Abstract
Background
Pharmacotherapy in the elderly population is complicated by several factors that increase the risk of drug‐related harms and less favourable effectiveness. The concept of medication review is a key element in improving the quality of prescribing and in preventing adverse drug events. Although there is no generally accepted definition of medication review, it can be broadly defined as a systematic assessment of pharmacotherapy for an individual patient that aims to optimise patient medication by providing a recommendation or by making a direct change. Medication review performed in adult hospitalised patients may lead to better patient outcomes.
Objectives
We examined whether delivery of a medication review by a physician, pharmacist or other healthcare professional leads to improvement in health outcomes of hospitalised adult patients compared with standard care.
Search methods
We searched the Specialised Register of the Cochrane Effective Practice and Organisation of Care (EPOC) Group; the Cochrane Central Register of Controlled Trials (CENTRAL); MEDLINE; EMBASE; and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) to November 2014, as well as International Pharmaceutical Abstracts and Web of Science to May 2015. In addition, we searched reference lists of included trials and relevant reviews. We searched trials registries and contacted experts to identify additional published and unpublished trials. We applied no language restrictions.
Selection criteria
We included randomised controlled trials (RCTs) of medication review in hospitalised adult patients. We excluded trials of outclinic and paediatric patients. Our primary outcome was all‐cause mortality, and secondary outcomes included hospital readmissions, emergency department contacts and adverse drug events.
Data collection and analysis
Two review authors independently included trials, extracted data and assessed trials for risk of bias. We contacted trial authors for clarification of data and for additional unpublished data. We calculated risk ratios for dichotomous data and mean differences for continuous data (with 95% confidence intervals (CIs)). The GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach was used to assess the overall certainty of evidence for the most important outcomes.
Main results
We identified 6600 references (4647 references in our initial review) and included 10 trials (3575 participants). Follow‐up ranged from 30 days to one year. Nine trials provided mortality data (3218 participants, 466 events), with a risk ratio of 1.02 (95% CI 0.87 to 1.19) (low‐certainty evidence). Seven trials provided hospital readmission data (2843 participants, 1043 events) with a risk ratio of 0.95 (95% CI 0.87 to 1.04) (high‐certainty evidence). Four trials provided emergency department contact data (1442 participants, 244 events) with a risk ratio of 0.73 (95% CI 0.52 to 1.03) (low‐certainty evidence). The estimated reduction in emergency department contacts of 27% (with a CI ranging from 48% reduction to 3% increase in contacts) corresponds to a number needed to treat for an additional beneficial outcome of 37 for a low‐risk population and 12 for a high‐risk population over one year. Subgroup and sensitivity analyses did not significantly alter our results.
Authors' conclusions
We found no evidence that medication review reduces mortality or hospital readmissions, although we did find evidence that medication review may reduce emergency department contacts. However, because of short follow‐up ranging from 30 days to one year, important treatment effects may have been overlooked. High‐quality trials with long‐term follow‐up (i.e. at least up to a year) are needed to provide more definitive evidence for the effect of medication review on clinically important outcomes such as mortality, readmissions and emergency department contacts, and on outcomes such as adverse events. Therefore, if used in clinical practice, medication reviews should be undertaken as part of a clinical trial with long‐term follow‐up.
Plain language summary
Reassessment of drugs given to hospitalised adult patients to improve patients’ health
Review question
This updated Cochrane systematic review studies the evidence for performing in‐hospital medication review (defined as a systematic reassessment by a healthcare professional of an individual patients's medication with suggestions for improvement). We aimed to assess whether medication review may improve the health of adult patients.
Background
Elderly patients are often prescribed several drugs despite a generally higher risk of adverse events and sometimes lesser treatment effectiveness in this population.
Search date
To find relevant trials, we searched electronic medical literature databases up to May 2015.
Study characteristics
We included 10 randomised controlled trials with a total of 3575 participants.
Key results
We found that medication review does not seem to prevent death and hospital readmissions, but that it might reduce emergency department contacts.
Certainty of the evidence
Our confidence in results across studies ranged from low to high. We found no evidence that medication review in hospitalised patients makes a difference towards preventing mortality (low‐certainty evidence) or hospital readmissions (high‐certainty evidence), but we found that medication review may have a preventive effect on reducing the number of emergency department contacts (low‐certainty evidence). In the included trials, participants were followed for a short time (ranging from 30 days to one year). Therefore, important long‐term treatment effects may have been overlooked. We suggest that further research with long‐term patient follow‐up and examination of specific methods of medication review should be undertaken before this intervention is implemented in clinical practice.
Summary of findings
Summary of findings for the main comparison. Medication review compared with standard care for hospitalised adult patients.
| Medication review compared with standard care for hospitalised adult patients | ||||||
|
Patient or population: hospitalised adult patients Intervention: medication review Comparison: standard care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard care | Medication review | |||||
|
Mortality (all‐cause) 1 year |
Low‐risk population | RR 1.02 (0.87 to 1.19) | 3218 (9 trials) | ⊕⊕⊝⊝ Lowb,c |
NA | |
| 200 per 1000a | 204 per 1000 (174 to 238) | |||||
| High‐risk population | ||||||
| 400 per 1000a | 408 per 1000 (348 to 476) | |||||
|
Hospital readmission (all‐cause) 1 year |
Low‐risk population | RR 0.95 (0.87 to 1.04) | 2843 (7 trials) |
⊕⊕⊕⊕ High |
NA | |
| 300 per 1000a | 285 per 1000 (261 to 312) | |||||
| High‐risk population | ||||||
| 600 per 1000a | 570 per 1000 (522 to 624) | |||||
|
Hospital emergency department contacts (all‐cause) 1 year |
Low‐risk population | RR 0.73 (0.52 to 1.03) | 1442 (4 trials) | ⊕⊕⊝⊝ Lowd,e | Equal to number needed to treat of 12 for the high‐risk population and 37 for the low‐risk population |
|
| 100 per 1000a | 73 per 1000 (52 to 103) | |||||
| High‐risk population | ||||||
| 300 per 1000a | 219 per 1000 (156 to 309) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; NA: Not applicable; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low certainty: We are very uncertain about the estimate | ||||||
aRisk for the high‐risk population of the control group was based on data from Gillespie 2009, one of the 3 trials with 12 months of follow‐up (all outcomes) and with greatest risk in the control group. For the low‐risk population, the 12 months of follow‐up for Scullin 2007 (mortality) and the 3 months of follow‐up for Lisby 2010 (hospital emergency department contacts) were extrapolated to determine 12‐month risk. All trials had almost similar control group risks for hospital readmissions, and the low‐risk group was based on half the risk of 12 months of follow‐up for Gillespie 2009
bSubgroup analysis comparing 'high'‐ and 'low'‐risk populations revealed a small trend toward increased mortality in the low‐risk group compared with the high‐risk group (P value = 0.08) (downgraded 1 category)
cFollow‐up ranged from 3 to 12 months for mortality. Short follow‐up may be inadequate to detect the effect on changes in prophylactic medication (indirectness of evidence) (downgraded 1 category)
d'Risk of bias' assessments determined that 3 of 4 trials in the analysis (Gillespie 2009; Lisby 2010; Lisby 2015) had overall 'high risk' of bias (downgraded 1 category)
eThe confidence interval overlapped 1 (downgraded 1 category)
Background
Evidence links polypharmacy (defined as the use of many drugs) to increased risk of preventable interactions and adverse drug events (e.g. falls) (Bourgeois 2010; Hallas 1996; Obreli‐Neto 2012; Rothschild 2000; Ziere 2006), poorer drug adherence (Pasina 2014), use of inappropriate medications (Beers 1997; Hanlon 2004), a greater economic burden (Classen 1997), emergency department visits and hospital admissions (Kongkaew 2008; Schneeweiss 2002; Zed 2008), drug‐related deaths and overall mortality (Ebbesen 2001; Gnjidic 2012). Thus, it is critical in the patient with multi‐morbidity to discern between appropriate polypharmacy (e.g. as often the case treating conditions such as hypertension, diabetes and chronic pain) and inappropriate polypharmacy leading to unfavourable health and economic consequences (Aronson 2006; Hajjar 2007; Page 2010; Routledge 2004; Spinewine 2007b). The existence and recognition of inappropriate polypharmacy are of particular concern for the elderly population, for whom age‐related physiological changes, a greater degree of frailty and multiple coexisting conditions have been associated with increased risk of adverse drug events (ElDesoky 2007; Mangoni 2004). Additionally, adherence to and efficacy of drug treatment are generally reduced in elderly patients (Hughes 2004; Zulman 2011). The problem of inappropriate pharmacotherapy is expected to grow in the future as new drugs are introduced, as new uses for old drugs are found and as individuals in most parts of the world live longer and have increased risk of chronic medical conditions (CDC 2011; Christensen 2009; European Communities 2006; Pefoyo 2015).
Substantial efforts have been made to characterise and improve the appropriateness of prescribing for the elderly (Patterson 2012; Spinewine 2007b). Medication review constitutes such an attempt to improve the quality of prescribing and to prevent adverse drug events. There is no generally accepted definition of medication review, but it can be defined as a systematic assessment of the pharmacotherapy of an individual patient that aims to evaluate and optimise medication by providing a recommendation or by making a direct change. Medication review involves evaluating the therapeutic efficacy and harms of each drug in relation to the individual patient and conditions being treated. Other issues, such as adherence, interactions, biochemical monitoring and patient preferences and understanding of the condition, should also be considered and addressed when appropriate (Zermansky 2001). It is also important to include medication reconciliation (i.e. identifying the most accurate list of medications a patient is taking and using that list to provide correct pharmacotherapy), especially during transitions in care (Joint Commission 2012; Rogers 2006; Steurbaut 2010). To aid the process of reviewing patient medication, several criteria have been formulated to identify potentially inappropriate medications, especially for elderly people (Beers 1991; Beers 1997; Fick 2003; Gallagher 2008a; Hanlon 1992; Holt 2010; Laroche 2007a; McLeod 1997; Naugler 2000; O'Mahony 2015; Samsa 1994). However, the applicability and effects in clinical practice for these various measures remain uncertain (Bregnhøj 2009; Gallagher 2008b; Lozano‐Montoya 2015; Lund 2010; Ryan 2009; Spinewine 2007b).
Randomised trials of medication review have been summarised in recent systematic reviews (Holland 2008; Nkansah 2010; Royal 2006). The systematic reviews investigating medication review most often included trials with elderly people in primary care and failed to show effects on morbidity or mortality. Trials often involved pharmacist‐led medication reviews that ranged from 'hands‐on' clinical evaluation of hospital inpatient medication to informational approaches to physicians in outpatient clinics or primary care (Holland 2008; Nkansah 2010; Royal 2006). It is important to note that some pharmacist‐led medication reviews may be restricted because they are not directly linked to changes in clinical care (Spinewine 2007b). Physicians do not always implement pharmacists' suggestions (Chen 2007; Mannheimer 2006; Spinewine 2007b), and older patients may be reluctant to accept pharmacists' suggestions (Salter 2007) or may prefer to have their medications reviewed by a physician (Jones 1997). However, some evidence indicates that inpatient medication reviews by pharmacists in close contact with physicians might lead to fewer readmissions and lower morbidity (Gillespie 2009). Hospitalised patients likely represent a more frail patient group compared with primary care patients (Laroche 2007b); therefore, we investigated whether medication reviews affect hard clinical endpoints in hospitalised patients. This is an update of a previous meta‐analysis on this subject (Christensen 2013).
Description of the condition
Inappropriate pharmacotherapy is a major cause of patient morbidity and mortality. Inappropriate pharmacotherapy includes situations where medicines are prescribed without correct indication or dosage, in unfavorable combination with certain patient conditions, or combined with other interacting medicines that may increase the risk of treatment failure or adverse effects. Also included in the term 'inappropriate pharmacotherapy' is the presence of unacceptable adverse effects, lack of necessary biochemical monitoring of pharmacotherapy and poor adherence to pharmacotherapy, as well as underprescribing (i.e. not prescribing despite indication for pharmacotherapy).
Description of the intervention
Any medication review of a patient's list of medicines delivered by a healthcare professional with the aim of improving the pharmacotherapy (i.e. optimising effectiveness, minimising harms and/or costs of the prescribed medication).
How the intervention might work
More appropriate prescribing could improve effectiveness, reduce adverse events and improve adherence to, and thereby appropriateness of, drug therapy (i.e. ensure that treatment is properly indicated and monitored, and that the individual patient receives the right drug and dosage), possibly leading to reduced morbidity and mortality.
Why it is important to do this review
Medication reviews are performed in many parts of the world, but it is unclear whether medication reviews for hospitalised adult patients reduce patient morbidity and mortality. In addition, the best method for medication review is at present unknown. Through analysis of collective scientific evidence from randomised controlled trials (RCTs), we will clarify whether medication review can reduce mortality, hospital readmissions, emergency department contacts or adverse drug events among patients. We will also examine whether some methods of medication review are more effective than others. The results of this systematic review could encourage optimisation of current practices in this complex and important area. In addition, future research could be pointed in a more favourable direction.
Objectives
We examined whether delivery of a medication review by a physician, pharmacist or other healthcare professional leads to greater improvement in health outcomes of hospitalised adult patients compared with standard care.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) in any language, published or unpublished, with randomisation on an individual level or on an aggregated level (i.e. cluster‐RCTs).
Types of participants
We included hospitalised patients (i.e. patients admitted to a hospital).
We excluded outpatients and patients seen in the emergency department but not admitted to a hospital, as well as patients admitted to a paediatric department.
Types of interventions
We included any medication review of a patient's medicines delivered by a healthcare professional with the aim of improving pharmacotherapy for the patient (i.e. optimising the balance of effectiveness, harms and costs of the prescribed medication). We defined medication review as any systematic assessment of the pharmacotherapy of an individual patient that aims to evaluate and optimise patient medication by providing a recommendation or by making a direct change in prescriptions. We included trials comparing medication review with usual care or comparing two or more types of medication review.
We excluded:
trials aimed solely at increasing the patient's knowledge about current medication, improving adherence or reducing costs;
trials in which the results of medication review were to be implemented after discharge (e.g. letter to patient's general practitioner); and
trials reviewing only portions of a patient's medication related to a specific condition or to a single class of drugs (e.g. dealing only with diabetes or heart failure medication).
Types of outcome measures
Primary outcomes
Mortality (all‐cause).
Secondary outcomes
Hospital readmission (all‐cause).
Hospital readmission (due to adverse drug events).
Hospital emergency department contacts (all‐cause).
Hospital emergency department contacts (due to adverse drug events).
Mortality (due to adverse drug events).
Adverse drug events.
We included any trial that reported data on either primary or secondary outcomes.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases for trials.
The Cochrane Effective Practice and Organisation of Care (EPOC) Group Specialised Register, November 2014.
CENTRAL (The Cochrane Library, November 2014).
MEDLINE, 1946 to November 2014, In‐Process & Other Non‐indexed Citations, Ovid.
EMBASE, 1980 to November 2014, Ovid SP.
CINAHL (Cumulative Index to Nursing and Allied Health Literature), 1980 to November 2014, EbscoHost.
International Pharmaceutical Abstracts, 1970 to May 2015, Ovid.
The search strategies (Appendix 1) were developed for Ovid MEDLINE and were adapted for the other databases. We used the Cochrane RCT Sensitivity/Precision‐Maximizing Filter to limit our search to RCTs (Lefebvre 2011).
Searching other resources
We searched the reference lists of all included trials and relevant review articles for additional trials. We searched MEDLINE (PubMed, May 2015) for relevant papers by authors (first and last) of included trials, and Web of Science (ISI Web of Knowledge, May 2015) for papers that cited any of the included trials. We contacted content experts in the field and corresponding authors of included trials to identify additional trials.
Unpublished trials
To identify conference abstracts of unpublished trials, we searched EMBASE and International Pharmaceutical Abstracts as described above.
In addition, we searched the following clinical trial registries (May 2015) to identify unpublished and ongoing trials.
ClinicalTrials.gov, US National Institutes of Health (NIH), www.clinicaltrials.gov.
Current Controlled Trials, www.controlled‐trials.com.
International Clinical Trials Registry Platform (ICTRP), World Health Organization (WHO), www.who.int/trialsearch.
Data collection and analysis
Selection of studies
We (MC, AL) independently selected all trials for inclusion in two rounds. First, we screened titles and abstracts for potentially includable articles. Then we screened the full text of all potential articles for inclusion. We resolved disagreements by discussion.
Data extraction and management
We (MC, AL) independently and unblinded extracted data for all included trials . We resolved disagreements by discussion.
Data included:
study characteristics: author name, publication year, journal name, methods of randomisation;
participants: number of participants, country, age, gender, type of department, morbidities, medication history, inclusion and exclusion criteria;
intervention: description of medication review, profession of reviewer (pharmacist, physician, other), explanation of how medication could be changed (recommendation by letter to patient's attending physician, meeting between pharmacist and physician, assessment by physician with direct change of prescription);
control: any co‐interventions that could influence the change in prescription;
outcome: outcome assessor, timing of outcomes; and
other characteristics: funding source.
Assessment of risk of bias in included studies
We (MC, AL) independently and unblinded assessed each trial and outcome for risk of bias using the 'Risk of bias assessment' of The Cochrane Collaboration (Higgins 2011). In addition, we evaluated contamination bias (EPOC 2015) and assessed the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other biases. We resolved disagreements by discussion.
Measures of treatment effect
For dichotomous data, we used risk ratios (RRs), and for continuous data, we used mean differences (MDs).
Dealing with missing data
We contacted authors of all included trials by email requesting missing data. One study author provided us with raw data (Gillespie 2009), and one provided tabulated data for two trials (Lisby 2010; Lisby 2015).
Assessment of heterogeneity
We assessed statistical heterogeneity by using I2.
Assessment of reporting biases
We assessed publication bias by using a funnel plot for our primary outcome (all‐cause mortality).
Data synthesis
We analysed all data by performing intention‐to‐treat analysis using available case analysis. In some trial reports, patients who died in‐hospital were excluded from the analysed population, but we retained these patients in our analysis. With Review Manager 5 (RevMan 2012), we calculated pooled RRs and estimated 95% confidence intervals (CIs) by using the random‐effects model with the Mantel‐Haenszel method for dichotomous data. In our original review, we used a fixed‐effect model, but because of large clinical heterogeneity in settings, patient populations and methodology of medication reviews in identified trials, we used a random‐effects model for this update. We calculated absolute risk reduction and number needed to treat for an additional beneficial outcome for outcomes with a clinically significant effect for low‐risk and high‐risk populations (see Table 1). For continuous data, we calculated pooled MDs and estimated 95% CIs using the random‐effects model with the inverse variance method.
We used the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach to assess the overall certainty of evidence (Guyatt 2008). We constructed a Table 1 for mortality (all‐cause), hospital readmissions (all‐cause) and hospital emergency department contacts (all‐cause), as these were the most reliable and patient‐relevant outcomes.
Subgroup analysis and investigation of heterogeneity
We planned to explore our findings by performing the following prespecified subgroup analyses.
Trials including only patients with high risk of medication errors and adverse drug events (study inclusion and exclusion criteria defined patient population as a high‐risk population (e.g. elderly patients, patients with multiple co‐medications)).
Trials in which the medication review was performed by a person or team with the capability to change the patient's medication directly (as opposed to a medication review carried out by healthcare professionals who were not allowed to change the patient's medications, but who recommended changes to an in‐hospital tending physician).
Trials in which the medication review was done using a validated method (e.g. Beers’ criteria (Beers 1997), START/STOPP criteria (Gallagher 2008a)).
To avoid multiplicity issues, we restricted these analyses to the dichotomous outcomes of mortality (all‐cause), hospital readmissions (all‐cause) and hospital emergency department contacts (all‐cause).
Originally we planned to investigate the intervention effect by performing a sensitivity analysis of trials at low risk of bias. However, in keeping with recent recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011), and with the goal of testing for subgroup differences, we instead conducted a subgroup analysis to compare low risk of bias trials with high risk of bias trials. We defined low risk of bias trials as trials with low risk of selection bias, detection bias and selective reporting, and all other trials as having high risk of bias.
Sensitivity analysis
We intended to perform a sensitivity analysis of only cluster‐RCTs, but none of the identified trials were cluster‐randomised. For some trials and outcomes, the reported denominator was different from what was to be expected from available case analysis. We therefore performed another sensitivity analysis while assuming that data were available for all patients if otherwise not directly stated. To test the robustness of our findings, we performed a sensitivity analysis in which we reanalysed all outcomes by using a fixed‐effect instead of a random‐effects model.
Results
Description of studies
Results of the search
In this update, we identified 1953 references (Figure 1) through our searches. By reading titles and abstracts, we eliminated 1943 references, as they were not relevant to the review. We obtained full papers for 10 references, excluded seven studies (Ahmad 2012; Bondesson 2013; Bonnet‐Zamponi 2013; Connor 2012; Frankenthal 2014; Marusic 2013; Sjoberg 2013) and included three new trials (Bladh 2011; Dalleur 2014; Farris 2014). We included in this update one of the trials (Scullin 2007) excluded from our original review (Christensen 2013) on the basis of comments received after its publication.
1.
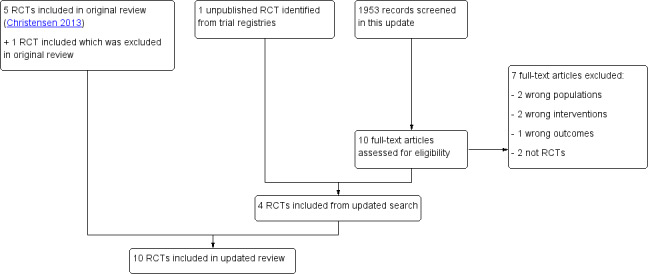
Study flow diagram.
We contacted the authors of two unpublished trials identified in our original review (ISRCTN08043800; NCT00844025). One trial author responded that the trial had not yet been submitted for publication (ISRCTN08043800), and the other trial author did not respond to our message. We identified four unpublished trials (Bonnerup 2014; Loffler ongoing; NCT01467128; NCT01504672) by searching trial registries. We contacted authors of all four trials and received a reply from all trial authors. One trial had finished enrolment (NCT01504672) but follow‐up data were still being collected, one trial was ongoing and completion was planned for November 2016 (Loffler ongoing) and one trial was finished and could be included, as the author supplied us with data that had been published in the form of a PhD thesis (Bonnerup 2014). As only participants with high risk of prescription error received a medication review intervention in the trial, we included only data from the subgroup of high‐risk participants in control and intervention groups. The last trial was finished and the manuscript had been submitted for publication (NCT01467128), but because of lack of resources, study authors had not collected follow‐up data and so the trial was excluded.
In summary, with five trials included in our original review, four newly identified trials and inclusion of one previously excluded trial, this review now includes 10 trials (see Characteristics of included studies).
