Abstract
Intranasal inoculation with mouse hepatitis virus strain JHM (MHV-JHM) results in acute meningoencephalitis. We found NOS II mRNA expression in brains of acutely infected animals on days 5 through 7 after infection. In situ hybridization and immunohistochemistry demonstrated NOS II message and protein in infiltrating macrophages. Persistent infection with MHV-JHM results in chronic demyelinating encephalomyelitis. NOS II mRNA was detected in persistently infected spinal cords. In situ hybridization and immunohistochemistry showed expression of NOS II in astrocytes in and around demyelinated lesions. These results suggest the role of NO release in acute versus persistent infection with this virus, and its contribution to the resulting pathology, may be different.
Keywords: Nitric oxide, Virus, Demyelination, Astrocyte, Macrophage, Immunohistochemistry
1. Introduction
Nitric oxide (NO) production in the CNS results from the activity of nitric oxide synthases (NOS), of which three isoforms have been identified to date. These enzymes vary in their cellular distribution and regulation. Constitutively expressed NOS types I and III have relatively restricted cellular distributions in the CNS to neurons and endothelial cells, respectively, and their activities are regulated by intracellular calcium levels. These isoforms are thought to synthesize NO important for such physiological processes as intercellular signalling and vasoregulation. In contrast, CNS cells do not express NOS type II (also referred to as inducible or iNOS) unless appropriately activated (Simmons and Murphy, 1992; Corradin et al., 1993; Nunokawa et al., 1993; Minc-Golomb et al., 1994; Borgerding and Murphy, 1995). Common to all cells presently described which express NOS II in vitro is the requirement for transcriptional induction by exposure to specific proinflammatory cytokines or bacterial endotoxins. Cellular release of NO with NOS II expression is, therefore, thought to be important primarily under pathophysiological conditions when such inducers are present, for example in CNS infections.
A detailed understanding of the immune mechanisms responsible for viral clearance and for persistent viral infection in the CNS is currently lacking. Increasing evidence has accumulated to support the idea that activation of NOS II expression and NO production is an important component in the immune response to specific CNS infections, possibly contributing to the resulting pathology (Cross et al., 1993; Koprowski et al., 1993; Campbell et al., 1994; Dighiero et al., 1994; Van Dam et al., 1995). Phagocytic cells expressing NOS II and producing NO have been shown to be cytotoxic for cells infected with intracellular pathogens (Adams et al., 1990; Chao et al., 1993; Vouldoukis et al., 1995) and murine macrophages induced with interferon (IFN)-g to express NOS II have been shown to inhibit replication of ectromelia, vaccinia, and herpes simplex-1 viruses in vitro (Karupiah et al., 1993). Recently, productive Vesicular Stomatitis Virus (VSV) infection has been shown to be inhibited in a susceptible neuronal cell line by exposure to NO (Bi and Reiss, 1995). Additionally, a conducive environment for NOS II induction is created with acute and chronic viral CNS infections. Production of a variety of cytokines by exogenous and endogenous cells occurs in increased amounts, including IFN-γ, tumor necrosis factor (TNF)-α, and interleukin (IL)-1 (Frei et al., 1988; Benveniste, 1992; Schneider-Schaulies et al., 1993). The release of NO with acute and chronic viral infection may, therefore, function protectively to clear virus from the CNS. However, since the cytotoxic effects of NO (or of peroxynitrite, formed by its interaction with superoxide anion) are non-specific, NOS II induction in the CNS could have detrimental or lethal effects, not only on invading pathogens and infected cells but also on nearby uninfected endogenous cells (Chao et al., 1992; Merrill et al., 1993; Dawson et al., 1994; Hewett et al., 1994). Such nonspecific cytotoxicity may be particularly important in the cellular mechanisms responsible for CNS damage and dysfunction following infection by viruses that are neither cytolytic nor infective of neurons (Koprowski, 1994).
The neurotropic JHM strain of mouse hepatitis virus (MHV-JHM) is a member of the coronavirus family, enveloped RNA viruses with positive-sense RNA genomes. The outcome of infection with this virus in mice depends on the route of inoculation, genetic susceptibility, and host immune response (Compton et al., 1993). Acute CNS infection with this virus in susceptible animals results in lytic infection of a variety of endogenous parenchymal cells, producing a rapidly fatal meningoencephalitis. Previous studies have reported the importance of CD4+ and CD8+ cell infiltration in the clearance of MHV virus, with cytokines as possible mediators (Sussman et al., 1989; Williamson and Stohlman, 1990; Korner et al., 1991; Yamaguchi et al., 1991; Pearce et al., 1994).
In addition to acute encephalitis, infection with this virus causes acute and chronic demyelinating diseases (Lampert et al., 1973; Weiner, 1973; Nagashima et al., 1978; Sorensen et al., 1980; Stohlman and Weiner, 1981; Lavi et al., 1984; Erlich et al., 1987; Perlman et al., 1987; Wang et al., 1992) with pathological features similar to human multiple sclerosis. Previously, we have described the expression of multiple cytokines and NOS II protein in the CNS of mice acutely and persistently infected with MHV-JHM (Sun et al., 1995). Here we report NOS II expression during acute MHV-JHM infection, describing the time course and cellular distribution of both NOS II mRNA and protein with acute MHV-JHM meningoencephalitis. Differences between the cellular distribution of NOS II expression with acute and persistent MHV-JHM infections are also confirmed, suggesting different roles for the NO released.
2. Materials and methods
2.1. Animals
A total of 51 mice were used in this study. Thirty-eight mice were used for NOS II mRNA detection by RT-PCR, and 13 were used for NOS II in situ hybridization and immunohistochemistry studies. For the acute encephalitis studies, pathogen-free adult C57BL/6 mice (Jackson Laboratories, Bar Harbor, Maine or Harlan Laboratories, Indianapolis, IN) were infected intranasally with MHV-JHM (5×104 PFU). At 3, 4, 5, 6 and 7 days post-infection, mice were intracardially perfused with PBS and brains were harvested. Tissue was either frozen at −70°C immediately or placed in Histochoice MB fixative (Amresco, Solon, OH) overnight prior to paraffin embedding. Control animals were uninfected animals housed in separate cages. For the persistent infection studies, C57BL/6 dams were immunized and their offspring inoculated intranasally with MHV-JHM as described previously (Perlman et al., 1987). Mice subsequently exhibiting hindlimb paralysis were sacrificed and spinal cord tissues were handled as described above. Control animals were infected but asymptomatic.
