP) in spores. Notably, the loss of a key SP repair protein markedly decreased spore UV222 resistance. UV222-treated B. subtilis spores germinated relatively normally, and the generation of colonies from these germinated spores was not salt sensitive. The latter two findings suggest that UV222 does not kill spores by general protein damage, and thus, the new results are consistent with the notion that DNA damage is responsible for the killing of spores and cells by UV222.
IMPORTANCE Spores of a variety of bacteria are resistant to common decontamination agents, and many of them are major causes of food spoilage and some serious human diseases, including anthrax caused by spores of Bacillus anthracis. Consequently, there is an ongoing need for efficient methods for spore eradication, in particular methods that have minimal deleterious effects on people or the environment. UV radiation at 254 nm (UV254) is sporicidal and commonly used for surface decontamination but can cause deleterious effects in humans. Recent work, however, suggests that 222-nm UV (UV222) may be less harmful to people than UV254 yet may still kill bacteria and at lower fluences than UV254. The present work has identified the damage by UV222 that leads to the killing of growing cells and spores of some bacteria, many of which are human pathogens, and UV222 also inactivates a herpesvirus.
INTRODUCTION
Spores of bacteria of Bacillus and Clostridium species are of major concern in the food, medical product, and health care industries as a consequence of spores’ ubiquity in the environment, their dormancy, their extreme resistance, and the ability of spores of some species to cause food spoilage and human disease (1–3). Because of these spore properties, there is continuing interest in methods to inactivate spores in a safe manner while minimizing damage to either the environment or materials with which spores are associated (4–6). The need for such decontamination methods is further exacerbated by the increased prevalence of antibiotic-resistant bacteria as well as the potential use of spores of some strains of Bacillus anthracis as agents of bioterrorism or biowarfare.
Two decontamination agents that have had long use are γ-radiation and UV radiation at 254 nm (UV254) (1, 2, 6, 7). However, γ-radiation has disadvantages, needing specialized and expensive equipment, and there is often consumer concern about γ-irradiated foods. UV254 is also used for surface decontamination as it can kill dormant spores as well as growing cells, although spores are more resistant. Until recently, almost all UV decontamination was done with UV254, a specific emission band of mercury that coincides approximately with the maximal wavelength for UV absorption by DNA. This radiation kills bacterial spores and cells through the generation of specific DNA damage (1–3, 6–8). In growing or stationary-phase cells, this damage is predominantly the generation of pyrimidine dimers, including cyclobutane pyrimidine dimers (CPDs) between adjacent pyrimidines as well as some pyrimidine(6-4) photoproducts (6-4PPs). However, in more UV-resistant spores, UV254 generates minimal amounts of CPDs or 6-4PPs in DNA but rather generates a spore-specific photoproduct, SP (α-thyminyl-5,6-dihydrothymine), between adjacent thymine residues (1, 2, 7, 8). These types of UV254-mediated damage in DNA can be repaired by a variety of enzymes, although some of these lesions can be mutagenic. The mutagenic effects of UV254, which arise from the miscoding properties of pyrimidine dimers, are thus of potential concern when people are exposed to this radiation; indeed, UV radiation can cause DNA damage and skin cancer in animals (8).
Given the concerns about UV254 noted above, there is increasing interest in using 222-nm UV radiation (UV222) for decontamination (9–19). Importantly, while UV222 is absorbed well by nucleic acids, it is also well absorbed by proteins, which are much more abundant in cells than nucleic acids, and kills bacteria and spores, reportedly more rapidly than UV254 (12, 13). However, there have been no definitive studies on how UV222 kills growing bacteria and spores, and the factors that are important in allowing cells and spores to resist the effects of this radiation have not been identified. In this work, we have investigated (i) the UV222 killing of growing cells and spores of multiple species and Bacillus subtilis with and without specific proteins important for UV254 resistance; (ii) the role of spores’ huge depot of a 1:1 chelate of Ca2+ and dipicolinic acid (CaDPA) (∼25% of the spore core dry weight) in spore UV222 resistance, as CaDPA is involved in spore UV254 resistance; and (iii) DNA photoproducts generated in spores and cells by UV222. B. subtilis spore killing by UV222 was compared to the killing of spores of Bacillus cereus and Bacillus thuringiensis Al Hakam, an accepted surrogate for B. anthracis spores (20); spores of Clostridioides difficile, an emerging human health problem (21); growing and stationary-phase cells of B. subtilis and methicillin-resistant Staphylococcus aureus (MRSA); and herpes simplex virus (HSV). (i) Analysis of UV222 mutagenesis in B. subtilis growing cells and spores, (ii) identification of the DNA damage generated by UV222 in growing cells and spores, (iii) analysis of the effects of the loss of specific DNA repair proteins or protective spore components on cell or spore resistance to UV222, and (iv) analysis of the germination and outgrowth of UV222-irradiated spores have together indicated that this radiation likely kills growing cells and spores by damage to DNA.
RESULTS
Killing and mutagenesis of spores and cells by UV222.
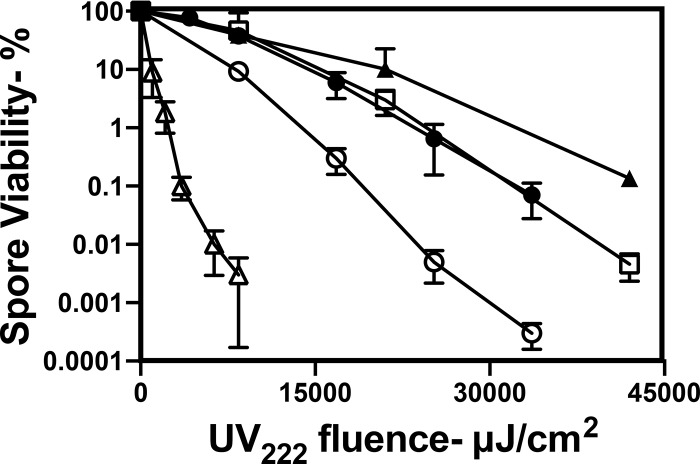
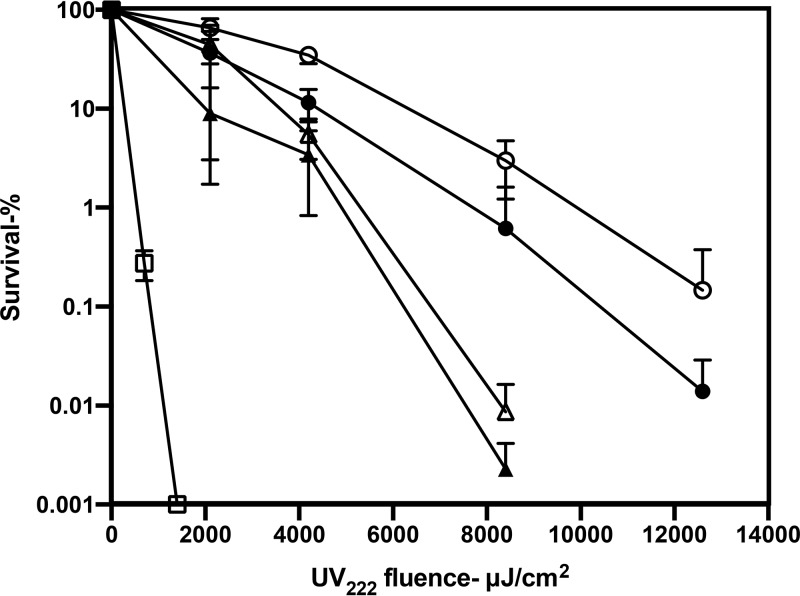
As expected (12, 13, 22–24), UV222 irradiation killed wild-type spores of a number of species, and we found that B. cereus spores were the least resistant and that C. difficile spores were the most resistant (Fig. 1). UV222 killing of B. thuringiensis Al Hakam spores, an accepted surrogate for B. anthracis spores, was slower than that of B. subtilis spores. Importantly, the relative rates of killing of B. subtilis spores at concentrations of ∼106 spores/ml to 108 spores/ml (optical densities of 0.01 to 1) by either UV222 or UV254 were relatively similar, although UV222 was more effective (Fig. 2 and see below). The latter result showed that even with 108 spores/ml, there was minimal shielding of either UV222 or UV254. Given the higher light scattering of spores than the equivalent levels for growing or stationary-phase cells, there is most likely no shielding of any cells or spores at the concentrations used in all these irradiations. The use of L broth agar plates with 1 M NaCl for the determination of wild-type B. subtilis spore viability after UV222 treatment gave the same level of spore killing as a function of UV222 fluence as did the use of L broth agar plates with the usual 0.15 M NaCl (data not shown). This finding suggests that UV222 does not kill spores by damage to one or more essential spore proteins, as this type of spore damage often makes spores’ return to vegetative growth, termed spore outgrowth, salt sensitive (25, 26).
FIG 1.
UV222 killing of spores of different species. Spores of different species were treated with UV222, and spore survival was measured, all as described in Materials and Methods. Symbols: ○, B. subtilis PS533 (wild type); ●, B. thuringiensis Al Hakam; △, B. cereus T; ▲, C. difficile 43593; □, C. difficile JIR8094. Data shown are averages from duplicate determinations ± standard deviations in one experiment. This experiment was repeated 3 times, and the same relative rates of killing of spores of different species were seen.
FIG 2.