Included studies
Setting
The ten trials included 3575 participants in total and reported follow‐up from 30 days to one year. Trial reports were published between 2006 and 2015; two studies were conducted in the USA (Farris 2014; Schnipper 2006) and the remaining eight in Europe (Belgium, Denmark, Ireland Northern Ireland and Sweden). Six trials included participants admitted to departments of internal medicine (Bladh 2011; Bonnerup 2014; Dalleur 2014; Gillespie 2009; Lisby 2010; Scullin 2007); one to departments of internal medicine, family medicine, cardiology and orthopaedics (Farris 2014); one to a tertiary medical centre admitted via the emergency department (Gallagher 2011); one to an orthopaedic ward (Lisby 2015); and one to the general medicines service (Schnipper 2006).
Participants
Five trials listed age as an inclusion criterion (two trials (Gallagher 2011; Lisby 2015) used 65 years or older, one used 70 years or older (Lisby 2010), one used 75 years or older (Dalleur 2014) and one used 80 years or older (Gillespie 2009). In general, participants were elderly with a mean age around 80 years in all trials except three, in which participant age was 59, 61 and 70 years, respectively (Farris 2014; Schnipper 2006; Scullin 2007). The proportion of women among included participants ranged from 53% to 71%, and the mean number of drugs per participant ranged from seven to 11.
Types of interventions
The medication review was performed by a pharmacist in four trials (Bladh 2011; Farris 2014; Gillespie 2009; Schnipper 2006), by a team of both pharmacists and pharmacy technicians in one (Scullin 2007), by a physician in two (Dalleur 2014; Gallagher 2011), by a pharmacist or a physician specialised in clinical pharmacology in one (Bonnerup 2014) and by a team of both pharmacists and physicians specialised in clinical pharmacology in two (Lisby 2010; Lisby 2015). In two trials the medication review was done using the validated Screening Tool of Older Persons’ potentially inappropriate Prescriptions (STOPP) (Dalleur 2014; Gallagher 2011); the latter trial also used the Screening Tool to Alert to Right Treatment (START). One trial described that the medication review was done via a computer decision support system (MiniQ) (Bladh 2011). In the remaining seven trials, the medication review was performed primarily by a pharmacist, who performed medication reconciliation and systematically reviewed the medication. In four trials, the medication review ended with a written recommendation to the prescribing physicians (Bonnerup 2014; Dalleur 2014; Lisby 2010; Lisby 2015); in three it was discussed with the prescribing physicians (Bladh 2011; Gallagher 2011; Schnipper 2006); and three provided no description of how recommendations were communicated to prescribing physicians (Farris 2014; Gillespie 2009; Scullin 2007). Seven trials provided additional interventions besides medication review for the intervention group. One trial included drug counselling (Lisby 2010); one included a discharge letter to the general practitioner (GP) (Dalleur 2014); one included drug counselling and a discharge letter to the GP (Bladh 2011); two included patient education, drug counselling, a discharge letter to the GP and telephone follow‐up (Farris 2014; Gillespie 2009); one included telephone follow‐up (Schnipper 2006); and one included a comprehensive integrated medicines management service including drug counselling and in‐patient monitoring (Scullin 2007). Two trials reported that the medication review resulted in a recommendation for drug changes for 58% (Gallagher 2011) and 60% of patients (Schnipper 2006). The proportion of suggested medication review recommendations that were followed by the prescribing physicians varied between trials: 18% (Lisby 2015), 39% (Lisby 2010), 41% (Bladh 2011), 65% (Bonnerup 2014),75% (Gillespie 2009) and 94% (Gallagher 2011).
Excluded studies
See Characteristics of excluded studies for the complete list of excluded studies with reasons.
Risk of bias in included studies
Risk of bias in the included trials is described in the Characteristics of included studies section (see Figure 2 and Figure 3 for graphical displays).
2.
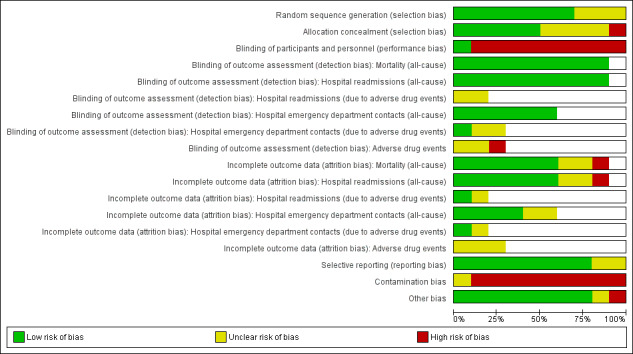
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. White spaces in this figure represent instances where it was not possible to make a judgement regarding objective or non‐objective outcomes.
3.
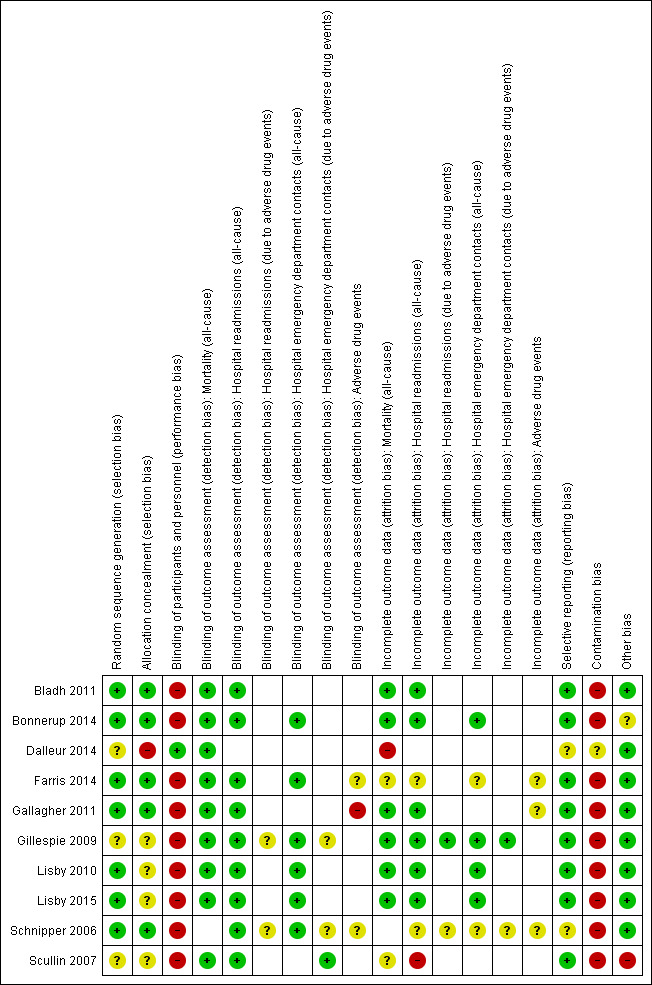
Risk of bias summary: review authors' judgements about each risk of bias item for each included study. White spaces in this figure represent instances where it was not possible to make a judgement regarding objective or non‐objective outcomes.
Allocation
All 10 trials were randomised at an individual level; two trials used fixed blocks of 10 in each group (Gillespie 2009; Scullin 2007), and one trial used random blocks of maximum 20 (Bonnerup 2014). Seven trials had adequate randomisation sequence generation, of which five also had adequate allocation concealment and thus low risk of selection bias (Bladh 2011; Bonnerup 2014; Farris 2014; Gallagher 2011; Schnipper 2006) and two did not describe who included participants; risk of selection bias was therefore unclear (Lisby 2010; Lisby 2015). Of the remaining three trials, two did not describe how the randomisation sequence was generated or who included participants (Gillespie 2009; Scullin 2007) but used a fixed block size, which could have led to deciphering of the sequence, thus conferring unclear risk of selection bias. The final trial did not adequately describe how the randomisation sequence was generated, but as a study nurse both generated the sequence and included participants, risk of selection bias was high (Dalleur 2014).
Blinding
Nine trials described directly or indirectly that participants or personnel were not blinded, leading to high risk of performance bias in all trials. The last trial randomised patients to two geriatric teams, with one doing medication reviews using STOPP criteria. As participants and personnel were unaware of which team used STOPP, risk of performance bias was low (Dalleur 2014).
Three trials were described as providing blinded assessment for readmissions (Farris 2014; Gillespie 2009; Schnipper 2006), and two for hospital emergency department contacts (Farris 2014; Schnipper 2006). However, we judged it unlikely that awareness of group assignments would lead to risk of detection bias for these objective outcomes. Both trials assessing hospital readmissions due to adverse drug events provided blinded outcome assessment (Gillespie 2009; Schnipper 2006), as did one trial assessing hospital emergency department contacts due to adverse drug events (Schnipper 2006); one study did not describe this (Gillespie 2009). Of the three trials assessing adverse drug events, two provided blinded outcome assessment (Farris 2014; Schnipper 2006) and the remaining one (Gallagher 2011) was not blinded and had high risk of detection bias. For trials with blinded assessment of hospital readmissions due to adverse drug events, hospital emergency department contacts due to adverse drug events and adverse events, we judged risk as unclear, as participants were aware of group assignment and had knowledge of the drug adverse event profile; this could have led to differences in reporting of adverse events.
Incomplete outcome data
Six of nine trials reporting on mortality had low risk of attrition bias: One trial (Gillespie 2009) described no loss to follow‐up, five (Bladh 2011; Bonnerup 2014; Gallagher 2011; Lisby 2010; Lisby 2015) did not describe loss to follow‐up and all data seem to have been available from registries. In contrast, one trial (Dalleur 2014) reported only mortality follow‐up data for 66 out of 158 participants, leading to high risk of attrition bias, and for two trials (Farris 2014; Scullin 2007), it was unclear whether mortality data for participants lost to follow‐up were available, leading to unclear risk of attrition bias. Nine trials reported other outcomes; of these, one trial (Gillespie 2009) described no loss to follow‐up for all outcomes, four trials measured outcomes that should be available in registry data (Bladh 2011; Bonnerup 2014; Lisby 2010; Lisby 2015), two trials (Farris 2014; Schnipper 2006) showed discrepancies between reported participants lost to follow‐up and participants excluded from analysis; thus, we judged risk as unclear. One trial (Scullin 2007) described loss to follow‐up of around 1% in the manuscript but around 9% on the basis of reported tabular data; thus it was judged as high risk. Finally, one trial (Gallagher 2011) measured outcomes by using registry data and contact with general practitioners and participants without reporting how often data were not available. As adverse events, such as falls, could lead to loss to follow‐up, we judged this outcome as unclear.
Selective reporting
We judged one trial (Schnipper 2006) as having unclear risk for selective reporting of all‐cause mortality, as it did not report on the outcome, although the data seem to have been available. Also one trial (Dalleur 2014) was unclear for selective reporting of all‐cause readmissions and emergency department contacts, as the co‐authors had previously assessed these outcomes in similar trials.
Other potential sources of bias
The funnel plot for all‐cause mortality showed no sign of publication bias (Figure 4). Nine trials were judged as having high risk of contamination bias, as they were not cluster‐randomised. One trial (Dalleur 2014), which was also randomised at the individual level, was judged as having unclear risk of contamination bias. The medication review intervention (i.e. applying STOPP criteria) was blinded to personnel, and study authors stated that contamination bias was thus avoided. However, intervention group recommendations may also have been applied to control patients at the same hospital. From one trial (Bonnerup 2014), we included only a subgroup of participants with high risk of prescription errors. Including only this subgroup confers unclear risk of bias, as assessment of risk score for prescription errors was performed after allocation to groups (i.e. may introduce unbalance). Finally, one trial (Scullin 2007) was judged as having high risk of bias in relation to data analysis. First, a difference of 20 participants was noted between treatment arms, and this should not have been possible because the trial randomised in blocks of 10 in each arm. Second, data from a surgical ward were excluded from the analysis without an explanation.
4.
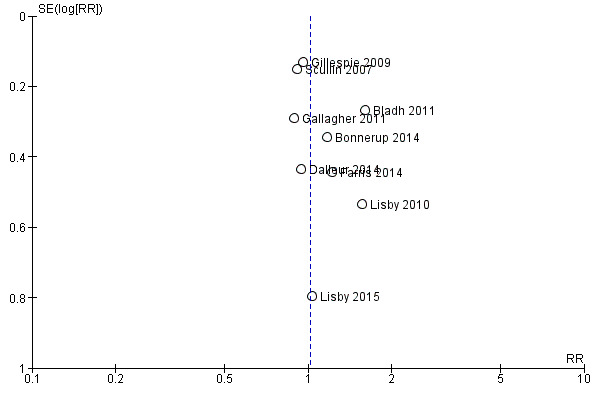
Funnel plot of comparison: 1 Primary outcome: 1.1 Mortality (all‐cause).
Effects of interventions
See: Table 1
See Table 1 for main comparisons.
Mortality (all‐cause)
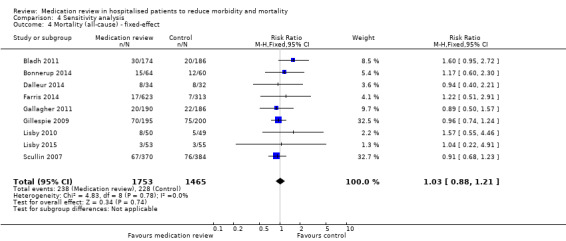
See Analysis 1.1. Nine trials with data from 3218 participants and follow‐up from three to 12 months reported all‐cause mortality. During follow‐up, 238 participants in the medication review group died as did 228 in the control group (RR 1.02, 95% CI 0.87 to 1.19) (low‐certainty evidence). We did not observe any heterogeneity.
1.1. Analysis.
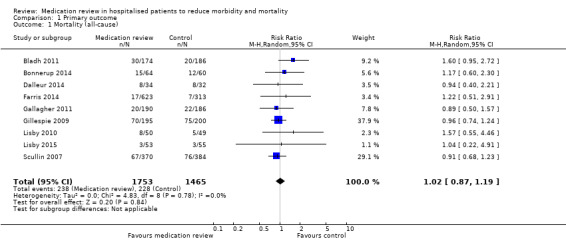
Comparison 1 Primary outcome, Outcome 1 Mortality (all‐cause).
Hospital readmissions (all‐cause)
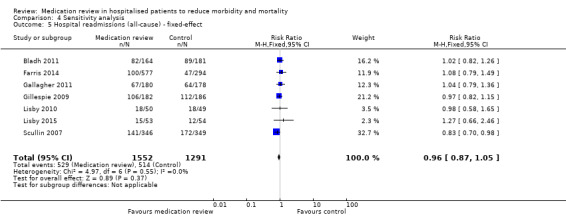
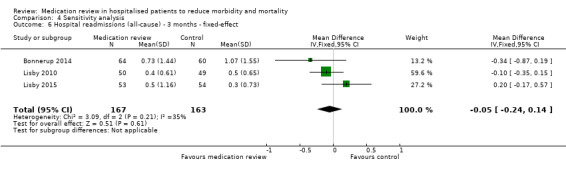
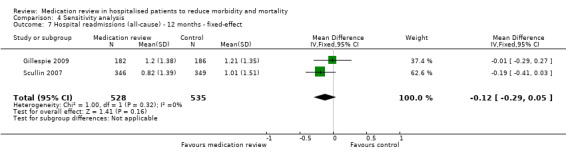
See Analysis 2.1; Analysis 2.2; and Analysis 2.3. Seven trials with data from 2843 participants and follow‐up from three to 12 months reported on hospital readmissions. During follow‐up, 529 participants in the medication review group and 514 in the control group had one or more hospital readmissions (RR 0.95, 95% CI 0.87 to 1.04) (high‐certainty evidence). We did not observe any heterogeneity. Three trials reported continuous data from 330 participants with three months of follow‐up. There was no difference in the number of readmissions per participant (mean difference (MD) ‐0.05, 95% CI ‐0.31 to 0.21; I2 = 35%). Two trials reported continuous data from 1063 participants with 12 months of follow‐up. There was no difference in the number of readmissions per participant (mean difference (MD) ‐0.12, 95% CI ‐0.29 to 0.05). We did not observe any heterogeneity.
2.1. Analysis.
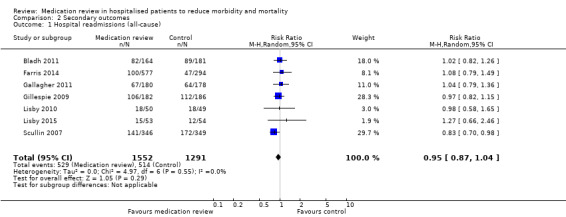
Comparison 2 Secondary outcomes, Outcome 1 Hospital readmissions (all‐cause).
2.2. Analysis.
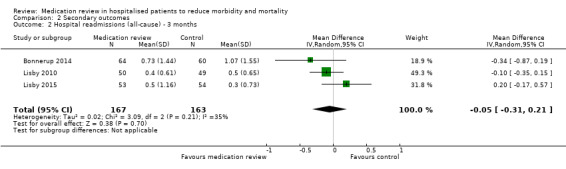
Comparison 2 Secondary outcomes, Outcome 2 Hospital readmissions (all‐cause) ‐ 3 months.
2.3. Analysis.
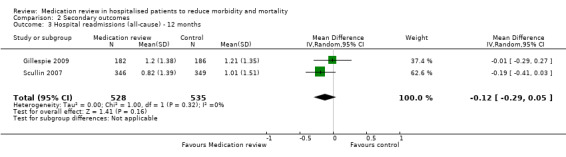
Comparison 2 Secondary outcomes, Outcome 3 Hospital readmissions (all‐cause) ‐ 12 months.
One trial (Schnipper 2006) with data from 176 participants and 30 days of follow‐up reported hospital readmissions and hospital emergency department contacts as a composite. During follow‐up, 28 participants in the medication review group and 25 in the control group had one or more hospital readmissions or hospital emergency department contacts (RR 1.02, 95% CI 0.65 to 1.61).
Hospital readmissions (due to adverse drug events)
One trial (Gillespie 2009) with data from 368 participants and 12 months of follow‐up reported on hospital readmissions due to adverse drug events. During follow‐up, nine participants in the medication review group and 33 in the control group had one or more hospital readmissions due to adverse drug events, yielding a relative risk reduction of 72% favouring medication review (RR 0.28, 95% CI 0.14 to 0.57). The trial also reported continuous data and found fewer readmissions due to adverse drug events per participant in the medication review group (MD ‐0.19, 95% CI ‐0.28 to ‐0.10).
One trial (Schnipper 2006) with data from 176 participants and 30 days of follow‐up reported hospital readmissions and hospital emergency department contacts due to adverse drug events as a composite. During follow‐up, four participants in the medication review group and seven in the control group had one or more hospital readmissions or hospital emergency department contacts due to adverse drug events (RR 0.52, 95% CI 0.16 to 1.72).
Hospital emergency department contacts (all‐cause)
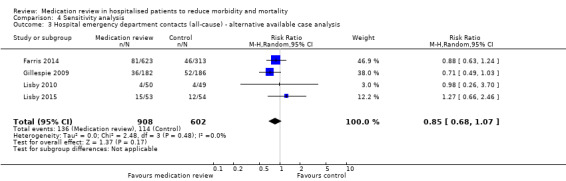
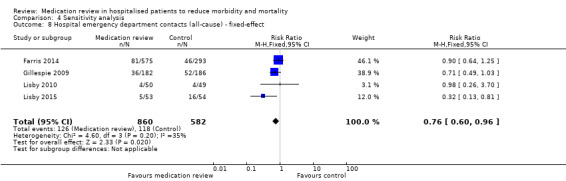
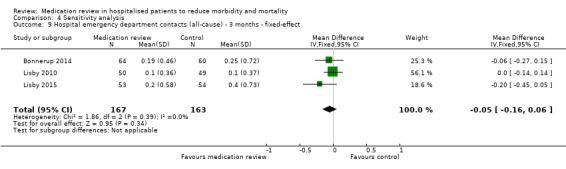
See Analysis 2.4 and Analysis 2.5. Four trials with data from 1442 participants and follow‐up from three to 12 months reported on hospital emergency department contacts. During follow‐up, 126 participants in the medication review group and 118 in the control group had one or more contacts, yielding a relative risk reduction of 27% favouring medication review (RR 0.73, 95% CI 0.52 to 1.03) (low‐certainty evidence; I2= 35%). Three trials reported continuous data from 330 participants with three months of follow‐up. Data show no difference in the number of emergency department contacts per participant (MD ‐0.05, 95% CI ‐0.16 to 0.06). We did not observe any heterogeneity. One trial (Gillespie 2009) reported continuous data from 368 participants with 12 months of follow‐up. There were fewer emergency department contacts per participant in the medication review group (MD ‐0.23, 95% CI ‐0.43 to ‐0.03).
2.4. Analysis.
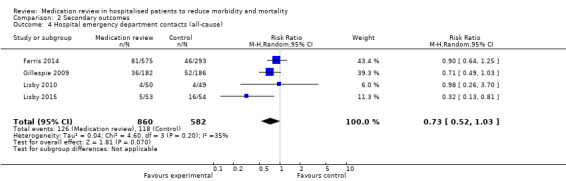
Comparison 2 Secondary outcomes, Outcome 4 Hospital emergency department contacts (all‐cause).
2.5. Analysis.
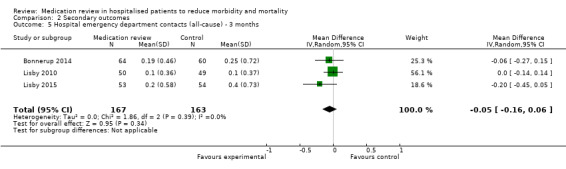
Comparison 2 Secondary outcomes, Outcome 5 Hospital emergency department contacts (all‐cause) ‐ 3 months.
Hospital emergency department contacts (due to adverse drug events)
One trial (Gillespie 2009) of 368 participants with 12 months of follow‐up reported on emergency department contacts due to adverse drug events. During follow‐up, four participants in the medication review group and nine in the control group had one or more contacts due to adverse drug events (RR 0.45, 95% CI 0.14 to 1.45). The trial also reported continuous data and found no differences in emergency department contacts due to adverse drug events (MD ‐0.03, 95% CI ‐0.07 to 0.01).
Mortality (due to adverse drug events)
No trials reported data for this outcome.
Adverse drug events
One trial (Schnipper 2006) with data from 152 participants and 30 days of follow‐up reported adverse drug events. During follow‐up, 14 participants in the medication review group and 12 in the control group had one or more adverse drug events (RR 1.08, 95% CI 0.53 to 2.18). One trial (Gallagher 2011) with data from 382 participants and six months of follow‐up reported on falls as an adverse drug event. During follow‐up, 11 participants in the medication review group and 16 in the control group had one or more falls (RR 0.69, 95% CI 0.33 to 1.46). One trial (Farris 2014) reported on adverse events and adverse drug events as a composite. We were unable to get separate data on adverse drug events from the author and did not include data for this outcome.
