Abstract
Group 2 innate lymphoid cells (ILC2s) are increasingly recognized as a key controller of type 2 inflammation, and are well known to be highly elevated in human airway type 2 inflammatory diseases including allergic rhinitis, chronic rhinosinusitis with nasal polyps, and asthma. ILC2-mediated production of type 2 cytokines initiates and amplifies airway inflammation via activation of eosinophils, B cells, mast cells, macrophages, fibroblasts, and epithelial cells in these diseases. ILC2s require at least three major signals to fully activate and robustly produce type 2 cytokines. IL-1 family cytokines (IL-1β, IL-18, IL-33), IL-25, and TNF superfamilies (TNF, TL1A, GITR-L, RANK-L) activate the NF-κB and AP-1 pathways that initiate production of IL-5 and IL-13. Lipid mediators (LTC4, LTD4, PGD2) and neuropeptide NMU promote production of IL-4 through the NFAT pathway. IL-2 and IL-7 family cytokines (IL-2, IL-7, IL-9, TSLP) activate the STAT5 pathway that induces survival of ILC2s and enhances cytokine production. The activation of STAT5 is necessary to potently induce cytokine- and lipid mediator-mediated production of type 2 cytokines. Inhibitory pathways for ILC2s have also become clearer. Type I and II interferons and IL-27 inhibit ILC2 functions through the activation of STAT1. Suppression mediated via β2-adrenergic receptor agonists, PGE2, and PGI2 occurs through cAMP and PKA. Glucocorticoid, testosterone, IL-10, and TGF-β are also able to inhibit ILC2-mediated production of type 2 cytokines. Blockage of ILC2 activators, activation of inhibitory pathways of ILC2s, and suppression of ILC2-mediated pathways including type 2 cytokines (IL-5, IL-13, IL-4Ra) may become therapeutic strategies for airway type 2 inflammatory diseases.
Key Words: allergic rhinitis, asthma, chronic rhinosinusitis with nasal polyps, ILC2, type 2 inflammation
Abbreviations: ADRB2, β2-adrenergic receptor; AR, androgen receptor; AREG, amphiregulin; CGRP, calcitonin gene-related peptide; CRS, chronic rhinosinusitis; CRSsNP, CRS without nasal polyps; CRSwNP, CRS with nasal polyps; CysLT1R, cysteinyl leukotriene receptor 1; γc, IL-2R common γ chain; GITR, glucocorticoid-induced TNFR-related protein; ICOS, inducible T-cell costimulator; IFN, interferon; ILC, innate lymphoid cell; ILC2, group 2 ILC; LT, leukotriene; MAPK, mitogen-activated protein kinase; NMU, neuromedin U; NP, nasal polyp; PG, prostaglandin; RANK, receptor activator of NF-κB; RANK-L, RANK ligand; SCC, solitary chemosensory cell; TL1A, TNF-like cytokine 1A; TNFRSF, TNF receptor superfamily; TNFSF, TNF superfamily; TSLP, thymic stromal lymphopoietin; VIP, vasoactive intestinal peptide
Innate Lymphoid Cells
Innate lymphoid cells (ILCs) are increasingly recognized as a key component of both innate immunity, to protect from pathogens, and pathological processes for many diseases. ILCs are classified into three major subsets: group 1 ILCs (ILC1s), ILC2s and ILC3s, and each ILC group has several subsets.1, 2, 3 All ILCs develop from common innate lymphoid progenitors, which are differentiated from the common lymphoid progenitors.2 Although ILCs lack antigen receptors (T-cell receptor [TCR], B-cell receptor [BCR]), ILCs produce high levels of helper T-cell cytokines via antigen-independent stimuli including cytokines and lipid mediators. ILC1s are characterized by the expression of T-bet and production of the helper T type 1 (Th1) cytokine interferon (IFN)-γ. ILC2s express GATA3 and produce Th2 cytokines including IL-5 and IL-13. ILC3s are characterized by the expression of RORγt (retinoic acid receptor-related orphan nuclear receptor γ, thymus-specific isoform) and the production of type 3 (Th17) cytokines including IL-17A and IL-22. Therefore, ILCs are recognized as the innate counterpart of T lymphocytes in that ILC1s, ILC2s, and ILC3s mirror CD4+ Th1 cells, Th2 cells, and Th17 cells, respectively.
ILC2s
The presence of ILC2s was initially implicated in mice by showing that IL-25 and IL-33 induce type 2 cytokines in RAG-deficient mice.4, 5 These studies suggested that non-B/non-T cells are responsible for IL-25- and IL-33-mediated production of type 2 cytokines. In 2010, four independent groups characterized type 2 cytokine-producing non-B/non-T cells in mice, and these cells are now categorized as a single population termed ILC2s by nomenclature.6 Human ILC2s are currently recognized as CD45+ lymphocytes that are Lineage (CD1a, CD3, CD4, CD16, CD19, CD34, CD94, CD303, FcεRI) negative CD127+CD161+CRTH2+.1, 7
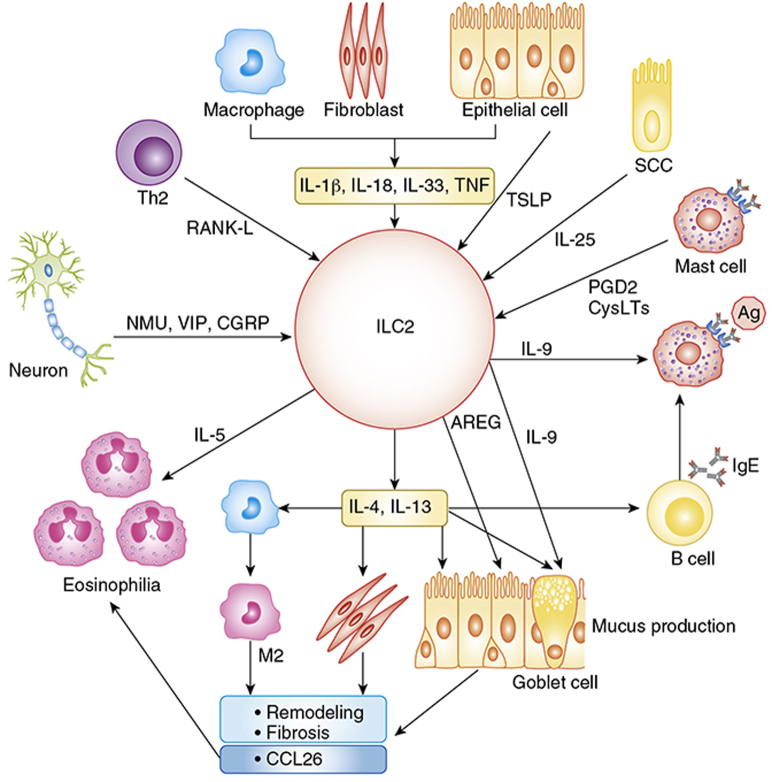
ILC2-mediated inflammation is mainly via the production of type 2 cytokines including IL-4, IL-5, IL-9, and IL-13 (Fig 1).1, 8, 9, 10 IL-5 primarily induces eosinophilia. IL-4 and IL-13 are key factors that control IgE responses in B cells, mucus production and remodeling in epithelial cells, induction of eosinophil chemoattractants including eotaxin-3 (CCL26) in epithelial cells and fibroblasts, and the induction of M2 macrophages. IL-9 promotes mast cell growth and goblet cell metaplasia. ILC2s not only produce type 2 cytokines but also release many other factors including amphiregulin (AREG), which controls fibrosis and tissue repair and remodeling.1, 8 Therefore, accumulation and activation of ILC2s is now considered a key event for many type 2 inflammatory diseases (Fig 1).