Killing of different concentrations of B. subtilis spores by UV222 and UV254. B. subtilis PS533 spores at an OD600 of 1 (● and ○), 0.1 (▲ and △), or 0.01 (■ and □) were irradiated with UV222 (●, ▲, and ■) or UV254 (○, △, and □), and duplicate samples of various dilutions were spotted onto L broth agar plates to determine spore survival as described in Materials and Methods. Data shown are averages of duplicate determinations ± standard deviations in one experiment. This experiment was repeated twice, with the same relative rates of killing of spores seen at different concentrations and with different wavelengths of UV radiation.
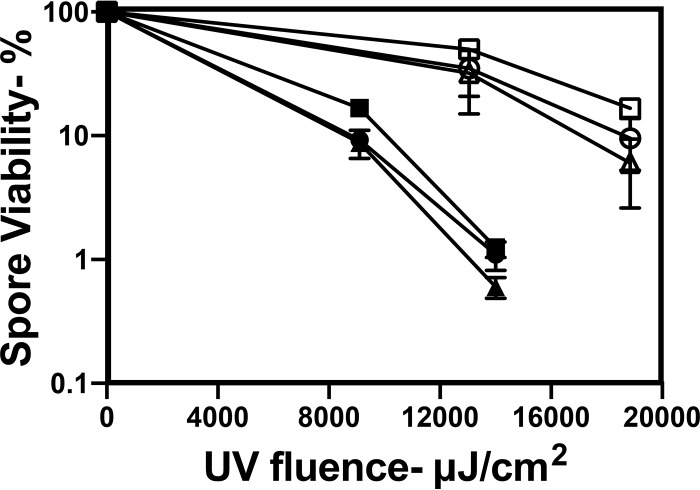
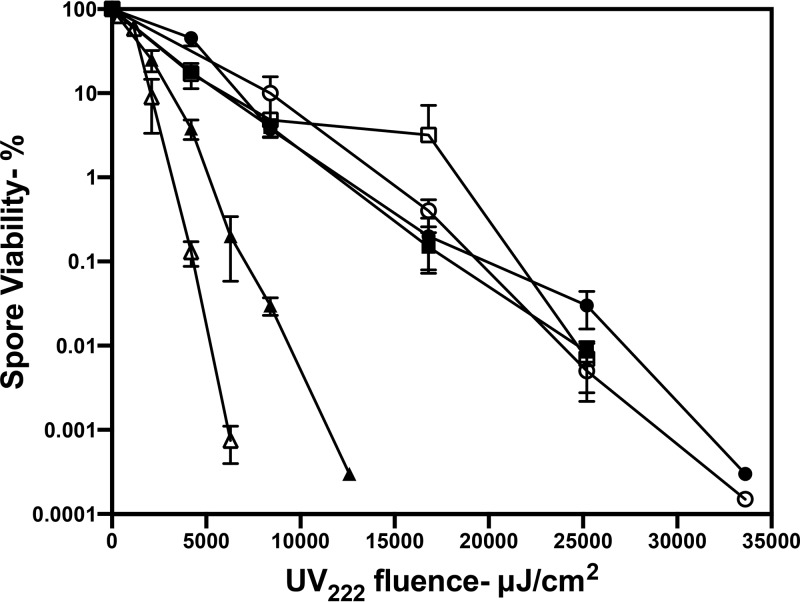
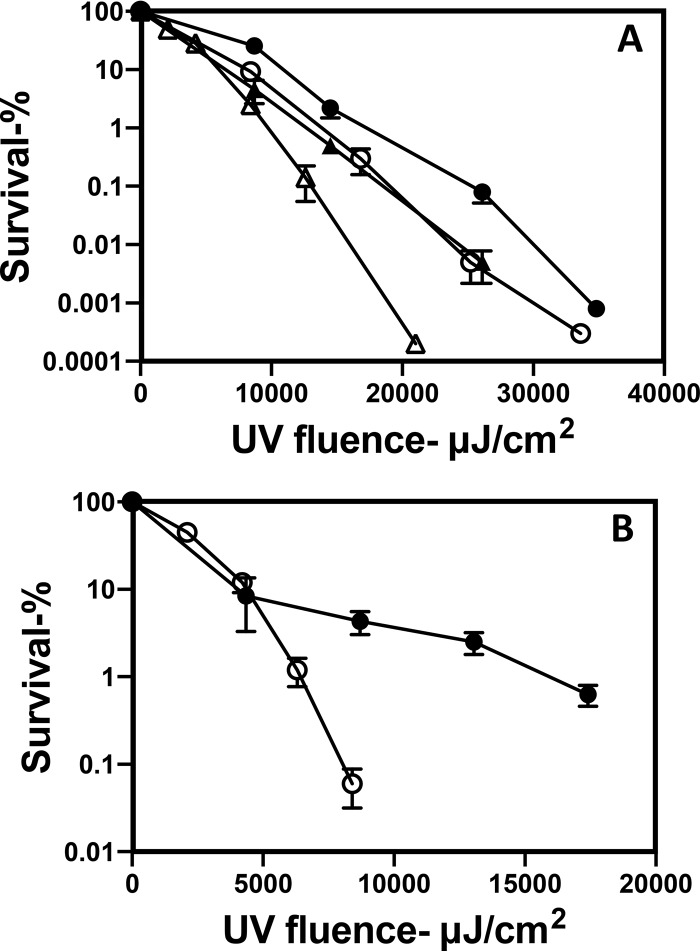
Analysis of the UV222 killing of B. subtilis spores with and without components involved in spore resistance to different agents found that the loss of a moderate amount of the spore coat in cotE spores had no effect on spore resistance to UV222, and the loss of almost all of the spore coat in cotE gerE spores perhaps decreased spore UV222 resistance slightly, while the loss of the DNA-protective α/β-type small, acid-soluble spore proteins (SASPs) or the important DNA repair protein RecA decreased spore UV222 resistance markedly (Fig. 3). CaDPA-less spores of two B. subtilis strains that cannot either synthesize DPA (strain FB122) or take up CaDPA into the spore core (strain PS3406) exhibited slightly lower UV222 resistance than wild-type spores, although FB122 spores sporulated with exogenous DPA, which accumulated wild-type levels of DPA (data not shown), exhibited UV222 resistance identical to that of these wild-type spores (Fig. 3 and Fig. 4A). DPA-less C. difficile spores were also more UV222 sensitive than wild-type C. difficile spores (Fig. 4B). Surprisingly, dpaAB-complemented C. difficile spores were more UV222 resistant than wild-type spores (Fig. 4B). Although the reason for the latter finding is not clear, the mutant spores prepared with dpaAB complemented on the chromosome had ∼25% more DPA than did the wild-type spores (data not shown).
FIG 3.
UV222 killing of spores of strains of B. subtilis with defects in possible protective components. Spores of isogenic B. subtilis strains were treated with UV222, and spore survival was measured as described in Materials and Methods. Symbols: ○, PS533 (wild type); ●, PS3328 (cotE); △, PS578 (α− β−); ▲, PS2318 (recA); ■, PS4150 (cotE gerE); □, FB122 (spoVF sleB), prepared with DPA. Data shown are averages from duplicate determinations ± standard deviations in one experiment. This experiment was repeated twice, with the same relative rates of killing of spores of different species seen.
FIG 4.
Effects of loss of DPA on B. subtilis and C. difficile spore UV resistance. (A) Purified spores of strain PS832 (wild type) (●) and DPA-less spores of strains FB122 (spoVAF sleB) (▲) and PS3406 (spoVA sleB) (■) were irradiated with UV222, and spore survival was determined as described in Materials and Methods. (B) Purified spores of C. difficile JIR8094, either the wild type (○ and ●), its dpaAB derivative (△ and ▲), or the complemented dpaAB derivative (□), were irradiated with UV222 (○, △, and □) or UV254 (● and ▲), and spore survival was determined as described in Materials and Methods. Data shown are averages from duplicate determinations ± standard deviations in one experiment. This experiment was repeated twice, and the same relative rates of killing of spores of different species/strains were seen.
