Abstract
Background
Osteoarthritis is the most common form of joint disease and the leading cause of pain and physical disability in the elderly. Transcutaneous electrical nerve stimulation (TENS), interferential current stimulation and pulsed electrostimulation are used widely to control both acute and chronic pain arising from several conditions, but some policy makers regard efficacy evidence as insufficient.
Objectives
To compare transcutaneous electrostimulation with sham or no specific intervention in terms of effects on pain and withdrawals due to adverse events in patients with knee osteoarthritis.
Search methods
We updated the search in CENTRAL, MEDLINE, EMBASE, CINAHL and PEDro up to 5 August 2008, checked conference proceedings and reference lists, and contacted authors.
Selection criteria
Randomised or quasi‐randomised controlled trials that compared transcutaneously applied electrostimulation with a sham intervention or no intervention in patients with osteoarthritis of the knee.
Data collection and analysis
We extracted data using standardised forms and contacted investigators to obtain missing outcome information. Main outcomes were pain and withdrawals or dropouts due to adverse events. We calculated standardised mean differences (SMDs) for pain and relative risks for safety outcomes and used inverse‐variance random‐effects meta‐analysis. The analysis of pain was based on predicted estimates from meta‐regression using the standard error as explanatory variable.
Main results
In this update we identified 14 additional trials resulting in the inclusion of 18 small trials in 813 patients. Eleven trials used TENS, four interferential current stimulation, one both TENS and interferential current stimulation, and two pulsed electrostimulation. The methodological quality and the quality of reporting was poor and a high degree of heterogeneity among the trials (I2 = 80%) was revealed. The funnel plot for pain was asymmetrical (P < 0.001). The predicted SMD of pain intensity in trials as large as the largest trial was ‐0.07 (95% CI ‐0.46 to 0.32), corresponding to a difference in pain scores between electrostimulation and control of 0.2 cm on a 10 cm visual analogue scale. There was little evidence that SMDs differed on the type of electrostimulation (P = 0.94). The relative risk of being withdrawn or dropping out due to adverse events was 0.97 (95% CI 0.2 to 6.0).
Authors' conclusions
In this update, we could not confirm that transcutaneous electrostimulation is effective for pain relief. The current systematic review is inconclusive, hampered by the inclusion of only small trials of questionable quality. Appropriately designed trials of adequate power are warranted.
Plain language summary
Transcutaneous electrostimulation for osteoarthritis of the knee
This summary of a Cochrane review presents what we know from research about the effect of transcutaneous electrostimulation on osteoarthritis of the knee.
The review shows that in people with osteoarthritis:
‐ We are uncertain whether transcutaneous electrostimulation affects pain or your ability to use your knee because of the very low quality of the evidence. ‐ Transcutaneous electrostimulation may not have any side effects. We often do not have precise information about side effects and complications. This is particularly true for rare but serious side effects.
What is osteoarthritis and what is transcutaneous electrostimulation?
Osteoarthritis (OA) is a disease of the joints, such as your knee. When the joint loses cartilage, the bone grows to try and repair the damage. Instead of making things better, however, the bone grows abnormally and makes things worse. For example, the bone can become misshapen and make the joint painful and unstable. This can affect your physical function or ability to use your knee.
Transcutaneous electrostimulation, such as TENS, is a kind of pain relief typically using electrical currents applied to the skin. Transcutaneous electrostimulation machines are typically small, battery‐operated machines with 2 electrodes attached. Electrodes are wires that send the electrical current. Usually, you connect two electrodes from the machine to your skin on the painful area. Your doctor or physiotherapist will show you how to use it, and most machines can be used at home.
Best estimate of what happens to people with osteoarthritis who use transcutaneous electrostimulation up to 4 weeks after using it:
Pain
‐ People who used electrostimulation had an improvement in their pain of about 2 on a scale from 0 (no pain) to 10 (extreme pain) 4 weeks after using it.
‐ People who used a fake electrostimulation machine or just took their usual treatments had an improvement in their pain of about 2 on a scale from 0 (no pain) to 10 (extreme pain) 4 weeks after using it.
‐ People had no more average improvement when using electrostimulation, and no more people responded to treatment with electrostimulation compared with people who used a fake electrostimulation machine or just took their usual treatments (difference of 0%).
Physical Function
‐ People who used electrostimulation had an improvement in their physical function of about 2 on a scale from 0 (no disability) to 10 (extreme disability) 4 weeks after using it.
‐ People who used a fake electrostimulation machine or just took their usual treatments had an improvement in their physical function of about 1 on a scale from 0 (no disability) to 10 (extreme disability) 4 weeks after using it.
‐ People using electrostimulation had 1 unit more improvement in their knee function when compared to people who used a fake electrostimulation machine or just took their usual treatments.
Another way of saying this is:
‐ 29 people out of 100 who used electrostimulation respond to treatment (29%).
‐ 26 people out of 100 who used a fake electrostimulation machine or just took their usual treatments respond to treatment (26%).
‐ 3 more people respond to treatment with electrostimulation compared with people who used a fake electrostimulation machine or just took their usual treatments (difference of 3%).
Dropouts or withdrawals from the trial because of side effects
‐ 2 people out of 100 who used electrostimulation dropped out or withdrew from the trial because of side effects (2%).
‐ 2 people out of 100 who used a fake electrostimulation machine or just took their usual treatments dropped out of the trial because of side effects (2%).
‐ There was no difference in the number of people who dropped out of the trial because of side effects (difference of 0%). This could be the result of chance.
Side effects
‐ 15 people out of 100 who used electrostimulation experienced side effects (15%).
‐ 15 people out of 100 who used a fake electrostimulation machine or just took their usual treatments experienced side effects (15%).
‐ There was no difference in the number of people who experience side effects (difference of 0%). This could be the result of chance.
Summary of findings
for the main comparison.
| Any type of transcutaneous electrostimulation compared with sham or no intervention for osteoarthritis of the knee | ||||||
|
Patient or population: patients with osteoarthritis Settings: physical therapy practice of outpatient clinic Intervention: any type of transcutaneous applied electrostimulation Comparison: sham or no specific intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk* | Corresponding risk | |||||
| Sham or no specific intervention | Any type of transcutaneous electrostimulation | |||||
|
Pain Various pain scales Median follow‐up: 4 weeks |
‐1.8 cm change on 10 cm VAS1 29% improvement |
‐2.0 cm change
(Δ ‐0.2 cm, ‐1.2 to 0.8 cm)2 33% improvement (Δ +4%, ‐13% to +20%)3 |
SMD ‐0.07 (‐0.46 to 0.32) | 726 (16 studies) | +OOO very low4 | Little evidence of beneficial effect (NNT: not statistically significant) The estimated pain in the intervention group of large trials was derived from meta‐regression using the standard error as independent variable |
|
Function Various validated function scales Median follow‐up: 4 weeks |
‐1.2 units on WOMAC
(range 0 to 10)1 21% improvement |
‐2.3 units on WOMAC
(Δ ‐1.1, ‐1.6 to ‐0.6)5 41% improvement (Δ +20%, +11% to +29%)6 |
SMD ‐0.34 (‐0.54 to ‐0.14) | 407 (9 studies) | +OOO very low7 | NNT: 10 (95% CI 7 to 22)8 |
|
Number of patients experiencing any adverse event Median follow‐up: 4 weeks |
150 per 1000 patient‐years1 | 153 per 1000 patient‐years (80 to 296) | RR 1.02 (0.53 to 1.97) | 175 (3 studies) | ++OO low9 | No evidence of harmful effect (NNH: not statistically significant) |
|
Number of patients withdrawn or dropped out because of adverse events Median follow‐up: 4 weeks |
17 per 1000 patient‐years1 | 16 per 1000 patient‐years (3 to 102) | RR 0.97 (0.16 to 6.00) | 363 (8 studies) | +++O moderate10 | No evidence of harmful effect (NNH: not statistically significant) |
|
Number of patients experiencing any serious adverse event Median follow ‐up: 4 weeks |
4 per 1000 patient‐years1 | 1 per 1000 patient‐years (0 to 29) | RR 0.33 (0.02 to 7.32) | 195 (4 studies) | ++OO low11 | No evidence of harmful effect (NNH: not statistically significant) |
| *The basis for the assumed risk in the safety outcomes (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GRADE: GRADE Working Group grades of evidence (see explanations); NNT: number needed to treat; NNH: number needed to harm; RR: risk ratio; SMD: standardised mean difference | ||||||
GRADE Working Group grades of evidence High quality (++++): Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality (+++O): Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality (++OO): Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality (+OOO): We are very uncertain about the estimate.
1 Median reduction as observed across control groups in large osteoarthritis trials (Nuesch 2009). 2 Standardised mean differences (SMDs) were back‐transformed onto a 10 cm visual analogue scale (VAS) on the basis of a typical pooled SD of 2.5 cm in trials that assessed pain using a VAS, and expressed as change based on an assumed standardised reduction of 0.72 standard deviation units in the control group. 3 The median observed pain score at baseline across control groups in large osteoarthritis trials was 6.1 cm on a 10 cm VAS (Nuesch 2009). 4 Downgraded (3 levels) because the effect was estimated from a meta‐regression model using the standard error as independent variable and because included trials were generally of low quality and small sample size: only 2 out of 16 trials used adequate concealment of allocation, only 3 performed analyses according to the intention‐to‐treat principle, and the presence of large between trial heterogeneity. 5 Standardised mean differences (SMDs) were back‐transformed onto a 0 to 10 standardised WOMAC function score on the basis of a typical pooled SD of 2.1 in trials that assessed function on WOMAC function scale and expressed as change based on an assumed standardised reduction of 0.58 standard deviation units in the control group. 6 The median observed standardised WOMAC function score at baseline across control groups in large osteoarthritis trials was 5.6 units (Nuesch 2009). 7 Downgraded (3 levels) because included trials were generally of low quality and small sample size: 1 out of 9 studies used adequate concealment of allocation methods, only 2 performed analyses according to the intention‐to‐treat principle, presence of moderate between trial heterogeneity, 9 out of 18 studies reported this outcome, likely leading to selective outcome reporting bias. 8 Absolute response risks for function in the control groups were assumed 26% (see Methods section). 9 Downgraded (2 levels) because the confidence interval crosses no difference in the pooled estimate, 1 out of 3 studies included all patients in this analysis, 3 out of 18 studies reported this outcome, likely leading to selective outcome reporting bias. 10 Downgraded (1 level) because the confidence interval of the pooled estimate is wide and crossed no difference, 8 out of 18 studies reported this outcome, possibly leading to selective outcome reporting bias. 11 Downgraded (2 levels) because 4 out of 18 studies reported this outcome, possibly leading to selective outcome reporting bias, the confidence interval of the pooled estimate is wide and crossed no difference.
Background
Osteoarthritis is an age‐related condition, occurring more frequently in women than in men. Its prevalence, causal associations and outcomes vary markedly according to the joint site affected (Jüni 2006). Osteoarthritis is characterised by focal areas of loss of articular cartilage in synovial joints, accompanied by subchondral bone changes, osteophyte formation at the joint margins, thickening of the joint capsule and mild synovitis (Solomon 1997). The objectives of management of knee osteoarthritis are to relieve pain and to maintain or improve function. Different modalities in physiotherapy have been suggested to improve the clinical course of knee osteoarthritis, with potentially fewer adverse effects than medical treatment (Bjordal 2007; Jamtvedt 2008), but some policy makers consider the evidence for effectiveness to be insufficient (Gezondheidsraad 1999).
Transcutaneous electrostimulation, the application of any electrical current through the skin with the aim of pain modulation, is a frequently used modality in knee osteoarthritis (Carroll 2001; Osiri 2000). It is based on the 'Gate‐Control Theory' of pain perception as described by Melzack and Wall (Melzack 1965). The theory suggests that the stimulation of large diameter, (A‐beta) primary sensory afferent cutaneous fibres activates inhibitory interneurons in the spinal cord dorsal horn and, thereby, may attenuate the transmission of nociceptive signals from small diameter A‐delta and C fibres. Other suggested mechanisms include a stimulation of β endorphin production (Andersson 1976; Grimmer 1992; Mayer 1989) and even the potential for articular cartilage repair (Fary 2008; Haddad 2007).
Several types of electrostimulation are available. Conventional transcutaneous electrical nerve stimulation (TENS), in its narrow sense, uses moderate to high frequency current of 40 to 150 Hz and 50 to 100 µsec pulse width, typically at a low intensity, to stimulate sensory fibres. Several other types of TENS were subsequently developed, which differ in intensity, pulse width or frequency. Acupuncture‐like TENS (AL TENS) uses a low frequency current of 0.5 to 10 Hz and a pulse width of > 150 µsec at a high intensity to stimulate both motor and sensory fibres. The stimulation may be painful, and the intensity of the current will depend on the patient's individual pain tolerance. Burst TENS was developed to minimise patients' discomfort, as experienced with AL TENS. It uses short bursts of high frequency current of typically 80 to 100 Hz, which are repetitively applied at low intensity and a burst frequency of around 5 Hz, to stimulate motor and sensory fibres. The intensity used is slightly higher than used with conventional TENS. Brief TENS uses a high frequency current of more than 100 Hz and 150 to 250 µsec pulse width at the maximal intensity tolerated by the patient to stimulate not only motor and sensory, but also nociceptor fibres. Modulation TENS combines several of the modalities above, typically using alternations of low and high frequency currents (Brosseau 2004; Sluka 2003). Classical interferential current stimulation simultaneously uses two non‐modulated biphasic pulsed currents applied with two sets of electrodes with four electrical poles; one current is fixed at approximately 4000 Hz and the other ranging typically from 4000 to 4100 Hz. The superimposition of the two currents results in a new frequency with a range from 1 to 100 Hz (Wadsworth 1980). Modulated interferential current stimulation uses directed currents between two electrical poles and vectorially sums currents in the tissue, with a carrier frequency typically set at 4000 Hz, a beat frequency at 80 Hz, and a modulation frequency set between 0 to 150 Hz. The effective frequency is defined by the sum of beat and modulation frequency and varies between 80 and 230 Hz. The high frequency of the carrier currents in inferential current stimulation leads to a considerably lower impedance of skin and subcutaneous tissue as compared with conventional TENS and minimises patients' discomfort. Lastly, pulsed electrostimulation applies high frequency current of 100 Hz and a pulse width of 640 to 1800 µsec, typically using knee garments with flexible, embedded electrodes and a small battery‐operated generator, allowing application times of several hours rather than 15 to 60 minutes, as is the case for any other of the modalities described above.
Objectives
We set out to compare transcutaneous electrostimulation with sham or no specific intervention in terms of effects on pain and function and safety outcomes in patients with knee osteoarthritis and to explore whether potential variation between trials could be explained by characteristics of the electrostimulation, by biases affecting individual trials or by publication bias.
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised controlled trials with a control group receiving a sham intervention or no intervention.
Types of participants
Studies including at least 75% of patients with clinically and/or radiologically confirmed osteoarthritis of the knee.
Types of interventions
Any type of transcutaneous electrostimulation with electrodes set to stimulate nerves supplying the knee joint area aiming at pain relief. We did not consider transcutaneous electrostimulation aiming at muscle strength enhancement, such as neuromuscular electrostimulation, and electrostimulation not directly aimed at stimulating nerves of the knee joint area, such as transcranial applications or transcutaneous spinal electroanalgesia. There were no restrictions related to the type of electrode used.
Types of outcome measures
Main outcomes
Main outcomes were pain intensity as the effectiveness outcome (Altman 1996; Pham 2004) and withdrawals or drop‐outs because of adverse events as the safety outcome. If data on more than one pain scale were provided for a trial, we referred to a previously described hierarchy of pain‐related outcomes (Jüni 2006; Reichenbach 2007) and extracted data on the pain scale that is highest on this hierarchy:
Global pain
Pain on walking
WOMAC osteoarthritis index pain subscore
Composite pain scores other than WOMAC
Pain on activities other than walking
Rest pain or pain during the night
WOMAC global algofunctional score
Lequesne osteoarthritis index global score
Other algofunctional scale
Patient's global assessment
Physician's global assessment
If pain outcomes were reported at several time points, we extracted the estimate at the end of the treatment period.
Secondary outcomes
Secondary outcomes were function, the number of patients experiencing any adverse event and patients experiencing any serious adverse events. We defined serious adverse events as events resulting in hospitalisation, prolongation of hospitalisation, persistent or significant disability, congenital abnormality/birth defect of offspring, life‐threatening events or death.
If data on more than one function scale were provided for a trial, we extracted data according to the hierarchy presented below.
Global disability score
Walking disability
WOMAC disability subscore
Composite disability scores other than WOMAC
Disability other than walking
WOMAC global scale
Lequesne osteoarthritis index global score
Other algofunctional scale
Patient’s global assessment
Physician’s global assessment
If function outcomes were reported at several time points, we extracted the estimate at the end of the treatment period. For safety outcomes, we extracted end of trial data.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2008, issue 3), MEDLINE and EMBASE through the Ovid platform (www.ovid.com), CINAHL through EBSCOhost, Physiotherapy Evidence Database (PEDro, http://www.pedro.fhs.usyd.edu.au/, from 1929 onwards), all from implementation to 5 August 2008, using a combination of keywords and text words related to electrostimulation combined with keywords and text words related to osteoarthritis and a validated filter for controlled clinical trials (Dickersin 1994). The search strategy is presented in Appendix 1 and Appendix 2.
Searching other sources
We manually searched conference proceedings, used Science Citation Index to retrieve reports citing relevant articles, contacted content experts and trialists and screened reference lists of all obtained articles, including related reviews. Finally, we searched several clinical trial registries (www.clinicaltrials.gov, www.controlled‐trials.com, www.actr.org.au, www.umin.ac.jp/ctr) to identify ongoing trials.
The last update of the manual search was on 2 February 2009.
Data collection and analysis
Selection of studies
Two review authors evaluated independently all titles and abstracts for eligibility (see Figure 1). We resolved disagreements by discussion. We applied no language restrictions. If multiple reports described the same trial, we considered all.
1.
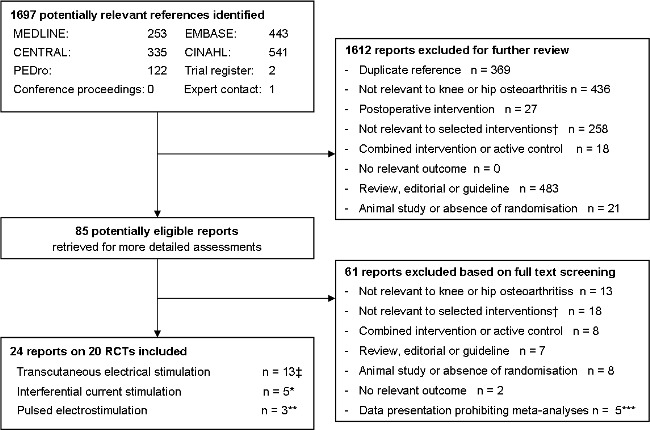
Flow chart
Data collection
Two review authors (AR and EN, RS or LK) extracted trial information independently using a standardised, piloted data extraction form accompanied by a codebook. We resolved disagreements by consensus or discussion with a third author (SR or PJ). We extracted the type of electrostimulation, including the mode of function (types of stimulator and electrode), the pulse form (intensity, rate and width), the electrode placement site and the frequency and duration of treatment. Other data extracted included the type of control intervention used, patient characteristics (gender, average age, duration of symptoms, type of joint), characteristics of pain, function and safety outcomes, design, trial size, trial duration (defined as time from randomisation until end of follow up), type and source of financial support and publication status. When necessary, we approximated means and measures of dispersion from figures in the reports. For cross‐over trials, we extracted data from the first period only. Whenever possible, we used results from an intention‐to‐treat analysis. If effect sizes could not be calculated, we contacted the authors for additional data.
Quality assessment
Two review authors (AR and EN, RS or LK) independently assessed randomisation, blinding, selective outcome reporting and handling of incomplete outcome data in the analyses (Higgins 2008; Jüni 2001). We resolved disagreements by consensus or discussion with a third author (SR or PJ). We assessed two components of randomisation: generation of allocation sequences and concealment of allocation. We considered generation of sequences adequate if it resulted in an unpredictable allocation schedule; mechanisms considered adequate included random‐number tables, computer‐generated random numbers, minimisation, coin tossing, shuffling of cards and drawing of lots. Trials using an unpredictable allocation sequence were considered randomised; trials using potentially predictable allocation mechanisms, such as alternation or the allocation of patients according to date of birth, were considered quasi‐randomised. We considered allocation concealment adequate if the investigators responsible for patient selection were unable to suspect before allocation which treatment was next; methods considered adequate included central randomisation and sequentially numbered, sealed, opaque envelopes. We considered blinding of patients adequate if a sham intervention was used that was identical in appearance from the control intervention. Transcutaneous electrostimulation generally does not allow blinding of therapists, whereas pain as the main effectiveness outcome is patient‐reported by definition. Therefore, we did not assess blinding of therapists and outcome assessors. We considered handling of incomplete outcome data adequate if all randomised patients were included in the analysis (intention‐to‐treat principle). Finally, we used GRADE to describe the quality of the overall body of evidence (Higgins 2008; Guyatt 2008), defined as the extent of confidence in the estimated treatment benefits and harms.
