Abstract
Two fragments, S66 and S55, of the S glycoprotein of the newly identified canine coronavirus type I (CCoV type I), were expressed in a procariotic system. The purified recombinant proteins of 350 and 366 amino acids in length, respectively, were employed to develop an enzyme-linked immunosorbent assay (ELISA) for the detection of CCoV type I antibodies in dog sera. Four canine sera-positive for CCoV type II, four sera-positive for CCoV type I and 10 negative control sera were examined. Only the sera-positive for CCoV type I strongly reacted with both the proteins, whereas the sera-positive for CCoV type II showed low reactivity in the ELISA test. As CCoV type I seems to be not cultivable in cell cultures, the recombinant fragments of the S protein represent a unique method to study, preliminarily, the immunological and the pathogenetic characteristics of this new virus.
Keywords: Canine coronavirus, Genotype I, Recombinant S protein
1. Introduction
Canine coronavirus (CCoV) is an enveloped virus of dogs associated with moderate to severe enteritis in young pups. CCoV is included in the group I of Coronaviridae family with transmissible gastroenteritis virus (TGEV) of swine, porcine epidemic diarrhoea virus (PEDV), porcine respiratory coronavirus (PRCoV), feline coronaviruses (FCoVs) and human coronavirus 229E (HCoV 229E). Coronavirus virions contain a single molecule of linear, positive sense RNA ranging from 27.6 to 31 kb. The genome has a 5′-terminal cap followed by a leader sequence of 65–98 nucleotides (nt) and an untranslated region of 200–400 nt. At the 3′ end of the genome there is an untranslated region of 200–500 nt followed by a poly(A) tail. The virion RNA functions as an mRNA and is infectious. It contains approximately 7–10 functional genes, 4 or 5 of which encode structural proteins. The genes are arranged in the order 5′-replicase–(HE)–S–E–M–N–3′, with a variable number of other genes that are believed to be non-structural and largely non-essential (Lai and Holmes, 2001).
The S protein, a large surface glycoprotein ranging from 1160 to 1452 amino acids (aa), is responsible for attachment to cells, haemagglutination, membrane fusion and induction of neutralizing antibodies. This large protein can be divided into three structural domains. The large external domain at the N-terminus is further divided into two sub-domains S1 and S2. The S1 sub-domain includes the N-terminal half of the molecule and forms the globular portion of the spikes. It contains sequences that are responsible for binding to specific receptors on the membrane of susceptible cells. S1 sequences are variable, containing various degrees of deletions and substitutions in different coronavirus strains or isolates. Mutations in the S1 region have been associated with altered antigenicity and pathogenicity. In contrast, S2 sequences are more conserved and contain two heptad repeat motifs that suggest a coiled-coil structure (Lai and Holmes, 2001).
Recently, an evident genetic diversity between the S gene of reference CCoV strains and of the newly identified CCoV type I strain Elmo/02 was observed, suggesting strongly the existence of a novel genotype of CCoV (CCoV type I) in dogs (Pratelli et al., 2003a). Sequence analysis of the S gene of the strain Elmo/02 revealed low nucleotide (49–51%) and amino acid (44%) identity to the reference CCoV (CCoV type II) and FCoV type II strains, while the highest correlation (73% nt and 74–75% aa) was found with FCoVs type I. The S protein of CCoV type I is characterised by the presence of a stretch of basic residues RRARR that is indicative of a potential cleavage of the protein (Pratelli et al., 2003a). A similar basic motif is present, approximately in the same position, in the coronaviruses belonging to groups II and III, but it is absent in the coronaviruses of group I, where CCoV type I is included.
The expression in Escherichia coli of two fragments of the S gene of CCoV type I strain Elmo/02 and the use of the recombinant proteins in serological tests are described.
