Abstract
Background
Adolescent substance use is a major problem in and of itself, and because it acts as a risk factor for other problem behaviours. As substance use during adolescence can lead to adverse and often long‐term health and social consequences, it is important to intervene early in order to prevent progression to more severe problems. Brief interventions have been shown to reduce problematic substance use among adolescents and are especially useful for individuals who have moderately risky patterns of substance use. Such interventions can be conducted in school settings. This review set out to evaluate the effectiveness of brief school‐based interventions for adolescent substance use.
Objectives
To evaluate the effectiveness of brief school‐based interventions in reducing substance use and other behavioural outcomes among adolescents compared to another intervention or assessment‐only conditions.
Search methods
We conducted the original literature search in March 2013 and performed the search update to February 2015. For both review stages (original and update), we searched 10 electronic databases and six websites on evidence‐based interventions, and the reference lists of included studies and reviews, from 1966 to February 2015. We also contacted authors and organisations to identify any additional studies.
Selection criteria
We included randomised controlled trials that evaluated the effects of brief school‐based interventions for substance‐using adolescents.
The primary outcomes were reduction or cessation of substance use. The secondary outcomes were engagement in criminal activity and engagement in delinquent or problem behaviours related to substance use.
Data collection and analysis
We used the standard methodological procedures outlined by The Cochrane Collaboration, including the GRADE approach for evaluating the quality of evidence.
Main results
We included six trials with 1176 adolescents that measured outcomes at different follow‐up periods in this review. Three studies with 732 adolescents compared brief interventions (Bls) with information provision only, and three studies with 444 adolescents compared Bls with assessment only. Reasons for downgrading the quality of evidence included risk of bias of the included studies, imprecision, and inconsistency. For outcomes that concern substance abuse, the retrieved studies only assessed alcohol and cannabis. We generally found moderate‐quality evidence that, compared to information provision only, BIs did not have a significant effect on any of the substance use outcomes at short‐, medium‐, or long‐term follow‐up. They also did not have a significant effect on delinquent‐type behaviour outcomes among adolescents. When compared to assessment‐only controls, we found low‐ or very low‐quality evidence that BIs reduced cannabis frequency at short‐term follow‐up in one study (standardised mean difference (SMD) ‐0.83; 95% confidence interval (CI) ‐1.14 to ‐0.53, n = 269). BIs also significantly reduced frequency of alcohol use (SMD ‐0.91; 95% CI ‐1.21 to ‐0.61, n = 242), alcohol abuse (SMD ‐0.38; 95% CI ‐0.7 to ‐0.07, n = 190) and dependence (SMD ‐0.58; 95% CI ‐0.9 to ‐0.26, n = 190), and cannabis abuse (SMD ‐0.34; 95% CI ‐0.65 to ‐0.02, n = 190) at medium‐term follow‐up in one study. At long‐term follow‐up, BIs also reduced alcohol abuse (SMD ‐0.72; 95% CI ‐1.05 to ‐0.40, n = 181), cannabis frequency (SMD ‐0.56; 95% CI ‐0.75 to ‐0.36, n = 181), abuse (SMD ‐0.62; 95% CI ‐0.95 to ‐0.29, n = 181), and dependence (SMD ‐0.96; 95% CI ‐1.30 to ‐0.63, n = 181) in one study. However, the evidence from studies that compared brief interventions to assessment‐only conditions was generally of low quality. Brief interventions also had mixed effects on adolescents' delinquent or problem behaviours, although the effect at long‐term follow‐up on these outcomes in the assessment‐only comparison was significant (SMD ‐0.78; 95% CI ‐1.11 to ‐0.45).
Authors' conclusions
We found low‐ or very low‐quality evidence that brief school‐based interventions may be more effective in reducing alcohol and cannabis use than the assessment‐only condition and that these reductions were sustained at long‐term follow‐up. We found moderate‐quality evidence that, when compared to information provision, brief interventions probably did not have a significant effect on substance use outcomes. It is premature to make definitive statements about the effectiveness of brief school‐based interventions for reducing adolescent substance use. Further high‐quality studies examining the relative effectiveness of BIs for substance use and other problem behaviours need to be conducted, particularly in low‐ and middle‐income countries.
Keywords: Adolescent; Humans; Schools; Adolescent Behavior; Adolescent Behavior/psychology; Motivational Interviewing; Psychotherapy, Brief; Psychotherapy, Brief/methods; Randomized Controlled Trials as Topic; Substance‐Related Disorders; Substance‐Related Disorders/rehabilitation
Plain language summary
Can brief interventions delivered in schools reduce substance use among adolescents?
Review question: We reviewed evidence on the effects of brief school‐based interventions for substance use and substance‐related problem behaviours among adolescents. We found six studies.
Background: Adolescents worldwide are known to use both legal and illegal substances, which can lead to other problems. These high rates of substance use are concerning, as early initiation of substance use is a risk factor for substance use disorders in later life, and alcohol and illegal drugs have been associated with years lost due to disability among youth aged 10 to 24 years.
We wanted to learn whether brief school‐based interventions had an effect on substance misuse in adolescents. Brief interventions are short programmes that aim to help reduce or stop substance use. This review updates a previous review published in 2014.
Search date: The evidence is current to February 2015.
Study characteristics: We included six studies in this review, with 1176 adolescents overall. The mean age of adolescents was 16.9 years. We were interested in studies with short‐, medium‐, and long‐term follow‐up periods to assess whether any effects were due to the brief intervention. The studies compared brief intervention programmes with two major kinds of comparison or control groups: 1) an information provision only (general health promotion materials and harm reduction information) group and 2) an assessment‐only group, where adolescents received no intervention but were evaluated on substance use and other behaviour at follow‐up appointments at different time periods following delivery of the intervention. Three studies with 732 adolescents compared brief interventions with information provision only, while the other three, with 444 adolescents, compared brief interventions with assessment only.
Trials were either conducted in the United States or the United Kingdom.
Delivery of the interventions was individual or group face‐to‐face feedback across high schools and further education colleges. All interventions were up to four sessions in length.
Our primary outcome was abstinence or reduction of substance use behaviour, and our secondary outcomes were engagement in criminal activity related to substance use and engagement in delinquent‐type behaviours related to substance use.
Key results: For outcomes that concern substance use, the studies assessed use of alcohol and cannabis. When compared to information provision, brief interventions are probably not more efficacious in reducing substance use or delinquent behaviour. When compared to assessment‐only controls, the interventions may have some significant effects on substance use and behaviours. At short‐term follow‐up, brief interventions significantly reduced cannabis frequency in one study. At medium‐term follow‐up, brief interventions significantly reduced frequency of alcohol use, alcohol abuse and dependence symptoms, and cannabis abuse symptoms in one study. At long‐term follow‐up, brief interventions significantly reduced alcohol abuse, cannabis frequency, and cannabis abuse and dependence symptoms in one study.
The pattern of results indicates that adolescents who received a brief intervention generally did better in reducing their alcohol and cannabis use than adolescents who received no intervention at all. However, adolescents who received a brief intervention did not seem to do better in reducing their alcohol and cannabis use than adolescents who received information‐only interventions. It is therefore premature to make definitive statements about the effectiveness of brief school‐based interventions for reducing adolescent substance use.
Quality of evidence: Overall, the evidence was of moderate or low quality, with two outcomes found to have very low quality of evidence. There were three major issues across the studies: 1) there was no blinding of adolescents, 2) there was uncertainty as to whether participant allocation to study groups was concealed, and 3) a small total number of adolescents and number of events. None of the included studies reported information about funding source or conflicts of interest.
Summary of findings
Summary of findings for the main comparison. Brief intervention compared to information provision for substance‐using adolescents.
| Brief intervention compared to information provision for substance‐using adolescents | ||||||
| Patient or population: Substance‐using adolescents Settings: High schools or further education training colleges Intervention: Brief intervention Comparison: Information provision | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Estimate effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Information provision | Brief intervention | |||||
| Alcohol frequency Self report questionnaires Medium‐term follow‐up: 4 to 6 months | See comment | The standardised mean alcohol frequency in the intervention groups was 0.01 standard deviations lower (0.20 lower to 0.18 higher) | SMD ‐0.01 (‐0.20 to 0.18) | 434 (2 studies) | ⊕⊕⊕⊝ moderate1 | Number of days of alcohol use |
|
Alcohol quantity
Self report questionnaires Medium‐term follow‐up: 4 to 6 months |
See comment | The standardised mean alcohol quantity in the intervention groups was 0.14 standard deviations lower (0.33 lower to 0.05 higher) | SMD ‐0.14 (‐0.33 to 0.05) | 434 (2 studies) | ⊕⊕⊕⊝ moderate1 | Number of standard alcohol units |
| Cannabis dependence Self report questionnaires Short‐term follow‐up: 1 to 3 months | See comment | The standardised mean cannabis dependence score in the intervention groups was 0.09 standard deviations lower (0.27 lower to 0.09 higher) |
SMD ‐0.09 (‐0.27 to 0.09) | 470 (2 studies) | ⊕⊕⊕⊝ moderate1 | Mean dependence score |
| Cannabis frequency Self report questionnaires Short‐term follow‐up: 1 to 3 months | See comment | The mean cannabis frequency in the intervention groups was 0.07 standard deviations lower (0.25 lower to 0.11 higher) | SMD ‐0.07 (‐0.25 to 0.11) | 470 (2 studies) | ⊕⊕⊕⊝ moderate1 | Number of days cannabis use |
|
Secondary outcomes related to substance use
Self report questionnaires Short‐term follow‐up: 1 to 3 months |
See comment | The mean behavioural outcomes related to substance use in the intervention groups was ‐0.01 standard deviations lower (0.19 lower to 0.17 higher) | SMD ‐0.01 (‐0.19 to 0.17) | 470 (2 studies) | ⊕⊕⊕⊝ moderate1 | Interactional Problems Score |
| *The basis for the assumed risk (e.g. the mean control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the estimate effect of the intervention (and its 95% CI). The estimate effects for certain outcomes were not estimable due to only one study assessing the specific outcome, or extremely high levels of heterogeneity making effects across studies difficult to compare. CI: confidence interval; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Risk of bias (‐1): It was not possible to blind the participants in all of the included studies. There was also uncertainty in two of the studies about allocation concealment and blinding of outcome assessor (Walker 2011; Werch 2005).
Summary of findings 2. Brief intervention compared to assessment only for substance‐using adolescents.
| Brief intervention compared to assessment only for substance‐using adolescents | ||||||
| Patient or population: Substance‐using adolescents Settings: High schools or further education colleges Intervention: Brief intervention Comparison: Assessment only | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Estimate effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Assessment only | Brief intervention | |||||
|
Alcohol frequency Self report questionnaires Medium‐term follow‐up: 4 to 6 months |
See comment | The standardised mean alcohol frequency in the intervention groups was 0.91 standard deviations lower (1.21 lower to 0.61 lower) | SMD ‐0.91 (‐1.21 to ‐0.61) | 242 (2 studies) | ⊕⊕⊝⊝ low1, 2 | Number of days of alcohol use |
|
Alcohol quantity
Self report questionnaires Medium‐term follow‐up: 4 to 6 months |
See comment | The standardised mean alcohol quantity in the intervention groups was 0.16 standard deviations lower (0.45 lower to 0.14 higher) | SMD ‐0.16 (‐0.45 to 0.14) |
179 (1 study) |
⊕⊕⊝⊝ low1,2 | Number of standard alcohol units |
| Cannabis dependence Self report questionnaires Medium‐term follow‐up: 4 to 6 months | See comment | The mean cannabis dependence in the intervention groups was 0.56 standard deviations lower (0.57 lower to 0.06 higher) | SMD ‐0.26 (‐0.57 to 0.36) | 190 (1 study) | ⊕⊕⊝⊝ low1, 2 | Mean dependence score |
| Cannabis frequency Self report questionnaires Long‐term follow‐up: 7 to 12 months | See comment | The mean cannabis frequency in the intervention groups was 0.54 standard deviations lower (0.77 lower to 0.31 higher) | SMD ‐0.54 (‐0.77 to ‐0.31) | 338 (2 studies) | ⊕⊕⊝⊝ low1,2 | Number of days of cannabis use |
|
Secondary outcomes related to substance use Self report questionnaires Medium‐term follow‐up: 4 to 6 months |
See comment | The mean mean behavioural outcomes related to substance use in the intervention groups was 0.65 standard deviations lower (1.58 lower to 0.28 higher) | SMD ‐0.65 (‐1.58 to 0.28) | 242 (2 studies) | ⊕⊕⊝⊝ low1, 2 | Interactional Problems Score |
| *The basis for the assumed risk (e.g. the mean control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the estimate effect of the intervention (and its 95% CI). The estimate effects for certain outcomes were not estimable due to only one study assessing the specific outcome, or extremely high levels of heterogeneity making effects across studies difficult to compare. CI: confidence interval; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Risk of bias (‐1): It was not possible to blind the participants in all of the included studies. There was no allocation concealment in two of the included studies, and it was unclear whether the outcome assessor was blinded (Winters 2007b; Winters 2012). The other study was also not free of selective reporting bias (McCambridge 2004). 2Imprecision (‐1): The confidence intervals contained the null value of zero and the upper or lower confidence limit crosses an effect size of 0.5 in either direction; the sample size was also small for medium‐term follow‐up.
Background
Description of the condition
Substance use among adolescents refers to the use of licit substances (including alcohol and prescription or over‐the‐counter medicines) and illicit drugs (cannabis, heroin, cocaine, amphetamines, methaqualone, hallucinogenic drugs). Globally, alcohol and cannabis (after tobacco) are the most commonly used substances among young people (Hingson 2006; UNODC 2012), and alcohol initiation is occurring at earlier ages, which is associated with substance dependence and other related problems later on in life (Hingson 2006). Middle and secondary or high school is an especially high‐risk period for the initiation of substance use as adolescents transition from one type of schooling to another and face numerous challenges (Jackson 2013). School surveys conducted in different regions of the world, such as Europe (Hibell 2012), Australia (White 2012), the United States (Johnston 2015), and South Africa (Reddy 2013), have reported a high prevalence of alcohol use among young people as well as high levels of other drug use. For example, a study of adolescent drug use across 35 European countries reported that 70% of students reported lifetime alcohol use (in some countries this was as high as 95%), while 18% had engaged in illicit drug use (Hibell 2012). An Australian school survey similarly indicated that 84% of students reported lifetime use of alcohol, 14.8% reported lifetime use of cannabis, and 17.3% reported lifetime inhalant use (White 2012). In the United States national Monitoring the Future survey, lifetime and past 30‐day use of alcohol was 46% and 23% respectively, while lifetime and past 30‐day use of cannabis was 31% and 14% respectively (Johnston 2015). In addition, the national Youth Risk Behavior Survey (YRBS) in the United States reported that the lifetime prevalences for alcohol, cannabis, prescription drugs, and inhalants were 66%, 41%, 28%, and 9% respectively (Kann 2013). The most recent South African national YRBS found lifetime prevalence rates of 49% for alcohol use, 13% for cannabis use, and 12% for inhalants or prescription drug use (Reddy 2013).