Data synthesis
We summarised continuous outcomes using standardised mean differences (SMD), with the differences in mean values at the end of treatment across treatment groups divided by the pooled standard deviation. If differences in mean values at the end of the treatment were unavailable, we used differences in mean changes. If some of the required data were unavailable, we used approximations as previously described (Reichenbach 2007). A SMD of ‐0.20 standard deviation units can be considered a small difference between experimental and control group, a SMD of ‐0.50 a moderate difference, and ‐0.80 a large difference (Cohen 1988; Jüni 2006). SMDs can also be interpreted in terms of the percent of overlap of the experimental group's scores with the scores of the control group. A SMD of ‐0.20 indicates an overlap in the distributions of pain or function scores in about 85% of cases, a SMD of ‐0.50 in approximately 67% and a SMD of ‐0.80 in about 50% of cases (Cohen 1988; Jüni 2006). On the basis of a median pooled SD of 2.5 cm found in large‐scale osteoarthritis trials that assessed pain using a 10 cm visual analogue scale (VAS) (Nuesch 2009), SMDs of ‐0.20, ‐0.50 and ‐0.80 correspond to approximate differences in pain scores between experimental and control groups of 0.5, 1.25 and 2.0 cm on a 10 cm VAS. SMDs for function were back transformed to a standardised WOMAC disability score (Bellamy 1995) ranging from 0 to 10 on the basis of a median pooled SD of 2.1 units observed in large‐scale osteoarthritis (Nuesch 2009). We expressed binary outcomes as relative risks.
We used standard inverse‐variance random‐effects meta‐analysis (DerSimonian 1986) to combine trials overall and stratified according to gross categories of electrostimulation (TENS, interferential current stimulation or pulsed electrostimulation). We quantified heterogeneity between trials using the I2 statistic (Higgins 2003), which describes the percentage of variation across trials that is attributable to heterogeneity rather than to chance and the corresponding χ2 test. I2 values of 25%, 50% and 75% may be interpreted as low, moderate and high between‐trial heterogeneity, although the interpretation of I2 depends on the size and number of trials included (Rucker 2008). The association between trial size and treatment effects was investigated in funnel plots, plotting effect sizes on the vertical axis against their standard errors on the horizontal axis. We assessed asymmetry by the asymmetry coefficient: the difference in effect size per unit increase in standard error (Sterne 2001), which is mainly a surrogate for sample size, and used uni‐variable meta‐regression analysis to predict treatment effects in trials as large as the largest trials included in the meta‐analysis, using the standard error as the explanatory variable (Shang 2005). In view of the biased nature of the predominantly small trials included in the meta‐analysis of pain intensity, we considered the predicted estimates of effectiveness more reliable than the pooled estimates. For the analysis on the effectiveness outcomes pain and function, we differentiated between TENS, interferential current stimulation and pulsed electrostimulation. Then, we performed effectiveness analyses stratified by the following trial characteristics: concealment of allocation, use of a sham intervention in the control group, blinding of patients, analysis in accordance with the intention‐to‐treat principle, trial size, difference in the use of analgesic cointerventions, specific type of electrostimulation, duration of stimulation per session, number of sessions per week, duration of electrostimulation per week as an overall measure of treatment intensity, and duration of treatment period. A cut‐off of 200 patients was used to distinguish between small and large trials; a sample size of 100 patients per group will yield more than 80% power to detect a small to moderate SMD of ‐0.40 at a two‐sided P of 0.05. For the analysis according to specific type of stimulation, we distinguished between high frequency TENS, burst TENS, modulation TENS, low frequency TENS, interferential current stimulation or pulsed electrostimulation. We classified conventional TENS and brief TENS as high frequency TENS. Cut‐offs of 20 and 60 minutes were used for the duration of electrostimulation per session, corresponding to the typical treatment duration in physical therapy, and the optimum stimulation duration suggested by Cheing 2003. A cut‐off of four weeks was used for the overall duration of the treatment period (time from randomisation to last session), in line with the previous version on this review. Cut‐offs of three and seven were used for the number of sessions per week; one and five hours for the duration of electrostimulation per week, corresponding to the distribution of tertiles. We used uni‐variable random‐effects meta‐regression models to determine whether treatment effects were affected by these factors (Thompson 1999). Then, we converted SMDs of pain intensity and function to odds ratios (Chinn 2000) to derive numbers needed to treat (NNT) to cause one additional treatment response on pain or function as compared with control, and numbers needed to harm (NNH) to cause one additional adverse outcome. We defined treatment response as a 50% improvement in scores (Clegg 2006), which corresponds to an average decrease of 1.2 standard deviation units. Based on the median standardised pain intensity at baseline of 2.4 standard deviation units and the median standardised decrease in pain scores of 0.72 standard deviation units observed in large osteoarthritis trials (Nuesch 2009), we calculated that a median of 31% of patients in the control group would achieve an improvement of pain scores of 50% or more. This percentage was used as the control group response rate to calculate NNTs for treatment response on pain. Based on the median standardised WOMAC function score at baseline of 2.7 standard deviation units and the median standardised decrease in function scores of 0.58 standard deviation units (Nuesch 2009), 26% of patients in the control group would achieve a reduction in function of 50% or more. Again, this percentage was used as the control group response rate to calculate NNTs for treatment response on function. We used median risks of 150 patients with adverse events per 1000 patient‐years, four patients with serious adverse events per 1000 patient‐years and 17 drop‐outs due to adverse events per 1000 patient‐years observed in placebo groups in large osteoarthritis trials (Nuesch 2009) to calculate NNHs for safety outcomes. We performed analyses in RevMan version 5 (RevMan 2008) and STATA version 10.1 (StataCorp, College Station, Texas). All P values are two‐sided.
Results
Description of studies
We identified 1697 references to articles and considered 85 to be potentially eligible (Figure 1). Twenty‐two reports describing 18 completed trials in 813 patients and two protocols describing uncompleted trials (Fary 2008; Palmer 2007) met our inclusion criteria. Six trials evaluated high frequency TENS (Bal 2007; Cetin 2008; Cheing 2002;Cheing 2003;Law 2004a;Smith 1983), one high frequency and burst TENS (Grimmer 1992), one high frequency TENS and interferential current stimulation (Adedoyin 2005), one low frequency, high frequency and modulation TENS with alternating low and high frequency current (Law 2004), one burst TENS (Fargas‐Babjak 1989), two low frequency TENS (Ng 2003;Yurtkuran 1999), four interferential current stimulation (Adedoyin 2002; Defrin 2005; Itoh 2008; Quirk 1985), and three evaluated pulsed electrostimulation (Fary 2008; Garland 2007; Zizic 1995). The protocol of Palmer 2007 did not specify which type of TENS would be used.
The description of the uncompleted trials can be found in the 'Characteristics of ongoing studies' table. Of the completed trials, 17 trials used a parallel group and one a 2 x 2 factorial design (Itoh 2008). Twelve trials used a sham intervention in the control group, five used no intervention (Adedoyin 2005; Cetin 2008; Itoh 2008; Quirk 1985; Ng 2003) and one trial had both a sham and a no intervention control (Cheing 2002). Standardised co‐interventions, provided in both experimental and control groups, were used in five trials with no intervention controls (Adedoyin 2005; Cetin 2008; Cheing 2002; Ng 2003; Quirk 1985) and in two trials with a sham intervention (Adedoyin 2002; Bal 2007). Cetin 2008 used hot packs and exercise, Adedoyin 2002 dietary advice and exercise, Quirk 1985, Cheing 2002 and Adedoyin 2005 exercise, Bal 2007 used infra‐red therapy and Ng 2003 an educational pamphlet. In addition, Itoh 2008 assigned 50% of patients to acupuncture using a factorial design.
Characteristics of the currents varied considerably, even within a specific type of electrostimulation. In the three trials evaluating low frequency TENS, pulse width and pulse frequency ranged from 200 μsec and 2 Hz to 1000 μsec and 4 Hz, with intensities set to reach a comfortable level in one (Law 2004), and resulting in muscle contraction in two trials (Ng 2003; Yurtkuran 1999). In trials of high frequency TENS, pulse width and pulse frequency ranged from 80 μsec and 32 Hz (Smith 1983) to 200 μsec and 100 Hz (Cheing 2003), with the majority of intensities described as strong but comfortable. In trials of burst TENS, Fargas‐Babjak 1989 used a pulse frequency of 200 Hz, a train length of 125 μsec and a repetition frequency of 4 Hz with intensity increased up to the patients' limits of tolerability, while Grimmer 1992 used a pulse frequency of 80 Hz, an unclear train length and pulse width and a repetition frequency of 3 Hz, with the intensity resulting in a strong, tolerable tingling sensation and visible, but comfortable muscle contraction. In the five trials of interferential current stimulation, the beat frequency ranged from 30 to 130 Hz and intensities resulted typically in tingling sensations in four trials (Adedoyin 2002; Adedoyin 2005; Itoh 2008; Quirk 1985), and pain in one (Defrin 2005). The two trials of pulsed electrostimulation were the only ones to use intensities below the sensory threshold (Garland 2007; Zizic 1995). The trials used the same device, which produces monophasic, spike‐shaped pulses in a frequency of 100 Hz. The intensity of the current was initially increased until a tingling sensation was felt and subsequently reduced until this sensation disappeared.
The trials differed in type, number and localisation of electrodes used (see 'Characteristics of included studies'). The median duration of electrostimulation per session was 25 minutes (range 15 minutes to 8.2 hours), with a duration of 15 to 20 minutes in 10 trials (Adedoyin 2005; Adedoyin 2002; Cetin 2008; Cheing 2003; Defrin 2005; Itoh 2008; Quirk 1985; Ng 2003; Smith 1983; Yurtkuran 1999), 30 to 40 minutes in six (Bal 2007; Cheing 2003; Fargas‐Babjak 1989;Grimmer 1992;Law 2004a;Law 2004) and 60 minutes or more in 4 trials (Cheing 2002;Cheing 2003;Garland 2007;Zizic 1995). The median number of treatment sessions per week was 3.5 (range 1 to 14), with up to three sessions per week in eight trials (Adedoyin 2002; Adedoyin 2005; Cetin 2008; Defrin 2005; Grimmer 1992; Itoh 2008; Quirk 1985; Smith 1983), four to six in seven (Bal 2007; Cheing 2002; Cheing 2003; Law 2004; Law 2004a; Ng 2003; Yurtkuran 1999) and seven or more in three trials (Fargas‐Babjak 1989;Garland 2007;Zizic 1995). This resulted in a median duration of electrostimulation of 1.5 hours per week (range 15 minutes to 57.4 hours). The median length of the treatment period was four weeks (range one day to 12 weeks).
All but one trial explicitly included patients with knee osteoarthritis only, with the diagnosis based on clinical and/or radiographic evidence. Fargas‐Babjak 1989 included patients with either knee or hip osteoarthritis, and failed to report the percentage of patients with knee osteoarthritis, but it was considered likely that this percentage was above 75%. The majority of patients had a clinical severity requiring simple non‐surgical treatments (Jüni 2006). In one trial of pulsed electrostimulation, the majority of patients (41 out of 58) were candidates for total knee arthroplasty, however (Garland 2007). The description of patient characteristics was generally poor. Only four trials (Bal 2007;Garland 2007; Law 2004a; Yurtkuran 1999) reported the average disease duration, which ranged from two to 8.4 years.
Four cross‐over trials could not be included because of incomplete reporting, which did not allow the distinction between treatment phases (Lewis 1984;Lewis 1985;Lewis 1994; Taylor 1981). All but Lewis 1985 were included in the previous version of this review (Osiri 2000). Three other trials were excluded because of an active control intervention using another type of electrostimulation (Burch 2008; Jensen 1991;Volklein 1990). Detailed reasons for exclusion are displayed in 'Characteristics of excluded studies'.
Risk of bias in included studies
Figure 2 summarises the methodological characteristics and source of funding of included trials. One trial reported both adequate sequence generation and adequate concealment of allocation (Garland 2007), five trials reported only adequate sequence generation (Itoh 2008; Law 2004; Law 2004a; Ng 2003; Smith 1983) and one trial reported adequate concealment, but provided insufficient detail on the generation of allocation sequence (Grimmer 1992). Two trials were quasi‐randomised, one used alternation to allocate patients to experimental and control intervention (Adedoyin 2002), the other allocated patients according to hospital registration number (Bal 2007). In the remaining nine trials, low quality of reporting hampered any judgement regarding sequence generation and concealment of allocation.
2.
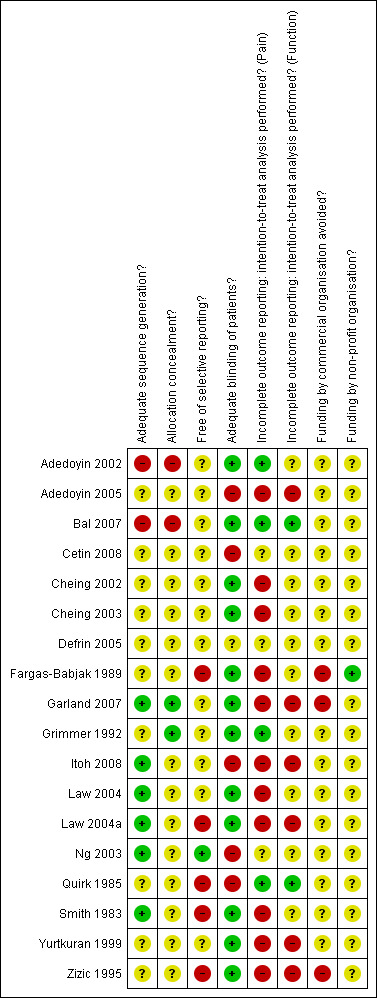
Methodological characteristics and source of funding of included trials. (+) indicates low risk of bias, (?) unclear and (‐) a high risk of bias on a specific item.
Six trials (Fargas‐Babjak 1989; Garland 2007; Grimmer 1992; Law 2004; Law 2004a; Zizic 1995) were described as double‐blind. Thirteen trials used sham interventions, all using identical devices in experimental and control groups (Adedoyin 2002; Bal 2007; Cheing 2002; Cheing 2003; Defrin 2005; Fargas‐Babjak 1989; Garland 2007; Grimmer 1992; Law 2004a; Law 2004; Smith 1983; Yurtkuran 1999; Zizic 1995). In 10 out of 13 trials, sham devices had broken leads so that no current could pass, whereas the indicator light or digital display of intensity control functioned normally. In the two pulsed electrostimulation trials, all patients were instructed to increase the intensity until a tingling sensation was felt, after which they were asked to reduce intensity just below the perception (sensory) level. Pulsed electrostimulation sham devices were adapted with an automatic shut‐off as soon as the amplitude was reduced (Garland 2007;Zizic 1995). Only the sham device used in Defrin 2005 was not considered to lead to adequate patient blinding, as the sham device was described as shut off. Only the two trials of pulsed electrostimulation, however, which used currents below the sensory threshold, were deemed to have fully credible blinding of patients (Garland 2007; Zizic 1995).
Sixteen out of 18 completed trials contributed to the analysis of pain outcomes. Of these, only three trials (Adedoyin 2002; Bal 2007;Grimmer 1992), which had analysed all randomly assigned patients, were considered to have an intention‐to‐treat analysis of pain outcomes at end of treatment. In three trials (Cetin 2008;Defrin 2005;Ng 2003) it was unclear whether exclusions of randomised patients from the analysis had occurred, in five trials (Fargas‐Babjak 1989;Garland 2007;Law 2004;Law 2004a;Yurtkuran 1999) exclusions were reported, but their percentage remained unclear and in the remaining six trials the median reported exclusion rate was 7% in the experimental and 11.5% in the control groups (range 0% to 25% in both experimental and control groups). Two out of nine trials contributing to the analysis of function outcomes were considered to have an intention‐to‐treat analysis (Bal 2007;Quirk 1985). In one trial (Cetin 2008) it was unclear whether exclusions of randomised patients from the analysis had occurred, in three trials (Garland 2007;Law 2004a;Yurtkuran 1999) exclusions were reported, but their percentage remained unclear and in the remaining three trials the median reported exclusion rate was 11.5% in experimental and 12% in control groups (range 0% to 25% in experimental, and 11% to 25% in control groups, respectively).
Only three trials explicitly specified primary outcomes (Adedoyin 2002;Itoh 2008;Zizic 1995), although one of these specified more than two (Zizic 1995). Only one trial reported a sample size calculation (Adedoyin 2005). None of the trials had a sufficient sample size of at least 200 patients overall to achieve sufficient power for detecting a small to moderate SMD. Only three trials reported their source of funding: one was supported by a non‐profit organisation and a commercial body (Fargas‐Babjak 1989), the other two by a commercial body only (Garland 2007;Zizic 1995).
For the effectiveness outcomes pain and function, the quality of the evidence (Guyatt 2008) was classified as very low in view of the risk of bias in the included, predominantly small trials of questionable quality, the large heterogeneity between trials, the potential for selective reporting of function outcomes and the exploratory nature of the model used to predict SMDs of pain in trials as large as the largest trials ('Table 1'). For the safety outcomes, the quality of the evidence (Guyatt 2008) was classified as moderate to low, again because of the predominantly small trials of questionable quality, the small number of trials reporting the outcomes and the small number of events resulting in imprecise estimates.
Effects of interventions
See: Table 1
Knee pain
Sixteen trials with 18 comparisons (726 patients) contributed to the meta‐analysis of pain outcomes (Figure 3). The analysis suggested an overall large SMD of ‐0.86 (95% CI ‐1.23 to ‐0.49), which corresponds to a difference in pain scores of 2.1 cm on a 10 cm VAS between electrostimulation and control, favouring electrostimulation. Within the types of electrostimulation, a very large effect was found for interferential current stimulation (SMD ‐1.20, 95% CI ‐1.99 to ‐0.42), a large effect in TENS (SMD ‐0.85, 95% CI ‐1.36 to ‐0.34) and a moderate effect in pulsed electrostimulation (SMD ‐0.41, 95% CI ‐0.77 to ‐0.05). However, interaction tests provided little evidence for differences between different types. Pooling all types of electrostimulation, an I2 of 80% indicated a high degree of between‐trial heterogeneity (P for heterogeneity < 0.001), which was not substantially reduced when pooling types of electrostimulation separately. Four trials (Cheing 2003;Defrin 2005; Law 2004;Law 2004a) showed unrealistically large SMDs of twice to three times the magnitude of what would be expected for total joint replacement (Jüni 2006). The funnel plot appeared asymmetrical (Figure 4, P for asymmetry < 0.001) and the corresponding asymmetry coefficient was ‐7.6 (95% CI ‐10.6 to ‐4.5). This coefficient indicates that the benefit of electrostimulation increases by 7.6 standard deviation units for each unit increase in the standard error of the SMD, which is mainly a surrogate for sample size. The predicted SMD in trials as large as the largest trial (Zizic 1995, n = 71, standard error = 0.24) was ‐0.07 (95% CI ‐0.46 to 0.32), which corresponds to a difference in pain scores of 0.2 cm on a 10 cm VAS between electrostimulation and control. Referring to a median pain intensity of 6.1 cm in placebo groups at baseline, this corresponds to a difference of 4% improvement (95% CI ‐13% to +20%) between electrostimulation and control ('Table 1').
3.
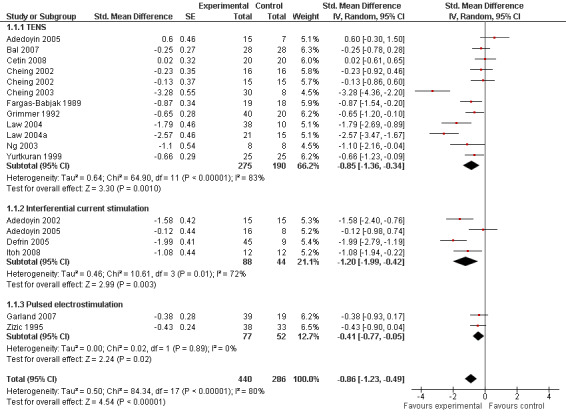
Forest plot of 16 trials comparing the effects of any type of transcutaneous electrostimulation and control (sham or no intervention) on knee pain. Values on x‐axis denote standardised mean differences. The plot is stratified according to type of electrostimulation. Law 2004 reported on knee level, we inflated the standard error with sqrt(number knees)/sqrt(number patients) to correct for clustering of knees within patients. Adedoyin 2005 and Cheing 2002 contributed with two comparisons each. In Adedoyin 2005, the standard error was inflated and the number of patients in the control group was halved to avoid duplicate counting of patients when including 2 both comparisons in the overall meta‐analysis. Data relating to the 3, 2, 3 and 4 active intervention arms in Cheing 2003, Grimmer 1992, Law 2004 and Defrin 2005, respectively, were pooled.
