Abstract
The neurotropic rabies virus (RABV) is a prototype member of the Mononegavirales order of viruses and is the most significant human pathogen of the Rhabdoviridae family. A reverse genetics system for RABV was established almost 20 years ago, providing a paradigm for other Mononegavirales members as well. The availability of engineered recombinant viruses opened a new era to study common aspects of Mononegavirales biology and specific aspects of the unique lifestyle and pathogenesis of individual members. Above all, the knowledge gained has allowed engineering of beneficial biomedical tools such as viral vectors, vaccines, and tracers. In this chapter, the development of the classical rabies virus reverse genetics approach is described, and some of the most exciting biomedical applications for recombinant RABV and other Mononegavirales are briefly addressed.
Keywords: Newcastle Disease Virus, Rabies Virus, Vesicular Stomatitis Virus, Reverse Genetic, Hepatitis Delta Virus
Mononegavirales: A Huge Diversity of Similar Viruses
The order Mononegavirales, or nonsegmented negative strand RNA viruses (NNSV), comprises the families of Rhabdoviridae, Paramyxoviridae, Filoviridae, and Bornaviridae. Members of the Rhabdoviridae family have the broadest host range among the Mononegavirales and infect plants, insects, fish, aquatic, aerial, and terrestrial animals, and humans (for reviews, see Fu 2005; Pringle 2005). Remarkably, the only globally important human pathogen among rhabdoviruses is the rabies virus (RABV) of the genus Lyssavirus, which causes rabies encephalitis, a long-known, most dangerous and feared zoonotic disease. In spite of the availability of potent vaccines for animals and humans and effective postexposure treatments, rabies still causes more than 50,000 human deaths per year in rural areas of Asia and Africa, where it is mostly transmitted by rabid dogs. RABV is a typical member of the Mononegavirales in terms of virus organization and gene expression, and in this respect is closely related to the insect-transmitted vesicular stomatitis virus (VSV), a well-studied prototype rhabdovirus of the Vesiculovirus genus. However, the biology of RABV is unique in terms of direct transmission between mammals, a strict neurotropism, and an extremely broad host species range. These features are key to rabies biology, transmission, and pathogenesis, and represent severe obstacles in terms of RABV eradication.
In contrast to the Rhabdoviridae, numerous members of the Paramyxoviridae family have evolved to become human pathogens, such as the measles, mumps, parainfluenza, respiratory syncytial, Hendra, and Nipah viruses. An extremely valuable model paramyxovirus has always been Sendai virus (murine parainfluenza virus type 1; hemagglutinating virus of Japan, HVJ), the study of which has uncovered numerous traits of paramyxovirus biology (Nagai et al. 2011), and the use of which as a biomedical tool is highly promising (see other chapters in this volume). Filoviruses such as Ebola and Marburg virus and Bornaviruses are animal viruses, which have not (yet) managed to become established in the human population (Lamb 2007).
As different as individual members, genera, or families of Mononegavirales may be in terms of shape, biology, and pathogenesis, they all are but variations of a common theme of viral genome and particle organization and mode of gene expression. The single 10- to 20-kb RNA genomes and antigenomes of Mononegavirales exist in the form of a permanent helical ribonucleoprotein (RNP), in which the RNA is enclosed in a nucleoprotein (N, or NP). The information encoded is expressed by sequential and polar transcription of discrete subgenomic mRNAs from the RNPs (Fig. 1.1) (for detailed review, see Whelan et al. 2004). The polymerase seems to be an RNP-dependent RNP polymerase for replication of RNPs and an RNP-dependent RNA polymerase for transcription of subgenomic mRNAs. It is composed of a large (L) multifunctional catalytic protein and a noncatalytic cofactor, mostly named phosphoprotein (P). The existence of such P protein distinguishes Mononegavirales from related segmented NSV such as bunyaviruses and arenaviruses. The most highly conserved N and L proteins of the Mononegavirales families still share common sequence blocks, whereas P proteins are much more variable. In addition to N, P, and L, Mononegavirales encode at least one matrix protein (M) and at least one transmembrane glycoprotein (e.g., G, GP, F), which make up the viral envelope, and which are predominantly engaged in formation of RNP-containing virions and infection of new target cells, respectively. Notably, the order of these five minimal genes (3′-N-P-M-G-L-5′) is strictly conserved in all Mononegavirales genomes (Fig. 1.1). This conservation is thought to reflect the stoichiometric need of the individual proteins, which is brought about through sequential and polar transcription. The modular organization of Mononegavirales genomes facilitates the acquisition of additional genes, mostly to internal positions, to specifically adapt to novel hosts and environments. In addition, individual genes of Mononegavirales may encode multiple gene products, as illustrated by the accessory proteins expressed from the paramyxovirus “P” gene (Sakaguchi et al. 2008). The versatility of the common NNSV blueprint is illustrated by the fact that Mononegavirales have conquered different kingdoms of life, including plants and animals, as well as numerous niches within a single species.
Fig. 1.1.
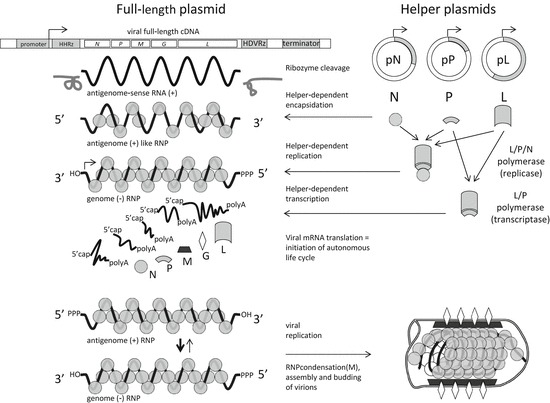
Recovery of Mononegavirales from plasmids. The standard protocol for recovery of Mononegavirales from cDNA involves simultaneous transfection of at least four plasmids into a cell, one comprising the full-length cDNA of the virus and three expression plasmids encoding helper virus proteins N, P, and L. The viral cDNA is oriented to give rise to a positive strand (antigenomic) RNA and is flanked by autocatalytic ribozyme sequences to produce the correct viral 5′- and 3′-ends upon transcription of the RNA by T7 RNA polymerase (encoded in transgenic cells, or from transfected plasmids) or by an endogenous cellular polymerase such as polymerase II. The virus-like RNA must be encapsulated into N protein to mimic a ribonucleoprotein (RNP) and to be recognized by the viral polymerase, which can act as a replicase (L–P–N complex) producing complementary RNPs, or as a transcriptase (L–P complex), producing nonencapsidated subgenomic mRNAs. Because the viral N, P, M, G, and L mRNAs are produced from the genome RNP, an autonomous infection cycle is initiated. Viral replication gives rise to an excess of genome RNPs, which can be used for assembly of novel virions
Synthetic Biology or “Reverse Genetics” of Viruses
Viruses and phages can be regarded as highly mobile genetic elements that need only a cell to translate the encoded information into protein. In the past, they have played a major role both as research objects and as tools in the development of modern gene technology. They are also at the forefront of synthetic biology. In fact, introducing authentic or modified nucleic acids or cDNA of viruses into a cell in many cases leads to initiation of an infectious cycle and ultimately in the creation of viruses never existing previously in nature. This technique was initially found to work for DNA viruses such as SV40 (Goff and Berg 1976), retroviruses (Rothenberg et al. 1977), and RNA phages (Taniguchi et al. 1978), and for RNA viruses such as poliovirus (Racaniello and Baltimore 1981), now known as “positive” strand RNA viruses. Importantly, the RNA of the positive strand RNA viruses, or the RNA derived from the introduced cDNA, can be immediately used for translation of all virus proteins. The negative strand RNA virus particles, however, were found to comprise RNA complementary to mRNA, and a specific polymerase (which is not present in cells) to transcribe individual subgenomic mRNAs upon entry into cells (Baltimore et al. 1970; Baltimore 1971). Obviously, the cDNA or cDNA-derived RNAs of Mononegavirales are not infectious because they are not suitable for translation of the necessary viral proteins by the cellular machinery. It was therefore noted by Racaniello and Baltimore (Racaniello and Baltimore 1981) that “It is uncertain whether the application of recombinant DNA techniques to negative-strand RNA viruses (5) [referring to Baltimore 1971] or viruses with multiple genome RNA’s will also yield infectious cDNA.” The term “reversed genetics” for this approach was introduced by Charles Weissmann and colleagues, describing methods for generating point mutations at predetermined sites of RNA or DNA genomes, specifically with Qbeta phage RNA as a template (Weissmann et al. 1979). Fortunately, in spite of inherent drawbacks, reversed genetics has become available for negative strand RNA viruses as well, including members of all Mononegavirales virus families, the Rhabdoviridae, Paramyxoviridae, Filoviridae, and Bornaviridae.