Subgroup analysis and investigation of heterogeneity
Six trials used age as an inclusion criterion (three older than 65, one older than 70, one older than 75 and one older than 80 years), two used number of drugs as an inclusion criterion (minimum four drugs) and one included patients for medication review if they had high risk of prescription errors based on an algorithm. Three trials (Bladh 2011; Farris 2014; Schnipper 2006) did not have any risk factors for medication errors and adverse drug events as part of the inclusion criteria. Reporting of the data in one trial (Schnipper 2006) precluded its inclusion in the subgroup analysis.
Comparison between subgroups revealed a small trend toward increased mortality in the low‐risk group compared with the high‐risk group (P value = 0.08) (Analysis 3.1), but no differences in effects on readmissions (Analysis 3.2) or on hospital emergency department contacts (Analysis 3.3).
3.1. Analysis.
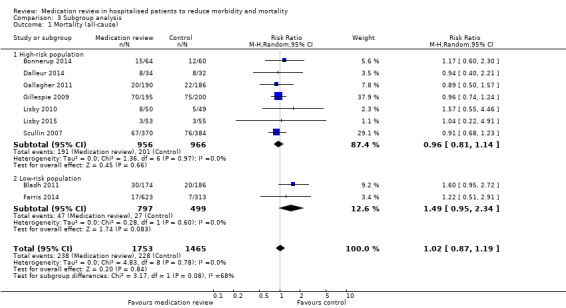
Comparison 3 Subgroup analysis, Outcome 1 Mortality (all‐cause).
3.2. Analysis.
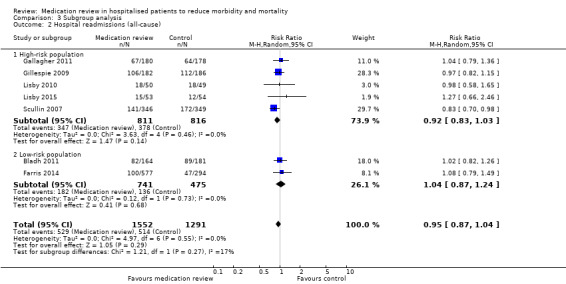
Comparison 3 Subgroup analysis, Outcome 2 Hospital readmissions (all‐cause).
3.3. Analysis.
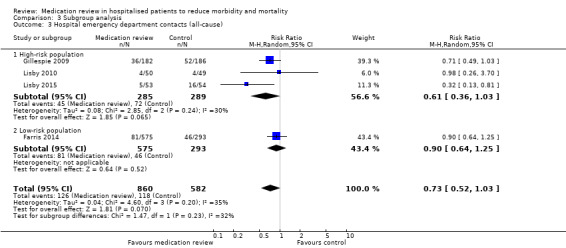
Comparison 3 Subgroup analysis, Outcome 3 Hospital emergency department contacts (all‐cause).
In none of the trials was the person or team performing the medication review allowed to change the medication directly; therefore, we could not explore this in a separate subgroup analysis.
In two trials (Dalleur 2014; Gallagher 2011), medication review was performed through validated methods. Comparison between subgroups with and without validated methods revealed no difference in effect (Analysis 3.4; Analysis 3.5).
3.4. Analysis.
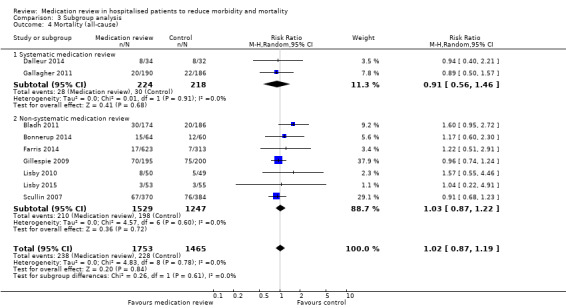
Comparison 3 Subgroup analysis, Outcome 4 Mortality (all‐cause).
3.5. Analysis.
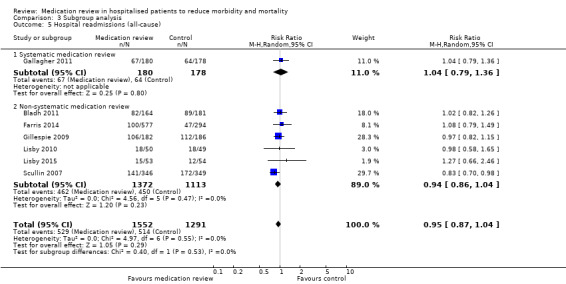
Comparison 3 Subgroup analysis, Outcome 5 Hospital readmissions (all‐cause).
We judged four trials (Bladh 2011; Bonnerup 2014; Farris 2014; Gallagher 2011) as having low overall risk of bias. Comparison between subgroups of trials with low overall risk of bias and with high overall risk of bias revealed no difference in effect (Analysis 3.6; Analysis 3.7; Analysis 3.8).
3.6. Analysis.
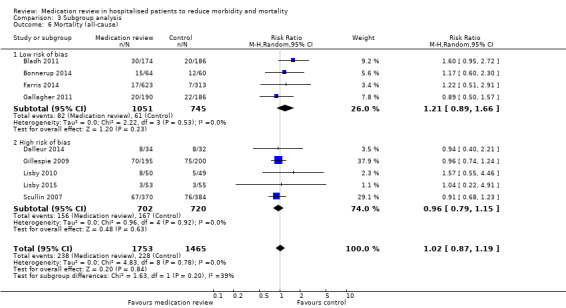
Comparison 3 Subgroup analysis, Outcome 6 Mortality (all‐cause).
3.7. Analysis.
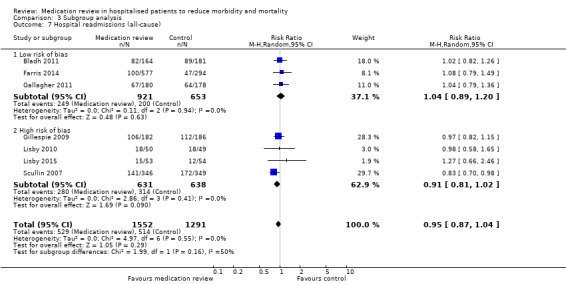
Comparison 3 Subgroup analysis, Outcome 7 Hospital readmissions (all‐cause).
3.8. Analysis.
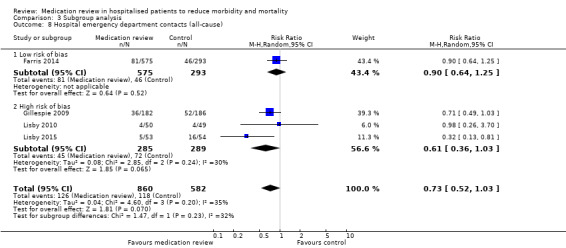
Comparison 3 Subgroup analysis, Outcome 8 Hospital emergency department contacts (all‐cause).
Sensitivity analysis
For some trials, we could calculate a different available case analysis for mortality and readmission outcomes, but our reanalysis was similar to our main analysis (Analysis 4.1; Analysis 4.2; Analysis 4.3). Our reanalysis of all outcomes based on a fixed‐effect model did not change our results (Analysis 4.4; Analysis 4.5; Analysis 4.6; Analysis 4.7; Analysis 4.8; Analysis 4.9).
4.1. Analysis.
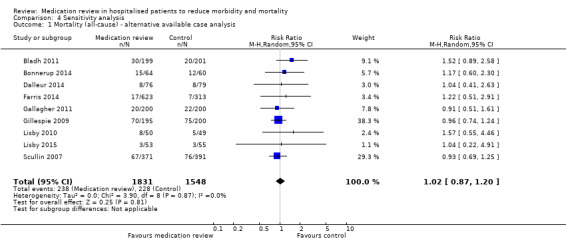
Comparison 4 Sensitivity analysis, Outcome 1 Mortality (all‐cause) ‐ alternative available case analysis.
4.2. Analysis.
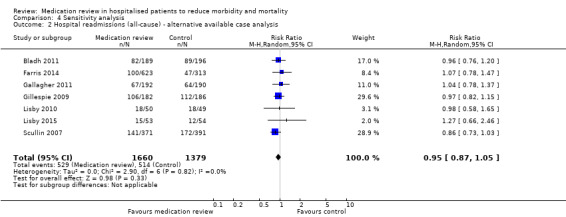
Comparison 4 Sensitivity analysis, Outcome 2 Hospital readmissions (all‐cause) ‐ alternative available case analysis.
4.3. Analysis.
Comparison 4 Sensitivity analysis, Outcome 3 Hospital emergency department contacts (all‐cause) ‐ alternative available case analysis.
4.4. Analysis.
Comparison 4 Sensitivity analysis, Outcome 4 Mortality (all‐cause) ‐ fixed‐effect.
4.5. Analysis.
Comparison 4 Sensitivity analysis, Outcome 5 Hospital readmissions (all‐cause) ‐ fixed‐effect.
4.6. Analysis.
Comparison 4 Sensitivity analysis, Outcome 6 Hospital readmissions (all‐cause) ‐ 3 months ‐ fixed‐effect.
4.7. Analysis.
Comparison 4 Sensitivity analysis, Outcome 7 Hospital readmissions (all‐cause) ‐ 12 months ‐ fixed‐effect.
4.8. Analysis.
Comparison 4 Sensitivity analysis, Outcome 8 Hospital emergency department contacts (all‐cause) ‐ fixed‐effect.
4.9. Analysis.
Comparison 4 Sensitivity analysis, Outcome 9 Hospital emergency department contacts (all‐cause) ‐ 3 months ‐ fixed‐effect.
Discussion
Summary of main results
We found no evidence suggesting that medication review reduces mortality (low‐certainty evidence) or hospital readmissions (high‐certainty evidence), but found that medication review may reduce the number of emergency department contacts compared with standard care (low‐certainty evidence). The estimated reduction in emergency department contacts of 27% (with a confidence interval ranging from 48% reduction to 3% increase in contacts) corresponds to a number needed to treat (to prevent one emergency department contact) of 37 for a low‐risk population and 12 for a high‐risk population over one year. The specific type of medication review provided did not seem to influence the results. Despite consistent results suggesting no effect, a beneficial or detrimental effect on mortality or readmissions cannot be ruled out because estimates were uncertain and follow‐up was short.
Overall completeness and applicability of evidence
This review focused primarily on patient‐relevant outcomes such as mortality, readmissions and emergency department contacts. Adverse drug events are often linked causally to all of these outcomes (Budnitz 2011; Hallas 1996), and an intervention reducing the inappropriateness of patient medication would, in contrast to our findings, be assumed to have a beneficial affect on all. Therefore, the possible intervention effect on emergency department contacts could be considered somewhat paradoxical, when no effect on readmissions was observed. This discrepancy might be explained by the large beneficial effect particularly in one trial (Gillespie 2009), which was crucial for the observed pooled estimate. However, it is possible that emergency department contacts represent a more sensitive outcome measure of adverse drug events than readmission or mortality, or that the duration of follow‐up simply was too short to reveal an effect on these outcomes. Along these lines, it is important to note that medication review as a general rule of thumb includes the addition of relevant prophylactic medicines (e.g. statins, antihypertensives), which mainly confer beneficial effects on hospital admissions and mortality after several years of treatment (Gutierrez 2012; Wright 2009). Thus, beneficial effects of a reduction in inappropriate underprescribing will likely not have an effect on admission and mortality in studies with shorter follow‐up. Whereas the occurrence or prevention of adverse drug events would be expected to have a shorter time frame, longer follow‐up in these trials is thus crucial for a true evaluation of the effects of medication review.
The size of the suggested effect on emergency department contacts depends on baseline risk in the population receiving the medication review. All trials included older participants receiving multiple medications and, based on the trial populations, had numbers needed to treat for an additional beneficial outcome of 12 for a high‐risk population and 37 for a low‐risk population to prevent one emergency department contact over one year. As medication review is time‐consuming, the question is whether the intervention is cost‐effective. In one trial, authors estimated the costs of the medication review as USD 170 per patient (Gillespie 2009). If their figure is used as an estimate of the costs, it would cost between USD 2040 and USD 6290 to avoid one emergency department contact in a year. However, in addition to possible cost‐savings from reducing the number of emergency department contacts, a reduction in unnecessary medications could reduce costs. A future cost‐effectiveness analysis based on data from our systematic review could clarify in which subpopulations medication review is cost‐effective.
The two trials (Gillespie 2009; Schnipper 2006) reporting emergency department contacts or readmissions attributable to adverse drug events stipulated that medication review conferred sizeable reductions in these outcomes. In the trial by Gillespie et al, participants receiving medication review had their risk of drug‐related readmissions lowered by 72% (nine vs 33 participants), but this was not reflected by a similar absolute reduction in participants with all‐cause readmissions (106 vs 112 participants). This difference perhaps illustrates some of the problems associated with the causality assessment of adverse drug events. Adverse events are rarely drug specific but are often general symptoms or illnesses that could have many causes (e.g. dizziness or gastric ulcer). In the context of polypharmacy, it is easy to associate medicines with symptoms, particularly if the drug is inappropriate and the symptom is a known adverse effect of that drug. It follows that any intervention that results in patients taking fewer inappropriate drugs may, solely by reducing the possibility of attributing a symptom to a drug, lead to fewer of these assessed drug‐related outcomes. Additionally, despite the fact that outcome assessors were blinded to group assignments, participants' knowledge of assignments could have resulted in unmasking during the participant interview, thereby introducing detection bias. Another complex issue is that the medication review resulting in discontinuation of medicines might lead to alleviation of adverse events at the expense of undertreatment of certain conditions. For example, less use of antihypertensive agents could lead to fewer readmissions due to dizziness but more readmissions due to stroke. The effect of medication review on readmissions due to adverse drug events (with no effect on all‐cause readmissions) should therefore be viewed with caution.
Previous admissions are a major risk factor for subsequent admissions (Epstein 2011; Hasan 2010; Marcantonio 1999). The decision to exclude trials of medication review performed in primary care, outpatient clinics, emergency departments or paediatric departments was taken to limit the study population to patients with demonstrated high risk of hospital admissions. In general, trial populations consisted of elderly participants receiving polypharmacy and with multimorbidity. The 10 included trials differed slightly with regards to the content of the medication review. Applying explicit criteria for reviewing medication could improve the applicability and reviewer independency of study findings (Dalleur 2014; Gallagher 2011), whereas interventions depending on few reviewers using unvalidated methods (Farris 2014; Gillespie 2009; Lisby 2010; Lisby 2015; Schnipper 2006) may introduce problems in relation to generalisability. Furthermore, co‐interventions such as telephone contact with patients or general practitioners (Bladh 2011; Dalleur 2014; Farris 2014; Gallagher 2011; Schnipper 2006) are resource demanding, and the added effect of including them as part of the medication review is not known. We chose to exclude trials of interventions aimed solely at increasing patient knowledge or adherence, interventions that were to be implemented after discharge, or interventions in which the medication review was related to only a portion of a participant's medication, because such interventions might have a lesser effect on clinical outcomes, thereby introducing heterogeneity.
Medication review may have an impact on other outcomes such as number of drugs prescribed, adherence, drug knowledge and/or patient satisfaction. However, these outcomes do not capture the potentially harmful effects of medication review resulting from undertreatment and because of their subjective nature are more prone to bias; therefore, we excluded them from our review.
Quality of the evidence
Our review was based on a very comprehensive literature search and was further strengthened by the inclusion of unpublished data. We included data from 10 trials with around 3600 participants, most of whom had a presumed high risk of adverse drug events.
However, some limitations must be considered. Most included trials had some problems related to risk of bias, and some had problems due to inadequate reporting. The nature of the intervention precluded blinding of participants, which may introduce performance bias. Similarly, as previously stated, it can be questioned whether detection bias for the drug‐related outcomes can actually be prevented. We judged outcomes to have low risk of bias when outcome assessors were unaware of group assignment, but whether this was sufficient to prevent detection bias is debatable. Two trials described drug counselling, which may in some form have taken place as part of the medication review in the other trials. Knowledge of adverse events from prescribed drugs, for example, dizziness from antihypertensive agents, may lead participants to focus on these problems when presenting a broader problem during an emergency department contact or readmission. This may result in underestimation of the intervention effect on adverse drug events. However, it could also work the other way around, as participants knowing the adverse events of a drug would not focus on those particular symptoms during the participant interview.
As described previously, the effect on all‐cause outcomes is therefore preferred as the result of lower risk of bias. Although mortality was not the primary outcome in any of the trials, only one trial did not report on this outcome (Schnipper 2006). Our funnel plot showed no sign of publication bias. In all trials, medication review was delivered by a special team of dedicated persons, and participants in control groups were treated by the same healthcare providers as were those in the intervention groups. Although it seems unlikely that participants in control groups should have received a similar intervention, some contamination bias might have occurred (e.g. increasing physicians' and nurses' focus on appropriate pharmacotherapy), thereby introducing bias towards the null. Remarkably, we did not identify any cluster‐randomised controlled trials of medication review, which by their design could minimise contamination bias.
Trials were conducted in different settings and employed different co‐interventions. The longest follow‐up was one year and was assessed in only two of the trials (Gillespie 2009; Scullin 2007). The short duration of follow‐up should be a caveat when interpreting the results of this review, while bearing in mind that many drugs are used for preventive purposes to avoid long‐term events (e.g. cardiovascular mortality). Likewise, the confidence intervals included both possible beneficial and harmful effects of the intervention, making any conclusions about the effects uncertain. However, the narrow confidence intervals suggest that any effect is likely to be small.
Agreements and disagreements with other studies or reviews
We attempted to examine the effects of medication review on hospitalised adult patients. A recent review (Hohl 2015) failed to identify an effect of early in‐hospital pharmacist‐led medication review on health outcomes. In contrast to our analyses, the review included only trials in which medication review was initiated within 24 hours of emergency department presentation or within 72 hours of admission, and also included trials in which medication review was investigated using quasi‐randomised methods. A systematic review (Holland 2008) that included patients from both primary and secondary care, of younger age and receiving fewer drugs on average, found no effects of medication review on mortality and readmissions. Likewise, two systematic reviews (Nkansah 2010; Royal 2006) included trials with participants at lower risk, and reported no effects on mortality and readmissions. A recent systematic review (Wallerstedt 2014) assessed medication review for nursing home residents and found no beneficial effect on mortality nor on hospitalisation.
Authors' conclusions
Implications for practice.
The likely beneficial effect of medication review for preventing emergency department contacts provides an argument for implementing medication review for elderly hospitalised patients (e.g. as part of geriatric care). Despite increased use of medication review in recent years, we advocate that further research should be conducted before staff members are employed to undertake medication review. First, we do not know in which form or for which patients medication reviews are most effective. Second, we do not know the long‐term treatment effects of the intervention. And third, we do not know whether medication reviews are actually cost‐effective. Thus, if medication reviews are implemented, this should be done in the context of rigorous evaluation.
Implications for research.
On the basis of available data, we cannot exclude the possibility of a beneficial or harmful effect of medication review on mortality and on hospital contacts or readmissions. Trials generally had short follow‐up and, because many used registry data for assessment of mortality and readmissions, follow‐up studies of these trials are strongly urged. Implementation of recommendations (i.e. actual changes in prescriptions) were highly variable among trials. We recommend that future trials focus on high‐risk populations, ensure that the team performing the medication review includes members who are allowed to change patient medications, use well‐described methods when conducting the medication review, have long‐term follow‐up and randomise on a cluster level. Future trials preferably could use a factorial design to assess the effects of various co‐interventions included in medication review trials, for example, medicines counselling, telephone follow‐up and information on the patient’s general practitioner.
What's new
| Date | Event | Description |
|---|---|---|
| 26 August 2015 | New citation required but conclusions have not changed | Five new trials were added; the review now describes 10 included trials. We included contamination bias as a domain in the risk of bias assessment |
| 18 November 2014 | New search has been performed | New searches were performed; 5 new trials were identified |
History
Protocol first published: Issue 2, 2011 Review first published: Issue 2, 2013
| Date | Event | Description |
|---|---|---|
| 4 March 2014 | Feedback has been incorporated | Minor amendments were made |
Acknowledgements
We thank Michelle Fiander (Trials Search Co‐ordinator) at the EPOC group for developing the search strategy and identifying studies for our initial review, and Sharlini Yogasingam (Assistant Trials Search Co‐ordinator) and Tamara Rader (Trials Search Co‐ordinator) at the EPOC group for assisting with our update. We thank Daphna Y Stark for translation of an article written in Hebrew for our initial review. We thank Dorthe Krogsgaard Bonnerup, Hans Garmo, Marianne Lisby, Ulrika Gillespie and their co‐authors for supplying us with additional trial data.
Appendices
Appendix 1. Search strategies
MEDLINE
Ovid MEDLINE <update 18 November 2014>
1 Pharmacy service, hospital/ [ML]
2 ((PHARMACEUTICAL CARE or PHARMACY or PHARMACIES or PHARMACIST? or PRESCRIBING) and (inpatient? or hospital$ or WARD? or UNIT or UNITS)).ti.
3 ((PHARMACEUTICAL CARE or PHARMACY or PHARMACIES or PHARMACIST? or PRESCRIBING) adj2 (inpatient? or hospital$ or WARD? or UNIT or UNITS)).ab.
4 Medication Systems, Hospital/ [ML]
5 ((medication? or prescribing or prescription? or dispensing) adj2 system?).ti,ab. and (hospital$ or WARD or WARDS or (CARE adj2 UNIT?) or INPATIENT?).ti,hw.
6 (stopp or beer's criteria).ti,ab. [Term added Aug 2011]
7 or/1‐6 [Hosp Pharm/Med Systems]
8 exp Hospitals/ or exp Hospital Units/ [ML]
9 (hospital$ or WARD or WARDS).ti.
10 Hospitalization/ [ML]
11 hospital$.ab.
12 "length of stay"/ or Patient admission/ or Patient discharge/ or Patient readmission/ or Patient transfer/ [ML]
13 ((patient? or hospital$).ti,hw. and (discharg$ or admission? or admitting or readmission? or readmit$ or transfer?).ti.) or "length of stay".ti.
14 (((patient? or hospital?) adj2 (discharg$ or admission? or admitting or readmission? or transfer?)) or "length of stay").ab.
15 Inpatients/ [ML]
16 (inpatient? or in‐patient?).ti.
17 exp HOSPITAL DEPARTMENTS/ or HOSPITAL SHARED SERVICES/ [ML]
18 MEDICAL STAFF, HOSPITAL/ or HOSPITALISTS/ [ML]
19 or/8‐18 [Hospitals/Hospitalization/Inpatients]
20 (pharmacy or pharmacies or pharmacist? or prescription? or prescribing).ti.
21 (pharmacist‐led or pharma$ initiated or ((driven or lead or led) adj2 pharmacist?)).ab.
22 (PRESCRIBING adj2 PATTERN?).ab.
23 ("physician‐pharmacist?" or "doctor‐pharmacist?").ti,ab.