4.
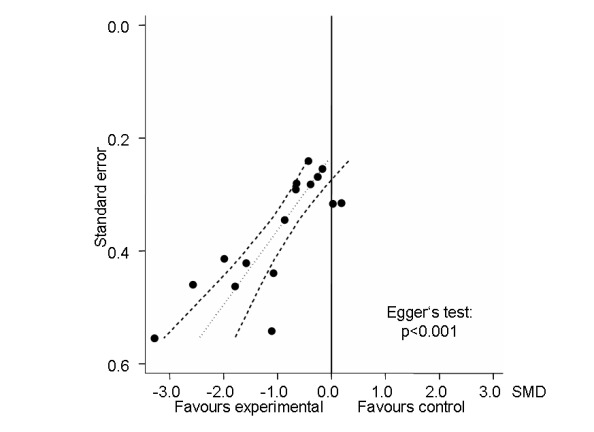
Funnel plot for effects on knee pain. Numbers on x‐axis refer to standardised mean differences (SMDs), on y‐axis to standard errors of SMDs.
Table 3 presents results from stratified analyses. Estimates of SMD varied to some degree depending on concealment of allocation, adequacy of patient blinding, use of analgesic cointerventions and characteristics of electrostimulation, but 95% CIs of SMDs were wide and tests of interaction and tests for trend not statistically significant. There was little evidence to suggest that SMDs depended on the type of electrostimulation used (P for interaction = 0.94). Contrary to what would be expected in the presence of relevant placebo effects, we found some evidence towards larger benefits of electrostimulation in trials with a sham intervention as compared with trials without (P for interaction = 0.12). In addition, there was some evidence for larger benefits of electrostimulation associated with short durations of the overall treatment period of less than four weeks as compared with four weeks or more (P for interaction = 0.14). The analysis could not be stratified according to sample size, because none of included trials reached the pre‐specified sample size of 200 patients to be considered as adequately sized.
1. Results of stratified analyses of pain outcomes.
| Variable | N of trials | N of patients (experimental) | N of patients (control) | Pain intensity | Heterogeneity | P for interaction |
| n | n | n | SMD (95% CI) | I2 (%) | ||
| All trials | 16 | 440 | 286 | ‐0.86 (‐1.23 to ‐0.49) | 80% | |
| Allocation concealment | 0.47 | |||||
| Adequate | 2 | 79 | 39 | ‐0.52 (‐0.91 to ‐0.13) | 0% | |
| Inadequate or unclear | 14 | 361 | 247 | ‐1.03 (‐1.49 to ‐0.57) | 84% | |
| Type of control intervention* | 0.12 | |||||
| Sham intervention | 12 | 354 | 216 | ‐1.13 (‐1.59 to ‐0.67) | 82% | |
| No control intervention | 5 | 86 | 70 | ‐0.31 (‐0.80 to 0.19) | 58% | |
| Blinding of patients | 0.37 | |||||
| Adequate | 11 | 309 | 205 | ‐1.05 (‐1.52 to ‐0.59) | 82% | |
| Inadequate or unclear | 6 | 131 | 79 | ‐0.63 (‐1.31 to 0.05) | 81% | |
| Use of analgesic cointerventions | 0.36 | |||||
| Similar between groups | 4 | 124 | 83 | ‐0.57 (‐1.16 to 0.02) | 74% | |
| Not similar or unclear | 12 | 316 | 23 | ‐1.10 (‐1.60 to ‐0.59) | 84% | |
| Intention‐to‐treat analysis | 0.73 | |||||
| Yes | 3 | 83 | 63 | ‐0.76 (‐1.43 to ‐0.09) | 72% | |
| No or unclear | 13 | 357 | 223 | ‐1.00 (‐1.48 to ‐0.53) | 84% | |
| Type of ES** | 0.94 | |||||
| High frequency TENS | 8 | 177 | 139 | ‐0.82 (‐1.51 to ‐0.12) | 86% | |
| Burst TENS | 2 | 39 | 38 | ‐0.85 (‐1.32 to ‐0.38) | 0% | |
| Modulation TENS | 1 | 13 | 3 | ‐1.41 (‐2.92 to 0.10) | N/A | |
| Low frequency TENS | 3 | 46 | 40 | ‐0.82 (‐1.29 to ‐0.34) | 0% | |
| Interferential current stimulation | 4 | 88 | 44 | ‐1.20 (‐1.99 to ‐0.42) | 71% | |
| Pulsed ES | 2 | 77 | 52 | ‐0.41 (‐0.77 to ‐0.05) | 0% | |
| Duration of ES per session† | 0.69‡ | |||||
| ≤ 20 minutes | 8 | 166 | 112 | ‐0.95 (‐1.55 to ‐0.35) | 78% | |
| 30 to 40 minutes | 6 | 156 | 99 | ‐1.45 (‐2.28 to ‐0.62) | 85% | |
| ≥ 60 minutes | 4 | 118 | 91 | ‐0.47 (‐0.96 to 0.02) | 58% | |
| Number of sessions per week | 0.90‡ | |||||
| ≤ 3 | 6 | 163 | 91 | ‐0.81 (‐1.48 to ‐0.14) | 82% | |
| 4 to 6 | 7 | 182 | 125 | ‐1.33 (‐2.11 to ‐0.54) | 88% | |
| ≥ 7 | 3 | 96 | 70 | ‐0.51 (‐0.83 to ‐0.19) | 0% | |
| Duration of ES per week*** | 0.74‡ | |||||
| ≤1 hour | 5 | 123 | 71 | ‐0.85 (‐1.72 to 0.01) | 86% | |
| > 1 to 5 hours | 8 | 180 | 122 | ‐1.42 (‐2.11 to ‐0.74) | 81% | |
| > 5 hours | 5 | 137 | 109 | ‐0.53 (‐0.96 to ‐0.11) | 55% | |
| Duration of treatment period | 0.14 | |||||
| < 4 weeks | 7 | 190 | 114 | ‐1.39 (‐2.13 to ‐0.66) | 86% | |
| ≥ 4 weeks | 9 | 250 | 172 | ‐0.64 (‐1.06 to ‐0.22) | 75% |
ES: electrostimulation; *In Cheing 2002, two independent comparisons contributed in the two different strata. **Adedoyin 2005, Grimmer 1992 and Law 2004 contributed to two, two and three different strata: high‐frequency TENS and interferential current stimulation, high‐frequency TENS and burst, and high‐, low‐frequency and modulation TENS, respectively. † = Cheing 2003 contributed to all three different strata, with the same 8 control patients displayed in each stratum. ‡ = P values from test for trend.
Withdrawals or drop‐outs because of adverse events
Eight trials (348 patients) contributed to the meta‐analysis of patients withdrawn or dropped out because of adverse events (Figure 5). Of these, four TENS trials and one interferential current stimulation trial reported that no withdrawals or drop‐outs due to adverse events had occurred, neither in experimental nor in control groups, therefore relative risks could not be estimated. In the remaining three trials, there was no evidence that transcutaneous electrostimulation is unsafe (relative risk 0.97), but 95% confidence intervals were wide and ranged from 0.16 to 6.00. Pooling all types of electrostimulation, an I2 of 20% indicated a low degree of between‐trial heterogeneity (P for heterogeneity = 0.29).
5.
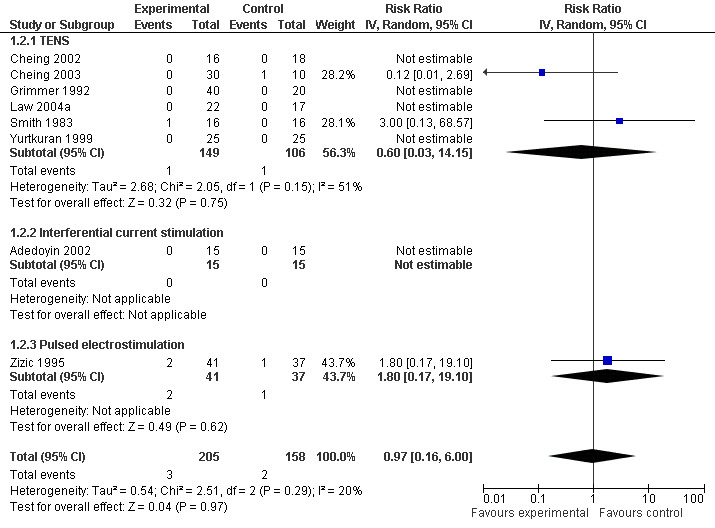
Forest plot of 8 trials comparing patients withdrawn or dropped out because of adverse events between any transcutaneous electrostimulation and control (sham or no intervention). Values on x‐axis denote risk ratios. Risk ratios could not be estimated in 5 trials, because no drop‐out occurred in either group. The plot is stratified according to type of electrostimulation. Data relating to the 3 and 2 active intervention arms in Cheing 2003 and Grimmer 1992, respectively, were pooled.
Function
Nine trials (407 patients) contributed to the meta‐analysis of function. The analysis suggested a small SMD of ‐0.34 (95% CI ‐0.54 to ‐0.14, Figure 6), which corresponds to a difference in function scores of 0.7 units on a standardised WOMAC disability scale ranging from 0 to 10, favouring electrostimulation. Referring to a median function score of 5.6 units in placebo groups at baseline, this corresponds to a difference of 20% improvement (95% CI +11% to +29%) between electrostimulation and control ('Table 1'). The estimated difference in the percentage of treatment responders between patients allocated to electrostimulation and patients allocated to placebo of 3% translated into an NNT to cause one additional treatment response on function of 10 (95% CI 7 to 22) ('Table 1'). Differences between types of electrostimulation were not statistically significant. An I2 of 0% suggested no between‐trial heterogeneity (P for heterogeneity = 0.57). The funnel plot did not appear asymmetrical (Figure 7, P for asymmetry = 0.52). The corresponding asymmetry coefficient was 1.4 (95% CI, ‐3.5 to 6.3).
6.
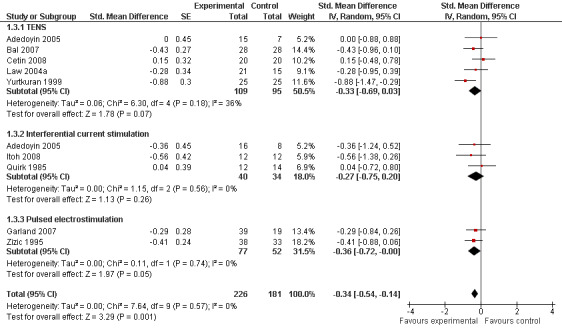
Forest plot of 9 trials comparing the effects of any type of transcutaneous electrostimulation and control (sham or no intervention) on function. Values on x‐axis denote standardised mean differences. The plot is stratified according to type of electrostimulation. In Adedoyin 2005, the standard error was inflated and the number of patients in the control group was halved to avoid duplicate counting of patients when including both comparisons in the overall meta‐analysis.
7.
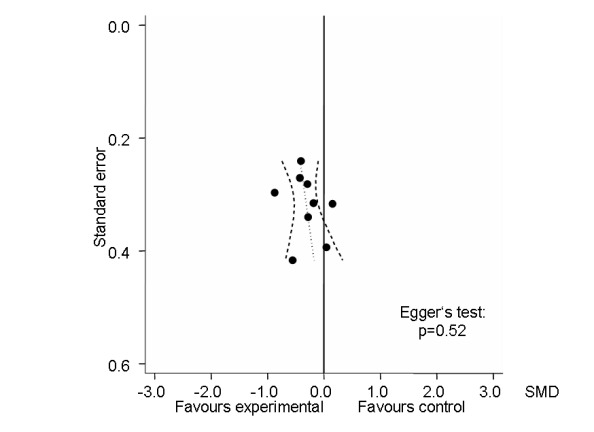
Funnel plot for effects on functioning of the knee. Numbers on x‐axis refer to standardised mean differences (SMDs), on y‐axis to standard errors of SMDs.
Table 4 presents results from stratified analyses. Estimates of SMD varied to some degree depending on type of control intervention, adequacy of patient blinding, characteristics of electrostimulation and overall treatment period, but 95% CIs of SMDs were wide and tests for interaction and tests for trend not statistically significant. There was little evidence to suggest that SMDs depended on the type of electrostimulation used (P for interaction = 0.32). Again, the analysis could not be stratified according to sample size, because none of included trials reached the pre‐specified sample size of 200 patients to be considered as adequately sized.
2. Results of stratified analyses of function.
| Variable | N of trials | N of patients (experimental) | N of patients (control) | Function | Heterogeneity | P for interaction |
| SMD (95% CI) | I2 (%) | |||||
| All trials | 9 | 226 | 181 | ‐0.34 (‐0.54 to ‐0.14) | 0% | |
| Allocation concealment | 0.88 | |||||
| Adequate | 1 | 39 | 19 | ‐0.29 (‐0.85 to 0.26) | N/A | |
| Inadequate or unclear | 8 | 187 | 162 | ‐0.34 (‐0.56 to ‐0.12) | 5% | |
| Type of control intervention | 0.14 | |||||
| Sham intervention | 5 | 151 | 120 | ‐0.46 (‐0.70 to ‐0.21) | 0% | |
| No control intervention | 4 | 75 | 61 | ‐0.10 (‐0.45 to 0.24) | 0% | |
| Blinding of patients | 0.14 | |||||
| Adequate | 5 | 151 | 120 | ‐0.46 (‐0.70 to ‐0.21) | 0% | |
| Inadequate or unclear | 4 | 75 | 61 | ‐0.10 (‐0.45 to 0.24) | 0% | |
| Use of analgesic cointerventions | 0.95 | |||||
| Similar between groups | 2 | 69 | 48 | ‐0.33 (‐0.70 to 0.05) | 0% | |
| Not similar or unclear | 7 | 157 | 133 | ‐0.34 (‐0.60 to ‐0.08) | 15% | |
| Intention‐to‐treat analysis | 0.76 | |||||
| Yes | 2 | 40 | 42 | ‐0.28 (‐0.71 to 0.16) | 0% | |
| No or unclear | 7 | 186 | 139 | ‐0.35 (‐0.58 to ‐0.12) | 5% | |
| Type of ES** | 0.32 | |||||
| High frequency TENS | 4 | 84 | 70 | ‐0.18 (‐0.50 to 0.14) | 0% | |
| Burst TENS | 0 | |||||
| Modulation TENS | 0 | |||||
| Low frequency TENS | 1 | 25 | 25 | ‐0.88 (‐1.46 to ‐0.30) | N/A | |
| Interferential current stimulation | 3 | 40 | 34 | ‐0.27 (‐0.75 to 0.20) | 0% | |
| Pulsed ES | 2 | 77 | 52 | ‐0.36 (‐0.72 to ‐0.00) | 0% | |
| Duration of ES per session | 0.80‡ | |||||
| ≤ 20 minutes | 5 | 100 | 86 | ‐0.29 (‐0.69 to 0.11) | 44% | |
| 30 to 40 minutes | 2 | 49 | 43 | ‐0.37 (‐0.79 to 0.04) | 0% | |
| ≥ 60 minutes | 2 | 77 | 52 | ‐0.36 (‐0.72 to ‐0.00) | 0% | |
| Number of sessions per week | 0.32‡ | |||||
| ≤ 3 | 4 | 75 | 61 | ‐0.10 (‐0.45 to 0.24) | 0% | |
| 4 to 6 | 3 | 74 | 68 | ‐0.54 (‐0.88 to ‐0.20) | 2% | |
| ≥ 7 | 2 | 77 | 52 | ‐0.36 (‐0.72 to ‐0.00) | 0% | |
| Duration of ES per week | 0.32‡ | |||||
| ≤ 1 hour | 4 | 75 | 61 | ‐0.10 (‐0.45 to 0.24) | 0% | |
| > 1 to 5 hours | 3 | 74 | 68 | ‐0.54 (‐0.88 to ‐0.20) | 2% | |
| > 5 hours | 2 | 77 | 52 | ‐0.36 (‐0.72 to ‐0.00) | 0% | |
| Duration of treatment period | 0.18 | |||||
| < 4 weeks | 3 | 74 | 68 | ‐0.54 (‐0.88 to ‐0.20) | 2% | |
| ≥ 4 weeks | 6 | 152 | 113 | ‐0.23 (‐0.47 to 0.02) | 0% |
ES: electrostimulation; **Adedoyin 2005 contributed to two different strata: high‐frequency TENS and interferential current stimulation; ‡ = P values from test for trend.
Other safety outcomes
Three trials (175 patients) contributed to the meta‐analysis of patients experiencing any adverse event (Figure 8) and four trials (195 patients) to the meta‐analysis of patients experiencing any serious adverse event (Figure 9). In general, there was no evidence to suggest that electrostimulation is unsafe, but 95% CIs were wide and results inconclusive.
8.
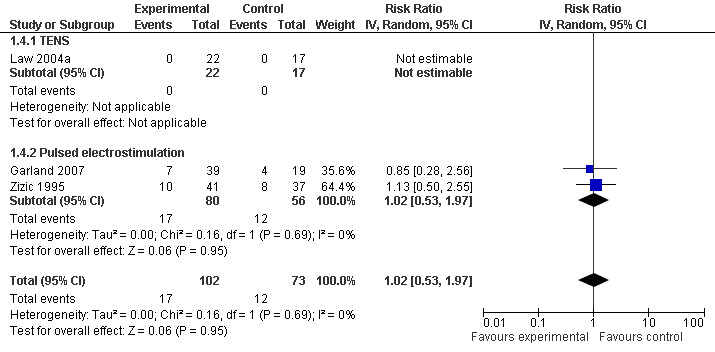
Forest plot of 3 trials comparing patients experiencing any adverse event between any transcutaneous electrostimulation and control (sham or no intervention). Values on x‐axis denote risks ratios. The risk ratio in one TENS trial could not be estimated because no adverse event occurred in either group. The plot is stratified according to type of electrostimulation.
9.
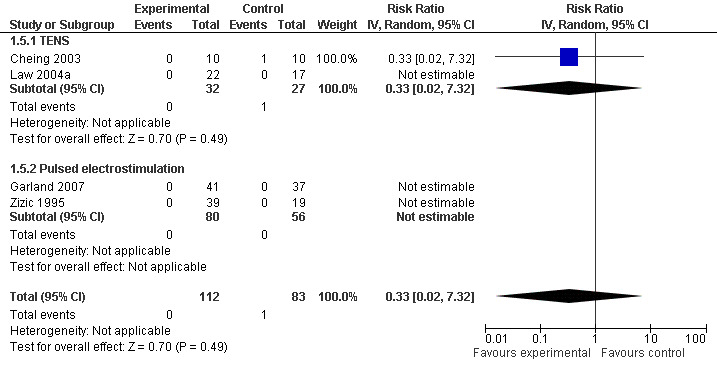
Forest plot of 4 trials comparing patients experiencing any serious adverse event between any transcutaneous electrostimulation and control (sham or no intervention). Values on x‐axis denote risk ratios. Risk ratios could not be estimated in 3 trials, because no serious adverse event occurred in either group. The plot is stratified according to type of electrostimulation. Data relating to the 3 active intervention arms in Cheing 2003 were pooled.
Discussion
Summary of main results
Our systematic review of trials comparing any type of transcutaneous electrostimulation with a sham or non‐intervention control revealed a lack of adequately sized, methodologically sound and appropriately reported trials and a moderate to high degree of heterogeneity between trials, which made the interpretation of results difficult, particularly for joint pain as the primary therapeutic target of transcutaneous electrostimulation. In an attempt to minimise biases associated with small trials of questionable quality, we used meta‐regression to predict effects of transcutaneous electrostimulation on pain and found the predicted effect sizes for pain negligibly small. The rates of withdrawals or drop‐outs due to adverse events were comparable in experimental and control groups, but 95% CIs were wide and therefore inconclusive.
Quality of the evidence
An inspection of funnel plots and a formal analysis of asymmetry indicated asymmetry for knee pain, but not for function, which suggested the presence of biases associated with small sample size particularly when estimating the effects of electrostimulation on knee pain. Asymmetrical funnel plots should be seen not only as an indication of publication bias, but as a generic tool for examination of small study effects: the tendency for the smaller studies to show larger treatment effects, possibly due to a combination of publication bias, selective reporting of outcomes and methodological problems particularly in small trials (Nuesch 2009a; Sterne 2000). If reporting is inadequate, as was the case in our systematic review, then the standard error as a proxy for study size may be a more precise measure of trial quality than formal assessments of methodological quality. When modelling effects expected in trials as large as the largest trial included in our systematic review, we found effects on pain near null ‐0.07 (95% CI ‐0.46 to 0.32), which were clearly smaller than the pooled SMD actually found for pain in the meta‐analysis ‐0.86 (95% CI ‐1.23 to ‐0.49). The effect of electrostimulation on function was small, but potentially clinically relevant, and the accumulated evidence appeared less affected by biases associated with small sample size.
