Abstract
Respiratory syncytial virus (RSV) is an important human respiratory pathogen with narrow species tropism. Limited availability of human pathologic specimens during early RSV-induced lung disease and ethical restrictions for RSV challenge studies in the lower airways of human volunteers has slowed our understanding of how RSV causes airway disease and greatly limited the development of therapeutic strategies for reducing RSV disease burden. Our current knowledge of RSV infection and pathology is largely based on in vitro studies using nonpolarized epithelial cell-lines grown on plastic or in vivo studies using animal models semipermissive for RSV infection. Although these models have revealed important aspects of RSV infection, replication, and associated inflammatory responses, these models do not broadly recapitulate the early interactions and potential consequences of RSV infection of the human columnar airway epithelium in vivo. In this chapter, the pro et contra of in vitro models of human columnar airway epithelium and their usefulness in respiratory virus pathogenesis and vaccine development studies will be discussed. The use of such culture models to predict characteristics of RSV infection and the correlation of these findings to the human in vivo situation will likely accelerate our understanding of RSV pathogenesis potentially identifying novel strategies for limiting the severity of RSV-associated airway disease.
Keywords: Respiratory Syncytial Virus, Airway Epithelial Cell, Airway Epithelium, Respiratory Syncytial Virus Infection, Respiratory Virus
Why Study RSV Infection of Human Columnar Airway Epithelium In Vitro?
The predominant site of RSV infection in the immunocompetent human respiratory tract is the columnar epithelium lining the conducting airways. Columnar airway epithelium serves as an important innate barrier in the respiratory tract and possesses highly specialized functions due to the distinct features of diverse columnar epithelial cell-types present throughout the airways. How RSV infection affects the integrity and innate defenses of the human airway epithelium resulting in airway disease is still not clearly defined. Whether outcomes of RSV infection of nonpolarized epithelial cell-lines or semipermissive animal models in vivo reflect those of RSV infection of human columnar airway epithelial cells is currently under investigation. Although in vivo human challenge protocols are available and are an excellent model system for assessing consequences of RSV infection and testing the efficacy of RSV vaccine candidates and anti-virals, these studies are limited to inoculation of the nasal epithelium and provide little information on RSV pathogenesis in lower airway regions. Human in vivo challenge studies are also cost-prohibitive to most in the RSV field; they require specialized facilities, personnel and appropriate review by regulatory agencies. Therefore, more cost effective and widely available models of human columnar airway epithelium have been sought to further understand how RSV infection affects the normal functions of the airway epithelium and how altering epithelium function influences RSV pathogenesis. Such in vitro models also hold great utility for preclinical testing of infection and growth phenotypes of live, attenuated RSV vaccine candidates and assessing the efficacy of RSV antiviral approaches.
Culture models of human columnar airway epithelium developed by several groups are gaining acceptance for studying particular aspects of respiratory virus infection and airway innate defenses (Pickles et al. 1998; Zhang et al. 2002; Sims et al. 2006; Zabner et al. 2000; Wright et al. 2005; Villenave et al. 2012; Ilyushina et al. 2012). Technical aspects associated with isolation and culture of human airway epithelial cell cultures will not be further discussed here and the reader is referred to a comprehensive description of these protocols (Fulcher et al. 2005). The availability of airway cell culture models relies on an abundant primary source of airway epithelial cells isolated from the lungs of human donors. In our laboratory, human airway epithelial cell cultures (HAE) are generated from epithelial cells isolated from human nasal or tracheobronchial epithelium obtained from cadaver airways or excess airway tissues after lung transplantation or elective surgery. An alternative source of human airway cells is from volunteers willing to undergo harvest of epithelial cells by scraping or brushing of nasal or tracheobronchial epithelium. Regardless of the method of procurement, isolated epithelial cells can then be grown using appropriate culture conditions to generate differentiated cultures of columnar airway epithelium. In our experience and that of others, HAE cultures exhibit morphologic and physiologic properties similar to those of the human columnar airway epithelium in vivo.
What is the Cellular Composition of HAE Cultures and What Airway Regions do they Represent?
To appreciate the usefulness of human columnar airway cultures it is important to describe the diversity of airway epithelial cells distributed in the human airways in vivo and how HAE reproduce this cell diversity in vitro.
Respiratory columnar epithelium lines the nasopharyngeal (upper conducting airways) and the tracheal, bronchial, and bronchiolar airways (lower conducting airways). The respiratory epithelium also lines the alveolar lung regions but does not possess morphologic features of a columnar epithelium. Although ciliated airway epithelial cells are predominant throughout the conducting airways of all species, both ciliated cell density and the phenotype of nonciliated cells are airway region and species dependent (Harkema et al. 1994). For humans, the cartilaginous conducting airway epithelium (nasal, tracheobronchial, bronchial) is a pseudostratified columnar epithelium with predominant ciliated cells interspersed with variable numbers of mucin-secreting cells. In these airway regions, columnar cells overlie basal epithelial cells directly attached to the underlying basement membrane. Ciliated cells are also prominent in the human small, noncartilaginous bronchiolar airways but their numbers decrease as the bronchioles approach the airspaces of the alveolar regions. In the cartilaginous airways, nonciliated columnar cells largely represent the morphology and properties of classic mucin-secreting cells (Goblet cells) whereas, the nonciliated columnar cells in the bronchiolar regions are most often identified as Clara cells. Distal to the conducting airways, the lung epithelium consists of Type I and Type II pneumocytes.
