Highlights
-
•
Detection and characterization of novel viruses is often hampered by the lack of adequately stored materials.
-
•
Formalin-fixed paraffin embedded (FFPE) tissues can be used to detect known viral sequences.
-
•
The application of FFPE tissues for detection of novel viral sequences is currently unclear.
-
•
Sequence-independent amplification and next-generation was performed on FFPE tissues.
-
•
Sequences of known viruses and a novel rotavirus were detected, with relatively low sensitivity but standard accuracy.
Keywords: Virus discovery, Metagenomics, Formalin, Histopathology
Abstract
Detection and characterization of novel viruses is hampered frequently by the lack of properly stored materials. Especially for the retrospective identification of viruses responsible for past disease outbreaks, often only formalin-fixed paraffin-embedded (FFPE) tissue samples are available. Although FFPE tissues can be used to detect known viral sequences, the application of FFPE tissues for detection of novel viruses is currently unclear. In the present study it was shown that sequence-independent amplification in combination with next-generation sequencing can be used to detect sequences of known and unknown viruses, although with relatively low sensitivity. These findings indicate that this technique could be useful for detecting novel viral sequences in FFPE tissues collected from humans and animals with disease of unknown origin, when other samples are not available. In addition, application of this method to FFPE tissues allows to correlate with the presence of histopathological changes in the corresponding tissue sections.
1. Introduction
Novel viruses continue to cause outbreaks of disease in humans and animals with recent outbreaks of MERS coronavirus and the influenza A/H7N9 virus as important examples (Zaki et al., 2012, Gao et al., 2013). Rapid identification of novel viruses is a crucial first step in mitigating potential effects of future viral threats and emerging novel viral outbreaks.
Novel technologies have increased the chance and speed of detection of novel viruses in samples collected from humans and animals. Especially next-generation sequencing played an important role in the discovery and characterization of various novel viruses (van Boheemen et al., 2012, Grard et al., 2012, Delwart, 2012, Allander et al., 2005, Lipkin and Firth, 2013). The combination of sequence-independent amplification in combination with next-generation sequencing and subsequent analysis of obtained sequence data by BLAST searches allows generating an enormous amount of sequence data without previous selection by using specific primers. Using these techniques, various previously unknown viruses could be identified and subsequently characterized (van Boheemen et al., 2012, Grard et al., 2012, Delwart, 2012, Allander et al., 2005, Lipkin and Firth, 2013). Unfortunately, identification of novel viruses is hampered often by the absence of adequately stored samples. This holds especially true for the retrospective identification of viruses responsible for past disease outbreaks. From these tissues, often only formalin-fixed paraffin-embedded (FFPE) tissue samples are available.
The procedure of formalin (formaldehyde dissolved in an aqueous solution) fixation, processing, paraffin embedding, sectioning and staining is used widely for histopathological evaluation of tissues (for review, see Srinivasan et al., 2002). The use of formalin fixation allows preservation of tissues at room temperature while maintaining structures of cells and other components. Formalin fixes mainly by cross-linking of macromolecules, including DNA and RNA (Srinivasan et al., 2002), which complicates the extraction of intact DNA and RNA from FFPE tissues. However, novel techniques have been developed to extract nucleic acids from FFPE tissues and have allowed to detect known viral sequences in formalin-fixed paraffin-embedded (FFPE) tissue samples. An interesting example is the recovery of the 1918 ‘Spanish’ influenza A/H1N1 virus (Tumpey et al., 2005, Taubenberger et al., 1997). Using RNA extracted from a FFPE lung tissue sample of a victim of the 1918 pandemic, this virus could be characterized and subsequently recovered by reverse genetics (Tumpey et al., 2005, Taubenberger et al., 1997). In addition to the recovery of the 1918 influenza A virus, sequences of various other known viruses were detected in FFPE tissues using recently developed techniques (Cimino et al., 2014, Baldwin et al., 2014, Duncavage et al., 2011).