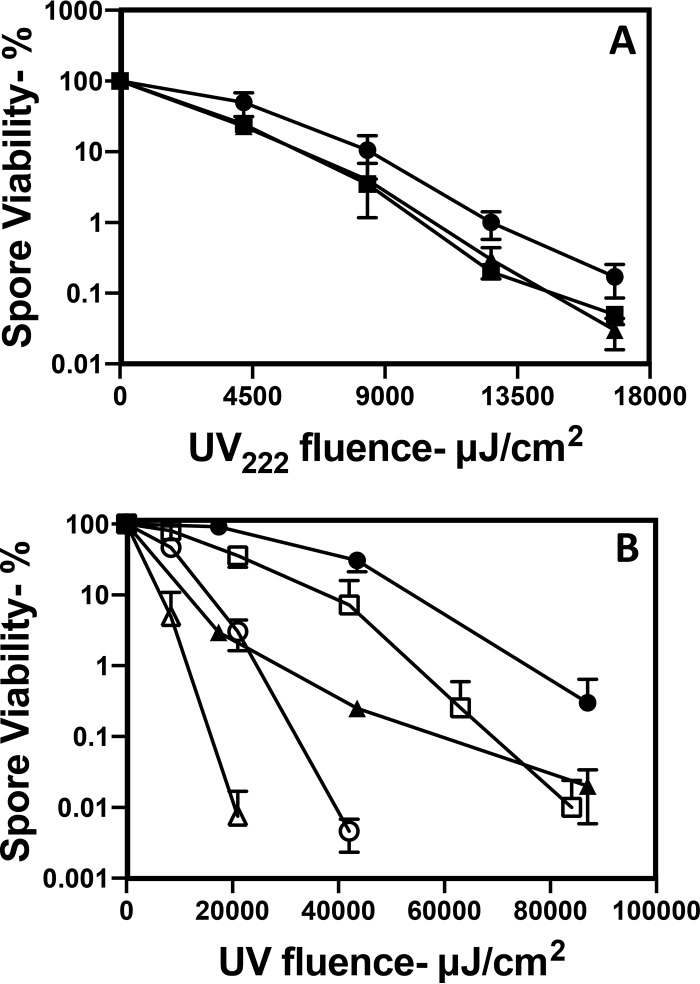
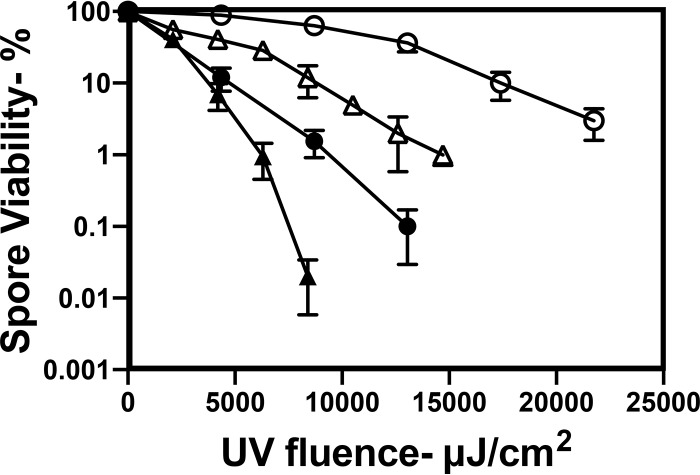
Previous studies have shown that log-phase cells of B. subtilis are more sensitive to UV254 than are dormant spores (2). Consistent with those studies, we observed that growing B. subtilis cells were more sensitive to UV222 than dormant spores (compare Fig. 5 with Fig. 1). The UV222 killing of stationary-phase B. subtilis cells was also slightly faster than that of log-phase cells (Fig. 5), and as found with B. subtilis spores, log-phase recA B. subtilis cells were much more UV222 sensitive than were wild-type cells (Fig. 5). Growing and stationary-phase MRSA cells were more UV222 sensitive than B. subtilis cells, with MRSA stationary-phase cells being the most UV222 sensitive (Fig. 5). Comparison of B. subtilis spore and cell killing by UV222 and UV254 found that UV222 killing was ∼2-fold more effective (Fig. 6A), and this was also the case with C. difficile spores (Fig. 4B). Notably, UV222 also inactivated HSV, again more efficiently than UV254 (Fig. 6B).
FIG 5.
UV222 killing of log- and stationary-phase cells of various species and strains. Log-phase and stationary-phase cells of various species and strains were isolated and UV222 treated, and cell survival was measured, all as described in Materials and Methods. Symbols: ○, log-phase cells of B. subtilis PS533 (wild type); ●, stationary-phase cells of B. subtilis PS533; □, log-phase cells of B. subtilis PS2318 (recA); △, log-phase cells of S. aureus; ▲, stationary-phase cells of S. aureus. Data shown are averages from duplicate determinations ± standard deviations in one experiment. This experiment was repeated twice, and the same relative rates of killing of cells of different species were seen.
FIG 6.
UV222 and UV254 killing of wild-type B. subtilis spores and growing cells and HSV inactivation. Dormant spores and log-phase growing cells of wild-type PS533 B. subtilis (A) or HSV (B) was irradiated for various times, and spore and growing cell survival and HSV inactivation were measured as described in Materials and Methods. Results for HSV inactivation are averages from two independent experiments. Symbols: ○ and △, irradiated with UV222; ● and ▲, irradiated with UV254; ○ and ●, spores or HSV; △ and ▲, growing cells. Data shown are averages from duplicate determinations ± standard deviations in one experiment. This experiment was repeated twice, and the same relative rates of killing of spores, cells, and virus, and at both wavelengths, were seen.
UV254 not only kills B. subtilis spores and cells but also generates high levels of mutants in the survivors (27, 28). Consequently, large numbers of individual survivors of UV222 treatment giving 93 to 96% killing of growing cells and spores of wild-type B. subtilis PS533, as well as PS578 spores lacking the great majority of the DNA-protective α/β-type SASPs (these spores are termed α− β−), were tested for the acquisition of mutations giving rise to auxotrophy (aux) or asporogeny (spo) (Table 1). As expected, untreated wild-type growing cells or spores had a minimal level of aux or spo mutants, while untreated α− β− spores had a slightly higher level of such mutants, as seen previously (29). Notably, the growing cells or spores surviving UV222 irradiation had acquired a high level of aux, spo, or both mutations (Table 1), similar to what has been seen previously with growing cells or spores treated with many DNA-damaging agents, including UV254 (27, 29).
TABLE 1.
Mutagenesis and killing of Bacillus subtilis cells and spores by UV222a
| Sample type | Level of killing (%) | No. of colonies examined | No. of mutants |
Total proportion of mutants (%) | ||
|---|---|---|---|---|---|---|
| aux | spo | aux spo | ||||
| Growing PS533 wild-type cells | 0 | 400 | 0 | 1 | 0 | 0.3 |
| Growing PS533 wild-type cells | 95 | 290 | 11 | 10 | 2 | 8 |
| Wild-type PS533 spores | 0 | 400 | 1 | 0 | 0 | 0.3 |
| Wild-type PS533 spores | 96 | 400 | 10 | 15 | 3 | 7 |
| α− β− PS578 spores | 0 | 400 | 2 | 2 | 0 | 1 |
| α− β− PS578 spores | 93 | 400 | 18 | 17 | 5 | 10 |
Log-phase cells and dormant spores of B. subtilis strains were or were not UV222 irradiated, and levels of cell/spore killing were determined as described in Materials and Methods. Large numbers of colonies from survivors of the irradiation were obtained on L broth agar plates and toothpicked onto Spizizen’s minimal medium and sporulation plates for assessment of mutants that were auxotrophic (aux) or asporogenous (spo) or that had both mutations, all as described in Materials and Methods.
Identification and quantitation of damage in DNA from UV222-treated B. subtilis cells and spores and importance of repair of one lesion in spore UV222 resistance.
The results described above strongly suggested that UV222 kills B. subtilis cells and spores by DNA damage. To determine if UV222 treatment of cells or spores generates specific photoproducts, DNA from untreated B. subtilis wild-type and α− β− spores and wild-type log-phase cells as well as cells and spores killed to various levels by UV222 were isolated, and photoproducts in the irradiated DNA were identified and quantitated (Table 2) (note that DNA was extracted from both dead and live cells and spores in populations). Almost all of the UV222 photoproducts found in this work were those previously found to be generated by UV254 (2, 29). These were almost exclusively SP in B. subtilis wild-type spores and a mixture of SP and CPDs in B. subtilis α− β− spores, with CPDs and 6-4PPs being the only bipyrimidine photoproducts found in growing wild-type cells. We also examined UV222-treated cell and spore DNA for an increase in strand breaks. These experiments used B. subtilis PS533 that carries the high-copy-number plasmid pUB110 (30). However, analysis of DNA from UV222-treated cells and spores revealed minimal, if any, strand breaks in either the chromosomal or plasmid DNA (Fig. 7 and data not shown).
TABLE 2.
Levels of photoproducts in UV222-irradiated B. subtilis spores and growing cellsa
| Sample type | UV fluence (mJ/cm2) | No. of molecules/106 basesb
|
||||||
|---|---|---|---|---|---|---|---|---|
| TT-CPD | TT-6-4PP | TC-CPD | TC-6-4PP | CT-CPD | CC-CPD | SP | ||
| WT cells | 0 | ND | ND | ND | ND | ND | ND | ND |
| WT cells | 8.4 | 25 | 1.3 | ND | 4.5 | ND | ND | ND |
| WT cells | 17 | 64 | 1.2 | 2.2 | 5.2 | 2.3 | ND | ND |
| α− β− spores | 0 | ND | ND | ND | ND | ND | ND | ND |
| α− β− spores | 8.4 | 78 | ND | ND | ND | ND | ND | 831 |
| α− β− spores | 17 | 144 | 0.6 | ND | ND | ND | ND | 1,521 |
| WT spores | 0 | ND | ND | ND | ND | ND | ND | 102 |
| WT spores | 8.4 | 0.8 | 0.1 | 0.1 | ND | ND | ND | 10,878 |
| WT spores | 17 | 4.3 | 0.8 | 6.1 | 3 | 3.1 | 0.9 | 67,036 |
B. subtilis PS533 (wild-type [WT]) log-phase cells and PS533 and PS578 (α− β−) spores were or were not UV222 irradiated, DNA was isolated and hydrolyzed, and photoproducts were analyzed and quantitated as described in Materials and Methods.
ND, not detectable and <0.1 molecules/106 bases.
FIG 7.