These high rates of substance use among adolescents are cause for concern, not only because the early initiation of substance use is a risk factor for substance use disorders in later life (Winters 2008), but also because of its association with increased morbidity and mortality among young people. For example, the most recent Global Burden of Disease study found that alcohol (7%) and illegal drugs (2%) were two of the main risk factors for incident disability‐adjusted life‐years for youth aged 10 to 24 years (Gore 2011).
It is important to intervene early with adolescents who use substances as substance use is often associated with a number of other problem behaviours including withdrawal from school involvement, drinking and driving, violent behaviour, and general delinquency. These kinds of behavioural outcomes have been consistently associated with adolescent substance use in studies throughout the world (Feldstein 2006; Hallfors 2006; Plüddemann 2010; Storr 2007). For example, the YRBS in the United States found that 10% of high school students had driven a car or vehicle after alcohol use in the past month (Kann 2013), while in South Africa, this was reported to be 13% (Reddy 2013). Studies also show that substance use can play a role in criminal behaviour. In a recent study, youth offenders reported that they committed crimes in order to finance their drug habit (Leoschut 2007). Some also reported that substance use gave them the courage to commit their crimes, or an excuse if they were apprehended. Ward 2007 also suggested that when young people are under the influence of substances they may not be able to monitor or self regulate their behaviour as well as when they are sober.
Adolescents who become involved with the legal system due to substance use are more likely to associate with deviant networks and be disadvantaged in terms of education and employment. They are also more likely to participate in criminal activity during adulthood (Mulvey 2010). Adolescents involved in the criminal justice system often have more psychiatric problems and are more in need of drug treatment in adulthood than their peers who are not involved in the criminal justice system (Kutcher 2009; Lanctôt 2007). For example, Corneau 2004 estimated that 12% of institutionalised adolescents need drug treatment as adults. Furthermore, substance‐using adolescents who are involved in the criminal justice system are more likely to have negative interpersonal relationships, including violent intimate partner relationships (Lanctôt 2007). If an intervention can take place early on with these adolescents, it may be able to prevent the development of some of these negative consequences.
Description of the intervention
Brief interventions (BIs)
Brief interventions (BIs) are targeted, time‐limited, low‐threshold services that aim to reduce substance use and its associated risks, as well as prevent progression to more severe levels of use and potential negative consequences (Babor 2007). In general, BIs are delivered in person and provide information or advice, increase motivation not to use substances, and teach behaviour change skills with the aim of reducing substance use. The way that BIs have been defined and delivered has varied in the literature in terms of number of sessions provided, length of the intervention sessions, and format of delivery (Young 2012). It is thus important to recognise common elements used to define BI. One such component is the screening of potential participants. Although screening has formed part of BIs in other settings, it often does not take place in schools, with a few exceptions (Hallfors 2006). A second common element of BIs is their short length, as they generally last between one and five intervention sessions (Moyer 2002; Tevyaw 2004).
In addition to advice‐giving, the common elements of successful BIs are referred to by the acronym FRAMES, and include provision of the following:
Feedback on behaviour and its consequences to the client;
Responsibility for change as the responsibility of the individual;
Advice for change;
Menu of options for change;
Empathy;
Self efficacy for change (Bien 1993).
These kinds of interventions were developed based upon the theoretical assumption that people are not always ready to change their patterns of substance use. In such cases, straightforward advice‐giving is of limited use, and the adolescents need to recognise for themselves that their behaviour is problematic and identify their own reasons for wanting to change their behaviour. The development of this brief method was guided by a number of principles: it should be useable in time‐limited consultations; the training of practitioners should take between 12 and 15 hours; interviewers should be able to raise the subject of behaviour change in a sensitive and respectful manner; and the method itself should be flexible, meaning that it can be used with individuals at various stages of readiness to change (Rollnick 1995). Most BIs rely on principles of motivational interviewing, in Winters 2007a, or brief motivational enhancement therapy, in Tevyaw 2004, which focus on building adolescents' readiness to change their behaviours. This technique provides personalised feedback on substance use together with a motivational‐interviewing counselling style (Miller 2002).
Relevance for adolescents
BIs have been identified as useful for individuals who have moderately risky patterns of substance use (Barry 1999). This makes this type of intervention relevant for use with adolescents, who for the most part have not yet developed substance dependence. BIs seem to be better suited for those adolescents who are less set in a delinquent lifestyle and who are not institutionalised (Brunelle 2000). Tevyaw 2004 characterises BI methods as accepting adolescents as individuals, instead of confronting them and their behaviour or lecturing them as their teachers, parents, and other authority figures may do. BIs could therefore be a more effective strategy for building rapport and a collaborative therapeutic relationship with adolescents than other confrontational forms of interacting with adolescents. Furthermore, the methods are seen as a cost‐effective alternative to traditional, lengthier treatments of adolescents who use substances (Tevyaw 2004).
Ideal conditions: what we do and do not know
BIs have traditionally been used in healthcare and substance abuse treatment settings (Bien 1993), but studies have suggested that their use could be expanded to other settings, such as schools (Winters 2007a). There are a number of advantages of school‐based BIs for substance‐using adolescents. Firstly, adolescents usually are not dependent on substances yet, although a number of them may exhibit mild or moderate use, which makes them good candidates for BI. Secondly, research has shown that BIs can be conducted during school or after‐school hours, making the intervention very accessible to students. Finally, the growing volume of BI material on how to conduct BI sessions means that they can often be run by staff available to schools, and not just health professionals (Winters 2007a). There is also some research suggesting that BIs may work in other settings as well, such as family interventions for school‐going adolescents in terms of alcohol and cannabis use (Spoth 2001). Recent research has also suggested that web‐based BI programmes may be useful in reducing substance use in young adults (Bingham 2010). Despite the promise of school‐based BI programmes, meta‐analyses of school‐based interventions have not yet been conducted.
How the intervention might work
The goals of BIs are to assess substance use in adolescents, provide advice on these behaviours, facilitate behaviour change with regards to substance use, and motivate the adolescents to receive further treatment if necessary (Bien 1993). The primary focus of these types of interventions is to systematically target problematic behaviours (Tevyaw 2004), using a motivational‐interviewing framework.
The theoretical basis for BIs is grounded in client‐centred therapy, behavioural therapy, and the transtheoretical model of behaviour change. The transtheoretical model of behaviour change argues that readiness for change develops along a series of stages rather than as a fixed event that either occurs or does not occur. These steps are pre‐contemplation, contemplation, preparation, action, and maintenance, and individuals usually move between these stages before reaching termination (Prochaska 1993). From this perspective, motivation is seen as a state that can be altered rather than a trait that is inherent and cannot be changed. Since BIs are typically organised around a developmental theory of normative and non‐normative patterns of substance use, this is an appropriate theoretical orientation for a behaviour change strategy aimed at adolescents (Winters 2007a).
Why it is important to do this review
Brief interventions are recognised as an appropriate treatment for adolescents who use substances, yet there have been only a few reviews of the effectiveness of BI for adolescent substance use. Tait 2003 conducted a systematic review of 11 studies of BIs for adolescent substance use and found that BI was effective in reducing alcohol use among adolescents, but not in reducing polysubstance use. Only two of these studies were conducted in schools (with one conducted by nurses over the telephone); these two studies showed moderate effect sizes of between 0.38 and 0.52. BIs also did not have a significant effect on drinking in the last seven days. In their review of brief motivational interventions among adolescents, Tevyaw 2004 reported significant reductions in alcohol‐related problems such as drinking and driving, traffic violations and, to a lesser extent, drinking rates. While the reviewed studies were conducted in a number of settings, including emergency rooms and colleges, not many of these settings were high schools. Furthermore, existing reviews were conducted a number of years ago and have not been updated. It is useful to re‐examine the evidence in an updated review.
No existing Cochrane reviews examine the effectiveness of BIs for reducing substance use among high school (or the equivalent of high school) students, while a recent systematic review that addressed alcohol use among adolescents only included two studies conducted in a high school setting. These results were inconclusive, as in one study the BI was effective, but in the other it was ineffective (Patton 2014). Furthermore, there are no reviews that address BIs for substance use as a primary outcome and related behavioural outcomes (for example problem behaviours) as secondary outcomes. The current review is the first to examine both outcomes.
Objectives
To evaluate the effectiveness of brief school‐based interventions in comparison to another intervention or assessment only on reducing substance use and related behavioural outcomes among adolescents.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials that evaluated the effects of BIs on substance use as well as on behavioural outcomes associated with adolescent substance use. We excluded studies that recruited adolescents from anywhere else other than an educational setting.
Types of participants
Participants were adolescents under the age of 19 who were attending high school, secondary school, or a further education training college that provided alternative schooling or vocational training for adolescents between 16 and 18 years of age, and who used alcohol or other drugs, or both, but did not meet the criteria for substance dependence. In addition, adolescents had faced negative behavioural consequences due to their substance use.
Types of interventions
Experimental intervention
The intervention should have been labelled as a BI, but could also have been defined as motivational interviewing, brief skills‐orientation, motivational enhancement, or other specific types of BIs that were up to four sessions long and used BI principles to facilitate change. The focus should have been on building the individual's motivation to change. The BIs could have been offered as a stand‐alone option, integrated with other intervention efforts, or as a precursor to other treatments. Only BIs that were offered to individuals in a face‐to‐face modality were included in this review.
Control intervention
The control could have been no intervention, placebo, assessment only, or other types of interventions or education.
Types of outcome measures
Primary outcomes
Abstinence or reduction of substance use behaviour.
The outcome measures could have been self reported measures, including dichotomous and continuous outcomes. In addition, substance use could have been measured with standardised measures of substance use that are appropriate for adolescents such as the Alcohol Diagostic Interview (ADI), Adolescent Drug Abuse Diagnosis (ADAD), Adolescent Drug Involvement Scale (ADIS), Adolescent Alcohol and Drug Involvement Scale (AADIS), and Personal Experience Inventory (PEI), which are all self report measures.
Any biological testing could also have been included, such as urinalysis for drug use and breathalyser tests for alcohol use.
Secondary outcomes
Engagement in criminal activity (such as theft, drug and alcohol crimes, property crimes) related to substance use.
Engagement in delinquent‐type behaviours (such as drinking and driving, aggression and fighting, bullying, carrying weapons to school, buying and selling drugs, gang involvement, truancy, suspension and expulsion, and disobeying rules in general) related to substance use.
It was not expected that the included BIs would have adverse effects on the primary or secondary outcomes.
Search methods for identification of studies
Included studies were published from 1966 onwards, the year that BIs were first introduced.
Electronic searches
We obtained relevant trials from searching the following sources:
CDAG Specialized Register (February 2015);
Cochrane Central Register of Controlled Trials (CENTRAL in the Cochrane Library, issue 2, 2015);
PubMed (January 1966 to February 2015);
EMBASE (1974 to March 2013);
PsycINFO (January 1966 to February 2015);
ERIC (Education Resources Information Center) (January 1966 to February 2015);
ISAP (Index to South African Periodicals), Social Science Index (January 1966 to February 2015);
Academic Search Premier (January 1966 to March 2013);
LILACS (2004 to March 2013);
Alcohol and Alcohol Problems Science Database (1972 to March 2013);
Web of Science Social Science Citation Index (January 1966 to March 2013).
We developed a detailed search strategy for each database. The search strategy combined the subject search with the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials in PubMed, sensitivity maximising version (2008 revision), as referenced in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
The subject search utilised a combination of controlled vocabulary and free text terms based on the strategy for searching PubMed. We adapted this search strategy as appropriate for the other databases (see Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7 for all searches). We applied no language restrictions.
Searching other resources
We contacted relevant authors and searched citations in all relevant papers to obtain information on potential additional randomised controlled trials. We also searched for other unpublished studies and assessed relevant conference proceedings for additional references. We searched the following websites:
http://nrepp.samhsa.gov/
http://sbirt.samhsa.gov/core_comp/brief_int.htm
http://motivationalinterview.org
Current Controlled Trials (http://www.controlled‐trials.com/)
ClinicalTrials.gov
Trialsjournal.com
Data collection and analysis
Selection of studies
Two review authors (TC and BM) assessed the title, abstract, and keywords of all the papers from the electronic searches against the eligibility criteria for this review, and retrieved the full texts of studies deemed potentially eligible. These included randomised controlled trial or clinical trials and substance use, alcohol use, drug use (and related terms), alcohol or drug use or substance use reduction strategies (and related terms), problem behaviours (including but not limited to aggression, fighting, suspension, expulsion, weapon‐carrying), interventions, school staff or settings or both (and related terms). If the title, abstract, and keywords did not provide enough information to make an informed decision with regards to inclusion of the paper, the full text of the paper was obtained.
Two review authors (TC and BM) assessed the full texts of potentially relevant studies for inclusion. A third review author (JL) was on hand to resolve any disagreements, however there were no disagreements about the inclusion of studies.
Data extraction and management
Two review authors (TC and BM) independently extracted data using a piloted data extraction form based on the Cochrane Collaborative Drugs and Alcohol Review Group's extraction form and subsequently entered the data into The Cochrane Collaboration software Review Manager 5.1 for analysis (the data extraction form is available on request from TC) (RevMan 2014). We extracted data from studies on the following information: study design and method, allocation process, participant data, intervention, and outcomes. When information was missing from the original studies on outcomes or other important information, we contacted the corresponding author via e‐mail in order to request additional data. Certain statistics were not readily available in the articles; if authors were not able to provide this information to us we calculated them from existing data, consulting the Cochrane Handbook for Systematic Reviews of Interventions for guidance (Higgins 2011).
Assessment of risk of bias in included studies
Two review authors independently assessed potential biases resulting from the trial design. Any discrepancies between the review authors were resolved by discussion.
We performed the 'Risk of bias' assessment for trials included in this review using the criteria recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The recommended approach for assessing risk of bias in studies included in a Cochrane review is a two‐part tool addressing seven specific domains, namely sequence generation and allocation concealment (selection bias), blinding of participants and providers (performance bias), blinding of outcome assessor (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other source of bias. The first part of the tool involves describing what was reported to have happened in the study. The second part of the tool involves assigning a judgement relating to the risk of bias for that entry, in terms of low, high, or unclear risk. To make these judgments we used the criteria indicated by the Cochrane Handbook for Systematic Reviews of Interventions adapted to the addiction field.
If the first two review authors struggled to make a judgement, we contacted the author of the article in an attempt to obtain more information about the particular bias domain, and only if it was still unclear did we assign it a judgement of 'unclear'.
For other domains, we examined the following:
appropriateness of the statistical tests used in data analysis;
compliance with the intervention(s);
validity and reliability of outcome measures.
For a detailed description of the criteria used to assess risk of bias, please see Appendix 8.
Grading of evidence
We assessed the overall quality of the evidence for the primary outcome using the Grading of Recommendation, Assessment, Development and Evaluation (GRADE) system. The Grading of Recommendation, Assessment, Development and Evaluation Working Group developed a system for grading the quality of evidence that takes into account issues not only related to internal validity but also to external validity, such as directness of results (GRADE 2004; Guyatt 2008; Guyatt 2011; Schünemann 2006). The 'Summary of findings' tables present the main findings of a review in a transparent and simple tabular format. In particular, they provide key information concerning the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data on the main outcomes.
