Cardiac cell therapy using adult cells with stem or progenitor-like properties has been under clinical investigation for over 15 years. However, the cumulative results from dozens of trials suggest a slight, transient benefit at best1, 2. The underlying rationale for these trials was derived from studies in small and large animals where a more consistent functional benefit has been observed3, albeit by still unclear mechanisms. Most trials to-date used whole unfractionated bone marrow mononuclear cells (MNCs) delivered systemically via the circulation, usually by intracoronary infusion1, 2. This strategy was also based on prior rodent studies in which infused adult stem cells were reported to home to the injured heart and restore contractile function, in part through direct regeneration of myocardium. Our results here and some recent data in the field show that infused “progenitor” cells simply perish and do not penetrate the capillary network of the heart to generate new cardiac myocytes; thus cell infusion is now proposed to work through temporary secretion of rejuvenating factors from these cells within the circulation or when lodged in other tissues4. Here we critically re-evaluated the hypothesis that function of the injured rodent heart can be improved with systemic infusion of unfractionated bone marrow MNCs, versus MNCs delivered by direct intraparenchymal injection.
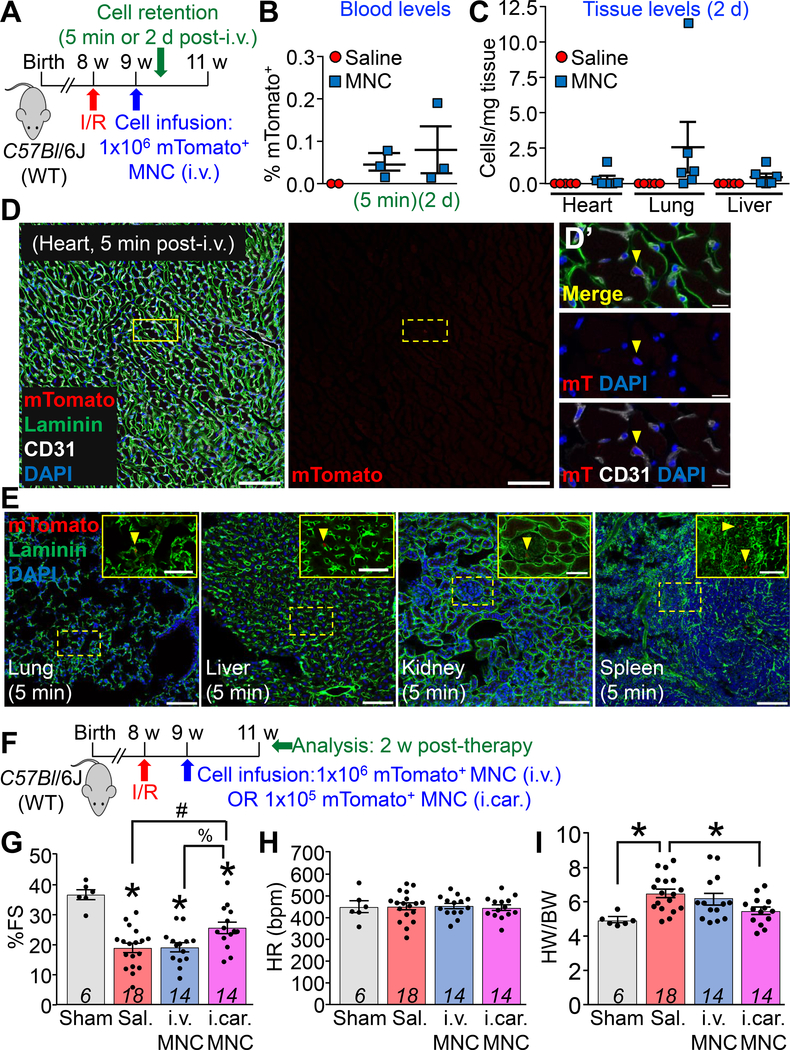
Eight week-old male and female C57Bl/6J (wild-type) mice received acute ischemia/reperfusion (I/R) injury (120 min. of ischemia) followed by MNC therapy or saline at 1 week post-I/R, to mimic clinical use of cell therapy in patients with pre-existing myocardial infarction1, 2 (Figure, A). All animal procedures were conducted in accordance with institutional guidelines and were approved by the IACUC. For in vivo tracking, MNCs were isolated from strain-matched Rosa26-mTomato/mGFP reporter mice that express ubiquitous membrane-localized TdTomato (mTomato). MNCs were isolated from an equal number of 8 week-old male and female mice via Ficoll density centrifugation and delivered intravenously in sterile saline via tail vein at a dose of 1×106 cells, in line with clinical studies of systemic infusion1, 2. We first assessed MNC retention by detection of mTomato using flow cytometry. mTomato+ MNCs in circulation accounted for less than 0.2% of all blood cells, either 5 min after infusion or by 2 days post-infusion (Figure, B). MNCs were largely absent from all tissues examined, including heart and lung (Figure, C), accounting for less than three cells per milligram tissue weight, or roughly 450 MNCs in one entire mouse heart. Interestingly, confocal microscopy showed the few rare mTomato+ MNCs observed in the heart were always within CD31+ capillaries and not free within the parenchyma (Figure, D). Similarly, minimal retention was observed in other organs including lung, liver, kidney, and spleen (Figure, E). Thus, MNCs infused into mice with acute I/R injury are rapidly cleared within minutes and are not retained at physiologically meaningful levels in any tissue, injured or otherwise.