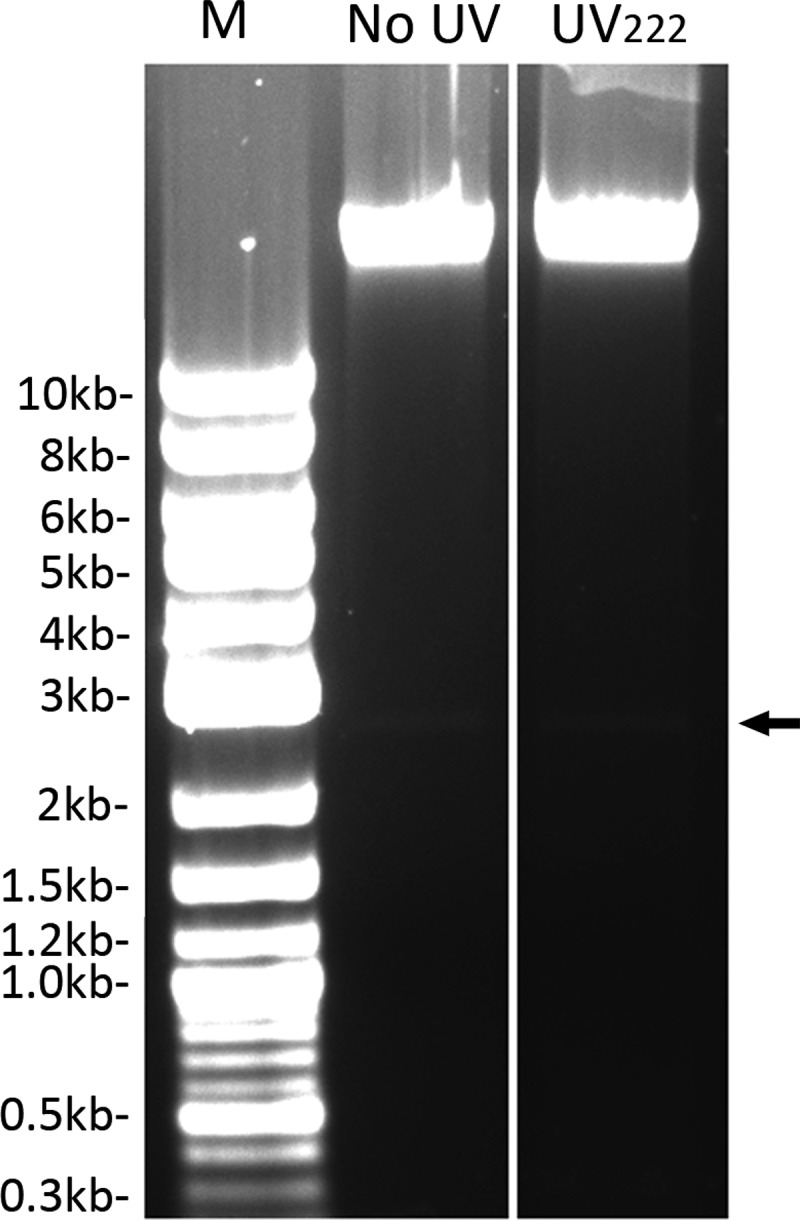
Agarose gel electrophoretic analysis of DNA from untreated or UV222-treated B. subtilis wild-type spores. Total DNA was purified from ∼6 mg (dry weight) of B. subtilis PS533 (wild-type) spores that were either untreated or UV222 irradiated, giving 98% spore killing, as described in Materials and Methods. Approximately 1 μg of each purified DNA was run on an agarose gel plus ethidium bromide alongside 2 μg of DNA size markers, with their sizes shown in kilobases, and the gel was photographed. The samples run in the various lanes are DNA size markers (M), untreated spores’ DNA, and UV222-treated spores’ DNA. The arrow to the right denotes the migration position of supercoiled plasmid pUB110.
With photoproducts generated by UV222 in spore and cell DNA identified, it was then possible to examine the effects of the loss of a DNA repair protein important for the repair of the most abundant of the photoproducts formed in spores (2, 31, 32). The specific DNA repair protein targeted was Spl, which monomerizes SP in spore DNA. As expected, the loss of Spl from spores resulted in a significant increase in the rate of killing of the mutant spores by either UV222 or UV254, the latter as reported previously (31, 32) (Fig. 8).
FIG 8.
UV222 and UV254 killing of wild-type and spl B. subtilis spores. Isogenic wild-type and spl spores were irradiated with UV222 and UV254, and spore survival at various times was determined as described in Materials and Methods. Symbols: △ and ○, wild type; ▲ and ●, spl; ○ and ●, UV254 irradiated; △ and ▲, UV222 irradiated. Data shown are averages from duplicate determinations ± standard deviations in one experiment. This experiment was repeated twice, and the same relative rates of killing of spores of all strains and at the two wavelengths were seen.
Germination of UV222-irradiated spores.
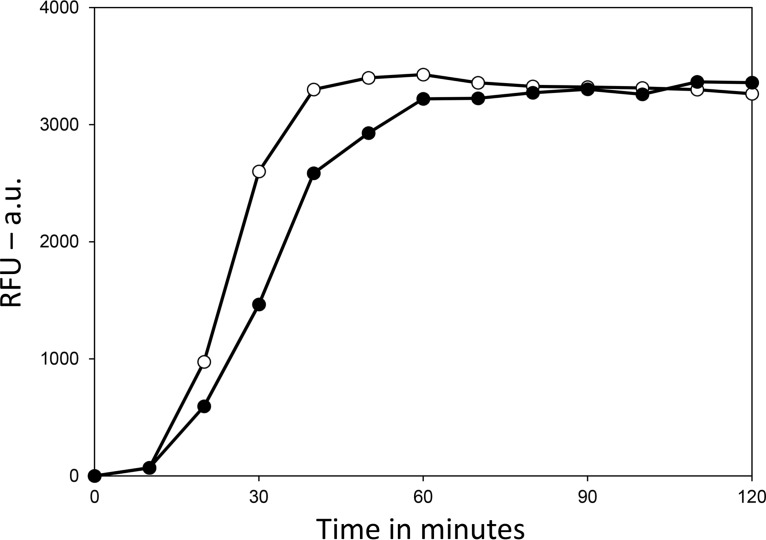
Spores clearly lose viability upon UV222 irradiation. However, it is formally possible that UV222 could damage one or more proteins essential for spore germination, and spores that cannot germinate will not appear viable. To test this possibility, untreated wild-type B. subtilis spores as well as UV222-treated spores killed ∼99%, as assessed by the ability of spores to form colonies on an L broth agar plate, were germinated with l-valine, which triggers germination through a specific spore germinant receptor, with further completion of germination also requiring the SpoVA channel for DPA as well as cortex-lytic enzymes (CLEs) that degrade spores’ peptidoglycan cortex during spore germination (33) (Fig. 9). The results of this experiment showed clearly that the great majority of spores killed ∼99% by UV222 both released DPA and completed germination when exposed to a germinant receptor (GR)-dependent germinant, and the rate of germination of the UV222-irradiated spore population was only slightly lower than that for the unirradiated spores. Note that it is well established that spore populations killed to high degrees with a variety of agents, including UV radiation and wet heat, are still able to germinate; however, they never go on to begin vegetative growth (25, 30).
FIG 9.
Germination of untreated and UV222-killed B. subtilis wild-type spores. Spores of B. subtilis PS533 (wild type), either without or after UV222 irradiation giving ∼99% killing, were germinated with l-valine, and spore germination was monitored by the fluorescence in relative fluorescence units (RFU) of released DPA with Tb3+. Symbols: ○, unirradiated spores; ●, UV222-irradiated spores. Analysis of the spores by phase-constant microscopy at the end of the experiment showed that >85% of both types of spores had germinated completely (data not shown). a.u., arbitrary units.
DISCUSSION
The work in this communication leads to a number of conclusions about (i) the effects of UV222 on spores, cells, and viruses; (ii) how cells and spores are inactivated by UV222; and (iii) what is involved in spore and cell resistance to UV222. These conclusions about UV222 were also compared with those made previously about UV254. First, as is the case for UV254, UV222 also inactivates spores and growing cells of all species tested as well as at least one virus. Indeed, as found previously (11–13), UV222 was more effective in killing growing bacteria or spores than UV254. Notably, a number of known human pathogens, including HSV, MRSA, and spores of a B. anthracis surrogate and C. difficile, were all killed or inactivated by UV222 at lower fluences than with UV254. Thus, UV222 could be a useful addition to the overall decontamination arsenal.
Second, the available evidence indicates that the lethal damage generated in growing cells and spores by UV222 is, like that by UV254, DNA damage. This is indicated by the large number of mutations in B. subtilis cells and spores surviving UV222 treatment; the increased UV222 sensitivity of cells and spores that lack the major DNA repair protein RecA; the increased UV222 sensitivity of B. subtilis spores that lack the DNA-protective α/β-type SASPs; the generation of known lethal and mutagenic DNA damage by UV222, including SP in dormant spores and CPDs in growing cells and α− β− spores; and the decreased UV222 resistance of spores deficient in SP repair. That UV222 is not killing spores by massive protein damage is indicated by the lack of high-salt sensitivity of spores surviving UV222 treatment, as spores surviving treatments by agents that damage proteins, such as wet heat, are sensitized to high salt concentrations in recovery media (25, 26). In addition, a spore population killed ∼99% germinated almost as well as untreated spores, indicating that there is minimal, if any, major damage to crucial germination proteins, including CLEs in spores’ outer layers, the CaDPA channel in the spore’s inner membrane, and the low-copy-number nutrient GRs also in the inner membrane. Spores killed >95% by UV254 also germinate relatively normally (30). It must be noted, however, that while the experiments in this work found no evidence that general damage to protein is involved in UV222 killing of spores, it is certainly possible that damage to one or more DNA repair proteins or DNA damage responses might be important in the ultimate death of spores by lethal DNA damage (see below). While experimental analysis of the latter possibility was beyond the scope of this work, it is notable that there is evidence that the initial UV and γ-radiation damage that leads to cell and perhaps spore killing is to one or more crucial proteins involved in DNA repair (34–37) (see below).