The GRADE system uses the following criteria for assigning grades of evidence:
High: further research is very unlikely to change our confidence in the estimate of effect.
Moderate: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low: any estimate of effect is very uncertain.
Grading is decreased for the following reasons:
Serious (‐1) or very serious (‐2) limitation to study quality.
Important inconsistency (‐1).
Some (‐1) or major (‐2) uncertainty about directness.
Imprecise or sparse data (‐1).
High probability of reporting bias (‐1).
Grading is increased for the following reasons:
Strong evidence of association ‐ significant risk ratio of > 2 (0.5) based on consistent evidence from two or more observational studies, with no plausible confounders (+1).
Very strong evidence of association ‐ significant risk ratio of > 2 (< 0.5) based on direct evidence with no major threats to validity (+2).
Evidence of a dose response gradient (+1).
All plausible confounders would have reduced the effect (+1).
Measures of treatment effect
We compared the outcomes of the experimental and control groups at different follow‐up appointments. We categorised the findings into short‐term follow‐up appointments (one to three months), medium‐term follow‐up appointments (four to 11 months), and long‐term follow‐up appointments (12 months and longer). We assessed dichotomous outcome measures by calculating the risk ratio with the 95% confidence interval, while for continuous outcome measures the standardised mean difference with 95% confidence interval was the treatment measure used as the summary statistic. It is common in meta‐analysis for studies assess the same outcome but measure it in a variety of ways, so the same outcome may be measured with different scales (Higgins 2011). If standard deviations for the mean values were not provided, we used the standard errors that were provided and employed the calculation in the Cochrane Handbook for Systematic Reviews of Interventions to change them to standard deviations (Higgins 2011).
Unit of analysis issues
The analysis of clinical trials needs to take into account the level at which randomisation occurred. While this can be on an individual basis, cluster‐randomised trials have groups of individuals (for example schools, community) as opposed to individuals as the unit of analysis. The review authors originally planned to measure the intracluster correlation coefficient (ICC) in these studies and then use the ICC to measure the design effect, which is an inflation factor that is used to increase the statistical power of the study (Campbell 2000). However, as the authors of the cluster‐randomised trials used the Huber‐White estimator of variance to control for the effects of clustered recruitment, further calculations were not necessary. While the review authors had decided to use a conversion rate of 4.29 (30 days/7) where outcomes across studies used different measurement times other than monthly frequency, doing any additional conversions was unnecessary as the measures in the studies were of monthly use (for example frequency of use, quantity of use).
Dealing with missing data
We contacted the original investigators of the included studies up to three times to request any missing data (missing studies, outcomes, summary data, individuals, and study‐level characteristics). We needed to decide whether the data were missing at random (not related to the actual data) or not missing at random (related to the actual data). When study data were assumed to be missing at random, only the available data were analysed. For data that were not missing at random, this needed to be addressed by performing a sensitivity analysis or, if this was not possible, by replacing missing data with specified values (Higgins 2011). The imputation of missing data with specific replacement values was not needed for the studies included in this review.
Assessment of heterogeneity
We assessed the extent of heterogeneity across the studies using the Chi² test and I² statistic and looking at whether the P values were statistically significant (Higgins 2011), with a P value of 0.10 or less showing significant heterogeneity.
Assessment of reporting biases
We planned to use funnel plots (plots of the effect estimate from each study against the sample size or effect standard error) in an attempt to assess any publication bias. More specifically, we planned to examine the funnel plots for asymmetry as an indication of publication bias. However, asymmetrical funnel plots are not always caused by publication bias, and publication bias does not always cause asymmetrical funnel plots (Higgins 2011). This was not possible for the current review because fewer than 10 studies were included.
Data synthesis
We performed a meta‐analysis was performed, as there were more than two individual trials with comparable intervention methods and outcomes that could be analysed. We used random‐effects models based on the fact that we expected different types of interventions to be included in the review and combined in the meta‐analysis (such as interventions of different duration and using different follow‐up measures).
Subgroup analysis and investigation of heterogeneity
Although we originally had planned to conduct subgroup analyses for studies with low and unclear risk of bias and, if possible, for different ages, gender, and school grades for adolescent study participants, this was not possible. Only a small number of studies were included in the meta‐analysis, and the results were not reported by these variables of interest.
Sensitivity analysis
We decided that if there was significant unexplained heterogeneity and more than 10 studies were included in the analysis, we would perform a sensitivity analysis to consider if the following had an impact on effect size:
studies conducted in settings other than traditional high or secondary schools (e.g. alternative high schools, reform school);
studies that utilised quasi‐experimental designs (as long as an experimental and a control group were included);
studies that had attrition rates of more than 20%.
Since we included only six studies in the review, these sensitivity analyses were unnecessary.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies.
Results of the search
This is an update of a Cochrane review first published in February 2014. In the first edition of this review, we identified through bibliographic searches 1037 potentially relevant articles after removing duplicates. We excluded 1010 studies on the basis of title and abstract, and retrieved 27 articles in full text for more detailed evaluation. We excluded 21 of these; the remaining six trials (in eight articles) satisfied all the criteria for inclusion in the review (see Figure 1 for flowchart).
1.
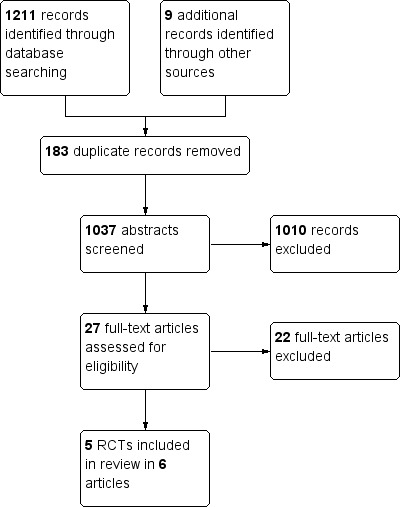
Study flow diagram Carney 2014.
In the present update, we identified an additional 1264 records, giving us a total of 939 reports after removing 325 duplicates. We excluded 910 of these reports on the basis of title and abstract. We retrieved 29 articles in full text for more detailed evaluation, of which we excluded 28. We included no new trials in the review, although we included one additional article that reported on the long‐term follow‐up of one trial (See Figure 2). We have summarised the reasons for exclusion in the Characteristics of excluded studies table. The six randomised controlled trials (RCTs) from the original review (reported in eight separate articles) met our inclusion criteria and are described in detail in the Characteristics of included studies table.
2.
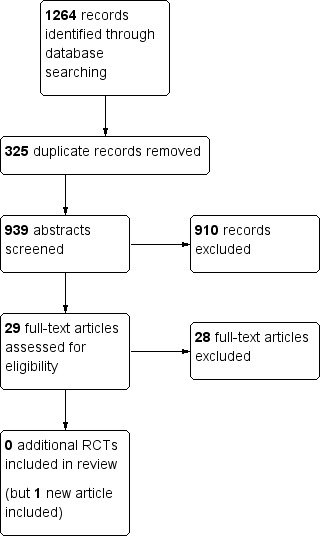
Study flow diagram for updated review.
Included studies
We identified six studies (reported in eight articles) that were published between 2004 and February 2015 for inclusion in this review. These studies at their start included a total of 1176 adolescents. The total number of adolescents that were analysed at the follow‐up appointments varied according to the length of follow‐up period of the studies (short‐term follow‐up: n = 470; medium‐term follow‐up: n = 855; long‐term follow‐up: n = 529). All six studies were RCTs, of which two were cluster‐RCTs (McCambridge 2004; McCambridge 2008). All interventions were provided on a face‐to‐face individual basis.
Four out of the six studies included only adolescents engaging in cannabis or alcohol use or abuse, whereas the other two studies included adolescents engaging in any form of substance abuse.
Types of comparison
Brief intervention versus information provision, three studies, 732 adolescents at baseline (McCambridge 2008; Walker 2011; Werch 2005).
Brief intervention versus assessment only, three studies, 444 adolescents at baseline (McCambridge 2004; Winters 2007b; Winters 2012).
Location
All of the studies were based in educational settings. Four were based in public secondary schools (Walker 2011; Werch 2005; Winters 2007b; Winters 2012), while two were based in further education colleges, which provided alternative schooling and training for adolescents 16 to 18 years of age (McCambridge 2004; McCambridge 2008). The former four studies were conducted in the United States (Walker 2011; Werch 2005; Winters 2007b; Winters 2012), while the latter two studies were conducted in the United Kingdom (McCambridge 2004; McCambridge 2008).
Length and description of intervention
The six interventions met the criteria for brief interventions (BIs). Adolescents received some or all of the following: screening, motivational interviewing, information provision and discussion, brochures, and follow‐up appointments. Three of the studies provided adolescents with a single BI session (McCambridge 2004; McCambridge 2008; Werch 2005), while the other three studies held two intervention sessions with the adolescents (Walker 2011; Winters 2007b; Winters 2012).
Screening and outcomes measures
All six of the studies used self report measures. To measure substance abuse some studies used established screening and diagnostic tools such as the Global Appraisal of Individual Needs Interview (GAIN‐I) (Walker 2011), Alcohol Use Disorders Identification Test (AUDIT) (McCambridge 2004; McCambridge 2008), Timeline Followback (TLFB) interview (Winters 2007b; Winters 2012), Severity of Dependence Scale (SDS) (McCambridge 2008), and Substance Use Disorder Manual of the Adolescent Diagnostic Interview (ADI) (Winters 2007b; Winters 2012). Other studies used substance use questionnaires such as the Alcohol Beverage Youth Survey (Werch 2005). A combination of instruments was also used to measure alcohol behaviours. There was consistency regarding the measures of alcohol and cannabis frequency (number of days used) and quantity (number of units used). The Fagerström Test was also used in one study to measure nicotine dependence (McCambridge 2008).
Measures of behavioural outcomes were less clear and seemed to ask about the general consequences of the adolescents' drug use. McCambridge 2008 used a measure that assessed interactional problems, and was adapted from its original use for adolescents who had alcohol problems to include those who used drugs. Walker 2011 used the Marijuana Problem Inventory to measure problem behaviours associated with cannabis use. Two of the other studies used the Personal Consequences Scale, which measured legal, health, motor vehicle, social, and family problems experienced due to substance use (Winters 2007b; Winters 2012).
Length of follow‐up
The trials differed in terms of outcomes measured at follow‐up. While some of the trials conducted short‐term follow‐up appointments, such as McCambridge 2004, McCambridge 2008, and Walker 2011 at three months, they also conducted medium‐ and longer‐term follow‐ups. McCambridge 2008 also conducted six‐month follow‐up appointments, while Walker 2011 also conducted 12‐month follow‐ups. Two trials only reported one medium‐term follow‐up, at four months (Werch 2005), and six months (Winters 2007b), respectively. The remaining study reported outcomes at both six months and 12 months (Winters 2012).
Secondary population group
Two of the trials reported a secondary population group, namely the parents of the adolescents who used substances (Winters 2007b; Winters 2012). This made up a third experimental group, where both adolescents and parents received the intervention. While we considered these secondary population groups to be important, we did not compare them in the meta‐analysis, as the four other studies only had one experimental group with adolescents as the population, and no other interventions that worked with parents. However, we have written up these findings in the text of the review.
Excluded studies
We excluded 29 potentially eligible studies that were obtained and read in full. We excluded three of these studies because the length of the interventions did not fit the criteria for brief intervention, while another seven were prevention studies and not early‐intervention studies. Ten of the studies were not school based; they were either based at college level or in the community. Finally, we excluded some studies for methodological reasons, such as being pilot/feasibility studies and having no control group, not being RCTs, or not containing any information about interventions.
Risk of bias in included studies
Figure 3 provides a summary of the 'Risk of bias' assessments for all the studies. Figure 4 provides a summary of the risk of bias for each study and in each area.
3.
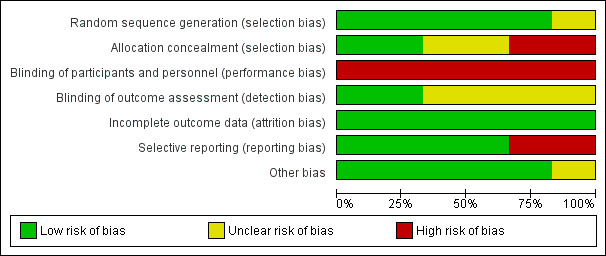
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.
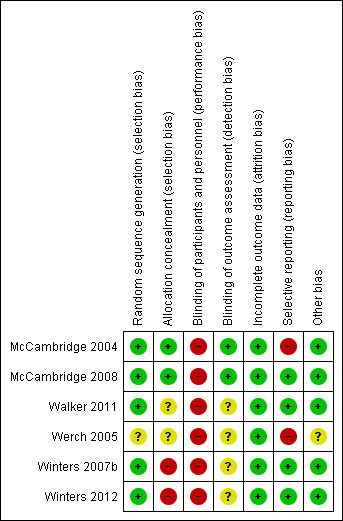
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Generation of randomisation sequence
We judged sequence generation as adequate in all but one of the studies (Werch 2005), which referred to random allocation, but this was not clarified and we were not able to contact the authors for further information. We therefore found the level of bias for this study to be unclear.
Concealment of allocation
Concealment of allocation was adequate in two of the studies (McCambridge 2004; McCambridge 2008). It was unclear in two of the studies (Walker 2011; Werch 2005), and once again contacting the authors proved unsuccessful. In the remaining two studies, allocation concealment did not take place (Winters 2007b; Winters 2012). Communication with the authors revealed that this was not done because it was believed that it would negatively affect study participation, so we judged these two studies to be at high risk of selection bias.
Blinding
Performance bias
This review reports on psychological interventions such as motivational interviewing, where it was not possible to blind the participants or staff who worked on the study to the intervention. The risk of performance bias can actually influence the outcomes if they are self reported and not objective. Because all the six included studies used self‐report measures, all the studies were judges at high risk of performance bias.
Detection bias
Outcome assessors were blinded to study condition in two of the studies (McCambridge 2004; McCambridge 2008). In four studies there was insufficient information to evaluate the risk of bias in terms of blinding (Walker 2011; Werch 2005; Winters 2007b; Winters 2012), and we could not contact the authors.
Incomplete outcome data
We reported all six of the studies to have low risk of bias because either the rates of attrition were low, or factors associated with attrition were identified and controlled for in both groups in the original analysis.
Selective reporting
Four of the studies were free of selective reporting, and reported on all prespecified outcomes (McCambridge 2008; Walker 2011; Winters 2007b; Winters 2012). One of the trials did not report on all longer‐term outcomes as the findings were no longer significant (McCambridge 2004), and the sixth study did not report all outcomes (Werch 2005), so we judged these two studies to be at high risk of selective reporting.
Other potential sources of bias
We identified no other sources of bias (appropriateness of statistical tests used in data analysis; compliance with the intervention(s); validity and reliability of outcome measures).
Effects of interventions
Due to high levels of heterogeneity, we could not combine the effects across studies for some of the outcomes. While a meta‐analysis of results across one study was not possible (see Table 1; Table 2), we have reported the effect of the intervention compared to the control group below.
