Abstract
Infection of mice with mouse hepatitis virus (MHV) strain A59 results in acute encephalitis, hepatitis, and chronic demyelinating disease. T lymphocytes play an important role in MHV infection, and costimulatory signals are an important component of T cell function. To elucidate the role of the main costimulatory molecule, CD28, in MHV pathogenesis and demyelination, we examined the kinetics of MHV-A59 infection in CD28 knockout mice. MHV-A59-infected CD28 knockout mice developed acute encephalitis and hepatitis, and the same degree of chronic demyelination as normal C57Bl/6 (B6) mice. Thus, CD28, the costimulatory T cell molecule, is not required for MHV infection and MHV-induced demyelination.
Keywords: CD28, Coronaviruses, Demyelination, Multiple sclerosis
1. Introduction
Multiple sclerosis (MS) is the main inflammatory demyelinating disease of the central nervous system. The cause of MS is unknown, but it has been postulated that myelin damage is immune-mediated, possibly as an autoimmune process (Lovett-Racke et al., 1998). Epidemiological studies suggested that MS might be associated with viral triggers similar to other examples of autoimmune diseases (Allen and Brankin, 1993). Animal models of virus-induced demyelination provide useful tools for studying potential mechanisms that link demyelination with viral infections (Lavi et al., 1999).
Mouse hepatitis virus (MHV), a single-stranded enveloped RNA murine coronavirus member of the Nidovirales order, is one of the most extensively studied laboratory models for viral-induced chronic demyelinating disease in rodents Lavi et al., 1984b, Perlman et al., 1990, Stohlman and Weiner, 1981, Wege et al., 1982, Weiner, 1973. The immune system plays a major role in MHV pathogenesis. Macrophages and T lymphocytes play an important role in the recovery from MHV-induced viral infection (Wijburg et al., 1996). Antiviral antibodies or CD8+ T lymphocytes may protect infected animals from lethal encephalitis, but both CD4+ and CD8+ T lymphocytes are required for effective viral clearance. CD4+ and cytotoxic T lymphocytes (CTLs) play a pivotal role in protection against MHV-A59 infection (Heemskerk et al., 1995). Both CD8+ cytotoxic T cells and the CD4+ helper T cells can protect mice from a lethal MHV-4 infection in the central nervous system (Yamaguchi et al., 1991). While the pathogenesis of MHV-induced demyelination is still poorly understood, it may be due to a proinflammatory T cell-mediated pathology, as in MS (Martin and McFarland, 1996). However, it is not certain whether the immune system causes demyelination by direct cytotoxic response against infected oligodendrocytes, or if demyelination is the result of an indirect “bystander” autoimmune phenomenon through molecular mimicry or antigenic spreading. It has been shown that T cells are required and can modify the course of demyelination in JHM–MHV-induced demyelination; however, the role of T cells in MHV-induced demyelination is still not clear. Although neither T cells nor B cells are absolutely required for MHV-induced demyelination (Matthews et al., 2002), the role of CD4 and CD8 cells is redundant but not identical in MHV-induced demyelination (Wu et al., 2000). It has been proposed that CD8 cells serve as initiators of a “bystander” effect that triggers demyelination (Haring et al., 2002).
T cell activation requires two signals. The first signal is triggered by the interaction between the T cell receptor (TCR) and the antigen. This interaction produces both the antigen-specific components of the immune response as well as genetic (MHC) restriction. The second signal, termed costimulation, is triggered by the interaction of an accessory receptor on the T cell with its ligand on the antigen-presenting cell. The presence or absence of costimulation determines the outcome of TCR engagement Boise et al., 1995, Gimmi et al., 1993, Harding et al., 1992, Schwartz, 1990. Several receptor–ligand interactions are capable of producing costimulation. However, the primary signal appears to be through the interaction of T cell CD28 with its ligand, either B7.1 (CD80) or B7.2 (CD86) Arima et al., 1996, Edmead et al., 1997, Karandikar et al., 1996. Studies with CD28 knockout (CD28−/−) mice have demonstrated the importance of CD28 in T cell-mediated immune responses (Bachmaier et al., 1996). The inflammatory demyelinating autoimmune disorder experimental allergic encephalomyelitis (EAE) in rats, a CD4+ T cell-mediated disease that serves as a prototypic model for MS, critically requires the costimulatory B7/CD28 pathway early in the disease. Some immune responses have an absolute requirement for CD28. For example, CD28−/− mice do not produce tumor necrosis factor-alpha (TNFα) when challenged with toxic shock syndrome toxin-1 and do not develop fatal toxic shock syndrome (Saha et al., 1996). However, other immune responses remain either partially or completely intact, suggesting that other costimulatory pathways, through other receptors, may play a significant role (Lucas et al., 1995). To investigate whether CD28-costimulatory signal is required for induction of MHV-induced demyelination in vivo, we studied infection in CD28 knockout mice with a demyelinating strain, MHV-A59.