Sequence-independent amplification in combination with next-generation sequencing has been used to detect novel viruses on different samples, but whether this technique can be applied for the detection of known and unknown viruses in FFPE samples is currently unclear (van den Brand et al., 2012, Bodewes et al., 2013a). In the present study, the possible use of sequence-independent amplification in combination with next-generation sequencing to identify viral sequences in FFPE tissues was evaluated. To this end, sequence-independent amplification in combination with next-generation sequencing was performed on FFPE tissue sections of the bursa of Fabricius of herring gulls (Larus argentatus) infected with gull adenovirus (Bodewes et al., 2013a) and of the lungs of ferrets (Mustela putorius furo) experimentally inoculated with influenza A/H1N1(2009)pdm virus (Bodewes et al., 2013b) to prove the principle of this technique.
2. Materials and methods
2.1. Collection and processing of tissue samples
Cloacal bursas were collected from two herring gulls that were found dead in the Netherlands in 2001. Bursal tissues were stored in 10% (v/v) neutral buffered formalin solution and embedded in paraffin according to standard procedures after fixation for 1–2 days. Both bursal tissues were embedded in separate cassettes and contained no other tissues (Supplementary Fig. 1). Adenovirus was detected in these tissues by a gull adenovirus real-time PCR and electron microscopy as described previously (Duncavage et al., 2011).
Lung tissues were collected from two ferrets that were euthanized four days after intratracheal inoculation with the influenza A/H1N1(2009)pdm virus (influenza A/Netherlands/602/2009) in 2011 and stored in 10% (v/v) neutral buffered formalin solution as described previously (Bodewes et al., 2013b). Eleven days after fixation, lung tissues were embedded in paraffin according to standard procedures, with each processing cassette containing about 1.5 cross-sections of a lung lobe and no other tissues (Supplementary Fig. 1).
All paraffin-embedded tissues were sectioned at 3 μm and examined by light microscopy after staining with hematoxylin and eosin. In addition, sequential slides of lungs of ferrets were stained with a monoclonal antibody directed against the influenza A virus nucleoprotein using an immunoperoxidase method as described previously (Rimmelzwaan et al., 2001).
2.2. Sequence-independent amplification and next-generation sequencing of tissue samples
The microtome, blade and surroundings were sprayed with RNaseZap (Sigma). After removal of a few tissue sections, two consecutive, 10-μm-thick, tissue sections were collected. Immediately after collection, tissue sections were deparaffinized in xylene, and both RNA and DNA were extracted from each sample using the RNEasy FFPE kit and QIAamp DNA FFPE Tissue Kit (both Qiagen) respectively, according to the instructions of the manufacturer.
Extracted RNA and DNA were processed for random PCR in combination with next-generation sequencing as described previously (van den Brand et al., 2012, van Leeuwen et al., 2010). In brief, first- and second-strand syntheses and random PCR amplification were performed. Random PCR products from the RNA and DNA fractions were pooled, purified using the MinElute PCR purification kit (Qiagen) and subsequently prepared for next-generation sequencing on a 454 GS Junior instrument according to the instructions of the manufacturer (454 Life Science, Roche).
Obtained reads were analyzed by a bioinformatics virus discovery pipeline as described previously using standard parameters (Schurch et al., 2014). In brief, this virus discovery pipeline consists of a combination of removal of multiplex identifier adaptors and primer sequences, quality trimming, iterative exhaustive assembly, filtering of low complexity sequences, and a BLASTN and BLASTX search of the remaining singletons and contigs. For comparison, a set of swab samples collected from harbor seals (Phoca vitulina) as run in parallel (Bodewes et al., Novel gammaherpesvirus in harbor seals, submitted for publication). Extraction of RNA and DNA of these samples and subsequent preparation for deep sequencing was performed as described previously (van den Brand et al., 2012, van Leeuwen et al., 2010).
2.3. Detection of adenovirus DNA and influenza A virus RNA by real-time (RT-) PCR
Extracted DNA of the FFPE herring gull tissues was tested for the presence of gull adenovirus DNA by a gull adenovirus-specific real-time PCR (Bodewes et al., 2013a). Extracted RNA of the FFPE ferret tissues was tested for the presence of influenza A virus RNA by a Matrix real-time RT-PCR as described previously (Munster et al., 2007).
