Abstract
INTRODUCTION:
Bloating is one of the most common gastrointestinal complaints. Evidence has linked fiber and sodium to bloating; however, randomized trials examining these diet components are lacking. Here, we used a randomized trial to examine the effects of the high-fiber DASH diet and dietary sodium intake on abdominal bloating. We hypothesized that both the high-fiber DASH diet and higher sodium intake would increase bloating.
METHODS:
The DASH–Sodium trial (1998–1999) randomized healthy adults to a high-fiber (32 g/d) DASH or low-fiber (11 g/d) Western diet (control). On their assigned diet, participants ate 3 sodium levels (50, 100, and 150 mmol/d at 2100 kcal) in 30-day periods in random order, with 5-day breaks between each period. The participants reported the presence of bloating at baseline and after each feeding period. Statistical analyses included log-binomial models to evaluate the risk of bloating.
RESULTS:
Of 412 participants (mean age 48 years; 57% women; 57% black), 36.7% reported bloating at baseline. Regardless of the diet, high sodium intake increased the risk of bloating (risk ratio = 1.27; 95% confidence interval: 1.06–1.52; P = 0.01). The high-fiber DASH diet also increased the risk of bloating over all sodium levels (risk ratio = 1.41; 95% confidence interval: 1.22–1.64; P < 0.001). The effect of high-fiber DASH on bloating was greater in men than in women (P for interaction = 0.001).
DISCUSSION:
Higher dietary sodium increased bloating, as did the high-fiber DASH diet. Although healthful high-fiber diets may increase bloating, these effects may be partially mitigated by decreasing dietary sodium intake. Future research is needed to explore mechanisms by which sodium intake and diet can influence bloating.
INTRODUCTION
Bloating is one of the most commonly reported symptoms of gastrointestinal (GI) discomfort (1-4), reported by over 30% of adults in a recent survey of U.S. adults (5) and by over 90% of adults with irritable bowel syndrome (6-11). Although the causes of bloating are likely myriad, diet is often implicated.
A high-fiber diet has been shown in small studies to increase gas production and retention (12), which may keep adults from choosing this healthful dietary pattern. Identification of nonhealthful nutrients that cause bloating is needed to inform dietary advice for the relief of GI gas retention without compromising health.
Sodium is among the most ubiquitously consumed nutrients in a typical U.S. diet. In addition to causing elevated blood pressure (BP), among other health problems, high sodium intake has been linked to GI bloating (13-18). The most convincing evidence supporting this notion derives from a double-blind crossover trial, in which oral administration of oral sodium bicarbonate increased bloating (18). Whether the symptoms result from the sodium or the bicarbonate is unclear. In addition, whether bloating would occur in the setting of a low-fiber typical American diet or a high-fiber healthful diet has not been demonstrated.
In this context, we analyzed data from the DASH–Sodium randomized controlled trial to examine the effects of dietary sodium and the high-fiber DASH diet vs a low-fiber control diet on the risk of bloating. We hypothesized that higher sodium, and the high-fiber DASH diet, would increase abdominal bloating.
METHODS
Trial overview
The DASH-Sodium trial was a multicenter trial, completed in November 1999 and sponsored by the National Heart, Lung, and Blood Institute. This trial tested the effects of 3 sodium intake levels in 2 separate diets (32 g high-fiber DASH diet and 11 g low-fiber control diet) in adults with high BP. Based on 2,100 kcal consumption, these sodium levels were low (goal of 50 mmol/d), intermediate (goal of 100 mmol/d), and high (goal of 150 mmol/d). Participants who were smaller or less active received less sodium and food than those who were larger or more active to account for total energy requirements. The DASH diet had low total fat, saturated fat, and cholesterol and was rich in low-fat dairy products, fruits, and vegetables. There were also more whole grains, nuts, fish, and poultry, and less sugary items and red meat in the DASH diet than the typical Western diet (see Table, Supplementary Digital Content 1, http://links.lww.com/AJG/A189 for a detailed description of both diets). The IRB at Johns Hopkins University School of Medicine approved secondary analyses of the DASH-Na trial.