2. Materials and methods
2.1. Virus, mice
Plaque-purified MHV-A59 virus was used in this study Budzilowicz et al., 1985, Lavi et al., 1984a, Lavi et al., 1984b. Viral stock had titers of 107–108 pfu/ml. The virus was propagated and titrated on murine L2 cells in DMEM with 10% fetal bovine serum (FBS). CD28−/− mice backcrossed onto the C57BL/6 strain (12th backcross generation) were obtained from the Jackson Laboratory (Bar Harbor, ME) and bred by intercrossing at the University of Pennsylvania animal facilities. The phenotype of the mice used in the study had been confirmed by polymerase chain reaction (PCR) analysis. Control C57BL/6 (CD28+/+) mice were also obtained from the Jackson Laboratory. Mice were 4 weeks of age when experiments were initiated. This research adhered to the “Guide for Laboratory Animal Facilities and Care,” Institute of Laboratory Animal Resources, National Research Council, DHHS, Publication No. (NIH) 86-23 (1985).
2.2. Viral infection
CD28−/− knockout C57BL/6 mice and wild-type C57BL/6 mice were inoculated intracerebrally (i.c.) with 1000 pfu of MHV-A59. Mice were monitored daily for signs of disease and mortality. Disease signs included ruffled fur, hunched position, lack of mobility, and lethargy.
2.3. Histology
Mice were sacrificed at various intervals postinoculation (days 1, 3, 5, 7, and 30) and were perfused intracardially with phosphate-buffered saline (PBS) and 10% phosphate-buffered formalin. Organs were removed and fixed in 10% buffered formalin for at least an additional 48 h. Tissues prepared for histology were embedded in paraffin, and 5-mm sections were stained with hematoxylin and eosin (H&E). Spinal cord sections were stained with Luxol Fast Blue for myelin. For each animal, five coronal sections of the brain and at least five sections of the cervical, thoracic, and lumber spinal cord were examined as previously described Das Sarma et al., 2000, Das Sarma et al., 2001.
2.4. Viral titration
During the acute phase of disease (days 1–7), mice were perfused with sterile PBS and specimens of brain and liver were removed aseptically and kept frozen at −80°C. These samples were homogenized and tested for viral titers by plaque assay as previously described Das Sarma et al., 2000, Das Sarma et al., 2001.
3. Results
Following i.c. injection of mice with 1000 pfu of MHV-A59, both groups of mice developed an acute disease, consisting of acute hepatitis and encephalitis, as seen histologically. Viral titers in the brain and liver were not significantly different in the two groups of mice (Fig. 1) . By day 30 postinfection, both the MHV-A59-infected CD28−/− mice and the wild-type CD28+/+ mice exhibited the same degree of mild symptoms of chronic paralytic demyelinating disease. All five CD28−/− mice injected with 1000 pfu of MHV-A59 developed chronic demyelinating lesions in 28/92 of the spinal cord quadrants, as did all five normal B6 mice in 35/88 spinal cord quadrants following injection with the same dose of virus.
Fig. 1.
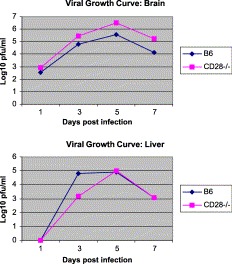
Growth curves of viral titers in livers and brains of mice infected with MHV-A59 and tested in either B6 or CD28−/− knockout mice.