2.4. Confirmation of the partial sequence of the gull rotavirus VP6 gene by Sanger sequencing
The sequence of the partial VP6 gene of the gull rotavirus obtained by 454 sequencing was confirmed by Sanger sequencing. To this end, intestine and duplicate bursal tissue collected from the same herring gull in which the novel rotavirus was detected and stored at −70 °C, were defrosted and tissues were homogenized as described previously (Bodewes et al., 2013a). Subsequently, nucleic acids were extracted using the High Pure viral nucleic acid kit (Roche) and cDNA was prepared using random hexamers. Using forward primer GCAATTGAACAGCGAGTGGCGG and reverse primer GTTTCCATCCCGCATGAAGTCG, the partial VP6 gene of gull rotavirus was obtained as described previously (Bodewes et al., 2013a).
2.5. Sequence alignment and phylogenetic analysis
Alignment of sequences obtained by next-generation sequencing was performed in ClustalOmega (Sievers et al., 2011). Subsequent phylogenetic analysis and pairwise identity analysis of rotavirus sequences was performed in MEGA6 (Tamura et al., 2013) using the Neighbour-Joining method with 1,000 bootstrap replicates and otherwise default parameters.
3. Results
3.1. Microscopic observations
By histopathological examination of the bursa of Fabricius of the herring gulls multiple enlarged cells with enlarged nuclei in the cortex of a few follicles, typical of infection with gull adenovirus were detected as described previously (Bodewes et al., 2013a) (Supplementary Fig. 2A, B). Histological examination of lungs of ferrets revealed a moderate, multifocal, necrotizing bronchointerstitial pneumonia, characterized by the presence of inflammatory cells in the lumina and walls of alveoli and bronchioles as described previously (Bodewes et al., 2013b). The presence of influenza A virus-infected cells was demonstrated by immunohistochemistry (Supplementary Fig. 3A, B) (Bodewes et al., 2013b).
3.2. Next generation sequencing
By next-generation sequencing, between 13,214 and 26,259 reads were obtained of each analyzed sample of which at least 93% passed quality check and was further analyzed in a virus discovery pipeline (Table 1 ). Compared to other samples that were analyzed in the same sequencing run, a similar number of reads was obtained, although the number of large sequence reads was somewhat reduced (Fig. 1 ). Reads were analysed by BLASTN and BLASTX analysis and categorized according to their taxonomy. Most reads detected in the ferret tissues were categorized as eukaryotic sequences (66% and 69%). Most reads detected in the gull tissues were categorized as unknown (48% both tissues) while also a substantial proportion was categorized as eukaryotic sequence (35% both tissues) (Table 2 ).
Table 1.
Overview of next-generation sequencing data.
| Tissue | Total reads | Number of analyzed reads | Read length (nt) |
|---|---|---|---|
| Ferret lung tissue 1 | 13,214 | 12,494 | 20–512 |
| Ferret lung tissue 2 | 26,259 | 24,996 | 20–535 |
| Gull bursa tissue 1 | 17,233 | 16,110 | 20–520 |
| Gull bursa tissue 2 | 15,452 | 14,419 | 20–503 |
Fig. 1.
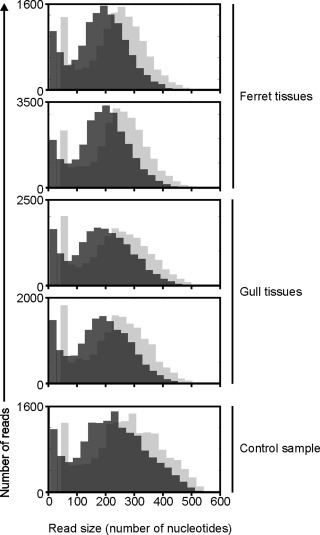
Distribution of read size obtained by 454 sequencing of FFPE tissues. Distribution of read length obtained by next-generation sequencing of each sample before (light grey) and after (dark grey) quality trimming and removal of adaptors and primer sequences. A representative swab control sample (Bodewes et al., 2014) that was analysed in the same run, is also indicated.
Table 2.