1. Comparison of BI to information provision
Primary outcomes
See Table 1
Alcohol frequency: Two studies measured alcohol frequency at different follow‐up periods. One study measured alcohol frequency at both short‐ and medium‐term follow‐ up (McCambridge 2008), while the other measured alcohol frequency only at medium‐term follow‐up (Werch 2005). There were a total of 269 adolescents at short‐term follow‐up and 434 adolescents at medium‐term follow‐up. We found no significant difference between BI and information provision for both of the follow‐up periods, with a standardised mean difference (SMD) of ‐0.05 (95% confidence interval (CI) ‐0.29 to 0.19) at short‐term follow‐up (one study) and SMD of ‐0.01 (95% CI ‐0.20 to 0.18) I² = 0%, Chi² = 0.34, P = 0.56, at medium‐term follow‐up (two studies). See Analysis 1.1.
1.1. Analysis.
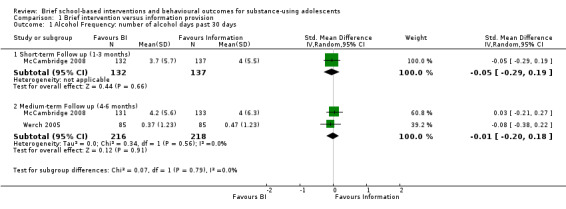
Comparison 1 Brief intervention versus information provision, Outcome 1 Alcohol Frequency: number of alcohol days past 30 days.
Alcohol quantity: Two studies measured alcohol quantity at different follow‐up periods. McCambridge 2008 measured alcohol quantity at both short‐ and medium‐term follow‐up. Werch 2005 measured alcohol frequency only at medium‐term follow‐up. There were a total of 269 adolescents at short‐term follow‐up and 434 adolescents at medium‐term follow‐up. We found no significant difference in both of the follow‐up periods, with SMD of 0.02 (95% CI ‐0.22 to 0.26) at short‐term follow‐up (one study) and SMD of ‐0.14 (95% CI ‐0.33 to 0.05) Chi² = 0.62, P = 0.43, I² = 0%, at medium‐term follow‐up (two studies).
Cannabis quantity: One study with 269 adolescents at short‐term follow‐up and 264 adolescents at medium‐term follow‐up reported on quantity of cannabis use (McCambridge 2008). The SMD was ‐0.00 (95% CI ‐0.24 to 0.24) at short‐term follow‐up and ‐0.15 (95% CI ‐0.39 to 0.09) at medium‐term follow‐up. See Analysis 1.3.
1.3. Analysis.
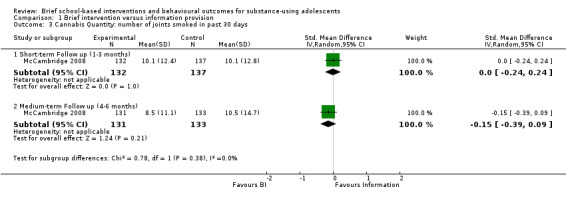
Comparison 1 Brief intervention versus information provision, Outcome 3 Cannabis Quantity: number of joints smoked in past 30 days.
Cannabis dependence: Two studies reported on cannabis dependence (McCambridge 2008; Walker 2011). Both studies reported on this outcome at short‐term follow‐up (n = 470). The SMD was ‐0.09, which was not significant (95% CI ‐0.27 to 0.09). There was no heterogeneity (Chi² = 0.45, P = 0.50, I² = 0%). Only one of the studies reported this outcome at medium‐term follow‐up (n = 264) (McCambridge 2008), and the SMD was 0.06 (95% CI ‐0.18 to 0.30). Walker 2011 also measured cannabis dependence at long‐term follow‐up appointments (n = 186). The SMD was ‐0.09 (95% CI ‐0.38 to 0.20). See Analysis 1.4.
1.4. Analysis.
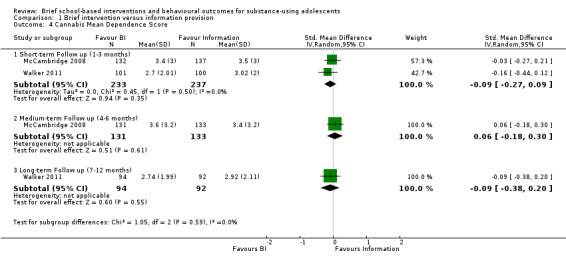
Comparison 1 Brief intervention versus information provision, Outcome 4 Cannabis Mean Dependence Score.
Cannabis frequency: Two studies reported on cannabis frequency (McCambridge 2008; Walker 2011). Both reported on this outcome at short‐term follow‐up (n = 470). The SMD was ‐0.07, which was not significant (95% CI ‐0.25 to 0.11) Chi² = 0.43, P = 0.51, I² = 0%. McCambridge 2008 also reported cannabis frequency at medium‐term follow‐up (n = 264), and the SMD was ‐0.06 (95% CI ‐0.30 to 0.18). Walker 2011 also measured cannabis frequency at long‐term follow‐up appointments (n = 186). The SMD was ‐0.02 (95% CI ‐0.31 to 0.26). See Analysis 1.5.
1.5. Analysis.
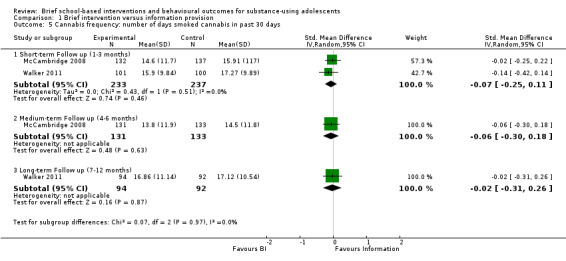
Comparison 1 Brief intervention versus information provision, Outcome 5 Cannabis frequency: number of days smoked cannabis in past 30 days.
Secondary outcomes
The information pooled in the meta‐analysis for the secondary outcomes included engagement in criminal activity and delinquent‐type behaviours associated with alcohol or cannabis use, or both, such as drug selling, drug‐related crime, and arrests for being intoxicated. Two studies reported on our secondary outcomes at different follow‐up periods (McCambridge 2008; Walker 2011). Both studies reported on our secondary outcomes at short‐term follow‐up (n = 470) (McCambridge 2008; Walker 2011). The SMD was ‐0.01 (95% CI ‐0.19 to 0.17) Chi² = 0.23, P = 0.63, I² = 0%. McCambridge 2008 reported on our secondary outcomes at medium‐term follow‐up (n = 264). The SMD was ‐0.13 (95% CI ‐0.37 to 0.11). Walker 2011 reported on our secondary outcomes at long‐term follow‐up (n = 186); the SMD was ‐0.10 (95% CI ‐0.39 to 0.19). See Analysis 1.6.
1.6. Analysis.
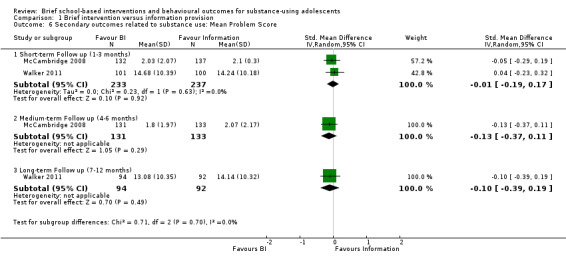
Comparison 1 Brief intervention versus information provision, Outcome 6 Secondary outcomes related to substance use: Mean Problem Score.
2. Comparison of BI to assessment only
Primary outcomes
See Table 2
Alcohol frequency: Two studies measured alcohol frequency (Winters 2007b; Winters 2012). At medium‐term follow‐up for these studies with 242 adolescents in total, there was a significant difference in favour of BI: SMD ‐0.91 (95% CI ‐1.21 to ‐0.61), with very little heterogeneity (I² = 5%, Chi² = 1.06, P = 0.30). Only the Winters 2012 study measured alcohol frequency at long‐term follow‐up (n = 170), but the SMD was ‐0.20, which was not significant (95% CI ‐0.53 to 0.14). See Analysis 2.1.
2.1. Analysis.
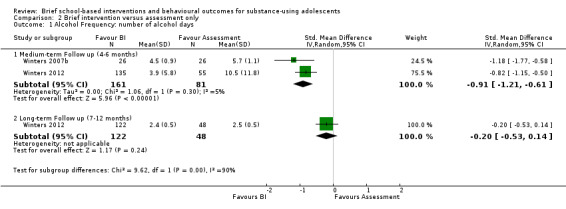
Comparison 2 Brief intervention versus assessment only, Outcome 1 Alcohol Frequency: number of alcohol days.
Alcohol quantity: One study with 179 adolescents at medium‐term follow‐up and 162 adolescents at long‐term follow‐up measured alcohol quantity (McCambridge 2004). At medium‐term follow‐up, there was not a significant difference between the group that received the intervention and the group that received an assessment only (SMD ‐0.16; 95% CI ‐0.45 to 0.14). At long‐term follow‐up, this difference was also not significant (SMD ‐0.16; 95% CI ‐0.47 to 0.15). See Analysis 2.2.
2.2. Analysis.
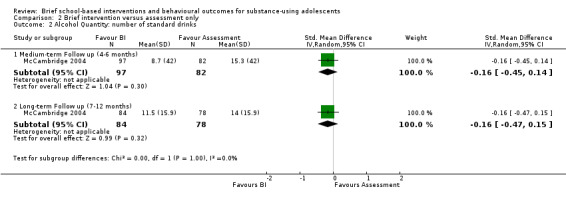
Comparison 2 Brief intervention versus assessment only, Outcome 2 Alcohol Quantity: number of standard drinks.
Alcohol abuse:Winters 2012 reported the number of alcohol abuse symptoms among 190 adolescents at medium‐term follow‐up and 170 adolescents at long‐term follow‐up. There were significant differences in favour of BI at both medium‐term (SMD ‐0.38, 95% CI ‐0.70 to ‐0.07) and long‐term follow‐up (SMD ‐0.72, 95% CI ‐1.07 to ‐0.38). See Analysis 2.3.
2.3. Analysis.
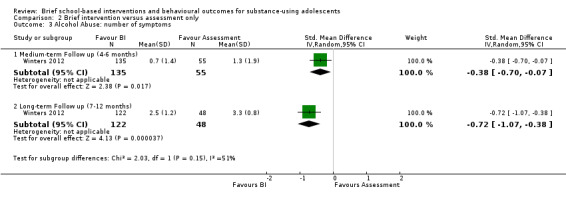
Comparison 2 Brief intervention versus assessment only, Outcome 3 Alcohol Abuse: number of symptoms.
Alcohol dependence: Only one study reported the number of alcohol dependence symptoms (Winters 2012), among 190 adolescents at medium‐term follow‐up and 170 adolescents at long‐term follow‐up. While the difference was significant at medium‐term follow‐up (SMD ‐0.58, 95% CI ‐0.90 to ‐0.26) in favour of BI, it was not significant at long‐term follow‐up (SMD ‐0.13, 95% CI ‐0.47 to ‐0.20). See Analysis 2.4.
2.4. Analysis.
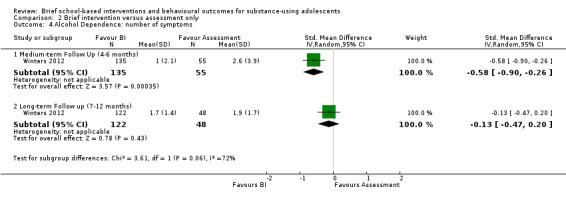
Comparison 2 Brief intervention versus assessment only, Outcome 4 Alcohol Dependence: number of symptoms.
Cannabis frequency: Three studies reported on cannabis frequency (McCambridge 2004; Winters 2007b; Winters 2012). Only McCambridge 2004 measured cannabis frequency at short‐term follow‐up (n = 179), and the SMD was ‐0.83, which was significant (95% CI ‐1.14 to ‐0.53) in favour of BI. Both Winters 2007b and Winters 2012 measured cannabis frequency at medium‐term follow‐up (n = 242), but the difference was not significant (SMD ‐0.23, 95% CI ‐0.50 to 0.05) Chi² = 0.56, P = 0.45, I² = 0%. McCambridge 2004 and Winters 2012 measured this outcome at long‐term follow‐up (n = 338), and the difference was significant in favour of BI (SMD ‐0.54, 95% CI ‐0.77 to ‐0.31) Chi² = 0.12, P = 0.73, I² = 0%. See Analysis 2.5.
2.5. Analysis.
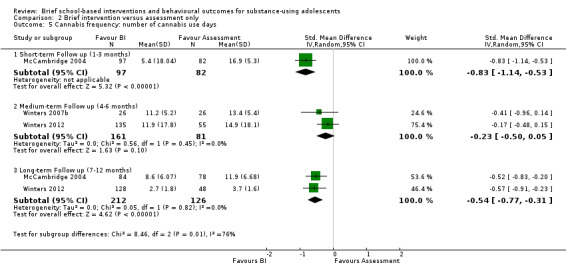
Comparison 2 Brief intervention versus assessment only, Outcome 5 Cannabis frequency: number of cannabis use days.
Cannabis abuse:Winters 2012 reported the number of cannabis abuse symptoms among 190 adolescents at medium‐term follow‐up and 170 adolescents at long‐term follow‐up. The differences were significant at both medium‐term (SMD ‐0.34, 95% CI ‐0.65 to ‐0.02) and long‐term follow‐up (SMD ‐0.62, 95% CI ‐0.96 to ‐0.28) in favour of BI. See Analysis 2.6.
2.6. Analysis.
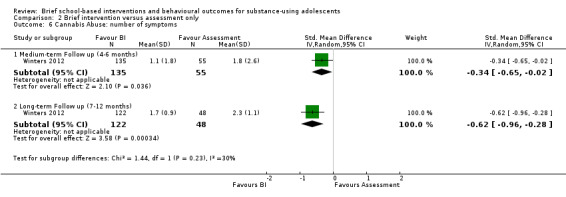
Comparison 2 Brief intervention versus assessment only, Outcome 6 Cannabis Abuse: number of symptoms.
Cannabis dependence: Only one study reported the number of alcohol dependence symptoms (Winters 2012), among 190 adolescents at medium‐term follow‐up and 170 adolescents at long‐term follow‐up. While the difference was not significant at medium‐term follow‐up (SMD ‐0.26, 95% CI ‐0.57 to 0.06), it was significant at long‐term follow‐up (SMD ‐0.96, 95% CI ‐1.32 to ‐0.62) in favour of BI. See Analysis 2.7.
2.7. Analysis.
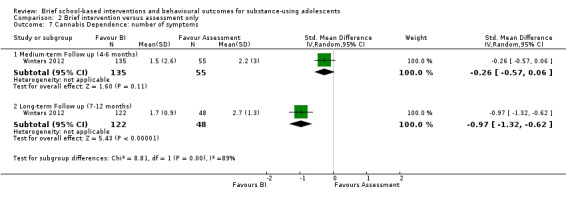
Comparison 2 Brief intervention versus assessment only, Outcome 7 Cannabis Dependence: number of symptoms.
Secondary outcomes
Two studies with a total of 242 adolescents at medium‐term follow‐up and a total of 170 adolescents at long‐term follow‐up measured engagement in delinquent‐type behaviours or engagement in criminal activity, which were secondary outcomes for this review (Winters 2007b; Winters 2012). There were not significant differences at medium‐term follow‐up (SMD ‐0.65, 95% CI ‐1.58 to 0.28) Chi² = 7.75, P = 0.005, I² = 87%, but there was a significant difference at long‐term follow‐up in Winters 2012 (SMD ‐0.78, 95% CI ‐1.13 to ‐0.44) in favour of BI. See Analysis 2.8.