4. Discussion
CD28 can regulate immune responses by attenuating T cell activation. Previous studies demonstrated a requirement for B7-mediated costimulation for the initiation of the encephalitogenic immune response. For example, blockade of B7.1 and B7.2 with the soluble receptor CTLA4 Ig ameliorates EAE following immunization with myelin basic protein (MBP), and suppresses the ability of MBP-reactive T cells to transfer EAE to naive synergic recipients Arima et al., 1996, Hurwitz et al., 1997. Therefore, we had anticipated that the absence of CD28 signaling would result in a diminished or absent immune response and less demyelination following MHV-A59 infection of CD28−/− mice. This hypothesis, however, can only be indirectly investigated since mice susceptible to MHV-A59 (B6) are resistant to EAE, and mice susceptible to EAE (SJL) are resistant to MHV-A59. The induction of MHV-A59-induced demyelination is therefore performed in a different mouse strain than EAE. Nevertheless, our studies showed that unlike EAE, CD28 costimulation is not required for MHV-A59-induced demyelination, and both sets of animals develop demyelination in 100% of the mice. We therefore suggest that MHV-induced demyelination may use different cellular and molecular immune-mediated mechanisms than the ones used in EAE.
Although the CD28/B7 interaction is extremely important for immunological responses, there are examples other than MHV-induced demyelination where CD28 is not necessary for certain viral-induced immunological responses. Alternative costimulatory pathways may be used in those circumstances (Karandikar et al., 1996). For example, infection of CD28−/− mice with lymphocytic choric-meningitis virus induces a cytotoxic T cell response and delayed-type hypersensitivity. Thus, CD28 is not required for these viral-induced, cell-mediated immune responses (Shahinian et al., 1993).
In the infection of HIV, T cell–T cell contact causes the presentation alloantigen to fresh uninfected CD4+ T cells, leading to increased proliferation and virus spread to the activated cells. CTLA4 Ig blocks both of these events. Thus, chronic activation of HIV-1-infected CD4+ T cells reduces expression of CD28 and increases expression of B7, thereby enabling these T cells to become antigen-presenting cells for uninfected CD4+ T cells. This might be another mechanism for HIV-1 transmission via T cell–T cell contact (Haffar et al., 1993).
It is well established that adhesion molecules are required for interaction between virus-specific CTLs and target cells, but the CD28/B7 pathway seems not to be required for cytotoxicity mediated by activated virus-specific CTLs. CD8+ virus-specific CTLs can utilize either the CD2/LFA-3 or the LFA-1/ICAM-1 adhesion pathway (de Waal Malefyt et al., 1993).
Infection of CD28−/− mice with MHV-A59 would be a valuable tool to further elucidate the role of costimulatory events by CD28-dependent and -independent mechanisms in the generation of immune responses against pathogens and tumors as well as in the course of autoimmune diseases. The study of these animals could help to determine where immunosuppression by disruption of CD28/B7 interaction can be effective as a treatment strategy as well as when T cell activation is dependent on other mechanisms of costimulation (Shahinian et al., 1993).
Acknowledgements
This work was supported, in part, by a grant from the National Multiple Sclerosis Society (RG-2615) and a PHS grant (NS30606). We thank Dr. Peter Perrin for some of the CD28 knockout mice and Elsa Aglow for excellent histology expertise.