Figure 1.
Group 2 innate lymphoid cell (ILC2s) contribute to airway inflammation via the production of type 2 cytokines and amphiregulin. ILC2s produce type 2 cytokines in response to stimulation with several cytokines such as TNF and IL-1 family cytokines from epithelial cells, fibroblasts, and macrophages; TSLP from epithelial cells; IL-25 from SCCs and RANK-L from Th2 cells; lipid mediators from mast cells; and neuropeptides from neurons. IL-5 promotes eosinophilia and IL-9 induces goblet cell metaplasia and mast cell growth. AREG controls fibrosis and remodeling in epithelial cells. IL-4 and IL-13 activate macrophages, B cells, fibroblasts, epithelial cells, and goblet cells to induce eosinophil recruitment, mucus production, remodeling, fibrosis, and IgE-mediated reactions. Ag = antigen; AREG = amphiregulin; CGRP = calcitonin gene-related peptide; CysLTs = cysteinyl leukotrienes; M2 = M2 macrophage; NMU = neuromedin U; PGD2 = prostaglandin D2; RANK-L = receptor activator of NF-κB ligand; SCC = solitary chemosensory cell; Th2 = helper T type 2; TNF = tumor necrosis factor; TSLP = thymic stromal lymphopoietin; VIP = vasoactive intestinal peptide.
Activation of ILC2s by Cytokines
ILC2s express a wide variety of cytokine receptors on the cell surface. IL-25 and IL-33 were initially identified as major type 2 cytokine inducers in ILC2s, and receptors for IL-25 (IL-17RA and IL-17RB [IL-25R]) and IL-33 (IL-1RL1 [IL-33R] and IL-1RAP) are highly expressed on ILC2s. IL-25 is an IL-17 family cytokine that is released from epithelium, especially from a unique population of epithelial cells called tuft cells in the gut and solitary chemosensory cells (SCCs) and brush cells in the airway.11, 12, 13 IL-33 is released from epithelial cells and other cells including fibroblasts and endothelial cells.8 IL-25 and IL-33 activate AP-1 and nuclear factor (NF)-κB pathways via mitogen-activated protein kinases (MAPKs) and TRAF6, respectively.14, 15, 16 In addition to IL-33, receptors for other IL-1 family cytokines, IL-1R1 and IL-18R, are expressed on ILC2s and IL-1β and IL-18 induce IL-5 and IL-13 in ILC2s.17, 18, 19, 20
Members of the TNF superfamily (TNFSF) also provide strong signals in ILC2s. TNF-like cytokine 1A (TL1A [TNFSF15]) induces production of IL-5 and IL-13 in ILC2s via death receptor 3 (DR3 [TNF receptor superfamily 25; TNFRSF25]).21, 22 Nagashima et al23 found the expression of glucocorticoid-induced TNFR-related protein (GITR [TNFRSF18]) on ILC2s and an agonistic antibody against GITR enhanced IL-33-mediated induction of IL-5 and IL-13 in human ILC2s. Since many TNFRSFs, including DR3 and GITR, share the NF-κB and MAPK signal pathways, our group24 recently screened TNFRSFs on ILC2s and ligands (TNFSFs) that activate human ILC2s. We discovered that TNF receptor II (TNFRII [TNFRSF1B]) and receptor activator of NF-κB (RANK [TNFRSF11A]) are expressed on human ILC2s; and that their ligands, TNF and RANK ligand (RANK-L [TNFSF11]), induce production of type 2 cytokines in human ILC2s. These results suggest that IL-25, IL-1 family cytokines, and TNFSFs are major activators and inducers of type 2 cytokines in ILC2s, mainly via NF-κB and AP-1 (MAPK) pathways (Fig 2).
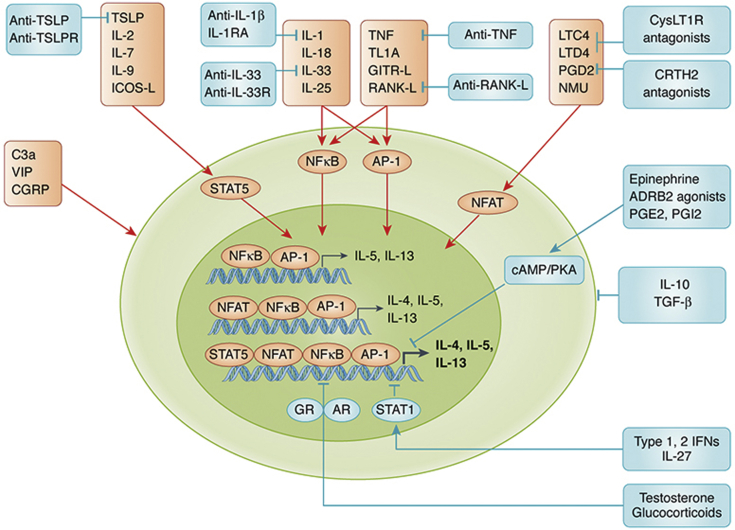
Figure 2.