24 ((IMPROV$ or OPTIMI?ING or OPTIMI?E? or OPTIMAL$) and (DOSING or DOSAGE or PHARMAC$ or PRESCRIB$ or PRESCRIPT$)).ti. or ((IMPROV$ or OPTIMI?ING or OPTIMI?E? or OPTIMAL$) adj2 (PHARMACEUTICAL CARE or PHARMACY or PRESCRIB$ or PRESCRIPT$)).ab.
25 ((pharmaceutical adj (care or consult$)) or (pharmacist? adj2 (care or consult$ or intervention? or managed))).ab.
26 (((prescription? or prescribing or medication?) adj4 review$) or (pharmacist? adj2 review$)).ti,ab.
27 ((drug therapy or drug regime? or medication? or medicineS or pharmacy or pharmacist? or pharmaceutical or PRESCRIB$ or prescription?) adj2 (audit$ or monitor$ or RECONCIL$ or review?)).ti,ab.
28 ((medication? or prescrib$ or pharmac$) adj2 (manage? or management or service? or system?)).ti,ab.
29 (("drug therapy" or dosage? or dose? or medication? or PRESCRIPTION? or PRESCRIB$ or PHARMACIST? or PHARMACEUTICAL CARE) adj2 (managing or management or monitor$)).ti,ab.
30 (drug? review? or drug? assess$ or drug? audit? or drug? reconcil$).ti,ab.
31 ("drug utili?ation" adj2 (review? or reconcil$ or audit?)).ab. or ("drug utili?ation" and (review? or reconcil$ or audit?)).ti.
32 Medication adherence/ [ML]
33 Pharmacists/ or Pharmacists' Aides/ [ML]
34 Pharmaceutical Services/ or Drug Information Services/ [ML]
35 Clinical Pharmacy Information Systems/ [ML]
36 Prescriptions/ or Drug Prescriptions/ or Pharmaceutical Preparations/ or Drug Therapy/ or Drug Dosage Calculations/ or Electronic Prescribing/ or Medication Systems/ [ML]
37 Drug Monitoring/ or Medication Therapy Management/ [ML]
38 Drug Therapy/ or Drug Therapy, Computer‐Assisted/ [ML]
39 POLYPHARMACY/ or POLYPHARM$.ti. [ML]
40 MEDICATION ERRORS/ [ML]
41 Drug utilization review/ [ML]
42 Drug Utilization/ [ML]
43 inappropriate prescribing/ [Term added Aug 2011]
44 ((Medication? or prescrib$ or prescription? or drug therap$) adj2 assessment?).ti,ab. [Term added Aug 2011]
45 (inappropriate$ adj2 (medicine? or medication? or prescrib$ or drug?)).ti,ab. [Term added Aug 2011]
46 or/20‐45 [PHARMA/DRUG CONCEPTS ‐‐combine with hospital concepts]
47 (randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.
48 exp animals/ not humans.sh.
49 47 not 48 [Cochrane RCT Filter 6.4.d Sens/Precision Maximizing]
50 7 and 49 [Hosp Pharma & RCT]
51 19 and 46 and 49 [Hospitals & Pharma/Drug sets & RCT]
52 50 or 51
53 limit 52 to yr="1980 ‐Current"
54 (2012$ or 2013$ or 2014$).ed,ep,dp. [Entry date, E‐pub date, Pub Date]
55 (198$ or 199$ or 2$).ep. [Electronic publication date 1980 to present]
56 (201108$ or 201109$ or 20111$).ed,dp. [August 2011‐Dec2011]
57 52 and 54
58 (52 and 55) not 57
59 (52 and 56) not (or/57‐58)
60 52 and 2011$.dp,ep,yr,ed. [2011 all date search]
61 60 not (or/57‐59)
62 57 or 58 or 59 or 61 [Results to export Jan 7 2013 update search]
63 remove duplicates from 62
EMBASE
Ovid EMBASE <update 18 November 2014>
1 *hospital pharmacy/ not outpatient?.ti. [EM]
2 hospital? pharmacy.ti.
3 ((pharmaceutical care or pharmacist? or prescribing) adj4 (inpatient? or hospital$ or ward? or ICU or intensive care or (emergency adj2 (room? or department? or unit or units)))).ti.
4 ((pharmaceutical care or pharmacist? or prescribing) adj3 (inpatient? or hospital$ or ward? or ICU or intensive care or (emergency adj2 (room? or department? or unit or units)))).ab.
5 ((medication? or prescribing or prescription? or dispensing) adj2 system?).ti,ab. and (hospital$ or ward or wards or (care adj2 unit?) or inpatient?).ti,hw.
6 (medication? adj4 (review$ or audit$)).ti. and (hospital$ or ward or wards or (care adj2 unit?) or inpatient?).ti,hw.
7 (stopp or beer's criteria).ti,ab. [Term added Aug 2011]
8 or/1‐7 [Hosp Medication Rev or Hosp Pharm‐‐combine with Filters]
9 ((medication? or medicine?) adj4 (review or audit)).ti.
10 ((medication? or medicine?) adj2 (review or audit)).ab.
11 (((prescription? or prescribing) adj4 review$) or (pharmacist? adj2 review$)).ti,ab.
12 ((drug formulary or drug therapy or drug regime? or medication? or medicines or pharmacy or pharmacist? or pharmaceutical or prescrib$ or prescription?) adj3 (audit$ or monitor$ or reconcil$)).ti,ab.
13 (drug? review? or drug? assess$ or drug? audit? or drug? reconcil$).ti,ab.
14 ("drug utili?ation" adj2 (reconcil$ or audit?)).ab. or ("drug utili?ation" adj4 (reconcil$ or audit?)).ti. [line moved]
15 inappropriate prescribing/ [Term added Aug 2011]
16 ((Medication? or prescrib$ or prescription? or drug therap$) adj2 assessment?).ti,ab. [Term added Aug 2011]
17 (inappropriate$ adj2 (medicine? or medication? or prescrib$ or drug?)).ti,ab. [Term added Aug 2011]
18 or/9‐17 [Medication Review/Audit]
19 exp *Hospital/ [EM]
20 exp *Ward/ [EM]
21 (hospital$ or WARD or WARDS).ti.
22 *Hospitalization/ [EM]
23 *Hospital care/ or *Intensive care/ [EM]
24 *"length of stay"/ or *hospital admission/ or *Hospital discharge/ or *Hospital readmission/ or *Patient transport/ [EM]
25 (((patient? or hospital$) and (discharg$ or admission? or admitting or readmission? or readmit$ or transfer?)) or "length of stay").ti.
26 (((patient? or hospital?) adj2 (discharg$ or admission? or admitting or readmission? or transfer?)) or "length of stay").ab.
27 *hospital patient/ [EM]
28 (inpatient? or in‐patient?).ti.
29 *Hospital service/ [EM]
30 *Hospital personnel/ or *Hospital physician/ or *Medical staff/ or *Resident/ [EM]
31 or/19‐30 [Hospitals/Hospitalization/Inpatients]
32 (pharmacy or pharmacies or pharmacist? or prescription? or prescribing).ti.
33 (pharmacist‐led or pharma$ initiated or ((driven or lead or led) adj2 pharmacist?)).ab.
34 (prescribing adj2 pattern?).ab.
35 ("physician‐pharmacist?" or "doctor‐pharmacist?").ti,ab.
36 ((improv$ or optimi?ing or optimi?e? or optimal$) and (dosing or dosage or pharmac$ or prescrib$ or prescript$)).ti. or ((improv$ or optimi?ing or optimi?e? or optimal$) adj2 (pharmaceutical care or pharmacy or prescrib$ or prescript$)).ab.
37 ((pharmaceutical adj (care or consult$)) or (pharmacist? adj2 (care or consult$ or intervention? or managed))).ab.
38 ((medication? or prescrib$ or pharmac$) adj2 (manage? or management or service? or system?)).ti,ab.
39 (("drug therapy" or dosage? or dose? or medication? or PRESCRIPTION? or PRESCRIB$ or PHARMACIST? or PHARMACEUTICAL CARE) adj2 (managing or management or monitor$)).ti,ab. (11654)
40 *Patient compliance/ and (medication? or pharmac$ or drug? or prescrib$ or prescription?).ti.
41 *Pharmacist/ or *Pharmacy technician/ [EM]
42 *Pharmaceutical care/ [EM]
43 *medical information system/ and (medication? or pharmac$ or drug? or prescrib$ or prescription?).ti,hw. [EM]
44 *Prescription/ [EM]
45 *Medication therapy management/ or *Recommended drug dose/ or *Optimal drug dose/ [EM]
46 *Polypharmacy/ or POLYPHARM$.ti. [EM]
47 *Medication error/ [EM]
48 *"drug use"/ [EM]
49 *Drug utilization/ [EM]
50 *DRUG FORMULARY/
51 or/32‐50 [Pharmacy/Prescribing/Med Use]
52 medical audit/
53 *medical audit/ or *monitoring/ [EM]
54 monitoring/
55 (audit? or monitoring or reconcil$).ti.
56 or/52,54‐55 [Monitoring/Audit broad]
57 randomized controlled trial/ or controlled study/ or controlled clinical trial/ [EM]
58 pretest posttest control group design/
59 clinical study/ or major clinical study/ or clinical trial/
60 multicenter study/
61 random$.ti. or (randomi?ed or randomly).ab. or controlled.ti.
62 (clinical study/ or major clinical study/ or clinical trial/) and random$.ti.
63 crossover‐procedure/ or double‐blind procedure/ or single‐blind procedure/ [EM]
64 or/57‐63 [Trials Filter EM]
65 (animal model? or animal experiment? or animal study? or animal trial? or canine or feline or bovine or cow or cows or mice or dog? or cat or cats or rabbit? or rat or rats or veterinar$).ti. or (animal or veterinary).hw. [EM]
66 (editorial or letter or note or "review" or trade or survey).pt. [EM]
67 systematic review/ or meta‐analysis/ or (systematic adj3 review).ti. or (meta‐analy$ or metaanaly$).ti. or (literature adj2 review).ti.
68 64 not (or/65‐67) [EPOC RCT Filter EM]
69 18 and 31 [Drug Review/Audit & Hosp]
70 31 and 51 and 56 [Hosp & Pharma & Monitoring‐‐Broad search]
71 (or/69‐70) and 68 [RCT Results 2]
72 8 and 68 [Med Rev Hosp & RCT Results 1]
73 72 or 71 [RCT Results]
74 (20113$ or 20114$ or 20115$ or 2012$ or 2013$ or 2014$).em. [Entry week Aug 2011 to Nov 2014]
75 ("2011" or "2012" or "2013" or "2014").yr.
76 73 and (74 or 75) [Results Nov 18, 2014]
77 remove duplicates from 76
The Cochrane Library
The Cochrane Library<update 18 November 2014>, Wiley
#1 ("PHARMACEUTICAL CARE" near/2 inpatient* or PHARMACY near/2 inpatient* or PHARMACIES near/2 inpatient* or PHARMACIST* near/2 inpatient* or PRESCRIBING near/2 inpatient*):ab or (stopp or (Beer N2 criteria)):ti,ab
#2 ("PHARMACEUTICAL CARE" near/2 hospital*or PHARMACY near/2 hospital* or PHARMACIES near/2 hospital* or PHARMACIST* near/2 hospital* or PRESCRIBING near/2 hospital*):ab
#3 ("PHARMACEUTICAL CARE" near/2 WARD* or PHARMACY near/2 WARD* or PHARMACIES near/2 WARD* or PHARMACIST* near/2 WARD* or PRESCRIBING near/2 WARD*):ab
#4 ("PHARMACEUTICAL CARE" near/2 UNIT or PHARMACY near/2 UNIT or PHARMACIES near/2 UNIT or PHARMACIST* near/2 UNIT or PRESCRIBING near/2 UNIT):ab
#5 ("PHARMACEUTICAL CARE" near/2 UNITS or PHARMACY near/2 UNITS or PHARMACIES near/2 UNITS or PHARMACIST* near/2 UNITS or PRESCRIBING near/2 UNITS):ab
#6 (medication* near/2 system* or prescribing near/2 system* or prescription* near/2 system* or dispensing near/2 system*):ti,kw and (hospital* or WARD or WARDS or INPATIENT* or CARE near/2 UNIT*):ti,kw
#7 MeSH descriptor: [Pharmacy Service, Hospital] this term only
#8 MeSH descriptor: [Medication Systems, Hospital] this term only
#9 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8)
#10 MeSH descriptor: [Hospitalization] explode all trees
#11 MeSH descriptor: [Inpatients] this term only
#12 MeSH descriptor: [Hospital Departments] explode all trees
#13 MeSH descriptor: [Hospital Shared Services] this term only
#14 MeSH descriptor: [Hospital Units] explode all trees
#15 MeSH descriptor: [Medical Staff, Hospital] explode all trees
#16 (hospital* or WARD or WARDS):ti
#17 hospital*:ab
#18 (patient* or hospital*):ti,kw and (discharge* or admission* or admitting or readmission* or readmit* or transfer*):ti or "length of stay":ti
#19 (Patient* near/2 discharg* or Patient* near/2 admission* or Patient* near/2 admitting or Patient* near/2 readmission* or Patient* near/2 transfer*) or "length of stay":ab
#20 (hospital* near/2 discharg* or hospital* near/2 admission* or hospital near/2 admitting or hospital near/2 readmission* or hospital near/2 transfer*) or "length of stay":ab
#21 (inpatient* or in‐patient*):ti
#22 (#10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21)
#23 (pharmacy or pharmacies or pharmacist* or prescription* or prescribing):ti
#24 ("pharmacist‐led" or "pharma* initiated" or pharmacist* near/2 driven or pharmacist* near/2 lead or pharmacist* near/2 led):ab
#25 Prescribing near/2 Pattern*:ab
#26 ("physician‐pharmacist*" or "doctor‐pharmacist*"):ti,ab
#27 (IMPROV* or OPTIMI*ING or OPTIMI*E* or OPTIMAL*):ti and (DOSING or DOSAGE or PHARMAC* or PRESCRIB* or PRESCRIPT*):ti
#28 (IMPROV* near/2 "PHARMACEUTICAL CARE" or OPTIMI*ING near/2 "PHARMACEUTICAL CARE" or OPTIMI*E* near/2 "PHARMACEUTICAL CARE" or OPTIMAL* near/2 "PHARMACEUTICAL CARE"):ab
#29 (IMPROV* near/2 PHARMACY or OPTIMI*ING near/2 PHARMACY or OPTIMI*E* near/2 PHARMACY or OPTIMAL* near/2 PHARMACY):ab
#30 (IMPROV* near/2 PRESCRIB* or OPTIMI*ING near/2 PRESCRIB* or OPTIMI*E* near/2 PRESCRIB* or OPTIMAL* near/2 PRESCRIB*):ab
#31 (IMPROV* near/2 PRESCRIPT* or OPTIMI*ING near/2 PRESCRIPT* or OPTIMI*E* near/2 PRESCRIPT*or OPTIMAL* near/2 PRESCRIPT*):ab
#32 "pharmaceutical care" or "pharmaceutical consult*" or (pharmacist* near/2 care or pharmacist* near/2 consult* or pharmacist* near/2 intervention* or pharmacist* near/2 managed):ab
#33 (prescription* near/4 review* or prescribing near/4 review* or medication* near/4 review*OR pharmacist* near/2 review*):ti,ab
#34 ("drug therapy" near/2 audit* or "drug regime*" near/2 audit* or medication* near/2 audit* or medicine* near/2 audit* or pharmacy near/2 audit* or pharmacist* near/2 audit* or pharmaceutical near/2 audit* or PRESCRIB* near/2 audit* or prescription* near/2 audit*):ti,ab
#35 ("drug therapy" near/2 monitor* or "drug regime*" near/2 monitor* or medication* near/2 monitor* or medicine* near/2 monitor* or pharmacy near/2 monitor* or pharmacist* near/2 monitor* or pharmaceutical near/2 monitor* or PRESCRIB* near/2 monitor* or prescription* near/2 monitor*):ti,ab
#36 ("drug therapy" near/2 RECONCIL* or "drug regime*" near/2 RECONCIL* or medication* near/2 RECONCIL* or medicine* near/2 RECONCIL* or pharmacy near/2 RECONCIL* or pharmacist* near/2 RECONCIL* or pharmaceutical near/2 RECONCIL* or PRESCRIB* near/2 RECONCIL* or prescription* near/2 RECONCIL*):ti,ab
#37 ("drug therapy" near/2 review* or "drug regime*" near/2 review* or medication* near/2 review* or medicine* near/2 review* or pharmacy near/2 review* or pharmacist* near/2 review* or pharmaceutical near/2 review* or PRESCRIB* near/2 review* or prescription* near/2 review*):ti,ab
#38 (medication* near/2 manage* or prescrib* near/2 manage* or phamac* near/2 manage*):ti,ab
#39 (medication* near/2 management or prescrib* near/2 management or pharmac* near/2 management):ti,ab
#40 (medication* near/2 service* or prescrib* near/2 service* or pharmac* near/2 service*):ti,ab
#41 (medication* near/2 system* or prescrib* near/2 system* or pharmac* near/2 system*):ti,ab
#42 ("drug therapy" near/2 managing or dosage* near/2 managing or dose* near/2 managing or medication* near/2 managing or PRESCRIPTION* near/2 managing or PRESCRIB* near/2 managing or PHARMACIST* near/2 managing or "PHARMACEUTICAL CARE" near/2 managing):ti,ab
#43 ("drug therapy" near/2 management or dosage* near/2 management or dose* near/2 management or medication* near/2 management or PRESCRIPTION* near/2 management or PRESCRIB* near/2 management or PHARMACIST* near/2 management or "PHARMACEUTICAL CARE" near/2 management):ti,ab
#44 ("drug therapy" near/2 monitor* or dosage* near/2 monitor* or dose* near/2 monitor* or medication* near/2 monitor* or PRESCRIPTION* near/2 monitor* or PRESCRIB* near/2 monitor* or PHARMACIST* near/2 monitor* or "PHARMACEUTICAL CARE" near/2 monitor*):ti,ab
#45 ("drug* review*" or "drug* assess*" or "drug* audit*" or "drug* reconcil*"):ti,ab
#46 ("drug utili*ation" near/2 review* or "drug utili*ation" near/2 reconcil* or "drug utili*ation" near/2 audit*):ab
#47 (review* or reconcil* or audit*):ti and "drug utili*ation":ti
#48 MeSH descriptor: [Medication Adherence] this term only
#49 MeSH descriptor: [Pharmacists] this term only
#50 MeSH descriptor: [Pharmacists' Aides] explode all trees
#51 MeSH descriptor: [Pharmaceutical Services] this term only
#52 MeSH descriptor: [Drug Information Services] this term only
#53 MeSH descriptor: [Clinical Pharmacy Information Systems] this term only
#54 MeSH descriptor: [Prescriptions] this term only
#55 MeSH descriptor: [Drug Prescriptions] this term only
#56 MeSH descriptor: [Drug Dosage Calculations] this term only
#57 MeSH descriptor: [Pharmaceutical Preparations] this term only
#58 MeSH descriptor: [Electronic Prescribing] this term only
#59 MeSH descriptor: [Medication Systems] this term only
#60 MeSH descriptor: [Drug Monitoring] this term only
#61 MeSH descriptor: [Medication Therapy Management] this term only
#62 MeSH descriptor: [Drug Therapy] this term only
#63 MeSH descriptor: [Drug Therapy, Computer‐Assisted] this term only
#64 MeSH descriptor: [Medication Errors] this term only
#65 MeSH descriptor: [Drug Utilization Review] this term only
#66 MeSH descriptor: [Drug Utilization] this term only
#67 MeSH descriptor: [Polypharmacy] this term only
#68 Polypharm*:ti
#69 Polypharmacy or polypharm*:ti
#70 MeSH descriptor: [Inappropriate Prescribing] this term only
#71 ((Medication or medications or prescrib* or prescription or prescriptions or drug therap*) near/2 assessment):ti,ab
#72 (inappropriate* near/2 (medicine or medicines or medication or medications or prescrib* or drug or drugs)):ti,ab
#73 (#23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 or #57 or #58 or #59 or #60 or #61 or #62 or #63 or #64 or #65 or #66 or #67 or #68 or #69 or #70 or #71 or #72)
#74 (#9 or (#22 and #73))
#75 (medication near/2 review) (Word variations have been searched)
#76 hospital* or inpatient*:ti,ab,kw
#77 #75 and #76 in Cochrane Reviews (Reviews and Protocols)
CINAHL
EbscoHost CINAHL <update 18 November 2014>
S1 (MH "Pharmacy Service")
S2 TI ( pharmaceutical care or pharmacy or pharmacies or pharmacist* or prescribing )
S3 (MH "Medication Systems") OR TI (medication* n2 system) or (prescribing n2 system) or (prescription* n2 system) or (dispensing n2 system) OR TI (medication* n2 systems) or (prescribing n2 systems) or (prescription* n2 systems) or (dispensing n2 systems) OR TI ((medication N2 assessment) or (prescrib* N2 assessment) or (prescription N2 assessment) or (drug therap* N2 assessment)) OR AB ((medication N2 assessment) or (prescrib* N2 assessment) or (prescription N2 assessment) or (drug therap* N2 ass ...