Recovery of Mononegavirales from cDNA
Rescue and Use of Model Minigenomes
Successful rescue of viruses from recombinant RNA relies on the expression of viral proteins from the viral RNA. In NNSV, expression of the encoded proteins is achieved by sequential transcription of individual mRNAs from the negative strand RNP. The proposed stop–restart model suggests entry of the polymerase exclusively at the 3′-end of the genomic (negative strand) RNP and the sequential transcription of a short nonmodified leader RNA specified by the 3′-end of the genome and of 5′-capped and 3′-polyadenylated mRNAs. The ends of the subgenomic RNAs are specified by conserved transcription stop/polyadenylation and restart signals at the gene borders. New RNPs are only formed upon accumulation of sufficient N protein, which apparently switches the transcriptase form of the polymerase to a replicase form of the polymerase. In this “replication” mode of the polymerase, synthesis of RNA and encapsidation into N protein is mechanistically linked, and the transcription signals are ignored.
The expression of viral proteins such as the polymerase components L and P is straightforward by using so-called “helper” plasmids or virus vectors providing the proteins in trans (Fig. 1.1). In contrast, the encapsidation of viral RNA by N protein, that is, the reconstitution of a functional RNP from premade RNA and N protein, also called “illegitimate encapsidation,” turned out to be more difficult. Such artificial RNP must be able to serve as a template for the polymerase. Early experimental work mostly involved short defective interfering (DI) RNAs, or minigenomes comprising only the noncoding terminal promoter regions of Mononegavirales genomes. Initial encouraging success was obtained with the segmented influenza virus by transfection of an in vitro packaged genome segment (Luytjes et al. 1989), and for the Mononegavirales with Sendai virus (Park et al. 1991) by transfection of an in vitro-transcribed minigenome RNA into Sendai helper virus-infected cells, which resulted in expression of the minigenome-encoded reporter gene.
A major breakthrough was then the establishment of helper virus-free systems, in which DI- or minigenome RNAs and virus proteins were simultaneously expressed within cells from transfected circular plasmids (Pattnaik et al. 1992; Pattnaik and Wertz 1990, 1991). The system employed vaccinia virus (vv)-encoded phage T7 RNA polymerase and plasmids equipped with T7 RNA polymerase promoter and terminator sequences (Fuerst et al. 1986). The critical ends of the model genome RNAs were generated by self-cleaving ribozymes (Fig. 1.1), such as the antigenome ribozyme of hepatitis delta virus (HDVagrz) (Perrotta and Been 1990; Sharmeen et al. 1988). The vv/T7 protein and RNA expression system paved the way for similar progress with minus strand RNA minigenomes of all families of the Mononegavirales. This achievement provided immediate experimental access to the clarification of fundamental and longstanding questions in the Mononegavirales field, including the identity and function of cis-acting signal sequences such as terminal promoters and transcription signals, or other RNA-based regulatory mechanisms, just to mention the rule of six (Calain and Roux 1993; Vulliemoz and Roux 2001), as well as the contribution of individual proteins to gene expression and replication. Moreover, minigenome systems are valuable tools for high-throughput screening of antivirals and provide a safe possibility to study aspects of highly dangerous viruses (Biacchesi 2011; Conzelmann 2004; Marriott and Easton 1999; Theriault et al. 2005; Whelan et al. 2004).
Rescue of Recombinant Viruses from cDNA
Although minigenome recovery in the described system worked reliably for many virus species, the desired generation of recombinant nondeficient viruses using the same approaches seemed to be out of reach for some time. Finally, an approach in which positive sense antigenome RNA rather than genome sense RNA of RABV was employed for initial encapsidation resulted in the successful recovery of the first negative strand RNA virus entirely from cDNA in our laboratory (Schnell et al. 1994). In fact, this seemingly counterintuitive “positive approach” circumvents lethal common problems associated with genome sense RNA expression and encapsidation and was immediately applicable for recovery of other Mononegavirales as well, including rinderpest virus, respiratory syncytial virus, parainfluenza virus type 3, Sendai virus, SV5 or parainfluenza virus type 5, measles virus, VSV (Baron and Barrett 1997; Collins et al. 1995; Durbin et al. 1997; Garcin et al. 1995; He et al. 1997; Hoffman and Banerjee 1997; Kato et al. 1996; Lawson et al. 1995; Radecke et al. 1995; Whelan et al. 1995), and even of segmented NSV such as Bunyamwera virus (Bridgen and Elliott 1996). Although initial encapsidation of antigenome RNA requires that an extra step of replication is supported by the coexpressed N, P, and L helper proteins (see Fig. 1.1), it avoids deleterious hybridization of viral genome RNA and the complementary helper protein mRNAs expressed simultaneously in the same cell (Conzelmann 1996; Roberts and Rose 1998; Schnell et al. 1994). The resulting dsRNA is assumed not only to interfere with RNA encapsidation and helper protein translation but also to trigger innate antiviral immunity (Randall and Goodbourn 2008; Rieder and Conzelmann 2011). Although most laboratories confirmed a failure of Mononegavirales genome sense RNA rescue, in two highly optimized systems viruses could be rescued from both genome and antigenome RNAs, namely Sendai virus (Kato et al. 1996) and HPIV-3 (Durbin et al. 1997). In the former work, the magnitude of the antisense problem was nicely illustrated, as rescue efficiency with Sendai virus negative strand RNA was at least 100 fold less effective than with positive strand RNA (Kato et al. 1996).
Technical Improvement of Virus Rescue Systems
The principle of the “classical” vaccinia virus/T7 virus rescue system turned out to work for viruses of all Mononegavirales families, including nonmammalian species such as fish rhabdoviruses (Biacchesi 2011). Variations mostly included the use of other sources of T7 RNA polymerase, such as the host range-restricted vaccinia virus MVA-T7, or other poxvirus species, alleviating the need for active removal of the T7-encoding helper viruses just by passaging the recoveries in nonpermissive cells (Conzelmann 2004). In addition, helper virus-free systems have been developed, based on transient expression of T7 RNA polymerase from plasmids or in stable cell lines. Particularly, BSR T7/5 cells (Buchholz et al. 1999) are being widely used, as this cell clone combines the advantage of high-level T7 RNA polymerase expression, and of having a defect in the IRF3 gene, such that the antiviral interferon response is not induced (Conzelmann 2004). In the absence of vaccinia virus capping and polyadenylation enzymes, T7 RNA polymerase transcripts have 5′-triphosphate ends, which is advantageous for generating virus-like antigenomes, but detrimental for helper protein translation. Therefore, T7 protein expression constructs should include an upstream IRES element, or helper proteins should be expressed from polymerase II promoter-driven plasmids.
Of particular importance for rescue of Mononegavirales are the ends of the RNA to be packaged, as already noticed in many minigenome systems. Particularly, a precise 3′-end was found critical for initial recognition by the viral polymerase and replication, whereas some extra nonviral nucleotides at the 5′-end were well tolerated (Pattnaik et al. 1992). Therefore, in most Mononegavirales rescue systems T7 promoter transcripts have been employed that comprise three extra G residues to facilitate transcription initiation by T7 RNA polymerase (Conzelmann and Schnell 1994; Pattnaik et al. 1992). However, precise 5′-ends of the transcripts do more than compensate for lower transcript levels, as illustrated by highly efficient rescue of Sendai virus RNA lacking extra G residues (Kato et al. 1996). More recently, hammerhead ribozymes (Blount and Uhlenbeck 2002) are being increasingly employed for generation of exact 5′-ends of transcripts (Fig. 1.1). A direct comparison of RABV strain SAD L16 cDNA constructs yielding transcripts comprising three extra G residues or possessing correct 5′-ends generated by a hammerhead ribozyme revealed a tenfold advantage of the latter (Ghanem et al. 2012). In this work it was also noticed that the cleavage efficiency of the HDV agrz used so far in almost all rescue systems is very low in transfected cells, and that only 10 % of the RNA has the right 3′-end available for initiation of replication. Exchange with a longer and more effective HDV ribozyme (SC) cleaving 90 % of intracellular transcripts again increased rescue efficiency more than tenfold. Moreover, the combination of hammerhead and SC HDVagrz in full-length clones of the RV SAD L16 (pSAD HH-L16-SC) had a synergistic effect and improved rescue by 100 fold, yielding the most efficient RABV rescue plasmid so far (Ghanem et al. 2012).