Schematic of activation and inhibition pathways for ILC2s. IL-1 family cytokines, IL-25, and TNFSFs weakly induce IL-5 and IL-13 via activation of NF-κB and AP-1. Activation of NFAT by lipid mediators and NMU is required for induction of IL-4. IL-2 and IL-7 family cytokines and ICOS-L act as costimulators and potently enhance cytokine- and lipid mediator-mediated induction of IL-4, IL-5, and IL-13 via the activation of STAT5 in ILC2s. Although C3a, VIP, and CGRP activate ILC2s, at least in mice, their signaling pathways in ILC2s have not been elucidated. Testosterone and glucocorticoids suppress NF-κB-mediated induction of type 2 cytokines via activation of nuclear receptors, AR and GR, respectively. IFN- and IL-27-mediated inhibition of ILC2s occurs via the STAT1 pathway. The activation of cAMP and PKA is involved in ADRB2 agonist-, PGE2-, and PGI2-mediated inhibition. IL-10 and TGF-β also suppress functions of ILC2s. Furthermore, monoclonal antibodies and receptor antagonists against activation pathways of ILC2s are commercially available or in clinical trials. ADRB2 = β2-adrenergic receptor; AR = androgen receptor; cAMP = cyclic AMP; CRTH2 = chemoattractant receptor-homologous molecule expressed on Th2 cells; CysLT1R = cysteinyl leukotriene receptor 1; GITR-L = glucocorticoid-induced TNFR-related protein ligand; GR = glucocorticoid receptor; ICOS-L = inducible T-cell costimulatory ligand; IFN = interferon; IL-1RA = IL-1 receptor antagonist; IL-33R = IL-33 receptor; LTC4, LTD2 = leukotrienes C4 and D2; NF-κB = nuclear factor-κB; NFAT = nuclear factor of activated T cells; PGD2, PGE2, PGI2 = prostaglandins D2, E2, and I2; PKA = protein kinase A; STAT1, STAT5 = signal transducer and activator of transcription 1 and 5; TL1A = TNF-like cytokine 1A; TNFSF = TNF superfamily; TSLPR = thymic stromal lymphopoietin receptor. See Figure 1 legend for expansion of other abbreviations.
Although administration of IL-25 or IL-33 induces potent type 2 inflammation in mice in vivo, IL-25 or IL-33 alone is a weak inducer of IL-5 and IL-13 in ILC2s in vitro.4, 5, 25, 26, 27 This suggests that these cytokines are important initiators of ILC2-mediated type 2 inflammation; however, similar to T lymphocytes, ILC2s may require costimulatory signals that fully activate ILC2s. ILC2s also express receptors for IL-2 and IL-7 family cytokines including IL-2 (IL-2Rα, IL-2Rβ, and IL-2R common γ [γc]), IL-7 (IL-7Rα and γc), IL-9 (IL-9R and γc), and thymic stromal lymphopoietin (TSLP) (IL-7Rα and CRLF2 [TSLPR]).8 IL-2, IL-7, IL-9, and TSLP activate ILC2s through similar signaling pathways including STAT1, STAT3, and STAT5, and the activation of STAT5 is considered a key pathway that promotes ILC2 functions.8 Although these cytokines alone do not induce full activation, they are able to enhance IL-1β-, IL-18-, IL-25-, IL-33-, TNF-, and RANK-L-mediated production of type 2 cytokines in ILC2s.17, 18, 19, 24, 26 This suggests that IL-2, IL-7, IL-9, and TSLP act as costimulators and enhancers for ILC2-mediated type 2 inflammation via activation of STAT5 (Fig 2).
Activation of ILC2s by Lipid Mediators
Lipid mediators including leukotrienes (LTs) and prostaglandins (PGs) play important roles in homeostasis and inflammation. Importantly, CRTH2, a receptor for PGD2, and cysteinyl leukotriene receptor 1 (CysLT1R), a receptor for LTC4 and LTD4, are highly expressed on ILC2s and their ligands (PGD2, LTC4, and LTD4) induce ILC2 migration and production of type 2 cytokines including IL-4, IL-5, and IL-13 in human ILC2s.28, 29, 30, 31, 32, 33 Activation of CRTH2 and CysLT1R increases cytosolic Ca2+ levels and activate the NFAT pathway. Interestingly, unlike cytokines such as IL-33, PGD2 and CysLTs potently induce production of IL-4 in ILC2s.28, 29 This indicates that activation of NFAT may be necessary to induce ILC2-mediated production of IL-4. In Th2 cells, engagement of TCR activates three major transcription factors (NFAT, NF-κB, and AP-1) to drive profound production of IL-4, IL-5, and IL-13.32 Based on these findings, von Moltke et al32 proposed that ILC2s also require these three transcription factors and that a combination of cytokines (that activate NF-κB and AP-1) and lipid mediators (that activate NFAT) mimics TCR signaling by inducing all type 2 cytokines including IL-4 in ILC2s (Fig 2).
Activation of ILC2s by Other Factors
In addition to cytokines and lipid mediators, inducible T-cell costimulator (ICOS) and receptors for complement C3a and neuropeptides including neuromedin U (NMU), vasoactive intestinal peptide (VIP), and calcitonin gene-related peptide (CGRP) were found on ILC2s.34, 35, 36, 37, 38, 39 C3a, NMU, and VIP directly induce production of type 2 cytokines in ILC2s, and at least NMU-mediated responses are via MAPK and NFAT pathways in mice.36, 37, 38 Although CGRP alone does not, it significantly enhances the production of IL-5 in the presence of IL-25 or IL-33 in mouse ILC2s.39 ICOS ligand induces the cell survival of ILC2s via the activation of STAT5.34 These results suggest that C3a, NMU, and VIP are direct activators and that ICOS ligand and CGRP may play a costimulatory role in ILC2-mediated type 2 inflammation. However, the role of complement and neuropeptides has not been investigated in human ILC2s and will require further studies.
Plasticity and Heterogeneity of ILC2s
ILCs are known to display plasticity and heterogeneity.1, 3 Although IL-1β and IL-18 induce production of type 2 cytokines in ILC2s, IL-1β plus IL-12 or IL-18 plus IL-12 have the capacity to transdifferentiate human ILC2s into IFN-γ-producing ILC1s.17, 18, 40 Furthermore, notch, CysLTs, and a combination of IL-1β, IL-23, and transforming growth factor (TGF)-β seem to convert ILC2s into an IL-17-producing ILC3-like phenotype.3, 41, 42
Recently, Ricardo-Gonzalez et al19 performed single-cell RNA sequencing on ILC2s sorted from bone marrow, lung, fat, gut, and skin of mice and found that expression of IL-18R1, IL-25R, and IL-33R in ILC2s was enriched in particular tissues although other common ILC2 markers such as GATA3, IL-7Rα, and TSLPR were expressed in the majority of ILC2s across tissues. They found that IL-33R is abundant on bone marrow, lung, and fat ILC2s; IL-25R is on gut ILC2s; and IL-18R1 is highly expressed on skin ILC2s.19 However, whether ILC2s express distinct activating receptors in human tissue is not known and future study is required.