The methodological quality and the quality of reporting was poor. Insufficient information was noted in several randomised controlled trials about the treatment assignment procedure and concealment of allocation. Primary outcomes were specified in only three trials. Although several studies reported blinding of patients, complete blinding is difficult to achieve due to the sensory differences between treatment and placebo, as well as unintended communication between patient and evaluator (Deyo 1990). Only Grimmer 1992 and Bal 2007 mentioned the inclusion of patients to be restricted to those without prior TENS experience; another two trials were likely to have achieved adequate blinding of patients with currents below the sensory threshold used in the experimental group, which were likely to be indistinguishable from the sham intervention also for patients with treatment experience (Garland 2007, Zizic 1995). The majority of papers did not provide adequate information regarding withdrawals, drop‐outs and losses to follow up, nor indicated whether patients with incomplete clinical data were included in the data analysis. Several trials omitted to describe adverse events, which is of concern.
Potential biases in the review process
Our review is based on a broad literature search, and it seems unlikely that we missed relevant trials. Trial selection and data extraction, including quality assessment, were done independently by two authors to minimise bias and transcription errors. Components used for quality assessment are validated and reported to be associated with bias (Jüni 2001; Wood 2008).
As with any systematic review, our study is limited by the quality of included trials. As indicated above, trials generally suffered from poor methodological quality, inadequate reporting and small sample size. Some trials (Cheing 2003; Defrin 2005; Law 2004a) showed unrealistically large SMDs of twice to three times the magnitude of what would be expected for total joint replacement (Jüni 2006). Including these trials in the meta‐analysis is likely to result in an overestimation of the benefits of transcutaneous electrostimulation.
Agreements and disagreements with other studies or reviews
Interestingly, there are nearly as many systematic reviews and meta‐analyses on transcutaneous electrostimulation in osteoarthritis as randomised trials. Here, we will focus mainly on the similarities and differences between ours and the previous version of this review (Osiri 2000), which included seven transcutaneous electrical nerve stimulation (TENS) trials. We updated the search and used broader selection criteria, which resulted in 14 additional trials; 11 trials used TENS as the experimental treatment, four interferential current stimulation, one both TENS and interferential current stimulation, and two pulsed electrostimulation. As in the review of Osiri 2000, both parallel group and cross‐over RCTs were included. For the cross‐over studies, we only collected data from the first intervention phase in order to eliminate carry‐over effects, whereas Osiri and colleagues included pooled data over all phases. We excluded three previously included cross‐over trials, because the investigators were unable to provide data from the first phase only. In this update, we performed a more detailed quality assessment of component trials, followed by a detailed exploration of sources of variation between trials, including concealment of allocation, blinding, intention‐to‐treat analysis, characteristics of electrostimulation, and the investigation of funnel plots. To analyse continuous data, Osiri and colleagues used weighted mean differences or SMDs of the change from baseline scores, whereas we used SMDs of end of treatment scores and based our conclusions on treatment effects on pain predicted in uni‐variable meta‐regression models by using the standard error as the explanatory variable. In addition, fixed‐effect models were used in the previous version unless there was statistically significant heterogeneity between trials based on χ2 testing. Model selection based on the mechanistic application of heterogeneity tests should be avoided, however. Here, we used random‐effects models, which will generally be more conservative in terms of the estimated precision, but will be more affected by small study effects than a fixed‐effect model, which makes an exploration of sources of variation, including different types of bias, mandatory. Results from the previous and current versions are therefore not directly comparable. Nevertheless, pooled SMDs for pain were favourable in our and the previous review (Osiri 2000), with us reporting a pooled SMD of ‐0.86 (95% CI ‐1.23 to ‐0.49), whereas Osiri 2000 reported a SMD of ‐0.45 (95% CI ‐0.70 to ‐0.19), with confidence intervals overlapping widely. Although both Osiri and we acknowledge the risk of bias in summary estimates, Osiri concluded that transcutaneous electrostimulation is "shown to be effective in pain control over placebo". We disagree with these conclusions: when modelling effects expected in trials as large as the largest trial included, we found the SMD of pain near null and clinically irrelevant (‐0.07, 95% CI ‐0.46 to 0.32). Osiri 2000 recorded function separately for the outcomes 'stiffness of the knee', '50‐foot walking time', 'quadriceps muscle strength' and 'knee flection' with only one trial contributing to each of the categories. We choose a different approach, using a hierarchy developed to minimise the impact of selective reporting of outcomes and to allow for a synthesis of evidence across different studies using divergent definitions of function. Our effect sizes and conclusion concerning function are less favourable compared to those made by Osiri 2000. In this version, we also summarised safety data and found no evidence to suggest that electrostimulation is unsafe. Finally, unlike Osiri 2000, we also included trials of interferential current stimulation and pulsed electrostimulation. One of the two trials of pulsed electrostimulation (Zizic 1995) is covered in another Cochrane Review by Hulme 2002 on electromagnetic fields, even though the device used (BioniCare BIO‐1000) does not generate electromagnetic fields, but electric currents (Regence Medical Policy 2009).
Authors' conclusions
Implications for practice.
Despite more than 20 years of clinical research, there is a lack of adequate evidence to support the use of any type of transcutaneous electrostimulation in patients with knee osteoarthritis. The effects on both knee pain and function are potentially clinically relevant and deserve further clinical evaluation.
Implications for research.
The current systematic review is inconclusive, hampered by the inclusion of only small trials of questionable quality (Nuesch 2009a). Adequately sized randomised parallel‐group trials in about 2 x 100 patients with knee osteoarthritis are necessary to determine whether a specific type of transcutaneous electrostimulation is indeed associated with a clinically relevant benefit on pain. A sample size of 2 x 100 patients will yield more than 80% power to detect a small to moderate SMD of ‐0.40 at a two‐sided P of 0.05, which corresponds to a difference of 1 cm on a 10 cm visual analogue scale (VAS) between experimental and control intervention. The trials should enrol patients without prior experience of any type of transcutaneous electrostimulation or evaluate success of blinding at the end of trial, use adequate concealment of allocation, experimental and sham interventions that are close to indistinguishable and an intention‐to‐treat analysis. Transcutaneous electrical nerve stimulation (TENS) devices are marketed as small, inexpensive, easy‐to‐use home units, but in the majority of trials TENS was administered by a therapist in a practice or hospital setting. Future research may focus on the effectiveness of self‐administered TENS, with accurate recording of the duration of electrostimulation per day to assess compliance and enable the exploration of possible dose‐effect relationships.
What's new
| Date | Event | Description |
|---|---|---|
| 8 October 2009 | Amended | Number needed to treat for function were corrected |
| 1 May 2008 | Amended | CMSG ID C094‐R |
History
Review first published: Issue 4, 2000
| Date | Event | Description |
|---|---|---|
| 17 July 2009 | New search has been performed | 14 additional trials included |
| 17 July 2009 | New citation required and conclusions have changed | Change in authors and conclusions. Updated search and wider selection criteria, which resulted in 14 additional trials; more detailed quality assessment of component trials; exclusion of results from cross‐over trials if treatment phases could not be distinguished; use of end of trial estimates to calculate SMDs; detailed exploration of sources of variation between trials, including concealment of allocation, blinding, intention‐to‐treat analysis, characteristics of electrostimulation, and investigations of funnel plots; use of a random‐effects model. |
| 30 April 2008 | Amended | Converted to new review format |
Acknowledgements
We thank the Cochrane Musculoskeletal editorial team and Henk van Zutphen for valuable comments, and Malcolm Sturdy for database support. The authors are grateful to Serpil Bal, Gladys Cheing and Pearl Law for providing additional information concerning design and outcome data. We thank Beverly Lewis, Daniel Lewis and Mark Hallett who replied to our queries and attempted to locate files of trials published approximately 20 years ago, but were unable to provide additional outcome data.
Appendices
Appendix 1. MEDLINE, EMBASE and CINAHL search strategy
| OVID MEDLINE | OVID EMBASE | CINAHL through EBSCOhost |
|
search terms for design 1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized controlled trial.sh. 4. random allocation.sh. 5. double blind method.sh. 6. single blind method.sh. 7. clinical trial.pt. 8. exp clinical trial/ 9. (clin$ adj25 trial$).ti,ab. 10. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab. 11. placebos.sh. 12. placebo$.ti,ab. 13. random$.ti,ab. 14. research design.sh. 15. comparative study.sh. 16. exp evaluation studies/ 17. follow up studies.sh. 18. prospective studies.sh. 19. (control$ or prospectiv$ or volunteer$).ti,ab. |
search terms for design 1. randomized controlled trial.sh. 2. randomization.sh. 3. double blind procedure.sh. 4. single blind procedure.sh. 5. exp clinical trials/ 6. (clin$ adj25 trial$).ti,ab. 7. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab. 8. placebo.sh. 9. placebo$.ti,ab. 10. random$.ti,ab. 11. methodology.sh. 12. comparative study.sh. 13. exp evaluation studies/ 14. follow up.sh. 15. prospective study.sh. 16. (control$ or prospectiv$ or volunteer$).ti,ab. |
Search terms for design 1. (MH "Clinical Trials+") 2. (MH "Random Assignment") 3. (MH "Double‐Blind Studies") or (MH "Single‐Blind Studies") 4. TX (clin$ n25 trial$) 5. TX (sing$ n25 blind$) 6. TX (sing$ n25 mask$) 7. TX (doubl$ n25 blind$) 8. TX (doubl$ n25 mask$) 9. TX (trebl$ n25 blind$) 10. TX (trebl$ n25 mask$) 11. TX (tripl$ n25 blind$) 12. TX (tripl$ n25 mask$) 13. (MH "Placebos") 14. TX placebo$ 15. TX random$ 16. (MH "Study Design+") 17. (MH "Comparative Studies") 18. (MH "Evaluation Research") 19. (MH "Prospective Studies+") 20. TX (control$ or prospectiv$ or volunteer$) 21. S1 or S2 or (…….) or S20 |
|
Search terms for Osteoarthritis 20. osteoarthriti$.ti,ab,sh. 21. osteoarthro$.ti,ab,sh. 22. gonarthriti$.ti,ab,sh. 23. gonarthro$.ti,ab,sh. 24. coxarthriti$.ti,ab,sh. 25. coxarthro$.ti,ab,sh. 26. arthros$.ti,ab. 27. arthrot$.ti,ab. 28. ((knee$ or hip$ or joint$) adj3 (pain$ or ach$ or discomfort$)).ti,ab. 29. ((knee$ or hip$ or joint$) adj3 stiff$).ti,ab. |
Search terms for Osteoarthritis 17. osteoarthriti$.ti,ab,sh. 18. osteoarthro$.ti,ab,sh. 19. gonarthriti$.ti,ab,sh. 20. gonarthro$.ti,ab,sh. 21. coxarthriti$.ti,ab,sh. 22. coxarthro$.ti,ab,sh. 23. arthros$.ti,ab. 24. arthrot$.ti,ab. 25. ((knee$ or hip$ or joint$) adj3 (pain$ or ach$ or discomfort$)).ti,ab. 26. ((knee$ or hip$ or joint$) adj3 stiff$).ti,ab. |
Search terms for Osteoarthritis 22. osteoarthriti$ 23. (MH "Osteoarthritis") 24. TX osteoarthro$ 25. TX gonarthriti$ 26. TX gonarthro$ 27. TX coxarthriti$ 28. TX coxarthro$ 29. TX arthros$ 30. TX arthrot$ 31. TX knee$ n3 pain$ 32. TX hip$ n3 pain$ 33. TX joint$ n3 pain$ 34. TX knee$ n3 ach$ 35. TX hip$ n3 ach$ 36. TX joint$ n3 ach$ 37. TX knee$ n3 discomfort$ 38. TX hip$ n3 discomfort$ 39. TX joint$ n3 discomfort$ 40. TX knee$ n3 stiff$ 41. TX hip$ n3 stiff$ 42. TX joint$ n3 stiff$ 43. S22 or S23 or S24….or S42 |
|
Search terms for TENS 30. exp electric stimulation therapy/ 31. (electric$ adj (nerve or therapy)).tw. 32. (electric$ adj (stimulation or muscle)).tw. 33. electrostimulation.tw. 34. electroanalgesia.tw. 35. (tens or altens).tw. 36. electroacupuncture.tw. 37. neuromusc$ electric$.tw. 38. high volt.tw. 39. pulsed.tw. 40. (electric$ adj25 current).tw. 41. (electromagnetic or electrotherap$).tw. 42. iontophoresis.tw. 43. transcutaneous nerve stimulation.tw. |
Search terms for TENS 27. exp electric stimulation therapy/ 28. (electric$ adj (nerve or therapy).tw. 29. (electric$ adj (stimulation or muscle)).tw. 30. electrostimulation.tw. 31. electroanalgesia.tw. 32. (tens or altens).tw. 33. electroacupuncture.tw. 34. neuromusc$ electric$.tw. 35. high volt.tw. 36. pulsed.tw. 37. electric current.sh. 38. (electric$ adj25 current).tw 39. (electromagnetic or electrotherap$).tw. 40. iontophoresis.tw. 41. transcutaneous nerve stimulation.tw. |
Search terms for TENS 44. (MH "Electric Stimulation+") 45. TX (electric$ n1 nerve) 46. TX (electric$ n1 therapy) 47. TX (electric$ n1 stimulation) 48. TX (electric$ n1 muscle) 49. TX electrostimulation 50. TX electroanalgesia 51. TX tens 52. TX altens 53. TX electroacupuncture 54. TX neuromusc$ electric$ 55. TX high volt 56. TX pulsed 57. TX (electric$ n25 current) 58. TX ( (electromagnetic or electrotherap$) ) 59. TX iontophoresis 60. TX transcutaneous nerve stimulation 61. S44 or S45 or …. S60 |
|
Combining terms 44. or/1‐19 45. or/20‐29 46. or/30‐40 47. and/44‐46 48. animal/ 49. animal/ and human/ 50. 48 not 49 51. 47 not 50 |
Combining terms 42. or/1‐16 43. or/17‐26 44. or/27‐37 45. and/42‐44 46. animal/ 47. animal/ and human/ 48. 46 not 47 49. 45 not 48 |
Combining terms S21 and S43 and S61 |
Appendix 2. CENTRAL and PEDro search strategy
| CENTRAL | PEDro |
|
Search terms for Osteoarthritis #1. (osteoarthritis* OR osteoarthro* OR gonarthriti* OR gonarthro* OR coxarthriti* OR coxarthro* OR arthros* OR arthrot* OR ((knee* OR hip* OR joint*) near/3 (pain* OR ach* OR discomfort*)) OR ((knee* OR hip* OR joint*) near/3 stiff*)) in Clinical Trials #2. MeSH descriptor Osteoarthritis explode all trees Search terms for TENS #3. MeSH descriptor Electric Stimulation Therapy explode all trees #4. electric* near/ (nerve or therapy) in Clinical Trials #5. electric* near/ (stimulation or muscle) in Clinical Trials #6. electrostimulation in Clinical Trials #7. electroanalgesia in Clinical Trials #8. tens or altens in Clinical Trials #9. electroacupuncture in Clinical Trials #10. neuromusc* electric* in Clinical Trials #11. high volt in Clinical Trials #12. pulsed in Clinical Trials #13. (electric* near/25 current) in Clinical Trials #14. (electromagnetic or electrotherap*) in Clinical Trials #15. iontophoresis in Clinical Trials #16. transcutaneous nerve stimulation in Clinical Trials Combining terms #17. (#3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16) #18. (#1 OR #2) #19. (#17 AND #18) in Clinical Trials |
1. Electro in title or abstract 2. Method: clinical trial 3. Body part: thigh or hip 4. Body part lower leg or knee Combination 1. and 2. and 3. Combination 1. and 2. and 4. 1. TENS in title or abstract 2. Method: clinical trial 3. Body part: thigh or hip 4. Body part lower leg or knee Combination 1. and 2. and 3. Combination 1. and 2. and 4. Combine all |
Data and analyses
Comparison 1. Any type of transcutaneous electrostimulation versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 16 | 726 | Std. Mean Difference (Random, 95% CI) | ‐0.86 [‐1.23, ‐0.49] |
| 1.1 TENS | 11 | 465 | Std. Mean Difference (Random, 95% CI) | ‐0.85 [‐1.36, ‐0.34] |
| 1.2 Interferential current stimulation | 4 | 132 | Std. Mean Difference (Random, 95% CI) | ‐1.20 [‐1.99, ‐0.42] |
| 1.3 Pulsed electrostimulation | 2 | 129 | Std. Mean Difference (Random, 95% CI) | ‐0.41 [‐0.77, ‐0.05] |
| 2 Number of patients withdrawn or dropped out because of adverse events | 8 | 363 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.16, 6.00] |
| 2.1 TENS | 6 | 255 | Risk Ratio (IV, Random, 95% CI) | 0.60 [0.03, 14.15] |
| 2.2 Interferential current stimulation | 1 | 30 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Pulsed electrostimulation | 1 | 78 | Risk Ratio (IV, Random, 95% CI) | 1.80 [0.17, 19.10] |
| 3 Function | 9 | 407 | Std. Mean Difference (Random, 95% CI) | ‐0.34 [‐0.54, ‐0.14] |
| 3.1 TENS | 5 | 204 | Std. Mean Difference (Random, 95% CI) | ‐0.33 [‐0.69, 0.03] |
| 3.2 Interferential current stimulation | 3 | 74 | Std. Mean Difference (Random, 95% CI) | ‐0.27 [‐0.75, 0.20] |
| 3.3 Pulsed electrostimulation | 2 | 129 | Std. Mean Difference (Random, 95% CI) | ‐0.36 [‐0.72, ‐0.00] |
| 4 Number of patients experiencing any adverse event | 3 | 175 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.53, 1.97] |
| 4.1 TENS | 1 | 39 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Pulsed electrostimulation | 2 | 136 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.53, 1.97] |
| 5 Number of patients experiencing any serious adverse event | 4 | 195 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.02, 7.32] |
| 5.1 TENS | 2 | 59 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.02, 7.32] |
| 5.2 Pulsed electrostimulation | 2 | 136 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
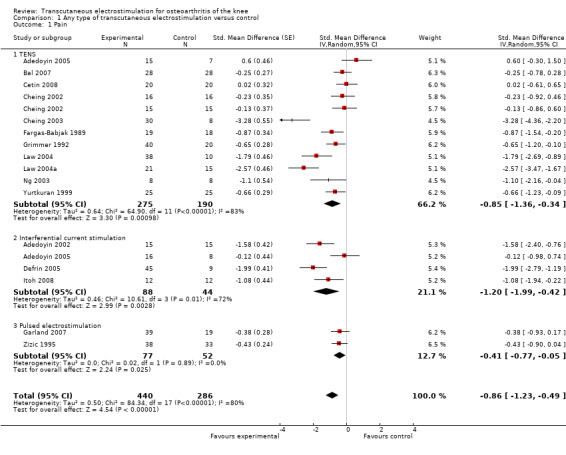
Comparison 1 Any type of transcutaneous electrostimulation versus control, Outcome 1 Pain.
1.2. Analysis.
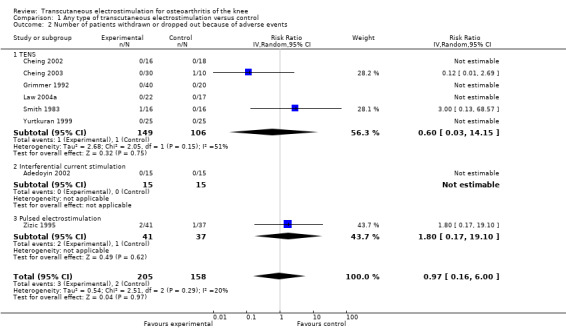
Comparison 1 Any type of transcutaneous electrostimulation versus control, Outcome 2 Number of patients withdrawn or dropped out because of adverse events.
1.3. Analysis.
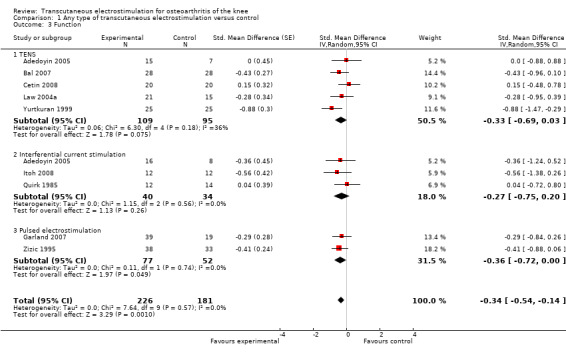
Comparison 1 Any type of transcutaneous electrostimulation versus control, Outcome 3 Function.