Figure: Bone Marrow Mononuclear Cells Delivered by Vascular Infusion Do Not Persist or Improve Cardiac Function in Ischemia/reperfusion-injured Mice.
A. Experimental scheme and timeline for cell retention studies using genetically labeled bone marrow mononuclear cells (MNCs) isolated from Rosa26-mTomato mice on the C57Bl/6J background. 1×106 mTomato+ MNCs or sterile saline was infused intravenously (i.v.) by tail vein into C57Bl/6J (wild-type) mice, 1 week after ischemia/reperfusion (I/R) injury via 120 min of left coronary artery ischemia. Male and female mice were used in all experiments and for MNC isolations. B. Quantitative flow cytometry analysis of mTomato fluorescence from peripheral blood of post-I/R C57Bl/6J mice infused with MNCs or saline. mTomato+ MNCs accounted for less than 0.2% of all circulating nucleated blood cells at 5 min or 2 d after cell infusion. C. Quantitative flow cytometry analysis of single-cell suspensions prepared from dissociated whole hearts, lungs, or livers of post-I/R C57Bl/6J mice infused with MNCs or saline, 2 d post-infusion. mTomato+ MNCs accounted for less than 3 cells per milligram of tissue across each organ. D, E. Representative confocal micrographs showing histological sections from hearts (D) or lungs, livers, kidneys, and spleens (E) from n=3 C57Bl/6J mice infused at 1 week post-I/R with mTomato+ MNCs and harvested for analysis 5 min after infusion. Immunohistochemistry was performed against laminin (green) and CD31 (white) along with endogenous mTomato fluorescence (red). Nuclei were visualized with DAPI (blue). Scale bars in D = 100 μm. Yellow dashed lines in D denote a rare mTomato+ MNC (mT) within the cardiac vasculature as shown in D’ with high magnification insets. Scale bars in D’ = 10 μm. Images in E show additional organs from the mice in D. Scale bars = 100 μm. Yellow dashed lines denote rare mTomato+ MNCs, shown in enlarged insets above. Inset scale bars = 50 μm. F. Experimental scheme and timeline for post-I/R functional studies in C57Bl/6J mice following MNC delivery. At 1 week post-I/R, animals received either intravenous infusion (i.v.) of 1×106 mTomato+ MNCs, direct intramyocardial injection (i.car) of 1×105 mTomato+ MNCs (1/10th the infusion dose, over two injection sites flanking the infarct border zone), or sterile saline. Animals that received infusion versus intramyocardial injection of saline showed no difference in cardiac function or structure post-I/R, so these groups were combined (denoted as Sal.). G-I. Experimental groups as described in F were assessed by 2D M-mode echocardiography, 2 weeks after cell therapy (3 weeks post-I/R). Left ventricular fractional shortening (%FS; G) was significantly decreased in all post-I/R groups versus sham-operated controls at 2 weeks. Direct intramyocardial injection of MNCs but not MNC infusion attenuated this cardiac dysfunction. *p<0.05 versus Sham; #p<0.05 versus Sal.; %p<0.05 versus i.v. MNC, all by one-way ANOVA with Tukey’s multiple comparisons test. Heart rates (H) were equivalent in all groups. Cardiac hypertrophy (I) as assessed via gravimetric analysis of heart weight/body weight (HW/BW) ratio was attenuated at 2 weeks post-therapy by MNC direct injection, but not MNC infusion. *p<0.05 versus Sal. by Kruskal-Wallis with Dunn’s multiple comparisons test.