Presence of ILC2s in Airway Inflammatory Diseases
Animal studies have revealed the importance of ILC2s in airway type 2 inflammatory diseases.3, 9, 10 Many groups also have identified accumulation and activation of ILC2s in human airway diseases. We summarize here the presence of ILC2s and their activators in three human airway type 2 inflammatory diseases.
ILC2s in Allergic Rhinitis
Allergic rhinitis is characterized by IgE-mediated type I hypersensitivity with elevation of Th2 cells and type 2 cytokines in nasal mucosa, and is one of the most common allergic diseases in the world.43 ILC2s in peripheral blood and nasal curettage samples are increased after nasal allergen challenge in patients with allergic rhinitis, and ILC2 numbers in these nasal curettage samples positively correlate with eosinophil numbers and IL-5 concentrations.44, 45 Peripheral blood ILC2s are increased during pollen season in patients with seasonal allergic rhinitis.46, 47 However, patients with seasonal allergic rhinitis who received allergen-specific immunotherapy did not show seasonal elevation of blood ILC2s.46 These results indicate that ILC2s are elevated locally and systemically in patients with allergic rhinitis and that the accumulation of ILC2s is controlled at least in part by allergic reactions.
During IgE-mediated reactions, mast cells quickly release lipid mediators including LTs and PGD2 that are elevated in the nasal mucosa of patients with allergic rhinitis.43, 45, 48 These lipid mediators may play an initial role in recruitment and activation of ILC2s in allergic rhinitis. In the case of epithelial-derived cytokines, IL-33 is detected in the nasal mucosa but it is still not clear whether IL-33 is elevated in allergic rhinitis.48 Limited studies have found that IL-25 is elevated in allergic rhinitis.49 Many groups have found that TSLP is significantly elevated in allergic rhinitis.48, 49, 50 These results suggest that lipid mediators, IL-25, and TSLP may play critical roles in ILC2-mediated type 2 inflammation in allergic rhinitis.
ILC2s in Chronic Rhinosinusitis With Nasal Polyps
Chronic rhinosinusitis (CRS) is a common chronic inflammatory disease of the human upper airway and sinuses, and is frequently divided into two main phenotypes: CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP). In Western countries, CRSwNP is well known to be characterized by type 2 inflammation with eosinophilia and the presence of high levels of type 2 cytokines such as IL-5 and IL-13.51, 52 Nasal polyps (NPs) are one of the first tissues in which human ILC2s were discovered, and many groups have reported the elevation of ILC2s in NPs.7, 35, 52, 53, 54, 55 Our group has characterized the presence of all major ILC subsets in CRS and found that ILC2s are the predominant ILCs in NPs and are significantly elevated in NPs compared with sinus mucosa of control subjects and patients with CRSsNP.35 In Asia, about 50% of patients with CRSwNP presented noneosinophilic inflammation in NPs.52 Tojima et al55 found that ILC2s are elevated only in eosinophilic NPs in Japan. In contrast to allergic rhinitis and asthma, blood ILC2s do not seem to be elevated in patients with CRSwNP.35, 55 These results suggest that ILC2s may play a role in type 2 inflammation in eosinophilic CRSwNP, but ILC2 activation may be restricted to the nasal mucosa of these patients.
Although our group also found that ILC2s are not only elevated but also activated and releasing type 2 cytokines in NPs in vivo, factors that activate ILC2s in NPs were not clear.35 Our group56 recently examined the presence of IL-25, IL-33, and TSLP in CRSwNP in Chicago and reviewed these cytokines in worldwide published CRS studies. We found that IL-25 is almost undetectable, IL-33 is present but shows mixed results, and that only TSLP consistently shows significant elevation in NPs. However, TSLP alone does not induce type 2 cytokines in ILC2s. This suggests that IL-25- and IL-33-independent ILC2 activators may play a role in CRSwNP. Our group24 recently screened other potential ILC2 activators and found that RANK-L is significantly elevated in NPs. We also found that RANK-L is expressed on Th2 cells in NPs; and that NP Th2 cells enhance ILC2-mediated production of type 2 cytokines via the RANK-L-mediated pathway. In addition to RANK-L and TSLP, several groups found elevation of lipid mediators including LTC4 and PDG2 in NPs, especially in patients with CRSwNP who have intolerance to inhibitors of cyclooxygenase-1.30, 57 These reports suggest that RANK-L and lipid mediators may be key activators and that TSLP is a costimulator to control ILC2-mediated type 2 inflammation in CRSwNP.
Although IL-33 may not be elevated,56 IL-33 is present in NPs and IL-33 can act in synergy with other elevated factors including lipid mediators and TSLP. This implies the possibility that IL-33 together with TSLP and/or lipid mediators may play a role in type 2 inflammation in CRSwNP. This possibility may also be true in allergic rhinitis. Furthermore, Kohanski et al12 recently found that IL-25 is expressed in a minor subset of epithelial cells called SCCs and these cells are increased in NPs. This may suggest that IL-25 plays a role in the activation of ILC2s in a limited area of NPs. Further studies require examination of the direct role of IL-25 and IL-33 in CRSwNP and allergic rhinitis.
ILC2s in Asthma
Asthma is a heterogeneous lower airway inflammatory disease and is frequently divided into two phenotypes: type 2 high (eosinophilic) asthma and type 2 low (neutrophilic) asthma. Many groups have clearly shown that ILC2s are increased in blood and sputum in patients with asthma, especially in type 2 high severe eosinophilic asthma.27, 58, 59, 60 Smith et al59 also found that IL-5+IL-13+ ILC2s are elevated in the sputum of patients with uncontrolled asthma. In the case of ILC2 activators, genome-wide association studies showed that IL-33, IL-33R, and TSLP are strongly associated with asthma.61, 62, 63 Several groups showed that IL-25, IL-33, and TSLP are elevated in patients with asthma and that they are all increased in the bronchial epithelium after inhalational allergen challenge in subjects with atopic asthma.64, 65, 66, 67, 68 In addition, lipid mediators including LTC4, LTD4, and PGD2 are well known to be involved in asthma and leukotriene receptor antagonists are used to treat asthma. These results suggest that ILC2s are highly elevated and activated in patients with asthma and that epithelial-derived cytokines and lipid mediators may play a key role in ILC2-mediated type 2 inflammation.