Participants
Four clinical centers in the United States had randomized 412 participants (age ≥ 22 years) in the trial. For each participant, systolic BP was required to be between 120 and 159 mm Hg and diastolic BP to be between 80 and 95 mm Hg while not on hypertension medications. Exclusion criteria included diabetes mellitus, anemia, inflammatory bowel disease, pregnancy, renal insufficiency, history of cardiovascular event, use of insulin, poorly controlled dyslipidemia, and consumption of >14 alcoholic beverages per week. Written informed consent was obtained from all study participants.
Controlled feeding
Participants were provided with all meals and snacks during the run-in period and 3 intervention periods. During the run-in period, which lasted 2 weeks, participants followed the control diet with a high sodium level. Subsequently, participants were randomly assigned to either the control or DASH diet, using a parallel-arm design. Then, in a 3-period, crossover design, participants followed their assigned diet for 30 days at each of the 3 sodium levels (three 30-day periods). Each participant’s calorie content was adjusted to maintain a constant weight throughout the trial.
Outcome measure: bloating
The participants completed a symptoms questionnaire at the end of the run-in period and at the end of each feeding period. On the questionnaire, they were asked whether they felt “bloating/uncomfortably full.” There were 4 options that participants could pick: none (symptom did not occur), mild (symptom occurred but did not interfere with usual activities), moderate (occurrence of symptom somewhat interfered with usual activities), or severe (occurrence of symptom resulted in an inability to perform usual activities).
Other covariates
Data collection on other covariates occurred during screening (baseline) and during each feeding period. BP measurements were taken from the right arm of participants while in a seated position. Using height and weight measurements, body mass index (BMI) was derived. Urine collected over a 24-hour period was used to determine urine sodium levels.
Statistical analysis
We characterized the demographic and clinical factors of the study population using proportions and means (SD). We used histograms to illustrate the distribution of the severity score. To evaluate the effect of sodium intake on bloating, we made comparisons between intermediate vs low sodium, high vs intermediate sodium, and high vs low sodium overall and by the assigned diet (DASH vs control). To evaluate the effect of diet on bloating, we made comparisons between the DASH vs control diet overall and by strata of sodium level (low, intermediate, and high). To evaluate the combined diet-sodium effects, we compared high sodium in the control diet vs low sodium in the DASH diet. We modeled the risk of any bloating using log-binomial models with a log link, binomial family, and an exchangeable covariance structure.
To assess effect modification of baseline covariates on the association between sodium intake and bloating, as well as between diet and bloating, we stratified analyses by age (<60, ≥60 years), sex (men, women), race (white, black), obesity (BMI≥30, <30 kg/m2), and hypertension (BP≥140/90, <140/90 mm Hg). We chose these categories a priori based on the distribution of baseline data (age) and established clinical cut points (BMI or hypertension). We used interaction terms to test for differences across strata. We performed all analyses using Stata/SE 14.0 (Stata Corporation LP, College Station, TX). We considered a P value of < 0.05 as statistically significant. Missing data were rare (19 of 809 visits or <3%) and evenly distributed across treatments.
RESULTS
Of the 412 trial participants, 204 were assigned to the control diet and 208 to the DASH diet. The demographic and clinical characteristics were similar in both groups (Table 1).
Table 1.
Baseline characteristics overall and by diet
| Overall (N = 412) | Control diet (N = 204) | DASH diet (N = 208) | |
|---|---|---|---|
| Age, yr (SD) | 48.2 (10.0) | 49.1 (10.4) | 47.4 (9.6) |
| Women, % | 56.8 | 54.4 | 59.1 |
| Black, % | 56.8 | 56.4 | 57.2 |
| Hypertensiona, % | 40.8 | 40.7 | 40.9 |
| Blood pressure, mm Hg | |||
| Systolic (SD) | 134.8 (9.5) | 135.4 (9.4) | 134.2 (9.6) |
| Diastolic (SD) | 85.7 (4.5) | 85.8 (4.1) | 85.6 (4.8) |
| BMI, kg/m2 (SD) | 29.2 (4.8) | 29.5 (5.0) | 28.8 (4.7) |
| BMI ≥ 30, % | 38.8 | 40.2 | 37.5 |
| Urinary sodiumb, mmol/d (SD) | 155.03 (75.4) | 152.5 (71.8) | 157.6 (78.9) |
| Any bloating, %c | 36.7 | 39.2 | 34.1 |
BMI, body mass index.
