Abstract
Astrocytes are the most abundant cell type in the central nervous system and have diverse functions in blood–brain barrier maintenance, neural circuitry formation and function, and metabolic regulation. To better understand the diverse roles of astrocytes, we will summarize what is known about astrocyte development and the challenges limiting our understanding of this process. We will also discuss new approaches and technologies advancing the field.
Keywords: Glia, Astrocyte, Gliogenesis, Neurodevelopment
Introduction
The central nervous system (CNS) is composed of multiple highly specialized cell types, including neurons, astrocytes, oligodendrocytes, microglia, and endothelial cells, each with different functions that are highly adapted to local circuitries 1– 3. Astrocytes are the most abundant glial cells in the CNS, representing between 20 and 40% of total cells in the brain 4, 5. Astrocytes were first described by Rudolf Virchow as a homogenous connective tissue supporting nervous system elements. Later, Ramon y Cajal and others investigated the cellular structure of the brain and observed that different brain regions contain morphologically distinct neuronal and glial cell types.
Over the past century, astrocytes have emerged as a bridge to the outside world for neurons with diverse physiological roles in neural development, neural circuit function, neurotransmission, blood–brain barrier formation, and metabolic support. Astrocytes form the neurovascular unit between neurons and endothelial cells, and their end-feet structure maintains brain homeostasis by regulating water, amino acid, and neurotransmitter uptake. Astrocytes also have the ability to monitor the ongoing local activity of synaptic circuits with their elaborate processes ensheathing synapses and forming tripartite synapses. Astrocytes express neurotransmitter receptors that sense synaptic activity and respond to it by elevating astrocytic Ca 2+ and secreting neuroactive molecules back to synapses. Despite these known roles, how astrocytes develop and mature to form functional neurovascular circuits to carry out these diverse functions remains relatively unknown 1– 4.
Previous studies have shed light on how neurons and oligodendrocytes develop in a stepwise fashion, where they specify, undergo terminal differentiation, and enter the postmitotic stage. Since the initial observation by Ramon y Cajal, hundreds of types of neurons have been identified and functionally characterized, while astrocytes are still broadly classified as either protoplasmic or fibrous. The lack of markers and tools to access the precursor and intermediate stages of astrocyte development has hindered characterization of their development and heterogeneity. Further complicating their characterization, astrocytes are more plastic than neurons and proliferate after specification. However, with their critical and diverse functions that actively regulate neuronal function, it is essential to understand where, when, and how astrocytes are generated during development.
Stages of astrocyte development
Neural stem cells (NSCs), or radial glia, generate astrocytes through complex intrinsic and extrinsic cellular processes. This sequential series of events results in mature astrocytes that actively participate in CNS physiology. Conserved mechanisms regulate gliogenesis in the spinal cord and brain, although the process begins early, at embryonic day 11.5 (E11.5), in the spinal cord and later, at E18, in the brain. Whereas spinal cord astrocytes are derived from the ventricular zone (VZ), forebrain astrocytes are from the ventricular–subventricular zone (V-SVZ) 6. Below, we discuss recent progress toward understanding astrocyte development and maturation, beginning with patterning and specification, proliferation, and maturation.
Astrocyte specification and developmental patterning
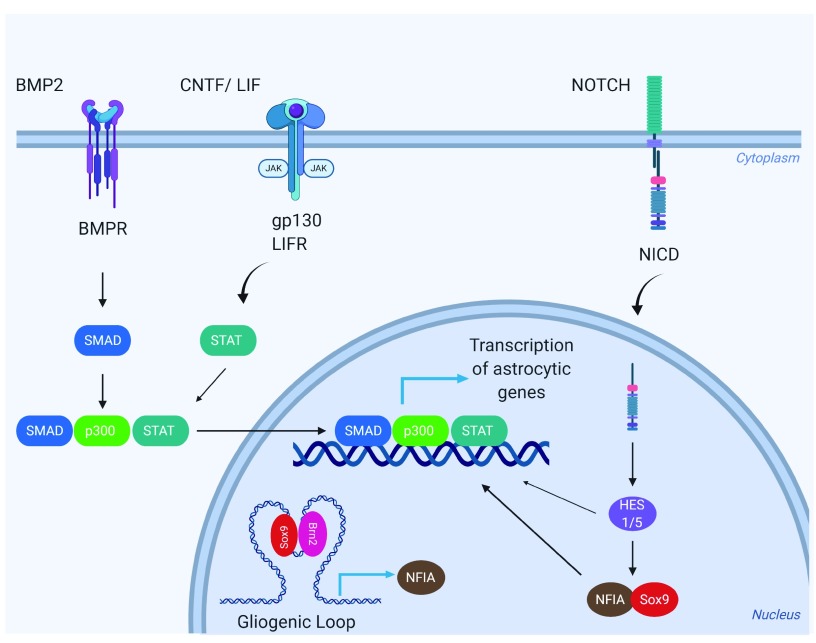
During CNS development, neurons are specified from NSCs before glial cells, and radial glia serve as a scaffold for this process. Signaling pathways and dynamic transcription factor expression control these cell fate decisions. Previous studies have focused on the spinal cord VZ, where the timing of the gliogenic switch in NSCs is clearly defined. In the VZ, neurogenesis ceases at E11.5 and gliogenesis commences at E12.5, and transcription factors sex-determining region Y-box 9 (Sox9) and nuclear factor-I A (NFIA) play critical roles during this developmental interval. Sox9 and brain-specific homeobox/POU domain protein 2 (Brn2) regulate NFIA induction and glial specification 7. NFIA is both necessary and sufficient for embryonic gliogenesis 8, and the association between Sox9 and NFIA regulates genes essential for astrocyte migration and maturation 9 ( Figure 1). In neocortical development, zinc finger- and BTB domain-containing protein 20 (Zbtb20) was shown to promote astrocyte specification while suppressing the production of oligodendrocyte precursors (OPCs), and knockdown of either NFIA or Sox9 suppresses Zbtb20 activity 10.
Figure 1. Molecular signaling pathways involved in astrocytogenesis.
Neural stem cells (NSCs) express astrocytic genes in response to several signaling molecules, including bone morphogenic protein (BMP) families, the leukemia inhibitory factor/ciliary neurotrophic factor (LIF/CNTF), and Notch pathway. BMPs signal primarily through SMAD, whereas LIF/CNTF activates the JAK/STAT pathways. The active SMAD–STAT complex bridged by p300 goes directly into the nucleus, binds to the promoter, and activates astrocytic genes such as GFAP and S100. Another important pathway that regulates astrocytogenesis is the Notch pathway. Notch ligands will activate Notch receptors and activate the expression of Hes genes, Hes1/5. Hes1, Hes5, and activated forms of Notch receptors induce the expression of astrocytic genes and glial promoting transcription factor nuclear factor-I A (NFIA). In early development, a pre-formed gliogenic loop serves to facilitate the association between sex-determining region Y-box 9 (Sox9) and brain-specific homeobox/POU domain protein 2 (Brn2), which also drives expression of NFIA. NFIA is both necessary and sufficient for the induction of astrocytic genes.
Notch signaling is another important regulator of cell differentiation. In early development, Notch activation maintains the NSC pool in addition to inducing NFIA. NFIA maintains the continued inhibition of neurogenesis through induction of the Notch effector Hes5, further clarifying the role of Notch signaling in early gliogenesis 8. NFIA associates with astrocytic gene promoters such as GFAP to disrupt their methylation through association with DNA methylating dissociation of the DNA methylating enzyme 1 (Dnmt1) 11, 12. Loss of function of Notch effectors such as Hes5 leads to loss of glia, and gain of function produces more glia; however, Notch activity alone is insufficient to drive precocious astrocytic differentiation; thus, Notch signaling is permissive and not instructive for gliogenesis 13 ( Figure 1).
During early development, the embryonic spinal cord is patterned throughout the dorsoventral axis by a combination of morphogens—sonic hedgehog (Shh), BMPs, and Wnts—which regulate the combinatorial expression of homeodomain transcription factors 14. These patterning principles govern what type of progenitor cells will be generated from.
The first evidence of patterning during astrocyte development emerged in 2005, when Muroyama et al. showed that the basic helix-loop-helix (bHLH) transcription factor Scl/tal1 (stem cell leukemia, Tal) promotes astrogenesis via a cross-antagonistic relationship with the oligodendrocyte transcription factor Olig2 15. At the p2 and pMN domain boundary, the interaction between Scl and Olig2 allowed p2 domain to generate astrocyte precursors while the pMN domain produced oligodendrocyte precursors 15.