For human ciliated airway epithelium models, three anatomical airway regions are routinely used to obtain primary epithelial cells; the nasal epithelium, the nasopharyngeal epithelium (adenoids), and the tracheobronchial epithelium. HAE cultures derived from cells procured from these regions result in the generation of a pseudostratified mucociliary columnar epithelium in which ciliated cells and mucin-secreting (Goblet) cells make up the vast majority of columnar cells. Nonciliated columnar cells with morphological features of Clara cells are usually scarce in these culture models likely reflecting the low numbers of Clara cells present in the proximal airway tissues used for harvesting the epithelial cells.
RSV Infects Human Ciliated Airway Epithelium In Vivo and In Vitro
Histopathologic specimens of RSV-infected lung tissues have immunolocalized RSV antigen to columnar airway epithelium of the large conducting airways and in ciliated cells and nonciliated cells (Clara cells) in the bronchiolar regions (Johnson et al. 2007; Welliver et al. 2008; Neilson and Yunis 1990). Although RSV antigen can be detected by immunohistologic studies in alveolar regions, data supporting RSV infection of alveolar epithelial cells in immunocompetent patients are less robust than for RSV infection of the bronchiolar regions (Neilson and Yunis 1990). Although Type II pneumocytes can be infected by RSV, especially in immunocompromised patients, the frequency and timing of infection of bronchiolar versus alveolar epithelium and its relationship to outcomes of disease remain unclear.
Human airway epithelial cell cultures derived from cartilaginous airway regions have been used by us and by others to show RSV robustly infects ciliated cells (Fig. 1 Zhang et al. 2002; Villenave et al. 2012). However, ciliated cell tropism in these models is not unique to RSV since parainfluenza viruses (human PIV1-5, Sendai virus), human/avian influenza viruses, and coronaviruses exhibit preferential infection of ciliated cells in these models (Zhang et al. 2005, 2012; Scull et al. 2009; Sims et al. 2005; Villenave et al. 2012; Bartlett et al. 2008a; Schaap-Nutt et al. 2010). Indeed, by comparing infection of HAE with different respiratory viruses we and others have found human influenza viruses are the only respiratory viruses so far tested that infect both ciliated and nonciliated columnar cells of HAE (Scull et al. 2009; Matrosovich et al. 2004). The significance of expanded virus tropism for both ciliated and nonciliated cells with regards to outcomes of influenza virus infection is unknown but we speculate influenza virus evolution in human hosts towards nonciliated columnar cells may provide an advantage to the virus against innate host defenses.
Fig. 1.
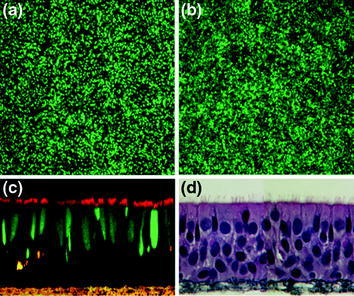
Human airway epithelial cell cultures infected by RSV and PIV3 expressing GFP. (a + b) En face XY views of HAE infected with RSV (a) or PIV3 (b) and infected cells detected by expression of GFP (green). c Confocal XZ microscopy of HAE infected with PIV3 shows GFP (green) is localized to columnar cells positive for β-tubulin IV immunoreactivity (red) indicating PIV3 infects ciliated cells. d Histological cross-section of HAE demonstrating the morphologic characteristics of a pseudostratified mucociliary airway epithelium
For RSV infection of HAE, ciliated cells are the exclusive target for infection (Zhang et al. 2002). An estimated 60–90 % of lumenal surface cells in HAE are ciliated cells (Zhang et al. 2009), with the remainder of columnar cells resembling mucin-secreting Goblet cells found in human airway epithelium in vivo (Fig. 2). However, other less common “secretory columnar cell-types” are occasionally identified in HAE although these columnar cell-types are not commonly found in human airways in vivo (Fig. 2). Thus, for the most part, the columnar cell composition of HAE closely mimics columnar cell density and phenotype of the columnar epithelium of the human nasal and tracheobronchial airway regions in vivo. In our experience, we have not routinely identified Clara cells in HAE; an expected finding given the isolation of cells from large airway regions where Clara cells are scarce. Isolation of airway epithelial cells from human bronchiolar airways where Clara cell density is high has not yet successfully resulted in differentiated cultures with columnar epithelial cells displaying morphologic characteristics of Clara cells in vivo. Primary cultures for human alveolar Type II pneumocytes are available and currently await studies with RSV infection (Bove et al. 2011).
Fig. 2.
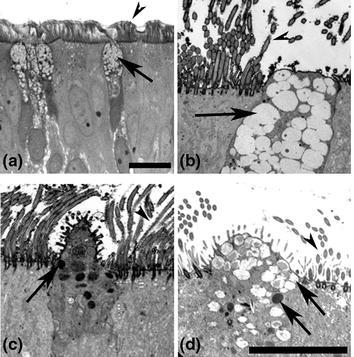
Morphological features of columnar epithelial cells in HAE. a Histological section of HAE showing ciliated and nonciliated columnar epithelial cells with distribution similar to human tracheobronchial airway epithelium in vivo. b–d Transmission electron micrographs detailing morphological characteristics of nonciliated columnar epithelial cells in HAE. b A frequently identified nonciliated columnar cell containing clear mucin-rich granules and resembling a Goblet cell. c An infrequently identified nonciliated columnar epithelial cell with electron dense granules and resembling a Serous cell. d An infrequently identified nonciliated columnar epithelial cell with mixed phenotypes of mucin-secreting and serous-like cell features. Arrowheads indicate cilia of ciliated columnar cells and long arrows indicate clear and/or dense granules in nonciliated columnar epithelial cells. Scale bars represent 20 μm
Is RSV Infection of Human Ciliated Cells Different from Infection of NonPolarized Epithelial Cell-Lines?