Defined in the trial as a systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg.
N = 408 (Control Diet, N = 204; DASH Diet, N = 204).
Obtained at the end of run-in on the control diet, prerandomization.
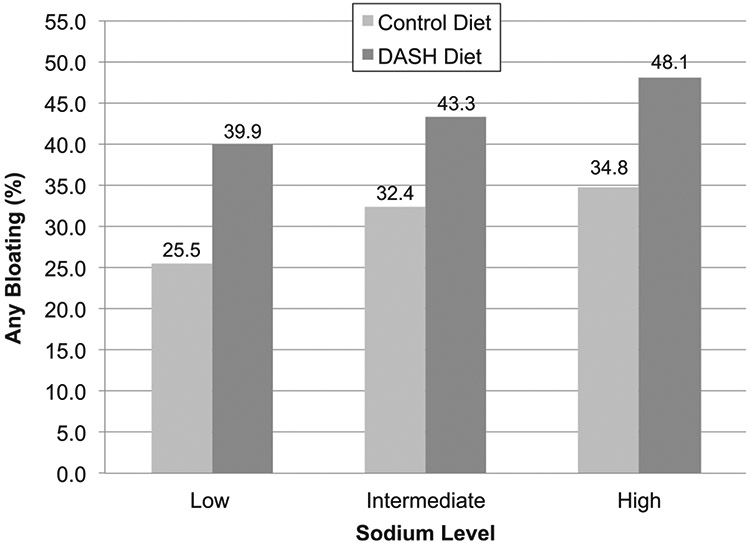
In Figure 1, we show the percentage of bloating at the end of the diet period according to the sodium intake level and assigned diet. The highest occurrence of bloating was in the high sodium group for both the control diet (34.8%) and the DASH diet (48.1%). The lowest occurrence of bloating was in the low sodium group for both the control diet (25.5%) and the DASH diet (39.9%).
Figure 1.
Bloating by diet and sodium level.
The occurrence and severity of bloating by sodium level and diet are displayed in Table 2. At the high level of sodium intake, more participants experienced severe bloating in the DASH diet than in the control diet (8 vs 4; 4.0% vs 2.1%). At the intermediate level of sodium, there was no difference in severe bloating by diet (5 participants [2.5%] on each diet). At the low sodium level, 9 participants (4.5%) on the DASH diet vs 4 participants (2.1%) on the control diet reported severe bloating.
Table 2.
Occurrence and severity of bloating by sodium diet and level, n (%)
| Mild | Moderate | Severe | |
|---|---|---|---|
| Level of sodium | |||
| Low | |||
| Control (n = 194) | 37 (19.1) | 11 (5.7) | 4 (2.1) |
| DASH (n = 200) | 50 (25.0) | 24 (12.0) | 9 (4.5) |
| Intermediate | |||
| Control (n = 197) | 41 (20.8) | 20 (10.2) | 5 (2.5) |
| DASH (n = 200) | 62 (31.0) | 23 (11.5) | 5 (2.5) |
| High | |||
| Control (n = 195) | 51 (26.2) | 16 (8.2) | 4 (2.1) |
| DASH (n = 201) | 63 (31.3) | 29 (14.4) | 8 (4.0) |
Overall, high vs low sodium intake increased the risk of experiencing bloating (risk ratio [RR] = 1.27; 95% confidence interval [CI]: 1.06, 1.52; P = 0.010) (Table 3). Regardless of the sodium intake level, the DASH diet vs the control diet increased the risk of bloating (RR = 1.41; 95% CI: 1.22, 1.64; P < 0.001). Although there was no statistical evidence of a sodium-diet interaction on bloating (P for interaction = 0.55), we present the stratum-specific effects, according to strata of the other factor, in Table 3.
Table 3.