When expressed in different VZ domains, the transcription factors Pax6 and Nkx6.1 yield three distinct astrocyte subtypes (VA1, VA2, and VA3, dorsal to ventral) that arise from the p1, p2, and p3 progenitor domains, respectively 16. These populations are further demarcated by differential expression of Reelin and Slit. Tsai et al. used lineage tracing with Cre recombinases to specifically target these domains and demonstrated that astrocytes arise from different segmental domains throughout the brain and spinal cord 17. When specific astrocyte populations were ablated with a Cre-dependent diphtheria toxin A (DTA) approach, the authors observed that neighboring astrocytes from other progenitor domains were unable to migrate, suggesting that there are region-specific neuron–astrocyte interactions following developmental patters. This domain-specific identity is also observed in forebrain astrocytes, which showed dorsal-ventral restriction depending on the developing region they derived from, as a result of strictly radial migration 17. Overall, these studies illuminate the transcriptional code determining astrocytes’ regional identity and suggest that patterning also may lead to functional diversity.
Before terminal differentiation, astrocyte precursors migrate from VZ and SVZ along the radial glial processes. Newborn astrocytes continue to divide locally after migration. Initial studies identified these targeted precursor astrocytes with Glast 18, a glutamate aspartate transporter whose induction coincides with the gliogenic switch. A recent study identified new glial markers for the intermediate stages of astrocyte development using a Glast reporter mouse, including Asef, Gpr37l1, Mfge8, and Tom1l1 19. Asef is a guanine exchange factor that has a role in cell migration, and functional studies in vivo have shown that Asef is required for AQP4 expression on the end-feet of spinal cord astrocytes 19.
Our understanding of the stepwise specification and migration of astrocytes is improving with newly developed tools for cell lineage mapping, spatial and temporal profiling, and functional studies. However, several important questions remain. For instance, which other factors trigger the gliogenic switch in NSCs is an ongoing area of investigation. Furthermore, owing to the lack of markers for astrocyte precursors, it has been difficult to dissect the signaling mechanisms responsible for astrocyte specification and migration. Whether we can use the recently identified and functionally characterized intermediate markers to address these remains an open question.
Astrocyte differentiation
The differentiation trajectory of astrocytes in the postnatal mouse brain remains somewhat controversial 10, 20, 21. In one study, Nagao et al. identified astrocyte-lineage restricted progenitors using astrocyte lineage-specific marker Zbtb20 along with markers that label both astrocyte and oligodendrocyte precursors, Sox9 and Olig2 10. Using immune co-staining, the group identified the presence of Zbtb20 +/Sox9 +/Olig2 + triple-positive cells in the mouse postnatal day 3 (P3) neocortex and demonstrated that Zbtb20 +/Olig2 + cells were devoid of Sox10, a marker of OPCs 10. However, in other studies, several groups have identified progenitor cells that exhibited bipotent signatures, expressing cell markers of both the astrocytic (Aldh1l1, AldoC, Aqp4, and Slc1a3) and oligodendrocytic (Pdgfra, Olig1, and Olig2) lineages by microarray, immunofluorescent co-staining, and single-cell sequencing 20, 21. This inconsistency may stem from the current use of a single marker (for example, Aldh1l1 only or GFAP only) to identify astrocytes. Similar to the hematopoietic system, specific lineages may be better characterized by combinatorial codes of multiple cell markers. Recent advances in single-cell sequencing technology should enable us to discover and validate combinations of cell markers that better demarcate the intermediate stages of the astrocyte lineage.
Astrocytes express canonical markers before initiating terminal differentiation. One of the markers expressed at a later stage is glial fibrillary acidic protein (GFAP), which provides structural stability and motility to astrocytes. Several groups generated GFAP-Cre mouse lines to target astrocytes, but these lines also target neural progenitor cells 11– 13, 18, 22. Although GFAP is commonly used as a marker, it is weakly expressed in protoplasmic astrocytes in rodents. Other currently used astrocytic markers include calcium-binding protein S100 23, glutamate transporter GLAST 24, aldolase C 25, CD44 26, glutamine synthase (GS), and fatty acid-binding protein FABP7. Aquaporin 4 (AQP4) and connexins 30 (Cx30) have been used as astrocyte end-feet markers 27, 28. These markers are less specific than GFAP, as all of them also can be expressed in neurons, oligodendrocytes, or ependymal cells 14. Glutamate transporter, EAAT2, also known as GLT-1, is expressed in astrocytes and neurons, although 80% of total EAAT2 protein is expressed in astrocytes in the hippocampus 29. These markers can be used in a combinatorial approach. For example, Miller et al. used a specific promoter region of GLT-1 to generate a reporter mouse that targets gray matter astrocytes in the cerebral cortex, which interestingly are absent in the hippocampus 30. The authors further analyzed the transcriptome of this astrocytic population and determined its unique molecular profile, identifying a pathway-specific to it with the expression of the genes norrin and LRG6, which have roles in dendritic spine maintenance in this population 30. (Also, see the “Molecular maturation” section).
Recent genetic profiling studies identified aldehyde dehydrogenase family 1, member L1 (ALDH1L1), a metabolic enzyme, as the most homogenously expressed astrocyte marker throughout the brain. Aldh1L1 was reportedly used to stain cortical astrocytes, whereas hippocampal astrocytes are widely stained with GFAP 31. Morel et al. also used a combinatorial approach with the Aldh1L1-GFP transgene reporter mouse with the EAAT2-tdtomato mouse line and identified a tdT −eGFP + astrocyte population that is selectively localized at layers I and II in the cortex 31. Furthermore, this population of cells showed increased resting membrane potential and resistance and reduced potassium channel Kir4.1 expression 31. The recently developed Aldh1L1-CreER transgenic mice should allow astrocyte-specific manipulations 32.
Transcription factors NFIA and SOX9 are also non–stage-specific astrocytes markers, although NFIA also is expressed in oligodendrocyte precursor cells and some neurons. On the other hand, Sox9 is not expressed in neurons and recently was identified as an astrocyte-specific marker in the adult brain. Henceforth, Sox9 may be an important tool to access astrocytes in the adult brain 32– 34.
GFAP expression is used as an indicator of astrocyte maturation and has given significant insight into the mechanisms regulating astrocyte differentiation. Previously, it was used to identify the JAK-STAT pathway and BMPs and Notch signaling as central players controlling astrocyte differentiation from precursor cells 35, 36. Recently, a large-scale interchromosomal interaction study identified Brahma-related gene 1 (BRG1), an ATP-dependent chromatin remodeling factor, as clustering with the GFAP gene and regulating GFAP expression 37.
In addition to these general astrocyte markers, astrocyte markers for anatomically distinct populations have been identified in recent studies. Molofsky et al. used the Aldhl1L1-GFP transgene reporter mouse to identify distinct molecular differences between dorsal and ventral astrocytes and identified Sema3a, an axon guidance protein, as being highly expressed in ventral astrocytes 38. Ventral region motor neurons α–MN failed to maintain axon initial segment orientation following loss of astrocytic Sema3a, affecting their survival and function. That study was the first to show how positional cues by diverse astrocytes maintain specific circuitry 38. Inwardly rectifying potassium channel, Kir4.1, also was shown to be enriched in astrocytes of the spinal cord ventral horn, which support the survival and function of motor neurons 39. Another study compared adult striatal and hippocampal astrocytes and identified μ-crystallin Crym as a striatal astrocyte-specific marker 2. Although the functional significance of this protein is unknown, it is the first marker that defines a region-specific astrocyte population in the brain. These studies indicate that the molecular and anatomical properties of astrocyte subpopulations may yield insight into their function.
Although our knowledge of astrocyte biology continues to expand, how mature astrocytes are formed and differentiate to carry out their diverse roles remains unclear. Historically, GFAP has been used to understand how external inputs affect astrocyte maturation, even though it is not the most comprehensive astrocyte marker in rodents. In the future, identifying region-specific astrocyte markers may allow us to study specific circuits. In addition, we have not yet identified a developmental endpoint for astrocytes. In the CNS, terminally differentiated neurons and oligodendrocytes are postmitotic while GFAP-expressing astrocytes retain their ability to proliferate (discussed in further detail in the next section).