2. Materials and methods
2.1. DNA techniques and protein expression
The newly identified CCoV type I strain Elmo/02 was employed in the present study (Pratelli et al., 2003a). About the three-fourths of the sequence of S gene, approximately 3347 nucleotides, were determined using primer pair sets designed with the CODEHOPE strategy (Rose et al., 1998), as described previously (Pratelli et al., 2003a). In order to over express and purify a large region of the S gene in E. coli, two pairs of primers were designed to amplify two different portions of the S gene, of 1050 bp (S66) and 1098 bp (S55), respectively (Fig. 1 ). Both forward primers contained in position 5′ the enzyme restriction site BamHI whereas both reverse primers contained the enzyme restriction site HindIII. The 1050 bp (S66) and 1098 bp (S55) gene fragments encode proteins of 350 and 366 aa with estimated molecular masses of 38.5 and 40 kDa, respectively. The amplified products were cloned into pCR®2.1-TOPO® vectors (TOPO TA Cloning®, Invitrogen, Milan, Italy). The nucleotide sequence of each insert was subjected to sequence analysis (Genome Express: Labo Grenoble, France) to confirm that no mutations had occurred during the amplification reaction. The cloned sequences were subsequently removed from the pCR®2.1-TOPO® vectors at the BamHI and HindIII restriction sites and transferred to a pQE-30 expression vector (Qiagen, Chatsworth, CA, USA). The constructs were used to transform E. coli DH5α cells containing pREP-4 repressor plasmid (lacIq). Cloning into pQE-30 makes it possible to produce recombinant proteins linked to a polyhistidine (His) stretch that binds strongly to nickel-chelated columns (Qiagen). PQE-30 derivatives were selected in LB agar plates supplemented with 100 μg/ml ampicillin and 50 μg/ml kanamycin. Plasmid DNA was extracted with Qiagen Plasmid mini Kit (Qiagen).
Fig. 1.
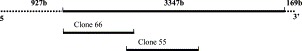
Schematic representation of the S gene of CCoV type I. The amplified region (3347 bp) and the two cloned fragments (S66 and S55) are indicated as thick lines.
Recombinant E. coli clones were grown in LB medium containing 2 mM isopropyl-1-thio-β-d-galactoside (IPTG) at 37 °C for 18 h. Bacteria were harvested by centrifugation at 2000×g for 10 min and washed twice with STE buffer (0.1 M NaCl, 10 mM Tris–HCl, 1 mM EDTA, pH 8.0). Pellets were resuspended in lysis buffer B (8 M urea, 0.1 M NaH2PO4, 0.01 M Tris–HCl, pH 8.0) at room temperature for 1 h with gently rocking. The His-tagged proteins were then extracted with the Qiagen Kit following the manufacturer’s instructions.
2.2. SDS-PAGE and immunoblotting
SDS-PAGE was performed according to the procedure of Laemmli (Laemmli, 1970). The apparent molecular mass of the recombinant proteins was determined using the marker Precision Plus Protein Standards, All Blue (Biorad, Hercules, USA). Electrophoresed proteins were transferred to nitrocellulose membranes of 0.45 μm pore size (Sigma, St. Louis, MO) in a Trans-Blot-semidry-apparatus (Biorad) as described by the manufacturer. The blots were incubated 2 h at room temperature in PBS-2% skim milk (Difco, Detroit, MI, USA) and then incubated 1 h at 37 °C with an anti-His monoclonal antibody. After several washes with PBS-2% skim milk, the blots were incubated for at least 1 h at 37 °C in phosphatase-conjugated anti-mouse IgG (Sigma). After washings, the blots were developed with bromochloroindolyl-phopshate/nitroblue tetrazolium (BCIP/NBT, Promega, Madison, WI) in alkaline phosphatase buffer (100 mM NaCl, 5 mM MgCl2, 100 mM Tris, pH 9.5).
2.3. Dog serum samples
Eighteen canine serum samples were employed in the present study. As representative of serum samples positive to CCoV type II (classical strain), four sera (nn 1–4) were collected from dogs vaccinated with an inactivated commercial CCoV type II vaccine and challenged subsequently with a field CCoV type II strain (Pratelli et al., 2003b). As representative of serum samples with antibodies to CCoV type I, four samples (nn 5–8) were collected from dogs naturally infected with CCoV type I, as confirmed by a specific PCR assay (Pratelli et al., 2002a). As negative control, 10 samples (nn 9–18) were collected from dogs kept under strict isolation that resulted sero-negative to CCoV type II by both enzyme-linked immunosorbent assay (ELISA) and western blotting assays (Pratelli et al., 2002b, Elia et al., 2002) and virus-negative to both CCoV type I and CCoV type II by specific PCR assays (Pratelli et al., 1999) for at least three times at 1-week-intervals.