1.4. Analysis.
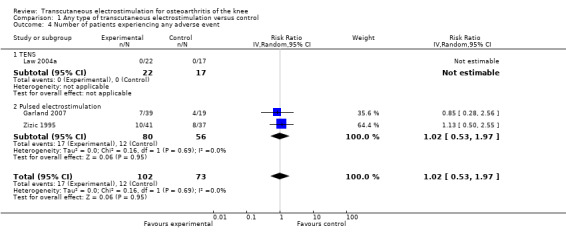
Comparison 1 Any type of transcutaneous electrostimulation versus control, Outcome 4 Number of patients experiencing any adverse event.
1.5. Analysis.
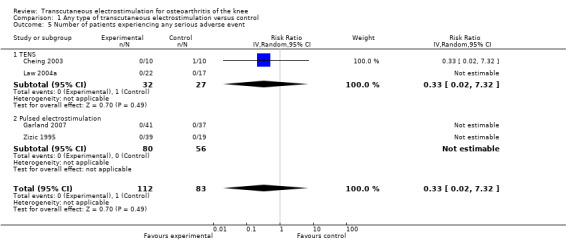
Comparison 1 Any type of transcutaneous electrostimulation versus control, Outcome 5 Number of patients experiencing any serious adverse event.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adedoyin 2002.
| Methods | Quasi‐randomised trial using alternation for the allocation of patients 2‐arm parallel group design Trial duration: 4 weeks No power calculation reported | |
| Participants | 30 patients randomised 30 patients with knee OA reported at baseline Study joints: 30 knees Number of females: 20 of 30 (67%) Average age: 59 years Average BMI: 28 kg/m2 | |
| Interventions | Experimental intervention: interferential current stimulation, dietary advice and exercise, twice per week
Control intervention: Sham interferential current stimulation, dietary advice and exercise, twice per week
Duration of treatment period: 4 weeks
Analgesics not allowed Device: Enraf‐Nonius Endomed 5921 (4 pole) Self‐administered: no Waveform: interferential Pulse width: not applicable Pulse frequency: amplitude‐modulated frequency of 100 Hz for 15 min (beat frequency), 80 Hz for last 5 min (beat frequency) Amplitude: above sensory threshold, up to appreciable sensation Duration of stimulation per session: 20 minutes Electrodes: 4 electrodes covered with padding Placement: 2 latero‐medial, 2 antero‐posterior |
|
| Outcomes | Extracted pain outcome: global pain after 4 weeks, described as "Pain perception (VAS)"
No function outcome reported Primary outcome: global pain (VAS) |
|
| Notes | All subjects from black Nigerian population | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Alternation |
| Allocation concealment? | High risk | Alternation |
| Free of selective reporting? | Unclear risk | Trial protocol not accessible, methods section not explicit about pre‐specified outcomes |
| Adequate blinding of patients? | Low risk | Sham device: identical in appearance, not increasing intensity, flash light on, patient in position unable to read level of intensity |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? Pain | Low risk | — |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? Function | Unclear risk | Not applicable, no function outcome reported |
| Funding by commercial organisation avoided? | Unclear risk | No information provided |
| Funding by non‐profit organisation? | Unclear risk | No information provided |
Adedoyin 2005.
| Methods | Randomised controlled trial 3‐arm parallel group design Trial duration: 4 weeks Power calculation reported | |
| Participants | 51 patients randomised 46 patients with knee OA reported at baseline Study joints: 46 knees Number of females: 28 of 46 (61%) Average age: 55 years Average BMI: 28 kg/m2 | |
| Interventions |
Comparison 1
Experimental intervention: TENS and exercise twice per week
Control intervention: exercise, twice per week Comparison 2 Experimental intervention: interferential current stimulation and exercise, twice per week Control intervention: exercise, twice per week Duration of treatment period: 4 weeks Analgesics not allowed, patients confirmed not to take analgesics TENS Device: Endomed 5921D Self‐administered: no Waveform: not reported Pulse width: 200 ms Pulse frequency: 80 Hz Amplitude: above sensory threshold, strong but comfortable Duration of stimulation per session: 20 minutes Electrodes: 2 electrodes 8 x 6 cm Placement: Each side of affected knee joint, aligned longitudinally along length of limb Interferential Current Stimulation Device: Endomed 5921D (2 pole) Waveform: interferential Pulse width: not applicable Pulse frequency: 80 Hz (beat) Amplitude: above sensory threshold: strong but comfortable, strong tingling sensation without muscle contraction Duration of stimulation per session: 20 minutes Electrodes: 2 electrodes 8 x 6 cm Placement: each side of affected knee joint, aligned longitudinally along length of limb |
|
| Outcomes | Extracted pain outcome: pain on activities other than walking after 4 weeks, described as "Pain recorded while standing (10‐point pain rating scale with 0 “no pain”, 5 “moderate pain” and 10 “worst pain imaginable”)"
Extracted function outcome: WOMAC global scale after 4 weeks (Likert) No primary outcome reported |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided |
| Allocation concealment? | Unclear risk | No information provided |
| Free of selective reporting? | Unclear risk | Trial protocol not accessible, methods section not explicit about pre‐specified outcomes |
| Adequate blinding of patients? | High risk | No sham intervention |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? Pain | High risk | 15 out of 15 (100%) in TENS group, 16 out of 19 (84%) in interferential current stimulation group, 15 out of 17 (88%) in control group analysed |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? Function | High risk | See above |
| Funding by commercial organisation avoided? | Unclear risk | No information provided |
| Funding by non‐profit organisation? | Unclear risk | No information provided |
Bal 2007.
| Methods | Quasi‐randomised single centre controlled trial with allocation according to hospital registration number 2‐arm parallel group design Trial duration: 13 weeks No power calculation reported | |
| Participants | 56 patients randomised 56 patients with knee OA reported at baseline Study joints: 56 knees Number of females: 50 of 56 (89%) Average age: 57 years Average BMI: 31 kg/m2 Average disease duration: 2 years | |
| Interventions | Experimental intervention: TENS and infra‐red therapy, 5 times per week
Control intervention: sham TENS and infra‐red therapy, 5 times per week
Duration of treatment period: 2 weeks
Unclear whether analgesics were allowed and the intake was assessed Device: PlusMED 1‐904 Self‐administered: no Waveform: not reported Pulse width: 140 µsec Pulse frequency: 80 Hz Amplitude: above sensory threshold, not up to maximum tolerance, no muscle contractions observed* Duration of stimulation per session: 40 minutes Electrodes: 4, type unclear Placement: acupuncture points: ST36, GB34, SP10, SP9, ST34 |
|
| Outcomes | Extracted pain outcome: WOMAC pain subscore after 13 weeks (Likert)
Extracted function outcome: WOMAC disability subscore after 13 weeks (Likert) No primary outcome reported |
|
| Notes | Article in Turkish, outcome assessment done by AR and RS assisted by a native Turkish researcher. Serpil Bal verified all extracted data. *as indicated by Serpil Bal in personal communication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | The published report only stated that there was a random allocation of patients to comparison groups. In personal communication, investigator Serpil Bal stated that the patients were allocated according to last digit of their hospital registration number. Patients with even numbers were assigned to TENS group, patients with odd numbers to a sham intervention. |
| Allocation concealment? | High risk | No, the same investigator responsible of randomisation was giving interventions, as indicated by Serpil Bal in personal communication |
| Free of selective reporting? | Unclear risk | Trial protocol not accessible, methods section not explicit about pre‐specified outcomes, we have been unable to sort out this item with investigator Serpil Bal |
| Adequate blinding of patients? | Low risk | Trial is described as single blind study using sham device PlusMED 1‐904, indistinguishable from real TENS unit. Sham device had broken leads, no current passed but flashing light was on. None of the patients had prior experience with TENS. |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? Pain | Low risk | All subjects were available for end of treatment measurements, as indicated by Serpil Bal in personal communication |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? Function | Low risk | All subjects were available for end of treatment measurements, as indicated by Serpil Bal in personal communication |
| Funding by commercial organisation avoided? | Unclear risk | No information provided |
| Funding by non‐profit organisation? | Unclear risk | No information provided |
Cetin 2008.
| Methods | Randomised controlled trial 5‐arm parallel group design Trial duration: 8 weeks No power calculation reported | |
| Participants | 100 patients randomised 100 patients with knee OA reported at baseline Study joints: 100 knees Number of females: 100 of 100 (100%) Average age: 60 years Average BMI: 28 kg/m2 | |
| Interventions | Experimental intervention: TENS + hot packs + isokinetic exercise, 3 times per week
Control intervention: hot packs + isokinetic exercise, 3 times per week
Duration of treatment period: 8 weeks
Analgesics allowed, unclear whether intake was similar between groups Device: MED911 Self‐administered: no Waveform: not reported Puls width: 60 msecs Pulse frequency: 60‐100 Hz Amplitude: above sensory threshold, increased to point of seeing no contraction, while patient felt comfortable Duration of stimulation per session: 20 minutes Electrodes: not reported Electrode placement: around painful areas |
|
| Outcomes | Extracted pain outcome: pain on walking after 8 weeks, described as "Knee pain severity after a 50‐m walk (VAS)"
Extracted function outcome: Lequesne OA index global score after 8 weeks (Likert) No primary outcome reported |
|
| Notes | Only 2 arms qualified for inclusion in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided |
| Allocation concealment? | Unclear risk | No information provided |
| Free of selective reporting? | Unclear risk | Trial protocol not accessible, methods section not explicit about pre‐specified outcomes |
| Adequate blinding of patients? | High risk | No sham intervention |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? Pain | Unclear risk | No information provided |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? Function | Unclear risk | No information provided |
| Funding by commercial organisation avoided? | Unclear risk | No information provided |
| Funding by non‐profit organisation? | Unclear risk | No information provided |
Cheing 2002.
| Methods | Randomised controlled trial 4‐arm parallel group design Trial duration: 8 weeks Randomisation stratified according to age, gender, BMI No power calculation reported | |
| Participants | 66 patients randomised 62 patients with knee OA reported at baseline Study joints: 62 knees Number of females: 53 of 62 (85%) Average age: 64 years Average BMI: 28 kg/m2 | |
| Interventions |
Comparison 1
Experimental intervention: 60 min TENS, 5 times per week
Control intervention: sham TENS, 5 times per week Comparison 2 Experimental intervention: TENS plus exercise, 5 times per week Control intervention: exercise alone, 5 times per week Duration of treatment period: 4 weeks Analgesics allowed, unclear whether intake was similar between groups Device: MAXIMA III (dual channel) Self‐administered: unclear, most likely not Waveform: square Pulse width: 140 µsec Pulse frequency: 80 Hz Amplitude: above sensory threshold, tingling sensation, 3 to 4 times above sensory threshold Duration of stimulation per session: 60 minutes Electrodes: 4 electrodes of 4 x 4 cm Placement: at acupuncture points: ST35, SP9, GB34, extra 31,32 (one electrode covering both extra 32 and ST35) |
|
| Outcomes | Extracted pain outcome: global pain after 8 weeks, described as "Intensity of subjective pain sensation (Baseline score on 0‐10 cm VAS was standardised to be 100% in each of the groups. Follow up values were expressed as mean decrease in % from baseline)".
No function outcome reported No primary outcome reported |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided |
| Allocation concealment? | Unclear risk | No information provided |
| Free of selective reporting? | Unclear risk | Trial protocol not accessible, methods section not explicit about pre‐specified outcomes |
| Adequate blinding of patients? | Low risk | Comparison 1: Yes, sham device identical in appearance to real TENS unit, no current passed but indicator light was lit up Comparison 2: No, no sham intervention |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? Pain | High risk | Comparison 1: 16 out of 16 (100%) randomised to experimental and 16 out of 18 (89%) randomised to control group were analysed Comparison 2: 15 out of 17 (88%) randomised to experimental and 15 out of 15 (100%) randomised to control group were analysed |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? Function | Unclear risk | Not applicable |
| Funding by commercial organisation avoided? | Unclear risk | No information provided |
| Funding by non‐profit organisation? | Unclear risk | No information provided |
Cheing 2003.
| Methods | Randomised controlled trial 4‐arm parallel group design Trial duration: 4 weeks Randomisation stratified according to gender No power calculation reported | |
| Participants | 40 patients randomised 38 patients with knee OA reported at baseline Study joints: 38 knees Number of females: 34 of 38 (89%) Average age: 66 years | |
| Interventions | Experimental intervention: 20 min TENS in group 1, 40 min TENS in group 2, 60 min TENS in group 4, 5 times per week
Control intervention: sham TENS, 5 times per week
Duration of treatment period: 2 weeks
Unclear whether analgesics were allowed and whether intake was similar between groups Device: ITO 120Z TENS (dual channel) Self‐administered: no Waveform: not reported Pulse width: 200 µsec Pulse frequency: 100 Hz Amplitude: above sensory threshold, strong but comfortable Duration of stimulation per session: 20 minutes Electrodes: 4 of 2 x 3 cm rubber electrodes Placement: 4 acupuncture points extra 31,32, ST35, GB34, SP9 |
|
| Outcomes | Extracted pain outcome: pain on walking after 4 weeks, described as "pain during walking (VAS)"
No function outcome reported No primary outcome reported |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided |
| Allocation concealment? | Unclear risk | No information provided |
| Free of selective reporting? | Unclear risk | Trial protocol not accessible, methods section not explicit about pre‐specified outcomes |
| Adequate blinding of patients? | Low risk | Sham device: electronic circuit disconnected, no current passed, but indicator light on |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? Pain | High risk | 30 out of 30 (100%) randomised to experimental and 8 out of 10 (80%) randomised to control group were analysed |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? Function | Unclear risk | Not applicable |
| Funding by commercial organisation avoided? | Unclear risk | No information provided |
| Funding by non‐profit organisation? | Unclear risk | No information provided |
Defrin 2005.
| Methods | Randomised controlled trial 6‐arm parallel group design Trial duration: 4 weeks No power calculation reported | |
| Participants | 62 patients randomised 62 patients with knee OA reported at baseline Study joints: 62 knees Average age: 67 years | |
| Interventions | Experimental intervention: noxious adjusted interferential current stimulation in group 1, noxious unadjusted interferential current stimulation in group 2, innocuous adjusted interferential current stimulation in group 3, innocuous unadjusted interferential current stimulation in group 4, 3 times per week
Control intervention: sham interferential current stimulation, 3 times per week
Duration of treatment period: 4 weeks
Analgesics allowed, unclear whether intake was similar between groups. Device: Uniphy: Phyaction electrical stimulator Self‐administered: no Waveform: interferential Pulse width: not applicable Pulse frequency: 30 to 60 Hz (beat) Amplitude: above sensory threshold, 2 groups 30% above pain threshold; 2 groups 30% below pain threshold Duration of stimulation per session: 20 minutes Electrodes: 2 of 8 x 6 cm wet sponge electrodes Placement: medial and lateral aspects of the knee, 2 cm from outer margins of patella |
|
| Outcomes | Extracted pain outcome: global pain after 4 weeks, described as "chronic pain intensity (VAS)"
No function outcome reported No primary outcome reported |
|
| Notes | 1 out of 6 trial arms, the no‐intervention control group was excluded in the review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided |
| Allocation concealment? | Unclear risk | No information provided |
| Free of selective reporting? | Unclear risk | Trial protocol not accessible, methods section not explicit about pre‐specified outcomes |
| Adequate blinding of patients? | Unclear risk | Use of sham device: Uniphy‐Phyaction electrical stimulator, however the device described as shut‐off |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? Pain | Unclear risk | No information provided |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? Function | Unclear risk | Not applicable |
| Funding by commercial organisation avoided? | Unclear risk | No information provided |
| Funding by non‐profit organisation? | Unclear risk | No information provided |
Fargas‐Babjak 1989.
| Methods | Randomised controlled trial 2‐arm parallel group design Trial duration: 13 weeks No power calculation reported | |
| Participants | 56 patients randomised 56 patients with knee OA reported at baseline Study joints: 56 joints, most likely > 75% knees Average age; gender, BMI: not reported | |
| Interventions | Experimental intervention: burst TENS, twice per day
Control intervention: sham TENS, twice per day
Duration of treatment period: 6 weeks
Analgesics allowed, but change of dosage prohibited. Unclear whether analgesics were assessed and whether intake was similar between groups. Device: Codetron Self‐administered: yes Waveform: square Pulse width: 1000 µsec Pulse frequency: 200 Hz, train length of 125 ms, repetition frequency of 4 Hz (25 pulses per train) Amplitude: above sensory threshold, highest intensity that could be tolerated without inducing frank pain Duration of stimulation per session: 30 minutes Electrodes: 7 carbon rubber (self‐adhesive) Karaya Pads electrodes of 2 x 3 cm Placement: 10 acupuncture points: GV14, GV4, GB30, GB34, SP13, B1 60, ST36, B1 40, SP9, LI4 and 3 extra tender points |
|
| Outcomes | Extracted pain outcome: global pain after 13 weeks described as "Pain improvement (percentage pain improvement based on VAS)"
No function outcome reported No primary outcome reported |
|
| Notes | *Investigators named their intervention AL‐TENS, but we coded it burst TENS in the analyses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided |
| Allocation concealment? | Unclear risk | No information provided |
| Free of selective reporting? | High risk | Quote: "Full details of this (Percent Improvement Pain Scale) are reported elsewhere". Investigators however failed to provide reference. |
| Adequate blinding of patients? | Low risk | Use of sham device: Codetron, identical in appearance, set at frequency of 0.2 Hz with a threshold electrical stimulus of 0.5 mA, which caused a sensation on the skin but failed causing the deep muscle afferent stimulation |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? Pain | High risk | 56 patients randomised but only 19 analysed in the experimental, and 18 analysed in the control group |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? Function | Unclear risk | Not applicable |
| Funding by commercial organisation avoided? | High risk | Sponsor: Electronic Health Machines |
| Funding by non‐profit organisation? | Low risk | NRC grant no: 689 |
Garland 2007.
| Methods | Randomised multicentre controlled trial 2‐arm parallel group design Number of participating centres: 3 Trial duration: 12 weeks Randomisation stratified according to study site No power calculation reported | |
| Participants | 100 patients randomised 58 patients with knee OA reported at baseline; 41 out of 58 candidates for total knee arthroplasty Study joints: 58 knees Number of females: 38 of 58 (66%) Average age: 66 Disease duration: 8.4 years | |
| Interventions | Experimental intervention: pulsed electrical stimulation
Control intervention: sham intervention
Duration of treatment period: 12 weeks
Analgesics allowed and intake assessed, but unclear whether intake was similar. Device: BIO‐1000 Self‐administered: yes Waveform: unclear Pulse width: unclear Pulse frequency: 100 Hz Amplitude: below sensory threshold, initial increase of amplitude up to 12 Volt until a tingling sensation was felt then reduction of the amplitude until this sensation disappeared Duration of stimulation per session: 8.2 hours in active group, 7.8 hours in sham group (mean daily application time) Electrodes: flexible electrodes embedded in garment, type not reported Electrode placement: negative electrode at patella, positive over anterior distal thigh |
|
| Outcomes | Extracted pain outcome: global pain after 12 weeks, described as "Considering your pain and symptoms in your study joint how are you doing today? (VAS)"
Extracted function outcome: WOMAC disability subscore after 12 weeks (VAS) No primary outcome reported |
|
| Notes | *Due to major protocol violations, all 42 randomised patient of one site were excluded by Garland et al | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random number table |
| Allocation concealment? | Low risk | Central randomisation |
| Free of selective reporting? | Unclear risk | Quote: "Total WOMAC scores were not a defined outcome in the protocol, but are shown in Tables II(a)‐(d)." |
| Adequate blinding of patients? | Low risk | Use of sham device: BIO‐1000, indistinguishable from active device, with automatic shut‐off as soon as amplitude is reduced (all patients were instructed to reduce intensity just below perception level). Further adjustments required all devices to be restarted. |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? Pain | High risk | Due to major protocol violations, all 42 randomised patient of 1 site were excluded by original authors. From the other site, all patients randomised were included in the analysis. |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? Function | High risk | See above |
| Funding by commercial organisation avoided? | High risk | Sponsor: BioniCare Medical Technologies |
| Funding by non‐profit organisation? | Unclear risk | No information provided |
Grimmer 1992.