The use of polymerases such as T7 RNA polymerase has the advantage of allowing transcription of transfected plasmids in the cytoplasm and therefore is ideal for the generation of viruses with a cytoplasmic replication cycle. Specifically, potential problems with nuclear export of RNA transcripts synthesized by cellular polymerases such as RNA Pol II are circumvented. Nevertheless, CMV promoter-driven rescue has been achieved for several cytoplasmic mammalian and fish rhabdoviruses (Ammayappan et al. 2010; Biacchesi 2011; Huang et al. 2010; Inoue et al. 2003; Ming et al. 2009; Orbanz and Finke 2010; Tao et al. 2010), as well as paramyxoviruses (Martin et al. 2006), suggesting the possibility to conditionally express viruses from host cell genome-encoded DNA in cultured cells or in animals.
Genetic Engineering of Mononegavirales Genomes
Even the highly optimized Mononegavirales rescue systems remain less effective by orders of magnitude than positive strand RNA rescue. The major bottleneck is most probably a very poor rate of illegitimate RNA encapsidation by the N protein. Although this precludes a few applications, such as the use of Mononegavirales as cloning vectors for genetic libraries, it is sufficient for rescue of individual viruses, even severely attenuated viruses or gene-defective viruses, which require additional complementation in trans. Nonetheless, the possibilities of genetic manipulation of individual Mononegavirales as well as the applications are enormous as a result of their particular genome organization, lack of packaging size, the stability of their RNA genomes, and the lack of recombination with host sequences.
Mutation of Virus Functions: Host Immune Escape
In the past two decades the possibility of site-directed mutagenesis of Mononegavirales has brought about hundreds of publications and a wealth of information on the structure, function, biology, virus–host interplay, and pathogenesis of individual Mononegavirales. One recent common topic is the interplay of viruses with the host innate immune system. This field has been fueled by tremendous progress in the identification of pattern recognition receptors (PRR) and details of the signaling pathways leading to the expression of type I and III interferons (IFN) and of proinflammatory cytokines.
IFN and proinflammatory cytokines are activated by two PRR families recognizing non-self viral RNA, the endosomal transmembrane Toll-like receptors (TLR 3, 7/8) and the cytoplasmic (RIG-I)-like helicases RIG-I (retinoic acid inducible gene-I, also known as DDX58) and MDA5 (melanoma differentiation-associated gene 5, also known as IFIH1 or helicard) (Kato et al. 2011; Kawasaki et al. 2011). The pathways for IFN induction merge in the activation of interferon regulatory factors (IRF) 3 and IRF7, which control transcription of type I and III IFN genes (Honda et al. 2006; Onoguchi et al. 2007; Yoneyama et al. 1998). The pathways for induction of proinflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukins lead to canonical activation of NF-κB, which also supports transcription of the early IFNs (IFN-β, IFN-α4).
It turns out that Mononegavirales infection of cells is generally and effectively sensed by the ubiquitous RIG-I, which recognizes viral triphosphate RNAs (Cui et al. 2008; Hornung et al. 2006), that is, the leader RNA and possibly non-encapsidated genome RNAs of Mononegavirales (Gerlier and Lyles 2011). Specific dsRNA patterns recognized by MDA5 remain elusive, and the usually minor contribution of MDA5 may differ for both virus species and host cell types (Bruns and Horvath 2012; Loo and Gale 2011). RIG-I or MDA5 activation by viral RNAs leads to their association with the mitochondrial protein IPS-1 (also known as MAVS, VISA, or Cardif), via CARD domains, and to the recruitment of a signaling complex in which IRF3 is phosphorylated by the kinases TBK-1 and IKKi (also known as IKKε) (Fitzgerald et al. 2003; Sharma et al. 2003). Ser386-phosphorylated IRF3 dimerizes and is imported into the nucleus where it drives transcription of IFN-β mRNA in conjunction with NF-κB and AP1 transcription factors (Ford and Thanos 2010). The secreted IFN acts on cells by activating JAK/STAT signaling pathways (Platanias 2005), which activate hundreds of IFN-stimulated genes (ISG), many of which have antiviral and immunomodulatory activities (Schoggins et al. 2011; Theofilopoulos et al. 2005).
Notably, even viruses with a very small coding capacity, such as the Mononegavirales, have evolved means to interfere with both IFN induction and IFN-mediated response and with ISG function, illustrating the power of the IFN system and the high evolutionary pressure for viruses to evolve suitable countermeasures (Gerlier and Lyles 2011; Goodbourn and Randall 2009; Randall and Goodbourn 2008; Versteeg and Garcia-Sastre 2010). Proper encapsidation of the viral RNA by N proteins into RNPs may be a common advantageous trait of Mononegavirales and largely prevent recognition by PRRs, but this requires a well-balanced synthesis of RNA and protein. If this delicate balance is disturbed, possibly in cell types not supporting RNA and protein synthesis equally well, or by overproduced DI RNAs, virus infection is easily recognized. In addition to shielding viral RNA, Mononegavirales have developed means to actively interfere with signal transduction. In mammalian Mononegavirales, these IFN antagonists are typically encoded by the P genes.
The RABV P protein, in addition to its functions as an N chaperone and polymerase cofactor, was found to have important roles in counteracting multiple specific steps of IFN gene expression, IFN-induced STAT signaling, and the function of antiviral proteins (Rieder and Conzelmann 2011). Specifically, RABV P targets the activation of the transcription factors IRF3 and IRF7 and prevents their phosphorylation by the kinases TBK-1 and IKKi. The IRFs are therefore not able to dimerize, and their import into the nucleus and transcription of the IFN genes is precluded (Brzózka et al. 2005). In addition, STAT-mediated transcription of ISGs is precluded in the presence of RABV P. After binding of IFN to the IFN receptor, STAT1 and STAT2 are phosphorylated at specific tyrosine residues by receptor-associated Janus kinases, which is a prerequisite for hetero-dimerization, association with IRF9, and nuclear import of the STATs (Platanias 2005). Most remarkably, RABV P binds exclusively to tyrosine-phosphorylated STAT1 and STAT2, whereas in nonactivated cells an association of P with any STAT is not apparent (Brzózka et al. 2006). Targeting of an already activated form of STAT is unprecedented and may appear critical in terms of timing. However, such conditional emergency activity may allow P to perform its many other functions in virus replication. The high relevance in the virus context of both IFN antagonistic functions was recently illustrated by generating recombinant viruses expressing low levels of P (Brzózka et al. 2005), P mutants lacking specifically the IRF inhibitory function (Rieder et al. 2011), or viruses defective in STAT inhibition (Ito et al. 2010).
The P genes of paramyxoviruses such as Sendai virus encode, in addition to P, “accessory” proteins such as V or C, which largely take over IFN escape functions (Nagai et al. 2011; Sakaguchi et al. 2008) (see 10.1007/978-4-431-54556-9_2 for details). The V proteins of paramyxoviruses are translated from an edited P mRNA and possess a specific short C-terminal domain (V-CTD) of conserved structure and which is involved in binding and inhibiting a variety of target proteins involved in IFN induction (MDA5, IKKα, and IRF7), IFN signaling (STAT1, STAT2, Janus kinases), and NF-κB pathways (p65/RelA) (Gerlier and Lyles 2011; Goodbourn and Randall 2009; Motz et al. 2013; Schuhmann et al. 2011). The C proteins, which are expressed from an alternative reading frame of the P gene, seem to be involved in actively counteracting IFN (Sparrer et al. 2012), but the C proteins of some viruses such as the measles virus may also be active in controlling viral RNA synthesis and preventing dsRNA accumulation, thereby limiting IFN responses (Boonyaratanakornkit et al. 2011; Pfaller et al. 2013). Notably, V and C proteins from different paramyxovirus species and genera may differ in their affinity to individual targets, probably reflecting adaptation to counteract pathways important in specific hosts, organs, and cell types (Versteeg and Garcia-Sastre 2010).