2.2. Cells and virus
RAW 264.7, a mouse macrophage cell line, was obtained from the American Type Culture Collection (Rockville, MD) and grown in Dulbecco's MEM with 10% FCS and 50 μg/ml gentamicin at 37°C in 5% CO2/95% air. Cells exposed 5 h to 50 U/ml murine IFN-γ (GibcoBRL, Grand Island, NY) and 500 ng/ml lipopolysaccharide (LPS, Sigma, St. Louis, MO) were used as a source of NOS II RNA for RT-PCR. MHV-JHM used in all studies was grown and titered as described previously (Perlman et al., 1987).
2.3. RNA extraction
Total RNA was extracted from 200–400 mg (wet weight) mouse brain or spinal cord using TRISOLV (Biotecx Laboratories, Houston, TX) according to manufacturer's instructions. Total RNA was quantitated by absorbance at 260 nm. Total RNA used for RT-PCR was first treated with 2 U of RNase-free DNase (Promega, Madison, WI) for 1 h at 37°C to remove possible genomic DNA contamination.
2.4. RT-PCR/southern blot
A non-quantitative RT-PCR method for the detection of NOS II RNA using NOS II specific primers, followed by Southern blotting using a 32P-end-labeled internal oligonucleotide probe, was performed as described previously (Grzybicki et al., 1996). The expected PCR product was 441 bp. Beta-actin primers were synthesized using published sequences (Montgomery and Dallman, 1991) and the predicted PCR product was 571 bp.
2.5. In situ hybridization
Mouse brain or spinal cord tissues were harvested as described above and placed in Histochoice MB fixative (AMRESCO, Solon, OH) overnight followed by dehydration and embedding in paraffin. The exact chemical composition of Histochoice MB fixative is protected by the manufacturer; however, it is a non-crosslinking fixative/preservative, and the manufacturer's directions were followed exactly for tissue fixation. 15 μm sagittal (brain) or horizontal (spinal cord) sections were cut and mounted onto silanized (Sigma, St. Louis, MO) Superfrost Plus (Fisher Scientific, Pittsburgh, PA) slides, dried overnight, and stored at −20°C. NOS II probes were 665 bp 35S-labeled single-stranded antisense or sense RNA, and their synthesis and the in situ hybridization procedure used to detect NOS II message was a modification of a method described by Perlman et al. (1987)and has been described previously (Grzybicki et al., 1996). Briefly, after de-paraffinization in xylenes and rehydration in graded ethanols, sections were pretreated with 25 μg/ml Proteinase K (Gibco BRL Grand Island, NY) in 0.1 M Tris, pH 8, and 0.05 M EDTA. This was followed by immersion in 0.25% (vol/vol) acetic anhydride in 0.1 M triethanolamine and dehydration through graded ethanols. Sections were then hybridized with 1×106 cpm/section probe in a Tris-buffered hybridization solution in a humid chamber overnight at 55°C. After overnight hybridization, sections were washed in SSC and then treated with RNase A (20 μg/ml, BMB, Indianapolis, IN). Slides were washed in progressively stringent SSC washes, dehydrated through graded ethanols, and air-dried. Emulsion autoradiography was performed using Kodak NTB-2 emulsion, and slides were exposed for 14–16 days at 4°C before development. Slides were counterstained with with hematoxylin and eosin (H and E) or methyl green (0.25% aqueous), dehydrated, and mounted in Permount before examination by light microscopy.
2.6. Immunohistotochemistry
Brains or spinal cords were harvested and processed as described for in situ hybridization. NOS II was detected using an immunoperoxidase technique as described previously (Grzybicki et al., 1996). Briefly, sections were incubated with rabbit anti-murine NOS II Ab (diluted 1:100, Transduction Laboratories, Lexington, KY) followed by goat anti-rabbit biotinylated IgG secondary Ab (1:200, Sigma, St. Louis, MO). Specific protein was detected using an avidin-biotinylated horseradish peroxidase complex (ABC) kit used according to the manufacturer's instructions (Vector, Burlingame, CA). Staining reactions were performed with 3,3′-diaminobenzidine as substrate and enhanced in the presence of nickel chloride. Before being mounted, sections were counterstained in methyl green (0.25% aqueous). Specificity of the commercial NOS II polyclonal antibody was confirmed by immunoblotting. Antibody was preabsorbed overnight against IFN-g/LPS-activated RAW cells fixed with Histochoice. This preabsorbed antibody (1:1000) was then immunoblotted against NOS II-upregulated murine whole brain protein samples separated on 7.5% SDS-PAGE gels. Parallel immunoblots were run using non-preabsorbed NOS II polyclonal antibody and NOS II murine monoclonal antibody (Transduction Laboratories, Lexington, KY). Immunoblots using non-preabsorbed but not preabsorbed antibody revealed the presence of a single protein band of the expected size (130 kD) (data not shown).
GFAP detection was achieved using a murine monoclonal anti-GFAP Ab (1:200, Sigma, St. Louis, MO) and a goat anti-mouse biotinylated polyvalent secondary Ab (1:200, Sigma, St. Louis, MO). Macrophages/microglia were detected with a rat anti-mouse macrophage antibody (F4/80 Cl:A3-1, 1:50, Serotec, Indianapolis, IN) and virus with a mouse monoclonal antibody raised against the N protein of MHV-JHM (Mab5B188.2, 1:500), kindly provided by M. Buchmeier, Scripps Research Institute. These antibodies were utilized with biotinylated goat anti-rat and goat anti-mouse antibodies, respectively (1:200, Sigma, St. Louis, MO). The controls employed for all antibodies were reactions lacking primary antibody.
2.7. Histology
Sections from CNS tissue used for in situ hybridization analysis or immunocytochemistry were stained with H and E and/or luxol fast blue (LFB) in order to verify pathological changes consistent with acute meningoencephalitis or to detect areas of demyelination.
3. Results
3.1. Induction of NOS II mRNA in the CNS of mice with MHV-JHM infection
For the acute encephalitis experiments, 50,000 PFU of MHV-JHM were intranasally inoculated into a total of 14 unimmunized, adult C57BL/6 mice. Mice surviving to day 5 began to exhibit clinical signs of infection (hunching, ruffled fur, irritability and lethargy) which progressed to a moribund state by day 7. Mice were sacrificed 3, 4, 5, 6, and 7 days after infection and brain tissues were analyzed for NOS II mRNA. Southern analysis of RT-PCR products revealed the presence of NOS II mRNA in tissues of 5/5 mice 7 days after infection, 3/3 mice 6 days after infection, and 1/2 mice 5 days after infection. NOS II mRNA was not detected in the brains of two mice 4 and 3 days after infection. Uninfected control brains (2/2) did not express NOS II mRNA (Fig. 1 ).
Fig. 1.