The effects of sodium intake and diet on the occurrence of bloating
| Bloating | ||
|---|---|---|
| RR (95% CI) | P | |
| Sodium effects | ||
| Sodium effects overalla | ||
| Intermediate vs low sodium | 1.16 (0.96, 1.39) | 0.127 |
| High vs intermediate sodium | 1.10 (0.93, 1.30) | 0.286 |
| High vs low sodium | 1.27 (1.06, 1.52) | 0.010 |
| Sodium effects on the control dieta | ||
| Intermediate vs low sodium | 1.27 (0.93, 1.73) | 0.128 |
| High vs intermediate sodium | 1.08 (0.82, 1.41) | 0.600 |
| High vs low sodium | 1.37 (1.01, 1.84) | 0.042 |
| Sodium effects on the DASH dieta | ||
| Intermediate vs low sodium | 1.08 (0.86, 1.36) | 0.487 |
| High vs intermediate sodium | 1.11 (0.90, 1.37) | 0.326 |
| High vs low sodium | 1.20 (0.97, 1.50) | 0.095 |
| Diet effects (DASH vs control) | ||
| Low sodium | 1.57 (1.17, 2.09) | 0.002 |
| Medium sodium | 1.34 (1.04, 1.72) | 0.024 |
| High sodium | 1.38 (1.09, 1.75) | 0.007 |
| All sodium levels | 1.41 (1.22, 1.64) | <0.001 |
| Combined effects | ||
| High sodium in the control diet vs low sodium in DASH diet | 0.87 (0.68, 1.12) | 0.287 |
CI, confidence interval; RR, risk ratio.
A test for a diet-sodium interaction was nonsignificant, P =0.55.
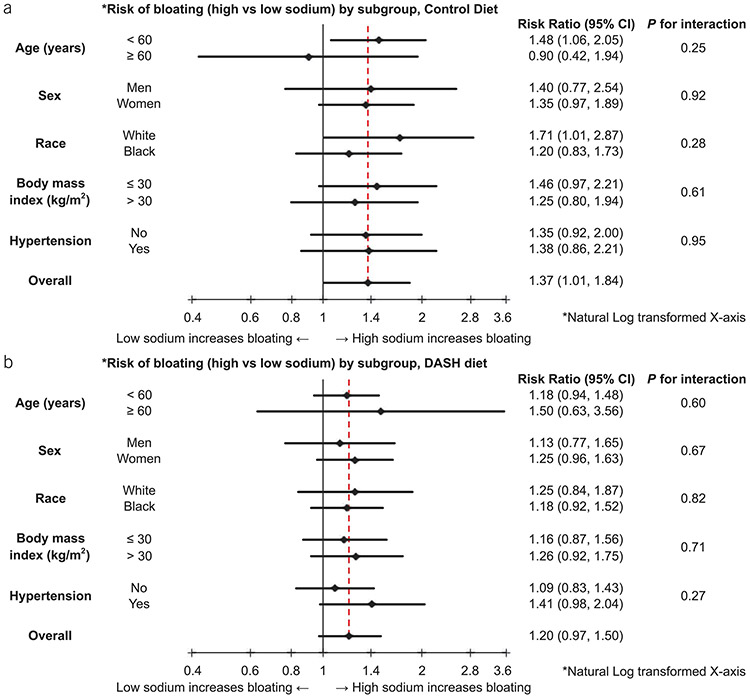
The effects of high vs low sodium intake and diet on bloating were also investigated through a stratified analysis by age, sex, race, BMI, and hypertension, but no interactions were found (Figure 2).
Figure 2.
(a) Risk of bloating (high vs low sodium) by subgroup, in the control diet. (b) Risk of bloating (high vs low sodium) by subgroup, in the DASH diet. CI, confidence interval.
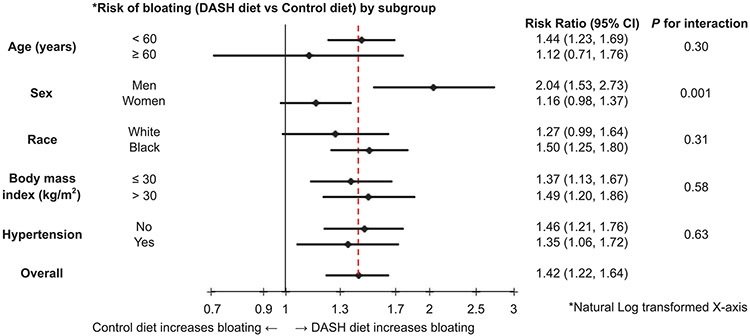
In another stratified analysis by age, sex, race, BMI, and hypertension, the effects of the DASH diet vs the control diet averaging over all sodium levels were analyzed (Figure 3). The one significant diet interaction found was sex. Men experienced over 2 times the risk of bloating on the DASH vs the control diet (RR = 2.04; 95% CI: 1.53, 2.73), whereas bloating in women was not significantly affected by the diet (RR = 1.16; 95% CI: 0.98, 1.37; P for interaction = 0.001).