In striking contrast to RABV and paramyxoviruses, innate immune escape of the insect-transmitted rhabdovirus VSV seems not to be determined by P gene functions. In VSV, rather, the M protein seems to represent the major factor, by shutting down host gene transcription and mRNA export (Rajani et al. 2012 and references therein).
The study of the structure of immune-stimulating RNAs of Mononegavirales and of the viral inhibitory mechanisms is not only shedding light on the nature of the innate immune system but offers directions toward the development of better attenuated and more immunogenic live vaccines. The elimination or modification of viral IFN antagonists by reverse genetics is yielding viruses that activate both innate and adaptive immune responses better than the wild-type (wt) virus.
Reorganization of Genomes: Vaccines, Vectors, Tracers, and Other Tools
The modular organization of genes in the Mononegavirales genomes (Fig. 1.1) allows easy deletion or insertion of extra genes without disturbing the expression machinery or virus formation. Gene cassettes comprising the transcriptional signals for transcription start and stop can be inserted between any of the viral genes, and in some viruses even in the 3′- and 5′-terminal positions. The major effect of extra transcription units in most viruses seems to be restricted to a modest transcriptional attenuation of the downstream genes. Thus, for example, up to four extra genes have been introduced successfully into the genomes of Sendai virus to simultaneously express multiple transcription factors for cell reprogramming (Nishimura et al. 2011). Transcriptional attenuation can be compensated by equipping the downstream genes with gene borders known to cause less attenuation than others (Finke et al. 2000). Alternative approaches include construction of single multi-cistronic transcription units encoding multiple proteins, by using picornaviral IRES elements or 2A-like sequences (Marschalek et al. 2009). By manipulation of the genome and antigenome promoters, even rabies and Sendai viruses with an ambisense gene expression strategy have been recovered. These viruses express the set of viral proteins from the viral genome RNA and extra proteins from the viral antigenome RNA (Finke and Conzelmann 1997; Le Mercier et al. 2002).
As the position in the viral genome determines the relative expression levels, shifting of genes to different positions is a versatile and commonly used tool to study dose effects of both viral and foreign genes. In combination with other approaches such as gene deletion, the gene shift approach has been used in the case of RABV to identify so far unappreciated roles of M and P proteins as a regulator of viral transcription (Finke et al. 2003) or as an interferon antagonist (Brzózka et al. 2005), respectively.
Vectors for Vaccines and Therapeutic Genes
The ability to express foreign or heterologous genes and antigens is the basis for the development of heterologous or multivalent vaccines. Particularly, many Mononegavirales are well known to induce strong humoral, cellular, and mucosal immune responses. Established human vaccine virus strains, such as the measles Schwarz strain of the Edmonston lineage, or animal viruses that do not cause disease in humans such as Sendai virus or Newcastle disease virus, seem to be immediately suited as promising carriers. In fact, numerous viral antigens from the major agents threatening human health, including human immunodeficiency virus (HIV)-1, Ebola virus, influenza virus, and SARS virus, hepatitis C virus (HCV), and HBV, have been expressed from these viruses (Brandler et al. 2010; Geisbert and Feldmann 2011; Khattar et al. 2011; Yu et al. 2010), and the first human trials are awaited. Also, recombinant RABV is being exploited as a vector vaccine, particularly for immunization against HIV-1 (Gomme et al. 2011), because completely attenuated RABV variants are now available. The RABV G protein is the major virulence factor of RABV and is responsible for the pronounced neurotropism and neuroinvasiveness of the virus. Mutations affecting the arginine 333 residue of the G protein dramatically alter the cell tropism and render RABV avirulent, even after intracerebral injection (Mebatsion 2001). Similarly, RABV lacking the IRF3 inhibitory function are completely attenuated after infection in mouse brains (Rieder et al. 2011).
Envelope Switching
A particular advantage of Mononegavirales is their amenability to envelope switching. As confirmed early by gene deletion mutants, the G proteins of the rhabdoviruses RABV and VSV are not essential for virus formation and budding of virions, although they may contribute to the efficiency of the process (Mebatsion et al. 1996; Schnell et al. 1998). Moreover, they can be entirely replaced by foreign type I transmembrane proteins. By using viral proteins active in receptor binding and membrane fusion, virions with a novel tropism or host range can be generated (Johnson et al. 1997; Mebatsion and Conzelmann 1996; Schnell et al. 1996). Even viruses specifically targeting cells infected with another virus can be generated (Mebatsion et al. 1997; Schnell et al. 1997). Natural paramyxoviruses have been long known for phenotypic mixing, or formation of pseudo-type viruses in coinfections (Kimura 1973), and are similarly amenable to artificial envelope swapping (for a recent paper, see Mourez et al. 2011). However, the requirements for incorporation of heterologous envelope proteins may differ for viruses. In contrast to VSV, which readily incorporates numerous type I transmembrane proteins (Schnell et al. 1998), efficient incorporation into RABV requires a C-tail sequence and structure similar to that of RABV G (Mebatsion and Conzelmann 1996).
Envelope swapping is a strategy extremely useful for various approaches. For vaccination purposes, sequential application of viruses possessing different envelopes avoids immune recognition and allows repeated use of the same vector backbone. In addition, the glycoproteins of highly dangerous viruses can be studied safely by using pseudo-type virions. Appropriate de- and retargeting of viruses is especially important in the field of oncolytic virotherapy. Envelope switching can involve the generation of chimeric “surrogate” viruses, in which a novel G-protein gene replaces the autologous gene, or of pseudo-type viruses, where the G gene is deleted and the protein is provided in trans. The latter is a safe approach, as single-round vectors are being generated that can infect a single cell but cannot spread further.
Oncolytic Virotherapy
A highly promising field for some recombinant Mononegavirales is oncolytic virotherapy, which means the use of viruses to selectively infect and damage cancerous tissues without causing harm to normal tissues (Russell et al. 2012). Preferred replication in transformed cells is partly caused by their genetic defects in innate immune responses, such as impaired STAT signaling or induction of apoptosis, that is, in major antiviral mechanisms. Viruses with defects in their IFN antagonists (such as paramyxovirus V proteins, or VSV M protein) therefore may replicate effectively in such cancer cells, whereas they are attenuated in normal tissue in which the antiviral mechanisms are intact. Some viruses, such as Newcastle disease virus and mumps virus, seem to have a strong natural preference for cancer cells, but other viruses such as measles, VSV, or Sendai virus can be readily engineered to make them more cancer specific (Cattaneo et al. 2008; Kinoh et al. 2009). Engineering of oncolytic viruses may include retargeting by destroying the natural receptor-binding domains of the viral envelope proteins in combination with displaying polypeptide ligands on the surface to facilitate infection of tumor cells overexpressing the targeted receptor. An outstanding series of retargeted measles viruses has been created accordingly, targeting various antigens overexpressed on tumor cells (Cattaneo et al. 2008). An alternative approach is complete envelope switching, that is, the complete exchange of the viral surface proteins. Promising examples of envelope switching include the use of oncolytic VSV carrying the envelope of LCMV, enhancing the infectivity for glioma cells and minimizing neurotropism (Muik et al. 2011). In the case of Sendai virus, tumor specificity could be enhanced by exploiting the need for proteolytic activation of the fusion protein (F). Specifically, the F-cleavage site was modified in such a way that it was cleaved by a protease highly expressed only in tumor cells (Morodomi et al. 2012).
Monosynaptic Tracing of Neurons with RABV: Envelope Swapping in Situ
The large group of Mononegavirales comprises viruses with highly diverse biological traits and thus represents an almost unlimited source of tools for all kinds of basic research and biomedical applications. In the past two decades of Mononegavirales reverse genetics, Sendai virus has emerged as a prime example for the broad and huge potential of this virus, as impressively illustrated in detail in the following chapters. Major inherent advantages of Sendai virus include its apathogenicity for humans and broad host and tissue range. Also, however, virulent viruses and, more explicitly, specific pathogenic traits of these viruses can be exploited for research. One example is the unparalleled neurotropism of RABV, which makes this virus a fierce pathogen but also offers unique possibilities for the study of the nervous system. RABV is entirely adapted to the nervous system of its hosts and—importantly—is completely dependent on the integrity of the peripheral nervous system to reach the brain where it replicates best and where it can elicit behavioral changes of the host to facilitate virus transmission within the population. Upon peripheral infection, RABV is taken up by the axon ends of neurons via endocytosis and is retrogradely transported in axonal transport vesicles toward the cell body (Klingen et al. 2008). Upon membrane fusion and release of the RNP into the cytoplasm, replication takes place without severe effects on the viability and function of neurons (Lafon 2011). Remarkably, newly assembled virions seem to be released exclusively at functional synapses where they can enter second-order neurons at the presynaptic membrane (Astic et al. 1993; Ugolini 1995). For trans-synaptic transmission, the RABV G protein is required (Etessami et al. 2000).