A third conclusion is that spore protection against UV222 involves both α/β-type SASPs in dormant spores as well as the repair of DNA damage when spores germinate and outgrow. Again, this is what has been found for dormant-spore resistance to UV254, with a major factor in the effects of α/β-type SASPs on spore UV254 resistance being the conversion of spore DNA from the normal B conformation of growing and sporulating cell DNA to the A-like conformation of spore DNA when the DNA is saturated with these DNA binding proteins (2, 38). This conformational change alters the α/β-type SASP-saturated spore DNA’s photochemistry such that SP is by far the major UV254 photoproduct and not the CPDs and 6-4PPs generated in growing cells. Indeed, the same major photoproducts were found in UV222-irradiated growing cells and dormant wild-type spores in this work, with less SP and significant CPDs in UV222-irradiated α− β− spores, as seen previously (39). The loss of almost all spore coat protein in cotE gerE (PS4150) spores also perhaps increased spore UV222 sensitivity slightly; presumably, the large amount of UV222-absorbing coat protein in wild-type spores shields DNA in the spore core, while UV254 is much less well absorbed by coat protein relative to these wavelengths’ absorption by DNA. Notably, B. cereus spores that have minimal pigment in the spores’ outer layers were the most UV222 sensitive of all the wild-type spores tested.
As noted above, a major conclusion from this work was that UV254 and UV222 generate the same major photoproducts in wild-type spores and growing cells, although the relative efficacies of these two wavelengths in photoproduct generation in spores were not rigorously examined. This similarity in UV222 and UV254 photoproduct generation was not surprising since previous work showed that UV at wavelengths well below 254 nm generates SP in dormant Bacillus spores and CPDs and 6-4PPs in growing cells (23, 24). More quantitative comparisons were made in cell-free DNA, where an action spectrum from 220 to 365 nm showed that the efficiencies of the formation of CPDs and 6-4PPs were similar at 222 and 254 nm (40). Another study comparing 195- and 245-nm radiation reached a similar conclusion for CPDs (41). Interestingly, the same work showed that the yield of strand breaks was ∼40 times higher at the lower wavelength as the result of photoionization. This is in contrast to our present observation of the lack of obvious strand breaks in UV222-exposed spores. In addition, we also failed to observe any significant increase in the level of the oxidized DNA base 8-oxo-7,8-dihydroguanine in spores, which is a known ionization product of DNA (42). The latter two results suggest that there is strong protection against radical formation and oxidative stress damage in spores, which may be related to spores’ known radioresistance and the minimal levels of water in the spore core, where the spore DNA is located. Collectively, our data demonstrate that UV222 can induce DNA lesions similar to those induced by UV254 and kills a range of infectious agents, including vegetative cells, bacterial spores, and viruses, regardless of their drug sensitivity.
One surprising result in this work concerned the role of the spore core’s huge CaDPA depot, ∼25% of the core dry weight, in UV222 resistance. Previous studies showed that spore CaDPA sensitizes spores to UV254, perhaps by the absorption of this radiation by CaDPA and the transfer of the energy to DNA (43). However, the present work found that the loss of CaDPA actually decreased B. subtilis UV222 resistance slightly and C. difficile spore UV222 resistance even more so. Importantly, one possible explanation for the higher effectiveness of spore killing by UV222 is based on the higher absorption of DPA at 222 nm than at 254 nm, which suggests that this absorbed energy is transferred to pyrimidine bases in DNA to increase the formation of potentially lethal photoproducts in spores (12, 13, 37, 43). While this rationale is not unreasonable, the lower UV222 resistance of CaDPA-less B. subtilis and C. difficile spores is not consistent with this explanation, nor will this rationale explain the more effective UV222 inactivation of growing cells and viruses, which lack DPA. A second, and perhaps more plausible, explanation for the overall greater cidal activity of UV222 than of UV254 is based on the facts that (i) the absorption of UV222 by proteins is greater than that of UV254 and (ii) UV radiation can cause significant damage to protein (35–37). Thus, damage to one or more crucial proteins important in DNA damage repair will potentially be greater with UV222 than with UV254; perhaps, this protein damage is the initiating event leading to increased levels of unrepaired lesions in cell, spore, or virus DNA, and the increased DNA damage then leads to cell and spore killing and HSV inactivation. The second explanation is certainly logical and has been suggested previously (12, 13, 35, 36) but will need further work to determine if there is indeed a causal connection between the cidal activity of UV222 and specific protein damage, as has also been suggested for UV254 (35, 36).
MATERIALS AND METHODS
Bacterial strains and virus used.
Seven of the Bacillus strains used in this work are isogenic with strain PS832, a laboratory 168 wild-type strain. These strains are (i) PS533 (30), also termed the wild type but carrying plasmid pUB110 giving resistance to kanamycin (10 μg/ml); (ii) PS578 (30), identical to PS533 but with deletions of the sspA and sspB genes encoding ∼85% of spores’ DNA-protective α/β-type SASPs (these α− β− spores are invariably much more sensitive to many DNA-damaging agents than are wild-type spores) (2, 3); (iii) PS2318 (30), identical to PS533 but lacking an intact recA gene controlling much of the DNA repair activity; (iv) PS3328 (44), identical to PS832 but lacking the cotE gene, the product of which is important for the assembly of some layers of the spore coat (45); (v) PS4150 (46), identical to PS832 but lacking the CotE and GerE proteins crucial for the assembly of almost all of the coat layers of B. subtilis spores which contains ∼50% of the total spore protein as well as a number of pigments (45); (vi) FB122 (44), identical to PS832 but lacking the spoVF operon essential for the synthesis of dipicolinic acid (DPA) in the mother cell compartment of the sporulating cell as well as the sleB gene encoding one of B. subtilis spores’ two redundant CLEs (33); and (vii) PS3406 (47), identical to PS832 but lacking the products of the spoVA operon essential for DPA uptake into the developing spore as well as SleB. DPA is synthesized in the mother cell compartment of the sporulating cell by the SpoVF DPA synthase and is taken up by the developing spore as a 1:1 complex with Ca2+ (CaDPA) (1) by the SpoVA protein complex to ∼25% of the spore core dry weight. While CaDPA-less B. subtilis spores that retain SleB very rapidly germinate spontaneously, CaDPA-less spoVF sleB and spoVA sleB spores are stable and can be isolated and purified (33, 44, 47). Two additional B. subtilis 168 strains are congenic with each other, BP130, lacking the spl gene for the SP lyase important for SP repair after spores germinate, and its wild-type 168 counterpart (31, 32). The spores of the latter two strains were prepared and purified as described previously (31). Bacillus cereus strain T (Bacillus Genetic Stock Center code 6A1) and B. thuringiensis Al Hakam (20) were also used, as were wild-type C. difficile strains JIR8094 (630 Δerm) and 43593. Two C. difficile JIR8094 derivatives were also used, which have an erm cassette replacing the dpaAB operon encoding DPA synthase. One of these strains carries plasmid pMTL84151 (dpaAB deletion mutant), while the other carries pMTL84151 with the intact dpaAB operon (dpaAB-complemented strain) in the pyrE locus (48); only the complemented strain accumulates CaDPA. Unlike the situation with CaDPA-less spores of B. subtilis, CaDPA-less C. difficile spores do not germinate spontaneously (33, 48). The S. aureus strain used was 43300 (49, 50) and is resistant to methicillin. The HSV strain used was HSV-1 KOS, obtained from P. A. Schaeffer, which was originally isolated from a lip lesion (51).
Preparation and purification of spores, log- and stationary-phase cells, and HSV.
Spores of Bacillus strains were routinely prepared on 2× Schaeffer's glucose (SG) medium agar plates at 37°C in the absence of exogenous DPA, harvested after 2 to 3 days, and purified as described previously, including centrifugation through high-density solutions of 50% (wt/vol) HistoDenz for CaDPA-replete spores and 45% HistoDenz for CaDPA-less spores, since the latter spores have a lower core wet density than CaDPA-replete spores (52–54). All Bacillus spore preparations used were >98% free of growing and sporulating cells, germinated spores, and cell wall debris, as seen by phase-contrast microscopy, and were stored in water at 4°C, protected from light.
C. difficile sporulation induction and spore purification were performed according to a previously described protocol, with some modifications (48). To induce sporulation, 2.5 ml of brain heart infusion (BHI) medium (Becton, Dickinson and Co., Franklin Lakes, NJ) was inoculated with C. difficile grown on BHI agar with 1.9 mM taurocholic acid (TA) and 0.1% l-cysteine in screw-cap test tubes. After 4 to 5 h of incubation at 37°C in an anaerobic cabinet (Coy Laboratory Products, Grass Lakes, MI), where anaerobic conditions were achieved using a gas of 85% N2, 5% CO2, and 10% H2, the culture was diluted 1:50 into 2.5 ml of fresh BHI broth. These cultures were incubated anaerobically at 37°C until they reached an optical density at 600 nm (OD600) of 0.35 to 0.75, when 120 μl was spread onto agar plates prepared with a mixture of 70% sporulation medium and 30% BHI broth (63 g/liter Bacto peptone, 3.5 g/liter protease peptone, 11.1 g/liter BHI medium, 1.5 g/liter yeast extract, 1.06 g/liter Tris base, 0.7 g/liter NH4SO4, 15 g/liter agar) (55). Plates were incubated anaerobically for 3 days at 37°C in an anaerobic chamber. Prior to harvesting of spores, a sample of the bacterial lawn was collected in a microcentrifuge tube, suspended in 100 μl of autoclaved water, and fixed with 100 μl of 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) (Fisher Scientific, Waltham, MA). Following fixation, the sample was pelleted by centrifugation at 15,000 rpm for 3 min, the supernatant fluid was removed, and the pellet was suspended in 100 μl of PBS. An aliquot of the latter suspension was examined by phase-contrast microscopy to verify that sporulation and spore release from sporangia were largely complete.