S4 TI ( hospital* OR inpatient ward or wards or intensive care or ICU or emergency department* or unit ) OR MW ( hospital* OR inpatient ward or wards or intensive care or ICU or emergency department* )
S5 (MH "Adolescent, Hospitalized") OR (MH "Aged, Hospitalized") OR (MH "Child, Hospitalized") OR (MH "Emergency Patients") OR (MH "Infant, Hospitalized") OR (MH "Inpatients")
S6 (MH "Hospitals+") OR (MH "Hospital Units+") OR TI ( inpatient* or hospital$ or WARD* or UNIT or UNITS )
S7 (MH "Hospitalization") OR (MH "Length of Stay") OR (MH "Patient Admission") OR (MH "Patient Discharge") OR (MH "Discharge Planning+") OR (MH "Patient Discharge Education") OR (MH "Early Patient Discharge") OR (MH "Transfer, Discharge") OR (MH "Patient Dumping") OR (MH "Readmission") OR (MH "Transfer, Intrahospital") S7
S8 (MH "Medication Reconciliation")
S9 TI ( (drug therapy N2 reconcil*) or (drug therapy N2 audit*) or (drug therapy N2 review*) ) or AB ( (drug therapy N2 reconcil*) or (drug therapy N2 audit*) or (drug therapy N2 review*) ) OR TI ( (medicine* N2 reconcil*) or (medicine* N2 audit*) or (medicine* N2 review*) ) or AB ( (medicine* N2 reconcil*) or (medicine* N2 audit*) or (medicine* N2 review*) )
S10 (MH "Nursing Audit") OR (MH "Audit")
S11 TI ( medication* or medicine* or drug therap* or prescrib* or prescript* or medication* ) or MW ( medication* or medicine* or drug therap* or prescrib* or prescript* or medication* )
S12 S10 and S11
S13 S1 or S2 or S3
S14 S4 or S5 or S6 or S7
S15 S8 or S9 or S12
S16 S13 and S14
S17 S14 and S15
S18 TI ( (multicent* n2 design*) or (multicent* n2 study) or (multicent* n2 studies) or (multicent* n2 trial*) ) or AB ( (multicent* n2 design*) or (multicent* n2 study) or (multicent* n2 studies) or (multicent* n2 trial*) )
S19 (MM "Clinical Trials+")
S20 TI ( “clinical study” or “clinical studies” ) or AB ( “clinical study” or “clinical studies” )
S21 TI random* or AB random*
S22 TI controlled or AB controlled
S23 TI ( “control* N1 clinical” or “control* N1 group*” or “control* N1 trial*” or “control* N1 study” or “control* N1 studies” or “control* N1 design*” or “control* N1 method*” ) or AB ( “control* N1 clinical” or “control* N1 group*” or “control* N1 trial*” or “control* N1 study” or “control* N1 studies” or “control* N1 design*” or “control* N1 method*” )
S24 S18 or S19 or S20 or S21 or S22 or S23
S25 TI ( (stopp or "beer's criteria") ) OR AB ( (stopp or "beer's criteria") )
S26 S16 or S17 or S25
S27 S24 and S26
S28 TI medication review*
S29 S27 or S28
S30 (MH "Pharmacy Service")
S31 TI ( pharmaceutical care or pharmacy or pharmacies or pharmacist* or prescribing )
S32 (MH "Medication Systems") OR TI (medication* n2 system) or (prescribing n2 system) or (prescription* n2 system) or (dispensing n2 system) OR TI (medication* n2 systems) or (prescribing n2 systems) or (prescription* n2 systems) or (dispensing n2 systems) OR TI ((medication N2 assessment) or (prescrib* N2 assessment) or (prescription N2 assessment) or (drug therap* N2 assessment)) OR AB ((medication N2 assessment) or (prescrib* N2 assessment) or (prescription N2 assessment) or (drug therap* N2 ass ...
S33 TI ( hospital* OR inpatient ward or wards or intensive care or ICU or emergency department* or unit ) OR MW ( hospital* OR inpatient ward or wards or intensive care or ICU or emergency department* )
S34 (MH "Adolescent, Hospitalized") OR (MH "Aged, Hospitalized") OR (MH "Child, Hospitalized") OR (MH "Emergency Patients") OR (MH "Infant, Hospitalized") OR (MH "Inpatients")
S35 (MH "Hospitals+") OR (MH "Hospital Units+") OR TI ( inpatient* or hospital$ or WARD* or UNIT or UNITS )
S36 (MH "Hospitalization") OR (MH "Length of Stay") OR (MH "Patient Admission") OR (MH "Patient Discharge") OR (MH "Discharge Planning+") OR (MH "Patient Discharge Education") OR (MH "Early Patient Discharge") OR (MH "Transfer, Discharge") OR (MH "Patient Dumping") OR (MH "Readmission") OR (MH "Transfer, Intrahospital")
S37 (MH "Medication Reconciliation")
S38 TI ( (drug therapy N2 reconcil*) or (drug therapy N2 audit*) or (drug therapy N2 review*) ) or AB ( (drug therapy N2 reconcil*) or (drug therapy N2 audit*) or (drug therapy N2 review*) ) OR TI ( (medicine* N2 reconcil*) or (medicine* N2 audit*) or (medicine* N2 review*) ) or AB ( (medicine* N2 reconcil*) or (medicine* N2 audit*) or (medicine* N2 review*) )
S39 (MH "Nursing Audit") OR (MH "Audit")
S40 TI ( medication* or medicine* or drug therap* or prescrib* or prescript* or medication* ) or MW ( medication* or medicine* or drug therap* or prescrib* or prescript* or medication* )
S41 S39 and S40
S42 S30 or S31 or S32
S43 S33 or S34 or S35 or S36
S44 S37 or S38 or S41
S45 S42 and S43
S46 S43 and S44
S47 TI ( (multicent* n2 design*) or (multicent* n2 study) or (multicent* n2 studies) or (multicent* n2 trial*) ) or AB ( (multicent* n2 design*) or (multicent* n2 study) or (multicent* n2 studies) or (multicent* n2 trial*) )
S48 (MM "Clinical Trials+"
S49 TI ( “clinical study” or “clinical studies” ) or AB ( “clinical study” or “clinical studies” )
S50 TI random* or AB random*
S51 TI controlled or AB controlled
S52 TI ( “control* N1 clinical” or “control* N1 group*” or “control* N1 trial*” or “control* N1 study” or “control* N1 studies” or “control* N1 design*” or “control* N1 method*” ) or AB ( “control* N1 clinical” or “control* N1 group*” or “control* N1 trial*” or “control* N1 study” or “control* N1 studies” or “control* N1 design*” or “control* N1 method*” )
S53 S47 or S48 or S49 or S50 or S51 or S52
S54 TI ( (stopp or "beer's criteria") ) OR AB ( (stopp or "beer's criteria")
S55 S45 or S46 or S54
S56 S53 and S55
S57 TI medication review*
S58 S56 or S57
EPOC Specialised Register
Reference Manager, EPOC Specialised Register <update 18 November 2014>
TI: {Medication} AND {review} OR
TI: {prescription} AND {review} OR
TI: {prescription} AND {audit} OR
TI: {medication} AND {audit} OR
TI: {medication} AND {reconcil} OR
TI: {prescription} AND {reconcil} OR
TI: {prescrib} AND {reconcil} OR
TI: {prescrib} AND {audit} OR
TI: {prescrib} AND {review} OR
TI: {pharmacist} AND {audit} OR
TI: {pharmacist} AND {review} OR
TI: {hospital pharmacist} OR
TI: {hospital AND prescribe} OR
AB: hospital prescribe OR
Keyword: (Pharmacy Service,Hospital*) OR
TI: (inappropriate OR assessment) AND
TI: (medication OR medicine OR drug OR prescrib OR prescrip)
NOTE: Due to the limited searching capabilities of RefMan, this strategy was searched in separate parts.
International Pharmaceutical Abstracts
Ovid International Pharmaceutical Abstracts <17 August 2011 to 12 May 2015>
1 Pharmacy service, hospital.mp.
2 ((PHARMACEUTICAL CARE or PHARMACY or PHARMACIES or PHARMACIST? or PRESCRIBING) and (inpatient? or hospital$ or WARD? or UNIT or UNITS)).ti.
3 ((PHARMACEUTICAL CARE or PHARMACY or PHARMACIES or PHARMACIST? or PRESCRIBING) adj2 (inpatient? or hospital$ or WARD? or UNIT or UNITS)).ab.
4 Medication Systems, Hospital.mp.
5 ((medication? or prescribing or prescription? or dispensing) adj2 system?).ti,ab. and (hospital$ or WARD or WARDS or (CARE adj2 UNIT?) or INPATIENT?).ti,hw.
6 (stopp or beer's criteria).ti,ab.
7 1 or 2 or 3 or 4 or 5 or 6
8 (hospital$ or WARD or WARDS).ti.
9 Hospitalization.mp.
10 hospital$.ab.
11 ("length of stay" or Patient admission or Patient discharge or Patient readmission or Patient transfer).mp.
12 ((patient? or hospital$).ti,hw. and (discharg$ or admission? or admitting or readmission? or readmit$ or transfer?).ti.) or "length of stay".ti.
13 Inpatients.mp. [mp=title, subject heading word, registry word, abstract, trade name/generic name]
14 (inpatient? or in‐patient?).ti.
15 HOSPITAL SHARED SERVICES.mp.
16 (MEDICAL STAFF, HOSPITAL or HOSPITALISTS).mp.
17 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16
18 (pharmacy or pharmacies or pharmacist? or prescription? or prescribing).ti.
19 (pharmacist‐led or pharma$ initiated or ((driven or lead or led) adj2 pharmacist?)).ab.
20 (PRESCRIBING adj2 PATTERN?).ab.
21 ("physician‐pharmacist?" or "doctor‐pharmacist?").ti,ab.
22 ((IMPROV$ or OPTIMI?ING or OPTIMI?E? or OPTIMAL$) and (DOSING or DOSAGE or PHARMAC$ or PRESCRIB$ or PRESCRIPT$)).ti. or ((IMPROV$ or OPTIMI?ING or OPTIMI?E? or OPTIMAL$) adj2 (PHARMACEUTICAL CARE or PHARMACY or PRESCRIB$ or PRESCRIPT$)).ab.
23 ((pharmaceutical adj (care or consult$)) or (pharmacist? adj2 (care or consult$ or intervention? or managed))).ab.
24 (((prescription? or prescribing or medication?) adj4 review$) or (pharmacist? adj2 review$)).ti,ab.
25 ((drug therapy or drug regime? or medication? or medicineS or pharmacy or pharmacist? or pharmaceutical or PRESCRIB$ or prescription?) adj2 (audit$ or monitor$ or RECONCIL$ or review?)).ti,ab.
26 ((medication? or prescrib$ or pharmac$) adj2 (manage? or management or service? or system?)).ti,ab.
27 (("drug therapy" or dosage? or dose? or medication? or PRESCRIPTION? or PRESCRIB$ or PHARMACIST? or PHARMACEUTICAL CARE) adj2 (managing or management or monitor$)).ti,ab.
28 (drug? review? or drug? assess$ or drug? audit? or drug?reconcil$).ti,ab.
29 ("drug utili?ation" adj2 (review? or reconcil$ or audit?)).ab. or ("drug utili?ation" and (review? or reconcil$ or audit?)).ti.
30 Medication adherence.mp.
31 (Pharmacists or Pharmacists' Aides).mp.
32 (Pharmaceutical Services or Drug Information Services).mp.
33 Clinical Pharmacy Information Systems.mp.
34 (Prescriptions or Drug Prescriptions or Pharmaceutical Preparations or Drug Therapy or Drug Dosage Calculations or Electronic Prescribing or Medication Systems).mp.
35 (Drug Monitoring or Medication Therapy Management).mp.
36 (Drug Therapy or Drug Therapy, Computer‐Assisted).mp.
37 POLYPHARMACY.mp. or POLYPHARM$.ti.
38 MEDICATION ERRORS.mp.
39 Drug utilization review.mp.
40 Drug Utilization.mp.
41 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40
42 (randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.
43 animals/ not humans.sh.
44 42 not 43
45 (((patient? or hospital?) adj2 (discharg$ or admission? or admitting or readmission? or transfer?)) or "length of stay").ab.
46 17 or 45
47 7 and 44
48 41 and 44 and 45
49 47 or 48
Data and analyses
Comparison 1. Primary outcome.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (all‐cause) | 9 | 3218 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.87, 1.19] |
Comparison 2. Secondary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hospital readmissions (all‐cause) | 7 | 2843 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.87, 1.04] |
| 2 Hospital readmissions (all‐cause) ‐ 3 months | 3 | 330 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.31, 0.21] |
| 3 Hospital readmissions (all‐cause) ‐ 12 months | 2 | 1063 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.29, 0.05] |
| 4 Hospital emergency department contacts (all‐cause) | 4 | 1442 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.52, 1.03] |
| 5 Hospital emergency department contacts (all‐cause) ‐ 3 months | 3 | 330 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.16, 0.06] |
Comparison 3. Subgroup analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (all‐cause) | 9 | 3218 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.87, 1.19] |
| 1.1 High‐risk population | 7 | 1922 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.81, 1.14] |
| 1.2 Low‐risk population | 2 | 1296 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.95, 2.34] |
| 2 Hospital readmissions (all‐cause) | 7 | 2843 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.87, 1.04] |
| 2.1 High‐risk population | 5 | 1627 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.83, 1.03] |
| 2.2 Low‐risk population | 2 | 1216 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.87, 1.24] |
| 3 Hospital emergency department contacts (all‐cause) | 4 | 1442 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.52, 1.03] |
| 3.1 High‐risk population | 3 | 574 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.36, 1.03] |
| 3.2 Low‐risk population | 1 | 868 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.64, 1.25] |
| 4 Mortality (all‐cause) | 9 | 3218 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.87, 1.19] |
| 4.1 Systematic medication review | 2 | 442 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.56, 1.46] |
| 4.2 Non‐systematic medication review | 7 | 2776 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.87, 1.22] |
| 5 Hospital readmissions (all‐cause) | 7 | 2843 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.87, 1.04] |
| 5.1 Systematic medication review | 1 | 358 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.79, 1.36] |
| 5.2 Non‐systematic medication review | 6 | 2485 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.86, 1.04] |
| 6 Mortality (all‐cause) | 9 | 3218 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.87, 1.19] |
| 6.1 Low risk of bias | 4 | 1796 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.89, 1.66] |
| 6.2 High risk of bias | 5 | 1422 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.79, 1.15] |
| 7 Hospital readmissions (all‐cause) | 7 | 2843 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.87, 1.04] |
| 7.1 Low risk of bias | 3 | 1574 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.89, 1.20] |
| 7.2 High risk of bias | 4 | 1269 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.81, 1.02] |
| 8 Hospital emergency department contacts (all‐cause) | 4 | 1442 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.52, 1.03] |
| 8.1 Low risk of bias | 1 | 868 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.64, 1.25] |
| 8.2 High risk of bias | 3 | 574 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.36, 1.03] |
Comparison 4. Sensitivity analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (all‐cause) ‐ alternative available case analysis | 9 | 3379 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.87, 1.20] |
| 2 Hospital readmissions (all‐cause) ‐ alternative available case analysis | 7 | 3039 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.87, 1.05] |
| 3 Hospital emergency department contacts (all‐cause) ‐ alternative available case analysis | 4 | 1510 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.68, 1.07] |
| 4 Mortality (all‐cause) ‐ fixed‐effect | 9 | 3218 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.88, 1.21] |
| 5 Hospital readmissions (all‐cause) ‐ fixed‐effect | 7 | 2843 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.87, 1.05] |
| 6 Hospital readmissions (all‐cause) ‐ 3 months ‐ fixed‐effect | 3 | 330 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.24, 0.14] |
| 7 Hospital readmissions (all‐cause) ‐ 12 months ‐ fixed‐effect | 2 | 1063 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.29, 0.05] |
| 8 Hospital emergency department contacts (all‐cause) ‐ fixed‐effect | 4 | 1442 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.60, 0.96] |
| 9 Hospital emergency department contacts (all‐cause) ‐ 3 months ‐ fixed‐effect | 3 | 330 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.16, 0.06] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bladh 2011.
| Methods | Randomised controlled trial | |
| Participants | A total of 400 participants were randomised ‐ 199 to medication review and 201 to control. Patients admitted to 2 internal medicine wards at a university hospital in Sweden. Median (IQR) age: medication review group 81 (72 to 87) years, control group 82 (75 to 86) years; 39% male; median number of drugs: 7 (+1 drug on demand) | |
| Interventions | Medication reviews were performed with a computer support system (MiniQ) that identified potentially inappropriate prescribing (PIP) according to 3 drug‐specific quality indicators, and included oral feedback to prescribing physicians. PIPs were (1) drugs that should be avoided in the elderly, for example, long‐acting benzodiazepines and drugs with anticholinergic action, (2) 3 or more psychotropic drugs (i.e. antipsychotics, anxiolytics, hypnotic‐sedatives and antidepressants) and (3) potentially serious drug‐drug interactions: Category D interactions according to the Pharmaceutical Specialities in Sweden (FASS) specifying drug combinations that should be avoided | |
| Outcomes | Primary: EQ‐5D index
Secondary: 'self rated global health' and 'attitudes towards the medication report' (evaluated by questionnaires sent to participants' GPs after discharge from hospital) PIP items: (1) drugs that should be avoided in the elderly, (2) 3 or more psychotropics and (3) potentially serious interactions Potential drug‐related problems (DRPs) identified only in the intervention group (mortality not reported as an outcome per se) All outcomes had 6 months of follow‐up |
|
| Notes | Funding: Swedish National Board of Health and Welfare | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised (1:1) to intervention or control group. Two persons without knowledge about the study protocol performed the randomisation |
| Allocation concealment (selection bias) | Low risk | A ward physician or nurse judged whether the medical condition of the patient allowed inclusion in the study. Sequentially numbered, sealed envelopes were opened after participant details were written and transferred to the assignment card via a carbon paper inside the envelope |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described, but probably not blinded |
| Blinding of outcome assessment (detection bias) Mortality (all‐cause) | Low risk | Mortality data were likely gathered from a non‐biased national register (through unique patient‐specific social security number) |
| Blinding of outcome assessment (detection bias) Hospital readmissions (all‐cause) | Low risk | Readmission data should be unbiased, as they were gathered from a non‐biased register |
| Incomplete outcome data (attrition bias) Mortality (all‐cause) | Low risk | No missing data were described; all data should be available from a non‐biased national register |
| Incomplete outcome data (attrition bias) Hospital readmissions (all‐cause) | Low risk | No missing data were described; all data should be available from a non‐biased national register |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were collected and were similar to information provided on www.clinicaltrials.gov |
| Contamination bias | High risk | No cluster‐randomisation |
| Other bias | Low risk | No evidence of other types of bias |
Bonnerup 2014.
| Methods | Randomised controlled trial | |
| Participants | A total of 375 participants were randomised ‐ 124 to high‐risk subgroup, 64 to medication review and 60 to control group. Patients admitted to 1 acute medical department at university hospital in Denmark. High‐risk subgroup: mean age of control group: 78.2 years; mean number of drugs: 11.0 | |
| Interventions | Patients presenting with risk of prescribing errors identified by a risk score called MERIS (ranging from 0 to 37). A MERIS score between 14 and 26 warranted a medication review by a clinical pharmacist, whereas a risk score ≥ 26 led to medication review by a clinical pharmacologist. Medication reviews consisted of (1) collecting information concerning the participant's drug treatment and the clinical status of the participant, (2) conducting a participant interview and (3) performing a critical examination of a participant's overall drug treatment. Recommendations or information arising from the medication reviews were delivered to hospital physicians as a note in the electronic medical record. If fast response was needed (e.g. if the participant was about to be discharged, if urgent action was required), the note was accompanied by direct contact with a physician | |
| Outcomes | Primary outcome: number of prescribing errors during participants' hospitalisation Secondary outcomes: healthcare utilisation (divided into all‐cause readmissions, contacts with general practitioners and visits to emergency departments), health‐related quality of life, mortality All outcomes had 90 days of follow‐up (after hospital discharge) . |
|
| Notes | Funding: not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was generated by a computer programme in the hospital pharmacy in random blocks of a maximum of 20 |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque and sealed envelopes containing randomisation codes were delivered to study pharmacists who allocated participants |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described, but probably not blinded |
| Blinding of outcome assessment (detection bias) Mortality (all‐cause) | Low risk | Mortality data were taken from a non‐biased national register (participants identifiable through unique patient‐specific social security numbers) |
| Blinding of outcome assessment (detection bias) Hospital readmissions (all‐cause) | Low risk | Readmission data were taken from a non‐biased national register |
| Blinding of outcome assessment (detection bias) Hospital emergency department contacts (all‐cause) | Low risk | Emergency department contacts were taken from a non‐biased national register |
| Incomplete outcome data (attrition bias) Mortality (all‐cause) | Low risk | No missing data were described; all data should be available from a non‐biased national register |
| Incomplete outcome data (attrition bias) Hospital readmissions (all‐cause) | Low risk | No missing data were described; all data should be available from a non‐biased national register |
| Incomplete outcome data (attrition bias) Hospital emergency department contacts (all‐cause) | Low risk | No missing data were described; all data should be available from a non‐biased national register |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were collected and were similar to information provided on www.clinicaltrials.gov |
| Contamination bias | High risk | No cluster‐randomisation |
| Other bias | Unclear risk | We included only a subgroup of patients with high risk of medication errors (i.e. MERIS risk score ≥ 14 as specified in the manuscript). Inclusion of only this subgroup of patients may induce bias, as risk score assessment was performed after allocation to groups (i.e. may introduce unbalance) |
Dalleur 2014.
| Methods | Randomised controlled trial | |
| Participants | A total of 158 participants were randomised ‐ 77 to medication review and 81 to control. Patients admitted to non‐geriatric medical wards at a teaching hospital in Belgium. Median (IQR) age: medication review 84 years (81 to 87), control 86 (81 to 89) years; 37% male; median number of drugs: 7 | |
| Interventions | Each participant's medications were routinely reviewed by the in‐patient consultation team geriatrician, who used an implicit approach (i.e. no explicit tool was used). In the intervention group, in addition to the usual in‐patient geriatric consultation team care, geriatricians performed the following 2 steps: (1) they applied 64 STOPP criteria to systematically screen the list of medications being taken by the pati ent on admission for potentially inappropriate medications (PIMs) (‘duplicate drug classes’ was not considered because the concept of duplication is perceived differently by clinicians), and (2) they made oral and written recommendations to the ward physician during hospitalisation for discontinuation of PIMs | |
| Outcomes | Primary: proportion of PIMs discontinued (or corrected in case of dosage‐related or duration‐related PIMs) between hospital admission and discharge (according to the discharge letter) Secondary: (1) characteristics associated with discontinuation of PIMs at discharge, (2) proportion of PIMs that were still discontinued 1 year after discharge and (3) clinical significance of STOPP‐related recommendations. Mortality after 1 year was also reported | |
| Notes | Funding: Dr Dalleur was funded by the Federal Public Service Health of the Belgian government as part of a national project on implementation of clinical pharmacy in hospitals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Eligible patients were allocated by the study nurse to control or intervention group by simple randomisation using drawing of lots (without matching for age or geriatric profile). No description of how drawing of lots was performed |
| Allocation concealment (selection bias) | High risk | Study nurse assigned participants and performed randomisation using an apparently open design, which could lead to lack of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Attending ward physician (responsible for prescriptions during hospitalisation and at discharge), outcome evaluator and participants were blinded to group assignment |
| Blinding of outcome assessment (detection bias) Mortality (all‐cause) | Low risk | Study nurse provided the outcome evaluator with a list of participants included in the study, which did not specify allocation group |
| Incomplete outcome data (attrition bias) Mortality (all‐cause) | High risk | Follow‐up data on mortality were not available for 58% of randomised participants |
| Selective reporting (reporting bias) | Unclear risk | No data on readmissions and emergency department contacts, which were reported in similar trials conducted by some co‐authors. No protocol available |
| Contamination bias | Unclear risk | No cluster‐randomisation. The paper states: "In order to avoid contamination bias, two of the four geriatricians involved in the inpatient geriatric consultation team during the study period were allocated to the intervention group because they used the STOPP criteria in their current practice, while the other two, who had never worked with the STOPP criteria, were allocated to the control group". However, as participants in the control group were treated at the same wards (as intervention group participants), this will not prevent contamination bias |
| Other bias | Low risk | No evidence of other types of bias |
Farris 2014.