Inhibition of ILC2 Activation
Since ILC2s are highly elevated and activated in type 2 airway inflammatory diseases, the inhibition of ILC2 mediators and activators could be a therapeutic option for these diseases. Several monoclonal antibodies against ILC2 mediators including IL-5 (mepolizumab, reslizumab), IL-5Rα (benralizumab), IL-13 (lebrikizumab, tralokinumab), and IL-4Ra (dupilumab) have been developed and some of them are already approved for treatment of type 2 airway inflammatory diseases.69 Furthermore, monoclonal antibodies and small-molecule antagonists against activation pathways of ILC2s including TSLP (tezepelumab), TSLPR (MK-8226), IL-33 (etokimab, REGN3500), IL-33R (MSTT1041A), and CRTH2 (AZD1981, fevipiprant) are in clinical trials for many type 2 inflammatory diseases (not limited to the airway). Although they are not currently used to target type 2 inflammatory diseases, monoclonal antibodies against other ILC2 activators including IL-1β (canakinumab), TNF (infliximab, adalimumab), and RANK-L (denosumab) as well as an IL-1R antagonist (IL-1RA) protein are commercially available for treatment of other diseases including rheumatoid arthritis and psoriasis. These biologics may have benefit in a subset of patients with type 2 inflammatory disease who present with elevation of these cytokines.
Activation of inhibitory pathways for ILC2s could represent other therapeutic options. Currently, a subset of cytokines (IL-10, IL-27, TGF-β, IFN-α, IFN-β, and IFN-γ), lipid mediators (PGE2 and PGI2), norepinephrine, and glucocorticoids are known to suppress ILC2 activation (Fig 2).70
Initial studies identified that regulatory T cells suppress the function of ILC2s via production of IL-10 and TGF-β in mice.71, 72 Subsequently, our group found that IL-10RA and TGFBR2 are expressed on human ILC2s, and IL-10 and TGF-β are able to suppress IL-33 plus TSLP-mediated production of IL-4, IL-5, and IL-13 in human ILC2s.73 We also found that IL-33-induced IL-9 is inhibited by IL-10 whereas TGF-β further enhanced production of IL-9.73 This indicates that the induction of IL-9 may be differently controlled in ILC2s compared with IL-4, IL-5, and IL-13, although NF-κB is involved in expression of all type 2 cytokines.
Several groups found receptors for type I IFN (IFNAR), type II IFN (IFNGR1), and IL-27 (IL-27RA) on ILC2s; and showed that their ligands IFN-α, IFN-β, IFN-γ, and IL-27 inhibit proliferation and production of type 2 cytokines in ILC2s in mice.74, 75, 76 Importantly, STAT1-deficient mice lose these functions by IFN-β, IFN-γ, or IL-27, indicating that the STAT1 pathway is a negative regulator of ILC2s.
Two lipid mediators are known to inhibit ILC2 function. Human ILC2s express receptors for PGE2 (EP2 and EP4) and PGI2 (IP); and PGE2 and PGI2 inhibit the proliferation and production of type 2 cytokines in ILC2s.77, 78 Since PGE2 and PGI2 both activate adenylate cyclase and cyclic AMP (cAMP)/protein kinase A (PKA) pathways, these pathways may be additional negative regulators of ILC2s.8
Moriyama et al79 found that murine ILC2s express the β2-adrenergic receptor (ADRB2) and that administration of an ADRB2 agonist, salmeterol, inhibits ILC2-mediated airway type 2 inflammation in mice. An endogenous ligand of ADRB2, epinephrine, activates adenylate cyclase and cAMP pathways, suggesting that inhibitory pathways involving ADRB2 may be similar to PGE2 and PGI2.
Inhibition of ILC2s by Hormone and Glucocorticoids
The prevalence of asthma is known to be higher in women than in men. Several groups found significant elevation of ILC2s in women compared with men in both people with asthma and mouse asthma models, suggesting that sex hormones may directly influence ILC2s in patients with asthma.80, 81 They also found that the androgen receptor (AR) is expressed on ILC2s; and that a ligand of AR, 5α-dihydrotestosterone, which is a downstream product of the male sex hormone testosterone, inhibits IL-33- and Alternaria extract-induced airway type 2 inflammation and ILC2-mediated production of type 2 cytokines in mice.80, 81 These results suggest that testosterone may be responsible for sex influences in patients with asthma by suppressing ILC2s.
Walford et al54 reported that the frequency of ILC2s in NPs is reduced by treatment with systemic glucocorticoid. Our group73 showed that dexamethasone strongly suppresses the production of IL-5 and IL-13 in NP-derived ILC2s ex vivo. These results suggest that treatment with glucocorticoids is a therapeutic option to directly inhibit ILC2-mediated inflammation in human diseases. However, several groups reported that ILC2s in patients with asthma are resistant to glucocorticoids and that TSLP abrogates the inhibitory activity of glucocorticoids in ILC2s in patients with severe asthma.66, 82 In contrast, our study clearly showed that dexamethasone strongly suppresses IL-33-mediated production of type 2 cytokines in human ILC2s even in the presence of TSLP.73 Future studies are required to clarify why TSLP induces steroid resistance in ILC2s but only in a subset of tissues and/or diseases.
Conclusion
Increasing evidence suggests that ILC2s and their activators are highly accumulated in human airway type 2 inflammatory diseases including allergic rhinitis, CRSwNP, and asthma; and that ILC2s significantly contribute to the production of type 2 cytokines including IL-5 and IL-13 in these diseases. Although there is almost no ILC2-targeting therapy currently, except treatment with glucocorticoids, mechanisms of activation and inhibition of ILC2s have become clearer in the past decade. In the future, this area of research may lead to the development of novel therapies for these diseases aimed at suppressing ILC2-mediated type 2 inflammation via blockages of ILC2 activators and stimulation of inhibitory pathways.
Acknowledgments
Financial/nonfinancial disclosures: The author has reported to CHEST the following: A. K. has received grants from the National Institutes of Health (R01 AI104733, R01 AI137174, R37 HL068546, and U19 AI106683), Janssen Research, and the Ernest S. Bazley Foundation and has received payment for a lecture from Janssen Research.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The author acknowledges Ms Julie Poposki for proofreading of this review.