| Methods | Randomised controlled trial 3‐arm parallel group design Trial duration: 1 day No power calculation reported | |
| Participants | 60 patients randomised 60 patients with knee OA reported at baseline Study joints: 60 knees Number of females: 37 of 60 (62%) Average age: 66 years | |
| Interventions | Experimental intervention: high frequency TENS, once only in group 1, burst TENS, once only in group 2
Control intervention: sham TENS, once only
Duration of treatment period: 1 day
Analgesics not allowed Device: Medtronic Neuromed Selectra (dual channel) Self‐administered: no Waveform: unclear Pulse width: unclear Pulse frequency: 80 Hz in group 1, 3 Hz trains of 7 80 Hz pulses in group 2 Amplitude: above sensory threshold, strong tolerable tingling paraesthesia Duration of stimulation per session: 30 minutes Electrodes: 4 carbon rubber silicone electrodes, 2 x 3 cm Placement: 4 acupuncture points around the knee: medial (SP9), lateral (GB33), posterior (UB40), anterior (SP10) |
|
| Outcomes | Extracted pain outcome: global pain immediately after first and only application, described as "Immediate pain relief (VAS)"
No function outcome reported No primary outcome reported |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "randomly allocated (by dice) into three groups of 20" |
| Allocation concealment? | Low risk | By a person independent of the study |
| Free of selective reporting? | Unclear risk | Insufficient information provided; no access to study protocol |
| Adequate blinding of patients? | Low risk | Sham device: Medtronic Neuromed Selectra, with non‐functioning leads. Patient were told that a very high frequency current was being tested and that no skin sensation would be felt. |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? Pain | Low risk | Degrees of freedom reported indicate that all randomised patients were included in the analysis |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? Function | Unclear risk | Not applicable |
| Funding by commercial organisation avoided? | Unclear risk | No information provided |
| Funding by non‐profit organisation? | Unclear risk | No information provided |
Itoh 2008.
| Methods | Randomised controlled trial 2 x 2 factorial design Trial duration: 10 weeks No power calculation reported | |
| Participants | 32 patients randomised 32 patients with knee OA reported at baseline Study joints: 32 knees Number of females: 21 of 32 (66%) | |
| Interventions | Experimental intervention: interferential current stimulation*, once per week
Control intervention: no intervention, optional use of poultice 16 out of 32 patients (50%) allocated to acupuncture using a factorial design; no evidence for an interaction between treatments Duration of treatment period: 5 weeks Analgesics allowed and intake assessed, but unclear whether intake was similar. Device: HV‐F3000 (single channel, 2 pole) Self‐administered: no Waveform: sinusoidal Pulse width: not applicable Pulse frequency: amplitude‐modulated frequency of 122 Hz (beat frequency) Amplitude: above sensory threshold, up to a tingling sensation, 2 to 3 times above sensory threshold Duration of stimulation per session: 15 minutes Placement: site of tenderness and opposite site Electrodes: 2 disposable electrodes different in size, 809 mm2 and 5688 mm2 |
|
| Outcomes | Extracted pain outcome: global pain after 10 weeks, described as "Pain intensity (VAS)"
Extracted function outcome: WOMAC global scale after 10 weeks (VAS) Primary outcomes: pain intensity, WOMAC global scale |
|
| Notes | *The investigators used the label TENS in their report, but from their description of the intervention it was clear that interferential current stimulation was applied | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated block randomisation. Quote "According to a block randomised allocation table (generated by Sample Size, version 2.0, Int), the enrolled patients were allocated to (1) the control (CT) group, (2) the acupuncture (ACP) group, (3) the transcutaneous electrical nerve stimulation (TENS) group or (4) the acupuncture and TENS (A&T) group." |
| Allocation concealment? | Unclear risk | No information provided |
| Free of selective reporting? | Unclear risk | Insufficient information provided, no access to study protocol |
| Adequate blinding of patients? | High risk | No sham intervention |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? Pain | High risk | 12 out of 16 (75%) randomised to experimental and 12 out of 16 (75%) randomised to control group were analysed |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? Function | High risk | See above |
| Funding by commercial organisation avoided? | Unclear risk | No information provided |
| Funding by non‐profit organisation? | Unclear risk | No information provided |
Law 2004.
| Methods | Randomised controlled trial 4‐arm parallel group design Trial duration: 4 weeks No power calculation reported | |
| Participants | 36 patients randomised 36 patients with knee OA reported at baseline Study joints: 48 knees* Number of females: 35 of 36 (97%) Average age: 82 years | |
| Interventions | Experimental intervention: 2 Hz TENS in group 1, 100 Hz TENS in group 2, modulation TENS with alternations between 2 to 100 Hz in group 3, 5 times per week in all groups
Control intervention: sham TENS, 5 times per week
Duration of treatment period: 2 weeks
Unclear whether analgesics were allowed and whether intake was similar between groups Device: Han Acupoint Nerve Stimulation LH204H Self‐administered: no Waveform: unclear Pulse width and frequency: 576 µsec and 2 Hz in group 1, 200 µsec and 100 Hz in group 2, 576/200 µsec and 2/100 Hz alternation in group 3 Amplitude: above sensory threshold, up to comfortable level, range 25 to 35 mA Duration of stimulation per session: 40 minutes Electrodes: 4 rubber electrodes of 4.5 x 3.8 cm Placement: 4 acupuncture points: ST35, LE4, SP9, GB34 |
|
| Outcomes | Extracted pain outcome: pain on walking after 4 weeks, described as "intensity of pain felt while walking (VAS)"
No function outcome reported No primary outcome reported |
|
| Notes | Outcome data were reported on knee level. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "Randomization was carried out by drawing lots from the randomization envelope." |
| Allocation concealment? | Unclear risk | No information provided |
| Free of selective reporting? | Unclear risk | Insufficient information provided; no access to study protocol |
| Adequate blinding of patients? | Low risk | Use of sham device: identical in appearance, internal circuit disconnected, no current passed, indicator light on, digital display of intensity control functioned normally. Quote: "Only therapists who administered treatment to the subjects knew the group allocation, while the subjects and the assessor were not given this information." |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? Pain | High risk | In total, 3 patients dropped out and were excluded from analysis, as indicated by Gladys Cheing and Pearl Law in personal communication |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? Function | Unclear risk | Not applicable |
| Funding by commercial organisation avoided? | Unclear risk | No information provided |
| Funding by non‐profit organisation? | Unclear risk | No information provided |
Law 2004a.
| Methods | Randomised controlled trial 2‐arm parallel group design Trial duration: 2 weeks Unstratified randomisation Multicentre trial with 2 centres No power calculation reported | |
| Participants | 39 patients randomised 39 patients with knee OA reported at baseline Study joints: 39 knees Number of females: 37 of 39 (95%) Average age: 75 years Average BMI: 27 kg/m2 Average disease duration: 7.6 years | |
| Interventions | Experimental intervention: TENS, 5 times per week
Control intervention: sham TENS, 5 times per week
Duration of treatment period: 2 weeks
Unclear whether analgesics were allowed and whether intake was similar between groups Device: ITO model 120Z (dual channel) Self‐administered: no Waveform: unclear Pulse width: 200 µsec Pulse frequency: 100 Hz Amplitude: above sensory threshold, up to a comfortable level, range 25‐35 mA Duration of stimulation per session: 40 minutes Electrodes: 4 rubber electrodes, 4.5 x 3.8 cm2 Placement: acupuncture points: ST35, LE4, SP9, GB34 |
|
| Outcomes | Extracted pain outcome: pain on walking after 2 weeks, described as "intensity of pain felt while walking (VAS)"**
Extracted function outcome: walking disability after 2 weeks, described as "Timed‐Up‐and‐Go test over 3 meters (seconds)" No primary outcome reported |
|
| Notes | **Only baseline values reported in the report. Contact established with investigators Law and Cheing, who provided end of treatment and follow‐up data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "'by drawing lots from the randomization envelope without replacement" |
| Allocation concealment? | Unclear risk | Quote : "(...) carried out by physiotherapists who performed the treatment" |
| Free of selective reporting? | High risk | No results reported for some outcomes mentioned in the methods section, including pain intensity on VAS |
| Adequate blinding of patients? | Low risk | Use of sham device: ITO model 120Z, no current delivered but flashing light on. Quote: "The assessors and subjects were blind to the group allocation. All subjects were told that when the indicator light of the TENS was blinking, it meant the machine was working properly. They might or might not feel any tingling sensation during treatment because the intensity of the current was small." |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? Pain | High risk | In total, 3 patients dropped out and were excluded from analysis, as indicated by Gladys Cheing and Pearl Law in personal communication |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? Function | High risk | See above |
| Funding by commercial organisation avoided? | Unclear risk | No information provided |
| Funding by non‐profit organisation? | Unclear risk | No information provided |
Ng 2003.
| Methods | Randomised controlled trial 3‐arm parallel group design Trial duration: 4 weeks Unstratified randomisation No power calculation reported | |
| Participants | 24 patients randomised 24 patients with knee OA reported at baseline Study joints: 24 knees Number of females: 23 of 24 (96%) Average age: 85 years | |
| Interventions | Experimental intervention: TENS, 4 times per week, with a total of 8 applications and educational pamphlet
Control intervention: educational pamphlet
Duration of treatment period: 2 weeks
Unclear whether analgesics were allowed and whether intake was similar between groups Device: ITO model F‐2 (dual channel) Self‐administered: no Waveform: unclear Pulse width: 200 µsec Pulse frequency: 2 Hz Amplitude: above sensory threshold, until strong, tolerable, stroking sensation, preferably evoking phasic muscle contraction Duration of stimulation per session: 20 minutes Electrode placement: acupuncture points ST35, EX‐LE‐4 Electrodes: 50 x 35 mm2 |
|
| Outcomes | Extracted pain outcome: global pain after 4 weeks, described as "pain (Numeric rating scale (NRS))"
No function outcome reported No primary outcome reported |
|
| Notes | 2 out of 3 trial arms qualified for inclusion in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Drawing lots. Quote: "Subjects were randomly assigned by drawing a piece of paper that designated each person to the EA, TENS, and control groups" |
| Allocation concealment? | Unclear risk | No information provided |
| Free of selective reporting? | Low risk | Quote: "In each evaluation session, three outcome measures were collected." The authors present results of all these 3 outcomes. |
| Adequate blinding of patients? | High risk | No sham intervention |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? Pain | Unclear risk | No information provided |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? Function | Unclear risk | Not applicable |
| Funding by commercial organisation avoided? | Unclear risk | No information provided |
| Funding by non‐profit organisation? | Unclear risk | No information provided |
Quirk 1985.
| Methods | Randomised controlled trial 3‐arm parallel group design* Trial duration: 26 weeks No power calculation reported | |
| Participants | 38 patients randomised 38 patients with knee OA reported at baseline Study joints: 38 knees Number of females: 29 of 38 (76%) Average age: 63 years | |
| Interventions | Experimental intervention: interferential current + exercise, interferential current stimulation: 3 times per week, exercise twice daily
Control intervention: exercise twice daily
Duration of treatment period: 4 weeks
Analgesics allowed, unclear whether intake was similar between groups Device: Endomed 433 and Vacutron 423 (unclear whether 2 or 4 pole) Self‐administered: no Waveform: interferential Pulse width: not applicable Pulse frequency: 0 to 100 Hz 10 minutes, 130 Hz last 5 minutes Amplitude: not reported Duration of stimulation per session: 15 minutes Electrodes: suction electrodes Placement: not reported |
|
| Outcomes | Extracted pain outcome: other after 26 weeks, described as "Pain composite score with items rest, post‐exercise and night pain (approach unclear; either VAS or verbal scoring technique modified after Newland)"**
Extracted function outcome: other algofunctional scale after 26 weeks, described as "Overall clinical condition scale developed by authors, which was based on 3 items for pain; rest‐, post‐exercise‐, night pain and 3 for function; gait, method of climbing stairs and using walking aids (most likely Likert)". No primary outcome reported |
|
| Notes | *1 trial arm, in which shortwave diathermy was given, was excluded, **only baseline values with standard error and P values for change from baseline per group reported. No contact could be established with the investigators. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided |
| Allocation concealment? | Unclear risk | No information provided |
| Free of selective reporting? | High risk | No results reported for some outcomes mentioned in the methods section, including maximum knee girth |
| Adequate blinding of patients? | High risk | No sham intervention |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? Pain | Low risk | Quote: "All patients completed their therapy and the first two assessments (baseline and end of treatment), while 92% completed the final assessment (3‐6 months after treatment)" |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? Function | Low risk | See above |
| Funding by commercial organisation avoided? | Unclear risk | No information provided |
| Funding by non‐profit organisation? | Unclear risk | No information provided |
Smith 1983.
| Methods | Randomised sham controlled trial 2‐arm parallel group design Trial duration: 8 weeks Randomisation stratified according to gender Multicentre trial with 2 centres No power calculation reported | |
| Participants | 32 patients randomised 30 patients with knee OA reported at baseline Study joints: 30 knees Number of females: 20 of 30 (67%) Average age: 68 years | |
| Interventions | Experimental intervention: TENS, twice per week*
Control intervention: sham TENS, twice per week*
Duration of treatment period: 4 weeks
Analgesics intake assessed and found to be similar between groups Device: RDG Tiger Pulse Self‐administered: no Waveform: square Pulse width: 80 µsec Pulse frequency: 32 to 50 Hz Amplitude: above sensory threshold, adjusted up to a comfortable tingling sensation Duration of stimulation per session: 20 minutes Electrodes: 4 Lec Tec pads applied with electrode jelly Placement: tender knee points or acupuncture points (SP9, xiyan and UB40) |
|
| Outcomes | Extracted pain outcome: global pain after 8 weeks, described as "Weekly pain score derived from daily pain recording (linear 7‐point scale)"**
No function outcome reported No primary outcome reported |
|
| Notes | *Preceded by 1 'standard' week without any treatment, **No pain outcome data presented, investigators were contacted, but we did not receive any reply. This study only contributed in safety analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated. Quote: "(...) assigned by random computer programme and effected by using sealed envelopes containing cards which defined the treatment (...)". |
| Allocation concealment? | Unclear risk | Sealed assignment envelopes, but unclear whether these were opaque and sequential |
| Free of selective reporting? | High risk | No results reported for some outcomes mentioned in the methods section, including sleep disturbance |
| Adequate blinding of patients? | Low risk | Use of sham device: RDG Tiger Pulse with broken electrode connection at jack point, no current passed but flashing light on. Quote: "Exactly the same procedure were followed for both the treatment and control groups". |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? Pain | High risk | 15 out of 16 (0.94) randomised to experimental and 15 out of 16 (0.94) randomised to control group were analysed |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? Function | Unclear risk | Not applicable |
| Funding by commercial organisation avoided? | Unclear risk | No information provided |
| Funding by non‐profit organisation? | Unclear risk | No information provided |
Yurtkuran 1999.
| Methods | Randomised controlled trial 4‐arm parallel group design Trial duration: 2 weeks No power calculation reported | |
| Participants | 100 patients randomised, 25 per group 100 patients with knee OA reported at baseline Study joints: 100 knees Number of females: 91 of 100 (91%) Average age: 58 years | |
| Interventions | Experimental intervention: TENS, 5 times per week
Control intervention: sham TENS, 5 times per week
Duration of treatment period: 2 weeks
Unclear whether analgesics were allowed and whether intake was similar between groups Device: MEA‐TENS (dual channel) Self‐administered: no Waveform: rectangular Pulse width: 1000 µsec Pulse frequency: 4 Hz* Amplitude: above sensory threshold, up to muscle contraction, just below pain tolerance threshold Duration of stimulation per session: 20 minutes Electrodes: 4 small MEA rubber electrodes Placement: 4 acupuncture points SP‐9, GB‐34, ST‐34, ST‐35 |
|
| Outcomes | Extracted pain outcome: global pain after 2 weeks described as "Overall present pain intensity at rest (Likert)"
Extracted function outcome: walking disability after 2 weeks, described as "50 foot walking time (in minutes)" No primary outcome reported |
|
| Notes | Two out of 4 groups, the electroacupuncture and ice massage groups, were excluded in this review. *Investigators named their intervention AL‐TENS, but we coded it low frequency TENS in our analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided |
| Allocation concealment? | Unclear risk | No information provided |
| Free of selective reporting? | Unclear risk | Trial protocol not accessible, methods section not explicit about pre‐specified outcomes |
| Adequate blinding of patients? | Low risk | Sham device: MEA‐TENS with broken lead at jack plug, no current passed but red indicator light on. Quote: "(...) treatment appeared to be done in the same way as the other groups without the subjects suspecting the nature of the stimulation". |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? Pain | High risk | Investigators reported that "no subject was withdrawn either active or placebo groups". However, the reported degrees of freedom indicate that 5 out of 100 patients were not included. It remained unclear to which of the 4 groups the excluded patients belonged. |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? Function | High risk | See above |
| Funding by commercial organisation avoided? | Unclear risk | No information provided |
| Funding by non‐profit organisation? | Unclear risk | No information provided |
Zizic 1995.
| Methods | Randomised controlled trial 2‐arm parallel group design Trial duration: 34 weeks Multicentre trial with 5 centres No power calculation reported | |
| Participants | 78 patients randomised 71 patients with knee OA reported at baseline Study joints: 71 knees Number of females: 33 of 71 (46%) | |
| Interventions | Experimental intervention: pulsed electrostimulation stimulation, daily application
Control intervention: sham pulsed electrostimulation, daily application
Duration of treatment period: 4 weeks
Analgesics allowed, intake assessed and found to be similar between groups. Device: Bionicare Stimulator BIO‐1000 Self‐administered: yes Waveform: monophasic, spiked Pulse width: unclear Pulse frequency: 100 Hz Amplitude: below sensory threshold, initial increase of amplitude until a tingling sensation was felt then reduction of the amplitude until this sensation disappeared Duration of stimulation: 6 to 10 hours per day Electrodes: 2, unclear whether positioned in knee garment Placement: one on knee, other on thigh directly above that knee |
|
| Outcomes | Extracted pain outcome: global pain after 34 weeks described as "Patient evaluation of pain of treated knee (Baseline based on 0‐10 VAS, follow‐up based on % change from baseline)"
Extracted function outcome: patient's global assessment after 34 weeks, described as "Patient evaluation of function of treated knee (Baseline based on 0‐10 VAS, follow‐up based on % change from baseline)" More than 2 primary outcomes reported (1 physician global evaluation; 2) VAS pain; 3) VAS function) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided |
| Allocation concealment? | Unclear risk | No information provided |
| Free of selective reporting? | High risk | No results reported for some outcomes mentioned in the methods, including walking time, tenderness and swelling |
| Adequate blinding of patients? | Low risk | Sham device: BIO‐1000, identical in appearance to active device, with automatic shut‐off as soon as amplitude is reduced (all patients were instructed to reduce intensity just below perception level) |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? Pain | High risk | 38 out of 41 (0.93) randomised to experimental and 33 out of 37 (0.89) randomised to control group were analysed |
| Incomplete outcome reporting: intention‐to‐treat analysis performed? Function | High risk | See above |
| Funding by commercial organisation avoided? | High risk | Sponsor: Murray Electronics |
| Funding by non‐profit organisation? | Unclear risk | No information provided |
BMI = body mass index min = minutes OA = osteoarthritis VAS = visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barr 2004 | Less than 50% of patients diagnosed with osteoarthritis of the knee |
| Bernau 1981 | Not a randomised controlled trial, use of active control groups. Additional description: comparing diadynamic electrostimulation df, diadynamic electrostimulation cf and galvanic current |
| Burch 2008 | Use of active control group. Additional description: randomised controlled trial comparing interferential current stimulation followed by patterned muscle stimulation and low‐current transcutaneous electrical nerve stimulation (TENS). |
| Cauthen 1975 | Not concerning osteoarthritis |
| Commandre 1977 | No randomised controlled trial (review) |
| Cottingham 1985a | Not transcutaneous but subcutaneous application |
| Cottingham 1985b | Not transcutaneous but subcutaneous application. Abstract referring to same RCT as described in Cottingham 1985a. |
| Durmus 2005 | Use of active control group (exercise) |
| Gaines 2001 | Neuromuscular electrostimulation primarily aiming at muscle strengthening |
| Gaines 2004 | Neuromuscular electrostimulation primarily aiming at muscle strengthening |
| Gibson 1989 | Most likely not a randomised controlled trial; percutaneous electrostimulation primarily aiming at muscle strengthening |
| Godfrey 1979 | Faradic electrostimulation with parameters set to increase muscle strength and use of active control (exercise plus low intensity (sham) faradic electrostimulation) |
| Grigor'eva 1992 | No relevant pain or function outcomes |
| Guven 2003 | High voltage galvanic electrostimulation for muscle strengthening |
| Hamilton 1959 | Only 34% of patients suffered OA; use of active controls. Additional description: cross‐over design evaluating faradic electrostimulation. |
| Huang 2000 | TENS as part of a combined experimental intervention. Additional description design: 3 groups, Group A receiving auricular acupuncture, diet control and aerobic exercise, Group B like A with addition of TENS and ultrasound, Group C receiving TENS and ultrasound; unclear whether allocation was at random. |
| Jensen 1991 | Use of active control: high frequency TENS versus low frequency TENS |
| Kang 2007 | Percutaneous electrostimulation |
| Katsnelson 2004 | Electrode placement not involving knee innervation: transcranial electrostimulation |
| Komarova 1998 | Electrode placement not involving knee innervation: transcranial electrostimulation |
| Lewis 1984 | Cross‐over RCT reporting pooled results after completion of all phases. Contact established with Daniel and Beverly Lewis, who were unable to provide results for the first phase (before cross‐over) |
| Lewis 1985 | RCT reporting P values of effect only. Contact established with Daniel and Beverly Lewis, who could not provide any additional outcome data, nor could they indicate whether the design concerned a cross‐over or a parallel RCT |
| Lewis 1988 | Published abstract addressing the same cross‐over RCT reported by Lewis 1994 |
| Lewis 1994 | Cross‐over RCT reporting pooled results after completion of all phases. Contact established with Daniel and Beverly Lewis, who were unable to provide results for the first phase (before cross‐over) |
| Lone 2003 | Not a randomised controlled study. Additional description: before‐after study design that was incorrectly labelled as randomised study by original authors. |
| Lund 2005 | Not concerning osteoarthritis |
| Macchione 1995 | Not a randomised controlled trial (review) |
| Matti 1987 | Not concerning osteoarthritis, not a randomised clinical trial. Tetanus‐like faradisation electrostimulation with exercise after surgical removal of meniscus, primarily aiming at muscle enhancement. Active control with 10 Hz sinusoidal current application and exercise. |
| Miranda‐Filloy 2005 | Electrical muscle stimulation using sport400 (Complex), primarily aiming at muscle strengthening |
| Mont 2006 | Not a randomised clinical trial. Description: comparative study with historical control evaluating pulsed electrostimulation. |
| Oldham 1995 | Neuromuscular electrostimulation primarily aiming at muscle strengthening |
| Oldham 1997 | Electrostimulation primarily aiming at muscle strengthening |
| Oosterhof 2008 | Mixed population, only 4 out of 163 patients reported to have knee, hip or ankle OA |
| Paillard 2005 | Not concerning osteoarthritis (healthy volunteers) |
| Picaza 1975 | Not concerning osteoarthritis and not a randomised controlled trial |
| Salaj 2001 | Not a randomised controlled trial, combined multiple interventions in both interventions and control group |
| Salim 1996 | Not a randomised controlled trial (review) |
| Sluka 1998 | Animal study |
| Sok 2007 | Concerns chronic knee pain. First author was contacted by email to verify how many patients had osteoarthritis. No response received. Additional description: article in Korean, using a TENS device, abstract however suggests that parameters were set to strengthen muscles. |
| Svarcova 1988a | Use of active control groups. Additional description: controlled trial with groups receiving either galvanic electrostimulation or YES ultrasound or pulsed shortwaves. Within these groups, half of the patients received ibuprofen, half received placebo ibuprofen. It was unclear whether allocation was at random. |
| Svarcova 1988b | See Svarcova 1988a. Double publication of the same study, including the same number of patient and outcome data. |
| Svarcova 1990 | Use of active control group. Additional description: galvanic electrostimulation versus electroacupuncture. |
| Talbot 2003 | Neuromuscular electrostimulation primarily aiming at muscle strengthening |
| Tam 2004 | No relevant pain or function outcomes used |
| Taylor 1981 | Incomplete presentation of data. Additional description: cross‐over randomised clinical trial presenting pooled results only. Contact established with Mark Hallett, who was unable to provide data concerning the first phase, before cross‐over. We were unable to contact the other authors. |
| Tulgar 1991 | Not concerning osteoarthritis |
| Volklein 1990 | Use of active control group. Additional description: random allocation of patients to 4 different types of diadynamic current. |
| Weiner 2007 | Not transcutaneous but periosteal (needle) application |
| Zivkovic 2005 | Use of active control group. Additional description: the combination of low‐energy laser, pulsed electromagnetic field and kinesitherapy was compared to the combination of electrotherapy, pulsed electromagnetic field and kinesitherapy. |
OA = osteoarthritis RCT = randomised controlled trial TENS = transcutaneous electrical nerve stimulation
Characteristics of ongoing studies [ordered by study ID]
Fary 2008.