References
- Allen I., Brankin B. Pathogenesis of multiple sclerosis—the immune diathesis and the role of viruses. J. Neuropathol. Exp. Neurol. 1993;52:95–105. doi: 10.1097/00005072-199303000-00001. [DOI] [PubMed] [Google Scholar]
- Arima T., Rehman A., Hickey W.F., Flye M.W. Inhibition by CTLA4Ig of experimental allergic encephalomyelitis. J. Immunol. 1996;156:4916–4924. [PubMed] [Google Scholar]
- Bachmaier K., Pummerer C., Shahinian A., Ionescu J., Neu N., Mak T.W., Penninger J.M. Induction of autoimmunity in the absence of CD28 costimulation. J. Immunol. 1996;157:1752–1757. [PubMed] [Google Scholar]
- Boise L.H., Minn A.J., Noel P.J., June C.H., Accavitti M.A., Lindsten T., Thompson C.B. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- Budzilowicz C.J., Wilczynski S.P., Weiss S.R. Three intergenic regions of mouse hepatitis virus strain A59 genome RNA contain a common nucleotide sequence that is homologous to the 3′ end of the viral mRNA leader sequence. J. Virol. 1985;53:834–840. doi: 10.1128/jvi.53.3.834-840.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das Sarma J., Fu L., Tsai J.C., Weiss S.R., Lavi E. Demyelination determinants map to the spike glycoprotein gene of coronavirus mouse hepatitis virus. J. Virol. 2000;74:9206–9213. doi: 10.1128/jvi.74.19.9206-9213.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das Sarma J., Fu L., Hingley S.T., Lai M.M., Lavi E. Sequence analysis of the S gene of recombinant MHV-2/A59 coronaviruses reveals three candidate mutations associated with demyelination and hepatitis. J. Neurovirol. 2001;7:432–436. doi: 10.1080/135502801753170282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Verma S., Bejarano M.T., Ranes-Goldberg M., Hill M., Spits H. CD2/LFA-3 or LFA-1/ICAM-1 but not CD28/B7 interactions can augment cytotoxicity by virus-specific CD8+ cytotoxic T lymphocytes. Eur. J. Immunol. 1993;23:418–424. doi: 10.1002/eji.1830230218. [DOI] [PubMed] [Google Scholar]
- Edmead C.E., Lamb J.R., Hoyne G.F. The T cell surface protein, CD28. Int. J. Biochem. Cell Biol. 1997;29:1053–1057. doi: 10.1016/s1357-2725(97)00012-5. [DOI] [PubMed] [Google Scholar]
- Gimmi C.D., Freeman G.J., Gribben J.G., Gray G., Nadler L.M. Human T-cell clonal anergy is induced by antigen presentation in the absence of B7 costimulation. Proc. Natl. Acad. Sci. U. S. A. 1993;90:6586–6590. doi: 10.1073/pnas.90.14.6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffar O.K., Smithgall M.D., Bradshaw J., Brady B., Damle N.K., Linsley P.S. Costimulation of T-cell activation and virus production by B7 antigen on activated CD4+ T cells from human immunodeficiency virus type 1-infected donors. Proc. Natl. Acad. Sci. U. S. A. 1993;90:11094–11098. doi: 10.1073/pnas.90.23.11094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding F.A., McArthur J.G., Gross J.A., Raulet D.H., Allison J.P. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992;356:607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- Haring J.S., Pewe L.L., Perlman S. Bystander CD8 T cell-mediated demyelination after viral infection of the central nervous system. J. Immunol. 2002;169:1550–1555. doi: 10.4049/jimmunol.169.3.1550. [DOI] [PubMed] [Google Scholar]
- Heemskerk M.H., Schilham M.W., Schoemaker H.M., Spierenburg G., Spaan W.J., Boog C.J. Activation of virus-specific major histocompatibility complex class II-restricted CD8+ cytotoxic T cells in CD4-deficient mice. Eur. J. Immunol. 1995;25:1109–1112. doi: 10.1002/eji.1830250438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz A.A., Sullivan T.J., Krummel M.F., Sobel R.A., Allison J.P. Specific blockade of CTLA-4/B7 interactions results in exacerbated clinical and histologic disease in an actively-induced model of experimental allergic encephalomyelitis. J. Neuroimmunol. 1997;73:57–62. doi: 10.1016/s0165-5728(96)00168-3. [DOI] [PubMed] [Google Scholar]