| Methods | Randomised controlled trial | |
| Participants | A total of 936 participants were randomised ‐ 314 to enhanced medication review, 315 to minimal medication review, 313 to control. Patients admitted to departments of internal medicine, family medicine, cardiology and orthopedics at a Midwestern academic health centre in the USA. Mean age: 61.0 years; mean number of drugs: 11.0 | |
| Interventions | Participants in the minimal and enhanced intervention groups received medication reconciliation at admission and pharmacist visits every 2 to 3 days for patient education during inpatient stay, discharge counselling and discharge medication list. Counselling was tailored for each participant and focused on goals of therapy, medication administration and barriers to adherence including cost and patient concerns. Participants in the enhanced intervention group also received a telephone call 3 to 5 days post discharge, and primary care physician and community pharmacist received a discharge care plan focused on medication changes and recommendations. The care plan was faxed to the primary care physician and to the community pharmacist within 24 hours of discharge but usually within 6 hours. The care plan included the discharge medication list, plans for dosage adjustments and monitoring and recommendations for preventing adverse drug events, with participant‐specific concerns such as adherence or cost issues highlighted | |
| Outcomes | Primary: medication appropriateness index (MAI) Secondary: adverse events, preventable adverse events as a composite variable of combined hospital readmission, emergency department visit or unscheduled general practitioner office visit during 30‐day and 90‐day follow‐up periods | |
| Notes | Funding: This study was supported by the National Heart, Lung and Blood Institute (1RO1 HL082711). Drs Carter, Kaboli and Christensen are also supported by the Comprehensive Access and Delivery Research and Evaluation (CADRE), Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service (HFP 04–149) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised according to a statistician‐generated randomisation scheme |
| Allocation concealment (selection bias) | Low risk | Allocated to groups by sequentially numbered envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and staff were aware of interventions, but not whether allocation was to minimal or enhanced group medication review |
| Blinding of outcome assessment (detection bias) Mortality (all‐cause) | Low risk | Records from the primary care provider and the pharmacy were obtained for all participants. Hospitalisation records were obtained from the university hospital and from community hospitals, when such an event occurred. Research staff was blinded, and readmission data should be complete |
| Blinding of outcome assessment (detection bias) Hospital readmissions (all‐cause) | Low risk | Trained research assistants contacted all participants by telephone to gather self reported adverse events and symptoms and self reported healthcare utilisation Primary care provider and pharmacy records were obtained for all participants. Hospitalisation records were obtained from the university hospital and from community hospitals when such an event occurred. Research staff was blinded when assessing readmission data |
| Blinding of outcome assessment (detection bias) Hospital emergency department contacts (all‐cause) | Low risk | Trained research assistants contacted all participants by telephone to gather self reported adverse events and symptoms and self reported healthcare utilisation Primary care provider and pharmacy records were obtained for all participants. Hospitalisation records were obtained from the university hospital and from community hospitals, when such an event occurred. Research staff was blinded when assessing emergency department contact assessment |
| Blinding of outcome assessment (detection bias) Adverse drug events | Unclear risk | Trained research assistants contacted all participants by telephone to gather self reported adverse events and symptoms and self reported healthcare utilisation Primary care provider and pharmacy records were obtained for all participants. Hospitalisation records were obtained from the university hospital and from community hospitals, when such an event occurred. Research staff was blinded. Nevertheless, coding of the causality of adverse events may be unblinded (e.g. by participants knowing their allocation and expressing this to the rating physician) |
| Incomplete outcome data (attrition bias) Mortality (all‐cause) | Unclear risk | Uneven loss to follow‐up in the 3 groups ‐ medication review 16/623 participants and control 5/313 participants ‐ might affect mortality results. Unclear whether participants lost to follow‐up had mortality data available (i.e. loss to follow‐up due to death) |
| Incomplete outcome data (attrition bias) Hospital readmissions (all‐cause) | Unclear risk | Discrepancies in the publication between the number of participants reported as study completers and the number of patients used in the calculation of hospital readmissions |
| Incomplete outcome data (attrition bias) Hospital emergency department contacts (all‐cause) | Unclear risk | Discrepancies in the publication between the number of participants reported as study completers and the number of patients used in the calculation of hospital emergency department contacts |
| Incomplete outcome data (attrition bias) Adverse drug events | Unclear risk | Discrepancies in the publication between the number of participants reported as study completers and the number of patients used in the calculation of adverse events |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were collected and were similar to information provided on www.clinicaltrials.gov |
| Contamination bias | High risk | Not cluster‐randomised |
| Other bias | Low risk | No evidence of other types of bias |
Gallagher 2011.
| Methods | Randomised controlled trial | |
| Participants | A total of 400 participants were randomised ‐ 200 to medication review and 200 to control. Participants were admitted via the emergency department under the care of attending physicians at a tertiary medical centre at the university hospital in Ireland. Median (IQR) age: medication review 75 (71 to 80) years, control 77 (71 to 82) years; 47% male; mean number of drugs: 7.7 | |
| Interventions | A primary research physician evaluated the pharmacotherapy of participants in the intervention group using specific criteria for potentially inappropriate prescribing and prescribing omissions (STOPP/START criteria). The research physician discussed with attending medical team and provided written communication of interventions within 24 hours after hospitalisation. Team members were not obliged to follow up. No reporting of co‐interventions | |
| Outcomes | Primary outcome measures: medication appropriateness index (MAI) and assessment of underutilization index (AOU)
Secondary outcome measures: mortality, frequency of general practitioner visits, hospital readmissions, falls All outcomes had 6 months of follow‐up |
|
| Notes | Funding: The study was funded by the Health Research Board of Ireland, Clinical Research Training Fellowship number CRT/2006/029 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation sequence was determined by an independently generated random numbers table using StatsDirect software |
| Allocation concealment (selection bias) | Low risk | The random numbers table was retained, independent of investigators, by a physician external to the study, who also assigned participants to groups using a sealed envelope system. Group allocation was concealed from the research physician and from participants until baseline data had been collected and inclusion criteria verified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study described as not blinded |
| Blinding of outcome assessment (detection bias) Mortality (all‐cause) | Low risk | Data collected by research physician aware of assignments, but this will likely not influence assessment of mortality |
| Blinding of outcome assessment (detection bias) Hospital readmissions (all‐cause) | Low risk | Data collected by research physician aware of assignments, but this will likely not influence assessment of hospital readmissions |
| Blinding of outcome assessment (detection bias) Adverse drug events | High risk | Data collected by research physician,who was aware of assignments. An interrater reliability analysis of outcome measurements was conducted to ensure no bias towards more favourable ratings in the intervention group as compared with the control group (n = 40). Nevertheless, the causality assessment of falls is highly subjective and may lead to bias |
| Incomplete outcome data (attrition bias) Mortality (all‐cause) | Low risk | No loss to follow‐up was described. Primary researcher obtained data from general practitioner, community pharmacist and hospital records. No description or evidence in publication of loss to follow‐up |
| Incomplete outcome data (attrition bias) Hospital readmissions (all‐cause) | Low risk | Primary researcher obtained data through contact with participants, their general practitioners or community pharmacists, and from hospital records. No description (or evidence in publication) of loss to follow‐up |
| Incomplete outcome data (attrition bias) Adverse drug events | Unclear risk | Assessment of falls (adverse events) was obtained by telephone contact with participants or their general practitioners. No description of how many times participants could not be contacted. Lack of contact could be related to falls and might not reveal whether participants had experienced a fall not requiring medical assistance |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were collected and were similar to information provided on www.clinicaltrials.gov |
| Contamination bias | High risk | No cluster‐randomisation |
| Other bias | Low risk | No evidence of other types of bias |
Gillespie 2009.
| Methods | Randomised controlled trial | |
| Participants | A total of 400 participants were randomised ‐ 199 to medication review and 201 to control. Participants were admitted to 2 acute internal medicine wards at a university hospital in Sweden. Mean age: 87 years; 41% male; mean number of drugs: 8.0 | |
| Interventions | The list of current medications was reconciled to ensure that the medication list was correct. Thereafter, a drug review was performed, and advice was given to participant's physician on drug selection, dosages and monitoring needs, with the final decision made by the physician in charge. Participants were educated and monitored throughout the admission process, and received discharge counselling Co‐interventions: Information about discharge medications (e.g. rationale for changes, therapeutic goals, monitoring needs for newly commenced drugs) was provided to primary care physicians (general practitioners) by study pharmacists. A follow‐up telephone call was made to participants 2 months after discharge |
|
| Outcomes | Primary outcome measure: frequency of hospital visits (emergency department and readmissions (total and drug related))
Secondary outcome measure: cost of hospital care
Mortality not stated as an outcome, but measured All outcomes had 12 months of follow‐up |
|
| Notes | Funding: This study was funded by Uppsala County Council, University Hospital of Uppsala, Uppsala University, Apoteket AB and Swedish Society of Pharmaceutical Sciences | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Allocated to groups using closed‐envelope technique. Randomisation was performed in blocks of 20 (each block contained 10 intervention and 10 control allocations). Unclear who included participants, but the 10 block arrangement and unblinding could make it possible to predict the group |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study described as not blinded |
| Blinding of outcome assessment (detection bias) Mortality (all‐cause) | Low risk | Mortality data were likely taken from a non‐biased national register (participants identifiable through unique patient‐specific social security number) |
| Blinding of outcome assessment (detection bias) Hospital readmissions (all‐cause) | Low risk | The 2 researchers responsible for analysing readmission data were blinded regarding the group to which participants had been randomised |
| Blinding of outcome assessment (detection bias) Hospital readmissions (due to adverse drug events) | Unclear risk | The physician in charge of the participant was required to document in the medical record whether readmissions were drug related. Physicians making this decision were blinded as to whether patients were study participants. Nevertheless, coding of the causality of hospital readmissions due to adverse events may be unblinded (e.g. by participants knowing their allocation and expressing this to the rating physician) |
| Blinding of outcome assessment (detection bias) Hospital emergency department contacts (all‐cause) | Low risk | Emergency department contact data were likely taken from a non‐biased national register |
| Blinding of outcome assessment (detection bias) Hospital emergency department contacts (due to adverse drug events) | Unclear risk | Blinding of outcome assessment was not described for emergency department contacts, and causality assessment of hospital readmissions due to adverse events may be unblinded by participants knowing their allocation and expressing this to the rating physician |
| Incomplete outcome data (attrition bias) Mortality (all‐cause) | Low risk | Describes that no participants were lost to follow‐up |
| Incomplete outcome data (attrition bias) Hospital readmissions (all‐cause) | Low risk | Describes that no participants were lost to follow‐up |
| Incomplete outcome data (attrition bias) Hospital readmissions (due to adverse drug events) | Low risk | Describes that no participants were lost to follow‐up |
| Incomplete outcome data (attrition bias) Hospital emergency department contacts (all‐cause) | Low risk | Describes that no participants were lost to follow‐up |
| Incomplete outcome data (attrition bias) Hospital emergency department contacts (due to adverse drug events) | Low risk | Describes that no participants were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were collected and were similar to information provided on www.clinicaltrials.gov |
| Contamination bias | High risk | No cluster‐randomisation |
| Other bias | Low risk | No evidence of other types of bias |
Lisby 2010.
| Methods | Randomised controlled trial | |
| Participants | A total of 100 participants were randomised ‐ 50 to medication review and 50 to control. Participants were admitted to an acute ward of internal medicine at a regional hospital in Denmark. Mean age: 79.2 years; 39% male; mean number of drugs: 10.2 | |
| Interventions | The intervention included clinical pharmacists and clinical pharmacologists (physicians) and was accomplished in 2 steps. First, a clinical pharmacist systematically collected information about participants' medication; second, collected medical histories were discussed with a clinical pharmacologist according to participants' entire medical records, including medical histories and laboratory test results. Discrepancies, inappropriate drugs, doses, routes, dosing schedules and inappropriate interactions between drugs were described in a note with recommendations for change. Ward physicians were not obliged to follow these recommendations. No co‐interventions were reported | |
| Outcomes | Primary outcome measure: length of hospital stay (hours) Secondary outcome measures: time to first admission, readmissions, emergency department visits, visits to outpatient care clinic, general practitioner visits, specialist visits, after‐hours care, quality of life assessment, mortality All outcomes had 3 months of follow‐up |
|
| Notes | Funding: ALIS, Amgros I/S, which is a publicly owned pharmaceutical procurement service for the 5 regional authorities in Denmark | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible patients were randomly assigned to intervention or control by a computer‐generated code |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study described as not blinded |
| Blinding of outcome assessment (detection bias) Mortality (all‐cause) | Low risk | Mortality data were likely gathered from a non‐biased national register (through unique patient‐specific social security number) |
| Blinding of outcome assessment (detection bias) Hospital readmissions (all‐cause) | Low risk | Readmission data were likely gathered from a non‐biased national register |
| Blinding of outcome assessment (detection bias) Hospital emergency department contacts (all‐cause) | Low risk | Hospital emergency department contact data were likely gathered from a non‐biased national register |
| Incomplete outcome data (attrition bias) Mortality (all‐cause) | Low risk | No loss to follow‐up was described; all data should be readily available from a non‐biased national register |
| Incomplete outcome data (attrition bias) Hospital readmissions (all‐cause) | Low risk | No loss to follow‐up was described; all data should be readily available from a non‐biased national register |
| Incomplete outcome data (attrition bias) Hospital emergency department contacts (all‐cause) | Low risk | No loss to follow‐up was described; all data should be readily available from a non‐biased national register |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were collected and were similar to information provided on www.clinicaltrials.gov |
| Contamination bias | High risk | No cluster‐randomisation |
| Other bias | Low risk | No evidence of other types of bias |
Lisby 2015.
| Methods | Randomised controlled trial | |
| Participants | In total, 108 participants were randomised ‐ 53 to medication review and 55 to control. Participants were admitted to an orthopaedic ward at a regional hospital in Denmark. Mean age: 80.5 years; 29% male; mean number of drugs: 6.7 | |
| Interventions | Systematic medication review by a clinical pharmacist and a clinical pharmacologist. A clinical pharmacist obtained medication history through medical records, electronic prescribing system, registry of drug purchase and interview after ward physician had prescribed in‐hospital medication. Subsequently, the case was discussed with a clinical pharmacologist, and a note with comments and recommendations for medication changes was prepared and handed directly to the physician responsible for the ward round. Ward physicians were not obliged to follow these recommendations. No co‐interventions were reported | |
| Outcomes | Primary outcome measures: time to first unscheduled physician contact (general practitioner, emergency department, ambulatory care or hospital) after discharge from the orthopaedic department
Secondary outcome measures: admission time, time to first readmission, number of readmissions, emergency department visits, visits to outpatient care clinic, general practitioner contacts if first contact with general practitioner included medication issues, contacts with physicians outside working hours, medical specialist contacts, quality of life assessment, mortality All outcomes had 3 months of follow‐up |
|
| Notes | Funding: The Health Insurance Foundation in Denmark | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible participants were randomly assigned to intervention or control by a computer‐generated code |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study described as not blinded |
| Blinding of outcome assessment (detection bias) Mortality (all‐cause) | Low risk | Mortality data were taken from a non‐biased national register (participants identifiable through unique patient‐specific social security number) |
| Blinding of outcome assessment (detection bias) Hospital readmissions (all‐cause) | Low risk | Readmission data were likely gathered from a non‐biased national register |
| Blinding of outcome assessment (detection bias) Hospital emergency department contacts (all‐cause) | Low risk | Emergency department contacts were likely gathered from a non‐biased national register |
| Incomplete outcome data (attrition bias) Mortality (all‐cause) | Low risk | No loss to follow‐up was described; all data should be readily available from a non‐biased national register |
| Incomplete outcome data (attrition bias) Hospital readmissions (all‐cause) | Low risk | No loss to follow‐up was described; all data should be readily available from a non‐biased national register |
| Incomplete outcome data (attrition bias) Hospital emergency department contacts (all‐cause) | Low risk | No loss to follow‐up was described; all data should be readily available from a non‐biased national register |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were collected and were similar to information provided on www.clinicaltrials.gov |
| Contamination bias | High risk | No cluster‐randomisation |
| Other bias | Low risk | No evidence of other types of bias |
Schnipper 2006.
| Methods | Randomised controlled trial | |
| Participants | A total of 178 participants were randomised ‐ 92 to medication review and 86 to control. Participants were admitted to the general medicine service at a university hospital in the USA Mean age: 59.3 years; 34% male; median number of drugs: 8.0 | |
| Interventions | The pharmacist intervention on the day of discharge consisted of several parts. First, discharge medication regimens were compared with preadmission regimens, and all discrepancies were reconciled with assistance of the medical team. Participants were screened for previous drug‐related problems, including non‐adherence, lack of efficacy and side effects. The pharmacist reviewed with the participant the indications, directions for use and potential adverse effects of each discharge medication, and discussed significant findings with the medical team Co‐interventions: follow‐up telephone call, during which the pharmacist compared the participant's self reported medication list with the discharge list, exploring any discrepancies The pharmacist also asked about medication adherence, possible adverse drug events and adherence with scheduled follow‐up and laboratory appointments. Significant findings were communicated to the participant's primary care physician |
|
| Outcomes | Primary outcome measure: preventable adverse drug events
Secondary outcome measures: all adverse drug events (preventable or not), participant satisfaction, health care utilisation (readmission + emergency department contact), medication adherence, medication discrepancies All outcomes had 30 days of follow‐up |
|
| Notes | Funding: This study was supported by the Division of General Medicine at Brigham and Women’s Hospital (BWH), Boston, MA, the Fish and Anderson Fundsat BWH and an unrestricted grant from the Merck Co Foundation, West Point, PA. Dr Schnipper is supported by Mentored Clinical Scientist Development Award HL072806 from the National Heart, Lung and Blood Institute, Bethesda, MD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performedby a computer‐generated algorithm |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes were opened only after patient consent was obtained |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study was described as not blinded |
| Blinding of outcome assessment (detection bias) Hospital readmissions (all‐cause) | Low risk | Outcomes were assessed by research assistants and manuscript authors blinded to treatment assignment |
| Blinding of outcome assessment (detection bias) Hospital readmissions (due to adverse drug events) | Unclear risk | Outcomes were assessed by research assistants and manuscript authors blinded to treatment assignment. Nevertheless, coding of the causality of hospital readmissions due to adverse events may be biased (e.g. by participants knowing their allocation and expressing this to the rating physician) |
| Blinding of outcome assessment (detection bias) Hospital emergency department contacts (all‐cause) | Low risk | Outcomes were assessed by research assistants and manuscript authors blinded to treatment assignment |
| Blinding of outcome assessment (detection bias) Hospital emergency department contacts (due to adverse drug events) | Unclear risk | Outcomes were assessed by research assistants and manuscript authors blinded to treatment assignment. Nevertheless, coding of the causality of hospital emergency department visits due to adverse events may be biased (e.g. by participants knowing their allocation and expressing this to the rating physician) |
| Blinding of outcome assessment (detection bias) Adverse drug events | Unclear risk | Outcomes were assessed by research assistants and manuscript authors blinded to treatment assignment. Nevertheless, coding of the causality of adverse events may be unblinded (e.g. by participants knowing their allocation and expressing this to the rating physician) |
| Incomplete outcome data (attrition bias) Hospital readmissions (all‐cause) | Unclear risk | Uneven loss to follow‐up: medication review 33/92 participants; control group 18/84 participants. Unclear how many participants lost to follow‐up had died |
| Incomplete outcome data (attrition bias) Hospital readmissions (due to adverse drug events) | Unclear risk | Uneven loss to follow‐up: medication review 33/92 participants; control group 18/84 participants. Unclear how many participants lost to follow‐up had died |
| Incomplete outcome data (attrition bias) Hospital emergency department contacts (all‐cause) | Unclear risk | Uneven loss to follow‐up: medication review 33/92 participants; control group 18/84 participants. Unclear how many participants lost to follow‐up had died |
| Incomplete outcome data (attrition bias) Hospital emergency department contacts (due to adverse drug events) | Unclear risk | Uneven loss to follow‐up: medication review 33/92 participants; control group 18/84 participants. Unclear how many participants lost to follow‐up had died |
| Incomplete outcome data (attrition bias) Adverse drug events | Unclear risk | Uneven loss to follow‐up: medication review 33/92 participants; control group 18/84 participants. Unclear how many participants lost to follow‐up had died |
| Selective reporting (reporting bias) | Unclear risk | No deaths reported, but some mortality data were likely to have been available (e.g. when hospital records of contacting spouses of participants were searched) |
| Contamination bias | High risk | No cluster‐randomisation |
| Other bias | Low risk | No evidence of other types of bias |
Scullin 2007.