2.4. Virus neutralization (VN) test
Serial two-fold dilutions starting from 1/2 of each serum sample were mixed with 100 TCID50 of CCoV type II strain 45/93 (Buonavoglia et al., 1994) in 96-well microtiter plates. The plates were incubated at room temperature for 90 min and then about 20,000 canine cells A72 were added to each well. The plates were read after 4 days of incubation at 37 °C, when the cytopathic effect was complete in the virus control cultures. The titre was expressed as the highest serum dilution neutralizing the virus.
2.5. Western blotting
The immunoblotting was carried out as described by Elia et al. (2002). Briefly, the two recombinant fragments of the S protein (S55 and S66) were resolved by SDS-PAGE and transferred to PVDF membranes. The membranes were blocked overnight at +4 °C with a 5% non-fat dry milk (Biorad). The PVDF membranes were washed three times at 10 min intervals with Tris buffered saline (TBS; Tris 25 mM, NaCl 200 mM, pH 7.4) containing 0.05% Tween 20 (TBS-TM) and then incubated for 2 h at room temperature with the canine sera.
Three wash cycles were carried out with TBS-TM and the membranes were incubated at room temperature for 2 h with a peroxidase-labelled goat anti-dog IgG (Sigma). After washing, a substrate solution, 3,3′-diaminobenzidine tetrahydrochloride (Sigma), was used to visualise the reaction.
2.6. ELISA
The ELISA was carried out as described by Pratelli et al. (2002b) with minor modifications. Briefly, 96-well microtiter plates were coated with 500 ng/well of the recombinant fragments of the S protein diluted in coating buffer and incubated overnight at +4 °C to ensure adequate antigen adsorption.
Plates were then washed four times with phosphate-buffered saline (PBS) containing 0.05% Tween 20 (PBS-T) and treated with blocking solution (0.2% gelatin in carbonate buffer) for 90 min at 37 °C. After repeated washes, 100 μl of each dog serum diluted 1:50 in PBS-T were added in duplicate and the plates were incubated for 90 min at 37 °C. The wash cycle was repeated four times, then goat anti-dog IgGs labelled with peroxidase (Sigma) were added and the plates were incubated for 60 min at 37 °C. After additional four wash cycles, 100 μl/well of freshly-prepared substrate 2,2′-azino-di-(3-ethylbenzothiazoline sulphonate) (ABTS) (Sigma) plus 25 μg/100 ml H2O2 were added and the plates were incubated for 25 min at room temperature. After adding the stop buffer solution (SDS 1%), the optical density (OD) was measured at 405 nm using an automatic ELISA reader (Biorad). The cut-off value was defined as the mean OD plus three standard deviations calculated from the 10 negative dog sera used as control.
3. Results
Immune staining with the anti-His monoclonal antibody identified polypeptides of the expected sizes (38.5 and 40 kDa) in the lysates of the bacterial cells transformed with clones S66 and S55, respectively (Fig. 2 ).
Fig. 2.
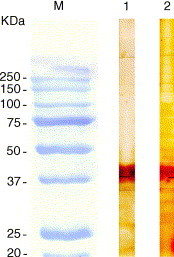
Immune-staining with anti-His monoclonal antibody. M, markers (precision plus protein standards, all blue, Biorad). Lane 1: recombinant polypeptide S55. Lane 2: recombinant polypeptide S66.
The reactivity of the recombinant polypeptides S66 and S55 was evaluated by Western blotting using dog sera-positive to either CCoV type I or to CCoV type II, as well as negative control sera. Both the 38.5 and 40 kDa polypeptides reacted only with the sera-positive to CCoV type I, while they were not recognized by the sera-positive to CCoV type II and by the negative control sera.
In the ELISA the cut-off point was determined as 0.024 when using the polypeptide S66 and 0.033 when using the polypeptide S55. The four sera-positive for CCoV type II displayed an OD median value of 0.034 when using the polypeptide S66 and 0.028 when using the polypeptide S55. Interestingly, the sera-positive for CCoV type I showed OD median values of 0.073 and 0.076 when using the polypeptides S66 and S55, respectively. The 10 negative control sera showed OD median values of 0.016 and 0.009 for the S66 and S55 polypeptides, respectively. The detailed results are shown in Table 1 .
Table 1.