C. difficile spores were harvested using sterile inoculation loops and suspended in 1 ml of 4°C water in a 2-ml microcentrifuge tube, and spores from two plates were combined in one microcentrifuge tube. Following incubation on ice for ≥1 h, cell debris and spores were pelleted by centrifugation at 14,000 rpm at 4°C for 5 min, the supernatant fluid was discarded, the pellet was suspended in 4°C water, and this procedure was repeated seven times. Spores were then incubated at 4°C overnight, and spores and cell debris were washed in 4°C water three more times. Following these washes, spores were treated with 27.2 Kunitz units of DNase I (Qiagen, Germantown, MD) at 37°C for 30 to 60 min in DNase I buffer (10 mM Tris-HCl, 2.5 mM MgCl2, and 0.5 mM CaCl2 [pH 7.6]). Following DNase I treatment, the samples were pelleted by centrifugation, washed once in 1 ml of 4°C water, and purified on high-density solutions of HistoDenz (Sigma-Aldrich, St. Louis, MO). For spores of C. difficile ATCC 43593, C. difficile JIR8094, and the C. difficile dpaAB mutant complemented with dpaAB (complemented strain), spores at an OD600 of ∼25 were suspended in 100 μl of 20% HistoDenz, and this suspension was layered on 900 μl of 50% HistoDenz in a microcentrifuge tube. For the CaDPA-less dpaAB mutant spores, which are reported to be less dense than spores that are CaDPA replete (48), spores were suspended in 450 μl of 20% HistoDenz and layered on 500 μl of 45% HistoDenz. The gradients were centrifuged at 15,000 rpm at 4°C for 10 min, the supernatant was discarded, and the pellet was washed three times with 4°C water. The purity of the final spores was determined by phase-contrast microscopy of PFA-fixed spores as detailed above and was >98%. DPA was extracted from C. difficile spores in boiling water and assayed in the extracts by the addition of TbCl3 and measurement of Tb3+-DPA fluorescence as described previously (56).
Cells of Bacillus strains and S. aureus were grown at 37°C in liquid L broth medium (10 g/liter tryptone, 5 g/liter yeast extract, 10 g/liter NaCl) with good aeration to mid-log phase (OD600 = 1.0) (log-phase cells) or for 14 h (stationary-phase cells). There was no apparent sporulation in B. subtilis stationary-phase cultures. Culture volumes of 1 to 2 ml were centrifuged in a microcentrifuge for ∼5 min, washed twice with 1 to 2 ml of sterile PBSa (25 mM KPO4 buffer [pH 7.4]–0.15 M NaCl), and suspended in a volume of PBSa to give cells at an OD600 of ∼1.0 just prior to UV irradiation. C. difficile strains were routinely grown anaerobically on prereduced brain heart infusion medium with 1.5% agar plus 1.9 mM TA and 0.1% (wt/vol) l-cysteine (BHI-TA plates) (48).
HSV was propagated in Vero cells as follows. A confluent monolayer of Vero cells on a 225-cm2 dish was inoculated with KOS at a multiplicity of infection (MOI) of 0.005 PFU/ml in 5 ml of serum-free Dulbecco’s modified Eagle medium (DMEM) (Gibco, Fisher Scientific). After 1 h of virus absorption at 37°C, the inoculum was removed, and 20 ml DMEM supplemented with 2.5% fetal bovine serum was added to the flask. Infected cells were incubated at 37°C for 2 to 3 days, until 100% of the cells displayed a cytopathic effect (CPE). Cells and media containing virus were collected and centrifuged at 125 × g for 5 min. The cell pellet was freeze-thawed 2 times and sonicated in a cup horn sonicator at 50% amplitude 3 times for 20 s each. The medium containing virus was combined with the sonicated cell pellet and centrifuged for 15 min at 1,000 × g, and the supernatant fluid containing the virus was aliquoted and stored at −80°C.
Irradiation with UV222 or UV254 and determination of spore or cell viability and virus recovery.
Spore and cell irradiation with UV222 were performed at 23°C with a CARE222 lamp (Ushio America, Cypress, CA), as shown in Fig. S1 in the supplemental material. The outputs of this lamp at wavelengths above 235 nm, including at 254 nm, were ≤1% of those at 222 nm (data not shown). Cells were in 1.5 ml of PBSa (cells) or PBSb (virus) (9 g/liter NaCl, 0.144 g/liter KH2PO4, 0.795 g/liter Na2HPO4 [pH 7.4]), and spores were in 1.5 ml of water in a round, sterile, 35-mm-diameter petri dish; the 1.5 ml covered the whole bottom of the petri dish. Spores and cells were routinely irradiated with UV222 and UV254 at an OD600 of ∼1, although similar killing curves were obtained with spores at OD600 values of 0.1 and 0.01, indicating that there is minimal shielding, at least of spores, during the irradiations (Fig. 2). The UV222 lamp was routinely 6.5 cm above the surface of the liquid, and the radiation intensity at the surface of the liquid was 140 μW/cm2, as measured with a recently calibrated Unimeter SNK005 meter (Ushio America). UV254 irradiation was performed with a UVG-11 lamp (Ultraviolet Products, San Gabriel, CA) that was routinely 11.1 cm above the surface of the liquid, and the radiation intensity was 290 μW/cm2, as measured with a recently factory-calibrated J225 Blak-Ray UV meter (Ultraviolet Products). UV254 irradiation was also done 14.5 cm above the surface of the liquid, where the radiation intensity was 170 μW/cm2, and this treatment gave the same relative spore viability-versus-fluence results as with a radiation intensity of 290 μW/cm2 (data not shown). For spore and cell analyses, samples (routinely 50 μl but 25 μl for C. difficile spores) were taken from the petri dish at various times and serially diluted 1/10 in either sterile PBSa (cells) or water (spores). For samples taken at time zero, the viable counts obtained were invariably those expected based on the viable counts in the spore or cell inocula (data not shown), indicating that there was minimal spore or cell adhesion to the petri dish in which irradiation was carried out. For determination of the viability of spores and all B. subtilis or S. aureus cells after irradiation, duplicate aliquots (10 μl) of various dilutions obtained as described above were spotted in duplicate onto L broth agar plates (Bacillus species and S. aureus) or prereduced BHI plates as described above (C. difficile spores), plates were incubated at 30°C or 37°C until no more colonies appeared (14 to 24 h), colonies were counted, and values for duplicates were averaged. However, CaDPA-less spores of B. subtilis strains FB122 and PS3406 do not germinate with nutrient germinants since they lack the CLE SleB as well as the CaDPA needed to activate the other redundant CLE, CwlJ (33, 44, 47). Therefore, aliquots of these irradiated spores were diluted 1/10 in 50 mM CaDPA at pH 7.4, incubated for 2 h at 23°C to germinate these spores (44, 47), and then further diluted; duplicate aliquots were spotted onto L broth agar plates; the plates were incubated; and the counts were averaged as described above. The spore/cell inactivation experiments in Fig. 1 to 5 and Fig. 6A were carried out at least twice, and the same relative rates of spore/cell inactivation were seen in all experiments (data not shown). In duplicate experiments, in which we examined if the outgrowth of UV222-irradiated spores was salt sensitive, dilutions of UV222-irradiated wild-type B. subtilis spores and growing cells were also spotted in duplicate onto LB agar plates containing 1 M NaCl, and plates were incubated and colonies were counted as described above.
For HSV irradiation, the KOS viral stock propagated in Vero cells was diluted in PBSb to a concentration of 1 × 108 PFU/ml. A total of 1.5 ml of diluted virus was added to a sterile 35-mm petri dish and exposed to UV light, and 20-μl samples were taken at various times during irradiation. Tenfold serial dilutions were prepared in DMEM and plated onto a monolayer of Vero cells in 24-well plates. After 30 min of incubation at 37°C, DMEM containing 2.5% fetal bovine serum and 2% human serum was added, and plates were incubated for 72 h at 37°C. Plates were then fixed with 8% formaldehyde and stained with 1% crystal violet, plaques were counted, and viral yields were calculated. All HSV work was done in a class II biological safety cabinet in a biosafety level 2 (BSL-2)-approved facility.
Assessment of mutagenesis and DNA damage in UV222-irradiated B. subtilis cells or spores.
For determination of the levels of mutagenesis caused by UV222, appropriate dilutions of log-phase cells or spores of B. subtilis PS533 (wild type) that were either not irradiated or inactivated to various degrees by UV222 were spread onto L broth agar plates with kanamycin (10 μg/ml) to obtain 100 to 300 colonies per plate, and plates were incubated overnight at 37°C. A total of 290 to 400 colonies from the latter plates were then toothpicked onto 2× SG sporulation medium and Spizizen’s minimal medium (57) agar plates, and the plates were incubated at 37°C for ∼3 days. Auxotrophic (aux) mutants were identified on the minimal medium plates as colonies that grew on 2× SG plates but not on minimal medium plates, and asporogenous (spo) mutants were identified by their colonies’ translucent appearance on 2× SG medium plates, in contrast to the opaque, crusty appearance of well-sporulated colonies (29).