| Methods | Randomised controlled trial | |
| Participants | A total of 762 participants were randomised ‐ 371 to medication review (integrated medicines management) and 391 to control. Participants were admitted to medical wards at 3 general hospitals in Northern Ireland. Mean age: 70.1 years; 47% male | |
| Interventions | A clinical pharmacist (aided by a pharmacy technician) constructed an accurate medication history by using a variety of sources and reviewed drug treatment daily, taking into account therapeutic goals, relevant clinical chemistry and haematology results and, when appropriate, therapeutic drug monitoring. The intervention also included medication counselling tailored to suit the needs of each individual participant | |
| Outcomes | Primary outcome measure: difference in length of hospital stay
Secondary outcome measures: time to hospital readmission, number of readmissions, healthcare practitioner satisfaction All outcomes had 30 days of follow‐up |
|
| Notes | Funding: Funding for this project was obtained from the Department of Health, Social Services and Public Safety (Northern Ireland), under its Executive Programme Fund scheme | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Allocated to groups using closed‐envelope technique. Randomisation was performed in blocks of 20 (each block contained 10 intervention and 10 control allocations), which could reveal allocation. No description of who includedparticipants |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described as blinded |
| Blinding of outcome assessment (detection bias) Mortality (all‐cause) | Low risk | Data collected by researchers aware of assignments, but this will likely not influence assessment of mortality |
| Blinding of outcome assessment (detection bias) Hospital readmissions (all‐cause) | Low risk | Data collected by researchers aware of assignments, but this will likely not influence assessment of readmissions |
| Blinding of outcome assessment (detection bias) Hospital emergency department contacts (due to adverse drug events) | Low risk | |
| Incomplete outcome data (attrition bias) Mortality (all‐cause) | Unclear risk | Discrepancies in the publication concerning reporting of mortality data, as 7 participants seem to be missing from the medication review group and 1 from the control group |
| Incomplete outcome data (attrition bias) Hospital readmissions (all‐cause) | High risk | Discrepancies in the publication concerning reporting of hospital readmission data. The publication states that 141 participants (40.8%) were readmitted in the medication review group vs 171 participants (49.3%) in the control group. However, when percentages were used to calculate the total number of participants, 25 participants seemed to be missing from the medication review group (346 vs 371), but only 1 was described as lost to follow‐up and 42 as missing from the control group (349 vs 391); only 7 were described as lost to follow‐up. This could have influenced the outcome of hospital readmissions |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes seem to have been reported |
| Contamination bias | High risk | No cluster‐randomisation |
| Other bias | High risk | Unequal randomisation with 371 participants assigned to the medication review group and 391 to the control group should not have been possible when block sizes were 20. This may have been possible if each block was per hospital, but this was not described. Very unclear reporting of data. Data not reported for surgical wards; no reason stated for excluding data. No protocol available |
Abbreviations:
AOU: Assessment of Underutilization index. DRP: Drug‐related problem. EQ‐5D: Standardised instrument of the EuroQol Group used to measure health outcomes. FASS: Pharmaceutical Specialities in Sweden. IQR: Interquartile range. MAI: Medication appropriateness index. PIM: Potentially inappropriate medication. PIP: Potentially inappropriate prescribing. START: Screening Tool to Alert to Right Treatment. STOPP: Screening Tool of Older Persons’ potentially inappropriate Prescriptions.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| [no author] 2001 | Not a randomised controlled trial |
| Ahmad 2012 | Intervention did not meet inclusion criteria (to be implemented after discharge) |
| Al Mazroui 2009 | Population did not meet inclusion criteria (included outpatients) |
| Al‐Rashed 2002 | Intervention did not meet inclusion criteria (only drug information) |
| Allen 1986 | Intervention did not meet inclusion criteria (geriatric team) |
| Bolas 2004 | Intervention did not meet inclusion criteria (only medication reconciliation) |
| Bondesson 2013 | Not a randomised controlled trial |
| Bonnet‐Zamponi 2013 | Not a randomised controlled trial (used a Zelen design by which consent is obtained after randomisation, leading to selection bias) |
| Burleson 2003 | Intervention did not meet inclusion criteria (medication history) |
| Burnett 2009 | Outcome did not meet inclusion criteria (medication appropriateness) |
| Cardinale 1993 | Not a randomised controlled trial |
| Ciechanover 1987 | Not a randomised controlled trial |
| Connor 2012 | Outcome did not meet inclusion criteria (no follow‐up data after discharge according to study authors) |
| Crotty 2004 | Intervention did not meet inclusion criteria (medication reconciliation) |
| Frankenthal 2014 | Population did not meet inclusion criteria (chronic geriatric facility) |
| Gattis 1999 | Population did not meet inclusion criteria (included outpatients) |
| Hellstrom 2011 | Not a randomised controlled trial |
| Kelly 2011 | Not a randomised controlled trial |
| Koehler 2009 | Intervention did not meet inclusion criteria (no medication review) |
| Lipton 1992 | Outcome did not meet inclusion criteria (medication appropriateness) |
| Marusic 2013 | Intervention did not meet inclusion criteria (counselling) |
| McMullin 1999 | Not a randomised controlled trial |
| Naughton 1994 | Intervention did not meet inclusion criteria (geriatric team) |
| NCT01467128 | Outcome did not meet inclusion criteria (no follow‐up data after discharge according to study authors) |
| Pope 2011 | Intervention did not meet inclusion criteria (medication review implemented after discharge) |
| Rainville 1999 | Intervention did not meet inclusion criteria (only heart failure medication reviewed) |
| Saltvedt 2005 | Outcome did not meet inclusion criteria (medication use) |
| Schmader 1997 | Not a randomised controlled trial |
| Schmader 2004 | Intervention did not meet inclusion criteria (geriatric team) |
| Sjoberg 2013 | Intervention did not meet inclusion criteria (medication review restricted to fall risk‐increasing drugs) |
| Smith 1996 | Not a randomised controlled trial |
| Spinewine 2007 | Not a randomised controlled trial (quasi‐randomised trial, used alternate randomisation) |
| Stowasser 2002 | Intervention did not meet inclusion criteria (medication reconciliation) |
| Walker 2009 | Not a randomised controlled trial |
Characteristics of ongoing studies [ordered by study ID]
ISRCTN08043800.
| Trial name or title | Pharmacists' review of medicine during admission to hospital |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Intervention group • Review and use of patient's own drugs by clinical pharmacist • Clinical pharmacist taking secondary medication history • Medication review by clinical pharmacist • Entry of proposed prescriptions into the electronic medication system by pharmacist, ready for approval by doctor The intervention takes place on the day the participant is admitted, and the duration of the intervention is approximately 1.5 hours Control group
|
| Outcomes | Primary outcome measures
Secondary outcome measures • Length of hospital stay • Number of readmissions during the first year after admission • Direct cost for the hospital |
| Starting date | March 2009 |
| Contact information | Principal Investigator: Trine R. H. Nielsen, Region Zealand Hospital Pharmacy, Denmark |
| Notes | www.isrctn.com (accessed May 2015). Trial ID: ISRCTN08043800 |
Loffler ongoing.
| Trial name or title | Optimizing polypharmacy among elderly hospital patients with chronic diseases ‐ study protocol of the cluster randomized controlled POLITE‐RCT trial |
| Methods | Cluster‐randomised controlled trial |
| Participants | Clusters
Inclusion criteria
Exclusion criteria
Participants Inclusion criteria
Exclusion criteria
|
| Interventions | During in‐patient treatment of participants affected by polypharmacy, a pharmacist specially trained in communication skills performs a narrative‐based medication review. Thus, 2 approaches are combined here: the face‐to‐face clinical “brown bag” medication review, and the patient‐centred approach of narrative medicine. Apart from detecting potentially inadequate medication, a major aim is to identify patient preferences and to include them ‐ when possible ‐ into a hierarchically structured list of evidence‐based medication recommendations. Thus, priorities for medication modification can be based on both 'objective' pharmaceutical considerations and 'subjective' participant preferences |
| Outcomes | The 2 independent main outcomes are (1) health‐related quality of life (EQ‐5D), and (2) the difference in the number of prescribed long‐term pharmaceutical agents between intervention and control groups at T3. Secondary outcomes are appropriateness of prescribed medication (PRISCUS list, Beers criteria, MAI), patient satisfaction (TSQM), patient empowerment (PEF‐FB‐9), patient autonomy (IADL), falls (frequency and severity), rehospitalisation and death. For all participants ensured with the largest public German health insurance provider (AOK), cost‐effectiveness will be analysed by the Scientific Institute of the AOK (WIdO) |
| Starting date | November 2013 |
| Contact information | Principal Investigator: Christin Löffler, Institute of General Practice, Rostock University Medical Center, Rostock, Germany |
| Notes | www.controlled‐trials.com (accessed May 2015). Trial ID: ISRCTN42003273 |
NCT00844025.
| Trial name or title | Pharmaceutical care and clinical outcomes for the elderly taking potentially inappropriate medication |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Patients in the intervention group will receive pharmaceutical care delivered by a clinical pharmacist, which includes medication review, medication reconciliation, patient education and recommended actions. Patients randomised to the usual care group will receive routine review of medication by ward‐based pharmacist and nurse |
| Outcomes | Primary outcome measure: number of unsolved drug‐related problems Secondary outcome measures: rate of adverse drug events during hospitalisation, number of potentially inappropriate medications |
| Starting date | February 2009 |
| Contact information | Principal Investigator: Liu Jen Wei, Shin Kong Wo Ho‐Su Memorial Hospital, Department of Pharmacy, Taipei, Taiwan |
| Notes | www.clinicaltrials.gov (accessed May 2015). Trial ID: NCT00844025 |
NCT01504672.
| Trial name or title | A randomized controlled pharmacist intervention study to reduce drug‐related problems and readmissions among old people with dementia |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Medication review In the intervention, the pharmacist will evaluate the following
|
| Outcomes | Primary outcome measure
Secondary outcome measures
|
| Starting date | January 2012 |
| Contact information | Principal Investigator: Hugo Lövheim, Umeå University, Umeå, Sweden |
| Notes | www.clinicaltrials.gov (accessed May 2015). Trial ID: NCT01504672 |
Abbreviations:
EQ‐5D: Standardised instrument of the EuroQol Group used to measure health outcomes. IADL: Instrumental activities of daily living. MAI: Medication appropriateness index. PEF‐FB‐9: "Fragebogen zur Partizipativen Ent‐scheidungsfindung (revidierte 9‐Item‐Fassung)" ‐ tool used to measure patient empowerment. TSQM: Treatment Satisfaction Questionnaire for Medication,
Differences between protocol and review
The domain of contamination bias was added in the update of this review.
Subgroup analysis and investigation of heterogeneity
We planned to explore our findings by performing the following prespecified subgroup analyses.
Trials including only patients with high risk of medication errors and adverse drug events (study inclusion and exclusion criteria defined patient population as a high‐risk population (e.g. elderly patients, patients with multiple co‐medications)).
Trials in which the medication review was performed by a person or team with the capability to change participants' medications directly (as opposed to medication review carried out by healthcare professionals who were not allowed to change participants' medications, but who recommended changes to a responsible in‐hospital tending physician).
Trials in which the medication review was done through a validated method (e.g. Beers’ criteria (Beers 1997), START/STOPP criteria (Gallagher 2008a)).
As a result of the limited number of trials and the need to avoid multiplicity issues, we restricted analyses to the dichotomous outcomes of mortality (all‐cause), hospital readmissions (all‐cause) and hospital emergency department contacts (all‐cause).
Originally we planned to investigate the intervention effect in a sensitivity analysis of trials with low risk of bias. However, to adhere to recent recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011), and to test for subgroup differences, we instead investigated this in a subgroup analysis comparing low risk of bias trials with high risk of bias trials. We defined low risk of bias trials as trials with low risk of selection bias, detection bias and selective reporting, and all other trials as having high risk of bias.
Sensitivity analysis
We intended to perform a sensitivity analysis by excluding cluster‐randomised trials, but none of the identified trials were cluster‐randomised.
Contributions of authors
Review authors contributed equally to development of the protocol, study inclusion, data extraction, 'Risk of bias' assessment, data analysis, interpretation of results and writing of the manuscript.
Sources of support
Internal sources
-
The Nordic Cochrane Centre, Denmark.
The centre provided research facilities for the initial review.
-
Department of Clinical Pharmacology, Bispebjerg Hospital, Denmark.
The department provided research facilities for the update.
External sources
-
TrygFonden, Denmark.
Both review authors were salaried by a grant from TrygFonden, a non‐profit foundation, for the initial review. Review authors did not receive funding for the update.
Declarations of interest
MC declares no conflicts of interest relevant to this review. AL declares no conflicts of interest relevant to this review.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bladh 2011 {published data only}
- Bladh L, Ottosson E, Karlsson J, Klintberg L, Wallerstedt SM. Effects of a clinical pharmacist service on health‐related quality of life and prescribing of drugs: a randomised controlled trial. BMJ Quality & Safety 2011;20(9):738‐46. [PUBMED: 21209140] [DOI] [PubMed] [Google Scholar]
Bonnerup 2014 {unpublished data only}
- Bonnerup DK, Lisby M, Sædder EA, Brock B, Truelshøj T, Sørensen CA, et al. Effects of stratified medication reviews in high‐risk patients at admission to hospital: a randomised controlled trial. Risk Stratification of Medication Reviews: Physicians’ Perspectives, Test of Algorithm and Interventions [PhD Thesis by Dorthe Bonnerup]. Aarhus, Denmark: Faculty of Health Sciences, Aarhus University, 2014:82‐100. [Google Scholar]
Dalleur 2014 {published data only}
- Dalleur O, Boland B, Losseau C, Henrard S, Wouters D, Speybroeck N, et al. Reduction of potentially inappropriate medications using the STOPP criteria in frail older inpatients: a randomised controlled study. Drugs & Aging 2014;31(4):291‐8. [PUBMED: 24566877] [DOI] [PubMed] [Google Scholar]
Farris 2014 {published data only}
- Farris KB, Carter BL, Xu Y, Dawson JD, Shelsky C, Weetman DB, et al. Effect of a care transition intervention by pharmacists: an RCT. BMC Health Services Research 2014;14:406. [PUBMED: 25234932] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gallagher 2011 {published data only (unpublished sought but not used)}
- Gallagher PF, O’Connor MN, O’Mahony D. Prevention of potentially inappropriate prescribing for elderly patients: a randomized controlled trial using STOPP/START criteria. Clinical Pharmacology and Therapeutics 2011;89(6):845‐54. [PUBMED: 21508941] [DOI] [PubMed] [Google Scholar]
Gillespie 2009 {published and unpublished data}
- Gillespie U, Alassaad A, Henrohn D, Garmo H, Hammarlund‐Udenaes M, Toss H, et al. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: a randomized controlled trial. Archives of Internal Medicine 2009;169(9):894‐900. [PUBMED: 19433702] [DOI] [PubMed] [Google Scholar]
Lisby 2010 {published and unpublished data}
- Lisby M, Thomsen A, Nielsen LP, Lyhne NM, Breum‐Leer C, Fredberg U, et al. The effect of systematic medication review in elderly patients admitted to an acute ward of internal medicine. Basic and Clinical Pharmacology and Toxicology 2010;106(5):422‐7. [PUBMED: 20059474] [DOI] [PubMed] [Google Scholar]
Lisby 2015 {published data only}
- Lisby M, Bonnerup DK, Brock B, Gregersen PA, Jensen J, Larsen ML, et al. Medication review and patient outcomes in an orthopedic department: a randomized controlled study. Journal of Patient Safety 2015;March 4:[Epub ahead of print]. [PUBMED: 25742062] [DOI] [PubMed] [Google Scholar]
Schnipper 2006 {published data only (unpublished sought but not used)}
- Schnipper JL, Kirwin JL, Cotugno MC, Wahlstrom SA, Brown BA, Tarvin E, et al. Role of pharmacist counselling in preventing adverse drug events after hospitalization. Archives of Internal Medicine 2006;166(5):565‐71. [PUBMED: 16534045] [DOI] [PubMed] [Google Scholar]
Scullin 2007 {published data only}
- Scullin C, Scott MG, Hogg A, McElnay JC. An innovative approach to integrated medicines management. Journal of Evaluation in Clinical Practice 2007;13(5):781‐8. [PUBMED: 17824872] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
[no author] 2001 {published data only}
- [no author]. Hospital pharmacists and the NSF for CHD. Pharmaceutical Journal 2001;267(7169):518. [Google Scholar]
Ahmad 2012 {published data only}
- Ahmad A, Nijpels G, Dekker JM, Kostense PJ, Hugtenburg JG. Effect of a pharmacist medication review in elderly patients discharged from the hospital. Archives of Internal Medicine 2012;172(17):1346‐7. [PUBMED: 22868274] [DOI] [PubMed] [Google Scholar]
Allen 1986 {published data only}
- Allen CM, Becker PM, McVey LJ, Saltz C, Feussner JR, Cohen HJ. A randomized, controlled clinical trial of a geriatric consultation team. Compliance with recommendations. JAMA 1986;255(19):2617‐21. [PUBMED: 3517396] [PubMed] [Google Scholar]
Al Mazroui 2009 {published data only}
- Al Mazroui NR, Kamal MM, Ghabash NM, Yacout TA, Kole PL, McElnay JC. Influence of pharmaceutical care on health outcomes in patients with Type 2 diabetes mellitus. British Journal of Clinical Pharmacology 2009;67(5):547‐57. [PUBMED: 19552750] [DOI] [PMC free article] [PubMed] [Google Scholar]
Al‐Rashed 2002 {published data only}
- Al‐Rashed SA, Wright DJ, Roebuck N, Sunter W, Chrystyn H. The value of inpatient pharmaceutical counselling to elderly patients prior to discharge. British Journal of Clinical Pharmacology 2002;54(6):657‐64. [PUBMED: 12492615] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bolas 2004 {published data only}
- Bolas H, Brookes K, Scott M, McElnay J. Evaluation of a hospital‐based community liaison pharmacy service in Northern Ireland. Pharmacy World and Science 2004;26(2):114‐20. [PUBMED: 15085948] [DOI] [PubMed] [Google Scholar]
Bondesson 2013 {published data only}
- Bondesson A, Eriksson T, Kragh A, Holmdahl L, Midlov P, Hoglund P. In‐hospital medication reviews reduce unidentified drug‐related problems. European Journal of Clinical Pharmacology 2013;69(3):647‐55. [PUBMED: 22955893] [DOI] [PubMed] [Google Scholar]
Bonnet‐Zamponi 2013 {published data only}
- Bonnet‐Zamponi D, d'Arailh L, Konrat C, Delpierre S, Lieberherr D, Lemaire A, et al. Drug‐related readmissions to medical units of older adults discharged from acute geriatric units: results of the Optimization of Medication in AGEd multicenter randomized controlled trial. Journal of the American Geriatrics Society 2013;61(1):113‐21. [PUBMED: 23252914] [DOI] [PubMed] [Google Scholar]
Burleson 2003 {published data only}
- Burleson BS. Impact of pharmacist‐conducted medication histories on number of pharmacist's interventions, drug cost, medication errors, re‐admission rates, and mortality during hospitalization of medicine patients. ASHP Midyear Clinical Meeting. 2003; Vol. 38:P‐610.
Burnett 2009 {published data only}
- Burnett KM, Scott MG, Fleming GF, Clark CM, McElnay JC. Effects of an integrated medicines management program on medication appropriateness in hospitalized patients. American Journal of Health‐System Pharmacy 2009;66(9):854‐9. [DOI] [PubMed] [Google Scholar]
Cardinale 1993 {published data only}
- Cardinale V. Clinical R.Ph.s in a team prove to be cost‐effective. Drug Topics 1993;Jan Suppl:1. [Google Scholar]
Ciechanover 1987 {published data only}
- Ciechanover M, Kohn D. A drug‐reduction trial in elderly patients. Harefuah 1987;112(11):543‐5. [PubMed] [Google Scholar]
Connor 2012 {unpublished data only}
- Connor MO, O'Sullivan D, Gallagher P, Byrne S, Eustace J, O'Mahony D. Prevention of adverse drug events in hospitalised older patients: a randomised controlled trial. Irish Journal of Medical Science. Cork, Ireland: Conference: 60th Annual Scientific Meeting of the Irish Gerontological Society, 2012:S181‐S223.
Crotty 2004 {published data only}
- Crotty M, Rowett D, Spurling L, Giles LC, Phillips PA. Does the addition of a pharmacist transition coordinator improve evidence‐based medication management and health outcomes in older adults moving from the hospital to a long‐term care facility? Results of a randomized, controlled trial. American Journal of Geriatric Pharmacotherapy 2004;2(4):257‐64. [PUBMED: 15903284] [DOI] [PubMed] [Google Scholar]
Frankenthal 2014 {published data only}
- Frankenthal D, Lerman Y, Kalendaryev E, Lerman Y. Intervention with the screening tool of older persons potentially inappropriate prescriptions/screening tool to alert doctors to right treatment criteria in elderly residents of a chronic geriatric facility: a randomized clinical trial. Journal of the American Geriatrics Society 2014;62(9):1658‐65. [PUBMED: 25243680] [DOI] [PubMed] [Google Scholar]
Gattis 1999 {published data only}
- Gattis WA, Hasselblad V, Whellan DJ, O'Connor CM. Reduction in heart failure events by the addition of a clinical pharmacist to the heart failure management team: results of the Pharmacist in Heart Failure Assessment Recommendation and Monitoring (PHARM) Study. Archives of Internal Medicine 1999;159(16):1939‐45. [PUBMED: 10493325] [DOI] [PubMed] [Google Scholar]
Hellstrom 2011 {published data only}
- Hellstrom LM, Bondesson A, Hoglund P, Midlov P, Holmdahl L, Rickhag E, et al. Impact of the Lund Integrated Medicines Management (LIMM) model on medication appropriateness and drug‐related hospital revisits. European Journal of Clinical Pharmacology 2011;67(7):741‐52. [PUBMED: 21318595] [DOI] [PubMed] [Google Scholar]
Kelly 2011 {published data only}
- Kelly DM, Frick EM, Hale LS. How the medication review can help to reduce risk of falls in older patients. JAAPA 2011;24(4):30‐4, 55. [PUBMED: 21534380] [DOI] [PubMed] [Google Scholar]
Koehler 2009 {published data only}
- Koehler BE, Richter KM, Youngblood L, Cohen BA, Prengler ID, Cheng D, et al. Reduction of 30‐day postdischarge hospital readmission or emergency department (ED) visit rates in high‐risk elderly medical patients through delivery of a targeted care bundle. Journal of Hospital Medicine 2009;4(4):211‐8. [PUBMED: 19388074] [DOI] [PubMed] [Google Scholar]
Lipton 1992 {published data only}
- Lipton HL, Bero LA, Bird JA, McPhee SJ. The impact of clinical pharmacists' consultations on physicians' geriatric drug prescribing. A randomized controlled trial. Medical Care 1992;30(7):646‐58. [PUBMED: 1614233] [DOI] [PubMed] [Google Scholar]
Marusic 2013 {published data only}
- Marusic S, Gojo‐Tomic N, Erdeljic V, Bacic‐Vrca V, Franic M, Kirin M, et al. The effect of pharmacotherapeutic counselling on readmissions and emergency department visits. International Journal of Clinical Pharmacy 2013;35(1):37‐44. [PUBMED: 23007693] [DOI] [PubMed] [Google Scholar]
McMullin 1999 {published data only}
- McMullin ST, Hennenfent JA, Ritchie DJ, Huey WY, Lonergan TP, Schaiff RA, et al. A prospective, randomized trial to assess the cost impact of pharmacist‐initiated interventions. Archives of Internal Medicine 1999;159(19):2306‐9. [PUBMED: 10547170] [DOI] [PubMed] [Google Scholar]
Naughton 1994 {published data only}
- Naughton BJ, Moran MB, Feinglass J, Falconer J, Williams ME. Reducing hospital costs for the geriatric patient admitted from the emergency department: a randomized trial. Journal of the American Geriatrics Society 1994;42(10):1045‐9. [PUBMED: 7930327] [DOI] [PubMed] [Google Scholar]
NCT01467128 {unpublished data only}
- NCT01467128. Adverse Drug Event (ADE) Incidence in Older Patients Following Hospital Admission and Pharmacist Review to Older Persons' Prescriptions and Its' Effect on ADE Reduction in Hospital: A Randomised Controlled Trial. www.clinicaltrials.gov/show/NCT01467128 (accessed May 2015).