Detection of antibodies to CCoVs in dog sera by an ELISA test with the recombinant polypeptides S66 and S55 and by virus neutralization test
| Dogs | Positivity to CCoV | ELISAa |
VN | |||
| S66 | Median value | S55 | Median value | |||
| 1 | Type II | 0.041 | 0.034 | 0.021 | 0.028 | 1:32 |
| 2 | Type II | 0.036 | 0.031 | 1:32 | ||
| 3 | Type II | 0.032 | 0.025 | 1:16 | ||
| 4 | Type II | 0.030 | 0.038 | 1:32 | ||
| 5 | Type I | 0.065 | 0.073 | 0.083 | 0.076 | <1:2 |
| 6 | Type I | 0.068 | 0.080 | <1:2 | ||
| 7 | Type I | 0.078 | 0.072 | <1:2 | ||
| 8 | Type I | 0.080 | 0.070 | <1:2 | ||
| 9–18 | Negative | 0.016 | 0.009 | <1:2 | ||
Cut-off values: 0.024 (S66), 0.033 (S55).
The VN test carried out on the negative control sera confirmed the absence of antibodies to CCoV. The four dog sera-positive to CCoV type I resulted negative. Otherwise, the four sera-positive to CCoV type II showed VN titres ranging from 1:32 (three dogs) to 1:16 (one dog) (Pratelli et al., 2003b).
4. Discussion
The identification of two distinct CCoV genotypes rises numerous intriguing questions such as the relative distribution of the two CCoV genotypes in dogs and their exact patho-biological role. In young pups CCoV causes diarrhoea, dehydration and occasional death. CCoV shedding in faeces occurs over a period of 6–14 days post-infection (Keenan et al., 1976), even if prolonged viral shedding has been demonstrated by PCR and nested-PCR in CCoV infected pups (Pratelli et al., 2001, Pratelli et al., 2002c).
Analysis with type-specific primer pairs, able to distinguish the two genotypes of CCoV, has demonstrated that 14.5% of CCoV infections are caused by CCoV type I (Elmo-like), 8.7% by CCoV type II (classical CCoV) and 76.8% by both the genotypes (Pratelli et al., in press). This indicates that: (i) most CCoV infections are caused by the two genotypes; (ii) CCoV type I is widespread in dogs. The wide diffusion of CCoV type I in dog population, underlines the need for more in-depth investigations on the pathogenetic role of the new CCoV genotype, on the biological implication of double infections and, finally, on the serologic correlations between the two CCoV genotypes. Unfortunately, failure to adapt CCoV type I to growth in cell cultures, complicates these studies.
Using the ELISA described in the present study, the negative control sera did not react with the two recombinant fragments of the S protein, while the sera-positive to CCoV type II resulted weakly positive to polypeptide S66 (OD median values of 0.034) and completely negative to S55. Interestingly, dog sera-positive to CCoV type I revealed high OD values with both the recombinant proteins.
By Western blotting both the negative control sera and the sera-positive to CCoV type II did not react with either of the recombinant polypeptides. In contrast, the sera-positive to CCoV type I reacted weakly with the recombinant polypeptides.
Of interest, the sera that tested positive for CCoV type I by ELISA, were negative by the VN test, suggesting that no cross-relationship exists between CCoV type I and II.
The results obtained by Western blotting and ELISA using the two recombinant fragments of the S protein of CCoV type I, even if tested on a small number of sera, indicates that those tests (the ELISA, in particular) may be considered as a valid system to study the unknown patho-biology of CCoV type I infection in dogs.
It is well known that FCoV, on the basis of its relationship to CCoV, can be distinguished into two serotypes, FCoV type I and II, by in vitro virus neutralization using either type-specific feline sera or monoclonal antibodies raised to the S protein (Herrewegh et al., 1998). Comparative sequence analysis of the genome of FCoVs type I and II and of CCoV has demonstrated that FCoV type II has arisen from a template switch between FCoV type I and CCoV, which took place between the S and M genes. Consequently, CCoV specific sera neutralize FCoV type II but fail to neutralize, or neutralize poorly, FCoV type I (Rottier, 1999). Failure of CCoV type I to grow in cell culture hinders the evaluation of serological correlation between CCoV type I and CCoV type II. From this perspective, the expression of the two fragments of the S protein of CCoV type I and their use to develop serological tests or to produce monoclonal antibodies currently represent a powerful and unique tool for research of the new genotype of CCoV.
Acknowledgements
This work was supported by grants from Ministry of University, Italy (project: Enteriti virali del cane).
References
- Buonavoglia, C., Marsilio, F., Cavalli, A., Tiscar, P.G., 1994. L’infezione da coronavirus del cane: indagine sulla presenza del virus in Italia. Not. Farm. Vet. No. 2/94, ed. SCIVAC.