Figure 3.
Risk of bloating (DASH diet vs control diet) by subgroup. CI, confidence interval.
DISCUSSION
In the DASH-Sodium trial, higher sodium intake and the high-fiber DASH diet increased bloating in additive fashion. The effects of sodium on bloating were consistent by diet (DASH vs control), and the effects for diet were similar across levels of sodium intake. The risk of bloating was similar for those consuming the low-fiber control diet at the high sodium level vs the high-fiber DASH diet at the low sodium level. The effect of the DASH diet on bloating was greater in men than in women.
Our findings on dietary sodium and bloating are largely consistent with another trial, which found that oral administration of sodium bicarbonate increased bloating in participants (18), and they are consistent with the biological effects of sodium on the gut. Higher sodium intake can promote water retention and suppress digestive efficiency, which can lead to bloating (19-22). In addition, rodent studies have shown that dietary sodium can change the composition of the gut microbiota, which could also increase bloating (23). Recent research has found that in both rodents and humans, a high-salt diet is associated with diminished abundance of Lactobacillus spp. in the gut (24). In a recent trial, Riezzo et al. (25) demonstrated that administration of Lactobacillus spp. in patients led to a significant improvement in intestinal symptoms, including bloating, constipation, and abdominal discomfort. Thus, it is plausible that the composition of the gut microbiome can mediate the effect of sodium on bloating, which is also supported by a recent review article by Li et al. (26).
Our finding of a high-fiber DASH diet causing more bloating than the low-fiber control diet is consistent with smaller studies showing that dietary fiber content influences gas production and retention (8,12,27-30). One trial involving 5 participants examined the effects of fiber on gas production and found that increased dietary fiber led to increased gas production (27). In a trial with 12 participants where gastric emptying was measured through real-time ultrasound, Bergmann et al. (28) found that increased dietary fiber led to delayed gastric emptying. As part of a separate trial, physiologic gas was jejunally infused into 10 healthy volunteers, and it was found that a high-fiber diet hindered intestinal transit by reducing bolus propulsion to the rectum (12). Finally, in a trial consisting of 63 participants, Ho et al. (30) found that consuming a high-fiber diet resulted in significantly more bloating than consuming a low- or no-fiber diet. High-fiber diets increase gas production through the fermentation of fiber by gut bacteria and promote gas retention by impeding intestinal gas transit (12,27,31).
On the DASH vs the control diet, men experienced more bloating than women. There is growing research that the gut microbiome differs by sex (32-37), which could lead to a difference in the processing and fermentation of dietary fiber in men vs women. In fact, one study found that women had a significantly lower abundance of Bacteroidetes in their gut microbiome than men (34). The Bacteroides genus (phylum: Bacteroidetes) has been found to be capable of metabolizing and fermenting many different types of fibers and glycans (38,39). Bacterial fermentation in the gut is known to lead to GI side effects such as bloating (40-43). Furthermore, another study found that dietary fiber supplementation in men increased the relative abundance of Bacteroidetes bacteria (44). Therefore, it is plausible that consuming the high-fiber DASH diet vs the low-fiber control diet may have modified the gut microbiota differently by sex.
There are several limitations to our study. First, bloating was not the primary outcome of the DASH–Sodium trial, but rather a secondary outcome. The presence and severity of bloating symptoms experienced was based on a single question. The question asked participants the extent to which they felt “bloating/uncomfortably full,” which could result in variable interpretations for different individuals. Second, each feeding period for each sodium intake level lasted 4 weeks; although we expect effects to be applicable beyond 4 weeks, the design of this trial did not permit an assessment of long-term effects. Third, some of the bloating effects from the DASH diet may have been secondary to mass effects. That is, to be isocaloric, the total mass of food in those consuming the DASH diet may have been greater than in those consuming the control diet, contributing to the finding that those consuming the DASH diet experienced more bloating in the trial than those consuming the control diet. Fourth, because the trial had a crossover design, there is the possibility of a carryover effect from the previous period. How-ever, we expect the carryover effects in our trial were negligible, if there were any, given the intervention periods lasted a maximum of 30 days with a 5-day washout period between interventions, which left about 30–35 days between the assessments of symptoms. In addition, the order of the intervention (low to high vs high to low sodium) yielded similar findings. Last, there was a relatively limited range of sodium intake because those in the high sodium group actually followed the average sodium intake in the United States, which could underestimate our results regarding the relationship of high sodium intake with abdominal bloating.