We also assessed whether delivery of MNCs could improve function of the injured heart as suggested in various human clinical trials1, 2. At 1 week post-I/R, mice were either infused with 1×106 MNCs (i.v.), or we delivered MNCs directly into either side of the infarct border zone via intramyocardial injection (i.car.) at a dose of 1×105 total MNCs, one-tenth the infusion amount (Figure, F). Systemic infusion of MNCs did not alter cardiac dysfunction or adverse remodeling compared to saline controls when assessed 2 weeks later (Figure, G–I). However, direct intramyocardial injection of 1/10th the dosage of the same MNCs significantly improved systolic function (Figure, G, H) and attenuated cardiac hypertrophy (Figure, I) post-I/R. Taken together these data indicate that the previously reported beneficial effects of MNC therapy in the rodent heart are not achieved with systemic infusion. In contrast, direct intramuscular delivery of MNCs flanking the infarcted region of the heart mildly, albeit significantly improved cardiac function over 2 weeks.
Our data do not support a mechanism where systemically infused stem or progenitor-like cells seed distal organs and produce paracrine-mediated cardiac rejuvenation (Figure, G). The miniscule amount of MNCs retained after infusion were trapped within the vasculature (Figure, B–E) and seemingly below a quantitative level that could have a global paracrine secretory effect on the heart, although local signaling via secreted factors could be occurring in regions where the few MNCs were present. In contrast, direct injection of MNCs on both sides of the infarct region was beneficial post-I/R in mice. For patient safety reasons, most clinical trials conducted to-date simply employed vascular infusion, with little understanding of the efficacy of such a route, especially because most animal model data used direct intraparenchymal injection. We have recently shown that direct injection of nearly any cell type into the parenchyma of the heart surrounding the injured region produces a localized acute immune response that further enhances healing by affecting the extracellular matrix and/or microvasculature5. Future uses of cardiac cell-based therapy in humans warrants careful re-examination as to why systemic vascular infusion would be selected, especially because we now understand that these cells do not make it through the capillaries, nor persist from a distal region as a global source of secreted paracrine factors.
Data Sharing
Requests of materials, datasets, and protocols used in this study should be directed to the corresponding author and will be made available to investigators upon reasonable request.
Acknowledgements
We thank Jeff Bailey and Victoria Summey in the Cincinnati Children’s Hospital Medical Center (CCHMC) Comprehensive Mouse and Cancer Core for assistance with intravenous infusions. All flow cytometric data were acquired using equipment maintained by the Research Flow Cytometry Core in the Division of Rheumatology at CCHMC.
Sources of Funding
This study was supported by grants from the National Institutes of Health (NIH) and by the Howard Hughes Medical Institute and American Heart Association MERIT award (to J.D.M.). R.J.V. was supported by a National Research Service Award from the NIH (F32 HL128083) and a Career Development Award from the American Heart Association (19CDA34670044).
Footnotes
Disclosures
None.
REFERENCES
- 1.Nguyen PK, Rhee JW and Wu JC. Adult Stem Cell Therapy and Heart Failure, 2000 to 2016: A Systematic Review. JAMA Cardiol. 2016;1:831–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher SA, Zhang H, Doree C, Mathur A and Martin-Rendon E. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev. 2015;9:CD006536. doi: 10.1002/14651858.CD006536.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tompkins BA, Balkan W, Winkler J, Gyongyosi M, Goliasch G, Fernandez-Aviles F and Hare JM. Preclinical Studies of Stem Cell Therapy for Heart Disease. Circ Res. 2018;122:1006–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wysoczynski M, Khan A and Bolli R. New Paradigms in Cell Therapy: Repeated Dosing, Intravenous Delivery, Immunomodulatory Actions, and New Cell Types. Circ Res. 2018;123:138–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vagnozzi RJ, Maillet M, Sargent MA, Khalil H, Johansen AK, Schwanekamp JA, York AJ, Huang V, Nahrendorf M, Sadayappan S, Molkentin JD. An acute immune response underlies the benefit of cardiac stem-cell therapy. Nature. 2019;doi: 10.1038/s41586-019-1802-2. [DOI] [PMC free article] [PubMed] [Google Scholar]