Overview of detected reads for each taxonomic category.
| Tissue | Viral (%) | Eukaryota (%) | Bacteria (%) | Archea (%) | No hit/unknowns (%) |
|---|---|---|---|---|---|
| Ferret lung tissue 1 | 0.07 | 66.33 | 0.05 | 14.55 | 19.00 |
| Ferret lung tissue 2 | 0.08 | 68.61 | 0.02 | 11.90 | 19.39 |
| Gull bursa tissue 1 | 0.02 | 35.06 | 0.63 | 16.22 | 48.07 |
| Gull bursa tissue 2 | 1.24 | 35.27 | 2.07 | 13.86 | 47.54 |
3.3. Detection of influenza A/H1N1(2009)pdm virus
By specific real-time PCR, influenza A virus Matrix RNA was detected in both FFPE lung tissues of ferrets inoculated with the influenza A/H1N1(2009)pdm virus (Ct-values 23.0 and 23.6; Table 3 ). By sequence-independent amplification in combination with next-generation sequencing of FFPE lung tissue sections, sequences were detected that were related most closely to all gene segments of influenza A/H1N1(2009)pdm virus. In the tissue of ferret one, nine individual reads similar to influenza A/H1N1(2009)pdm viruses were detected, which ranged in size from 101 to 311 nucleotides (mean size 203 nucleotides). In the tissue of ferret two, 18 reads were detected that were related most closely to influenza A/H1N1(2009)pdm virus. The obtained reads ranged in size from 111 to 287 nucleotides (mean size 192 nucleotides), with five contigs and three single reads. Alignment of the identified reads with influenza A/H1N1(2009)pdm virus revealed that 15 taxonomic units (both single reads and contigs) had an identity of 99% or higher on the nucleotide level, while two individual reads had a lower identity (98% and 87%).
Table 3.
Detected viral sequences.
| Tissue | Detected influenza A virus/adenovirus reads | Viral sequences/total reads (%) | Additional detected viral reads | Ct-value |
|---|---|---|---|---|
| Ferret lung tissue 1 | 9 | 0.07 | 0 | 23.6 |
| Ferret lung tissue 2 | 18 | 0.08 | 0 | 23.0 |
| Gull bursa tissue 1 | 3 | 0.02 | 0 | 27.4 |
| Gull bursa tissue 2 | 1 | 0.007 | 170 (rotavirus) | 26.4 |
3.4. Detection of adenovirus sequences
Gull adenovirus DNA was detected in both FFPE cloacal bursa tissues of the herring gulls by real-time PCR (Ct-values of 27.4 and 26.4; Table 2). In tissues of both herring gulls, reads were detected that were related most closely to adenoviruses. In the tissue of herring gull one, three reads (one contig of two reads and one single read) were detected that were related most closely to adenovirus, while in the tissue of herring gull two only one read was detected that was related most closely to an adenovirus. The contig was related most closely to the hexon gene of gull adenovirus (98% identity on the nucleotide level), while the two single reads were related most closely to regions of goose adenovirus 4 strain P29 (80% and 81% identity on the nucleotide level) for which the sequence of gull adenovirus has not been determined yet.
3.5. Detection of a novel rotavirus
In addition to sequences of the expected viruses, previously unknown viruses were detected by sequence independent amplification and next-generation sequencing in the FFPE cloacal bursa tissues of the herring gulls. In the tissue of herring gull one, two reads were detected that were related most closely to endogenous retroviruses (identity 23% and 46%), which are integrated most likely into the host genome. In tissue of herring gull two, 170 reads were detected that were related most closely to various gene segments of viruses belonging to the genus Rotavirus (subfamily Sedoreovirinae, family Reoviridae) (Fig. 2A). Among these 170 reads, there was one contig of 570 nucleotides consisting of in total six reads that was related most closely to segment 6, which encodes the inner capsid protein VP6. The nucleotide sequence of this partial gene was obtained by 454 sequencing on FFPE tissue was identical to the sequence obtained by Sanger sequencing using bursal tissue and intestine collected from the same animal and stored at −70 °C. Since demarcation of species among rotaviruses is mainly based on genetic differences in VP6 regions (Johne et al., 2011, Matthijnssens et al., 2012), the partial region of this novel virus, tentatively called herring gull rotavirus (Genbank accession number KP057507), was aligned with various other rotaviruses. Subsequent phylogenetic analysis revealed that the partial VP6 segment of gull rotavirus was related most closely to rotavirus G chicken/03V0567/DEU/2003, with pairwise identities of 65% and 59% on the nucleotide level and deduced amino acid level, respectively (Fig. 2B). The species demarcation cut-off value for rotaviruses is <60% nucleotide identity in the VP6 gene segment (Johne et al., 2011), indicating that the herring gull rotavirus belongs to group G rotaviruses.