2.8. Analysis.
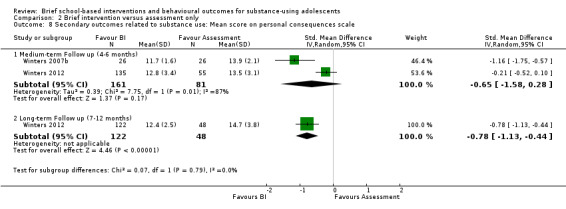
Comparison 2 Brief intervention versus assessment only, Outcome 8 Secondary outcomes related to substance use: Mean score on personal consequences scale.
McCambridge 2004 reported on these behaviours using dichotomous outcomes. At medium‐term follow‐up, adolescents in the control group were found to be almost twice as likely to have sold drugs to friends (risk ratio 0.38, 95% CI 0.23 to 0.66). This outcome was not reported at long‐term follow‐up.
Discussion
Summary of main results
We were interested in assessing whether brief intervention (BI) is more effective than assessment only or information provision in reducing alcohol and other drug use. We found low‐ or very low‐quality evidence that, compared to an assessment‐only control, BI may have an effect on the following alcohol outcomes: reduction of alcohol frequency at medium‐term follow‐up, alcohol abuse at medium‐ and long‐term follow‐up, and alcohol dependence at medium‐term follow‐up. In terms of outcomes related to cannabis use, BI may reduce cannabis frequency of use and cannabis abuse at both short‐ and medium‐term follow‐up, and on cannabis dependence at long‐term follow‐up. When comparing BI to the information‐only control, we found moderate‐quality evidence that the effects were generally not significant.
BIs did not change engagement in delinquent‐type behaviours or criminal activity for the experimental groups in comparison to both control comparisons (namely information provision and assessment only), except in the Winters 2012 study, which indicated that the intervention had a significant effect on these behavioural outcomes at long‐term follow‐up. Other findings from the McCambridge 2004 study, although not included in the meta‐analysis, also indicated that BIs led to reductions in drug selling to friends (odds ratio = 0.42, P = 0.008).
Overall completeness and applicability of evidence
This review included a small number of studies (n = 6). These studies covered a narrow age range, with the mean age range being from 15.4 to 18 years old, and three of the six studies reported a mean age of 17 to 18 years old (McCambridge 2004; McCambridge 2008; Werch 2005). This makes it somewhat difficult to generalise the results to students who are in early adolescence, who are at a different phase of social and cognitive development. Also, in the United Kingdom, where some of the studies were conducted (McCambridge 2004; McCambridge 2008), the minimum legal drinking age is 18 years (International Center for Alcohol Policies 2010), in comparison to the United States, where the legal drinking age is 21 years. Alcohol use is more acceptable and therefore may be more common among this age group in this context. Adolescents may need interventions that are tailored specifically for their age group, which the National Institute for Health and Care Excellence (NICE) guidelines for school‐based interventions for alcohol advise (NICE 2007).
While our secondary outcomes were described as those that measured criminal or delinquent behaviours, it was difficult to disentangle these behaviours from other interactional and social behaviours in the results, as many studies used scales with specific psychometric properties that made looking at a single item difficult (McCambridge 2004; McCambridge 2008; Walker 2011; Winters 2007b; Winters 2012). Additional research using rigorous methods measuring an array of these outcomes is needed before generalisations and specific recommendations can be made about whether BIs for substance use can curb delinquent behaviours.
Only three published programmes (adapted motivational interviewing, 'Teen Intervene', brief experimental alcohol beverage‐tailored programme) were used or adapted for use in the six studies included in this review. This limited variability in the interventions that were delivered might limit the generalisability of the findings further. These interventions were developed either in the United States or the United Kingdom, which might limit the applicability of the evidence to students in schools within high‐income countries. We are uncertain how applicable these findings are to low‐ and middle‐income countries, and in particular countries with different cultural and social norms around alcohol use.
Quality of the evidence
We used the GRADE approach to assess the quality of evidence for the five key outcomes at the follow‐up periods. See Table 1; Table 2.
The quality of the evidence for the outcomes varied from very low to moderate. We downgraded the evidence on account of risk of bias including that it was not possible to blind adolescents and providers, blinding of the outcome assessor was often unclear, and all outcomes were self report data, unexplained significant heterogeneity, and imprecision. Overall, the quality of the evidence seemed to be higher for the comparison BI versus information provision and lower for the comparison BI versus assessment only. For the first set of comparisons, namely BI versus information provision, we had insufficient information in one of the two studies to be certain about the risk of bias in a number of areas; we therefore downgraded the quality of the evidence to moderate. The quality of the evidence was therefore further downgraded. For the comparison BI versus assessment only, there were issues with risk of bias across all of the outcomes. There was also imprecision in some of the outcomes, which led to further downgrading of the evidence quality. There was a large amount of unexplained heterogeneity in addition to the issues with risk of bias and imprecision for two of the outcomes, resulting in the quality of the evidence being downgraded to very low.
Potential biases in the review process
We believe that we have identified all of the studies that focused on the effect of BIs on general substance use as a primary outcome, and behavioural outcomes related to substance use as secondary outcomes, that met our study design and adolescent inclusion criteria up to February 2015. We used a comprehensive search strategy designed with assistance from the Cochrane Drugs and Alcohol Review Group, and ensured that there was independent assessment for inclusion eligibility, risk of bias, and data extraction. We also attempted to locate possible unpublished literature, but were not very successful. A small possibility does exist that unpublished RCTs were excluded from the review. We also took into account that journal articles have strict word or page limits, and contacted authors for additional information where necessary. With one exception, the authors we contacted were very responsive and were able to provide the requested information. Certain questions about risk of bias remain unanswered in the studies whose authors we could not contact. We applied strict criteria in the process of grading the evidence and were transparent about the judgements that led to the decisions on how the studies were rated for the various outcomes.
Agreements and disagreements with other studies or reviews
We found a significant effect size for most comparisons between BI and assessment‐only control conditions. Tait 2003's review reported similar effect sizes, although the studies included in their review were conducted in multiple settings, while the studies included in this review were conducted in educational settings only. Similarly, although the Jensen 2011 review looked at motivational interviewing only and included studies that were again conducted in a number of settings, our effect sizes were comparable with their range of results. The meta‐analysis on alcohol use among adolescents by Watchel 2010, while conducted in clinical settings (and not school settings), found that motivational interviewing (one form of BI) was partially successful, with the most encouraging results being those related to harm minimisation (looking at harms associated with drinking).
In addition, the BIs included in the Watchel 2010 review were not particularly effective in reducing secondary behavioural outcomes such as health, legal, and social harms. Unfortunately, our effect sizes were not directly comparable to these previous reviews, as ours included standardised mean differences, while the other studies included Cohen's d for their effect sizes.
Authors' conclusions
Implications for practice.
The findings of this review suggest that there is low/moderate‐quality evidence that school‐based BIs are probably no more effective than information provision for alcohol and cannabis use and related delinquent‐type behaviours or criminal activity, at different follow‐up periods. The retrieved studies did not assess the impact of BI on other substances of abuse.
We found low‐ or very low‐quality evidence that BI may perform more favourably when compared to assessment only. Overall, BI did not seem to have a significant effect on alcohol quantity for either of the comparisons. BIs seem to reduce cannabis frequency and abuse at short‐term follow‐up, as well as cannabis frequency, abuse, and dependence and behavioural outcomes related to substance use at long‐term follow‐up. At medium‐term follow‐up, BIs seem to be more effective in reducing alcohol frequency, alcohol abuse, alcohol dependence, and cannabis abuse. It is premature to make any definitive statements about the effectiveness of brief school‐based interventions for reducing adolescent substance use.
Implications for research.
We suggest that further research is required, with an emphasis on improvement in study design, analysis, and reporting, in line with accepted guidelines (for example CONSORT 2010). There is also a need for corroborative studies that include biological measurements of alcohol or other drug use, as all of the studies included in this review used self report measures. The impact of BI on the abuse of substances other that alcohol and cannabis should also be addressed.
Recent studies have identified possible ways to blind participants and personnel in RCTs that assess non‐pharmological treatment (Boutron 2007), including placebo interventions and blinding participants to the study hypothesis. This could be explored further in the future.
Three of the studies in the current review had any kind of long‐term follow‐up (McCambridge 2004; Walker 2011; Winters 2012), which generally indicated that effects remained stable or that BIs had a significant effect on substance use and behavioural outcomes related to substance use. Further research should measure effectiveness over the long term with studies of higher quality. Our secondary outcomes, engagement in criminal activity and delinquent‐type behaviours, may show significant results if measured at 12 months' follow‐up, as these behaviours may take longer to change than substance use behaviours.
We were unable to address in this review how certain factors (for example age, gender, and school grade) interact with the intervention effects for adolescents, as it was not possible to conduct any subgroup analyses due to the small number of included studies. It is important that methodologically sound studies measure the effects of single components when added to the basic BI, such as peer influence and booster sessions. This will enable the best combination of intervention components to be used in real‐life school settings.
We did not identify any studies conducted in low‐ or middle‐income countries that met our inclusion criteria. Further well‐designed randomised trials of BIs are needed in low‐ and middle‐income settings.
What's new
| Date | Event | Description |
|---|---|---|
| 18 December 2015 | New citation required but conclusions have not changed | Conclusions not changed |
| 19 August 2015 | New search has been performed | Conducted an updated search and found new additional article of included study. Allowed for meta‐analysis by period of follow‐up |
Acknowledgements
The review authors would like to thank Elizabeth Pienaar and Joy Oliver of the South African Cochrane Centre (Cape Town, South Africa) and Zuzana Mitrova of the Cochrane Drugs and Alcohol Review Group (Rome, Italy) for their assistance with search strategies and other support for this review.
Appendices
Appendix 1. Cochrane Drugs and Alcohol Review Group Specialised Register
(adolescen* OR teenage* OR young OR student* OR juvenile OR school* OR class* OR kid OR kids OR youth OR underage)
AND
((brief AND intervention*) OR (brief AND therap*) OR (brief AND interview*) OR (minimal AND intervention*) OR (minimal AND therap*) OR (minimal AND interview*) OR (early AND intervention*) OR (early AND therap*) OR (early AND interview*) OR (motivat* AND intervention*) OR (motivat* AND therap*) OR (motivat* AND interview*) OR counselling OR counseling OR advice)
Appendix 2. CENTRAL search strategy
MeSH descriptor: [Substance‐Related Disorders] explode all trees
MeSH descriptor: [Drinking Behavior] explode all trees
binge
drink*
(abus* or consumption or misuse or use*):ti,ab
#1 or #2 or #3 or #4 or #5
(drug* or substance* or alcohol* or cannabis or amphetamine or cocaine or heroin or Methaqualone or prescription):ti,ab
#6 and #7
brief near/2 intervention
early near/2 intervention
minimal near/2 intervention
(BI or BMI):ti,ab
MeSH descriptor: [Counseling] explode all trees
((brief near/2 motivation*) near/2 interview*):ti,ab
#9 or #10 or #11 or #12 or #13 or #14
MeSH descriptor: [Adolescent] explode all trees
(adolescen* or teenage* or young or student* or juvenile):ti,ab
school* or class*
#16 or #17 or #18
#8 and #15 and #19
Appendix 3. PubMed search strategy
Substance‐related disorders [mesh]
Drinking behavior [mesh]
binge [tiab]
drink*[tiab]
abus*[tiab] OR consumption[tiab] OR misuse[tiab] OR use*[tiab]
#1 or #2 or #3 or #4 or #5
drug [tiab] OR substance [tiab] OR alcohol [tiab] OR cannabis[tiab] OR *amphetamine[tiab] OR cocaine[tiab] OR heroin [tiab] OR Methaqualone [tiab] OR prescription [tiab]
#6 AND #7
"Brief intervention" [tiab]
"early intervention"[tiab]
"minimal intervention"[tiab]
BI[tiab] OR BMI[tiab]
Counseling [mesh]
((brief[Title/Abstract]) AND motivation*[Title/Abstract]) AND interview*[Title/Abstract]
Motivation* [mesh:no exp]
#9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15
Adolescent [mesh]
((((adolescen*[Title/Abstract]) OR teenage*[Title/Abstract]) OR young[Title/Abstract]) OR student* [Title/Abstract] OR juvenile [Title/Abstract] kid[Title/Abstract] OR kids[Title/Abstract] OR youth[Title/Abstract] OR underage[Title/Abstract]
School* [tw] OR class* [tw]
#17 OR #18 OR #19
Randomized controlled trial [pt]
controlled clinical trial [pt]
random*[tiab]
placebo [tiab]
trial [tiab]
groups [tiab]
animals [mh] NOT humans [mh]
(#26) NOT #27
(((#7) AND #16) AND #17) AND #28
Appendix 4. EMBASE search strategy
'substance abuse'/syn OR abus*:ab,ti OR consumption:ab,ti OR misuse:ab,ti OR use*:ab,ti
'drinking behaviour' OR binge:ab,ti OR drink*:ab,ti
#1 OR #2
drug:ab,ti OR substance:ab,ti OR 'cannabis'/syn OR 'cocaine'/syn OR 'heroin'/syn OR 'methaqualone'/syn OR prescription:ab,ti OR alcohol:ab,ti OR 'amphetamine'/syn
#3 AND #4
'brief intervention':ab,ti OR 'brief interventions':ab,ti OR 'early intervention':ab,ti OR 'early interventions':ab,ti OR 'minimal intervention':ab,ti OR 'minimal interventions':ab,ti OR bi:ab,ti OR bmi:ab,ti
'counseling'/syn OR counselling:ab,ti
'motivation'/syn
brief:ab,ti AND motivation:ab,ti
interview*:ab,ti
#10 AND #11
#6 OR #7 OR #8 OR #9 OR #11
'adolescence'/syn OR adolescen*:ab,ti OR teenage*:ab,ti OR young*:ab,ti OR student*:ab,ti OR school*:ab,ti OR kid:ab,ti OR youth:ab,ti OR underage:ab,ti
random*:ti OR random*:ab OR factorial*:ti OR factorial*:ab OR cross?over*:ti OR cross?over:ab OR crossover*:ti OR crossover*:ab OR placebo*:ti OR placebo*:ab OR (doubl*:ti AND blind*:ti) OR (doubl*:ab AND blind*:ab) OR (singl*:ti AND blind*:ti) OR (singl*:ab AND blind*:ab) OR assign*:ti OR assign*:ab OR volunteer*:ti OR volunteer*:ab OR 'crossover procedure'/de OR 'crossover procedure'OR 'double‐blind procedure'/de OR 'double‐blind procedure' OR 'single‐blind procedure'/de OR 'single‐blind procedure' OR 'Randomized controlled trial'/de OR 'Randomized controlled trial' OR allocat*:ti OR allocat*:ab
#5 AND #13 AND #14 AND #15 AND [embase]/lim
Appendix 5. Web of Science search strategy
Timespan=2012‐06‐01 ‐ 2013‐03‐13. Databases=SCI‐EXPANDED, SSCI.