- Karandikar N.J., Vanderlugt C.L., Walunas T.L., Miller S.D., Bluestone J.A. CTLA-4: a negative regulator of autoimmune disease. J. Exp. Med. 1996;184:783–788. doi: 10.1084/jem.184.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi E., Gilden D.H., Highkin M.K., Weiss S.R. Persistence of MHV-A59 RNA in a slow virus demyelinating infection in mice as detected by in situ hybridization. J. Virol. 1984;51:563–566. doi: 10.1128/jvi.51.2.563-566.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi E., Gilden D.H., Wroblewska Z., Rorke L.B., Weiss S.R. Experimental demyelination produced by the A59 strain of mouse hepatitis virus. Neurology. 1984;34:597–603. doi: 10.1212/wnl.34.5.597. [DOI] [PubMed] [Google Scholar]
- Lavi E., Schwartz T., Jin Y.P., Fu L. Nidovirus infections: experimental model systems of human neurologic diseases. J. Neuropathol. Exp. Neurol. 1999;58:1197–1206. doi: 10.1097/00005072-199912000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett-Racke A.E., Trotter J.L., Lauber J., Perrin P.J., June C.H., Racke M.K. Decreased dependence of myelin basic protein-reactive T cells on CD28-mediated costimulation in multiple sclerosis patients. A marker of activated/memory T cells. J. Clin. Invest. 1998;101:725–730. doi: 10.1172/JCI1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas P.J., Bare C.V., Gress R.E. The human anti-murine xenogeneic cytotoxic response: II. Activated murine antigen-presenting cells directly stimulate human T helper cells. J. Immunol. 1995;154:3761–3770. [PubMed] [Google Scholar]
- Martin R., McFarland H. Experimental immunotherapies for multiple sclerosis. Springer Semin. Immunopathol. 1996;18:1–24. doi: 10.1007/BF00792605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews A.E., Lavi E., Weiss S.R., Paterson Y. Neither B cells nor T cells are required for CNS demyelination in mice persistently infected with MHV-A59. J. Neurovirol. 2002;8:257–264. doi: 10.1080/13550280290049697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S., Jacobsen G., Olson A.L., Afifi A. Identification of the spinal cord as a major site of persistence during chronic infection with a murine coronavirus. Virology. 1990;175:418–426. doi: 10.1016/0042-6822(90)90426-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha B., Harlan D.M., Lee K.P., June C.H., Abe R. Protection against lethal toxic shock by targeted disruption of the CD28 gene. J. Exp. Med. 1996;183:2675–2680. doi: 10.1084/jem.183.6.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R.H. A cell culture model for T lymphocyte clonal anergy. Science. 1990;248:1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- Shahinian A., Pfeffer K., Lee K.P., Kundig T.M., Kishihara K., Wakeham A., Kawai K., Ohashi P.S., Thompson C.B., Mak T.W. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993;261:609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- Stohlman S.A., Weiner L.P. Chronic central nervous system demyelination in mice after JHM virus infection. Neurology. 1981;31:38–44. doi: 10.1212/wnl.31.1.38. [DOI] [PubMed] [Google Scholar]
- Wege H., Siddell S., ter Meulen V. The biology and pathogenesis of coronaviruses. Adv. Virol. Immunol. 1982;99:165–200. doi: 10.1007/978-3-642-68528-6_5. [DOI] [PubMed] [Google Scholar]
- Weiner L.P. Pathogenesis of demyelination induced by a mouse hepatitis virus (JHM virus) Arch. Neurol. 1973;28:298–303. doi: 10.1001/archneur.1973.00490230034003. [DOI] [PubMed] [Google Scholar]
- Wijburg O.L., Heemskerk M.H., Sanders A., Boog C.J., Van Rooijen N. Role of virus-specific CD4+ cytotoxic T cells in recovery from mouse hepatitis virus infection. Immunology. 1996;87:34–41. [PMC free article] [PubMed] [Google Scholar]
- Wu G.F., Dandekar A.A., Pewe L., Perlman S. CD4 and CD8 T cells have redundant but not identical roles in virus-induced demyelination. J. Immunol. 2000;165:2278–2286. doi: 10.4049/jimmunol.165.4.2278. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Goto N., Kyuwa S., Hayami M., Toyoda Y. Protection of mice from a lethal coronavirus infection in the central nervous system by adoptive transfer of virus-specific T cell clones. J. Neuroimmunol. 1991;32:1–9. doi: 10.1016/0165-5728(91)90065-F. [DOI] [PMC free article] [PubMed] [Google Scholar]