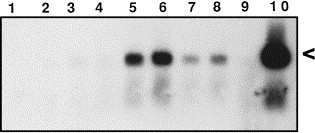
Expression of NOS II mRNA in the CNS of mice with acute or persistent MHV-JHM infection. For acute infection, unimmunized mice were sacrificed at different time points after intranasal inoculation with 50,000 PFU of MHV-JHM and whole brain was harvested. For persistent infection, mice suckled from immunized dams were intranasally inoculated with 50,000 PFU of MHV-JHM. Animals exhibiting hindlimb paralysis weeks later were sacrificed and spinal cord was harvested. RNA extraction followed by RT-PCR/Southern blotting was performed. The expected size cDNA product is 441 bp, indicated by the arrowhead. Lane 1, `no target' control; 2–6, acutely infected mice three, four, five, six, and seven days post-inoculation; 7 and 8, persistent infection with hindlimb paralysis; 9, uninfected control; 10, LPS+IFN-γ induced RAW cells (positive control).
Spinal cord tissue was harvested from eight C57BL/6 mice with persistent MHV-JHM infection exhibiting hindlimb paralysis and analyzed for NOS II mRNA. The length of time from inoculation to manifestation of clinical paralysis ranged from 30 to 65 days. Southern analysis of RT-PCR products revealed the presence of NOS II mRNA in spinal cords of 7/8 mice with hindlimb paralysis (Fig. 1). Spinal cord tissue from 1/4 infected but asymptomatic mice expressed NOS II mRNA by this method.
3.2. In situ localization of NOS II mRNA
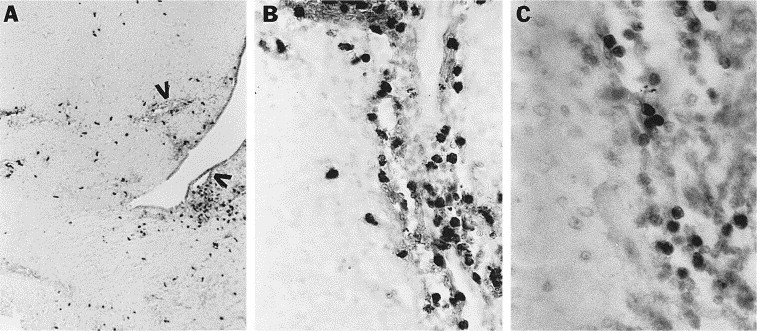
In situ hybridization analysis utilizing a 35S-labeled NOS II antisense cRNA probe was performed on multiple brain sections from one mouse sacrificed 7 days after acute infection, one mouse sacrificed 6 days after acute infection, and an uninfected control mouse. Changes consistent with diffuse acute encephalitis were evident on H and E staining of sections from these animals, including diffuse and extensive meningeal, perivascular, and parenchymal mononuclear infiltrates as well as multifocal areas of necrosis. NOS II mRNA evidenced by silver grain clustering was present in cells in brains on both days 6 and 7 after infection. These round cells were present in the meninges, clustered around blood vessels in the parenchyma, and scattered diffusely throughout the brain parenchyma (Fig. 2 A, frontal cortex). The morphological appearance of the positive cells as well as their pattern of distribution suggested that they were infiltrating mononuclear phagocytes. Confirmation of this cell type expression was achieved with demonstration of NOS II protein (see below). NOS II sense cRNA probe hybridization analysis on adjacent sections revealed only a background scattering of silver grains (Fig. 2B). NOS II antisense probe hybridizations performed on brain sections from an uninfected control mouse revealed no evident clustering of silver grains in any brain area (data not shown).
Fig. 2.

Cellular localization of NOS II mRNA in brains of mice acutely infected with MHV-JHM. In situ hybridization utilizing a 35S-labeled NOS II antisense (A) or sense (B) cRNA probe was performed on brain sections from acutely infected mice 7 days after inoculation. Numerous cells exhibit the presence of NOS II mRNA in A (examples indicated by arrows). Magnification, 400×.
In situ hybridization analysis was also performed on multiple sections at several levels in the spinal cord from two mice exhibiting hindlimb paralysis. Multifocal chronic-active demyelinated lesions were evident by H and E and LFB staining in the spinal cords of both of these mice (Fig. 3 ). Lesions were characterized by a well-demarcated edge with surrounding inflammatory cells and a gliotic center. Antisense probe hybridization revealed NOS II mRNA apparent by silver grain clustering present in the white and gray matter adjacent to demyelinated foci and scattered in demyelinated areas (Fig. 4 A). Such clustering was not evident after hybridization with the control sense probe, which resulted in only a background scattering of silver grains (Fig. 4B).
Fig. 3.

Demyelinated lesion in ventral spinal cord of mouse with persistent MHV-JHM infection and hindlimb paralysis. White matter (W) chronic active lesion in lower right is hypercellular due to infiltrating chronic inflammatory cells and has multifocal areas of myelin loss evident by LFB staining, seen as rarified areas in the tissue by H and E staining. Magnification, 100×.
Fig. 4.

Localization of NOS II mRNA in spinal cords of mice persistently infected with MHV-JHM. In situ hybridization utilizing a 35S-labeled NOS II antisense (A) or sense (B) cRNA was performed on cord sections from mice exhibiting hindlimb paralysis. Clustering of silver grains is present in the white matter and peripheral gray matter of the cervical spinal cord at the edge of a demyelinated lesion (E) and in the lesion (L) which is histologically similar to that shown in Fig. 3. Magnification, 25×.
3.3. Distribution and identity of cells expressing NOS II protein with acute infection
Immunohistochemical staining of multiple brain sections from each of two mice sacrificed at 3, 4, 5, 6, and 7 days after acute infection utilizing a polyclonal antibody to NOS II revealed the presence of positive cells at all time points. Similar to the general histological distribution pattern of cells expressing NOS II mRNA in acutely infected brains described above, the only cells positive for NOS II protein were round cells without processes present in the meninges, around vessels in the brain parenchyma, and scattered diffusely throughout the brain parenchyma (Fig. 5 A). Specifically, neurons and vascular endothelial cells did not stain positively in any sections. Control reactions lacking primary antibody were without positive cells in any brain region (data not shown). Additionally, reactions using preabsorbed NOS II antibody (see Materials and Methods) were also without positive cells in any brain region (data not shown).
Fig. 5.
Cellular localization of NOS II protein in brains of acutely infected mice. Immunohistochemical staining of brain sections utilizing a polyclonal NOS II antibody followed by counterstaining with methyl green was performed on brain sections from mice sacrificed 3–7 days after infection. (A, B) NOS II positive cells in a brain 5 days after infection. Positive cells are seen surrounding blood vessels and migrating into the parenchyma. Vessels are indicated by arrowheads in (A). (C) F4/80 positive cells have a similar pattern of staining to that seen in B. Magnifications: (A) 25×, (B) 250×, (C) 400×.