This exclusive trans-synaptic spread is unique among viruses and therefore natural RABV has been used for years as a “polysynaptic” tracer in specialized laboratories (Dum and Strick 2012). Direct synaptic connections of individual neurons, however, cannot be easily determined with conventional nonviral or viral tracers. The availability of RABV reverse genetics and progress in vector construction and virus retargeting, however, has more recently allowed the development of the first system for “monosynaptic” tracing of direct neuronal connections (Wickersham et al. 2007) (Fig. 1.2).
Fig. 1.2.
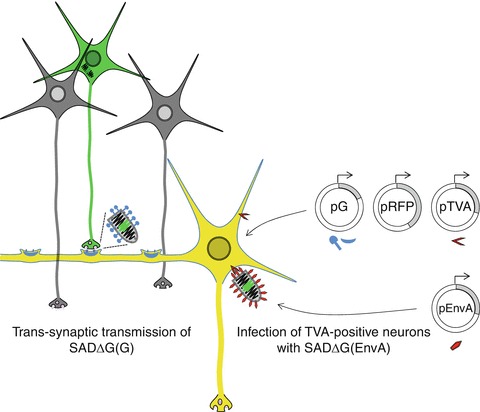
Monosynaptic tracing of neuronal circuits with rabies virus (RABV) ΔG. The exclusive trans-synaptic transmission of RABV is being exploited for mapping of direct connections between neurons. A starter neuron (yellow) that provides RABV G protein (blue) is transmitting a G gene-deleted RABV expressing eGFP (SAD ΔG-eGFP) via a synapse to presynaptic neurons (green). Because the G protein is not produced in the presynaptic neurons, the virus cannot be transmitted further. Selective infection of the postsynaptic starter neuron can be achieved, for example, by expression of a specific receptor (here, TVA), making cells permissive for infection with SAD ΔG-eGFP pseudo-typed with a retroviral envelope protein (EnvA)
The system involves targeted infection of a defined (postsynaptic) neuron with a G gene-deficient recombinant RABV (ΔG RABV) expressing a fluorescent protein such as green fluorescent protein (GFP). Specific targeting of the postsynaptic neuron is achieved, for example, by expression of the avian TVA receptor, and infection with ΔG RABV pseudo-typed with EnvA of an avian retrovirus that uses the TVA receptor for entry (Fig. 1.2). Additional expression of RABV G protein supports trans-synaptic transfer of the virus to synaptically connected (presynaptic) neurons. As no G is expressed in the presynaptic cells, the virus does not spread further. This safe ΔG RABV system is now being used widely by neurobiologists not only to dissect sensory and motor circuits in the nervous system of various animal models but also to read out and modulate the activity of single neurons and of neuronal circuits (Ginger et al. 2013; Wickersham and Feinberg 2012). It is expected that recombinant RABV in this way greatly contributes to the understanding of how our most complex brains work.
Conclusions
After an intricate birth phase, reverse genetics of Mononegavirales has become a standard technique to study this group of medically important viruses. Tremendous progress has been made subsequently in understanding the biology of these viruses, the interplay with their hosts, and the mechanisms of pathogenicity. It is becoming increasingly clear that the host innate immune system and specific viral counteractions profoundly shape virus–host relationships. Reverse genetics can now be used to make friends from foes, by converting the viruses to safe vaccines and biomedical tools. The group of Mononegavirales represents an exceptional source of diverse viruses for diverse applications, for example, rabies virus neurotracing. Sendai virus, in particular, has emerged in the past years as a cornucopia for many novel and innovative biomedical tools, as impressively illustrated in the following chapters of this volume.
Acknowledgments
Research in the author’s laboratory is funded by the German Research Foundation (DFG) through SFB 870 and Grako 1202, and the German Federal Ministry of Education and Research (BMBF) grant no. 01KI1016B. I thank Kerstin Schuhmann and Yoshiyuki Nagai for valuable comments on the manuscript.
Contributor Information
Yoshiyuki Nagai, Phone: +81-3-3518-2951, FAX: +81-3-3219-1061, Email: yoshi-nagai@riken.jp.
Karl-Klaus Conzelmann, Email: conzelma@lmb.uni-muenchen.de.
References
- Ammayappan A, Lapatra SE, Vakharia VN. A vaccinia-virus-free reverse genetics system for infectious hematopoietic necrosis virus. J Virol Methods. 2010;167:132–139. doi: 10.1016/j.jviromet.2010.03.023. [DOI] [PubMed] [Google Scholar]
- Astic L, Saucier D, Coulon P, Lafay F, Flamand A. The CVS strain of rabies virus as transneuronal tracer in the olfactory system of mice. Brain Res. 1993;619:146–156. doi: 10.1016/0006-8993(93)91606-s. [DOI] [PubMed] [Google Scholar]
- Baltimore D. Expression of animal virus genomes. Bacteriol Rev. 1971;35:235–241. doi: 10.1128/br.35.3.235-241.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D, Huang AS, Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus, II. An RNA polymerase in the virion. Proc Natl Acad Sci USA. 1970;66:572–576. doi: 10.1073/pnas.66.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron MD, Barrett T. Rescue of rinderpest virus from cloned cDNA. J Virol. 1997;71:1265–1271. doi: 10.1128/jvi.71.2.1265-1271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biacchesi S. The reverse genetics applied to fish RNA viruses. Vet Res. 2011;42:12. doi: 10.1186/1297-9716-42-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount KF, Uhlenbeck OC. The hammerhead ribozyme. Biochem Soc Trans. 2002;30:1119–1122. doi: 10.1042/bst0301119. [DOI] [PubMed] [Google Scholar]
- Boonyaratanakornkit J, Bartlett E, Schomacker H, Surman S, Akira S, Bae YS, Collins P, Murphy B, Schmidt A. The C proteins of human parainfluenza virus type 1 limit double-stranded RNA accumulation that would otherwise trigger activation of MDA5 and protein kinase R. J Virol. 2011;85:1495–1506. doi: 10.1128/JVI.01297-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandler S, Ruffie C, Najburg V, Frenkiel MP, Bedouelle H, Despres P, Tangy F. Pediatric measles vaccine expressing a dengue tetravalent antigen elicits neutralizing antibodies against all four dengue viruses. Vaccine. 2010;28:6730–6739. doi: 10.1016/j.vaccine.2010.07.073. [DOI] [PubMed] [Google Scholar]
- Bridgen A, Elliott RM. Rescue of a segmented negative-strand RNA virus entirely from cloned complementary DNAs. Proc Natl Acad Sci USA. 1996;93:15400–15404. doi: 10.1073/pnas.93.26.15400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns AM, Horvath CM. Activation of RIG-I-like receptor signal transduction. Crit Rev Biochem Mol Biol. 2012;47:194–206. doi: 10.3109/10409238.2011.630974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzózka K, Finke S, Conzelmann KK. Identification of the rabies virus alpha/beta interferon antagonist: phosphoprotein P interferes with phosphorylation of interferon regulatory factor 3. J Virol. 2005;79:7673–7681. doi: 10.1128/JVI.79.12.7673-7681.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzózka K, Finke S, Conzelmann KK. Inhibition of interferon signaling by rabies virus phosphoprotein P: activation-dependent binding of STAT1 and STAT2. J Virol. 2006;80:2675–2683. doi: 10.1128/JVI.80.6.2675-2683.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz UJ, Finke S, Conzelmann KK. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J Virol. 1999;73:251–259. doi: 10.1128/jvi.73.1.251-259.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calain P, Roux L. The rule of six, a basic feature for efficient replication of Sendai virus defective interfering RNA. J Virol. 1993;67:4822–4830. doi: 10.1128/jvi.67.8.4822-4830.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R, Miest T, Shashkova EV, Barry MA. Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded. Nat Rev Microbiol. 2008;6:529–540. doi: 10.1038/nrmicro1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins PL, Hill MG, Camargo E, Grosfeld H, Chanock RM, Murphy BR. Production of infectious human respiratory syncytial virus from cloned cDNA confirms an essential role for the transcription elongation factor from the 5′ proximal open reading frame of the M2 mRNA in gene expression and provides a capability for vaccine development. Proc Natl Acad Sci USA. 1995;92:11563–11567. doi: 10.1073/pnas.92.25.11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conzelmann KK. Genetic manipulation of non-segmented negative-strand RNA viruses. J Gen Virol. 1996;77(pt 3):381–389. doi: 10.1099/0022-1317-77-3-381. [DOI] [PubMed] [Google Scholar]