Our study also has several strengths. First, the study design allowed for a test of the effects of both diet (DASH and control) and sodium levels. Second, the trial was rigorously implemented with high levels of adherence and follow-up. Third, the study population enrolled a racially diverse, noninstitutionalized, cohort of middle-to-older aged men and women. Therefore, we believe these results to be applicable to the general, ambulatory population of U.S. adults.
Our findings have important clinical ramifications. Millions of U.S. adults visit health practitioners because of GI diseases, with bloating as one of the most common symptoms, reported by anywhere from approximately 15%–30% of the general population (3,40,45,46). Sodium intake in the United States is quite high (3,4,10) and is an unrecognized cause of bloating. Beyond the cardiovascular benefits of lowering sodium, our study demonstrates that lowering sodium intake can also improve GI bloating. This is meaningful for the general population and should be evaluated in subgroups with more pronounced and regular GI bloating.
Our results also show that although a high-fiber diet, such as the DASH diet, can increase the risk of bloating, decreasing sodium intake in such a diet may diminish some of these undesired effects. In fact, we showed that a high-fiber low-sodium diet does not cause significantly more bloating than a low-fiber high-sodium diet. This finding demonstrates that sodium reduction might improve compliance with a high-fiber diet, such as the DASH diet.
In conclusion, a high-fiber diet increased bloating symptoms, whereas sodium reduction lowered these effects. Sodium reduction represents an important dietary intervention to reduce bloating symptoms and could be used to enhance compliance with healthful high-fiber diets, such as the DASH diet. The mechanisms by which sodium intake and diet can influence bloating and related GI symptoms represent important areas for future research.
Supplementary Material
Study Highlights.
WHAT IS KNOWN
Bloating is one of the most common GI complaints.
Dietary fiber and sodium have been hypothesized to increase bloating, but no rigorous randomized controlled trials have investigated these factors.
WHAT IS NEW HERE
Higher sodium intake increased bloating in a dosedependent manner, regardless of the diet.
Even at the highest sodium level, participants consuming the control diet experienced less bloating than at baseline.
One possible explanation is that the control diet at the highest sodium level still provided lesssodium than participants’ freeliving diet before the feeding intervention.
The high-fiber DASH diet also increased bloating compared with the control diet, with a greater effect in men than in women.
ACKNOWLEDGEMENTS
We are indebted to the study participants for their sustained commitment to the DASH–Sodium trial; to the Almond Board of California, Beatrice Foods, Bestfoods, Cabot Creamery, C.B. Foods, Dannon, Diamond Crystal Specialty Foods, Elwood International, Hershey Foods, Hormel Foods, Kellogg, Lipton, McCormick, Nabisco U.S. Foods Group, Procter & Gamble, Quaker Oats, and Sun-Maid Growers for donating food; and to Frost Cold Storage for food storage.
Financial support: The study was supported by cooperative agreements and grants from the National Heart, Lung, and Blood Institute (U01-HL57173, to Brigham and Women’s Hospital; U01-HL57114, to Duke University; U01-HL57190, to Pennington Biomedical Research Institute; U01-HL57139 and K08 HL03857-01, to Johns Hopkins University; and U01-HL57156, to Kaiser Permanente Center for Health Research) and by the General Clinical Research Center Program of the National Center for Research Resources (M01-RR02635, to Brigham and Women’s Hospital, and M01-RR00722, to Johns Hopkins University). N.T.M. was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K01HL141589. S.P.J. was supported by NIH/NHLBI K23HL135273 and NIH/NHLBI R21HL144876.
Footnotes
This trial is registered at clinicaltrials.gov, number: NCT00000608.