Immunohistochemical staining was performed on sections adjacent to those found to contain NOS II positive cells utilizing F4/80 and GFAP antibodies in an effort to identify the cell type(s) expressing NOS II protein. The pattern and distribution of cells positive after staining with rat anti-mouse macrophage/microglia F4/80 antibody was similar to the NOS II positive cells (Fig. 5B and C). Although the expression of GFAP protein was noted to increase with progression of acute MHV-JHM infection indicating the presence of astrogliosis, the pattern and distribution of cells positive for GFAP was totally unlike the pattern and distribution of NOS II positive cells. Based on this comparison and morphology, cells expressing NOS II with acute MHV-JHM infection are most likely exogenous mononuclear inflammatory cells which infiltrate significantly only at late stages of the infection. No cells expressing NOS II mRNA or protein were seen which morphologically resembled neurons, astrocytes, or microglia.
3.4. Spatiotemporal expression of NOS II protein and virus with acute infection
Immunohistochemical staining was also performed on sections adjacent to those found to contain NOS II positive cells utilizing a monoclonal antibody against MHV-JHM in order to verify infection and to compare the spatial and temporal expression of NOS II protein with that of viral antigen.
On day 3 post-infection, rare cells positive for virus were seen in the olfactory bulb and olfactory cortex. Only rare cells positive for NOS II were seen at this time point, but they were also located in the olfactory bulb.
At 4 days after infection, virus was present in the olfactory bulb, olfactory nuclei, medial preoptic area, and in one focus of the pons. Cells positive for NOS II protein were also seen in the olfactory bulb and olfactory nuclei, but none were seen in the preoptic area or brainstem. Scattered positive cells were seen in the rostral frontal cortex.
At day 5 after infection, virus was present less abundantly in the olfactory areas, but was seen diffusely in the ventromedial brain, particularly in the ventral tegmental area (VTA) (Fig. 6 A). Both dorsal and ventral areas of the brainstem also contained virus. Cells positive for NOS II at this time point were also seen in the ventromedial brain, particularly the VTA (Fig. 6B), and in the basal ganglia and brainstem. However, NOS II positive cells were also abundant in the hippocampus, particularly in the meninges of the hippocampal area (Fig. 6C) and in the septal area. Many positive cells were also still present in the olfactory regions.
Fig. 6.
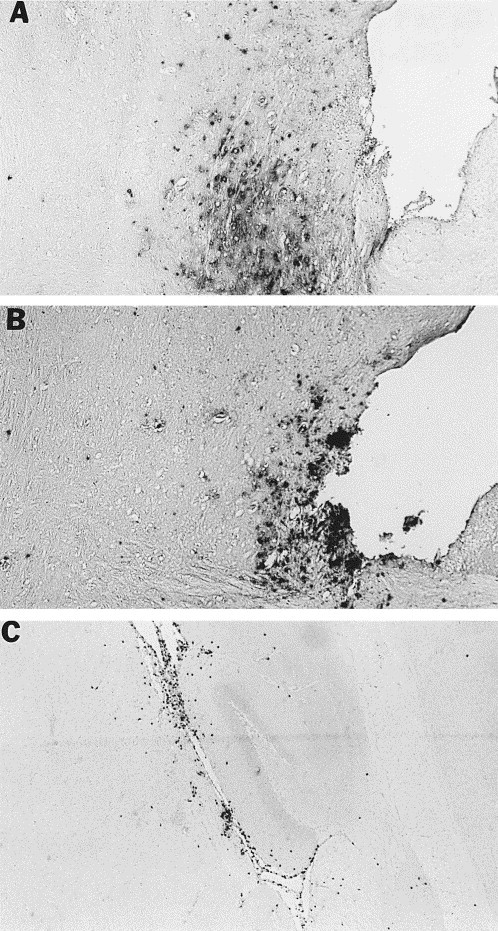
Cellular localization of MHV-JHM viral antigen (A) and NOS II protein (B) in VTA of an acutely infected mouse at 5 days post-infection after immunohistochemical staining utilizing a polyclonal NOS II antibody followed by counterstaining with methyl green. Viral antigen occurs in parenchymal patches, while NOS II positive cells have a conspicuous meningeal and perivascular distribution. NOS II positive cells in the parenchyma often occur singly. (C) NOS II positive cells in the meninges of the hippocampus. Magnification, 25×.
On day 6 after infection, virus was more diffusely located throughout the brain, with abundant virus in the entire ventral brain as well as in the subcortical gray matter, particularly the central gray region and the septal area. Virus was also abundant in the brainstem. Cells expressing NOS II were also present in the ventromedial brain, particularly the VTA, as well as in the septal area, subcortical gray matter, and brainstem, with NOS II expression at this time point particularly abundant in the midbrain. Unlike the distribution of virus, NOS II positive cells remained abundant in the hippocampus, especially in the meninges, and there were also scattered positive cells throughout the cortex.
In general, viral antigen appeared to move rostro-caudally with time after infection, and to be present in the brain multifocally, with patches of positive cells seen in the regions described above. The distribution of NOS II positive cells on the whole appeared to be more diffuse, with single cells and clusters of cells scattered in the parenchyma, conspicuously around vessels and in meninges in the regions described above. While there was some overlap between areas with cells positive for virus and areas with NOS II positive cells, their spatial and temporal patterns of expression did not appear to correlate directly.
3.5. Distribution and identity of cells expressing NOS II protein with persistent infection
Immunohistochemical staining of multiple spinal cord sections with chronic-active lesions from two persistently infected mice also revealed the presence of cells positive for NOS II protein. These cells were located in the white matter of the spinal cord, most conspicuously in and adjacent to areas of demyelination. Cells positive for NOS II protein were of medium size and possessed both thick and thin elongated processes, morphologically resembling astrocytes (Fig. 7 A and B). Based on shape alone, some of these cells could represent activated microglia; however, their size was generally too large for microglia, and the pattern of staining for F4/80 was unlike the pattern obtained with NOS II staining (see below). Therefore, despite the fact that infiltrating inflammatory cells were present in these lesions (see Fig. 3) they did not stain positively for NOS II protein. Control reactions lacking primary antibody were without positive cells in any cord region (data not shown).
Fig. 7.