Early studies of RSV infection of HAE revealed that although ciliated cells were robustly infected by RSV these cells were less susceptible to infection than human nonpolarized epithelial cell-lines, e.g., HEp-2 and A549 cells (unpublished observations and (Wright et al. 2005). One explanation for these observations is the greater abundance of extracellular glycocalyx present on the lumenal surface of ciliated cells when compared to that on the surfaces of nonpolarized epithelial cell-lines (Kesimer et al. 2009, 2012). It is expected RSV and other viruses that infect ciliated cells will have to negotiate the more complex glycocalyx structure and these viruses have likely evolved strategies to enable this. Indeed, we have shown columnar cell glycocalyx in HAE poses a significant diffusion barrier to respiratory viruses reaching the apical membranes of columnar cells (Stonebraker et al. 2004; Kesimer et al. 2012). The robust glycocalyx of the columnar airway epithelium is largely due to the abundance of several families of carbohydrate-rich molecules, including proteoglycans, glycolipids, and glycoproteins most notably, the highly O-glycosylated ‘tethered’ mucins MUC1, 4, and 16. These tethered mucins constitute a significant portion of the glycocalyx structure in human and murine airway epithelia (Kesimer et al. 2012; Stonebraker et al. 2004; Button et al. 2012).
Despite the barrier properties of the glycocalyx on HAE some respiratory viruses, including RSV, are relatively efficient at negotiating this carbohydrate-rich network. We have speculated the RSV G glycoprotein may serve to facilitate the passage of RSV into the glycocalyx layer. This idea was based on data showing recombinant RSV deleted the G protein (RSVΔG) infected nonpolarized epithelial cell-lines just as efficiently as wild-type virus suggesting the G protein was not required for infection (Karron et al. 1997). In contrast to these previous findings in nonpolarized epithelial cells, we recently showed RSV infection of HAE is significantly increased when the virus expresses a full-length G protein (Kwilas et al. 2009). While RSV efficiently infects and spreads in HAE, RSVΔG poorly infects ciliated cells and displays attenuated growth characteristics in HAE (Kwilas et al. 2009). Since RSV G has been previously described as a “mucin-like” glycoprotein with the potential for multiple O-glycosylation sites (Collins 1990), we speculate one function of G may be to facilitate the negotiation of RSV through the robust glycocalyx layer present on the lumenal surface of polarized airway epithelial cells. These studies highlight just one example of the utility of HAE to better model in vivo virus–host airway cell interactions when compared to nonpolarized epithelial cell-lines.
The importance of the RSV G protein for efficient infection of human ciliated cells is further supported by experiments showing RSV propagated in HEp-2 cells efficiently infects HAE whereas RSV propagated in VERO cells poorly infects HAE (Kwilas et al. 2009). However, in contrast to RSVΔG growth in HAE, only the initial inoculum of VERO cell-derived RSV was attenuated in HAE and subsequent rounds of infection within HAE cultures proceeded similarly to those after inoculation with RSV propagated in HEp-2 cells (Fig. 3). Subsequently, it was discovered the G glycoprotein of RSV propagated in HEp-2 cells had a molecular mass of 90 kD while the G from VERO cell-derived RSV was only 55 kD suggesting VERO-derived G was deficient is some component important for efficient infection of ciliated cells. Although the numbers of ciliated cells infected by VERO cell-derived RSV were lower than for HEp-2 derived RSV, progeny virions budding from ciliated cells infected by VERO-derived RSV quickly regained normal infection kinetics suggesting deficiencies in G function for RSV propagated in VERO cells were possibly due to altered post-transcriptional modification of G in VERO cells and not due to genetic alterations in the virus genome. The significance of the different molecular masses for G protein derived from different producer cell-lines remains to be determined but only became apparent using HAE as the target cells for infection. Precisely, how the RSV G protein may be modified in producer cell-lines to account for these differences in mass and infectivity for ciliated cells is under investigation.
Fig. 3.
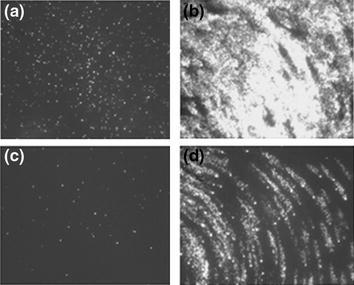
Decreased initial infection of HAE by RSV propagated in VERO cells compared to HEp-2 cells. HEp-2 or VERO cell derived RSV titered on cell-lines was inoculated at equal titer (106 PFU) onto HAE and GFP expression (white signal) assessed at 1 (a, c) and 2 (b, d) days pi. En face views of: a RSV (HEp2-derived) infected HAE at 1 day pi; b RSV (HEp-2-derived) infected HAE at 2 days pi; c RSV (VERO-derived) infected HAE at 1 day pi; and d RSV (VERO-derived) infected HAE at 2 days pi. One day after inoculation RSV (HEp-2) infects far more ciliated cells than RSV (VERO) and by day 2 pi, RSV (HEp-2) has spread throughout the culture while RSV (VERO) exhibits delayed spread. Note Spread of RSV infection in (d) demonstrates patterns of ‘comet-like’ spread of infection accounted for by the directionality of cilia beat in HAE
These findings associated with RSV G may also have technical implications for RSV vaccine development. Since VERO cells remain the only FDA-approved cell-line for propagating RSV vaccines for clinical use, the inoculating dose of vaccine candidates may exhibit reduced infection efficiency of ciliated cells of the human respiratory tract. For live, attenuated RSV vaccines that replicate similarly to wild-type RSV in the upper airways such an infection deficiency may not be a significant concern as further rounds of replication and infection would rapidly generate RSV expressing a fully functional G protein (see chapter by 10.1007/978-3-642-38919-1_4, this volume).