DNA was isolated from chemically decoated dormant spores or growing cells, with or without prior UV222 irradiation, as described previously (39, 40) but using Qiagen Genomic-Tip 20/G columns (Qiagen). Purified DNA samples of 1 to 3 μg were then hydrolyzed, and photoproducts were analyzed and quantitated by high-performance liquid chromatography coupled with tandem mass spectrometry, essentially as described previously (58). Analyses of strand breaks in DNA of UV222-irradiated spores and log-phase cells were performed using strain PS533. Total DNA was isolated from unirradiated and UV222-irradiated spores and cells, purified, and run on an agarose gel, and the gels were stained and photographed, all as described previously (59).
Measurement of germination of unirradiated and UV222-irradiated spores.
B. subtilis spores at an OD600 of 0.5 with or without UV222 irradiation giving ∼99% spore killing were germinated at 37°C with the germinant l-valine (33) in 200 μl of a solution containing 25 mM K-HEPES buffer (pH 7.4), 10 mM l-valine, and 50 μM TbCl3, as described previously (56). Germination was done in a multiwell fluorometer, and spore germination was monitored by the fluorescence of released DPA with Tb3+ as described previously (56). Aliquots of the germination incubation mixtures were also examined by phase-contrast microscopy to be sure that germinated spores not only had released DPA but also had undergone cortex peptidoglycan hydrolysis and thus had completed the germination process (33).
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Aimee Shen for providing C. difficile JIR8094, JIR8094 ΔdpaAB, and JIR8094 ΔdpaAB/dpaAB. We also thank Dennis Wright for providing S. aureus strain 43300 and Holger Claus and Ashish Mathur for helpful suggestions about the work and the manuscript.
This work was supported by a contract from UltraViolet Devices, Inc.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Setlow P, Johnson EA. 2019. Spores and their significance, p 23–64. In Doyle MP, Diez-Gonzalez F, Hill C (ed), Food microbiology, fundamentals and frontiers, 5th ed ASM Press, Washington, DC. [Google Scholar]
- 2.Setlow P. 2006. Spores of Bacillus subtilis: their resistance to radiation, heat and chemicals. J Appl Microbiol 101:514–525. doi: 10.1111/j.1365-2672.2005.02736.x. [DOI] [PubMed] [Google Scholar]
- 3.Setlow P. 2007. I will survive: DNA protection in bacterial spores. Trends Microbiol 15:172–180. doi: 10.1016/j.tim.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Kohler LJ, Quirk AV, Welkos SK, Cote CK. 2018. Incorporation of germination-induction into decontamination strategies for bacterial spores. J Appl Microbiol 124:2–14. doi: 10.1111/jam.13600. [DOI] [PubMed] [Google Scholar]
- 5.Wood JP, Adrion AC. 2019. Review of decontamination strategies for the inactivation of Bacillus anthracis and other spore-forming bacteria associated with building or outdoor materials. Environ Sci Technol 53:4045–4062. doi: 10.1021/acs.est.8b05274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbut F. 2015. How to eradicate Clostridium difficile from the environment. J Hosp Infect 89:287–295. doi: 10.1016/j.jhin.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Cadet J, Sage E, Douki T. 2005. Ultraviolet radiation-mediated damage to cellular DNA. Mutat Res 571:3–17. doi: 10.1016/j.mrfmmm.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Mullenders LHF. 2018. Solar UV damage to cellular DNA: from mechanisms to biological effects. Photochem Photobiol Sci 17:1842–1852. doi: 10.1039/c8pp00182k. [DOI] [PubMed] [Google Scholar]
- 9.Buonanno M, Randers-Pehrson G, Bigelow AW, Trivedi S, Lowy FD, Spotnitz HM, Hammer SM, Brenner DJ. 2013. 207-nm UV light—a promising tool for safe low-cost reduction of surgical site infections. I. In vitro studies. PLoS One 8:e76968. doi: 10.1371/journal.pone.0076968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buonanno M, Stanislauskas M, Ponnaiya B, Bigelow AW, Randers-Pehrson G, Xu Y, Shuryak I, Smilenov L, Owens DM, Brenner DJ. 2016. 207-nm UV light—a promising tool for safe low-cost reduction of surgical site infections. II. In vivo safety studies. PLoS One 11:e0138418. doi: 10.1371/journal.pone.0138418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buonanno M, Ponnaiya B, Welch D, Stanislauskas M, Randers-Pehrson G, Smilenov L, Lowy FD, Owens DM, Brenner DJ. 2017. Germicidal efficacy and mammalian skin safety of 222-nm UV light. Rad Res 187:493–501. doi: 10.1667/RR0010CC.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clauß M. 2006. Higher effectiveness of photoinactivation of bacterial spores, UV resistant vegetative bacteria and mold spores with 222 nm compared to 254 nm wavelength. Acta Hydrochim Hydrobiol 34:525–532. doi: 10.1002/aheh.200600650. [DOI] [Google Scholar]
- 13.Wang D, Oppenländer T, El-Din MG, Bolton JR. 2010. Comparison of the disinfection effects of vacuum-UV (VUV) and UV light on Bacillus subtilis spores in aqueous suspensions at 172, 222 and 254 nm. Photochem Photobiol 86:176–181. doi: 10.1111/j.1751-1097.2009.00640.x. [DOI] [PubMed] [Google Scholar]
- 14.Welch D, Buonanno M, Shuryak I, Randers-Pehrson G, Spotnitz HM, Brenner DJ. 2018. Effect of far ultraviolet light emitted from an optical diffuser on methicillin-resistant Staphylococcus aureus in vitro. PLoS One 13:e0202275. doi: 10.1371/journal.pone.0202275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welch D, Buonanno M, Grilj V, Shuryak I, Crickmore C, Bigelow AW, Randers-Pehrson G, Johnson GW, Brenner DJ. 2018. Far-UVC light: a new tool to control the spread of airborne-mediated microbial diseases. Sci Rep 8:2752. doi: 10.1038/s41598-018-21058-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ponnaiya B, Buonanno M, Welch D, Shuryak I, Randers-Pehrson G, Brenner DJ. 2018. Far-UVC light prevents MRSA infection of superficial wounds in vivo. PLoS One 13:e0192053. doi: 10.1371/journal.pone.0192053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narita K, Asano K, Morimoto Y, Igarashi T, Hamblin MR, Dai T, Nakane A. 2018. Disinfection and healing effects of 222-nm UVC light on methicillin-resistant Staphylococcus aureus infection in mouse wounds. J Photochem Photobiol B 178:10–18. doi: 10.1016/j.jphotobiol.2017.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaidzu S, Sugihara K, Sasaki M, Nishiaki A, Igarashi T, Tanito M. 2019. Evaluation of acute corneal damage induced by 222-nm and 254-nm ultraviolet light in Sprague-Dawley rats. Free Radic Res 53:611–617. doi: 10.1080/10715762.2019.1603378. [DOI] [PubMed] [Google Scholar]
- 19.Narita K, Asano K, Morimoto Y, Igarashi T, Nakane A. 2018. Chronic irradiation with 222-nm UVC light induces neither DNA damage nor epidermal lesions in mouse skin, even at high doses. PLoS One 13:e0201259. doi: 10.1371/journal.pone.0201259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buhr TL, Young AA, Bensman M, Minter ZA, Kennihan NL, Johnson CA, Bohmke MD, Borgers-Klonkowski E, Osborn EB, Avila SD, Theys AM, Jackson PJ. 2016. Hot, humid air decontamination of a C-130 aircraft contaminated with spores of two acrystalliferous Bacillus thuringiensis strains, surrogates for Bacillus anthracis. J Appl Microbiol 120:1074–1078. doi: 10.1111/jam.13055. [DOI] [PubMed] [Google Scholar]
- 21.Czepiel J, Dróżdż M, Pituch H, Kuijper EJ, Perucki W, Mielimonka A, Goldman S, Wultańska D, Garlicki A, Biesiada G. 2019. Clostridium difficile infection: review. Eur J Clin Microbiol Infect Dis 38:1211–1221. doi: 10.1007/s10096-019-03539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munakata N, Hieda K, Kobayashi K, Ito A, Ito T. 1986. Action spectra in ultraviolet wavelengths (150–250 nm) for inactivation and mutagenesis of Bacillus subtilis spores obtained with synchrotron radiation. Photochem Photobiol 44:385–390. doi: 10.1111/j.1751-1097.1986.tb04680.x. [DOI] [PubMed] [Google Scholar]
- 23.Donnellan JE Jr, Stafford RS. 1968. The ultraviolet photochemistry and photobiology of vegetative cells and spores of Bacillus megaterium. Biophys J 8:17–28. doi: 10.1016/S0006-3495(68)86471-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindberg C, Horneck G. 1991. Action spectra for survival and spore photoproduct formation of Bacillus subtilis irradiated with short-wavelength (200-300 nm) UV at atmospheric pressure and in vacuo. J Photochem Photobiol B 11:69–80. doi: 10.1016/1011-1344(91)80269-N. [DOI] [PubMed] [Google Scholar]
- 25.Coleman WH, Setlow P. 2009. Analysis of damage due to moist heat treatment of spores of Bacillus subtilis. J Appl Microbiol 106:1600–1607. doi: 10.1111/j.1365-2672.2008.04127.x. [DOI] [PubMed] [Google Scholar]