Fig. 2.
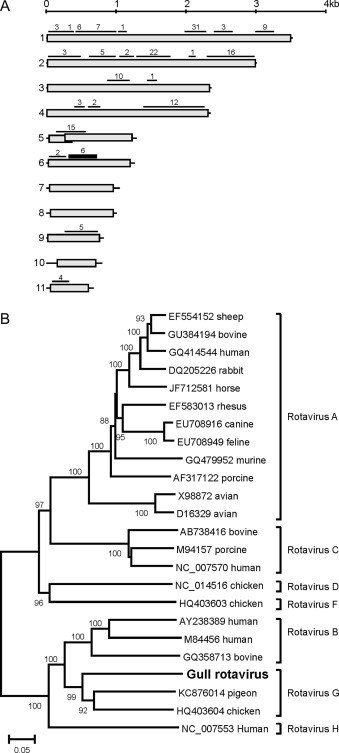
Sequence coverage mapping and phylogenetic analysis of gull rotavirus. (A) Sequence coverage mapping of reads detected in gull bursa tissue 2 that were related most closely to rotaviruses. The complete genome of rotavirus G chicken/03V0567/DEU/2003 was used as reference. Black lines indicate the presence reads that were related most closely to this region of the genome, the number above the black line indicates the number of reads that mapped to this region. (B) Phylogenetic neighbor-joining tree with 1000 bootstrap replicates of the partial segment 6 of gull rotavirus (570nt, corresponding to nt 319-885 of rotavirus G chicken/03V0567/DEU/2003) and various other rotaviruses. Only bootstrap values >70 are indicated.
4. Discussion
In the present study, the use of sequence-independent amplification in combination with next-generation sequencing to detect known and unknown viruses in FFPE tissues was evaluated. Using FFPE tissues from ferrets and herring gulls that were infected with either RNA viruses or DNA viruses, it was demonstrated that sequences of both known RNA and DNA viruses could be detected. In addition, this approach also allowed detection of a novel RNA virus, a rotavirus. These findings highlight the potential value of this technique in the identification of novel viral sequences in humans and animals with disease of presumed viral origin, for which only FFPE samples are available. The advantage of FFPE samples is that they are the most commonly collected and banked samples during pathological examination of people or animals that have died, that once embedding in paraffin takes place, the fixation process is stopped and the tissue remains in a stable state, and that samples may be kept indefinitely at room temperature. Furthermore, because FFPE samples have been produced in order to allow histopathological evaluation of tissues, any newly discovered virus can be directly correlated to the presence and character of histopathological changes in the tissue from which the viral RNA or DNA was obtained.
Next-generation sequencing has been applied previously on FFPE tissues to detect viral sequences using similar approaches (Cimino et al., 2014, Duncavage et al., 2011), but no previously unknown viral sequences have been detected and characterized yet to our knowledge.
A drawback of the present approach was that only a limited number of influenza A virus and adenovirus reads were detected, despite the presence of relatively high viral loads as detected by real time (RT-) PCR. These findings suggest that the combination of sequence-independent amplification and next-generation sequencing is rather insensitive compared to real time (RT-) PCR in FFPE tissues, which is in contrast to previous observations indicating a sensitivity approaching the sensitivity of real time (RT-) PCR in unfixed respiratory samples (Prachayangprecha et al., 2014). This insensitivity in FFPE tissues may be due to the impossibility to increase the relative amount of viral RNA and DNA by depletion of host nucleic acids with endonucleases, which is considered crucial for viral metagenomics studies (Delwart, 2013, Allander et al., 2001, Ng et al., 2009). The amount of viral reads could potentially be increased by selecting exactly the site of the lesion for RNA and DNA extraction and not the complete tissue section. This approach could be especially useful for well-demarcated, focal lesions, or for tissue changes that are characteristic for the presence of a virus infection, such as viral inclusion bodies. Another approach could be to apply a different sequencing platform (e.g. Illumina) which sequences to a higher depth and could increase the absolute number of viral reads.