Variability of Virus Infection in HAE Derived from Different Donors
In contrast to HAE, epithelial cell-lines offer unlimited and continuous use of genetically homogenous cell populations. HAE cultures are derived from individual donors with a strong possibility of genetic and environmental variability between donors. When using HAE there are two practical considerations when designing experiments. First, the total number of primary cells and hence, HAE cultures, derived from an individual donor will always be limiting. Human primary airway epithelial cells grown on plastic can be passaged several times to increase cell availability, but the ability to reproducibly differentiate these cells into ciliated columnar epithelium wanes in later passages. Attempts to generate ‘human primary epithelial cell-lines’ have successfully generated cultures of pseudostratified columnar airway epithelium however, the extent of ciliated cell differentiation in these cultures does not approach the ciliated cell densities achievable with freshly isolated primary epithelial cells (Fulcher et al. 2009). The second practical concern when using HAE relates to the potential for variability due to differences in host genetics and/or environmental exposures between individual donors. Currently, the only feasible method to determine variability in experimental outcomes between HAE derived from different donors is empirical testing of the specific experimental outcome, e.g., virus growth kinetics, across cultures derived from several different donors (i.e., 4–7 different donors).
In our experience, the most satisfactory approach to these experimental considerations is to generate sufficient numbers of HAE cultures (24–48 wells) from a single donor and plan experiments to include the necessary replicates of test and controls conditions within this cohort. HAE cultures derived from different donors can then be used in identical experiments and variability assessed. If variability is low, data can be aggregated across all HAE used. For example, PIV1 infection and growth kinetics in human ciliated cells of HAE was highly reproducible both between cultures derived from an individual donor and HAE derived from 5 different donors (Bartlett et al. 2008a, b). Similar data were obtained for influenza viruses (Scull et al. 2009) and PIV2 (Schaap-Nutt et al. 2012). However, in our experience, not all experimental outcomes are as consistent between donors. For example, although PIV2 infection kinetics between donors were identical, significant differences in inflammatory mediator production was determined between donors (Schaap-Nutt et al. 2012); thus, highlighting the requirement to confirm reproducibility of experimental outcomes both between cultures and donor sources.
Using HAE to Predict Respiratory Virus Growth Kinetics In Vivo
We have recently found striking differences between the growth kinetics of PIV serotypes 1–3 in HAE (Schaap-Nutt et al. 2012). In contrast, PIV1-3 grows with identical kinetics in nonpolarized epithelial cell-lines (HEp-2, LLC-MK2 and VERO cells). Why HAE reveal differences in PIV growth kinetics when epithelial cell-lines do not remains to be determined but requires detailed experimentation in ciliated cells to identify the underlying cause of this phenomenon.
The ability of HAE to discriminate between respiratory virus growth kinetics has also been shown for live, attenuated PIV, RSV, and influenza virus vaccine candidates (Schaap-Nutt et al. 2010; Bartlett et al. 2008a; Wright et al. 2005; Ilyushina et al. 2012). Generally, live, attenuated vaccine candidates exhibit a greater degree of growth attenuation in HAE models than in nonpolarized epithelial cell-lines and the degree of attenuation in HAE more closely mimics that measured in vivo. A human adenoid airway epithelium culture model was used to demonstrate that live, attenuated RSV vaccine growth kinetics in differentiated cultures, but not that in cell-lines, closely resembled the kinetics of vaccine growth in airways of human and nonhuman primates in vivo (Wright et al. 2005). Figure 4 illustrates similar experiments with a live, attenuated PIV2 vaccine that showed decreased virus growth in HAE compared to PIV2 wild-type at 32 °C (the temperature of the nasal cavity) whereas, cell-lines showed no differences in growth kinetics between the PIV2 vaccine and PIV2 wild-type (Schaap-Nutt et al. 2010). The discrepancy between differences in attenuation of the PIV2 vaccine in HAE versus cell-lines was more dramatic when experiments were performed at 37 °C (the temperature of the lower airways). While PIV2 wild-type grew well in HAE at 37 °C, the vaccine candidate was severely attenuated essentially showing zero growth. In contrast to these data in HAE, the vaccine candidate was only modestly attenuated compared to PIV2 wild-type in epithelial cell-lines at 37 °C. The relevance of these in vitro findings was demonstrated when PIV2 wild-type and vaccine candidate growth kinetics were determined in the upper and lower airways of nonhuman primates in vivo where the degree of growth attenuation for the vaccine candidate versus wild-type virus closely mimicked that in HAE but not in epithelial cell-lines. Recently, similar conclusions were reached for influenza virus vaccines where differentiated airway epithelium culture models far better predicted in vivo growth kinetics of vaccine candidates than epithelial cell-lines (Ilyushina et al. 2012).
Fig. 4.