| Trial name or title | ACTRNI2607000492459 |
| Methods | Double‐blind, randomised placebo‐controlled trial Randomisation method: computer‐generated block randomisation with stratification for gender, age and intensity of pain Concealment of allocation: by independent administrator Blinding: patients, those administering treatment/s, those assessing outcomes, those analysing results/data Sample size calculation: reported Analyses based on intention‐to‐treat principle Trial duration: 26 weeks Sponsored by: non‐profit organisation Arthritis Australia and Physiotherapy Research Foundation |
| Participants | 70 patients with primary knee OA to be randomised Study joints: 70 knees Selection criteria: persistent, stable pain for minimum of 3 months, at least 25 mm on a 100 mm VAS |
| Interventions | Experimental intervention: pulsed electrostimulation, daily
Control intervention: sham pulsed electrostimulation, daily
Duration of treatment period: 26 weeks
Analgesics allowed and measured with diary Device: Metron Digi‐10s, adapted by engineer Self‐administered: yes Waveform: pulsed, exponentially declining Pulse width: not reported Pulse frequency: 100 Hz Amplitude: below sensory threshold Duration of stimulation: minimally 7 hours per day Electrodes: not reported Electrode placement: not reported Sham device: identical in appearance |
| Outcomes | Primary outcomes: conflicting information reported in Australian/New Zealand clinical trial register (ANZCTR) and subsequent publication in BMC. In ANZCR reported as pain on VAS, in BMC more than 2 primary outcomes are reported; pain (VAS and WOMAC), function (WOMAC), and patient global assessment (VAS). Main time points of interest are reported consistently as baseline, 4, 16 and 26 weeks. Secondary outcomes: in ANZCTR reported as function (WOMAC) and patient global assessment (VAS); in BMC reported as stiffness (WOMAC 3.1), quality of life (SF‐36), global perceived effect scale (GPES), physical activity (Human Activity Profile (HAP) questionnaire plus accelerometers Safety outcomes: in BMC, the recording of adverse events was reported |
| Starting date | 26th of September 2007 |
| Contact information | Robyn E Fary Curtin University of Technology, School of Physiotherapy, Kent Street, Bentley, WA, 6102, Australia Tel: 08 9266 3667 Email: R.Fary@curtin.edu.au |
| Notes | Status at 17 July 2009: open to recruitment |
Palmer 2007.
| Trial name or title | ISRCTN12912789 |
| Methods | A randomised, sham‐controlled trial with 3 parallel arms Randomisation method: not reported Concealment of allocation: not reported Blinding: not reported Sample size calculation: not reported Analyses: not reported whether is based on intention‐to‐treat principle Trial duration: 6 weeks Sponsored by: not reported |
| Participants | 261 (87 in each arm) patients with primary knee OA to be randomised Study joints: knees Selection criteria: knee pain, radiographic (X‐ray) evidence of osteophytes, and at least 1 of the following 3 criteria: 50 years or older, morning stiffness that lasts for less than 30 minutes, crepitus on active movement |
| Interventions | Experimental intervention: TENS, as much as needed and group education including self‐efficacy and exercise training, once per week
Control intervention 1: Sham TENS, as much as needed and group education once per week, as described above
Control intervention 2: group education once per week, as described above
Duration of treatment period: 6 weeks
Analgesics: unclear wether analgesic intake is allowed and is measured Device: not reported Self‐administered: yes Waveform: not reported Pulse width: not reported Pulse frequency: not reported Amplitude: "strong but comfortable" tingling sensation Duration of stimulation: defined as "as much as needed" Electrodes: not reported Electrode placement: within or close to the site of pain Sham device: identical in appearance, displays are active but there is no current output |
| Outcomes | Primary outcome: WOMAC function subscale (at baseline, 3, 6, 12 and 24 weeks) Secondary outcomes: 1. Total WOMAC score and WOMAC pain and stiffness subscale scores (at baseline, 3, 6, 12 and 24 weeks) 2. Knee extensor torque (quadriceps strength) (at baseline, 3, 6, 12 and 24 weeks) 3. Patient global assessment of change (at 3, 6, 12 and 24 weeks) 4. Self‐efficacy for exercise (at baseline and 24 weeks) 5. Self‐reported exercise adherence (at baseline, 3, 6, 12 and 24 weeks) 6. Logged TENS usage time (at 6 weeks) |
| Starting date | 1 October 2007 |
| Contact information | Dr Shea Palmer Faculty of Health and Social Care University of the West of England Blackberry Hill Bristol BS16 1DD United Kingdom Tel +44 (0)117 328 8919 Email Shea.Palmer@uwe.ac.uk |
| Notes | Status at 17 July 2009: completed at 30 June 2009 |
OA = osteoarthritis TENS = transcutaneous electrical nerve stimulation VAS = visual analogue scale
Differences between protocol and review
Before embarking on this review, we generated a standard protocol for this and all other Cochrane Reviews performed by our group. The protocol was approved by the Editorial Board of the Cochrane Musculoskeletal Review Group (CMSG), but, as an update, did not result in a specific publication in the Cochrane database. We deviated from the standard protocol with respect to the selection of main outcomes and analysis. The main outcomes specified in the protocol were pain and function, as recommended for osteoarthritis trials. After approval of the standard protocol, the Editorial Board of CMSG reconvened several times to establish common views on how to conduct systematic reviews, and it was decided that the main outcomes of future reviews should reflect both effectiveness and safety. CMSG further agreed to recommend the use of a maximum of two main outcomes. Therefore, the CMSG Editorial Board and the authors of this review agreed to specify pain intensity and the number of drop‐outs or withdrawals due to adverse events as main outcomes for this update. Function was specified as one of the secondary outcomes. The protocol specified that our main analysis would be based on standardised mean differences (SMDs) derived from inverse‐variance random‐effects meta‐analysis. In view of the high degree of heterogeneity, the predominance of small trials of low methodological quality and the skewed funnel plot for pain intensity as one of the main outcomes, we refrained from presenting the SMD of pain as primary result in main body of text and summary of findings table, but reported results from uni‐variable meta‐regression analysis used to predict treatment effects in trials as large as the largest trials included in the meta‐analysis with the standard error as the explanatory variable. We acknowledge that this analysis is exploratory, however. In addition, we used 'Risk of bias' tables to present the methodological quality of included trials and a 'Summary of findings' table to present results.
Contributions of authors
Study conception: Rutjes, Jüni Protocol development: Rutjes, Nüesch, Hendriks, Kalichman, Reichenbach, Jüni Acquisition of data: Rutjes, Nüesch, Sterchi, Kalichman, Hendriks, Osiri, Brosseau, Reichenbach, Jüni Analysis and interpretation of data: Rutjes, Nüesch, Sterchi, Hendriks, Kalichman, Osiri, Brosseau, Reichenbach, Jüni Drafting of the manuscript: Rutjes Critical revision of the manuscript for important intellectual content: Rutjes, Nüesch, Sterchi, Hendriks, Kalichman, Reichenbach, Jüni Statistical analysis: Nüesch, Jüni, Rutjes Obtained funding: Reichenbach, Jüni
Dr Rutjes and Mrs Nüesch contributed equally to this article.
Sources of support
Internal sources
-
Institute of Social and Preventive Medicine, University or Bern, Switzerland.
Intramural grants
External sources
-
Swiss National Science Foundation, Switzerland.
National Research Program 53 on musculoskeletal health (grant numbers 4053‐40‐104762/3 and 3200‐066378)
Declarations of interest
None.
Edited (no change to conclusions)
References
References to studies included in this review
Adedoyin 2002 {published data only}
- Adedoyin RA, Olaogun MOB, Fagbeja OO. Effect of interferential current stimulation in management of osteo‐arthritic knee pain. Physiotherapy 2002;88(8):493‐9. [Google Scholar]
Adedoyin 2005 {published data only}
- Adedoyin RA, Olaogun MOB, Oyeyemi AL. Transcutaneous electrical nerve stimulation and interferential current combined with exercise for the treatment of knee osteoarthritis: a randomised controlled trial. Hong Kong Physiotherapy Journal 2005;23:13‐19. [Google Scholar]
Bal 2007 {published data only}
- Bal S, Turan Y, Grgan A. The effectiveness of transcutaneous electrical nerve stimulation in patients with knee osteoarthritis. Osteoporosis International 2005;16(suppl. 3):S94. [Expert contact] [Google Scholar]
- Bal S, Turan Y, Gurgan A. The effectiveness of transcutaneous electrical nerve stimulation in patients with knee osteoarthritis. Journal of Rheumatology and Medical Rehabilitation 2007;18(1):1‐5. [Google Scholar]
Cetin 2008 {published data only}
- Cetin N, Aytar A, Atalay A, Akman MN, Cetin Nuri, Aytar Aydan, et al. Comparing hot pack, short‐wave diathermy, ultrasound, and TENS on isokinetic strength, pain, and functional status of women with osteoarthritic knees: a single‐blind, randomized, controlled trial. American Journal of Physical Medicine & Rehabilitation 2008;87(6):443‐51. [DOI] [PubMed] [Google Scholar]
Cheing 2002 {published data only}
- Cheing GL, Hui‐Chan CW, Chan KM, Cheing Gladys LY, Hui‐Chan Christina WY, Chan KM. Does four weeks of TENS and/or isometric exercise produce cumulative reduction of osteoarthritic knee pain?. Clinical Rehabilitation 2002;16(7):749‐60. [DOI] [PubMed] [Google Scholar]
- Cheing GL, Hui‐Chan CW, Cheing Gladys LY, Hui‐Chan Christina WY. Would the addition of TENS to exercise training produce better physical performance outcomes in people with knee osteoarthritis than either intervention alone?. Clinical Rehabilitation 2004;18(5):487‐97. [DOI] [PubMed] [Google Scholar]
- Cheing GLY, Hui‐Chan CWY, Chan KM. Does four weeks of TENS and/or isometric exercise produce cumulative reduction of osteoarthritic knee pain?. Pain Reviews 2002;9(3/4):141‐51. [DOI] [PubMed] [Google Scholar]
Cheing 2003 {published data only}
- Cheing GL, Tsui AY, Lo SK, Hui‐Chan CW, Cheing Gladys LY, Tsui Amy YY, et al. Optimal stimulation duration of TENS in the management of osteoarthritic knee pain. Journal of Rehabilitation Medicine 2003;35(2):62–8. [DOI] [PubMed] [Google Scholar]
Defrin 2005 {published data only}
- Defrin R, Ariel E, Peretz C. Segmental noxious versus innocuous electrical stimulation for chronic pain relief and the effect of fading sensation during treatment. Pain 2005;115(1‐2):152–60. [DOI] [PubMed] [Google Scholar]
Fargas‐Babjak 1989 {published data only}
- Fargas‐Babjak A, Rooney P, Gerecz E, Fargas‐Babjak A, Rooney P, Gerecz E. Randomized trial of Codetron for pain control in osteoarthritis of the hip/knee. Clinical Journal of Pain 1989;5(2):137‐41. [DOI] [PubMed] [Google Scholar]
- Fargas‐Babjak AM, Pomeranz B. Acupuncture‐like stimulation with Codetron for rehabilitation of patients with chronic pain syndrome and osteoarthritis. Acupuncture & Electro‐Therapeutics Research 1992;17:95‐105. [DOI] [PubMed] [Google Scholar]
Garland 2007 {published data only}
- Garland D, Holt P, Harrington JT, Caldwell J, Zizic T, Cholewczynski J, et al. A 3‐month, randomized, double‐blind, placebo‐controlled study to evaluate the safety and efficacy of a highly optimized, capacitively coupled, pulsed electrical stimulator in patients with osteoarthritis of the knee. Osteoarthritis & Cartilage 2007;15(6):630‐7. [DOI] [PubMed] [Google Scholar]
Grimmer 1992 {published data only}
- Grimmer K. A controlled double blind study comparing the effects of strong burst mode TENS and high rate TENS on painful osteoarthritic knees. Australian Journal of Physiotherapy 1992;38(1):49‐56. [DOI] [PubMed] [Google Scholar]
Itoh 2008 {published data only}
- Itoh K, Hirota S, Katsumi Y, Ochi H, Kitakoji H. A pilot study on using acupuncture and transcutaneous electrical nerve stimulation (TENS) to treat knee osteoarthritis (OA). Chinesische Medizin 2008;3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Law 2004 {published data only}
- Law PP, Cheing GL. Optimal stimulation frequency of transcutaneous electrical nerve stimulation on people with knee osteoarthritis. Journal of Rehabilitation Medicine 2004;36(5):220–5. [DOI] [PubMed] [Google Scholar]
Law 2004a {published data only}
- Law PPW, Cheing GLY, Tsui AYY. Does transcutaneous electrical nerve stimulation improve the physical performance of people with knee osteoarthritis?. Journal of Clinical Rheumatology 2004;10(6):295–9. [DOI] [PubMed] [Google Scholar]
Ng 2003 {published data only}
- Ng MM, Leung MC, Poon DM, Ng MML, Leung Mason CP, Poon DMY. The effects of electro‐acupuncture and transcutaneous electrical nerve stimulation on patients with painful osteoarthritic knees: a randomized controlled trial with follow‐up evaluation. Journal of Alternative & Complementary Medicine 2003;9(5):641–9. [DOI] [PubMed] [Google Scholar]
Quirk 1985 {published data only}
- Quirk AS, Newman RJ, Newman KJ. An evaluation of interferential therapy, shortwave diathermy and exercise in the treatment of osteoarthrosis of the knee. Physiotherapy 1985;71:55‐7. [Google Scholar]
Smith 1983 {published data only}
- Smith CR, Lewith GT, Machin D, Smith CR, Lewith GT, Machin D. TNS and osteo‐arthritic pain. Preliminary study to establish a controlled method of assessing transcutaneous nerve stimulation as a treatment for the pain caused by osteo‐arthritis of the knee. Physiotherapy 1983;69(8):266‐8. [PubMed] [Google Scholar]
Yurtkuran 1999 {published data only}
- Yurtkuran M, Kocagil T, Yurtkuran M, Kocagil T. TENS, electroacupuncture and ice massage: comparison of treatment for osteoarthritis of the knee. American Journal of Acupuncture 1999;27(3‐4):133‐40. [PubMed] [Google Scholar]
Zizic 1995 {published data only}
- Zizic TM, Hoffman KC, Holt PA, Hungerford DS, O'Dell JR, Jacobs MA, et al. The treatment of osteoarthritis of the knee with pulsed electrical stimulation. Journal of Rheumatology 1995;22(9):1757‐61. [PubMed] [Google Scholar]
References to studies excluded from this review
Barr 2004 {published data only}
- Barr JO, Weissenbuehler SA, Cleary CK. Effectiveness and comfort of transcutaneous electrical nerve stimulation for older persons with chronic pain. Journal of Geriatric Physical Therapy 2004;27(3):93‐9. [Google Scholar]
Bernau 1981 {published data only}
- Bernau A, Kruppa G, Bernau A, Kruppa G. Low frequency electro‐stimulation and ultrasonic therapy (author's transl). Zeitschrift fur Orthopadie und Ihre Grenzgebiete 1981;119(1):126‐37. [DOI] [PubMed] [Google Scholar]
Burch 2008 {published data only}
- Burch FX, Tarro JN, Greenberg JJ, Carroll WJ. Evaluating the benefits of patterned stimulation in the treatment of osteoarthritis of the knee. A multi‐center, randomized, single‐blind, controlled study with an independent masked evaluator. Osteoarthritis & Cartilage 2008;16(8):865‐72. [DOI] [PubMed] [Google Scholar]
Cauthen 1975 {published data only}
- Cauthen J, Renner E. Transcutaneous and peripheral nerve stimulation for chronic pain states. Surgical Neurology 1975;4(1):102‐4. [PubMed] [Google Scholar]
Commandre 1977 {published data only}
- Commandre F, Guillemin R, Revelli G. Electrotherapy in osteoarticular inflammatory reactions. Electro Diagnostic Therapie 1977;14(2):37‐49. [PubMed] [Google Scholar]
Cottingham 1985a {published data only}
- Cottingham B, Phillips PD, Davies GK, Getty CJ, Cottingham B, Phillips PD, et al. The effect of subcutaneous nerve stimulation (SCNS) on pain associated with osteoarthritis of the hip. Pain 1985;22(3):243‐8. [DOI] [PubMed] [Google Scholar]
Cottingham 1985b {published data only}
- Cottingham B, Phillips PD, Davies GK, Getty CJM. The effects of peripheral electrical nerve stimulation on pain associated with osteoarthritis of the hip. American Journal of Surgery 1985;67‐B:152. [DOI] [PubMed] [Google Scholar]
Durmus 2005 {published data only}
- Durmus D, Alayli G, Canturk F. Effects of biofeedback assisted isometric exercise and electrical stimulation on pain, anxiety and depression scores in knee osteoarthritis. Turkiye Fiziksel Tip ve Rehabilitasyon Dergisi 2005;51(4):142‐5. [Google Scholar]
Gaines 2001 {published data only}
- Gaines JM. The effects of neuromuscular electrical stimulation on chronic knee pain and functional performance in older adults with osteoarthritis of the knee. PhD Thesis. Johns Hopkins University, 2001:269.