Footnotes
FUNDING/SUPPORT: This research was supported in part by NIH grants [R01 AI104733, R01 AI137174, R37 HL068546, and U19 AI106683] and by grants from Janssen Research and the Ernest S. Bazley Foundation.
References
- 1.Vivier E., Artis D., Colonna M. Innate lymphoid cells: 10 years on. Cell. 2018;174(5):1054–1066. doi: 10.1016/j.cell.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 2.Scoville S.D., Freud A.G., Caligiuri M.A. Cellular pathways in the development of human and murine innate lymphoid cells. Curr Opin Immunol. 2018;56:100–106. doi: 10.1016/j.coi.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krabbendam L., Bal S.M., Spits H., Golebski K. New insights into the function, development, and plasticity of type 2 innate lymphoid cells. Immunol Rev. 2018;286(1):74–85. doi: 10.1111/imr.12708. [DOI] [PubMed] [Google Scholar]
- 4.Fort M.M., Cheung J., Yen D. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15(6):985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 5.Kondo Y., Yoshimoto T., Yasuda K. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int Immunol. 2008;20(6):791–800. doi: 10.1093/intimm/dxn037. [DOI] [PubMed] [Google Scholar]
- 6.Spits H., Artis D., Colonna M. Innate lymphoid cells: a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13(2):145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 7.Mjosberg J.M., Trifari S., Crellin N.K. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12(11):1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 8.Kabata H., Moro K., Koyasu S. The group 2 innate lymphoid cell (ILC2) regulatory network and its underlying mechanisms. Immunol Rev. 2018;286(1):37–52. doi: 10.1111/imr.12706. [DOI] [PubMed] [Google Scholar]
- 9.Kubo M. Innate and adaptive type 2 immunity in lung allergic inflammation. Immunol Rev. 2017;278(1):162–172. doi: 10.1111/imr.12557. [DOI] [PubMed] [Google Scholar]
- 10.Doherty T.A., Broide D.H. Airway innate lymphoid cells in the induction and regulation of allergy. Allergol Int. 2019;68(1):9–16. doi: 10.1016/j.alit.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Moltke J., Ji M., Liang H.E., Locksley R.M. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature. 2016;529(7585):221–225. doi: 10.1038/nature16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kohanski M.A., Workman A.D., Patel N.N. Solitary chemosensory cells are a primary epithelial source of IL-25 in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2018;142(2):460–469.e467. doi: 10.1016/j.jaci.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bankova L.G., Dwyer D.F., Yoshimoto E. The cysteinyl leukotriene 3 receptor regulates expansion of IL-25-producing airway brush cells leading to type 2 inflammation. Sci Immunol. 2018;3(28):eaat9453. doi: 10.1126/sciimmunol.aat9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong C.K., Li P.W., Lam C.W. Intracellular JNK, p38 MAPK and NF-κB regulate IL-25 induced release of cytokines and chemokines from costimulated T helper lymphocytes. Immunol Lett. 2007;112(2):82–91. doi: 10.1016/j.imlet.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Xu M., Dong C. IL-25 in allergic inflammation. Immunol Rev. 2017;278(1):185–191. doi: 10.1111/imr.12558. [DOI] [PubMed] [Google Scholar]
- 16.Liew F.Y., Girard J.P., Turnquist H.R. Interleukin-33 in health and disease. Nat Rev Immunol. 2016;16(11):676–689. doi: 10.1038/nri.2016.95. [DOI] [PubMed] [Google Scholar]
- 17.Ohne Y., Silver J.S., Thompson-Snipes L. IL-1 is a critical regulator of group 2 innate lymphoid cell function and plasticity. Nat Immunol. 2016;17(6):646–655. doi: 10.1038/ni.3447. [DOI] [PubMed] [Google Scholar]
- 18.Bal S.M., Bernink J.H., Nagasawa M. IL-1β, IL-4 and IL-12 control the fate of group 2 innate lymphoid cells in human airway inflammation in the lungs. Nat Immunol. 2016;17(6):636–645. doi: 10.1038/ni.3444. [DOI] [PubMed] [Google Scholar]
- 19.Ricardo-Gonzalez R.R., Van Dyken S.J., Schneider C. Tissue signals imprint ILC2 identity with anticipatory function. Nat Immunol. 2018;19(10):1093–1099. doi: 10.1038/s41590-018-0201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simoni Y., Fehlings M., Kloverpris H.N. Human innate lymphoid cell subsets possess tissue-type based heterogeneity in phenotype and frequency. Immunity. 2017;46(1):148–161. doi: 10.1016/j.immuni.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meylan F., Hawley E.T., Barron L. The TNF-family cytokine TL1A promotes allergic immunopathology through group 2 innate lymphoid cells. Mucosal Immunol. 2014;7(4):958–968. doi: 10.1038/mi.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu X., Pappu R., Ramirez-Carrozzi V. TNF superfamily member TL1A elicits type 2 innate lymphoid cells at mucosal barriers. Mucosal Immunol. 2014;7(3):730–740. doi: 10.1038/mi.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagashima H., Okuyama Y., Fujita T. GITR cosignal in ILC2s controls allergic lung inflammation. J Allergy Clin Immunol. 2018;141(5):1939–1943.e1938. doi: 10.1016/j.jaci.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 24.Ogasawara N., Poposki J.A., Klingler A.I. Role of TNFSF11 and group 2 innate lymphoid cells in type 2 inflammation in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2018;141(2 suppl):AB1. [Google Scholar]
- 25.Moro K., Yamada T., Tanabe M. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature. 2010;463(7280):540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 26.Mjosberg J., Bernink J., Golebski K. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012;37(4):649–659. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 27.Bartemes K.R., Kephart G.M., Fox S.J., Kita H. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol. 2014;134(3):671–678.e674. doi: 10.1016/j.jaci.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xue L., Salimi M., Panse I. Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. J Allergy Clin Immunol. 2014;133(4):1184–1194. doi: 10.1016/j.jaci.2013.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doherty T.A., Khorram N., Lund S., Mehta A.K., Croft M., Broide D.H. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J Allergy Clin Immunol. 2013;132(1):205–213. doi: 10.1016/j.jaci.2013.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eastman J.J., Cavagnero K.J., Deconde A.S. Group 2 innate lymphoid cells are recruited to the nasal mucosa in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2017;140(1):101–108.e103. doi: 10.1016/j.jaci.2016.