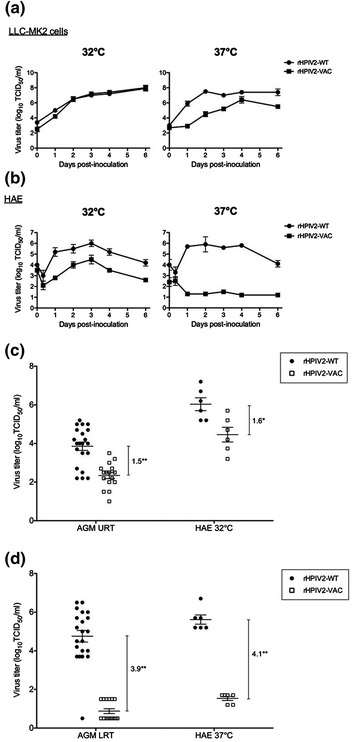
PIV2 vaccine candidate growth kinetics in HAE but not in epithelial cell-lines correlate with growth kinetics in the upper and lower airways of nonhuman primates in vivo. a Growth kinetics of rHPIV2-WT (closed circles) and rHPIV2-VAC (closed squares) in LLC-MK2 cells at 32 and 37 °C. b Single cycle growth curves in HAE inoculated with rHPIV2-WT (closed circles) or rHPIV2-VAC (closed squares) at either 32 or 37 °C. Virus titers were determined in the apical compartments at the indicated times pi. c Titers of rHPIV2-WT and rHPIV2-VAC in the upper respiratory tract (URT) of NHPs and in the apical wash of HAE incubated at 32 °C. d Virus titers in the lower respiratory tract (LRT) of NHPs and in the apical wash of HAE incubated at 37 °C. In c and d, peak virus titer in NHPs or HAEs is indicated by a closed circle (rHPIV2-WT) or open square (rHPIV2-VAC). * indicates statistical significance with P value <0.01, while ** indicates a P value <0.001. Adapted with permission from Schaap-Nutt et al. (2010)
Together, these published data with RSV, PIV, and influenza virus vaccine candidates strongly support the use of human columnar airway epithelium cultures for predicting growth attenuation of live, attenuated respiratory virus vaccine candidates. The capacity to predict in vivo growth attenuation in an appropriate human in vitro culture model will greatly benefit respiratory virus vaccine programs and reduce the number of animals required for initial testing of such vaccines.
The Epithelium is Only One Section of the Orchestra Playing the Immunologic Symphony in the Airways
The airway epithelium plays a central role in identifying, responding to, and resolving respiratory virus infections. The cellular cross-talk between epithelial cells, macrophages, dendritic cells, and infiltrating cells of the immune system is highly complex and ideally results in an appropriate, but not exaggerated, inflammatory response sufficient to clear virus infection. A significant drawback of human airway epithelium culture models for respiratory virus vaccine research is the lack of these other immune-related cell-types in this culture model.
Several groups have begun to develop the next generation of human airway cultures with co-cultures of other cellular components of the lung. Co-cultures of HAE (grown on the upper surface of the semipermeable membrane support) and dendritic cells (grown on the other side of the support) have been used to investigate cellular cross-talk between epithelial and dendritic cells (Ilona Jaspers, personal communication). Similar co-culture HAE models could be developed to include macrophages, fibroblasts, and immune cells normally recruited to the airways in response to infections.
Cultures containing only airway epithelial cells can be useful for assessing the inflammatory profile of a virus infection by measuring inflammatory mediators generated before and after virus infection. Global ‘whole culture’ analysis of secreted inflammatory mediators in culture washes can be performed at the protein level by ELISA or antibody/bead-based technologies. For example, the magnitude and duration of two secreted pro-inflammatory mediators, IL-8 and CXCL10 were measured by Luminex bead-based technology in the serosal media of RSV-infected HAE (Fig. 5). Alternatively, whole culture mRNA gene expression analysis using qRT-PCR provides a cost-effective approach for determining inflammatory responses to virus infections. These commonly used assays for measuring inflammatory mediator profiles measure protein secretion or changes in mRNA expression from whole HAE cultures in which differences in cell-types present, some infected, others not, can complicate interpretation of the data. Unfortunately, in our hands, attempts to isolate ciliated cell populations or RSV-infected ciliated cell populations from HAE by proteolytic dispersion or laser-capture dissection, significantly reduced mRNA integrity and invalidated conclusions drawn from these assays.
Fig. 5.