Potential competing interests: None.
REFERENCES
- 1.Ryu MS, Jung HK, Ryu J, et al. Clinical dimensions of bloating in functional gastrointestinal disorders. J Neurogastroenterol Motil 2016;22;509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agrawal A, Whorwell PJ. Review article: Abdominal bloating and distension in functional gastrointestinal disorders—epidemiology and exploration of possible mechanisms. Aliment Pharmacol Ther 2008;27;2–10. [DOI] [PubMed] [Google Scholar]
- 3.Sandler RS, Stewart WF, Liberman JN, et al. Abdominal pain, bloating, and diarrhea in the United States: Prevalence and impact. Dig Dis Sci 2000;45;1166–71. [DOI] [PubMed] [Google Scholar]
- 4.Johnsen R, Jacobsen BK, Førde OH. Associations between symptoms of irritable colon and psychological and social conditions and lifestyle. Br Med J (Clin Res Ed) 1986;292:1633–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drossman DA, Li Z, Andruzzi E, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci 1993;38:1569–80. [DOI] [PubMed] [Google Scholar]
- 6.Ringel Y, Williams RE, Kalilani L, et al. Prevalence, characteristics, and impact of bloating symptoms in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 2009;7:68–72; quiz 3. [DOI] [PubMed] [Google Scholar]
- 7.Chang L, Lee OY, Naliboff B, et al. Sensation of bloating and visible abdominal distension in patients with irritable bowel syndrome. Am J Gastroenterol 2001;96:3341–7. [DOI] [PubMed] [Google Scholar]
- 8.Agrawal A, Houghton LA, Reilly B, et al. Bloating and distension in irritable bowel syndrome: The role of gastrointestinal transit. Am J Gastroenterol 2009;104:1998–2004. [DOI] [PubMed] [Google Scholar]
- 9.Kanazawa M, Miwa H, Nakagawa A, et al. Abdominal bloating is the most bothersome symptom in irritable bowel syndrome with constipation (IBS-C): A large population-based internet survey in Japan. Biopsychosoc Med 2016;10:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Safaee A, Moghimi-Dehkordi B, Pourhoseingholi MA, et al. Bloating in irritable bowel syndrome. Gastroenterol Hepatol Bed Bench 2011;4:86–90. [PMC free article] [PubMed] [Google Scholar]
- 11.Lembo T, Naliboff B, Munakata J, et al. Symptoms and visceral perception in patients with pain-predominant irritable bowel syndrome. Am J Gastroenterol 1999;94:1320–6. [DOI] [PubMed] [Google Scholar]
- 12.Gonlachanvit S, Coleski R, Owyang C, et al. Inhibitory actions of a high fibre diet on intestinal gas transit in healthy volunteers. Gut 2004;53:1577–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meneton P, Jeunemaitre X, de Wardener HE, et al. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev 2005;85:679–715. [DOI] [PubMed] [Google Scholar]
- 14.He FJ, MacGregor GA. A comprehensive review on salt and health and current experience of worldwide salt reduction programmes. J Hum Hypertens 2009;23:363–84. [DOI] [PubMed] [Google Scholar]
- 15.Strazzullo P, D’Elia L, Kandala NB, et al. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. BMJ 2009; 339:b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mugavero KL, Gunn JP, Dunet DO, et al. Sodium reduction: An important public health strategy for heart health. J Public Health Manag Pract 2014;20:S1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Millen BE, Abrams S, Adams-Campbell L, et al. The 2015 dietary guidelines advisory committee scientific report: Development and major conclusions. Adv Nutr 2016;7:438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahle LE, Kelly PV, Eliot KA, et al. Acute sodium bicarbonate loading has negligible effects on resting and exercise blood pressure but causes gastrointestinal distress. Nutr Res 2013;33:479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang C, Huang Z, Yu K, et al. High-salt diet has a certain impact on protein digestion and gut microbiota: A sequencing and proteome combined study. Front Microbiol 2017;8:1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cappuccio FP. Cardiovascular and other effects of salt consumption. Kidney Int Suppl (2011) 2013;3:312–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rakova N, Kitada K, Lerchl K, et al. Increased salt consumption induces body water conservation and decreases fluid intake. J Clin Invest 2017. 