- Conzelmann KK. Reverse genetics of Mononegavirales. Curr Top Microbiol Immunol. 2004;283:1–41. doi: 10.1007/978-3-662-06099-5_1. [DOI] [PubMed] [Google Scholar]
- Conzelmann KK, Schnell M. Rescue of synthetic genomic RNA analogs of rabies virus by plasmid-encoded proteins. J Virol. 1994;68:713–719. doi: 10.1128/jvi.68.2.713-719.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui S, Eisenacher K, Kirchhofer A, Brzózka K, Lammens A, Lammens K, Fujita T, Conzelmann KK, Krug A, Hopfner KP. The C-terminal regulatory domain is the RNA 5′-triphosphate sensor of RIG-I. Mol Cell. 2008;29:169–179. doi: 10.1016/j.molcel.2007.10.032. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL (2012) Transneuronal tracing with neurotropic viruses reveals network macroarchitecture. Curr Opin Neurobiol doi:10.1016/j.conb.2012.12.002 [DOI] [PMC free article] [PubMed]
- Durbin AP, Hall SL, Siew JW, Whitehead SS, Collins PL, Murphy BR. Recovery of infectious human parainfluenza virus type 3 from cDNA. Virology. 1997;235:323–332. doi: 10.1006/viro.1997.8697. [DOI] [PubMed] [Google Scholar]
- Etessami R, Conzelmann KK, Fadai-Ghotbi B, Natelson B, Tsiang H, Ceccaldi PE. Spread and pathogenic characteristics of a G-deficient rabies virus recombinant: an in vitro and in vivo study. J Gen Virol. 2000;81:2147–2153. doi: 10.1099/0022-1317-81-9-2147. [DOI] [PubMed] [Google Scholar]
- Finke S, Conzelmann KK. Ambisense gene expression from recombinant rabies virus: random packaging of positive- and negative-strand ribonucleoprotein complexes into rabies virions. J Virol. 1997;71:7281–7288. doi: 10.1128/jvi.71.10.7281-7288.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finke S, Cox JH, Conzelmann KK. Differential transcription attenuation of rabies virus genes by intergenic regions: generation of recombinant viruses overexpressing the polymerase gene. J Virol. 2000;74:7261–7269. doi: 10.1128/jvi.74.16.7261-7269.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finke S, Mueller-Waldeck R, Conzelmann KK. Rabies virus matrix protein regulates the balance of virus transcription and replication. J Gen Virol. 2003;84:1613–1621. doi: 10.1099/vir.0.19128-0. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, Coyle AJ, Liao SM, Maniatis T. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- Ford E, Thanos D. The transcriptional code of human IFN-beta gene expression. Biochim Biophys Acta. 2010;1799:328–336. doi: 10.1016/j.bbagrm.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Fu ZF. Genetic comparison of the Rhabdoviruses from animals and plants. Curr Top Microbiol Immunol. 2005;292:1–24. doi: 10.1007/3-540-27485-5_1. [DOI] [PubMed] [Google Scholar]
- Fuerst TR, Niles EG, Studier FW, Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci USA. 1986;83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcin D, Pelet T, Calain P, Roux L, Curran J, Kolakofsky D. A highly recombinogenic system for the recovery of infectious Sendai paramyxovirus from cDNA: generation of a novel copy-back nondefective interfering virus. EMBO J. 1995;14:6087–6094. doi: 10.1002/j.1460-2075.1995.tb00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisbert TW, Feldmann H. Recombinant vesicular stomatitis virus-based vaccines against Ebola and Marburg virus infections. J Infect Dis. 2011;204(Suppl 3):S1075–S1081. doi: 10.1093/infdis/jir349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlier D, Lyles DS. Interplay between innate immunity and negative-strand RNA viruses: towards a rational model. Microbiol Mol Biol Rev. 2011;75:468–490. doi: 10.1128/MMBR.00007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanem A, Kern A, Conzelmann KK. Significantly improved rescue of rabies virus from cDNA plasmids. Eur J Cell Biol. 2012;91:10–16. doi: 10.1016/j.ejcb.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Ginger M, Haberl M, Conzelmann KK, Schwarz MK, Frick A. Revealing the secrets of neuronal circuits with recombinant rabies virus technology. Front Neural Circuits. 2013;7:2. doi: 10.3389/fncir.2013.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff SP, Berg P. Construction of hybrid viruses containing SV40 and lambda phage DNA segments and their propagation in cultured monkey cells. Cell. 1976;9:695–705. doi: 10.1016/0092-8674(76)90133-1. [DOI] [PubMed] [Google Scholar]
- Gomme EA, Wanjalla CN, Wirblich C, Schnell MJ. Rabies virus as a research tool and viral vaccine vector. Adv Virus Res. 2011;79:139–164. doi: 10.1016/B978-0-12-387040-7.00009-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodbourn S, Randall RE. The regulation of type I interferon production by paramyxoviruses. J Interferon Cytokine Res. 2009;29:539–547. doi: 10.1089/jir.2009.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Paterson RG, Ward CD, Lamb RA. Recovery of infectious SV5 from cloned DNA and expression of a foreign gene. Virology. 1997;237:249–260. doi: 10.1006/viro.1997.8801. [DOI] [PubMed] [Google Scholar]
- Hoffman MA, Banerjee AK. An infectious clone of human parainfluenza virus type 3. J Virol. 1997;71:4272–4277. doi: 10.1128/jvi.71.6.4272-4277.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, Takaoka A, Taniguchi T. Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity. 2006;25:349–360. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Hornung V, Ellegast J, Kim S, Brzózka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann KK, Schlee M, Endres S, Hartmann G. 5′-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- Huang Y, Tang Q, Nadin-Davis SA, Zhang S, Hooper CD, Ming P, Du J, Tao X, Hu R, Liang G. Development of a reverse genetics system for a human rabies virus vaccine strain employed in China. Virus Res. 2010;149:28–35. doi: 10.1016/j.virusres.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Inoue K, Shoji Y, Kurane I, Iijima T, Sakai T, Morimoto K. An improved method for recovering rabies virus from cloned cDNA. J Virol Methods. 2003;107:229–236. doi: 10.1016/s0166-0934(02)00249-5. [DOI] [PubMed] [Google Scholar]
- Ito N, Moseley GW, Blondel D, Shimizu K, Rowe CL, Ito Y, Masatani T, Nakagawa K, Jans DA, Sugiyama M. The role of interferon-antagonist activity of rabies virus phosphoprotein in viral pathogenicity. J Virol. 2010;84:6699–6710. doi: 10.1128/JVI.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JE, Schnell MJ, Buonocore L, Rose JK. Specific targeting to CD4+ cells of recombinant vesicular stomatitis viruses encoding human immunodeficiency virus envelope proteins. J Virol. 1997;71:5060–5068. doi: 10.1128/jvi.71.7.5060-5068.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A, Sakai Y, Shioda T, Kondo T, Nakanishi M, Nagai Y. Initiation of Sendai virus multiplication from transfected cDNA or RNA with negative or positive sense. Genes Cells. 1996;1:569–579. doi: 10.1046/j.1365-2443.1996.d01-261.x. [DOI] [PubMed] [Google Scholar]
- Kato H, Takahasi K, Fujita T. RIG-I-like receptors: cytoplasmic sensors for non-self RNA. Immunol Rev. 2011;243:91–98. doi: 10.1111/j.1600-065X.2011.01052.x. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Kawai T, Akira S. Recognition of nucleic acids by pattern-recognition receptors and its relevance in autoimmunity. Immunol Rev. 2011;243:61–73. doi: 10.1111/j.1600-065X.2011.01048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattar SK, Samal S, Devico AL, Collins PL, Samal SK. Newcastle disease virus expressing human immunodeficiency virus type 1 envelope glycoprotein induces strong mucosal and serum antibody responses in Guinea pigs. J Virol. 2011;85:10529–10541. doi: 10.1128/JVI.05050-11. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kimura Y. Phenotypic mixing of vesicular stomatitis virus with HVJ (Sendai virus) Jpn J Microbiol. 1973;17:373–381. doi: 10.1111/j.1348-0421.1973.tb00788.x. [DOI] [PubMed] [Google Scholar]