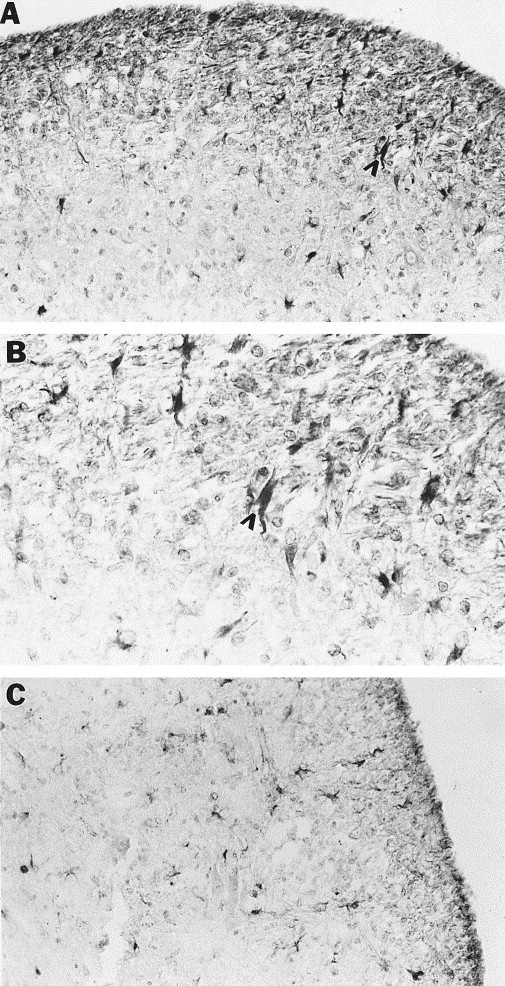
Cellular localization of NOS II protein in spinal cords of mice persistently infected with MHV-JHM. Immunohistochemical staining of brain sections utilizing a polyclonal NOS II antibody was performed on spinal cord sections of mice with hindlimb paralysis and chronic-active demyelinating lesions in the spinal cord. (A, B) NOS II positive cells were present in the lesion and around the lesion edge (B is a higher magnification of A). Arrowheads indicate one of many positive cells morphologically resembling astrocytes. (C) GFAP staining reveals a similar pattern to that seen in (A). Magnifications: (A, C) 100×, (B) 250×.
Immunohistochemical staining of cord sections adjacent to those found to contain NOS II positive cells was performed utilizing GFAP and F4/80 antibodies in an effort to identify the cell type(s) expressing NOS II protein. The expression of GFAP protein was increased in areas of demyelination, indicating the presence of astrogliosis with persistent MHV-JHM infection. The pattern and distribution of cells positive for GFAP was the same as that of NOS II positive cells in and adjacent to demyelinated foci (Fig. 7C). The pattern and distribution of cells positive for F4/80 was unlike that of NOS II positive cells (data not shown). Therefore, a population of activated astrocytes appears to be the major source of NOS II under these conditions. These results confirm and extend our earlier findings (Sun et al., 1995).
Immunohistochemical staining was performed on sections adjacent to those positive for NOS II utilizing a monoclonal antibody against MHV-JHM to verify viral infection. Cells positive for virus were present in the spinal cord in both gray and white matter regions (data not shown).
4. Discussion
The present study reveals the time course and cellular distribution of NOS II message and protein in mice with acute MHV-JHM meningoencephalitis. Detailed descriptions of acute MHV infection from previous studies (Bailey et al., 1949; Cheever et al., 1949, Lampert et al., 1973; Weiner, 1973; Lavi et al., 1984; Perlman et al., 1987) show that inoculation of unimmunized mice results in the presence of viral antigen in the brain by day 3 after infection, increasing progressively until death. Infected cells are neurons and other parenchymal cells, with approximately 35% of infected cells identified as astrocytes (Perlman and Ries, 1987). MHV-JHM produces a lytic infection, directly destroying infected cells. Pathological changes seen with acute infection are not unlike other acute encephalitides: leptomeningeal, perivascular, and parenchymal inflammatory infiltrates are present, with proliferating astrocytes and activated microglia surrounding neurons. One unique feature of this acute viral infection is the presence of abundant necrosis.
NOS II mRNA expression in acute infection appears to correlate with the severity of clinical signs, appearing in some mice on day 5 after infection and in all mice on days 6 and 7 after infection. These observations suggest that NOS II induction and NO release may contribute to the pathogenesis of the disease; however, in this rapidly progessive infection it is more likely that CNS dysfunction is a result of neuronal and general parenchymal damage caused directly by the virus. Non-specific cytotoxic effects on cells adjacent to infiltrated macrophages in acute MHV-JHM infection may produce some CNS damage. This would be expected since activated phagocytes also produce superoxide anion which could interact with NO to produce peroxynitrite. However, the observation that significant numbers of NOS II positive macrophages are present in the brain parenchyma only at late stages of the disease when extensive CNS damage has already occurred, suggests that the amount of NO-associated injury is probably not enough to significantly alter the course of the disease. One would predict, therefore, that administration of a specific NOS II inhibitor would not affect the clinical course of this acute infection. Infiltrating macrophages also express NOS II in Borna and Rabies encephalitides, but in these cases the NO released is thought to contribute significantly to CNS damage and therefore clinical disease (Zheng et al., 1993; Van Dam et al., 1995).
In this model, infected resident CNS cells do not appear to express NOS II, and NOS II expression is also not completely spatially correlated with virus. These observations could be explained by the sensitivity limit of the assay; it is possible that neurons, glia, or other cells may be producing low levels of NOS II which are undetectable by immunohistochemistry. This explanation is not likely since NOS II was demonstrated in endogenous cells with persistent infection. Alternatively, it may take longer than expected (based on in vitro data) to induce astrocytes or other CNS cells to express NOS II in vivo. More likely, a complex system of regulation for NOS II expression by soluble mediators and/or virus may exist in vivo. Therefore, NO release does not appear to be a component of the innate immune response to acute infection with this virus or to play a significant role in viral clearance. This is unlike Lymphocytic Choriomeningitis Virus (LCMV) infection, in which infiltrating macrophages also express NOS II. Here, administration of antimacrophage agents before LCMV infection results in a significant increase and prolongation of viremia, suggesting a role for macrophage NO release in clearance of virus (Cross et al., 1993).
Other reports have shown NOS II induction in virus-infected glial cells in vivo (Dighiero et al., 1994) and a recent study describes NOS II expression in non-infected but nearby glial cells in VSV-infected brain, expression coinciding with decreased viral titers (Bi et al., 1995). These studies, together with the observations reported here, suggest that, while NOS II induction occurs in a variety of acute CNS viral infections (leading to the hypothesis that consequent NO release may be a generalized anti-viral defense mechanism), NO release actually plays a role in the innate immune response only in specific acute CNS viral infections.