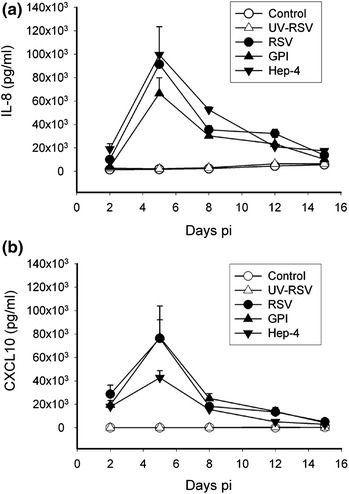
Inflammatory mediator secretion from HAE after infection with RSV. HAE cultures were inoculated with recombinant RSV A2 expressing GFP (RSV, solid circles), recombinant RSV A2 (GPI, solid upright triangles), biologically derived RSV A2 (Hep-4, solid inverted triangles), ultraviolet light-inactivated RSV expressing GFP (UV-RSV, open upright triangles) or mock-inoculate (Control, open circles) and over time samples of serosal media harvested. Luminex bead-based technology was used to detect and measure 23 different pro-inflammatory analytes in the samples. (a) and (b) show concentrations of the pro-inflammatory mediators IL-8 and CXCL10 harvested in the serosal media, respectively. Of the 23 analytes probed only IL-8, CXCL10, IL-6, and RANTES were increased above baseline levels by RSV infection. Analytes probed for but not increased by RSV infection, were: IL-1α/β, IL-2, IL-3, IL-4, IL-5, IL-7, IL-9, IL-10, IL-12p40, IL-13, IL-15, Eotaxin, GM-CSF, IFNγ, IFNα2, TNFα, MCP-1, MIP-1α, and EGF. Data represent n = 5 replicate cultures derived from one donor. NOTE: Extent of inflammatory mediator secretion paralleled RSV titers. At maximal secretion (day 5) despite viral titers being equal there are differences in the extent of IL-8 and CXCL10 induced by the different RSV inoculates. For example, the GPI virus does not induce as much IL-8 as the recombinant expressing GFP or Hep-4. In contrast, for CXCL10 secretion, Hep-4 does not induce as much CXCL10 secretion as the other two RSV inoculates. Since cultures are derived from the same donor, host genetic variability is unlikely to account for these differences. Rather, these differences may be influenced by how host cells respond to subtle differences the virus genome sequences
How Well are Ciliated Airway Epithelial Cells from Different Species Infected by Human RSV?
The development of differentiated columnar airway epithelial cell models from species other than human has allowed studies on species tropism of respiratory viruses. In addition to human cultures, we have also generated tracheal airway ciliated columnar epithelial cell cultures from bat, mouse, hamster, ferret, dog, cow, pig, and nonhuman primate. Although culture conditions need to be tailored for each species we have successfully obtained sufficient ciliated cells from all these species for comparative virus infection studies. Comparative in vitro versus in vivo morphologic studies indicate, depending on the species, that the proportion of ciliated versus nonciliated cells in airway cultures reflects the cellular distribution in the tracheal epithelium in vivo.
The use of differentiated cultures from different species also allows confirmation of the predicted tropism of respiratory viruses for columnar cells (Fig. 6). Using these models, we find human RSV robustly infects human ciliated cells and to a lesser extent, ciliated cells from nonhuman primates. In our hands, RSV poorly infects ciliated cells from hamsters and mice corroborating our in vivo studies in these semipermissive animal models (unpublished observations). Human PIV3 (and PIV1) shows a broader species tropism in vitro with robust infection of human, nonhuman primate, and hamster ciliated cells. These observations match the expected tropism of human PIV3 for humans, NHPs, and hamsters in vivo. While human PIVs only modestly infect mouse ciliated cells, Sendai virus (mouse PIV1) shows broad species tropism for ciliated cells robustly infecting cells in mouse, hamster, NHP, and human cultures.
Fig. 6.
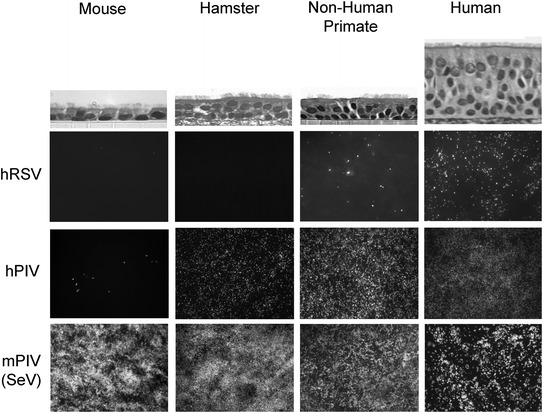
In vitro models of ciliated tracheobronchial airway epithelium derived from multiple species for investigating respiratory virus tropism. (Top row) Histological sections of cultures of ciliated airway epithelium derived from cells isolated from the trachea of mouse, hamster, nonhuman primates, and humans. Ciliated and nonciliated cells are present in cultures from all species tested. (Lower panels) En face views of cultures of ciliated airway epithelium for each species inoculated with recombinant, GFP expressing, human RSV (hRSV), human PIV3 (hPIV), or mouse PIV1/Sendai virus (mPIV1/SeV). Infected cells were identified 3 days pi by monitoring GFP expression (white signal). NOTE: RSV infects human ciliated cells well and nonhuman primate cells modestly but does not infect hamster or mouse ciliated cells. In contrast, Sendai virus (SeV) robustly infects ciliated cells from all species tested. Human PIV3 infects human, nonhuman primate, and hamster ciliated cells well but poorly infects mouse ciliated cells. These in vitro data correlate with the known in vivo tropism of SeV for airways of all these species and for hPIV3 for human, nonhuman primates, and hamster airways
Conclusions and Future Directions
Human ciliated airway epithelium cultures closely resemble the morphologic and physiologic characteristics of the human ciliated airway epithelium in vivo; the primary infection site of many respiratory viruses including RSV. Existing evidence already indicates differentiated airway epithelium culture models more accurately predict in vivo virus-host airway cell interactions, virus growth kinetics and consequences of infection than nonpolarized epithelial cell-lines. The ability to compare virus infection in ciliated airway epithelium in vitro versus in vivo, e.g., for PIV infection of hamster airways, will strengthen the concept that airway cultures can be used as a precursor and/or surrogate model for in vivo studies. For RSV, direct comparison between infection of HAE in vitro versus in vivo is a significant challenge due to the inability to perform detailed experiments on humans especially in lower airway regions. Therefore, more emphasis should be placed on correlating the consequences of RSV infection of human airway epithelium culture models to histopathologic findings in lower airways of patients naturally infected by RSV.