Gaines 2004 {published data only}
- Gaines JM, Metter EJ, Talbot LA. The effect of neuromuscular electrical stimulation on arthritis knee pain in older adults with osteoarthritis of the knee. Applied Nursing Research 2004;17(3):201‐6. [DOI] [PubMed] [Google Scholar]
Gibson 1989 {published data only}
- Gibson JN, Morrison WL, Scrimgeour CM, Smith K, Stoward PJ, Rennie MJ, et al. Effects of therapeutic percutaneous electrical stimulation of atrophic human quadriceps on muscle composition, protein synthesis and contractile properties. European Journal of Clinical Investigation 1989;19(2):206‐12. [DOI] [PubMed] [Google Scholar]
Godfrey 1979 {published data only}
- Godfrey CM, Jayawardena H, Quance TA, Welsh P. Comparison of electro‐stimulation and isometric exercise in strengthening the quadriceps muscle. Physiotherapy Canada 1979;31(5):265‐7. [Google Scholar]
Grigor'eva 1992 {published data only}
- Grigor'eva VD, Suzdal'nitskii DV, Strel'tsova EN, Nikolaeva TG, Grigor'eva VD, Suzdal'nitskii DV, et al. The effect of cryo‐ and cryoelectrotherapy on regional hemodynamics in coxarthrosis patients. Voprosy Kurortologii, Fizioterapii i Lechebnoi Fizicheskoi Kultury 1992;Sep‐Dec(5‐6):49‐54. [PubMed] [Google Scholar]
Guven 2003 {published data only}
- Guven Z, Coskun U, Gunduz O, Kaptan A. The effect of high voltage galvanic stimulation on quadriceps femoris muscle strength knee osteoarthritis. Journal of Rheumatology and Medical Rehabilitation 2003;14(2):72‐9. [Google Scholar]
Hamilton 1959 {published data only}
- Hamilton D, Bywaters E, Please N. A controlled trial of various forms of physiotherapy in arthritis. British Medical Journal 1959;1(5121):542‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2000 {published data only}
- Huang MH, Chen CH, Chen TW, Weng MC, Wang WT, Wang YL, et al. The effects of weight reduction on the rehabilitation of patients with knee osteoarthritis and obesity. Arthritis Care & Research 2000;13(6):398‐405. [DOI] [PubMed] [Google Scholar]
Jensen 1991 {published data only}
- Jensen H, Zesler R, Christensen T, Jensen H, Zesler R, Christensen T. Transcutaneous electrical nerve stimulation (TNS) for painful osteoarthrosis of the knee. International Journal of Rehabilitation Research 1991;14(4):356‐8. [DOI] [PubMed] [Google Scholar]
Kang 2007 {published data only}
- Kang RW, Lewis PB, Kramer A, Hayden JK, Cole BJ, Kang Richard W, et al. Prospective randomized single‐blinded controlled clinical trial of percutaneous neuromodulation pain therapy device versus sham for the osteoarthritic knee: a pilot study. Orthopedics 2007;30(6):439‐45. [DOI] [PubMed] [Google Scholar]
Katsnelson 2004 {published data only}
- Katsnelson Y, Khokhlov A, Tsvetkov V, Bartoo G, Bartoo M, Katsnelson Y, et al. Temporary pain relief using transcranial electrotherapy stimulation: results of a randomized, double‐blind pilot study. Conference Proceedings: Annual International Conference of the IEEE Engineering in Medicine & Biology Society 2004;6:4087‐90. [DOI] [PubMed] [Google Scholar]
Komarova 1998 {published data only}
- Komarova LA, Kir'ianova VV, Zabolotnykh II, Zabolotnykh VA, Komarova LA, Kir'ianova VV, et al. The use of transcranial electrotherapy in the rehabilitation of osteoarthrosis patients. Voprosy Kurortologii, Fizioterapii i Lechebnoi Fizicheskoi Kultury 1998;Sep‐Oct(5):27‐9. [PubMed] [Google Scholar]
Lewis 1984 {published data only}
- Lewis D, Lewis B, Sturrock RD, Lewis D, Lewis B, Sturrock RD. Transcutaneous electrical nerve stimulation in osteoarthrosis: a therapeutic alternative?. Annals of the Rheumatic Diseases 1984;43(1):47‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 1985 {published data only}
- Lewis B. Analgesic efficacy of transcutaneous electrical nerve stimulation compared with a non‐steroidal anti‐inflammatory drug in osteoarthritis [abstract]. Australian and New Zealand Journal of Medicine Suppl 1985;15:189. [Google Scholar]
Lewis 1988 {published data only}
- Lewis B, Lewis D, Cumming G. The analgesic efficacy of transcutaneous electrical nerve stimulation (TENS) compared with a non‐steroidal anti‐inflammatory drug (naprosyn) in painful osteoarthritis (OA) of the knee [abstract]. Australian and New Zealand Journal of Medicine Suppl 1988;18:224. [Google Scholar]
Lewis 1994 {published data only}
- Lewis B, Lewis D, Cumming G. The comparative analgesic efficacy of transcutaneous electrical nerve stimulation and a non‐steroidal anti‐inflammatory drug for painful osteoarthritis. British Journal of Rheumatology 1994;33(5):455‐60. [DOI] [PubMed] [Google Scholar]
Lone 2003 {published data only}
- Lone AR, Wafai ZA, Buth BA, Wani TA, Koul PA, Khan SH. Analgesic efficacy of transcutaneous electrical nerve stimulation compared with diclofenac sodium in osteo‐arthritis of the knee. Physiotherapy 2003;89(8):478‐85. [Google Scholar]
Lund 2005 {published data only}
- Lund I, Lundeberg T, Kowalski J, Sandberg L, Norrbrink Budh C, Svensson E. Evaluation of variations in sensory and pain threshold assessments by electrocutaneous stimulation. Physiotherapy Theory & Practice 2005;21(2):81‐92. [DOI] [PubMed] [Google Scholar]
Macchione 1995 {published data only}
- Macchione RA. Electrotherapeutic modalities: TENS. Chiropractic Journal 1995;9(7):18‐19. [Google Scholar]
Matti 1987 {published data only}
- Matti A, Felicetti G, Maini M, Zelaschi F. Low‐frequency muscular electrogymnastics; biologic action on parameters of muscle function [Italian]. Riabilitazione 1987;20(4):241‐9. [Google Scholar]
Miranda‐Filloy 2005 {published data only}
- Miranda‐Filloy JA, Barbazan Alvarez C, Monteagudo Sanchez B, Graña Gil J, Galdo Fernandez YF. Effect of transcutaneous electrical quadriceps muscle stimulation in knee osteoarthritis symptomatology [Spanish]. Rehabilitacion 2005;39(4):167‐70. [Google Scholar]
Mont 2006 {published data only}
- Mont MA, Hungerford DS, Caldwell JR, Ragland PS, Hoffman KC, He YD, et al. Pulsed electrical stimulation to defer TKA in patients with knee osteoarthritis. Orthopedics 2006;29(10):887‐92. [DOI] [PubMed] [Google Scholar]
Oldham 1995 {published data only}
- Oldham JA, Howe TE, Petterson T, Smith GP, Tallis RC. Electrotherapeutic rehabilitation of the quadriceps in elderly osteoarthritic patients: a double blind assessment of patterned neuromuscular stimulation. Clinical Rehabilitation 1995;9(1):10‐20. [Google Scholar]
Oldham 1997 {published data only}
- Oldham J, Howe T. The effectiveness of placebo muscle stimulation in quadriceps muscle rehabilitation: a preliminary evaluation... including commentary by Draper P. Clinical Effectiveness in Nursing 1997;1(1):25‐30. [Google Scholar]
Oosterhof 2008 {published data only}
- Oosterhof J, Samwel HJ, Boo TM, Wilder‐Smith OH, Oostendorp RA, Crul BJ, et al. Predicting outcome of TENS in chronic pain: a prospective, randomized, placebo controlled trial. Pain 2008;136(1‐2):11‐20. [DOI] [PubMed] [Google Scholar]
Paillard 2005 {published data only}
- Paillard T, Lafont C, Soulat JM, Montoya R, Costes‐Salon MC, Dupui P. Short‐term effects of electrical stimulation superimposed on muscular voluntary contraction in postural control in elderly women. Journal of Strength & Conditioning Research 2005;19(3):640‐6. [DOI] [PubMed] [Google Scholar]
Picaza 1975 {published data only}
- Picaza J, Cannon B, Hunter S. Pain suppression by peripheral nerve stimulation. Part I. Observations with transcutaneous stimuli. Surgical Neurology 1975;4(1):105‐14. [PubMed] [Google Scholar]
Salaj 2001 {published data only}
- Salaj R. Osteoarthritis and its treatment in the sanatorium for chronic diseases in Hostinne. Rehabilitace a Fyzikalni Lekarstvi 2001;8(3):115‐18. [Google Scholar]
Salim 1996 {published data only}
- Salim M. Transcutaneous electrical nerve stimulation (TENS) in chronic pain. Alternative Therapies in Clinical Practice 1996;3(4):33‐5. [Google Scholar]
Sluka 1998 {published data only}
- Sluka K, Bailey K, Bogush J, Olson R, Ricketts A. Treatment with either high or low frequency TENS reduces the secondary hyperalgesia observed after injection of kaolin and carrageenan into the knee joint. Pain 1998;77(1):97‐102. [DOI] [PubMed] [Google Scholar]
Sok 2007 {published data only}
- Sok SR, Kim KB, Sok Sohyune R, Kim Kwuy Bun. Effects of muscle electric stimulation on chronic knee pain, activities of daily living, and living satisfaction for Korean elderly women. Daehan Ganho Haghoeji 2007;37(3):305‐12. [DOI] [PubMed] [Google Scholar]
Svarcova 1988a {published data only}
- Svarcova J, Zvarova J, Kouba A, Trnavsky K. Does physiotherapy affect the pain in activated arthrosis? [Beeinflusst Physiotherapie den Schmerz bei aktivierter Arthrose?]. Zeitschrift für Physiotherapie 1988;40:333‐6. [Google Scholar]
Svarcova 1988b {published data only}
- Svarcova J, Trnavsky K, Zvarova J, Svarcova J, Trnavsky K, Zvarova J. The influence of ultrasound, galvanic currents and shortwave diathermy on pain intensity in patients with osteoarthritis. Scandinavian Journal of Rheumatology ‐ Supplement 1988;67:83‐5. [DOI] [PubMed] [Google Scholar]
Svarcova 1990 {published data only}
- Svarcova J, Zvarova J, Pichova A, Kouba A, Simacek K, Uhlemann C, et al. Comparison of the analgesic effects of electroacupuncture and of galvanic current in patients with activated osteoarthrosis (a controlled clinical study) [German]. Zeitschrift fur Physiotherapie 1990;42(6):375‐8. [Google Scholar]
Talbot 2003 {published data only}
- Talbot LA, Gaines JM, Ling SM, Metter EJ. A home‐based protocol of electrical muscle stimulation for quadriceps muscle strength in older adults with osteoarthritis of the knee. Journal of Rheumatology 2003;30(7):1571‐8. [PubMed] [Google Scholar]
Tam 2004 {published data only}
- Tam S, Cheing GLY, Hui‐Chan CWY. Predicting osteoarthritic knee rehabilitation outcome by using a prediction model developed by data mining techniques. International Journal of Rehabilitation Research 2004;27(1):65‐9. [DOI] [PubMed] [Google Scholar]
Taylor 1981 {published data only}
- Taylor P, Hallett M, Flaherty L, Taylor P, Hallett M, Flaherty L. Treatment of osteoarthritis of the knee with transcutaneous electrical nerve stimulation. Pain 1981;11(2):233‐40. [DOI] [PubMed] [Google Scholar]
Tulgar 1991 {published data only}
- Tulgar M, McGlone F, Bowsher D, Miles J. Comparative effectiveness of different stimulation modes in relieving pain. Part II. A double‐blind controlled long‐term clinical trial. Pain 1991;47(2):157‐62. [DOI] [PubMed] [Google Scholar]
Volklein 1990 {published data only}
- Volklein R, Callies R. Changes in pain by different types of diadynamic current in gonarthrosis and lumbar syndrome. Zeitschrift fur Physiotherapie 1990;42(2):113‐18. [Google Scholar]
Weiner 2007 {published data only}
- Weiner DK, Rudy TE, Morone N, Glick R, Kwoh CK. Efficacy of periosteal stimulation therapy for the treatment of osteoarthritis‐associated chronic knee pain: an initial controlled clinical trial. Journal of the American Geriatrics Society 2007;55(10):1541‐7. [DOI] [PubMed] [Google Scholar]
Zivkovic 2005 {published data only}
- Zivkovic. Different physiotherapy programs for patients with knee osteoarthritis. EULAR. 2005.
References to ongoing studies
Fary 2008 {published data only}
- Fary RE, Carroll GJ, Briffa TG, Gupta R, Briffa NK, Fary Robyn E, et al. The effectiveness of pulsed electrical stimulation (E‐PES) in the management of osteoarthritis of the knee: a protocol for a randomised controlled trial. BMC Musculoskeletal Disorders 2008;9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Palmer 2007 {published data only}
- Palmer S. Effects of transcutaneous electrical nerve stimulation (TENS) and exercise on knee osteoarthritis (OA): a randomised controlled trial. ISTRCTN 2007.
Additional references
Altman 1996
- Altman R, Brandt K, Hochberg M, Moskowitz R, Bellamy N, Bloch DA, et al. Design and conduct of clinical trials in patients with osteoarthritis: recommendations from a task force of the Osteoarthritis Research Society. Results from a workshop. Osteoarthritis Cartilage 1996;4(4):217‐43. [DOI] [PubMed] [Google Scholar]
Andersson 1976
- Andersson SA, Hansson G, Holmgren E, Renberg O. Evaluation of the pain suppression effect of different frequencies of peripheral electrical stimulation in chronic pain conditions. Acta Orthopaedica Scandinavia 1976;47:149‐57. [DOI] [PubMed] [Google Scholar]
Bellamy 1995
- Bellamy N. Outcome measurement in osteoarthritis clinical trials. Journal of Rheumatology 1995;22(Suppl. 43):49‐51. [PubMed] [Google Scholar]
Bjordal 2007
- Bjordal JM, Johnson MI, Lopes‐Martins RA, Bogen B, Chow R, Ljunggren AE. Short‐term efficacy of physical interventions in osteoarthritic knee pain. A systematic review and meta‐analysis of randomised placebo‐controlled trials. BMC Musculoskeletal Disorders 2007;8:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brosseau 2004
- Brosseau L, Yonge K, Marchand S, Robinson V, Osiri M, Wells G, et al. Efficacy of transcutaneous electrical nerve stimulation for osteoarthritis of the lower extremities: a meta‐analysis. Physical Therapy Reviews 2004;9:213‐33. [Google Scholar]
Carroll 2001
- Carroll D, Moore RA, McQuay HJ, Fairman F, Tramer M, Leijon G. Transcutaneous electrical nerve stimulation (TENS) for chronic pain. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD003222] [DOI] [PubMed] [Google Scholar]
Chinn 2000
- Chinn S. A simple method for converting an odds ratio to effect size for use in meta‐analysis. Statistics in Medicine 2000;19(22):3127‐31. [DOI] [PubMed] [Google Scholar]
Clegg 2006
- Clegg DO, Reda DJ, Harris CL, Klein MA, O'Dell JR, Hooper MM, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. New England Journal of Medicine 2006;354(8):795‐808. [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd Edition. Hillsdale, NJ: Lawrence Earlbaum Associates, 1988. [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Deyo 1990
- Deyo RA, Wash NE, Schoenfeld LS, Ramamurthy S. Can trials of physical treatments be blinded: the example of transcutaneous electrical nerve stimulation for chronic pain. American Journal of Physical Medicine and Rehabilitation 1990;69:6‐10. [DOI] [PubMed] [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309(6964):1286‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gezondheidsraad 1999
- Gezondheidsraad. Efficacy of physical therapy: electrostimulation, laser therapy, ultrasound therapy (own translation) [De effectiviteit van fysische therapie: electrotherapie, lasertherapie, ultrageluidbehandeling]. Den Haag: Gezondheidsraad 1999.
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haddad 2007
- Haddad JB, Obolensky AG, Shinnick P. The biologic effects and the therapeutic mechanism of action of electric and electromagnetic field stimulation on bone and cartilage: new findings and a review of earlier work. Journal of Alternative and Complementary Medicine (New York, N.Y.) 2007;13(5):485‐90. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org.
Hulme 2002
- Hulme J, Robinson V, DeBie R, Wells G, Judd M, Tugwell P. Electromagnetic fields for the treatment of osteoarthritis. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD003523] [DOI] [PubMed] [Google Scholar]
Jamtvedt 2008
- Jamtvedt G, Dahm KT, Christie A, Moe RH, Haavardsholm E, Holm I, et al. Physical therapy interventions for patients with osteoarthritis of the knee: an overview of systematic reviews. Physical Therapy 2008;88(1):123‐36. [DOI] [PubMed] [Google Scholar]
Jüni 2001
- Jüni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ (Clinical Research Ed.) 2001;323(7303):42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jüni 2006
- Jüni P, Reichenbach S, Dieppe P. Osteoarthritis: rational approach to treating the individual. Best Practice & Research. Clinical Rheumatology 2006;20(4):721‐40. [DOI] [PubMed] [Google Scholar]
Mayer 1989
- Mayer DJ, Prince DD. The neurobiology of pain. In: Snyder‐Mackper L, Robinson A editor(s). Clinical Electrophysiology, Electrotherapy and Electrophysiologic Testing. 1st Edition. Baltimore, MD: Williams & WIlkins, 1989:141‐201. [Google Scholar]
Melzack 1965
- Melzack R, Wall P. Pain mechanisms: a new theory. Science 1965;150:971‐7. [DOI] [PubMed] [Google Scholar]
Nuesch 2009
- Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Bürgi E, Scherer M, et al. The effects of the exclusion of patients from the analysis in randomised controlled trials: meta‐epidemiological study. BMJ (in press). [DOI] [PMC free article] [PubMed]
Nuesch 2009a
- Nüesch E, Juni P. Commentary: Which meta‐analyses are conclusive?. International Journal of Epidemiology 2009;38(1):298‐303. [DOI] [PubMed] [Google Scholar]
Pham 2004
- Pham T, Heijde D, Altman RD, Anderson JJ, Bellamy N, Hochberg M, et al. OMERACT‐OARSI initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthritis Cartilage 2004;12(5):389‐99. [DOI] [PubMed] [Google Scholar]
Regence Medical Policy 2009
- Regence Medical Policy. Durable medical equipment section. Electrical stimulation for the treatment of arthritis. Available from: http://blue.regence.com/trgmedpol/dme/dme70.html issue Accessed 14 July 2009.
Reichenbach 2007
- Reichenbach S, Sterchi R, Scherer M, Trelle S, Burgi E, Burgi U, et al. Meta‐analysis: chondroitin for osteoarthritis of the knee or hip. Annals of Internal Medicine 2007;146(8):580‐90. [DOI] [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration, 2008.
Rucker 2008
- Rucker G, Schwarzer G, Carpenter JR, Schumacher M. Undue reliance on I2 in assessing heterogeneity may mislead. BMC Medical Research Methodology 2008;8(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shang 2005
- Shang A, Huwiler‐Muntener K, Nartey L, Juni P, Dorig S, Sterne JA, et al. Are the clinical effects of homoeopathy placebo effects? Comparative study of placebo‐controlled trials of homoeopathy and allopathy. Lancet 2005;366(9487):726‐32. [DOI] [PubMed] [Google Scholar]
Sluka 2003
- Sluka KA, Walsh D. Transcutaneous electrical nerve stimulation: basic science mechanisms and clinical effectiveness. The Journal of Pain 2003;4(3):109‐21. [DOI] [PubMed] [Google Scholar]
Solomon 1997
- Solomon L. Clinical features of osteoarthritis. In: Kelly WN, Harris ED Jr, Ruddy S, Sledge CB editor(s). Textbook of Rheumatology. 5th Edition. Vol. 2, Philadelphia: WB Saunders, 1997:1383‐93. [Google Scholar]
Sterne 2000
- Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta‐analysis: power of statistical tests and prevalence in the literature. Journal of Clinical Epidemiology 2000;53(11):1119‐29. [DOI] [PubMed] [Google Scholar]
Sterne 2001
- Sterne JA, Egger M. Funnel plots for detecting bias in meta‐analysis: guidelines on choice of axis. Journal of Clinical Epidemiology 2001;54(10):1046‐55. [DOI] [PubMed] [Google Scholar]
Thompson 1999
- Thompson SG, Sharp SJ. Explaining heterogeneity in meta‐analysis: a comparison of methods. Statistics in Medicine 1999;18(20):2693‐708. [DOI] [PubMed] [Google Scholar]
Wadsworth 1980
- Wadsworth H, Chanmugan A. Electrophysical agents in physiotherapy. Marrickville, Australia: Science Press, 1980:347‐72. [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Osiri 2000
- Osiri M, Welch V, Brosseau L, Shea B, McGowan J, Tugwell P, et al. Transcutaneous electrical nerve stimulation for knee osteoarthritis. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD002823] [DOI] [PubMed] [Google Scholar]