Fragmentation of sequences during formalin fixation is also considered a major drawback of the use of FFPE tissues (Masuda et al., 1999, von Ahlfen et al., 2007, Hewitt et al., 2008). However, only minor differences in sequence length were observed between FFPE tissue and control samples in the present study. In addition, various other currently available deep-sequencing technologies produce only relatively shorter reads and for these applications the presence of a reduced number of large reads does not have any impact. Furthermore, the accuracy of deep sequencing on FFPE tissues was evaluated. After stringent quality trimming, most obtained viral taxonomic units for adeno- and influenza viruses and the partial VP6 gene of gull rotavirus had an error rate of 1% or less compared to the viral sequence in the tissue, which is similar to the error rate of 454 sequencing of nucleic acids extracted from other sample sources (Gilles et al., 2011, Shao et al., 2013).
In addition to sequences of known viruses, a relatively high number of reads were detected that were related most closely to rotaviruses in the cloacal bursa tissue of one of the herring gulls. Phylogenetic analysis suggested that this rotavirus belongs to the rotaviruses of group G, to which also rotaviruses detected in pigeons and chickens belong (Johne et al., 2011, Phan et al., 2013). No intestine was present in the tissue blocks used in the present study, suggesting that the rotavirus was present in the bursal tissue. However, additional studies are necessary to exclude that this was indeed no contamination and to elucidate the potential pathogenicity of this virus.
In conclusion, sequence-independent amplification in combination with next-generation sequencing of FFPE tissues might be a valuable approach to detect sequences of (unknown) viruses when no other samples are available and when a high viral load is expected. Of interest, the application of this method to FFPE tissues allows a direct correlation with the presence of histopathological changes in the corresponding tissue sections.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jviromet.2015.02.002.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- Allander T., Emerson S.U., Engle R.E., Purcell R.H., Bukh J. A virus discovery method incorporating DNase treatment and its application to the identification of two bovine parvovirus species. Proc. Natl. Acad. Sci. U. S. A. 2001;98:11609–11614. doi: 10.1073/pnas.211424698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allander T., Tammi M.T., Eriksson M., Bjerkner A., Tiveljung-Lindell A., Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc. Natl. Acad. Sci. U. S. A. 2005;102:12891–12896. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin D.A., Feldman M., Alwine J.C., Robertson E.S. Metagenomic assay for identification of microbial pathogens in tumor tissues. MBio. 2014;5:e01714-14. doi: 10.1128/mBio.01714-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodewes R., van de Bildt M.W., Schapendonk C.M., van Leeuwen M., van Boheemen S., de Jong A.A., Osterhaus A.D., Smits S.L., Kuiken T. Identification and characterization of a novel adenovirus in the cloacal bursa of gulls. Virology. 2013;440:84–88. doi: 10.1016/j.virol.2013.02.011. [DOI] [PubMed] [Google Scholar]
- Bodewes R., Kreijtz J.H., van Amerongen G., Hillaire M.L., Vogelzang-van Trierum S.E., Nieuwkoop N.J., van Run P., Kuiken T., Fouchier R.A., Osterhaus A.D., Rimmelzwaan G.F. Infection of the upper respiratory tract with seasonal influenza A(H3N2) virus induces protective immunity in ferrets against infection with A(H1N1)pdm09 virus after intranasal, but not intratracheal, inoculation. J. Virol. 2013;87:4293–4301. doi: 10.1128/JVI.02536-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodewes R., Sánchez Contreras G.J., Rubio García A., Hapsari R., van de Bildt M.W., Kuiken T., Osterhaus A.D. Identification of DNA sequences that imply a novel gammaherpesvirus in seals. J. Gen. Virol. 2014 doi: 10.1099/vir.0.000029. pii: vir.0.000029. [Epub ahead of print] PMID: 25524165. [DOI] [PubMed] [Google Scholar]