Acknowledgments
The author thanks Drs. Peter Collins and Mark Peeples for many years of enjoyable and fruitful collaboration and for teaching a cell biologist about the wonders of respiratory viruses. Thanks also to members of the laboratory who contributed to the thoughts and experiments discussed here, in particular, Drs. Liqun Zhang, Meg Hennessey, Racheal Liesman. I would also like to thank the Directors and teams of the UNC Cystic Fibrosis Center Tissue Culture Core, the UNC Morphology and Morphometry Core, and the Michael Hooker Microscopy Facility for supplying reagents and technical expertise. Portions of these studies were funded by grants from the UNC University Research Council, the Cystic Fibrosis Foundation, and the National Institutes of Health (R01HL103940, R01 HL77844, P50HL084934).
Contributor Information
Larry J. Anderson, Email: larry.anderson@emory.edu
Barney S. Graham, Email: bgraham@mail.nih.gov
Raymond J. Pickles, Email: raymond_pickles@med.unc.edu
References
- Bartlett EJ, Cruz AM, Esker J, Castano A, Schomacker H, Surman SR, Hennessey M, Boonyaratanakornkit J, Pickles RJ, Collins PL, Murphy BR, Schmidt AC. Human parainfluenza virus type 1 C proteins are nonessential proteins that inhibit the host interferon and apoptotic responses and are required for efficient replication in nonhuman primates. J Virol. 2008;82(18):8965–8977. doi: 10.1128/JVI.00853-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett EJ, Hennessey M, Skiadopoulos MH, Schmidt AC, Collins PL, Murphy BR, Pickles RJ. Role of interferon in the replication of human parainfluenza virus type 1 wild type and mutant viruses in human ciliated airway epithelium. J Virol. 2008;82(16):8059–8070. doi: 10.1128/JVI.02263-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bove PF, Grubb BR, Okada SF, Ribeiro CM, Rogers TD, Randell SH, O’Neal WK, Boucher RC. Human alveolar type II cells secrete and absorb liquid in response to local nucleotide signaling. J Biol Chem. 2011;285(45):34939–34949. doi: 10.1074/jbc.M110.162933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button B, Cai LH, Ehre C, Kesimer M, Hill DB, Sheehan JK, Boucher RC, Rubinstein M. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science. 2012;337(6097):937–941. doi: 10.1126/science.1223012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins PL. O glycosylation of glycoprotein G of human respiratory syncytial virus is specified within the divergent ectodomain. J Virol. 1990;64(8):4007–4012. doi: 10.1128/jvi.64.8.4007-4012.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-differentiated human airway epithelial cell cultures. Methods Mol Med. 2005;107:183–206. doi: 10.1385/1-59259-861-7:183. [DOI] [PubMed] [Google Scholar]
- Fulcher ML, Gabriel SE, Olsen JC, Tatreau JR, Gentzsch M, Livanos E, Saavedra MT, Salmon P, Randell SH. Novel human bronchial epithelial cell lines for cystic fibrosis research. Am J Physiol Lung Cell Mol Physiol. 2009;296(1):L82–L91. doi: 10.1152/ajplung.90314.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkema J, Mariassy A, St. George J, Hyde DM, Plopper CG (1994) Epithelial cells of the conducting airways: a species comparison. In: Farmer SG, Hay DWP (eds) The airway epithelium, vol 55. Lung Biology in Health and Disease. Marcel Dekker, New York, pp 3–39
- Ilyushina NA, Ikizler MR, Kawaoka Y, Rudenko LG, Treanor JJ, Subbarao K, Wright PF (2012) Comparative study of influenza virus replication in MDCK cells and in primary cells derived from adenoids and airway epithelium. J Virol [DOI] [PMC free article] [PubMed]
- Johnson JE, Gonzales RA, Olson SJ, Wright PF, Graham BS. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod Pathol. 2007;20(1):108–119. doi: 10.1038/modpathol.3800725. [DOI] [PubMed] [Google Scholar]
- Karron RA, Buonagurio DA, Georgiu AF, Whitehead SS, Adamus JE, Clements-Mann ML, Harris DO, Randolph VB, Udem SA, Murphy BR, Sidhu MS. Respiratory syncytial virus (RSV) SH and G proteins are not essential for viral replication in vitro: clinical evaluation and molecular characterization of a cold-passaged, attenuated RSV subgroup B mutant. Proc Natl Acad Sci USA. 1997;94(25):13961–13966. doi: 10.1073/pnas.94.25.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesimer M, Ehre C, Burns KA, Davis CW, Sheehan JK, Pickles RJ (2012) Molecular organization of the mucins and glycocalyx underlying mucus transport over mucosal surfaces of the airways. Mucosal Immunol [DOI] [PMC free article] [PubMed]
- Kesimer M, Kirkham S, Pickles RJ, Henderson AG, Alexis NE, Demaria G, Knight D, Thornton DJ, Sheehan JK. Tracheobronchial air-liquid interface cell culture: a model for innate mucosal defense of the upper airways? Am J Physiol Lung Cell Mol Physiol. 2009;296(1):L92–L100. doi: 10.1152/ajplung.90388.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwilas S, Liesman RM, Zhang L, Walsh E, Pickles RJ, Peeples ME. Respiratory syncytial virus grown in Vero cells contains a truncated attachment protein that alters its infectivity and dependence on glycosaminoglycans. J Virol. 2009;83(20):10710–10718. doi: 10.1128/JVI.00986-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrosovich MN, Matrosovich TY, Gray T, Roberts NA, Klenk HD. Human and avian influenza viruses target different cell types in cultures of human airway epithelium. Proc Natl Acad Sci USA. 2004;101(13):4620–4624. doi: 10.1073/pnas.0308001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson KA, Yunis EJ. Demonstration of respiratory syncytial virus in an autopsy series. Pediatr Pathol. 1990;10(4):491–502. doi: 10.3109/15513819009067138. [DOI] [PubMed] [Google Scholar]