Astrocyte proliferation
Neurogenesis is complete in most regions of the brain at birth, and the same number of neurons is maintained throughout life 40. On the other hand, concurrent with brain growth during this period, the number of glial cells increases six- to eight-fold during the first three weeks of postnatal development 41. Given the diverse functions of astrocytes, which facilitate maturation of the neuronal network during this critical developmental period 42, it is important to understand where, when, and how astrocytes proliferate to reach their final population size.
Over the past few decades, it has become clear that cortical astrocytes are generated from four main sources in successive yet overlapping chronological order 43– 45. Each stage of development takes place in a specific anatomical region; astrocyte differentiation first occurs from radial glia in the VZ of late embryonic-perinatal brain (1), followed by astrocytes in the cortex of postnatal brain (2), progenitor cells in the SVZ of postnatal and adult brain (3), and NG2 cells in the cortex of postnatal or adult brain or both (4). However, as discussed above, astrocytes in the developing spinal cord are generated mainly from radial glia and astrocyte progenitors derived from radial glia at an earlier developmental stage. Below, we will focus on developmental astrocytogenesis from the VZ and local proliferation in the cortex, which are major sources of astrocytogenesis and together contribute to about 80% of cortical astrocytes 45. Meanwhile, we will briefly describe the process of astrocyte proliferation in the spinal cord. For SVZ and NG2 cell-derived astrocytes in the cortex, please see reviews 43– 45.
Astrocyte generation in the ventricular zone
Radial glia are bipolar cells with their soma residing in the VZ of the embryonic CNS. Radial glia extend and anchor one branch to the ventricular wall and the other to either the pial surface or blood vessels 46– 50. During the late embryonic stage, radial glia are the major source of astrocytogenesis, and their bipolar structure provides a scaffold for the migration of newborn astrocyte precursor cells 50.
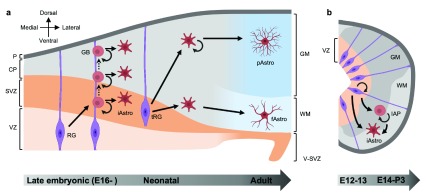
Astrocytogenesis from radial glia in the developing cortex occurs in two waves. In the first wave, glia progenitors or glioblasts are derived from the asymmetrical division of radial glia. Glioblasts are proliferative glia progenitors found between the late embryonic (E16–E18) and perinatal stage in the mouse cortex 50, 51. Upon generation, these glia progenitors migrate radially from the VZ/SVZ and undergo several rounds of proliferation on their way out, giving rise to multiple clusters of astrocytes in the same cortical column of the postnatal cortex 52– 54. The second wave, which occurs at the terminal stage of differentiation, results from the direct transformation of radial glia. Between the late embryonic and early perinatal stage, radial glia detach their anchorage from VZ and lift their soma toward the pial surface, resulting in unipolar transitional radial glia (tRG). These tRG undergo terminal differentiation to give rise to protoplasmic and fibrous astrocytes in the gray matter and white matter of cortex, respectively ( Figure 2a). Using retroviral-mediated lineage tracing, organotypic slice culture, and confocal time-lapse imaging on rat brain slices at E16, Noctor et al. demonstrated direct evidence that individual radial glial cells transform into astrocytes at the terminal stage of differentiation 49. The presence of tRG-derived astrocytes has been seen across several different species, including monkey, ferret, human, and rodents, suggesting an evolutionarily conserved mechanism that controls terminal differentiation of radial glia into astrocytes 46– 50.
Figure 2. Astrocytogenesis from the ventricular/subventricular zones and outer cortical layers in the mouse developing central nervous system (CNS).
( a) In the developing cortex, radial glia (RG) first give rise to glioblasts (GBs) during the late embryonic to perinatal period. Glioblasts undergo several rounds of division while migrating out along radial glia, resulting in clusters of astrocytes in the developing cortex. At the terminal stage of radial glial differentiation, radial glia detach from the ventricular zone and form unipolar transitional radial glia (tRG), which give rise to protoplasmic and fibrous astrocytes in the gray matter and white matter of the cortex, respectively. During the early postnatal period, differentiated astrocytes in the outer cortical layer undergo symmetric division and generate daughter astrocytes that exhibit astrocytic morphology and functions. ( b) In the developing spinal cord, radial glia first proliferate during embryonic day 12 (E12) to 13, giving rise to radial glial pool which differentiates into astrocytes between E14 and postnatal day 3 (P3). Alternatively, radial glial cells differentiate into intermediate astrocyte precursors (IAPs), which proliferate during E14 to P3 and undergo terminal differentiation, ultimately giving rise to astrocytes. The progression from embryonic stage to adult is shown from left to right below each panel. Straight arrows indicate differentiation or maturation from one cell type to another. Circular arrows indicate proliferation. Dashed arrows indicate migration. CP, cortical plate; fAstro, fibrous astrocyte; GM, gray matter; iAstro, immature astrocyte; P, pia mater; pAstro, protoplasmic astrocyte; SVZ, subventricular zone; VZ, ventricular zone; WM, white matter.
Parallel to findings in the developing cortex, astrocytes in the spinal cord are also derived from radial glia, albeit earlier, between E14 and P3 17, 55, 56. Using Aldh1L1-GFP labeling, Tien et al. found two types of proliferative astrocyte precursors that exhibit distinct morphology 56. Radial glia in the VZ were the first precursors identified, and the second were astrocyte progenitors called intermediate astrocyte precursors (IAP) 56. These IAPs are generated first from radial glia before migrating out of the VZ into the mantle zone, where they undergo several rounds of division before terminally differentiating into spinal cord astrocytes ( Figure 2b).
Overall, these studies demonstrate that a conserved mechanism exists between the embryonic cortex and spinal cord, wherein radial glial cells first give rise to lineage-restricted glia progenitors, which then undergo multiple rounds of division to give rise to astrocytes in the postnatal CNS.
Astrocyte proliferation in the outer cortical layer
Early lineage-tracing studies with VZ/SVZ progenitors identified clusters of astrocytes in the postnatal cortex, indicating local proliferation of cells during or after migration or both 51, 52, 57. Although the field long hypothesized that these clusters derived from glioblasts 50, 51, 56, one study found that local proliferation of terminally differentiated astrocytes in the outer cortical layer (I–IV) of the early mouse postnatal brain (P0–P2) may have contributed to some of these clusters 58 ( Figure 2a). These parental cells exhibited astrocyte characteristics, including morphology, gap junction connectivity, and expression of astrocyte markers; however, they underwent symmetrical division to give rise to two functional daughter astrocytes. This symmetrical division was at its highest rate before P6 and decreases over time, resulting in the generation of 50% of total astrocytes at P28 58. Consistently, Moroni et al. identified, in the rat postnatal cortex, astrocytes that expressed differentiated astrocyte markers S100b or Aldh1L1 that co-expressed proliferation marker Ki67 59. The proliferation rate of these cells increased from P1 to P10 and decreased thereafter 59. The consistent timeline between mouse and rat suggests a conserved mechanism regulating the proliferative potential of these differentiated astrocytes. Conversely, in the adult SVZ, lineage tracing demonstrated that adult SVZ-derived astrocytes are mostly postmitotic with no signs of local proliferation 60. These studies suggested that astrocytes derived from different sources may have different proliferative potential once differentiated. Henceforth, it will be important to delineate the mechanisms that endow postnatal astrocytes with their unique proliferative potential.
Despite the above discoveries, several unanswered questions remain in the field. For example, since not all differentiated astrocytes proliferate 58, it will be important to uncover which mechanisms drive the transition between dividing to non-dividing astrocytes. Interestingly, Ge et al. demonstrated that dividing astrocytes exhibit slight changes in their membrane properties compared with surrounding non-dividing astrocytes, suggesting that dividing astrocytes retain or regain proliferative activity, which could result from local environmental cues or cell-intrinsic molecular mechanisms or both 58. Indeed, several previous studies identified clusters of astrocytes residing around specific structures of the brain, including blood vessels, the pial surface, and corpus callosum, or specific layers of the cortex 58, 61, indicating the presence of local environmental cues. As of today, we still know very little about the cell-intrinsic mechanisms underlying the local proliferation of differentiated astrocytes. Recent studies identified that YAP (yes-associated protein), a transcription co-factor of the Hippo signaling pathway, is required for the proliferation of astrocytes in postnatal neocortex via cooperation with the BMP-SMAD signaling pathway 62. However, more detailed study on the in vivo brain is needed. With new single-cell sequencing techniques, we should be able to delineate the transcriptomes of these dividing astrocytes and thus identify signaling pathways and associated environmental cues that alter gene expression to confer these dividing astrocytes with proliferative activity.