The observation that NOS II induction does not occur in astrocytes in acutely infected mice also points to a lack of direct correlation between astrogliosis and NOS II induction. While it is clear that astrocyte `activation' occurs with acute MHV-JHM infection, as seen by an increase in GFAP staining over the disease course, NOS II is not induced in these activated astrocytes. One role astrocytes may be playing during acute infection, which could contribute to the pathogenesis of this disease, is that of producers of chemokines, molecules contributing to the influx of inflammatory cells into the CNS (Vanguri and Farber, 1994; Hurwitz et al., 1995).
In this study, 7/8 spinal cords of mice with hindlimb paralysis and 1/4 infected but asymptomatic mice contained NOS II mRNA by PCR. The observation that one of the mice with clinical symptoms did not appear to express NOS II mRNA is most likely due to inter-animal variation in time course and quantitative expression of NOS II. One control animal also expressed NOS II; this expression could have been induced by some component of the experimental manipulation. Alternatively, viral infection in this animal may have induced a subclinical inflammatory response which nevertheless induced NOS II. Performing these experiments on larger numbers of animals would clarify these points. The fact that we observed a consistent pattern of NOS II expression by PCR in the animals studied which was confirmed by in situ hybridization and immunohistochemical studies supports the conclusion that NOS II is upregulated in persistently infected mice with hindlimb paralysis.
The chronic demyelinating process occurring with persistent MHV-JHM infection has been well described (Bailey et al., 1949; Cheever et al., 1949; Nagashima et al., 1978; Sorensen et al., 1980; Stohlman and Weiner, 1981; Lavi et al., 1984; Erlich et al., 1987; Perlman et al., 1987; Wang et al., 1992; Perlman and Ries, 1987) and exhibits similarities to human multiple sclerosis. Oligodendrocytes and astrocytes are target cells for persistent infection with MHV-JHM (Sun et al., 1995; Perlman et al., 1988; Bailey et al., 1949; Cheever et al., 1949; Perlman and Ries, 1987), and a recent report identifies astrocyte-to-astrocyte spread of virus as an important component of the establishment of persistent infection in the spinal cord white matter (Sun and Perlman, 1995). In contrast to acute infection these astrocytes express NOS II, suggesting that virus-infected cells may be transcriptionally induced in an effort to clear virus. Upregulation of NOS II transcription may be stimulated either directly by virus or after exposure to proinflammatory cytokines (IFN-γ, TNF-α) released locally by other infected or activated cells. The ongoing presence of virus in the spinal cord with demyelination indicates that, if NOS II induction with NO release is an anti-viral defense mechanism under these conditions, it is not entirely successful; however, NO cytotoxicity may significantly limit an infection which would otherwise be overwhelming. In vitro studies are needed to clarify whether astrocytes can be induced to express NOS II and release NO in response to persistent MHV-JHM viral infection alone, but our observations suggest this as a possibility.
A CNS milieu is created in rodents persistently infected with MHV-JHM and in other models of demyelinating diseases in which proinflammatory cytokines (IFN-γ, IL-1, TNF-α) known to induce NOS II in vitro are produced in significant quantities (Sun et al., 1995; Hartung et al., 1992; Bauer et al., 1993; Renno et al., 1995). Under these conditions, it has been hypothesized that NOS II induction and sustained NO release could mediate the destruction of myelin and oligodendrocytes via a `bystander' effect and/or by raising intracellular levels of cGMP which increases the susceptibility of cells to TNF-a toxicity (Sherman et al., 1992). Astrocytic expression of NOS II in demyelinated lesions from persistently infected animals supports this hypothesis. Therefore, the administration of a specific NOS II inhibitor might be predicted to prevent or ameliorate development of pathological changes in the spinal cord and clinical symptoms in mice with persistent infection, as reported by Cross et al. (1993)for experimental allergic encephalomyelitis.
In summary, the results of this study suggest a role for NOS II expression and NO release as part of the acquired immune response to acute MHV-JHM infection but do not support a significant role in viral clearance. Due to the relatively late temporal expression of NOS II, this NO release probably does not contribute significantly to the pathogenesis of acute disease. In contrast, NOS II expression and NO release may be a component of the innate immune response to persistent MHV-JHM infection and, via a `bystander' effect, contribute to CNS injury. The lack of specific NOS II inhibitors has so far precluded definitive testing of this hypothesis. However, the availability of NOS II-deficient mice (MacMicking et al., 1995; Wei et al., 1995) now provides an opportunity to clarify roles for NOS II induction and NO release in MHV-JHM and other CNS viral diseases.
Acknowledgements
We thank Ning Sun, Lecia Pewe, Sherry Kardos, and Angela Loihl for assistance in harvesting and processing tissues used in this study, and Qiao-wen Xie and Carl Nathan for the NOS II cDNA. Support was received from NIH Project Grants NS 29226 (S.M.) and NS 24401(S.P.), and training Grant NS 07294 (D.G.).