Topic=(((((drug or substance* or alcohol or *amphetamine* or cocaine or marijuana or cannabis or heroin or Methaqualone) same (misuse or abuse* or addict* or consumption or use*))))) AND Topic=(((brief NEAR/3 intervention*) OR (brief NEAR/3 therap*) OR (brief NEAR/3 interview*) OR (minimal NEAR/3 intervention*) OR (minimal NEAR/3 therap*) OR (minimal NEAR/3 interview*) OR (early NEAR/3 intervention*) OR (early NEAR/3 therap*) OR (early NEAR/3 interview*) OR (motivat* NEAR/3 intervention*) OR (motivat* NEAR/3 therap*) OR (motivat* NEAR/3 interview*) OR (counselling or counseling or advice))) AND Topic=((adolescen* or teenage* or young or student* or juvenile or school* or class* or kid or kids or youth or underage)) AND Topic=((randomi* OR randomly OR placebo* OR trial*))
Appendix 6. LILACS search strategy
((((([MH] ("substance‐related disorders")) or ([MH] ("drinking behavior")) or ((binge)) or ((drink$)) or (("abus$" or "consumption" or "misuse" or "use$")) or (("drug" or "substance" or "alcohol" or "cannabis" or "amphetamine" or "cocaine" or "heroin" or "methaqualone" or "prescription")))) and ((((("brief " or "early" or "minimal") and "intervention")) or (("bi" or "bmi")) or ([MH] ("counseling")) or ([MH]"COUNSELING") or ([MH] ("motivation")))))) and ((([MH] ("adolescent")) or ([MH] ("adolescen$" or "teenage$" or "young" or "student$" or "juvenile" or "school" or "class$" or " kid " or " youth " or " underage "))))
Appendix 7. ETOH search strategy
("TI" ct (counseling/counseling/brief&intervention/brief intervention*/early intervention/minimal intervention*/ interview*/BI/BMI) & (adolescen*/teenage*/young*/student*/school*)) OR ("AB" ct (counseling/counseling/brief&intervention/brief intervention*/early intervention/minimal intervention*/ interview*/BI/BMI) & (adolescen*/teenage*/young*/student*/school*)) AND ("TI" / "AU" / "AB" / "CG" / "FS" / "MJ" / "MN" / "ID" ct clinical trial/random*/assign*/allocat*/crossover/factorial*/control*W2 study/ control* W2 trial*/single W2 blind*/ double W2 blind*/triple W2 blind*) "
Appendix 8. Criteria for judging risk of bias
| Item | Judgement | Description |
| 1. Random sequence generation (selection bias) |
Low risk | The investigators describe a random component in the sequence generation process such as: random‐number table; computer random‐number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation |
| High risk | The investigators describe a non‐random component in the sequence generation process such as: odd or even date of birth; date (or day) of admission; hospital or clinic record number; alternation; judgement of the clinician; results of a laboratory test or a series of tests; availability of the intervention | |
| Unclear risk | Insufficient information about the sequence generation process to permit judgement of low or high risk | |
| 2. Allocation concealment (selection bias) |
Low risk | Investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based, and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes |
| High risk | Investigators enrolling participants could possibly foresee assignments because one of the following methods was used: open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. if envelopes were unsealed or nonopaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure | |
| Unclear risk | Insufficient information to permit judgement of low or high risk. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement | |
| 3. Blinding of participants and providers (performance bias) | Low risk | No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken |
| High risk | No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk | |
| 4. Blinding of outcome assessor (detection bias) |
Low risk |
No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken |
| High risk | No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding Blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk | |
| 5. Incomplete outcome data (attrition bias) for all outcomes except retention in treatment or drop‐out |
Low risk |
No missing outcome data Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias) Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size Missing data have been imputed using appropriate methods All randomised participants are reported/analysed in the group they were allocated to by randomisation irrespective of non‐compliance and co‐interventions (intention to treat) |
| High risk | Reasons for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk is enough to induce clinically relevant bias in intervention effect estimate For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes is enough to induce clinically relevant bias in observed effect size ‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk (e.g. number randomised not stated, no reasons for missing data provided; number of dropouts not reported for each group) | |
| 6. Selective reporting (reporting bias) |
Low risk | The study protocol is available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon) |
| High risk | Not all of the study’s prespecified primary outcomes have been reported One or more primary outcomes is reported using measurements, analysis methods, or subsets of the data (e.g. subscales) that were not prespecified One or more reported primary outcomes were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect) One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis The study report fails to include results for a key outcome that would be expected to have been reported for such a study |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk | |
| 7. Other bias |
Low risk | The study appears to be free of other sources of bias |
| High risk | There is at least one important risk of bias. For example, the study:
|
|
| Unclear risk | Insufficient information to assess whether an important risk of bias exists; or insufficient rationale or evidence that an identified problem will introduce bias |
Data and analyses
Comparison 1. Brief intervention versus information provision.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Alcohol Frequency: number of alcohol days past 30 days | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Short‐term Follow up (1‐3 months) | 1 | 269 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.29, 0.19] |
| 1.2 Medium‐term Follow up (4‐6 months) | 2 | 434 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.20, 0.18] |
| 2 Alcohol Quantity: number of standard drinks in past 30 days | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Short‐term Follow up (1‐3 months) | 1 | 269 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.22, 0.26] |
| 2.2 Medium‐term Follow up (4‐6 months) | 2 | 434 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.33, 0.05] |
| 3 Cannabis Quantity: number of joints smoked in past 30 days | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Short‐term Follow up (1‐3 months) | 1 | 269 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.24, 0.24] |
| 3.2 Medium‐term Follow up (4‐6 months) | 1 | 264 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.39, 0.09] |
| 4 Cannabis Mean Dependence Score | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Short‐term Follow up (1‐3 months) | 2 | 470 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.27, 0.09] |
| 4.2 Medium‐term Follow up (4‐6 months) | 1 | 264 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.18, 0.30] |
| 4.3 Long‐term Follow up (7‐12 months) | 1 | 186 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.38, 0.20] |
| 5 Cannabis frequency: number of days smoked cannabis in past 30 days | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Short‐term Follow up (1‐3 months) | 2 | 470 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.25, 0.11] |
| 5.2 Medium‐term Follow up (4‐6 months) | 1 | 264 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.30, 0.18] |
| 5.3 Long‐term Follow up (7‐12 months) | 1 | 186 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.31, 0.26] |
| 6 Secondary outcomes related to substance use: Mean Problem Score | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Short‐term Follow up (1‐3 months) | 2 | 470 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.19, 0.17] |
| 6.2 Medium‐term Follow up (4‐6 months) | 1 | 264 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.37, 0.11] |
| 6.3 Long‐term Follow up (7‐12 months) | 1 | 186 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.39, 0.19] |
1.2. Analysis.
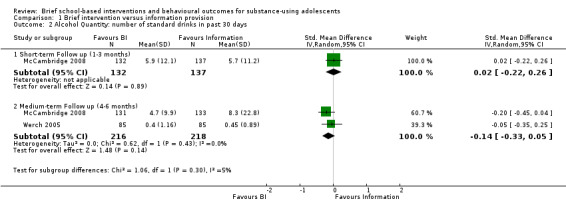
Comparison 1 Brief intervention versus information provision, Outcome 2 Alcohol Quantity: number of standard drinks in past 30 days.
Comparison 2. Brief intervention versus assessment only.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Alcohol Frequency: number of alcohol days | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Medium‐term Follow up (4‐6 months) | 2 | 242 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.21, ‐0.61] |
| 1.2 Long‐term Follow up (7‐12 months) | 1 | 170 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.53, 0.14] |
| 2 Alcohol Quantity: number of standard drinks | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Medium‐term Follow up (4‐6 months) | 1 | 179 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.45, 0.14] |
| 2.2 Long‐term Follow up (7‐12 months) | 1 | 162 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.47, 0.15] |
| 3 Alcohol Abuse: number of symptoms | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Medium‐term Follow up (4‐6 months) | 1 | 190 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.70, ‐0.07] |
| 3.2 Long‐term Follow up (7‐12 months) | 1 | 170 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.07, ‐0.38] |
| 4 Alcohol Dependence: number of symptoms | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Medium‐term Follow Up (4‐6 months) | 1 | 190 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐0.90, ‐0.26] |
| 4.2 Long‐term Follow up (7‐12 months) | 1 | 170 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.47, 0.20] |
| 5 Cannabis frequency: number of cannabis use days | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Short‐term Follow up (1‐3 months) | 1 | 179 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐1.14, ‐0.53] |
| 5.2 Medium‐term Follow up (4‐6 months) | 2 | 242 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.50, 0.05] |
| 5.3 Long‐term Follow up (7‐12 months) | 2 | 338 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.77, ‐0.31] |
| 6 Cannabis Abuse: number of symptoms | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Medium‐term Follow up (4‐6 months) | 1 | 190 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.65, ‐0.02] |
| 6.2 Long‐term Follow up (7‐12 months) | 1 | 170 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐0.96, ‐0.28] |
| 7 Cannabis Dependence: number of symptoms | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Medium‐term Follow up (4‐6 months) | 1 | 190 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.57, 0.06] |
| 7.2 Long‐term Follow up (7‐12 months) | 1 | 170 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐1.32, ‐0.62] |
| 8 Secondary outcomes related to substance use: Mean score on personal consequences scale | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Medium‐term Follow up (4‐6 months) | 2 | 242 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.58, 0.28] |
| 8.2 Long‐term Follow up (7‐12 months) | 1 | 170 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.13, ‐0.44] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
McCambridge 2004.
| Methods | Cluster‐randomised controlled trial | |
| Participants | Number of participants: 179 City and country: London, England Type of setting: Urban School setting: Alternative campus (further education training) Gender: 46% female, 54% male Mean age: 18.0 years Inclusion criteria: 16 or older, attending FET, weekly or more use of cannabis Exclusion criteria: Younger than 16, older than 19; less than weekly use cannabis; literacy (low levels); not English speaking |
|
| Interventions | Number of adolescents allocated to each group: 97 allocated to experimental condition, 82 allocated to control condition Brief intervention: Motivational intervention versus information and advice‐giving Dosage: 1 session Type of delivery: Face‐to‐face (individual) Timing: 1 hour |
|
| Outcomes | Follow‐up at 3 months and 12 months (2005 study) Measures: Severity of Dependence Scale, The Drug Attitudes Scale Primary outcomes:
Secondary outcomes:
|
|
| Notes | Only alcohol, cannabis frequency outcomes were measured at 12 months' follow‐up Funding: Action on Addiction for 12 12 months' follow‐up assessments. Conflict of interest: Information not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was non‐computerised and consisted of a colleague not involved in the study allocating clusters randomly to either the intervention or control condition. Stratification by college was applied in order to control for local variations in drug use |
| Allocation concealment (selection bias) | Low risk | Complete concealment was mentioned by the authors |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding is not possible for the type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As 1 interventionist was the study principal investigator, a second independent interviewer who was blind to study condition was employed to conduct 3 months' follow‐ups, and an additional interviewer who was blind to initial group allocation was employed for 12 months' follow‐ups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition analyses conducted, and no difference was found between groups. Various factors associated with attrition in both groups were identified and controlled for in the analysis. In addition, follow‐up rates were provided for 3 months' follow‐up (experimental group: 92.4%; control group: 86.3%) and 12 months' follow‐up (experimental group: 80%; control group: 82%) |
| Selective reporting (reporting bias) | High risk | All outcomes discussed and reported on at 3 months' follow‐up, although at 12 months' follow‐up there was some unplanned deterioration of the intervention effect, so certain outcomes were not reported on |
| Other bias | Low risk | No other sources of bias identified |
McCambridge 2008.
| Methods | Randomised controlled trial | |
| Participants | Number of participants: 326 City and country: Inner London, England Type of setting: Urban School setting: Alternative campus (further education training) Gender: 69% female, 31% male Mean age: 18.0 years Inclusion criteria: 16 or older, attending FET, weekly or more use of cannabis Exclusion criteria: Younger than 16, older than 19; less than weekly use cannabis; literacy (low levels); not English speaking |
|
| Interventions | Number of adolescents allocated to each group: 164 to experimental group, 162 to control group Brief intervention: Motivational intervention versus information and advice‐giving Dosage: 1 session Type of delivery: Face‐to‐face (individual) Timing: 1 hour |
|
| Outcomes | Follow‐up at 3 months Measures: Severity of Dependence Scale, Cannabis Problems Questionnaire, Alcohol Use Disorders Identification (AUDIT), Fagerström Test Primary outcomes:
Secondary outcomes:
|
|
| Notes | Funding: Wellcome Trust for a Health Services Research Fellowship (071301), the Big Lottery Fund, and Action on Addiction Conflict of interest: Information not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised individual randomisation was undertaken by the local clinical trials unit, stratifying by college |
| Allocation concealment (selection bias) | Low risk | Recruitment and baseline data collection took place first, and then individual researchers were informed by telephone or e‐mail about selection to preserve allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding is not possible for the type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blind to the study conditions |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition analyses were conducted, and there were no differences between groups in attrition or follow‐up rates ( experimental group: 85% at 3 months' follow‐up, 83% at 6 months' follow‐up; control group: 80% at 3 months' follow‐up, 80% at 6 months' follow‐up). Various factors associated with attrition were identified in both groups and controlled for in the analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes discussed, attrition and differences in practitioner effects were also addressed |
| Other bias | Low risk | None reported |
Walker 2011.
| Methods | Randomised controlled trial | |
| Participants | Number of participants: 205 City and country: Seattle, Washington, USA Type of setting: Urban School setting: Alternative campus (further education training) Gender: 69% female, 31% male Mean age: 18.0 years Inclusion criteria: 16 or older, attending FET, weekly or more use of cannabis Exclusion criteria: Younger than 16, older than 19; less than weekly use cannabis; literacy (low levels); not English speaking |
|
| Interventions | Number of adolescents allocated to each group: 103 to experimental group, 102 to control group Brief intervention: Motivational Enhancement Therapy versus information and advice‐giving Dosage: 2 sessions Type of delivery: Face‐to‐face (individual) Timing: 45 to 50 minutes |
|
| Outcomes | Follow‐up at 3 months and 12 months Measures: Global Appraisal of Individual Needs‐I, Marijuana Problem Inventory Primary outcomes:
Secondary outcomes:
|
|
| Notes | Funding: National Institute on Drug Abuse (RO1DA014296) Conflict of interest: Information not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was conducted using randomisation tables per school |
| Allocation concealment (selection bias) | Unclear risk | Information not provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding is not possible for the type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | This was not clearly discussed, and it was unknown who delivered the baseline and follow‐up appointments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition levels were low (experimental group: 85% at 3 months' follow‐up, 83% at 12 months' follow‐up; control group: 80% at 3 months' follow‐up, 80% at 12 months' follow‐up), and no difference was found between groups in attrition. While no differences were found in attrition across the treatment conditions, an intention‐to‐treat analysis was still conducted |
| Selective reporting (reporting bias) | Low risk | The focus of the intervention was cannabis‐related outcomes, therefore, while cannabis, alcohol, and other drug frequency and quantity measures were included to assess if there were any differences between treatment groups at baseline, only outcomes related to cannabis were provided postintervention |
| Other bias | Low risk | None reported |
Werch 2005.