Studies during the embryonic stage have delineated gliogenesis during development, but our understanding of postnatal astrocytogenesis is still in the early stage. Answering the above questions should shed light on the mechanisms controlling postnatal astrocyte proliferation, which may be further applied in different contexts, including reactive gliosis and glioma.
Astrocyte maturation
For more than 100 years, astrocytes have been divided into two main subtypes—protoplasmic or fibrous—on the basis of differences in their cellular morphologies and location. During the late phase of astrocyte proliferation, astrocytes undergo morphological and molecular maturation to develop their characteristic “spongiform” morphology and tiny distal processes called perisynaptic astrocytic processes (PAPs). Protoplasmic astrocytes of the gray matter have PAPs with several stem branches, giving rise to fine branches that ensheathe neural synapses and form direct contact with blood vessels. Fibrous astrocytes of white matter have an elongated morphology and are in contact with myelinated axonal tracts and nodes of Ranvier 63. Concurrent with morphological maturation, both astrocyte types begin to express functional proteins, including channels and receptors in their membrane, and secrete synaptogenic factors. Below, we summarize the current understanding of morphological and molecular astrocyte maturation and discuss their functional implications.
Morphological maturation
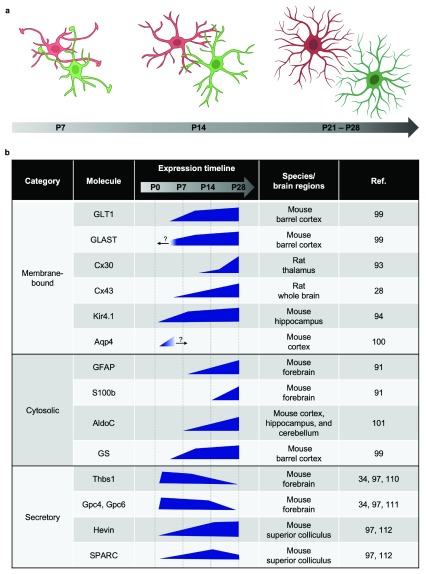
Early studies in postnatal rats delineated the stages of astrocyte morphogenesis in the hippocampus by using intracellular dye filling in fixed brain slices 64. At P7 and P14, astrocytes appear smaller and less ramified, and a dozen long processes stick out from soma and end with filopodia-like structures. The territory of these astrocytes is not well defined, and long extending branches often invade the “territory” of neighboring astrocytes. By P21, most of the filopodia-like structures disappear and fine distal processes appear, resulting in more ramified astrocytes with clear territories and processes with limited overlap among neighboring cells. Concurrently, from P7 to P14, astrocyte morphology becomes more homogeneous, and by P28, spongiform, highly ramified protoplasmic astrocytes are abundant ( Figure 3a).
Figure 3. Maturation of astrocytes during postnatal development demonstrated by progressive changes of morphology and gene expression.
( a) Morphology of astrocytes matures in a stepwise manner. At postnatal day 7 (P7), astrocytes exhibit few branches and the overall morphology is simple and territories are small. Long protrusions are often seen extending into the territories of the neighboring astrocytes. The boundaries of territories are not well-defined yet. At postnatal day 14 (P14), the number of branches become greater and astrocyte territories become bigger. More branch points grow out of existing branches, resulting in more complex morphology. Long protrusions are seen less, and the boundaries of territories start to emerge. At postnatal day 21 to day 28 (P21–P28), astrocytes develop their characteristic “spongiform” complex morphology, and there is minimal overlap between neighboring astrocytes and clear boundary of each territory. ( b) Genes that are induced during astrocyte maturation. Blue polygons indicate expression levels of the genes during development. Question marks indicate that the expression level beyond the developmental stage is unknown.
As astrocyte morphology matures, a specialized structure called PAP also appears at the distal ends of astrocytes. PAPs are extremely fine (<50 nm) distal processes positioned near synapses 65, 66. This arrangement allows communication between astrocytes and synapses and originally formed the basis of the concept of the “tripartite synapse” 64, 67, 68. To facilitate crosstalk with the synapse, PAPs exhibit high surface-to-volume ratios, providing ample space for the expression of channels, transporters, and receptors in the membranes, including glutamate transporters GLAST and GLT1 69, potassium channel Kir4.1 70, and glutamate receptors mGluR 3 and 5 71 (see the “Molecular maturation” section). With this specialized anatomical arrangement and protein localization, PAPs maintain homeostasis of the local environment at the synaptic cleft 69, 70 and also actively crosstalk with synapses and regulate their function 68, 71.
Given the close relationship between astrocyte processes and synapses, it is not surprising that neurons or neuronal activity or both participate in astrocyte morphogenesis and PAP plasticity 41, 72– 82. Previous studies demonstrated that astrocytes exhibit reduced territory and neuropil infiltration in dark-reared animals, providing evidence for the involvement of neuronal activity in astrocyte morphogenesis 73, 74. Several subsequent studies demonstrated that the neurotransmitter glutamate is one molecular mechanism underlying this activity-dependent morphogenesis 75– 77. For example, using a VGluT1 KO mouse model in which VGluT1 + synaptic activity is silenced, Morel et al. found that excitatory synaptic activity is required for the growth of astrocyte territory and for perisynaptic astrocyte processes to ensheathe synapses 75. Mechanistically, this crosstalk is mediated by an astrocytic glutamate receptor mGluR5-dependent signaling pathway and downstream intracellular Ca 2+ activity 75. The above findings suggest that the local environment guides astrocyte maturation, resulting in astrocyte processes that facilitate neighboring synapses.
Interestingly, a similar mechanism influences the structural plasticity of PAPs in the adult brain. Using time-lapse imaging on organotypic brain slices and anesthetized adult mouse, Bernardinelli et al. found that PAPs are highly motile at a time scale of several minutes 76. This structural plasticity is regulated by synaptic activity and is dependent on astrocyte metabotropic glutamate receptor mGluR1/5. Moreover, astrocytic Ca 2+ signaling, presumably downstream of mGluR1/5, is both necessary and sufficient for PAP motility. In that study, changes in PAP motility enhanced astrocytic coverage of the synapse and spine stability 76. Currently, whether glutamate also regulates PAP maturation and motility during development remains unknown. However, the conserved function of glutamate in controlling astrocyte morphology during development and PAP plasticity in adulthood suggests that glutamate might also play a role in PAP development during the postnatal period.
Neurons also regulate astrocyte morphogenesis and PAP plasticity via contact-dependent mechanisms, including neuroligin–neurexin 73, Notch signaling 72, and EphA4/ephrine-A3 79, 80, 83. In the case of neuroligin–neurexin interaction, direct contact between astrocytic neuroligins and presynaptic neurexin is required and sufficient for astrocyte morphogenesis and PAP development in vitro and in vivo 73. Moreover, the morphological effect of astrocytic neuroligins is associated with the development of local excitatory synapses. In the case of EphA4/ephrine-A3, neuronal EphA4 and astrocytic ephrin-A3 interact to maintain the proper structure of spine morphology and also are required for proper expression of glutamate transporters in the astrocytic PAPs that surround the spine 79, 80, 83. Together, the above studies reveal a contact-dependent reciprocal mechanism between astrocytic synapses and PAPs and demonstrate that astrocyte morphogenesis and synaptic formation and function are tightly linked components that coordinate during the critical period of postnatal synaptogenesis.
Although the biological consequences of neuron–astrocyte interactions are well characterized, the downstream mechanisms by which their contact results in morphological changes or PAP plasticity are still limited. Interestingly, there is a plethora of evidence demonstrating the involvement of actin filaments and their regulators in astrocyte morphological changes, including Arp2/3, N-WASP, small GTPase Rho and Rac, and the effector ROCK, though mostly in vitro 84– 87. Henceforth, testing whether similar mechanisms exist under physiological conditions in vivo upon neuron–astrocyte contact and mediate astrocyte morphological maturation during development would be necessary.