Pope 2011 {published data only}
- Pope G, Wall N, Peters CM, O'Connor M, Saunders J, O'Sullivan C, et al. Specialist medication review does not benefit short‐term outcomes and net costs in continuing‐care patients. Age and Ageing 2011;40(3):307–12. [PUBMED: 20817937] [DOI] [PubMed] [Google Scholar]
Rainville 1999 {published data only}
- Rainville EC. Impact of pharmacist interventions on hospital readmissions for heart failure. American Journal of Health‐System Pharmacy 1999;56(13):1339‐42. [PUBMED: 10683133] [PubMed] [Google Scholar]
Saltvedt 2005 {published data only}
- Saltvedt I, Spigset O, Ruths S, Fayers P, Kaasa S, Sletvold O. Patterns of drug prescription in a geriatric evaluation and management unit as compared with the general medical wards: a randomised study. European Journal of Clinical Pharmacology 2005;61(12):921‐8. [PUBMED: 16307267] [DOI] [PubMed] [Google Scholar]
Schmader 1997 {published data only}
- Schmader KE, Hanlon JT, Landsman PB, Samsa GP, Lewis IK, Weinberger M. Inappropriate prescribing and health outcomes in elderly veteran outpatients. The Annals of Pharmacotherapy 1997;31(5):529‐33. [PUBMED: 9161643] [DOI] [PubMed] [Google Scholar]
Schmader 2004 {published data only}
- Schmader KE, Hanlon JT, Pieper CF, Sloane R, Ruby CM, Twersky J, et al. Effects of geriatric evaluation and management on adverse drug reactions and suboptimal prescribing in the frail elderly. American Journal of Medicine 2004;116(6):394‐401. [PUBMED: 15006588] [DOI] [PubMed] [Google Scholar]
Sjoberg 2013 {published data only}
- Sjoberg C, Wallerstedt SM. Effects of medication reviews performed by a physician on treatment with fracture‐preventing and fall‐risk‐increasing drugs in older adults with hip fracture ‐ a randomized controlled study. Journal of the American Geriatrics Society 2013;61(9):1464‐72. [PUBMED: 24028354] [DOI] [PubMed] [Google Scholar]
Smith 1996 {published data only}
- Smith DM, Cox MR, Brizendine EJ, Hui SL, Freedman JA, Martin DK, et al. An intervention on discharge polypharmacy. Journal of the American Geriatrics Society 1996;44(4):416‐9. [PUBMED: 8636588] [DOI] [PubMed] [Google Scholar]
Spinewine 2007 {published data only (unpublished sought but not used)}
- Spinewine A, Swine C, Dhillon S, Lambert P, Nachega JB, Wilmotte L, et al. Effect of a collaborative approach on the quality of prescribing for geriatric inpatients: a randomized, controlled trial. Journal of the American Geriatrics Society 2007;55(5):658‐65. [PUBMED: 17493184] [DOI] [PubMed] [Google Scholar]
Stowasser 2002 {published data only}
- Stowasser DA, Collins DM, Stowasser M. A randomised controlled trial of medication liaison services ‐ patient outcomes. Journal of Pharmacy Practice and Research 2002;32:133‐40. [Google Scholar]
Walker 2009 {published data only}
- Walker PC, Bernstein SJ, Tucker Jones JN, Piersma J, Kim HW, Regal RE, et al. Impact of a pharmacist‐facilitated hospital discharge program: a quasi‐experimental study. Archives of Internal Medicine 2009;169(21):2003‐10. [PUBMED: 19933963] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ISRCTN08043800 {unpublished data only}
- ISRCTN08043800. Pharmaceutical Optimization of the Medication Process During Admission to Hospital: A Multicentre, Randomised, Controlled Trial. www.isrctn.com/ISRCTN42003273 (accessed May 2015).
Loffler ongoing {unpublished data only}
- Loffler C, Drewelow E, Paschka SD, Frankenstein M, Eger J, Jatsch L, et al. Optimizing Polypharmacy Among Elderly Hospital Patients With Chronic Diseases ‐ Study Protocol of the Cluster Randomized Controlled POLITE‐RCT Trial. Implementation Science 2014;9:151. [PUBMED: 25287853] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00844025 {unpublished data only}
- NCT00844025. Pharmaceutical Care and Clinical Outcomes for the Elderly Taking Potentially Inappropriate Medication. www.clinicaltrials.gov/show/NCT00844025 (accessed May 2015).
NCT01504672 {unpublished data only}
- NCT01504672. A Randomized Controlled Pharmacist Intervention Study to Reduce Drug‐related Problems and Readmissions Among Old People With Dementia. www.clinicaltrials.gov/show/NCT01504672 (accessed May 2015).
Additional references
Aronson 2006
- Aronson JK. Polypharmacy, appropriate and inappropriate. The British Journal of General Practice 2006; Vol. 56, issue 528:484‐5. [PUBMED: 16834872] [PMC free article] [PubMed]
Beers 1991
- Beers MH, Ouslander JG, Rollingher I, Reuben DB, Brooks J, Beck JC. Explicit criteria for determining inappropriate medication use in nursing home residents. UCLA Division of Geriatric Medicine. Archives of Internal Medicine 1991;151(9):1825‐32. [PUBMED: 1888249] [PubMed] [Google Scholar]
Beers 1997
- Beers MH. Explicit criteria for determining potentially inappropriate medication use by the elderly. An update. Archives of Internal Medicine 1997;157(14):1531‐6. [PUBMED: 9236554] [PubMed] [Google Scholar]
Bourgeois 2010
- Bourgeois FT, Shannon MW, Valim C, Mandl KD. Adverse drug events in the outpatient setting: an 11‐year national analysis. Pharmacoepidemiology and Drug Safety 2010;19(9):901‐10. [PUBMED: 20623513] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bregnhøj 2009
- Bregnhøj L, Thirstrup S, Kristensen MB, Bjerrum L, Sonne J. Combined intervention programme reduces inappropriate prescribing in elderly patients exposed to polypharmacy in primary care. European Journal of Clinical Pharmacology 2009;65(2):199‐207. [PUBMED: 18807252] [DOI] [PubMed] [Google Scholar]
Budnitz 2011
- Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. The New England Journal of Medicine 2011;365(21):2002‐12. [PUBMED: 22111719] [DOI] [PubMed] [Google Scholar]
CDC 2011
- Centers for Disease Control and Prevention. Health, United States, 2011. Atlanta: Centers for Disease Control and Prevention, 2012. [Google Scholar]
Chen 2007
- Chen TF, Almeida Neto AC. Exploring elements of interprofessional collaboration between pharmacists and physicians in medication review. Pharmacy World and Science 2007;29(6):574‐6. [PUBMED: 17479354] [DOI] [PubMed] [Google Scholar]
Christensen 2009
- Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet 2009;374(9696):1196‐208. [PUBMED: 19801098] [DOI] [PMC free article] [PubMed] [Google Scholar]
Classen 1997
- Classen DC, Pestotnik SL, Evans RS, Lloyd JF, Burke JP. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA 1997;277(4):301‐6. [PUBMED: 9002492] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Ebbesen 2001
- Ebbesen J, Buajordet I, Erikssen J, Brors O, Hilberg T, Svaar H, et al. Drug‐related deaths in a department of internal medicine. Archives of Internal Medicine 2001;161(19):2317‐23. [PUBMED: 11606147] [DOI] [PubMed] [Google Scholar]
ElDesoky 2007
- ElDesoky ES. Pharmacokinetic‐pharmacodynamic crisis in the elderly. American Journal of Therapeutics 2007;14(5):488‐98. [PUBMED: 17890940] [DOI] [PubMed] [Google Scholar]
EPOC 2015
- EPOC. Suggested risk of bias criteria for EPOC reviews: suggested risk of bias criteria for EPOC reviews. http://epoc.cochrane.org/sites/epoc.cochrane.org/files/uploads/ (accessed November 2015).
Epstein 2011
- Epstein AM, Jha AK, Orav EJ. The relationship between hospital admission rates and rehospitalizations. The New England Journal of Medicine 2011;365(24):2287‐95. [PUBMED: 22168643] [DOI] [PubMed] [Google Scholar]
European Communities 2006
- European Communities. Population statistics 2006. www.epp.eurostat.ec.europa.eu/cache/ITY_OFFPUB/KS‐EH‐06‐001/EN/KS‐EH‐06‐001‐EN.PDF (accessed 1 April 2012).
Fick 2003
- Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Archives of Internal Medicine 2003;163(22):2716‐24. [PUBMED: 14662625] [DOI] [PubMed] [Google Scholar]
Gallagher 2008a
- Gallagher P, Ryan C, Byrne S, Kennedy J, O'Mahony D. STOPP (Screening Tool of Older Person's Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. International Journal of Clinical Pharmacology and Therapeutics 2008;46(2):72‐83. [PUBMED: 18218287] [DOI] [PubMed] [Google Scholar]
Gallagher 2008b
- Gallagher P, O'Mahony D. STOPP (Screening Tool of Older Persons' potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers' criteria. Age and Ageing 2008;37(6):673‐9. [PUBMED: 18829684] [DOI] [PubMed] [Google Scholar]
Gnjidic 2012
- Gnjidic D, Hilmer SN, Blyth FM, Naganathan V, Waite L, Seibel MJ, et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community‐dwelling older men at risk of different adverse outcomes. Journal of Clinical Epidemiology 2012;65(9):989‐95. [PUBMED: 22742913] [DOI] [PubMed] [Google Scholar]
Gutierrez 2012
- Gutierrez J, Ramirez G, Rundek T, Sacco RL. Statin therapy in the prevention of recurrent cardiovascular events: a sex‐based meta‐analysis. Archives of Internal Medicine 2012;172(12):909‐19. [PUBMED: 22732744] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist G, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, Schünemann HJ, for the GRADE Working Group. Rating quality of evidence and strength of recommendations GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hajjar 2007
- Hajjar ER, Cafiero AC, Hanlon JT. Polypharmacy in elderly patients. The American Journal of Geriatric Pharmacotherapy 2007;5(4):345‐51. [PUBMED: 18179993] [DOI] [PubMed] [Google Scholar]
Hallas 1996
- Hallas J. Drug related hospital admissions in subspecialities of internal medicine. Danish Medical Bulletin 1996;43(2):141‐55. [PUBMED: 8741207] [PubMed] [Google Scholar]
Hanlon 1992
- Hanlon JT, Schmader KE, Samsa GP, Weinberger M, Uttech KM, Lewis IK, et al. A method for assessing drug therapy appropriateness. Journal of Clinical Epidemiology 1992;45(10):1045‐51. [PUBMED: 1474400] [DOI] [PubMed] [Google Scholar]
Hanlon 2004
- Hanlon JT, Artz MB, Pieper CF, Lindblad CI, Sloane RJ, Ruby CM, et al. Inappropriate medication use among frail elderly inpatients. The Annals of Pharmacotherapy 2004;38(1):9‐14. [PUBMED: 14742785] [DOI] [PubMed] [Google Scholar]
Hasan 2010
- Hasan O, Meltzer DO, Shaykevich SA, Bell CM, Kaboli PJ, Auerbach AD, et al. Hospital readmission in general medicine patients: a prediction model. Journal of General Internal Medicine 2010;25(3):211‐9. [PUBMED: 20013068] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hohl 2015
- Hohl CM, Wickham ME, Sobolev B, Perry JJ, Sivilotti ML, Garrison S, et al. The effect of early in‐hospital medication review on health outcomes: a systematic review. British Journal of Clinical Pharmacology 2015;80(1):51‐61. [PUBMED: 25581134] [DOI] [PMC free article] [PubMed] [Google Scholar]
Holland 2008
- Holland R, Desborough J, Goodyer L, Hall S, Wright D, Loke YK. Does pharmacist‐led medication review help to reduce hospital admissions and deaths in older people? A systematic review and meta‐analysis. British Journal of Clinical Pharmacology 2008;65(3):303‐16. [PUBMED: 18093253] [DOI] [PMC free article] [PubMed] [Google Scholar]
Holt 2010
- Holt S, Schmiedl S, Thurmann PA. Potentially inappropriate medications in the elderly: the PRISCUS list. Deutsches Arzteblatt international 2010;107(31‐32):543‐51. [PUBMED: 20827352] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hughes 2004
- Hughes CM. Medication non‐adherence in the elderly: how big is the problem?. Drugs & Aging 2004;21(12):793‐811. [PUBMED: 15382959] [DOI] [PubMed] [Google Scholar]
Joint Commission 2012
- Joint Commission. Comprehensive Accreditation Manual for Hospitals (CAMH): The Official Handbook. www.jcrinc.com/Joint‐Commission‐Requirements/ (accessed 19 December 2011).
Jones 1997
- Jones D, Seymour R, Woodhouse K. Use of pharmacists by older people in the community. Archives of Gerontology and Geriatrics 1997;24(1):9‐13. [PUBMED: 15374131] [DOI] [PubMed] [Google Scholar]
Kongkaew 2008
- Kongkaew C, Noyce PR, Ashcroft DM. Hospital admissions associated with adverse drug reactions: a systematic review of prospective observational studies. The Annals of Pharmacotherapy 2008;42(7):1017‐25. [PUBMED: 18594048] [DOI] [PubMed] [Google Scholar]
Laroche 2007a
- Laroche ML, Charmes JP, Merle L. Potentially inappropriate medications in the elderly: a French consensus panel list. European Journal of Clinical Pharmacology 2007;63(8):725‐31. [PUBMED: 17554532] [DOI] [PubMed] [Google Scholar]
Laroche 2007b
- Laroche ML, Charmes JP, Nouaille Y, Picard N, Merle L. Is inappropriate medication use a major cause of adverse drug reactions in the elderly?. British Journal of Clinical Pharmacology 2007;63(2):177‐86. [PUBMED: 17166186] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Lozano‐Montoya 2015
- Lozano‐Montoya I, Velez‐Diaz‐Pallares M, Delgado‐Silveira E, Montero‐Errasquin B, Cruz Jentoft AJ. Potentially inappropriate prescribing detected by STOPP‐START criteria: are they really inappropriate?. Age and Ageing 2015;44(5):861‐6. [PUBMED: 26175348] [DOI] [PubMed] [Google Scholar]
Lund 2010
- Lund BC, Carnahan RM, Egge JA, Chrischilles EA, Kaboli PJ. Inappropriate prescribing predicts adverse drug events in older adults. The Annals of Pharmacotherapy 2010;44(6):957‐63. [PUBMED: 20460558] [DOI] [PubMed] [Google Scholar]
Mangoni 2004
- Mangoni AA, Jackson SH. Age‐related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. British Journal of Clinical Pharmacology 2004;57(1):6‐14. [PUBMED: 14678335] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mannheimer 2006
- Mannheimer B, Ulfvarson J, Eklof S, Bergqvist M, Andersen‐Karlsson E, Pettersson H, et al. Drug‐related problems and pharmacotherapeutic advisory intervention at a medicine clinic. European Journal of Clinical Pharmacology 2006;62(12):1075‐81. [PUBMED: 17066294] [DOI] [PubMed] [Google Scholar]
Marcantonio 1999
- Marcantonio ER, McKean S, Goldfinger M, Kleefield S, Yurkofsky M, Brennan TA. Factors associated with unplanned hospital readmission among patients 65 years of age and older in a Medicare managed care plan. The American Journal of Medicine 1999;107(1):13‐7. [PUBMED: 10403347] [DOI] [PubMed] [Google Scholar]
McLeod 1997
- McLeod PJ, Huang AR, Tamblyn RM, Gayton DC. Defining inappropriate practices in prescribing for elderly people: a national consensus panel. CMAJ 1997;156(3):385‐91. [PUBMED: 9033421] [PMC free article] [PubMed] [Google Scholar]
Naugler 2000
- Naugler CT, Brymer C, Stolee P, Arcese ZA. Development and validation of an improving prescribing in the elderly tool. The Canadian Journal of Clinical Pharmacology 2000;7(2):103‐7. [PUBMED: 10958706] [PubMed] [Google Scholar]
Nkansah 2010
- Nkansah N, Mostovetsky O, Yu C, Chheng T, Beney J, Bond CM, et al. Effect of outpatient pharmacists' non‐dispensing roles on patient outcomes and prescribing patterns. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD000336.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Mahony 2015
- O'Mahony D, O'Sullivan D, Byrne S, O'Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age and Ageing 2015;44(2):213‐8. [PUBMED: 25324330] [DOI] [PMC free article] [PubMed] [Google Scholar]
Obreli‐Neto 2012
- Obreli‐Neto PR, Nobili A, Oliveira Baldoni A, Guidoni CM, Lyra Junior DP, Pilger D, et al. Adverse drug reactions caused by drug‐drug interactions in elderly outpatients: a prospective cohort study. European Journal of Clinical Pharmacology 2012;68(12):1667‐76. [PUBMED: 22644345] [DOI] [PubMed] [Google Scholar]
Page 2010
- Page RL 2nd, Linnebur SA, Bryant LL, Ruscin JM. Inappropriate prescribing in the hospitalized elderly patient: defining the problem, evaluation tools, and possible solutions. Clinical Interventions in Aging 2010;5:75‐87. [PUBMED: 20396637] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pasina 2014
- Pasina L, Brucato AL, Falcone C, Cucchi E, Bresciani A, Sottocorno M, et al. Medication non‐adherence among elderly patients newly discharged and receiving polypharmacy. Drugs & Aging 2014;31(4):283‐9. [PUBMED: 24604085] [DOI] [PubMed] [Google Scholar]
Patterson 2012
- Patterson SM, Hughes C, Kerse N, Cardwell CR, Bradley MC. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD008165.pub2; PUBMED: 22592727] [DOI] [PubMed] [Google Scholar]
Pefoyo 2015
- Pefoyo AJ, Bronskill SE, Gruneir A, Calzavara A, Thavorn K, Petrosyan Y, et al. The increasing burden and complexity of multimorbidity. BMC Public Health 2015;15:415. [PUBMED: 25903064] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rogers 2006
- Rogers G, Alper E, Brunelle D, Federico F, Fenn CA, Leape LL, et al. Reconciling medications at admission: safe practice recommendations and implementation strategies. Joint Commission Journal on Quality and Patient Safety/Joint Commission Resources 2006;32(1):37‐50. [PUBMED: 16514938] [DOI] [PubMed] [Google Scholar]
Rothschild 2000
- Rothschild JM, Bates DW, Leape LL. Preventable medical injuries in older patients. Archives of Internal Medicine 2000;160(18):2717‐28. [PUBMED: 11025781] [DOI] [PubMed] [Google Scholar]
Routledge 2004
- Routledge PA, O'Mahony MS, Woodhouse KW. Adverse drug reactions in elderly patients. British Journal of Clinical Pharmacology 2004;57(2):121‐6. [PUBMED: 14748810] [DOI] [PMC free article] [PubMed] [Google Scholar]
Royal 2006
- Royal S, Smeaton L, Avery AJ, Hurwitz B, Sheikh A. Interventions in primary care to reduce medication related adverse events and hospital admissions: systematic review and meta‐analysis. Quality and Safety in Health Care 2006;15(1):23‐31. [PUBMED: 16456206] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ryan 2009
- Ryan C, O'Mahony D, Kennedy J, Weedle P, Byrne S. Potentially inappropriate prescribing in an Irish elderly population in primary care. British Journal of Clinical Pharmacology 2009;68(6):936‐47. [PUBMED: 20002089] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salter 2007
- Salter C, Holland R, Harvey I, Henwood K. "I haven't even phoned my doctor yet." The advice giving role of the pharmacist during consultations for medication review with patients aged 80 or more: qualitative discourse analysis. BMJ 2007;334(7603):1101. [PUBMED: 17449504] [DOI] [PMC free article] [PubMed] [Google Scholar]
Samsa 1994
- Samsa GP, Hanlon JT, Schmader KE, Weinberger M, Clipp EC, Uttech KM, et al. A summated score for the medication appropriateness index: development and assessment of clinimetric properties including content validity. Journal of Clinical Epidemiology 1994;47(8):891‐6. [PUBMED: 7730892] [DOI] [PubMed] [Google Scholar]
Schneeweiss 2002
- Schneeweiss S, Hasford J, Gottler M, Hoffmann A, Riethling AK, Avorn J. Admissions caused by adverse drug events to internal medicine and emergency departments in hospitals: a longitudinal population‐based study. European Journal of Clinical Pharmacology 2002;58(4):285‐91. [PUBMED: 12136375] [DOI] [PubMed] [Google Scholar]
Spinewine 2007b
- Spinewine A, Schmader KE, Barber N, Hughes C, Lapane KL, Swine C, et al. Appropriate prescribing in elderly people: how well can it be measured and optimised?. Lancet 2007;370(9582):173‐84. [PUBMED: 17630041] [DOI] [PubMed] [Google Scholar]
Steurbaut 2010
- Steurbaut S, Leemans L, Leysen T, Baere E, Cornu P, Mets T, et al. Medication history reconciliation by clinical pharmacists in elderly inpatients admitted from home or a nursing home. The Annals of Pharmacotherapy 2010;44(10):1596‐603. [PUBMED: 20736427] [DOI] [PubMed] [Google Scholar]
Wallerstedt 2014
- Wallerstedt SM, Kindblom JM, Nylen K, Samuelsson O, Strandell A. Medication reviews for nursing home residents to reduce mortality and hospitalization: systematic review and meta‐analysis. British Journal of Clinical Pharmacology 2014;78(3):488‐97. [PUBMED: 24548138] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wright 2009
- Wright JM, Musini VM. First‐line drugs for hypertension. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD001841.pub2; PUBMED: 19588327] [DOI] [PubMed] [Google Scholar]
Zed 2008
- Zed PJ, Abu‐Laban RB, Balen RM, Loewen PS, Hohl CM, Brubacher JR, et al. Incidence, severity and preventability of medication‐related visits to the emergency department: a prospective study. Canadian Medical Association Journal 2008;178(12):1563‐9. [PUBMED: 18519904] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zermansky 2001
- Zermansky AG, Petty DR, Raynor DK, Freemantle N, Vail A, Lowe CJ. Randomised controlled trial of clinical medication review by a pharmacist of elderly patients receiving repeat prescriptions in general practice. BMJ (Clinical research ed.) 2001;323(7325):1340‐3. [PUBMED: 11739221] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ziere 2006
- Ziere G, Dieleman JP, Hofman A, Pols HA, Cammen TJ, Stricker BH. Polypharmacy and falls in the middle age and elderly population. British Journal of Clinical Pharmacology 2006;61(2):218‐23. [PUBMED: 16433876] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zulman 2011
- Zulman DM, Sussman JB, Chen X, Cigolle CT, Blaum CS, Hayward RA. Examining the evidence: a systematic review of the inclusion and analysis of older adults in randomized controlled trials. Journal of General Internal Medicine 2011;26(7):783‐90. [PUBMED: 21286840] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Christensen 2011
- Christensen M, Lundh A. Medication review of hospitalized patients to prevent morbidity and mortality. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD008986] [DOI] [PMC free article] [PubMed] [Google Scholar]
Christensen 2013
- Christensen M, Lundh A. Medication review in hospitalised patients to reduce morbidity and mortality. The Cochrane Database of Systematic Reviews 2013;2:CD008986. [PUBMED: 23450593] [DOI] [PubMed] [Google Scholar]