- 26.Cortezzo DE, Koziol-Dube K, Setlow B, Setlow P. 2004. Treatment with oxidizing agents damages the inner membrane of spores of Bacillus subtilis and sensitizes the spores to subsequent stress. J Appl Microbiol 97:838–852. doi: 10.1111/j.1365-2672.2004.02370.x. [DOI] [PubMed] [Google Scholar]
- 27.Setlow B, Parish S, Zhang P, Li YQ, Neely WC, Setlow P. 2014. Mechanisms of killing of spores of Bacillus anthracis in a high-temperature gas environment, and analysis of DNA damage generated by various decontamination treatments of spores of Bacillus anthracis, Bacillus subtilis and Bacillus thuringiensis. J Appl Microbiol 116:805–814. doi: 10.1111/jam.12421. [DOI] [PubMed] [Google Scholar]
- 28.Jensen RA, Haas FL. 1963. Analysis of ultraviolet light-induced mutagenesis by DNA transformation in Bacillus subtilis. Proc Natl Acad Sci U S A 50:1109–1116. doi: 10.1073/pnas.50.6.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fairhead H, Setlow B, Setlow P. 1993. Prevention of DNA damage in spores and in vitro by small, acid-soluble proteins from Bacillus species. J Bacteriol 175:1367–1374. doi: 10.1128/jb.175.5.1367-1374.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Setlow B, Setlow P. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J Bacteriol 178:3486–3495. doi: 10.1128/jb.178.12.3486-3495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Djouiai B, Thwaite JE, Laws TR, Commichau FM, Setlow B, Setlow P, Moeller R. 2018. Role of DNA repair and protective components in Bacillus subtilis spore resistance to inactivation by 400-nm-blue light. Appl Environ Microbiol 84:e01604-18. doi: 10.1128/AEM.01604-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Setlow P, Li L. 2015. Photochemistry and photobiology of the spore photoproduct: a 50-year journey. Photochem Photobiol 91:1263–1290. doi: 10.1111/php.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Setlow P, Wang S, Li Y-Q. 2017. Germination of spores of the orders Bacillales and Clostridiales. Annu Rev Microbiol 71:459–477. doi: 10.1146/annurev-micro-090816-093558. [DOI] [PubMed] [Google Scholar]
- 34.Granger AC, Gaidamakova EK, Matrosova VY, Daly MJ, Setlow P. 2011. Effects of Mn and Fe levels on Bacillus subtilis spore resistance and effects of Mn2+, other divalent cations, orthophosphate, and dipicolinic acid on protein resistance to ionizing radiation. Appl Environ Microbiol 77:32–40. doi: 10.1128/AEM.01965-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daly MJ. 2012. Death by protein damage in irradiated cells. DNA Repair (Amst) 11:12–21. doi: 10.1016/j.dnarep.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 36.Radman M. 2016. Protein damage, radiation sensitivity and aging. DNA Repair (Amst) 44:186–192. doi: 10.1016/j.dnarep.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 37.Beck S, Hull NM, Poepping C, Linden KG. 2017. Wavelength-dependent damage to adenoviral proteins across the germicidal UV spectrum. Env Sci Technol 52:223–229. doi: 10.1021/acs.est.7b04602. [DOI] [PubMed] [Google Scholar]
- 38.Lee KS, Bumbaca D, Kosman J, Setlow P, Jedrzejas MJ. 2008. Structure of a protein-DNA complex essential for DNA protection in spores of Bacillus species. Proc Natl Acad Sci U S A 105:2806–2811. doi: 10.1073/pnas.0708244105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Douki T, Setlow B, Setlow P. 2005. Effects of the binding of α/β-type small, acid-soluble spore proteins on the photochemistry of DNA in spores of Bacillus subtilis and in vitro. Photochem Photobiol 81:163–169. doi: 10.1562/2004-08-18-RA-278. [DOI] [PubMed] [Google Scholar]
- 40.Matsunaga T, Hieda K, Nikaido O. 1991. Wavelength dependent formation of thymine dimers and (6-4) photoproducts in DNA by monochromatic ultraviolet light ranging from 150 to 365 nm. Photochem Photobiol 54:403–410. doi: 10.1111/j.1751-1097.1991.tb02034.x. [DOI] [PubMed] [Google Scholar]
- 41.Gurzadyan GG, Gorner H, Schulte-Frohlinde D. 1993. Photolesions and biological inactivation of plasmid DNA on 254 nm irradiation and comparison with 193 nm laser irradiation. Photochem Photobiol 58:477–485. doi: 10.1111/j.1751-1097.1993.tb04918.x. [DOI] [PubMed] [Google Scholar]
- 42.Melvin T, Cunniffe S, Papworth D, Roldan-Arjona T, O’Neill P. 1997. Irradiation of DNA with 193 nm light yields formamidopyrimidine-DNA glycosylase (Fpg) protein-sensitive lesions. Photochem Photobiol 65:660–665. doi: 10.1111/j.1751-1097.1997.tb01908.x. [DOI] [PubMed] [Google Scholar]
- 43.Douki T, Setlow B, Setlow P. 2005. Photosensitization of DNA by dipicolinic acid, a major component of spores of Bacillus species. Photochem Photobiol Sci 4:591–597. doi: 10.1039/b503771a. [DOI] [PubMed] [Google Scholar]
- 44.Paidhungat M, Ragkousi K, Setlow P. 2001. Genetic requirements for induction of germination of spores of Bacillus subtilis by Ca2+-dipicolinate. J Bacteriol 183:4886–4893. doi: 10.1128/JB.183.16.4886-4893.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McKenney PT, Driks A, Eichenberger P. 2013. The Bacillus subtilis endospore: assembly and functions of the spore coat. Nat Rev Microbiol 11:33–44. doi: 10.1038/nrmicro2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghosh S, Setlow B, Wahome PG, Cowan AE, Plomp M, Malkin AJ, Setlow P. 2008. Characterization of spores of Bacillus subtilis that lack most coat layers. J Bacteriol 190:6741–6748. doi: 10.1128/JB.00896-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tovar-Rojo F, Chander M, Setlow B, Setlow P. 2002. The products of the spoVA operon are involved in dipicolinic acid uptake into developing spores of Bacillus subtilis. J Bacteriol 184:584–587. doi: 10.1128/jb.184.2.584-587.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Donnelly ML, Fimlaid KA, Shen A. 2016. Characterization of Clostridium difficile spores lacking either SpoVAC or dipicolinic acid synthetase. J Bacteriol 198:1694–1707. doi: 10.1128/JB.00986-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keshipeddy S, Reeve SM, Anderson AC, Wright DL. 2015. Nonracemic antifolates stereoselectively recruit alternate cofactors and overcome resistance in S. aureus. J Am Chem Soc 137:8983–8990. doi: 10.1021/jacs.5b01442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reeve SM, Scocchera EW, G-Dayanadan N, Keshipeddy S, Krucinska J, Hajian B, Ferreira J, Nailor M, Aeschlimann J, Wright DL, Anderson AC. 2016. MRSA isolates from United States hospitals carry dfrG and dfrK resistance genes and succumb to propylargyl-linked antifolates. Cell Chem Biol 23:1458–1467. doi: 10.1016/j.chembiol.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith KO. 1964. Relationship between the envelope and the infectivity of herpes simplex virus. Proc Soc Exp Biol Med 115:814–816. doi: 10.3181/00379727-115-29045. [DOI] [PubMed] [Google Scholar]
- 52.Paidhungat M, Setlow B, Driks A, Setlow P. 2000. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J Bacteriol 182:5505–5512. doi: 10.1128/jb.182.19.5505-5512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nicholson WL, Setlow P. 1990. Sporulation, germination and outgrowth, p 391–450. In Harwood CR, Cutting SM (ed), Molecular biological methods for Bacillus. John Wiley & Sons, Chichester, United Kingdom. [Google Scholar]
- 54.Setlow P. 2019. Observations on research with spores of Bacillales and Clostridiales species. J Appl Microbiol 126:348–358. doi: 10.1111/jam.14067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Putnam EE, Nock AM, Lawley TD, Shen A. 2013. SpoIVA and SipL are Clostridium difficile spore morphogenetic proteins. J Bacteriol 195:1214–1225. doi: 10.1128/JB.02181-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yi X, Setlow P. 2010. Studies of the commitment step in the germination of spores of Bacillus species. J Bacteriol 192:3424–3433. doi: 10.1128/JB.00326-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spizizen J. 1958. Transformation of biochemically deficient strains of Bacillus subtilis by deoxyribonucleate. Proc Natl Acad Sci U S A 44:1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nicholson WL, Schuerger AC, Douki T. 2018. The photochemistry of unprotected DNA and DNA inside Bacillus subtilis spores exposed to simulated Martian surface conditions of atmospheric composition, temperature, pressure and solar radiation. Astrobiology 18:393–402. doi: 10.1089/ast.2017.1721. [DOI] [PubMed] [Google Scholar]
- 59.Setlow B, Sun D, Setlow P. 1992. Studies of the interaction between DNA and α/β-type small, acid-soluble spore proteins: a new class of DNA binding protein. J Bacteriol 174:2312–2322. doi: 10.1128/jb.174.7.2312-2322.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.