- Cimino P.J., Zhao G., Wang D., Sehn J.K., Lewis J.S., Jr., Duncavage E.J. Detection of viral pathogens in high grade gliomas from unmapped next-generation sequencing data. Exp. Mol. Pathol. 2014;96:310–315. doi: 10.1016/j.yexmp.2014.03.010. [DOI] [PubMed] [Google Scholar]
- Delwart E. Animal virus discovery: improving animal health, understanding zoonoses, and opportunities for vaccine development. Curr. Opin. Virol. 2012;2:344–352. doi: 10.1016/j.coviro.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwart E. A roadmap to the human virome. PLoS Pathog. 2013;9:e1003146. doi: 10.1371/journal.ppat.1003146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncavage E.J., Magrini V., Becker N., Armstrong J.R., Demeter R.T., Wylie T., Abel H.J., Pfeifer J.D. Hybrid capture and next-generation sequencing identify viral integration sites from formalin-fixed, paraffin-embedded tissue. J. Mol. Diagn. 2011;13:325–333. doi: 10.1016/j.jmoldx.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R., Cao B., Hu Y., Feng Z., Wang D., Hu W., Chen J., Jie Z., Qiu H., Xu K., Xu X., Lu H., Zhu W., Gao Z., Xiang N., Shen Y., He Z., Gu Y., Zhang Z., Yang Y., Zhao X., Zhou L., Li X., Zou S., Zhang Y., Li X., Yang L., Guo J., Dong J., Li Q., Dong L., Zhu Y., Bai T., Wang S., Hao P., Yang W., Zhang Y., Han J., Yu H., Li D., Gao G.F., Wu G., Wang Y., Yuan Z., Shu Y. Human infection with a novel avian-origin influenza A (H7N9) virus. N. Engl. J. Med. 2013;368:1888–1897. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- Gilles A., Meglecz E., Pech N., Ferreira S., Malausa T., Martin J.F. Accuracy and quality assessment of 454 GS-FLX Titanium pyrosequencing. BMC Genomics. 2011;12:245. doi: 10.1186/1471-2164-12-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grard G., Fair J.N., Lee D., Slikas E., Steffen I., Muyembe J.J., Sittler T., Veeraraghavan N., Ruby J.G., Wang C., Makuwa M., Mulembakani P., Tesh R.B., Mazet J., Rimoin A.W., Taylor T., Schneider B.S., Simmons G., Delwart E., Wolfe N.D., Chiu C.Y., Leroy E.M. A novel rhabdovirus associated with acute hemorrhagic fever in central Africa. PLoS Pathog. 2012;8:e1002924. doi: 10.1371/journal.ppat.1002924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt S.M., Lewis F.A., Cao Y., Conrad R.C., Cronin M., Danenberg K.D., Goralski T.J., Langmore J.P., Raja R.G., Williams P.M., Palma J.F., Warrington J.A. Tissue handling and specimen preparation in surgical pathology: issues concerning the recovery of nucleic acids from formalin-fixed, paraffin-embedded tissue. Arch. Pathol. Lab. Med. 2008;132:1929–1935. doi: 10.5858/132.12.1929. [DOI] [PubMed] [Google Scholar]
- Johne R., Otto P., Roth B., Lohren U., Belnap D., Reetz J., Trojnar E. Sequence analysis of the VP6-encoding genome segment of avian group F and G rotaviruses. Virology. 2011;412:384–391. doi: 10.1016/j.virol.2011.01.031. [DOI] [PubMed] [Google Scholar]
- Lipkin W.I., Firth C. Viral surveillance and discovery. Curr. Opin. Virol. 2013;3:199–204. doi: 10.1016/j.coviro.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda N., Ohnishi T., Kawamoto S., Monden M., Okubo K. Analysis of chemical modification of RNA from formalin-fixed samples and optimization of molecular biology applications for such samples. Nucleic Acids Res. 1999;27:4436–4443. doi: 10.1093/nar/27.22.4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthijnssens J., Otto P.H., Ciarlet M., Desselberger U., Van Ranst M., Johne R. VP6-sequence-based cutoff values as a criterion for rotavirus species demarcation. Arch. Virol. 2012;157:1177–1182. doi: 10.1007/s00705-012-1273-3. [DOI] [PubMed] [Google Scholar]