Molecular maturation
As astrocytes mature, their transcriptional profiles change dramatically to exhibit stage-specific signatures 14, 19, 21, 34, 88, 89. The genes that are induced during astrocyte maturation can be categorized into three groups on the basis of their locations: (1) membrane-bound proteins, including GLT1, Cx43, Cx30, Kir4.1, and Aqp4; (2) cytosolic proteins, including GFAP, S100b, AldoC, and GS; and (3) secretory proteins, including Thbs1, Gpc4, Gpc6, Hevin, and SPARC 28, 42, 90– 101 ( Figure 3b).
The membrane-bound proteins necessary for astrocytic maturation include channels and receptors, and this group of genes is enriched in subcellular structures such as PAPs and end-feet. Expression of these genes offers astrocytes their characteristic functions during postnatal development. For example, the glutamate transporter GLT1 mediates glutamate uptake and regulates glutamate availability in the synaptic cleft, thus regulating synaptic transmission 102, 103. Cx43 and Cx30 form gap junctions to connect neighboring cells, allowing the exchange of molecules (water, glucose, metabolites, and neurotransmitters) and ions (Ca 2+, K +, and Na +) over long distances 104, 105. Kir4.1 is the major potassium channel of astrocytes and plays an essential role in buffering extracellular potassium built up during action potential. Aqp4 is the most abundant water channel in the brain and is important for water balance and also contributes to synaptic plasticity and learning/memory in the CNS 27, 106. During the morphological maturation of astrocytes, neurons often induce these genes 72, 75, 107– 109.
The second group of genes is composed of enzymes and cytoskeleton proteins that serve as markers of mature astrocytes. However, each one of these genes labels only a portion of astrocytes, and there is some overlap in expression with other cell types. Comprehensive characterization and immunohistochemical validation of the timeline and astrocyte specificity of these markers during development remain open avenues of investigation. Furthermore, whether and how cytosolic proteins serve as members of combinatorial codes in different brain regions are interesting areas for future research. (Also, see the “Astrocyte differentiation” section.)
Astrocytic neuroactive molecules that regulate synaptogenesis make up the genetic signature of the third and final stage of astrocyte maturation. These molecules include Thbs1, Gpc4, Gpc6, Hevin, and SPARC, each of which regulates a different phase of synaptogenesis. These genes are concurrently expressed in astrocytes at corresponding developmental stages. For example, Thbs1, which is required for the formation of silent synapses, and Gpc4/6, which is required for inducing postsynaptic active synapses, have the highest expression between P0 and P14 in developing astrocytes and are down-regulated thereafter. On the other hand, SPARC, an inhibitor of hevin and a negative regulator of synaptogenesis, is elevated only around P14 and remains expressed thereafter 34, 110– 112. The temporal control of these synaptogenic genes suggests a well-defined mechanism that regulates different phases of synaptogenesis.
Despite the above findings, most studies to date have focused on neuron–astrocyte crosstalk via secretory molecules and membrane proteins (that is, glutamate, BDNF, mGluR, Eph–Ephrine, neuroligin–neurexin), and very little investigation of the upstream transcriptional mechanisms governs their expression. Thus, how transcription programs reciprocally respond to neuronal signaling is an important area of future research.
Borrowing a concept from neurons, astrocytes may respond to external environmental cues and tailor their course of maturation to the local environment via transcriptional regulation. With more advanced technology, including single-cell sequencing, super-resolution microscopy, in vivo live imaging, membrane-bound optical sensors for neurotransmitters and ions, fluorescence resonance energy transfer techniques, and three-dimensional culture systems that reproduce astrocyte morphology in vivo, we should be able to increase our understanding of the mechanisms that mediate crosstalk between neurons and astrocytes, and that facilitate maturation of astrocytes into the right subtype, at the right time and place.
In the future, it will be interesting to answer the following questions: (1) Do any cell types other than neurons (for example, neighboring astrocytes, endothelial cells, oligodendrocytes, or oligodendrocyte precursors) contribute to astrocyte maturation? (2) How does transient Ca 2+, an indicator of mature astrocyte activity, develop over this postnatal period? (3) Does Ca 2+ activity also mediate astrocyte–neuron crosstalk to facilitate the co-maturation of PAPs and synapses? When addressing these questions, we should bear in mind that astrocytes are very responsive cells that preserve certain progenitor properties even in their mature form, a characteristic supported by recent studies looking into the transformation of reactive astrocytes in response to injury and trans-differentiation into neurons in vivo 113, 114. Thus, the astrocytic developmental program may be defined by proliferation, differentiation, and maturation over time, and their final refinement is regulated by the local environment, which gives rise to the optimal number, function, and morphology of astrocytes.
Closing remarks
Since Virchow first discovered astrocytes more than a hundred years ago, much progress has been made in our understanding of the processes by which they are specified, migrate, proliferate, and mature. However, there remain significant gaps in our characterization of these processes and our knowledge regarding molecular mechanisms underlying astrocyte phenotypes. Some crucial outstanding questions include (1) which upstream signaling pathways regulate the proliferation of astrocytes in the postnatal cortex? (2) Do transcription factors serve as immediate early genes that govern astrocyte maturation in accordance with the local synaptic environment? (3) Is Ca 2+ activity mature during the period of astrocytic morphological and molecular maturation? With the recent explosion of knowledge and tools in the glial field, the future is bright, and we are looking forward to a better understanding of astrocyte development and the mechanisms underlying it.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Sarah Kucenas, Department of Biology, University of Virginia, Charlottesville, VA, USA
Anna Molofsky, Weill Institute for Neurosciences, University of California, San Francisco, San Francisco, CA, USA
Funding Statement
This work was supported by the National Institutes of Health (NINDS NS071153, NIMH AG054111) and the National Multiple Sclerosis Society (RG-1501-02756). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 2 approved]
References
- 1. John Lin C-C, Yu K, Hatcher A, et al. : Identification of diverse astrocyte populations and their malignant analogs. Nat Neurosci. 2017;20(3):396–405. 10.1038/nn.4493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chai H, Diaz-Castro B, Shigetomi E, et al. : Neural Circuit-Specialized Astrocytes: Transcriptomic, Proteomic, Morphological, and Functional Evidence. Neuron. 2017;95(3):531–549.e9. 10.1016/j.neuron.2017.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 3. Morel L, Chiang MSR, Higashimori H, et al. : Molecular and Functional Properties of Regional Astrocytes in the Adult Brain. J Neurosci. 2017;37(36):8706–17. 10.1523/JNEUROSCI.3956-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Herculano-Houzel S: The glia/neuron ratio: How it varies uniformly across brain structures and species and what that means for brain physiology and evolution. Glia. 2014;62(9):1377–91. 10.1002/glia.22683 [DOI] [PubMed] [Google Scholar]
- 5. von Bartheld CS, Bahney J, Herculano-Houzel S: The search for true numbers of neurons and glial cells in the human brain: A review of 150 years of cell counting. J Comp Neurol. 2016;524(18):3865–95. 10.1002/cne.24040 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 6. Bayraktar OA, Fuentealba LC, Alvarez-Buylla A, et al. : Astrocyte development and heterogeneity. Cold Spring Harb Perspect Biol. 2014;7(1):a020362. 10.1101/cshperspect.a020362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Glasgow SM, Carlson JC, Zhu W, et al. : Glia-specific enhancers and chromatin structure regulate NFIA expression and glioma tumorigenesis. Nat Neurosci. 2017;20(11):1520–8. 10.1038/nn.4638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deneen B, Ho R, Lukaszewicz A, et al. : The transcription factor NFIA controls the onset of gliogenesis in the developing spinal cord. Neuron. 2006;52(6):953–68. 10.1016/j.neuron.2006.11.019 [DOI] [PubMed] [Google Scholar]