References
- Adams, L.B., Hibbs, J.B., Taintor, R.R. and Krahenbuhl, J.L. (1990) Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from L-arginine. J. Immunol. 144, 2725–2729. [PubMed]
- Benveniste, E.N. (1992) Inflammatory cytokines within the central nervous system:sources, function, and mechanism of action. Am. J. Physiol. 263, C1–C16. [DOI] [PubMed]
- Bailey, O.T., Pappenheimer, A.M., Sargent, F., Cheever, M.D. and Daniels, J.B. (1949) A murine virus (JHM) causing disseminated encephalomyelitis with extensive destruction of myelin II. Pathology. J. Exp. Med. 90, 195–211. [DOI] [PMC free article] [PubMed]
- Bauer, J., Berkenbosch, F., Van Dam, A. and Dijkstra, C.D. (1993) Demonstration of interleukin-1b in Lewis rat brain during experimental allergic encephalomyelitis by immunocytochemistry at the light and ultrastructural level. J. Neuroimmunol. 48, 13–22. [DOI] [PubMed]
- Bi, Z., Barna, M., Komatsu, T. and Reiss, C.S. (1995) Vesicular stomatitis virus infection of the central nervous system activates both innate and acquired immunity. J. Virol. 69, 6466–6472. [DOI] [PMC free article] [PubMed]
- Bi, Z. and Reiss, C.S. (1995) Inhibition of Vesicular Stomatitis Virus infection by nitric oxide. J. Virol. 69, 2208–2213. [DOI] [PMC free article] [PubMed]
- Borgerding, R. and Murphy, S. (1995) Expression of inducible NO synthase in cerebral endothelial cells is regulated by cytokine-activated astrocytes. J. Neurochem. 65, 1342–1347. [DOI] [PubMed]
- Campbell, I.L., Samimi, A. and Chiang, C. (1994) Expression of the inducible nitric oxide synthase. Correlation with neuropathology and clinical features in mice with lymphocytic choriomeningitis. J. Immunol. 153, 3622–3629. [PubMed]
- Corradin, S.B., Mauel, J., Donini, D., Quattrocchi, E. and Ricciardi-Castagnoli, P. (1993) Inducible nitric oxide synthase activity of cloned murine microglial cells. Glia 7, 255–262. [DOI] [PubMed]
- Chao, C.C., Molitor, T.W. and Hu, S. (1993) Neuroprotective role of IL-4 against activated microglia. J. Immunol. 151, 1473–1481. [PubMed]
- Chao, C.C., Shuxian, H., Molitor, T.W., Shaskan, E.G. and Paterson, P. (1992) Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J. Immunol. 149, 2736–2741. [PubMed]
- Cheever, F.S., Daniels, J.B., Pappenheimer, A.M. and Bailey, O.T. (1949) A murine virus (JHM) causing disseminated encephalomyelitis with extensive destruction of myelin I. Isolation and biological properties of the virus. J. Exp. Med. 90, 181–194. [DOI] [PMC free article] [PubMed]
- Compton, S.R., Barthold, S.W. and Smith, A.L. (1993) The cellular and molecular pathogenesis of coronaviruses. Lab. Anim. Sci. 43, 15–28. [PubMed]
- Cross, A.H., Misko, T.P., Lin, R.F., Hickey, W.F., Trotter, J.L. and Tilton, R.G. (1993) Aminoguanidine, an inhibitor of inducible nitric oxide synthase, ameliorates experimental autoimmune encephalomyelitis in SJL mice. J. Clin. Invest. 93, 2684–2690. [DOI] [PMC free article] [PubMed]
- Dawson, V.L., BrahmBhatt, H.P., Mong, J.A. and Dawson, T.M. (1994) Expression of inducible nitric oxide synthase causes delayed neurotoxicity in primary mixed neuronal–glial cortical cultures. Neuropharmacol. 33, 1425–1430. [DOI] [PubMed]
- Dighiero, P., Reux, I., Hauw, J., Fillet, A.M., Courtois, Y. and Goureau, O. (1994) Expression of inducible nitric oxide synthase in cytomegalovirus-infected glial cells of retinas from AIDS patients. Neurosc. Lett. 166, 31–34. [DOI] [PubMed]
- Erlich, S.S., Fleming, J.O., Stohlman, S.A. and Weiner, L.P. (1987) Experimental Neuropathology of chronic demyelination induced by a JHM virus variant (DS). Arch. Neurol. 44, 839–842. [DOI] [PubMed]
- Frei, K., Leist, T.P., Meager, A., Gallo, P., Leppert, D., Zinkkernagel, R.M. and Fontana, A. (1988) Production of B cell stimulatory factor-2 and interferon-γ in the central nervous system during viral meningitis and encephalitis. Evaluation in a murine model of infection and in patients. J. Exp. Med. 168, 449–453. [DOI] [PMC free article] [PubMed]
- Grzybicki, D., Gebhart, G.F. and Murphy, S. (1996) Expression of nitric oxide synthase type II in the spinal cord under conditions producing thermal hyperalgesia. J. Chem. Neuroanat. 10, 221–229. [DOI] [PMC free article] [PubMed]
- Hartung, H., Jung, S., Stoll, G., Zielasek, J., Schmidt, B., Archelos, J.J. and Toyka, K.V. (1992) Inflammatory mediators in demyelinating disorders of the CNS and PNS. J. Neuroimmunol. 40, 197–210. [DOI] [PubMed]
- Hewett, S.J., Csernansky, C.A. and Choi, D.W. (1994) Selective potentiation of NMDA-induced neuronal injury following induction of astrocytic iNOS. Neuron 13, 487–494. [DOI] [PubMed]
- Hurwitz, A.A., Lyman, W.D. and Berman, J.W. (1995) Tumor necrosis factor a and transforming growth factor b upregulate astrocyte expression of monocyte chemoattractant protein-1. J. Neuroimmunol. 57, 193–198. [DOI] [PubMed]
- Karupiah, G., Xie, Q.W., Buller, R.M.L., Nathan, C., Duarte, C. and MacMicking, J.D. (1993) Inhibition of viral replication by interferon-g-induced nitric oxide synthase. Science 261, 1445–1448. [DOI] [PubMed]
- Koprowski, H. (1994) Are CNS lesions produced by free radicals? Ann. N.Y. Acad. Sci. 724, 396–398. [DOI] [PubMed]
- Koprowski, H., Zheng, Y.M., Heber-Katz, E., Frasier, N., Rorke, L., Fu, Z.F., Hanlon, C. and Dietzschold, B. (1993) In vivo expression of inducible nitric oxide synthase in experimentally induced neurologic diseases. Proc. Natl. Acad. Sci. USA 90, 3024–3027. [DOI] [PMC free article] [PubMed]
- Korner, H., Schliephake, A., Winter, J., Zimprich, F., Lassman, H., Sedgwick, J., Siddell, S. and Wege, H. (1991) Nucleocapsid or spike protein-specific CD4+ T lymphocytes protect against coronavirus-induced encephalomyelitis in the absence of CD8+ T cells. J. Immunol. 147, 2317–2323. [PubMed]
- Lampert, P.W., Sims, J.K. and Kniazeff, A.J. (1973) Mechanism of demyelination in JHM encephalomyelitis. Acta Neuropath. 24, 76–85. [DOI] [PMC free article] [PubMed]
- Lavi, E., Gilden, D.H., Wroblewska, Z., Rorke, L.B. and Weiss, S.R. (1984) Experimental demyelination produced by the A59 strain of mouse hepatitis virus. Neurology 34, 597–603. [DOI] [PubMed]