| Methods | Randomised controlled trial of experiment | |
| Participants | Number of participants: 201 City and country: Northeast Florida, USA Type of setting: Urban School setting: Public high school Gender: 39.4% female, 60.6% male Mean age: 16.04 years Inclusion criteria: Age 14 to 19, Grade 9 to 12, 9 or more days of cannabis use in past 30 days Exclusion criteria: Not fluent in English, had thought disorder, refused to accept randomisation to a condition |
|
| Interventions | Number of adolescents allocated to each group: 100 to experimental group, 101 to control group Brief intervention: Screening, one‐on‐one risk reduction consultations, tip sheets with key messages from consultation, provided individual feedback, prevention messages were linked to different kinds of alcohol versus minimal intervention control Dosage: 1 session Type of delivery: Face‐to‐face (individual) Timing: School hours |
|
| Outcomes | Follow‐up at 4 months Primary outcomes:
|
|
| Notes | Funding: National Institute on Alcohol Abuse and Alcoholism (AA9283) Conflict of interest: Information not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Although the investigators discussed the random allocation of participants, this is not clearly explained |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding is not possible for the type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Equal distribution among groups, intention‐to‐treat analysis not necessary. 15 (10%) participants in the experimental group and 16 (13.7%) of participants in the control group dropped out of the study |
| Selective reporting (reporting bias) | High risk | Results were not indicative of all outcomes |
| Other bias | Unclear risk | Unknown, as first author is no longer working in the field of adolescent health research |
Winters 2007b.
| Methods | Randomised controlled trial | |
| Participants | Number of participants: 53 (79 including adolescents' parents who also received intervention) City and country: Minnesota, USA Type of setting: Urban School setting: Public junior/high school Gender: 35% female, 65% male Mean age: 15.5 years Inclusion criteria: Age 13 to 17, meets diagnostic criteria for 1 or more substance abuse disorders, agrees to participation with parents Exclusion criteria: referred to a treatment programme, meets diagnostic criteria for DSM‐IV substance use dependence, currently in treatment programme, reported acute psychiatric or medical problem/condition |
|
| Interventions | Number of adolescents allocated to each group: 26 to experimental group, 27 to control group (26 to parent experimental group, which is not relevant to the current review) Brief intervention: Motivational interviewing style session 1: obtain information about adolescents' substance use and consequences, address willingness to change, look at goals with regards to abstinence, reduction; session 2: some focus on progress to reaching goal, barriers; parenting session: address substance use problem, parent attitudes and behaviours, monitoring and supervision, versus assessment only (control) Dosage: 2 sessions adolescents, 1 session parents Type of delivery: Face‐to‐face (individual) Timing: 1 hour per session, after school hours |
|
| Outcomes | Follow‐up at 6 months Measures: Adolescent Diagnostic Interview, Timeline Followback, Personal Consequences Scale, Treatment Services Review Primary outcomes:
Secondary outcomes:
|
|
| Notes | Funding: Robert Wood Johnson Foundation (Grant 38324) and National Institute on Drug Abuse (K02DA15347) Conflict of interest: Information not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random selection by computer random‐number generator, no differences in groups at baseline |
| Allocation concealment (selection bias) | High risk | No concealment from investigators, which could introduce selection bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding is not possible for the type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is unclear if the outcome assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was only 1 attrition case (out of 27) in the control group (3.7%) |
| Selective reporting (reporting bias) | Low risk | Published report contains all outcomes that were discussed |
| Other bias | Low risk | None reported |
Winters 2012.
| Methods | Randomised controlled trial | |
| Participants | Number of participants: (315 including adolescents' parents who also received intervention) City and country: Minnesota, USA Type of setting: Urban School setting: Public junior/high school Gender: 48% female, 52% male Mean age: 16.3 years Inclusion criteria: Age 13 to 17, meets diagnostic criteria for 1 or more substance abuse disorders, agrees to participation with parents Exclusion criteria: referred to a treatment programme, meets diagnostic criteria for DSM‐IV substance use dependence, currently in treatment programme, reported acute psychiatric or medical problem/condition |
|
| Interventions | Number of adolescents allocated to each group: 136 allocated to experimental group, 56 allocated to control group (123 allocated to adolescent‐parent condition not relevant for this review) Brief intervention: Motivational interviewing style session 1: obtain information about adolescents' substance use and consequences, address willingness to change, look at goals with regards to abstinence, reduction; session 2: some focus on progress to reaching goal, barriers; parenting session: address substance use problem, parent attitudes and behaviours, monitoring and supervision, versus assessment only (control) Dosage: 2 sessions adolescents, 1 session parents Type of delivery: Face‐to‐face (individual) Timing: 1 hour per session, after school hours |
|
| Outcomes | Follow‐up at 6 months and 12 months (2014 study) Measures: Adolescent Diagnostic Interview, Timeline Followback, Personal Consequences Scale, Treatment Services Review Primary outcomes:
Secondary outcomes:
|
|
| Notes | Funding: National Institute on Health (DA017492, AA14866, K02‐DA15347, and P50‐DA027841) Conflict of interest: Information not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random selection by computer random‐number generator |
| Allocation concealment (selection bias) | High risk | No concealment from investigators, which could introduce selection bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding is not possible for the type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is unclear if the outcome assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No difference in 6 months' follow‐up attrition, which was very low. The follow‐up rate for the experimental group was 98.5% and for the control group was 98.2% |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | None reported |
DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition FET: further education training
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Apsler 2006 | Length of intervention |
| Armitage 2014 | Brief intervention was not delivered face‐to‐face |
| Baer 1992 | College based intervention |
| Barnett 2012 | Included prevention, only general substance use outcomes |
| Bear 2008 | Not school‐based |
| Cirillo 1998 | Length of intervention |
| Conrod 2013 | Not all participants used substances, focused on those at risk of substance use |
| D'Amico 2002 | Prevention, not intervention |
| D'Amico 2013 | Not school‐based |
| de Gee 2014 | Not school‐based |
| Dennis 2004 | Not school‐based |
| Doumas 2014 | Web‐based intervention, not delivered face‐to‐face |
| Gray 2005 | Quasi‐experimental study, no randomisation (a consideration if not enough randomised controlled trials were found) |
| Hecht 2003 | Prevention, not early intervention |
| Marsden 2006 | Target population were not all students, was not school‐based |
| Martin 2008 | Not school‐based |
| Newbury‐Birch 2014 | Feasibility study, no measure of intervention effect |
| Peleg 2001 | Prevention, not early intervention |
| Saunders 2004 | College‐based interventions |
| Sinha 2003 | Age of target population, not school‐based |
| Spoth 2001 | Target population were not only adolescents, intervention aimed at entire family |
| Srisurapanont 2007 | Not school‐based |
| Thaker 2008 | Prevention, not early intervention |
| Tubman 2002 | Pilot study, no comparison group (no abstract included so full ‐text article required) |
| Wagner 2014 | Brief treatment, more than 4 sessions |
| Werch 1999 | Prevention, not early intervention |
| Werch 2010 | Majority of students did not use any substances |
| Williams 2007 | Target population were receiving substance use treatment, not school‐based |
| Wu 2003 | Length of intervention, not school‐based |
Differences between protocol and review
We made the following changes to the present update from the previous version.
We removed the tobacco frequency outcomes, as this was not listed in the protocol and does not fall under the Cochrane Drugs and Alcohol Review Group.
We included an additional article (Winters 2012), which allowed for the analysis of outcomes by follow‐up period.
We changed 'Summary of findings' tables and GRADE quality of evidence accordingly.
We assessed risk of performance bias.
Contributions of authors
Tara Carney developed the data extraction form, was responsible for conducting the meta‐analysis and overseeing the drafting of the review, and is the contact review author. Tara Carney and Bronwyn Myers read all titles and abstracts that resulted from the search process and selected possibly relevant studies, and then Tara Carney obtained full copies of these studies, which both of these review authors used to undertake data extraction. Bronwyn Myers participated in the writing of the review and gave critical feedback on the drafts of the reviews. Johann Louw was available to assist in any of these decisions if necessary, participated in the design and writing of the review, and gave critical feedback on the drafts of the reviews. Charles Okwundu assisted with the meta‐analysis of the extracted data as well as the Results and Discussion sections. All review authors reviewed and commented on the drafts and final version of the review.
Sources of support
Internal sources
South African Medical Research Council, South Africa.
External sources
-
Open Society Foundation for South Africa, South Africa.
Grant received
Declarations of interest
Tara Carney: None known. Bronwyn Myers: None known. Johann Louw: None known. Charles Okwundu: None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
McCambridge 2004 {published data only}
- *McCambridge J, Strang J. The efficacy of single‐session motivational interviewing in reducing drug consumption and perceptions of drug‐related risk and harm among young people: results from a multisite cluster randomized trial. Addiction 2004;99:39‐52. [DOI] [PubMed] [Google Scholar]
- McCambridge J, Strang J. Deterioration over time in effect of motivational interviewing in reducing drug consumption and related risk among young people. Journal of Substance Abuse Treatment 2005;100:470‐8. [DOI] [PubMed] [Google Scholar]
McCambridge 2008 {published data only}
- McCambridge J, Slym, RL, Strang, J. Randomized controlled trial of motivational interviewing compared with drug information and advice for early intervention among young cannabis users. Addiction 2008;103:1809‐18. [DOI] [PubMed] [Google Scholar]
Walker 2011 {published data only}
- Walker DD, Stephens R, Roffman R, DeMarce J, Lozano B, Towe S, et al. Randomized controlled trial of motivational enhancement therapy with nontreatment‐seeking adolescent cannabis users: a further test of the teen marijuana check‐up. Psychology of Addictive Behaviors 2011;25(3):474‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Werch 2005 {published data only}
- Werch C, Jobli E, Moore, MJ, DiClemente CC, Dore, H, Brown CH. A brief experimental alcohol beverage‐tailored program for adolescents. Journal of Studies on Alcohol 2005;65:284‐90. [DOI] [PubMed] [Google Scholar]
Winters 2007b {published data only}
- Winters KC, Leitten W. Brief intervention for drug‐abusing adolescents in a school setting. Psychology of Addictive Behaviors 2007;21(2):249–54. [DOI] [PubMed] [Google Scholar]
Winters 2012 {published data only}
- Winters KC, Fahnhorst T, Botzet A, Lee S, Lalone B. Brief intervention for drug‐abusing adolescents in a school setting: Outcomes and mediating factors. Journal of Substance Abuse Treatment 2012;42:279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters KC, Lee S, Botzet A, Fahnhorst T, Nicholson A. One‐year outcomes and mediators of a brief intervention for drug abusing adolescents. Psychology of Addictive Behaviors 2014;28(2):464‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Apsler 2006 {published data only}
- Apsler R, Formica S, Fraster B, McMahan R. Promoting positive adolescent development for at‐risk students with a student assistance program. The Journal of Primary Prevention 2006;27(6):533‐54. [DOI] [PubMed] [Google Scholar]
Armitage 2014 {published data only}
- Armitage CJ, Rowe R, Arden MA, Harris PR. A brief psychological intervention that reduces adolescent alcohol consumption. Journal of Consulting and Clinical Psychology 2014;82(3):546‐50. [DOI] [PubMed] [Google Scholar]
Baer 1992 {published data only}
- Baer S, Marlatt GA, Kivlahan DR, Fromme K, Larimer ME, Williams E. An experimental test of three methods of alcohol risk reduction with young adults. Journal of Consulting and Clinical Psychology 1992;60(6):974‐9. [DOI] [PubMed] [Google Scholar]
Barnett 2012 {published data only}
- Barnett E, Spruijt‐Metz D, Unger JB, Sun P, Rohrbach LA, Sussman S. Boosting a teen substance use prevention program with motivational interviewing. Substance Use and Misuse 2012;47(4):418‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bear 2008 {published data only}
- Bear JS, Beadnell B, Garrett SB, Hartzler B, Wells EA, Peterson PL. Adolescent change language within a brief motivational intervention and substance use outcomes. Psychology of Addictive Behaviors 2008;22(4):570–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cirillo 1998 {published data only}
- Cirillo KJ, Pruitt BE, Colwell B, Kingery PM, Hurley RS, Ballard D. School violence: Prevalence and intervention strategies for at‐risk adolescents. Adolescence 1998;33(130):319‐30. [PubMed] [Google Scholar]
Conrod 2013 {published data only}
- Conrod PJ, O'Leary‐Barrett M, Newton N, Topper L, Castellanos‐Ryan, MacKie C, et al. Effectiveness of a selective, personality‐targeted prevention program for adolescent alcohol use and misuse: a cluster randomized controlled trial. JAMA Psychiatry 2013;70(3):334‐42. [DOI] [PubMed] [Google Scholar]
D'Amico 2002 {published data only}
- D'Amico EJ, Fromme K. Brief prevention for adolescent risk‐taking behavior. Addiction 2002;97:563‐74. [DOI] [PubMed] [Google Scholar]
D'Amico 2013 {published data only}
- D'Amico EJ, Hunter SB, Miles JNV, Ewing BA, Osilla KC. A randomized controlled trial of a group motivational interviewing intervention for adolescents with a first time alcohol or drug offense. Journal of Substance Abuse Treatment 2013;45(5):400‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Gee 2014 {published data only}
- Gee EA, Verdurmen JEE, Bransen E, Jonge JM, Schippers GM. A randomized controlled trial of a brief motivational enhancement for non‐treatment‐seeking adolescent cannabis users. Journal of Substance Abuse Treatment 2014;47(3):181‐8. [DOI] [PubMed] [Google Scholar]
Dennis 2004 {published data only}