- Munster V.J., Baas C., Lexmond P., Waldenstrom J., Wallensten A., Fransson T., Rimmelzwaan G.F., Beyer W.E., Schutten M., Olsen B., Osterhaus A.D., Fouchier R.A. Spatial, temporal, and species variation in prevalence of influenza A viruses in wild migratory birds. PLoS Pathog. 2007;3:e61. doi: 10.1371/journal.ppat.0030061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng T.F., Manire C., Borrowman K., Langer T., Ehrhart L., Breitbart M. Discovery of a novel single-stranded DNA virus from a sea turtle fibropapilloma by using viral metagenomics. J. Virol. 2009;83:2500–2509. doi: 10.1128/JVI.01946-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan T.G., Vo N.P., Boros A., Pankovics P., Reuter G., Li O.T., Wang C., Deng X., Poon L.L., Delwart E. The viruses of wild pigeon droppings. PLoS ONE. 2013;8:e72787. doi: 10.1371/journal.pone.0072787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prachayangprecha S., Schapendonk C.M., Koopmans M.P., Osterhaus A.D., Schurch A.C., Pas S.D., van der Eijk A.A., Poovorawan Y., Haagmans B.L., Smits S.L. Exploring the potential of next-generation sequencing in detection of respiratory viruses. J. Clin. Microbiol. 2014;52:3722–3730. doi: 10.1128/JCM.01641-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmelzwaan G.F., Kuiken T., van Amerongen G., Bestebroer T.M., Fouchier R.A., Osterhaus A.D. Pathogenesis of influenza A (H5N1) virus infection in a primate model. J. Virol. 2001;75:6687–6691. doi: 10.1128/JVI.75.14.6687-6691.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurch A.C., Schipper D., Bijl M.A., Dau J., Beckmen K.B., Schapendonk C.M., Raj V.S., Osterhaus A.D., Haagmans B.L., Tryland M., Smits S.L. Metagenomic survey for viruses in Western Arctic Caribou, Alaska, through iterative assembly of taxonomic units. PLoS ONE. 2014;9:e105227. doi: 10.1371/journal.pone.0105227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao W., Boltz V.F., Spindler J.E., Kearney M.F., Maldarelli F., Mellors J.W., Stewart C., Volfovsky N., Levitsky A., Stephens R.M., Coffin J.M. Analysis of 454 sequencing error rate, error sources, and artifact recombination for detection of low-frequency drug resistance mutations in HIV-1 DNA. Retrovirology. 2013;10:18. doi: 10.1186/1742-4690-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers F., Wilm A., Dineen D., Gibson T.J., Karplus K., Li W., Lopez R., McWilliam H., Remmert M., Soding J., Thompson J.D., Higgins D.G. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan M., Sedmak D., Jewell S. Effect of fixatives and tissue processing on the content and integrity of nucleic acids. Am. J. Pathol. 2002;161:1961–1971. doi: 10.1016/S0002-9440(10)64472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubenberger J.K., Reid A.H., Krafft A.E., Bijwaard K.E., Fanning T.G. Initial genetic characterization of the 1918 Spanish influenza virus. Science. 1997;275:1793–1796. doi: 10.1126/science.275.5307.1793. [DOI] [PubMed] [Google Scholar]
- Tumpey T.M., Basler C.F., Aguilar P.V., Zeng H., Solorzano A., Swayne D.E., Cox N.J., Katz J.M., Taubenberger J.K., Palese P., Garcia-Sastre A. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science. 2005;310:77–80. doi: 10.1126/science.1119392. [DOI] [PubMed] [Google Scholar]
- van Boheemen S., de Graaf M., Lauber C., Bestebroer T.M., Raj V.S., Zaki A.M., Osterhaus A.D., Haagmans B.L., Gorbalenya A.E., Snijder E.J., Fouchier R.A. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. MBio. 2012:3. doi: 10.1128/mBio.00473-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brand J.M., van Leeuwen M., Schapendonk C.M., Simon J.H., Haagmans B.L., Osterhaus A.D., Smits S.L. Metagenomic analysis of the viral flora of pine marten and European badger feces. J. Virol. 2012;86:2360–2365. doi: 10.1128/JVI.06373-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen M., Williams M.M., Koraka P., Simon J.H., Smits S.L., Osterhaus A.D. Human picobirnaviruses identified by molecular screening of diarrhea samples. J. Clin. Microbiol. 2010;48:1787–1794. doi: 10.1128/JCM.02452-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Ahlfen S., Missel A., Bendrat K., Schlumpberger M. Determinants of RNA quality from FFPE samples. PLoS ONE. 2007;2:e1261. doi: 10.1371/journal.pone.0001261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