- MacMicking, J.D., Nathan, C., Hom, G., Chartrain, N., Fletcher, D.S., Trumbauer, M., Stevens, K., Xie, Q., Sokol, K., Hutchinson, N., Chen, H. and Mudgett, J.S. (1995) Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell 81, 641–650. [DOI] [PubMed]
- Merrill, J.E., Ignarro, L.J., Sherman, M.P., Melinek, J. and Lane, T.E. (1993) Microglial cell cytotoxicity of oligodendrocytes is mediated through nitric oxide. J Immunol. 151, 2132–2141. [PubMed]
- Minc-Golomb, D., Tsarfaty, I. and Schwartz, J.P. (1994) Expression of inducible nitric oxide synthase by neurons following exposure to endotoxin and cytokine. Br. J. Pharmacol. 112, 720–722. [DOI] [PMC free article] [PubMed]
- Montgomery, R.A. and Dallman, M.J. (1991) Analysis of cytokine gene expression during fetal thymic ontogeny using the polymerase chain reaction. J. Immunol. 147, 554–560. [PubMed]
- Nagashima, K., Wege, H., Meyermann, R. and ter Meulen, V. (1978) Corona virus induced subacute demyelinating encephalomyelitis in rats: a morphological analysis. Acta Neuropathol. 44, 63–70. [DOI] [PMC free article] [PubMed]
- Nunokawa, Y., Ishida, N. and Tanaka, S. (1993) Cloning of the inducible nitric oxide synthase in rat vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 191, 89–94. [DOI] [PubMed]
- Pearce, B.D., Hobbs, M.V., McGraw, T.S. and Buchmeier, M.J. (1994) Cytokine induction during T-cell-mediated clearance of mouse hepatitis virus from neurons in vivo. J. Virol. 68, 5483–5495. [DOI] [PMC free article] [PubMed]
- Perlman, S., Jacobsen, G. and Moore, S. (1988) Regional localization of virus in the central nervous system of mice persistently infected with murine coronavirus JHM. Virology 166, 328–338. [DOI] [PMC free article] [PubMed]
- Perlman, S. and Ries, D. (1987) The astrocyte is a target cell in mice persistently infected with mouse hepatits virus, strain JHM. Microb. Path. 3, 309–314. [DOI] [PMC free article] [PubMed]
- Perlman, S., Schelper, R., Bolger, E. and Ries, D. (1987) Late onset, symptomatic, demyelinating encephalomyelitis in mice infected with MHV-JHM in the presence of maternal antibody. Microb. Path. 2, 185–194. [DOI] [PMC free article] [PubMed]
- Renno, T., Krakowski, M., Piccirillo, C., Lin, J. and Owens, T. (1995) TNF-a expression by resident microglia and infiltrating leukocytes in the central nervous system of mice with experimental allergic encephalomyelitis. J Immunol. 154, 944–953. [PubMed]
- Schneider-Schaulies, J., Schneider-Schaulies, S. and Ter Meulen, V. (1993) Differential induction of cytokines by primary and persistent measles virus infection in human glial cells. Virology 195, 219–228. [DOI] [PubMed]
- Sherman, M.P., Griscavage, J.M. and Ignarro, L.J. (1992) Nitric Oxide-mediated neuronal injury in multiple sclerosis. Medical Hypotheses 39, 143–146. [DOI] [PubMed]
- Simmons, M.L. and Murphy, S. (1992) Induction of nitric oxide synthase in glial cells. J. Neurochem. 59, 897–905. [DOI] [PubMed]
- Sorensen, O., Perry, D. and Dales, S. (1980) In vivo and in vitro models of demyelinating diseases III. JHM virus infection of rats. Arch Neurol. 37, 478–484. [DOI] [PubMed]
- Stohlman, S.A. and Weiner, L.P. (1981) Chronic central nervous system demyelination in mice after JHM virus infection. Neurol. 31, 38–44. [DOI] [PubMed]
- Sun, N., Grzybicki, D., Castro, R.F., Murphy, S. and Perlman, S. (1995) Activation of astrocytes in the spinal cord of mice chronically infected with a neurotropic coronavirus. Virology 213, 482–493. [DOI] [PMC free article] [PubMed]
- Sun, N. and Perlman, S. (1995) Spread of a neurotropic coronavirus to spinal cord white matter via neurons and astrocytes. J. Virol. 69, 633–641. [DOI] [PMC free article] [PubMed]
- Sussman, M.A., Shubin, R.A., Kyuwa, S. and Stohlman, S.A. (1989) T-cell-mediated clearance of mouse hepatitis virus strain JHM from the central nervous system. J. Virol. 63, 3051–3056. [DOI] [PMC free article] [PubMed]
- Van Dam, A.M., Bauer, J., Man-A-Hing, W.K.H., Marquette, C., Tilders, F.J.H. and Berkenbosch, F. (1995) Appearance of inducible nitric oxide synthase in the rat central nervous system after rabies virus infection and during experimental allergic encephalomyelitis but not after peripheral administration of endotoxin. J. Neurosci. Res. 40, 251–260. [DOI] [PubMed]
- Vanguri, P. and Farber, J.M. (1994) IFN and virus-inducible expression of an immediate early gene, crg-2/IP-10, and a delayed gene, I-Aa, in astrocytes and microglia. J. Immunol. 152, 1411–1418. [PubMed]
- Vouldoukis, I., Riveros-Moreno, V., Dugas, B., Ouaaz, F., Becherel, P., Debre, P., Moncada, S. and Mossalayi, M.D. (1995) The killing of Leishmania major by human macrophages is mediated by nitric oxide induced after ligation of the FceRII/CD23 surface antigen. Proc. Natl. Acad. Sci. 92, 7804–7808. [DOI] [PMC free article] [PubMed]
- Wang, F.I., Hinton, D.R., Gilmore, W., Trousdale, M.D. and Fleming, J.O. (1992) Sequential infection of glial cells by the murine hepatits virus JHM strain (MHV-4) leads to a characteristic distribution of demyelination. Lab. Invest. 66, 744–754. [PubMed]
- Wei, X., Charles, I.G., Sith, A., Ure, J., Feng, G., Huang, F., Xu, D., Muller, W., Moncada, S. and Liew, F. (1995) Altered immune responses in mice lacking inducible nitric oxide synthase. Nature 375, 408–411. [DOI] [PubMed]
- Weiner, L.P. (1973) Pathogenesis of demyelination induced by a mouse hepatitis virus (JHM virus). Arch. Neurol. 28, 298–303. [DOI] [PubMed]
- Williamson, J.S.P. and Stohlman, S.A. (1990) Effective clearance of mouse hepatitis virus from the central nervous system requires both CD4+ and CD8+ T cells. J. Virol. 64, 4589–4592. [DOI] [PMC free article] [PubMed]
- Yamaguchi, K., Goto, N., Kyuwa, S., Hayami, M. and Toyoda, Y. (1991) Protection of mice from a lethal coronavirus infection in the central nervous system by adoptive transfer of virus-specific T cell clones. J. Neuroimmunol. 32, 1–9. [DOI] [PMC free article] [PubMed]
- Zheng, Y.M., Schafer, M.K.H., Weihe, E., Sheng, H., Corisdeo, S., Fu, Z.F., Koprowski, H. and Dietzschold, B. (1993) Severity of neurological signs and degree of inflammatory lesions in the brains of rats with borna disease correlate with the induction of nitric oxide synthase. J. Virol. 67, 5786–5791. [DOI] [PMC free article] [PubMed]