- Dennis M, Godley SH, Diamond G, Tims FM, Babor T, Donaldson J, et al. The cannabis youth treatment (CYT) experiment: main findings from two randomized trials. Journal of Substance Abuse Treatment 2004;27:197–213. [DOI] [PubMed] [Google Scholar]
Doumas 2014 {published data only}
- Doumas DM, Hausheer R, Esp S, Cuffee C. Reducing alcohol use among 9th grade students: 6 month outcomes of a brief, Web‐based intervention. Journal of Substance Abuse Treatment 2014;47(1):102‐5. [DOI] [PubMed] [Google Scholar]
Gray 2005 {published data only}
- Gray E, McCambridge J, Strang J. The effectiveness of motivational interviewing delivered by youth workers in reducing drinking, cigarette and cannabis smoking among young people: a quasi‐experimental study. Alcohol and Alcoholism 2005;40(6):535–9. [DOI] [PubMed] [Google Scholar]
Hecht 2003 {published data only}
- Hecht ML, Marsiglia FF, Elek E, Wagstaff, DA, Kulis S, Dustman P, et al. Culturally grounded substance use prevention: An evaluation of the keepin’ it R.E.A.L. curriculum. Prevention Science 2003;4(4):233‐48. [DOI] [PubMed] [Google Scholar]
Marsden 2006 {published data only}
- Marsden J, Stillwell G, Barlow H, Boys A, Taylor C, Hunt N, et al. An evaluation of a brief motivational intervention among young ecstasy and cocaine users: no effect on substance and alcohol use outcomes. Addiction 2006;101:1014‐26. [DOI] [PubMed] [Google Scholar]
Martin 2008 {published data only}
- Martin G, Copeland J. The adolescent cannabis check‐up: Randomized trial of a brief intervention for young cannabis users. Journal of Substance Abuse Treatment 2008;34:407‐14. [DOI] [PubMed] [Google Scholar]
Newbury‐Birch 2014 {published data only}
- Newbury‐Birch D, O'Neil S, O'Donnell A, Coulton S, Howel D, McColl E, et al. A pilot feasiblity C‐RCT of screening and brief alcohol intervention in young people aged 14‐15 in a high school setting: Sips Jr‐high. Alcoholism: Clinical and Experimental Research 2014;38(S1):127A. [Google Scholar]
Peleg 2001 {published data only}
- Peleg A, Neumann L, Friger M, Peleg R, Sperber AD. Outcomes of a brief alcohol abuse prevention program for Israeli high school students. Journal of Adolescent Health 2001;28:263–9. [DOI] [PubMed] [Google Scholar]
Saunders 2004 {published data only}
- Saunders JB, Kypri K, Walters ST, Laforge RG, Larimer ME. Approaches to brief intervention for hazardous drinking in young people. Alcoholism: Clinical and Experimental Research 2004;28(2):322‐9. [DOI] [PubMed] [Google Scholar]
Sinha 2003 {published data only}
- Sinha R, Easton C, Renee‐Aubin L, Carroll KM. Engaging young probation‐referred marijuana‐abusing individuals in treatment: a pilot trial. The American Journal on Addictions 2003;12:314‐23. [PubMed] [Google Scholar]
Spoth 2001 {published data only}
- Spoth RL, Redmond C, Shin C. Randomized trial of brief family interventions for general populations: adolescent substance use outcomes 4 years following baseline. Journal of Consulting and Clinical Psychology 2001;69(4):627‐42. [DOI] [PubMed] [Google Scholar]
Srisurapanont 2007 {published data only}
- Srisurapanont M, Sombatmai S, Boripuntakul T. Brief intervention for students with methamphetamine use disorders: a randomized controlled trial. The American Journal on Addictions 2007;16:111‐6. [DOI] [PubMed] [Google Scholar]
Thaker 2008 {published data only}
- Thaker S, Steckler V, Khataopush S, Rose J, Hallforts D. Program characteristics and organizational factors affecting the implementation of a school‐based indicated prevention program. Health Education Research 2007;23(2):238‐48. [DOI] [PubMed] [Google Scholar]
Tubman 2002 {published data only}
- Tubman JG, Wagner EF, Gil AG, Pate KN. Brief motivational intervention for substance‐abusing delinquent adolescents: guided self‐change as a social work practice innovation. Health and Social Work 2002;27(3):208‐12. [DOI] [PubMed] [Google Scholar]
Wagner 2014 {published data only}
- Wagner EF, Hospital MM, Graziano JN, Morris SL, Gil AG. A randomized controlled trial of guided self‐change with minority adolescents. Journal of Consulting and Clinical Psychology 2014;82(6):1128‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Werch 1999 {published data only}
- Werch CE, Pappas DM, Carlson JM, DiClemente CC. Six‐month outcomes of an alcohol prevention program for inner‐city youth. American Journal of Health Promotion 1999;13(4):237‐40. [DOI] [PubMed] [Google Scholar]
Werch 2010 {published data only}
- Werch CE, Bian H, Diclemente CC, Moore MJ, Thombs D, Ames SC, et al. A brief image‐based prevention intervention for adolescents. Psychology of Addictive Behaviours 2010;24(1):170‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2007 {published data only}
- Williams AV, Meyer E, Pechansky F. Development of a therapeutic game for relapse prevention and motivation for change in young drug users [Desenvolvimento de um jogo terapêutico para prevenção da recaída e motivação para mudança em Jovens usuários de drogas]. Psicologia: Teoria e Pesquisa 2007;23(4):407‐14. [Google Scholar]
Wu 2003 {published data only}
- Wu Y, Stanton BF, Galbraith J, Kaljee L, Cottrell L, Li X, et al. Sustaining and broadening intervention impact: a longitudinal randomized trial of 3 adolescent risk reduction approaches. Pediatrics 2003;111(1):e32‐8. [DOI] [PubMed] [Google Scholar]
Additional references
Babor 2007
- Babor TF, McRee BG, Kassebaum PA, Grimaldi PL, Ahmed K, Bray J. Screening, Brief Intervention, and Referral to Treatment (SBIRT). Substance Abuse 2007;28(3):7‐30. [DOI] [PubMed] [Google Scholar]
Barry 1999
- Barry KL. Brief Interventions and Brief Therapies for Substance Abuse. Treatment Improvement Protocol (TIP) Series 34. Center for Substance Abuse Treatment, 1999. [Google Scholar]
Bien 1993
- Bien TH, Miller WR, Tonigan SJ. Brief interventions for alcohol problems: a review. Addiction 1993;88:315‐36. [DOI] [PubMed] [Google Scholar]
Bingham 2010
- Bingham CR, Barretto AI, Walton MA, Bryant CM, Shope JT, Raghunathan TE. Efficacy of a web‐based, tailored, alcohol prevention/intervention program for college students: initial findings. Journal of American College Health 2010;58(4):349‐56. [DOI] [PubMed] [Google Scholar]
Boutron 2007
- Boutron I, Guittet L, Estellat C, Moher D, Hrobjartsson A, Ravaud P. Reporting methods of blinding in randomized trials assessing nonpharmacological treatments. PL oS Medicine 2007;4(2):370‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brunelle 2000
- Brunelle N, Brochu S, Cousineau MM. Drug‐crime relations among drug‐consuming juvenile delinquents: a tripartite model and more. Contemporary Drug Problems 2000;27:835‐66. [Google Scholar]
Campbell 2000
- Campbell MK, Mollison J, Steen N, Grimshaw JM, Eccles M. Analysis of cluster randomised trials in primary care: a practical approach. Family Practice 2000;17(2):192‐6. [DOI] [PubMed] [Google Scholar]
CONSORT 2010
- CONSORT 2010. Consolidated Standards of Reporting Trials. Library for health research reporting. http://www.equator‐network.org/resource‐centre/library‐of‐health‐research‐reporting/ (accessed 16 July 2010).
Corneau 2004
- Corneau M, Lanctôt N. Mental health outcomes of adjudicated males and females: the aftermath of juvenile delinquency. Criminal Behavior and Mental Health 2004;14:251‐62. [DOI] [PubMed] [Google Scholar]
Feldstein 2006
- Feldstein SW, Miller WR. Substance use and risk‐taking among adolescents. Journal of Mental Health 2006;15(3):633‐43. [Google Scholar]
Gore 2011
- Gore FM, Bloem PJN, Patton GC, Ferguson J, Joseph V, Coffey C, et al. Global burden of disease in young people aged 10–24 years: a systematic analysis. The Lancet 2011;377:2093–102. [DOI] [PubMed] [Google Scholar]
GRADE 2004
- The GRADE working group. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64:383‐94. [DOI] [PubMed] [Google Scholar]
Hallfors 2006
- Hallfors D, Cho H, Brodish PH, Flewelling R, Khatapoush S. Identifying high school students "at risk" for substance use and other problems: implication for prevention. Substance Use and Misuse 2006;41:1‐15. [DOI] [PubMed] [Google Scholar]
Hibell 2012
- Hibell B, Guttormsson U. The 2011 ESPAD Report: Substance Use Among Students in 36 European Countries. ESPAD Report 2012.
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011].. The Cochrane Collaboration. www.cochrane‐handbook.org 2011.
Hingson 2006
- Hingson RW, Heeren T, Winter MR. Age of alcohol‐dependence onset: associations with severity of dependence and seeking treatment. Pediatrics 2006;11(3):e755‐63. [DOI: 10.1542/peds.2006‐0223] [DOI] [PubMed] [Google Scholar]
International Center for Alcohol Policies 2010
- International Center for Alcohol Policies. Washington, DC: International Center for Alcohol Policies; 2010. ICAP Blue Book Module 12: Legal Age Limits. Practical Guides for Alcohol Policy and Prevention Approaches.
Jackson 2013
- Jackson K, Schulenberg J. Alcohol use during the transition from middle school to high school: national panel data on prevalence and moderators. Developmental Psychology 2013;49(11):2147‐58. [DOI: 10.1037/a0031843] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jensen 2011
- Jensen CD, Cushing CC, Aylward BS, Craig JT, Sorell DM, Steele RG. Effectiveness of motivational interviewing interventions for adolescent substance use behavior change: a meta‐analytic review. Journal of Consulting and Clinical Psychology 2011;79(4):433‐40. [DOI] [PubMed] [Google Scholar]
Johnston 2015
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future National Survey Results on Drug Use: 1975‐2014: Overview, Key Findings on Adolescent Drug Use. Ann Arbor: Institute for Social Research, The University of Michigan, 2015. [Google Scholar]
Kann 2013
- Kann L, Kinchen S, Shanklin SL, Flint KH, Hawkins J, Harris WA, et al. Youth risk behavior surveillance — United States, 2013. Morbidity and Mortality Weekly Report. Surveillence Summaries 2014;63(Supplement 4):1‐168. [PubMed] [Google Scholar]
Kutcher 2009
- Kutcher S, McDougall A. Problems with access to adolescent mental health care can lead to dealings with the criminal justice system. Paediatrics & Child Health 2009;14(1):15‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lanctôt 2007
- Lanctôt N, Cernkovich SA, Giordano PC. Delinquent behaviour, official delinquency and gender consequences for adulthood functioning and well‐being. Criminology 2007;45(1):131‐57. [Google Scholar]
Leoschut 2007
- Leoschut L, Bonora A. Offender Perspective on Violent Crime. Someone Stole My Smile: An Exploration Into the Causes of Youth Violence in South Africa 2007; Vol. Monograph Series, No. 3.
Miller 2002
- Miller WR, Rollnick S. Motivational Interviewing: Preparing People to Change Addictive Behavior. 2nd Edition. New York: Guilford Press, 2002. [Google Scholar]
Moyer 2002
- Moyer A, Finney JW, Swearingen CE, Vergun P. Brief interventions for alcohol problems: a meta‐analytic review of controlled investigations in treatment‐seeking and non‐treatment seeking populations. Addiction 2002;97:279‐92. [DOI] [PubMed] [Google Scholar]
Mulvey 2010
- Mulvey EP, Schubert CA, Chassin LA. Substance Use and Delinquent Behavior Among Serious Adolescent Offenders. Juvenile Justice 2010:Washington, DC: Office of Juvenile Justice and Delinquency Prevention.
NICE 2007
- National Institute for Health and Clinical Excellence. School‐based interventions on alcohol. NICE Public Health Guidance Report 2007; Vol. 7:1‐46.
Patton 2014
- Patton R, Deluca P, Kaner E, Newbury‐Birch D, Phillips T, Drummond C. Alcohol screening and brief intervention for adolescents: The how, what and where of reducing alcohol consumption and related harm among young people. Alcohol & Alcoholism 2014;49(2):207‐12. [DOI: 10.1093/alcalc/agt165] [DOI] [PMC free article] [PubMed] [Google Scholar]
Plüddemann 2010
- Plüddemann A, Flisher AJ, Mathews C, Parry CDH, Lombard CA. Methamphetamine use, aggressive behaviour and other mental health issues among high‐school students in Cape Town, South Africa. Drug and Alcohol Dependence 2010;109:14‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prochaska 1993
- Prochaska JO, DeClemente CC, Norcross JC. In search of how people change: applications to addictive behaviors. Journal of Addictions Nursing 1993;15(1):2‐16. [DOI] [PubMed] [Google Scholar]
Reddy 2013
- Reddy SP, James S, Sewpaul R, Sifunda S, Ellahebokus A, Kambaran NS, et al. Umthente Uhlaba Usamila – The 3rd South African National Youth Risk Behaviour Survey 2011. Cape Town: South African Medical Research Council, 2013. [Google Scholar]
RevMan 2014
- Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rollnick 1995
- Rollnick S, Miller WR. What is motivational interviewing?. Behavioural and Cognitive Psychotherapy 1995;23:325‐34. [DOI] [PubMed] [Google Scholar]
Schünemann 2006
- Schünemann HJ, Jaeschke R, Cook D, Bria W, El‐Solh A, Ernst A, et al. An official ATS statement: grading the quality of evidence and strength of recommendations in ATS guidelines and recommendations. American Journal of Respiratory and Critical Care Medicine 2006;174:605‐14. [DOI] [PubMed] [Google Scholar]
Storr 2007
- Storr CL, Accornero VH, Crum RM. Profiles of disruptive behaviour: association with recent drug consumption among adolescents. Addictive Behaviors 2007;32:248‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tait 2003
- Tait RJ, Hulse GK. A systematic review of the effectiveness of brief interventions with substance using adolescents by type of drug. Drug and Alcohol Review 2003;22:337‐46. [DOI] [PubMed] [Google Scholar]
Tevyaw 2004
- Tevyaw TO'L, Monti PM. Motivational enhancement and other brief interventions for adolescent substance abuse: Foundations, applications, and evaluations. Addiction 2004;99:63‐75. [DOI] [PubMed] [Google Scholar]
UNODC 2012
- United Nations on Drugs, Crime. Cannabis: a short review. United Nations on Drugs and Crime; 2012. Youth Report.
Ward 2007
- Ward CL, Martin E, Theron C, Distiller GB. Factors affecting resilience in children exposed to violence. South African Journal of Psychology 2007;37(1):165‐87. [Google Scholar]
Watchel 2010
- Watchel T, Staniford M. The effectiveness of brief interventions in the clinical setting in reducing alcohol misuse and binge drinking in adolescents: a critical review of the literature. Journal of Clinical Nursing 2010;19:605‐20. [DOI] [PubMed] [Google Scholar]
White 2012
- White V, Bariola E. Australian secondary school students’ use of tobacco, alcohol, and over‐the‐counter and illicit substances in 2011. Victoria, Australia: Drug Strategy Branch Australian Government, Department of Health and Ageing; December 2012..
Winters 2007a
- Winters KC, Leitten W, Wagner E, O’Leary Tevyaw T. Use of brief interventions for drug abusing teenagers within a middle and high school setting. Journal of School Health 2007;77(4):196‐206. [DOI] [PubMed] [Google Scholar]
Winters 2008
- Winters KC, Lee CS. Likelihood of developing an alcohol and cannabis use disorder during youth: association with recent use and age. Drug and Alcohol Dependence 2008;92:239‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 2012
- Young MM, Stevens A, Porath‐Waller A, Pirie T, Garritty C, Skidmore B, et al. Effectiveness of brief interventions as part of the screening, brief intervention and referral to treatment (SBIRT) model for reducing the non‐medical use of psychoactive substances: a systematic review protocol. Systematic Reviews 2012;1(22):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Carney 2014
- Carney T, Myers BJ, Louw J, Okwundu CI. Brief school‐based interventions and behavioural outcomes for substance‐using adolescents. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD008969.pub2] [DOI] [PubMed] [Google Scholar]