- Kinoh H, Inoue M, Komaru A, Ueda Y, Hasegawa M, Yonemitsu Y. Generation of optimized and urokinase-targeted oncolytic Sendai virus vectors applicable for various human malignancies. Gene Ther. 2009;16:392–403. doi: 10.1038/gt.2008.167. [DOI] [PubMed] [Google Scholar]
- Klingen Y, Conzelmann KK, Finke S. Double-labeled rabies virus: live tracking of enveloped virus transport. J Virol. 2008;82:237–245. doi: 10.1128/JVI.01342-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafon M. Evasive strategies in rabies virus infection. Adv Virus Res. 2011;79:33–53. doi: 10.1016/B978-0-12-387040-7.00003-2. [DOI] [PubMed] [Google Scholar]
- Lamb RA. Mononegavirales. In: Knipe DM, Howley PM, editors. Fields virology. Philadelphia: Lippincott; 2007. pp. 1357–1362. [Google Scholar]
- Lawson ND, Stillman EA, Whitt MA, Rose JK. Recombinant vesicular stomatitis viruses from DNA. Proc Natl Acad Sci USA. 1995;92:4477–4481. doi: 10.1073/pnas.92.10.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Mercier P, Garcin D, Hausmann S, Kolakofsky D. Ambisense Sendai viruses are inherently unstable but are useful to study viral RNA synthesis. J Virol. 2002;76:5492–5502. doi: 10.1128/JVI.76.11.5492-5502.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo YM, Gale M., Jr Immune signaling by RIG-I-like receptors. Immunity. 2011;34:680–692. doi: 10.1016/j.immuni.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luytjes W, Krystal M, Enami M, Pavin JD, Palese P. Amplification, expression, and packaging of foreign gene by influenza virus. Cell. 1989;59:1107–1113. doi: 10.1016/0092-8674(89)90766-6. [DOI] [PubMed] [Google Scholar]
- Marriott AC, Easton AJ. Reverse genetics of the Paramyxoviridae. Adv Virus Res. 1999;53:321–340. [PubMed] [Google Scholar]
- Marschalek A, Finke S, Schwemmle M, Mayer D, Heimrich B, Stitz L, Conzelmann KK. Attenuation of rabies virus replication and virulence by picornavirus internal ribosome entry site elements. J Virol. 2009;83:1911–1919. doi: 10.1128/JVI.02055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Staeheli P, Schneider U. RNA polymerase II-controlled expression of antigenomic RNA enhances the rescue efficacies of two different members of the Mononegavirales independently of the site of viral genome replication. J Virol. 2006;80:5708–5715. doi: 10.1128/JVI.02389-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebatsion T. Extensive attenuation of rabies virus by simultaneously modifying the dynein light chain binding site in the P protein and replacing Arg333 in the G protein. J Virol. 2001;75:11496–11502. doi: 10.1128/JVI.75.23.11496-11502.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebatsion T, Conzelmann KK. Specific infection of CD4+ target cells by recombinant rabies virus pseudotypes carrying the HIV-1 envelope spike protein. Proc Natl Acad Sci USA. 1996;93:11366–11370. doi: 10.1073/pnas.93.21.11366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebatsion T, Konig M, Conzelmann KK. Budding of rabies virus particles in the absence of the spike glycoprotein. Cell. 1996;84:941–951. doi: 10.1016/s0092-8674(00)81072-7. [DOI] [PubMed] [Google Scholar]
- Mebatsion T, Finke S, Weiland F, Conzelmann KK. A CXCR4/CD4 pseudotype rhabdovirus that selectively infects HIV-1 envelope protein-expressing cells. Cell. 1997;90:841–847. doi: 10.1016/s0092-8674(00)80349-9. [DOI] [PubMed] [Google Scholar]
- Ming PG, Huang Y, Tang Q, Du JL, Tao XY, Yan JX, Hu RL. Construction and analysis of full-length cDNA clone of rabies virus street strain. Bing Du Xue Bao. 2009;25:17–22. [PubMed] [Google Scholar]
- Morodomi Y, Yano T, Kinoh H, Harada Y, Saito S, Kyuragi R, Yoshida K, Onimaru M, Shoji F, Yoshida T, Ito K, Shikada Y, Maruyama R, Hasegawa M, Maehara Y, Yonemitsu Y. BioKnife, a uPA activity-dependent oncolytic Sendai virus, eliminates pleural spread of malignant mesothelioma via simultaneous stimulation of uPA expression. Mol Ther. 2012;20:769–777. doi: 10.1038/mt.2011.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motz C, Schuhmann KM, Kirchhofer A, Moldt M, Witte G, Conzelmann KK, Hopfner KP. Paramyxovirus V proteins disrupt the fold of the RNA sensor MDA5 to inhibit antiviral signaling. Science. 2013;339:690–693. doi: 10.1126/science.1230949. [DOI] [PubMed] [Google Scholar]
- Mourez T, Mesel-Lemoine M, Combredet C, Najburg V, Cayet N, Tangy F. A chimeric measles virus with a lentiviral envelope replicates exclusively in CD4+/CCR5+ cells. Virology. 2011;419:117–125. doi: 10.1016/j.virol.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Muik A, Kneiske I, Werbizki M, Wilfingseder D, Giroglou T, Ebert O, Kraft A, Dietrich U, Zimmer G, Momma S, Laer D. Pseudotyping vesicular stomatitis virus witn lymphocytic choriomeningitis virus glycoproteins enhances infectivity for glioma cells and minimizes neurotropism. J Virol. 2011;85:5679–5684. doi: 10.1128/JVI.02511-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y, Takakura A, Irie T, Yonemitsu Y, Gotoh B. Sendai virus: evolution from mouse pathogen to a state-of-the-art tool in virus research and biotechnology. In: Samal SK, editor. The biology of paramyxoviruses. Norfolk: Caister Academic; 2011. pp. 115–173. [Google Scholar]
- Nishimura K, Sano M, Ohtaka M, Furuta B, Umemura Y, Nakajima Y, Ikehara Y, Kobayashi T, Segawa H, Takayasu S, Sato H, Motomura K, Uchida E, Kanayasu-Toyoda T, Asashima M, Nakauchi H, Yamaguchi T, Nakanishi M. Development of defective and persistent Sendai virus vector: a unique gene delivery/expression system ideal for cell reprogramming. J Biol Chem. 2011;286:4760–4771. doi: 10.1074/jbc.M110.183780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoguchi K, Yoneyama M, Takemura A, Akira S, Taniguchi T, Namiki H, Fujita T. Viral infections activate types I and III interferon genes through a common mechanism. J Biol Chem. 2007;282:7576–7581. doi: 10.1074/jbc.M608618200. [DOI] [PubMed] [Google Scholar]
- Orbanz J, Finke S. Generation of recombinant European bat lyssavirus type 1 and inter-genotypic compatibility of lyssavirus genotype 1 and 5 antigenome promoters. Arch Virol. 2010;155:1631–1641. doi: 10.1007/s00705-010-0743-8. [DOI] [PubMed] [Google Scholar]
- Park KH, Huang T, Correia FF, Krystal M. Rescue of a foreign gene by Sendai virus. Proc Natl Acad Sci USA. 1991;88:5537–5541. doi: 10.1073/pnas.88.13.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattnaik AK, Wertz GW. Replication and amplification of defective interfering particle RNAs of vesicular stomatitis virus in cells expressing viral proteins from vectors containing cloned cDNAs 42. J Virol. 1990;64:2948–2957. doi: 10.1128/jvi.64.6.2948-2957.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattnaik AK, Wertz GW. Cells that express all five proteins of vesicular stomatitis virus from cloned cDNAs support replication, assembly, and budding of defective interfering particles. Proc Natl Acad Sci USA. 1991;88:1379–1383. doi: 10.1073/pnas.88.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattnaik AK, Ball LA, LeGrone AW, Wertz GW. Infectious defective interfering particles of VSV from transcripts of a cDNA clone. Cell. 1992;69:1011–1020. doi: 10.1016/0092-8674(92)90619-n. [DOI] [PubMed] [Google Scholar]
- Perrotta AT, Been MD. The self-cleaving domain from the genomic RNA of hepatitis delta virus: sequence requirements and the effects of denaturant. Nucleic Acids Res. 1990;18:6821–6827. doi: 10.1093/nar/18.23.6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller CK, Radeke MJ, Cattaneo R, Samuel CE (2013) Measles virus C protein impairs production of defective copyback double-stranded viral RNA and activation of protein kinase R. J Virol. Oct 23. [Epub ahead of print] PubMed PMID: 24155404 [DOI] [PMC free article] [PubMed]
- Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- Pringle CR. Mononegavirales. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. Virus taxonomy. Eighth report of the International Committee on the taxonomy of viruses. London: Elsevier/Academic; 2005. pp. 609–614. [Google Scholar]
- Racaniello VR, Baltimore D. Cloned poliovirus complementary DNA is infectious in mammalian cells. Science. 1981;214:916–919. doi: 10.1126/science.6272391. [DOI] [PubMed] [Google Scholar]
- Radecke F, Spielhofer P, Schneider H, Kaelin K, Huber M, Dotsch C, Christiansen G, Billeter MA. Rescue of measles viruses from cloned DNA. EMBO J. 1995;14:5773–5784. doi: 10.1002/j.1460-2075.1995.tb00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajani KR, Pettit Kneller EL, McKenzie MO, Horita DA, Chou JW, Lyles DS. Complexes of vesicular stomatitis virus matrix protein with host Rae1 and Nup98 involved in inhibition of host transcription. PLoS Pathog. 2012;8:e1002929. doi: 10.1371/journal.ppat.1002929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall RE, Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol. 2008;89:1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- Rieder M, Conzelmann KK. Interferon in rabies virus infection. Adv Virus Res. 2011;79:91–114. doi: 10.1016/B978-0-12-387040-7.00006-8. [DOI] [PubMed] [Google Scholar]
- Rieder M, Brzózka K, Pfaller CK, Cox JH, Stitz L, Conzelmann KK. Genetic dissection of interferon-antagonistic functions of rabies virus phosphoprotein: inhibition of interferon regulatory factor 3 activation is important for pathogenicity. J Virol. 2011;85:842–852. doi: 10.1128/JVI.01427-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A, Rose JK. Recovery of negative-strand RNA viruses from plasmid DNAs: a positive approach revitalizes a negative field. Virology. 1998;247:1–6. doi: 10.1006/viro.1998.9250. [DOI] [PubMed] [Google Scholar]
- Rothenberg E, Smotkin D, Baltimore D, Weinberg RA. In vitro synthesis of infectious DNA of murine leukaemia virus. Nature (Lond) 1977;269:122–126. doi: 10.1038/269122a0. [DOI] [PubMed] [Google Scholar]
- Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30:658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi T, Kato A, Kiyotani K, Yoshida T, Nagai Y. Studies on the paramyxovirus accessory genes by reverse genetics in the Sendai virus-mouse system. Proc Jpn Acad Ser B Phys Biol Sci. 2008;84:439–451. doi: 10.2183/pjab/84.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell MJ, Mebatsion T, Conzelmann KK. Infectious rabies viruses from cloned cDNA. EMBO J. 1994;13:4195–4203. doi: 10.1002/j.1460-2075.1994.tb06739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell MJ, Buonocore L, Kretzschmar E, Johnson E, Rose JK. Foreign glycoproteins expressed from recombinant vesicular stomatitis viruses are incorporated efficiently into virus particles. Proc Natl Acad Sci USA. 1996;93:11359–11365. doi: 10.1073/pnas.93.21.11359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell MJ, Johnson JE, Buonocore L, Rose JK. Construction of a novel virus that targets HIV-1-infected cells and controls HIV-1 infection. Cell. 1997;90:849–857. doi: 10.1016/s0092-8674(00)80350-5. [DOI] [PubMed] [Google Scholar]
- Schnell MJ, Buonocore L, Boritz E, Ghosh HP, Chernish R, Rose JK. Requirement for a non-specific glycoprotein cytoplasmic domain sequence to drive efficient budding of vesicular stomatitis virus. EMBO J. 1998;17:1289–1296. doi: 10.1093/emboj/17.5.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, Bieniasz P, Rice CM. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature (Lond) 2011;472:481–485. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhmann KM, Pfaller CK, Conzelmann KK. The measles virus V protein binds to p65 (RelA) to suppress NF-{kappa}B activity. J Virol. 2011;85:3162–3171. doi: 10.1128/JVI.02342-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, tenOever BR, Grandvaux N, Zhou GP, Lin R, Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300:1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- Sharmeen L, Kuo MY, Dinter-Gottlieb G, Taylor J. Antigenomic RNA of human hepatitis delta virus can undergo self-cleavage. J Virol. 1988;62:2674–2679. doi: 10.1128/jvi.62.8.2674-2679.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrer KM, Pfaller CK, Conzelmann KK. Measles virus C protein interferes with beta interferon transcription in the nucleus. J Virol. 2012;86:796–805. doi: 10.1128/JVI.05899-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T, Palmieri M, Weissmann C. QB DNA-containing hybrid plasmids giving rise to QB phage formation in the bacterial host. Nature (Lond) 1978;274:223–228. doi: 10.1038/274223a0. [DOI] [PubMed] [Google Scholar]
- Tao L, Ge J, Wang X, Zhai H, Hua T, Zhao B, Kong D, Yang C, Chen H, Bu Z. Molecular basis of neurovirulence of flury rabies virus vaccine strains: importance of the polymerase and the glycoprotein R333Q mutation. J Virol. 2010;84:8926–8936. doi: 10.1128/JVI.00787-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–335. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- Theriault S, Groseth A, Artsob H, Feldmann H. The role of reverse genetics systems in determining filovirus pathogenicity. Arch Virol Suppl. 2005;19:157–177. doi: 10.1007/3-211-29981-5_13. [DOI] [PubMed] [Google Scholar]
- Ugolini G. Specificity of rabies virus as a transneuronal tracer of motor networks: transfer from hypoglossal motoneurons to connected second-order and higher order central nervous system cell groups. J Comp Neurol. 1995;356:457–480. doi: 10.1002/cne.903560312. [DOI] [PubMed] [Google Scholar]
- Versteeg GA, Garcia-Sastre A. Viral tricks to grid-lock the type I interferon system. Curr Opin Microbiol. 2010;13:508–516. doi: 10.1016/j.mib.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulliemoz D, Roux L. “Rule of six:” how does the Sendai virus RNA polymerase keep count? J Virol. 2001;75:4506–4518. doi: 10.1128/JVI.75.10.4506-4518.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann C, Weber H, Taniguchi T, Muller W, Meyer F. Reversed genetics: a new approach to the elucidation of structure–function relationship. Ciba Found Symp. 1979;66:47–61. doi: 10.1002/9780470720486.ch4. [DOI] [PubMed] [Google Scholar]
- Whelan SP, Ball LA, Barr JN, Wertz GT. Efficient recovery of infectious vesicular stomatitis virus entirely from cDNA clones. Proc Natl Acad Sci USA. 1995;92:8388–8392. doi: 10.1073/pnas.92.18.8388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan SP, Barr JN, Wertz GW. Transcription and replication of nonsegmented negative-strand RNA viruses. Curr Top Microbiol Immunol. 2004;283:61–119. doi: 10.1007/978-3-662-06099-5_3. [DOI] [PubMed] [Google Scholar]
- Wickersham IR, Feinberg EH. New technologies for imaging synaptic partners. Curr Opin Neurobiol. 2012;22:121–127. doi: 10.1016/j.conb.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Wickersham IR, Lyon DC, Barnard RJ, Mori T, Finke S, Conzelmann KK, Young JA, Callaway EM. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron. 2007;53:639–647. doi: 10.1016/j.neuron.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama M, Suhara W, Fukuhara Y, Fukuda M, Nishida E, Fujita T. Direct triggering of the type I interferon system by virus infection: activation of a transcription factor complex containing IRF-3 and CBP/p300. EMBO J. 1998;17:1087–1095. doi: 10.1093/emboj/17.4.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Feng X, Shu T, Matano T, Hasegawa M, Wang X, Li H, Li Z, Zhong R, Zeng Y. Comparison of the expression and immunogenicity of wild-type and sequence-modified HIV-1 gag genes in a recombinant Sendai virus vector. Curr HIV Res. 2010;8:199–206. doi: 10.2174/157016210791111133. [DOI] [PubMed] [Google Scholar]