- Elia G., Decaro N., Tinelli A., Martella V., Pratelli A., Buonavoglia C. Evaluation of antibody response to canine coronavirus infection in dogs by Western blotting analysis. New Microbiol. 2002;25:275–280. [PubMed] [Google Scholar]
- Herrewegh A.A.P.M., Smeenk I., Horzinek M.C., Rottier P.J.M., de Groot R.J. Feline coronavirus type II strains 79-1683 and 79-1146 originate from a double recombination between feline coronavirus type I and canine coronavirus. J. Virol. 1998;72:4508–4514. doi: 10.1128/jvi.72.5.4508-4514.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan K.P., Jervis H.R., Marchwicki R.H., Binn L.N. Intestinal infection of neonatal dogs with canine coronavirus 1–71: studies by virologic, histologic, histochemical and immunofluorescent techniques. Am. J. Vet. Res. 1976;37:247–256. [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai, M.M.C., Holmes, K.V., 2001. Coronaviridae: the viruses and their replication. In: Knipe, D.M., Howley, P.M., Griffin, D.E., Lamb, R.A., Martin, M.A., Roizman, B., Strais, S.E. (Eds.), Fields Virology, vol. 1. Lippincott Williams & Wilkins, Philadelphia, pp. 1163–1185.
- Pratelli A., Tempesta M., Greco G., Martella V., Buonavoglia C. Development of a nested PCR for the detection of canine coronavirus. J. Virol. Methods. 1999;80:11–15. doi: 10.1016/S0166-0934(99)00017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratelli A., Martella V., Elia G., Tempesta M., Guarda F., Capucchio M.T., Carmichael L.E., Buonavoglia C. Severe enteric disease in an animal shelter associated with dual infections by canine adenovirus type 1 and canine coronavirus. J. Vet. Med. B. 2001;48:385–392. doi: 10.1046/j.1439-0450.2001.00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratelli A., Tinelli A., Decaro N., Camero M., Elia G., Gentile A., Buonavoglia C. PCR assay for the detection and the identification of atypical canine coronavirus in dogs. J. Virol. Methods. 2002;106:209–213. doi: 10.1016/S0166-0934(02)00165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratelli A., Elia G., Martella V., Palmieri A., Cirone F., Tinelli A., Corrente M., Buonavoglia C. Prevalence of canine coronavirus antibodies in dogs in the south of Italy by an enzyme-linked immunosorbent assay. J. Virol. Methods. 2002;102:67–71. doi: 10.1016/S0166-0934(01)00450-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratelli A., Elia G., Martella V., Tinelli A., Decaro N., Marsilio F., Buonavoglia D., Tempesta M., Buonavoglia C. M gene evolution of canine coronavirus in naturally infected dogs. Vet. Rec. 2002;151:758–761. [PubMed] [Google Scholar]
- Pratelli A., Martella V., Decaro N., Tinelli A., Camero M., Cirone F., Elia G., Cavalli A., Corrente M., Greco G., Buonavoglia D., Gentile M., Tempesta M., Buonavoglia C. Genetic diversity of a canine coronavirus detected in pups with diarrhoea in Italy. J. Virol. Methods. 2003;110:9–17. doi: 10.1016/S0166-0934(03)00081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratelli A., Tinelli A., Decaro N., Cirone F., Elia G., Roperto S., Tempesta M., Buonavoglia C. Efficacy of an inactivated canine coronavirus vaccine in pups. New Microbiol. 2003;26:151–155. [PubMed] [Google Scholar]
- Pratelli, A., Decaro, N., Tinelli, A., Martella, V., Elia, G., Tempesta, M., Cirone, F., Buonavoglia, C., 2004. Two genotypes of canine coronavirus are simultaneously detected in the faecal samples of dogs with diarrhoea. J. Clin. Microbiol., in press. [DOI] [PMC free article] [PubMed]
- Rose T.M., Schultz E.R., Henikoff J.G., Pietrokovski S., McCallum C.M., Henikoff S. Consensus-degenerate hybrid oligonucleotide primers for amplification of distantly related sequences. NAR. 1998;26:1628–1635. doi: 10.1093/nar/26.7.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottier P.J. The molecular dynamics of feline coronaviruses. Vet. Microbiol. 1999;69:117–125. doi: 10.1016/S0378-1135(99)00099-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