- 9. Kang P, Lee HK, Glasgow SM, et al. : Sox9 and NFIA Coordinate a Transcriptional Regulatory Cascade during the Initiation of Gliogenesis. Neuron. 2012;74(1):79–94. 10.1016/j.neuron.2012.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 10. Nagao M, Ogata T, Sawada Y, et al. : Zbtb20 promotes astrocytogenesis during neocortical development. Nat Commun. 2016;7:11102. 10.1038/ncomms11102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bajenaru ML, Zhu Y, Hedrick NM, et al. : Astrocyte-specific inactivation of the neurofibromatosis 1 gene ( NF1) is insufficient for astrocytoma formation. Mol Cell Biol. 2002;22(14):5100–13. 10.1128/mcb.22.14.5100-5113.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garcia ADR, Doan NB, Imura T, et al. : GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7(11):1233–41. 10.1038/nn1340 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 13. Gregorian C, Nakashima J, Le Belle J, et al. : Pten deletion in adult neural stem/progenitor cells enhances constitutive neurogenesis. J Neurosci. 2009;29(6):1874–86. 10.1523/JNEUROSCI.3095-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Molofsky AV, Krenick R, Ullian E, et al. : Astrocytes and disease: A neurodevelopmental perspective. Genes Dev. 2012;26(9):891–907. 10.1101/gad.188326.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Muroyama Y, Fujiwara Y, Orkin SH, et al. : Specification of astrocytes by bHLH protein SCL in a restricted region of the neural tube. Nature. 2005;438(7066):360–3. 10.1038/nature04139 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 16. Hochstim C, Deneen B, Lukaszewicz A, et al. : Identification of positionally distinct astrocyte subtypes whose identities are specified by a homeodomain code. Cell. 2008;133(3):510–22. 10.1016/j.cell.2008.02.046 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 17. Tsai HH, Li H, Fuentealba LC, et al. : Regional Astrocyte Allocation Regulates CNS Synaptogenesis and Repair. Science. 2012;337(6092):358–62. 10.1126/science.1222381 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Slezak M, Göritz C, Niemiec A, et al. : Transgenic mice for conditional gene manipulation in astroglial cells. Glia. 2007;55(15):1565–76. 10.1002/glia.20570 [DOI] [PubMed] [Google Scholar]
- 19. Chaboub LS, Manalo JM, Lee HK, et al. : Temporal Profiling of Astrocyte Precursors Reveals Parallel Roles for Asef during Development and after Injury. J Neurosci. 2016;36(47):11904–17. 10.1523/JNEUROSCI.1658-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weng Q, Wang J, Wang J, et al. : Single-Cell Transcriptomics Uncovers Glial Progenitor Diversity and Cell Fate Determinants during Development and Gliomagenesis. Cell Stem Cell. 2019;24(5):707–723.e8. 10.1016/j.stem.2019.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. Molofsky AV, Glasgow SM, Chaboub LS, et al. : Expression profiling of Aldh1l1-precursors in the developing spinal cord reveals glial lineage-specific genes and direct Sox9-Nfe2l1 interactions. Glia. 2013;61(9):1518–32. 10.1002/glia.22538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhuo L, Theis M, Alvarez-Maya I, et al. : hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis. 2001;31(2):85–94. 10.1002/gene.10008 [DOI] [PubMed] [Google Scholar]
- 23. Ghandour MS, Langley OK, Labourdette G, et al. : Specific and artefactual cellular localizations of S 100 protein: An astrocyte marker in rat cerebellum. Dev Neurosci. 1981;4(1):66–78. 10.1159/000112742 [DOI] [PubMed] [Google Scholar]
- 24. Shibata T, Yamada K, Watanabe M, et al. : Glutamate Transporter GLAST Is Expressed in the Radial Glia–Astrocyte Lineage of Developing Mouse Spinal Cord. J Neurosci. 1997;17(23):9212–9. 10.1523/JNEUROSCI.17-23-09212.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Staugaitis SM, Zerlin M, Hawkes R, et al. : Aldolase C/Zebrin II Expression in the Neonatal Rat Forebrain Reveals Cellular Heterogeneity within the Subventricular Zone and Early Astrocyte Differentiation. J Neurosci. 2001;21(16):6195–205. 10.1523/JNEUROSCI.21-16-06195.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu Y, Wu Y, Lee JC, et al. : Oligodendrocyte and astrocyte development in rodents: An in situ and immunohistological analysis during embryonic development. Glia. 2002;40(1):25–43. 10.1002/glia.10111 [DOI] [PubMed] [Google Scholar]
- 27. Szu JI, Binder DK: The Role of Astrocytic Aquaporin-4 in Synaptic Plasticity and Learning and Memory. Front Integr Neurosci. 2016;10:8. 10.3389/fnint.2016.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yamamoto T, Vukelic J, Hertzberg EL, et al. : Differential anatomical and cellular patterns of connexin43 expression during postnatal development of rat brain. Brain Res Dev Brain Res. 1992;66(2):165–80. 10.1016/0165-3806(92)90077-a [DOI] [PubMed] [Google Scholar]
- 29. Furness DN, Dehnes Y, Akhtar AQ, et al. : A quantitative assessment of glutamate uptake into hippocampal synaptic terminals and astrocytes: New insights into a neuronal role for excitatory amino acid transporter 2 (EAAT2). Neuroscience. 2008;157(1):80–94. 10.1016/j.neuroscience.2008.08.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miller SJ, Philips T, Kim N, et al. : Molecularly defined cortical astroglia subpopulation modulates neurons via secretion of Norrin. Nat Neurosci. 2019;22(5):741–52. 10.1038/s41593-019-0366-7 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 31. Morel L, Men Y, Chiang MSR, et al. : Intracortical astrocyte subpopulations defined by astrocyte reporter Mice in the adult brain. Glia. 2019;67(1):171–81. 10.1002/glia.23545 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Srinivasan R, Lu TY, Chai H, et al. : New Transgenic Mouse Lines for Selectively Targeting Astrocytes and Studying Calcium Signals in Astrocyte Processes In Situ and In Vivo. Neuron. 2016;92(6):1181–95. 10.1016/j.neuron.2016.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sun W, Cornwell A, Li J, et al. : SOX9 Is an Astrocyte-Specific Nuclear Marker in the Adult Brain Outside the Neurogenic Regions. J Neurosci 2017;37(17):4493–507. 10.1523/JNEUROSCI.3199-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Cahoy JD, Emery B, Kaushal A, et al. : A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28(1):264–78. 10.1523/JNEUROSCI.4178-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. Bonni A, Sun Y, Nadal-Vicens M, et al. : Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science. 1997;278(5337):477–83. 10.1126/science.278.5337.477 [DOI] [PubMed] [Google Scholar]
- 36. Barnabé-Heider F, Wasylnka JA, Fernandes KJL, et al. : Evidence that Embryonic Neurons Regulate the Onset of Cortical Gliogenesis via Cardiotrophin-1. Neuron. 2005;48(2):253–65. 10.1016/j.neuron.2005.08.037 [DOI] [PubMed] [Google Scholar]
- 37. Ito K, Noguchi A, Uosaki Y, et al. : Gfap and Osmr regulation by BRG1 and STAT3 via interchromosomal gene clustering in astrocytes. Mol Biol Cell. 2018;29(2):209–19. 10.1091/mbc.E17-05-0271 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 38. Molofsky AV, Kelley KW, Tsai HH, et al. : Astrocyte-encoded positional cues maintain sensorimotor circuit integrity. Nature. 2014;509(7499):189–94. 10.1038/nature13161 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 39. Kelley KW, Ben Haim L, Schirmer L, et al. : Kir4.1-Dependent Astrocyte-Fast Motor Neuron Interactions Are Required for Peak Strength. Neuron. 2018;98(2):306–319.e7. 10.1016/j.neuron.2018.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 40. van Dyck LI, Morrow EM: Genetic control of postnatal human brain growth. Curr Opin Neurol. 2017;30(1):114–24. 10.1097/WCO.0000000000000405 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 41. Bandeira F, Lent R, Herculano-Houzel S: Changing numbers of neuronal and non-neuronal cells underlie postnatal brain growth in the rat. Proc Natl Acad Sci U S A. 2009;106(33):14108–13. 10.1073/pnas.0804650106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Allen NJ, Eroglu C: Cell Biology of Astrocyte-Synapse Interactions. Neuron. 2017;96(3):697–708. 10.1016/j.neuron.2017.09.056 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Kriegstein A, Alvarez-Buylla A: The Glial Nature of Embryonic and Adult Neural Stem Cells. Annu Rev Neurosci. 2009;32:149–84. 10.1146/annurev.neuro.051508.135600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tabata H: Diverse subtypes of astrocytes and their development during corticogenesis. Front Neurosci. 2015;9:114. 10.3389/fnins.2015.00114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ge WP, Jia JM: Local production of astrocytes in the cerebral cortex. Neuroscience. 2016;323:3–9. 10.1016/j.neuroscience.2015.08.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schmechel DE, Rakic P: A golgi study of radial glial cells in developing monkey telencephalon: Morphogenesis and transformation into astrocytes. Anat Embryol. 1979;156(2):115–52. 10.1007/bf00300010 [DOI] [PubMed] [Google Scholar]