- Pickles RJ, McCarty D, Matsui H, Hart PJ, Randell SH, Boucher RC. Limited entry of adenovirus vectors into well-differentiated airway epithelium is responsible for inefficient gene transfer. J Virol. 1998;72(7):6014–6023. doi: 10.1128/jvi.72.7.6014-6023.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaap-Nutt A, Scull MA, Schmidt AC, Murphy BR, Pickles RJ. Growth restriction of an experimental live attenuated human parainfluenza virus type 2 vaccine in human ciliated airway epithelium in vitro parallels attenuation in African green monkeys. Vaccine. 2010;28(15):2788–2798. doi: 10.1016/j.vaccine.2010.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaap-Nutt A, Liesman R, Bartlett EJ, Scull MA, Collins PL, Pickles RJ, Schmidt AC. Human parainfluenza virus serotypes differ in their kinetics of replication and cytokine secretion in human tracheobronchial airway epithelium. Virology. 2012;433(2):320–328. doi: 10.1016/j.virol.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scull MA, Gillim-Ross L, Santos C, Roberts KL, Bordonali E, Subbarao K, Barclay WS, Pickles RJ. Avian Influenza virus glycoproteins restrict virus replication and spread through human airway epithelium at temperatures of the proximal airways. PLoS Pathog. 2009;5(5):e1000424. doi: 10.1371/journal.ppat.1000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims AC, Baric RS, Yount B, Burkett SE, Collins PL, Pickles RJ. Severe acute respiratory syndrome coronavirus infection of human ciliated airway epithelia: role of ciliated cells in viral spread in the conducting airways of the lungs. J Virol. 2005;79(24):15511–15524. doi: 10.1128/JVI.79.24.15511-15524.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims AC, Yount B, Burkett SE, Baric RS, Pickles RJ. SARS CoV replication and pathogenesis in human airway epithelial cultures. Adv Exp Med Biol. 2006;581:535–538. doi: 10.1007/978-0-387-33012-9_97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stonebraker JR, Wagner D, Lefensty RW, Burns K, Gendler SJ, Bergelson JM, Boucher RC, O’Neal WK, Pickles RJ. Glycocalyx restricts adenoviral vector access to apical receptors expressed on respiratory epithelium in vitro and in vivo: role for tethered mucins as barriers to lumenal infection. J Virol. 2004;78(24):13755–13768. doi: 10.1128/JVI.78.24.13755-13768.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villenave R, Thavagnanam S, Sarlang S, Parker J, Douglas I, Skibinski G, Heaney LG, McKaigue JP, Coyle PV, Shields MD, Power UF. In vitro modeling of respiratory syncytial virus infection of pediatric bronchial epithelium, the primary target of infection in vivo. Proc Natl Acad Sci USA. 2012;109(13):5040–5045. doi: 10.1073/pnas.1110203109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welliver TP, Reed JL, Welliver RC., Sr Respiratory syncytial virus and influenza virus infections: observations from tissues of fatal infant cases. Pediatr Infect Dis J. 2008;27(10 Suppl):S92–S96. doi: 10.1097/INF.0b013e318168b706. [DOI] [PubMed] [Google Scholar]
- Wright PF, Ikizler MR, Gonzales RA, Carroll KN, Johnson JE, Werkhaven JA. Growth of respiratory syncytial virus in primary epithelial cells from the human respiratory tract. J Virol. 2005;79(13):8651–8654. doi: 10.1128/JVI.79.13.8651-8654.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabner J, Seiler M, Walters R, Kotin RM, Fulgeras W, Davidson BL, Chiorini JA. Adeno-associated virus type 5 (AAV5) but not AAV2 binds to the apical surfaces of airway epithelia and facilitates gene transfer. J Virol. 2000;74(8):3852–3858. doi: 10.1128/JVI.74.8.3852-3858.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Bukreyev A, Thompson CI, Watson B, Peeples ME, Collins PL, Pickles RJ. Infection of ciliated cells by human parainfluenza virus type 3 in an in vitro model of human airway epithelium. J Virol. 2005;79(2):1113–1124. doi: 10.1128/JVI.79.2.1113-1124.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Button B, Gabriel SE, Burkett S, Yan Y, Skiadopoulos MH, Dang YL, Vogel LN, McKay T, Mengos A, Boucher RC, Collins PL, Pickles RJ. CFTR delivery to 25 % of surface epithelial cells restores normal rates of mucus transport to human cystic fibrosis airway epithelium. PLoS Biol. 2009;7(7):e1000155. doi: 10.1371/journal.pbio.1000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Collins PL, Lamb RA, Pickles RJ. Comparison of differing cytopathic effects in human airway epithelium of parainfluenza virus 5 (W3A), parainfluenza virus type 3, and respiratory syncytial virus. Virology. 2012;421(1):67–77. doi: 10.1016/j.virol.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Peeples ME, Boucher RC, Collins PL, Pickles RJ. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J Virol. 2002;76(11):5654–5666. doi: 10.1128/JVI.76.11.5654-5666.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