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pelly V.S., Kannan Y., Coomes S.M. IL-4-producing ILC2s are required for the differentiation of TH2 cells following Heligmosomoides polygyrus infection. Mucosal Immunol. 2016;9(6):1407–1417. doi: 10.1038/mi.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Moltke J., O’Leary C.E., Barrett N.A., Kanaoka Y., Austen K.F., Locksley R.M. Leukotrienes provide an NFAT-dependent signal that synergizes with IL-33 to activate ILC2s. J Exp Med. 2017;214(1):27–37. doi: 10.1084/jem.20161274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salimi M., Stoger L., Liu W. Cysteinyl leukotriene E4 activates human group 2 innate lymphoid cells and enhances the effect of prostaglandin D2 and epithelial cytokines. J Allergy Clin Immunol. 2017;140(4):1090–1100.e1011. doi: 10.1016/j.jaci.2016.12.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maazi H., Patel N., Sankaranarayanan I. ICOS:ICOS-ligand interaction is required for type 2 innate lymphoid cell function, homeostasis, and induction of airway hyperreactivity. Immunity. 2015;42(3):538–551. doi: 10.1016/j.immuni.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poposki J.A., Klingler A.I., Tan B.K. Group 2 innate lymphoid cells are elevated and activated in chronic rhinosinusitis with nasal polyps. Immun Inflamm Dis. 2017;5(3):233–243. doi: 10.1002/iid3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gour N., Smole U., Yong H.M. C3a is required for ILC2 function in allergic airway inflammation. Mucosal Immunol. 2018;11(6):1653–1662. doi: 10.1038/s41385-018-0064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cardoso V., Chesne J., Ribeiro H. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature. 2017;549(7671):277–281. doi: 10.1038/nature23469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nussbaum J.C., Van Dyken S.J., von Moltke J. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502(7470):245–248. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sui P., Wiesner D.L., Xu J. Pulmonary neuroendocrine cells amplify allergic asthma responses. Science. 2018;360(6393):eaan8546. doi: 10.1126/science.aan8546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silver J.S., Kearley J., Copenhaver A.M. Inflammatory triggers associated with exacerbations of COPD orchestrate plasticity of group 2 innate lymphoid cells in the lungs. Nat Immunol. 2016;17(6):626–635. doi: 10.1038/ni.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang K., Xu X., Pasha M.A. Cutting edge: notch signaling promotes the plasticity of group-2 innate lymphoid cells. J Immunol. 2017;198(5):1798–1803. doi: 10.4049/jimmunol.1601421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai T., Qiu J., Ji Y. IL-17-producing ST2+ group 2 innate lymphoid cells play a pathogenic role in lung inflammation. J Allergy Clin Immunol. 2019;143(1):229–244.e229. doi: 10.1016/j.jaci.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wise S.K., Lin S.Y., Toskala E. International consensus statement on allergy and rhinology: allergic rhinitis. Int Forum Allergy Rhinol. 2018;8(2):108–352. doi: 10.1002/alr.22070. [DOI] [PubMed] [Google Scholar]
- 44.Doherty T.A., Scott D., Walford H.H. Allergen challenge in allergic rhinitis rapidly induces increased peripheral blood type 2 innate lymphoid cells that express CD84. J Allergy Clin Immunol. 2014;133(4):1203–1205. doi: 10.1016/j.jaci.2013.12.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dhariwal J., Cameron A., Trujillo-Torralbo M.B. Mucosal type 2 innate lymphoid cells are a key component of the allergic response to aeroallergens. Am J Respir Crit Care Med. 2017;195(12):1586–1596. doi: 10.1164/rccm.201609-1846OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lao-Araya M., Steveling E., Scadding G.W., Durham S.R., Shamji M.H. Seasonal increases in peripheral innate lymphoid type 2 cells are inhibited by subcutaneous grass pollen immunotherapy. J Allergy Clin Immunol. 2014;134(5):1193–1195.e1194. doi: 10.1016/j.jaci.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 47.Shamji M.H., Layhadi J.A., Achkova D. Role of IL-35 in sublingual allergen immunotherapy. J Allergy Clin Immunol. 2019;143(3):1131–1142. doi: 10.1016/j.jaci.2018.06.041. [DOI] [PubMed] [Google Scholar]
- 48.Scadding G. Cytokine profiles in allergic rhinitis. Curr Allergy Asthma Rep. 2014;14(5):435. doi: 10.1007/s11882-014-0435-7. [DOI] [PubMed] [Google Scholar]
- 49.Xu G., Zhang L., Wang D.Y. Opposing roles of IL-17A and IL-25 in the regulation of TSLP production in human nasal epithelial cells. Allergy. 2010;65(5):581–589. doi: 10.1111/j.1398-9995.2009.02252.x. [DOI] [PubMed] [Google Scholar]
- 50.Mou Z., Xia J., Tan Y. Overexpression of thymic stromal lymphopoietin in allergic rhinitis. Acta Otolaryngol. 2009;129(3):297–301. doi: 10.1080/00016480802225884. [DOI] [PubMed] [Google Scholar]
- 51.Tan B.K., Klingler A.I., Poposki J.A. Heterogeneous inflammatory patterns in chronic rhinosinusitis without nasal polyps in Chicago, Illinois. J Allergy Clin Immunol. 2017;139(2):699–703.e697. doi: 10.1016/j.jaci.2016.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kato A. Immunopathology of chronic rhinosinusitis. Allergol Int. 2015;64(2):121–130. doi: 10.1016/j.alit.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shaw J.L., Fakhri S., Citardi M.J. IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med. 2013;188(4):432–439. doi: 10.1164/rccm.201212-2227OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walford H.H., Lund S.J., Baum R.E. Increased ILC2s in the eosinophilic nasal polyp endotype are associated with corticosteroid responsiveness. Clin Immunol. 2014;155(1):126–135. doi: 10.1016/j.clim.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tojima I., Kouzaki H., Shimizu S. Group 2 innate lymphoid cells are increased in nasal polyps in patients with eosinophilic chronic rhinosinusitis. Clin Immunol. 2016;170:1–8. doi: 10.1016/j.clim.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 56.Ogasawara N., Klingler A.I., Tan B.K. Epithelial activators of type 2 inflammation: elevation of thymic stromal lymphopoietin, but not IL-25 or IL-33, in chronic rhinosinusitis with nasal polyps in Chicago, Illinois. Allergy. 