- 47. Voigt T: Development of glial cells in the cerebral wall of ferrets: Direct tracing of their transformation from radial glia into astrocytes. J Comp Neurol. 1989;289(1):74–88. 10.1002/cne.902890106 [DOI] [PubMed] [Google Scholar]
- 48. deAzevedo LC, Fallet C, Moura-Neto V, et al. : Cortical radial glial cells in human fetuses: Depth-correlated transformation into astrocytes. J Neurobiol. 2003;55(3):288–98. 10.1002/neu.10205 [DOI] [PubMed] [Google Scholar]
- 49. Noctor SC, Martínez-Cerdeño V, Ivic L, et al. : Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7(2):136–44. 10.1038/nn1172 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 50. Burns KA, Murphy B, Danzer SC, et al. : Developmental and post-injury cortical gliogenesis: A Genetic fate-mapping study with Nestin-CreER mice. Glia. 2009;57(10):1115–29. 10.1002/glia.20835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Magavi S, Friedmann D, Banks G, et al. : Coincident generation of pyramidal neurons and protoplasmic astrocytes in neocortical columns. J Neurosci. 2012;32(14):4762–72. 10.1523/JNEUROSCI.3560-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Levison SW, Goldman JE: Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron. 1993;10(2):201–12. 10.1016/0896-6273(93)90311-e [DOI] [PubMed] [Google Scholar]
- 53. Levison SW, Chuang C, Abramson BJ, et al. : The migrational patterns and developmental fates of glial precursors in the rat subventricular zone are temporally regulated. Development. 1993;119(3):611–22. [DOI] [PubMed] [Google Scholar]
- 54. Luskin MB, McDermott K: Divergent lineages for oligodendrocytes and astrocytes originating in the neonatal forebrain subventricular zone. Glia. 1994;11(3):211–26. 10.1002/glia.440110302 [DOI] [PubMed] [Google Scholar]
- 55. Hirano M, Goldman JE: Gliogenesis in rat spinal cord: Evidence for origin of astrocytes and oligodendrocytes from radial precursors. J Neurosci Res. 1988;21(2-4):155–67. 10.1002/jnr.490210208 [DOI] [PubMed] [Google Scholar]
- 56. Tien AC, Tsai HH, Molofsky AV, et al. : Regulated temporal-spatial astrocyte precursor cell proliferation involves BRAF signalling in mammalian spinal cord. Development. 2012;139(14):2477–87. 10.1242/dev.077214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Price J, Thurlow L: Cell lineage in the rat cerebral cortex: a study using retroviral-mediated gene transfer. Development. 1988;104(3):473–82. [DOI] [PubMed] [Google Scholar]
- 58. Ge WP, Miyawaki A, Gage FH, et al. : Local generation of glia is a major astrocyte source in postnatal cortex. Nature. 2012;484(7394):376–80. 10.1038/nature10959 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 59. Moroni RF, Deleo F, Regondi MC, et al. : Proliferative cells in the rat developing neocortical grey matter: new insights into gliogenesis. Brain Struct Funct. 2018;223(9):4053–66. 10.1007/s00429-018-1736-8 [DOI] [PubMed] [Google Scholar]
- 60. Sohn J, Orosco L, Guo F, et al. : The subventricular zone continues to generate corpus callosum and rostral migratory stream astroglia in normal adult mice. J Neurosci.. 2015;35(9):3756–63. 10.1523/JNEUROSCI.3454-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. García-Marqués J, López-Mascaraque L: Clonal Identity Determines Astrocyte Cortical Heterogeneity. Cereb Cortex. 2013;23(6):1463–72. 10.1093/cercor/bhs134 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 62. Huang Z, Hu J, Pan J, et al. : YAP Stabilizes SMAD1 and Promotes BMP2-induced Neocortical Astrocytic Differentiation. Development. 2016;143(13):2398–409. 10.1242/dev.130658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Haim LB, Rowitch DH: Functional Diversity of Astrocytes in Neural Circuit Regulation. Nat Rev Neurosci. 2017;18(1):31–41. 10.1038/nrn.2016.159 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 64. Bushong EA, Martone ME, Ellisman MH: Maturation of Astrocyte Morphology and the Establishment of Astrocyte Domains During Postnatal Hippocampal Development. Int J Dev Neurosci. 2004;22(2):73–86. 10.1016/j.ijdevneu.2003.12.008 [DOI] [PubMed] [Google Scholar]
- 65. Octeau JC, Chai H, Jiang R, et al. : An Optical Neuron-Astrocyte Proximity Assay at Synaptic Distance Scales. Neuron. 2018;98(1):49–66.e9. 10.1016/j.neuron.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 66. Derouiche A, Frotscher M: Peripheral Astrocyte Processes: Monitoring by Selective Immunostaining for the Actin-Binding ERM Proteins. Glia. 2001;36(3):330–41. 10.1002/glia.1120 [DOI] [PubMed] [Google Scholar]
- 67. Freeman MR: Specification and Morphogenesis of Astrocytes. Science. 2010;330(6005):774–8. 10.1126/science.1190928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Savtchouk I, Volterra A: Gliotransmission: Beyond Black-and-White. J Neurosci. 2018;38(1):14–25. 10.1523/JNEUROSCI.0017-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 69. Chaudhry FA, Lehre KP, van Lookeren Campagne M, et al. : Glutamate transporters in glial plasma membranes: highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron. 1995;15(3):711–20. 10.1016/0896-6273(95)90158-2 [DOI] [PubMed] [Google Scholar]
- 70. Higashi K, Fujita A, Inanobe A, et al. : An Inwardly Rectifying K(+) Channel, Kir4.1, Expressed in Astrocytes Surrounds Synapses and Blood Vessels in Brain. Am J Physiol Cell Physiol. 2001;281(3):C922–31. 10.1152/ajpcell.2001.281.3.C922 [DOI] [PubMed] [Google Scholar]
- 71. Lavialle M, Aumann G, Anlauf E, et al. : Structural plasticity of perisynaptic astrocyte processes involves ezrin and metabotropic glutamate receptors. Proc Natl Acad Sci U S A. 2011;108(31):12915–9. 10.1073/pnas.1100957108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hasel P, Dando O, Jiwaji Z, et al. : Neurons and neuronal activity control gene expression in astrocytes to regulate their development and metabolism. Nat Commun. 2017;8:15132. 10.1038/ncomms15132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Stogsdill JA, Ramirez J, Liu D, et al. : Astrocytic neuroligins control astrocyte morphogenesis and synaptogenesis. Nature. 2017;551(7679):192–197. 10.1038/nature24638 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 74. Müller CM: Dark-rearing retards the maturation of astrocytes in restricted layers of cat visual cortex. Glia. 1990;3(6):487–94. 10.1002/glia.440030607 [DOI] [PubMed] [Google Scholar]
- 75. Morel L, Higashimori H, Tolman M, et al. : VGluT1 + neuronal glutamatergic signaling regulates postnatal developmental maturation of cortical protoplasmic astroglia. J Neurosci. 2014;34(33):10950–62. 10.1523/JNEUROSCI.1167-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bernardinelli Y, Randall J, Janett E, et al. : Activity-Dependent Structural Plasticity of Perisynaptic Astrocytic Domains Promotes Excitatory Synapse Stability. Curr Biol. 2014;24(15):1679–88. 10.1016/j.cub.2014.06.025 [DOI] [PubMed] [Google Scholar]