127:1932–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullivan SN Functional abdominal bloating with distention. ISRN Gastroenterol 2012;2012:721820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weidemann BJ, Voong S, Morales-Santiago FI, et al. Dietary sodium suppresses digestive efficiency via the Renin-angiotensin system. Sci Rep 2015;5:11123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilck N, Matus MG, Kearney SM, et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 2017;551:585–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riezzo G, Orlando A, D’Attoma B, et al. Randomised double blind placebo controlled trial on Lactobacillus reuteri DSM 17938: Improvement in symptoms and bowel habit in functional constipation. Benef Microbes 2018;9:51–60. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Sun F, Guo Y, et al. High-salt diet gets involved in gastrointestinal diseases through the Reshaping of gastroenterological milieu. DIG 2018. [Epub ahead of print Oct 5, 2018.] [DOI] [PubMed]
- 27.Marthinsen D, Fleming SE. Excretion of breath and flatus gases by humans consuming high-fiber diets. J Nutr 1982;112:1133–43. [DOI] [PubMed] [Google Scholar]
- 28.Bergmann JF, Chassany O, Petit A, et al. Correlation between echographic gastric emptying and appetite: Influence of psyllium. Gut 1992;33:1042–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foley A, Burgell R, Barrett JS, et al. Management strategies for abdominal bloating and distension. Gastroenterol Hepatol (N Y) 2014; 10:561–71. [PMC free article] [PubMed] [Google Scholar]
- 30.Ho KS, Tan CY, Mohd Daud MA, et al. Stopping or reducing dietary fiber intake reduces constipation and its associated symptoms. World J Gastroenterol 2012;18:4593–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanson CF, Winterfeldt EA. Dietary fiber effects on passage rate and breath hydrogen. Am J Clin Nutr 1985;42:44–8. [DOI] [PubMed] [Google Scholar]
- 32.Haro C, Rangel-Zúñiga OA, Alcalá-Díaz JF, et al. Intestinal microbiota is influenced by gender and body mass index. PLoS One 2016;11:e0154090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fransen F, van Beek AA, Borghuis T, et al. The impact of gut microbiota on gender-specific differences in immunity. Front Immunol 2017;8:754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dominianni C, Sinha R, Goedert JJ, et al. Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PLoS One 2015;10:e0124599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mueller S, Saunier K, Hanisch C, et al. Differences in fecal microbiota in different european study populations in relation to age, gender, and country: A cross-sectional study. Appl Environ Microbiol 2006;72:1027–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yurkovetskiy L, Burrows M, Khan AA, et al. Gender bias in autoimmunity is influenced by microbiota. Immunity 2013;39:400–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de la Cuesta-Zuluaga J, Kelley ST, Chen Y, et al. Age- and sex-dependent patterns of gut microbial diversity in human adults. mSystems 2019;4(4). pii:e00261–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martens EC, Lowe EC, Chiang H, et al. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biol 2011;9:e1001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scott KP, Martin JC, Duncan SH, et al. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. FEMS Microbiol Ecol 2014;87:30–40. [DOI] [PubMed] [Google Scholar]
- 40.Seo AY, Kim N, Oh DH. Abdominal bloating: Pathophysiology and treatment. J Neurogastroenterol Motil 2013;19:433–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holscher HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017;8:172–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sawicki CM, Livingston KA, Obin M, et al. Dietary fiber and the human gut microbiota: Application of evidence mapping methodology Nutrients 2017;9:E125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iovino P, Bucci C, Tremolaterra F, et al. Bloating and functional gastrointestinal disorders: Where are we and where are we going? World J Gastroenterol 2014;20:14407–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holscher HD, Caporaso JG, Hooda S, et al. Fiber supplementation influences phylogenetic structure and functional capacity of the human intestinal microbiome: Follow-up of a randomized controlled trial. Am J Clin Nutr 2015;101:55–64. [DOI] [PubMed] [Google Scholar]
- 45.Jiang X, Locke GR III, Choung RS, et al. Prevalence and risk factors for abdominal bloating and visible distention. Gut 2008;57:756–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lacy BE, Gabbard SL, Crowell MD. Pathophysiology, evaluation, and treatment of bloating. Gastroenterol Hepatol (N Y) 2011;7:729–39. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.