2018;73(11):2251–2254. doi: 10.1111/all.13552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cahill K.N., Bensko J.C., Boyce J.A., Laidlaw T.M. Prostaglandin D2: a dominant mediator of aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2015;135(1):245–252. doi: 10.1016/j.jaci.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu T., Wu J., Zhao J. Type 2 innate lymphoid cells: a novel biomarker of eosinophilic airway inflammation in patients with mild to moderate asthma. Respir Med. 2015;109(11):1391–1396. doi: 10.1016/j.rmed.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 59.Smith S.G., Chen R., Kjarsgaard M. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J Allergy Clin Immunol. 2016;137(1):75–86.e78. doi: 10.1016/j.jaci.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 60.Chen R., Smith S.G., Salter B. Allergen-induced increases in sputum levels of group 2 innate lymphoid cells in subjects with asthma. Am J Respir Crit Care Med. 2017;196(6):700–712. doi: 10.1164/rccm.201612-2427OC. [DOI] [PubMed] [Google Scholar]
- 61.Torgerson D.G., Ampleford E.J., Chiu G.Y. Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nat Genet. 2011;43(9):887–892. doi: 10.1038/ng.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.He J.Q., Hallstrand T.S., Knight D. A thymic stromal lymphopoietin gene variant is associated with asthma and airway hyperresponsiveness. J Allergy Clin Immunol. 2009;124(2):222–229. doi: 10.1016/j.jaci.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 63.Moffatt M.F., Gut I.G., Demenais F. A large-scale, consortium-based genome-wide association study of asthma. N Engl J Med. 2010;363(13):1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Prefontaine D., Nadigel J., Chouiali F. Increased IL-33 expression by epithelial cells in bronchial asthma. J Allergy Clin Immunol. 2010;125(3):752–754. doi: 10.1016/j.jaci.2009.12.935. [DOI] [PubMed] [Google Scholar]
- 65.Shikotra A., Choy D.F., Ohri C.M. Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J Allergy Clin Immunol. 2012;129(1):104–111.e101-109. doi: 10.1016/j.jaci.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 66.Liu S., Verma M., Michalec L. Steroid resistance of airway type 2 innate lymphoid cells from patients with severe asthma: the role of thymic stromal lymphopoietin. J Allergy Clin Immunol. 2018;141(1):257–268.e256. doi: 10.1016/j.jaci.2017.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Y.H., Angkasekwinai P., Lu N. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med. 2007;204(8):1837–1847. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang W., Li Y., Lv Z. Bronchial allergen challenge of patients with atopic asthma triggers an alarmin (IL-33, TSLP, and IL-25) response in the airways epithelium and submucosa. J Immunol. 2018;201(8):2221–2231. doi: 10.4049/jimmunol.1800709. [DOI] [PubMed] [Google Scholar]
- 69.Bel E.H., Ten Brinke A. New anti-eosinophil drugs for asthma and COPD: targeting the trait! Chest. 2017;152(6):1276–1282. doi: 10.1016/j.chest.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 70.Hurrell B.P., Shafiei Jahani P., Akbari O. Social networking of group two innate lymphoid cells in allergy and asthma. Front Immunol. 2018;9:2694. doi: 10.3389/fimmu.2018.02694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morita H., Arae K., Unno H. An interleukin-33-mast cell-interleukin-2 axis suppresses papain-induced allergic inflammation by promoting regulatory T cell numbers. Immunity. 2015;43(1):175–186. doi: 10.1016/j.immuni.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rigas D., Lewis G., Aron J.L. Type 2 innate lymphoid cell suppression by regulatory T cells attenuates airway hyperreactivity and requires inducible T-cell costimulator-inducible T-cell costimulator ligand interaction. J Allergy Clin Immunol. 2017;139(5):1468–1477.e1462. doi: 10.1016/j.jaci.2016.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ogasawara N., Poposki J.A., Klingler A.I. IL-10, TGF-β, and glucocorticoid prevent the production of type 2 cytokines in human group 2 innate lymphoid cells. J Allergy Clin Immunol. 2018;141(3):1147–1151.e1148. doi: 10.1016/j.jaci.2017.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Duerr C.U., McCarthy C.D., Mindt B.C. Type I interferon restricts type 2 immunopathology through the regulation of group 2 innate lymphoid cells. Nat Immunol. 2016;17(1):65–75. doi: 10.1038/ni.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moro K., Kabata H., Tanabe M. Interferon and IL-27 antagonize the function of group 2 innate lymphoid cells and type 2 innate immune responses. Nat Immunol. 2016;17(1):76–86. doi: 10.1038/ni.3309. [DOI] [PubMed] [Google Scholar]
- 76.Maazi H., Banie H., Aleman Muench G.R. Activated plasmacytoid dendritic cells regulate type 2 innate lymphoid cell-mediated airway hyperreactivity. J Allergy Clin Immunol. 2018;141(3):893–905.e896. doi: 10.1016/j.jaci.2017.04.043. [DOI] [PubMed] [Google Scholar]
- 77.Maric J., Ravindran A., Mazzurana L. Prostaglandin E2 suppresses human group 2 innate lymphoid cell function. J Allergy Clin Immunol. 2018;141(5):1761–1773.e1766. doi: 10.1016/j.jaci.2017.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou W., Toki S., Zhang J. Prostaglandin I2 signaling and inhibition of group 2 innate lymphoid cell responses. Am J Respir Crit Care Med. 2016;193(1):31–42. doi: 10.1164/rccm.201410-1793OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moriyama S., Brestoff J.R., Flamar A.L. β2-Adrenergic receptor-mediated negative regulation of group 2 innate lymphoid cell responses. Science. 2018;359(6379):1056–1061. doi: 10.1126/science.aan4829. [DOI] [PubMed] [Google Scholar]
- 80.Cephus J.Y., Stier M.T., Fuseini H. Testosterone attenuates group 2 innate lymphoid cell-mediated airway inflammation. Cell Rep. 2017;21(9):2487–2499. doi: 10.1016/j.celrep.2017.10.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Laffont S., Blanquart E., Savignac M. Androgen signaling negatively controls group 2 innate lymphoid cells. J Exp Med. 2017;214(6):1581–1592. doi: 10.1084/jem.20161807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kabata H., Moro K., Fukunaga K. Thymic stromal lymphopoietin induces corticosteroid resistance in natural helper cells during airway inflammation. Nat Commun. 2013;4:2675. doi: 10.1038/ncomms3675. [DOI] [PubMed] [Google Scholar]