- 77. Genoud C, Quairiaux C, Steiner P, et al. : Plasticity of astrocytic coverage and glutamate transporter expression in adult mouse cortex. PLoS Biol. 2006;4(11):e343. 10.1371/journal.pbio.0040343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Holt LM, Hernandez RD, Pacheco NL, et al. : Astrocyte morphogenesis is dependent on BDNF signaling via astrocytic TrkB.T1. Elife. 2019;8: pii: e44667. 10.7554/eLife.44667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Murai KK, Nguyen LN, Irie F, et al. : Control of hippocampal dendritic spine morphology through ephrin-A3/EphA4 signaling. Nat Neurosci. 2003;6(2):153–60. 10.1038/nn994 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 80. Filosa A, Paixão S, Honsek SD, et al. : Neuron-glia communication via EphA4/ephrin-A3 modulates LTP through glial glutamate transport. Nat Neurosci. 2009;12(10):1285–92. 10.1038/nn.2394 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 81. Garrett AM, Weiner JA: Control of CNS synapse development by {gamma}-protocadherin-mediated astrocyte-neuron contact. J Neurosci. 2009;29(38):11723–31. 10.1523/JNEUROSCI.2818-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Medvedev N, Popov V, Henneberger C, et al. : Glia selectively approach synapses on thin dendritic spines. Philos Trans R Soc Lond B Biol Sci. 2014;369(1654):20140047. 10.1098/rstb.2014.0047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Carmona MA, Murai KK, Wang L, et al. : Glial ephrin-A3 regulates hippocampal dendritic spine morphology and glutamate transport. Proc Natl Acad Sci U S A. 2009;106(30):12524–9. 10.1073/pnas.0903328106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Racchetti G, D'Alessandro R, Meldolesi J: Astrocyte stellation, a process dependent on Rac1 is sustained by the regulated exocytosis of enlargeosomes. Glia. 2012;60(3):465–75. 10.1002/glia.22280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Murk K, Blanco Suarez EM, Cockbill LM, et al. : The antagonistic modulation of Arp2/3 activity by N-WASP, WAVE2 and PICK1 defines dynamic changes in astrocyte morphology. J Cell Sci. 2013;126(Pt 17):3873–83. 10.1242/jcs.125146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. John GR, Chen L, Rivieccio MA, et al. : Interleukin-1beta induces a reactive astroglial phenotype via deactivation of the Rho GTPase-Rock axis. J Neurosci. 2004;24(11):2837–45. 10.1523/JNEUROSCI.4789-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nishida H, Okabe S: Direct astrocytic contacts regulate local maturation of dendritic spines. J Neurosci. 2007;27(2):331–40. 10.1523/JNEUROSCI.4466-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 88. Li J, Khankan RR, Caneda C, et al. : Astrocyte-to-astrocyte contact and a positive feedback loop of growth factor signaling regulate astrocyte maturation. Glia. 2019;67(8):1571–1597. 10.1002/glia.23630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhang Y, Sloan SA, Clarke LE, et al. : Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron. 2016;89(1):37–53. 10.1016/j.neuron.2015.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Baba H, Nakahira K, Morita N, et al. : GFAP gene expression during development of astrocyte. Dev Neurosci. 1997;19(1):49–57. 10.1159/000111185 [DOI] [PubMed] [Google Scholar]
- 91. Raponi E, Agenes F, Delphin C, et al. : S100B expression defines a state in which GFAP-expressing cells lose their neural stem cell potential and acquire a more mature developmental stage. Glia. 2007;55(2):165–77. 10.1002/glia.20445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Furuta A, Rothstein JD, Martin LJ: Glutamate Transporter Protein Subtypes Are Expressed Differentially during Rat CNS Development. J Neurosci. 1997;17(21):8363–75. 10.1523/JNEUROSCI.17-21-08363.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Nagy JI, Patel D, Ochalski PAY, et al. : Connexin30 in rodent, cat and human brain: Selective expression in gray matter astrocytes, co-localization with connexin43 at gap junctions and late developmental appearance. Neuroscience. 1999;88(2):447–68. 10.1016/s0306-4522(98)00191-2 [DOI] [PubMed] [Google Scholar]
- 94. Seifert G, Hüttmann K, Binder DK, et al. : Analysis of astroglial K+ channel expression in the developing hippocampus reveals a predominant role of the Kir4.1 subunit. J Neurosci. 2009;29(23):7474–88. 10.1523/JNEUROSCI.3790-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Higashimori H, Sontheimer H: Role of Kir4.1 channels in growth control of glia. Glia. 2007;55(16):1668–79. 10.1002/glia.20574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Engel M, Do-Ha D, Muñoz SS, et al. : Common pitfalls of stem cell differentiation: A guide to improving protocols for neurodegenerative disease models and research. Cell Mol Life Sci. 2016;73(19):3693–709. 10.1007/s00018-016-2265-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Farhy-Tselnicker I, Allen NJ: Astrocytes, neurons, synapses: A tripartite view on cortical circuit development. Neural Dev. 2018;13(1):7. 10.1186/s13064-018-0104-y [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 98. Nwaobi SE, Lin E, Peramsetty SR, et al. : DNA methylation functions as a critical regulator of Kir4.1 expression during CNS development. Glia. 2014;62(3):411–27. 10.1002/glia.22613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Voutsinos-Porche B, Knott G, Tanaka K, et al. : Glial Glutamate Transporters and Maturation of the Mouse Somatosensory Cortex. Cereb Cortex. 2003;13(10):1110–21. 10.1093/cercor/13.10.1110 [DOI] [PubMed] [Google Scholar]
- 100. Fallier-Becker P, Vollmer JP, Bauer H-C: Onset of aquaporin-4 expression in the developing mouse brain. Int J Dev Neurosci. 2014;36:81–9. 10.1016/j.ijdevneu.2014.06.001 [DOI] [PubMed] [Google Scholar]
- 101. Walther EU, Dichgans M, Maricich SM, et al. : Genomic sequences of aldolase C (Zebrin II) direct lacZ expression exclusively in non-neuronal cells of transgenic mice. Proc Natl Acad Sci U S A. 1998;95(5):2615–20. 10.1073/pnas.95.5.2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Rose CR, Felix L, Zeug A, et al. : Astroglial Glutamate Signaling and Uptake in the Hippocampus. Front Mol Neurosci. 2018;10:41. 10.3389/fnmol.2017.00451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Pannasch U, Freche D, Dallérac G, et al. : Connexin 30 sets synaptic strength by controlling astroglial synapse invasion. Nat Neurosci. 2014;17(4):549–58. 10.1038/nn.3662 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 104. Lanciotti A, Brignone M, Bertini E, et al. : Astrocytes: Emerging stars in leukodystrophy pathogenesis. Transl Neurosci. 2013;4(2):356. 10.2478/s13380-013-0118-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Mayorquin C, Rodriguez AV, Sutachan JJ, et al. : Connexin-Mediated Functional and Metabolic Coupling Between Astrocytes and Neurons. Front Mol Neurosci. 2018;11:118. 10.3389/fnmol.2018.00118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Woo J, Kim JE, Im JJ, et al. : Astrocytic water channel aquaporin-4 modulates brain plasticity in both mice and humans: a potential gliogenetic mechanism underlying language-associated learning. Mol Psychiatry. 2018;23(4):1021–30. 10.1038/mp.2017.113 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 107. Yang Y, Gozen O, Watkins A, et al. : Presynaptic regulation of astroglial excitatory neurotransmitter transporter GLT1. Neuron. 2009;61(6):880–94. 10.1016/j.neuron.2009.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Swanson RA, Liu J, Miller JW, et al. : Neuronal Regulation of Glutamate Transporter Subtype Expression in Astrocytes. J Neurosci. 1997;17(3):932–40. 10.1523/JNEUROSCI.17-03-00932.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Koulakoff A, Ezan P, Giaume C: Neurons control the expression of connexin 30 and connexin 43 in mouse cortical astrocytes. Glia. 2008;56(12):1299–311. 10.1002/glia.20698 [DOI] [PubMed] [Google Scholar]
- 110. Christopherson KS, Ullian EM, Stokes CCA, et al. : Thrombospondins Are Astrocyte-Secreted Proteins that Promote CNS Synaptogenesis. Cell. 2005;120(3):421–33. 10.1016/j.cell.2004.12.020 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 111. Allen NJ, Bennett ML, Foo LC, et al. : Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature. 2012;486(7403):410–4. 10.1038/nature11059 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 112. Kucukdereli H, Allen NJ, Lee AT, et al. : Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proc Natl Acad Sci U S A. 2011;108(32):E440–9. 10.1073/pnas.1104977108 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 113. Liu Y, Miao Q, Yuan J, et al. : Ascl1 Converts Dorsal Midbrain Astrocytes into Functional Neurons In Vivo. J Neurosci. 2015;35(25):9336–55. 10.1523/JNEUROSCI.3975-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Sofroniew MV: Astrogliosis. Cold Spring Harb Perspect Biol. 2015;7(2):a020420. 10.1101/cshperspect.a020420 [DOI] [PMC free article] [PubMed] [Google Scholar]